Key Points
Questions
What is the drug survival of adalimumab, ustekinumab, secukinumab, guselkumab, ixekizumab that is associated with effectiveness and safety in patients with psoriasis in the UK and Ireland, and are there any patient factors that are associated with survival of each biologic differently that could help treatment stratification (ie, effect modifiers)?
Findings
In this cohort study of 16 122 treatment courses in patients with psoriasis, guselkumab had the highest overall drug survival associated with the effectiveness and safety of the included biologics. Psoriatic arthritis, nail involvement, previous biologic exposure, and ethnicity were effect modifiers for biologics and their survival in association with treatment effectiveness.
Meaning
The results of this cohort study on longer-term treatment persistence, safety, and tolerability of biologics will be potentially important for patients and their clinicians when making an informed decision to initiate treatment with a particular biologic therapy.
Abstract
Importance
Drug survival of biologic therapies for psoriasis is a proxy for longer-term treatment effectiveness and safety. Patient factors that are associated with the survival of each biologic differently (effect modifiers) may inform the decision to choose between biologics.
Objective
To assess the drug survival associated with the effectiveness and safety of commonly used biologics for psoriasis in the UK and Ireland and identify effect modifiers for these biologics and their survival.
Design, Setting, and Participants
We conducted a prospective cohort study of patients with psoriasis using data from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) between November 2007 and August 2021.
Exposures
Adalimumab, ustekinumab, secukinumab, guselkumab, ixekizumab.
Main Outcomes and Measures
We conducted a survival analysis and fitted separate flexible parametric models for drug survival as a proxy for effectiveness and safety.
Results
A total of 16 122 treatment courses were included: 6607 (41.0%) in which treatment with adalimumab was initiated, 5405 (33.5%) with ustekinumab, 2677 (16.6%) with secukinumab, 730 (4.5%) with guselkumab, and 703 (4.4%) with ixekizumab. The crude survival functions at year 1 for measures of effectiveness for treatment with adalimumab was 0.81 (95% CI, 0.80-0.82), 0.89 for ustekinumab (95% CI, 0.88-0.89), 0.86 for secukinumab (95% CI, 0.85-0.87), 0.94 for guselkumab (95% CI, 0.92-0.96), and 0.86 for ixekizumab (95% CI, 0.83-0.89). The adjusted survival curves from the multivariable model for effectiveness showed that treatment with guselkumab had the higher survival (adjusted hazard ratio, 0.13; 95% CI, 0.03-0.56) and adalimumab had the lower survival (adjusted hazard ratio, 2.37; 95% CI, 2.03-2.76) compared with ustekinumab. Secukinumab and ixekizumab had similar survival curves over time. Psoriatic arthritis, previous biologic exposure, nail involvement, and ethnicity were effect modifiers for survival in association with treatment effectiveness. The crude survival functions at year 1 for safety were 0.91 for treatment with adalimumab (95% CI, 0.90-0.91), 0.94 for ustekinumab (95% CI, 0.94-0.95), 0.94 for secukinumab (95% CI, 0.92-0.94), 0.96 for guselkumab (95% CI, 0.94-0.98), and 0.92 for ixekizumab (95% CI, 0.89-0.94). Guselkumab, ustekinumab, and secukinumab had similar adjusted survival curves for safety, while adalimumab (adjusted hazard ratio, 1.66; 95% CI, 1.46-1.89) and ixekizumab (adjusted hazard ratio, 1.52; 95% CI, 1.13-2.03) had lower survival compared with ustekinumab.
Conclusions and Relevance
The results of this cohort study suggest that guselkumab had the highest drug survival in BADBIR of the included biologics for treatment persistence that was associated with effectiveness, and guselkumab had highest drug survival for safety compared with other biologics except ustekinumab. Psoriatic arthritis, nail involvement, previous biologic exposure, and ethnicity were effect modifiers for biologics and their survival in association with treatment effectiveness. This information on longer-term treatment persistence, safety, and tolerability may help patients and their clinicians make an informed decision to initiate treatment with a biologic therapy.
This cohort study examines the drug survival associated with the effectiveness and safety of commonly used biologics for psoriasis in the UK and Ireland.
Introduction
Network meta-analyses of randomized clinical trials in psoriasis have suggested that newer biologics that target interleukin (IL)-23 p19 and IL-17 generally have a higher treatment efficacy compared with the older biologics that target tumor necrosis factor(TNF)–α and ustekinumab.1 However, drug response and safety of treatment with biologics for psoriasis in clinical trials do not fully reflect the routine clinical setting.2,3
Drug survival is a proxy measure for the effectiveness, safety, adherence, and tolerability of a medicine. Effect modification describes whether the effect of a treatment differs in groups of patients with different characteristics, in contrast to confounding, which represents a distortion of the true treatment effect by another variable. Identifying effect modifiers for the biologics used for psoriasis management and their drug survival in routine clinical practice may help patients and clinicians identify the best treatment option, potentially avoiding or delaying treatment failure. We previously reported on the drug survival of adalimumab, secukinumab, and ustekinumab using earlier data from the British Association of Dermatologists Biologics and Immunomodulators (BADBIR), a large, representative, national, and prospective psoriasis registry in the United Kingdom and the Republic of Ireland, and identified that prior experience with biologics and psoriatic arthritis (PsA) were effect modifiers between biologics and drug survival associated with the effectiveness of treatment.4 Several studies have since published conflicting results on the drug survival of ixekizumab, an IL-17A inhibitor,5,6,7,8 and guselkumab, an IL-23 p19 inhibitor.6 The objectives for this cohort study using data from BADBIR were to report on the drug survival in association with the effectiveness and safety of treatment with ixekizumab and guselkumab, as well as adalimumab, ustekinumab, and secukinumab, in a larger up-to-date data snapshot and identify effect modifiers for the association between biologic and drug survival associated with effectiveness and safety.
Methods
Data Source and Study Population
The structure, study design, and baseline characteristics of the patients recruited for BADBIR have been reported previously.9,10 BADBIR is a large, ongoing pharmacovigilance registry of patients with psoriasis in the UK and Republic of Ireland that was established in September 2007. To date, 165 secondary care dermatology centers have contributed data to BADBIR. Patients are recruited to 3 different cohorts depending on the drug of initiation: nonbiologic systemic therapies, oral small molecules, and biologic therapies. Data are collected every 6 months for the first 3 years, then annually thereafter. Details of the biologic therapies, including initiation and discontinuation dates and reasons for discontinuation and gaps in treatment, are obtained during follow-up visits. Data from the start of the registry until August 1, 2021, were used in this study. BADBIR was approved in by the National Health Service Research Ethics Committee North-West England. All participants provided written informed consent for their participation in the registry.
Data Analysis
Patients eligible for this study had chronic plaque psoriasis and were recruited or switched into the biologic cohort after initiating treatment with either adalimumab (Humira; AbbVie), ustekinumab (Stelara; Janssen), secukinumab (Cosentyx; Novartis), guselkumab (Tremfya; Janssen), or ixekizumab (Taltz; Lilly). In the absence of evidence for the interexchangeability of biosimilars compared with originators, only originators were included. Risankizumab, tildrakizumab, and brodalumab were not included because of comparatively lower numbers (n < 500). Patients contributed data to the study if at least 1 follow-up visit had occurred. We included data from patients who were receiving treatment with any of these therapies and counted each treatment course separately (ie, a patient who received treatment with adalimumab then ustekinumab would contribute a treatment course each for adalimumab and ustekinumab). We used the same definition for drug survival as in our previous studies,4,11 with discontinuation of therapy, which was defined as any gap in treatment for more than 90 days. We evaluated discontinuation associated with ineffectiveness or adverse events (AEs); discontinuation because of other reasons was censored, along with patients, at the last available follow-up date.
We performed a descriptive summary of the baseline characteristics of the biologic cohorts and reported the number of missing values in each. We performed a Kaplan-Meier survival analysis for each biologic and reported survival functions at 1 and 2 years. We stratified by discontinuation associated with ineffectiveness or AEs.
Model Development
We fitted 2 models, the first on the outcome of biologic discontinuation associated with ineffectiveness and the second on discontinuation associated with an AE. We used a 2-tier predictor selection process. The first tier identified covariates that were consistently found to be associated with biologic drug survival in psoriasis across different studies. These were age, sex, body mass index (calculated as weight in kilograms divided by height in meters squared), previous biologic treatments (a categorical variable of 0 for first-line therapy, 1 for second-line therapy, and 2 for third or further lines of therapy), and PsA for the biologic effectiveness model; these were age and sex in the biologic safety model. We included other covariates in the second tier, which were identified as a priori potential predictors for drug survival or discontinuation from our previous studies and a systematic review.12 These covariates were race and ethnicity (Asian [Chinese, Indian, and Pakistani], White, and Other racial and ethnic minority groups [which included Black British individuals]), baseline psoriasis area and severity index (PASI) score, smoking, alcohol intake, chronic obstructive pulmonary disease, type 1 diabetes, dyslipidemia, number of comorbid conditions, time-dependent concomitant use of methotrexate or cyclosporine, waist circumference, nail psoriasis, palmoplantar psoriasis, flexural psoriasis, scalp psoriasis, and unstable psoriasis. We included the biologics as a categorical variable and used ustekinumab as the main comparator to report the analyses, as ustekinumab was the drug with highest survival in our previous analyses.4,11
We fitted flexible parametric Royston-Palmar models using the stpm2 command in Stata, which allows modeling nonproportional effects of covariates,13 as we previously found the relative drug survival of secukinumab and ustekinumab to vary over time.4 The number of knots for the restricted cubic spline function was selected to give the smallest Akaike information criterion and the Bayesian information criterion. Missing data were accounted for with 20 multiply-imputed data sets. We used the mfpmi command in Stata14 to test the second-tier covariates for inclusion into the model using backward stepwise regression (P value of .10 as the cutoff) along with testing for fractional polynomial transformation for continuous predictors to account for nonlinearity. We investigated for potential effect modification by the categorical covariates that were selected in these models for the association between biologic therapy and subsequent drug survival using sequential likelihood ratio tests that compared models with and without an interaction term between the covariate and biologic. We reported the hazard ratios from the 2 models and plotted population-averaged survival curves for each biologic overlaid on corresponding crude Kaplan-Meier curves.
Sensitivity Analysis
Several sensitivity analyses were performed. We performed a complete case analysis to account for missing data instead of using multiple imputation. We restricted the data to people who were initiating treatment with biologic therapies between January 2018 and December 2021, as this was a period when all biologic therapies assessed in this study were available in BADBIR, and fitted the negative outcome and AE models. We conducted separate analyses that restricted the cohorts to second or third and subsequent lines of biologic therapy. We tested previous failure of individual or class of biologics in association with ineffectiveness or AEs for effect modification between biologic and outcomes of drug discontinuation associated with ineffectiveness and safety, respectively, in these 2 restricted cohorts using sequential likelihood ratio tests with and without the interaction term. Race and ethnicity were not included in these analyses because of data sparsity. All analyses were performed using Stata, version 16.1 (StataCorp). The study was reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Results
A total of 16 122 treatment courses were eligible for inclusion, with 6607 (41.0%) receiving treatment with adalimumab, 5405 (33.5%) ustekinumab, 2677 (16.6%) secukinumab, 730 (4.5%) guselkumab, and 703 (4.4%) ixekizumab. The overall median age of the cohort at initiation of therapy was 46.0 years (IQR, 36.0-55.0 years), with a median body mass index of 30.4 (IQR, 26.5-35.4) and a median PASI score of 12.7 (IQR, 9.5-18.0). The baseline characteristics of the cohort separated by biologic therapy, along with the proportion of missing data, are presented in Table 1. Notable differences between the biologic cohorts included proportion of patients with PsA (treatment with adalimumab, 29.2%; secukinumab, 34.4%; ustekinumab, 23.5%; ixekizumab, 41.0%; guselkumab. 26.0%) and the proportion of biologic-naive patients (treatment with adalimumab, 74.8%; secukinumab, 35.8%; ustekinumab, 46.5%; ixekizumab, 18.2%; guselkumab, 23.6%).
Table 1. Baseline Demographic and Disease Characteristics of the Biologic Cohorts.
| Baseline demographic and disease characteristic | No. (%) | ||||
|---|---|---|---|---|---|
| Adalimumab (n = 6607) | Secukinumab (n = 2677) | Ustekinumab (n = 5405) | Ixekizumab (n = 703) | Guselkumab (n = 730) | |
| Age, median (IQR), y | 45.0 (35.0-54.0) | 47.0 (37.0-56.0) | 46.0 (36.0-55.0) | 46.0 (36.0-56.0) | 48.0 (36.0-56.0) |
| Female sex | 2762 (41.8) | 1144 (42.7) | 2277 (42.1) | 309 (44.0) | 318 (43.6) |
| Race and ethnicity | |||||
| Asian | 266 (4.0) | 162 (6.1) | 278 (5.1) | 41 (5.8) | 45 (6.2) |
| Missinga | 818 (12.4) | 365 (13.6) | 556 (10.3) | 117 (16.6) | 115 (15.8) |
| Otherb | 233 (3.5) | 118 (4.4) | 207 (3.8) | 41 (5.8) | 32 (4.4) |
| White | 5290 (80.1) | 2032 (75.9) | 4364 (80.7) | 504 (71.7) | 538 (73.7) |
| BMI | |||||
| Median (IQR) | 29.8 (26.1-34.4) | 31.0 (27.1-36.2) | 30.8 (26.4-36.0) | 31.4 (27.3-36.7) | 31.6 (27.5-37.7) |
| Missinga | 1178 (17.8) | 634 (23.7) | 970 (17.9) | 194 (27.6) | 253 (34.7) |
| Waist circumference | |||||
| Median (IQR), cm | 100.0 (90.0-111.0) | 103.0 (93.0-115.0) | 102.5 (91.0-114.0) | 104.0 (94.0-115.0) | 104.0 (94.0-118.0) |
| Missinga | 1448 (21.9) | 876 (32.7) | 1352 (25.0) | 270 (38.4) | 311 (42.6) |
| Alcohol intake by categories | |||||
| No alcohol intake | 1778 (26.9) | 715 (26.7) | 1612 (29.8) | 168 (23.9) | 166 (22.7) |
| Lower-risk drinking (<21 U/wk for men, <14 U/wk for women) | 2717 (41.1) | 852 (31.8) | 2073 (38.4) | 167 (23.8) | 176 (24.1) |
| Hazardous drinking (21-49 U/wk for men, 14- 34 U/wk for women) | 588 (8.9) | 190 (7.1) | 409 (7.6) | 31 (4.4) | 46 (6.3) |
| Harmful drinking (≥50 U/wk for men, ≥35 U/wk for women) | 89 (1.3) | 20 (0.7) | 69 (1.3) | 5 (0.7) | 6 (0.8) |
| Missinga | 1435 (21.7) | 900 (33.6) | 1242 (23.0) | 332 (47.2) | 336 (46.0) |
| Smoking status | |||||
| Never | 3072 (46.5) | 1234 (46.1) | 2307 (42.7) | 326 (46.4) | 323 (44.2) |
| Previous | 2031 (30.7) | 860 (32.1) | 1789 (33.1) | 222 (31.6) | 258 (35.3) |
| Current | 1504 (22.8) | 583 (21.8) | 1309 (24.2) | 155 (22.0) | 149 (20.4) |
| Missinga | 1354 (20.5) | 742 (27.7) | 1081 (20.0) | 275 (39.1) | 264 (36.2) |
| Disease duration, median (IQR), y | 20.0 (12.0-30.0) | 20.0 (12.0-30.0) | 20.0 (12.0-30.0) | 21.0 (14.0-31.0) | 21.0 (13.0-31.0) |
| DLQI score | |||||
| Baseline, median (IQR) | 17.0 (11.0-23.0) | 16.0 (11.0-22.0) | 16.0 (10.0-22.0) | 15.0 (9.0-24.0) | 14.0 (8.0-20.0) |
| Missing | 3030 (45.9) | 1375 (51.4) | 2615 (48.4) | 408 (58.0) | 391 (53.6) |
| PASI score | |||||
| Baseline, median (IQR) | 13.3 (10.2-18.5) | 12.2 (8.0-17.5) | 12.6 (9.2-17.8) | 11.4 (6.8-16.8) | 11.0 (7.0-16.0) |
| Missinga | 912 (13.8) | 492 (18.4) | 774 (14.3) | 126 (17.9) | 130 (17.8) |
| Psoriatic arthritis | 1928 (29.2) | 922 (34.4) | 1269 (23.5) | 288 (41.0) | 190 (26.0) |
| Psoriasis | |||||
| Nail | 3683 (55.7) | 1414 (52.8) | 2847 (52.7) | 419 (59.6) | 376 (51.5) |
| Palmoplantar | 1234 (18.7) | 532 (19.9) | 1058 (19.6) | 151 (21.5) | 139 (19.0) |
| Scalp | 4674 (70.7) | 1880 (70.2) | 3876 (71.7) | 519 (73.8) | 522 (71.5) |
| Flexural | 2472 (37.4) | 944 (35.3) | 2024 (37.4) | 286 (40.7) | 280 (38.4) |
| Unstable | 756 (11.4) | 326 (12.2) | 587 (10.9) | 85 (12.1) | 78 (10.7) |
| No. of previous biologic therapies | |||||
| None | 4943 (74.8) | 959 (35.8) | 2512 (46.5) | 128 (18.2) | 172 (23.6) |
| 1 | 1278 (19.3) | 898 (33.5) | 2021 (37.4) | 205 (29.2) | 193 (26.4) |
| ≥2 | 386 (5.8) | 820 (30.6) | 872 (16.1) | 370 (52.6) | 365 (50.0) |
| During follow-up | |||||
| Any treatment with methotrexate | 1004 (15.2) | 280 (10.5) | 559 (10.3) | 52 (7.4) | 33 (4.5) |
| Any treatment with cyclosporine | 344 (5.2) | 78 (2.9) | 243 (4.5) | 29 (4.1) | 11 (1.5) |
| Any treatment with fumaric acid esters | 48 (0.7) | 9 (0.3) | 26 (0.5) | 0 | 1 (0.1) |
| Any treatment with apremilast | 10 (0.2) | 26 (1.0) | 20 (0.4) | 7 (1.0) | 2 (0.3) |
| No. of comorbid conditions | |||||
| None | 1594 (24.1) | 655 (24.5) | 1418 (26.2) | 139 (19.8) | 184 (25.2) |
| 1-2 | 3638 (55.1) | 1457 (54.4) | 2730 (50.5) | 376 (53.5) | 374 (51.2) |
| 3-4 | 1134 (17.2) | 457 (17.1) | 1001 (18.5) | 156 (22.2) | 139 (19.0) |
| ≥5 | 241 (3.6) | 108 (4.0) | 256 (4.7) | 32 (4.6) | 33 (4.5) |
| COPD | 107 (1.6) | 57 (2.1) | 118 (2.2) | 13 (1.8) | 18 (2.5) |
| Diabetes | 564 (8.5) | 295 (11.0) | 570 (10.5) | 86 (12.2) | 88 (12.1) |
| Dyslipidaemia | 638 (9.7) | 212 (7.9) | 517 (9.6) | 78 (11.1) | 60 (8.2) |
| Hypertension | 1563 (23.7) | 625 (23.3) | 1377 (25.5) | 171 (24.3) | 171 (23.4) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); COPD, chronic obstructive pulmonary disease; DLQI, Dermatology Life Quality Index; PASI, baseline psoriasis area and severity index.
The amount of missing data given for covariates with missing data.
Other included Black British individuals.
The overall median follow-up time was 2.1 years. Ustekinumab, with a median of 2.7 years (IQR, 1.2-4.6 years), had the longest accrued follow-up time, followed by adalimumab (median, 2.3 years [IQR, 0.8, 4.8 years]), secukinumab (median, 1.9 years [IQR, 0.9-3.0 years]), ixekizumab (median, 1.3 years [IQR, 0.6-2.0 years]), and then guselkumab (median, 1.1 years [IQR, 0.6-1.7 years]). The survival functions for the biologic cohorts are listed in Table 2. Guselkumab had the highest crude drug survival across outcomes.
Table 2. Survival Function at Years 1 and 2 for the Biologic Cohorts Stratified by Reason for Discontinuationa.
| Reasons for drug discontinuation | Adalimumab (n = 6607) | Secukinumab (n = 2677) | Ustekinumab (n = 5405) | Ixekizumab (n = 703) | Guselkumab (n = 730) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total participants/No. of discontinuations | Survival function (95% CI) | Total participants/No. of discontinuations | Survival function (95% CI) | Total participants/No. of discontinuations | Survival function (95% CI) | Total participants/No. of discontinuations | Survival function (95% CI) | Total participants/No. of discontinuations | Survival function (95% CI) | |
| Overall | ||||||||||
| Year 1 | 4693/1629 | 0.75 (0.74-0.76) | 1942/467 | 0.81 (0.80-0.83) | 4304/789 | 0.85 (0.84-0.86) | 410/119 | 0.80 (0.77-0.83) | 408/75 | 0.88 (0.85-0.90) |
| Year 2 | 3533/903 | 0.60 (0.59-0.61) | 1262/342 | 0.66 (0.64-0.68) | 3308/561 | 0.73 (0.72-0.75) | 170/78 | 0.61 (0.57-0.66) | 99/23 | 0.80 (0.76-0.84) |
| Ineffectiveness | ||||||||||
| Year 1 | 4377/1164 | 0.81 (0.80-0.82) | 1859/336 | 0.86 (0.85-0.87) | 4152/582 | 0.89 (0.88-0.89) | 392/80 | 0.86 (0.83-0.89) | 406/34 | 0.94 (0.92-0.96) |
| Year 2 | 3345/260 | 0.76 (0.75-0.77) | 1201/205 | 0.75 (0.74-0.77) | 3184/245 | 0.83 (0.82-0.84) | 165/39 | 0.75 (0.71-0.79) | 99/6 | 0.92 (0.89-0.94) |
| Adverse events | ||||||||||
| Year 1 | 4524/561 | 0.91 (0.90-0.91) | 1920/160 | 0.94 (0.92-0.94) | 4235/280 | 0.94 (0.94-0.95) | 404/51 | 0.92 (0.89-0.94) | 407/23 | 0.96 (0.94-0.98) |
| Year 2 | 3411/136 | 0.88 (0.87-0.89) | 1247/54 | 0.90 (0.89-0.92) | 3254/123 | 0.91 (0.91-0.92) | 166/13 | 0.87 (0.84-0.90) | 99/10 | 0.93 (0.91-0.95) |
Survival serves as a proxy for effectiveness and safety.
Flexible Parametric Modeling Results
Univariable analysis for each covariate is presented in eTable 1 in Supplement 1. Separate interaction terms between biologic choice and PsA; nail involvement, biologic line of therapy, and race and ethnicity were included in the multivariable analysis for the effectiveness model; dyslipidemia was included in an interaction term with biologic choice for the safety model.
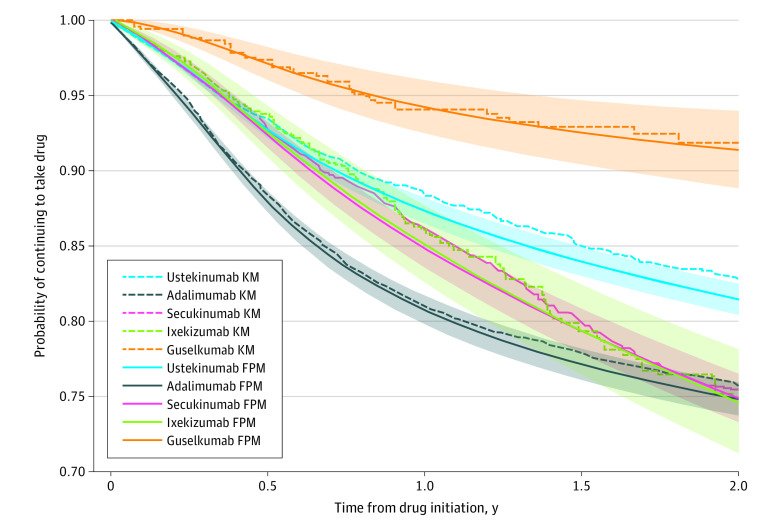
The hazard ratios from the multivariable effectiveness model are presented in Table 3 and eTable 2 in Supplement 1; the adjusted survival curves are presented in Figure 1. The adjusted survival curves from the multivariable model for effectiveness in Figure 1 show that guselkumab had the highest survival and adalimumab the lowest survival. Secukinumab and ixekizumab have nearly identical survival curves over time and reached a similar estimate of survival at 2 years as adalimumab. eFigures 1, 2, and 3 in Supplement 1 show the survival curves of the biologics stratified by PsA status, nail involvement, and race and ethnicity, respectively. The results were similar, showing the highest drug survival in people receiving treatment with guselkumab regardless of strata. People without PsA or nail involvement had the highest persistence with treatment with ustekinumab, while neither PsA nor nail involvement stratified for drug survival of secukinumab, ixekizumab, or adalimumab. Adalimumab appeared to have lower drug survival compared with ustekinumab in Asian patients (hazard ratio [HR], 3.10; 95% CI, 2.24-4.29) than in White individuals (HR, 2.36; 95% CI, 2.02-2.76) or individuals of other racial and ethnic minority groups (HR, 2.09; 95% CI, 1.39-3.15; Table 3).
Table 3. Final Multivariable Flexible Parametric Survival Model for Drug Discontinuation Associated With Ineffectivenessa.
| Covariate | Hazard ratio (95% CI) |
|---|---|
| Age | 0.99 (0.99-1.00) |
| Female sex | 1.11 (1.04-1.19)b |
| Baseline Psoriasis Area and Severity Index | 1.01 (1.01-1.02)b |
| BMIc | 0.01 (0.01-0.03)b |
| Palmoplantar psoriasis | 1.10 (1.01-1.20)b |
| Diabetes | 1.22 (1.09-1.35)b |
| COPD | 1.26 (1.00-1.58) |
| Flexural psoriasis | 1.06 (0.99-1.14) |
| Methotrexate use | 1.19 (1.05-1.33)b |
| Cyclosporine use | 3.00 (2.55-3.53)b |
| Psoriatic arthritis (in patients receiving treatment with ustekinumab) | 1.18 (1.03-1.35)b |
| Nail involvement (in patients receiving treatment with ustekinumab) | 1.23 (1.09-1.38)b |
| Previous biologic experience (compared with biologic-naive patients) | |
| 1 Previous biologic (in patients receiving treatment with ustekinumab) | 1.62 (1.42-1.85)b |
| 2 Previous biologics (in patients receiving treatment with ustekinumab) | 1.69 (1.43-2.00)b |
| Race and ethnicity (compared with White individuals) | |
| Asian | 1.21 (0.96-1.52) |
| Otherd | 1.20 (0.89-1.64) |
| Biologic therapies | |
| Biologic-naive White patients with no PsA and nail involvement | |
| Ustekinumab | 1 [Reference] |
| Adalimumab | 2.36 (2.02-2.76)b |
| Secukinumab | 1.22 (0.96-1.53) |
| Ixekizumab | 0.51 (0.22-1.20) |
| Guselkumab | 0.14 (0.03-0.61)b |
| In patients with PsA | |
| Ustekinumab | 1 [Reference] |
| Adalimumab | 1.81 (1.46-2.23)b |
| Secukinumab | 0.99 (0.74-1.32) |
| Ixekizumab | 0.41 (0.16-1.02) |
| Guselkumab | 0.07 (0.02-0.37)b |
| In patients with 1 previous biologic | |
| Ustekinumab | 1 [Reference] |
| Adalimumab | 1.51 (1.26-1.82)b |
| Secukinumab | 1.38 (1.11-1.72)b |
| Ixekizumab | 0.84 (0.50-1.41) |
| Guselkumab | 0.20 (0.08-0.55)b |
| In patients with ≥2 previous biologics | |
| Ustekinumab | 1 [Reference] |
| Adalimumab | 2.36 (1.85-3.03)b |
| Secukinumab | 1.85 (1.46-2.35)b |
| Ixekizumab | 2.07 (1.39-3.08)b |
| Guselkumab | 0.78 (0.41-1.50) |
| In patients with nail involvement | |
| Ustekinumab | 1 [Reference] |
| Adalimumab | 1.91 (1.65-2.21)b |
| Secukinumab | 1.09 (0.87-1.37) |
| Ixekizumab | 0.40 (0.17-0.92)b |
| Guselkumab | 0.10 (0.02-0.44)b |
| In Asian patients | |
| Ustekinumab | 1 [Reference] |
| Adalimumab | 3.10 (2.24-4.29)b |
| Secukinumab | 0.89 (0.57-1.37) |
| Ixekizumab | 0.43 (0.14-1.26) |
| Guselkumab | 0.04 (0.00-0.46)b |
| In patients of other racial and ethnic minority groups | |
| Ustekinumab | 1 [Reference] |
| Adalimumab | 2.09 (1.39-3.15)b |
| Secukinumab | 0.73 (0.43-1.25) |
| Ixekizumab | 0.46 (0.15-1.42) |
| Guselkumab | 0.13 (0.02-0.89)b |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); COPD, chronic obstructive pulmonary disease; PsA, psoriatic arthritis.
Comparisons of model fit statistics suggested that 4 knots and 1 knot be placed for the restricted cubic splines to model the baseline hazard and the time-dependent effect of biologic treatment, respectively. For the interactions, the effects of the biologic therapies are compared with each other in each covariate strata.
Statistically significant results (P < .05).
BMI transformation = (BMI/10)−2.
Other race and ethnicity included Black British individuals.
Figure 1. Overlaid Kaplan-Meier (KM) Survival Curves and the Flexible Parametric Model (FPM) Survival Curves for Discontinuation Associated With Ineffectiveness for All Biologic Cohorts During 2 Years.
The FPM curve is calculated using the Stata stpm2 meansurv command, which calculates the population-averaged survival curve in which a predicted survival curve is obtained for each participant and all the survival curves in a population are averaged. Shaded areas represent 95% CIs from the FPM curve. The y-axis starts from 0.70 for presentation clarity purposes.
eFigure 2 in Supplement 1 shows the biologic survival curves stratified by line of biologic therapy. The treatments with the highest survival included biologic-naive and second-line patients receiving treatment with guselkumab, and biologic-naive patients receiving treatment with ixekizumab. The treatments with the lowest survival included third or subsequent line patients using adalimumab, secukinumab, and ixekizumab, with the IL-17A inhibitors having higher survival initially but converging with adalimumab at 2 years.
The multivariable safety model is presented in eTable 3 and eFigure 4 in Supplement 1. Guselkumab, ustekinumab, and secukinumab had similar survival curves, while adalimumab (HR, 1.66; 95% CI, 1.46-1.89) and ixekizumab (HR, 1.52; 95% CI, 1.13-2.03) had lower survival for safety compared with ustekinumab. eFigure 4 in Supplement 1 shows the stratified biologic survival curves by dyslipidemia status. The stratified ixekizumab survival curves suggest that the persistence of those with dyslipidemia was higher than those without dyslipidemia, while the reverse was found for the other biologics.
Sensitivity Analyses
The complete case analysis yielded similar results to the multiple imputation analysis (unpublished data; Yiu ZZN, 2022). The effectiveness analysis involving the cohort starting from 2018 onwards are presented in eTable 4 and eFigure 5 in Supplement 1, and the corresponding safety analyses are presented in eTable 5 and eFigure 6 in Supplement 1, which were similar to the analyses in the full data set. The drug survival for safety for ixekizumab compared with adalimumab in eFigure 6 in Supplement 1 was not statistically significant (HR, 1.26; 95% CI, 0.86-1.84; results obtained from lincom command after the multivariable model fitted in eTable 4 in Supplement 1). There was also no significant difference between guselkumab and ustekinumab in this model.
In the second-line biologic sensitivity analyses, we found that previous failure of treatment with adalimumab associated with ineffectiveness reduced the survival of second-line ustekinumab (eFigure 7 in the Supplement; previous failure of TNF inhibitors produced near identical curves), while previous failure of treatment with ustekinumab associated with ineffectiveness was associated with reduced survival of all second-line biologics apart from guselkumab (eFigure 8 in Supplement 1). For safety, previous failure of treatment with adalimumab owing to AEs was associated with reduced survival of second-line ustekinumab and secukinumab (eFigure 9 in Supplement 1). In the third-line biologic sensitivity analysis, we found that patients who were receiving treatment with ustekinumab third line who had previous failure with IL-17 inhibitors owing to AEs had a lower drug survival than the other cohorts, but there was significant uncertainty in this estimate (eFigure 10 in Supplement 1).
Discussion
In this cohort study, the overall drug survival for all included biologics associated with effectiveness and safety was high. The results suggest that guselkumab had the highest drug survival of the included biologics in association with effectiveness, and guselkumab had highest drug survival for safety compared with other biologics, except ustekinumab. The results suggest that PsA, previous biologic experience, nail involvement, and race and ethnicity were effect modifiers for biologics and the risk of discontinuation associated with ineffectiveness. The sensitivity analyses found that the relative survival for the included biologics did not change substantially with time.
To our knowledge, this is the largest observational study assessing treatment with guselkumab and ixekizumab in patients with psoriasis to date. We used a flexible parametric survival multivariable regression model that allowed us to investigate and interpret the association of each covariate and assess for effect modification regarding the outcome. This would not be possible for analyses using methods such as propensity score or certain machine learning approaches that do not present coefficients for each covariate, or for alternative methods, such as a Fine and Gray model with subhazard ratios, that are difficult to interpret and/or have unreasonable assumptions. To our knowledge, there have been few consistent clinical or biomarkers that have acted as effect modifiers reported in the literature thus far. This may be because of (1) few true underlying phenotypic covariates that differentiate treatment response to a significant degree, and the fact that the higher the overall treatment response of present or new biologics the more likely we will not find such a covariate; or (2) the high costs of routinely collecting data on a biomarkers in clinical practice to validate in an observational study. In this article, we have described 4 potentially promising clinical markers of response that, if replicated in other large cohorts, may be helpful for treatment stratification in psoriasis.
Insights Into Lasting Associations With Effectiveness of Treatment
Consistent with our previous findings, PsA was an effect modifier for biologic survival in association with treatment effectiveness and, unsurprisingly given the close mechanistic association between nail psoriasis and PsA, nail involvement acted similarly to PsA (Table 3; eFigure 2 in Supplement 1). The number of prior exposures to biologics was an important effect modifier for drug survival in association with effectiveness of treatment for the 5 biologics. The drug survival of ixekiuzmab in biologic-naive patients was not significantly different from biologic-naive patients who were receiving treatment with guselkumab (HR, 3.89; 95% CI, 0.73-20.73; same model as Table 3 but with guselkumab as the comparator), while biologic-naive patients receiving treatment with secukinumab had similar drug survival to biologic-naive patients using ustekinumab (Table 3). However, patients receiving treatment with either IL-17A inhibitors as a third or subsequent-line therapy had a high attrition rate (Figure 2C). This could be a function of the selection of patients with more complex psoriasis endotypes who experienced treatment failures with TNF and/or the IL-12/23 p40 inhibitor ustekinumab, for which the disease pathogenesis was not solely driven through IL-17A. As the number of biologic-naive patients receiving treatment with ixekizumab and secukinumab were low, the difference in treatment response in these subgroups with different prior exposures to biologics potentially explains why the attrition of both drugs were relatively steep overall.
Figure 2. Overlaid Kaplan-Meier (KM) Survival Curves and the Population-Averaged Flexible Parametric Model (FPM) Survival Curves for the Biologic Therapies of First-Line, Second-Line, and Third or Subsequent Lines for Discontinuation Associated With Ineffectiveness During 2 Years.

Shaded areas represent 95% CIs from FPM curve. The y-axis starts from 0.60 (A and B) or 0.5 (C) for presentation clarity purposes.
Similarly, Asian people in this cohort discontinued use of adalimumab more often. There is early evidence that baseline blood and skin immune mediators can differentiate psoriasis endotypes for response to treatment with adalimumab,15,16 and our previous work found that White patients had a higher probability of achieving PASI 90 across biologic therapies.17 Further studies of drug survival in large observational cohorts on brodalumab or bimekizumab that also target IL-17F in this patient population may help elucidate this hypothesis. Another potential reason for the higher attrition rate for IL-17A inhibitors could be because of the pharmacokinetics and pharmacodynamics in association with the dosing regimen in unselected populations. The OPTIMISE trial18 found that in a subgroup of patients who had achieved PASI 75 but not PASI 90 while receiving treatment with secukinumab at week 24 (206 of 1513 [13.6%]), more patients who underwent a twice weekly dosing regimen (56.5%) compared with the 4 times weekly licensed dosing regimen (46.5%) achieved PASI 90 at week 52; similarly, more patients lost a PASI 75 response in the 4 times weekly group (47.4%) compared with the twice weekly group (41.3%), suggesting that the maintenance 4 times weekly administration regimen was insufficient to maintain an effective response in some patients. The development of antidrug antibodies is uncommon in people treated with IL-17A inhibitors; therefore, it is unlikely to be an explanation.19,20
We found that a history of previous first-line ustekinumab failure associated with ineffectiveness was adversely associated with all evaluated biologics apart from guselkumab (eFigure 8 in Supplement 1). The latter finding is consistent with the reported treatment effect of guselkumab that was reported in the NAVIGATE trial,21 in which patients treated with ustekinumab who did not achieve an investigator global assessment of 0 of 1 by week 16 had better treatment effect by switching to using guselkumab.
The finding that patients who were receiving treatment with ixekizumab with dyslipidemia had a higher drug survival for safety than those without dyslipidemia is likely spurious and can be explained by selection bias and competing risks, which describes an event that changes the chance of another event. As most of those with dyslipidemia who were receiving treatment with ixekizumab (71 [71.7%]) were receiving their third or subsequent line of therapy, which itself is associated with a high chance of discontinuation associated with ineffectiveness, most of these patients would have discontinued use because of poor treatment effects before they had the chance to discontinue because of AEs, leading to an apparent low discontinuation rate for AEs in this subgroup.
Comparisons With Other Studies
This study’s relative drug survival results are broadly consistent with the published literature. Torres et al6 conducted a multinational cohort study in Europe and North America that included 651 and 398 treatment courses of ixekiuzmab and guselkumab, respectively, and reported survival functions of 0.92 for guselkumab, with discontinuations restricted to those associated with ineffectiveness, compared with 0.84 for ixekizumab and 0.78 for secukinumab at 24 months. In contrast to the present study’s findings, they found that ixekizumab (HR, 1.03; 95% CI, 0.81-1.31) was not different to ustekinumab in a Cox regression model. Lockshin et al7 reported the overall drug survival of ixekizumab in 552 people of 0.68 (95% CI, 0.54-0.79) at 24 months in the CorEvitas (formerly Corrona) Psoriasis Registry and found a lower risk of discontinuation compared with a merged TNF inhibitor (adalimumab, certolizumab, etanercept) group (HR, 0.36; 95% CI, 0.27-0.47). Graier et al8 reported the overall drug survival of ixekizumab in 406 patients in the Austrian Psoriasis Registry and found that drug survival at 12 months was 0.86 (95% CI, 0.82-0.90) for ixekizumab. They also showed in the time-restricted analysis that the ixekizumab and ustekinumab Kaplan-Meier curves diverged after 1 year with ixekizumab having lower drug survival after 1 year compared with ustekinumab. Egeberg et al22 reported the drug survival of ixekizumab and secukinumab from DERMBIO with a small sample size (ixekizumab, n = 62) and short follow-up with no available 1-year survival, making it difficult to compare this study with our results.
Apart from the possibility of a true difference in the effect of biologics between different populations, there are 3 main reasons for discrepancies between the published studies and the present study:
Analysis of drug survival not separated by discontinuation reason: overall drug survival is hard to interpret given the effect of many reasons for discontinuation that can be associated with the results23 (eg, financial factors).
Analysis not stratifying for the effect of previous biologic treatment history, which was strongly associated with secukinumab4 and ixekizumab drug survival in the present study.
Analyses not testing for proportional hazards. Although the Kaplan-Meier curves from studies reported by Graier et al6 and Torres et al8 appear to cross at several points for the different biologics, no testing of the proportional hazard assumption was done. This is potentially a problem if the finding that IL-17A inhibitors had a higher attrition rate for second, third, or subsequent line therapy is generalizable, as the Cox model assumes constant relative effects over time.
Limitations
Missing data are a limitation, with the guselkumab and ixekizumab cohorts having a higher proportion of missing data compared with the other 3 biologics assessed (Table 1). This is partially because of the ongoing COVID pandemic. Many patients were reviewed by remote consultation; thus, in-person assessments, such as the PASI, could not be performed; and there was redeployment of clinical and research staff to COVID-related activities. These limitations have the potential to introduce bias in favor of the more recently initiated biologics, as it is more likely for clinicians to continue administering the medication in a remote consultation or arrange a subsequent follow-up to assess the patient in person if the drug response was starting to fail. However, a sensitivity analysis restricting the cohort to those who initiated treatment from 2018 onwards did not change this study’s findings materially. As data in BADBIR were taken from the UK and the Republic of Ireland, there is a potential that the findings are not fully generalizable to other populations with different background patient characteristics. For example, biologics are fully reimbursed by the National Health Service if used in accordance with national guidance, such as recommendations from the National Institute for Health and Care Excellence, reserving these treatments for patients with moderate to severe psoriasis (ie, PASI score >10, Dermatology Life Quality Index score ≥10). Hence, it is unlikely that a change in personal financial circumstances for the patient would be associated with treatment discontinuation of biologics in the UK. This study’s results may not be applicable to patients with mild to moderate disease. We also had limited sample size for the newer biologics; therefore, we did not have enough power to investigate how failure of treatment with secukinumab, ixekizumab, or guselkumab is associated with subsequent drug survival for other biologics. We did not robustly investigate the risk of AEs for the included biologics, as it was more appropriate to conduct a tailored analysis for the different serious AEs with different relevant potential confounders.
Conclusions
In this cohort study, guselkumab had the highest drug survival in BADBIR of the included biologics for treatment persistence associated with effectiveness, and guselkumab had highest drug survival for safety compared with other biologics except ustekinumab. We identified PsA, nail involvement, previous biologic exposure, and race and ethnicity as factors that had a differential association with drug survival associated with effectiveness that was dependent on the individual biologic therapy. This information on longer-term treatment effects, safety, and tolerability, along with other factors, such as background comorbidities and patient values, may help patients and their clinicians make an informed decision to initiate treatment with a particular biologic therapy.
eTable 1. Hazard ratios from univariable analysis, fitting each covariate as the exposure and discontinuation of treatment due to ineffectiveness or adverse events as the outcomes
eTable 2. Final multivariable flexible parametric survival model for drug discontinuation due to ineffectiveness, presenting only the interactions.
eTable 3. Final multivariable prognostic model for drug survival (discontinuation due to adverse events)
eTable 4. Final multivariable flexible parametric survival model for drug discontinuation due to ineffectiveness in restricted cohort 2018-2021
eTable 5. Final multivariable prognostic model for drug survival (discontinuation due to adverse events) in the restricted cohort 2018-2021
eTable 6. Final multivariable flexible parametric survival model for drug discontinuation due to ineffectiveness in 2nd line ustekinumab, secukinumab, ixekizumab and guselkumab, stratified by prior experience of adalimumab failure due to ineffectiveness
eTable 7. Final multivariable flexible parametric survival model for drug discontinuation due to ineffectiveness in 2nd line adalimumab, secukinumab, ixekizumab and guselkumab, stratified by prior experience of ustekinumab failure due to ineffectiveness
eTable 8. Final multivariable flexible parametric survival model for drug discontinuation due to adverse events in 2nd line ustekinumab, secukinumab, ixekizumab and guselkumab, stratified by prior experience of adalimumab failure due to adverse events
eTable 9. Final multivariable flexible parametric survival model for drug discontinuation due to adverse events in 3rd line ustekinumab, adalimumab and guselkumab, stratified by prior experience of IL-17 inhibitor failure due to adverse events
eFigure 1. Population-averaged survival curves from the flexible parametric model for all biologic therapies with or without psoriatic arthritis for discontinuation due to ineffectiveness
eFigure 2. Population-averaged survival curves from the flexible parametric model for all biologic therapies with or without nail involvement for discontinuation due to ineffectiveness
eFigure 3. Population-averaged survival curves from the flexible parametric model for the patients with White (3a), Asian (2b), and other ethnicities (2c) for discontinuation due to ineffectiveness over 2 years
eFigure 4. Graph showing the overlaid Kaplan-Meier survival curve and the flexible parametric model survival curve for discontinuation due to adverse events for all biologic cohorts over 2 years
eFigure 5. Graph showing the overlaid Kaplan-Meier survival curve and the population-averaged flexible parametric model survival curve for discontinuation due to ineffectiveness for all biologic cohorts over 2 years
eFigure 6. Graph showing the overlaid Kaplan-Meier survival curve and the population-averaged flexible parametric model survival curve for discontinuation due to adverse events for all biologic cohorts over 2 years
eFigure 7. Population-averaged survival curves from the flexible parametric model for effectiveness in 2nd line ustekinumab, secukinumab, ixekizumab and guselkumab with or without previous experience of adalimumab failure due to ineffectiveness
eFigure 8. Population-averaged survival curves from the flexible parametric model for effectiveness in 2nd line adalimumab, secukinumab, ixekizumab and guselkumab with or without previous experience of ustekinumab failure due to ineffectiveness
eFigure 9. Population-averaged survival curves from the flexible parametric model for safety in 2nd line ustekinumab, secukinumab, ixekizumab and guselkumab with or without previous experience of adalimumab failure due to adverse events
eFigure 10. Population-averaged survival curves from the flexible parametric model for safety in 3rd line ustekinumab, adalimumab, and guselkumab with or without previous experience of IL-17 inhibitor failure due to adverse events
Nonauthor collaborators
References
- 1.Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2021;4:CD011535. doi: 10.1002/14651858.CD011535.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mason KJ, Barker JNWN, Smith CH, et al. ; BADBIR Study Group . Comparison of drug discontinuation, effectiveness, and safety between clinical trial eligible and ineligible patients in BADBIR. JAMA Dermatol. 2018;154(5):581-588. doi: 10.1001/jamadermatol.2018.0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yiu ZZN, Mason KJ, Hampton PJ, et al. ; BADBIR Study Group . Randomized trial replication using observational data for comparative effectiveness of secukinumab and ustekinumab in psoriasis: a study from the British Association of Dermatologists Biologics and Immunomodulators Register. JAMA Dermatol. 2021;157(1):66-73. doi: 10.1001/jamadermatol.2020.4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yiu ZZN, Mason KJ, Hampton PJ, et al. ; BADBIR Study Group . Drug survival of adalimumab, ustekinumab and secukinumab in patients with psoriasis: a prospective cohort study from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR). Br J Dermatol. 2020;183(2):294-302. doi: 10.1111/bjd.18981 [DOI] [PubMed] [Google Scholar]
- 5.Blauvelt A, Shi N, Somani N, et al. Comparison of two-year treatment adherence, persistence, discontinuation, reinitiation, and switching between psoriasis patients treated with ixekizumab or secukinumab in real-world settings. J Am Acad Dermatol. 2022;86(3):581-589. doi: 10.1016/j.jaad.2021.06.878 [DOI] [PubMed] [Google Scholar]
- 6.Torres T, Puig L, Vender R, et al. Drug survival of IL-12/23, IL-17 and IL-23 inhibitors for psoriasis treatment: a retrospective multi-country, multicentric cohort study. Am J Clin Dermatol. 2021;22(4):567-579. doi: 10.1007/s40257-021-00598-4 [DOI] [PubMed] [Google Scholar]
- 7.Lockshin B, Cronin A, Harrison RW, et al. Drug survival of ixekizumab, TNF inhibitors, and other IL-17 inhibitors in real-world patients with psoriasis: the Corrona Psoriasis Registry. Dermatol Ther. 2021;34(2):e14808. doi: 10.1111/dth.14808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graier T, Salmhofer W, Jonak C, et al. Biologic drug survival rates in the era of anti-interleukin-17 antibodies: a time-period-adjusted registry analysis. Br J Dermatol. 2021;184(6):1094-1105. doi: 10.1111/bjd.19701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burden AD, Warren RB, Kleyn CE, et al. ; BADBIR Study Group . The British Association of Dermatologists’ Biologic Interventions Register (BADBIR): design, methodology and objectives. Br J Dermatol. 2012;166(3):545-554. doi: 10.1111/j.1365-2133.2012.10835.x [DOI] [PubMed] [Google Scholar]
- 10.Iskandar IY, Ashcroft DM, Warren RB, et al. Demographics and disease characteristics of patients with psoriasis enrolled in the British Association of Dermatologists Biologic Interventions Register. Br J Dermatol. 2015;173(2):510-518. doi: 10.1111/bjd.13908 [DOI] [PubMed] [Google Scholar]
- 11.Warren RB, Smith CH, Yiu ZZN, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol. 2015;135(11):2632-2640. doi: 10.1038/jid.2015.208 [DOI] [PubMed] [Google Scholar]
- 12.Mourad A, Straube S, Armijo-Olivo S, Gniadecki R. Factors predicting persistence of biologic drugs in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2019;181(3):450-458. doi: 10.1111/bjd.17738 [DOI] [PubMed] [Google Scholar]
- 13.Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21(15):2175-2197. doi: 10.1002/sim.1203 [DOI] [PubMed] [Google Scholar]
- 14.Morris TP, White IR, Carpenter JR, Stanworth SJ, Royston P. Combining fractional polynomial model building with multiple imputation. Stat Med. 2015;34(25):3298-3317. doi: 10.1002/sim.6553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andres-Ejarque R, Ale HB, Grys K, et al. ; PSORT Consortium . Enhanced NF-κB signaling in type-2 dendritic cells at baseline predicts non-response to adalimumab in psoriasis. Nat Commun. 2021;12(1):4741. doi: 10.1038/s41467-021-25066-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yiu ZZN, Barker JNWN, Barnes MR, et al. ; PSORT Consortium . Meeting report: psoriasis stratification to optimize relevant therapy showcase. J Invest Dermatol. 2021;141(8):1872-1878. doi: 10.1016/j.jid.2021.02.746 [DOI] [PubMed] [Google Scholar]
- 17.Warren RB, Marsden A, Tomenson B, et al. ; PSORT Consortium and on behalf of the BADBIR Study Group . Identifying demographic, social and clinical predictors of biologic therapy effectiveness in psoriasis: a multicentre longitudinal cohort study. Br J Dermatol. 2019;180(5):1069-1076. doi: 10.1111/bjd.16776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reich K, Puig L, Szepietowski JC, et al. Secukinumab dosing optimization in patients with moderate-to-severe plaque psoriasis: results from the randomized, open-label OPTIMISE study. Br J Dermatol. 2020;182(2):304-315. doi: 10.1111/bjd.18143 [DOI] [PubMed] [Google Scholar]
- 19.Reich K, Jackson K, Ball S, et al. Ixekizumab pharmacokinetics, anti-drug antibodies, and efficacy through 60 weeks of treatment of moderate to severe plaque psoriasis. J Invest Dermatol. 2018;138(10):2168-2173. doi: 10.1016/j.jid.2018.04.019 [DOI] [PubMed] [Google Scholar]
- 20.Thomas LW, Lee EB, Wu JJ. Systematic review of anti-drug antibodies of IL-17 inhibitors for psoriasis. J Dermatolog Treat. 2019;30(2):110-116. doi: 10.1080/09546634.2018.1473552 [DOI] [PubMed] [Google Scholar]
- 21.Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178(1):114-123. doi: 10.1111/bjd.15750 [DOI] [PubMed] [Google Scholar]
- 22.Egeberg A, Bryld LE, Skov L. Drug survival of secukinumab and ixekizumab for moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2019;81(1):173-178. doi: 10.1016/j.jaad.2019.03.048 [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Doval I, Dávila-Seijo P. How real are ‘real-life studies’ in psoriasis, and the uncertain meaning of drug persistence. Br J Dermatol. 2019;180(1):15-16. doi: 10.1111/bjd.17104 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Hazard ratios from univariable analysis, fitting each covariate as the exposure and discontinuation of treatment due to ineffectiveness or adverse events as the outcomes
eTable 2. Final multivariable flexible parametric survival model for drug discontinuation due to ineffectiveness, presenting only the interactions.
eTable 3. Final multivariable prognostic model for drug survival (discontinuation due to adverse events)
eTable 4. Final multivariable flexible parametric survival model for drug discontinuation due to ineffectiveness in restricted cohort 2018-2021
eTable 5. Final multivariable prognostic model for drug survival (discontinuation due to adverse events) in the restricted cohort 2018-2021
eTable 6. Final multivariable flexible parametric survival model for drug discontinuation due to ineffectiveness in 2nd line ustekinumab, secukinumab, ixekizumab and guselkumab, stratified by prior experience of adalimumab failure due to ineffectiveness
eTable 7. Final multivariable flexible parametric survival model for drug discontinuation due to ineffectiveness in 2nd line adalimumab, secukinumab, ixekizumab and guselkumab, stratified by prior experience of ustekinumab failure due to ineffectiveness
eTable 8. Final multivariable flexible parametric survival model for drug discontinuation due to adverse events in 2nd line ustekinumab, secukinumab, ixekizumab and guselkumab, stratified by prior experience of adalimumab failure due to adverse events
eTable 9. Final multivariable flexible parametric survival model for drug discontinuation due to adverse events in 3rd line ustekinumab, adalimumab and guselkumab, stratified by prior experience of IL-17 inhibitor failure due to adverse events
eFigure 1. Population-averaged survival curves from the flexible parametric model for all biologic therapies with or without psoriatic arthritis for discontinuation due to ineffectiveness
eFigure 2. Population-averaged survival curves from the flexible parametric model for all biologic therapies with or without nail involvement for discontinuation due to ineffectiveness
eFigure 3. Population-averaged survival curves from the flexible parametric model for the patients with White (3a), Asian (2b), and other ethnicities (2c) for discontinuation due to ineffectiveness over 2 years
eFigure 4. Graph showing the overlaid Kaplan-Meier survival curve and the flexible parametric model survival curve for discontinuation due to adverse events for all biologic cohorts over 2 years
eFigure 5. Graph showing the overlaid Kaplan-Meier survival curve and the population-averaged flexible parametric model survival curve for discontinuation due to ineffectiveness for all biologic cohorts over 2 years
eFigure 6. Graph showing the overlaid Kaplan-Meier survival curve and the population-averaged flexible parametric model survival curve for discontinuation due to adverse events for all biologic cohorts over 2 years
eFigure 7. Population-averaged survival curves from the flexible parametric model for effectiveness in 2nd line ustekinumab, secukinumab, ixekizumab and guselkumab with or without previous experience of adalimumab failure due to ineffectiveness
eFigure 8. Population-averaged survival curves from the flexible parametric model for effectiveness in 2nd line adalimumab, secukinumab, ixekizumab and guselkumab with or without previous experience of ustekinumab failure due to ineffectiveness
eFigure 9. Population-averaged survival curves from the flexible parametric model for safety in 2nd line ustekinumab, secukinumab, ixekizumab and guselkumab with or without previous experience of adalimumab failure due to adverse events
eFigure 10. Population-averaged survival curves from the flexible parametric model for safety in 3rd line ustekinumab, adalimumab, and guselkumab with or without previous experience of IL-17 inhibitor failure due to adverse events
Nonauthor collaborators