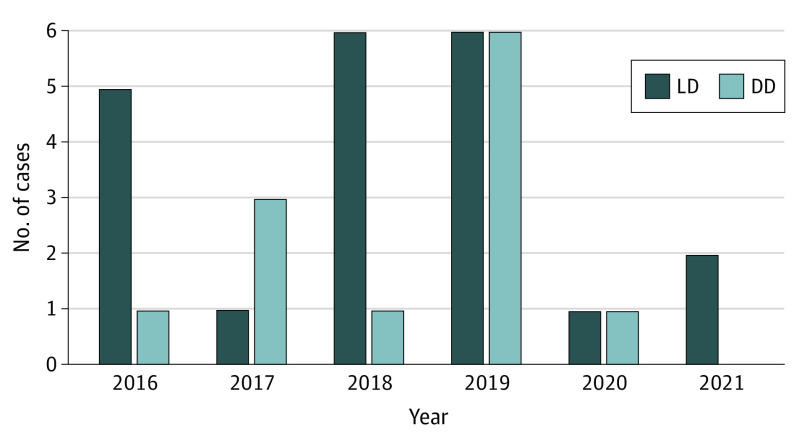Key Points
Question
What are the initial outcomes of the first 5 years of uterus transplant in the US?
Findings
In this cohort study including 33 uterus transplant recipients, US centers had high rates of technical success, with 74% graft survival (23 of 31 recipients) at 1 year. More than 80% of recipients with a viable graft at 1 year after transplant (19 of 23) achieved at least 1 live birth; uterus transplant was shown to be safe for the recipient, living donor, and child.
Meaning
Uterus transplant may be a viable method for women with uterine-factor infertility to have children.
This cohort study reviews transplant and birth outcomes of uterus transplant recipients in the US since the first case in 2016.
Abstract
Importance
Uterus transplant is a viable surgical treatment for women affected by absolute uterine-factor infertility, which affects 1 in 500 women.
Objective
To review transplant and birth outcomes of uterus transplant recipients in the US since the first case in 2016.
Design, Setting, and Participants
In this cohort study, 5 years of uterus transplant outcome data were collected from the 3 centers performing uterus transplants in the US: Baylor University Medical Center, Dallas, Texas; Cleveland Clinic, Cleveland, Ohio; and University of Pennsylvania, Philadelphia. A total of 33 women with absolute uterine-factor infertility who underwent uterus transplant between February 2016 and September 2021 were included.
Main Outcomes and Measures
Graft survival, live birth, and neonatal outcome.
Results
Of the 33 included uterus transplant recipients, 2 (6%) were Asian, 1 (3%) was Black, 1 (3%) was South Asian, and 29 (88%) were White; the mean (SD) age was 31 (4.7) years; and the mean (SD) body mass index (calculated as weight in kilograms divided by height in meters squared) was 24 (3.6). Most uterus transplant recipients (31 of 33 [94%]) had a congenitally absent uterus (Mayer-Rokitansky-Küster-Hauser syndrome), and 21 of 33 (64%) received organs from living donors. Mean (range) follow-up was 36 (1-67) months. There was no donor or recipient mortality. One-year graft survival was 74% (23 of 31 recipients). Through October 2021, 19 of 33 recipients (58%) had delivered 21 live-born children. Among recipients with a viable graft at 1 year, the proportion with a live-born child was 83% (19 of 23). The median (range) gestational age at birth of neonates was 36 weeks 6 days (30 weeks, 1 day to 38 weeks), and the median (range) birth weight was 2860 (1310-3940) g (median [range], 58th [6th-98th] percentile). No congenital malformations were detected.
Conclusions and Relevance
Uterus transplant is a surgical therapy that enables women with uterine-factor infertility to successfully gestate and deliver children. Aggregate data from US centers demonstrate safety for the recipient, living donor, and child. These data may be used to counsel women with uterine-factor infertility on treatment options.
Introduction
Uterus transplant is a treatment for women affected by absolute uterine-factor infertility (AUFI), previously considered untreatable. AUFI is a major cause of female infertility, reported to affect 1 in 500 women.1,2 Primary care clinicians are typically the front line of care when AUFI is suspected or diagnosed. Given the life-framing nature of the diagnosis, appropriate counseling is challenging. Many are told that family-building options are limited to use of a gestational carrier or adoption.3 Uterus transplant uniquely restores reproductive ability and enables gestation and childbirth.4 Increased data regarding outcomes following uterus transplant are essential to counsel individuals with AUFI to evaluate all available pathways to parenthood.
The United States Uterus Transplant Consortium (USUTC) was formed in 2019 by centers performing uterus transplant in the US: Baylor University Medical Center (BUMC), Dallas, Texas; Cleveland Clinic (CCF), Cleveland, Ohio; and the University of Pennsylvania (UPenn), Philadelphia. These centers account for approximately half of worldwide cases and more than half of live births after uterus transplant.4,5,6,7 USUTC have been instrumental in the evolution of uterus transplant worldwide in proposing nomenclature and standardizing reporting of outcomes, establishing Current Procedural Terminology codes,5 developing novel surgical techniques for deceased donor (DD)8,9,10 and fully robotic living donor (LD) hysterectomies,11 allowing early embryo transfer (ET),12 and using the superior uterine veins for graft survival and live birth (LB).13,14 Since the first US uterus transplant at CCF in 2016 and the first LB at BUMC in 2017, to our knowledge, there has yet to be a comprehensive report examining the 33 uterus transplant and 21 LBs performed in the US. The granularity of this report, summarizing the outcomes in the US over the past 5 years, will be instrumental to establish best practices for the development and growth of uterus transplant in the US and abroad.
Methods
The uterus transplant clinical research trials were approved by institutional review boards at the respective centers and registered.15,16,17 Written informed consent was obtained from all participants. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Population and Data Collection
We examined donor, recipient, and offspring outcomes following all uterus transplants performed in the US between 2016 and 2021. Participant criteria were derived from the American Society for Reproductive Medicine statement on uterus transplant.18 Center-specific criteria are detailed on ClinicalTrials.gov.15,16,17 The follow-up period was defined as time from uterus transplant to the most recent encounter. All participants underwent extensive evaluation by a multidisciplinary transplant team, including an independent LD advocate.
Outcomes
Primary recipient outcomes were patient survival, allograft survival (30 days, 1 year, and 3 years), and LB. Graft failure was defined as hysterectomy performed for any reason or death of recipient prior to birth of a child. Primary efficacy outcome was the proportion of recipients achieving at least 1 LB. Primary LD outcome was patient survival. Primary offspring outcomes were gestational age and weight percentile at delivery. Secondary outcomes included graft ischemic time (cold ischemia time [CIT]; warm ischemia time [WIT]); acute cellular rejection (ACR) seen on histologic examination of cervical biopsies; kidney function, measured by serum creatinine; menstruation; infection; clinical pregnancy19; miscarriage19; and vaginal stricture (narrowing of the vaginal anastomosis).
Statistical Analysis
Detailed data on all participants were collected and maintained by the centers. Race demographic data used for demographic purpose were self-reported, with options defined by the investigators. Coded data stripped of all protected health information from the centers were combined, and statistical analysis was performed using Stata version 15.1 (StataCorp). Log-rank statistical tests were used. Significance was set at P < .05, and where applicable, P values were 1-tailed.
Results
Transplant Volumes and Primary Outcomes
From 2016 to 2021, 33 uterus transplants were performed (22 at BUMC, 8 at CCF, and 3 at UPenn). A total of 21 donors (64%) were LDs, and 12 (36%) were DDs (Figure 1). Negative cross-matches were obtained in all cases. No mortality of recipients or LDs was recorded.
Figure 1. Annual Volume of Uterus Transplant in the US Between February 2016 and September 2021.
Living donor (LD) and deceased donor (DD) transplants are displayed separately.
Although success following uterus transplant is measured in ability to achieve an LB, technically successful transplant (viable graft at postoperative day 30)5 is an essential milestone. Per this definition, 25 of 33 uterus transplants (76%) in this cohort were technically successful. The overall 1-year graft survival rate was 74% (23 of 31; 2 recipients excluded because of recent [less than 1 year] uterus transplant), including 74% (14 of 19) after LD transplant and 75% (9 of 12) after DD transplant (Figure 2).
Figure 2. Uterus Transplants per Year in the US (2016-2021) and Uterine Graft Survival .
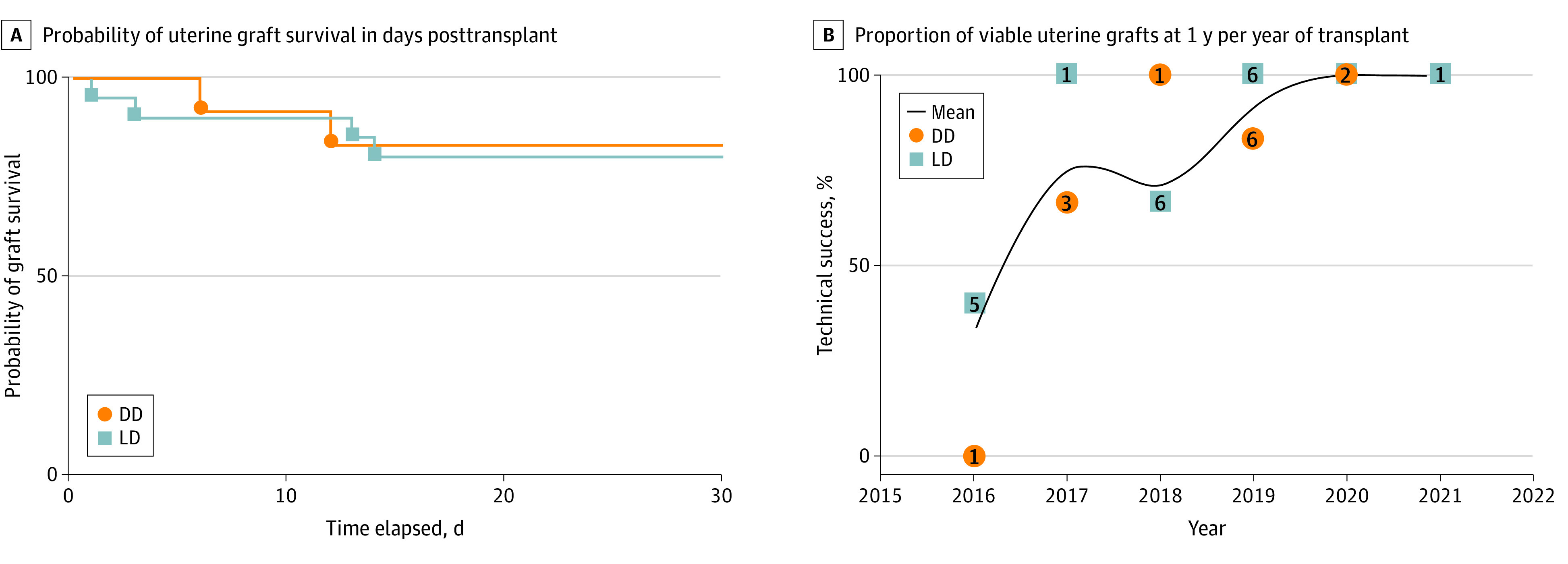
A, Probability of uterine graft survival from living donors (LDs) and deceased donors (DDs). B, Proportion of viable uterine grafts at 1 year. Numbers indicate the number of transplants per year. The trend line indicates the mean percentage of technical success for all recipients.
At the time of submission, the proportion of patients who achieved at least 1 LB following uterus transplant was 58% (19 of 33). Currently, 19 of 23 recipients (83%) with 1-year graft survival achieved at least 1 LB (7 of 9 [78%] after DD transplant and 12 of 14 [86%] after LD transplant).
Patient Characteristics
Recipients
The indication for transplant was Mayer-Rokitansky-Küster-Hauser syndrome in 31 recipients and prior hysterectomy in 2 recipients (Table). Mean (SD) recipient age at time of uterus transplant was 31 (4.7) years, and the mean (SD) body mass index (calculated as weight in kilograms divided by height in meters squared) was 24 (3.6). All recipients with Mayer-Rokitansky-Küster-Hauser syndrome used self-dilation to further develop the vaginal canal prior to surgery. Two recipients had a single kidney. Six recipients had at least 1 child (through adoption, gestational carrier, or marriage) prior to uterus transplant.
Table. Uterus Transplant Recipient and Donor Demographic Data in the US.
| Characteristic | No. (%) | |
|---|---|---|
| Recipients (n = 33) | Donors (n = 33) | |
| Indication for uterus transplant | ||
| MRKH | 31 (94) | NA |
| Hysterectomy | 2 (6) | NA |
| Age at uterus transplant, mean (SD), y | 31 (4.7) | 35 (7.3) |
| BMI at uterus transplant, mean (SD)a | 24 (3.6) | 25 (4.5) |
| Age at embryo generation, mean (SD), y | 30 (4.2) | NA |
| Race | ||
| Asian | 2 (6) | 0 |
| Black | 1 (3) | 1 (3) |
| South Asian | 1 (3) | 0 |
| White | 29 (88) | 32 (97) |
| Previous children | 6 (18) | 31 (94) |
| Parity, median (range) | 0 (0-0) | 2 (0-7) |
| Geographic region (origin) | ||
| Northeast | 6 (18) | 6 (18) |
| Southeast | 4 (12) | 2 (6) |
| West | 6 (18) | 1 (3) |
| Southwest | 12 (37) | 16 (49) |
| Midwest | 2 (6) | 8 (24) |
| International | 3 (9) | 0 |
| Donor type | ||
| Living | NA | 21 (64) |
| Deceased | NA | 12 (36)b |
Abbreviations: BMI, body mass index; MRKH, Mayer-Rokitansky-Küster-Hauser syndrome; NA, not applicable.
Calculated as weight in kilograms divided by height in meters squared.
Distance traveled for organ procurement ranged from 0 miles (when donor and recipient procedures were performed at the same center) to approximately 1100 miles.
Donors
A total of 21 uteri were from LDs and 12 were from DDs (Table). The vast majority (20 of 21 [95%]) of LDs were nondirected. The mean (SD) age at donation was 37.7 (6.5) years for LDs and 31.5 (7.6) years for DDs. The mean (SD) body mass index of all donors was 25 (4.5). Most donors (31 of 33) had 1 or more full-term LBs (median [range] of 2 [0-7] LBs). Of parous donors, 3 had a history of 1 prior cesarean delivery, and 4 had a history of 2 prior cesarean deliveries.
Surgical Parameters
The organ procurement technique in LDs was similar to a radical hysterectomy and was performed either as an open procedure (13 [62%]) or as a complete (7 [33%]) or partial (1 [5%]) robotic-assisted procedure. In a multiorgan procurement, the uterus is prepared early, and removal occurs prior to retrieval of other solid organs10 or last.9
The mean CIT was longer among DDs (332.4 minutes; 95% CI, 267.3-397.5) than in LDs (219.7 minutes; 95% CI, 188.2-251.1; P < .001). Uteri from LDs had longer mean WIT (63.3 minutes; 95% CI, 55.2-71.3) compared with uteri from DDs (47.5 minutes; 95% CI, 29.2-65.8; P = .06). WIT was greater in recipients with graft loss than in recipients with 1-year graft survival (70.8 minutes [95% CI, 49.0-92.5] vs 52.0 minutes [95% CI, 43.0-61.0]; P = .05).
The uterine arteries, with either a whole segment (12 of 12 DD grafts and 18 of 20 LD grafts) or patches (2 of 20 LD grafts) of the internal iliac arteries were used bilaterally for anastomosis. Among all transplants, 14 (43%) used a combination of superior and inferior uterine veins. Only inferior uterine veins and only superior uterine veins were used in 12 (36%) and 7 (21%), respectively. No ovarian veins were used for anastomosis.
Immunosuppression
All recipients received induction therapy with Thymoglobulin (Sanofi) and corticosteroids. At 1-year follow-up, the most common regimen was a combination of tacrolimus and azathioprine, either with (11 of 25 [44%]) or without (14 of 25 [56%]) corticosteroids. Center-specific target trough levels of tacrolimus have been previously published.20,21
Complications After Uterus Transplant
Postoperative complications occurred at a similar rate in recipients after LD and DD uterus transplant.
Graft Loss
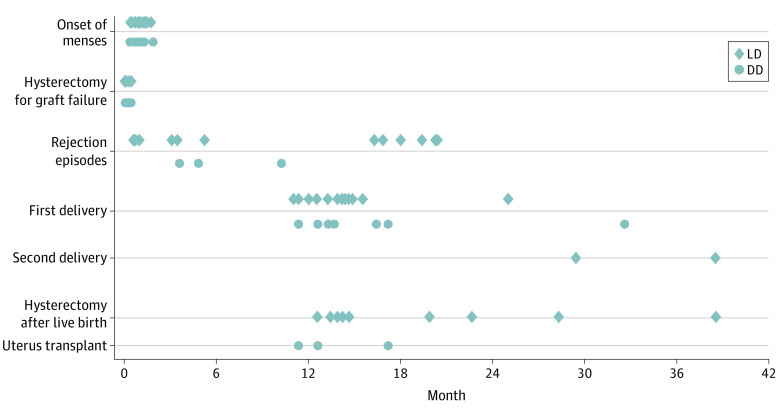
Graft loss occurred in 8 recipients (26%; LD, 5 [24%]; DD, 3 [25%]). The leading cause for graft loss was thrombosis of the graft artery or vein in the immediate postoperative period. The median (range) time to graft failure requiring hysterectomy was 7 (0-14) days (Figure 3). No graft hysterectomy has been performed with technically successful uterus transplant prior to delivery of a live-born child. Prevention to mitigate graft loss include anticoagulation, intraoperative assessment of patency of anastomotic sites by Doppler velocimetry, and ultrasonography monitoring postoperatively, as previously described20 (eTable 1 in the Supplement).
Figure 3. Timing of Events After Uterus Transplant.
Living donor (LD) and deceased donor (DD) transplants are displayed separately.
Infection
Infection occurred in 10 recipients (30%; LD, 7 [33%]; DD, 3 [25%]). Most common was urinary tract infection (4 [12%]). Two recipients (6%) developed cytomegalovirus viremia outside of pregnancy after a median (range) time of 79 (10-148) days22 (eTables 1 and 2 in the Supplement). Three recipients experienced COVID-19 infections, in one case despite receiving 2 doses of the mRNA-based vaccine. The other cases occurred in unvaccinated recipients; one before pregnancy and one during pregnancy (gestational week 22). COVID-19 infection was not associated with acute sequelae or pregnancy or delivery complications.
Kidney Function
Median (range) serum creatinine level was 0.73 (0.54-0.89) mg/dL (to convert to micromoles per liter, multiply by 88.4) before uterus transplant and 0.87 (0.49-1.04) mg/dL 6 months after graft removal with hysterectomy (P = .003). Median (range) peak serum creatinine levels during nonpregnancy and pregnancy were 1.08 (0.75-1.89) mg/dL and 1.17 (0.61-1.67) mg/dL, respectively.
Rejection Episodes
Surveillance for ACR is achieved with histologic examination of cervical biopsies.23 All centers used the same histological criteria for rejection.23 A total of 10 recipients with 1-year graft survival (43%) experienced at least 1 episode of ACR requiring treatment. The rate of ACR was 57% (8 of 14) among LDs and 22% (2 of 9) among DDs (P = .10). Most ACR episodes (9 of 10 [90%]) resolved with steroid cycles (500 to 1000 mg intravenously daily for 3 days). In 2 instances, additional treatment had to be given. In 1 recipient, ACR resolved with a course of Thymoglobulin. The other recipient showed both ACR and antibody-mediated rejection and received multimodal therapy (1000 mg of methylprednisolone [Solu-Medrol; Pfizer] followed by 5 daily doses of Thymoglobulin [1 mg/kg per dose]).20 Because of de novo class 2 donor-specific alloantibody, 5 sessions of plasmapheresis and 6 doses of intravenous immunoglobulin (0.5 g/kg) was given. Tacrolimus was increased (12 ng/mL), mycophenolate mofetil restarted, and prednisone increased (20 mg daily). ACR was seen in 2 pregnancies (9.5%) and resolved with temporary steroid treatment.
Vaginal Strictures
Stricture at the vaginal anastomosis is a common complication after uterus transplant. Methods to reduce the stricture rate is an area of active investigation.24 Visibility of and access to the cervix is important for rejection monitoring, prevention of infection, and ET. Most strictures can be resolved with self-dilation; however, severe strictures may require surgical intervention. Surgical correction is associated with genitourinary injury and should only be pursued when nonsurgical dilation fails.25 A total of 18 recipients (72%) developed vaginal strictures. Half of the strictures were managed with nonsurgical dilation, and half required surgical intervention.
Reproductive Outcome
Menses
Menstrual bleeding is the first clinical sign of graft function, as it requires regeneration of the endometrium, a patent uterine cavity, and outflow tract. The mean (range) time to the first menstrual cycle was 30 (10-59) days. The mean time to menses was not statistically significantly different following DD (29 days; 95% CI, 18.5-40.4) and LD (31 days; 95% CI, 24.4-37.1; P = .82) (Figure 3).
ET, Clinical Pregnancy, and Miscarriage
Pregnancy following uterus transplant relies on assisted reproductive technology, as the fallopian tubes are not transplanted. Prior to uterus transplant, recipients create embryos through in vitro fertilization (IVF) that can be transferred (frozen ET [FET]) after uterus transplant. Six recipients exhausted all previously frozen embryos before completing childbearing, which required additional IVF cycles after uterus transplant. A total of 23 recipients underwent a total of 59 ETs, and thus far, 19 recipients have had at least 1 live-born child (83%). The LB rate per ET in this cohort was 36% (21 of 59). First ET was performed after a mean (range) of 178 (106-335) days. Of those who had an LB, 17 of 19 (89%) achieved pregnancy after their first or second ET, and 2 (11%) required 3 or more ETs to achieve pregnancy. Of the recipients who achieved pregnancy, 5 (19%) experienced miscarriage, with most (3 [60%]) occurring during the first trimester. Miscarriages resolved without medical or surgical intervention. Four women with 1-year graft survival have yet to achieve a LB despite 4 or more (range, 4 to 8) ETs each. Four recipients who have already achieved an LB are planning to undergo additional ETs or are currently pregnant with a second child. Because of these ongoing efforts, numbers are subject to change.
Pregnancy Complications
In pregnancies resulting in an LB, the most common complication was gestational hypertension (5 of 21 pregnancies [24%]), gestational diabetes (3 [12%]), and preeclampsia (3 [12%]). None of the recipients who developed preeclampsia had a congenital kidney malformation. Complications each affecting one patient in this cohort included placenta accreta, subchorionic hematoma, preterm premature rupture of the membranes, and polyhydramnios. All pregnancy-related complications were successfully managed following standard obstetrical care.
Delivery
At the time of submission, 19 recipients with 1-year graft survival (83%) have delivered at least 1 LB child. Two recipients have delivered 2 children. The median (range) time interval from uterus transplant to first and second delivery was 14 (11-35) months and 34 (29-38) months, respectively. The median (range) time to delivery was 15 (12-35) months after DD and 14 (11-25) months after LD (P = .20) (Figure 3).
Of the 21 pregnancies, 8 (38%) were delivered as planned between 37 and 39 weeks of gestation. More than half of the neonates (13 [63%]) were delivered for obstetric or maternal indications that occurred earlier than 37 weeks. Two neonates were born very preterm (before 32 weeks’ gestation). The most common obstetric indication resulting in preterm delivery (PTD) was preeclampsia (n = 3). Additional indications for PTD included maternal acute kidney injury (n = 2), abnormal placentation (n = 1), subchorionic hematoma (n = 1), gestational hypertension (n = 1), gestational diabetes (n = 1), preterm premature rupture of the membranes (n = 1), preterm labor (n = 4), or scheduled (n = 2). All deliveries were performed by cesarean section owing to concerns for vaginal anastomosis dehiscence or damage to the neovagina and surrounding structures during labor.
Neonatal Outcomes
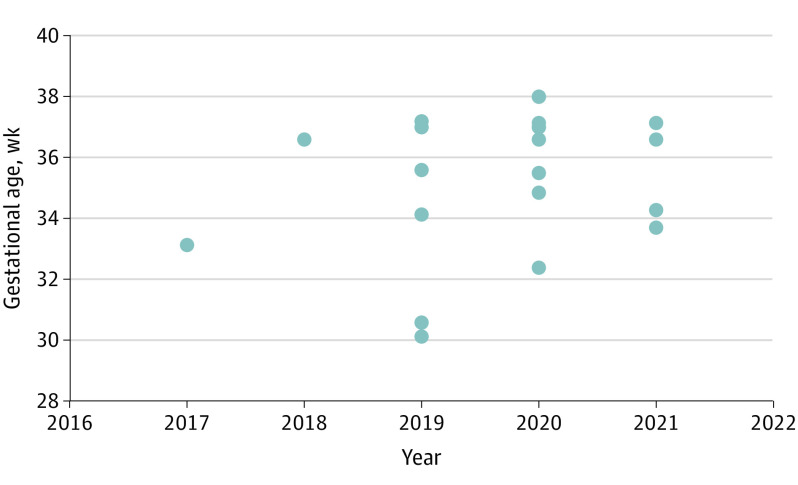
All neonates were live born at a median (range) gestational age of 36 weeks 6 days (30 weeks, 1 day to 38 weeks). More than half (11 [52%]) were born after 36 weeks’ gestation. Although the rate of very PTD (before 32 weeks’ gestation) decreased in the past 2 years, an unadjusted regression analysis confirmed that this association was not statistically significant (Figure 4). Neonates had a median (range) birth weight of 2860 (1310-3940) g (median [range], 58th [6th-98th] percentile). Nearly half of neonates (10 [47%]) spent at least 1 day in the neonatal intensive care unit (median [range] duration, 18 [2-41] days). No congenital malformations were detected. Sex was evenly distributed (10 [48%] female and 11 [52%] male).
Figure 4. Gestational Age at Delivery by Year of Delivery.
Differences in gestational age and year of delivery were not statistically significant (P = .40). Each dot represents a live birth.
Graft Hysterectomy
After a successful outcome (LB of at least 1 child), recipients undergo a graft hysterectomy. Several factors contribute to timing of hysterectomy: recipient/couple preference, complications of immunosuppression, and maternal/obstetrical complications. In this cohort, 11 grafts (57%) were removed at the time of cesarean delivery and 8 (43%) later. Graft hysterectomy following delivery of the first child was medically indicated in 11 recipients (60%) and based on patient preference in 8 recipients (40%). Medical indications for hysterectomy included acute kidney injury, preeclampsia, abnormal placentation, and gestational diabetes. The median (range) time to hysterectomy following a successful outcome was 15 (12-38) months posttransplant (Figure 4).
Discussion
The first 5 years of clinical experience of uterus transplant in the US demonstrate technical feasibility and efficacy in helping patients with AUFI to carry and deliver an LB child. In aggregate, US centers performing uterus transplant have achieved high rates of technical success (76%). Although 1-year graft survival following uterus transplant (76%) is lower than the current graft survival following other solid organ transplants (SOTs), such as kidney transplant (92%), the longevity of the field is different (less than 10 years compared with 70 years).26 In most surgical innovations, a learning curve is expected in which success rates improve and the rate of complications decreases with experience. This trend was seen in liver transplant, where 1-year survival changed from 66% in 1986 (23 years after the first transplant) to 92% in 2015.27 The learning curve in uterus transplant is already evident, with most graft failures occurring early in a center’s experience. Because all graft failures have been within 2 weeks, the data suggests that if a transplanted uterus is viable at this time, there is a high likelihood of LB (83% expected to increase as recipients continue ET attempts).
Determining an appropriate reference population in uterus transplant is challenging. Outcome data for IVF/FET cycles performed are available through the Society for Assisted Reproductive Technology’s Clinic Outcome Reporting System (SART-CORS); however, this is not linked with organ transplant data. Outcome data for pregnant SOT recipients is reported by voluntary registries, case reports, and retrospective cohort studies; however, less than 5% of these pregnancies are achieved using IVF.28 Therefore, comparisons between uterus transplant pregnancy and neonatal outcomes with these populations must be interpreted with caution.
SART-CORS reports an LB rate of 41.3% per FET in women younger than 35 years.29 Similar LB rates per FET were observed in our cohort (36%). Importantly, the proportion of recipients who have achieved LB is 83%, which is similar if not superior to the estimated cumulative LB rate for patients younger than 35 years after 6 cycles of IVF without uterus transplant (65% to 86%).30
Uterus transplant patients appear to experience increased rates of pregnancy complications, including PTD (63% vs 11% of all pregnancies). Both SOT receipt and use of IVF generally are associated with an increased risk of PTD (more than 50% in SOT recipients).31,32,33 Most PTD observed in our cohort was iatrogenic. The pathophysiology underlying spontaneous PTD remains poorly understood; possibly certain factors contributing to PTD (eg, kidney dysfunction and abnormal placentation) are shared by SOT and IVF populations, thereby putting uterus transplant recipients at even greater risk. Antenatal complications seen after SOT are often attributed to underlying medical conditions. Because uterus transplant recipients lack significant comorbidities, the rate of antenatal complications after uterus transplant may uncover mechanisms underlying complications in other pregnant SOT recipients.
Prior to this report, comparisons between LD and DD uterus transplants were limited by a modest number of uterus transplants performed worldwide and the small proportion of DD transplants. While hundreds of uterus transplant will be needed to report noninferiority in a cumulative LB rate with statistical confidence, to our knowledge, this is the first report with aggregated and robust outcome data from multiple centers using both donor types.34 Notably, there were no differences in graft survival or LB rate between donor types in this cohort. While the mean CIT was significantly longer for DD uterus transplants, this did not appear to affect clinical outcomes. In LD kidney transplants, the primary advantage over DD transplants is long-term graft function.35,36,37 An increased CIT is hypothesized to negatively affect long-term graft survival. In uterus transplant, long-term graft function is of lesser importance given the ephemeral nature.
Possible and observed complications in LDs is a concern and discussed in detail elsewhere.38 In brief, 23% of LDs experienced a grade 3 Clavien-Dindo complication, with the most common complication being ureteral injury (14%). The major disadvantage of DD is shortage of eligible donors and inability to time the operation.
Limitations
Historically, limitations of uterus transplant studies have included small cohorts and limited follow-up time. Although this report has similar limitations, it is the first comprehensive report of surgical, reproductive, and neonatal outcomes in a cohort of this size and reflects the first 5 years of complete experience with uterus transplant in the US. Future work should address fetal development and postnatal behavioral milestones, which has limited reported data to date39; follow-up of mental, physical, and psychological well-being of recipients, LDs, and children; and possible long-term complications, such as sensitization precluding future blood transfusions or organ transplants.
It is important to highlight that most of these transplants were performed as part of clinical research trials with strict participant criteria and regulatory oversight. Some individuals with AUFI are poor candidates for uterus transplant, as their risk of complications would outweigh their chance of achieving LB. Although our results cannot be generalized, these data suggest that uterus transplant is effective in achieving pregnancy and LB in selected patients with AUFI.
Conclusions
This comprehensive report of outcomes following uterus transplant in the US demonstrates that uterus transplant is safe for mother and child; success is reproducible and not limited to single centers; success is achieved with both DDs and LDs; and success rate is comparable with the most effective infertility treatments. For these reasons, uterus transplant should be considered a clinical reality in the US and presented as an option for individuals with AUFI interested in parenthood. Our data serve as a guide for health care professionals caring for patients affected by AUFI, institutions seeking to expand treatment options for patients, and patients interested in pursuing uterus transplant at a recognized center within the USUTC.
eTable 1. Patient Complication List
eTable 2. Cytomegalovirus (CMV) Viremia Infection Cases
References
- 1.Johannesson L, Järvholm S. Uterus transplantation: current progress and future prospects. Int J Womens Health. 2016;8:43-51. doi: 10.2147/IJWH.S75635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johannesson L, Kvarnström N, Mölne J, et al. Uterus transplantation trial: 1-year outcome. Fertil Steril. 2015;103(1):199-204. doi: 10.1016/j.fertnstert.2014.09.024 [DOI] [PubMed] [Google Scholar]
- 3.Richards EG, Agatisa PK, Davis AC, et al. Framing the diagnosis and treatment of absolute uterine factor infertility: insights from in-depth interviews with uterus transplant trial participants. AJOB Empir Bioeth. 2019;10(1):23-35. doi: 10.1080/23294515.2019.1572672 [DOI] [PubMed] [Google Scholar]
- 4.Jones BP, Saso S, Bracewell-Milnes T, et al. Human uterine transplantation: a review of outcomes from the first 45 cases. BJOG. 2019;126(11):1310-1319. doi: 10.1111/1471-0528.15863 [DOI] [PubMed] [Google Scholar]
- 5.Johannesson L, Testa G, Flyckt R, et al. Guidelines for standardized nomenclature and reporting in uterus transplantation: an opinion from the United States Uterus Transplant Consortium. Am J Transplant. 2020;20(12):3319-3325. doi: 10.1111/ajt.15973 [DOI] [PubMed] [Google Scholar]
- 6.Johannesson L, Wall A, Tzakis A, et al. Life underneath the VCA umbrella: perspectives from the US Uterus Transplant Consortium. Am J Transplant. 2021;21(5):1699-1704. doi: 10.1111/ajt.16445 [DOI] [PubMed] [Google Scholar]
- 7.Tummers P, Göker M, Dahm-Kahler P, et al. Meeting report: first state-of-the-art meeting on uterus transplantation. Transplantation. 2019;103(3):455-458. doi: 10.1097/TP.0000000000002561 [DOI] [PubMed] [Google Scholar]
- 8.Richards EG, Flyckt R, Tzakis A, Falcone T. Uterus transplantation: organ procurement in a deceased donor model. Fertil Steril. 2018;110(1):183. doi: 10.1016/j.fertnstert.2018.04.014 [DOI] [PubMed] [Google Scholar]
- 9.D’Amico G, Quintini C, Eghtesad B, et al. Uterus recovery from deceased donor: simple technique securing safety of vital organs and uterus graft. J Am Coll Surg. 2021;232(3):e1-e6. doi: 10.1016/j.jamcollsurg.2020.11.007 [DOI] [PubMed] [Google Scholar]
- 10.Testa G, Anthony T, McKenna GJ, et al. Deceased donor uterus retrieval: a novel technique and workflow. Am J Transplant. 2018;18(3):679-683. doi: 10.1111/ajt.14476 [DOI] [PubMed] [Google Scholar]
- 11.Johannesson L, Koon EC, Bayer J, et al. Dallas UtErus Transplant Study: early outcomes and complications of robot-assisted hysterectomy for living uterus donors. Transplantation. 2021;105(1):225-230. doi: 10.1097/TP.0000000000003211 [DOI] [PubMed] [Google Scholar]
- 12.Johannesson L, Wall A, Putman JM, Zhang L, Testa G, Diaz-Garcia C. Rethinking the time interval to embryo transfer after uterus transplantation—DUETS (Dallas UtErus Transplant Study). BJOG. 2019;126(11):1305-1309. doi: 10.1111/1471-0528.15860 [DOI] [PubMed] [Google Scholar]
- 13.Ramani A, Testa G, Ghouri Y, et al. DUETS (Dallas UtErus Transplant Study): complete report of 6-month and initial 2-year outcomes following open donor hysterectomy. Clin Transplant. 2020;34(1):e13757. doi: 10.1111/ctr.13757 [DOI] [PubMed] [Google Scholar]
- 14.Testa G, McKenna GJ, Gunby RT Jr, et al. First live birth after uterus transplantation in the United States. Am J Transplant. 2018;18(5):1270-1274. doi: 10.1111/ajt.14737 [DOI] [PubMed] [Google Scholar]
- 15.Uterine transplantation and pregnancy induction in women affected by absolute uterine infertility. ClinicalTrials.gov identifier: NCT02656550. Updated March 8, 2022. Accessed March 11, 2022. https://clinicaltrials.gov/ct2/show/NCT02656550
- 16.Uterine transplantation for the treatment of uterine factor infertility. ClinicalTrials.gov identifier: NCT02573415. Updated May 6, 2022. Accessed March 11, 2022. https://clinicaltrials.gov/ct2/show/NCT02573415
- 17.The University of Pennsylvania Uterus Transplant for Uterine Factor Infertility Trial (UNTIL) . ClinicalTrials.gov identifier: NCT03307356. Updated March 21, 2022. Accessed March 11, 2022. https://clinicaltrials.gov/ct2/show/NCT03307356
- 18.Practice Committee of the American Society for Reproductive Medicine; Practice Committee of the American Society for Reproductive Medicine . American Society for Reproductive Medicine position statement on uterus transplantation: a committee opinion. Fertil Steril. 2018;110(4):605-610. doi: 10.1016/j.fertnstert.2018.06.017 [DOI] [PubMed] [Google Scholar]
- 19.Zegers-Hochschild F, Adamson GD, Dyer S, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108(3):393-406. doi: 10.1016/j.fertnstert.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 20.Flyckt R, Falcone T, Quintini C, et al. First birth from a deceased donor uterus in the United States: from severe graft rejection to successful cesarean delivery. Am J Obstet Gynecol. 2020;223(2):143-151. doi: 10.1016/j.ajog.2020.03.001 [DOI] [PubMed] [Google Scholar]
- 21.Johannesson L, Testa G, Putman JM, et al. Twelve live births after uterus transplantation in the Dallas UtErus Transplant Study. Obstet Gynecol. 2021;137(2):241-249. doi: 10.1097/AOG.0000000000004244 [DOI] [PubMed] [Google Scholar]
- 22.Rosenzweig M, Wall A, Spak CW, Testa G, Johannesson L. Pregnancy after CMV infection following uterus transplantation: a case report from the Dallas Uterus Transplant Study. Transpl Infect Dis. 2021;23(4):e13653. doi: 10.1111/tid.13653 [DOI] [PubMed] [Google Scholar]
- 23.Agarwal A, Johannesson L, Findeis SK, et al. Clinicopathological analysis of uterine allografts including proposed scoring of ischemia reperfusion injury and T-cell-mediated rejection—Dallas UtErus Transplant Study: a pilot study. Transplantation. 2022;106(1):167-177. doi: 10.1097/TP.0000000000003633 [DOI] [PubMed] [Google Scholar]
- 24.Rehmer JM, Ferrando CA, Flyckt R, Falcone T. Techniques for successful vaginal anastomosis in the uterine transplantation patient. Fertil Steril. 2021;115(3):802-803. doi: 10.1016/j.fertnstert.2020.05.017 [DOI] [PubMed] [Google Scholar]
- 25.Chmel R, Novackova M, Janousek L, et al. Revaluation and lessons learned from the first 9 cases of a Czech uterus transplantation trial: four deceased donor and 5 living donor uterus transplantations. Am J Transplant. 2019;19(3):855-864. doi: 10.1111/ajt.15096 [DOI] [PubMed] [Google Scholar]
- 26.Foroutan F, Friesen EL, Clark KE, et al. Risk factors for 1-year graft loss after kidney transplantation: systematic review and meta-analysis. Clin J Am Soc Nephrol. 2019;14(11):1642-1650. doi: 10.2215/CJN.05560519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rana A, Ackah RL, Webb GJ, et al. No gains in long-term survival after liver transplantation over the past three decades. Ann Surg. 2019;269(1):20-27. doi: 10.1097/SLA.0000000000002650 [DOI] [PubMed] [Google Scholar]
- 28.Moritz MJ, Constantinescu S, Coscia LA. Transplant Pregnancy Registry International (TPRI) 2019 Annual Report. Transplant Pregnancy Registry International; 2020. [Google Scholar]
- 29.Society for Assisted Reproductive Technology . Final national summary report for 2019. Accessed March 3, 2022. https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx
- 30.Malizia BA, Hacker MR, Penzias AS. Cumulative live-birth rates after in vitro fertilization. N Engl J Med. 2009;360(3):236-243. doi: 10.1056/NEJMoa0803072 [DOI] [PubMed] [Google Scholar]
- 31.McKay DB, Josephson MA. Pregnancy in recipients of solid organs—effects on mother and child. N Engl J Med. 2006;354(12):1281-1293. doi: 10.1056/NEJMra050431 [DOI] [PubMed] [Google Scholar]
- 32.Blencowe H, Cousens S, Chou D, et al. ; Born Too Soon Preterm Birth Action Group . Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(suppl 1):S2. doi: 10.1186/1742-4755-10-S1-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cavoretto P, Candiani M, Giorgione V, et al. Risk of spontaneous preterm birth in singleton pregnancies conceived after IVF/ICSI treatment: meta-analysis of cohort studies. Ultrasound Obstet Gynecol. 2018;51(1):43-53. doi: 10.1002/uog.18930 [DOI] [PubMed] [Google Scholar]
- 34.Walter JR, O’Neill KE. Reproductive technology considerations in uterus transplant. Clin Obstet Gynecol. 2022;65(1):68-75. doi: 10.1097/GRF.0000000000000684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim MS, Kim DK, Myoung SM, et al. Chronologically different impacts of immunologic and non-immunologic risk factors on renal allograft function. Clin Transplant. 2005;19(6):742-750. doi: 10.1111/j.1399-0012.2005.00414.x [DOI] [PubMed] [Google Scholar]
- 36.van der Hoeven JA, Molema G, Ter Horst GJ, et al. Relationship between duration of brain death and hemodynamic (in)stability on progressive dysfunction and increased immunologic activation of donor kidneys. Kidney Int. 2003;64(5):1874-1882. doi: 10.1046/j.1523-1755.2003.00272.x [DOI] [PubMed] [Google Scholar]
- 37.Siddiqi N, McBride MA, Hariharan S. Similar risk profiles for post-transplant renal dysfunction and long-term graft failure: UNOS/OPTN database analysis. Kidney Int. 2004;65(5):1906-1913. doi: 10.1111/j.1523-1755.2004.00589.x [DOI] [PubMed] [Google Scholar]
- 38.Testa G, McKenna GJ, Bayer J, et al. The evolution of transplantation from saving lives to fertility treatment: DUETS (Dallas UtErus Transplant Study). Ann Surg. 2020;272(3):411-417. doi: 10.1097/SLA.0000000000004199 [DOI] [PubMed] [Google Scholar]
- 39.York JR, Testa G, Gunby RT, et al. Neonatal outcomes after uterus transplantation: Dallas Uterus Transplant Study. Am J Perinatol. Published online April 20, 2021. doi: 10.1055/s-0041-1727212 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Patient Complication List
eTable 2. Cytomegalovirus (CMV) Viremia Infection Cases