Abstract
Vision plays a crucial role in guiding locomotion in complex environments. However, the coordination between the gaze and stride is not well understood. We investigated this coordination in cats walking on a flat surface in darkness or light, along a horizontal ladder and on a pathway with small stones. We recorded vertical and horizontal eye movements and 3-D head movement, and calculated where gaze intersected the walkway. The coordination of gaze shifts away from the animal, gaze shifts toward, fixations, constant gaze, and slow gaze with strides was investigated. We found that even during walking on the flat surface in the darkness, all gaze behaviours were coordinated with strides. Gaze shifts and slow gaze toward started in the beginning of each forelimb’s swing and ended in its second half. Fixations peaked throughout the beginning and middle of swing. Gaze shifts away began throughout the second half of swing of each forelimb and ended when both forelimbs were in stance. Constant gaze and slow gaze away occurred in the beginning of stance. However, not every behaviour occurred during every stride. Light had a small effect. The ladder and stones typically increased the coordination and caused gaze behaviours to occur 3% earlier in the cycle. At faster speeds, the coordination was often tighter and some gaze behaviours occurred 2–16% later in the cycle. The findings indicate that the coordination of gaze with strides is not vision-driven, but is a part of the whole body locomotion synergy; the visual environment and locomotor task modulate it.
Keywords: eye, eye movement, fixation, head, ladder, locomotion, obstacles, saccade
Introduction
Vision plays a crucial role in guiding locomotion in complex environments. It is the only sensory input available to most animals providing detailed information about distant obstacles in their path. However, how gaze is used during locomotion is not well understood.
There are three major types of gaze behaviours: fixations on objects, constant gaze and gaze shifts. Fixations and constant gaze are believed to be the gaze behaviours during which visual information is gathered (e.g. Land & Hayhoe, 2001). Fixations occur when a subject focuses on a point during locomotion. Constant gaze, also described as ‘travel fixation’, occurs when a subject looks a fixed distance ahead during locomotion (Patla & Vickers, 1997, 2003; Fowler & Sherk, 2003) and images of objects travel across the retina in a constant pattern. Many studies suggest that such ‘optic flow’ provides useful information about both the objects in the environment and the subject’s own movement (Gibson, 1958; Lee, 1980; Sherk & Fowler, 2001). Gaze shifts, also known as gaze saccades, are rapid gaze movements, during which visual sampling is significantly suppressed (Bridgeman et al. 1975; reviewed in Wurtz, 2008). Little is known about the timing of gaze behaviours within the stride cycle as until recently it has been difficult to accurately record gaze behaviour in freely moving subjects.
In our previous study in cats, we used a wearable scleral search coil-based eye-tracking technology that allows a high frequency and accurate recordings of eye movements in freely walking subjects (Ogorodnikov et al. 2006). We combined this technology with an active marker-based head recording system to characterize the gaze behaviour of cats walking over terrains of different complexity: on a flat surface in the darkness and light, on a horizontal ladder, and along a pathway with many scattered small stones (Rivers et al. 2014). We calculated where the cat gaze intercepts the walking surface during locomotion. Based on the velocity of the gaze along the walkway relative to the cat’s velocity along the walkway, we subdivided all gazes that intercepted the surface into six behaviours: gaze shifts away and toward the animal, gaze fixations, constant gaze and ‘slow gaze’ (slow gaze drifts) away and toward. These definitions differ from those typically used in oculomotor physiology and allow description of the relationship of the gaze either to the subject (e.g. in degrees of eye rotation in the orbit) or to the viewed object (e.g. the gaze path on a painting) in that they allow simultaneous characterization of the gaze behaviour in respect to both the subject and the environment. This is particularly useful for a study of a behaviour during which the position of the subject and the view of the environment both change constantly. We found that fixations and gaze shifts dominate the cats’ gaze behaviour during all locomotor tasks tested. We have also noted that during complex tasks such as walking on a horizontal ladder, gaze behaviours predominantly followed a ‘gaze stepping’ pattern characterized by a repeated cycle of forward gaze shift followed by fixation (Rivers et al. 2014).
In this study we have asked the following two questions: (1) are there preferred phases within the stride cycle when particular gaze behaviours are used? (2) If there are such phases, do the complexity of the walking surface and the speed of walking influence them?
We have chosen the cat as the subject because biomechanics of cat locomotion are very well researched, including mechanics and muscle physiology of the body and limbs (Manter et al. 1938; Rossignol, 1996; Trank et al. 1996; McFadyen et al. 1999; Prilutsky et al. 2005; Drew et al. 2008; Beloozerova et al. 2010), the head and neck (Vidal et al. 1986; Graf et al. 1995a,b; reviewed in Graf et al. 1997; and Zubair et al. 2016), and the oculomotor system (reviewed, for example, in Takahashi & Shinoda, 2018). Based on literature and our raw data previously obtained in experiments with the horizontal ladder (Rivers et al. 2014), we hypothesized that gaze fixations occur during the swing phase of a forelimb, while gaze shifts away from the animal occur during the transition from the swing to stance phase. Our results supported these hypotheses in respect to walking along the ladder. They also revealed that gaze shifts away and fixations are related to stride phases in a similar manner on the flat surface in darkness and light, and on the pathway cluttered with small stones. We also found that other gaze behaviours, such as constant gaze, gaze shifts toward the animal and slow gaze, are also coordinated with strides. In addition, we conducted an analysis of gaze behaviour sequencing during individual strides and found that gaze stepping and several other gaze patterns indeed repeatedly occur.
Further, since both humans and cats look closer to themselves when walking on more complex surfaces (Marigold & Patla, 2007; Rivers et al. 2014), we hypothesized that the specific phase of the stride when they visually inspect the pathway might depend on the characteristics of the surface. Our results supported this expectation as we found that the stride-related modulation of gaze behaviours typically increased on complex surfaces as the behaviours concentrated more tightly within their preferred stride phases. They also slightly shifted within the phase as compared to the flat surface. At the same time, our results demonstrated that the sequence of gaze behaviours during the stride and the general preferred phases of individual gaze behaviours seen on the flat surface are remarkably robust and preserved on complex surfaces. This robustness was also evidenced by a rather small effect that walking speed had on the gaze–stride coordination. When walking faster, cats looked further away, and their gaze behaviours were often better coordinated with the stride, and sometimes occurred slightly later in the stride cycle. However, the basic pattern of the gaze–stride coordination was unaltered.
These results suggest that gaze behaviour during locomotion is not vision-driven, but is a part of the whole body locomotor synergy determined by the central locomotor mechanism. The results indicate that the visual environment and behavioural task modulate the coordination between the gaze and stride. Brief accounts of parts of this study have been published in abstract form (Rivers et al. 2010, Chu et al. 2016).
Methods
We will first describe locomotion tasks of different complexity that we studied, which included walking on the flat surface in the complete darkness and in the light, walking on a horizontal ladder and walking along a pathway cluttered with small stones. We will then shortly outline the conventional surgical procedures used to implant an eye coil electrode and a head base to allow recording of eye and head movement. We will next define coordinate frames and describe the calculation of the point where the gaze intercepted the walking surface. Based on the velocity of this point along the surface, we will define six gaze behaviours that we have analysed. They included rapid gaze shifts away and toward the animal, gaze fixations, constant gaze, and ‘slow gaze’ away and toward, with the latter behaviour including slow gaze drifts that did not qualify as either of the first three gaze behaviours. Finally, we will describe our analysis of the relationship of gaze behaviours with strides, including statistics.
Recordings were obtained from three adult cats, two females: cat 1 (3.7 kg) and cat 3 (3.0 kg), and a male, cat 2 (4.0 kg). The cats were purchased from a certified commercial class B dealer. The data were collected during our study of gaze behaviours, some results of which have already been reported (Rivers et al. 2014). A complete description of experimental methods is provided in that publication (Rivers et al. 2014). These cats were also used for other studies of locomotor biomechanics as well as in studies of the activity of neurons in the cortex and thalamus during locomotion. All cats were used for the study of the head movement during walking by Zubair and colleagues (2016). In addition, cat 1 was used in Beloozerova et al. (2010), Marlinski et al. (2012b), Marlinski and Beloozerova (2014), Farrell et al. (2014, 2015), and Favorov et al. (2015); cat 2 was used in Marlinski et al. (2012a), Armer et al. (2013) and Stout and Beloozerova (2013); and cat 3 was used in Favorov et al. (2015). At the end of all studies, cats were euthanized using methods approved by the American Veterinary Medical Association (AVMA 2013 guidelines, www.avma.org/issues/animal_welfare/euthanasia.pdf).
All experiments were conducted in accordance with NIH guidelines and with the approval of the Barrow Neurological Institute Animal Care and Use Committee. The authors understand the ethical principles under which The Journal of Physiology operates and confirm that this study complies with the animal ethics checklist as published in Grundy (2015).
Locomotion tasks
To determine whether cats have preferred phases within the stride for particular gaze behaviours and if there are such phases, whether the complexity of the walking surface influences them, we presented cats with four locomotor tasks of different complexity (Fig. 1).
Figure 1. Locomotion tasks.
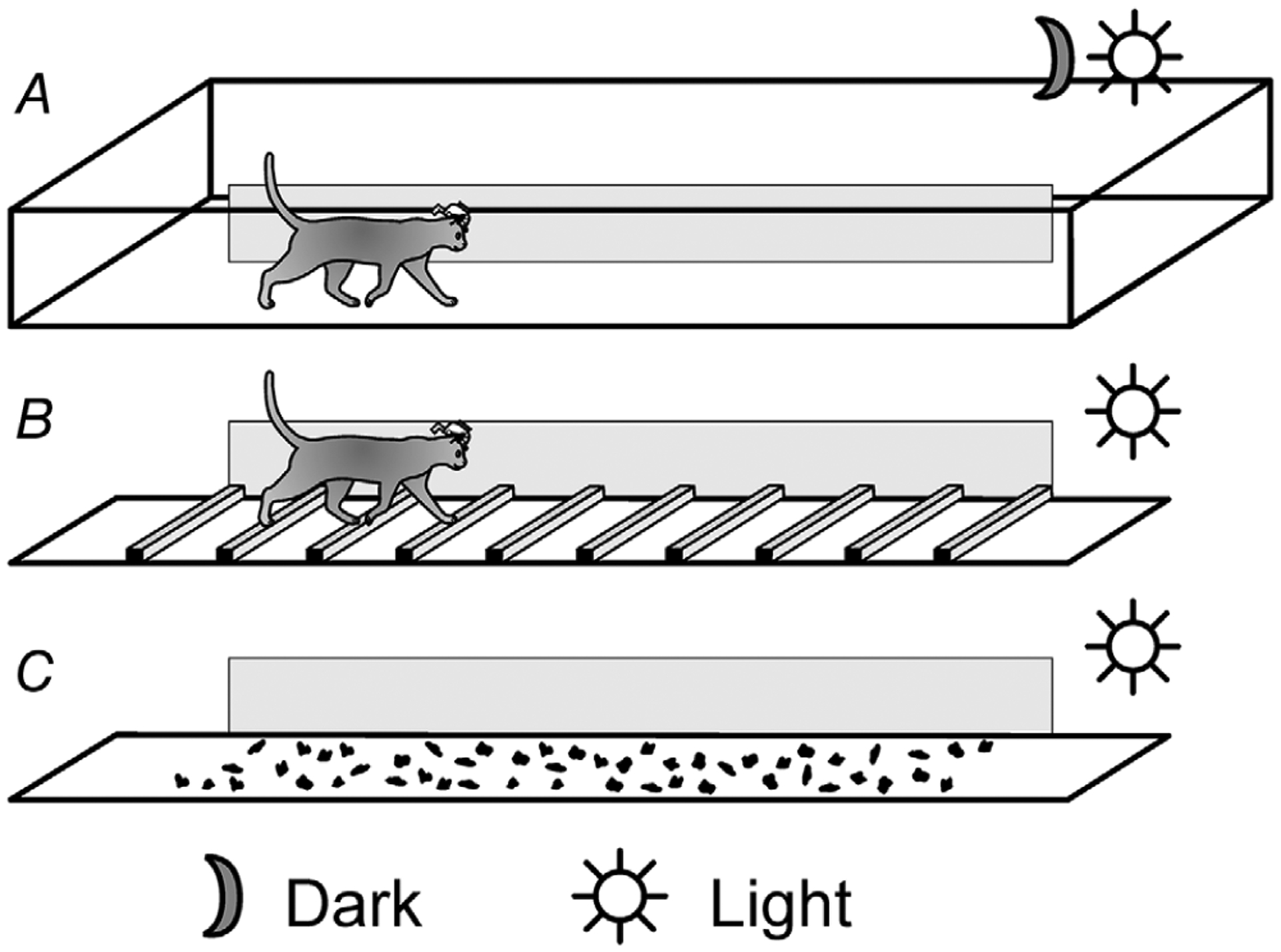
A, cats walked in a 2.5-by-0.6 m chamber separated into two corridors. The exterior wall of one of the corridors was transparent, and data were collected in this test corridor. Cats walked on a flat surface of the test corridor in both complete darkness and under normal laboratory illumination. B, a horizontal ladder could be placed in the test corridor, and cats walked on top of its crosspieces under normal laboratory illumination. C, fifty-one small stones, which were pieces of quartz landscaping gravel 1–2 cm high and 1.5–4 cm in diameter, could be placed in the test corridor, and cats walked on the stone-cluttered surface under normal laboratory illumination.
Walking on the flat surface in complete darkness (Fig. 1A). Recordings during this task provided background information on the relationship of gaze behaviours with strides during locomotion that was not related to sampling of visual information from the environment. For this task, all sources of visible light inside the room were either extinguished or covered. Monitors of the computers controlling recording equipment were placed in a separate room. On randomly selected trials, the lights in the experimental room were turned off, triggered by the cat passing by an LED at the beginning of the test corridor, and stayed off until the cat reached the end of the corridor in approximately 4 s.
Walking on the flat surface in the light (Fig. 1A). Recordings during this task provided information on relationship of gaze behaviours with strides when visual information was available but not necessary for successful locomotion. In trials randomly alternated with those presented in the dark, the lights stayed on and cats walked on the same surface illuminated by the overhead bulbs. Since cats could successfully walk on the flat surface in the dark, visual information was not required for this task. Nevertheless, when the lights were on, there were many potential areas of visual interest for the cats, from the walkway’s surface to items in the laboratory.
Walking along a horizontal ladder (Fig. 1B). During selected trials under the same illumination as the ‘flat-light’ task, a ladder was placed in the test corridor, and the cats walked atop the crosspieces. The ladder’s crosspieces were spaced 25 cm apart, and had flat tops 5 cm wide, which were elevated 8 cm above the floor of the chamber. The crosspieces of the ladder were placed at regular intervals and, because of extensive training, the cats were very familiar with the task. Cats were unable, however, to complete this task in complete darkness (Beloozerova & Sirota, 2003). Thus, recordings during the ladder walking task gave information on gaze behaviours during walking along a complex surface, on which visual information was required for successful stepping.
Walking along a stone-cluttered pathway (Fig. 1C). Recordings during this task provided information about the relationship of gaze behaviours with strides during walking on another highly complex surface where visual information was needed for successful locomotion. Unlike the ladder, the stone-cluttered pathway was irregularly structured and variable on a day-to-day basis. For this task, 51 small stones (pieces of quartz landscaping gravel 1–2 cm high and 1.5–4 cm in diameter) were placed pseudo-randomly on the surface of the walkway in the test corridor. The stones were not attached to the walkway. They occupied 6.7% of the walking surface, and were sufficiently far apart that the cat could comfortably step in-between them. Four different cardboard templates, each 60 cm long, were placed in the walkway, and stones were placed on the walkway’s floor through holes in the templates. A different configuration of templates was used on different recording days. Thus, the cats could not become as familiar with a particular layout of stones as they were with locations of crosspieces of the ladder.
Cats walked at a self-selected speed during all tasks. To determine whether their gazing behaviour is influenced by the speed of walking, fast and slow strides from each recording session were analysed separately and results were compared.
Cats were adapted to the experimental situation and trained to perform all locomotion tasks for at least 1 month before data collection was initiated. Food was used as positive reinforcement (Skinner, 1938; Pryor, 1975). During periods of training and data collection, cats received all their food during training or experimental sessions, and most of this food was canned wet cat food; water was provided ad libitum in the home cage. The walking chamber was 2.5 m long by 0.6 m wide and was divided by a longitudinal wall into two corridors, a test and return corridor (Fig. 1A). The outer wall of the test corridor was transparent to allow recording of cat movements (see below). Cats passed through these two corridors sequentially and repeatedly in the counter-clockwise direction. The passage of the cat through the corridors was monitored using photo-sensors paired with infrared light-emitting diodes (LEDs) with emission wavelengths of 850–900 nm, which is outside the visible spectral range of the cat (Guenther & Zrenner, 1993). The passage through the return corridor was always accomplished in the light, and no obstructions were presented in that corridor. The food reward was always given in the same corner of the return corridor. The floor of the chamber was covered by an electro-conductive rubberized material that allowed recording of foot contact with the floor during walking using electro-mechanical feet sensors (see below). Cats were accustomed to wearing a cotton jacket, an LED and electro-mechanical sensor on forelimb paws for recording the swing and stance phases of the stride (see below), and a light backpack with preamplifiers.
Recording gaze of walking cats
Surgical procedures.
After cats were trained, surgery was performed under isoflurane anaesthesia using aseptic procedures to implant a head base and an eye coil electrode. Surgical procedures are described in detail in our previous publications (Prilutsky et al. 2005; Rivers et al. 2014). In summary, anaesthesia was induced using ketamine (8 mg kg−1), which was followed by 2–5% isofluorane mixed with oxygen (flow rate 0.8 l min−1) administered by inhalation for the length of the surgical procedure. To form the head base, the skin and fascia were retracted from the dorsal surface of the skull. Stainless steel screws were inserted at 10 points around the circumference of the skull. The screw heads were then embedded into a plastic cast to form the head base. The top of the head base was made parallel to the stereotaxic horizontal plane. An eye coil was implanted in the left eye using a conventional technique (Robinson, 1963). The conjunctiva was cut around the iris, and a three-turn coil 21 mm in diameter made of Teflon-coated stainless steel wire (Cooner Wire, Chatsworth, CA, USA; AS-634) was positioned symmetrically around it and sewn to the sclera at three or four points. The leads of the coil were led subcutaneously along the lateral aspect of the head and connected to a connector on the head base. The cats used for this study were also implanted with devices to allow neuronal activity recordings during other experiments. The total weight of all head implants and protective cap was approximately 100 g. Immediately after the surgery and then 12 h thereafter an analgesic buprenorphine was administered intramuscularly. For the duration of experiments, triple antibiotic bacitracin–neomycin–polymyxin ointment was applied to wound margins around the head base 3–4 times a week.
Coordinate frames.
In walking subjects, gaze direction results from a combination of the head position and rotation in space and eye rotation in the orbit. Therefore, a ‘head-in-space,’ or ‘global’ coordinate frame (X, Y, Z), and a head-related ‘eye-in-head’ (XH, YH, ZH) coordinate frame were defined (Fig. 2). The head-in-space coordinate frame originated from the bottom left corner of the test corridor. The X-axis ran along the outer transparent side of it parallel to the floor of the corridor. The Y-axis was perpendicular to the X-axis, and ran along the width of the corridor. The Z-axis was orthogonal to the XY plane and was directed vertically. The ‘eye-in-head’ (XH, YH, ZH) coordinate frame originated from the centre of the left eyeball, which was assumed to always coincide with the centre of the left orbit. This coordinate frame was defined in accordance with the adopted Fick scheme in stereotaxical planes as follows: the XH (roll) axis was parallel to the rostro-caudal line, the YH (pitch) axis was orthogonal to the rostro-caudal line and parallel to the inter-aural line (positive rotation was upward), and the ZH (yaw) axis was orthogonal to those two and was directed upward (positive rotation was to the cat’s left).
Figure 2. Coordinate frames.
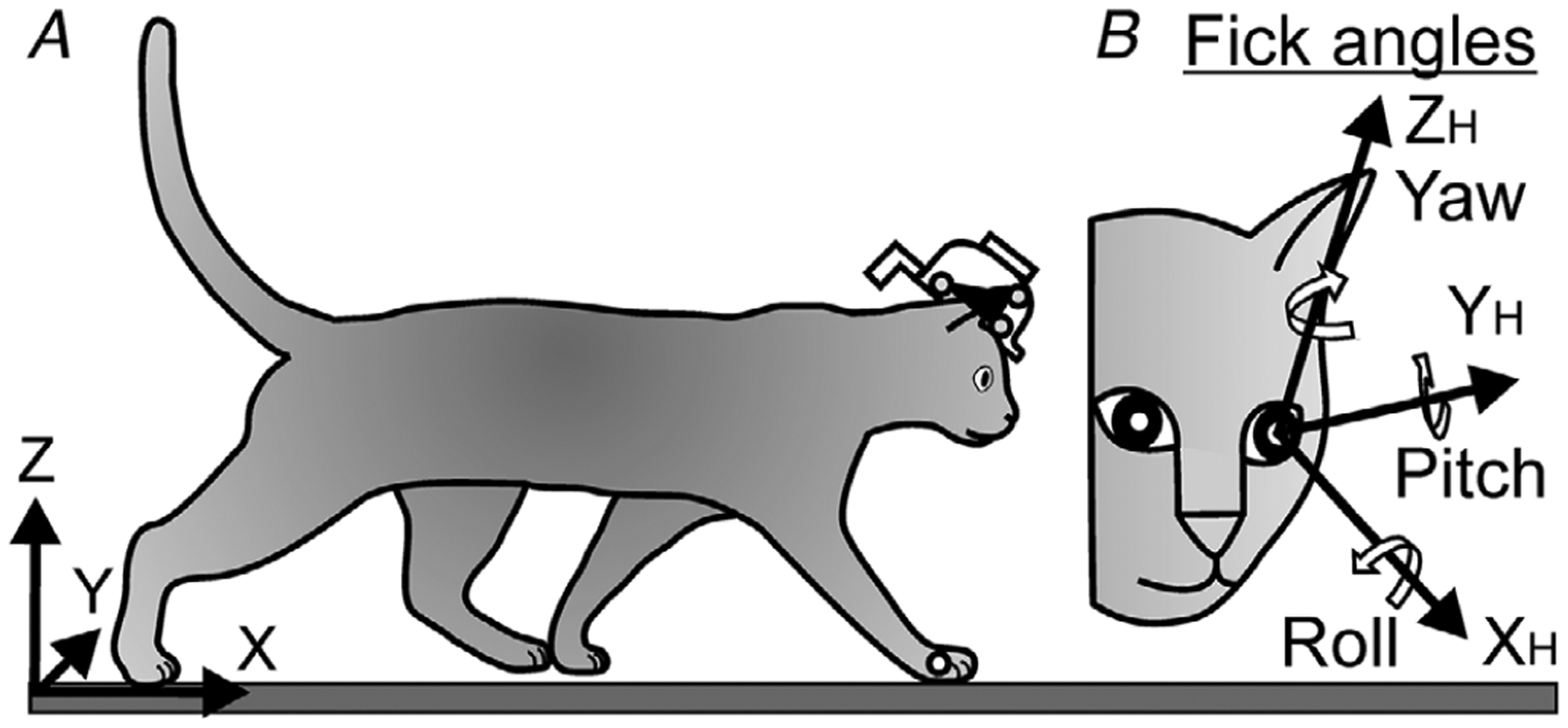
A, a test corridor-related global (X, Y, Z; ‘head-in-space’) coordinate system was used to describe the spatial location and rotation of the head. The head was defined as the centre of the left eye.
Approximate positions of LEDs on the head implant and right wrist are indicated by small circles. B, the Fick coordinate system (XH, YH, ZH; ‘eye-in-head’; Fick, 1854) was used to describe the orientation of the eye in the head.
Eye movement recording.
Movement of the left eye in the orbit was recorded using a modified scleral coil technique (Ogorodnikov et al. 2006; Rivers et al. 2014). A high-frequency magnetic field generator (10 and 11 MHz) was positioned on the head base. The generator’s emitting antenna was placed behind the head of the cat and oriented orthogonally to the eye coil. The signal from the coil was decoded and pre-amplified using an electronic module positioned on the head base. The total weight of this equipment, excluding the head implant described above, was approximately 100 g. The voltage output from the pre-amplifier was sampled at a frequency of 200 Hz and recorded using a data acquisition system, Power1401/Spike2 (Cambridge Electronic Design, Cambridge, UK). Only vertical and horizontal (pitch/yaw) eye movement components according to the adopted Fick scheme (Fick, 1854; Haslwanter, 1995) were recorded (Fig. 2B). The eye roll does not affect the direction of gaze but would have required an implantation of an additional eye coil to be measured, and therefore it was neither measured nor included in the calculations. For calibration of the signal, cats were trained to follow a target on a screen of a computer while their head was held stationary (see below). A detailed description of the calibration technique is given in the preceding paper (Rivers et al. 2014).
Head movement recording.
The position and rotation of the head in the chamber (in ‘global’ X, Y, Z coordinates, Fig. 2A) was recorded using the computerized, active-marker three-dimensional real-time motion capture and analysis system Visualeyez (VZ-4000, Phoenix Technologies Inc., Vancouver, BC, Canada). Three wide-angle, six-chip infrared LEDs with wavelengths of 755–785 nm, which is outside of the visible range for cats (Guenther & Zrenner, 1993), were placed on the head implant in a non-collinear fashion 3–8 cm apart (Fig. 2A). Before each locomotion test, the cat was seated next to the chamber in a comfortable position and its head was fixed to an external frame with zero degrees angle in yaw, zero degrees angle in roll, and −17 deg angle in pitch (nose down) in the global (X, Y, Z) reference frame. To calculate the position of the orbit in the global coordinate system, the VZSoft program was used to create an ‘object’ based on the three LEDs on the head (schematically shown as a black triangle on the cat’s head in Fig. 2A). The ‘object’ function finds coordinates of the centre of mass of a triangle formed by non-collinear LEDs, and these coordinates can be then transposed to the known position of the centre of the orbit. To calculate the head rotation values, the VZSoft program was used to create a ‘rigid body’ based on the LEDs. According to manufacturer’s specifications, the VZ system error for recording position of a LED was 0.5 mm.
Recording during locomotion.
After calibration was completed, the cat was released into the walking chamber where it continuously walked through corridors, stopping only briefly after each round in one of the corners for food reward. Signals from the eye coil decoder on the head base (yaw and pitch) were led to a connector in the cat’s backpack and then through a cable to an analog-to-digital converter board, and recorded using the Power1401/Spike2 system. To record swing and stance phases of the step cycle, an electromechanical sensor was placed under the right and left forelimb paws (e.g. Beloozerova & Sirota, 1993; Prilutsky et al. 2005, Favorov et al. 2015). A sensor consisted of a ~0.1 mm thin metal plate ~10 mm in diameter, which was attached to the sole of the paw with electro-isolative adhesive tape. A voltage of 2–5 mV was applied to the plate, and the fall of the voltage resulting from contact of the foot with the electro-conductive cover of the floor was recorded. The start and finish of electrical contact was taken as the start and finish of the stance phase of a stride. In addition, an LED similar to those on the head base was placed on the right metacarpophalangeal joint (base of the fifth metacarpal) to record foot position in three-dimensional space (Fig. 2A). Signals from all LEDs and the foot electromechanical sensors were sampled at a frequency of 200 Hz. All signals were saved to a computer hard disk. Synchronization between the VZ-4000 and Power1401/Spike2 systems was achieved through a linked electrical channel.
Calculation of gaze–surface intersect point.
To determine the gaze intersection with the walkway’s surface, a rotation matrix algorithm was employed. First, the direction vector of the eye in the head (line of sight) was found using the eye rotation values in the head coordinate system. Next, this vector was rotated by head rotation values within the global coordinate system to obtain the spatial gaze direction vector. Then, using head coordinates in space, the intersect point of the gaze direction vector with the walking surface was found. These rotation calculations were carried out using a custom MATLAB program (The Mathworks, Natick, MA, USA). Further details and an example calculation are provided in the previous publication (Rivers et al. 2014). Gaze behaviours were analysed only as they were observed in the sagittal (pitch) plane, and only for the periods of time when gaze intersected the walking surface.
Classification of gaze behaviours
To classify gaze behaviours, first a modified algorithm for detecting microsaccades (Engbert & Mergenthaler, 2006; Otero-Millan et al. 2014) was used to determine all inflection points on the line of sequential gaze–surface intersect positions plotted against time (Fig. 3A). An inflection point was recognized when the change in gaze velocity (acceleration) along the surface exceeded ±5 cm s−2 for more than 30 ms. Between each neighbouring inflection point, the velocity of the gaze moving along the surface of the XY plane was divided by the velocity of the cat walking during that time to obtain a relative ratio. Based on this gaze/cat velocity proportion, gaze behaviour was classified into six categories: gaze shift away, gaze shift toward, fixation, constant gaze, slow gaze away and slow gaze toward (Fig. 3A).
Figure 3. Graphical illustration of the definition of gaze behaviours, and example plots.
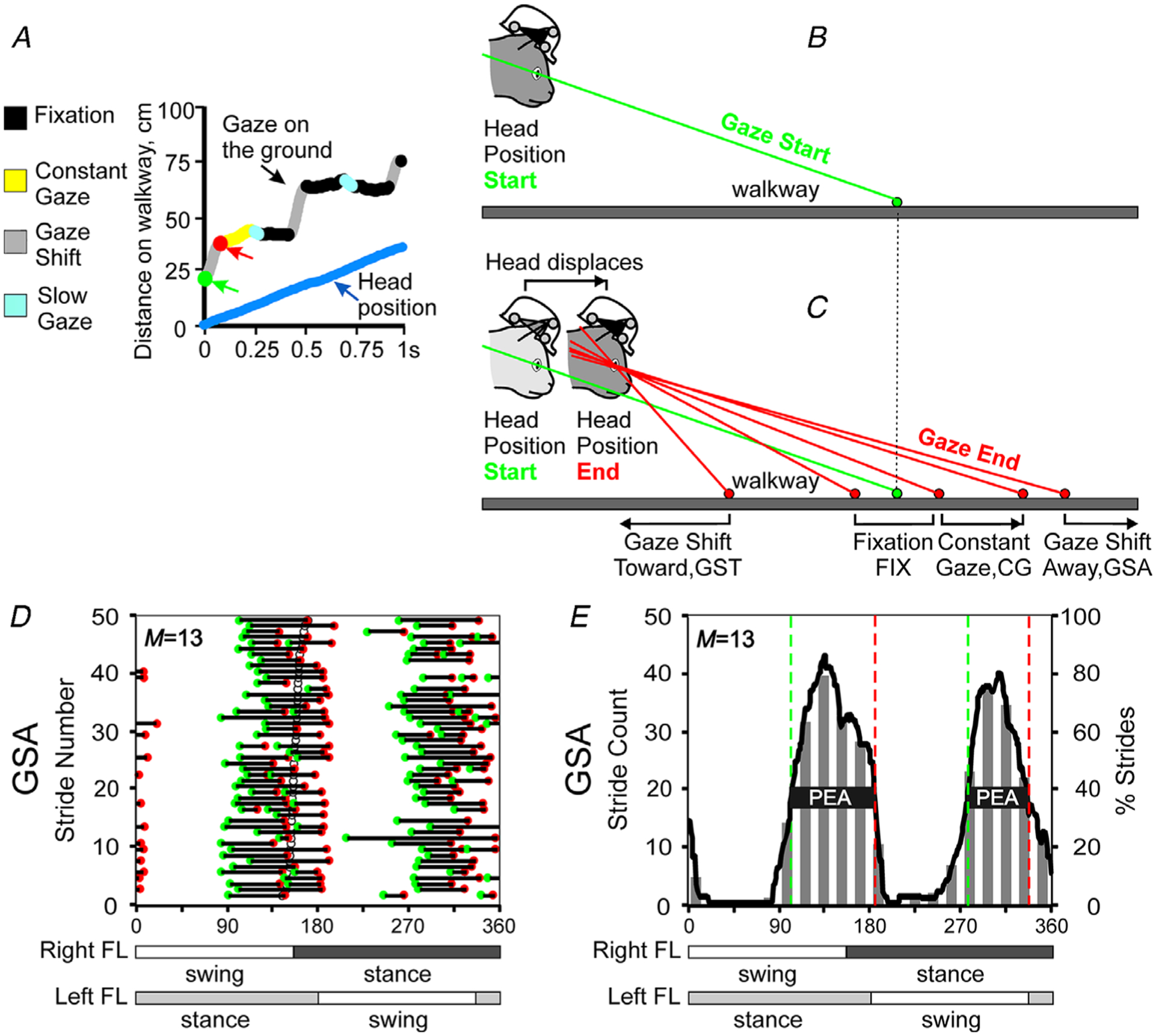
A–C, gaze behaviours were classified based on the relative ratio of gaze intercept point longitudinal velocity and cat head longitudinal velocity (see text for detail). A, an example of sequential positions of the gaze along the walkway, and the four gaze behaviours indicated with different colours. Fixation (black) occurs when the gaze is not moving along the ground. Constant gaze (yellow) occurs when the gaze and the cat (‘head position’ blue line) are moving at approximately same speed. Gaze shift (grey) occurs when the gaze is moving along the walkway much faster or much slower than the cat. Slow gaze (light blue) encompasses the remainder of the gaze behaviour. The start and end of the first gaze behaviour, which was a gaze shift, are highlighted with green and red circles and arrows. B and C, the start (green) and end (red) position of a gaze behaviour on the walkway surface are shown for the gaze shift toward (GST), fixation (FIX), constant gaze (CG) and gaze shift away (GSA). D, an example graphical representation plot of the phases of starts (green circles) and ends (red circles) of GSAs of cat 2 during 50 strides along the ladder. Horizontal black lines indicate phase duration of the gaze behaviour during each stride. Strides are rank-ordered according to the duty factor of the right forelimb strides; duty factor is the percentage of the stride occupied by the stance phase. The strides with a smaller duty factor are plotted progressively higher in the plot. The phase of each stride, in which stance started, is shown by an open circle. The value of the coefficient of modulation, M, characterizing the magnitude of fluctuation in probability of the gaze events occurrence, is shown in the top left corner (see Methods for the M calculation). E, a 20-bin bar graph (left ordinate) and a 360-bin line graph (right ordinate) of the fluctuation in the probability of the occurrence of gaze events during the stride. Vertical interrupted green and red lines indicate the average phase of the beginnings and ends of the gaze behaviours, respectively. The horizontal black bar shows the ‘period of elevated activity’ (PEA), defined as the portion of the cycle in which the activity level of the behaviour is equal to or exceeds the minimal activity by 50% of the difference between the maximal and minimal activity in the histogram (see Methods for details). Swing and stance phases of the right forelimb (FL, recorded) and left forelimb (extrapolated) are shown under panels D and E.
Gaze shift away (GSA): the gaze on the surface was moving forward much faster than the cat, and the gaze/cat velocity ratio was greater than 2.
Gaze shift toward (GST): the gaze on the surface was moving much slower than the cat, and the gaze/cat velocity ratio was less than −2.
Fixation (FIX): the gaze was not moving much along the surface, and the gaze/cat velocity ratio was 0 ± 0.5.
Constant gaze (CG): the gaze on the surface and the cat were moving at approximately the same speed, and the gaze/cat velocity ratio was between 0.5 and 1.5.
Slow gaze away (SGA): the gaze was moving along the surface away from the cat, but was not moving fast enough to be classified as a gaze shift away. The gaze/cat velocity ratio was between 1.5 and 2.
Slow gaze toward (SGT): the gaze was moving along the surface toward the cat, but was not moving fast enough to be classified as a gaze shift toward. The gaze/cat velocity ratio was between −0.5 and −2.
Figure 3B and C schematically depict the start (Fig. 3B) and end positions (Fig. 3C) of the gaze shifts, fixation and constant gaze on the walking surface.
Analysis of the relationship of gaze behaviours with strides
From each passage along the test corridor, two or three strides made in the middle of the corridor were selected for the analysis. The full movement cycle of one limb (e.g. beginning of swing to beginning of next swing of the same limb) is referred to as a ‘step cycle’ or ‘stride’ (used interchangeably); and one half of such a cycle is referred to as a ‘step.’ For each selected stride, the stance-to-stride duration ratio (the stride duty factor), which characterizes the temporal structure of the stride, was calculated. For each cat performing each locomotor task, the strides whose durations and duty factors fell within ±2 SD of the mean for a recording day were selected for further analyses. A two-sample Student’s t test was employed to compare stride durations of a cat between two locomotor tasks, with n being the total number of strides. To compare stride durations across the three cats, a one-way ANOVA with a Tukey HSD post hoc analysis was performed.
The step cycle was time-normalized, allowing for comparison of phases of gaze behaviours among cycles of different durations. The stride phase of the start and end of each gaze event were calculated, and plots were constructed to visualize the phase positions of episodes of the behaviour during a locomotor task (Fig. 3D). For further analysis, the step cycle was subdivided into 20 equal bins. Gaze behaviours which occupied more than half of a bin were considered as occupants of that bin. Counts of strides with a gaze behaviour across all bins were plotted as histograms (Fig. 3E, the bar graph and left ordinate). To determine whether a gaze behaviour was modulated in the rhythm of strides, a chi-square test (χ2 test) was used to compare its histogram with a histogram of the same number of gaze behaviours split equally across the 20 bins (http://ccnmtl.columbia.edu/projects/qmss/the_chisquare_test/about_the_chisquare_test.html). The coefficient of modulation, M, characterizing the magnitude of fluctuation in probability of the gaze events’ occurrence, was calculated as M (%) = (Nmax − Nmin)/N × 100, where Nmax and Nmin are the numbers of strides with the gaze event in the maximal and minimal histogram bins and N is the total number of events in the histogram. In 90% of measurements the value of M was between 5 and 25.
To determine the ‘period of elevated activity’ (PEA) of a stride-modulated gaze behaviour, the stride cycle was divided into 360 bins (i.e. degrees of the locomotor cycle, 360 rather than 20 bins were used to enhance accuracy). For each stride, if a bin was at least half occupied by a gaze behaviour, it was considered to contain that behaviour. The proportion of the strides during which the behaviour occurred was calculated for each bin (Fig. 3E, the line graph and right ordinate). The PEA was defined as the portion of the cycle in which the activity level met or exceeded the minimal activity by 50% of the difference between the maximal and minimal activity in the histogram (Fig. 3E). PEAs were smoothed by removing all peaks and troughs 20 deg or less.
To compare M values and the phase of the starts and ends of PEAs across cats, locomotor tasks and experimental days, a measure of variability in the phase was calculated using a Monte Carlo Analysis (MCA) method (Berg, 2004). All strides for a given condition (n) were pooled. Strides were then repeatedly and randomly selected, and the bin numbers occupied by the specific gaze behaviour in each stride were duplicated into a separate ‘simulation’ pool. After this process was repeated for half the number of strides (n) in the original data set, all data were compiled into a histogram, and a ‘simulation dataset’ was complete. In total, 1000 simulations for each condition were performed. PEAs were calculated for each simulation, and the standard deviation was reported for the condition as a whole. PEA metrics across all simulations fell into a normal distribution. A two-tailed t test was used to compare the phase of a PEA beginning and end, and its duration in different conditions. The phase of the PEA was defined as the phase of the stride between the PEA’s beginning and end.
The sequences of gaze behaviours during individual strides were analysed. First, the frequency of each combination of two consecutive gaze behaviours (e.g. GST followed by FIX, GSA followed by CG) was computed. The series of GST–FIX–GSA–CG was considered to feature three behavioural pairs: GST–FIX, FIX–GSA and GSA–CG. Second, the frequency of all combinations of three consecutive gaze behaviours (e.g. GST followed by FIX followed by GSA) was computed. To determine whether the observed frequency of a combination of gaze behaviours can be explained by chance, a MCA method was used (Berg, 2004). All gaze events observed during strides included in the analysis for each locomotor task on an experimental day were randomized to form a ‘replica’ cluster of data. One thousand ‘replica’ clusters were created in total, and, for each subset, the frequencies of all gaze behaviour combinations were calculated. The mean and SD of these frequencies were calculated across all 1000 replica subsets. For each combination, the 95% confidence interval was computed with boundaries of mean ± 2 SD. If the actual observed frequency of a combination was outside this range, the frequency of the combination was considered statistically significantly different from chance.
To determine whether the speed of walking influences gaze behaviour, we first analysed whether it affects the frequency of individual gaze behaviours. For this analysis, we subdivided strides by speed using 10 cm s−1 bins up to 110 cm s−1 and for each bin, determined the proportion of total time that strides were occupied by the corresponding gaze behaviour. Logistic regression analysis was then used to assess the relationship between the probability for a gaze behaviour to occur during a stride and the speed of walking. We also analysed in an analogous manner, using linear regression analysis, the distances from the cat where the gaze behaviour started, how long it lasted, and, for the gaze shifts, constant gaze and slow gaze, the amplitude (i.e. the distance on the surface travelled). To determine whether the coordination of gaze behaviours with strides was influenced pared gaze behaviour during strides with durations within 2 SD below an average for this day and task (classified as ‘fast’) and within 2 SD above the average (classified as ‘slow’). The duration of the thus selected fast and slow strides differed by 8–25% with P < 0.0001. A two-tailed t test was used to compare the gaze behaviour’s coefficient of modulation, M, the phase of its PEA beginning and end, and the PEA’s duration between the fast and slow strides.
The analysis was not blind to the experimental condition.
Results
Data for this study were collected on 13 experimental days 4–15 months after a cat underwent the implantation surgery. Data from cat 1 were recorded on five days: walking on the flat surface in the darkness and light, and along the ladder were recorded within a 1.5 month period, and walking along the stone-cluttered pathway was recorded 6 months later. Data from cat 2 were recorded on three experimental days within a 10 day period and included walking on the flat surface in the darkness and light, and along the ladder. Finally, data from cat 3 were recorded on five experimental days across a 1 month period, and cat 3 was tested in all four locomotor tasks. For each cat performing each walking task, 96 ± 49 (mean ± SD) strides from 39 ± 18 passages along the test corridor were included in the analyses. A total of 11,104 gaze events were observed and analysed during these strides; their numbers per cat per task are shown in Table 1.
Table 1.
The number of strides and gaze behaviours analyzed
| Behaviour | Cat no. | Flat surface in dark | Flat surface in light | Ladder | Stones |
|---|---|---|---|---|---|
| Strides | 1 | 135 | 125 | 205 | 86 |
| 2 | 113 | 184 | 49 | 0 | |
| 3 | 106 | 202 | 124 | 373 | |
| Total | 493 | 511 | 378 | 459 | |
| Gaze shifts away | 1 | 242 | 141 | 367 | 210 |
| 2 | 136 | 134 | 127 | 0 | |
| 3 | 151 | 129 | 112 | 288 | |
| Total | 529 | 404 | 606 | 498 | |
| Gaze shifts toward | 1 | 214 | 167 | 84 | 118 |
| 2 | 88 | 136 | 51 | 0 | |
| 3 | 182 | 107 | 57 | 104 | |
| Total | 484 | 410 | 192 | 222 | |
| Fixations | 1 | 119 | 143 | 792 | 324 |
| 2 | 225 | 218 | 198 | 0 | |
| 3 | 359 | 343 | 261 | 786 | |
| Total | 703 | 704 | 1251 | 1110 | |
| Constant gaze | 1 | 98 | 65 | 297 | 80 |
| 2 | 75 | 96 | 76 | 0 | |
| 3 | 167 | 161 | 132 | 457 | |
| Total | 340 | 322 | 505 | 537 | |
| Slow gaze away | 1 | 47 | 25 | 95 | 43 |
| 2 | 15 | 31 | 33 | 0 | |
| 3 | 97 | 90 | 42 | 241 | |
| Total | 159 | 146 | 170 | 284 | |
| Slow gaze toward | 1 | 113 | 104 | 312 | 72 |
| 2 | 132 | 136 | 80 | 0 | |
| 3 | 109 | 128 | 101 | 241 | |
| Total | 354 | 368 | 493 | 313 |
Walking on the flat surface in the dark
Vision was not available during this task, nor was it required for successful walking on a flat surface (Beloozerova & Sirota, 2003). In the dark, cat 1 walked at a pace of approximately 0.5 m s−1, taking 790 ± 80 ms-long strides (mean ± SD; Fig. 4A). Cat 2 was slightly faster at a pace of about 0.55 m s−1, with strides of 740 ± 40 ms duration (P < 0.0001, t test). Cat 3 was the fastest, taking 630 ± 80 ms long strides and walking at a pace of approximately 0.65 m s−1 (P < 0.0001, t test).
Figure 4. The pace of walking and frequency of gaze behaviours.
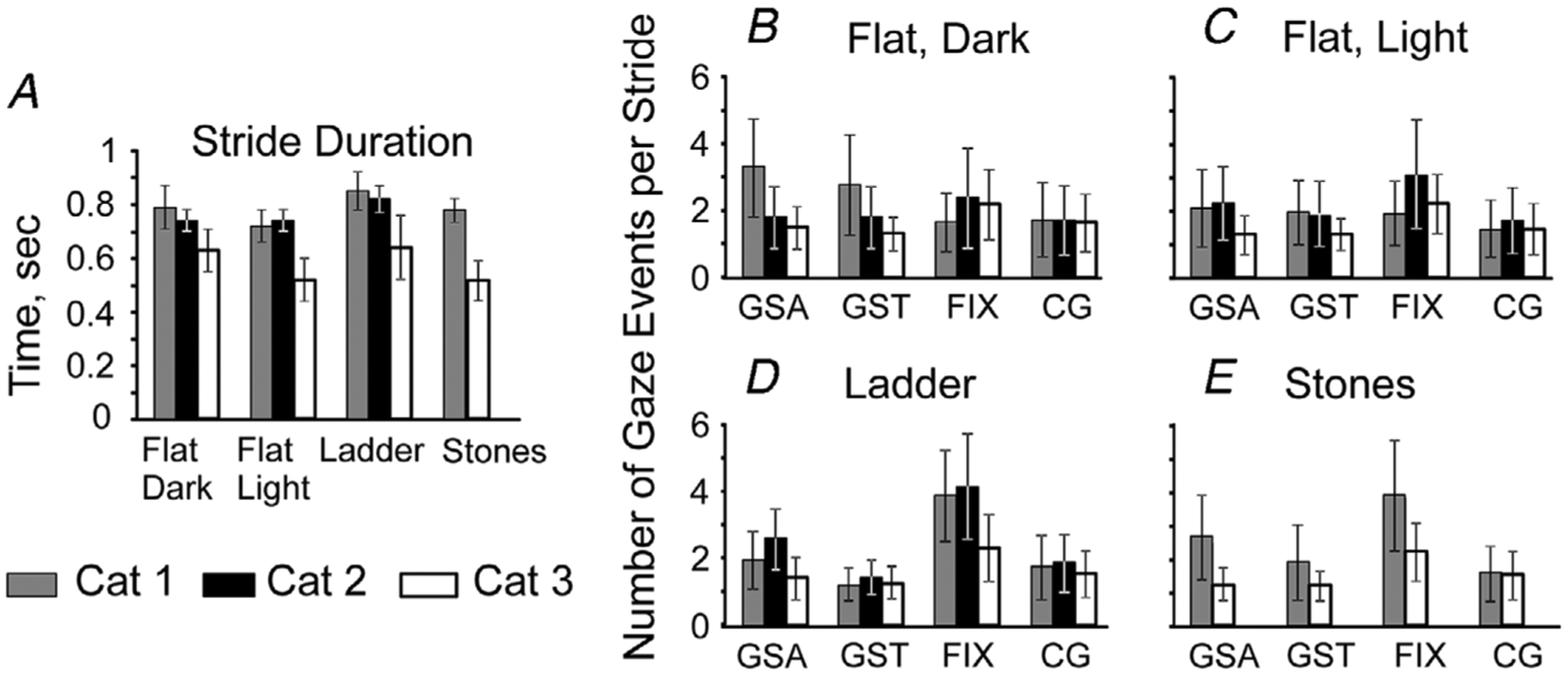
A, average stride durations for each cat performing each locomotion task. B–E, frequency of gaze behaviours per stride for each locomotor task. Only the strides during which the gaze behaviour was expressed at least once were considered. When data from two recording days were available, they were averaged between days. Error bars represent standard deviation.
As we reported previously, during walking in the darkness, cats directed their gaze at the invisible surface of the walkway 34–48% of the total walking time (Rivers et al. 2014). During strides selected for this study according to the criteria described above, cats’ behaviour varied. During some strides, cats looked at the walkway all the time, while during other strides they looked only fragmentarily, or did not look at all (Fig. 5, top row). Cats 1 and 3 behaved differently on different days, looking at the walkway more on one day and less on another. On the days when cats looked more, they looked at the walkway during nearly every stride (black and grey in Fig. 5, top row). Even on the days when cats looked less at the walkway, they still looked at it during 45–60% of strides (Fig. 6, top row). On one of the testing days, cats 2 and 3 showed a preference to look at the walkway during the swing rather than stance phase of a forelimb (P < 0.002, χ2 test), but had no preference on the other day.
Figure 5. Time spent looking at the flat surface of the walkway in the darkness (top row) and light (bottom row).
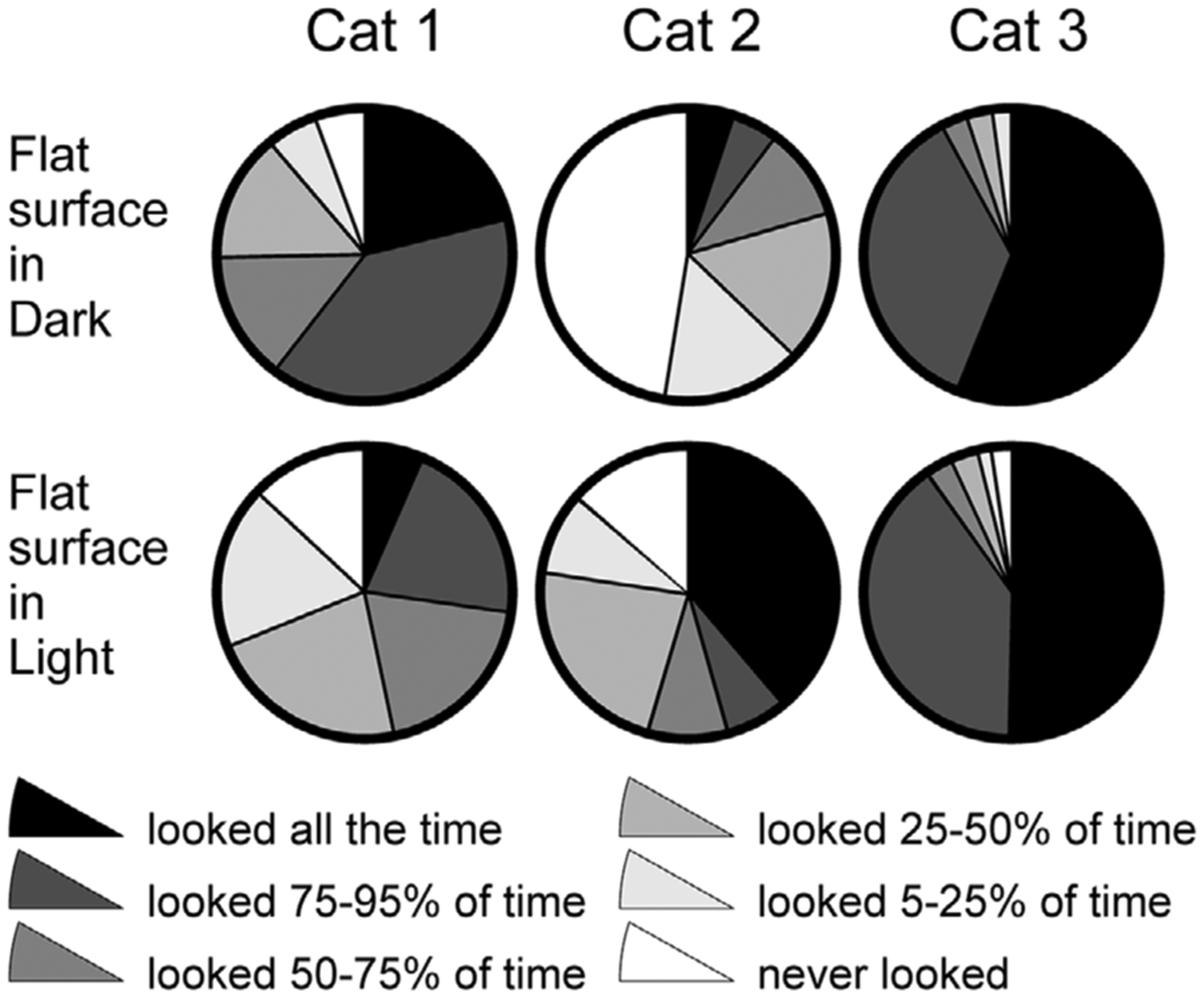
For cats 1 and 3, data from experimental days when they looked more at the walkway are shown. Data from the days when these cats looked less at the walkway are shown in Fig. 6. Black designates the percentage of strides during which cats looked at the walkway 95–100% of the stride time. Grey shades designate strides during which cats looked at the walkway for different percentages of the stride time. White designates strides during which cats did not look at the walkway.
Figure 6. Time spent looking at the flat surface of the walkway in the darkness (top row) and light (bottom row).
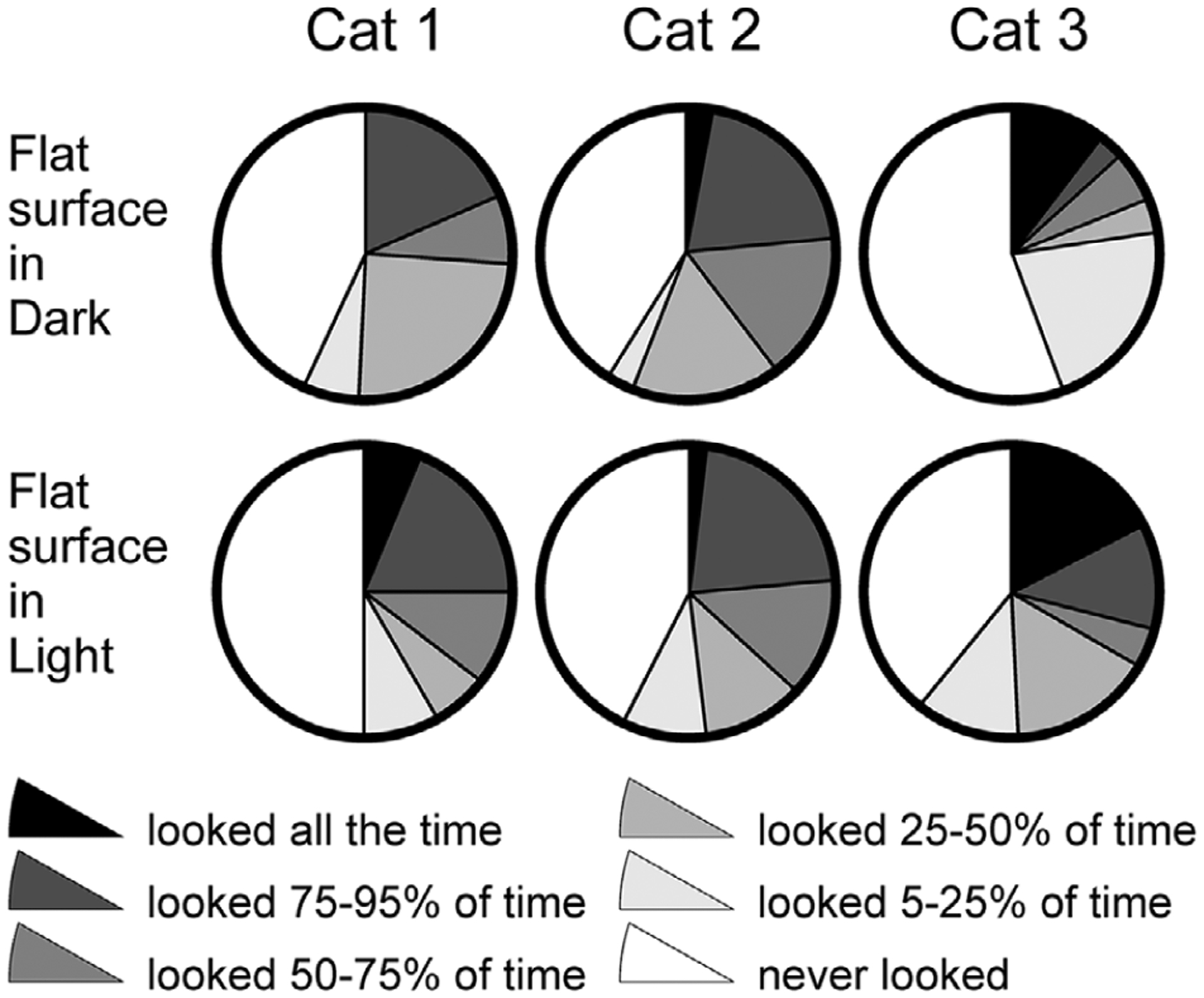
For cats 1 and 3, data from experimental days when they looked less at the walkway are shown. Data from the days when these cats looked more at the walkway are shown in Fig. 5. Black designates percentage of strides, during which cats looked at the walkway 95–100% of the stride time. Grey shades designate strides during which cats looked at the walkway for the specific proportion of the stride time. White designates strides during which cats did not look at the walkway.
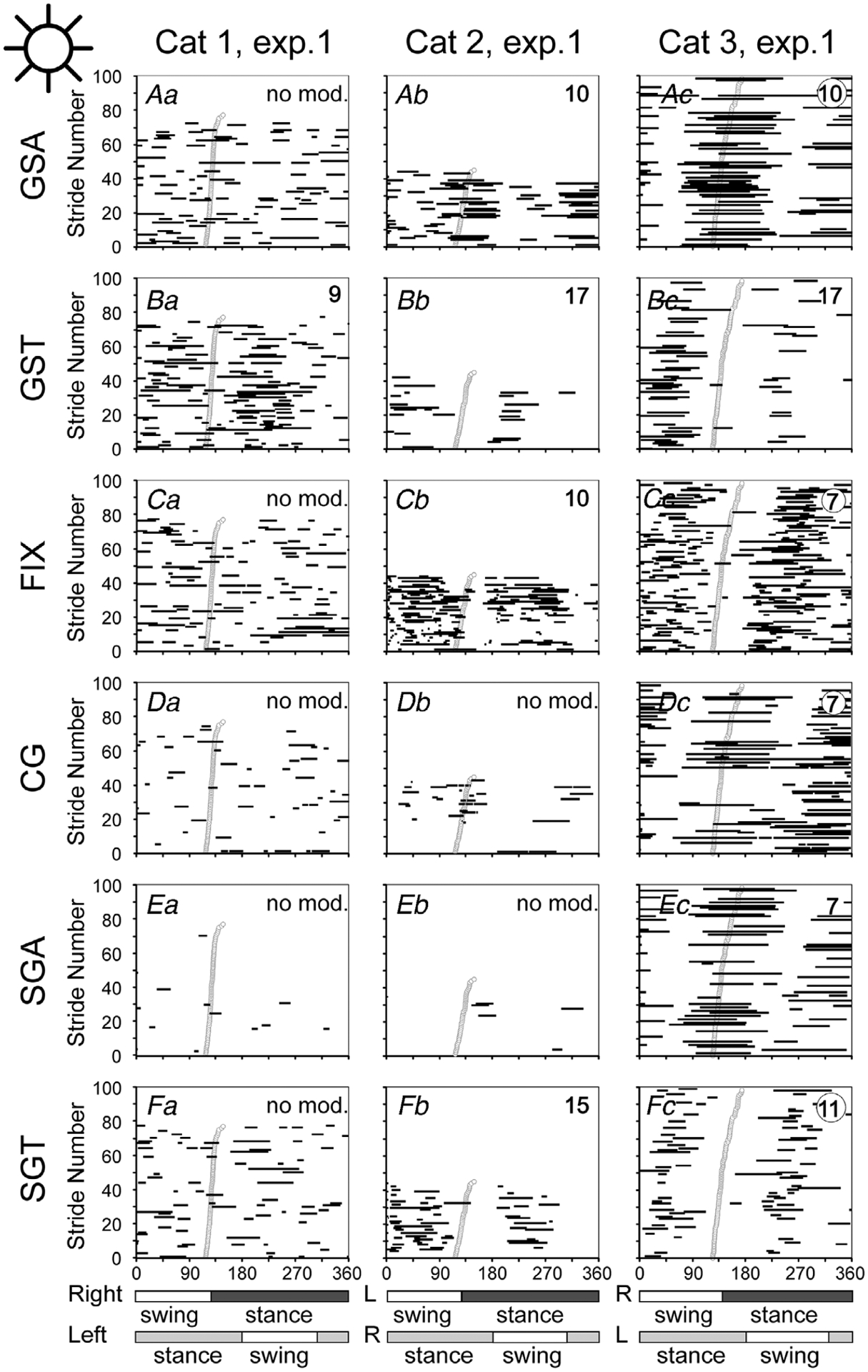
When cats looked at the walking surface, their specific gaze behaviours typically occurred during particular phases of the stride, that is, they were ‘modulated’ in the rhythm of strides. Figures 7 and 8 show occurrences of gaze behaviours in respect to the phases of the step cycle for all cats walking on the flat surface in the darkness.
Figure 7. Gaze coordination with strides during walking on a flat surface in the dark.
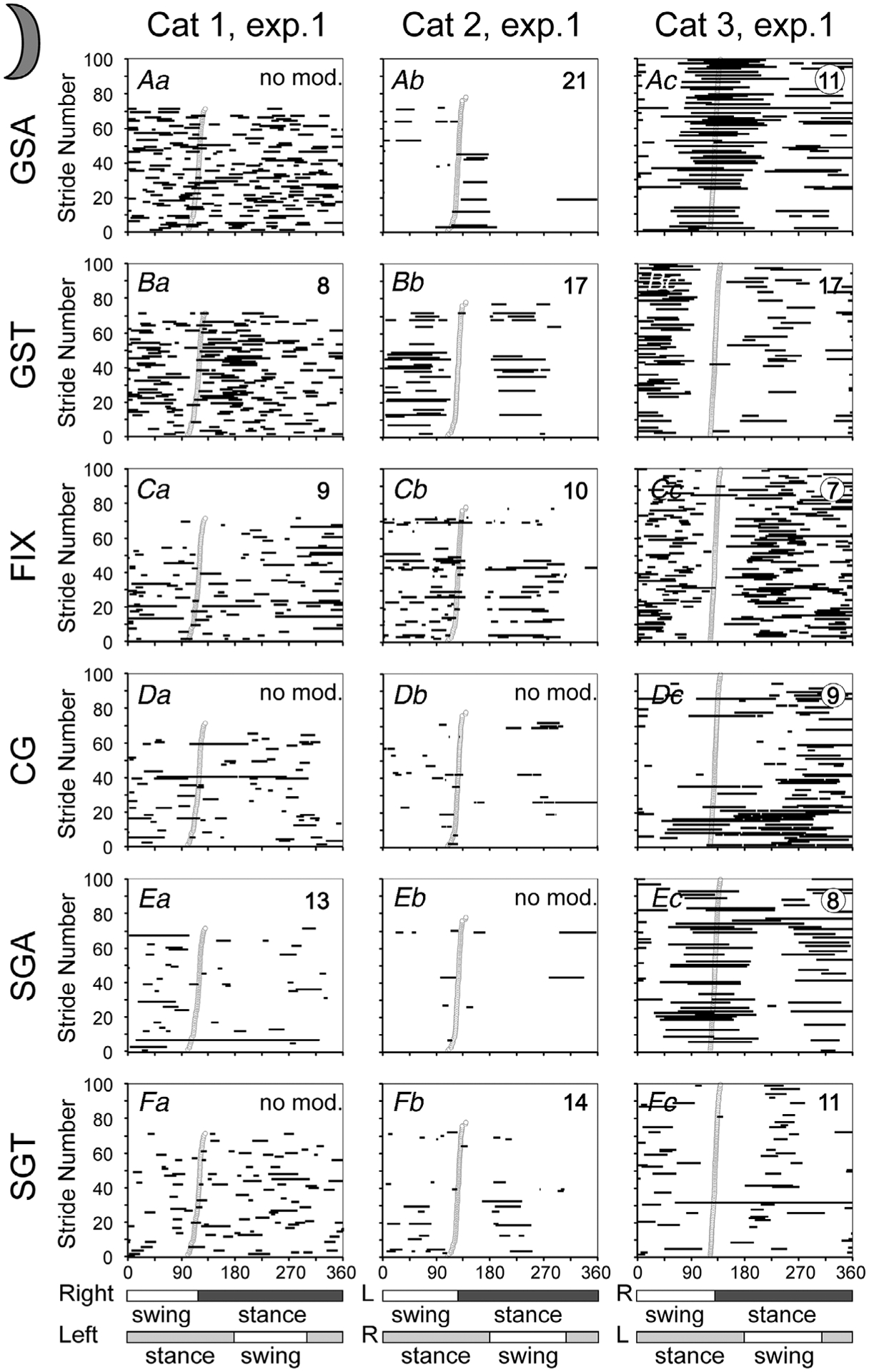
For cats 1 and 3, data from experimental days when they looked more at the walking surface are shown. Data from experimental days when these cats looked less at the walkway, as well data from the second experimental day for cat 2 are shown in Fig. 8. Aa–c, graphical representation (horizontal black lines) of the phases of gaze shifts away (GSA) during the stride. The beginning of stance during each stride is shown by an open circle. Strides are rank-ordered according to the stride duty factor; strides with smaller duty factors are plotted progressively higher in the plot. For cat 3, for which more than 100 strides were available, only 100 with duty factors in the middle of the distribution are shown in each plot. The value of the coefficient of the stride-related modulation, M, is indicated in the top right corner. Ba–c, phases of gaze shifts toward the cat (GST). Ca–c, phases of fixations (FIX). Da–c, phases of constant gaze (CG). Ea–c, phases of slow gaze away (SGA). Fa–c, phases of slow gaze toward (SGT). For cats 1 and 3, data are presented with respect to the step cycle of the right forelimb. For cat 2, data are shown with respect to the left forelimb, because the electromechanical sensor on the right forelimb was less effective during this test. Other designations as in Fig. 3D.
Figure 8. Gaze coordination with strides during walking on the flat surface in the dark.
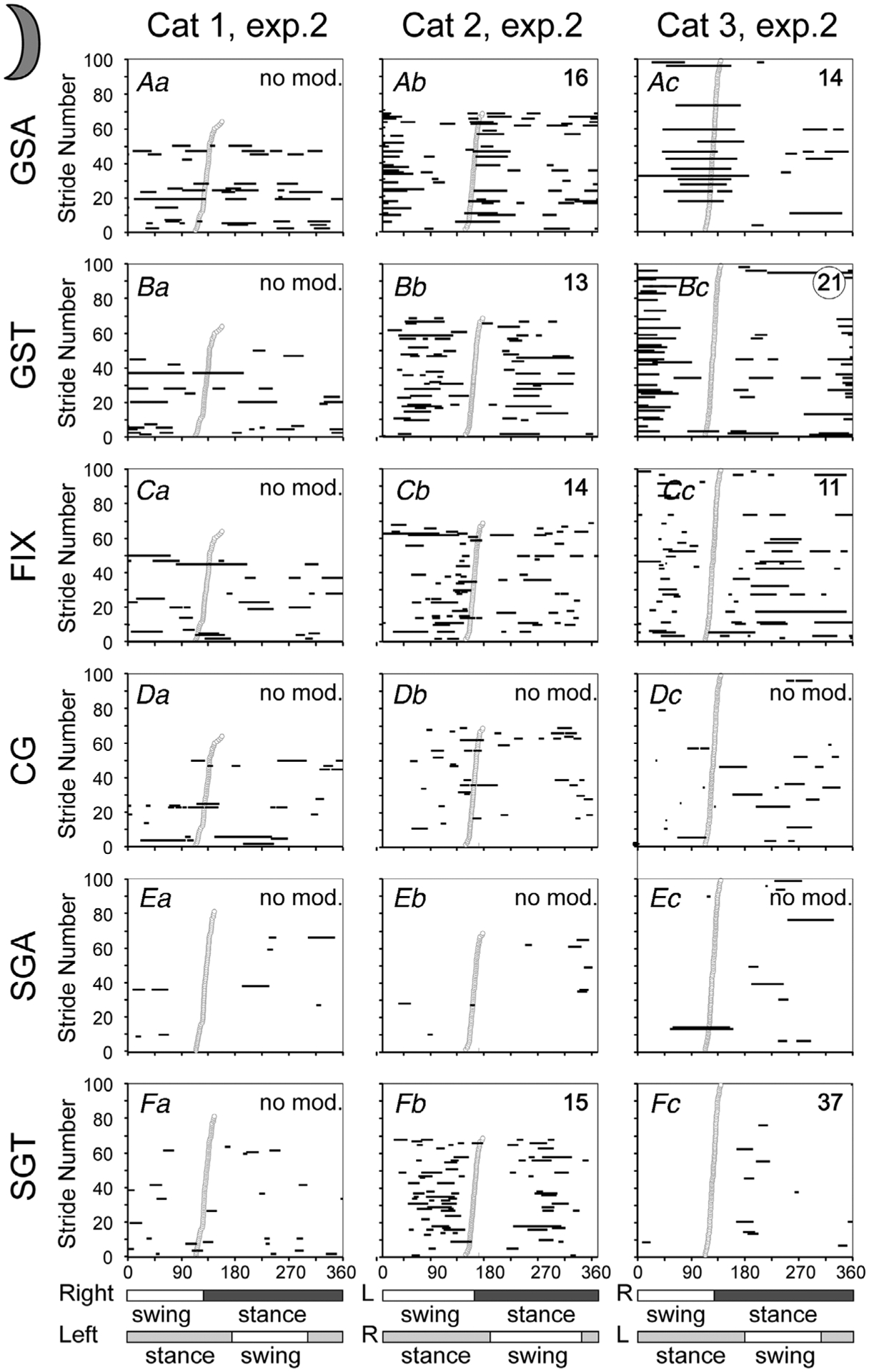
Data from experimental days when cats 1 and 3 looked less at the walkway are shown. Data from experimental days when these cats looked more at the walking surface are shown in Fig. 7. Aa–c, graphical representation (horizontal black lines) of the phases of gaze shifts away (GSA) during the stride. Strides are rank-ordered according to the duty factor, with those having a smaller factor plotted progressively higher in the plot. For Cat 3, for which more than 100 strides were available, only 100 with duty factors in the middle of the distribution are shown in each plot. Ba–c, phases of gaze shifts toward the cat (GST). Ca–c, phases of fixations (FIX). Da–c, phases of constant gaze (CG). Ea–c, phases of slow gaze away (SGA). Fa–c, phases of slow gaze toward (SGT). Other designations as in Figs 3D and 7.
Gaze shifts away (GSAs).
Cats made one to five gaze shifts away along the walking surface during many strides in the darkness (Figs 4B, 7Aa–c and 8Aa–c). Cats 1, 2 and 3 made these shifts during 25%, 50–70% and 17% of strides, producing 2.8, 1.5–2.1 and 1.4 shifts per stride on average, respectively, even on the days when cats 1 and 3 looked less at the walkway. On the days when they looked more, they made similar number of gaze shifts away during 80% and 55% of strides, respectively.
Gaze shifts away of cats 2 and 3, but not cat 1, were modulated in the rhythm of strides (P < 0.0001, χ2 test; Figs 7A–c and 8A–c). Cat 2 typically made gaze shifts away during the beginning of the stance phase of a forelimb (Figs 7Ab and 8Ab), and cat 3 made them during the transition phase from swing to stance (Figs 7Ac and 8Ac). The coefficient of modulation, M, ranged between 16 and 21 and between 11 and 14, respectively. The phases of the beginnings and ends of the periods of elevated activity, PEAs, of gaze shifts away were consistent between the days for cat 2 and were within 1/10 of the stride for cat 3. The PEAs lasted 10–15% and 28% of the stride for the two cats, respectively, and the duration was consistent between days. Cat 3 made more gaze shifts away with the right forelimb movement compared to the left, while cat 2 on one of the days made more gaze shifts away with the left limb.
Gaze shifts toward.
Cats also made one to four gaze shifts toward during many strides in the darkness (Figs 4B, 7Ba–c and 8Ba–c). Cats 1, 2 and 3 made these shifts during 25%, 40–65% and 42% of strides, producing 1.8, 1.7–1.9 and 1.3 shifts per stride on average, respectively, even on the days when cats 1 and 3 looked less at the walkway. On the days when they looked more, they made gaze shifts toward during 87% and 65% of strides, 2.4 and 1.4 shifts per stride on average, respectively.
Gaze shifts toward of all cats were modulated in the rhythm of strides (P < 0.0001, χ2 test; Figs 7Ba–c and 8Ba–c), albeit in cat 1 the modulation was observed on only one of two days. M ranged between 8 and 21. Cats typically made gaze shifts toward during the beginning-to-middle of the swing phase of each forelimb (Figs 7Ba–c and 8Ba–c), much earlier in the step cycle than gaze shifts away (Figs 7Aa–c and 8Aa–c). The PEAs lasted 10–29%, 17–24% and 11–19% of the stride for cats 1, 2 and 3, respectively. In cat 3, the PEAs ended later in the swing phase on the day when the cat looked more at the walkway (P < 0.0001, MCA and t test), while starting in the same phase. Cat 3 made more gaze shifts toward when it was moving the right forelimb compared to the left, while cats 1 and 2 made more gaze shifts toward with the left limb.
Fixations.
Even in the darkness, cats fixated their gaze on the invisible walkway 1–4 times during many strides (Figs 4B, 7Ca–c and 8Ca–c). Cats 1, 2 and 3 fixated during 23%, 47–51% and 36% of strides, making 1.7; 2.0–2.7 and 2.0 fixations per stride on average, respectively, even on the days when cats 1 and 3 looked less at the walkway. On the days when they looked more, they fixated on the walkway during 79% and 91% of strides, respectively, making a similar number of fixations per stride.
Like gaze shifts toward, gaze fixations of all cats were modulated in the rhythm of strides (P < 0.05, χ2 test; Figs 7Ca–c and 8Ca–c). The M ranged between 7 and 14. Cats 2 and 3 made most gaze fixations in the middle and end of the swing phase of a forelimb, a phase when gaze shifts toward mostly ended while gaze shifts away have not yet began. Cat 1 fixated most often during the end of the stance and beginning of swing phase, also after gaze shifts toward largely ended. Across cats, the PEAs of gaze fixations lasted 13–17% of the stride. In cats 2 and 3, they started earlier in the swing phase on the day when cats looked more at the walkway (P < 0.0001, MCA and t test), while ending in the same phase.
Constant gaze.
Constant gaze was less frequently observed compared to gaze shifts and fixations (Rivers et al. 2014); however, cats still showed it 1–4 times during a number of strides (Figs 4B, 7Da–c and 8Da–c). Cats 1, 2 and 3 had episodes of constant gaze in 21%, 26–37% and 19% of strides, 2.1, 1.6–1.9 and 1.5 per stride on average, respectively, even on the days when cats 1 and 3 looked less at the walkway. On the days when they looked more, they had approximately the same number of constant gaze episodes during 62% and 58% of strides, respectively. Episodes of constant gaze were not modulated with strides, except in cat 3 on the day when it looked more at the walkway and had many constant gaze episodes at the end of the stance phase (Fig. 7Dc).
Slow gaze away and toward.
The coordination of slow gaze away and toward with the step cycle was generally similar to that of their faster counterparts, the gaze shifts away and toward (Figs 7E and F and 8E and F). However, on some experimental days, there were not enough of these behaviours observed to allow an evaluation.
Influence of walking speed on gaze behaviour in the dark.
The proportion of all individual gaze behaviour in the total gaze except that of fixations depended on the speed of walking in the darkness (Table 2). The dependence, however, was weak. Constant gaze depended the strongest with the odds for it to occur during a stride decreasing by a factor of 0.96 [exp(slope)] for every 1 cm s−1 increase in speed (P < 0.0001, logistic regression analysis). The odds for the gaze shifts away also decreased, by a factor of 0.98. On the other hand, the odds for gaze shifts toward and slow gaze in both directions to occur increased by a factor 1.02–1.03. Other parameters of constant gaze, which were evaluated using linear regression analysis, also depended on speed: the distance from the cat where constant gaze commenced increased by 1.3 cm, its duration decreased by 5 ms, and amplitude decreased by 0.1 cm for 1 cm s−1 of speed increase. Gaze shifts and slow gaze toward also started 1.7 cm and 1.6 cm further away for every 1 cm s−1 increase in speed, respectively. Additionally, gaze shifts away and gaze fixations became 2 ms and 5 ms shorter, respectively. Statistical evaluations of trends for all behaviours are shown in Table 2.
Table 2.
Dependence of the proportion of gaze behaviours in total gaze, and start distance, duration and amplitude of gaze behaviours on the speed of walking (the slope of averaged data across cats, P < 0.05)
| Parameter | Gaze behaviour | Flat surface in dark | Flat surface in light | Ladder | Stones |
|---|---|---|---|---|---|
| Proportion in total gaze | GSA | −0.024 | — | −0.023 | −0.009 |
| GST | 0.029 | −0.010 | −0.012 | −0.035 | |
| FIX | — | 0.003 | 0.002 | −0.001 | |
| CG | −0.038 | — | 0.023 | 0.023 | |
| SGA | 0.026 | 0.018 | 0.009 | 0.023 | |
| SGT | 0.015 | 0.007 | 0.017 | −0.010 | |
| Start distance | GSA | 1.146 | 0.744 | 0.455 | 0.131 |
| GST | 1.684 † | 1.134 | 0.895 | 0.507 | |
| FIX | 0.722 | 0.676 | 0.359 | −0.037 | |
| CG | 1.300 † | 0.373 | 0.352 | −0.122 | |
| SGA | 0.639 | 0.297 | 0.694 | −0.141 | |
| SGT | 1.560 | 0.517 | 0.318 | 0.019 | |
| Duration | GSA | −0.002 | — | — | 0.001 |
| GST | −0.001 | 0.001 | — | 0.001 | |
| FIX | −0.005 | −0.001 | −0.001 | −0.001 | |
| CG | −0.005† | — | 0 | 0.002 | |
| SGA | — | — | 0 | — | |
| SGT | — | −0.001 | 0 | 0 | |
| Amplitude | GSA | 0.340 | 0.170 | 0.480 | |
| GST | — | −0.393 | 0.281 | 0.474 | |
| CG | −0.113 | 0.248 | 0.072 | 0.257 † | |
| SGA | — | 0.479 † | 0.031 | 0.419 † | |
| SGT | — | — | 0.048 | 0.065 |
The dash indicates absence of correlation.
Values for the strong (r = ±0.5–1) and moderate (r = ±0.3–0.5) correlations between the start distance, duration and amplitude and the speed of walking are shown in bold and the strong ones are shown with †.
The M values of nearly all gaze behaviours in cats 1 and 2 did not differ between fast and slow strides (P > 0.05, MCA and t test). Only gaze shifts toward and occasionally slow gaze toward were more modulated during faster strides. At variance, the gaze behaviour of cat 3 changed notably with speed. The stride-related modulation of constant gaze increased while that of gaze shifts toward and slow gaze away decreased with increasing speed (P < 0.0001, MCA and t test). In addition, constant gaze, gaze shifts away, slow gaze away and fixations occurred later in the cycle (by 15–20% of the step cycle the first two, and by 5% the latter two; P < 0.0001, MCA and t test), and gaze shifts away became more numerous. Also, during faster strides cat 3 distributed all its gaze behaviours more equally between strides of the right and left forelimbs (had less ‘limb preference’).
Walking on the flat surface in the light
During walking in the light, vision was available but not necessary for successful locomotion as on the flat surface cats walked successfully in the darkness. In the light, cat 2 walked at the same pace as in the darkness, while cats 1 and 3 walked faster (Fig. 4A, P < 0.02, t test). Cat 1 walked at 0.61 m s−1 with 720 ± 60 ms-long strides, and cat 3 walked at 0.84 m s−1 with 520 ± 80 ms-long strides.
During walking on the flat surface in the light, cats differed in the amount of time they spent looking at the walkway. Cat 1 only looked at it 22% of time, significantly less than during walking in the darkness, while cat 2 looked 62% of time, significantly more than in the darkness, and cat 3 spent the same time looking at the walkway in both conditions (Rivers et al. 2014). During strides selected for the study according to the criteria described above, similarly to walking in the darkness, cats could look at the walkway constantly, only fragmentarily, or not at all (Fig. 5, bottom row). All cats behaved differently on different days, looking at the walkway more on one day and less on the other day. On the day when they looked more, they looked at the walkway during the great majority of strides (black and grey in Fig. 5, bottom row). Even on the days when cats looked less at the walkway, they still looked at it during 50–60% of strides (Fig. 6, bottom row). Cats 1 and 3 looked equally throughout the entire stride cycle, while cat 2 looked more during the swing than stance phase of a forelimb (P > 0.002, χ2 test), but only on the day when it looked less at the walkway overall.
When cats looked at the walking surface, their gaze behaviours, as in the darkness, were modulated in the rhythm of strides. Figures 9 and 10 show gaze behaviours of all cats walking on the flat surface in the light in respect to the phases of the step cycle.
Figure 9. Gaze coordination with strides during walking on a flat surface in the light.
Data from experimental days when cats looked more at the walkway are shown. For cat 3, for which more than 100 strides were available, only 100 are shown in each plot. Data from experimental days when cats looked less at the walkway are shown in Fig. 10. Aa–c, phases of GSAs. Ba–c, phases of GSTs. Ca–c, phases of FIXs. Da–c, phases of CG. Ea–c, phases of SGAs. Fa–c, phases of SGTs. Designations as in Fig. 7.
Figure 10. Gaze coordination with strides during walking on the flat surface in the light.

Data from experimental days when cats looked less at the walkway are shown. For cat 2, for which more than 100 strides were available, only 100 are shown in each plot. Data from experimental days when cats looked more at the walkway are shown in Fig. 9. Aa–c, phases of GSAs. Ba–c, phases of GSTs. Ca–c, phases of FIXs. Da–c, phases of CG. Ea–c, phases of SGAs. Fa–c, phases of SGTs. Designations as in Figs 7 and 8.
Gaze shifts away.
Even on the days when cats looked less at the walking surface, they made one to four gaze shifts away along it during many strides (43–46% of strides) producing 1.3–2.6 shifts per stride on average; and on the days when they looked more, they made these shifts during 50–76% of strides, approximately same number per stride (Figs 4C, 9Aa–c and 10Aa–c).
As in the darkness, gaze shifts away were modulated in the rhythm of strides only in cats 2 and 3 (P < 0.0001, χ2 test; Figs 9Aa–c and 10Aa–c). M ranged between 10 and 13 and between 9 and 10, respectively, in these cats. For both, it was smaller than in the darkness (P < 0.0001, MCA and t test). The stride phases of gaze shifts away were, however, not significantly different (P > 0.05, MCA and t test; compare Figs 7Ab and c and 6Ab and c). The PEAs of gaze shifts away in cat 2 lasted 10–20% of the stride, longer on the day when the cat looked more at the walkway (P < 0.001, MCA and t test), and they lasted ~30% of the stride consistently in cat 3. For both cats, the PEA’s duration was similar to that in the darkness (P > 0.05, MCA and t test). Cat 2 did not have a limb preference for gaze shifts away, while cat 3 on one testing day, as in the darkness, made more gaze shifts away when it was about to stand on the right forelimb compared to the left. Although the modulation of gaze shifts away in cat 1 did not reach the level of statistical significance, on the day when the cat looked less at the walkway, a concentration of away gaze shifts in the early stance phase was visible in the plot (Fig. 10Aa).
Gaze shifts toward.
Cats made one to four gaze shifts toward during many strides in the light (Figs 4C, 9Ba–c and 10Ba–c). On the days when they looked less at the walkway, they made gaze shifts toward during 44%, 39% and 35% of strides, producing 2.0, 1.9 and 1.3 shifts per stride on average for cats 1, 2 and 3, respectively. On the days when they looked more, they made gaze shifts toward during 82%, 38% and 44% of strides, respectively, approximately the same number per stride.
Gaze shifts toward of all cats were modulated in the rhythm of strides (P < 0.0001, χ2 test; Figs 9Ba–c and 10Ba–c). M ranged between 9 and 17. For all cats, M was similar to that in the darkness (P > 0.05, MCA and t test), except on one day for cat 3 when M was smaller. Cats typically made gaze shifts toward during the beginning-to-middle of the swing phase of a forelimb (Fig. 9B). For cats 1 and 3 they were slightly later in the cycle compared to where these cats made gaze shifts toward in the darkness (by 5–10% of the cycle, P < 0.004, MCA and t test; compare to Fig. 7Ba and c). The PEAs of gaze shifts toward lasted 15–24%, 9–18% and 16–17% of the stride in cats 1, 2 and 3, respectively. In cat 2, the PEA was shorter on the day when the cat looked more at the walkway ending 10% of the stride earlier (P < 0.05, MCA and t test). The duration of PEAs was similar to that in the darkness in all cats (P > 0.05, MCA and t test). Cat 3 made more gaze shifts toward when it was moving the right forelimb compared to the left, similarly to its behaviour in respect to gaze shifts away, while cat 1 favoured the left limb.
Fixations.
Cats fixated gaze on the walkway 1–5 times during many strides on the flat surface in the light (Figs 4C, 9Ca–c and 10Ca–c). On the days when they looked less at the walkway, cats 1, 2 and 3 fixated during 42%, 47% and 49% of strides, producing 2.0, 2.4 and 2.3 fixations per stride on average, respectively. On the days when they looked more, cats 1 and 3 made approximately same number of fixations per stride during 71% and 91% of strides, respectively, while cat 2 made almost twice as many fixations (4.0 ± 1.6) during 84% of strides.
Like gaze shifts away, gaze fixations were modulated in the rhythm of strides only in cats 2 and 3 (P < 0.05, χ2 test; Figs 9Ca–c and 10Ca–c). M ranged between 7 and 11. For both cats, M was similar to that in the darkness (P > 0.05, MCA and t test), except for cat 2 on the day when it looked less at the walkway, when M was smaller (P < 0.0001, MCA and t test). Cats fixated gaze on the walking surface throughout the entire swing phase. PEAs of fixations partly overlapped with PEAs of gaze shifts toward (Fig. 9Bb and c). For both cats, the phases were similar to those in the darkness. In both conditions, however, the PEAs of cat 2 started earlier in the swing phase on the days when the cat looked more at the walkway (P < 0.0001, MCA and t test; compare Figs 9Cc and 10Cc). Across two cats, PEAs lasted 13–28% of the stride. This was similar to their duration in the darkness for the days when cats looked more at the surface in both conditions, but tended to be longer on the days when cats looked less (Figs 10Cb and c and 8Cb and c).
Constant gaze.
On the days when they looked less at the walkway, cats 1, 2 and 3 had constant gaze during 25%, 35% and 33% of strides, 1.3, 1.7 and 1.0 episodes per stride on average, respectively; and on the days when they looked more, they had these episodes during 42%, 44% and 70% of strides, respectively, approximately same number per stride (Figs 4C, 9Da–c and 10Da–c). As in the darkness, constant gaze episodes were not modulated with the strides, except in cat 3 on the day when it looked more at the walkway and, as in darkness, had many episodes of constant gaze at the end of the stance phase (Fig. 9Dc).
Slow gaze away and toward.
The coordination of slow gaze away and toward behaviours with the stride was generally similar to that of gaze shifts away and toward, respectively (Figs 9E and F and 10E and F). However, for slow gaze away of cats 2 and 3 there were not enough behaviours observed on one of the testing days to allow for an evaluation.
Influence of walking speed on gaze behaviour in the light.
The proportion of all individual gaze behaviour in the total gaze except that of gaze shifts away and constant gaze depended on the speed of walking in the light (Table 2, logistic regression analysis). As in the darkness, the dependence was weak. The odds for a behaviour to occur during a stride changed by a factor between 0.99 and 1.02 for every 1 cm s−1 change in walking speed. The distances from the cat where gaze shifts away and toward, and fixations started moderately correlated with speed increasing by 0.7, 1.1 and 0.7 cm for every 1 cm s−1 of speed increase, respectively. The durations of gaze behaviours were either not or weakly correlated with speed, changing at most 1 ms for 1 cm s−1 increase in speed. The amplitudes of away slow gaze and constant gaze correlated with speed showing 0.5 and 0.25 cm increase per 1 cm s−1 of speed increase, respectively. Statistical evaluations of these trends are given in Table 2 (linear regression analysis).
As in the darkness, the coordination of gaze behaviours with strides in cat 1 was not significantly different between fast and slow strides (P > 0.05, MCA and t test). In cat 2, gaze shifts toward were more modulated during faster strides on one day, and fixations were less modulated though more numerous on the other day (P < 0.0001, MCA and t test). However, all gaze behaviours of cat 3 depended on speed. The modulation of fixations was greater during faster strides, while that of all other behaviours except constant gaze was smaller (P < 0.0001, MCA and t test). In addition, all gaze behaviours except gaze shifts away occurred later in the cycle by 6–16% (P < 0.0001, MCA and t test), and slow gaze toward lasted longer (P < 0.002, MCA and t test).
Walking along the horizontal ladder
The horizontal ladder presented cats with a highly uneven but regularly structured surface, which, despite being very familiar to the cats, required that they see it for successful locomotion (Beloozerova & Sirota, 2003). Cats never missed stepping on a crosspiece; however, they walked slower on the ladder than on the flat surface in the light (Fig. 4A; P < 0.001, t test). On the ladder, cats 1, 2 and 3 took 850 ± 70, 820 ± 50 and 640 ± 120 ms-long strides, walking at a pace of 0.59, 0.62 and 0.80 m s−1, respectively.
When walking along the ladder, cats looked at it significantly more than at the flat surface. Cats 1 and 2 looked at the ladder (i.e. the plane along the top of the ladder’s crosspieces) 95–100% of the stride time during 80% of strides, and cat 3 did so during 60% of strides. Out of 378 strides analysed for all cats, there were only four, all by cat 3, during which the cat did not look at the ladder. Cats looked at the ladder equally during the swing and stance phases of the stride. Their specific gaze behaviours, however, occurred in particular phases of the stride, that is, were modulated in the rhythm of strides (Fig. 11).
Figure 11. Gaze coordination with strides during walking on the horizontal ladder.
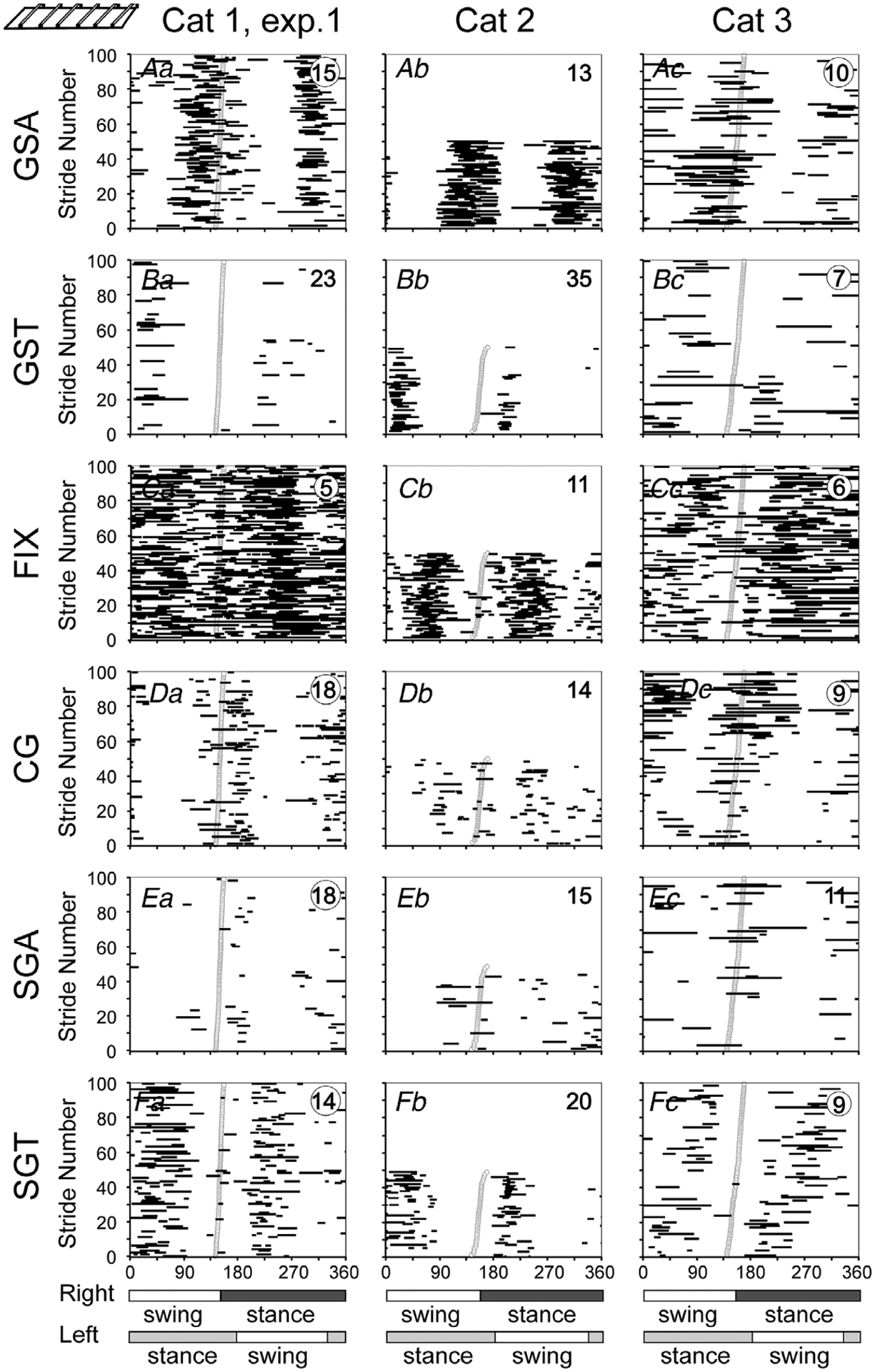
Aa–c, phases of GSAs. Ba–c, phases of GSTs. Ca–c, phases of FIXs. Da–c, phases of CG. Ea–c, phases of SGAs. Fa–c, phases of SGTs. Data are presented with respect to the step cycle of the right forelimb. Designations as in Fig. 7.
Gaze shifts away.
While walking on the ladder, cats made one to four gaze shifts away during the great majority of strides (93%, 100% and 65% of strides by cats 1, 2 and 3, respectively, Figs 4D and 11Aa–c). Gaze shifts away of all cats were modulated in the rhythm of strides (P < 0.0001, χ2 test; Fig. 11Aa–c). M for cat 1, whose gaze shifts away were not modulated on the flat surface, was as high as 15 (Fig. 11Aa). M for cat 2 was greater than on the flat surface in the light on the day when the cat looked more at the surface, but smaller than in the darkness (P < 0.0001, MCA and t test). For cat 3, the M was similar to that on the flat surface in the light, and smaller than in the darkness (P < 0.0001, MCA and t test).
Cats typically made gaze shifts away during transition from the swing to stance phase of each forelimb (Fig. 11Aa–c). The peak was at the end of the swing phase. For both cats 2 and 3, whose gaze shifts away were modulated on the flat surface, this was earlier in the cycle compared to when cats made these shifts on the flat surface by 3–14% of the stride cycle (P < 0.05, MCA and t test; compare to Fig. 9Ab and Ac). Across cats, individual PEAs of gaze shifts away lasted 15–25% of the stride, approximately the same as during walking on the flat surface. Cats 1 and 2 did not have a limb preference, while cat 3, similarly to its behaviour on the flat surface, made more gaze shifts away when it was about to stand on the right forelimb compared to the left.
Gaze shifts toward.
Cats made one to three gaze shifts toward along the ladder during many strides (30–43%, 71% and 36% of strides by cats 1, 2 and 3, respectively, Figs 4D and 11Ba–c). Gaze shifts toward of all cats were modulated with strides (P < 0.05, χ2 test; Fig. 11Ba–c). M ranged between 7 and 35. For cats 1 and 2 it was substantially greater than on the flat surface, but was smaller than on the flat surface for cat 3 (P < 0.0001, MCA and t test). Cats typically made gaze shifts toward in the beginning of the swing phase of a forelimb (Fig. 11B), the same phase as on the flat surface (P > 0.05, MCA and t test). PEAs lasted 6–10% of the stride and tended to be shorter than on the flat surface.
Fixations.
When the ladder made vision necessary for successful locomotion, fixations became the most frequent gaze behaviour of all cats. Cats fixated on the plane of the tops of the ladder’s crosspieces during nearly every stride (99%, 98% and 91% of strides for cats 1, 2 and 3, respectively; Fig. 11Ca–c). They made one to eight fixations per stride: 3.9, 4.1 and 2.3 on average, respectively (Fig. 4D). Like gaze shifts, fixations were modulated with the strides in all cats (P < 0.01, χ2 test; Fig. 11Ca–c). M for cat 1, whose fixations were not modulated on the flat surface, was 4–5 (Fig. 11Aa). M for cat 2 was greater than on the flat surface in the light (P < 0.0001, MCA and t test), but not more than in the darkness. M for cat 3 was smaller than on the flat surface in any condition (P < 0.0001, MCA and t test). Although cats could fixate on the ladder throughout the entire stride, they made most fixations during the middle of the swing phase of a forelimb after the gaze shifts toward mostly ended but before the gaze shifts away began. The PEAs lasted 9–22% of the stride in cat 1 and 12–15% in cats 2 and 3. In cat 2 they started in the same phase as on the flat surface on the day when cats looked more at it, but ended earlier (P < 0.05, MCA and t test). For both cats 2 and 3, the PEAs of fixations were shorter by approximately 3% of the cycle or 6% of the swing phase compared to their duration on the flat surface in the light (P < 0.02, MCA and t test; compare, for example, Fig. 9Cb and c with Fig. 11Cb and c).
Constant gaze.
Cats used constant gaze 1–4 times during many strides on the ladder (80%, 82% and 58% of strides by cats 1, 2 and 3, respectively, Figs 4D and 11Da–c). Unlike on the flat surface, constant gaze episodes of all cats were modulated in the rhythm of strides (P < 0.05, χ2 test; Fig. 11Da–c). M ranged between 9 and 18. Cat 1 had most constant gaze episodes in the beginning of the stance phase of a forelimb; cat 2 had them slightly later, in the middle of stance, while cat 3 had most constant gaze episodes earlier in the cycle, during the transition from the swing to stance phase. This was the same stride phase where this cat had most of its constant gaze during walking on the flat surface in the light. The PEAs of constant gaze lasted 6–10% of the stride in cats 1 and 2, and 18–24% of the stride in cat 3.
Slow gaze away and toward.
In cats 1 and 2, slow gaze away was more often observed when they walked on the ladder than on the flat surface, and it was modulated with strides in all cats (Figs 11Ea–c). M ranged between 11 and 18. In cat 1, slow gaze away typically occurred in the beginning of the stance phase, slightly later than gaze shifts away (P < 0.0001, MCA and t test; compare Fig. 11Ea and Fig. 11Aa), while in cats 2 and 3 it occurred during the same phase as gaze shifts away. Across cats, PEAs of slow gaze away lasted 7–15% of the stride.
Slow gaze toward was also modulated with strides in all cats. M for cat 1, whose slow gaze toward was not modulated on the flat surface, was 14–17 (Fig. 11Fa). M for cat 2 was greater than on the flat surface, but was smaller for cat 3 (P < 0.0001, MCA and t test). Cats used slow gaze toward during the same stride phases as gaze shifts toward. The PEAs of slow gaze toward in cats 2 and 3 were earlier in the step cycle than they were on the flat surface (by ~40 deg or 10% of the stride cycle; P < 0.0001, MCA and t test; compare Figs 11Fb and c to Figs 9Fb and c). Across cats, the PEAs lasted 6–19% of the stride, approximately as long as on the flat surface.
Influence of walking speed on gaze behaviour on the ladder.
Unlike during walking on the flat surface, on the ladder the frequency of all gaze behaviours depended on the speed of walking, although the dependence was weak (Table 2, logistic regression analysis). The odds for gaze shifts to occur during a stride decreased by a factor of 0.98–0.99 for every 1 cm s−1 change in walking speed, while those for other behaviours increased by a factor ranging between 1.002 and 1.02. The distance from the cat where gaze behaviours started increased with speed for all gaze behaviours except slow gaze toward: for every 1 cm s−1 increase in speed it increased for gaze shifts away and toward by 0.5 and 0.9 cm, respectively, and for fixations and constant gaze by 0.4 cm. The duration and amplitude of gaze behaviours were, at most, only weakly correlated with speed, with the sole exception of the constant gaze amplitude, which was, however, very small. Statistical evaluations of the trends are shown in Table 2 (linear regression analysis).
On the ladder, the now well stride-related gaze behaviours of cat 1 (Fig. 11) prominently depended on speed. Gaze shifts away were more modulated during faster strides on both days, and constant gaze and slow gaze were too on one day (P < 0.0001, MCA and t test; see Fig. 13Aa and b and Da and b), while gaze shifts toward and fixations were less modulated on one of the days (P < 0.0001, MCA and t test; Fig. 13Ba and b and Ca and b). In addition, gaze shifts away occurred later in the cycle by 2.5% (P < 0.0001, MCA and t test; Fig. 13Aa and b), and gaze shifts toward were more evenly distributed between strides of the right and left forelimb (Fig. 13Ba and b). In cat 2, gaze shifts away and constant gaze were also more modulated during faster strides (P < 0.0001, MCA and t test), while other behaviours did not depend on speed.
Cat 3 was different from other cats in that the modulation of gaze shifts away in this cat was smaller during faster strides whereas that of gaze shifts toward was greater (P < 0.0001, MCA and t test). This cat had all gaze behaviours later in the cycle during faster strides: by 5% for fixation and constant gaze and by 13–16% for gaze shifts and slow gaze (P < 0.0001, MCA and t test), and had them more equally distributed between strides of the right and left forelimb. In addition, cat 3 had more constant gaze and slow gaze away during faster strides.
Walking along the stone-cluttered pathway
Cats 1 and 3 were also tested on the pathway cluttered with many small stones. Unlike the ladder, which presented cats with a highly uneven but regularly structured and familiar surface, the stone-cluttered pathway challenged them with irregularly placed obstacles whose layouts were new every day. The stones occupied only 6.7% of the surface; however, if the cat did not see them and adjusted steps to avoid them, in 58.2% of strides it would step on a stone with one of the four paws (the estimation is detailed in Appendix). Cats, however, stepped on a stone only in 1–2% of strides. This indicates that cats did adjust strides to avoid stepping on or too near the stones. Both cats walked faster on the stony pathway than on the ladder, with cat 3 being as fast as on the flat surface (t test, Fig. 4A).
When walking along the stone-cluttered pathway cats looked at it even more than they looked at the ladder. Cat 1 looked at the pathway 95–100% of the stride time during 80% of strides, and cat 3 did so during 89–98% of strides. Out of the total 459 strides analysed for the two cats, there were only two, both by cat 1, during which the cat did not look at the pathway. Cats looked at the stony pathway equally during the swing and stance phases of the stride. Their specific gaze behaviours, however, were modulated in the rhythm of strides (Fig. 12).
Figure 12. Gaze coordination with strides during walking on the stony surface.
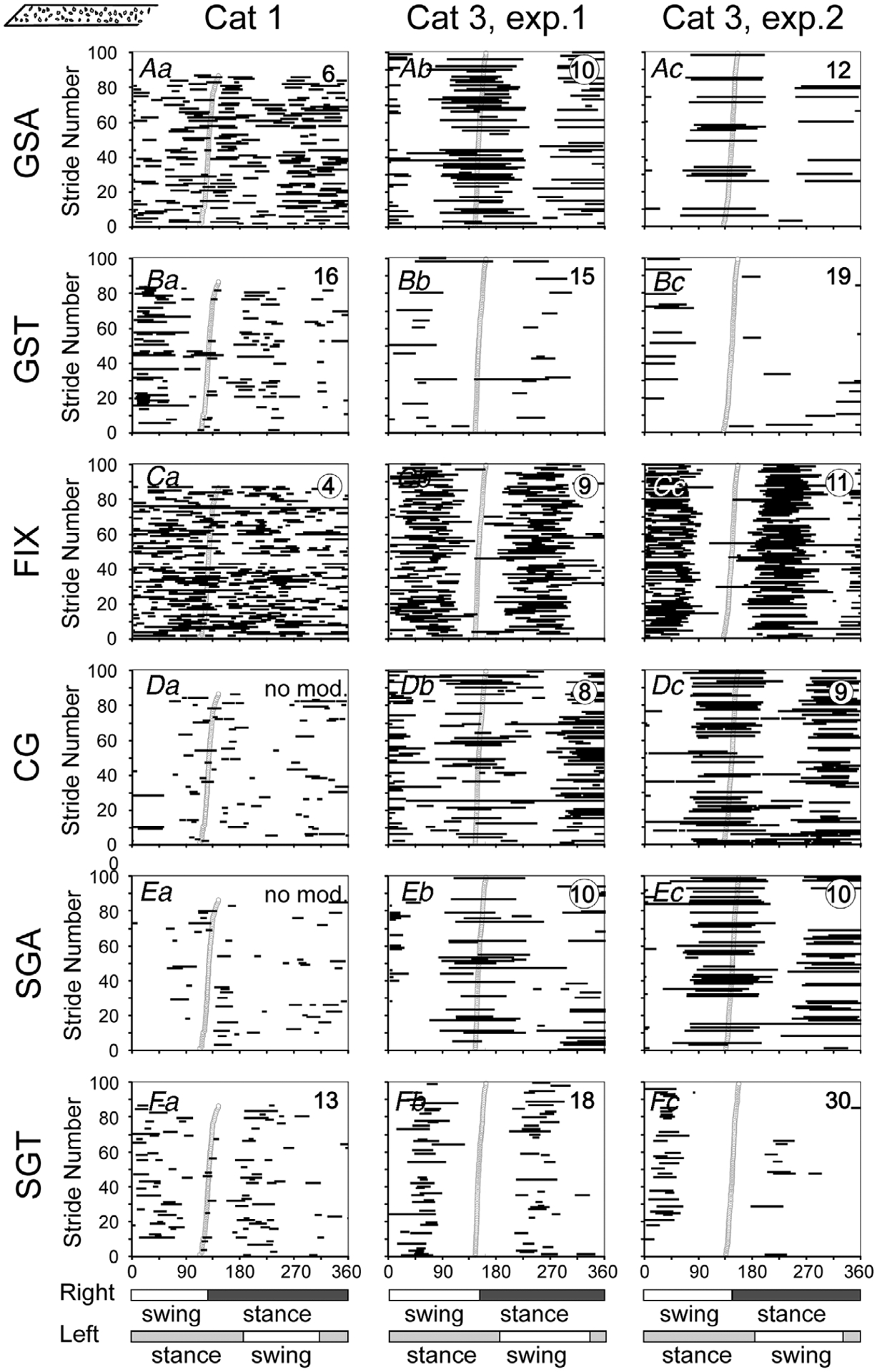
Aa–c, phases of GSAs. Ba–c, phases of GSTs. Ca–c, phases of FIXs. Da–c, phases of CG. Ea–c, phases of SGAs. Fa–c, phases of SGTs. Data are presented with respect to the step cycle of the right forelimb. Designations as in Fig. 7.
Gaze shifts away.
Cats made one to four gaze shifts away along the stone-cluttered pathway during many strides (92% and 26–76% of strides by cats 1 and 3, respectively, Figs 4E and 12Aa–c). Gaze shifts away of both cats were modulated in the rhythm of strides (P < 0.0001, χ2 test; Fig. 12Aa–c). M ranged from 6 to 12, and for both cats was greater than on the flat surface in the light (P < 0.005, MCA and t test). Compared to the ladder, M was greater for cat 3 (P < 0.04, MCA and t test), but smaller for cat 1 (P < 0.0001, MCA and t test).
Both cats made gaze shifts away during the transition from the swing to stance phase of each forelimb (Figs 12Aa–c). In cat 1, short gaze shifts away first occurred prior to the foot–ground contact and then again in the beginning of stance (Fig. 12Aa). In cat 3, longer uninterrupted gaze shifts spanned the transition from the swing to stance phase (Figs 12Ab–c). For cat 3, the PEA was a little earlier in the cycle compared to the flat surface in the light, by approximately 6% of the cycle (P < 0.002, MCA and t test; compare Figs 12Ac and 9Ac). Compared to the ladder, however, it was slightly later in the cycle for both cats (P < 0.001, MCA and t test; compare Fig. 12Aa with 11Aa and Figs 12Ab and c with 11Ac). The PEAs lasted approximately 30% of the stride, and for cat 3, this was similar to the duration seen on the flat surface (P > 0.05, MCA and t test). For both cats, however, PEAs were longer than on the ladder (P < 0.05, MCA and t test). Similar to its behaviour during all other tasks, cat 3 made more gaze shifts away with the right forelimb movement as compared to the left limb.
Gaze shifts toward.
Cats made one to three gaze shifts toward during many strides on the stone-cluttered pathway (62% and 27–59% of strides by cats 1 and 3, respectively, Figs 4E and 12Bb–c). Gaze shifts toward of both cats were modulated with the strides (P < 0.0001, χ2 test; Fig. 12Ba–c). M ranged between 15 and 19. For cat 1, M was greater than on the flat surface in both light and dark, and for cat 3 it was greater than on the flat surface in the light (P < 0.0001, MCA and t test). Compared to the ladder task, M was smaller for cat 1, but greater for cat 3 (P < 0.0001, MCA and t test). Both cats made gaze shifts toward during the beginning of the swing phase of each forelimb (Figs 12Ba–c), which was the same phase in which they made these shifts on the flat surface and ladder (P > 0.05, MCA and t test; compare to Figs 7Ba–c, 9Ba–c and 11Ba–c). The PEAs lasted 11–16% of the stride, similar to that on the flat surface, but, for cat 1, longer than on the ladder (P < 0.05, MCA and t test).
Fixations.
As during walking on the ladder, gaze fixations were the most frequent gaze behaviour of both cats on the stone-cluttered pathway. Cats fixated gaze on the walkway during nearly every stride (97% and 91–97% of strides by cats 1 and 3, respectively, Figs 12Ca–c) and made one to six fixations per stride (Fig. 4E). Like gaze shifts, gaze fixations of both cats were modulated in the rhythm of strides (P < 0.05, χ2 test; Figs 12Ca–c). In cat 1, M was small, similar to that on the ladder (P > 0.05, MCA and t test). In cat 3, M ranged between 9 and 11, and was greater than on the flat surface on the day when the cat looked more at it and greater than on the ladder (P < 0.0001, MCA and t test).
Although cat 1 fixated gaze on the stone-cluttered pathway throughout the entire stride, it made most fixations at the end of the swing phase of the right forelimb and in the beginning-through-middle of the left forelimb swing. This phase in respect to the left forelimb was similar to that observed on the ladder, but in respect to the right forelimb, it was later in the cycle by ~20% of the cycle (P < 0.0001, MCA and t test). Cat 3 made almost all fixations in the beginning-through-middle of the swing phase of each forelimb, the same phase as on the flat surface and the ladder on one of the ladder testing days (P > 0.05, MCA and t test). On the other ladder testing day, however, cat 3 made fixations ~10% earlier in the cycle (P < 0.0001, MCA and t test).The PEAs of fixations lasted 11–26% of the stride in both cats, which was similar to their duration on both the flat surface and ladder (P > 0.05, MCA and t test).
Constant gaze.
Cats 1 and 3 had constant gaze during 60% and 80% of strides, respectively, 1.6 episodes per stride on average (Figs 4E and 12Db and c). Constant gaze episodes of only cat 3 were modulated with the strides (P < 0.0001, χ2 test; Fig. 12Da–c). M was 8–9, similar to the value seen on the flat surface and ladder (P > 0.05, MCA and t test). As during walking on the ladder, constant gaze occurred primarily during the transition from the swing to the stance phase; however, the PEA was approximately 10% of the stride earlier in the cycle compared to the ladder (P < 0.0001, MCA and t test), and tended to be earlier than on the flat surface. The duration of the PEAs was 20–25% of the stride, similar to that seen on the flat surface and ladder (P > 0.05, MCA and t test).
Slow gaze away and toward.
Slow gaze away was modulated with strides also only in cat 3 (P < 0.0001, MCA and t test). M was greater than on the flat surface but smaller than on the ladder (P < 0.0001, MCA and t test). Slow gaze away typically occurred during transition from the swing to stance phase (Fig. 12Ec), the same phase as gaze shifts away and constant gaze. This was earlier in the cycle compared to the flat surface in the light (P < 0.01, MCA and t test; Fig. 9Ec), and same phase as on the ladder (P > 0.05, MCA and t test; Fig. 11Ec). Similar to walking on the flat surface, the PEAs occupied 20–30% of the stride, substantially more that on the ladder (P < 0.05, MCA and t test; compare to Fig. 11Ec). Although the modulation of slow gaze away in cat 1 did not reach the level of statistical significance, a concentration of slow gaze away episodes around the transition from the swing to stance phase was visible in the plot (Fig. 12Ea), similar to the ladder (Fig. 12Ea).
Slow gaze toward was modulated with strides in both cats (P < 0.001, MCA and t test). M ranged between 13 and 18–30 (Figs 12Fa–c). For cat 1, M was similar to that seen on the ladder (P > 0.05, MCA and t test), but was greater than on the ladder for cat 3 (P < 0.0001, MCA and t test). For cat 3, whose slow gaze toward was also modulated with strides on the flat surface, M was also greater than on the flat surface in the light on the day when the cat looked more at the surface (P < 0.007, MCA and t test). Slow gaze toward occurred during the same phase of the stride as gaze shifts toward, namely, in the beginning of the swing phase of each forelimb. This was the same phase as on the ladder, and the same phase as on the flat surface in the light for cat 3 (P > 0.05, MCA and t test). For cat 3, the PEAs of slow gaze toward tended to be shorter than on the flat surface or ladder.
Influence of walking speed on gaze behaviour on the stony pathway.
The frequency of gaze shifts and constant gaze significantly depended on the speed of walking but the dependence was weak (Table 2, logistic regression analysis). The odds for gaze shifts, fixations and slow gaze toward to occur during a stride decreased by a factor 0.97–0.99 for every 1 cm s−1 change in walking speed, while those for constant gaze and slow gaze away increased by a factor of 1.02. Unlike on the ladder, the start distance of gaze behaviours did not depend on walking speed on the stony pathway. But as on the ladder, the duration of most gaze behaviours did not correlate with speed either; only constant gaze showed a moderate correlation, increasing in duration by 2 ms per 1 cm s−1 increase in speed (Table 2). The amplitude of constant gaze and slow gaze away both strongly correlated with speed, increasing by 0.3 cm and 0.4 cm for each 1 cm s−1 increase in speed, respectively. The amplitude of gaze shifts away and slow gaze toward correlated moderately, increasing by 0.5 cm and 0.07 cm for 1 cm s−1 increase in speed, respectively. Statistical evaluations of the trends are given in Table 2, linear regression analysis).
In cat 1 gaze shifts toward, constant gaze and slow gaze away were more modulated during faster strides (P < 0.0001, MCA and t test). Gaze shifts away, however, were less modulated (P < 0.0001, MCA and t test), and fixations and slow gaze toward did not depend on speed. Gaze behaviour of cat 3 was similar to that of cat 1 in that gaze shifts toward were also more modulated during faster strides (P < 0.0001, MCA and t test; Fig. 13Bc and d). Cat 3’s behaviours were different in other aspects, however, as its gaze shifts away, rather than being less modulated, were modulated more during faster strides, while constant gaze was less modulated (P < 0.0001, MCA and t test; Fig. 13Ac and d, and Dc and d) and slow gaze away did not depend on speed. In addition, similar to walking on the ladder, fixations and constant gaze occurred later in the cycle during faster strides in this cat by 1.5% of the step cycle, and constant gaze lasted longer (P < 0.0001, MCA and t test; Fig. 13Cc and d, and Dc and d).
Figure 13. Gaze coordination with strides during slow and fast strides on complex surfaces.
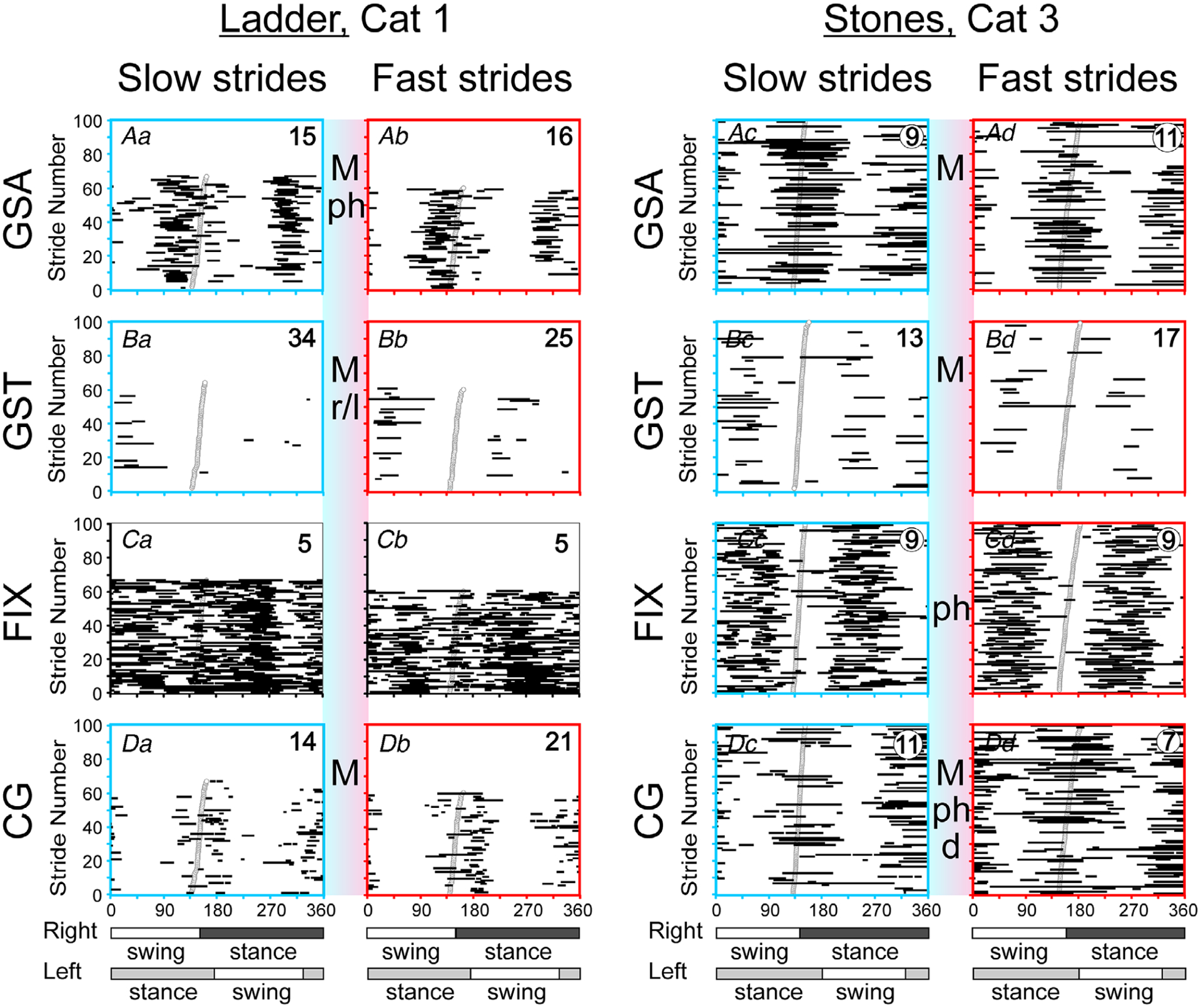
Aa–d, phases of GSAs. Ba–d, phases of GSTs. Ca–d, phases of FIXs. Da–d, phases of CGs. The left two columns show data for cat 1 walking on the ladder; the leftmost column shows data for slow strides, and the next shows data for fast strides. The right two columns show data for cat 3 walking on the stone-cluttered pathway; the left column of the two shows data for slow strides, and the right column shows data for fast strides. Definitions for slow and fast strides are provided in Methods. Data are presented with respect to the step cycle of the right forelimb. The frames of the plots for gaze behaviours that were different between the slow and fast strides in the stride-related modulation (M), phase (ph), PEA duration (d), and/or distribution between strides of the right and left forelimb (r/l) are coloured, and the parameters in which they differed are stated between the frames. Other designations as in Fig. 7.
Sequence of gaze behaviours during individual strides
Based on phase comparisons of the gaze behaviours, as described above (Figs 7–12), a general sequence of behaviours in the step cycle can be derived. This sequence is depicted in Fig. 14 for cats 1 and 2 walking on the ladder and cat 3 walking along the stone-cluttered pathway. This pattern was also evident during other tests (Figs 7–12 and 15A–D). At the beginning of the swing phase of a forelimb, the gaze shifted toward the cat both rapidly (GST) and slowly (SGT) (Fig. 14A, top panel). A third of the way into the swing phase, these behaviours peaked and were then replaced by gaze fixations, which dominated the middle of the swing phase (Fig. 14A, the second from top panel). During the last third of the swing phase, the gaze shifted away from the cat, both rapidly (GSA) and slowly (SGA) (Fig. 14A, the third from top panel). Finally, during the beginning of the stance phase (the forelimbs’ double-support period), constant gaze (CG) was the dominant behaviour (Fig. 14A, bottom panel). This cycle then repeated with the start of the other forelimb’s swing. Thus, data pooled across strides suggest that gaze shifts and slow gaze toward were followed by fixations (GST–FIX, SGT–FIX), fixations were followed by gaze shifts and slow gaze away (FIX–GSA, FIX–SGA), gaze shifts and slow gaze away in turn were followed by constant gaze (GSA–CG, SGA–CG), and, in completing the cycle, constant gaze was followed by gaze shifts and slow gaze toward (CG–GST, CG–SGT). To determine whether these general behavioural sequences also occurred during individual strides, we conducted an analysis of sequences of pairs and triplets of individual gaze behaviours.
Figure 14. Sequences of gaze behaviours.
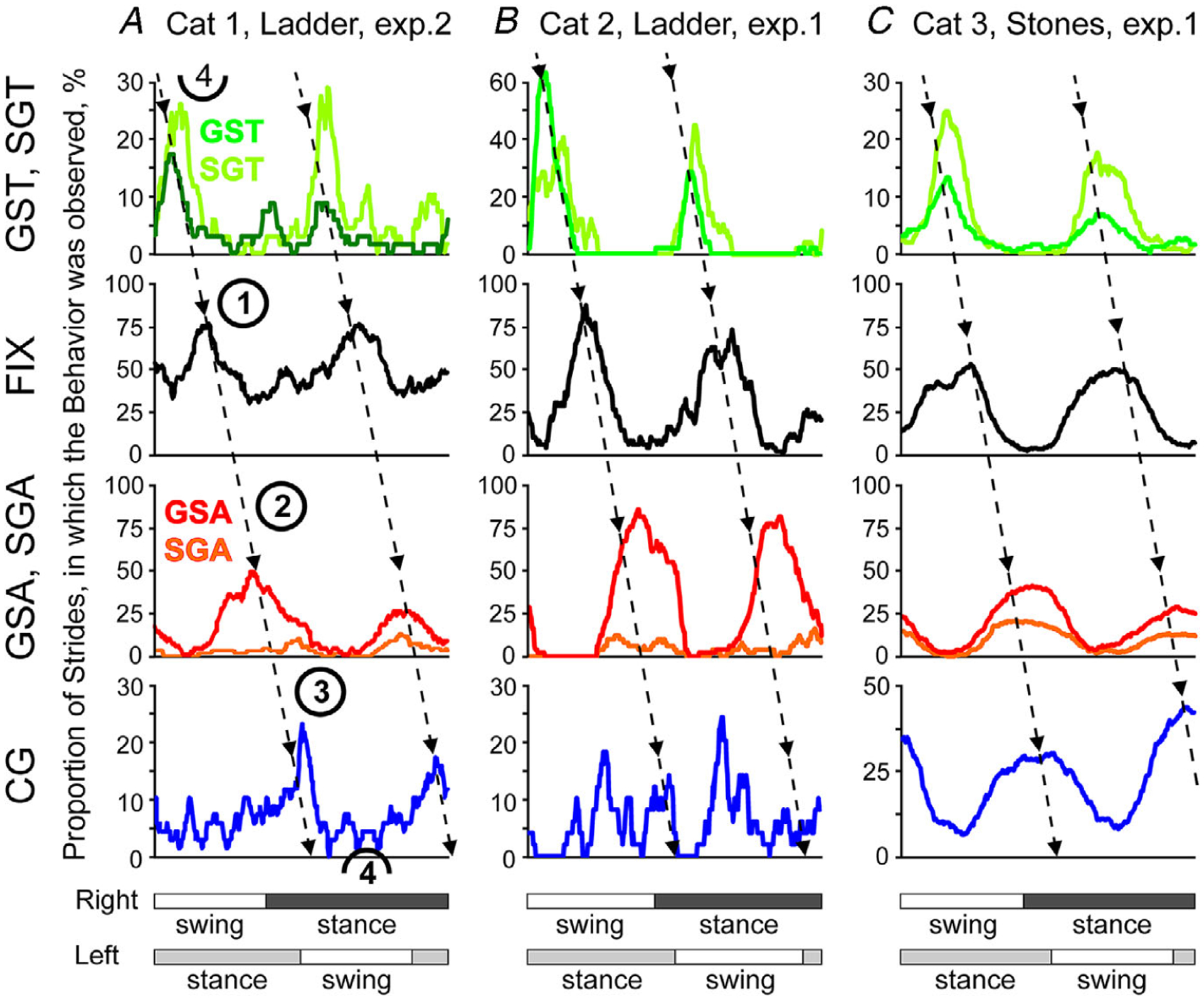
Frequency distributions of gaze behaviours in the step cycle of ladder walking in ladder experiment 2 with cat 1 (A), ladder experiment 1 with cat 2 (B), and stone-cluttered pathway experiment 1 with cat 3 (C). Arrows highlight a relative phase shift of a behaviour shown on a graph further down the panel. In column A, sequential number of a behavioural pair in respect to the beginning of the swing phase is shown in an open circle; see text for detail.
Figure 15. Relationship of gaze behaviours to the movement of the head, trunk and limbs during walking on the flat surface.
A–D, frequency distributions of gaze behaviours in the step cycle of flat surface walking in the darkness in experiment 1 with cat 3.
White and black horizontal bars below D show swing and stance phases of the right forelimb, respectively. In A and C, horizontal lines show the phases of gaze shifts during individual strides (same plots as in Fig. 7). E–G, averaged head pitch rotation (E), head vertical translation (F), and vertical trunk translation (G) during walking in the darkness. Adapted from Fig. 11C, F and I in Zubair et al. (2016). Positive peaks in the head pitch rotation and head and trunk vertical translations are indicated with vertical dashed lines. Light grey background indicates stance phase. The dark grey vertical bar highlights the difference in the average beginning of stance between these papers; this difference is due to different stride selection. H and I, swing and stance stride phases for each limb and representative cat body configurations during walking on the flat surface in the light. Adapted from Fig. 3B in Farrell et al. (2014). In H, white and black horizontal bars show swing and stance phases, respectively. Standard deviations for the time of phase transitions are indicated. Hind, hindlimb; Fore, forelimb. Limb support pattern 2–3–2–3–2–3–2–3 indicates the number of limbs in support during each stride phase; phases are indicated by numbers 1–8 and cat silhouettes in I. On cat silhouettes, the base of support is shown as a light grey area, and paw prints indicate paws on the ground that form the base of support; large black and white circle, centre of mass; black and red diamonds, extrapolated centre of mass. The animal is considered dynamically unstable if the extrapolated centre of mass is outside of the base of support, which occurs during stride phases 3 and 7.
Sequences of gaze behaviours during walking on the flat surface in the dark.
When cats walked on the flat surface in the darkness, the first pair-wise sequence, the GST–FIX sequence (Fig. 14A, top two panels), occurred more often than would be expected by chance in cats 2 and 3, but only on one of the testing days (P < 0.05, MCA). In addition, the SGT–FIX sequence occurred more often than expected in cat 2 on both days, and in cat 1 on one day. Interestingly, the ‘reversed’ FIX–SGT sequence also occurred more often than expected in cats 2 and 3 on one day, suggesting that fixations were not very stable on this day and gaze often drifted toward the cat as the cat progressed forward. Indeed, the FIX–SGT–FIX triplet was seen more often than expected by chance for cat 2 on both days and for cat 3 on one day. Fixations, however, were rarely followed by the gaze shift toward, the other possible reversed sequence.
The second sequence, the FIX–GSA sequence, or the ‘gaze stepping’ sequence (Rivers et al. 2014; Fig. 14A, middle two panels), was observed more often than expected by chance in cats 1 and 3, but only on the day when they looked less at the walkway. In addition, the FIX–SGA sequence was more frequent than expected for cat 1 on one day (P < 0.05, MCA). Combining the first two behavioural pairs, the triplet SGT–FIX–GSA was seen more often than expected by chance for cats 1 and 3 on one testing day, and the SGT–FIX–SGA sequence was more frequent for cats 1 and 2 on one day (P < 0.05, MCA). Also, the triplet GST–FIX–GSA was more frequent than expected by chance for cat 3 on one day and the triplet GST–FIX–SGA was more frequent for cat 2 on one day (P < 0.05, MCA).
The third sequences, the GSA–CG and SGA–CG sequences (Fig. 14A, bottom two panels) both occurred more often than expected by chance in cat 3 on the day when it looked less at the walkway, and the SGA–CG sequence was more frequent than expected in cat 1 on such a day. Interestingly, the reversed CG–SGA sequence also occurred more often than expected by chance in cats 2 and 3 on one day, suggesting that on that day constant gaze was not very stable and, at the end of the swing phase, the gaze often drifted away more rapidly than it should have moved to maintain constant gaze as the cat progressed forward. Combining the second and third behavioural pairs, the triplet FIX–GSA–CG was observed more often than expected for cats 1 and 3 on one testing day.
Finally, the last pair-wise sequences, the CG–GST and CG–SGT sequences (Fig. 14A, bottom and top panels), occurred at or below the chance level. On the other hand, the GSA–GST sequence, which skips constant gaze, was observed more often than expected by chance for cat 1 on both days, and for cats 2 and 3 on one day.
Sequences of gaze behaviours during walking on the flat surface in the light.
The sequences of gaze behaviours during individual strides on the flat surface in the light were largely similar to those in the dark. One notable difference was that the frequency of the first behavioural pair, the GST–FIX sequence, in cat 1 increased to above the chance level on one day, and the frequency of the SGT–FIX sequence increased in cat 3 (P < 0.05, MCA). In addition, in the light the FIX–GSA, GSA–CG and SGA–CG sequences did not occur more frequently than by chance, which they did in the darkness. However, the triplet combining the first two behavioural pairs, the SGT–FIX–GSA sequence, was still seen at above the chance level on nearly all tests and the GST–FIX–GSA sequence was more frequent than would be expected by chance on both days for cat 3 and on one day for cat 2.
Sequences of gaze behaviours during walking along the horizontal ladder.
When cats had to step accurately on crosspieces of the horizontal ladder the GST–FIX sequence was seen more often than would be expected by chance only in cat 2. However, the SGT–FIX sequence occurred more often than expected by chance in all cats (P < 0.05, MCA). Unlike on the flat surface, the reversed FIX–SGT sequence typically occurred at the chance level, suggesting that fixations, most often preceded by slow gaze toward on the ladder, were more stable than on the flat surface.
Also in contrast with what was seen on the flat surface, the FIX–GSA ‘gaze stepping’ sequence occurred more often than would be expected by chance in all cats, being indeed the most frequently occurring sequence observed on the ladder. Consequently, the SGT–FIX–GSA sequence was more frequent than expected by chance in all cats, and the GST–FIX–GSA triplet was more frequent for cat 2.
The GSA–CG and SGA–CG sequences and the CG–GST and CG–SGT sequences typically occurred at or below the chance level, just as they did on the flat surface in the light. Unlike on the flat surface, however, where the GSA-GST sequence was seen more often than expected by chance in all cats, on the ladder this sequence was seen less frequently and occurred above the chance level only in cat 2.
Sequences of gaze behaviours during walking along the stone-cluttered pathway.
When cats had to step accurately in-between many haphazardly placed small stones, the sequences of their gaze behaviours during individual strides largely resembled those seen on the flat surface. The GST–FIX sequence occurred more often than expected by chance in cat 1 and the SGT–FIX sequence was more frequent than by chance in cat 3 (P < 0.05, MCA). The reversed FIX–SGT sequence occurred more often than expected by chance in cat 3, suggesting that the fixations were unstable. Indeed, the triplet FIX–SGT–FIX was seen at above the chance level in this cat. In contrast with walking on the ladder, the FIX–GSA ‘gaze-stepping’ sequence occurred at the chance level in both cats, while the FIX–SGA sequence was above the chance level in cat 3. The triplet sequence SGT–FIX–GSA, however, was seen more often than would be expected by chance in cat 3. Similar to the ladder, the GSA-GST sequence occurred above the chance level in only one of the two cats.
Discussion
Gaze coordination with strides on the flat surface
Gaze behaviour as a part of the full body locomotor synergy.
The first finding of this study is that gaze behaviours are synchronized with strides even during walking in the darkness, when vision is not available (Figs 7 and 8). We took great care to ensure that the room was absolutely dark for cats whose sensitivity to light is greater than that of humans (detailed in Rivers et al. 2014). Although the walkway was invisible, all cats directed their gaze at it a fair amount of the time (Figs 5 and 6). Most gaze behaviours of cats 2 and 3 and some behaviours of cat 1 were modulated in the rhythm of strides (Figs 7 and 8). This indicates that a substantial coordination of gaze behaviours with stride is determined not by vision. There are three groups of other factors that can coordinate the gaze with strides during locomotion in the darkness: mechanical factors, signals from peripheral receptors other than retina, and central efference copy/corollary discharge signals (a replica of the motor command sent to the muscles, which is also sent elsewhere, is termed an ‘efference copy’ signal; von Holst & Mittelstaedt, 1950). We wish to argue that the efference copy signals from the central locomotor mechanism play the leading role in determining gaze behaviour in walking cats, and that the gaze–stride coordination arises as a part of the whole body locomotor synergy, which includes the limbs, body, head, and eyes.
Close coordination of gaze with movements of the body and limbs during a variety of everyday activities, including locomotion, has been demonstrated for humans in several studies (Imai et al. 2001; Mulavara & Bloomberg, 2002–2003; Mulavara et al. 2005; Land, 2004, 2009; Matthis & Fajen, 2013; Matthis et al. 2017). Previous studies have suggested that during walking in environments with a high demand on visual guidance of stepping, human eye movements may be programmed jointly with limb movements (Hollands et al. 1995; Hollands & Marple-Horvat, 1996, 2001). A close coupling of the body and limb locomotor movements with eye movements was also shown and investigated in detail in swimming frogs (Stehouwer, 1987; Combes et al. 2008; Lambert et al. 2012; von Uckermann et al. 2013). Experiments in the decerebrate cat revealed that the locomotion-related discharges of vestibulo-, reticulo-, and rubro-spinal neurons controlling limbs and body during locomotion are significantly determined by the efference copy signals from the spinal locomotor central pattern generator (CPG; Orlovsky, 1970, 1972a,b; reviewed in Orlovsky et al. 1999).
In our prior study of head movement in the cat, we found that head translations, rotations, as well as linear and angular velocities and accelerations are all well-coordinated with the limb’s step cycle during walking, even in the darkness (Zubair et al. 2016). A strong relationship between head movement and step cycle was also described in humans, non-human primates, and horses (Pozzo et al. 1990; Hirasaki et al. 2004; Dunbar et al. 2008; Xiang et al. 2008; Cohen et al. 2009). In cats, vertical displacements of the head partly result from vertical displacements of the trunk, which reaches its highest position in the middle of the swing phase of each forelimb (Fig. 15G), and with a lag of 20–90 deg the head is at its highest in the middle of the second half of the swing phase (Fig. 15F). Head pitch oscillations follow vertical head translations with a lag of 40–90 deg (Fig. 15E), and the head rotates maximally nose-up at the end of the swing phase of each forelimb (Zubair et al. 2016). Due to the relatively small phase lag of the pitch rotation in respect to the head vertical displacement, pitch movement of the head cannot be explained as a reflexive response of the head vertical translation. This and several other observations, which included the fact that in the decerebrate cat most vestibulo-spinal neurons greatly reduce their responses to vestibular stimulation during locomotion (Orlovsky & Pavlova, 1971; reviewed in Arshavsky et al. 1986) indicating that locomotion-related signals can block signals arriving from the vestibular endorgans much like other signals can (Roy & Cullen, 2004; Marlinski & McCrea, 2009; reviewed in, for example, Cullen, 2012), as well as the fact that the dominant frequencies of the head rotational movements during locomotion are very dissimilar from the resonant frequency of the head (Zubair et al. 2016), lead us to conclude that head rotational movements in the walking cat are not, or at least not primarily, caused by reflexive responses to linear head movements as well as not by mechanical factors. We suggested that signals from the centrally generated locomotor synergy must be the main drivers for head movements during walking (see discussion in Zubair et al. 2016 for more details). A similar conclusion was reached earlier by Cohen and colleagues (2009) regarding the head movement of quadrupedally walking cynomolgus monkeys.
With respect to the eyes, in Xenopis tadpoles and juvenile frogs locomotion-related rhythmicity can be readily observed in oculomotor nerves of an isolated spinal-brainstem preparation (Stehouwer, 1987; Combes et al. 2008; Lambert et al. 2012; von Uckermann et al. 2013). In such a preparation, there are no sensory inputs from the vestibular apparatus, body or eyes. Therefore, the spinal locomotor CPG, in addition to sending signals to the limbs and body, must be also sending signals to the eyes. This is likely to be true for higher vertebrates as well. Prominent anatomical connections exist between the cerebellum and oculomotor nuclei (Gacek, 1977; reviewed in Takahashi & Shinoda, 2018), and several lines of evidence indicate that the cerebellar output signals are heavily based on information that the cerebellum receives from the spinal locomotor CPG (reviewed in Arshavsky et al. 1986; Orlovsky et al. 1999).
In walking subjects, gaze results from a combination of body, head and eye movement. Gaze changes during walking are typically produced by a combination of simultaneous eye saccades and head movements (Guitton et al. 1984; Freedman, 2008). As data reviewed above suggest that efference copies of the spinally generated locomotion pattern influence both head and eye movement, they thus suggest that the resulting gaze behaviour is also influenced by the spinal locomotor CPG. In a recent review, Straka and colleagues (2018) discuss how the locomotor and oculomotor systems may interact. They note that in Xenopus the locomotor system explicitly controls eye movements by way of a very powerful efference copy signal, and takes over the oculomotor system to assure that the visual image is stable on the retina. Although we do not yet have comparable data on the nervous mechanism of interaction of these two motor systems in the cat, behavioural findings of this study indicatethatlocomotor influenceon theoculomotor system in the cat is not as strong.
Phase windows for gaze behaviours are determined, but their use is optional.
The central locomotor influence, if present in walking cats, appears to only offer an opportunity for a gaze behaviour to occur during a particular phase of the stride rather than directing it to occur or prohibiting it from occurring in any phase. Indeed, cats accomplished many strides in the darkness without a particular gaze behaviour, or with a behaviour occurring with the step of only one forelimb but not the other (e.g. Fig. 7Ac and Bb). Each gaze behaviour could take place during more strides on one day and fewer strides on another day (Figs 7–10), and most gaze behaviours were occasionally seen outside of their main phase.
The phases for gaze behaviours appear to be coordinated with head movement. We have previously found that when cats walk on a complex surface, 64–70% of gaze shifts toward the cat start when the head is rotating down, while most gaze shifts away are made when the head is rotating up (Rivers et al. 2010). On the flat surface as well, gaze shifts toward coincide with the head rotation nose-down (Fig. 15A and E), and gaze shifts away coincide with the head rotation nose-up (Fig. 15C and E). However, while the starts of gaze shifts can be explained by the head vertical translation and rotation, their ends cannot as both the toward and away gaze shifts typically continue past the times of the head movement assisting them (Fig. 15A, C and E). Therefore, eye movement must be responsible for the gaze’s continuous shifting during these periods, and we believe that it is the signal from the central locomotor mechanism that encourages eyes to move. During fast voluntary coordinated movement of the head and eyes to a visual target, activation of eye and neck muscles on the same side of the body is nearly synchronous (Bizzi et al. 1971; Vidal et al. 1982; Guitton et al. 1990), and Bizzi and colleagues (1971) suggested that a parallel central motor command activates both groups of muscles. During locomotion such command may come from the spinal locomotor CPG. On the other hand, during non-express saccades, gaze shifts to known targets, and in several other circumstances, activation of the eye and neck musculature occurs separately (e.g. Blakemore & Donaghy, 1980; Guitton et al. 1984; Corneil et al. 2004, reviewed in Freedman, 2008), indicating that a neural mechanism exists to allow the eyes to perform a gaze shift even when the head and body are not assisting.
Like humans, cats have unstable phases in the locomotor cycle when their projected centre of mass moves outside of the base of support and the body is falling forward (phases 3 and 7 in Fig. 15H and I; Farrell et al. 2014, 2015). This sagittal instability apparently helps to propel the body forward by exploiting the body’s inertia in a manner analogous to humans (Winter, 1995; Zijlstra & Hof, 2003). Cats differ from humans in what they do with their gaze during these unstable stride phases. Rather than fixating on where the immediate next foothold will occur as people do (Matthis et al. 2017; Barton et al. 2017), cats throw their gaze forward (Fig. 15C). When the forelimb catches the body, the gaze continues shifting away and also travels forward in the constant gaze (Fig. 15C and D) during now the most stable phase of the stride (phases 4 and 8; Farrell et al. 2014, 2015). When the swing of the other forelimb begins, the gaze shifts back toward the cat and only then fixates on a site (Fig. 15A and B; Rivers et al. 2014). This difference in the gaze strategy is likely related to the difference in the body biomechanics between cats and humans, and we discuss it shortly below.
The utility of locomotor control as a suggestive influence over gaze appears to be obvious. It should be useful for the acting in concert visuo-locomotor system to set standard times at which visual information needed for locomotion arrives to the network, as well as indicate times during which it would be safe to not have visual information. By the presumed efference copy signal, the central locomotor mechanism thus opens a phase ‘window’ for gaze fixations (intermingled with gaze shifts toward) in the beginning and middle of each forelimb’s swing to obtain visual information. The window for gaze shifts away (intermingled with the constant gaze) occurs in the end of swing and beginning of stance of each forelimb, indicating that it is safe to move gaze to a new location during this time without hampering behaviour (visual sampling is suppressed during gaze shifts; Wurtz, 2008). Thus, there are four periods during the step cycle when visual information can be potentially obtained: one in the middle of the swing phase of each forelimb, when it can be obtained using gaze fixations (phases 1 and 5 in Fig. 15H and I), and one during each double-stance phase of the forelimbs (phases 4 and 8 in Fig. 15H and I), when it can be obtained using constant gaze. The stride phases 2–3 and 6–7 are the phases when the gaze is shifted forward. It is important that such gaze–stride coordination is maintained even in conditions in which visual control of locomotion cannot be used such as during walking in the darkness. This way the system is always ready to process visual information as soon as it becomes available.
Light and walking speed have small effect on the gaze behaviour on the flat surface; the robustness of the phase windows for gaze behaviours.
The central locomotor mechanism appears to continue setting the phases for gaze behaviours when animals walk on the flat surface in the light. For all cats, the coordination of all gaze behaviours with the step cycle was quite similar to that observed in the darkness (compare Figs 7 and 9). The only difference was that the stride-related modulation of gaze shifts away, and occasionally gaze shifts toward and fixations was smaller (e.g. Figs 7Ab and c, and 9Ab and c), and gaze shifts toward could start slightly later in the swing phase (Figs 7Bc and 9Bc). Cats still followed the pattern for the gaze coordination with strides set in the darkness, although not as accurately. We have also previously found that when walking in the light cats make relatively more gaze shifts away than in the darkness (Rivers et al. 2014). Thus, cats could select how they use the pattern by selecting how often they use a particular behaviour. The slight delay in the phase of gaze shifts toward in cat 3 likely resulted from this cat walking faster in the light and perhaps paying more attention to the flat walking surface compared to other cats, and below we discuss how this could have affected the phase. Overall, it is remarkable how well the pattern of the gaze coordination with strides was preserved in the light. It must be important to have it, albeit in a slightly ‘blurred’ form, even in the condition when visual control of locomotion is not required. This way the system is always ready to process visual information as soon as any obstacles appear on the pathway.
The robustness of the gaze pattern and the strictness of the phase windows for individual gaze behaviours were also evidenced by a rather small influence that a change in walking speed had on the gaze–stride coordination. The only effects of speed observed in cats 1 and 2, both in the darkness and light, were that gaze shifts toward were more modulated with faster strides and slow gaze toward occasionally was too, while fixations were occasionally less modulated but more numerous. There was no change in the phase of gaze behaviours despite an 8–25% change in the stride duration. A substantial effect that the speed had on the gaze behaviour of cat 3 can be probably explained by the fact that cat 3 looked more at the surface (Fig. 5), and perhaps looked more intensively at small objects that are always present on the surface such as small pieces of dirt than other cats did, thus effectively treating the flat surface as a complex one. In the next section we discuss why gaze behaviours may be slightly delayed when the cat walks faster on a complex surface.
When walking faster, cats used constant gaze slightly more, looked further away and had longer gaze shifts away and fixations (Table 2). However, the basic pattern of coordination of gaze behaviours with the phases of the stride was unaltered in all cats. Even the phase shifts observed in cat 3 were confined to 5–20% of the step cycle. The robustness of the phases of gaze behaviours with changing speed can be indeed useful because it assures that the gaze behaviours stay in the proper phases of the stride regardless of speed and visual information always arriving at just the right time.
Albeit small, the effects of light and walking speed demonstrate that the gaze behaviours during locomotion can be adjusted by external conditions and cat behaviour; however, they also reveal how strict the limits for the adjustments are in regard to the phase of the step cycle. Walking on the flat surface is not controlled by vision on a stride-by-stride basis, however, and therefore it is impossible to understand why particular stride phases are designated for particular gaze behaviours when considering flat surface walking. It is during walking on complex surfaces, when vision is used for guiding strides, that we can hope to understand the function of individual gaze behaviours, and the significance of whether they occur during particular phases of the stride.
Gaze coordination with strides increases on complex surfaces
We have examined cat gaze behaviour on two complex surfaces: a regularly structured and familiar surface of a horizontal ladder and a surface cluttered with many small haphazardly placed stones. Both tasks required vision. For the ladder, we have previously shown that vision is needed by showing that cats are unable to walk on the ladder in complete darkness (Beloozerova & Sirota, 2003). We tested cats that were very well trained for walking on the ladder in the light by having completed thousands of passages prior to testing, and were also accustomed to short periods of darkness while on the ladder. We found that when the lights were turned off for 3–5 s while the cat was on the ladder, cats typically immediately stopped and proceeded only when the lights were brought back on. We concluded that cats needed to see the ladder to step successfully on the crosspieces, and that visual information that guides steps on the ladder is acquired and used on a step-by-step basis. For walking on the stone-cluttered pathway, calculations showed that if cats would not adjust strides, they would have hit the stones more frequently than they did (Appendix). Since vision is the only sense that cats could have used to learn about locations of the stones, we concluded that they must have used vision to guide steps on the stony pathway.
The pattern of gaze behaviours seen on the flat surface in the darkness is maintained on complex surfaces.
While walking on either complex surface, cats almost constantly looked at the surface. Their gaze behaviours followed the same pattern as during walking on the flat surface, and the stride-related modulation in most instances was greater than on the flat surface in the light (Figs 11 and 12). This indicates that when the environment presents visual objects, consideration of which is important for successful locomotion, cats adjust their gaze behaviour in a very specific manner. They follow the gaze behaviour pattern set on the flat surface in the darkness with a greater accuracy (a greater M) than on the flat surface in the light. Cats did adjust the timing of the gaze behaviours slightly by making gaze shifts away slightly earlier in the cycle on both complex surfaces (by 3–14% of the stride; Fig. 13Aa and b), fixating slightly earlier while on the ladder (by 1.3–5% of the stride; Fig. 13Cc and d), and engaging constant gaze slightly earlier on the stony pathway (by 1.7–5.6% of the stride; Fig. 13Dc and d). These earlier engagements of gaze behaviours may be explained by the need to be more proactive with the gaze on a complex surface to detect important objects earlier. However, the fact that only small changes in the phases of any gaze behaviours were observed shows that the phase windows for behaviours set in the darkness are rather strict. The fact that the timing of fixations was adjusted during walking on the ladder, while the timing of constant gaze was adjusted on the stony pathway points to potentially different roles of these gaze behaviours for successful walking on different surfaces.
During walking on the ladder, in addition to typically increasing the stride-related modulation, gaze fixations were shorter by approximately 6% of the swing phase, and gaze shifts toward the cat also tended to be shorter (Figs 9 and 11). Of two types of adaptations to locomotion on the ladder, an increase in the stride-related modulation of the activity and shortening of the period of the activity, both are common for neurons in brain areas that are concerned with visuo-motor processing during locomotion such as motor and somatosensory cortex, and ventro-lateral thalamus (e.g. Beloozerova et al. 2010; Marlinski et al. 2012a,b; Stout & Beloozerova, 2013; Favorov et al. 2015). It should be noted that the cats used for the present study of the gaze–stride coordination were also used among other subjects for the above five cited studies on the activity of neurons in the cortex and thalamus. It is possible that some of the neurons, the activity of which during locomotion is described in those studies, were influencing the movement of the head and/or the eyes.
The biomechanics of locomotion shape the pattern of gaze behaviours.
Why are particular gaze behaviours used during particular phases of the stride? For humans, it was suggested that biomechanics of the body and limbs determine the critical phase for visual control of stepping on complex terrains (Matthis & Fajen, 2013, Matthis et al. 2015, 2017; reviewed in Barton et al. 2017; see also Matthis et al. 2018). This phase for humans is the end of stance when the leg pushes off; the characteristics of the push significantly determine the next landing site for this foot. It is believed that the push off is guided by concurrently obtained visual information from the intended landing site. Indeed, when vision was briefly denied during the end of stance, the stance was often prolonged (Hollands & Marple-Horvat, 1996). In our previous experiments with cats, we have found, however, that a denial of visual information during the stance phase of ladder locomotion has little effect on performance, but a denial during the swing phase has a significant effect (Nguyen et al. 2019). We removed light for 200–600 ms during different phases of the stride while cats walked on the ladder and found that if the darkness fell on the swing phase of a forelimb, the following stance of this limb became longer.
We believe that in cats too ‘the biomechanics of walking shape the use of visual information during locomotion over complex terrain’ as Matthis and colleagues (2015) suggested for humans. Cat body biomechanics, however, are different from those of humans. Supported by four legs rather than two, the cat’s body is more stable during walking than that of humans. This offers cats an opportunity to be less concerned with the maintenance of stability during walking by meticulously planning the coming step at the push off, but instead look further along the pathway and plan steps in advance with a greater efficacy in respect to other aspects.
When walking on complex surfaces, cats look on average 75 cm ahead (Fowler & Sherk, 2003; Wilkinson & Sherk, 2005; Rivers et al. 2014). On the ladder with crosspieces spaced 25 cm apart, this is the future landing site of the forelimb that is about to take off to swing onto its preceding target (Fig. 16). If lights would go off, the swing that is about to take the limb to crosspiece no. 5 would not be affected and thus must be already planned. The next swing to crosspiece no. 7, however, would be delayed by a prolongation of stance on crosspiece no. 5. This indicates that fixation of gaze on crosspiece no. 7 while transferring the forelimb to its preceding target, crosspiece no. 5, does in fact gather visual information about that distant crosspiece no. 7. This information will not be used until later, however, not until after this limb completes the transfer to crosspiece no. 5, and also the other forelimb completes its transfer to crosspiece no. 6, as well as the hindlimbs perform their steps. Until then, which is about 0.7 s into the future, visual information about crosspiece no. 7 for the right forelimb transfer will be stored in working memory.
Figure 16. Schematic illustration of gaze movement during first half of the swing phase of a forelimb.
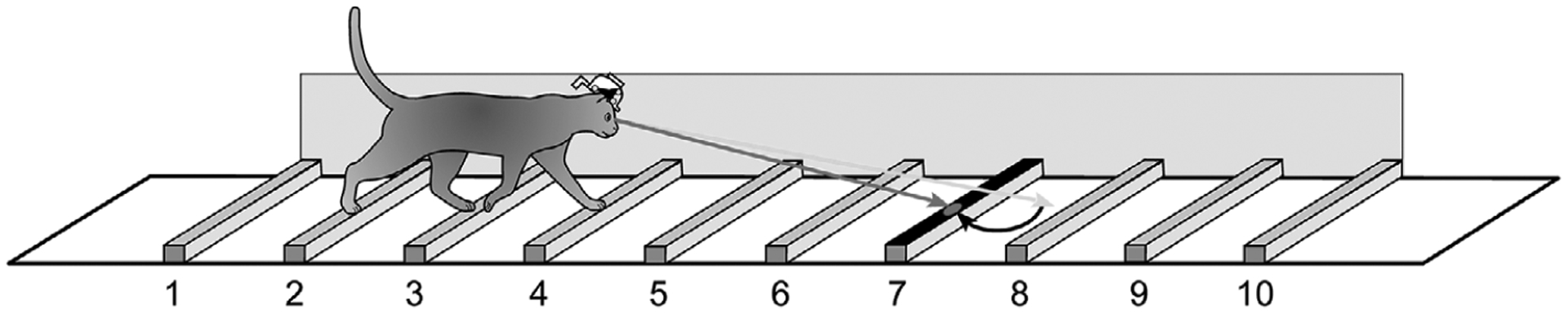
The crosspieces of the ladder are numbered in the sequence in which the cat encounters them. The green arrow shows a typical direction of the gaze at the start of the swing phase. The red arrow shows at which crosspiece the cat would usually look in the middle of the swing phase. This crosspiece is highlighted with its top shown in black.
We want to suggest that such proactive sampling of visual information may allow cats to better plan the path on a complex terrain. For example, in our prior study we found that cats always avoid single small obstacles by walking around them rather than by stepping over, an approach that preserves the locomotor rhythm (Chu et al. 2017). If such preservation is impossible in a constrained space, cats prefer to adjust movement of one limb only, a strategy that minimizes disturbance of the whole body locomotor synergy. In addition, cats prefer to deal with different aspects of the stride’s adaptation such as the adaptation of the stride’s length, height and duration separately by distributing different adjustments between successive strides (Chu et al. 2017). To implement any of these strategies, information on the path further ahead than the landing side for the limb that is about to lift is required. Cats appear to be taking advantage of the significant stability of their four-legged body by planning further ahead while assuming a risk that the memory will fail them as they can afford to be less accurate with limb placement than humans.
It appears that the most straightforward approach by which cats could fixate gaze on an object during the mid-swing of a forelimb would be to shift the gaze forward to the object in the beginning of the swing phase, fixate on the object during the middle of the phase, and then shift gaze forward again at the beginning of the swing of the other forelimb. Indeed, in our first study of gaze behaviour during walking in the cat (Rivers et al. 2014), we observed that on complex surfaces cats use a ‘gaze stepping’ pattern, in which fixations and gaze shifts away follow each other sequentially. This observation was not formally investigated in that earlier study, however. Here, we have shown that on the ladder gaze stepping is in fact a statistically significantly more frequent behaviour than would be expected if gaze shifts away and fixations were independent of each other. We also found that gaze stepping occurs also on the flat surface, including in the darkness. Moreover, the analysis revealed that gaze stepping can have an interesting fine structure.
The gaze stepping strategy applied straightforwardly requires acquisition of some visual information about the future fixation target while fixating on a previous target, since visual perception is suppressed during gaze shifts. Such acquisition could be accomplished using peripheral vision. Although feasible and probably often used, this is not the strategy that cats used at all times. They also often used a finer approach that can be termed a ‘fisherman’ or ‘netting’ strategy. In this strategy, the gaze is thrown forward as a fisherman would cast a net, but instead of immediately fixating on a target as strictly applied gaze stepping dictates, the gaze continues floating forward in constant gaze, another gaze behaviour during which visual information can be effectively obtained (Gibson, 1958; Lee, 1980). Only after a while does the gaze start shifting and dragging back to the cat ‘netting’ objects for fixation, objects that the cat already had a chance to observe during constant gaze. Consequently, for a gaze fixation to occur in the middle of the swing phase, the drag has to take place before it in the beginning of the phase, and casting the net has to occur before, in the end of the preceding stance of that limb, the forelimb’s double support phase.
Surface features and walking speed have limited effect on the pattern of gaze behaviours on the complex surface; the limited flexibility of the phase windows for gaze behaviours.
The two complex surfaces that we have used, the horizontal ladder and the stone-cluttered pathway, differed in several aspects: the regularity of the objects, the familiarity of the objects’ layout, the need to adjust only the length of the stride on the ladder (Beloozerova et al. 2010) or also the height and direction of the strides on the stony pathway (Chu et al. 2017). In addition, these surfaces differed in what cats had to do with the objects: to step on them on the ladder or to avoid them on the stony pathway. Despite being so different in their demands on the animal, the ladder and stone-cluttered pathway had rather similar effects on the gaze coordination with strides. Neither of them altered the basic pattern of the coordination seen on the flat surface, and the main effect for both was that the strength of the coordination in most instances increased. While some gaze behaviours were seen slightly earlier in the cycle during both tasks (by 1.3–14% of the step cycle), all gaze behaviours stayed within the same general stride phase and in the same phase relationship to each other. This similarity of the gaze adjustments to different visuo-motor demands indicates that it is critically important that any of the gaze adjustments are accomplished within very precise phase limits of the stride. The significant similarity of gaze behaviours on very different surfaces supports the suggestion that the basic pattern of the coordination of gaze behaviours with strides is set not by the visual environment encountered by the cat, or a particular visuo-motor task, but by the basic locomotor rhythm governing the whole body locomotor synergy. We want to suggest that these phase limits as well as the necessity for the gaze behaviours to be more focused within them for successful locomotion on the complex terrain are dictated by the way visual information for guiding steps is processed through the vision-to-motor network of the brain.
The robustness of the gaze pattern on the complex surface, and the strictness of the phase windows for individual gaze behaviours were also evidenced by a rather small influence that a change in walking speed had on the gaze–stride coordination on the complex surface. The main effect was that many gaze behaviours were more modulated during faster strides. This is quite understandable because a better coordination of gaze behaviours with strides provides more accurately phased information and should be useful when time for information processing at higher speeds is smaller. There was very little change in the phase of gaze behaviours despite a substantial change in the stride duration, however. A small but consistent shift of some gaze behaviours to later in the step cycle at faster speeds, which was observed mostly in cat 3, may be explained by existence of an absolute time requirement for visual information processing, which cats have to comply with in addition to fitting their gaze behaviours into the set step cycle phase windows. This absolute time requirement may be the factor that limits how fast it is possible to go on a complex surface without compromising performance. Indeed, at a certain speed the absolute time requirement may demand that the gaze behaviours shift outside of their locomotor-synergy set phases; however, such shifting is not feasible in the neuronal processing network.
A solution may be in engaging constant gaze more at higher speeds. We found that on complex surfaces cats shifted gaze slightly less and used constant gaze slightly more as they walked faster (Table 2). There was no such trend on the flat surface, which means that it was not the frequency of stepping that caused the trend, but instead the demand on vision and visuo-motor coordination on the complex surface. While fixating effectively on objects when moving at a high speed may be problematic, analysis of the optic flow is more tolerant of a high speed. In addition, when walking on the ladder faster, cats directed their gaze further ahead, but not far enough to maintain the ‘time to impact’, the time that will elapse before their limbs reach the visually inspected crosspiece. By using constant gaze, more distant objects can be effectively assessed using peripheral vision.
The main errors of this study resulted from projection of the two-dimensional gaze movement along the walking surface onto the line where this surface intersected with the sagittal plane (X, Z in Fig. 2). Although when walking cats move both the head and eyes in the vertical direction much more than in the lateral direction (Rivers et al. 2014 for eye movement, and Fowler & Sherk, 2003; Zubair et al. 2016 for head movement), such projection likely caused misclassification of slower oblique gaze shifts into the ‘slow gaze’ group and possibly the rare strictly lateral gaze shifts and slow gaze events into gaze fixations.
In summary, our findings indicate that the coordination of gaze behaviours with strides is not vision-driven, but is a part of the whole body locomotion synergy. The visual environment and locomotor task modulate the coordination. These findings may be relevant to developing diagnostic tools and rehabilitation approaches for patients with locomotor and visuo-motor deficits as they illuminate the tight coupling between the stride and the gaze.
Key points.
Vision plays a crucial role in guiding locomotion in complex environments, but the coordination between gaze and stride is not well understood.
The coordination of gaze shifts, fixations, constant gaze and slow gaze with strides in cats walking on different surfaces were examined.
It was found that gaze behaviours are coordinated with strides even when walking on a flat surface in the complete darkness, occurring in a sequential order during different phases of the stride.
During walking on complex surfaces, gaze behaviours are typically more tightly coordinated with strides, particularly at faster speeds, only slightly shifting in phase.
These findings indicate that the coordination of gaze behaviours with strides is not vision-driven, but is a part of the whole body locomotion synergy; the visual environment and locomotor task modulate it. The results may be relevant to developing diagnostic tools and rehabilitation approaches for patients with locomotor deficits.
Acknowledgements
The authors are deeply indebted to Mr Neet Shah for assistance with data collection and processing, and to Mr Peter Wettenstein for outstanding engineering assistance. The authors are grateful to Dr Maxim Volgushev for review of the manuscript and useful suggestions.
Funding
The study was supported by NIH grant R01 NS-058659 and NSF grant 1656882 to I.N.B. and by Barrow Neurological Foundation.
Biographies
Humza Nisar Zubair is an undergradute student at Arizona State University (ASU). He conducts neurophysiology research involving cats in the Beloozerova laboratory. His research focuses on the neural control of visually guided locomotion. His expertise includes data science, medical image computing and time series analysis. He is studying medicinal chemistry and biomedical sciences at ASU. He has presented at conferences and published three first-authored papers in peer-reviewed journals. He is a recipient of the Goldwater Scholarship. He aspires to become a physician scientist.

Kevin Chu is a PhD student in Electrical and Computer Engineering at Duke University. His current research uses machine learning to mitigate the effects of reverberation and noise in cochlear implants (CIs). This work has the potential to improve the intelligibility of CI users in adverse listening environments. Previously, he worked in the Beloozerova laboratory at Barrow Neurological Institute, where he studied the gaze and kinematics of cats during walking.

Appendix: Estimation of the probability of the cat’s stepping on a stone on the stone-cluttered pathway if strides are not adjusted
The area of the test corridor was 6222 cm2 with 51 pseudo-randomly placed small stones with diameters ranging between 1.5 and 4 cm. They were sufficiently far apart for the cat’s paw to easily fit in between any of them. The area of the cat paw was considered to be 3 cm2 (e.g. de Carvalho et al., 2015). The following simplification was used for this estimate: we assumed that the cat is equally likely to step anywhere on the walkway for a given stride. Therefore, we added the radius of the cat’s paw (1.5 cm) to the radius of each stone, and calculated the area of all circles which such combined radiuses occupy. We found that it is 19.6% of the surface, meaning that there is a 19.6% chance for each of the four paws to touch a stone if strides are not adjusted to avoid them. The total chance that any of the four paws will touch a stone in a given stride is therefore 1 – (1 – 0.196)4 = 58.2%.
Footnotes
Competing interests
Authors have no competing interests to report.
References
- AMVA (2013). www.avma.org/issues/animal_welfare/euthanasia.pdf
- Armer MC, Nilaweera WU, Rivers TJ, Dasgupta NM & Beloozerova IN (2013). Effect of light on the activity of motor cortex neurons during locomotion. Behav Brain Res 250, 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky YI, Gelfand IM & Orlovsky GN (1986). Cerebellum and Rhythmical Movements. Springer-Verlag, Berlin, Heidelberg. [Google Scholar]
- Beloozerova IN & Sirota MG (1993). The role of the motor cortex in the control of accuracy of locomotor movements in the cat. J Physiol 461, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloozerova IN & Sirota MG (2003). Integration of motor and visual information in the parietal area 5 during locomotion. J Neurophysiol 90, 961–971. [DOI] [PubMed] [Google Scholar]
- Beloozerova IN, Farrell BJ, Sirota MG & Prilutsky BI (2010). Differences in movement mechanics, electromyographic, and motor cortex activity between accurate and non-accurate stepping. J Neurophysiol 103, 2285–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg BA (2004). Markov Chain Monte Carlo Simulations and Their Statistical Analysis. World Scientific: Hackensack, NJ, USA. [Google Scholar]
- Bizzi E, Kalil RE & Tagliasco V (1971) Eye-head coordination in monkeys, evidence for centrally patterned organization. Science 173, 452–454. [DOI] [PubMed] [Google Scholar]
- Blakemore C & Donaghy M (1980) Co-ordination of head and eyes in the gaze changing behaviour of cats. J Physiol 300, 317–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgeman B, Hendry D & Stark L (1975). Failure to detect displacement of the visual world during saccadic eye movements. Vision Res 15, 719–722. [DOI] [PubMed] [Google Scholar]
- Barton SL, Matthis JS & Fajen BR (2017). Visual regulation of gait: Zeroing in on a solution to the complex terrain problem. J Exp Psychol Hum Percept Perform 43, 1773–1790. [DOI] [PubMed] [Google Scholar]
- Chu KMI, Seto SH, Beloozerova IN & Marlinski V (2017). Strategies for obstacle avoidance during walking in the cat. J Neurophysiol 118, 817–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu KMI, Zubair HN, Johnson JL, Rivers TJ & Beloozerova IN (2016). Gaze coordination with strides during walking in the cat. Neuroscience Meeting Planner, Program No. 715.09. Society for Neuroscience, Washington, DC. [Google Scholar]
- Cohen B, Xiang Y, Yakushin SB, Kunin M, Raphan T, Minor L & Della Santina CC (2009). Effect of canal plugging on quadrupedal locomotion in monkey. Ann N Y Acad Sci 1164, 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes D, Le Ray D, Lambert FM, Simmers J & Straka H (2008). An intrinsic feed-forward mechanism for vertebrate gaze stabilization. Curr Biol 18, 241–243. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E & Munoz DP (2004). Visual responses on neck muscles reveal selective gating that prevents express saccades. Neuron 42, 831–841. [DOI] [PubMed] [Google Scholar]
- Cullen KE (2012). The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci 35(3), 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho WD, Rosalino LM, Dalponte JC, Santos B, Harumi Adania C & Lustosa Esbérard CE (2015). Can footprints of small and medium sized felids be distinguished in the field? Evidences from Brazil’s Atlantic Forest. Trop Conserv Sci 8, 760–777. [Google Scholar]
- Drew T, Kalaska J, Krouchev N (2008). Muscle synergies during locomotion in the cat: a model for motor cortex control. J Physiol 586, 1239–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar DC, Macpherson JM, Simmons RW & Zarcades A (2008). Stabilization and mobility of the head, neck and trunk in horses during overground locomotion: comparisons with humans and other primates. J Exp Biol 211, 3889–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engbert R & Mergenthaler K (2006.) Microsaccades are triggered by low retinal image slip. Proc Natl Acad Sci U S A 103, 7192–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell BJ, Bulgakova MA, Beloozerova IN, Sirota MG & Prilutsky BI (2014). Body stability and muscle and motor cortex activity during walking with wide stance. J Neurophysiol 112, 504–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell BJ, Bulgakova MA, Sirota MG, Prilutsky BI & Beloozerova IN (2015). Accurate stepping on a narrow path: mechanics, EMG, and motor cortex activity in the cat. J Neurophysiol 114, 2682–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favorov OV, Nilaweera WU, Miasnikov AA & Beloozerova IN (2015). Activity of somatosensory-responsive neurons in high subdivisions of SI cortex during locomotion. J Neurosci 35, 7763–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick AE (1854). Die bewegungen des menschlichenaugapfels. Zeitschrift fur rationale Medizin 4, 109–128. [Google Scholar]
- Fowler GA & Sherk HA (2003). Gaze during visually-guided locomotion in cats. Behav Brain Res 139, 83–96. [DOI] [PubMed] [Google Scholar]
- Freedman EG (2008). Coordination of the eyes and head during visual orienting. Exp Brain Res 190, 369–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacek RR (1977). Location of brain stem neurons projecting to the oculomotor nucleus in the cat. Exp Neurol 57, 725–749. [DOI] [PubMed] [Google Scholar]
- Gibson JJ (1958). Visually controlled locomotion and visual orientation in animals. Br J Psychol 49, 182–194. [DOI] [PubMed] [Google Scholar]
- Graf W, de Waele C & Vidal PP (1995a). Functional anatomy of the head-neck movement system of quadrupedal and bipedal mammals. J Anat 186, 55–74. [PMC free article] [PubMed] [Google Scholar]
- Graf W, de Waele C, Vidal PP, Wang DH & Evinger C (1995b). The Orientation of the cervical vertebral column in unrestrained awake animals. II. Movement strategies. Brain Behav Evol 45, 209–231. [DOI] [PubMed] [Google Scholar]
- Graf W, Keshner E, Richmond FJ, Shinoda Y, Statler K & Uchino Y (1997). How to construct and move a cat’s neck. J Vestib Res 7, 219–237. [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther E & Zrenner E (1993). The spectral sensitivity of dark- and light-adapted cat retinal ganglion cells. J Neurosci 13, 1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitton D, Douglas RM & Volle M (1984). Eye-head coordination in cats. J Neurophysiol 52, 1030–1050. [DOI] [PubMed] [Google Scholar]
- Guitton D, Munoz DP & Galiana HL (1990). Gaze control in the cat: Studies and modeling of the coupling between orienting eye and head movements in different behavioral tasks. J Neurophysiol 64, 509–531. [DOI] [PubMed] [Google Scholar]
- Haslwanter T (1995). Mathematics of three-dimensional eye rotations. Vision Res 35, 1727–1739. [DOI] [PubMed] [Google Scholar]
- Hirasaki E & Kumakura H (2004). Head movements during locomotion in a gibbon and Japanese macaques. Neuroreport 15, 643–647. [DOI] [PubMed] [Google Scholar]
- Hollands MA, Marple-Horvat DE, Henkes S & Rowan AK (1995). Human eye movements during visually guided stepping. J Mot Behav 27, 155–163. [DOI] [PubMed] [Google Scholar]
- Hollands MA & Marple-Horvat DE (1996.) Visually guided stepping under conditions of step cycle-related denial of visual information. Exp Brain Res 109, 343–356. [DOI] [PubMed] [Google Scholar]
- Hollands MA & Marple-Horvat DE (2001). Coordination of eye and leg movements during visually guided stepping. J Mot Behav 33, 205–216. [DOI] [PubMed] [Google Scholar]
- Imai T, Moore ST, Raphan T & Cohen B (2001). Interaction of the body, head, and eyes during walking and turning. Exp Brain Res 136, 1–18. [DOI] [PubMed] [Google Scholar]
- Lambert FM, Combes D, Simmers J & Straka H (2012). Gaze stabilization by efference copy signaling without sensory feedback during vertebrate locomotion. Curr Biol 22, 1649–1658. [DOI] [PubMed] [Google Scholar]
- Land MF (2004). The coordination of rotations of the eyes, head and trunk in saccadic turns produced in natural situations. Exp Brain Res 159, 151–160. [DOI] [PubMed] [Google Scholar]
- Land MF (2009). Vision, eye movements, and natural behavior. Vis Neurosci 26, 51–62. [DOI] [PubMed] [Google Scholar]
- Land MF & Hayhoe M (2001). In what ways do eye movements contribute to everyday activities? Vision Res 41, 3559–3565. [DOI] [PubMed] [Google Scholar]
- Lee DN (1980). The optic flow field: the foundation of vision. Philos Trans R Soc Lond B Biol Sci 290, 169–179. [DOI] [PubMed] [Google Scholar]
- Manter JT (1938). The dynamics of quadrupedal walking. J Exp Biol 15, 522–540. [DOI] [PubMed] [Google Scholar]
- Marigold DS & Patla AE (2007). Gaze fixation patterns for negotiating complex ground terrain. Neuroscience 144, 302–313. [DOI] [PubMed] [Google Scholar]
- Marlinski V & Beloozerova IN (2014). Burst firing of neurons in the thalamic reticular nucleus during locomotion. J Neurophysiol 112, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlinski V & McCrea RA (2009). Self-motion signals in vestibular nuclei neurons projecting to the thalamus in the alert squirrel monkey. J Neurophysiol 101(4), 1730–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlinski V, Nilaweera WU, Zelenin PV, Sirota MG & Beloozerova IN (2012a). Signals from the ventrolateral thalamus to the motor cortex during locomotion. J Neurophysiol 107, 455–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlinski V, Sirota MG & Beloozerova IN (2012b). Differential gating of thalamo-cortical signals by reticular nucleus of thalamus during locomotion. J Neurosci 32, 15823–15836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthis JS, Barton SL & Fajen BR (2015). The biomechanics of walking shape the use of visual information during locomotion over complex terrain. J Vis 15, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthis JS, Barton SL & Fajen BR (2017). The critical phase for visual control of human walking over complex terrain. Proc Natl Acad Sci U S A 114, E6720–E6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthis JS & Fajen BR (2013). Humans exploit the biomechanics of bipedal gait during visually guided walking over complex terrain. Proc R Soc B Biol Sci 1762, 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthis JS, Yates JL & Hayhoe MM (2018). Gaze and the control of foot placement when walking in natural terrain. Curr Biol 28, 1224–1233.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadyen BJ, Lavoie S & Drew T (1999). Kinetic and energetic patterns for hindlimb obstacle avoidance during cat locomotion. Exp Brain Res 125, 502–510. [DOI] [PubMed] [Google Scholar]
- Mulavara AP & Bloomberg JJ (2002–2003). Identifying head-trunk and lower limb contributions to gaze stabilization during locomotion. J Vestib Res 12, 255–269. [PubMed] [Google Scholar]
- Mulavara AP, Houser J, Miller C & Bloomberg JJ (2005). Full-body gaze control mechanisms elicited during locomotion: effects of VOR adaptation. J Vestib Res 15, 279–289. [PubMed] [Google Scholar]
- Nguyen CT, Iyer GS, Volgushev M & Beloozerova IN (2019). Denial of visual information during swing phase of the stride prolongs the following stance in cats. Neuroscience Meeting Planner Program No. 227.07. Society for Neuroscience, Chicago, IL. [Google Scholar]
- Ogorodnikov D, Tarasenko S, Yakushin S, Cohen B & Raphan T (2006). Head fixed field coil system for measuring eye movements in freely moving monkeys. Conf Proc IEEE Eng Med Bio Soc 1, 5567–5570. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN (1970). Influence of the cerebellum on the reticulo-spinal neurons during locomotion. Biophysics 15, 928–936. [Google Scholar]
- Orlovsky GN (1972a). Activity of vestibulospinal neurons during locomotion. Brain Res 46, 85–98. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN (1972b). Activity of rubrospinal neurons during locomotion. Brain Res 46, 99–112. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN, Deliagina TG & Grillner S (1999). Neuronal Control of Locomotion. From Mollusc to Man. Oxford University Press, New York. [Google Scholar]
- Orlovsky GN & Pavlova GA (1971). Vestibular responses in neurons of descending pathways during locomotion. Neurophysiologia 4, 311–316 [in Russian]. [Google Scholar]
- Otero-Millan J, Alba Castro JL, Macknik SL & Martinez-Conde S (2014). Unsupervised clustering method to detect microsaccades. J Vis 14, 18. [DOI] [PubMed] [Google Scholar]
- Patla AE & Vickers JN (1997). Where and when do we look as we approach and step over an obstacle in the travel path? Neuroreport 8, 3661–3665. [DOI] [PubMed] [Google Scholar]
- Patla AE & Vickers JN (2003). How far ahead do we look when required to step on specific locations in the travel path during locomotion? Exp Brain Res 148, 133–138. [DOI] [PubMed] [Google Scholar]
- Pozzo T, Berthoz A & Lefort L (1990). Head stabilization during various locomotor tasks in humans. I. Normal subjects. Exp Brain Res 82, 97–106. [DOI] [PubMed] [Google Scholar]
- Prilutsky BI, Sirota MG, Gregor RJ & Beloozerova IN (2005). Quantification of motor cortex activity and full-body biomechanics during unconstrained locomotion. J Neurophysiol 94, 2959–2969. [DOI] [PubMed] [Google Scholar]
- Pryor K (1975). Lads Before the Wind: Adventures in Porpoise Training. Harper & Row, New York. [Google Scholar]
- Rivers TJ, Shaw NA, Sirota MG & Beloozerova IN (2010). The relationship between vertical gaze shifts and stride cycle in freely walking cats. 2010 Neuroscience Meeting Planner. Programme No. 278.3. Society for Neuroscience, Washington, DC. [Google Scholar]
- Rivers TJ, Sirota MG, Guttentag AI, Ogorodnikov DA, Shah NA & Beloozerova IN (2014). Gaze shifts and fixations dominate gaze behavior of walking cats. Neuroscience 275, 477–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA (1963). A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng 10, 137–145. [DOI] [PubMed] [Google Scholar]
- Rossignol S (1996). Neural control of stereotypic limb movements. In: Handbook of Physiology, Section12, Exercise: Regulation and Integration of Multiple Systems, ed. Rowell LB & Sheperd TJ, pp. 173–216. Oxford University Press, New York. [Google Scholar]
- Roy JE & Cullen KE (2004). Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei. J Neurosci 24(9), 2102–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherk H & Fowler GA (2001). Visual analysis and image motion in locomoting cats. Eur J Neurosci 13, 1239–1248 [DOI] [PubMed] [Google Scholar]
- Skinner BF (1938). The Behavior of Organisms: An Experimental Analysis. Appleton-Century-Crofts Inc., New York. [Google Scholar]
- Stehouwer DJ (1987). Effect of tectotomy and decerebration on spontaneous and elicited behavior of tadpoles and juvenile frogs. Behav Neurosci 101, 378–384. [DOI] [PubMed] [Google Scholar]
- Stout EE & Beloozerova IN (2013). Differential responses of fast and slow conducting pyramidal tract neurons to changes in accuracy demands during locomotion. J Physiol 591, 2647–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straka H, Simmers J & Chagnaud BP (2018). A new perspective on predictive motor signaling. Curr Biol 28, R232–R243. [DOI] [PubMed] [Google Scholar]
- Takahashi M & Shinoda Y (2018). Brain stem neural circuits of horizontal and vertical saccade systems and their frame of reference. Neuroscience 392, 281–328. [DOI] [PubMed] [Google Scholar]
- Trank TV, Chen C & Smith JL (1996). Forms of forward quadrupedal locomotion. I. A comparison of posture, hindlimb kinematics, and motor patterns for normal and crouched walking. J Neurophysiol 76, 2316–2326. [DOI] [PubMed] [Google Scholar]
- Vidal PP, Roucoux A & Berthoz A (1982). Horizontal eye position-related activity in neck muscles of the alert cat. Exp Brain Res 46, 448–453. [DOI] [PubMed] [Google Scholar]
- von Holst E & Mittelstaedt H (1950). Das reafferenzprinzip. Naturwissenschaften 37, 464–476. [Google Scholar]
- von Uckermann G, Le Ray D, Combes D, Straka H & Simmers J (2013). Spinal efference copy signaling and gaze stabilization during locomotion in juvenile Xenopus frogs. J Neurosci 33, 4253–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal PP, Graf W & Berthoz A (1986). The orientation of the cervical vertebral column in unrestrained awake animals. I. Resting position. Exp Brain Res 61(3), 549–559. [DOI] [PubMed] [Google Scholar]
- Wilkinson EJ & Sherk HA (2005). The use of visual information for planning accurate steps in a cluttered environment. Behav Brain Res 164, 270–274. [DOI] [PubMed] [Google Scholar]
- Winter DA (1995). Human balance and posture control during standing and walking. Gait Posture 3, 193–214. [Google Scholar]
- Wurtz RH (2008). Neuronal mechanisms of visual stability. Vision Res 48, 2070–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Yakushin SB, Kunin M, Raphan T & Cohen B (2008). Head stabilization by vestibulocollic reflexes during quadrupedal locomotion in monkey. J Neurophysiol 100, 763–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra W & Hof AL (2003). Assessment of spatio-temporal gait parameters from trunk accelerations during human walking. Gait Posture 18, 1–10. [DOI] [PubMed] [Google Scholar]
- Zubair HN, Beloozerova IN, Sun H & Marlinski V (2016). Head movement during walking in the cat. Neuroscience 332, 101–120. [DOI] [PMC free article] [PubMed] [Google Scholar]


