Abstract
Enterococci and Escherichia coli are opportunistic pathogens of poultry and are associated with embryo and neonatal chick mortality. We have recently demonstrated that 56% of dead broiler chicken embryos in commercial hatcheries in western Canada were due to the coinfection of Enterococcus species and E. coli. The objective of this study was to investigate the host-pathogen interactions of Enterococcus faecalis and E. coli in developing chicken embryos. Embryonating eggs at 12 d of incubation were dipped in a solution of E. faecalis and/or E. coli for 30 s to expose the eggshell to study the migration and colonization of E. faecalis and E. coli in the internal organs of chicken embryos and subsequent neonatal chicken mortality following hatch. A multidrug-resistant E. faecalis isolate from a dead chicken embryo and an E. faecalis isolate from a case of yolk sac infection were able to colonize the internal organs of chicken embryos rapidly compared to an E. faecalis isolate from a healthy chicken without affecting viability or hatchability of embryos. Although E. faecalis colonized internal organs of chicken embryos, no evidence of inflammation of these organs nor the expression of virulence genes of E. faecalis was observed. Although E. faecalis and E. coli alone did not affect the viability of embryos, a significantly high neonatal chicken mortality (27%) was observed following exposure of embryos to both E. faecalis and E. coli. Upregulation of IL-1 and CXCR4 was evident 48 h before peak mortality of neonatal chickens; this could suggest a possible link of cytokine dysregulation to increased mortality in coinfected neonatal chickens. However, further studies are warranted to investigate this issue vis-à-vis coinfection with E. faecalis and E. coli in chicken embryos and neonatal chickens.
Key words: Enterococcus faecalis, Escherichia coli, coinfection, chicken embryos, neonatal chicken
Abbreviations: BA, Blood Agar; BHI, Brain Heart Infusion; MDR, Multidrug Resistant; SEM, Scanning Electron Microscope; SPF, Specific Pathogen Free; TEM, Transmission Electron Microscope
INTRODUCTION
Bacterial infections of embryonating chicken eggs are frequently associated with embryo mortality and clinical disease in neonatal chicks (Berrang et al., 1999; Cortés et al., 2004; Olsen et al., 2012). However, the mechanism of such host-pathogen interactions is unclear. Bacterial colonization of chicken embryos starts early during embryo development, and the composition of bacterial species changes during the incubation of fertile eggs (Ding et al., 2017). Although various infectious and non-infectious causes lead to chicken embryo mortality, bacterial infections are recognized as the leading cause of embryo and neonatal chick mortality (Al-Sadi et al., 2000; Kalita et al., 2013; Babaca, 2014). Chicken embryo mortality and yolk sac infections of neonatal chickens due to enterococci and Escherichia coli are the most common and economically important pathogens. These bacterial infections lead to acute and chronic diseases during the entire growth period of broiler chickens, including increased mortality due to septicemia in neonatal chickens, chronic joint diseases, poor performance, poor feed conversion efficiency, loss of uniformity of the flock, and downgrading and increased condemnations at processing (Jassim et al., 1996; Razmyar and Zamani, 2016).
Historically, E. coli-associated yolk sac infections accounted for most embryo death compared to Enterococcus species (Babaca, 2014). Avian pathogenic E. coli causes extraintestinal tract infections in neonatal broiler chickens, predominantly yolk sac infections, omphalitis, respiratory infections, swollen head syndrome, pericarditis, airsacculitis, perihepatitis, arthritis, and osteomyelitis, septicemia, and cellulitis (Mellata, 2013). Enterococcus species are the most abundant inhabitant of the normal gastrointestinal flora of chickens (Devriese et al., 1991; Fertner et al., 2011). However, recently, Enterococcus-associated yolk sac infections in poultry have become an emerging problem in the poultry industry worldwide, including in Canada. E. faecalis has been associated with amyloid arthropathy in table-egg layers, and E. cecorum-associated osteomyelitis and spondylitis in broiler chickens and broiler breeder parents were the primary economically important pathogens in the poultry industry (Landman, 1999; Jung and Rautenschlein, 2014). E. hirae and E. durans have been associated with encephalomalacia and endocarditis in broiler breeders and young broiler chicks with high mortality (Abe et al., 2006).
Enterococci are opportunistic pathogens and cause nosocomial infections in humans, such as bacteremia, septicemia, valvular endocarditis, urinary tract infections, and intra-abdominal and pelvic infections (Moellering Jr, 1992). Among different Enterococcus species, E. faecalis, and E. faecium are responsible for the majority of infections in people (Noskin et al., 1995). Therapeutic failures associated with multidrug resistant (MDR) enterococci, such as vancomycin-resistant enterococci, cause high mortality among immunocompromised and debilitated patients (Noskin et al., 1995; Jean et al., 2001; Arias and Murray, 2012). In polymicrobial infections, synergistic interactions between various microorganisms are involved in causing diseases in the host (Hughes and Winter, 2016). Studies of wound infections in a mouse model suggest that E. faecalis promotes E. coli biofilm formation under low-iron availability, thus facilitating polymicrobial infections (Hughes and Winter, 2016).
We have recently reported that Enterococcus species, followed by E. coli were the predominant species isolated from dead chicken embryos in poultry hatcheries in western Canada (Karunarathna et al., 2017). Furthermore, we found that 56% of E. coli positive dead embryos had coinfection with Enterococcus species suggesting a potential synergism between Enterococcus species and E. coli that may increase embryo mortality (Karunarathna et al., 2017). A recent study reported that coinfection of chicken embryos with Enterococcus species and E. coli contributes to the development and increased severity of colibacillosis and enhanced embryo death (Walker et al., 2020a). Despite recent advances, whether E. faecalis and E. coli coinfections of chicken embryos could increase neonatal chicken mortality remains to be investigated. Therefore, the objective of this study was to mimic embryonic coinfections with E. faecalis and E. coli and examine their impact on posthatch mortality in neonatal chicks.
MATERIALS AND METHODS
Selection of Enterococcus and E. coli Isolates
Three E. faecalis isolates were used in these experiments. The first isolate was recovered from the rectum of a healthy 32-wk-old broiler breeder chicken and was used as the control isolate. This isolate was resistant to tylosin. The second isolate was recovered from the yolk sac of an early dead embryo from a broiler hatchery (Karunarathna et al., 2017) and determined to be multidrug resistant (MDR) to bacitracin, ceftiofur, erythromycin, lincomycin, neomycin, tetracycline, triple sulfa, and tylosin. The third isolate was recovered from the yolk sac of a 3-day-old neonatal broiler chicken that died of a yolk sac infection. This field isolate was resistant to lincomycin and tylosin.
A field isolate of E. coli from a turkey with septicemia was used as previously described. This isolate belongs to serogroup O2, is nonhemolytic, serum-resistant, produces aerobactin, K1 capsule, and Type 1 pili (Gunawardana et al., 2014).
Preparation of E. faecalis and E. coli for Experimental Challenge
Each isolate of E. faecalis was streaked on 5% Columbia sheep blood agar (BA) (Oxoid, Nepean, Ontario, Canada) and incubated aerobically at 37°C for 24 h. A single colony was inoculated in 100 mL Todd Hewitt broth (THB) and incubated at 37°C for 12 to 13 h on a shaker. According to growth curve analysis, each E. faecalis isolate reached 1 × 109 colony forming units (cfu)/mL at the end of the incubation period. Following incubation, a 1:100 dilution was made in THB, then incubated at 37°C for 4 h on a shaker to bring E. faecalis to the logarithmically growing phase at the concentration of 1 × 109 cfu/mL.
E. coli was streaked on 5% BA (Oxoid, Nepean, Ontario, Canada) and incubated aerobically at 37°C for 24 h. A single colony of E. coli was inoculated in 100 mL of Luria broth (LB) (Difco LB broth Miller; Becton, Dickinson, and Company, Sparks, MD) in a 250 mL Erlenmeyer flask. The culture was grown at 37°C for 12 h, shaking at 150 rpm. This stationary phase culture contained approximately 1 × 109 cfu/mL.
For E. faecalis and E. coli coinfection experiments, 1.5 L of THB containing E. faecalis at the required concentration was mixed with 1.5 L of THB containing E. coli with corresponding concentration according to each experiment. E. faecalis and E. coli culture preparations were cooled to 10°C and placed in 6 L plastic containers.
Viable bacterial counts of E. faecalis or E. coli were determined by plating serial dilutions of bacterial cultures in duplicate before and after each experiment on either m-Enterococcus agar or MacConkey agar (Oxoid, Nepean, Ontario, Canada) to enumerate E. faecalis or E. coli, respectively.
Ethics Statement
The animal experiment was approved by the University Committee on Animal Care and Supply Animal Research Ethics Board at the University of Saskatchewan and conducted following the guidelines of the Canadian Council on Animal Care.
EXPERIMENTAL DESIGN
Experiment A.E. faecalis infection, pathology and host cytokine gene expression in chicken embryos
The objectives of this experiment were to explore the migration of enterococci from the eggshell to internal organs, histopathology and electron microscopy of chicken embryos during incubation, expression of virulence factors of enterococci, and expression of host cytokines. The bacterial inoculation was performed using a well-established egg-dipping model (Sauter and Petersen, 1969, 1974; Mayes and Takeballi, 1983; Landman et al., 1999b; Jones et al., 2002; De Reu et al., 2006). An E. faecalis incubating egg infection model was used with few modifications (Landman et al., 1999a). Briefly, a temperature gradient was maintained between specific-pathogen-free (SPF) (Canadian Food Inspection Agency, Nepean, ON, Canada) incubating eggs at 12 d of incubation (37°C) and bacterial broth (10°C) to facilitate entry of E. faecalis into incubating eggs. Eggs were candled to determine embryo viability prior to E. faecalis infection. Viable eggs were divided into 5 groups (n = 60) as (1) nondip; (2) THB dip; (3) control E. faecalis; (4) MDR E. faecalis; and (5) field E. faecalis. Three liters of the logarithmically growing phase of E. faecalis at 1 × 109 cfu/mL were prepared for each E. faecalis isolate and maintained at 10°C during the experiment. Eggs from each group were immersed in the respective bacterial solution for 30 s and held at room temperature to air dry. All eggs were placed in incubators until they hatched. Swab samples were taken from the outer shell, inner shell, shell membrane, amnion at 6 and 8 d postinfection; additional swabs were collected from the intestine, liver, lung, and yolk at 48 h, 6, and 8 d postinfection from 5 viable embryos per group at each time point. The presence of bacteria and bacterial counts were enumerated from the direct culture method. Tissue samples from the intestine and liver were collected from 3 live embryos at 6 d postinfection for total RNA extraction to detect the expression of host cytokines (IL-1β and IL-8) and chemokines (CXCR4 and MIP-1α). The expression levels of putative virulence genes of E. faecalis (agg, gelE, cylM, cylB, cylA, efaAfs, cob, and ccf) were studied using samples from the intestine, liver, and yolk collected at 48 h, 6, and 8 d postinfection. Yolk, liver, and lung samples were collected at 6, and 8 d postinfection for histopathology. Yolk samples were collected from 5 live embryos at 6 d postinfection for electron microscopy.
Experiment B. E. faecalis and E. coli coinfection of chicken embryos and subsequent mortality and pathology in neonatal chickens
The objectives of this experiment were to explore the colonization of E. faecalis and E. coli in chicken embryos and subsequent mortality and pathology in neonatal chickens. The MDR E. faecalis isolate obtained from the yolk sac of an early dead embryo and the E. coli isolate described above were used in this experiment. Viable SPF eggs at 12 d of incubation were divided into 9 groups (n = 60) as (1) nondip; (2) THB dip; (3) E. faecalis (1 × 109 cfu/mL); (4) E. coli (1 × 109 cfu/mL); (5) E. coli (1 × 106 cfu/mL); (6) E. coli (1 × 103 cfu/mL); (7) E. faecalis and E. coli (1 × 109 and 1 × 103 cfu/mL); (8) E. faecalis and E. coli (1 × 109 and 1 × 106 cfu/mL); and (9) E. faecalis and E. coli (1 × 109 and 1 × 109 cfu/mL). Incubating SPF eggs were exposed to a logarithmically growing phase of E. faecalis, and a stationary phase of E. coli as described in experiment A. Bacterial swabs were taken from the yolk at 48 hr, 6, and 8 d postinfection from 5 viable embryos per group at each time point. Following hatch, neonatal chicks from five groups [nondip, E. faecalis alone (1 × 109 cfu/mL), E. coli alone (1 × 109 cfu/mL), E. faecalis together with E. coli (1 × 109 and 1 × 103 cfu/mL) and E. faecalis together with E. coli (1 × 109 and 1 × 106 cfu/mL)] (n = 15/group) were monitored for clinical signs and mortality for one week following hatch. Tissues from the yolk sac, liver, lung, and heart from any dead chicks following hatch were collected for histopathology.
Experiment C. E. faecalis and E. coli coinfection in chicken embryos and subsequent mortality and host cytokine gene expression in embryos and neonatal chickens
The objectives of this experiment were to explore host cytokine gene expression of chicken embryos and neonatal chickens and neonatal chicken mortality following exposure of E. faecalis and E. coli to chicken embryos. The MDR E. faecalis isolate and E. coli were used in this experiment as in experiment B. Viable SPF eggs were exposed to E. faecalis and E. coli as in experiment B, at 12 d of incubation (n = 100) as (1) nondip; (2) E. faecalis alone (1 × 109 cfu/mL); (3) E. coli alone (1 × 106 cfu/mL) and (4) E. faecalis and E. coli (1 × 109 and 1 × 106 cfu/mL). Following hatch, neonatal chicks (n = 40/group) were monitored for two weeks for clinical signs and mortality. Tissue samples from the lungs were collected from 3 live embryos and 3 live chicks at 48 hr, 6, and 8 d postinfection, and 3 and 10 d posthatch to obtain total RNA for host cytokine (IL-β) and chemokine (CXCR4) gene expression.
Bacterial Isolation and Identification from Embryos and Neonatal Chickens
Swab samples were plated on 5% Columbia sheep BA, m-Enterococcus and MacConkey agar and incubated at 37°C for 24 to 48 h. Semiquantitative analyses on plates were conducted on a scale from 0 to 4+ (Gunawardana et al., 2014). To study bacterial growth in enriched cultures, swabs were inoculated in 3 mL of THB and incubated overnight at 37°C in a shaking incubator. Bacterial isolates were identified using Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) as previously described (van Veen et al., 2010). The antimicrobial susceptibility profile of 12 antimicrobials (i.e., ampicillin, bacitracin, chloramphenicol, enrofloxacin, erythromycin, florfenicol, gentamycin, neomycin, penicillin, spectinomycin, trimethoprim-sulfamethoxazole, and tylosin) were conducted using the disk diffusion method (Kirby Bauer), interpretation of results were done according to CLSI guidelines (Clinical and Laboratory Standards Institute, 2013). Antibiograms of input and output pools of bacterial isolates were compared at each time point and organ (2 isolates/group/time point) to identify the bacteria recovered from embryos to confirm the causality.
Histopathological Examination
Histopathology of the yolk sac, liver, and lungs was examined from 3 live embryos per group at 6 and 8 d postinfection from experiment A. The yolk sac, liver, lung, and heart from any dead chicks following hatch were collected from the experiment B. Tissue sections were preserved in 10% neutral buffered formalin, embedded in paraffin, sectioned in 5 μm, and stained with hematoxylin and eosin (H&E) and Gram stain.
Scanning Electron Microscopy and Transmission Electron Microscopy
Electron microscopy of yolk sacs of embryos from all the groups was performed 6 d after the E. faecalis infection (experiment A). Tissue sections for transmission electron microscopy (TEM) were treated with 1% osmium tetroxide, then dehydrated through a graded ethanol series to propylene oxide and subsequently infiltrated with epoxy resin by a gradual exchange. The blocked specimen was polymerized at 60°C overnight. Blocks were sectioned to 90 nm on a Leica Ultracut ultramicrotome and mounted on 200 mesh copper grids. Images were collected using the Hitachi HT7700 TEM. Scanning electron microscopy (SEM) samples were paraffin-embedded and affixed to glass slides by baking at 60°C. Samples were dewaxed in xylene and rinsed in 100% ethanol, then sputter-coated with 10 nm of gold. Images were collected using the Hitachi SU8010.
Molecular Screening of Putative Virulence Determinants of E. faecalis
Total genomic DNA from E. faecalis isolates was extracted using Qiagen kit–DNeasy. Blood and tissue kits were used according to the manufacturer's instructions. Extracted DNA sample concentrations were determined by spectrophotometry at the wavelength of 260 nm and 280 nm. Virulence gene targets and polymerase chain reaction (PCR) primers are listed in Table 1. Primers were designed as previously described (Özmen Toğay et al., 2010; Yildiz and Turkyilmaz, 2015). All PCR reactions were carried out in a final volume of 50 µL reaction mixture containing 100 ng of DNA, 10X PCR buffer, 3.5 mM MgCl2, 0.4 mM each of the four dNTPs (Fermentas, Rockville, Maryland, USA), 0.8 μM of each primer and 2 units of Taq DNA polymerase (Fermentas, Rockville, Maryland, USA). Samples were subjected to an initial cycle of denaturation (95°C for 5 min), annealing (at an appropriate temperature for 30 s; Table 1), and elongation (72°C for 1 min), followed by 35 cycles in the thermocycler. Reference strain E. faecalis ATCC 29212 was used as a positive control. The amplification products were analyzed by electrophoresis on 1.0% agarose gel at 100 V for 40 min in Tris-acetate-EDTA buffer and revealed in ethidium bromide (20 µg/mL).
Table 1.
Oligonucleotide primers used to amplify putative virulence determinants (Experiment A: E. faecalis infection, pathology, and host cytokine gene expression in chicken embryos).
| Virulence gene | Primer(5-3) | Product size (bp) | Annealing temp (°C) |
|---|---|---|---|
| Ragg-F | AAGAAAAAGAAGTAGACCAAC | 1553 | 55 |
| Ragg-R | AAACGGCAAGACAAGTAAATA | ||
| RgelE-F | ACCCCGTATCATTGGTTT | 419 | 55 |
| RgelE-R | ACGCATTGCTTTTCCATC | ||
| RcylM-F | CTGATGGAAAGAAGATAGTAT | 742 | 53 |
| RcylM-R | TGAGTTGGTCTGATTACATTT | ||
| RcylB-F | ATTCCTACCTATGTTCTGTTA | 843 | 53 |
| RcylB-R | AATAAACTCTTCTTTTCCAAC | ||
| RcylA-F | TGGATGATAGTGATAGGAAGT | 517 | 53 |
| RcylA-R | TCTACAGTAAATCTTTCGTCA | ||
| RefaAfs-F | GACAGACCCTCACGAATA | 705 | 53 |
| RefaAfs-R | AGTTCATCATGCTGTAGTA | ||
| Rcob-F | AACATTCAGCAAACAAAGC | 1405 | 53 |
| Rcob-R | TTGTCATAAAGAGTGGTCAT | ||
| Rccf-F | GGGAATTGAGTAGTGAAGAAG | 543 | 53 |
| Rccf-R | AGCCGCTAAAATCGGTAAAAT |
RT-qPCR Based Determination of Putative Virulence Gene Expression in E. faecalis During Embryo Infection
Intestine and liver samples were collected in 500 μL RNAlater from 3 live embryos per group at 48 h, 6, and 8 d postinfection from the experiment A. Total RNA was extracted using Qiagen RNeasy Mini Kit according to the manufacturer's instructions. cDNA was prepared using QuantiTect Reverse Transcription kit according to the manufacturer's instructions using random primers. Amplification, detection, and real-time analysis were performed using the ABI Prism 7700 Sequence Detection System (Applied Biosystems, Waltham, Massachusetts, USA). SYBR Green I (Applied Biosystems, Waltham, Massachusetts, USA) was used for the detection of the amplified product. Primers were selected for virulence gene expression level analysis as previously described (Shepard and Gilmore, 2002). Amplification was carried out in a total volume of 20 µL containing 0.5 × SYBR Green master mix, and 2 µL of 1:5 diluted cDNA. The reactions were cycled 40 times under the following parameters: 95 C for 5 min initial cycle of denaturation followed by 95°C for 5 min denaturation, 60°C for 20 s annealing, 72°C for 30 s extension steps. At the end of the PCR, the temperature was increased from 60 to 96°C at a rate of 0.5°C/min, and the fluorescence was measured every 5 s to construct the melting curve. 23S rRNA gene was used as the positive control and primers were used as previously reported (Shepard and Gilmore, 2002). A nontemplate control (NTC) was run with every assay, and all determinations were performed at least in duplicates to demonstrate reproducibility.
Determination of Host Cytokine Gene Expression in Chicken Embryos Following E. faecalis and E. coli Infection
Total RNA extraction and cDNA synthesis were conducted as described above. Host cytokine gene expression was determined in the intestine and liver for experiment A and the lung for experiment C using real-time PCR (Mx3000P qPCR system, Agilent Technologies, Santa Clara, California, USA) and TaqMan probes. Host cytokine gene expression was conducted at 48 h, 6, and 8-d postinfection in embryos and 3 and 10 d following hatch in experiment C. The respective primer-probes and 18S rRNA amplification in the same tube (20 μL total volume) were performed using Prime Time-Gene Expression Master Mix (IDT, Redwood City, California, USA), 2 μL of cDNA template, and primers and probes as described in Table 2.
Table 2.
Primers and probes for host cytokine gene expression detection by RT-qPCR (Experiment A: E. faecalis infection, pathology, and host cytokine gene expression in chicken embryos and Experiment C: E. faecalis and E. coli coinfection in chicken embryos and subsequent mortality and host cytokine gene expression in embryos and neonatal chickens).
| Primers/probe | Sequence |
|---|---|
| IL-1 Forward IL-1 Reverse IL-1 Probe |
5-GCTCTACATGTCGTGTGTGATGAG-3 5-TGTCGATGTCCCGCATGA-3 5-(FAM)-CCACACTGCAGCTGGAGGAAGCC-(ZEN/IBFQ)-3 |
| IL-8 Forward IL-8 Reverse IL-8 Probe |
5-GCCCTCCTCCTGGTTTCAG-3 5-TGGCACCGCAGCTCATT-3 5-(FAM)-TCTTTACCAGCGTCCTACCTTGCGACA-(ZEN/IBFQ)-3 |
| MIP-1 Forward MIP-1 Reverse MIP-1 Probe |
5-GGCAGACTACTACGAGACCAACAG-3 5-ACGGCCCTTCCTGGTGAT-3 5-(FAM)-ACACAACACCAGCATGAGGGCACTG-(ZEN/IBFQ)-3 |
| CXCR-4 Forward CXCR-4 Reverse CXCR-4 Probe |
5-TGCTGCCTCAATCCAATTCTT-3 5-CAAGGCATTTTGTGCTGATGTT-3 5-(FAM)-ACGCCTTCCTGGGTGCCAAGTTC-(ZEN/IBFQ)-3 |
Statistical Analysis
Fisher's exact test was performed to determine the significance of differences in hatchability and mortality among groups. One-way ANOVA was conducted to determine the difference in cytokine and chemokine gene expression among groups.
RESULTS
Experiment A. E. faecalis infection, pathology, and host cytokine gene expression in chicken embryos
E. faecalis egg-shell penetration, and colonization in internal organs of embryos
Hatchability and embryo mortality were conducted between days 12 and 21 of incubation. No significant difference in hatchability or embryo mortality was observed among groups of hatching eggs [hatchability = nondip (85%); THB dip (80%); control E. faecalis (79%); MDR E. faecalis (94%); and field E. faecalis (92%)] (P > 0.05). At 6 d postinfection, the MDR E. faecalis isolate was recovered from the outer shell (20%), inner shell (60%), shell membrane (20%) and amnion (60%) of embryos. The field E. faecalis isolate was recovered from the inner shell (60%), shell membrane (40%) and amnion (60%) while control E. faecalis isolate was recovered from the inner shell (20%), shell membrane (20%) and amnion (20%) of embryos (Figure 1). At 8 d postinfection, the MDR E. faecalis isolate was recovered from the inner shell (40%) and shell membrane (40%) of incubating eggs. The field E. faecalis isolate was recovered from the inner shell (20%) and shell membrane (60%) while control E. faecalis isolate was recovered from the outer shell (20%) and inner shell (20%) of incubating eggs. Swabs were not collected from the amnion at 20 d following incubation since the amniotic fluid was not sufficient in the amniotic cavity on 20 d of incubation (Figure 1).
Figure 1.
Isolation of E. faecalis from the surface of the eggshell, inside of the eggshell and shell membrane at 6 and 8 days following exposure of incubating eggs to E. faecalis. (Amnion was not collected at 20 days following incubation since amniotic fluid was not sufficient for bacterial isolations.) (Experiment A: E. faecalis infection, pathology and host cytokine gene expression in chicken embryos).
No E. faecalis was isolated from nondip and THB group at any time point. E. faecalis load and rate of E. faecalis colonization in internal organs following egg infection was demonstrated in Figure 2 by direct culture. At 48 h postinfection, neither of the MDR E. faecalis isolate, field or control isolates were recovered from any internal organs of embryos. At 6 d postinfection, the MDR isolate was recovered from the yolk (40%), liver (20%), intestine (20%), and lung (40%), while the control isolate was recovered only from the yolk (20%). The field isolate was not recovered from any internal organs. At 8 d postinfection, the MDR isolate was recovered from the yolk (20%), while control E. faecalis isolate was not recovered from any internal organs. The field E. faecalis isolate was recovered from the yolk (40%), liver (20%), lung (40%), and intestines (20%). The antimicrobial susceptibility profile of the isolate remained unchanged before and after challenge at all time points.
Figure 2.
Isolation of E. faecalis from the yolk, intestine, liver and lung following 48 h, 6, and 8 d after exposure of incubating eggs to E. faecalis. (E. faecalis isolation was conducted in m-Enterococcus agar). Rate of systemic colonization of MDR E. faecalis was higher compared to other E. faecalis isolates. The field isolate of E. faecalis was detected predominantly at 8 days postinfection from all organs. (Experiment A: E. faecalis infection, pathology and host cytokine gene expression in chicken embryos).
Histopathology and Electron Microscopy
Histopathologic examination of tissue sections of the yolk, lung, and liver revealed colonization of Gram-positive cocci in the yolk, lung, and liver (Figure 3). There was no evidence of inflammation in any of the tissues in any of the groups exposed to E. faecalis. TEM and SEM demonstrated colonization of E. faecalis and biofilm-like structures attached to epithelial cells of the yolk sac at 6 d following exposure of MDR E. faecalis isolate (Figure 4).
Figure 3.
Gram and H&E stained sections of yolk, lung and liver 6 days following infection with MDR E. faecalis. Aggregates of gram positive cocci (black arrows) in the yolk, lung and liver. (A; x20 and B; x100= yolk; C; x20 and D; x60 = lung; E; x20 and F; x60 = liver) (Experiment A: E. faecalis infection, pathology and host cytokine gene expression in chicken embryos).
Figure 4.
Electron microscopic demonstration of MDR E. faecalis in the yolk sac 6 days following exposure to E. faecalis. TEM image (A) = (× 2,000, × 8,000 magnification) illustrates heavy colonization of E. faecalis in the yolk sac. SEM image (B) = (× 2,000, × 8,000 magnification) illustrates biofilm like structure of E. faecalis attached to epithelial cells of yolk sac (Yellow arrow). (Experiment A: E. faecalis infection, pathology and host cytokine gene expression in chicken embryos).
Expression of Virulence Genes of E. faecalis in Chicken Embryos During Egg Incubation
All putative virulence genes (i.e., agg, gelE, cylM, cylB, cylA, efaAfs, cob, and ccf) were present in all three E. faecalis isolates used. The amplified genomic DNA products of putative virulence genes of MDR E. faecalis isolate are shown in Figure 5. The presence of E. faecalis in the intestine, liver, and yolk sac from respective groups was confirmed by PCR amplification of cpn60. However, the virulence genes of E. faecalis were not expressed in any of the three E. faecalis isolates recovered from any internal organs from any of the groups during the experiment.
Figure 5.
(A) Amplified genomic DNA products of putative virulence genes of MDR E. faecalis isolate. (B) Amplified genomic DNA products of putative virulence genes of MDR E. faecalis isolate (A). Amplified cpn60 gene target from cDNA obtained from intestine (I), liver (L), and yolk (Y) at 48 h and 6 d post infection. (Experiment A: E. faecalis infection, pathology and host cytokine gene expression in chicken embryos).
Host Cytokine and Chemokine Gene Expression in Chicken Embryos Following E. faecalis Infection
The housekeeping gene, 18S rRNA, was stable across all the time points and all samples tested. U.pregulation of IL-1β, IL-8, CXCR4, and MIP-1α was significantly high in the intestines of embryos infected with MDR E. faecalis isolate at 6 d post-infection (P < 0.01). In contrast, no significant upregulation or downregulation of IL-1β, IL-8, CXCR4, and MIP-1α were noted in the intestine of chicken embryos infected with either field E. faecalis isolate or control E. faecalis isolate (P > 0.05) (Figure 6). Upregulation of IL-1β and CXCR4 was significantly high in the liver of embryos infected with MDR E. faecalis isolate at 6 d postinfection (P < 0.05). Upregulation of CXCR4 was significantly high in the liver of embryos infected with field E. faecalis isolate at 6 d postinfection (P < 0.05). In contrast, no significant upregulation or downregulation of IL-1β, IL-8, CXCR4, and MIP-1α were noted in the liver of chicken embryos infected with control E. faecalis isolate (P > 0.05) (Figure 7).
Figure 6.
Cytokine and chemokine gene expression in the intestines at 6 d postinfection with E. faecalis. Upregulation of IL-β, IL-8, CXCR4, and MIP-1α was significantly high in the intestine of embryos infected with MDR E. faecalis at 6 d postinfection (P < 0.01) (n = 3). (Experiment A: E. faecalis infection, pathology and host cytokine gene expression in chicken embryos).
Figure 7.
Cytokine and chemokine gene expression in the liver at 6 d postinfection with E. faecalis. Upregulation of IL-β and CXCR4 were significantly high in the liver of embryos infected with MDR E. faecalis at 6 d postinfection (P < 0.05) (n = 3). (Experiment A: E. faecalis infection, pathology and host cytokine gene expression in chicken embryos).
Experiment B. E. faecalis and E. coli coinfection of chicken embryos and subsequent mortality and pathology in neonatal chickens
E. faecalis or E. coli isolation from the yolk of chicken embryos exposed to E. faecalis and/or E. coli
There was no significant difference in hatchability or embryo mortality among groups of chicken embryos exposed to E. faecalis and E. coli, THB or the group not exposed to bacteria [hatchability = nondip (71%); THB dip (62%); E. faecalis (1 × 109 cfu/mL) (65%); E. coli (1 × 109 cfu/mL) (85%); E. coli (1 × 106 cfu/mL) (82%); E. coli (1 × 103 cfu/mL) (58%); E. faecalis and E. coli (1 × 109 and 1 × 103 cfu/mL) (95%); E. faecalis and E. coli (1 × 109 and 1 × 106 cfu/mL) (83%); and E. faecalis and E. coli (1 × 109 and 1 × 109 cfu/mL) (81%)] (P > 0.05). Colonization of E. faecalis and/or E. coli in the yolk at different time points during the incubation period is shown in Figure 8. At 48 h postinfection, E. coli was isolated from 20% (1 × 106 E. coli cfu/mL), 20% (1 × 109 E. coli cfu/mL), 20% (1 × 109 E. faecalis and 1 × 106 E. coli cfu/mL), and 20% (1 × 109E. faecalis and 1 × 109 E. coli cfu/mL) of embryos. E. faecalis was isolated from 40% (1 × 109 E. faecalis and 1 × 106 E. coli cfu/mL) of embryos. At 6 d postinfection, no E. coli was isolated from E. coli alone groups or in combination with E. faecalis. E. faecalis was isolated from 60% (1 × 109 E. faecalis cfu/mL) and 20% (1 × 109 E. faecalis and 1 × 106 cfu/mL) of embryos. At 8 d postinfection, E. coli was isolated from 20% (1 × 103 E. coli cfu/mL), 100% (1 × 106 E. coli cfu/mL), 20% (1 × 109 E. coli cfu/mL), 20% (1 × 109 E. faecalis and 1 × 106 E. coli cfu/mL) and 40% (1 × 109 E. faecalis and 1 × 109 E. coli cfu/mL) of embryos. E. faecalis was isolated from 20% (1 × 109 E. faecalis and 1 × 106 E. coli cfu/mL) and 20% (1 × 109 E. faecalis and 1 × 109 E. coli cfu/mL) of embryos. No bacteria were isolated from groups not exposed to bacteria at any time point.
Figure 8.
Isolation of E. faecalis (Ef) and E. coli (Ec) from the yolk at 48 h, 6, and 8 d following exposure of incubating eggs to different doses of E. faecalis, E. coli or co infection with E. faecalis and E. coli. (Experiment B: E. faecalis and E. coli coinfection in chicken embryos and subsequent mortality and pathology in neonatal chickens).
Percentage of bacterial recovery from the shell membrane, amnion, and yolk resulting from enrichment culture were shown in Table 3. The group of embryos exposed to 1 × 109 E. faecalis cfu/mL had 0%, 40%, and 20% E. faecalis recovery rate from the shell membrane at 48 h, 6 d, and 8 d respectively. Similarly, the rate of recovery of E. faecalis from the yolk was 0%, 80%, and 80% at 48 h, 6 d, and 8 d, respectively, while rate of E. faecalis recovery from the amnion was 0% and 40% at 48 h and 6 d, respectively.
Table 3.
Isolation percentages of E. faecalis and/or E. coli from chicken embryos in enrichment culture.
| Group-time point |
Shell membrane |
Amnion |
Yolk |
|||
|---|---|---|---|---|---|---|
| E. faecalis (%) | E. coli (%) | E. faecalis (%) | E. coli (%) | E. faecalis (%) | E. coli (%) | |
| E. faecalis 109 CFU/mlL– 48 h | 0 | 0 | 0 | |||
| E. faecalis 109 CFU/mL – 6 d | 40 | 40 | 80 | |||
| E. faecalis 109 CFU/mL – 8 d | 20 | 80 | ||||
| E.coli103 CFU/mL – 48 h | 0 | 0 | 0 | |||
| E.coli103 CFU/mL – 6 d | 0 | 0 | 0 | |||
| E.coli103 CFU/mL – 8 d | 0 | 20 | ||||
| E.coli106 CFU/mL – 48 h | 0 | 20 | 20 | |||
| E.coli106 CFU/mL – 6 d | 40 | 20 | 20 | |||
| E.coli106 CFU/mL – 8 d | 80 | 100 | ||||
| E.coli109 CFU/mL – 48 h | 0 | 20 | 20 | |||
| E.coli109 CFU/mL – 6 d | 20 | 0 | 20 | |||
| E.coli109 CFU/mL – 8 d | 40 | 60 | ||||
| E. faecalis + E.coli (109/103) – 48 h | 40 | 0 | 40 | 0 | 40 | 0 |
| E. faecalis + E.coli (109/103) – 6 d | 60 | 0 | 80 | 0 | 60 | 0 |
| E. faecalis + E.coli (109/103) – 8 d | 60 | 20 | 60 | 0 | ||
| E. faecalis + E.coli (109/ 106) – 48hrs | 20 | 0 | 20 | 20 | 20 | 20 |
| E. faecalis + E.coli (109/106) – 6 d | 0 | 0 | 0 | 0 | 40 | 20 |
| E. faecalis + E.coli (109/106) – 8 d | 0 | 20 | 60 | 80 | ||
| E. faecalis + E.coli (109/109) – 48 h | 20 | 20 | 20 | 20 | 20 | 20 |
| E. faecalis + E.coli (109/109) – 6d | 20 | 20 | 20 | 40 | 80 | 60 |
| E. faecalis + E.coli (109/109) – 8 d | 40 | 40 | 20 | 20 | ||
Enrichment cultures were obtained from shell membrane, amnion and yolk sac at different time points following exposure to E. faecalis and/or E. coli. The empty boxes indicate no isolation was attempted. (Experiment B: E. faecalis and E. coli coinfection in chicken embryos and subsequent mortality and pathology in neonatal).
The embryos exposed to 1 × 103 E. coli cfu/mL had no bacterial recovery from the shell membrane, amnion, and yolk from any time point except 20% E. coli recovery was obtained from the yolk at 8 d. The group of embryos exposed to 1 × 106 E. coli cfu/mL had 0%, 40%, and 80% E. coli recovery rate from the shell membrane at 48 h, 6 d, and 8 d respectively. Similarly, rate of recovery of E. coli from the yolk was 20%, 20%, and 100% at 48 h, 6 d, and 8 d, respectively while rate of E. coli recovery from the amnion was 20% and 20% at 48 h and 6 d, respectively. The group of embryos exposed to 1 × 109 E. coli cfu/mL had 0%, 20%, and 40% E. coli recovery rates from the shell membrane at 48 h, 6 d, and 8 d, respectively. Similarly, rates of recovery of E. coli from the yolk were 20%, 20%, and 60% at 48 h, 6 d, and 8 d, respectively, while rates of E. coli recovery from the amnion were 20% and 0% at 48 h and 6 d, respectively.
The embryos coinfected with 1 × 109 E. faecalis and 1 × 103 E. coli cfu/mL had 40%, 60% and 60% E. faecalis and 0%, 0%, and 20% E. coli recovery from the shell membrane at 48 h, 6 d, and 8 d, respectively. Similarly, rates of recovery of bacteria from the yolk were 40%, 60% and 60% E. faecalis and 0%, 0%, and 0% E. coli at 48 h, 6 d, and 8 d, respectively, while rates of bacterial recovery from the amnion were 40% and 80% E. faecalis and 0% and 0% E. coli at 48 h and 6 d, respectively.
The embryos coinfected with 1 × 109 E. faecalis and 1 × 106 E. coli cfu/mL had 20%, 0%, and 0% E. faecalis and 0%, 0%, and 20% E. coli recovery from the shell membrane at 48 h, 6 d, and 8 d, respectively. Similarly, rates of recovery of bacteria from the yolk were 20%, 40%, and 60% E. faecalis and 20%, 20%, and 80% E. coli at 48 h, 6 d, and 8 d, respectively. The rate of bacterial recovery from the amnion was 20%, and 0% E. faecalis and 20% and 0% E. coli at 48 h and 6 d, respectively.
The embryos coinfected with 1 × 109 E. faecalis and 1 × 109 E. coli cfu/mL had 20%, 20%, and 40% E. faecalis and 20%, 20%, and 40% E. coli recovery from the shell membrane at 48 h, 6 d, and 8 d, respectively. Similarly, rates of recovery of bacteria from the yolk were 20%, 80%, and 20% E. faecalis and 20%, 60%, and 20% E. coli at 48 h, 6 d and 8 d, respectively, while rates of bacterial recovery from the amnion were 20% and 20% E. faecalis and 20% and 40% E. coli at 48 h and 6 d, respectively.
Neonatal Chick Mortality
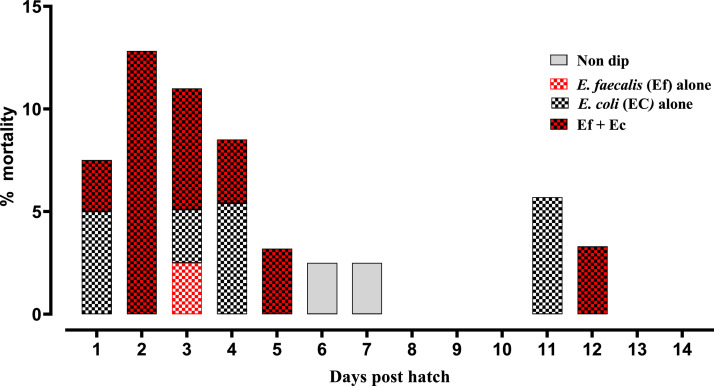
The highest neonatal chick mortality of 13.33% (2 of 15) was observed in the group of chicken embryos exposed to 1 × 109 cfu/mL E. faecalis with 1 × 10 6 cfu/mL E. coli (Figure 9). The second highest neonatal chick mortality of 6.67% (1 of 15) was observed in the group of chicken embryos exposed to 1 × 109 cfu/mL E. faecalis with 1 × 10 3 cfu/mL E. coli. Neonatal chick mortality of 6.67% (1 of 15) was also observed in the group of chicken embryos exposed to 1 × 109 cfu/mL E. coli. No clinical signs or mortality was observed in any of the other groups of chicken embryos exposed to E. faecalis or E. coli.
Figure 9.
Cumulative neonatal chick mortality during day 0–7 posthatch. Ef; E. faecalis, Ec; E. coli (Experiment B: E. faecalis and E. coli coinfection in chicken embryos and subsequent mortality and pathology in neonatal).
Gross and Histopathological Lesions of Neonatal Chickens that Died Following Hatch
Macroscopic and microscopic examination of dead chicks revealed yolk sacculitis, pericarditis, and perihepatitis. The liver stained with Gram stain revealed Gram-negative rods representing E. coli in hepatic sinusoids. Epicarditis, pericarditis, myocarditis, along with infiltration of heterophils and macrophages were prominent in the heart. Gram-negative rods were prominent in the heart, and blood vessels of the lungs. Yolk sac membranes were thick and congested and multifocal areas had infiltration of heterophils and macrophages around necrotic debris. Gram-positive cocci and Gram-negative rods were present in the yolk sac of 2 of 3 dead birds. E. faecalis and E. coli were isolated from tissue with inflammation.
Experiment C. E. faecalis and E. coli coinfection in chicken embryos and subsequent mortality and host cytokine gene expression in embryos and neonatal chickens
Neonatal Chick Mortality
There was no significant difference in hatchability or embryo mortality among groups of chicken embryos exposed to E. faecalis and E. coli or the group not exposed to bacteria [hatchability = nondip (64%); E. faecalis alone (1 × 109 CFU/mL) (59%); E. coli alone (1 × 106 CFU/mL) (55%) and E. faecalis and E. coli (1 × 109 and 1 × 106 CFU/mL) (65%)] (P > 0.05). After hatching, the highest cumulative mortality of 27.5% was observed in the group coinfected with E. faecalis and E. coli (P < 0.01). The cumulative mortality of groups infected with E. coli alone, E. faecalis alone, and nondipped were 17.5%, 2.5%, and 5%, respectively. The highest mortality of 12.82% was observed at 2 d posthatch in the group coinfected with E. faecalis and E. coli (Figure 10). No macroscopic lesions were found in any of the dead birds observed until 9 d posthatch. Dead birds in groups infected with E. coli alone or E. faecalis and E. coli coinfected group on 10 to 11 d posthatch had pericarditis, airsacculitis, perihepatitis, peritonitis, or yolk sacculitis.
Figure 10.
Neonatal chick mortality 1–2 wk posthatch. Neonatal chicks following coinfection of incubating eggs with E. faecalis and E. coli had cumulative mortality of 27.5%. Of 27.5% cumulative mortality in the group coinfected with E. faecalis with E. coli had a mortality of 12.82% on 2 d following hatch. (Experiment C: E. faecalis and E. coli coinfection in chicken embryos and subsequent mortality and host cytokine gene expression in embryos and neonatal chickens).
Host Cytokine and Chemokine Gene Expression in Chicken Embryos and Neonatal Chicks Following E. faecalis and/E. coli Infection
Upregulation of IL-1β was significantly high in chicken embryos at 48 h postinfection with E. coli alone group (P < 0.01). Similarly, expression of IL-1β was significantly high at 8 d postchallenge (prior to hatch) in E. faecalis alone group (P < 0.01) and in the group coinfected with E. faecalis and E. coli (P < 0.0001). No significant upregulation or downregulation of IL-1β was noted in any other time points with E. faecalis, E. coli, or coinfected groups (Figure 11). Upregulation of CXCR4 was significantly high at 8 d postchallenge in E. faecalis alone group (P < 0.05) and in the group coinfected with the E. faecalis and E. coli (P < 0.0001). No significant upregulation or downregulation of CXCR4 was noted in any other time points with E. faecalis, E. coli, or coinfected groups (Figure 11).
Figure 11.
Cytokine gene expression in lungs at different time points following infection with E. faecalis, E. coli or coinfection of E. faecalis and E. coli. P.C; Postchallenge, P. H; Posthatch. (n = 3). No significant difference among groups for IL-1 and CXCR4 expression in lungs (P > 0.05) but there was an upward trend in IL-1 and CXCR4 at 8 days postinfection. (Experiment C: E. faecalis and E. coli coinfection in chicken embryos and subsequent mortality and host cytokine gene expression in embryos and neonatal chickens).
DISCUSSION
The incidences of E. coli and Enterococcus species associated mortality in broiler chicken embryos and neonatal chickens have increased in the broiler chicken industry worldwide, including in western Canada, in recent years (Karunarathna et al., 2017). E. faecalis was isolated from the yolk sac of the majority of dead embryos, followed by E. coli, in a study conducted in Canada (Karunarathna et al., 2017). Bacterial contamination of fertile eggs of broiler breeder parents occurs throughout the production cycle, starting at broiler breeder farms, during egg storage, and incubation in commercial hatcheries. Vertical transmission of certain bacterial species from the hen's reproductive tract to their progeny is hypothesized in several studies, particularly with avian pathogenic E. coli isolated from neonatal broilers with omphalitis and yolk sac infections (Giovanardi et al., 2005). A study demonstrating that E. faecalis exposure via egg albumen led to arthritis in their progeny indicated possible vertical transmission (Landman et al., 1999a). Although it has been reported that chicken embryo mortality and yolk sac infections due to E. faecalis and E. coli were commonplace in the commercial poultry industry, the role of E. faecalis on E. coli associated yolk sac infections in chicken embryos and neonatal chickens is not clear. This study aimed to explore the impact of embryonic coinfections with E. faecalis and E. coli on the mortality of embryos and neonatal chickens.
We have conducted 3 experiments to demonstrate host-pathogen interactions of E. faecalis and E. coli in chicken embryos and neonatal chickens by exposing embryonated eggs to E. faecalis and E. coli. The first experiment was to explore the rate of E. faecalis migration and colonization in chicken embryos, cytokine and chemokine responses, and investigate the E. faecalis-associated pathology of embryos at microscopic and electron microscopic levels. The second experiment was to explore bacterial migration and colonization rates in chicken embryos following coinfection of chicken embryos with E. faecalis and E. coli and subsequent neonatal chicken mortality. The third experiment was to measure cytokine and chemokine responses of embryos and neonatal chickens and neonatal chicken mortality following coinfection of chicken embryos with E. faecalis and E. coli. To the best of our knowledge, this is the first study to demonstrate the role of E. faecalis on E. coli-associated pathology in chicken embryos and neonatal chickens by exposing E. faecalis and E. coli on eggshells of embryonated SPF eggs.
In the first experiment, we demonstrated that E. faecalis was able to penetrate through the eggshell to the inner shell, shell membrane and amnion during the incubation period without causing a negative effect on hatchability. The E. faecalis load and the rate of bacterial colonization were higher with the MDR E. faecalis isolate from a dead embryo and with the field E. faecalis isolate from a case of yolk sac infection compared to the control E. faecalis isolate from a healthy adult broiler breeder chicken. Similarly, colonization of E. faecalis in the liver, intestine, yolk, and lung was higher with the MDR E. faecalis isolate and with the field E. faecalis isolate compared to the control E. faecalis isolate from a healthy chicken, particularly 6 d and 8 d following infection. Although E. faecalis was isolated and the presence of E. faecalis was confirmed by histopathology in the yolk, liver and lung, no inflammation was noted on histopathology around E. faecalis colonized areas in any of those internal organs 6 d following E. faecalis exposure in embryonated eggs. Furthermore, biofilm-like structures were noted in the yolk with the MDR E. faecalis isolate 6 d following exposure of embryonated eggs to E. faecalis. This may be a mechanism used by E. faecalis to evade the immune system of chicken embryos. In our study, the MDR E. faecalis isolate had virulence factors associated genes that play roles in adhesion, colonization, and cell damage, as evidenced by PCR amplification of these genes in vitro cultured bacteria. We could detect very low amplification of cpn60 genes in vivo following E. faecalis infection; however, we failed to detect the expression of virulence-related genes in vivo in embryonated eggs. It is quite possible that the levels of expression of these genes may be low enough to get detected by the conventional PCR, or we missed the time to collect the samples when these genes were transiently expressed (mRNA) by the bacteria. Therefore, our study can not rule out the role of these virulence factors in bacterial pathogenesis.
Increased levels of IL-1β, IL-8, CXCR4, and MIP-1α gene expression were noted in the liver or intestines of embryos 6 d following exposure with MDR E. faecalis isolate (P < 0.01). In contrast, CXCR4 was increased in the liver with field E. faecalis isolate (P < 0.05). No cytokine or chemokine mRNA expression was detected in the liver or intestines with control E. faecalis isolate. Our data indicate that the pathogenic but not the non-pathogenic strains of E. faecalis may stimulate IL-1β cytokine expression following infection. A recent study supports our data that reported that the pathogenic E. faecalis strain could upregulate IL-1β by activating NLRP3 inflammasome (Ran et al., 2021). In our study, we found upregulation of both IL-1β and CXCR4 following MDR E. faecalis infection. A study has reported that IL-1β can upregulate CXCR4 (Sun et al., 2015); we hypothesize it may also be the case here, but it remains to be determined experimentally. Interestingly, our study did not see mRNA expression of IL-8 and MIP-1, whose expression largely depends on NF-kappaB activation. It is possible that E. faecalis down-regulates some proinflammatory responses of the host to escape from the immune system. It has been demonstrated in a mouse model of catheter-associated urinary tract infection that E. faecalis was able to subvert macrophage activation by preventing the NF-κB signaling pathway, which controls the transcription of genes responsible for immune regulation and proinflammatory cytokines and chemokines that regulate recruitment and activation of immune cells(Tien et al., 2017). Future studies will investigate if our observation of no upregulation of host IL-8 and MIP-1 α in our study is also linked to the NF-κB signaling dysregulations.
Although we have seen in our experiments that MDR E. faecalis isolate and field E. faecalis isolate had a higher colonization rate in internal organs compared to control E. faecalis isolate from a healthy chicken, all E. faecalis isolates were able to penetrate eggshell and colonize internal organs of chicken embryos, thus indicating the ability of E. faecalis to infect chicken embryos following fecal contamination of eggshells irrespective of genetic differences of E. faecalis isolates. Although we have not seen embryo mortality associated with E. faecalis in this study, we have recently demonstrated that 56% of dead broiler chicken embryos have coinfection of Enterococcus species including E. faecalis with E. coli in a field study conducted in poultry hatcheries in western Canada (Karunarathna et al., 2017). It is likely that certain virulent E. faecalis isolates together with E. coli potentially cause chicken embryo mortality. A recent study using a broiler chicken embryo lethal assay coinfection of embryonated eggs with avian pathogenic E. coli with E. faecalis resulted in increased mortality of chicken embryos compared to embryonated eggs infected with E. faecalis alone or E. coli alone (Walker et al., 2020b). The differences in the method of bacterial infection could explain the discrepancy between our and this recent study. In our study, we have used eggshell bacteria application to facilitate embryonic infection (here, the number of bacteria penetrating eggshells will be in low number). In contrast, Walker et al. (Walker et al., 2020b) directly injected a substantially large number of bacteria into embryonated eggs to simulate the bacterial infection of embryos.
In the second experiment, we demonstrated coinfection of embryonated SPF eggs with E. faecalis with E. coli leading to colonization of yolk with E. faecalis and E. coli, particularly close to hatch. E. faecalis and E. coli colonization was measured by both direct and enrichment culture methods in yolk, shell membrane and amnion. Both culture methods confirmed that, on average, less than 50% of embryos were colonized with E. faecalis or E. coli. Although no significant colonization or mortality was observed in infected embryos, increased mortality of neonatal chickens was observed in the group coinfected with E. faecalis and E. coli compared to the groups infected with either E. faecalis or E. coli alone. Based on our data, we hypothesized that E. faecalis might have supported the pathogenicity of E. coli during the coinfection of embryos with E. faecalis and E. coli.
Therefore, a third experiment was designed to look for a possible mechanism for the increased mortality of neonatal chicks after coinfection with E. faecalis and E. coli. The third experiment found significantly high mortality of neonatal chickens in the group exposed to E. faecalis and E. coli during embryonic life. Moreover, in the third experiment, we found substantially high IL-1 and CXCR4 mRNA expression in the lung, particularly a day before hatch. Birds that died of E. faecalis and E. coli were septicaemic and had severe pericarditis, airsacculitis, or polyserositis. The enhanced expression of CXCR4 has been linked with neonatal sepsis in humans and used as a biomarker for neonatal sepsis (Tunc et al., 2015). Therefore, our findings could suggest that increased mortality of neonatal chickens in the group that were coinfected with E. faecalis and E. coli could be due to an excessive cytokine IL-1 and chemokine receptor CXCR4 resulting in sepsis, contributing to high mortality of neonatal chickens 24 h following hatch.
In summary, our findings suggest that E. faecalis can penetrate the eggshell, evade immune barriers in the egg, and colonize systemically. The coinfection of embryonated eggs with E. faecalis and E. coli leads to enhanced pathogenicity resulting in increased mortality of neonatal chickens. However, more studies are required to investigate the role of the cytokine IL-1 and chemokine receptor CXCR4 in the resulting septicemia and bacterial pathogenesis during E. faecalis and E. coli coinfection in chickens.
ACKNOWLEDGMENTS
The authors greatly appreciate the assistance of animal care technicians at the Animal Care Unit, Western College of Veterinary Medicine, University of Saskatchewan for their service. Financial support for this research was provided by grants from the Alberta Agriculture and Forestry, Alberta, Canada (Grant # 2018F141R), and the Natural Sciences and Engineering Research Council of Canada (NSERC-Discovery 420261).
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- Abe Y., Nakamura K., Yamada M., Yamamoto Y. Encephalomalacia with Enterococcus durans infection in the brain stem and cerebral hemisphere in chicks in Japan. Avian Dis. 2006;50:139–141. doi: 10.1637/7419-080805R.1. [DOI] [PubMed] [Google Scholar]
- Al-Sadi H., Basher H., Ismail H. Bacteriologic and pathologic studies on dead-in-shell chicken embryos. Iraqi J. Vet. Sci. 2000;13:297–307. [Google Scholar]
- Arias C.A., Murray B.E. The rise of the Enterococcus: beyond vancomycin resistance. Nat. Rev. Microbiol. 2012;10:266–278. doi: 10.1038/nrmicro2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaca Z. Epidemiological and bacteriological studies on dead-in-shell embryos. J. Vet. Sci. Technol. 2014;5:2. [Google Scholar]
- Berrang M., Cox N., Frank J., Buhr R. Bacterial penetration of the eggshell and shell membranes of the chicken hatching egg: a review. J. Appl. Poult. Res. 1999;8:499–504. [Google Scholar]
- Clinical and Laboratory Standards Institute . VET01-S2 Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals. 2nd informational supplement. Clinical and Laboratory Standard Institute; Wayne, PA: 2013. [Google Scholar]
- Cortés C.R., Isaías G.T., Cuello C.L., Flores J.M.V., Anderson R.C., Campos C.E. Bacterial isolation rate from fertile eggs, hatching eggs, and neonatal broilers with yolk sac infection. Revista Latinoamericana de Microbiologia. 2004;46:12–16. [PubMed] [Google Scholar]
- De Reu K., Grijspeerdt K., Messens W., Heyndrickx M., Uyttendaele M., Debevere J., Herman L. Eggshell factors influencing eggshell penetration and whole egg contamination by different bacteria, including Salmonella enteritidis. Int. J. Food Microbiol. 2006;112:253–260. doi: 10.1016/j.ijfoodmicro.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Devriese L., Hommez J., Wijfels R., Haesebrouck F. Composition of the enterococcal and streptococcal intestinal flora of poultry. J. Appl. Microbiol. 1991;71:46–50. [PubMed] [Google Scholar]
- Ding J., Dai R., Yang L., He C., Xu K., Liu S., Zhao W., Xiao L., Luo L., Zhang Y. Inheritance and establishment of gut microbiota in chickens. Front. Microbiol. 2017;8:1967. doi: 10.3389/fmicb.2017.01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertner M.E., Olsen R.H., Bisgaard M., Christensen H. Transmission and genetic diversity of Enterococcus faecalis among layer chickens during hatch. Acta Veterinaria Scandinavica. 2011;53:56. doi: 10.1186/1751-0147-53-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanardi D., Campagnari E., Ruffoni L.S., Pesente P., Ortali G., Furlattini V. Avian pathogenic Escherichia coli transmission from broiler breeders to their progeny in an integrated poultry production chain. Avian Pathol. 2005;34:313–318. doi: 10.1080/03079450500179046. [DOI] [PubMed] [Google Scholar]
- Gunawardana T., Foldvari M., Zachar T., Popowich S., Chow-Lockerbie B., Ivanova M.V., Tikoo S., Kurukulasuriya S., Willson P., Gomis S. Protection of neonatal broiler chickens following in ovo delivery of oligodeoxynucleotides containing CpG motifs (CpG-ODN) formulated with carbon nanotubes or liposomes. Avian Dis. 2014;59:31–37. doi: 10.1637/10832-032814-reg. [DOI] [PubMed] [Google Scholar]
- Hughes E.R., Winter S.E. Enterococcus faecalis: E. coli's Siderophore-Inducing Sidekick. Cell Host Microbe. 2016;20:411–412. doi: 10.1016/j.chom.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassim E., Grossman M., Koops W., Luykx R. Multiphasic analysis of embryonic mortality in chickens. Poult. Sci. 1996;75:464–471. doi: 10.3382/ps.0750464. [DOI] [PubMed] [Google Scholar]
- Jean S.S., Fang C.T., Wang H.K., Hsue P., Chang S.C., Luh K.T. Invasive infections due to vancomycin-resistant enterococci in adult patients. J. Microbiol. Immunol. Infect. = Wei mian yu gan ran za zhi. 2001;34:281–286. [PubMed] [Google Scholar]
- Jones D.R., Anderson K.E., Curtis P.A., Jones F.T. Microbial contamination in inoculated shell eggs: I. Effects of layer strain and hen age. Poult. Sci. 2002;81:715–720. doi: 10.1093/ps/81.5.715. [DOI] [PubMed] [Google Scholar]
- Jung A., Rautenschlein S. Comprehensive report of an Enterococcus cecorum infection in a broiler flock in Northern Germany. BMC Vet. Res. 2014;10:311. doi: 10.1186/s12917-014-0311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalita N., Pathak N., Ahmed M., Saikia G. Various causes related to dead-in-shell embryos of crossbred (PB-2 x Indigenous) chicken egg. Vet. World. 2013;6:774–777. [Google Scholar]
- Karunarathna R., Popowich S., Wawryk M., Chow-Lockerbie B., Ahmed K.A., Yu C., Liu M., Goonewardene K., Gunawardana T., Kurukulasuriya S. Increased Incidence of enterococcal infection in nonviable broiler chicken embryos in Western Canadian hatcheries as detected by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry. Avian Dis. 2017;61:472–480. doi: 10.1637/11678-052317-Reg.1. [DOI] [PubMed] [Google Scholar]
- Landman W.J.M. Amyloid arthropathy in chickens: (Summary of thesis, Utrecht University, faculty of veterinary medicine, 1998) Vet. Q. 1999;21:78–82. doi: 10.1080/01652176.1999.9694998. [DOI] [PubMed] [Google Scholar]
- Landman W., Mekkes D., Chamanza R., Doornenbal P., Gruys E. Arthropathic and amyloidogenic Enterococcus faecalis infections in brown layers: a study on infection routes. Avian Pathol. 1999;28:545–557. doi: 10.1080/03079459994335. [DOI] [PubMed] [Google Scholar]
- Landman W.J., Mekkes D.R., Chamanza R., Doornenbal P., Gruys E. Arthropathic and amyloidogenic Enterococcus faecalis infections in brown layers: a study on infection routes. Avian Pathol. 1999;28:545–557. doi: 10.1080/03079459994335. [DOI] [PubMed] [Google Scholar]
- Mayes F.J., Takeballi M.A. Microbial contamination of the hen's egg: a review. J. Food Prot. 1983;46:1092–1098. doi: 10.4315/0362-028X-46.12.1092. [DOI] [PubMed] [Google Scholar]
- Mellata M. Human and avian extraintestinal pathogenic Escherichia coli: infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathogens Dis. 2013;10:916–932. doi: 10.1089/fpd.2013.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering R.C., Jr. Emergence of Enterococcus as a significant pathogen. Clin. Infect. Dis. 1992;14:1173–1176. doi: 10.1093/clinids/14.6.1173. [DOI] [PubMed] [Google Scholar]
- Noskin G.A., Peterson L.R., Warren J.R. Enterococcus faecium and Enterococcus faecalis bacteremia: acquisition and outcome. Clin. Infect. Dis. 1995;20:296–301. doi: 10.1093/clinids/20.2.296. [DOI] [PubMed] [Google Scholar]
- Olsen R.H., Frantzen C., Christensen H., Bisgaard M. An investigation on first-week mortality in layers. Avian Dis. 2012;56:51–57. doi: 10.1637/9777-051011-Reg.1. [DOI] [PubMed] [Google Scholar]
- Özmen Toğay S., Celebi Keskin A., Açık L., Temiz A. Virulence genes, antibiotic resistance and plasmid profiles of Enterococcus faecalis and Enterococcus faecium from naturally fermented Turkish foods. J. Appl. Microbiol. 2010;109:1084–1092. doi: 10.1111/j.1365-2672.2010.04763.x. [DOI] [PubMed] [Google Scholar]
- Ran S., Huang J., Liu B., Gu S., Jiang W., Liang J. Enterococcus Faecalis activates NLRP3 inflammasomes leading to increased interleukin-1 beta secretion and pyroptosis of THP-1 macrophages. Microb. Pathog. 2021;154 doi: 10.1016/j.micpath.2021.104761. [DOI] [PubMed] [Google Scholar]
- Razmyar J., Zamani A.H. An outbreak of yolk sac infection and dead-in-shell mortality in common canary (Serinus canaria) caused by Klebsiella pneumoniae. Iranian J. Vet. Res. 2016;17:141. [PMC free article] [PubMed] [Google Scholar]
- Sauter E.A., Petersen C.F. The effect of egg shell quality on penetration by Pseudomonas fluorescens. Poult. Sci. 1969;48:1525–1528. doi: 10.3382/ps.0481525. [DOI] [PubMed] [Google Scholar]
- Sauter E.A., Petersen C.F. The effect of egg shell quality on penetration by various salmonellae. Poult. Sci. 1974;53:2159–2162. doi: 10.3382/ps.0532159. [DOI] [PubMed] [Google Scholar]
- Shepard B.D., Gilmore M.S. Differential expression of virulence-related genes in Enterococcus faecalis in response to biological cues in serum and urine. Infect. Immun. 2002;70:4344–4352. doi: 10.1128/IAI.70.8.4344-4352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Zhu D., Wang G., Wang D., Zhou H., Liu X., Jiang M., Liao L., Zhou Z., Hu J. Pro-Inflammatory cytokine IL-1beta up-regulates CXC chemokine receptor 4 via Notch and ERK signaling pathways in tongue squamous cell carcinoma. PloS One. 2015;10 doi: 10.1371/journal.pone.0132677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien B.Y.Q., Goh H.M.S., Chong K.K.L., Bhaduri-Tagore S., Holec S., Dress R., Ginhoux F., Ingersoll M.A., Williams R.B., Kline K.A. Enterococcus faecalis promotes innate immune suppression and polymicrobial catheter-associated urinary tract infection. Infection Immun. 2017;85:1–14. doi: 10.1128/IAI.00378-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunc T., Cekmez F., Cetinkaya M., Kalayci T., Fidanci K., Saldir M., Babacan O., Sari E., Erdem G., Cayci T., Kul M., Kavuncuoglu S. Diagnostic value of elevated CXCR4 and CXCL12 in neonatal sepsis. J. Maternal-Fetal Neonatal Med. 2015;28:356–361. doi: 10.3109/14767058.2014.916683. [DOI] [PubMed] [Google Scholar]
- van Veen S.Q., Claas E., Kuijper E.J. High-throughput identification of bacteria and yeast by matrix-assisted laser desorption ionization-time of flight mass spectrometry in conventional medical microbiology laboratories. J. Clin. Microbiol. 2010;48:900–907. doi: 10.1128/JCM.02071-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G.K., Suyemoto M.M., Gall S., Chen L., Thakur S., Borst L.B. The role of Enterococcus faecalis during co-infection with avian pathogenic Escherichia coli in avian colibacillosis. Avian Pathol. 2020;49:589–599. doi: 10.1080/03079457.2020.1796926. [DOI] [PubMed] [Google Scholar]
- Walker G.K., Suyemoto M.M., Gall S., Chen L., Thakur S., Borst L.B. The role of Enterococcus faecalis during co-infection with avian pathogenic Escherichia coli in avian colibacillosis. Avian Pathol. 2020;49:589–599. doi: 10.1080/03079457.2020.1796926. [DOI] [PubMed] [Google Scholar]
- Yildiz O., Turkyilmaz S. Investigation of virulence genes of Enterococcus faecalis strains isolated from mastitic bovine milk. Israel J. Vet. Med. 2015;70:4. [Google Scholar]