Abstract

High-porosity activated carbon (AC) was prepared from low-cost coconut shells (CS) using KOH as an activating agent with different KOH/char mass ratios. To cut down the amount of KOH used for AC preparation, freezing pretreatment for a certain number of times was carried out on CS before carbonization, which resulted in the maximum increase in the specific surface area (SBET) and total pore volume of 92.8 and 44.4%, respectively, in the resultant biochar. For the sake of description, AC from CS undergoing x times of freezing pretreatment and with a KOH/char ratio of y is denoted as AC-xy. The SBET values of AC-13 and AC-24 are 193 and 166 m2 g–1 larger than that of AC-05 (2217 m2 g–1), respectively. At a current density of 0.25 A g–1, the specific gravimetric capacitance (Cg) values of AC-05, AC-13, and AC-24 are 386, 403, and 425 F g–1. Moreover, a symmetric supercapacitor based on AC-24 exhibits a high energy density of 14.7 Wh kg–1 at a power density of 120 W kg–1. The energy density retention rate of AC-24 is 71.1% with the power density increased by about 110 times, indicating excellent rate capability. Additionally, a capacitance retention rate of about 95% after 3000 cycles implies an outstanding cycle lifetime of an AC-24-based capacitor. The freezing strategy developed here provides a novel route for low-cost and eco-friendly production of AC from biomass wastes for high-performance supercapacitors.
1. Introduction
Among all carbon-based electrode materials, activated carbon (AC) is the most used one for supercapacitors. For a long time, various coal-based materials or woods have been the main precursors for AC. However, due to challenges such as resources, cost, and environmental issues, it is imperative to develop alternative raw materials.1 Biomass has been widely utilized in a large number of fields because of its advantages such as easy access, special structure, environmental friendliness, and low price.2 Particularly, the unique crystal structure of biomass-based carbon materials determines the rapid transport of electrolyte ions in electrodes, which makes them very promising supercapacitor electrode materials.3 Substantial studies have demonstrated biomass-based carbonaceous materials as a competitive raw material in supercapacitor applications, such as paddy,4 banana leaves,5 rice husk,6 garlic seeds,7 dragon fruit peels,8 oak seeds,9 etc. Among all biowaste-based carbonaceous materials, AC fabricated from a lignocellulosic precursor is preferable because of its large specific surface area, tunable porosity, high chemical stability, and good electrical conductivity.10,11 As an easily available and inexpensive lignocellulosic biomass, coconut shell (CS) is an ideal precursor of AC for supercapacitor electrodes.12−14
For AC preparation, the most used activating agents are steam, CO2, ZnCl2, and KOH, among which KOH is more advantageous since more localized reactions take place with the precursor when KOH is applied and thus is beneficial to obtain high porosity.15,16 However, generally a high KOH/carbon ratio is required to achieve fully activated AC. This will increase the cost of preparation, corrode the instruments, and pollute the environment. The common way to reduce KOH usage is to optimize the carbonization and activation parameters, but the effect is very limited. Other methods to this problem have also been reported, such as oxidizing or expanding the precursor to make it easier to get activated.17,18 Those methods have reduced the usage of KOH to varying degrees. In this study, a simple and efficient freezing strategy was developed to modify the structure of CS and thus the resultant biochar. The freezing pretreatment reduced the difficulty of the following activation process and greatly cut down the KOH usage.
2. Experimental Section
2.1. Materials
CS were collected from Sanya Hainan (China). HCl, KOH, Na2SO4, carbon black (Super Li, Timcal Switzerland), poly(tetrafluoroethylene) (D210C, Daikin Japan), and nickel foam (110 PPI, Liyuan China) were supplied by Sinopharm Chemical Reagent Co. (China). Except for CS and nickel foam, all reagents were of analytical reagent (AR) and used as received.
2.2. Freezing Pretreatment of CS
After dirt and residual flesh were removed, CS were rinsed and dried and then broken to sizes less than 10 mm with a mechanical crusher. The broken CS were then soaked in deionized water for sufficient time, frozen at −20 °C adequately, and then dried thoroughly to obtain frozen CS. The freezing pretreatment was repeated a certain number of times.
2.3. Preparation of ACs
Carbonization of CS and frozen CS was carried out at 500 °C for 2 h to obtain biochar-x (BC-x) with x representing the number of times coconut shells were frozen. The activating process was conducted by mixing KOH and BC-x at a KOH/BC-x mass ratio of y, followed by heating the mixture in a crucible oven first at 500 °C for 1 h and successfully at 800 °C for 3 h. The product was boiled in HCl aqueous solution, then rinsed and dried thoroughly, and finally labeled as AC-xy with y representing the mass ratio of KOH to BC-x and x the number of times CS were frozen.
2.4. Preparation of Electrodes
Nickel foams, 10 mm in diameter, were first sonicated with alcohol and then rinsed with deionized water. AC, carbon black, and poly(tetrafluoroethylene) were mixed with a mass proportion of 75, 20, and 5% to form a slurry. The slurry was then evenly spread on the nickel foams and dried. Pelletlike electrodes were prepared by pressing the nickel foams loaded with AC mixture under a pressure of 2 × 106 Pa. Excluding the nickel foam, the average mass of the electrode was about 10 mg.
2.5. Characterization Method
A thermogravimetric (TG) test was conducted in a N2 atmosphere using an STA 2500 (Netzsch, German) between 25 and 900 °C at a scan rate of 10 °C min–1. X-ray diffraction (XRD) patterns were obtained using a Rigaku Ultima IV diffractometer within the range of 10–90°. Raman measurements were carried out on a Thermo-DXR-2xi in the range of 500 to 3000 nm–1 with a 532 nm wavelength laser. Scanning electron microscopy (SEM) investigations were conducted on a Hitachi SU8020. Transmission electron microscopic (TEM) images were obtained with a JEOL JEM-2100F microscope operated at 200 kV. The porosity of the samples was characterized using a Micromeritics ASAP2000 with N2 used as an adsorbate. Before testing, all samples were degassed at 300 °C for 12 h. The Brunauer–Emmett–Teller method was used to calculate the specific surface area. The specific surface area of micropores (SMicro) and volume of micropores (VMicro) were calculated by the t-plot method. Pore size distribution (PSD) of the samples was obtained by the density functional theory method using the N2 adsorption data assuming a slit pore geometry.
2.6. Electrochemical Measurements
Cyclic voltammetry (CV) was performed on the Zahner IM6EX electrochemical workstation within the voltage range of −0.4 to 0.8 V at scan rates from 2 to 20 mV s–1, with Pt pellets and the Ag/AgCl electrode used as counter and reference electrodes, respectively. The electrolyte was 0.5 mol L–1 Na2SO4 aqueous solution. Before measurement, the working electrode was soaked in the electrolyte for over 24 h. The galvanostatic charge–discharge (GCD) test was conducted at various constant current densities using a two-electrode configuration on a battery analyzer model (Landian, China). The specific gravimetric capacitance (Cg (F g–1)) was calculated from the discharge curve according to eq 1
| 1 |
where I (A) is the discharge current, m (g) is the average mass of a single electrode, Δt (s) is the discharge time, and ΔV (V) is the voltage change during discharge.19 The energy density and power density of the supercapacitors were calculated by applying eqs 2 and 3
| 2 |
| 3 |
where E (Wh kg–1) is the energy density, ΔV (V) is the cell voltage excluding the IR drop during the discharging process, P (W kg–1) is the power density, Cg (F g–1) is the specific gravimetric capacitance, and Δt (s) is the discharge time.20,21
3. Results and Discussion
3.1. Structure and Morphology
The purpose of TG analysis is to find the suitable temperature for the CS pyrolysis process. Figure 1a illustrates the weight depletion of CS caused by dehydration and compound decomposition. The curve can be roughly divided into three sections. The mass decrease of about 10% can be attributed to dehydration in CS, which took place under 200 °C.22 The greatest mass loss of 40% occurred in the range of 200 to 500 °C, which included the decomposition of semicellulose from 225 to 325 °C, the cellulose between 300 and 375 °C, and the lignin between 250 and 500 °C.12,23−25 For this reason, the carbonization temperature of CS and frozen CS was set at 500 °C. The mass loss between 500 and 900 °C corresponds to the lignin decomposition.25,26 Due to the wide temperature range of lignin decomposition, it is difficult to identify the decomposing peak.27,28
Figure 1.
TG and derivative thermogravimetric (DTG) of CS (a) and XRD patterns of biochars (BCs) (b).
As shown in Figure 1b, all samples possess two broad peaks at about 23 and 42°, which can be interpreted as the (002) reflection of the turbostratic carbon and the (100) diffraction of graphitic carbon, respectively, indicating that all BCs are amorphous.29,30 Compared with the (002) peak of graphitic carbon at around 25°, there is a slight shift to the small diffraction direction for the diffraction peak at around 23°, indicating a low graphitization degree of BCs.
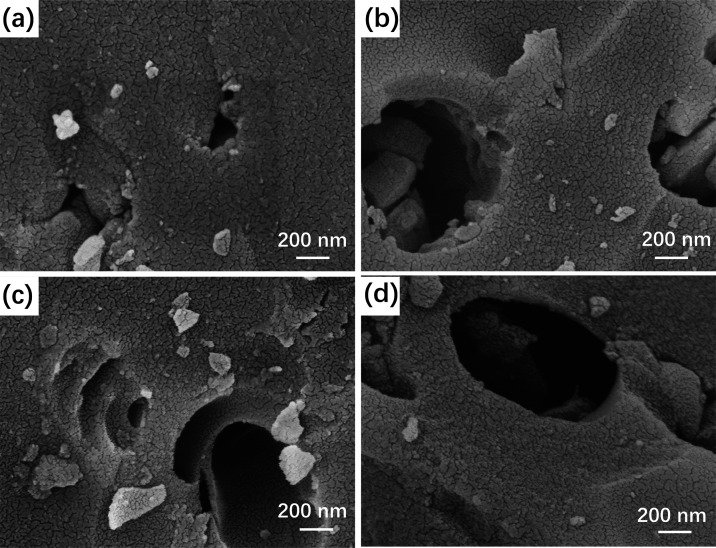
As shown in Figure 2, there are a certain number of macropores of different shapes and sizes on the surface of each sample. Large quantities of narrow-sized cracks can be seen evenly distributed on the external surface of the particles and the inner surface of the macropores. The cracks are vital for KOH to permeate the BCs adequately and subsequently obtain fully activated BCs.
Figure 2.
SEM images of BC-0 (a), BC-1 (b), BC-2 (c), and BC-3 (d).
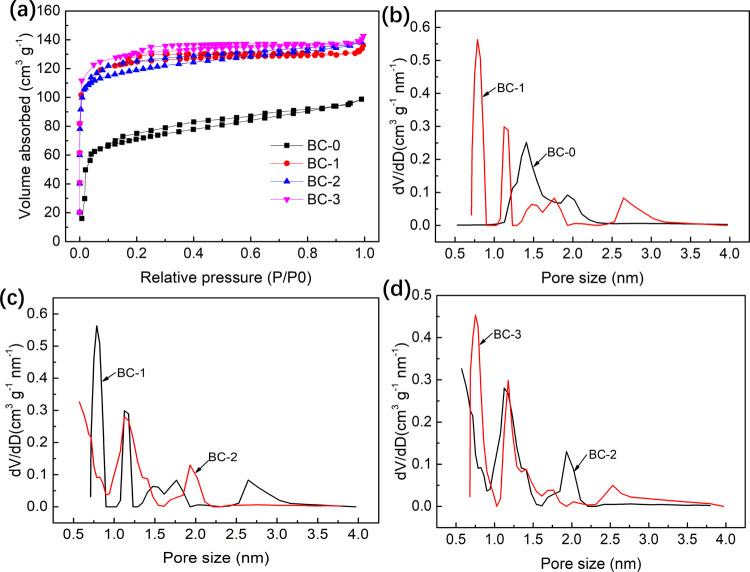
Figure 3a illustrates the N2 adsorption and desorption of BCs. All samples possess a typical type-IV isotherm. The N2 adsorption increases sharply in the low relative pressure region (P/P0 < 0.05), demonstrating that all samples possess abundant micropores.31 In the high relative pressure region, the amount of N2 absorbed continues to increase, indicating the presence of a certain amount of mesopores.32 The adsorption capacity of any BC-x (x > 0) is much higher than that of BC-0, indicating that freezing pretreatment increased the porosity of the BC significantly. However, the difference in N2 adsorption capacity between samples of BC-x (x > 0) is not remarkable, implying that the growth of SBET and Vtotal in BC-x (x > 0) was mainly contributed by the first freezing pretreatment. The purpose of further freezing was to adjust the pore structure with a slight change in SBET and Vtotal.
Figure 3.
Adsorption and desorption isotherms of BCs (a). PSD comparison of BC-0 and BC-1 (b), BC-1 and BC-2 (c), and BC-2 and BC-3 (d).
As shown in Figure 3b–d, the pore network of all BCs is mainly composed of micropores (<2 nm) and narrow mesopores (2–4 nm). The pore size distribution of BC-0 is confined to a narrow range of 1.1 to 2.3 nm, with a bimodal distribution pattern concentrated at 1.5 and 2.0 nm, respectively. By contrast, BC-1 demonstrates a multimodal distribution pattern with two narrow but strong distribution peaks at pore sizes of 0.8 and 1.1 nm and a wide but weak distribution peak at 2.6 nm (Figure 3b). This indicates that the first freezing pretreatment not only broadened the size of the original pores in CS but also created new narrow-sized pores. That is why the SBET and Vtotal of BC-1 are 1.83 and 1.41 times that of BC-0, yet the average pore diameter (Daver) of BC-1 is 0.57 nm narrower than that of BC-0 (Table 1).
Table 1. Porosity of BCs and ACs.
| samples | SBET (m2 g–1) | Rsa (%) | Vtotal (cm3 g–1) | RVb (%) | Daver (nm) |
|---|---|---|---|---|---|
| BC-0 | 263 | 74.5 | 0.153 | 53.6 | 2.32 |
| BC-1 | 482 | 95.0 | 0.211 | 86.7 | 1.75 |
| BC-2 | 469 | 89.8 | 0.214 | 77.1 | 1.82 |
| BC-3 | 507 | 94.7 | 0.221 | 86.4 | 1.74 |
| AC-05 | 2217 | 7.4 | 1.64 | 18.9 | 2.96 |
| AC-13 | 2410 | 38.0 | 2.02 | 28.9 | 3.35 |
| AC-24 | 2383 | 9.0 | 1.86 | 19.2 | 3.12 |
Rs, Ratio of SMicro–SBET.
Rv, Ratio of VMicro–Vtotal.
Unlike the main distribution peak of BC-1 centered at 0.8 nm, the vast majority of pores of BC-2 are distributed in the region between 0.9 and 1.5 nm (Figure 3c), which leads to a wider Daver. Thus, the SBET, Rs, and RV of BC-1 are all lower than those of BC-2 (Table 1).
Compared with BC-2, the main distribution peaks of BC-3 shifted to the narrow-size direction (Figure 3d), which led to lower Daver but higher SBET, RV, and Rs. Overall, the difference between the samples of BC-x (x > 0) lies not in SBET or Vtotal but in Rs, RV, and Daver (Table 1).
Figure 4a shows the relationship between SBET and KOH usage of all ACs. As an activating agent, KOH acts to selectively react with carbon atoms in biochar to form pores. Therefore, the amount of KOH has a significant influence on the porosity of the resultant AC. It can be seen, in most cases, that the same KOH usage resulted in a significant difference in the SBET of ACs undergoing different times of freezing. This indicates that the porosity of AC-xy strongly depends on the structure of BC-x, and the freezing pretreatment has a great influence on the structure of BC-x.
Figure 4.
SBET of ACs (a). PSD of BC-0 and BC-2 (b) and BC-1 and BC-3 (c). Cg of ACs (d).
According to Figure 4a, the variation trend of SBET with KOH can be roughly divided into two categories: fluctuation and consistent growth. Accordingly, ACs can be divided into two groups, with AC-0y and AC-2y in the fluctuation group and AC-1y and AC-3y in the consistent growth group. The reason for this may be that the corresponding BC of each AC in the same group has a similar porosity structure. For instance, both BC-0 and BC-2 have a strong distribution peak in the range of about 1.00 to 1.75 nm and a weak peak centralized at about 2.00 nm (Figure 4b). On the other hand, for the consistent growth group, both BC-1 and BC-3 have two strong distribution peaks at the narrow-size end and several weak peaks at the wide-size end, with the two strong distribution peaks centralized at almost the same pore size (Figure 4c).
The Cg of all ACs is illustrated in Figure 4d, which was calculated from GCD curves at a current density of 0.25 A g–1. It can be seen that the Cg of AC-0y increases significantly with the growth of y, reaching a maximum at y = 5, indicating that a large amount of KOH is needed to achieve full activation.33 To cut down KOH usage, freezing pretreatment was introduced to prepare BCs with suitable porosity. It can be found that for any given y (y < 5), the Cg of any AC-xy (x > 0) is higher than that of AC-0y, and the Cg of AC-13, AC-14, and AC-24 is greater than that of AC-05.
As seen in Figure 4d, BC was subjected to different times of freezing following different Cg developing paths. For any given y, there is no obvious relation between Cg and the number of freezing times (x). Because BCs were subjected to different times of freezing, they possessed quite different porosities. In terms of Cg, the optimized BC for y of 2, 3, 4, and 5 was BC-3, BC-1, BC-2, and BC-0, respectively.
Generally, a large specific surface area is accompanied by high charge storage capability at the pore/electrolyte interface. But it is not true for a wide range of ACs.34,35 As seen in the comparison in Figure 4a–d, for all samples, there is no obvious relation between SBET and Cg. It is difficult to speculate the Cg of any AC-xy simply according to its SBET. For instance, the SBET of AC-03 and AC-13 was almost the same, yet the Cg of AC-13 was 142% of that of AC-03. On the other hand, the SBET of AC-12 was only 69% of that of AC-22; however, they possess almost the same Cg. For charge storage of electric double-layer capacitors, SBET and PSD were of the same importance to the carbons.33,36,37 Micropores with sizes narrower than those of electrolyte ions cannot be occupied and thus make no contribution to the capacitance.38 Apparently, pores with a size of less than 1 nm have a significant effect on SBET improvement, yet it is difficult to form a double layer in pores less than 0.5 nm.39,40 The most appropriate pore is the one whose size is slightly larger than that of the electrolyte ions.39−41
Compared with AC-14, AC-13 is apparently preferable because it possesses higher Cg with less KOH usage. Therefore, samples with high economic efficiency and greater Cg than AC-05 are AC-13 and AC-24. At a current density of 0.25 A g–1, the Cg values of AC-05, AC-13, and AC-24 were 386, 403, and 425 F g–1, respectively. This means that by performing freezing treatment for one time, KOH consumption can be reduced by 40% with a Cg increase of 4.4%, while freezing pretreatment for two times can bring a KOH usage reduction of 20% and a Cg growth of 10.1%.
The porosity parameters of AC-05, AC-13, and AC-24 are illustrated in Table 1. The SBET values of AC-13 and AC-24 were, respectively, 193 and 166 m2 g–1 higher than that of AC-05, and the Vt values of AC-13 and AC-24 were 0.38 and 0.22 cm3 g–1 larger than that of AC-05, implying that the freezing of CS has an important effect on the subsequent activation. The reason for this is primarily that water absorbed in coconut shells during the soaking process expanded during freezing pretreatment, enlarged the existing pores, and created new pores in CS. These pores were retained in the biochar after the carbonizing process to promote KOH diffusion and improve the activation efficiency.
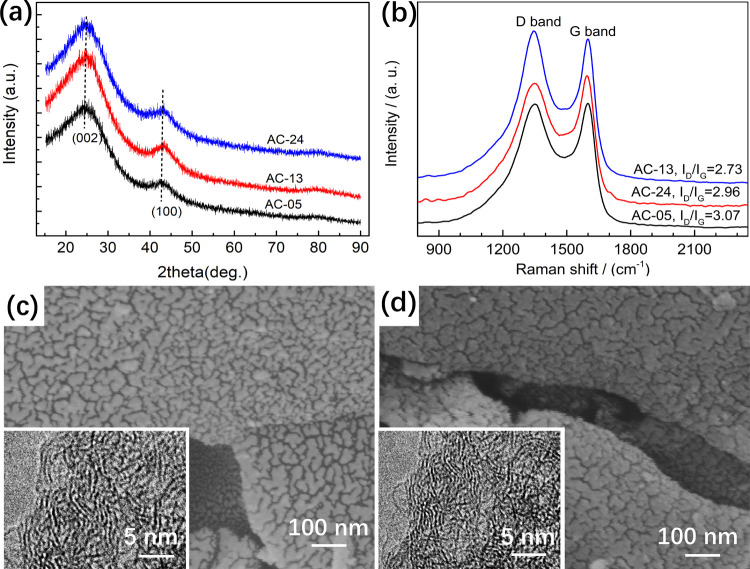
XRD patterns of ACs are shown in Figure 5a. Similar to BCs, the structure of all ACs is also amorphous, in agreement with that reported in previous studies.42−44 However, ACs possess stronger diffraction peaks than BCs. Furthermore, the fraction peaks of ACs shift to a greater 2θ angle direction, indicative of enhanced graphitization degree due to activation at a high temperature.45
Figure 5.
XRD patterns of ACs (a). Raman spectra of ACs (b). SEM and TEM (inset) images of AC-05 (c) and AC-24 (d).
As shown in Figure 5b, the Raman peak observed at about 1350 cm–1 can be attributed to the D band, which corresponds to the stretching modes of disordered carbon atoms at the edge of the graphite layer. Meanwhile, the peak detected at around 1600 cm–1 can be assigned to the G band, which is associated with the stretching modes of sp2-hybridized carbon atoms in the carbon rings or chains.13 Moreover, the ratio of the integrated area of the D peak to that of the G peak (ID/IG) can reflect the degree of disorder of the carbonaceous materials.13 The larger the ID/IG ratio, the higher the degree of disorder. The ID/IG ratios of AC-13, AC-24, and AC-05 were 2.73, 2.96, and 3.07, respectively. This is mainly because the larger the amount of KOH used, the more defects will be generated due to the selective reaction between KOH and the biochar.
As shown in Figure 5c,d, both AC-05 and AC-24 were activated fully and uniformly. They were filled with pores of different sizes. Most of the pores were of a cracklike shape. The cracks were interconnected and evenly distributed on the external surface of the AC particles and the inner surface of large-size pores. TEM images revealed that both samples were disordered. AC-13 possesses SEM and TEM images similar to those of AC-05 and AC-24.
3.2. Electrochemical Performance
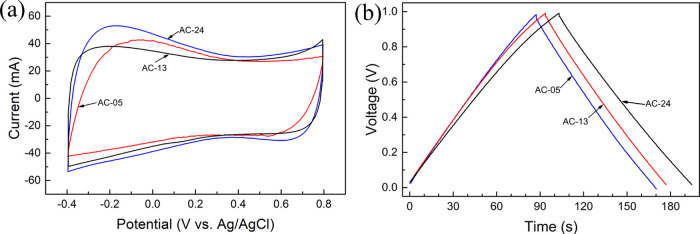
The CV testing results are illustrated in Figure 6a. All ACs exhibited typical capacitive behavior with an almost perfect rectangular shape. A typical GCD profile at a current density of 1 A g–1 is shown in Figure 6b. The triangular symmetric distribution profile of all samples implies excellent capacitive characterization, in agreement with the CV results. The Cg values of AC-05, AC-13, and AC-24 at a current density of 1 A g–1 were 360, 377, and 397 F g–1, respectively. The voltage of the supercapacitor always drops suddenly when discharge begins. The magnitude of voltage drop can effectively reflect the size of the internal resistance.46 Apparently, there is no obvious ohmic drop on the curves of all ACs, characteristic of low equivalent series resistance (ESR). The ESR values of supercapacitors based on AC-05, AC-13, and AC-24 were 0.32, 0.27, and 0.18 Ω, respectively.
Figure 6.
CV curves of ACs at a scan rate of 20 mV s–1 (a). GCD curves of ACs at a current density of 1 A g–1 (b).
As shown in Table 2, the SBET and Cg values of AC-24 were compared with literature values. The SBET of AC-24 was greater or comparable to the other biomass-derived ACs for supercapacitors. Yet the Cg of AC-24 was higher than those of the other ACs. As-prepared AC-24 is one of the best samples observed thus far in biomass-derived ACs.
Table 2. Comparison of Properties of Variable Biomass-Derived Carbon Materials for Supercapacitors.
| materials | SBET(m2 g–1) | electrolyte | Cg (F g–1) | measure current (A g–1) | ref |
|---|---|---|---|---|---|
| miscanthus grass | 1816 | 6 M KOH | 203 | 0.05 | (47) |
| waste paper | 2341 | 1 M H2SO4 | 286 | 0.5 | (48) |
| coffee waste | 2330 | 1 M Na2SO4 | 84 | 1 | (49) |
| litchi shell | 1122 | 6 M KOH | 220 | 0.1 | (50) |
| soybean meal | 1175 | 3 M KOH | 330 | 0.5 | (30) |
| peanut bran | 2565 | 3 M KOH | 188 | 0.04 | (51) |
| coconut kernel | 1200 | 1 M H2SO4 | 173 | 0.25 | (52) |
| sugar cane bagasse | 1788 | 1 M H2SO4 | 300 | 0.25 | (53) |
| coconut shell | 1998 | 1.5 M H2SO4 | 132 | 1 | (13) |
| coconut shell | 3512 | 6 M KOH | 325 | 0.1 | (33) |
| coconut shell | 2440 | 0.5 M H2SO4 | 246 | 0.5 | (54) |
| coconut shell | 1874 | 6 M KOH | 268 | 1 | (55) |
| AC-24 | 2383 | 0.5 M Na2SO4 | 397 | 1 | this work |
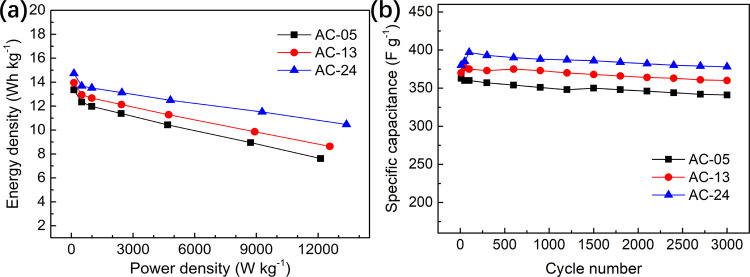
Figure 7a shows the Ragone plots of ACs. All samples showed high energy density and excellent rate capability, especially AC-24. At a power density of 120 W kg–1, the energy density of AC-24 was 14.7 Wh kg–1. When the power density increased by about 110 times, the energy density retention rate was 71.1%. It can also be found that the whole curve of AC-24 was over that of AC-05 and AC-13. This can be attributed to the wide Daver (3.12 nm) and a high fraction (91%) of the external surface area of AC-24.
Figure 7.
Ragone plots of ACs (a), and the cycling stability of ACs at a current density of 1 A g–1 (b).
Long-term cycling stability is another key in determining the electrochemical properties of the electrode materials. As shown in Figure 7b, the capacitance retention rate of all samples was over 95% after 3000 cycles, indicative of great potential application for supercapacitor electrodes.
4. Conclusions
In summary, freezing pretreatment of CS for a certain number of times before carbonization increased the SBET and Vtotal of the resultant BC to varying degrees. Freezing pretreatment for three times resulted in the maximum increase in the specific surface area and total pore volume by 92.8 and 44.4%, respectively, in the resultant BC. However, too many or too few times of freezing are not suitable for obtaining biochar with proper porosity. Furthermore, the coordination between the amount of KOH and the porosity of biochar is of great importance for the preparation of high-performance AC.
Compared with AC-05, which has a Cg of 386 F g–1 at a current density of 0.25 A g–1, AC-13 consumed 60% as much KOH and possessed a Cg of 17 F g–1 higher. With a KOH usage of 80% as much as that of AC-05, AC-24 achieved a Cg of 425 F g–1. At a power density of 120 W kg–1, the AC-24-based symmetric supercapacitor exhibited a high energy density of 14.7 Wh kg–1. Even with the power density increasing by about 110 times, the energy density retention rate was 71.1%, showing excellent rate capacity. After 3000 cycles, the capacitance retention rate was still over 95%. This work indicates that the freezing strategy is an effective way to cut down on activating agent usage and promote environmental protection.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 60901051), Shenzhen Foundation for Science and Technology Development Guided by the Central Government (No. 2021Szvup053), and Science and Technology Foundation of Jiangxi Province Department of Education (No. GJJ170323).
The authors declare no competing financial interest.
References
- Mensah-Darkwa K.; Zequine C.; Kahol P. K.; Gupta R. K. Supercapacitor energy storage device using biowastes: a sustainable approach to green energy. Sustainability 2019, 11, 414 10.3390/su11020414. [DOI] [Google Scholar]
- Deng J.; Li M.; Wang Y. Biomass-derived carbon: synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854. 10.1039/C6GC01172A. [DOI] [Google Scholar]
- Shaker M.; Ghazvini A. A. S.; Cao W. Q.; Riahifar R.; Ge Q. Biomass-derived porous carbons as supercapacitor electrodes - A review. New Carbon Mater. 2021, 36, 546–572. 10.1016/S1872-5805(21)60038-0. [DOI] [Google Scholar]
- Yuan Y. D.; Sun Y.; Feng Z. C.; Li X. J.; Yi R. W.; Sun W.; Zhao C. Z.; Yang L. Nitrogen-doped hierarchical porous activated carbon derived from paddy for high-performance supercapacitors. Materials 2021, 14, 318 10.3390/ma14020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy C. K.; Shah S. S.; Reaz A. H.; Sultana S.; Chowdhury A.; Firoz S. H.; Zahir M. H.; Qasem M. A. A.; Aziz M. A. Preparation of Hierarchical Porous Activated Carbon from Banana Leaves for High-performance Supercapacitor: Effect of Type of Electrolytes on Performance. Chem. - Asian J. 2021, 16, 296–308. 10.1002/asia.202001342. [DOI] [PubMed] [Google Scholar]
- Cai J.; Zhang D.; Ding W. P.; Zhu Z. Z.; Wang G. Z.; He J. R.; Wang H. B.; Fei P.; Si T. L. Promising Rice-Husk-Derived Carbon/Ni(OH)2 Composite Materials as a High-Performing Supercapacitor Electrode. ACS Omega 2020, 5, 29896–29902. 10.1021/acsomega.0c04117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. S.; Chen Q. Y.; Gong Y. N.; Wang H.; Li D. L.; Zhang Y. P.; Fu Q.; Pan C. X. ″One-Step″ Carbonization Activation of Garlic Seeds for Honeycomb-like Hierarchical Porous Carbon and Its High Supercapacitor Properties. ACS Omega 2020, 5, 29913–29921. 10.1021/acsomega.0c04190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandla D.; Wu X. D.; Zhang F. M.; Wu C. R.; Tan D. Q. High-Performance and High-Voltage Supercapacitors Based on N-Doped Mesoporous Activated Carbon Derived from Dragon Fruit Peels. ACS Omega 2021, 6, 7615–7625. 10.1021/acsomega.0c06171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghei S. A.; Zare M. H.; Ahmadi M.; Sadeghi M. H.; Marjani A.; Shirazian S.; Ghadiri M. Synthesis of Multi-application Activated Carbon from Oak Seeds by KOH Activation for Methylene Blue Adsorption and Electrochemical Supercapacitor Electrode. Arabian J. Chem. 2021, 14, 102958 10.1016/j.arabjc.2020.102958. [DOI] [Google Scholar]
- Zhang C.; Lin S.; Peng J.; Hong Z.; Wang Y.; Jin X. Preparation of Highly Porous Carbon through Activation of NH4Cl Induced Hydrothermal Microsphere Derivation of Glucose. RSC Adv. 2017, 7, 6486–6491. 10.1039/C6RA26141H. [DOI] [Google Scholar]
- Peng C.; Yan X. B.; Wang R. T.; Lang J. W.; Ou Y. J.; Xue Q. J. Promising Activated Carbons Derived from Waste Tea-leaves and Their Application in High Performance Supercapacitors Electrodes. Electrochim. Acta 2013, 87, 401–408. 10.1016/j.electacta.2012.09.082. [DOI] [Google Scholar]
- Taer E.; et al. Activated Carbon Electrode Made From Coconut Husk Waste For Supercapacitor Application. Int. J. Electrochem. Sci. 2018, 13, 12072–12084. 10.20964/2018.12.19. [DOI] [Google Scholar]
- Keppetipola N. M.; Dissanayake M.; Dissanayake P.; Karunarathne B.; Dourges M. A.; Talaga D.; Servant L.; Olivier C.; Toupance T.; Uchida S.; Tennakone K.; Kumara G. R. A.; Cojocaru L. Graphite-type Activated Carbon from Coconut Shell: A Natural Source for Eco-friendly Non-volatile Storage Devices. RSC Adv. 2021, 11, 2854–2865. 10.1039/D0RA09182K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omokafe S. M.; Adeniyi A. A.; Igbafen E. O.; Oke S. R.; Olubambi P. A. Fabrication of Activated Carbon from Coconut Shells and its Electrochemical Properties for Supercapacitors. Int. J. Electrochem. Sci. 2020, 11, 10854–10865. 10.20964/2020.11.10. [DOI] [Google Scholar]
- LIllo-Rodenas M. A.; Cazorla-Amoros D.; Linares-Solano A. Understanding Chemical Reactions between Carbons and NaOH and KOH: An Insight into the Chemical Activation Mechanism. Carbon 2003, 41, 267–275. 10.1016/S0008-6223(02)00279-8. [DOI] [Google Scholar]
- Jain A.; Tripathi J. S. K. Fabrication and Characterization of Energy Storing Supercapacitor Devices Using Coconut Shell Based Activated Charcoal Electrode. Mater. Sci. Eng.: B 2014, 183, 54–60. 10.1016/j.mseb.2013.12.004. [DOI] [Google Scholar]
- Deng M.-g.; Wang R.-q. The Effect of the HClO4 Oxidization of Petroleum Coke on the Properties of the Resulting Activated Carbon for Use in Supercapacitor. New Carbon Mater. 2013, 28, 262–265. 10.1016/S1872-5805(13)60080-3. [DOI] [Google Scholar]
- Deng M. G.; Wang R. Q. Effect of Petroleum Coke Expanding by Perchloric Acid on the Performance of the Resulted Activated Carbon. Funct. Mater. Lett. 2014, 07, 1350066. 10.1142/S1793604713500665. [DOI] [Google Scholar]
- Chen M.; Kang X.; Wumaier T.; Dou J.; Gao B.; Han Y.; Xu G.; Liu Z.; Zhang L. Preparation of Activated Carbon from Cotton Stalk and Its Application in Supercapacitor. J. Solid State Electrochem. 2013, 17, 1005–1012. 10.1007/s10008-012-1946-6. [DOI] [Google Scholar]
- Song S. J.; Ma F. W.; Wu G.; Ma D.; Geng W. D.; Wan J. F. Facile Self-templating Large Scale Preparation of Biomass-derived 3D Hierarchical Porous Carbon for Advanced Supercapacitors. J. Mater. Chem. A 2015, 3, 18154–18162. 10.1039/C5TA04721H. [DOI] [Google Scholar]
- Xie L.; Sun G.; Su F.; Guo X.; Kong Q.; Li X.; Huang X.; Wan L.; song W.; Li K.; Lv C.; Chen C. M. Hierarchical Porous Carbon Microtubes Derived from Willow Catkins for Supercapacitor Applications. J. Mater. Chem. A 2016, 4, 1637–1646. 10.1039/C5TA09043A. [DOI] [Google Scholar]
- Paris O.; Zollfrank C.; Zickler G. A. Decomposition and Carbonisation of Wood Biopolymers-A Microstructural Study of Softwood Pyrolysis. Carbon 2005, 43, 53–66. 10.1016/j.carbon.2004.08.034. [DOI] [Google Scholar]
- Sun K.; Leng C. Y.; Jiang J. C.; Bu Q.; Lin G. F.; Lu X. C.; Zhu G. Z. Microporous Activated Carbons from Coconut Shells Produced by Self-activation Using the Pyrolysis Gases Produced from Them, That Have an Excellent Electric Double Layer Performance. New Carbon Mater. 2017, 32, 451–459. 10.1016/S1872-5805(17)60134-3. [DOI] [Google Scholar]
- Brebu M.; Vasile C. Thermal Degradation of Lignin-A Review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Tsamba A. J.; Yang W.; Blasiak W. Pyrolysis Characteristics and Global Kinetics of Coconut and Cashew Nut Shells. Fuel Process. Technol. 2006, 87, 523–530. 10.1016/j.fuproc.2005.12.002. [DOI] [Google Scholar]
- Mi J.; Wang X. R.; Fan R. J.; Qu W. H.; Li W. C. Coconut-Shell-Based Porous Carbons with a Tunable Micro/Mesopore Ratio for High-Performance Supercapacitors. Energy Fuel 2012, 26, 5321–5329. 10.1021/ef3009234. [DOI] [Google Scholar]
- Vamvuka D.; Kakaras E.; Ksatanaki E.; Grammeis P. Pyrolysis Characteristics and Kinetics of Biomass Residuals Mixtures with Lignite. Fuel 2003, 82, 1949–1960. 10.1016/S0016-2361(03)00153-4. [DOI] [Google Scholar]
- Ksatanaki E.; Vamvuika D.; Grammelis P.; Kakaras E. Thermogravimetric Studies of the Behavior of Lignite-biomass Blends during Devolatilization. Fuel Process. Technol. 2002, 77–78, 159–166. 10.1016/S0378-3820(02)00049-8. [DOI] [Google Scholar]
- Moreno-Fernández G.; Gómez-Urbano L. J.; Enterría M.; Rojo T.; Carriazo D. Flat-shaped Carbon–graphene Microcomposites as Electrodes for High Energy Supercapacitors. J. Mater. Chem. A 2019, 7, 14646–14655. 10.1039/C9TA03295A. [DOI] [Google Scholar]
- Zhao H.; Xing B.; Zhang C.; Huang G.; Liu Q.; Yi G.; Jia J.; Ma M.; Chen Z.; Zhang C. Efficient Synthesis of Nitrogen and Oxygen Co-doped Hierarchical Porous Carbons Derived from Soybean Meal for High-performance Supercapacitors. J. Alloys Compd. 2018, 766, 705–715. 10.1016/j.jallcom.2018.06.267. [DOI] [Google Scholar]
- Xie Z.; Shang X.; Yan J.; Hussain T.; Nie P.; Liu J. Biomass-derived Porous Carbon Anode for High-Performance Capacitive Deionization. Electrochim. Acta 2018, 290, 666–675. 10.1016/j.electacta.2018.09.104. [DOI] [Google Scholar]
- Zhang K.; Liu M.; Si M.; Wang Z.; Zhuo S.; Chai L.; Shi Y. Polyhydroxyalkanoate-Modified Bacterium Regulates Biomass Structure and Promotes Synthesis of Carbon Materials for High-Performance Supercapacitors. ChemSusChem 2019, 12, 1732–1742. 10.1002/cssc.201802894. [DOI] [PubMed] [Google Scholar]
- Hou B.; Zhang T.; Yan R.; Li D.; Mo Y.; Yin L.; Chen Y. High Specific Surface Area Activated Carbon with Well-balanced Micro/Mesoporosity for Ultrahigh Supercapacitive Performance. Int. J. Electrochem. Sci. 2016, 11, 9007–9018. 10.20964/2016.11.01. [DOI] [Google Scholar]
- Lozano-Castelló D.; Cazorla-Amorós D.; Linares-Solano A.; Shiraishi S.; Kurihara H.; Oya A. Influence of Pore Structure and Surface Chemistry on Electric Double Layer Capacitance in Non-aqueous Electrolyte. Carbon 2003, 41, 1765–1775. 10.1016/S0008-6223(03)00141-6. [DOI] [Google Scholar]
- Kierzek K.; Frackowiak E.; Lota G.; Gryglewicz G.; Machnikowski J. Erratum to “Electrochemical Capacitors Based on Highly Porous Carbons Prepared by KOH Activation”. Electrochim. Acta 2004, 49, 1169–1170. 10.1016/j.electacta.2004.01.005. [DOI] [Google Scholar]
- Dai Y.; Jiang H.; Hu Y.; Fu Y.; Li C. Controlled Synthesis of Ultrathin Hollow Mesoporous Carbon Nanospheres for Supercapacitor Applications. Ind. Eng. Chem. Res. 2014, 53, 3125–3130. 10.1021/ie403950t. [DOI] [Google Scholar]
- Gao Y.; Zhang W.; Yue Q.; Gao B.; Sun Y.; Kong J.; Zhao P. Simple Synthesis of Hierarchical Porous Carbon from Enteromorpha Prolifera by a Self-template Method for Supercapacitor Electrodes. J. Power Sources 2014, 270, 403–410. 10.1016/j.jpowsour.2014.07.115. [DOI] [Google Scholar]
- Eliad L.; Salitra G.; Soffer A.; Aurbach D. Ion Sieving Effects in the Electrical Double Layer of Porous Carbon Electrodews: Estimating Effective Ion Size in Electrolytic Solutions. J. Phys. Chem. B 2001, 105, 6880–6887. 10.1021/jp010086y. [DOI] [Google Scholar]
- Chmiola J.; Yushin G.; Gogosti Y.; Portet C.; Simon P.; Taberna P. Anomalous Increase in Carbon Capacitance at Pore Sizse Less Than 1 Nanometer. Science 2006, 313, 1760–1763. 10.1126/science.1132195. [DOI] [PubMed] [Google Scholar]
- Pandolfo A. G.; Hollenkamp A. F. Carbon Properties and Their Role in Supercapacitors. J. Power Sources 2006, 157, 11–27. 10.1016/j.jpowsour.2006.02.065. [DOI] [Google Scholar]
- Huang J.; Sumpter B. G.; Meunier V. Theoretical Model for Nanoporous Carbon Supercapacitors. Angew. Chem. 2008, 120, 530–534. 10.1002/ange.200703864. [DOI] [PubMed] [Google Scholar]
- Cao L.; Li H.; Xu Z.; Gao R.; Wang S.; Zhang G.; Jiang S.; Xu W.; Hou H. Camellia Pollen-Derived Carbon with Controllable N Content for High-Performance Supercapacitors by Ammonium Chloride Activation and Dual N-doping. ChemNanoMat 2021, 7, 34–43. 10.1002/cnma.202000531. [DOI] [Google Scholar]
- Chen W.; Luo M.; Yang K.; Zhou X. Microwave-assisted KOH Activation from Lignin into Hierarchically Porous Carbon with Super High Specific Surface Area by Utilizing the Dual Roles of Inorganic Salts: Microwave Absorber and Porogen. Microporous Mesoporous Mater. 2020, 300, 110178 10.1016/j.micromeso.2020.110178. [DOI] [Google Scholar]
- Chen W.; Luo M.; Yang K.; Zhou X. Simple Pyrolysis of Alginate-based Hydrogel Cross-linked by Bivalent Ions into Highly Porous Carbons for Energy Storage. Int. J. Biol. Macromol. 2020, 158, 265–274. 10.1016/j.ijbiomac.2020.04.123. [DOI] [PubMed] [Google Scholar]
- Yang L.; Wu D.; Wang T.; Jia D. B/N-codoped Carbon Nanosheets Derived from the Self-Assembly of Chitosan-Amino Acid Gels for Greatly Improved Supercapacitor Performances. ACS Appl. Mater. Interfaces 2020, 12, 18692–18704. 10.1021/acsami.0c01655. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Wang X.; Su J.; Wang X.; Jiang L.; Wu H.; Wu C. The Effects of Surfactant Template Concentration on the Supercapacitive Behaviors of Hierarchically Porous Carbons. J. Power Sources 2012, 199, 402–408. 10.1016/j.jpowsour.2011.10.070. [DOI] [Google Scholar]
- Yakaboylu G. A.; Jiang C. L.; Yumak T.; Zondlo J. W.; Wang J. X.; Sabolsky E. M. Engineered Hierarchical Porous Carbons for Supercapacitor Applications through Chemical Pretreatment and Activation of Biomass Precursors. Renewable Energy 2021, 163, 276–287. 10.1016/j.renene.2020.08.092. [DOI] [Google Scholar]
- Puthusseri D.; Aravindan V.; Anothumakkool B.; Kurungot S.; Madhavi S.; Ogale S. From Waste Paper Basket to Solid State and Li-HEC Ultracapacitor Electrodes: A Value Added Journey for Shredded Office Paper. Small 2014, 10, 4395–4402. 10.1002/smll.201401041. [DOI] [PubMed] [Google Scholar]
- Adan-Mas A.; Alcaraz L.; Arevalo-Cid P.; Lopez-Gomez F. A.; Montemor F. Coffee-derived Activated Carbon from Second Biowaste for Supercapacitor Applications. Waste Manage. 2021, 120, 280–289. 10.1016/j.wasman.2020.11.043. [DOI] [PubMed] [Google Scholar]
- Zhao N.; Zhang P.; Luo D.; Xiao W.; Deng L.; Qiao F. Direct Production of Porous Carbon Nanosheets/Particle Composites from Wasted Litchi Shell for Supercapacitors. J. Alloys Compd. 2019, 788, 677–684. 10.1016/j.jallcom.2019.02.304. [DOI] [Google Scholar]
- Kang W.; Lin B.; Huang G.; Zhang C.; Yao Y.; Hou W.; Xu B.; Xing B. Peanut Bran Derived Hierarchical Porous Carbon for Supercapacitor. J. Mater. Sci.: Mater. Electron. 2018, 29, 6361–6368. 10.1007/s10854-018-8615-1. [DOI] [Google Scholar]
- Kishore B.; Shanmughasundaram D.; Penki T. R.; Munichandraiah N. Coconut Kernel-derived Activated Carbon as Electrode Material for Electrical Double-layer Capacitors. J. Appl. Electrochem. 2014, 44, 903–916. 10.1007/s10800-014-0708-9. [DOI] [Google Scholar]
- Rufford T. E.; Hulicova-Jurcakova D.; Khosla K.; Zhu Z.; Lu G. Q. Microstructure and Electrochemical Double-layer Capacitance of Carbon Electrodes Prepared by Zinc Chloride Activation of Sugar Cane Bagasse. J. Power Sources 2010, 195, 912–918. 10.1016/j.jpowsour.2009.08.048. [DOI] [Google Scholar]
- Jain A.; Xu C.; Jayaraman S.; Balasubramanian R.; Lee J. Y.; Srinivasan M. P. Mesoporous Activated Carbons with Enhanced Porosity by Optimal Hydrothermal Pre-treatment of Biomass for Supercapacitor Applications. Microporous Mesoporous Mater. 2015, 218, 55–61. 10.1016/j.micromeso.2015.06.041. [DOI] [Google Scholar]
- Sun L.; Tian C.; Li M.; Meng X.; Wang L.; Wang R.; Yin J.; Fu H. From Coconut Shell to Porous Graphene-like Nanosheets for High-power Supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470. 10.1039/c3ta10897j. [DOI] [Google Scholar]