Abstract

A simple and efficient method for the stereoselective synthesis of nojirimycin α-C-glycoside derivatives has been developed using a bicyclic carbamate-type sp2-iminosugar, whose preparation on a gram scale has been optimized, as the starting material. sp2-iminosugar O-glycosides or anomeric esters serve as excellent precursors of acyliminium cations, which can add nucleophiles, including C-nucleophiles. The stereochemical outcome of the reaction is governed by stereoelectronic effects, affording the target α-anomer with total stereoselectivity. Thus, the judicious combination of C-allylation, carbamate hydrolysis, cross-metathesis, and hydrogenation reactions provides a very convenient entry to iminosugar α-C-glycosides, which have been transformed into N,C-biantennary derivatives by reductive amination or thiourea-forming reactions. The thiourea adducts undergo intramolecular cyclization to bicyclic iminooxazolidine iminosugar α-C-glycosides upon acid treatment, broadening the opportunities for molecular diversity. A preliminary evaluation against a panel of commercial glycosidases validates the approach for finely tuning the inhibitory profile of glycomimetics.
Introduction
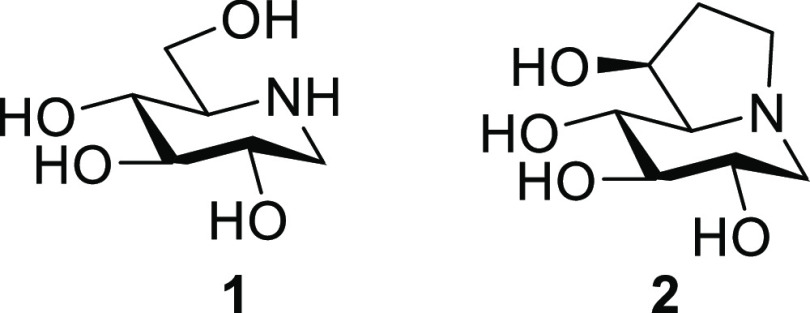
Nitrogen-in-the-ring sugar mimetics (iminosugars) have played a prominent role in our understanding of the mechanisms underlying the metabolism and biological functions of carbohydrates during the last five decades.1−6 By interfering in the biosynthesis or processing of glycans and glycoconjugates, iminosugars enable the regulation of pathological processes resulting from the dysfunction of the intervening enzymes, namely glycosyl hydrolases (glycosidases)7 and glycosyl transferases.8 These processes cause from major global health problems, for instance diabetes9 or cystic fibrosis,10 to highly disabling rare syndromes such as the lysosomal storage disorders Gaucher, Pompe, GM1-gangliosidosis, and Fabry diseases.11−14 Disrupting the activity of carbohydrate-processing enzymes in pathogens can be further exploited in fighting infectious diseases: e.g., viral infections.15,16 Innate immune response signaling routes,17,18 cell proliferation and metastasis,19 or age-related neurodegenerative disorders, e.g. Alzheimer and Parkinson diseases,20,21 can also be targeted with iminosugars. The recent reports on the promise of the piperidine- and indolizidine-type iminosugars 1-deoxynojirimycin (DNJ; 1) and castanospermine (CS; 2), respectively, and some of their derivatives for treating patients infected with SARS-CoV-2, the causative agent of the Covid-19 worldwide pandemic, attest to the unrelenting interest in the therapeutic potential of this amazing family of glycomimetics (Figure 1).22−27
Figure 1.
Structures of the bioactive iminosugars 1-deoxynojirimycin (DNJ, 1) and castanospermine (CS, 2).
The sustained success of iminosugars in glycobiology research and drug discovery programs lies, to a great extent, on the large structural variability provided by the naturally occurring representatives and the hundreds of synthetic analogues reported over the years. Most efforts on the chemistry side have been directed to devise efficient strategies matching the stereochemical and substitution profiles of the monosaccharides.28,29−31 Replicating the glycosidic linkage distinctive of glycoconjugates, however, has proven much more challenging. The intrinsic instability of aminoacetal functionalities makes impractical the synthesis of iminosugar O-glycosides or of analogues having other heteroatom substituents at the pseudoanomeric position. Instead, aglycon-like appendages have been incorporated through N-substitution32−39 and C-branching approaches,40−47 which accounts for the two major subclasses of iminosugar derivatives on record.
The combination of N-substitution and C-branching in the same iminosugar scaffold appears to be an obvious strategy for finely tuning the biological activity.48 Surprisingly, the reported examples of biantennary N-substituted iminosugar C-glycosides are rather limited.49−51 An apparent reason is the complexity in accessing the C-glycoside precursors in pure diastereoisomeric form. Most of the current protocols involve intramolecular reductive amination47,52 or aza-Wittig cyclization reactions,47,50,53 with variable diastereoselectivity outcomes, or the use of 1-C-activated precursors that are themselves obtained as mixtures of diastereomers (Figure 2, left panel).47,54 Sharply different, the 5N,6O-oxomethylidenenojirimycin derivative 3(55,56) (ONJ), a member of the sp2-iminosugar subgroup,57 is a chemically stable and configurationally defined compound that can be functionalized at the pseudoanomeric position via the corresponding acyliminium cation with total α-stereoselectivity (Figure 2, right panel).58−62 Since the cyclic carbamate ring can be considered as a protecting group of the vicinal amino alcohol fragment,63 we conceived that 3 could provide a reliable access to iminosugar α-C-glycosides and then to N-substituted-α-C-glycosides, in both the classical and sp2-iminosugar series, thereby offering opportunities for molecular diversity. As a proof of concept, here we report (a) an optimized synthesis of 3 on a gram scale, (b) its transformation into NJ α-C-glycosides (I) by allylation–carbamate hydrolysis–cross-metathesis sequences, (c) the synthesis of N-alkyl (II) and N-(N’-alkylthiocarbamoyl) (III) biantennary derivatives via an intermolecular reductive amination or a thiourea-forming reaction, respectively, and (d) the cyclization of the last two species to access bicyclic isothiourea analogues (IV; Figure 2, right panel). The potential of the strategy to customize the glycosidase inhibition pattern of the glycomimetics is also discussed.
Figure 2.
Retrosynthetic analysis commonly used in the synthesis of iminosugar C-glycosides and N,C-biantennary iminosugars via (A) intramolecular reductive amination, (B) aza-Wittig reaction, or (C) 1-C-activated precursors the and strategy proposed in this work starting from the bicyclic carbamate sp2-iminosugar ONJ (3).
Results and Discussion
Synthesis
Compound 3 has been previously prepared on a 500 mg scale from commercial 1,2-O-isopropylidene-α-d-glucuronolactone (4) in 17% overall yield through a nine-step route involving, as the key transformations, nucleophilic substitution of chlorine by azide and OH-6 deprotection to render the d-gluco vicinal azido alcohol intermediate 5 (25% over five steps; Scheme 1, route A).55,56 We have now optimized this critical double-inversion sequence by using triflate as the leaving group and trifluoroacetate and azide as the nucleophiles.64 Conducting both substitution reactions on the lactone scaffold avoids the need for additional OH-6 protection/deprotection, considerably increasing the efficiency of the 4 to 5 conversion (55% over three steps; Scheme 1, route B). The whole synthetic scheme has been reproducibly scaled up by 20-fold with no significant difference in product yield, providing a very convenient access to the reducing sp2-iminosugar 3 in gram quantities (43% over eight steps from commercial 9; see the Supporting Information for a detailed description of reaction conditions and synthetic intermediates).
Scheme 1. Optimized Synthesis of the sp2-Iminosugar Precursor 3 and Its Transformation into the Known NJ α-C-Glycosides 13 and 16.
En route toward the synthesis of ONJ pseudo-C-glycosides, the incorporation of a pseudoanomeric methoxy group followed by O-benzylation of the secondary alcohols was first considered. N-Acylated aminal derivatives are known to be suitable precursors for acyliminium cation species, which can undergo the addition of a variety of nucleophiles, including carbon nucleophiles.65−67 Accordingly, a Fischer-type pseudoglycosidation reaction of 3 with methanol, using an acid resin as the promoter, afforded the corresponding ONJ methyl α-O-glycoside 6 (69% yield), which upon conventional benzylation afforded the tri-O-benzyl derivative 7 (91% yield). C-Allylation of 7 by treatment with allyltrimethylsilane (AllTMS) proceeded smoothly in acetonitrile at room temperature in the presence of trimethylsilyl triflate to give the pivotal ONJ allyl α-C-glycoside 8 in excellent yield. No trace of the β diastereomer was detected in either the O- or the C-glycosylation reaction mixtures. However, the relatively modest yield of the O-glycosylation reaction was judged to be a limitation. As an alternative, peracetate 9 was prepared and attempted as a pseudoglycosyl donor for C-glycosylation. Gratifyingly, the reaction with AllTMS in acetonitrile at 80 °C, using boron trifluoride etherate as a promoter, provided the corresponding allyl α-C-glycoside 10 in a remarkable 91% yield. It is worth noting that the α stereoselectivity was preserved despite the participating character of the acetyl group vicinal to the pseudoanomeric center. Compound 10 was next transformed into 8 by standard deacetylation (→11)/benzylation protocols (Scheme 1).
Hydrolysis of the cyclic carbamate functionality in 8 was successfully effected by treatment with barium hydroxide in a mixture of methanol and water at 70 °C.68−70 Partition of the reaction mixture between ethyl acetate and water allowed a very convenient separation of the organic product from the inorganic salts, which was instead very problematic when the base-sensitive triacetate 10 was used as the precursor. The allyl tri-O-benzyl-NJ α-C-glycoside 12 was thus obtained in 93% yield. Palladium(0)-catalyzed hydrogenation next delivered the known iminosugar propyl α-C-glycoside 13.53,71,72 Interestingly, protection of the amino group in 12 followed by cross-metathesis with terminal alkenes and final hydrogenation enables a general entry to NJ alkyl α-C-glycosides. As a proof of concept, the potent β-glucocerebrosidase inhibitor 16(72) was synthesized through this procedure via the corresponding N-benzyloxycarbonyl 14 and unsaturated C-glycoside 15 (mixture of E and Z isomers in a 5:1 ratio) intermediates in excellent yield (Scheme 1).
Having a general strategy to access iminosugar α-C-glycosides in hand, we undertook the preparation of N,C-biantennary nojirimycin derivatives. Attempts to selectively N-alkylate 13 with butyl and octyl bromides using sodium carbonate as a base failed to provide the corresponding tertiary amines in pure form. Alternatively, reductive amination of the partially protected precursor 12 with butanal and octanal furnished the desired N-alkyl iminosugars 17 and 18 in 70–90% yield, which after catalytic hydrogenation afforded the target fully unprotected N,C-dialkyliminosugars 19 and 20. The propyl and octyl NJ α-C-glycosides 13 and 16 were also readily transformed into the corresponding N-(N′-butyl)thiocarbamoyl (21 and 27) and N-(N′-octyl)thiocarbamoyl adducts (22 and 28) by a thiourea-forming reaction, a click-type conjugation chemistry,73,74 with butyl and octyl isothiocyanates, respectively. The thiocarbamoylated propyl α-C-glycoside series was additionally expanded with the corresponding N′-benzyl, N′-phenethyl, N′-p-methoxybenzyl and N′-p-trifluoromethylbenzyl congeners 23–26 to test the generality of the approach and assess the effect of an aromatic moiety on the glycosidase inhibitory properties (Scheme 2).
Scheme 2. Synthesis of NJ N-Alkyl α-C-Glycosides 19 and 20, N-(N′-Alkylthiocarbamoyl) α-C-Glycosides 21–28, and Bicyclic N′-Alkyl Iminothiazolidine α-C-Glycosides 29–32.
Intramolecular cyclization of 21, 22, 27, and 28 was achieved by heating an acid (HCl) methanolic solution, affording the bicyclic iminothiazolidines 29–32 as a novel family of N,C- biantennary glycomimetics (Scheme 2).75
Effect of Structural Modifications in Nojirimycin α-C-Glycosides on the Glycosidase Inhibitory Profile
The synthesized compounds were assayed against a panel of glycosidases covering a range of substrate configuration specificities (α- and β-d-glucosidases, α- and β-d-mannosidases and α- and β-d-galactosidases; glcases, Manases, and Galases, respectively). Given the stereochemical complementarity of the ONJ derivatives with d-glucopyranosides, α (baker’s yeast, rice, or Aspergillus niger)- and β-glcases (Thermotoga maritima, almonds, or bovine liver) from diverse origins were included to assess potential selectivity biases. Table 1 displays the corresponding inhibition constant (Ki) values.
Table 1. Inhibition Constants (Ki, μM) for the Nojirimycin N,C-Biantennary Derivatives 19–26 and 27–32, in Comparison with Data for the Nojirimycin α-C-Glycosides 13 and 16a.
| compd | α-Glcase baker’s yeast | α-Glcase rice | amyloglcase A. niger | β-Glcase T. maritima | β-Glcase almonds | β-Glcase bovine | α-Galase coffee |
|---|---|---|---|---|---|---|---|
| 13 | 92 ± 8 | 11 ± 1 | 67 ± 5 | 657 ± 65 | nib | ni | ni |
| 19 | ni | ni | ni | ni | ni | 117 ± 12 | ni |
| 20 | ni | 25 ± 3 | ni | ni | ni | 358 ± 35 | 130 ± 12 |
| 21 | ni | 8.2 ± 0.6 | ni | ni | ni | 136 ± 11 | ni |
| 22 | ni | 11 ± 0.7 | ni | ni | ni | ni | ni |
| 23 | ni | 583 ± 0.2 | 154 ± 12 | ni | ni | ni | ni |
| 24 | ni | 72 ± 6 | 455 ± 40 | ni | ni | ni | ni |
| 25 | ni | 340 ± 30 | 842 ± 75 | ni | ni | ni | ni |
| 26 | ni | 104 ± 9 | ni | ni | ni | ni | ni |
| 16 | 10.7 ± 0.9 | 0.38 ± 0.04 | 12.3 ± 2 | 29 ± 3 | 28 ± 2 | 32 ± 3 | 87 ± 9 |
| 27 | 119 ± 10 | 3.3 ± 0.5 | 92 ± 8 | 293 ± 25 | ni | 169 ± 15 | 508 ± 35 |
| 28 | ni | 4.3 ± 0.6 | 455 ± 40 | ni | ni | ni | ni |
| 29 | 439 ± 35 | ni | ni | 16 ± 1 | 75 ± 5 | 177 ± 14 | ni |
| 30 | ni | ni | ni | 280 ± 25 | ni | ni | ni |
| 31 | ni | ni | 842 ± 80 | ni | ni | 134 ± 10 | ni |
| 32 | ni | ni | 305 ± 28 | ni | ni | ni | ni |
The inhibition was competitive in all cases. No inhibitory activity was detected for any of the compounds at 2 mM against jack bean α-mannosidase, Helix pomatia β-mannosidase, or E. coli β-galactosidase at 2 mM concentration.
No inhibition observed at 2 mM concentration.
The α-C-Propyl NJ 13 is a micromolar inhibitor of the three α-glucosidases tested in this work, exhibiting full configurational and anomeric selectivity and a higher affinity for the rice enzyme (Ki = 11 ± 1 μM). The concurrent presence of the N-butyl substituent in 19 totally abolished inhibition of the α-glucosidases, promoting instead weak inhibition of bovine β-glucosidase (Ki = 117 ± 12 μM). Interestingly, the corresponding N-octyl derivative 20 recovered the ability to inhibit rice α-glucosidase (Ki = 25 ± 3 μM), showing in this case total selectivity among the α-glucosidase isoenzymes. It also weakly inhibited bovine β-glucosidase and green coffee bean α-galactosidase. The neutral N-thiocarbamoyl α-C-propyl cognates 21 and 22 preserved the selectivity toward rice α-glucosidase and exhibited even higher inhibitory potencies. Indeed, the N′-octyl derivative 22 (Ki = 11 ± 0.7 μM) was as potent as the parent compound 13 and did not show significant inhibition of any other of the tested enzymes at 2 mM concentration. When α-C-octyl NJ 16 and the N-(N′-propyl)- and N-(N′-octyl)thiocarbamoyl analogues 27 and 28 were compared, a similar narrowing effect in the spectra of responsive glycosidases was observed, the latter being also a powerful and selective inhibitor of rice α-glucosidase (Ki = 4.3 ± 0.6 μM). The effect of the N′-substituent nature on the inhibitory activity of thiourea-type NJ C-glycosides became much more evident when the above data were compared with data for the aromatic-bearing derivatives 23–26: a drastic increase in the Ki values against rice α-glucosidase was observed in all cases (Ki > 70 μM), accompanied by weak inhibition of amyloglucosidase in the case of compounds 23–25. Transformation of the neutral monocyclic thiourea core into a basic bicyclic isothiourea has been previously found to be a powerful strategy to adjust the enzyme inhibition selectivity of sp2-iminosugar glycomimetics. In the α-C-glycoside series, we encountered that cyclization of 21, 22, 27, and 28 into the corresponding iminothiazolidines 29–32 generally shifted the anomeric selectivity from the α- to the β-glucosidases, compound 29 being a strong/modest inhibitor of the three β-glucosidase isoenzymes monitored in this study (Ki = 16 ± 1, 75 ± 5 and 177 ± 14 μM for the T. maritima, almonds, and bovine liver isoenzymes, respectively).
Conclusions
We have developed an efficient tactic for the stereoselective synthesis of iminosugar α-C-glycosides that exploits the unique reactivity and stereoelectronic properties of sp2-iminosugars. The procedures are purposely conceived to be general and translatable to other configurational patterns. Unlike classical iminosugars, reducing carbamate-type bicyclic sp2-iminosugars can be transformed into stable O-glucosides or anomeric esters that serve as suitable precursors for acyliminium cations. The rich chemistry of these intermediates enables mimicking the underlying chemistry of glycosyl oxocarbenium cation species, which has been applied to the case of C-glycosidation. An optimized preparation of the nojirimycin-related sp2-iminosugar ONJ (3) demonstrates the feasibility of the strategy. A very efficient reaction sequence employing C-allylation, carbamate hydrolysis, and cross-metathesis was then implemented to access nojirimycin α-C-glycosides, from which N,C-biantennary glycomimetics were readily prepared by reductive amination with aldehydes or by coupling with isothiocyanates. NJ α-C-glycoside thiourea adducts have been next transformed into N′-substituted iminothiazolidines, the first representatives of bicyclic N,C-biantennary iminosugars. A main advantage of the procedure is its rather low synthetic cost, high versatility, and suitability for molecular-diversity-oriented approaches. As a proof of concept, compounds with high inhibition potency and selectivity toward rice α-glcase have been identified.
Experimental Section
General Methods
Reagents and solvents were commercial grade and were used without further purification. Optical rotations were measured using a sodium lamp (λ = 589 nm) at 22 °C in 1 cm or 1 dm tubes. Unit for ε values from UV spectra: mM–1 cm–1. NMR experiments were performed at 300 (75.5) and 500 (125.7) MHz. 1-D TOCSY as well as 2-D COSY and HSQC experiments were carried out to assist in signal assignments. TLC was performed on precoated plates, silica gel 30F-245. Column chromatography was conducted on Chromagel (silice 60 AC.C 70–200 μm). Elemental analyses were performed at the Servicio de Microanálisis del Instituto de Investigaciones Químicas de Sevilla, Spain. ESI mass spectra were recorded for 0.1 pM samples using 50% aqueous MeCN at 0.1 mL min–1 as the mobile phase. (1R)-2,3,4-Tri-O-acetyl-1-C-allyl-5N,6O-oxomethylidene-1-deoxynojirimycin (10)76 and (1R)-1-C-allyl-5N,6O-oxomethylidene-1-deoxynojirimycin (11)76 were synthesized using previously described procedures.
(1R)-1-O-Methyl-5N,6O-oxomethylidenenojirimycin (6)
A solution of ONJ (3; 95 mg, 0.46 mmol) in MeOH (2.2 mL) was treated with Amberlite IRA 120 H+ ion-exchange resin, stirred at RT under Ar for 24 h, filtered, and chromatographed using 70/10/1 → 20/10/1 DCM/MeOH/H2O as eluent. Yield: 61 mg (60%; white amorphous solid). Rf = 0.62 (40/10/1 DCM/MeOH/H2O). [α]D +53.5 (c 1.0 in MeOH). 1H NMR (300 MHz, CD3OD): δ 4.85 (d, 1 H, J1,2 = 4.0 Hz, H-1), 4.70 (bs, 3 H, OH), 4.49 (t, 1 H, J5,6a = J6a,6b = 8.6 Hz, H-6a), 4.18 (dd, 1 H, J5,6b = 6.1 Hz, H-6b), 3.46 (ddd, 1 H, J4,5 = 9.4 Hz, H-5), 3.52 (dd, 1 H, J2,3 = J3,4 = 9.4 Hz, H-3), 3.35 (dd, 1 H, H-2), 3.30 (s, 3 H, OMe), 3.25 (t, 1 H, H-4). 13C NMR (75.5 MHz, CD3OD): δ 158.9 (CO), 84.6 (C-1), 75.7 (C-4), 74.4 (C-3), 73.0 (C-2), 68.5 (C-6), 56.3 (OMe), 54.7 (C-5). ESIMS: m/z 242.1 [M + Na]+. Anal. Calcd for C8H13NO6: C, 43.84; H, 5.98; N, 6.39. Found: C, 43.96; H, 6.11; N, 6.21.
(1R)-2,3,4-Tri-O-benzyl-1-O-methyl-5N,6O-oxomethylidenenojirimycin (7)
A solution of 6 (189 mg, 0.86 mmol) in anhydrous DMF (4.3 mL), under Ar at 0 °C, was treated with 95% NaH (155 mg, 6.45 mmol; slow addition) and stirred for 10 min. Benzyl bromide (613 μL, 5.16 mmol) was added dropwise and stirring was continued at RT for 24 h. The reaction mixture was quenched with water (6 mL) and extracted with Et2O (5 × 5 mL), and the combined organic extracts were washed with water (2 × 10 mL), dried (MgSO4), concentrated, and chromatographed using 1/5 → 1/3 → 1/1 EtOAc/cyclohexane as eluent. Yield: 383 mg (91%; white amorphous solid). Rf = 0.42 (1/2 EtOAc/cyclohexane). [α]D +86.8 (c 1.0 in DCM). 1H NMR (300 MHz, CDCl3): δ 7.30–7.15 (m, 15 H, Ph), 4.95 (d, 1 H, J1,2 = 3.9 Hz, H-1), 4.94, 4.72 (2 d, 2 H, 2JH,H = 10.7 Hz, CHPh), 4.81, 4.52 (2 d, 2 H, 2JH,H = 11.7 Hz, CHPh), 4.66 (s, 2 H, CH2Ph), 4.24 (t, 1 H, J6a,6a = J5,6a = 8.6 Hz, H-6a), 3.90 (t, 1 H, J2,3 = J3,4 = 9.2 Hz, H-3), 3.69 (m, 1 H, H-5), 3.59 (dd, 1 H, J5,6b = 6.6 Hz, H-6b), 3.44 (dd, 1 H, H-2), 3.31 (s, 3 H, OMe), 3.27 (t, 1 H, H-4). 13C NMR (75.5 MHz, CDCl3): δ 156.4 (CO), 138.4–127.8 (Ph), 81.6 (C-3), 80.9 (C-4), 80.7 (C-1), 79.8 (C-2), 76.1, 74.8, 73.0 (CH2Ph), 67.0 (C-6), 56.1 (OMe), 52.3 (C-5). ESIMS: m/z 512.3 [M + Na]+. Anal. Calcd for C29H31NO6: C, 71.15; H, 6.38; N, 2.86. Found: C, 71.25; H, 6.50; N, 2.76.
(1R)-1-C-Allyl-2,3,4-tri-O-benzyl-5N,6O-oxomethylidene-1-deoxynojirimycin (8)
From 7
A solution of 7 (105 mg, 0.2 mmol) in anhydrous MeCN (1.1 mL) at RT, under Ar, was treated with allylTMS (341 μL, 2.2 mmol), stirred for 30 min, and cooled to 0 °C. TMSOTf (195 μL, 1.1 mmol) was added dropwise, and the mixture was further stirred for 19 h at RT, diluted with DCM (10 mL), and washed with saturated aqueous NaHCO3 (2 × 10 mL), water (10 mL), and brine (10 mL). The organic extracts were dried (MgSO4), concentrated, and chromatographed (1/4 EtOAc/cyclohexane). Yield: 108 mg (98%; white amorphous solid).
From 11
A solution of 11 (607 mg, 2.65 mmol) in anhydrous DMF (14 mL), under Ar at 0 °C, was treated with 95% NaH (477 mg, 19.9 mmol, slow addition) and stirred for 10 min. Benzyl bromide (1.9 mL, 15.9 mmol) was added dropwise, and stirring was continued at RT for 24 h. The reaction mixture was quenched by addition of water (6 mL) and extracted with Et2O (5 × 5 mL), and the combined organic extracts were washed with water (2 × 10 mL), dried (MgSO4), concentrated, and chromatographed using 1/5 → 1/3 → 1/1 EtOAc/cyclohexane as eluent. Yield: 1.29 g (97%; white amorphous solid). Rf = 0.50 (1/2 EtOAc/cyclohexane). [α]D +74.9 (c 1.0 in DCM). 1H NMR (300 MHz, CDCl3): δ 7.30–7.15 (m, 15 H, Ph), 5.71–5.60 (m, 1 H, CH=CH2), 5.05–4.99 (m, 2 H, CH = CH2), 4.93, 4.70 (2 d, 2 H, 2JH,H = 10.3 Hz, CHPh), 4.82, 4.52 (2 d, 2 H, 2JH,H = 11.4 Hz, CHPh), 4.62, 4.56 (2 d, 2 H, 2JH,H = 11.4 Hz, CHPh), 4.17 (m, 2 H, H-1, H-6a), 3.68 (t, 1 H, J2,3 = J3,4= 9.5 Hz, H-3), 3.68 (dd, 1 H, J6a,6b = 9.1 Hz, J5,6b = 4.5 Hz, H-6b), 3.57 (dd, 1 H, J1,2 = 5.9 Hz, H-2), 3.52 (m, 1 H, H-5), 3.26 (dd, 1 H, J4,5= 8.8 Hz, H-4), 2.59 (m, 1 H, CHaHbCH = CH2), 2.21–2.09 (m, 1 H, CHaHbCH=CH2). 13C NMR (75.5 MHz, CDCl3): δ 156.9 (CO), 138.3–137.6 (Ph), 133.9 (CH=CH2), 128.7–127.8 (Ph), 117.8 (CH = CH2), 81.9 (C-3), 80.8 (C-4), 79.5 (C-2), 75.8, 75.1, 72.9 (CH2Ph), 66.1 (C-6), 52.9 (C-5), 50.7 (C-1), 30.3 (CH2CH=CH2). ESIMS: m/z 522.4 [M + Na]+. Anal. Calcd for C31H33NO5: C, 74.53; H, 6.66; N, 2.80. Found: C, 74.70; H, 6.83; N, 2.87.
(1R)-1-C-Allyl-2,3,4-tri-O-benzyl-1-deoxynojirimycin (12)
To a solution of 8 (171 mg, 0.34 mmol) in MeOH/H2O (5/1, 3.6 mL) was added Ba(OH)2·8H2O (1.07 g, 3.4 mmol). The suspension was heated at 70 °C for 18 h, diluted with 1/1 H2O/EtOAc (20 mL), and filtered, and the organic phase was separated. The aqueous layer was extracted with EtOAc (3 × 10 mL), and the combined organic extracts were dried (MgSO4), concentrated, and chromatographed using 2/1 → 4/1 → 1/0 EtOAc/cyclohexane as eluent. Yield: 149 mg (93%; white amorphous solid). Rf = 0.34 (2/1 EtOAc/cyclohexane). [α]D +34.3 (c 1.0 in DCM). IR (ATR): νmax 3333, 1750, 1065 cm–1. 1H NMR (400 MHz, CDCl3): δ 7.39–7.28 (m, 15 H, Ph), 5.75 (m, 1 H, CH=CH2), 5.16–5.10 (m, 2 H, CH=CH2), 4.96, 4.82 (2 d, 2 H, 2JH,H = 10.9 Hz, CHPh), 4.92, 4.66 (2 d, 2 H, 2JH,H = 10.7 Hz, CHPh), 4.71, 4.67 (2 d, 2 H, 2JH,H = 11.6 Hz, CHPh), 3.78 (t, 1 H, J2,3 = J3,4= 8.4 Hz, H-3), 3.72 (dd, 1 H, J1,2 = 5.0, H-2), 3.69 (m, 1 H, H-6a), 3.66 (dd, 1 H, J6a,6b = 11.0 Hz, J5,6b = 4.8, H-6b), 3.42 (dd, 1 H, J4,5 = 9.1 Hz, H-4), 3.24 (m, 1 H, H-1), 2.91 (m, 1 H, H-5), 2.50 (m, 1 H, CHaHbCH=CH2), 2.33 (m, 1 H, CHaHbCH=CH2), 2.20 (bs, 2 H, OH, NH). 13C NMR (100.6 MHz, CDCl3): δ 138.7–127.6 (Ph), 135.6 (CH=CH2), 117.9 (CH=CH2), 82.5 (C-3), 81.1 (C-2), 79.4 (C-4), 75.4, 75.1, 72.6 (CH2Ph), 62.7 (C-6), 54.2 (C-5), 53.0 (C-1), 30.5 (CH2CH=CH2). ESIMS: m/z 473.26 [M + Na]+. Anal. Calcd for C30H35NO4: C, 76.08; H, 7.45; N, 2.96. Found: C, 74.21; H, 7.51; N, 2.96.
Synthesis of (1R)-1-C-Propyl-1-deoxynojirimycin (13)
A solution of 12 (0.32 mmol) in degassed MeOH (3.0 mL) was acidified with HCl (300 μL, 6.0 M) and hydrogenated at atmospheric pressure (balloon) for 24 h using 10% Pd/90% C (30 mg). The mixture was filtered (Celite), concentrated, and chromatographed using 10/1/1 → 10/2/1 MeCN/H2O/NH4OH as eluent. Yield: 66 mg (quantitative; colorless oil). The analytical and spectroscopical data of 13 were in agreement with those in the literature.71
Synthesis of (1R)-1-C-Allyl-2,3,4-tri-O-benzyl-N-benzyloxycarbonyl-1-deoxynojirimycin (14)
To a solution of 12 (322 mg, 0.68 mmol) in anhydrous DCM (13.8 mL), under Ar, at RT were added DIPEA (470 μL, 2.72 mmol) and benzyl chloroformate (530 μL, 3.74 mmol). The mixture was stirred for 3 h, diluted with DCM (10 mL), and washed with water (10 mL), and the organic extracts were dried (MgSO4), concentrated, and chromatographed using 1/5 EtOAc/cyclohexane as eluent. Yield: 384 mg (93%; colorless oil). Rf = 0.50 (1/3 EtOAc/cyclohexane). [α]D −27.0 (c 0.8 in DCM). 1H NMR (300 MHz, CDCl3): δ 7.44–7.28 (m, 20 H, Ph), 5.62 (m, 1 H, CH=CH2), 5.16, 5.10 (2 d, 2 H, 2JH,H = 12.4 Hz, CHPh), 5.05 (m, 2 H, CH=CH2), 4.90, 4.82 (2 d, 2 H, 2JH,H = 10.4 Hz, CHPh), 4.87, 4.81 (2 d, 2 H, 2JH,H = 11.0 Hz, CHPh), 4.66, 4.61 (2 d, 2 H, 2JH,H = 11.6 Hz, CHPh), 4.57 (m, 1 H, H-1), 4.12 (m, 2 H, H-6a, H-6b), 3.80 (m, 2 H, H-3, H-4), 3.61 (dd, 1 H, J2,3 = 8.4 Hz, J1,2 = 6.1 Hz, H-2), 3.33 (m, 1 H, H-5), 2.57 (dt, 1 H, 2JHa,Hb = 15.0 Hz, JHa,1 = JHa,CH = 5.2 Hz, CHaHbCH=CH), 2.40 (ddd, 1 H, J1′b,1 = 10.9 Hz, J1′b,2′ = 8.5 Hz, CHaHbCH=CH2). 13C NMR (75.5 MHz, CDCl3): δ 155.9 (CO), 138.6–136.2 (Ph), 134.1 (CH=CH2), 129.4–127.6 (Ph), 117.4 (CH=CH2), 82.7 (C-3), 79.5 (C-2), 77.8 (C-4), 75.1, 74.9, 72.7, 67.6 (CH2Ph), 59.3 (C-6), 57.2 (C-5), 54.4 (C-1), 30.0 (CH2CH=CH2). ESIMS: m/z 630.3 [M + Na]+. Anal. Calcd for C38H41NO6: C, 75.10; H, 6.80; N, 2.30. Found: C, 75.21; H, 6.93; N, 2.19.
(1R)-2,3,4-Tri-O-benzyl-N-benzyloxycarbonyl-1-(octa-2-en-1-yl)-1-deoxynojirimycin (15)
To a solution of 14 (98 mg, 0.16 mmol) in anhydrous degassed DCM (2.3 mL), under Ar, were added hept-1-ene (113 μL, 0.8 mmol), 1,4-BQ (3.5 mg, 0.032 mmol), and the Grubbs II catalyst (14 mg, 0.016 mmol), and the mixture was stirred under Ar at RT for 5 h. A second portion of Grubbs II catalyst (7 mg, 0.008 mmol) was added, and stirring was continued for 18 h. The solvent was evaporated, and the residue was chromatographed using 1/6 EtOAc/cyclohexane as eluent to afford 15 as a mixture of E/Z isomers (5/1 ratio). Yield: 80 mg (74%; colorless oil). Rf = 0.32 (1/4 EtOAc/cyclohexane). 1H NMR (500 MHz, CDCl3): δ 7.31–7.18 (m, 20 H, Ph), 5.34 (m, 1 H, =CH), 5.11 (m, 1 H, =CH), 5.05, 4.99 (2 d, 2 H, 2JH,H = 12.4 Hz, CHPh), 4.74 (m, 4 H, CHPh), 4.52, 4.50 (2 d, 2 H, 2JH,H = 11.7 Hz, CHPh), 4.47 (m, 1 H, H-1), 4.00 (m, 2 H, H-6a, H-6b), 3.68 (m, 2 H, H-3, H-4), 3.48 (dd, 1 H, J2,3 = 8.7 Hz, J1,2 = 6.1 Hz, H-2), 3.18 (m, 1 H, H-5), 2.40–2.16 (m, 2 H, CH2CH=CH), 1.84 (m, 2 H, CH2CH=CH), 1.18 (m, 6 H, CH2), 0.80 (t, 3 H, 3JH,H = 7.0 Hz, CH3). 13C NMR (125.7 MHz, CDCl3): δ 155.9 (CO), 138.7–136.2 (Ph), 133.8, 132.8 (=CH), 128.6–127.6 (Ph), 125.0, 124.4 (=CH), 82.8, 82.7 (C-3), 79.6, 79.5 (C-2), 77.9 (C-4), 75.2–67.4 (CH2Ph), 59.2 (C-6), 57.3, 57.2 (C-5), 55.1, 54.7 (C-1), 32.6 (CH2CH=CH), 31.5, 31.3, 29.3, 29.1 (CH2), 28.7 (CH2CH=CH), 27.6, 22.6, 22.5 (CH2), 14.1 (CH3). ESIMS: m/z 700.4 [M + Na]+. Anal. Calcd for C43H51NO6: C, 76.19; H, 7.58; N, 2.07. Found: C, 76.07; H, 7.44; N, 1.85.
Synthesis of (1R)-1-C-Octyl-1-deoxynojirimycin (16)
A solution of 15 (0.32 mmol) in degassed MeOH (3.0 mL) was acidified with HCl (300 μL, 6.0 M) and hydrogenated at atmospheric pressure for 24 h using 10% Pd/90% C (30 mg). The mixture was filtered (Celite), concentrated, and chromatographed using 10/1/1 → 10/2/1 MeCN/H2O/NH4OH as eluent to afford 16. Yield: 88 mg (quantitative; colorless oil). The analytical and spectroscopical data of 16 were in agreement with those reported in the literature.72
General Procedure for the Synthesis of (1R)-N-Alkyl-1-C-allyl-2,3,4-tri-O-benzyl-1-deoxynojirimycin Derivatives 17 and 18
A solution of 12 (60 mg, 0.13 mmol) and the corresponding aldehyde (1.3 mmol, 10 equiv) in 5/1 MeCN/MeOH (1.3 mL), under Ar, was acidified to pH 5–6 with AcOH (3 μL). Sodium sulfate (32 mg, 0.26 mmol, 2 equiv) and sodium cyanoborohydride (33 mg, 0.52 mmol, 4 equiv) were added, the mixture was heated to 65 °C for 18 h and then diluted with saturated aqueous NaHCO3 (15 mL) and extracted with Et2O (3 × 15 mL). The combined organic phases were dried (MgSO4), filtered, concentrated, and chromatographed with the indicated eluent.
(1R)-1-C-Allyl-2,3,4-tri-O-benzyl-N-butyl-1-deoxynojirimycin (17)
Column chromatography (1/4 EtOAc/cyclohexane). Yield: 48 mg (70%; colorless oil). Rf = 0.51 (1/3 EtOAc/cyclohexane). [α]D +6.4 (c 0.8 in DCM). IR (ATR): νmax 1637, 1453, 1092, 698 cm–1. 1H NMR (500 MHz, CDCl3): δ 7.28–7.18 (m, 15 H, Ph), 5.68 (m, 1 H, CH=CH2), 4.98–4.91 (m, 2 H, CH=CH2), 4.84, 4.71 (2 d, 2 H, 2JH,H = 10.9 Hz, CHPh), 4.78, 4.48 (2 d, 2 H, 2JH,H = 11.0 Hz, CHPh), 4.61, 4.54 (2 d, 2 H, 2JH,H = 11.6 Hz, CHPh), 3.72 (dd, 1 H, J6a,6b = 10.8 Hz, J5,6a = 4.5 Hz, H-6a), 3.69–3.64 (m, 2 H, H-2, H-3), 3.50 (dd, 1 H, J5,6b = 7.5 Hz, H-6b), 3.39 (m, 1 H, H-4), 3.03 (m, 1 H, H-1), 2.84 (ddd, 1 H, J4,5 = 10.3 Hz, H-5), 2.51 (m, 1 H, CHaHbN), 2.40 (m, 2 H, CHaHbN, CHaHbCH=CH2), 2.21 (m, 2 H, CHaHbCH=CH2, OH), 1.18 (m, 4 H, CH2), 0.81 (t, 3 H, 3JH,H = 7.3 Hz, CH3). 13C NMR (125.7 MHz, CDCl3): δ 138.8–127.6 (Ph), 136.9 (CH=CH2), 115.9 (CH=CH2), 83.7 (C-2), 79.0 (C-3), 78.5 (C-4), 75.4, 75.1, 72.9 (CH2Ph), 59.5 (C-6), 58.3 (C-5), 56.9 (C-1), 46.5 (CH2N), 31.6 (CH2), 29.6 (CH2CH=CH2), 20.3 (CH2), 14.0 (CH3). ESIMS: m/z 530.33 [M + H]+. HRMS (ESI) [M + H]+ calcd for C34H44NO4m/z 530.3265; found m/z 530.3258.
(1R)-1-C-Allyl-2,3,4-tri-O-benzyl-N-octyl-1-deoxynojirimycin (18)
Column chromatography (1/5 EtOAc/cyclohexane). Yield: 69 mg (90%; colorless oil). Rf = 0.49 (1/5 EtOAc/cyclohexane). [α]D +16.5 (c 1.0 in CHCl3). IR (ATR): νmax 925, 2854, 1453, 1027, 749, 696 cm–1. 1H NMR (500 MHz, CDCl3): δ 7.28–7.18 (m, 15 H, Ph), 5.68 (m, 1 H, CH=CH2), 4.98–4.91 (m, 2 H, CH=CH2), 4.84, 4.71 (2 d, 2 H, 2JH,H = 10.9 Hz, CHPh), 4.78, 4.48 (2 d, 2 H, 2JH,H = 11.0 Hz, CHPh), 4.61, 4.54 (2 d, 2 H, 2JH,H = 11.6 Hz, CHPh), 3.72 (dd, 1 H, J6a,6b = 10.9 Hz, J5,6a = 4.5 Hz, H-6a), 3.69–3.64 (m, 2 H, H-2, H-3), 3.50 (dd, 1 H, J5,6b = 7.5 Hz, H-6b), 3.39 (m, 1 H, H-4), 3.03 (m, 1 H, H-1), 2.84 (ddd, 1 H, J4,5 = 10.2 Hz, H-5), 2.50 (m, 1 H, CHaHbN), 2.37 (m, 2 H, CHaHbN, CHaHbCH=CH2), 2.22 (m, 2 H, CHaHbCH = CH2, OH), 1.30–1,10 (m, 12 H, CH2), 0.82 (t, 3 H, 3JH,H = 6.9 Hz, CH3). 13C NMR (125.7 MHz, CDCl3): δ 138.8–127.6 (Ph), 136.9 (CH=CH2), 115.9 (CH=CH2), 83.7 (C-2), 79.0 (C-3), 78.5 (C-4), 75.4, 75.1, 72.9 (CH2Ph), 59.5 (C-6), 58.3 (C-5), 56.9 (C-1), 46.7 (CH2N), 31.8 (CH2), 29.6–29.3 (CH2, CH2CH=CH2), 27.1, 22.7 (CH2), 14.1 (CH3). ESIMS: m/z 586.39 [M + H]+, 589.40 [M + Na]+. HRMS (ESI) [M + H]+ calcd for C34H44NO4m/z 586.3891; found m/z 586.3885.
General Procedure for the Synthesis of (1R)-N-Alkyl-1-C-propyl-1-deoxynojirimycin Hydrochloride Derivatives 19 and 20
A solution of 17 or 18 (0.094 mmol) in degassed MeOH (1.0 mL) was acidified with HCl (94 μL, 6.0 M) and hydrogenated at atmospheric pressure for 24 h using 10% Pd/C (9 mg). The mixture was filtered (Celite), concentrated, and chromatographed with the indicated eluent.
(1R)-1-C-Propyl-N-butyl-1-deoxynojirimycin Hydrocloride (19)
Column chromatography (40/10/1 DCM/MeOH/H2O). Yield: 27 mg (97%; white amorphous solid). Rf = 0.49 (60/10/1 DCM/MeOH/H2O). [α]D −6.5 (c 1.0 in MeOH). 1H NMR (500 MHz, 5/1 CD3OD/CDCl3): δ 3.76 (dd, 1 H, J6a,6b = 11.5 Hz, J5,6a = 4.2 Hz, H-6a), 3.70 (m, 1 H, H-6b), 3.61 (dd, 1 H, J2,3 = 9.3 Hz, J1,2 = 5.4 Hz, H-2), 3.37 (t, 1 H, J3,4 = 9.3 Hz, H-3), 3.31 (t, 1 H, J4,5 = 9.3 Hz, H-4), 2.94 (bs, 1 H, H-1), 2.63 (m, 3 H, H-5, CH2N), 1.50–1.19 (m, 8 H, CH2), 0.85 (t, 3 H, 3JH,H = 7.1 Hz, CH3), 0.84 (t, 3 H, 3JH,H = 7.4 Hz, CH3). 13C NMR (125.7 MHz, 5/1 CD3OD/CDCl3): δ 76.6 (C-3), 72.4 (C-2), 71.5 (C-4), 61.0 (C-1, C-5, C-6), 48.8 (CH2N), 27.3, 22.4, 21.3 (CH2), 14.6, 14.4 (CH3). ESIMS: m/z 262.20 [M + H – Cl]+. HRMS (ESI) [M + H – Cl]+ calcd for C13H28ClNO4m/z 262.2013; found m/z 262.2014.
(1R)-1-C-Propyl-N-octyl-1-deoxynojirimycin Hydrochloride (20)
Column chromatography (80/10/1 DCM/MeOH/H2O). Yield: 27 mg (80%; white amorphous solid). Rf = 0.23 (80/10/1 DCM/MeOH/H2O). [α]D +16.6 (c 1.0 in MeOH). 1H NMR (500 MHz, CD3OD): δ 3.85 (dd, 1 H, J6a,6b = 11.5 Hz, J5,6a = 4.1 Hz, H-6a), 3.78 (dd, 1 H, J5,6b = 5.5 Hz, H-6b), 3.68 (dd, 1 H, J2,3 = 9.1 Hz, J1,2 = 5.4 Hz, H-2), 3.44 (t, 1 H, J3,4 = 9.0 Hz, H-3), 3.37 (t, 1 H, J4,5 = 9.0 Hz, H-4), 3.01 (m, 1 H, H-1), 2.71 (m, 3 H, H-5, CH2N), 1.60–1.31 (m, 16 H, CH2), 0.93 (t, 3 H, 3JH,H = 7.0 Hz, CH3), 0.89 (t, 3 H, 3JH,H = 6.9 Hz, CH3). 13C NMR (125.7 MHz, CDCl3): δ 76.7 (C-3), 72.5 (C-2), 71.7 (C-4), 61.2 (C-1, C-5, C-6),48.9 (CH2N), 33.0, 30.6, 30.4, 28.3, 27.4, 23.9, 23.7, 22.5 (CH2), 14.7, 14.4 (CH3). ESIMS: m/z 318.26 [M + H – Cl]+. HRMS (ESI) [M + H – Cl]+ calcd for C17H36ClNO4m/z 318.2639; found m/z 318.2639.
General Procedure for the Synthesis of (1R)-N-(N′-Alkylthiocarbamoyl)-1-C-propyl-1-deoxynojirimycin Derivatives 21–26
To a solution of 13 (66 mg, 0.32 mmol) in pyridine (5.0 mL) were added Et3N (53 μL, 0.38 mmol) and the isothiocyanate reagent (0.35 mmol, 1.1 equiv). The mixture was stirred at RT for 18 h, concentrated, coevaporated with toluene, and chromatographed with the indicated eluent. Line broadening was observed in the 1H and 13NMR spectra of these compounds and even line splitting in the case of the 13C NMR spectrum of compound 25; this effect is not a sign of the presence of impurities but the consequence of slow rotation about the N–C(=S) thiourea bonds.
(1R)-N-(N′-Butylthiocarbamoyl)-1-C-propyl-1-deoxynojirimycin (21)
Column chromatography (100/10/1 → 80/10/1 DCM/MeOH/H2O). Yield: 80 mg (78%; colorless oil). Rf = 0.27 (80/10/1 DCM/MeOH/H2O). UV (MeOH): 253 nm (εmM 11.7). [α]D −236.1 (c 1.0 in MeOH). 1H NMR (300 MHz, CD3OD): δ 3.92 (m, 4 H, H-4, H-5, H-6a, H-6b), 3.55 (m, 3 H, H-1, CH2NH), 3.39 (m, 2 H, H-2, H-3), 2.02 (m, 1 H, CHaHbCH2CH3), 1.76 (m, 1 H, CHaHbCH2CH3), 1.61 (m, 2 H, CH2), 1.42 (m, 2 H, CH2), 1.33 (m, 2 H, CH2), 0.97 (t, 3 H, 3JH,H = 7.3 Hz, CH3), 0.96 (t, 3 H, 3JH,H = 7.3 Hz, CH3). 13C NMR (100.6 MHz, CD3OD): δ 186.4 (CS), 79.0 (C-2), 75.9 (C-1), 71.2 (C-3, C-4), 62.0 (C-5), 60.3 (C-6), 46.8 (CH2NH), 32.0, 29.7, 21.2, 20.5 (CH2), 14.2, 14.0 (CH3). ESIMS: m/z 343.3 [M + Na]+. Anal. Calcd for C14H28N2O4S: C, 52.47; H, 8.81; N, 8.74; S, 10.00. Found: C, 52.36; H, 9.00; N, 8.70; S, 9.88.
(1R)-N-(N′-Octylthiocarbamoyl)-1-C-propyl-1-deoxynojirimycin (22)
Column chromatography (20/1 → 7/1 DCM/MeOH). Yield: 89 mg (74%; colorless oil). Rf = 0.48 (15/1 DCM/MeOH). UV (MeOH): 253 nm (εmM 9.3). [α]D −221.4 (c 1.0 in MeOH). 1H NMR (300 MHz, CD3OD): δ 3.92 (m, 4 H, H-4, H-5, H-6a, H-6b), 3.54 (m, 3 H, H-1, CH2NH), 3.56 (m, 2 H, H-2, H-3), 2.03 (m, 1 H, CHaHbCH2CH3), 1.76 (m, 1 H, CHaHbCH2CH3), 1.61 (m, 2 H, CH2), 1.34 (m, 12 H, CH2), 0.97 (t, 3 H, 3JH,H = 7.3 Hz, CH3), 0.92 (t, 3 H, 3JH,H = 7.0 Hz, CH3). 13C NMR (75.5 MHz, CD3OD): δ 186.1 (CS), 79.0 (C-2), 75.7 (C-1), 71.0 (C-3, C-4), 61.9 (C-5), 60.3 (C-6), 47.0 (CH2NH), 33.0, 30.4, 30.3, 30.0, 29.6, 28.2, 23.7, 20.5 (CH2), 14.4, 14.3 (CH3). ESIMS: m/z 399.4 [M + Na]+. Anal. Calcd for C18H36N2O4S: C, 57.41; H, 9.64; N, 7.44; S, 8.51. Found: C, 57.44; H, 9.72; N, 7.65; S, 8.30.
(1R)-N-(N′-Benzylthiocarbamoyl)-1-C-propyl-1-deoxynojirimycin (23)
Column chromatography (100/10/1 DCM/MeOH/H2O). Yield: 70 mg (62%; colorless oil). Rf = 0.57 (70/10/1 DCM/MeOH/H2O). UV (MeOH): 252 nm (εmM 13.1). [α]D −254.4 (c 1.1 in MeOH). 1H NMR (500 MHz, CD3CN): δ 7.37–7.26 (m, 5 H, Ph), 4.83, 4.74 (2 d, 2 H, 2JH,H = 14.3 Hz, CH2NH), 4.01 (bs, 1 H, H-4), 3.94–3.87 (m, 3 H, H-5, H-6a, H-6b), 3.57 (m, 1 H, H-1), 3.41 (m, 2 H, H-2, H-3), 1.96 (m, 1 H, CHaHbCH2CH3), 1.70 (m, 1 H, CHaHbCH2CH3), 1.31 (m, 2 H, CH2), 0.92 (t, 3 H, 3JH,H = 7.4 Hz, CH3). 13C NMR (125.7 MHz, CD3CN): δ 185.0 (CS), 138.0, 128.2, 127.6, 127.0 (Ph), 77.6 (C-2), 74.5 (C-1), 69.6 (C-3, C-4), 60.6 (C-6), 59.2 (C-5), 49.4 (CH2NH), 28.3 (CH2), 19.1 (CH2CH3), 12.9 (CH3). ESIMS: m/z 377.1 [M + Na]+. Anal. Calcd for C17H26N2O4S: C, 57.60; H, 7.39; N, 7.90; S, 9.04. Found: C, 57.45; H, 7.12; N, 7.69; S, 8.80.
(1R)-N-[N′-(2-Phenyl)ethylthiocarbamoyl]-1-C-propyl-1-deoxynojirimycin (24)
Column chromatography (100/10/1 → 80/10/1 DCM/MeOH/H2O). Yield: 86 mg (73%; colorless oil). Rf = 0.42 (70/10/1 DCM/MeOH/H2O). UV (MeOH): 251 nm (εmM 10.5). [α]D −240.1 (c 1.0 in MeOH). 1H NMR (500 MHz, CD3CN): δ 8.19 (bs, 1 H NH), 7.36–7.22 (m, 5 H, Ph), 4.17 (bs, 1 H, OH), 3.92 (bs, 1 H, H-4), 3.83–3.73 (m, 5 H, CH2NH, H-5, H-6a, H-6b), 3.49 (m, 1 H, H-1), 3.31 (dd, 1 H, J2,3 = 9.4 Hz, J1,2 = 6.1 Hz, H-2), 3.26 (m, 1 H, H-3), 2.93 (t, 2 H, 3JH,H = 7.5 Hz, CH2Ph), 1.97 (m, 1 H, CHaHbCH2CH3), 1.66 (m, 1 H, CHaHbCH2CH3), 1.23 (m, 2 H, CH2), 0.90 (t, 3 H, 3JH,H = 7.3 Hz, CH3). 13C NMR (125.7 MHz, CD3CN): δ 185.9 (CS), 139.9, 129.5, 129.1, 126.9 (Ph), 78.2 (C-2), 75.1 (C-1), 70.3 (C-3, C-4), 61.4 (C-6), 58.7 (C-5), 47.5 (CH2NH), 35.0 (CH2Ph), 28.8 (CH2), 19.8 (CH2CH3), 13.8 (CH3). ESIMS: m/z 391.2 [M + Na]+, 369.2 [M + H]+. Anal. Calcd for C18H28N2O4S: C, 58.67; H, 7.66; N, 7.60; S, 8.70. Found: C, 58.77; H, 7.78 N, 7.42; S, 8.46.
(1R)-N-(N′-p-Methoxybenzylthiocarbamoyl)-1-C-propyl-1-deoxynojirimycin (25)
Column chromatography (100/10/1 DCM/MeOH/H2O). Yield: 107 mg (87%; colorless oil). Rf = 0.45 (80/10/1 DCM/MeOH/H2O). UV (MeOH): 252 nm (εmM 15.0). [α]D −309.9 (c 1.0 in MeOH). 1H NMR (500 MHz, CD3CN): δ 8.48 (bs, 1 H NH), 7.28–6.92 (m, 4 H, Ph), 4.72, 4.65 (2 dd, 2 H, 2JH,H = 14.6 Hz, 3JH,H = 5.2 Hz, CH2NH), 4.24 (bs, 1 H, OH), 4.01 (bs, 1 H, H-4), 3.84–3.80 (m, 3 H, H-5, H-6a, H-6b), 3.79 (s, 3 H, OMe), 3.68 (bs, 2 H, OH), 3.53 (m, 1 H, H-1), 3.35 (dd, 1 H, J2,3 = 9.5 Hz, J1,2 = 6.0 Hz, H-2), 3.31 (m, 1 H, H-3), 2.33 (bs, 1 H, OH), 2.01 (m, 1 H, CHaHbCH2CH3), 1.68 (m, 1 H, CHaHbCH2CH3), 1.26 (m, 2 H, CH2), 0.90 (t, 3 H, 3JH,H = 7.3 Hz, CH3). 13C NMR (125.7 MHz, CD3CN): δ 186.0 (CS), 159.7, 131.1, 130.0, 114.6 (Ph), 78.4 (C-2), 75.4 (C-1), 70.5 (C-3, C-4), 61.7 (C-6), 59.0 (C-5), 55.6 (OMe), 49.6 (CH2NH), 29.1 (CH2), 19.9 (CH2CH3), 14.0 (CH3). ESIMS: m/z 407.2 [M + Na]+, 385.2 [M + H]+. Anal. Calcd for C18H28N2O5S: C, 56.23; H, 7.34; N, 7.29; S, 8.34. Found: C, 55.99; H, 7.20; N, 7.04; S, 8.01.
(1R)-N-(N′-p-Trifluoromethylbenzylthiocarbamoyl)-1-C-propyl-1-deoxynojirimycin (26)
Column chromatography (100/10/1 → 80/10/1 DCM/MeOH/H2O). Yield: 88 mg (65%; colorless oil). Rf = 0.54 (80/10/1 DCM/MeOH/H2O). UV (MeOH 253 nm (εmM 13.2). [α]D −190.6 (c 1.0 in MeOH). 1H NMR (300 MHz, CD3CN): δ 8.459 (bs, 1 H NH), 7.60, 7.44 (2 d, 4 H, 3JH,H = 8.1 Hz, Ph), 4.80 (d, 2 H, 3JH,H = 5.6 Hz, CH2NH), 3.94 (bs, 1 H, H-4), 3.83–3.78 (m, 3 H, H-5, H-6a, H-6b), 3.46 (m, 1 H, H-1), 3.28 (m, 2 H, H-2, H-3), 1.97 (m, 1 H, CHaHbCH2CH3), 1.65 (m, 1 H, CHaHbCH2CH3), 1.22 (m, 2 H, CH2), 0.84 (t, 3 H, 3JH,H = 7.4 Hz, CH3). 13C NMR (125.7 MHz, CD3CN): δ 186.5 (CS), 144.1, 128.7, 125.8 (Ph), 78.2 (C-2), 75.1 (C-1), 70.3 (C-3, C-4), 61.4 (C-6), 59.3 (C-5), 49.1 (CH2NH), 28.9 (CH2), 19.8 (CH2CH3), 13.8 (CH3). ESIMS: m/z 445.14 [M + Na]+, 423.16 [M + H]+. Anal. Calcd for C18H25F3N2O4S: C, 51.18; H, 5.97; N, 6.63; S, 7.59. Found: C, 50.88; H, 5.76; N, 6.37; S, 7.31.
General Procedure for the Synthesis of (1R)-5-N,6-S-(N′-Alkyliminomethylidene)-1-C-propyl-6-thio-1-deoxynojirimycin Hydrochloride Derivatives 29 and 30
To a solution of 21 or 22 (0.14 mmol) in MeOH (5.0 mL) was added concentrated HCl (pH 1), and the reaction mixture was stirred at RT for 18 h and evaporated. The residue was coevaporated with MeOH (3 × 10 mL) and chromatographed with the indicated eluent.
(1R)-5-N,6-S-(N′-Butyliminomethylidene)-1-C-propyl-6-thio-1-deoxynojirimycin Hydrochloride (29)
Column chromatography (70/10/1 → 60/10/1 → 40/10/1 DCM/MeOH/H2O). Yield: 37 mg (78%; white amorphous solid). Rf = 0.30 (70/10/1 DCM/MeOH/H2O). [α]D +23.9 (c 1.0 in MeOH). 1H NMR (300 MHz, CD3OD): δ 4.28 (ddd, 1 H, J1,CH = 11.3 Hz, J1,2 = 5.0 Hz, J1,CH = 3.2 Hz, H-1), 4.15 (ddd, 1 H, J4,5 = 10.0 Hz, J5,6a = 8.4 Hz, J5,6b = 4.9 Hz, H-5), 3.70 (dd, 1 H, J6a,6b = 11.6 Hz, H-6a), 3.50 (dd, 1 H, H-6b), 3.49 (m, 2 H, H-2, H-3), 3.32 (m, 3 H, H-4, CH2NH), 1.85 (m, 1 H, CHaHbCH2CH3), 1.57 (m, 3 H, CHaHbCH2CH3, CH2), 1.26 (m, 4 H, CH2), 0.89 (t, 3 H, 3JH,H = 7.2 Hz, CH3), 0.88 (t, 3 H, 3JH,H = 7.3 Hz, CH3). 13C NMR (75.5 MHz, CD3OD): δ 172.5 (CN), 74.8 (C-3), 73.8 (C-4), 71.2 (C-2), 65.1 (C-5), 59.6 (C-1), 49.8 (CH2NH), 32.1 (CH2), 31.1 (C-6), 28.3, 20.8, 20.2 (CH2), 14.2, 13.9 (CH3). ESIMS: m/z 303.3 [M - Cl]+. Anal. Calcd for C14H27ClN2O3S: C, 49.62; H, 8.03; N, 8.27; S, 9.56. Found: C, 49.86; H, 8.30; N, 8.43; S, 9.54.
(1R)-5-N,6-S-(N′-Octyliminomethylidene)-1-C-propyl-6-thio-1-deoxynojirimycin Hydrochloride (30)
Column chromatography (50/10/1 DCM/MeOH/H2O). Yield: 55 mg (quant; white amorphous solid). Rf = 0.44 (50/10/1 DCM/MeOH/H2O). [α]D +48.4 (c 1.0 in MeOH). 1H NMR (500 MHz, CD3OD): δ 4.35 (ddd, 1 H, J1,CH = 11.4 Hz, J1,2 = 5.2 Hz, J1,CH = 3.9 Hz, H-1), 3.98 (m, 1 H, H-5), 3.60 (dd, 1 H, J6a,6b = 11.5 Hz, J5,6a = 7.9 Hz, H-6a), 3.57 (t, 1 H, J2,3 = J3,4 = 9.6 Hz, H-3), 3.53 (dd, 1 H, H-2), 3.46 (dd, 1 H, J5,6b = 4.0 Hz, H-6b), 3.30 (m, 3 H, H-4, CH2NH), 1.90 (m, 1 H, CHaHbCH2CH3), 1.63 (m, 3 H, CHaHbCH2CH3, CH2), 1.35 (m, 12 H, CH2), 0.98 (t, 3 H, 3JH,H = 7.4 Hz, CH3), 0.92 (t, 3 H, 3JH,H = 7.2 Hz, CH3). 13C NMR (125.7 MHz, CD3OD): δ 167.2 (CN), 75.2 (C-3), 73.7 (C-4), 71.5 (C-2), 63.1 (C-5), 58.2 (C-1), 52.7 (CH2NH), 32.9, 31.0, 30.3 (CH2), 30.2 (C-6), 28.3, 27.9, 23.6, 20.4 (CH2), 14.4, 14.3 (CH3). ESIMS: m/z 359.4 [M - Cl]+. Anal. Calcd for C18H35ClN2O3S: C, 54.73; H, 8.93; N, 7.09; S, 8.12. Found: C, 54.49; H, 9.12; N, 6.86; S, 7.81.
General Procedure for the Synthesis of (1R)-N-(N′-Alkylthiocarbamoyl)-1-C-octyl-1-deoxynojirimycin Derivatives 27 and 28
To a solution of 16 (55 mg, 0.2 mmol) in DMF (2.0 mL) were added Et3N (56 μL, 0.4 mmol) and the isothiocyanate reagent (0.24 mmol, 1.2 equiv). The mixture was stirred at 40 °C for 18 h, concentrated, and chromatographed using 100/10/1 DCM/MeOH/H2O as eluent.
(R)-N-(N′-Butylthiocarbamoyl)-1-C-octyl-1-deoxynojirimycin (27)
Yield: 55 mg (70%; colorless oil). Rf = 0.38 (100/10/1 DCM/MeOH/H2O). UV (MeOH): 252 nm (εmM 14.1). [α]D −212.5 (c 1.0 in MeOH). 1H NMR (500 MHz, CD3CN, 303 K): δ 8.16 (bs, 1 H, NH), 4.54 (bs, 1 H, OH), 4.18 (m, 1 H, H-6a), 3.97 (m, 1 H, H-4), 3.82 (m, 2 H, H-5, H-6b), 3.63–3.40 (m, 6 H, H-1, OH, CH2NH), 3.29 (m, 2 H, H-2, H-3), 2.01 (m, 1 H, CHaHbCH2), 1.76 (m, 1 H, CHaHbCH2), 1.59 (m, 2 H, CH2), 1.40 (m, 2 H, CH2), 1.37–1.22 (m, 12 H, CH2), 0.97 (t, 3 H, 3JH,H = 7.4 Hz, CH3), 0.92 (t, 3 H, 3JH,H = 7.1 Hz, CH3). 13C NMR (125.7 MHz, CD3CN, 303 K): δ 185.7 (CS), 77.8 (C-2), 74.8 (C-1), 70.1 (C-3, C-4), 61.1 (C-5), 58.2 (C-6), 45.5 (CH2NH), 31.6, 30.7, 29.2, 29.1, 28.9, 26.3, 26.0, 22.3, 19.9 (CH2), 13.3, 13.0 (CH3). ESIMS: m/z 413.2 [M + Na]+. Anal. Calcd for C19H38N2O4S: C, 58.43; H, 89.81; N, 7.17; S, 8.21. Found: C, 58.31; H, 9.66; N, 6.92; S, 8.01.
(1R)-N-(N′-Octylthiocarbamoyl)-1-C-octyl-1-deoxynojirimycin (28)
Yield: 63 mg (70%; colorless oil). Rf = 0.41 (100/10/1 DCM/MeOH/H2O). UV (MeOH): 251 nm (εmM 11.6). [α]D −178.6 (c 1.0 in MeOH). 1H NMR (500 MHz, 17:3 CD3CN-CDCl3): δ 8.15 (bs, 1 H, NH), 4.57 (bs, 1 H, OH), 4.18 (m, 1 H, H-6a), 4.00 (bs, 1 H, H-4), 3.79 (m, 2 H, H-5, H-6b), 3.59–3.44 (m, 6 H, H-1, OH, CH2NH), 3.34 (dd, 1 H, J2,3 = 9.5 Hz, J1,2 = 6.0 Hz, H-2), 2.78 (bt, 1 H, H-3), 2.08 (m, 1 H, CHaHbCH2), 1.77 (m, 1 H, CHaHbCH2), 1.60 (m, 2 H, CH2), 1.35–1.24 (m, 22 H, CH2), 0.93 (t, 3 H, 3JH,H = 7.0 Hz, CH3), 0.92 (t, 3 H, 3JH,H = 7.1 Hz, CH3). 13C NMR (125.7 MHz, 17:3 CD3CN-CDCl3): δ 184.8 (CS), 77.7 (C-2), 74.3 (C-1), 69.4 (C-3, C-4), 60.6 (C-5), 57.6 (C-6), 45.3 (CH2NH), 31.2, 31.1, 28.8, 28.7, 28.6, 28.5, 28.1, 26.3, 25.8, 25.5, 22.0, 21.9 (CH2), 13.1 (CH3). ESIMS: m/z 469.3 [M + Na]+. Anal. Calcd for C23H46N2O4S: C, 61.84; H, 10.38 N, 6.27; S, 7.18. Found: C, 61.69; H, 10.30; N, 6.04; S, 6.89.
General Procedure for the Synthesis of (1R)-5-N,6-S-(N′-Alkyliminomethylidene)-1-C-octyl-6-thio-1-deoxynojirimycin Hydrochloride Derivatives 31 and 32
To a solution of 27 or 28 (0.14 mmol) in MeOH (5.0 mL) was added concentrated HCl (pH 1), and the reaction mixture was further stirred at RT for 18 h. The solvent was removed, and the residue was coevaporated several times with MeOH (neutral pH) and purified by column chromatography with the eluent indicated in each case.
(1R)-5-N,6-S-(N′-Butyliminomethylidene)-1-C-octyl-6-thio-1-deoxynojirimycin Hydrochloride (31)
Column chromatography (60/10/1 DCM/MeOH/H2O. Yield: 46 mg (80%; white amorphous solid). Rf = 0.64 (60/10/1 DCM/MeOH/H2O). [α]D +35.9 (c 1.0 in MeOH). 1H NMR (500 MHz, CD3OD): δ 4.32 (ddd, 1 H, J1,CH = 11.3 Hz, J1,2 = 5.1 Hz, J1,CH = 4.0 Hz, H-1), 4.05 (bs, 1 H, H-5), 3.66 (dd, 1 H, J6a,6b = 11.2 Hz, J5,6a = 8.5 Hz, H-6a), 3.58 (t, 1 H, J2,3 = J3,4 = 9.5 Hz, H-3), 3.55 (dd, 1 H, H-2), 3.49 (dd, 1 H, J5,6b = 4.3 Hz, H-6b), 3.40–3.30 (m, 3 H, H-4, CH2NH), 1.98 (m, 1 H, CHaHbCH2CH3), 1.65 (m, 3 H, CHaHbCH2CH3, CH2), 1.45–1.30 (m, 14 H, CH2), 0.99 (t, 3 H, 3JH,H = 7.4 Hz, CH3), 0.93 (t, 3 H, 3JH,H = 7.1 Hz, CH3). 13C NMR (125.7 MHz, CD3OD): δ 169.4 (CN), 75.3 (C-3), 73.9 (C-4), 71.6 (C-2), 63.7 (C-5), 58.8 (C-1), 51.8 (CH2NH), 33.0, 32.9, 30.7, 30.5 (CH2), 30.4 (C-6), 27.3, 26.2, 23.7, 21.1 (CH2), 14.4, 14.1 (CH3). ESIMS: m/z 373.3 [M – Cl]+. HRMS (ESI) [M + Na]+ calcd for C19H37ClN2O3NaS m/z 431.2106; found m/z 431.2103.
(1R)-5-N,6-S-(N′-Octyliminomethylidene)-1-C-octyl-6-thio-1-deoxynojirimycin Hydrochloride (32)
Column chromatography (70/10/1 DCM/MeOH/H2O). Yield: 4659 mg (90%; white amorphous solid). Rf = 0.45 (70/10/1 DCM/MeOH/H2O). [α]D +24.8 (c 1.0 in MeOH). 1H NMR (500 MHz, 6/1 CD3OD/CDCl3): δ 4.20 (ddd, 1 H, J1,CH = 11.3 Hz, J1,2 = 5.3 Hz, J1,CH = 4.0 Hz, H-1), 3.87 (m, 1 H, H-5), 3.50 (m, 1 H, H-6a), 3.47 (t, 1 H, J2,3 = J3,4 = 9.7 Hz, H-3), 3.43 (dd, 1 H, H-2), 3.35 (dd, 1 H, J6a,6b = 11.5 Hz, J5,6b = 4.3 Hz, H-6b), 3.27–3.15 (m, 3 H, H-4, CH2NH), 1.86 (m, 1 H, CHaHbCH2CH3), 1.51 (m, 3 H, CHaHbCH2CH3, CH2), 1.22 (m, 22 H, CH2), 0.80 (t, 3 H, 3JH,H = 7.2 Hz, CH3), 0.79 (t, 3 H, 3JH,H = 7.0 Hz, CH3). 13C NMR (125.7 MHz, 6/1 CD3OD/CDCl3) δ 167.0 (CN), 75.3 (C-3), 74.0 (C-4), 71.6(C-2), 63.5 (C-5), 58.6 (C-1), 52.6 (CH2NH), 33.1, 33.0, 31.1, 30.8, 30.6 (CH2), 30.5 (C-6), 30.4, 28.1, 27.4, 26.3, 23.8, 23.7 (CH2), 14.7, 14.6 (CH3). ESIMS: m/z 429.3 [M - Cl]+. HRMS (ESI) [M + Na]+ calcd for C23H45ClN2O3NaS m/z 487.2732; found m/z 431.2723.
Inhibitory Activity Screening toward Commercial Glycosidases
Kinetic Ki values were determined from the residual activities of the enzymes at their optimal pHs using o (for β-galactosidase from E. coli)- or p-nitrophenyl α- or β-d-glycopyranoside (for other glycosidases) in the presence of increasing concentrations of the inhibitors. Approximate values were first obtained using a fixed concentration of substrate close to the KM value for the corresponding glycosidase. Lineweaver–Burk plots and a double-reciprocal analysis provided accurate Ki values and confirmed the inhibition mode.76,77
Acknowledgments
This work was financially supported by MCIN/AEI/10.13039/501100011033 and “ERDF A way of making Europe” (grant nos. PID2019-105858RB-I00 and RTI2018-097609-B-C21) and the Junta de Andalucía (Grant number P20_00166). I.H.-G. and M.G.-C. are FPU and FPI fellows, respectively (grant nos. FPU17/03147 and BES-2017-079676, funded by MCIN/AEI/10.13039/501100011033 and by “ESF Investing in your future”).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c01469.
Optimized synthesis of the sp2-iminosugar precursor 3, 1H and 13C NMR spectra for all compounds, and experimental details for glycosidase inhibition studies (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Paparella A. S.; Aboulache P. L.; Harijan R. K.; Potts K. S.; Tyler P. C.; Schramm V. L. Inhibition of Clostridium Difficile TcdA and TcdB Toxins with Transition State Analogues. Nat. Commun. 2021, 12, 6285. 10.1038/s41467-021-26580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matassini C.; Warren J.; Wang B.; Goti A.; Cardona F.; Morrone A.; Bols M. Imino- and Azasugar Protonation Inside Human Acid β-Glucosidase, the Enzyme that is Defective in Gaucher Disease. Angew. Chem., Int. Ed. 2020, 59, 10466–10469. 10.1002/anie.202002850. [DOI] [PubMed] [Google Scholar]
- González-Cuesta M.; Ortiz Mellet C.; García Fernández J. M. Carbohydrate Supramolecular Chemistry: Beyond the Multivalent Effect. Chem. Commun. 2020, 56, 5207–5222. 10.1039/D0CC01135E. [DOI] [PubMed] [Google Scholar]
- Howard E.; Cousido-Siah A.; Lepage M. L.; Schneider J. P.; Bodlenner A.; Mitschler A.; Meli A.; Izzo I.; Alvarez H. A.; Podjarny A.; Compain P. Structural Basis of Outstanding Multivalent Effects in Jack Bean α-Mannosidase Inhibition. Angew. Chem., Int. Ed. 2018, 57, 8002–8006. 10.1002/anie.201801202. [DOI] [PubMed] [Google Scholar]
- Lahav D.; Liu B.; van den Berg R. J. B. H. N.; van den Nieuwendijk A. M. C. H.; Wennekes T.; Ghisaidoobe A. T.; Breen I.; Ferraz M. J.; Kuo C.-L.; Wu L.; Geurink P. P.; Ovaa H.; van der Marel G. A.; van der Stelt M.; Boot R. G.; Davies G. J.; Aerts J. M. H. G.; Overkleeft H. S. A Fluorescence Polarization Activity-Based Protein Profiling Assay in the Discovery of Potent, Selective Inhibitors for Human Nonlysosomal Glucosylceramidase. J. Am. Chem. Soc. 2017, 139, 14192–14197. 10.1021/jacs.7b07352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillelund V. H.; Jensen H. H.; Liang X.; Bols M. Recent Developments of Transition-State Analogue Glycosidase Inhibitors of Non-Natural Product Origin. Chem. Rev. 2002, 102, 515–554. 10.1021/cr000433k. [DOI] [PubMed] [Google Scholar]
- Wadood A.; Ghufran M.; Khan A.; Azam S. S.; Jelani M.; Uddin R. Selective Glycosidase Inhibitors: A Patent Review (2012-Present). Int. J. Biol. Macromol. 2018, 111, 82–91. 10.1016/j.ijbiomac.2017.12.148. [DOI] [PubMed] [Google Scholar]
- Conforti I.; Marra A. Iminosugars as Glycosyltransferase Inhibitors. Org. Biomol. Chem. 2021, 19, 5439–5475. 10.1039/D1OB00382H. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Ma S. Recent Advances in Synthetic α-Glucosidase Inhibitors. ChemMedChem. 2017, 12, 819–829. 10.1002/cmdc.201700216. [DOI] [PubMed] [Google Scholar]
- Esposito A.; D’Alonzo D.; De Fenza M.; De Gregorio E.; Tamanini A.; Lippi G.; Dechecchi M. C.; Guaragna A. Synthesis and Therapeutic Applications of Iminosugars in Cystic Fibrosis. Int. J. Mol. Sci. 2020, 21, 3353. 10.3390/ijms21093353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Fernández E. M.; García Fernandez J. M.; Ortiz Mellet C. Glycomimetic-Based Pharmacological Chaperones for Lysosomal Storage Disorders: Lessons from Gaucher, GM1-Gangliosidosis and Fabry Diseases. Chem. Commun. 2016, 52, 5497–5515. 10.1039/C6CC01564F. [DOI] [PubMed] [Google Scholar]
- Parenti G.; Andria G.; Valenzano K. J. Pharmacological Chaperone Therapy: Preclinical Development, Clinical Translation, and Prospects for the Treatment of Lysosomal Storage Disorders. Mol. Ther. 2015, 23, 1138–1148. 10.1038/mt.2015.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt F. M. Emptying the stores: Lysosomal Diseases and Therapeutic Strategies. Nat. Rev. Drug Discovery 2018, 17, 133–150. 10.1038/nrd.2017.214. [DOI] [PubMed] [Google Scholar]
- Stütz A. E.; Thonhofer M.; Weber P.; Wolfsgruber A.; Wrodnigg T. M. Pharmacological Chaperones for β-Galactosidase Related to GM1-Gangliosidosis and Morquio B: Recent Advances. Chem. Rec. 2021, 21, 2980–2989. 10.1002/tcr.202100269. [DOI] [PubMed] [Google Scholar]
- Alonzi D. S.; Scott K. S.; Dwek R. A.; Zitzmann N. Iminosugar Antivirals: the Therapeutic Sweet Spot. Biochem. Soc. Trans. 2017, 45, 571–582. 10.1042/BST20160182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWald L. E.; Starr C.; Butters T.; Treston A.; Warfield K. L. Iminosugars: A Host-Targeted Approach to Combat Flaviviridae Infections. Antiviral Res. 2020, 184, 104881. 10.1016/j.antiviral.2020.104881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain F.; Andreana P. R. Developments in Carbohydrate-Based Cancer Therapeutics. Pharmaceuticals 2019, 12, 84. 10.3390/ph12020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.; Ye X.-S. Iminosugars as Immunomodulating Agents: Synthesis and Biological Activities of 1-Deoxynojirimycin and Related Compounds. Isr. J. Chem. 2015, 55, 336–346. 10.1002/ijch.201400150. [DOI] [Google Scholar]
- Sayce A. C.; Martinez F. O.; Tyrrell B. E.; Perera N.; Hill M. L.; Dwek R. A.; Miller J. L.; Zitzmann N. Pathogen-Induced Inflammation is Attenuated by the Iminosugar MON-DNJ via Modulation of the Unfolded Protein Response. Immunology 2021, 164, 587–601. 10.1111/imm.13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes J. P. B.; Silva L.; Lüdtke D. S. An Overview on the Synthesis of Carbohydrate-Based Molecules with Biological Activity Related to Neurodegenerative Diseases. RSC Med. Chem. 2021, 12, 2001–2015. 10.1039/D1MD00217A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa T. C. S.; Fernandez-Villalba E.; Izura V.; Lucas-Ochoa A. M.; Menezes-Filho N. J.; Santana R. S.; de Oliveira M. D.; Araújo M. F.; Estrada C.; Silva V. D. A.; Costa S. L.; Herrero M. T. Combined 1-Deoxynojirimycin and Ibuprofen Treatment Decreases Microglial Activation, Phagocytosis and Dopaminergic Degeneration in MPTP-Treated Mice. J. Neuroimmune Pharmacol. 2021, 16, 390–402. 10.1007/s11481-020-09925-8. [DOI] [PubMed] [Google Scholar]
- Wang H.; Shen Y.; Zhao L.; Ye Y. 1-Deoxynojirimycin and its Derivatives: A Mini Review of the Literature. Curr. Med. Chem. 2021, 28, 628–643. 10.2174/0929867327666200114112728. [DOI] [PubMed] [Google Scholar]
- Azeez S. A.; Alhashim Z. G.; Al Otaibi W. M.; Alsuwat H. S.; Ibrahim A. M.; Almandil N. B.; Borgio J. F. State-of-the-Art Tools to Identify Druggable Protein Ligand of SARS-Cov-2. Arch. Med. Sci. 2020, 16, 497–507. 10.5114/aoms.2020.94046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S.; Roy A. In Silico Analysis of Selected Alkaloids Against Main Protease (M(Pro)) of SARS-Cov-2. Chem. Biol. Interact. 2020, 332, 109309. 10.1016/j.cbi.2020.109309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekharan S.; Bonotto R. M.; Alves L. N.; Kazungu Y.; Poggianella M.; Martinez-Orellana P.; Skoko N.; Polez S.; Marcello A. Inhibitors of Protein Glycosylation are Active Against the Coronavirus Severe Acute Respiratory Syndrome Coronavirus SARS-Cov-2. Viruses 2021, 13, 808. 10.3390/v13050808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke E. C.; Nofchissey R. A.; Ye C.; Bradfute S. B. The Iminosugars Celgosivir, Castanospermine and UV-4 Inhibit SARS-Cov-2 Replication. Glycobiology 2021, 31, 378–384. 10.1093/glycob/cwaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitner E. B.; Achdout H.; Avraham R.; Politi B.; Cherry L.; Tamir H.; Yahalom-Ronen Y.; Paran N.; Melamed S.; Erez N.; Israely T. Glucosylceramide Synthase Inhibitors Prevent Replication of SARS-Cov-2 and Influenza Virus. J. Biol. Chem. 2021, 296, 100470. 10.1016/j.jbc.2021.100470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri R.; Ansari A. A.; Vankar Y. D. Recent Developments in Design and Synthesis of Bicyclic Azasugars, Carbasugars and Related Molecules as Glycosidase Inhibitors. Chem. Soc. Rev. 2013, 42, 5102–5118. 10.1039/c3cs35525j. [DOI] [PubMed] [Google Scholar]
- Harit V. K.; Ramesh N. G. Amino-Functionalized Iminocyclitols: Synthetic Glycomimetics of Medicinal Interest. RSC Adv. 2016, 6, 109528–109607. 10.1039/C6RA23513A. [DOI] [Google Scholar]
- Nicolas C.; Martin O. R. Glycoside Mimics from Glycosylamines: Recent Progress. Molecules 2018, 23, 1612. 10.3390/molecules23071612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhara D.; Dhara A.; Bennett J.; Murphy P. V. Cyclisations and Strategies for Stereoselective Synthesis of Piperidine Iminosugars. Chem. Rec. 2021, 21, 2958–2979. 10.1002/tcr.202100221. [DOI] [PubMed] [Google Scholar]
- Meanwell M.; Fehr G.; Ren W.; Adluri B.; Rose V.; Lehmann J.; Silverman S. M.; Rowshanpour R.; Adamson C.; Bergeron-Brlek M.; Foy H.; Challa V. R.; Campeau L.-C.; Dudding T.; Britton R. Diversity-Oriented Synthesis of Glycomimetics. Commun. Chem. 2021, 4, 96. 10.1038/s42004-021-00520-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftikhar M.; Lu Y.; Zhou M. An Overview of Therapeutic Potential of N-Alkylated 1-Deoxynojirimycin Congeners. Carbohydr. Res. 2021, 504, 108317. 10.1016/j.carres.2021.108317. [DOI] [PubMed] [Google Scholar]
- Klunda T.; Hricovíni M.; Sesták S.; Kóna J.; Polákova M. Selective Golgi α-Mannosidase II Inhibitors: N-Alkyl Substituted Pyrrolidines with a Basic Functional Group. New J. Chem. 2021, 45, 10940–10951. 10.1039/D1NJ01176F. [DOI] [Google Scholar]
- Wolfsgruber A.; Thonhofer M.; Weber P.; Nasseri S. A.; Fischer R.; Schalli M.; Stütz A. E.; Withers S. G.; Wrodnigg T. M. N-Alkylated Iminosugar Based Ligands: Synthesis and Inhibition of Human Lysosomal β-Glucocerebrosidase. Molecules 2020, 25, 4618. 10.3390/molecules25204618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F.; Yin Z.; Chen J.; Nie X.; Lin P.; Lu T.; Wang M.; Peng D. Design, Synthesis, and Activity Evaluation of Novel N-Benzyl Deoxynojirimycin Derivatives for Use as α-Glucosidase Inhibitors. Molecules 2019, 24, 3309. 10.3390/molecules24183309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamoner L. O. B.; Aragao-Leoneti V.; Carvalho I. Iminosugars: Effects of Stereochemistry, Ring Size, and N-Substituents on Glucosidase Activities. Pharmaceuticals 2019, 12, 108–122. 10.3390/ph12030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X.; Gupta V.; Yang Y.; Zhu J.-Y.; Carlson E. J.; Kingsley C.; Tash J. S.; Schonbrunn E.; Hawkinson J.; Georg G. I. Structure-Activity Studies of N-Butyl-1-Deoxynojirimycin (NB-DNJ) Analogues: Discovery of Potent and Selective Aminocyclopentitol Inhibitors of GBA1 and GBA2. ChemMedChem. 2017, 12, 1977–1984. 10.1002/cmdc.201700558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg R. J.; van Rijssel E. R.; Ferraz M. J.; Houben J.; Strijland A.; Donker-Koopman W. E.; Wennekes T.; Bonger K. M.; Ghisaidoobe A. B.; Hoogendoorn S.; van der Marel G. A.; Codee J. D.; Overkleeft H. S.; Aerts J. M. F. G. Synthesis and Evaluation of Hybrid Structures Composed of Two Glucosylceramide Synthase Inhibitors. ChemMedChem. 2015, 10, 2042–2062. 10.1002/cmdc.201500407. [DOI] [PubMed] [Google Scholar]
- Ghisaidoobe A.; Bikker P.; de Bruijn A. C.; Godschalk F. D.; Rogaar E.; Guijt M. C.; Hagens P.; Halma J. M.; Van’t Hart S. M.; Luitjens S. B.; van Rixel V. H.; Wijzenbroek M.; Zweegers T.; Donker-Koopman W. E.; Strijland A.; Boot R.; van der Marel G.; Overkleeft H. S.; Aerts J. M. F. G.; van den Berg R. J. Identification of Potent and Selective Glucosylceramide Synthase Inhibitors from a Library of N-Alkylated Iminosugars. ACS Med. Chem. Lett. 2011, 2, 119–123. 10.1021/ml100192b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Désiré J.; Foucart Q.; Poveda A.; Gourlaouen G.; Shimadate Y.; Kise M.; Proceviat C.; Ashmus R.; Vocadlo D. J.; Jiménez-Barbero J.; Kato A.; Blériot Y. Synthesis, Conformational Analysis and Glycosidase Inhibition of Bicyclic Nojirimycin C-Glycosides Based on an Octahydrofuro[3,2-b]pyridine Motif. Carbohydr. Res. 2022, 511, 108491. 10.1016/j.carres.2021.108491. [DOI] [PubMed] [Google Scholar]
- Lu T.-T.; Shimadate Y.; Cheng B.; Kanekiyo U.; Kato A.; Wang J.-Z.; Li Y.-X.; Jia Y.-M.; Fleet G. W. J.; Yu C.-Y. Synthesis and Glycosidase Inhibition Of 5-C-Alkyl-DNJ and 5-C-Alkyl-L-Ido-DNJ Derivatives. Eur. J. Med. Chem. 2021, 224, 113716. 10.1016/j.ejmech.2021.113716. [DOI] [PubMed] [Google Scholar]
- Li S.; Jaszczyk J.; Pannecoucke X.; Poisson T.; Martin O. R.; Nicolas C. Stereospecific Synthesis of Glycoside Mimics Through Migita-Kosugi-Stille Cross-Coupling Reactions of Chemically and Configurationally Stable 1-C-Tributylstannyl Iminosugars. Adv. Synth. Catal. 2021, 363, 470–483. 10.1002/adsc.202000886. [DOI] [Google Scholar]
- Lumbroso A.; Berthonneau C.; Beaudet I.; Quintard J.-P.; Planchat A.; García-Moreno M. I.; Ortiz Mellet C.; Le Grognec E. A Versatile Stereocontrolled Synthesis of 2-Deoxyiminosugar C-Glycosides and their Evaluation as Glycosidase Inhibitors. Org. Biomol. Chem. 2021, 19, 1083–1099. 10.1039/D0OB02249G. [DOI] [PubMed] [Google Scholar]
- Zhu S.; Jagadeesh Y.; Tran A. T.; Imaeda S.; Alisdair Boraston A.; Alonzi D. S.; Poveda A.; Zhang Y.; Désiré J.; Charollais-Thoenig J.; Demotz S.; Kato A.; Butters T.; Jiménez-Barbero J.; Sollogoub M.; Blériot Y. Iminosugar C-Glycosides Work as Pharmacological Chaperones of NAGLU, a Glycosidase Involved in MPS IIIB Rare Disease. Chem. - Eur. J. 2021, 27, 11291–11297. 10.1002/chem.202101408. [DOI] [PubMed] [Google Scholar]
- Dehoux-Baudoin C.; Génisson Y. C-Branched Imino Sugars: Synthesis and Biological Relevance. Eur. J. Org. Chem. 2019, 2019, 4765–4777. 10.1002/ejoc.201900605. [DOI] [Google Scholar]
- Foucart Q.; Marrot J.; Désiré J.; Blériot Y. Site-Selective Debenzylation of C-Allyl Iminosugars Enables Their Stereocontroled Structure Diversification at the C-2 Position. Org. Lett. 2019, 21, 4821–4825. 10.1021/acs.orglett.9b01712. [DOI] [PubMed] [Google Scholar]
- Compain P.; Chagnault V.; Martin O. R. Tactics and Strategies for the Synthesis of Iminosugar C-Glycosides: A Review. Tetrahedron Asymmetry 2009, 20, 672–711. 10.1016/j.tetasy.2009.03.031. [DOI] [Google Scholar]
- Clemente F.; Matassini C.; Giachetti S.; Goti A.; Morrone A.; Martínez-Bailén M.; Orta S.; Merino P.; Cardona F. Piperidine Azasugars Bearing Lipophilic Chains: Stereoselective Synthesis and Biological Activity as Inhibitors of Glucocerebrosidase (GCase). J. Org. Chem. 2021, 86, 12745–12761. 10.1021/acs.joc.1c01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangarasu A. K.; Shainy Sambyal S.; Kumar H. M. S.; Lankalapalli R. S. Design, Synthesis, and Preliminary Immunopotentiating Activity of New Analogues of Nojirimycin. Carbohydr. Res. 2022, 511, 108479. 10.1016/j.carres.2021.108479. [DOI] [PubMed] [Google Scholar]
- Wennekes T.; Bonger K. M.; Vogel K.; van den Berg R. J. B. N.; Strijland A.; Donker-Koopman W. E.; Aerts J. M. F. G.; van der Marel G. A.; Overkleeft H. S. The Development of an Aza-C-Glycoside Library Based on a Tandem Staudinger/Aza-Wittig/Ugi Three-Component Reaction. Eur. J. Org. Chem. 2012, 2012, 6420–6454. 10.1002/ejoc.201200923. [DOI] [Google Scholar]
- Wennekes T.; van den Berg R. J. B. N.; Boltje T. J.; Donker-Koopman W. E.; Kuijper B.; van der Marel G. A.; Strijland A.; Verhagen C. P.; Aerts J. M. F. G.; Overkleeft H. S. Synthesis and Evaluation of Lipophilic Aza-C-glycosides as Inhibitors of Glucosylceramide Metabolism. Eur. J. Org. Chem. 2010, 2010, 1258–1283. 10.1002/ejoc.200901208. [DOI] [Google Scholar]
- Clemente F.; Matassini C.; Cardona F. Reductive Amination Routes in the Synthesis of Piperidine Iminosugars. Eur. J. Org. Chem. 2020, 2020, 4447–4462. 10.1002/ejoc.201901840. [DOI] [Google Scholar]
- Zoidl M.; Gonzalez Santana A.; Torvisco A.; Tysoe C.; Siriwardena A.; Withers S. G.; Wrodnigg T. M. The Staudinger/Aza-Wittig/Grignard Reaction as Key Step for the Concise Synthesis of 1-C-Alkyl-Iminoalditol Glycomimetics. Carbohydr. Res. 2016, 429, 62–70. 10.1016/j.carres.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Fuchss T.; Streicher H.; Schmidt R. R. A General Method for the Synthesis of C-Glycosides of Nojirimycin. Liebigs Ann. Recueil 1997, 1997, 1315–1321. 10.1002/jlac.199719970706. [DOI] [Google Scholar]
- Jiménez Blanco J. L.; Díaz Pérez V. M.; Ortiz Mellet C.; Fuentes J.; García Fernández J. M.; Díaz Arribas J. C.; Cañada F. J. N-Thiocarbonyl Azasugars: a New Family of Carbohydrate Mimics with Controlled Anomeric Configuration. Chem. Commun. 1997, 1969–1970. 10.1039/a705755e. [DOI] [Google Scholar]
- Díaz Pérez V. M.; García Moreno M. I.; Ortiz Mellet C.; Fuentes J.; J Díaz Arribas J. C.; Cañada F.; García Fernández J. M. Generalized Anomeric Effect in Action: Synthesis and Evaluation of Stable Reducing Indolizidine Glycomimetics as Glycosidase Inhibitors. J. Org. Chem. 2000, 65, 136–143. 10.1021/jo991242o. [DOI] [PubMed] [Google Scholar]
- Sánchez-Fernández E. M.; García Moreno M. I.; García Fernández J. M.; Ortiz Mellet C.. sp2-Iminosugars as Chemical Mimics for Glycodrug Design. In Small Molecule Drug Discovery Methods: Molecules and Applications; Trabocchi A., Lenci E., Eds.; Elsevier: 2020; Chapter 7, pp 197–224. [Google Scholar]
- Sánchez-Fernández E. M.; Rísquez-Cuadro R.; Chasseraud M.; Ahidouch A.; Ortiz Mellet C.; Ouadid-Ahidouch H.; García Fernández J. M. Synthesis of N-, S-, and C-Glycoside Castanospermine Analogues with Selective Neutral α-Glucosidase Inhibitory Activity as Antitumour Agents. Chem. Commun. 2010, 46, 5328–5330. 10.1039/c0cc00446d. [DOI] [PubMed] [Google Scholar]
- Herrera-González I.; Sánchez-Fernández E. M.; Sau A.; Nativi C.; García Fernández J. M.; Galán M. C.; Ortiz Mellet C. Stereoselective Synthesis of Iminosugar 2-Deoxy(Thio)Glycosides from Bicyclic Iminoglycal Carbamates Promoted by Cerium(IV) Ammonium Nitrate and Cooperative Brønsted Acid-Type Organocatalysis. J. Org. Chem. 2020, 85, 5038–5047. 10.1021/acs.joc.0c00324. [DOI] [PubMed] [Google Scholar]
- Guillen-Poza P. A.; Sánchez-Fernández E. M.; Artigas G.; García Fernández J. M.; Hinou H.; Ortiz Mellet C.; Nishimura S.-I.; Garcia-Martin F. Amplified Detection of Breast Cancer Autoantibodies Using MUC1-Based Tn Antigen Mimics. J. Med. Chem. 2020, 63, 8524–8533. 10.1021/acs.jmedchem.0c00908. [DOI] [PubMed] [Google Scholar]
- Bermejo I. A.; Navo C. D.; Castro-López J.; Guerreiro A.; Jiménez-Moreno E.; Sánchez-Fernández E. M.; García-Martín F.; Hinou H.; Nishimura S.-I.; García Fernández J. M.; Ortiz Mellet C.; Avenoza A.; Busto J. H.; Bernardes G. J. L.; Hurtado-Guerrero R.; Peregrina J. M.; Corzana F. Synthesis, Conformational Analysis and in Vivo Assays Of An Anti-Cancer Vaccine that Features an Unnatural Antigen Based on an sp2-Iminosugar Fragment. Chem. Sci. 2020, 11, 3996–4006. 10.1039/C9SC06334J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueder N.; Allan G.; Telliez M.-S.; Hague F.; García Fernández J. M.; Sánchez-Fernández E. M.; Ortiz Mellet C.; Ahidouch A.; Ouadid-Ahidouch H. sp2-Iminosugar α-Glucosidase Inhibitor 1-C-Octyl-2-Oxa-3-Oxocastanospermine Specifically Affected Breast Cancer Cell Migration Through Stim1, B1-Integrin, And FAK Signaling Pathways. J. Cell. Physiol. 2017, 232, 3631–3640. 10.1002/jcp.25832. [DOI] [PubMed] [Google Scholar]
- Katz S. J.; Bergmeier S. C. Convenient Methods for the Hydrolysis of Oxazolidinones to Vicinal Aminoalcohols. Tetrahedron Lett. 2002, 43, 557–559. 10.1016/S0040-4039(01)02218-3. [DOI] [Google Scholar]
- Best D.; Wang C.; Weymouth-Wilson A. C.; Clarkson E. A.; Wilson F. X.; Nash R. J.; Miyauchi S.; Kato A.; Fleet G. W. J. Looking Glass Inhibitors: Scalable Syntheses of DNJ, DMDP, and (3R)-3-Hydroxy-L-Bulgecinine from D-Glucuronolactone and of L-DNJ, L-DMDP, And (3S)-3-Hydroxy-D-Bulgecinine from L-Glucuronolactone. DMDP Inhibits β-Glucosidases and β-Galactosidases whereas L-DMDP Is a Potent and Specific Inhibitor of I-Glucosidases. Tetrahedron: Assymetry 2010, 21, 311–319. 10.1016/j.tetasy.2010.01.017. [DOI] [Google Scholar]
- Wu P.; Nielsen T. E. Scaffold Diversity from N-Acyliminium Ions. Chem. Rev. 2017, 117, 7811–7856. 10.1021/acs.chemrev.6b00806. [DOI] [PubMed] [Google Scholar]
- Yazici A.; Pyne S. G. Intermolecular Addition Reactions of N-Acyliminium Ions (Part I). Synthesis 2009, 2009, 339–368. 10.1055/s-0028-1083325. [DOI] [Google Scholar]
- Yazici A.; Pyne S. G. Intermolecular Addition Reactions of N-Acyliminium Ions (Part II). Synthesis 2009, 2009, 513–541. 10.1055/s-0028-1083346. [DOI] [Google Scholar]
- de la Fuente A.; Rísquez-Cuadro R.; Verdaguer X.; García Fernández J. M.; Nanba E.; Higaki K.; Ortiz Mellet C.; Riera A. Efficient Stereoselective Synthesis of 2-Acetamido-1,2-Dideoxyallonojirimycin (DAJNAc) and sp2-Iminosugar Conjugates: Novel Hexosaminidase Inhibitors with Discrimination Capabilities Between the Mature and Precursor Forms of the Enzyme. Eur. J. Med. Chem. 2016, 121, 926–938. 10.1016/j.ejmech.2015.10.038. [DOI] [PubMed] [Google Scholar]
- de la Fuente A.; Mena-Barragán T.; Farrar-Tobar R. A.; Verdaguer X.; García Fernández J. M.; Ortiz Mellet C.; Riera A. Stereoselective Synthesis of 2-Acetamido-1,2-Dideoxynojirimycin (DNJNAc) and Ureido-DNJNAc Derivatives as New Hexosaminidase Inhibitors. Org. Biomol. Chem. 2015, 13, 6500–6510. 10.1039/C5OB00507H. [DOI] [PubMed] [Google Scholar]
- de la Fuente A.; Mena-Barragán T.; Verdaguer X.; García Fernández J. M.; Ortiz Mellet C.; Riera A. Stereoselective Synthesis of 2-Acetamido-1,2-dideoxyallonojirimycin (DAJNAc), a New Potent Hexosaminidase Inhibitor. Org. Lett. 2013, 15, 3638–3641. 10.1021/ol401517x. [DOI] [PubMed] [Google Scholar]
- Cipolla L.; La Ferla B.; Peri F.; Nicotra F. A New Procedure for the Synthesis of C-Glycosides of Nojirimycin. Chem. Commun. 2000, 1289–1290. 10.1039/b003877f. [DOI] [Google Scholar]
- Godin G.; Compain P.; Martin O. R.; Ikeda K.; Yu L.; Asano N. α-1-C-Alkyl-1-Deoxynojirimycin Derivatives as Potent and Selective Inhibitors of Intestinal Isomaltase: Remarkable Effect of the Alkyl Chain Length on Glycosidase Inhibitory Profile. Bioorg. Med. Chem. 2004, 14, 5991–5995. 10.1016/j.bmcl.2004.09.086. [DOI] [PubMed] [Google Scholar]
- Shao S.; Zhou Q.; Si J.; Tang J.; Liu X.; Wang M.; Gao J.; Wang K.; Xu R.; Shen Y. A Non-Cytotoxic Dendrimer with Innate and Potent Anticancer and Anti-Metastatic Activities. Nat. Biomed. Eng. 2017, 1, 745–757. 10.1038/s41551-017-0130-9. [DOI] [PubMed] [Google Scholar]
- Carbajo-Gordillo A. I.; Jiménez Blanco J. L.; Benito J. M.; Lana H.; Marcelo G.; Di Giorgio C.; Przybylski C.; Hinou H.; Ceña V.; Ortiz Mellet C.; Mendicuti F.; Tros de Ilarduya C.; García Fernández J. M. Click Synthesis of Size- and Shape-Tunable Star Polymers with Functional Macrocyclic Cores for Synergistic DNA Complexation and Delivery. Biomacromolecules 2020, 21, 5173–5188. 10.1021/acs.biomac.0c01283. [DOI] [PubMed] [Google Scholar]
- González-Cuesta M.; Sidhu P.; Ashmus R. A.; Males A.; Proceviat C.; Madden Z.; Rogalski J. C.; Busmann J. A.; Foster L. J.; García Fernández J. M.; Davies G. J.; Ortiz Mellet C.; Vocadlo D. J. Bicyclic Picomolar OGA Inhibitors Enable Chemoproteomic Mapping of Its Endogenous Post-translational Modifications. J. Am. Chem. Soc. 2022, 144, 832–844. 10.1021/jacs.1c10504. [DOI] [PubMed] [Google Scholar]
- Sánchez-Fernández E. M.; García-Moreno M. I.; García-Hernández R.; Padrón J. M.; García Fernández J. M.; Gamarro F.; Ortiz Mellet C. Thiol-ene “Click” Synthesis and Pharmacological Evaluation of C-Glycoside sp2-Iminosugar Glycolipids. Molecules 2019, 24, 2882. 10.3390/molecules24162882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.