Abstract
Chronic rotator cuff tears are debilitating diseases which significantly affect patients’ quality of life and pose substantial financial burden to the society. The intraoperative reparability of injured tendon and postoperative probability of tendon retear are highly associated with the quality of torn muscles, specifically, the severity of muscle atrophy and fatty infiltration. Animal models that reproduce the characteristic muscle pathology after rotator cuff injury have been developed and used to provide insight into the underlying biology and pathophysiology. In this review, we briefly summarize the current information obtained from preclinical animal studies regarding the degenerative change of cuff muscle subsequent to tendon release and/or suprascapular nerve denervation. Importantly, we focus on the potential translational therapeutic targets or agents for the prevention or reversal of muscle atrophy and fatty infiltration. While further studies are warranted to assess the safety and efficacy of novel therapies derived from these preclinical animal research, we believe that their clinical translation for the treatment of rotator cuff disorders is on the horizon.
The Translational potential of this article
Novel therapeutic strategies described in this review from preclinical animal studies hold a great translational potential for preventing or reversing rotator cuff muscle pathology, while further assessments on their safety and efficacy are warranted.
Keywords: Rotator cuff tear, Animal model, Muscle atrophy, Fatty infiltration, Tenotomy, Suprascapular nerve denervation
1. Introduction
Chronic rotator cuff tears (RCTs) are characterized by retracted tendon, muscle atrophy, fatty infiltration and fibrosis [1,2]. While the underlying mechanisms are not fully understood, increasing evidence have suggested the critical role of tendon tear and suprascapular neuropathy in rotator cuff muscular changes [[3], [4], [5]]. More importantly, the severity of muscle atrophy and fatty infiltration is closely correlated with the clinical outcomes and repair integrity [[6], [7], [8], [9]]. In a prospective cohort study, Gladstone et al. [9] found that preoperative poorer muscle quality of the infraspinatus significantly affected postoperative functional scores. Similarly, Liem et al. [10] noted that higher degree of preoperative supraspinatus atrophy along with Goutallier stage 2 fatty infiltration were positive predictors of a retear of the repaired tendon. For patients with Goutallier stage 3 or 4 fatty infiltration, it has also been shown that higher percentage of intramuscular fat was related to inferior clinical results [11]. In addition, previous studies have demonstrated that even after a successful repair, the initial fatty infiltration and muscle atrophy were irreversible [7,9,12]. Currently, there are no treatment or rehabilitation protocols that specifically target muscle degeneration after RCTs or tendon repair [13,14]. Although neuromuscular electrical stimulation has been used for improving muscle impairment in patients with hemiplegic shoulder subluxation [15], only a few reports have demonstrated its efficacy in enhancing peak force production of the infraspinatus [16] and reducing muscle atrophy of the deltoid [17] after rotator cuff repair. Therefore, a better understanding of the pathophysiologic process involved in muscular degeneration may contribute to early diagnosis, timely management and improved prognosis.
Animal models including rat, dog, sheep, rabbit and other mammals have been widely used to reveal the etiology, pathogenesis of rotator cuff disorders, and to assess novel treating strategies for RCTs [[18], [19], [20]]. The selection of an appropriate animal model and the development of new animal models mimicking the features of rotator cuff injury in humans are an essential prerequisite for potential clinical translation [21]. Although a number of well-established animal shoulder models have been reported previously, the majority of them were focused on investigating surgical techniques, suture or augmentation materials for cuff tears [[22], [23], [24], [25]]. Recently, animal models simulating fatty infiltration and atrophy within rotator cuff muscles has gained increasing attention as researchers have gradually realized the importance of clarifying these changes [[26], [27], [28], [29], [30], [31], [32]]. In this regard, we provide a review of the animal models that have been utilized for the purpose of reproducing degenerative muscular changes after RCTs, as well as aiding in the understanding of involved molecular or cellular processes and the development of novel translational therapies.
2. Process of muscle degeneration after chronic RCTs
The progression of muscle pathology following a chronic tear can be categorized into early, middle and late stages [33]. During the early stage, the primary muscular change is atrophy which is characterized by decreased muscle cross-sectional area (CSA) and muscle length. Beginning at the middle stage, muscular change at the micro-level is seen including alterations in the type and size of muscle fiber, as well as in the length of sarcomere. In addition, the accumulation of fat and connective tissue within muscle, namely fatty infiltration and fibrosis, are frequently observed. With ongoing atrophy, fibrosis and fatty infiltration of the tissue, the mechanical and biological microenvironment of torn muscle is severely deteriorated which in turn compromises its regenerative capacity.
3. Muscle response to tendon release
The detachment of tendon and subsequent musculotendinous unit retraction is thought to be the primary reason for irreversible changes in rotator cuff musculature. In a cadaver study, it has been shown that cuff tears shortened the length and increased the pennation angle of muscle fibers of the supraspinatus and infraspinatus [34]. Through reattaching the sheep infraspinatus tendon after 40 weeks of tendon release, Meyer et al. [35] assessed the changes in muscle structure and property at 75 weeks (35 weeks after tendon repair). The infraspinatus muscle retracted 9% of its entire length, the pennation angle and the mean muscle fiber length was increased and shortened approximately 50%, respectively. Histological analysis further showed a significant increase of intramuscular fat and fibrous tissue. Since tendon release is associated with shortened fiber length and increased pennation angle which allowed intramuscular fat accumulation, the restoration of pennation angle and length of muscle fibers may hold the potential to prevent or reverse architectural muscle changes. Therefore, the effect of a device allowing continuous extension of the retracted tendon on reversal of muscle changes was investigated [36]. The sheep infraspinatus tendon was detached for 16 weeks before mounting the device on the scapular spine to exert transcutaneously elongation of 1 mm per day for 6 weeks, the tendon was then repaired back to its insertion. Twelve weeks after the repair, the pennation angle of successfully elongated muscles was comparable to the controls. In contrast, the pennation angle of the failed elongated muscles was increased by around 25°. In terms of fatty infiltration, successfully elongated muscles remained at Goutallier stage 1 while a rapid progression to Goutallier stage 2 was seen in failed elongated muscles. In addition, muscle atrophy was improved in the sheep with successful traction compared to the sheep with failed traction [36]. Of note is that only two-thirds of sheep had successful traction (8 of 12 sheep), suggesting this technique needs further refinement to maximize its efficacy.
In small animal models such as mouse [37], rat [38] and rabbit [28], the progression of muscle atrophy, fatty infiltration and fibrosis after tenotomy of the rotator cuff tendon has also been noted. In rabbits after supraspinatus tenotomy, the loss of muscle mass was started at 2 weeks, and continues over time with a reduction of 25% at 16 weeks. Decreased muscle fiber CSA was seen at 4 and 16 weeks, with a 26.5% smaller CSA at 16 weeks. By 4 weeks after tenotomy, there were increased collagen content and fat accumulation which reached the largest difference compared to sham side at 8 weeks and 16 weeks, respectively. Additionally, while significant increased intrafascicular fat accumulation was observed at 1, 4 and 16 weeks, a significant difference in interfascicular fat accumulation was only seen 1 week after tenotomy [28]. Liu et al. [38] established a rat massive RCTs model with tenotomy of the supraspinatus, infraspinatus and teres minor tendons. The average wet weight of the supraspinatus and infraspinatus muscle lost 25.4% and 28.9% compared to the control side 2 weeks after tenotomy, respectively. At 6 weeks, the muscle wet weight loss was 13.2% and 28.3% for the supraspinatus and infraspinatus, respectively. Although no significant differences were seen in either whole muscle CSA or muscle fiber CSA post-tenotomy, increased intrafibrillar space, collagenous connective tissue and fat accumulation was found in the affected muscles. In a following study, Liu et al. [37] created a mouse model simulating massive RCTs by transecting the supraspinatus and infraspinatus tendons. Compared to the sham control, decrease of muscle wet weight, muscle volume, and increase of intramuscular fat volume and connective tissue was demonstrated in both supraspinatus and infraspinatus muscles 12 weeks after surgery.
Besides architectural and histological changes, the functional change of torn cuff muscle has been reported [39]. Using staircase test, Sevivas et al. [40] found that the motor control function of the forepaw was significantly affected in rats with chronic massive RCTs. Similar impairment of the forepaw function, which was indicated by worse ambulatory parameters and total range of motion, was also noted after tendon detachment in a rat model [41]. Moreover, Fabis’ et al. [42] studied the twitch tension and fatigue index of rabbit supraspinatus muscle after 6, 12 and 24 weeks of tenotomy. The results showed that significant decrease of both twitch tension and fatigue was began as early as 6 weeks. The twitch tension of the detached muscle expressed as a percentage of the normal muscle was 58.2%, 34.5% and 32.7% at 6, 12, and 24 weeks post-tenotomy. Similar trend was noted for the fatigue index which decreased from 45.4% to 26.7%, and from 42.5% to 18.5% after 2 and 4 min of stimulation, respectively, at 6 and 24 weeks post-tenotomy. Their findings are consistent with previous animal studies which indicate tendon detachment could influence the contractile properties of corresponding muscles [43,44].
4. Muscle response to suprascapular neuropathy
Suprascapular nerve innervates the supraspinatus and infraspinatus and may subject to injury results from retracted RCTs. Previous cadaver studies have shown that the motor branch of suprascapular nerve was tensioned due to supraspinatus retraction [45] and the anatomic course of suprascapular nerve at the spinoglenoid notch was altered when the supraspinatus/infraspinatus tendon was torn [46]. Several clinical reports have indicated the prevalence of suprascapular neuropathy (SSN) secondary to tendon tears [[47], [48], [49], [50]]. Mallon et al. [49] found SSN in the supraspinatus and/or infraspinatus muscles by electromyography (EMG) in 8 patients with massive RCTs. Costouros et al. [50] demonstrated that 38% (7 of 26) patients with massive RCTs had isolated SSN using EMG/nerve conduction velocity (Fig. 1).
Fig. 1.
Schematic illustration showing injury to the suprascapular nerve following medial retraction of torn supraspinatus and infraspinatus tendons. Reprinted with permission from Ref. [50].
Since SSN is often accompanied with tendon retraction, whether the muscle pathology after RCTs results from the mutual effect of tendon tear and suprascapular denervation, or whether they play a different role in muscle changes is not clear. In a cohort study, Shi et al. [51] revealed that SSN was marginally and significantly associated with supraspinatus and infraspinatus tendon injury, respectively. However, only 3 out of 18 and 3 out of 13 patients with full-thickness retracted supraspinatus and infraspinatus tear, showed a positive EMG of SSN, respectively. In addition, there was no correlation between fatty degeneration and SSN in both the supraspinatus and infraspinatus muscles. Beeler et al. [52] selected 20 shoulders from patients who had chronic RCTs with no signs of SSN, and 17 shoulders from patients who had EMG evidence of SSN without RCTs. A comparison of the degree of fatty infiltration and muscle atrophy, especially the muscle border, pattern and extent of fatty infiltration, was performed between these two groups on magnetic resonance imaging (MRI) scans. They identified distinct muscle changes following chronic RCTs and SSN. In the RCTs group, the muscle border smoothness was correlated with the severity of fatty infiltration, which was not observed in the SSN group. The morphological pattern of fatty infiltration in the RCTs group differed from that in the SSN group. Additionally, the perineurovascular fat surrounding the suprascapular nerve was markedly increased in the RCTs group compared to the SSN group.
Although chronic RCTs and SSN led to different MRI appearances of fatty infiltration and muscle atrophy, it is difficult to discriminate the muscle pathology when tendon tear and denervation happens concomitantly given the low practicability of muscle or nerve biopsies in patients. Accordingly, by creating denervation alone or together with tendon release in animal models could help decouple and elucidate the respective effect of RCTs and SSN. Previous small animal studies have shown that combined nerve injury aggravated the degeneration of affected cuff muscles [37,38,53]. Kim et al. [53] investigated the change of supraspinatus and infraspinatus muscles in rats underwent tenotomy or tenotomy with suprascapular nerve neurotomy. At 8 weeks after surgery, a significant muscle weight loss was seen in the supraspinatus and infraspinatus muscles with combined tenotomy and neurotomy compared to other groups. Histologically, greater number of adipocytes, severer muscle atrophy and higher level of intramuscular fat were observed in the tenotomized and neurotomized muscles. Similar muscle weight loss and muscle volume reduction was reported in a mouse massive RCTs model with combined tendon transection and suprascapular denervation at 12 weeks. Additionally, this combination was associated with the highest degree of fibrosis and fatty infiltration in both supraspinatus and infraspinatus muscles and infraspinatus alone, respectively [37].
For large animal models, Gerber et al. [32] harvested infraspinatus muscles from 18 sheep after tendon transection, suprascapular nerve transection or combined procedures. In contrast to the mean amount of intramuscular fat which showed no differences among three groups, the mean muscle volume of infraspinatus was markedly reduced after either denervation or combined procedures compared to transection alone at 6 and 16 weeks postoperatively. Furthermore, muscle denervation led to decreased pennation angle, lengthened fascicle, reduced mean fiber CSA, increased area of hybrid-type fibers, as well as a slow-to fast-type fiber transformation, which not only differed from that in tenotomized muscles but also contributed to more severe muscle atrophy. Notably, the addition of neurectomy significantly increased the muscle stiffness with a mean excursion of 7 ± 2 mm obtained under a maximum force of 100 N, whereas an excursion of 1 cm was obtained with a mean force of 61 ± 3 N in the tenotomy group [32].
To better mimic the clinical scenario that suprascapular nerve injury occurs from musculotendinous traction after RCTs, Wieser et al. [29] released the infraspinatus tendon in 6 sheep before neurectomy 8 weeks later (T/N group). Another 6 sheep had neurectomy first and tenotomy 8 weeks later (N/T group). MRI data were collected at 8 and 16 weeks after the index surgery. While the reduction of muscle volume was less severe in the T/N group than in the N/T group at 8 weeks, secondary neurectomy exacerbated muscle atrophy in tenotomized muscles from 8 to 16 weeks. Regarding fatty infiltration, the accumulation of intramuscular fat was comparable between the two groups postoperatively, though there was a trend toward more intramuscular fat in the T/N group than in the N/T group at 16 weeks. Furthermore, the musculotendinous retraction, pennation angel and muscle fiber length were not different between these two groups postoperatively [29].
Collectively, the results from Gerber et al. [32] and Wiser et al. [29] suggested that additional SSN significantly affect muscle atrophy, regardless of a single-stage tenoneurectomy or a secondary neurectomy in the sheep model, which resembles suprascapular nerve injury seen in a massive RCTs or after tendon repair. The increased muscle stiffness also replicates the clinical setting of chronic RCTs repair where the repairability of tendon is compromised by high tissue tension. In a rat model of massive RCTs with or without chemical denervation, passive mechanical testing at single fiber or fiber bundle level was performed on supraspinatus and infraspinatus muscles. It was found that single fiber stiffness was not influenced by RCTs or combined denervation, whereas fiber bundle stiffness was markedly increased in the tenotomy plus denervation rats after 8 weeks of surgery. The passive mechanics of fiber bundle was weakly correlated with collagen content [54]. Consequently, it is postulated that increased muscle stiffness is owing to increased extracellular matrix production of collagen, and thus causing abnormal fiber bundle stiffness.
5. Therapeutics
In order to develop novel therapeutic strategies for preventing or alleviating muscle pathology after RCTs, exploration of the mechanisms that mediate the atrophy and fatty infiltration of torn muscles is required. A variety of potential therapeutic targets or agents have been reported including proteases, anabolic steroids, growth factors, stem cells, molecular signaling pathways, and mitochondrial metabolism.
5.1. Proteases
It has been suggested that the change of protease activity after RCTs regulates the ECM remodeling and potential tissue damage. For example, cathepsin L, one of papain-family cysteine proteases have been reported to associate with skeletal muscle atrophy [55]. In a rat model of massive RCTs with combined suprascapular nerve denervation, the protease activity in the supraspinatus muscle was evaluated along with histological analysis at 1, 3 and 12 weeks postoperatively. A significant upregulation of cathepsin L was noted in injured supraspinatus muscle compared to control at 1 week. Interestingly, unlike the upregulation of cathepsin L in injured supraspinatus tendon with simultaneous altered collagen structure and cellular infiltration, no noticeable damage to the supraspinatus muscle was observed, indicating that cathepsin L could serve as an alarmin for later tissue damage [56].
Another protease, calpain, is capable of releasing sarcomeres by disrupting their anchors at the Z-disk. Previous studies have demonstrated the use of calpain inhibitors such as calpastatin, nitric oxide and calpeptin could preserve muscle proteins [[57], [58], [59]]. In a recent study by Ruoss et al. [27], the activity of calpain after infraspinatus tendon release and the effect of calpain inhibition on muscle atrophy and musculotendinous unit retraction was examined in a sheep model. Compared to the control group, the administration of calpeptin with and without sildenafil which promotes nitric oxide, protected the released muscle from atrophy and slow-to-fast fiber shift for 2 weeks, but this effect did not continue to 4 and 6 weeks (Fig. 2). The musculotendinous unit retraction was significantly reduced by calpeptin plus sildenafil at 30 min after tendon release, and by either calpeptin or calpeptin plus sildenafil at 4 weeks after tendon release. Mechanistically, the protective effect of calpeptin was found through maintaining FAK-pY397 levels and preventing cleavage of talin, a calpain substrate. Taken together, selectively targeting certain protease activities may mitigate muscle atrophy and tendon retraction, consequently increasing the tendon reparability and success rates of surgical repair.
Fig. 2.
The effect of calpain inhibition on muscle volume (A), muscle length (B) and muscle CSA (C) at 30 min, 2, 4, and 6 weeks postoperatively (CALP, n = 5; CALPSILD, n = 6). Time effects: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 versus 30 min; +p < 0.05, ++p < 0.01, +++p < 0.001 versus 2 weeks; &p < 0.05, &&&p < 0.001 versus 4 weeks. Group effects: #p < 0.05 versus CONTROL; $p < 0.05, $$$p < 0.001 versus operated shoulder at 30 min (D) The distribution of contractile, fatty and non-fatty connective tissue among groups prior to, and 2, 4, and 6 weeks after surgery (E) The slow-to-fast fiber shift was delayed with calpain inhibition for 2 weeks compared to the CONTROL group (CALP, n = 5; CALPSILD, n = 6). Time effects: ∗p < 0.05, ∗∗p < 0.01 versus PRE; +p < 0.05, ++p < 0.01 versus 2 weeks. Values are presented as means ± SD. CALP: calpeptin-treated; CALPSILD: calpeptin and sildenafil-treated. Adapted and reprinted with permission from Ref. [27].
5.2. Anabolic steroids and growth factors
Anabolic steroids, one of various pharmacological substances which affects muscle development, growth and repair [[60], [61], [62]], holds a great potential for clinical translation. In a rabbit supraspinatus tenotomy model with musculotendinous retraction for 6 weeks, the amount of tendon retraction was significantly reduced in animals received nandrolone decanoate during the 6 weeks compared to controls. On CT images and histological sections, no apparent fatty infiltration was observed in the nandrolone decanoate treated animals but in the untreated animals. In addition, the radiographic muscular CSA and the work of affected muscle was mostly preserved in rabbits with systemic administration of nandrolone decanoate [63].
Given the successful application of nandrolone decanoate on preventing detrimental effects of chronic tendon retraction on the rabbit muscle, researchers further assessed its efficacy in a series of following studies in sheep. Based on their previous results [36,63], Gerber et al. [64] hypothesized that the use of either nandrolone decanoate or insulin-like growth factor (IGF), a factor critically involved in muscle growth, in combination with continuous musculotendinous relengthening would be able to reverse muscle alterations after chronic tendon retraction. After 16 weeks of tendon release, the relengthening of the musculotendinous unit was started and the sheep were given no treatment, 150 mg of nandrolone decanoate or 267 μg of IGF. While musculotendinous retraction was similar among groups before relengthening, only small regain of length was achieved by continuous traction in each group which was lost after repair in the IGF group. Neither nandrolone nor IGF together with continuous traction improved fatty infiltration, muscle atrophy and muscle work capability [64]. These different results to the rabbit model are probably owing to the timing of nandrolone decanoate administration. Once degenerative changes of the retracted muscle are established, which is the case in the sheep model, pharmacological intervention is ineffective in regenerating the architecture or function of muscle.
To confirm whether the timing of pharmacological simulation is a key factor in reversing or preventing the degeneration of retracted muscle, Gerber et al. [65] used 18 sheep which had tendon repair 16 weeks after the infraspinatus tenotomy. Right after tendon release or tendon repair, 6 sheep were injected with 150 mg of nandrolone decanoate every week, respectively. The rest 6 sheep were served as controls. Similar trend of muscle volume loss on MRI were observed in the controls and sheep with weekly nandrolone injection after tendon release, nonetheless, a significant postoperative increase of muscle volume was seen in sheep with weekly nandrolone injection immediately after tendon repair. Consistent with previous rabbit study [63], substantially diminished fatty infiltration was seen in sheep which started nandrolone injection at the time of tendon release. An interesting phenomenon worth exploring is that postoperative regain of muscle volume is absent when nandrolone decanoate was given after tendon release, which is unexpected as longer period of nandrolone decanoate application should have more potent beneficial effects. The authors postulated this might be related to the down-regulation of androgen receptors and subsequent decreased responsiveness to anabolic steroids.
More recently, using the same experimental design as Gerber et al. [65], Flück et al. [31] studied the effects of nandrolone decanoate on lipid-related gene expression in detached infraspinatus muscle. They found an up-regulation of 227 lipid species along with increased expression of peroxisome proliferator-activated receptor gamma 2 (PPARG2), an adipocyte differentiation marker, in the affected muscle. The administration of nandrolone decanoate after tendon release lessened fatty accumulation, mitigated loss of area percentage of muscle fibers. Additionally, the loss of responsiveness to nandrolone decanoate in retracted muscle at the time of repair was associated with an overall down-regulation of gene transcripts. It is also worth noting that the expression of PPARG isoforms was not affected by nandrolone decanoate, indicating the prevention of lipid accumulation is not directly by inhibiting adipocyte differentiation but rather through other pathways regulating lipid biosynthesis.
5.3. Stem cells
Various sources of stem cells have been investigated in enhancing rotator cuff healing and regeneration, among which mesenchymal stem cells (MSCs) derived from bone marrow or adipose tissue are most common [[66], [67], [68], [69]]. In a rabbit model of chronic RCTs, Oh et al. [70] injected allogenic adipose-derived stem cells (ADSCs) into the repaired subscapularis muscle near the musculotendinous unit. On hematoxylin and eosin staining, the mean proportion of fatty infiltration was significant lower in the ADSCs treated muscles than that in the saline treated controls, which was concomitant with decreased degree of Oil red O staining for fat in the ADSCs group.
In a sheep model of chronic infraspinatus tear, Brandão et al. [71] evaluated the effects of autologous bone marrow-derived stem cells (BMSCs) on muscle regeneration. After 60 days of tenotomy, BMSCs isolated from the medulla or saline solution was applied to the injury site. In the BMSCs group, grade 1 fatty infiltration was seen in the injured area compared to grades 4 and 5 fatty infiltration in the saline group 34 days after tendon repair. Furthermore, satellite cells were present in the BMSCs treated samples but not in the control samples 34 days after tendon repair. The important role of satellite cells in muscle regeneration and growth have been widely acknowledged [[72], [73], [74]]. Since injured cuff muscle has fewer satellite cells [75], the presence of satellite cells with the use of BMSCs may be a positive signal for reversal of muscle degeneration.
In addition to ADSCs and BMSCs, another subpopulation of stem cells resides in the adipose tissue, namely perivascular stem cells (PSCs), have displayed protective effects on released muscle in a mouse model of massive RCTs [30]. A total of 90 immunodeficient mice were subjected to one of the following surgeries: sham, tenotomy of the supraspinatus and infraspinatus (TT), or TT plus suprascapular denervation (TT + DN). At the time of surgery or 2 weeks post-surgery, PSCs isolated from human white adipose tissue were injected to the supraspinatus muscle. Muscle specimens were obtained 6 weeks postoperatively for the analysis of muscle atrophy, fibrosis, and fatty infiltration. Compared with respective controls, there was reduced muscle weight loss in mice received PSCs at either time point in the TT group or at 2 weeks postoperatively in the TT + DN group. Additionally, the muscle fiber CSA was significantly greater in mice treated with PSCs at either time point in both the TT and TT + DN groups. The pericytes and adventitial cells of the PSCs led to decreased fibrosis in the TT + DN group when applied at either time point or 2 weeks after surgery, respectively. In the TT group, pericytes injected at either time point or adventitial cells injected at the time of surgery resulted in less fatty infiltration, respectively.
Considering the easy procurement and isolation of PSCs [76], the application of PSCs possesses great promise for clinical translation. Nevertheless, future studies are needed to reveal the underlying mechanism of PSCs-mediated muscle regeneration, specifically, whether it is due to direct differentiation of PSCs into myofibers or through paracrine effects of PSCs.
5.4. Signaling pathway regulators
Several signaling pathways have been implicated in the development of muscle atrophy and fatty infiltration after RCTs. Of these, the Akt/mammalian target of rapamycin (mTOR) signaling pathway has been widely studied given its known role in regulating protein synthesis of skeletal muscle in respond to mechanical loading, as well as its expression change in denervated muscles [77,78]. Liu et al. [79] reported a different pattern of Akt/mTOR activity in the setting of tendon transection or suprascapular denervation in rat. At 2 weeks postoperatively, significant decreased and increased activity of Akt/mTOR signaling pathway within atrophied muscle was observed after tendon transection and suprascapular denervation, respectively. The expression of two atrophy-related genes, muscle RING finger protein 1 (MuRF-1) and muscle atrophy F box (MAFbx), was markedly increased only in the denervated muscle which was in agreement with previous studies [80]. There was also reduced protein activity of ribosome protein S6 kinase 1 (S6K1), an Akt/mTOR downstream effector, following tendon transection. These results suggested that tendon tear induced muscle atrophy was correlated with inactivation of the Akt/mTOR signaling pathway and subsequent decrease of protein synthesis through downregulating S6K1. In contrast, suprascapular denervation induced muscle atrophy was likely a result of increased protein degradation via upregulation of MuRF-1 and MAFbx expressions.
Following the above study, Joshi et al. [81] explored the role of Akt/mTOR signaling pathway in fatty infiltration after massive RCTs combined with suprascapular nerve injury in rat. They found significant upregulation of Akt/mTOR signaling and gene expression of peroxisome proliferator activated receptor gamma (PPARγ) and fatty acid synthase (FASN). By inhibiting mTOR pathway with rapamycin, there was a notable decrease in mTOR activity, protein levels of sterol regulatory element binding protein 1 (SREBP-1) and PPARγ, gene expression of SREBP-1, PPARγ, FASN, as well as reduced fatty infiltration.
Given the differential Akt/mTOR signaling in tendon transection or nerve denervation induced muscle atrophy [79], the corresponding molecular pathway involved in protein synthesis and degradation remains to be revealed. Using the rat model, Joshi et al. [82] examined the regulatory role of two key proteolytic systems, the ubiquitin–proteasome pathway and autophagy, in muscle atrophy following RCTs. As expected, muscle atrophy caused by tendon transection or nerve denervation had different mechanisms of protein degradation, specifically, the ubiquitin-proteasome pathway was mainly responsible for denervated muscle atrophy, while autophagy was mainly responsible for atrophy of tenotomized muscle. Interestingly, the protein synthesis was upregulated in atrophied muscle after both surgical interventions, which contradicted previous findings that inactivation of the Akt/mTOR pathway was observed after tendon transection. This discrepancy may indicate that Akt/mTOR signaling is not the solely pathway regulating the protein synthesis following tendon rupture. Additionally, the increased protein synthesis might be a compensating response to the progressive muscle atrophy.
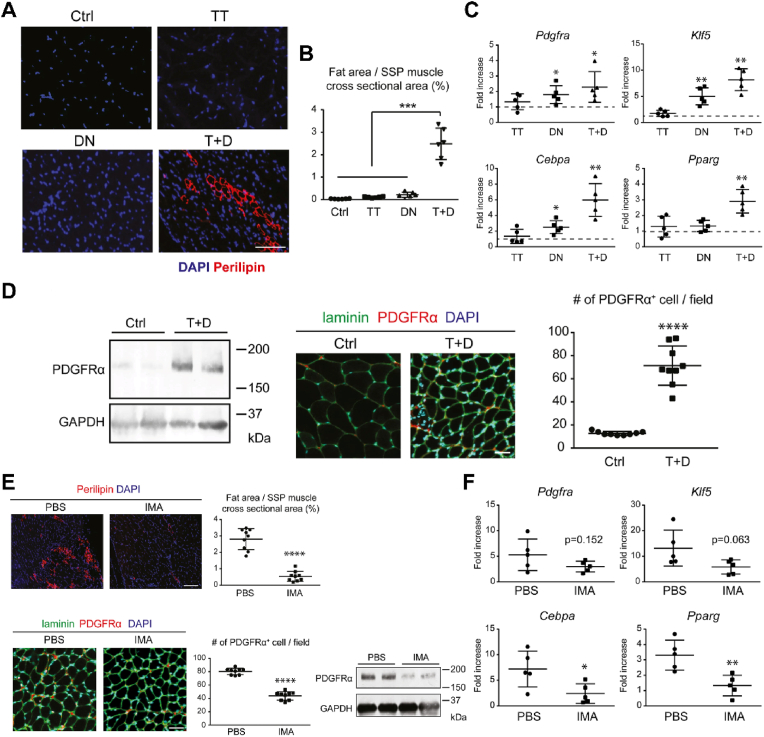
Recently, the role of two signaling pathways, platelet-derived growth factor receptor (PDGFR) and Poly (adenosine 5′-diphosphate-ribose) polymerase 1 (PARP-1), in alleviating fatty infiltration after RCTs has been elucidated in mice. After a combined procedure of transecting all cuff tendons, resecting suprascapular nerve, and removing the humerus head, Shirasawa et al. [83] generated a mouse model of muscular fatty infiltration. They found a significant increased expression of transcripts for adipocyte markers including Klf5, Cebpa and Pparg, postoperatively. There was also a remarkable upregulation of the transcripts for Pdgfra, a marker for PDGFRα+ MSCs, at 1 week after the surgery. As previous studies have demonstrated that PDGFRα+ MSCs are capable of differentiating towards adipocyte lineage [84], and their numbers were significantly increased after the surgery, the authors therefore used imatinib to block PDGFRα signaling to see whether fatty infiltration could be attenuated. The results showed that gene expression of late adipocyte markers such as Cebpa and Pparg was markedly decreased, as well as the number of PDGFRα+ MSCs (Fig. 3). The suppression of adipogenic differentiation was further corroborated by a notable histological reduction of the fatty tissue ratio after imatinib treatment.
Fig. 3.
(A) T + D contributes to fatty infiltration within the supraspinatus muscle demonstrated by immunofluorescence staining for perilipin and DAPI. Scale bar = 50 μm (B) The proportional areas of fat were significantly higher in the T + D group. n = 3 mice/group (C) The transcripts expression of adipocyte markers. n = 5 mice/group (D) Increase of PDGFRα+ MSCs in the T+D group shown by Western blotting, immunofluorescent images and semi-quantitative counting of PDGFRα+ cells per microscopic field (214 μ m × 214 μ m). Scale bar = 30 μm, n = 3 mice/group (E) The effect of imatinib on the suppression of fatty infiltration illustrated by immunofluorescence staining for perilipin with DAPI (scale bar = 100 μm) or laminin and PDGFRα with DAPI (scale bar = 30 μm) (n = 3 mice/group), as well as Western blotting (2 mice/group) (F) The transcripts expression of adipocyte markers after imatinib treatment. n = 5 mice/group. Values are presented as means ± SD. TT, tendon transection; DN, suprascapular nerve denervation; T + D, tendon transection and denervation. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. Adapted and reprinted with permission from Ref. [83].
Instead of using an inhibitor, Kuenzler et al. [26] evaluated the effect of PARP-1 on the reversal of muscular changes in a PARP-1 knockout (KO) mouse model after combined tendon transection and nerve denervation. Twelve weeks postoperatively, the PARP-1 KO mice had less tendon and muscle retraction on macroscopic and MRI measurements compared with wild-type (WT) mice. The wet weight of the supraspinatus muscle returned to almost normal in the PARP-1 KO mice but remained lower in the WT mice 12 weeks after the surgery. Histologically, while the pennation angle was significantly increased in the WT mice through the whole postoperative period compared with the uninjured controls, it remained unchanged in the PARP-1 KO mice postoperatively. The fatty infiltration analyzed by histology and MRI quantification both demonstrated a significant decrease in the PARP-1 KO mice compared with the WT mice 12 weeks post-surgery. After either 1 week or 6 weeks, the PARP-1 KO mice had a significant lower expression of genes mediating adipogenesis, muscle degeneration, apoptosis and inflammation. It has been previously shown that PARP-1 is a pivotal regulator of cellular homeostasis [85], apoptosis [86], muscle regeneration [87], and adipogenesis [88]. Therefore, a complete block of PARP-1 signaling as seen in this PARP-1 KO mouse study would potentially impede a cluster of downstream pathways that govern the irreversible muscle alterations, leading to improved muscular architecture after RCTs.
Since inflammatory cascade is critically involved in the degeneration of injured muscles [89], the inhibition of inflammation-related pathways could exert beneficial effects. Using a rat model of chronic RCTs, Oak et al. [90] examined the mechanical properties of repaired tendon after administrating licofelone, an anti-inflammatory drug which inhibits 5-lipoxygenase (5-LOX) cyclooxygenase (COX)-1, and COX-2, for 2 weeks. They found that animals received licofelone demonstrated a significant increase in maximum load to failure, maximum stress and peak stiffness, compared with controls. However, the contractility of muscle fibers did not improve but showed a 23% reduction after licofelone treatment, which may be attributed to licofelone-induced muscle fiber remodeling.
Except for blocking signaling pathways, the activation of certain pathways may contribute to improved histological and mechanical properties of chronically torn muscles. It was found that by activating hypoxia-inducible factor-1 alpha (HIF-1α) signaling with GSK1120360 A, an inhibitor of HIF prolyl 4-hydroxylases, the expression of genes related to fat accumulation and muscle fibrosis was significantly downregulated in treated animals compared with controls. There was approximate a 50% decrease of collagen content within the treated muscle. Biomechanical testing further demonstrated enhanced displacement to failure, peak stiffness, and maximum stress to failure of the repaired enthesis at 2 weeks postoperatively [91].
5.5. Mitochondrial metabolism
Muscle degeneration after RCTs involves two major components: muscle atrophy and fatty infiltration. The interplay between these two components, especially the underlying biological processes linking decreased myofibrillar content and increased lipid accumulation remains unknown. It has been indicated that the reduced muscle fiber area had a linear correlation with the fatty accumulation in the affected muscle after tendon release [31]. However, an unanswered question is whether fiber degradation-related free amino acids serve as substrates for lipid synthesis, and how this process is regulated. The close relationship between mitochondrial dysfunction and disuse muscle atrophy has been well defined [92,93]. The reduction of mitochondrial metabolism may contribute to lipid accumulation and atrophy in rat or human skeletal muscle [[94], [95], [96]]. In a recent study by Flück et al. [97], the mitochondrial-related gene expression, metabolite and lipid levels, and the effect of l-carnitine treatment on mitigating early muscle degeneration were assessed using a sheep infraspinatus tenotomy model. Significant increase of the area-percentage for lipid-positive connective tissue within tenotomized muscle was seen at 16 weeks, while the area-percentage for lipid-free connective tissue started to increase at 6 weeks and remained elevated at 16 weeks. During the first 4 weeks, there was a notable slow-to-fast type shift demonstrated by measurements of the area-percentage of slow-type and fast-type fibers. Through RNA sequencing, they identified 25 gene transcripts with downregulated expression 2 weeks after tenotomy, among which 17 were related to contraction, 4 were related to oxygen or caron dioxide metabolism, and 4 were related to cell regulation. Furthermore, the expression of mitochondrial proteins was decreased at 2, 4, and 6 weeks, but not at 16 weeks after tenotomy. With the administration of l-carnitine 1 week before tenotomy, the reduction in muscle volume was significantly improved at 2 weeks compared to the untreated sheep. Additionally, the protein levels of NDUFA9 and ATP5A1, constituents of the mitochondrial respiratory chain, tended to increase after tenotomy.
Metabolically, the administration of l-carnitine led to global decreased levels of lipid in tenotomized muscle for classes of fatty acyls, glycerolipids, and sterol lipids. However, the levels of glycerophospholipids in tenotomized muscle were increased after l-carnitine treatment compared to untreated sheep. There were 8 metabolites with levels being partially corrected by l-carnitine treatment after tendon release, one of which is associated with mitochondrial β-oxidation, and two of which are associated with the class of amino acids. In contrast, the levels of four metabolites related to high-energy reserves through oxidative phosphorylation, which are ATP, adenosine 5′-diphosphate, adenosine 2′-phosphate and phosphocreatine, were further decreased after l-carnitine treatment. Altogether, this study unveiled the role of l-carnitine administration in preventing early muscle atrophy, which may be translated into clinical practice for the treatment of rotator cuff disease.
6. Future directions
Animal models play a vital role in bridging experimental studies and clinical trials. There are various well-established animal models of chronic RCTs that recapitulate the characteristic degenerative changes of human muscle, which help us to gain a deeper understanding of the underlying biology and pathophysiology. Nevertheless, none of these animal models are able to recapitulate all of the features of the human condition, given their inherent structural or functional differences to humans. Additionally, the time points for repairing chronically torn rotator cuff vary from animal to animal, study to study, which could be a confounding factor in evaluating the outcomes. In this respect, cautions are needed to interpret the results from animal studies for translational purposes.
To answer research questions and confirm hypotheses, the selection of an appropriate animal model is very important. For instance, muscle fiber types are commonly classified by myosin heavy chain (MyHC) isoforms. Previous studies have suggested that there is a slow-to-fast type shift of muscle fibers after tendon release or denervation [27,32]. In order to reveal the mechanism behind this shift, a suited animal model should have similar composition of muscle fiber types to humans. While rat model has been widely used in creating RCTs-related muscle atrophy, less than 10% of rat supraspinatus and infraspinatus muscles are MyHC I fibers compared to 44% in human rotator cuff muscles. The MyHC composition of fast-twitch fibers was also different between rat and human rotator cuff muscles. These differences are thus need to be taken into account when interpreting results on pathological muscular changes. Further, several findings from human studies worth exploring on animal models have yet to be done. By collecting intraoperative tendon samples from patients with full-thickness rotator cuff tears, Lakemeier et al. [98] found a significant higher expression of matrix metalloproteinases (MMPs) 1 and 9 in torn tendons, and lower expression of MMP 3 when compared with healthy controls. The expression of MMP 9 was positively correlated with the degree of tendon retraction, whereas such correlation was absent for MMPs 1 and 3. Another study by Meyer et al. [3] showed a highly asymmetric atrophy of torn supraspinatus muscle where primary atrophy occurs on the fascial muscle portion, and the scapular portion mainly undergoes fatty infiltration. The mechanisms leading to these findings are required to be revealed using animal models.
Finally, and most importantly, the potential side effects, optimal dose, delivery timing, period and methods of protease inhibitors, anabolic steroids, stem cells or other agents warrants future studies and serve as the cornerstone for translating these therapeutics to human patients. For example, although the application of nandrolone decanoate demonstrated promising effects on suppressing fatty infiltration and preserving muscle function in rabbits, it may lead to wound infection or other complications [63]. Likewise, systemic administration of calpain inhibitors or sildenafil might have adverse effects on other organs. In terms of stem cells, the source, the isolation and expansion approach, the way of application, and the risk of immune response are all need to be taken into account. In addition, the microenvironment of the injured muscle which plays a vital role in guiding stem cell fate may differ among patients. A suitable microenvironment that leads implanted cells toward a myogenic pathway instead of a fibrotic or adipogenic pathway might depend on the appropriate selection of patients and timing of implantation. Last, while many signaling pathways involved in the development of muscle pathology which could serve as therapeutic targets, each pathway may orchestrate numerous biological processes other than those related to degenerative muscle changes. Thus, the identification of an ideal pathway by which not only maximum beneficial effects could be achieved but also associated with minimum undesirable consequences, requires future efforts.
7. Conclusions
In summary, animal models mimicking muscle atrophy and fatty infiltration after RCTs are effective tools for providing insight into the molecular and cellular mechanisms that result in progressive muscle deterioration. Novel therapeutic strategies for increasing the reparability of torn rotator cuff and decreasing the postoperative retear rate may derived from these preclinical animal studies which demonstrated the possibility of preventing or reversing muscle degeneration. It is believed that after further research to assess the safety and efficacy of these novel approaches, their clinical translation will be in the near future.
Declaration of competing interest
The author(s) have no conflicts of interest relevant to this article.
Acknowledgment
This work was supported by National Natural Science Foundation of China (No. 81902221), Natural Science Foundation of Hunan Province (2019JJ30035) and NIH/NIAMS (AR73811).
Contributor Information
Weihong Zhu, Email: zhuweihong@csu.edu.cn.
Chunfeng Zhao, Email: zhaoc@mayo.edu.
References
- 1.Meyer D.C., Farshad M., Amacker N.A., Gerber C., Wieser K. Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. Am J Sports Med. 2012;40(3):606–610. doi: 10.1177/0363546511429778. [DOI] [PubMed] [Google Scholar]
- 2.Goutallier D., Postel J.M., Bernageau J., Lavau L., Voisin M.C. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78–83. [PubMed] [Google Scholar]
- 3.Meyer D.C., Pirkl C., Pfirrmann C.W., Zanetti M., Gerber C. Asymmetric atrophy of the supraspinatus muscle following tendon tear. J Orthop Res. 2005;23(2):254–258. doi: 10.1016/j.orthres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Melis B., DeFranco M.J., Chuinard C., Walch G. Natural history of fatty infiltration and atrophy of the supraspinatus muscle in rotator cuff tears. Clin Orthop Relat Res. 2010;468(6):1498–1505. doi: 10.1007/s11999-009-1207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strauss E.J., Kingery M.T., Klein D., Manjunath A.K. The evaluation and management of suprascapular neuropathy. J Am Acad Orthop Surg. 2020;28(15):617–627. doi: 10.5435/JAAOS-D-19-00526. [DOI] [PubMed] [Google Scholar]
- 6.Goutallier D., Postel J.M., Gleyze P., Leguilloux P., Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003;12(6):550–554. doi: 10.1016/s1058-2746(03)00211-8. [DOI] [PubMed] [Google Scholar]
- 7.Deniz G., Kose O., Tugay A., Guler F., Turan A. Fatty degeneration and atrophy of the rotator cuff muscles after arthroscopic repair: does it improve, halt or deteriorate? Arch Orthop Trauma Surg. 2014;134(7):985–990. doi: 10.1007/s00402-014-2009-5. [DOI] [PubMed] [Google Scholar]
- 8.Gerber C., Fuchs B., Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505–515. doi: 10.2106/00004623-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Gladstone J.N., Bishop J.Y., Lo I.K., Flatow E.L. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 10.Liem D., Lichtenberg S., Magosch P., Habermeyer P. Magnetic resonance imaging of arthroscopic supraspinatus tendon repair. J Bone Joint Surg Am. 2007;89(8):1770–1776. doi: 10.2106/JBJS.F.00749. [DOI] [PubMed] [Google Scholar]
- 11.Burkhart S.S., Barth J.R., Richards D.P., Zlatkin M.B., Larsen M. Arthroscopic repair of massive rotator cuff tears with stage 3 and 4 fatty degeneration. Arthroscopy. 2007;23(4):347–354. doi: 10.1016/j.arthro.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs B., Gilbart M.K., Hodler J., Gerber C. Clinical and structural results of open repair of an isolated one-tendon tear of the rotator cuff. J Bone Joint Surg Am. 2006;88(2):309–316. doi: 10.2106/JBJS.E.00117. [DOI] [PubMed] [Google Scholar]
- 13.Sgroi T.A., Cilenti M. Rotator cuff repair: post-operative rehabilitation concepts. Curr Rev Musculoskelet Med. 2018;11(1):86–91. doi: 10.1007/s12178-018-9462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikolaidou O., Migkou S., Karampalis C. Rehabilitation after rotator cuff repair. Open Orthop J. 2017;11:154–162. doi: 10.2174/1874325001711010154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nussbaum E.L., Houghton P., Anthony J., Rennie S., Shay B.L., Hoens A.M. Neuromuscular electrical stimulation for treatment of muscle impairment: critical review and recommendations for clinical practice. Physiother Can. 2017;69(5):1–76. doi: 10.3138/ptc.2015-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinold M.M., Macrina L.C., Wilk K.E., Dugas J.R., Cain E.L., Andrews J.R. The effect of neuromuscular electrical stimulation of the infraspinatus on shoulder external rotation force production after rotator cuff repair surgery. Am J Sports Med. 2008;36(12):2317–2321. doi: 10.1177/0363546508322479. [DOI] [PubMed] [Google Scholar]
- 17.Lee G.J., Cho H., Ahn B.H., Jeong H.S. Effects of electrical muscle stimulation for preventing deltoid muscle atrophy after rotator cuff repair: preliminary results of a prospective, randomized, single-blind trial. Clin Shoulder Elb. 2019;22(4):195–202. doi: 10.5397/cise.2019.22.4.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carpenter J.E., Hankenson K.D. Animal models of tendon and ligament injuries for tissue engineering applications. Biomaterials. 2004;25(9):1715–1722. doi: 10.1016/s0142-9612(03)00507-6. [DOI] [PubMed] [Google Scholar]
- 19.Longo U.G., Forriol F., Campi S., Maffulli N., Denaro V. Animal models for translational research on shoulder pathologies: from bench to bedside. Sports Med Arthrosc Rev. 2011;19(3):184–193. doi: 10.1097/JSA.0b013e318205470e. [DOI] [PubMed] [Google Scholar]
- 20.Mannava S., Plate J.F., Tuohy C.J., Seyler T.M., Whitlock P.W., Curl W.W., et al. The science of rotator cuff tears: translating animal models to clinical recommendations using simulation analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1610–1619. doi: 10.1007/s00167-012-2145-9. [DOI] [PubMed] [Google Scholar]
- 21.Derwin K.A., Baker A.R., Iannotti J.P., McCarron J.A. Preclinical models for translating regenerative medicine therapies for rotator cuff repair. Tissue Eng B Rev. 2010;16(1):21–30. doi: 10.1089/ten.teb.2009.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Q., Yu Y., Reisdorf R.L., Qi J., Lu C.K., Berglund L.J., et al. Engineered tendon-fibrocartilage-bone composite and bone marrow-derived mesenchymal stem cell sheet augmentation promotes rotator cuff healing in a non-weight-bearing canine model. Biomaterials. 2019;192:189–198. doi: 10.1016/j.biomaterials.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 23.Rossbach B.P., Gulecyuz M.F., Kempfert L., Pietschmann M.F., Ullamann T., Ficklscherer A., et al. Rotator cuff repair with autologous tenocytes and biodegradable collagen scaffold: a histological and biomechanical study in sheep. Am J Sports Med. 2020;48(2):450–459. doi: 10.1177/0363546519892580. [DOI] [PubMed] [Google Scholar]
- 24.Tokunaga T., Ide J., Arimura H., Nakamura T., Uehara Y., Sakamoto H., et al. Local application of gelatin hydrogel sheets impregnated with platelet-derived growth factor BB promotes tendon-to-bone healing after rotator cuff repair in rats. Arthroscopy. 2015;31(8):1482–1491. doi: 10.1016/j.arthro.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Pan J., Liu G.M., Ning L.J., Zhang Y., Luo J.C., Huang F.G., et al. Rotator cuff repair using a decellularized tendon slices graft: an in vivo study in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2015;23(5):1524–1535. doi: 10.1007/s00167-014-2923-7. [DOI] [PubMed] [Google Scholar]
- 26.Kuenzler M.B., Nuss K., Karol A., Schar M.O., Hottiger M., Raniga S., et al. Neer Award 2016: reduced muscle degeneration and decreased fatty infiltration after rotator cuff tear in a poly(ADP-ribose) polymerase 1 (PARP-1) knock-out mouse model. J Shoulder Elbow Surg. 2017;26(5):733–744. doi: 10.1016/j.jse.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Ruoss S., Kindt P., Oberholzer L., Rohner M., Jungck L., Abdel-Aziz S., et al. Inhibition of calpain delays early muscle atrophy after rotator cuff tendon release in sheep. Phys Rep. 2018;6(21) doi: 10.14814/phy2.13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vargas-Vila M.A., Gibbons M.C., Wu I.T., Esparza M.C., Kato K., Johnson S.D., et al. Progression of muscle loss and fat accumulation in a rabbit model of rotator cuff tear. J Orthop Res. 2021 doi: 10.1002/jor.25160. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wieser K., Grubhofer F., Hasler A., Gotschi T., Beeler S., Meyer D., et al. Muscle degeneration induced by sequential release and denervation of the rotator cuff tendon in sheep. Orthop J Sports Med. 2021;9(8) doi: 10.1177/23259671211025302. 23259671211025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eliasberg C.D., Dar A., Jensen A.R., Murray I.R., Hardy W.R., Kowalski T.J., et al. Perivascular stem cells diminish muscle atrophy following massive rotator cuff tears in a small animal model. J Bone Joint Surg Am. 2017;99(4):331–341. doi: 10.2106/JBJS.16.00645. [DOI] [PubMed] [Google Scholar]
- 31.Fluck M., Ruoss S., Mohl C.B., Valdivieso P., Benn M.C., von Rechenberg B., et al. Genomic and lipidomic actions of nandrolone on detached rotator cuff muscle in sheep. J Steroid Biochem Mol Biol. 2017;165(Pt B):382–395. doi: 10.1016/j.jsbmb.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Gerber C., Meyer D.C., Fluck M., Valdivieso P., von Rechenberg B., Benn M.C., et al. Muscle degeneration associated with rotator cuff tendon release and/or denervation in sheep. Am J Sports Med. 2017;45(3):651–658. doi: 10.1177/0363546516677254. [DOI] [PubMed] [Google Scholar]
- 33.Meyer G.A., Ward S.R. Developmental biology and regenerative medicine: addressing the vexing problem of persistent muscle atrophy in the chronically torn human rotator cuff. Phys Ther. 2016;96(5):722–733. doi: 10.2522/ptj.20150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomioka T., Minagawa H., Kijima H., Yamamoto N., Abe H., Maesani M., et al. Sarcomere length of torn rotator cuff muscle. J Shoulder Elbow Surg. 2009;18(6):955–959. doi: 10.1016/j.jse.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Meyer D.C., Hoppeler H., von Rechenberg B., Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004–1007. doi: 10.1016/j.orthres.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Gerber C., Meyer D.C., Frey E., von Rechenberg B., Hoppeler H., Frigg R., et al. Neer Award 2007: reversion of structural muscle changes caused by chronic rotator cuff tears using continuous musculotendinous traction. An experimental study in sheep. J Shoulder Elbow Surg. 2009;18(2):163–171. doi: 10.1016/j.jse.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Liu X., Laron D., Natsuhara K., Manzano G., Kim H.T., Feeley B.T. A mouse model of massive rotator cuff tears. J Bone Joint Surg Am. 2012;94(7):e41. doi: 10.2106/JBJS.K.00620. [DOI] [PubMed] [Google Scholar]
- 38.Liu X., Manzano G., Kim H.T., Feeley B.T. A rat model of massive rotator cuff tears. J Orthop Res. 2011;29(4):588–595. doi: 10.1002/jor.21266. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y., Fu S.C., Leong H.T., Ling S.K., Oh J.H., Yung P.S. Evaluation of animal models and methods for assessing shoulder function after rotator cuff tear: a systematic review. J Orthop Translat. 2021;26:31–38. doi: 10.1016/j.jot.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sevivas N., Serra S.C., Portugal R., Teixeira F.G., Carvalho M.M., Silva N., et al. Animal model for chronic massive rotator cuff tear: behavioural and histologic analysis. Knee Surg Sports Traumatol Arthrosc. 2015;23(2):608–618. doi: 10.1007/s00167-014-3441-3. [DOI] [PubMed] [Google Scholar]
- 41.Perry S.M., Getz C.L., Soslowsky L.J. Alterations in function after rotator cuff tears in an animal model. J Shoulder Elbow Surg. 2009;18(2):296–304. doi: 10.1016/j.jse.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fabis J., Kordek P., Bogucki A., Mazanowska-Gajdowicz J. Function of the rabbit supraspinatus muscle after large detachment of its tendon: 6-week, 3-month, and 6-month observation. J Shoulder Elbow Surg. 2000;9(3):211–216. [PubMed] [Google Scholar]
- 43.Fabis J., Kordek P., Bogucki A., Synder M., Kolczynska H. Function of the rabbit supraspinatus muscle after detachment of its tendon from the greater tubercle. Observations up to 6 months. Acta Orthop Scand. 1998;69(6):570–574. doi: 10.3109/17453679808999257. [DOI] [PubMed] [Google Scholar]
- 44.Bjorkenheim J.M. Structure and function of the rabbit's supraspinatus muscle after resection of its tendon. Acta Orthop Scand. 1989;60(4):461–463. doi: 10.3109/17453678909149320. [DOI] [PubMed] [Google Scholar]
- 45.Albritton M.J., Graham R.D., Richards R.S., 2nd, Basamania C.J. An anatomic study of the effects on the suprascapular nerve due to retraction of the supraspinatus muscle after a rotator cuff tear. J Shoulder Elbow Surg. 2003;12(5):497–500. doi: 10.1016/s1058-2746(03)00182-4. [DOI] [PubMed] [Google Scholar]
- 46.Massimini D.F., Singh A., Wells J.H., Li G., Warner J.J. Suprascapular nerve anatomy during shoulder motion: a cadaveric proof of concept study with implications for neurogenic shoulder pain. J Shoulder Elbow Surg. 2013;22(4):463–470. doi: 10.1016/j.jse.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 47.Boykin R.E., Friedman D.J., Higgins L.D., Warner J.J. Suprascapular neuropathy. J Bone Joint Surg Am. 2010;92(13):2348–2364. doi: 10.2106/JBJS.I.01743. [DOI] [PubMed] [Google Scholar]
- 48.Boykin R.E., Friedman D.J., Zimmer Z.R., Oaklander A.L., Higgins L.D., Warner J.J. Suprascapular neuropathy in a shoulder referral practice. J Shoulder Elbow Surg. 2011;20(6):983–988. doi: 10.1016/j.jse.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 49.Mallon W.J., Wilson R.J., Basamania C.J. The association of suprascapular neuropathy with massive rotator cuff tears: a preliminary report. J Shoulder Elbow Surg. 2006;15(4):395–398. doi: 10.1016/j.jse.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 50.Costouros J.G., Porramatikul M., Lie D.T., Warner J.J. Reversal of suprascapular neuropathy following arthroscopic repair of massive supraspinatus and infraspinatus rotator cuff tears. Arthroscopy. 2007;23(11):1152–1161. doi: 10.1016/j.arthro.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 51.Shi L.L., Boykin R.E., Lin A., Warner J.J. Association of suprascapular neuropathy with rotator cuff tendon tears and fatty degeneration. J Shoulder Elbow Surg. 2014;23(3):339–346. doi: 10.1016/j.jse.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 52.Beeler S., Ek E.T., Gerber C. A comparative analysis of fatty infiltration and muscle atrophy in patients with chronic rotator cuff tears and suprascapular neuropathy. J Shoulder Elbow Surg. 2013;22(11):1537–1546. doi: 10.1016/j.jse.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 53.Kim H.M., Galatz L.M., Lim C., Havlioglu N., Thomopoulos S. The effect of tear size and nerve injury on rotator cuff muscle fatty degeneration in a rodent animal model. J Shoulder Elbow Surg. 2012;21(7):847–858. doi: 10.1016/j.jse.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sato E.J., Killian M.L., Choi A.J., Lin E., Esparza M.C., Galatz L.M., et al. Skeletal muscle fibrosis and stiffness increase after rotator cuff tendon injury and neuromuscular compromise in a rat model. J Orthop Res. 2014;32(9):1111–1116. doi: 10.1002/jor.22646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deval C., Mordier S., Obled C., Bechet D., Combaret L., Attaix D., et al. Identification of cathepsin L as a differentially expressed message associated with skeletal muscle wasting. Biochem J. 2001;360(Pt 1):143–150. doi: 10.1042/0264-6021:3600143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trevino E.A., McFaline-Figueroa J., Guldberg R.E., Platt M.O., Temenoff J.S. Full-thickness rotator cuff tear in rat results in distinct temporal expression of multiple proteases in tendon, muscle, and cartilage. J Orthop Res. 2019;37(2):490–502. doi: 10.1002/jor.24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shenkman B.S., Belova S.P., Lomonosova Y.N., Kostrominova T.Y., Nemirovskaya T.L. Calpain-dependent regulation of the skeletal muscle atrophy following unloading. Arch Biochem Biophys. 2015;584:36–41. doi: 10.1016/j.abb.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 58.Goll D.E., Neti G., Mares S.W., Thompson V.F. Myofibrillar protein turnover: the proteasome and the calpains. J Anim Sci. 2008;86(14 Suppl):E19–E35. doi: 10.2527/jas.2007-0395. [DOI] [PubMed] [Google Scholar]
- 59.Koh T.J., Tidball J.G. Nitric oxide inhibits calpain-mediated proteolysis of talin in skeletal muscle cells. Am J Physiol Cell Physiol. 2000;279(3):C806–C812. doi: 10.1152/ajpcell.2000.279.3.C806. [DOI] [PubMed] [Google Scholar]
- 60.Bhasin S., Storer T.W., Berman N., Callegari C., Clevenger B., Phillips J., et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335(1):1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- 61.Ferrando A.A., Tipton K.D., Doyle D., Phillips S.M., Cortiella J., Wolfe R.R. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol. 1998;275(5):E864–E871. doi: 10.1152/ajpendo.1998.275.5.E864. [DOI] [PubMed] [Google Scholar]
- 62.Sinha-Hikim I., Cornford M., Gaytan H., Lee M.L., Bhasin S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J Clin Endocrinol Metab. 2006;91(8):3024–3033. doi: 10.1210/jc.2006-0357. [DOI] [PubMed] [Google Scholar]
- 63.Gerber C., Meyer D.C., Nuss K.M., Farshad M. Anabolic steroids reduce muscle damage caused by rotator cuff tendon release in an experimental study in rabbits. J Bone Joint Surg Am. 2011;93(23):2189–2195. doi: 10.2106/JBJS.J.01589. [DOI] [PubMed] [Google Scholar]
- 64.Gerber C., Meyer D.C., Von Rechenberg B., Hoppeler H., Frigg R., Farshad M. Rotator cuff muscles lose responsiveness to anabolic steroids after tendon tear and musculotendinous retraction: an experimental study in sheep. Am J Sports Med. 2012;40(11):2454–2461. doi: 10.1177/0363546512460646. [DOI] [PubMed] [Google Scholar]
- 65.Gerber C., Meyer D.C., Fluck M., Benn M.C., von Rechenberg B., Wieser K. Anabolic steroids reduce muscle degeneration associated with rotator cuff tendon release in sheep. Am J Sports Med. 2015;43(10):2393–2400. doi: 10.1177/0363546515596411. [DOI] [PubMed] [Google Scholar]
- 66.Degen R.M., Carbone A., Carballo C., Zong J., Chen T., Lebaschi A., et al. The effect of purified human bone marrow-derived mesenchymal stem cells on rotator cuff tendon healing in an athymic rat. Arthroscopy. 2016;32(12):2435–2443. doi: 10.1016/j.arthro.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 67.Gulotta L.V., Kovacevic D., Packer J.D., Deng X.H., Rodeo S.A. Bone marrow-derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39(6):1282–1289. doi: 10.1177/0363546510395485. [DOI] [PubMed] [Google Scholar]
- 68.Kim Y.S., Sung C.H., Chung S.H., Kwak S.J., Koh Y.G. Does an injection of adipose-derived mesenchymal stem cells loaded in fibrin glue influence rotator cuff repair outcomes? A clinical and magnetic resonance imaging study. Am J Sports Med. 2017;45(9):2010–2018. doi: 10.1177/0363546517702863. [DOI] [PubMed] [Google Scholar]
- 69.Rothrauff B.B., Smith C.A., Ferrer G.A., Novaretti J.V., Pauyo T., Chao T., et al. The effect of adipose-derived stem cells on enthesis healing after repair of acute and chronic massive rotator cuff tears in rats. J Shoulder Elbow Surg. 2019;28(4):654–664. doi: 10.1016/j.jse.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 70.Oh J.H., Chung S.W., Kim S.H., Chung J.Y., Kim J.Y. 2013 Neer Award: effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model. J Shoulder Elbow Surg. 2014;23(4):445–455. doi: 10.1016/j.jse.2013.07.054. [DOI] [PubMed] [Google Scholar]
- 71.Brandao A.G.M., Consalter A., Leite J.D.S., Zamprogno H., Ferreira A.M.R. Muscle regeneration through therapy with estromal stem cells in injury of infraespinhosus muscle of sheep. Acta Cir Bras. 2018;33(3):231–237. doi: 10.1590/s0102-865020180030000005. [DOI] [PubMed] [Google Scholar]
- 72.Anderson J.E. A role for nitric oxide in muscle repair: nitric oxide-mediated activation of muscle satellite cells. Mol Biol Cell. 2000;11(5):1859–1874. doi: 10.1091/mbc.11.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hara M., Tabata K., Suzuki T., Do M.K., Mizunoya W., Nakamura M., et al. Calcium influx through a possible coupling of cation channels impacts skeletal muscle satellite cell activation in response to mechanical stretch. Am J Physiol Cell Physiol. 2012;302(12):C1741–C1750. doi: 10.1152/ajpcell.00068.2012. [DOI] [PubMed] [Google Scholar]
- 74.Seale P., Rudnicki M.A. A new look at the origin, function, and "stem-cell" status of muscle satellite cells. Dev Biol. 2000;218(2):115–124. doi: 10.1006/dbio.1999.9565. [DOI] [PubMed] [Google Scholar]
- 75.Isaac C., Gharaibeh B., Witt M., Wright V.J., Huard J. Biologic approaches to enhance rotator cuff healing after injury. J Shoulder Elbow Surg. 2012;21(2):181–190. doi: 10.1016/j.jse.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 76.Crisan M., Corselli M., Chen W.C., Peault B. Perivascular cells for regenerative medicine. J Cell Mol Med. 2012;16(12):2851–2860. doi: 10.1111/j.1582-4934.2012.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bodine S.C., Stitt T.N., Gonzalez M., Kline W.O., Stover G.L., Bauerlein R., et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3(11):1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 78.Dogra C., Changotra H., Wergedal J.E., Kumar A. Regulation of phosphatidylinositol 3-kinase (PI3K)/Akt and nuclear factor-kappa B signaling pathways in dystrophin-deficient skeletal muscle in response to mechanical stretch. J Cell Physiol. 2006;208(3):575–585. doi: 10.1002/jcp.20696. [DOI] [PubMed] [Google Scholar]
- 79.Liu X., Joshi S.K., Samagh S.P., Dang Y.X., Laron D., Lovett D.H., et al. Evaluation of Akt/mTOR activity in muscle atrophy after rotator cuff tears in a rat model. J Orthop Res. 2012;30(9):1440–1446. doi: 10.1002/jor.22096. [DOI] [PubMed] [Google Scholar]
- 80.Kimura N., Kumamoto T., Oniki T., Nomura M., Nakamura K., Abe Y., et al. Role of ubiquitin-proteasome proteolysis in muscle fiber destruction in experimental chloroquine-induced myopathy. Muscle Nerve. 2009;39(4):521–528. doi: 10.1002/mus.21223. [DOI] [PubMed] [Google Scholar]
- 81.Joshi S.K., Liu X., Samagh S.P., Lovett D.H., Bodine S.C., Kim H.T., et al. mTOR regulates fatty infiltration through SREBP-1 and PPARgamma after a combined massive rotator cuff tear and suprascapular nerve injury in rats. J Orthop Res. 2013;31(5):724–730. doi: 10.1002/jor.22254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Joshi S.K., Kim H.T., Feeley B.T., Liu X. Differential ubiquitin-proteasome and autophagy signaling following rotator cuff tears and suprascapular nerve injury. J Orthop Res. 2014;32(1):138–144. doi: 10.1002/jor.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shirasawa H., Matsumura N., Shimoda M., Oki S., Yoda M., Tohmonda T., et al. Inhibition of PDGFR signaling prevents muscular fatty infiltration after rotator cuff tear in mice. Sci Rep. 2017;7:41552. doi: 10.1038/srep41552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uezumi A., Fukada S., Yamamoto N., Takeda S., Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12(2):143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 85.Pirinen E., Canto C., Jo Y.S., Morato L., Zhang H., Menzies K.J., et al. Pharmacological Inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metabol. 2014;19(6):1034–1041. doi: 10.1016/j.cmet.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hong S.J., Dawson T.M., Dawson V.L. Nuclear and mitochondrial conversations in cell death: PARP-1 and AIF signaling. Trends Pharmacol Sci. 2004;25(5):259–264. doi: 10.1016/j.tips.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 87.Huang D., Wang Y., Wang L., Zhang F., Deng S., Wang R., et al. Poly(ADP-ribose) polymerase 1 is indispensable for transforming growth factor-beta Induced Smad3 activation in vascular smooth muscle cell. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0027123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Erener S., Hesse M., Kostadinova R., Hottiger M.O. Poly(ADP-ribose)polymerase-1 (PARP1) controls adipogenic gene expression and adipocyte function. Mol Endocrinol. 2012;26(1):79–86. doi: 10.1210/me.2011-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gumucio J.P., Davis M.E., Bradley J.R., Stafford P.L., Schiffman C.J., Lynch E.B., et al. Rotator cuff tear reduces muscle fiber specific force production and induces macrophage accumulation and autophagy. J Orthop Res. 2012;30(12):1963–1970. doi: 10.1002/jor.22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oak N.R., Gumucio J.P., Flood M.D., Saripalli A.L., Davis M.E., Harning J.A., et al. Inhibition of 5-LOX, COX-1, and COX-2 increases tendon healing and reduces muscle fibrosis and lipid accumulation after rotator cuff repair. Am J Sports Med. 2014;42(12):2860–2868. doi: 10.1177/0363546514549943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gumucio J.P., Flood M.D., Bedi A., Kramer H.F., Russell A.J., Mendias C.L. Inhibition of prolyl 4-hydroxylase decreases muscle fibrosis following chronic rotator cuff tear. Bone Joint Res. 2017;6(1):57–65. doi: 10.1302/2046-3758.61.BJR-2016-0232.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ji L.L., Yeo D. vol. 8. 2019. p. F1000Res. (Mitochondrial dysregulation and muscle disuse atrophy). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Powers S.K., Wiggs M.P., Duarte J.A., Zergeroglu A.M., Demirel H.A. Mitochondrial signaling contributes to disuse muscle atrophy. Am J Physiol Endocrinol Metab. 2012;303(1):E31–E39. doi: 10.1152/ajpendo.00609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stevenson E.J., Giresi P.G., Koncarevic A., Kandarian S.C. Global analysis of gene expression patterns during disuse atrophy in rat skeletal muscle. J Physiol. 2003;551(Pt 1):33–48. doi: 10.1113/jphysiol.2003.044701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen Y.W., Gregory C.M., Scarborough M.T., Shi R., Walter G.A., Vandenborne K. Transcriptional pathways associated with skeletal muscle disuse atrophy in humans. Physiol Genom. 2007;31(3):510–520. doi: 10.1152/physiolgenomics.00115.2006. [DOI] [PubMed] [Google Scholar]
- 96.Hoeks J., Schrauwen P. Muscle mitochondria and insulin resistance: a human perspective. Trends Endocrinol Metabol. 2012;23(9):444–450. doi: 10.1016/j.tem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 97.Fluck M., Fitze D., Ruoss S., Valdivieso P., von Rechenberg B., Bratus-Neuenschwander A., et al. Down-regulation of mitochondrial metabolism after tendon release primes lipid accumulation in rotator cuff muscle. Am J Pathol. 2020;190(7):1513–1529. doi: 10.1016/j.ajpath.2020.03.019. [DOI] [PubMed] [Google Scholar]
- 98.Lakemeier S., Braun J., Efe T., Foelsch C., Archontidou-Aprin E., Fuchs-Winkelmann S., et al. Expression of matrix metalloproteinases 1, 3, and 9 in differing extents of tendon retraction in the torn rotator cuff. Knee Surg Sports Traumatol Arthrosc. 2011;19(10):1760–1765. doi: 10.1007/s00167-010-1367-y. [DOI] [PubMed] [Google Scholar]