Abstract
Chemotherapy-induced peripheral neuropathy (CIPN) is a somatosensory small fiber axonopathy in cancer patients receiving any of a variety of widely-use antitumor agents. CIPN can lead to long-lasting neuropathic pain that limits the dose or length otherwise life-saving cancer therapy. Accumulating evidence over the last two decade indicates that many chemotherapeutic agents cause mitochondrial injury in the peripheral sensory nerves by disrupting mitochondrial structure and bioenergetics, increasing nitro-oxidative stress and altering mitochondrial transport, fission, fusion and mitophagy. The accumulation of abnormal and dysfunctional mitochondria in sensory neurons are linked axonal growth defects resulting in loss of intraepidermal nerve fibers in the hands and feet, increased spontaneous discharge and the sensitization of peripheral sensory neurons that provoke and promote changes in the central nervous system that establish chronic neuropathic pain. This has led to the propose mitotoxicity theory of CIPN. Strategies that improve mitochondrial function have shown success in preventing and reversing CIPN in pre-clinical animal models and have begun to show some progress toward translation to the clinic. In this review, we will review the evidence for, the causes and effects of and current strategies to target mitochondrial dysfunction in CIPN.
Keywords: Chemotherapy-induced neuropathy, neuropathic pain, mitochondrial dysfunction, bioenergetics, nitro-oxidative stress, mitochondrial transport
1. Introduction
Chemotherapy-induced peripheral neuropathy that leads to a long-lasting bilateral neuropathic pain state (CIPN) is a common adverse side effect that develops in patients receiving treatment with first-line anticancer drugs for breast, gastrointestinal, lung, ovarian and testicular cancers and multiple myeloma [30, 93]. These agents include taxanes (e.g., paclitaxel and docetaxel) [30, 93] that disrupt microtubule depolymerization [38, 75], vinca alkaloids (e.g., vincristine) [30, 93] that disrupt microtubule polymerization [6], platinum-based antineoplastic agents (e.g., cisplatin and oxaliplatin) [30, 93] that disrupt DNA replication [43], proteasome inhibitors (e.g., bortezomib) [30, 65, 93] and targeted monoclonal antibody therapies (e.g., brentuximab and trastuzumab) [93]. However, despite their diverse mechanisms of action in cancer, these agents induce a somatosensory small fiber axonopathy hallmarked by reductions in the density of intraepidermal nerve fibers (IENF) of axon terminal that innervate the cutaneous layer of glabrous skin [88, 102, 108], which may or may not be accompanied by axonal atrophy, axonal demyelination and/or neuronal degeneration of sensory neurons in the dorsal root ganglia [7, 36]. An increase in the incidence of low frequency, irregularly-patterned spontaneous discharges in the sensory neurons [100, 103] also develops with this axonopathy and is thought to initiate and contribute to changes in the processing and amplification of pain sensations that lead to the transition to a chronic pain state [7, 50].
Evidence over the last two decades has shown that these features are associated with abnormal and dysfunctional mitochondria in peripheral sensory neurons (Figure 1). Mitochondria play an essential role in neuronal bioenergetics, calcium buffering, lipid and protein biosynthesis and antioxidant status that drive the health, growth and synaptic function of neurons [16, 20, 45]. Strategies discussed in this review that improve or prevent mitochondrial abnormalities in peripheral sensory neurons have shown effectiveness in preclinical studies in reducing pain symptoms during CIPN or preventing the development of CIPN altogether. This has led to a propose hypothesis that chemotherapeutic agents are mitotoxic in the peripheral sensory neurons and the ensuing mitochondrial dysfunction drives the development and maintenance of CIPN [7].
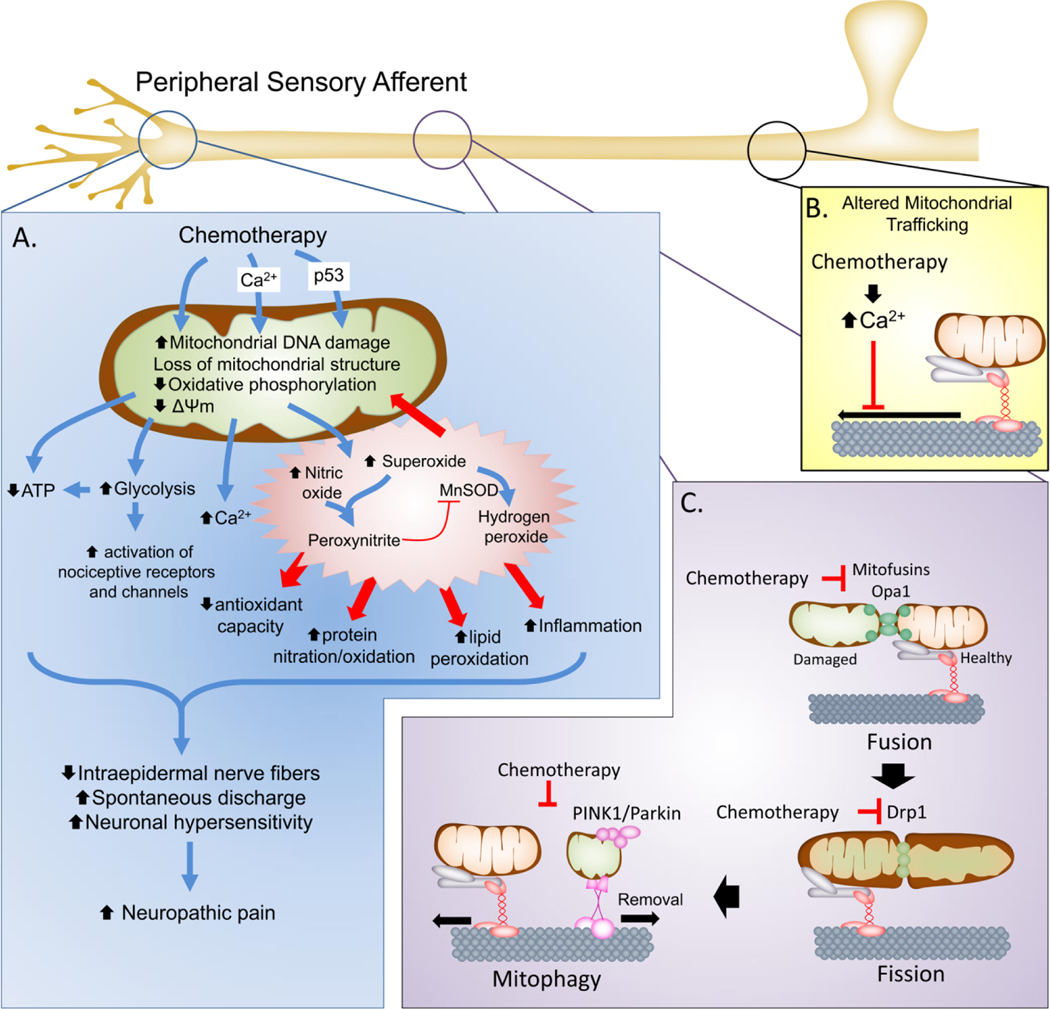
Figure 1. Mitochondrial dysfunction in chemotherapy-induced neuropathy.
A. In the peripheral sensory neurons, chemotherapeutic agents can cause direct or indirect (e.g., through Ca2+ influx or p53 accumulation) injury to mitochondria that include damage to mitochondrial DNA, loss of mitochondrial morphology and disruption of oxidative phosphorylation and mitochondrial membrane potential. This leads to reduced ATP production and increased reliance on glycolysis that result the net reduction of cellular bioenergetic capacity to respond to increase cellular activity and potential activation of nociceptive receptors and channels. Moreover, mitochondrial dysfunction also leads to increased production of SO and PN to drive nitroxidative stress that initiate protein, lipid and nucleic acid modification to further drive nitro-oxidative stress and mitochondrial dysfunction as well as contribute to neuronal signaling pathways and inflammation. Collectively this leads to reduced axonal growth, loss of IENFs and increased spontaneous discharge and neuronal hypersensitivity that contribute to establishment of the chronic neuropathic pain state. B. Chemotherapeutics also contribute to mitochondrial dysfunction by altering the axonal transport of mitochondria to the axon terminals, depriving the axonal terminals of bioenergetics support. C. The health of mitochondria is maintained in part by mitochondrial fission and fusion processes that allow healthy mitochondria to fuse and mix with damage mitochondria to sort the damaged areas for removal (mitophagy). In addition to altering transport, chemotherapeutics may down regulate the expression of mitochondrial fusion (mitofusins and Opa1) and fission (Drp1) proteins to disrupt the renewal of mitochondria in the axonal terminals and prevent mitophagy resulting in the accumulation of abnormal, dysfunctional mitochondria.
2. Evidence for Mitochondrial Dysfunction in CIPN
2.1. Abnormal mitochondrial morphology.
A greater incidence of abnormal swollen and vacuolated mitochondria in peripheral nerve sensory axons was first noted in myelinated A-fibers and unmyelinated C-fibers in the saphenous nerves in rats following paclitaxel treatment prior to the development of CIPN and at peak hypersensitivity, but not during the resolution of CIPN [29]. Abnormal mitochondria have been observed in the saphenous nerves [39, 41, 71], sciatic nerves [99] and the sensory axons of the dorsal root [103] in rodents treated with paclitaxel. Moreover, direct paclitaxel treatment of mouse sciatic nerve explants increased axonal mitochondrial swelling as early as one day after treatment [74]. Similar increases in abnormal mitochondria have been reported peripheral sensory axons in animal models of vincristine- [15, 17, 104], oxaliplatin- [102], cisplatin- [61] and bortezomib- [108] induced painful peripheral neuropathy. Increased abnormal mitochondria in small distal cutaneous nerves have also been reported in skin biopsies of female non-diabetic patients receiving treatment with the microtubule-stabilizing epothilone chemotherapy, ixabepilone [25].
The occurrence of abnormal mitochondria morphologies following chemotherapeutics is largely restricted to the sensory axons [99, 103] with little evidence of abnormal mitochondria associated with paclitaxel in motor axons [103], Schwann cells [99, 103] or in the spinal cord [99]. This has led to the suggestion that abnormal mitochondrial morphologies are largely a sensory axon phenomenon during the development of CIPN [7]. However, increased numbers of swollen and vacuolated mitochondria have been reported in the dorsal root ganglia (DRG) cell bodies following paclitaxel [5] or cisplatin treatments [61, 77] and in Remak Schwann cells during ixabepilone treatment [25].
2.1. Dysfunctional mitochondrial bioenergetics.
Mitochondrial swelling and vacuolization disrupts the maintenance of the proton gradient and impairs mitochondrial ATP production [31]. Ex vivo preparations of sciatic nerve axons from rats with CIPN following paclitaxel, oxaliplatin, or bortezomib treatment exhibited significant reductions in the oxygen consumption rates (OCR) and ATP production after maximal stimulation of Complex I-mediated and Complex II-mediated respiration prior to the development of CIPN (day 7) and lasting at least three weeks after the last dose of chemotherapy [39, 103, 108]. This suggested functional impairment of mitochondria during CIPN. Recent studies by Maj et al. in mice with cisplatin-induced neuropathy confirmed compromised OCR, indicating compromised respiration, in isolated DRG cells and tibia nerves at baseline, during the production of ATP and at maximal respiratory capacity [61]. The spare respiratory capacity, which indicates the ability of mitochondria to respond to energy demands, was reduced in DRG cells, but not tibial nerves [61]. That same year, Duggett et al., demonstrated DRG cells isolated from rats treated with paclitaxel had significant reductions in maximal respiratory capacity and spare respiratory capacity after paclitaxel, but only prior to the pain manifestation [23]. ATP formation and ion exchange in the mitochondria is determined by the transmembrane potential energy established by the chemical hydrogen ion gradient (ΔpH) and charge gradient (mitochondrial membrane potential; ΔΨm) resulting from the proton pumping activity of Complex I, II and IV proteins within the mitochondrial oxidative phosphorylation chain (OXPHOS) on the inner mitochondrial membrane [109]. In sciatic nerve mitochondria from rats with CIPN following oxaliplatin, protein levels and activities of the OXPHOS Complex I, II and IV were reduced and the ΔΨm and ATP production was compromised [1, 3]. Similar reductions of ΔΨm have recently been reported in DRG neurons isolated from mice treated with one dose of cisplatin [61] and in segmental motor neurons of Drosophila larvae treated with cisplatin [78].
Much of a neuron’s bioenergetics expenditure is in maintaining membrane potential, neurite growth and the formation and stability synapses [84]. Impairment of mitochondrial ATP production in the peripheral sensory neurons has been posited as one mechanism by which mitochondrial dysfunction contributes to CIPN [7] (Figure 1A). This has been supported by findings that treatments supporting mitochondrial function attenuate CIPN, whereas, mitochondrial toxins exacerbate CIPN. For example, acetyl-L-carnitine (ALCAR) administration in paclitaxel-treated rats prevents and reverses mechano-hypersensitivities [29, 41]. ALCAR is metabolized in the mitochondria to yield carnitine for fatty acid transport and acetyl-CoA production to generate nicotinamide adenosine dinucleotide (NADH) for ATP production via the oxidative phosphorylation [82]. ALCAR prevented swelling and vacuolation of mitochondria in primary afferent C-fibers, but not A-fibers, in paclitaxel-treated rats [41]. ALCAR also attenuated spontaneous discharge in rats treated with vincristine- [100], paclitaxel- [100] and oxaliplatin- [102] induced peripheral neuropathy. This was accompanied by reductions of sensory axonal Complex I & II respiration and ATP production and attenuated the development of mechano-hypersensitivity in rats treated with paclitaxel, oxaliplatin, or bortezomib [107, 108]. In contrast, inhibition of the ATPase with oligomycin increased spontaneous discharge in animals with CIPN following paclitaxel [101]. Moreover, nicotinamide adenine nucleotide (NAD+) levels in the hind paw and sciatic nerves, but not the DRG, have been shown to be significantly reduced in animals treated with paclitaxel [53]. The depletion of NAD+ corresponded with mitochondrial dysfunction, reduced IENF and neuropathic pain, which was prevented by the stimulation of the NAD+ salvage pathway using a pharmacological activator of nicotinamide phosphoribosyl transferase (NAMPT) [53].
Other strategies that are thought to improve mitochondrial OXPHOS also show that ability to attenuate chemotherapy-induced neuropathic pain, IENF loss and spontaneous discharge. For example, deletion of the exchange factor directly activated by cyclic AMP 1 (EPAC1) prevented paclitaxel-induced reductions in mitochondrial OCR [89]. Its inhibition prevented the loss of IENF and the development of pain. The proposed mechanism of action is through EPAC1 translocation to the plasma membrane in response to increased cyclic AMP [89]. This is then thought to activate PKCε and cause its accumulation in mitochondria where it can inhibit OXPHOS [89]. The loss of mitochondrial ATP has been suggested to reduce the capacity of neurons to maintain the Na+/K+ ATPase to maintain membrane potential allowing a slow leak of Na+ to trigger spontaneous discharge [7, 69, 102]. However, quantitative measurements of ATP in brain neurons suggests that maintaining resting membrane potential for a neuron through the Na+/K+ ATPase is easily maintained by ATP produced through glycolysis [81]. Oxidative phosphorylation is then stimulated by neuronal activity to meet the additional bioenergetic burden associated with maintaining synaptic integrity, organelle transport and protein synthesis [81, 84]. In animals treated with repeated paclitaxel, Duggett et al., found evidence of enhanced basal glycolysis and maximal glycolytic ATP production in peripheral sensory neurons during peak pain in absence of altered respiration or respiratory capacity [23]. This suggests that sensory neurons switch from a reliance on oxidative phosphorylation to less efficient ATP production through glycolysis for their bioenergetics needs [23] (Figure 1A). The implications of such a switch would be that glycolysis would no longer be sufficient to maintain basal Na+/K+ ATPase activity and facilitating spontaneous discharge as its ATP production would be directed to areas normally supplied by oxidative phosphorylation. Ludman and Melemedjian found a similar switch towards glycolysis in the DRG cells from animals with bortezomib-induced neuropathic pain [57]. However, in their model, they found that pyruvate dehydrogenase kinase 1 (PDHK1) and lactate dehydrogenase expression increased leading to reduced pyruvate dehydrogenase-mediated conversion of pyruvate to acetyl-CoA and increased pyruvate conversion to its downstream metabolite lactate by lactate dehydrogenase [57]. Instead of CIPN being driven by a lack of ATP production due to glycolysis, they proposed that CIPN pain stemmed from the release of lactate and protons extracellular space that allowed lactate to potentiate voltage-gated sodium channels and immune signaling while the acidification of the extracellular space triggered proton-sensitive ion channels, such as acid-sensing ion channels, transient receptor potential cation channel subfamily V member 1 (TrpV1) and ATP-gated P2X receptor cation channels that have been associated with nociception [57] (Figure 1A). To support their model, pharmacological inhibition of PDHK1 and lactate dehydrogenase attenuated spontaneous bortezomib-induced pain behaviors in mice [57].
3. Mitochondrial dysfunction and nitro-oxidative stress.
3.1. Evidence of mitochondrial nitro-oxidative stress in CIPN.
Nitro-oxidative stress is the imbalance between the production of reactive oxygen species (ROS; e.g. superoxide and hydrogen peroxide) and reactive nitrogen species (RNS; e.g., peroxynitrite) and the cellular antioxidant capacity bolstered by a number of antioxidant enzymes that include glutathione peroxidases (Gpx), catalase, cytosolic copper/zinc superoxide dismutase (Cu/Zn-SOD) and the mitochondrial manganese superoxide dismutase (MnSOD) [9, 40, 70, 80]. This imbalance leads to uncontrolled release of ROS/RNS that can undergo oxidative, nitrosylative and nitrative reactions with proteins, lipids and nucleic acids [9, 40, 70, 80]. Nitro-oxidative stress has been implicated in the development of CIPN (Figure 1A). For example, increased levels of lipid peroxidation and protein oxidation products have been found in plasma, sciatic nerves and spinal cord in animals with oxaliplatin-induced neuropathic pain [21]. Increased lipid peroxidation products have also been reported in the sciatic nerve of animals with vincristine- [68], cisplatin- [85] and oxaliplatin- [2] induced neuropathic pain. The DNA oxidation product 8-OH-dG increased in animal sciatic nerve and spinal cord with oxaliplatin treatment [21]. In neuronal cell cultures, oxaliplatin increased cellular ROS and mitochondrial superoxide production [1, 3], lipid peroxidation [2] and nitrite [2] formation. Cisplatin also induced cellular and mitochondrial production of ROS/RNS in SH-5Y5Y neuroblastoma [18] and N2a cells [79]. Direct measurements of ROS/RNS are difficult to do in vivo; however, studies using indicator dyes have reported increased ROS/RNS in the DRG of animals with paclitaxel-induced neuropathic pain [24, 94]. Moreover, when nitro-oxidative stress was augmented in rats using auranofin, an inhibitor of the mitochondrial antioxidant thioredoxin (Trx)-thioredoxin reductase (TrxR) system, paclitaxel- and oxaliplatin-induced neuropathic pain in rats was exacerbated [101].
Mitochondria are a major source of superoxide production, which is primarily generated by electron leak at complex I and complex III of oxidative phosphorylation chain [92]. Platinum-based chemotherapeutics form DNA adducts in nuclear and mitochondrial DNA causing intrastrand nucleotide adducts that impact protein production [49, 51]. In the nucleus, these adducts can be efficiently repaired by the nuclear excision repair mechanisms [49]; whereas, little or no repair of these adducts occurs in mitochondria [49, 51]. This may lead to inadequate levels of oxidative phosphorylation proteins, which produce further superoxide [70]. Paclitaxel also causes disruptions in the mitochondrial ΔΨm through changes in mitochondrial structure, mitochondrial calcium flux and/or mitochondrial permeability transition pore [13]. However, once superoxide is formed in the mitochondria, it will undergo a dismutation reaction by MnSOD to hydrogen peroxide that then oxidizes reduced glutathione via glutathione peroxidases or degraded by catalase to generate water and oxygen [37, 40, 59]. Reductions in the levels of glutathione have been reported in the sciatic nerve of rats with vincristine-induced neuropathic pain [68]. Once superoxide exceeds these antioxidant mechanisms, it can cause lipid peroxidation, protein and DNA oxidation or undergo diffusion-limited reaction with nitric oxide to form peroxynitrite [9, 40, 70, 80]. Peroxynitrite will nitrate tyrosine-34 on MnSOD via a Mn-catalyzed process that reduces its activity by 80% and impairing antioxidant capacity [59, 60]. In CIPN models, we have found significant increases in tyrosine nitrated MnSOD in saphenous nerves of rats treated with paclitaxel, oxaliplatin or bortezomib [39]. Reductions in MnSOD have been reported by others in the sciatic nerve of paclitaxel-treated rats and N2a cells [2]. Such reductions in MnSOD activity and other mitochondrial antioxidant systems can lead to reinforcement of superoxide and peroxynitrite formation by further impairing ATP synthesis [80] or indirectly via protein kinase C activation and subsequent triggering of the cellular nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex, which will produce a burst of cellular superoxide [4, 14].
3.2. Targeting chemotherapy-induced mitochondrial nitro-oxidative stress.
Strategies that target nitro-oxidative stress have been successful in animal and cellular models in combating chemotherapy-induced neurotoxicity. In addition to their metabolic functions, ALCAR [97], α-lipoic acid [73], ascorbic acid [54] and α-tocopherol [54] can also act as antioxidants. Intravenous and intradermal injections of ALCAR, α-lipoic acid, or ascorbic acid dose-dependently reversed oxaliplatin-induced hyperalgesia [44]. Alpha-tocopherol and the plant-based polyphenolic flavonoid, silibinin, reduced oxaliplatin-induced lipid peroxidation and protein oxidation products in the plasma and sciatic nerves of rats and attenuated the formation of 8-OH-dG in the sciatic nerve [21]. Other studies used the mitochondrial-targeted alpha-tocopherol, MitoVitE, to attenuate paclitaxel-induced neuropathic pain [64]. Rosmarinic acid is a plant polyphenol that has can act as an antioxidant or stimulate PPARγ signaling [35]. PPARγ activation increases mitochondrial function, mitochondrial biogenesis and the transcription of a number of antioxidant enzymes through action with retinoid X receptor or by stimulating the antioxidant response transcription factor nuclear factor E2-related factor 2 (Nrf2) [48]. In N2a cells, oxaliplatin-induced lipid peroxidation and nitrite formation were reduced and antioxidant Nrf2 and MnSOD levels increased with rosmarinic acid [2]. Rosmirinic acid also improved mitochondrial function, reduced inflammation in the sciatic nerves and blocked the development of oxaliplatin-induced pain in animals [2]. However, there has been limited success thus far in human CIPN clinical trials using these approaches [83, 105].
More directed antioxidant pharmacological strategies using ROS/RNS scavengers, SOD mimetics and peroxynitrite decomposition catalysts have shown success attenuating chemotherapy-induced neurotoxic effects in cellular and animal models. For example, n-tert-Butyl-a-phenylnitrone (PBN), a global free-radical scavenger, and TEMPOL, a non-selective nitroxyl antioxidant [67] attenuated the development of and reverse established paclitaxel-induced neuropathic pain in rats [28]. In cisplatin-treated mice, PBN attenuated reductions in conductance velocity and increases in electro-stimulated action potential in the tibial nerve [87]. The active metabolite of amifostine, WR-1065, is a ROS/RNS scavenger [32] and activates MnSOD [66]. In neurons, WR1065 attenuated cisplatin-induced ROS, reduced neurite outgrowth and apoptosis. [79]. The SOD mimetic, MnL4, reduced superoxide production and lipid peroxidation in SH-SY5Y neuronal cells and attenuated decreased oxaliplatin-induced mechano-hyperalgesia and allodynia and cold allodynia in rats [22]. Mangafodipir, a manganese-based contrast dye, and its calcium-substituted derivative, calmangafodipir (PLEDOx), have SOD mimetic properties [12, 19]. In animal models of oxaliplatin-induced neuropathic pain, mangafodipir reduced the level of oxidized proteins in the serum in mice. Both compounds prevented peripheral nerve damage and attenuated the development of mechanical and cold hypersensitivity in mice [12, 19]. In Phase II trials with 22 cancer patients whose oxaliplatin-treatment was ceased due to the development of grade 2 or greater CIPN, 77% patients that resumed at least 4 cycles of oxaliplatin therapy with a cotreatment of mangafodipir showed improved pain outcomes [19]. When compared to non-responding patients, those with improved pain had reduced oxidized proteins products and increased SOD activity in their serum [19]. In Phase II clinical trials, calmangafodipir also appeared to provide favorable pain outcomes in patients with grade 2 or higher oxaliplatin-induced neuropathy [33] and is now currently in Phase III trials for CIPN (NCT03654729) [76]. In our own studies, we have found that using a compound with both SOD mimetic and peroxynitrite-decomposition catalytic activities prevented increased abnormal mitochondria in the saphenous nerves of paclitaxel-treated rats [39]. This compound also prevented MnSOD nitration and inactivation and protected ATP production in the saphenous nerves of rats treated with paclitaxel, oxaliplatin or bortezomib [39].
Other strategies attempt to address nitro-oxidative stress in CIPN by indirectly blocking the production of ROS/RNS. In DRG neurons treated with cisplatin, meclizine improved ATP production and neurite outgrowth by shifting the cells towards glycolysis and the pentose phosphate pathway to replenish depleted NADPH and antioxidant glutathione [34]. In mouse models of paclitaxel-induced neuropathic pain, matrix metalloproteases 9 (MMP9) levels increased in the DRG and intrathecal administration of a monoclonal antibody, MMP9 mAb, attenuated ROS/RNS production and stimulated Cu/Zn SOD in the DRG that resulting in reduced IENF loss and neuropathic pain [94]. The authors suggested that blocking MMP9 attenuated inflammation to reduce oxidative stress [94]. However, expression of matrix metalloprotease 9 is stimulated by oxidative stress and recent work shows that oxidative stress can drive MMP9 to translocate to mitochondria and act on substrates such as connexin-43, hsp 60 and 70 and drive mitochondrial dysfunction [42]. In other studies, the Szeto–Schiller (SS) peptides, SS-31 (Elamipretide) [95] and SS-20 [96] have been shown to attenuate pain and IENF loss in mice treated with oxaliplatin. SS peptides interact with cardiolipin [10, 98], which is a phospholipid of the inner mitochondrial membrane that maintains cristae structure and stabilizes cytochrome c for the OXPHOS [56]. Cardiolipin peroxidation disrupts the OXPHOS, increases ROS/RNS generation, mitochondrial swelling and the release of cytochrome c [10, 72, 98]. SS-31 is in early and late phase clinical trials for cardiological, opthalmological and neurological mitochondrial diseases (NCT02805790, NCT02976038, NCT02693119 and NCT03323749).
Other studies targeting the oxidative stress-sensitive PARP/p53 pathway have also reduced mitochondrial dysfunction and nerve injury. The selective serotonin and norepinephrine reuptake inhibitor, duloxetine, attenuated peroxidation and PARP/p53-dependent apoptosis in primary DRG rat neurons after paclitaxel [55]. The p53 inhibitor, pifithrin α, in cisplatin-treated animals prevented the accumulation of p53 in the mitochondria and preserved mitochondrial membrane potential, ATP production and normal morphology in the DRG [61]. This was associated with reductions in pain and loss of IENF [61].
4. Axonal mitochondrial transport and mitochondrial dysfunction.
Axonal transport of protein, messenger RNA and mitochondria from the cell soma to the axonal end terminal and nodes of Ranvier are critical to maintaining healthy mitochondrial pools and energy supply necessary for proper neurotransmission, axonal growth and synaptic function [62, 86]. Mitochondria will travel anterograde along axonal microtubules via kinesin motor complexes and anchor at a regions with high intracellular calcium concentrations detected by the Miro1 protein in the motor complex [62, 86]. To maintain anchored mitochondria function in the presence of increasing protein turnover, oxidative stress and accumulation of mitochondrial DNA errors, younger mitochondria traveling along the axon will fuse their outer and inner membranes via mitofusins and Opa1 proteins with older mitochondria, mix contents and sort regions of mitochondrial damage that then are removed by mitochondrial fission processes directed by Drp1 [62]. In damaged mitochondria fractions, the PTEN-induced kinase 1 (PINK1), which is usually translocated to and sequestered within the inner mitochondrial membrane, accumulates on the outer membrane to recruit the Parkin complex and ubiquitinate the damaged mitochondrial fraction for mitophagy [62, 86]. Disruption along any of these pathways leads to mitochondrial dysfunction and nitro-oxidative stress [62, 86].
There is growing evidence that dysregulated mitochondrial trafficking and fission/fusion may contribute to mitochondrial dysfunction and neuropathic pain during CIPN (Figures 1B,C). Smith et al., demonstrated that microtubule-stabilizing chemotherapeutics (paclitaxel and ixabepilone) reduced anterograde mitochondrial movement in human neuroblastoma cells and mouse sciatic nerves [91]. In mice with cisplatin-induced neuropathic pain, the levels of mitofusin-2 have been found to be reduced in both the DRG and tibial nerves; implicating reduced anterograde trafficking. Moreover, the levels of fission/fusion mRNA, Opa1 and Drp1, were reduced in the tibial nerve [11]. Inhibiting mitochondrial fission with a Drp1 inhibitor was found to attenuate oxaliplatin-induced neuropathic pain [27].
In addition to abnormal trafficking and fission/fusion, mitophagy is also altered by chemotherapeutics. For example, cisplatin activated PINK1/parkin mitophagy, but blocked its late stages in PC12 cells. Reducing parkin in these cells increased cisplatin toxicity in mitochondria and drove further depletion of ATP levels; whereas increasing parkin expression increased neurite outgrowth [106]. Similar beneficial effects were observed in the Drosophila CIPN model when PINK1 was overexpressed [46]. Restoration of NAD+ production by overexpressing nicotinamide nucleotide adenylyltransferase 1 (Nmnat1) restored the fission/fusion rates in DRG neurons treated with vincristine [8] . This prevented the slowing of mitochondrial velocity down the axon, mitochondrial fragmentation and neurodegeneration induced by vincristine [8] .
Histone deacetylase 6 (HDAC6) deacetylates α-tubulin to destabilize microtubules necessary for mitochondrial trafficking [63, 90]. The HDAC6 inhibitors, ACY-1083 [47] and ACY-1215 [58] have shown beneficial effects on mitochondrial function and CIPN in cisplatin-treated mice. ACY-1083 prevented and reversed the development of cisplatin-induced mechano-allodynia and IENF loss, while increasing mitochondrial mass and restoring mitochondrial bioenergetics in the tibial nerves [47]. Similar effects were observed with ACY-1215 [58]. This suggested that HDAC6 inhibition may have improved mitochondrial trafficking [47, 58]. However, HDAC6 has a number of mechanisms that could alter the development of CIPN [52]. When HDAC6 was specifically knocked out in the DRG had little or no effect on CIPN [58]. When ACY-1215 was tested in Rag2 knockout mice that are T cell depleted, ACY-1215 lost its effects, suggesting that the beneficial effects of these compounds may be on inflammatory processes.
5. Conclusions and future directions.
A substantial body of evidence has accumulated over the last 30 years to suggest a vital role of mitochondria in the development of CIPN. Mitochondrial dysfunction does occur in the sensory afferents and rectify their function improves the health, axonal growth and regulation of neurotransmission of sensory afferents to prevent and reduce peripheral and central sensitization that leads to chronic neuropathic pain. This has opened new research for novel therapeutic pharmacological approaches to treat CIPN for which there are currently limited options for clinicians and patients [26]. Moreover, several peripheral neuropathies of various etiologies (e.g., diabetes, human immunodeficiency virus and nucleoside reverse transcriptase inhibitors) share similar mitochondria defects with in the peripheral afferents [7]. Despite the rapid expansion in our knowledge of mitochondrial dysfunction and CIPN over the last decade, the limited success in clinical trials thus far for strategies that target mitochondrial dysfunction suggests that substantial gaps remain in our understanding of how chemotherapy triggers mitochondrial dysfunction and how that mitochondrial dysfunction drives CIPN. Questions as to what triggers mitochondrial dysfunction and whether these effects are due to chemotherapeutic agent on the mitochondria or due to pathological changes in the cell soma in the DRG are among the questions that still remain. Further understanding of how chemotherapeutics cause mitochondrial dysfunction and the role of mitochondrial dysfunction has on CIPN is necessary for the development of strategies to combat this devastating adverse side-effect of otherwise life-saving therapies.
Highlights.
Chemotherapies induce mitochondrial dysfunction in peripheral sensory nerves.
Mitochondrial dysfunction alters the bioenergetics and nitro-oxidative state.
These alterations reduce axonal outgrowth and abnormal sensory neuron activation.
As a result, a painful neuropathy emerges for which treatments are limited.
Strategies improving mitochondrial function may be useful in treating this pain.
Funding.
This review was supported by funds from the NIH/National Cancer Institute (NIH RO1 CA169519; D.S.).
Footnotes
Declaration of Competing Interest. The authors declare no conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References.
- [1].Areti A, Komirishetty P, Akuthota M, Malik RA, Kumar A, Melatonin prevents mitochondrial dysfunction and promotes neuroprotection by inducing autophagy during oxaliplatin-evoked peripheral neuropathy, J Pineal Res 62 (2017) n/a-N.PAG. [DOI] [PubMed] [Google Scholar]
- [2].Areti A, Komirishetty P, Kalvala AK, Nellaiappan K, Kumar A, Rosmarinic Acid Mitigates Mitochondrial Dysfunction and Spinal Glial Activation in Oxaliplatin-induced Peripheral Neuropathy, Mol Neurobiol 55 (2018) 7463–7475. [DOI] [PubMed] [Google Scholar]
- [3].Areti A, Komirishetty P, Kumar A, Carvedilol prevents functional deficits in peripheral nerve mitochondria of rats with oxaliplatin-evoked painful peripheral neuropathy, Toxicol Appl Pharmacol 322 (2017) 97–103. [DOI] [PubMed] [Google Scholar]
- [4].Balafanova Z, Bolli R, Zhang J, Zheng Y, Pass JM, Bhatnagar A, Tang XL, Wang O, Cardwell E, Ping P, Nitric oxide (NO) induces nitration of protein kinase Cepsilon (PKCepsilon ), facilitating PKCepsilon translocation via enhanced PKCepsilon -RACK2 interactions: a novel mechanism of no-triggered activation of PKCepsilon, The Journal of biological chemistry 277 (2002) 15021–15027. [DOI] [PubMed] [Google Scholar]
- [5].Barriere DA, Rieusset J, Chanteranne D, Busserolles J, Chauvin MA, Chapuis L, Salles J, Dubray C, Morio B, Paclitaxel therapy potentiates cold hyperalgesia in streptozotocin-induced diabetic rats through enhanced mitochondrial reactive oxygen species production and TRPA1 sensitization, Pain 153 (2012) 553–561. [DOI] [PubMed] [Google Scholar]
- [6].Below JM, Das J, Vincristine. StatPearls, Vol. 2020, StatPearls Publishing, Treasure Island, FL, 2020. [Google Scholar]
- [7].Bennett GJ, Doyle T, Salvemini D, Mitotoxicity in distal symmetrical sensory peripheral neuropathies, Nature reviews. Neurology 10 (2014) 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Berbusse GW, Woods LC, Vohra BP, Naylor K, Mitochondrial Dynamics Decrease Prior to Axon Degeneration Induced by Vincristine and are Partially Rescued by Overexpressed cytNmnat1, Front Cell Neurosci 10 (2016) 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O, Oxidative stress and antioxidant defense, World Allergy Organ J 5 (2012) 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Birk AV, Chao WM, Bracken C, Warren JD, Szeto HH, Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis, British journal of pharmacology 171 (2014) 2017–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bobylev I, Joshi AR, Barham M, Neiss WF, Lehmann HC, Depletion of Mitofusin-2 Causes Mitochondrial Damage in Cisplatin-Induced Neuropathy, Mol Neurobiol 55 (2018) 1227–1235. [DOI] [PubMed] [Google Scholar]
- [12].Canta A, Chiorazzi A, Pozzi E, Fumagalli G, Monza L, Meregalli C, Carozzi VA, Rodriguez-Menendez V, Oggioni N, Nasstrom J, Marmiroli P, Cavaletti G, Calmangafodipir Reduces Sensory Alterations and Prevents Intraepidermal Nerve Fibers Loss in a Mouse Model of Oxaliplatin Induced Peripheral Neurotoxicity, Antioxidants (Basel) 9 (2020) 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Canta A, Pozzi E, Carozzi VA, Mitochondrial Dysfunction in Chemotherapy-Induced Peripheral Neuropathy (CIPN), Toxics 3 (2015) 198–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chakraborti T, Das S, Chakraborti S, Proteolytic activation of protein kinase Calpha by peroxynitrite in stimulating cytosolic phospholipase A2 in pulmonary endothelium: involvement of a pertussis toxin sensitive protein, Biochemistry 44 (2005) 5246–5257. [DOI] [PubMed] [Google Scholar]
- [15].Chen XJ, Wang L, Song XY, Mitoquinone alleviates vincristine-induced neuropathic pain through inhibiting oxidative stress and apoptosis via the improvement of mitochondrial dysfunction, Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 125 (2020) 110003. [DOI] [PubMed] [Google Scholar]
- [16].Cheng A, Hou Y, Mattson MP, Mitochondria and neuroplasticity, ASN neuro 2 (2010) e00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chine VB, Au NPB, Ma CHE, Therapeutic benefits of maintaining mitochondrial integrity and calcium homeostasis by forced expression of Hsp27 in chemotherapy-induced peripheral neuropathy, Neurobiology of disease 130 (2019) 104492. [DOI] [PubMed] [Google Scholar]
- [18].Chiou CT, Wang KC, Yang YC, Huang CL, Yang SH, Kuo YH, Huang NK, Liu Jun Zi Tang-A Potential, Multi-Herbal Complementary Therapy for Chemotherapy-Induced Neurotoxicity, Int J Mol Sci 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Coriat R, Alexandre J, Nicco C, Quinquis L, Benoit E, Chereau C, Lemarechal H, Mir O, Borderie D, Treluyer JM, Weill B, Coste J, Goldwasser F, Batteux F, Treatment of oxaliplatin-induced peripheral neuropathy by intravenous mangafodipir, J Clin Invest 124 (2014) 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Devine MJ, Kittler JT, Mitochondria at the neuronal presynapse in health and disease, Nat Rev Neurosci 19 (2018) 63–80. [DOI] [PubMed] [Google Scholar]
- [21].Di Cesare Mannelli L, Zanardelli M, Failli P, Ghelardini C, Oxaliplatin-induced neuropathy: oxidative stress as pathological mechanism. Protective effect of silibinin, The journal of pain : official journal of the American Pain Society 13 (2012) 276–284. [DOI] [PubMed] [Google Scholar]
- [22].Di Cesare Mannelli L, Zanardelli M, Landini I, Pacini A, Ghelardini C, Mini E, Bencini A, Valtancoli B, Failli P, Effect of the SOD mimetic MnL4 on in vitro and in vivo oxaliplatin toxicity: Possible aid in chemotherapy induced neuropathy, Free radical biology & medicine 93 (2016) 67–76. [DOI] [PubMed] [Google Scholar]
- [23].Duggett NA, Griffiths LA, Flatters SJL, Paclitaxel-induced painful neuropathy is associated with changes in mitochondrial bioenergetics, glycolysis, and an energy deficit in dorsal root ganglia neurons, Pain 158 (2017) 1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Duggett NA, Griffiths LA, McKenna OE, de Santis V, Yongsanguanchai N, Mokori EB, Flatters SJ, Oxidative stress in the development, maintenance and resolution of paclitaxel-induced painful neuropathy, Neuroscience 333 (2016) 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ebenezer GJ, Carlson K, Donovan D, Cobham M, Chuang E, Moore A, Cigler T, Ward M, Lane ME, Ramnarain A, Vahdat LT, Polydefkis M, Ixabepilone-induced mitochondria and sensory axon loss in breast cancer patients, Ann Clin Transl Neurol 1 (2014) 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Farquhar-Smith P, Chemotherapy-induced neuropathic pain, Curr Opin Support Palliat Care 5 (2011) 1–7. [DOI] [PubMed] [Google Scholar]
- [27].Ferrari LF, Chum A, Bogen O, Reichling DB, Levine JD, Role of Drp1, a key mitochondrial fission protein, in neuropathic pain, The Journal of neuroscience : the official journal of the Society for Neuroscience 31 (2011) 11404–11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fidanboylu M, Griffiths LA, Flatters SJ, Global inhibition of reactive oxygen species (ROS) inhibits paclitaxel-induced painful peripheral neuropathy, PloS one 6 (2011) e25212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Flatters SJL, Bennett GJ, Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction, Pain 122 (2006) 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Flatters SJL, Dougherty PM, Colvin LA, Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): a narrative review, British journal of anaesthesia 119 (2017) 737–749. [DOI] [PubMed] [Google Scholar]
- [31].Garcia GC, Bartol TM, Phan S, Bushong EA, Perkins G, Sejnowski TJ, Ellisman MH, Skupin A, Mitochondrial morphology provides a mechanism for energy buffering at synapses, Sci Rep 9 (2019) 18306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Giannopoulou E, Papadimitriou E, Amifostine has antiangiogenic properties in vitro by changing the redox status of human endothelial cells, Free radical research 37 (2003) 1191–1199. [DOI] [PubMed] [Google Scholar]
- [33].Glimelius B, Manojlovic N, Pfeiffer P, Mosidze B, Kurteva G, Karlberg M, Mahalingam D, Buhl Jensen P, Kowalski J, Bengtson M, Nittve M, Nasstrom J, Persistent prevention of oxaliplatin-induced peripheral neuropathy using calmangafodipir (PledOx((R))): a placebo-controlled randomised phase II study (PLIANT), Acta Oncol 57 (2018) 393–402. [DOI] [PubMed] [Google Scholar]
- [34].Gorgun MF, Zhuo M, Englander EW, Cisplatin Toxicity in Dorsal Root Ganglion Neurons Is Relieved by Meclizine via Diminution of Mitochondrial Compromise and Improved Clearance of DNA Damage, Mol Neurobiol 54 (2017) 7883–7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Han J, Wang D, Ye L, Li P, Hao W, Chen X, Ma J, Wang B, Shang J, Li D, Zheng Q, Rosmarinic Acid Protects against Inflammation and Cardiomyocyte Apoptosis during Myocardial Ischemia/Reperfusion Injury by Activating Peroxisome Proliferator-Activated Receptor Gamma, Frontiers in pharmacology 8 (2017) 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Han Y, Smith MT, Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN), Frontiers in pharmacology 4 (2013) 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Holley AK, Bakthavatchalu V, Velez-Roman JM, St Clair DK, Manganese superoxide dismutase: guardian of the powerhouse, Int J Mol Sci 12 (2011) 7114–7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Horwitz SB, Taxol (paclitaxel): mechanisms of action, Ann Oncol 5 Suppl 6 (1994) S3–6. [PubMed] [Google Scholar]
- [39].Janes K, Doyle T, Bryant L, Esposito E, Cuzzocrea S, Ryerse J, Bennett GJ, Salvemini D, Bioenergetic deficits in peripheral nerve sensory axons during chemotherapy-induced neuropathic pain resulting from peroxynitrite-mediated post-translational nitration of mitochondrial superoxide dismutase, Pain 154 (2013) 2432–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Janes K, Neumann WL, Salvemini D, Anti-superoxide and anti-peroxynitrite strategies in pain suppression, Biochim Biophys Acta 1822 (2012) 815–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jin HW, Flatters SJ, Xiao WH, Mulhern HL, Bennett GJ, Prevention of paclitaxel-evoked painful peripheral neuropathy by acetyl-L-carnitine: effects on axonal mitochondria, sensory nerve fiber terminal arbors, and cutaneous Langerhans cells, Exp Neurol 210 (2008) 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jobin PG, Butler GS, Overall CM, New intracellular activities of matrix metalloproteinases shine in the moonlight, Biochim Biophys Acta Mol Cell Res 1864 (2017) 2043–2055. [DOI] [PubMed] [Google Scholar]
- [43].Johnstone TC, Park GY, Lippard SJ, Understanding and improving platinum anticancer drugs--phenanthriplatin, Anticancer Res 34 (2014) 471–476. [PMC free article] [PubMed] [Google Scholar]
- [44].Joseph EK, Chen X, Bogen O, Levine JD, Oxaliplatin acts on IB4-positive nociceptors to induce an oxidative stress-dependent acute painful peripheral neuropathy, The journal of pain : official journal of the American Pain Society 9 (2008) 463–472. [DOI] [PubMed] [Google Scholar]
- [45].Kann O, Kovacs R, Mitochondria and neuronal activity, American journal of physiology. Cell physiology 292 (2007) C641–657. [DOI] [PubMed] [Google Scholar]
- [46].Kim YY, Yoon JH, Um JH, Jeong DJ, Shin DJ, Hong YB, Kim JK, Kim DH, Kim C, Chung CG, Lee SB, Koh H, Yun J, PINK1 alleviates thermal hypersensitivity in a paclitaxel-induced Drosophila model of peripheral neuropathy, PloS one 15 (2020) e0239126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Krukowski K, Ma J, Golonzhka O, Laumet GO, Gutti T, van Duzer JH, Mazitschek R, Jarpe MB, Heijnen CJ, Kavelaars A, HDAC6 inhibition effectively reverses chemotherapy-induced peripheral neuropathy, Pain 158 (2017) 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kvandova M, Majzunova M, Dovinova I, The role of PPARgamma in cardiovascular diseases, Physiological research 65 (2016) S343–S363. [DOI] [PubMed] [Google Scholar]
- [49].Larsen NB, Rasmussen M, Rasmussen LJ, Nuclear and mitochondrial DNA repair: similar pathways?, Mitochondrion 5 (2005) 89–108. [DOI] [PubMed] [Google Scholar]
- [50].Latremoliere A, Woolf CJ, Central sensitization: a generator of pain hypersensitivity by central neural plasticity, The journal of pain : official journal of the American Pain Society 10 (2009) 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].LeDoux SP, Wilson GL, Beecham EJ, Stevnsner T, Wassermann K, Bohr VA, Repair of mitochondrial DNA after various types of DNA damage in Chinese hamster ovary cells, Carcinogenesis 13 (1992) 1967–1973. [DOI] [PubMed] [Google Scholar]
- [52].Li Y, Shin D, Kwon SH, Histone deacetylase 6 plays a role as a distinct regulator of diverse cellular processes, The FEBS journal 280 (2013) 775–793. [DOI] [PubMed] [Google Scholar]
- [53].LoCoco PM, Risinger AL, Smith HR, Chavera TS, Berg KA, Clarke WP, Pharmacological augmentation of nicotinamide phosphoribosyltransferase (NAMPT) protects against paclitaxel-induced peripheral neuropathy, Elife 6 (2017) e29626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lu JM, Lin PH, Yao Q, Chen C, Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems, J Cell Mol Med 14 (2010) 840–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lu Y, Zhang P, Zhang Q, Yang C, Qian Y, Suo J, Tao X, Zhu J, Duloxetine Attenuates Paclitaxel-Induced Peripheral Nerve Injury by Inhibiting p53-Related Pathways, J Pharmacol Exp Ther 373 (2020) 453–462. [DOI] [PubMed] [Google Scholar]
- [56].Lu YW, Claypool SM, Disorders of phospholipid metabolism: an emerging class of mitochondrial disease due to defects in nuclear genes, Front Genet 6 (2015) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ludman T, Melemedjian OK, Bortezomib-induced aerobic glycolysis contributes to chemotherapy-induced painful peripheral neuropathy, Molecular pain 15 (2019) 1744806919837429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ma J, Trinh RT, Mahant ID, Peng B, Matthias P, Heijnen CJ, Kavelaars A, Cell-specific role of histone deacetylase 6 in chemotherapy-induced mechanical allodynia and loss of intraepidermal nerve fibers, Pain 160 (2019) 2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Macmillan-Crow LA, Cruthirds DL, Invited review: manganese superoxide dismutase in disease, Free radical research 34 (2001) 325–336. [DOI] [PubMed] [Google Scholar]
- [60].MacMillan-Crow LA, Thompson JA, Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite, Archives of biochemistry and biophysics 366 (1999) 82–88. [DOI] [PubMed] [Google Scholar]
- [61].Maj MA, Ma J, Krukowski KN, Kavelaars A, Heijnen CJ, Inhibition of Mitochondrial p53 Accumulation by PFT-mu Prevents Cisplatin-Induced Peripheral Neuropathy, Frontiers in molecular neuroscience 10 (2017) 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Mandal A, Drerup CM, Axonal Transport and Mitochondrial Function in Neurons, Front Cell Neurosci 13 (2019) 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, SeigneurinBerny D, Osada H, Komatsu Y, Nishino N, Khochbin S, Horinouchi S, Yoshida M, In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation, The EMBO journal 21 (2002) 6820–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].McCormick B, Lowes DA, Colvin L, Torsney C, Galley HF, MitoVitE, a mitochondria-targeted antioxidant, limits paclitaxel-induced oxidative stress and mitochondrial damage in vitro, and paclitaxel-induced mechanical hypersensitivity in a rat pain model, British journal of anaesthesia 117 (2016) 659–666. [DOI] [PubMed] [Google Scholar]
- [65].Mujtaba T, Dou QP, Advances in the understanding of mechanisms and therapeutic use of bortezomib, Discov Med 12 (2011) 471–480. [PMC free article] [PubMed] [Google Scholar]
- [66].Murley JS, Kataoka Y, Miller RC, Li JJ, Woloschak G, Grdina DJ, SOD2-mediated effects induced by WR1065 and low-dose ionizing radiation on micronucleus formation in RKO human colon carcinoma cells, Radiat Res 175 (2011) 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang ZQ, Salvemini D, On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies, British journal of pharmacology 140 (2003) 445–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Muthuraman A, Jaggi AS, Singh N, Singh D, Ameliorative effects of amiloride and pralidoxime in chronic constriction injury and vincristine induced painful neuropathy in rats, European journal of pharmacology 587 (2008) 104–111. [DOI] [PubMed] [Google Scholar]
- [69].Nasu S, Misawa S, Nakaseko C, Shibuya K, Isose S, Sekiguchi Y, Mitsuma S, Ohmori S, Iwai Y, Beppu M, Shimizu N, Ohwada C, Takeda Y, Fujimaki Y, Kuwabara S, Bortezomib-induced neuropathy: axonal membrane depolarization precedes development of neuropathy, Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 125 (2014) 381–387. [DOI] [PubMed] [Google Scholar]
- [70].Nayak AP, Kapur A, Barroilhet L, Patankar MS, Oxidative Phosphorylation: A Target for Novel Therapeutic Strategies Against Ovarian Cancer, Cancers (Basel) 10 (2018) 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Nieto FR, Cendan CM, Canizares FJ, Cubero MA, Vela JM, Fernandez-Segura E, Baeyens JM, Genetic inactivation and pharmacological blockade of sigma-1 receptors prevent paclitaxel-induced sensory-nerve mitochondrial abnormalities and neuropathic pain in mice, Molecular pain 10 (2014) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ott M, Zhivotovsky B, Orrenius S, Role of cardiolipin in cytochrome c release from mitochondria, Cell death and differentiation 14 (2007) 1243–1247. [DOI] [PubMed] [Google Scholar]
- [73].Packer L, Witt EH, Tritschler HJ, Alpha-Lipoic Acid as a Biological Antioxidant, Free Radical Bio Med 19 (1995) 227–250. [DOI] [PubMed] [Google Scholar]
- [74].Park JY, Jang SY, Shin YK, Koh H, Suh DJ, Shinji T, Araki T, Park HT, Mitochondrial swelling and microtubule depolymerization are associated with energy depletion in axon degeneration, Neuroscience 238 (2013) 258–269. [DOI] [PubMed] [Google Scholar]
- [75].Pienta KJ, Preclinical mechanisms of action of docetaxel and docetaxel combinations in prostate cancer, Seminars in oncology 28 (2001) 3–7. [DOI] [PubMed] [Google Scholar]
- [76].PledPharma, Preventive Treatment of Oxaliplatin Induced Peripheral Neuropathy in Metastatic Colorectal Cancer (POLAR-M) (POLAR-M) (NCT03654729). In: U.S.N.L.o. Medicine; (Ed.). [Google Scholar]
- [77].Podratz JL, Knight AM, Ta LE, Staff NP, Gass JM, Genelin K, Schlattau A, Lathroum L, Windebank AJ, Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons, Neurobiology of disease 41 (2011) 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Podratz JL, Lee H, Knorr P, Koehler S, Forsythe S, Lambrecht K, Arias S, Schmidt K, Steinhoff G, Yudintsev G, Yang A, Trushina E, Windebank A, Cisplatin induces mitochondrial deficits in Drosophila larval segmental nerve, Neurobiology of disease 97 (2017) 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Popovic J, Klajn A, Paunesku T, Ma Q, Chen S, Lai B, Stevanovic M, Woloschak GE, Neuroprotective Role of Selected Antioxidant Agents in Preventing Cisplatin-Induced Damage of Human Neurons In Vitro, Cellular and molecular neurobiology 39 (2019) 619–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Radi R, Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine, Proceedings of the National Academy of Sciences of the United States of America 115 (2018) 5839–5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Rangaraju V, Calloway N, Ryan TA, Activity-driven local ATP synthesis is required for synaptic function, Cell 156 (2014) 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Scafidi S, Fiskum G, Lindauer SL, Bamford P, Shi D, Hopkins I, McKenna MC, Metabolism of acetyl-L-carnitine for energy and neurotransmitter synthesis in the immature rat brain, J Neurochem 114 (2010) 820–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Schloss JM, Colosimo M, Airey C, Masci PP, Linnane AW, Vitetta L, Nutraceuticals and chemotherapy induced peripheral neuropathy (CIPN): a systematic review, Clin Nutr 32 (2013) 888–893. [DOI] [PubMed] [Google Scholar]
- [84].Seager R, Lee L, Henley JM, Wilkinson KA, Mechanisms and roles of mitochondrial localisation and dynamics in neuronal function, Neuronal Signal 4 (2020) NS20200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Sharawy N, Rashed L, Youakim MF, Evaluation of multi-neuroprotective effects of erythropoietin using cisplatin induced peripheral neurotoxicity model, Exp Toxicol Pathol 67 (2015) 315–322. [DOI] [PubMed] [Google Scholar]
- [86].Sheng ZH, The Interplay of Axonal Energy Homeostasis and Mitochondrial Trafficking and Anchoring, Trends in cell biology 27 (2017) 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Shim HS, Bae C, Wang J, Lee KH, Hankerd KM, Kim HK, Chung JM, La JH, Peripheral and central oxidative stress in chemotherapy-induced neuropathic pain, Molecular pain 15 (2019) 1744806919840098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Siau C, Xiao W, Bennett GJ, Paclitaxel- and vincristine-evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells, Exp Neurol 201 (2006) 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Singhmar P, Huo X, Li Y, Dougherty PM, Mei F, Cheng X, Heijnen CJ, Kavelaars A, Orally active Epac inhibitor reverses mechanical allodynia and loss of intraepidermal nerve fibers in a mouse model of chemotherapy-induced peripheral neuropathy, Pain 159 (2018) 884–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Skultetyova L, Ustinova K, Kutil Z, Novakova Z, Pavlicek J, Mikesova J, Trapl D, Baranova P, Havlinova B, Hubalek M, Lansky Z, Barinka C, Human histone deacetylase 6 shows strong preference for tubulin dimers over assembled microtubules, Sci Rep 7 (2017) 11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Smith JA, Slusher BS, Wozniak KM, Farah MH, Smiyun G, Wilson L, Feinstein S, Jordan MA, Structural Basis for Induction of Peripheral Neuropathy by Microtubule-Targeting Cancer Drugs, Cancer research 76 (2016) 5115–5123. [DOI] [PubMed] [Google Scholar]
- [92].St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD, Topology of superoxide production from different sites in the mitochondrial electron transport chain, The Journal of biological chemistry 277 (2002) 44784–44790. [DOI] [PubMed] [Google Scholar]
- [93].Staff NP, Grisold A, Grisold W, Windebank AJ, Chemotherapy-induced peripheral neuropathy: A current review, Annals of neurology 81 (2017) 772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Tonello R, Lee SH, Berta T, Monoclonal Antibody Targeting the Matrix Metalloproteinase 9 Prevents and Reverses Paclitaxel-Induced Peripheral Neuropathy in Mice, The journal of pain : official journal of the American Pain Society 20 (2019) 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Toyama S, Shimoyama N, Ishida Y, Koyasu T, Szeto HH, Shimoyama M, Characterization of acute and chronic neuropathies induced by oxaliplatin in mice and differential effects of a novel mitochondria-targeted antioxidant on the neuropathies, Anesthesiology 120 (2014) 459–473. [DOI] [PubMed] [Google Scholar]
- [96].Toyama S, Shimoyama N, Szeto HH, Schiller PW, Shimoyama M, Protective Effect of a Mitochondria-Targeted Peptide against the Development of Chemotherapy-Induced Peripheral Neuropathy in Mice, ACS chemical neuroscience 9 (2018) 1566–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wang S, Xu J, Zheng J, Zhang X, Shao J, Zhao L, Hao J, Anti-Inflammatory and Antioxidant Effects of Acetyl-L-Carnitine on Atherosclerotic Rats, Med Sci Monit 26 (2020) e920250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Wu J, Zhang M, Hao S, Jia M, Ji M, Qiu L, Sun X, Yang J, Li K, Mitochondria-Targeted Peptide Reverses Mitochondrial Dysfunction and Cognitive Deficits in Sepsis-Associated Encephalopathy, Mol Neurobiol 52 (2015) 783–791. [DOI] [PubMed] [Google Scholar]
- [99].Wu Y, Li J, Zhou J, Feng Y, Dynamic long-term microstructural and ultrastructural alterations in sensory nerves of rats of paclitaxel-induced neuropathic pain, Chin Med J (Engl) 127 (2014) 2945–2952. [PubMed] [Google Scholar]
- [100].Xiao WH, Bennett GJ, Chemotherapy-evoked neuropathic pain: Abnormal spontaneous discharge in A-fiber and C-fiber primary afferent neurons and its suppression by acetyl-L-carnitine, Pain 135 (2008) 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Xiao WH, Bennett GJ, Effects of mitochondrial poisons on the neuropathic pain produced by the chemotherapeutic agents, paclitaxel and oxaliplatin, Pain 153 (2012) 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Xiao WH, Zheng H, Bennett GJ, Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel, Neuroscience 203 (2012) 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Xiao WH, Zheng H, Zheng FY, Nuydens R, Meert TF, Bennett GJ, Mitochondrial abnormality in sensory, but not motor, axons in paclitaxel-evoked painful peripheral neuropathy in the rat, Neuroscience 199 (2011) 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Xu J, Wang W, Zhong XX, Feng Y, Wei X, Liu XG, EXPRESS: Methylcobalamin ameliorates neuropathic pain induced by vincristine in rats: Effect on loss of peripheral nerve fibers and imbalance of cytokines in the spinal dorsal horn, Molecular pain 12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Zaki SM, Mohamed EA, Motawie AG, Abdel Fattah S, N-acetylcysteine versus progesterone on the cisplatin-induced peripheral neurotoxicity, Folia morphologica 77 (2018) 234–245. [DOI] [PubMed] [Google Scholar]
- [106].Zhang Y, Liu Q, Li Y, Li C, Zhu Y, Xia F, Xu S, Li W, PTEN-Induced Putative Kinase 1 (PINK1)/Parkin-Mediated Mitophagy Protects PC12 Cells Against Cisplatin-Induced Neurotoxicity, Med Sci Monit 25 (2019) 8797–8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Zheng H, Xiao WH, Bennett GJ, Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy, Exp Neurol 232 (2011) 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Zheng H, Xiao WH, Bennett GJ, Mitotoxicity and bortezomib-induced chronic painful peripheral neuropathy, Exp Neurol 238 (2012) 225–234. [DOI] [PubMed] [Google Scholar]
- [109].Zorova LD, Popkov VA, Plotnikov EY, Silachev DN, Pevzner IB, Jankauskas SS, Babenko VA, Zorov SD, Balakireva AV, Juhaszova M, Sollott SJ, Zorov DB, Mitochondrial membrane potential, Anal Biochem 552 (2018) 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]