Abstract
This study measured the metabolizable energy of soybean meal (SBM) and evaluated effects of soybean meal specific enzymes supplementation in corn-soybean diets on growth performance, intestinal digestion properties and energy values of 28-day-old broilers. A total of 336 one-day-old male AA broiler chickens were distributed to 7 groups in a completely random design. The birds were given 7 diets containing 6 diets with different combined soybean meals and a fasting treatment, 8 replicates per treatment and 6 birds per replicate (Trial 1). A total of 672 one-day-old male AA broiler chickens were randomly allocated to 7 dietary treatments including a control diet and 6 diets supplemented with 300 mg/kg α-galactosidase, 200 mg/kg β-mannanase, and 300 mg/kg protease individually or in combination (Trial 2). Apparent metabolizable energy (AME) of broilers was measured from d 25 to 27 in both trial 1 and trial 2. The results showed that AME values of combined soybean meals averaged 2,894 kcal/kg. Dietary β-mannanase and protease supplementation increased body weight gain (P < 0.05) during d 0 to 14, whereas did not affect the growth performance (P > 0.05) during d 14 to 28. Addition of β-mannanase in combination with other enzymes significantly increased lipase and trypsin content (P < 0.05) in ileum. In addition, dietary β-mannanase and protease supplementation individually or in combination enhanced trypsin enzyme content in jejunum (P < 0.05). The β-mannanase enzyme enhanced villus height and villus height to crypt depth ratio (P < 0.05) of ileum compared with control diet. Moreover, supplementation of enzyme except for protease enhanced raffinose and stachyose degradation ratio (P < 0.05). Dietary β-mannanase supplementation individually or in combination enhanced AME and AMEn values (P < 0.05). This study demonstrated that dietary enzyme supplementation especially β-mannanase improved intestinal digestion properties and contributed to high energy values.
Key words: energy value, broiler, enzyme, soybean meal
INTRODUCTION
As soybean meal (SBM) dominates the protein feed market for broilers in china, there has been considerable interest in improving nutrient utilization of soybean meal in broilers. Many companies tend to add soybean hull to soybean meal to formulate combined soybean meal with a certain protein content recommended by industrial standard. However, soybean meal with hull contains an appreciable amount of non-soluble polysaccharides (NSP), especially α-galactoside and β-mannan. β-mannan is a polysaccharide containing repeated units of mannose and glucose, which participate in purposeless energy consuming immune response (Hsiao et al., 2006; Latham et al., 2018). α-Galactosidase that specific for galacto-oligosaccharides in soybean meal has been shown to increase dietary energy values (Llamas-Moya et al., 2020). The NSP in feed may reduce nutrients utilization due to physical hinderance and intestinal physiological changes. Therefore, dietary carbohydrase addition may enhance the nutrition utilization of SBM for poultry. Although protein degradability is relatively high in broiler chickens, some protein still residue in the intestinal due to antinutritional factors that inhibit digestion. Protease may enhance the mRNA abundance of oligopeptide transporter, which contributes to improving the absorption efficiency of peptide and amino acid (Zuo et al., 2015). Therefore, dietary protease is needed to supplement endogenous enzyme for efficient digestion, especially when low quality-protein is included in feed.
Complex relationship exists in various components in feed such as protein, fat, fiber and carbohydrates. Additionally, there are studies illustrated that difference in the ingredients and chemical composition could significantly influence the apparent metabolizable energy values (AME) for broiler chickens (Aderibigbe et al., 2020). Intestinal metabolism for nutrient digestion and absorption had been estimated to account for 20 to 36% of energy use in chickens (Cant et al., 1996). Therefore, exogenous enzyme was expected to release energy in substrates and provide a competitive strategy to enhance nutrient and energy utilization in soybean meal. There are many studies focused on the effects of enzyme on animals, but ultimately divided into 2 different views. Woyengo et al. (2016) reported a limited effect of multienzyme on energy digestibility and energy values. However, Meng and Slominski (2005) illustrated that multi-carbohydrase enzyme enhanced nutrient utilization probably due to cell wall degrading. Furthermore, a complex blend for multiple enzyme might depolymerize the NSP, ultimately leading to predictable enhancement in nutrient and energy utilization (Slominski, 2011). Hence, we evaluated the energy content of combined soybean meal and explored the effects of soybean meal specific enzymes on growth performance, intestinal digestion and energy utilization of 28 d broilers.
MATERIALS AND METHODS
Experiment 1: AME Measurement of Soybean Meal
Experiment 1 was conducted to determine the metabolizable energy of soybean meal. Both experiments 1 and 2 were conducted in Zhuozhou city, Hebei Province, China. The experiment procedure was approved by Animal Welfare and Ethical Committee of China Agriculture University. Soybean hull and soybean meal was purchased by local supplier. Six different combined soybean meals consisted of soybean hull (CP = 10%) and soybean meal with different CP content (LP-SBM, low protein soybean meal, CP = 43.97%; HP-SBM1, high protein soybean meal 1, CP = 47.58%; HP-SBM2, high protein soybean meal 2, CP = 47.64%). The composition and nutrient content of combined soybean meal was exhibited in Table 1. The semi-purified diets were based on combined soybean meal and glucose and formulated to contain averaged 20% CP (Table 2). A total of 336 one-day-old male AA broiler chickens were placed in an environmentally controlled room and given a common commercial diet until d 21. There were 7 different treatments that included 6 combined soybean meal diets and a fasting treatment for endogenous measurement. Each treatment comprised of 8 replicates, with 6 birds per replicate. After 3 d dietary adaption period, birds were fasted for 24 h and then fed test diets from d 25 to 27, during which feces were collected as described by Zduńczyk et al. (2020).
Table 1.
Ingredient and chemical composition of combined soybean meal in experiment 1 (%).
| Items | Combined soybean meal |
Mean | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Ingredients (as fed basis)1 | |||||||
| LP-SBM | 93.79 | 100 | |||||
| HP-SBM1 | 94.32 | 100 | |||||
| HP-SBM2 | 94.48 | 100 | |||||
| Soybean hull | 6.21 | 5.68 | 5.52 | ||||
| Total | 100 | 100 | 100 | 100 | 100 | 100 | |
| Nutrient levels (air-dry basis)2 | |||||||
| DM3 | 89.32 | 89.40 | 91.59 | 90.84 | 90.45 | 90.02 | 90.01 |
| GE (Kcal/kg) | 4,109 | 4,147 | 4,224 | 4,185 | 4,243 | 4,171 | 4,181 |
| CP | 42.29 | 43.97 | 45.31 | 45.79 | 47.58 | 47.64 | 45.43 |
| EE | 2.16 | 2.14 | 2.53 | 1.46 | 2.40 | 1.32 | 2.00 |
| CF | 9.05 | 7.58 | 5.16 | 4.46 | 3.77 | 3.14 | 5.53 |
| NDF | 16.11 | 13.60 | 10.06 | 8.97 | 8.07 | 6.96 | 10.63 |
| ADF | 10.55 | 8.57 | 5.76 | 5.00 | 4.16 | 3.39 | 6.24 |
| Ash | 5.92 | 6.07 | 6.57 | 5.92 | 6.36 | 5.99 | 6.14 |
| NFE | 29.91 | 29.64 | 32.02 | 33.22 | 30.28 | 31.99 | 31.18 |
| PS | 76.12 | 78.00 | 77.33 | 79.76 | 79.44 | 81.83 | 78.75 |
| TI (mg/kg) | 3.69 | 4.61 | 4.19 | 4.73 | 3.69 | 4.34 | 4.21 |
| Carbohydrate levels (air-dry basis)2 | |||||||
| Gal (mg/kg) | 38.07 | 29.66 | 21.72 | 22.48 | 14.46 | 13.53 | 23.32 |
| Man (mg/kg) | 3.43 | 2.14 | 9.62 | 12.30 | 11.82 | 8.97 | 8.04 |
| Lac (mg/kg) | 150.84 | 153.42 | 115.77 | 111.09 | 112.29 | 117.76 | 126.86 |
| Suc (g/kg) | 40.94 | 41.11 | 47.47 | 59.53 | 61.39 | 48.72 | 49.86 |
| Raf (g/kg) | 13.36 | 13.56 | 12.07 | 12.81 | 13.09 | 12.36 | 12.88 |
| Sta (g/kg) | 33.81 | 33.30 | 42.87 | 51.03 | 52.12 | 43.58 | 42.79 |
LP-SBM (low protein soybean meal, crude protein = 43.97%), HP-SBM1 (high protein soybean meal 1, crude protein = 47.58%), HP-SBM2 (high protein soybean meal 2, crude protein = 47.64%).
Nutrient levels were measured values.
Abbreviations: ADF, acid detergent fiber; CF, crude fiber; CP, crude protein; DM, dry matter; EE, ether extract; Gal, galactose; GE, gross energy; Lac, lactose; Man, mannose; NDF, neutral detergent fiber; NFE, nitrogen free extract; PS, protein solubility; Raf, raffinose; Sta, Stachyose; Suc, sucrose; TI, trypsin inhibitor
Table 2.
The composition of semi-purified diets containing combined soybean meal in experiment 1 (%, air-dry basis).
| Ingredients | Diets |
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Combined soybean meal | 48.78 | 46.51 | 45.45 | 44.44 | 43.48 | 42.55 |
| Glucose | 47.22 | 49.49 | 50.55 | 51.56 | 52.52 | 53.45 |
| CaHP04 | 1.70 | 1.70 | 1.70 | 1.70 | 1.70 | 1.70 |
| Limestone | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Titanium dioxide | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| NaCl | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Choline chloride | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Vitamin premix1 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Antioxidant | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| Trace element premix2 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
The Vitamin premix provided the following per kg of diets: Vitamin A, 9 500 IU; Vitamin D3, 362.5 ug; Vitamin E, 30 IU; Vitamin K3, 2.65 mg; Vitamin B1, 2 mg, Vitamin B2, 6 mg; Vitamin B6, 6 mg, Vitamin B12, 0.025 mg; Biotin, 0.0325 mg; Folic acid, 1.25 mg; Pantothenic acid, 12 mg; Nicotinic acid, 50 mg.
The trace element premix provided the following per kg of diets: Cu, 8 mg; Zn, 75 mg; Fe, 80 mg; Mn, 100 mg; Se, 0.15 mg; I, 0.35 mg.
Experiment 2: Improvement of AME Through Enzyme Supplemention
In experiment 2, a total of 672 one-day-old male AA broiler chickens were randomly assigned to 7 dietary treatments that replicate 8 times with a pen housing 12 birds. Broilers were raised under similar temperature and lighting program followed by Mohammadigheisar et al. (2018). Birds on days 1 to 14 and 14 to 28 were fed different basal diets that formulated to exceed the recommended nutrient requirements for broiler chickens (NRC, 1994) (Table 3). Diets of experiment 2 were formulated with or without α-galactosidase (300 mg/kg), β-mannanase (200 mg/kg), and protease (300 mg/kg; Table 4). Enzymes were added individually or in suitable combination without substituting any of ingredients. The fecal collection was performed for AME measurement, which was the same as experiment 1.
Table 3.
Ingredient and chemical composition of basal diet in experiment 2 (air-dry basis, %).
| Items | 1–14 d | 14–28 d |
|---|---|---|
| Ingredient | ||
| Corn | 55.00 | 61.52 |
| Soybean meal | 31.11 | 23.35 |
| Soybean oil | 2.92 | 3.12 |
| Corn gluten meal | 3.50 | 4.85 |
| Flour | 3.00 | 3.00 |
| Calcium hydrophosphate | 1.88 | 1.60 |
| Limestone | 1.00 | 0.90 |
| Zeolite powder | 0.20 | 0.20 |
| DL-Methionine | 0.20 | 0.14 |
| Lysine | 0.45 | 0.47 |
| NaCl | 0.25 | 0.35 |
| Choline chloride | 0.25 | 0.25 |
| Vitamin premix | 0.02 | 0.02 |
| Trace element premix1 | 0.20 | 0.20 |
| Antioxidants | 0.02 | 0.03 |
| Total | 100.00 | 100.00 |
| Nutrient levels2 | ||
| ME(Kcal/kg)3 | 3020 | 3120 |
| CP | 21.45 | 19.52 |
| Lys | 1.30 | 1.15 |
| Met | 0.55 | 0.47 |
| Ca | 0.90 | 0.78 |
| aP | 0.44 | 0.39 |
The trace element premix provided the following per kg of diets: Cu, 8 mg; Zn, 75 mg; Fe, 80 mg; Mn, 100 mg; Se, 0.15 mg; I, 0.35 mg.
Nutrient levels were calculated values.
Abbreviations: ME, metabolizable energy; CP, crude protein; aP, available phosphorus.
Table 4.
Dietary multi-enzyme supplementation in experiment 2.
| Treatment | Enzyme | Content (mg/kg) |
|---|---|---|
| Con | None | |
| GAS | α-galactosidase | 300 |
| MAS | β-mannanase | 200 |
| PRO | Protease | 300 |
| GAS+PRO | α-galactosidase+ Protease | 300 + 300 |
| MAS+PRO | β-mannanase+ Protease | 200 + 300 |
| GAS+MAS+PRO | α-galactosidase+β-mannanase +Protease | 300 + 200 +300 |
Abbreviations: Con, control diet; GAS, α-galactosidase; MAS, β-mannanase; PRO, Protease; GAS+PRO, α-galactosidase+Protease; MAS+PRO, β-mannanase+Protease; GAS+MAS+PRO, α-galactosidase+β-mannanase +Protease.
Chemical Analysis and Calculations
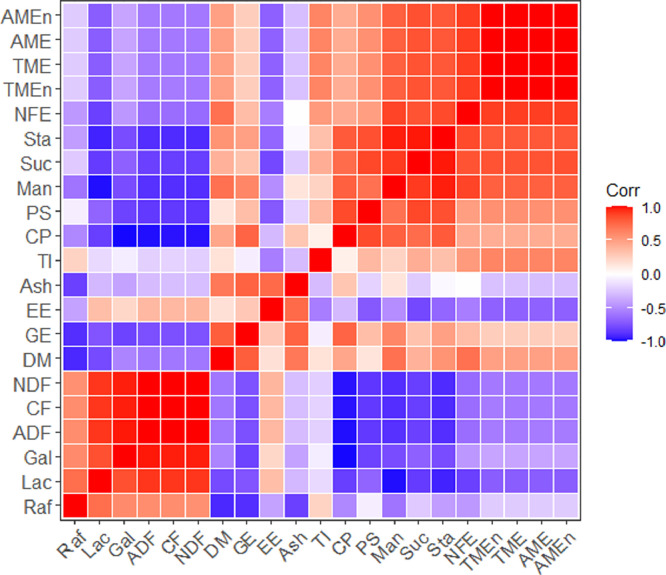
Diets and ingredients were collected and air-dried in oven at 65°C for 72 h. Feces were collected daily and subsequently frozen in −20°C before being oven dried and pooled. The diets, feces, and ingredients samples were ground finely in a centrifugal mill through a 0.75-mm screen to homogenize the material. Samples were analyzed for crude protein using Kjeldahl method with Foss KT200 and gross energy content using bomb calorimetry (IKA-C3000, Germany) on dry matter basis. Combined soybean meal was further analyzed for crude fiber (CF), Neutral detergent fiber (NDF) and acid detergent fiber (ADF) by automatic fiber analyzer (ANKOM A2000i, Parr Instrument Company, Moline, IL) according to Van Soest and Wine (1967). The nitrogen-free extract (NFE) was calculated by other nutrients: NFE (%, air-dry basis) = DM-Ash-CP-EE-CF. Protein solubility (PS) was calculated as the ratio of protein dissolved in 0.2% KOH to total protein and measured by Foss KT200. The trypsin inhibitor (TI) was measured using ELISA (eBioscience, Moline, IL) according to the method by Xue et al. (2021). The correlation of nutrients and energy values among 6 combined soybean meal was shown in Figure 1.
Figure 1.
Correlations of nutrients and energy values in 6 different combined soybean meal. Heatmap of correlation analysis showing the relationship of nutrients and energy values in 6 combined soybean meals. Positive relationship and negative relationship were shown in red and blue, respectively. Abbreviations: ADF, acid detergent fiber; AME, apparent metabolizable energy; AMEn, nitrogen corrected metabolizable energy; CF, crude fiber; CP, crude protein; Corr, correlation; DM, dry matter; EE, ether extract; Gal, galactose; GE, gross energy; Lac, lactose; Man, mannose; NDF, neutral detergent fiber; NFE, nitrogen free extract; PS, protein solubility; Raf, raffinose; Suc, sucrose; Sta, Stachyose; TI, trypsin inhibitor; TME, true metabolizable energy; TMEn, nitrogen corrected metabolizable energy.
Apparent metabolizable energy (AMEn) of diets was determined by total feces collection method and followed the study of Bourdillon et al. (1990). Nitrogen corrected AMEn of diets and ingredients were calculated using 8.22 kcal as the N correction ratio. True metabolizable energy (TME) was calculated considering endogenous energy loss, as the equation of Wu et al. (2020).
TME = (energy intake-energy excretion + endogenous energy loss) / feed intake
AME of combined soybean meals were calculated subtracting the contribution of AME from glucose (3,083.7 Kcal/kg) as followed:
AMEs represented the AME for combined soybean meal, while AMEd and AMEg was the AMEn of diet and glucose, respectively. X and Y were the percentage of glucose and combined soybean meal in diet. Apparent nutrient digestibility was calculated as followed:
Apparent nutrient digestibility = (nutrient intake- nutrient excretion) / nutrient intake. True nutrient digestibility took into account endogenous nutrient loss based on apparent nutrient digestibility, as the equation described by Parsons (1985).
Sugar degradation ratio was calculated as followed:
Sample Preparation and Analyses
Upon AME measurement completion in experiment 2, a bird from each replicate at 28 days old was weighed and euthanized for blood samples that drawn from inferior pterygoid vein and centrifuged at 3,000 r/min for 15 min. The total protein (TP), albumin (ALB), globulin (GLB), albumin to globulin (A/G), cholesterol (TC), triglyceride (TG),and uric acid (UA) of serum were analyzed using fully automatic biochemical analyzer (7020, HITACHI, Japan). Digesta was collected from jejunum and ileum by gently squeezing the content into a container, and subsequently stored in −80°C for assay. The jejunum and ileum digesta samples were homogenized in appropriate buffers and centrifuged to obtain a clear supernatant. Enzyme activities including lipase, amylase and trypsin were determined using commercially available assay kit (eBioscience, Moline, IL) according to the instructions. The mid-jejunum and mid-ileum segments were collected and flushed with phosphate buffered saline and fixed in 10% buffered formalin before stained with hematoxylin and eosin. Villi height and crypt depth were measured with 6 replicates per sample as previously described (Adebowale et al., 2019) under computer-assisted microscopy (DM750, Leica, Frankfurt, Germany).The oligosaccharides content were measured using high efficiency liquid chromatography (Agilent 1260, American) (Zhu et al., 2020).
Statistical Analysis
All data were expressed as means and analyzed via one way ANOVA using SPSS 24.0. Duncan's method was used to make multiple comparisons of main effects of enzymes. Pearson procedure of R Gui 4.1.1 was used for analyzing the relationship between nutrients and metabolizable energy. Principal-component analysis (PCA) of individuals and variables of experiment 2 were analyzed using R Gui 4.1.1. To test the hypotheses, P < 0.05 was considered significant.
RESULTS AND DISCUSSION
Experiment 1: AME Content of Soybean Meal in 25–27 d Broilers
To obtain the comprehensive energy values of soybean meal for commercial needs, we formulated combined soybean meal using soybean hull and soybean meal with different protein levels. As expected, the chemical composition among the 6 combined soybean meals was variable (Table 1). With the addition of high-fiber (43%) soybean hull in soybean meal, the fiber content increased, thereby diluting the protein content (Kornegay, 1981). The measured protein content of 6 combined soybean meals were consistent with formulated protein gradient. Crude protein of the combined soybean meal ranged from 42.29 to 47.64% averaging 45.43%, EE from 1.32 to 2.53%, gross energy from 4,109 kcal/kg to 4,243 kcal/kg, CF from 3.14 to 9.05%, NDF from 6.96 to 16.11%, and ADF from 3.39 to 10.55%. The range of nutritional value was fully expected to simulating soybean meal use in the commercial field. TI was generally considered as antinutritional factor of soybean meal and high amounts resulted in low protein and AA digestibility in soybean meal-based diets (Wedekind et al., 2020). The combined soybean meal contained considerable amounts of TI averaged 4.21 mg/kg, which was close to the range of values (3.20–5.32 mg/kg) as previously reported (Woyengo et al., 2016). The concentration of sucrose, raffinose, and stachyose in soybean meals were consistent with Baker et al. (2010) and Lopez et al. (2020). There was a positive correlation between mannose, sucrose, and stachyose, and they were negatively correlated with lactose, ADF, NDF, and CF (Figure 1).
As shown in Table 5, apparent and true digestibility of DM and CP were increased with the generally improved CP content of combined soybean meal (P < 0.05), which was consistent with Yu et al., 2020. The differences of CP content in combined soybean meals could influence digestibility because of differing proportions of endogenous N contributions (Nyachoti et al., 1997). In our study, GE content was highest in combined soybean meal 3 and 5 (P < 0.05) probably owing to lower CF and appropriate CP and EE content (Table 1). In addition, there was a negative correlation between raffinose and GE (Figure 1). It had been illustrated that raffinose could reduce nutrient digestibility and the growth performance of animals (Zeng et al., 2021). Furthermore, the DM and GE digestibility of combined soybean meal 1 were significantly decreased compared with other soybean meals. Interestingly, CP digestibility in our study was lower than that in other studies (Attia et al., 2021), but similarly to that obtained by Liu et al. (2021). Woyengo et al. (2019) reported that apparent ideal digestibility of CP was higher than apparent total tract digestibility. In addition, the different method used for CP digestibility (total collection method and indicator method) could explain the difference. The CP digestibility of combined soybean meal 1 was significantly lower than that of combined soybean meal 5 and 6 (P < 0.05) while did not show difference with combined soybean meal 2, 3, and 4. This could be linked to the lowest CP content in combined soybean meal 1 (Liu et al., 2021; McCaffertya et al., 2022). In addition, GE of soybean meal was positively related to CP (r = 0.76), which was shown in Figure 1.
Table 5.
Nutrient digestibility and energy values of diets containing combined soybean meal in broiler chickens (Experiment 1) treatment.
| Apparent digestibility (%) |
True digestibility (%) |
Energy values (kcal/kg) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DM | CP | GE | DM | CP | GE | AME | AMEn | TME | TMEn | |
| 1 | 70.77d | 47.73b | 73.24f | 74.31d | 56.20c | 75.52f | 2,742c | 2,697c | 2,826c | 2,771c |
| 2 | 72.44c | 48.80ab | 74.86e | 76.13c | 56.98bc | 77.24e | 2,774c | 2,728c | 2,862c | 2,805c |
| 3 | 74.51b | 48.31ab | 76.98d | 78.23b | 57.12bc | 79.36d | 2,862bc | 2,817bc | 2,950bc | 2,893bc |
| 4 | 75.15b | 49.49ab | 82.08a | 78.72b | 57.94abc | 83.94a | 3,204a | 3,158a | 3,292a | 3,237a |
| 5 | 76.79a | 50.32a | 78.57c | 80.43a | 59.80a | 80.98c | 2,833bc | 2,785bc | 2,919bc | 2,862bc |
| 6 | 77.24a | 50.36a | 79.93b | 80.73a | 58.96ab | 82.43b | 2,948b | 2,905b | 3,032b | 2,977b |
| SEM | 0.363 | 0.291 | 0.459 | 0.367 | 0.317 | 0.453 | 2,894 | 2,848 | 2,980 | 2,924 |
| P-value | <0.001 | 0.035 | <0.001 | <0.001 | 0.006 | <0.001 | 24.61 | 24.61 | 24.61 | 24.61 |
| Linear | <0.001 | 0.341 | <0.001 | <0.001 | 0.288 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Quadratic | <0.001 | 0.581 | <0.001 | <0.001 | 0.460 | <0.001 | 0.006 | 0.006 | 0.004 | 0.004 |
Abbreviations: AME, apparent metabolizable energy; AMEn, nitrogen corrected apparent metabolizable energy; CP, crude protein; DM, dry matter; GE, gross energy; TME, true metabolizable energy; TMEn, nitrogen corrected apparent metabolizable energy.
Means with different superscripts within the same row are significantly different (P < 0.05).
AME values among 6 combined soybean meals ranged from 2,742 Kcal/kg to 3,204 Kcal/kg, averaging 2,894 Kcal/kg (Table 5). The AMEn values were slightly lower than AME due to the nitrogen correction, and the same as TME and TMEn. Noteworthy, the AME values measured in our study were lower than that from Goebel and Stein (2011) values 3,980 Kcal/kg. Velayudhan et al. (2015) reported a higher ME value of 3,827 Kcal/kg DM for extruded-expelled soybean meal. The inconsistencies could be explained by the variations in animals, ingredients and changes during feed mixing and detection procedure. The TME values of 6 combined soybean meals evaluated varying from 2,826 to 3,292 Kcal/kg, averaging 2,980 Kcal/kg. In general, AME values of combined soybean meals were lower than TME values. The TME values could prove to be advantageous, which took metabolic fecal and endogenous urinary energy into account (Oba et al., 2019). Furthermore, the concentrations of metabolizable energy progressively increased with 6 combined soybean meal addition (P < 0.01). The energy values reached maximum values in combined soybean meal 4 (P < 0.01). The energy utilization of ingredients depended on the balance of energy-containing components and antinutritional factors that impeded absorption and utilization. Concentration of protein in combined soybean meal 4 may fulfill the essential protein and AA requirement of broilers. In addition, marked improvement in energy values after addition of enzyme may, therefore, be associated with significant changes in CP and EE digestibility. Energy values were positively correlated with mannose, sucrose, stachyose, and NFE (r = 0.78, 0.83, 0.81, and 0.90, respectively) in 6 combined soybean meals (Figure 1). NDF, ADF, and CF were negatively correlated with energy values which is shown in Figure 1. It is plausible that low NDF content in soybean meal could increase the utilization of energy values. This was linked to release of oil trapped in fibrous matrix (Kiarie et al., 2020). However, even SBM6 contained the lowest NDF content and the highest CP level, its AME was not the highest in the study. This may help explain the results that the high-protein diet could lead to wastage of resources and higher CP content was not always positively related to energy (Liu et al., 2020). The concentration of AME was approximately 28% lower in poultry than that in swine, partly attributed to low digestibility of the oligosaccharides in the gut of poultry. Therefore, exogenous enzyme supplementation could be regarded as a potential strategy to minimize the antinutritional effect of NSP and enhance energy utilization in broilers.
Experiment 2: Enzyme Supplementation Specific to Soybean Meal Improved Energy Values of Broilers
The results of growth performance on broilers at d 0 to 14 and d 14 to 28 were presented in Table 6. The feed intake and feed conversion ratio did not change as enzyme supplementation, but body weight gain at 0 to 14 d of broilers was improved (P < 0.01) in diets containing protease or β-mannanase. It had been reported that the immaturity of digestive tract in younger birds may contribute to relatively poor nutrient digestibility (Jin et al., 1998). Therefore, young birds with immature digestive systems were sensitive to dietary enzymes. Consistent with our study, the beneficial influence of diets containing β-mannanase was shown in body weight (Williams et al., 2014; Li et al., 2010). Exogenous β-mannanase could degrade β-mannan fibers, ultimately restoring and improving growth performance of animals (Vangroenweghe et al., 2021). Overall, the diets supplemented with enzyme in our experiment had little effects on performance (P > 0.05) except for BWG at 0 to 14 d. This was in line with Mohammadigheisar et al. (2018) who illustrated that the benefits of enzyme can only be fully realized upon reducing nutrient density in feed. In addition, the unaffected feed intake and feed conversion ratio as a response to enzyme supplementation were in agreement with some studies (Shalash et al., 2009; Slominski et al., 2011), whereas contrary to Toghyani et al. (2017).
Table 6.
Effect of dietary enzyme supplementation on growth performance of broiler chickens in experiment 2.
| Treatment1 | 0–14 d |
14–28 d |
||||
|---|---|---|---|---|---|---|
| BWG (g)2 | FI (g) | FCR | BWG(g) | FI (g) | FCR | |
| Con | 218c | 365 | 1.69 | 815 | 1,302 | 1.62 |
| GAS | 224bc | 371 | 1.65 | 807 | 1,308 | 1.59 |
| MAS | 227ab | 374 | 1.65 | 806 | 1,296 | 1.61 |
| PRO | 231a | 373 | 1.62 | 807 | 1,320 | 1.62 |
| GAS+PRO | 220c | 362 | 1.64 | 802 | 1,290 | 1.61 |
| MAS+PRO | 222bc | 367 | 1.65 | 818 | 1,309 | 1.60 |
| GAS+MAS+PRO | 223bc | 370 | 1.66 | 815 | 1,327 | 1.61 |
| SEM | 0.907 | 1.170 | 0.005 | 4.035 | 4.405 | 0.005 |
| P-value | 0.003 | 0.082 | 0.077 | 0.940 | 0.322 | 0.872 |
Means with different superscripts within the same row are significantly different (P < 0.05).
Con, control diet; GAS, α-galactosidase; MAS, β-mannanase; PRO, Protease; GAS+PRO, α-galactosidase+ Protease; MAS+PRO; β-mannanase+ Protease; GAS+MAS+PRO, α-galactosidase+β-mannanase +Protease.
Abbreviations: BWG, body weight gain; FI, feed intake; FCR, feed conversion ratio.
Several investigations focused on the effects of enzyme supplementation on immune response of broilers but did not find promising results (Jang et al., 2020). We hypothesized that serum parameters associated with nutrient metabolism might show differences due to enzyme supplementation. As shown in Table 7, the GLB content of serum was highest in GAS + PRO group, and lowest in MAS group (P < 0.05). However, there was no significant difference in ALB and A/G, indicating comparable immune function among different diets (Chung et al., 2020). The GAS, GAS + PRO, and MAS + PRO supplementation also increased (P < 0.05) TP content in serum. Interestingly, diets with enzyme other than GAS + MAS + PRO supplementation increased TG content in blood (P < 0.05). The improvement of TP and TG within normal range was attributed to enhancement of nutrients digestion and absorption (Wang et al., 2020). Previous study had shown that multienzymes enhanced dietary ratio of energy to protein and caused high energy excess, ultimately depositing as body fat (Musigwa et al., 2021). This may potentially explain the impact of enzymes on TG content in serum. It's noticeable that UA, a parameter of protein utilization, was not affected by protease supplementation (P > 0.05). Previous study on the effects of dietary protease on urea nitrogen resulted in contradictory (Yu et al., 2020; Zuo et al., 2015). This could be attributed to the variation of enzyme specificity, difference in diet composition and chemical characteristics existing among feed ingredients.
Table 7.
Effect of dietary enzyme supplementation on blood metabolites of broiler chickens in experiment 2.
| Treatment1 | TP (g/L)2 | ALB (g/L) | GLB (g/L) | A/G | TC (mmol/L) | TG (mmol/L) | UA (umol/L) |
|---|---|---|---|---|---|---|---|
| Con | 31.42c | 13.34 | 18.78bc | 0.67 | 4.50 | 0.66b | 448.13 |
| GAS | 33.41ab | 13.80 | 19.61abc | 0.71 | 4.47 | 0.89a | 434.88 |
| MAS | 31.27c | 12.41 | 18.54c | 0.66 | 4.48 | 0.83a | 412.50 |
| PRO | 31.71bc | 12.98 | 18.74bc | 0.69 | 4.67 | 0.85a | 459.25 |
| GAS+PRO | 33.74a | 13.55 | 20.19a | 0.66 | 4.54 | 0.84a | 475.13 |
| MAS+PRO | 34.16a | 13.69 | 19.95ab | 0.68 | 4.51 | 0.80a | 408.75 |
| GAS+MAS+PRO | 33.11abc | 13.21 | 19.90ab | 0.67 | 4.46 | 0.76ab | 434.38 |
| SEM | 0.271 | 0.135 | 0.170 | 0.006 | 0.039 | 0.019 | 10.356 |
| P-value | 0.008 | 0.087 | 0.023 | 0.178 | 0.845 | 0.019 | 0.616 |
Means with different superscripts within the same row are significantly different (P < 0.05).
Con, control diet; GAS, α-galactosidase; MAS, β-mannanase; PRO, Protease; GAS+PRO, α-galactosidase+ Protease; MAS+PRO; β-mannanase+ Protease; GAS+MAS+PRO, α-galactosidase+β-mannanase +Protease.
Abbreviations: A/G, albumin to globulin; ALB, albumin; GLB, globulin; TC, cholesterol; TG, triglyceride; TP, total protein; UA, uric acid.
The NSP was considered as constitute of cell walls that encapsulated nutrients and impeded the access of digestive enzymes to their substrates in digestive tract (Wenk, 2001). Interactions between intestinal digestion properties and energy utilization were associated with enzyme administration (Latham et al., 2018). In addition, endogenous enzymes in the small intestine adapted to the energy levels of digesta and refreshed the digestive enzyme system (Ma et al., 2020; Yang et al., 2017). The digestive enzyme activity in jejunum and ileum of broilers in response to exogenous enzyme supplementation were shown in Table 8. Numerically improvement of lipase activity (P = 0.056) that attributed to MAS supplementation was observed in jejunum. Supplementation of β-mannanase and protease individually or in combination improved trypsin activity in jejunum (P < 0.05). Furthermore, MAS+PRO and GAS + MAS + PRO added in diets significantly enhanced activity of lipase and trypsin (P < 0.05) in ileum. It had been well documented that dietary addition of carbohydrase resulted in improvement of trypsin activity and intestinal morphology, impeding diarrhea in weaned piglets (Shang et al., 2018). Protease improved protein digestibility by hydrolyzing protein to free AA and peptides that were available for absorption in the small intestine (Shahir et al., 2016). As shown in supplementary data 1, endogenous lipase and trypsin positively correlated to the energy values. These results indicated that the enzyme addition to the diets may stimulate the synthesis of endogenous digestive enzymes, which resulted in better digestion and high energy values.
Table 8.
Effect of dietary enzyme supplementation on digestive enzyme activities of broiler chickens on d 28 (Experiment 2).
| Treatment1 | Jejunal enzyme activities |
Ileal enzyme activities |
||||
|---|---|---|---|---|---|---|
| Lipase (U/gprot) | Amylase (U/mgrot) | Trypsin (U/mgrot) | Lipase (U/gprot) | Amylase (U/mgrot) | Trypsin (U/mgrot) | |
| Con | 479.34 | 35.23 | 21,684.22c | 166.23c | 37.12 | 16,204.15c |
| GAS | 427.02 | 30.52 | 20,638.12c | 202.15bc | 43.68 | 16,208.82c |
| MAS | 543.70 | 40.32 | 29,094.52a | 174.72c | 28.15 | 16,058.16c |
| PRO | 335.58 | 29.93 | 28,354.21ab | 196.96bc | 27.25 | 15,710.49c |
| GAS+PRO | 437.19 | 36.89 | 23,984.07bc | 230.25abc | 31.70 | 16,997.15bc |
| MAS+PRO | 443.05 | 33.84 | 27,404.09ab | 248.35ab | 38.84 | 19,641.32a |
| GAS+MAS+PRO | 287.28 | 36.03 | 23,301.66bc | 281.59a | 27.25 | 18,581.83ab |
| SEM | 12.880 | 1.762 | 234.690 | 9.326 | 2.327 | 330.210 |
| P-value | 0.056 | 0.771 | 0.001 | 0.003 | 0.420 | 0.003 |
Means with different superscripts within the same row are significantly different (P < 0.05).
Con, control diet; GAS, α-galactosidase; MAS, β-mannanase; PRO, Protease; GAS+PRO, α-galactosidase+ Protease; MAS+PRO; β-mannanase+ Protease; GAS+MAS+PRO, α-galactosidase+β-mannanase +Protease.
Dietary structure affected by enzyme supplementation played critical role in stimulating villi development, changing digesta passage rate, and enhancing gut motility (Sanchez et al., 2019). Dietary enzyme affected gastrointestinal physiology, mainly through the inhibition of NSP and release of available nutrient. As shown in Table 9, jejunal morphology was not affected by different enzyme supplementation (P > 0.05), but villi height, crypt depth, and VCR were all changed in ileum (P < 0.05). The villi height was increased in ileum with GAS, MAS, and GAS + MAS + PRO supplementation (P < 0.05). Improved VCR corresponding with decreased crypt depth (P < 0.05) was observed due to PRO and GAS+PRO supplementation. Furthermore, GAS+MAS+PRO supplementation decreased crypt depth while MAS increased VCR in ileum when compared with control group (P < 0.05). As expected, energy values were positively related to VCR while negatively correlated with crypt depth in ileum (Supplementary data 1.). It was well established that protease in diets could improve VCR and facilitate the intestinal development (Zuo et al., 2015). In addition, intestinal morphology was improved by carbohydrase supplementation mainly due to reduced viscosity of digesta (Jang et al., 2020). Similarly, Attia et al. (2021) also stated that the supplementation of multi-carbohydrase could additionally or synergistically improve gut microbiota and intestinal histomorphology. Overall, it appeared that addition of enzyme resulted in different degree of enhancement in ileum morphology, thus improving the intestinal integrity.
Table 9.
Effect of dietary enzyme supplementation on intestinal morphological structure of broiler chickens on d 28 (Experiment 2).
| Treatment1 | Jejunal morphology |
Ileal morphology |
||||
|---|---|---|---|---|---|---|
| VH (um)2 | CD (um) | VCR | VH (um) | CD (um) | VCR | |
| Con | 1,067.52 | 195.70 | 5.97 | 529.86c | 152.51a | 4.10b |
| GAS | 1,205.98 | 185.71 | 6.37 | 756.74a | 148.98ab | 4.52ab |
| MAS | 1,189.55 | 170.64 | 6.87 | 717.33ab | 132.17abc | 5.17a |
| PRO | 1,293.63 | 180.12 | 6.50 | 646.40abc | 126..53c | 5.22a |
| GAS+PRO | 1,100.96 | 163.58 | 6.54 | 619.51bc | 119.90c | 5.04a |
| MAS+PRO | 1,185.81 | 175.74 | 6.44 | 644.62abc | 132.26abc | 4.46ab |
| GAS+MAS+PRO | 1,192.53 | 165.42 | 7.40 | 662.24ab | 128.64bc | 4.51ab |
| SEM | 21.880 | 3.605 | 0.145 | 17.180 | 2.769 | 0.107 |
| P-value | 0.133 | 0.187 | 0.241 | 0.022 | 0.023 | 0.040 |
Means with different superscripts within the same row are significantly different (P < 0.05).
Con, control diet; GAS, α-galactosidase; MAS, β-mannanase; PRO, Protease; GAS+PRO, α-galactosidase+ Protease; MAS+PRO; β-mannanase+ Protease; GAS+MAS+PRO, α-galactosidase+β-mannanase +Protease.
Abbreviations: H, villi height; VCD, crypt depth; VCR, villus height to crypt depth ratio.
Dietary enzyme supplementation derived energy from soybean meal, through access to substrates including protein and non-starch polysaccharides. Multienzyme supplementation was reported to increase the nutrient efficiency and degradation rate of sugar thus enhancing energy values of diets (Lee et al., 2019). In our study, there were no significant effects observed in DM and CP digestibility (P > 0.05) that attributed to enzyme supplementation (Table 10). Yu et al. (2007) reported that protease supplementation was beneficial in improving nutrient digestibility, which was inconsistent with our study. Other studies illustrated that protease isolated from different microbiome could contribute to different results (Ghazi et al., 2002). Furthermore, different methods (fecal collection method and indicator method) used may explain the difference in nutrient digestibility. Compared with control diet, dietary enzyme supplementation other than protease increased the degradation rate of raffinose and stachyose (P < 0.05), and the values were higher when enzyme supplemented in combination. Diets supplemented with enzyme may increase nutrients transport efficiency, thus improving the sugar degradation rate. Gallardo et al. (2020) suggested that carbohydrase supplementation in corn-soybean based diets reduced the negative effects of structural carbohydrates. Potential interaction between structural and nonstructural carbohydrates in soybean meal may affect effective enzymatic digestion (Abella et al., 2020). Broilers fed with MAS, MAS + PRO improved (P < 0.05) the AME and AMEn values, whereas GAS+MAS+PRO only improved (P < 0.05) AME values. Results from other study demonstrated positive impacts of β-mannanase on overall broiler performance and intestinal viscosity (Latham et al., 2018). We hypothesized that reducing intestinal viscosity helped feeds to pass through the intestine and be quickly digested, thereby reducing caloric production and improving energy utilization. In addition, the higher AME content in diets supplemented with β-mannanase attributed to the hydrolysis of β-mannan to monomeric sugars and the release of the energy-yielding nutrients (Woyengo et al., 2019). However, there was no significant effect on energy values in GAS group (P > 0.05). Appropriate additive dosage of GAS should probably be reconsidered when applied to broilers. The dosage of enzymes was essential to achieve optimum response in broilers (Rao et al., 2021). As expected, the positive effects of GAS on metabolizable energy were shown when added with other enzymes. As previously mentioned, AME values of corn-soybean meal diets were increased due to dietary inclusion of NSPase (Woyengo et al., 2010). Moreover, Musigwa et al. (2021) suggested that addition of multi-carbohydrase was beneficial to diets containing low energy levels. As shown in Supplementary data 1, sugar degradation ratio was positively correlated with metabolizable values, implying a strong relationship between available nutrients and energy values. Overall, dietary supplementation with MAS+PRO and GAS+MAS+PRO was clearly distinguished with control group and achieved overall improvement on sugar degradation, intestinal health, and energy values (Supplementary data 2). Dietary supplementation of MAS individually obtained optimal intestinal health and energy values, whereas supplementation of GAS had no marked deviations compared with control diet. The lower AME values in our study as compared with NRC recommendation (3,199 Kcal/kg) may be related to the intensive selection of fast-growing birds with high efficiency. Marked increment in AME in the current study, caused by the enzyme supplementation may therefore be associated with the reduced digesta viscosity and improvement of nutrient utilization and intestinal morphology. The addition of NSP enzymes to diets accelerated feed passage rate and reduced substances residue in the gut, thereby reducing excess energy expenditure due to gut motility (Wu et al., 2004). Further study should focus on reducing intestinal maintenance energy and heat increment by NSP enzyme supplementation.
Table 10.
Effect of dietary enzyme supplementation on nutrient digestibility, sugar degradation rate and energy value of broiler chickens on d 28 (Experiment 2).
| Treatment1 | Nutrient digestibility (%) |
Sugar degradation rate (%) |
Energy value (Kcal/kg) |
||||
|---|---|---|---|---|---|---|---|
| DM2 | CP | Sucrose | Raffinose | Stachyose | AME | AMEn | |
| Con | 75.52 | 60.49 | 99.62 | 92.50d | 93.15d | 3,029b | 2,993b |
| GAS | 75.27 | 58.92 | 99.70 | 95.94bc | 96.39bc | 3,029b | 2,993b |
| MAS | 76.53 | 61.13 | 99.65 | 96.98b | 96.84b | 3,110a | 3,063a |
| PRO | 75.65 | 58.91 | 99.71 | 94.40cd | 94.38cd | 3,077ab | 3,032ab |
| GAS+PRO | 76.22 | 60.59 | 99.49 | 99.72a | 99.80a | 3,086ab | 3,041ab |
| MAS+PRO | 76.81 | 60.06 | 99.67 | 99.72a | 99.98a | 3,139a | 3,094a |
| GAS+MAS+PRO | 76.07 | 57.48 | 99.51 | 99.72a | 99.96a | 3,079a | 3,036ab |
| SEM | 0.200 | 0.381 | 0.021 | 0.497 | 0.494 | 9.08 | 8.60 |
| P-value | 0.378 | 0.203 | 0.105 | <0.001 | <0.001 | 0.006 | 0.015 |
Means with different superscripts within the same row are significantly different (P < 0.05).
Con, control diet; GAS, α-galactosidase; MAS, β-mannanase; PRO, Protease; GAS+PRO, α-galactosidase+ Protease; MAS+PRO; β-mannanase+ Protease; GAS+MAS+PRO, α-galactosidase+β-mannanase +Protease.
Abbreviations: AME, apparent metabolizable energy; AMEn, nitrogen corrected apparent metabolizable energy; CP, crude protein; DM, dry matter.
CONCLUSIONS
To conclude, the AME value of 6 combined soybean meals that averaged 2,894 Kcal/kg was positively related to content of stachyose and sucrose. Dietary supplementation of enzyme that specific to soybean meal improved sugar degradation rate, intestinal morphology and released energy without influence of growth performance. Broilers that fed with β-mannanase achieved optimal energy values, which proximately attributed to accelerated nutrients utilization and improved intestinal health. It is necessary to further investigate optimal enzyme dosage to achieve full energy values.
ACKNOWLEDGMENTS
This work was supported by China Agriculture Research System (CARS-41-G11) and by the 2115 Talent Development Program of China Agricultural University.
DISCLOSURES
The authors declare that there is no conflict of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2022.101978.
Appendix. Supplementary materials
Supplementary data 1. Principal-component analysis graph (PCA) of variables.
Variables factor map showing the relationship among AME and ileal enzyme activities, morphology, sugar degradation rate and energy values. AME values was selected from the variables as supplementary variables for exploratory data analysis. An acute angle or obtuse angle indicated a positive or negative correlation with AME, respectively. Contrib represents the contribution of a variable to the principal component.
Abbreviations: IEA_Amy, ileal enzyme activities of amylase; IEA_Try, ileal enzyme activities of trypsin; IEA_Lip, ileal enzyme activities of lipase; Star_DR, degradation ratio of stachyose; Raff_DR, degradation ratio of raffinose; SUC_DR, degradation ratio of sucrose; IM_VH, villi height in ileum; IM_CD, crypt depth in ileum, IM_VCR, villus height to crypt depth ratio in ileum.
Supplementary data 2. Principal-component analysis (PCA) graph of individuals.
PCA analysis was performed by differences in ileal enzyme activities and morphology, sugar degradation rate and energy values among 7 diets to screen out the optimal individuals.
1, CON;2, GAS; 3, MAS;4, PRO; 5, GAS+PRO; 6, MAS+PRO; 7, GAS+MAS+PRO.
REFERENCES
- Abella L.B., Agbisit E.M., Jr, Sulabo R.C. Effect of multi-enzyme supplementation on energy concentration, nutrient and fiber digestibilities and growth performance of nursery pigs fed diets with cassava meal. Philipp J. Vet. Anim. Sci. 2020;46:42–53. [Google Scholar]
- Adebowale T., Shunshun J., Yao K. The effect of dietary high energy density and carbohydrate energy ratio on digestive enzymes activity, nutrient digestibility, amino acid utilization and intestinal morphology of weaned piglets. J. Anim. Physiol. An. N. 2019;103:1492–1502. doi: 10.1111/jpn.13123. [DOI] [PubMed] [Google Scholar]
- Aderibigbe A., Cowieson A.J., Sorbara J.O., Adeola O. Growth phase and dietary alpha-amylase supplementation effects on nutrient digestibility and feedback enzyme secretion in broiler chickens. Poult. Sci. 2020;99:6867–6876. doi: 10.1016/j.psj.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia G.A., Metwally A.E., Beheiry R.R., Farahat M.H. Effect of a multicarbohydrase supplementation to diets varying in metabolisable energy level on the performance, carcase traits, caecal microbiota, intestinal morphology, and nutrient digestibility in broiler chickens. Ital. J. Anim. Sci. 2021;20:215–225. [Google Scholar]
- Baker K.M., Kim B.G., Stein H.H. Amino acid digestibility in conventional, high-protein, or low-oligosaccharide varieties of full-fat soybeans and in soybean meal by weanling pigs. Anim. Feed Sci. Tech. 2010;162:66–73. [Google Scholar]
- Bourdillon A., Carré B., Conan L., Duperray J., Huyghebaert G., Leclercq B., Lessire M., McNab J., Wiseman J. European reference method for the in vivo determination of metabolisable energy with adult cockerels: reproducibility, effect of food intake and comparison with individual laboratory methods. Br. Poult. Sci. 1990;31:557–565. doi: 10.1080/00071669008417287. [DOI] [PubMed] [Google Scholar]
- Cant J.P., McBride B.W., Croom W.J., Jr The regulation of intestinal metabolism and its impact on whole animal energetics. J. Anim. Sci. 1996;74:2541–2553. doi: 10.2527/1996.74102541x. [DOI] [PubMed] [Google Scholar]
- Gallardo C., Dadalt J.C., Trindade Neto M.A. Carbohydrases and phytase with rice bran, effects on amino acid digestibility and energy use in broiler chickens. Animal (Cambridge, England) 2020;14:482–490. doi: 10.1017/S1751731119002131. [DOI] [PubMed] [Google Scholar]
- Ghazi S., Rooke J.A., Galbraith H., Bedford M.R. The potential for the improvement of the nutritive value of soya-bean meal by different proteases in broiler chicks and broiler cockerels. Br. Poult. Sci. 2002;43:70–77. doi: 10.1080/00071660120109935. [DOI] [PubMed] [Google Scholar]
- Goebel K.P., Stein H.H. Phosphorus digestibility and energy concentration of enzyme-treated and conventional soybean meal fed to weanling pigs1. J. Anim. Sci. 2011;89:764–772. doi: 10.2527/jas.2010-3253. [DOI] [PubMed] [Google Scholar]
- Chung E.L.T., Kamalludin M.H., Jesse F.F.A., Reduan M.F.H., Ling L.W., Mahzan N.M., Henipah N.N.M.M., Loh T.C., Idrus Z. Health performance and blood profile changes in commercial broilers supplemented with dietary monocalcium phosphate. Comp. Clin. Pathol. 2020;29:573–579. [Google Scholar]
- Hsiao H.Y., Anderson D.M., Dale N.M. Levels of beta-mannan in soybean meal. Poult Sci. 2006;85:1430–1432. doi: 10.1093/ps/85.8.1430. [DOI] [PubMed] [Google Scholar]
- Jang J.C., Kim K.H., Jang Y.D., Kim Y.Y. Effects of dietary beta-mannanase supplementation on growth performance, apparent total tract digestibility, intestinal integrity, and immune responses in weaning pigs. Animals (Basel) 2020;10:703. doi: 10.3390/ani10040703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C.F., Kim J.H., Han In.K., Jung H.J., Kwon C.H. Effects of various fat sources and lecithin on the growth performance and nutrient utilization in pigs weaned at 21 days of age. AJAS. 1998;11:176–184. [Google Scholar]
- Kornegay Soybean hull digestibility by sows and feeding value for growing -finishing swine. J. Anim. Sci. 1981;53:138–145. [Google Scholar]
- Kiarie E.G., Parenteau I.A., Zhu C., Ward N.E., Cowieson A.J. Digestibility of amino acids, energy, and minerals in roasted full-fat soybean and expelled-extruded soybean meal fed to growing pigs without or with multienzyme supplement containing fiber-degrading enzymes, protease, and phytase. J. Anim. Sci. 2020;98:1–10. doi: 10.1093/jas/skaa174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham R.E., Williams M.P., Walters H.G., Carter B., Lee J.T. Efficacy of beta-mannanase on broiler growth performance and energy utilization in the presence of increasing dietary galactomannan. Poult. Sci. 2018;97:549–556. doi: 10.3382/ps/pex309. [DOI] [PubMed] [Google Scholar]
- Llamas-Moya S., Girdler C.P., Shalash S.M.M., Atta A.M., Gharib H.B., Morsy E.A., Salim H.M., Awaad M.H.H., Elmenawey M. Effect of a multicarbohydrase containing α-galactosidase enzyme on the performance, carcass yield, and humoral immunity of broilers fed corn–soybean meal–based diets of varying energy density. J. Appl. Poult. Res. 2020;29:142–151. [Google Scholar]
- Lee J.W., Patterson R., Rogiewicz A., Woyengo T.A. Nutrient digestibility of multi-enzyme supplemented low-energy and AA diets for grower pigs1. J. Anim. Sci. 2019;97:2979–2988. doi: 10.1093/jas/skz178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Chen X., Chen Y., Li Z., Cao Y. Effects of β-mannanase expressed by Pichia pastoris in corn–soybean meal diets on broiler performance, nutrient digestibility, energy utilization and immunoglobulin levels. Anim. Feed Sci. Tech. 2010;159:59–67. [Google Scholar]
- Liu W., Yan X.G., Yang H.M., Zhang X., Wu B., Yang P.L., Ban Z.B. Metabolizable and net energy values of corn stored for 3 years for laying hens. Poult. Sci. 2020;99:3914–3920. doi: 10.1016/j.psj.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Xing K., Ning R., Carné S., Wu X., Nie W. Impact of combined α-galactosidase and xylanase enzymes on growth performance, nutrients digestibility, chyme viscosity, and enzymes activity of broilers fed corn–soybean diets. J. Anim. Sci. 2021;99:1–11. doi: 10.1093/jas/skab088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez D.A., Lagos L.V., Stein H.H. Digestible and metabolizable energy in soybean meal sourced from different countries and fed to pigs. Anim. Feed Sci. Tech. 2020;268 [Google Scholar]
- Ma J., Yang T., Yang M., Yan Z., Zhao L., Yao L., Chen J., Chen Q., Tan B., Li T. Effects of dietary amylose/amylopectin ratio and amylase on growth performance, energy and starch digestibility, and digestive enzymes in broilers. J. Anim. Physiol. An. N. 2020;104:928–935. doi: 10.1111/jpn.13338. [DOI] [PubMed] [Google Scholar]
- McCaffertya K.W., Choctb M., Musigwab S., Morganb N.K., Cowiesonc A.J., Moss A.F. Protease supplementation reduced the heat increment of feed and improved energy and nitrogen partitioning in broilers fed maize-based diets with supplemental phytase and xylanase. Anim. Nutr. 2022;10:19–25. doi: 10.1016/j.aninu.2021.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X., Slominski B.A. Nutritive values of corn, soybean meal, canola meal, and peas for broiler chickens as affected by a multicarbohydrase preparation of cell wall degrading enzymes. Poult. Sci. 2005;84:1242–1251. doi: 10.1093/ps/84.8.1242. [DOI] [PubMed] [Google Scholar]
- Mohammadigheisar M., Kim H.S., Kim I.H. Effect of inclusion of lysolecithin or multi-enzyme in low energy diet of broiler chickens. J. Appl. Anim. Res. 2018;46:1198–1201. [Google Scholar]
- Musigwa S., Morgan N., Swick R.A., Cozannet P., Kheravii S.K., Wu S.B. Multi-carbohydrase enzymes improve feed energy in broiler diets containing standard or low crude protein. Anim. Nutr. 2021;7:496–505. doi: 10.1016/j.aninu.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyachoti C.M., de Lange C.F.M., Schulze H. Estimating endogenous amino acid flows at the terminal ileum and true ileal amino acid digestibilities in feedstuffs for growing pigs using the homoarginine method. J. Anim. Sci. 1997;75:3206–3213. doi: 10.2527/1997.75123206x. [DOI] [PubMed] [Google Scholar]
- Oba P.M., Utterback P.L., Parsons C.M., de Godoy M.R.C., Swanson K.S. Chemical composition, true nutrient digestibility, and true metabolizable energy of chicken-based ingredients differing by processing method using the precision-fed cecectomized rooster assay1. J. Anim. Sci. 2019;97:998–1009. doi: 10.1093/jas/sky461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson C.M. Influence of cecectomy on digestibility of amino acids by roosters fed distillers’ dried grains with solubles. J. Agric. Sci. 1985;104:469–472. [Google Scholar]
- Rao S.V.R., Raju M.V.L.N., Nagalakshmi D., Prakash B., Paul S.S. Effect of supplementation of graded concentrations of xylanase and α-amylase on performance, slaughter variables, and energy digestibility in broiler chickens fed corn-soybean meal–based diet. J. Appl. Poult. Res. 2021;30 [Google Scholar]
- Sanchez J., Thanabalan A., Khanal T., Patterson R., Slominski B.A., Kiarie E. Growth performance, gastrointestinal weight, microbial metabolites and apparent retention of components in broiler chickens fed up to 11% rice bran in a corn-soybean meal diet without or with a multi-enzyme supplement. Anim. Nutr. 2019;5:41–48. doi: 10.1016/j.aninu.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski B.A. Recent advances in research on enzymes for poultry diets. Poult. Sci. 2011;90:2013–2023. doi: 10.3382/ps.2011-01372. [DOI] [PubMed] [Google Scholar]
- Shalash S.M.M., Ali M.N., Sayed M.A.M., El-Gabry H.E., Shabaan M. Novel method for improving the utilization of corn dried distillers grains with solubles in broiler diets. Int. J. Poult. Sci. 2009;8:545–552. [Google Scholar]
- Shahir M.H., Rahimi R., Taheri H.R., Heidariniya A., Baradaran N., Asadi Kermani Z. Effect of protein source and protease addition on performance, blood metabolites and nutrient digestibility of turkeys fed on low-protein diets from 28 to 55 d post hatch. Br. Poult. Sci. 2016;57:390–396. doi: 10.1080/00071668.2016.1172202. [DOI] [PubMed] [Google Scholar]
- Shang Q.H., Ma X.K., Li M., Zhang L.H., Hu J.X., Piao X.S. Effects of α-galactosidase supplementation on nutrient digestibility, growth performance, intestinal morphology and digestive enzyme activities in weaned piglets. Anim. Feed Sci. Tech. 2018;236:48–56. [Google Scholar]
- Toghyani M., Wu S.B., Perez-Maldonado R.A., Iji P.A., Swick R.A. Performance, nutrient utilization, and energy partitioning in broiler chickens offered high canola meal diets supplemented with multicomponent carbohydrase and mono-component protease. Poult. Sci. 2017;96:3960–3972. doi: 10.3382/ps/pex212. [DOI] [PubMed] [Google Scholar]
- Van Soest P.J., Wine R.H. Use of detergents in the analysis of fibrous feeds. IV. Determination of plant cell-wall constituents. J. AOAC Int. 1967;50:50–55. [Google Scholar]
- Velayudhan D.E., Heo J.M., Nyachoti C.M. Net energy content of dry extruded-expelled soybean meal fed with or without enzyme supplementation to growing pigs as determined by indirect calorimetry. J. Anim. Sci. 2015;93:3402–3409. doi: 10.2527/jas.2014-8514. [DOI] [PubMed] [Google Scholar]
- Vangroenweghe F., Poulsen K., Thas O. Supplementation of a β-mannanase enzyme reduces post-weaning diarrhea and antibiotic use in piglets on an alternative diet with additional soybean meal. Porcine Health Manag. 2021;7:8. doi: 10.1186/s40813-021-00191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang Q., Dai C., Li J., Huang P., Li Y., Ding X., Huang J., Hussain T., Yang H. Effects of dietary energy on growth performance, carcass characteristics, serum biochemical index, and meat quality of female Hu lambs. Anim. Nutr. 2020;6:499–506. doi: 10.1016/j.aninu.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk C. The role of dietary fiber in the digestive physiology of the pig. Anim. Feed Sci. Tech. 2001;90:21–33. [Google Scholar]
- Wedekind K.J., Chen J., Yan F., Escobar J., Vazquez-Anon M. Efficacy of a mono-component protease is affected by trypsin inhibitor concentration in soybean meal. Anim. Feed Sci. Tech. 2020;265 [Google Scholar]
- Williams M.P., Brown B., Rao S., Lee J.T. Evaluation of beta-mannanase and nonstarch polysaccharide-degrading enzyme inclusion separately or intermittently in reduced energy diets fed to male broilers on performance parameters and carcass yield. J. Appl. Poult. Res. 2014;23:715–723. [Google Scholar]
- Woyengo T.A., Slominski B.A., Jones R.O. Growth performance and nutrient utilization of broiler chickens fed diets supplemented with phytase alone or in combination with citric acid and multicarbohydrase. Poult. Sci. 2010;89:2221–2229. doi: 10.3382/ps.2010-00832. [DOI] [PubMed] [Google Scholar]
- Woyengo T.A., Patterson R., Levesque C.L. Nutritive value of extruded or multi-enzyme supplemented cold-pressed soybean cake for pigs1. J. Anim. Sci. 2016;94:5230–5238. doi: 10.2527/jas.2016-0822. [DOI] [PubMed] [Google Scholar]
- Woyengo T.A., Bogota K.J., Noll S.L., Wilson J. Enhancing nutrient utilization of broiler chickens through supplemental enzymes. Poult. Sci. 2019;98:1302–1309. doi: 10.3382/ps/pey452. [DOI] [PubMed] [Google Scholar]
- Wu Y.B., Ravindran V., Thomas D.G., Birtles M.J., Hendriks W.H. Influence of phytase and xylanase, individually or in combination, on performance, apparent metabolisable energy, digestive tract measurements and gut morphology in broilers fed wheat-based diets containing adequate level of phosphorus. Br. Poult. Sci. 2004;45:76–84. doi: 10.1080/00071660410001668897. [DOI] [PubMed] [Google Scholar]
- Wu S.B., Choct M., Pesti G. Historical flaws in bioassays used to generate metabolizable energy values for poultry feed formulation: a critical review. Poult. Sci. 2020;99:385–406. doi: 10.3382/ps/pez511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue C., Li Y., Lv H., Zhang L., Bi C., Dong N., Shan A., Wang J. Oleanolic acid targets the gut-liver axis to alleviate metabolic disorders and hepatic steatosis. J. Agric. Food Chem. 2021;69:7884–7897. doi: 10.1021/acs.jafc.1c02257. [DOI] [PubMed] [Google Scholar]
- Yang J., Yang L., Wang Y., Zhai S., Wang S., Yang Z., Wang W. Effects of dietary protein and energy levels on digestive enzyme activities and electrolyte composition in the small intestinal fluid of geese. Anim. Sci. J. 2017;88:294–299. doi: 10.1111/asj.12557. [DOI] [PubMed] [Google Scholar]
- Yu B., Wu S.T., Liu C.C., Gauthier R., Chiou P.W.S. Effects of enzyme inclusion in a maize–soybean diet on broiler performance. Anim. Feed Sci. Tech. 2007;134:283–294. [Google Scholar]
- Yu J., Yu G.X., Yu B., Zhang Y., He J., Zheng P., Mao X.B., Luo J.Q., Huang Z.Q., Luoa Y.H., Yana H., Wanga Q., Wanga H., Chen D.W. Dietary protease improves growth performance and nutrient digestibility in weaned piglets fed diets with different levels of soybean meal. Livest. Sci. 2020;241:104179. [Google Scholar]
- Zduńczyk Z., Jankowski J., Mikulski D., Zduńczyk P., Juśkiewicz J., Slominski B.A. The effect of NSP-degrading enzymes on gut physiology and growth performance of turkeys fed soybean meal and peas-based diets. Anim. Feed Sci. Tech. 2020;263 [Google Scholar]
- Zeng Z., Zhang Y., He J., Yu J., Mao X., Zheng P., Luo Y., Luo J., Huang Z., Yu B., Chen D.W. Effects of soybean raffinose on growth performance, digestibility, humoral immunity and intestinal morphology of growing pigs. Anim. Nutr. 2021;7:393–399. doi: 10.1016/j.aninu.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Liu J.Z., Liu H.Y., Yang G.Q. Soybean oligosaccharide, stachyose, and raffinose in broilers diets: effects on odor compound concentration and microbiota in cecal digesta. Poult. Sci. 2020;99:3532–3539. doi: 10.1016/j.psj.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J.J., Ling B.M., Long L., Li T.J., Lahaye L., Yang C.B., Feng D.Y. Effect of dietary supplementation with protease on growth performance, nutrient digestibility, intestinal morphology, digestive enzymes and gene expression of weaned piglets. Anim. Nutr. 2015;1:276–282. doi: 10.1016/j.aninu.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data 1. Principal-component analysis graph (PCA) of variables.
Variables factor map showing the relationship among AME and ileal enzyme activities, morphology, sugar degradation rate and energy values. AME values was selected from the variables as supplementary variables for exploratory data analysis. An acute angle or obtuse angle indicated a positive or negative correlation with AME, respectively. Contrib represents the contribution of a variable to the principal component.
Abbreviations: IEA_Amy, ileal enzyme activities of amylase; IEA_Try, ileal enzyme activities of trypsin; IEA_Lip, ileal enzyme activities of lipase; Star_DR, degradation ratio of stachyose; Raff_DR, degradation ratio of raffinose; SUC_DR, degradation ratio of sucrose; IM_VH, villi height in ileum; IM_CD, crypt depth in ileum, IM_VCR, villus height to crypt depth ratio in ileum.
Supplementary data 2. Principal-component analysis (PCA) graph of individuals.
PCA analysis was performed by differences in ileal enzyme activities and morphology, sugar degradation rate and energy values among 7 diets to screen out the optimal individuals.
1, CON;2, GAS; 3, MAS;4, PRO; 5, GAS+PRO; 6, MAS+PRO; 7, GAS+MAS+PRO.