Assouly K, Smit AL, Stegeman I, et al. Cochlear implantation for tinnitus in adults with bilateral hearing loss: protocol of a randomised controlled trial. BMJ Open 2021;11:e043288. doi: 10.1136/bmjopen-2020-043288
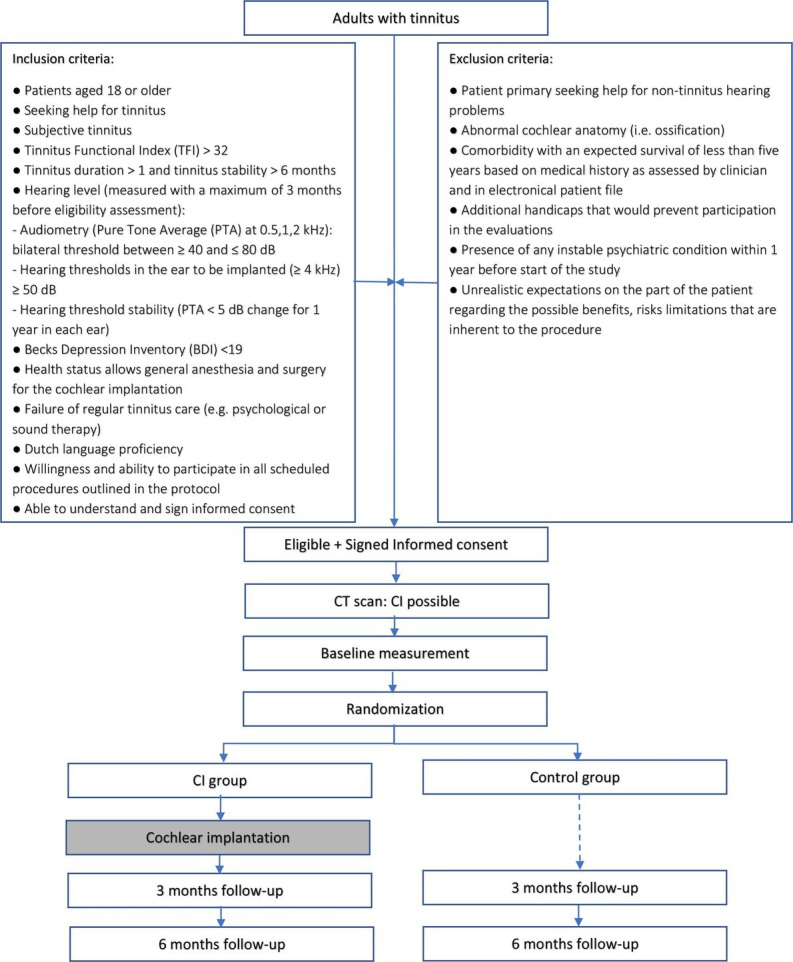
An amendment to the protocol resulted in the change of one inclusion criteria of the study. The inclusion criteria ‘pure tone average at 0.5, 1, 2 and 4 kHz: bilateral threshold between 50 and ≤75 dB’ has been changed to ‘pure tone average at 0.5,1,2 kHz: bilateral threshold between ≥40 and≤80 dB and hearing thresholds in the ear to be implanted (≥4 kHz)≥50 dB’. The correction of the inclusion criteria does not change the aim and the design of the study, but for clarification, the following correction are noted:
The inclusion criteria in the ‘Methods and analysis’ section of the Abstract should be: (Tinnitus Functional Index (TFI) >32, Beck’s Depression Index <19, pure tone average at 0.5,1,2 kHz bilateral threshold between ≥40 and≤80 dB and hearing thresholds in the ear to be implanted (≥4 kHz)≥50 dB).
-
In the Method and analysis section, in the Inclusion criteria paragraph, the hearing level criteria should be:
Audiometry (Pure Tone Average (PTA) at 0.5,1,2 kHz: bilateral threshold between ≥40 and≤80 dB.
Hearing thresholds in the ear to be implanted (≥4 kHz)≥50 dB
Hearing threshold stability (PTA <5 dB change for 1 year in each ear).’
Figure 1 has been updated with the correction of the inclusion criteria.