Abstract
Objective:
This study aimed to evaluate the effectiveness of three levels of exercise on weight regain subsequent to clinically meaningful weight loss (WL).
Methods:
Adults with overweight or obesity (n = 298) initiated a 3-month behavioral WL intervention, which included reduced energy intake, increased exercise, and weekly behavioral counseling. Participants achieving ≥5% WL (n = 235) began a 12-month behavioral WL maintenance intervention and were randomized to 150 min/wk (n = 76), 225 min/wk (n = 80), or 300 min/wk (n = 79) of partially supervised moderate-to-vigorous–intensity exercise.
Results:
Participants randomized to 150, 225, and 300 minutes of exercise completed 129 ± 30, 153 ± 49 and 179 ± 62 min/wk of exercise (supervised + unsupervised), respectively. Mean WL at 3 months (9.5 ± 3.1 kg) was similar across randomized groups (P = 0.68). Weight change across 12 months was 1.1 ± 6.5 kg, 3.2 ± 5.7 kg, and 2.8 ± 6.9 kg in the 150, 225, and 300 min/wk groups, respectively. Intent-to-treat analysis revealed no significant overall trend across the three treatment groups (P = 0.09), effects for group (P = 0.08), or sex (P = 0.21).
Conclusions:
This study found no evidence for an association between the volume of moderate-to-vigorous–intensity exercise and weight regain across 12 months following clinically relevant WL. Further, results suggest that exercise volumes lower than those currently recommended for WL maintenance, when completed in conjunction with a behavioral weight-maintenance intervention, may minimize weight regain over 12 months.
Introduction
Comprehensive lifestyle interventions (≥14 sessions in 6 months) that assist individuals with overweight and obesity in adhering to a reduced-energy-intake diet and increased physical activity (PA) result in clinically meaningful short-term (6-month) weight loss (WL) of ~5% to 10% of baseline weight (1). However, 40% to 65% of adults with overweight and obesity participating in weight-management interventions will not maintain WL of ≥5% of initial body weight at ≥2 years from baseline (1).
WL increases the propensity for weight regain through decreases in sympathetic tone, leptin, insulin, lean body mass, and bioactive thyroid hormones, increases in ghrelin, and changes in substrate use, which result in altered energy balance by impacting energy intake and energy expenditure (resting metabolic rate and PA) (2,3). Exercise may counter the biological changes associated with WL that promote weight regain by increasing energy expenditure and resetting the balance between energy intake and expenditure at the reduced body weight (4).
Recommendations for WL maintenance from the American Heart Association (AHA), American College of Cardiology (ACC), and The Obesity Society (TOS) suggest a minimum of monthly contact with a trained interventionist to assist participants with adherence to high levels of PA (200-300 min/wk), the reduced-energy diet needed to maintain lower body weight, and regular self-monitoring of weight (1). Other groups including the International Association for the Study of Obesity (5), the European College of Sport Science (6), and the American College of Sports Medicine (7) have recommended 60 to 90 min/d (300-450 min/wk) of moderate-intensity PA to maintain WL. Exercise recommendations for weight maintenance are based primarily on results from cross-sectional (8–10) and nonrandomized studies (11–15) or retrospective analyses of randomized trials. These studies suggest that relatively high volumes of exercise (>200 min/wk) are required to minimize weight regain (16–18).
The Midwest Exercise Trial for the Prevention of Weight Regain (MET-POWeR) evaluated the effectiveness of three different volumes of aerobic exercise, with a 150 min/wk group (G150), a 225 min/wk group (G225), and a 300 min/wk group (G300), on the prevention of weight regain over 12 months subsequent to clinically meaningful WL (≥5%) in a sample of adult men and women with overweight or obesity. We expected the greatest, intermediate, and least weight regain in G150, G225, and G300, respectively.
Methods
Design
A detailed description of the rationale and design for (MET-POWeR) has been published (19). Adults with overweight or obesity (n = 298) initiated a 3-month behavioral WL intervention (−3 to 0 months). Participants achieving clinically meaningful WL (≥5%,) began a 12-month behavioral weight-maintenance intervention (0-12 months) conducted in accordance with AHA/ACC/TOS guidelines (1) and they were randomized to one of three moderate-to-vigorous–intensity exercise groups: G150, G225 or G300. The primary outcome was weight change from 0 to 12 months (i.e., weight at 12 months – weight at 0 months). Approval for this study was obtained from the Human Subjects Committee at the University of Kansas Medical Center. The study began in November 2012. Participant recruitment and data collection were completed in April 2016 and January 2017, respectively. All participants provided written informed consent and they were compensated monetarily for time and travel associated with outcome evaluations (up to $600) and received monetary bonuses for completing ≥80% of their prescribed exercise minutes over 3-month periods (1-3 months, 4-6 months, 7-9 months, and 10-12 months) (up to $450).
Participant inclusion/exclusion
Participants were adults (BMI = 25-44.9 kg/m2, age 21-55 years) who were able to exercise and willing to be randomized to one of three exercise groups. Clearance from their primary care physician was required. Exclusion criteria included participating in a research project involving WL or exercise in the previous 6 months; currently participating in a regular exercise program (i.e., >500 kcal/wk) of planned activity assessed by questionnaire (20); not being weight stable (±4.5 kg) for 3 months prior to intake; being pregnant during the previous 6 months, currently lactating, or planned pregnancy in the following 15 months; having a serious medical risk such as type 1 diabetes, cancer, or recent cardiac event (heart attack, angioplasty, etc.); having an eating disorder, current treatment for psychological issues, or taking psychotropic medications; taking medications known to affect weight; adhering to specialized diets; not having access to grocery shopping and meal preparation (military, college students with cafeteria plan, etc.).
WL intervention (−3 to 0 months)
Participants completed a group-based, in-person lifestyle intervention that included a reduced-energy diet, increased exercise, and behavioral strategies to facilitate adherence to dietary and exercise recommendations (1).
Diet.
Energy intake was reduced to 1,200 to 1,500 kcal/d for women and 1,500 to 1,800 kcal/d for men as recommended by AHA/ACC/TOS guidelines (1). Participants were asked to use a combination of two commercially available portion-controlled entrées (180-270 kcal each), three low-calorie shakes (~100 kcal each), at least five servings of fruits and vegetables, and ad libitum noncaloric beverages to achieve their prescribed level of energy intake. The portion-controlled entrées and low-calorie shakes were provided by the trial only during WL (−3 to 0 months).
Behavioral sessions.
Participants attended weekly 60-minute in-person sessions based on Social Cognitive Theory to promote adherence to diet and exercise recommendations (). Strategies included goal setting, self-monitoring, self-efficacy, and the manipulation of the environment to promote behavioral change. Health educators delivered interactive 30- to 45-minute lessons on topics including nutrition, exercise, and behavior-change strategies, with the remaining time devoted to discussion and problem solving. Participants were asked to complete weekly homework assignments designed to increase self-efficacy for both diet and exercise, provide practice of behavioral skills, and self-monitor daily diet (study-designed Web form) and exercise (paper-and-pencil log). Body weight, for participant feedback only, was assessed at weekly behavioral sessions. All self-monitoring data were available to health educators for discussion with participants during behavioral sessions. Participant attendance at behavioral sessions was tracked by health educators. Weekly health-educator meetings, plus a review of tape recordings of behavioral sessions in a 20% random sample of health educators, were conducted to assure quality and standardization of presentations across health educators.
Exercise.
Exercise progressed from 10 min/d and 5 d/wk at 65% of the age-predicted maximal heart rate (HRmax = 220 – age in years) to the goal of 20 min/d and 5 d/wk at 70% of the HRmax (22) at week 7 and remained at this level through the completion of the 3-month WL intervention. Participants were asked to complete three of five sessions per week under supervision by research staff. Supervised exercise was performed in dedicated facilities at either the University of Kansas Medical Center or University of Kansas–Lawrence that were open Monday through Friday from 6 to 10 am and 4 to 8 pm to accommodate participant schedules. Treadmill walking was the primary mode of supervised exercise; however, one session per week of supervised exercise using an alternative mode (elliptical, bike, etc.) was permitted to provide variety and prevent overuse injuries. The duration and intensity of all exercise sessions (supervised + unsupervised) were verified by heart-rate (HR) monitors (RS 400; Polar Electro, Woodbury, New York). Each exercise session was preceded by a brief (5-minute) warm-up. Participants were asked to increase exercise intensity to reach their prescribed target HR prior to starting the HR monitor and to remain at the target intensity for the duration of each exercise session. Trained research staff checked participants’ exercise HR every 10 minutes during all supervised exercise sessions and instructed participants to increase or decrease exercise intensity if their HR was not within ±4 beats/min of their target HR. Participants were asked to document unsupervised exercise using a logging sheet provided by the trial. Prior to each supervised exercise session, research staff downloaded HR data (Polar HR Software) and reviewed exercise log data from all unsupervised exercise sessions completed since the last exercise laboratory visit. Participants who were not in compliance with the exercise prescriptions were counseled regarding behavioral strategies designed to improve compliance.
Weight-maintenance intervention (0-12 months)
Participants who lost ≥5% of their baseline weight (−3 to 0 months) began a 12-month maintenance intervention that included energy intake to maintain WL and increased exercise and behavioral strategies to facilitate adherence to these recommendations (1). Participants were stratified by sex and magnitude of WL (5%-9.9%, 10%-14.9%, and ≥15%) and were computer-randomized to exercise groups (G150, G225, G300) in a 1:1:1 ratio by the study statistician (MSM). Participant blinding to group assignment was not possible. However, investigators and research assistants were blinded at the level of outcome assessments, data entry, and data analysis.
Diet.
Energy intake was prescribed as resting metabolic rate estimated using the equation of Mifflin and St. Jeor (using participant weight at month 0) multiplied by 1.2 to account for activities of daily living (23). Daily meal plans including suggested servings of grains, proteins, fruits, vegetables, dairy, and fats, based on participants energy requirements and in compliance with the United States Department of Agriculture and Health and Human Services Dietary Guidelines for Americans, were provided to all participants (24). Continued consumption of a minimum of two portion-controlled entrées, three low-calorie shakes, and five servings of fruits and vegetables per day were encouraged, but not required, and portion-controlled entrées and shakes were no longer provided by the trial. Participants were asked to purchase portion-controlled entrées and shakes with acceptable energy and macronutrient content available at local supermarkets from a list provided by the trial.
Exercise.
Exercise groups represented a spectrum of exercise volume from that recommended by the US Department of Health and Human Services to improve health in adults (150 min/wk) (25), which, although not specifically recommend for weight maintenance, have been interpreted as such, to 300 min/wk as recommend by professional organizations to promote WL maintenance (5–7). Exercise progressed from 100 min/wk to the prescribed goal (150, 225, or 300 min/wk at 70% of HRmax) at month 2 and remained at the prescribed goal for the duration of the 12-month intervention. The average exercise minutes across the 12-month maintenance intervention was calculated as the average exercise minutes per week (supervised + unsupervised) divided by the number of weeks with exercise data. Requirements for exercise mode, supervision, and HR monitoring were identical to those for exercise during WL, as previously described.
Behavioral sessions.
Group sessions were held weekly during the first 3 months (in person) and twice per month (phone conference call) over the final 9 months of the maintenance intervention. Delivery of weight-maintenance interventions via phone conference calls removes the burden of travel and has been shown to provide weight maintenance equivalent to interventions delivered in-person (26). The session format during maintenance was identical to that during WL; however, topics such as meal planning, environmental control, eating on the go, and maintaining motivation for diet and exercise were included. Session attendance was tracked by health educators. Participants self-monitored diet and exercise as previously described; however, over the final 9 months when behavioral sessions were conducted by phone, body weight was self-reported using an online Web form.
Outcome assessments
All outcomes were assessed by trained research staff in our laboratories. Staff completed reliability testing for height and waist-circumference assessments. An interrater correlation of ≥0.90 was required prior to data collection. At baseline (−3 months), participants completed a health history and provided demographic information, including age, sex, ethnicity, education, and income.
Anthropometrics (weight/height/waist circumference)
Weight, height, and waist circumference were recorded before (−3 months) and after WL (0 months) and at 3, 6, 9, and 12 months during maintenance. Weight was obtained using a digital scale accurate to ±0.1 kg (model #PS6600; Befour Inc., Saukville, Wisconsin) between the hours of 6 and 10 am, after an overnight fast. Participants were weighed prior to breakfast and after attempting to void wearing a standard hospital gown. Height was measured using a stadiometer (model PE-WM-60-84; Perspective Enterprises, Portage, Michigan). Waist circumference was assessed using procedures described by Lohman et al. (27).
Energy intake/total daily moderate-to-vigorous PA
Energy intake was assessed by 3-day food records (2 weekdays/1 weekend day) before and after WL (−3 and 0 months) and at 3, 6, 9, and 12 months during maintenance. Data from the 3-day food records were entered in the Nutrition Data System for Research (version 2014; University of Minnesota, Minneapolis, Minnesota) for calculation of energy intake. Moderate-to-vigorous PA (MVPA) was assessed using an ActiGraph GT3+ (ArchiMed Co., Lyon, Auvergne-Rhone-Alpes, France) worn by participants for 7 consecutive days at −3, 0, 6, and 12 months. Data, collected in 60-second epochs, were processed using the protocol for adults used in the 2003 to 2004 and 2005 to 2006 cycles of the National Health and Nutrition Examination Survey (28) with a custom program developed by our group.
Analysis
The primary analysis, a two-factor ANOVA (SAS Proc GLM; SAS Institute, Cary, North Carolina) to evaluate the main effect of treatment group and/or sex on weight change during the weight-maintenance intervention (0-12 months) was conducted using intent-to-treat principles with multiple imputation for missing weight data at 12 months. Missing weights were not related to treatment or demographic characteristics; therefore, a traditional method (i.e., Monte Carlo Markov Chain multiple imputation (29) [SAS Proc MI, k = 10]) was used to impute missing weight. A two-factor ANOVA was also used for an analysis of completers-only and an analysis of completers who performed ≥80% of prescribed exercise minutes. Two-factor ANOVA was also used to compare secondary outcomes (i.e., BMI, waist circumference, weekly exercise minutes, energy intake, and total daily MVPA over 12 months) across the three randomized groups. All secondary outcome analyses were performed on participants who completed the 12-month trial.
Power and sample size
The power and sample size were discussed in detail in our previously published rationale and design paper (19). Briefly, sample size estimates were based on an expected weight change (0-12 months) of 8 kg, 5 kg, and 2 kg with a common SD of 6 kg and a type 1 error rate of 5%. With these assumptions and using a two-factor ANOVA on weight change over 12 months, our randomized groups (G150, n = 76; G225, n = 80; G300, n = 79) provided greater than 90% power to detect an overall trend across the three treatment groups. However, our sample included an inadequate number of men (G150, n = 14; G225, n = 14, G300, n = 15) to provide adequate power to assess sex differences in weight change in response to the exercise intervention; thus, the results for the impact of sex on weight regain should be cautiously interpreted.
Results
Participants
Two hundred thirty-five of 298 participants who participated in the WL intervention achieved ≥5% WL at 0 months (78.9%) and were randomized to the three exercise groups: G150, n = 76 (14 men); G225, n = 80 (14 men); and G300, n = 79 (15 men). There were no baseline differences (−3 months) in weight, age, sex, minority status, education, or income between participants who did or did not achieve ≥5% WL. Complete data (i.e., participants with weight data at 0 and 12 months) were available for 74.4% of the randomized sample (175/235). The completion rate did not differ by randomized group: G150, n = 53 (70%); G225, n = 60 (75%); G300, n = 62 (78%). Reasons for missing data included inability to contact or schedule participants for 12-month outcome testing (n = 27), participants indicating they were no longer interested in participating in the intervention (n = 28), and relocation outside the area (n = 5). Weight, age, sex, minority status, education, and income at month 0 did not differ significantly between completers (n = 175) or those without 12-month data (n = 60). For details, see Figure 1 and Tables 1 and 2.
Figure 1.
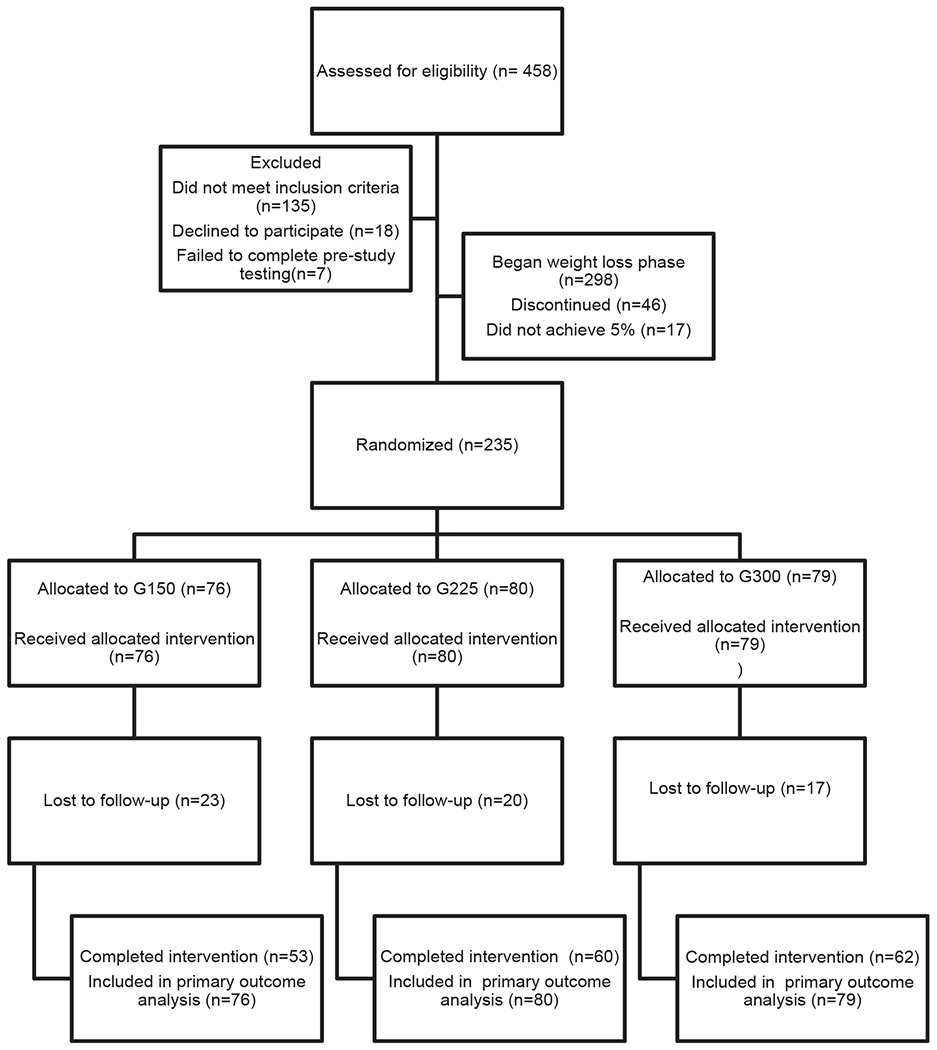
MET-POWeR Consolidated Standards of Reporting Trials (CONSORT) diagram. G150, group at 150 min/wk; G225, group at 225 min/wk; G300, group at 300 min/wk.
TABLE 1.
Demographic characteristics
| Initiated weight loss (n = 298) | Initiated weight maintenance (n = 235) | |
|---|---|---|
| Age (y) a | 41.3 (9.0) | 42.3 (8.3) |
| Weight (kg) | 98.5 (17.7) | 89.4 (16.0) |
| BMI (kg/m2) | 35.2 (5.1) | 32.0 (4.7) |
| Waist circumference (cm) | 99.5 (12.6) | 93.0 (11.1) |
| Women, % | 82 | 82 |
| Minority, % b | 32 | 31 |
| Education (y) | 15.1 (3.7) | 15.2 (3.8) |
| Annual household income, % | ||
| ≥$39,000 | 19.5 | 15.3 |
| >$39,000 to ≤$79,000 | 41.0 | 37.5 |
| >$79,000 | 32.4 | 32.8 |
| No response | 20.1 | 14.5 |
Mean (SD).
Nonwhite.
TABLE 2.
Demographic characteristics by randomized group and sex
| G150 |
G225 |
G300 |
||||
|---|---|---|---|---|---|---|
| Men (n = 14) | Women (n = 62) | Men (n = 14) | Women (n = 66) | Men (n = 15) | Women (n = 64) | |
| Age (y) a | 41.9 (7.0) | 42.2 (8.3) | 43.3 (8.3) | 42.7 (8.1) | 43.7 (8.9) | 41.5 (8.9) |
| Weight (kg) | 98.7 (15.9) | 86.0 (14.6) | 105.7 (16.8) | 86.9 (15.6) | 98.0 (19.8) | 87.5 (13.3) |
| % Weight loss (−3 to 0 months) | −10.8 (4.1) | −9.4 (3.0) | −10.6 (4.3) | −9.1 (2.9) | −10.3 (3.3) | −9.0 (2.6) |
| BMI (kg/m2) | 31.4 (4.3) | 32.1 (4.8) | 32.3 (4.4) | 31.9 (4.9) | 31.0 (4.5) | 32.3 (4.4) |
| Waist (cm) | 101.1 (11.6) | 90.9 (10.3) | 104.7 (9.6) | 91.0 (10.2) | 100.9 (12.9) | 90.8 (10.4) |
| Minority, % b | 21.4 | 27.4 | 21.4 | 37.9 | 20.0 | 33.9 |
| Education (y) | 14.4 (4.7) | 15.7 (3.8) | 15.4 (4.4) | 14.9 (3.5) | 15.7 (2.8) | 15.1 (3.9) |
| Annual household income, % | ||||||
| ≤$39,000 | 7.1 | 21.0 | 7.1 | 16.7 | 0.0 | 15.6 |
| >$39,000 to ≤$79,000 | 35.7 | 33.9 | 28.6 | 36.4 | 46.7) | 42.2 |
| >$79,000 | 50.0 | 32.3 | 50.0 | 33.3 | 40.0 | 23.1 |
| No response | 7.1 | 12.9 | 14.3 | 13.6 | 13.3 | 18.8 |
Mean (SD).
Nonwhite.
G150, group at 150 min/wk; G225, group at 225 min/wk; G300, group at 300 min/wk.
Weight, BMI, and waist circumference
Mean WL (−3 to 0 months) was 9.3 ± 3.6 kg and did not differ significantly across randomized groups: G150 = 9.6 ± 4.1 kg, G225 = 9.3 ± 3.6 kg, and G300 = 9.1 ± 3.0 kg (P = 0.68). Mean weight regain across 12 months, irrespective of randomization group, was 2.4 ± 6.4 kg (women = 2.6 ± 6.5 kg, men = 1.5 ± 6.5 kg). An intent-to-treat analysis evaluating the overall trend across treatment groups, using imputed data for participants with missing weight at 12 months, found no group-by-sex interaction. Thus, the interaction term was removed from the final model, which revealed no significant overall trend across the three treatment groups (P = 0.09), effects for group (P = 0.08), or sex (P = 0.21). A completer-only analysis including only participants with weight at 12 months also indicated no significant between-group differences in weight change across the maintenance intervention (0-12 months): G150 = 1.1 ± 6.5 kg (n = 53); G225 = 3.2 ± 5.7 kg (n = 60); and G300 = 2.8 ± 6.9 kg (n = 62) (P = 0.21). An analysis including a subset of completers who performed ≥80% of prescribed exercise minutes (i.e., 120 [n = 31], 180 [n = 25], and 240 [n = 11] min/wk) also revealed no significant between-group differences in weight change across the 12-month maintenance intervention: G150 = −0.4 ± 6.1 kg (n = 31); G225 = −0.4 ± 5.0 kg (n = 25); and G300 = 2.9 ± 5.3 kg (n = 11). Agreement of results across these three analytic approaches provides support for our conclusion of no association between exercise volume and weight regain across 12 months following clinically relevant WL. An insufficient sample size across intervention groups precluded completion of a per-protocol analysis of weight change across 12 months. There were no significant between-group changes in BMI (P = 0.26) or waist circumference (P = 0.20) across 12 months. For details, see Figure 2 and Table 3.
Figure 2.
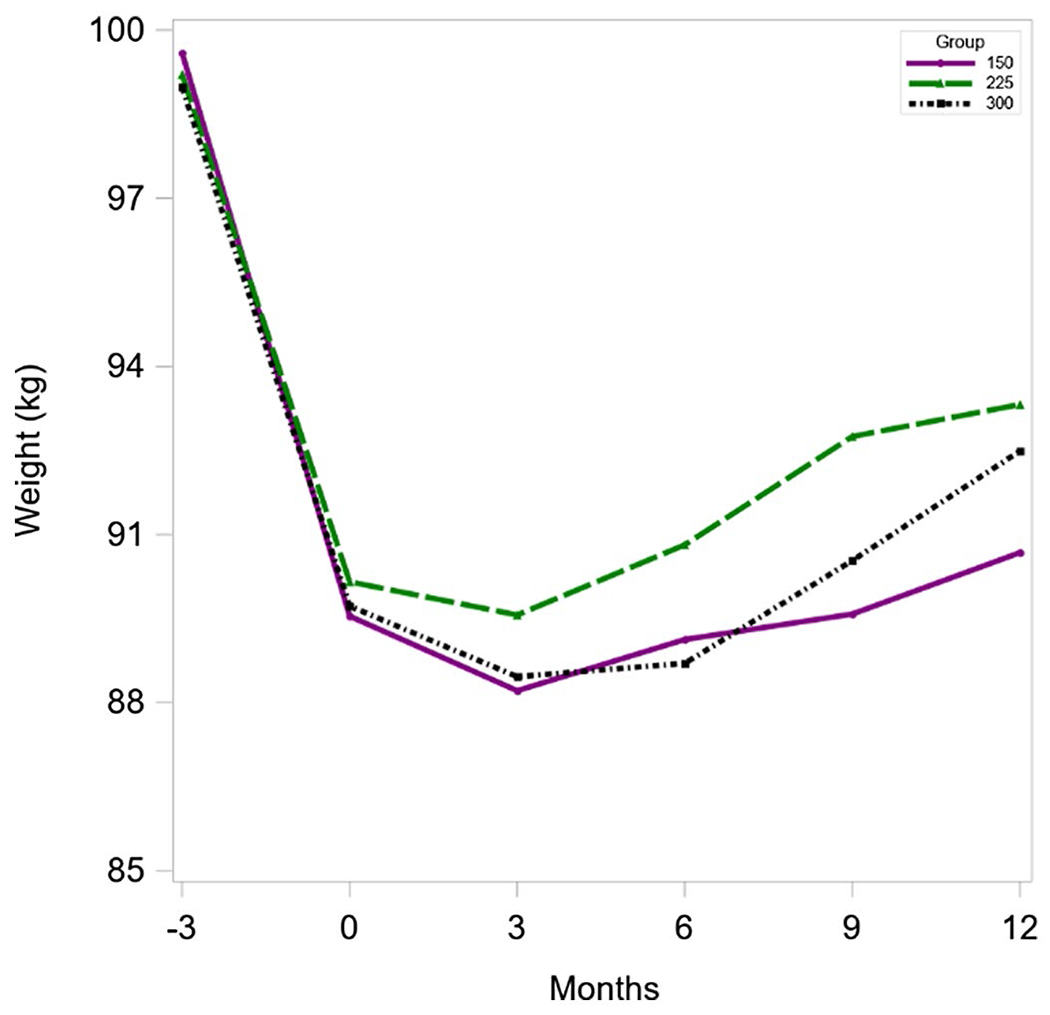
Body weight at −3 to 12 months by randomized group. 150, 150 min/wk; 225, 225 min/wk; 300, 300 min/wk. [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 3.
Change in weight, BMI, and waist circumference over a 12-month weight-maintenance intervention by randomized group and sex
| G150 |
G225 |
G300 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 53) | Women (n = 43) | Men (n = 10) | Total (n = 60) | Women (n = 50) | Men (n = 10) | Total (n = 62) | Women (n = 50) | Men (n = 12) | |
| Weight (kg) | 1.1 (6.5) | 1.2 (6.8) | 0.9 (5.5) | 3.2 (5.7) | 3.1 (5.4) | 3.2 (7.6) | 2.8 (6.9) | 3.3 (6.9) | 0.5 (6.6) |
| Weight (%) | 1.2 (7.1) | 1.2 (7.5) | 1.3 (5.0) | 3.4 (6.3) | 3.5 (5.9) | 3.3 (8.2) | 3.1 (7.4) | 3.6 (7.5) | 0.9 (7.0) |
| BMI (kg/m2) a | 0.4 (2.4) | 0.1 (1.7) | 1.0 (2.3) | 1.1 (2.0) | 1.1 (1.9) | 1.0 (2.3) | 1.0 (2.4) | 1.2 (2.4) | 0.03 (2.2) |
| Waist circumference (cm) b | 0.2 (6.5) | 0.2 (7.0) | 0.3 (4.6) | 2.0 (6.8) | 2.9 (6.8) | 2.9 (6.8) | 2.3 (5.9) | 3.0 (5.8) | −0.8 (5.6) |
Data given as mean (SD).
Height unavailable for three participants at 12 months.
Waist circumference unavailable for two participants at 12 months.
G150, group at 150 min/wk; G225, group at 225 min/wk; G300, group at 300 min/wk.
Individual data for weight change (kilograms) from 0 to 12 months by randomization group is presented in Figure 3. Across the 12-month maintenance intervention ~30% of participants continued to lose weight, ~30% regained <5%, ~30% regained 5% to 10%, and ~10% regained >10% of the weight that was initially lost. At 12 months, 88% of participants who completed the trial (154 of 175) lost ≥5% of their initial body weight (−3 months).
Figure 3.
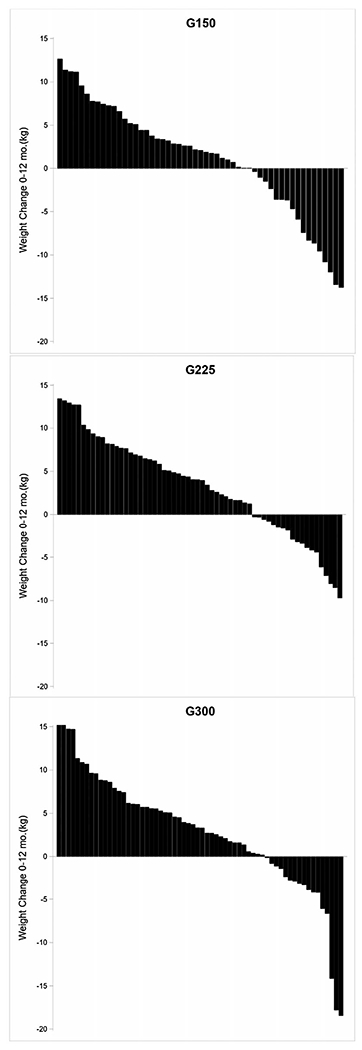
Individual weight change across the 12-month maintenance intervention. G150, group at 150 min/wk; G225, group at 225 min/wk; G300, group at 300 min/wk.
Exercise completed
Participants randomized to G150, G225, and G300 completed 129 ± 30, 153 ± 49, and 179 ± 62 minutes of exercise (supervised + unsupervised) each week, respectively. Individual data for average minutes of weekly exercise across 12 months is presented in Figure 4. Weekly exercise was significantly higher in G300 than in both G150 and G225 and was higher in G225 than in G150 (all P< 0.0001). Weekly exercise minutes did not differ by sex (P = 0.63), and there was no significant group-by-sex interaction (P = 0.67). The number of completed supervised (P = 0.16) or unsupervised exercise sessions (P = 0.054) did not differ by exercise group. Approximately 60% of weekly exercise minutes were completed under supervision. The intensity of all exercise sessions (supervised + unsupervised) (G150 = 76%, G225 = 75%, G300 = 76%) was slightly higher than prescribed (70% HRmax). However, the average exercise intensity across the 12-month maintenance intervention was in the vigorous-intensity range (i.e., 77%-95% of HRmax (22) in 25% of participants and was similar across randomized groups (G150 = 23%, G225 = 27%, G300 = 26%). Completion of prescribed exercise minutes was poor, with 11, 2, and 1 participant averaging 150, 225, and 300 min/wk across the 12-month intervention.
Figure 4.
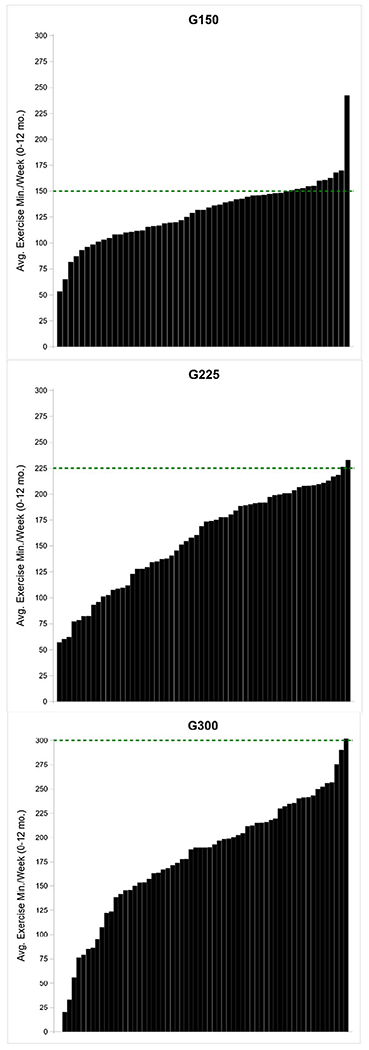
Individual average weekly minutes of exercise across the 12-month maintenance intervention. G150, group at 150 min/wk; G225, group at 225 min/wk; G300, group at 300 min/wk. [Color figure can be viewed at wileyonlinelibrary.com]
Behavioral sessions
Participants attended ~70% of the 30 scheduled behavioral sessions across 12 months: G150 = 72.4%, G225 = 67.9%, and G300 = 69.2%. Behavioral session attendance did not differ by group (P = 0.78) or sex (P = 0.61), with no group-by-sex interaction (P = 0.93).
Energy intake/MVPA
There were no between-group differences in average energy intake (G150 = 1,634 ± 480, G225 = 1,458 ± 414, and G300 = 1,627 ± 497 kcal/d; P = 0.18) or average total daily MVPA across the 12-month maintenance intervention (G150 = 33 ± 17, G225 = 29 ± 13, and G300 = 30 ± 17 min/d; P = 0.29).
Discussion
This trial was designed and powered to evaluate the effect of the volume of MVPA on weight regain across 12 months subsequent to clinically meaningful WL (≥5%). We expected significant differences in weight regain across the three exercise groups, with the highest, intermediate, and least regain in G150, G225, and G300, respectively. Contrary to our expectations, no significant between-group differences in weight regain across 12 months were evident in either our intent-to-treat or completer analyses. The average weight regain across the three randomized groups was minimal, ranging from 1.1 to 3.2 kg, with considerable interindividual variation. Eighty-eight percent of participants who completed the trial (−3 to 12 months) lost ≥5% of their initial body weight.
Our findings of small weight regain and no significant differences in regain across exercise groups suggest that a minimal volume of exercise completed in conjunction with participation in a behavioral weight-maintenance program may provide successful weight maintenance. Previous trials employing randomized (30), nonrandomized, and cross-sectional designs (13,31) have also reported exercise volumes lower than those currently recommended as being associated with WL maintenance. For example, Fogelholm et al. (30) randomized 74 women who completed a 12-week WL intervention to one of three groups: control (no increase in exercise) or partially supervised walking programs (50%-60% of HR reserve) targeted to expend 4.2 MJ/wk (120-180 min) or 8.4 MJ/wk (240-360 min) completed in conjunction with a 40-week behavioral maintenance intervention consisting of weekly group sessions. Mean 12-week WL was 13.1 ± 3.5 kg. No significant between-group differences in weight change were observed across the maintenance intervention (i.e., mean body weight increased in controls [2.0 kg] and was relatively stable in both exercise groups (4.2 MJ/wk = −0.6 kg, 8.4 MJ/wk = −0.7 kg; P = 0.06). Mekary et al. (13) examined the association between leisure-time PA, assessed by questionnaire, and 6-year maintenance of intentional WL in 4,558 female participants in the Nurses’ Health Study II who were 26 to 45 years of age in 1991 and had lost >5% of their body weight in the previous 2 years. An increase of only 30 min/d in leisure-time PA over 6 years was associated with less weight regain (−1.36 kg), particularly among women with overweight (−2.45 kg) compared with women who remained sedentary. A report based on 3,591 participants in the National Weight Control Registry (NWCR), which consists of >10,000 individuals who have lost at least 13.6 kg (30 lb.) and have maintained WL for at least 1 year, demonstrated that 25% of participants reported low PA (<1,000 kcal/wk) and successful WL maintenance (31). These participants did not rely more on dietary strategies to achieve or maintain WL compared with participants reporting higher levels of PA, suggesting lower levels of PA than currently recommended may be associated with WL maintenance.
In contrast to our results, results from numerous cross-sectional studies (8,10,32,33), nonrandomized trials (11–13,15), and retrospective analysis of long-term randomized WL trials (16–18) suggest that relatively high volumes of exercise (>200 min/wk) are required for long-term WL or to minimize weight regain following WL. For example, several reports from the NWCR indicated that participants engaged in 60 to 90 min/d of moderate-intensity exercise (32,33). Two more recent cross-sectional studies compared PA assessed by accelerometer (8) or PA energy expenditure assessed by doubly labelled water (10) in weight maintainers, as defined by the NWCR, with controls with normal weight or overweight. Results from both studies suggest that high levels of PA (60-90 min/d moderate, 30-45 min/d vigorous) were important in WL maintenance. Secondary analysis from randomized trials have also suggested the need for high volumes of exercise for long-term WL (16,18). For example, Jakicic et al. (18) found no significant between-group differences in exercise energy expenditure or WL in 201 women who completed a 24-month behavioral WL intervention that included a reduced-energy diet with randomization to one of four groups based on levels of exercise energy expenditure (1,000 vs. 2000 kcal/wk) and exercise intensity (moderate = 50%-65% HRmax; vigorous = 70%-85% of HRmax). However, a secondary analysis indicated that women sustaining WL of ≥10% of baseline body weight at 24 months, irrespective of randomization group, engaged in higher levels of self-reported PA (~275 min/wk) compared with those women sustaining WL < 10% of baseline weight. The inability to assess cause/effect (i.e., to answer whether increased PA results in less weight regain or whether increased weight regain results in lower PA), reliance on self-reported PA, and selection bias associated with secondary analysis suggest that results from studies employing these designs should be cautiously interpreted.
The degree to which the participation in increased exercise and/or a behavioral WL maintenance program is responsible for the minimal weight regain shown in this trial and others is unknown. We are unaware of trials that have specifically evaluated the differential impact of exercise or participation in a behavioral weight-maintenance program on WL maintenance. However, the results from several trials, in addition to the current trial and the previously described trial of Fogelholm et al. (30), demonstrate that behavioral weight-maintenance interventions, which prescribed relatively low volumes of exercise (120-200 min/wk), have reported minimal weight regain over 12 to 30 months (34–36), suggesting an important contribution of the behavioral component. For example, Perri et al. (34) randomized 234 women who had lost an average of 10 kg over 6 months to a behavioral weight-maintenance intervention consisting of 26 sessions, delivered in a group face-to-face format, through individual phone calls, or through educational newsletter (control). Self-reported PA averaged ~120 min/wk across 12 months. Weight regain was significantly lower in both the face-to-face format (1.3 kg) and the individual phone arms (1.2 kg) than in control participants (3.7 kg). Trials designed to delineate the relative contributions of exercise, participation in a behavioral program, the volumes of exercise, and the frequency of behavioral sessions associated with reduced weight regain would be an important addition to the weight-maintenance literature.
Strengths of this trial include the use of a randomized design, with randomization following clinically meaningful WL, partial supervision of prescribed exercise, documentation of duration and intensity of exercise by HR monitoring, inclusion of a behavioral weight-maintenance program, blinding of outcome assessments, interrater reliability for height and waist-circumference outcomes, and adequate statistical power to address our primary aim. Limitations include failure to achieve our goals for prescribed weekly exercise minutes across randomized groups and the lack of a sufficient sample of men to adequately address sex differences in response to the weight-maintenance intervention. Previous randomized trials designed to identify the volume of exercise associated with successful long-term WL or weight maintenance have also been unable to achieve participant compliance to the prescribed exercise protocols (18,37–39). Compliance with exercise prescriptions decreases with increased exercise volume (40). The difficulty in achieving long-term compliance with recommendations for higher volumes of exercise presents a major challenge to the successful completion of non–laboratory-based effectiveness trials addressing the issue of exercise volume required for WL maintenance. This suggests that laboratory-based efficacy trials with randomization to exercise groups following clinically relevant WL, in which compliance with the targeted exercise volumes is required for continued participation, may be required to answer continuing questions regarding the volume of exercise necessary for successful WL maintenance.
In conclusion, we observed no evidence for an association between the volume of MVPA and weight regain across 12 months following clinically relevant WL in men and women who previously had overweight or obesity. Further, our results suggest that exercise volumes lower than those currently recommended for weight maintenance (G150 = 129 ± 30, G225 = 153 ± 49, and G300 = 179 ± 62 min/wk), when completed in conjunction with a behavioral weight-maintenance intervention, may minimize weight regain over 12 months. The importance of exercise for weight maintenance over longer time frames (i.e., >12 months), participant factors (age, sex, ethnicity, etc.), and intervention-program components (exercise volume, behavioral session frequency, etc.) associated with the high interindividual variability in weight regain are important topics for future investigations.
Study Importance.
What is already known?
Exercise may counter the changes associated with weight loss (WL) that promote weight regain by increasing energy expenditure and resetting the balance between energy intake and expenditure at the reduced body weight.
Current exercise recommendations for the prevention of weight regain (200-450 min/wk) are based on cross-sectional, nonrandomized studies or retrospective analyses of randomized trials.
What does this study add?
We found no evidence for an association between exercise volume and weight regain across 12 months following clinically relevant WL.
Exercise volumes lower than currently recommended, when completed in conjunction with a behavioral weight-maintenance intervention, may minimize weight regain over 12 months.
How might these results change the direction of research?
Current recommendations may overstate the volume of exercise associated with WL maintenance. Laboratory-based efficacy trials with randomization to exercise groups may be required to adequately evaluate the volume required. The degree to which participation in increased exercise and/or a behavioral counseling is responsible for minimizing regain is an important topic for future investigations.
Acknowledgments
Beginning January 1, 2021 deidentified data, a data dictionary, the study protocol, and the statistical analysis plans will be available on request to the primary investigator (JED). Please contact the primary investigator to complete a use agreement for access to these resources.
Funding agencies:
Supported by NHLBI grant R01-HL11842 (JED), NIDDK grant F32-DK103493 (ANS-R), and grant KL2-TR002367 (ANS-R).
Footnotes
Disclosure: The authors declared no conflict of interest.
Clinical trial registration: ClinicalTrials.gov identifier NCT01664715.
References
- 1.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014;129(25 suppl 2):S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond) 2015;39:1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacLean PS, Bergouignan A, Cornier MA, Jackman MR. Biology’s response to dieting: the impetus for weight regain. Am J Physiol Regul Integr Comp Physiol 2011;301:R581–R600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacLean PS, Higgins JA, Wyatt HR, et al. Regular exercise attenuates the metabolic drive to regain weight after long-term weight loss. Am J Physiol Regul Integr Comp Physiol 2009;297:R793–R802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saris WHM, Blair SN, van Baak MA, et al. How much physical activity is enough to prevent unhealthy weight gain? Outcome of the IASO 1st stock conference and consensus statement. Obes Rev 2003;4:101–114. [DOI] [PubMed] [Google Scholar]
- 6.Fogelholm M, Stallknecht B, Van Baak M. ECSS position statement: Exercise and obesity. Eur J Sport Sci 2006;6:15–24. [Google Scholar]
- 7.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine position stand: appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 2009;41:459–471. [DOI] [PubMed] [Google Scholar]
- 8.Ostendorf DM, Lyden K, Pan Z, et al. Objectively measured physical activity and sedentary behavior in successful weight loss maintainers. Obesity (Silver Spring) 2018;26:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas JG, Bond DS, Phelan S, Hill JO, Wing RR. Weight-loss maintenance for 10 years in the National Weight Control Registry. Am J Prev Med 2014;46:17–23. [DOI] [PubMed] [Google Scholar]
- 10.Ostendorf DM, Caldwell AE, Creasy SA, et al. Physical activity energy expenditure and total daily energy expenditure in successful weight loss maintainers. Obesity (Silver Spring) 2019;27:496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoeller DA, Shay K, Kushner RF. How much physical activity is needed to minimize weight gain in previously obese women? Am J Clin Nutr 1997;66:551–556. [DOI] [PubMed] [Google Scholar]
- 12.Field AE, Wing RR, Manson JE, Spiegelman DL, Willett WC. Relationship of a large weight loss to long-term weight change among young and middle-aged US women. Int J Obes (Lond) 2001;25:1113–1121. [DOI] [PubMed] [Google Scholar]
- 13.Mekary RA, Feskanich D, Hu FB, Willett WC, Field AE. Physical activity in relation to long-term weight maintenance after intentional weight loss in premenopausal women. Obesity (Silver Spring) 2010;18:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss EC, Galuska DA, Kettel Khan L, Gillespie C, Serdula MK. Weight regain in U.S. adults who experienced substantial weight loss, 1999-2002. Am J Prev Med 2007;33:34–40. [DOI] [PubMed] [Google Scholar]
- 15.Wilson P Physical activity and dietary determinants of weight loss success in the US general population. Am J Public Health 2016;106:321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakicic JM, Winters C, Lang W, Wing RR. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women: a randomized trial. JAMA 1999;282:1554–1560. [DOI] [PubMed] [Google Scholar]
- 17.Tate DF, Jeffery RW, Sherwood NE, Wing RR. Long-term weight losses associated with prescription of higher physical activity goals: are higher levels of physical activity protective against weight regain? Am J Clin Nutr 2007;85:954–959. [DOI] [PubMed] [Google Scholar]
- 18.Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch Intern Med 2008;168:1550–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szabo AN, Washburn RA, Sullivan DK, et al. The Midwest Exercise Trial for the Prevention Of Weight Regain: MET POWeR. Contemp Clin Trials 2013;36:470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor HL, Jacobs DR Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 1978;31:741–755. [DOI] [PubMed] [Google Scholar]
- 21.Bandura A Social Foundations of Thought and Action: A Social Cognitive Theory. Prentice-Hall; 1986. [Google Scholar]
- 22.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand: quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334–1359. [DOI] [PubMed] [Google Scholar]
- 23.Mifflin MD, St. Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990;51:241–247. [DOI] [PubMed] [Google Scholar]
- 24.US Department of Health and Human Services; US Department of Agriculture. Dietary Guidelines for Americans 2015-2020. 8th ed. US Department of Health and Human Services; 2015. [Google Scholar]
- 25.US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. US Department of Health and Human Services; 2018. [Google Scholar]
- 26.Donnelly JE, Goetz J, Gibson C, et al. Equivalent weight loss for weight management programs delivered by phone and clinic. Obesity (Silver Spring) 2013;21:1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Human Kinetics Books; 1988. [Google Scholar]
- 28.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 2008;40:181–188. [DOI] [PubMed] [Google Scholar]
- 29.Enders CK. Applied Missing Data Analysis. Guilford Press; 2010. [Google Scholar]
- 30.Fogelholm M, Kukkonen-Harjula K, Nenonen A, Pasanen M. Effects of walking training on weight maintenance after a very-low-energy diet in prememopausal obese women: a randomized controlled trial. Arch Intern Med 2000;160:2177–2184. [DOI] [PubMed] [Google Scholar]
- 31.Catenacci VA, Odgen L, Phelan S, et al. Dietary habits and weight maintenance success in high versus low exercisers in the National Weight Control Registry. J Phys Activ Health 2014;11:1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catenacci VA, Ogden LG, Stuht J, et al. Physical activity patterns in the National Weight Control Registry. Obesity (Silver Spring) 2008;16:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Catenacci VA, Grunwald GK, Ingebrigtsen JP, et al. Physical activity patterns using accelerometry in the National Weight Control Registry. Obesity (Silver Spring) 2011;19:1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perri MG, Limacher MC, Durning PE, et al. Extended-care programs for weight maintenance in rural communities: the Treatment of Obesity in Underserved Rural Settings (TOURS) randomized trial. Arch Intern Med 2008;168:2347–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perri MG, Shankar MN, Daniels MJ, et al. Effect of telehealth extended care for maintenance of weight loss in rural US communities: a randomized clinical trial. JAMA Netw Open 2020;3:e206764. doi: 10.1001/jamanetworkopen.2020.6764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy RL, Jeffery RW, Langer SL, et al. Maintenance-tailored therapy vs. standard behavior therapy for 30-month maintenance of weight loss. Prev Med 2010;51:457–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeffery RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome? Am J Clin Nutr 2003;78:684–689. [DOI] [PubMed] [Google Scholar]
- 38.Byrne NM, Meerkin JD, Laukkanen R, Ross R, Fogelholm M, Hills AP. Weight loss strategies for obese adults: personalized weight management program vs. standard care. Obesity (Silver Spring) 2006;14:1777–1788. [DOI] [PubMed] [Google Scholar]
- 39.Colley RC, Hills AP, O’Moore-Sullivan TM, Hickman IJ, Prins JB, Byrne NM. Variability in adherence to an unsupervised exercise prescription in obese women. Int J Obes (Lond) 2008;32:837–844. [DOI] [PubMed] [Google Scholar]
- 40.Schutz Y, Nguyen DM, Byrne NM, Hills AP. Effectiveness of three different walking prescription durations on total physical activity in normal- and overweight women. Obes Facts 2014;7:264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]


