This cohort study explores whether pregnancy-related predisposing factors for offspring neurodevelopmental conditions are associated with maternal genetic liability for attention-deficit/hyperactivity disorder (ADHD), autism, and schizophrenia
Key Points
Question
Does maternal genetic liability for attention-deficit/hyperactivity disorder, autism, and schizophrenia predict exposure to pregnancy factors hypothesized to be causal for neurodevelopmental conditions in offspring?
Findings
In this cohort study of 14 539 mothers and 14 897 fathers, associations between polygenic scores for attention-deficit/hyperactivity disorder, autism, and schizophrenia and 37 pregnancy-related predisposing factors were assessed. Higher genetic liability in mothers was found to be modestly but robustly associated with likelihood of experiencing several of the pregnancy-related factors associated with offspring neurodevelopmental conditions.
Meaning
Observed associations between some pregnancy-related factors and offspring neurodevelopmental conditions are likely subject to genetic confounding and may not be causal.
Abstract
Importance
Several maternal exposures during pregnancy are considered predisposing factors for offspring neurodevelopmental conditions. However, many of these exposures may be noncausal and biased by maternal genetic liability.
Objective
To assess whether pregnancy-related predisposing factors for offspring neurodevelopmental conditions are associated with maternal genetic liability for attention-deficit/hyperactivity disorder (ADHD), autism, and schizophrenia and to compare associations for maternal genetic liability with those for paternal genetic liability, which could indicate that paternal exposures are not suitable negative controls for maternal exposures.
Design, Setting, and Participants
The Norwegian Mother, Father and Child Cohort Study (MoBa) is a population-based pregnancy cohort that recruited parents from June 1999 to December 2008. Polygenic scores (PGS) for ADHD, autism, and schizophrenia were derived in mothers and fathers. The associations between maternal PGS and 37 pregnancy-related measures were estimated, and these results were compared with those from paternal PGS predicting paternal measures during the mother’s pregnancy. Analysis took place between March 2021 and March 2022.
Exposures
PGS for ADHD, autism, and schizophrenia, calculated (using discovery effect size estimates and threshold of P < .05) from the largest available genome-wide association studies.
Main Outcomes and Measures
Self-reported pregnancy-related measures capturing lifestyle behaviors, metabolism, infectious and autoimmune diseases, other physical health conditions, and medication use.
Results
Data were available for up to 14 539 mothers (mean [SD] age, 30.00 [4.45] years) and 14 897 fathers (mean [SD] age, 32.46 [5.13] years) of European ancestry. Modest but robust associations were observed between specific pregnancy-related measures and maternal PGS, including ADHD PGS with asthma (odds ratio [OR], 1.15 [95% CI, 1.06-1.25]), smoking (OR, 1.26 [95% CI, 1.19-1.33]), prepregnancy body mass index (β, 0.25 [95% CI, 0.18-0.31]), pregnancy weight gain (β, 0.20 [95% CI, 0.10-0.30]), taking folate (OR, 0.92 [95% CI, 0.88-0.96]), and not taking supplements (OR, 1.09 [95% CI, 1.04-1.14]). Schizophrenia PGS was associated with coffee consumption (OR, 1.09 [95% CI, 1.05-1.12]), smoking (OR, 1.12 [95% CI, 1.06-1.19]), prepregnancy body mass index (β, −0.18 [95% CI, −0.25 to −0.11]), and pregnancy weight gain (β, 0.17 [95% CI, 0.07-0.27]). All 3 PGSs associated with symptoms of depression/anxiety (ADHD: OR, 1.15 [95% CI, 1.09-1.22]; autism: OR, 1.13 [95% CI, 1.06-1.19]; schizophrenia: OR, 1.13 [95% CI, 1.07-1.20]). Associations were largely consistent for maternal and paternal PGS, except ADHD PGS and smoking (fathers: OR, 1.13 [95% CI, 1.09-1.17]).
Conclusions and Relevance
In this study, genetic liability to neurodevelopmental conditions that is passed from mothers to children was associated with several pregnancy-related factors and may therefore confound associations between these pregnancy-related factors and offspring neurodevelopment that have previously been thought to be causal. It is crucial that future study designs account for genetic confounding to obtain valid causal inferences so that accurate advice can be given to pregnant individuals.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (hereafter, autism) are common neurodevelopmental conditions.1 Schizophrenia, although with later onset, shares many of the same features, and is hypothesized to have a neurodevelopmental origin. ADHD, autism, and schizophrenia are all highly heritable,2 although their etiology is complex and likely to include a combination of genetic, environmental, and stochastic factors.1 As development of these conditions is thought to begin early, prenatal factors have been investigated widely as potential predisposing factors, often through observational studies.3,4,5,6,7,8 However, it is unclear whether the observed associations are causal. Observed associations may reflect confounding by unknown or unmeasured shared familial factors that influence maternal exposure and offspring neurodevelopmental outcomes.9,10 Genetic confounding, when the same genetic factors are independently associated with both the exposure and outcome, has consistently been shown to explain the association between smoking during pregnancy and risk for ADHD in children using different causally informative designs.11,12,13,14,15 Determining if exposure to prenatal factors is causal is important because misleading evidence about the causes of neurodevelopmental conditions can result in unnecessary worry for pregnant individuals and hinder attention to more appropriate intervention targets.
Genome-wide association studies (GWAS) have revealed ADHD, autism, and schizophrenia to be highly polygenic.16,17,18 Individual common genetic liability can be expressed by a composite measure, called a polygenic score (PGS), summarizing the association of all the risk-increasing genetic variants identified in the GWAS.19 By using PGS for genetic liability to neurodevelopmental conditions to predict pregnancy exposures, we can test whether shared genetics transmitted from mother to offspring can partially explain associations in observational studies.20
Comparison of maternal and paternal PGS associations with the same pregnancy-related factors is informative given that paternal exposures are commonly used as negative controls to strengthen causal inference about intrauterine effects of maternal exposures.21 Under the assumption that maternal and paternal associations between exposures and neurodevelopmental outcomes in the child are similarly influenced by shared genetics between parent and offspring (and other confounding familial factors, eg, socioeconomic status), similar magnitudes of parental factor–offspring outcome associations would suggest that the maternal factor does not have a causal intrauterine effect. However, different magnitudes of association between maternal vs paternal PGS with exposures would suggest that the use of paternal exposures as a negative control may not be appropriate. For example, some negative control studies have found stronger associations between prenatal substance use (smoking, alcohol, and caffeine) and offspring ADHD for mothers compared with fathers,15,22 despite broader triangulation of evidence suggesting no causal effects.12,13,15 During pregnancy, the substance use of the pregnant individual is a more severe phenotype than that of their nonpregnant partner, owing to the strong pressure on pregnant individuals to quit. Therefore, a higher genetic liability for substance use might be required for pregnant individuals to continue to smoke compared with their partners.
In the current study, we tested for the association between maternal and paternal PGS for ADHD, autism, and schizophrenia with maternal pregnancy-related exposures and co-occurring paternal exposures in the Norwegian Mother, Father and Child Cohort Study (MoBa).23 The large sample size of mothers and fathers with genetic data allows us to include rare pregnancy-related factors such as vitamin B12 insufficiency and use of depression medication. We also compare PGS associations with maternal exposures before and during pregnancy.
Methods
Sample
We used data from MoBa, a population-based pregnancy cohort study conducted by the Norwegian Institute of Public Health.23 Participants were recruited from all over Norway from June 1999 to December 2008. Individuals were classified as mothers or fathers based on whether they returned the questionnaire for mothers or fathers. The genetic data quality control identified all of the mothers and fathers in the sample used in this article as chromosomally female and male, respectively. Mothers consented in writing to participate in 41% of the pregnancies. The cohort includes approximately 114 500 children, 95 200 mothers, and 75 200 fathers. The current study is based on version 12 of the quality-assured data files released for research in January 2019. The establishment of MoBa and initial data collection was based on a license from the Norwegian Data Protection Agency and approval from the Regional Committees for Medical and Health Research Ethics. The MoBa cohort is now based on regulations related to the Norwegian Health Registry Act. The current study was approved by the Regional Committees for Medical and Health Research Ethics (2016/1702). Blood samples were collected from both parents during pregnancy and from children (umbilical cord) at birth.24 A range of pregnancy-related factors have been captured through questionnaires and the Medical Birth Registry of Norway, a national health registry containing information about all births in Norway. Genotyping and quality control are described in eAppendix 1 in the Supplement and previously.25 Quality-controlled genotype data were available for 14 804 mothers and 15 198 fathers of European ancestry.
Polygenic Scores
Of the neurodevelopmental conditions, only ADHD and autism had available GWAS summary statistics of large sample sizes (>10 000 cases). Schizophrenia was additionally included because of its commonly shared features of atypical attention, activity and impulse regulation, social communication and behavioral flexibility, hypothesized neurodevelopmental origin, and shared observational associations with prenatal exposures and to allow comparison with results from a study in the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort.26
Maternal and paternal PGS for ADHD, autism, and schizophrenia were calculated using PRSice version 2.027 as the weighted sum of single-nucleotide variants (only common sequence variants, ie, single-nucleotide polymorphisms) associated with ADHD, autism and schizophrenia in the discovery GWAS.20 We excluded single-nucleotide variants in approximate linkage disequilibrium (r2 < 0.25 within a 500-kb sliding window) and in the major histocompatibility complex region owing to complex linkage disequilibrium structure. PGSs were adjusted for genotyping batch and the top 10 principal components to adjust for population stratification. Standardized residual scores were used in all analyses. Risk alleles were identified in GWAS for ADHD (individuals with ADHD, n = 20 183; controls, n = 35 191),16 autism (individuals with autism, n = 18 381; controls, n = 27 969)17 and schizophrenia (individuals with schizophrenia, n = 36 989; controls, n = 113 075).18 Our primary single-nucleotide variant inclusion P value threshold was less than .05, selected for comparability with relevant previous studies of these PGS.20,28 eAppendix 2 in the Supplement shows the number of single-nucleotide variants included in each PGS and histograms and correlations between PGS.
Pregnancy-Related Factors
Pregnancy-related factors were chosen after a literature review of early life exposures that have been reported to be associated with neurodevelopmental conditions (Table 1; eAppendix 3 in the Supplement). Broadly, these exposures related to maternal lifestyle and health behaviors, metabolism, immune system, other physical health conditions, and medication use. We excluded pregnancy-related factors with fewer than 100 cases based on our power calculation (eAppendix 3 in the Supplement). Where the same measures were reported by the fathers (during the mother’s pregnancy), we included these in the father’s analysis (eAppendix 3 in the Supplement).
Table 1. Sample Overview and Comparison of Maternal Pregnancy-Related Factors in the Full and Genotyped Norwegian Mother, Father and Child Cohort Study Cohort.
| Characteristic | Full sample, No. (%) | Genotyped sample, No. (%) | P valuea | ||
|---|---|---|---|---|---|
| No | Yes | No | Yes | ||
| Female child | 48 453 (51.2) | 46 180 (48.8) | 7418 (51.0) | 7113 (49) | .70 |
| Male child | 46180 (48.8) | 48453 (51.2) | 7113 (49.0) | 7418 (51.0) | .70 |
| Behavior and lifestyle | |||||
| Cigarette smoking | 78 208 (89.7) | 9008 (10.3) | 13 286 (91.6) | 1219 (8.4) | 8.38 × 10−17 |
| Alcohol consumption | 59 826 (68.6) | 27 390 (31.4) | 9921 (68.4) | 4584 (31.6) | .58 |
| Binge drinking | 74 841 (86.1) | 12 077 (13.9) | 12 365 (85.3) | 2138 (14.7) | .001 |
| Coffee consumption | 48 942 (56.3) | 37 976 (43.7) | 8302 (57.2) | 6201 (42.8) | .01 |
| Binge coffee drinking | 85 242 (98.1) | 1676 (1.9) | 14 307 (98.6) | 196 (1.4) | 3.78 × 10−8 |
| No supplements taken | 69 842 (80.4) | 17 076 (19.6) | 11 878 (81.9) | 2625 (18.1) | 3.00 × 10−7 |
| Folate supplement before pregnancy | 49 868 (57.9) | 36 211 (42.1) | 7877 (54.4) | 6616 (45.6) | 1.06 × 10−21 |
| Folate supplement during pregnancy | 17 863 (20.8) | 68 216 (79.2) | 2334 (16.1) | 12 159 (83.9) | 1.23 × 10−51 |
| Metabolic conditions | |||||
| Type 2 diabetes (including gestational diabetes) | 93 543 (98.7) | 1265 (1.3) | 14 365 (98.8) | 174 (1.2) | .13 |
| High blood pressure (including preeclampsia) | 83 138 (87.7) | 11 670 (12.3) | 12 549 (86.3) | 1990 (13.7) | 4.17 × 10−8 |
| Hyperthyroidism/hypothyroidism | 84 461 (98.1) | 1618 (1.9) | 14 252 (98.3) | 241 (1.7) | .04 |
| Infectious and autoimmune diseases | |||||
| Upper respiratory tract infections | 75 768 (86.9) | 11 448 (13.1) | 12 666 (87.3) | 1839 (12.7) | .08 |
| Lower respiratory tract infections | 84 625 (97.0) | 2591 (3) | 14 116 (97.3) | 389 (2.7) | .03 |
| Urinary tract infection | 78 843 (90.4) | 8373 (9.6) | 13 107 (90.4) | 1398 (9.6) | .88 |
| Fever | 73 832 (84.7) | 13 384 (15.3) | 12 271 (84.6) | 2234 (15.4) | .85 |
| Asthma | 83 360 (95.9) | 3558 (4.1) | 13 924 (96.0) | 579 (4) | .52 |
| Psoriasis | 84 600 (98.3) | 1479 (1.7) | 14 233 (98.2) | 260 (1.8) | .46 |
| Type 1 diabetes | 94 432 (99.6) | 376 (0.4) | 14 484 (99.6) | 55 (0.4) | .76 |
| Other autoimmune disease | 84 827 (98.5) | 1252 (1.5) | 14 276 (98.5) | 217 (1.5) | .66 |
| Other physical health conditions | |||||
| Vaginal bleeding | 94 640 (99.8) | 168 (0.2) | 14 513 (99.8) | 26 (0.2) | >.99 |
| Vitamin B12 insufficiency | 84 346 (98.0) | 1733 (2) | 14 229 (98.2) | 264 (1.8) | .08 |
| Anemia/low hemoglobin in early pregnancy | 83 601 (97.1) | 2478 (2.9) | 14 130 (97.5) | 363 (2.5) | .003 |
| Indication for medicine use | |||||
| Depression/anxiety | 77 251 (88.9) | 9667 (11.1) | 13 180 (90.9) | 1323 (9.1) | 5.42 × 10−17 |
| Depression medication | 85 868 (98.8) | 1050 (1.2) | 14 361 (99.0) | 142 (1) | .006 |
| Depression or anxiety medication | 85 627 (98.5) | 1291 (1.5) | 14 328 (98.8) | 175 (1.2) | .003 |
| Pain | 24 432 (28.1) | 62 487 (71.9) | 3946 (27.2) | 10 557 (72.8) | .008 |
| Migraine | 89 935 (94.9) | 4873 (5.1) | 13 753 (94.6) | 786 (5.4) | .12 |
| Headache | 67 347 (78.2) | 18 733 (21.8) | 11 320 (78.1) | 3173 (21.9) | .68 |
| Epilepsy | 85 816 (99.7) | 263 (0.3) | 14 456 (99.7) | 37 (0.3) | .26 |
| Pain medication | 81 093 (93.3) | 5825 (6.7) | 13 588 (93.6) | 915 (6.3) | .04 |
| Fever medication | 84 579 (98.3) | 1500 (1.7) | 14 243 (98.3) | 250 (1.7) | .89 |
| Pain or fever medication | 79 763 (91.8) | 7155 (8.2) | 13 369 (92.2) | 1134 (7.8) | .049 |
| Paracetamol use | 42 927 (49.4) | 43 991 (50.6) | 7031 (48.5) | 7472 (51.5) | .02 |
| Ibuprofen use | 80 433 (92.5) | 6485 (7.5) | 13 362 (92.1) | 1141 (7.9) | .04 |
P values from χ2 test comparing the genotyped sample with the nongenotyped sample. Multiple testing correction threshold was P < .002. Unless otherwise specified, the variables were measured during pregnancy.
Statistical Analysis
We restricted the sample to 1 observation per mother, keeping the firstborn child in MoBa. The multiple testing corrected threshold for significance was determined to be P < .002 for all analyses (eAppendix 4 in the Supplement). Analysis took place between March 2021 and March 2022.
PGS Validation
We assessed whether the ADHD PGS predicted ADHD behaviors in the MoBa parents (eAppendix 7 in the Supplement). No direct phenotypic measure of autism or schizophrenia behaviors was available for the parents. However, autism and schizophrenia PGSs have both been validated in previous samples.29,30,31
Primary Analyses
Associations of maternal ADHD, autism, and schizophrenia PGS with pregnancy-related factors were assessed using linear regression for continuous measures and logistic regression for binary outcomes in Stata version 15.1 (StataCorp). Effect estimates are presented per 1-SD increase in PGS. Analyses were repeated for paternal PGS on available paternal pregnancy-related factors.
Secondary Analyses
To assess consistency, where possible, we estimated the association between maternal neurodevelopmental PGS and relevant exposures before pregnancy and at specific trimesters of the pregnancy.
Sensitivity Analyses
PGS constructed using P value thresholds .0005, .005, .05, .10, and .50 were derived for sensitivity analyses as they provide different balance between levels of variance explained and inclusion of pleiotropic variants. We also conducted 2 analyses to investigate the potential effect of missing data. First, inverse probability weighting on missing maternal genetic data was applied to account for sampling bias in the genotyped data set (eAppendix 5 in the Supplement). Second, we used multiple imputation (n = 100) with chained equations to impute missing data in the PGS and pregnancy-related factors (eAppendix 6 in the Supplement).
Results
Sample Overview
Data were available for up to 14 539 mothers and 14 897 fathers. To account for differences between the genotyped and nongenotyped samples (Table 1) and missing data, additional analyses were conducted (eAppendices 5 and 6 in the Supplement).
Maternal PGS and Pregnancy-Related Exposures
Effect sizes for all associations of maternal PGS with maternal pregnancy-related exposures are shown in Table 2. Maternal ADHD PGS was associated with younger age at childbirth (of first included MoBa child), higher odds of smoking during pregnancy, higher body mass index (BMI) before pregnancy, and higher pregnancy weight gain. Higher maternal ADHD PGS was associated with lower odds of taking supplements (including folate) during pregnancy. Additionally, mothers with higher ADHD PGS were more likely to have asthma and depression/anxiety symptoms. There was weak evidence of association with higher odds of migraine and pain during pregnancy.
Table 2. Association of Maternal PGS for ADHD, Autism, and Schizophrenia With Pregnancy-Related Factors.
| Characteristic | No. | ADHD PGS | Autism PGS | Schizophrenia PGS | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||
| Behavior and lifestyle | |||||||
| Maternal agea | 14 532 | −0.21 (−0.28 to −0.14) | 6.18 × 10−9 | 0.08 (0.01 to 0.15) | .04 | 0.05 (−0.02 to 0.12) | .18 |
| Cigarette smoking | 14 505 | 1.26 (1.19 to 1.33) | 2.2 × 10−14 | 0.99 (0.94 to 1.05) | .78 | 1.12 (1.06 to 1.19) | 1.10 × 10−4 |
| Alcohol consumption | 14 505 | 0.96 (0.92 to 0.99) | .01 | 1.04 (1.01 to 1.08) | .02 | 1.03 (1.00 to 1.07) | .09 |
| Binge drinking | 14 503 | 0.98 (0.94 to 1.02) | .36 | 1.01 (0.96 to 1.06) | .71 | 1.05 (1.00 to 1.10) | .04 |
| Coffee consumption | 14 503 | 0.98 (0.95 to 1.02) | .29 | 1.03 (1.00 to 1.07) | .04 | 1.09 (1.05 to 1.12) | 8.92 × 10−7 |
| Binge coffee drinking | 14 503 | 1.20 (1.05 to 1.38) | .01 | 1.00 (0.87 to 1.16) | .95 | 1.15 (1.00 to 1.33) | .047 |
| No supplements taken | 14 503 | 1.09 (1.04 to 1.14) | 7.04 × 10−5 | 1.01 (0.97 to 1.05) | .59 | 0.94 (0.90 to 0.98) | .002 |
| Folate supplement before pregnancy | 14 493 | 0.96 (0.93 to 0.99) | .01 | 1.02 (0.99 to 1.06) | .20 | 1.00 (0.97 to 1.03) | .98 |
| Folate supplement during pregnancy | 14 493 | 0.92 (0.88 to 0.96) | 3.23 × 10−4 | 0.98 (0.94 to 1.03) | .44 | 1.02 (0.98 to 1.07) | .36 |
| Metabolic conditions | |||||||
| Body mass index before pregnancya | 14 166 | 0.25 (0.18 to 0.31) | 7.88 × 10−13 | 0.07 (−0.00 to 0.13) | .05 | −0.18 (−0.25 to −0.11) | 2.26 × 10−7 |
| Weight gaina | 12 268 | 0.20 (0.10 to 0.30) | 9.63 × 10−5 | 0.01 (−0.09 to 0.11) | .88 | 0.17 (0.07 to 0.27) | .001 |
| Type 2 diabetes (including gestational diabetes) | 14 539 | 0.92 (0.80 to 1.07) | .29 | 1.05 (0.91 to 1.22) | .52 | 0.97 (0.83 to 1.12) | .66 |
| High blood pressure (including preeclampsia) | 14 539 | 1.00 (0.96 to 1.05) | .92 | 0.99 (0.94 to 1.04) | .69 | 1.00 (0.96 to 1.05) | .93 |
| Hyperthyroidism/hypothyroidism | 14 493 | 1.12 (0.99 to 1.27) | .08 | 0.99 (0.88 to 1.13) | .94 | 1.02 (0.90 to 1.16) | .78 |
| Infectious and autoimmune diseases | |||||||
| Upper respiratory tract infections | 14 505 | 1.04 (0.99 to 1.09) | .13 | 1.00 (0.96 to 1.05) | .85 | 1.01 (0.96 to 1.06) | .80 |
| Lower respiratory tract infections | 14 505 | 1.02 (0.93 to 1.13) | .64 | 1.04 (0.94 to 1.15) | .40 | 1.01 (0.91 to 1.12) | .86 |
| Urinary tract infection | 14 505 | 1.06 (1.01 to 1.12) | .03 | 1.08 (1.02 to 1.14) | .005 | 1.06 (1.00 to 1.12) | .053 |
| Fever | 14 505 | 1.03 (0.98 to 1.07) | .28 | 0.99 (0.95 to 1.04) | .68 | 1.03 (0.98 to 1.07) | .26 |
| Asthma | 14 503 | 1.15 (1.06 to 1.25) | 8.59 × 10−4 | 1.07 (0.99 to 1.16) | .10 | 0.95 (0.88 to 1.04) | .26 |
| Psoriasis | 14 493 | 0.98 (0.87 to 1.11) | .79 | 1.02 (0.91 to 1.16) | .71 | 0.89 (0.79 to 1.01) | .06 |
| Type 1 diabetes | 14 539 | 0.92 (0.71 to 1.20) | .53 | 0.97 (0.74 to 1.26) | .80 | 1.01 (0.77 to 1.31) | .97 |
| Other autoimmune disease | 14 493 | 0.94 (0.82 to 1.07) | .35 | 1.04 (0.91 to 1.19) | .54 | 0.94 (0.82 to 1.07) | .35 |
| Other physical health conditions | |||||||
| Vaginal bleeding | 14 539 | 1.25 (0.85 to 1.83) | .26 | 0.84 (0.57 to 1.24) | .38 | 1.18 (0.80 to 1.73) | .41 |
| Vitamin B12 insufficiency | 14 493 | 1.03 (0.91 to 1.16) | .68 | 0.92 (0.81 to 1.04) | .17 | 1.03 (0.92 to 1.17) | .59 |
| Anemia/low hemoglobin in early pregnancy | 14 493 | 0.91 (0.82 to 1.01) | .08 | 0.98 (0.89 to 1.09) | .77 | 1.04 (0.94 to 1.16) | .42 |
| Indication for medicine use | |||||||
| Lifetime depression | 14 075 | 1.12 (1.08 to 1.17) | 3.73 × 10−9 | 1.12 (1.08 to 1.16) | 9.70 × 10−9 | 1.16 (1.11 to 1.20) | 3.11 × 10−13 |
| Depression/anxiety symptoms | 14 503 | 1.15 (1.09 to 1.22) | 5.48 × 10−7 | 1.13 (1.06 to 1.19) | 3.62 × 10−5 | 1.13 (1.07 to 1.20) | 1.71 × 10−5 |
| Depression medication | 14 503 | 1.17 (0.99 to 1.38) | .06 | 1.03 (0.87 to 1.21) | .723 | 1.43 (1.21 to 1.69) | 2.76 × 10−5 |
| Depression or anxiety medication | 14 503 | 1.11 (0.96 to 1.29) | .17 | 1.04 (0.89 to 1.20) | .63 | 1.37 (1.18 to 1.59) | 3.72 × 10−5 |
| Pain | 14 503 | 1.05 (1.02 to 1.09) | .004 | 1.01 (0.97 to 1.05) | .58 | 1.02 (0.99 to 1.06) | .19 |
| Migraine | 14 539 | 1.12 (1.04 to 1.20) | .002 | 1.10 (1.02 to 1.18) | .009 | 0.98 (0.92 to 1.06) | .68 |
| Headache | 14 493 | 1.04 (1.00 to 1.08) | .07 | 0.98 (0.94 to 1.02) | .27 | 1.01 (0.97 to 1.05) | .60 |
| Epilepsy | 14 493 | 0.82 (0.59 to 1.13) | .22 | 0.82 (0.60 to 1.14) | .24 | 0.94 (0.68 to 1.30) | .71 |
| Pain medication | 14 503 | 1.07 (1.00 to 1.15) | .04 | 1.04 (0.97 to 1.11) | .29 | 1.05 (0.98 to 1.12) | .16 |
| Paracetamol use | 14 503 | 1.03 (1.00 to 1.07) | .04 | 1.02 (0.98 to 1.05) | .31 | 0.99 (0.95 to 1.02) | .41 |
| Ibuprofen use | 14 503 | 0.99 (0.93 to 1.05) | .81 | 1.06 (1.00 to 1.12) | .07 | 0.95 (0.89 to 1.01) | .08 |
| Fever medication | 14 493 | 1.01 (0.89 to 1.14) | .88 | 1.01 (0.89 to 1.14) | .88 | 0.93 (0.82 to 1.06) | .29 |
| Pain or fever medication | 14 503 | 1.06 (0.99 to 1.12) | .08 | 1.02 (0.96 to 1.09) | .45 | 1.02 (0.96 to 1.08) | .53 |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; OR, odds ratio; PGS, polygenic scores.
Effect estimates for maternal age at birth, body mass index prepregnancy, and weight gain during pregnancy are shown as β per 1-SD increase in PGS. Multiple testing corrected P value threshold was <.002. Measures occurred during pregnancy unless otherwise specified.
Maternal autism PGS was associated with higher odds of experiencing depression/anxiety symptoms (and weak evidence for migraine and urinary tract infection) during pregnancy. There was little evidence for other associations of autism PGS with maternal health or lifestyle during pregnancy.
Maternal schizophrenia PGS was associated with higher odds of coffee consumption and cigarette smoking during pregnancy, lower prepregnancy BMI, and higher pregnancy weight gain. Schizophrenia PGS was associated with higher odds of depression/anxiety symptoms during pregnancy and of taking medication for depression/anxiety. There was only weak evidence of association between schizophrenia PGS and higher odds of taking supplements during pregnancy.
Comparison of Maternal and Paternal PGS Associations
Sixteen of the pregnancy-related exposures were also measured in fathers during the mother’s pregnancy. We compared the magnitude of the maternal exposure PGS association with the magnitude of the paternal exposure PGS association (Figure and eAppendix 8 in the Supplement). Nonoverlapping confidence intervals were observed only for 2 associations: (1) maternal ADHD PGS was associated with higher odds of maternal smoking in pregnancy compared with paternal ADHD PGS predicting odds of father smoking and (2) maternal schizophrenia PGS was associated with higher maternal coffee consumption during pregnancy, while there was no association in fathers.
Figure. Comparison of Maternal and Paternal Polygenic Score (PGS) With Pregnancy-Related Factors.
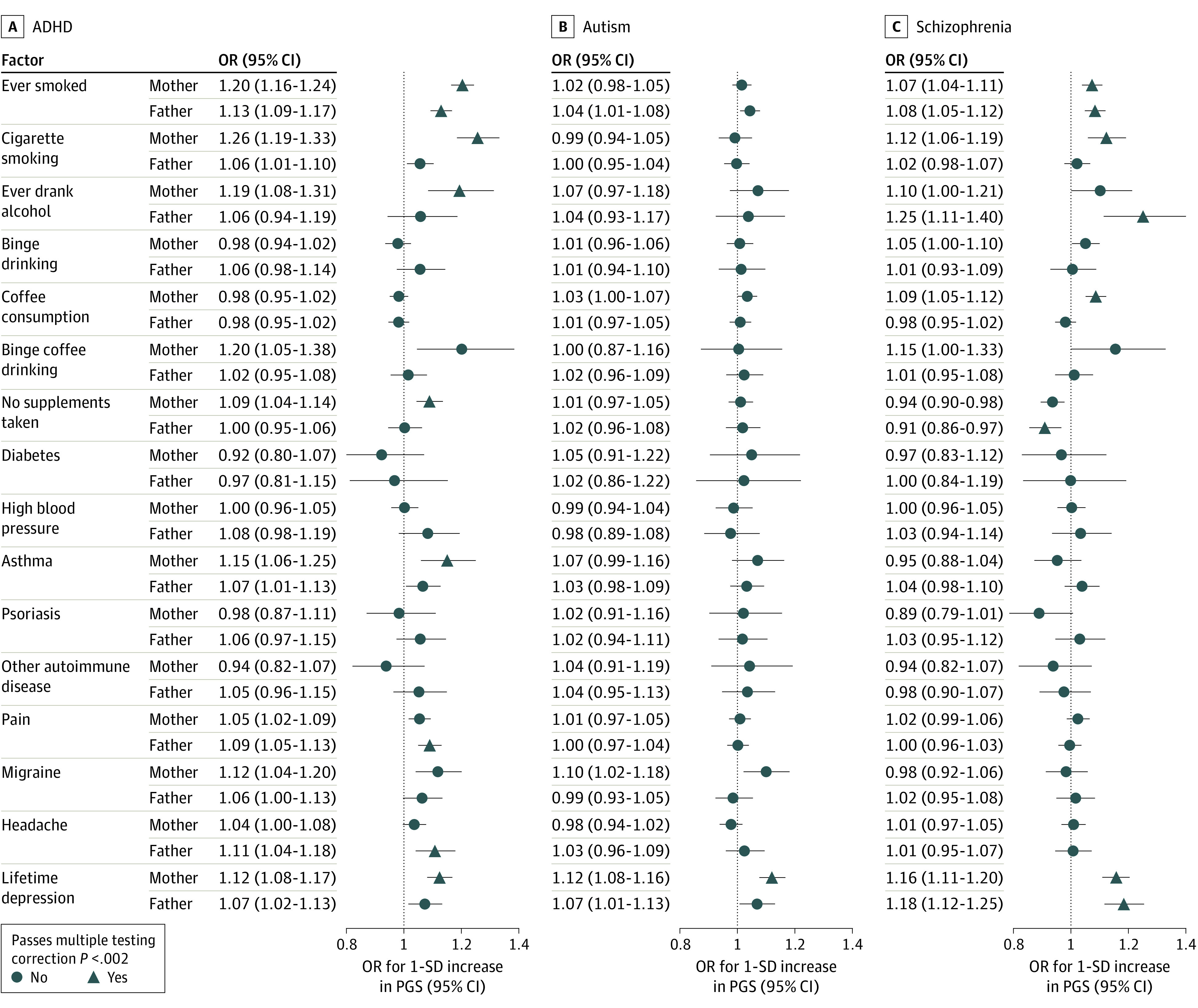
ADHD indicates attention-deficit/hyperactivity disorder; OR, odds ratio.
Secondary Analyses: Exposures Before and at Different Stages of Pregnancy
The associations of ADHD PGS with smoking, migraine, and depression before pregnancy were consistent with the associations during pregnancy (Table 3). However, mothers with higher ADHD PGS were more likely to have ever drank alcohol, whereas there was little evidence for an association with alcohol consumption during pregnancy. The associations of schizophrenia PGS with smoking and depression during pregnancy were also seen for ever smoking and lifetime depression. Autism PGS was associated with lifetime depression and depression/anxiety symptoms during pregnancy. Stratifying by trimester, differences were found for pain, with evidence that mothers with high ADHD PGS were more likely to experience pain only during the first trimester.
Table 3. Associations of Maternal PGS for ADHD, Autism, and Schizophrenia With Exposures by Timing of Exposurea.
| Characteristic | No. | ADHD PGS | Autism PGS | Schizophrenia PGS | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||
| Cigarette smoking | |||||||
| Ever | 14 252 | 1.20 (1.16-1.24) | 4.00 × 10−28 | 1.02 (0.98-1.05) | .35 | 1.07 (1.04-1.11) | 2.61 × 10−5 |
| During pregnancy | 14 505 | 1.26 (1.19-1.33) | 2.29 × 10−14 | 0.99 (0.94-1.05) | .78 | 1.12 (1.06-1.19) | 1.10 × 10−4 |
| Trimester 1 | 12 984 | 1.26 (1.19-1.34) | 2.43 × 10−13 | 0.99 (0.93-1.06) | .86 | 1.11 (1.04-1.18) | .001 |
| Trimester 2 | 13 768 | 1.33 (1.24-1.43) | 6.36 × 10−16 | 1.01 (0.94-1.08) | .76 | 1.09 (1.02-1.17) | .01 |
| Trimester 3 | 12 700 | 1.28 (1.19-1.38) | 7.36 × 10−11 | 1.00 (0.93-1.08) | .95 | 1.03 (0.96-1.11) | .43 |
| Alcohol consumption | |||||||
| Ever | 14 182 | 1.19 (1.08-1.31) | 2.93 × 10−4 | 1.07 (0.97-1.18) | .15 | 1.10 (1.00-1.21) | .048 |
| During pregnancy | 14 505 | 0.96 (0.92-0.99) | .01 | 1.04 (1.01-1.08) | .02 | 1.03 (1.00-1.07) | .09 |
| Trimester 1 | 14 495 | 0.95 (0.92-0.99) | .006 | 1.05 (1.02-1.09) | .004 | 1.03 (1.00-1.07) | .07 |
| Trimester 2 | 13 881 | 0.99 (0.93-1.05) | .67 | 1.07 (1.01-1.14) | .02 | 1.00 (0.94-1.06) | >.99 |
| Trimester 3 | 13 105 | 0.96 (0.91-1.01) | .09 | 1.06 (1.00-1.11) | .04 | 1.02 (0.96-1.07) | .58 |
| High blood pressure | |||||||
| Before pregnancy | 13 664 | 1.02 (0.97-1.07) | .48 | 1.06 (1.01-1.12) | .02 | 1.02 (0.97-1.08) | .39 |
| During pregnancy (including preeclampsia) | 14 539 | 1.00 (0.96-1.05) | .92 | 0.99 (0.94-1.04) | .69 | 1.00 (0.96-1.05) | .93 |
| Trimester 1 | 14 493 | 1.01 (0.85-1.20) | .92 | 1.07 (0.90-1.26) | .47 | 1.02 (0.86-1.22) | .80 |
| Trimester 2 | 13 498 | 1.06 (0.98-1.14) | .17 | 0.96 (0.88-1.04) | .27 | 0.98 (0.91-1.06) | .68 |
| Trimester 3 | 13 105 | 0.99 (0.94-1.04) | .68 | 1.00 (0.95-1.05) | .93 | 1.02 (0.97-1.08) | .44 |
| Vaginal bleeding | |||||||
| During pregnancy | 14 539 | 1.25 (0.85-1.83) | .26 | 0.84 (0.57-1.24) | .38 | 1.18 (0.80-1.73) | .41 |
| Trimester 1 | 14 539 | 1.06 (0.97-1.16) | .21 | 1.00 (0.92-1.10) | .94 | 1.00 (0.92-1.10) | .94 |
| Trimester 2 | 14 539 | 1.05 (0.91-1.21) | .51 | 1.10 (0.95-1.26) | .19 | 1.06 (0.92-1.23) | .40 |
| Trimester 3 | 14 539 | 0.93 (0.77-1.11) | .43 | 1.04 (0.86-1.24) | .71 | 0.96 (0.80-1.16) | .70 |
| Urinary tract infections | |||||||
| Before pregnancy | 14 493 | 1.01 (0.98-1.05) | .50 | 1.01 (0.97-1.05) | .61 | 1.02 (0.98-1.06) | .30 |
| During pregnancy | 14 505 | 1.06 (1.01-1.12) | .02 | 1.08 (1.02-1.14) | .005 | 1.06 (1.00-1.12) | .053 |
| Trimester 1 | 14 493 | 1.04 (0.97-1.11) | .29 | 1.08 (1.01-1.15) | .03 | 1.03 (0.96-1.10) | .39 |
| Trimester 2 | 13 881 | 1.09 (1.01-1.18) | .03 | 1.12 (1.04-1.21) | .004 | 1.09 (1.01-1.18) | .03 |
| Trimester 3 | 13 881 | 1.05 (0.88-1.24) | .61 | 1.07 (0.90-1.27) | .44 | 1.15 (0.96-1.37) | .12 |
| Fever | |||||||
| During pregnancy | 14 505 | 1.03 (0.98-1.07) | .28 | 0.99 (0.95-1.04) | .68 | 1.03 (0.98-1.07) | .26 |
| Trimester 1 | 14 493 | 1.03 (0.94-1.14) | .50 | 1.04 (0.94-1.14) | .48 | 1.11 (1.00-1.22) | .04 |
| Trimester 2 | 14 503 | 1.02 (0.97-1.07) | .54 | 1.00 (0.95-1.05) | .87 | 1.01 (0.96-1.06) | .69 |
| Trimester 3 | 13 128 | 1.12 (1.01-1.25) | .04 | 0.98 (0.88-1.09) | .66 | 1.04 (0.93-1.16) | .50 |
| Pain | |||||||
| During pregnancy | 14 503 | 1.05 (1.02-1.09) | .004 | 1.01 (0.97-1.05) | .58 | 1.02 (0.99-1.06) | .19 |
| Trimester 1 | 14 493 | 1.06 (1.02-1.09) | .001 | 1.03 (0.99-1.06) | .11 | 1.01 (0.98-1.05) | .55 |
| Trimester 2 | 14 503 | 0.91 (0.82-1.00) | .053 | 1.01 (0.91-1.11) | .91 | 0.99 (0.90-1.10) | .90 |
| Trimester 3 | 13 881 | 1.03 (1.00-1.07) | .07 | 1.01 (0.97-1.04) | .73 | 1.03 (1.00-1.07) | .07 |
| Migraine | |||||||
| Ever | 14 493 | 1.08 (1.03-1.14) | .004 | 1.05 (1.00-1.11) | .055 | 0.99 (0.94-1.04) | .65 |
| Before pregnancy | 14 539 | 1.08 (1.02-1.13) | .007 | 1.05 (1.00-1.11) | .06 | 0.97 (0.92-1.03) | .34 |
| During pregnancy | 14 539 | 1.12 (1.04-1.20) | .002 | 1.10 (1.02-1.18) | .009 | 0.98 (0.92-1.06) | .68 |
| Depression/anxiety symptoms | |||||||
| During pregnancy | 14 503 | 1.15 (1.09-1.22) | 5.48 × 10−7 | 1.13 (1.06-1.19) | 3.62 × 10−5 | 1.13 (1.07-1.20) | 1.71 × 10−5 |
| Trimester 1 | 14 392 | 1.15 (1.08-1.22) | 2.98 × 10−5 | 1.14 (1.07-1.21) | 9.10 × 10−5 | 1.12 (1.05-1.20) | 4.22 × 10−4 |
| Trimester 2-3 | 13 826 | 1.15 (1.06-1.24) | 8.29 × 10−4 | 1.13 (1.04-1.23) | .002 | 1.12 (1.03-1.22) | .006 |
| Depression medication | |||||||
| During pregnancy | 14 503 | 1.17 (0.99-1.38) | .06 | 1.03 (0.87-.21) | .72 | 1.43 (1.21-1.69) | 2.76 × 10−5 |
| Trimester 1 | 14 493 | 1.16 (0.97-1.39) | .11 | 1.04 (0.87-1.25) | .65 | 1.46 (1.21-1.75) | 6.47 × 10−5 |
| Trimester 2 | 14 503 | 1.32 (1.04-1.66) | .02 | 1.00 (0.79-1.26) | .995 | 1.32 (1.04-1.68) | .02 |
| Paracetamol use | |||||||
| During pregnancy | 14 503 | 1.03 (1.00-1.07) | .04 | 1.02 (0.98-1.05) | .31 | 0.99 (0.95-1.02) | .41 |
| Trimester 1-2 | 14 493 | 1.03 (0.99-1.06) | .11 | 1.01 (0.98-1.05) | .41 | 0.98 (0.95-1.01) | .24 |
| Trimester 2-3 | 13 881 | 1.03 (0.99-1.07) | .19 | 1.00 (0.96-1.04) | .91 | 1.00 (0.96-1.04) | .91 |
| Ibuprofen use | |||||||
| During pregnancy | 14 503 | 0.99 (0.93-1.05) | .81 | 1.06 (1.00-1.12) | .07 | 0.95 (0.89-1.01) | .08 |
| Trimester 1-2 | 14 493 | 0.99 (0.93-1.06) | .85 | 1.07 (1.00-1.13) | .04 | 0.96 (0.90-1.02) | .17 |
| Trimester 2-3 | 13 881 | 0.94 (0.78-1.13) | .51 | 0.85 (0.70-1.02) | .09 | 0.87 (0.72-1.06) | .17 |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; OR, odds ratio; PGS, polygenic scores.
Multiple testing corrected P < .002. Trimesters were defined as 0-12 weeks, first trimester; 13-28 weeks, second trimester; and ≥29 weeks, third trimester.
Sensitivity Analyses
Results using PGSs derived at different P value thresholds were consistent with our primary analysis of using a P value threshold of less than .05 (eAppendix 9 in the Supplement). Results from inverse probability weighting and multiple imputation analyses were consistent with our primary results using complete case data (eAppendices 3, 5, 6, and 10 in the Supplement).
Discussion
We examined the association between parental genetic liability to ADHD, autism, and schizophrenia and a wide range of pregnancy-related factors previously observed to be associated with these conditions in offspring. Mothers with a higher ADHD PGS were more likely to be younger at age of childbirth, smoke during pregnancy, have higher BMI, gain more weight during pregnancy, have asthma and depression/anxiety symptoms, and less likely to take folate or other supplements. Our findings were broadly in line with findings from ALSPAC20 and the UK Biobank.28 Concordant results across these 2 UK-based cohorts represent a cross-cultural replication, strengthening the evidence that some pregnancy-related factors are associated with ADHD genetic liability and emphasizing the need to consider genetic confounding as a potential explanation for parent-offspring associations.
Evidence for an association with PGS does not exclude a causal effect. Genetic liability for neurodevelopmental conditions in parents might increase liability in their offspring through direct genetic effects and increase the likelihood of causal pregnancy-related exposures. Future study designs should attempt to partition genetic confounding from causal effects, triangulating different genetically informative approaches such as within-family Mendelian randomization, sibling comparison, and children-of-twins designs.32,33,34 Our findings suggest potential effects of parental genetic liability to ADHD, autism, and schizophrenia on pregnancy-related factors. Even if these pregnancy-related factors are not causal for offspring neurodevelopment, many of them are still known to be causal for other offspring health outcomes (eg, low birth weight35). Consequently, future studies should determine whether expecting parents with neurodevelopmental conditions require specific support during pregnancy (eg, to quit smoking and regulate weight gain).
In the case of smoking, studies using different causally informative designs have found that smoking during pregnancy is unlikely to increase the likelihood of ADHD outcomes in offspring via causal mechanisms.12,36,37,38 Current findings suggest that ADHD is more likely to increase the risk of smoking during pregnancy, rather than the other way around.
The paternal ADHD PGS association with paternal smoking during pregnancy was of smaller magnitude than the maternal ADHD PGS association with maternal smoking during pregnancy. Thus, for smoking (and caffeine consumption, where disparity was also observed), there are implications for the validity of paternal negative control studies. Such studies assume that associations between maternal and paternal smoking with offspring outcomes will be similarly biased by familial factors such as shared genetics, and therefore any difference between the 2 is likely due to intrauterine effects.13 However, we show that associations between maternal and offspring outcomes are more at risk of bias by genetic confounding than associations involving paternal smoking.
Maternal schizophrenia PGS was associated with higher likelihood of smoking and coffee consumption during pregnancy. There is both phenotypic and genetic correlation between coffee consumption and smoking,39 making it challenging to account for pleiotropy. Schizophrenia PGS was also associated with lower BMI, consistent with findings from ALSPAC20 and the UK Biobank.28 We found a novel association between schizophrenia PGS and increased pregnancy weight gain. These findings are important given that exposures such as smoking during pregnancy were thought to play a causal role in schizophrenia.40 The findings suggest that observational studies of pregnancy-related exposures and offspring schizophrenia need to be regarded with caution.
Autism PGS, as well as ADHD PGS and schizophrenia PGS, were associated with higher odds of experiencing depression/anxiety symptoms during pregnancy, as reported previously in ALSPAC.20 Major depression is also genetically correlated with neurodevelopmental conditions.41 The associations we found between schizophrenia PGS and depression/antidepressant medication use in pregnancy highlight the importance of genetically informative designs in studies of prenatal exposure to antidepressants and child neurodevelopment.42
There was evidence for an association between ADHD PGS and an increased risk of asthma. Comorbidity between asthma and ADHD has been demonstrated previously.43,44 Asthma and ADHD are also genetically correlated,45 with ADHD PGS previously found to predict asthma risk in the UK Biobank.28 Shared genetic liability between asthma and ADHD could be due to immunological mechanisms as ADHD is also positively associated with other allergic diseases.46
There was some weak evidence of ADHD PGS and autism PGS association with migraine. ADHD-migraine comorbidity has been reported in children and adults,47 and migraine is genetically correlated with ADHD.48 Migraine could represent a mediating or confounding mechanism between the association of ADHD and paracetamol use.49
Strengths and Limitations
Our study has several strengths, including large sample size and availability of many pregnancy-related factors. We were able to compare maternal and paternal PGS associations on the same pregnancy-related factors. We found relatively consistent associations, suggesting that genetic confounding may contribute to some associations between pregnancy-related factors and offspring ADHD, autism, and schizophrenia.
Our study was limited by the small amount of variance explained by neurodevelopmental PGS, especially for autism because of its low common single-nucleotide variant heritability.16,17,18,50 Therefore, even where there was no evidence for an association in the current study, it is difficult to exclude associations of small magnitudes. The majority of the prenatal exposure PGS associations identified were of small magnitude. However, given that the PGS only explains a small proportion of the variance in the heritability, these estimates do not capture the full extent of genetic confounding. Consequently, only adjusting for parental PGS in observational studies is unlikely to sufficiently control for genetic confounding. An important next step (when the MoBa offspring are older) is to incorporate offspring phenotypic and genetic information and triangulate different designs to quantify the true extent of genetic confounding in associations between pregnancy exposures and neurodevelopmental outcomes.51
We confirmed that ADHD PGS was associated with ADHD behaviors in MoBa mothers and fathers, and previous studies have shown that PGS for ADHD, autism, and schizophrenia predict signs of these conditions in the general population.29,31,52 Owing to lack of power, we were not able to investigate other neurodevelopmental conditions, such as Tourette syndrome (cases, n = 4819; controls, n = 9488).53 When larger GWAS become available, these investigations can be extended.
We relied on self-reports for many of the pregnancy-related factors. For some exposures (eg, smoking), this might have led to reporting bias. However, results were consistent with paternal exposures during pregnancy, which does not tend to be considered as harmful. Paternal associations of ADHD PGS with smoking were in fact lower than maternal associations, which might indicate that mother’s reporting was not biased by stigma.
As with all cohort studies, MoBa is subject to certain selection biases, for example, underrepresentation of younger parents and those with less education.23,54,55,56 Thus, generalizability of results to populations not well-represented in MoBa should not be assumed. However, it is also worth noting that most measures used, and the blood samples from which genotype data arise, were collected during pregnancy, meaning that selective attrition is not a likely source of bias in these results. Genotyping in MoBa prioritized full trios, which likely contributed to differences between the full and genotyped sample. We performed sensitivity analyses using inverse probability weighting and multiple imputation and the results were consistent, suggesting this selection bias did not substantially impact our findings.
Conclusions
Our study demonstrates associations of ADHD genetic liability with several pregnancy-related factors that have been considered predisposing factors for offspring ADHD. Schizophrenia genetic liability also showed associations with some pregnancy-related factors, including lower prepregnancy BMI, higher pregnancy weight gain, and increased smoking during pregnancy. Autism genetic liability showed few associations with pregnancy-related factors beyond depression. Our findings suggest that pregnant individuals with high ADHD or schizophrenia genetic liability are at increased risk of adverse pregnancy-related exposures. Furthermore, our results indicate that observed associations between asthma, depression, smoking, BMI, pregnancy weight gain, and reduced likelihood of taking supplements with offspring ADHD as well as coffee consumption, smoking, BMI, and higher pregnancy weight gain with schizophrenia in the offspring are likely to be, at least in part, due to shared genetic liability, highlighting the need for genetically informative study designs for causal inference.
eAppendix 1. MoBa genetic data generation and quality control
eAppendix 2. Polygenic risk score thresholds
eAppendix 3. Pregnancy-related factors
eAppendix 4. Principal Component Analysis (PCA)
eAppendix 5. Inverse Probability Weighting (IPW)
eAppendix 6. Multiple Imputation (MI)
eAppendix 7. ADHD symptom measures in adulthood in MoBa
eAppendix 8. Paternal PGS sample overview and results
eAppendix 9. PGS associations at different P value thresholds
eAppendix 10. Comparison of complete case, inverse probability weighted and multiple imputation results
eReferences
References
- 1.Thapar A, Cooper M, Rutter M. Neurodevelopmental disorders. Lancet Psychiatry. 2017;4(4):339-346. doi: 10.1016/S2215-0366(16)30376-5 [DOI] [PubMed] [Google Scholar]
- 2.Thapar A, Riglin L. The importance of a developmental perspective in psychiatry: what do recent genetic-epidemiological findings show? Mol Psychiatry. 2020;25(8):1631-1639. doi: 10.1038/s41380-020-0648-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C, Geng H, Liu W, Zhang G. Prenatal, perinatal, and postnatal factors associated with autism: a meta-analysis. Medicine (Baltimore). 2017;96(18):e6696. doi: 10.1097/MD.0000000000006696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JH, Kim JY, Lee J, et al. Environmental risk factors, protective factors, and peripheral biomarkers for ADHD: an umbrella review. Lancet Psychiatry. 2020;7(11):955-970. doi: 10.1016/S2215-0366(20)30312-6 [DOI] [PubMed] [Google Scholar]
- 5.Sciberras E, Mulraney M, Silva D, Coghill D. Prenatal risk factors and the etiology of ADHD: review of existing evidence. Curr Psychiatry Rep. 2017;19(1):1. doi: 10.1007/s11920-017-0753-2 [DOI] [PubMed] [Google Scholar]
- 6.Davies C, Segre G, Estradé A, et al. Prenatal and perinatal risk and protective factors for psychosis: a systematic review and meta-analysis. Lancet Psychiatry. 2020;7(5):399-410. doi: 10.1016/S2215-0366(20)30057-2 [DOI] [PubMed] [Google Scholar]
- 7.Bölte S, Girdler S, Marschik PB. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol Life Sci. 2019;76(7):1275-1297. doi: 10.1007/s00018-018-2988-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis J, Eyre H, Jacka FN, et al. A review of vulnerability and risks for schizophrenia: beyond the two hit hypothesis. Neurosci Biobehav Rev. 2016;65:185-194. doi: 10.1016/j.neubiorev.2016.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133-1163. doi: 10.1002/sim.3034 [DOI] [PubMed] [Google Scholar]
- 10.Davey Smith G, Ebrahim S. Epidemiology: is it time to call it a day? Int J Epidemiol. 2001;30(1):1-11. doi: 10.1093/ije/30.1.1 [DOI] [PubMed] [Google Scholar]
- 11.Skoglund C, Chen Q, D’Onofrio BM, Lichtenstein P, Larsson H. Familial confounding of the association between maternal smoking during pregnancy and ADHD in offspring. J Child Psychol Psychiatry. 2014;55(1):61-68. doi: 10.1111/jcpp.12124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thapar A, Rice F, Hay D, et al. Prenatal smoking might not cause attention-deficit/hyperactivity disorder: evidence from a novel design. Biol Psychiatry. 2009;66(8):722-727. doi: 10.1016/j.biopsych.2009.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langley K, Heron J, Davey Smith G, Thapar A. Maternal and paternal smoking during pregnancy and risk of ADHD symptoms in offspring: testing for intrauterine effects. Am J Epidemiol. 2012;176(3):261-268. doi: 10.1093/aje/kwr510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obel C, Zhu JL, Olsen J, et al. The risk of attention deficit hyperactivity disorder in children exposed to maternal smoking during pregnancy: a re-examination using a sibling design. J Child Psychol Psychiatry. 2016;57(4):532-537. doi: 10.1111/jcpp.12478 [DOI] [PubMed] [Google Scholar]
- 15.Haan E, Sallis HM, Zuccolo L, et al. Prenatal smoking, alcohol and caffeine exposure and maternal-reported attention deficit hyperactivity disorder symptoms in childhood: triangulation of evidence using negative control and polygenic risk score analyses. Addiction. 2022;117(5):1458-1471. doi: 10.1111/add.15746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demontis D, Walters RK, Martin J, et al. ; ADHD Working Group of the Psychiatric Genomics Consortium (PGC); Early Lifecourse & Genetic Epidemiology (EAGLE) Consortium; 23andMe Research Team . Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51(1):63-75. doi: 10.1038/s41588-018-0269-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grove J, Ripke S, Als TD, et al. ; Autism Spectrum Disorder Working Group of the Psychiatric Genomics Consortium; BUPGEN; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium; 23andMe Research Team . Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. 2019;51(3):431-444. doi: 10.1038/s41588-019-0344-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schizophrenia Working Group of the Psychiatric Genomics Consortium . Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi: 10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013;9(3):e1003348. doi: 10.1371/journal.pgen.1003348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leppert B, Havdahl A, Riglin L, et al. Association of maternal neurodevelopmental risk alleles with early-life exposures. JAMA Psychiatry. 2019;76(8):834-842. doi: 10.1001/jamapsychiatry.2019.0774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Easey KE, Timpson NJ, Munafò MR. Association of prenatal alcohol exposure and offspring depression: a negative control analysis of maternal and partner consumption. Alcohol Clin Exp Res. 2020;44(5):1132-1140. doi: 10.1111/acer.14324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nomura Y, Marks DJ, Halperin JM. Prenatal exposure to maternal and paternal smoking on attention deficit hyperactivity disorders symptoms and diagnosis in offspring. J Nerv Ment Dis. 2010;198(9):672-678. doi: 10.1097/NMD.0b013e3181ef3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magnus P, Birke C, Vejrup K, et al. Cohort profile update: the Norwegian mother and child cohort study (MoBa). Int J Epidemiol. 2016;45(2):382-388. doi: 10.1093/ije/dyw029 [DOI] [PubMed] [Google Scholar]
- 24.Paltiel L, Anita H, Skjerden T, et al. The biobank of the Norwegian Mother and Child Cohort Study: present status. Nor Epidemiol. 2014;24(1-2). doi: 10.5324/nje.v24i1-2.1755 [DOI] [Google Scholar]
- 25.Helgeland Ø, Vaudel M, Juliusson PB, et al. Genome-wide association study reveals dynamic role of genetic variation in infant and early childhood growth. Nat Commun. 2019;10(1):4448. doi: 10.1038/s41467-019-12308-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:21. [Google Scholar]
- 27.Euesden J, Lewis CM, O’Reilly PF. PRSice: Polygenic Risk Score software. Bioinformatics. 2015;31(9):1466-1468. doi: 10.1093/bioinformatics/btu848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leppert B, Millard LAC, Riglin L, et al. A cross-disorder PRS-pheWAS of 5 major psychiatric disorders in UK Biobank. PLoS Genet. 2020;16(5):e1008185. doi: 10.1371/journal.pgen.1008185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones HJ, Stergiakouli E, Tansey KE, et al. Phenotypic manifestation of genetic risk for schizophrenia during adolescence in the general population. JAMA Psychiatry. 2016;73(3):221-228. doi: 10.1001/jamapsychiatry.2015.3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson EB, St Pourcain B, Anttila V, et al. ; iPSYCH-SSI-Broad Autism Group . Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. Nat Genet. 2016;48(5):552-555. doi: 10.1038/ng.3529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stergiakouli E, Davey Smith G, Martin J, et al. Shared genetic influences between dimensional ASD and ADHD symptoms during child and adolescent development. Mol Autism. 2017;8:18. doi: 10.1186/s13229-017-0131-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies NM, Howe LJ, Brumpton B, Havdahl A, Evans DM, Davey Smith G. Within family Mendelian randomization studies. Hum Mol Genet. 2019;28(R2):R170-R179. doi: 10.1093/hmg/ddz204 [DOI] [PubMed] [Google Scholar]
- 33.McAdams TA, Hannigan LJ, Eilertsen EM, Gjerde LC, Ystrom E, Rijsdijk FV. Revisiting the children-of-twins design: improving existing models for the exploration of intergenerational associations. Behav Genet. 2018;48(5):397-412. doi: 10.1007/s10519-018-9912-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davey Smith G, Richmond RC, Pingault JB. Combining human genetics and causal inference to understand human disease and development. In: Cold Spring Harbor Perspectives in Medicine Collection. CSHL Press; 2021. [Google Scholar]
- 35.Tyrrell J, Huikari V, Christie JT, et al. ; Early Growth Genetics (EGG) Consortium . Genetic variation in the 15q25 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) interacts with maternal self-reported smoking status during pregnancy to influence birth weight. Hum Mol Genet. 2012;21(24):5344-5358. doi: 10.1093/hmg/dds372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haan E, Sallis HM, Zuccolo L, et al. Prenatal smoking, alcohol and caffeine exposure and ADHD risk in childhood: parental comparisons and polygenic risk score (PRS) analyses. medRxiv. Published online March 26, 2021. doi: 10.1101/2021.03.25.21254087 [DOI]
- 37.Thapar A, Rice F. Family-based designs that disentangle inherited factors from pre- and postnatal environmental exposures: in vitro fertilization, discordant sibling pairs, maternal versus paternal comparisons, and adoption designs. Cold Spring Harb Perspect Med. 2021;11(3):a038877. doi: 10.1101/cshperspect.a038877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gustavson K, Ystrom E, Stoltenberg C, et al. Smoking in pregnancy and child ADHD. Pediatrics. 2017;139(2):e20162509. doi: 10.1542/peds.2016-2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gage SH, Jones HJ, Taylor AE, Burgess S, Zammit S, Munafò MR. Investigating causality in associations between smoking initiation and schizophrenia using Mendelian randomization. Sci Rep. 2017;7(1):40653. doi: 10.1038/srep40653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunter A, Murray R, Asher L, Leonardi-Bee J. The effects of tobacco smoking, and prenatal tobacco smoke exposure, on risk of schizophrenia: a systematic review and meta-analysis. Nicotine Tob Res. 2020;22(1):3-10. doi: 10.1093/ntr/nty160 [DOI] [PubMed] [Google Scholar]
- 41.Wray NR, Ripke S, Mattheisen M, et al. ; eQTLGen; 23andMe; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium . Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50(5):668-681. doi: 10.1038/s41588-018-0090-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El Marroun H, White T, Verhulst FC, Tiemeier H. Maternal use of antidepressant or anxiolytic medication during pregnancy and childhood neurodevelopmental outcomes: a systematic review. Eur Child Adolesc Psychiatry. 2014;23(10):973-992. doi: 10.1007/s00787-014-0558-3 [DOI] [PubMed] [Google Scholar]
- 43.Holmberg K, Lundholm C, Anckarsäter H, Larsson H, Almqvist C. Impact of asthma medication and familial factors on the association between childhood asthma and attention-deficit/hyperactivity disorder: a combined twin- and register-based study: Epidemiology of Allergic Disease. Clin Exp Allergy. 2015;45(5):964-973. doi: 10.1111/cea.12529 [DOI] [PubMed] [Google Scholar]
- 44.Cortese S, Sun S, Zhang J, et al. Association between attention deficit hyperactivity disorder and asthma: a systematic review and meta-analysis and a Swedish population-based study. Lancet Psychiatry. 2018;5(9):717-726. doi: 10.1016/S2215-0366(18)30224-4 [DOI] [PubMed] [Google Scholar]
- 45.Zhu Z, Zhu X, Liu CL, et al. Shared genetics of asthma and mental health disorders: a large-scale genome-wide cross-trait analysis. Eur Respir J. 2019;54(6):1901507. doi: 10.1183/13993003.01507-2019 [DOI] [PubMed] [Google Scholar]
- 46.Miyazaki C, Koyama M, Ota E, et al. Allergic diseases in children with attention deficit hyperactivity disorder: a systematic review and meta-analysis. BMC Psychiatry. 2017;17(1):120. doi: 10.1186/s12888-017-1281-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansen TF, Hoeffding LK, Kogelman L, et al. Comorbidity of migraine with ADHD in adults. BMC Neurol. 2018;18(1):147. doi: 10.1186/s12883-018-1149-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anttila V, Bulik-Sullivan B, Finucane HK, et al. ; Brainstorm Consortium . Analysis of shared heritability in common disorders of the brain. Science. 2018;360(6395):eaap8757. doi: 10.1126/science.aap8757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stergiakouli E, Thapar A, Davey Smith G. Association of acetaminophen use during pregnancy with behavioral problems in childhood: evidence against confounding. JAMA Pediatr. 2016;170(10):964-970. doi: 10.1001/jamapediatrics.2016.1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.St Pourcain B, Robinson EB, Anttila V, et al. ; iPSYCH-SSI-Broad Autism Group . ASD and schizophrenia show distinct developmental profiles in common genetic overlap with population-based social communication difficulties. Mol Psychiatry. 2018;23(2):263-270. doi: 10.1038/mp.2016.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pingault JB, Rijsdijk F, Schoeler T, et al. Genetic sensitivity analysis: adjusting for genetic confounding in epidemiological associations. PLoS Genet. 2021;17(6):e1009590. doi: 10.1371/journal.pgen.1009590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin J, Hamshere ML, Stergiakouli E, O’Donovan MC, Thapar A. Genetic risk for attention-deficit/hyperactivity disorder contributes to neurodevelopmental traits in the general population. Biol Psychiatry. 2014;76(8):664-671. doi: 10.1016/j.biopsych.2014.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu D, Sul JH, Tsetsos F, et al. ; Tourette Association of America International Consortium for Genetics, the Gilles de la Tourette GWAS Replication Initiative, the Tourette International Collaborative Genetics Study, and the Psychiatric Genomics Consortium Tourette Syndrome Working Group . Interrogating the genetic determinants of Tourette’s syndrome and other tic disorders through genome-wide association studies. Am J Psychiatry. 2019;176(3):217-227. doi: 10.1176/appi.ajp.2018.18070857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biele G, Gustavson K, Czajkowski NO, et al. Bias from self selection and loss to follow-up in prospective cohort studies. Eur J Epidemiol. 2019;34(10):927-938. doi: 10.1007/s10654-019-00550-1 [DOI] [PubMed] [Google Scholar]
- 55.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C; MoBa Study Group . Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol. 2006;35(5):1146-1150. doi: 10.1093/ije/dyl170 [DOI] [PubMed] [Google Scholar]
- 56.Nilsen RM, Vollset SE, Gjessing HK, et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23(6):597-608. doi: 10.1111/j.1365-3016.2009.01062.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. MoBa genetic data generation and quality control
eAppendix 2. Polygenic risk score thresholds
eAppendix 3. Pregnancy-related factors
eAppendix 4. Principal Component Analysis (PCA)
eAppendix 5. Inverse Probability Weighting (IPW)
eAppendix 6. Multiple Imputation (MI)
eAppendix 7. ADHD symptom measures in adulthood in MoBa
eAppendix 8. Paternal PGS sample overview and results
eAppendix 9. PGS associations at different P value thresholds
eAppendix 10. Comparison of complete case, inverse probability weighted and multiple imputation results
eReferences


