Abstract
Meloidogyne enterolobii and M. floridensis are virulent species that can overcome root-knot nematode resistance in economically important crops. Our objectives were to determine the effects of temperature on the infectivity of second-stage juveniles (J2) of these two species and determine differences in duration and thermal-time requirements (degree-days [DD]) to complete their developmental cycle. Florida isolates of M. enterolobii and M. floridensis were compared to M. incognita race 3. Tomato cv. BHN 589 seedlings following inoculation were placed in growth chambers set at constant temperatures of 25°C, and 30°C, and alternating temperatures of 30°C to 25°C (day–night). Root infection by the three nematode species was higher at 30°C than at 25°C, and intermediate at 30°C to 25°C, with 33%, 15%, and 24% infection rates, respectively. There was no difference, however, in the percentages of J2 that infected roots among species at each temperature. Developmental time from infective J2 to reproductive stage for the three species was shorter at 30°C than at 25°C, and 30°C to 25°C. The shortest time and DD to egg production for the three species were 13 days after inoculation (DAI) and 285.7 DD, respectively. During the experimental timeframe of 29 d, a single generation was completed at 30°C for all three species, whereas only M. floridensis completed a generation at 30°C to 25°C. The number of days and accumulated DD for completing the life cycle (from J2 to J2) were 23 d and 506.9 DD for M. enterolobii, and 25 d and 552.3 DD for M. floridensis and M. incognita, respectively. Exposure to lower (25°C) and intermediate temperatures (30°C to 25°C) decreased root penetration and slowed the developmental cycle of M. enterolobii and M. floridensis compared with 30°C.
Keywords: degree-day, development, ecology, life cycle, pacara earpod tree root-knot nematode, peach root-knot nematode, temperature
Meloidogyne enterolobii (= M. mayaguensis) is an emerging root-knot nematode (RKN) species that is highly virulent on agricultural crops (Castagnone-Sereno, 2012) including many economically important vegetable and field crops (Yang and Eisenback, 1983; Carneiro et al., 2001; Brito et al., 2014; Villar-Luna et al., 2016). This nematode species has been increasingly detected worldwide predominantly in warmer climates, with the highest reported occurrence in South America, and thus it is now considered as a major threat to crop production (Philbrick et al., 2020; Collett et al., 2021). In addition to its high reproduction rate and causing severe root galling on host roots, M. enterolobii is also a cause for concern due to its ability to develop on crops that are typically resistant to M. arenaria, M. incognita, and M. javanica (Castagnone-Sereno, 2012). Some RKN-resistant crops that are known to be affected by M. enterolobii include sweet potato, soybean (Fargette and Braaksma, 1990), tomato (Mi-1 gene), bell pepper (N gene), and sweet pepper (Tabasco gene) (Brito et al., 2007; Kiewnick et al., 2009). Recently, it was also found to infect RKN-resistant sweet potato in North Carolina, South Carolina, and Louisiana (Ye, et al., 2013; Anonymous 2018; Rutter et al., 2019), and soybean and cotton in North Carolina (Ye, et al., 2013). M. enterolobii has been reported to infect many economically important crops in Brazil such as guava (Carneiro et al., 2001), and most recently, sweet potato (Silva, et al., 2021).
The resistance-breaking ability of M. floridensis (peach RKN) on economically important crops (Handoo et al., 2004) suggests this species as an important pathogen in agriculture, but which has so far been reported only in the USA. M. floridensis is of concern in Florida agriculture because of its ability to reproduce on cvs. Nemaguard, Okinawa, Nemared, and Guardian peach rootstocks (Nyczepir et al., 1998; Stanley et al., 2009), which are resistant to both M. javanica and M. incognita (Sharpe et al., 1969; Sherman et al., 1991). A virulent isolate of M. floridensis (MFGnv14) was found recently infecting peach rootstock, cv. Flordaguard (Maquilan et al., 2018; Qiu et al., 2022). Flordaguard rootstock was bred specifically to ensure root-knot disease protection for the Florida peach industry (Sherman et al., 1991). Field infestations of M. floridensis were first noted on tomato (Church, 2007), and later cucumber, eggplant, snap bean, and lilac tasselflower (Emilia sonchifolia) (Brito et al., 2005). In addition to Florida, there have been reports of this nematode severely infecting RKN-resistant peach-almond hybrid rootstock, Hansen 536 and Bright’s Hybrid®5 in California (Westphal et al., 2019), peach rootstock cv. Guardian in South Carolina orchards (Reighard et al., 2019), and tomato in Georgia (Marquez et al., 2020).
Temperature is an important factor affecting nematode development, infection rates, reproduction, survival, and migration (Madulu and Trudgill, 1994; Trudgill and Perry, 1994; Thompson et al., 2015; Leitao et al., 2021). Studies on thermal requirements of RKNs are important because of the poikilothermic nature of these pathogens, whereby temperature has a direct influence on their ecological adaptation (Trudgill et al., 2005). Thermal requirements of RKN species have been reported for M. incognita (Triantaphyllou and Hirschmann, 1960), M. hapla (Trudgill and Perry, 1994), M. javanica and M. arenaria, (Madulu and Trudgill, 1994; Dávila-Negrón and Dickson, 2013), M. hispanica (Maleita et al., 2012), and M. chitwoodi and M. fallax (Khan et al., 2014). However, for both emerging species M. enterolobii and M. floridensis, the temperature required for their development and life cycle completion is unknown. A recent review reports that there has been little research reported on the biology of M. enterolobii (Collett et al., 2021). Therefore, the objectives of the present study were to determine the effects of temperature on the life cycle and temporal variations of progression from infective to reproductive stage and emergence of second generation of J2 of M. enterolobii, concurrently with M. floridensis and compared to M. incognita. Ultimately, this study will provide better insight into the temperature-dependent biology and ecological adaptation of M. enterolobii as well as M. floridensis in regions where M. incognita is also most likely to establish successfully.
Materials and Methods
Nematode culture and second-stage juvenile (J2) inocula
The nematode isolates were reared on tomato cv. BHN 589 in the greenhouse (21 ± 8°C). M. enterolobii, M. floridensis, and M. incognita race 3 identification were confirmed using isozyme phenotypes, DNA analysis, and host differentials, respectively (Dickson et al., 1971; Brito et al., 2008; Smith et al., 2015; Subbotin, 2019). Nematode eggs were extracted from infected roots according to established protocol (Hussey and Barker, 1973) with further modifications (Boneti and Ferraz, 1981). Egg suspension was poured through a wire mesh lined with moist filter paper inside a 140-mm × 25-mm polystyrene petri dish and maintained at room temperature. After 24 hr to 48 hr, second-stage juveniles were collected and used for the experiments.
Preparation and maintenance of plant materials
Root penetration and development of the three Meloidogyne spp. were studied on tomato cv. BHN 589 seedlings. Seeds were germinated in a 38-cell seedling tray containing fine-grade vermiculite in a greenhouse (21 ± 8°C). Germinated seedlings were transplanted into 125 ml pots containing autoclaved sand (100%), fertilized weekly with 0.21% (w/v) 24N– 8P–16 K solution, Miracle-Gro (Marysville, OH). Four-to five-leaf-stage seedlings were transplanted to 251-ml polystyrene foam cups filled with autoclaved sand (100% sand). The test units were then placed in each of three growth chambers set at 30°C, 25°C, and 30°C to 25°C and maintained for 1 week before inoculation. During the experimental period, individual plants received 40 ml of water daily or as needed and fertilized biweekly as above.
Growth chamber
Three growth chambers (Percival I-36LL; Percival Scientific, Perry, IA) were each set at 30°C, 25°C, or alternating 30°C to 25°C with a 12-hr light period at 30°C and a 12-hr dark period at 25°C. Lighting was provided by fluorescent lamps (65 μmol ⋅ m−2 ⋅ s−1). Temperature in chambers were recorded with two pendant data loggers (HOBO MX2202; Onset Computer, Bourne, MA) set to record hourly averages from 5-min sampling intervals. Hourly temperatures were averaged from the two data loggers before calculating the degree-days (DD) as described below.
Nematode inoculation, penetration, and life cycle observations
Nematode inocula (200 J2/tomato seedling for M. enterolobii and M. incognita, and 100 J2/tomato seedling for M. floridensis) were pipetted into three 2-cm-deep holes around the seedling stem base and then holes were pinched closed. The low hatching obtained for M. floridensis at the time of inoculation resulted in using a different number of J2; therefore, J2 root penetration was calculated based on percentage and not numbers of nematodes that penetrated. After 48 hr from inoculation, the seedlings were removed from containers and sand around the roots was washed away under running tap water to eliminate non-penetrated juveniles. The seedlings were again transplanted into fresh autoclaved sand in 251-ml polystyrene foam cups and returned to the growth chambers. They were incubated up to 29 d in each of three growth chambers with its designated temperature treatment. Nematode development was examined at 2-d intervals between 5 d and 29 d after inoculation (DAI) with a total of 13 intervals. At each interval, two infected plants were arbitrarily collected to represent each of the three RKN species per temperature regime. A total of 78 seedlings (2 × 3 × 13) were examined for each RKN species, giving a grand total of 234 evaluated seedlings. Roots were gently rinsed and subjected to a root clearing-staining method (Byrd et al., 1983). The number of nematodes at each developmental stage (Figs. 1, 2) was observed and counted. The number of J2 that had penetrated at 5 DAI was used as a baseline for calculating the percentage of M. enterolobii, M. floridensis, and M. incognita J2 present in roots over the 29-d observation period. When globose females were detected for the first time, the root samples were stained (Thies et al., 2002) to aid with visualization of egg masses, which would indicate the emergence of egg-laying females. The egg masses were also checked to avoid missed counting of egg-laying attributable to egg masses that may have been dislodged during the process of clearing and staining of internal root tissues. Presence of egg masses, therefore, corresponded to the number of egg-laying females that were present before they were dislodged during the root-clearing and staining processes. The stained root systems were immersed in glycerol before different developmental stages were examined individually under the stereomicroscope (Zeiss Stereo Discovery.V8, Oberkochen, Germany).
Figure 1.

Growth stages of second-stage juveniles (J2) of Meloidogyne spp. (A) vermiform, early J2 with no swelling; (B, C, D) mid-stage J2 with early swelling and conoid tail; and (E, F) late J2 with swollen body, rounded terminus. Scale bars: A–E = 50 μm; and F = 10 μm.
Figure 2.

Developmental stages of Meloidogyne spp.; (A) third-stage juvenile (J3); (B) anterior part of a J3; (C) fourth-stage juvenile (J4); (D) anterior part of a J4; (E) female; and (F) egg-laying globose female. Scale bars: A, E, and F = 50 μm; B, C, and D = 10 μm.
Classification of nematode developmental stages
The number of nematodes in each developmental stage was recorded. They were assigned as J2 and succeeding stages up to new-generation vermiform J2. The J2 present in the roots beginning at 5 DAI were classified further into three growth stages based on their body shape as follows: (i) early stage J2 − vermiform with no swelling, (ii) mid-stage J2 − with early swelling and conoid tail, and (iii) late J2 − with swollen body and rounded terminus. Third- and fourth-stage juveniles were distinguished based on the cuticle layers in the anterior part of their body as previously described (Triantaphyllou and Hirschmann, 1960; Dávila-Negrón and Dickson, 2013). To distinguish between stages J3 and J4, the nematodes were handpicked and mounted in glycerin on glass slides (25 mm × 75 mm × 1 mm) for observation at ×40 magnification individually under the stereomicroscope.
Data collection and analyses
Observations were made on whole root systems from two tomato plants for each treatment, for a total of 18 observations at each time point between 5 d to 29 d. For each, the total number of nematodes present in whole root system was used as the baseline for calculating the percentage of nematodes at each of the following developmental stages: J2, J3, J4, females, and egg-laying females. The percentages were averaged for the two observations and the resulting values were plotted on the graph. The percentage of penetrated J2 at 5 DAI was calculated by dividing the number of J2 embedded in the roots by the number of inoculated J2. To assess the main and interaction effects of RKN species and temperature treatment on nematode infectivity, data on proportion of penetrated J2 at 5 DAI were subjected to two-way (RKN species × temperature treatment) analysis of variance (ANOVA) using SigmaPlot. Tukey’s HSD test (P ≤ 0.05) was used to compare means. To calculate the accumulated degree-days (ADD) for vermiform J2 to reach each successive developmental stage or to complete a generation, the daily difference between mean temperature in the growth chamber and the base temperature (Tb) was summed over the number of DAI. The mean Tb of M. incognita was 9.8°C when inoculated on okra (Dávila-Negrón and Dickson, 2013), 10.1°C on tomato (Ploeg and Maris, 1999), and 10.1°C on clover (Vrain et al., 1978). To date, there have been no temperature-based models developed for M. enterolobii and M. floridensis to estimate the Tb but we suspect similarities with the heat requirements for M. incognita development and those of other tropical and subtropical RKN species (Ferris et al., 1978; Maleita et al., 2012); thus, we followed Tyler’s (1933) calculation of heat units for RKN development, wherein each centigrade above 10°, acting for 1 hr, is counted as one effective unit.
Results
Temperature effects on root penetration
No significant interaction was found between RKN species and temperature treatments for the number of J2 that penetrated the root system. Based on the average of the three nematode species evaluated, the percentage of J2 that penetrated the whole root system of tomato at 5 DAI ranged from 15% to 33% under the three temperature regimes. Root invasion of J2 in tomato roots was affected by temperature regardless of the species (Fig. 3). For all three species, the percentage of J2 penetrating roots at 5 DAI was greater at 30°C than at 25°C, and intermediate at 30°C to 25°C (P = 0.022).
Figure 3.

Percentage of Meloidogyne spp. second-stage juveniles (J2) penetrating tomato roots grown under different temperature (25°C, 30°C, or 30-25°C [12-hr light period at 30°C and 12-hr dark period at 25°C]) at 5 DAI. Bars represent the grand means ± SE of the three Meloidogyne spp. (M. enterolobii, M. floridensis, and M. incognita race 3). The percentage of penetrated J2 was calculated by dividing the total number of nematodes observed in the whole root system at 5 DAI over the initial inoculum concentration. Bars with different letter(s) indicate significant differences based on Tukey’s HSD test (P ≤ 0.05). DAI, days after inoculation.
Temperature effects on post-penetration development
During the time span of 29 d, the developmental rates of M. enterolobii, M. floridensis, and M. incognita varied in response to temperatures (Fig. 4). Regardless of the species, only J2 were observed inside roots at 5 DAI and 7 DAI at 25°C (Fig. 4A) and 30°C to 25°C (Fig. 4C), respectively. At 7 DAI, however, development into J3 had begun for all three species at 30°C (Fig. 4B), 2 d ahead of those at 25°C, and 30°C to 25°C with greater J3 numbers in the latter. At 11 DAI at 25°C, development into J4 and females occurred concomitantly with the increase in the numbers of J3. At 11 DAI at 30°C to 25°C, however, there was a corresponding decrease in the numbers of J3 as they increasingly developed into J4 and females; the same occurred 2 d earlier (9 DAI) at 30°C. The percentage of females increased over time at all three temperatures (Fig. 4), but occurred faster, and the number of females was greater at 30°C (Fig. 4B). At 30°C, the number of females increased from 40% to 80% for M. enterolobii and 60% to 95% for M. incognita between 9 DAI and 13 DAI, whereas for M. floridensis an increase of 70% to 80% occurred 2 d earlier (9–11 DAI). At 15 DAI under the same temperature (Fig. 4B), the number of females reached more than 90% for all three species. M. floridensis was the first to reach female stage (9 DAI), but for all three species egg-laying females were observed at 17 DAI. Egg-laying females were first observed at 13 DAI under 30°C (Fig. 4B) and at 17 DAI under 30°C to 25°C (Fig. 4C) for all three species, with, however, greater numbers occurring for M. floridensis. At 25°C (Fig. 4A), egg-laying females were first seen at 21 DAI for M. enterolobii, and at 17 DAI for M. floridensis and M. incognita. Predominance of the egg-laying female stage was apparent 17 DAI at 30°C for all three species (Fig. 4B), 19 DAI at 30°C to 25°C for M. floridensis and M. incognita (Fig. 4C), 21 DAI at 30°C to 25°C for M. enterolobii (Fig. 4C), 23 DAI at 25°C for M. enterolobii and M. floridensis (Fig. 4A), and 21 DAI at 25°C for M. incognita (Fig. 4A).
Figure 4.
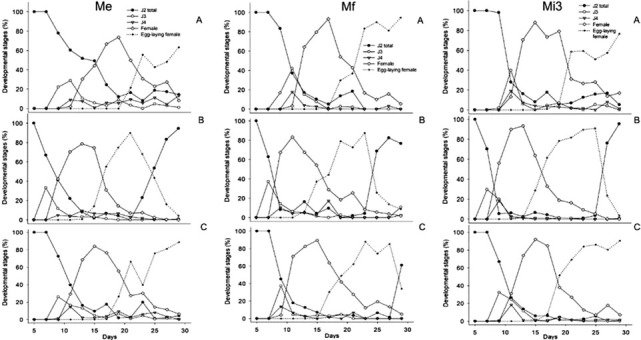
Percentage of Meloidogyne enterolobii (Me), M. floridensis (Mf), and M. incognita race 3 (Mi3) developmental stages on tomato grown in a growth chamber for 29 DAI at 25°C (A), 30°C (B), and 30–25°C (C) (12-hr light period at 30°C and 12-hr dark period at 25°C). Percentage of nematodes in each developmental stage was based on the total number of nematodes observed in the whole root system. A total of 78 root systems per RKN species were subjected to analysis. The increase of J2 at 30°C at 23 DAI for M. enterolobii, at 25 DAI for M. floridensis and M. incognita, and at 30–25°C at 29 DAI for M. floridensis represent the earliest observation of vermiform juveniles from the second generation. RKN, root-knot nematode; DAI, days after inoculation.
DD required for development and life cycle completion
Cumulative days (CD) and ADD (DD; Tb = 10°C) required for the first observation of each different development stage from infective J2 to new-generation vermiform J2 in tomato at 25°C, 30°C, and 30°C to 25°C are shown in Table 1. At 25°C, M. enterolobii required more DD (308.3) to develop into egg-laying females compared with M. incognita and M. floridensis (248.1). At 30°C, the three species reached all developmental stages faster than at other temperatures (Fig. 4), but there was no difference in DD required for development from J3 to egg-laying female among the three species (Table 1).
Table 1.
CD and ADD required for first observation of each developmental stage of Meloidogyne enterolobii (Me), M. floridensis (Mf), and M. incognita race 3 (Mi3) on tomato inoculated with second-stage juveniles at different temperatures.
| Meloidogyne spp. | Developmental stagesa | Temperature (°C) | |||||
|---|---|---|---|---|---|---|---|
| 25 | 30 | 30–25b | |||||
|
|
|||||||
| CD | ADDc | CD | ADD | CD | ADD | ||
| M. enterolobii | J3 | 9 | 130.6 | 7 | 152.6 | 9 | 144.1 |
| J4 | 11 | 159.4 | 9 | 195.7 | 9 | 144.1 | |
| Female | 11 | 159.4 | 9 | 195.7 | 11 | 176.1 | |
| Egg-laying female | 21 | 308.3 | 13 | 285.7 | 17 | 272.7 | |
| New-vermiform J2 | nad | na | 23 | 506.9 | na | na | |
| M. floridensis | J3 | 9 | 130.6 | 7 | 152.6 | 9 | 144.1 |
| J4 | 11 | 159.4 | 9 | 195.7 | 9 | 144.1 | |
| Female | 11 | 159.4 | 9 | 195.7 | 9 | 144.1 | |
| Egg-laying female | 17 | 248.1 | 13 | 285.7 | 17 | 272.7 | |
| New-vermiform J2 | na | na | 25 | 552.3 | 29 | 468.8 | |
| M. incognita | J3 | 9 | 130.6 | 7 | 152.6 | 9 | 144.1 |
| J4 | 11 | 159.4 | 9 | 195.7 | 9 | 144.1 | |
| Female | 11 | 159.4 | 9 | 195.7 | 11 | 176.1 | |
| Egg-laying female | 17 | 248.1 | 13 | 285.7 | 17 | 272.7 | |
| New-vermiform J2 | na | na | 25 | 552.3 | na | na | |
J3 = third-stage juvenile; J4 = fourth-stage juvenile; new-vermiform J2 = second-stage vermiform juvenile from the second generation.
bTemperature alternated between 30°C and 25°C (12-hr light period at 30°C and 12-hr dark period at 25°C).
cADD above a threshold temperature [base temperature (Tb = 10°C)].
dna = none observed within the 29-d period.
DD, degree-days; ADD, accumulated degree-days; CD, cumulative days.
At 25°C with 425.4 DD (Tb =10°C), the three species were not able to complete their life cycle (J2–J2) within 29 d, as ascertained based on the absence of new generation of vermiform J2 inside roots (Table 1). Similar results were observed at 30°C to 25°C for M. enterolobii and M. incognita, but not for M. floridensis, wherein the life cycle completion (J2– J2) occurred at 29 DAI (468.8 DD). However, at 30°C, the three species completed their life cycle within 23 DAI to 25 DAI with 506.9 DD for M. enterolobii, and 552.3 DD for both M. floridensis and M. incognita. At this temperature, new-vermiform juveniles from the second generation could be observed in the roots after 12 d (266.6 DD30) for M. floridensis and M. incognita and 10 d (221.2 DD) for M. enterolobii from the first occurrence of egg-laying females. At 30°C to 25°C, it occurred after 12 d (196.1 DD) for M. floridensis, but not for the other two species.
Discussion
The infectivity and rates of development of M. enterolobii, M. floridensis, and M. incognita on tomato roots were affected by temperature. Greater numbers of J2, females, and egg-laying females were observed at 30°C than at 25°C or 30°C to 25°C. The infectivity in host roots requires considerable activity by the J2, and elevated temperatures would increase their activity (Tyler, 1933) up to a certain threshold, beyond which higher temperatures would be harmful or lethal to the juveniles (Wang and McSorley, 2008).
Our results indicate that all three species accelerate their developmental rate with increasing temperature and there was no difference in their development time from J2 to egg-laying females at 30°C. At this temperature, there was a reduction in the number of days taken to reach J3, J4, females, and egg-laying females, whereas lower temperature (25°C) delayed progression of the J2 into the reproductive stages, which is consistent with previous studies (Dávila-Negrón and Dickson, 2013; Vela et al., 2014).
Egg-laying females were observed at 13 DAI at 30°C for all three species, similar to previous findings for M. incognita on tomato at the same temperature (Davide and Triantaphyllou, 1967), but differed by 2 d (15 DAI) in another study on okra (Dávila-Negrón and Dickson, 2013). In the present study, egg-laying females were predominant at 17 DAI at 30°C for all three species and reached 90% between 21 d and 25 d. Dávila-Negrón and Dickson (2013) reported a smaller number of egg-laying females (60%) for M. incognita at the same temperature by the end of their observation (31 d). However, the differences in these results may be related to the different nematode isolates and to the host used in our experiment.
The duration of the life cycle of M. enterolobii, M. floridensis, and M. incognita was affected by temperature, as reported for other RKNs (Zhang and Schmitt, 1995; Ploeg and Maris, 1999; Maleita et al., 2012; Khan et al., 2014; Vela et al., 2014). For M. incognita, the life cycle (from J2 to J2) was completed on tomato plants in 20 d and 27 d at average temperatures of 30°C and 25°C, respectively (Ploeg and Maris, 1999). In the present study, M. incognita was able to complete its life cycle in 25 d at 30°C, but not at 25°C within our timeframe of 29 DAI.
Meloidogyne floridensis also completed its life cycle in 25 d at 30°C, whereas M. enterolobii completed it at 23 DAI. The alternating temperature (30°C to 25°C) affected the length of the life cycle for the three species by delaying their development into egg-laying females. When exposed to fluctuating temperatures, only M. floridensis completed its cycle at 29 DAI when new-generation vermiform J2 were observed in roots. These findings may be attributed to differences in days required for embryogenesis and hatching (Dávila-Negrón and Dickson, 2013). To our knowledge, this is the first detailed report of the development and duration of M. enterolobii and M. floridensis life cycle completion on tomato; and, given the global distribution of M. enterolobii and increased distribution of M. floridensis in the USA, these are worth further investigation under other diurnal temperature ranges and with a broader timespan.
The base temperature (Tb) and thermal requirements or DD have varied only slightly among studies on development and life cycle of RKNs because of adaptation of these species to similar warmer climates (Trudgill et al., 2005). The calculated values of Tb and thermal-time requirements for egg mass formation on tomato (Tb = 9.8, DD = 300; Dávila-Negrón and Dickson, 2013) and on cucumber (Tb = 12.2, DD = 294; Giné et al., 2014) were similar to those reported for M. incognita. In our study, using 10°C as base temperature for calculating the DD (Tyler, 1933; Trudgill, 1995), the results from M. enterolobii and M. floridensis (DD = 248–308) lie within close ranges as that from M. incognita. Similar values were also reported for the life cycle of M. incognita (Tb = 10.1, DD = 400; Ploeg and Maris, 1999), M. javanica and M. hapla (Tb = 12.9, DD = 350; Tb = 8.25, DD = 554, respectively; Trudgill and Perry, 1994), and M. hispanica (Tb = 10.22, DD = 515.46; Maleita et al., 2012) on tomato, and for M. incognita and M. javanica on cucumber (Tb = 11.4, DD = 500; Giné et al., 2014). Using 10°C as Tb, the values of DD calculated for the three species (M. enterolobii = 506.9; M. floridensis and M. incognita = 552.3) for life cycle completion from J2 to new generation of J2 at 30°C were similar to those reported for other RKN species. These results confirm that M. enterolobii and M. floridensis can reproduce in climates optimal for M. incognita, reportedly the most widespread RKN species worldwide (Taylor and Sasser, 1978), and further suggest that climates closer to or at 30°C could favor a shorter generation time for M. enterolobii.
Acknowledgement
The authors thank Dr. Silvia Vau for support in obtaining images.
Literature Cited
- Declaration of emergency. Office of Agriculture and environmental sciences horticulture and quarantine. Department of Agriculture and forestry. State of Louisiana, Baton Rouge, LA. 2018. https://www.doa.la.gov/osr/EMR/2019/1906EMR020.pdf Anonymous. [Accessed on March 14, 2022]
- Boneti J. I. S., Ferraz S.. Modificação do método de Hussey & Barker para extração de ovos de Meloidogyne exigua de raízes de cafeeiro. Fitopatologia Brasileira. 1981;6:553. [Google Scholar]
- Brito J. A., Ramandeep K., Cetintas R., Stanley J. D., Mendes M. L., McAvoy E. J., Powers T. O., Dickson D. W.. Identification and isozyme characterization of Meloidogyne spp. infecting horticultural and agronomic crops and weed plants in Florida. Nematology. 2008;10:757–766. [Google Scholar]
- Brito J. A., Stanley J. D., Cetintas R., Hamill J., Dickson D. W.. A new root-knot nematode infecting vegetables in Florida. Journal of Nematology. 2005;37:359–360. (Abstr.) [Google Scholar]
- Brito J. A., Stanley J. D., Kaur R., Cetintas R., Di Vito M., Thies J. A., Dickson D. W.. Effects of the Mi-1 N and Tabasco genes on infection and reproduction of Meloidogyne mayaguensis on tomato and pepper genotypes. Journal of Nematology. 2007;39:327–332. [PMC free article] [PubMed] [Google Scholar]
- Brito J. A., Vau S. J., Dickson D. W.. Host status of selected sweet potato cultivars to Meloidogyne enterolobii. Journal of Nematology. 2014;46:140. (Abstr.) [Google Scholar]
- Byrd D.W.. Kirkpatrick T., Barker K. R.. An improved technique for clearing and staining plant tissues for detection of nematodes. Journal of Nematology. 1983;15:142–143. Jr. [PMC free article] [PubMed] [Google Scholar]
- Carneiro R. M. D. G., Moreira W. A., Almeida M. R. A., Gomes A. C. M. M.. Primeiro registro de Meloidogyne mayaguensis em goiabeira no Brasil. Nematologia Brasileira. 2001;25:223–228. [Google Scholar]
- Castagnone-Sereno P.. Meloidogyne enterolobii (= M. mayaguensis): Profile of an emerging, highly pathogenic, root-knot nematode species. Nematology. 2012;14:133–138. [Google Scholar]
- Church G. T.. First report of the root-knot nematode Meloidogyne floridensis on tomato (Lycopersicon esculentum) in Florida. Plant Disease. 2007;89:527. doi: 10.1094/PD-89-0527B. [DOI] [PubMed] [Google Scholar]
- Collett R. L., Marais M., Daneel M., Rashidifard M., Fourie H. Meloidogyne enterolobii, a threat to crop production with particular reference to sub-Saharan Africa: an extensive, critical and updated review. Nematology 23:247–285. 2021. [DOI]
- Davide R. G., Triantaphyllou A. C.. Influence of the environment on development and sex differentiation of root-knot nematodes. I. Effect of infection density, age of the host plant and soil temperature. Nematologica. 1967;13:102–110. [Google Scholar]
- Dávila-Negrón M., Dickson D. W.. Comparative thermal-time requirements for development of Meloidogyne arenaria M. incognita, and M. javanica, at constant temperatures. Nematropica. 2013;43:152–163. [Google Scholar]
- Dickson D. W., Huisingh D., Sasser J. N.. Dehydrogenases, acid and alkaline phosphatases, and esterases for chemotaxonomy of selected Meloidogyne Ditylenchus Heterodera, and Aphelenchus spp. Journal of Nematology. 1971;3:1–16. [PMC free article] [PubMed] [Google Scholar]
- Fargette M., Braaksma R.. Use of the esterase phenotype in the taxonomy of the genus Meloidogyne. 3. A study of some “ B ” race lines and their taxonomic position. Revue de Nématologie. 1990;13:375–386. [Google Scholar]
- Ferris H., Du Vernay H. S., Small R. H.. Development of a soil-temperature data base on Meloidogyne arenaria for a simulation model. Journal of Nematology. 1978;10:39–42. [PMC free article] [PubMed] [Google Scholar]
- Giné A., López-Gómez M., Vela M. D., Ornat C., Talavera M., Verdejo-Lucas S., Sorribas F. J.. Thermal requirements and population dynamics of root-knot nematodes on cucumber and yield losses under protected cultivation. Plant Pathology. 2014;63:1446–1453. [Google Scholar]
- Handoo Z. A., Nyczepir A. P., Esmenjaud D., van der Beek J. G., Castagnone-Sereno P., Carta L. K., Skantar A. M., Higgins J. A.. Morphological, molecular, and differential-host characterization of Meloidogyne floridensis n. sp. (Nematode: Meloidogynidae), a root-knot nematode parasitizing peach in Florida. Journal of Nematology. 2004;36:20–35. [PMC free article] [PubMed] [Google Scholar]
- Hussey R. S., Barker K. R.. Comparison of methods for collecting inocula of Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Khan A., Wesemael W., Moens M.. Influence of temperature on the development of the temperate root-knot nematodes Meloidogyne chitwoodi and M. fallax. Russian Journal of Nematology. 2014;22:1–9. [Google Scholar]
- Kiewnick S., Dessimoz M., Franck L.. Effects of the Mi-1 and the N root-knot nematode-resistance gene on infection and reproduction of Meloidogyne enterolobii on tomato and pepper cultivars. Journal of Nematology. 2009;41:134–139. [PMC free article] [PubMed] [Google Scholar]
- Leitao D. A. H. S., Pedrosa E. M. R., Dickson D. W., Oliveira A. K. S., Rolim M. M.. Temperature: A driving factor for Meloidogyne floridensis migration toward different hosts. Journal of Nematology. 2021;53:1–10. doi: 10.21307/jofnem-2021-074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madulu J. D., Trudgill D. L.. Influence of temperature on the development and survival of Meloidogyne javanica. Nematologica. 1994;40:230–243. [Google Scholar]
- Maleita C., Curtis R., Abrantes I.. Thermal requirements for the embryonic development and life cycle of Meloidogyne hispanica. Plant Pathology. 2012;61:1002–1010. [Google Scholar]
- Maquilan M. A. D., Olmstead M. A., Dickson D. W., Chaparro J. X.. Inheritance of resistance to the peach root-knot nematode (Meloidogyne floridensis) in interspecific crosses between peach (Prunus persica) and its wild relative (Prunus kansuensis) Plant Breeding. 2018;137:805–813. [Google Scholar]
- Marquez J., Forghani F., Hajihassani A. First report of the root-knot nematode. Meloidogyne floridensis, on tomato in Georgia, USA. Plant Disease. 2020. https://apsjournals.apsnet.org/doi/10.1094/PDIS-10-20-2286-PDN
- Nyczepir A. P., Esmenjaud D., Eisenback J. D.. Pathogenicity of Meloidogyne sp. (FL-isolate) on Prunus in the southeastern United States and France. Journal of Nematology. 1998;30:509. (Abstr.) [Google Scholar]
- Philbrick A. N., Adhikari T. B., Louws F. J., Gorny A. M.. Meloidogyne enterolobii, a major threat to tomato production: current status and future prospects for its management. Frontiers in Plant Science. 2020;11:606395. doi: 10.3389/fpls.2020.606395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploeg A. T., Maris P. C.. Effects of temperature on the duration of the life cycle of a Meloidogyne incognita population. Nematology. 1999;1:389–393. [Google Scholar]
- Qiu S., Maquilan M. A., Chaparro J. X., Brito J. A., Beckman T. G., Dickson D. W.. Susceptibility of Flordaguard peach rootstock to a resistant-breaking population of Meloidogyne floridensis and two populations of Meloidogyne arenaria. Journal of Nematology. 2022;53:e2021–111. doi: 10.21307/jofnem-2021-111. https:// doi.org/10.21307/jofnem-2021-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reighard G. L., Henderson W. G., Scott S. O., Subbotin S. A.. First report of the root-knot nematode, Meloidogyne floridensis infecting Guardian® peach rootstock in South Carolina, USA. Journal of Nematology. 2019;51:1–3. doi: 10.21307/jofnem-2019-061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter W. B., Skantar A. M., Handoo Z. A., Mueller J. D., Aultman S. P., Agudelo P.. Meloidogyne enterolobii found infecting root-knot nematode resistant sweet potato in South Carolina, United States. Plant Disease. 2019;103:775. [Google Scholar]
- Sharpe R. H., Hesse C. O., Lownsberry B. A., Perry V. G., Hansen C. J.. Breeding peaches for root-knot nematode resistance. Journal of the American Society for Horticultural Sciences. 1969;94:209–212. [Google Scholar]
- Sherman W. B., Lyrene P. M., Sharpe R. H.. Flordaguard peach rootstock. HortScience. 1991;26:427–428. [Google Scholar]
- Silva E. M., Souza Pollo A., Nascimento D. D., Ferreira R. J., Duarte S. R., Fernandes J. P. P., Soares P. L. M. First report of root-knot nematode Meloidogyne enterolobii infecting sweetpotato in the state of Rio Grande do Norte, Brazil. Plant Disease. 2021. [DOI]
- Smith T., Brito J. A., Han H., Kaur R., Cetintas R., Dickson D. W.. Identification of the peach root-knot nematode, Meloidogyne floridensis, using mtDNA PCR-RFLP. Nematropica. 2015;45:138–143. [Google Scholar]
- Stanley J. D., Brito J. A., Kokalis-Burelle N., Frank J. H., Dickson D. W.. Biological evaluation and comparison of four Florida isolates of Meloidogyne floridensis. Nematropica. 2009;39:255–271. [Google Scholar]
- Subbotin S. A.. Recombinase polymerase amplification assay for rapid detection of the root-knot nematode Meloidogyne enterolobii. Nematology. 2019;21:243–251. doi: 10.2478/jofnem-2024-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Sasser J. N. Biology, identification, and control of root-knot nematodes. Department of Plant Pathology, North Carolina State University and the United States Agency for International Development. Raleigh: North Carolina State University Graphics; 1978. [Google Scholar]
- Thies J. A., Merrill S. B., Corley E. L.. Red food coloring stain: New, safer procedures for staining nematodes in roots and egg masses on root surfaces. Journal of Nematology. 2002;34:179–181. [PMC free article] [PubMed] [Google Scholar]
- Thompson J. P., Clewett T. G., O’Reilly M. M.. Temperature response of root-lesion nematode (Pratylenchus thornei) reproduction on wheat cultivars has implications for resistance screening and wheat production. Annals of Applied Biology. 2015;167:1–10. [Google Scholar]
- Triantaphyllou A. C., Hirschmann H.. Post-infection development of Meloidogyne incognita Chitwood, 1949 (Nematoda: Heteroderidae) Annales de L’Institut Phytopathologique Benaki. 1960;3:1–11. [Google Scholar]
- Trudgill D. L.. An assessment of the relevance of thermal time relationships to nematology. Fundamental and Applied Nematology. 1995;18:407–417. [Google Scholar]
- Trudgill D. L., Perry J. N.. Thermal time and ecological strategies – a unifying hypothesis. Annals of Applied Biology. 1994;125:521–532. [Google Scholar]
- Trudgill D. L., Honek A., Li D., Van Straalen N. M.. Thermal time - concepts and utility. Annals of Applied Biology. 2005;146:1–14. [Google Scholar]
- Tyler J.. Development of the root-knot nematode as affected by temperature. Hilgardia. 1933;7:391–415. [Google Scholar]
- Vela M. D., Giné A., López-Gómes M., Sorribas F. J., Ornat C., Verdejo-Lucas S., Talavera M.. Thermal time requirements of root-knot nematodes on zucchini-squash and population dynamics with associated yield losses on spring and autumn cropping cycles. European Journal of Plant Pathology. 2014;140:481–490. [Google Scholar]
- Villar-Luna E., Goméz-Rodriguez O., Rojas-Martínez R. I., Zavaleta-Mejía E.. Presence of Meloidogyne enterolobii on Jalapeño pepper (Capsicum annuum L.) in Sinaloa, Mexico. Helminthologia. 2016;53:155–160. [Google Scholar]
- Vrain T. C., Barker K. R., Holtzman G. I.. Influence of low temperature and rate of development of Meloidogyne incognita and M. hapla larvae. Journal of Nematology. 1978;10:166–171. [PMC free article] [PubMed] [Google Scholar]
- Wang K.-H., McSorley R.. Exposure time to lethal temperatures for Meloidogyne incognita suppression and its implication for soil solarization. Journal of Nematology. 2008;40:7–12. [PMC free article] [PubMed] [Google Scholar]
- Westphal A., Maung Z. T. Z., Doll D. A., Yaghmour M. A., Chitambar J. J., Subbotin S. A.. First report of the peach root-knot nematode, Meloidogyne floridensis infecting almond on root-knot nematode-resistant ‘Hansen 536’ and ‘Bright’s Hybrid 5’ rootstocks in California, USA. Journal of Nematology. 2019;51:1–3. doi: 10.21307/jofnem-2019-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Eisenback J. D.. Meloidogyne enterolobii n. sp. (Meloidogynidae), a root-knot nematode parasitizing pacara earpod tree in China. Journal of Nematology. 1983;15:381–391. [PMC free article] [PubMed] [Google Scholar]
- Ye W., Koening S., Zhuo K., Liao J.. First report of Meloidogyne enterolobii on cotton and soybean in North Carolina, United States. Plant Disease. 2013;97:1262. doi: 10.1094/PDIS-03-13-0228-PDN. [DOI] [PubMed] [Google Scholar]
- Zhang F., Schmitt D. P.. Embryogenesis and postinfection development of Meloidogyne konaensis. Journal of Nematology. 1995;27:103–108. [PMC free article] [PubMed] [Google Scholar]


