Abstract
Objective
We performed a retrospective observational study to demonstrate the surgical risks and long-term prognoses of intramedullary tumors in Japan using a multicenter registry authorized by the Neurospinal Society of Japan.
Methods
Data from 1,033 consecutive patients with intramedullary tumors, treated between 2009 and 2020, were collected from 58 centers. Patients with spinal lipomas or myxopapillary ependymomas were excluded. Patient characteristics, clinical presentations, imaging characteristics, treatments, and outcomes were analyzed. The modified McCormick scale was used to classify functional status. Survival was described using Kaplan-Meier curves, and multivariable logistic regression analyses were performed.
Results
The mean age of the patients was 48.4 years. Data of 361 ependymomas, 196 hemangioblastomas, 168 astrocytic tumors, 160 cavernous malformations, and the remaining 126 cases including subependymomas, metastases, schwannomas, capillary hemangiomas, and intravascular B-cell lymphomas were analyzed. Twenty-two patients were undiagnosed. The mean follow-up duration was 46.1 ± 38.5 months. Gross total tumor removal was achieved in 672 tumors (65.1%). On the modified McCormick scale, 234 patients (22.7%) had worse postoperative grades at the time of discharge. However, neurological status gradually improved. At 6 months postoperatively, 251 (27.5%), 500 (54.9%), and 160 patients (17.6%) had improved, unchanged, and worsened grades, respectively. Preoperative functional status, gross total tumor removal, and histopathological type were significantly associated with mortality and functional outcomes.
Conclusion
Our findings demonstrate better postoperative functional outcomes in patients with fewer preoperative neurological deficits. Degree of resection, postoperative treatments, and prognoses are closely related to the histology of intramedullary tumors.
Keywords: Intramedullary tumors, Astrocytoma, Ependymoma, Cavernous angioma, Hemangioblastoma
INTRODUCTION
Intramedullary spinal cord tumors are rare. However, they significantly affect patients’ daily life by causing neurological dysfunction and mortality [1-3]. When treating patients with intramedullary tumors, referring to standardized treatment protocols would be ideal in order to decide when and how to perform surgical interventions. However, such protocols are available but scarce [4,5], as planning prospective randomized studies have been difficult because of the rarity and varied clinical courses of these tumors.
Thus, this study aimed to present the clinical course and surgical outcomes of intramedullary tumors by analyzing the available data from Japanese neurosurgical centers. Here, we developed a multicenter registry of intramedullary tumors authorized by the Neurospinal Society of Japan. In addition to the epidemiological and clinical characteristics, we determined the factors associated with improved survival and functional outcomes.
MATERIALS AND METHODS
1. Ethics
This was a multicenter cohort study authorized by the Neurospinal Society of Japan. The study protocol was approved by the Institutional Review Board of Tohoku University Hospital (2021-1-130) and the participating centers. As this was a retrospective and noninvasive study, the requirement for written informed consent from patients was waived. Instead, a public notice that provided information on this study was given on individual center websites.
2. Patient Selection
The inclusion criterion was consecutive patients with intramedullary spinal cord tumors treated surgically at 58 centers between 2009 and 2020. The exclusion criteria were patients with the spinal lipoma or the myxopapillary ependymoma. Patients who underwent their first surgery at different hospitals or before 2008 were also excluded.
3. Baseline Characteristics
Clinical characteristics including age, sex, height, weight, past medical history (including neurofibromatosis, von Hippel-Lindau disease, and brain tumors), clinical presentations, and duration of the disease were anonymously extracted from the patients’ medical records. Modified McCormick scales [6] (grade I, normal gait; grade II, mild gait disturbance not requiring support; grade III, gait with support; grade IV, assistance required; and grade V, wheelchair needed) were periodically analyzed, allowing comparisons between the preoperative and postoperative status. Specifically, the grades at discharge and 6 months postoperatively were compared to those before surgery. Radiological data were collected from preoperative and postoperative images, including lesion level, lesion length, magnetic resonance imaging (MRI), and computed tomography findings. From the surgical records, surgical approaches, degree of excision, operative time, blood loss, and complications were assessed. The surgeons’ years of experience and certification status in the Neurospinal Society of Japan were also reported from each facility. Pathological diagnoses and immunohistochemical markers were extracted from the pathological records. Information was also recorded on the postoperative clinical course, including adjuvant radiochemotherapy, presence of recurrence and dissemination, and treatment modalities for recurrence.
4. Functional and Performance Grades
Using the modified McCormick scale, when a patient remained at the same grade, we termed the pattern “stable.” Changes of at least one level when compared to the preoperative status were described as “improved” or “worsened” as appropriate.
5. Statistical Analysis
All data were analyzed for completeness and accuracy, and anonymized prior to being scrutinized. The survival period, defined as the number of months from surgery to death, was censored at the last available follow-up or cutoff study date (December 31, 2020) for those who were still alive. Kaplan-Meier curves were created to estimate overall survival for the entire cohort as well as survival in subgroups classified based on histological diagnosis. Risk factors for mortality and factors indicating better functional outcomes were identified using multivariable logistic regression analyses across different demographic characteristics, tumor types, and surgical interventions after controlling for potential confounders. The effects are presented as odds ratios with associated 95% confidence intervals. The multivariable models were adjusted for all included factors. Each preliminary model was then entered into the final logistic regression model. Goodness of fit was assessed using the Hosmer-Lemeshow test. Statistical analyses were performed using IBM SPSS Statistics ver. 26.0 (IBM Co., Armonk, NY, USA).
RESULTS
1. Patient Demographics
In total, 1,033 individual cases were identified. The mean age of the patients at the time of surgery was 48.4 years (range, 0–88 years). Patient demographics are summarized in Table 1. Of the cases, 560 were men (54.2%), and 473 were women (45.8%), indicating a slight male predominance. A clinical diagnosis of neurofibromatosis type 1 was present in 6 cases (0.6%), and neurofibromatosis type 2 was present in 16 cases (1.6%). Seventy-four patients (7.1%) were diagnosed with von Hippel-Lindau disease. The mean duration of symptoms was 22.8 months, with limb paresthesia being the most common presenting symptom (85.1%), followed by weakness (66.2%), and gait disturbance (57.2%). The patients also presented with head, neck, or back pain (41.2%), limb pain (40.9%), or bladder and/or bowel disturbances (33.0%).
Table 1.
Summary of patient demographics among 1,033 cases of the intramedullary spinal cord tumors
| Variable | Value |
|---|---|
| Age on admission (yr) | 48.4 (0–88) |
| Sex | |
| Men | 560 (54.2) |
| Women | 473 (45.8) |
| Cases with hereditary disease | |
| Neurofibromatosis, type 1 | 6 (0.6) |
| Neurofibromatosis, type 2 | 16 (1.6) |
| von Hippel-Lindau disease | 74 (7.1) |
| Mean duration of the symptoms (mo) | 22.8 |
| Clinical presentations | |
| Head, neck or back pain | 426 (41.2) |
| Limb pain | 424 (40.9) |
| Limb paresthesia | 882 (85.1) |
| Limb weakness | 686 (66.2) |
| Gait disturbance | 593 (57.2) |
| Bladder/bowel disturbance | 342 (33.0) |
Values are presented as mean (range) or number (%) unless otherwise indicated.
The tumors were distributed across the following spinal levels (Table 2): 465 cervical (44.9%), cervical and thoracic (127% and 12.4%, respectively), 333 thoracic (32.2%), and thoracic and lumbar (108% and 10.5%, respectively). Based on MRI findings, the tumors were classified as cystic (8.2%), solid (50.1%), mixed (25.6%), or hemorrhagic (15.1%). The tumor size was measured based on sagittal images. The average tumor length was 39.1 mm. Concomitant brain tumors were observed in 130 cases (12.5%).
Table 2.
Tumor characteristics of the 1,033 cases of the intramedullary spinal cord tumors
| Characteristic | Value |
|---|---|
| Tumor levels | |
| Cervical | 465 (44.9) |
| Cervical and thoracic | 127 (12.4) |
| Thoracic | 333 (32.2) |
| Thoracic and lumbar | 108 (10.5) |
| Tumor characteristics | |
| Cystic | 85 (8.2) |
| Solid | 518 (50.1) |
| Mixed | 265 (25.6) |
| Hemorrhagic | 156 (15.1) |
| Unclassified | 9 (0.9) |
| Tumor length | 39.1 (3–500) |
| Intracranial tumors, concomitant | 130 cases |
Values are presented as number (%) or mean (range) unless otherwise indicated.
2. Histopathology and Tumor Characteristics
Among the 1,033 intramedullary spinal cord tumor cases, 361 were ependymomas, 196 were hemangioblastomas, 168 were astrocytic tumors, and 160 were cavernous malformations. The remaining 126 cases comprised subependymomas, metastases, capillary hemangiomas, lymphomas, and schwannomas (Table 3). Twenty-two cases remained undiagnosed, even after histological evaluation.
Table 3.
Histopathological types of the 1,033 intramedullary tumors in Japan, data arranged in decreasing order of frequency
| Type | No. of cases (%) |
|---|---|
| Ependymoma | 361 (35.0) |
| Hemangioblastoma | 196 (18.9) |
| Astrocytoma | 168 (16.2) |
| Cavernous malformations | 160 (15.4) |
| Subependymoma | 22 (2.1) |
| Metastasis | 21 (2.0) |
| Capillary hemangioma | 16 (1.5) |
| Lymphoma | 13 (1.3) |
| Schwannoma | 12 (1.2) |
| Embryonal tumors (medulloblastoma, PNET, ATRT) | 8 (0.8) |
| Solitary fibrous tumor | 5 (0.5) |
| Germ Cell tumors (germinoma, mature teratoma, yolk sac tumor) | 5 (0.5) |
| Gangliocytoma, Ganglioglioma | 5 (0.5) |
| Neurenteric cyst | 4 (0.4) |
| Rosette-forming glioneuronal tumor | 3 (0.3) |
| Malignant peripheral nerve sheath tumor | 2 (0.2) |
| Neurofibroma | 1 (0.1) |
| Pineoblastoma | 1 (0.1) |
| Paraganglioma | 1 (0.1) |
| Glioependymal cyst | 1 (0.1) |
| Meningioma | 1 (0.1) |
| Granuloma | 2 (0.2) |
| Sclerosing epithelioid fibrosarcoma | 1 (0.1) |
| Dermoid cyst | 1 (0.1) |
| Central neurocytoma | 1 (0.1) |
| Undiagnosed | 22 (2.1) |
PNET, primitive neuroectodermal tumor; ATRT, atypical teratoid/rhabdoid tumor.
3. Surgical Treatment
As demonstrated in Table 4, surgery was performed via the posterior approach in almost all cases (1,023 operations, 99.0%). Ten surgeries (1.0%) were performed anteriorly. Most surgeons (94.5%) had > 10 years of experience. In 88.3% of the cases, the main operators were certified spine surgeons of the Neurospinal Society of Japan. The mean operative time was 399.9± 173.2 minutes. The mean blood loss was 186.0± 247.0 mL. Overall, 65.1% of the cases were treated with gross total resection, while 13.0% and 14.1% of the lesions received subtotal and partial removal, respectively. Biopsy was performed in 7.8% of the cases to confirm the histological diagnosis. The frequency of total resection varied among different histological subtypes. For example, 91.8% of hemangioblastomas were completely removed, whereas total resection was performed in 88.8% and 74.8% of cavernous malformations and ependymomas, respectively. However, only 10.7% of astrocytomas underwent total resection. Postoperative radiation therapy was performed in 130 cases (12.6%), including 13 cases (1.3%) with whole-spinal irradiation. Chemotherapy was administered using temozolomide alone in 57 patients and temozolomide and bevacizumab in 24 cases. Methotrexate, monoclonal antibodies, and other chemotherapeutic agents have been used to treat metastatic tumors, lymphomas, and embryonal and germ cell tumors.
Table 4.
Surgical details and the results of the 1,033 intramedullary tumor cases
| Characteristic | Value |
|---|---|
| Surgical approaches | |
| Posterior | 1,023 (99.0) |
| Anterior | 10 (1.0) |
| Surgeons’ experience | |
| > 10 years | 976 (94.5) |
| < 10 years | 57 (5.5) |
| Main operator | |
| Board-certified spine surgeons | 912 (88.3) |
| Operation time (min) | 399.9 ± 173.2 |
| Blood loss (mL) | 186.0 ± 247.0 |
| Degrees of removal | |
| Total | 672 (65.1) |
| Subtotal | 134 (13.0) |
| Partial | 146 (14.1) |
| Biopsy | 81 (7.8) |
| Total removals | |
| Ependymoma | 270/361 (74.8) |
| Hemangioblastoma | 180/196 (91.8) |
| Cavernous malformations | 142/160 (88.8) |
| Astrocytoma | 18/168 (10.7) |
| Others | 54/126 (42.9) |
| Postoperative radiation | |
| Local | 117 (11.3) |
| Whole spine | 13 (1.3) |
| Chemotherapy | |
| Temozolomide only | 57 (5.5) |
| Temozolomide and bevacizumab | 24 (2.3) |
Values are presented as number (%) or mean±standard deviation.
4. Postoperative Course
Immediately postoperatively, 286 patients (27.7%) experienced symptom improvement, 333 (32.2%) remained stable, and 414 (40.1%) experienced worsening of their symptoms (Table 5). On the modified McCormick scale, 153 (14.8%), 646 (62.5%), and 234 patients (22.7%) had improved, unchanged, and worsened grades postoperatively (at discharge), respectively. In the same scale at 6 months postoperatively, 251 (27.6%), 500 (54.9%), and 160 patients (17.6%) had improved, unchanged, and worsened grades, respectively.
Table 5.
Postoperative course and the complications
| Characteristic | No. (%) |
|---|---|
| Symptoms at discharge | |
| Improved | 286 (27.7) |
| Unchanged | 333 (32.2) |
| Worsened | 414 (40.1) |
| Modified McCormick Scales at discharge (comparison with the preoperative status) | |
| Improved | 153 (14.8) |
| Unchanged | 646 (62.5) |
| Worsened | 234 (22.7) |
| Modified McCormick Scales 6 months after the operations (comparison with the preoperative status) | |
| Improved | 251 (27.6) |
| Unchanged | 500 (54.9) |
| Worsened | 160 (17.6) |
| Complications | |
| CSF leak | 28 (2.7) |
| Postoperative hematoma | 8 (0.8) |
| Infection | 9 (0.9) |
| DVT, pulmonary embolism | 2 (0.2) |
| Relapse of the tumors | |
| Local recurrence | 42 (4.1) |
| CSF dissemination | 10 (1.0) |
| Local recurrence and CSF disseminations at the same time | 27 (2.6) |
CSF, cerebrospinal fluid; DVT, deep venous thrombosis.
Postoperative complications included cerebrospinal fluid (CSF) leakage in 28 patients, hematoma in 8, infection in 9, and pulmonary embolism in 2. Sixty-nine patients experienced local recurrence, which included 42 astrocytomas, 12 ependymomas, 6 cavernous malformations, and 4 hemangioblastomas. Among them, 27 patients also had evidence of CSF dissemination at the time of recurrence, while 10 patients experienced only CSF dissemination. Of the 37 patients who experienced CSF disseminations, 29 and 5 patients were originally diagnosed as astrocytoma and ependymoma, respectively. The mean time interval before recurrence and/or dissemination postoperatively was 23.8± 28.2 months. As treatment, 24 patients including 11 astrocytomas, 5 ependymomas, 4 cavernomas, and 2 hemangioblastomas underwent reoperations. Thirteen and 21 patients received radiation therapy and chemotherapy, respectively. Among them, 27 and 5 patients were originally diagnosed as astrocytoma and ependymoma, respectively.
5. Mortality and Risk Factors
From the 1,033 patients, we excluded 55 patients due to loss of follow-up. Among the 978 patients, 871 (89.1%), and 841 (86.0%) survived longer than 5 and 10 years, respectively. Overall survival is depicted in the Kaplan-Meier curves (Fig. 1). Survival varied according to histological type, as illustrated in Fig. 2. Five-year survival rates of patients with ependymomas, hemangioblastomas, astrocytomas, and cavernous malformations were 96.5%, 96.6%, 59.4%, and 99.1%, respectively. Patients with astrocytoma had worse survival than those in the other histological groups. The multivariable analyses indicated that lesser degrees of tumor removal, worse preoperative modified McCormick scales, and histopathological types of the tumors were associated with mortality (Table 6). Younger age was also a risk factor, although the statistical significance was marginal.
Fig. 1.
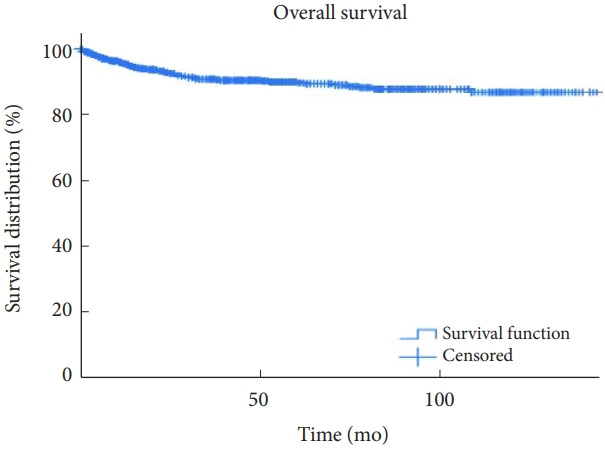
Overall survival is demonstrated using Kaplan-Meier curves of those who underwent surgical interventions for intramedullary tumors.
Fig. 2.
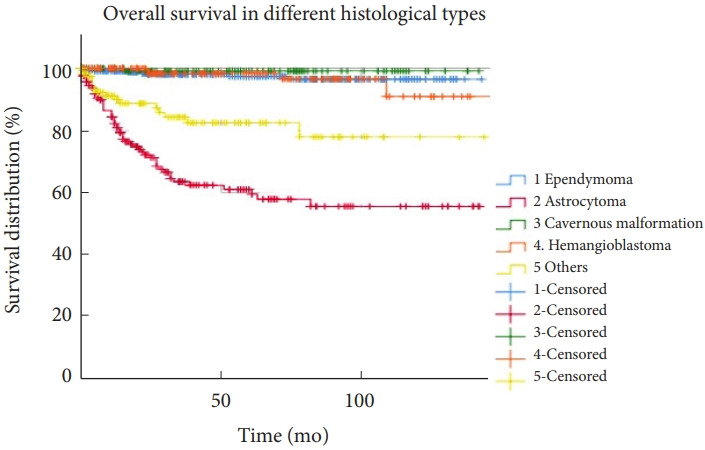
Overall survival is demonstrated using Kaplan-Meier curves for tumors with different histological diagnoses. Astrocytomas had worse survival than the other histological types.
Table 6.
Multiple logistic regression modeling with clinical factors indicating mortality (n=978, 55 cases were lost of follow up)
| Variable | Multivariable analysis |
||
|---|---|---|---|
| OR | 95% CI | p-value | |
| Age | 0.99 | 0.97–0.99 | 0.046 |
| Male sex† | 1.29 | 0.75–2.22 | 0.36 |
| Surgery | |||
| Biopsy | Reference | ||
| Partial removal | 0.64 | 0.32–1.28 | 0.21 |
| Subtotal removal | 0.56 | 0.24–1.31 | 0.18 |
| Total removal | 0.12 | 0.04–0.33 | < 0.001 |
| Modified McCormick Scales (III, IV, V)‡ | 4.82 | 2.62–8.88 | < 0.001 |
| Histopathology | |||
| Ependymoma | Reference | ||
| Astrocytoma | 6.20 | 2.53–15.20 | < 0.001 |
| Cavernous malformations | 0.30 | 0.04–2.44 | 0.26 |
| Hemangioblastoma | 1.59 | 0.44–5.72 | 0.48 |
| Others | 3.31 | 1.29–8.49 | 0.01 |
| Locations | 0.89 | 0.72–1.11 | 0.30 |
| Hosmer and Lemeshow goodness of fit test | 0.146 | ||
OR, odds ratio; CI, confidence interval.
OR was calculated with Female as reference.
OR was calculated with modified McCormick Scales I and II as reference.
6. Functional Outcomes and Associated Factors
The patients’ grades on the McCormick scale preoperatively and postoperatively are depicted in Fig. 3. The proportion of patients with McCormick grades I and II decreased at discharge. However, this proportion increased again, indicating improvement compared to the preoperative status 6 months postoperatively. Functional status further improved at 12 months and was maintained thereafter. Further analyses were performed on 896 patients who were followed up for > 6 months or died 6 months postoperatively. Total tumor removal, better preoperative modified McCormick grades, and histopathologic type of the tumor were also associated with better functional outcomes at 6 months postoperatively (Table 7). Younger age is also associated with better functional outcomes. However, statistical significance was marginal.
Fig. 3.
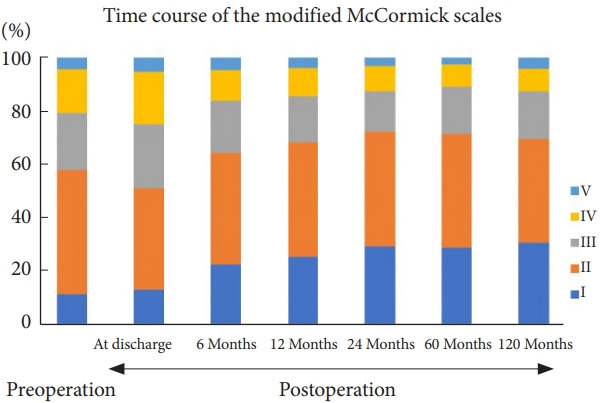
Time course of neurological function expressed using the modified McCormick scale. The proportions of patients with McCormick grades I and II decreased immediately postoperatively (at discharge). However, the proportions increased at 6 months postoperatively. The surgical results were better than the preoperative status. The improved functional status was maintained thereafter.
Table 7.
Multiple logistic regression modeling with clinical factors indicating the better functional outcomes (n=896 with 6-month follow-up)
| Variables | Multivariable analysis |
||
|---|---|---|---|
| OR | 95% CI | p-value | |
| Age | 0.98 | 0.97–0.99 | 0.046 |
| Male sex† | 1.02 | 0.73–1.44 | 0.90 |
| Surgery | |||
| Biopsy | Reference | ||
| Partial removal | 1.04 | 0.47–2.31 | 0.92 |
| Subtotal removal | 1.78 | 0.76–4.19 | 0.19 |
| Total removal | 3.66 | 1.62–8.23 | 0.002 |
| Modified McCormick Scales (I, II)‡ | 14.26 | 10.03–20.29 | < 0.001 |
| Histopathology | |||
| Ependymoma | Reference | ||
| Astrocytoma | 0.41 | 0.22–0.77 | 0.006 |
| Cavernous malformations | 1.20 | 0.72–1.98 | 0.49 |
| Hemangioblastoma | 0.60 | 0.35–1.05 | 0.08 |
| Others | 0.60 | 0.35–1.05 | 0.08 |
| Locations | 0.91 | 0.79–1.05 | 0.21 |
| Hosmer and Lemeshow goodness of fit test | 0.469 | ||
OR, odds ratio; CI, confidence interval.
OR was calculated with Female as reference.
OR was calculated with Modified McCormick scales III, IV, V as reference.
DISCUSSION
In this study, where we successfully collected real clinical data of 1,033 intramedullary tumor cases from 58 certified facilities authorized by the Neurospinal Society of Japan, the main operators were board-certified spine surgeons of the society in 88.3% of the cases [4,7]. To the best of our knowledge, this is the first multicenter collaborative study on surgically treated intramedullary spinal cord tumors in Japanese neurosurgical centers authorized by the Neurospinal Society of Japan. Moreover, our report is one of the largest studies to present details of surgically treated intramedullary tumors, including 361 ependymomas, 196 hemangioblastomas, 168 astrocytic tumors, and 160 cavernous malformations.
The overall incidence of intramedullary spinal cord tumors was reported as 0.31 or 0.35 per 100,000 persons in the United States [1,2]. If we apply this number to the Japanese population (125,000,000), we could expect approximately 340 new cases of intramedullary tumors annually in Japan. In fact, the Japanese Neurosurgical Registry has reported 266 surgical cases of intramedullary tumors annually, accounting for 27.3% and 1.29% of all spinal and intracranial tumors, respectively [3].
Because of the rarity of these tumors, information regarding standard treatment protocols has been sparse [5]. When relevant studies in the literature were reviewed, almost all the reports were retrospective in nature and were classified as providing low-quality evidence [7-9]. Under these circumstances, we still believe that retrospective case reviews could play a meaningful role, especially since we included a large number of cases exceeding those of previous reports.
1. Long-term Results
In this study, the 5-year and 10-year survival rates were 89.1% and 86.0%, respectively. Furthermore, 71.5% and 69.4% of the patients had grade I or II on the modified McCormick scale and were functionally fully independent at 5 and 10 years postoperatively, respectively. Our results were better than those of other reports [10,11]. This could be because the majority of our cases (75.1%) were low-grade lesions, including ependymomas, hemangioblastomas, cavernous malformations, subependymomas, capillary hemangiomas, and schwannomas. Furthermore, the proportion of the astrocytoma was important, which was low at 16.2% in our study. Constantini et al. [10] have reported the long-term clinical results of intramedullary tumors, especially in children and young adults. They had 164 intramedullary tumors, of which 79.3% and 46.3% of the tumors were low-grade lesions and astrocytic tumors, respectively. In their study, 76.8% of patients underwent total tumor removal. The mean follow-up time was 85.1 ± 4.4 months, with 5- and 10-year survival rates of 76% and 70%, respectively. As an example of an adult case series, Raco et al. [11] have reported 202 patients whose mean age was 42.3 years old. In their follow-up, 61.8% of the patients had a stable or improved neurological status. The 5- and 10-year survival rates were 73% and 42%, respectively. They had astrocytomas in 42.6% of cases, which might have influenced the results and led to worse prognoses.
2. Preoperative Neurological Status
Among the various factors, we identified the preoperative modified McCormick scale, the degree of surgical resection of the tumor, and tumor histology as strong indicators of the functional status at 6 months and mortality in the multivariable analyses. The surgical results of intramedullary tumors have been published in the literature (Table 8). However, simply comparing different studies would not be ideal because they included different types of tumors in various proportions. However, across all previous studies, the authors have agreed that preoperative neurological status was a strong predictor of postoperative morbidity [9,11-18]. Here, the preoperative McCormick grade had a significant impact on both postoperative (6 months) functional status and mortality. When patients underwent surgical removal of intramedullary tumors while they were functionally independent, surgery was more likely to provide a better clinical course. These observations support early surgical interventions for intramedullary tumors [11,16,19]. Early recognition of symptoms and prompt MRI evaluation are important for the proper management of intramedullary tumors.
Table 8.
Previous studies demonstrating surgical results for the intramedullary tumors
| Study | Intramedullary tumor cases | Complete resection | Postoperative deterioration | Prognostic factors | |
|---|---|---|---|---|---|
| Cooper and Epstein, [12] 1985 | 29 Cases | 72% | 28% | Satisfactory neurological status before surgery | |
| -14 Ependymomas | Histological type | ||||
| -11 Astrocytomas | Complete removal | ||||
| Cristante and Herrmann, [17] 1994 | 69 Cases | 55.1% | 29.4%–31.5% | Preoperative neurologic deficit | |
| -34 Ependymomas | |||||
| -28 Astrocytomas | |||||
| Constantini et al., [10] 2000 | 164 Cases | 76.8% | 23.% | Histological type | |
| -19 Ependymomas | Preoperative functions | ||||
| -76 Astrocytomas | Patients with shunts | ||||
| Sandalcioglu et al., [13] 2005 | 78 Cases | 83.3% | 34.6% | Preoperative neurological condition | |
| -32 Ependymomas | Histological differentiation | ||||
| -15 Astrocytomas | |||||
| Raco et al., [11] 2005 | 202 Cases | 57.8% | 38.2% | Functional status at surgery | |
| -86 Astrocytomas | Histological type | ||||
| -68 Ependymomas | Extent of surgical removal | ||||
| Woodworth et al., [14] 2007 | 78 Cases | Not addressed | 19.2% | Serum glucose levels | |
| -27 Ependymomas | Preoperative ambulatory status | ||||
| -23 Astrocytomas | Preoperative radiation therapy | ||||
| Matsuyama et al., [15] 2009 | 106 Cases | 56.0% | 31.5% | Good preoperative neurological status | |
| -46 Ependymomas | Total resections | ||||
| -12 Astrocytomas | |||||
| -16 Hemangioblastomas | |||||
| -17 Cavernous malformations | |||||
| Klekamp, [19] 2013 | 250 Cases | 61.2% | 19.5% | Surgical experience | |
| -99 Ependymomas | Preoperative status | ||||
| -76 Astrocytomas | Thoracic tumors | ||||
| -28 Hemangioblastomas | Tumor hemorrhage | ||||
| -13 Cavernous malformations | Recurrent tumors | ||||
| Kumar and Banerjee, [9] 2014 | 43 Cases | 69.8% | 23.3% | Preoperative neurological grade | |
| -21 Ependymomas | Gross tumor resections | ||||
| -12 Astrocytomas | High histological grades | ||||
| -5 Hemangioblastomas | |||||
| Boström et al., [16] 2014 | 70 Cases | 64.3% | 14.3% | Degree of resection | |
| -39 Ependymomas | Preoperative status | ||||
| -11 Astrocytomas | |||||
| -5 Hemangioblastomas | |||||
| -3 Cavernous Malformations | |||||
| Hongo et al., [18] 2019 | 49 Cases | 49.0% | Not addressed | Histological type | |
| -32 Ependymomas | Gross total resection for ependymoma | ||||
| -17 Astrocytomas | |||||
3. Surgical Resections of the Intramedullary Tumors
The degree of surgical removal of intramedullary tumors also had a positive impact on clinical results. Following complete tumor resection, patients had better prognoses and functional outcomes in our analyses. Several other studies support the importance of achieving higher degrees of removal of intramedullary tumors (Table 8) [11,12,15,16,18]. These results are encouraging for neurosurgeons aiming for total resection as the primary goal of treatment for intramedullary tumors.
However, recognizing that complete resection is not always possible is important. Gross total resection was only possible in 65.1% of the cases in our study. Our data clearly indicated that the proportion of total resection varied among different histological subtypes. In cases of hemangioblastomas, cavernous malformations, and ependymomas, 91.8%, 88.8%, and 74.8% of the tumors, respectively, were completely removed. However, total resection could only be performed in 10.7% of the astrocytomas (Table 4). In ependymomas, cavernous malformations, and hemangioblastomas, we could expect dissecting margins between the lesions and the normal spinal cord, making radical resection a reasonable approach [20-23]. Instead, astrocytomas tend to be more infiltrative, lacking a good plane of dissection [15,24]. A recent study has indicated that surgical removal of intramedullary astrocytomas could be associated with higher rates of neurological complications [25].
During each surgery, surgeons must decide whether to continue or stop resection. As neurosurgeons, we should know the microanatomy of the spinal cord and train in microsurgical techniques so that we can remove tumors without damaging the spinal cord function when there is a surgical dissection plane [26]. If there is no plane, making the difficult decision to discontinue the surgical resection is crucial. Currently, spinal cord monitoring is mandatory when performing intramedullary tumor surgery [27]. The recordings of motor and sensory evoked potentials provide useful information regarding the functional integrity of the spinal cord tracts [28]. Rijs et al. [29] have recently performed a meta-analysis that included 1266 intramedullary spinal cord tumor patients who underwent surgery with intraoperative monitoring. These included 727 ependymomas, 173 astrocytomas, 129 hemangioblastomas, and 20 cavernous malformations. Altogether, 855 (72%) and 242 patients (21%) underwent gross total and subtotal resection, respectively. According to the criteria defined by the respective studies, the motor and sensory evoked potentials predicted the emergence of new postoperative neurologic morbidities in 83.8% and 80.8% of the cases, respectively [29]. Nonetheless, judgments based on spinal cord monitoring may have false-positives and false-negatives in predicting postoperative neurological function [30,31]. Especially, rates of the false-positives were reported as high as 50% and 59% in the 2 recent studies from Japan analyzing intraoperative motor evoked potential recordings [30,31]. Since the critical points in the neuromonitoring in predicting new postoperative deficits were different among studies and have not been established, neurosurgeons should still take responsibility for deciding the degree of surgical resection in each case.
4. Surgical Morbidity
Early and radical surgical interventions are associated with better postoperative outcomes after intramedullary tumor surgery. However, at the same time, our study indicated that surgical resection of intramedullary tumors can be challenging. Among the 1,033 patients, 414 (40.1%) experienced worsening of symptoms immediately postoperatively. An analysis based on the modified McCormick scale indicated that 234 patients (22.7%) demonstrated deterioration at discharge. Previous studies have reported consistent results for the risk of postoperative neurological deterioration, ranging from 14.8% to 38.2% (Table 8) [9,11-17,19].
Over the last century, substantial advances have been made in microsurgical techniques, including operating microscopes and intraoperative spinal cord monitoring [30,32,33]. These accomplishments have led to increased safety and effectiveness in intramedullary tumor resection. Several studies have reported that surgical results have improved over time. Klekamp [19] reviewed 278 intramedullary tumors operated on from 1990 to 2012. They divided the study periods into 3 categories: prior to 1990 (n = 67), from 1991 to 2000 (n = 92), and from 2001 to 2012 (n= 87). In these periods, permanent morbidity occurred in 27.9%, 19.8%, and 11.9% of the patients, respectively. In a series of 164 intramedullary tumors over 15 years, Constantini et al. [10] have reported a surgical morbidity rate of 23.8%. However, in the latter half of the study period, the incidence of deterioration by more than one grade on the modified McCormick scale was 5.6%.
Importantly, our data indicate that we could expect gradual recovery and improvement of functional outcomes, despite deterioration immediately postoperatively. These findings are encouraging for surgeons and patients. Although 22.7% of the patients had worse grades immediately postoperatively, this rate improved to 17.6% at 6 months postoperatively. Klekamp [19] have reported worsening of neurological status in 61.5% of patients postoperatively. However, the deficits were transient in 41.5% of the patients. The permanent surgical morbidity rate was 19.5%. Cristante and Herrmann [17] also presented detailed information on postoperative neurological deterioration and delayed recovery in 86 cases of intramedullary tumors, including 34 ependymomas and 28 astrocytomas. Overall, postoperative functional deterioration of the upper and lower extremities was observed in 65.4% and 55.1% of patients, respectively. After a mean follow-up period of 54 months, significant neurological improvements were evident, while 31.5% and 29.4% of the patients continued to have worse upper and lower extremity function, respectively, compared to the preoperative status. Although neurological recovery could be expected over time postoperatively, intramedullary tumor resection poses a potential risk of damaging the neural circuits. The risk of surgery and the expected time course of neurological recovery presented in this study constitute important information for both surgeons and patients.
5. Limitations
This study had some limitations. First, this study was retrospective in nature, and only surgically treated cases were sampled. Therefore, we were unable to include all intramedullary spinal cord tumors treated in Japan. In particular, we could not assess the clinical course of tumors that were treated conservatively. Second, a discussion on the individual histological types of tumors was beyond the scope of this study. For example, we were unable to discuss the roles of adjuvant radiation therapy and chemotherapy in the functional outcomes and survival of malignant astrocytic tumors. Last, our study included 22 cases of intramedullary subependymomas, and we were unable to analyze the clinical characteristics of these intramedullary tumors separately. Consequently, each histological tumor subtype, including these rare lesions, will be further investigated and reported in different studies.
CONCLUSION
These are the first results of a multicenter collaborative study on surgically treated intramedullary spinal cord tumors authorized by the Neurospinal Society of Japan. The degree of resection and postoperative functional outcomes were closely related to the histology of intramedullary tumors. Early surgical interventions aimed at higher degrees of tumor resection, while the patients were functionally independent, were considered a reasonable approach. Simultaneously, the risks associated with intramedullary surgery should be reaffirmed.
Acknowledgments
Portions of this work were presented in abstract form at the 36th Annual Meeting of the Neurospinal Society of Japan, 2021. We thank the investigators of intramedullary spinal cord tumors in the Neurospinal Society of Japan for data registration and analysis (listed in Appendix).
Appendix. Investigators of intramedullary spinal cord tumors in the Neurospinal Society of Japan
Masahito Hara and Masahiro Aoyama; Aichi Medical University. Taku Sugawara; Akita Cerebrospinal and Cardiovascular Center. Hiroaki Shimizu; Akita University. Kotaro Ogihara; Iwakuni Clinical Center. Atsushi Sugawara; Iwate Medical University. Phyo Kim and Kazushige Itoki; Utsunomiya Brain and spinal cord center. Seiji Matsui and Seiji Shigekawa; Ehime University. Noritsugu Kunihiro; Osaka City General Hospital. Kentaro Naito; Osaka City University. Shinji Yamamoto; Ohnishi Neurological Center. Takao Yasuhara; Okayama University. Motoyuki Iwasaki; Otaru City Hospital. Yasuyuki Miyoshi; Kawasaki Medical School. Hideki Hayashi; Kitano Hospital. Nakayama Noriyuki and Toru Iwama; Gifu University. Daisuke Umebayashi; Kyoto Prefectural University of Medicine. Hiroshi Nakagawa and Manabu Sumiyoshi; Kushiro Kojinkai Memorial Hospital. Yasukazu Hijikata: Spine and Low Back Pain Center, Kitasuma Hospital. Hisaaki Uchikado; Kurume University. Hitoshi Fukuda; Kochi University. Tomoaki Nakai and Takashi Sasayama; Kobe University. Kazuhiko Mishima; Saitama Medical University, International Medical Center. Shunsuke Yano and Toru Sasamori; Sapporo Azabu Neurosurgical Hospital. Nobuhiro Mikuni and Yukinori Akiyama; Sapporo Medical University. Tsuyoshi Hara; Juntendo University. Gakuji Gondo; Shonan Kamakura General Hospital. Mitsuhiro Yoshida; Yokkaichi Municipal Hospital. Hideki Komatani and Yuichi Takahashi; Shin Komonji Hospital. Hisaharu Goto and Node Yasuhiro; Shin-yurigaoka General Hospital. Mizuki Watanabe; Seirei Hamamatsu General Hospital. Yasunobu Ito; Tokyo General Hospital. Yoshitaka Hirano; Southern TOHOKU Research Institute for Neuroscience. Teiji Tominaga; Tohoku University. Hirokazu Takami; Tokyo University. Jun Karakama; Tokyo Medical and Dental University. Hiroki Ohashi; The Jikei University School of Medicine. Naoyuki Harada; Toho University. Tetsuro Shingo and Satoshi Kawajiri; Dokkyo Medical University. Tomohiro Yamauchi; Tomakomai City Hospital. Tetsuji Uno; Tottori University. Keisuke Takai and So Fujimoto; Tokyo Metropolitan Neurological Hospital. Yasufumi Otake; Nakamura Memorial Hospital. Yasuhiro Takeshima and Hiroyuki Nakase; Nara Medical University. Akihiko Saito; Niigata City hospital. Daijiro Morimoto and Kyongsong Kim; Nippon Medical School. Tatsuya Ohtonari; Brain Attack Center, Ota Memorial Hospital. Hiroto Kageyama; Hyogo Medical University. Takafumi Mitsuhara; Hiroshima University. Yosuke Kuromi; Fukushima Medical University. Toshiyuki Takahashi and Ryo Kanematsu; Fujieda Heisei Memorial Hospital. Tatsushi Inoue; Fujita Health University. Toshitaka Seki and Kazuyoshi Yamazaki; Hokkaido University. Izumi Koyanagi; Hokkaido Neurosurgical Memorial Hospital. Kazuhisa Yoshifuji; Hokkaido Medical Center for Child Health and Rehabilitation. Masashi Fujimoto; Mie University. Misao Nishikawa; Moriguchi-Ikuno Memorial Hospital. Takashi Yagi and Hiroyuki Kinouchi; University of Yamanashi. Hidetoshi Murata; Yokohama City University. Mari Kitayama; Wakayama Medical University.
Footnotes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study was financially supported by the Neurospinal Society of Japan.
Author Contribution
Conceptualization: TE, MM, RK, SU, TT, KH, MH; Data curation: TE, TI, MM, K Ito, TT, Investigators of Intramedullary Spinal Cord Tumors Japan; Formal analysis: TE, TI, RK, K Ito, Investigators of Intramedullary Spinal Cord Tumors Japan; Funding acquisition: MM, SU, KH, MH; Methodology: TE, SU, TT, KH; Project administration: TE, RK, KH, MH; Visualization: TE; Writing - original draft: TE, TI; Writing - review & editing: TE, T Inoue, MM, RK, K Ito, SU, TT, KH, MH, Investigators of Intramedullary Spinal Cord Tumors Japan.
REFERENCES
- 1.Schellinger KA, Propp JM, Villano JL, et al. Descriptive epidemiology of primary spinal cord tumors. J Neurooncol. 2008;87:173–9. doi: 10.1007/s11060-007-9507-z. [DOI] [PubMed] [Google Scholar]
- 2.Duong LM, McCarthy BJ, McLendon RE, et al. Descriptive epidemiology of malignant and nonmalignant primary spinal cord, spinal meninges, and cauda equina tumors, United States, 2004-2007. Cancer. 2012;118:4220–7. doi: 10.1002/cncr.27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Report of Japan Neurosurgery Registry (2015 - 2017) Neurol Med Chir (Tokyo) 2019;59(Spec):13–81. doi: 10.2176/nmc.si.2019-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tobin MK, Geraghty JR, Engelhard HH, et al. Intramedullary spinal cord tumors: a review of current and future treatment strategies. Neurosurg Focus. 2015;39:E14. doi: 10.3171/2015.5.FOCUS15158. [DOI] [PubMed] [Google Scholar]
- 5.Juthani RG, Bilsky MH, Vogelbaum MA. Current management and treatment modalities for intramedullary spinal cord tumors. Curr Treat Options Oncol. 2015;16:39. doi: 10.1007/s11864-015-0358-0. [DOI] [PubMed] [Google Scholar]
- 6.McCormick PC, Torres R, Post KD, et al. Intramedullary ependymoma of the spinal cord. J Neurosurg. 1990;72:523–32. doi: 10.3171/jns.1990.72.4.0523. [DOI] [PubMed] [Google Scholar]
- 7.Harrop JS, Ganju A, Groff M, et al. Primary intramedullary tumors of the spinal cord. Spine (Phila Pa 1976) 2009;34(22 Suppl):S69–77. doi: 10.1097/BRS.0b013e3181b95c6f. [DOI] [PubMed] [Google Scholar]
- 8.Chamberlain MC, Tredway TL. Adult primary intradural spinal cord tumors: a review. Curr Neurol Neurosci Rep. 2011;11:320–8. doi: 10.1007/s11910-011-0190-2. [DOI] [PubMed] [Google Scholar]
- 9.Kumar R, Banerjee S. Management and functional outcome of intramedullary spinal cord tumors: a prospective clinical study. Asian J Neurosurg. 2014;9:177–81. doi: 10.4103/1793-5482.146591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Constantini S, Miller DC, Allen JC, et al. Radical excision of intramedullary spinal cord tumors: surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg. 2000;93(2 Suppl):183–93. doi: 10.3171/spi.2000.93.2.0183. [DOI] [PubMed] [Google Scholar]
- 11.Raco A, Esposito V, Lenzi J, et al. Long-term follow-up of intramedullary spinal cord tumors: a series of 202 cases. Neurosurgery. 2005;56:972–81. [PubMed] [Google Scholar]
- 12.Cooper PR, Epstein F. Radical resection of intramedullary spinal cord tumors in adults. Recent experience in 29 patients. J Neurosurg. 1985;63:492–9. doi: 10.3171/jns.1985.63.4.0492. [DOI] [PubMed] [Google Scholar]
- 13.Sandalcioglu IE, Gasser T, Asgari S, et al. Functional outcome after surgical treatment of intramedullary spinal cord tumors: experience with 78 patients. Spinal Cord. 2005;43:34–41. doi: 10.1038/sj.sc.3101668. [DOI] [PubMed] [Google Scholar]
- 14.Woodworth GF, Chaichana KL, McGirt MJ, et al. Predictors of ambulatory function after surgical resection of intramedullary spinal cord tumors. Neurosurgery. 2007;61:99–105. doi: 10.1227/01.neu.0000279729.36392.42. discussion 105-6. [DOI] [PubMed] [Google Scholar]
- 15.Matsuyama Y, Sakai Y, Katayama Y, et al. Surgical results of intramedullary spinal cord tumor with spinal cord monitoring to guide extent of resection. J Neurosurg Spine. 2009;10:404–13. doi: 10.3171/2009.2.SPINE08698. [DOI] [PubMed] [Google Scholar]
- 16.Boström A, Kanther NC, Grote A, et al. Management and outcome in adult intramedullary spinal cord tumours: a 20- year single institution experience. BMC Res Notes. 2014;7:908. doi: 10.1186/1756-0500-7-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cristante L, Herrmann HD. Surgical management of intramedullary spinal cord tumors: functional outcome and sources of morbidity. Neurosurgery. 1994;35:69–76. doi: 10.1227/00006123-199407000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Hongo H, Takai K, Komori T, et al. Intramedullary spinal cord ependymoma and astrocytoma: intraoperative frozensection diagnosis, extent of resection, and outcomes. J Neurosurg Spine. 2018;30:133–9. doi: 10.3171/2018.7.SPINE18230. [DOI] [PubMed] [Google Scholar]
- 19.Klekamp J. Treatment of intramedullary tumors: analysis of surgical morbidity and long-term results. J Neurosurg Spine. 2013;19:12–26. doi: 10.3171/2013.3.SPINE121063. [DOI] [PubMed] [Google Scholar]
- 20.Karikari IO, Nimjee SM, Hodges TR, et al. Impact of tumor histology on resectability and neurological outcome in primary intramedullary spinal cord tumors: a single-center experience with 102 patients. Neurosurgery. 2011;68:188–97. doi: 10.1227/NEU.0b013e3181fe3794. [DOI] [PubMed] [Google Scholar]
- 21.Hoshimaru M, Koyama T, Hashimoto N, et al. Results of microsurgical treatment for intramedullary spinal cord ependymomas: analysis of 36 cases. Neurosurgery. 1999;44:264–9. doi: 10.1097/00006123-199902000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Mehta GU, Asthagiri AR, Bakhtian KD, et al. Functional outcome after resection of spinal cord hemangioblastomas associated with von Hippel-Lindau disease. J Neurosurg Spine. 2010;12:233–42. doi: 10.3171/2009.10.SPINE09592. [DOI] [PubMed] [Google Scholar]
- 23.Ren J, Hong T, He C, et al. Surgical approaches and longterm outcomes of intramedullary spinal cord cavernous malformations: a single-center consecutive series of 219 patients. J Neurosurg Spine. 2019;31:123–32. doi: 10.3171/2018.12.SPINE181263. [DOI] [PubMed] [Google Scholar]
- 24.Epstein FJ, Farmer JP, Freed D. Adult intramedullary spinal cord ependymomas: the result of surgery in 38 patients. J Neurosurg. 1993;79:204–9. doi: 10.3171/jns.1993.79.2.0204. [DOI] [PubMed] [Google Scholar]
- 25.Babu R, Karikari IO, Owens TR, et al. Spinal cord astrocytomas: a modern 20-year experience at a single institution. Spine (Phila Pa 1976) 2014;39:533–40. doi: 10.1097/BRS.0000000000000190. [DOI] [PubMed] [Google Scholar]
- 26.Takami T, Naito K, Yamagata T, et al. Surgical management of spinal intramedullary tumors: radical and safe strategy for benign tumors. Neurol Med Chir (Tokyo) 2015;55:317–27. doi: 10.2176/nmc.ra.2014-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kothbauer KF. Intraoperative neurophysiologic monitoring for intramedullary spinal-cord tumor surgery. Neurophysiol Clin. 2007;37:407–14. doi: 10.1016/j.neucli.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Sala F, Palandri G, Basso E, et al. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study. Neurosurgery. 2006;58:1129–43. doi: 10.1227/01.NEU.0000215948.97195.58. [DOI] [PubMed] [Google Scholar]
- 29.Rijs K, Klimek M, Scheltens-de Boer M, et al. Intraoperative neuromonitoring in patients with intramedullary spinal cord tumor: a systematic review, meta-analysis, and case series. World Neurosurg. 2019;125:498–510. doi: 10.1016/j.wneu.2019.01.007. e2. [DOI] [PubMed] [Google Scholar]
- 30.Kurokawa R, Kim P, Itoki K, et al. False-positive and falsenegative results of motor evoked potential monitoring during surgery for intramedullary spinal cord tumors. Oper Neurosurg (Hagerstown) 2018;14:279–87. doi: 10.1093/ons/opx113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi M, Imagama S, Kobayashi K, et al. Validity of the alarm point in intraoperative neurophysiological monitoring of the spinal cord by the monitoring working group of the Japanese Society for Spine Surgery and related research: a prospective multicenter cohort study of 1934 cases. Spine (Phila Pa 1976) 2021;46:E1069–76. doi: 10.1097/BRS.0000000000004065. [DOI] [PubMed] [Google Scholar]
- 32.Sciubba DM, Liang D, Kothbauer KF, et al. The evolution of intramedullary spinal cord tumor surgery. 2009;65(6 Suppl):84–91. doi: 10.1227/01.NEU.0000345628.39796.40. discussion 91-2. [DOI] [PubMed] [Google Scholar]
- 33.Abe H. History of spinal surgery in Japan - from the pioneering period to the progressive era (1911-2017) Neurospine. 2019;16:155–83. doi: 10.14245/ns.1938154.077. [DOI] [PMC free article] [PubMed] [Google Scholar]


