Abstract

The prevalence of hypertension reported around the world is increasing and is an important public health challenge. This study was designed to explore the disease’s genetic variations and to identify new hypertension-related genes and target proteins. We analyzed 22 publicly available Affymetrix cDNA datasets of hypertension using an integrated system-level framework involving differential expression genetic (DEG) analysis, data mining, gene enrichment, protein–protein interaction, microRNA analysis, toxicogenomics, gene regulation, molecular docking, and simulation studies. We found potential DEGs after screening out the extracellular proteins. We studied the functional role of seven shortlisted DEGs (ADM, EDN1, ANGPTL4, NFIL3, MSR1, CEBPD, and USP8) in hypertension after disease gene curation analysis. The expression profiling and cluster analysis showed significant variations and enriched GO terms. hsa-miR-365a-3p, hsa-miR-2052, hsa-miR-3065-3p, hsa-miR-603, hsa-miR-7113-3p, hsa-miR-3923, and hsa-miR-524-5p were identified as hypertension-associated miRNA targets for each gene using computational algorithms. We found functional interactions of source DEGs with target and important gene signatures including EGFR, AGT, AVP, APOE, RHOA, SRC, APOB, STAT3, UBC, LPL, APOA1, and AKT1 associated with the disease. These DEGs are mainly involved in fatty acid metabolism, myometrial pathways, MAPK, and G-alpha signaling pathways linked with hypertension pathogenesis. We predicted significantly disordered regions of 71.2, 48.8, and 45.4% representing the mutation in the sequence of NFIL3, USP8, and ADM, respectively. Regulation of gene expression was performed to find upregulated genes. Molecular docking analysis was used to evaluate Food and Drug Administration-approved medicines against the four DEGs that were overexpressed. For each elevated target protein, the three best drug candidates were chosen. Furthermore, molecular dynamics (MD) simulation using the target’s active sites for 100 ns was used to validate these 12 complexes after docking. This investigation establishes the worth of systems genetics for finding four possible genes as potential drug targets for hypertension. These network-based approaches are significant for finding genetic variant data, which will advance the understanding of how to hasten the identification of drug targets and improve the understanding regarding the treatment of hypertension.
1. Introduction
Hypertension (HTN) is considered a major health problem associated with its high risk of cardiovascular abnormalities.1,2 The high prevalence of hypertension has been reported in both economically developed and developing countries, affecting more than one billion individuals worldwide.3,4 However, the prevalence ratio is diverse according to geological conditions and is not uniform.5 The present management protocols of HTN are inadequate to control cardiovascular complications because of shortfalls in prevention, diagnosis, and control of the disorder.6 The understanding of pharmacoepidemiology plays a significant role in awareness and reducing hypertension-associated morbidity and mortality. The etiology of HTN is not clearly understood; however, a number of factors including diet, lifestyle, and genetics may contribute to the pathogenesis.7 Although there are established recommendations, implementation is difficult due to less awareness, patient and physician compliance, and health care issues.8
In this study, we carry out the differential analysis of HTN-related Affymetrix datasets to find the genetic reasons for the disease. The most common techniques involve comparative genomics to proteomic-level analysis, genome-wide scanning, differential screening, and systems biology approaches. Many small changes in gene expression and polymorphisms of genes are associated with the progression of the disease.9 These genetic variations lead toward post-translational modifications (PTM) including more than 400 types of chemical alterations at the amino acid level. PTM sites of the disordered proteins are responsible for altering motifs and ultimately disease development.10
Simulation-based analysis improves therapeutic strategies by providing innovative ways for medical sciences to cope with diseases using data in a virtual environment. Therefore, these machine learning programs can help to find the potential target proteins.11 Meta-genomics covers the broader spectrum of genetic analysis, which results in better outcomes in clinical sciences.12 The virtual-based approach is inexpensive, safe, and time-effective to analyze the complex samples of the patient.11 The major objectives of our integrative framework are to (i) determine the DEGs associated with the pathogenesis of hypertension, (ii) map their role in physiological and biochemical functions, (iii) carry out expression profiling, (iv) identify functional interactors and gene signatures of the disease, (v) analyze the mutation of core proteins and other regulatory motifs, and (vi) determine potential targets by drug–gene networks through molecular docking and simulation studies (Figure 1).
Figure 1.
Schematic diagram and framework of the study.
2. Results
2.1. Differential Analysis and Normalization
We obtained 22 hypertension-related Affymetrix (CELL format) cDNA datasets. AffyBatch has 712 × 712, 1164 × 1164, 1050 × 1050, and 732 × 732 array sizes (Table 1). The density estimation of data is shown by the histograms representing expression after normalization. The array distributions have similar shapes and ranges, indicating that the data is of good quality. The right-hand distribution of the array reveals a high background level (Figure 2). RNA quality, sequence biases, and RNA degradation were all examined in cDNA datasets. The use of low-quality RNA samples in genome sequencing is wasteful in genomic analysis. It is unclear if transcript degradation happens reliably in low-quality RNA samples, in which case data normalization can counteract the effects of degradation, or whether different RNA samples degrade at different rates, thereby biasing expression measures. As a result, we examined the RNA quality for differential expression analysis to ensure that the dataset is reliable for detecting transcriptional variation in original samples. By applying discrimination measures for statistical and algorithmic analysis, the normalization procedure was used to standardize sample handling techniques and to estimate the best RNA variability threshold. Individual probes in each probe set are positioned at the 5′-end of the target RNA molecule. A 3′/5′ intensity gradient has been demonstrated to affect the competitive binding of a probe to its target. Because the RNA is of poor quality, just a small amount of it is hybridized into the array. The total signal output level is reduced as a result of the low hybridization. The 3′/5′ intensity gradient, on the other hand, falls as the saturation level rises. A probe set corresponding to the transcripts is located at the target gene’s 3′-end. “AffyRNAdeg” generates a statistical summary for each batch array to assess RNA degradation levels and their importance (Figure 3). Table 2 is a list of the tools, information, databases, and web servers that were used in this inquiry.
Table 1. List of Human Affymetrix cDNA Datasets.
| S. no. | dataset accession | total samples | tissues | conditions | platform | size of arrays | AffyIDs | ref |
|---|---|---|---|---|---|---|---|---|
| 1 | GSE3356 | 09 | coronary smooth muscle | case vs control | GPL96 [HG-U133A] Affymetrix Human Genome U133A Array | 712 × 712 | 22,283 | (13) |
| 2 | GSE6489 | 06 | endothelial cells | case vs control | GPL570 [HG-U133_Plus_2] Affymetrix Human Genome U133 | 1164 × 1164 | 54,675 | (14) |
| 3 | GSE6573 | 06 | adipose, decidua, and placenta tissue | case vs control | GPL570 [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array | 1164 × 1164 | 54,675 | (15) |
| 4 | GSE10767 | 07 | arterial hypertension | case vs control | GPL570 [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array | 1164 × 1164 | 54,675 | (16) |
| 5 | GSE11341 | 12 | endothelial cells | case vs control | GPL96 [HG-U133A] Affymetrix Human Genome U133A Array | 712 × 712 | 22,283 | (17) |
| 6 | GSE17814 | 18 | endothelial cells | case vs control | GPL9099 Affymetrix GeneChip Human Genome U133 Plus 2.0 Array | 1164 × 1164 | 54,675 | (18) |
| 7 | GSE19136 | 12 | human left mammary artery | case vs control | GPL570 [HG-U133_Plus_2] Affymetrix Human Genome U133 | 1164 × 1164 | 54,675 | (19) |
| 8 | GSE22255 | 40 | blood mononuclear cells | case vs control | GPL570 [HG-U133_Plus_2] Affymetrix Human Genome U133 | 1164 × 1164 | 54,675 | (20) |
| 9 | GSE22356 | 38 | blood mononuclear cells | case vs control | GPL570 [HG-U133_Plus_2] Affymetrix Human Genome U133 | 1164 × 1164 | 54,675 | (21) |
| 10 | GSE24752 | 06 | peripheral blood cells | case vs control | GPL570 [HG-U133_Plus_2] Affymetrix Human Genome U133 | 1164 × 1164 | 54,675 | (22) |
| 11 | GSE28345 | 08 | kidney | case vs control | GPL6244 [HuGene-1_0-st] Affymetrix Human Gene 1.0 ST Array | 1050 × 1050 | 32,321 | (23) |
| 12 | GSE28360 | 14 | kidney | case vs control | GPL6244 [HuGene-1_0-st] Affymetrix Human Gene 1.0 ST Array | 1050 × 1050 | 32,321 | (23) |
| 13 | GSE37455 | 41 | kidney | case vs control | GPL11670 Affymetrix Human Genome U133 Plus 2.0 Array | 1164 × 1164 | 54,675 | (24) |
| 14 | GSE38783 | 24 | endothelial cell | case vs control | GPL570 [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array | 1164 × 1164 | 54,675 | (25) |
| 15 | GSE42955 | 29 | heart | case vs control | GPL6244 [HuGene-1_0-st] Affymetrix Human Gene 1.0 ST Array | 1050 × 1050 | 32,321 | (25) |
| 16 | GSE67492 | 06 | heart | case vs control | GPL6244 [HuGene-1_0-st] Affymetrix Human Gene 1.0 ST Array | 1050 × 1050 | 32,321 | (26) |
| 17 | GSE69601 | 06 | blood samples | case vs control | GPL6244 [HuGene-1_0-st] Affymetrix Human Gene 1.0 ST Array | 1050 × 1050 | 32,321 | (27) |
| 18 | GSE70456 | 16 | endothelial Cell | case vs control | GPL15207 Affymetrix | 732 × 732 | 49,495 | (28) |
| 19 | GSE71994 | 40 | peripheral blood mononuclear cells | case vs control | GPL6244 [HuGene-1_0-st] Affymetrix Human Gene 1.0 ST Array | 1050 × 1050 | 32,321 | (29) |
| 20 | GSE87493 | 32 | blood mononuclear cells | case vs control | GPL6244 [HuGene-1_0-st] Affymetrix Human Gene 1.0 ST Array | 1050 × 1050 | 32,321 | (30) |
| 21 | GSE113439 | 26 | lung tissue | case vs control | GPL6244 [HuGene-1_0-st] Affymetrix | 1050 × 1050 | 32,321 | (31) |
| 22 | GSE124114 | 18 | trabecular meshwork cells | case vs control | GPL6244 [HuGene-1_0-st] Affymetrix Human Gene 1.0 ST Array | 1050 × 1050 features | 32,321 | (32) |
Figure 2.
Normalization and differential analysis. The histogram shows the density of the data analyzed. Normally, the proportions of the clusters have comparable shapes. Significant levels of background shifted the intensities of the different arrays toward the right.
Figure 3.
RNA degradation plot produced by the AffyRNAdeg representation 5′ to 3′ pattern, indicating an assessment of the degradation and severity level.
Table 2. Tools, Databases, and Software Used in This Study.
| databases/software/tools | accessibility | utility |
|---|---|---|
| CELLO subcellular localization predictor | http://cello.life.nctu.edu.tw/ | subcellular localization prediction |
| DAVID bioinformatics tool | http://david.abcc.ncifcrf.gov | functional annotation tool |
| STRING database | http://string-db.org/ | for known and predicted protein/COGs interaction |
| NCBI | http://blast.ncbi.nlm.nih.gov/ | biomedical and genomic information source |
| KEGG database | http://www.genome.jp/ | pathway analysis and comparison |
| Cytoscape version 3.6.0 | http://www.cytoscape.org/ | for network analysis and visualization |
| FunRich tool 3.1.3 | http://funrichweb.org/ | for significant association of DEGs in biological pathways |
| CIMminer | https://discover.nci.nih.gov | for cluster analysis regarding their expression value |
| ActiveDriverDB | https://www.activedriverdb.org/ | for mutation analysis |
| WikiPathways | https://www.wikipathways.org/ | pathway analysis and comparison |
| oPOSSUM version 3.0 | http://opossum.cisreg.ca/oPOSSUM3/ | prediction of regulatory motifs |
| miRDB | http://mirdb.org./ | to explore the functional annotation |
2.2. Finding Potential Drug Targets
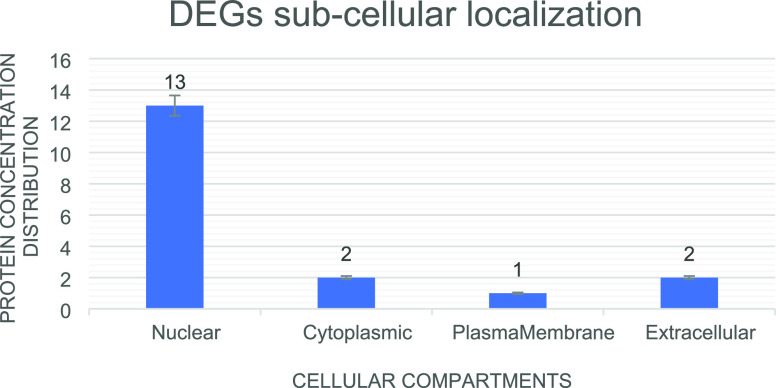
We identified 30 DEGs in every individual dataset by pairwise comparison. From the list of top-ranked genes, 18 common DEGs were sorted out (Table S1). The protein subcellular localization predicted that GREM2 and SCN2B are extracellular; TPBG is membrane-bound; DUSP6 and HDDC2 are cytoplasmic; and DDIT4, CEBPD, EDN1, NFIL3, ANGPTL4, BHLHE40, C10orf10, ADM, MSR1, LRR1, c6orf15, ETS2, and USP8 are nuclear (Figure 4). Based on data mining, seven hypertension-related DEGs were shortlisted (>200 literature count) including ADM, EDN1, ANGPTL4, NFIL3, MSR1, CEBPD, and USP8 (Figure 5).
Figure 4.
Subcellular localization of differential expressed genes and DEG distribution among the cellular compartments.
Figure 5.
Disease–gene curation. The bar graph indicates the disease–gene mapping (hypertension-potential genes) using online databases.
2.3. Cluster and Functional Enrichment Analysis
We evaluated the expression of these seven DEGs to detect the complete description of molecular functions. The profiling showed variations and comparative expression of the gene in hypertension. We assessed the similarity index between disease–gene interactions through cluster analysis and observed hypertension-related enriched terms33−36 (Figure 6). Pathway-enriched relations specify a substantial association of hypertension with DEGs in the biological pathways involving hypoxia and oxygen homeostasis of HIF-I, an affinity for calcitonin-like ligands, and PIK3, EGF, and ARF6 signaling (Figure 7).
Figure 6.
Cluster analysis of seven hypertension-related DEGs with Euclidean distance (binning method: quantile lines show the limits of the clusters in the degree of the tree).
Figure 7.
Pathway enrichment analysis indicates the percentage of DEGs in the biological pathway using FunRich tool.
2.4. Identifying Regulatory Motifs and MiRNAs Targets
Seven hypertension-related DEGs were used for de novo examination to find the regulatory motifs and the transcriptional factors including Nkx2-5, HOXA5, ARID3A, Pdx1, MZF1_1-4, SPIB, Prrx2, ZEB1, ZNF354C, SRY, and ELF5 (Table S2). Mainly miRNAs including hsa-miR-365a-3p, hsa-miR-2052, hsa-miR-3065-3p, hsa-miR-603, hsa-miR-7113-3p, hsa-miR-3923, and hsa-miR-524-5p were predicted for gene ADM, USP8, ANGPTL4, NFIL3, EDN1, EDN1, and CEBPD, respectively (Table 3).
Table 3. MiRNA-Targets of Hypertension-Associated Genes.
| serial no. | gene symbol | gene description | target scorea | microRNA name | total hits | miRNA sequence | seed location | 3’-UTR length |
|---|---|---|---|---|---|---|---|---|
| 1 | ADM | adrenomedullin | 97 | hsa-miR-365a-3p | 71 | UAAUGCCCCUAAAAAUCCUUAU | 531 | 777 |
| 2 | USP8 | ubiquitin specific peptidase 8 | 94 | hsa-miR-2052 | 140 | UGUUUUGAUAACAGUAAUGU | 213 | 2008 |
| 3 | ANGPTL4 | angiopoietin-like 4 | 85 | hsa-miR-3065-3p | 22 | UCAGCACCAGGAUAUUGUUGGAG | 381, 387 | 489 |
| 4 | NFIL3 | nuclear factor, interleukin 3 regulated | 97 | hsa-miR-603 | 49 | CACACACUGCAAUUACUUUUGC | 254 | 320 |
| 5 | EDN1 | endothelin 1 | 97 | hsa-miR-7113-3p | 112 | CCUCCCUGCCCGCCUCUCUGCAG | 389 | 1139 |
| 6 | MSR1 | macrophage scavenger receptor 1 | 100 | hsa-miR-3923 | 235 | AACUAGUAAUGUUGGAUUAGGG | 1184, 1870, 1888, 1900, 1906 | 2207 |
| 7 | CEBPD | CCAAT enhancer binding protein delta | 98 | hsa-miR-524-5p | 37 | CUACAAAGGGAAGCACUUUCUC | 210, 264, 303, 368 | 415 |
Highly reliable score ≥ 80, least reliable score ≤ 50.
2.5. Mutation Analysis
We performed a mutation analysis of the seven DEGs. The NFIL-3 protein has 21 PTM sites, and at chromosome number 9, it showed recurrent mutations as a negative-strand representing 71.21% as a disordered region encoding approximately 462 different protein residues. The isoform NFIL-3 N103S showed a proximal mutation at position 103, with amino acid residues N and S. CEBPD exhibited a 52.04% sequence region as disordered and have six (06) PTM sites with 18 mutations and 269 residues at chromosome number 8. CEBPD G186D, CEBPD R195Q, and CEBPD P257A isoforms were investigated by CEBPD mutational enrichment analysis at positions 186, 195, and 257, respectively. USP8 revealed 48.84% of the sequence disordered, which has 42 PTM sites with 118 protein residues and shows 137 mutations at chromosome 15. USP8 T351A showed the direct mutation. Other isoforms like USP8 R638T, USP8 R638K, and USP8 R638G showed mutations at position 638. Similarly, ADM genes have 24 mutations at chromosome number 11 on the positive strand with 185 residues and 12 PTM regions. The ADM S178N isoform showed a direct mutation at position 178, while ANGPTL4 showed 86 mutations on the positive strand of chromosome 19 with 406 protein residues (Figure 8).
Figure 8.
Mutation analysis indicates the post-translational change in human genes/proteins using the ActiveDriverDB database. Needle plots demonstrate the PTM sites in our proteins (shown in legend color codes). The y axis indicates the mutation count while the x axis demonstrates the position of the amino acid sequence. Pinhead shading means the mutation effect, and x axis shading shows the kind of PTM related to the mutation area.
2.6. Protein Network Analysis
In the protein–protein network, a total of 72 nodes and 64 edges were retrieved from the STRING database. The PPI network was principally characterized by three nodes: source nodes (light pink color) to target nodes (light gray color), while the remaining light-yellow nodes represent the other gene signatures (Figure 9A). The network topological properties were analyzed through a network analyzer. In this network, the topological parameters in the network were measured between the nodes and edges and characterized the qualitative gene pattern (Figure 9B). Shortlisted DEGs interact with other proteins like CALCRL, RAMP2, RAMP1, RAMP3, CALCA, ADM2, AVP, MAPK1, EDNRB, EDNRA, ECE1, AGT, AKT1, RHOA, SRC, LPL, PPARA, PPARGC1A, PARGC1A, PPARG, FABP4, RXRA, PER2, ARNTL, BHLHE40, CRY2, CRY1, COL4A2, APOE, APOB, APOA1, COL1A2, CALR, COL3A1, HSP90B1, COL4A1, HSP90B1STAT3, KLF5, STAM2, EGFR, UBC, and HGS are important in disease phenotype. After disease gene mapping using the PubMed database, we estimated that 40 target genes have the potential for a drug target in hypertension. Among them, EGFR, AGT, AVP, APOE, RHOA, SRC, APOB, STAT3, UBC, LPL, APOA1, and AKT1 are the enriched terms (Figure 9C).
Figure 9.
Gene network analysis. (A) Protein–protein interaction network. Interaction of seeder/source nodes (light pink) with target nodes (light gray). (B) Topological properties of the network were analyzed by a network analyzer. (C) Disease–gene mapping.
2.7. Pathway Analysis and Associated Mechanisms
We analyzed the role of potential drug targets in the associated pathways to identify the mechanism of action of these molecules in hypertension. It was also found that these proteins have a major role in integrated pathway regulation including fatty acid metabolism, melatonin, transcriptional cascade, regulating adipogenesis, prostaglandin synthesis and regulation, binding and uptake of ligands by scavenger receptors, plasma lipoprotein assembly, myometrial relaxation, and contraction pathways, PPAR signaling pathway, relaxin signaling pathway, MAPK signaling pathway, and G alpha (s) signaling events. The synthesis of these biomolecules would be affected by the regular expression patterns of respected genes, while dysregulation of these pathways results in positive anomalies of hypertension (Figure 10). We found 31 signaling pathways associated with hypertension.
Figure 10.
Pathway analysis and molecular mechanisms in hypertension. The pathways have been mapped using KEGG and Wiki Pathways. Color codes are used to describe the reaction steps of the pathway model.
2.8. Toxicogenomics
We found the adverse effects of environmental and pharmaceutical chemicals on disease progression and ultimately on human health. Based on the activity, binding, and expression patterns, we observed the effect of chemicals that either increase or decrease gene activity at cellular levels (Figure 11). The effect of chemicals on either level expression (increase or decrease) has been shown in light green and yellow color nodes. The effect of cotreatment expression is indicated by light gray.
Figure 11.
Toxicogenomic analysis of differentially expressed genes by the Comparative Toxicogenomics Database (CTD) helps to study the chemical genome to phenome relationships.
2.9. Upregulated Genes
The predicted log_2-fold change of the score of the seven shortlisted genes obtained from the Expression Atlas data are given in Table 4. These results were further verified in a study2 in which ADM, USP8, ANGPTL4, and EDNI were up-regulated in the peripheral blood samples of hypertensive patients (Figure 12).
Table 4. Regulation Gene Expression via Expression Atlas.
| S. no. | gene | log_2-fold change | gene regulation |
|---|---|---|---|
| 1 | ADM | 1.1 | upregulated |
| 2 | ANGPTL4 | 3.1 | upregulated |
| 3 | EDN1 | 1.1 | upregulated |
| 4 | USP8 | 1.2 | upregulated |
| 5 | MSR1 | –1.4 | downregulated |
| 6 | CEBPD | –1.1 | downregulated |
| 7 | NFIL3 | –2.3 | downregulated |
Figure 12.

Based on the fold variations in gene expression and abnormal expression levels of differentially expressed genes in hypertension patients and controls.
2.10. Molecular Docking
MOE docked FDA-approved medicines with four upregulated genes in hypertension against a specific binding pocket, and all of the complexes were graded based on the energy function score (S-Score), RMSD plots, non-covalent interaction strength, hydrogen bonding, and maximal accommodation with the binding pocket. Out of the 99 library medications, 12 were reused as the best for simulation. CID:65999 had a minimum binding score of −10.2 kcal/mol in ADM (PDB: 4RWF), CID:135409642 had a score of −10.1 kcal/mol, and CID:3749 had a value of −9.9 kcal/mol (Figure 13A–C, respectively). The top three ranking medications, CID:2450, CID:65999, and CID:71301, all had minimum binding scores of −9.1, −9.1, and −8.6 kcal/mol, respectively. In the case of USP8 (PDB:2GF0) (Figure 13D–F, respectively). Top-ranked medicines with a minimum binding score of −8 kcal/mol in ANGPTL4 (PDB: 6EUB) were CID:110635, CID:135409642, and CID:3157. (Figure 13G,H,I). EDN1(PDB: 6DK5) with CID:65999, CID:110635, and CID:3749 had the lowest binding scores, with −9, −8.7, and −8.5 kcal/mol, respectively, (Figure 13J–L). All 12 complexes show the highest hydrogen bonding, van der Waals interaction, and other hydrophobic interactions with the binding pocket residues.
Figure 13.
(A–C) 2D interactions of CID:65999, CID:135409642, and CID:3749 with 4WRF. (D–F) 2D interactions of CID:2450, CID:65999, and CID:71301 with 2GFO. (G–I) 2D interactions of CID:110635, CID:135409642, and CID:3157 with 6EUB. (J–L) 2D interactions of CID:65999, CID:110635, and CID:3749 with 6DK5.
2.11. Molecular Dynamic (MD) Simulation
With the Desmond simulation package, MD simulations were run for 100 ns per complex after the docking measurements. The root mean square deviation (RMSD), root mean square fluctuation (RMSF), and protein–ligand interaction (PLI) parameters were determined using MD trajectories. We ran MD simulations with similar parameters for each of our twelve substances to test the outcome of the MD simulations. The results of each complex’s simulation were demonstrated to be repeatable, and they are displayed below.
2.11.1. RMSD Analysis
Both ligand and protein in the 4RWF–CID:65999 complex reached equilibrium at 40 ns and remained stable throughout the simulation. After establishing stability, ligand RSMD indicated that fluctuation changes were maintained within 1.6 Å (Figure 14A). Similarly, the 4RWF complex with the CID:135409642 ligand RSMD becomes stable at 10 nm and stays stable for 100 ns. After 40 ns, there was considerable variability in protein RSMD, although it was within acceptable limits (Figure 14B). RMSD indicated good stability up to 80 ns for compound 4RWF with the CID:3749 ligand (Figure 14C). RMSD changes stayed within 1.5 Å over the simulation period, which is acceptable for tiny, globular proteins like 4RWF. Simultaneously, the 2GFO/CID:2540 ligand complex (Figure 14D) reached equilibrium within 10 ns and remained stable throughout the simulation. The RMSD fluctuated by 1.6 Å for the first 10 ns and then stayed steady for the next 100 ns. Similarly, after 20 ns, the complex of 2GFO and CID:65999 ligand (Figure 14E) remained stable throughout the simulation up to 100 ns. The complex remained steady throughout the simulation up to 100 ns, with just a slight fluctuation of 0.15 nm after 50 ns up to 60 ns. Furthermore, in the 2GFO–CID:71301 ligand complex (Figure 14F), the stability was shown by RMSD up to 100 ns after 45 ns. During the simulation period, changes in the RMSD values stayed within 1.5 Å, which is acceptable for tiny, globular proteins like 2GFO. Furthermore, the CID:110635 ligand–6EUB complex (Figure 15A) demonstrated stabilization up to 50 ns and showed 2 Å variations between 50 and 55 ns during simulation, and it became stable at 60 ns and stayed constant up to 100 ns. Similarly, the 6EUB complex with ligand CID:135409642 (Figure 15B) showed minor fluctuations of 1.8 Å between 55 and 60 ns while remaining stable throughout the simulation time. When compared to the 6EUB combination with the CID:3157 ligand (Figure 15C), they stay stable for 65 ns. RMSD values stayed within 2 Å over the simulation period, which is ideal for tiny, globular proteins such as 6EUB. Figure 14 shows the 6D5K RMSD plot, which shows that complexes stayed intact throughout the experiment. The CID:65999 ligand–6D5K complex (Figure 15D) showed modest variance up to 15 ns at first but thereafter demonstrated simulated stability of up to 100 ns. Similarly, the 6D5K complex with ligand CID:110635 (Figure 15E) showed stability up to 40 ns during the simulation, with a flip of the ligand between 40 and 50 ns due to variations in protein structure, but the ligand remained constant after that. Both the ligand and the protein are initially unstable in the 6D5K complex with the CID:3749 ligand. They reached equilibrium after 20 ns; however, their RSMD is exceedingly high, indicating relative instability (Figure 15F).
Figure 14.
RMSD plot of 4RWF protein with three ligands, (A) CID:65999, (B) CID:135409642, and (C) CID:3749, respectively. RMSD plot of the 2GFO protein with three ligands, (D) CID:2540, (E) CID:65999, and (F) CID:71301, respectively. The x axis depicts the simulation’s time frame (in seconds). The protein RMSD variation is shown on the right y axis, while the RMSD variation of the ligand is shown on the left y axis.
Figure 15.
RMSD plot of 6EUB protein with three ligands, (A) CID:110635, (B) CID:135409642, and (C) CID:3157, respectively. RMSD plot of the 6D5K protein with three ligands, (D) CID:65999, (E) CID:110635, and (F) CID:3749, respectively. The x axis depicts the simulation’s time frame (in seconds). The protein RMSD variation is shown on the right y axis, while the RMSD variation of the ligand is shown on the left y axis.
2.11.2. RMSF Analysis
The deviation of a particle in a macromolecule is defined by the RMSF. It defines the flexibility and rigidity of protein structures. The residues with higher peaks are found in loop areas or N- and C-terminal zones, as N and C are the most fluctuating in MD trajectories. Low RMSF values of binding site residues indicate that the ligand binding to the protein is stable. RMSF values of the protein complexes (4RWF bound with CID:65999, 135,409,642, and 3749), (2GFO bound with CID:2540, 65,999, and 71,301), (6EUB bound with CID:110635, 135,409,642, and 3157), and (6D5K bound with CID:65999, 110,635, and 3749) with different ligands are shown in Figure 16.
Figure 16.
RMSF plot analysis of complex protein concerning ligands. (A) Plot of 4RWF–CID:65999, (B) plot of 4RWF–CID:135409642, (C) plot of 4RWF–CID:3749, (D) plot of 2GFO–CID:2540, (E) plot of 2GFO–CID:65999, (F) plot of 2GFO–CID:71301, (G) plot of 6EUB–CID:110635, (H) plot of 6EUB–CID:135409642, (I) plot of 6EUB–CID:3157, (J) plot of 6D5K–CID:65999, (K) plot of 6D5K–CID:110635, and (L) plot of 6D5K–CID:3749, respectively.
During the simulation procedure, the RMSF measured the distinct variations in protein residues. During the modeling of 4RWF with corresponding ligands, substantial changes (between residues 480–490 with CID:65999, 170–180 with CID:135409642, and 480–500 with CID:3749) were detected (Figure 16A–C). Furthermore, during the modeling of 2GFO with respective ligands, substantial changes (between residues 130–135 with CID:2540, 130–135 and 240–255 with CID:135409642, and 120–130 and 200–220 with CID:71301) were detected (Figure 16D–F). Other discrepancies were detected during the modeling of 6EUB with relevant ligands (between residues 150–155 and 175–185 with CID:110635, 150–160 and 175–185 with CID:135409642, and 150–160 and 175–190 with CID:3157) (Figure 16G–I). Moreover, during the modeling of 6D5K with relevant ligands, other oscillations (between residues 20–25 with 6D5K–CID:65999, 22–27 with CID:110635, and 3–7 and 20–25 with CID:3749) were detected (Figure 16J–L).
2.11.3. Protein–Ligand Interactions
Throughout the simulation, protein interactions with the ligand were observed. Hydrogen bonds, hydrophobic, ionic, and water bridges are the four forms of protein–ligand interactions. Exploring the “Simulation Interactions Diagram” tab reveals more precise subtypes for each interaction type. In ligand binding, hydrogen bonding (H-bonds) and hydrophobic interactions are important. Because of their considerable influence on drug selectivity, metabolization, and adsorption, hydrogen-bonding properties should be considered in drug design. Water bridges and ionic interaction are also vital in the formation of complex proteins structure.
LYS-17, ASP-43, GLU-113, and TRP-232 were the most active residues in the 4RWF–CID:65999 complex, forming tight hydrogen bonds, while ASP-16, LYS-44, GLU-155, and ASN-229 were the amino acid residues that formed water bridges. Figure 17A shows the minimal contribution of TRP-64, ALA-65, TYR-212, and TYR-157 residues to hydrophobic interaction with ligand atoms. Strong hydrogen bonds were established by the most active residues in the PL complex of 4RWF–CID:135409642, such as ASN-14, LYS-17, GLU-113, and TRP-232, whereas TRP-64, ALA-65, TYR-157, PHE-158, and TYR-212 were five amino acid residues that contributed to strong hydrophobic interactions Figure 17B. Although strong hydrogen bonds were formed for the 4RWF–CID:3749 complex, ASN-229, GLY-230, and TRP-232, whereas LEU-115, GLU-115, and TYR-157 formed hydrophobic interactions with the relevant ligand atoms (Figure 17C). The most active residues, such as SER-953 and ASN-981, established strong hydrogen bonds, while LYS-996, ILE-998, PHE-971, and PHE-930 contributed to strong hydrophobic contacts in the protein–ligand complex 2GFO–CID:2540 (Figure 17D). LYS-996 and LYS-1012 residues displayed hydrogen bonding, while PHE-930, VAL-934, PHE-971, and TYR-1016 residues were crucial in the ligand’s hydrophobic interaction. The development of water bridges for the 2GFO–CID:65999 complex involved SER-953, LYS-976, THR-978, and ASP-979 (Figure 17E). Although strong hydrogen bonds were formed for the 2GFO–CID:71301 complex, ASP-878, ASN-918, and PHE-946 were formed, while LEU-874, ILE-922, VAL-923, and PHE-930 established a hydrophobic connection with the relevant ligand atoms (Figure 17F). The most active residues in the 6EUB–CID:110635 combination established excellent hydrogen bonds: LEU-213 and LYS-261. The hydrophobic interaction of LEU-201, PHE-212, PRO-251, HIS-252, PHE-255, and LEU-257 residues with ligand atoms adds to the overall contribution (Figure 18A). Strong hydrogen bonds were generated by the most active residues in the 6EUB–CID:135409642 complex, such as THR-315, THR-316, SER-323, and HIS-333. The residues of amino acids LEU-322, PRO-325, LEU-335, and PHE-351 resulted in significant hydrophobic interactions (Figure 18B). For the 6EUB–CID:3157 complex, SER-323, GLN-331, ASP-332, ASP-334, and PHE-351 established strong hydrogen bonds. Along with ALA-299, PRO-325, LEU-335, and ARG-336,337, the hydrophobic interaction with the relevant ligand atoms was established (Figure 18C). Hydrogen bonds were produced by the most active residues, such as SER-2 and LYS-9, in the 6DK5-CID:65999 complex, while hydrophobic interactions with ligand atoms were contributed by amino acid residues TRP-21, LEU-6, VAL-12, and HIS-16 (Figure 18D). For the 6DK5-CID:110635protein-ligand complex, the most active residues, such as TRP-13 and ILE-19, formed tight hydrogen bonds, while TRP-21, PHE-14, HIS-16, and LEU-17 amino acid residues formed hydrophobic contacts (Figure 18E). SER-5, ILE-19, and 20 performed a significant function in hydrogen bonding in 6DK5–CID:3749, whereas MET-7, PHE-14, ILE-19, TYR-13, and 21 showed hydrophobic interaction (Figure 18F).
Figure 17.
Protein interaction analysis (PIA). (A) PIA plot of 4RWF–CID:65999, (B) PIA plot of 4RWF–CID:135409642, (C) PIA plot of 4RWF–CID:3749, (D) PIA plot of 2GFO–CID:2540, (E) PIA plot of 2GFO–CID:65999, and (F) PIA plot of 2GFO–CID:71301.
Figure 18.
Protein interaction analysis (PIA). (A) PIA plot of 6EUB–CID:110635, (B) PIA plot of 6EUB–CID:135409642, (C) PIA plot of 6EUB–CID:3157, (D) PIA plot of 6DK5–CID:65999, (E) PIA plot of 6DK5–CID:110635, and (F) PIA plot of 6DK5–CID:3749.
3. Discussion
In recent years, the advent of technological development has been making remarkable changes in biological sciences. This study shows the association of genetic variation in hypertension. We found a list of hypertension-related genes based on differential expression and systems biology analysis. The system-level framework gives us a consistent development of the meta-analysis of cDNA microarray datasets to identify DEGs.37 We separated seven common genes as potential medication targets, to be specific ADM, EDN1, ANGPTL4, NFIL3, MSR1, CEBPD, and USP8 (p < 0.05), from the rundown of 18 DEGs dependent on physicochemical and functional examination. The profiling showed variations and comparative expression of the gene in hypertension. The pathway-enriched study showed a significant association between hypertension with DEGs. The dysregulation of HIF-I and calcitonin has been studied in hypertension.38,39 PIK3 and ARF6 signaling is considered a novel target in hypertension.40,41 Some studies indicate that abnormal EGF signaling is linked to the development of cardiovascular pathology.42 This study showed significant enrichment of four upregulated (ADM, ANGPTL4, USP8, and EDNI) and three downregulated genes. We found the role of regulatory motifs of these genes in heart pathogenesis, control of cell cycle progression, regulation of β-cell development, myocardial fibrosis, hypertensive nephrosclerosis, and blood pressure modulation. MicroRNAs are involved in the regulation of human physiological processes by transcription events, and dysregulation of these expressions results in many diseases.43 miR-365b-3p appears to play a role in coronary artery smooth muscle cell proliferation and migration, through its direct target gene ADAMTS1.44 Altered responses to hsa-miR-3065-3p, hsa-miR-603, and hsa-miR-3923 may result in cardiovascular pathophysiology.45,46 hsa-miR-524-5p is a circulating miRNA that has been found to be considerably downregulated in patients with heart disease as compared to controls.47 The inherited mutation encodes genetic variations in the relation of genotype to phenotype. Thousands of SNVs are reported to be the cause of disease. Amino acid substitution may have a significant role in cell differentiation and growth in correspondence to missense mutations as post-translational modifications (PTM).10 Mutations can interfere at different levels leading to the emergence of some toxic confirmations and have a major role in protein function both in normal and pathological states. A change in PTM reveals the disease progression48 and is responsible for functional diversity.49 In the PPI, we recognized that these conceivable potential drug targets are practically connected with other associated protein targets including EGFR, AGT, AVP, APOE, RHOA, STAT3, SRC, APOB, UBC, LPL, APOA1, and AKT1. Of these proteins, EGFR is involved in the molecular mechanism of Ang II-mediated cerebrovascular remodeling,50 AGT polymorphism, and APoB is associated with hypertension.51,52 Vasopressin has several functions via its three distinct receptors, V1a, V1b, and V2. Among them, AVP is one potent vascular constrictor by regulating the vascular tone and fluid through its V1aR and/or V2R. The RhoA/Rho-kinase signaling pathway increases the vasoconstriction characteristic of hypertension. The increase in vascular peripheral resistance is partly due to vascular constriction mediated by the calcium-independent activation of the small G-protein RhoA and a downstream target, Rho-kinase.53 Src/STAT3, AKT1, and epidermal growth factor (EGF) activation plays important roles in cell proliferation and results in the pathobiology of pulmonary arterial hypertension characterized by the enhanced pulmonary artery smooth muscle cell.60,61 The allelic association of ApoB and LPL has a strong genetic association with hypertensive individuals.54
The synthesis of these biomolecules would be affected by the regular expression patterns of respective DEGs while dysregulation of these pathways results in positive anomalies of hypertension. Melatonin involves an antioxidant activity with its potential role in mitochondrial physiology in the cardiovascular system. Melatonin regulates blood pressure by central and peripheral mechanisms, in addition to the action on the renin–angiotensin system.55 The prostacyclin pathway was studied for the treatment of pulmonary arterial hypertension (PAH), which is a chronic and progressive disease resulting in right ventricular failure and death.56 Elevated blood pressure activates mitogen-activated protein (MAP) kinases, resulting in a rapid and transient induction of MKP-1 mRNA followed by elevated MKP-1 protein expression in the aorta.57 The reno-protective effects of peroxisome proliferator-activated receptors (PPARS) produce ligand-induced blood-pressure-lowering effects, protective effects on endothelial function, and vasodilating effects on glomerular efferent arterioles.58 Similarly, relaxin contributes to sympathetic overdrive and hypertension via the PI3K-Akt pathway.59 We find that genes linked directly with blood pressure regulation by binding with G-protein coupled receptors have a sympathomimetic-like action and indirectly, through communication with other genes, are effective in hypertension. The toxicogenomic analysis helps to understand gene interaction with the chemicals that may be the reason for disease progression. This analysis suggests the mechanism of action of chemicals and their effect on the disease state influenced by environmental exposure.60 We used an FDA-approved hypertensive medication with a specific binding site to dock against four target proteins that have previously been identified as overexpressed DEGs in hypertensive people.2 These goals were chosen based on several variables, including their relative importance and the availability of the relevant literature. The binding energy was used to compute the optimal inhibitory potential of these docked ligands; thus, a ligand with low binding energy is favored, as a low binding score is directly linked to greater binding affinity.61 We select three of the best ligands for each target for additional molecular dynamic modeling to determine the stability of the complexes up to 100 nm. The stability of all 12 complexes has been maintained. Because of loop development, the 6EUB–CID:3157 complex showed higher deviation, but changes in the RMSD value remained within limits. The complex 6D5K–CID:3749 had a high RSMD score, indicating relative instability. Furthermore, all protein complexes with their respective ligands had RMSF values of <3. The higher peaks’ residues follow the MD trajectories’ loop areas or N- and C-terminal regions. The reduced RMSF values of binding site residues and ligand atoms demonstrate the stability of ligands bound to these proteins. Such analysis not only improves our comprehension of disease pathophysiology but is also useful in drug discovery.62
4. Methods
4.1. Accession to cDNA Datasets
The goal of the study was to find differentially expressed genes (DEGs) in hypertension to diagnose its cause. The accession number, sample numbers, and other traits of a human expression dataset of hypertension were retrieved from the Gene Expression Omnibus (GEO) database. The DEGs were discovered using the Affymetrix U133Plus2.0 array platform and the annotation probe HGU133plus2. To examine the measurable outcomes, various Bioconductor programs with the R-platform were utilized (Affy, AffyQCReport, AffyRNADegradation, AnnotationDbi, Annotate, Biobase, Limma, and HGU133a2cdf).63
4.2. Normalization and Differential Investigation
For normalization, the pheno-data files were organized in an acceptable format.64 The Array Quality Metric was used to standardize the dataset to the median level of expression for each gene set. By utilizing the following equation, the Robust Multi-Array Analyses (RMA) were employed for background correction to quantify perfect matches (PM) and mismatches (MM).65−67
where PM is a perfect match, BG is the background caused by optical noise, and S is the nonspecific binding; ijk is the signal for probe, j of the probe set k on array i.
The PM data show a mix of both ″BG″ and the expression signal ″E″. The dataset was analyzed using the array quality measure, which was normalized to the median level of expression with a cutoff value of p value less than 0.15.65,68 AffyRNAdeg, summary AffyRNAdeg, and plotAffyRNAdeg were used to verify the quality of RNA in the samples for RNA degradation analysis.69,70 The genes that were chosen were based on their p values and scores. P value = 0.05, absolute log-fold change (log FC) > 1, FDR = 0.05 (false discovery rate), and average expression level (AEL) = 40% were used as cutoff values.
4.3. Subcellular Localization Prediction
The top-ranked DEGs of each dataset were compared by the Compare-Two list tool to identify the common genes.71 The appropriate subcellular protein provides the idea of normal human functions, and the unusual localization of proteins shows the pathogenesis of different human diseases.72 We predicted the subcellular location of DEGs using CELLO version 2.5.73
4.4. Data Mining and Disease–Gene Curation
In biomedical research, literature-based text mining is a significant step to extract information (DEG–disease interaction) based on the research entities.74 We investigated disease interactions curated from online data sources including the Comparative Toxicogenomics Database (CTD), Online Mendelian Inheritance in Man (OMIM), PubMed, and MeSH of shortlisted DEGs to filter specific genes.75
4.5. Enrichment and Cluster Analysis
The function of the gene gives us the information to understand the signaling pathway at the cellular level. We performed the expression profiling of each dataset to understand gene expression variations in different datasets using online web-based databases DAVID, FunRich, and Enrich Annotation tool.76,77 We performed cluster analysis regarding their expression values in each sample of hypertension-associated DEGs to evaluate expression profiling using an online one matrix CIMminer tool.78
4.6. Prediction of Regulatory Motifs and Hypertension-Associated MiRNA Targets
Hypertension mechanisms can be revealed by understanding the regulation functions at the transcription level. Various genes are influenced by miRNAs in the signaling cascade. MiRNAs play an important regulatory role in gene expression and disease etiology. oPOSSUM tool version 3.0 was used to find the regulatory motifs and transcription factors of target genes with default parameters (matrix threshold of 85% with a cutoff value 0.4). Hypertension-related miRNA targets were predicted by the MiR database.79
4.7. Mutation Analysis
The human genome with nucleotide variations (SNVs) is associated with many diseases. The mutation was analyzed to find specific variants. We performed mutation analysis of DEGs by the ActiveDriverDB database. It is used to identify protein posttranslational modification (PTM) sites.10
4.8. Protein–Protein Interaction Analysis
Protein–protein interactions (PPIs) play a key role in cellular functions. Dysregulation in normal protein networks may be the reason for the disease.80 PPIs are the functional interaction of proteins that are used to explore the variations in biological function.81,82 This biological network shows the difference in activity in both physiological and pathological conditions. The functional interactions of the source gene were retrieved from STRING.v.10.83 Furthermore, the roles of the target genes in hypertension were curated from different databases including PubMed, CTD, and OMIM. Cytoscape software version 3.6 was used to visualize the network to explore the role of both source (DEGs) and target proteins in hypertension.84 The entire network is important to find potential hypertension-linked gene signatures as their abnormality is directly related to the disease phenotype.
4.9. Pathway Analysis
Pathway analysis is important to analyze the metabolic networks to understand the underlying functional mechanisms. The KEGG and WIKI databases were used to analyze the available pathways of target genes.85−87 We constructed an integrated network of pathway models using Cystoscope software. This interactive model reveals the function of each gene in pathways.
4.10. Toxicogenomic
The possible cause of human diseases may be the chemicals in the environment. We retrieved the available gene–disease information to analyze the chemical–gene interactions using the Comparative Toxicogenomics Database.88
4.11. Regulation of Gene Expression
ExAtlas database (https://www.ebi.ac.uk/gxa/home) and hypertensive patient blood samples2 were used to predict the gene regulation, i.e., either upregulated or downregulated.
4.12. FDA-Approved Antihypertensive Drug Interaction with Upregulated Genes
4.12.1. Protein and Ligand Preparation for Docking
The crystal structures of ADM (PDB: 4RWF), USP8 (PDB: 2GFO), EDN (PDB: 6DK5), and ANGPTL4 (PDB: 6EUB) proteins were obtained from the Protein Data Bank (www.rcsb.com).89 The sequence was obtained from the Uniprot database.90 Using Chimera software,91 all heteroatoms and water in the PDB data were eliminated and stored as PDB files for docking. The Drug Bank92 and PubChem database5 were used to obtain a library of 99 FDA-approved hypertensive medications. The Molecular Operating Environment (MOE) software was used to create the protein and drug library. Adding hydrogens to proteins and ligands using the protonate 3D approach in MOE followed by energy minimization was used to prepare them for docking. The AMBER99 force-field was used to remove further non-bounded structures after this energy minimization stage.
4.12.2. Molecular Docking
MOE docked a large library of hypertensive medicines to four elevated proteins in hypertension with a specific binding site. The triangular matcher algorithm (TMA) was used to produce 1000 optimal poses for each docked molecule by using the default ligand-placement approach and the London dG scoring function.93 Using the force field refinement approach, which determines binding affinity using the generalized born solvation (GBS) model,9,10 the top 10 best poses were chosen based on the energy function score and root mean square deviation (RMSD).94,95
4.13. Molecular Dynamics
Molecular dynamics (MD) simulations provide information about the dynamic behavior of protein–ligand complexes in a virtual graphical environment, displaying the free energy landscape that approximates the native state of the protein in the body. As a result, MD simulation is better for checking the precise ligand-protein interaction profile. The top-ranked medicines were tested against the four proteins using MD simulations. The MD simulation for the twelve complexes was done using the Desmond suite, and each complex was run at 100 ns utilizing the OPLS-2005 force field with an NVIDIA RTX IO: GPU – Dell Xeon series 6th generation 4 core system. To begin, protein–ligand complexes were created using the Protein Production Wizard, which included an optimization and minimization step. Second, each complex was assigned to a grid box, and NA+/Cl– ions were introduced to neutralize the system. Finally, simulations were run at 300 K and 1000 frames, with all other parameters set to default.96
5. Conclusions
This integrative gene expression analysis is significant to understanding the genetic variations. From Affymetrix cDNA datasets, we found seven DEGs as potential drug targets for hypertension. Functional analysis revealed the significant role of these DEGs in the pathological mechanisms of hypertension. Mutation analysis showed significant disordered regions in these molecules. These genes have functional interaction with the target and other gene signatures including EGFR, AGT, AVP, APOE, RHOA, SRC, APOB, STAT3, UBC, LPL, and AKT1 linked to hypertension. The associated pathways involving melatonin, MAPK, PPARs, and relaxin have been found in disease etiology. Among the seven DEGs we found, four genes were upregulated. We analyzed and repurposed the 99 FDA-approved hypertensive drugs to find potential drug targets against upregulated hypertensive genes. This system-level genomic analysis helps us to find drug targets and improve the understanding of the treatment of hypertension.
Acknowledgments
The authors wish to thank the University of Oradea, Oradea, Romania for financial support in publishing this paper.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c02277.
Preliminary investigation of common and related differentially expressed genes of each microarray dataset; DEGs utilized for de novo examination to anticipate the regulatory motifs (PDF)
Author Contributions
F.A. has written the manuscript. S.S.M. has done a formal analysis. S.Q.A. has rechecked the paper for any errors. S.S.H. and S.B. have done manuscript editing and preparation. A.K. and S.A.M. have done the supervision of all of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Bromfield S.; Muntner P. High blood pressure: the leading global burden of disease risk factor and the need for worldwide prevention programs. Curr. Hypertens. Rep. 2013, 15, 134–136. 10.1007/s11906-013-0340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali F.; Khan A.; Muhammad S. A.; Hassan S. S. u. J. G. Quantitative Real-Time Analysis of Differentially Expressed Genes in Peripheral Blood Samples of Hypertension Patients. Genes 2022, 13, 187. 10.3390/genes13020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R.; Suh I.; Singh V.; Chaithiraphan S.; Laothavorn P.; Sy R.; Babilonia N.; Rahman A.; Sheikh S.; Tomlinson B.; Sarraf-Zadigan N. Hypertension and stroke in Asia: prevalence, control and strategies in developing countries for prevention. J. Hum. Hypertens. 2000, 14, 749–763. 10.1038/sj.jhh.1001057. [DOI] [PubMed] [Google Scholar]
- Gupta R. Hypertension in India--definition, prevalence and evaluation. J. Indian Med. Assoc. 1999, 97, 74–80. [PubMed] [Google Scholar]
- Mills K. T.; Bundy J. D.; Kelly T. N.; Reed J. E.; Kearney P. M.; Reynolds K.; Chen J.; He J. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 2016, 134, 441–450. 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen M. H.; Angell S. Y.; Asma S.; Boutouyrie P.; Burger D.; Chirinos J. A.; Damasceno A.; Delles C.; Gimenez-Roqueplo A.-P.; Hering D.; López-Jaramillo P.; Martinez F.; Perkovic V.; Rietzschel E. R.; Schillaci G.; Schutte A. E.; Scuteri A.; Sharman J. E.; Wachtell K.; Wang J. G. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet 2016, 388, 2665–2712. 10.1016/S0140-6736(16)31134-5. [DOI] [PubMed] [Google Scholar]
- Mills K. T.; Stefanescu A.; He J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 1–15. 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwlaat R.; Schwalm J.-D.; Khatib R.; Yusuf S. Why are we failing to implement effective therapies in cardiovascular disease?. Eur. Heart J. 2013, 34, 1262–1269. 10.1093/eurheartj/ehs481. [DOI] [PubMed] [Google Scholar]
- Puddu P.; Puddu G. M.; Cravero E.; Ferrari E.; Muscari A. The genetic basis of essential hypertension. Acta Cardiol. 2007, 62, 281–293. [DOI] [PubMed] [Google Scholar]
- Krassowski M.; Paczkowska M.; Cullion K.; Huang T.; Dzneladze I.; Ouellette B. F. F.; Yamada J. T.; Fradet-Turcotte A.; Reimand J. ActiveDriverDB: human disease mutations and genome variation in post-translational modification sites of proteins. Nucleic Acids Res. Spec. Publ. 2018, 46, D901–D910. 10.1093/nar/gkx973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makransky G.; Bonde M. T.; Wulff J. S.; Wandall J.; Hood M.; Creed P. A.; Bache I.; Silahtaroglu A.; Nørremølle A. Simulation based virtual learning environment in medical genetics counseling: an example of bridging the gap between theory and practice in medical education. BMC Med. Educ. 2016, 16, 1–9. 10.1186/s12909-016-0620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaghie W. C.; Issenberg S. B.; Petrusa E. R.; Scalese R. J. A critical review of simulation-based medical education research: 2003–2009. Med. Educ. 2010, 44, 50–63. 10.1111/j.1365-2923.2009.03547.x. [DOI] [PubMed] [Google Scholar]
- Wolf S. C.; Sauter G.; Jobst J.; Kempf V. A.; Risler T.; Brehm B. R. Major differences in gene expression in human coronary smooth muscle cells after nebivolol or metoprolol treatment. Int. J. Cardiol. 2008, 125, 4–10. 10.1016/j.ijcard.2007.02.045. [DOI] [PubMed] [Google Scholar]
- Bull T. M.; Meadows C. A.; Coldren C. D.; Moore M.; Sotto-Santiago S. M.; Nana-Sinkam S. P.; Campbell T. B.; Geraci M. W. Human herpesvirus-8 infection of primary pulmonary microvascular endothelial cells. Am. J. Respir. Cell Mol. Biol. 2008, 39, 706–716. 10.1165/rcmb.2007-0368OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herse F.; Dechend R.; Harsem N. K.; Wallukat G.; Janke J. r.; Qadri F.; Hering L.; Muller D. N.; Luft F. C.; Staff A. C. Dysregulation of the circulating and tissue-based renin-angiotensin system in preeclampsia. Hypertension 2007, 49, 604–611. 10.1161/01.HYP.0000257797.49289.71. [DOI] [PubMed] [Google Scholar]
- West J.; Cogan J.; Geraci M.; Robinson L.; Newman J.; Phillips J. A.; Lane K.; Meyrick B.; Loyd J. Gene expression in BMPR2 mutation carriers with and without evidence of pulmonary arterial hypertension suggests pathways relevant to disease penetrance. BMC Med. Genet. 2008, 1, 1–11. 10.1186/1755-8794-1-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello C. M.; Howell K.; Cahill E.; McBryan J.; Konigshoff M.; Eickelberg O.; Gaine S.; Martin F.; McLoughlin P. Lung-selective gene responses to alveolar hypoxia: potential role for the bone morphogenetic antagonist gremlin in pulmonary hypertension. Am. J. Physiol. - Lung Cell. Mol. Physiol. 2008, 295, L272–L284. 10.1152/ajplung.00358.2007. [DOI] [PubMed] [Google Scholar]
- Wójtowicz A.; Babu S. S.; Li L.; Gretz N.; Hecker M.; Cattaruzza M. Zyxin mediation of stretch-induced gene expression in human endothelial cells. Circ. Res. 2010, 107, 898–902. 10.1161/CIRCRESAHA.110.227850. [DOI] [PubMed] [Google Scholar]
- Tang Z.; Gu J.; Sun P.; Zhao J.; Zhao Y. Identification of functional modules induced by bare-metal stents and paclitaxel-eluting stents in coronary heart disease. Exp. Ther. Med. 2018, 15, 3801–3808. 10.3892/etm.2018.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug T.; Gabriel J. P.; Taipa R.; Fonseca B. V.; Domingues-Montanari S.; Fernandez-Cadenas I.; Manso H.; Gouveia L. O.; Sobral J.; Albergaria I.; Gaspar G.; Jiménez-Conde J.; Rabionet R.; Ferro J. M.; Montaner J.; Vicente A. M.; Silva M. R.; Matos I.; Lopes G.; Oliveira S. A. TTC7B emerges as a novel risk factor for ischemic stroke through the convergence of several genome-wide approaches. J. Cereb. Blood Flow Metab. 2012, 32, 1061–1072. 10.1038/jcbfm.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbano M. G.; Meadows C. A.; Coldren C. D.; Jenkins T. J.; Edwards M. G.; Collier D.; Huber W.; Mack D. G.; Fontenot A. P.; Geraci M. W.; Bull T. M. Altered immune phenotype in peripheral blood cells of patients with scleroderma-associated pulmonary hypertension. Clin Transl Sci 2010, 3, 210–218. 10.1111/j.1752-8062.2010.00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkor M. T.; Meng F. B.; Xing S. Y.; Zhang M. C.; Guo J. R.; Zhu X. X.; Yang P. Microarray analysis of differential gene expression profile in peripheral blood cells of patients with human essential hypertension. Int. J. Med. Sci. 2011, 8, 168. 10.7150/ijms.8.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques F. Z.; Campain A. E.; Tomaszewski M.; Zukowska-Szczechowska E.; Yang Y. H. J.; Charchar F. J.; Morris B. J. Gene expression profiling reveals renin mRNA overexpression in human hypertensive kidneys and a role for microRNAs. Hypertension 2011, 58, 1093–1098. 10.1161/HYPERTENSIONAHA.111.180729. [DOI] [PubMed] [Google Scholar]
- Guo F.; Zhang W.; Su J.; Xu H.; Yang H. Prediction of drug positioning for quan-du-zhong capsules against hypertensive nephropathy based on the robustness of disease network. Front. Pharmacol. 2019, 10, 49. 10.3389/fphar.2019.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo P. C.; Onat D.; Harxhi A.; Demmer R. T.; Hayashi Y.; Jelic S.; LeJemtel T. H.; Bucciarelli L.; Kebschull M.; Papapanou P.; Uriel N.; Schmidt A. M.; Sabbah H. N.; Jorde U. P. Peripheral venous congestion causes inflammation, neurohormonal, and endothelial cell activation. Eur. Heart J. 2014, 35, 448–454. 10.1093/eurheartj/eht456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemnes A. R.; Brittain E. L.; Trammell A. W.; Fessel J. P.; Austin E. D.; Penner N.; Maynard K. B.; Gleaves L.; Talati M.; Absi T.; DiSalvo T.; West J. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014, 189, 325–334. 10.1164/rccm.201306-1086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani K.; Kawabe J.; Morikawa H.; Akahoshi T.; Hashizume M.; Shiomi S. Comprehensive screening of gene function and networks by DNA microarray analysis in Japanese patients with idiopathic portal hypertension. Mediators Inflamm. 2015, 2015, 1. 10.1155/2015/349215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad K. S.; Elinoff J. M.; Wang S.; Gairhe S.; Ferreyra G. A.; Cai R.; Sun J.; Solomon M. A.; Danner R. L. Raf/ERK drives the proliferative and invasive phenotype of BMPR2-silenced pulmonary artery endothelial cells. Am. J. Physiol. - Lung Cell. Mol. Physiol. 2016, 310, L187–L201. 10.1152/ajplung.00303.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil G. A.; de Almeida Silva-Cutini M.; Moraes F. d. S. A.; Pereira T. d. M. C.; Vasquez E. C.; Lenz D.; Bissoli N. S.; Endringer D. C.; de Lima E. M.; Biancardi V. C. The benefits of soluble non-bacterial fraction of kefir on blood pressure and cardiac hypertrophy in hypertensive rats are mediated by an increase in baroreflex sensitivity and decrease in angiotensin-converting enzyme activity. Nutrition 2018, 51, 66–72. 10.1016/j.nut.2017.12.007. [DOI] [PubMed] [Google Scholar]
- Strojny W.; Drozdz D.; Fijorek K.; Korostynski M.; Piechota M.; Balwierz W.; Pietrzyk J. A.; Kwinta P.; Siedlar M.; Skoczen S. Looking for new diagnostic tools and biomarkers of hypertension in obese pediatric patients. Blood Press Monit. 2017, 22, 122–130. 10.1097/MBP.0000000000000242. [DOI] [PubMed] [Google Scholar]
- Mura M.; Cecchini M. J.; Joseph M.; Granton J. T. Osteopontin lung gene expression is a marker of disease severity in pulmonary arterial hypertension. Respirology 2019, 24, 1104–1110. 10.1111/resp.13557. [DOI] [PubMed] [Google Scholar]
- Faralli J. A.; Desikan H.; Peotter J.; Kanneganti N.; Weinhaus B.; Filla M. S.; Peters D. M. Genomic/proteomic analyses of dexamethasone-treated human trabecular meshwork cells reveal a role for GULP1 and ABR in phagocytosis. Mol. Vis. 2019, 25, 237. [PMC free article] [PubMed] [Google Scholar]
- Cui Y.; Zhu Z.; Qi X.; Li H.; Wu Y.; Chen W.; Liu Y. Relationship between circulating concentration of Ang II, ADM and ADT and left ventricular hypertrophy in hypertension. Am. J. Transl. Res. 2019, 11, 3167. [PMC free article] [PubMed] [Google Scholar]
- Vadapalli S.; Rani H. S.; Sastry B.; Nallari P. Endothelin-1 and endothelial nitric oxide polymorphisms in idiopathic pulmonary arterial hypertension. Int. J. Mol. Epidemiol. Genet. 2010, 1, 208. [PMC free article] [PubMed] [Google Scholar]
- Folsom A. R.; Peacock J. M.; Demerath E.; Boerwinkle E. Variation in ANGPTL4 and risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Metabolism 2008, 57, 1591–1596. 10.1016/j.metabol.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller F. B. AI (Artificial Intelligence) and Hypertension Research. Curr. Hypertens. Rep. 2020, 22, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awan F. M.; Naz A.; Obaid A.; Ali A.; Ahmad J.; Anjum S.; Janjua H. A. Identification of circulating biomarker candidates for hepatocellular carcinoma (HCC): an integrated prioritization approach. PLoS One 2015, 10, e0138913 10.1371/journal.pone.0138913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y.-L.; Law T. C. Chronic hypoxia-and monocrotaline-induced elevation of hypoxia-inducible factor-1α levels and pulmonary hypertension. J. Biomed. Sci. 2004, 11, 315–321. [DOI] [PubMed] [Google Scholar]
- Kumar A.; Potts J. D.; DiPette D. J. Protective role of α-calcitonin gene-related peptide in cardiovascular diseases. Front. Physiol. 2019, 10, 821. 10.3389/fphys.2019.00821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevale D.; Lembo G. PI3Kγ in hypertension: a novel therapeutic target controlling vascular myogenic tone and target organ damage. Cardiovasc. Res. 2012, 95, 403–408. 10.1093/cvr/cvs166. [DOI] [PubMed] [Google Scholar]
- Ikeda S.; Ushio-Fukai M.; Zuo L.; Tojo T.; Dikalov S.; Patrushev N. A.; Alexander R. W. Novel role of ARF6 in vascular endothelial growth factor–induced signaling and angiogenesis. Circ. Res. 2005, 96, 467–475. 10.1161/01.RES.0000158286.51045.16. [DOI] [PubMed] [Google Scholar]
- Staruschenko A.; Palygin O.; Ilatovskaya D. V.; Pavlov T. S. Epidermal growth factors in the kidney and relationship to hypertension. Am. J. Physiol. Renal Physiol. 2013, 305, F12–F20. 10.1152/ajprenal.00112.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillet A.; Saad C.; Ferry G.; Anouar Y.; Vergne N.; Lecroq T.; Dubessy C. Improving bioinformatics prediction of microRNA targets by ranks aggregation. Front. Genet. 2020, 10, 1330. 10.3389/fgene.2019.01330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S.; Zhang L.; Zhao Z.; Fu W.; Fu K.; Liu G.; Jia W. J. O. R.. MicroRNA-92a-3p inhibits the cell proliferation, migration and invasion of Wilms tumor by targeting NOTCH1. 2018, 40 ( (2), ), 571–578. [DOI] [PMC free article] [PubMed]
- Huang X.; Wang W. Association of MEF2A gene 3’UTR mutations with coronary artery disease. Genet Mol Res 2015, 14, 11073–11078. 10.4238/2015.September.21.20. [DOI] [PubMed] [Google Scholar]
- Fichtlscherer S.; De Rosa S.; Fox H.; Schwietz T.; Fischer A.; Liebetrau C.; Weber M.; Hamm C. W.; Röxe T.; Müller-Ardogan M. Circulating microRNAs in patients with coronary artery disease. Circ. Res. 2010, 107, 677–684. 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- Yamada R.; Okumura S.; Kono Y.; Miyazaki A.; Niwa Y.; Ito T.; Ueda S.; Ishiguro T.; Yoshinaga M.; Fujiwara W. J. F. m. j.. Effect of cardiac rehabilitation on circulating microRNA expression in heart failure: a preliminary study. 2020. [DOI] [PMC free article] [PubMed]
- Thygesen C.; Boll I.; Finsen B.; Modzel M.; Larsen M. R. Characterizing disease-associated changes in post-translational modifications by mass spectrometry. Expert Rev. Proteom. 2018, 15, 245–258. 10.1080/14789450.2018.1433036. [DOI] [PubMed] [Google Scholar]
- Polak-Iwaniuk A.; Harasim-Symbor E.; Gołaszewska K.; Chabowski A. How hypertension affects heart metabolism. Front. Physiol. 2019, 10, 435. 10.3389/fphys.2019.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S.-L.; Umesalma S.; Baumbach G. L. Epidermal growth factor receptor is critical for angiotensin II–mediated hypertrophy in cerebral arterioles. Hypertension 2015, 65, 806–812. 10.1161/HYPERTENSIONAHA.114.04794. [DOI] [PubMed] [Google Scholar]
- Kolovou V.; Lagou E.; Mihas C.; Vasiliki G.; Katsiki N.; Kollia A.; Triposkiadis F.; Degiannis D.; Mavrogeni S.; Kolovou G. Angiotensinogen (AGT) M235T, AGT T174M and angiotensin-1-converting enzyme (ACE) I/D gene polymorphisms in essential hypertension: effects on ramipril efficacy. Open Cardiovasc. Med. J. 2015, 9, 118. 10.2174/1874192401509010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frossard P. M.; Obineche E. N.; Lestringant G. G. Association of an apolipoprotein B gene marker with essential hypertension. Hypertension 1999, 33, 1052–1056. 10.1161/01.HYP.33.4.1052. [DOI] [PubMed] [Google Scholar]
- Chitaley K.; Weber D. S.; Webb R. C. RhoA/Rho-kinase, vascular changes, and hypertension. Curr. Hypertens. Rep. 2001, 3, 139–144. 10.1007/s11906-001-0028-4. [DOI] [PubMed] [Google Scholar]
- Das B.; Pawar N.; Saini D.; Seshadri M. Genetic association study of selected candidate genes (ApoB, LPL, Leptin) and telomere length in obese and hypertensive individuals. BMC Med. Genet. 2009, 10, 99. 10.1186/1471-2350-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltatu O. C.; Amaral F. G.; Campos L. A.; Cipolla-Neto J. Melatonin, mitochondria and hypertension. Cell. Mol. Life Sci. 2017, 74, 3955–3964. 10.1007/s00018-017-2613-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubarak K. K. A review of prostaglandin analogs in the management of patients with pulmonary arterial hypertension. Respir. Med. 2010, 104, 9–21. 10.1016/j.rmed.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Xu Q.; Fawcett T. W.; Gorospe M.; Guyton K. Z.; Liu Y.; Holbrook N. J. Induction of mitogen-activated protein kinase phosphatase-1 during acute hypertension. Hypertension 1997, 30, 106–111. 10.1161/01.HYP.30.1.106. [DOI] [PubMed] [Google Scholar]
- Sugawara A.; Uruno A.; Kudo M.; Matsuda K.; Yang C. W.; Ito S. Effects of PPARγ on hypertension, atherosclerosis, and chronic kidney disease. Endocr. J. 2010, 57, 847–852. 10.1507/endocrj.K10E-281. [DOI] [PubMed] [Google Scholar]
- Sun H.-J.; Chen D.; Han Y.; Zhou Y.-B.; Wang J.-J.; Chen Q.; Li Y.-H.; Gao X.-Y.; Kang Y.-M.; Zhu G.-Q. Relaxin in paraventricular nucleus contributes to sympathetic overdrive and hypertension via PI3K-Akt pathway. Neuropharmacology 2016, 103, 247–256. 10.1016/j.neuropharm.2015.12.023. [DOI] [PubMed] [Google Scholar]
- Mattingly C. J.; Rosenstein M. C.; Colby G. T.; Forrest J. Jr.; Boyer J. The Comparative Toxicogenomics Database (CTD): a resource for comparative toxicological studies. J. Exp. Zool. 2006, 305, 689–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali F.; Fang H. L.; Shah F. A.; Muhammad S. A.; Khan A.; Li S. J. I. i. M. U. Reprofiling analysis of FDA approved drugs with upregulated differential expression genes found in hypertension. Genes 2022, 100895. 10.1016/j.imu.2022.100895. [DOI] [Google Scholar]
- Moe S. M.; Chen N. X. Mechanisms of vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 2008, 19, 213–216. 10.1681/ASN.2007080854. [DOI] [PubMed] [Google Scholar]
- Muhammad S. A.; Raza W.; Nguyen T.; Bai B.; Wu X.; Chen J. Cellular signaling pathways in insulin resistance-systems biology analyses of microarray dataset reveals new drug target gene signatures of type 2 diabetes mellitus. Front. Physiol. 2017, 8, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyanskaya O.; Cantor M.; Sherlock G.; Brown P.; Hastie T.; Tibshirani R.; Botstein D.; Altman R. B. Missing value estimation methods for DNA microarrays. Bioinformatics 2001, 17, 520–525. 10.1093/bioinformatics/17.6.520. [DOI] [PubMed] [Google Scholar]
- Bolstad B. M.; Irizarry R. A.; Åstrand M.; Speed T. P. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003, 19, 185–193. 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Fujita A.; Sato J. R.; de Oliveira Rodrigues L.; Ferreira C. E.; Sogayar M. C. Evaluating different methods of microarray data normalization. BMC bioinf. 2006, 7, 469. 10.1186/1471-2105-7-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obenchain V.; Lawrence M.; Carey V.; Gogarten S.; Shannon P.; Morgan M. VariantAnnotation: a Bioconductor package for exploration and annotation of genetic variants. Bioinformatics 2014, 30, 2076–2078. 10.1093/bioinformatics/btu168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z.; Irizarry R. A.; Gentleman R.; Martinez-Murillo F.; Spencer F. A model-based background adjustment for oligonucleotide expression arrays. JASA. 2004, 99, 909–917. 10.1198/016214504000000683. [DOI] [Google Scholar]
- Affymetrix I. Affymetrix Microarray Suite User Guide. Santa Clara 2000, 295–316. [Google Scholar]
- Manual A., Affymetrix Mircoarray Suite User Guide version 5.0. Santa Clara, CA2001.
- Muhammad S. A.; Fatima N.; Paracha R. Z.; Ali A.; Chen J. Y. A systematic simulation-based meta-analytical framework for prediction of physiological biomarkers in alopecia. J. Biol. Res. 2019, 26, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung M.-C.; Link W. Protein localization in disease and therapy. J. Cell Sci. 2011, 124, 3381–3392. 10.1242/jcs.089110. [DOI] [PubMed] [Google Scholar]
- Lu Z.; Szafron D.; Greiner R.; Lu P.; Wishart D. S.; Poulin B.; Anvik J.; Macdonell C.; Eisner R. Predicting subcellular localization of proteins using machine-learned classifiers. Bioinformatics 2004, 20, 547–556. 10.1093/bioinformatics/btg447. [DOI] [PubMed] [Google Scholar]
- Rebholz-Schuhmann D.; Oellrich A.; Hoehndorf R. Text-mining solutions for biomedical research: enabling integrative biology. Nat. Rev. Genet. 2012, 13, 829–839. 10.1038/nrg3337. [DOI] [PubMed] [Google Scholar]
- Choi O.; Deng K. K.; Kim N.-J.; Ross L. Jr.; Surampalli R. Y.; Hu Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074. 10.1016/j.watres.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Huang T.-T.; Gonzales C. B.; Gu F.; Hsu Y.-T.; Jadhav R. R.; Wang C.-M.; Redding S. W.; Tseng C.-E.; Lee C.-C.; Thompson I. M.; Chen H. R.; Huang T. H. M.; Kirma N. B. Epigenetic deregulation of the anaplastic lymphoma kinase gene modulates mesenchymal characteristics of oral squamous cell carcinomas. Carcinogenesis 2013, 34, 1717–1727. 10.1093/carcin/bgt112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathan M.; Keerthikumar S.; Ang C. S.; Gangoda L.; Quek C. Y.; Williamson N. A.; Mouradov D.; Sieber O. M.; Simpson R. J.; Salim A.; Bacic A.; Hill A. F.; Stroud D. A.; Ryan M. T.; Agbinya J. I.; Mariadason J. M.; Burgess A. W.; Mathivanan S. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 2015, 15, 2597–2601. 10.1002/pmic.201400515. [DOI] [PubMed] [Google Scholar]
- Scherf U.; Ross D. T.; Waltham M.; Smith L. H.; Lee J. K.; Tanabe L.; Kohn K. W.; Reinhold W. C.; Myers T. G.; Andrews D. T.; Scudiero D. A.; Eisen M. B.; Sausville E. A.; Pommier Y.; Botstein D.; Brown P. O.; Weinstein J. N. A gene expression database for the molecular pharmacology of cancer. Nat. Genet. 2000, 24, 236–244. 10.1038/73439. [DOI] [PubMed] [Google Scholar]
- Abbas S. Z.; Qadir M. I.; Muhammad S. A. Systems-level differential gene expression analysis reveals new genetic variants of oral cancer. Sci. Rep. 2020, 10, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmanov U.; Emili A. Protein-protein interaction networks: probing disease mechanisms using model systems. Genome Med. 2013, 5, 37. 10.1186/gm441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad S. A.; Ahmed S.; Ali A.; Huang H.; Wu X.; Yang X. F.; Naz A.; Chen J. Prioritizing drug targets in Clostridium botulinum with a computational systems biology approach. Genomics 2014, 104, 24–35. 10.1016/j.ygeno.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Rachlin J.; Cohen D. D.; Cantor C.; Kasif S.. Biological context networks: a mosaic view of the interactome. Mol. Syst. Biol. 2006, 2 (). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D.; Franceschini A.; Kuhn M.; Simonovic M.; Roth A.; Minguez P.; Doerks T.; Stark M.; Muller J.; Bork P. The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 2010, 39, D561–D568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. S.; Smoot M.; Cerami E.; Kuchinsky A.; Landys N.; Workman C.; Christmas R.; Avila-Campilo I.; Creech M.; Gross B.; Hanspers K.; Isserlin R.; Kelley R.; Killcoyne S.; Lotia S.; Maere S.; Morris J.; Ono K.; Pavlovic V.; Pico A. R.; Vailaya A.; Wang P. L.; Adler A.; Conklin B. R.; Hood L.; Kuiper M.; Sander C.; Schmulevich I.; Schwikowski B.; Warner G. J.; Ideker T.; Bader G. D. Integration of biological networks and gene expression data using Cytoscape. Nat. Protoc. 2007, 2, 2366–2382. 10.1038/nprot.2007.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergholdt R.; Brorsson C.; Palleja A.; Berchtold L. A.; Fløyel T.; Bang-Berthelsen C. H.; Frederiksen K. S.; Jensen L. J.; Størling J.; Pociot F. Identification of novel type 1 diabetes candidate genes by integrating genome-wide association data, protein-protein interactions, and human pancreatic islet gene expression. Diabetes 2012, 61, 954–962. 10.2337/db11-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Califano S.; Schettino V.; Neto N., Lattice dynamics of molecular crystals. Springer Science & Business Media: 2012; Vol. 26. [Google Scholar]
- Kutmon M.; Riutta A.; Nunes N.; Hanspers K.; Willighagen E. L.; Bohler A.; Mélius J.; Waagmeester A.; Sinha S. R.; Miller R.; Coort S. L.; Cirillo E.; Smeets B.; Evelo C. T.; Pico A. R. WikiPathways: capturing the full diversity of pathway knowledge. Nucleic Acids Res. 2016, 44, D488–D494. 10.1093/nar/gkv1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly C. J.; Colby G. T.; Forrest J. N.; Boyer J. L. The Comparative Toxicogenomics Database (CTD). Environ. Health Perspect. 2003, 111, 793–795. 10.1289/ehp.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman J. L.; Lin D.; Jiang J.; Manning N. O.; Prilusky J.; Ritter O.; Abola E. E. J. A. C. S. D. B. C.. Protein Data Bank (PDB): database of three-dimensional structural information of biological macromolecules. 1998, 54 ( (6), ), 1078–1084. [DOI] [PubMed]
- research, U. C. J. N. a UniProt: a hub for protein information. 2015, 43 ( (D1), ), D204–D212. [DOI] [PMC free article] [PubMed]
- Goddard T. D.; Huang C. C.; Ferrin T. E. J. S.. Software extensions to UCSF chimera for interactive visualization of large molecular assemblies. 2005, 13 ( (3), ), 473–482. [DOI] [PubMed]
- Ma’ayan A.; Jenkins S. L.; Goldfarb J.; Iyengar R. J. M. S. J. o. M. A. J. o. T.; Translational P. M. A. J. o.; Medicine P.. Network analysis of FDA approved drugs and their targets. 2007, 74 ( (1), ), 27–32. [DOI] [PMC free article] [PubMed]
- Podvinec M.; Schwede T.; Peitsch M., Docking for neglected diseases as community efforts. In Computational Structural Biology: Methods and Applications, World Scientific: 2008; pp. 683–704, 10.1142/9789812778789_0025. [DOI] [Google Scholar]
- Kandeel M.; Abdelrahman A. H.; Oh-Hashi K.; Ibrahim A.; Venugopala K. N.; Morsy M. A.; Ibrahim M. A. Repurposing of FDA-approved antivirals, antibiotics, anthelmintics, antioxidants, and cell protectives against SARS-CoV-2 papain-like protease. J. Biomol. Struct. Dyn. 2021, 1–5136. 10.1080/07391102.2020.1784291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borreguero J. M.; Lynch V. E. Molecular dynamics force-field refinement against quasi-elastic neutron scattering data. J. Chem. Theory Comput. 2016, 12, 9–17. 10.1021/acs.jctc.5b00878. [DOI] [PubMed] [Google Scholar]
- Sarma P.; Shekhar N.; Prajapat M.; Avti P.; Kaur H.; Kumar S.; Singh S.; Kumar H.; Prakash A.; Dhibar D. P. J. J. o. B. S.. Dynamics, In-silico homology assisted identification of inhibitor of RNA binding against 2019-nCoV N-protein (N terminal domain). 2020, 1–9. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.