Abstract
We report on the reaction of ethylene-terminated heteroatoms (C2X; X = N, O, and S) with CS2/CO2 using Mukaiyama reagent (2-chloro-1-methylpyridinium iodide, CMPI) as a promoter for the preparation of imidazolidin-2-one, oxazolidin-2-one, 1,3-dioxolan-2-one, 1,3-dithiolan-2-one, and their thione counterparts at ambient temperature and pressure. Spectroscopic measurements, viz., 1H/13C nuclear magnetic resonance (NMR) and ex situ attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy methods verified the reaction of CS2/CO2 with the ethylene-based substrates and subsequently the formation of cyclic products. The experimental data indicated the formation of the enol-form of imidazolidin-2-one and oxazolidin-2-one, while the keto-form was obtained for their thione correspondents. Furthermore, density functional theory calculations revealed the stability of the keto- over the enol-form for all reactions and pointed out the solvent effect in stabilizing the latter.
1. Introduction
The utilization of CO2 as a raw material for the production of intermediates/fine chemicals/value-added products is considered an attractive research theme that could be addressed in terms of market demands, economic feasibility, efficiency of using resources, and sustainability implications.1−5 One of the common routes for CO2 fixation is the cycloaddition (or copolymerization) of CO2 with epoxide,6 episulfide, or aziridine.7,8 Moreover, it could be used for the synthesis of profitable products such as (a)cyclic urea/carbamate, isocyanate, carbonate, and their thione counterparts (in the case of CS2) when reacted with simple amines or bifunctionalized scaffolds including diamines, amino alcohols, diols, and others.9−22
According to literature reports, olidine compounds were obtained by reacting phosgene23 or urea with diamines24 or amino alcohols.25 Also, they were prepared upon catalyzing the reaction by Zn–Zr oxide,26 triphenyl stibine oxide,13,14 thiol/Fe4S4 cluster12 or under catalyst-free conditions.15 Moreover, ethylene urea or 2-pyrrolidone was used as the promoter for olidine synthesis.27,28 (Thio)olidine compounds were synthesized using different sulfur-containing reagents (such as CS2, thiophosgene, and isothiocyanate)29 or elemental sulfur (S8) with formaldehyde aminals,30 silver carbene complex,31 or chloroform.32 In addition, olanes, e.g., cyclic carbonates, were prepared by the reaction of diols with CO2 using a wide range of inorganic33−36 and organic catalysts37,38 or promoters.18,19 For more details on the synthesis of olidine/olane compounds and their thiol analogues, see Table S1, Supporting Information.
The problems associated with the common (thio)carbonylating agents such as phosgenes39 and isocyanates40 as well as conventional processes make it necessary to search for benign alternatives and safer methods to avoid potential catastrophes such as in Bhopal, India, 198441 and to eliminate toxic/hazardous byproducts.42 Thus, green approaches are directed toward microwave-assisted,43 solvent-free methods44 by employing green carbonylating agents.45−47 Interestingly, Dondoni group reacted CS2 with monofunctionalized amine in the presence of triethylamine (Et3N) and 2-chloro-1-methylpyridinium iodide, [Mukaiyama reagent (CMPI), Scheme 1] to prepare isothiocyanate.48
Scheme 1. Chemical Structure of CMPI.

In this context, N-alkyl-2-halopyridinium salts have been used as promoters for the synthesis of different compounds49,50 such as esters,51−53 ketenes,54,55 lactones,56,57 lactams,58−60 peptides,61 amides,62 carbodiimides,63 thiocyanates,64 pyrazole derivatives,65 and different polymeric materials.66
The success story of CMPI lies in the fact that it acts as an oxygen sink, converting the bad leaving group into a good one upon a simple nucleophilic addition–elimination reaction. This might be a plausible gateway for synthetic chemists to use it as a (thio)carbonylation promoter. To our knowledge, there is no precedented route for the preparation of tailor-made, benign, non-phosgene intermediates, viz., isocyanates from carbamates using CMPI, thus eliminating the toxicity associated with well-known hazardous materials.
As an augmentation to our research efforts on CO2 capture using biorenewables,67−72 biomaterials,73,74 and small organic molecules/oligomers,75,76 as well as CO2 utilization catalyzed by poly(ionic liquid)s,77 organocatalysts,78 and metal oxide/inorganic complexes,79−81 we provide a platform for the synthesis of olidines and olanes, including imidazolidin-2-one, oxazolidin-2-one, 1,3-dioxolan-2-one, 1,3-dithiolan-2-one, and their sulfur counterparts upon reacting CO2 (CS2) with ethylenediamine (en), monoethanolamine (MEA), ethylene glycol (EG), and ethane-1,2-dithiol (EDT), respectively, in the presence of CMPI as shown in Scheme 2.
Scheme 2. Proposed Synthesis of Functionalized Olidine/Olane Using CMPI as a (Thio)carbonylation Promoter.
2. Results and Discussion
2.1. Synthesis of Imidazolidin-2-one (1)
Upon bubbling CO2 in en/DCM solution for 60 min, a white precipitate was obtained. As shown in Figure 1, the 13C NMR spectrum of the product measured in deuterium oxide (D2O) indicated the formation of an ammonium carbamate adduct as inferred from the chemical shift at 164.5 ppm (blue trace).82 The decomposition of the obtained adduct into ammonium bicarbonate was detected due to the emergence of a new peak at 160.4 ppm.83 This was verified using labeled sodium bicarbonate (NaH13CO3) (Figure S1, Supporting Information). The formation of the carbamate adduct was further supported by ex situ ATR-FTIR measurements of the precipitate that indicated the appearance of a new peak at 1660 cm–1, which was assigned for the carbonyl group of the CO2 adduct,82 as well as the disappearance of the asymmetric stretching frequency peak of the primary amine group upon carbamation (Figure S2, Supporting Information).
Figure 1.
13C NMR spectra of en dissolved in D2O before (red trace) and after bubbling (blue trace) with CO2.
In order to attain compound 1, the carbamate adduct was mixed with a solution of CMPI/DCM under N2 atmosphere. Afterward, a yellow precipitate was obtained, which was dissolved in deuterated dimethyl sulfoxide (DMSO-d6) and analyzed using 13C NMR spectroscopy. For the sake of comparison, the spectra of the starting materials are shown in Figure 2. The chemical shifts associated with C-3 to C-7 of CMPI (green trace) showed an upfield shift upon the formation of the pyridinium carbamate salt; however, the C-2 peak was downfield shifted from 146.9 to ca. 152.7 ppm (purple trace) due to the inductive effect of the activated carbamate.
Figure 2.
13C NMR spectra measured in DMSO-d6 of en (red trace), CMPI (green trace), pyridinium carbamate salt (purple trace), and the reaction mixture (black trace).
Later on, Et3N was added to the reaction mixture to drive the intramolecular attack of the terminal amine on the carbamate carbonyl by neutralizing the salt (vide supra) and subsequently deprotonating the proposed cyclic product 1 (black trace). The analysis of the reaction mixture verified the formation of (a) N-methyl-2-pyridinoate, as deduced from the chemical shift of the C-2 peak from 152.7 to 143.0 ppm and (b) the enol-form of 1, as C3′ was upfield-shifted from 158.8 to 152.7 ppm. This was fortified by the new chemical shift of the protons located at carbons labeled 3–7 upon the formation of the pyridinium carbamate salt (Figure S3, Supporting Information). A representative reaction mechanism is proposed in Scheme 3.
Scheme 3. Postulated Reaction Mechanism for en to Yield Ethylene Urea.
The reaction is mediated via CMPI, which acts as the oxygen-sink promoter.
The presumed reaction product 1 was separated from the reaction mixture, exploiting its ability to capture CO2 in non-aqueous media.76 Herein, the reaction crop was dissolved in DMSO, then sodium hydride (NaH) was added for activation purposes. The solution was bubbled with CO2 until it became turbid. Upon adding concentrated hydrochloric acid to the filtrate dissolved in D2O, 13C NMR measurement confirmed the exclusive existence of 1 in the keto-form (Figure 3).
Figure 3.
13C NMR spectrum measured in D2O for 1.
2.2. Synthesis of Imidazolidine-2-thione (2)
Using the same reaction conditions, 2 was obtained using CS2 instead of CO2, where the formation of the ammonium ethylenethiocarbamate adduct was confirmed in DMSO-d6 by the emergence of a new peak at 183.5 ppm (green trace, Figure 4).84 This was supported by the ATR-FTIR measurements, which were consistent with the previously obtained spectrum of the carbamate adduct (Figure S4, Supporting Information).
Figure 4.
Partial 13C NMR spectra of en (red trace), CS2 (gray trace), and en after reaction with CS2 (green trace), CMPI (blue trace), thiopyridinium salt (purple trace), and the reaction mixture (black trace) measured in DMSO-d6.
However, the addition of CMPI to the thiocarbamate adduct produced the enol form of the thiopyridinium salt as deduced from the chemical shifts associated with C3′ at 164.5 ppm (purple trace, Figure 4) and the thiol proton at 1.21 ppm (Figure S5, Supporting Information). In this respect, Nguyen and co-workers85 reported on the stability of thiones and thiols in polar aprotic solvents and in the gas phase. The double bond between the carbon atom and the high electronegative heteroatom is energetically more stable than C=S. This makes our results show good agreement with the literature. In the cyclization step (upon adding Et3N), N-methyl-2-pyridinethione acts as the leaving group to generate the keto- and enol-forms of 2 as indicated by peaks emerging at 183.4 [thione (−C=S)] and 152.7 [thiol (−C–SH)] ppm, respectively (black trace, Figure 4). Once again, the desired product was obtained upon activating the reaction crop by NaH, bubbling CO2,76 and then dissolving the obtained precipitate in D2O. This was confirmed by 13C NMR measurements of the solution together with the decarbonized species as shown in Figure 5 (green and black traces, respectively).
Figure 5.
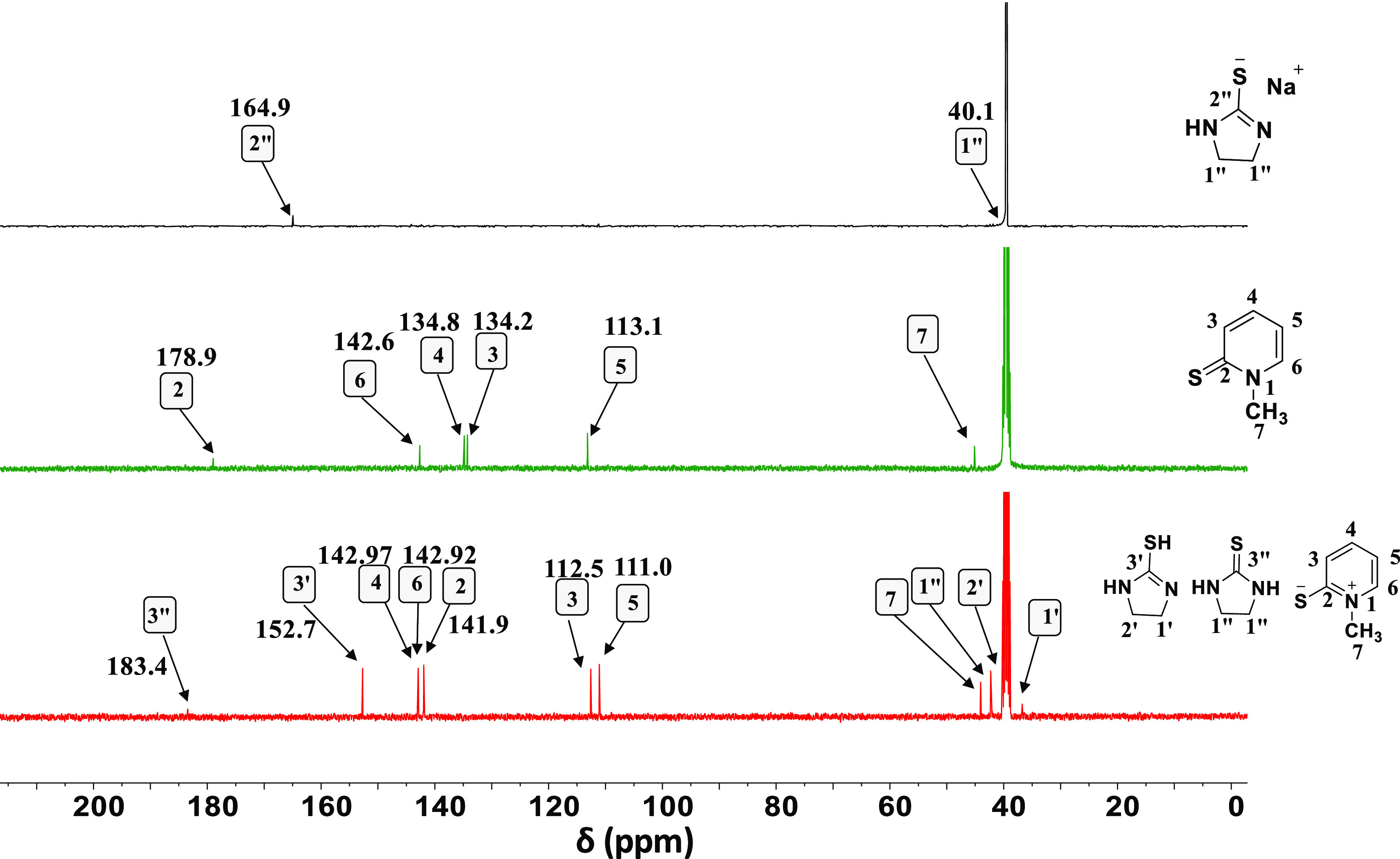
13C NMR spectra of the reaction mixture (red trace), N-methyl-2-pyridinethione (green trace), and sodium ethylene thiourea (black trace).
2.3. Synthesis of Oxazolidin-2-one (3)
As shown in Scheme 4, the reaction of primary amines with CO2 in non-aqueous media leads to the formation of an unstable zwitterionic intermediate that rapidly rearranges through intramolecular proton transfer to the produce carbamic acid (following a 1:1 mechanism). In the presence of an excessive amount of amine, the latter is rapidly deprotonated to form an ammonium carbamate adduct via a 2:1 mechanism. If DMSO is used as a reaction medium, carbamic acid is expected to be the more favored product due to hydrogen-bonding interactions.86 In our case, bubbling MEA/DMSO-d6 solution with CO2 resulted in a new peak at 158.5 ppm, which indicated the formation of carbamic acid, with a small amount of ammonium carbamate adduct (green trace, Figure S6, Supporting Information). This was fortified by the emergence of a new peak at 1700 cm–1 in the IR spectrum of the product (Figure S7, Supporting Information).
Scheme 4. General Reaction Scheme of Primary Amines with CO2 in a Non-Aqueous Solvent.
In order to prepare 3, Et3N and CMPI were added to the MEA-CO2 adduct solution in DCM under N2 atmosphere, where a white precipitate was collected after 1 h, which contained a mixture of oxazolidin-2-one (enol form) and N-methyl-2-pyridinoate as inferred from the chemical shift observed at 152.9 ppm together with an upfield shift of C-3 to C-7 of CMPI (black and purple traces, Figure S6, Supporting Information) as well as their protons (black trace, Figure S8, Supporting Information).
2.4. Synthesis of Oxazolidine-2-thione (4)
In contrast to the MEA/CO2 scenario, analyzing the viscous mixture MEA/CS2 showed that thiocarbamate was the major product over the carbamic acid counterpart as demonstrated by the new peaks that emerged at 188.7 and 202.5 ppm, with the presence of a small amount of unreacted MEA (green trace, Figure S9, Supporting Information). This was confirmed by the newly emerged peaks at 2.88 and 3.58 ppm in the 1H NMR spectrum (Figure S10, Supporting Information).
In order to obtain the thione target product, the carbamate adduct was reacted with CMPI in DCM. Analyzing the formed yellow precipitate indicated a mixture of 4 and N-methylpyridine-2-thione (black trace, Figure S9, Supporting Information), where the chemical shift of C=S in the product was observed at 188.6 ppm and that of pyridone was observed at 178.9 ppm. This finding was supported by an upfield shift of the protons located at carbons labeled 3–7 in the 1H NMR spectrum (black trace, Figure S10, Supporting Information).
It is worth mentioning that these oxazolidine compounds do not absorb CO2 successfully as the former diazolidine. This could be attributed to the resonance stabilization of the active enolate species and the less nucleophilic character of oxygen in comparison with nitrogen in cyclic urea. In the case of oxazolidine-2-thione, presumably, the presence of oxygen weakens the ability of the anion to react with the electrophilic carbon of CO2. Unfortunately, several separation strategies such as metathesis with silver salts, extraction, thin layer (conventional and reversed phase) as well as column chromatography have been employed with no success in separating the oxazolidine compounds and the associated pyridinoate/pyridone.
2.5. Synthesis of 1,3-Dioxolan-2-one (5)
In order to synthesize olanes, 5 was obtained by a two-step reaction, first, the activated EG with Et3N was bubbled with CO2 for 2 h to generate triethylammonium 2-hydroxyethyl carbonate, which was characterized by NMR and IR analyses. The 13C NMR spectrum measured in DMSO-d6 indicated the formation of the adduct, as inferred by the chemical shifts at 61.0, 66.4, and 158.0 ppm (green trace, Figure S11, Supporting Information). These results were supported using 1H NMR analysis by the appearance of new peaks at 3.35 and 4.56 ppm together with ATR-FTIR measurement by the emergence of three new peaks at 1637, 1390, and 1288 cm–1 attributed to asymmetric and symmetric stretchings of (C=O), respectively87 (Figures S12 and 13, Supporting Information).
Second, CMPI was added together with Et3N to the carbonate adduct in 5 mL of acetonitrile (CH3CN) as the solvent. A white precipitate was collected after 3 h, which was referred to as 5, and it was deduced from the chemical shifts at 69.5 and 159.1 ppm, with N-methyl-2-pyridinoate shown as black traces in Figure S11, Supporting Information. The 1H NMR spectrum indicated the appearance of a new peak at 5.04 ppm, which was assigned to C-1′ of ethylene carbonate (EC) (black trace, Figure S12, Supporting Information).
2.6. Synthesis of 1,3-Dioxolane-2-thione (6)
The synthesis of 6 started with the reaction of CS2 with activated EG in DMSO-d6 to form a carbonodithioate adduct. The 13C NMR spectrum indicated the formation of the adduct, as inferred from the chemical shifts at 61.0, 66.4, and 184.8 ppm which were assigned to C-1, C-2, and C-3 (C=S) of the product, respectively (Figure 6). These data were supported by 1H NMR, with two new emerging peaks at 3.61 (C-1) and 4.22 (C-2) ppm, respectively, (green trace, S15, Supporting Information). Then, CMPI was added with another mole of Et3N to the carbonodithioate adduct dissolved in 5 mL of CH3CN solution, which was evaporated in a later stage to give a clear yellow solution containing 1,3-dioxolane-2-thione and N-methylpyridin-2-thione. 13C NMR indicated the formation of the former by the emergence of chemical shifts centered at 69.5 and 174.3 ppm, while the latter was as inferred from the peak at 179.1 ppm, which was assigned to C-2 (C=S) (black traces, Figure S14, Supporting Information). In the 1H NMR spectrum, formation of 6 was indicated by the appearance of a new peak at 3.63 ppm, assigned to C-1′ (black trace, Figure S15, Supporting Information).
Figure 6.

13C NMR spectra of the reaction crop of oxazolidin-2-one (red trace), oxazolidine-2-thione (green trace), 1,3-dioxolan-2-one (purple trace), and 1,3-dioxolane-2-thione (black trace).
2.7. Synthesis of 1,3-Dithiolan-2-one/-thione (7 and 8)
Starting with ethanedithiol, 7 was prepared, which contained trace amounts of the disulfide bridge compound (2,2′-disulfanediylbis(ethan-1-ol)) as a result of oxidation; the addition of Et3N resulted in the reverse formation of the starting material as proven in the NMR spectrum (blue trace, Figures S16 and S17, Supporting Information).88,89 The mechanism of the reduction process is proposed in Scheme S1, Supporting Information.
Afterward, dithiol was activated using NaH, followed by the formation of the sodium carbonothioate adduct upon bubbling the solution with CO2. The spectrum of the latter white precipitate showed the emergence of two new peaks at 2.71 and 2.85 ppm for the product once dissolved in DMSO-d6 (green trace, Figure S16, Supporting Information). This was further supported by ATR-FTIR measurement by the appearance of a new peak at 1637 cm–1 attributed to the asymmetric stretching of (C=O) (Figure S18, Supporting Information). Finally, the whitish adduct was further dissolved in CH3CN to which solid CMPI was added and stirred for about an hour till the formation of a white precipitate. The crude product was collected by filtration, which was later referred to as a mixture of 7, and it was inferred from the chemical shifts at 30.4 and 157.6 ppm together with N-methyl-2-pyridinolate shown in Figure S17 (black traces, Supporting Information). This was confirmed by 1H NMR analysis by the appearance of a new peak at 3.91 ppm for C-1′ of 7 (black traces, Figure S16, Supporting Information). In the case of CS2, a yellowish precipitate was formed for adducts and the final product, as inferred from the chemical shift values shown in Figures S19 and S20 (Supporting Information). The chemical shift of the starting materials and the obtained products (1–8) is summarized in the Supporting Information.
2.8. Density Functional Theory (DFT) Calculations
The quantum chemical calculations were used to understand the stability of the keto/enol-forms in the different investigated reactions using the B3LYP/6-311++G(d,p) level of theory90 in Gaussian 09.91 The applied method has been used previously to predict reliable geometries and vibrational frequencies of hydrogen-bonded systems.92 In order to investigate the solvent effects, the calculations were also carried out in DCM and DMSO at the same level using the polarizable continuum model. Table 1 lists the thermodynamic parameters for the tautomerism (relative to the enol-form) of the olidine products. The calculated free energy values indicated higher stability for the keto-compared to the enol-forms in DCM as well as in DMSO, with a slight preference in the latter. The experimental data indicated the formation of the enol-form of the olidine, whereas the olidine-thione counterpart was found in the keto-form. This can be understood in terms of the greater bond energy of C=O than C=N in the case of urea (1) and urethane (3), while C=S has lower energy than C=N for both thiourea (2) and thiourethane (4).93 The discrepancy between the experimental and theoretical data can be understood by applying explicit DMSO molecules to unveil the effect of solvent on tautomerization. In general, the free energy values were lower than those calculated using in the absence of explicit solvent (DMSO) molecules. For 1 and 2, the energy values indicated higher stability of the keto-from of the latter (−20.02 kcal/mol) compared to the former (−16.60 kcal/mol). This might be explained by the stabilization of the enol-form (1) upon the formation of hydrogen bonding with DMSO, which highlights the role of solvent in assisting tautomerization.
Table 1. Calculated Thermodynamic Parametersa for the keto-(k)/enol-Forms (e) of the Final Products; Values are Given in kcal/mol.
| entry | substrate | ΔErel | ΔHrel | TΔSrel | ΔGrel (k–e) |
|---|---|---|---|---|---|
| 1 | imidazolidin-2-one | –22.60 [−22.86]b | –22.74 [−23.01]b | –0.69 [−0.69]b | –22.05 [−22.31]b |
| 2 | imidazolidine-2-thione | –19.84 [−20.36]b | –20.14 [−20.57]b | –1.15 [−0.85]b | –18.99 [−19.71]b |
| 3 | oxazolidin-2-one | –20.53 [−21.50]b | –20.70 [−21.71]b | –0.50 [−0.55]b | –20.20 [−21.15]b |
| 4 | oxazolidine-2-thione | –14.99 [−16.11]b | –15.35 [−16.45]b | –0.86 [−0.78]b | –14.49 [−15.66]b |
| 5 | imidazolidin-2-one-DMSO | –16.18c | –15.99c | 0.60c | –16.60c |
| 6 | imidazolidine-2-thione-DMSO | –19.08c | –18.65c | 1.36c | –20.02c |
| 7 | oxazolidin-2-one-DMSO | –16.14c | –16.01c | 0.70c | –16.71c |
| 8 | oxazolidine-2-thione-DMSO | –17.64c | –17.97c | –1.36c | –16.61c |
Calculated using the B3LYP/6-311++G(d,p) basis set, and the values are given relative to the enol-form in all reactions.
Values are calculated in DCM, and values in brackets are calculated in DMSO.
Values calculated in the presence of explicit DMSO molecules.
The relative energy difference between keto/enol forms of the olidine-thione compounds was also calculated in the presence of N-methyl pyridonate/N-methyl-2-pyridinethione as the reaction mixture (Table 2). The values again revealed higher stability for the keto-over its enol-forms, with a lower energy difference in the case of 3 and 4 compared to 1 and 2. The optimized structures are given in Tables S2 and S3.
Table 2. Calculated Thermodynamic Parametersa (in DCM) for the keto-/enol-Product Mixed with Pyridone; Values are Given in kcal/mol.
| sample | ΔErel | ΔHrel | TΔSrel | ΔGrel |
|---|---|---|---|---|
| imidazolidin-2-one | –19.66 | –19.78 | –0.65 | –19.12 |
| imidazolidine-2-thione | –19.19 | –19.25 | 0.77 | –20.03 |
| oxazolidin-2-one | –16.01 | –15.96 | –0.01 | –15.95 |
| oxazolidine-2-thione | –15.76 | –15.95 | –0.66 | –15.28 |
Calculated using the B3LYP/6-311++G(d,p) basis set, and the values are given relative to the enol-form in all reaction systems.
3. Conclusions
We introduced a novel methodology for the synthesis of different heterocyclic compounds by reacting a set of ethylene-terminated heteroatoms (C2X; X = N, O, and S) with CO2 and CS2 using Mukaiyama reagent as a promoter in a basic medium under ambient conditions. The resulting intermediates/products were verified using a combination of 1H/13C NMR and ex situ ATR-FTIR spectroscopy methods. Notably, the formation of the enol-products was favored in the case of urea and urethane, while the keto-forms were obtained for the corresponding sulfur heterocyclic compounds. DFT calculations highlighted the effect of DMSO on the keto–enol tautomerization.
4. Experimental Section
4.1. Materials
All chemicals were used without purification. Ethylenediamine (99%), ethylene glycol (99%), Mukaiyama reagent (CMPI, 97%), triethylamine (99.5%), and dichloromethane (99.9%) were purchased from Loba Chemie, TEDIA, Aldrich, Fisher, and AZ chem, respectively. Ethanolamine (98%) and DMSO-d6 (99.5 atom % D) were acquired from Sigma-Aldrich, ethane-1,2-dithiol (98%) was obtained from Fluka, carbon disulfide (CS2, 99%) was bought from Panreac Quimica, CO2 and N2 (industrial grade) were purchased from Advanced Technical Gases Co. (Amman, Jordan). Unless otherwise stated, all isolated reaction products (intermediates) during the synthesis of 1–8 were identified by 1H/13C NMR and ex situ ATR-FTIR spectroscopic measurements to verify the presumed structures.
4.2. Instruments
1H and 13C nuclear NMR spectra were measured using AVANCE-III 400 MHz (1H: 400.13 MHz, 13C: 100.61 MHz) equipped with a FTNMR Nano Bay spectrometer (Bruker, Switzerland). Ex situ ATR-FTIR spectra were recorded using a Bruker Vertex 70-FT-IR spectrometer at RT coupled with a Vertex Pt-ATR-FTIR accessory. Elemental analysis (EA) was performed using a CHN elemental analyzer EA3000 instrument (Euro Vector, Italy).
4.3. Synthesis of Imidazolidin-2-one (1)
en (2.25 mmol) was dissolved in 20 mL of DCM, and the solution was directly bubbled with CO2 for 60 min using a needle. The adduct appeared as a white precipitate, which was collected by decanting the DCM, washed with diethylether (10 mL × 2), and dried at RT (yield 89%). To the suspension of the latter carbamate in DCM, CMPI (2.70 mmol) was added. The reaction was carried out under N2 gas for 2 h at RT (yield 81%). Then, Et3N (6.75 mmol) was dropwise added and left to stir for 2 h to yield the enol-pyridinoate adduct, which was dissolved in 3.0 mL DMSO, and activated with 2.25 mmol NaH. Then, the solution was bubbled with CO2 for 1 h and the salt was collected after filtration. Finally, a drop of concentrated HCl was added and stirred for 1 h to form 1. EA (%) calculated for C6H16N4O4: N, 26.92; C, 34.61; H, 7.69. Found: N, 26.13; C, 34.08; H, 8.03. C9H13N3O2: N, 21.52; C, 55.37; H, 6.71. Found: N, 21.47; C, 55.80; H, 6.54. C3H6N2O: N, 32.54; C, 41.85; H, 7.08. Found: N, 32.49; C, 42.28; H, 7.25.
4.4. Synthesis of Imidazolidine-2-thione (2)
Following the same procedure of 1, the thiocarbamate adduct was formed by adding an equal molar ratio of CS2 to en (1.49 mmol) in 20 mL DCM, and the reaction was carried out under N2 gas for 3 h (yield 98%). CMPI (1.78 mmol) was added to a suspension of the latter, where a yellow precipitate was obtained after 2 h (yield 77%). The addition of Et3N (4.47 mmol) resulted in the formation of 2 a yellow precipitate, which was separated from the reaction mixture by activation with NaH, bubbling CO2 and acidification with HCl.
4.5. Synthesis of Carbamic Acid and Oxazolidin-2-one (3)
MEA (1.95 mmol) was bubbled with CO2 gas without any solvent for 30 min. Upon adding a mixture of 5.85 mmol of Et3N and 1.95 mmol of CMPI in 5.0 mL of DCM. Oxazolidin-2-one/pyridinoate was collected after stirring the reaction mixture for 1 h under N2 atmosphere. Note: in order to confirm the entity of carbamic versus carbamate in the initial step spectroscopically, MEA (1.95 mmol) was dissolved in 1.0 mL DMSO-d6 and bubbled with CO2 for 30 min. EA (%) calculated for C9H12N2O3: N, 14.28; C, 55.09; H, 6.16. Found: N, 14.24; C, 55.07; H, 6.12.
4.6. Synthesis of Thiocarbamic Acid and Oxazolidine-2-thione (4)
The same procedure used for 3 was followed using CS2 (1.95 mmol) as a thiocarbonylating agent to synthesize 4, where oxazolidine-2-thione/pyridinethione was separated as a yellowish precipitate.
4.7. Synthesis of 1,3-Dioxolan-2-one (5)
EG (1.6 mmol) was activated using Et3N (2.4 mmol) to form the alkoxide, which was bubbled with CO2 and kept stirring for 2 h until a gummy product was obtained, which was identified as the carbonate adduct. Et3N (2.4 mmol) was added to the adduct to deprotonate the other terminus of EG resulting in a clear solution, to which CMPI (1.9 mmol) and 5.0 was added followed by addition of 5.0 mL CH3CN were addd, and the reaction was left under N2 gas for 3 h at RT with continues stirring. The adduct of 5 1,3-Dioxolan-2-one/pyridinoate was collected upon evaporating acetonitrile as a white solid product. EA (%) calculated for C9H11NO4: N, 7.10; C, 54.82; H, 5.62. Found: N, 6.90; C, 54.80; H, 5.59.
4.8. Synthesis of 1,3-Dioxolane-2-thione (6)
The same procedure used for 5 was followed using CS2 (1.60 mmol) as a thiocarbonylating agent to synthesize 6. 1,3-Dioxolane-2-thione/pyridinethione was separated as a yellowish precipitate.
4.9. Synthesis of 1,3-Dithiolan-2-one (7)
Et3N (2.4 mmol) was added to EDT (1.6 mmol) to reduce the disulfide bond involved in the starting material. Afterward, NaH (2.4 mmol) was added to deprotonate EDT. The solution was bubbled with CO2 for 1 h using a needle to produce the sodium triethylammonium carbonothioate adduct. CMPI (1.9 mmol) was added, followed by a consecutive addition of 5.0 mL of CH3CN. After 1 h, 1,3-dithiolan-2-one/pyridinoate was obtained.
4.10. Synthesis of 1,3-Dithiolane-2-thione (8)
The same procedure used for synthesis of 7 was followed, after the reduction with Et3N (2.4 mmol), deprotonation with NaH (2.4 mmol), and reacting with CS2 (1.6 mmol) for 1 h, the carbonotrithioate adduct was produced. Then, CMPI (1.9 mmol) and CH3CN (5.0 mL) were added to the reaction mixture. After stirring for 2 h, 1,3-dithiolane-2-thione/pyridinethione was produced.
Acknowledgments
A.K.Q. and A.F.E. are thankful to Scientific Research and Innovation Support Fund (SRISF), grant number: (BAS/1/5/2021) for funding this work. A.H.S. is thankful to the faculty of graduate studies at HU for partially funding this work.
Glossary
Abbreviations
- CMPI
Mukaiyama reagent
- en
ethylenediamine
- MEA
monoethanolamine
- EG
ethylene glycol
- EDT
ethane-1,2-dithiol
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c01774.
1H/13C NMR and ATR-FTIR spectroscopic data and DFT-optimized structures (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Pal T. K.; De D.; Bharadwaj P. K. Metal-Organic Frameworks for the Chemical Fixation of CO2 into Cyclic Carbonates. Coord. Chem. Rev. 2020, 408, 213173. 10.1016/j.ccr.2019.213173. [DOI] [Google Scholar]
- Kumar A.; Sharma S.. Chemo-Biological Systems for CO2 Utilization; CRC Press, 2020. [Google Scholar]
- van Heek J.; Arning K.; Ziefle M. Reduce, reuse, recycle: Acceptance of CO2-utilization for plastic products. Energy Pol. 2017, 105, 53–66. 10.1016/j.enpol.2017.02.016. [DOI] [Google Scholar]
- Arning K.; Offermann-van Heek J.; Linzenich A.; Kaetelhoen A.; Sternberg A.; Bardow A.; Ziefle M. Same or Different? Insights on Public Perception and Acceptance of Carbon Capture and Storage or Utilization in Germany. Energy Pol. 2019, 125, 235–249. 10.1016/j.enpol.2018.10.039. [DOI] [Google Scholar]
- Xu S.; Carter E. A. 2-Pyridinide as an Active Catalytic Intermediate for CO2 Reduction on p-GaP Photoelectrodes: Lifetime and Selectivity. J. Am. Chem. Soc. 2018, 140, 8732–8738. 10.1021/jacs.8b03774. [DOI] [PubMed] [Google Scholar]
- Kamphuis A. J.; Picchioni F.; Pescarmona P. P. CO2-Fixation into Cyclic and Polymeric Carbonates: Principles and Applications. Green Chem. 2019, 21, 406–448. 10.1039/c8gc03086c. [DOI] [Google Scholar]
- Deacy A. C.; Kilpatrick A. F. R.; Regoutz A.; Williams C. K. Understanding Metal Synergy in Heterodinuclear Catalysts for the Copolymerization of CO2 and Epoxides. Nat. Chem. 2020, 12, 372–380. 10.1038/s41557-020-0450-3. [DOI] [PubMed] [Google Scholar]
- Huang J.; Worch J. C.; Dove A. P.; Coulembier O. Update and Challenges in Carbon Dioxide-Based Polycarbonate Synthesis. ChemSusChem 2020, 13, 469–487. 10.1002/cssc.201902719. [DOI] [PubMed] [Google Scholar]
- Liu H.; Yang P.; Li Z.; Wen Q.; Li X.; Zhu C.; Jiao P.; Zhuang W.; Wu J.; Ying H. Thermodynamics, Characterization, and Polymorphic Transformation of 1,5-Pentanediamine Carbonate. Ind. Eng. Chem. Res. 2020, 59, 10185–10194. 10.1021/acs.iecr.0c00365. [DOI] [Google Scholar]
- Riemer D.; Hirapara P.; Das S. Chemoselective Synthesis of Carbamates using CO2 as Carbon Source. ChemSusChem 2016, 9, 1916–1920. 10.1002/cssc.201600521. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Pan S.-Y.; Li H.; Cai J.; Olabi A. G.; Anthony E. J.; Manovic V. Recent Advances in Carbon Dioxide Utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799. 10.1016/j.rser.2020.109799. [DOI] [Google Scholar]
- Kodaka M.; Tomohiro T.; Lee A. L.; Okuno H. Carbon Dioxide Fixation Forming Oxazolidone Coupled with a Thiol/Fe4S4 Cluster Redox System. Chem. Commun., J. Chem. Soc. 1989, 1479–1481. 10.1039/c39890001479. [DOI] [Google Scholar]
- Matsuda H.; Baba A.; Nomura R.; Kori M.; Ogawa S. Improvement of the Process in the Synthesis of 2-Oxazolidinones from 2-Amino Alcohols and Carbon Dioxide by Use of Triphenylstibine Oxide as Catalyst. Ind. Eng. Chem. Res. 1985, 24, 239–242. 10.1021/i300018a013. [DOI] [Google Scholar]
- Nomura R.; Yamamoto M.; Matsuda H. Preparation of Cyclic Ureas from Carbon Dioxide and Diamines Catalyzed by Triphenylstibine Oxide. Ind. Eng. Chem. Res. 1987, 26, 1056–1059. 10.1021/ie00066a002. [DOI] [Google Scholar]
- Bhanage B. M.; Fujita S.-i.; Ikushima Y.; Arai M. Synthesis of Cyclic Ureas and Urethanes from Alkylene Diamines and Amino Alcohols with Pressurized Carbon Dioxide in the Absence of Catalysts. Green Chem. 2003, 5, 340–342. 10.1039/b300778b. [DOI] [Google Scholar]
- Da Silva E.; Dayoub W.; Mignani G.; Raoul Y.; Lemaire M. Propylene Carbonate Synthesis from Propylene Glycol, Carbon Dioxide and Benzonitrile by Alkali Carbonate Catalysts. Catal. Commun. 2012, 29, 58–62. 10.1016/j.catcom.2012.08.030. [DOI] [Google Scholar]
- Vieville C.; Yoo J. W.; Pelet S.; Mouloungui Z. Synthesis of Glycerol Carbonate by Direct Carbonatation of Glycerol in Supercritical CO2 in the Presence of Zeolites and Ion Exchange Resins. Catal. Lett. 1998, 56, 245–247. 10.1023/a:1019050205502. [DOI] [Google Scholar]
- McGuire T. M.; López-Vidal E. M.; Gregory G. L.; Buchard A. Synthesis of 5- to 8-membered cyclic carbonates from diols and CO2: A one-step, atmospheric pressure and ambient temperature procedure. J. CO2 Util. 2018, 27, 283–288. 10.1016/j.jcou.2018.08.009. [DOI] [Google Scholar]
- Gregory G. L.; Ulmann M.; Buchard A. Synthesis of 6-membered cyclic carbonates from 1,3-diols and low CO2 pressure: a novel mild strategy to replace phosgene reagents. RSC Adv. 2015, 5, 39404–39408. 10.1039/c5ra07290e. [DOI] [Google Scholar]
- Wu Y.; Yang Y.-Q.; Hu Q. A Facile Access to Chiral 4-Isopropyl-, 4-Benzyl-, and 4-Phenyloxazolidine-2-Thione. J. Org. Chem. 2004, 69, 3990–3992. 10.1021/jo049799d. [DOI] [PubMed] [Google Scholar]
- Ortiz A.; Sansinenea E. The Synthetic Versatility of Oxazolidinethiones. J. Sulfur Chem. 2007, 28, 109–147. 10.1080/17415990601139699. [DOI] [Google Scholar]
- Delaunay D.; Toupet L.; Corre M. L. Reactivity of .beta.-Amino Alcohols with Carbon Disulfide Study on the Synthesis of 2-Oxazolidinethiones and 2-Thiazolidinethiones. J. Org. Chem. 1995, 60, 6604–6607. 10.1021/jo00125a059. [DOI] [Google Scholar]
- Puschin N. A.; Mitić R. V. Über die Verbindungen des Phosgens mit Hexamethylentetramin, m-Toluidin und Äthylendiamin. Ann. Chem. 1937, 532, 300. 10.1002/jlac.19375320125. [DOI] [Google Scholar]
- Schweitzer C. E. Ethyleneurea. I. Synthesis from Urea and Ethylenediamine. J. Org. Chem. 1950, 15, 471–474. 10.1021/jo01149a005. [DOI] [Google Scholar]
- lose W. J. Anticonvulsant Drugs. IV. Some 2-Oxazolidones1. J. Am. Chem. Soc. 1951, 73, 95–98. 10.1021/ja01145a035. [DOI] [Google Scholar]
- More G. S.; Srivastava R. Synthesis of amino alcohols, cyclic urea, urethanes, and cyclic carbonates and tandem one-pot conversion of an epoxide to urethanes using a Zn-Zr bimetallic oxide catalyst. Sustain. Energy Fuels 2021, 5, 1498–1510. 10.1039/d0se01912g. [DOI] [Google Scholar]
- Jin S.-J.; Khan Y.; Maeng J. H.; Kim Y. J.; Hwang J.; Cheong M.; Lee J. S.; Kim H. S. Efficient Catalytic Systems for the Carboxylation of Diamines to Cyclic Ureas Using Ethylene Urea as a Promoter. Appl. Catal., B 2017, 209, 139–145. 10.1016/j.apcatb.2017.02.079. [DOI] [Google Scholar]
- Hwang J.; Han D.; Oh J. J.; Cheong M.; Koo H.-J.; Lee J. S.; Kim H. S. Efficient Non-Catalytic Carboxylation of Diamines to Cyclic Ureas Using 2-Pyrrolidone as a Solvent and a Promoter. Adv. Synth. Catal. 2019, 361, 297–306. 10.1002/adsc.201800945. [DOI] [Google Scholar]
- Bogatskii A. V.; Luk’yanenko N. G.; Kirichenko T. I. Cyclic thioureas (review). Chem. Heterocycl. Compd. 1983, 19, 577–589. 10.1007/bf00523064. [DOI] [Google Scholar]
- Denk M. K.; Gupta S.; Brownie J.; Tajammul S.; Lough A. J. C–H Activation with Elemental Sulfur: Synthesis of Cyclic Thioureas from Formaldehyde Aminals and S8. Chem.—Eur. J. 2001, 7, 4477–4486. . [DOI] [PubMed] [Google Scholar]
- Paas M.; Wibbeling B.; Fröhlich R.; Hahn F. E. Silver and Rhodium Complexes of Stable, Monomeric Imidazolidin-2-ylidenes: Synthesis, Reactivity and Decomposition Pathway. Eur. J. Inorg. Chem. 2006, 158–162. 10.1002/ejic.200500777. [DOI] [Google Scholar]
- Tan W.; Wei J.; Jiang X. Thiocarbonyl Surrogate via Combination of Sulfur and Chloroform for Thiocarbamide and Oxazolidinethione Construction. Org. Lett. 2017, 19, 2166–2169. 10.1021/acs.orglett.7b00819. [DOI] [PubMed] [Google Scholar]
- Tomishige K.; Yasuda H.; Yoshida Y.; Nurunnabi M.; Li B.; Kunimori K. Novel Route to Propylene Carbonate: Selective Synthesis from Propylene Glycol and Carbon Dioxide. Catal. Lett. 2004, 95, 45–49. 10.1023/b:catl.0000023720.39110.4e. [DOI] [Google Scholar]
- Tomishige K.; Tamura M.; Nakagawa Y. CO2 Conversion with Alcohols and Amines into Carbonates, Ureas, and Carbamates over CeO2 Catalyst in the Presence and Absence of 2-Cyanopyridine. Chem. Rec. 2019, 19, 1354–1379. 10.1002/tcr.201800117. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Sun N.; Wang S.; Li F.; Wang Y. Synthesis of Propylene Carbonate from Carbon Dioxide and 1,2-Propylene Glycol over Zinc Acetate Catalyst. Ind. Eng. Chem. Res. 2008, 47, 1365–1369. 10.1021/ie070789n. [DOI] [Google Scholar]
- Du Y.; He L.-N.; Kong D.-L. Magnesium-Catalyzed Synthesis of Organic Carbonate from 1,2-Diol/Alcohol and Carbon Dioxide. Catal. Commun. 2008, 9, 1754–1758. 10.1016/j.catcom.2008.02.004. [DOI] [Google Scholar]
- Huang S.; Ma J.; Li J.; Zhao N.; Wei W.; Sun Y. Efficient Propylene Carbonate Synthesis from Propylene Glycol and Carbon Dioxide via Organic Bases. Catal. Commun. 2008, 9, 276–280. 10.1016/j.catcom.2007.06.008. [DOI] [Google Scholar]
- Wu L.-X.; Wang H.; Tu Z.-Y.; Ding B.-B.; Xiao Y.; Lu J.-X. Synthesis of Cyclic Carbonates from CO2 and Diols via Electrogenerated N-Heterocyclic Carbenes. Int. J. Electrochem. Sci. 2012, 7, 11540–11549. [Google Scholar]
- Cucinell S. A.; Arsenal E. Review of the Toxicity of Long-Term Phosgene Exposure. Arch. Environ. Health: Int. J. 1974, 28, 272–275. 10.1080/00039896.1974.10666485. [DOI] [PubMed] [Google Scholar]
- Bengtström L.; Salden M.; Stec A. A. The Role of Isocyanates in Fire Toxicity. Fire Sci. Rev. 2016, 5, 4. 10.1186/s40038-016-0013-2. [DOI] [Google Scholar]
- Bowonder B. The Bhopal Incident: Implications for Developing Countries. Environ. Syst. Decis. 1985, 5, 89–103. 10.1007/bf02235978. [DOI] [Google Scholar]
- Dunlap K. L.Phosgene. Encyclopedia of Polymer Science and Technology; Wiley, 2002; Vol. 3. [Google Scholar]
- A Li W.; Jie X.; Wang C.; Dilworth J. R.; Xu C.; Xiao T.; Edwards P. P. MnOx-Promoted, Coking-Resistant Nickel-Based Catalysts for Microwave-Initiated CO2 Utilization. Ind. Eng. Chem. Res. 2020, 59, 6914–6923. 10.1021/acs.iecr.9b06558. [DOI] [Google Scholar]; B Qaroush A. K.; Alsayyed A. W.; Eftaiha A. F.; Al-Qaisi F. M.; Salameh B. A. Green Microwave-Assisted Synthesis of Cyclic/Acyclic Ureas from Propylene Carbonate. ChemistrySelect 2022, 7 (20), e202200478 10.1002/slct.202200478. [DOI] [Google Scholar]
- Bahadori M.; Marandi A.; Tangestaninejad S.; Moghadam M.; Mirkhani V.; Mohammadpoor-Baltork I. Ionic Liquid-Decorated MIL-101(Cr) via Covalent and Coordination Bonds for Efficient Solvent-Free CO2 Conversion and CO2 Capture at Low Pressure. J. Phys. Chem. C 2020, 124, 8716–8725. 10.1021/acs.jpcc.9b11668. [DOI] [Google Scholar]
- Paz J.; Pérez-Balado C.; Iglesias B.; Muñoz L. Carbon Dioxide as a Carbonylating Agent in the Synthesis of 2-Oxazolidinones, 2-Oxazinones, and Cyclic Ureas: Scope and Limitations. J. Org. Chem. 2010, 75, 3037–3046. 10.1021/jo100268n. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Yang S.; Ebrahimiasl S.; Arshadi S.; Hosseinian A. Synthesis of six-membered cyclic carbamates employing CO2 as building block: A review. J. CO2 Util. 2019, 33, 37–45. 10.1016/j.jcou.2019.05.004. [DOI] [Google Scholar]
- Niemi T.; Repo T. Antibiotics from Carbon Dioxide: Sustainable Pathways to Pharmaceutically Relevant Cyclic Carbamates. Eur. J. Org. Chem. 2019, 1180–1188. 10.1002/ejoc.201801598. [DOI] [Google Scholar]
- Barghash R. F.; Massi A.; Dondoni A. Synthesis of thiourea-tethered C-glycosyl amino acids via isothiocyanate-amine coupling. Org. Biomol. Chem. 2009, 7, 3319–3330. 10.1039/b908156a. [DOI] [PubMed] [Google Scholar]
- Novosjolova I. The Mukaiyama Reagent: An Efficient Condensation Agent. Synlett 2013, 24, 135–136. 10.1055/s-0032-1317530. [DOI] [Google Scholar]
- Shapiro J.; Sonberg J.; Schafer B.; Williams C.; Ferris H.; Reinheimer E.; Van Wynsberghe A.; Kriley C.; Majireck M. Synthesis, Characterization, and Computational Modeling of N-(1-Ethoxyvinyl)pyridinium Triflates, an Unusual Class of Pyridinium Salts. Molecules 2018, 23, 413. 10.3390/molecules23020413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaiyama T.; Usui M.; Shimada E.; Saigo K. A Convenient Method for the Synthesis of Carboxylic Esters. Chem. Lett. 1975, 4, 1045–1048. 10.1246/cl.1975.1045. [DOI] [Google Scholar]
- Mukaiyama T. New Synthetic Reactions Based on the Onium Salts of Aza-Arenes [New synthetic methods (29)]. Angew. Chem., Int. Ed. Engl. 1979, 18, 707–721. 10.1002/anie.197907073. [DOI] [Google Scholar]
- Zhao H.; Song Z.; Cowins J.; Olubajo O. Microwave-Assisted Esterification of N-Acetyl-L-Phenylalanine Using Modified Mukaiyama’s Reagents: A New Approach Involving Ionic Liquids. Int. J. Mol. Sci. 2008, 9, 33–44. 10.3390/ijms9010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk R.; Abelman M.; Jellison K. Generation of Ketenes from Carboxylic Acids Using the Mukaiyama Reagent (1-Methyl-2-Chloropyridinium Iodide). Synlett 1989, 1989, 36–37. 10.1055/s-1989-34706. [DOI] [Google Scholar]
- Morrill L. C.; Smith A. D. Organocatalytic Lewis Base Functionalisation of Carboxylic Acids, Esters and Anhydrides via C1-Ammonium or Azolium Enolates. Chem. Soc. Rev. 2014, 43, 6214–6226. 10.1039/c4cs00042k. [DOI] [PubMed] [Google Scholar]
- Mukaiyama T.; Usui M.; Saigo K. The Facile Synthesis of Lactones. Chem. Lett. 1976, 5, 49–50. 10.1246/cl.1976.49. [DOI] [Google Scholar]
- Motozaki T.; Sawamura K.; Suzuki A.; Yoshida K.; Ueki T.; Ohara A.; Munakata R.; Takao K.-i.; Tadano K.-i. Total Synthesis of (+)-Tubelactomicin A. 2. Synthesis of the Upper-Half Segment and Completion of the Total Synthesis. Org. Lett. 2005, 7, 2265–2267. 10.1021/ol050763x. [DOI] [PubMed] [Google Scholar]
- Huang H.; Iwasawa N.; Mukaiyama T. A Convenient Method for the Construction of β-lactam Compounds from β-amino Acids using 2-Chloro-1-Methylpyridinium Iodide as Condensing Reagent. Chem. Lett. 1984, 13, 1465–1466. 10.1246/cl.1984.1465. [DOI] [Google Scholar]
- Teulade-Fichou M.-P.; Vandromme L.; Monchaud D. Beneficial Effect of Mukaiyama Reagent on Macrobislactamization Reactions. Synlett 2006, 3423–3426. 10.1055/s-2006-956483. [DOI] [Google Scholar]
- Rashidi M.; Islami M. R.; Esmaeili-Mahani S. Design and Stereoselective Synthesis of Novel β-lactone and β-lactams as Potent Anticancer Agents on Breast Cancer Cells. Tetrahedron 2018, 74, 835–841. 10.1016/j.tet.2017.12.044. [DOI] [Google Scholar]
- Bionda N.; Pitteloud J.-P.; Pitteloud J.-P.; Cudic P. Solid-phase Synthesis of Fusaricidin/LI-F Class of Cyclic Lipopeptides: Guanidinylation of Resin-Bound Peptidyl Amines. Pept. Sci. 2013, 100, 160–166. 10.1002/bip.22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.; Luo G. Facile Synthesis of Acyl Sulfonamides from Carboxyic Acids Using the Mukaiyama Reagent. Tetrahedron Lett. 2019, 60, 268–271. 10.1016/j.tetlet.2018.12.030. [DOI] [Google Scholar]
- Kong K. H.; Tan C. K.; Lin X.; Lam Y. A Versatile Thiouronium-Based Solid-Phase Synthesis of 1,3,5-Triazines. Chem. Eur. 2012, 18, 1476–1486. 10.1002/chem.201102097. [DOI] [PubMed] [Google Scholar]
- Mokhtari B.; Azadi R.; Mardani E. 2-Chloro-1-Methylpyridinium Iodide, an Efficient Reagent for the Conversion of Alcohols into Alkyl Thiocyanates Both under Solvent and Solvent-Free Conditions. Tetrahedron Lett. 2012, 53, 491–493. 10.1016/j.tetlet.2011.11.050. [DOI] [Google Scholar]
- Van der Plas S. E.; Kelgtermans H.; De Munck T.; Martina S. L. X.; Dropsit S.; Quinton E.; De Blieck A.; Joannesse C.; Tomaskovic L.; Jans M.; Christophe T.; van der Aar E.; Borgonovi M.; Nelles L.; Gees M.; Stouten P.; Van Der Schueren J.; Mammoliti O.; Conrath K.; Andrews M. Discovery of N-(3-Carbamoyl-5,5,7,7-Tetramethyl-5,7-Dihydro-4H-Thieno[2,3-c]Pyran-2-Yl)-LH-Pyrazole-5-Carboxamide (GLPG1837), a Novel Potentiator Which Can Open Class III Mutant Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Channels to a High Extent. J. Med. Chem. 2018, 61, 1425–1435. 10.1021/acs.jmedchem.7b01288. [DOI] [PubMed] [Google Scholar]
- Ištuk Z. M.; Vujasinovi I.; Čikoš A.; Kragol G. Regioselective 2-Imino-1, 3-thiazolidine vs. 2-Imino-1, 3-oxazolidine Formation from the Vicinal Sec-Amino Alcohol of Desosamine. Eur. J. Org. Chem. 2013, 4666–4673. 10.1002/ejoc.201300266. [DOI] [Google Scholar]
- Eftaiha A. F.; Alsoubani F.; Assaf K. I.; Troll C.; Rieger B.; Khaled A. H.; Qaroush A. K. An Investigation of Carbon Dioxide Capture by Chitin Acetate/DMSO Binary System. Carbohydr. Polym. 2016, 152, 163–169. 10.1016/j.carbpol.2016.06.092. [DOI] [PubMed] [Google Scholar]
- Qaroush A. K.; Alshamaly H. S.; Alazzeh S. S.; Abeskhron R. H.; Assaf K. I.; Eftaiha A. F. Inedible Saccharides: A Platform for CO2 Capturing. Chem. Sci. 2018, 9, 1088–1100. 10.1039/c7sc04706a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftaiha A. F.; Alsoubani F.; Assaf K. I.; Nau W. M.; Troll C.; Qaroush A. K. Chitin-acetate/DMSO as a Supramolecular Green CO2-phile. RSC Adv. 2016, 6, 22090–22093. 10.1039/c6ra03022j. [DOI] [Google Scholar]
- Qaroush A. K.; Assaf K. I.; Bardaweel S. K.; Al-Khateeb A. a.; Alsoubani F.; Al-Ramahi E.; Masri M.; Brück T.; Troll C.; Rieger B.; Eftaiha A. F. Chemisorption of CO2 by Chitosan Oligosaccharide/DMSO: Organic Carbamato-Carbonato Bond Formation. Green Chem. 2017, 19, 4305–4314. 10.1039/c7gc01830d. [DOI] [Google Scholar]
- Eftaiha A. F.; Qaroush A. K.; Alsoubani F.; Pehl T. M.; Troll C.; Rieger B.; Al-Maythalony B. A.; Assaf K. I. A Green Sorbent for CO2 Capture: α-Cyclodextrin-based Carbonate in DMSO solution. RSC Adv. 2018, 8, 37757–37764. 10.1039/c8ra08040b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftaiha A. F.; Qaroush A. K.; Abu-Daabes M. A.; Alsyouri H. M.; Assaf K. I. New Metrics of Green Sorbents for CO2 Capturing. Adv. Sustainable Syst. 2020, 4, 1900121. 10.1002/adsu.201900121. [DOI] [Google Scholar]
- Assaf K. I.; Qaroush A. K.; Mustafa F. M.; Alsoubani F.; Pehl T. M.; Troll C.; Rieger B.; Eftaiha A. F. Biomaterials for CO2 Harvesting: From Regulatory Functions to Wet Scrubbing Applications. ACS Omega 2019, 4, 11532–11539. 10.1021/acsomega.9b00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftaiha A. F.; Mustafa F. M.; Alsoubani F.; Assaf K. I.; Qaroush A. K. A Catecholamine Neurotransmitter: Epinephrine as a CO2 Wet Scrubbing Agent. Chem. Commun. 2019, 55, 3449–3452. 10.1039/c8cc09572h. [DOI] [PubMed] [Google Scholar]
- A Eftaiha A. F.; Qaroush A. K.; Okashah I. K.; Alsoubani F.; Futter J.; Troll C.; Rieger B.; Assaf K. I. CO2 activation through C-N, C-O and C-C bond formation. Phys. Chem. Chem. Phys. 2020, 22, 1306–1312. 10.1039/c9cp05961j. [DOI] [PubMed] [Google Scholar]; B Qaroush A. K.; Assaf K. I.; Al-Khateeb A.; Alsoubani F.; Nabih E.; Troll C.; Rieger B.; Eftaiha A. F. Pentaerythritol-Based Molecular Sorbent for CO2 Capturing: A Highly Efficient Wet Scrubbing Agent Showing Proton Shuttling Phenomenon. Energy Fuels 2017, 31, 8407–8414. 10.1021/acs.energyfuels.7b01125. [DOI] [Google Scholar]; C Eftaiha A. F.; Qaroush A. K.; Al-shami B. O.; Assaf K. I. Chemisorption of CO2 by Diamine-Tetraamido Macrocyclic Motifs: A Theoretical Study. Org. Biomol. Chem. 2021, 19, 3873–3881. 10.1039/d1ob00180a. [DOI] [PubMed] [Google Scholar]
- A Eftaiha A. F.; Qaroush A. K.; Alsayyed A. W.; Alsoubani F.; Assaf K. I. The Eternal Battle to Combat Global Warming:(Thio) Urea as a CO2 Wet Scrubbing Agent. Phys. Chem. Chem. Phys. 2020, 22, 11829. 10.1039/d0cp00629g. [DOI] [PubMed] [Google Scholar]; B Eftaiha A. F.; Qaroush A. K.; Assaf K. I.; Alsoubani F.; Markus Pehl T.; Troll C.; El-Barghouthi M. I. Bis-tris propane in DMSO as a Wet Scrubbing Agent: Carbamic Acid as a Sequestered CO2 Species. New J. Chem. 2017, 41, 11941–11947. 10.1039/c7nj02130e. [DOI] [Google Scholar]; C Qaroush A. K.; Saleh M. I.; Alsyouri H. M.; Abu-Daabes M. A.; Eftaiha A. F.; Assaf K. I.; Abu-Zaid R.; Abu-Surrah A. S.; Troll C.; Rieger B. In Situ Activation of Green Sorbents for CO2 Capture upon End Group Backbiting. Phys Chem Chem Phys 2022, 24 (20), 12293–12299. 10.1039/D2CP00837H. [DOI] [PubMed] [Google Scholar]
- Eftaiha A. F.; Qaroush A. K.; Hasan A. K.; Assaf K. I.; Melhem M.; Maythalony B.; Usman M. Cross-Linked, Porous Imidazolium-Based Poly (Ionic Liquid)s for CO2 Capture and Utilisation. New J. Chem. 2021, 45, 16452. 10.1039/d1nj02946k. [DOI] [Google Scholar]
- A Qaroush A. K.; Hasan A. K.; Hammad S. B.; Assaf K. I.; Alsoubani F.; Eftaiha A. F. Mechanistic Insights for CO2 Utilization Using Sustainable Catalysis. New J. Chem 2021, 45, 22280. 10.1039/d1nj04757d. [DOI] [Google Scholar]; B Eftaiha A. F.; Qaroush A. K.; Hasan A. K.; Helal W.; Al-Qaisi F. M. CO2 Fixation into Cyclic Carbonates Catalyzed by Single-Site Aprotic Organocatalysts. React. Chem. Eng. 2022, 10.1039/D2RE00157H. [DOI] [Google Scholar]
- A Qaroush A. K.; Alsoubani F. A.; Al-Khateeb A. M.; Nabih E.; Al-Ramahi E.; Khanfar M. F.; Assaf K. I.; Eftaiha A. a. F. An Efficient Atom-Aconomical Chemoselective CO2 Cycloaddition using Lanthanum Oxide/Tetrabutyl Ammonium Bromide. Sustain. Energy Fuels 2018, 2, 1342–1349. 10.1039/c8se00092a. [DOI] [Google Scholar]; B Al-Qaisi F. M.; Qaroush A. K.; Smadi A. H.; Alsoubani F.; Assaf K. I.; Repo T.; Eftaiha A. F. CO2 Coupling with Epoxides Catalysed by using One-Oot Synthesised, in situ Activated Zinc Ascorbate under Ambient Conditions. Dalton Trans. 2020, 49, 7673–7679. 10.1039/d0dt01329c. [DOI] [PubMed] [Google Scholar]
- Assaf K. I.; Qaroush A. K.; Okashah I. K.; Al-Qaisi F. a. M.; Alsoubani F.; Eftaiha A. F. Activation of β-diketones for CO2 Capture and Utilization. React. Chem. Eng. 2021, 6, 2364–2375. 10.1039/d1re00278c. [DOI] [Google Scholar]
- Sunjuk M.; Abu-Surrah A. S.; Al-Ramahi E.; Qaroush A. K.; Saleh A. Selective Coupling of Carbon Dioxide and Epoxystyrene via Salicylaldimine-, Thiophenaldimine-, and Quinolinaldimine-Iron(II), Iron(III), Chromium(III), and Cobalt(III)/Lewis Base Catalysts. Transition Met. Chem. 2013, 38, 253–257. 10.1007/s11243-012-9685-1. [DOI] [Google Scholar]
- Hwang J.; Han D.; Oh J. J.; Cheong M.; Koo H.-J.; Lee J. S.; Kim H. S. Efficient Non-Catalytic Carboxylation of Diamines to Cyclic Ureas Using 2-Pyrrolidone as a Solvent and a Promoter. Adv. Synth. Catal. 2019, 361, 297–306. 10.1002/adsc.201800945. [DOI] [Google Scholar]
- Kortunov P. V.; Siskin M.; Paccagnini M.; Thomann H. CO2 Reaction Mechanisms with Hindered Alkanolamines: Control and Promotion of Reaction Pathways. Energy Fuels 2016, 30, 1223–1236. 10.1021/acs.energyfuels.5b02582. [DOI] [Google Scholar]
- Budhram R. S.; Uff B. C.; Jones R. A.; Jones R. O. 13C NMR spectra of 2,3-dihydro-1H-pyrrolo[1,2-c]imidazol-1,3-dione and its thione analogues. Org. Magn. Reson. 1980, 13, 89–91. 10.1002/mrc.1270130203. [DOI] [Google Scholar]
- Delaere D.; Raspoet G.; Nguyen M. T. Thiol–Thione Tautomerism in Thioformic Acid: Importance of Specific Solvent Interactions. J. Phys. Chem. A 1999, 103, 171–177. 10.1021/jp983298c. [DOI] [Google Scholar]
- Kortunov P. V.; Siskin M.; Baugh L. S.; Calabro D. C. In Situ Nuclear Magnetic Resonance Mechanistic Studies of Carbon Dioxide Reactions with Liquid Amines in Non-Aqueous Systems: Evidence for the Formation of Carbamic Acids and Zwitterionic Species. Energy Fuels 2015, 29, 5940–5966. 10.1021/acs.energyfuels.5b00985. [DOI] [Google Scholar]
- Yang D.; Lv M.; Chen J. Efficient non-aqueous solvent formed by 2-piperidineethanol and ethylene glycol for CO2 absorption. Chem. Commun. 2019, 55, 12483–12486. 10.1039/c9cc06320j. [DOI] [PubMed] [Google Scholar]
- Dmitrenko O.; Thorpe C.; Bach R. D. Mechanism of SN2 Disulfide Bond Cleavage by Phosphorus Nucleophiles. Implications for Biochemical Disulfide Reducing Agents. J. Org. Chem. 2007, 72, 8298–8307. 10.1021/jo071271w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. S.; Trethewey K. R. Photosensitised Oxidation of Amines: Mechanism of Oxidation of Triethylamine. J. Chem. Soc., Perkin Trans. 2 1977, 173–178. 10.1039/p29770000173. [DOI] [Google Scholar]
- Yamada H. Comparison of Solvation Effects on CO2 Capture with Aqueous Amine Solutions and Amine-Functionalized Ionic Liquids. J. Phys. Chem. B 2016, 120, 10563–10568. 10.1021/acs.jpcb.6b07860. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; et al. Gaussian 09; Gaussian. Inc.: Wallingford CT, 2009, Vol. 121, pp 150–166.
- Özdemir N.; Türkpençe D. Theoretical Investigation of Thione-Thiol Tautomerism, Intermolecular Double Proton Transfer Reaction and Hydrogen Bonding Interactions in 4-Ethyl-5-(2-Hydroxyphenyl)-2H-1, 2, 4-Triazole-3 (4H)-Thione. Comput. Theor. Chem. 2013, 1025, 35–45. 10.1016/j.comptc.2013.10.001. [DOI] [Google Scholar]
- Huheey J. E.; Keiter E. A.; Keiter R. L.. Inorganic Chemistry: Principles of Structure and Reactivity; HarperCollins College Publishers: New York, NY, 1993. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.










