Abstract
Background
The change from prescription to over-the-counter (OTC) status of oral antihistamines may raise concerns about drug safety due to the possibility of misuse/abuse. In most European countries, oral antihistamines are available without prescription, whereas in Italy, only <10-tablet packs are available OTC.
Objectives
To evaluate the safety profile of fexofenadine after OTC switch in Italy in a real-world setting, and to compare its safety profile to that of other European countries where larger pack sizes are available. To compare the safety of fexofenadine, cetirizine, and loratadine in Italy. To examine safety/efficacy across Europe with a systematic review.
Methods
This case-by-case analysis used the US Food and Drug Administration (FDA) adverse event reporting system (FAERS) to extract data of the adverse events (AEs) related to fexofenadine, loratadine and cetirizine in Italy January 2010–June 2020. The year 2016 was taken as the index date (removal of prescription requirement) for evaluation of the reporting trend of AEs of fexofenadine in Italy and make a comparison pre/post-OTC switch. A comparison of AEs with other European countries where fexofenadine is sold OTC in large packs >20 tablets (Belgium, Portugal, Switzerland, Finland, Hungary) was made. The rate at which an AE related to oral antihistamines occurred was estimated by calculation of the reporting rate (number of cases/[defined daily dose/1000 inhabitants per day]) on the basis of IQVIA sales data using the Italian Institute of Statistics database. A systematic review of the literature was also performed.
Results
There were 3760 reports of AEs with a suspected association with fexofenadine; of these, eight were reported from Italy. There was a slightly increasing trend per year, in line with a general reporting trend of other drugs. In European countries where fexofenadine is available, the impact of OTC switch on AE reporting activity was negligible: from 2016, the reporting rate increased slightly and then normalized at 3.01, an incidence value similar to that recorded before the OTC switch (3.7 in 2015). Of 22 studies included in the systematic review, 18 (82%) positively evaluated the OTC use of oral antihistamines, confirming an acceptable safety/tolerability profile.
Conclusion
There was no difference in number of AEs reported for fexofenadine pre/post-OTC switch, indicating a similar safety profile. Spontaneous reporting systems are a valuable source of real-world data and support the OTC provision of oral antihistamines in Europe and fexofenadine in Italy, in addition to supporting the use of larger pack sizes in Italy.
Keywords: Fexofenadine, Second-generation oral antihistamines, Pharmacovigilance, Food and drug administration adverse event reporting system (FAERS), Over-the-counter (OTC)
Introduction
The process of switching prescription drugs to over-the-counter (OTC) drugs has had an important impact on the global pharmaceutical market, and the sales of OTC drugs are expected to increase until at least 2024.1 The OTC availability of drugs may make them a more convenient and affordable option. In regards to allergy medication, a clear shift has been seen in favour of the use of OTC medicines, rising from 66% in 2009 to 75% in 2015.2 In order to qualify for OTC status, a medication must have a well-established safety profile with a wide safety margin, be effective and be appropriate for self-medication.3
Oral antihistamines have several indications for use, including the treatment of allergic rhinitis, allergic conjunctivitis and urticaria.4,5 Fexofenadine is a second-generation antihistamine which lacks cognitive, cardiac and sedative adverse effects.6,7 Common adverse drug reactions include headache, nausea and dizziness, but no dose-related trends in adverse effects have been noted with oral fexofenadine use.8 Fexofenadine was first commercialised in Europe in the 1990s as a prescription medicine and, starting from 2008, it became available as an OTC medication in most European countries, recently also in Italy (2016). Other oral second-generation antihistamines available as OTC on the Italian and European market include cetirizine and loratadine, both dispensed as behind-the-counter medications (i.e., without prescription but requiring prior consultation with the pharmacist) since 2002 and 2003, respectively. Depending on the country, oral antihistamines can be available as OTC in packs of 7–100 tablets, however in Italy, only small packs of 7–10 tablets are marketed.
The prescription requirement for oral antihistamine medications is strictly related to safety concerns, inappropriate use or the potential for abuse, as was observed with first-generation antihistamines.9 For example, common side effects of these medications include sedation, decreased cognitive function, poor sleep quality, dry mouth, dizziness and orthostatic hypotension.10,11 Furthermore, recreational misuse of diphenhydramine has been reported in several studies, and intoxication is associated with psychotic symptoms, disorientation and restlessness.9 In severe cases of dependence and toxicity, diphenhydramine has also been linked to cardiac abnormalities.12 The Canadian Society of Allergy and Clinical Immunology (CSACI) recommend that the newer generation of antihistamines should be first-line treatment in the context of allergic rhinitis and urticaria, due to their superior safety profile. However, first-generation antihistamines are still over utilised because of their OTC status and long history of use.11
To support the CSACI recommendation and to promote the OTC use of newer antihistamines, it is therefore of great importance to assess the safety of these medications after the switch to OTC products. This type of analysis can be done by examining available safety reports of the medication of interest before and after the removal of the prescription requirement. On this note, spontaneous reporting systems, such as the Food and Drug Administration adverse event reporting system (FAERS), represent a valuable source to obtain real-world data on the efficacy and safety profile of specific drugs. Such reporting systems are used for detecting novel drug-related safety events, identifying possible mechanisms of adverse events (unexpected events that occur during treatment) and serious adverse events (unexpected events that occur during treatment that are life-threatening, cause hospitalisation or prolong existing hospitalisation, or result in death), exploring potential drug-drug interactions, and comparing therapeutic options; all these are valuable information for the assessment of the prescription requirement.13, 14, 15, 16
The main objective of this study was to evaluate the impact of the switch to non-prescription status (including both OTC or behind-the-counter) on the safety profile of fexofenadine in Italy, in comparison to other countries where large packs of fexofenadine are available without prescription via analysis of the FAERS database. This study also aims to compare the profile of fexofenadine with other second-generation antihistamines (loratadine and cetirizine) in Italy, and to evaluate the safety of these second-generation antihistamines across Europe with the use of a systematic literature review.
Methods
Study design and data sources
This study was designed as a case-by-case study in order to carry out a case-level clinical review. Data were obtained from the FAERS. The database is designed to support the Food and Drug Administration's post-marketing safety surveillance program for drug and therapeutic biologic products. The FAERS database is publicly accessible and allows to locate countries around the world from where cases are reported. The FAERS receives approximately 1.5 million adverse event reports, product complaints and medication error reports per year from sources including health care professionals, consumers and companies. These reports concern drugs, vaccines and medical devices for human use. Adverse events are recorded in the FAERS using the Medical Dictionary for Regulatory Activities (MedDRA®) preferred terms.17
Data extraction
All Italian reports of serious adverse events in which at least one drug of interest (fexofenadine, cetirizine or loratadine) was considered the primary suspect, secondary suspect, or an interacting drug, were retrieved from the FAERS database for the period January 1, 2010 to June 30, 2020 (date of extraction: August 2020). Reports involving fexofenadine from Belgium, Finland, Portugal, Switzerland, and Hungary were also included for comparison of adverse events (Table 1). The statistical program R was used to extract, organize and clean data, in order to remove reports that lacked the information necessary for analysis. Duplicates were systematically removed through an ad hoc script created for the R data managing programming software: reports with same sex, age, primary suspect drug, date of adverse event occurrence, adverse event, and reporting country were considered as a single unique case. Subsequent manual data cleaning was performed to detect all cases that presented the same adverse events and suspected drugs but displayed in a different order and therefore not recognized by the script.
Table 1.
Serious adverse events reported in Italy and other European countries for fexofenadine between 1 January 2010–30 June 2020.
| Personal data |
Role of the drug |
||||||
|---|---|---|---|---|---|---|---|
| Number of AEs | Female N (%) | Male N (%) | Age mean (SD) | Primary suspect N (%) | Secondary suspect N (%) | Interacting N (%) | |
| Italy | 13 | 11 (85) | 2 (15) | 34.2 (21.1) | 7 (54) | 6 (46) | 0 (0) |
| Belgium | 2 | 2 (100) | 0 (0) | 46.5 (2.1) | 2 (100) | 0 (0) | 0 (0) |
| Finland | 3 | 2 (67) | 1 (33) | 58.7 (21.2) | 3 (100) | 0 (0) | 0 (0) |
| Portugal | 4 | 2 (50) | 2 (50) | 41.8 (20.5) | 4 (100) | 0 (0) | 0 (0) |
| Switzerland | 5 | 4 (80) | 1 (20) | 41.8 (22.6) | 3 (60) | 2 (40) | 0 (0) |
SD, standard deviation.
Hungary is not shown as there were no suspected AEs reported
In order to bypass the main intrinsic spontaneous reporting system database biases, the analysis was carried out only on reports with complete information on patient age and sex. Cases reporting data extracted from literature were also excluded.
Data analyses
Italy: adverse event reports for fexofenadine before and after the index date
The reporting trend of adverse events related to fexofenadine in Italy was evaluated to make a comparison pre-versus post-OTC switch. The legal status of fexofenadine in Italy switched in 2013 with the brand name Fexallegra® (120 mg fexofenadine tablets),18 and entered the market as OTC in 2016, which is considered the index date of this study. During the period analysed, cetirizine and loratadine were already available in Italy without prescription.
A case-by-case analysis was performed for each adverse drug reaction report involving fexofenadine as the suspected or interacting drug in order to carefully evaluate the expectedness of the reported adverse event, the causal correlation between drug use and reported adverse events (via use of Naranjo's algorithm, or the Adverse Drug Reaction Probability Scale), and the likely occurrence of relevant drug-drug interactions. Potential pharmacological interactions involved in the onset of the reported adverse event were checked with Clinical Pharmacology powered by ClinicalKey®. Naranjo's algorithm, or the Adverse Drug Reaction Probability Scale, is a validated method to standardize the assessment of causal relationships between identified untoward clinical events and a drug, using a simple questionnaire to assign probability scores. The Adverse Drug Reaction Probability Scale consists of 10 questions that are answered as either Yes, No, or Do Not Know. Different point values (−1, 0, +1 or +2) are assigned to each answer. Total scores range from −4 to +13; causative: score ≥9; probable: 5–8; possible: 1–4; doubtful: ≤0.19
Italy vs Europe: serious adverse event reports for fexofenadine
Italian fexofenadine safety data extracted from FAERS were compared with data extracted for other European countries: Belgium, Portugal, Switzerland, Finland, Hungary. These countries were selected because of the availability of large packs of fexofenadine OTC, up to 20–100 tablets.
Drug utilisation analysis of oral antihistamines in Italy
Drug utilisation analysis and reporting rates were calculated on the basis of IQVIA sales data of each molecule as OTC (fexofenadine, loratadine, cetirizine) and the annual population data available from the Italian National Institute of Statistics (Istat) database.20 Istat provides official statistics on the population, economy and environment of Italy. Data covering the period 2010–2020 were extracted from the database on November 3rd, 2020. The reporting rate was calculated as follows:
The defined daily dose (DDD), is, the assumed average maintenance dose per day for a drug used for its main indication in adults, as defined by the World Health Organisation. This value gives a rough estimate of consumption and not an exact picture of actual use. The DDD is different to the prescribed daily dose, it allows the assessment of trends in drug utilisation at an international level, and provides a fixed unit of measurement independent of price, currencies and package size.21
Systematic review evaluating the safety and efficacy of OTC oral antihistamines in Europe
PubMed, EMBASE and Web of Science were searched for relevant publications. The search string included two domains in order to systematically evaluate the efficacy and safety profile related to the OTC use of the drugs of interest: 1) the drug of interest, including synonyms and pharmacological class, and 2) terms attributable to OTC use of the drug and any outcomes suggestive of onset of adverse events, non-adherence to treatment, or inappropriateness of use (Supplementary Methods). All European studies evaluating the safety and efficacy of OTC oral antihistamines, across all study types and patient populations, were included.
Results
Italy: adverse event reports for fexofenadine and other oral antihistamines
A total of 3760 reports of serious adverse events with suspected association with fexofenadine were identified in FAERS during the analysed time frame; of those, 13 (0.3%) were of serious adverse events from Italy (Table 1). Adverse event occurrence was more often seen in female versus male patients. After duplicate removal, eight suspected serious adverse drug reactions (adverse events causally related to a drug) remained for analysis (Table 2). In five suspected adverse drug reactions, fexofenadine was found to be the only suspected drug. Five reported at least one unexpected event not mentioned in the patient leaflet. Two were reported prior to the index date.
Table 2.
Reports of suspected adverse drug reactions involving fexofenadine.
| Year | Age | Sex | Suspected drug | Therapeutic indication for fexofenadine | Adverse reaction |
|---|---|---|---|---|---|
| 2011 | 70 | Female | Fexofenadine Paroxetine Atenolol Ramipril Delorazepam Simvastatin Ezetimibe |
Allergic reaction | Retrograde Amnesiaa, Bradyphreniaa, Psychomotor Retardationsa, Abnormal Behavioura, Sleepiness, Disorientation, Agitation |
| 2015 | 36 | Female | Fexofenadine Desometasone/Tobramycin |
Not specified | Ocular oedema, Labial oedema |
| 2017 | 29 | Male | Fexofenadine | Allergic rhinitis | Glomerulonephritisa, Weight increasea |
| 2017 | 21 | Female | Fexofenadine | Allergic rhinitis | Chest oppression, Tachycardia, Throat constriction |
| 2018 | 30 | Female | Fexofenadine | Not specified | Anxietya, Diarrhoeaa, Insomniaa, Nervousness, Nightmares, Tachycardia |
| 2018 | 23 | Female | Fexofenadine | Seasonal allergy | Dry moutha, Flusha, Palpitation, Pharynx Oedema, Tongue oedema |
| 2019 | 22 | Female | Fexofenadine | Allergic reaction | Sense of oppression, Tachycardia |
| 2019 | 16 | Female | Fexofenadine Cetirizine | Not specified | Depressiona, Misusea, Maniaa, Panic Attacka, Event not evaluablea |
Cases are listed chronologically according to year the report was submitted to the FDA.
FDA, food and drug administration.
Unexpected events
All adverse event reports were categorized as possible for causality based on the application of Naranjo's algorithm.19 All adverse event reports oscillated between 1 and 3 suggestive of a weak correlation.
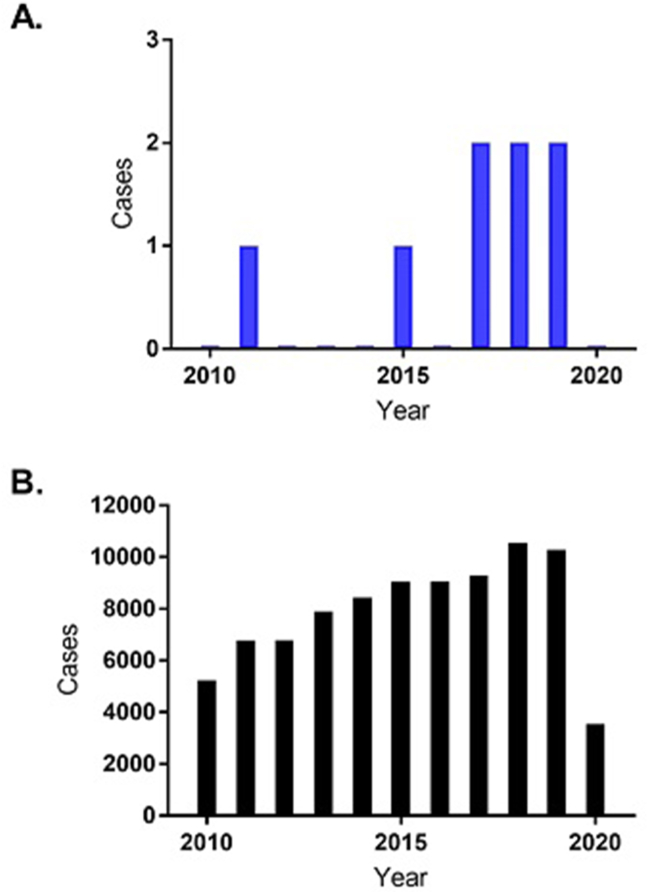
The number of reports related to adverse drug reactions from fexofenadine in Italy ranged from 0 to a maximum of 2 per year (Fig. 1A); these data suggested a slightly increasing trend from 2010 to 2019, although, as they remained in the order of a couple of serious adverse events per year, overall they supported a favourable safety profile for fexofenadine OTC. Additionally, despite the small sample size for fexofenadine which makes comparison of Fig. 1A and B difficult, fexofenadine's adverse event trend was in line with the general reporting trend from any drug in the FAERS database over the study period (Fig. 1B).
Fig. 1.
Number of annual reports of adverse drug reactions in Italy between 2010–June 2020 for (A) fexofenadine vs (B) any type of drug
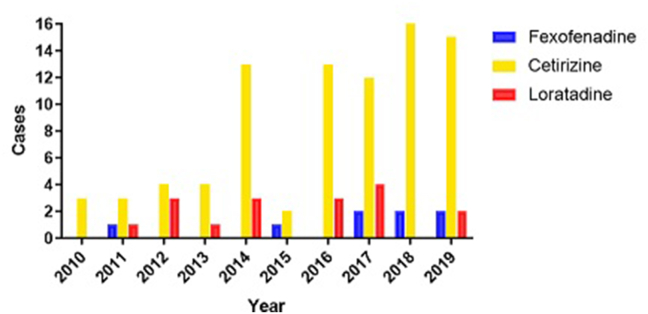
In comparison to loratadine (N = 16) and cetirizine (N = 42), the reporting trend of adverse events for fexofenadine remained relatively similar; an increasing reporting trend was recorded only for cetirizine (Fig. 2). Assessment of potential drug-drug interactions showed no relevant pharmacokinetic or pharmacodynamic interactions between fexofenadine and the other drugs involved in the reported cases.
Fig. 2.
Number of annual reports of AEs for fexofenadine, cetirizine and loratadine in Italy (2010–2019)
Italy vs Europe: adverse event reports for fexofenadine
The number of reports of serious adverse events from European countries where fexofenadine is available as OTC in large packs (Belgium, Finland, Portugal, Switzerland) was smaller than that from Italy; between 2 and 5 reports per country throughout the analysed timeframe (Table 1).
Drug utilisation analysis of oral antihistamines across Italy
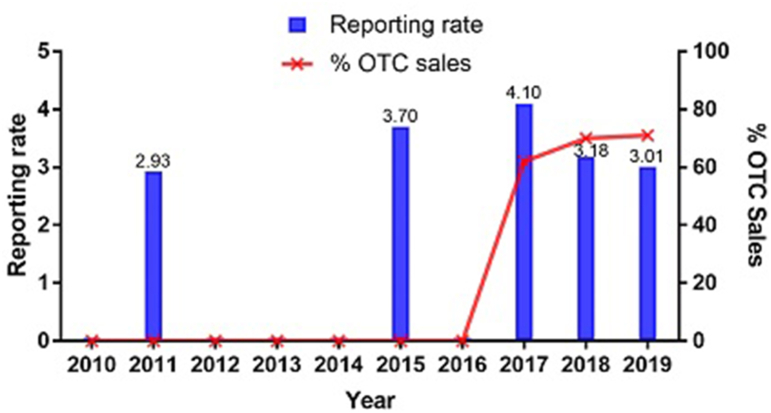
Despite the increase in sales of fexofenadine as an OTC treatment, its annual reporting rates showed only a slight increase from 2016 (the index date of transition to OTC). It plateaued at 3.01 in 2019, a reporting rate similar to that detected before the switch to OTC: 3.7 in 2015 (Fig. 3). Reporting rates for loratadine and cetirizine were below three across the whole of the study period (Supplementary Figure 1).
Fig. 3.
Annual reporting rate of fexofenadine in Italy (2010–2019). OTC, over-the-counter
Systematic review evaluating the safety and efficacy of OTC oral antihistamines
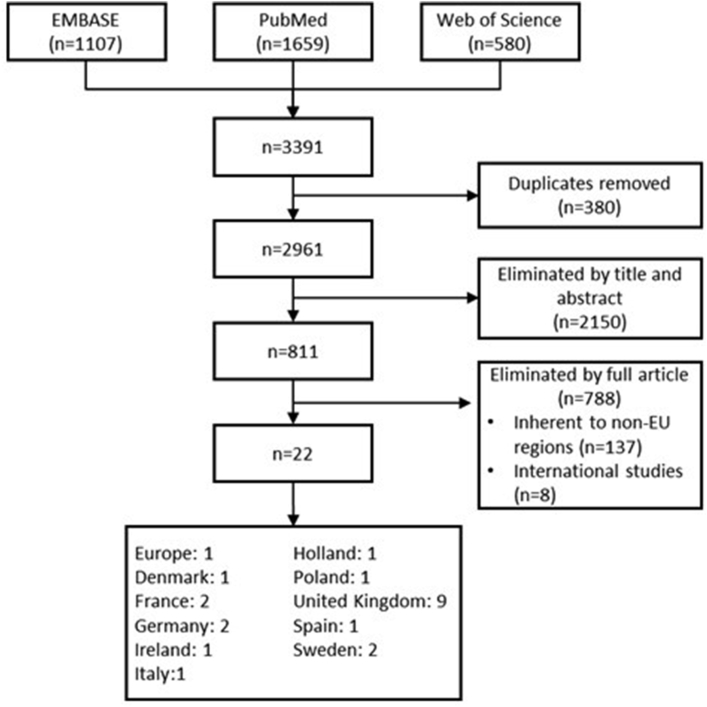
A total of 22 studies (retrospective observational surveys, interviews and expert opinions) were included in the systematic review (Fig. 4).1,3,5,22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 Of these, 18 (82%) were favourable or confirmed an acceptable safety and tolerability profile for OTC use of oral antihistamines.3,5,22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37
Fig. 4.
Screening of systematic review results. EU, European Union
Some safety issues were raised, including possible risk of misuse and abuse and an increased risk of medication error with OTC drugs in children. Nevertheless, the safety profile of oral antihistamines in the paediatric population was considered acceptable.25 Two of the studies recruited pregnant women and did not report clinically relevant adverse drug reactions related to the use of second-generation antihistamines during pregnancy (Supplementary Table 1).26,27
Five studies reported favourable opinion on oral antihistamines held by healthcare professionals including pharmacists.1,3,23,32,35
Discussion
In this study, the small number of adverse events reported for fexofenadine and other oral antihistamines between 2010 and 2019 served as an indication of an excellent safety profile of these OTC medicines when provided in both smaller packs, as in Italy, and in larger packs, as in some other European countries. There were slightly more adverse events reported for fexofenadine following the switch from prescription to OTC, however, this could be expected due to the increase in sales of fexofenadine. Reporting activity increased in Italy over the time studied, possibly reflective of a general intensification of pharmacovigilance activities. Taken together, these findings are consistent with the overall safety of second-generation antihistamines, an outcome that has been recognized since their introduction.41
FAERS is one of the largest and most comprehensive spontaneous reporting system databases for obtaining real-world data on the safety/efficacy profile of specific drugs. In 2016, the 21st Century Cures Act, designed to aid medical product development and innovation,42 placed additional focus on the use of these types of real-world data to support decision making.43, 44, 45
OTC sales of fexofenadine have been increasing in Italy since 2016, but despite this, the impact of the switch to OTC on the reporting of serious adverse events does not suggest a change in the safety profile of the product. Similarly, absolute data on the number of total serious annual adverse event reports for loratadine and cetirizine also remained low throughout the analysed time frame.
Furthermore, in a separate analysis during this study of the total serious and non-serious adverse events reported to the EudraVigilance public dashboard,46 low numbers were recorded over a wider time frame 2001–2020, supporting the favourable safety profile of oral antihistamines (7410: cetirizine; 3611: fexofenadine; 3273: loratadine).
In line with previous evidence surrounding sex differences in the risk of adverse events, female patients in this study had a higher occurrence of adverse events in all countries. It is widely acknowledged that women have a nearly two-fold greater risk of experiencing adverse events, and of being hospitalised due to adverse event occurrence, than men across all drug classes.47 However, due to the small sample size used in the current study, no firm conclusions can be drawn in regards to the drugs of interest.
Under-reporting is a well-known phenomenon with spontaneous reporting systems such as FAERS, as there is a reliance on individuals to report adverse events. However, in our study which analyses serious adverse events, under-reporting is unlikely to be a significant source of bias as patients are far more likely to report them. For example, a study analysing a similar spontaneous reporting system, the French Pharmacovigilance Database, demonstrated that the reporting of serious adverse events was much less affected by under-reporting than non-serious adverse events.48,49
Overall, the systematic review showed the favourable opinion of oral antihistamines held by various healthcare professionals, including pharmacists. The analysis of studies conducted in Europe revealed a wide use of OTC oral antihistamines in clinical practice, good management of the disease and highlighted a favourable opinion on their use in self-medication or on the advice of a pharmacist.
In Italy, only small packs of 7–10 tablets of oral antihistamines are available without prescription (OTC or behind-the-counter) which could lead to difficulties in adherence as patients are forced to return repeatedly to the pharmacy and may choose to discontinue with the therapy. For example, in patients with chronic idiopathic urticaria, the daily dose of oral antihistamines can be increased up to four times the usual dose.50 As such, the availability of large packs of fexofenadine without the need for a prescription could represent a practical advantage for patients receiving long-term or high dosage treatment, without safety concerns.
Limitations to this study include that the number of relevant reports covered by the study was too small to allow targeted sub-analyses. Due to the small sample size, it was not possible to make adjustments in a pre-post study. In addition, the nature of real-world studies can be limiting due to the lack of direct control and heterogeneity of populations.
However, strengths of this study also lie in the real-world nature of the analysis51 that allows application of the results to everyday clinical practice. This is especially important for antihistamines since they are widely recommended for many conditions.50,52 Italy is a reliable model to show the impact of switching oral antihistamines to OTC medication, as data were recorded before and after the switch using just one spontaneous reporting system (FAERS).
Conventional sources of real-world data (e.g. electronic health records, patient registries, and claims databases) continue to emerge as valuable sources of clinical evidence related to the complex pharmacological management of patients. Real-world evidence has been an important factor in prescription drug development and commercial decision-making for years, however, there is currently little evidence to support the role of real-world evidence within the OTC drug landscape. In part, this is due to the fact that many of these real-world data sources are unlikely to capture data relating to non-prescription medicines, or the outcomes associated with their use. Therefore, patient-generated health data may be particularly relevant for exploring the OTC drug landscape in the real-world, as consumers take the primary role in managing their pharmacological therapy without clinical supervision. Opportunities for real-world studies include the validation of OTC drug safety and efficacy, monitoring real world outcomes, and examination of when/why consumers purchase and use these products; consequently, such studies may have a powerful impact on decision-making surrounding non-prescription medicines.53
Conclusions
The results of this study suggest there is no impact on the safety profile of fexofenadine when switched to no prescription requirement status, as there was negligible impact on the number of AEs reported. Spontaneous reporting systems are a valuable source of real-world data and support the OTC provision of oral antihistamines in Europe and fexofenadine in Italy, in addition to supporting the use of larger oral antihistamine pack sizes in Italy. Future studies should utilise a range of real-world data sources to better explore and characterise the use of OTC drugs in daily clinical practice.
Abbreviations
AE, adverse event; FAERS, Food and Drug Administration adverse event reporting system; Istat, Italian Institute of Statistics; OTC, over-the-counter.
Funding and role of funding source
This study and preparation of this article were funded by Sanofi.
Availability of data and materials
Data are available from the corresponding author upon request.
Contributions
Conceptualisation, CC, MV, MCU, AC, GP; Methodology, CC, VB, MG, MV, MCU; Validation, CC, VB, MG, MV, MCU, AC, GP; Formal Analysis, CC, VB, MG, AC; Investigation, CC, VB, MG; Resources, CC, VB, MG; Data Curation, CC, VB, MG; Writing – original draft, CC; Writing – review and editing, CC, VB, MG, MV, MCU, GP; Visualisation, GP; Software, VB.
All authors approved the final version of this article for publication.
Disclosures
C. Carnovale, V. Battini, M. Gringeri and G. Passalacqua have no conflict of interest to declare. M. Volontè, A. Chiarenza and M.C. Uboldi are Sanofi Employees and may hold shares and/or stock options in the company.
Ethics approval
N/A.
Acknowledgements
This study was sponsored by Sanofi. Editorial support was provided by Kezia Pittaway, MSc, and Ella Palmer, PhD, CMPP, of inScience Communications, Springer Healthcare Ltd, UK, and was funded by Sanofi.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2022.100658.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zaprutko T., Kopciuch D., Ratajczak P., et al. The prescription to over-the-counter switches and double registration of medicines - the perspective of pharmacists from the Greater Poland. Clin Exp Allergy. 2019;76:907–912. [Google Scholar]
- 2.Association CHP . 2022. Rx-to-OTC Switch.https://www.chpa.org/our-issues/otc-medicines/rx-otc-switch Available from: [Google Scholar]
- 3.Stippler A., Eckstein N., Kroth E. To switch or not to switch—first Germany-wide study from the perspective of pharmacists in the European environment. J Public Health. 2021;29(1):9–17. [Google Scholar]
- 4.Devillier P., Roche N., Faisy C. Clinical pharmacokinetics and pharmacodynamics of desloratadine, fexofenadine and levocetirizine: a comparative review. Clin Pharmacokinet. 2008;47(4):217–230. doi: 10.2165/00003088-200847040-00001. [DOI] [PubMed] [Google Scholar]
- 5.Bilkhu P.S., Wolffsohn J.S., Naroo S.A. A review of non-pharmacological and pharmacological management of seasonal and perennial allergic conjunctivitis. Contact Lens Anterior Eye: J Br Contact Lens Assoc. 2012;35(1):9–16. doi: 10.1016/j.clae.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Hindmarch I., Shamsi Z., Stanley N., Fairweather D.B. A double-blind, placebo-controlled investigation of the effects of fexofenadine, loratadine and promethazine on cognitive and psychomotor function. Br J Clin Pharmacol. 1999;48(2):200–206. doi: 10.1046/j.1365-2125.1999.00993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pratt C., Brown A.M., Rampe D., et al. Cardiovascular safety of fexofenadine HCl. Clin Exp Allergy. 1999;29(Suppl 3):212–216. doi: 10.1046/j.1365-2222.1999.0290s3212.x. [DOI] [PubMed] [Google Scholar]
- 8.EMC . 2018. Fexofenadine Hydrochloride 120mg Film-Coated Tablets.https://www.medicines.org.uk/emc/product/3487/smpc#gref Available from: [Google Scholar]
- 9.Schifano F., Chiappini S., Miuli A., et al. Focus on over-the-counter drugs' misuse: a systematic review on antihistamines, cough medicines, and decongestants. Front Psychiatr. 2021;12 doi: 10.3389/fpsyt.2021.657397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper R.J. Over-the-counter medicine abuse - a review of the literature. J Subst Use. 2013;18(2):82–107. doi: 10.3109/14659891.2011.615002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fein M.N., Fischer D.A., O'Keefe A.W., Sussman G.L. CSACI position statement: newer generation H1-antihistamines are safer than first-generation H1-antihistamines and should be the first-line antihistamines for the treatment of allergic rhinitis and urticaria. Allergy Asthma Clin Immunol. 2019;15(1):61. doi: 10.1186/s13223-019-0375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen T.Y., Yeh Y.W., Kuo S.C., Chen C.Y., Lin T.P., Chang C.C. Diphenhydramine dependence through deep intramuscular injection resulting in myonecrosis and prolonged QT interval. J Clin Pharm Therapeut. 2014;39(3):325–327. doi: 10.1111/jcpt.12142. [DOI] [PubMed] [Google Scholar]
- 13.FDA U . 2018. FDA Adverse Event Reporting System (FAERS) Public Dashboard.https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html Available from: [Google Scholar]
- 14.Carnovale C., Mazhar F., Pozzi M., Gentili M., Clementi E., Radice S. A characterization and disproportionality analysis of medication error related adverse events reported to the FAERS database. Expet Opin Drug Saf. 2018;17(12):1161–1169. doi: 10.1080/14740338.2018.1550069. [DOI] [PubMed] [Google Scholar]
- 15.Carnovale C., Mazhar F., Arzenton E., et al. Bullous pemphigoid induced by dipeptidyl peptidase-4 (DPP-4) inhibitors: a pharmacovigilance-pharmacodynamic/pharmacokinetic assessment through an analysis of the vigibase®. Expet Opin Drug Saf. 2019;18(11):1099–1108. doi: 10.1080/14740338.2019.1668373. [DOI] [PubMed] [Google Scholar]
- 16.Mazhar F., Pozzi M., Gentili M., et al. Association of hyponatraemia and antidepressant drugs: a pharmacovigilance-pharmacodynamic assessment through an analysis of the US food and drug administration adverse event reporting system (FAERS) database. CNS Drugs. 2019;33(6):581–592. doi: 10.1007/s40263-019-00631-5. [DOI] [PubMed] [Google Scholar]
- 17.MedDRA. Medical Dictionary for Regulatory Activities [Available from: https://www.meddra.org/.
- 18.Provvedimenti relativi a taluni medicinali per uso umano [Available from: https://www.gazzettaufficiale.it/do/gazzetta/downloadPdf?dataPubblicazioneGazzetta=20131213&numeroGazzetta=292&tipoSerie=SG&tipoSupplemento=SO&numeroSupplemento=84&estensione=pdf&edizione=0.
- 19.Naranjo C.A., Busto U., Sellers E.M., et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Therapeut. 1981;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 20.National Institute of Statistics I . 2021. Istat.https://www.istat.it/en/analysis-and-products/databases Available from: [Google Scholar]
- 21.Organisation WH . 2021. Defined Daily Dose (DDD)https://www.who.int/tools/atc-ddd-toolkit/about-ddd Available from: [Google Scholar]
- 22.Pedersen M., Brabrand M. One fourth of acutely admitted patients use over-the-counter-drugs 24 hours prior to hospitalisation. Danish Med J. 2014;61(2):A4789. [PubMed] [Google Scholar]
- 23.Faure S. [Stings and bites in summer: what prevention in the pharmacy?] Actualit Pharm. 2010;497(33) [Google Scholar]
- 24.Deters M. Human H1-antihistamine exposures reported to the poisons information centre Erfurt from 2007 to 2016. Clin Toxicol. 2018;56(6):518–519. [Google Scholar]
- 25.Cassidy N., Duggan E., Williams D.J., Tracey J.A. The epidemiology and type of medication errors reported to the National Poisons Information Centre of Ireland. Clin Toxicol. 2011;49(6):485–491. doi: 10.3109/15563650.2011.587193. [DOI] [PubMed] [Google Scholar]
- 26.Tuccori M., Convertino I., Galiulo M., et al. IntenSive monitoring program of TeratOgen and non-teratogen risk of drugs during pregnancy: the STORK project. Drug Saf. 2017;40(10):937–1045. [Google Scholar]
- 27.van Gelder M.M.H.J., de Jong L.A.A., Te Winkel B., et al. Assessment of medication use during pregnancy by Web-based questionnaires, pharmacy records and serum screening. Reprod Toxicol. 2019;84:93–97. doi: 10.1016/j.reprotox.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Borres M.P., Bråkenhielm G., Irander K. How many teenagers think they have allergic rhinoconjunctivitis and what they do about it. Ann Allergy Asthma Immunol. 1997;78(1):29–34. doi: 10.1016/S1081-1206(10)63367-4. [DOI] [PubMed] [Google Scholar]
- 29.Carlsten A., Wennberg M., Bergendal L. The influence of Rx-to-OTC changes on drug sales. Experiences from Sweden 1980-1994. J Clin Pharm Therapeut. 1996;21(6):423–430. doi: 10.1111/j.1365-2710.1996.tb00042.x. [DOI] [PubMed] [Google Scholar]
- 30.Thompson A.J. Fexofenadine - why is it still not available OTC in the UK? Prescriber. 2019;30(11):34–35. [Google Scholar]
- 31.Alexandroff A.B., Harman K.E. Urticaria: an evidence-based update. Conference report. Br J Dermatol. 2010;163(2):275–278. doi: 10.1111/j.1365-2133.2010.09884.x. [DOI] [PubMed] [Google Scholar]
- 32.Marshall S. An update on hay fever treatments. Pharm J. 2009;282(7549):489–492. [Google Scholar]
- 33.Petróczi A., Naughton D.P. Popular drugs in sport: descriptive analysis of the enquiries made via the Drug Information Database (DID) Br J Sports Med. 2009;43(11):811. doi: 10.1136/bjsm.2008.052894. [DOI] [PubMed] [Google Scholar]
- 34.Sinclair H., Bond C., Largue G., Price D., Hannaford P. Community pharmacy provision of allergic rhinitis treatments: a longitudinal study of patient reported outcome. Int J Pharm Pract. 2010;13(4):249–256. [Google Scholar]
- 35.Duff N. The direct supply of medicines pilot in Scotland: a local view. Pharm J. 2003;270:26. [Google Scholar]
- 36.Watura J.C. Nut allergy in schoolchildren: a survey of schools in the Severn NHS Trust. Arch Dis Child. 2002;86(4):240–244. doi: 10.1136/adc.86.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grewar J., MacDonald T.M. Hay fever symptoms and over-the-counter remedies: a community pharmacy study. Int J Pharm Pract. 1998;6(1):22–99. [Google Scholar]
- 38.Stull D.E., Gavriel S. Use of, satisfaction with, and willingness to switch prescription and over-the-counter treatments for chronic urticaria: an online survey. The patient. 2009;2(3):151–157. doi: 10.2165/11314870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 39.Estève E., Ah-Toye C., Nseir A., Martin L. [Use of non prescription drugs for acute dermatoses. A prospective study of 111 hospitalized patients] Ann Dermatol Vénéréol. 2005;132(4):372–373. doi: 10.1016/s0151-9638(05)79287-5. [DOI] [PubMed] [Google Scholar]
- 40.Carranza Caricol F. Availability and coexistence of advertising OTC drugs with other specialities of similar composition. Pharm Care Espana. 2006;8(2):46–54. [Google Scholar]
- 41.Passalacqua G., Bousquet J., Bachert C., et al. The clinical safety of H1-receptor antagonists. An EAACI position paper. Allergy. 1996;51(10):666–675. doi: 10.1111/j.1398-9995.1996.tb02109.x. [DOI] [PubMed] [Google Scholar]
- 42.FDA . 2020. 21st Century Cures Act.https://www.fda.gov/regulatory-information/selected-amendments-fdc-act/21st-century-cures-act Available from: [Google Scholar]
- 43.US Food and Drug Administration Real World Evidence. https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence Available from:
- 44.Levi L. Stress and distress in response to psychosocial stimuli. Laboratory and real life studies on sympathoadrenomedullary and related reactions. Acta Med Scand Suppl. 1972;528:1–166. [PubMed] [Google Scholar]
- 45.Roche N., Anzueto A., Bosnic Anticevich S., et al. The importance of real-life research in respiratory medicine: manifesto of the respiratory Effectiveness group: endorsed by the international primary care respiratory group and the world allergy organization. Eur Respir J. 2019;54(3) doi: 10.1183/13993003.01511-2019. [DOI] [PubMed] [Google Scholar]
- 46.DAP EMA Europe [Available from: https://dap.ema.europa.eu/analytics/saw.dll?PortalPages.
- 47.Nakagawa K., Kajiwara A. [Female sex as a risk factor for adverse drug reactions] Nihon Rinsho Japanese J Clin Med. 2015;73(4):581–585. [PubMed] [Google Scholar]
- 48.Moulis G., Sommet A., Durrieu G., Bagheri H., Lapeyre-Mestre M., Montastruc J.L. Trends of reporting of 'serious' vs. 'non-serious' adverse drug reactions over time: a study in the French PharmacoVigilance Database. Br J Clin Pharmacol. 2012;74(1):201–204. doi: 10.1111/j.1365-2125.2012.04185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hasford J., Goettler M., Munter K.H., Müller-Oerlinghausen B. Physicians' knowledge and attitudes regarding the spontaneous reporting system for adverse drug reactions. J Clin Epidemiol. 2002;55(9):945–950. doi: 10.1016/s0895-4356(02)00450-x. [DOI] [PubMed] [Google Scholar]
- 50.Zuberbier T., Aberer W., Asero R., et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73(7):1393–1414. doi: 10.1111/all.13397. [DOI] [PubMed] [Google Scholar]
- 51.Blonde L., Khunti K., Harris S.B., Meizinger C., Skolnik N.S. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35(11):1763–1774. doi: 10.1007/s12325-018-0805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bousquet J., Schünemann H.J., Togias A., et al. Next-generation allergic rhinitis and its impact on asthma (ARIA) guidelines for allergic rhinitis based on grading of recommendations assessment, development and evaluation (GRADE) and real-world evidence. J Allergy Clin Immunol. 2020;145(1):70–80. doi: 10.1016/j.jaci.2019.06.049. e3. [DOI] [PubMed] [Google Scholar]
- 53.Csoke E., Landes S., Francis M.J., Ma L., Teotico Pohlhaus D., Anquez-Traxler C. How can real-world evidence aid decision making during the life cycle of nonprescription medicines? Clin Trans Sci. 2022;15(1):43–54. doi: 10.1111/cts.13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author upon request.