Abstract
Background and Aim:
Resection for giant hepatocellular carcinoma (HCC) (≥10 cm) is deemed safe and feasible. However, a super-giant HCC (≥15 cm) poses unique technical complexity for hepatectomy with limited data suggesting feasibility and oncologic efficiency. This study aims to evaluate the short-term and long-term outcomes of hepatectomy in patients with super-giant HCC.
Methods:
A retrospective review was conducted on patients with super-giant HCC who underwent hepatectomy from 2011 to 2021. We report perioperative and oncologic outcomes such as length of stay (LOS), 30-day readmission, 90-day mortality, and cumulative survival rate.
Results:
Of the 18 patients, the median tumor diameter was 172.5 mm (range 150–250). The most common risk factor was chronic hepatitis B virus (HBV) infection (n=7, 38.9%). Most of the patients were Barcelona Clinic Liver Cancer (BCLC) Stage B (n=14, 77.8%) and Hong Kong Liver Cancer (HKLC) Stage IIb (n=15, 83.3%). Extended right hepatectomy was the most common procedure. The median LOS was 11 days (range 3–90). The most common post-operative complication was pneumonia (n=4, 22.2%). Fourteen patients were discharged well without any need for invasive therapy (n=7, 38.9% no complications, n=1, 5.6% Clavien Grade I, n=6, 33.3% Clavien Grade II). Thirty-day readmission rate was 5.6% (n=1) and 90-day mortality rate was 5.6% (n=1). There were 12 patients (66.7%) with microvascular invasion and three patients (16.7%) with macrovascular invasion. Most patients had Grade III (poorly differentiated) HCC (n=9, 50%). At a median follow-up of 11 months (range 2–95), 12 (66.7%) patients had local recurrence, and 9 (50%) developed distant metastasis. The 1-, 2-, and 3-year cumulative disease-free survival (DFS) was 36%, 18%, and 18%, respectively. The 1-, 2-, and 3-year cumulative overall survival was 49% and 39%, and 29%, respectively.
Conclusion:
Primary hepatic resection is safe in patients with super-giant HCC. However, long-term outcomes are poor, and high tumor volume may be associated with inferior oncological outcomes in HCC.
Relevance for Patients:
The presentation of super-giant HCCs may be asymptomatic and some patients are diagnosed late with limited treatment options. In some centers, this group of patients are denied surgical resection and recommended for only locoregional therapies like TACE. This paper demonstrates that hepatic resection is safe and may be an option in patients who present at an advanced stage with a high tumor burden.
Keywords: hepatectomy, hepatocellular carcinoma, large, outcomes, super-giant, survival
1. Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer death globally [1]. The median overall survival (OS) of HCC patients is approximately 6–20 months [2]. Despite effective surveillance programs, many patients continue to be diagnosed at an advanced stage with a high tumor burden. The management of HCC patients is guided according to the stage of the disease. In patients with intermediate stage and beyond, the BCLC staging system advocates locoregional therapeutic options like TACE or systemic therapy instead of surgery [3-5]. However, many Asian centers have reported acceptable survival outcomes with surgical resection, so long as patients have good functional status, sufficient future liver remnant (FLR), and preserved liver function [6-9]. Part of the differences in recommendation is related to the etiology of HCC, with HBV predominant in Asia. This is reflected in a retrospective study by Selby et al. [10] on 766 HCC patients with predominant HBV etiology. Survival outcomes improved when patients with BCLC Stage C HCC were managed according to recommendations based on the HKLC system. Thus, the management of HCC patients should consider patient-related, disease-related, and treatment-related factors and be discussed in a multidisciplinary team. Strict adherence to clinical practice guidelines (CPG) and staging system-based recommendations may compromise outcomes [11] and should be avoided. Moreover, these guidelines are not without limitations and should always be guiding but not binding.
Tumor size is often an essential determinant of most malignancies’ staging, prognostication, and management options. However, in the context of HCC, a myriad of other factors are considered, and size alone plays a less significant role. A study by Chen et al. [11] evaluated recent internationally published CPGs on the management of HCC, and none of the 22 CPGs included tumor size in the treatment decisions. Hence, many surgeons continue to offer liver resections despite a large tumor size. A larger tumor poses many technical challenges for a surgeon, including their propensity to cause anatomical distortions, the increased proximity to hepatic hilum limiting safe dissection, and more difficulty in mobilization and manipulation, causing a high risk of rupture and tumor seeding [12]. Recent studies have shown promising outcomes after hepatic resection of giant HCC (≥10 cm) with 5-year survival rates exceeding 25% [13] and no significant difference in OS compared to patients with smaller HCC [14].
However, most of these reports included patients with a median tumor diameter of <15 cm. A super-giant HCC (≥15 cm) poses even more technical complexity as large tumors are associated with a higher risk of vascular invasion [15], increased intraoperative blood loss, more difficulty obtaining clear surgical margins [16], and increased risk of insufficient FLR causing post-hepatectomy liver failure (PHLF).
The volume of a tumor is proportional to size. Applying the mathematical formula for the volume of a sphere (4/3 π r3), a tumor with a 5 cm, 10 cm, and 15 cm size has a volume of 65.5 ml, 524 ml, and 1767 ml, respectively. Thus, the 15 cm HCC is about 3 times the volume of 10cm HCC. In solid organ malignancies such as breast cancer, prostate cancer, and nasopharyngeal cancer, high tumor volume (Tv) is associated with a worse prognosis [17-19]. For HCC, it is likely that though the size is not included in the staging criteria, the volume burden of the tumor could be associated with the abovementioned risks and predict inferior outcomes. There are currently limited data to suggest the feasibility, safety, and oncologic efficiency of hepatectomy of super-giant HCC. Therefore, this study aims to evaluate short-term and long-term outcomes of hepatectomy in patients with super-giant HCC in our institution.
2. Materials and Methods
2.1. Patient selection
A retrospective review was conducted on patients who underwent hepatic resection of super-giant HCC from January 2011 to February 2020 in Tan Tock Seng Hospital, Singapore. A lesion ≥5 cm in diameter is defined as a large lesion, and a lesion ≥10 cm in diameter is defined as a giant lesion [12]. This study defines super-giant HCC as histologically proven HCC ≥15 cm in diameter.
2.2. Pre-operative assessment
Patients were diagnosed using cross-sectional imaging with triphasic liver protocol computed tomography (CT) or magnetic resonance imaging scan. Radiological parameters including size and location of the tumor, number of lesions, presence of satellite nodules, vascular invasion, tumor thrombus, liver echotexture, and signs of portal hypertension were collected.
All patients were discussed at a multidisciplinary tumor board. Liver resection was offered as curative therapy after evaluating the patient’s performance status and ensuring adequate FLR. The performance status was assessed using the Eastern Cooperative Oncology Group score and existing comorbidities were screened. The hepatic reserve was determined using Child’s Pugh scoring, Indocyanine Green (ICG) clearance, and CT Volumetry. An ICG retention ≥15% at 15 min was considered a contraindication to resection of super-giant HCC. Routine pre-operative blood tests, chest X-ray, and electrocardiogram were performed. In addition, alpha-fetoprotein (AFP) levels and hepatitis serologies were evaluated. Once listed for surgery, patients were reviewed at the preadmission evaluation and counseling clinic by the anesthesia team, dieticians, physiotherapists, and case managers [20].
2.3. Surgical technique
The surgical technique of our unit is previously reported [21]. All patients received surgical infection prophylaxis and a low central venous pressure anesthesia. A reverse “L” incision was typically made. Liver mobilization with a portal sling was routinely performed. Blood loss was controlled intraoperatively with Pringle’s maneuver whenever necessary. Intraoperative ultrasound was used to confirm the location of lesions, evaluate for additional tumors, and delineate resection margins. The extent of hepatectomy was classified according to the number of anatomical segments resected. Parenchymal transection was achieved using energy devices such as Cavitron Ultrasonic Surgical Aspirator (CUSA) (SonoSurg, Olympus, Tokyo, Japan), Harmonic™ (Johnson and Johnson Medical Devices Companies, USA), SonoSurg™ (Olympus, Tokyo, Japan), and LigaSure™ (Covidien, Minneapolis, USA) with dolphin tip. Tubular structures of >3 mm were ligated or clipped, and major pedicles were stapled with a vascular stapling device. Drains were placed before abdominal closure. Intraoperative details regarding the length of operation, blood loss, and number of pints of blood transfusion were recorded.
2.4. Follow-up and analysis
The number of tumors, liver cirrhosis, vascular or perineural invasion, and surgical margins was evaluated on histological assessment. Surgical margins were considered positive if viable tumor cells were seen within 1 mm on microscopy. According to the HCC pathologic grading system issued in 2010 by the World Health Organization, the tumor was graded to classify digestive system tumors. The different grades include Grades I (well-differentiated), II (moderately differentiated), III (poorly differentiated), and IV (Undifferentiated).
Postoperatively, the patients were monitored in the high dependency unit (HDU) for a minimum of 24 h, or the intensive care unit, depending on intraoperative progress and comorbidities. All patients were managed under a standardized liver resection care pathway. They were started on incentive spirometry with physiotherapy and enteral feeding as early as possible. Surgical drain outputs were charted strictly and drains were removed when the output was <50 ml/24 h or at the surgeon’s discretion. The length of stay (LOS), post-operative complications, readmission within 30 days, and 90-day mortality were recorded.
Patients were typically followed up with 3-monthly CT scans and AFP for surveillance and laboratory testing for liver function (liver panel and coagulation panel) for the first 2 years. Subsequently, the interval can be increased to 6-monthly and annually. Recurrence was defined as new or growing liver lesions on imaging with characteristic appearances of HCC. The presence of metastasis was also evaluated on CT scans. Multidisciplinary teams confirmed the diagnosis of recurrent or metastatic disease. The date of disease relapse (recurrence or metastasis) and date of death were recorded and disease-free interval and length of survival were measured. The duration of follow-up was recorded and the last day of follow-up for the surviving patients in the study was taken to be 1st December 2021.
Survival data were analyzed using Kaplan-Meier Curve and were compared with life tables. P<0.05 was considered as significant for the statistical association.
3. Results
3.1. Patient and tumor characteristics
Of the 18 patients, the median age was 65.5 years (range 55–77), and 17 patients (94.4%) were male. Half of the patients (n=9, 50%) had no risk factor, while the most common risk factor was chronic HBV infection (n=7, 38.9%). The demographics and clinical profile of the patients are shown in Table 1.
Table 1. Demographics and clinical profile of patients with super-giant HCC.
| Median age, years (range) | 65.5 (55–77) |
| Gender, n (%) | |
| Male | 17 (94.4) |
| Female | 1 (5.6) |
| Risk factors, n (%) | |
| HBV | 7 (38.9) |
| Alcohol and HBV | 2 (11.1) |
| No risk factors | 9 (50.0) |
| Comorbidities, n (%) | |
| Hypertension | 9 (50.0) |
| Diabetes mellitus | 8 (44.4) |
| Hyperlipidemia | 7 (38.9) |
| Stroke | 2 (11.1) |
| Ischemic heart disease | 4 (22.2) |
| Chronic renal disease | 3 (16.7) |
| Pulmonary disease | 2 (11.1) |
| Child-Pugh score, n (%) | |
| A | 17 (94.4) |
| B | 1 (5.6) |
| C | 0 |
| BCLC stage, n (%) | |
| A | 0 |
| B | 14 (77.8) |
| C | 4 (22.2) |
| HKLC stage, n (%) | |
| I, IIa | 0 |
| IIb | 15 (83.3) |
| IIIa | 1 (5.6) |
| IIIb | 2 (11.1) |
| Iva, IVb | 0 |
| Va, Vb | 0 |
| Pre-operative treatment, n (%) | |
| TACE | 1 (5.6)* |
| None | 17 (94.4) |
This patient also received portal vein embolization. HBV, hepatitis B virus; BCLC, Barcelona Clinic for Liver Cancer; HKLC, Hong Kong liver cancer; TACE, transarterial chemoembolization.
When staged according to the BCLC and HKLC staging systems, the most of the patients were BCLC Stage B (n=14, 77.8%) and HKLC Stage IIb (n=15, 83.3%). Four patients (22.2%) were BCLC Stage C. One patient (5.6%) was HKLC Stage IIIa and two patients were HKLC Stage IIIb (11.1%). In addition, one patient underwent pre-operative TACE and portal vein embolization in the same setting. Extended right hepatectomy was the most commonly performed procedure (n=9, 11.1%). The median operative time was 447 min (range 264–760). Median blood loss was 1200 ml (500–11,000), with one patient experiencing 11 L of blood loss due to iatrogenic intraoperative middle hepatic vein injury. The intraoperative data are shown in Table 2.
Table 2. Intraoperative data.
| Median operative time, min (range) | 447 (264–760) |
| Median blood loss, ml (range) | 1200 (500–11000) |
| Median units of blood transfusions (range) | 2 (1–14) |
| Type of liver resection, n (%) | |
| Right hepatectomy | 4 (22.2) |
| Extended right hepatectomy | 9 (50.0) |
| Left hepatectomy | 1 (5.6) |
| Left lateral sectionectomy | 2 (11.1) |
| Extended left hepatectomy | 2 (11.1) |
3.2. Post-operative outcomes
Postoperatively, the median LOS was 11 days (range 3–90), with 4 days (range 0–20) in an HDU. Post-operative complications were classified according to the Clavien-Dindo classification. As a result, 14 patients were discharged well without any need for invasive therapy (n=7, 38.9% no complications, n=1, 5.6% Clavien Grade I, n=6, 33.3% Clavien Grade II). The post-operative outcomes and histopathological data of patients are highlighted in Table 3.
Table 3. Post-operativeTable outcomes and histopathological data.
| Post-operative outcomes | |
| Post-operative length of stay, days (range) | |
| Median length of stay in hospital | 11 (7-45) |
| Median length of stay in HDU | 4 (1-30) |
| Median length of stay in ICU | 0 (0-20) |
| Post-operative complications, liver-specific, n (%) | |
| Post hepatectomy liver failure | 1 (5.6) |
| Bile leak | 1 (5.6) |
| Liver abscess | 0 |
| Bleeding | 0 |
| Post-operative complications, general, n (%) | |
| Pneumonia | 4 (22.2) |
| Myocardial infarction | 3 (16.7) |
| Ileus | 3 (16.7) |
| Intra-abdominal abscess | 2 (11.1) |
| Urinary tract infection | 1 (5.6) |
| Stroke | 0 |
| Acute kidney injury | 2 (11.1) |
| Deep vein thrombosis | 0 |
| Pulmonary embolism | 0 |
| Superficial surgical site infection | 0 |
| Others^ | 7 (38.8) |
| Clavien Dindo classification, n (%) | |
| Grade I | 1 (5.6) |
| Grade II | 6 (33.3) |
| Grade IIIa | 2 (11.1) |
| Grade IIIb | 0 |
| Grade IVa | 0 |
| Grade IVb | 1 (5.6) |
| Grade V | 1 (5.6) |
| Readmission within 30 days | 1 (5.6) |
| 30-day mortality, n (%) | 0 |
| 90-day mortality, n (%) | 1 (5.6) |
| Histopathological data | |
| Vascular invasion, n (%) | |
| Yes | |
| Microvascular invasion | 12 (66.7) |
| Macrovascular invasion | 3 (16.7) |
| No | 3 (16.7) |
| Perineural invasion, n (%) | |
| No | 18 (100) |
| Presence of tumor capsule, n (%) | |
| Yes | 13 (72.2) |
| No | 5 (27.8) |
| Presence of tumor rupture, n (%) | |
| Yes | 2 (11.1) |
| No | 16 (88.9) |
| Presence of multiple tumors, n (%) | |
| Yes | |
| ≤3 tumors | 1 (5.6) |
| >3 tumors | 4 (22.2) |
| No | 13 (72.2) |
| Presence of liver cirrhosis, n (%) | |
| Yes | 1 (5.6) |
| No | 17 (94.4) |
| Tumor differentiation | |
| Grade I (Well-differentiated) | 0 |
| Grade II (Moderately-differentiated) | 8 (44.4) |
| Grade III (Poorly-differentiated) | 9 (50.0) |
| Grade IV (Undifferentiated, Anaplastic) | 1 (5.6) |
^ Other complications include anemia requiring blood transfusion with no active bleeding source bacteremia with no apparent source, scrotal swelling from hypoalbuminemia, polyarticular gout flare, ex-drain site bleeding, vocal cord palsy with right arytenoid granuloma, and post-operative delirium. HDU, high dependency unit; ICU, intensive care unit.
The most common post-operative complication was pneumonia (n=4, 22.2%), with two patients developing a complication of parapneumonic effusion requiring radiologic guided pigtail catheter pleural drainage. Three patients (16.7%) developed myocardial infarction, heart failure, and acute pulmonary edema. Two patients (11.1%) developed intra-abdominal abscess requiring radiologically guided drainage and a prolonged course of intravenous antibiotics. Finally, two patients (11.1%) developed acute kidney injury.
Regarding liver-specific complications, one patient (5.6%) developed a bile leak which resolved spontaneously. One patient (5.6%) developed PHLF and was demised on post-operative day 70. There was no patient with an unplanned return to theatre within 30 days. Thirty-day readmission rate was 5.6% (n=1) and 30-day mortality rate was 0%. Ninety-day mortality rate was 5.6% (n=1).
The median tumor diameter was 172.5 mm (range 150–250) on histology. There were 12 patients (66.7%) with microvascular invasion and three patients (16.7%) with macrovascular invasion. None of the patients had a perineural invasion. Five patients (27.8%) had multifocal tumors, with four (22.2%) having more than three lesions. Two of the patients (11.1%) had tumor rupture. Only one (5.6%) patient had the presence of liver cirrhosis noted on histology. Most patients had Grade III (poorly differentiated) HCC (n=9, 50%), 8 patients (44.4%) with Grade II (moderately differentiated) HCC, and 1 (5.6%) with Grade 4 (undifferentiated) HCC. Histology of all tumors revealed HCC and an additional sarcomatoid component in one patient.
3.3. Disease and survival outcomes
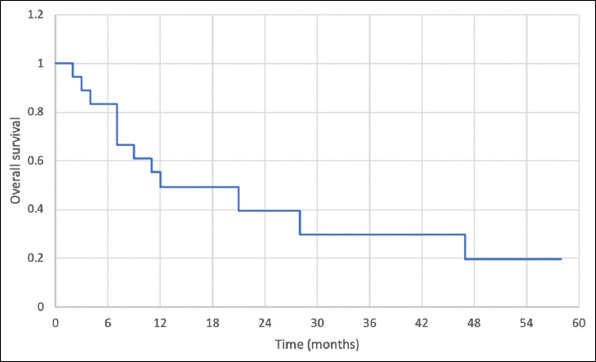
The survival data are shown in Figures 1 and 2. At a median follow-up of 11 months (range 2–95), 12 (66.7%) patients died. Twelve (66.7%) patients had local recurrence and 9 (50%) developed distant metastasis. The median DFS was 5 months and the median OS was 12 months. The 1-, 2-, and 3-year cumulative DFS was 36%, 18%, and 18%, respectively. The 1-, 2-, and 3-year cumulative OS was 49%, 39%, and 29%, respectively.
Figure 1. Disease-free survival in patients who underwent hepatic resection of super-giant hepatocellular carcinoma.
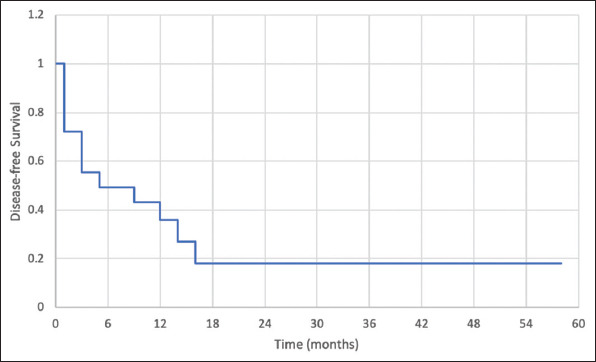
Figure 2. Overall survival in patients who underwent hepatic resection of super-giant hepatocellular carcinoma.
4. Discussion
There are no agreed criteria to define the terminology of liver tumor size. Various authors have reported “large” and “giant” to suggest a cutoff of 5 cm and 10 cm [14]. We frequently observe large tumors in our clinical practice, with a local study by Lei et al. [22] on 244 patients with hepatic resection reporting a median tumor diameter of 56 mm (range, 1–200 mm). Thus, we coined the term super-giant HCC for this study. There is a paucity of hepatic resection outcomes for super-giant HCCs (≥15 cm), with only one report of surgical resection of HCC more than 15 cm size [23]. This study has shown that hepatic resection of selected patients with super-giant HCC is safe but associated with poor survival, with a median OS of 12-month and a 3-year cumulative OS of 29%. These results are inferior to standard reports of survival outcomes for hepatic resection in large or giant tumors (>10 cm) and warrant further discussion.
Patients with super-giant HCC do not qualify for liver transplantation, and thus resection remains the primary curative treatment option. However, surgical resection of a giant or super giant HCC is associated with a high risk of recurrence as large size implies aggressive tumor biology with a higher incidence of intrahepatic metastasis and portal venous invasion [15,24,25]. In a multicenter study including 300 HCC patients with HCC ≥10 cm, Pawlik et al. [13] reported 5% mortality and actuarial 5-year survival of 27%. Chen et al. [26] also reported one of the largest single-center experiences of hepatic resection for HCC ≥10 cm and demonstrated 30-day mortality of 2.2% and a 5-year OS rate of 18.2%. In a comparative study by Zhou et al. [27] including 1227 HCC patients with HCC >10 cm and 2349 patients with HCC <10 cm, the authors concluded that patients with HCC >10 cm were younger, more symptomatic, with a poorer tumor differentiation, a higher operative mortality rate (4.5% vs. 2.3%, P<0.001), and a much lower 5-year OS rate (26.2% (n=621) vs 54.3% (n=2039), P<0.01). The only study on super-giant HCCs (>15 cm) was by Huang et al. [23]. They concluded that outcomes of patients with >15 cm HCC were worse when compared to operated patients within the European Association for Study of Liver, 2001 and American Association for Study of Liver Diseases, 2005 criteria. The perioperative morbidity, mortality, and 3-year OS were 30.2%, 3.6%, and 23%, respectively. The most common cause of death was HCC recurrence (72%). Our study reported perioperative mortality of 5.6% and 3-year OS of 29%, which is comparable. Current staging systems, such as the HKLC [28], do not specify an upper limit for the size as a contraindication for surgical resection. However, it is known that as the tumor size increases, the risks of local and distant recurrence increase as well [15,27,28]. Therefore, it is only reasonable to expect that the survival outcomes will gradually become poorer as surgeons push the limits of resection.
Another concept to explain the difference between 10 cm and 15 cm HCC is total Tv – which has both technical and oncological implications. In our study, we did not compute the total Tv based on exact dimensions; but if we assume that HCC is spherical, applying the sphere volume formula (v=4/3 πr3), it is evident that 15cm HCC will have about 3 times more Tv compared to 10 cm HCC. From a technical standpoint, a super-giant HCC poses unique challenges: Lack of working space, increased risk of tumor cell seeding or spillage from surgical manipulation, larger parenchymal transection surface, and higher risk of bleeding. From an oncological standpoint, some studies have shown that total Tv is a more reliable prognostic marker in HCCs compared to size alone in both liver transplant and surgical resection [29,30]. In patients with nasopharyngeal carcinoma [17], Tv increased with the disease stage and predicted all survival endpoints. In breast cancer patients, the effect of Tv is well reported. Hwang et al. [19] have reported a comprehensive method of calculating Tv in a study including 8996 breast cancer patients. They defined tumor size as the maximum tumor dimension and Tv was calculated by the equation of (4π×r1×r2×r3)/3; r1, r2, and r3 were defined as half of the largest, intermediate, and shortest dimension of the tumor, respectively. The authors concluded that Tv was a significant prognostic factor and superior to T stage alone.
More than half of the patients in our cohort suffered early local or distant recurrence within 6 months. The prognosis for local and distant recurrence patients is poor, with a median DFS of 4.9–7 months. Moreover, the burden of intrahepatic metastasis in a small remnant liver often precludes patients from locoregional therapies, and there is a lack of effective systemic treatment [31-33]. At present, apart from markers of tumor biology, for example, multifocality, vascular invasion, and AFP levels, we have no reliable way of predicting those who would develop metastasis. Some biomarkers such as AFP-L3, des-γ-carboxy prothrombin, and tumor-associated signatures such as micro-RNAs have been proposed to be effective for monitoring recurrence [34]. However, most are still not used in the clinical setting due to insufficient validity and reliability. More research is needed to develop and validate effective biomarkers in HCCs to predict those likely to develop early distant metastasis in which surgery is deemed futile.
As hepatic resection for super-giant HCC has inferior OS outcomes, it is important to determine if locoregional therapeutic options should be a preferred recommendation for patients. Alternatives to surgical resection include locoregional therapies such as TACE or trans-arterial radioembolization, which are palliative options. These locoregional therapies often prove ineffective [35-37]. Even if they may control the progression of the disease, it is unlikely that these therapies will be able to palliate the pain and discomfort associated with tumor size. Furthermore, some centers deem HCCs ≥10 cm as a relative contraindication for locoregional therapies, and many patients are not even presented with this option. However, for patients who were offered locoregional therapies, Cui et al. [35] reported a median OS of only 8.0 months (TACE-cryoablation) and 5.0 months (TACE alone) for HCCs ≥15 cm. This is considerably lower than our study’s median OS of 12.0 months after surgery. The risks of major complications following TACE, such as hepatic abscess, hepatic failure, and tumor rupture, were also reported to be higher as tumor size increases [38]. Thus, in the absence of effective alternative therapies, surgeons must guide patients to choose in their best interests, and frequently a shared decision for hepatic resection is made. Therefore, in this group of patients with super-giant HCC, although long-term survival outcomes from surgery are less than ideal, surgery still offers better long-term outcomes and should still be an option to discuss with patients.
Resection of super-giant HCCs is technically challenging and major hepatectomy is usually needed. Our study shows that hepatectomy can be performed safely with acceptable short-term perioperative outcomes. Measures to ensure safe hepatectomy include careful patient selection, low central venous pressure anesthesia, meticulous surgical techniques, and the use of surgical technology [39]. Despite widespread adoption of laparoscopy and expanding indications, minimal access surgery is not reported in super-giant HCC. This is mainly due to a lack of operating space within the abdominal cavity. Operative field exposure is paramount for safe surgical conduct, and this is best achieved with a generous reverse L incision. If necessary, a Mercedes Benz extension of the incision can be performed. The use of self-retaining surgical retractor systems, for example, Omni-Tract® (Integra Lifesciences, Princeton, USA), Kent retractor® (Takasago Medical Industry Company Limited, Tokyo, Japan), and Thompson retractor® (Surgi-One Medical Technologies Incorporated, Ontario, Canada), helps maintain good exposure to the operative field. It is advisable to obtain exposure for possible control of suprahepatic and infrahepatic vena cava in case of massive hemorrhage during surgery. An anterior approach should be adopted to avoid mobilization and rupture of large tumors. Surgical instruments such as (CUSA® - Integra Lifesciences, Princeton, USA) should be used to effectively dissect the tumor off major veins or hepatic ducts and obtain a clear margin. Continuous close communication with anesthetists is also crucial, for example, ensuring a low central venous pressure during parenchymal transection to reduce blood loss.
The importance of careful patient selection is highlighted in our case series. In our study, we have one patient with PHLF. This patient had a large tumor (17 cm) in the right hepatic lobe and a few satellite nodules (>3) on the same side. There was also a tumor thrombus in the right main portal vein. Although his pre-operative ICG clearance was acceptable at 13.5% and he was Child’s Pugh Class A, his liver was noted to be cirrhotic intraoperatively. He underwent right hepatectomy and developed post-operative intra-abdominal sepsis with PHLF and was demised on a post-operative day 70. At the time of his demise, he was noted to have metastasis at the resection margin and lungs. In retrospect, due to the large tumor burden associated with poor prognostic features like multiple satellite nodules and portal vein invasion, locoregional therapies or systemic therapies should have been recommended to this patient.
There are no data that post-operative adjuvant systemic chemotherapy improves survival outcomes in patients following curative resection of HCC [40-44]. However, some of the newer immunotherapeutic agents such as cytokine-induced killer cells and NK cells have shown promising results in the treatment of advanced HCC [45,46]. It has been shown recently that HCC and HCC-associated Kupffer cells exhibit upregulation of programmed death receptor-1 (PD-1) and programmed death-ligand 1 in the tumor infiltrating lymphocytes [47-49], which have generated new interest in using these checkpoint inhibitors as adjuvant therapy following resection. The CheckMate 9DX trial evaluating the use of adjuvant Nivolumab (PD-1 inhibitor) in patients with HCC who are at high risk of recurrence after curative resection or ablation is currently underway and the results are anticipated [50]. Based on our results, super-giant HCC patients should be considered for these clinical trials.
The results of our study should be considered with its limitations, mainly related to its small sample size and short median follow-up. Moreover, data were only obtained from a single center, limiting results to other centers or populations. A comparative group of patients with super-giant HCCs who underwent non-surgical treatment will also be helpful to compare the survival between both groups.
5. Conclusion
This study shows that primary hepatic resection can be performed safely in patients with super-giant HCCs with acceptable short-term survival outcomes. Long-term outcomes are poor but considerably better compared to locoregional therapy. Early recurrence remains a significant problem and further studies are necessary to study the role of Tv in the prognostication of HCC.
Acknowledgments
This paper and the research behind it would not have been possible without the tremendous support of the general surgery department at Tan Tock Seng General Hospital for their support for this paper and sharing their invaluable input toward this subject. Their enthusiasm in guiding, vast knowledge in this field, and attentional to detail have been an exceptional help from the initial dating extraction to the final draft of this paper.
I also thank the rest of the peer reviewers at Hepatobiliary and Pancreatic Diseases International. The expertise has improved this study in innumerable ways and elevated the quality of this paper with their valuable feedback.
Conflict of Interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. I declare no conflict of interest.
Ethical Approval
This study was approved by the Ethics Committee of Tan Tock Seng Hospital, Written informed consent was obtained from all participants.
References
- [1].Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020:GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- [2].Cancer of the Liver Italian Program (CLIP) Investigators. A New Prognostic System for Hepatocellular Carcinoma:A Retrospective Study of 435 Patients. Hepatology. 1998;28:751–5. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- [3].Llovet JM, Brú C, Bruix J. Prognosis of Hepatocellular Carcinoma:The BCLC Staging Classification. Semin Liver Dis. 1999;19:329–38. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- [4].Bruix J, Sherman M. Management of Hepatocellular Carcinoma:An Update. Hepatology. 2011;53:1020. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical Management of Hepatocellular Carcinoma. Conclusions of the Barcelona-2000 EASL Conference. J Hepatol. 2001;35:421–30. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- [6].Ng KK, Vauthey JN, Pawlik TM, Lauwers GY, Regimbeau JM, Belghiti J, et al. Is Hepatic Resection for Large or Multinodular Hepatocellular Carcinoma Justified?Results from a Multi-institutional Database. Ann Surg Oncol. 2005;12:364–73. doi: 10.1245/ASO.2005.06.004. [DOI] [PubMed] [Google Scholar]
- [7].Yang LY, Fang F, Ou DP, Wu W, Zeng ZJ, Wu F. Solitary Large Hepatocellular Carcinoma:A Specific Subtype of Hepatocellular Carcinoma with Good Outcome after Hepatic Resection. Ann Surg. 2009;249:118–23. doi: 10.1097/SLA.0b013e3181904988. [DOI] [PubMed] [Google Scholar]
- [8].Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, et al. A Snapshot of the Effective Indications and Results of Surgery for Hepatocellular Carcinoma in Tertiary Referral Centers:Is it Adherent to the EASL/AASLD Recommendations?An Observational Study of the HCC East-West Study Group. Ann Surg. 2013;257:929–37. doi: 10.1097/SLA.0b013e31828329b8. [DOI] [PubMed] [Google Scholar]
- [9].Furuta T, Sonoda T, Matsumata T, Kanematsu T, Sugimachi AK. Hepatic Resection for a Hepatocellular Carcinoma Larger than 10 cm. J Surg Oncol. 1992;51:114–7. doi: 10.1002/jso.2930510210. [DOI] [PubMed] [Google Scholar]
- [10].Selby LK, Tay RX, Woon WW, Low JK, Bei W, Shelat VG, et al. Validity of the Barcelona Clinic Liver Cancer and Hong Kong Liver Cancer Staging Systems for Hepatocellular Carcinoma in Singapore. J Hepatobiliary Pancreat Sci. 2017;24:143–52. doi: 10.1002/jhbp.423. [DOI] [PubMed] [Google Scholar]
- [11].Dai T, Deng M, Ye L, Lin G, Liu R, Deng Y, et al. Nomograms Based on Clinicopathological Factors and Inflammatory Indicators for Prediction of Early and Late Recurrence of Hepatocellular Carcinoma after Surgical Resection for Patients with Chronic Hepatitis B. Ann Transl Med. 2021;9:12. doi: 10.21037/atm-20-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shelat VG, Cipriani F, Basseres T, Armstrong TH, Takhar AS, Pearce NW, et al. Pure Laparoscopic Liver Resection for Large Malignant Tumors:Does Size Matter? Ann Surg Oncol. 2015;22:1288–93. doi: 10.1245/s10434-014-4107-6. [DOI] [PubMed] [Google Scholar]
- [13].Pawlik TM, Poon RT, Abdalla EK, Zorzi D, Ikai I, Curley SA, et al. Critical Appraisal of the Clinical and Pathologic Predictors of Survival after Resection of Large Hepatocellular Carcinoma. Arch Surg. 2005;140:450–8. doi: 10.1001/archsurg.140.5.450. [DOI] [PubMed] [Google Scholar]
- [14].Thng Y, Tan JK, Shridhar IG, Chang SK, Madhavan K, Kow AW. Outcomes of Resection of Giant Hepatocellular Carcinoma in a Tertiary Institution:Does Size Matter? HPB. 2015;17:988–93. doi: 10.1111/hpb.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wu G, Wu J, Wang B, Zhu X, Shi X, Ding Y. Importance of Tumor Size at Diagnosis as a Prognostic Factor for Hepatocellular Carcinoma Survival:A Population-based Study. Cancer Manage Res. 2018;10:4401. doi: 10.2147/CMAR.S177663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen JH, Wei CK, Lee CH, Chang CM, Hsu TW, Yin WY. The Safety and Adequacy of Resection on Hepatocellular Carcinoma Larger than 10 cm:A Retrospective Study Over 10 Years. Ann Med Surg. 2015;4:193–9. doi: 10.1016/j.amsu.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liang SB, Teng JJ, Hu XF, Yang XL, Luo M, Fang XN, et al. Prognostic Value of Total Tumor Volume in Patients with Nasopharyngeal Carcinoma Treated with Intensity-modulated Radiotherapy. BMC Cancer. 2017;17:1. doi: 10.1186/s12885-017-3480-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chung BI, Tarin TV, Ferrari M, Brooks JD. Comparison of Prostate Cancer Tumor Volume and Percent Cancer in Prediction of Biochemical Recurrence and Cancer Specific Survival. Urol Oncol. 2011;29:314–8. doi: 10.1016/j.urolonc.2009.06.017. [DOI] [PubMed] [Google Scholar]
- [19].Hwang KT, Han W, Lee SM, Choi J, Kim J, Rhu J, et al. Prognostic Influence of 3-dimensional Tumor Volume on Breast Cancer Compared to Conventional 1-dimensional Tumor Size. Ann Surg Treat Res. 2018;95:183–91. doi: 10.4174/astr.2018.95.4.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chan KS, Wang B, Tan YP, Chow JJ, Ong EL, Junnarkar SP, et al. Sustaining a Multidisciplinary Single-institution, Postoperative Mobilization Clinical Practice Improvement Program Following Hepatopancreatobiliary Surgery during the COVID-19 Pandemic:Prospective Cohort Study. JMIR Perioperat Med. 2021;4:e30473. doi: 10.2196/30473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kabir T, Ye M, Noor NA, Woon W, Junnarkar SP, Shelat VG. Preoperative Neutrophil-to-lymphocyte Ratio Plus Platelet-to-lymphocyte Ratio Predicts the Outcomes after Curative Resection for Hepatocellular Carcinoma. Int J Hepatol. 2019;2019:4239463. doi: 10.1155/2019/4239463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lei GY, Shen L, Junnarkar SP, Huey CT, Low J, Shelat VG. Predictors of 90-Day Mortality Following Hepatic Resection for Hepatocellular Carcinoma. Visc Med. 2021;37:102–9. doi: 10.1159/000510811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Huang J, Hernandez-Alejandro R, Croome KP, Zeng Y, Wu H, Chen Z. Hepatic Resection for Huge (>15 cm) Multinodular HCC with Macrovascular Invasion. J Surg Res. 2012;178:743–50. doi: 10.1016/j.jss.2012.04.058. [DOI] [PubMed] [Google Scholar]
- [24].Shah SA, Greig PD, Gallinger S, Cattral MS, Dixon E, Kim RD, et al. Factors Associated with Early Recurrence after Resection for Hepatocellular Carcinoma and Outcomes. J Am Coll Surg. 2006;202:275–83. doi: 10.1016/j.jamcollsurg.2005.10.005. [DOI] [PubMed] [Google Scholar]
- [25].Germani G, Gurusamy K, Garcovich M, Toso C, Fede G, Hemming A, et al. Which Matters Most:Number of Tumors, Size of the Largest Tumor, or Total Tumor Volume. Liver Transpl. 2011;17:S58–66. doi: 10.1002/lt.22336. [DOI] [PubMed] [Google Scholar]
- [26].Chen XP, Qiu FZ, Wu ZD, Zhang BX. Hepatectomy for Huge Hepatocellular Carcinoma in 634 Cases. World J Gastroenterol. 2006;12:4652. doi: 10.3748/wjg.v12.i29.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhou XD, Tang ZY, Ma ZC, Wu ZQ, Fan J, Qin LX, et al. Surgery for Large Primary Liver Cancer more than 10 cm in Diameter. J Cancer Res Clin Oncol. 2003;129:543–8. doi: 10.1007/s00432-003-0446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer Staging System with Treatment Stratification for Patients with Hepatocellular Carcinoma. Gastroenterology. 2014;146:1691–700. doi: 10.1053/j.gastro.2014.02.032. [DOI] [PubMed] [Google Scholar]
- [29].Toso C, Meeberg G, Hernandez-Alejandro R, Dufour JF, Marotta P, Majno P, et al. Total Tumor Volume and Alpha-fetoprotein for Selection of Transplant Candidates with Hepatocellular Carcinoma:A Prospective Validation. Hepatology. 2015;62:158–65. doi: 10.1002/hep.27787. [DOI] [PubMed] [Google Scholar]
- [30].Zakaria HM, Macshut M, Gaballa NK, Sherif AE, Abdel-Samea ME, Abdel-Samiee M, et al. Total Tumor Volume as a Prognostic Value for Survival Following Liver Resection in Patients with Hepatocellular Carcinoma. Retrospective Cohort Study. Ann Med Surg. 2020;54:47–53. doi: 10.1016/j.amsu.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Okusaka T, Okada S, Ishii H, Nose H, Nagahama H, Nakasuka HE, et al. Prognosis of Hepatocellular Carcinoma Patients with Extrahepatic Metastases. Hepatogastroenterology. 1997;44:251–7. [PubMed] [Google Scholar]
- [32].Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, et al. Clinical Features and Prognosis of Patients with Extrahepatic Metastases from Hepatocellular Carcinoma. World J Gastroenterol. 2007;13:414. doi: 10.3748/wjg.v13.i3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Si MS, Amersi F, Golish SR, Ortiz JA, Zaky J, Finklestein D, et al. Prevalence of Metastases in Hepatocellular Carcinoma:Risk Factors and Impact on Survival. Am Surg. 2003;69:879–85. [PubMed] [Google Scholar]
- [34].Pan Y, Chen H, Yu J. Biomarkers in Hepatocellular Carcinoma:Current Status and Future Perspectives. Biomedicines. 2020;8:576. doi: 10.3390/biomedicines8120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cui W, Fan W, Huang K, Wang Y, Lu M, Yao W, et al. Large Hepatocellular Carcinomas:Treatment with Transarterial Chemoembolization Alone or in Combination with Percutaneous Cryoablation. Int J Hyperthermia. 2018;35:239–45. doi: 10.1080/02656736.2018.1493235. [DOI] [PubMed] [Google Scholar]
- [36].Dong J, Zhai X, Chen Z, Liu Q, Ye H, Chen W, et al. Treatment of Huge Hepatocellular Carcinoma using Cinobufacini Injection in Transarterial Chemoembolization:A Retrospective Study. Evid Based Complement Alternat Med. 2016;2016:2754542. doi: 10.1155/2016/2754542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chaikajornwat J, Tanasoontrarat W, Phathong C, Pinjaroen N, Chaiteerakij R. Clinical Outcome of Yttrium-90 Selective Internal Radiation Therapy (Y-90 SIRT) in Unresectable Hepatocellular Carcinoma:Experience from a Tertiary Care Center. Liver Res. 2021;6:30–8. [Google Scholar]
- [38].Kim GH, Kim JH, Shim JH, Ko HK, Chu HH, Shin JH, et al. Chemoembolization for Single Large Hepatocellular Carcinoma with Preserved Liver Function:Analysis of Factors Predicting Clinical Outcomes in a 302 Patient Cohort. Life. 2021;11:840. doi: 10.3390/life11080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chok KS. Surgical Strategy for Huge and Advanced Hepatocellular Carcinoma in Hong Kong. Hepatoma Res. 2017;3:189–95. [Google Scholar]
- [40].Yamamoto M, Arii S, Sugahara K, Tobe T. Adjuvant Oral Chemotherapy to Prevent Recurrence after Curative Resection for Hepatocellular Carcinoma. Br J Surg. 1996;83:336–40. doi: 10.1002/bjs.1800830313. [DOI] [PubMed] [Google Scholar]
- [41].Ono T, Yamanoi A, El Assal ON, Kohno H, Nagasue N. Adjuvant Chemotherapy after Resection of Hepatocellular Carcinoma Causes Deterioration of Long-term Prognosis in Cirrhotic Patients:Metaanalysis of Three Randomized Controlled Trials. Cancer. 2001;91:2378–85. [PubMed] [Google Scholar]
- [42].Wang J, He XD, Yao N, Liang WJ, Zhang YC. A Meta-analysis of Adjuvant Therapy after Potentially Curative Treatment for Hepatocellular Carcinoma. Can J Gastroenterol. 2013;27:351–63. doi: 10.1155/2013/417894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lai EC, Lo CM, Fan ST, Liu CL, Wong J. Postoperative Adjuvant Chemotherapy after Curative Resection of Hepatocellular Carcinoma:A Randomized Controlled Trial. Arch Surg. 1998;133:183–8. doi: 10.1001/archsurg.133.2.183. [DOI] [PubMed] [Google Scholar]
- [44].Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, et al. Adjuvant Sorafenib for Hepatocellular Carcinoma after Resection or Ablation (STORM):A Phase 3, Randomised, Double-blind, Placebo-controlled Trial. Lancet Oncol. 2015;16:1344–54. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- [45].Hui D, Qiang L, Jian W, Ti Z, Da-Lu K. A Randomized, Controlled Trial of Postoperative Adjuvant Cytokine-induced Killer Cells Immunotherapy after Radical Resection of Hepatocellular Carcinoma. Dig Liver Dis. 2009;41:36–41. doi: 10.1016/j.dld.2008.04.007. [DOI] [PubMed] [Google Scholar]
- [46].Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, et al. Adjuvant Immunotherapy with Autologous Cytokine-induced Killer Cells for Hepatocellular Carcinoma. Gastroenterology. 2015;148:1383–91. doi: 10.1053/j.gastro.2015.02.055. [DOI] [PubMed] [Google Scholar]
- [47].Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer Cell Suppression of CD8+T Cells in Human Hepatocellular Carcinoma is Mediated by B7-H1/Programmed Death-1 Interactions. Cancer Res. 2009;69:8067–75. doi: 10.1158/0008-5472.CAN-09-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, et al. PD-1 and PD-L1 Upregulation Promotes CD8+T-cell Apoptosis and Postoperative Recurrence in Hepatocellular Carcinoma Patients. Int J Cancer. 2011;128:887–96. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]
- [49].Lu LC, Lee YH, Chang CJ, Shun CT, Fang CY, Shao YY, et al. Increased Expression of Programmed Death-ligand 1 in Infiltrating Immune Cells in Hepatocellular Carcinoma Tissues after Sorafenib Treatment. Liver Cancer. 2019;8:110–20. doi: 10.1159/000489021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Exposito MJ, Akce M, Alvarez JM, Assenat E, Balart LA, Baron AD, et al. CA209-9DX:Phase III, Randomized, Double-blind Study of Adjuvant Nivolumab vs Placebo for Patients with Hepatocellular Carcinoma (HCC) at High Risk of Recurrence after Curative Resection or Ablation. Ann Oncol. 2018;29:267–8. [Google Scholar]


