Abstract
Vitamin D deficiency has been associated with the risk of multiple sclerosis, disease activity and progression. Results from in vitro experiments, animal models and analysis of human samples from randomized controlled trials provide comprehensive data illustrating the pleiotropic actions of Vitamin D on the immune system. They globally result in immunomodulation by decreasing differentiation of effector T and B cells while promoting regulatory subsets. Vitamin D also modulates innate immune cells such as macrophages, monocytes and dendritic cells, and acts at the level of the blood–brain barrier reducing immune cell trafficking. Vitamin D exerts additional activity within the central nervous system reducing microglial and astrocytic activation. The immunomodulatory role of Vitamin D detected in animal models of multiple sclerosis has suggested its potential therapeutic use for treating multiple sclerosis. In this review, we focus on recent published data describing the biological effects of Vitamin D in animal models of multiple sclerosis on immune cells, blood–brain barrier function, activation of glial cells and its potential neuroprotective effects. Based on the current knowledge, we also discuss optimization of therapeutic interventions with Vitamin D in patients with multiple sclerosis, as well as new technologies allowing in-depth analysis of immune cell regulations by vitamin D.
Keywords: vitamin D, multiple sclerosis, cytokines, lymphocytes, immunomodulation
Galoppin et al. provide a comprehensive analysis of the biological effects of Vitamin D (VitD) in animal models and patients with multiple sclerosis, especially focusing on regulation of immune cells, blood–brain barrier function, activation of glial cells and therapeutic trials. They discuss optimization of therapeutic interventions with VitD in multiple sclerosis.
Graphical Abstract
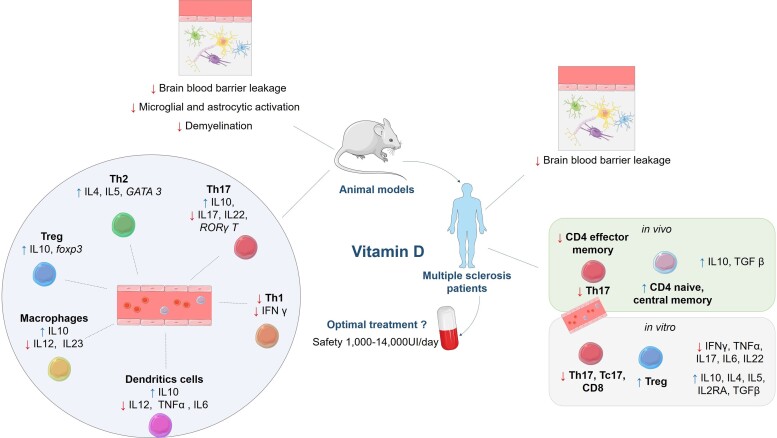
Graphical Abstract.
Introduction
Multiple sclerosis is a chronic inflammatory and neurodegenerative disease of the CNS especially affecting women (3:1 sex ratio). Eighty-five per cent of patients develop multiple sclerosis with a clinically isolated syndrome corresponding to an acute demyelination of the optic nerves, brainstem or spinal cord, followed by recovery. Most multiple sclerosis patients experience subsequent relapses (relapsing-remitting multiple sclerosis—RRMS) with some later converting to secondary progressive multiple sclerosis (SPMS), characterized by gradual neurological deterioration, with or without superimposed relapses. In 15% of cases, neurological symptoms progress from onset (primary progressive multiple sclerosis), with equal frequency in males and females.
Epidemiological studies have highlighted a familial aggregation of multiple sclerosis potentially explained by several environmental and genetic risk factors.1 Family studies assessing risks to relatives of multiple sclerosis patients showed that first-degree relatives are generally at 10–25 times greater risk of developing multiple sclerosis than the general population, but this risk could be lower.2 Several major histocompatibility complex (MHC) loci—especially HLA DRB1*15:01—are the most important genetic risk factors for developing multiple sclerosis with odd ratios around three. Moreover, genome wide association studies (GWAS) studies have identified more than 200 susceptibility variants other than MHC that implicate multiple innate and adaptive immune pathways distributed across the cellular components of the immune system and brain-resident immune cells, highlighting the importance of altered peripheral immune cells and glial cells responses in multiple sclerosis pathophysiology.3
The main environmental risk factors for multiple sclerosis include smoking,4,5 obesity,6 Epstein-Barr Virus infection,7,8 sun exposure9,10 and vitamin D (VitD) deficiency.11 The latter has been recognized as a major risk factor for multiple sclerosis, with circulating levels modulated by specific genetic variants, skin colour and sun exposure, especially in early years of life.2 Many reports have highlighted the clinical correlation of circulating VitD levels in multiple sclerosis with clinical disease activity12,13 and of disability progression.9,14 Moreover, VitD concentrations are significantly lower in females compared with males15 and lack of sunlight and VitD deficiency seem to further increase the risk of multiple sclerosis in females than in males.16
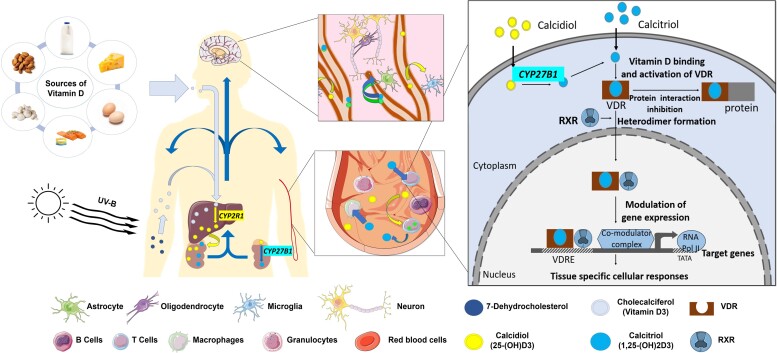
There are different sources of VitD. Cholecalciferol (VitD3) is a lipophilic cholesterol derivative (Fig. 1) synthesized in the skin from 7-dehydrocholesterol under the effect of UV radiation. It is also provided by animal food sources, especially oily fish. Cholecalciferol, which is biologically inactive, is stored in adipose tissue and transformed in the liver into 25-hydroxyvitamin D3 [25(OH)D3, calcidiol], which is the main circulating form of VitD. Calcidiol is then hydroxylated in the kidney to form 1,25-dihydroxyvitamin D3 [1,25(OH)2D3, calcitriol], which is the active metabolite of VitD.17 VitD metabolites circulate ubiquitously, bound to VitD binding protein (DBP), its transporter. Calcitriol acts intracellularly as a transcription factor by binding to the VitD receptor (VDR), which heterodimerizes with retinoid-X-receptor (RXR).18,19 Upon translocation of this heterodimer to the nucleus, VitD recognizes VitD responsive elements (VDREs) in the genomic DNA and modulates the expression of over 200 genes. Most of these are associated with immune functions and VitD metabolism, highlighting the key role of VitD deficiency in numerous inflammatory diseases and cancer.1
Figure 1.
Sources, metabolism and cellular mode of action of VitD. VitD is synthesized in the skin under the effect of UV radiation and is also provided by food sources. Cholecalciferol (inactive form) is stored in adipose tissue and transformed in the liver into 25-hydroxyvitamin D3 [25(OH)D3, calcidiol—main circulating form]. Calcidiol is then hydroxylated in the kidney to form 1,25-dihydroxyvitamin D3 [1,25(OH)2D3, calcitriol—active metabolite]. VitD metabolites circulate ubiquitously, bound to its transporter: Vit DBP and is able to cross the blood–brain barrier to modulate CNS-resident cells (neurons, astrocytes, oligodendrocytes and microglia). Calcitriol acts intracellularly as a transcription factor by binding to the cytoplasmic VDR which heterodimerizes with RXR. Upon translocation of this heterodimer to the nucleus, VitD/VDR/RXR complex binds to the VDRE in the genomic DNA and modulates the expression of target genes. Drawings of the individual cell types were adapted from Servier Medical Art (http://smart.servier.com/), licenced under a Creative Common Attribution 3.0 Generic License.
The pleiotropic actions of VitD on the immune system globally result in immunomodulation by decreasing differentiation of effector T and B cells while promoting regulatory subsets.20 VitD is also known to modulate cells of the innate immune system such as macrophages, monocytes and dendritic cells (DCs), that additionally contribute to multiple sclerosis pathophysiology.20 Furthermore, VitD acts in the CNS by regulating microglial and astrocytic activation.21 The immunomodulatory role of VitD in animal models of multiple sclerosis has suggested its potential therapeutic use in multiple sclerosis. In this context, a comprehensive analysis of data concerning the in vitro and in vivo effects of VitD in multiple sclerosis is mandatory to optimize its use in clinical practice. In this review, we focus on recent published data describing the biological effects of VitD in animal models of multiple sclerosis, on immune cells, blood–brain barrier (BBB) function, activation of glial cells and its potential neuroprotective effects. Based on the current knowledge, we discuss the optimization of therapeutic interventions with VitD in multiple sclerosis patients.
Immune cells and animal models
VitD ameliorates experimental autoimmune encephalomyelitis
Experimental autoimmune encephalitis (EAE), the main animal model of multiple sclerosis, is based on immunization of susceptible animals (especially rodents) with myelin antigens to trigger an autoinflammatory response in the CNS. Used in multiple strains or genotypes of rodents, it has been largely developed to recapitulate different aspects of the disease (clinical course, lesion localization or pathological patterns) and has significantly contributed to our understanding of the immunopathologic features of multiple sclerosis.22
EAE can be actively induced (active EAE) by the immunization of animals with peptides in emulsion of incomplete Freund’s adjuvant containing heat killed Mycobacterium tuberculosis (H37Ra) in combination with pertussis toxin. Peptide injection triggers activation of myelin antigen-specific T cells and leads to CNS autoimmunity.23 Alternatively, passive EAE is induced by the adoptive transfer of pre-activated myelin-specific T cells into naïve syngeneic recipients. The development of clinical EAE can be quantified using different clinical EAE scoring systems.23,24
Most used EAE models include immunization with peptides from myelin oligodendrocyte glycoprotein (MOG) (e.g. MOG35−55) in C57BL/6 mice,25–30 with myelin basic protein (MBP) in B10.PL mice or in Lewis rats29,31–35 or with proteolipid protein (PLP) (e.g. PLP139–151) in SJL mice.27,28,36
The efficacy of calcitriol in active EAE models has been analysed in a prophylactic or curative approach. Most studies show a clear protective effect of VitD in EAE. In the prophylactic method, a supplementation of calcitriol is added to the food before induction of EAE (generally, 50 ng/day for females and 200 ng/day for males).32 In the curative method, calcitriol is administrated after the onset of clinical signs by intraperitoneal injection (50–300 ng), which may be combined with a supplementation in food (50 ng/day). Prophylactic administration of calcitriol inhibited EAE development, and therapeutic administration decreased the severity of active and passive EAE in B10.PL, C57BL/6 and SJL/J.25–27,31,32,34,36,37 Moreover, cholecalciferol also reduced EAE severity in prevention.29,33,35 In contrast, cholecalciferol alone failed to reverse the disease in the therapeutic setting in the MBP B10.PL mouse EAE model, while the combination of cholecalciferol and calcitriol did.38
An interesting study showed that the protective effect of VitD in EAE is transient as removal of VitD resulted in an increased severity of the disease in MOG-induced EAE in C57BL/6 mice.26 This study also suggested that calcitriol does not inhibit the production of autoreactive CD4+ T cells but that it may block their migration to the CNS. Indeed, there are fewer mononuclear cells infiltrating the CNS tissue after the induction of active EAE in mice and rats treated with cholecalciferol or calcitriol.25,27,33,34,37 Calcitriol may decrease the migration of encephalitogenic T cells via the regulation of adhesion molecules involved in their trafficking into the CNS, as suggested by the lowered CXCR3 expression on CD4+ T cells in calcitriol-treated mice (MOG-induced EAE in the C57BL/6 model).26 In vitro, calcitriol reduced CCR6 expression and migration of Th17 cells.25 Furthermore, reduced secretion of pro-inflammatory interleukin (IL)-17A and IL-22 and increased production of anti-inflammatory IL-10 by Foxp3+ CD4+ CD25high regulatory T cells (Tregs) was detected in the brain of PLP139–151 EAE SJL/J mice treated with calcitriol.27
Several important studies have demonstrated the key role of the VDR in mediating the protective effect of VitD in EAE. VDR knockout (VDR KO) C57BL/6 mice with deleted exons coding for the first or second zinc finger of the DNA binding domain (VDRtm1Ska-KO and VDRtm1Mbd-KO, respectively) have been used.39,40 In the absence of VDR, calcitriol does not confer a protection to active EAE in C57BL/6 VDRtm1Ska-KO mice.41 Furthermore, calcitriol failed to inhibit EAE in C57BL/6 mice lacking functional VDR specifically in CD4+ T lymphocytes,41 demonstrating that VDR expression in CD4+ T cells is key for its protective effect.42 Moreover, both in vitro and in vivo, an increase in autoreactive Th17 responses and a decrease in tolerogenic CD103+ CD11c+ DCs were observed in VDR KO mice.43 Taken together, VDR seems indispensable to mediate the anti-inflammatory responses mediated by VitD.
Circulating lymphocytes
CD4+ Th1 and Th17 cells
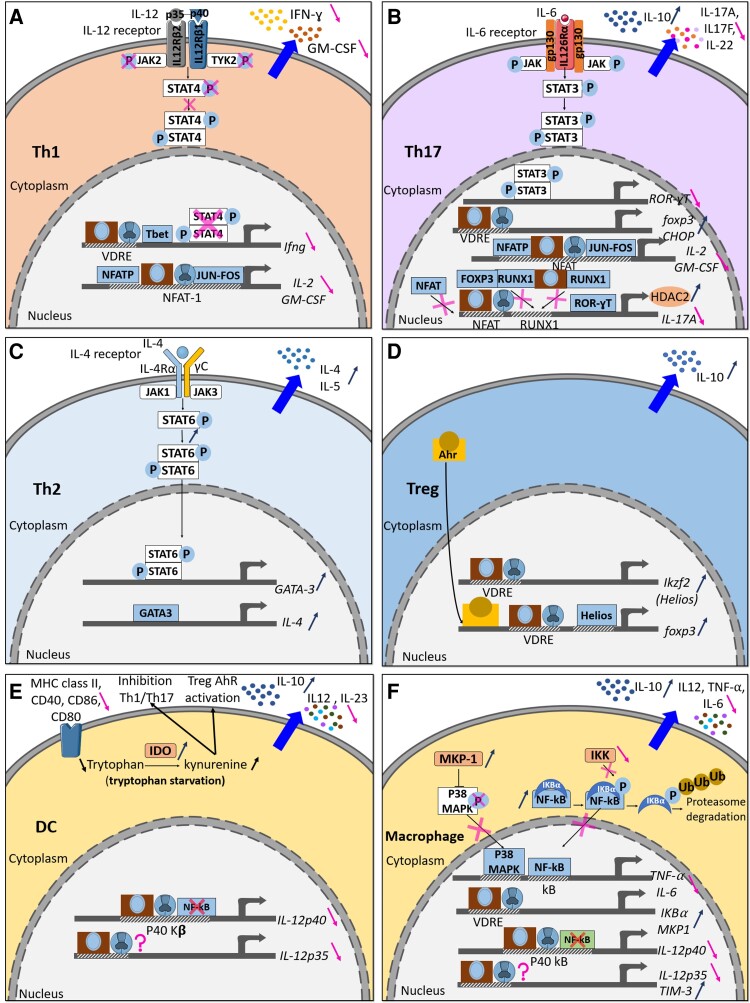
Most immunomodulatory effects of VitD have been well described in CD4+ T lymphocytes. Calcitriol exerts strong and direct immunomodulatory effects when added to CD4+ T-cell cultures in vitro, by inhibiting effector Th1 and Th17 and promoting Th2 and Tregs calcitriol inhibits the differentiation of naïve CD62L+CD4+ T cells into Th1 and Th17 cells25,44 and reduces the production of interferon gamma (IFN-γ) by Th1 cells.45–47 In Jurkat T cells, the VDR/RXR complex was found to bind to a silencer region in the human IFN-γ promoter46 (Fig. 2A). However, it is important to note that the decreased IFN-γ production could also result from an indirect action of calcitriol on IL-12 production by DCs and macrophages,28,48 as further described below, as IL-12 promotes IFN-γ production and Th1 differentiation via the activation of STAT449 (Fig. 2E). In vitro treatment of activated T cells from SJL/J mice with calcitriol inhibits IL-12-induced tyrosine phosphorylation of STAT3, STAT4 and of the kinases JAK2 and TYK228 (Fig. 2A). Therefore, the modulation of the JAK-STAT signalling pathway by VitD impairs the development of Th1 cells.
Figure 2.
Immunomodulatory effects of VitD in mice. VitD has both a direct and indirect immunosuppressive effect on Th1 (A), Th17 (B), Th2 (C), Treg (D), DC (E) and macrophage (F).
Several studies have shown that calcitriol also inhibits IL-17A, IL-22 and IL-17F production by Th17 cells from EAE mice, both in vivo and in vitro.27,50,51 Calcitriol reduces the level of mRNA that encodes IL-17A, IL-17F and IL-22, suggesting that inhibition of cytokines in Th17 cells occurs via transcriptional modulation51 (Fig. 2B). The nuclear factor of activated T cells (NFAT)-AP-1 complex is important for the induction of IL-17 and IL-2 expression in CD4+ T cells.52 In one study using HUT102 cells and Jurkat T cells treated with calcitriol, there is a competition of calcitriol–VDR–RXR complex and NFAT to NFAT-binding sites in the gene coding IL-17A. However, in mice, the repression of IL-17A is independent of NFAT (Fig. 2B). Furthermore, in both human cell lines and in mice, binding of the calcitriol–VDR–RXR complex leads to the transcriptional repression of IL-17A expression through the recruitment of Histone Deacetylase 2.27 In a similar fashion, IL-2 and GM-CSF are inhibited.53,54 The VDR–RXR complex interferes with the formation of the NFATp–Jun–Fos transcriptional complex on the promoter of the IL-2 gene. RUNX1 has a key role in the differentiation of Th17 cells and the transcription of IL-17A. VDR binds to RUNX1 and prevents its binding to the IL-17a gene promoter. In addition, there is the formation of an inhibitory complex between FOXP3 and RUNX1, which blocks the activation of IL-17A (Fig. 2B). However, Chang et al. found no significance difference of the level of IL-17A mRNA upon addition of calcitriol in MOG35–55 EAE in C57BL/6 mice. They proposed that the reduction of production of cytokines results from a translational regulation. In vitro, C/EBP homologous protein, endoplasmic reticulum stress sensor is induced by calcitriol in Th17 cells.50
CD4+ Th2 cells
In contrast to Th1 and Th17, several studies have shown that calcitriol favours Th2 differentiation.30,37,44 Following the addition of calcitriol to CD4+ T cells from BALB/C and C57BL/6 mice, a significant increase in GATA-3, a Th2-specific transcription factor, and c-maf was observed (Fig. 2C). Indeed, the Th2 signature cytokines IL-4 and IL-5 were upregulated by calcitriol while expression of IFN-γ and IL-17 was decreased. Moreover, STAT6 is important for calcitriol to confer a protection of MOG35–55 EAE in CD57BL/6 mice (Fig. 2C).
Regulatory T cells
Tregs play an important role in maintaining peripheral tolerance.55 Impaired Foxp3+ CD4+ CD25high Treg have been identified as a key feature of multiple sclerosis.56 Many studies have highlighted the role of VitD in promoting Tregs, and of the secretion of anti-inflammatory IL-10. Calcitriol and cholecalciferol increased the numbers of Tregs in the periphery and in the spinal cord of MOG35–55 induced EAE in C57BL/6 mice and MBP-induced EAE in B10.PL mice and Lewis rats.27,33,37,38 Moreover, calcitriol increased their production of IL-1057 (Fig. 2D). Indeed, key studies have shown that a functional IL-10 pathway is necessary for 1,25(OH)2D3 to inhibit EAE.51,58,59 Few studies have also reported an important role of VitD in Foxp3 regulation. The increase in Tregs is due to increased transcription of the foxp3 gene as VDRE are present in the murine Foxp3 promoter.27 Calcitriol also indirectly increased FOXP3 expression through the modulation of Helios, a positive regulator of FOXP3, and a marker of thymic-derived natural Tregs.60 Injection of calcitriol (200 ng in oil) or the combination of calcitriol and cholecalciferol (gavage 5 μg in oil) increased Helios+Foxp3+ Treg in MOG35–55 EAE in C57BL/6 mice and MBP EAE in B10.PL mice.38,61 VitD increases the expression of Ikzf2 (encoding Helios) in CD4+ T cells in a VDR-dependent mechanism,61 and Helios was shown to bind to the foxp3 promoter and increase its expression, like the aryl-hydrocarbon receptor (Ahr) does62,63 (Fig. 2D).
In addition, there is evidence that VitD signalling plays a greater role in maintaining T-cell tolerance in females than in males. In this interesting study, a cooperative loop between VitD and oestrogen has been described as calcitriol promotes oestradiol (E2) that reduces transcription of Cyp24a1, the catabolic enzyme for active vitamin, and increases VDR in T cells, which in turn, promotes Treg development.29,64 Therefore, lowered VitD and sex-specific VitD signalling may contribute to the breakdown in T-cell self-tolerance and increased multiple sclerosis incidence in females.
B cells
Much of the current literature has focused on CD4+ T cells. However, understanding the role of VitD on B cells is essential, given the high efficacy of anti-CD20 treatments in multiple sclerosis.65 VitD has been shown to modulate human B cells, by regulating their proliferation, cytokine secretion and differentiation into plasma cells.20 However, as far as we know, no study analysed the direct role of VitD on B cells in EAE. One study reported the indirect effect of calcitriol through tolerogenic DCs in B cells.66 The transfer of tolerogenic DCs induced by calcitriol in MOG35–55 EAE in C57BL/6 mice increased the proportion of CD19+ CD5+ CD1d+ regulatory B cells (Breg) in the spleen. Breg play an important role in MOGG35–55 EAE C57BL/6 mice by increasing the production of IL-10.67
Innate immune cells
Dendritic cells
DCs are antigen presenting cells (APCs) that participate in the initiation and development of multiple sclerosis.68 In the periphery, DCs activate naïve CD4+ T cells into CD4+ Th1 and Th17 cells, that in turn cross the BBB and are reactivated in the perivascular and subarachnoid CNS spaces by resident APCs allowing them to infiltrate the CNS parenchyma.69,70
Besides the established role of VitD in T cells, a clear role in DCs has also been clearly demonstrated. Addition of calcitriol generates tolerogenic DCs. Most studies have generated tolerogenic DCs in vitro and transferred them to MOG35–55 EAE in C57BL/6 or MBP73–86 EAE in Lewis rats.66,71 Tolerogenic DCs are generated by adding GM-CSF (10 ng/ml) and/or IL-4 (10 ng/ml) and calcitriol (1–10 nM) or cholecalciferol (1 nM) to progenitor bone marrow cells from female CD57BL/6 mice or Lewis rats. They are characterized by a decreased expression of MHC Class II, CD40, CD86 and CD80 (co-stimulatory molecules), decreased IL-12, IL-23 and increased IL-10 production compared with untreated DCs66,71,72 (Fig. 2E). DCs are generally pulsed with MOG35–55 peptide before transfer in EAE models at different time points: before immunization (preventive), after immunization (preclinical) and after the onset of the disease (therapeutic). This approach allows to determine the direct impact of VitD on DCs and its indirect effect on the adaptive immune response. The administration of tolerogenic DCs after immunization and after the onset of the disease led to a reduction of the severity of the disease in MOG35–55 EAE in C57BL/6 mice.66,71 In the preventive approach, tolerogenic DCs abrogated EAE in MOG35–55 EAE in C57BL/6 mice and Lewis rat.33,71 Furthermore, treatment of EAE by tolerogenic DCs led to an increase in Tregs and Bregs and secretion of IL-10,33,66,71 with a reduced infiltration of autoreactive Th1 and Th17 cells into the spinal cord66 and in a reduction of CD11c+ DCs66,73 (Fig. 2E).
Mechanistically, cholecalciferol and calcitriol increased the expression of indoleamine 2,3-dioxygenase (IDO), an enzyme that converts tryptophan to kynurenine (Fig. 2E). The inhibition of IDO on tolerogenic DCs abrogated the effect of cholecalciferol on MBP73–86 EAE course in Lewis rats.33 IDO+-tolerogenic DCs led to the generation of Treg through two mechanisms: tryptophan deficiency74 and the activation of AhR on CD4+ T cells by kynurenine, first metabolite of IDO.63,75
As mentioned earlier, it is also demonstrated that VitD reduces IL-12 production by myeloid cells. It inhibits IL-12p35 and p40 subunits at the transcriptional level in DCs and macrophages (Fig. 2E and F). Furthermore, calcitriol inhibits the mRNA expression of the IL-12p40 subunit in a VDR-dependent manner. Calcitriol may interfere with the binding of nuclear factor-κB (NF-kB) on the NF-kB binding site in the p40 promoter by competition with VDR–RXR heterodimers48 or by downregulation of the activation of the NF-kB signalling pathway in DCs and macrophages.76
Monocytes/macrophages
Similarly to DCs, VitD modulates their function. In macrophages, calcitriol inhibits the production of tumour necrosis factor-α (TNFα) by reducing the activity of a major transcription factor (NF-kB), through the increase of inhibitor of kB-α (IkB-α) that sequesters NF-kB in the cytoplasm of these cells (Fig. 2F). The phosphorylation of IkB-α by IkB kinase (IKK) leads to its ubiquitination and degradation by the proteasome. It allows the release of NF-kB that can translocate to the nucleus where it binds to kB site on TNFα promoter and activates its expression. Calcitriol binds to the promoter of IkB-α and upregulates its mRNA expression, leading to an increase of IkB-α in the cytosol. In the presence of calcitriol, IkB-α mRNA is more stable. Calcitriol also decreases IKK phosphorylating activity on IkB-α, reducing IkB-α protein degradation and reducing production of TNFα.76–78 In addition, calcitriol induces MAPK phosphatase-1 (MKP-1), which dephosphorylates p38 and reduces p38 MAPK activation in monocytes/macrophages (Fig. 2F) and the production of TNFα and IL-6,79 a critical cytokine for the differentiation of Th17 cells. This leads to the phosphorylation of STAT3 and the activation of the transcription of retinoic acid-related orphan receptor. Furthermore, calcitriol promotes M2 macrophage polarization by increasing the expression of T-cell immunoglobulinmucin3 (Tim-3), resulting in a decrease in TNFα and IL-6 and an increase in IL-1080 (Fig. 2F). So, VitD indirectly inhibits Th17 cells and Th1 through the inhibition of production of IL-6 and TNFα by macrophages.
Altogether, these studies indicate that calcitriol inhibits EAE in a preventive approach and ameliorates EAE in a therapeutic approach. Clear evidence show that calcitriol reduces effector Th1 and Th17 while promoting Tregs, and decreases the infiltration of auto-aggressive T cells into the CNS. The reduced EAE depends on the expression of VDR in CD4+ T cells. VitD has both a direct and indirect immunosuppressive effect on immune cells through the increase in tolerogenic immune responses (IDO+-DCs, Treg and Breg) and in the anti-inflammatory cytokine IL-10. However, most studies focused on calcitriol, the active form of VitD, and relatively few animal model studies used cholecalciferol,29,33,35 the main VitD metabolite administered to multiple sclerosis patients.
Immunomodulation in multiple sclerosis patients
While in vivo studies in EAE provided key molecular insights into the mode of action of VitD, several studies have analysed the effects of VitD on human lymphocytes from healthy donors or multiple sclerosis patients. Most studies determined the ability of VitD to modulate cell phenotype, proliferation and cytokine secretion in vitro. Several reports focused on the in vivo effects of VitD by directly assessing the levels of cytokines in serum or plasma of multiple sclerosis patients treated with high-dose VitD, or by analysing the proportions of key immune cell populations involved in multiple sclerosis pathophysiology (e.g. Treg, Th1, Th17).
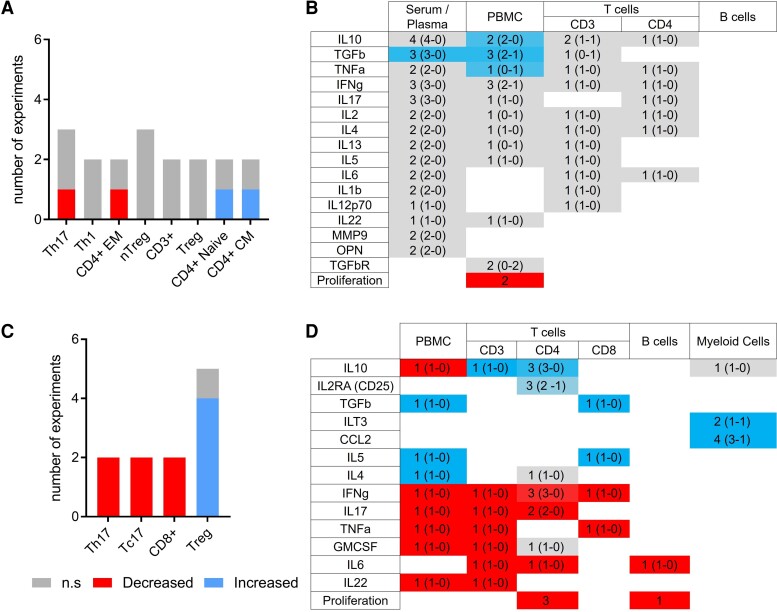
An in-depth search of the recent literature (since 2010) identified 28 publications investigating the effects of VitD on peripheral immune cells and cytokines in multiple sclerosis patients. Fig. 3 summarizes the in vivo (Fig. 3A and B, Supplementary Fig. 1 and Table 1) and in vitro (Fig. 3C and D, Supplementary Fig. 1 and Table 2) studies analysing the changes observed in cell populations and expression of biomarkers in multiple sclerosis patients treated with high-dose VitD (range 7000–20 000 UI/day cholecalciferol) in therapeutic trials81–94 (Supplementary Table 1) or by adding active VitD (10–100 nM calcitriol) in vitro to peripheral blood mononuclear cells (PBMCs) or purified T cells obtained from multiple sclerosis patients83,95–106 (Supplementary Table 2).
Figure 3.
Main effects and modulations of VitD treatment on immune cells in multiple sclerosis. Effects and modulations of VitD investigated in more than one in vivo (A and B) and in vitro (C and D) studies. Graphs (A and C) represent the number of studies showing significantly increased (blue), decreased (red) or non-significantly modified (grey) cell populations after treatment by VitD. Heat-map (B and D) representing the modifications of circulating and secreted cytokines levels studied after VitD treatment/supplementation. Studies showing a significant increase (n = a), a significant decrease (n = b) or an absence of variation (n = c) were scored as follows: [(a × 2) − (b × 2)]/[(a × 2) + (b × 2) + c]. Red represents a global decrease, blue represents a global increase and grey non-significant or discrepant results. In each case, X (Y–Z) represents the total number of tests (X) and the number of tests realized using FACS/ELISA (Y) or PCR (Z).
In only one out of three in vivo studies looking at modifications of cell populations, VitD induced a significant decrease in circulating Th17 cells82,84,85 (Fig. 3A). Different studies focusing on other immune cell populations showed a significant decrease of CD4+ effector/memory T cells and a significant increase in circulating CD4+ naïve and CD4+ central memory T cells85 (Fig. 3A). Concerning the modifications of circulating and secreted cytokine levels in multiple sclerosis patients treated with high-dose VitD, a significant increase of IL-10, transforming growth factor beta (TGFβ) and TNFα was reported in total PBMCs47,83,86,87 and TGFβ in serum,47,88,90 as well as a significant decrease in proliferation in PBMCs86,94 (Fig. 3B). However, no significant effect of VitD was observed in cytokine secretion by T and B cells sorted by fluorescence activated cell sorting (FACS).
In contrast, in vitro studies showed a significant decrease in pro-inflammatory T-cell subsets, especially for those secreting IL-17 (Tc17 and Th17), and in CD8+ T cells97,100 (Fig. 1C, Supplementary Table 2). In four out of five studies, VitD induced a significant increase in circulating Treg.95,97,98 These studies indicate a significant increase of IL-2RA in CD4+ T cells, TGFβ and IL-5 in CD8+ T cells and in total PBMCs, ILT3 and CCL2 in myeloid cells, and IL-4 in total PBMCs83,95–98,100,103–106 and a significant decrease of pro-inflammatory cytokines such as IFN-γ, IL-17, TNFα, GM-CSF, IL-22, IL-6 in PBMCs, and in T-cell subtypes95–98,100,101 (Fig. 3D). VitD also induces a significant decrease of IL-6 in B cells.102 However, contrary to in vivo data, IL-10 is decreased in total PBMCs in vitro.83
Altogether, VitD decreases proliferation of CD4+ T cells and B cells and reduces cell populations secreting pro-inflammatory cytokines (IFN-γ, IL-17, TNFα, IL-6, GM-CSF, IL-22) while increasing the proportion of cell populations secreting anti-inflammatory cytokines (such as IL-10 and TGFβ). In vitro, the decrease in IL-17 secretion in CD4+ T cells is in accordance with the decrease in Th17 under VitD treatment (Fig. 3C and D). Similarly, the decrease of IFN-γ and TNFα secretion in CD8+ T cells reflects the decrease in CD8+ T cells, while the increase of IL-10 secretion and CD25 expression in CD4+ T cells correlates with the increase in Treg. However, in vivo studies provide inconclusive data on the modulation of immune cell populations as compared with the cytokine secretion regulation. However, CD8+ T cells, B cells and myeloid cells were not analysed in in vivo studies in multiple sclerosis patients and little is known on VitD effects on cytokine secretion profiles in specific subpopulations like Th1, Th2, Th17, Treg in this disease.
VitD actions in the CNS
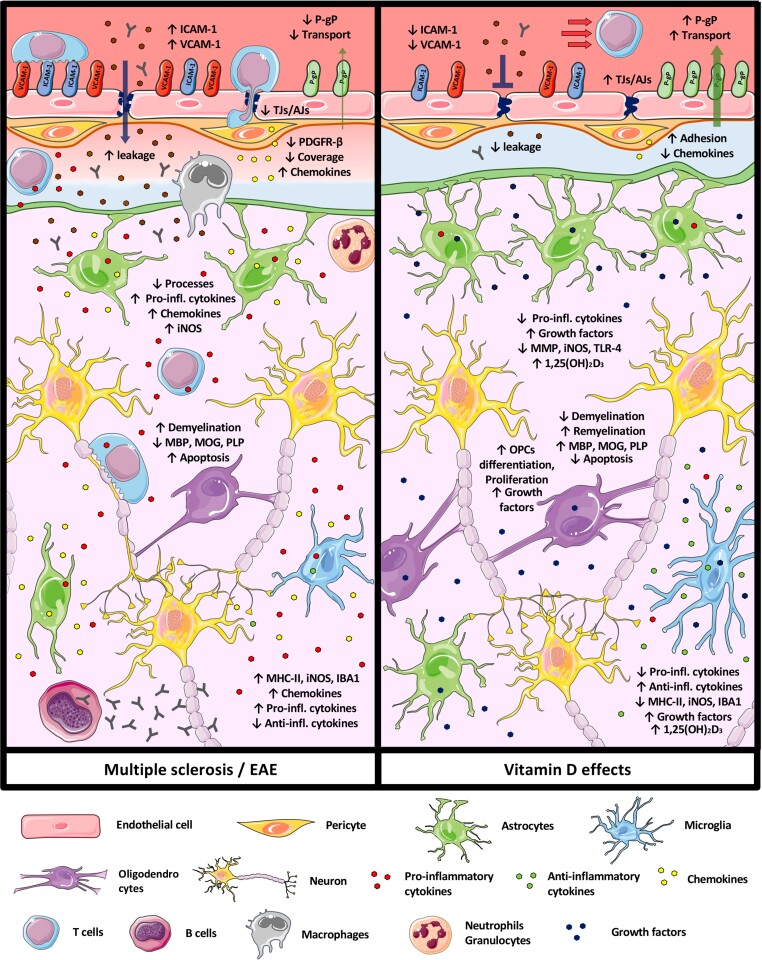
Immune regulations induced by VitD in the periphery may also protect the CNS from the inflammatory insult by local protection of the BBB and reduction of glial activation in the brain parenchyma. We particularly highlighted the effects of VitD on cellular mechanisms and cell types targeted by inflammation in multiple sclerosis (Fig. 4).
Figure 4.
VitD modulates different cellular and molecular mechanisms of CNS-resident cells and of the blood–brain barrier involved in multiple sclerosis pathology. (Left) Schematic representation of the cellular and molecular mechanisms involved in multiple sclerosis/EAE pathology at the level of CNS-resident cells and the BBB. (Right) Schematic representation VitD impact on cellular and molecular mechanisms involved in multiple sclerosis/EAE pathology at the level of CNS-resident cells and the BBB. Changes in the cellular and molecular mechanisms are given as (↑) increased and (↓) decreased. Drawings of the individual cell types were adapted from Servier Medical Art (http://smart.servier.com/), licenced under a Creative Common Attribution 3.0 Generic License.
At the BBB level
Endothelial cells
The CNS is an immune-privileged site with unique anatomic, cellular and molecular elements that limit both physiologic and pathophysiologic inflammation and preserve CNS homeostasis.107 The endothelial BBB of the CNS microvessels is composed of endothelial cells adjoined continuously by tight junctions (TJs), surrounded by pericytes and astrocyte end-feet that tightly ensheath the vessel, and possess the essential function of strictly controlling the cellular and molecular exchanges between the blood and the CNS.70,108 It is well established that BBB breakdown is an early hallmark of multiple sclerosis pathogenesis which is detected by gadolinium enhanced (Gd) MRI in the CNS of multiple sclerosis patients.109,110 Impaired BBB properties during multiple sclerosis are suggested by the observation of interrupted immunostaining for TJ and adherens junction (AJ) proteins, such as claudin-5 and VE-cadherin, respectively,111–116 as well as leakage of fibrinogen and immunoglobulin G (IgG) outside of blood vessels in post-mortem CNS tissue of multiple sclerosis patients117 (Fig. 4, Supplementary Table 3).
BBB endothelial cells express VDR, and VitD has been suggested to provide beneficial effects in multiple sclerosis by protecting BBB integrity as shown in vitro and in vivo (Table 1).
Table 1.
Main cellular and molecular effects of VitD on CNS cells
| BBB (see Supplementary Table 3) | Pericytes (see Supplementary Table 4) | Astrocytes (see Supplementary Table 5) | Microglia (see Supplementary Table 6) | Oligodendrocytes (see Supplementary Table 7) | |
|---|---|---|---|---|---|
| VitD metabolism | |||||
| VDR | ↑ in vitro118,119 n.s. in vitro120,121 ↑ in vivo122,123 |
↑ in vitro124,125 | ↑ in vitro126–128 n.s. in vitro121 |
↑ in vitro121,129–131 | ↑ in vitro132–134 |
| Cyp24a1 | ↑ in vitro118 n.s. in vitro121 ↑ in vivo135,136 |
↑ in vitro121,127 | ↑ in vitro121,127,129 | ||
| Cyp24b1 | n.s. in vitro121,127 | ||||
| Growth factors | |||||
| BDNF | ↑ in vivo137,138 | ||||
| NGF | ↑ in vitro139,140 | ||||
| Signalling | |||||
| p-AKT | ↑ in vitro141,142 | ||||
| p-ERK | ↑ in vitro141,142 | ||||
| NF-kb Activation | ↓ in vitro143,144 | ||||
| Cell viability | ↑ in vitro141,144 | n.s. in vitro145–147 ↑ in vitro148 |
|||
| Leakage | |||||
| Claudin-5 | ↑ in vitro143,144 | ||||
| ZO-1 | ↑ in vitro143,144 ↑ in vivo149,150 |
||||
| TEER | ↑ in vitro143,144 | ||||
| Brain efflux | ↑ in vivo135,151 | ||||
| Permeability | ↓ in vivo26,122,137,138,149,150 | ||||
| Trafficking | |||||
| Mrp1 | n.s. in vivo120,152 | ||||
| P-gP | ↑ in vivo120,135,136,151,152 | ||||
| BCRP | n.s. in vivo135,136 | ||||
| Oxidative stress | |||||
| NO-production | ↑ in vitro141,142 | ↓ in vitro145,147,148,153 n.s. in vitro154 |
|||
| ROS production | ↓ in vitro141,142 | ||||
| iNOS | ↓ in vitro129,131,148,155 n.s. in vitro153 |
||||
| SOD | ↑ in vivo137,156 | ||||
| Arg1 | ↑ in vitro131,155 | ||||
| Cytokines | |||||
| IL-10 | ↑ in vitro129,130 | ||||
| TNFa | ↓ in vitro131,145,147,153,154 n.s. in vitro148,155 ↓ in vivo157,158 |
||||
| IL-1b | ↓ in vitro131,148,155 | ||||
| IL-6 | ↓ in vitro129,131,147,148,153–155 | ||||
| Myelin | |||||
| Myelination | ↑ in vivo34,159 | ||||
| MBP | ↑ in vivo159,160 | ||||
| Demyelination | ↓ in vivo34,159–162 | ||||
| CNS insult | |||||
| Osteopontin | ↑ in vivo122,123 | ||||
| GFAP | ↓ in vivo163–166 ↑ in vivo161,167,168 n.s. in vivo169 |
||||
| Radio clinical parameters | |||||
| Brain oedema | ↓ in vivo122,137,150 | ||||
| Neurological deficit | ↓ in vivo122,137,150 n.s. in vivo170 |
||||
| New Gd-enhancing lesions | ↓ multiple sclerosis Patients171–173 n.s. multiple sclerosis patients86 |
||||
Molecules/mechanisms/endpoints showing concordant variation after VitD stimulation in more than one study for each cell types are represented in this table. See Supplementary Tables 3–7 for experimental details. Arg1, Arginase-1; BBB, blood–brain barrier; BCRP, breast cancer resistance protein; BDNF, brain-derived neurotrophic factor; GFAP, glial fibrillary acidic protein; IL, interleukin; iNOS, inducible nitric oxide synthase; MBP, myelin basic protein; Mrp1, multidrug resistance-associated protein 1; NGF, neural growth factor; NO, nitric oxide; P-gP, permeability-glycoprotein; ROS, reactive oxygen species; SOD, superoxide dismutase; TEER, transepithelial/trans-endothelial electrical resistance; TNF-a, tumour necrosis factor alpha; VDR, vitamin D receptor.
For instance, 1,25(OH)2D3 prohibited the loss of TJs protein expression and decreased trans-endothelial electrical resistance (TEER) in brain microvascular endothelial cells (BMECs) incubated with TNFα or serum from RRMS patients in vitro.143 Furthermore, 1,25(OH)2D3 reduced endothelial cells apoptosis rate,137,141,157 suggesting a potential beneficial role of VitD in sustaining BBB endothelial cells survival during multiple sclerosis. With respect to in vivo data, 1,25(OH)2D3 treatment prohibited downregulation of zonula occludens-1 (ZO-) in spinal cord homogenates and reduced the extravasation of sodium fluorescein, Evans blue and IgG into the CNS of mice suffering from EAE.26,149 Similarly, different randomized clinical trials (RCTs) on the effect of daily oral high dose of cholecalciferol in multiple sclerosis patients showed significantly reduced numbers of new Gd or new/enlarging T2 lesions compared with placebo group.171–173
Additional evidence of VitD impact on preventing BBB breakdown comes from the research fields of ischaemic stroke and obesity. For instance, 1,25(OH)2D3 reduced the expression of matrix metalloproteinase-9 (MMP-9) and the permeability for fluorescein isothiocyanate (FITC)-dextran, as also increased TEER and TJs expression in a mouse brain endothelial cell line (bEnd3) cells under hypoxic conditions.144 Furthermore, 1,25(OH)2D3 treatment prior experimental stoke reduced Evans blue extravasation and infarct volumes in the CNS of different animal models for ischaemic stroke.122,137,170 Similarly, oral administration of cholecalciferol reduced BBB permeability for Evans blue in the hippocampus of obese rats,138 which do also possess an altered BBB.174–177 Furthermore, increased MMP-9 expression, IgG leakage and decreased immunostaining for TJs were observed in VitD-deficient rats after transient middle cerebral artery occlusion.123
Next to the presence of complex TJs, an additional property of the BBB is the presence of specific endothelial transporters, such as the efflux pump P-glycoprotein (P-gp), which actively removes unwanted compounds from the brain.178 Interestingly, P-gp expression was reduced in BMECs in active lesions of multiple sclerosis patients179 and P-gp transport function was significantly reduced during EAE in rats,179 suggesting reduced P-gp function at the BBB in multiple sclerosis. To the best of our knowledge, there are no studies investigating the impact of VitD on BBB transporters in multiple sclerosis. However, in the context of Alzheimer’s disease, different studies have demonstrated that VitD can modulate the expression of BBB transporters. In fact, 1,25(OH)2D3 increased P-gp expression and transport activity in BMECs in vitro and in vivo.118,135 Furthermore, 1,25(OH)2D3 pre-treatment reduced amyloid-β accumulation in the parenchyma of two different Alzheimer’s disease mouse models.136 Although the role of BBB transporters in multiple sclerosis pathogenesis still needs further investigation, these observations provide some evidence on how VitD could potentially be beneficial for BBB function in multiple sclerosis.
Besides BBB breakdown and changes in expression of BBB transporters, post-mortem brain tissues of multiple sclerosis patients have also shown increased adhesion molecules expression such as intercellular adhesion molecule (ICAM)-1 and vascular cell-adhesion molecule (VCAM)-1, accompanied by an increased infiltration of leucocytes into the CNS parenchyma.114,180 To this end, 1,25(OH)2D3 treatment reduced ICAM-1 and VCAM-1 expression on BMECs incubated with TNFα or serum from acute phase RRMS patients in vitro,143 highlighting the potential of VitD to modulate immune cell migration to the CNS by acting directly on the BBB endothelium. In line with this hypothesis, many studies have shown that VitD pre-treatment reduces immune cell infiltration into the CNS during EAE.26,50,149,160,163,181,182 However, the exact mechanisms by which VitD might modulate the BBB to control immune cell migration into the CNS still have to be elucidated. Furthermore, the effect of VitD on the expression of other important BBB adhesion molecules, which contribute to the recruitment of immune cells into the CNS have not been investigated yet.114,183–187
Taken together, these observations suggest a beneficial effect of VitD supplementation on BBB integrity during different neuroinflammatory processes. Still, there is currently no evidence of a direct effect of VitD on BBB endothelial cells in vivo during EAE nor in multiple sclerosis patients. Further investigations are required to clarify if VitD can exert a direct or indirect effect on the BBB endothelium and how this may affect the course of multiple sclerosis.
Brain pericytes
Pericytes are mural cells associated with the walls of blood capillaries188 and play a crucial role in sustaining BBB integrity.189–195 Indeed, BBB permeability is inversely proportional to pericyte coverage,196,197 and their loss leads to increased perivascular immune cells infiltration.198,199
The specific role of brain pericytes in EAE or multiple sclerosis has not been addressed in depth. Nevertheless, many studies have demonstrated the contribution of pericytes in mediating different neuroinflammatory processes.199–203 Furthermore, atypical symptoms and sudden death were observed in pericyte-deficient mice during EAE, underscoring a protective role of pericytes in neuroinflammation.204 During development, brain capillaries secrete platelet derived growth factor (PDGF)-β to recruit PDGF-receptor-beta (PDGFR-β) expressing pericytes to their wall.190,205 Mice suffering from EAE were reported to have reduced numbers of PDGFR-β+ pericytes, and lower expression of PDGFR-β expression in pericytes.206,207 With respect to multiple sclerosis patients, CNS biopsies showed that active lesions harbour higher numbers of proliferative and quiescent perivascular cells (including pericytes) compared with inactive ones, but that these are lower in chronic lesions when compared with healthy tissue.208 This indicates that the inflammatory status of the CNS plays an important role in determining the number of pericytes associated with microvessels.
Recent studies showed that brain pericytes lack the expression of the oxidative enzyme CYP27B1 and can therefore not synthesize 1,25(OH)2D3,197,209,210 suggesting that pericytes rely rather on paracrine or circulating 1,25(OH)2D3. Accordingly, only a few studies have investigated the effects of VitD on brain pericytes (Fig. 4 and Table 1, Supplementary Table 4).
Brain pericytes express VDR and its expression is increased upon 1,25(OH)2D3 exposure124,125,143,210 (Table 1). In addition, 1,25(OH)2D3 induced an anti-inflammatory phenotype in brain pericytes by downregulating the expression of chemokines like CCL2 in vitro.210 In line with this, 1,25(OH)2D3 increased the activity of the antioxidant enzyme γ-glutamyl transferase in brain pericytes in vitro.211 Furthermore, primary brain pericytes isolated from 1,25(OH)2D3 treated wild type (WT) mice showed reduced proliferation and migration in vitro but had increased adhesion to extracellular matrix proteins compared with cells isolated from VDR KO mice.125 The latter might ultimately help strengthening BBB integrity.
The effect of VitD on pericyte coverage in multiple sclerosis has to the best of our knowledge not yet been investigated. Nevertheless, it has been shown that vessels of tumours transplanted into VDR KO mice have less pericytes coverage compared with those in WT mice, suggesting that pericyte attachment might be partially dependent on VDR.212 In contrast, the endothelial cell/pericyte ratio was reduced in full retinal mounts of VDR KO mice compared with those of WT mice.213
Although the above-listed studies show variable effects of VitD on pericytes, further investigations are necessarily required to establish the therapeutic potential of VitD at this level.
Biological effects on glial cells
Astrocytes
Astrocytes are among the most crucial modulators of brain function. They maintain neurotransmitters homoeostasis, cerebral blood flow, and provide essential nutrients and growth factors to the entire CNS.214 During development astrocytes play a major role in the maturation of the BBB by inducing for example the expression of TJs in CNS capillaries.215 Furthermore, astrocytes contribute to making the CNS an immune-privileged site by forming a second barrier called the glia limitans, which ensheaths the entire CNS parenchyma and, along with the BBB, prevents unrestricted entrance of immune cells.107,216
It has been shown that astrocytes are early responders in nascent white matter lesions of multiple sclerosis patients217,218 and in the CNS of mice after induction of EAE.219–221 Indeed, they can react promptly to inflammatory stimuli such as TNFα and IL-1β by amplifying neuroinflammation in a NFkB-dependent manner,222 and by recruiting leucocytes to the CNS via the expression of inflammatory chemokines, cell-adhesion molecules and nitric oxide synthase (iNOS).223,224 In multiple sclerosis, astrocytes attain an hypertrophic morphology and a reduced number of cytoplasmic processes, leading to the retraction of the glia limitans and thereby increasing the chance of immune cell infiltration in the CNS.217 On the other hand, activated astrocytes also exert beneficial effects by recruiting microglia and promoting clearance of myelin debris in the CNS of mice suffering from EAE.225 This directs us toward the possibility of a dual role of astrocytes in multiple sclerosis, by not only sustaining but also preventing the development of neuroinflammation.216
Many studies have shown that astrocytes express CYP24A1, CYP27B1 and VDR.121,126,127,132,226–228 These observations suggest that astrocytes can not only synthetize 1,25(OH)2D3 but also respond to it in an autocrine or paracrine manner. To this end, different studies on effects of VitD on astrocytes and their contribution in multiple sclerosis/EAE and other neuroinflammatory diseases have been conducted (Fig. 4 and Table 1, Supplementary Table 5).
Regarding inflammation, a recent in vitro study showed that 25(OH)D3 leads to a significant decrease in the expression of pro-inflammatory cytokines such as TNFα and IL-1β, as also TLR4 in activated astrocytes.226 Similarly, reduced TLR4 expression in the CNS of mice suffering from traumatic brain injury after combined 1,25(OH)2D3 and progesterone treatment was observed.164 Furthermore, a VitD2 analogue could inhibit NF-kB signalling in astrocytes of mice suffering from EAE, leading also to reduced clinical score.163 Taken together, these results point toward a beneficial effect of VitD treatment in multiple sclerosis by potentially reducing the pro-inflammatory response of astrocytes in the CNS.
With respect to CNS astrocytes density and GFAP expression, VitD treatment gave discrepant results in different neuroinflammatory animal models.161,163–169 For example, the VitD2 analogue reduced the populations of GFAP+ astrocytes in the spinal cord of mice suffering from EAE,163 while 1,25(OH)2D3 increased the number of GFAP+ astrocytes in the CNS of mice with cuprizone induced toxic demyelination.161 1,25(OH)2D3 was also shown to rescue cell death by decreasing ROS production, iNOS and p53 activity in astrocytes in vitro.229,230 Similarly, VitD treatment decreased immunostaining for iNOS+ astrocytes in the CNS of EAE rats, suggesting VitD as a potential antioxidant treatment in multiple sclerosis, since ROS production is well established in the CNS of multiple sclerosis patients.231
Concerning cell survival, growth factors such as NGF and VEGF have been shown to be upregulated upon 1,25(OH)2D3 treatment in activated astrocytes in vitro.128,226(p3),139 However, no changes in the expression of their receptors were observed.126 Furthermore, an analogue of 1,25(OH)2D3 decreased the proliferation, migration and metalloprotease activity of astrocytes in vitro.232
Altogether, these observations suggest a beneficial effect of VitD supplementation in reducing astrocyte activation and increasing their growth factor production in different models of multiple sclerosis.
Microglial cells
Microglia are the tissue-resident macrophages of the CNS parenchyma233,234 and play a key role in the brain and spinal cord by promoting synapse formation/refinement, oligodendrogenesis and myelin synthesis.235 In the CNS, microglia remain selfsustained by proliferation and build the first line of defence in the CNS parenchyma.236 In fact, microglia are very efficient in surveying the parenchymal environment for pathogens, cytokines, chemokines, neurotransmitters, neuromodulators and damaged cells.237 When a potential danger is recognized, microglia get activated assuming a more amoeboid-like appearance, and start clearing the surrounding environment by phagocytosis, an inherent characteristic of these cells that permits the maintenance of CNS homeostasis.238,239 Furthermore, they also secrete different growth factors such as TGF-β to promote cell survival.240
Microglia are recognized as a key player in many different inflammatory and neurodegenerative disorders. In the context of multiple sclerosis, microglia are abundant immune cells present in active lesions.241 Within active lesions, microglia play a critical role in antigen presentation, recruitment of T cells, ROS production and release of pro-inflammatory cytokines, which further amplify neuroinflammation.239,242 On the other hand, microglia are equally crucial for clearing myelin debris and enabling remyelination.243 These observations suggest that microglia play both a detrimental but also a beneficial role in multiple sclerosis pathology.
With respect to VitD, it has been demonstrated that microglia can synthetize calcitriol in vitro,244 since they express both CYP27A1 and CYP27B1.121,127,129,145,245 Along with BBB endothelial cells, pericytes and astrocytes, microglia also express VDR and can respond in either an autocrine or paracrine manner to calcitriol.121,127,129,130,145,153,245 Interestingly, a recent study showed that local synthesis of calcitriol by myeloid cells (microglia included) was essential for VitD-mediated EAE resistance.246 Several in vitro and in vivo studies in the broader context of neuroinflammation have also investigated the impact of VitD treatment on microglia (Fig. 4 and Table 1, Supplementary Table 6). Calcidiol and calcitriol can reduce microglia activation and antigen presentation by downregulating the expression of Iba1, MHC-II, CD86 and TLR-4 in vitro and in vivo in different neuroinflammatory models, including EAE.131,146,149,155,158,162,165,246,247 This was confirmed also by the observation of increased microglia activation in VitD-deficient mice.146 On the other hand, different in vitro and in vivo studies showed increased CD163, CD206, CD204 expression on microglia after calcitriol treatment, suggesting a switch toward an anti-inflammatory phenotype.131,165,170 Furthermore, calcidiol and calcitriol treatment reduced the production of pro-inflammatory cytokine such as IL-6, IL-1β, TNF-α, IFN-γ and MIP-1α, and on the other hand increased the expression of anti-inflammatory cytokines such as TGF-β1, IL-10, IL-4, IFN-α and IFN-β in microglia in vitro and in vivo in different neuroinflammatory models, including EAE.129–131,147,148,153–155,158,165,248
Along with a reduction in pro-inflammatory cytokine secretion, different in vitro studies have demonstrated that calcitriol can also reduce the production of ROS in microglia.131,145,147,148,153 This might result from the downregulation of iNOS expression in microglia after cholecalciferol, calcidiol and calcitriol treatment, as shown in vitro and in vivo.129,131,148,155,165,230,247,249 Furthermore, calcitriol increased microglia’s TGF-β expression153,165 pointing toward the possibility of a beneficial effect of VitD treatment in maintaining cell survival in the CNS of multiple sclerosis patients. Modulation of microglia phagocytosis by calcitriol has been also proposed by two different studies in vitro,154,248 however, with discrepant results.
Altogether, the current evidence suggests that VitD may alleviate the pro-inflammatory phenotype of microglial cells promoting an anti-inflammatory milieu in the CNS by directly modulating microglia activity during multiple sclerosis.
Oligodendrocytes
Oligodendrocytes are a type of neuroglial cells of the CNS, which provide support and insulation to axons by ensheathing them with multiple lamellae of their membrane, the myelin.250 They arise from oligodendrocyte precursor cells (OPCs)251 and are the last cell type generated in the CNS during development.252 Axon myelination is essential for fast and efficient signal transmission between neurons and proper neuronal function in the CNS.253 Furthermore, oligodendrocytes provide essential metabolic support to neighbouring cells by releasing different growth factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1).254
Two different types of oligodendrocyte dysfunction, namely an immune-mediated oligodendrocyte dysfunction and a primary oligodendrogliopathy255 with subsequent demyelination and neurodegeneration accompanied by oligodendrocyte apoptosis have been observed in multiple sclerosis.256 In the first case, lymphocytes257,258 and auto-antibodies targeting myelin proteins (e.g. MBP, MOG and PLP)259–261 induce direct cytotoxicity to myelin and oligodendrocytes. In the second case, oligodendrocyte apoptosis as a response to stress precedes both inflammation and the recruitment of immune cells to the lesion site.262 Nevertheless, demyelinating plaques can sometimes undergo remyelination resulting in partial recovery of the neurological function in RRMS.263 This process is mainly sustained by OPCs which after activation, proliferate and migrate to the damaged area, and differentiate into mature oligodendrocytes to replace harmed myelin sheaths.264 On the other hand, in case of progressive multiple sclerosis, remyelination is impaired and neurological function is irreversibly lost.265
In contrast to microglia, oligodendrocytes lack the expression of CYP27B1 and are therefore unable to synthetize 1,25(OH)2D3.121 Nevertheless, oligodendrocytes and OPCs express VDR suggesting they can respond to calcitriol.121,127,132–134,266 To this end, different in vitro and in vivo studies investigated the impact of VitD treatment of oligodendrocytes and myelin in the context of multiple sclerosis (Fig. 4 and Table 1, Supplementary Table 7).
In the last two decades, multiple studies have demonstrated that cholecalciferol and 1,25(OH)2D3 treatment have a beneficial effect on myelination by reducing demyelination and increasing remyelination, as also restoring neurological function in the CNS in different animal models of multiple sclerosis including EAE.34,159–162,266 Indeed, animals suffering from neurodegeneration showed increased expression of MBP, MOG and PLP upon cholecalciferol or calcitriol treatment compared with vehicle control.159,160,266,267 Similarly, calcitriol treatment reduced OPC proliferation and lead to higher oligodendrocyte differentiation with consequent increase in MBP production in vitro.133,266 In addition, rats suffering from ethidium–bromide induced demyelination showed decreased caspase-3 activation in the CNS when treated with calcitriol, suggesting also a beneficial effect of VitD on oligodendrocyte apoptosis in multiple sclerosis.159 Along the beneficial effects of VitD on myelination, different in vitro studies showed that calcitriol could also increase the expression of growth factors (e.g. nerve growth factor in oligodendrocytes132,134 and BDNF in neural stem cells133 and therefore potentially sustain cell survival in the CNS).
Altogether, despite potential indirect effects of VitD on oligodendrocytes and the lack of evidence for a role of VitD on oligodendrocytes in multiple sclerosis brains, these results highlight VitD as a promising candidate to reducing demyelination (Table 1).
Globally, VitD protects the CNS from inflammation at the cellular level (BBB and glia) through the modulation of multiple mechanisms including cytokine and growth factors secretion, cell signalling, response to oxidative stress, BBB leakage and trafficking. The most robust effects of VitD in the CNS are summarized in Table 1.
VitD therapy in multiple sclerosis patients
Despite all previous data about the potential immunological regulation and neuroprotective effects of VitD in multiple sclerosis models and multiple sclerosis patients, controversial results have been obtained from clinical trials evaluating VitD supplementation in multiple sclerosis patients. Kimball et al. evaluated tolerance and safety of escalating doses of active VitD ranging from 28 000 to 280 000 IU/week in an open-label study including 12 RRMS patients over a total of 28 weeks.172 Despite final mean serum concentrations of 25(OH)D of 386 ± 157 nmol/l, they did not observe any severe adverse events (SAEs) on this very small cohort. Since then, several SAEs (e.g. severe gastric symptoms, pseudo-brain tumour and seizures, tonic–clonic seizures, severe hypercalcemia) were observed at extremely high doses (100 000 IU/day for 1–36 months), suggesting that excessive supplementation with VitD can be dangerous despite a plateau concentration close to 400 nmol/l and that treatment duration might play a role in VitD toxicity.268 Moreover, little is known about the immunological effects of very high levels of circulating VitD. In animal models, the higher VitD dose, the higher immunomodulatory effect is generally observed, although excessive VitD treatment achieving serum levels over 250 nmol/L resulted in pro-inflammatory effects through lymphocyte activation and demyelination and myeloid infiltration into the brain, in addition to perturbations of phosphocalcic homeostasis.269
Since the original study by Mahon et al. in 2003, we identified more than 20 published case–control studies and double-blind RCTs aiming at evaluating the potential clinical and radiological benefits of VitD supplementation in multiple sclerosis patients.47,81–94,171,173,270–276 Some of these were recently reviewed and showed limited improvement in multiple sclerosis clinical and/or MRI features including fatigue, expanded disability status scale, relapses, T2-lesions, non-evidence of disease activity (NEDA-3) and in retinal nerve fibre layer thickness.21,277 These trials also evaluated safety of VitD supplementation at more physiological dosages (<15 000 IU/day) and did not observe a significant increase of AEs or SAEs in VitD-treated groups compared with placebo groups (CHOLINE,275 SOLAR171). Importantly, phosphocalcic homeostasis seemed respected at this level of supplementation, as no cases of calciuria and hypercalcemia were observed (SOLAR).
The safety range of VitD levels has been defined by an expert consensus in 2010 as of 30–100 ng/ml (equivalent to 75–250 nmol/l), considering that in healthy subjects who have spent prolonged periods in a sunny environment, measured 25(OH)D concentrations rarely exceed 100 ng/ml.278 In populations with light skin frequently exposed to the sunlight, the upper limit of circulating VitD levels is located between 75 and 150 nmol/l, providing an optimal range to reach after VitD supplementation, as defined by experts of bone and calcium homeostasis in order to prevent osteoporosis and fractures.279 However, so far, there are no recommendations for multiple sclerosis patients on potential VitD supplementation to achieve optimal levels for immune modulation and clinical effect.
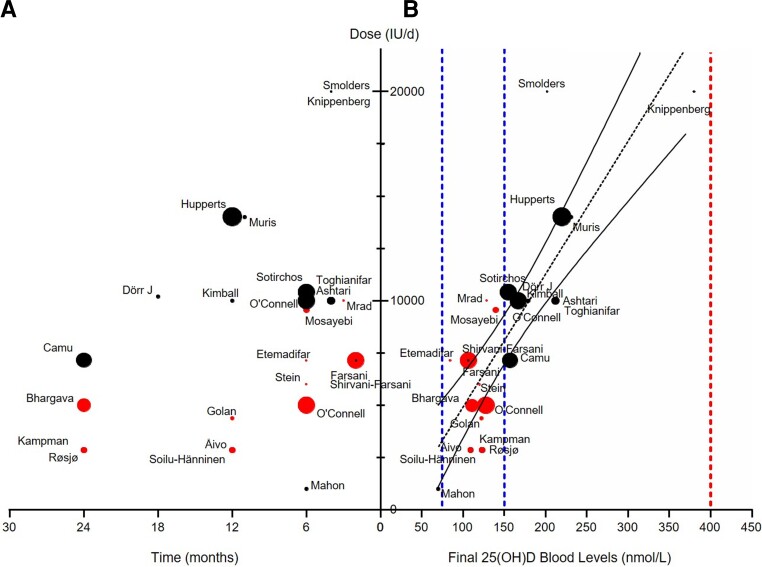
In order to define VitD dosing to achieve optimal VitD therapeutic range after supplementation, we analysed 24 therapeutic trials accounting for 742 treated patients and providing their final VitD levels. When plotting the time of exposure with the daily dose of VitD supplementation of the treated group, we observed that protocols were very heterogeneous with treatment duration ranging from 2 to 24 months and dosages ranging from 1000 to 20 000 UI/day (Fig. 5A). However, we observed a strong correlation between the daily dose and the final circulating VitD levels of treated patients (Spearman r = 0.803, P < 0.0001, Fig. 5B), indicating that a daily dose of VitD between 3000 and 11 000 IU for 3–24 months achieves final blood VitD levels within the 75–150 nmol/l recommended range (red dots, Fig. 5A and B). Even at the higher doses used in the SOLAR study171 where patients received 14 000 UI/day for 12 months, VitD levels above 200 nmol/l were obtained without occurrence of SAEs after 1 year. This potentially increases the safety of higher VitD supplementation reaching up to 250 nmol/l for short periods. However, the exact concentrations of VitD in multiple sclerosis needed to properly restore immune homeostasis over the long term remains to be determined as well as the optimal risk/benefit balance for VitD supplementation. Given the weak clinical and radiological effects of VitD treatment in short-term RCTs involving multiple sclerosis patients, further analysis of the in vivo immunological efficacy of VitD are warranted to refine the objectives of chronic VitD supplementation in multiple sclerosis patients.
Figure 5.
Correlation between daily dose of VitD and the final blood levels of 25(OH)D from different Phase 2 and Phase 3 RCTs in RRMS patients. (A) Representations of the different clinical studies on the effect of VitD in multiple sclerosis patients according to the dose administered (IU/day) as a function of time (months). (B) Representations of the different clinical studies in multiple sclerosis patients according to the final 25(OH)D blood levels (nmol/l) as a function of time (months). Dots are proportional to the number of patients in each study. Red dots represent studies with final blood VitD levels in the range 75–150 nmol/l as determined by Souberbielle et al., 2019; other studies are represented in black. Linear regression shows a significant correlation between daily dose of VitD and the final blood levels of 25(OH)D [r2 = 0687; 25(OH)D = daily dose/63.5 + 22.5].
Discussion and perspectives
While it is indisputable that VitD exerts strong immunomodulatory effects, it is still debated whether VitD supplementation is efficacious in multiple sclerosis. Data from animal models and in vitro studies provide important information on VitD effects to optimize its clinical application. Despite the large number of studies published in the field of multiple sclerosis so far, sound review of the literature revealed that analysis of VitD modulation of the immune system only focused on subpopulations of PBMCs and cytokine secretion, principally by FACS, ELISA and RT-PCR. FACS relies on designed panels of antibodies coupled to serial wavelength fluorophores to target markers of cell surface (and also intracellular markers) and analyse ± sort cells of interest, providing an efficient tool to study immune cell populations. However, when dealing with complex immunological diseases like multiple sclerosis, limited numbers of lasers and channels does not provide the ability to study subpopulations of a given sample in a row. The recent development of high-parameter FACS equipped with 4–7 lasers allows the detection of up to 18–30 markers in each cell that is much more in line with the investigation of several subpopulations.
New and innovative techniques developed recently could also be of high interest to further discriminate lymphocyte subsets of importance in multiple sclerosis pathophysiology, regulation of which by treatments is mandatory to efficiently reduce inflammation and disease activity. Taking advantage of antibody coupling to non-radioactive isotopes of heavy metals that can be detected by mass spectrometry, cytometry by time of flight (CyTOF) enhances the study and analysis of very specific types of cells.280 This technique allows the detection of a large number of cell types and the assessment of the immune cell activation state (around 60 markers) and overcomes the limitations of the use of fluorophores in flow cytometry. Using this technique, Couloume et al.281 detected an increased abundance of a T-bet expressing B-cells subset and an increased abundance of a CD206+ classical monocytes subset in the blood of multiple sclerosis patients with early multiple sclerosis. Johansson et al.282 used CyTOF and identified an multiple sclerosis-associated B-cell population using an unsupervised clustering of all CSF cells characterized by the expression of CD49d, CD69, CD27, CXCR3 and also found that proteins involved in neural plasticity were reduced in multiple sclerosis. Comparing multiple sclerosis to other inflammatory and non-inflammatory conditions of the CNS, Galli et al.283 identified an expanded T-helper cell subset characterized by the expression of GM-CSF and the C–X–C chemokine receptor Type 4 (CRCX4), revealing Th cells as the major contributor to GM-CSF production in the peripheral immune compartment of multiple sclerosis patients, with highest GM-CSF production in the effector memory fraction. Using intracellular staining after permeabilisation of cells, they combined surface and intracellular markers for mass cytometry and showed that the vast majority of GM-CSF producing Th cells co-expressed TNFα and IL-2.283 Although intracellular markers can be used for additional analyses, one limitation of CyTOF is that cells are directly discarded at the end of the analysis and cannot be sorted for complementary functional tests in vitro. Another limitation of CyTOF is the important signal variation over time and among centres, precluding multicentre studies unless a reference sample is used to normalize the data and ensure reliable comparisons. Finally, CyTOF is limited by the targeted investigation of specific cell populations and markers using a panel of antibodies designed by the experimenter, precluding discovery of novel markers of new cell subtypes potentially playing an important role in multiple sclerosis. Despite these limitations, CyTOF emerges as a very innovative technique with a high potential to investigate immune regulation induced by VitD in multiple sclerosis patients.
Another approach to decipher changes in immune cell profiles and activation is RNA sequencing (RNAseq). This technique, relying on the identification and quantification of gene transcripts (coding RNA) in each sample is used to analyse qualitatively and quantitatively gene expression changes. It can also be used to investigate non-coding RNAs potentially playing the role of regulators of gene expression. Comparing the linear and circular transcriptomes of leucocytes from 50 multiple sclerosis patients and 20 healthy controls (HC), Iparraguirre et al.284 found 689 upregulated and 683 downregulated transcripts in multiple sclerosis patients, most of them related to immune processes including neutrophil activation involved in immune response, neutrophil degranulation and granulocyte activation. Investigating circular RNA (circRNA) known as non-coding RNA regulating various immune responses, they observed a global upregulation of circRNAs in multiple sclerosis patients compared with HCs, with similar circRNA profiles between multiple sclerosis types. Long non-coding RNAs (lncRNAs) were also investigated in multiple sclerosis patients versus HCs by Han et al.,285 who identified 1438 downregulated and 945 upregulated lncRNAs, some of which were found to influence the antigen processing/presentation and MAPK signalling pathway in multiple sclerosis. To provide more informative data regarding immune regulation, RNAseq can be used to investigate the modifications of specific lymphocyte populations sorted by FACS. Fernandes et al.286 reported a detailed gene expression by RNAseq profiling analysis of FACS-sorted CD4+ and CD8+ T cells from HCs, RRMS and SPMS patients. Their analysis revealed 149 differentially expressed genes in both cell types, confirming previous findings (human-leucocyte antigen G) and discovering several novel genes and processes involved in multiple sclerosis, like IgG heavy chain and neurofilament-light chain. Looking at the effects of VitD in THP-1 cells (the human acute monocytic leukaemia cell line) by RNAseq, Neme et al.287 identified 1284 out of 14 402 expressed genes (8.9% of the expressed transcriptome of THP-1 cells) regulated by 1,25(OH)2D3. In human PBMCs from HCs exposed to a bolus of VitD (80 000 UI), they also observed that 702 genes were significantly regulated, including genes involved in general protein translation, monocyte differentiation and cellular growth control, suggesting that this approach could be applied to multiple sclerosis patients.288
More recently, single cell RNAseq (scRNAseq) has emerged as a powerful method to analyse modifications of cell populations and activation state. Compared with CyTOF, scRNAseq data are much more highly dimensional. Compared with bulk RNAseq that provides data from a collection of mixed cell populations to which we cannot point to the individual contribution, ScRNAseq directly reveals all the transcripts expressed by a cell, thus ensuring a qualitative and quantitative analysis of gene changes of all cell subsets of the sample. In EAE, using a combination of scRNAseq and other techniques, Wheeler et al.289 identified a reduced NRF2-driven gene expression and increased MAFG and MAT2α signalling in astrocytes, potentially promoting pro-inflammatory genomic programmes in astrocytes via GM-CSF, produced by inflammatory T cells recruited to the CNS in multiple sclerosis and EAE. Using scRNAseq to establish a detailed molecular description of microglia from HCs and multiple sclerosis patients, Masuda et al.290 observed that the microglial profile from HCs was enriched for transcripts indicative of a homeostatic phenotype, while multiple sclerosis patients showed multiple activated subsets potentially indicating engagement with infiltrating autoreactive T cells, matrix remodelling or reduction of cytotoxic CD8+ T-cell functions. Esaulova et al.291 used scRNAseq to profile individual cells of CSF and blood from two subjects with RRMS and one with anti-MOG associated disorder and identified myeloid cell types expressing a microglial signature. Finally, using scRNAseq and FACS coupled to bulk RNAseq, Ramesh et al.292 analysed paired CSF and blood B-cell subsets from multiple sclerosis patients, showing that B cells primarily clustered based on their subset type and the body compartment from which they were isolated. Independent analyses across technologies demonstrated that NF-κB and cholesterol biosynthesis pathways were activated, and specific cytokine and chemokine receptors were upregulated in CSF memory B cells.
Altogether, scRNAseq has a high potential to investigate the effects of VitD in immune cells in multiple sclerosis patients. However, scRNAseq has limitations, especially concerning the identification of cell subtypes according to the gene transcripts alone, especially concerning CD4+ T cells that show very little expression of clusters of differentiation genes. To overcome this caveat, the use of antibodies generally used for characterization of cell subtypes by FACS has been barcoded (adjunction of a specific RNA sequence on the Fc fragment of the protein) to ensure faithful selection of the target population for genomic analysis. This method called Cellular Indexing of Transcriptomes and Epitopes by sequencing (CITE-seq) allows accurate characterization of both phenotype and transcriptome in a scRNAseq experiment.293–295
All these data strongly support the accuracy of CYTOF/CITE-seq and scRNAseq to analyse the effects of VitD on the whole PBMC population to improve our understanding of the pleiotropic effects of VitD while keeping detailed analysis of key populations modified by VitD.
Conclusion
Despite conflicting results, the overall effect of VitD supplementation in multiple sclerosis patients and animal models of multiple sclerosis results in a global modulation of inflammation. Inconclusive results from the clinical studies analysing the effect of VitD supplementation as add-on therapy in small numbers of multiple sclerosis patients or during short periods do not exclude a potential protective effect in vivo. Despite an excellent clinical safety profile of VitD, excessive doses are associated with SAEs, limiting the therapeutic range of VitD much below the high doses generally used in vitro. Finally, well-powered multicentre randomized controlled trials of VitD monotherapy versus placebo are still needed to bring significant data highlighting the therapeutic effectiveness of VitD in multiple sclerosis, and to refine the optimal posology to reach clinical effects and allow generalization of VitD supplementation in multiple sclerosis. The ongoing RCT D-lay multiple sclerosis (NCT01817166) included 316 multiple sclerosis patients within 3 months from disease onset to show the ability of high-dose cholecalciferol versus placebo for 2 years to reduce disease activity. This study will also provide useful biological samples to decipher the complex modifications of immune cell hierarchy and functions by coupling high-throughput technologies such as high-parameter flow cytometry and CITE-seq.
Data sharing is not applicable to this article as no new data were created or analysed in this study. Statistical analysis is not applicable to this article.
Supplementary Material
Abbreviations
- BBB =
blood–brain barrier
- CITE-seq =
Cellular Indexing of Transcriptomes and Epitopes by sequencing
- CyTOF =
cytometry by time of flight
- DCs =
dendritic cells
- EAE =
experimental autoimmune encephalomyelitis
- FACS =
fluorescence activated cell sorting
- ICAM-1 =
intercellular adhesion molecule-1
- IFN-γ =
interferon gamma
- IgG =
immunoglobulin G
- IL =
interleukin
- KO =
knockout
- MBP =
myelin basic protein
- MHC =
major histocompatibility complex
- MMP9 =
matrix metalloproteinase-9
- MOG =
myelin oligodendrocyte glycoprotein
- NFAT =
nuclear factor of activated T cells
- NF-kB =
nuclear factor-κB
- OPCs =
oligodendrocyte precursor cells
- PBMC =
peripheral blood mononuclear cells
- PDGF =
platelet derived growth factor
- P-gp =
P-glycoprotein
- PLP =
proteolipid protein
- RCTs =
randomized clinical trials
- RNAseq =
RNA sequencing
- RRMS =
relapsing-remitting multiple sclerosis
- RXR =
retinoid-X-receptor
- SAEs =
severe adverse events
- scRNAseq =
single cell RNA sequencing
- SPMS =
secondary progressive multiple sclerosis
- TEER =
trans-endothelial electrical resistance
- TJs =
tight junctions
- TGF-β =
transforming growth factor beta
- TNFα =
tumour nuclear factor alpha
- VCAM-1 =
vascular cell-adhesion molecule-1
- VDR =
vitamin D receptor
- VDRE =
vitamin D receptor element
- VitD =
vitamin D
- WT =
wild type
Contributor Information
Manon Galoppin, IGF, University Montpellier, CNRS, INSERM, Montpellier, France.
Saniya Kari, Toulouse Institute for Infectious and Inflammatory Diseases (Infinity), INSERM UMR1291 – CNRS UMR5051 – Université Toulouse III, 31024 Toulouse cedex 3, France.
Sasha Soldati, Theodor Kocher Institute, University of Bern, Bern, Switzerland.
Arindam Pal, Theodor Kocher Institute, University of Bern, Bern, Switzerland.
Manon Rival, IGF, University Montpellier, CNRS, INSERM, Montpellier, France; Department of Neurology, Nîmes University Hospital, University Montpellier, Nîmes, France.
Britta Engelhardt, Theodor Kocher Institute, University of Bern, Bern, Switzerland.
Anne Astier, Toulouse Institute for Infectious and Inflammatory Diseases (Infinity), INSERM UMR1291 – CNRS UMR5051 – Université Toulouse III, 31024 Toulouse cedex 3, France.
Eric Thouvenot, IGF, University Montpellier, CNRS, INSERM, Montpellier, France; Department of Neurology, Nîmes University Hospital, University Montpellier, Nîmes, France.
Funding
This research has been supported by Agence Nationale de la Recherche (ANR) grant ANR-19-CE14-0043 to A.A., E.T., and Swiss National Science Foundation (SNSF) grant 310030E_189312 to B.E.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain Communications online.
References
- 1. Ramagopalan SV, Heger A, Berlanga AJ, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: Associations with disease and evolution. Genome Res. 2010;20(10):1352–1360. 10.1101/gr.107920.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Westerlind H, Ramanujam R, Uvehag D, et al. Modest familial risks for multiple sclerosis: A registry-based study of the population of Sweden. Brain. 2014;137(Pt 3):770–778. 10.1093/brain/awt356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. International Multiple Sclerosis Genetics Consortium . Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science. 2019;365(6460):eaav7188. 10.1126/science.aav7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. D’hooghe MB, Haentjens P, Nagels G, De Keyser J. Alcohol, coffee, fish, smoking and disease progression in multiple sclerosis. Eur J Neurol. 2012;19(4):616–624. 10.1111/j.1468-1331.2011.03596.x. [DOI] [PubMed] [Google Scholar]
- 5. Hedström AK, Hillert J, Olsson T, Alfredsson L. Smoking and multiple sclerosis susceptibility. Eur J Epidemiol. 2013;28(11):867–874. 10.1007/s10654-013-9853-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Munger KL, Chitnis T, Ascherio A. Body size and risk of MS in two cohorts of US women. Neurology. 2009;73(19):1543–1550. 10.1212/WNL.0b013e3181c0d6e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-barr virus associated with multiple sclerosis. Science. 2022;375(6578):296–301. 10.1126/science.abj8222. [DOI] [PubMed] [Google Scholar]
- 8. Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: The role of infection. Ann Neurol. 2007;61(4):288–299. 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- 9. Ostkamp P, Salmen A, Pignolet B, et al. Sunlight exposure exerts immunomodulatory effects to reduce multiple sclerosis severity. Proc Natl Acad Sci U S A. 2021;118(1):e2018457118. 10.1073/pnas.2018457118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lucas RM, Byrne SN, Correale J, Ilschner S, Hart PH. Ultraviolet radiation, vitamin D and multiple sclerosis. Neurodegener Dis Manag. 2015;5(5):413–424. 10.2217/nmt.15.33. [DOI] [PubMed] [Google Scholar]
- 11. Ascherio A, Munger KL, Simon KC. Vitamin D and multiple sclerosis. Lancet Neurol. 2010;9(6):599–612. 10.1016/S1474-4422(10)70086-7. [DOI] [PubMed] [Google Scholar]
- 12. Simpson S, Taylor B, Blizzard L, et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol. 2010;68(2):193–203. [DOI] [PubMed] [Google Scholar]
- 13. Mowry EM Jr, Waubant E, McCulloch CE, et al. Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann Neurol. 2012;72(2):234–240. 10.1002/ana.23591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thouvenot E, Orsini M, Daures JP, Camu W. Vitamin D is associated with degree of disability in patients with fully ambulatory relapsing-remitting multiple sclerosis. Eur J Neurol. 2015;22(3):564–569. 10.1111/ene.12617. [DOI] [PubMed] [Google Scholar]
- 15. Muscogiuri G, Barrea L, Somma CD, et al. Sex differences of vitamin D status across BMI classes: An observational prospective cohort study. Nutrients. 2019;11(12):3034. 10.3390/nu11123034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Orton SM, Wald L, Confavreux C, et al. Association of UV radiation with multiple sclerosis prevalence and sex ratio in France. Neurology. 2011;76(5):425–431. 10.1212/WNL.0b013e31820a0a9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. VanAmerongen BM, Dijkstra CD, Lips P, Polman CH. Multiple sclerosis and vitamin D: An update. Eur J Clin Nutr. 2004;58(8):1095–1109. 10.1038/sj.ejcn.1601952. [DOI] [PubMed] [Google Scholar]
- 18. Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-Dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221(4616):1181–1183. 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 19. Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D3 receptor in the immune system. Arch Biochem Biophys. 2000;374(2):334–338. 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- 20. Peelen E, Knippenberg S, Muris AH, et al. Effects of vitamin D on the peripheral adaptive immune system: A review. Autoimmun Rev. 2011;10(12):733–743. 10.1016/j.autrev.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 21. Smolders J, Torkildsen Ø, Camu W, Holmøy T. An update on vitamin D and disease activity in multiple sclerosis. CNS Drugs. 2019;33(12):1187–1199. 10.1007/s40263-019-00674-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simmons KM, Beaudin SG, Narvaez CJ, Welsh J. Gene signatures of 1,25-dihydroxyvitamin D3 exposure in normal and transformed mammary cells: Genomic profiles of vitamin D treated mammary cells. J Cell Biochem. 2015;116(8):1693–1711. 10.1002/jcb.25129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burrows DJ, McGown A, Jain SA, et al. Animal models of multiple sclerosis: From rodents to zebrafish. Mult Scler J. 2019;25(3):306–324. 10.1177/1352458518805246. [DOI] [PubMed] [Google Scholar]
- 24. Tietz SM, Zwahlen M, Haghayegh Jahromi N, et al. Refined clinical scoring in comparative EAE studies does not enhance the chance to observe statistically significant differences: Technical comment. Eur J Immunol. 2016;46(10):2481–2483. 10.1002/eji.201546272. [DOI] [PubMed] [Google Scholar]
- 25. Chang JHH, Cha HRR, Lee DSS, Seo KY, Kweon MNN. 1,25-Dihydroxyvitamin D3 inhibits the differentiation and migration of TH17 cells to protect against experimental autoimmune encephalomyelitis. PLoS One. 2010;5(9):e12925–e12925. 10.1371/journal.pone.0012925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grishkan IV, Fairchild AN, Calabresi PA, Gocke AR. 1,25-Dihydroxyvitamin D3 selectively and reversibly impairs T helper-cell CNS localization. Proc Natl Acad Sci U S A. 2013;110(52):21101–21106. 10.1073/pnas.1306072110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Joshi S, Pantalena LC, Liu XK, et al. 1,25-Dihydroxyvitamin D3 ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol Cell Biol. 2011;31(17):3653–3669. 10.1128/MCB.05020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Muthian G, Raikwar HP, Rajasingh J, Bright JJ. 1,25 dihydroxyvitamin-D3 modulates JAK–STAT pathway in IL-12/IFNγ axis leading to Th1 response in experimental allergic encephalomyelitis. J Neurosci Res. 2006;83(7):1299–1309. 10.1002/jnr.20826. [DOI] [PubMed] [Google Scholar]
- 29. Nashold FE, Spach KM, Spanier JA, Hayes CE. Estrogen controls vitamin D3-mediated resistance to experimental autoimmune encephalomyelitis by controlling vitamin D3 metabolism and receptor expression. J Immunol. 2009;183(6):3672–3681. 10.4049/jimmunol.0901351. [DOI] [PubMed] [Google Scholar]
- 30. Sloka S, Silva C, Wang J, Yong V. Predominance of Th2 polarization by vitamin D through a STAT6-dependent mechanism. J Neuroinflammation. 2011;8(1):56–56. 10.1186/1742-2094-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A. 1996;93(15):7861–7864. 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cantorna MT, Woodward WD, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 is a positive regulator for the two anti-encephalitogenic cytokines TGF-β1 and IL-4. J Immunol. 1998;160(11):5314–5319. [PubMed] [Google Scholar]
- 33. Farias AS, Spagnol GS, Bordeaux-Rego P, et al. Vitamin D3 induces IDO+ tolerogenic DCs and enhances treg, reducing the severity of EAE. CNS Neurosci Ther. 2013;19(4):269–277. 10.1111/cns.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nashold FE, Miller DJ, Hayes CE. 1,25-dihydroxyvitamin D3 treatment decreases macrophage accumulation in the CNS of mice with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2000;103(2):171–179. 10.1016/S0165-5728(99)00247-7. [DOI] [PubMed] [Google Scholar]
- 35. Spach KM, Hayes CE. Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J Immunol. 2005;175(6):4119–4126. 10.4049/jimmunol.175.6.4119. [DOI] [PubMed] [Google Scholar]
- 36. Lemire JM, Archer DC. 1,25-Dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest. 1991;87(3):1103–1107. 10.1172/JCI115072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haghmorad D, Yazdanpanah E, Jadid Tavaf M, et al. Prevention and treatment of experimental autoimmune encephalomyelitis induced mice with 1, 25-dihydroxyvitamin D3. Neurol Res. 2019;41(10):943–957. 10.1080/01616412.2019.1650218. [DOI] [PubMed] [Google Scholar]
- 38. Nashold FE, Nelson CD, Brown LM, Hayes CE. One calcitriol dose transiently increases Helios+ FoxP3+ T cells and ameliorates autoimmune demyelinating disease. J Neuroimmunol. 2013;263(1–2):64–74. 10.1016/j.jneuroim.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 39. Yoshizawa T, Handa Y, Uematsu Y, et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16(4):391–396. 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- 40. Li YC, Pirro AE, Amling M, et al. Targeted ablation of the vitamin D receptor: An animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997;94(18):9831–9835. 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mayne CG, Spanier JA, Relland LM, Williams CB, Hayes CE. 1,25-Dihydroxyvitamin D3 acts directly on the T lymphocyte vitamin D receptor to inhibit experimental autoimmune encephalomyelitis. Eur J Immunol. 2011;41(3):822–832. 10.1002/eji.201040632. [DOI] [PubMed] [Google Scholar]
- 42. Nashold FE, Hoag KA, Goverman J, Hayes CE. Rag-1-dependent cells are necessary for 1,25-dihydroxyvitamin D3 prevention of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2001;119(1):16–29. 10.1016/S0165-5728(01)00360-5. [DOI] [PubMed] [Google Scholar]
- 43. Bruce D, Yu S, Ooi JH, Cantorna MT. Converging pathways lead to overproduction of IL-17 in the absence of vitamin D signaling. Int Immunol. 2011;23(8):519–528. 10.1093/intimm/dxr045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Staeva-Vieira TP, Freedman LP. 1,25-Dihydroxyvitamin D3 inhibits IFN-γ and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J Immunol. 2002;168(3):1181–1189. 10.4049/jimmunol.168.3.1181. [DOI] [PubMed] [Google Scholar]
- 45. Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HFJ, O’Garra A. 1α,25-Dihydroxyvitamin D3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J Immunol. 2001;167(9):4974–4980. 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 46. Cippitelli M, Santoni A. Vitamin D3: A transcriptional modulator of the interferon-gamma gene. Eur J Immunol. 1998;28(10):3017–3030. . [DOI] [PubMed] [Google Scholar]
- 47. Mahon BD, Gordon SA, Cruz J, Cosman F, Cantorna MT. Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J Neuroimmunol. 2003;134(1–2):128–132. 10.1016/S0165-5728(02)00396-X. [DOI] [PubMed] [Google Scholar]
- 48. D’Ambrosio D, Cippitelli M, Cocciolo MG, et al. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101(1):252–262. 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3(2):133–146. 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 50. Chang SH, Chung Y, Dong C. Vitamin D suppresses Th17 cytokine production by inducing C/EBP homologous protein (CHOP) expression. J Biol Chem. 2010;285(50):38751–38755. 10.1074/jbc.C110.185777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Palmer MT, Lee YK, Maynard CL, et al. Lineage-specific effects of 1,25-dihydroxyvitamin D3 on the development of effector CD4 T cells. J Biol Chem. 2011;286(2):997–1004. 10.1074/jbc.M110.163790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hermann-Kleiter N, Baier G. NFAT pulls the strings during CD4+ T helper cell effector functions. Blood. 2010;115(15):2989–2997. 10.1182/blood-2009-10-233585. [DOI] [PubMed] [Google Scholar]
- 53. Alroy I, Towers TL, Freedman LP. Transcriptional repression of the interleukin-2 gene by vitamin D3: Direct inhibition of NFATp/AP-1 complex formation by a nuclear hormone receptor. Mol Cell Biol. 1995;15(10):5789–5799. 10.1128/MCB.15.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Towers TL, Staeva TP, Freedman LP. A two-hit mechanism for vitamin D3-mediated transcriptional repression of the granulocyte-macrophage colony-stimulating factor gene: Vitamin D receptor competes for DNA binding with NFAT1 and stabilizes c-jun. Mol Cell Biol. 1999;19(6):4191–4199. 10.1128/MCB.19.6.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11(1):7–13. 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 56. Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+ CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199(7):971–979. 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang X. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+ CD4+ regulatory T cells. Int Immunol. 2004;16(2):249–256. 10.1093/intimm/dxh029. [DOI] [PubMed] [Google Scholar]
- 58. Spach KM, Nashold FE, Dittel BN, Hayes CE. IL-10 Signaling is essential for 1,25-dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. J Immunol. 2006;177(9):6030–6037. 10.4049/jimmunol.177.9.6030. [DOI] [PubMed] [Google Scholar]
- 59. Stohlman SA, Pei L, Cua DJ, Li Z, Hinton DR. Activation of regulatory cells suppresses experimental allergic encephalomyelitis via secretion of IL-10. J Immunol. 1999;163(11):6338–6344. [PubMed] [Google Scholar]
- 60. Thornton AM, Korty PE, Tran DQ, et al. Expression of Helios, an ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184(7):3433–3441. 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Moore JR, Hubler SL, Nelson CD, Nashold FE, Spanier JA, Hayes CE. 1,25-Dihydroxyvitamin D3 increases the methionine cycle, CD4+ T cell DNA methylation and Helios+ Foxp3+ T regulatory cells to reverse autoimmune neurodegenerative disease. J Neuroimmunol. 2018;324:100–114. 10.1016/j.jneuroim.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 62. Getnet D, Grosso JF, Goldberg MV, et al. A role for the transcription factor Helios in human CD4+ CD25+ regulatory T cells. Mol Immunol. 2010;47(7–8):1595–1600. 10.1016/j.molimm.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185(6):3190–3198. 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Spanier JA, Nashold FE, Mayne CG, Nelson CD, Hayes CE. Vitamin D and estrogen synergy in vdr-expressing CD4+ T cells is essential to induce Helios+ FoxP3+ T cells and prevent autoimmune demyelinating disease. J Neuroimmunol. 2015;286:48–58. 10.1016/j.jneuroim.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 65. Comi G, Bar-Or A, Lassmann H, et al. Role of B cells in multiple sclerosis and related disorders. Ann Neurol. 2021;89(1):13–23. 10.1002/ana.25927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xie Z, Chen J, Zheng C, et al. 1,25-dihydroxyvitamin D3-induced dendritic cells suppress experimental autoimmune encephalomyelitis by increasing proportions of the regulatory lymphocytes and reducing T helper type 1 and type 17 cells. Immunology. 2017;152(3):414–424. 10.1111/imm.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3(10):944–950. 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 68. Grigoriadis N, van Pesch V. A basic overview of multiple sclerosis immunopathology. Eur J Neurol. 2015;22:3–13. 10.1111/ene.12798. [DOI] [PubMed] [Google Scholar]
- 69. Engelhardt B, Sorokin L. The blood–brain and the blood–cerebrospinal fluid barriers: Function and dysfunction. Semin Immunopathol. 2009;31(4):497–511. 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- 70. Marchetti L, Engelhardt B. Immune cell trafficking across the blood–brain barrier in the absence and presence of neuroinflammation. Vasc Biol. 2020;2(1):H1–H18. 10.1530/VB-19-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mansilla MJ, Sellès-Moreno C, Fàbregas-Puig S, et al. Beneficial effect of tolerogenic dendritic cells pulsed with MOG autoantigen in experimental autoimmune encephalomyelitis. CNS Neurosci Ther. 2015;21(3):222–230. 10.1111/cns.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Adorini L, Penna G. Dendritic cell tolerogenicity: A key mechanism in immunomodulation by vitamin D receptor agonists. Hum Immunol. 2009;70(5):345–352. 10.1016/j.humimm.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 73. Besusso D, Saul L, Leech MD, et al. 1,25-Dihydroxyvitamin D3-conditioned CD11c+ dendritic cells are effective initiators of CNS autoimmune disease. Front Immunol. 2015;6:575–575. 10.3389/fimmu.2015.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fallarino F, Grohmann U, You S, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor ζ-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176(11):6752–6761. 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 75. Quintana FJ, Basso AS, Iglesias AH, et al. Control of treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 76. Chen Y, Zhang J, Ge X, Du J, Deb DK, Li YC. Vitamin D receptor inhibits nuclear factor κB activation by interacting with IκB kinase β protein. J Biol Chem. 2013;288(27):19450–19458. 10.1074/jbc.M113.467670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cohen-Lahav M, Shany S, Tobvin D, Chaimovitz C, Douvdevani A. Vitamin D decreases NFκB activity by increasing IκBα levels. Nephrol Dial Transplant. 2006;21(4):889–897. 10.1093/ndt/gfi254. [DOI] [PubMed] [Google Scholar]
- 78. Wu S, Xia Y, Liu X, Sun J. Vitamin D receptor deletion leads to reduced level of IkappaBalpha protein through protein translation, protein–protein interaction, and post-translational modification. Int J Biochem Cell Biol. 2010;42(2):329–336. 10.1016/j.biocel.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang Y, Leung DYM, Richers BN, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188(5):2127–2135. 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liang S, Cai J, Li Y, Yang R. 1,25-Dihydroxy-Vitamin D3 induces macrophage polarization to M2 by upregulating T-cell Ig-mucin-3 expression. Mol Med Rep. 2019;19(5):3707–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Knippenberg S, Smolders J, Thewissen M, et al. Effect of vitamin D3 supplementation on peripheral B cell differentiation and isotype switching in patients with multiple sclerosis. Mult Scler. 2011;17(12):1418–1423. 10.1177/1352458511412655. [DOI] [PubMed] [Google Scholar]
- 82. Mrad MF, El Ayoubi NK, Esmerian MO, Kazan JM, Khoury SJ. Effect of vitamin D replacement on immunological biomarkers in patients with multiple sclerosis. Clin Immunol. 2017;181:9–15. 10.1016/j.clim.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 83. Muris AH, Smolders J, Rolf L, Thewissen M, Hupperts R, Damoiseaux J. Immune regulatory effects of high dose vitamin D3 supplementation in a randomized controlled trial in relapsing remitting multiple sclerosis patients receiving IFNβ; the SOLARIUM study. J Neuroimmunol. 2016;300:47–56. 10.1016/j.jneuroim.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 84. Smolders J, Peelen E, Thewissen M, et al. Safety and T cell modulating effects of high dose vitamin D3 supplementation in multiple sclerosis. PLoS One. 2010;5(12):e15235. 10.1371/journal.pone.0015235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sotirchos ES, Bhargava P, Eckstein C, et al. Safety and immunologic effects of high- vs low-dose cholecalciferol in multiple sclerosis. Neurology. 2016;86(4):382–390. 10.1212/WNL.0000000000002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mosayebi G, Ghazavi A, Ghasami K, Jand Y, Kokhaei P. Therapeutic effect of vitamin D3 in multiple sclerosis patients. Immunol Invest. 2011;40(6):627–639. 10.3109/08820139.2011.573041. [DOI] [PubMed] [Google Scholar]
- 87. Shirvani-Farsani Z, Behmanesh M, Mohammadi SM, Naser Moghadasi A. Vitamin D levels in multiple sclerosis patients: Association with TGF-β2, TGF-βRI, and TGF-βRII expression. Life Sci. 2015;134:63–67. 10.1016/j.lfs.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 88. Åivo J, Hänninen A, Ilonen J, Soilu-Hänninen M. Vitamin D3 administration to MS patients leads to increased serum levels of latency activated peptide (LAP) of TGF-beta. J Neuroimmunol. 2015;280:12–15. 10.1016/j.jneuroim.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 89. Ashtari F, Toghianifar N, Zarkesh-Esfahani SH, Mansourian M. Short-term effect of high-dose vitamin D on the level of interleukin 10 in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled clinical trial. Neuroimmunomodulation. 2015;22(6):400–404. 10.1159/000439278. [DOI] [PubMed] [Google Scholar]
- 90. Røsjø E, Steffensen LH, Jørgensen L, et al. Vitamin D supplementation and systemic inflammation in relapsing-remitting multiple sclerosis. J Neurol. 2015;262(12):2713–2721. 10.1007/s00415-015-7902-5. [DOI] [PubMed] [Google Scholar]
- 91. Golan D, Halhal B, Glass-Marmor L, et al. Vitamin D supplementation for patients with multiple sclerosis treated with interferon-beta: A randomized controlled trial assessing the effect on flu-like symptoms and immunomodulatory properties. BMC Neurol. 2013;13(1):60. 10.1186/1471-2377-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Toghianifar N, Ashtari F, Zarkesh-Esfahani SH, Mansourian M. Effect of high dose vitamin D intake on interleukin-17 levels in multiple sclerosis: A randomized, double-blind, placebo-controlled clinical trial. J Neuroimmunol. 2015;285:125–128. 10.1016/j.jneuroim.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 93. Farsani ZS, Behmanesh M, Sahraian MA. Interleukin-10 but not transforming growth factor-β1 gene expression is up-regulated by vitamin D treatment in multiple sclerosis patients. J Neurol Sci. 2015;350(1–2):18–23. 10.1016/j.jns.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 94. Kimball S, Vieth R, Dosch HM, et al. Cholecalciferol plus calcium suppresses abnormal PBMC reactivity in patients with multiple sclerosis. J Clin Endocrinol Metab. 2011;96(9):2826–2834. 10.1210/jc.2011-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Correale J, Ysrraelit MC, Gaitan MI. Immunomodulatory effects of vitamin D in multiple sclerosis. Brain. 2009;132(5):1146–1160. 10.1093/brain/awp033. [DOI] [PubMed] [Google Scholar]
- 96. Correale J, Ysrraelit MC, Gaitán MI. Gender differences in 1,25 dihydroxyvitamin D3 immunomodulatory effects in multiple sclerosis patients and healthy subjects. J Immunol. 2010;185(8):4948–4958. 10.4049/jimmunol.1000588. [DOI] [PubMed] [Google Scholar]
- 97. da Costa DSMM, Hygino J, Ferreira TB, et al. Vitamin D modulates different IL-17-secreting T cell subsets in multiple sclerosis patients. J Neuroimmunol. 2016;299:8–18. 10.1016/j.jneuroim.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 98. Kickler K, Ni Choileain S, Williams A, Richards A, Astier AL. Calcitriol modulates the CD46 pathway in T cells. PLoS One. 2012;7(10):e48486. 10.1371/journal.pone.0048486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Killick J, Hay J, Morandi E, et al. Vitamin D/CD46 crosstalk in human T cells in multiple sclerosis. Front Immunol. 2020;11:598727. 10.3389/fimmu.2020.598727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lysandropoulos AP, Jaquiéry E, Jilek S, Pantaleo G, Schluep M, Du Pasquier RA. Vitamin D has a direct immunomodulatory effect on CD8+ T cells of patients with early multiple sclerosis and healthy control subjects. J Neuroimmunol. 2011;233(1–2):240–244. 10.1016/j.jneuroim.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 101. Peelen E, Muris AH, Damoiseaux J, et al. GM-CSF production by CD4+ T cells in MS patients: Regulation by regulatory T cells and vitamin D. J Neuroimmunol. 2015;280:36–42. 10.1016/j.jneuroim.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 102. Haas J, Schwarz A, Korporal-Kuhnke M, Faller S, Jarius S, Wildemann B. Hypovitaminosis D upscales B-cell immunoreactivity in multiple sclerosis. J Neuroimmunol. 2016;294:18–26. 10.1016/j.jneuroim.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 103. Sanseverino I, Rinaldi AO, Purificato C, et al. CCL2 induction by 1,25(OH)2D3 in dendritic cells from healthy donors and multiple sclerosis patients. J Steroid Biochem Mol Biol. 2014;144:102–105. 10.1016/j.jsbmb.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 104. Waschbisch A, Sanderson N, Krumbholz M, et al. Interferon beta and vitamin D synergize to induce immunoregulatory receptors on peripheral blood monocytes of multiple sclerosis patients. PLoS One. 2014;9(12):e115488. 10.1371/journal.pone.0115488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bartosik-Psujek H, Tabarkiewicz J, Pocinska K, Stelmasiak Z, Rolinski J. Immunomodulatory effects of vitamin D on monocyte-derived dendritic cells in multiple sclerosis. Mult Scler. 2010;16(12):1513–1516. 10.1177/1352458510379611. [DOI] [PubMed] [Google Scholar]
- 106. Berge T, Leikfoss IS, Brorson IS, et al. The multiple sclerosis susceptibility genes TAGAP and IL2RA are regulated by vitamin D in CD4+ T cells. Genes Immun. 2016;17(2):118–127. 10.1038/gene.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol. 2017;18(2):123–131. 10.1038/ni.3666. [DOI] [PubMed] [Google Scholar]
- 108. Mohseni S, Tabatabaei-Malazy O, Shadman Z, Khashayar P, Mohajeri-Tehrani M, Larijani B. Targeting dyslipidemia with antioxidative vitamins C, D, and E; a systematic review of meta-analysis studies: Dyslipidemia and antioxidative vitamins. J Diabetes Metab Disord. 2021;20(2):2037–2047. 10.1007/s40200-021-00919-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kermode AG, Thompson AJ, Tofts P, et al. Breakdown of the blood–brain barrier precedes symptoms and other MRI signs of new lesions in multiple sclerosis. Pathogenetic and clinical implications. Brain. 1990;113(Pt 5):1477–1489. 10.1093/brain/113.5.1477. [DOI] [PubMed] [Google Scholar]
- 110. Lassmann H. Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front Immunol. 2019;9:3116. 10.3389/fimmu.2018.03116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Plumb J, McQuaid S, Mirakhur M, Kirk J. Abnormal endothelial tight junctions in active lesions and normal-appearing white matter in multiple sclerosis. Brain Pathol. 2002;12(2):154–169. 10.1111/j.1750-3639.2002.tb00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kirk J, Plumb J, Mirakhur M, McQuaid S. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood–brain barrier leakage and active demyelination. J Pathol. 2003;201(2):319–327. 10.1002/path.1434. [DOI] [PubMed] [Google Scholar]
- 113. Leech S, Kirk J, Plumb J, McQuaid S. Persistent endothelial abnormalities and blood–brain barrier leak in primary and secondary progressive multiple sclerosis. Neuropathol Appl Neurobiol. 2007;33(1):86–98. 10.1111/j.1365-2990.2006.00781.x. [DOI] [PubMed] [Google Scholar]
- 114. Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta. 2011;1812(2):252–264. 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 115. Alvarez JI, Saint-Laurent O, Godschalk A, et al. Focal disturbances in the blood–brain barrier are associated with formation of neuroinflammatory lesions. Neurobiol Dis. 2015;74:14–24. 10.1016/j.nbd.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 116. Padden M, Leech S, Craig B, Kirk J, Brankin B, McQuaid S. Differences in expression of junctional adhesion molecule-A and beta-catenin in multiple sclerosis brain tissue: Increasing evidence for the role of tight junction pathology. Acta Neuropathol. 2007;113(2):177–186. 10.1007/s00401-006-0145-x. [DOI] [PubMed] [Google Scholar]
- 117. Vos CM, Geurts JJ, Montagne L, et al. Blood–brain barrier alterations in both focal and diffuse abnormalities on postmortem MRI in multiple sclerosis. Neurobiol Dis. 2005;20(3):953–960. 10.1016/j.nbd.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 118. Durk MR, Chan GN, Campos CR, et al. 1α,25-Dihydroxyvitamin D3-liganded vitamin D receptor increases expression and transport activity of P-glycoprotein in isolated rat brain capillaries and human and rat brain microvessel endothelial cells. J Neurochem. 2012;123(6):944–953. 10.1111/jnc.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Guo YX, He LY, Zhang M, Wang F, Liu F, Peng WX. 1,25-Dihydroxyvitamin D3 regulates expression of LRP1 and RAGE in vitro and in vivo, enhancing Aβ1-40 brain-to-blood efflux and peripheral uptake transport. Neuroscience. 2016;322:28–38. 10.1016/j.neuroscience.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 120. Alam C, Hoque MT, Finnell RH, Goldman ID, Bendayan R. Regulation of reduced folate carrier (RFC) by vitamin D receptor at the blood–brain barrier. Mol Pharm. 2017;14(11):3848–3858. 10.1021/acs.molpharmaceut.7b00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Landel V, Stephan D, Cui X, Eyles D, Feron F. Differential expression of vitamin D-associated enzymes and receptors in brain cell subtypes. J Steroid Biochem Mol Biol. 2018;177:129–134. 10.1016/j.jsbmb.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 122. Enkhjargal B, McBride DW, Manaenko A, et al. Intranasal administration of vitamin D attenuates blood–brain barrier disruption through endogenous upregulation of osteopontin and activation of CD44/P-gp glycosylation signaling after subarachnoid hemorrhage in rats. J Cereb Blood Flow Metab. 2017;37(7):2555–2566. 10.1177/0271678X16671147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sayeed I, Turan N, Stein DG, Wali B. Vitamin D deficiency increases blood–brain barrier dysfunction after ischemic stroke in male rats. Exp Neurol. 2019;312:63–71. 10.1016/j.expneurol.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 124. El-Atifi M, Dreyfus M, Berger F, Wion D. Expression of CYP2R1 and VDR in human brain pericytes: The neurovascular vitamin D autocrine/paracrine model. Neuroreport. 2015;26(5):245–248. 10.1097/WNR.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 125. Jamali N, Song YS, Sorenson CM, Sheibani N. 1,25(OH)2D3 regulates the proangiogenic activity of pericyte through VDR-mediated modulation of VEGF production and signaling of VEGF and PDGF receptors. FASEB Bioadv. 2019;1(7):415–434. 10.1096/fba.2018-00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Naveilhan P, Neveu I, Baudet C, et al. 1,25-Dihydroxyvitamin D3 regulates the expression of the low-affinity neurotrophin receptor. Brain Res Mol Brain Res. 1996;41(1–2):259–268. 10.1016/0169-328X(96)00103-9. [DOI] [PubMed] [Google Scholar]
- 127. Smolders J, Schuurman KG, van Strien ME, et al. Expression of vitamin D receptor and metabolizing enzymes in multiple sclerosis affected brain tissue. J Neuropathol Exp Neurol. 2013;72(2):91. 10.1097/NEN.0b013e31827f4fcc. [DOI] [PubMed] [Google Scholar]
- 128. Neveu I, Naveilhan P, Baudet C, Brachet P, Metsis M. 1,25-dihydroxyvitamin D3 regulates NT-3, NT-4 but not BDNF mRNA in astrocytes. Neuroreport. 1994;6(1):124–126. 10.1097/00001756-199412300-00032. [DOI] [PubMed] [Google Scholar]
- 129. Boontanrart M, Hall SD, Spanier JA, Hayes CE, Olson JK. Vitamin D3 alters microglia immune activation by an IL-10 dependent SOCS3 mechanism. J Neuroimmunol. 2016;292:126–136. 10.1016/j.jneuroim.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 130. He J, Guo X, Liu ZQ, Yang PC, Yang S. Vitamin D inhibits the staphylococcal enterotoxin B-induced expression of tumor necrosis factor in microglial cells. Immunol Res. 2017;65(4):913–919. 10.1007/s12026-017-8930-2. [DOI] [PubMed] [Google Scholar]
- 131. Cui C, Xu P, Li G, et al. Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II-exposed microglial cells: Role of renin-angiotensin system. Redox Biol. 2019;26:101295. 10.1016/j.redox.2019.101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Baas D, Prufer K, Ittel ME, et al. Rat oligodendrocytes express the vitamin D3 receptor and respond to 1,25-dihydroxyvitamin D3. Glia. 2000;31(1):59–68. . [DOI] [PubMed] [Google Scholar]
- 133. Shirazi HA, Rasouli J, Ciric B, Rostami A, Zhang GX. 1,25-Dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation. Exp Mol Pathol. 2015;98(2):240–245. 10.1016/j.yexmp.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Mengozzi M, Hesketh A, Bucca G, Ghezzi P, Smith CP. Vitamins D3 and D2 have marked but different global effects on gene expression in a rat oligodendrocyte precursor cell line. Mol Med. 2020;26(1):32. 10.1186/s10020-020-00153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Durk MR, Fan J, Sun H, et al. Vitamin D receptor activation induces P-glycoprotein and increases brain efflux of quinidine: An intracerebral microdialysis study in conscious rats. Pharm Res. 2015;32(3):1128–1140. 10.1007/s11095-014-1524-y. [DOI] [PubMed] [Google Scholar]
- 136. Durk MR, Han K, Chow EC, et al. 1α,25-Dihydroxyvitamin D3 reduces cerebral amyloid-β accumulation and improves cognition in mouse models of Alzheimer’s disease. J Neurosci. 2014;34(21):7091–7101. 10.1523/JNEUROSCI.2711-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Sadeghian N, Shadman J, Moradi A, Ghasem Golmohammadi M, Panahpour H. Calcitriol protects the blood–brain barrier integrity against ischemic stroke and reduces vasogenic brain edema via antioxidant and antiapoptotic actions in rats. Brain Res Bull. 2019;150:281–289. 10.1016/j.brainresbull.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 138. Hajiluian G, Nameni G, Shahabi P, Mesgari-Abbasi M, Sadigh-Eteghad S, Farhangi MA. Vitamin D administration, cognitive function, BBB permeability and neuroinflammatory factors in high-fat diet-induced obese rats. Int J Obes (Lond). 2017;41(4):639–644. 10.1038/ijo.2017.10. [DOI] [PubMed] [Google Scholar]
- 139. Hahn M, Lorez H, Fischer G. Effect of calcitriol in combination with corticosterone, interleukin-1beta, and transforming growth factor-beta1 on nerve growth factor secretion in an astroglial cell line. J Neurochem. 1997;69(1):102–109. 10.1046/j.1471-4159.1997.69010102.x. [DOI] [PubMed] [Google Scholar]
- 140. Neveu I, Naveilhan P, Jehan F, et al. 1,25-Dihydroxyvitamin D3 regulates the synthesis of nerve growth factor in primary cultures of glial cells. Mol Brain Res. 1994;24(1-4):70–76. 10.1016/0169-328X(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 141. Uberti F, Lattuada D, Morsanuto V, et al. Vitamin D protects human endothelial cells from oxidative stress through the autophagic and survival pathways. J Clin Endocrinol Metab. 2014;99(4):1367–1374. 10.1210/jc.2013-2103. [DOI] [PubMed] [Google Scholar]
- 142. Molinari C, Uberti F, Grossini E, et al. 1α,25-Dihydroxycholecalciferol Induces nitric oxide production in cultured endothelial cells. Cell Physiol Biochem. 2011;27(6):661–668. 10.1159/000330075. [DOI] [PubMed] [Google Scholar]
- 143. Takahashi S, Maeda T, Sano Y, et al. Active form of vitamin D directly protects the blood–brain barrier in multiple sclerosis. Clin Exp Neuroimmunol. 2017;8(3):244–254. 10.1111/cen3.12398. [DOI] [Google Scholar]
- 144. Won S, Sayeed I, Peterson BL, Wali B, Kahn JS, Stein DG. Vitamin D prevents hypoxia/reoxygenation-induced blood–brain barrier disruption via vitamin D receptor-mediated NF-kB signaling pathways. PLoS One. 2015;10(3):e0122821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Hur J, Lee P, Kim MJ, Cho YW. Regulatory effect of 25-hydroxyvitamin D3 on nitric oxide production in activated microglia. Korean J Physiol Pharmacol. 2014;18(5):397–402. 10.4196/kjpp.2014.18.5.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Alessio N, Belardo C, Trotta MC, et al. Vitamin D deficiency induces chronic pain and microglial phenotypic changes in mice. Int J Mol Sci. 2021;22(7):3604. 10.3390/ijms22073604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kurtys E, Eisel ULM, Verkuyl JM, Broersen LM, Dierckx R, de Vries EFJ. The combination of vitamins and omega-3 fatty acids has an enhanced anti-inflammatory effect on microglia. Neurochem Int. 2016;99:206–214. 10.1016/j.neuint.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 148. Dulla YA, Kurauchi Y, Hisatsune A, Seki T, Shudo K, Katsuki H. Regulatory mechanisms of vitamin D3 on production of nitric oxide and pro-inflammatory cytokines in microglial BV-2 cells. Neurochem Res. 2016;41(11):2848–2858. 10.1007/s11064-016-2000-3. [DOI] [PubMed] [Google Scholar]
- 149. de Oliveira LRC, Mimura LAN, Fraga-Silva TFC, et al. Calcitriol prevents neuroinflammation and reduces blood–brain barrier disruption and local macrophage/microglia activation. Front Pharmacol. 2020;11:161. 10.3389/fphar.2020.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Yang J, Wang K, Hu T, Wang G, Wang W, Zhang J. Vitamin D3 supplement attenuates blood–brain barrier disruption and cognitive impairments in a rat model of traumatic brain injury. Neuromolecular Med. 2021;23(4):491–499. 10.1007/s12017-021-08649-z. [DOI] [PubMed] [Google Scholar]
- 151. Chow EC, Durk MR, Cummins CL, Pang KS. 1Alpha,25-dihydroxyvitamin D3 up-regulates P-glycoprotein via the vitamin D receptor and not farnesoid X receptor in both fxr(−/−) and fxr(+/+) mice and increased renal and brain efflux of digoxin in mice in vivo. J Pharmacol Exp Ther. 2011;337(3):846–859. 10.1124/jpet.111.179101. [DOI] [PubMed] [Google Scholar]
- 152. Peng HB, Noh K, Pan SR, et al. Human amyloid-β40 kinetics after intravenous and intracerebroventricular injections and calcitriol treatment in rats in vivo. Drug Metab Dispos. 2020;48(10):944–955. 10.1124/dmd.120.090886. [DOI] [PubMed] [Google Scholar]
- 153. Lefebvre d’Hellencourt C, Montero-Menei CN, Bernard R, Couez D. Vitamin D3 inhibits proinflammatory cytokines and nitric oxide production by the EOC13 microglial cell line. J Neurosci Res. 2003;71(4):575–582. 10.1002/jnr.10491. [DOI] [PubMed] [Google Scholar]
- 154. Djukic M, Onken ML, Schutze S, et al. Vitamin d deficiency reduces the immune response, phagocytosis rate, and intracellular killing rate of microglial cells. Infect Immun. 2014;82(6):2585–2594. 10.1128/IAI.01814-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Lee PW, Selhorst A, Lampe SG, Liu Y, Yang Y, Lovett-Racke AE. Neuron-specific vitamin D signaling attenuates microglia activation and CNS autoimmunity. Front Neurol. 2020;11:19. 10.3389/fneur.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Ekici F, Ozyurt B, Erdogan H. The combination of vitamin D3 and dehydroascorbic acid administration attenuates brain damage in focal ischemia. Neurol Sci. 2009;30(3):207–212. 10.1007/s10072-009-0038-6. [DOI] [PubMed] [Google Scholar]
- 157. Dehghani L, Meamar R, Etemadifar M, et al. Can vitamin d suppress endothelial cells apoptosis in multiple sclerosis patients? Int J Prev Med. 2013;4(Suppl 2):S211–S215. [PMC free article] [PubMed] [Google Scholar]
- 158. Kim JS, Ryu SY, Yun I, et al. 1alpha,25-dihydroxyvitamin D3 protects dopaminergic neurons in rodent models of Parkinson’s disease through inhibition of microglial activation. J Clin Neurol. 2006;2(4):252–257. 10.3988/jcn.2006.2.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Goudarzvand M, Javan M, Mirnajafi-Zadeh J, Mozafari S, Tiraihi T. Vitamins E and D3 attenuate demyelination and potentiate remyelination processes of hippocampal formation of rats following local injection of ethidium bromide. Cell Mol Neurobiol. 2010;30(2):289–299. 10.1007/s10571-009-9451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Shirazi HA, Rasouli J, Ciric B, Wei D, Rostami A, Zhang GX. 1,25-Dihydroxyvitamin D3 suppressed experimental autoimmune encephalomyelitis through both immunomodulation and oligodendrocyte maturation. Exp Mol Pathol. 2017;102(3):515–521. 10.1016/j.yexmp.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Nystad AE, Wergeland S, Aksnes L, Myhr KM, Bo L, Torkildsen O. Effect of high-dose 1.25 dihydroxyvitamin D3 on remyelination in the cuprizone model. APMIS. 2014;122(12):1178–1186. 10.1111/apm.12281. [DOI] [PubMed] [Google Scholar]
- 162. Wergeland S, Torkildsen O, Myhr KM, Aksnes L, Mork SJ, Bo L. Dietary vitamin D3 supplements reduce demyelination in the cuprizone model. PLoS One. 2011;6(10):e26262. 10.1371/journal.pone.0026262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Zhang D, Qiao L, Fu T. Paricalcitol improves experimental autoimmune encephalomyelitis (EAE) by suppressing inflammation via NF-κB signaling. Biomed Pharmacother. 2020;125:109528. 10.1016/j.biopha.2019.109528. [DOI] [PubMed] [Google Scholar]
- 164. Tang H, Hua F, Wang J, et al. Progesterone and vitamin D combination therapy modulates inflammatory response after traumatic brain injury. Brain Inj. 2015;29(10):1165–1174. 10.3109/02699052.2015.1035330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Calvello R, Cianciulli A, Nicolardi G, et al. Vitamin D treatment attenuates neuroinflammation and dopaminergic neurodegeneration in an animal model of Parkinson’s disease, shifting M1 to M2 microglia responses. J Neuroimmune Pharmacol. 2017;12(2):327–339. 10.1007/s11481-016-9720-7. [DOI] [PubMed] [Google Scholar]
- 166. Spach KM, Pedersen LB, Nashold FE, et al. Gene expression analysis suggests that 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by stimulating inflammatory cell apoptosis. Physiol Genomics. 2004;18(2):141–151. 10.1152/physiolgenomics.00003.2004. [DOI] [PubMed] [Google Scholar]
- 167. Gomez-Pinedo U, Cuevas JA, Benito-Martín MS, et al. Vitamin D increases remyelination by promoting oligodendrocyte lineage differentiation. Brain Behav. 2020;10(1):e01498. 10.1002/brb3.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Malinic M, Dozudic JG. Effects of vitamin D on the expression of markers of principal neurons, interneurons and astrocytes in cerebral cortex and hippocampus in gerbils exposed to transient global cerebral ischemia: PS231. Porto Biomed J. 2017;2(5):209. 10.1016/j.pbj.2017.07.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Hua F, Reiss JI, Tang H, et al. Progesterone and low-dose vitamin D hormone treatment enhances sparing of memory following traumatic brain injury. Horm Behav. 2012;61(4):642–651. 10.1016/j.yhbeh.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Evans MA, Kim HA, Ling YH, et al. Vitamin D3 supplementation reduces subsequent brain injury and inflammation associated with ischemic stroke. Neuromolecular Med. 2018;20(1):147–159. 10.1007/s12017-018-8484-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Hupperts R, Smolders J, Vieth R, et al. Randomized trial of daily high-dose vitamin D3 in patients with RRMS receiving subcutaneous interferon β-1a. Neurology. 2019;93(20):e1906–e1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Kimball SM, Ursell MR, O’Connor P, Vieth R. Safety of vitamin D3 in adults with multiple sclerosis. Am J Clin Nutr. 2007;86(3):645–651. 10.1093/ajcn/86.3.645. [DOI] [PubMed] [Google Scholar]
- 173. Soilu-Hänninen M, Åivo J, Lindström BM, et al. A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon β-1b in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2012;83(5):565–571. 10.1136/jnnp-2011-301876. [DOI] [PubMed] [Google Scholar]
- 174. Chang HC, Tai YT, Cherng YG, et al. Resveratrol attenuates high-fat diet-induced disruption of the blood–brain barrier and protects brain neurons from apoptotic insults. J Agric Food Chem. 2014;62(15):3466–3475. 10.1021/jf403286w. [DOI] [PubMed] [Google Scholar]
- 175. Davidson TL, Monnot A, Neal AU, Martin AA, Horton JJ, Zheng W. The effects of a high-energy diet on hippocampal-dependent discrimination performance and blood–brain barrier integrity differ for diet-induced obese and diet-resistant rats. Physiol Behav. 2012;107(1):26–33. 10.1016/j.physbeh.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Elahy M, Lam V, Pallebage-Gamarallage MM, Giles C, Mamo JC, Takechi R. Nicotine attenuates disruption of blood–brain barrier induced by saturated-fat feeding in wild-type mice. Nicotine Tob Res. 2015;17(12):1436–1441. 10.1093/ntr/ntv044. [DOI] [PubMed] [Google Scholar]
- 177. Pallebage-Gamarallage M, Lam V, Takechi R, Galloway S, Clark K, Mamo J. Restoration of dietary-fat induced blood–brain barrier dysfunction by anti-inflammatory lipid-modulating agents. Lipids Health Dis. 2012;11:117. 10.1186/1476-511X-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Löscher W, Potschka H. Blood–brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2(1):86–98. 10.1602/neurorx.2.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Kooij G, van Horssen J, de Lange EC, et al. T lymphocytes impair P-glycoprotein function during neuroinflammation. J Autoimmun. 2010;34(4):416–425. 10.1016/j.jaut.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 180. Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 181. Chiuso-Minicucci F, Ishikawa LLW, Mimura LAN, et al. Treatment with vitamin D/MOG association suppresses experimental autoimmune encephalomyelitis. PLoS One. 2015;10(5):e0125836. 10.1371/journal.pone.0125836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Hochmeister S, Aeinehband S, Dorris C, et al. Effect of vitamin D on experimental autoimmune neuroinflammation is dependent on haplotypes comprising naturally occurring allelic variants of CIITA (Mhc2ta). Front Neurol. 2020;11:600401. 10.3389/fneur.2020.600401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Cayrol R, Wosik K, Berard JL, et al. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol. 2008;9(2):137–145. 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
- 184. Lyck R, Lécuyer MA, Abadier M, et al. ALCAM (CD166) is involved in extravasation of monocytes rather than T cells across the blood–brain barrier. J Cereb Blood Flow Metab. 2017;37(8):2894–2909. 10.1177/0271678X16678639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Larochelle C, Cayrol R, Kebir H, et al. Melanoma cell adhesion molecule identifies encephalitogenic T lymphocytes and promotes their recruitment to the central nervous system. Brain. 2012;135(Pt 10):2906–2924. 10.1093/brain/aws212. [DOI] [PubMed] [Google Scholar]
- 186. Ifergan I, Kebir H, Terouz S, et al. Role of ninjurin-1 in the migration of myeloid cells to central nervous system inflammatory lesions. Ann Neurol. 2011;70(5):751–763. 10.1002/ana.22519. [DOI] [PubMed] [Google Scholar]
- 187. Odoardi F, Sie C, Streyl K, et al. T cells become licensed in the lung to enter the central nervous system. Nature. 2012;488(7413):675–679. 10.1038/nature11337. [DOI] [PubMed] [Google Scholar]
- 188. Brown LS, Foster CG, Courtney JM, King NE, Howells DW, Sutherland BA. Pericytes and neurovascular function in the healthy and diseased brain. Front Cell Neurosci. 2019;13:282. 10.3389/fncel.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Stratman AN, Davis GE. Endothelial cell–pericyte interactions stimulate basement membrane matrix assembly: Influence on vascular tube remodeling, maturation, and stabilization. Microsc Microanal. 2012;18(1):68–80. 10.1017/S1431927611012402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Armulik A, Genove G, Betsholtz C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193–215. 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 191. Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114(24):5091–5101. 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–566. 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood–brain barrier. Nature. 2010;468(7323):557–561. 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 194. Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277(5323):242–245. 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 195. Quaegebeur A, Segura I, Carmeliet P. Pericytes: Blood–brain barrier safeguards against neurodegeneration? Neuron. 2010;68(3):321–323. 10.1016/j.neuron.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 196. Shepro D, Morel NM. Pericyte physiology. FASEB J. 1993;7(11):1031–1038. 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- 197. He L, Vanlandewijck M, Mae MA, et al. Single-cell RNA sequencing of mouse brain and lung vascular and vessel-associated cell types. Sci Data. 2018;5:180160. 10.1038/sdata.2018.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Ogura S, Kurata K, Hattori Y, et al. Sustained inflammation after pericyte depletion induces irreversible blood-retina barrier breakdown. JCI Insight. 2017;2(3):e90905. 10.1172/jci.insight.90905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Rustenhoven J, Jansson D, Smyth LC, Dragunow M. Brain pericytes as mediators of neuroinflammation. Trends Pharmacol Sci. 2017;38(3):291–304. 10.1016/j.tips.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 200. Thomas H, Cowin AJ, Mills SJ. The importance of pericytes in healing: Wounds and other pathologies. Int J Mol Sci. 2017;18(6):1129. 10.3390/ijms18061129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Duan L, Zhang XD, Miao WY, et al. PDGFRbeta cells rapidly relay inflammatory signal from the circulatory system to neurons via chemokine CCL2. Neuron. 2018;100(1):183–200.e8. 10.1016/j.neuron.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 202. Herland A, van der Meer AD, FitzGerald EA, Park TE, Sleeboom JJ, Ingber DE. Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood–brain barrier on a chip. PLoS One. 2016;11(3):e0150360. 10.1371/journal.pone.0150360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Rustenhoven J, Aalderink M, Scotter EL, et al. TGF-beta1 regulates human brain pericyte inflammatory processes involved in neurovasculature function. J Neuroinflammation. 2016;13:37. 10.1186/s12974-016-0503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Torok O, Schreiner B, Schaffenrath J, et al. Pericytes regulate vascular immune homeostasis in the CNS. Proc Natl Acad Sci U S A. 2021;118(10):e2016587118. 10.1073/pnas.2016587118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Girolamo F, de Trizio I, Errede M, Longo G, d’Amati A, Virgintino D. Neural crest cell-derived pericytes act as pro-angiogenic cells in human neocortex development and gliomas. Fluids Barriers CNS. 2021;18(1):14. 10.1186/s12987-021-00242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Grygorowicz T, Dabrowska-Bouta B, Struzynska L. Administration of an antagonist of P2X7 receptor to EAE rats prevents a decrease of expression of claudin-5 in cerebral capillaries. Purinergic Signal. 2018;14(4):385–393. 10.1007/s11302-018-9620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Tuzun E, Şekerdağ E, Atak D, et al. The Role of Pericytes in the Pathophysiology of Multiple Sclerosis (P2.2-075). Neurology [Internet]. 2019;92(15 Supplement). Available from: https://n.neurology.org/content/92/15_Supplement/P2.2-075. [Google Scholar]
- 208. Iacobaeus E, Sugars RV, Tornqvist Andren A, et al. Dynamic changes in brain mesenchymal perivascular cells associate with multiple sclerosis disease duration, active inflammation, and demyelination. Stem Cells Transl Med. 2017;6(10):1840–1851. 10.1002/sctm.17-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Vanlandewijck M, He L, Mae MA, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554(7693):475–480. 10.1038/nature25739. [DOI] [PubMed] [Google Scholar]
- 210. Nissou MF, Guttin A, Zenga C, Berger F, Issartel JP, Wion D. Additional clues for a protective role of vitamin D in neurodegenerative diseases: 1,25-dihydroxyvitamin D3 triggers an anti-inflammatory response in brain pericytes. J Alzheimers Dis. 2014;42(3):789–799. 10.3233/JAD-140411. [DOI] [PubMed] [Google Scholar]
- 211. Nataf S, Garcion E, Darcy F, Chabannes D, Muller JY, Brachet P. 1,25 Dihydroxyvitamin D3 exerts regional effects in the central nervous system during experimental allergic encephalomyelitis. J Neuropathol Exp Neurol. 1996;55(8):904–914. 10.1097/00005072-199608000-00006. [DOI] [PubMed] [Google Scholar]
- 212. Chung I, Han G, Seshadri M, et al. Role of vitamin D receptor in the antiproliferative effects of calcitriol in tumor-derived endothelial cells and tumor angiogenesis in vivo. Cancer Res. 2009;69(3):967–975. 10.1158/0008-5472.CAN-08-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Jamali N, Wang S, Darjatmoko SR, Sorenson CM, Sheibani N. Vitamin D receptor expression is essential during retinal vascular development and attenuation of neovascularization by 1, 25(OH)2D3. PLoS One. 2017;12(12):e0190131. 10.1371/journal.pone.0190131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Sofroniew MV, Vinters HV. Astrocytes: Biology and pathology. Acta Neuropathol. 2010;119(1):7–35. 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Liebner S, Dijkhuizen RM, Reiss Y, Plate KH, Agalliu D, Constantin G. Functional morphology of the blood–brain barrier in health and disease. Acta Neuropathol. 2018;135(3):311–336. 10.1007/s00401-018-1815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Agrawal S, Anderson P, Durbeej M, et al. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med. 2006;203(4):1007–1019. 10.1084/jem.20051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Brosnan CF, Raine CS. The astrocyte in multiple sclerosis revisited. Glia. 2013;61(4):453–465. 10.1002/glia.22443. [DOI] [PubMed] [Google Scholar]
- 218. Ponath G, Ramanan S, Mubarak M, et al. Myelin phagocytosis by astrocytes after myelin damage promotes lesion pathology. Brain. 2017;140(2):399–413. 10.1093/brain/aww298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Wang D, Ayers MM, Catmull DV, Hazelwood LJ, Bernard CCA, Orian JM. Astrocyte-associated axonal damage in pre-onset stages of experimental autoimmune encephalomyelitis. Glia. 2005;51(3):235–240. 10.1002/glia.20199. [DOI] [PubMed] [Google Scholar]
- 220. Pham H, Ramp AA, Klonis N, et al. The astrocytic response in early experimental autoimmune encephalomyelitis occurs across both the grey and white matter compartments. J Neuroimmunol. 2009;208(1–2):30–39. 10.1016/j.jneuroim.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 221. D’Amelio FE, Smith ME, Eng LF. Sequence of tissue responses in the early stages of experimental allergic encephalomyelitis (EAE): Immunohistochemical, light microscopic, and ultrastructural observations in the spinal cord. Glia. 1990;3(4):229–240. 10.1002/glia.440030402. [DOI] [PubMed] [Google Scholar]
- 222. Shih RH, Wang CY, Yang CM. NF-kappaB Signaling pathways in neurological inflammation: A mini review. Front Mol Neurosci. 2015;8:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Sheng WS, Hu S, Feng A, Rock RB. Reactive oxygen species from human astrocytes induced functional impairment and oxidative damage. Neurochem Res. 2013;38(10):2148–2159. 10.1007/s11064-013-1123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Lee SJ, Benveniste EN. Adhesion molecule expression and regulation on cells of the central nervous system. J Neuroimmunol. 1999;98(2):77–88. 10.1016/S0165-5728(99)00084-3. [DOI] [PubMed] [Google Scholar]
- 225. Colombo E, Cordiglieri C, Melli G, et al. Stimulation of the neurotrophin receptor TrkB on astrocytes drives nitric oxide production and neurodegeneration. J Exp Med. 2012;209(3):521–535. 10.1084/jem.20110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Jiao KP, Li SM, Lv WY, Jv ML, He HY. Vitamin D3 repressed astrocyte activation following lipopolysaccharide stimulation in vitro and in neonatal rats. Neuroreport. 2017;28(9):492–497. 10.1097/WNR.0000000000000782. [DOI] [PubMed] [Google Scholar]
- 227. Eyles DW, Liu PY, Josh P, Cui X. Intracellular distribution of the vitamin D receptor in the brain: Comparison with classic target tissues and redistribution with development. Neuroscience. 2014;268:1–9. 10.1016/j.neuroscience.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 228. de la Fuente AG, Errea O, van Wijngaarden P, et al. Vitamin D receptor–retinoid X receptor heterodimer signaling regulates oligodendrocyte progenitor cell differentiation. J Cell Biol. 2015;211(5):975–985. 10.1083/jcb.201505119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Molinari C, Morsanuto V, Ghirlanda S, et al. Role of combined lipoic acid and vitamin D3 on astrocytes as a way to prevent brain ageing by induced oxidative stress and iron accumulation. Oxid Med Cell Longev. 2019;2019:2843121. 10.1155/2019/2843121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Garcion E, Nataf S, Berod A, Darcy F, Brachet P. 1,25-Dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Brain Res Mol Brain Res. 1997;45(2):255–267. 10.1016/S0169-328X(96)00260-4. [DOI] [PubMed] [Google Scholar]
- 231. Ohl K, Tenbrock K, Kipp M. Oxidative stress in multiple sclerosis: Central and peripheral mode of action. Exp Neurol. 2016;277:58–67. 10.1016/j.expneurol.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Ferronato MJ, Alonso EN, Salomon DG, et al. Antitumoral effects of the alkynylphosphonate analogue of calcitriol EM1 on glioblastoma multiforme cells. J Steroid Biochem Mol Biol. 2018;178:22–35. 10.1016/j.jsbmb.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 233. Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10(12):1538–1543. 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 234. Mildner A, Schmidt H, Nitsche M, et al. Microglia in the adult brain arise from ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10(12):1544–1553. 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 235. Hagemeyer N, Hanft KM, Akriditou MA, et al. Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol. 2017;134(3):441–458. 10.1007/s00401-017-1747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91(2):461–553. 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 238. Sierra A, Encinas JM, Deudero JJ, et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7(4):483–495. 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Olson JK. Immune response by microglia in the spinal cord. Ann N Y Acad Sci. 2010;1198:271–278. 10.1111/j.1749-6632.2010.05536.x. [DOI] [PubMed] [Google Scholar]
- 240. Jurga AM, Paleczna M, Kuter KZ. Overview of general and discriminating markers of differential microglia phenotypes. Front Cell Neurosci. 2020;14:198. 10.3389/fncel.2020.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Bogie JFJ, Mailleux J, Wouters E, et al. Scavenger receptor collectin placenta 1 is a novel receptor involved in the uptake of myelin by phagocytes. Sci Rep. 2017;7(1):44794. 10.1038/srep44794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: Pathology and pathogenesis. Nat Rev Neurol. 2012;8(11):647–656. 10.1038/nrneurol.2012.168. [DOI] [PubMed] [Google Scholar]
- 243. Voß EV, Škuljec J, Gudi V, et al. Characterisation of microglia during de- and remyelination: Can they create a repair promoting environment? Neurobiol Dis. 2012;45(1):519–528. 10.1016/j.nbd.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 244. Neveu I, Naveilhan P, Menaa C, Wion D, Brachet P, Garabédian M. Synthesis of 1,25-dihydroxyvitamin D3 by rat brain macrophages in vitro. J Neurosci Res. 1994;38(2):214–220. 10.1002/jnr.490380212. [DOI] [PubMed] [Google Scholar]
- 245. Cui LY, Chu SF, Chen NH. The role of chemokines and chemokine receptors in multiple sclerosis. Int Immunopharmacol. 2020;83:106314. 10.1016/j.intimp.2020.106314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Spanier JA, Nashold FE, Nelson CD, Praska CE, Hayes CE. Vitamin D3-mediated resistance to a multiple sclerosis model disease depends on myeloid cell 1,25-dihydroxyvitamin D3 synthesis and correlates with increased CD4(+) T cell CTLA-4 expression. J Neuroimmunol. 2020;338:577105. 10.1016/j.jneuroim.2019.577105. [DOI] [PubMed] [Google Scholar]
- 247. Garcion E, Sindji L, Nataf S, Brachet P, Darcy F, Montero-Menei CN. Treatment of experimental autoimmune encephalomyelitis in rat by 1,25-dihydroxyvitamin D3 leads to early effects within the central nervous system. Acta Neuropathol. 2003;105(5):438–448. 10.1007/s00401-002-0663-0. [DOI] [PubMed] [Google Scholar]
- 248. Clarke J, Yaqubi M, Futhey NC, et al. Vitamin D regulates MerTK-dependent phagocytosis in human myeloid cells. J Immunol. 2020;205(2):398–406. 10.4049/jimmunol.2000129. [DOI] [PubMed] [Google Scholar]
- 249. Garcion E, Sindji L, Montero-Menei C, Andre C, Brachet P, Darcy F. Expression of inducible nitric oxide synthase during rat brain inflammation: Regulation by 1,25-dihydroxyvitamin D3. Glia. 1998;22(3):282–294. . [DOI] [PubMed] [Google Scholar]
- 250. Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8(6):451–465. 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- 251. Cameron-Curry P, Le Douarin NM. Oligodendrocyte precursors originate from both the dorsal and the ventral parts of the spinal cord. Neuron. 1995;15(6):1299–1310. 10.1016/0896-6273(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 252. Thomas JL, Spassky N, Perez Villegas EM, et al. Spatiotemporal development of oligodendrocytes in the embryonic brain. J Neurosci Res. 2000;59(4):471–476. . [DOI] [PubMed] [Google Scholar]
- 253. Stadelmann C, Timmler S, Barrantes-Freer A, Simons M. Myelin in the central nervous system: Structure, function, and pathology. Physiol Rev. 2019;99(3):1381–1431. 10.1152/physrev.00031.2018. [DOI] [PubMed] [Google Scholar]
- 254. Du Y, Dreyfus CF. Oligodendrocytes as providers of growth factors. J Neurosci Res. 2002;68(6):647–654. 10.1002/jnr.10245. [DOI] [PubMed] [Google Scholar]
- 255. Nakahara J, Aiso S, Suzuki N. Autoimmune versus oligodendrogliopathy: The pathogenesis of multiple sclerosis. Arch Immunol Ther Exp (Warsz). 2010;58(5):325–333. 10.1007/s00005-010-0094-x. [DOI] [PubMed] [Google Scholar]
- 256. Lubetzki C, Stankoff B. Demyelination in multiple sclerosis. Handb Clin Neurol. 2014;122:89–99. 10.1016/B978-0-444-52001-2.00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Na SY, Cao Y, Toben C, et al. Naive CD8 T-cells initiate spontaneous autoimmunity to a sequestered model antigen of the central nervous system. Brain. 2008;131(Pt 9):2353–2365. [DOI] [PubMed] [Google Scholar]
- 258. Lassmann H. Multiple sclerosis: Lessons from molecular neuropathology. Exp Neurol. 2014;262(Pt A):2–7. 10.1016/j.expneurol.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 259. Ramanathan S, Dale RC, Brilot F. Anti-MOG antibody: The history, clinical phenotype, and pathogenicity of a serum biomarker for demyelination. Autoimmun Rev. 2016;15(4):307–324. 10.1016/j.autrev.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 260. Hecker M, Fitzner B, Wendt M, et al. High-density peptide microarray analysis of IgG autoantibody reactivities in serum and cerebrospinal fluid of multiple sclerosis patients. Mol Cell Proteomics. 2016;15(4):1360–1380. 10.1074/mcp.M115.051664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Linington C, Bradl M, Lassmann H, Brunner C, Vass K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol. 1988;130(3):443–454. [PMC free article] [PubMed] [Google Scholar]
- 262. Cui QL, Rone M, Khan D, et al. Oligodendrogliopathy in multiple sclerosis: Relation to low glycolytic metabolic rate of oligodendrocytes (I10.004). Neurology. 2016;86(Suppl. 16):I10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Patani R, Balaratnam M, Vora A, Reynolds R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol Appl Neurobiol. 2007;33(3):277–287. 10.1111/j.1365-2990.2007.00805.x. [DOI] [PubMed] [Google Scholar]
- 264. Lytle JM, Chittajallu R, Wrathall JR, Gallo V. NG2 cell response in the CNP-EGFP mouse after contusive spinal cord injury. Glia. 2009;57(3):270–285. 10.1002/glia.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Dulamea AO. Role of oligodendrocyte dysfunction in demyelination, remyelination and neurodegeneration in multiple sclerosis. Adv Exp Med Biol. 2017;958:91–127. 10.1007/978-3-319-47861-6_7. [DOI] [PubMed] [Google Scholar]
- 266. De La Fuente AG, Lange S, Silva ME, et al. Pericytes stimulate oligodendrocyte progenitor cell differentiation during CNS remyelination. Cell Rep. 2017;20(8):1755–1764. 10.1016/j.celrep.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Mashayekhi F, Salehi Z. Administration of vitamin D3 induces CNPase and myelin oligodendrocyte glycoprotein expression in the cerebral cortex of the murine model of cuprizone-induced demyelination. Folia Neuropathol. 2016;54(3):259–264. 10.5114/fn.2016.62535. [DOI] [PubMed] [Google Scholar]
- 268. Fragoso YD, Adoni T, Damasceno A, et al. Unfavorable outcomes during treatment of multiple sclerosis with high doses of vitamin D. J Neurol Sci. 2014;346(1–2):341–342. 10.1016/j.jns.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 269. Häusler D, Torke S, Peelen E, et al. High dose vitamin D exacerbates central nervous system autoimmunity by raising T-cell excitatory calcium. Brain. 2019;142(9):2737–2755. 10.1093/brain/awz190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Etemadifar M, Janghorbani M. Efficacy of high-dose vitamin D3 supplementation in vitamin D deficient pregnant women with multiple sclerosis: Preliminary findings of a randomized-controlled trial. Iran J Neurol. 2015;14(2):67–73. [PMC free article] [PubMed] [Google Scholar]
- 271. Stein MS, Liu Y, Gray OM, et al. A randomized trial of high-dose vitamin D2 in relapsing-remitting multiple sclerosis. Neurology. 2011;77(17):1611–1618. 10.1212/WNL.0b013e3182343274. [DOI] [PubMed] [Google Scholar]
- 272. Bhargava P, Steele SU, Waubant E, et al. Multiple sclerosis patients have a diminished serologic response to vitamin D supplementation compared to healthy controls. Mult Scler. 2016;22(6):753–760. 10.1177/1352458515600248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. O’Connell K, Sulaimani J, Basdeo SA, et al. Effects of vitamin D3 in clinically isolated syndrome and healthy control participants: A double-blind randomised controlled trial. Mult Scler J Exp Transl Clin. 2017;3(3):205521731772729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Kampman MT, Steffensen LH, Mellgren SI, Jørgensen L. Effect of vitamin D3 supplementation on relapses, disease progression, and measures of function in persons with multiple sclerosis: Exploratory outcomes from a double-blind randomised controlled trial. Mult Scler. 2012;18(8):1144–1151. 10.1177/1352458511434607. [DOI] [PubMed] [Google Scholar]
- 275. Camu W, Lehert P, Pierrot-Deseilligny C, et al. Cholecalciferol in relapsing-remitting MS: A randomized clinical trial (CHOLINE). Neurol Neuroimmunol Neuroinflamm. 2019;6(5):e597. 10.1212/NXI.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Dörr J, Bäcker-Koduah P, Wernecke KD, et al. High-dose vitamin D supplementation in multiple sclerosis – results from the randomized EVIDIMS (efficacy of vitamin D supplementation in multiple sclerosis) trial. Mult Scler J Exp Transl Clin. 2020;6(1):205521732090347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Feige J, Moser T, Bieler L, Schwenker K, Hauer L, Sellner J. Vitamin D supplementation in multiple sclerosis: A critical analysis of potentials and threats. Nutrients. 2020;12(3):783. 10.3390/nu12030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Souberbielle JC, Body JJ, Lappe JM, et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun Rev. 2010;9(11):709–715. 10.1016/j.autrev.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 279. Souberbielle JC, Cormier C, Cavalier E, et al. La supplémentation en vitamine D en France chez les patients ostéoporotiques ou à risque d’ostéoporose : Données récentes et nouvelles pratiques. Revue du Rhumatisme. 2019;86(5):448–452. 10.1016/j.rhum.2019.02.014. [DOI] [Google Scholar]
- 280. Fernández-Zapata C, Leman JKH, Priller J, Böttcher C. The use and limitations of single-cell mass cytometry for studying human microglia function. Brain Pathol. 2020;30(6):1178–1191. 10.1111/bpa.12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Couloume L, Ferrant J, Le Gallou S, et al. Mass cytometry identifies expansion of T-bet+ B cells and CD206+ monocytes in early multiple sclerosis. Front Immunol. 2021;12:653577. 10.3389/fimmu.2021.653577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Johansson D, Rauld C, Roux J, et al. Mass cytometry of CSF identifies an MS-associated B-cell population. Neurol Neuroimmunol Neuroinflamm. 2021;8(2):e943. 10.1212/NXI.0000000000000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Galli E, Hartmann FJ, Schreiner B, et al. GM-CSF and CXCR4 define a T helper cell signature in multiple sclerosis. Nat Med. 2019;25(8):1290–1300. 10.1038/s41591-019-0521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Iparraguirre L, Alberro A, Sepúlveda L, et al. RNA-Seq profiling of leukocytes reveals a sex-dependent global circular RNA upregulation in multiple sclerosis and 6 candidate biomarkers. Hum Mol Genet. 2020;29(20):3361–3372. 10.1093/hmg/ddaa219. [DOI] [PubMed] [Google Scholar]
- 285. Han Z, Xue W, Tao L, Lou Y, Qiu Y, Zhu F. Genome-wide identification and analysis of the eQTL lncRNAs in multiple sclerosis based on RNA-seq data. Brief Bioinform. 2020;21(3):1023–1037. 10.1093/bib/bbz036. [DOI] [PubMed] [Google Scholar]
- 286. Fernandes SJ, Morikawa H, Ewing E, et al. Non-parametric combination analysis of multiple data types enables detection of novel regulatory mechanisms in T cells of multiple sclerosis patients. Sci Rep. 2019;9(1):11996. 10.1038/s41598-019-48493-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Neme A, Nurminen V, Seuter S, Carlberg C. The vitamin D-dependent transcriptome of human monocytes. J Steroid Biochem Mol Biol. 2016;164:180–187. 10.1016/j.jsbmb.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 288. Neme A, Seuter S, Malinen M, et al. In vivo transcriptome changes of human white blood cells in response to vitamin D. J Steroid Biochem Mol Biol. 2019;188:71–76. 10.1016/j.jsbmb.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 289. Wheeler MA, Clark IC, Tjon EC, et al. MAFG-driven astrocytes promote CNS inflammation. Nature. 2020;578(7796):593–599. 10.1038/s41586-020-1999-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Masuda T, Sankowski R, Staszewski O, et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature. 2019;566(7744):388–392. 10.1038/s41586-019-0924-x. [DOI] [PubMed] [Google Scholar]
- 291. Esaulova E, Cantoni C, Shchukina I, et al. Single-cell RNA-seq analysis of human CSF microglia and myeloid cells in neuroinflammation. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e732. 10.1212/NXI.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Ramesh A, Schubert RD, Greenfield AL, et al. A pathogenic and clonally expanded B cell transcriptome in active multiple sclerosis. Proc Natl Acad Sci U S A. 2020;117(37):22932–22943. 10.1073/pnas.2008523117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Stoeckius M, Hafemeister C, Stephenson W, et al. Large-scale simultaneous measurement of epitopes and transcriptomes in single cells. Nat Methods. 2017;14(9):865–868. 10.1038/nmeth.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Xu G, Liu Y, Li H, Liu L, Zhang S, Zhang Z. Dissecting the human immune system with single cell RNA sequencing technology. J Leukoc Biol. 2020;107(4):613–623. 10.1002/JLB.5MR1019-179R. [DOI] [PubMed] [Google Scholar]
- 295. Peterson VM, Zhang KX, Kumar N, et al. Multiplexed quantification of proteins and transcripts in single cells. Nat Biotechnol. 2017;35(10):936–939. 10.1038/nbt.3973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.