Abstract
Objective
To assess the impact of iterative changes in preoperative and postoperative biopsy techniques on the outcomes of men undergoing the precision prostatectomy procedure. Precision prostatectomy is a novel surgical treatment for prostate cancer that aims to maximally preserve erectogenic nerves via partial preservation of the prostate capsule.
Design
Retrospective.
Setting
Single tertiary care center.
Participants
This study included 120 patients who consented to undergo prostate cancer treatment with the precision prostatectomy procedure. Patients were originally enrolled in one of two separate prospective protocols studying precision prostatectomy.
Interventions
Preoperatively, 60 patients were screened with transrectal (TR) biopsy and 60 were screened by transperineal (TP) biopsy. Ultimately, 117 patients underwent precision prostatectomy. Of the 43 postoperative biopsies, 19 were TR; 17 were TP with ultrasound; and 7 were TP with microultrasound (mUS).
Main outcome measures
Preoperatively, we evaluated whether the transition to TP biopsy was associated with differences in postoperative treatment failure defined as a neoplasm-positive postoperative biopsy. Postoperative biopsies were compared with respect to their ability to sample the remnant tissue, specifically percentage of cores positive for prostate tissue.
Results
Preoperatively, 9/60 (15%) positive postoperative biopsies occurred in the TR group and 6/60 (10%) in the TP group; Kaplan-Meier survival estimates did not differ between groups (p=0.69 by log rank). Postoperatively, the numbers of cores positive for prostate tissue were 99/160 (62%), 63/107 (59%), and 36/39 (92%) in the TR biopsy, TP with ultrasound, and TP with mUS groups, respectively; this difference was statistically significant versus the rate in the TR and standard TP groups (p=0.0003 and 0.0002).
Conclusion
We found no significant improvement in patient screening, preoperatively—though limited by small sample size and relatively short follow-up. The incorporation of high-frequency mUS for postoperative biopsies improved the ability to sample the remnant tissue with a higher efficiency.
Keywords: Robotic Surgical Procedures
What is already known about this subject?
Precision prostatectomy is a novel surgical subtotal procedure that removes ~95% of the prostate with complete removal of the side with a lesion and spares a sliver of prostatic capsule and the seminal vesicle on the contralateral side.
Transrectal (TR) biopsies have been used to screen patients for candidacy for the procedure by sampling the prostatic tissue planned to be left in situ and postoperatively to sample the prostatic remnant in the setting of elevated prostate-specific antigen.
What are the new findings?
Transitioning from TR biopsies to standard transperineal (TP) biopsy preoperatively and to high-resolution microultrasound-guided TP biopsy postoperatively served as important facets that may have improved patient screening for the precision prostatectomy and were shown to better assess for residual cancer postoperatively.
How might these results affect future research or surgical practice?
Optimization of the oncological/functional trade-off via refined biopsy procedures within the precision prostatectomy may increase adoptability.
Introduction
Whole gland treatment with either radical prostatectomy or radiation therapy represents the standard of care for the management of grade group 2 or higher clinically localized prostate cancer.1 2 Although effective at eradicating disease, these treatments are associated with high rates of urinary and sexual side effects. For example, in the ProtecT trial, which compared active surveillance, radical prostatectomy, and radiation therapy, only 14.6% of men reported the ability to obtain an erection firm enough for intercourse 1 year following radical prostatectomy (preoperative baseline rate of 65.7%).3 Similarly, in men who underwent radiation therapy, this figure was 37.6% (baseline of 68.4%). Furthermore, in a large meta-analysis examining continence outcomes, Ficarra et al found 1-year incontinence rates for robotic radical prostatectomy ranged from 4% up to 31% using a ‘no pad’ definition.4 These same findings have been corroborated in other high-quality reports in the urological literature.5 6
The unfavorable side effect profile of whole gland treatments has motivated the development of targeted, or focal, treatments for prostate cancer, which aim to avoid damage to the anatomical structures that allow for erectile function and urinary control. To date, these efforts have largely focused on the use of ablative technologies such as high-intensity focus ultrasound, cryotherapy, photodynamic therapy, and laser ablation.7–9 Candidates for prostate cancer focal therapy typically have one to two regions of cancer identified on a prostate biopsy performed with the guidance of MRI.10 Unfortunately, due to the imperfect sensitivity of MRI for detecting sites of clinically significant prostate cancer11 as well as issues related to the limited sampling density that can be achieved with prostate biopsy, 10%–40% of men treated for a focal tumour ultimately harbour multifocal sites of disease.9 As a result, the 5-year retreatment rates for prostate focal therapy have historically been unacceptably high in the range of 20%–30%.12–14
To address the issues outlined previously, we have developed a novel surgical technique—known as the precision prostatectomy procedure—that aims to remove ~95% of the prostate while maximally preserving the erectogenic nerves that run alongside the prostate capsule.15–17 During this procedure, men undergo a standard radical prostatectomy on one side along with a contralateral subtotal prostatectomy, leaving the patient with several millimetre rims of tissue that contains the erectogenic nerves. We have previously reported the highly favorable results of 88 patients who underwent this novel procedure.17 Notably, by 12 months postoperatively, 90% of preoperatively potent men reported a return of erections sufficient for intercourse. Furthermore, at 36 months of follow-up, only 7% of patients were found to harbour clinically significant prostate cancer in their remnant prostate tissue, far less than the historical outcomes with focal ablative therapies.
As with focal therapy, a key component for selecting candidates for the precision prostatectomy procedure is ensuring that the untreated portion of the patient’s prostate is free from any cancer. This is accomplished by performing a preoperative diagnostic biopsy aimed at sampling the periphery of the prostate, concentrating on the area that will be left in situ. Similarly, when assessing the oncological success of this procedure, a postoperative biopsy is required to evaluate for evidence of residual disease in men with a rising or elevated prostate-specific antigen (PSA) level.
Over the course of developing the precision prostatectomy procedure, we have made iterative changes to our techniques for performing both preoperative and postoperative biopsies. This included transitioning from a transrectal (TR) to a transperineal (TP) approach for preoperative prostate biopsy, a method which is known to be associated with a lower risk of infectious complications as well as improved sampling of the peripheral and anterior zones of the prostate.18 Additionally, we have implemented the use of high-frequency microultrasound (mUS) while performing postoperative TP prostate biopsies to improve the visualization of the small-volume remnant tissue. The aim of this study was to use the Innovation, Development, Exploration, Assessment, Long-Term study (IDEAL) model, put forth by the Balliol Colloquium,19 20 to assess the impact of these iterative changes to our biopsy techniques on the outcomes of men undergoing the novel precision prostatectomy procedure.
Methods
All patients included in this retrospective IDEAL 2b analysis were prospectively enrolled in one of two separate research protocols studying the precision prostatectomy procedure (HFH-IRB#12 507 for IDEAL stage 1 and HFH-IRB#14 531 for IDEAL stage 2b). Patients who were eligible for precision prostatectomy had a unilateral lesion with grade group ≤3 prostate cancer, a serum PSA ≤15 ng/mL, clinical stage ≤cT2, and preoperative erectile function score (International Index of Erectile Function score −5) of ≥17 out of 25. Patients were permitted to have grade group ≤2 disease on the contralateral side so long as it was not contained within the capsular region to be left in situ at the time of the precision prostatectomy.
As a first component of our study, we evaluated whether our transition from a TR to TP approach was associated with differences in treatment failure defined by either intraoperative conversion to radical prostatectomy or a postoperative biopsy containing prostate cancer. In the second component of our study, we compared three methods of prostate biopsy (TR, TP, and mUS-guided biopsy) with respect to their ability to adequately sample the prostatic remnant tissue, using percentage of cores positive for prostate tissue as the primary endpoint.
Preoperative TR prostate biopsies were performed with ultrasound guidance alone or with ultrasound/MRI fusion using the UroNav platform (Invivo, Gainesville, Florida, USA), and TP prostate biopsies were performed with or without MRI guidance using the KOELIS Trinity biopsy platform (KOELIS, Grenoble, France; figure 1).21 MRIs were infrequently obtained and MRI fusion only occurred when there was an outside MRI obtained prior to presentation. Postoperative biopsies were performed with both methods but more recently evolved to perform TP biopsies with the guidance of high-frequency mUS (ExactVu, Exact Imaging, Markham, Canada; figure 2).
Figure 1.
A) Three-dimensional reconstructed view of the KOELIS Trinity system used for preoperative TP biopsies. In this case, the MRI targets are the yellow spheres and the needle throws are the green cylinders around the circumferential edge of the prostate. (B) Ultrasound image of a preoperative TP needle biopsy sampling the prostate. TP, transperineal.
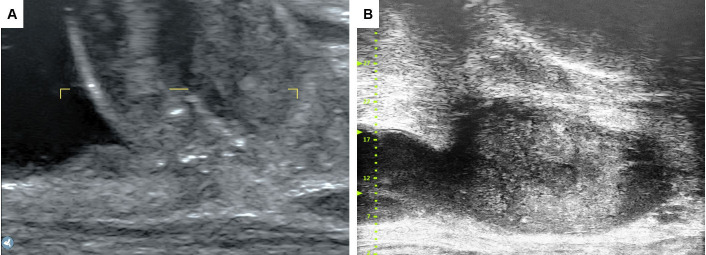
Figure 2.
(A) Traditional ultrasound imaging of the prostatic remnant. (B) High-resolution microultrasound image of the prostatic remnant.
For-cause postoperative biopsies were performed on violation of the American Urological Association definition of biochemical failure (BCF) for radical prostatectomy,22 and intraoperative frozen section biopsies were performed solely at the discretion of the treating surgeon. They were obtained always in the early stages of biopsy development but were omitted in the setting of preoperative TP capsular biopsy that sampled the remnant. The number of post-treatment biopsies was based on surgeon judgment informed by adequate tissue on needle cores. Biopsy needle throws were repeated until it was felt that the remnant was adequately sampled and there was sufficient tissue sampled. The prostate biopsies, both preoperatively and postoperatively, were performed by two attending surgeons (MM and WJ). There was minimal resident and fellow involvement, all of which was appropriately supervised.
The χ2 test and Mann-Whitney U test were used to detect differences in categorical and non-parametric continuous variables, respectively. Additionally, in our comparison of the preoperative biopsy techniques, we used the Kaplan-Meier method to estimate the cumulative rates of treatment failure. Groups were compared using the log-rank statistic. P values of 0.05 or less were considered to indicate statistical significance. All statistical analysis was performed in IBM SPSS Statistics for Windows V.27.0.
Results
Between December 2016 and May 2021, 120 patients were brought to the operating room with the intent of performing a precision prostatectomy procedure. The baseline characteristics of the study cohort are shown in table 1. In total, 60 (50%) of the 120 men were screened using TR prostate biopsy and 60 (50%) were screened with the TP approach. Prebiopsy MRIs were obtained from 23 patients, and of those, only 12 patients had an MRI target and underwent fusion biopsy. Three patients (2.5%) were converted to radical surgery based on positive intraoperative frozen section. Of the 117 patients who underwent the precision prostatectomy, 46 patients experienced BCF, stringently defined as two consecutive PSA values above 0.2 ng/dL after postoperative PSA nadir; 29 of these patients were screened by TR ultrasounds and 17 were screened by TP ultrasound. Of the 46 BCRs, 27 received postoperative biopsies. The remaining 19 patients omitted biopsies via shared decision making based on elevated but stable (not increasing) PSA level, deemed to be due to benign tissue left in situ. In total, 9 (15%) patients in the TR group and 6 (10%) patients in the TP group met the definition of treatment failure. Kaplan-Meier survival estimates did not differ significantly between groups (p=0.69 by log-rank without adjustment). Figure 3 shows the survival curves for up to 4 years for the TR group and 2.5 years for the TP group. The disparity in follow-up is due to the lead-time in the transition to TP-screened patients. Other endpoints used to compare the efficacy of the preoperative biopsy included the proportion of positive surgical margins (12/60 (20%) of the TR biopsies and 16/60 (27%) of the TP biopsies resulted in a positive margin on the side of tissue preservation), clinically significant margins, focal margins, and multifocal margins in the specimen removed via precision prostatectomy—all of which did not reach a statistical significant difference between biopsy techniques, with a p value of >0.4 for all comparisons.
Table 1.
Baseline characteristics of 120 patients who underwent screening for precision prostatectomy with either TR biopsy or TP biopsy
| TR biopsy (n=60) |
TP biopsy (n=60) |
|
| Age (years), median (IQR) | 57.5 (53.0–64.0) | 62.0 (57.0–66.3) |
| BMI (kg/m2), median (IQR) | 28.4 (26.0–31.0) | 28.5 (25.2–30.9) |
| Race, n (%) | ||
| African–American | 8 (13) | 16 (27) |
| Asian | 1 (2) | 1 (2) |
| Hispanic | 2 (3) | 1 (2) |
| Caucasian | 45 (75) | 37 (62) |
| Other/unknown | 4 (7) | 5 (8) |
| Preoperative PSA (ng/mL), median (IQR) | 5.4 (3.8–6.3) | 5.9 (4.6–8.2) |
| Biopsy Gleason group, n (%) | ||
| 1 | 19 (31) | 8 (13) |
| 2 | 37 (62) | 34 (57) |
| 3 | 4 (7) | 18 (30) |
| Clinical T stage, n (%) | ||
| T1 | 41 (68) | 55 (92) |
| T2 | 19 (32) | 4 (7) |
| T3 | 0 (0) | 1 (2) |
| Clinical National Comprehensive Cancer Network (NCCN) risk, n (%) | ||
| Low | 19 (32) | 8 (13) |
| Intermediate | 40 (67) | 52 (87) |
| High | 1 (2) | 0 (0) |
BMI, body mass index; PSA, prostate-specific antigen; TP, transperineal; TR, transrectal.
Figure 3.
Kaplan-Meier survival curve comparing transperineal and transrectal preoperative screening biopsy, with respect to their ability to avoid treatment failure defined as neoplasm-positive postoperative biopsy.
The primary outcome for judging the adequacy of our postoperative biopsy technique was the percentage of biopsy cores positive for prostate tissue. As our biopsy method evolved in time (ie, TR to TP with standard ultrasound and then to TP with ExactVu), we saw an increase in our ability to sample the prostate remnant. With the TR technique, 99 of 160 (62%) collection cores contained prostate tissue. Similarly, with the standard TP technique, 63 of 107 (59%) cores contained the target tissue. In contrast, on implementing TP biopsy with high-frequency mUS guidance, the proportion of cores containing prostate tissue rose dramatically to 36 of 39 (92%). The difference between groups was highly significant (both comparisons p<0.001). As a secondary endpoint, we evaluated the number of remnant biopsy cores taken per procedure as a marker of procedural efficiency. Surgeons took a median of 9.0 (IQR 6.0–9.0), 5.0 (IQR: 4.0–7.5), and 6.0 (IQR 4.0–6.5) cores with the TR, standard TP, and TP with mUS biopsy procedures, respectively. There was improved efficiency with the standard TP and mUS-guided technique, as compared with the TR biopsy (p=0.01, 0.03), but there was no difference between the two TP techniques (p=1.0).
Discussion
In this study, we examined the impact of iterative changes to our preoperative and postoperative biopsy techniques on the outcomes of patients undergoing the precision prostatectomy procedure. Our preoperative change from TR to TP biopsy did not statistically improve patient screening for the precision prostatectomy. Postoperatively, this change did not improve diagnostic remnant sampling in terms of number of cores containing prostate tissue. We did, however, find an improved diagnostic yield on implementing the postoperative use of high-frequency mUS guidance, which improved our efficiency of postbiopsy tissue sampling (ie, less cores needed to adequately sample the gland).
Historically, diagnostic prostate biopsy has been performed via a TR approach. Because this procedure requires the biopsy needle to puncture the rectal wall on its trajectory to the prostate, TR prostate biopsy carries with it a substantial risk of infectious complications.23–25 In contrast, TP prostate biopsy, which is performed percutaneously, is associated with a marked reduction in postbiopsy infections.26–30 Based on the historically favorable safety profile with TP prostate biopsy, we opted to incorporate this technique into our clinical practice. A second motivation for this change was the purported benefits of improved prostate sampling with the TP approach. More specifically, TP prostate biopsy is better suited for anterior zone sampling, an area of the gland where prostate cancers are inadequately sampled with TR prostate biopsy.31–33 TP prostate biopsy also appears to allow for improved sampling of small Prostate Imaging Reporting & Data System (PI-RADS) of three and four lesions regardless of anatomical location as well as higher rates of disease reclassification among men on active surveillance for low-risk prostate cancer.34
Based on the data presented earlier and other similar results, we hypothesized that the transition to TP prostate biopsy would allow for improved sampling of the gland ahead of the precision prostatectomy procedure and in turn lead to a lower risk of post-treatment failure. Given the results of our analysis, this does not appear to be true. Although there was a 33% reduction in the hazard of postoperative treatment failure, this difference did not meet the conventional threshold for statistical significance. It is, however, possible that the relatively small number of patients and the lag in follow-up for the TP group made this analysis prone to type II error. Moreover, the TR group had a higher proportion of men with grade group 1 prostate cancer, placing them at an overall lower risk of recurrence.
The role of post-treatment biopsy among patients undergoing the precision prostatectomy procedure is distinct from its preoperative counterpart. The indication for a postoperative biopsy is any rise in PSA concerning for treatment failure. However, during the precision prostatectomy, a sliver of prostatic tissue is intentionally left in situ, so a non-zero postoperative PSA is possible in cases of treatment success. Given this was a new procedure, we assumed a conservative posture with respect to BCF, adopting the American Urological Association (AUA) definition after radical prostatectomy. Although there is some PSA-producing prostate tissue in situ, the median PSA at 24 months was 0.0 IQR (0.0–0.3) in the first 88 patients.17 Thus, we believe that the AUA definition is stringent but appropriate; as the data matures, the biopsy criteria may evolve as patients with stable but elevated PSA may not need a biopsy. Nevertheless, it is critical to be able to adequately sample the prostatic remnant to discern a benign PSA elevation from one due to cancer. Although seemingly a simple task, because of the small size of the remnant prostate tissue (ie, ~1 to 5 g) and the limited resolution of most TR ultrasound units, this has proven challenging.
Initial attempts at targeting of the capsular remnant via a TR approach resulted in a median of 9.0 cores taken per case with 62% of cores positive for prostate tissue. Our switch to the TP approach improved this significantly, with a median of 5.0 cores taken per case and 59% of cores positive for prostate tissue. This was refined with the introduction of the ExactVu high-resolution mUS system, which provided superlative real-time image resolution by virtue of its use of a 29 MHz linear TR probe to the image the prostate. In contrast, most standard TR ultrasound probes allow for imaging at a maximum of 14–16 MHz. TP prostate biopsy with ExactVu guidance allowed for precise targeting of remnant tissue and resulted in a median of 6.0 cores per cases with 92% cores positive for prostate tissue. Importantly, there were fewer cores taken per case with the mUS-guided biopsy. This is because the surgeon was more confident that they properly sampled the remnant intraoperatively when using the mUS and TP biopsies as opposed to the standard US-guided TR biopsies. Previously, more needle throws were required to acquire tissue cores due to poorer visualization with the lower definition. The lower number of cores speaks to the efficacy of the mUS visualization, though there could be some learning curve bias as it was adopted in the latter stages of technique development. The increased efficiency’s relation to sampling accuracy has clinical implications in the setting of treatment failure. When the entire remnant is more confidently sampled, then clinically significant cancer is more confidently ruled out when the biopsy is negative. This in turn informs the postoperative management decision in the setting of PSA rise, as it can be ascribed to secretion from benign prostate tissue. As an adjunct, the use of MRI in the postoperative setting was considered but never implemented, given the inability to produce real-time actionable images. Moreover, there is evidence that mUS is equivalent or better at detecting clinically significant cancer than MRI-fusion biopsies.35–38
This study has several limitations worthy of mention. These include its retrospective design and small sample size. Furthermore, changes made to our postoperative biopsy technique may have influenced our readout of success in terms of the pretreatment biopsy technique. More specifically, because pretreatment biopsy success was measured by post-treatment biopsy positivity for cancer, improvements in the post-treatment biopsy method may have skewed our analysis. It is plausible that shortcomings in our initial post-treatment biopsy method led to false-negative biopsy results in the pretreatment TR prostate biopsy group leading to incorrectly accepting the null hypothesis in our analysis of pretreatment biopsy technique.
Conclusion
In this retrospective IDEAL phase IIb study, we evaluated the impact of iterative changes to our pretreatment and post-treatment biopsy techniques on the outcomes of men undergoing the novel precision prostatectomy procedure. The transition from TR to TP prostate biopsy did not improve the selection of candidates for precision prostatectomy or our ability to postoperatively sample the remnant biopsy tissue. However, our incorporation of high-frequency mUS for postoperative biopsies did lead to an improved ability to sample the remnant prostate tissue with a higher degree of efficiency. Based on the results of this analysis, we feel no additional iterative changes to our biopsy technique are warranted and the presented biopsy methods (ie, preoperative TP biopsy with KOELIS Trinity and postoperative TP biopsy with ExactVu) will be employed in future planned randomized trials comparing precision prostatectomy to other forms of prostate cancer treatment such as ablative focal therapy and radical prostatectomy.
Footnotes
Twitter: @GrauerRalph, @MohitButaney
Contributors: Conception or design of the work: RG, AS, MB, MG, FA WJ and MM. Data collection: RG, AS, MB, PO, GF and RHC. Data analysis and interpretation: RG, MG, AS and MM. Drafting the article: RG, MG, AS and MB. Critical revision of the article: RG, MG, AS and MM. Final approval of the version to be published: all authors. Guarantor: MM
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
The data that support the findings of this study are available from the corresponding author, RG, upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by HFH-IRB#12507 for IDEAL stage 1 and HFH-IRB#14531 for IDEAL stage 2b. The participants gave informed consent to participate in the study before taking part.
References
- 1.Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part I: risk stratification, shared decision making, and care options. J Urol 2018;199:683–90. 10.1016/j.juro.2017.11.095 [DOI] [PubMed] [Google Scholar]
- 2.Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 2017;71:618–29. 10.1016/j.eururo.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 3.Donovan JL, Hamdy FC, Lane JA, et al. Patient-Reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016;375:1425–37. 10.1056/NEJMoa1606221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ficarra V, Novara G, Rosen RC, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol 2012;62:405–17. 10.1016/j.eururo.2012.05.045 [DOI] [PubMed] [Google Scholar]
- 5.Resnick MJ, Koyama T, Fan K-H, et al. Long-Term functional outcomes after treatment for localized prostate cancer. N Engl J Med 2013;368:436–45. 10.1056/NEJMoa1209978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman KE, Penson DF, Zhao Z, et al. Patient-Reported outcomes through 5 years for active surveillance, surgery, brachytherapy, or external beam radiation with or without androgen deprivation therapy for localized prostate cancer. JAMA 2020;323:149–63. 10.1001/jama.2019.20675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahdoot M, Lebastchi AH, Turkbey B, et al. Contemporary treatments in prostate cancer focal therapy. Curr Opin Oncol 2019;31:200–6. 10.1097/CCO.0000000000000515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebastchi AH, Gill IS, Abreu AL. A focus on focal therapy for prostate cancer. JAMA Surg 2021;156:881–2. 10.1001/jamasurg.2021.3181 [DOI] [PubMed] [Google Scholar]
- 9.Hopstaken JS, Bomers JGR, Sedelaar MJP, et al. An updated systematic review on focal therapy in localized prostate cancer: what has changed over the past 5 years? Eur Urol 2022;81:5-33. 10.1016/j.eururo.2021.08.005 [DOI] [PubMed] [Google Scholar]
- 10.Lebastchi AH, George AK, Polascik TJ, et al. Standardized Nomenclature and surveillance methodologies after focal therapy and partial gland ablation for localized prostate cancer: an international multidisciplinary consensus. Eur Urol 2020;78:371–8. 10.1016/j.eururo.2020.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815–22. 10.1016/S0140-6736(16)32401-1 [DOI] [PubMed] [Google Scholar]
- 12.Ganzer R, Hadaschik B, Pahernik S, et al. Prospective multicenter phase II study on focal therapy (Hemiablation) of the prostate with high intensity focused ultrasound. J Urol 2018;199:983–9. 10.1016/j.juro.2017.10.033 [DOI] [PubMed] [Google Scholar]
- 13.Guillaumier S, Peters M, Arya M, et al. A multicentre study of 5-year outcomes following focal therapy in treating clinically significant nonmetastatic prostate cancer. Eur Urol 2018;74:422–9. 10.1016/j.eururo.2018.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bass R, Fleshner N, Finelli A, et al. Oncologic and functional outcomes of partial gland ablation with high intensity focused ultrasound for localized prostate cancer. J Urol 2019;201:113–9. 10.1016/j.juro.2018.07.040 [DOI] [PubMed] [Google Scholar]
- 15.Sood A, Jeong W, Taneja K, et al. The precision prostatectomy: an ideal stage 0, 1 and 2A study. BMJ Surg Interv Health Technol 2019;1:e000002. 10.1136/bmjsit-2019-000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sood A, Jeong W, Keeley J, et al. Subtotal surgical therapy for localized prostate cancer: a single-center precision prostatectomy experience in 25 patients, and SEER-registry data analysis. Transl Androl Urol 2021;10:3155–66. 10.21037/tau-20-1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sood A, Jeong W, Palma-Zamora I, et al. Description of surgical technique and oncologic and functional outcomes of the precision prostatectomy procedure (ideal stage 1-2b study). Eur Urol 2022;81:396–406. 10.1016/j.eururo.2021.10.017 [DOI] [PubMed] [Google Scholar]
- 18.Bhanji Y, Allaway MJ, Gorin MA. Recent advances and current role of Transperineal prostate biopsy. Urol Clin North Am 2021;48:25–33. 10.1016/j.ucl.2020.09.010 [DOI] [PubMed] [Google Scholar]
- 19.Barkun JS, Aronson JK, Feldman LS, et al. Evaluation and stages of surgical innovations. Lancet 2009;374:1089–96. 10.1016/S0140-6736(09)61083-7 [DOI] [PubMed] [Google Scholar]
- 20.McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the ideal recommendations. Lancet 2009;374:1105–12. 10.1016/S0140-6736(09)61116-8 [DOI] [PubMed] [Google Scholar]
- 21.Knaub RJ, Allaf ME, Gorin MA. Freehand Transperineal prostate biopsy with three-dimensional ultrasound Organ-Based tracking. J Endourol 2021;35:S-7–S-16. 10.1089/end.2021.0569 [DOI] [PubMed] [Google Scholar]
- 22.Lowrance WT, Breau RH, Chou R, et al. Advanced prostate cancer: AUA/ASTRO/SUO guideline Part I. J Urol 2021;205:14–21. 10.1097/JU.0000000000001375 [DOI] [PubMed] [Google Scholar]
- 23.Rodríguez LV, Terris MK. Risks and complications of transrectal ultrasound guided prostate needle biopsy: a prospective study and review of the literature. J Urol 1998;160:2115–20. 10.1016/S0022-5347(01)62255-9 [DOI] [PubMed] [Google Scholar]
- 24.Raaijmakers R, Kirkels WJ, Roobol MJ, et al. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology 2002;60:826–30. 10.1016/S0090-4295(02)01958-1 [DOI] [PubMed] [Google Scholar]
- 25.Liss MA, Ehdaie B, Loeb S, et al. An update of the American urological association white paper on the prevention and treatment of the more common complications related to prostate biopsy. J Urol 2017;198:329–34. 10.1016/j.juro.2017.01.103 [DOI] [PubMed] [Google Scholar]
- 26.Xiang J, Yan H, Li J, et al. Transperineal versus transrectal prostate biopsy in the diagnosis of prostate cancer: a systematic review and meta-analysis. World J Surg Oncol 2019;17:31. 10.1186/s12957-019-1573-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pepe P, Aragona F. Prostate biopsy: results and advantages of the transperineal approach--twenty-year experience of a single center. World J Urol 2014;32:373–7. 10.1007/s00345-013-1108-1 [DOI] [PubMed] [Google Scholar]
- 28.Iremashvili VV, Chepurov AK, Kobaladze KM, et al. Periprostatic local anesthesia with pudendal block for transperineal ultrasound-guided prostate biopsy: a randomized trial. Urology 2010;75:1023–7. 10.1016/j.urology.2009.09.083 [DOI] [PubMed] [Google Scholar]
- 29.Pradere B, Veeratterapillay R, Dimitropoulos K, et al. Nonantibiotic strategies for the prevention of infectious complications following prostate biopsy: a systematic review and meta-analysis. J Urol 2021;205:653–63. 10.1097/JU.0000000000001399 [DOI] [PubMed] [Google Scholar]
- 30.Bennett HY, Roberts MJ, Doi SAR, et al. The global burden of major infectious complications following prostate biopsy. Epidemiol Infect 2016;144:1784–91. 10.1017/S0950268815002885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schouten MG, van der Leest M, Pokorny M, et al. Why and where do we miss significant prostate cancer with multi-parametric magnetic resonance imaging followed by magnetic resonance-guided and transrectal ultrasound-guided biopsy in Biopsy-naïve men? Eur Urol 2017;71:896–903. 10.1016/j.eururo.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 32.Pepe P, Garufi A, Priolo G, et al. Transperineal versus transrectal MRI/TRUS fusion targeted biopsy: detection rate of clinically significant prostate cancer. Clin Genitourin Cancer 2017;15:e33–6. 10.1016/j.clgc.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 33.Ber Y, Segal N, Tamir S, et al. A noninferiority within-person study comparing the accuracy of transperineal to transrectal MRI-US fusion biopsy for prostate-cancer detection. Prostate Cancer Prostatic Dis 2020;23:449–56. 10.1038/s41391-020-0205-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer AR, Mamawala M, Winoker JS, et al. Transperineal prostate biopsy improves the detection of clinically significant prostate cancer among men on active surveillance. J Urol 2021;205:1069–74. 10.1097/JU.0000000000001523 [DOI] [PubMed] [Google Scholar]
- 35.Abouassaly R, Klein EA, El-Shefai A, et al. Impact of using 29 MHz high-resolution micro-ultrasound in real-time targeting of transrectal prostate biopsies: initial experience. World J Urol 2020;38:1201–6. 10.1007/s00345-019-02863-y [DOI] [PubMed] [Google Scholar]
- 36.Claros OR, Tourinho-Barbosa RR, Fregeville A, et al. Comparison of initial experience with transrectal magnetic resonance imaging cognitive guided Micro-Ultrasound biopsies versus established Transperineal robotic ultrasound magnetic resonance imaging fusion biopsies for prostate cancer. J Urol 2020;203:918–25. 10.1097/JU.0000000000000692 [DOI] [PubMed] [Google Scholar]
- 37.Klotz L, Lughezzani G, Maffei D, et al. Comparison of micro-ultrasound and multiparametric magnetic resonance imaging for prostate cancer: a multicenter, prospective analysis. Can Urol Assoc J 2021;15:E11–16. 10.5489/cuaj.6712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sountoulides P, Pyrgidis N, Polyzos SA, et al. Micro-Ultrasound-Guided vs multiparametric magnetic resonance Imaging-Targeted biopsy in the detection of prostate cancer: a systematic review and meta-analysis. J Urol 2021;205:1254–62. 10.1097/JU.0000000000001639 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, RG, upon reasonable request.