Four novel piperazinium salts are reported, and based on 1-phenylpiperazinium as a common cation in the asymmetric units that include additionally water molecules, and different P-substituent benzoate anions or a trifluoroacetate anion. They are hydrated and there are three crystallized as 1:1 salts while the fourth is a 2:2 salt. Their crystal packing depends on strong ribbons or sheets stabilized by hydrogen bonds of type N—H⋯O and O—H⋯O and other interactions as C—H⋯O, C—H⋯π and C—H⋯F in the trifluoroacetate-based one.
Keywords: crystal structure, hydrogen bonding, piperazine, biological activity
Abstract
In this study, four new piperazinium salts, namely, 4-phenylpiperazin-1-ium 4-ethoxybenzoate monohydrate, C9H9O3·C10H15N2·H2O (I); 4-phenylpiperazin-1-ium 4-methoxybenzoate monohydrate, C10H15N2·C8H7O3·H2O (II); 4-phenylpiperazin-1-ium 4-methylbenzoate monohydrate, C10H15N2·C8H7O2·H2O (III); and 4-phenylpiperazin-1-ium trifluoroacetate 0.12 hydrate, C10H15N2·C2F3O2·0.12H2O (IV), have been synthesized. The single-crystal structures of these compounds reveal that all of them crystallize in the triclinic P
 space group and the crystal packing of (I)–(III) is built up of ribbons formed by a combination of hydrogen bonds of type N—H⋯O, O—H⋯O and other weak interactions of type C—H⋯O and C—H⋯π, leading to a three-dimensional network. In the crystal of (IV), the cations and the anions are connected by C—H⋯O, N—H⋯O and C—H⋯F hydrogen bonds and by C—H⋯π interactions, forming sheets which in turn interact to maintain the crystal structure by linking through the oxygen atoms of water molecules and van der Waals interactions, giving the whole structure.
space group and the crystal packing of (I)–(III) is built up of ribbons formed by a combination of hydrogen bonds of type N—H⋯O, O—H⋯O and other weak interactions of type C—H⋯O and C—H⋯π, leading to a three-dimensional network. In the crystal of (IV), the cations and the anions are connected by C—H⋯O, N—H⋯O and C—H⋯F hydrogen bonds and by C—H⋯π interactions, forming sheets which in turn interact to maintain the crystal structure by linking through the oxygen atoms of water molecules and van der Waals interactions, giving the whole structure.
1. Chemical context
Piperazines are among the most important building blocks in today’s drug discovery efforts and are found in biologically active compounds across a number of different therapeutic areas (Brockunier et al., 2004 ▸; Bogatcheva et al., 2006 ▸). For a review on the current pharmacological and toxicological information for piperazine derivative, see Elliott (2011 ▸). Various pharmacological properties of phenylpiperazines and their derivatives have been discussed by several authors (Cohen et al., 1982 ▸; Conrado et al., 2010 ▸; Neves et al., 2003 ▸; Hanano et al., 2000 ▸). The design and synthesis of phenylpiperazine derivatives as potent anticancer agents for prostate cancer have been described (Demirci et al., 2019 ▸). Many pharmaceutical compounds are derived from 1-phenylpiperazine, viz., oxypertine, trazodone, nefazodone, etc.
The crystal structures of 2-(4-methyl-2-phenylpiperazin-4-ium-1-yl)pyridine-3-carboxylate dehydrate (Li et al., 2008 ▸), 1-chloro-2-(4-phenylpiperazin-1-yl)-ethanone (Xu & Jing, 2009 ▸), 4-phenylpiperazin-1-ium dihydrogen phosphate (Essid et al., 2010 ▸) and 1-phenylpiperazine-1,4-diium bis(hydrogen sulfate) (Marouani et al., 2010 ▸) have been reported, as have those of 4-phenylpiperazin-1-ium 6-chloro-5-ethyl-2,4-dioxopyrimidin-1-ide and 4-phenylpiperazin-1-ium 6-chloro-5-isopropyl-2,4-dioxopyrimidin-1-ide (Al-Alshaikh et al., 2015 ▸). We have reported the crystal structures of some salts of 4-methoxyphenylpiperazine (Kiran Kumar et al., 2019a
▸), six 1-aroyl-4-(4-methoxyphenyl)piperazines (Kiran Kumar et al., 2019b
▸), 2-methoxyphenylpiperazine (Harish Chinthal et al., 2020 ▸) and the recreational drug N-(4-methoxyphenyl)piperazine (MeOPP) and three of its salts (Kiran Kumar et al., 2020a
▸).

In view of the importance of piperazines in general and the use of 1-phenylpiperazine in particular, the present paper reports the crystal structure studies of some salts of 1-phenylpiperazine with organic acids viz., 4-phenylpiperazin-1-ium 4-ethoxybenzoate monohydrate, C9H9O3·C10H15N2·H2O (I); 4-phenylpiperazin-1-ium 4-methoxybenzoate monohydrate, C10H15N2·C8H7O3·H2O (II); 4-phenylpiperazin-1-ium 4-methylbenzoate monohydrate, C10H15N2·C8H7O2·H2O (III); and 4-phenylpiperazin-1-ium trifluoroacetate 0.12 hydrate C10H15N2·C2F3O2·0.12H2O (IV).
2. Structural commentary
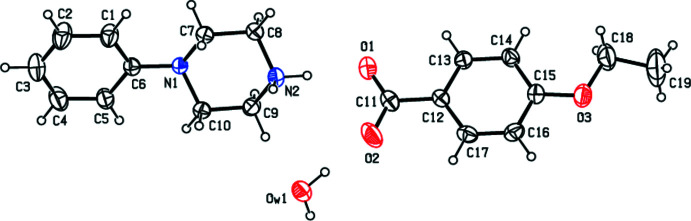
The asymmetric unit of the compound (I), (Fig. 1 ▸), consists of a 4-phenylpiperazin-1-ium cation, a 4-ethoxybenzoate anion and one water molecule. The aromatic ring of the cation is essentially planar while the protonated piperazine ring adopts a chair conformation, with puckering parameters (Cremer & Pople, 1975 ▸) Q T = 0.553 (2) Å, θ = 175.0 (2)° and φ = 15 (3)°. In compound (II) the asymmetric unit (Fig. 2 ▸) comprises a 4-phenylpiperazin-1-ium cation, a 4-methoxybenzoate anion and one water molecule. The aromatic ring of the cation is essentially planar while the protonated piperazine ring adopts a chair conformation, with puckering parameters Q T = 0.5614 (18) Å, θ = 175.89 (17)° and φ = 346 (3)°. Compound (III) presents an asymmetric unit (Fig. 3 ▸) composed of a 4-phenylpiperazin-1-ium cation, a 4-methylbenzoate anion and one water molecule. The aromatic ring of the cation is essentially planar but the protonated piperazine ring adopts a distorted chair conformation, with puckering parameters Q T = 0.5486 (19) Å, θ = 9.38 (19)° and φ = 167.9 (13)°. On the other hand, the asymmetric unit of (IV) (Fig. 4 ▸) contains two 4-phenylpiperazin-1-ium cations (A1 with N1, A2 with N3) and two trifluoroacetate anions (B1 with F1, B2 with F4) and a 0.12 occupancy water molecule. The aromatic rings of the cations (A1, A2) are essentially planar while the protonated piperazine rings adopt a chair conformation for cation A1, with puckering parameters (Cremer & Pople, 1975 ▸) Q T = 0.552 (4) Å, θ = 0.0 (4)° and φ = 207 (14)°, and a distorted chair conformation for the cation A2, with puckering parameters Q T = 0.559 (5) Å, θ = 6.6 (4)° and φ = 168 (4)°.
Figure 1.
The independent components of compound (I) showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Figure 2.
The independent components of compound (II) showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Figure 3.
The independent components of compound (III) showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Figure 4.
The independent components of compound (IV) showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level. (Atom splitting is omitted for clarity.)
3. Supramolecular features
In the crystal structure of (I), the cation pairs are connected across two water molecules by C—H⋯O and N—H⋯O hydrogen bonds, forming an
 (10) ring motif in which the anions and cations are linked through the water molecules by O—H⋯O and N—H⋯O hydrogen bonds, forming ribbons along the a-axis direction (Table 1 ▸, Fig. 5 ▸
a). In addition, a set of C—H⋯π interactions, through the benzene rings of the anions and the cations, connect the molecules together in ribbons along the a-axis direction (Table 1 ▸, Fig. 5 ▸
b). The C—H⋯O, N—H⋯O, O—H⋯O hydrogen bonds and C—H⋯π interactions together form a three-dimensional network, contributing to the stabilization of the crystal structure.
(10) ring motif in which the anions and cations are linked through the water molecules by O—H⋯O and N—H⋯O hydrogen bonds, forming ribbons along the a-axis direction (Table 1 ▸, Fig. 5 ▸
a). In addition, a set of C—H⋯π interactions, through the benzene rings of the anions and the cations, connect the molecules together in ribbons along the a-axis direction (Table 1 ▸, Fig. 5 ▸
b). The C—H⋯O, N—H⋯O, O—H⋯O hydrogen bonds and C—H⋯π interactions together form a three-dimensional network, contributing to the stabilization of the crystal structure.
Table 1. Hydrogen-bond geometry (Å, °) for (I) .
Cg1 and Cg3 are the centroids of the C12–C17 and C1–C6 benzene rings, respectively.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—HN1⋯OW1i | 0.89 (2) | 1.94 (2) | 2.817 (3) | 167 (2) |
| N2—HN2⋯O1 | 0.93 (2) | 1.80 (2) | 2.724 (3) | 174 (2) |
| OW1—HW1⋯O2 | 0.88 (3) | 1.75 (3) | 2.630 (3) | 178 (4) |
| OW1—HW2⋯O1ii | 0.91 (3) | 1.89 (3) | 2.789 (3) | 167 (3) |
| C9—H9A⋯OW1 | 0.97 | 2.52 | 3.308 (3) | 138 |
| C1—H1⋯Cg1iii | 0.93 | 2.91 | 3.607 (3) | 133 |
| C5—H5⋯Cg1iv | 0.93 | 2.79 | 3.570 (3) | 142 |
| C18—H18B⋯Cg3v | 0.97 | 2.88 | 3.737 (4) | 148 |
Symmetry codes: (i)
 ; (ii)
; (ii)
 ; (iii)
; (iii)
 ; (iv)
; (iv)
 ; (v)
; (v)
 .
.
Figure 5.
Parts of the crystal structure of compound (I) showing (a) the formation of a cyclic hydrogen-bonded
 (10) aggregate and (b) a general view of C—H⋯π interactions parallel to [100]. Hydrogen bonds and C—H⋯π interactions are drawn as dashed lines.
(10) aggregate and (b) a general view of C—H⋯π interactions parallel to [100]. Hydrogen bonds and C—H⋯π interactions are drawn as dashed lines.
In the crystal structure of (II), the cations, the anions and the water molecules are connected by C—H⋯O, N—H⋯O and O—H⋯O hydrogen bonds, forming ribbons along the a-axis direction (Table 2 ▸, Fig. 6 ▸ a). Furthermore, the cations interact via C—-H⋯π interactions through the benzene ring of the anion, forming ribbons along the b-axis direction (Table 2 ▸, Fig. 6 ▸ b). The C—H⋯O, N—H⋯O, O—H⋯O hydrogen bonds and C—H⋯π interactions together form a three-dimensional network, contributing to the stabilization of the crystal structure.
Table 2. Hydrogen-bond geometry (Å, °) for (II) .
Cg3 is the centroid of the C12–C17 benzene ring.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—HN1⋯OW1i | 0.93 (2) | 1.91 (2) | 2.815 (2) | 166 (2) |
| OW1—HW1⋯O1ii | 0.84 (2) | 1.80 (2) | 2.633 (2) | 175 (2) |
| N2—HN2⋯O2 | 0.93 (2) | 1.81 (2) | 2.7350 (19) | 176 (2) |
| OW1—HW2⋯O2iii | 0.85 (2) | 1.96 (2) | 2.7876 (19) | 168 (2) |
| C8—H8B⋯OW1ii | 0.97 | 2.53 | 3.331 (2) | 140 |
| C1—H1⋯Cg3ii | 0.93 | 2.76 | 3.549 (2) | 144 |
| C5—H5⋯Cg3iv | 0.93 | 2.86 | 3.625 (2) | 140 |
Symmetry codes: (i)
 ; (ii)
; (ii)
 ; (iii)
; (iii)
 ; (iv)
; (iv)
 .
.
Figure 6.
Parts of the crystal structure of compound (II) showing (a) the formation of hydrogen-bonded ribbons parallel to [010] and (b) a general view of the C—H⋯π interactions parallel to [010]. Hydrogen bonds and C—H⋯π interactions are drawn as dashed lines.
In the crystal structure of (III), the cations, the anions and the water molecules are connected by C—H⋯O, N—H⋯O and O—H⋯O hydrogen bonds, forming ribbons along the a-axis direction (Table 3 ▸, Fig. 7 ▸). There are no C—H⋯π interactions or π-π stacking interactions. The crystal structure is stabilized by C—H⋯O, N—H⋯O, O—H⋯O hydrogen bonds and van der Waals interactions between the ribbons, which run along the a-axis direction.
Table 3. Hydrogen-bond geometry (Å, °) for (III) .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| OW1—HW1⋯O2i | 0.89 (3) | 1.90 (3) | 2.782 (2) | 171 (4) |
| OW1—HW2⋯O1 | 0.84 (2) | 1.92 (3) | 2.751 (2) | 172 (3) |
| N2—HN1⋯O1ii | 0.90 (2) | 1.94 (2) | 2.819 (2) | 164 (2) |
| N2—HN2⋯O2 | 0.92 (2) | 1.80 (2) | 2.7207 (19) | 176 (2) |
| C8—H8A⋯OW1 | 0.97 | 2.33 | 3.116 (3) | 138 |
Symmetry codes: (i)
 ; (ii)
; (ii)
 .
.
Figure 7.
Part of the crystal structure of compound (III) showing the formation of a hydrogen-bonded chain of rings parallel to [001]. Hydrogen bonds are drawn as dashed lines.
In the crystal structure of (IV), the cations and the anions are connected by C—H⋯O, N—H⋯O and C—H⋯F hydrogen bonds (Table 4 ▸, Fig. 8 ▸ a) and C—H⋯π interactions, generating sheets parallel to the (100) plane (Table 4 ▸, Fig. 8 ▸). These sheets further interact to maintain the crystal structure by linking through the oxygen atoms of water molecules and by van der Waals interactions. As shown in Table 4 ▸, the main interactions in the structure of (IV) involve the oxygen atoms of carboxylate groups, while the 0.12 fraction of the water molecule contributes with one interaction of the type C—H⋯O and it is weak in comparison to the other oxygen-based ones.
Table 4. Hydrogen-bond geometry (Å, °) for (IV) .
Cg2 is the centroid of the C1–C6 phenyl ring.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H21⋯O1i | 0.88 | 1.91 | 2.790 (4) | 174 |
| N2—H22⋯O3 | 0.87 | 2.04 | 2.860 (4) | 157 |
| N2—H22⋯O4 | 0.87 | 2.47 | 3.164 (5) | 137 |
| N4—H41⋯O4ii | 0.86 | 1.95 | 2.759 (6) | 156 |
| N4—H42⋯O2iii | 0.89 | 1.90 | 2.758 (4) | 164 |
| C18—H18A⋯F5′iii | 0.97 | 2.53 | 3.273 (18) | 134 |
| C18—H18B⋯Ow1 | 0.97 | 2.08 | 2.929 (15) | 145 |
| C19—H19B⋯O3iv | 0.97 | 2.59 | 3.420 (5) | 144 |
| C20—H20A⋯F5iv | 0.97 | 2.64 | 3.468 (8) | 144 |
| C16—H16⋯Cg2v | 0.93 | 2.99 | 3.745 (4) | 140 |
Symmetry codes: (i)
 ; (ii)
; (ii)
 ; (iii)
; (iii)
 ; (iv)
; (iv)
 ; (v)
; (v)
 .
.
Figure 8.
Parts of the crystal structure of compound (IV) showing (a) a general view of the C—H⋯O, N—H⋯O and C—H⋯F hydrogen bonds and C—H⋯π interactions and (b) the molecular packing of (IV) down the a-axis. Hydrogen bonds and C—H⋯π interactions are drawn as dashed lines.
4. Database survey
A search of the Cambridge Structural Database (Version 2020.3, last update February 2022; Groom et al., 2016 ▸) for an unsubstituted 4-phenylpiperazin-1-ium cation and para-substituted benzoate anion involved in the reported salts (I)–(III) gave no hits. However, searching for a branched phenyl piperazinium cation and para-substituted benzoate anion gave comparable hits, namely; 4-(4-methoxyphenyl)piperazin-1-ium 4-fluorobenzoate monohydrate, 4-(4-methoxyphenyl)piperazin-1-ium 4-chlorobenzoate monohydrate, 4-(4-methoxyphenyl)piperazin-1-ium 4-bromobenzoate monohydrate (FOVPOY, FOVPUE, FOVQAL; Kiran Kumar et al., 2019a ▸), 4-(4-methoxyphenyl)piperazin-1-ium 4-iodobenzoate monohydrate (KUJPUD; Kiran Kumar et al., 2020b ▸). They exhibit a methoxy group as a substituent in the 4-phenylpiperazin-1-ium cation while the reported compounds (I)–(IV) have no substituent. They also crystallize as monohydrates, and their crystal structures are based on differently sized chains of rings formed via a combination of hydrogen bonds of the type N–H⋯O and O–H⋯O and other weak interactions of types C—H⋯O and C—H⋯π to form sheets. In 4-(4-methoxyphenyl)piperazin-1-ium 4-aminobenzoate monohydrate (IHIMEU; Kiran Kumar et al., 2020a ▸) the presence of the amino substituent in the anion, which acts as both a donor and as an acceptor of hydrogen bonds, makes the supramolecular assembly of this compound more complex than those reported here. A search for 4-phenylpiperazin-1-ium and acetate derivatives involved in the reported compound (IV) gave no hits.
5. Synthesis and crystallization
For the synthesis of salts (I)–(IV), a solution of commercially available (from Sigma–Aldrich) 1-phenylpiperazine (100 mg, 0.62 mol) in methanol (10 ml) was mixed with equimolar solutions of the appropriate organic acids in methanol (10 ml) viz., 4-ethoxybenzoic acid (103 mg, 0.62 mol) for (I), 4-methoxybenzoic acid (94 mg, 0.62 mol) for (II), 4-methylbenzoic acid (84 mg, 0.62 mol) for (III) and trifluoroacetic acid (71 mg, 0.62 mol) for (IV). The corresponding solutions were stirred for 15 min at room temperature and allowed to stand at the same temperature. X-ray quality crystals were formed on slow evaporation in a week for all compounds, where ethanol:ethylacetate (1:1) was used for crystallization. The corresponding melting points were 353–355 K for (I), 368–370 K for (II), 338–340 K for (III) and 385–387 K for (IV).
6. Refinement
Crystal data, data collection and structure refinement details are summarized in Table 5 ▸. All H atoms bonded to C atoms were fixed geometrically and treated as riding with C—H = 0.93 Å (aromatic), C—H = 0.96 Å (methyl) or 0.97 Å (methylene), with U iso(H) = 1.2U eq(C) or 1.5U eq(C). For the H atoms bonded to the N and O atoms, the atomic coordinates were refined with U iso(H) = 1.2U eq(N) and 1.5U eq(O), [for (I), N2—HN2 = 0.931 (19), N2—HN1 = 0.888 (17) Å and OW1—HW2 = 0.91 (3), OW1—HW1 = 0.88 (3) Å; for (II), N2—HN1 = 0.927 (16), N2—HN2 = 0.931 (18) Å and OW—HW1 = 0.840 (19), OW1—HW2 = 0.85 (2) Å; for (III), N2—HN1 = 0.900 (16), N2—HN2 = 0.918 (17) Å and OW1—HW1 = 0.89 (3), OW1—HW2 = 0.84 (2) Å and for (IV), N2—H22 = 0.87 (2) and N2—H21 = 0.88 (3) Å]. In (IV), the atoms of the CF3 groups of two trifluoroacetate anions (B1, B2) are disordered over two sets of sites with site occupancies of 0.737 (3) and 0.263 (3). The corresponding bond distances in the disordered groups were restrained to be equal. The U ij components of these atoms were restrained to be equal and were restrained to approximate isotropic behaviour. The OW1 water molecule was refined with a resulting occupation factor of 0.245 (10) and the H atoms of the water molecule were placed geometrically.
Table 5. Experimental details.
| (I) | (II) | (III) | (IV) | |
|---|---|---|---|---|
| Crystal data | ||||
| Chemical formula | C10H15N2 +·C9H9O3 −·H2O | C10H15N2 +·C8H7O3 −·H2O | C10H15N2 +·C8H7O2 −·H2O | C10H15N2 +·C2F3O2 −·0.123H2O |
| M r | 346.42 | 332.39 | 316.39 | 278.47 |
| Crystal system, space group | Triclinic, P

|
Triclinic, P

|
Triclinic, P

|
Triclinic, P

|
| Temperature (K) | 293 | 293 | 293 | 293 |
| a, b, c (Å) | 6.1635 (5), 7.5946 (6), 20.458 (2) | 6.2039 (4), 7.5565 (7), 18.614 (1) | 6.1175 (5), 7.6225 (7), 18.452 (1) | 9.6544 (6), 9.9029 (6), 15.2090 (9) |
| α, β, γ (°) | 79.545 (7), 86.521 (7), 83.791 (7) | 81.799 (7), 87.020 (7), 84.852 (7) | 97.421 (9), 90.403 (8), 92.405 (8) | 79.621 (6), 86.579 (6), 70.603 (6) |
| V (Å3) | 935.38 (14) | 859.53 (11) | 852.40 (12) | 1349.10 (15) |
| Z | 2 | 2 | 2 | 4 |
| Radiation type | Mo Kα | Mo Kα | Mo Kα | Mo Kα |
| μ (mm−1) | 0.09 | 0.09 | 0.08 | 0.12 |
| Crystal size (mm) | 0.48 × 0.42 × 0.1 | 0.48 × 0.48 × 0.32 | 0.5 × 0.4 × 0.08 | 0.48 × 0.48 × 0.36 |
| Data collection | ||||
| Diffractometer | Oxford Diffraction Xcalibur | Oxford Diffraction Xcalibur | Oxford Diffraction Xcalibur | Oxford Diffraction Xcalibur |
| Absorption correction | Multi-scan (CrysAlis RED; Oxford Diffraction, 2009 ▸) | Multi-scan (CrysAlis RED; Oxford Diffraction, 2009 ▸) | Multi-scan (CrysAlis RED; Oxford Diffraction, 2009 ▸) | Multi-scan (CrysAlis RED; Oxford Diffraction, 2009 ▸) |
| T min, T max | 0.623, 1.000 | 0.520, 1.000 | 0.837, 1.000 | 0.724, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 5989, 3429, 2159 | 5360, 3142, 2322 | 5354, 3126, 2248 | 9220, 4940, 2777 |
| R int | 0.022 | 0.016 | 0.013 | 0.014 |
| (sin θ/λ)max (Å−1) | 0.602 | 0.602 | 0.602 | 0.602 |
| Refinement | ||||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.054, 0.124, 1.08 | 0.045, 0.125, 1.06 | 0.046, 0.128, 1.03 | 0.070, 0.235, 1.07 |
| No. of reflections | 3424 | 3139 | 3118 | 4927 |
| No. of parameters | 244 | 230 | 226 | 375 |
| No. of restraints | 2 | 4 | 4 | 4 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.15, −0.15 | 0.2, −0.17 | 0.16, −0.16 | 0.42, −0.28 |
Supplementary Material
Crystal structure: contains datablock(s) global, I, II, III, IV. DOI: 10.1107/S2056989022006004/dj2048sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989022006004/dj2048Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989022006004/dj2048Isup6.cml
Structure factors: contains datablock(s) II. DOI: 10.1107/S2056989022006004/dj2048IIsup3.hkl
Supporting information file. DOI: 10.1107/S2056989022006004/dj2048IIsup7.cml
Structure factors: contains datablock(s) III. DOI: 10.1107/S2056989022006004/dj2048IIIsup4.hkl
Supporting information file. DOI: 10.1107/S2056989022006004/dj2048IIIsup8.cml
Structure factors: contains datablock(s) IV. DOI: 10.1107/S2056989022006004/dj2048IVsup5.hkl
Supporting information file. DOI: 10.1107/S2056989022006004/dj2048IVsup9.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
NM is grateful to the University of Mysore for research facilities. HSY thanks the UGC for a BSR Faculty fellowship for three years. SGG gratefully acknowledges financial support from the Spanish Ministerio de Ciencia e Innovación (PID2020–113558RB-C41) and Gobierno del Principado de Asturias (AYUD/2021/50997).
supplementary crystallographic information
4-Phenylpiperazin-1-ium 4-ethoxybenzoate monohydrate (I). Crystal data
| C10H15N2+·C9H9O3−·H2O | Z = 2 |
| Mr = 346.42 | F(000) = 372 |
| Triclinic, P1 | Dx = 1.23 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 6.1635 (5) Å | Cell parameters from 2352 reflections |
| b = 7.5946 (6) Å | θ = 3.0–27.8° |
| c = 20.458 (2) Å | µ = 0.09 mm−1 |
| α = 79.545 (7)° | T = 293 K |
| β = 86.521 (7)° | Plate, colourless |
| γ = 83.791 (7)° | 0.48 × 0.42 × 0.1 mm |
| V = 935.38 (14) Å3 |
4-Phenylpiperazin-1-ium 4-ethoxybenzoate monohydrate (I). Data collection
| Oxford Diffraction Xcalibur diffractometer | 2159 reflections with I > 2σ(I) |
| ω scans | Rint = 0.022 |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009) | θmax = 25.4°, θmin = 3.0° |
| Tmin = 0.623, Tmax = 1.000 | h = −7→7 |
| 5989 measured reflections | k = −9→9 |
| 3429 independent reflections | l = −24→14 |
4-Phenylpiperazin-1-ium 4-ethoxybenzoate monohydrate (I). Refinement
| Refinement on F2 | Secondary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.054 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.124 | w = 1/[σ2(Fo2) + (0.0392P)2 + 0.2859P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.08 | (Δ/σ)max < 0.001 |
| 3424 reflections | Δρmax = 0.15 e Å−3 |
| 244 parameters | Δρmin = −0.15 e Å−3 |
| 2 restraints | Extinction correction: SHELXL2018/3 (Sheldrick 2015b), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 constraints | Extinction coefficient: 0.0058 (17) |
| Primary atom site location: structure-invariant direct methods |
4-Phenylpiperazin-1-ium 4-ethoxybenzoate monohydrate (I). Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
4-Phenylpiperazin-1-ium 4-ethoxybenzoate monohydrate (I). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.1990 (4) | 0.7508 (4) | 0.79338 (12) | 0.0626 (7) | |
| H1 | 0.069252 | 0.809989 | 0.776125 | 0.075* | |
| C2 | 0.2437 (5) | 0.7539 (4) | 0.85869 (13) | 0.0782 (9) | |
| H2 | 0.142853 | 0.814295 | 0.884641 | 0.094* | |
| C3 | 0.4331 (5) | 0.6699 (4) | 0.88559 (14) | 0.0759 (9) | |
| H3 | 0.462163 | 0.672073 | 0.929504 | 0.091* | |
| C4 | 0.5786 (5) | 0.5827 (4) | 0.84658 (14) | 0.0753 (8) | |
| H4 | 0.709095 | 0.525999 | 0.864083 | 0.09* | |
| C5 | 0.5363 (4) | 0.5771 (3) | 0.78183 (12) | 0.0579 (7) | |
| H5 | 0.638106 | 0.515837 | 0.756445 | 0.069* | |
| C6 | 0.3432 (3) | 0.6615 (3) | 0.75355 (10) | 0.0407 (5) | |
| C7 | 0.1046 (3) | 0.7635 (3) | 0.66062 (11) | 0.0484 (6) | |
| H7A | 0.130663 | 0.888733 | 0.656771 | 0.058* | |
| H7B | −0.01969 | 0.742138 | 0.691398 | 0.058* | |
| C8 | 0.0502 (3) | 0.7292 (3) | 0.59343 (11) | 0.0496 (6) | |
| H8A | 0.005085 | 0.609001 | 0.59798 | 0.059* | |
| H8B | −0.07032 | 0.814458 | 0.576145 | 0.059* | |
| C9 | 0.4290 (3) | 0.6216 (3) | 0.57366 (10) | 0.0480 (6) | |
| H9A | 0.555062 | 0.634835 | 0.543053 | 0.058* | |
| H9B | 0.392361 | 0.498713 | 0.578625 | 0.058* | |
| C10 | 0.4839 (3) | 0.6594 (3) | 0.64012 (10) | 0.0447 (6) | |
| H10A | 0.602615 | 0.572985 | 0.657958 | 0.054* | |
| H10B | 0.5333 | 0.778458 | 0.634186 | 0.054* | |
| C11 | 0.2356 (4) | 0.7988 (3) | 0.37750 (13) | 0.0504 (6) | |
| C12 | 0.1740 (3) | 0.8093 (3) | 0.30715 (11) | 0.0409 (5) | |
| C13 | −0.0108 (3) | 0.7381 (3) | 0.29259 (11) | 0.0462 (6) | |
| H13 | −0.09987 | 0.685018 | 0.327326 | 0.055* | |
| C14 | −0.0669 (4) | 0.7436 (3) | 0.22762 (11) | 0.0496 (6) | |
| H14 | −0.191724 | 0.694349 | 0.218988 | 0.06* | |
| C15 | 0.0633 (4) | 0.8223 (3) | 0.17610 (11) | 0.0480 (6) | |
| C16 | 0.2462 (4) | 0.8988 (3) | 0.18948 (12) | 0.0547 (6) | |
| H16 | 0.332264 | 0.955074 | 0.154649 | 0.066* | |
| C17 | 0.3005 (4) | 0.8916 (3) | 0.25403 (12) | 0.0521 (6) | |
| H17 | 0.424178 | 0.942678 | 0.262421 | 0.063* | |
| C18 | −0.1614 (5) | 0.7562 (5) | 0.09410 (14) | 0.0931 (10) | |
| H18A | −0.293805 | 0.818359 | 0.110377 | 0.112* | |
| H18B | −0.155291 | 0.63023 | 0.114607 | 0.112* | |
| C19 | −0.1605 (8) | 0.7747 (7) | 0.02032 (17) | 0.178 (2) | |
| H19A | −0.195823 | 0.898767 | 0.00101 | 0.268* | |
| H19B | −0.266871 | 0.703796 | 0.008494 | 0.268* | |
| H19C | −0.018288 | 0.73364 | 0.003915 | 0.268* | |
| N1 | 0.2969 (3) | 0.6493 (2) | 0.68767 (8) | 0.0382 (4) | |
| N2 | 0.2420 (3) | 0.7474 (3) | 0.54621 (10) | 0.0452 (5) | |
| O1 | 0.1211 (3) | 0.7133 (2) | 0.42359 (8) | 0.0622 (5) | |
| O2 | 0.3970 (3) | 0.8727 (3) | 0.38724 (10) | 0.0885 (7) | |
| O3 | 0.0253 (3) | 0.8330 (2) | 0.11014 (8) | 0.0681 (5) | |
| OW1 | 0.7230 (4) | 0.8819 (2) | 0.46338 (9) | 0.0591 (5) | |
| HN1 | 0.275 (4) | 0.860 (2) | 0.5421 (11) | 0.056 (7)* | |
| HN2 | 0.208 (4) | 0.729 (3) | 0.5044 (9) | 0.067 (8)* | |
| HW1 | 0.612 (5) | 0.879 (4) | 0.4385 (15) | 0.102 (11)* | |
| HW2 | 0.842 (5) | 0.825 (4) | 0.4445 (15) | 0.102 (12)* |
4-Phenylpiperazin-1-ium 4-ethoxybenzoate monohydrate (I). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0719 (17) | 0.0673 (18) | 0.0465 (15) | 0.0151 (14) | −0.0066 (13) | −0.0156 (13) |
| C2 | 0.103 (2) | 0.079 (2) | 0.0522 (17) | 0.0150 (18) | −0.0020 (16) | −0.0248 (15) |
| C3 | 0.106 (2) | 0.076 (2) | 0.0510 (17) | −0.0055 (18) | −0.0223 (17) | −0.0207 (15) |
| C4 | 0.0735 (18) | 0.090 (2) | 0.0636 (18) | 0.0027 (16) | −0.0267 (15) | −0.0145 (17) |
| C5 | 0.0561 (15) | 0.0696 (18) | 0.0486 (15) | 0.0031 (13) | −0.0116 (12) | −0.0147 (13) |
| C6 | 0.0467 (12) | 0.0353 (13) | 0.0414 (13) | −0.0092 (10) | −0.0045 (10) | −0.0067 (10) |
| C7 | 0.0399 (12) | 0.0595 (16) | 0.0441 (13) | 0.0007 (11) | −0.0025 (10) | −0.0078 (11) |
| C8 | 0.0407 (12) | 0.0622 (16) | 0.0445 (14) | −0.0045 (11) | −0.0053 (10) | −0.0053 (11) |
| C9 | 0.0480 (13) | 0.0512 (15) | 0.0423 (13) | −0.0011 (11) | 0.0026 (10) | −0.0057 (11) |
| C10 | 0.0396 (12) | 0.0496 (14) | 0.0435 (13) | −0.0024 (10) | −0.0018 (10) | −0.0062 (11) |
| C11 | 0.0524 (14) | 0.0427 (14) | 0.0593 (17) | 0.0050 (12) | −0.0162 (13) | −0.0190 (12) |
| C12 | 0.0398 (12) | 0.0373 (13) | 0.0475 (13) | −0.0007 (10) | −0.0072 (10) | −0.0121 (10) |
| C13 | 0.0494 (13) | 0.0462 (14) | 0.0432 (13) | −0.0111 (11) | −0.0024 (11) | −0.0048 (11) |
| C14 | 0.0502 (13) | 0.0519 (15) | 0.0490 (14) | −0.0163 (11) | −0.0091 (11) | −0.0063 (12) |
| C15 | 0.0556 (14) | 0.0469 (15) | 0.0412 (14) | −0.0042 (11) | −0.0052 (11) | −0.0065 (11) |
| C16 | 0.0534 (14) | 0.0566 (16) | 0.0533 (16) | −0.0136 (12) | 0.0088 (12) | −0.0063 (12) |
| C17 | 0.0409 (12) | 0.0533 (16) | 0.0659 (17) | −0.0098 (11) | −0.0026 (12) | −0.0174 (13) |
| C18 | 0.111 (2) | 0.121 (3) | 0.0572 (18) | −0.046 (2) | −0.0218 (17) | −0.0148 (18) |
| C19 | 0.242 (6) | 0.251 (6) | 0.063 (2) | −0.137 (5) | −0.039 (3) | −0.007 (3) |
| N1 | 0.0374 (9) | 0.0419 (11) | 0.0350 (10) | −0.0028 (8) | −0.0021 (8) | −0.0062 (8) |
| N2 | 0.0548 (12) | 0.0428 (13) | 0.0390 (11) | −0.0088 (10) | −0.0079 (9) | −0.0058 (9) |
| O1 | 0.0706 (11) | 0.0703 (12) | 0.0465 (10) | −0.0048 (10) | −0.0127 (9) | −0.0108 (9) |
| O2 | 0.0835 (13) | 0.1109 (17) | 0.0825 (14) | −0.0368 (12) | −0.0321 (11) | −0.0215 (12) |
| O3 | 0.0816 (12) | 0.0792 (13) | 0.0450 (10) | −0.0182 (10) | −0.0052 (9) | −0.0080 (9) |
| OW1 | 0.0593 (11) | 0.0598 (12) | 0.0604 (12) | −0.0122 (10) | −0.0135 (10) | −0.0092 (9) |
4-Phenylpiperazin-1-ium 4-ethoxybenzoate monohydrate (I). Geometric parameters (Å, º)
| C1—C6 | 1.377 (3) | C11—O2 | 1.237 (3) |
| C1—C2 | 1.385 (3) | C11—O1 | 1.267 (3) |
| C1—H1 | 0.93 | C11—C12 | 1.497 (3) |
| C2—C3 | 1.365 (4) | C12—C13 | 1.383 (3) |
| C2—H2 | 0.93 | C12—C17 | 1.391 (3) |
| C3—C4 | 1.362 (4) | C13—C14 | 1.386 (3) |
| C3—H3 | 0.93 | C13—H13 | 0.93 |
| C4—C5 | 1.375 (3) | C14—C15 | 1.373 (3) |
| C4—H4 | 0.93 | C14—H14 | 0.93 |
| C5—C6 | 1.395 (3) | C15—O3 | 1.370 (3) |
| C5—H5 | 0.93 | C15—C16 | 1.386 (3) |
| C6—N1 | 1.416 (3) | C16—C17 | 1.372 (3) |
| C7—N1 | 1.465 (2) | C16—H16 | 0.93 |
| C7—C8 | 1.508 (3) | C17—H17 | 0.93 |
| C7—H7A | 0.97 | C18—O3 | 1.425 (3) |
| C7—H7B | 0.97 | C18—C19 | 1.490 (4) |
| C8—N2 | 1.482 (3) | C18—H18A | 0.97 |
| C8—H8A | 0.97 | C18—H18B | 0.97 |
| C8—H8B | 0.97 | C19—H19A | 0.96 |
| C9—N2 | 1.484 (3) | C19—H19B | 0.96 |
| C9—C10 | 1.504 (3) | C19—H19C | 0.96 |
| C9—H9A | 0.97 | N2—HN1 | 0.887 (16) |
| C9—H9B | 0.97 | N2—HN2 | 0.930 (16) |
| C10—N1 | 1.462 (3) | OW1—HW1 | 0.88 (3) |
| C10—H10A | 0.97 | OW1—HW2 | 0.91 (3) |
| C10—H10B | 0.97 | ||
| C6—C1—C2 | 121.2 (2) | O2—C11—C12 | 118.0 (2) |
| C6—C1—H1 | 119.4 | O1—C11—C12 | 118.2 (2) |
| C2—C1—H1 | 119.4 | C13—C12—C17 | 117.6 (2) |
| C3—C2—C1 | 121.1 (3) | C13—C12—C11 | 121.3 (2) |
| C3—C2—H2 | 119.4 | C17—C12—C11 | 121.1 (2) |
| C1—C2—H2 | 119.4 | C12—C13—C14 | 121.8 (2) |
| C4—C3—C2 | 118.4 (3) | C12—C13—H13 | 119.1 |
| C4—C3—H3 | 120.8 | C14—C13—H13 | 119.1 |
| C2—C3—H3 | 120.8 | C15—C14—C13 | 119.4 (2) |
| C3—C4—C5 | 121.3 (3) | C15—C14—H14 | 120.3 |
| C3—C4—H4 | 119.4 | C13—C14—H14 | 120.3 |
| C5—C4—H4 | 119.4 | O3—C15—C14 | 124.4 (2) |
| C4—C5—C6 | 121.1 (2) | O3—C15—C16 | 115.8 (2) |
| C4—C5—H5 | 119.4 | C14—C15—C16 | 119.8 (2) |
| C6—C5—H5 | 119.4 | C17—C16—C15 | 120.1 (2) |
| C1—C6—C5 | 116.9 (2) | C17—C16—H16 | 119.9 |
| C1—C6—N1 | 122.23 (19) | C15—C16—H16 | 119.9 |
| C5—C6—N1 | 120.8 (2) | C16—C17—C12 | 121.2 (2) |
| N1—C7—C8 | 112.74 (18) | C16—C17—H17 | 119.4 |
| N1—C7—H7A | 109 | C12—C17—H17 | 119.4 |
| C8—C7—H7A | 109 | O3—C18—C19 | 107.7 (3) |
| N1—C7—H7B | 109 | O3—C18—H18A | 110.2 |
| C8—C7—H7B | 109 | C19—C18—H18A | 110.2 |
| H7A—C7—H7B | 107.8 | O3—C18—H18B | 110.2 |
| N2—C8—C7 | 110.70 (17) | C19—C18—H18B | 110.2 |
| N2—C8—H8A | 109.5 | H18A—C18—H18B | 108.5 |
| C7—C8—H8A | 109.5 | C18—C19—H19A | 109.5 |
| N2—C8—H8B | 109.5 | C18—C19—H19B | 109.5 |
| C7—C8—H8B | 109.5 | H19A—C19—H19B | 109.5 |
| H8A—C8—H8B | 108.1 | C18—C19—H19C | 109.5 |
| N2—C9—C10 | 110.47 (18) | H19A—C19—H19C | 109.5 |
| N2—C9—H9A | 109.6 | H19B—C19—H19C | 109.5 |
| C10—C9—H9A | 109.6 | C6—N1—C10 | 115.21 (16) |
| N2—C9—H9B | 109.6 | C6—N1—C7 | 115.50 (17) |
| C10—C9—H9B | 109.6 | C10—N1—C7 | 111.72 (16) |
| H9A—C9—H9B | 108.1 | C8—N2—C9 | 109.60 (17) |
| N1—C10—C9 | 112.16 (17) | C8—N2—HN1 | 106.9 (15) |
| N1—C10—H10A | 109.2 | C9—N2—HN1 | 109.4 (15) |
| C9—C10—H10A | 109.2 | C8—N2—HN2 | 110.8 (15) |
| N1—C10—H10B | 109.2 | C9—N2—HN2 | 112.2 (15) |
| C9—C10—H10B | 109.2 | HN1—N2—HN2 | 108 (2) |
| H10A—C10—H10B | 107.9 | C15—O3—C18 | 117.7 (2) |
| O2—C11—O1 | 123.7 (2) | HW1—OW1—HW2 | 107 (3) |
| C6—C1—C2—C3 | 0.6 (4) | O3—C15—C16—C17 | −178.7 (2) |
| C1—C2—C3—C4 | 0.2 (5) | C14—C15—C16—C17 | 1.8 (4) |
| C2—C3—C4—C5 | −0.7 (5) | C15—C16—C17—C12 | −0.4 (4) |
| C3—C4—C5—C6 | 0.5 (4) | C13—C12—C17—C16 | −1.2 (3) |
| C2—C1—C6—C5 | −0.8 (4) | C11—C12—C17—C16 | 178.9 (2) |
| C2—C1—C6—N1 | 177.0 (2) | C1—C6—N1—C10 | 143.0 (2) |
| C4—C5—C6—C1 | 0.3 (4) | C5—C6—N1—C10 | −39.3 (3) |
| C4—C5—C6—N1 | −177.6 (2) | C1—C6—N1—C7 | 10.3 (3) |
| N1—C7—C8—N2 | −54.6 (3) | C5—C6—N1—C7 | −171.9 (2) |
| N2—C9—C10—N1 | 56.7 (2) | C9—C10—N1—C6 | 172.59 (17) |
| O2—C11—C12—C13 | −176.6 (2) | C9—C10—N1—C7 | −53.0 (2) |
| O1—C11—C12—C13 | 4.1 (3) | C8—C7—N1—C6 | −173.71 (18) |
| O2—C11—C12—C17 | 3.3 (3) | C8—C7—N1—C10 | 52.0 (2) |
| O1—C11—C12—C17 | −176.0 (2) | C7—C8—N2—C9 | 57.2 (2) |
| C17—C12—C13—C14 | 1.6 (3) | C10—C9—N2—C8 | −58.4 (2) |
| C11—C12—C13—C14 | −178.6 (2) | C14—C15—O3—C18 | −0.4 (4) |
| C12—C13—C14—C15 | −0.3 (3) | C16—C15—O3—C18 | −179.9 (2) |
| C13—C14—C15—O3 | 179.1 (2) | C19—C18—O3—C15 | −176.7 (3) |
| C13—C14—C15—C16 | −1.4 (3) |
4-Phenylpiperazin-1-ium 4-ethoxybenzoate monohydrate (I). Hydrogen-bond geometry (Å, º)
Cg1 and Cg3 are the centroids of the C12–C17 and C1–C6 benzene rings, respectively.
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—HN1···OW1i | 0.89 (2) | 1.94 (2) | 2.817 (3) | 167 (2) |
| N2—HN2···O1 | 0.93 (2) | 1.80 (2) | 2.724 (3) | 174 (2) |
| OW1—HW1···O2 | 0.88 (3) | 1.75 (3) | 2.630 (3) | 178 (4) |
| OW1—HW2···O1ii | 0.91 (3) | 1.89 (3) | 2.789 (3) | 167 (3) |
| C9—H9A···OW1 | 0.97 | 2.52 | 3.308 (3) | 138 |
| C1—H1···Cg1iii | 0.93 | 2.91 | 3.607 (3) | 133 |
| C5—H5···Cg1iv | 0.93 | 2.79 | 3.570 (3) | 142 |
| C18—H18B···Cg3v | 0.97 | 2.88 | 3.737 (4) | 148 |
Symmetry codes: (i) −x+1, −y+2, −z+1; (ii) x+1, y, z; (iii) −x, −y+2, −z+1; (iv) −x+1, −y+1, −z+1; (v) −x, −y+1, −z+1.
4-Phenylpiperazin-1-ium 4-methoxybenzoate monohydrate (II). Crystal data
| C10H15N2+·C8H7O3−·H2O | Z = 2 |
| Mr = 332.39 | F(000) = 356 |
| Triclinic, P1 | Dx = 1.284 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 6.2039 (4) Å | Cell parameters from 2855 reflections |
| b = 7.5565 (7) Å | θ = 3.1–27.8° |
| c = 18.614 (1) Å | µ = 0.09 mm−1 |
| α = 81.799 (7)° | T = 293 K |
| β = 87.020 (7)° | Prism, colourless |
| γ = 84.852 (7)° | 0.48 × 0.48 × 0.32 mm |
| V = 859.53 (11) Å3 |
4-Phenylpiperazin-1-ium 4-methoxybenzoate monohydrate (II). Data collection
| Oxford Diffraction Xcalibur diffractometer | 2322 reflections with I > 2σ(I) |
| ω scans | Rint = 0.016 |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009) | θmax = 25.3°, θmin = 3.1° |
| Tmin = 0.520, Tmax = 1.000 | h = −7→5 |
| 5360 measured reflections | k = −9→9 |
| 3142 independent reflections | l = −22→22 |
4-Phenylpiperazin-1-ium 4-methoxybenzoate monohydrate (II). Refinement
| Refinement on F2 | Secondary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.045 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.125 | w = 1/[σ2(Fo2) + (0.0613P)2 + 0.1503P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.06 | (Δ/σ)max < 0.001 |
| 3139 reflections | Δρmax = 0.2 e Å−3 |
| 230 parameters | Δρmin = −0.16 e Å−3 |
| 4 restraints | Extinction correction: SHELXL2018/3 (Sheldrick 2015b), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 constraints | Extinction coefficient: 0.032 (4) |
| Primary atom site location: structure-invariant direct methods |
4-Phenylpiperazin-1-ium 4-methoxybenzoate monohydrate (II). Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
4-Phenylpiperazin-1-ium 4-methoxybenzoate monohydrate (II). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | −0.0254 (3) | 0.4086 (3) | 0.19209 (10) | 0.0516 (5) | |
| H1 | −0.120858 | 0.476382 | 0.219629 | 0.062* | |
| C2 | −0.0695 (3) | 0.3996 (3) | 0.12102 (11) | 0.0625 (6) | |
| H2 | −0.194898 | 0.46027 | 0.101533 | 0.075* | |
| C3 | 0.0686 (3) | 0.3024 (3) | 0.07854 (10) | 0.0597 (6) | |
| H3 | 0.039957 | 0.298402 | 0.030279 | 0.072* | |
| C4 | 0.2497 (3) | 0.2117 (3) | 0.10901 (10) | 0.0593 (6) | |
| H4 | 0.343702 | 0.143909 | 0.08102 | 0.071* | |
| C5 | 0.2964 (3) | 0.2181 (3) | 0.18024 (9) | 0.0512 (5) | |
| H5 | 0.420732 | 0.154912 | 0.199433 | 0.061* | |
| C6 | 0.1594 (2) | 0.3180 (2) | 0.22353 (8) | 0.0361 (4) | |
| C7 | 0.0222 (3) | 0.3258 (2) | 0.34797 (8) | 0.0419 (4) | |
| H7A | −0.026461 | 0.205915 | 0.354319 | 0.05* | |
| H7B | −0.096084 | 0.40906 | 0.32889 | 0.05* | |
| C8 | 0.0795 (3) | 0.3708 (2) | 0.42029 (9) | 0.0456 (4) | |
| H8A | 0.116889 | 0.494094 | 0.414858 | 0.055* | |
| H8B | −0.044531 | 0.359357 | 0.454053 | 0.055* | |
| C9 | 0.4547 (3) | 0.2652 (3) | 0.39777 (9) | 0.0485 (5) | |
| H9A | 0.574598 | 0.182967 | 0.416336 | 0.058* | |
| H9B | 0.499661 | 0.386146 | 0.392734 | 0.058* | |
| C10 | 0.3979 (3) | 0.2229 (3) | 0.32457 (9) | 0.0445 (4) | |
| H10A | 0.520581 | 0.241283 | 0.29068 | 0.053* | |
| H10B | 0.36928 | 0.097655 | 0.328948 | 0.053* | |
| C11 | 0.2655 (3) | 0.2146 (2) | 0.63263 (10) | 0.0474 (5) | |
| C12 | 0.3208 (3) | 0.2073 (2) | 0.71052 (9) | 0.0389 (4) | |
| C13 | 0.1897 (3) | 0.1251 (2) | 0.76647 (10) | 0.0479 (5) | |
| H13 | 0.067829 | 0.072733 | 0.755273 | 0.057* | |
| C14 | 0.2386 (3) | 0.1207 (3) | 0.83782 (10) | 0.0496 (5) | |
| H14 | 0.149875 | 0.065178 | 0.874461 | 0.06* | |
| C15 | 0.4186 (3) | 0.1982 (2) | 0.85567 (9) | 0.0442 (4) | |
| C16 | 0.5528 (3) | 0.2784 (2) | 0.80102 (9) | 0.0453 (4) | |
| H16 | 0.675183 | 0.329713 | 0.812349 | 0.054* | |
| C17 | 0.5021 (3) | 0.2808 (2) | 0.72949 (9) | 0.0424 (4) | |
| H17 | 0.592994 | 0.333766 | 0.6929 | 0.051* | |
| C18 | 0.6395 (4) | 0.2628 (4) | 0.94845 (12) | 0.0858 (8) | |
| H13A | 0.643815 | 0.248303 | 1.000449 | 0.129* | |
| H13B | 0.76663 | 0.201465 | 0.928951 | 0.129* | |
| H13C | 0.63455 | 0.388125 | 0.929616 | 0.129* | |
| N1 | 0.2079 (2) | 0.33487 (18) | 0.29565 (7) | 0.0359 (3) | |
| N2 | 0.2652 (2) | 0.2489 (2) | 0.44963 (8) | 0.0434 (4) | |
| O1 | 0.1058 (3) | 0.1395 (3) | 0.61995 (9) | 0.0873 (6) | |
| O2 | 0.3847 (2) | 0.29717 (19) | 0.58441 (7) | 0.0598 (4) | |
| O3 | 0.4517 (2) | 0.1895 (2) | 0.92816 (7) | 0.0620 (4) | |
| OW1 | 0.2271 (2) | 0.8777 (2) | 0.46022 (8) | 0.0579 (4) | |
| HN1 | 0.230 (3) | 0.131 (2) | 0.4565 (11) | 0.07* | |
| HW1 | 0.120 (3) | 0.865 (3) | 0.4356 (11) | 0.07* | |
| HN2 | 0.303 (3) | 0.270 (3) | 0.4956 (9) | 0.07* | |
| HW2 | 0.336 (3) | 0.822 (3) | 0.4413 (12) | 0.07* |
4-Phenylpiperazin-1-ium 4-methoxybenzoate monohydrate (II). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0534 (11) | 0.0579 (12) | 0.0441 (10) | 0.0127 (9) | −0.0106 (8) | −0.0169 (9) |
| C2 | 0.0654 (13) | 0.0739 (14) | 0.0491 (11) | 0.0143 (11) | −0.0228 (10) | −0.0166 (10) |
| C3 | 0.0820 (14) | 0.0654 (13) | 0.0347 (10) | −0.0036 (11) | −0.0147 (10) | −0.0145 (9) |
| C4 | 0.0717 (13) | 0.0660 (13) | 0.0411 (10) | 0.0070 (11) | 0.0022 (9) | −0.0202 (9) |
| C5 | 0.0535 (11) | 0.0597 (12) | 0.0399 (10) | 0.0105 (9) | −0.0032 (8) | −0.0137 (8) |
| C6 | 0.0405 (9) | 0.0344 (9) | 0.0346 (8) | −0.0042 (7) | −0.0035 (7) | −0.0071 (7) |
| C7 | 0.0388 (9) | 0.0515 (11) | 0.0352 (9) | 0.0009 (8) | −0.0006 (7) | −0.0083 (7) |
| C8 | 0.0518 (10) | 0.0495 (11) | 0.0354 (9) | 0.0016 (8) | 0.0004 (8) | −0.0104 (8) |
| C9 | 0.0425 (10) | 0.0619 (12) | 0.0419 (10) | −0.0015 (8) | −0.0105 (8) | −0.0090 (8) |
| C10 | 0.0385 (9) | 0.0569 (11) | 0.0381 (9) | 0.0049 (8) | −0.0053 (7) | −0.0113 (8) |
| C11 | 0.0484 (11) | 0.0464 (11) | 0.0508 (11) | 0.0051 (8) | −0.0161 (9) | −0.0190 (9) |
| C12 | 0.0387 (9) | 0.0356 (9) | 0.0443 (9) | 0.0023 (7) | −0.0086 (7) | −0.0131 (7) |
| C13 | 0.0366 (9) | 0.0485 (11) | 0.0617 (12) | −0.0058 (8) | −0.0057 (8) | −0.0157 (9) |
| C14 | 0.0437 (10) | 0.0551 (12) | 0.0500 (11) | −0.0060 (8) | 0.0047 (8) | −0.0079 (9) |
| C15 | 0.0450 (10) | 0.0485 (10) | 0.0398 (9) | 0.0020 (8) | −0.0042 (8) | −0.0110 (8) |
| C16 | 0.0461 (10) | 0.0501 (11) | 0.0430 (10) | −0.0103 (8) | −0.0101 (8) | −0.0112 (8) |
| C17 | 0.0447 (10) | 0.0437 (10) | 0.0400 (9) | −0.0072 (8) | −0.0036 (7) | −0.0075 (7) |
| C18 | 0.0785 (16) | 0.136 (2) | 0.0509 (13) | −0.0228 (16) | −0.0171 (11) | −0.0274 (14) |
| N1 | 0.0347 (7) | 0.0416 (8) | 0.0320 (7) | 0.0006 (6) | −0.0034 (5) | −0.0091 (6) |
| N2 | 0.0553 (9) | 0.0450 (8) | 0.0319 (7) | −0.0061 (7) | −0.0089 (6) | −0.0082 (6) |
| O1 | 0.0815 (11) | 0.1184 (15) | 0.0727 (11) | −0.0352 (10) | −0.0327 (9) | −0.0230 (10) |
| O2 | 0.0721 (9) | 0.0700 (10) | 0.0402 (7) | −0.0038 (7) | −0.0139 (7) | −0.0144 (7) |
| O3 | 0.0641 (9) | 0.0866 (11) | 0.0365 (7) | −0.0067 (8) | −0.0047 (6) | −0.0117 (7) |
| OW1 | 0.0595 (9) | 0.0631 (9) | 0.0544 (8) | −0.0097 (7) | −0.0177 (7) | −0.0108 (7) |
4-Phenylpiperazin-1-ium 4-methoxybenzoate monohydrate (II). Geometric parameters (Å, º)
| C1—C2 | 1.377 (3) | C10—H10A | 0.97 |
| C1—C6 | 1.393 (2) | C10—H10B | 0.97 |
| C1—H1 | 0.93 | C11—O1 | 1.234 (2) |
| C2—C3 | 1.371 (3) | C11—O2 | 1.263 (2) |
| C2—H2 | 0.93 | C11—C12 | 1.499 (2) |
| C3—C4 | 1.368 (3) | C12—C17 | 1.381 (2) |
| C3—H3 | 0.93 | C12—C13 | 1.396 (3) |
| C4—C5 | 1.380 (3) | C13—C14 | 1.373 (3) |
| C4—H4 | 0.93 | C13—H13 | 0.93 |
| C5—C6 | 1.388 (2) | C14—C15 | 1.383 (3) |
| C5—H5 | 0.93 | C14—H14 | 0.93 |
| C6—N1 | 1.4165 (19) | C15—O3 | 1.367 (2) |
| C7—N1 | 1.469 (2) | C15—C16 | 1.386 (2) |
| C7—C8 | 1.502 (2) | C16—C17 | 1.381 (2) |
| C7—H7A | 0.97 | C16—H16 | 0.93 |
| C7—H7B | 0.97 | C17—H17 | 0.93 |
| C8—N2 | 1.485 (2) | C18—O3 | 1.424 (3) |
| C8—H8A | 0.97 | C18—H13A | 0.96 |
| C8—H8B | 0.97 | C18—H13B | 0.96 |
| C9—N2 | 1.484 (2) | C18—H13C | 0.96 |
| C9—C10 | 1.509 (2) | N2—HN1 | 0.924 (15) |
| C9—H9A | 0.97 | N2—HN2 | 0.938 (16) |
| C9—H9B | 0.97 | OW1—HW1 | 0.847 (16) |
| C10—N1 | 1.467 (2) | OW1—HW2 | 0.850 (16) |
| C2—C1—C6 | 121.26 (16) | C9—C10—H10B | 109.1 |
| C2—C1—H1 | 119.4 | H10A—C10—H10B | 107.9 |
| C6—C1—H1 | 119.4 | O1—C11—O2 | 124.33 (18) |
| C3—C2—C1 | 121.01 (18) | O1—C11—C12 | 117.62 (19) |
| C3—C2—H2 | 119.5 | O2—C11—C12 | 118.06 (16) |
| C1—C2—H2 | 119.5 | C17—C12—C13 | 117.75 (16) |
| C4—C3—C2 | 118.25 (17) | C17—C12—C11 | 121.49 (16) |
| C4—C3—H3 | 120.9 | C13—C12—C11 | 120.77 (16) |
| C2—C3—H3 | 120.9 | C14—C13—C12 | 120.75 (16) |
| C3—C4—C5 | 121.67 (17) | C14—C13—H13 | 119.6 |
| C3—C4—H4 | 119.2 | C12—C13—H13 | 119.6 |
| C5—C4—H4 | 119.2 | C13—C14—C15 | 120.56 (17) |
| C4—C5—C6 | 120.65 (17) | C13—C14—H14 | 119.7 |
| C4—C5—H5 | 119.7 | C15—C14—H14 | 119.7 |
| C6—C5—H5 | 119.7 | O3—C15—C14 | 116.20 (16) |
| C5—C6—C1 | 117.15 (15) | O3—C15—C16 | 124.10 (16) |
| C5—C6—N1 | 122.11 (14) | C14—C15—C16 | 119.69 (16) |
| C1—C6—N1 | 120.68 (14) | C17—C16—C15 | 119.06 (16) |
| N1—C7—C8 | 111.59 (14) | C17—C16—H16 | 120.5 |
| N1—C7—H7A | 109.3 | C15—C16—H16 | 120.5 |
| C8—C7—H7A | 109.3 | C16—C17—C12 | 122.16 (16) |
| N1—C7—H7B | 109.3 | C16—C17—H17 | 118.9 |
| C8—C7—H7B | 109.3 | C12—C17—H17 | 118.9 |
| H7A—C7—H7B | 108 | O3—C18—H13A | 109.5 |
| N2—C8—C7 | 110.40 (13) | O3—C18—H13B | 109.5 |
| N2—C8—H8A | 109.6 | H13A—C18—H13B | 109.5 |
| C7—C8—H8A | 109.6 | O3—C18—H13C | 109.5 |
| N2—C8—H8B | 109.6 | H13A—C18—H13C | 109.5 |
| C7—C8—H8B | 109.6 | H13B—C18—H13C | 109.5 |
| H8A—C8—H8B | 108.1 | C6—N1—C10 | 115.62 (12) |
| N2—C9—C10 | 110.34 (14) | C6—N1—C7 | 114.90 (12) |
| N2—C9—H9A | 109.6 | C10—N1—C7 | 111.53 (12) |
| C10—C9—H9A | 109.6 | C9—N2—C8 | 109.67 (13) |
| N2—C9—H9B | 109.6 | C9—N2—HN1 | 108.7 (13) |
| C10—C9—H9B | 109.6 | C8—N2—HN1 | 110.6 (13) |
| H9A—C9—H9B | 108.1 | C9—N2—HN2 | 110.0 (13) |
| N1—C10—C9 | 112.30 (13) | C8—N2—HN2 | 113.2 (13) |
| N1—C10—H10A | 109.1 | HN1—N2—HN2 | 104.5 (18) |
| C9—C10—H10A | 109.1 | C15—O3—C18 | 117.68 (16) |
| N1—C10—H10B | 109.1 | HW1—OW1—HW2 | 106 (2) |
| C6—C1—C2—C3 | −0.7 (3) | C13—C14—C15—C16 | −1.1 (3) |
| C1—C2—C3—C4 | 1.2 (3) | O3—C15—C16—C17 | −179.35 (16) |
| C2—C3—C4—C5 | −0.9 (3) | C14—C15—C16—C17 | 0.7 (3) |
| C3—C4—C5—C6 | 0.0 (3) | C15—C16—C17—C12 | 0.5 (3) |
| C4—C5—C6—C1 | 0.6 (3) | C13—C12—C17—C16 | −1.4 (2) |
| C4—C5—C6—N1 | −176.76 (18) | C11—C12—C17—C16 | 178.68 (15) |
| C2—C1—C6—C5 | −0.2 (3) | C5—C6—N1—C10 | −7.1 (2) |
| C2—C1—C6—N1 | 177.14 (18) | C1—C6—N1—C10 | 175.65 (16) |
| N1—C7—C8—N2 | −57.13 (19) | C5—C6—N1—C7 | −139.28 (17) |
| N2—C9—C10—N1 | 55.4 (2) | C1—C6—N1—C7 | 43.5 (2) |
| O1—C11—C12—C17 | 177.14 (17) | C9—C10—N1—C6 | 172.93 (14) |
| O2—C11—C12—C17 | −3.0 (2) | C9—C10—N1—C7 | −53.34 (19) |
| O1—C11—C12—C13 | −2.8 (2) | C8—C7—N1—C6 | −171.86 (13) |
| O2—C11—C12—C13 | 177.07 (16) | C8—C7—N1—C10 | 54.06 (18) |
| C17—C12—C13—C14 | 1.0 (2) | C10—C9—N2—C8 | −57.61 (19) |
| C11—C12—C13—C14 | −179.05 (15) | C7—C8—N2—C9 | 58.76 (19) |
| C12—C13—C14—C15 | 0.2 (3) | C14—C15—O3—C18 | 178.00 (19) |
| C13—C14—C15—O3 | 178.99 (15) | C16—C15—O3—C18 | −1.9 (3) |
4-Phenylpiperazin-1-ium 4-methoxybenzoate monohydrate (II). Hydrogen-bond geometry (Å, º)
Cg3 is the centroid of the C12–C17 benzene ring.
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—HN1···OW1i | 0.93 (2) | 1.91 (2) | 2.815 (2) | 166 (2) |
| OW1—HW1···O1ii | 0.84 (2) | 1.80 (2) | 2.633 (2) | 175 (2) |
| N2—HN2···O2 | 0.93 (2) | 1.81 (2) | 2.7350 (19) | 176 (2) |
| OW1—HW2···O2iii | 0.85 (2) | 1.96 (2) | 2.7876 (19) | 168 (2) |
| C8—H8B···OW1ii | 0.97 | 2.53 | 3.331 (2) | 140 |
| C1—H1···Cg3ii | 0.93 | 2.76 | 3.549 (2) | 144 |
| C5—H5···Cg3iv | 0.93 | 2.86 | 3.625 (2) | 140 |
Symmetry codes: (i) x, y−1, z; (ii) −x, −y+1, −z+1; (iii) −x+1, −y+1, −z+1; (iv) −x+1, −y, −z+1.
4-Phenylpiperazin-1-ium 4-methylbenzoate monohydrate (III). Crystal data
| C10H15N2+·C8H7O2−·H2O | Z = 2 |
| Mr = 316.39 | F(000) = 340 |
| Triclinic, P1 | Dx = 1.233 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 6.1175 (5) Å | Cell parameters from 2877 reflections |
| b = 7.6225 (7) Å | θ = 3.0–27.8° |
| c = 18.452 (1) Å | µ = 0.08 mm−1 |
| α = 97.421 (9)° | T = 293 K |
| β = 90.403 (8)° | Plate, colourless |
| γ = 92.405 (8)° | 0.5 × 0.4 × 0.08 mm |
| V = 852.40 (12) Å3 |
4-Phenylpiperazin-1-ium 4-methylbenzoate monohydrate (III). Data collection
| Oxford Diffraction Xcalibur diffractometer | 2248 reflections with I > 2σ(I) |
| ω scans | Rint = 0.013 |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009) | θmax = 25.4°, θmin = 3.1° |
| Tmin = 0.837, Tmax = 1.000 | h = −7→7 |
| 5354 measured reflections | k = −7→9 |
| 3126 independent reflections | l = −22→22 |
4-Phenylpiperazin-1-ium 4-methylbenzoate monohydrate (III). Refinement
| Refinement on F2 | Secondary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.046 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.128 | w = 1/[σ2(Fo2) + (0.0582P)2 + 0.2086P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.03 | (Δ/σ)max < 0.001 |
| 3118 reflections | Δρmax = 0.16 e Å−3 |
| 226 parameters | Δρmin = −0.16 e Å−3 |
| 4 restraints | Extinction correction: SHELXL2018/3 (Sheldrick 2015b), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 constraints | Extinction coefficient: 0.011 (3) |
| Primary atom site location: structure-invariant direct methods |
4-Phenylpiperazin-1-ium 4-methylbenzoate monohydrate (III). Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
4-Phenylpiperazin-1-ium 4-methylbenzoate monohydrate (III). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.5691 (4) | 0.8192 (4) | 0.17484 (12) | 0.0759 (7) | |
| H1 | 0.680156 | 0.871649 | 0.206032 | 0.091* | |
| C2 | 0.5967 (5) | 0.8079 (4) | 0.10029 (13) | 0.0979 (9) | |
| H2 | 0.725499 | 0.854112 | 0.082348 | 0.117* | |
| C3 | 0.4416 (5) | 0.7314 (4) | 0.05273 (13) | 0.0932 (8) | |
| H3 | 0.462879 | 0.722711 | 0.002561 | 0.112* | |
| C4 | 0.2536 (5) | 0.6676 (4) | 0.08016 (13) | 0.0966 (9) | |
| H4 | 0.144014 | 0.6157 | 0.048248 | 0.116* | |
| C5 | 0.2219 (4) | 0.6782 (3) | 0.15497 (11) | 0.0776 (7) | |
| H5 | 0.091389 | 0.633226 | 0.172178 | 0.093* | |
| C6 | 0.3793 (3) | 0.7539 (2) | 0.20421 (9) | 0.0472 (4) | |
| C7 | 0.5393 (3) | 0.8071 (3) | 0.32686 (9) | 0.0497 (4) | |
| H7A | 0.626347 | 0.904686 | 0.31147 | 0.06* | |
| H7B | 0.626834 | 0.703106 | 0.320724 | 0.06* | |
| C8 | 0.4853 (3) | 0.8506 (3) | 0.40599 (10) | 0.0534 (5) | |
| H8A | 0.619196 | 0.865746 | 0.435007 | 0.064* | |
| H8B | 0.410139 | 0.960943 | 0.413335 | 0.064* | |
| C9 | 0.1390 (3) | 0.6889 (3) | 0.38632 (10) | 0.0554 (5) | |
| H9A | 0.062182 | 0.798326 | 0.394853 | 0.067* | |
| H9B | 0.04531 | 0.594989 | 0.401599 | 0.067* | |
| C10 | 0.1858 (3) | 0.6476 (3) | 0.30616 (9) | 0.0502 (5) | |
| H10A | 0.241603 | 0.529612 | 0.296889 | 0.06* | |
| H10B | 0.050244 | 0.647749 | 0.278608 | 0.06* | |
| C11 | 0.3129 (3) | 0.7088 (2) | 0.62385 (9) | 0.0445 (4) | |
| C12 | 0.2060 (3) | 0.7367 (2) | 0.69737 (9) | 0.0413 (4) | |
| C13 | 0.2989 (3) | 0.6749 (3) | 0.75734 (10) | 0.0535 (5) | |
| H13 | 0.428845 | 0.615675 | 0.752062 | 0.064* | |
| C14 | 0.2008 (4) | 0.7003 (3) | 0.82503 (11) | 0.0656 (6) | |
| H14 | 0.265661 | 0.657329 | 0.864556 | 0.079* | |
| C15 | 0.0081 (4) | 0.7885 (3) | 0.83492 (11) | 0.0611 (5) | |
| C16 | −0.0837 (3) | 0.8490 (3) | 0.77515 (11) | 0.0583 (5) | |
| H16 | −0.214079 | 0.907628 | 0.780509 | 0.07* | |
| C17 | 0.0129 (3) | 0.8250 (2) | 0.70719 (10) | 0.0484 (4) | |
| H17 | −0.052163 | 0.868519 | 0.667839 | 0.058* | |
| C18 | −0.0996 (5) | 0.8177 (4) | 0.90877 (13) | 0.0968 (9) | |
| H18A | −0.171921 | 0.927853 | 0.913714 | 0.145* | |
| H18B | 0.009588 | 0.821099 | 0.946613 | 0.145* | |
| H18C | −0.204829 | 0.722525 | 0.912877 | 0.145* | |
| N1 | 0.3441 (2) | 0.77371 (19) | 0.28025 (7) | 0.0434 (4) | |
| N2 | 0.3444 (2) | 0.7069 (2) | 0.43010 (8) | 0.0487 (4) | |
| O1 | 0.4844 (2) | 0.62506 (19) | 0.61816 (7) | 0.0632 (4) | |
| O2 | 0.2244 (2) | 0.77468 (19) | 0.57233 (7) | 0.0601 (4) | |
| OW1 | 0.7937 (3) | 0.7241 (4) | 0.52207 (11) | 0.1232 (9) | |
| HW1 | 0.926 (4) | 0.748 (5) | 0.542 (2) | 0.177 (17)* | |
| HW2 | 0.702 (4) | 0.704 (4) | 0.5539 (13) | 0.119 (11)* | |
| HN1 | 0.415 (3) | 0.605 (2) | 0.4225 (11) | 0.061 (6)* | |
| HN2 | 0.310 (3) | 0.731 (3) | 0.4786 (9) | 0.065 (6)* |
4-Phenylpiperazin-1-ium 4-methylbenzoate monohydrate (III). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0620 (13) | 0.114 (2) | 0.0494 (12) | −0.0113 (13) | 0.0106 (10) | 0.0080 (12) |
| C2 | 0.0883 (18) | 0.148 (3) | 0.0556 (15) | −0.0138 (18) | 0.0233 (13) | 0.0120 (15) |
| C3 | 0.123 (2) | 0.109 (2) | 0.0456 (13) | −0.0055 (18) | 0.0201 (15) | 0.0043 (13) |
| C4 | 0.130 (2) | 0.106 (2) | 0.0480 (13) | −0.0340 (18) | −0.0161 (14) | 0.0036 (13) |
| C5 | 0.0895 (16) | 0.0936 (17) | 0.0466 (12) | −0.0337 (13) | −0.0042 (11) | 0.0105 (11) |
| C6 | 0.0561 (11) | 0.0456 (10) | 0.0397 (9) | 0.0045 (8) | 0.0038 (8) | 0.0042 (7) |
| C7 | 0.0473 (10) | 0.0550 (11) | 0.0456 (10) | −0.0123 (8) | 0.0008 (8) | 0.0070 (8) |
| C8 | 0.0581 (11) | 0.0569 (12) | 0.0440 (10) | −0.0065 (9) | −0.0031 (8) | 0.0045 (8) |
| C9 | 0.0439 (10) | 0.0734 (13) | 0.0494 (11) | −0.0025 (9) | 0.0087 (8) | 0.0105 (9) |
| C10 | 0.0383 (9) | 0.0668 (12) | 0.0446 (10) | −0.0056 (8) | 0.0005 (7) | 0.0064 (8) |
| C11 | 0.0429 (10) | 0.0454 (10) | 0.0432 (10) | −0.0062 (8) | 0.0042 (7) | 0.0012 (7) |
| C12 | 0.0413 (9) | 0.0376 (9) | 0.0439 (9) | −0.0047 (7) | 0.0046 (7) | 0.0025 (7) |
| C13 | 0.0525 (11) | 0.0601 (12) | 0.0479 (11) | 0.0081 (9) | 0.0012 (8) | 0.0054 (9) |
| C14 | 0.0798 (15) | 0.0739 (14) | 0.0439 (11) | 0.0046 (11) | −0.0013 (10) | 0.0111 (9) |
| C15 | 0.0742 (14) | 0.0597 (12) | 0.0479 (11) | −0.0009 (10) | 0.0167 (10) | 0.0012 (9) |
| C16 | 0.0577 (12) | 0.0554 (12) | 0.0614 (12) | 0.0098 (9) | 0.0173 (9) | 0.0025 (9) |
| C17 | 0.0525 (10) | 0.0458 (10) | 0.0476 (10) | 0.0047 (8) | 0.0056 (8) | 0.0072 (8) |
| C18 | 0.125 (2) | 0.108 (2) | 0.0567 (14) | 0.0110 (17) | 0.0378 (15) | 0.0048 (13) |
| N1 | 0.0436 (8) | 0.0487 (8) | 0.0379 (8) | −0.0025 (6) | 0.0016 (6) | 0.0068 (6) |
| N2 | 0.0519 (9) | 0.0577 (10) | 0.0371 (8) | 0.0057 (8) | 0.0054 (7) | 0.0068 (7) |
| O1 | 0.0546 (8) | 0.0720 (9) | 0.0633 (9) | 0.0149 (7) | 0.0170 (6) | 0.0047 (7) |
| O2 | 0.0557 (8) | 0.0833 (10) | 0.0426 (7) | 0.0032 (7) | 0.0096 (6) | 0.0120 (7) |
| OW1 | 0.0607 (12) | 0.250 (3) | 0.0691 (12) | 0.0102 (15) | 0.0088 (10) | 0.0577 (15) |
4-Phenylpiperazin-1-ium 4-methylbenzoate monohydrate (III). Geometric parameters (Å, º)
| C1—C2 | 1.379 (3) | C10—H10A | 0.97 |
| C1—C6 | 1.385 (3) | C10—H10B | 0.97 |
| C1—H1 | 0.93 | C11—O1 | 1.249 (2) |
| C2—C3 | 1.349 (4) | C11—O2 | 1.260 (2) |
| C2—H2 | 0.93 | C11—C12 | 1.504 (2) |
| C3—C4 | 1.356 (4) | C12—C13 | 1.385 (2) |
| C3—H3 | 0.93 | C12—C17 | 1.386 (2) |
| C4—C5 | 1.388 (3) | C13—C14 | 1.384 (3) |
| C4—H4 | 0.93 | C13—H13 | 0.93 |
| C5—C6 | 1.375 (3) | C14—C15 | 1.383 (3) |
| C5—H5 | 0.93 | C14—H14 | 0.93 |
| C6—N1 | 1.411 (2) | C15—C16 | 1.374 (3) |
| C7—N1 | 1.461 (2) | C15—C18 | 1.512 (3) |
| C7—C8 | 1.497 (2) | C16—C17 | 1.384 (2) |
| C7—H7A | 0.97 | C16—H16 | 0.93 |
| C7—H7B | 0.97 | C17—H17 | 0.93 |
| C8—N2 | 1.481 (2) | C18—H18A | 0.96 |
| C8—H8A | 0.97 | C18—H18B | 0.96 |
| C8—H8B | 0.97 | C18—H18C | 0.96 |
| C9—N2 | 1.481 (2) | N2—HN1 | 0.900 (15) |
| C9—C10 | 1.504 (2) | N2—HN2 | 0.918 (15) |
| C9—H9A | 0.97 | OW1—HW1 | 0.886 (19) |
| C9—H9B | 0.97 | OW1—HW2 | 0.839 (18) |
| C10—N1 | 1.462 (2) | ||
| C2—C1—C6 | 121.3 (2) | C9—C10—H10B | 109 |
| C2—C1—H1 | 119.3 | H10A—C10—H10B | 107.8 |
| C6—C1—H1 | 119.3 | O1—C11—O2 | 124.44 (16) |
| C3—C2—C1 | 121.6 (2) | O1—C11—C12 | 118.17 (16) |
| C3—C2—H2 | 119.2 | O2—C11—C12 | 117.37 (15) |
| C1—C2—H2 | 119.2 | C13—C12—C17 | 118.11 (16) |
| C2—C3—C4 | 118.1 (2) | C13—C12—C11 | 120.69 (15) |
| C2—C3—H3 | 120.9 | C17—C12—C11 | 121.19 (15) |
| C4—C3—H3 | 120.9 | C14—C13—C12 | 120.81 (17) |
| C3—C4—C5 | 121.2 (2) | C14—C13—H13 | 119.6 |
| C3—C4—H4 | 119.4 | C12—C13—H13 | 119.6 |
| C5—C4—H4 | 119.4 | C15—C14—C13 | 121.14 (19) |
| C6—C5—C4 | 121.4 (2) | C15—C14—H14 | 119.4 |
| C6—C5—H5 | 119.3 | C13—C14—H14 | 119.4 |
| C4—C5—H5 | 119.3 | C16—C15—C14 | 117.78 (17) |
| C5—C6—C1 | 116.25 (18) | C16—C15—C18 | 120.7 (2) |
| C5—C6—N1 | 121.84 (18) | C14—C15—C18 | 121.5 (2) |
| C1—C6—N1 | 121.81 (17) | C15—C16—C17 | 121.68 (18) |
| N1—C7—C8 | 112.51 (14) | C15—C16—H16 | 119.2 |
| N1—C7—H7A | 109.1 | C17—C16—H16 | 119.2 |
| C8—C7—H7A | 109.1 | C16—C17—C12 | 120.47 (17) |
| N1—C7—H7B | 109.1 | C16—C17—H17 | 119.8 |
| C8—C7—H7B | 109.1 | C12—C17—H17 | 119.8 |
| H7A—C7—H7B | 107.8 | C15—C18—H18A | 109.5 |
| N2—C8—C7 | 110.19 (15) | C15—C18—H18B | 109.5 |
| N2—C8—H8A | 109.6 | H18A—C18—H18B | 109.5 |
| C7—C8—H8A | 109.6 | C15—C18—H18C | 109.5 |
| N2—C8—H8B | 109.6 | H18A—C18—H18C | 109.5 |
| C7—C8—H8B | 109.6 | H18B—C18—H18C | 109.5 |
| H8A—C8—H8B | 108.1 | C6—N1—C7 | 116.19 (14) |
| N2—C9—C10 | 110.84 (14) | C6—N1—C10 | 116.09 (14) |
| N2—C9—H9A | 109.5 | C7—N1—C10 | 113.11 (13) |
| C10—C9—H9A | 109.5 | C8—N2—C9 | 108.70 (15) |
| N2—C9—H9B | 109.5 | C8—N2—HN1 | 108.9 (13) |
| C10—C9—H9B | 109.5 | C9—N2—HN1 | 109.5 (13) |
| H9A—C9—H9B | 108.1 | C8—N2—HN2 | 111.2 (13) |
| N1—C10—C9 | 112.96 (15) | C9—N2—HN2 | 108.7 (13) |
| N1—C10—H10A | 109 | HN1—N2—HN2 | 109.8 (18) |
| C9—C10—H10A | 109 | HW1—OW1—HW2 | 111 (3) |
| N1—C10—H10B | 109 | ||
| C6—C1—C2—C3 | −0.7 (5) | C13—C14—C15—C16 | 0.4 (3) |
| C1—C2—C3—C4 | 1.1 (5) | C13—C14—C15—C18 | −179.7 (2) |
| C2—C3—C4—C5 | −0.8 (5) | C14—C15—C16—C17 | −0.6 (3) |
| C3—C4—C5—C6 | 0.1 (4) | C18—C15—C16—C17 | 179.5 (2) |
| C4—C5—C6—C1 | 0.4 (4) | C15—C16—C17—C12 | 0.7 (3) |
| C4—C5—C6—N1 | 176.6 (2) | C13—C12—C17—C16 | −0.5 (3) |
| C2—C1—C6—C5 | −0.1 (4) | C11—C12—C17—C16 | 179.76 (17) |
| C2—C1—C6—N1 | −176.3 (2) | C5—C6—N1—C7 | 162.90 (19) |
| N1—C7—C8—N2 | 56.4 (2) | C1—C6—N1—C7 | −21.0 (3) |
| N2—C9—C10—N1 | −53.3 (2) | C5—C6—N1—C10 | 26.2 (3) |
| O1—C11—C12—C13 | 1.6 (2) | C1—C6—N1—C10 | −157.72 (19) |
| O2—C11—C12—C13 | −177.16 (17) | C8—C7—N1—C6 | 172.21 (15) |
| O1—C11—C12—C17 | −178.61 (16) | C8—C7—N1—C10 | −49.8 (2) |
| O2—C11—C12—C17 | 2.6 (2) | C9—C10—N1—C6 | −173.76 (14) |
| C17—C12—C13—C14 | 0.3 (3) | C9—C10—N1—C7 | 48.2 (2) |
| C11—C12—C13—C14 | −179.94 (18) | C7—C8—N2—C9 | −60.59 (19) |
| C12—C13—C14—C15 | −0.3 (3) | C10—C9—N2—C8 | 59.1 (2) |
4-Phenylpiperazin-1-ium 4-methylbenzoate monohydrate (III). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| OW1—HW1···O2i | 0.89 (3) | 1.90 (3) | 2.782 (2) | 171 (4) |
| OW1—HW2···O1 | 0.84 (2) | 1.92 (3) | 2.751 (2) | 172 (3) |
| N2—HN1···O1ii | 0.90 (2) | 1.94 (2) | 2.819 (2) | 164 (2) |
| N2—HN2···O2 | 0.92 (2) | 1.80 (2) | 2.7207 (19) | 176 (2) |
| C8—H8A···OW1 | 0.97 | 2.33 | 3.116 (3) | 138 |
Symmetry codes: (i) x+1, y, z; (ii) −x+1, −y+1, −z+1.
4-Phenylpiperazin-1-ium trifluoroacetate 0.123-hydrate (IV). Crystal data
| C10H15N2+·C2F3O2−·0.123H2O | Z = 4 |
| Mr = 278.47 | F(000) = 580.9 |
| Triclinic, P1 | Dx = 1.371 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.6544 (6) Å | Cell parameters from 3827 reflections |
| b = 9.9029 (6) Å | θ = 2.6–27.7° |
| c = 15.2090 (9) Å | µ = 0.12 mm−1 |
| α = 79.621 (6)° | T = 293 K |
| β = 86.579 (6)° | Prism, colourless |
| γ = 70.603 (6)° | 0.48 × 0.48 × 0.36 mm |
| V = 1349.10 (15) Å3 |
4-Phenylpiperazin-1-ium trifluoroacetate 0.123-hydrate (IV). Data collection
| Oxford Diffraction Xcalibur diffractometer | 2777 reflections with I > 2σ(I) |
| ω scans | Rint = 0.014 |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2009) | θmax = 25.3°, θmin = 2.6° |
| Tmin = 0.724, Tmax = 1.000 | h = −11→11 |
| 9220 measured reflections | k = −11→11 |
| 4940 independent reflections | l = −18→18 |
4-Phenylpiperazin-1-ium trifluoroacetate 0.123-hydrate (IV). Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: structure-invariant direct methods |
| R[F2 > 2σ(F2)] = 0.070 | Hydrogen site location: mixed |
| wR(F2) = 0.235 | H-atom parameters constrained |
| S = 1.07 | w = 1/[σ2(Fo2) + (0.1128P)2 + 0.3819P] where P = (Fo2 + 2Fc2)/3 |
| 4927 reflections | (Δ/σ)max < 0.001 |
| 375 parameters | Δρmax = 0.42 e Å−3 |
| 4 restraints | Δρmin = −0.28 e Å−3 |
| 0 constraints |
4-Phenylpiperazin-1-ium trifluoroacetate 0.123-hydrate (IV). Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
4-Phenylpiperazin-1-ium trifluoroacetate 0.123-hydrate (IV). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| C1 | 0.2559 (3) | 0.7294 (3) | 0.51366 (18) | 0.0565 (7) | |
| C2 | 0.1392 (4) | 0.7473 (3) | 0.5737 (2) | 0.0733 (9) | |
| H2 | 0.043765 | 0.775704 | 0.552306 | 0.088* | |
| C3 | 0.1634 (5) | 0.7235 (4) | 0.6648 (3) | 0.0913 (12) | |
| H3 | 0.083976 | 0.737161 | 0.703837 | 0.11* | |
| C4 | 0.3019 (6) | 0.6803 (4) | 0.6979 (2) | 0.0945 (12) | |
| H4 | 0.317436 | 0.664341 | 0.759246 | 0.113* | |
| C5 | 0.4172 (5) | 0.6606 (4) | 0.6405 (3) | 0.0939 (11) | |
| H5 | 0.512209 | 0.630205 | 0.66299 | 0.113* | |
| C6 | 0.3958 (4) | 0.6850 (4) | 0.5497 (2) | 0.0746 (9) | |
| H6 | 0.476639 | 0.671553 | 0.511724 | 0.089* | |
| C7 | 0.3582 (4) | 0.7522 (4) | 0.3646 (2) | 0.0748 (9) | |
| H7A | 0.439678 | 0.665346 | 0.385364 | 0.09* | |
| H7B | 0.386552 | 0.835603 | 0.369838 | 0.09* | |
| C8 | 0.3289 (5) | 0.7565 (4) | 0.2686 (2) | 0.0924 (11) | |
| H8A | 0.41407 | 0.762586 | 0.232966 | 0.111* | |
| H8B | 0.311597 | 0.667892 | 0.261853 | 0.111* | |
| C9 | 0.0709 (5) | 0.8815 (5) | 0.2926 (3) | 0.1078 (14) | |
| H9A | 0.044836 | 0.796648 | 0.287542 | 0.129* | |
| H9B | −0.01211 | 0.967225 | 0.272441 | 0.129* | |
| C10 | 0.1038 (4) | 0.8774 (5) | 0.3876 (2) | 0.0933 (12) | |
| H10A | 0.121556 | 0.966325 | 0.393067 | 0.112* | |
| H10B | 0.019188 | 0.872467 | 0.424128 | 0.112* | |
| C11 | 0.7162 (3) | 0.7837 (3) | 0.34093 (18) | 0.0568 (7) | |
| C12 | 0.6756 (3) | 0.9091 (3) | 0.3787 (2) | 0.0682 (8) | |
| H12 | 0.637995 | 0.999179 | 0.342289 | 0.082* | |
| C13 | 0.6902 (4) | 0.9023 (4) | 0.4692 (2) | 0.0777 (9) | |
| H13 | 0.660653 | 0.987687 | 0.492938 | 0.093* | |
| C14 | 0.7475 (4) | 0.7719 (5) | 0.5247 (2) | 0.0786 (10) | |
| H14 | 0.758672 | 0.767891 | 0.585529 | 0.094* | |
| C15 | 0.7878 (4) | 0.6476 (4) | 0.4884 (2) | 0.0856 (10) | |
| H15 | 0.826468 | 0.558096 | 0.525259 | 0.103* | |
| C16 | 0.7723 (4) | 0.6527 (4) | 0.3984 (2) | 0.0760 (9) | |
| H16 | 0.799969 | 0.566471 | 0.375625 | 0.091* | |
| C17 | 0.8021 (5) | 0.6701 (4) | 0.2099 (2) | 0.0988 (13) | |
| H17A | 0.807268 | 0.577457 | 0.246337 | 0.119* | |
| H17B | 0.898326 | 0.680875 | 0.21027 | 0.119* | |
| C18 | 0.7624 (6) | 0.6706 (5) | 0.1152 (3) | 0.1280 (18) | |
| H18A | 0.837937 | 0.594874 | 0.090434 | 0.154* | |
| H18B | 0.670336 | 0.65126 | 0.114843 | 0.154* | |
| C19 | 0.6330 (5) | 0.9270 (5) | 0.0978 (2) | 0.0940 (11) | |
| H19A | 0.540215 | 0.909315 | 0.096567 | 0.113* | |
| H19B | 0.622668 | 1.021169 | 0.061705 | 0.113* | |
| C20 | 0.6691 (4) | 0.9279 (4) | 0.1919 (2) | 0.0773 (9) | |
| H20A | 0.756179 | 0.95605 | 0.191813 | 0.093* | |
| H20B | 0.58882 | 0.999996 | 0.216184 | 0.093* | |
| C21 | 0.2670 (4) | 0.2041 (4) | 0.1833 (2) | 0.0776 (9) | |
| C22 | 0.2420 (8) | 0.3636 (6) | 0.1772 (3) | 0.1240 (19) | 0.736 (3) |
| C22' | 0.2420 (8) | 0.3636 (6) | 0.1772 (3) | 0.1240 (19) | 0.264 (3) |
| C23 | 0.1345 (7) | 0.8145 (4) | 0.0390 (2) | 0.0940 (13) | |
| C24 | 0.1356 (7) | 0.7597 (5) | −0.0474 (3) | 0.1064 (14) | 0.736 (3) |
| C24' | 0.1356 (7) | 0.7597 (5) | −0.0474 (3) | 0.1064 (14) | 0.264 (3) |
| N1 | 0.2321 (3) | 0.7528 (2) | 0.42089 (15) | 0.0596 (6) | |
| N2 | 0.1985 (4) | 0.8842 (3) | 0.23622 (18) | 0.0888 (9) | |
| N3 | 0.6944 (3) | 0.7878 (3) | 0.24907 (15) | 0.0687 (7) | |
| N4 | 0.7485 (4) | 0.8143 (4) | 0.05995 (19) | 0.1087 (12) | |
| O1 | 0.2367 (4) | 0.1448 (3) | 0.25394 (17) | 0.1168 (10) | |
| O2 | 0.3178 (4) | 0.1492 (4) | 0.11854 (18) | 0.1323 (12) | |
| O3 | 0.2462 (4) | 0.8383 (4) | 0.05509 (19) | 0.1252 (11) | |
| O4 | 0.0222 (4) | 0.8316 (3) | 0.08423 (17) | 0.1113 (10) | |
| F1 | 0.3669 (7) | 0.3949 (7) | 0.1721 (5) | 0.202 (3) | 0.736 (3) |
| F2 | 0.1775 (9) | 0.4242 (5) | 0.2477 (4) | 0.184 (3) | 0.736 (3) |
| F3 | 0.1706 (8) | 0.4421 (5) | 0.1039 (4) | 0.179 (3) | 0.736 (3) |
| F1' | 0.302 (2) | 0.407 (2) | 0.0998 (15) | 0.202 (3) | 0.264 (3) |
| F2' | 0.282 (3) | 0.3950 (17) | 0.2311 (15) | 0.184 (3) | 0.264 (3) |
| F3' | 0.102 (2) | 0.4303 (16) | 0.1642 (11) | 0.179 (3) | 0.264 (3) |
| F4 | 0.2609 (7) | 0.6549 (8) | −0.0615 (4) | 0.175 (2) | 0.736 (3) |
| F5 | 0.1187 (10) | 0.8524 (4) | −0.1159 (2) | 0.181 (3) | 0.736 (3) |
| F6 | 0.0480 (7) | 0.6817 (8) | −0.0460 (4) | 0.153 (2) | 0.736 (3) |
| F4' | 0.238 (2) | 0.789 (2) | −0.1015 (13) | 0.175 (2) | 0.264 (3) |
| F5' | 0.157 (4) | 0.6370 (19) | −0.0412 (9) | 0.181 (3) | 0.264 (3) |
| F6' | −0.0072 (19) | 0.8256 (19) | −0.0913 (10) | 0.153 (2) | 0.264 (3) |
| OW1 | 0.5122 (12) | 0.6320 (14) | 0.0361 (10) | 0.150 (8) | 0.245 (10) |
| H21 | 0.207318 | 0.969376 | 0.238227 | 0.18* | |
| H22 | 0.187596 | 0.886717 | 0.179696 | 0.18* | |
| H41 | 0.826598 | 0.838953 | 0.053835 | 0.18* | |
| H42 | 0.722096 | 0.810784 | 0.005806 | 0.18* | |
| HW1A | 0.572085 | 0.618747 | −0.007306 | 0.225* | 0.245 (10) |
| HW1B | 0.441675 | 0.707638 | 0.015874 | 0.225* | 0.245 (10) |
4-Phenylpiperazin-1-ium trifluoroacetate 0.123-hydrate (IV). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.065 (2) | 0.0490 (15) | 0.0531 (16) | −0.0184 (13) | 0.0022 (14) | −0.0040 (12) |
| C2 | 0.075 (2) | 0.069 (2) | 0.068 (2) | −0.0195 (16) | 0.0111 (17) | −0.0051 (15) |
| C3 | 0.116 (3) | 0.074 (2) | 0.075 (2) | −0.025 (2) | 0.033 (2) | −0.0120 (18) |
| C4 | 0.141 (4) | 0.079 (2) | 0.056 (2) | −0.028 (2) | −0.001 (2) | −0.0089 (17) |
| C5 | 0.107 (3) | 0.098 (3) | 0.072 (2) | −0.025 (2) | −0.026 (2) | −0.0087 (19) |
| C6 | 0.064 (2) | 0.089 (2) | 0.066 (2) | −0.0204 (17) | −0.0069 (16) | −0.0074 (16) |
| C7 | 0.073 (2) | 0.086 (2) | 0.0618 (18) | −0.0228 (17) | 0.0105 (16) | −0.0132 (16) |
| C8 | 0.122 (3) | 0.098 (3) | 0.062 (2) | −0.039 (2) | 0.010 (2) | −0.0215 (19) |
| C9 | 0.099 (3) | 0.139 (4) | 0.079 (3) | −0.044 (3) | −0.030 (2) | 0.017 (2) |
| C10 | 0.065 (2) | 0.128 (3) | 0.063 (2) | −0.010 (2) | −0.0084 (17) | 0.0067 (19) |
| C11 | 0.0597 (18) | 0.0649 (18) | 0.0512 (15) | −0.0254 (14) | 0.0087 (13) | −0.0168 (13) |
| C12 | 0.086 (2) | 0.0636 (18) | 0.0605 (17) | −0.0280 (16) | 0.0031 (15) | −0.0183 (14) |
| C13 | 0.089 (2) | 0.093 (2) | 0.066 (2) | −0.0390 (19) | 0.0122 (18) | −0.0374 (19) |
| C14 | 0.076 (2) | 0.119 (3) | 0.0513 (17) | −0.044 (2) | 0.0031 (16) | −0.019 (2) |
| C15 | 0.092 (3) | 0.093 (3) | 0.063 (2) | −0.024 (2) | −0.0075 (18) | −0.0006 (18) |
| C16 | 0.091 (2) | 0.065 (2) | 0.066 (2) | −0.0172 (16) | 0.0037 (17) | −0.0140 (15) |
| C17 | 0.167 (4) | 0.071 (2) | 0.064 (2) | −0.042 (2) | 0.028 (2) | −0.0283 (17) |
| C18 | 0.221 (6) | 0.115 (4) | 0.074 (3) | −0.078 (4) | 0.039 (3) | −0.050 (2) |
| C19 | 0.118 (3) | 0.118 (3) | 0.0538 (19) | −0.051 (2) | 0.0026 (19) | −0.0131 (19) |
| C20 | 0.094 (3) | 0.076 (2) | 0.0602 (18) | −0.0257 (18) | −0.0026 (17) | −0.0121 (16) |
| C21 | 0.102 (3) | 0.086 (2) | 0.0458 (17) | −0.0309 (19) | −0.0063 (17) | −0.0135 (16) |
| C22 | 0.194 (6) | 0.122 (4) | 0.068 (3) | −0.074 (4) | −0.044 (3) | 0.011 (3) |
| C22' | 0.194 (6) | 0.122 (4) | 0.068 (3) | −0.074 (4) | −0.044 (3) | 0.011 (3) |
| C23 | 0.159 (4) | 0.066 (2) | 0.052 (2) | −0.032 (2) | 0.011 (3) | −0.0113 (16) |
| C24 | 0.172 (5) | 0.084 (3) | 0.070 (2) | −0.047 (3) | 0.028 (3) | −0.028 (2) |
| C24' | 0.172 (5) | 0.084 (3) | 0.070 (2) | −0.047 (3) | 0.028 (3) | −0.028 (2) |
| N1 | 0.0591 (15) | 0.0674 (15) | 0.0519 (13) | −0.0234 (12) | −0.0030 (11) | −0.0028 (11) |
| N2 | 0.136 (3) | 0.082 (2) | 0.0530 (15) | −0.0447 (19) | −0.0139 (17) | −0.0015 (13) |
| N3 | 0.094 (2) | 0.0709 (16) | 0.0501 (13) | −0.0357 (14) | 0.0087 (12) | −0.0184 (12) |
| N4 | 0.162 (3) | 0.127 (3) | 0.0538 (16) | −0.066 (2) | 0.0207 (19) | −0.0303 (18) |
| O1 | 0.205 (3) | 0.0908 (18) | 0.0635 (16) | −0.0618 (19) | 0.0128 (17) | −0.0133 (13) |
| O2 | 0.163 (3) | 0.164 (3) | 0.0675 (16) | −0.038 (2) | 0.0098 (17) | −0.0474 (18) |
| O3 | 0.161 (3) | 0.157 (3) | 0.0719 (18) | −0.062 (3) | 0.0133 (19) | −0.0395 (18) |
| O4 | 0.154 (3) | 0.121 (2) | 0.0633 (15) | −0.0477 (19) | 0.0290 (17) | −0.0313 (14) |
| F1 | 0.249 (6) | 0.248 (6) | 0.198 (6) | −0.196 (5) | −0.015 (4) | −0.040 (4) |
| F2 | 0.289 (8) | 0.078 (3) | 0.159 (4) | −0.016 (4) | 0.037 (5) | −0.053 (2) |
| F3 | 0.278 (7) | 0.117 (3) | 0.142 (4) | −0.095 (3) | −0.104 (5) | 0.068 (3) |
| F1' | 0.249 (6) | 0.248 (6) | 0.198 (6) | −0.196 (5) | −0.015 (4) | −0.040 (4) |
| F2' | 0.289 (8) | 0.078 (3) | 0.159 (4) | −0.016 (4) | 0.037 (5) | −0.053 (2) |
| F3' | 0.278 (7) | 0.117 (3) | 0.142 (4) | −0.095 (3) | −0.104 (5) | 0.068 (3) |
| F4 | 0.227 (5) | 0.149 (4) | 0.137 (4) | −0.024 (4) | 0.046 (3) | −0.082 (4) |
| F5 | 0.411 (11) | 0.084 (2) | 0.0504 (17) | −0.087 (4) | −0.014 (3) | 0.0007 (16) |
| F6 | 0.226 (5) | 0.152 (4) | 0.118 (3) | −0.092 (4) | 0.009 (3) | −0.061 (3) |
| F4' | 0.227 (5) | 0.149 (4) | 0.137 (4) | −0.024 (4) | 0.046 (3) | −0.082 (4) |
| F5' | 0.411 (11) | 0.084 (2) | 0.0504 (17) | −0.087 (4) | −0.014 (3) | 0.0007 (16) |
| F6' | 0.226 (5) | 0.152 (4) | 0.118 (3) | −0.092 (4) | 0.009 (3) | −0.061 (3) |
| OW1 | 0.082 (10) | 0.146 (13) | 0.219 (18) | −0.019 (8) | −0.031 (9) | −0.048 (11) |
4-Phenylpiperazin-1-ium trifluoroacetate 0.123-hydrate (IV). Geometric parameters (Å, º)
| C1—C6 | 1.388 (4) | C17—C18 | 1.512 (5) |
| C1—C2 | 1.392 (4) | C17—H17A | 0.97 |
| C1—N1 | 1.408 (3) | C17—H17B | 0.97 |
| C2—C3 | 1.384 (5) | C18—N4 | 1.485 (6) |
| C2—H2 | 0.93 | C18—H18A | 0.97 |
| C3—C4 | 1.359 (6) | C18—H18B | 0.97 |
| C3—H3 | 0.93 | C19—N4 | 1.465 (5) |
| C4—C5 | 1.357 (5) | C19—C20 | 1.495 (4) |
| C4—H4 | 0.93 | C19—H19A | 0.97 |
| C5—C6 | 1.375 (5) | C19—H19B | 0.97 |
| C5—H5 | 0.93 | C20—N3 | 1.451 (4) |
| C6—H6 | 0.93 | C20—H20A | 0.97 |
| C7—N1 | 1.445 (4) | C20—H20B | 0.97 |
| C7—C8 | 1.494 (5) | C21—O1 | 1.197 (4) |
| C7—H7A | 0.97 | C21—O2 | 1.211 (4) |
| C7—H7B | 0.97 | C21—C22' | 1.502 (7) |
| C8—N2 | 1.489 (5) | C21—C22 | 1.502 (7) |
| C8—H8A | 0.97 | C22—F3 | 1.321 (6) |
| C8—H8B | 0.97 | C22—F1 | 1.337 (7) |
| C9—N2 | 1.464 (5) | C22—F2 | 1.343 (7) |
| C9—C10 | 1.488 (5) | C22'—F2' | 1.06 (2) |
| C9—H9A | 0.97 | C22'—F3' | 1.306 (19) |
| C9—H9B | 0.97 | C22'—F1' | 1.346 (19) |
| C10—N1 | 1.466 (4) | C23—O3 | 1.224 (5) |
| C10—H10A | 0.97 | C23—O4 | 1.228 (5) |
| C10—H10B | 0.97 | C23—C24' | 1.506 (6) |
| C11—C16 | 1.386 (4) | C23—C24 | 1.506 (6) |
| C11—C12 | 1.390 (4) | C24—F5 | 1.238 (5) |
| C11—N3 | 1.417 (3) | C24—F6 | 1.319 (7) |
| C12—C13 | 1.379 (4) | C24—F4 | 1.342 (7) |
| C12—H12 | 0.93 | C24'—F5' | 1.151 (17) |
| C13—C14 | 1.369 (5) | C24'—F4' | 1.32 (2) |
| C13—H13 | 0.93 | C24'—F6' | 1.459 (17) |
| C14—C15 | 1.368 (5) | N2—H21 | 0.8818 |
| C14—H14 | 0.93 | N2—H22 | 0.8672 |
| C15—C16 | 1.377 (5) | N4—H41 | 0.8621 |
| C15—H15 | 0.93 | N4—H42 | 0.8861 |
| C16—H16 | 0.93 | OW1—HW1A | 0.8501 |
| C17—N3 | 1.471 (4) | OW1—HW1B | 0.8501 |
| C6—C1—C2 | 116.9 (3) | N4—C18—H18B | 109.8 |
| C6—C1—N1 | 122.0 (3) | C17—C18—H18B | 109.8 |
| C2—C1—N1 | 121.1 (3) | H18A—C18—H18B | 108.2 |
| C3—C2—C1 | 120.8 (3) | N4—C19—C20 | 110.7 (3) |
| C3—C2—H2 | 119.6 | N4—C19—H19A | 109.5 |
| C1—C2—H2 | 119.6 | C20—C19—H19A | 109.5 |
| C4—C3—C2 | 120.8 (3) | N4—C19—H19B | 109.5 |
| C4—C3—H3 | 119.6 | C20—C19—H19B | 109.5 |
| C2—C3—H3 | 119.6 | H19A—C19—H19B | 108.1 |
| C5—C4—C3 | 119.2 (4) | N3—C20—C19 | 112.8 (3) |
| C5—C4—H4 | 120.4 | N3—C20—H20A | 109 |
| C3—C4—H4 | 120.4 | C19—C20—H20A | 109 |
| C4—C5—C6 | 121.0 (4) | N3—C20—H20B | 109 |
| C4—C5—H5 | 119.5 | C19—C20—H20B | 109 |
| C6—C5—H5 | 119.5 | H20A—C20—H20B | 107.8 |
| C5—C6—C1 | 121.2 (3) | O1—C21—O2 | 127.2 (4) |
| C5—C6—H6 | 119.4 | O1—C21—C22' | 115.1 (4) |
| C1—C6—H6 | 119.4 | O2—C21—C22' | 117.7 (4) |
| N1—C7—C8 | 112.5 (3) | O1—C21—C22 | 115.1 (4) |
| N1—C7—H7A | 109.1 | O2—C21—C22 | 117.7 (4) |
| C8—C7—H7A | 109.1 | F3—C22—F1 | 103.5 (5) |
| N1—C7—H7B | 109.1 | F3—C22—F2 | 108.8 (6) |
| C8—C7—H7B | 109.1 | F1—C22—F2 | 100.9 (6) |
| H7A—C7—H7B | 107.8 | F3—C22—C21 | 112.7 (4) |
| N2—C8—C7 | 110.4 (3) | F1—C22—C21 | 112.9 (6) |
| N2—C8—H8A | 109.6 | F2—C22—C21 | 116.7 (4) |
| C7—C8—H8A | 109.6 | F2'—C22'—F3' | 112.1 (15) |
| N2—C8—H8B | 109.6 | F2'—C22'—F1' | 110.0 (16) |
| C7—C8—H8B | 109.6 | F3'—C22'—F1' | 103.4 (13) |
| H8A—C8—H8B | 108.1 | F2'—C22'—C21 | 116.5 (11) |
| N2—C9—C10 | 110.4 (3) | F3'—C22'—C21 | 106.9 (7) |
| N2—C9—H9A | 109.6 | F1'—C22'—C21 | 107.0 (10) |
| C10—C9—H9A | 109.6 | O3—C23—O4 | 127.7 (4) |
| N2—C9—H9B | 109.6 | O3—C23—C24' | 115.5 (5) |
| C10—C9—H9B | 109.6 | O4—C23—C24' | 116.8 (5) |
| H9A—C9—H9B | 108.1 | O3—C23—C24 | 115.5 (5) |
| N1—C10—C9 | 112.1 (3) | O4—C23—C24 | 116.8 (5) |
| N1—C10—H10A | 109.2 | F5—C24—F6 | 111.9 (6) |
| C9—C10—H10A | 109.2 | F5—C24—F4 | 104.5 (6) |
| N1—C10—H10B | 109.2 | F6—C24—F4 | 96.7 (5) |
| C9—C10—H10B | 109.2 | F5—C24—C23 | 115.4 (4) |
| H10A—C10—H10B | 107.9 | F6—C24—C23 | 113.1 (4) |
| C16—C11—C12 | 116.8 (3) | F4—C24—C23 | 113.6 (5) |
| C16—C11—N3 | 121.0 (2) | F5'—C24'—F4' | 106.2 (16) |
| C12—C11—N3 | 122.0 (3) | F5'—C24'—F6' | 103.6 (18) |
| C13—C12—C11 | 121.1 (3) | F4'—C24'—F6' | 109.6 (11) |
| C13—C12—H12 | 119.4 | F5'—C24'—C23 | 116.0 (8) |
| C11—C12—H12 | 119.4 | F4'—C24'—C23 | 110.3 (9) |
| C14—C13—C12 | 121.1 (3) | F6'—C24'—C23 | 110.7 (6) |
| C14—C13—H13 | 119.4 | C1—N1—C7 | 116.1 (2) |
| C12—C13—H13 | 119.4 | C1—N1—C10 | 115.0 (2) |
| C15—C14—C13 | 118.3 (3) | C7—N1—C10 | 110.5 (2) |
| C15—C14—H14 | 120.8 | C9—N2—C8 | 110.4 (3) |
| C13—C14—H14 | 120.8 | C9—N2—H21 | 104.1 |
| C14—C15—C16 | 121.2 (3) | C8—N2—H21 | 115 |
| C14—C15—H15 | 119.4 | C9—N2—H22 | 115.8 |
| C16—C15—H15 | 119.4 | C8—N2—H22 | 108.2 |
| C15—C16—C11 | 121.4 (3) | H21—N2—H22 | 103.4 |
| C15—C16—H16 | 119.3 | C11—N3—C20 | 115.9 (2) |
| C11—C16—H16 | 119.3 | C11—N3—C17 | 114.7 (3) |
| N3—C17—C18 | 111.9 (4) | C20—N3—C17 | 111.7 (2) |
| N3—C17—H17A | 109.2 | C19—N4—C18 | 109.0 (3) |
| C18—C17—H17A | 109.2 | C19—N4—H41 | 108.2 |
| N3—C17—H17B | 109.2 | C18—N4—H41 | 116.5 |
| C18—C17—H17B | 109.2 | C19—N4—H42 | 109.5 |
| H17A—C17—H17B | 107.9 | C18—N4—H42 | 106.4 |
| N4—C18—C17 | 109.5 (3) | H41—N4—H42 | 107.1 |
| N4—C18—H18A | 109.8 | HW1A—OW1—HW1B | 104.5 |
| C17—C18—H18A | 109.8 | ||
| C6—C1—C2—C3 | 0.8 (4) | O3—C23—C24—F5 | −75.6 (8) |
| N1—C1—C2—C3 | 179.7 (3) | O4—C23—C24—F5 | 103.6 (7) |
| C1—C2—C3—C4 | −0.8 (5) | O3—C23—C24—F6 | 153.8 (6) |
| C2—C3—C4—C5 | 0.1 (6) | O4—C23—C24—F6 | −26.9 (7) |
| C3—C4—C5—C6 | 0.6 (6) | O3—C23—C24—F4 | 44.9 (7) |
| C4—C5—C6—C1 | −0.5 (6) | O4—C23—C24—F4 | −135.8 (6) |
| C2—C1—C6—C5 | −0.2 (5) | O3—C23—C24'—F5' | 102 (2) |
| N1—C1—C6—C5 | −179.0 (3) | O4—C23—C24'—F5' | −79 (2) |
| N1—C7—C8—N2 | 55.4 (4) | O3—C23—C24'—F4' | −18.9 (12) |
| N2—C9—C10—N1 | −57.1 (5) | O4—C23—C24'—F4' | 160.3 (11) |
| C16—C11—C12—C13 | −0.3 (5) | O3—C23—C24'—F6' | −140.4 (8) |
| N3—C11—C12—C13 | 176.4 (3) | O4—C23—C24'—F6' | 38.8 (9) |
| C11—C12—C13—C14 | 1.1 (5) | C6—C1—N1—C7 | −10.1 (4) |
| C12—C13—C14—C15 | −1.1 (5) | C2—C1—N1—C7 | 171.1 (3) |
| C13—C14—C15—C16 | 0.3 (5) | C6—C1—N1—C10 | −141.4 (3) |
| C14—C15—C16—C11 | 0.5 (6) | C2—C1—N1—C10 | 39.8 (4) |
| C12—C11—C16—C15 | −0.5 (5) | C8—C7—N1—C1 | 171.8 (3) |
| N3—C11—C16—C15 | −177.2 (3) | C8—C7—N1—C10 | −54.8 (4) |
| N3—C17—C18—N4 | 56.6 (5) | C9—C10—N1—C1 | −170.6 (3) |
| N4—C19—C20—N3 | −55.9 (4) | C9—C10—N1—C7 | 55.5 (4) |
| O1—C21—C22—F3 | −132.4 (6) | C10—C9—N2—C8 | 56.8 (4) |
| O2—C21—C22—F3 | 49.2 (8) | C7—C8—N2—C9 | −55.8 (4) |
| O1—C21—C22—F1 | 110.7 (6) | C16—C11—N3—C20 | −165.9 (3) |
| O2—C21—C22—F1 | −67.7 (6) | C12—C11—N3—C20 | 17.6 (4) |
| O1—C21—C22—F2 | −5.5 (8) | C16—C11—N3—C17 | −33.3 (4) |
| O2—C21—C22—F2 | 176.1 (6) | C12—C11—N3—C17 | 150.2 (3) |
| O1—C21—C22'—F2' | 47.1 (18) | C19—C20—N3—C11 | −174.6 (3) |
| O2—C21—C22'—F2' | −131.3 (17) | C19—C20—N3—C17 | 51.5 (4) |
| O1—C21—C22'—F3' | −79.2 (9) | C18—C17—N3—C11 | 173.3 (3) |
| O2—C21—C22'—F3' | 102.4 (10) | C18—C17—N3—C20 | −52.1 (4) |
| O1—C21—C22'—F1' | 170.6 (10) | C20—C19—N4—C18 | 59.6 (4) |
| O2—C21—C22'—F1' | −7.8 (12) | C17—C18—N4—C19 | −60.0 (5) |
4-Phenylpiperazin-1-ium trifluoroacetate 0.123-hydrate (IV). Hydrogen-bond geometry (Å, º)
Cg2 is the centroid of the C1–C6 phenyl ring.
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H21···O1i | 0.88 | 1.91 | 2.790 (4) | 174 |
| N2—H22···O3 | 0.87 | 2.04 | 2.860 (4) | 157 |
| N2—H22···O4 | 0.87 | 2.47 | 3.164 (5) | 137 |
| N4—H41···O4ii | 0.86 | 1.95 | 2.759 (6) | 156 |
| N4—H42···O2iii | 0.89 | 1.90 | 2.758 (4) | 164 |
| C18—H18A···F5′iii | 0.97 | 2.53 | 3.273 (18) | 134 |
| C18—H18B···Ow1 | 0.97 | 2.08 | 2.929 (15) | 145 |
| C19—H19B···O3iv | 0.97 | 2.59 | 3.420 (5) | 144 |
| C20—H20A···F5iv | 0.97 | 2.64 | 3.468 (8) | 144 |
| C16—H16···Cg2v | 0.93 | 2.99 | 3.745 (4) | 140 |
Symmetry codes: (i) x, y+1, z; (ii) x+1, y, z; (iii) −x+1, −y+1, −z; (iv) −x+1, −y+2, −z; (v) −x+1, −y+1, −z+1.
Funding Statement
Funding for this research was provided by: Ministerio de Ciencia e Innovación (grant No. PID2020-113558RB-C41 to S. Garcia-Granda, M. S. M. Abdelbaky); Gobierno del Principado de Asturias (grant No. AYUD/2021/50997 to S. Garcia-Granda, M. S. M. Abdelbaky).
References
- Al-Alshaikh, M. A., El-Emam, A. A., Al-Deeb, O. A., Abdelbaky, M. S. M. & Garcia-Granda, S. (2015). Acta Cryst. E71, 956–959. [DOI] [PMC free article] [PubMed]
- Bogatcheva, E., Hanrahan, C., Nikonenko, B., Samala, R., Chen, P., Gearhart, J., Barbosa, F., Einck, L., Nacy, C. A. & Protopopova, M. (2006). J. Med. Chem. 49, 3045–3048. [DOI] [PMC free article] [PubMed]
- Brockunier, L. L., He, J., Colwell, L. F. Jr, Habulihaz, B., He, H., Leiting, B., Lyons, K. A., Marsilio, F., Patel, R. A., Teffera, Y., Wu, J. K., Thornberry, N. A., Weber, A. E. & Parmee, E. R. (2004). Bioorg. Med. Chem. Lett. 14, 4763–4766. [DOI] [PubMed]
- Cohen, M. R., Hinsch, E., Palkoski, Z., Vergona, R., Urbano, S. & Sztokalo, J. (1982). J. Pharmacol. Exp. Ther. 223, 110–119. [PubMed]
- Conrado, D. J., Verli, H., Neves, G., Fraga, C. A., Barreiro, E. J., Rates, S. M. & Dalla-Costa, T. (2010). J. Pharm. Pharmacol. 60, 699–707. [DOI] [PubMed]
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
- Demirci, S., Hayal, T. B., Kıratlı, B., Şişli, H. B., Demirci, S., Şahin, F. & Doğan, A. (2019). Chem. Biol. Drug Des. 94, 1584–1595. [DOI] [PubMed]
- Elliott, S. (2011). Drug Test Anal. 3, 430–438. [DOI] [PubMed]
- Essid, M., Marouani, H., Rzaigui, M. & Al-Deyab, S. S. (2010). Acta Cryst. E66, o2244–o2245. [DOI] [PMC free article] [PubMed]
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Hanano, T., Adachi, K., Aoki, Y., Morimoto, H., Naka, Y., Hisadome, M., Fukuda, T. & Sumichika, H. (2000). Bioorg. Med. Chem. Lett. 10, 875–879. [DOI] [PubMed]
- Harish Chinthal, C., Kavitha, C. N., Yathirajan, H. S., Foro, S., Rathore, R. S. & Glidewell, C. (2020). Acta Cryst. E76, 1779–1793. [DOI] [PMC free article] [PubMed]
- Kiran Kumar, H., Yathirajan, H. S., Foro, S. & Glidewell, C. (2019a). Acta Cryst. E75, 1494–1506. [DOI] [PMC free article] [PubMed]
- Kiran Kumar, H., Yathirajan, H. S., Harish Chinthal, C., Foro, S. & Glidewell, C. (2020a). Acta Cryst. E76, 488–495. [DOI] [PMC free article] [PubMed]
- Kiran Kumar, H., Yathirajan, H. S., Harish Chinthal, C., Foro, S. & Glidewell, C. (2020b). CSD Communication (refcode KUJPUD). CCDC, Cambridge, England.
- Kiran Kumar, H., Yathirajan, H. S., Sagar, B. K., Foro, S. & Glidewell, C. (2019b). Acta Cryst. E75, 1253–1260. [DOI] [PMC free article] [PubMed]
- Li, A.-J., Zhang, X.-H., Sun, W.-Q., Zhou, X.-Q. & Liu, D.-Z. (2008). Acta Cryst. E64, o1234. [DOI] [PMC free article] [PubMed]
- Macrae, C. F., Sovago, I., Cottrell, S. J., Galek, P. T. A., McCabe, P., Pidcock, E., Platings, M., Shields, G. P., Stevens, J. S., Towler, M. & Wood, P. A. (2020). J. Appl. Cryst. 53, 226–235. [DOI] [PMC free article] [PubMed]
- Marouani, H., Rzaigui, M. & Al-Deyab, S. S. (2010). Acta Cryst. E66, o2613. [DOI] [PMC free article] [PubMed]
- Neves, G., Fenner, R., Heckler, A. P., Viana, A. F., Tasso, L., Menegatti, R., Fraga, C. A. M., Barreiro, E. J., Dalla-Costa, T. & Rates, S. M. K. (2003). Braz. J. Med. Biol. Res. 36, 625–629. [DOI] [PubMed]
- Oxford Diffraction (2009). CrysAlis CCD and CrysAlis RED. Oxford Diffraction Ltd, Abingdon, England.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Spek, A. L. (2020). Acta Cryst. E76, 1–11. [DOI] [PMC free article] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Xu, Y.-J. & Jing, F. (2009). Acta Cryst. E65, o1211. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I, II, III, IV. DOI: 10.1107/S2056989022006004/dj2048sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989022006004/dj2048Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989022006004/dj2048Isup6.cml
Structure factors: contains datablock(s) II. DOI: 10.1107/S2056989022006004/dj2048IIsup3.hkl
Supporting information file. DOI: 10.1107/S2056989022006004/dj2048IIsup7.cml
Structure factors: contains datablock(s) III. DOI: 10.1107/S2056989022006004/dj2048IIIsup4.hkl
Supporting information file. DOI: 10.1107/S2056989022006004/dj2048IIIsup8.cml
Structure factors: contains datablock(s) IV. DOI: 10.1107/S2056989022006004/dj2048IVsup5.hkl
Supporting information file. DOI: 10.1107/S2056989022006004/dj2048IVsup9.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report