Abstract
Background and Aim:
Lymph node transfer surgery (LNTS) is indicated in secondary lymphedema (LE) patients who do not respond to conservative therapy. Animal models are the spearhead of LE research and were used to pioneer most of the surgical interventions currently in practice. We conducted a systematic review of the literature to explore animal models dedicated to LNTS to compare different species, techniques, and outcomes.
Methods:
Four databases were searched: PubMed, Cumulative Index of Nursing and Allied Health Literature, Scopus, and Web of Science. We used the Preferred Reporting Items for Systematic Reviews and Meta-Analysis as our basis of organization.
Results:
Avascular lymph node graft (ALNG) and vascularized lymph node transfer (VLNT) effectively treated LE and lead to better outcomes than controls. Whole ALNGs are superior to fragmented ALNGs. Larger fragments are more likely to be reintegrated into the lymphatic system than small fragments. VLNT was superior to whole and fragmented ALNG. Increasing the number of VLNT resulted in better outcomes. Adipose-derived stem cells improved outcomes of VLNT; vascular endothelial growth factor C and D and platelet-rich plasma improved outcomes for ALNG. Cryopreservation of lymph nodes (LNs) did not affect outcomes for ALNG. The critical ischemia and venous occlusion time for LN flaps were 4-5 and 4 h, respectively. The critical time for reperfusion injury was 2 h. Some of the novel models included venous LNT, and cervical adipocutaneous flap to groin.
Conclusion:
Current evidence from animals favors VLNT over other surgical interventions. Several pharmacological therapies significantly improved outcomes of ALNG and VLNT.
Relevance to Patients:
LE is a chronic condition affecting millions of patients worldwide. LNTS is becoming more popular as a LE treatment. Animal models have led the LE research for decades and developing new models for LE are essential for LE research. This systematic review aims to summarize the existing animal models dedicated to LNTS. We believe that this review is critical to guide researchers in the selection of the model that is best fit for their hypothesis-driven experiments.
Keywords: lymphedema, animal models, lymphedema surgery, systematic review
1. Introduction
Lymphedema (LE) is a chronic progressive and debilitating condition caused by lymphatic transport dysfunction [1]. LE can be further classified into primary and secondary LE according to its etiology [1]. The pathology in primary LE is exclusively limited to the lymphatic vasculature and can be categorized into hypoplastic or hyperplastic [2,3]. Primary LE is predominately caused by three disorders: Congenital hereditary LE (Milroy disease), familial LE praecox (Meigs disease), and LE tarda [1].
Secondary LE is caused by damage or obstruction of the lymphatic system by infections, trauma, malignancy, morbid obesity, surgery, and irradiation [4]. The most common cause of LE worldwide is filariasis (also known as elephantiasis), a parasitic disease caused by the mosquito-borne nematode Wuchereria bancrofti and affects over 90 million people [5]. In the developed world, almost all LE cases are caused by malignancy or its surgical and radiological sequelae [1]. Treatment of many cancers, including breast cancer, gynecological and urological cancers, have been found to cause LE [1]. Breast cancer treatment is highly associated with LE, with 49% and 28% of patients developing upper limb LE following mastectomy and lumpectomy, respectively [6].
The clinical course of LE is usually indolent and develops over months or years, starting with an accumulation of edematous fluid in the subcutaneous tissue, leading to chronic inflammation and further fluid accumulation. As a result, tissue fibrosis may then develop, leading to the characteristic pitting sign [7]. If left untreated, lymphostatic elephantiasis then develops; at this stage, the pitting disappears, and trophic skin changes as acanthosis and warty overgrowth take place [7]. Elephantiasis leads to recurrent cellulitis as the accumulated subcutaneous fluid facilitates bacterial proliferation [1]. A rare yet, life-threatening complication of chronic LE is the development of cutaneous malignancies as lymphangiosarcoma, lymphoma, and Kaposi sarcoma [8]. The risk for developing cutaneous angiosarcoma is 10% in patients suffering LE for 10 or more years, and the prognosis is poor with a 5-year survival of <10% [9,10].
While there are several modalities for the treatment of LE, no single method has demonstrated superiority over the other [11], and in general, there is no strong evidence on single treatment yielding high long-term cure rates [12]. Complete decongestive therapy combines manual lymphatic drainage, compressive therapy, skin hygiene, and exercise and is currently considered the standard of care [11,13]. Combining these techniques reduces limb swelling by increasing lymphatic contractility and flow. Benzopyrones are the most frequently used pharmacological treatments for LE and have been proven effective in a randomized controlled clinical trial [1]. Their long-term use, however, is limited due to drug-induced hepatotoxicity [14,15]. Diuretics are also another viable option; however, they are used less frequently as they are less effective and can induce fibrosis which may worsen LE in the long run [16,17].
Surgical treatment is indicated when conservative therapies do not slow down the progression of LE as expected. Three major surgical approaches are currently in practice: Resection procedures, microsurgical and supermicrosurgical interventions, and liposuction [1]. Resection procedures involve the excision of subcutaneous tissue with or without the overlying skin. Unfortunately, although effective, such interventions leave the patient liable to numerous complications, including recurrence, infections, esthetic disfigurement, eczema, unstable scars, and lymphatic fistulae [18]. Microsurgical interventions aim at restoring the function of the lymphatic system. Lymphovenous anastomosis are effective in treating LE if used early in the disease before fibrosis takes place [6]. Lymph node (LN) transplantation is another microsurgical intervention that can reduce LE when combined with post-operative compressive therapy. Unlike other organ transplantations, the transplanted LNs are autologous; therefore, no rejection is expected. A series of patients who underwent lymphatic grafting followed by compressive therapy showed up to 30% reduction in arm volume after 8 years of follow-up [19]. The mechanism of LN grafting is not entirely understood, however, some theories have tried to explain the therapeutic effect. One theory is that the transplanted lymphatic tissue releases growth factors (GFs) that induce lymphangiogenesis [6]. Another theory is the lymphatic pump theory; it is thought that the transplanted LNs act as a mechanical pump draining the accumulated fluids [20]. Finally, liposuction has recently been introduced as a viable therapy with favorable long-term outcomes [21]. Liposuction targets subcutaneous tissue as resection procedures but with fewer post-operative complications and better esthetic outcomes [21].
Animal models have been the spearhead of LE research for decades and they have significantly contributed to our understanding of the underlying pathology and the possible treatments of LE [22]. The first animal model of avascular lymph node grafts (ALNGs) and vascularized lymph node transfer (VLNT) were described in the late 1960s and 1970s, respectively [23,24]. Since then, several models were introduced describing new techniques, proposing a different combination of therapies, and evaluating different outcomes. This systematic review explores the animal models dedicated to lymph node transfer surgery (LNTS) to compare different species, techniques, and outcomes. The goal of the paper is to provide researchers and surgeons with a brief summary on the different animal models in LNTS and assess their outcomes. Furthermore, we aim to guide researchers in the selection of the experimental model that is a best fit for their hypothesis-driven experiment.
2. Methods
2.1. Information sources, search strategy, and eligibility criteria
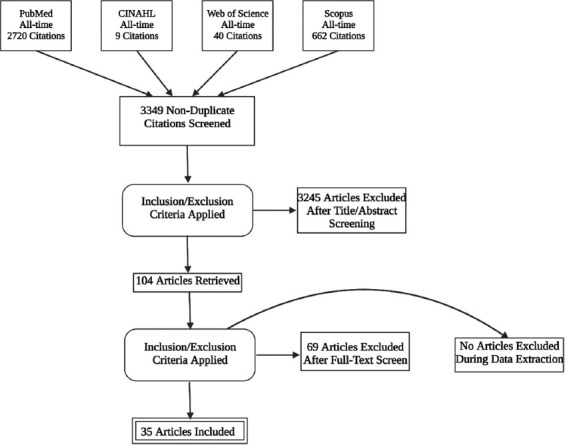
We have used four electronic databases to conduct an all-time search: PubMed (including MEDLINE), Cumulative Index of Nursing and Allied Health Literature, Scopus, and Web of Science. In addition, we used the Preferred Reporting Items for Systematic Reviews and Meta-Analysis as our basis of organization (Figure 1) [25]. The search terms were adjusted according to each database, and Boolean expressions were used to create a complex search string to conduct our search. Details on search terms used for every database are provided in the supplementary material. We ran our search in August 2021 with the following inclusion criteria: (1) Animal studies, (2) LN transplantation surgery, (3) attempt to evaluate outcomes, (4) English language, and (5) full text available. We excluded editorials, reviews, letters to the editor, and conference papers. We have also excluded descriptive studies that do not evaluate the outcomes.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analysis flow chart diagram. Created using Biorender.Com.
2.2. Study selection and data collection process
The first two authors independently performed the search and removed the duplicates using EndNote (Clarivate Analytics). After filtering the studies based on titles, abstracts were then screened according to the aforementioned eligibility criteria. The remaining studies were screened based on full-text readings. Finally, the third author solved any conflicts between the first two authors.
2.3. Data items and summary measures
We have included all studies reported in the English language and describing LN transplantation surgery in animal models with an attempt to evaluate the outcomes of the intervention.
3. Results
After the initial search yielded 3349 publications, 104 underwent abstract and full-text screening resulting in 35 studies included in our final analysis. Table 1 summarizes the included studies chronologically, with the earliest at the top (n = 35). For each study in our sample, we have summarized the methodology of LE induction, evaluation, and treatment. We have also reported the maximum follow-up time in weeks, the pharmacological treatments combined with surgery, and a summary of outcomes for every study.
Table 1. Summary of the included studies.
| Author (year) | Animal (model) | Number | LE induction | LE quantification/Lymph flow evaluation | Follow up (wks) | Surgical technique | Other treatment | Summary/outcome |
|---|---|---|---|---|---|---|---|---|
| Tilak and Howard [23] (1965) | Mongrel dogs (hind limb) | 4 | PLN. § | Lymphangiogram. Histological examination |
20 | ALNG | N/A | LNs were hard to examine. Unknown success rates. |
| Shesol et al., [24] (1979) | Lewis albino rats (hind limb) | 60 | PLN | Radioactive scan measuring lymph flow in popliteal region before and after intervention. | 1 | Lymphadenectomy Lymphadenectomy+ ipsilateral inguinal LN island flap. Lymphadenectomy+ LN free flap. Lymphadenectomy+ ALNG. Ipsilateral inguinal LN island flap without lymphadenectomy. |
N/A | 0/6 15/29 5/10 0/9 0/6 |
| Pabst et al., [55] (1988) | Gottingen minipigs | 6 | ILN or mesenteric lymohadenectomy. § | Histological examination | 24 | Fragmented ALNG to greater omentum. Fragmented ALNG to subcutaneous inguinal region. |
Killed Pasteurella multocida and Bordetella brochoseptica | No regeneration. Regeneration detected in all sections. |
| Chen et al., [52] (1990) | Dogs (hind limb) | 10 | Irradiation followed by skin stripping and PLN | Linear measurements, radionucleide scan, lymphangiography, histological examination. | 24 | VLNT of superficial inguinal LN + lymphatic anastomosis. VLNT of superficial inguinal LN without lymphatic anastomosis. |
N/A | Measurements: improvement after surgery, no difference between groups. Isotope study: improvement after surgery, no difference between groups. Histology: preserved size and architecture of transferred LNs in both groups (9/10). |
| Tammela et al., [50] (2007) | Mouse (upper limb) | 20 | Axillary lymphadenectomy. | Fluorescent dextran micro-lymphangiography. | 8 | LN allograft LN allograft + VEGF-C |
VEGF-C | Dextran detected in 22% (2/9) of mice. Dextran detected in 82% (9/11) of mice. |
| Tobbia et al., [58] (2009) | Dorset sheep (hind limb) | 50 | PLN. § | Transport rate of iodine 125 HSA to blood (%injected/hr) | 12 | VLNT ALNG Sham control |
N/A | 15.7 ± 1.0 percent /hour 12.6 ± 0.8 percent /hour 16.1± 0.7 percent / hour VLNT are superior to ALNG. |
| Hadamitzky et al., [42] (2009) | Lewis rat | 44 | Bilateral ILN. § |
Proportion of regenerated LNs (%) by histological examination. | 4 | Whole ALNG Fragmented ALNG Fragmented ALNG+ sheep RBCs Fragmented ALNG+ PRP Control |
PRP, Sheep RBCs. |
81.8% 63.6% 50% 100% 0% |
| Blum et al., [54] (2010) | Gottingen minipigs | 26 | ILN. § |
Tc-99 m-NC-SPECT/CT | 32 | Bilateral lymphadenectomy+ fragmented ALNG on one side. Bilateral lymphadenectomy+ fragmented ALNG on both sides |
Sheep RBCs | Impaired lymph flow and dermal back flow on the control side. Good lymph flow and less dermal backflow. |
| Lahteenvuo et al., [56] (2011) | Pigs | 19 | Damage to lymphatic vasculature around inguinal LNs. § |
Lymphangiography Histological examination. |
2 | ALNG+ VEGF-C ALNG+VEGF-D ALNG+ LacZ (control) |
VEGF-C VEGF-D |
↑ lymphatic growth ↑ lymphatic growth |
| Sommer et al., [41] (2012) | Lewis rats | 41 | ILN and PLN+ irradiation. § |
Proportion of regenerated LNs (%) by histological examination. | 4 | Fragmented ALNG Fragmented ALNG+ VEGF-C |
VEGF-C | 59% 74% |
| Uygur et al., [40] (2013) | Sprague-Dawley rats | 10 | N/A | laser-assisted ICG angiography. Histological examination. |
N/A | VLNT (a novel model of vascularized cervical LN flap) | N/A | Good perfusion of LN flap. Well-established vascular network. |
| Cheng et al., [39] (2014) | Sprague-Dawley rats | 18 | N/A | ICG fluorescence | N/A | VLNT Cutaneous flap devoid of LNs |
N/A | Dye detected. Dye not detected. |
| Schindewolff et al., [38] (2014) | Lewis rats (hind limb) | 109 | ILN and PLN. § |
Proportion of regenerated LNs (%) by histological examination. | 4 | Fragmented ALNG Fragmented ALNG+ saline Fragmented ALNG+ VEGF-C (abdominal wall) Fragmented ALNG+ VEGF-C (thigh) |
VEGF-C | 70% 70% 80% ≤85% |
| Kwiecien et al., [37] (2015) | Lewis rats | 10 | N/A | Histological examination | 1 | VLNT (a novel model of axillary LN flap to groin) | N/A | No signs of ischemia or necrosis. |
| Tervala et al., [49] (2015) | Immunodeficient nude mice (recepient) DsRed reporter mice (donor) |
50 25 |
Axillary lymphadenectomy. § | Proportion of surviving LNs by histological examination. | 12 | ALNG+ VEGF-C ALNG+ VEGF-D ALNG+ VEGF-C156S ALNG+ VEGF-A ALNG+ LacZ (control) |
VEGF-C, VEGF-D, VEGF-C156S, and VEGF-A | 8/10 8/10 6/9 1/6 0/10 |
| Suami et al., [51] (2016) | Mongrel dogs | 2 | Unilateral axillary and neck lymphadenectomy+ irradiation | Limb circumference Indocyanine lymphangiography |
56 | VLNT | N/A | Slight decrease. New lymphatic collaterals. VLNT may stimulate formation of new lymph vessels. |
| Visconti et al., [35] (2016) | Wistar rats | 15 | N/A | Methylene blue Histological examination |
4 | VLNT (a novel model of cervical lymph node-adipo-cutaneous flap to groin) | N/A | Restored lymph flow 100% of flaps viable. |
| Visconti et al., [36] (2016) | Wistar rats | 8 | ILN. § | Methylene blue Histological examination |
6 1/2 | VLNT ( a novel model of cervical venous LN flap) | N/A | Patent anastomosis. ↓↓stromal compartment, preserved sinus. |
| Shioya et al., [48] (2016) | Mice (hind limb) | 8 | Subiliac and PLN+ irradiation | Paw volume Indocyanine fluorescent |
8 | ALNG Control |
N/A | ↓paw volume, functional LN with collateral pathway. ↑paw volume, dermal backflow pattern. |
| Huang et al., [47] (2016) | FLT-4 DTR mouse (hind limb) | NR | subdermal hindlimb diphtheria toxin injections+ PLN. | Hind limb thickness | 15 | ALNG Sham surgery (control) |
N/A | 4.8%↑ 31.1%↑ |
| Ito et al., [34] (2016) | Sprague-Dawley rats | 18 | N/A | ICG fluorescence | N/A | LN containing groin flap LN devoid DIEP flap |
N/A | ICG detected in pedicle vein in 58.3% 0.0% |
| Nguyen et al., [33] (2016) | Sprague-Dawley rats (hind limb) | 18 | ILN and PLN+ irradiation | Volumetric analysis using micro-CT imaging | 12 | Single VLNT Three VLNT No VLNT (control) |
N/A | -8.9% ± 5.2% -9.1% ± 3.1% 7.6% ± 3.3% |
| Hayashida et al., [46] (2017) | C57BL/6J mice (hind limb) | 20 | PLN+ irradiation | Paw volume | 2 | VLNT VLNT+ADSC Skin flap Skin flap+ ADSC |
ADSC | 0.284 to 0.453 0.241 to 0.265 0.294 to 0.480 0.312 to 0.438 |
| Aydogdu et al., [32] (2017) | Sprague-Dawley rats | 30 | PLN | Limb measurement | 12 | VLNT Control |
N/A | ↓↓ |
| Najjar et al., [31] (2018) | Sprague- Dawley rats | 7 | ILN | ICG fluorescence Uptake by LN. |
37 | VLNT | N/A | ICG uptake by LN in 5/7 |
| Kwiecien et al., [30] (2018) | Sprague-Dawley rats | 14 | N/A | Time to ICG fluorescence detection in axillary vein (in seconds) | N/A | Control (no VLNT) 2 VLNT 4 VLNT |
N/A | 229s 79s 56s |
| Maeda et al., [45] (2018) | C57BL/6N mice | 36 | PLN | ICG fluorescence to detect whether lymph directed to inguinal LNs. | 4 | ALNG Control (no ALNG) |
N/A | 2/18 8/18 |
| Yang et al., [29] (2018) | Sprague- Dawley rats | 12 | N/A | ICG uptake, laser doppler, histological examination to evaluate effect of ischemia on LNs | N/A | Clamping of vascular pedicle of groin LN flap for 0,1,2,3,4,5,6,7 hours. | N/A | The critical ischemia time for vascularized LNs is 5 hours in the tested animal model. |
| Hadamitzky et al., [53] (2018) | Gottinger Minipig |
8 | Right groin lymphadenectomy | SPECT-CT, histological examination to assess LN regeneration rate (%) | 24 | LN resection and immediate replantation (ALNG) LN resection and implantation after cryopreservation for 1 mo |
N/A | 75% 67% |
| Penuela et al., [57] (2019) | New Zealand White rabbit (hind limb) | 8 | PLN | Hind limb volume. | 4 | VLNT | N/A | Basal: (51.94 ± 11.23) Transfer day: (73.40 ± 26.47) Final: (50.13 ± 12) |
| Ishikawa et al., [44] (2019) | Mouse | 25 | PLN. § | ICG lymphography | 4 | VLNT (a novel model of inguinal LN bearing flap containing superficial caudal epigastric vessels) | N/A | Reconnection of LN with afferent lymphatics in 16/25 |
| Ishikawa et al., [43] (2019) | C57BL/6 N mice | 50 | PLN. § | Afferent lymphatic reconnection by histological examination. | 4 | pedicled vascularized LNT pedicled nonvascularized LNT ALNG |
N/A | 13/20 11/15 7/15 |
| Perrault et al., [28] (2020) | Prox1-EGFP reporter rats | 48 | N/A | histological examination to evaluate effect of ischemia and reperfusion on LNs | 5 days | LN containing groin flaps subjected to ischemia for either 1, 2, 4, or 8 hours and harvested after 0 hours, 24 hours, and 5 days. | N/A | Critical time for ischemia: 4 hrs. Critical time for reperfusion injury:2hrs |
| Tinhofer et al., [27] (2020) | Lewis rats | 27 | N/A | laser doppler, histological examination. | N/A | Groin LN flap+ clamping of femoral artery and vein for 1,3,4 or 5 hours. | N/A | Critical venous occlusion time: 4 hrs. |
| Frueh et al., [26] (2020) | Lewis rats | 44 | N/A | Western blot and histological examination. | N/A | Vascular LN flaps+ ischemia for 45 or 120 mins+ reperfusion for 24 hrs VLNT (control) |
N/A | Ischemia for 120 mins lead to marked ↓ in cellularity compared to control. |
LE: Lymphedema, ALNG: Avascular lymph node graft, LN: Lymph node, PLN: Popliteal lymphadenectomy, §: Model of acute lymphatic damage without lymphedema, ILN: Inguinal lymphadenectomy, VEGF: Vascular endothelial growth factor, VLNT: Vascular lymph node transfer, CT: Computed tomography, ICG: Indocyanine green, ADSC: Adipose-derived stem cells, PRP: Platelet-rich plasma, LNT: Lymph node transfer
3.1. Synthesis of results
Rodents were the most frequently used animal model, this included 18 studies using rat models [24,26-42] and eight using mice [43-50]. Canine models were used in three studies [23,51,52], pigs were used 4 times [53-56], while rabbits [57] and sheep [58] were used once.
Twenty-five studies attempted to induce lymphatic damage with or without resulting in clinically detectable LE [23,24,31-33,36,38,41-58]. Surgical lymphadenectomy was the most frequent method used to induce lymphatic damage in the recipient site. Popliteal lymphadenectomy (PLN) and inguinal lymphadenectomy (ILN) were the most commonly used methods to induce LE. In five studies, surgical lymphadenectomy was combined with irradiation to mediate lymphatic damage [33,41,46,48,52]. One study used a combination of subdermal diphtheria toxin injection and irradiation to induce LE in the hind limb of an FLT-4 DTR mouse model [47]. A combination of surgery and irradiation was more reliable than surgery alone in producing chronic stable LE [52].
ALNG was reported in at least one group of animals in 16 studies [23,24,38,41-43,45,47-50,53-56,58]. VLNT, either free or pedicled, was attempted in 22 studies [24,26-37,39,40,43,44,46,51,52,57,58]. Four studies reported surgical models of composite flaps containing or devoid (as a control) of LNs as a means of VLNT [27-29,34], and two studies reported the use of cutaneous flaps for comparison with other interventions [39,46].
Variable pharmacological modalities were combined with the surgical interventions. Vascular endothelial growth factor (VEGF) was used in combination with ALNG in four studies [38,41,49,50,56]; adipose-derived stem cells (ADSCs) were combined with VLNT in one study [46]; while platelet-rich plasma (PRP) was used in combination with ALNG in another study [42]. In addition, sheep red blood cells were investigated in two studies for their theoretical regenerative effect on transplanted LNs through antigenic stimulation [42,54], while Pasteurella multocida and Bordetella bronchiseptica were used in one study for the same purpose [55].
Both ALNG and VLNT were effective in treating LE as they were associated with better outcomes than controls. Whole ALNGs were superior to fragmented ALNG due to the impact of fragmentation on regeneration of the ALNG [42]. Larger fragments were more likely to be reintegrated into the lymphatic system when to small fragments [54]. VLNT was superior to the whole and fragmented ALNG [24,39,58]. Increasing the number of VLNT was associated with better outcomes [30,33]. Composite flaps with LNs were found superior to those without LNs in treating LE [34]. ADSC improved outcomes of VLNT [46], while VEGF-C and D improved the outcomes for ALNG [38,49,50]. PRP was associated with favorable outcomes when combined with ALNG [42]. Immune challenge by peripheral injection of sheep RBCs did not improve the outcomes for fragmented ALNG [42,54]. Cryopreservation of ALNG for 1 month did not significantly affect outcomes compared to fresh grafts [53]. The critical ischemia and venous occlusion time for LN flaps (LNF) were 4-5 and 4 h, respectively [28,29], and the critical time for reperfusion injury was 2 h [28]. One study described a novel model of venous LNT [36], and another one described a new model of the cervical adipocutaneous flap to groin [35].
4. Discussion
Animal models are the keystone of translational research, and their importance becomes unparalleled when alternatives are faced with technical challenges and complex uncertainties. As our understanding of the complexity of LE mechanisms and pathophysiology grows, the need for reliable experimental animal models increases [59]. Variable mammalian animal models have been used to help treat LE. The earliest among these endeavors dates back to the early 1930s, when Homans et al. described a model of chronic LE in dogs mediated through complex lymphatic ligation and injection of sclerosing solution [60]. Thirty years later, Tilak and Howard [23] pioneered the first animal model for non-vascularized LN grafts. The next big step was achieved by Shesol et al. [23,24] who introduced the first animal model of successful VLNT in 1979. Since then, variable models have been tested, proposing a spectrum of surgical techniques and combinations with pharmacological therapies and evaluating different outcomes.
With the staggering growth in cancer incidence along with the advances in surgical and radiation oncology, we are witnessing an unprecedented exponential increase in the prevalence of secondary LE. Although LE is more likely to develop after aggressive surgical ablation of LNs and radiation therapy [61], there is also a risk with minimal interventions such as sentinel LN biopsy [62]. As a result, LE animal models have gained popularity as a trusted method to explore and establish new surgical approaches for LE management.
The studies included in this review could be classified to three major types: Studies comparing different surgical techniques (Figure 1), studies evaluating pharmacological and surgical interventions (Figure 2), and studies reporting novel surgical techniques (Figure 3). However, we preferred to construct our discussion based on the animal model used due to the significant differences that exist between rodents and other animal models.
Figure 2. Studies comparing different surgical techniques. (a) VLNT versus ALNG [24,42,43,58]; (b) Whole ALNG versus Fragmented ALNG [23,42]; (c) Few versus Multiple VLNTs [30,33]; (d) VLNT versus Skin Flap [39,46]; (e) VLNT +/− Lymphatic Anastomosis [52]; (f) Fresh versus Cryopreserved ALNG [53]. Created using Biorender.Com.
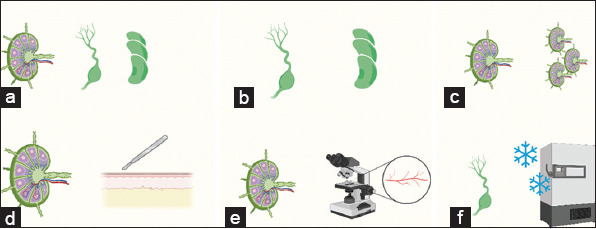
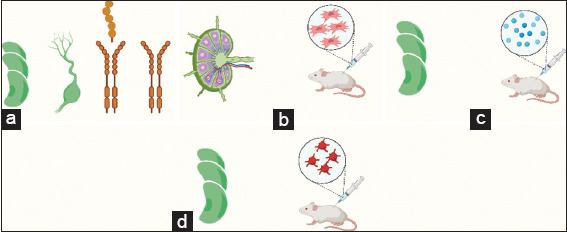
Figure 3. Studies evaluating pharmacological interventions. (a) ALNG +/− VEGF [38,41,49,50,56]; (b) VLNT+/− ADSC [46]; (c) ALNG +/− Antigenic Stimulation [42,54,55]; (d) ALNG +/− PRP [42]. Created using Biorender.Com.
4.1. Rodent models
Rats and mice are the most frequent and reliable models in LE research as they are affordable, easy to house, breed, handle, and permit a range of potential experimental interventions. Moreover, they offer a wide range of transgenic and knockout strains [63]. The first VLNT and ALNG rodent models were reported by Shesol et al. [24]; lymphatic damage was induced in Lewis albino rats through PLN. In two groups of rats, a mass of inguinal LNs was then transferred to the popliteal region either as a pedicled flap of the superficial epigastric vessels or as a free flap by microvascular anastomosis. In the third group, ALNG was transferred to the popliteal region. Seven days later, India ink or radioactive gold particles were injected in the distal part of the hind limb to evaluate the LN function and lymph flow after the transplantation. The results showed that VLNT in the form of pedicled or free flap was superior to ALNG. However, pedicled and free flap outcomes were not significantly different from each other. Despite the poor outcomes of ALNG reported by Shesol et al., ALNG was later found to be better than control in multiple studies [24,45,47,48].
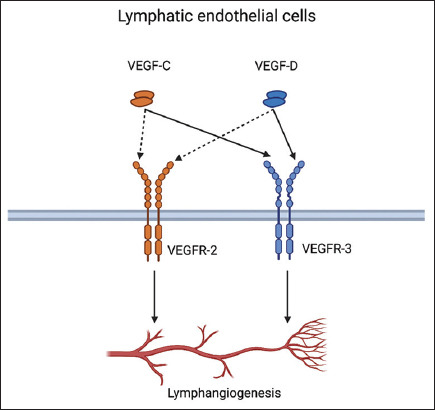
Since VEGFs heavily regulate lymphangiogenesis and angiogenesis, these factors have gained significant attention in many research fields such as cancer and LE treatment [64,65]. VEGFs exhibit their regulatory action through a group of tyrosine kinase receptors known as vascular endothelial growth factor receptors (VEGFRs) expressed exclusively in blood and lymphatic endothelial cells. VEGF-A induces angiogenesis through VEGFR-1 and VEGFR-2, while VEGF-C and VEGF-D mainly induce lymphangiogenesis by binding to VEGFR-3 (Figure 4) [64]. Furthermore, after being proteolytically processed, VEGF-C and VEGF-D smaller particles can bind to VEGFR-2 to induce angiogenesis as well [66,67]. The high hopes associated with VEGFs as a promising LE treatment do not come without some justified concerns. The potential risk of tumor metastasis and growth (by enhancing angiogenesis) remains the most feared among these concerns [68].
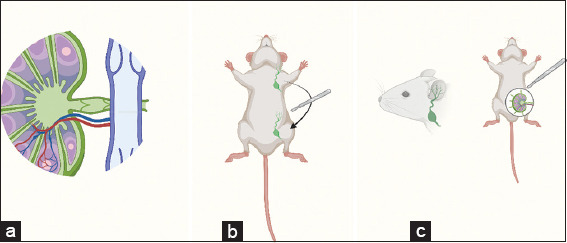
Figure 4. Studies reporting novel surgical techniques. (a) Cervical Venous Lymph Node Flap [36]; (b) Axillary VLNT to Groin [37]; (c) Cervical Lymph Node Flap to Groin [35]. Created using Biorender.Com.
In 2001, Mäkinen et al. successfully inhibited lymphangiogenesis in a transgenic mouse model by inhibiting VEGF-C and VEGF-D signaling [69]. The animals developed a clinical picture similar to Milroy disease in humans, including edema, thickening of the skin, and increased deposition of subcutaneous fat [69]. Five years later, Tammela et al. explored the potential effects of VEGF on survival of ALNG and lymph vessel maturation. Axillary LNs were surgically removed after they were visualized by Evans blue microlymphangiography. To evaluate the potential benefits of GF on the maturation of lymph vessels, the mice were divided into three groups after lymphadenectomy: the first group received VEGF-C injection at the operation site; the second group received VEGF-D, while the third group received LacZ injection and served as a control group. Lymphatic vessel function was evaluated by injecting fluorescent high-molecular-weight dextran, near-infrared quantum dots, or Evans blue solution into the mouse forepaws. At 2 months, significant improvement in lymphatic vessel function was noticed in VEGF-C and VEGF-D groups, and further improvement was documented at 6 months using the same methods. To evaluate the effect of VEGF-C on ALNG, one group of mice received ALNG from DsRed reporter mice with adenoviral gene transfer vectors encoding full-length human VEGF-C, while the other group received non-treated transplants. Evaluation using fluorescent dextran microlymphangiography at 2 months postoperatively revealed superiority of the VEGF-C-treated LNs over controls. Sommer et al., Schindewolff et al., and Tervala et al. confirmed the regenerative effect of VEGF-C on ALNG reported by Tammela et al. in their experiments using Lewis rats and immunodeficient mice models [38,41,49,50].
The potential regenerative effects of PRP and antigenic stimulation by sheep RBCs in rats were first investigated by Hadamitzky et al. [42]. Platelets are rich in GFs; their α-granules are storage sites for VEGFs, platelet-derived GF, transforming GF-β, insulin GF, and platelet-derived angiogenesis factor [70,71]. On the other hand, sheep RBCs are potent antigens that induce follicular development in the LN of minipigs [55]. For their purpose, Hadamitzky et al. harvested the inguinal LNs from 32 female Lewis rats and implanted them into a subcutaneous inguinal pouch. The animals were divided into four groups: They received whole ALNG, fragmented ALNG, fragmented ALNG with sheep RBCs, and fragmented ALNG with PRP. Histological examination of the transplanted LNs 1 month later showed that fragmentation of LNs negatively affected regeneration while PRP significantly improved the regeneration of the transplanted LNs. The outcomes in the sheep RBCs group were not favorable.
In the pursuit of exploring the feasibility of vascularized cervical LNF, Uygur et al. [40] used Sprague-Dawley rats to successfully raise a LN flap on a vascular pedicle composed of the common carotid artery and external jugular vein. The length of the vascular pedicles ranged from 2.5 to 3 cm, and each flap contained 5-6 LNs. The vascularity of the flap was tested by injecting methylene blue dye; the dye was distributed equally in the LNs, confirming adequate vascularity of the flap.
In 2016, Visconti et al. reported two novel models of the cervical adipocutaneous flap to the groin and venous LN flap in Wistar rats [35,36]. In the first study, an innominate septum-based LN-adipocutaneous flap was harvested and transferred to the inguinal region (after ILN) through anastomosis to either the femoral vessels (microsurgery model) or the superficial epigastric artery and vein (super microsurgery model). The innominate septum is a well-defined adipofascial structure located between the anterior belly of the digastric muscle and the masseter muscle and was described for the 1st time in the same study. After 30 days, methylene blue confirmed restored lymph flow in the recipient area, and histological examination revealed viable LNs in all the examined specimens [35]. In the second study, cervical LNFs were harvested and transferred to the inguinal region (after ILN) along the femoral vein in a flow-through fashion. What motivated establishing this model were the possible advantages of venous LNFs over arteriovenous LNFs. The venous LNFs were easier to harvest and salvage the continuity of the arterial system. In addition, the venous flaps were proven effective in restoring lymph flow in the recipient area. However, several histological changes were reported, including marked loss of the stromal compartment.
In general, stem cells were found to have great potential in regenerative medicine, and the therapeutic effects of ADSC on LE have been reported multiple times in literature [72,73]. These therapeutic effects are mediated through two pathways: Paracrine and differentiation pathway [74]. Through the paracrine pathway, some stem cells secrete mesenchymal-related factors. At the same time, other stem cells could differentiate into lymphatic endothelial cell precursor cells through both paracrine and differentiation pathways. Both effects are thought to contribute to lymphangiogenesis and potential improvement in LE [74]. Hayashida et al. investigated a combination of VLNT and ADSCs in a mouse model. The results of the experiment showed that ADSCs with VLNT were superior to VLNT alone. Therefore, ADSCs are capable of improving the clinical outcomes of VLNT [46].
The effect of the number of VLNT was investigated in two studies. In the first study [33], Sprague-Dawley rats underwent ILN, PLN, and irradiation to induce LE. The rats were then divided into three groups: A control group, single VLNT, and a group that received three VLNTs. The LE was quantified by volumetric analysis using micro-CT imaging. The group with three VLNTs had better outcomes when compared to a single VLNT. However, both groups were superior to the control group. The second study used the same model; however, outcomes were evaluated through calculating time to ICG fluorescence detection in axillary vein. ICG was detected faster in the group with the higher number of VLNTs, confirming the results from the first study [30].
In transplantation surgery, time is a vital factor for the procedure’s success as time translates to ischemia. Ischemia is defined as inadequate tissue oxygenation [75], and critical ischemia time is the maximum time an organ or tissue can survive ischemia without contracting irreversible damage [76]. Critical ischemia time differs according to the individual tissue characteristics as metabolic rate and temperature [29] but usually ranges from 45 min to 3 h [77]. The clinical signs of ischemia in the transplanted organ are usually carefully monitored intraoperatively to avoid unfavorable outcomes. However, identifying these signs in LNs can be challenging as the LNs are usually enclosed in a cushion of fat that masks any visual signs of ischemia [28]. Despite its clinical importance, few studies aimed to evaluate the critical ischemia time for LNF [28,29]. These studies used a similar technique of femoral artery clamping to induce ischemia in LN containing groin flaps. The critical ischemia time was 4 h in one study [28] and 5 h in another one [29]. The critical time to reperfusion injury was also evaluated in two studies and was found to be 2 h [26,28]. An exciting finding of Yang et al.’s experiment was that ischemia below the cutoff of 5 h actually improved lymphatic drainage and perfusion of vascularized LNF [29].
Inadequate venous drainage of VLNT can lead to VLNT failure or malfunction. This is due to venous congestion resulting in impaired perfusion and ischemia [27]. The critical time of venous obstruction is the maximum time of venous obstruction before irreversible damage occurs in the transplanted organ. It was evaluated by Tinhofer et al. in a rat model by clamping the femoral vein of a groin flap and was found to be 4 h [27].
4.2. Other animal models
Several models other than rodents have been used in LE research, these include minipigs, rabbits, dogs, and sheep. Although rodents are considered reliable models in LE research, some limitations in these models mandated the use of other species. The most significant limitation in rodents models is their small size, and hence, the small distance spanned by the vessels while regenerating. This may give a false impression regarding the accuracy of time required for lymphatic anastomosis to occur in humans [56].
Canine models were the first to be used in LNTS. They have lately attracted attention again for their potentials in post-breast cancer LE research. Suami et al. meticulously investigated the lymphatic system of the forearm of Mongrel dogs [78]. Their anatomic study revealed several similarities between the canine forelimb and the human upper limb. The lymphatic system was divided into superficial and deep systems by deep fascia. The lymphatic vessels were similar in diameter in both species. One dominant LN drained the medial side of the upper extremity and anterior upper torso.
The first animal model for ALNG was a canine model presented by Tilak and Howard who used Mongrel dogs to conduct their research [23]. They induced acute lymphatic damage in the dogs by PLN and then transferred ALNG to the same region. Since LE did not develop in the animals after PLN, the clinical outcomes could not be measured. Histological examination of the transplanted nodes was attempted after 4 weeks of follow-up. However, the hard consistency of the transplanted nodes did not permit preparing the nodes for examination, and therefore, the experiment’s success could not be evaluated [23].
The sole canine model for VLNT was described by Suami et al. [50,51] who induced lymphatic damage in a pair of Mongrel dogs by axillary lymphadenectomy and irradiation, resulting in clinical LE in one dog. After 1 year, a vascular LN flap based on the superficial epigastric artery and vein was transferred to the axillary region. The outcomes were evaluated by measuring the limb circumference and indocyanine lymphangiography. The flap successfully reduced limb circumference at the 1-month follow-up. Furthermore, new lymphatic collaterals were detected after surgery, suggesting that VLNT may induce new lymph vessel formation [51].
The first animal model to investigate the effect of antigenic stimulation on the regeneration of transplanted LNs was reported by Pabst and Rothkötter [55]. ALNG was implanted in the greater omentum and subcutaneously in Gottingen minipigs. After that, antigen challenge was conducted by subcutaneously injecting the transplanted nodes’ drainage areas with killed P. multocida and B. bronchiseptica. For the subcutaneous ALNG group, the antigen challenge successfully stimulated regeneration and differentiation of the LN fragments. On microscopic examination, many macrophages with cell debris were detected in the periphery of the fragments while a thick, highly vascular capsule-like structure was seen around the transplanted fragments.
Blum et al. [54] used minipigs to study the effect of fragmented ALNGs on regeneration of lymph vessels and restoration of lymph flow following bilateral ILN. Their experiment evaluated the effect of the size of the fragments on transplanted ALNGs and compared unilateral versus bilateral LN grafting. The experiment proved that larger fragments were more likely they were to be reintegrated into the lymphatic system than smaller fragments. The results also proved that bilateral ALNG was associated with better restoration of lymphatic function and less dermal backflow – a pathognomonic sign of LE.
The effect of VEGF on lymphatic vessel regeneration in pigs was evaluated by Lähteenvuo et al. [56]. Vasculature around inguinal LNs was destroyed followed by an ALNG transfer with VEGF-C or VEGF-D. Both GFs were found to induce lymphatic growth more than the control group. An interesting side effect of VEGF-D was a transient increase in seroma accumulation in the operated inguinal region. This side effect could be problematic in humans as the seroma may complicate wound healing, especially in immunocompromised cancer patients who developed LE after surgery or irradiation.
Hadamitzky et al. conducted another interesting study in minipigs to study the effect of cryopreservation of LNs on the survival of ALNG [53]. The study successfully demonstrated that cryopreservation of LNs for 1 month before grafting did not affect the regenerative capacity or the drainage function of the transplanted ALNGs. This concept could have a critical futuristic clinical implication since artificial LN engineering applies cryopreservative techniques on the lymphatic tissue to allow laboratory manipulation [79]. Before this study, it was not clear if cryopreservation could negatively affect the quality of the lymphatic tissue.
Tobbia et al. [58] used a sheep model to compare ALNG and VLNT. First, acute lymphatic damage was conducted by PLN. The animals were then divided into three groups: ALNG, VLNT, and the third group served as a control group with sham surgery. The lymph flow was evaluated by measuring the transport rate of iodine 125 human serum albumin to the blood after being injected in the hind limb. The results favored VLNT over ALNG, and both techniques were superior to control, confirming the findings from the previous animal studies.
5. Conclusion
Although animal models are not without flaws, they are indispensable in LE research. Animal models were successfully used to explore the potential surgical treatments for LE as ALNG and VLNT before their use in humans. Results from animal studies suggest that VLNT is superior to whole and fragmented ALNG; increasing the number of VLNT results in better outcomes; and composite flaps with LNs are superior to those without LNs.
Animal models are used to pioneer new surgical techniques for LE surgery. They are also used to explore the potentials of growth hormone and stem cell therapy. Although rodent’s hind limb is considered the most reliable model, new promising models are still being explored and reported. Moreover, they provide surgeons with readily available and cost-effective microsurgical training models.
This study is not without limitations. As most systematic reviews, this study is liable to the potential bias of misinterpreting data and results, and bias in the study selection process. Furthermore, the included studies introduced a wide range of surgical and pharmacological interventions. The variability of the included studies makes it challenging to reach a definitive conclusion. However, the primary aim of this review is to inform the reader about the different models for LNTS and not to reach definitive conclusions on the best model.
Acknowledgments
This study was funded in part by the Mayo Clinic Center for Regenerative Medicine. Figures 1-5 were created using BioRender.com.
Figure 5. VEGF-C and VEGF-D induce lymphangiogenesis through VEGFR-2 and VEGFR-3. Created using Biorender.Com.
Conflict of Interest
None of the authors have any conflicts of interest.
References
- [1].Warren AG, Brorson H, Borud LJ, Slavin SA. Lymphedema:A Comprehensive Review. Ann Plastic Surg. 2007;59:464–72. doi: 10.1097/01.sap.0000257149.42922.7e. [DOI] [PubMed] [Google Scholar]
- [2].Kitsiou-Tzeli S, Vrettou C, Leze E, Makrythanasis P, Kanavakis E, Willems P. Milroy's Primary Congenital Lymphedema in a Male Infant and Review of the Literature. In vivo (Athens, Greece) 2010;24:309–14. [PubMed] [Google Scholar]
- [3].Bolletta A, Di Taranto G, Chen SH, Elia R, Amorosi V, Chan JC, et al. Surgical Treatment of Milroy Disease. J Surg Oncol. 2020;121:175–81. doi: 10.1002/jso.25583. [DOI] [PubMed] [Google Scholar]
- [4].Wheeler ES, Chan V, Wassman R, Rimoin DL, Lesavoy MA. Familial Lymphedema Praecox:Meige's Disease. Plastic Reconstruct Surg. 1981;67:362–4. doi: 10.1097/00006534-198103000-00016. [DOI] [PubMed] [Google Scholar]
- [5].Szuba A, Shin WS, Strauss HW, Rockson S. The Third Circulation:Radionuclide Lymphoscintigraphy in the Evaluation of Lymphedema. J Nuclear Med. 2003;44:43–57. [PubMed] [Google Scholar]
- [6].Becker C, Vasile JV, Levine JL, Batista BN, Studinger RM, Chen CM, et al. Microlymphatic Surgery for the Treatment of Iatrogenic Lymphedema. Clin Plastic Surg. 2012;39:385–98. doi: 10.1016/j.cps.2012.08.002. [DOI] [PubMed] [Google Scholar]
- [7].International Society of Lymphology. The Diagnosis and Treatment of Peripheral Lymphedema. Consensus Document of the International Society of Lymphology. Lymphology. 2003;36:84–91. [PubMed] [Google Scholar]
- [8].Ruocco V, Schwartz RA, Ruocco E. Lymphedema:An Immunologically Vulnerable Site for Development of Neoplasms. J Am Acad Dermatol. 2002;47:124–7. doi: 10.1067/mjd.2002.120909. [DOI] [PubMed] [Google Scholar]
- [9].Grada AA, Phillips TJ. Lymphedema:Pathophysiology and Clinical Manifestations. J Am Acad Dermatol. 2017;77:1009–20. doi: 10.1016/j.jaad.2017.03.022. [DOI] [PubMed] [Google Scholar]
- [10].Sharma A, Schwartz RA. Stewart-Treves Syndrome:Pathogenesis and Management. J Am Acad Dermatol. 2012;67:1342–8. doi: 10.1016/j.jaad.2012.04.028. [DOI] [PubMed] [Google Scholar]
- [11].Oremus M, Dayes I, Walker K, Raina P. Systematic Review:Conservative Treatments for Secondary Lymphedema. BMC Cancer. 2012;12:6. doi: 10.1186/1471-2407-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hadamitzky C, Pabst R, Gordon K, Vogt PM. Surgical Procedures in Lymphedema Management. J Vasc Surg Venous Lymphat Disord. 2014;2:461–8. doi: 10.1016/j.jvsv.2014.02.001. [DOI] [PubMed] [Google Scholar]
- [13].Cormier JN, Rourke L, Crosby M, Chang D, Armer J. The Surgical Treatment of Lymphedema:A Systematic Review of the Contemporary Literature (2004-2010) Ann Surg Oncol. 2012;19:642–51. doi: 10.1245/s10434-011-2017-4. [DOI] [PubMed] [Google Scholar]
- [14].Casley-Smith JR, Morgan RG, Piller NB. Treatment of Lymphedema of the Arms and Legs with 5,6-benzo-alpha.-pyrone. N Engl J Med. 1993;329:1158–63. doi: 10.1056/NEJM199310143291604. [DOI] [PubMed] [Google Scholar]
- [15].Loprinzi CL, Sloan J, Kugler J. Coumarin-induced Hepatotoxicity. J Clin Oncol. 1997;15:3167–8. doi: 10.1200/JCO.1997.15.9.3167. [DOI] [PubMed] [Google Scholar]
- [16].Paskett ED, Stark N. Lymphedema:Knowledge, Treatment, and Impact Among Breast Cancer Survivors. Breast J. 2000;6:373–8. doi: 10.1046/j.1524-4741.2000.99072.x. [DOI] [PubMed] [Google Scholar]
- [17].Rockson SG, Miller LT, Senie R, Brennan MJ, Casley-Smith JR, Földi E, et al. American Cancer Society Lymphedema Workshop. Workgroup III:Diagnosis and Management of Lymphedema. Cancer. 1998;83:2882–5. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2882::aid-cncr45>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- [18].Gloviczki P. Principles of Surgical Treatment of Chronic Lymphoedema. Int Angiol. 1999;18:42–6. [PubMed] [Google Scholar]
- [19].Weiss M, Baumeister RG, Hahn K. Post-therapeutic Lymphedema:Scintigraphy Before and after Autologous Lymph Vessel Transplantation:8 Years of Long-term follow-up. Clin Nucl Med. 2002;27:788–92. doi: 10.1097/00003072-200211000-00007. [DOI] [PubMed] [Google Scholar]
- [20].Lin CH, Ali R, Chen SC, Wallace C, Chang YC, Chen HC, et al. Vascularized Groin Lymph Node Transfer using the Wrist as a Recipient Site for Management of Postmastectomy Upper Extremity Lymphedema. Plastic Reconstruct Surg. 2009;123:1265–75. doi: 10.1097/PRS.0b013e31819e6529. [DOI] [PubMed] [Google Scholar]
- [21].Brorson H, Svensson H. Liposuction Combined with Controlled Compression Therapy Reduces Arm Lymphedema more Effectively than Controlled Compression Therapy Alone. Plast Reconstruct Surg. 1998;102:1058–67. [PubMed] [Google Scholar]
- [22].Hadamitzky C, Pabst R. Acquired Lymphedema:An Urgent Need for Adequate Animal Models. Cancer Res. 2008;68:343–5. doi: 10.1158/0008-5472.CAN-07-2454. [DOI] [PubMed] [Google Scholar]
- [23].Tilak SP, Howard JM. Regeneration and Autotransplantation of Lymph Nodes. Ann Surg. 1965;161:441–6. doi: 10.1097/00000658-196503000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shesol BF, Nakashima R, Alavi A, Hamilton RW. Successful Lymph Node Transplantation in Rats, with Restoration of Lymphatic Function. Plast Reconstruct Surg. 1979;63:817–23. [PubMed] [Google Scholar]
- [25].Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-analyses of Studies that Evaluate Healthcare Interventions:Explanation and Elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Frueh FS, Jelvani B, Scheuer C, Körbel C, Kim BS, Giovanoli P, et al. Short-term Molecular and Cellular Effects of Ischemia/Reperfusion on Vascularized Lymph Node Flaps in Rats. PLoS One. 2020;15:e0239517. doi: 10.1371/journal.pone.0239517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tinhofer IE, Yang CY, Chen C, Cheng MH. Impacts of Arterial Ischemia or Venous Occlusion on Vascularized Groin Lymph Nodes in a Rat Model. J Surg Oncol. 2020;121:153–62. doi: 10.1002/jso.25518. [DOI] [PubMed] [Google Scholar]
- [28].Perrault DP, Lee GK, Bouz A, Sung C, Yu R, Pourmoussa AJ, et al. Ischemia and Reperfusion Injury in Superficial Inferior Epigastric Artery-based Vascularized Lymph Node Flaps. PLoS One. 2020;15:e0227599. doi: 10.1371/journal.pone.0227599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yang CY, Ho OA, Cheng MH, Hsiao HY. Critical Ischemia Time, Perfusion, and Drainage Function of Vascularized Lymph Nodes. Plast Reconstruct Surg. 2018;142:688–97. doi: 10.1097/PRS.0000000000004673. [DOI] [PubMed] [Google Scholar]
- [30].Kwiecien GJ, Gharb BB, Tadisina KK, Madajka M, Drazba J, Zins JE, et al. Quantity of Lymph Nodes in the Vascularized Lymph Node Transfer Influences Its Lymphaticovenous Drainage. J Reconstruct Microsurg. 2018;34:41–6. doi: 10.1055/s-0037-1606320. [DOI] [PubMed] [Google Scholar]
- [31].Najjar M, Lopez MM, Jr, Ballestin A, Munabi N, Naides AI, Noland RD, et al. Reestablishment of Lymphatic Drainage after Vascularized Lymph Node Transfer in a Rat Model. Plast Reconstruct Surg. 2018;142:503e–8. doi: 10.1097/PRS.0000000000004760. [DOI] [PubMed] [Google Scholar]
- [32].Aydogdu IO, Demir A, Keles MK, Yapici O, Yildizy L, Demirtas Y. Pedicled Vascularized Lymph Node Transfer Treats Lymphedema in Rat Hind Limb:A Simple Experimental Study Design. Lymphology. 2017;50:27–35. [PubMed] [Google Scholar]
- [33].Nguyen DH, Chou PY, Hsieh YH, Momeni A, Fang YH, Patel KM, et al. Quantity of Lymph Nodes Correlates with Improvement in Lymphatic Drainage in Treatment of Hind Limb Lymphedema with Lymph Node Flap Transfer in Rats. Microsurgery. 2016;36:239–45. doi: 10.1002/micr.22388. [DOI] [PubMed] [Google Scholar]
- [34].Ito R, Zelken J, Yang CY, Lin CY, Cheng MH. Proposed Pathway and Mechanism of Vascularized Lymph Node Flaps. Gynecol Oncol. 2016;141:182–8. doi: 10.1016/j.ygyno.2016.01.007. [DOI] [PubMed] [Google Scholar]
- [35].Visconti G, Brunelli C, Mulè A, Franceschini G, Chen HC, Masetti R, et al. Septum-based Cervical Lymph-node Free Flap in Rat:A New Model. J Surg Res. 2016;201:1–12. doi: 10.1016/j.jss.2015.09.027. [DOI] [PubMed] [Google Scholar]
- [36].Visconti G, Constantinescu T, Chen PY, Salgarello M, Franceschini G, Masetti R, et al. The Venous Lymph Node Flap:Concepts, Experimental Evidence, and Potential Clinical Implications. J Reconstruct Microsurg. 2016;32:625–31. doi: 10.1055/s-0036-1584527. [DOI] [PubMed] [Google Scholar]
- [37].Kwiecien GJ, Uygur S, Korn J, Gharb BB, Madajka M, Djohan R, et al. Vascularized Axillary Lymph Node Transfer:A Novel Model in the Rat. Microsurgery. 2015;35:662–7. doi: 10.1002/micr.22472. [DOI] [PubMed] [Google Scholar]
- [38].Schindewolffs L, Breves G, Buettner M, Hadamitzky C, Pabst R. Vegf-c Improves Regeneration and Lymphatic Reconnection of Transplanted Autologous Lymph Node Fragments:An Animal Model for Secondary Lymphedema Treatment. Immunity Inflamm Dis. 2014;2:152–61. doi: 10.1002/iid3.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cheng MH, Huang JJ, Wu CW, Yang CY, Lin CY, Henry SL, et al. The Mechanism of Vascularized Lymph Node Transfer for Lymphedema:Natural Lymphaticovenous Drainage. Plast Reconstruct Surg. 2014;133:192e–8. doi: 10.1097/01.prs.0000437257.78327.5b. [DOI] [PubMed] [Google Scholar]
- [40].Uygur S, Ozturk C, Bozkurt M, Kwiecien G, Madajka M, Siemionow M. A New Vascularized Cervical Lymph Node Transplantation Model:An Anatomic Study in Rats. Ann Plast Surg. 2013;71:671–4. doi: 10.1097/01.sap.0000438013.15453.8c. [DOI] [PubMed] [Google Scholar]
- [41].Sommer T, Buettner M, Bruns F, Breves G, Hadamitzky C, Pabst R. Improved Regeneration of Autologous Transplanted Lymph Node Fragments by VEGF-C Treatment. Anat Rec (Hoboken) 2012;295:786–91. doi: 10.1002/ar.22438. [DOI] [PubMed] [Google Scholar]
- [42].Hadamitzky C, Blum KS, Pabst R. Regeneration of Autotransplanted Avascular Lymph Nodes in the Rat is Improved by Platelet-rich Plasma. J Vasc Res. 2009;46:389–96. doi: 10.1159/000194269. [DOI] [PubMed] [Google Scholar]
- [43].Ishikawa K, Funayama E, Maeda T, Hayashi T, Murao N, Osawa M, et al. Changes in High Endothelial Venules in Lymph Nodes after Vascularized and Nonvascularized Lymph Node Transfer in a Murine Autograft Model. J Surg Oncol. 2019;119:700–7. doi: 10.1002/jso.25365. [DOI] [PubMed] [Google Scholar]
- [44].Ishikawa K, Maeda T, Funayama E, Hayashi T, Murao N, Osawa M, et al. Feasibility of Pedicled Vascularized Inguinal Lymph Node Transfer in a Mouse Model:A Preliminary Study. Microsurgery. 2019;39:247–54. doi: 10.1002/micr.30394. [DOI] [PubMed] [Google Scholar]
- [45].Maeda T, Yamamoto Y, Iwasaki D, Hayashi T, Funayama E, Oyama A, et al. Lymphatic Reconnection and Restoration of Lymphatic Flow by Nonvascularized Lymph Node Transplantation:Real-Time Fluorescence Imaging Using Indocyanine Green and Fluorescein Isothiocyanate-dextran. Lymphatic Res Biol. 2018;16:165–73. doi: 10.1089/lrb.2016.0070. [DOI] [PubMed] [Google Scholar]
- [46].Hayashida K, Yoshida S, Yoshimoto H, Fujioka M, Saijo H, Migita K, et al. Adipose-derived Stem Cells and Vascularized Lymph Node Transfers Successfully Treat Mouse Hindlimb Secondary Lymphedema by Early Reconnection of the Lymphatic System and Lymphangiogenesis. Plast Reconstruct Surg. 2017;139:639–51. doi: 10.1097/PRS.0000000000003110. [DOI] [PubMed] [Google Scholar]
- [47].Huang JJ, Gardenier JC, Hespe GE, Nores GD, Kataru RP, Ly CL, et al. Lymph Node Transplantation Decreases Swelling and Restores Immune Responses in a Transgenic Model of Lymphedema. PLoS One. 2016;11:e0168259. doi: 10.1371/journal.pone.0168259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shioya R, Furukawa H, Murao N, Hayashi T, Oyama A, Funayama E, et al. Prevention of Lymphedematous Change in the Mouse Hindlimb by Nonvascularized Lymph Node Transplantation. Ann Plast Surg. 2016;76:442–5. doi: 10.1097/SAP.0000000000000428. [DOI] [PubMed] [Google Scholar]
- [49].Tervala TV, Hartiala P, Tammela T, Visuri MT, Ylä-Herttuala S, Alitalo K, et al. Growth Factor Therapy and Lymph Node Graft for Lymphedema. J Surg Res. 2015;196:200–7. doi: 10.1016/j.jss.2015.02.031. [DOI] [PubMed] [Google Scholar]
- [50].Tammela T, Saaristo A, Holopainen T, Lyytikkä J, Kotronen A, Pitkonen M, et al. Therapeutic Differentiation and Maturation of Lymphatic Vessels after Lymph Node Dissection and Transplantation. Nat Med. 2007;13:1458–66. doi: 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- [51].Suami H, Scaglioni MF, Dixon KA, Tailor RC. Interaction between Vascularized Lymph Node Transfer and Recipient Lymphatics after Lymph Node Dissection-a Pilot Study in a Canine Model. J Surg Res. 2016;204:418–27. doi: 10.1016/j.jss.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Chen HC, O'Brien BM, Rogers IW, Pribaz JJ, Eaton CJ. Lymph Node Transfer for the Treatment of Obstructive Lymphoedema in the Canine Model. Br J Plast Surg. 1990;43:578–86. doi: 10.1016/0007-1226(90)90123-h. [DOI] [PubMed] [Google Scholar]
- [53].Hadamitzky C, Peric H, Theobald SJ, Gratz KF, Spohr H, Pabst R, et al. Effect of Cryopreservation on Lymph Node Fragment Regeneration after Autologous Transplantation in the Minipig Model. Innov Surg Sci. 2018;3:139–46. doi: 10.1515/iss-2018-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Blum KS, Hadamitzky C, Gratz KF, Pabst R. Effects of Autotransplanted Lymph Node Fragments on the Lymphatic System in the Pig Model. Breast Cancer Res Treat. 2010;120:59–66. doi: 10.1007/s10549-009-0367-4. [DOI] [PubMed] [Google Scholar]
- [55].Pabst R, Rothkötter HJ. Regeneration of Autotransplanted Lymph Node Fragments. Cell Tissue Res. 1988;251:597–601. doi: 10.1007/BF00214008. [DOI] [PubMed] [Google Scholar]
- [56].Lähteenvuo M, Honkonen K, Tervala T, Tammela T, Suominen E, Lähteenvuo J, et al. Growth Factor Therapy and Autologous Lymph Node Transfer in Lymphedema. Circulation. 2011;123:613–20. doi: 10.1161/CIRCULATIONAHA.110.965384. [DOI] [PubMed] [Google Scholar]
- [57].Penuela RF, Arazo LC, Ayala JM. Outcomes in Vascularized Lymph Node Transplantation in Rabbits:A Reliable Model for Improving the Surgical Approach to Lymphedema. Lymphat Res Biol. 2019;17:413–7. doi: 10.1089/lrb.2018.0038. [DOI] [PubMed] [Google Scholar]
- [58].Tobbia D, Semple J, Baker A, Dumont D, Johnston M. Experimental Assessment of Autologous Lymph Node Transplantation as Treatment of Postsurgical Lymphedema. Plast Reconstruct Surg. 2009;124:777–86. doi: 10.1097/PRS.0b013e3181b03787. [DOI] [PubMed] [Google Scholar]
- [59].Frueh FS, Gousopoulos E, Rezaeian F, Menger MD, Lindenblatt N, Giovanoli P. Animal Models in Surgical Lymphedema Research--a Systematic Review. J Surg Res. 2016;200:208–20. doi: 10.1016/j.jss.2015.07.005. [DOI] [PubMed] [Google Scholar]
- [60].Homans J, Drinker CK, Field M. Elephantiasis and the Clinical Implications of its Experimental Reproduction in Animals. Ann Surg. 1934;100:812–32. doi: 10.1097/00000658-193410000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hayes SC, Johansson K, Stout NL, Prosnitz R, Armer JM, Gabram S, et al. Upper-body Morbidity after Breast Cancer:Incidence and Evidence for Evaluation, Prevention, and Management within a Prospective Surveillance Model of Care. Cancer. 2012;118:2237–49. doi: 10.1002/cncr.27467. [DOI] [PubMed] [Google Scholar]
- [62].McLaughlin SA, Wright MJ, Morris KT, Giron GL, Sampson MR, Brockway JP, et al. Prevalence of Lymphedema in Women with Breast Cancer 5 Years after Sentinel Lymph Node Biopsy or Axillary Dissection:Objective Measurements. J Clin Oncol. 2008;26:5213–9. doi: 10.1200/JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Jun H, Lee JY, Kim JH, Noh M, Kwon TW, Cho YP, et al. Modified Mouse Models of Chronic Secondary Lymphedema:Tail and Hind Limb Models. Ann Vasc Surg. 2017;43:288–95. doi: 10.1016/j.avsg.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tammela T, Alitalo K. Lymphangiogenesis:Molecular Mechanisms and Future Promise. Cell. 2010;140:460–76. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- [65].Ferrara N, Kerbel RS. Angiogenesis as a Therapeutic Target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- [66].Stacker SA, Stenvers K, Caesar C, Vitali A, Domagala T, Nice E, et al. Biosynthesis of vascular endothelial growth factor-D involves proteolytic processing which generates non-covalent homodimers. J Biol Chem. 1999;274:32127–36. doi: 10.1074/jbc.274.45.32127. [DOI] [PubMed] [Google Scholar]
- [67].Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, et al. Proteolytic Processing Regulates Receptor Specificity and Activity of VEGF-C. EMBO J. 1997;16:3898–911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Scavelli C, Weber E, Aglianò M, Cirulli T, Nico B, Vacca A, et al. Lymphatics at the crossroads of angiogenesis and lymphangiogenesis. J Anat. 2004;204:433–49. doi: 10.1111/j.0021-8782.2004.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Mäkinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, et al. Inhibition of Lymphangiogenesis with Resulting Lymphedema in Transgenic Mice Expressing Soluble VEGF Receptor-3. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- [70].van den Dolder J, Mooren R, Vloon AP, Stoelinga PJ, Jansen JA. Platelet-rich Plasma:Quantification of Growth Factor Levels and the Effect on Growth and Differentiation of Rat Bone Marrow Cells. Tissue Eng. 2006;12:3067–73. doi: 10.1089/ten.2006.12.3067. [DOI] [PubMed] [Google Scholar]
- [71].Stacker SA, Farnsworth RH, Karnezis T, Shayan R, Smith DP, Paavonen K, et al. Molecular Pathways for Lymphangiogenesis and their Role in Human Disease. Novartis Found Symp. 2007;281:38–43. doi: 10.1002/9780470062128.ch4. [DOI] [PubMed] [Google Scholar]
- [72].Gousopoulos E, Proulx ST, Bachmann SB, Scholl J, Dionyssiou D, Demiri E, et al. Regulatory T Cell Transfer Ameliorates Lymphedema and Promotes Lymphatic Vessel Function. JCI Insight. 2016;1:e89081. doi: 10.1172/jci.insight.89081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Shimizu Y, Shibata R, Shintani S, Ishii M, Murohara T. Therapeutic Lymphangiogenesis with Implantation of Adipose-derived Regenerative Cells. J Am Heart Assoc. 2012;1:e000877. doi: 10.1161/JAHA.112.000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Dai T, Jiang Z, Cui C, Sun Y, Lu B, Li H, et al. The Roles of Podoplanin-Positive/Podoplanin-Negative Cells from Adipose-Derived Stem Cells in Lymphatic Regeneration. Plastic Reconstruct Surg. 2020;145:420–31. doi: 10.1097/PRS.0000000000006474. [DOI] [PubMed] [Google Scholar]
- [75].Kerrigan CL, Stotland MA. Ischemia Reperfusion Injury:A Review. Microsurgery. 1993;14:165–75. doi: 10.1002/micr.1920140307. [DOI] [PubMed] [Google Scholar]
- [76].Kerrigan CL, Daniel RK. Critical Ischemia Time and the Failing Skin Flap. Plastic Reconstruct Surg. 1982;69:986–9. doi: 10.1097/00006534-198206000-00014. [DOI] [PubMed] [Google Scholar]
- [77].Siemionow M, Arslan E. Ischemia/Reperfusion Injury:A Review in Relation to Free Tissue Transfers. Microsurgery. 2004;24:468–75. doi: 10.1002/micr.20060. [DOI] [PubMed] [Google Scholar]
- [78].Suami H, Shin D, Chang DW. Mapping of Lymphosomes in the Canine Forelimb:Comparative Anatomy between Canines and Humans. Plastic Reconstruct Surg. 2012;129:612–20. doi: 10.1097/PRS.0b013e3182402c6d. [DOI] [PubMed] [Google Scholar]
- [79].Tan JK, Watanabe T. Artificial Engineering of Secondary Lymphoid Organs. Adv Immunol. 2010;105:131–57. doi: 10.1016/S0065-2776(10)05005-4. [DOI] [PubMed] [Google Scholar]


