Abstract
Curcumin is a polyphenolic chemical derived from the rhizomes of Curcuma longa. It has been used throughout the Indian subcontinent for medicinal purposes, religious events, and regional cuisine. It has various pharmacological benefits owing to its anti-inflammatory and antioxidant properties. Its neuroprotective effects on the brain and peripheral nerves have been demonstrated in several in vivo neuronal tissue studies. Because of these functional properties of curcumin, it is considered to have great potential for use in the treatment of spinal cord injuries (SCIs). Numerous immunopathological and biochemical studies have reported that curcumin can help prevent and alleviate subsequent secondary injuries, such as inflammation, edema, free radical damage, fibrosis, and glial scarring, after a primary SCI. Furthermore, following SCI, curcumin administration resulted in better outcomes of neurological function recovery as per the Basso, Beattie, and Bresnahan locomotor rating scale. However, to date, its utility in treating SCIs has only been reported in laboratories. More studies on its clinical applications are needed in the future for ensuring its bioavailability across the blood-brain barrier and for verifying the safe dose for treating SCIs in humans.
Keywords: Antioxidant, Curcumin, Inflammation, Neuroprotective agent, Recovery of function, Spinal cord injury
INTRODUCTION
Spinal cord injuries (SCIs) have 2 phases—primary and secondary injuries [1]. A primary injury is caused by mechanical insult and structural damage, whereas a secondary injury is a sequence of systemic and local neurochemical and physiological alterations. Subsequent edema, ischemia, inflammation, cytokine production, free radical damage, glial scar formation, apoptosis, and necrosis contribute toward the development of secondary injuries [2]. A primary injury is immediate and irreversible; in contrast, a secondary injury worsens with time and necessitates therapeutic intervention. Thus, preventing or aggressively treating secondary injuries is the mainstay of care for acute SCIs [3,4].
Curcumin is a promising therapeutic drug for SCI treatment because it reduces the incidence of secondary injuries. It is a yellow extract derived from Curcuma longa that is frequently used as a spice and food-coloring ingredient in India (Fig. 1). Curcumin has antioxidant and nonsteroidal anti-inflammatory pharmacological properties [5,6]. Preclinical and clinical trials have revealed its various pharmacological activities, including its anti-inflammatory, antibacterial, anticancer, and neuroprotective effects on neurodegenerative disorders. Curcumin also has hepatoprotective, nephroprotective, cardioprotective, neuroprotective, hypoglycemic, and antirheumatic activities, and its neuroprotective activity against several neurodegenerative disorders is gaining researchers’ attention [7]. As an anti-inflammatory agent, curcumin suppresses the production of many proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1, IL-8, and monocyte chemoattractant protein 1 [8,9]. In a recent study, curcumin inhibited the hypoxia-induced upregulation of glial fibrillary acidic protein (GFAP) and neurofilament-H following hypoxia and downregulated the expression of proinflammatory cytokines, such as TNF-α and IL-1 [10]. It also suppresses glial scar formation and GFAP expression, contributing toward the development of a more favorable environment for neurological recovery (Fig. 2) [11].
Fig. 1.

Curcuma longa plant and powder. Curcumin is a yellow substance produced by Curcuma longa. Curcumin is the primary curcuminoid found in turmeric, a member of the ginger family. It is marketed as an herbal supplement, cosmetic ingredient, food-flavoring agent, and food colorant.
Fig. 2.
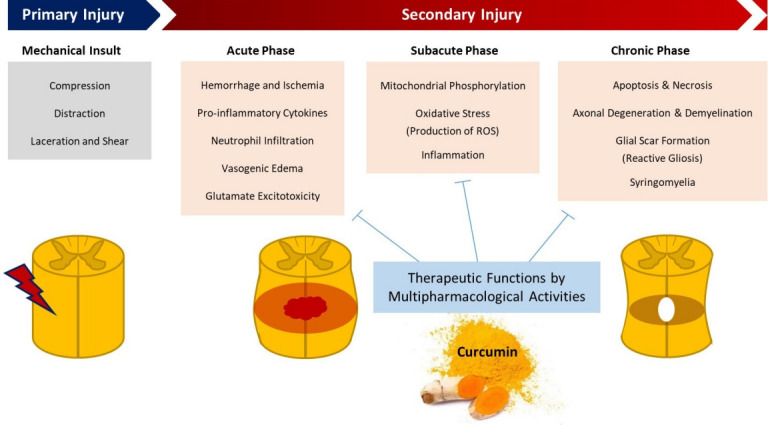
Pathophysiological process following spinal cord injury and the targeted therapeutic function of curcumin. ROS, reactive oxygen species.
This study aimed to consolidate the knowledge essential for spine surgeons and related clinicians to understand how curcumin can alleviate secondary injuries observed in SCIs. Herein, we discuss the basics of neuroprotective effects and accumulate experimental evidence regarding the neuroscience of curcumin.
PHARMACOLOGY
Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] is a complex pharmacophore that has the potential to serve as an antioxidant, chelate metals, and trigger the Michael reaction [12]. Additionally, it is a hydrophobic molecule with a strong affinity toward cellular membranes and consists of 2 ferulic acid residues connected by a methylene bridge [13]. The structure of the molecule is symmetrical. The 3 major components of curcumin molecules are the keto-enol tautomer in the middle, flexible α,β-unsaturated β-diketo linker, and terminal o-methoxyphenolic groups (Fig. 3).
Fig. 3.
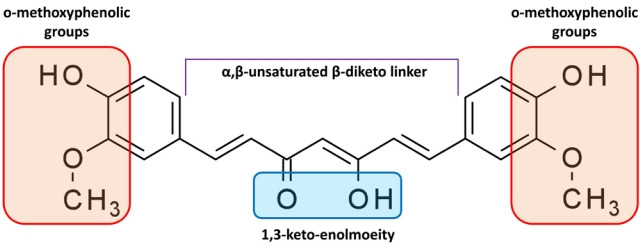
Molecular structure of curcumin. The keto-enol tautomer in the center, flexible α,β-unsaturated β-diketo linker, and terminal o-methoxyphenolic groups make up the curcumin molecule, which has an asymmetric structure.
The structure of curcumin contains various functional groups (diketo group, carbon-carbon double bonds, and phenyl rings). Thus, curcumin is a unique and strong antioxidant. Structure-activity correlations have shown that the β-diketone (keto-enol) moiety acts as a chelator of cationic metals in protein-binding sites and as a Michael reaction acceptor for nucleophilic compounds that form covalent bonds with curcumin, such as reduced selenocysteine and sulfhydryl [14]. The antioxidant activity of curcumin is dependent on the phenolic hydroxyl group [15]. This group and methylene hydrogen are essential for curcumin’s free radical scavenging activity, which involves electron transfer or H-atom abstraction from reactive oxygen species (ROS) and nitrogen species (Fig. 4) [16].
Fig. 4.
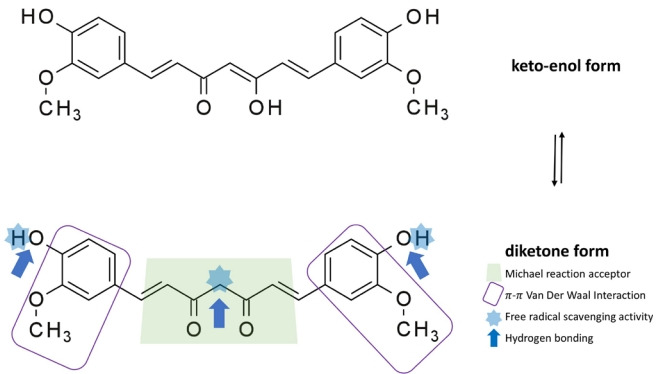
Curcumin has a wide range of interactions. Curcumin contains a complex pharmacophore that can function as an antioxidant; chelate metals; and facilitate Michael reactions (used in the mild formation of C–C bonds), hydrogen-bonding interactions, π-π van der Waals interactions, and free radical scavenging.
The wide range of interactions of curcumin might explain why it binds to various proteins. Curcumin affects the function of roughly 100 biological targets in various ways [17], including the modification of the phosphorylation state of cellular proteins [18]. Curcumin has effects at doses above the micromolar level in general. This low binding affinity has aided various attempts to use a structure-based drug design to improve the efficacy of curcumin.
ANTI-INFLAMMATORY EFFECT
One of the most promising alternatives for primary SCI treatment is an anti-inflammatory multimodal neuroprotection strategy [19]. Spinal cord edema, which is accompanied by acute inflammation and precedes fibrosis, plays a critical role in neurological impairment. This provides a fundamental explanation for the clinical use of corticosteroids in patients with SCIs. Curcumin is an anti-inflammatory molecule that suppresses transcription factors including nuclear factor-kappa B (NF-κB) and signal transducer and activator of transcription (STAT) in the upstream signaling pathways of inflammatory mediators such as prostaglandins, cytokines, and chemokines, resulting in the global inhibition of the inflammation network [20]. Given the fundamental nature of NF-κB signaling pathway activation to neuroinflammation and SCI pathophysiology [21], modulating NF-κB signaling could help minimize inflammation, lessen the severity of secondary injury, and maintain neuronal function.
Curcumin can also directly bind to inflammatory mediators and enzymes involved in downstream inflammatory pathways, such as IL-1 converting enzyme, TNF-α, TNF-α converting enzyme, p38 mitogen-activated protein kinase, myeloid differentiation protein-2, 1-acid glycoprotein, and glycogen synthase kinase-3 beta [14,22]. Additionally, curcumin suppresses the production of transforming growth factor-beta (TGF-β)1, TGF-β2, and sex-determining region Y-box transcription factor 9. It also facilitates the development of a microenvironment appropriate for nerve development [23]. Curcumin suppresses the upregulation of aquaporin 4 and GFAP and the atypical activation of the Janus kinase–STAT signaling pathway associated with SCI [24]. Recent in vivo studies on the anti-inflammatory function of curcumin are summarized in Table 1.
Table 1.
Anti-inflammatory properties of curcumin: list of recent evidentiary studies
| Study | Specimen/sample size/SCI method | Study design (experimental groups) | Curcumin treatment method | Summary of results |
|---|---|---|---|---|
| Zu et al., [24] 2014 | Male Sprague-Dawleyrats/N = 64/Striking bar falling (diameter: 3 mm) 5-cm height (150 gcf) on T8 level | Sham/DMSO (n = 16) | 40 mg/kg | Curcumin increased gray–white matter interface, tissue edema/AQP-4 expression, and GFAP/pJAK-STAT expression |
| Sham/Curcumin (n = 16) | Single IP injection | |||
| SCI/DMSO (n = 16) | 30 min after SCI | Moderately improved BBB scores | ||
| SCI/Curcumin (n = 16) | ||||
| Wang et al., [59] 2014 | Female BALB/c mice/N = No description/10-g force clip for 3 sec, extradural on T9 level | Sham | 50 mg/kg | Curcumin decreased tissue expression of GFAP and Iba-1 and increased NF-200 |
| SCI/DMSO | Single IP injection | Decreased levels of IL-1β, NO, and NF-κB | ||
| SCI/Curcumin | Immediately after SCI | Increased neuromotor scores (Basso mouse scale) | ||
| Lin et al., [60] 2015 | Wild-type C57BL/6JNarl mice/ N = 18/Weight dropped | Sham control (n = 6) | 40 mg/kg | Curcumin attenuated the downregulation of CISD2 in SCI and LPS-treated astrocytes. |
| Guide was lifted up to 4 mm to perform a hemitransection | SCI (n = 6) | Single IP injection | (CISD2 exerts antiapoptotic and anti-inflammatory effects on neural cells) | |
| SCI+Curcumin (n = 6) | 30 min after SCI | |||
| Yuan et al., [23] 2015 | Female Sprague-Dawley rats/N = No description/Aneurysm clip (fixed force of 50 g) for 60 secon T9 level | Sham | Various dose of curcumin (300, 100, and 30 mg/kg) | Curcumin inhibited the expression of proinflammatory cytokines (TNF-α, IL-1β, and NF-κb) |
| SCI | ||||
| SCI+Curcumin 30 | IP injection once per day for 7 days | Reduced the expression of the intracellular components and GFAP through its anti-inflammatory effects. | ||
| SCI+Curcumin 100 | Suppressed reactive gliosis. | |||
| SCI+Curcumin 300 | Inhibited the generation of TGF-β1, TGF-β2, and SOX-9 | |||
| SCI+Methylprednisolone | Improved BBB scores | |||
| Ni et al., [61] 2015 | Male Sprague-Dawley rats/N = 48/30-g force extradural com- pression with a clip for 30 sec on T8–9 level | Sham (n = 16) | 100 mg/kg | Curcumin modulated the TLR4/NF-κB inflammatory signaling pathway and significantly ameliorated SCI-induced spinal cord edema and apoptosis. |
| SCI (n = 16) | IP injections at 15 min after SCI | |||
| SCI+Curcumin (n = 16) | BBB scores significantly increased | |||
| Yuan et al., [9] 2017 | Female Sprague-Dawley rats/N = 280/50-g force clip compres- sion for 60 sec on T9 level | Sham (n = 70) | 100 mg/kg | Curcumin regulated both the NF-κB and SOX-9 signaling pathways. |
| SCI (n = 70) | IP injections | Downregulated the expression of chemokines, MCP-1, RANTES, and CXCL10, released by astrocytes. | ||
| SCI+Curcumin (n = 70) | Immediately after surgery and once every 24 hr for 7 days | |||
| SCI+DMSO (n = 70) | Decreased macrophage and T-cell infiltration | |||
| Ruzicka et al., [40] 2018 | Wistar rats/N = 135/Balloon-in- duced compression using Fogarty catheter on T8 level | Saline (n = 34) | High dose once a week (60 mg/kg diluted in olive oil) intrathecally 4 times (immediately after SCI followed up for 3subsequent weeks), low-dose IP injection daily (6 mg/kg diluted in olive oil) (immediately after SCI and on the 28th day) | The combined therapy facilitated axonal sprouting and modulated the expression of proregenerative factors and production of inflammatory responses. |
| Curcumin (n = 27) | ||||
| MSC (n = 28) | Both curcumin and curcumin combined with MSC therapy improved BBB score and the combined treatment group showed additional improvement in advanced locomotor performance. | |||
| Curcumin+MSC (n = 26) | ||||
| Ruzicka et al., [62] 2018 | Wistar rats/N=131/Ballooninduced compression using | Behavioral group study: | Curcumin 6 mg/kg, | Curcumin and EGCG alone or in combination increased axonal sprouting, decreased glial scar formation, and altered the levels of macrophage inflammatory protein 1-alpha, interleukin-1β, interleukin-4, and interleukin-6. |
| Saline (n=10) | EGCG 17 mg/kg | |||
| Fogarty catheter for 5 min | Curcumin (n=13) | IP daily | ||
| EGCG (n=19) | Curcumin 60 mg/kg | |||
| Curcumin+EGCG (n=9) | EGCG 17 mg/kg | All treatments displayed significant behavioral recovery (BBB score) with no obvious synergistic effect after the administration of the combined therapy of curcumin and ECGC | ||
| Cytokine group study: | IM weekly for 28 days | |||
| Saline (n=20) | ||||
| Curcumin (n=20) | ||||
| EGCG (n=20) | ||||
| Curcumin+EGCG (n=20) | ||||
| Lee et al., [4] 2019 | Sprague-Dawley rats/N=35/Clip with 30-g force for 2 min | Sham (n=32) | 200 mg/kg/day for 8 weeks, IP | SCI+hyperglycemia+curcumin group: SOD activity increased, malondialdehyde and ED-1 macrophage marker levels decreased, IL-6, IL-8, TNF-α, phosphorylated extracellular signal-regulated kinase, phosphorylated JNK, and phosphorylated p38 levels decreased, |
| SCI only (n=32) | ||||
| SCI+Hyperglycemia (n=32) | ||||
| SCI+Hyperglycemia+Curcumin (n=32) | Better BBB score | |||
| Yardım et al., [63] 2021 | Male Sprague-Dawley rats/N=35/PTX-induced SCI | Control (n=7) | 100 mg/kg or 200 mg/kg | Curcumin reduced mRNA expression levels of NF-κB, TNF-α, IL-6, iNOS, and GFAP and increased the levels of Nrf2, HO-1, and NQO1. Curcumin suppressed the activation of apoptotic and autophagic pathways by increasing Bcl-2 and Bcl-xL and decreasing p53, caspase-3, Apaf-1, LC3A, LC3B, and beclin-1 mRNA expression levels |
| Curcumin (n=7) | Oral daily for 10 days | |||
| PTX (n=7) | ||||
| PTX+Curcumin100 (n=7) | ||||
| PTX+Curcumin200 (n=7) |
SCI, spinal cord injury; DMSO, dimethyl sulfoxide; AQP-4, aquaporin 4; GFAP, glial fibrillary acidic protein; pJAK-STAT, phosphorylated Janus kinase-signal transducer and activator of transcription; BBB, Basso, Beattie, Bresnahan; BALB, Bagg albino; IP, intraperitoneal; NF-200, neurofilament-200; IL, interleukin; NO, nitric oxide; NF-κB, nuclear factor kappa B; LPS, lipopolysaccharide; TNF, tumor necrosis factor; TGF, transforming growth factor; SOX-9, sex-determining region Y-box transcription factor 9; MCP-1, monocyte chemoattractant protein-1; RANTES, regulated upon activation, normal T cell expressed and presumably secreted; CXCL10, C-X-C motif chemokine ligand 10; MSC, mesenchymal stem cells; EGCG, epigallocatechin gallate; IM, intramuscular; iNOS, inducible nitric oxide synthase; Nrf2, nuclear erythroid 2-related factor 2; HO-1, hemeoxygenase 1; NQO1, NAD(P)H:quinone oxidoreductase 1; PTX, paclitaxel; LC3A, light chain 3 A; LC3B, light chain 3 B.
ANTIOXIDANT EFFECT
Numerous experiments have been conducted to determine the antioxidant capabilities of curcumin for SCI treatment. There is a strong correlation between SCI and inflammation-induced free radical formation [25]. Curcumin is a potent antioxidant that is reported to be superior to vitamin E, resveratrol, and other commonly used antioxidants [26]. Curcumin interacts with ROS directly and also acts as an activator of antioxidant signaling systems, making its effects more comprehensive and long lasting [27]. Curcumin can also neutralize free radicals via electron transfer and/or H-atom donation. The antioxidant ability of curcumin is influenced by 3 distinct functional dissociation groups and a β-diketone site [28,29].
Curcumin binds to lipid radicals in cell membranes and converts them into phenoxyl radicals. Because phenoxy is more polar than curcumin, it diffuses to the membrane surface, where it can be repaired by any water-soluble antioxidant such as ascorbic acid. Thus, curcumin can protect cell membranes against oxidative damage by acting as a lipid radical scavenger [30]. It has the potential to enhance the activity of antioxidant enzymes, such as plasma catalase, erythrocyte superoxide dismutase (SOD), and plasma glutathione peroxidase [31]. Furthermore, malondialdehyde (MDA), which is the end product of lipid peroxidation, is a reliable marker of oxidative stress-mediated lipid peroxidation [32]. Curcumin decreased MDA based on a fixed-effects model (n= 56; pooled mean difference [MD]= -1.00; 95% confidence interval [CI], -1.59 to -0.42; p= 0.00008) in a meta-analysis of 4 studies on MDA levels [8].
Additionally, curcumin could stimulate antioxidant protection genes via the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway [33]. It increases intracellular antioxidant defense responses by activating the Nrf2/antioxidant response element pathway, which results in the development of several antioxidants and detoxification and cytoprotective proteins [34]. Recently, Ni et al. showed that SCI caused a considerable increase in labile Zn and inflammatory cytokines in an injured rat’s spinal cord, and curcumin decreased the accumulation of labile Zn [35]. Zn is important in decreasing oxidative stress and generating inflammatory cytokines. Recent studies that demonstrate the antioxidant function of curcumin are summarized in Table 2.
Table 2.
Antioxidant property of curcumin: list of recent evidentiary studies
| Study | Specimen/sample size/SCI method | Study design (experimental groups) | Curcumin treatment method | Summary of results |
|---|---|---|---|---|
| Akar et al., [64] 2017 | Wistar rats/N=40/Spinal cord ischemia induced by clamping the aorta | Sham (n=10) | 100 mg/kg, IP at 30 min before ischemia | Decreased MDA levels in the spinal cord |
| Ischemia–reperfusion (n=10) | Increased SOD and GPx levels caused by curcumin | |||
| Curcumin (n=10) | Dissolved in 5 N NaOH | Neurological outcome scores were significantly better when compared with those of the IR group. | ||
| Solvent (n=10) | ||||
| Xi et al., [65] 2019 | Sprague-Dawley rats/N=24/A hammer was dropped on T8 level | Sham/control (n=8) | 80 mg/kg/day, IP | Oxidative stress and apoptosis (caspase-3 activity and B cell lymphoma 2-associated X protein levels) were suppressed |
| SCI (n=8) | Tetrahydrocurcumin for 2 weeks | Tetrahydrocurcumin inhibits oxidative stress response by regulating FOXO4 in SCI model rats. | ||
| Tetrahydrocurcumin treatment (n=8) | Tetrahydrocurcumin increased the BBB scores | |||
| Daverey et al., [10] 2020 | Male Wistar rats/N=18/30-mm spinal cord section/Hypoxia | Sham | One hour incubation with 50 μM curcuminin 95% N2 and 5% CO2 | Curcumin inhibited hypoxia-induced HIF1-α expression and tissue damage by improving the morphology of astrocytes and remarkably reducting vacuolation. |
| Hypoxia | ||||
| Hypoxia+Curcumin | ||||
| Sham | It inhibited the hypoxia-induced upregulation of GFAP and neurofilament-H (NF-H) after hypoxia and downregulated the expression of proinflammatory cytokines such as TNF-α and IL-1. | |||
| Hypoxia | ||||
| Hypoxia+Curcumin | ||||
| Hypoxia+BAY11-7082 | Curcumin exerted its neuroprotective effect through cross-talk between the NF-κB and Nrf2 signaling pathways. | |||
| Daverey and Agraw-al, [66] 2020 | Human astrocytes | Human astrocytes | Human astrocytes: | Riluzole protects white matter injury by the activation of Nrf2/HO-1 and caspase 9. |
| Male Wistar rats/N=21/30-mm spinal cord section/Hypoxia | Sham | Riluzole (1 μM) | ||
| Hypoxia | Curcumin (1 μM) | Curcumin’s neuroprotective effect is mediated through the inhibition of HIF-1α, GFAP, NF-H, and caspase 9. | ||
| Hypoxia+Curcumin | Rat SCI model (white | |||
| Hypoxia+Riluzole | matter injury) | |||
| Hypoxia+Riluzole+Curcumin | Riluzole (10 μM) | Curcumin is more effective than riluzole in reducing GFAP and NF-H injury. | ||
| Curcumin (50 μM) |
SCI, spinal cord injury; IP, intraperitoneal; MDA, Malondialdehyde; SOD, serum superoxide dismutase; IR, ischemia–reperfusion; FOXO4, forkhead box protein O4; BBB, Basso, Beattie, Bresnahan; HIF1-α, Hypoxia inducible factor 1-α; GFAP, glial fibrillary acidic protein; TNF, tumor necrosis factor; IL, interleukin; Nrf2, nuclear erythroid 2-related factor 2; HO-1, hemeoxygenase 1; NF-H, neurofilament protein-H.
STEM CELL PROLIFERATION
While research on stem cells for SCI treatment is ongoing, there still exist significant barriers in achieving excellent therapeutic results. It is critical to promote neural stem cell (NSC) proliferation for treating SCI, and several investigations have reported the contribution of curcumin to this process [36-38]. An improved therapeutic effect can be achieved by modifying stem cell proliferation and differentiation and reducing the inflammatory microenvironment in injured regions [39].
Curcumin enhances the functional recovery of SCIs when combined with NSC or mesenchymal stem cell (MSC) therapy [37,40]. In a study by Ormond et al. [2] the combination of curcumin and NSC therapy led to a significant recovery of severe SCIs in vivo, which was evidenced by better functional locomotor recovery, body weight, and soleus muscle mass. Wanjiang et al. [41] confirmed that in combination with MSC therapy, curcumin suppressed human umbilical cord-derived MSC (hUC-MSC) apoptosis via the ERK1/2 signaling pathway, and the combined curcumin and hUC-MSC therapy improved the motor function of rats with SCIs. A study by Bonilla et al. [42] evaluated a combination therapy comprising human NSCs derived from induced pluripotent stem cells (iPSC-NSCs), human MSCs, and a pH-responsive polyacetal–curcumin nanoconjugate (PA–C) that allows the sustained release of curcumin. The combination of stem cell transplantation and PA–C therapy exerted higher neuroprotective effects compared with individual therapies. Representative studies showing that curcumin enhances stem cell proliferation are summarized in Table 3.
Table 3.
Recent studies showing proliferation enhancement through the combination of curcumin and stem cells
| Study | Stem cell types and specimen/SCI method | Study design (experimental groups) | Curcumin treatment method | Summary of results |
|---|---|---|---|---|
| Son et al., [36] 2014 | Neural progenitor cell (NPC) from the spinal cord of Sprague-Dawley rats | Examine cellular proliferation (MTS assay) in control (no curcumin) and curcumin groups at 6 different dose levels | In culture medium at 0.1, 0.5, 1, 10, 20, and 50 μM | Lower dosage (0.1, 0.5, 1 μM) of curcumin increased SC-NPC proliferation. |
| However, higher dosage decreased SC-NPC proliferation. | ||||
| Curcumin stimulates the proliferation of SC-NPCs via the MAP kinase signaling pathway, especially involving the p-ERK and p-38 proteins. | ||||
| Requejo- Aguilar et al., [39] 2017 | Ependymal stem/progenitor cells of the spinal cord (EpSP- Ci) of Sprague-Dawley rats | PA (as vehicle) (n=12) | Intrathecal administration | PA–C enhances neuroprotection, increases axonal growth |
| PA–curcumin–Cy5.5 (n=15) | PA–curcumin–Cy5.5 (10 μM) (combination treatment: ep-SPCs and a pH-responsive polymer–curcumin conjugate) | PA–C can improve functional recovery in acute SCI | ||
| Contusion 250 kdyn | Also enhances functional recovery in a rodent model of chronic SCI. | |||
| Infinite Horizon Impactor | *PA (polyacetal): enhances blood bioavailability and stability and provides a means for highly localized delivery. | |||
| Bang et al., [37] 2018 | Neural stem/progenitor cells de- rived from Sprague-Dawley rats N = 60/Clip with a closing force of 30 g & a 2-min com- pression | Sham (n=20) | Implanting indwelling intrathecal catheters/A concentration of 1 μmol/L for curcumin | SCI-Curcumin group: |
| SCI-Curcumin (n=20) | The co-immunoreactivity of nestin/BrdU was higher | |||
| SCI-Vehicle (n=20) | The GFAP immunoreactivity and lesion cavity was lower | |||
| The BBB score was better (up to 14 days) | ||||
| Wanjiang et al., [41] 2020 | hUC-MSC/Female Sprague- Dawley rats/N = 180/50-g aneurysm clip compression on for 60 sec on T9 level | Sham (n=30) | IP, 100 mg of curcumin, dissolved in 1 mL of DMSO and 0.5 mL of NS | Curcumin suppressed hUC-MSC apoptosis through the ERK1/2 signaling pathway |
| SCI+Veh (n=30) | ||||
| SCI+cur (n=30) | 1st injection: 30 min after the operation | The combination of curcumin and hUC-MSC therapies improved motor function after SCI in rats. | ||
| SCI+hUC-MSC (n=30) | ||||
| SCI+cur+hUC-MSC (n=30) | Once/day for 14 days. | |||
| SCI+cur+hUC-MSC+U0126 (n=30) | ||||
| Bonilla et al., [42] 2021 | Induced pluripotent stem cells (iPSC-NSC) & Human MSC Female Sprague-Dawley rats/200 kdyne contusion on T8 level iPCS-NSC (n = 8) | Control (n=16) | A pH-responsive polyacetal–curcumin nanoconjugate (PA–C) delivery into the intrathecal space in contusive SCI with stem cell transplantation. | PA–C-treated or PA–C and iPSC-NSC + MSC-treated groups: Smaller scars, whereas PA–C and iPSC-NSC + MSC therapy induced the preservation of β-III tubulin-positive axons. |
| MSC (n=11) | ||||
| iPCS-NSC+MSC (n=11) | ||||
| PA-C (n=6) | iPSC-NSC + MSC transplantation fostered the preservation of motoneurons and myelinated tracts, whereas PA–C therapy polarized microglia into an anti-inflammatory phenotype. | |||
| iPSC-NSC+MSC+PA-C (n=7) | ||||
| Elkhenany et al., [67] 2021 | Human induced neural progeni- tor cells (iNPC) Female Sprague-Dawley rats/200 kdyn Infinite Horizon Impactor on T8 level | HA_PM_iNPC (non SCI) (n=3) | PM-embedded curcumin | PM-embedded iNPCs and CURC with PPY fibers supported a significant increase in neuropreservation (as measured by higher βIII tubulin staining of neuronal fibers) and decrease in the injured area (as measured by the lack of GFAP staining). |
| HA_PPY_PM_iNPC (non SCI) (n=3) | ||||
| HA_PM_CURC (n=3) | ||||
| HA_PPY_PM_CURC (n=3)HA_PM_iNPC (n=3) | ||||
| HA_PPY_PM_iNPC (n=3) | *HA: hyaluronic acid | |||
| HA_PM_CURC_iNPC (n=3) | *PM: Corning® PuraMatrixTM peptide hydrogel | |||
| HA_PPY_PM_CURC_iNPC (n=3) | *PPY: polypyrrole-coated fibers |
SCI, spinal cord injury; SC-NPC, spinal cord neural progenitor cell; MAP, mitogen-activated protein; p-ERK, phospho-extracellular signal-regulated kinase; PA, polyacetal; PA-C polyacetal-curcumin; epSPC, ependymal stem/progenitor cells of the spinal cord; GFAP, glial fibrillary acidic protein; BBB, Basso, Beattie, Bresnahan; hUC-MSC, umbilical cord mesenchymal stem cell; IP, intraperitoneal; DMSO, Dimethyl sulfoxide; ERK, extracellular signal-regulated kinase; CURC, curcumin.
NEUROLOGICAL FUNCTIONAL IMPROVEMENT
Neurological function was found to be improved by curcumin in a random-effects model of a comprehensive meta-analysis [8]. The magnitude of the effect, as measured by the Basso, Beattie, and Bresnahan (BBB) locomotor rating scale, was significantly increased when the curcumin dosage was elevated (4 trials; total, 132 rats; pooled MD=3.09; 95% CI, 3.40–4.45; p=0.04) [8]. Several studies on functional recovery had the advantage of adopting a uniform scale (BBB score) for interstudy comparisons, making aggregated results reliable. In an animal trial, 60 Wistar rats were randomly assigned to receive either curcumin therapy (30 rats) or placebo (30 rats) [43]. Curcumin therapy (immediately applied to the injured spinal cord surface and then administered intraperitoneally daily) resulted in behavioral recovery within the first week following SCI, as shown by better BBB and plantar scores for sensory function. This functional improvement was induced by the anti-inflammatory effects of curcumin. In another animal study investigating antioxidative characteristics and functional recovery, the curcumin-treated group demonstrated enhanced locomotor scores (BBB scores), increased SOD levels, reduced MDA levels, and reduced macrophage markers following SCI [3].
One study found that curcumin was more effective than methylprednisolone, which is frequently used in clinical practice to treat secondary injury in patients with SCIs 2 weeks after primary injury based on BBB scores [44]. The study concluded that curcumin has a greater therapeutic potential than methylprednisolone, showing a longer duration of action in SCIs. Thus, in vivo studies on rats and their BBB scores offer substantial evidence of the efficacy of curcumin in causing functional recovery after SCIs. In many in vivo studies, neurological function was assessed using BBB scores (Tables 1–3). Other studies that evaluated functional recovery using BBB scores are summarized in Table 4.
Table 4.
Other studies showing the property of curcumin in promoting neurological functional recovery as evaluated by measuring the BBB score
| Study | Specimen/sample size/SCI method | Study design (experimental groups) | Curcumin treatment method | Summary of results |
|---|---|---|---|---|
| Kim et al., [3] 2014 | Male Sprague-Dawley rats/N = 36/clipping 30-g force for 2 min on T9 level | Sham (n=12) | 200 mg/kg IP daily for 7 days | Curcumin group: Higher BBB scores 7–14 days after surgery (by antiinflammatory and antioxidant action/ED-1, MDA, and SOD were measured) |
| SCI/vehicle (n=12) | ||||
| SCI/curcumin (n=12) | ||||
| Machova Urdzikova et al., [43] 2015 | Male Wistar rats/N = 60/or balloon compression using Fogarty catheter (2 Fr) on T8 level | Control (n=30) | 60 mg/kg Epidural locally | Curcumin group: Improved behavioral recovery (BBB scores and plantar sensory performance scores) within the first week following SCI (by anti-inflammatory action/NF-κB, MIP1a,IL4, IL1b, IL2, IL6, IL12p70, TNF-α, and RANTES were measured) |
| Curcumin (n=30) | Immediately after injury and 6 mg/kg in olive oil IP daily for 1–28 days | |||
| Liu et al., [44] 2018 | Male Sprague-Dawley rats/N = 60/10-g rod dropped, from 25-mm height on T9–10 level | SCI-Curcumin (n=27) | 200 mg/kg IP daily for 56 days | SCI-Curcumin group: Improvement in the BBB score |
| SCI-MP (n=27) | MP-treated group better within the first 14 days | |||
| Sham group (n=6) | Cur-treated group better from 21–49 days after SCI | |||
| Paralleled BBB scores of the 2 treatment groups on 56 days after SCI (by anti-inflammatory action/Bax, Bcl-2, Caspase-3, and GFAP were measured) | ||||
| Luo et al., [68] 2021 | Female Sprague-Dawley rats/N = 24/2-mm segment of the spinal cord removed at T9 level | Control (n=6). | FC/FI-Cur hydrogel was implanted into the lesion area. | FC/FI-Cur hydrogel group: Significantly promoted BBB walking score (by anti-inflammatory action/immunofluorescence staining of antibodies CD68, S100, neurofilament 200, GFAP, myelin basic protein, etc. were measured) |
| FC hydrogel (n=6) | ||||
| FC/FI hydrogel (n=6) | FC: Fmoc-grafted chitosan | |||
| FC/FI-Cur hydrogel (n=6) | FI: Fmoc peptide |
BBB, Basso, Beattie, Bresnahan; SCI, spinal cord injury; ED-1, CD68/SR-D1 antibody (marker for activated macrophages); MDA, malondialdehyde; SOD, superoxide dismutase; NF-κB, nuclear factor kappa B; MIP1a, macrophage inflammatory protein-1 alpha; IL, interleukin; TNF-α, tumor necrosis factor-alpha; RANTES, regulated upon activation, normal T cell expressed and presumably secreted; MP, methylprednisolone; IP, intraperitoneal; GFAP, glial fibrillary acidic protein.
OBSTACLES AND FUTURE DIRECTION
Although curcumin has been reported to be a promising neuroprotective agent, its practical applications are limited owing to a number of issues. Curcumin has limited bioavailability because of its low water solubility, poor absorption, rapid metabolism, and fast elimination. It cannot cross the blood-brain barrier, making it unsuitable for use in treating central nervous system injuries, including SCIs. Toxicity is also an issue. Curcumin induces damage to the DNA both in vitro and in vivo [45]. Curcumin acts as a dose-dependent antioxidant to inhibit ROS as well as a pro-oxidant to produce ROS [46,47]. Superoxide anion and hydrogen peroxide are the 2 types of ROS that may play an important role in carcinogenesis [48]. At high doses, curcumin can react with the thiol groups of cysteine residues [49], causing DNA damage or p53 inactivation [50,51].
However, these limitations are being addressed by encapsulating curcumin into nanoformulations. Encapsulation of curcumin into nanocarriers via various methods is an appropriate and effective strategy to increase its bioavailability because this method expands its solubility, promotes long-term circulation and retention in the body, and overcomes the physiological barriers of curcumin [52,53]. These nanoformulations can increase the half-life of curcumin in plasma and significantly reduce the administration dosage, resolving the toxicity issue associated with the use of high-dose curcumin.
Among the diverse nanocarriers developed for therapeutic applications, polymer therapeutics are the most successful polymeric nanomedicines [54]. For example, a combination therapy comprising ependymal stem/progenitor cells of the spinal cord and a pH-responsive polymer–curcumin conjugate for SCIs has been reported previously [39]. According to the study, conjugating curcumin with a pH-responsive polymeric carrier main chain, a polyacetal, improved its blood bioavailability and stability and facilitated a highly targeted curcumin distribution. PA–C also enhanced neuroprotection, axonal development, and functional recovery in acute SCIs. Recently, a new method that improves bioavailability by combining curcumin with extracellular vesicles was reported [55]. In the study, 120-nm engineered extracellular vesicles derived from primary M2 macrophages were used and nerve growth factors and curcumin were combined. The extracellular vesicles could effectively accumulate curcumin at the site of SCIs and inhibit uncontrollable inflammatory responses induced by secondary injury.
Further translational research is required to use curcumin in therapeutic settings. Oral intake of curcumin is insufficient for it to penetrate the blood-brain barrier; therefore, studies are needed to determine an effective administration method that would allow curcumin to act directly on injured neuronal tissues in the spinal cord. Furthermore, investigations on the safety of high-dose curcumin administration are required. In addition to nanotechnology, it is necessary to try various methods to enhance curcumin bioavailability. A strategy such as megadose administration might be crucial, and doses of previously commercialized curcumin products should be considered. Continuous or intermittent curcumin therapy has been administered in most animal investigations throughout the acute phase, which occurs within 24 hours of the primary injury, and the chronic phase, which occurs after the primary injury. Therefore, future research should focus on determining the most beneficial timing and duration of administration.
LIMITATIONS
The lack of information on certain aspects of curcumin is a limitation of this paper. For example, we did not investigate the bioactivity of curcumin in astrocytes [56]. It also lacks detailed information on several signaling pathways that are regulated by curcumin, including the Nrf2/heme oxygenase 1 pathway [30], the mammalian target of rapamycin [57], and TGF-β–SOX9 pathway [58]. As a result, there are constraints in providing cutting-edge knowledge regarding curcumin therapy. However, the goal of this review paper is to provide clinicians with basic and comprehensive information regarding the role of curcumin in SCI treatment. The authors believe that this review paper would prove to be helpful to neurospinal clinicians.
CONCLUSION
Curcumin is a neuroprotective polyphenolic compound that has benefits such as pluripotency, oral safety, long usage history, and low cost. Several animal experiments have shown that curcumin can minimize secondary injury following primary SCIs through its anti-inflammatory, antioxidant, and stem cell mobilization properties. Curcumin is an influential therapeutic agent that can potentially treat catastrophic secondary injuries in the spinal cord, including inflammation, edema, free radical injury, fibrosis, and glial scar formation. It can enhance neurological function in rats, as measured using the BBB locomotor rating scale. Studies exploring ways to overcome its limited bioavailability have recently begun. More translational investigations on curcumin are necessary to facilitate its use in clinical settings.
Footnotes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: IH, KK; Data curation: SL; Methodology: DC, KK; Project administration: IH, KK; Visualization: SL; Writing - original draft: SL; Writing - review & editing: DC, IH, KK.
REFERENCES
- 1.Lee YS, Kim KT, Kwon BK. Hemodynamic management of acute spinal cord injury: a literature review. Neurospine. 2021;18:7–14. doi: 10.14245/ns.2040144.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ormond DR, Shannon C, Oppenheim J, et al. Stem cell therapy and curcumin synergistically enhance recovery from spinal cord injury. PLoS One. 2014;9:e88916. doi: 10.1371/journal.pone.0088916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim KT, Kim MJ, Cho DC, et al. The neuroprotective effect of treatment with curcumin in acute spinal cord injury: laboratory investigation. Neurol Med Chir (Tokyo) 2014;54:387–94. doi: 10.2176/nmc.oa.2013-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee YS, Cho DC, Kim CH, et al. Effect of curcumin on the inflammatory reaction and functional recovery after spinal cord injury in a hyperglycemic rat model. Spine J. 2019;19:2025–39. doi: 10.1016/j.spinee.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Gautam SC, Gao X, Dulchavsky S. In: The molecular targets and therapeutic uses of curcumin in health and disease. Aggarwal BB, Surh YJ, Shishodia S, editors. Boston (MA): Springer US; 2007. Immunomodulation by curcumin; pp. 321–41. [Google Scholar]
- 6.Das L, Vinayak M. Long term effect of curcumin in restoration of tumour suppressor p53 and phase-II antioxidant enzymes via activation of Nrf2 signalling and modulation of inflammation in prevention of cancer. PLoS One. 2015;10:e0124000. doi: 10.1371/journal.pone.0124000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naqvi S, Panghal A, Flora SJS. Nanotechnology: a promising approach for delivery of neuroprotective drugs. Front Neurosci. 2020;14:494. doi: 10.3389/fnins.2020.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao M, Yang L, Wang J, et al. Neurological recovery and antioxidant effects of curcumin for spinal cord injury in the rat: a network meta-analysis and systematic review. J Neurotrauma. 2015;32:381–91. doi: 10.1089/neu.2014.3520. [DOI] [PubMed] [Google Scholar]
- 9.Yuan J, Liu W, Zhu H, et al. Curcumin inhibits glial scar formation by suppressing astrocyte-induced inflammation and fibrosis in vitro and in vivo. Brain Res. 2017;1655:90–103. doi: 10.1016/j.brainres.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Daverey A, Agrawal SK. Curcumin protects against white matter injury through NF-κB and Nrf2 cross talk. J Neurotrauma. 2020;37:1255–65. doi: 10.1089/neu.2019.6749. [DOI] [PubMed] [Google Scholar]
- 11.Machova Urdzikova L, Karova K, Ruzicka J, et al. The antiinflammatory compound curcumin enhances locomotor and sensory recovery after spinal cord injury in rats by immunomodulation. Int J Mol Sci. 2015;17:49. doi: 10.3390/ijms17010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minassi A, Sánchez-Duffhues G, Collado JA, et al. Dissecting the pharmacophore of curcumin. Which structural element is critical for which action? J Nat Prod. 2013;76:1105–12. doi: 10.1021/np400148e. [DOI] [PubMed] [Google Scholar]
- 13.Pérez-Lara A, Corbalán-García S, Gómez-Fernández JC. Curcumin modulates PKCα activity by a membrane-dependent effect. Arch Biochem Biophys. 2011;513:36–41. doi: 10.1016/j.abb.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Gupta SC, Prasad S, Kim JH, et al. Multitargeting by curcumin as revealed by molecular interaction studies. Nat Prod Rep. 2011;28:1937–55. doi: 10.1039/c1np00051a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selvam C, Jachak SM, Thilagavathi R, et al. Design, synthesis, biological evaluation and molecular docking of curcumin analogues as antioxidant, cyclooxygenase inhibitory and anti-inflammatory agents. Bioorg Med Chem Lett. 2005;15:1793–7. doi: 10.1016/j.bmcl.2005.02.039. [DOI] [PubMed] [Google Scholar]
- 16.Priyadarsini KI, Maity DK, Naik GH, et al. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic Biol Med. 2003;35:475–84. doi: 10.1016/s0891-5849(03)00325-3. [DOI] [PubMed] [Google Scholar]
- 17.Das J, Pany S, Panchal S, et al. Binding of isoxazole and pyrazole derivatives of curcumin with the activator binding domain of novel protein kinase C. Bioorg Med Chem. 2011;19:6196–202. doi: 10.1016/j.bmc.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Bill MA, Nicholas C, Mace TA, et al. Structurally modified curcumin analogs inhibit STAT3 phosphorylation and promote apoptosis of human renal cell carcinoma and melanoma cell lines. PLoS One. 2012;7:e40724. doi: 10.1371/journal.pone.0040724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song YH, Agrawal NK, Griffin JM, et al. Recent advances in nanotherapeutic strategies for spinal cord injury repair. Advanced Drug Delivery Reviews. 2019;148:38–59. doi: 10.1016/j.addr.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandran B, Goel A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother Res. 2012;26:1719–25. doi: 10.1002/ptr.4639. [DOI] [PubMed] [Google Scholar]
- 21.Xie XK, Xu ZK, Xu K, et al. DUSP19 mediates spinal cord injury-induced apoptosis and inflammation in mouse primary microglia cells via the NF-kB signaling pathway. Neurol Res. 2020;42:31–8. doi: 10.1080/01616412.2019.1685068. [DOI] [PubMed] [Google Scholar]
- 22.Elumalai M, Muthaiah R, Alf MA. Identification of curcumin targets in neuroinflammatory pathways: molecular docking scores with GSK-3β, p38 MAPK, COX, ICE and TACE enzymes. Acta Pol Pharm. 2012;69:237–45. [PubMed] [Google Scholar]
- 23.Yuan J, Zou M, Xiang X, et al. Curcumin improves neural function after spinal cord injury by the joint inhibition of the intracellular and extracellular components of glial scar. J Surg Res. 2015;195:235–45. doi: 10.1016/j.jss.2014.12.055. [DOI] [PubMed] [Google Scholar]
- 24.Zu J, Wang Y, Xu G, et al. Curcumin improves the recovery of motor function and reduces spinal cord edema in a rat acute spinal cord injury model by inhibiting the JAK/STAT signaling pathway. Acta Histochem. 2014;116:1331–6. doi: 10.1016/j.acthis.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369–78. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- 26.Bengmark S. Curcumin, an atoxic antioxidant and natural NFkappaB, cyclooxygenase-2, lipooxygenase, and inducible nitric oxide synthase inhibitor: a shield against acute and chronic diseases. JPEN J Parenter Enteral Nutr. 2006;30:45–51. doi: 10.1177/014860710603000145. [DOI] [PubMed] [Google Scholar]
- 27.Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–25. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 28.Yu T, Dohl J, Elenberg F, et al. Curcumin induces concentration-dependent alterations in mitochondrial function through ROS in C2C12 mouse myoblasts. J Cell Physiol. 2019;234:6371–81. doi: 10.1002/jcp.27370. [DOI] [PubMed] [Google Scholar]
- 29.Panahi Y, Ahmadi Y, Teymouri M, et al. Curcumin as a potential candidate for treating hyperlipidemia: a review of cellular and metabolic mechanisms. J Cell Physiol. 2018;233:141–52. doi: 10.1002/jcp.25756. [DOI] [PubMed] [Google Scholar]
- 30.Jin W, Botchway BOA, Liu X. Curcumin can activate the Nrf2/HO-1 signaling pathway and scavenge free radicals in spinal cord injury treatment. Neurorehabil Neural Repair. 2021;35:576–84. doi: 10.1177/15459683211011232. [DOI] [PubMed] [Google Scholar]
- 31.DiSilvestro RA, Joseph E, Zhao S, et al. Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr J. 2012;11:79. doi: 10.1186/1475-2891-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balci M, Namuslu M, Devrim E, et al. Effects of computer monitor-emitted radiation on oxidant/antioxidant balance in cornea and lens from rats. Mol Vis. 2009;15:2521–5. [PMC free article] [PubMed] [Google Scholar]
- 33.Daverey A, Agrawal SK. Pre and post treatment with curcumin and resveratrol protects astrocytes after oxidative stress. Brain Res. 2018;1692:45–55. doi: 10.1016/j.brainres.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Scapagnini G, Vasto S, Abraham NG, et al. Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol Neurobiol. 2011;44:192–201. doi: 10.1007/s12035-011-8181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ni H, Jin W, Yuan B, et al. Curcumin inhibits the increase of labile zinc and the expression of inflammatory cytokines after traumatic spinal cord injury in rats. J Surg Res. 2014;187:646–52. doi: 10.1016/j.jss.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 36.Son S, Kim KT, Cho DC, et al. Curcumin stimulates proliferation of spinal cord neural progenitor cells via a mitogenactivated protein kinase signaling pathway. J Korean Neurosurg Soc. 2014;56:1–4. doi: 10.3340/jkns.2014.56.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bang WS, Kim KT, Seo YJ, et al. Curcumin increase the expression of neural stem/progenitor cells and improves functional recovery after spinal cord injury. J Korean Neurosurg Soc. 2018;61:10–8. doi: 10.3340/jkns.2017.0203.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mujoo K, Nikonoff LE, Sharin VG, et al. Curcumin induces differentiation of embryonic stem cells through possible modulation of nitric oxide-cyclic GMP pathway. Protein Cell. 2012;3:535–44. doi: 10.1007/s13238-012-2053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Requejo-Aguilar R, Alastrue-Agudo A, Cases-Villar M, et al. Combined polymer-curcumin conjugate and ependymal progenitor/stem cell treatment enhances spinal cord injury functional recovery. Biomaterials. 2017;113:18–30. doi: 10.1016/j.biomaterials.2016.10.032. [DOI] [PubMed] [Google Scholar]
- 40.Ruzicka J, Urdzikova LM, Kloudova A, et al. Anti-inflammatory compound curcumin and mesenchymal stem cells in the treatment of spinal cord injury in rats. Acta Neurobiol Exp (Wars) 2018;78:358–74. [PubMed] [Google Scholar]
- 41.Wanjiang W, Xin C, Yaxing C, et al. Curcumin improves human umbilical cord-derived mesenchymal stem cell survival via ERK1/2 signaling and promotes motor outcomes after spinal cord injury. Cell Mol Neurobiol. 2022;42:1241–52. doi: 10.1007/s10571-020-01018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonilla P, Hernandez J, Giraldo E, et al. Human-induced neural and mesenchymal stem cell therapy combined with a curcumin nanoconjugate as a spinal cord injury treatment. Int J Mol Sci. 2021;22:5966. doi: 10.3390/ijms22115966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machova Urdzikova L, Karova K, Ruzicka J, et al. The antiinflammatory compound curcumin enhances locomotor and sensory recovery after spinal cord injury in rats by immunomodulation. Int J Mol Sci. 2015;17:49. doi: 10.3390/ijms17010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu X, Zhang Y, Yang Y, et al. Therapeutic effect of curcumin and methylprednisolone in the rat spinal cord injury. Anat Rec (Hoboken) 2018;301:686–96. doi: 10.1002/ar.23729. [DOI] [PubMed] [Google Scholar]
- 45.Burgos-Morón E, Calderón-Montaño JM, Salvador J, et al. The dark side of curcumin. Int J Cancer. 2010;126:1771–5. doi: 10.1002/ijc.24967. [DOI] [PubMed] [Google Scholar]
- 46.Aggeli I-K, Koustas E, Gaitanaki C, et al. Curcumin acts as a pro-oxidant inducing apoptosis via JNKs in the isolated perfused rana ridibunda heart. J Exp Zool A Ecol Genet Physiol. 2013;319:328–39. doi: 10.1002/jez.1797. [DOI] [PubMed] [Google Scholar]
- 47.Jantawong C, Priprem A, Intuyod K, et al. Curcumin-loaded nanocomplexes: acute and chronic toxicity studies in mice and hamsters. Toxicol Rep. 2021;8:1346–57. doi: 10.1016/j.toxrep.2021.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.López-Lázaro M. Dual role of hydrogen peroxide in cancer: possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007;252:1–8. doi: 10.1016/j.canlet.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 49.Ahsan H, Parveen N, Khan NU, et al. Pro-oxidant, anti-oxidant and cleavage activities on DNA of curcumin and its derivatives demethoxycurcumin and bisdemethoxycurcumin. Chem Biol Interact. 1999;121:161–75. doi: 10.1016/s0009-2797(99)00096-4. [DOI] [PubMed] [Google Scholar]
- 50.Banerjee A, Kunwar A, Mishra B, et al. Concentration dependent antioxidant/pro-oxidant activity of curcumin: studies from AAPH induced hemolysis of RBCs. Chem Biol Interact. 2008;174:134–9. doi: 10.1016/j.cbi.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 51.Moos PJ, Edes K, Mullally JE, et al. Curcumin impairs tumor suppressor p53 function in colon cancer cells. Carcinogenesis. 2004;25:1611–7. doi: 10.1093/carcin/bgh163. [DOI] [PubMed] [Google Scholar]
- 52.Yallapu MM, Jaggi M, Chauhan SC. Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov Today. 2012;17:71–80. doi: 10.1016/j.drudis.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fonseca-Santos B, Dos Santos AM, Rodero CF, et al. Design, characterization, and biological evaluation of curcumin-loaded surfactant-based systems for topical drug delivery. Int J Nanomedicine. 2016;11:4553–62. doi: 10.2147/IJN.S108675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duncan R, Gaspar R. Nanomedicine(s) under the microscope. Mol Pharm. 2011;8:2101–41. doi: 10.1021/mp200394t. [DOI] [PubMed] [Google Scholar]
- 55.Zhang C, Li D, Hu H, et al. Engineered extracellular vesicles derived from primary M2 macrophages with anti-inflammatory and neuroprotective properties for the treatment of spinal cord injury. J Nanobiotechnology. 2021;19:373. doi: 10.1186/s12951-021-01123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eghbaliferiz S, Farhadi F, Barreto GE, et al. Effects of curcumin on neurological diseases: focus on astrocytes. Pharmacol Rep. 2020;72:769–82. doi: 10.1007/s43440-020-00112-3. [DOI] [PubMed] [Google Scholar]
- 57.Lin J, Huo X, Liu X. “mTOR signaling pathway”: a potential target of curcumin in the treatment of spinal cord injury. BioMed Res Int. 2017;2017:1634801. doi: 10.1155/2017/1634801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan J, Botchway BOA, Zhang Y, et al. Curcumin can improve spinal cord injury by inhibiting TGF-β-SOX9 signaling pathway. Cell Mol Neurobiol. 2019;39:569–75. doi: 10.1007/s10571-019-00671-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang YF, Zu JN, Li J, et al. Curcumin promotes the spinal cord repair via inhibition of glial scar formation and inflammation. Neurosci Lett. 2014;560:51–6. doi: 10.1016/j.neulet.2013.11.050. [DOI] [PubMed] [Google Scholar]
- 60.Lin CC, Chiang TH, Chen WJ, et al. CISD2 serves a novel role as a suppressor of nitric oxide signalling and curcumin increases CISD2 expression in spinal cord injuries. Injury. 2015;46:2341–50. doi: 10.1016/j.injury.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 61.Ni H, Jin W, Zhu T, et al. Curcumin modulates TLR4/NFκB inflammatory signaling pathway following traumatic spinal cord injury in rats. J Spinal Cord Med. 2015;38:199–206. doi: 10.1179/2045772313Y.0000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruzicka J, Urdzikova LM, Svobodova B, et al. Does combined therapy of curcumin and epigallocatechin gallate have a synergistic neuroprotective effect against spinal cord injury? Neural Regen Res. 2018;13:119–27. doi: 10.4103/1673-5374.224379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yardım A, Kandemir FM, Çomaklı S, et al. Protective effects of curcumin against paclitaxel-induced spinal cord and sciatic nerve injuries in rats. Neurochem Res. 2021;46:379–95. doi: 10.1007/s11064-020-03174-0. [DOI] [PubMed] [Google Scholar]
- 64.Akar İ, İnce İ, Arici A, et al. The protective effect of curcumin on a spinal cord ischemia-reperfusion injury model. Ann Vasc Surg. 2017;42:285–92. doi: 10.1016/j.avsg.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 65.Xi J, Luo X, Wang Y, et al. Tetrahydrocurcumin protects against spinal cord injury and inhibits the oxidative stress response by regulating FOXO4 in model rats. Exp Ther Med. 2019;18:3681–7. doi: 10.3892/etm.2019.7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Daverey A, Agrawal SK. Neuroprotective effects of Riluzole and Curcumin in human astrocytes and spinal cord white matter hypoxia. Neurosci Lett. 2020;738:135351. doi: 10.1016/j.neulet.2020.135351. [DOI] [PubMed] [Google Scholar]
- 67.Elkhenany H, Bonilla P, Giraldo E, et al. A hyaluronic acid demilune scaffold and polypyrrole-coated fibers carrying embedded human neural precursor cells and curcumin for surface capping of spinal cord injuries. Biomedicines. 2021;9:1928. doi: 10.3390/biomedicines9121928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo J, Shi X, Li L, et al. An injectable and self-healing hydrogel with controlled release of curcumin to repair spinal cord injury. Bioact Mater. 2021;6:4816–29. doi: 10.1016/j.bioactmat.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]


