Abstract
The reproductive span (RS) of organisms could be affected by different factors during their lifetime. In the model nematode, Caenorhabditis elegans, RS is affected by both genetic and environmental factors. However, none of the factors identified so far were related to environmental bacteria, which may incidentally appear anywhere in the habitats of C. elegans. We aimed to find environmental bacteria that could affect the RS of C. elegans and related species. We tested 109 bacterial isolates and found that Microbacterium sp. CFBb37 increased the RS and lifespan of C. elegans but reduced its brood size. We studied the effect of M. sp. CFBb37 on the RS of Caenorhabditis briggsae, Caenorhabditis tropicalis, and another Rhabditidae family species, Protorhabditis sp., and found similar trends of RS extension in all three cases, suggesting that this bacterial species may induce the extension of RS broadly among Caenorhabditis species and possibly for many other Rhabditidae. This work will facilitate future research on the mechanism underlying the bacterial extension of RS of nematodes and possibly other animals.
Keywords: bacteria, brood size, Caenorhabditis nematodes, lifespan, Microbacterium sp., physiology, Protorhabditis sp., reproductive span
Caenorhabditis elegans is a free-living nematode species that feeds primarily on bacteria (Darby, 2005; Samuel et al., 2016; Schulenburg and Felix, 2017). In the laboratory C. elegans is commonly fed Escherichia coli OP50, but this is not its natural food (Stiernagle, 2006). Previous studies of the habitats where C. elegans are found, such as soil, and rotting fruits and leaves, have found a diverse set of associated microorganisms, including fungi and bacteria (Dirksen et al., 2016; Zhang et al., 2017a; Dirksen et al., 2020; Stuhr and Curran, 2020; Zimmermann et al., 2020). Among these, the dominant taxa are Proteobacteria, Bacteroidetes, Firmicutes, and Actinobacteria (Samuel et al., 2016; Zecic et al., 2019). Although some of the associated microbes are detrimental to C. elegans, such as through the production of toxins (Gravato-Nobre and Hodgkin, 2005; Pukkila-Worley and Ausubel, 2012; Kumar et al., 2020), many of the bacterial species can serve as food.
Food quality and composition can affect animal physiology. Several studies have described the influence of bacterial diets, both of different E. coli strains and other species, on the growth and development rates, lifespan, and reproduction of C. elegans (Darby, 2005; Gracida and Eckmann, 2013; MacNeil et al., 2013; Frezal and Felix, 2015; Samuel et al., 2016; Zhang et al., 2017b; Kumar et al., 2020; Stuhr and Curran, 2020). Lifespan effects can be through nutritional differences among the different bacteria or the products of bacterial metabolism such as folate metabolism or nitric oxide production (Clark and Hodgkin, 2014; Kumar et al., 2020). With respect to reproduction, worms fed with Comamonas DA1877, Methylobacterium, or Sphingomonas bacteria had smaller brood sizes relative to OP50, while those fed with Xanthomonas bacteria or various other E. coli strains had comparable brood sizes (MacNeil et al., 2013; Stuhr and Curran, 2020). There is also evidence that bacterial noncoding RNAs can affect C. elegans physiology and behavior (Liu et al., 2012; Kaletsky et al., 2020).
One area that has little been studied is the effect of bacterial diets on the reproductive span (RS) of C. elegans. RS is defined as the duration in which a living organism has reproductive ability and is closely related to the concept of reproductive aging (Luo et al., 2009; Templeman and Murphy, 2018). In C. elegans, a recent study observed reduced RS when worms were fed with Methylobacterium or E. coli HB101 (Stuhr and Curran, 2020), while another study found extended RS when fed with Staphylococcus epidermidis (Madhu et al., 2019). In these studies, E. coli is a gut bacteria, Methylobacterium was isolated as a lab contaminant (Stuhr and Curran, 2020), and S. epidermidis is typically part of the human skin flora, so these bacteria are unlikely natural food for C. elegans. Thus, an open question is whether bacterial species that are natural food for C. elegans could affect RS. Decreases in RS may simply reflect deleterious interactions (e.g., toxins), however, a particularly interesting and less easily explained effect would be an increase in RS. This latter scenario may imply that some microbe–nematode interactions go beyond predator–prey, hinting at greater inter-taxa ecological complexity.
At the genetic level, RS is regulated by two signaling pathways. The first is the insulin/IGF-signaling (IIS) pathway. Mutations in daf-2 (which encodes the IIS receptor tyrosine kinase) can extend RS (Hughes et al., 2007). Second, mutations of genes within the Transforming Growth Factor-β (TGF-β) Sma/Mab signaling pathway also extend RS, at least in part through the prolonged production of healthy oocytes (Luo et al., 2009; Templeman and Murphy, 2018). It is unknown whether bacteria would affect RS through these or other genetic pathways.
In this study, we wanted to determine whether any environmental bacteria could extend the RS of C. elegans and, possibly, related species. Therefore, we first isolated 109 natural strains of environmental bacteria and tested their influence on the reproduction of C. elegans. We found that one bacterial strain, Microbacterium sp. CFBb37, strongly extended both lifespan and RS, but reduced the offspring number, of C. elegans. Microbacterium sp. CFBb37 also extended RS in three other Rhabditidae species (two other Caenorhabditis species and a Protorhabditis sp.), suggesting that the extension of RS by this strain may be general across Rhabditidae. As this was a modest screen, our results suggest that more environmental bacterial species may be found that can extend RS. Additionally, our identification of Microbacterium sp. CFBb37 provides a potential entry point to study the molecular-genetic mechanisms for RS extension in both the bacteria and C. elegans.
Materials and Methods
Worm strains and maintenance
C. elegans Bristol var N2; C. tropicalis (BRC20400) (Le et al., 2021); C. briggsae CFB233 (a new wild isolate); and Protorhabditis sp. CFB231 (a new wild isolate). Information on the new wild isolates is given in Table S1. Worms were cultured in a 19 °C ± 1 °C incubator, unless otherwise noted. Worm strains were grown on Nematode Growth Media (NGM) plates seeded with either the E. coli OP50 control or test bacteria. Bacteria were cultured in a modified Luria-Bertani (LB) Broth at room temperature overnight prior to seeding the plates.
Chemicals and media
NaCl (Bio Basic Canada Inc., 7647-14-5), peptone (TM MEDIA, 1506), agar (TM MEDIA, 242M), nutrient agar (TM MEDIA, TM341), yeast extract (Bio Basic Canada Inc., G0961), cholesterol powder (Across Organics, 110190250), CaCl2 (Fisher, 10043-52-4), MgSO4 (Fisher, 10034-99-8), KH2PO4 (Merck, 7778-77-0), and K2HPO4 (Fisher, 7758-11-4). Modified LB media: 5 g of yeast extract, 1 g of nutrient agar, and 10 g of NaCl in 1 L of distilled water (Sambrook and Russell, 2001). NGM was prepared following the standard protocol (Stiernagle, 2006).
Isolation of wild nematodes and environmental bacteria
C. briggsae CFB233 and Protorhabditis sp. CFB231 were isolated as previously described (Le et al., 2021) as part of a larger survey from Cat Tien National Park in northern Vietnam. In short, rotting vegetation was plated onto OP50-seeded NGM petri plates and gravid adult nematodes that resembled C. elegans were isolated. For each strain, the sexual system (e.g., male-female or self-fertile hermaphrodites) was determined by singling out several L4 female individuals onto OP50-seeded NGM plates and then checking for self-progeny. PCR and sequencing of a fragment of the 18S rDNA using the primers SSU18A and SSU26R (Barriere and Felix, 2006) were used to assign the species (or genus) of the strain.
Environmental bacteria were isolated as previously described (Le et al., 2021) from Cat Tien and Cuc Phuong (southern Vietnam) National Parks in November 2019. In brief, rotting vegetation was diluted 10,000-fold in sterile water and then a 100–200 μl aliquot was plated onto an LB-agar plate. Bacteria colonies were scored for four qualitative plate phenotypes: size (big, medium, or small), color (transparent, red, or strong yellow), thickness (thin or thick), and prevalence (majority or minority). If a plate qualitatively contained fairly uniform phenotypes, possibly represented by one species, then two random colonies were picked. However, if a plate showed diverse bacterial phenotypes, then one colony of each putative type was picked (which was usually three to four). Twelve bacterial strains were identified to the species (or genus) level based on comparing the PCR amplified 16S rDNA sequence (primers QUGP-Fn5 and QUGP-Rn2, Vingataramin and Frost, 2015) to the NCBI nr database by BLAST (Zhang et al., 2000; Morgulis et al., 2008).
Characterization of the reproductive system of Protorhabditis sp. CFB231
Reproductive organ morphology was examined using a microscope (ZEISS, VMI 0560) equipped with differential interference contrast (DIC) optics. 4′,6-diamidino-2-phenylindole (DAPI) was used to stain nuclei, especially those of potential sperm, as follows. Young adults (N = 15) were placed into 20 μl of 100% methanol (in a 1.5-ml Eppendorf tube) and then incubated at 4 °C for 30 min. Next, filter paper was touched to the methanol liquid surface for ∼5 s to wick away most of the methanol. Then, 15 μl of 200 ng/ml DAPI was added into the tube and the worms were incubated at room temperature (approximately 25 °C) for 30 min. Filter paper was used to wick away most of the DAPI solution, and then a drop of water was added into the tube. DAPI stained worms were observed at 63× magnification with an inverted microscope (ZEISS, VMI 0560) equipped with a camera (Axiocam 105 color) and a fluorescent light source.
Testing the effect of different bacteria on RS
Three experiments were conducted to test the effect of bacteria on RS. The first experiment was a screen to identify candidate bacteria that affected the RS of C. elegans. To do this, gravid worms grown on OP50-seeded plates were bleached to release eggs (Stiernagle, 2006), which were then hatched on bacteria-free NGM plates overnight. After, the fresh L1 stage worms (P0) were washed off each plate with distilled water and approximately 10–15 worms were placed onto control OP50-seeded and test bacteria-seeded NGM plates. Each plate was then observed daily. We recorded the first and last day when we observed eggs on the plate, which was usually starting on day 3 and ending sometime between days 7 and 10. RS was then the number of days with eggs being laid. Because this was a screen, the P0 were not transferred to new plates and the effect of bacteria on RS were qualitatively judged as “similar to” or “different from” the OP50 control. All 109 bacterial strains and the OP50 control were tested in one big batch. Bacteria candidates that potentially extended RS (N = 16 strains) were screened a second time in an identical fashion.
In the second experiment the effect of bacteria strain on RS was determined more precisely. Here, only four bacteria strains were tested: Microbacterium sp. CFBb37 (screen suggested extended RS); CFBb9 and CFBb17 (both no obvious effect on RS) and the control OP50. In this experiment, RS was determined for second-generation C. elegans exposed to a test bacteria species. Parents (P0) were grown on the test bacteria species (since the egg or L1 stage), possibly providing some sort of acclimatization. Adult P0 (N = 10) worms were then transferred to a fresh test bacteria-seeded (or control OP50-seeded) plate, and allowed to lay eggs (F1) at room temperature. After 1–3 h, all P0 worms were removed and then the 6–15 F1s were cultured as a pool on the same plate at 19 ± 1 °C. The numbers of replicate pools for each bacterial strain were CFBb9 (N = 4), CFBb17 (N = 4, CFBb37 (N = 3), and OP50 (N = 5). After reaching adulthood, F1's were transferred daily to new plates. RS for each pool was then the number of days (plates) with eggs (F2's); RS for each bacterial strain was the average days across the replicate pools. The number of F2 progeny was also counted (see brood size assay below).
The third experiment differed from the second experiment in three ways. First, only one F1 was placed on each plate to eliminate any potential confounding effects from having pools of F1s per plate. Additionally, only Microbacterium sp. CFBb37 (N = 40 replicates) and OP50 (N = 41) were tested. Finally, RS was determined differently. A day was considered to be positive for brood if a one-sample t-test of brood number was significantly greater than zero (i.e., P < 0.05).
Brood size assays
Brood sizes were determined as previously described (Le et al., 2021) with minor modifications. In brief, eggs on each plate were allowed to hatch and develop until the L2–L4 stages. To facilitate counting, the F2 offspring were sometimes killed with mild heat, which preserved the worm's shape, by passing the plates briefly over the flame of an alcohol lamp. Depending on the experiment, total or daily brood sizes are reported. Brood sizes of test bacteria were compared with that of OP50 using the paired t-test.
Lifespan assays
Lifespan assays were conducted as previously described (Le et al., 2021). In brief, gravid P0 worms acclimated to test or control OP50 bacteria (since the egg or L1 stage) were transferred to a fresh bacteria-seeded NGM plate and allowed to lay eggs (F1) for 2 h before being removed from the plate. The next day, 20 F1 L1 or L2 worms were transferred to a new test or control OP50 bacteria-seeded plate. The F1 were transferred to new plates every 1 or 2 days until they died. A worm was scored as dead if it did not move when tapped with a worm pick. Lifespan was analyzed using the log-rank (Mantel–Cox) test (Kassambara, 2020).
Assaying the presence of eggs in the uterus
This assay was designed to simplify determining RS by looking for eggs directly in the uteri of the focal adult worms. In other words, it did not require looking for eggs on a plate (i.e., RS experiment one, above), which may be rare near the end of the RS period, or moving the parent worm each day and then waiting for progeny to hatch and develop. To do this, a generation of worms (P0) was first acclimated to the test bacteria or OP50. P0 eggs were obtained by sodium hypochlorite bleaching gravid adults (Stiernagle, 2006); the eggs were hatched into L1 larvae on unseeded NGM plates, and then 90–100 L1 were transferred to culture on test (or control OP50) bacteria-seeded NGM plates. Gravid P0 adults were bleached for F1 eggs, which were hatched on the test bacteria-seeded NGM plate overnight at 20 °C. The next day, 50 L1 were transferred onto a new test or OP50 bacteria-seeded NGM plate. Every day after reaching adulthood, groups of 10–20 F1 adults were placed on a glass slide containing one drop of water and then overlain with a glass coverslip (Shaham, 2006). The number of eggs within each adult (i.e., from both uteruses) was counted. Comparison of the egg counts between the test bacteria and OP50 were analyzed using a paired t-test. To determine RS on the pooled adults, a day was considered to be positive for eggs if a one-sample t-test of egg number was significantly greater than zero (i.e., P < 0.05). Due to insufficient F1 adults, the full RS was not determined for C. briggsae, C. tropicalis, and Protorhabditis sp. on CFBb37.
General statistics
All statistical analyses were done using R software (R Core Team, 2017).
Results
Isolation and screening of bacteria affecting RS of C. elegans
We hypothesized that the different bacteria encountered and eaten by C. elegans in the wild could affect its physiology, and in particular RS. To this end, we first isolated bacteria from rotting vegetation, substrates from which nematodes would often be found, from two national parks in Vietnam. We isolated 64 bacterial strains from 50 sampling sites in Cat Tien National Park and 45 strains from 44 sites in Cuc Phuong National Park (Tables S2 and S3). These 109 strains were qualitatively characterized based on colony size, color, thickness, and prevalence on the original screening plate (Table S3).
Next, we used a simple 1-plate assay (see “Methods”) to screen all 109 isolates for an effect on RS of C. elegans in comparison to the E. coli OP50 control. The RS of C. elegans on OP50 was about 3–4 days. Qualitatively, 108 strains also had similar RS in the 3- to 4-day range. One strain, CFBb37, seemed to extend RS to approximately 7 days. We note that we found a nematode, Caenorhabditis briggsae, co-occurring in the field sample containing CFBb37 (Table S2). We also noticed that this and six other bacterial strains reduced the body size and another delayed the onset of first brood production in C. elegans (Fig. S1 and Table S2). To determine their genus or species identity we sequenced the 16S rDNA barcode for these eight bacterial strains as well as four others without obvious effects on C. elegans (Table S2). This revealed that CFBb37 is a Microbacterium sp., which is in the Actinobacteria phylum. Most of the other strains belonged to the phylum Proteobacteria (n = 8) while the rest were in the phyla Bacteroidetes (n = 1) or Firmicutes (n = 2). We focused on Microbacterium sp. CFBb37 for the rest of this study.
Confirmation of RS extension by Microbacterium sp. CFBb37
To confirm the screening results, we determined more precisely the RS of C. elegans when fed with Microbacterium sp. CFBb37, the control OP50, and two additional identified strains (Acinetobacter sp. CFBb9 and Serratia sp. CFBb17). In this assay we moved pools of adults (n = 6–15) to fresh plates daily, which allowed determining both the presence of brood and counting the number of brood per day. The worms growing on OP50 as well as Acinetobacter sp. CFBb9 and Serratia sp. CFBb17 had similar RS durations, which was 4 days (P < 0.001; Table 1). In contrast, worms growing on Microbacterium sp. CFBb37 had an RS of 8 days (P < 0.001), confirming the RS extension from the screen. Qualitatively, average daily brood counts appeared to decline exponentially from day 2 onward (day 2, n = 47; day 7 and later, n < 4) (Table 1).
Table 1.
Average daily individual brood countsa for C. eleganson three environmental bacterial species and the OP50 control.
| Reproductive day number | Acinetobacter sp. CFBb9 (N = 4, n = 29)b | Serratia sp. CFBb17 (N = 4, n = 39)b | Microbacterium sp. CFBb37 (N = 3, n = 37)b | Escherichia coli OP50 (N = 5, n = 33)b | ||||
|---|---|---|---|---|---|---|---|---|
| Eggs laid per individual | Greater than zero P (one-sample t-test) | Eggs laid per individual | Greater than zero P (one-sample t-test) | Eggs laid per individual | Greater than zero P (one-sample t-test) | Eggs laid per individual | Greater than zero P (one-sample t-test) | |
| Day 1 | 41.24 | <0.001 | 69.30 | <0.001 | 35.16 | <0.001 | 151.69 | <0.001 |
| Day 2 | 162.34 | <0.001 | 165.74 | <0.001 | 47.00 | <0.001 | 95.39 | <0.001 |
| Day 3 | 44.51 | <0.001 | 39.23 | <0.001 | 24.08 | <0.001 | 2.96 | <0.001 |
| Day 4 | 2.07 | <0.001 | 0.95 | <0.001 | 13.59 | <0.001 | 1.12 | <0.001 |
| Day 5 | 0 | 0 | 8.86 | <0.001 | 0 | |||
| Day 6 | 0 | 0 | 6.16 | <0.001 | 0 | |||
| Day 7 | 3.51 | <0.001 | ||||||
| Day 8 | 1.49 | <0.001 | ||||||
| Day 9 | 1.02 | <0.001 | ||||||
| Day 10 | 0.24 | <0.001 | ||||||
| Day 11 | 0.51 | <0.001 | ||||||
| Day 12 | 0 | |||||||
| Day 13 | 0 | |||||||
| Average brood | 250.16 | 275.22 | 141.62 | 251.16 | ||||
| size per wormc | ||||||||
| RS (days)d | 4 | 4 | 11 | 3.8 | ||||
First, an average brood number per individual was calculated per pool, then the average of the pools was reported.
N, number of pools tested; n, total numbers of individuals tested.
Average brood size per worm in pools is the sum of all the daily brood sizes.
RS is the number of days with average daily individual brood counts >0 based on one-sample t-test.
RS, reproductive span.
In the above assay, the number of parents per plate varied both within and among bacterial strains. While unlikely to be of major effect, we decided to eliminate the potential confounding effects of parental interactions and differing parental density by re-examining brood presence (and brood sizes, see below) using one parental worm per plate. We found that the average RS for worms growing on Microbacterium sp. CFBb37 was 79% greater than on OP50 (7.29 ± 0.25 days; mean ± 1 standard error (SE) versus 4.08 ± 0.16; P < 0.001; Fig. 1A and S1; Table 2). The combined data clearly indicate that CFBb37 induces RS extension in C. elegans.
Figure 1.
RS of individual nematodes on Microbacterium sp. CFBb37 and the E. coli OP50 control. (A) C. elegans, (B) C. briggsae, (C) C. tropicalis, and (D) Protorhabditis sp. on CFBb37 had longer RS than on OP50 (P < 0.001, log-rank test). Full RS data in Table 2. RS, reproductive span.
Table 2.
Total average brood and RS of tested nematode species.
| Reproductive day number | OP50 | CFBb37 | Different between the strains P (paired t-test) | ||
|---|---|---|---|---|---|
| Eggs laid per day (mean ± SE) | Greater than zero P (one-sample t-test) | Eggs laid per day (mean ± SE) | Greater than zero P (one-sample t-test) | ||
| C. elegans | |||||
| Day 1 | 32.68 ± 5.04 | <0.001 | 3.76 ± 1.74 | <0.001 | <0.01 |
| Day 2 | 149.75 ± 4.71 | <0.001 | 24.64 ± 2.53 | <0.001 | <0.001 |
| Day 3 | 78.55 ± 6.46 | <0.001 | 23.49 ± 2.12 | <0.001 | <0.001 |
| Day 4 | 3.25 ± 0.81 | <0.001 | 22.05 ± 2.31 | <0.001 | <0.001 |
| Day 5 | 1.40 ± 0.77 | >0.05 | 15.10 ± 1.75 | <0.001 | <0.001 |
| Day 6 | 0.58 ± 0.55 | >0.05 | 7.93 ± 1.14 | <0.001 | <0.001 |
| Day 7 | 0.05 ± 0.03 | >0.05 | 4.39 ± 0.79 | <0.001 | <0.001 |
| Day 8 | 0.03 ± 0.03 | >0.05 | 1.88 ± 0.51 | <0.001 | <0.01 |
| Day 9 | 0 | 0.56 ± 0.23 | <0.05 | N.A. | |
| Day 10 | 0.07 ± 0.04 | >0.05 | N.A. | ||
| Day 11 | 0.03 ± 0.03 | >0.05 | N.A. | ||
| Day 12 | 0 | ||||
| n a | 40 | 41 | |||
| Average brood size per wormb | 266.28 ± 6.42 | 137.63 ± 5.74 | <0.001 | ||
| RS (day)c | 4.08 ± 0.16 | 7.29 ± 0.25 | <0.001 (log-rank test) | ||
| C. briggsae | |||||
| Day 1 | 11.59 ± 1.33 | <0.001 | 2.11 ± 0.34 | <0.001 | <0.001 |
| Day 2 | 18.21 ± 1.89 | <0.001 | 3.80 ± 0.57 | <0.001 | <0.001 |
| Day 3 | 22.56 ± 3.46 | <0.001 | 3.17 ± 0.56 | <0.001 | <0.001 |
| Day 4 | 12.00 ± 2.52 | <0.001 | 2.03 ± 0.50 | <0.001 | <0.001 |
| Day 5 | 0.88 ± 0.28 | <0.01 | 2.30 ± 0.50 | <0.001 | 0.0109 |
| Day 6 | 0.16 ± 0.10 | >0.05 | 1.69 ± 0.44 | <0.001 | <0.01 |
| Day 7 | 0 | 0.50 ± 0.17 | <0.001 | N.A. | |
| Day 8 | 0.22 ± 0.07 | <0.01 | N.A. | ||
| Day 9 | 0.08 ± 0.08 | >0.05 | N.A. | ||
| Day 10 | 0.08 ± 0.08 | >0.05 | N.A. | ||
| Day 11 | 0 | ||||
| n a | 32 | 36 | |||
| Average brood size per wormb | 65.40 ± 7.09 | 16.00 ± 1.88 | <0.001 | ||
| RS (day)c | 3.97 ± 0.25 | 5.61 ± 0.39 | <0.001 (log-rank test) | ||
| C. tropicalis | |||||
| Day 1 | 4.37 ± 0.78 | <0.001 | 4.75 ± 0.65 | <0.001 | 1.0 |
| Day 2 | 4.81 ± 1.37 | <0.001 | 4.79 ± 0.92 | <0.001 | 0.8652 |
| Day 3 | 1.22 ± 0.39 | <0.01 | 7.20 ± 1.71 | <0.001 | <0.01 |
| Day 4 | 0.37 ± 0.24 | >0.05 | 3.88 ± 1.05 | <0.01 | <0.01 |
| Day 5 | 0 | 3.29 ± 0.92 | <0.01 | N.A. | |
| Day 6 | 0.95 ± 0.29 | <0.01 | N.A. | ||
| Day 7 | 0.33 ± 0.14 | <0.05 | N.A. | ||
| Day 8 | 0.04 ± 0.04 | >0.05 | N.A. | ||
| Day 9 | 0 | N.A. | |||
| n a | 27 | 24 | |||
| Average brood size per wormb | 10.78 ± 1.90 | 25.25 ± 3.59 | <0.01 | ||
| RS (day)c | 2.37 ± 0.20 | 4.96 ± 0.36 | <0.001 (log-rank test) | ||
| Protorhabditis sp. | |||||
| Day 1 | 45.00 ± 5.02 | <0.001 | 2.70 ± 0.30 | <0.001 | <0.001 |
| Day 2 | 95.15 ± 7.18 | <0.001 | 4.21 ± 0.49 | <0.001 | <0.001 |
| Day 3 | 18.06 ± 4.95 | <0.001 | 3.23 ± 0.52 | <0.001 | <0.01 |
| Day 4 | 2.00 ± 0.69 | <0.01 | 3.12 ± 0.56 | <0.001 | >0.05 |
| Day 5 | 0.88 ± 0.70 | >0.05 | 2.38 ± 0.43 | <0.001 | N.A. |
| Day 6 | 0.24 ± 0.18 | >0.05 | 1.36 ± 0.28 | <0.001 | N.A. |
| Day 7 | 0.06 ± 0.06 | >0.05 | 1.09 ± 0.24 | <0.001 | N.A. |
| Day 8 | 0 | 1.00 ± 0.24 | <0.001 | N.A. | |
| Day 9 | 1.17 ± 0.33 | <0.01 | N.A. | ||
| Day 10 | 0.53 ± 0.18 | <0.01 | N.A. | ||
| Day 11 | 0.53 ± 0.19 | <0.01 | N.A. | ||
| Day 12 | 0.42 ± 0.14 | <0.01 | N.A. | ||
| Day 13 | 0.14 ± 0.06 | <0.05 | N.A. | ||
| Day 14 | 0.10 ± 0.07 | >0.05 | N.A. | ||
| Day 15 | 0.06 ± 0.04 | >0.05 | N.A. | ||
| 0 | |||||
| n a | 34 | 47 | |||
| Average brood size per wormb | 161.38 ± 10.05 | 22.08 ± 2.26 | <0.01 | ||
| RS (day)c | 3.59 ± 0.20 | 6.68 ± 0.61 | <0.001 (log-rank test) | ||
n, the total number of tested individuals in each test.
Average brood size per worm was the total brood of all hermaphrodites (n) divided by n.
Average RS. N.A., not applicable.
RS, reproductive span; SE, standard error.
Daily count of eggs in the uterus of C. elegans
Our assays for RS above were sometimes difficult (e.g., looking for rare laid eggs near the end of the RS period) or required moving the worms to new plates everyday as well as waiting for the progeny to hatch. A simplification of the RS assay would be to directly look for eggs in the uteruses (“uterus egg assay”) of the focal adult worm at 400× magnification under a DIC compound microscope each day. The presence of at least one egg would indicate fertility. The lack of eggs could indicate reproductive senescence or that any eggs were recently laid. Examining many adult worms each day should help distinguish between the two possibilities, although there would still be a bias for incorrectly scoring a batch of worms (i.e., day) as reproductively senescent.
To test whether the uterus egg assay gives comparable results to our original RS assays, we placed C. elegans L1s on either CFBb37 or OP50 and raised them to adulthood. Then, each day we counted the number of eggs in the uterus of 29–37 worms. For each day, if this number of eggs was greater than zero (using a one-sample t-test), then we considered that day to be a fertile day (i.e., not reproductively senescent).
Using this assay, the worms on CFBb37 had an RS of 8 days while those on OP50 had an RS of 4 days (Table S5). The results from counting eggs in the uteruses were qualitatively concordant with the earlier brood-based assays (7.29 and 4.08 days, respectively; Table 2). Thus, although this approach has an inherent bias for reproductive senescence (i.e., scoring shorter RS), we suggest that the simpler uterus egg assay provides a reasonably good estimate for the RS, and can distinguish differences in RS, at least for those separated by several days.
Reduction of brood size by Microbacterium sp. CFBb37
While confirming the RS extension by Microbacterium sp. CFBb37 on pools of C. elegans, we noticed that this bacterial strain reduced the average total brood size compared with OP50 (141.6 vs 251.2; P < 0.001; Table 1). As above, to remove the potential confounding effects of pooled worms, we recounted the brood sizes using one parental worm per plate. We found that the average total brood size on Microbacterium sp. CFB37 was less than on OP50 (137.63 mean ± 5.74 SE versus 266.28 ± 6.42; P < 0.001, Table 2). Partitioning the data into daily brood sizes over the first 8 days (i.e., the number of days with data for both bacterial strains, Table 2) revealed that worms fed with OP50 had greater brood sizes than those fed with Microbacterium sp. CFB37 during the first 3 days (29–125 more eggs/day) while the opposite (2–19 fewer eggs/day) occurred during the last 5 days (all P < 0.001, Table 2). Although there were more days where worms growing on CBFb37 had greater brood sizes than OP50, the magnitude of the differences was smaller during these 5 days, explaining the lower average total brood sizes for Microbacterium sp. CFB37.
Lifespan extension by Microbacterium sp. CFBb37
Bacterial diet has been shown to affect the (somatic) lifespan of C. elegans in many cases (Zhang et al., 2017; Kumar et al., 2020). Thus, we tested whether Microbacterium sp. CFBb37 could also alter the C. elegans lifespan. We found that worms fed with Microbacterium sp. CFBb37 lived longer than those fed with OP50 (26.77 ± 0.86 days, mean ± 1 SE, versus 17.26 ± 0.57; P < 0.001; Fig. 2 and Table S4).
Figure 2.
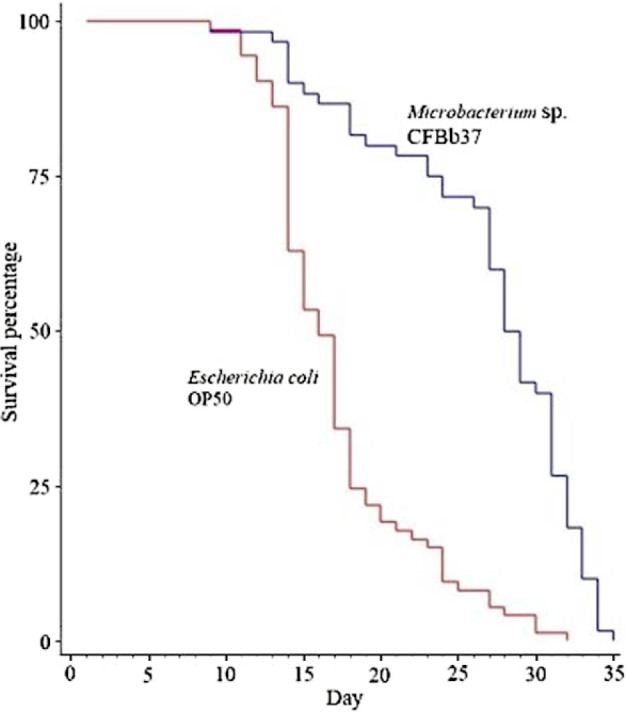
Lifespan assays of C. elegans on Microbacterium sp. CFBb37 and the OP50 control. C. elegans grown on CFBb37 survived longer than OP50 (P < 0.001, log-rank test). Detailed lifespan data are in Tables S4 and S5.
To determine at what general “time period” lifespan extension was occurring, we partitioned the total lifetime into the time spent in the larval, reproductive, and post-reproductive periods. For Microbacterium sp. CFBb37, C. elegans spent an average of 2.89 days as larvae, 7.29 days as reproductive adults, and 16.59 days as post-reproductive adults. On OP50, these durations were 2.05, 4.08, and 11.13 days, respectively. Thus, worms fed with CFBb37 spent longer periods of time in all three stages. This suggests that the total lifespan of the worms on CFBb37 was likely a combination of slower growth as larvae plus lifespan extension during reproductive and post-reproductive stages.
Extension of RS in closely related species
The extension of RS by Microbacterium sp. CBFb37 (compared with E. coli OP50) in C. elegans could be a species-specific effect. Alternatively, Microbacterium sp. CBFb37 may also extend RS in other worm species. We tested this by examining RS using both the uterus egg assay and the presence of brood assay for two additional Caenorhabditis species (C. briggsae CFB233 and C. tropicalis BRC20400) and another Rhabditidae family species, Protorhabditis sp. CFB231. C. briggsae CFB233 and Protorhabditis sp. CFB231 were isolated from two different field samples that were each also the source of two bacterial strains (Tables S2 and S3). The sexual system of Protorhabditis sp. CFB231 could be self-fertile hermaphrodites, because their spermathecae contain sperm (Fig. S2). However, other parthenogenetic Protorhabditis species also produce sperm (Fradin et al., 2017; Grosmaire et al., 2019), so additional genetic analysis will be needed to determine the sexual system of this species.
For all three species and for both assays, we found that feeding on Microbacterium sp. BCFb37 extended the RS compared with OP50 (all P < 0.001, log-rank test; Figures 1B–D and S1; Tables 2 and S5). For C. briggsae the RS extension was 1.64 additional days (+41%), for C. tropicalis 2.59 days (+109%), and for Protorhabditis sp. 3.09 days (+86%). Together, these results suggest that Microbacterium sp. CBFb37 can likely extend RS broadly across the Caenorhabditis genus and perhaps, at least, across the Rhabditidae family.
Brood sizes of closely related species on Microbacterium sp. CFBb37
The extension of RS in C. elegans by Microbacterium sp. CBFb37 was associated with a decrease in brood size. We next examined whether the extension of RS in the three other species was also associated with a consistent decrease in brood size. We found that the brood sizes of both C. briggsae (16.00 ± 1.88 Microbacterium sp. CBFb37 versus 65.40 ± 7.09 E. coli OP50; P < 0.001; Table 2) and Protorhabditis sp. (22.08 ± 2.26 versus 161.38 ± 10.05; P < 0.01; Table 2) on Microbacterium sp. CFBb37 was less than on E. coli OP50. However, C. tropicalis brood size on Microbacterium sp. CFBb37 was more than on E. coli OP50 (25.25 ± 3.59 versus 10.78 ± 1.90; P < 0.01; Table 2). Together, these results suggest that the interaction between Microbacterium sp. CFBb37 and the nematodes is not simple.
Discussion
The primary goal of this study, and to the best of our knowledge, was to investigate the effect on RS by natural isolates of bacteria that are found in environments where nematodes can be typically found. From 109 wild bacterial isolates in Vietnam, we succeeded in finding one, Microbacterium sp. CFBb37, which extended RS in C. elegans as well as three other related species. RS extension, however, did not correlate with brood size in a consistent way with three species having fewer progeny (C. elegans, C. briggsae, and Protorhabditis sp.) and one with more (C. tropicalis).
Many studies have investigated various factors that reduce RS in C. elegans. While no environmental bacteria have been previously tested, one study did find that another E. coli strain and a laboratory bacterial contaminant reduced the RS of C. elegans (Stuhr and Curran, 2020). Similarly, worms cultured in axenic media (presumably with suboptimal nutrient conditions) (Croll et al., 1977) or at higher temperatures (Klass, 1977) also had reduced RS compared with the standard conditions.
Extension of RS has been observed in C. elegans. Worms grown on S. epidermidis (Madhu et al., 2019); at lower temperatures (Klass, 1977; Huang et al., 2004); in the presence of the pharmacological reagents, ethosuximide (Hughes et al., 2007) or metformin (Onken and Driscoll, 2010); and with the addition of vitamin E (Harrington and Harley, 1988) or trehalose (Honda et al., 2010), exhibited longer RS. Extended RS is also seen with some mutants such as eat-2, which causes dietary restriction, and in certain IIS pathway and TGF-β Sma/Mab genes (Huang et al., 2004; Hughes et al., 2007; Hughes et al., 2011). These RS extensions are from ∼12% to ∼116%. Greater RS extension (∼25% to ∼160%) was found for 32 genes in an RNAi screen (Wang et al., 2014). With the caveat that the setup of these and our experiments differed, the 79% RS extension we observed for Microbacterium sp. CFBb37 is larger than most of these factors or reduction-of-function phenotypes.
As mentioned earlier, we found that Microbacterium sp. CFBb37 extended RS in two other Caenorhabditis species and a Protorhabditis sp. The magnitude of RS extension for these species was relatively large, ranging from 41% to 109%. Protorhabditis sp is part of a clade that is sister to the Caenorhabditis genus and together they are part of the “Eurhabditis” group. These results suggest that Microbacterium sp. CFBb37 could extend RS broadly among Eurhabditis, and possibly for many other Rhabditidae (Fig. 3). Microbacterium sp. CFBb37 was also co-isolated with C. briggsae, indicating that these two species likely encounter each other in natural settings. Overall, this leads us to suggest that this microbe–nematode interaction represents an ecologically relevant, and possibly important, interaction.
Figure 3.

Summary of RS extension in the Caenorhabditis species (orange) and the outgroup Protorhabditis sp. (light blue). Red lines indicate inferred ancestral evolutionary lineages with extended RS. We suggest that other species within the genus Caenorhabditis are likely to have extended RS but this is untested. For simplification, some species are not listed and are grouped together in triangles, with the numbers of known species indicated. Phylogeny is adapted from Kiontke and Fitch (2005) and Felix et al. (2014) with the position of Protorhabditis sp. CFB231 placed next to P. sp. 1 species based on the 18S ribosomal RNA gene sequence (Zhang et al., 2000; Morgulis et al., 2008). Protorhabditis sp. CFB231 is self-fertile but the sexual system, hermaphroditic or parthenogenetic, is unknown (indicated by a question mark). RS, reproductive span.
The mechanism by which Microbacterium sp. CFBb37 extends RS remains to be determined. Because RS extension occurs in several species, it likely operates through a conserved pathway. One possibility is dietary restriction, or starvation. Starved adults form elevated rates of bags of worms, or “worm bags”, often resulting in internal hatching of eggs (Angelo and Van Gilst, 2009). We observed very few worm bags on both E. coli OP50 and Microbacterium sp. CFBb37, suggesting that the RS extension we see is different from simple starvation. Another possibility is that Microbacterium sp. CFBb37 produces a presumably weak toxin, or alternatively provides less of a limiting nutrient. This could plausibly explain the slower larval development and reduced brood sizes, the latter of which might be the indirect consequence of reduced oocyte quality or compromised oogenesis (Kadandale and Singson, 2004; Templeman and Murphy, 2018; Templeman et al., 2020). Given the identification of many genes that increase RS, a third possibility could be that Microbacterium sp. CFBb37 somehow acts through one or more of these genes. Future studies will be needed to determine the molecular-genetic bases for Microbacterium sp. CFBb37-mediated RS extension.
In C. elegans, RS extension by Microbacterium sp. CFBb37 is associated with increased lifespan but fewer progeny compared with the standard E. coli OP50 (Table 2 and Table S4). This result is consistent with a frequently observed, apparent tradeoff between lifespan and progeny production (Maklakov and Immler, 2016; Scharf et al., 2021). However, there are exceptions to this negative relationship, including the composition of the cultivation media (Le et al., 2021). Our results with C. tropicalis are another example where the tradeoff does not always hold (Fig. 2; Table 2 and Table S4). Although a caveat is that brood sizes were low on both E. coli OP50 (∼11) and Microbacterium sp. CFBb37 (∼25), possibly indicating that C. tropicalis is not in a healthy state on either of these bacteria.
Conclusions
We have found that C. elegans cultured on a bacteria species, Microbacterium sp. CFBb37, extended RS and lifespan but reduced brood size. Within the family Rhabditidae, two Caenorhabditis species and another relatively close species, Protorhabditis sp. also exhibited RS extension and altered progeny production. Additionally, Microbacterium sp. CFBb37 was co-isolated with C. briggsae. Together, these results suggest that Microbacterium sp. CFBb37 potentially prolongs RS and affects brood size generally in other Rhabditidae nematodes and this microbe–nematode interaction is likely ecologically relevant.
Appendix
Figure S1.
Size comparison of the four tested nematode species grown on Microbacterium sp. CFBb37 and Escherichia coli OP50. Images are representative samples.
Figure S2.

Protorhabditis sp. CFB231 make sperm. (A) DIC image of spermathecal region (outlined). One oocyte is apparently transiting the spermatheca. (B) DAPI staining for nuclei. (C) Merged images of DIC and DAPI. DIC, differential interference contrast.
Table S1.
Wild-type isolates of nematodes in Cat Tien National Park.
| Species name | Strain number | GenBank accession No. | Best BLAST hita | Query coverage | Identity (%) | Bit score (max/total) | E value |
|---|---|---|---|---|---|---|---|
| Caenorhabditis briggsae | CFB233 | MZ457559 | Caenorhabditis briggsae AF16 | 98% | 100% | 1,544/1,544 | 0.0 |
| Protorhabditis sp. | CFB231 | MZ474671 | Protorhabditis sp. 1 GVDU-2019 | 90% | 95.35 | 1,229/1,229 | 0.0 |
Query date March 25, 2022.
Table S2.
Bacterial strains isolated from rotting vegetation samples collected in Cat Tien National Park and their effects on nematodes.
| Straina | Species | Isolation site | GenBank accession No. | Phenotype |
|---|---|---|---|---|
| CFBb3 | Pseudomonas sp. | 11°23′54.4″N 107°20′21.4″E |
OM456346 | Late laying egg |
| CFBb9b | Acinetobacter sp. | 11°24′20.9″N 107°24′22.3″E |
MZ467312 | Normal |
| CFBb11 | Acinetobacter sp. | 11°24′20.9″N 107°24′22.3″E |
OM456348 | Small body |
| CFBb17 | Serratia sp. | 11°24′21.4″N 107°24′21.4″E |
MZ457333 | Small body |
| CFBb35 | Serratia sp. | 11°27′5″N 107°27′25.6″E |
OM629173 | Normal |
| CFBb37 | Microbacterium sp | 11°27′5″N 107°27′25.6″E |
MZ453404 | RS; small body |
| CFBb39 | Sphingobacterium sp. | 11°27′17.9″N 107°22′5.6″E |
OM892000 | Normal |
| CFBb40 | Bacillus sp. | 11°27′17.9″N 107°22′5.6″E |
OM456347 | Normal |
| CFBb42 | Serratia sp. | 11°26′47.8″N 107°26′19.8″E |
OM456350 | Small body |
| CFBb53 | Bacillus cereus | 11°26′12.1″N 107°25′25.7″E |
OM883917 | Small body |
| CFBb54 | Enterobacter sp. | 11°26′12.1″N 107°25′25.7″E |
OM456349 | Small body |
| CFBb57 | Serratia sp. | 11°23′26″N 107°21′1.8″E |
OM892003 | Small body |
Gray highlights bacteria isolated from the same sites as the two new nematode strains isolated from this study (C. briggsae CFB233 [light gray] and Protorhabditis sp. CFB231 [dark gray]).
Strains were isolated in this study unless indicated.
From Le et al., 2021.
RS, reproductive span.
Table S3.
Bacterial isolates with description of colonial morphology, and geography of sample sites.
| Cat Tien National Park | Cuc Phuong National Park | ||||||
|---|---|---|---|---|---|---|---|
| Strain | Morphology of colonya | North | East | Strain | Morphology of colonya | North | East |
| CFBb1 | Small | 11°23′57.5″ | 107°20′21.4″ | CFBb65 | Big | 20°17′40.4″ | 105°39′46.6″ |
| CFBb2 | Big | 11°23′54.4″ | 107°20′21.4″ | CFBb66 | Big | – | – |
| CFBb3 | Small | – | – | CFBb67 | Small | – | – |
| CFBb4 | Irregular | 11°24′22.8″ | 107°24′22″ | CFBb68 | Small | – | – |
| CFBb5 | Irregular | – | – | CFBb69 | Big | 20°17′40″ | 105°39′57.5″ |
| CFBb6 | Big | – | – | CFBb70 | Small | – | – |
| CFBb7 | Big | – | – | CFBb71 | Small | 20°17′41.3″ | 105°39′59.6″ |
| CFBb8 | Small | – | – | CFBb72 | Small | – | – |
| CFBb9 | Big | 11°24′20.9″ | 107°24′22.3″ | CFBb73 | Big | – | – |
| CFBb10 | Irregular | 11°24′21.4″ | 107°24′25.6″ | CFBb74 | Big | – | – |
| CFBb11 | Big | 11°24′20.9″ | 107°24′22.3″ | CFBb75 | Small | – | – |
| CFBb12 | Small | 11°24′20.9″ | 107°24′22.1″ | CFBb76 | Irregular | 20°20′56.7″ | 105°35′48.1″ |
| CFBb13 | Big | 11°24′19.6″ | 107°24′22.1″ | CFBb77 | Big | – | – |
| CFBb14 | Small | – | – | CFBb78 | Small | 20°15′51.2″ | 105°41′57.6″ |
| CFBb15 | Big | 11°24′19″ | 107°24′22.2″ | CFBb79 | Big | 20°20′56.6″ | 105°35′47.4″ |
| CFBb16 | Big | 11°24′18.6″ | 107°24′22.3″ | CFBb80 | Small | – | – |
| CFBb17 | Irregular | 11°24′21.4″ | 107°24′21.4″ | CFBb81 | Irregular | – | – |
| CFBb18 | Big | – | – | CFBb82 | Big | 20°15′7.6″ | 105°42′45″ |
| CFBb19 | Big | 11°28′46.4″ | 107°22′52.9″ | CFBb83 | Small | – | – |
| CFBb20 | Small | – | – | CFBb84 | Big | 20°14′53.5″ | 105°42′34.3″ |
| CFBb21 | Medium | 11°23′18.7″ | 107°21′3.4″ | CFBb85 | Small | – | – |
| CFBb22 | Medium | – | – | CFBb86 | Small | – | – |
| CFBb23 | Big | 11°29′7.2″ | 107°22′58″ | CFBb87 | Medium | 20°14′59.8″ | 105°42′28.1″ |
| CFBb24 | Small | – | – | CFBb88 | Medium | – | – |
| CFBb25 | Medium | 11°27′56.1″ | 107°22′43.5″ | CFBb89 | Big | – | – |
| CFBb26 | Big, yellow | 11°27′0.9″ | 107°21′27.1″ | CFBb90 | Small | – | – |
| CFBb27 | Small | – | – | CFBb91 | Big | 20°14′56.7″ | 105°42′21.1″ |
| CFBb28 | Big | 11°27′1.5″ | 107°21′27.5″ | CFBb92 | Big | – | – |
| CFBb29 | Small | – | – | CFBb93 | Big | 20°14′39.1″ | 105°42′25.3″ |
| CFBb30 | Big, red | 11°26′56.6″ | 107°21′35.9″ | CFBb94 | Small | – | – |
| CFBb31 | Big | – | – | CFBb95 | Big | 20°20′56.7″ | 105°35′46.5″ |
| CFBb32 | Big | 11°23′18.8″ | 107°21′3.6″ | CFBb96 | Small | – | – |
| CFBb33 | Small | – | – | CFBb97 | Medium | 20°20′58.4″ | 105°35′36.5″ |
| CFBb34 | Big | 11°27′31.9″ | 107°20′43.1″ | CFBb98 | Medium | – | – |
| CFBb35 | Big | 11°27′5″ | 107°27′25.6″ | CFBb99 | Big | – | – |
| CFBb36 | Irregular | 11°27′3″ | 107°21′51.7″ | CFBb100 | Big | – | – |
| CFBb37 | Small | 11°27′5″ | 107°27′25.6″ | CFBb101 | Small | – | – |
| CFBb38 | Small | – | – | CFBb102 | Big | 20°21′0.5″ | 105°35′36.3″ |
| CFBb39 | Big | 11°27′17.9″ | 107°22′5.6″ | CFBb103 | Big | – | – |
| CFBb40 | Big | 11°27′17.9″ | 107°22′5.6″ | CFBb104 | Small | – | – |
| CFBb41 | Small | 11°27′17.9″ | 107°22′5.6″ | CFBb105 | Small | – | – |
| CFBb42 | Big | 11°26′47.8″ | 107°26′19.8″ | CFBb106 | Big | – | – |
| CFBb43 | Small | 11°26′47.8″ | 107°26″19.8″ | CFBb107 | Small | – | – |
| CFBb44 | Big | 11°27′50.4″ | 107°27′43.5″ | CFBb108 | Big | 20°20′54.1″ | 105°35′48.5″ |
| CFBb45 | Irregular | 11°27′51.8″ | 107°27′42.1″ | CFBb109 | Small | – | – |
| CFBb46 | Big | – | – | ||||
| CFBb47 | Small | – | – | ||||
| CFBb48 | Big | 11°23′19.3″ | 107°21′3.7″ | ||||
| CFBb49 | Small | – | – | ||||
| CFBb50 | Big, pink | 11°26′14.5″ | 107°25′24.2″ | ||||
| CFBb51 | Small | – | – | ||||
| CFBb52 | Big | 11°26′12.1″ | 107°25′25.7″ | ||||
| CFBb53 | Small | 11°26′12.1″ | 107°25′25.7″ | ||||
| CFBb54 | Small | 11°26′12.1″ | 107°25′25.7″ | ||||
| CFBb55 | Big | 11°25′23″ | 107°25′41.4″ | ||||
| CFBb56 | Small | – | – | ||||
| CFBb57 | Medium | 11°23′26″ | 107°21′1.8″ | ||||
| CFBb58 | Big | 11°24′49″ | 107°25′27.7″ | ||||
| CFBb59 | Big | – | – | ||||
| CFBb60 | Small | – | – | ||||
| CFBb61 | Small | – | – | ||||
| CFBb62 | Big | – | – | ||||
| CFBb63 | Medium | 11°24′48.2″ | 107°25′25.9″ | ||||
| CFBb64 | Small | 11°23′39.7″ | 107°21′47.9″ | ||||
Dashes indicate the same site above.
Colonies had circular shapes and white color, otherwise noted as “irregular” and specific color.
Table S4.
Counts of surviving and lifespan of C. elegans on Microbacterium sp. CFBb37 and the OP50 control.
| Day of lifespan | Number of surviving worms | |
|---|---|---|
| OP50 | CFBb37 | |
| 1 | 73 | 60 |
| 2 | 73 | 60 |
| 3 | 73 | 60 |
| 4 | 73 | 60 |
| 5 | 73 | 60 |
| 6 | 73 | 60 |
| 7 | 73 | 60 |
| 8 | 73 | 60 |
| 9 | 72 | 59 |
| 10 | 72 | 59 |
| 11 | 69 | 59 |
| 12 | 66 | 59 |
| 13 | 63 | 58 |
| 14 | 46 | 54 |
| 15 | 39 | 53 |
| 16 | 36 | 52 |
| 17 | 25 | 52 |
| 18 | 18 | 49 |
| 19 | 16 | 48 |
| 20 | 14 | 48 |
| 21 | 13 | 47 |
| 22 | 12 | 47 |
| 23 | 11 | 45 |
| 24 | 7 | 43 |
| 25 | 6 | 43 |
| 26 | 6 | 42 |
| 27 | 4 | 36 |
| 28 | 3 | 30 |
| 29 | 3 | 25 |
| 30 | 1 | 24 |
| 31 | 1 | 16 |
| 32 | 0 | 11 |
| 33 | 6 | |
| 34 | 1 | |
| 35 | 0 | |
| Censored | 7 | 0 |
| Lifespan (days) (mean ± 1SE) | 17.26 ± 0.57 | 26.77 ± 0.86 |
| P (log-rank test) | <0.001 | |
SE, standard error.
Table S5.
Counts of daily uterus eggs of close nematode species.
| Nematode | Reproductive day number | OP50 | CFBb37 | Different between the strains P (paired t-test) | ||||
|---|---|---|---|---|---|---|---|---|
| Number of tested hermaphrodites | Number of eggs in uterus/individual (mean ± SE) | Greater than zero P (one-sample t-test) | Number of tested hermaphrodites | Number of eggs in uterus/individual (mean ± SE) | Greater than zero P (one-sample t-test) | |||
| C. elegans | Day 1 | 32 | 3.94 ± 0.68 | <0.001 | 35 | 1.57 ± 0.34 | <0.001 | 0.0051 |
| Day 2 | 32 | 25.25 ± 1.12 | <0.001 | 31 | 3.13 ± 0.32 | <0.001 | <0.001 | |
| Day 3 | 29 | 5.38 ± 0.98 | <0.001 | 42 | 4.02 ± 0.33 | <0.001 | 0.06615 | |
| Day 4 | 30 | 1.13 ± 0.34 | <0.01 | 32 | 3.69 ± 0.33 | <0.001 | <0.001 | |
| Day 5 | 37 | 0.03 ± 0.03 | >0.05 | 34 | 3.5 ± 0.46 | <0.001 | <0.001 | |
| Day 6 | 37 | 3.22 ± 0.51 | <0.001 | |||||
| Day 7 | 34 | 2.29 ± 0.36 | <0.001 | |||||
| Day 8 | 35 | 0.43 ± 0.16 | <0.01 | |||||
| C. briggsae | Day 1 | 39 | 3.30 ± 0.73 | <0.001 | 39 | 0.51 ± 0.09 | <0.001 | <0.001 |
| Day 2 | ||||||||
| Day 3 | 34 | 4.09 ± 0.39 | <0.001 | 35 | 0.6 ± 0.15 | <0.001 | <0.001 | |
| Day 4 | 39 | 2.38 ± 0.24 | <0.001 | 32 | 0.81 ± 0.14 | <0.001 | <0.001 | |
| Day 5 | 35 | 0.2 ± 0.07 | <0.01 | 35 | 1.46 ± 0.15 | <0.001 | <0.001 | |
| Day 6 | 34 | 0.09 ± 0.05 | >0.05 | 31 | 1.45 ± 0.19 | <0.001 | <0.001 | |
| Day 7 | 32 | 0.63 ± 0.15 | <0.001 | |||||
| C. tropicalis | Day 1 | 33 | 2.09 ± 0.22 | <0.001 | 32 | 0.28 ± 0.09 | <0.001 | <0.001 |
| Day 2 | 32 | 3.47 ± 0.23 | <0.001 | 37 | 2.76 ± 0.32 | <0.001 | 0.2149 | |
| Day 3 | 38 | 1.87 ± 0.23 | <0.001 | 36 | 3.19 ± 0.4 | <0.001 | 0.0044 | |
| Day 4 | 28 | 0.64 ± 0.23 | <0.05 | 38 | 2.6 ± 0.24 | <0.001 | <0.001 | |
| Day 5 | 36 | 0.19 ± 0.13 | >0.05 | 34 | 2.94 ± 0.36 | <0.001 | <0.001 | |
| Day 6 | 36 | 1.53 ± 0.2 | <0.001 | |||||
| Day 7 | 27 | 1.63 ± 0.22 | <0.001 | |||||
| Day 8 | 17 | 0.82 ± 0.16 | <0.01 | |||||
| P. sp. | Day 1 | 34 | 3.23 ± 0.55 | <0.001 | 32 | 3.28 ± 0.26 | <0.001 | 0.595 |
| Day 2 | 36 | 3.67 ± 0.63 | <0.001 | 38 | 1.61 ± 0.18 | <0.001 | <0.001 | |
| Day 3 | 39 | 1.38 ± 0.24 | <0.001 | 36 | 1.58 ± 0.12 | <0.001 | 0.629 | |
| Day 4 | 36 | 0.19 ± 0.03 | <0.05 | 36 | 1.56 ± 0.12 | <0.001 | <0.001 | |
| Day 5 | 34 | 0.15 ± 0.03 | >0.05 | 40 | 1.80 ± 0.16 | <0.001 | <0.001 | |
| Day 6 | 35 | 1.06 ± 0.13 | <0.001 | <0.001 | ||||
| Day 7 | 53 | 0.81 ± 0.10 | <0.001 | <0.001 | ||||
SE, standard error.
Footnotes
Funding
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106.06-2019.27 to T. Son Le and by the Biodiversity Research Center, Academia Sinica to J. Wang. We thank Dr. Nguyen Lai Thanh, Center for Life Science Research, VNU University of Science, Hanoi for imaging the DAPI stained nematodes.
Literature Cited
- Angelo G., Van Gilst M. R.. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science. 2009;326:954–958. doi: 10.1126/science.1178343. [DOI] [PubMed] [Google Scholar]
- Barriere A., Felix M. A. The C. elegans Research Community, ed. WormBook; 2006. Isolation of C. elegans and related nematodes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L. C., Hodgkin J.. Commensals, probiotics and pathogens in the caenorhabditis elegans model. Cell Microbiol. 2014;16:27–38. doi: 10.1111/cmi.12234. [DOI] [PubMed] [Google Scholar]
- Croll N. A., Smith J. M., Zuckerman B. M.. The aging process of the nematode caenorhabditis elegans in bacterial and axenic culture. Experimental Aging Research. 1977;3:175–189. doi: 10.1080/03610737708257101. [DOI] [PubMed] [Google Scholar]
- Darby C. The C. elegans Research Community, ed. WormBook; 2005. Interactions with microbial pathogens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen P., Assie A., Zimmermann J., Zhang F., Tietje A. M., Marsh S. A., Felix M. A., Shapira M., Kaleta C., Schulenburg H., Samuel B. S.. Cembio – the caenorhabditis elegans microbiome resource. G3 (Bethesda) 2020;10:3025–3039. doi: 10.1534/g3.120.401309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen P., Marsh S. A., Braker I., Heitland N., Wagner S., Nakad R., Mader S., Petersen C., Kowallik V., Rosenstiel P., Felix M. A., Schulenburg H.. The native microbiome of the nematode caenorhabditis elegans: Gateway to a new host-microbiome model. BMC Biology. 2016;14:38. doi: 10.1186/s12915-016-0258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix M. A., Braendle C., Cutter A. D.. A streamlined system for species diagnosis in caenorhabditis (nematoda: Rhabditidae) with name designations for 15 distinct biological species. PLoS One. 2014;9:e94723. doi: 10.1371/journal.pone.0094723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin H., Kiontke K., Zegar C., Gutwein M., Lucas J., Kovtun M., Corcoran D. L., Baugh L. R., Fitch D. H. A., Piano F., Gunsalus K. C.. Genome architecture and evolution of a unichromosomal asexual nematode. Current Biology. 2017;27:2928–2939. doi: 10.1016/j.cub.2017.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezal L., Felix M. A.. C. elegans outside the petri dish. Elife. 2015;4:e05849. doi: 10.7554/eLife.05849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracida X., Eckmann C. R.. Fertility and germline stem cell maintenance under different diets requires nhr-114/hnf4 in C. elegans. Current Biology. 2013;23:607–613. doi: 10.1016/j.cub.2013.02.034. [DOI] [PubMed] [Google Scholar]
- Gravato-Nobre M. J., Hodgkin J.. Caenorhabditis elegans as a model for innate immunity to pathogens. Cell Microbiology. 2005;7:741–751. doi: 10.1111/j.1462-5822.2005.00523.x. [DOI] [PubMed] [Google Scholar]
- Grosmaire M., Launay C., Siegwald M., Brugiere T., Estrada-Virrueta L., Berger D., Burny C., Modolo L., Blaxter M., Meister P., Felix M. A., Gouyon P. H., Delattre M.. Males as somatic investment in a parthenogenetic nematode. Science. 2019;363:1210–1213. doi: 10.1126/science.aau0099. [DOI] [PubMed] [Google Scholar]
- Harrington L. A., Harley C. B.. Effect of vitamin E on lifespan and reproduction in caenorhabditis elegans. Mechanisms of Ageing and Development. 1988;43:71–78. doi: 10.1016/0047-6374(88)90098-x. [DOI] [PubMed] [Google Scholar]
- Honda Y., Tanaka M., Honda S.. Trehalose extends longevity in the nematode caenorhabditis elegans. Aging Cell. 2010;9:558–569. doi: 10.1111/j.1474-9726.2010.00582.x. [DOI] [PubMed] [Google Scholar]
- Huang C., Xiong C., Kornfeld K.. Measurements of age-related changes of physiological processes that predict lifespan of caenorhabditis elegans. Proceedings of the National Academy of Sciences U S A. 2004;101:8084–8089. doi: 10.1073/pnas.0400848101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. E., Evason K., Xiong C., Kornfeld K.. Genetic and pharmacological factors that influence reproductive aging in nematodes. PLoS Genetics. 2007;3:e25. doi: 10.1371/journal.pgen.0030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. E., Huang C., Kornfeld K.. Identification of mutations that delay somatic or reproductive aging of caenorhabditis elegans. Genetics. 2011;189:341–356. doi: 10.1534/genetics.111.130450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadandale P., Singson A.. Oocyte production and sperm utilization patterns in semi-fertile strains of caenorhabditis elegans. BMC Developmental Biology. 2004;4:3. doi: 10.1186/1471-213X-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletsky R., Moore R. S., Vrla G. D., Parsons L. R., Gitai Z., Murphy C. T.. C. elegans interprets bacterial non-coding RNAs to learn pathogenic avoidance. Nature. 2020;586:445–451. doi: 10.1038/s41586-020-2699-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassambara A., Kosinski M., Biecek P. survminer. Drawing survival curves using ‘ggplot2’. R package version 0.4.9. 2021. Available at: https://CRAN.R-project.org/package=survminer .
- Kiontke K., Fitch D. H. The C. elegans Research Community, ed. WormBook; 2005. The phylogenetic relationships of caenorhabditis and other rhabditids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klass M. R.. Aging in the nematode caenorhabditis elegans: Major biological and environmental factors influencing life span. Mechanisms of Ageing and Development. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Kumar A., Baruah A., Tomioka M., Iino Y., Kalita M. C., Khan M.. Caenorhabditis elegans: A model to understand host-microbe interactions. Cellular and Molecular Life Sciences. 2020;77:1229–1249. doi: 10.1007/s00018-019-03319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T. S., Nguyen T. T. H., Huong B. T., Nguyen H. G., Ha B. H., Nguyen V. S., Nguyen M. H., Nguyen H. H., Wang J.. Cultivation of caenorhabditis elegans on new cheap monoxenic media without peptone. Journal of Nematology. 2021;53 doi: 10.21307/jofnem-2021-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Wang X., Wang H. D., Wu J., Ren J., Meng L., Wu Q., Dong D., Kao T. Y., Ge Q., Wu Z. X., Yuh C. H., Shan G.. Escherichia coli noncoding RNAs can affect gene expression and physiology of caenorhabditis elegans. Nature Communications. 2012;3:1073. doi: 10.1038/ncomms2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S., Shaw W. M., Ashraf J., Murphy C. T.. TGF-β Sma/Mab signaling mutations uncouple reproductive aging from somatic aging. PLoS Genetics. 2009;5:e1000789. doi: 10.1371/journal.pgen.1000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil L. T., Watson E., Arda H. E., Zhu L. J., Walhout A. J.. Diet-induced developmental acceleration independent of tor and insulin in C. elegans. Cell. 2013;153:240–252. doi: 10.1016/j.cell.2013.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhu B., Salazar A., Gumienny T.. Caenorhabditis elegans egg-laying and brood-size changes upon exposure to serratia marcescens and staphylococcus epidermidis are independent of DBL-1 signaling. MicroPublication Biology. 2019;2019 doi: 10.17912/2r51-b476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maklakov A. A., Immler S.. The expensive germline and the evolution of ageing. Current Biology. 2016;26:R577–R586. doi: 10.1016/j.cub.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Morgulis A., Coulouris G., Raytselis Y., Madden T. L., Agarwala R., Schaffer A. A.. Database indexing for production megablast searches. Bioinformatics. 2008;24:1757–1764. doi: 10.1093/bioinformatics/btn322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken B., Driscoll M.. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans healthspan via AMPK, LKB1, and SKN-1. PLoS One. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukkila-Worley R., Ausubel F. M.. Immune defense mechanisms in the caenorhabditis elegans intestinal epithelium. Current Opinion in Immunology. 2012;24:3–9. doi: 10.1016/j.coi.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R foundation for statistical computing; 2017. [Google Scholar]
- Sambrook J., Russell D. W. Molecular cloning, a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Samuel B. S., Rowedder H., Braendle C., Felix M. A., Ruvkun G.. Caenorhabditis elegans responses to bacteria from its natural habitats. Proceedings of the National Academy of Sciences U S A. 2016;113:E3941–E3949. doi: 10.1073/pnas.1607183113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf A., Pohl F., Egan B. M., Kocsisova Z., Kornfeld K.. Reproductive aging in caenorhabditis elegans: From molecules to ecology. Frontiers in Cell and Developmental Biology. 2021;9 doi: 10.3389/fcell.2021.718522. 718522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenburg H., Felix M. A.. The natural biotic environment of caenorhabditis elegans. Genetics. 2017;206:55–86. doi: 10.1534/genetics.116.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham S. Ambros V. The C. elegans Research Community. WormBook; 2006. Methods in cell biology. WormBook. http://www.wormbook.org . [Google Scholar]
- Stiernagle T. The C. elegans Research Community. 2006. Maintenance of C. elegans. Wormbook. WormBook; pp. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhr N. L., Curran S. P.. Bacterial diets differentially alter lifespan and healthspan trajectories in C. elegans. Communications Biology. 2020;3:653. doi: 10.1038/s42003-020-01379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeman N. M., Cota V., Keyes W., Kaletsky R., Murphy C. T.. CREB non-autonomously controls reproductive aging through hedgehog/patched signaling. Developmental Cell. 2020;54:92–105. doi: 10.1016/j.devcel.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeman N. M., Murphy C. T.. Regulation of reproduction and longevity by nutrient-sensing pathways. Journal of Cell Biology. 2018;217:93–106. doi: 10.1083/jcb.201707168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingataramin L., Frost E. H.. A single protocol for extraction of gDNA from bacteria and yeast. Biotechniques. 2015;58:120–125. doi: 10.2144/000114263. [DOI] [PubMed] [Google Scholar]
- Wang M. C., Oakley H. D., Carr C. E., Sowa J. N., Ruvkun G.. Gene pathways that delay caenorhabditis elegans reproductive senescence. PLoS Genetics. 2014;10:e1004752. doi: 10.1371/journal.pgen.1004752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecic A., Dhondt I., Braeckman B. P.. The nutritional requirements of caenorhabditis elegans. Genes & Nutrition. 2019;14:15. doi: 10.1186/s12263-019-0637-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Berg M., Dierking K., Felix M. A., Shapira M., Samuel B. S., Schulenburg H.. Caenorhabditis elegans as a model for microbiome research. Frontiers in Microbiology. 2017a;8:485. doi: 10.3389/fmicb.2017.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Holdorf A. D., Walhout A. J.. C. Elegans and its bacterial diet as a model for systems-level understanding of host-microbiota interactions. Current Opinion in Biotechnology. 2017b;46:74–80. doi: 10.1016/j.copbio.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Schwartz S., Wagner L., Miller W.. A greedy algorithm for aligning DNA sequences. Journal of Computational Biology. 2000;7:203–214. doi: 10.1089/10665270050081478. [DOI] [PubMed] [Google Scholar]
- Zimmermann J., Obeng N., Yang W., Pees B., Petersen C., Waschina S., Kissoyan K. A., Aidley J., Hoeppner M. P., Bunk B., Sproer C., Leippe M., Dierking K., Kaleta C., Schulenburg H.. The functional repertoire contained within the native microbiota of the model nematode caenorhabditis elegans. The ISME Journal. 2020;14:26–38. doi: 10.1038/s41396-019-0504-y. [DOI] [PMC free article] [PubMed] [Google Scholar]