Abstract
Background
Individuals with severe asthma represent 5%–10% of the general asthmatic population. Despite the use of biologic drugs during clinical management, inadequate control of the disease has translated into high economic impact. In Mexico, however, these costs have not yet been assessed.
Methods
A retrospective cohort study was carried out in 2018 and 2019 at Regional Hospital Lic. Adolfo López Mateos, ISSSTE. The assessment of direct costs included pharmacological treatment, clinical tests, days of hospitalization, admissions to the emergency room, and scheduled consultations. The evaluation involved 2 groups of patients—with controlled severe asthma (CSA) and uncontrolled severe asthma (UCSA)—according to presence of exacerbations.
Results
60 patients (18–75 years old, 51 women) were included in the study. In 2018, 23 of them (38.3%) were categorized as belonging to the UCSA group; in 2019, 22 patients (36.7%) were in this condition (exacerbations: median = 1.5, maximum = 6). Of the 60 patients, 12 (20%) presented between 2 and 9 exacerbations in the study's two-year period (median = 3) after between 4 and 10 years (median = 7.8) of complementary anti-immunoglobulin E (IgE) therapy with omalizumab. The cost for all patients in the 2018–2019 period was 993,289.60 USD. The mean cost per patient was higher for those with UCSA (16,392 USD) than for those with CSA (16,246 USD, p = 0.02). We found a positive association between cost and exacerbations, with an increase of 350 USD per exacerbation (p˂0.0001). Our results indicate that 62% of patients respond to complementary anti-IgE treatment, while 38%—and especially 20%—do not respond optimally to this treatment.
Conclusions
Poor asthma control in this latter group of 38% of patients leads to lower quality of life and higher costs associated with pharmacological treatment.
Keywords: Severe asthma, Economic burden, Cost estimation, Biological drugs, Real-life study
Introduction
Asthma is one of the most common chronic noncommunicable diseases in children and adults. It is characterized by spasmodic contraction of the airway smooth muscle, variable airflow limitation, wheezing, and dyspnea. Asthma is classified as severe when high-intensity treatment is required for it to be kept under control, or when it remains uncontrolled despite treatment.1
Globally, the prevalence of asthma goes from 1% to 18%,2 with an average of 6.9%.3 The authors of the Global Burden of Disease study estimated that 358 million people suffered from asthma in 2015, an increase of 9.5% since 2005. Mexico has a prevalence of between 5% and 6.9%;4 it is higher in women (6.2%) than in men (3.3%).5
Patients with severe asthma comprise 5%–10% of the general asthmatic population. These patients experience the highest overall morbidity, as well as the greatest impact on quality of life. Moreover, the cost of treating individuals with severe asthma is considerably higher than the cost of treating patients in the general asthmatic population.6,7 Nevertheless, most low- and middle-income countries do not assess the economic costs of asthma.8
Corticosteroid drugs are the mainstay of standard asthma treatment.1,2,9,10 This treatment is based on a cycle of assessment and reassessment of symptoms, risk factors, comorbidities, side effects, and patient satisfaction.1,11
Biological drugs for the treatment of severe asthma—used as complementary therapies to standard treatments—have been shown to be effective, safe, and cost-effective. There are currently 5 biological drugs approved by the US Food and Drug Administration (FDA): benralizumab, dupilumab, mepolizumab, omalizumab, and reslizumab. Biologics encompass the different phenotypes of severe asthma, acting on key pathogenic pathways: interleukin 5 (IL-5), interleukin 4 and 3 (IL-4/IL-13), and IgE driven eosinophilic inflammation. The incorporation of biologics to the treatment of severe asthma represents an opportunity to carry out phenotype-specific interventions, which increases the prospect of more personalized treatments.9,12,13 The cost of treatments with biologics is substantially higher than the cost of standard corticosteroid treatments.11 This reinforces the need to achieve precision in diagnosis and in the phenotype-specific therapy.14
In Mexico, the response of patients with severe asthma to a three-year complementary anti-IgE biological therapy with omalizumab was evaluated. Patients belonged to the cohort of the Allergy and Immunology Regional Hospital Lic. Adolfo López Mateos, ISSSTE. In comparison with standard therapy, significant improvements were found regarding asthma control and forced expiratory volume in 1 s (FEV1). There was a drop in beclomethasone doses as well as in visits to the emergency room (from 95% to 19%), hospitalization days (from 38% to 2%), and intensive care (from 4% to 0%).9 However, an economic assessment of the treatment and how the cost varies depending on the level of asthma control has not been carried out so far. Therefore, in this study we seek to identify the direct cost of the treatment for severe asthma, both controlled and uncontrolled.
Materials and methods
A two-year retrospective cohort study was carried out in 2018 and 2019. The cohort was comprised by patients of the cohort of the Allergy and Immunology Regional Hospital Lic. Adolfo López Mateos, ISSSTE.9
Adult patients (18–75 years old) of both genders who met the criteria for severe asthma according to the Global Initiative for Asthma15 were selected. Patients diagnosed with occupational asthma, cystic fibrosis, heart failure class III or higher according to the New York Heart Association Functional Classification,16 severe pulmonary emphysema, disorders causing laryngeal or tracheal obstruction, carcinoid syndromes, hyperthyroidism, sleep apnea, and psychiatric disorders were excluded.
Standard treatments included corticosteroids, leukotriene modifiers, antihistamines, antibiotics, and drugs combined. Omalizumab (XOLAIR®) was administered subcutaneously to all patients every month. The omalizumab dose was calculated according to the manufacturer's guidelines, considering total IgE serum levels and the patient's weight (Supplemental Table 1) The patient received all pharmacological treatment monthly completely free of charge as they belong to the cohort.
The clinical variables collected were the following: comorbidities, respiratory rate (RR), heart rate (HR), FEV1 (expressed in %), peak expiratory flow (PEF, in %), leukocytes (in thousands/μL), eosinophils (in thousands/μL), proportion of patients with eosinophils ≥300 (thousands/μL), and immunoglobulin E (IgE, in mg/dL).
Cost estimate
The assessment of direct costs included the following: a) pharmacological treatment—doses and number of months per year of prescribed medication, b) clinical tests, c) days of hospitalization, d) admissions to the emergency room, and f) scheduled consultations.
All the information was obtained from patients’ clinical records. Direct healthcare costs were determined using information from providers of public health services from Allergy and Immunology Regional Hospital Lic. Adolfo López Mateos, ISSSTE. All costs were expressed in US dollars (USD) after being converted from Mexican pesos (MXN) using the official exchange rate (https://www.dof.gob.mx/indicadores) registered in July 2018 and July 2019. For our analysis, we estimated health care spending per patient, annually, and for the entire study period.
Patients were divided into 2 groups: controlled severe asthma (CSA)—that is, patients without exacerbations during the year in question—and uncontrolled severe asthma (UCSA)—patients who presented at least one exacerbation during the year. Exacerbations were defined as a worsening of symptoms requiring rescue treatment with systemic corticosteroids. The total number of exacerbations was calculated as the sum of deflazacort cycles used exclusively for the treatment of exacerbations.
Data analysis
The qualitative variables were expressed in absolute frequencies and percentages. Quantitative variables were expressed as medians (M), interquartile ranges (IQR, according to the values of quartiles 1 and 3, Q1-Q3), and minimums and maximums, since they did not present normal distribution after performing the Shapiro-Wilks test. The comparison of the frequency of comorbidities between both groups was carried out using the chi-squared test. For the remaining quantitative variables, the Mann-Whitney test was used. To detect those variables that most influenced the cost, a generalized linear model (GLM) was carried out, using the total cost in the 2018–2019 period as the response variable. The explanatory variables were gender, age, years of diagnosis, respiratory rate, heart rate, number of comorbidities, and number of exacerbations. Moreover, a second GLM was carried out with the aim of analyzing the most influential variables in the number of exacerbations. The explanatory variables were gender, age, years of diagnosis, respiratory rate, heart rate, and number of comorbidities. We assessed the relative performance of the models using the Akaike information criterion (AIC)17 and selected the model with the lowest AIC. All test and the GLMs were carried out using the R program.18
Results
This study included 60 patients between 18 and 75 years of age who met the inclusion criteria (M = 56, IQR = 47–64), of whom 51 were women and 9 men. Patients had between 3 and 10 years complementary anti-IgE therapies with Omalizumab (M = 7.6, IQR = 6.1–9.1). In 2018, 23 patients (38.3%) presented UCSA (exacerbations: M = 1.5, maximum = 3); in 2019, 22 patients (36.7%) were in this condition (exacerbations: M = 1.5, maximum = 6). Of the 60 patients, 12 (20%) presented exacerbations in the two-year study period (M = 3, minimum = 2, maximum = 9) after between 4 and 10 years (M = 7.8) of complementary therapy.
No patients were hospitalized or admitted to the emergency room in the 2018–2019 period, so there were no costs associated with these resources. All patients had 12 scheduled consultations per year, since the biological drug is administered subcutaneously, and the injection is applied at the hospital. The number of patients who underwent clinical tests varied by year and by type of analysis; less than 50% underwent annual clinical tests. The demographic and clinical characteristics of patients with CSA and UCSA, as well as the total numbers for both groups, are shown in Table 1. No differences were found in any of the variables analyzed except for PEF, which in 2018 was significantly lower in the UCSA group compared to the CSA group (Table 1).
Table 1.
Demographic and clinical characteristics of patients with controlled severe asthma (CSA) and uncontrolled severe asthma (UCSA).
| Total |
CSA |
UCSA |
P |
|
|---|---|---|---|---|
| M (Q1-Q3) | M (Q1-Q3) | M (Q1-Q3) | ||
| Age | 55.5 (44.6–64.4) | 55.5 (49.25–64.3) | 57.5 (44.8–63.34) | 0.74 |
| BMI (kg/m2) | 28.9 (25.7–32.5) | 27.8 (25.7–31.3) | 30.7 (25.6–33.8) | 0.3 |
| 2018 | n = 60 | n = 37 | n = 23 | |
| RR | 17 (16–18) | 16 (16–18.5) | 18 (16.18) | 0.36 |
| HR (bpm) | 76 (70–80) | 75 (70–80) | 76 (72–80) | 0.26 |
| 2019 | n = 60 | n = 38 | n = 22 | |
| RR | 16 (16–18) | 16 (16–18) | 17 (16–18) | 0.26 |
| HR | 72 (70.0–75.7) | 71.5 (70.0–75.0) | 73 (67.8–77.5) | 0.74 |
| 2018 | n = 40 | n = 23 | n = 17 | |
| FEV1 (%) | 80.0 (68.3–91.8) | 87.0 (71.0–93.0) | 73.0 (59.50–82.50) | 0.08 |
| PEF (%) | 88.0 (70.5–107.3) | 102.0 (82.0–113.0) | 76.0 (64.50–92.50) | 0.005 |
| n = 12 | n = 6 | n = 6 | ||
| IgE (mg/dL) | 606 (232–2153) | 606 (324–2568) | 730 (193–2863) | 0.7 |
| n = 22 | n = 15 | n = 7 | ||
| Leukocytes, thousands/μL | 6628 (8405–10245) | 6530 (8370–9200) | 6660 (10,080–11150) | 0.36 |
| Eosinophils, thousands/μL | 175 (430.0–648.5) | 160 (330.0–650.0) | 260 (460–648) | 0.6 |
| Eosinophils ˃300, thousands/μL, n (%) | 14 (63.6) | 8 (53.3) | 5 (71.4) | |
| 2019 | n = 40 | n = 23 | n = 17 | |
| FEV1 | 83.0 (68.0–90.8) | 83.0 (68.0–93.0) | 83.0 (67.5–89.0) | 0.58 |
| PEF | 92.0 (80.0–105.5) | 92.0 (82.0–109.0) | 93.0 (79.5–104.0) | 0.68 |
| n = 16 | n = 10 | n = 6 | ||
| IgE | 250 (172.0–440) | 280 (131–490) | 221 (160–502.5) | 0.98 |
| n = 24 | n = 14 | n = 10 | ||
| Leukocytes | 8505 (5725–10090) | 8640 (5688–10338) | 8190 (5698–10198) | 0.83 |
| Eosinophils | 371 (205–565) | 371 (258–693) | 355 (168–468) | 0.34 |
| Eosinophils ˃ 300, n (%) | 14 (58.3) | 9 (64.3) | 5 (50.0) |
UCSA was defined by the presence of at least one exacerbation in the year; BMI, body mass index; RR, respiratory rate; HR, heart rate; P, p-value resulting from the Mann-Whitney test; Q1 and Q3, quartiles 1 and 3 respectively
Of the comorbidities analyzed, diabetes, obesity, and aspirin-exacerbated respiratory disease were significantly more frequent in the UCSA group, while the opposite occurred with the frequency of rhinitis. In the remaining comorbidities that were analyzed, no significant differences were found between both groups (Table 2).
Table 2.
Absolute frequency (n) and proportion (%) of comorbidities in patients with controlled severe asthma (CSA) and uncontrolled severe asthma (UCSA).
| Total | CSA | UCSA | ||
|---|---|---|---|---|
| Comorbidities | n (%) | n (%) | n (%) | P |
| Diabetes | 8 (13.0) | 0 (0.0) | 8 (13.0) | 0.001 |
| HBP | 12 (20) | 3 (5.0) | 9 (15.0) | 0.110 |
| Rhinitis | 35 (58.3) | 22 (36.6) | 13 (21.6) | 0.001 |
| Rhinosinusitis | 5 (8.3) | 2 (2.2) | 3 (5.0) | 0.814 |
| GERD | 19 (31.6) | 10 (16.6) | 9 (15.0) | 0.420 |
| Obesity | 31 (51.6) | 11 (18.3) | 20 (33.0) | 0.124 |
| AD | 9 (15.0) | 6 (10.0) | 3 (5.0) | 0.156 |
| AERD | 6 (10.0) | 0 (0.0) | 6 (10.0) | 0.005 |
UCSA was defined by the presence of at least one exacerbation in the year; HBP, high blood pressure; GERD, gastroesophageal reflux disease; AD, atopic dermatitis; AERD, aspirin-exacerbated respiratory disease; P, p-value resulting from the Chi-squared test
The cost of the 60 patients with severe asthma during the 2018–2019 period was 993,289.60 USD (482,068.70 USD in 2018 and 511,220.90 USD in 2019). Nearly 100% of this cost corresponds to drug treatment expenses and scheduled consultations. The mean cost per patient in the 2018–2019 period was 16,301.10 USD. It was slightly but significantly higher for those with UCSA (16,392 USD) than for those with CSA (16,246 USD, p = 0.02). The mean cost per patient and a comparison between the CSA and UCSA groups for each year are shown in Table 3.
Table 3.
Mean cost per patient of controlled severe asthma (CSA) and uncontrolled severe asthma (UCSA).
| Cost (USD) |
|||||||
|---|---|---|---|---|---|---|---|
| Total, n = 60 | CSA, n = 37 | UCSA, n = 23 | |||||
| 2018 | Median (Q1-Q3) | Minimum-Maximum | Median (Q1-Q3) | Minimum-Maximum | Median (Q1-Q3) | Minimum-Maximum | P |
| Laboratories | 11.2 (0–25.3) | 0–54.2 | 11.23 (0–32.71) | 0–54.2 | 11.2 (0.0–25.3) | 0.0–40.2 | 0.092 |
| Medications | 6629.2 (6476.8–6778.1) | 3630.2–9058.8 | 6607.7 (6456.4–6712.6) | 6205.3–7211.6 | 6731.0 (6573.0–6922.0) | 3630.0–9059.0 | |
| Consultations | 1351.5 (1351.5–1351.5) | 1351.5–1351.5 | 1351.5 (1351.5–1351.5) | 1351.5–1351.5 | 1351.5 (1351.5–1351.5) | 1351.5–1351.5 | 0.053 |
| Total |
8002.1 (7843.5–8150.5) |
5006.9–10450.4 |
7967.3 (7814–8094.1) |
7572.4–8603.2 |
8094.0 (7924.0–8287.0) |
5007.0–10450.0 |
0.065 |
| 2019 |
CSA, n = 38 |
UCSA, n = 22 |
|||||
| Laboratories | 0.0 (0.0–26.2) | 0.0–56.1 | 0.0 (0.0–26.2) | 0–56.1 | 0.0 (0.0–30.0) | 0.0–56.1 | 0.93 |
| Medications | 6905.0 (6766.0–7013.0) | 6417.0–10593.0 | 6823.6 (6621.5–6891.9) | 6417.3–6954.3 | 7075.0 (7001.0–7376.0) | 6908.0–10593.0 | 0.001 |
| Consultations | 1399.1 (1399.1–1399.1) | 1399.1–1399.1 | 1399.1 (1399.1–1399.1) | 1399.1–1399.1 | 1399.1 (1399.1–1399.1) | 1399.1–1399.1 | |
| Total | 8321.0 (8193.0–8435.0) | 7816.0–11992.0 | 8239.2 (8039.3–8317.4) | 7816.4–8358.2 | 8482.0 (8426.0–8775.0) | 8359.0–11992.0 | 0.001 |
UCSA was defined by the presence of at least one exacerbation in the year; P, p-value resulting from the Mann-Whitney test; Q1 and Q3, quartiles 1 and 3 respectively
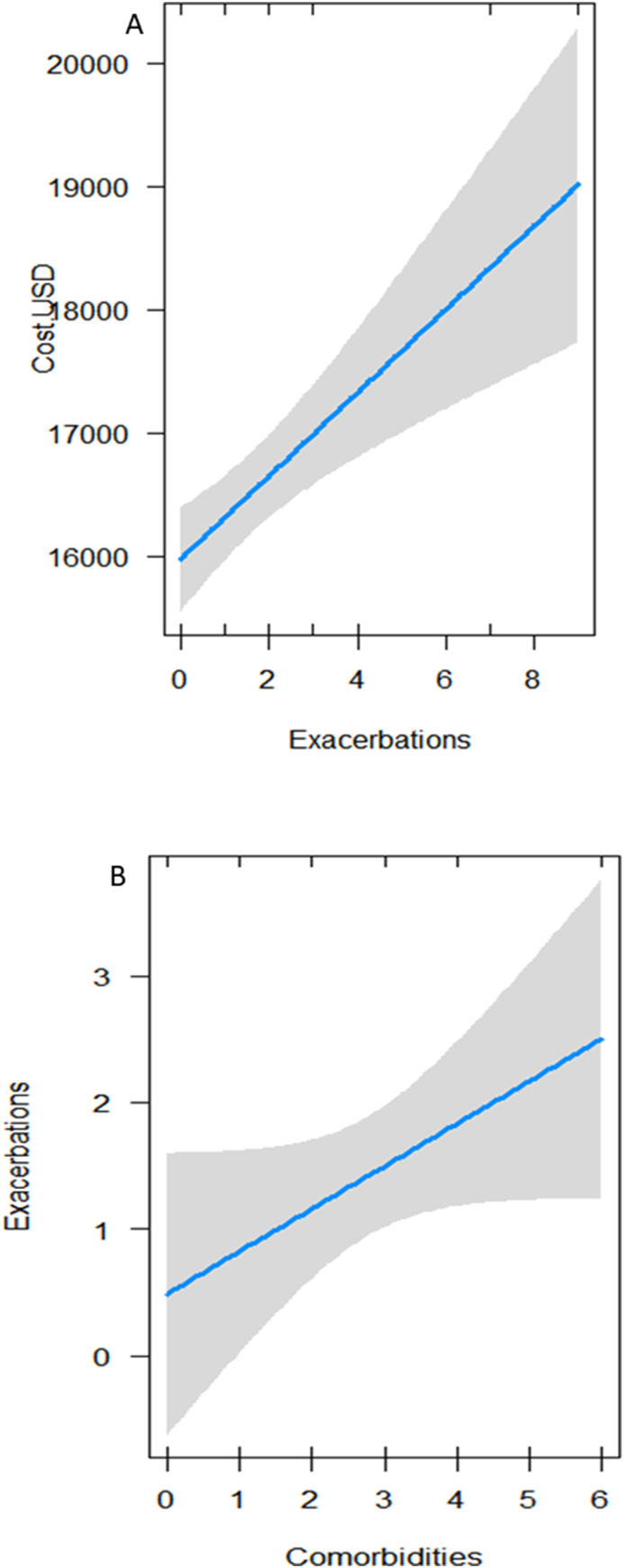
The GLM results indicated that the most influential variable on total cost in the study's two-year period was the number of exacerbations: there is an increase of 350 USD per exacerbation (Table 4A, Fig. 1a). Although the best model retained gender as an explanatory variable, it did not turn out to be significant. On the other hand, when analyzing the number of exacerbations as a response variable, it was positively associated to the number of comorbidities (Table 4B, Fig. 1b).
Table 4.
Association results: A) association of the total cost (2018–2019) with the number of exacerbations; B) association of the number of exacerbations with the number of comorbidities. Standard error (SE) and p-value (P)
| Estimates | SE | P | |
|---|---|---|---|
| A. Response variable: cost per patient | |||
| Intercept | 17370.4 | 812.9 | <2.0−16 |
| Gender | −712 | 437.5 | 0.11 |
| Number of exacerbations | 350.1 | 83 | 8.9−5 |
| B. Response variable: exacerbations | |||
| Intercept | 0.49 | 0.56 | 0.39 |
| Number of comorbidities | 0.34 | 0.19 | 0.04 |
Fig. 1.
A) Effect of the number of exacerbations on the total cost of severe asthma in the 2018–2019 period; B) effect of the number of comorbidities on the number of exacerbations in the 2018–2019 period
Discussion
In this study, the direct cost of CSA and UCSA was analyzed in 60 patients from the cohort of the Regional Hospital Lic. Adolfo López Mateos, ISSSTE.
Our analysis shows the substantial economic burden of severe asthma for the public health system and society. The average annual cost per patient of severe asthma is considerably higher than the cost of treatment for the general asthmatic population.7 In line with this general trend, our results showed that the average annual cost per patient of severe asthma in both 2018 (8002.10 USD) and 2019 (8321.00 USD) was substantially higher than the one reported in several countries in which prevalence and general costs were analyzed in the general asthma population. In the United States, in the 2008–2013 period, the cost per patient of severe asthma was 3266 USD; the highest expenses were medications and scheduled consultations.19 This is consistent with our findings, as we also identified a higher portion of spending being allocated to prescription drugs and scheduled visits. In South Korea, the average cost per patient in 2014 was 514.10 USD;20 in Canada, 157 USD;21 and in Europe, 1796 EUR.22 In Brazil, the mean cost per patient was 419.50 USD; however, in the case of patients with UCSA, the cost was more than double: 1283.70 USD.23
Given the high costs of treating severe asthma, studies about the effectiveness and cost-effectiveness of complementary therapies with biological drugs have become important when assessing the drop in the number of exacerbations and admissions to the emergency room.13 In this sense, multiple evaluations of both anti-IgE and anti-IL-5 therapies have been carried out.14,24 For example, according to an evaluation of 186 patients from 12 pulmonology services in Valencia, Spain, the direct cost of severe asthma per patient after 5 years of complementary anti-IgE therapy was 12,670.40 EUR. The cost-effectiveness per exacerbation avoided was 1789.30 EUR.25 Another evaluation of 220 patients from 15 pulmonology departments in Andalusia and Extremadura, also in Spain, found that the mean cost per patient was 12,690.00 EUR one year after starting complementary anti-IgE therapy; the cost-effectiveness per exacerbation avoided was 1712 EUR.26 Although the average costs per patient are higher than those reported in our study, it is important to consider that patients in the Spanish cohorts had days of hospitalization and visits to the emergency room.
The strongest existing evidence for complementary therapies is in the short term; beyond 4 years of treatment, the evidence is only starting to emerge.27 Our results provide evidence of the effectiveness of complementary with Omalizumab therapy in the long term—after 3–10 years of treatment (M = 7.6)—in 62% of patients. However, 38% of patients presented at least one exacerbation in one year, while 12 (20%) presented exacerbations in two consecutive years, with a minimum of 2 and a maximum of 9 (M = 3), after between 4 and 10 years (M = 7.75) of complementary therapy. Therefore, our results indicate that a considerable proportion of patients with severe asthma still require better control.
In a real-world setting, 30% of 196 patients with severe asthma showed excellent long-term adherence after four years of omalizumab therapy. High adherence to omalizumab was demonstrated to be associated with better outcomes and control of asthma.28 However, in our case, all patients had high adherence to omalizumab due to it was administrated monthly at the Hospital without cost. Thus, adherence did not interfere with the success of treatment.
Our results are in line with a comprehensive evaluation carried out in the United States on complementary therapies with biological drugs in which it was reported that 63% of patients (n = 3262) presented at least one asthma exacerbation per year (M = 1.3).29 Taken together, both studies—ours and that of Reibman et al.29 suggest that although biological drug therapies have led to substantial improvements in patients’ quality of life, adjustments in accuracy based on the asthma phenotype are still required. Our results also showed that at least 38% of the patients need a better assessment of asthma phenotypes and comorbidities. This is a fundamental step in the evaluation and management of patients with severe asthma to guide the optimal selection of biological therapies.30
Our study indicated that exacerbations per patient increased the cost of treatment by an average of 350 USD. The relationship between asthma control and increased cost has been widely documented for more than a decade in the United States and various countries in Europe, Asia, and South America.22,31, 32, 33, 34, 35 The strong inverse relationship between poor control of severe asthma and higher care costs, mainly derived from medications, reinforces the need for greater precision in the phenotypic diagnosis of asthma. The goal is to promote phenotype-specific therapies with higher cost-effectiveness for public health systems.29,36
This study has limitations that are worthy to mention. The first limitation is related to the limited use of biological therapy for asthma, consisting of anti-IgE with Omalizumab only. We expect in the future switching uncontrolled patients from anti-IgE to anti-IL-5 therapy may better control asthma and reduce exacerbations, leading to less cost. The second limitation was that due to the low number of patients with markers (IgE and eosinophils) of the key pathogenic pathways, we were not able to perform a deeper analysis on the number of exacerbations and the values of these markers due to the lack of statistical power, nor to introduce them into the models, even if all patients had pretreatment total serum IgE levels ≥ to 30 IU/mL. However, this did not affect the main objective of the study.
Conclusions
The overall cost of severe asthma for all 60 patients involved in this study during the 2018–2019 period was 993,289.60 USD. The mean cost per patient was slightly but significantly higher for those with UCSA (16,392 USD) than for those with CSA (16,246 USD, p = 0.02), due to an increase of 350 USD per exacerbation.
Our results indicate that 62% of patients respond to complementary anti-IgE treatment. However, the other 38%—and especially 20%—do not respond optimally to this treatment. These patients have poor asthma control and higher costs associated with pharmacological treatment. We strongly recommend the evaluation of adequate biological markers to achieve greater precision in the phenotypic diagnosis of asthma and, therefore, a more efficient therapy for patients with low asthma control. Personalizing treatments within the same cohort under GINA guidelines15 would be beneficial for the patient's health and, at the same time, increase cost-effectiveness in the public health system.
Abbreviations
CSA, controlled severe asthma; UCSA, uncontrolled severe asthma; IL-5, interleukin 5; IL-4, interleukin 4; IL-3, interleukin 3; IgE, immunoglobulin E; FEV1, forced expiratory volume in 1 second; RR, respiratory rate; HR, heart rate; PEF, peak expiratory flow; USD US dollars after; MXN Mexican pesos; M, medians; IQR interquartile ranges; Q1, quartile 1; Q3, quartile 3; GLM, generalized linear model; AIC, Akaike information criterion.
Funding
This research was funded by ISSSTE.
Availability of data and materials
Raw data were generated and derived data supporting the findings of this study are available from the corresponding author PCU upon request.
Authors’ contributions
PCU, JLP; the conception and design of the study, PCU, JLP; acquisition of data, or analysis, PCU, JLP, ACC, ERA interpretation of data, PCU, JLP, ACC, ERA Drafting the article or revising it critically for important intellectual content, PCU, JLP, ACC, ERA Final approval of the version to be submitted.
Ethics approval
The study was approved by the Regional Hospital Lic. Adolfo López Mateos’ (ISSSTE) Scientific and Ethics Committee, with registration number 211.2021.
Authors’ consent for publication
All authors accepted the manuscript in the current version form.
Declaration of competing interest
Paula Costa Urrutia received support for medical writing and article processing charges from AstraZeneca. The authors report no competing interests.
Acknowledgments
The authors would like to thank to the Regional Hospital Lic. Adolfo López Mateos' Department of Allergy and Immunology staff for logistic and technical support.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2022.100662.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Papi A., Brightling C., Pedersen S.E., Reddel H.K. Asthma. Lancet. 2018;391(10122):783–800. doi: 10.1016/S0140-6736(17)33311-1. [DOI] [PubMed] [Google Scholar]
- 2.Rehman A., Amin F., Sadeeqa S. Prevalence of asthma and its management: a review. J Pakistan Med Assoc. 2018;68(12):1823–1827. [PubMed] [Google Scholar]
- 3.Wang H., Naghavi M., Allen C., et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forno E., Gogna M., Cepeda A., et al. Asthma in Latin America. Thorax. 2015;70(9):898–905. doi: 10.1136/thoraxjnl-2015-207199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García-Sancho C., Fernández-Plata R., Martínez-Briseño D., Franco-Marina F., Pérez-Padilla J.R. Prevalencia y riesgos asociados con pacientes adultos con asma de 40 años o más de la Ciudad de México: estudio de base poblacional. Salud Publica Mex. 2012;54(4):425–432. doi: 10.1590/s0036-36342012000400013. [DOI] [PubMed] [Google Scholar]
- 6.Cisneros Serrano C., Melero Moreno C., Almonacid Sánchez C., et al. Normativa sobre asma grave no controlada. Arch Bronconeumol. 2015;51(5):235–246. doi: 10.1016/j.arbres.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Lötvall J., Akdis C.A., Bacharier L.B., et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127(2):355–360. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 8.Enilari O., Sinha S. The global impact of asthma in adult populatio. Ann Glob Heal. 2019;85(1):1–7. doi: 10.5334/aogh.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López Tiro J.J., Contreras E.A.C., del Pozo M.E.R., Vera J.G., Linnemann D.L. Real life study of three years omalizumab in patients with difficult-to-control asthma. Allergol Immunopathol. 2015;43(2):120–126. doi: 10.1016/j.aller.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Ramsahai J.M., Hansbro P.M., Wark P.A.B. Mechanisms and management of asthma exacerbations. Am J Respir Crit Care Med. 2019;199(4):423–432. doi: 10.1164/rccm.201810-1931CI. [DOI] [PubMed] [Google Scholar]
- 11.Anderson W.C., Szefler S.J. Cost-effectiveness and comparative effectiveness of biologic therapy for asthma: to biologic or not to biologic? Ann Allergy Asthma Immunol. 2019;122(4):367–372. doi: 10.1016/j.anai.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Miranda P. The effectiveness of omalizumab in the control of severe uncontrolled asthma in Latin America. An exploratory systematic review and meta-analysis. Rev Alerg Mex. 2020;67(1):19–24. doi: 10.29262/ram.v67i1.701. [DOI] [PubMed] [Google Scholar]
- 13.Almonacid-Sánchez C., Melero-Moreno C. Análisis económico de las terapias biológicas en asma grave. Farm Hosp. 2019;43(6):175–176. doi: 10.7399/fh.11342. [DOI] [PubMed] [Google Scholar]
- 14.Agache I., Beltran J., Akdis C., et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines - recommendations on the use of biologicals in severe asthma. Allergy Eur J Allergy Clin Immunol. 2020;75(5):1023–1042. doi: 10.1111/all.14221. [DOI] [PubMed] [Google Scholar]
- 15.GINA. Global Strategy for Asthma Management and Prevention; 2021. www.ginasthma.org.
- 16.Hurst J.W., Morris D.C., Alexander R.W. The use of the New York Heart Association's classification of cardiovascular disease as part of the patient's complete Problem List. Clin Cardiol. 1999;22(6):385–390. doi: 10.1002/clc.4960220604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burnham K.P., Anderson D.R. Multimodel inference understanding AIC and BIC in model selection. Socio Methods Res. 2004;33(2):261–304. doi: 10.1177/0049124104268644. [DOI] [Google Scholar]
- 18.Team TRDC. R: A language and environment for statistical computing. Published online 2008:2673.
- 19.Nurmagambetov T., Kuwahara Robin, Garbe Paul. 2017. The Economic Burden of Asthma in the United States, 2008–2013. Enhanced Reader.pdf. Published online. [DOI] [PubMed] [Google Scholar]
- 20.Lee E.W., Kim H.S., Kim W., Nam J.Y., Park J.H. Socioeconomic burden of disease due to asthma in South Korea. Asia Pac J Publ Health. 2020;32(4):188–193. doi: 10.1177/1010539520920524. [DOI] [PubMed] [Google Scholar]
- 21.Bedouch P., Marra C.A., FitzGerald J.M., Lynd L.D., Sadatsafavi M. Trends in asthma-related direct medical costs from 2002 to 2007 in British Columbia, Canada: a population based-cohort study. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0050949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Accordini S., Corsico A.G., Braggion M., et al. The cost of persistent asthma in Europe: an international population-based study in adults. Int Arch Allergy Immunol. 2013;160(1):93–101. doi: 10.1159/000338998. [DOI] [PubMed] [Google Scholar]
- 23.Costa E., Caetano R., Werneck G.L., Bregman M., Araújo D.V., Rufino R. Estimated cost of asthma in outpatient treatment: a real-world study. Rev Saude Publica. 2018;52:1–10. doi: 10.11606/S1518-8787.2018052000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farne H.A., Wilson A., Powell C., Bax L., Milan S.J. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;2017(9) doi: 10.1002/14651858.CD010834.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martínez-Moragón E., Climent M., Chiner E., et al. Effectiveness and pharmacoeconomic analysis of the treatment of severe asthma with omalizumab in clinical practice. Farm Hosp. 2019;43(3):101–109. doi: 10.7399/fh.11167. [DOI] [PubMed] [Google Scholar]
- 26.Entrenas Costa L.M., Casas-Maldonado F., Soto Campos J.G., et al. Economic impact and clinical outcomes of omalizumab add-on therapy for patients with severe persistent asthma: a real-world study. PharmacoEconomics - Open. 2019;3(3):333–342. doi: 10.1007/s41669-019-0117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karen M., MacDonald A.K., Benjamin Ortiz A.A., Lee C.S., Abraham I. Short- and long-term real-world effectiveness of omalizumab in severe allergic asthma: systematic review of 42 studies published 2008-2018. Expet Rev Clin Immunol. 2019;15(5):553–569. doi: 10.1080/1744666X.2019.1574571. [DOI] [PubMed] [Google Scholar]
- 28.Campisi R., Crimi C., Intravaia R., Strano S., Noto A. Adherence to omalizumab : a multicenter " real-world " study. World Allergy Organ J. 2020;13(2):100103. doi: 10.1016/j.waojou.2020.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reibman J., Tan L., Ambrose C., et al. Clinical and economic burden of severe asthma among US patients treated with biologic therapies. Ann Allergy Asthma Immunol. 2021;127(3):318–325. doi: 10.1016/j.anai.2021.03.015. e2. [DOI] [PubMed] [Google Scholar]
- 30.Gaffin Jonathan M., Castro Mario, Leonard B., Bacharier A.L.F. The role of comorbidities in difficult-to-control asthma in adults and children. World Allergy Organ J. 2022;10(2):397–408. doi: 10.1016/j.jaip.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan S.D., Rasouliyan L., Russo P.A., Kamath T., Chipps B.E. Extent, patterns, and burden of uncontrolled disease in severe or difficult-to-treat asthma. Allergy Eur J Allergy Clin Immunol. 2007;62(2):126–133. doi: 10.1111/j.1398-9995.2006.01254.x. [DOI] [PubMed] [Google Scholar]
- 32.Ivanova J.I., Bergman R., Birnbaum H.G., Colice G.L., Silverman R.A., McLaurin K. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol. 2012;129(5):1229–1235. doi: 10.1016/j.jaci.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 33.Inoue H., Kozawa M., Milligan K.L., Funakubo M., Igarashi A., Loefroth E. A retrospective cohort study evaluating healthcare resource utilization in patients with asthma in Japan. NPJ Prim Care Respir Med. 2019;29(1) doi: 10.1038/s41533-019-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flórez-Tanus Á., Parra D., Zakzuk J., Caraballo L., Alvis-Guzmán N. Health care costs and resource utilization for different asthma severity stages in Colombia: a claims data analysis 11 Medical and Health Sciences 1117 Public Health and Health Services. World Allergy Organ J. 2018;11(1):26. doi: 10.1186/s40413-018-0205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeiger R.S., Schatz M., Dalal A.A., et al. Utilization and costs of severe uncontrolled asthma in a managed-care setting. J Allergy Clin Immunol Pract. 2016;4(1):120–129. doi: 10.1016/j.jaip.2015.08.003. e3. [DOI] [PubMed] [Google Scholar]
- 36.García-Mochón L., Gil-Sierra M.D., Alegre-Del Rey E.J., Alarcón de la Lastra-Romero C., Sánchez-Hidalgo M. Evaluación económica y análisis de impacto presupuestario de mepolizumab en asma eosinofílica refractaria grave. Farm Hosp. 2019;43(6):187–193. doi: 10.7399/fh.11221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data were generated and derived data supporting the findings of this study are available from the corresponding author PCU upon request.