This randomized clinical trial investigates if the oral selective vasopressin 1a receptor antagonist, balovaptan, improves socialization and communication difficulties in children and adolescents with autism spectrum disorder.
Key Points
Question
Does balovaptan, an oral selective vasopressin 1a receptor antagonist, improve socialization and communication difficulties in children and adolescents with autism spectrum disorder (ASD)?
Findings
In this randomized clinical trial that included 167 children and adolescents in the primary analysis, there was no statistically significant difference observed between the balovaptan and placebo groups in the primary outcome measure, change from baseline on the Vineland-II two-domain composite score at week 24.
Meaning
Balovaptan was well tolerated in children with ASD but did not demonstrate efficacy in improvement of socialization and communication in this population, and these findings are important to guide future development efforts.
Abstract
Importance
There are no approved medications for the core symptoms of autism spectrum disorder (ASD), socialization and communication difficulties.
Objective
To evaluate the efficacy and safety of balovaptan, an oral selective vasopressin 1a receptor antagonist, compared with placebo in children and adolescents with ASD.
Design, Setting, and Participants
The aV1ation study was a randomized, double-blind, 24-week, parallel-group, placebo-controlled phase 2 trial. Between November 22, 2016, and September 3, 2019, individuals were screened and randomly assigned to treatment groups. The primary efficacy analysis population comprised participants taking age-adjusted balovaptan equivalent to a 10-mg adult dose and participants from the concurrently randomized placebo group. This multicenter trial took place across 41 sites in the US. Participants were aged 5 to 17 years with diagnosed ASD and an IQ of 70 or greater. Data were analyzed from April 8 to November 16, 2020.
Interventions
Participants were randomly assigned to daily 4-mg or 10-mg adult-equivalent balovaptan or placebo, until the 4-mg group was discontinued.
Main Outcomes and Measures
The primary end point was change from baseline on the Vineland-II two-domain composite (2DC; socialization and communication domains) score at week 24.
Results
Between November 2016 and September 2019, a total of 599 individuals were screened and 339 participants were randomly assigned to receive 4-mg balovaptan adult-equivalent dose (91 [26.8%]), 10-mg balovaptan adult-equivalent dose (126 [37.2%]), or placebo (122 [36.0%]). Primary analysis included 86 participants assigned to receive 10-mg balovaptan adult-equivalent dose and 81 assigned to receive placebo (mean [SD] age, 12.1 [3.4] years; 139 male participants [83.2%]). No statistically significant differences were observed between the balovaptan and placebo groups in change from baseline on the Vineland-II 2DC score at week 24 (difference in adjusted least-squares mean, −0.16; 90% CI, −2.56 to 2.23; P = .91). No improvements for balovaptan vs placebo were observed at week 24 for any secondary end points. Balovaptan was well tolerated with no emerging safety concerns. Similar proportions of participants reported adverse events (balovaptan, 66 of 86 [76.7%] vs placebo, 61 of 81 [75.3%]) and serious adverse events (balovaptan, 1 of 86 [1.2%] vs placebo, 4 of 81 [4.9%]).
Conclusions and Relevance
In this randomized clinical trial, balovaptan did not demonstrate efficacy in improvement of socialization and communication in this population with pediatric ASD. Balovaptan was well tolerated in children 5 years or older. Further development of robust, sensitive, and objective outcome measures may help to improve future studies in the assessment of therapies targeting communication and socialization in pediatric ASD.
Trial Registration
ClinicalTrials.gov Identifier: NCT02901431
Introduction
Autism spectrum disorder (ASD) is a common lifelong neurodevelopmental condition with an increasing global prevalence.1,2 The core symptoms of ASD are defined as difficulties in social communication/interaction and restrictive/repetitive behaviors.2 ASD is etiologically, biologically, and clinically heterogeneous; the genetic and environmental factors involved are still poorly understood.3 Current US Food and Drug Administration–approved medications do not target the core symptoms of ASD.4 There is an unmet need for approved pharmacologic therapies targeting the suspected underlying biologic pathways associated with the core symptoms of ASD.5
Social interaction and communication are partly modulated by neuropeptides, including vasopressin.6 Therapies targeting the arginine-vasopressin signaling pathway have shown potential to improve social abilities in mouse models of autism7 and in children with ASD.8 Promoter polymorphisms in the vasopressin 1a (V1a) gene have been associated with ASD in humans,9 and V1a receptor antagonists modulate anxiety and brain regions related to face emotion processing.10 Balovaptan is an orally administered, brain-penetrant, selective V1a receptor antagonist primarily metabolized by cytochrome P450 3A4 (CYP3A4).11 It was developed following preliminary evidence that V1a receptor antagonism improved social interaction in a rat model of autism12 and that intravenous balovaptan improved surrogate measurements of social communication in adults with ASD.13 In 2 phase 1, placebo-controlled, randomized clinical trials (RCTs) in healthy adults (NCT0376444914 and NCT0141896315), balovaptan demonstrated full oral absorption, high tolerability, and a stable and approximately linear, steady-state pharmacokinetic (PK) profile at once-daily doses of 10 to 50 mg that supported administration once per day with or without food.16 The Vasopressin Antagonist to Improve Social Communication in Autism (VANILLA) study (NCT0179344117) was a multicenter, double-blind, 12-week, phase 2 RCT of balovaptan 1.5 mg, 4 mg, and 10 mg compared with placebo in 223 men with ASD. No improvements were observed in the primary end point: change from baseline at week 12 in the caregiver-rated Social Responsiveness Scale, 2nd edition (SRS-2) total T score.18 However, both 4-mg and 10-mg balovaptan demonstrated a clinically meaningful and dose-dependent improvement on the secondary end point, change from baseline on the Vineland-II Adaptive Behavior Composite (ABC) score compared with placebo.18
We report the results of the phase 2 clinical trial, aV1ation, that evaluated the equivalent of a 10-mg adult dose of balovaptan compared with placebo in children (aged 5-12 years) and adolescents (aged 13-17 years) with ASD and an IQ of 70 or greater. The objective of the study was to assess the safety, tolerability, and the effect of balovaptan on social interaction and communication.
Methods
Study Design
This RCT was conducted in 41 sites across the US, including 21 academic sites, and in full accordance with the principles of the Declaration of Helsinki and the International Council of Harmonisation E6 guideline for Good Clinical Practice, or the relevant laws and regulations in the country in which the research was conducted. The protocol and written informed consent were approved by an institutional review board or ethics committee for each site. The study protocol, including the 5 protocol amendments and statistical analysis plan, are available in Supplements 1, 2, and 3. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
An initial PK study confirmed the estimated, age-adjusted balovaptan doses resulting in plasma exposures equivalent to 4 mg daily and 10 mg daily in adults for children and adolescents (eAppendixes 1, 2, and 3 in Supplement 2). Owing to exposures lower than targeted levels at interim analysis in both dosages and the need for the 10-mg balovaptan adult-equivalent dose to be adequately characterized, the 4-mg balovaptan adult-equivalent dose was discontinued and the 10-mg balovaptan adult-equivalent dose was continued as the single dose studied. The trial continued as a parallel, 2-group trial, with those participants entering after the final dose decision randomly assigned to receive either balovaptan, providing an exposure approximately equivalent to 10 mg in adults, or placebo. The screening period in the main study was up to 4 weeks followed by a randomized, double-blind, 24-week, parallel-group, placebo-controlled phase 2 trial, with an optional 52-week open-label extension and follow-up at least 6 weeks after last dose. Study visits and assessment details are in eAppendixes 1, 2, and 3 of Supplement 2.
Study medication (balovaptan or matching placebo) was manufactured by F. Hoffmann-La Roche Ltd, Basel, Switzerland, and consisted of tablets that were taken once per day in the morning swallowed whole with soft food or dispersed in liquid. To monitor compliance, participants or caregivers kept a diary of doses taken or missed.
Participants
Eligible participants were children and adolescents aged 5 to 17 years who met the criteria for ASD based on the DSM-5 or the International Statistical Classification of Diseases and Related Health Problems, 10th revision, confirmed by Autism Diagnostic Observational Schedule-2 (ADOS-2) criteria. Written informed consent forms were provided by all participants or the participant's legally authorized representative before participating in this study. Key inclusion criteria included Clinical Global Impression-Severity (CGI-S) score of 4 or greater (moderately ill), SRS-2 T score of 66 or greater, and IQ of 70 or greater as assessed by Wechsler Abbreviated Scale of Intelligence, 2nd edition or Wechsler Preschool & Primary Scale of Intelligence, 4th edition. Key exclusion criteria for participants were major changes in psychosocial intervention within 4 weeks prior to screening; unstable or uncontrolled clinically significant psychiatric and/or neurologic disorder; and/or suicidal behavior (eAppendixes 1, 2, 3, 4, 5, and 6 of Supplement 2).
Participant race and ethnicity data were gathered as standard practice and to report the diversity of study participants. Race and ethnicity were self-identified by participants or their parent/caregiver via questionnaire. Race categories included Asian, Black or African American, Native Hawaiian or Other Pacific Islander, White, multiple, or unknown. Ethnicity categories included Hispanic or Latinx, Not Hispanic or Latinx, not stated, or unknown.
Randomization and Masking
We randomly assigned participants using an interactive (voice/web) response system. Random allocation sequence was generated by the randomization provider (Almac). The main study cohort were stratified by age (5-12 years and 13-17 years) and sex. Initially, 3 treatment groups were randomized (placebo, 4-mg balovaptan adult-equivalent dose and 10-mg balovaptan adult-equivalent dose) in a 1:1:1 ratio with a block size of 6. Following a protocol amendment, the 4-mg group was discontinued, and randomization continued with 2 treatment groups in a 1:1 ratio and a block size of 4. The ratio between adolescents (aged 13-17 years) and children (aged 5-12 years) was planned to be approximately 1:1. The proportion of female participants was balanced in each treatment group by inclusion as a stratification factor in the interactive (voice/web) response system, and was limited to 20% or less of the entire study population to reflect the observed sex ratio in the general ASD population.19 Participants, investigators, and the sponsor were masked to the assigned study treatment. Access to potentially unblinding data (PK and safety) was restricted to the Internal Monitoring Committee, Scientific Oversight Committee, and an unblinded data acquisition specialist.
Outcomes
The primary end point was the change from baseline at week 24 in the Vineland-II two-domain composite (2DC) score. Prespecified secondary end points included change from baseline at 12 and 24 weeks on Vineland-II ABC score and Vineland-II communication, socialization, and daily living skills domain standard scores and change from baseline on the Vineland-II 2DC score after 12 weeks of treatment. Overall severity and change in overall ASD core symptoms were assessed by improvements in CGI-Improvement (CGI-I) and change from baseline in CGI-S. CGI-S scores range from 1 (no symptoms) to 7 (very severe symptoms). Responders in CGI-S were defined as individuals with a change from baseline of −1 or lower. In addition, at weeks 12 and 24, the Pediatric Quality of Life Inventory (PedsQL), version 4.0, generic core scale, was assessed, which included 23 items across the domains of physical functioning, emotional functioning, social functioning, and school functioning. Exploratory and safety end points are described in eAppendixes 1, 2, and 3 in Supplement 2.
Statistical Analysis
To ensure 80% power to detect statistical significance at 1-sided 5% significance level and a difference between active dose and placebo independently with an effect size of at least 0.4 and a withdrawal rate of 15% to 20%, 80 participants were required per treatment group (balovaptan, 10-mg equivalent, and placebo). A total sample size of approximately 160 participants with ASD and evaluable data at 24 weeks was required.
Interim analyses of PK and safety were carried out in the first cohort to confirm or adjust the final dose. The primary efficacy population consisted of the subset of the overall population including patients taking balovaptan, 10-mg adult-equivalent dose, and participants from the concurrently randomized placebo group in the corresponding randomization stage for whom baseline and at least 1 after-dose assessments were available. Participants with dose adjustments or interruptions, or who were receiving a different dose from the final dose for their age group, were excluded. Primary efficacy and safety interim analyses were carried out after approximately 80 participants (balovaptan, 10 mg, or placebo) had completed the 12-week visit without dose interruptions or adjustments to allow internal decisions. Participants in the first adolescent and child cohorts with dose adjustments or those who were receiving a different dose from the final dose for their age group were excluded from the primary analysis. Plasma concentrations of balovaptan were monitored at weeks 8, 12, and 24, or on early withdrawal, to assess whether plasma concentrations at steady state were maintained at expected levels.
The primary efficacy end point, the change from baseline of Vineland-II 2DC score, as well as the secondary end points expressed in terms of change from baseline on a continuous scale, were analyzed using mixed-model repeated measurements. The model included treatment, sex, and visit (week) as main effects, individual age and baseline score (where available) as covariates, and treatment by visit and baseline by visit as interaction terms, with visit week as a repeated effect. Missing data were handled by mixed-model repeated measurement without any data imputation or discard of data. Treatment differences between groups were estimated using least-squares mean (LSM) with 90% CIs. Owing to the exploratory nature of the study, there was no statistical correction for multiplicity.
The proportion of participants with at least a 1-point decrease (ie, improvement) from baseline on CGI-S was analyzed using logistic regression (eAppendixes 4, 5, and 6 in Supplement 2). Nominal P values were 2-sided and set at 90%. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute). Data were analyzed from April 8 to November 16, 2020.
Results
Participants
Between November 22, 2016, and September 3, 2019, a total of 599 individuals were screened and 339 participants were randomly assigned to receive 4-mg balovaptan adult-equivalent dose (91 [26.8%]), 10-mg balovaptan adult-equivalent dose (126 [37.2%]), or placebo (122 [36.0%]) (Figure 1). The primary efficacy analysis included 86 participants assigned to receive a balovaptan 10-mg adult-equivalent dose and 81 assigned to receive placebo (mean [SD] age, 12.1 [3.4] years; 139 male participants [83.2%]; 28 female participants [16.8%]). Participants from the following race categories were included: 10 Asian (6.0%), 8 Black or African American (4.8%), 1 Native Hawaiian or Other Pacific Islander (0.6%), 133 White (79.6%), 13 participants (7.8%) with multiple races, and 2 participants (1.2%) with unknown race. Participants from the following ethnic categories were included: 20 Hispanic or Latinx (12.0%), 144 not Hispanic or Latinx (86.2%), 2 not stated (1.2%), and 1 unknown (0.6%). The safety population comprised 86 participants taking age-adjusted balovaptan equivalent to a 10-mg adult dose and 81 participants from the concurrently randomized placebo group. Participants with dose adjustments or interruptions, or those who were taking a different dose than the final dose for their age group (ie, 10-mg adult-equivalent) were excluded. Baseline characteristics and ASD severity were well balanced between groups (Table 1; eAppendixes 1, 2, and 3 in Supplement 2). At week 24, 16 of 70 participants (22.9%) in the 10-mg balovaptan adult-equivalent group and 7 of 62 participants (11.3%) in the placebo group had a change in Vineland-II raters.
Figure 1. Consolidated Standards of Reporting Trials (CONSORT) Flow Diagram for the aV1ation Phase 2 Trial.
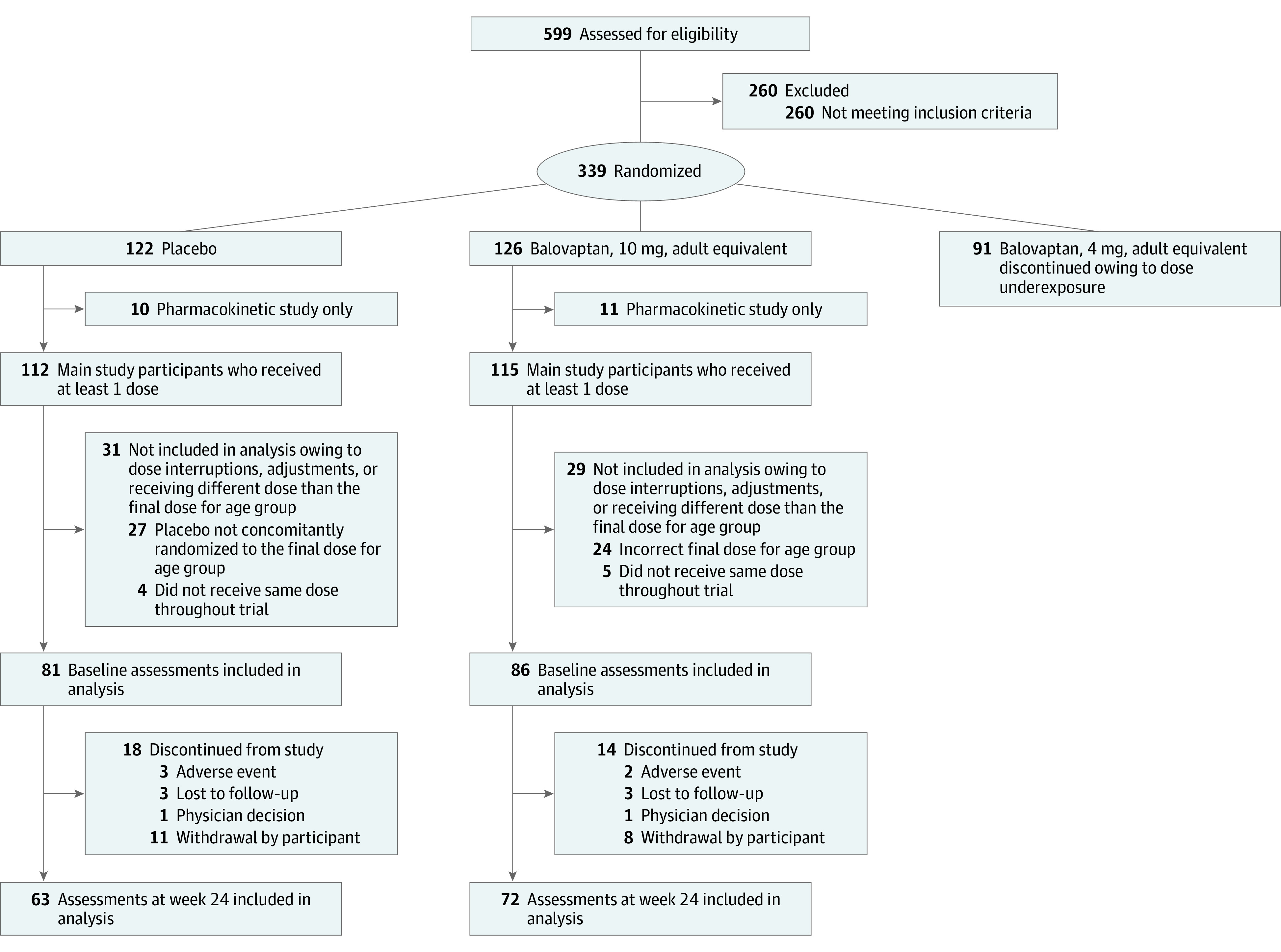
Numbers of participants who participated in both the pharmacokinetic study and main study: placebo (n = 2); balovaptan 10-mg adult-equivalent dose (n = 4); balovaptan, 4-mg adult-equivalent dose (n = 1). For assessments at week 24, n = number of nonmissing values at the respective visit.
Table 1. Participants’ Demographics and Baseline Characteristicsa.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Balovaptan, 10 mg (n = 86) | Placebo (n = 81) | Total (n = 167) | |
| Sex | |||
| Male | 72 (83.7) | 67 (82.7) | 139 (83.2) |
| Female | 14 (16.3) | 14 (17.3) | 28 (16.8) |
| Age, y | |||
| Mean (SD) | 11.9 (3.5) | 12.3 (3.4) | 12.1 (3.4) |
| Median (range) | 11.5 (5-17) | 12.0 (5-17) | 12.0 (5-17) |
| Age group | |||
| Children aged 5-7 y | 9 (10.5) | 9 (11.1) | 18 (10.8) |
| Children aged 8-12 y | 40 (46.5) | 35 (43.2) | 75 (44.9) |
| Adolescents aged 13-14 y | 9 (10.5) | 6 (7.4) | 15 (9.0) |
| Adolescents aged 15-17 y | 28 (32.6) | 31 (38.2) | 59 (35.3) |
| Race | |||
| Asian | 5 (5.8) | 5 (6.2) | 10 (6.0) |
| Black or African American | 6 (7.0) | 2 (2.5) | 8 (4.8) |
| Native Hawaiian or Other Pacific Islander | 1 (1.2) | 0 (0.0) | 1 (0.6) |
| White | 68 (79.1) | 65 (80.2) | 133 (79.6) |
| Multiple | 5 (5.8) | 8 (9.9) | 13 (7.8) |
| Unknown | 1 (1.2) | 1 (1.2) | 2 (1.2) |
| Ethnicity | |||
| Hispanic or Latinx | 7 (8.1) | 13 (16.0) | 20 (12.0) |
| Not Hispanic or Latinx | 78 (90.7) | 66 (81.5) | 144 (86.2) |
| Not stated | 0 (0.0) | 2 (2.5) | 2 (1.2) |
| Unknown | 1 (1.2) | 0 (0.0) | 1 (0.6) |
| BMIb | |||
| Mean (SD) | 21.19 (4.87) | 20.88 (4.98) | 21.04 (4.91) |
| Median (range) | 20.0 (13.8-34.3) | 19.8 (13.0-35.0) | 19.9 (1.0-35.0) |
| Participants with at least one | |||
| Ongoing medication | 77 (89.5) | 70 (86.4) | 147 (88.0) |
| Psychiatric comorbidity | 67 (77.9) | 71 (87.7) | 138 (82.6) |
| WASI-II IQc | |||
| Mean (SD) | 97.2 (16.2) | 98.5 (17.1) | 97.8 (16.6) |
| Median (range) | 94.0 (70-135) | 99.0 (70-140) | 97.5 (70-140) |
| CGI-S | |||
| CGI-S = 3d | 1 (1.2) | 1 (1.2) | 2 (1.2) |
| CGI-S = 4 | 60 (69.8) | 42 (51.9) | 102 (61.1) |
| CGI-S = 5 | 19 (22.1) | 33 (40.7) | 52 (31.1) |
| CGI-S = 6 | 6 (7.0) | 5 (6.2) | 11 (6.6) |
| SRS-2, Total T score | |||
| Mean (SD) | 82.8 (9.5) | 83.3 (9.1) | 83.1 (9.3) |
| Median (range) | 82.5 (66-111) | 82.0 (66-107) | 82.0 (66-111) |
| Aberrant Behavior Checklist, lethargy/social withdrawal | |||
| Mean (SD) | 12.7 (8.4) | 13.3 (7.7) | 13.0 (8.1) |
| Median (range) | 11.0 (0-34) | 13.0 (0-34) | 12.0 (0-34) |
| Vineland-II ABC scorec | |||
| Mean (SD) | 75.4 (10.4) | 72.5 (10.1) | 74.0 (10.3) |
| Median (range) | 74.0 (56-103) | 72.0 (50-105) | 73.0 (50-105) |
| Vineland-II 2DC scorec | |||
| Mean (SD) | 76.1 (10.7) | 72.1 (10.0) | 74.2 (10.5) |
| Median (range) | 74.0 (47-103) | 72.0 (48-98) | 73.0 (47-103) |
Abbreviations: ABC, Adaptive Behavior Composite; BMI, body mass index; CGI-S, Clinical Global Impression–Severity; SRS-2, Social Responsiveness Scale, 2nd edition; WASI-II, Wechsler Abbreviated Scale of Intelligence, 2nd edition.
Numbers shown are the aV1ation primary analysis population.
Calculated as weight in kilograms divided by height in meters squared.
IQ and Vineland-II ABC/two-domain composite score: placebo (n = 79), balovaptan (n = 85).
Participants’ baseline CSI-S scores met inclusion criteria of 4 or more at screening.
Primary Efficacy Outcome Measure
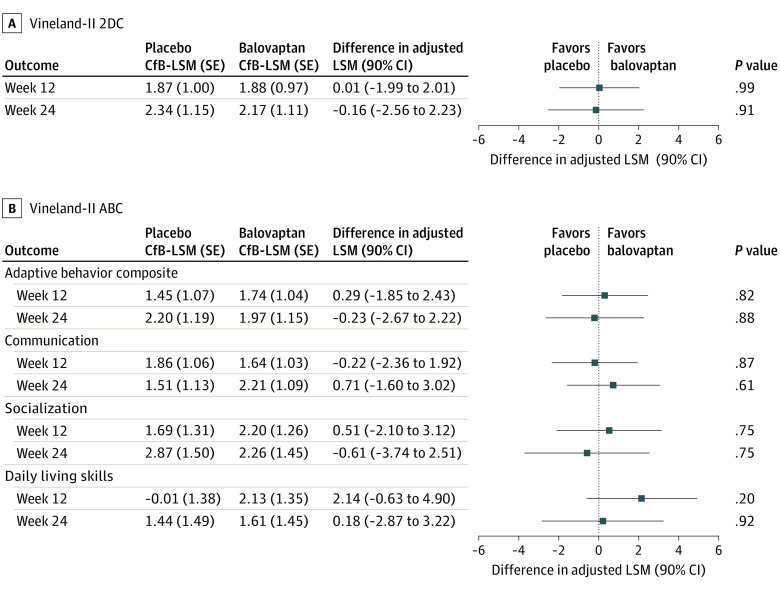
In the primary analysis, no statistically significant differences in change from baseline were observed on the Vineland-II 2DC score with balovaptan (LSM, 2.17; SE, 1.11) compared with placebo (LSM, 2.34; SE 1.15) at week 24 (difference in adjusted LSM, −0.16; 90% CI, −2.56 to 2.23; P = .91) (Figure 2A).
Figure 2. Treatment Difference in Vineland-II Two-Domain Composite (2DC) and Vineland-II Adaptive Behavior Composite (ABC) at 12 and 24 Weeks.
A, Vineland-II 2DC score and Vineland-II ABC (B) differences in adjusted least-squares mean (LSM) derived using mixed models for repeated measurement analysis are shown. At week 12: placebo (n = 66) and balovaptan (n = 70); at week 24: placebo (n = 61) and balovaptan (n = 67); week 24: socialization and daily living skills (n = 68). CfB indicates change from baseline.
Secondary Outcome Measures
No significant differences were seen in any of the secondary end points, including change from baseline on the Vineland-II 2DC score at week 12 (balovaptan: LSM, 1.88; SE 0.97 vs placebo: LSM, 1.87; SE, 1.00; difference in adjusted LSM, 0.01; 90% CI, −1.99 to 2.01; P = .99) (Figure 2A), change from baseline in Vineland-II ABC (Figure 2B), or any of the individual Vineland-II domain scores at 12 or 24 weeks (Figure 2B).
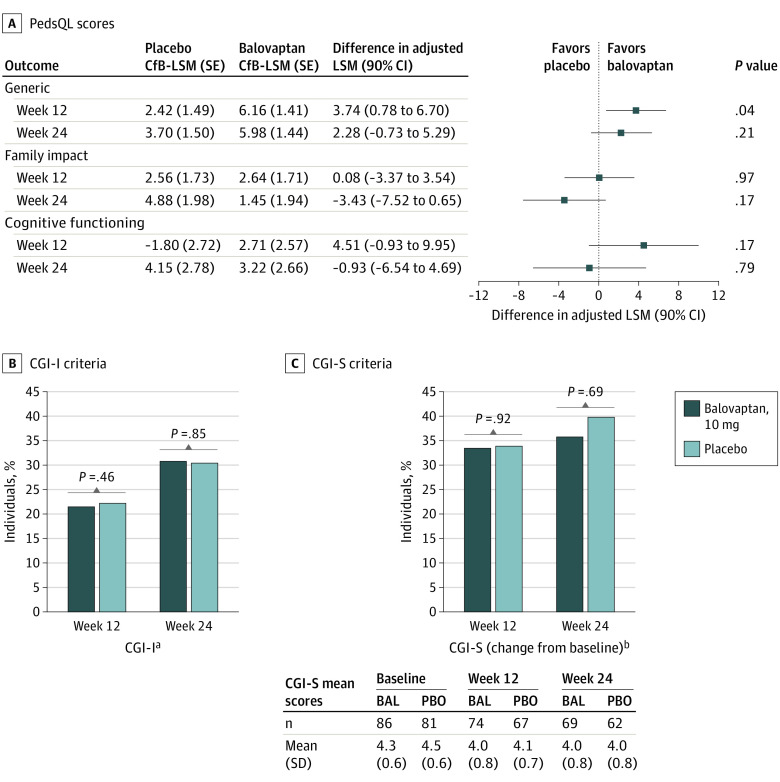
For the PedsQL generic core total score, an improvement was observed from baseline in both the balovaptan group (LSM, 5.98; SE, 1.44) and placebo group (LSM, 3.70; SE, 1.50) at week 24; however, there was no difference between treatment groups (difference in adjusted LSM, 2.28; 90% CI, −0.73 to 5.29; P = .21) (Figure 3A). No improvements were observed in the change from baseline in the PedsQL Cognitive Functioning Scale or the PedsQL Family Impact module at 24 weeks (Figure 3A). There was an isolated significant finding of improvement from baseline at week 12 favoring balovaptan on the PedsQL generic core scale (difference in adjusted LSM, 3.74; 90% CI, 0.78-6.70; P = .04), but this did not persist at week 24 and was not seen on the other PedsQL subscales described previously.
Figure 3. Treatment Differences for Pediatric Quality of Life Inventory (PedsQL) Scores and Clinical Global Impression (CGI) Responders .
A, Differences in adjusted least-squares mean (LSM) for PedsQL scores derived using mixed models for repeated measurement analysis are shown. Generic at week 12: placebo (n = 62) and balovaptan (n = 72); week 24: placebo (n = 59) and balovaptan (n = 65); family impact at week 12: placebo (n = 68) and balovaptan (n = 73); week 24: placebo (n = 62) and balovaptan (n = 67); cognitive functioning at week 12: placebo (n = 63) and balovaptan (n = 72); week 24: placebo (n = 59) and balovaptan (n = 65). B, CGI-Improvement (CGI-I) and C, CGI-Severity (CGI-S) week 12: placebo (n = 67) and balovaptan (n = 74); week 24: placebo (n = 62) and balovaptan (n = 68/69 [CGI-I/CGI-S]). BAL indicates balovaptan; CfB, change from baseline; PBO, placebo.
aPercentage of responders with scores of 1 (much improved) or 2 (very much improved).
b Percentage of individuals with an improvement in their rating of CGI-S of at least 1 point.
At weeks 12 and 24, there were no differences in the proportion of responders defined by CGI-I and CGI-S criteria between balovaptan and placebo, respectively (Figure 3B and 3C). No significant differences were observed in the Aberrant Behavior Checklist subscales between balovaptan and placebo at weeks 6, 12, 18, or 24 (eTables 1 and 2 in Supplement 2).
Safety
The proportion of participants reporting any adverse events (AEs) was similar across groups during the double-blind period. AEs were reported by 66 of 86 participants (76.7%) in the balovaptan group and 61 of 81 participants (75.3%) in the placebo group (Table 2). No balovaptan-associated safety signals emerged from review of laboratory vital sign and electrocardiogram data. Serious AEs occurred in 1 of 86 participants (1.2%) in the balovaptan group (suicidal ideation) and 4 of 81 participants (4.9%) in the placebo group (aggression, depression, intentional self-injury, viral gastroenteritis). Suicidality monitoring using the Columbia Suicide Severity Rating Scale did not reveal an imbalance in responses between study treatment groups (eTable 3 and eFigure in Supplement 2).
Table 2. Safety Dataa.
| Adverse event | No. (%) | |
|---|---|---|
| Balovaptan, 10 mg (n = 86) | Placebo (n = 81) | |
| Participants with ≥1 adverse event | 66 (76.7) | 61 (75.3) |
| Total No. of events | 339 | 188 |
| Participants with serious adverse events | 1 (1.2) | 4 (4.9) |
| Type of event | ||
| Suicidal ideation | 1 (1.2) | 0 (0.0) |
| Intentional self-injury | 0 (0.0) | 1 (1.2) |
| Aggression | 0 (0.0) | 1 (1.2) |
| Depression | 0 (0.0) | 1 (1.2) |
| Viral gastroenteritis | 0 (0.0) | 1 (1.2) |
| Deaths | 0 (0.0) | 0 (0.0) |
| Participants withdrawn from treatment owing to adverse events | 3 (3.5) | 4 (4.9) |
| Anxiety | 2 (2.3) | 0 (0.0) |
| Irritability | 1 (1.2) | 1 (1.2) |
| Troponin T increase | 0 (0.0) | 1 (1.2) |
| Weight increase | 0 (0.0) | 1 (1.2) |
| Headache | 0 (0.0) | 1 (1.2) |
| Most common adverse events (>5% in either group) | ||
| Nasopharyngitis | 21 (24.4) | 10 (12.3) |
| Influenza | 7 (8.1) | 5 (6.2) |
| Upper respiratory tract infection | 8 (9.3) | 4 (4.9) |
| Gastroenteritis | 5 (5.8) | 1 (1.2) |
| Headache | 14 (16.3) | 13 (16.0) |
| Diarrhea | 11 (12.8) | 4 (4.9) |
| Vomiting | 7 (8.1) | 6 (7.4) |
| Nausea | 5 (5.8) | 6 (7.4) |
| Abdominal discomfort | 6 (7.0) | 4 (4.9) |
| Abdominal pain upper | 6 (7.0) | 4 (4.9) |
| Oropharyngeal pain | 9 (10.5) | 6 (7.4) |
| Nasal congestion | 7 (8.1) | 4 (4.9) |
| Cough | 5 (5.8) | 5 (6.2) |
| Pyrexia | 6 (7.0) | 3 (3.7) |
| Irritability | 8 (9.3) | 4 (4.9) |
| Anxiety | 6 (7.0) | 1 (1.2) |
| Aggression | 5 (5.8) | 1 (1.2) |
| Decreased appetite | 5 (5.8) | 0 (0.0) |
No serious adverse events were considered related to the study treatment by the investigator, and all had resolved during the treatment period.
Discussion
In this multicenter RCT, no significant improvements were seen for balovaptan compared with placebo in pediatric ASD for the primary end point, change from baseline at week 24 in the Vineland-II 2DC score.18 Similarly, no benefit of balovaptan over placebo was observed in any secondary or exploratory end points, including Vineland-II standard scores, PedsQL, CGI-S, CGI-I, and the Aberrant Behavior Checklist, consistent with previous studies in adults.16,18 Balovaptan was well tolerated, and no balovaptan-associated safety issues were identified in children and adolescents, consistent with preceding clinical pharmacology studies in healthy volunteers and in the phase 2 VANILLA study in men with ASD.18 No safety pattern considered causally linked to balovaptan administration has become apparent to date. Of note, aV1ation is one of only a small number of well-powered pharmaceutical therapeutics’ trials in children and adolescents with ASD with a robust clinical trial design. These data may provide insights into this population, the role of vasopressin signaling in ASD, and the necessary considerations for appropriate trial design and outcome measures for pediatric participants.
As demonstrated in other ASD clinical trials, there was a robust placebo response for several outcome measures in this investigation.20 Similar improvements were experienced in both balovaptan and placebo treatment groups, further highlighting the placebo response. There is no criterion-standard outcome measure for evaluating improvement of the core symptoms of ASD in clinical trials,21 and many outcome measures have limitations.22,23 Assessments do not often assess ASD-specific symptoms or were not developed to measure change during treatment. All 3 commonly used measures, the Aberrant Behavior Checklist, the CGI, and the Vineland Adaptive Behavior Scales, have the potential for rater bias. Caregiver-reported measures may accentuate placebo effects, obscuring subtle changes that may happen in response to interventions.22 Clinician-rated measures, such as the Vineland Adaptive Behavior Scales and CGI scales, are heavily informed by caregiver reports. An unmet need remains to develop better outcome measures for ASD trials that focus on quantification of symptom improvement. An ideal outcome measure would be more closely related to the underlying neurobiological origins of ASD. Future trials may consider use of objective quantitative measures, such as digital biomarkers, behavioral task performance, electroencephalograms, or eye tracking to reduce expectation bias and treatment effect in placebo groups.
Expectation bias can affect participants, caregivers, and clinician raters, increasing the treatment effect seen in placebo groups.24 Expectation bias may have been more likely in this study owing to a demanding study schedule involving multiple laboratory assessments and the designation of balovaptan as a breakthrough therapy by the US Food and Drug Administration. Additionally, most study participants were taking medications at baseline and may have had positive prior experiences with medication, leading to increased expectation of balovaptan treatment benefit.
In the primary analysis population, approximately half of the participants were from academic research sites and half from private clinical research sites. In pediatric RCTs in major depressive disorder, the numbers and types of sites have been noted as important factors associated with placebo response.25 Randomizing individuals to a smaller number of larger-capacity sites with specialist expertise may reduce placebo response.
Lack of significant improvement from baseline in the primary or secondary outcome measures in the balovaptan group, particularly the CGI-I, suggests that balovaptan was not effective in this population of children and adolescents. Individuals with moderate social and communication challenges who were likely to have similar exposures to social opportunities were selected for by the eligibility criteria (SRS-2 T score ≥66, CGI-S score ≥4, and IQ ≥70); however, baseline factors can affect trial outcomes by preventing the demonstration of improvements and may also contribute to placebo response.25,26 It is worth noting that V1aduct, a phase 3, double-blind RCT in adults with ASD, also detected no benefit of balovaptan, 10 mg, on the Vineland-II 2DC primary end point.27 Although these negative results don’t support vasopressin signaling being a neurobiological driver in ASD, as ASD etiology is likely to be multifactorial, it remains possible that a subgroup of individuals with ASD may respond to vasopressin modulation, for example, children younger than 5 years who were not included in the study. The heterogeneity across autistic individuals may mean that there is no one-size-fits-all treatment. For example, genetic variation in V1a receptors has been observed,28 and it is possible that participants’ response to balovaptan may vary depending on their genetic background; however, future studies are required to determine the relationship between these factors. Further, randomization was not stratified for particular patterns of ASD symptoms, and the study was not powered for subgroup analyses; therefore, the study could not detect any treatment differences in specific subgroups of participants.
Importantly this work emphasizes ongoing challenges in the identification of treatments targeting core symptoms associated with ASD and highlights important study design considerations for future ASD trials and related disorders. Results from this trial can guide future drug development efforts in ASD based on candidate treatment mechanisms of action. As with balovaptan, several compounds with initial promise (eg, memantine, selective serotonin reuptake inhibitors, arbaclofen)29,30 have failed to demonstrate significant improvements in ASD symptoms when evaluated in large, multicenter RCTs. Contributing factors may include broad symptom and etiologic/mechanistic heterogeneity in ASD, life span developmental effects that may drive response, and the capacity and sensitivity of outcome measures to detect clinically meaningful differences.21,31 Additionally, placebo response rates of approximately 20% to 50% have been observed in several ASD clinical trials20,26 with limited information regarding causes. Elevated placebo response rates limit statistical power, making it more difficult to discern clinically meaningful change.
Limitations
A general limitation of this study was that the study population was not representative of the racial diversity of the US population, with a bias toward White, non-Latinx participants. The overall trial design of aV1ation may have contributed to difficulties in observing an effect of balovaptan. First, recruitment of older adolescents and children was separated to achieve the 2 objectives of confirming child and adolescent balovaptan 10-mg adult-equivalent dose exposure and determining the safety and efficacy at that exposure level. A smaller sample size than planned received the targeted adult-equivalent exposure of 10 mg, prompting study design changes to remove the 4-mg group when lower exposures were observed. With respect to developmental effects, research shows that early diagnosis and intervention in young children with ASD are most likely to have substantial long-term benefits in symptom improvement and skill development.32 There were few participants in the age range of 5 to 7 years in the study. Therefore, the study may not have had enough power to detect differences in the different age brackets owing to low sample sizes. The social communication behaviors that balovaptan aims to improve are often subtle, making them difficult to quantify, particularly over a relatively short period of time.23 Additionally, developmental gains in social communication are contingent upon recurring naturalistic opportunities for social learning.33
Conclusions
The phase 2 aV1ation RCT did not meet its primary efficacy end point of improvement in change from baseline in Vineland-II 2DC score with balovaptan treatment compared with placebo in children and adolescents with ASD and IQ of 70 or greater at 24 weeks. Balovaptan appeared to be well tolerated in children older than 5 years in this study, and no safety signals were detected. Results suggest that V1a receptor antagonism does not improve social and communication function in pediatric ASD.
Trial Protocol and Statistical Analysis Plan
eAppendix 1. Additional Methods
eAppendix 2. Procedures
eAppendix 3. Study Design
eAppendix 4. Statistical Analysis
eAppendix 5. Rationale for Safety Analysis Population
eAppendix 6. Rationale for Age-Adjusted Dosing
eTable 1. Baseline Autism Diagnostic Observation Schedule, 2nd Edition (ADOS-2) Scores
eTable 2. Summary of Participants Responding “Yes” During the 24-Week Double-Blind Period to the Columbia-Suicide Severity Rating Scale
eTable 3. Safety Evaluable Population Demographics and Baseline Characteristics
eFigure. Aberrant Behavior Checklist Mean Score
Data Sharing Statement
References
- 1.Hodges H, Fealko C, Soares N. Autism spectrum disorder: definition, epidemiology, causes, and clinical evaluation. Transl Pediatr. 2020;9(suppl 1):S55-S65. doi: 10.21037/tp.2019.09.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association Publishing; 2013. [Google Scholar]
- 3.Almandil NB, Alkuroud DN, AbdulAzeez S, AlSulaiman A, Elaissari A, Borgio JF. Environmental and genetic factors in autism spectrum disorders: special emphasis on data from Arabian studies. Int J Environ Res Public Health. 2019;16(4):658. doi: 10.3390/ijerph16040658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LeClerc S, Easley D. Pharmacological therapies for autism spectrum disorder: a review. P T. 2015;40(6):389-397. [PMC free article] [PubMed] [Google Scholar]
- 5.Howes OD, Rogdaki M, Findon JL, et al. Autism spectrum disorder: consensus guidelines on assessment, treatment and research from the British Association for Psychopharmacology. J Psychopharmacol. 2018;32(1):3-29. doi: 10.1177/0269881117741766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76(1):142-159. doi: 10.1016/j.neuron.2012.09.025 [DOI] [PubMed] [Google Scholar]
- 7.Grundschober CGA, Saxe M, Schnider P, Biemans B. A novel vasopressin V1a antagonist restores social behavior in the mouse cntnap2 KO model of autism. Presented at: International Society for Autism Research (INSAR) 2018 Annual Meeting; May 10, 2018; Rotterdam, Netherlands. [Google Scholar]
- 8.Parker KJ, Oztan O, Libove RA, et al. A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Sci Transl Med. 2019;11(491):eaau7356. doi: 10.1126/scitranslmed.aau7356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnider P, Bissantz C, Bruns A, et al. Discovery of balovaptan, a vasopressin 1a receptor antagonist for the treatment of autism spectrum disorder. J Med Chem. 2020;63(4):1511-1525. doi: 10.1021/acs.jmedchem.9b01478 [DOI] [PubMed] [Google Scholar]
- 10.Ratni H, Rogers-Evans M, Bissantz C, et al. Discovery of highly selective brain-penetrant vasopressin 1a antagonists for the potential treatment of autism via a chemogenomic and scaffold hopping approach. J Med Chem. 2015;58(5):2275-2289. doi: 10.1021/jm501745f [DOI] [PubMed] [Google Scholar]
- 11.Derks MGM, Wandel C, Young A, Bolt SK, Meyenberg C. Open-label assessment of the effects of itraconazole and rifampicin on balovaptan pharmacokinetics in healthy volunteers. Adv Ther. 2020;37(11):4720-4729. doi: 10.1007/s12325-020-01491-y [DOI] [PubMed] [Google Scholar]
- 12.Felix-Ortiz AC, Febo M. Gestational valproate alters BOLD activation in response to complex social and primary sensory stimuli. PLoS One. 2012;7(5):e37313. doi: 10.1371/journal.pone.0037313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umbricht D, Del Valle Rubido M, Hollander E, et al. A single dose, randomized, controlled proof-of-mechanism study of a novel vasopressin 1a receptor antagonist (RG7713) in high-functioning adults with autism spectrum disorder. Neuropsychopharmacology. 2017;42(9):1914-1923. doi: 10.1038/npp.2016.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.A study investigating the absolute oral bioavailability of balovaptan after single and multiple daily oral doses of balovaptan in healthy volunteers. ClinicalTrials.gov identifer: NCT03764449. Updated February 24, 2020. Accessed June 20, 2022. https://clinicaltrials.gov/ct2/show/NCT03764449
- 15.A study of RO5285119 in healthy volunteers. ClinicalTrials.gov identifer: NCT01418963. Updated November 2, 2016. Accessed June 20, 2022. https://clinicaltrials.gov/ct2/show/NCT01418963
- 16.Derks M, Lennon-Chrimes S, Guenther A, et al. Bioavailability and pharmacokinetic profile of balovaptan, a selective, brain-penetrant vasopressin 1a receptor antagonist, in healthy volunteers. Expert Opin Investig Drugs. 2021;30(8):893-901. doi: 10.1080/13543784.2021.1948009 [DOI] [PubMed] [Google Scholar]
- 17.A study of RG7314 to investigate efficacy and safety in individuals with autism spectrum disorders (ASDs). ClinicalTrials.gov identifier: NCT01793441. Updated February 3, 2017. Accessed June 20, 2022. https://clinicaltrials.gov/ct2/show/NCT01793441
- 18.Bolognani F, Del Valle Rubido M, Squassante L, et al. A phase 2 clinical trial of a vasopressin V1a receptor antagonist shows improved adaptive behaviors in men with autism spectrum disorder. Sci Transl Med. 2019;11(491):eaat7838. doi: 10.1126/scitranslmed.aat7838 [DOI] [PubMed] [Google Scholar]
- 19.Dietz PM, Rose CE, McArthur D, Maenner M. National and state estimates of adults with autism spectrum disorder. J Autism Dev Disord. 2020;50(12):4258-4266. doi: 10.1007/s10803-020-04494-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King BH, Dukes K, Donnelly CL, et al. Baseline factors predicting placebo response to treatment in children and adolescents with autism spectrum disorders: a multisite randomized clinical trial. JAMA Pediatr. 2013;167(11):1045-1052. doi: 10.1001/jamapediatrics.2013.2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolte EE, Diehl JJ. Measurement tools and target symptoms/skills used to assess treatment response for individuals with autism spectrum disorder. J Autism Dev Disord. 2013;43(11):2491-2501. doi: 10.1007/s10803-013-1798-7 [DOI] [PubMed] [Google Scholar]
- 22.McConachie H, Parr JR, Glod M, et al. Systematic review of tools to measure outcomes for young children with autism spectrum disorder. Health Technol Assess. 2015;19(41):1-506. doi: 10.3310/hta19410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anagnostou E, Jones N, Huerta M, et al. Measuring social communication behaviors as a treatment endpoint in individuals with autism spectrum disorder. Autism. 2015;19(5):622-636. doi: 10.1177/1362361314542955 [DOI] [PubMed] [Google Scholar]
- 24.Williams JB, Popp D, Kobak KA, Detke MJ. P-640—the power of expectation bias. Eur Psychiatry. 2012;27(1):1. doi: 10.1016/S0924-9338(12)74807-122153731 [DOI] [Google Scholar]
- 25.Bridge JA, Birmaher B, Iyengar S, Barbe RP, Brent DA. Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. Am J Psychiatry. 2009;166(1):42-49. doi: 10.1176/appi.ajp.2008.08020247 [DOI] [PubMed] [Google Scholar]
- 26.Siafis S, Çıray O, Schneider-Thoma J, et al. Placebo response in pharmacological and dietary supplement trials of autism spectrum disorder (ASD): systematic review and meta-regression analysis. Mol Autism. 2020;11(1):66. doi: 10.1186/s13229-020-00372-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacob S, Veenstra-VanderWeele J, Murphy D, et al. Efficacy and safety of balovaptan for socialisation and communication difficulties in autistic adults in North America and Europe: a phase 3, randomised, placebo-controlled trial. Lancet Psychiatry. 2022;9(3):199-210. doi: 10.1016/S2215-0366(21)00429-6 [DOI] [PubMed] [Google Scholar]
- 28.Tansey KE, Hill MJ, Cochrane LE, Gill M, Anney RJ, Gallagher L. Functionality of promoter microsatellites of arginine vasopressin receptor 1A (AVPR1A): implications for autism. Mol Autism. 2011;2(1):3. doi: 10.1186/2040-2392-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veenstra-VanderWeele J, Cook EH, King BH, et al. Arbaclofen in children and adolescents with autism spectrum disorder: a randomized, controlled, phase 2 trial. Neuropsychopharmacology. 2017;42(7):1390-1398. doi: 10.1038/npp.2016.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fosdick C, Wink L, McClellan L, Dominick K, Pedapati E, Erickson C. Pharmacologic treatment options for children and adolescents with autism spectrum disorder. Pharma J. Published online October 10, 2017. doi: 10.1211/PJ.2017.20203390 [DOI] [Google Scholar]
- 31.Anagnostou E. Clinical trials in autism spectrum disorder: evidence, challenges and future directions. Curr Opin Neurol. 2018;31(2):119-125. doi: 10.1097/WCO.0000000000000542 [DOI] [PubMed] [Google Scholar]
- 32.Pierce K, Courchesne E, Bacon E. To screen or not to screen universally for autism is not the question: why the task force got it wrong. J Pediatr. 2016;176:182-194. doi: 10.1016/j.jpeds.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paul R. Interventions to improve communication in autism. Child Adolesc Psychiatr Clin N Am. 2008;17(4):835-856, ix-x. ix–x. doi: 10.1016/j.chc.2008.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eAppendix 1. Additional Methods
eAppendix 2. Procedures
eAppendix 3. Study Design
eAppendix 4. Statistical Analysis
eAppendix 5. Rationale for Safety Analysis Population
eAppendix 6. Rationale for Age-Adjusted Dosing
eTable 1. Baseline Autism Diagnostic Observation Schedule, 2nd Edition (ADOS-2) Scores
eTable 2. Summary of Participants Responding “Yes” During the 24-Week Double-Blind Period to the Columbia-Suicide Severity Rating Scale
eTable 3. Safety Evaluable Population Demographics and Baseline Characteristics
eFigure. Aberrant Behavior Checklist Mean Score
Data Sharing Statement