Abstract
Despite numerous studies, the pathogenesis of ossification of the posterior longitudinal ligament (OPLL) is still unclear. Previous genetic studies proposed variations in genes related to bone and collagen as a cause of OPLL. It is unclear whether the upregulations of those genes are the cause of OPLL or an intermediate result of endochondral ossification process. Causal variations may be in the inflammation-related genes supported by clinical and updated genomic studies. OPLL demonstrates features of genetic diseases but can also be induced by mechanical stress by itself. OPLL may be a combination of various diseases that share ossification as a common pathway and can be divided into genetic and idiopathic. The phenotype of OPLL can be divided into continuous (including mixed) and segmental (including localized) based on the histopathology, prognosis, and appearance. Continuous OPLL shows substantial overexpression of osteoblast-specific genes, frequent upper cervical involvement, common progression, and need for surgery, whereas segmental OPLL shows moderate-to-high expression of these genes and is often clinically silent. Genetic OPLL seems to share clinical features with the continuous type, while idiopathic OPLL shares features with the segmental type. Further genomic studies are needed to elucidate the relationship between genetic OPLL and phenotype of OPLL.
Keywords: Ossification of posterior longitudinal ligament, Myelopathy, Genomics, NLRP1, BMP, SSH2
INTRODUCTION
Ossification of the posterior longitudinal ligament (OPLL; MIM 602475) is a hyperostotic condition that results in ectopic calcification of the posterior longitudinal ligament (PLL) and leads to a reduced range of motion and potential spinal cord compromise [1,2]. OPLL was first reported by Key in 1838 and described in detail by Tsukimoto in 1960 [3,4]. Two centuries have passed since the discovery of this disease, and nearly a century has passed since the specific form of the disease was confirmed, but we still do not know the exact cause, pathogenesis, and treatment of OPLL [5-8]. Numerous studies over the last several decades have suggested the involvement of multiple etiologic factors in the development of OPLL, including genetic factors, mechanical stress, nutrition, and secondary changes caused by systemic disease [9].
With regard to the pathogenesis of OPLL, on one hand, OPLL demonstrates racial differences in its incidence, which is especially high in East Asian males [10,11]. A previous study in North America showed that the prevalence of OPLL varied among races: 4.8% in Asian Americans, 1.3% in Caucasian Americans, 1.9% in Hispanic Americans, and 3.2% in Native Americans [12]. The prevalence of OPLL was found to be 26% in the parents and 29% in the siblings of probands from 347 OPLL families, which is significantly higher than that in the general population [13]. This findings imply that OPLL is a genetic disease. On the other hand, the nongenetic factors associated with OPLL include age, diabetes mellitus, obesity, exercise, and mechanical stress. The majority of OPLL cases occur after age 50, which means that OPLL is related to degenerative disease. Previous studies also reported that OPLL was associated with a vitamin A-rich diet, plasma pentosidine levels, and femoral neck bone mineral density [12,14,15].
Many investigators have performed case-control association studies, affected sibling-pair linkage studies, and candidate gene association studies, and they identified many genes or loci that are linked to OPLL susceptibility. However, replication studies have failed to verify these results, even in the same ancestry groups as the original studies [16]. Next-generation sequencing (NGS) has recently emerged, and the genetic odyssey of OPLL has moved to a new direction. The purpose of this study was to review systematically and summarize genetic and genomic studies on OPLL of the cervical spine, and to make a suggestion which will help us to guide our future OPLL studies.
MATERIALS AND METHODS
A comprehensive literature search was performed using PubMed, Embase, and the Cochrane Library for all journal articles published from January 2000 through October 2021. We also manually searched reference lists. Key words used in the search included “ossification of the posterior longitudinal ligament,” “genetics,” and “genomics.” The terms were searched individually or in combination. Appropriate articles for our review were selected based on scientific investigations on the genetic inheritance and susceptibility patterns of OPLL in humans. The search results were screened by title and abstract for the following exclusion criteria: duplicate studies; case reports, letters, comments, reviews, or technical notes; animal studies; and OPLL in the thoracic spine. After eliminating the excluded papers, fulltext articles were obtained, and studies were thoroughly screened again using the same exclusion criteria. We excluded the following articles: combined diseases such as diffuse idiopathic skeletal hyperostosis (DISH) and ankylosing spondylitis (AS); redundant papers; and those without any description of mean values, the standard deviation, or the number of patients in each group. We limited our results to articles in the English language. In the case of overlapping study populations, we excluded patients described twice or used the most recent publication.
RESULTS
1. Systematic Search and Identification of Relevant Papers
An initial literature search using the chosen subject headings identified 129 studies in PubMed, 138 studies in Embase, and 2 studies in the Cochrane Central Register of Controlled Trials. Among these 269 studies, 71 were duplicates and were thus excluded. After screening titles and abstracts, 86 of the 198 remaining papers were excluded from our analysis because they were case reports, review articles, letters, technical notes, or animal experiments. Ten papers written in Japanese or Chinese were excluded, and 6 studies that dealt with OPLL in the thoracic and lumbar spine were excluded. The remaining 96 studies were subjected to a full-text review, and another 35 were excluded. These 26 articles were excluded because the studies used a mixed group including patients with other rheumatic diseases such as AS (n = 21), no description of the standard deviation (n = 8), and indirect comparative studies or single group studies (n= 6). Finally, this systematic search found 39 gene-expression screening studies using real-time quantitative polymerase chain reaction, 6 genome-wide association studies (GWAS), 2 NGS studies, 7 proteomic tissue expression analyses, and 7 micro-RNA expression analyses. A detailed process is shown in Fig. 1.
Fig. 1.
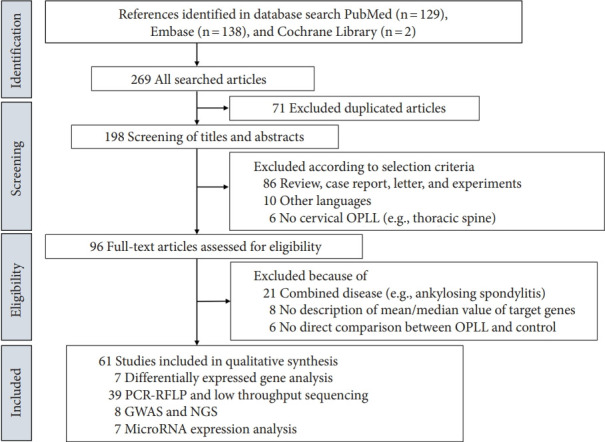
Flow diagram of the identification of relevant studies. Ossification of the posterior longitudinal ligament (OPLL) indicates ossification of the posterior longitudinal ligament. PCR, polymerase chain reaction; RFLP, restriction fragment length polymorphism; GWAS, genome-wide association study; NGS, next-generation sequencing.
2. Pathogenesis I: Review of Studies That Conducted Screening of a Few Genes
Many research groups have proposed that genes related to bone, collagen, and inflammation might be associated with the initiation and progression of OPLL by using several methods such as genetic linkage analysis, positional cloning, and association studies [17]. The expression levels of the osteoblast-specific genes encoding alkaline phosphatase (ALP), osteocalcin (OCN), and type I collagen (COL I), were upregulated in OPLL patients, as shown in Table 1 [18-23]. However, it remains unclear whether upregulated expression of bone and collagen-related genes is the cause of this disease or an intermediate result of the ossification process [21]. In addition, some ossification-related genes such as BMP, RUNX2, and TGFB families were introduced as causal genes in the development of OPLL based on genotyping [21,24-26]. The logic of these studies is that some suspected genetic variations that researchers assumed to be causal variations showed statistically significant differences in case-control studies. This may be valid if only a few variations demonstrate statistically significant differences between the 2 groups. However, there were thousands of variations show statistically significant differences in case-control studies. Even if, those variations were closely related with the OPLL, it is unknown whether they were causal variation or intermediate results.
Table 1.
Differential expression ratio of OPLL for the various osteogenic marker gene
| Study | No. of OPLL | No. of controls | ALP |
OCN |
COL I |
|||
|---|---|---|---|---|---|---|---|---|
| DE | p-value | DE | p-value | DE | p-value | |||
| Yamamoto et al. [21] 2002 | 5 | 5 | 1.55 | < 0.05 | N/D | N/D | N/D | N/D |
| Tsukahara et al. [22] 2006 | 3 | 3 | 1.76 | SD, 0.04 | 2.83 | 0.08 | 1.66 | 0.16 |
| Yang et al. [19,20] 2011 | 20 | 18 | 1.79 | < 0.01 | 1.72 | < 0.01 | 1.62 | < 0.01 |
| Tanaka et al. [23] 2011 | 18 | 14 | 1.8 | N/D | N/D | N/D | 1.1 | N/D |
| Yang et al. [18] 2020 | 21 | 16 | 2.71 | < 0.001 | 2.71 | < 0.001 | 3.31 | < 0.001 |
OPLL, ossification of the posterior longitudinal ligament; ALP, alkaline phosphatase; OCN, osteocalcin; COL I, type I collagen; DE, differential expression; SD, standard deviation; N/D, no description.
3. Pathogenesis II: Review of Genomic Variation Discovery Studies
Genomic variation detection can be divided into discovery and screening [27]. Discovery involves the identification of variations that are not yet known, while screening pertains to known variations [27]. Causative genetic variations of OPLL have not been clearly elucidated, and genomic studies must discover all genes, excluding the researcher’s prejudice. Nakajima et al. [16] performed the first GWAS study that discovered variant loci in the whole genome, and they identified 6 susceptibility loci for OPLL. Their subsequent study suggested that RSPO2 might be a susceptibility gene for OPLL based on genetic and functional data [28]. However, the RSPO2 gene did not show a significant difference in other researchers’ studies, including a whole-exome sequencing (WES) study [29].
Two WES studies that used the most up-to-date and precise methods to discover causal variations were published by researchers from Korea and China [29,30]. One paper included 28 patients with OPLL (67%, continuous/mixed type) and suggested that variants of the PTCH1 and COL17A1 genes might contribute to the development of OPLL [30]. PTCH1 is related to basal cell carcinoma, and no precise relationship was found between the results of the study and clinical findings. Seven years have passed since the paper was published, but no further study by the authors has yet been published. The other WES study included 74 OPLL patients (100% with the continuous/mixed type and a ≥ 40% occupying ratio) and 26 controls and was published this year [29]. A characteristic of this paper is that the patients group was selected very precisely and that it probably included more patients with OPLL caused by genetic diseases than other previous studies. The authors proposed that the causal variations of genetic OPLL might be auto-/local inflammation-related genes such as NLRP1, SSH2, and CYP4B1 [29]. Previous genetic studies also suggested that the inflammatory response were related to OPLL [31,32]. In a clinical study, serum level of C-reactive protein (CRP) as well-known inflammatory marker was higher in the OPLL group than in the control group, and the group with progression of OPLL showed much higher CRP levels than the group with nonprogression of OPLL [33]. The researchers suggested that inflammation might relate the development or progression of OPLL [33]. Genetic vulnerability of inflammation-related genes may be a causal factor of OPLL. Further biomarker studies are needed.
MiRNAs are small noncoding RNAs that regulate gene expression in numerous biological or pathological processes by binding with target mRNAs to affect their translation or stability, and they are thought to regulate cell reprogramming and differentiation [34]. Many miRNAs previously reported in network studies were addressed the association with osteogenesis [34]. Previous studies described significant upregulation of the expression of ossification-related RUNX2, ALP, and OSX genes by miR181a-5p, while the inhibition of miR-181a-5p by treatment with antagomir had the opposite effects [34,35]. Other investigators demonstrated that the GG genotype of miR-499 was associated with a significantly higher risk of OPLL in the segmental OPLL group [36]. More studies are needed to prove the pathogenesis of OPLL by miRNA.
4. Pathogenesis III: Role of Mechanical Stress in the Development/Progression of OPLL
It has been reported that the abnormal distribution of mechanical stress is closely correlated with the process of OPLL [37]. Mechanical stress could induce osteogenic differentiation of PLL fibroblasts in vitro, which is expressed as the upregulation of osteogenic markers, including OCN, ALP, and COL I [38,39]. Other researchers reported that numerous genes showed upregulated expression levels in response to mechanical stress, such as ALP, BMP2, BMP4, osteopontin, CBFA1, OCN, integrin-β1, and endothelin-1; furthermore, mechanical stress elevated prostacyclin synthesis in ligament cells derived from OPLL patients and induced osteogenic differentiation [12,40]. This evidence suggests that mechanical stress itself induces ossification of the ligament at highly stressed regions such as the low cervical spine.
One hand, a recent epidemiological study reported that the prevalence of OPLL has increased in North American and European populations from 0.1% to 2.5% [41]. The number of surgical cases of OPLL has tripled in 12 years across the United States (US) [41]. Because the incidence of genetic disease is hard to increase like this, the rapid increase of OPLL patients in the last decade may be related with mechanical stress. On the other hand, 7.5% of Asians and Pacific islander living in the US underwent surgery for the OPLL, which was higher than the 4.9% of all Americans [41]. Assuming that the lifestyle of the people living in US is similar, the fact that severe OPLL requiring surgery is especially frequent in Asians means that Asians have a genetic vulnerability under similar mechanical stress. This reflects that severe OPLL resulting from a genetic vulnerability needs to be distinguished from mild OPLL caused by mechanical stress.
5. Two Distinct Phenotypes of OPLL
One reason for the uncertain pathogenesis is that OPLL is a disease named based on a radiological finding regardless of cause and pathogenesis. According to the definition of the disease name, all patients showing any ossification or calcification at the PLL can be diagnosed with OPLL. The disease needs to be classified based on its cause and pathogenesis because ectopic osteogenesis (ossification and calcification) is frequently found even in healthy people in various organs and tissues, including connective tissues, blood vessels, and skeletal muscle [17,42,43]. In addition, some systemic diseases such as hypoparathyroidism, DISH, AS, and potentially schizophrenia sometimes induce paravertebral ligamentous ossification [12,14,15,44,45]. Therefore, clinically insignificant calcifications or ossification of PLL may be better excluded from the diagnosis of OPLL.
It is well known that OPLL can be classified into 4 types based on the shape of the ossified region using plain radiographs of the cervical spine in the lateral view: continuous, segmental, mixed, and localized. However, researchers suggested that it would be better to divide OPLL into only 2 types (continuous and segmental) based on the histopathology, prognosis, and appearance [44]. The mixed type of OPLL is regarded as a subcategory in which segmental ossification is added to the continuous type of OPLL. The localized type of OPLL is referred to as the circumscribed or unclassified type of OPLL, and is regarded as a kind of the segmental type of OPLL. Differential points between continuous/mixed and segmental/localized OPLL are described in Table 2.
Table 2.
Two different phenotypes of cervical OPLL
| Item | Continuous/mixed | Segmental/localized |
|---|---|---|
| Upper cervical (C2, 3) involve | Frequent | Rare |
| Lower cervical (C5/6/7) involve | Sometimes | Frequent |
| Mean age | Younger | Older |
| Progression rate | Fast | Slow |
| Need for surgical treatment | Sometimes | Rare |
OPLL, ossification of the posterior longitudinal ligament.
A previous study demonstrated that ALP activity in continuous OPLL, segmental OPLL, and non-OPLL was 2.56 ± 0.05, 1.21 ± 0.11, and 1.00 ± 0.05, respectively [17]. Other researchers also reported that the expression level of ALP, OCN, and COL I showed significant step-wide decrease, which was found most prominently in continuous OPLL, followed in order by segmental OPLL and non-OPLL [37,44,46-48]. The researchers stated that spinal ligament cells derived from continuous OPLL tended to be more easily mineralized than those from segmental OPLL patients [17]. From a clinical point of view, continuous OPLL requires careful observation of cervical myelopathy, whereas segmental OPLL is often clinically silent. A previous meta-analysis demonstrated that the continuous/mixed type accounted for 77% of the patients who underwent surgery due to cervical myelopathy with OPLL [10]. The progression rate of ossification was reported to be 75% in patients with continuous OPLL and 38% in those with segmental OPLL [44]. Another study showed 72% of patients with continuous/mixed OPLL had involvement in the upper cervical region such as C2 [29]. It may be necessary to distinguish clinically significant continuous OPLL from the asymptomatic segmental type based on genetic, biochemical, and clinical differences [29,44].
6. Suggestion for the Development of OPLL
We may summarize 2 mechanisms and 2 types of OPLL, as shown in Fig. 2. One is named idiopathic OPLL, which may be triggered by mechanical stress, comorbid diseases, and specific nutritional patterns. Mechanical stress to the cervical spine is usually focused on low cervical spine, high mobile segments [49,50]. Segmental OPLL usually occur at the segments, but continuous OPLL usually involve upper cervical spine. Segmental or localized OPLL may be developed by this mechanism. This type may show moderate over expression of ALP, OCN, and COL I. Rapid increase of surgery for OPLL over the past 10 years may mean that OPLL can be developed regardless of genetic vulnerability because it is difficult to triple the number of genetic diseases for 10 years [41]. The other is named genetic OPLL, which occurs in patients with variations in inflammatory genes such as NLRP1 and SSH2. Levels of inflammatory biomarkers such as CRP were found to remain high in OPLL patients and were positively associated with OPLL progression. Genetic OPLL may even show involvement in the upper cervical regions with less mechanical stress. In an epidemiologic study from US, Asian American underwent 53% more spine surgeries than the US overall. This may mean that genetic OPLL often seems to induce cervical myelopathy and more often requires surgical treatment than the idiopathic OPLL and genetic OPLL needs to surgical treatments more than idiopathic OPLL [51,52]. These features of genetic OPLL are observed in continuous OPLL. Although the genetic differences among types of ossification remain to be elucidated, genetic and genomic studies may provide new etiologic insights into how the type of OPLL relates to the causal genetic variation and the prognosis.
Fig. 2.
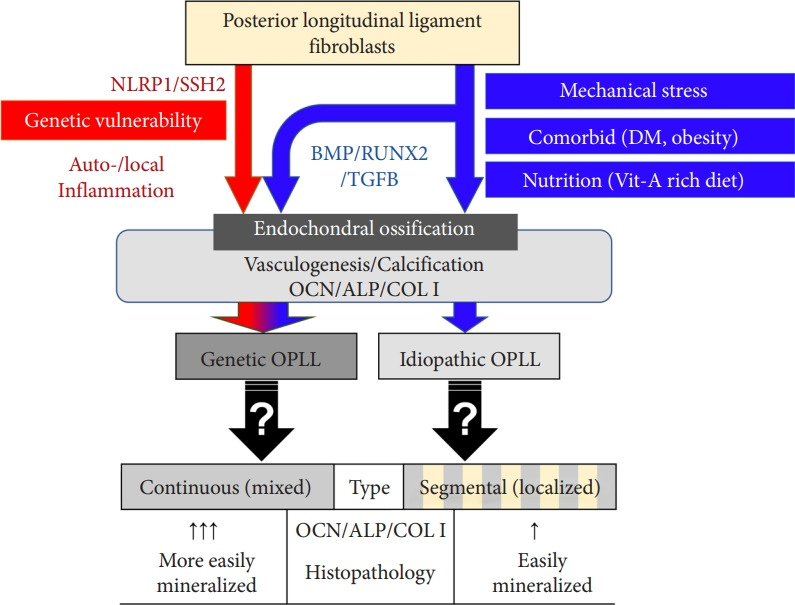
Hypothetic pathogenesis of ossification of the posterior longitudinal ligament (OPLL). The phenotype of OPLL is divided into continuous (including mixed) and segmental (including localized). The continuous type of OPLL demonstrates much higher expression of endochondral ossification genes encoding osteocalcin (OCN), alkaline phosphatase (ALP), and type I collagen (COL I) than the segmental type of OPLL. Genetic OPLL showed involvement of less stressed cervical region such as C2 and frequent surgical treatment, which is similar with continuous type of OPLL. DM, diabetes mellitus; Vit-A, vitamin A.
7. Limitations
There are some limitations that need to be acknowledged and addressed regarding the present study. The first limitation concerns a narrative review. We tried to search all genetic and genomic studies and offer clear evidence. We performed a systematic search and found 60 papers. Most of searched studies suggested misleading candidate genes that studied with single or a few genes and variants addressed one at a time by single team with small sample size [53]. The authors tried to summary all reported results without bias because following the systematic review protocol could rather lose objectivity. The second, there are few evidence that genetic OPLL make continuous type ossification. It is entirely our hypothesis. Although genetic OPLL showed similar feature with continuous type of OPLL, there are many things that are difficult to explain by the reason. Further studies are necessary to find out the difference in genetic variations by type of OPLL.
CONCLUSION
OPLL may develop from genetic vulnerabilities and idiopathic factors, including mechanical stress. It is not clear whether OPLL is a complex genetic disease or a combination of various diseases that share a process of calcification/ossification as a common pathway. Genetic OPLL seems to share clinical features with the continuous type, while idiopathic OPLL shares them with the segmental type. Further genomic studies with clear phenotypic classifications may provide further insights into the disease.
Footnotes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This work was supported by a National Research Foundation of Korea grant, funded by the Korean government (No. NRF-2021R1F1A1059136).
Author Contribution
Conceptualization: CL; Data curation: YIW, WTY, SWK; Formal analysis: CL; Funding acquisition: CL; Visualization: YIW; Writing - original draft: YIW; Writing - review & editing: YIW, CL, WTY, SWK, CHK, CKC.
REFERENCES
- 1.Lee JK, Ham CH, Kwon WK, et al. A new classification for cervical ossification of the posterior longitudinal ligament based on the coexistence of segmental disc degeneration. J Korean Neurosurg Soc. 2021;64:69–77. doi: 10.3340/jkns.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abiola R, Rubery P, Mesfin A. Ossification of the posterior longitudinal ligament: etiology, diagnosis, and outcomes of nonoperative and operative management. Global Spine J. 2016;6:195–204. doi: 10.1055/s-0035-1556580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Key CA. On paraplegia depending on disease of the ligaments of the spine. Guys Hosp Rep. 1838;3:17–34. [Google Scholar]
- 4.Tsukimoto H. A case report: autopsy of syndrome of compression of spinal cord owing to ossification within spinal canal of cervical spines. Nippon Geka Hokan. 1960;29:1003–7. [Google Scholar]
- 5.Kim SY, Hyun SJ, Kim KJ, et al. Surgical outcomes according to dekyphosis in patients with ossification of the posterior longitudinal ligament in the thoracic spine. J Korean Neurosurg Soc. 2020;63:89–98. doi: 10.3340/jkns.2018.0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin HK, Park JH. Posterior floating laminotomy as a new decompression technique for posterior cervical spinal fusion surgery. J Korean Neurosurg Soc. 2021;64:901–12. doi: 10.3340/jkns.2020.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee S, Roh SW, Jeon SR, et al. A prognostic factor for prolonged mechanical ventilator-dependent respiratory failure after cervical spinal cord injury : maximal canal compromise on magnetic resonance imaging. J Korean Neurosurg Soc. 2021;64:791–8. doi: 10.3340/jkns.2020.0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung JM, Jahng AL, Hyun SJ, et al. Comparison of spinal canal expansion following cervical laminoplasty based on the preoperative lamina angle : a simulation study. J Korean Neurosurg Soc. 2021;64:229–37. doi: 10.3340/jkns.2020.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Wei P, Chen Y, et al. Down-regulated expression of vimentin induced by mechanical stress in fibroblasts derived from patients with ossification of the posterior longitudinal ligament. Eur Spine J. 2014;23:2410–5. doi: 10.1007/s00586-014-3394-8. [DOI] [PubMed] [Google Scholar]
- 10.Lee CH, Sohn MJ, Lee CH, et al. Are there differences in the progression of ossification of the posterior longitudinal ligament following laminoplasty versus fusion?: a meta-analysis. Spine (Phila Pa 1976) 2017;42:887–94. doi: 10.1097/BRS.0000000000001933. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Son DW, Shin JJ, et al. Preoperative radiological parameters to predict clinical and radiological outcomes after laminoplasty. J Korean Neurosurg Soc. 2021;64:677–92. doi: 10.3340/jkns.2020.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan L, Gao R, Liu Y, et al. The pathogenesis of ossification of the posterior longitudinal ligament. Aging Dis. 2017;8:570–82. doi: 10.14336/AD.2017.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terayama K. Genetic studies on ossification of the posterior longitudinal ligament of the spine. Spine (Phila Pa 1976) 1989;14:1184–91. doi: 10.1097/00007632-198911000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Boody BS, Lendner M, Vaccaro AR. Ossification of the posterior longitudinal ligament in the cervical spine: a review. Int Orthop. 2019;43:797–805. doi: 10.1007/s00264-018-4106-5. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimura N, Nagata K, Muraki S, et al. Prevalence and progression of radiographic ossification of the posterior longitudinal ligament and associated factors in the Japanese population: a 3-year follow-up of the ROAD study. Osteoporos Int. 2014;25:1089–98. doi: 10.1007/s00198-013-2489-0. [DOI] [PubMed] [Google Scholar]
- 16.Nakajima M, Takahashi A, Tsuji T, et al. A genome-wide association study identifies susceptibility loci for ossification of the posterior longitudinal ligament of the spine. Nat Genet. 2014;46:1012–6. doi: 10.1038/ng.3045. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa KI. Pharmacological aspect of ectopic ossification in spinal ligament tissues. Pharmacol Ther. 2008;118:352–8. doi: 10.1016/j.pharmthera.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Lin Z, Chen J, et al. Autophagy in spinal ligament fibroblasts: evidence and possible implications for ossification of the posterior longitudinal ligament. J Orthop Surg Res. 2020;15:490. doi: 10.1186/s13018-020-02017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang HS, Lu XH, Chen DY, et al. Upregulated expression of connexin43 in spinal ligament fibroblasts derived from patients presenting ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976) 2011;36:2267–74. doi: 10.1097/BRS.0b013e31820ccfc6. [DOI] [PubMed] [Google Scholar]
- 20.Yang HS, Lu XH, Chen DY, et al. Mechanical strain induces Cx43 expression in spinal ligament fibroblasts derived from patients presenting ossification of the posterior longitudinal ligament. Eur Spine J. 2011;20:1459–65. doi: 10.1007/s00586-011-1767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto Y, Furukawa K, Ueyama K, et al. Possible roles of CTGF/Hcs24 in the initiation and development of ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976) 2002;27:1852–7. doi: 10.1097/00007632-200209010-00009. [DOI] [PubMed] [Google Scholar]
- 22.Tsukahara S, Ikeda R, Goto S, et al. Tumour necrosis factor alpha-stimulated gene-6 inhibits osteoblastic differentiation of human mesenchymal stem cells induced by osteogenic differentiation medium and BMP-2. Biochem J. 2006;398:595–603. doi: 10.1042/BJ20060027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka S, Kudo H, Asari T, et al. P2Y1 transient overexpression induced mineralization in spinal ligament cells derived from patients with ossification of the posterior longitudinal ligament of the cervical spine. Calcif Tissue Int. 2011;88:263–71. doi: 10.1007/s00223-010-9456-y. [DOI] [PubMed] [Google Scholar]
- 24.Chang F, Li L, Gao G, et al. Role of Runx2 polymorphisms in risk and prognosis of ossification of posterior longitudinal ligament. J Clin Lab Anal. 2017;31:e22068. doi: 10.1002/jcla.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li P, Liu Z, Liu J, et al. RUNX2 and IL-15RA polymorphisms associated with OPLL in the Han and Mongolian population. J Pak Med Assoc. 2020;70(Special Issue):98–104. [PubMed] [Google Scholar]
- 26.Kim KH, Kuh SU, Park JY, et al. Association between BMP2 and COL6A1 gene polymorphisms with susceptibility to ossification of the posterior longitudinal ligament of the cervical spine in Korean patients and family members. Genet Mol Res. 2014;13:2240–7. doi: 10.4238/2014.March.31.4. [DOI] [PubMed] [Google Scholar]
- 27.Kwok PY, Chen X. Detection of single nucleotide polymorphisms. Curr Issues Mol Biol. 2003;5:43–60. [PubMed] [Google Scholar]
- 28.Nakajima M, Kou I, Ohashi H, et al. Identification and functional characterization of RSPO2 as a susceptibility gene for ossification of the posterior longitudinal ligament of the spine. Am J Hum Genet. 2016;99:202–7. doi: 10.1016/j.ajhg.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee CH, Kim KT, Kim CH, et al. Unveiling the genetic variation of severe continuous/mixed-type ossification of the posterior longitudinal ligament by whole-exome sequencing and bioinformatic analysis. Spine J. 2021;21:1847–56. doi: 10.1016/j.spinee.2021.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Wei W, He HL, Chen CY, et al. Whole exome sequencing implicates PTCH1 and COL17A1 genes in ossification of the posterior longitudinal ligament of the cervical spine in Chinese patients. Genet Mol Res. 2014;13:1794–804. doi: 10.4238/2014.March.17.7. [DOI] [PubMed] [Google Scholar]
- 31.Chen D, Chen Y, Li T, et al. Role of Cx43-mediated NFsmall ka, CyrillicB signaling pathway in ossification of posterior longitudinal ligament: an in vivo and in vitro study. Spine (Phila Pa 1976) 2017;42:E1334–41. doi: 10.1097/BRS.0000000000002165. [DOI] [PubMed] [Google Scholar]
- 32.Yuan X, Guo Y, Chen D, et al. Long non-coding RNA MALAT1 functions as miR-1 sponge to regulate Connexin 43-mediated ossification of the posterior longitudinal ligament. Bone. 2019;127:305–14. doi: 10.1016/j.bone.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 33.Kawaguchi Y, Nakano M, Yasuda T, et al. Serum biomarkers in patients with ossification of the posterior longitudinal ligament (OPLL): inflammation in OPLL. PLoS One. 2017;12:e0174881. doi: 10.1371/journal.pone.0174881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu N, Zhang Z, Li L, et al. MicroRNA-181 regulates the development of ossification of posterior longitudinal ligament via epigenetic modulation by targeting PBX1. Theranostics. 2020;10:7492–509. doi: 10.7150/thno.44309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu C, Chen Y, Zhang H, et al. Integrated microRNA-mRNA analyses reveal OPLL specific microRNA regulatory network using high-throughput sequencing. Sci Rep. 2016;6:21580. doi: 10.1038/srep21580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim JJ, Shin DA, Jeon YJ, et al. Association of miR-146a, miR149, miR-196a2, and miR-499 polymorphisms with ossification of the posterior longitudinal ligament of the cervical spine. PLoS One. 2016;11:e0159756. doi: 10.1371/journal.pone.0159756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inamasu J, Guiot BH, Sachs DC. Ossification of the posterior longitudinal ligament: an update on its biology, epidemiology, and natural history. Neurosurgery. 2006;58:1027–39. doi: 10.1227/01.NEU.0000215867.87770.73. discussion -39. [DOI] [PubMed] [Google Scholar]
- 38.Shi L, Miao J, Chen D, et al. Endoplasmic reticulum stress regulates mechanical stress-induced ossification of posterior longitudinal ligament. Eur Spine J. 2019;28:2249–56. doi: 10.1007/s00586-019-06074-2. [DOI] [PubMed] [Google Scholar]
- 39.Furukawa KI. Current topics in pharmacological research on bone metabolism: molecular basis of ectopic bone formation induced by mechanical stress. J Pharmacol Sci. 2006;100:201–4. doi: 10.1254/jphs.fmj05004x4. [DOI] [PubMed] [Google Scholar]
- 40.Yoon HH, Lee HJ, Min J, et al. Optimal ratio of Wnt3a expression in human mesenchymal stem cells promotes axonal regeneration in spinal cord injured rat model. J Korean Neurosurg Soc. 2021;64:705–15. doi: 10.3340/jkns.2021.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernstein DN, Prong M, Kurucan E, et al. National trends and complications in the surgical management of ossification of the posterior longitudinal ligament (OPLL) Spine. 2019;44:1550–7. doi: 10.1097/BRS.0000000000003127. [DOI] [PubMed] [Google Scholar]
- 42.Lee S, Jung SK, Kim HB, et al. Postoperative non-pathological fever following posterior cervical fusion surgery : is laminoplasty a better preventive method than laminectomy? J Korean Neurosurg Soc. 2020;63:487–94. doi: 10.3340/jkns.2019.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JH, Han IH, Kim DH, et al. Spine computed tomography to magnetic resonance image synthesis using generative adversarial networks : a preliminary study. J Korean Neurosurg Soc. 2020;63:386–96. doi: 10.3340/jkns.2019.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kudo H, Furukawa K, Yokoyama T, et al. Genetic differences in the osteogenic differentiation potency according to the classification of ossification of the posterior longitudinal ligament of the cervical spine. Spine (Phila Pa 1976) 2011;36:951–7. doi: 10.1097/BRS.0b013e3181e9a8a6. [DOI] [PubMed] [Google Scholar]
- 45.Lee HJ, Kim IS, Hong JT. Physiologic cervical alignment change between cervical spine x-ray and computed tomography. J Korean Neurosurg Soc. 2021;64:784–90. doi: 10.3340/jkns.2020.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiba K, Yamamoto I, Hirabayashi H, et al. Multicenter study investigating the postoperative progression of ossification of the posterior longitudinal ligament in the cervical spine: a new computer-assisted measurement. J Neurosurg Spine. 2005;3:17–23. doi: 10.3171/spi.2005.3.1.0017. [DOI] [PubMed] [Google Scholar]
- 47.Kawaguchi Y, Kanamori M, Ishihara H, et al. Progression of ossification of the posterior longitudinal ligament following en bloc cervical laminoplasty. J Bone Joint Surg Am. 2001;83:1798–802. doi: 10.2106/00004623-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Hori T, Kawaguchi Y, Kimura T. How does the ossification area of the posterior longitudinal ligament thicken following cervical laminoplasty? Spine (Phila Pa 1976) 2007;32:E551–6. doi: 10.1097/BRS.0b013e31814614f3. [DOI] [PubMed] [Google Scholar]
- 49.Otluoglu GD, Konya D, Toktas ZO. The influence of mechanic factors in disc degeneration disease as a determinant for surgical indication. Neurospine. 2020;17:215–20. doi: 10.14245/ns.2040044.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin JJ, Kim B, Kang J, et al. Clinical, radiographic, and genetic analyses in a population-based cohort of adult spinal deformity in the older population. Neurospine. 2021;18:608–17. doi: 10.14245/ns.2142544.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papavero L, Schmeiser G, Kothe R, et al. Degenerative cervical myelopathy: a 7-letter coding system that supports decision-making for the surgical approach. Neurospine. 2020;17:164–71. doi: 10.14245/ns.1938010.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riew KD. Review of vertebral body sliding osteotomy for cervical myelopathy with rigid kyphosis. Neurospine. 2020;17:648–9. doi: 10.14245/ns.2040542.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ioannidis JP. The mass production of redundant, misleading, and conflicted systematic reviews and meta-analyses. Milbank Q. 2016;94:485–514. doi: 10.1111/1468-0009.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]


