Abstract
Aim
Semaglutide is a long-acting glucagon-like peptide-1 receptor agonist (GLP-1 RA) indicated for glycaemic management in adults with type 2 diabetes (T2D). Oral semaglutide administration can help decrease glycated haemoglobin (HbA1c) and body weight in people with uncontrolled T2D. We evaluated the efficacy and safety of oral semaglutide compared to that of subcutaneous semaglutide, placebo, and other GLP-1 RAs in the treatment of T2D.
Methods
Randomised controlled trials of subcutaneous and oral semaglutide for glycaemic control in adults with T2D were selected from the Cochrane Central Register of Controlled Trials and PubMed. Mean differences (MDs) and risk ratios with 95% confidence intervals (CIs) were used to synthesise the results, and oral and subcutaneous semaglutide formulations were indirectly compared using mixed treatment comparisons.
Results
Twelve studies were included in this review (6840 participants). Oral semaglutide (14.0 mg) significantly reduced HbA1c (MD, −1.30% [95%CI: -1.44, −1.16], P < 0.05) and body weight (MD, −3.17 kg [95%CI: -3.89, −2.45], P < 0.05) compared to placebo (MD, HbA1c: -0.32% [95%CI: -0.49, −0.15], P < 0.05; MD body weight: -2.56 kg [95%CI: -3.41, −1.71], P < 0.05), liraglutide (1.2 mg), exenatide ER (2.0 mg), and dulaglutide (1.5 mg). Oral semaglutide was slightly less effective than subcutaneous semaglutide in reducing HbA1c levels (MD: -0.26% [95%CI: -0.44, −0.07], P < 0.05) and body weight (MD: -1.08 kg [95%CI: -2.04, −0.12], P < 0.05). Oral semaglutide increased the incidence of adverse events (nausea, diarrhoea, dyspepsia, and vomiting) compared to placebo, liraglutide (1.2 mg), exenatide (ER, 2.0 mg), and dulaglutide 1.5 mg but not compared to subcutaneous semaglutide.
Conclusion
Oral semaglutide was non-inferior to subcutaneous semaglutide and superior to placebo and another GLP-1 RA in reducing HbA1c and body weight. It was superior to subcutaneous semaglutide and inferior to other GLP-1 RA comparators and placebo in terms of the incidence of adverse events. Thus, oral semaglutide provides a convenient administration route for patients who prefer oral treatments over injectable therapies.
Keywords: Semaglutide, GLP-1, Type 2 diabetes, Subcutaneous injection, HbA1c, Glycaemic control
Nomenclature
- AEs
adverse events
- CI
confidence interval
- GLP-1 RA
glucagon-like peptide-1 receptor agonist
- GRADE
Grading of Recommendations, Assessment, Development, and Evaluation
- MD
mean difference
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- SC
subcutaneous
- T2D
type 2 diabetes.
1. Introduction
The prevalence of type 2 diabetes (T2D) is increasing worldwide in correspondence to rising obesity and a sedentary lifestyle [1,2]. However, in most cases, diet and exercise fail to achieve long-term glycaemic control in people with T2D [3], thus necessitating individualised treatment approaches, such as pharmacotherapy [4,5]. A wide variety of therapies are currently available for the management of T2D, including oral (e.g. metformin and sulphonylurea) and injectable (e.g. insulin) drugs. However, many patients still struggle to achieve optimal glycaemic control [5]. A new therapy that can reduce the risk of both hypoglycaemia and weight gain is necessary [4].
Studies suggest that in addition to glycaemic control, the optimal treatment approach for T2D should include preventing weight gain and hypoglycaemic episodes and minimising the risk of cardiovascular disease events [6]. Patients with T2D should avoid weight gain and preferably lose weight, as weight loss has a beneficial effect on glycaemic control. Even moderate weight loss (5%–7% of body weight) is known to improve the control of blood glucose and reduce the risk of developing cardiovascular disease [7]. However, weight loss is particularly challenging for people with T2D treated with insulin or sulphonylurea [8].
Unlike many other medications for the management of T2D, glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are recommended for adults with T2D because of their beneficial effects, including enhanced glucose-dependent insulin secretion, inhibition of gastric emptying, and decreased appetite and calorie consumption [[9], [10], [11]]. However, they also increase the probability of gastrointestinal adverse events (AEs) such as nausea, vomiting, diarrhoea, and abdominal pain. Notwithstanding, GLP-1 RAs carry a low risk of hypoglycaemia with significant improvement in cardiovascular outcomes [12] and the potential to reduce glycated haemoglobin (HbA1c) and body weight [13]. GLP-1 RAs are recommended by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes as the first-line injectable therapy for adults with T2D with cardiovascular disease because of their effectiveness in HbA1c reduction and their cardiovascular benefits; they are not recommended in the presence of extreme and symptomatic hyperglycaemia [6].
Semaglutide (Novo Nordisk A/S, Bagsværd, Denmark) is a long-acting GLP-1 RA for the treatment of T2D in subcutaneous (SC) form, used once weekly. The efficiency and safety of SC semaglutide (0.5 mg and 1.0 mg) have been investigated in the Semaglutide Unabated Sustainability (SUSTAIN) trials, which showed significant reductions in HbA1c and body weight compared to placebo and a variety of active comparators, including other GLP-1 RAs (liraglutide, exenatide, exenatide release [ER], and dulaglutide) [[14], [15], [16], [17], [18], [19], [20], [21], [22], [23]]. Furthermore, SC semaglutide significantly reduced the risk of adverse cardiovascular events compared to placebo in adults with T2D [22].
Once-daily administration of liraglutide has been shown to be more effective than that of dulaglutide and albiglutide in promoting weight loss [24,25]. However, the next-generation GLP-1 RAs are mainly designed for once-weekly use [26]. Semaglutide was developed from a modified liraglutide profile by attaching long chain fatty acids to peptides to increase the half-life from 13 to 15 h (liraglutide) to 165 h to increase the effectiveness and suitability of semaglutide when used once weekly [26,27]. GLP-1 RAs are generally administered subcutaneously because of their extremely low bioavailability when administered orally, which leads to inadequate absorption through the gastrointestinal mucosa. An oral semaglutide form was developed and formulated with the absorption enhancer sodium N-(8-[2-hydroxylbenzoyl] amino) caprylate to enhance the passage of semaglutide through the gastric epithelial tissue and protect semaglutide from degradation [28]. Recently, a number of randomised controlled trials (RCTs) have examined the efficiency and safety of oral semaglutide in terms of reducing HbA1c and body weight in individuals with T2D as reported by the Peptide Innovation for Early Diabetes Treatment (PIONEER) studies [29,35]. However, there is lack of a comprehensive comparison between SC semaglutide and oral semaglutide. Therefore, we conducted a systematic review and meta-analysis including subgroup analysis of all RCTs that met our inclusion criteria to investigate the efficacy and safety of oral semaglutide compared to those of SC semaglutide, placebo, and other GLP-1 RA comparators (liraglutide (1.2 mg), exenatide ER (2.0 mg), and dulaglutide (1.5 mg)) for glycaemic control in adults with T2D.
2. Methodology
We performed a systematic review to identify RCTs of SC semaglutide and oral semaglutide for glycaemic control in adults with T2D using the Cochrane Central Register of Controlled Trials and the PubMed database from 2010 to February 2021. The following keywords were applied in the PubMed search ((‘semaglutide’ [Supplementary Concept] OR ‘oral semaglutide’[tw] OR ‘PIONEER’[tw]) AND (‘semaglutide’ [Supplementary Concept] OR ‘subcutaneous semaglutide’[tw] OR ‘SUSTAIN’[tw])) AND (‘Diabetes Mellitus, Type 2’[Mesh] OR ‘Diabetes Mellitus, Noninsulin-Dependent’[tw] OR ‘Diabetes Mellitus, Type II’[tw] OR ‘NIDDM’[tw] OR ‘Type 2 Diabetes’[tw]). The keywords (‘semaglutide PIONEER’ OR ‘semaglutide SUSTAIN’ AND ‘type 2 diabetes’) were used to search the Cochrane Central Register of Controlled Trials. The primary researcher undertook the initial search. The second researcher reviewed the selected papers following the title and abstract screen to determine whether they met the inclusion criteria. Any disagreement was resolved following discussion. The primary researcher led on the data extraction but with discussions with the second researcher.
The inclusion criteria were: 1) RCTs written in English that evaluated the use of once-weekly SC semaglutide or oral semaglutide compared to placebo or another GLP-1 RA comparator; 2) recruited adults with T2D (>18 years old); 3) RCTs with a duration of ≥20 weeks reporting one or more of the following outcomes: change from baseline HbA1c, body weight, AEs, and hypoglycaemic episodes. No restriction was applied to the background therapy, and no sex, race, or socioeconomic status restriction was placed. Given the lack of head-to-head trials comparing the efficacy of SC semaglutide versus oral semaglutide, we selected studies that used a placebo group or another GLP-1 RA comparator. Therefore, indirect comparison methods were used, such as direct comparison against a naïve state and adjusted indirect comparison with a common comparator. The rationale for the duration restriction of 20 weeks was the time required for the antidiabetic medication to have its full effect on HbA1c [33].
The exclusion criteria were: 1) RCTs that used a daily SC semaglutide intervention, 2) RCTs that compared semaglutide with another non-GLP-1 RA comparator without a pure control group (placebo group), 3) studies <20 weeks in duration, 4) studies that used unapproved doses of semaglutide, i.e. only 3, 7, and 14 mg for oral semaglutide and 0.5 and 1.0 mg for SC semaglutide.
2.1. Data extraction, quality assessment, and quality of evidence
The following data were recorded: study name, year of publication, intervention in each arm, study duration, background therapy, number of participants, mean age, mean diabetes duration, AEs, and the baseline mean HbA1c and body weight.
The primary outcomes were:
-
⁃
DCCT Unit (%) of change in HbA1c
The secondary outcomes were:
-
⁃
Change in weight (kg)
-
⁃
AEs
Quality assessment: The Cochrane Collaboration tool [34] was used to assess the quality of the included studies. Risk of bias was assessed in terms of random sequence generation and allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases. Every possible source of bias was rated as having a high, low, or unclear risk of bias. The domain was graded as involving unclear risk of bias if it could not be identified because of insufficient information.
Quality of evidence: To evaluate the quality of evidence, the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach was used [35]. The RCTs started with high quality of evidence, and, in this review, the quality of evidence was downgraded based on the risk of bias and high heterogeneity across studies in some of the outcomes (Table 1) (Appendix).
2.2. Statistical analysis
Statistical analysis was performed using the Review Manager software (RevMan v.5.4.1, Cochrane Collaboration, Oxford, UK), and data were pooled using mean differences (MDs) for continuous outcome variables and risk ratios (RRs) for dichotomous data with 95% confidence intervals (95% CIs) for standard meta-analysis and subgroup meta-analysis. A random-effect model was used to pool the data. Heterogeneity was measured using the I2 test and considered low if I2 was ≤25%, moderate if I2 > 25 to <75%, or high if I2 ≥ 75%. Because of different doses of semaglutide in both forms (SC and oral) in the experimental groups, subgroup analysis was performed only with placebo. Only the maximum dose was compared to the GLP-1 RA comparator because of the lack of data in some studies. A network meta-analysis was performed to indirectly compare oral semaglutide and SC semaglutide using the Stata SE 16.1 software (StataCorp LLC. 2019. Stata Statistical Software: Release 16. College Station, TX, USA) with mvmeta, a frequentist setting, using multivariate meta-analysis as described by White et al. [36] and White [37].
3. Results
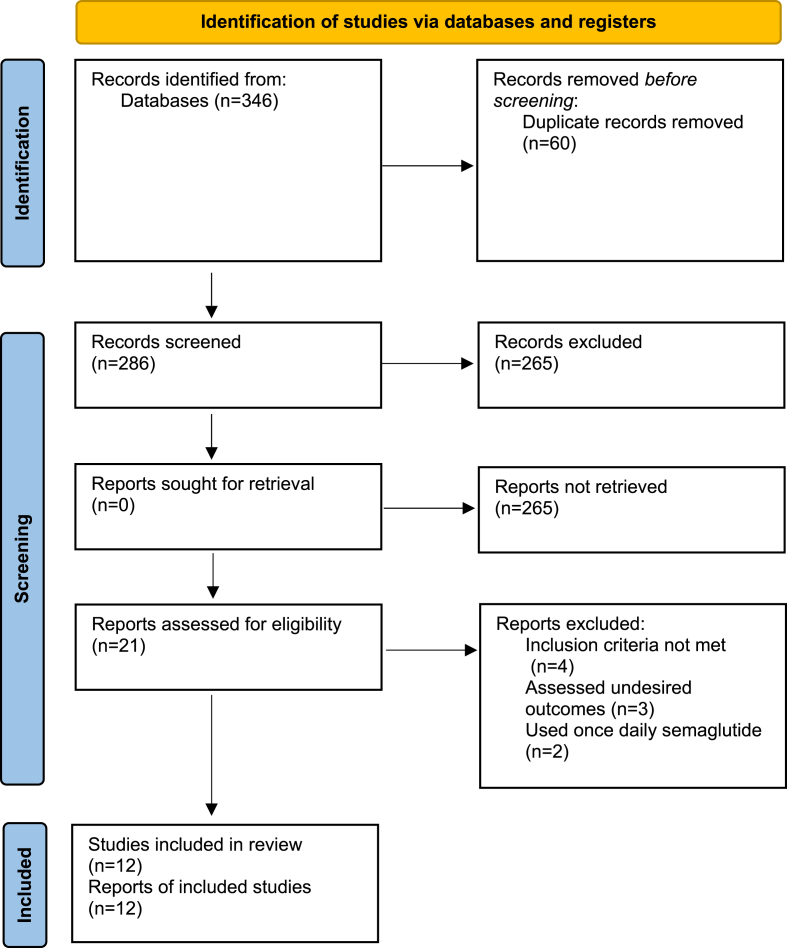
Twelve RCTs met the inclusion criteria and were included in this review (6840 participants; 3854 men, 2986 women). After applying the keywords in the databases, 346 articles were retrieved (179 from PubMed and 167 from Cochrane). After removing duplicates, 286 studies remained. After screening the title and applying filters, 21 records were screened for eligibility, and 9 articles were excluded, as the inclusion criteria were not met (4), assessed undesired outcomes (3), or used SC semaglutide once daily (2). The details of the PRISMA flowchart are presented in Fig. 1.
Fig. 1.
Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement [62].
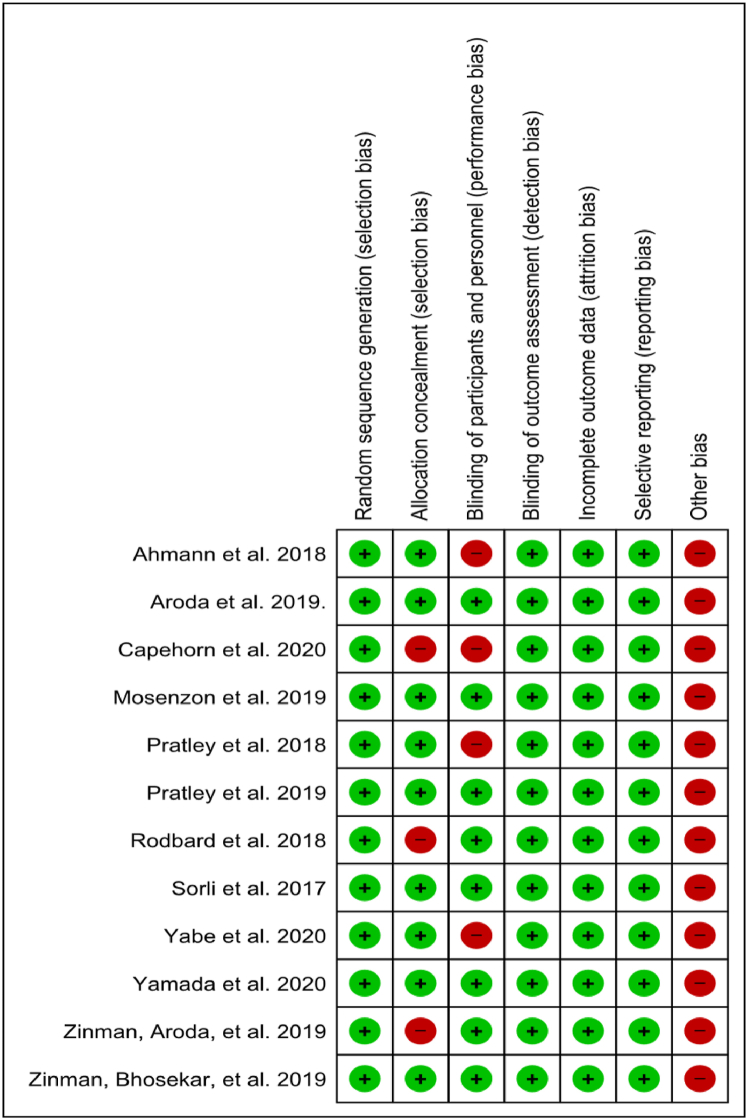
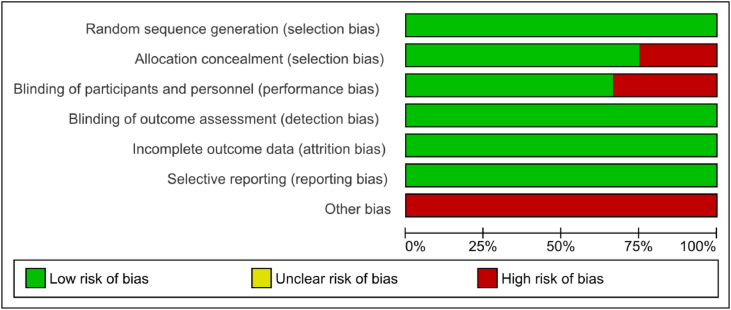
Of the included studies, six were judged to have a low risk of bias [14,20,29,30,32,38] and six were judged as having a high risk of bias due to issues in allocation concealment [18,23,39] and blinding of participants and personnel [16,19,23,31]. Other sources of bias were gauged as high in all included studies because all trials were funded by the drug manufacturer. Fig. 2 shows the risk of bias summary, and Fig. 3 shows the risk of bias graph.
Fig. 2.
Risk of bias summary of included studies.
Fig. 3.
Risk of bias graph of included studies, presented as percentages across all included studies.
Six studies compared SC semaglutide to placebo [14,18,20] or to another GLP-1 RA comparator [16,19,23] and six compared oral semaglutide with placebo [29,30,32,38,39] or another GLP-1 RA comparator [30,31,38]. Across the studies, the mean body mass index ranged from 26.2 to 33.8 kg/m2, the mean weight from 77.1 to 96.9 kg, and mean diabetes duration, from 3.5 to 15 years. The studies differed in terms of background medication, namely no glucose-lowering medication, metformin alone, sulfonylurea with or without metformin, basal insulin with or without metformin, and SGLT2 inhibitor with or without sulphonylurea or metformin. A summary of the included studies is presented in Table 1.
Table 1.
Summary of study characteristics.
| Study ID | Study duration (weeks) | Background therapy | Study arms | Number of participants (n) | Mean HbA1c (%) | Mean diabetes duration (years) | Mean age (years) |
|---|---|---|---|---|---|---|---|
| Ahmann et al., 2018 (SUSTAIN 3) [16] | 56 | Metformin and/or thiazolidinediones, and/or sulphonylureas | Semaglutide 1.0 mg (n = 404) Exenatide ER 2.0 mg (n = 405) |
809 | 8.3 ± 1.3 | 9.2 ± 5.5 | 56.6 ± 12.6 |
| Aroda et al., 2019 (PIONEER 1) [29] | 26 | Diet and exercise | Oral semaglutide 3 mg (n = 175) Oral semaglutide 7 mg (n = 175) Oral semaglutide 14 mg (n = 175) Placebo (n = 178) |
703 | 8.0 ± 0.7 | 3.5 ± 4.9 | 55 ± 11 |
| Capehorn et al., 2020 (SUSTAIN 10) [23] | 30 | SGLT-2 inhibitor as monotherapy or with sulphonylurea or metformin | Semaglutide 1.0 mg (n = 290) Liraglutide 1.2 mg (n = 287) |
577 | 8.2 ± 1.0 | 9.3 ± 5.9 | 59.5 ± 10.2 |
| Mosenzon et al., 2019 (PIONEER 5) [32] | 26 | Metformin, or sulphonylurea, or both, or basal insulin with or without metformin | Oral semaglutide 14 mg (n = 163) Placebo (n = 161) |
324 | 8.0 ± 0.7 | 14 ± 8 | 70 ± 8 |
| Pratley et al., 2018 (SUSTAIN 7) [19] | 40 | Metformin monotherapy | Semaglutide 0.5 mg (n = 301) Dulaglutide 0.75 mg (n = 299) Semaglutide 1.0 mg (n = 300) Dulaglutide 1.5 mg (n = 299) |
1199 | 8.2 ± 0.9 | 7.3 ± 5.6 | 55.6 ± 10.6 |
| Pratley et al., 2019 (PIONEER 4) [38] | 52 | Metformin with or without SGLT2 | Oral semaglutide 14 mg (n = 285) Liraglutide 1.8 mg (n = 284) Placebo (n = 142) |
711 | 8.0 ± 0.7 | 7.6 ± 5.5 | 56 ± 10 |
| Rodbard et al., 2018 (SUSTAIN 5) [18] | 30 | Basal insulin with or without metformin | Semaglutide 0.5 mg (n = 132) Semaglutide 1.0 mg (n = 131) Placebo (n = 133) |
396 | 8.4 ± 1.02 | 13.3 ± 7.6 | 58.8 ± 13.1 |
| Sorli et al., 2017 (SUSTAIN 1) [14] | 30 | diet and exercise | 0.5 mg semaglutide (n = 128) 1.0 mg semaglutide (n = 130) Placebo (n = 129) |
387 | 8.05 ± 0.85 | 4.18 ± 5.52 | 53.7 ± 11.3 |
| Yabe et al., 2020 (PIONEER 10) [31] | 52 | Sulphonylurea, glinide, thiazolidinedione, alpha-glucosidase inhibitor, or SGLT2 inhibitor | Oral semaglutide 3 mg (n = 131) Oral semaglutide 7 mg (n = 132) Oral semaglutide 14 mg (n = 130) Dulaglutide 0.75 mg (n = 65) |
458 | 8.3 ± 0.9 | 9.4 ± 6.3 | 58 ± 10 |
| Yamada et al., 2020 (PIONEER 9) [30] | 52 | Diet, exercise, or oral glucose-lowering drug monotherapy | Oral semaglutide 3 mg (n = 49) Oral semaglutide 7 mg (n = 49) Oral semaglutide 14 mg (n = 48) Placebo (n = 49) Liraglutide 0.9 mg (n = 48) |
243 | 8.3 ± 0.8 | 7.5 ± 5.6 | 59.4 ± 9.4 |
| Zinman et al., 2019a (PIONEER 8) [39] | 52 | Insulin with or without metformin | Oral semaglutide 3 mg (n = 184) Oral semaglutide 7 mg (n = 182) Oral semaglutide 14 mg (n = 181) Placebo (n = 184) |
731 | 8.2 ± 0.7 | 15.0 ± 8.1 | 61 ± 10 |
| Zinman et al., 2019b (SUSTAIN 9) [20] | 30 | SGLT-2 inhibitor as monotherapy or with sulphonylurea or metformin | Semaglutide 1.0 mg (n = 151) Placebo (n = 151) |
302 | 8.0 ± 0.8 | 9.7 ± 6.1 | 57.0 ± 9.5 |
3.1. Change in HbA1c
3.1.1. The SUSTAIN programme
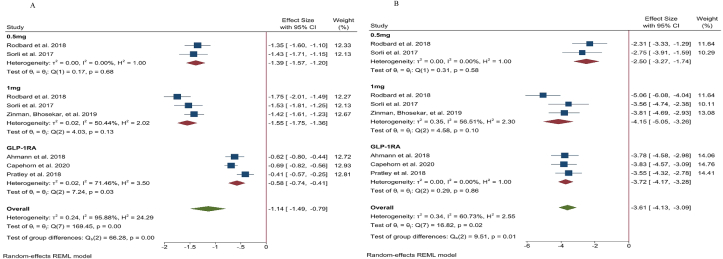
Three studies reported a difference in the % change in HbA1c between SC semaglutide and placebo [14,18,20] and three studies reported a difference between SC semaglutide and GLP-1 RA comparators [16,19,23]. The results for each study are presented in Table 2 (Appendix). Pooled data from these studies showed that 0.5 mg and 1.0 mg SC semaglutide led to a greater reduction in HbA1c (MD: -1.39 [95% CI: -1.57, −1.20], I2=0%, P < 0.00001; MD: -1.55% [95% CI: -1.75, −1.36], I2 = 50%, P < 0.00001, respectively) compared to placebo. SC semaglutide (1.0 mg) also significantly reduced HbA1c compared to another GLP-1 RA comparator (liraglutide [1.2 mg], exenatide ER [2.0 mg], and dulaglutide [1.5 mg]) (MD: -0.58% [95% CI: -0.75, −0.41], I2 = 71%, P < 0.00001) (Fig. 1A) (Appendix).
3.1.2. The PIONEER programme
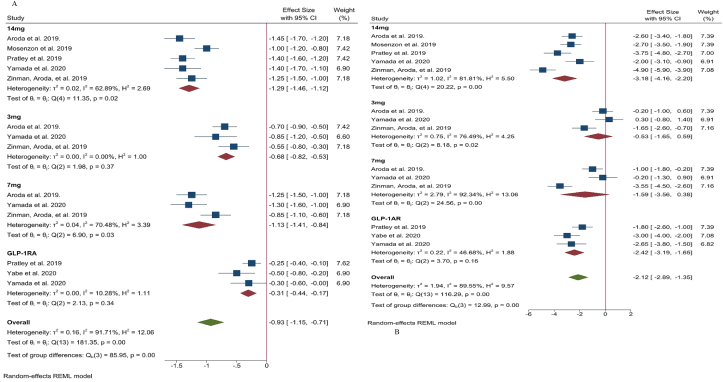
Five studies reported changes in HbA1c caused by oral semaglutide vs placebo [29,30,32,38,39] and three reported changes in HbA1c caused by oral semaglutide vs GLP-1 RA comparators [30,31,38]. The data for each study are presented in Table 3 (Appendix). The pooled analysis suggested that 3.0 mg, 7.0 mg, and 14.0 mg oral semaglutide led to greater reductions in HbA1c (MD: -0.68% [95% CI: -0.82, −0.53], I2 = 0%, P < 0.00001; MD: -1.13% [95% CI: -1.41, −0.84], I2 = 70%, P < 0.00001; MD: -1.29% [95% CI: -1.46, −1.12], I2 = 63%, P < 0.00001, respectively) compared to placebo. Moreover, 14.0 mg oral semaglutide was superior to another GLP-1 RA comparator (liraglutide [1.8 mg] and dulaglutide [0.75 mg]) in terms of reducing HbA1c (MD: -0.31% [95% CI: -0.44, −0.17], I2 = 10%, P < 0.0001) (Fig. 2A) (Appendix).
3.2. Bodyweight
3.2.1. The SUSTAIN programme
Information regarding body weight change was reported in six studies as shown in Table 2 (Appendix). Three studies compared SC semaglutide with placebo [14,18,20] and three studies compared SC semaglutide to another GLP-1 RA comparator [16,19,23]. Treatment with 0.5 mg and 1.0 mg SC semaglutide reduced bodyweight (MD: -2.50 kg [95% CI: -3.27, −1.74], I2 = 0%, P < 0.00001; MD: -4.15 kg [95% CI: -5.05, −3.26], I2 = 65%, P < 0.00001, respectively) compared to placebo as well as another GLP-1 RA comparator (MD: -3.72 kg [95% CI: -4.17, −3.28], I2 = 0%, P < 0.00001) (Fig. 2B) (Appendix).
3.2.2. The PIONEER programme
Six studies reported body weight changes from baseline as shown in Table 3 (Appendix). Five studies reported change in body weight between oral semaglutide and placebo [29,30,32,38,39] and three reported the difference compared to GLP-1 RA [30,31,38]. Oral semaglutide 3.0 mg, 7.0 mg, and 14.0 mg reduced bodyweight (MD: -0.53 kg [95% CI: -1.65, 0.59], I2 = 76%, P < 0.35; MD: -1.59 kg [95% CI: -3.56, 0.38], I2 = 92%, P < 0.1; MD: -3.18 kg [95% CI: -4.16, −2.20], I2 = 82%, P < 0.00001, respectively) compared to placebo. Moreover, 14.0 mg oral semaglutide was superior to another GLP-1 RA comparator in reducing body weight (MD: -2.42 kg [95% CI: -3.19, −1.65], I2 = 47%, P < 0.00001) (Fig. 2B) (Appendix).
3.3. Adverse events
Whilst total AEs reported are compared in this study, gastrointestinal side effects including nausea, diarrhoea, dyspepsia, and vomiting, were the most common AEs observed for both forms of semaglutide.
3.3.1. The SUSTAIN programme
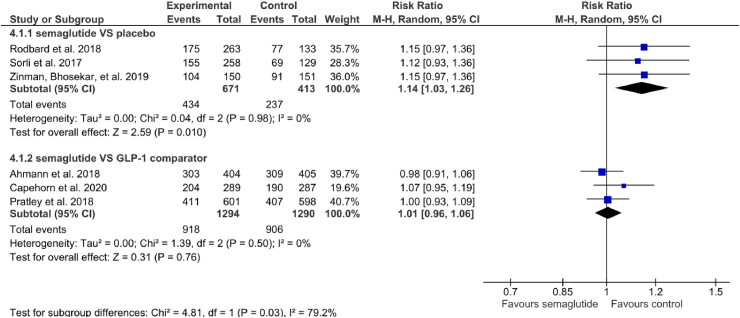
In terms of AEs, three studies compared SC semaglutide with placebo [14,18,20] and three studies compared SC semaglutide to another GLP-1 RA comparator [16,19,23]. The incidence of AEs was significantly higher for SC semaglutide than for placebo (RR: 1.14 [95% CI: 1.03. 1.26], I2 = 0%, P = 0.01) and another GLP-1 RA comparator (RR: 1.01 [95% CI: 0.96, 1.06], I2 = 0%, P = 0.76) (Fig. 3) (Appendix).
3.3.2. The PIONEER programme
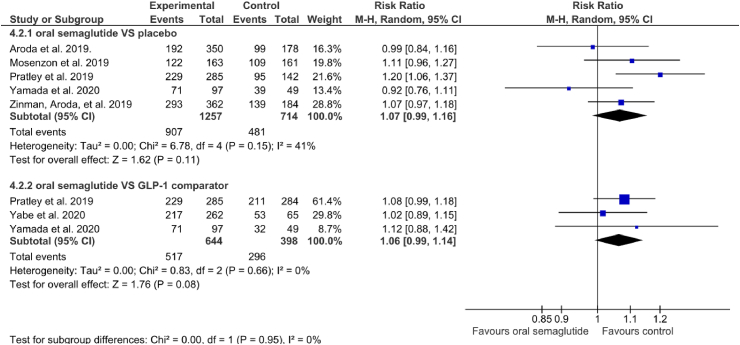
Six studies reported information on AEs. Five studies reported AE data for oral semaglutide compared to placebo [29,30,32,38,39] and three reported AEs for oral semaglutide compared to GLP-1 RA [30,31,38]. The incidence of AEs was not higher for oral semaglutide than for placebo (RR: 1.07 [95% CI: 0.99, 1.16], I2 = 41%, P = 0.11) or another GLP-1 RA comparator (RR: 1.06 [95% CI: 0.99, 1.14], I2 = 0%, P = 0.08) (Fig. 4) (Appendix).
3.4. Network meta-analysis
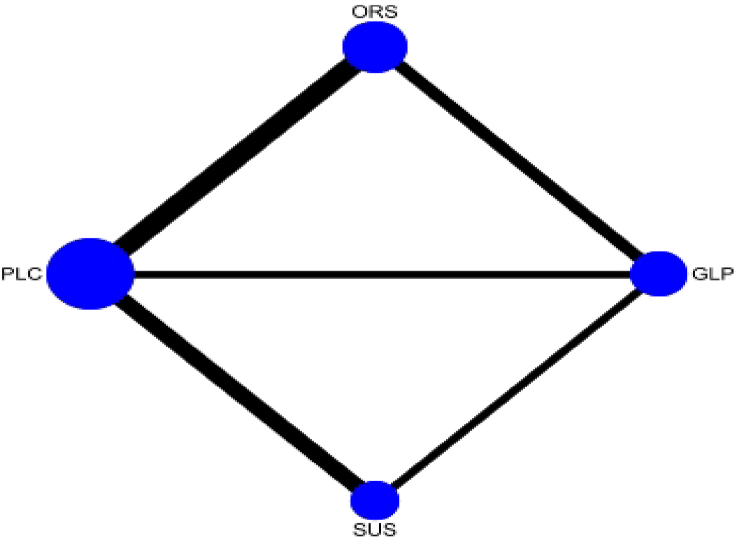
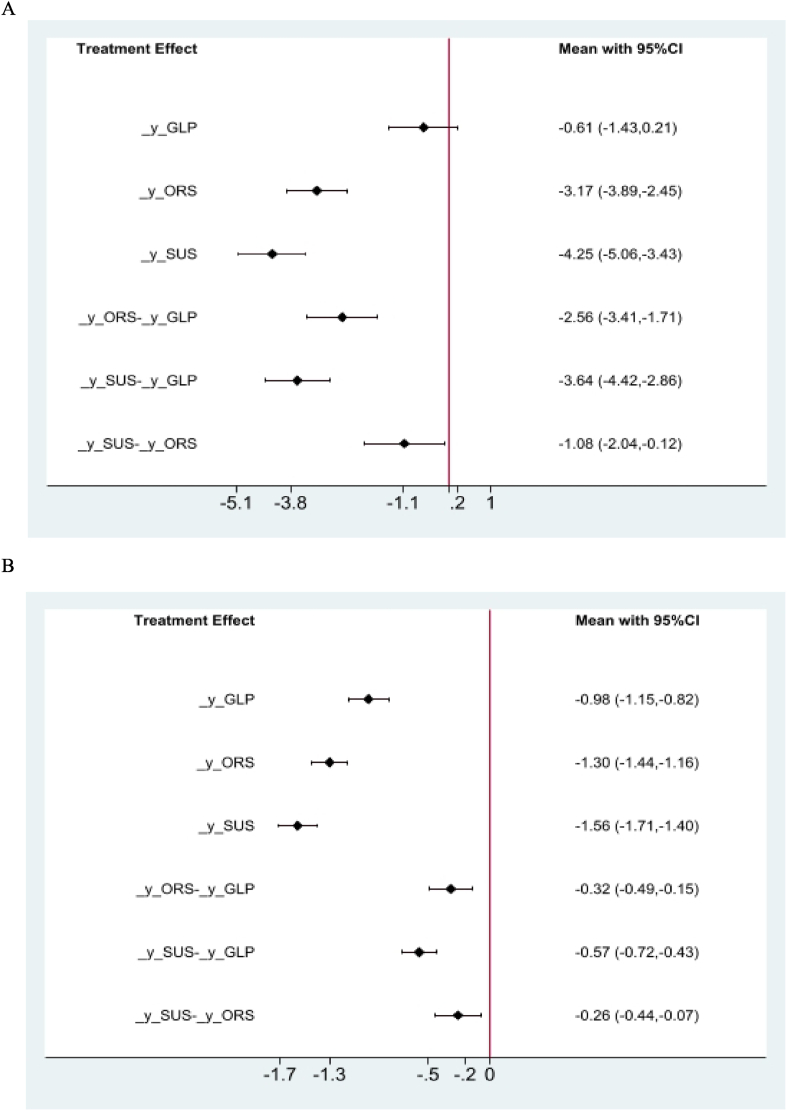
The network meta-analysis results for HbA1c, body weight, and AEs, using placebo as a reference, are presented in Table 2, Table 3. Fig. 4 shows the network of included trials used in the meta-analysis for HbA1c, body weight, and AEs. SC semaglutide was the most efficacious in lowering HbA1c and body weight (MD vs placebo, HbA1c: -1.56% [95% CI: -1.71, −1.40], P < 0.05; MD vs placebo, body weight: -4.25 kg [95% CI: -5.06, −3.43], P < 0.05). However, SC semaglutide was associated with an increase in AEs (RR vs placebo: 0.15 [95% CI: -0.20, 0.50], P > 0.05). SC semaglutide was superior to oral semaglutide in terms of reducing HbA1c and bodyweight (MD vs oral semaglutide, HbA1c: -0.26% [95% CI: -0.44, −0.07], P < 0.05; MD vs oral semaglutide, body weight: -1.08 kg [95% CI: -2.04, −0.12], P < 0.05). However, SC semaglutide was inferior to oral semaglutide with respect to the increase in AEs (RR vs placebo: 0.12 [95% CI: -0.30, 0.54], P > 0.05) (Fig. 5 A, B).
Table 2.
Network meta-analysis results of the mean difference in HbA1c (left lower half) and body weight (right upper half) in patients with the following interventions: ORS=Oral semaglutide, SUS=Subcutaneous semaglutide, GLP = GLP-1 RA comparator, and PLC=Placebo.
| Network comparison, HbA1c and body weight | |||
|---|---|---|---|
| SUS | −1.08 (−2.04, −0.12) | −3.64 (−4.42, −2.86) | −4.25 (−5.06, −3.43) |
| −0.26 (−0.44, −0.07) | ORS | −2.56 (−3.41, −1.71) | −3.17 (−3.89, −2.45) |
| −0.57 (−0.72, −0.43) | −0.32 (−0.49, −0.15) | GLP | −0.61 (−1.43, 0.21) |
| −1.56 (−1.71, −1.40) | −1.30 (−1.44, −1.16) | −0.98 (−1.15, −0.82) | PLC |
Table 3.
Network meta-analysis results of the risk ratio of adverse events in patients with the following interventions: ORS=Oral semaglutide, SUS=Subcutaneous semaglutide, GLP = GLP-1 RA comparator, and PLC=Placebo.
| Network comparison, adverse events | |||
|---|---|---|---|
| SUSrowhead | |||
| 0.12 (−0.30, 0.54) | ORS | ||
| 0.20 (−0.13, 0.53) | 0.08 (−0.32, 0.48) | GLP | |
| 0.15 (−0.20, 0.50) | 0.03 (−0.29, 0.36) | −0.05 (−0.42, 0.33) | PLC |
Fig. 4.
Interventions are presented in blue nodes. The size of the nodes is proportional to the number of studies included and the black lines show direct comparisons in the randomised control trials. The line thickness is directly proportional to the number of comparisons. Abbreviations: ORS = oral semaglutide, SUS = subcutaneous semaglutide, GLP = GLP-1 RA comparators, PLC = placebo.
Fig. 5.
Network meta-analysis of treatment effect on HbA1c (A) and body weight (B) using placebo as a reference. Data are presented as mean difference ±standard error, p < 0.05. Abbreviations: _ORS = oral semaglutide, _SUS = subcutaneous semaglutide, _GLP = GLP-1 RA comparator, _y = placebo.
4. Discussion
This systematic review and network meta-analysis summarised the evidence from 12 studies (6840 participants) that applied at least 26 weeks of intervention to test the efficacy and safety of SC and oral semaglutide for the control of HbA1c in adults with T2D. The results showed a low quality of evidence (according to the GRADE approach, Table 1) for the contribution of SC semaglutide (1.0 mg) in reducing HbA1c by 1.5% and body weight by 4.2 kg compared to placebo. Furthermore, there was low quality of evidence for the efficacy of SC semaglutide in reducing HbA1c and body weight compared to liraglutide (1.2 mg), exenatide ER (2.0 mg), and dulaglutide (1.5 mg) (−0.98%, −3.64 kg). However, there was moderate quality of evidence linking SC semaglutide with increased gastrointestinal AEs without increase in the incidence of hypoglycaemia compared to placebo, liraglutide (1.2 mg), exenatide ER (2.0 mg), and dulaglutide (1.5 mg).
These findings are consistent with those of Andreadis et al. [40] who reported that when compared to placebo, SC semaglutide (1.0 mg) was associated with a reduction in body weight (−4.11 kg) and HbA1c (1.5%) with no increase in the incidence of hypoglycaemic episodes. However, the researchers found that when SC semaglutide was tested against other diabetic agents, HbA1c was only reduced by 0.37% and body weight by −2.79 kg. This difference in the findings might be attributable to the inclusion of sitagliptin and insulin glargine as active comparators.
This review also showed low-quality evidence that oral semaglutide (14 mg) was associated with reduction in HbA1c and body weight compared to placebo (−1.3% and −3.2 kg, respectively) and another GLP-1 RA comparator (−0.31% and −2.4 kg, respectively), including liraglutide (1.2 mg), exenatide ER (2.0 mg), and dulaglutide (1.5 mg). The indirect comparison between SC semaglutide and oral semaglutide demonstrated that the former appears to have a slightly greater effect on HbA1c and body weight with increase in the incidence of gastrointestinal AEs. Nonetheless, clinically significant reductions in HbA1c and body weight were observed with semaglutide, regardless of the method of administration.
Early glycaemic control has significant health benefits for people with T2D [41]. Notably, there is growing evidence to support that inadequate glycaemic regulation in the early years following diagnosis increases the risk of developing complications associated with diabetes later in life [42]. Of these complications, microvascular events, mortality, and diabetic neuropathy may affect 50% of people with T2D [42]. Patients may also develop gastroparesis after they are diagnosed with autonomic neuropathy, which affects the vagus nerves [43]. Therefore, avoidance of clinical inertia and achieving glycaemic control as soon as possible after the diagnosis is highly recommended to avoid complications [44]. Across the included studies from the PIONEER programme, oral semaglutide was found to be superior to placebo and other GLP-1 RAs in terms of reducing HbA1c levels, regardless of diabetes duration. Furthermore, at least 50% of patients who were treated with oral semaglutide achieved an HbA1c < 7.0%, which is the optimal target set by the ADA [6]. Consequently, earlier initiation of GLP-1 RA medication, such as oral semaglutide, may be an effective treatment option. However, it is important to note that the use of oral semaglutide is generally not recommended or requires special consideration for women who are pregnant or breastfeeding and for children with T2D. In addition, oral semaglutide is not indicated for people with type 1 diabetes or patients with diabetic ketoacidosis [45].
Oral semaglutide should be considered in the context of providing a variety of treatment options for adults with T2D to potentially improve treatment adherence, especially by offering an alternative for patients with a preference for oral versus injectable therapy [46]. For those dissatisfied with their current treatment and/or outcomes, once weekly medication such as SC semaglutide is a more attractive option and could lead to better adherence, as such patients are usually highly motivated and interested in trying a new approach [47]. A recent review by Weiss et al. [48] reported a lower level of adherence among patients using daily doses of GLP-1 RAs than among those using GLP-1 RAs once a week. In contrast, when considering dosing frequency, discontinuation of the GLP-1 RA therapy was comparable for weekly and daily dose users at 12 months and higher among weekly dose users at 24 months [48]. Several retrospective studies from Italy, Germany, and the UK have reported higher rates of adherence with weekly dosing than with once- or twice-daily dosing [49,50]. However, Polonsky et al. [47] showed that when compared to patients who used injectable medications, a high percentage of those who had a preference for oral medication considered once-weekly injectable medication to be less favourable. Therefore, an oral formulation of semaglutide could be a superior option for patients who are hesitant to start injectable medication.
Gastrointestinal AEs were the most cited reason for the discontinuation of both forms of semaglutide and were reported by most of the included studies of the SUSTAIN and PIONEER programmes. In practice, many recommendations are available to manage these AEs. Gradual dose escalation of an oral semaglutide regimen is recommended, starting at 3 mg once daily for 1 month, followed by increase to 7 mg daily for the next month, and—in cases where additional glycaemic control is required—increase to 14 mg per day [9,29].
Gastroparesis or delayed gastric emptying may develop in people with T2D with poor blood glucose control [34] and can also be caused by GLP-1 RAs contributing to gastrointestinal AEs [52,53]. Patients should be aware of the dietary patterns that could contribute to such AEs and adopt dietary strategies to improve symptoms of delayed gastric emptying. This can be achieved primarily by providing the patient with the information needed to raise their awareness of food consistency, composition, and volume [54]. Liquid food usually has higher gastric mobility than solid food [55] with small particle size; for example, gastric emptying is faster with blended carrots than with chopped carrots [56]. Thus, adequate chewing may be sufficient to reduce food particles in mild cases [54]. Additionally, foods high in fat and fibre can cause prolonged gastric emptying, even in healthy individuals. Therefore, modification of these food groups is recommended in the population with T2D, alongside maintenance of normoglycaemia, which can also impair gastric emptying [57,58]. Finally, adjustment of meal size could be beneficial, as large meals require more time than small meals to exit the stomach [55]. Therefore, eating small frequent meals while avoiding large, high fat, and high fibre meals is recommended for patients who develop gastrointestinal AEs. In addition, the importance of drinking fluids and avoidance of alcoholic drinks should be emphasised to minimise the risk of dehydration that can develop because of gastrointestinal AEs [45]. When all dietary strategies fail to improve gastrointestinal symptoms, short-term use of antiemetics may be considered [59,60].
In the present review, the primary advantage of using mixed treatment comparisons is that they can include both direct and indirect information regarding drug treatment effects. Additionally, using these methods to compare drugs preserves the original randomisation and reduction of confounding factors and bias that may occur as a result of systematic differences between or among the trials being compared [61]. However, this statistical method is mainly limited by the use of two-stage techniques that depend on a normal approximation of the distribution of the estimated treatment effects. This limitation could lead to issues with count data, especially with small counts [37]. Another limitation of this review is that the results of the PIONEER and SUSTAIN programmes should be evaluated with caution because of the study designs and differences in baseline and background medications. However, these differences were taken into consideration in this review, and network meta-analysis was conducted by adjusting for specific inclusion criteria in terms of duration, comparators, and age of the participants, but without the restriction of background medications. Finally, searching only two databases could lead to publication bias; therefore, any conclusions from this review and network meta-analysis should be drawn carefully.
5. Conclusion
In summary, oral semaglutide was compared to SC semaglutide, placebo, and other GLP-1 RA comparators (liraglutide [1.2 mg], exenatide [ER, 2.0 mg], and dulaglutide [1.5 mg]) to assess their efficacy and safety in reducing the level of HbA1c and body weight in adults with T2D. Oral semaglutide was non-inferior to SC semaglutide and superior to placebo and another GLP-1 RA in reducing HbA1c and body weight. Moreover, oral semaglutide was superior to SC semaglutide and inferior to other GLP-1 RA comparators and placebo in terms of the incidence of AEs. However, oral semaglutide was well-tolerated in most studies, and the major AEs were gastrointestinal and included nausea, vomiting, and diarrhoea.
Oral semaglutide provides a new choice for the management of T2D and a convenient administration route for patients who prefer oral treatments over injectable therapies. By reducing the administrative burden on patients, oral semaglutide may enable more patients to improve their glycaemic control and reduce their body weight and encourage adherence to the medication. However, oral semaglutide should be carefully considered in pregnant or breastfeeding women and children with T2D. Dose escalation and dietary management could reduce AEs, which are the most common reason for discontinuation of GLP-1 RA medication. Further studies are required to confirm the findings of the PIONEER programme to enable their introduction to clinical practice. Additionally, future research should examine whether the availability of oral semaglutide will encourage earlier initiation and increased frequency of GLP-1 RA medication usage.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix.
Fig. 1.
The effect of subcutaneous semaglutide compared to placebo or GLP-1 RA comparator on HbA1c (A) and body weight (B). Data are presented as effect size with 95% CI, P < 0.05
Fig. 2.
The effect of oral semaglutide compared to placebo or GLP-1 RA comparator on HbA1c (A) and body weight (B). Data are presented as effect size with 95% CI, P < 0.05
Fig. 3.
Risk ratio of adverse events between the subcutaneous semaglutide and control arms.
Fig. 4.
Risk ratio of adverse events between the oral semaglutide and control arms.
Table 1.
Quality of evidence for the outcomes.
| SC semaglutide and oral semaglutide compared to placebo or GLP-1 RA comparator for HbA1c, body weight, and AEs | |||||
|---|---|---|---|---|---|
| Patient or population: HbA1c, body weight, and AEs Setting: Intervention: SC semaglutide and oral semaglutide Comparison: Placebo or GLP-1 RA comparator | |||||
| Outcomes | No. of participants (studies) Follow up |
Certainty of evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects |
|
| Risk with placebo or GLP-1 RA comparator | Risk difference with SC semaglutide and oral semaglutide | ||||
| Semaglutide vs placebo - semaglutide 1.0 mg vs placebo | 825 (3 RCTs) | 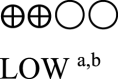 |
– | Mean semaglutide vs placebo - semaglutide 1.0 mg vs placebo was 8.07% | MD 1.55% lower (1.75 lower to 1.36 lower) |
| Semaglutide vs placebo - semaglutide 1.0 mg vs comparator | 1985 (3 RCTs) | 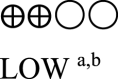 |
– | Mean semaglutide vs placebo - semaglutide 1.0 mg vs GLP-1 RA comparator was 7.13% | MD 0.58% lower (0.75 lower to 0.41 lower) |
| Oral semaglutide vs placebo - oral semaglutide 14 mg | 1566 (5 RCTs) | 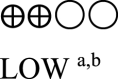 |
– | Mean oral semaglutide vs placebo - oral semaglutide 14 mg was 8.06% | MD 1.29% lower (1.47 lower to 1.11 lower) |
| Oral semaglutide vs placebo - oral semaglutide 14 mg vs GLP-1 comparator | 860 (3 RCTs) | 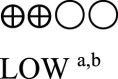 |
– | Mean oral semaglutide vs placebo - oral semaglutide 14 mg vs GLP-1 RA comparator was 7.03% | MD 0.31% lower (0.44 lower to 0.17 lower) |
| Body weight semaglutide vs placebo - semaglutide 1.0 mg vs placebo | 824 (3 RCTs) | 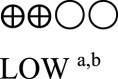 |
– | Mean body weight semaglutide vs placebo - semaglutide 1.0 mg vs placebo was 90.57 kg | MD 4.16 kg lower (5.05 lower to 3.26 lower) |
| Body weight semaglutide vs placebo - semaglutide 1.0 mg vs GLP-1 RA comparator | 1985 (3 RCTs) |  |
– | Mean body weight semaglutide vs placebo - semaglutide 1.0 mg vs GLP-1 RA comparator was 93.7 kg | MD 3.72 kg lower (4.17 lower to 3.28 lower) |
| Body weight oral semaglutide vs placebo or GLP-1 RA comparator - semaglutide 14 mg vs placebo | 1793 (5 RCTs) | 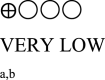 |
– | Mean body weight oral semaglutide vs placebo or GLP-1 RA comparator - semaglutide 14 mg vs placebo was 85.22 kg | MD 3.18 kg lower (4.12 lower to 2.24 lower) |
| Body weight oral semaglutide vs placebo or GLP-1 RA comparator - semaglutide 14 mg vs GLP-1 RA comparator | 860 (3 RCTs) | 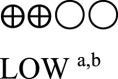 |
– | Mean body weight oral semaglutide vs placebo or GLP-1 RA comparator - semaglutide 14 mg vs GLP-1 RA comparator was 78.38 kg | MD 2.42 kg lower (3.18 lower to 1.66 lower) |
| AEs, semaglutide vs placebo or GLP-1 RA comparator - semaglutide vs placebo | 1084 (3 RCTs) |  |
RR 1.14 (1.03–1.26) | 574 per 1,000 | 80 more per 1,000 (17 more to 149 more) |
| AEs, semaglutide vs placebo or GLP-1 RA comparator - semaglutide vs GLP-1 RA comparator | 2584 (3 RCTs) |  |
RR 1.01 (0.96–1.06) | 702 per 1,000 | 7 more per 1,000 (28 fewer to 42 more) |
| AEs oral semaglutide vs placebo or GLP-1 RA comparator - oral semaglutide vs placebo | 1971 (5 RCTs) |  |
RR 1.07 (0.99–1.16) | 674 per 1,000 | 47 more per 1,000 (7 fewer to 108 more) |
| AEs oral semaglutide vs placebo or GLP-1 RA comparator - oral semaglutide vs GLP-1 RA comparator | 1042 (3 RCTs) |  |
RR 1.06 (0.99–1.14) | 744 per 1,000 | 45 more per 1,000 (7 fewer to 104 more) |
| *The risk in the intervention group (and its 95% confidence interval [95% CI]) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio; SC: subcutaneous, AE: adverse events. | |||||
|
GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimated effect. Moderate certainty: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimated effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimated effect. Very low certainty: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimated effect. | |||||
Explanations.
a. The quality was downgraded because some studies were not blinded.
b. The quality was downgraded because of high heterogeneity across studies.
Table 2.
The effect of subcutaneous semaglutide compared to that of placebo or GLP-1 RA comparator on HbA1c and body weight. Data are presented as mean difference and 95% CI.
| Study ID | HbA1c difference (%) [95% CI] | P value | Weight difference (kg) [95% CI] | P value |
|---|---|---|---|---|
| Ahmann et al., 2018 (SUSTAIN 3) [16] | GLP = -0.62 [-0.80, −0.44] | P < 0.0001 | GLP = -3.78 [-4.58, −2.98] | P < 0.0001 |
| Capehorn et al., 2020 (SUSTAIN 10) [23] | GLP = -0.69 [-0.82, −0.56] | P < 0.0001 | GLP = -3.83 [-4.57, −3.09] | P < 0.0001 |
| Pratley et al., 2018 (SUSTAIN 7) [19] | GLP = -0.41 [-0.57, −0.25] | P < 0.0001 | GLP = -3.55 [-4.32, −2.78] | P < 0.0001 |
| Rodbard et al., 2018 (SUSTAIN 5) [18] | 0.5 mg = -1.35 [-1.60, −1.10] 1.0 mg = -1.75 [-2.01, −1.49] |
P < 0.0001 | 0.5 mg = -2.31 [-3.30, −1.32] 1.0 mg = -5.06 [-6.08, −4.04] |
P < 0.0001 |
| Sorli et al., 2017 (SUSTAIN 1) [14] | 0.5 mg = -1.43 [-1.71, −1.15] 1.0 mg = -1.53 [-1.81, −1.25] |
P < 0.0001 | 0.5 mg = -2.75 [-3.92, −1.58] 1.0 mg = -3.56 [-4.74, −2.38] |
P < 0.0001 |
| Zinman et al., 2019b (SUSTAIN 9) [20] | 1.0 mg = -1.42 [-1.61, −1.23] | P < 0.0001 | 1.0 mg = -3.81 [-4.70, −2.92] | P < 0.0001 |
Table 3.
The effect of oral semaglutide compared to that of placebo or GLP-1 RA comparator on HbA1c. Data are presented as mean difference and 95% CI.
| Study ID | HbA1c difference (%) [95% CI] | P value | Weight difference (kg) [95% CI] | P value |
|---|---|---|---|---|
| Aroda et al., 2019 (PIONEER 1) [29] | 3.0 mg = -0.70 [-0.90, −0.50] 7.0 mg = -1.25 [-1.50, −1.00] 14.0 mg = -1.45 [-1.70, −1.20] |
P < 0.001 | 3.0 mg = -0.20 [-1.00, 0.60] 7.0 mg = -1.00 [-1.80, −0.20] 14.0 mg = -2.60 [-3.40, −1.80] |
3.0 mg = P = 0.71 7.0 mg = P = 0.01 14.0 mg = P < 0.001 |
| Mosenzon et al., 2019 (PIONEER 5) [32] | 14.0 mg = -1.00 [-1.20, −0.80] | P < 0.0001 | 14.0 mg = −2.70 [-3.50, −1.90] |
P < 0.0001 |
| Pratley et al., 2019 (PIONEER 4) [38] | 14.0 mg = -1.40 [-1.60, −1.20] GLP = -0.30 [-0.40, −0.10] |
14.0 mg = P = 0.0012 GLP = P < 0.0001 |
14.0 mg = -3.8 [-4.80, −2.70] GLP = -1.80 [-2.60, −1.00] |
P < 0.0001 |
| Yabe et al., 2020 (PIONEER 10) [31] | GLP = -0.50 [-0.80, −0.20] | P = 0.0007 | GLP = -3.00 [-4.00, −2.00] | P < 0.0001 |
| Yamada et al., 2020 (PIONEER 9) [30] | 3.0 mg = -0.85 [-1.20, −0.50] 7.0 mg = -1.30 [-1.60, −1.00] 14.0 mg = -1.40 [-1.70, −1.10] GLP = -0.30 [-0.70, 0.10] |
P < 0.0001 GLP = P = 0.1005 |
3.0 mg = 0.30 [-0.80, 1.40] 7.0 mg = -0.20 [-1.30, 0.90] 14.0 mg = -2.00 [-3.10, −0.90] GLP = -2.65 [-3.80, −1.50] |
3.0 mg = P = 0.5918 7.0 mg = P = 0.7021 14.0 mg = P = 0.0003 GLP = P < 0.0001 |
| Zinman et al., 2019a (PIONEER 8) [39] | 3.0 mg = -0.55 [-0.80, −0.30] 7.0 mg = -0.90 [-1.10, −0.60] 14.0 mg = -1.30 [-1.50, −1.00] |
P < 0.0001 | 3.0 mg = -1.65 [-2.60, −0.70] 7.0 mg = -3.55 [-4.50, −2.60] 14.0 mg = -4.90 [-5.90, −3.90] |
P < 0.001 |
References
- 1.Diabetes UK. Diabetes Prevalence 2019 | Diabetes UK [Internet]. Diabetes Prevalence UK, https://www.diabetes.org.uk/professionals/position-statements-reports/statistics/diabetes-prevalence-2019.
- 2.World Health Organization Diabetes. https://www.who.int/news-room/fact-sheets/detail/diabetes
- 3.Nathan D.M., Genuth S., Lachin J., Cleary P., Crofford O., Davis M., et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. Clin. Pediatr. 1994;33:378. doi: 10.1056/NEJM199309303291401. http://www.nejm.org/doi/abs/10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 4.Inzucchi S.E., Bergenstal R.M., Buse J.B., Diamant M., Ferrannini E., Nauck M., et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. http://care.diabetesjournals.org/lookup/ [DOI] [PubMed] [Google Scholar]
- 5.Turner R. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736%2898%2907019-6/fulltext [PubMed] [Google Scholar]
- 6.Davies M.J., D'Alessio D.A., Fradkin J., Kernan W.N., Mathieu C., Mingrone G., et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD) Diabetologia. 2018;61:2461–2498. doi: 10.1007/s00125-018-4729-5. https://pubmed.ncbi.nlm.nih.gov/30288571/ [DOI] [PubMed] [Google Scholar]
- 7.Pi-Sunyer F.X. The effects of pharmacologic agents for type 2 diabetes mellitus on body weight. Postgrad. Med. 2008;120:5–17. doi: 10.3810/pgm.2008.07.1785. https://pubmed.ncbi.nlm.nih.gov/18654064/ [DOI] [PubMed] [Google Scholar]
- 8.Abdi H., Azizi F., Amouzegar A. Insulin monotherapy versus insulin combined with other glucose-lowering agents in type 2 diabetes: a narrative review. Int. J. Endocrinol. Metabol. 2018;16 doi: 10.5812/ijem.65600. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6035366/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies M., Pieber T.R., Hartoft-Nielsen M.L., Hansen O.K.H., Jabbour S., Rosenstock J. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes a randomized clinical trial. JAMA. 2017;318:1460–1470. doi: 10.1001/jama.2017.14752. https://pubmed.ncbi.nlm.nih.gov/29049653/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Htike Z.Z., Zaccardi F., Papamargaritis D., Webb D.R., Khunti K., Davies M.J. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a systematic review and mixed-treatment comparison analysis. Diabetes Obes. Metabol. 2017;19:524–536. doi: 10.1111/dom.12849. https://pubmed.ncbi.nlm.nih.gov/27981757/ [DOI] [PubMed] [Google Scholar]
- 11.Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes. Metabol. 2016;18:203–216. doi: 10.1111/dom.12591. https://pubmed.ncbi.nlm.nih.gov/26489970/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meloni A.R., Deyoung M.B., Lowe C., Parkes D.G. GLP-1 receptor activated insulin secretion from pancreatic β-cells: mechanism and glucose dependence. Diabetes Obes. Metabol. 2013;15:15–27. doi: 10.1111/j.1463-1326.2012.01663.x. https://pubmed.ncbi.nlm.nih.gov/22776039/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buse J.B., Bergenstal R.M., Glass L.C., Heilmann C.R., Lewis M.S., Kwan A.Y.M., et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes. Ann. Intern. Med. 2011;154:103–112. doi: 10.7326/0003-4819-154-2-201101180-00300. https://pubmed.ncbi.nlm.nih.gov/21138825/ [DOI] [PubMed] [Google Scholar]
- 14.Sorli C., Harashima S.I., Tsoukas G.M., Unger J., Karsbøl J.D., Hansen T., et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5:251–260. doi: 10.1016/S2213-8587(17)30013-X. https://pubmed.ncbi.nlm.nih.gov/28110911/ [DOI] [PubMed] [Google Scholar]
- 15.Ahrén B., Masmiquel L., Kumar H., Sargin M., Karsbøl J.D., Jacobsen S.H., et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5:341–354. doi: 10.1016/S2213-8587(17)30092-X. https://pubmed.ncbi.nlm.nih.gov/28385659/ [DOI] [PubMed] [Google Scholar]
- 16.Ahmann A.J., Capehorn M., Charpentier G., Dotta F., Henkel E., Lingvay I., et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41:258–266. doi: 10.2337/dc17-0417. https://pubmed.ncbi.nlm.nih.gov/29246950/ [DOI] [PubMed] [Google Scholar]
- 17.Aroda V.R., Bain S.C., Cariou B., Piletič M., Rose L., Axelsen M., et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5:355–366. doi: 10.1016/S2213-8587(17)30085-2. https://pubmed.ncbi.nlm.nih.gov/28344112/ [DOI] [PubMed] [Google Scholar]
- 18.Rodbard H.W., Lingvay I., Reed J., De La Rosa R., Rose L., Sugimoto D., et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J. Clin. Endocrinol. Metab. 2018;103:2291–2301. doi: 10.1210/jc.2018-00070. https://pubmed.ncbi.nlm.nih.gov/29688502/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pratley R.E., Aroda V.R., Lingvay I., Lüdemann J., Andreassen C., Navarria A., et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6:275–286. doi: 10.1016/S2213-8587(18)30024-X. https://pubmed.ncbi.nlm.nih.gov/29397376/ [DOI] [PubMed] [Google Scholar]
- 20.Zinman B., Bhosekar V., Busch R., Holst I., Ludvik B., Thielke D., et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7:356–367. doi: 10.1016/S2213-8587(19)30066-X. https://pubmed.ncbi.nlm.nih.gov/30833170/ [DOI] [PubMed] [Google Scholar]
- 21.Lingvay I., Catarig A.M., Frias J.P., Kumar H., Lausvig N.L., le Roux C.W., et al. Efficacy and safety of once-weekly semaglutide versus daily canagliflozin as add-on to metformin in patients with type 2 diabetes (SUSTAIN 8): a double-blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7:834–844. doi: 10.1016/S2213-8587(19)30311-0. https://pubmed.ncbi.nlm.nih.gov/31540867/ [DOI] [PubMed] [Google Scholar]
- 22.Consoli A., Bain S.C., Davies M., Lingvay I., Bergan E.Q., Hansen O., et al. Semaglutide provides sustained reductions in body weight over 2 years in subjects with type 2 diabetes (SUSTAIN 6) Diabetologia. 2017;60:S4. https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01419078/full?highlightAbstract=semaglutid%7Csustain%7C6%7Csemaglutide [Google Scholar]
- 23.Capehorn M.S., Catarig A.M., Furberg J.K., Janez A., Price H.C., Tadayon S., et al. Efficacy and safety of once-weekly semaglutide 1.0 mg vs once-daily liraglutide 1.2 mg as add-on to 1–3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10) Diabetes Metab. 2020;46:100–109. doi: 10.1016/j.diabet.2019.101117. https://pubmed.ncbi.nlm.nih.gov/31539622/ [DOI] [PubMed] [Google Scholar]
- 24.Dungan K.M., Povedano S.T., Forst T., González J.G.G., Atisso C., Sealls W., et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384:1349–1357. doi: 10.1016/S0140-6736(14)60976-4. https://pubmed.ncbi.nlm.nih.gov/25018121/ [DOI] [PubMed] [Google Scholar]
- 25.Pratley R.E., Nauck M.A., Barnett A.H., Feinglos M.N., Ovalle F., Harman-Boehm I., et al. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2:289–297. doi: 10.1016/S2213-8587(13)70214-6. https://pubmed.ncbi.nlm.nih.gov/24703047/ [DOI] [PubMed] [Google Scholar]
- 26.Lau J., Bloch P., Schä L., Pettersson I., Spetzler J., Kofoed J., et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J. Med. Chem. 2015;58:7370–7380. doi: 10.1021/acs.jmedchem.5b00726. https://pubs.acs.org/sharingguidelines [DOI] [PubMed] [Google Scholar]
- 27.Kapitza C., Nosek L., Jensen L., Hartvig H., Jensen C.B., Flint A. Semaglutide, a once-weekly human GLP-1 analog, does not reduce the bioavailability of the combined oral contraceptive, ethinylestradiol/levonorgestrel. J. Clin. Pharmacol. 2015;55:497–504. doi: 10.1002/jcph.443. https://pubmed.ncbi.nlm.nih.gov/25475122/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckley S.T., Bækdal T.A., Vegge A., Maarbjerg S.J., Pyke C., Ahnfelt-Rønne J., et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aar7047. https://pubmed.ncbi.nlm.nih.gov/30429357/ [DOI] [PubMed] [Google Scholar]
- 29.Aroda V.R., Rosenstock J., Terauchi Y., Altuntas Y., Lalic N.M., Villegas E.C.M., et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42:1724–1732. doi: 10.2337/dc19-0749. https://pubmed.ncbi.nlm.nih.gov/31186300/ [DOI] [PubMed] [Google Scholar]
- 30.Yamada Y., Katagiri H., Hamamoto Y., Deenadayalan S., Navarria A., Nishijima K., et al. Dose-response, efficacy, and safety of oral semaglutide monotherapy in Japanese patients with type 2 diabetes (PIONEER 9): a 52-week, phase 2/3a, randomised, controlled trial. Lancet Diabetes Endocrinol. 2020;8:377–391. doi: 10.1016/S2213-8587(20)30075-9. https://pubmed.ncbi.nlm.nih.gov/32333875/ [DOI] [PubMed] [Google Scholar]
- 31.Yabe D., Nakamura J., Kaneto H., Deenadayalan S., Navarria A., Gislum M., et al. Safety and efficacy of oral semaglutide versus dulaglutide in Japanese patients with type 2 diabetes (PIONEER 10): an open-label, randomised, active-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2020;8:392–406. doi: 10.1016/S2213-8587(20)30074-7. https://pubmed.ncbi.nlm.nih.gov/32333876/ [DOI] [PubMed] [Google Scholar]
- 32.Mosenzon O., Blicher T.M., Rosenlund S., Eriksson J.W., Heller S., Hels O.H., et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:515–527. doi: 10.1016/S2213-8587(19)30192-5. https://pubmed.ncbi.nlm.nih.gov/31189517/ [DOI] [PubMed] [Google Scholar]
- 33.Nathan D.M., Turgeon H., Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia. 2007;50:2239–2244. doi: 10.1007/s00125-007-0803-0. https://pubmed.ncbi.nlm.nih.gov/17851648/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins J.P.T., Altman D.G.S.J., editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2015. www.cochrane-handbook.orghttp://ci.nii.ac.jp/naid/10027964982/en/ Version 5.1.0 [updated March 2011] [Google Scholar]
- 35.Puhan M.A., Schünemann H.J., Murad M.H., Li T., Brignardello-Petersen R., Singh J.A., et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349 doi: 10.1136/bmj.g5630. https://pubmed.ncbi.nlm.nih.gov/25252733/ [DOI] [PubMed] [Google Scholar]
- 36.White I.R., Barrett J.K., Jackson D., Higgins J.P.T. Consistency and inconsistency in network meta‐analysis: model estimation using multivariate meta‐regression. Res. Synth. Methods. 2012;3:111–125. doi: 10.1002/jrsm.1045. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4433772/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White I.R. Network meta-analysis. STATA J. 2015;15:951–985. https://journals.sagepub.com/doi/10.1177/1536867X1501500403 [Google Scholar]
- 38.Pratley R., Amod A., Hoff S.T., Kadowaki T., Lingvay I., Nauck M., et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394:39–50. doi: 10.1016/S0140-6736(19)31271-1. https://pubmed.ncbi.nlm.nih.gov/31186120/ [DOI] [PubMed] [Google Scholar]
- 39.Zinman B., Aroda V.R., Buse J.B., Cariou B., Harris S.B., Hoff S.T., et al. Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: the PioNEER 8 trial. Diabetes Care. 2019;42:2262–2271. doi: 10.2337/dc19-0898. https://pubmed.ncbi.nlm.nih.gov/31530667/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andreadis P., Karagiannis T., Malandris K., Avgerinos I., Liakos A., Manolopoulos A., et al. Semaglutide for type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes. Metabol. 2018;20:2255–2263. doi: 10.1111/dom.13361. https://pubmed.ncbi.nlm.nih.gov/29756388/ [DOI] [PubMed] [Google Scholar]
- 41.Santos Cavaiola T., Kiriakov Y., Reid T. Primary care management of patients with type 2 diabetes: overcoming inertia and advancing therapy with the use of injectables. Clin. Therapeut. 2019;41:352–367. doi: 10.1016/j.clinthera.2018.11.015. https://pubmed.ncbi.nlm.nih.gov/30655008/ [DOI] [PubMed] [Google Scholar]
- 42.Laiteerapong N., Ham S.A., Gao Y., Moffet H.H., Liu J.Y., Huang E.S., et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (the Diabetes & Aging study) Diabetes Care. 2019;42:416–426. doi: 10.2337/dc17-1144. https://pubmed.ncbi.nlm.nih.gov/30104301/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pop-Busui R., Lu J., Lopes N., Jones T.L.Z. Prevalence of diabetic peripheral neuropathy and relation to glycemic control therapies at baseline in the BARI 2D cohort. J. Peripher. Nerv. Syst. 2009;14:1–13. doi: 10.1111/j.1529-8027.2009.00200.x. https://pubmed.ncbi.nlm.nih.gov/19335534/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khunti K., Seidu S. Therapeutic inertia and the legacy of dysglycemia on the microvascular and macrovascular complications of diabetes. Diabetes Care. 2019;42:349–351. doi: 10.2337/dci18-0030. https://pubmed.ncbi.nlm.nih.gov/30787057/ [DOI] [PubMed] [Google Scholar]
- 45.Brunton S.A., Mosenzon O., Wright E.E. Integrating oral semaglutide into clinical practice in primary care: for whom, when, and how? Postgrad. Med. 2020;132:48–60. doi: 10.1080/00325481.2020.1798162. [DOI] [PubMed] [Google Scholar]
- 46.Thethi T.K., Pratley R., Meier J.J. Efficacy, safety and cardiovascular outcomes of once-daily oral semaglutide in patients with type 2 diabetes: the PIONEER programme. Diabetes Obes. Metabol. 2020;22:1263–1277. doi: 10.1111/dom.14054. https://pubmed.ncbi.nlm.nih.gov/32267058/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polonsky W.H., Fisher L., Hessler D., Bruhn D., Best J.H. Patient perspectives on once-weekly medications for diabetes. Diabetes Obes. Metabol. 2011;13:144–149. doi: 10.1111/j.1463-1326.2010.01327.x. https://pubmed.ncbi.nlm.nih.gov/21199266/ [DOI] [PubMed] [Google Scholar]
- 48.Weiss T., Carr R.D., Pal S., Yang L., Sawhney B., Boggs R., et al. Real-world adherence and discontinuation of glucagon-like peptide-1 receptor agonists therapy in type 2 diabetes mellitus patients in the United States. Patient Prefer. Adherence. 2020;14:2337–2345. doi: 10.2147/PPA.S277676. https://pubmed.ncbi.nlm.nih.gov/33273810/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Federici M.O., McQuillan J., Biricolti G., Losi S., Lebrec J., Richards C., et al. Utilization patterns of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus in Italy: a retrospective cohort study. Diabetes Ther. 2018;9:789–801. doi: 10.1007/s13300-018-0396-2. https://pubmed.ncbi.nlm.nih.gov/29525885/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilke T., Mueller S., Groth A., Berg B., Fuchs A., Sikirica M., et al. Non-persistence and non-adherence of patients with type 2 diabetes mellitus in therapy with GLP-1 receptor agonists: a retrospective analysis. Diabetes Ther. 2016;7:105–124. doi: 10.1007/s13300-015-0149-4. https://pubmed.ncbi.nlm.nih.gov/26695499/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakatani Y., Maeda M., Matsumura M., Shimizu R., Banba N., Aso Y., et al. Effect of GLP-1 receptor agonist on gastrointestinal tract motility and residue rates as evaluated by capsule endoscopy. Diabetes Metab. 2017;43:430–437. doi: 10.1016/j.diabet.2017.05.009. https://pubmed.ncbi.nlm.nih.gov/28648835/ [DOI] [PubMed] [Google Scholar]
- 53.Werner U. Effects of the GLP-1 receptor agonist lixisenatide on postprandial glucose and gastric emptying - preclinical evidence. J. Diabet. Complicat. 2014;28:110–114. doi: 10.1016/j.jdiacomp.2013.06.003. https://pubmed.ncbi.nlm.nih.gov/23992745/ [DOI] [PubMed] [Google Scholar]
- 54.Lomer M. Gastroparesis and nutrition. Adv. Nutr. Diet Gastroenterol. 2014:127–131. [Google Scholar]
- 55.Camilleri M. Integrated upper gastrointestinal response to food intake. Gastroenterology. 2006;131:640–658. doi: 10.1053/j.gastro.2006.03.023. https://pubmed.ncbi.nlm.nih.gov/16890616/ [DOI] [PubMed] [Google Scholar]
- 56.Olausson E.A., Alpsten M., Larsson A., Mattsson H., Andersson H., Attvall S. Small particle size of a solid meal increases gastric emptying and late postprandial glycaemic response in diabetic subjects with gastroparesis. Diabetes Res. Clin. Pract. 2008;80:231–237. doi: 10.1016/j.diabres.2007.12.006. https://pubmed.ncbi.nlm.nih.gov/18237818/ [DOI] [PubMed] [Google Scholar]
- 57.Hunt J.N., Knox M.T. A relation between the chain length of fatty acids and the slowing of gastric emptying. J. Physiol. 1968;194:327–336. doi: 10.1113/jphysiol.1968.sp008411. https://pubmed.ncbi.nlm.nih.gov/5639357/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holt S., Carter D.C., Tothill P., Heading R.C., Prescott L.F. Effect of gel fibre on gastric emptying and absorption of glucose and paracetamol. Lancet. 1979;313:636–639. doi: 10.1016/s0140-6736(79)91079-1. https://pubmed.ncbi.nlm.nih.gov/85872/ [DOI] [PubMed] [Google Scholar]
- 59.Ellero C., Han J., Bhavsar S., Cirincione B.B., DeYoung M.B., Gray A.L., et al. Prophylactic use of anti-emetic medications reduced nausea and vomiting associated with exenatide treatment: a retrospective analysis of an open-label, parallel-group, single-dose study in healthy subjects. Diabet. Med. 2010;27:1168–1173. doi: 10.1111/j.1464-5491.2010.03085.x. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3066409/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalra S., Das A.K., Sahay R.K., Baruah M.P., Tiwaskar M., Das S., et al. Consensus recommendations on GLP-1 RA use in the management of type 2 diabetes mellitus: South Asian Task Force. Diabetes Ther. 2019;10:1645–1717. doi: 10.1007/s13300-019-0669-4. http://link.springer.com/10.1007/s13300-019-0669-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim H., Gurrin L., Ademi Z., Liew D. Overview of methods for comparing the efficacies of drugs in the absence of head-to-head clinical trial data. Br. J. Clin. Pharmacol. 2014;77:116–121. doi: 10.1111/bcp.12150. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3895352/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]