Abstract
Drug-induced autoimmune hepatitis (DIAIH) is a specific phenotype of drug-induced liver injury that may lead to the devastating outcome of acute liver failure requiring liver transplantation. Drugs implicated in DIAIH include antimicrobials such as nitrofurantoin and minocycline, non-steroidal anti-inflammatory drugs, statins as well as anti-tumor necrosis agents. The clinical features of drug-induced liver injury are indistinguishable from idiopathic autoimmune hepatitis (AIH) as both may have positive AIH-related autoantibodies, elevated immunoglobulin G, as well as similar histopathological findings. In patients who show no clinical improvement, or there is progressive liver injury despite cessation of the suspected drug, a liver biopsy should be considered, whereby the presence of advance fibrosis on histology favors the diagnosis of idiopathic AIH. Empirical treatment with corticosteroids may be required in patients with non-resolving liver injury. A typical clinical scenario supportive of DIAIH includes a history of drug exposure with spontaneous resolution of liver injury after drug withdrawal and the absence of relapse after rapid steroid taper. In this article we report two cases of DIAIH secondary to Sorafenib and Atorvastatin along with a review of currently available literature. Early identification and treatment often lead to a favorable outcome in DIAIH.
Keywords: Drug-induced liver injury, Drug-induced autoimmune hepatitis, Autoimmune hepatitis, Review
Core Tip: Drug-induced autoimmune hepatitis is uncommon in clinical practice but may have devastating consequences. It is important to distinguish drug-induced autoimmune hepatitis from idiopathic autoimmune hepatitis as the former may not require prolong course of immunosuppressant. This minireview highlights the key differences between these two closely-linked entities.
INTRODUCTION
Idiosyncratic drug-induced liver injury (DILI) is rare and affects 14-19 per 100000 persons yearly[1,2]. Despite its relatively low incidence, it is a leading cause of acute liver failure in the United States[3], Europe[4,5], and Japan[6]. In patients with DILI, liver-related death and liver transplantation occur in 3.6%-10% of cases[7].
Drug-induced autoimmune hepatitis (DIAIH) is a specific phenotype of idiosyncratic DILI with features indistinguishable from idiopathic autoimmune hepatitis (AIH), as it shares serological markers and/or histological features with idiopathic AIH[8]. Various terms have been used synonymously with DIAIH, including immune-mediated DILI[9] and drug-induced AIH-like injury[10].
Due to its rare occurrence, it is difficult to estimate the frequency of DIAIH. In addition, studies use varying definitions of DIAIH and drug causality assessments, as well as having diverse patient populations with different follow-up periods (Tables 1 and 2). It is estimated that DIAIH accounts for 2%-18% of AIH cases[10-14], and 2.9%-8.8% of all DILI are due to DIAIH[15,16]. The increasing incidence of AIH has been in part attributed to prevalent use of anti-tumor necrosis factor agents[14].
Table 1.
Studies comparing drug-induced autoimmune hepatitis and drug-induced liver injury
|
Ref.
|
a: Study population; b: Study period; c: Follow-up period
|
a: Definition of DIAIH; b: Causality tool assessment
|
No. of DIAIH cases, % of all DILI
|
No. of DILI cases
|
Key findings for DIAIH (in addition to Table 3)
|
| Stephens et al[37], 2021, Spain | a: Prospective multicentre DILI database, n = 869; b: 1994-2018; c: Median 96-117 d in HC injury | a: Simplified AIH criteria; b: RUCAM (definite, highly probable, probable and possible) | 26, 2.9% | 843 | Culprit drugs: Statins (31%); antimicrobials (23%) |
| De Boer et al[15], 2015, United States | a: National prospective DILI database (n = 1322), subgroup of DILI secondary to Nitrofurantoin, hydralazine, Minocycline and methyldopa (n = 88); b: 2004-2014; c: 6 mo, 12 mo or 24 mo until normalization of LFT | a: Autoimmune (AI) DILI–AI score based on seropositivity for AIH antibodies and raised IgG); b: RUCAM (definite, highly probable and probable) | 47, 3.6% | Two groups: (a) 18 non-AI DILI due to 4 drugs; (b) 67 (reference cohort, DILI due to Augmentin, Isoniazid, Diclofenac) | Similar HLA-DRB1*03:01 (15%) and HLADRB1*04:01 (9%) percentage in patients with DILI compared to population controls from National Marrow Donor Program (12% and 9%, respectively) |
| Hisamochi et al[22], 2016, Japan | a: All DILI who underwent liver biopsy, n = 62; b: 1988-2010; c: Median 2290 d | a: Revised IAIHG criteria; b: RUCAM and JDD-W scale | 23, NA | 39 | Culprit drugs: CAM (69.6%); NSAIDs (8.7%). IgG reduction in 87%. 50% (8/16) relapsed (4 not treated with steroids, 2 previously received steroids and 2 on tapering dose of steroid dosage). Median time to relapse 283 d (range, 47-1090 d). Rise in IgG with relapse |
| Licata et al[16], 2014, Italy | a: Single centre hospitalized patients with DILI, n =136 (44 with liver biopsy); b: 2000-2011; c: Mean 26 mo (12-84 mo), at least 1 yr after stopping immunosuppressants | a: Simplified AIH score ≥ 6; b: RUCAM (definite, highly probable, probable and possible) | 12, 8.8% | 124 | Culprit drugs: NSAIDs (50%) - (Nimesulide/ketoprofen); Antimicrobials (25%) (Augmentin/Ceftriaxone); CAM (17%). 38.2% of all DILI patients had positive AIH antibodies but only 42.9% with positive antibodies have DIAIH. All DIAIH were treated with corticosteroids and all achieved remission at 15 mo. 58.3% (7/12) had addition of Azathioprine. One patient had a flare while on tapering prednisolone. In 41% (5/12), immunosuppressant was stopped after 2 yr, with no relapse |
DIAIH: Drug-induced autoimmune hepatitis; DILI: Drug-induced liver injury; RUCAM: The Roussel Uclaf Causality Assessment Model; NSAIDs: Non-steroidal anti-inflammatory drugs; AIH: Autoimmune hepatitis; IAIHG: International AIH Group; IgG: Immunoglobulin G; CAM: Complementary alternative medicines.
Table 2.
Studies comparing drug-induced autoimmune hepatitis and autoimmune hepatitis
|
Ref.
|
a: Study population; b: Study period; c: Follow-up period
|
a: Definition of DIAIH/AIH; b: Causality tool assessment
|
No. of DIAIH cases, % of all AIH
|
No. of AIH cases
|
Key findings for DIAIH (in addition to Table 4)
|
| Valgeirsson et al[14], 2019, Iceland | a: Population based AIH study, n = 71; b: 2006-2018; c: Median 4.8 yr | a: Simplified AIH score, if not fulfilled, Revised IAIHG score is used; or received corticosteroids; b: RUCAM score (highly probable, probable and possible) | 13, 18% (9/13 had liver biopsy) | 58 | Culprit drugs: Biologics (77%) - 80% were due to infliximab; Nitrofurantoin (15%) |
| Martínez-Casas et al[34], 2018, Columbia | a: Single centre retrospective review of AIH cases, n = 190; b: 2010-2016; c: Mean 47.4 mo | a: Simplified AIH score; b: RUCAM | 12, 6.3% | 178 | Culprit drugs: Nitrofurantoin (67%); NSAIDs (17%) |
| Wang et al[20], 2017, China | a: Single centre retrospective review of AIH and DILI patients; b: 2010-2014; c: NA | a: DILI with positive antibody; simplified AIH score; b: NA (DILI due to drugs/CAM within 6 mo of hospitalization) | 18 (12.4% of all DILI with positive antibody) | 52 | Culprit drugs: CAM, NSAIDs and antibiotics (no breakdown) |
| Yeong et al[32], 2016, United Kingdom | a: Single centre retrospective AIH cases, n = 82; b: 2005-2013; c: Median 86.3 mo (14.6% < 18 mo) | a: Revised IAIHG criteria; b: RUCAM (highly probable, probable) | 11, 13.4% | 71 | Culprit drugs: Nitrofurantoin (36.4%); Statins (36.4%); CAM (18%) |
| Weber et al[21], 2019, Germany | a: Single centre cohort of 288 acute liver injury patients who received corticosteroid for DILI/AIH, n = 44; b: 2013-2018; c: Median 19 mo in DILI; 23 mo in AIH | a: Simplified AIH score and revised IAIHG criteria; b: RUCAM | 22 | 22 | Culprit drugs: NSAIDs (27.3%); Statins (9%); Direct oral anticoagulants (9%) |
| Björnsson et al[13], 2010, United States | a: Single centre retrospective review of all AIH cases, n = 261; b: 1997-2007 | a: Simplified AIH score | 24, 9.2% (24/261) | 237 | Culprit drugs: Minocycline (45.8%); Nitrofurantoin (45.8%) |
DIAIH: Drug-induced autoimmune hepatitis; DILI: Drug-induced liver injury; RUCAM: The Roussel Uclaf Causality Assessment Model; NSAIDs: Non-steroidal anti-inflammatory drugs; AIH: Autoimmune hepatitis; IAIHG: International AIH Group; CAM: Complementary alternative medicines.
DRUGS ASSOCIATED WITH DIAIH
Multiple drugs that have been associated with DIAIH are classified into those with definite association (e.g., Minocycline, Nitrofurantoin, Infliximab), probable association (e.g., Diclofenac, Atorvastatin, Rosuvastatin, Etanercept), and possible association, depending on the cases reported and associations as summarized in the most recent American Association Society of Liver Disease AIH Practice Guidance[10].
DIAIH is classically associated with minocycline, nitrofurantoin, methyldopa, dihydralazine, and tienilic acid[17]. DILI with autoimmune phenotype defined as DILI with presence of AIH antibodies (antibodies to nuclear antigen, smooth muscle, and soluble liver antigen) occur in 83%, 74%, 60%, and 43% of nitrofurantoin, minocycline, methyldopa, and hydralazine related DILI cases, respectively[15]. Immuno-allergic phenotype characterized by any combination of rash, fever, facial edema, lymphadenopathy, and eosinophilia is common in DILI associated with these four drugs as well, ranging from 11%-27%[15]. Another important cause of DIAIH include statins, where DIAIH or DILI with immune features occurs in about 8.5%-27.2% of all statin related DILI[18,19].
In recent years, there has been a change in the predominant culprit drugs causing DIAIH to anti-tumor necrosis factor[14], statins, and non-steroidal anti-inflammatory drugs[16,20,21] with notable contribution from complementary alternative medicines in studies from Asia[22].
PATHOGENESIS OF DIAIH
Reactive metabolites generated from hepatic metabolism of drugs bind to cellular proteins such as components of CYP450, which is then recognized as neoantigens by heightened immunological response leading to AIH[11,23,24] as a result of misdirected immune response against self[25].
In this minireview, we highlight two recent cases of DIAIH induced by Sorafenib and Atorvastatin seen at our center. We also aim to review the current literature on DIAIH and discuss distinguishing features between DIAIH and AIH.
CASE DISCUSSION
Patient A
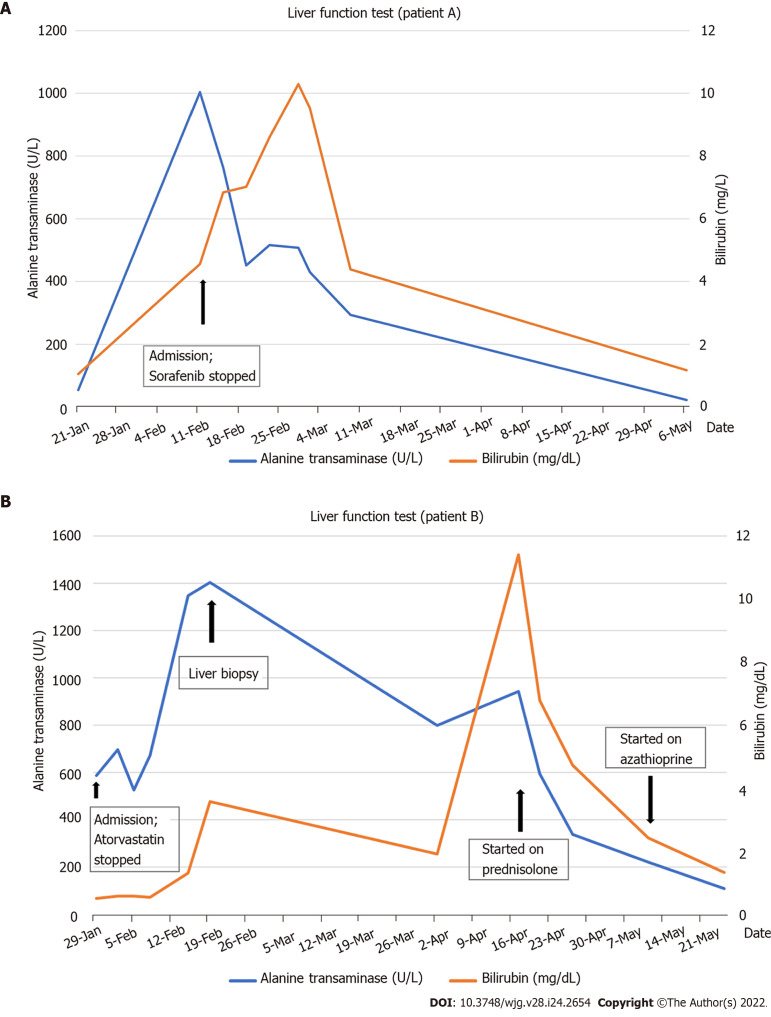
A 61-year-old man and teetotaler presented with a 1-week history of jaundice and malaise. He was on Atorvastatin 40 mg daily for 4 years for hyperlipidemia. He had a normal liver function test (LFT) prior to admission. He was started on Sorafenib 6 wk prior to presentation with jaundice for recurrent sarcoma of the left thigh. Clinical examination was unremarkable apart from scleral icterus. The LFT showed severe hepatocellular (HC) injury [bilirubin 4.56 mg/dL, alkaline phosphatase (ALP) 190 U/L, alanine transaminase (ALT) 1004 U/L, aspartate transaminase (AST) 790 U/L, international normalized ratio (INR) of 1.53]. Viral hepatitis screen, AIH-specific antibodies, and abdominal imaging were unremarkable. Of note, serum immunoglobulin G (IgG) was elevated (18.6 g/L). The liver biopsy showed features supportive of DILI and AIH (Figure 1). The Simplified AIH score was 6. The Roussel Uclaf Causality Assessment Model (RUCAM) score was 9 for Sorafenib and 6 for Atorvastatin. Diagnosis of Sorafenib-induced AIH was made. His LFT improved spontaneously with normalization of LFT 8 wk after stopping Sorafenib (Figure 2A).
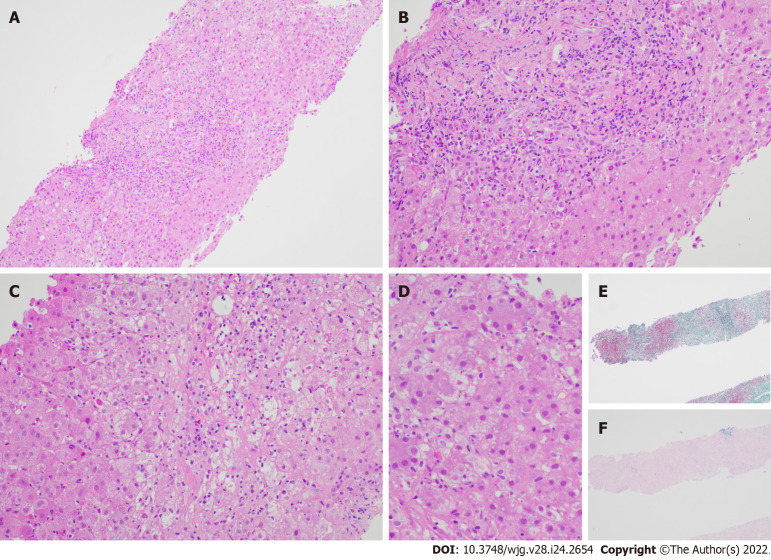
Figure 1.
Liver biopsy specimen for patient A. A: Low power view [hematoxylin & eosin (H&E) 100 ×] displays conspicuous portal and lobular inflammation with lobular disarray. Mild steatosis is also noted; B: Higher magnification of the portal tract (H&E 200 ×), zone 1, shows moderate chronic inflammation, lymphoplasmacytic predominantly, and rare eosinophils, with interface damage; C: At similar magnification (H&E 200 ×), the lobule including the perivenular region, e.g., zones 2 and 3, exhibits lobulitis characterized by aggregates of plasma cells, swollen hepatocytes with rosetting, Councilman bodies, and hepatocyte drop-out; D: High power view (H&E 400 ×) demonstrates rosetting of hepatocytes with droplets of orange-brown bile pigment; E and F: Histochemical stains Masson trichrome (E, 40 ×) showing collapse with mild early young fibrosis and Victoria blue (F, 40 ×) revealing paucity of elastic fibers, thus in keeping with subacute injury. Overall, the appearances are supportive of subacute drug-induced liver injury in association with autoimmune hepatitis histological pattern.
Figure 2.
Bilirubin and alanine transaminase trend for patients A and B. A: Patient A; B: Patient B.
Patient B
A 65-year-old man who was on Atorvastatin 40 mg daily presented with an incidental finding of acute HC pattern of liver injury 18 mo after initiation of Atorvastatin (albumin 39 g/L, globulin 39 g/L, bilirubin 0.64 mg/dL, ALP 107 IU/L, ALT 696 U/L, AST 381 U/L). Viral hepatitis screening, AIH-specific antibodies, and abdominal imaging were unremarkable. His serum IgG was normal (14.28 g/L). Atorvastatin was stopped, and an improvement in LFT was noted within the 1 wk (albumin ALT 474 U/L, AST 195 U/L). The RUCAM score for Atorvastatin was 4. As such, he was given the diagnosis of possible Atorvastatin-induced DILI. However, despite this initial improvement in his LFT, there was subsequent deterioration 2 wk after stopping Atorvastatin (albumin 34 g/L, globulin 43 g/L, ALP 137 U/L, ALT 1404 U/L, AST 676 U/L, INR 1.09). A liver biopsy was performed 3 wk after stopping Atorvastatin in view of worsening acute liver injury. This showed marked HC injury with histological features suggestive of AIH (Figure 3). Following the liver biopsy, there was again spontaneous improvement (ALT 699 U/L), but this did not persist. Two months after cessation of Atorvastatin, he had severe HC injury with jaundice (bilirubin 11.87 mg/dL, ALP 96 U/L, ALT 1095 U/L, AST 938 U/L, INR 1.33) and elevated IgG (28.35 g/L). Liver biopsy was repeated, and this again demonstrated features of AIH. He was then started on prednisolone with rapid improvement of LFT (Figure 2B). The final diagnosis was Atorvastatin-induced AIH.
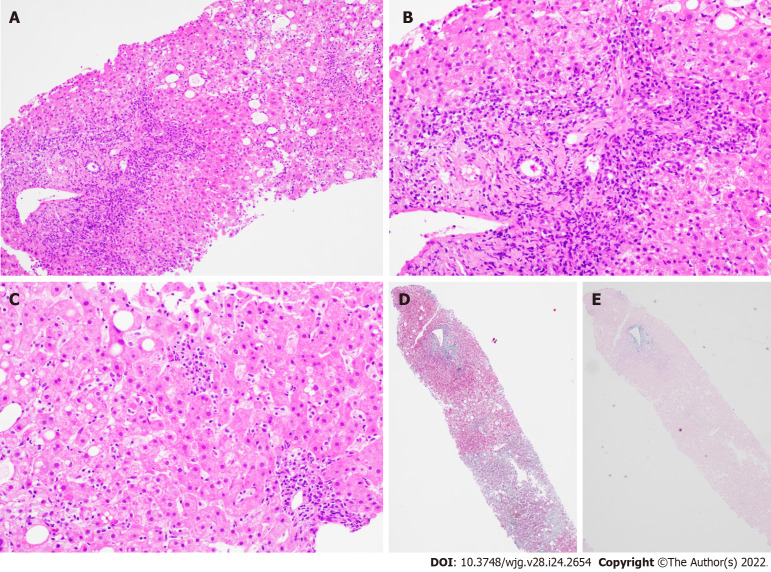
Figure 3.
Liver biopsy findings for patient B. A: Low power view [hematoxylin & eosin (H&E) 100 ×] shows portal and lobular inflammation with lobular disarray and mild steatosis; B: Higher magnification of the portal tract (H&E 200 ×) demonstrates moderate plasma cell-rich chronic inflammation with continuous interface damage; C: Lobulitis with aggregates of plasma cells and rosetting of hepatocytes is present in the lobule (H&E 200 ×); D and E: Masson trichrome (D, 40 ×) and Victoria blue (E, 40 ×) display mild early young fibrosis and paucity of elastic fibers, respectively. The absence of old mature type fibrosis suggested not a chronic injury. The autoimmune hepatitis histological pattern observed was therefore interpreted to be drug related, atorvastatin-induced.
WHEN TO SUSPECT DIAIH IN PATIENTS WHO PRESENTS WITH DILI
The cases described above highlight two possible presentations of DIAIH. The first patient (patient A) had DILI and histological features compatible with AIH on liver biopsy. Although Sorafenib has not been reported to be associated with DIAIH, the temporal sequence of this case presentation and subsequent spontaneous resolution after cessation of Sorafenib is in keeping with DIAIH.
DIAIH shares many similar characteristics with DILI without features of AIH. More than half of DIAIH present with acute liver injury associated with jaundice in 70%-75% of cases[16], which is similar to DILI. On top of that, rash may be present in 4.5% of DIAIH and 7.9% of DILI[26].
The following pointers are useful in identification of DIAIH in patients who present with DILI. The main differences between these two conditions are also summarized in Table 3: (1) DIAIH and AIH should always be considered as differentials in a patient with a hepatocellular pattern of DILI. DIAIH is rarely associated with a cholestatic/mixed picture, and it is only seen in 8% of cases[16]; (2) A detailed medication history with a focus on recent drug exposures including complementary alternative medicines is essential[10]; (3) The latency period of drug exposure in DIAIH is usually prolonged compared to other types of DILI, some with a latency period exceeding 1 year, e.g., nitrofurantoin and minocycline[15]; (4) Seropositivity for AIH antibodies, e.g., antinuclear antibody (ANA), anti-smooth muscle antibody, anti-liver kidney antibody, and elevated serum IgG suggest possible DIAIH. However, not all patients with DIAIH have detectable autoantibodies or elevated IgG. Similarly, a proportion of patients with DILI may have detectable AIH antibodies[15,16]; (5) In the presence of detectable AIH antibodies and elevated IgG, AIH scoring (either pre-treatment score for Revised International AIH Group criteria[27] or simplified AIH score[28]) is useful to assess for possible or probable AIH; (6) Liver-specific causality assessment tools may be used to ascertain the strength of association between drug exposure and clinical manifestation, e.g., RUCAM[29]; and (7) Liver biopsy is the cornerstone for the diagnosis of DIAIH and should be considered in the following scenarios: (a) Non resolving or worsening liver injury despite stopping possible culprit drugs; (b) Seropositivity of AIH antibodies, raised IgG, or possible AIH based on AIH scoring systems.
Table 3.
Comparison between drug-induced autoimmune hepatitis and drug-induced liver injury
|
Clinical features
|
DIAIH
|
DILI
|
| Demographics | ||
| Female, % | 62%[37] | 48% (P = 0.162)[37] |
| Age (yr), mean ± SD | 57± 17[37]; 59 ± 17[22] | 54 ± 18 (P = 0.550)[37]; 47 (P = 0.002)[22] |
| Clinical presentation | ||
| Jaundice, % | 69%[37]; 68%[15]; 66%[16] | 69% (P = 0.953)[37]; 56% (P = 0.4)[15]; 40%-47.6% (P = 0.2)[16] |
| Rash, % | 4.5%[37]; 19%[15] | 7.9% (P = 1.000)[37]; 22% (P = 0.7)[15] |
| Hepatocellular injury, % | 92%[37] | 57% (P = 0.002)[37] |
| Latency period (d), median (range) | 65 (27-274)[37]; 277 (8-7032)[15]; 4 (1-9)[16] | 27 (8-64) (P = 0.004)[37]; 100 (13-1572) (P = 0.03)[15]; 7-10 (5-50) (P = 0.7)[16] |
| Latency period (d), mean ± SD | 143 ± 188[22] | 32 ± 120 (P = 0.000)[22] |
| Culprit drug due to CAM, % | 70%[22] | 25% (P = 0.000)[22] |
| Biochemical results | ||
| ALT × ULN, mean ± SD | 28 ± 19[37] | 19 ± 22 (P = 0.0002)[37] |
| AST × ULN, mean ± SD | 24 ± 17[37] | 15 ± 21 (P = 0.0001)[37] |
| Autoimmune antibodies and serology | ||
| Detectable ANA, % | 88%[37]; 72%[15]; 52%[22] | 12 (P < 0.001)[37]; 22[15]; 15 (P = 0.003)[22] |
| Detectable ASMA, % | 44%[37]; 60%[15] | 8.9% (P < 0.001)[37]; 13%[15] |
| Detectable AMA, % | 4%[37] | 1.9% (P = 0.397)[37] |
| Detectable anti-LKM-1, % | 0%[37] | 1.1% (P = 1.000)[37] |
| Elevated IgG, % | 39%[15]; (25% > 1.1 × ULN)[15] | 9%[15] |
| Serum IgG (g/L), mean ± SD | 19.5 ± 10.7[37]; 1.07 × ULN ± 0.51[22] | 11.9 ± 4.6 (P < 0.001)[37]; 0.69 × ULN ± 0.28 (P = 0.000)[22] |
| Histopathology | ||
| Liver biopsy[16] | ||
| Severe portal inflammation, % | 100% | 56.2%-62.5% |
| Prominent portal plasma cells, % | 58.3% | 6.3%-12.5% |
| Rosette formation, % | 66.7% | 6.3%-12.5% |
| Severe focal necrosis, % | 66% | 6.3%-25% |
| Treatment and response to treatment | ||
| Corticosteroid therapy, % | 43%[15] | 61% (P = 0.3)[15] |
| Immunosuppressive therapy, (corticosteroid/Azathioprine), % | 58%[37]; 60.8%[22] | 9.9% (P < 0.001)[37]; 10.3% (P = 0.000)[22] |
| Outcomes | ||
| Mild/mod/severe DILI, % | 35%/45%/7.7%[37] | 31%/59%/6.2% (P = 0.784)1[37] |
| Outcomes (liver transplant/death), % | 3.8%/0%[37]; 6%/4%[15] | 2.1%/1.5% (P = 0.784)1[37]; 0/0 (P = 0.6 for liver transplant, P = 1.0 for death)[15] |
| Chronicity rate, % | 17%[15] | 21% (P = 0.70)[15] |
Combined comparisons of severity of drug-induced liver injury, mortality, and liver transplantation.
DIAIH: Drug-induced autoimmune hepatitis; DILI: Drug-induced liver injury; ALT: Alanine transaminase; AST: Aspartate transaminase; ULN: Upper limit of normal; CAM: Complementary alternative medicines; Anti-LKM: Anti-liver kidney antibody; SD: Standard deviation; IAIHG: International AIH Group.
Known culprit drugs of DIAIH such as nitrofurantoin may be overlooked as these agents are associated with longer latency period and have a lower ALT at presentation. A higher fibrosis stage or cirrhosis may be observed as a higher proportion of these patients are unknowingly continued on Nitrofurantoin prior to the diagnosis[30]. This underscores the importance of understanding the common culprits of DIAIH, where a significant proportion of DILI presents with DIAIH[15]. LiverTox® is an up-to-date online resource that provides information on hepatotoxicity caused by medications and supplements[31].
With the increasing use of targeted tumor therapies such as kinase inhibitor and immunotherapy, there are some case reports of DIAIH associated with these medications. To our knowledge, this is the first case report of Sorafenib-induced AIH. Imatinib, another type of kinase inhibitor, has also been reported to be associated with DIAIH[14,32].
Several studies have attempted to compare the differences between DIAIH and DILI (Table 1)[15,16,22], with key features of DIAIH including significantly longer duration of drug exposure and latency; higher ALT and AST; higher proportion of patients with positive ANA and SMA, and higher level of serum IgG, as summarized in Table 3.
Liver biopsy is key in differentiating DIAIH from DILI without features of AIH[10,33]. In patients with DIAIH, the histopathological features are similar to that of idiopathic AIH with significantly higher proportion of patients showing severe portal inflammation, prominent portal-plasma cells, rosettes, and severe focal necrosis as compared to other types of DILI[16].
Though there is no significant difference in the severity and outcomes of DIAIH compared to other types of DILI, it is crucial to identify DIAIH in patients who present with DILI, as DIAIH may require treatment with immunosuppressants if liver injury does not improve with cessation of possible culprit drugs[10,31,33]. A significantly higher proportion of patients (50% to 80%) with DIAIH are treated with corticosteroids/immunosuppressants as compared to non-DIAIH DILI, and DIAIH will need longer term follow up even when LFT normalizes as late relapses may occur in up to 50% of cases where immunosuppressants are stopped[22]. Majority of relapses occur within a year, but some may present late up to 3 years after the initial diagnosis, and risk factors for late disease recurrence are not clear.
DIFFERENTIATING DIAIH AND IDIOPATHIC AIH
The second case highlights the difficulty in differentiating DIAIH from idiopathic AIH. Both conditions have overlapping clinical presentations with HC pattern of liver injury and may have detectable ANA, SMA, and raised IgG in some cases[10,11]. A number of studies compared the difference between DIAIH and AIH (Table 2), with the key differences summarized in Table 4. Some useful features to distinguish between these two entities:
Table 4.
Comparison between drug-induced autoimmune hepatitis and autoimmune hepatitis
| Clinical features | DIAIH | AIH |
| Demographics | ||
| Female, % | 82%[32]; 91%[34] | 80% (P = 0.635)[32]; 92% (P = 0.95)[34] |
| Clinical presentation | ||
| Acute presentation | > 60%[14]; 55%[32]; 83%[34] | < 20%[14]; 47% (P = 0.618)[32]; 35% (P < 0.001)[34] |
| Hypersensitivity reaction (fever, rash, eosinophilia) | Up to 30%[14] | Unusual[14] |
| Cirrhosis at presentation, % | 0%[34] | 34.8% (P = 0.07)[34] |
| Temporal relationship with drugs | Positive | Negative |
| Concurrent AI disease | Unusual[14] | Present in 14%-44%[14] |
| Biochemical results | ||
| ALT (U/L), mean ± SD | 548 ± 335[20] | 227 ± 121 (P = 0.021)[20] |
| AST (U/L), mean ± SD | 460 ± 321[20] | 202 ± 57 (P = 0.018)[20] |
| Serology | ||
| IgG, mean ± SD (g/L) | 13.4 g/L[14]; 21.4 ± 7.5[34] | 18.6 g/L (P value non-significant)[14]; 24.3 ± 11.2 (P = 0.422)[34] |
| Pre-treatment score | ||
| RUCAM score, median (range) | 6 (3-10)[21] | 3.5 (0-7) (P < 0.01)[21] |
| Revised IAIHG score, median (range) | 9.5 (4-14)[21] | 13 (9-18)[21] |
| Simplified AIH score, median (range) | 4 (2-6)[21] | 5 (1-7) (P = 0.385)[21] |
| Histopathology | ||
| F3-F4, % | 33.3%[34] | 54.4% (P = 0.15)[34] |
| Typical histology (portal inflammation, interface hepatitis, plasma cells infiltrates) | 18.2%[20] | 54%[20] |
| Treatment and response to treatment | ||
| Time to biochemical remission, mean (mo) | 2[34] | 16.8 (P <0.001)[34] |
| Treatment with Azathioprine or Mycophenolic acid in addition to corticosteroids, % | 57%[13]; 15%[14]; 28%[20]; 20%[21] | 86% (P = 0.024)[13]; 83% (P < 0.001)[14]; 90% (P = 0.023)[20]; 85% (P < 0.01)[21] |
| Biochemical remission, % | 95%[21] | 77.3% (P = 0.08)[21] |
| Treatment discontinuation, % | 69%[14]; 100%[20]; 85%[21]; 25%[34] | 26% (P < 0.02)[14]; 25% (P = 0.013)[20]; 5% (P < 0.1)[21]; 3% (P < 0.001)[34] |
| Relapse rate, % | 0%[14]; 15%[21]; 60%[32]; 0%[34] | 43% (P = 0.022)[14]; 70% (P < 0.01)[21]; 83% (P = 0.538)[32]; 18% (P = 0.10)[34] |
| Time to relapse (wk), median (range) | 131 (37-216)[34] | 14 (1-155) (P = 0.033)[34] |
| Outcomes | ||
| Liver transplant/death, % | 0%/0%[32,34] | 2.8%/7% (P = 0.748 for liver transplant; p = 0.65 for death)[32]; 5.6%/2.8% (P = 0.40 for liver transplant; P = 0.55 for death)[34] |
DIAIH: Drug-induced autoimmune hepatitis; DILI: Drug-induced liver injury; ALT: Alanine transaminase; AST: Aspartate transaminase; RUCAM: The Roussel Uclaf Causality Assessment Model; SD: Standard deviation.
Clinical presentation
Majority (60%-83%) of DIAIH present with an acute presentation, whereas it is seen in less than 20%-35% of cases with idiopathic AIH[10,34]. Most studies of the two conditions do not demonstrate a significant difference in LFT levels[14,21,30,34], but ALT[13,30,34], AST[14,34], and bilirubin[14,34] tend to be higher in patients with DIAIH. Only one study[20] showed a significantly higher level of bilirubin, AST, and ALT in patients with DIAIH. There was no significant difference in the proportion of patients with detectable ANA (77-94%)[20,21] and elevated IgG (36%-59%)[14], although one study showed AIH had higher level of serum IgG compared to DIAIH; it was not, however, statistically significant[14]. Immuno-allergic presentation with skin rash, fever, lymphadenopathy, and eosinophilia favor DIAIH, as it may occur in up to 30% of DIAIH[10,11].
AIH scoring and causality assessment
In a cohort of 44 patients with DIAIH and AIH, the simplified AIH score was not useful in differentiating the two entities[21]. Of note, patients with AIH had significantly higher pre-treatment AIH score compared to patients with DIAIH. The sensitivity and the specificity for pre-treatment AIH score (using a cut off of ≥ 12 – probable AIH) was shown to be 59% and 82%, respectively. As for RUCAM, using a cut off of ≥ 6 (probable), the specificity reaches 91%, albeit with a low sensitivity at 32%. When these are used in combination, there is still a potential for misdiagnosis in up to 11% of patients.
Histopathology
Both DIAIH and AIH share similar histological findings (portal and periportal infiltrates of lymphocytes, lobular hepatitis, plasma cells, and eosinophils) with no clear differentiating features, except presence of advance fibrosis or cirrhosis favoring idiopathic AIH[11,34]. However, a significantly higher proportion of patients with AIH showed typical features of AIH (54%) as compared to DIAIH (18.2%)[20], specifically in terms of portal inflammation with interface hepatitis and plasma cell infiltrates. The same study also found that DIAIH tended to have more eosinophilic infiltrates, which was not noted in other studies[21].
Treatment and response to treatment
The cornerstone in the management of DIAIH is to stop the culprit drug. Spontaneous improvement of LFT may then occur, as was observed in patient A. The most consistent and significant differentiating feature between DIAIH and AIH in published case series thus far lies in the response to treatment (Table 4). In cases where liver injury does not resolve despite cessation of the culprit drug or where the degree of DILI is severe, corticosteroids are usually started as per the initial management of AIH. Most studies on DIAIH exclusively include patients who were treated with immunosuppressants[14,20,21,30]. Both DIAIH and AIH showed excellent response to corticosteroids, with remission rate exceeding 90% in most cases[21,30-34].
It should be borne in mind that there is discrepancy in literature regarding the mean time to achieve immunosuppression induced remission in DIAIH and AIH. While one study demonstrated similar 3 mo mean time to remission[21] in both conditions, another showed a significant difference, where DIAIH took a significantly shorter time than AIH to achieve remission[34] (2 mo vs 16.8 mo). This may be attributed to the heterogeneity of the study population. Resolution of DIAIH usually occurs after 1 mo (rarely up to 3 mo) of immunosuppression. Unlike in AIH, DIAIH rarely requires long term immunosuppression and has very low relapse rates after cessation of immunosuppressants over a long follow-up of up to 4 years[10,11,13-15,20,21,34] (Table 2). In cases where DIAIH relapses, the time to relapse is significantly longer compared to AIH[30], which implies that patients with DIAIH will require a longer period of follow-up after resolution of liver injury as compared to the usual DILI. Interestingly, despite similar time to remission in the two groups, Weber et al[21] showed that an early rapid response to corticosteroid treatment differentiates DIAIH from AIH with good sensitivity and specificity at 77%, respectively. The early rapid response was defined by 9% drop of ALT per day in the first week of corticosteroid therapy.
The use of steroids appears to be a promising means of differentiating DIAIH or AIH in the early course of disease with better sensitivity than RUCAM and AIH score[21]. This also has the potential benefit of avoiding long term immunosuppressive therapy in DIAIH. Also, in 30%-35% of DIAIH cases that were initially seropositive for ANA and SMA, the antibodies became undetectable at 6 mo following the initial DILI, which was independent of treatment with corticosteroids[15].
Major hepatology society guidelines recommend a similar approach in terms of corticosteroids initiation in non-resolving DIAIH and AIH[10,11]. However, there is no consensus regarding treatment duration and methods to confirm remission before discontinuation of immunosuppressants. Thus, the decision and timing to stop treatment should be assessed on case-by-case basis[10]. In fact, one study has shown success in tapering off immunosuppression within 3-17 mo without evidence of relapse[20].
Risk alleles and monocyte-derived hepatocyte like cells
Risk alleles for idiopathic AIH such as HLA DRB1 DRB1*03:01 or DRB1*04:01 are uncommon in DIAIH and, if this is detected, may favor a diagnosis of AIH[10]. The MetaHeps® test, which uses monocyte-derived hepatocyte-like cells, has been used to differentiate DIAIH from AIH with high sensitivity and specificity[21]. However, this test is not readily available, thereby limiting its utility in clinical practice. The lymphocyte toxicity assay described by Neuman et al[35] can sometimes be helpful in differentiating drug hypersensitivity syndromes from idiopathic AIH as well.
Outcomes
The long-term outcome of DIAIH is excellent with a survival rate between 90%-100% without liver transplantation[10]. This is in contrast with idiopathic AIH, where there is a high risk of relapse upon treatment withdrawal and risk of progression to cirrhosis, resulting in the need for liver transplantation[10,11,34]. It is also noteworthy that DIAIH can lead to chronic DILI with abnormal LFT lasting more than 6 mo in 17%-22% of cases[15,36].
CONCLUSION
In summary, it is important to differentiate between DIAIH and AIH in patients who present with DILI, as the management and outcome differ. Early recognition of DIAIH is key as the mainstay of management is cessation of culprit drug and in some cases, initiation of corticosteroids with the aim to avoid long-term immunosuppression.
ACKNOWLEDGEMENTS
We acknowledge all our colleagues in the Department of Gastroenterology and Hepatology and Pathology at Changi General Hospital who help manage the 2 patients described in the manuscript. We would also like to thank the 2 patients described here who readily consented to be described in this manuscript.
Footnotes
Conflict-of-interest statement: All authors have no relevant conflict of interest to report.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: August 5, 2021
First decision: September 4, 2021
Article in press: May 14, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Komori A, Japan; Malnick SDH, Israel S-Editor: Yan JP L-Editor: Filipodia P-Editor: Yan JP
Contributor Information
Chin Kimg Tan, Gastroenterology and Hepatology, Changi General Hospital, Singapore 529889, Singapore; Medicine Academic Clinical Programme, SingHealth Duke-NUS Academic Medical Centre, Singapore 529889, Singapore.
Danielle Ho, Gastroenterology and Hepatology, Changi General Hospital, Singapore 529889, Singapore.
Lai Mun Wang, Section of Pathology, Department of Laboratory Medicine, Changi General Hospital, Singapore 529889, Singapore; Pathology Academic Clinical Programme, SingHealth Duke-NUS Academic Medical Centre, Singapore 529889, Singapore.
Rahul Kumar, Gastroenterology and Hepatology, Changi General Hospital, Singapore 529889, Singapore; Medicine Academic Clinical Programme, SingHealth Duke-NUS Academic Medical Centre, Singapore 529889, Singapore. rahul.kumar@singhealth.com.sg.
References
- 1.Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–1425, 1425.e1. doi: 10.1053/j.gastro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Shen T, Liu Y, Shang J, Xie Q, Li J, Yan M, Xu J, Niu J, Liu J, Watkins PB, Aithal GP, Andrade RJ, Dou X, Yao L, Lv F, Wang Q, Li Y, Zhou X, Zhang Y, Zong P, Wan B, Zou Z, Yang D, Nie Y, Li D, Wang Y, Han X, Zhuang H, Mao Y, Chen C. Incidence and Etiology of Drug-Induced Liver Injury in Mainland China. Gastroenterology. 2019;156:2230–2241.e11. doi: 10.1053/j.gastro.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM U. S. Acute Liver Failure Study Group. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 4.Wei G, Bergquist A, Broomé U, Lindgren S, Wallerstedt S, Almer S, Sangfelt P, Danielsson A, Sandberg-Gertzén H, Lööf L, Prytz H, Björnsson E. Acute liver failure in Sweden: etiology and outcome. J Intern Med. 2007;262:393–401. doi: 10.1111/j.1365-2796.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- 5.Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol. 2005;40:1095–1101. doi: 10.1080/00365520510023846. [DOI] [PubMed] [Google Scholar]
- 6.Ohmori S, Shiraki K, Inoue H, Okano H, Yamanaka T, Deguchi M, Sakai T, Takase K, Nakano T, Tameda Y. Clinical characteristics and prognostic indicators of drug-induced fulminant hepatic failure. Hepatogastroenterology. 2003;50:1531–1534. [PubMed] [Google Scholar]
- 7.Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, Watkins PB, Navarro V, Barnhart H, Gu J, Serrano J United States Drug Induced Liver Injury Network. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. doi: 10.1053/j.gastro.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, Hunt CM, Wilke RA, Avigan M, Kaplowitz N, Bjornsson E, Daly AK. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89:806–815. doi: 10.1038/clpt.2011.58. [DOI] [PubMed] [Google Scholar]
- 9.Weiler-Normann C, Schramm C. Drug induced liver injury and its relationship to autoimmune hepatitis. J Hepatol. 2011;55:747–749. doi: 10.1016/j.jhep.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 10.Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, Vierling JM, Alsawas M, Murad MH, Czaja AJ. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases. Hepatology. 2020;72:671–722. doi: 10.1002/hep.31065. [DOI] [PubMed] [Google Scholar]
- 11.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63:971–1004. doi: 10.1016/j.jhep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 12.Gordon V, Adhikary R, Appleby V, Das D, Day J, Delahooke T, Dixon S, Elphick D, Hardie C, Hoeroldt B, Hooper P, Hutchinson J, Jones R, Khan F, Aithal GP, McGonigle J, Nelson A, Nkhoma A, Pelitari S, Prince M, Prosser A, Sathanarayana V, Savva S, Shah N, Saksena S, Thayalasekaran S, Vani D, Yeoman A, Gleeson D UK Multi-Centre AIH Audit Group. Diagnosis, presentation and initial severity of Autoimmune Hepatitis (AIH) in patients attending 28 hospitals in the UK. Liver Int. 2018;38:1686–1695. doi: 10.1111/liv.13724. [DOI] [PubMed] [Google Scholar]
- 13.Björnsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, Neuhauser M, Lindor K. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010;51:2040–2048. doi: 10.1002/hep.23588. [DOI] [PubMed] [Google Scholar]
- 14.Valgeirsson KB, Hreinsson JP, Björnsson ES. Increased incidence of autoimmune hepatitis is associated with wider use of biological drugs. Liver Int. 2019;39:2341–2349. doi: 10.1111/liv.14224. [DOI] [PubMed] [Google Scholar]
- 15.de Boer YS, Kosinski AS, Urban TJ, Zhao Z, Long N, Chalasani N, Kleiner DE, Hoofnagle JH Drug-Induced Liver Injury Network. Features of Autoimmune Hepatitis in Patients With Drug-induced Liver Injury. Clin Gastroenterol Hepatol. 2017;15:103–112.e2. doi: 10.1016/j.cgh.2016.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Licata A, Maida M, Cabibi D, Butera G, Macaluso FS, Alessi N, Caruso C, Craxì A, Almasio PL. Clinical features and outcomes of patients with drug-induced autoimmune hepatitis: a retrospective cohort study. Dig Liver Dis. 2014;46:1116–1120. doi: 10.1016/j.dld.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 17.Czaja AJ. Drug-induced autoimmune-like hepatitis. Dig Dis Sci. 2011;56:958–976. doi: 10.1007/s10620-011-1611-4. [DOI] [PubMed] [Google Scholar]
- 18.Russo MW, Scobey M, Bonkovsky HL. Drug-induced liver injury associated with statins. Semin Liver Dis. 2009;29:412–422. doi: 10.1055/s-0029-1240010. [DOI] [PubMed] [Google Scholar]
- 19.Perdices EV, Medina-Cáliz I, Hernando S, Ortega A, Martín-Ocaña F, Navarro JM, Peláez G, Castiella A, Hallal H, Romero-Gómez M, González-Jiménez A, Robles-Díaz M, Lucena MI, Andrade RJ. Hepatotoxicity associated with statin use: analysis of the cases included in the Spanish Hepatotoxicity Registry. Rev Esp Enferm Dig. 2014;106:246–254. [PubMed] [Google Scholar]
- 20.Wang H, Fu J, Liu G, Wang L, Yan A, Wang G. Drug induced autoimmune hepatitis (DIAIH): Pathological and clinical study. Biomed Res. 2017;28:6028–6034. [Google Scholar]
- 21.Weber S, Benesic A, Rotter I, Gerbes AL. Early ALT response to corticosteroid treatment distinguishes idiosyncratic drug-induced liver injury from autoimmune hepatitis. Liver Int. 2019;39:1906–1917. doi: 10.1111/liv.14195. [DOI] [PubMed] [Google Scholar]
- 22.Hisamochi A, Kage M, Ide T, Arinaga-Hino T, Amano K, Kuwahara R, Ogata K, Miyajima I, Kumashiro R, Sata M, Torimura T. An analysis of drug-induced liver injury, which showed histological findings similar to autoimmune hepatitis. J Gastroenterol. 2016;51:597–607. doi: 10.1007/s00535-015-1131-7. [DOI] [PubMed] [Google Scholar]
- 23.Beaune P, Dansette PM, Mansuy D, Kiffel L, Finck M, Amar C, Leroux JP, Homberg JC. Human anti-endoplasmic reticulum autoantibodies appearing in a drug-induced hepatitis are directed against a human liver cytochrome P-450 that hydroxylates the drug. Proc Natl Acad Sci U S A. 1987;84:551–555. doi: 10.1073/pnas.84.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourdi M, Larrey D, Nataf J, Bernuau J, Pessayre D, Iwasaki M, Guengerich FP, Beaune PH. Anti-liver endoplasmic reticulum autoantibodies are directed against human cytochrome P-450IA2. A specific marker of dihydralazine-induced hepatitis. J Clin Invest. 1990;85:1967–1973. doi: 10.1172/JCI114660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czaja AJ. Autoimmune hepatitis. Part A: pathogenesis. Expert Rev Gastroenterol Hepatol. 2007;1:113–128. doi: 10.1586/17474124.1.1.113. [DOI] [PubMed] [Google Scholar]
- 26.Liu ZX, Kaplowitz N. Immune-mediated drug-induced liver disease. Clin Liver Dis. 2002;6:755–774. doi: 10.1016/s1089-3261(02)00025-9. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ, Donaldson PT, Eddleston AL, Fainboim L, Heathcote J, Homberg JC, Hoofnagle JH, Kakumu S, Krawitt EL, Mackay IR, MacSween RN, Maddrey WC, Manns MP, McFarlane IG, Meyer zum Büschenfelde KH, Zeniya M. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 28.Czaja AJ. Performance parameters of the diagnostic scoring systems for autoimmune hepatitis. Hepatology. 2008;48:1540–1548. doi: 10.1002/hep.22513. [DOI] [PubMed] [Google Scholar]
- 29.Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1330. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 30.Yeong TT, Lim KH, Goubet S, Parnell N, Verma S. Natural history and outcomes in drug-induced autoimmune hepatitis. Hepatol Res. 2016;46:E79–E88. doi: 10.1111/hepr.12532. [DOI] [PubMed] [Google Scholar]
- 31. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012– . [PubMed] [Google Scholar]
- 32.Aliberti S, Grignani G, Allione P, Fizzotti M, Galatola G, Pisacane A, Aglietta M. An acute hepatitis resembling autoimmune hepatitis occurring during imatinib therapy in a gastrointestinal stromal tumor patient. Am J Clin Oncol. 2009;32:640–641. doi: 10.1097/COC.0b013e31802b4ef7. [DOI] [PubMed] [Google Scholar]
- 33.Chalasani NP, Maddur H, Russo MW, Wong RJ, Reddy KR Practice Parameters Committee of the American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Idiosyncratic Drug-Induced Liver Injury. Am J Gastroenterol. 2021;116:878–898. doi: 10.14309/ajg.0000000000001259. [DOI] [PubMed] [Google Scholar]
- 34.Martínez-Casas OY, Díaz-Ramírez GS, Marín-Zuluaga JI, Muñoz-Maya O, Santos O, Donado-Gómez JH, Restrepo-Gutiérrez JC. Differential characteristics in drug-induced autoimmune hepatitis. JGH Open. 2018;2:97–104. doi: 10.1002/jgh3.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neuman MG, Malkiewicz IM, Shear NH. A novel lymphocyte toxicity assay to assess drug hypersensitivity syndromes. Clin Biochem. 2000;33:517–524. doi: 10.1016/s0009-9120(00)00146-6. [DOI] [PubMed] [Google Scholar]
- 36.Castiella A, Zapata E, Lucena MI, Andrade RJ. Drug-induced autoimmune liver disease: A diagnostic dilemma of an increasingly reported disease. World J Hepatol. 2014;6:160–168. doi: 10.4254/wjh.v6.i4.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stephens C, Robles-Diaz M, Medina-Caliz I, Garcia-Cortes M, Ortega-Alonso A, Sanabria-Cabrera J, Gonzalez-Jimenez A, Alvarez-Alvarez I, Slim M, Jimenez-Perez M, Gonzalez-Grande R, Fernández MC, Casado M, Soriano G, Román E, Hallal H, Romero-Gomez M, Castiella A, Conde I, Prieto M, Moreno-Planas JM, Giraldez A, Moreno-Sanfiel JM, Kaplowitz N, Lucena MI, Andrade RJ Participating clinical centres. Comprehensive analysis and insights gained from long-term experience of the Spanish DILI Registry. J Hepatol. 2021;75:86–97. doi: 10.1016/j.jhep.2021.01.029. [DOI] [PubMed] [Google Scholar]