Abstract
Previously, it was shown that introns are required for efficient mRNA accumulation in Schizophyllum commune and that the presence of AT-rich sequences in the coding region of genes can result in truncation of transcripts in this homobasidiomycete. Here we show that intron-dependent mRNA accumulation and truncation of transcripts are two independent events that both affect expression of the bacterial hygromycin B resistance gene in S. commune.
In the homobasidiomycete Schizophyllum commune, introns are needed for efficient expression of at least some homologous and heterologous genes (5). Accumulation of mRNA of the SC3 and SC6 genes of S. commune, the ABH1 gene of Agaricus bisporus, and the GFP gene of Aequorea victoria did not occur in S. commune when cDNA coding sequences were introduced. In contrast, the mRNAs did accumulate when genomic sequences were used or when an intron was added to cDNA constructs. Run-on analysis with nuclei harboring intron-containing and intron-less transgenes showed that the introns affected a posttranscriptional event and that at least one intron was required for mRNA accumulation.
AT-rich stretches also hamper expression of heterologous genes in S. commune. When prokaryotic reporter genes (β-glucuronidase and β-galactosidase genes) or resistance genes (hygromycin B phosphotransferase and aminoglycoside phosphotransferase genes) (10, 11) were expressed in this fungus, no full-length transcripts were observed. Rather, they were truncated in the 5′ part of the coding sequence at the position of AT-rich stretches. Similar observations were made when the α-galactosidase (aglA) gene from Cyamopsis tetragonoloba or the hygromycin B resistance (hph) gene of Escherichia coli were expressed in Aspergillus niger (4) and Cryptococcus curvatus (J. Springer, unpublished data). In both cases the truncation was overcome by increasing the GC content of the AT-rich region.
In plants and animals, evidence exists that RNA splicing and 3′-end formation are coupled (12), especially when the sites of cleavage and polyadenylation are suboptimal (7). Analysis of 17 genes from homobasidiomycetes showed no conserved cleavage/polyadenylation sequence. In some cases a sequence resembling the eukaryotic consensus sequence for polyadenylation (AATAAA) was found (9), suggesting that coupling between RNA splicing and 3′-end formation may also occur in fungi.
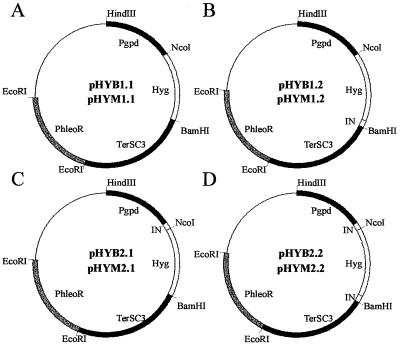
To determine whether introns affect hph mRNA accumulation and play a role in 3′-end formation of mRNA, we constructed plasmids in which the hph gene of E. coli (8) was regulated by the gpd promoter and SC3 terminator of S. commune (pHYB1.1) (Fig. 1). Alternatively, the hph gene was cloned in similar constructs that contained an artificial intron (as described in reference 5) directly downstream of the stop codon (pHYB1.2), the third intron of SC6 (5) directly upstream of the start codon (pHYB2.1), or a combination thereof (pHYB2.2) (Fig. 1). These constructs also contained the phleomycin resistance cassette, allowing direct or indirect selection on hygromycin B. The S. commune ΔSC3 strain, containing a disrupted SC3 gene (13, 14), was transformed with these constructs (10), and selection was carried out on phleomycin-containing medium. Northern blot analysis showed that none of the transformants (12 for each construct) accumulated hph mRNA (Fig. 2, lanes 17 to 20). Furthermore, the transformants were not resistant to hygromycin B. When the transformation mixture was plated directly on hygromycin B-containing medium no transformants could be selected. These results show that introduction of one or two introns in the hph expression cassette is not sufficient to effect accumulation of the hph mRNA transcripts.
FIG. 1.
Expression vectors used for transformation of S. commune. (A) Constructs pHYB1.1 and pHYM1.1, containing the unmodified and the modified hygromycin B gene, respectively, under regulation of the gpd promoter and SC3 terminator of S. commune. (B) Constructs pHYB1.2 and pHYM1.2, as for panel A with the artificial intron directly downstream of the stop codon. (C) Constructs pHYB2.1 and pHYM2.1, as for panel A with the third intron of SC6 directly upstream of the start codon. (D) Constructs pHYB2.2 and pHYM2.2, as for panel A with the artificial intron directly downstream of the stop codon and the third intron of SC6 directly upstream of the start codon. The artificial intron was made by annealing two complementary oligonucleotides, resulting in a double-stranded DNA fragment of 50 bp with receded ends complementary to those of BamHI. It contains consensus sequences for the splice and branch sites of S. commune introns. The sequences in between these three sites were randomly generated, although the average GC content for introns of S. commune (52%) was followed.
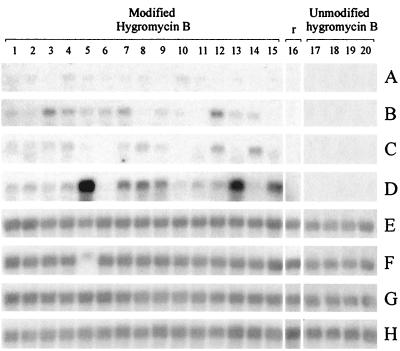
FIG. 2.
Effects of introns and codon modification on hygromycin B mRNA accumulation. The recipient S. commune ΔSC3 strain (r, lane 16) was transformed with the modified hygromycin B gene (lanes 1 to 15) or the unmodified hygromycin B gene (lanes 17 to 20). Shown are hygromycin B constructs without introns (A and E), constructs containing the third intron of SC6 directly upstream of the start codon (B and F), constructs containing an artificial intron directly downstream of the stop codon (C and G), and constructs containing both the third intron of SC6 and the artificial intron (D and H). The mRNA was hybridized with a 32P-labeled hygromycin B probe (A to D) or a 32P-labeled ribosomal probe (E to H). The software used was Corel PhotoPaint 8.0 and CorelDRAW 8.0.
Methylation of the hph gene also could prevent the expression of this gene in basidiomycetes (1, 6). However, it has been shown that the presence of homologous sequences in introduced constructs prevents extensive methylation of heterologous sequences (6). This result indicates that methylation of the hph gene in our system does not affect expression. Schuren et al. (11), however, suggest that low expression may be due to the presence of an AT-rich sequence in the hph gene.
We made constructs with a modified hph gene in which eight changes in an AT-rich stretch at the 5′ end of the gene were made to increase the GC content without affecting the encoded amino acids (Fig. 3). The S. commune ΔSC3 strain (13, 14) was transformed with these constructs, pHYM1.1, pHYM1.2, pHYM2.1, and pHYM2.2 (Fig. 1), and selection took place on phleomycin-containing medium. Northern blot analysis of 15 transformants containing the modified hph gene (pHYM1.1) showed a low hph mRNA signal of the expected size (1,020 nucleotides) (Fig. 2A, lanes 1 to 15). These results suggest that increasing the GC content in an AT-rich sequence in the hph gene prevents truncation of transcripts in S. commune. Introduction of the third intron of the SC6 gene directly upstream of the start codon or of the artificial intron directly downstream of the stop codon of the modified hph gene both increased the level of hph mRNA accumulation (Fig. 2B and C, lanes 1 to 15). Apparently, intron-dependent mRNA accumulation and truncation of transcripts at AT-rich stretches are independent processes that both affect expression of the hph gene in S. commune. When both introns were present in the hph construct, mRNA levels increased even further compared to those in the constructs containing a single intron (Fig. 2D, lanes 1 to 15). Previously, it was shown that addition of one intron outside the translational unit of SC3 cDNA was sufficient to increase SC3 mRNA accumulation to a level similar to that observed with the genomic SC3 gene, which contains five introns (5). Because the SC3 cDNA coding sequence is shorter (411 bp) than the hph gene (1,020 bp), we think that more than one intron may be needed for high mRNA accumulation for genes encoding longer transcripts.
FIG. 3.
The GC content of the AT-rich region between nucleotides 160 and 195 of the hygromycin B gene was increased by changing all A and T residues at the third position of the codons to C or G without affecting the encoded amino acid. The substitutions were introduced into the hygromycin B sequence by PCR. The 197 bp at the 5′ end of the coding sequence of hph were amplified by using an oligonucleotide primer corresponding to sense nucleotides 1 to 22 (accession no. Z32698) containing an NcoI site and to antisense nucleotides 158 to 197 containing the modified nucleotides and an AccI site. The remaining 865 bp of the coding sequence of hph were amplified by using oligonucleotide primers corresponding to sense nucleotides 158 to 197 containing the modified nucleotides and an AccI site and to antisense nucleotides 1001 to 1022 containing an additional BamHI site. Ligation of both fragments resulted in a 1,062-bp NcoI/BamHI fragment encompassing the complete modified hph gene. Note that these substitutions were introduced in an hph gene that had a C instead of a G at position 189.
All transformants (25 each) containing the modified hph gene in both the absence (pHYM1.1) and presence (pHYM1.2, pHYM2.1, and pHYM2.2) of introns were able to grow on medium containing up to 100 μg of hygromycin B ml−1. Transformants containing the third intron of SC6 upstream of the start codon (pHYM2.1) grew more slowly, indicating that they were less resistant. Yet, hph mRNA levels in transformants with an intron cloned upstream of the start codon or downstream of the stop codon were similar, showing that the differences in resistance were not due to differences in transcription level. The intron directly upstream of the start codon may be prone to incorrect splicing, resulting in part of the protein molecules being inactive and thus in reduced resistance.
We used plasmid pHYM1.1 to transform S. commune for direct selection on hygromycin B. In contrast to what occurred after selection on phleomycin, many false positives were obtained when hygromycin was used as a selection marker, as described earlier (6). No false positives were observed when 1 M sorbitol instead of 1 M MgSO4 was used for regeneration of protoplasts, and similar numbers of transformants (five [phyleomycin selection] and three [hygromycin B selection] per microgram of DNA) were obtained on hygromycin B-and phleomycin-containing media. All transformants selected on phleomycin grew on fresh phleomycin-containing plates, while 21 of 25 transformants also grew on hygromycin B-containing medium. Similarly, of 38 transformants selected on hygromycin B, 38 and 31 grew on fresh hygromycin B- and phleomycin-containing plates, respectively. Southern blot analysis of 10 transformants selected on hygromycin B confirmed the presence of one or more copies of the hph gene in the genome (data not shown).
Until now only the phleomycin resistance cassette could be used in S. commune as a selectable marker. The modified hph gene is now also available and is as efficient for transformant selection as the phleomycin resistance cassette. These results suggest that other inefficiently expressed prokaryotic genes (e.g., β-glucuronidase, β-galactosidase, and aminoglycoside phosphotransferase genes [11]) can also be successfully expressed in S. commune by replacing AT-rich sequences in the coding sequence and introduction of one or more introns. Although hygromycin B resistance has recently been observed in A. bisporus following transformation with an unmodified hph gene, using an Agrobacterium tumefaciens DNA transfer system (2), commercially important homobasidiomycetes like A. bisporus and Pleurotus ostreatus (oyster mushroom) have generally been difficult to transform using prokaryotic antibiotic resistance genes. Replacement of AT-rich sequences and introduction of one or more introns might increase levels of expression of these genes and may result in more efficient transformation.
Acknowledgments
This research was financially supported by The Netherlands Technology Foundation (STW) and is coordinated by the Life Sciences Foundation (SLW).
REFERENCES
- 1.Binz T, D'Mello N, Horgen P A. A comparison of DNA methylation levels in selected isolates of higher fungi. Mycologia. 1998;90:785–790. [Google Scholar]
- 2.de Groot M J A, Bundock P, Hooykaas P J J, Beijersbergen A G M. Agrobacterium tumefaciens-mediated transformation of filamentous fungi. Nat Biotechnol. 1998;16:839–842. doi: 10.1038/nbt0998-839. [DOI] [PubMed] [Google Scholar]
- 3.Dons J J M, de Vries O M H, Wessels J G H. Characterization of the genome of the basidiomycete Schizophyllum commune. Biochim Biophys Acta. 1979;563:100–112. doi: 10.1016/0005-2787(79)90011-x. [DOI] [PubMed] [Google Scholar]
- 4.Gouka R J, Punt P J, Hessing J G M, van den Hondel C A M J J. Analysis of heterologous protein production in defined recombinant Aspergillus awamori strains. Appl Environ Microbiol. 1996;62:1951–1957. doi: 10.1128/aem.62.6.1951-1957.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lugones L, Scholtmeijer K, Klootwijk R, Wessels J G H. Introns are necessary for mRNA accumulation in Schizophyllum commune. Mol Microbiol. 1999;32:681–689. doi: 10.1046/j.1365-2958.1999.01373.x. [DOI] [PubMed] [Google Scholar]
- 6.Mooibroek H, Kuipers A G J, Sietsma J H, Punt P J, Wessels J G H. Introduction of hygromycin B resistance into Schizophyllum commune: preferential methylation of donor DNA. Mol Gen Genet. 1990;222:41–48. doi: 10.1007/BF00283021. [DOI] [PubMed] [Google Scholar]
- 7.Nesic D, Maquat L E. Upstream introns influence the efficiency of final intron removal and RNA 3′-end formation. Genes Dev. 1994;8:363–375. doi: 10.1101/gad.8.3.363. [DOI] [PubMed] [Google Scholar]
- 8.Punt P J, Oliver R P, Dingemanse M A, Pouwels P H, van den Hondel C A M J J. Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene. 1987;56:117–124. doi: 10.1016/0378-1119(87)90164-8. [DOI] [PubMed] [Google Scholar]
- 9.Schuren F H J. Regulation of gene expression during fruit-body development in Schizophyllum commune. Ph.D. thesis. Haren, The Netherlands: University of Groningen; 1992. [Google Scholar]
- 10.Schuren F H J, Wessels J G H. Highly efficient transformation of the homobasidiomycete Schizophyllum commune to phleomycin resistance. Curr Genet. 1994;26:179–183. doi: 10.1007/BF00313808. [DOI] [PubMed] [Google Scholar]
- 11.Schuren F H J, Wessels J G H. Expression of heterologous genes in Schizophyllum commune is often hampered by the formation of truncated transcripts. Curr Genet. 1998;33:151–156. doi: 10.1007/s002940050321. [DOI] [PubMed] [Google Scholar]
- 12.Simpson G G, Filipowicz W. Splicing of precursors to mRNA in higher plants: mechanism, regulation and sub-nuclear organisation of the spliceosomal machinery. Plant Mol Biol. 1996;32:1–41. doi: 10.1007/BF00039375. [DOI] [PubMed] [Google Scholar]
- 13.van Wetter M-A, Schuren F H J, Schuurs T A, Wessels J G H. Targeted mutation of the SC3 hydrophobin gene of Schizophyllum commune affects formation of aerial hyphae. FEMS Microbiol Lett. 1996;140:265–269. [Google Scholar]
- 14.Wösten H A B, Schuren F H J, Wessels J G H. Interfacial self-assembly of a hydrophobin into an amphipathic membrane mediates fungal attachment to hydrophobic surfaces. EMBO J. 1994;13:5848–5854. doi: 10.1002/j.1460-2075.1994.tb06929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]