Abstract
Objective:
Obesity is associated with consumption of a western diet low in dietary fiber, while prebiotics reduce body weight. Fiber induces short-chain fatty acid (SCFA) production, and SCFA administration is beneficial to host metabolic homeostasis. However, the role of endogenous SCFA signaling in development of obesity is contentious. Therefore, the primary objective of this study is to evaluate the postprandial timecourse of short chain fatty acid (SCFA) production and uptake in healthy (chow), western diet-fed obese (HFD), and oligofructose-treated HFD-fed (HFD+OFS) rats.
Methods:
Male Sprague-Dawley rats were maintained on chow or HFD for 5 weeks, with or without supplementation of 10% OFS for 3 weeks. SCFAs were measured in the ileum, cecum, colon, portal vein and vena cava at 0, 2, 4, 6, and 8h postprandially.
Results:
Postprandial cecal and portal vein SCFAs were decreased in obese rats compared to lean chow controls, while no differences were observed in fasting SCFA concentrations. OFS supplementation increased SCFA levels in the cecum and portal vein during obesity. Butyrate levels were positively associated with portal GLP-1 and adiposity and with Roseburia relative abundance.
Conclusions:
The current study demonstrates that obesity is associated with reduced SCFA production, and that OFS supplementation increases SCFA levels. Additionally, postprandial butyrate production appears to be beneficial to host energy homeostasis.
Introduction
Increased consumption of a highly palatable “Western diet” that is characteristically high in fat and sugar and low in dietary fiber is a salient contributor to the current obesity epidemic (1). Increased overall fiber consumption leads to weight loss in individuals with obesity, and more specific dietary fiber treatments, like the inulin-type fructan oligofructose, decreases body weight and adiposity and improves glucose homeostasis via a gut microbiota-dependent mechanism (2). It is now generally accepted that the gut microbiota plays a role in metabolic homeostasis and metabolic disorders, as diabetes and obesity are associated with unique gut microbiome signatures (3), while both weight loss surgeries and glucose-lowering medications, like metformin, are known to beneficially shift the gut microbiota (4, 5).
Microbially-derived metabolites represent a potential mediator between diet, the gut microbiome, and host metabolic homeostasis (6). Among the many metabolites generated by the gut microbiome, short-chain fatty acids (SCFAs), mainly acetate, butyrate, and propionate, are some of the most well studied, given that they are microbial breakdown products of nondigestible carbohydrates and can be utilized by the host (7). Exogenous administration of either individual SCFAs, or a mixed cocktail, improves metabolic homeostasis in obese or HFD-fed rodents (8, 9). Although the mechanisms are not fully understood, SCFAs induce enteroendocrine cells (EECs) to release gut peptides known to influence food intake and glucose homeostasis, like GLP-1 and peptide YY (PYY) via activation of SCFA receptors free fatty acid receptor 2 (FFAR2, also known as GPR43) and free fatty acid receptor 3 (FFAR3, also known as GPR41) (10, 11). Despite this, the impact of endogenous SCFA production on host metabolic health is contentious. Some research indicates that SCFA levels are increased in individuals with obesity and decreased following weight loss (7), highlighting that production of SCFAs from the gut microbiota increases energy harvest from the diet. In line with this, glucose-stimulated insulin secretion, hyperphagia, and obesity during HFD feeding in mice was linked to increased circulating acetate levels (12). However, at least one study has found a decrease in fecal SCFAs in individuals with obesity (13) and type 2 diabetes (14), in accordance with the observed beneficial effects of exogenous SCFA administration (8, 9). The major issue with these studies is the methodology of measuring SCFA production. Many studies focus on fecal SCFA concentrations, which are likely a reflection of SCFAs that were produced but not absorbed. Additionally, these studies measure SCFA concentrations following an extended fast or without regard to meal consumption, and therefore cannot reliably assess the endogenous production of SCFAs (7, 15, 16, 17). Taken together, inconsistencies in the timing and location of sample collection limit the implications of the current body of evidence and underscore a need to fully characterize the postprandial production of SCFAs over time to develop a more accurate and standardized approach to measuring SCFA concentrations within studies. Furthermore, these discrepancies in sampling methods make it difficult to determine whether SCFAs are associated with metabolic disease or with the potential success of prebiotic dietary interventions.
Therefore, in the present study, we aimed to evaluate the postprandial timecourse of acetate, butyrate, and propionate production in both the cecum and colon, as well as in the hepatic portal vein and systemic circulation, in both lean and HFD-induced obese rats. This comprehensive study design aims to determine SCFA kinetics following a meal, providing valuable insight into the production and absorption of postprandial SCFAs, which has never been assessed. Further, compared to previous studies that have examined mostly fasting and fecal SCFA levels (7, 16, 18), this study provides a much more accurate and complete depiction of SCFA levels during obesity. Lastly, while previous research has demonstrated that prebiotic fibers, like inulin and oligofructose, improve energy and glucose homeostasis in both rodents and humans (19), production of SCFAs during oligofructose treatment has not been thoroughly examined.
Materials and Methods
Ethics statement.
All rats were housed and maintained in accordance with the University of Arizona Institutional Animal Care and Use Committee (IACUC). All protocols were approved by the IACUC.
Animal model and diets.
Male Sprague-Dawley rats (8–9 weeks old) were purchased from Charles River Laboratories (Wilmington). Rats were cohoused and maintained on a 12-h light/dark cycle with ad libitum chow (ENVIGO 2018 Teklad global 18% protein rodent diets), high fat diet (HFD; Research Diets D12451) or HFD with 10% oligofructose (HFD+OFS; BENEO-Orafti P95) in the drinking water. Dietary nutrient distribution and ingredients can be found in Table S1. OFS drinking water was made fresh 3x per week.
SCFA timecourse diet maintenance and terminal study.
Rats were weighed and randomly assigned to either chow (n=30) or HFD (n=61) treatment groups (t = 0 weeks) and maintained on these diets for 5 weeks. At t = 5 weeks, rats were weighed, and fat mass measured via an EchoMRI™ Body Composition Analyzer (Echo Medical Systems). HFD rats were randomly assigned to either receive OFS in their drinking water (n=31) or not (HFD; n=30). Rats were maintained on these diets for the remaining 3 weeks (Fig. 1a). At t = 8 weeks, overnight fasting body weight, fat mass, and blood were collected two days before terminal study. Adiposity was calculated as a proportion of fat mass to total body weight. Rats were randomly assigned for terminal postprandial tissue collections at timepoints 0, 2, 4, 6, and 8h post refeeding (n=4–6 per group, per timepoint). For the refeeding experiment, rats were overnight fasted, and the following morning given an isocaloric meal of their respective diet, then sacrificed according to their timepoint post refeeding. Rats were deeply anesthetized via isoflurane inhalation. Tissue collections included liver, visceral adipose, as well as ileum and colon mucosal scrapings and luminal intestinal contents from the ileum, cecum, and colon, that were quickly snap frozen and stored at −80°C. Plasma of portal vein and vena cava blood was collected with a DPPIV inhibitor cocktail (Millipore Sigma) and stored at −20°C.
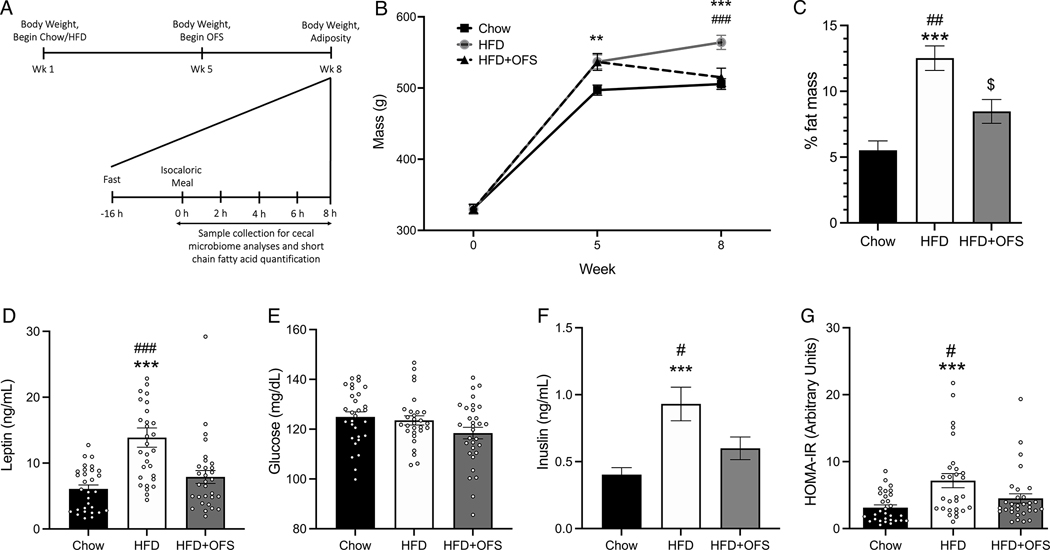
Figure 1. OFS attenuates HFD-induced body weight gain, adiposity, and dysregulated glucose homeostasis.
(A) Schematic of experimental design. (B) Body weight over time in chow (n=30), HFD (n=30), and HFD+OFS (n=31) rats. (C) % fat mass at 8 weeks. (D) Fasting plasma leptin, (E) glucose, (F) insulin levels at 8 weeks. (G) Calculated HOMA-IR. **p<0.01 chow vs HFD; ***p<0.001 chow vs HFD; #p<0.05 HFD vs HFS+OFS; ##p<0.01 HFD vs HFS+OFS; ###p<0.001 HFD vs HFS+OFS; $p<0.01 chow vs HFD+OFS. All data are presented as mean ± SEM.
SCFA collection and quantification.
Luminal intestinal contents and blood samples were sent to Metabolon Inc for SCFA extraction via bead beating and subsequent measurement of SCFA concentrations on a high-performance liquid chromatography (LC)-tandem mass-spectrometry (MS/MS) platform as previously described (20). Samples were analyzed in 96-well plates alongside two calibration curves per plate, as well as eight quality control samples for fecal samples and six quality control samples for plasma.
Measurement of plasma blood hormones.
Plasma insulin (Thermo Fisher Scientific), leptin (Millipore Sigma) and total portal vein plasma GLP-1 (Millipore Sigma) were all measured via ELISA according to the manufacturer’s protocol.
RNA isolation and qRT-PCR.
RNA isolation was performed for liver, ileal, and colon tissues using the PureLink™ RNA Mini Kit (Ambion) and for adipose by RNeasy Lipid Tissue Mini Kit (Qiagen) per the manufacturer’s protocol as previously described (4). cDNA synthesis was performed with SuperScript™ IV VILO™ Master Mix with ezDNase™ Enzyme (Invitrogen) per the manufacturer’s instruction with 500 ng RNA. qRT-PCR was performed with CFX96 Touch™ Real-Time PCR Detection System (BioRad Laboratories) using TaqMan™ Gene Expression Assays (ThermoFischer Scientific) for rat Ffar2 (Rn02345824_s1), Ffar3 (Rn02345824_s1), and 18s ribosomal RNA (Rn03928990_g1).
Microbiome sequencing and PICRUSt2 and SCFA correlation.
Cecal microbiome of each animal at all timepoints (0, 2, 4, 6, and 8h following meal consumption) was assessed based on the V4 fragment of 16S rRNA gene as published previously (21). Mechanical disruption of samples was performed with the high performance, high throughput TissueLyser II (MO BIO Laboratories) at 4 degrees Celsius, 2×10 minutes at 20Hz speed. Total DNA from cecal samples was purified using the QIAamp PowerFecal Pro DNA Kit (Qiagen) according to the manufacturer protocol. As a contamination control, an empty tube was processed and sequenced with samples. All samples, with unique barcoded reverse primer, were pooled into one sequencing library by taking 240 ng of amplicons from each sample. After removing unused primers (UltraClean PCR Clean-Up Kit, MoBio Laboratories) the library was diluted and denatured with 0.1N NaOH, and 5% of the PhiX Sequencing Control V3 (Illumina) was added to increase amplicon diversity. A 7.25 pM library was sequenced at the Microbiome Core at the Steele Children Research Center, University of Arizona on MiSeq platform (Illumina) using custom primers (22). For library preparation steps and sequencing, a non-template served as a negative control and a mock community as a positive control (ZymoBIOMICS Microbial Community DNA Standard). The sequencing reads were demultiplexed using the idemp script. Filtering, dereplication, chimera identification, and merging of paired-end reads were performed with DADA2 (23). The ASVs taxonomy was assigned using the Ribosomal Database Project (RDP) classifier (24) against SILVA database (release 132). Richness and Bray-Curtis dissimilarity based non-metric multidimensional scaling (NMDS) ordination were calculated using vegan package (25). DeSeq2 package was used to calculate differential abundance between experimental groups. Only taxa (family and genus level) significantly different (adjusted p<0.05, Wald test corrected for multiple testing using the FDR/Benjamini-Hochberg method) were presented. Spearman correlation for SCFA and taxa at the genus level was calculated with cor function form the stats package (26) and visualized with corplot package (27). To predict metagenome functions of the microbiome based on the 16S rRNA marker PICRUSt2 software was used followed by DeSeq2 to analyze PICRUSt2 output data (corrected p-value 0.05 or lower and the fold change at least 2 times were considered significant). The MetaCyc (28) pathways abundance was inferred based on the abundance of the EC numbers per sample stratified by the contributing amplicon sequence variants (ASVs).
Statistical analysis.
Statistical analyses and visualization were performed using GraphPad Prism 8 software (GraphPad Software, San Diego, CA, USA). Sample size of each experiment are indicated in figure legends and statistical significance defined at p≤0.05. Sample size for each experimental group was determined by power analysis from a pilot study with chow and HFD-fed rats. Body weight was analyzed by a one-way repeated measures ANOVA followed by Tukey post hoc multiple comparisons test. Adiposity, fasting plasma glucose, insulin, HOMA-IR, mRNA expression, and GLP-1 were analyzed by one-way ANOVA followed by Tukey post hoc. Comparisons of SCFA concentrations over time were conducted via two-way ANOVA (time x diet) with Tukey post hoc. Correlations of SCFA levels was done via simple linear regression. Alpha diversity data were analyzed by Kruskal-Wallis Rank Sum Test (one-way ANOVA on ranks, diet averaged across all timepoints). Analysis of Dissimilarities (beta diversity) between microbial communities were analyzed with Permutational Multivariate Analysis of Variance Using Distance Matrices test (vegan::adonis2 function). All data are presented as means ± SEM.
Results
Effect of HFD+OFS supplementation on body weight, adiposity, and plasma hormones
Prolonged high-fat feeding (5wk, Fig. 1a) significantly increased body weight and adiposity (Fig. 1b,c) compared to chow controls (Fig. 1b). 10% OFS supplementation during HFD significantly reduced body weight and adiposity compared to HFD rats (Fig. 1b,c). Further, obese HFD-fed rats had significantly higher fasting circulating leptin and insulin levels compared to both the lean chow and HFD+OFS rats (Fig. 1d,f). Despite no differences in fasting blood glucose concentrations (Fig. 1e), obese rats had significantly higher HOMA-IR compared to both chow and OFS-supplemented rats (Fig. 1g). Thus, 3wk of OFS supplementation decreases body weight and adiposity and improves glucose homeostasis during HFD-feeding, similar to previous findings (2, 29).
Gut microbiota composition
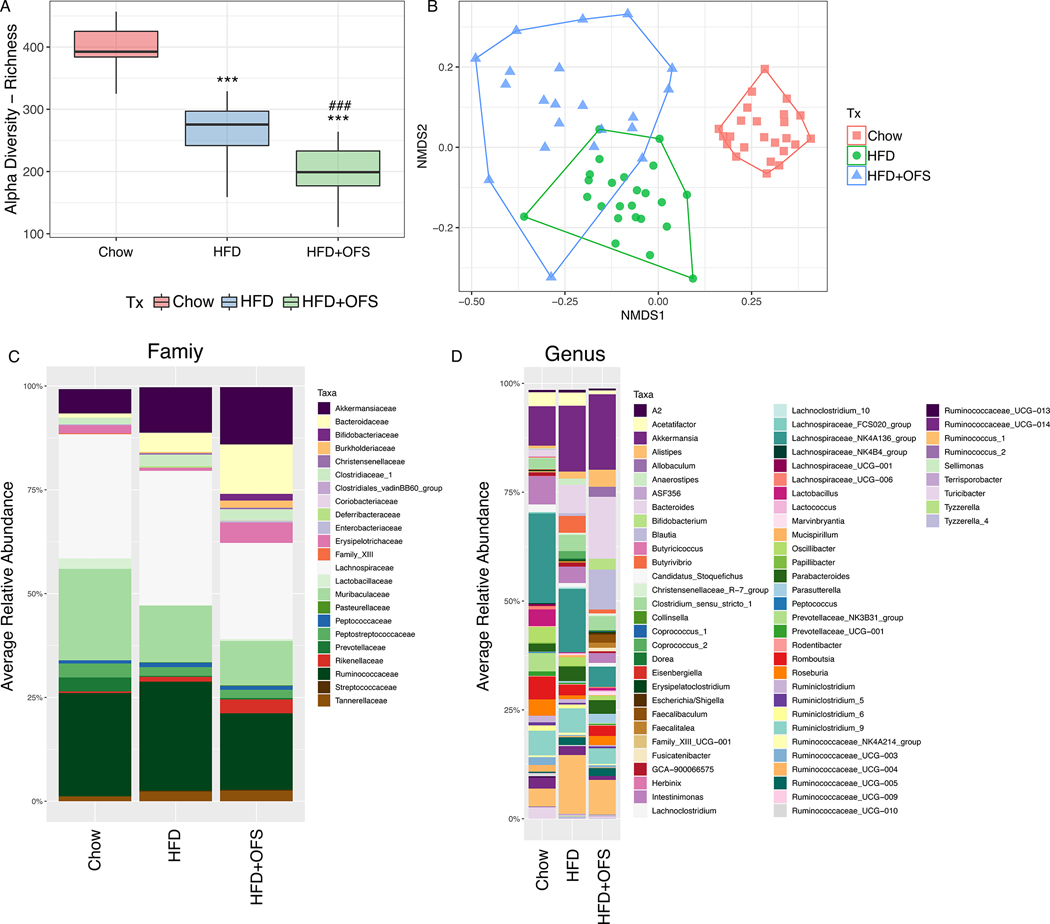
Obese rats, with or without OFS supplementation had decreased ASV species richness (alpha diversity) compared to lean rats after fasting and within all refeeding timepoints (Fig. 2a; Fig. S1a). Further, there was a significant effect of beta-diversity, with distinct microbiome clustering of chow, obese, and OFS-supplemented rats (Fig. 2b; ADONIS test for diet, p<0.001, R2=0.30317); however, there was no overall effect of postprandial time (Fig. S1b). Given the lack of difference across time in alpha and beta diversity, timepoints were combined within groups and DESeq2 analysis was used to compare differences at the family and genus level (Fig. 2c, d; Fig. S3, S4). We found significant differences (corrected p<0.05, Wald test) in bacterial families between lean and obese rats, including increased Clostridiaceae_1, Chistensenellaceae, Bacteroidaceae, and Prevotellaceae and decreased Lactobacillaceae, Eggerthellaceae, and Bifidobacteriaceae in HFD rats (Fig. 2c, Fig. S3a). Additionally, at the genus level, we found increased Blautia, Bacteroides, Prevotellaceae_UCG-001, Oscillibacter, and decreased Turicibacter, Lactobacillus, Ruminiclostridium, Roseburia, Bifidobacterium, among others in obesity (Fig. 2d, Fig. S3b). At the family level, OFS supplementation increased Erysipelotrichaceae, Bifidobacteriaceae, and Coriobacteriaceae levels and decreased Christensenellaceae, Rikenellaceae, Prevotellaceae, and Clostridiaceae_1 levels compared to obese-HFD rats (Fig. 2c, Fig. S4a). At the genus level, OFS supplementation increased Allobaculum, Bifidobacterium, Coinsella, Blautia, Lachnospiraceae_UCG-006, and decreased Alistipes, Ruminococcus_1, Lacnoclostridium, Prevotellaceae_UCG-001, Intestinimonas, Ruminoclostridium, and others compared to obese rats (Fig. 2d, Fig. S4b).
Figure 2. The effect of HFD and OFS supplementation on gut microbiota diversity and bacterial profile.
(A) Alpha diversity measured as an ASV species richness of the gut microbiota from the cecum of chow, HFD, and HFD+OFS rats (***p<0.001 compared to chow; ###p<0.001 compared to HFD, Wilcoxon rank sum test with continuity correction). (B) Bray-Curtis dissimilarity based NMDS of chow, HFD, and HFD+OFS rats (ADONIS, permutations = 999; p<0.001). (C) Average relative abundance of bacterial families from chow, HFD, and HFD+OFS rats. (D) Average relative abundance of bacterial genera from chow, HFD, and HFD+OFS rats. Groups were averaged over fasting and postprandial timepoints (0–8h following a meal) for all analyses; chow (n=24), HFD (n=24), and HFD+OFS (n=21).
Intestinal SCFA production
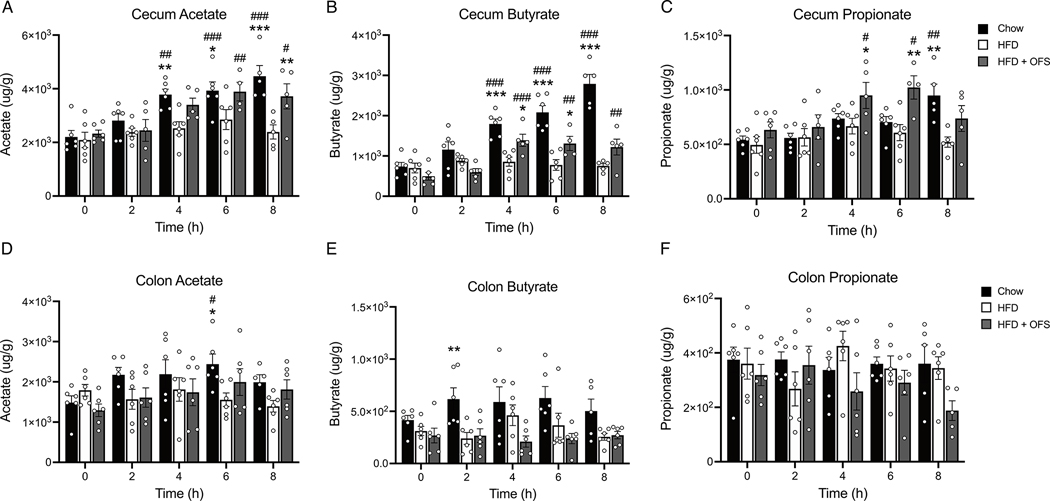
Acetate, butyrate, and propionate levels were extremely low in the ileum, with many samples below the detectable threshold and thus are not presented. There was no difference in fasting levels of acetate, propionate, or butyrate between groups in the cecum (Fig. 3a–c) or colon (Fig. 3d–f). However, refeeding resulted in increased postprandial levels of cecal acetate in lean chow-fed rats (4,6,8h) and OFS supplemented rats (6, 8h), but not HFD-induced obese rats (Fig. 3a). As such, cecal acetate was increased in lean rats (4,6,8h) and OFS supplemented rats (8h) compared to obese rats (Fig. 3a). Similarly, cecal butyrate levels increased postprandially only in lean chow-fed and OFS supplemented rats (4,6,8h) and levels were greater those in obese rats at those timepoints (Fig. 3b). Cecal propionate was increased postprandially in lean chow-fed rats (8h) and OFS supplemented rats (4,6h) and increased compared to obese rats at the same timepoints (Fig. 3c). Colonic SCFAs were relatively similar over time (Fig. 3d–f), with only lean rats exhibiting increased acetate at 6h postprandially (Fig. 3d) and increased butyrate at 2h compared to obese rats (Fig. 3e). Cecal butyrate, but not propionate or acetate, 4–8h following a meal, when significant postprandial increases were observed, was negatively correlated with body fat, independent of diet (Fig. S2a–c).
Figure 3. Chow feeding and OFS supplementation postprandially increase SCFAs in the cecum, but not the colon.
Postprandial time course (0–8h) of cecal (A) acetate, (B) butyrate, and (C) propionate in chow, HFD, and HFD+OFS rats (n=4–6 per group at each timepoint). Postprandial time course (0–8h) of colon (D) acetate, (E) butyrate, and (F) propionate in chow, HFD, and HFD+OFS rats (n=4–6 per group at each timepoint). *p<0.05 compared to HFD; **p<0.01 compared to HFD; ***p<0.001 compared to HFD; #p<0.05 compared to fasting (t=0h); ##p<0.01 compared to fasting (t=0h); ##p<0.001 compared to fasting (t=0h). All data are presented as mean ± SEM.
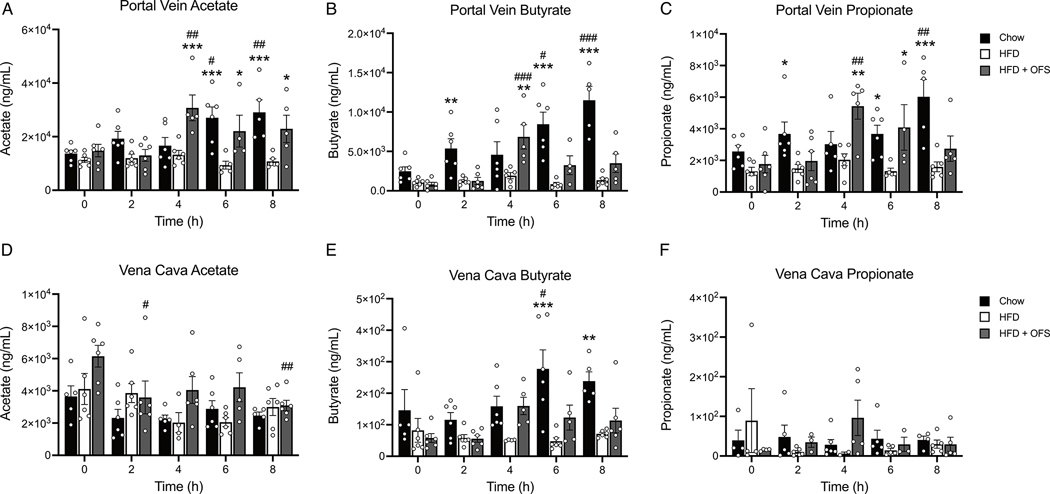
Circulating SCFA levels
There were no differences in fasted levels of any SCFA in portal vein or vena cava plasma (Fig. 4a–f) between the groups. Lean chow-fed rats had significant increases in postprandial portal vein acetate (6, 8h), butyrate (6, 8h), and propionate (8h; Fig. 4a–c) that were significantly higher than HFD-induced obese rats. OFS supplemented rats also had significant increases in portal vein acetate, butyrate, and propionate (Fig. 4a–c). Further, portal vein acetate, butyrate, and propionate were significantly higher in OFS supplemented rats compared to obese rats at 4–8h, 4h, and 4–6h following a meal, respectively (Fig. 4a–c). In vena cava, butyrate at 6 and 8h postprandially in lean rats that was significantly increased compared to obese (Fig. 4 d–f). Further, portal vein acetate, butyrate, and propionate levels were significantly negatively correlated with body fat independent of diet, with the strongest correlation between portal vein butyrate and body fat (Fig. S2g–i).
Figure 4. Chow feeding and OFS supplementation postprandially increase SCFAs in the portal vein, but not the vena cava.
Postprandial time course (0–8h) of portal vein plasma (A) acetate, (B) butyrate, and (C) propionate in chow, HFD, and HFD+OFS rats (n=4–6 per group at each timepoint). Postprandial time course (0–8h) of vena cava plasma (D) acetate, (E) butyrate, and (F) propionate in chow, HFD, and HFD+OFS rats (n=4–6 per group at each timepoint). *p<0.05 compared to HFD; **p<0.01 compared to HFD; ***p<0.001 compared to HFD; #p<0.05 compared to fasting (t=0h); ##p<0.01 compared to fasting (t=0h); ###p<0.001 compared to fasting (t=0h). All data are presented as mean ± SEM.
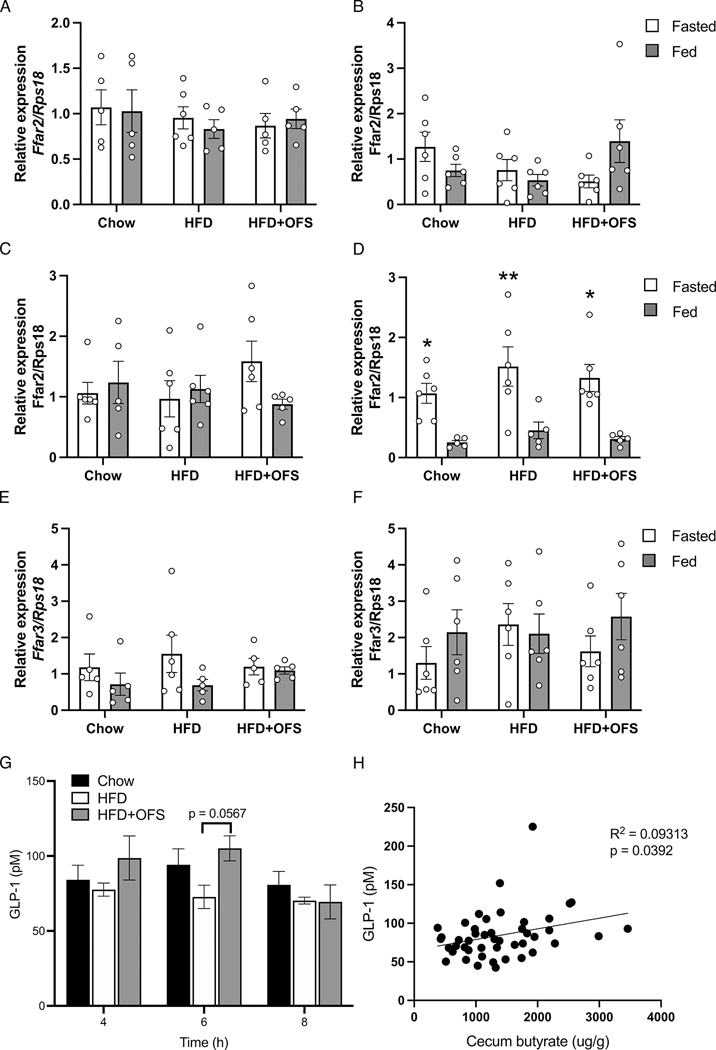
FFAR2/3 and GLP-1 secretion in chow, HFD, and HFD+OFS
The beneficial effects of SCFAs on metabolic homeostasis are mediated, at least in part, by FFAR2 and FFAR3 (30). However, we found no significant difference in Ffar2 or Ffar3 gene expression in any tissue (we were unable to detect Ffar3 in the liver or adipose tissue) between groups in either the fed or fasted state (Fig. 5a–f); however, adipose tissue Ffar2 gene expression was significantly decreased in the fed state compared to fasting in all three groups (Fig. 5d).
Figure 5. Expression of SCFA receptors and GLP-1 secretion are unaffected in chow fed, HFD-fed, or HFD+OFS rats.
Relative Ffar2 expression in tissue from the (A) ileum, (B) colon, (C) liver, and (D) adipose of chow, HFD, and HFD+OFS rats (n=5–6 per group). Relative Ffar3 expression in the (E) ileum and (F) colon of chow, HFD, and HFD+OFS rats (n=5–6 per group). (G) Total GLP-1 in portal vein plasma of chow, HFD, and HFD+OFS rats (n=5–6 per group) at 4, 6, and 8h postprandially. (H) Simple linear regression of portal vein plasma GLP-1 and cecum butyrate at 4–8h postprandially (p=0.0392). *p<0.05 fasted vs fed; **p<0.01 fasted vs fed. Data are presented as mean ± SEM.
SCFAs are known to induce gut peptide release in the distal intestine (10) and the beneficial effects of OFS on energy and glucose homeostasis are at least partly mediated by GLP-1 receptor signaling (2). Portal vein GLP-1 levels at 4–8h postprandially, when cecal SCFAs were highest, were modestly increased with OFS supplementation compared to obese rats (Fig. 5g), although this did not reach statistical significance (p=0.0567). However, postprandial cecal butyrate (4–8h) was positively correlated with portal GLP-1, independent of diet (Fig. 5h).
Gut microbiota functional data
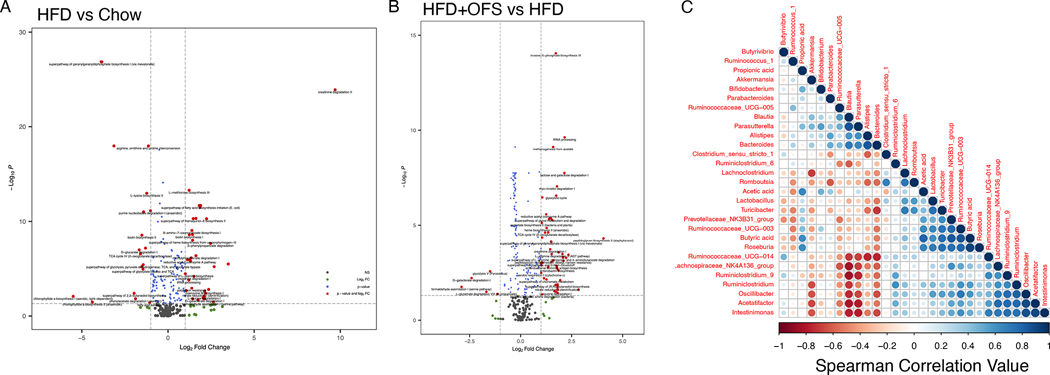
To assess the effect of diet on functional gut microbiome pathways, we performed a PICRUSt2 analysis on the cecal microbiota. A total of 61 functional pathways were significantly altered in the obese cecal microbiota, with 41 pathways upregulated and 20 pathways downregulated (Fig. 6a, Table S2). Predictably, lipid biosynthesis pathways were upregulated in the obese cecal microbiome, including the superpathway of fatty acid biosynthesis initiation (E. coli) (p<0.0001), oleate biosynthesis IV (anaerobic) (p<0.0001), palmitoleate biosynthesis I (from (5Z)-dodec-5-enoate) (p<0.0001), stearate biosynthesis II (bacteria and plants) (p<0.0001; Fig. 6a, Table S2). Pathways for carbohydrate utilization were downregulated in obesity, including lactose and galactose degradation I (p<0.0001) and lactose and galactose degradation I (p<0.0001; Fig. 6a, Table S2). Further, 67 functional pathways were significantly different in the cecal microbiome of OFS supplemented rats, with 61 upregulated with OFS supplementation and 6 downregulated (Fig. 6b, Table S3). Pathways resulting in acetate production were upregulated with OFS supplementation, including reductive acetyl coenzyme A pathway (p<0.0001) and hexitol fermentation to lactate, formate, ethanol and acetate (p<0.0001; Fig. 6b, Table S3). Of note, OFS supplementation also upregulated sucrose degradation IV (sucrose phosphorylase), used primarily by Bifidobacterium, as well as superpathway of L-tryptophan biosynthesis (p=0.0008; Fig. 6b, Table S3). Spearman’s correlation analysis shows that, independent of diet, cecal butyrate was significantly positively correlated with Roseburia (p<0.001), Ruminococcaceae_UCG-003 (p<0.001), Prevotellaceae_NK3B31_group (p<0.001), Turicibacter (p=0.004), Lactobacillus (p=0.008), Oscillibacter (p=0.014), Ruminiclostridium (p=0.019), and Intestinimonas (p=0.045), and negatively correlated with Bacteroides (p=0.033) (Fig. 6c, Table S4, S5).
Figure 6. Functional metagenomic profile of cecal bacteria in chow, HFD, and HFD+OFS rats.
(A) Functional analysis of differentially expressed pathways in (A) HFD vs chow and (B) HFD+OFS vs HFD cecal microbiota. Red dots represent pathways significantly different (p<0.05) with fold change >2; green dots represent pathways not statistically different with fold change >2; blue dots represent pathways statistically different with fold change <2. (C) Spearman correlation of gut bacteria and SCFAs from chow, HFD, and HFD+OFS rats.
Discussion
Despite the metabolic benefit of SCFA administration, it is still contentious as to the role of endogenous SCFA production in obesity, due to inconsistent experimental design across studies. Previous research utilizing SCFA measurements lacked uniformity in both timing and site, with several studies measuring concentrations of SCFA without taking timing of meal consumption into consideration or measuring in the cecum, feces, or even circulation (7, 15, 16, 17). For the first time, the current study addresses these methodological inconsistencies, performing an extensive timecourse study of SCFAs in obesity, and the impact of OFS supplementation in various tissue sites. Despite no differences in fasted levels of SCFAs between diets, we definitively demonstrate that obesity is associated with much lower levels of SCFA postprandially in the cecum and portal vein. The decrease in SCFAs in obese rats can likely be contributed to substrate availability, as chow contains slightly more fiber than the HFD used in this study, which is in line with human studies demonstrating that low dietary fiber consumption in the context of the Western diet is associated with obesity (31). Thus, despite the fact that differences in diets may have impacted SCFA production and that it would be interesting for future studies to utilize matched purified diets, these slight differences between chow and the western HFD are reasonable representations of the human obesity epidemic. Our data further indicate that fasting levels of SCFAs are likely a bad indicator of overall SCFA production, and the best time to accurately observe differences in SCFA levels in rats would be anywhere from 4–8h after a meal. Thus, overnight fasts should be avoided in animal studies examining SCFAs, and at the very least replaced with about a 5h fast after the end of a feeding cycle. Our data also indicate that SCFA are not consistently increased in the colon of any dietary group following a meal, which likely explains conflicting results of studies measuring fecal SCFAs in individuals with obesity and diabetes (7, 13, 14). Furthermore, while differences in SCFAs are observed in the portal vein plasma 4–8h after refeeding between diets, no real differences were found in the general circulation. Thus, it is also unlikely that measurements in the blood are a viable option for human studies. These data indicate that the timing and location of sample collection are imperative to reliably observe changes in SCFA production in the gut, and future research should take these implications for study design into consideration. For humans, it might be optimal to directly measure SCFA levels several hours after a meal to mimic the postprandial rise in the current study. However, the proper collection site for human studies may be difficult to discern, given the results of the current study. As an alternative to direct SCFA measurement, it might be more indicative to look at specific bacterial taxa that are known to increase SCFA production. For example, in the current study we observed several bacterial genera that are positively associated with SCFA production, most notably Roseburia, similar to other studies (32, 33).
OFS supplementation in obese rats rescued body weight and adiposity to the levels of lean chow-fed rats and improved HOMA-IR and reduced plasma insulin, similar to previous studies (19). We hypothesize that OFS supplementation decreased food intake to induce weight loss, as shown previously (29), possibly via SCFA induced neuronal activation (34). For the first time ever, we have shown that OFS supplementation increases SCFA levels in the cecum and portal vein compared to HFD-induced obese rats. This increase in endogenous SCFA levels suggest a role for SCFA production from OFS supplementation in control of energy and glucose homeostasis, although this remains to be explored further. OFS supplementation may exert beneficial effects on glucose and body weight regulation via promotion of GLP-1 (2, 29). We found a modest increase in portal vein GLP-1 6h postprandially with OFS supplementation; although, there was no effect at any other timepoint in any group, possibly due to the low sample size. However, GLP-1 secretion was positively correlated with cecal butyrate levels at 4–8h postprandially, suggesting that GLP-1 signaling may mediate some effect of OFS supplementation on glucose homeostasis and food intake (29). Further, FFAR2 and FFAR3 mediate the ability of SCFAs to induce GLP-1 release from EECs (11). However, there were no differences in expression of either receptor in the ileum or colon, similar to a lack of difference in Ffar2 and Ffar3 gene expression between small intestinal L-cells from chow and HFD mice (35). Given than FFAR2 and FFAR3 likely mediate the beneficial metabolic effects of SCFA, and that receptor expression is unchanged with HFD or OFS supplementation, this study highlights that restoration of SCFA alone may be sufficient to beneficially impact metabolism.
Because no changes in SCFA were observed in the small intestine in this study, and OFS remains mostly intact throughout the small intestine (36), we assessed the effects of HFD-induced obesity and OFS supplementation on the cecal gut microbiome. We found that obesity was associated with decreased Bifidobacterium genus, and this was increased with OFS supplementation, similar to previous studies (37). In rodent and human studies, probiotic supplementation with Bifidobacterium species reduced body weight gain (38, 39) and improved glucose tolerance (40). Interestingly, OFS supplementation also increased a sucrose degradation pathway specific to Bifidobacterium (41), highlighting a novel mechanism in which OFS could improve glucose homeostasis via breakdown of ingested sucrose. We also found decreased Lactobacillus in obese rats, a genus containing several species that have been researched extensively in probiotic supplementation for weight loss (42). Conversely, several bacteria were increased in HFD-induced obese rats. At the family level, Prevotellaceae were increased with high fat feeding and decreased in lean chow-fed and OFS supplemented rats; this family is known to be enriched in the gut of obese individuals and may contribute to obesity-associated hypertension (43).
We found that cecal and portal vein butyrate was negatively correlated with body fat, implicating the beneficial effects of butyrate on overall energy homeostasis and possibly in mediating the improvements with OFS treatment. Interestingly, there was a significant correlation between postprandial cecal butyrate levels and postprandial GLP-1 portal vein levels. Thus, it is likely that local butyrate action could mediate improvements in metabolic homoeostasis (10); however, it is possible that improvements in adiposity via butyrate are not mediated via gut peptide signaling but in other peripheral organs, like the liver or adipose tissue (34, 44). Additionally, we found that specific bacterial populations were correlated with butyrate production, indicating a role for these bacteria in the production of butyrate. Those that positively correlated with butyrate include Roseburia, a known butyrate-producing bacterial genus that has been previously implicated in the effect of prebiotic consumption on energy homeostasis in obese mice (33). Ruminococcaceae_UCG-003 was also found to correlate with cecal butyrate, and members of the Ruminoccaceae family, including F. prausnitzii, are known intestinal butyrate producers (45), indicating that this genus may require further investigation. Additionally, Lactobacillus was positively correlated with butyrate production, which produce lactate from glucose and other sugars, which can be further converted by the bacteria to butyrate (46). Thus, given the high amount of sugar in the HFD diet, Lactobacillus could provide an additional substrate for the eventual production of butyrate during OFS supplementation. Therefore, in addition to blunted postprandial cecal SCFA levels due to reduced substrate in HFD-feeding, a reduction in bacteria responsible for butyrate production likely plays a role in diminished SCFA production in obese rats. This was similar to our observations in the pathway analysis, as obesity was associated with reductions in bacteria associated with carbohydrate metabolism, while OFS supplementation increased pathways involved in fermentation of lactose and acetate, among others.
Conclusion
In conclusion, our data demonstrate that SCFA levels increase following a meal in healthy chow-fed rats but not in HFD-fed obese rats. Administration of OFS during obesity reduced adiposity and improved glucose homeostasis that was associated with increased levels of postprandial SCFAs in the cecum. Our data identifies butyrate production as a potential mediator of improvements in adiposity. In addition, the current study identifies the cecum as having the greatest differences in SCFA production between diets, which future studies show consider when the impact of endogenous SCFAs on energy and glucose homeostasis.
Supplementary Material
Study Importance Questions.
What is already known about this subject?
Obesity and western diet consumption are associated with shifts in the gut microbiome, and gut microbes produce metabolites that influence host metabolism, including short chain fatty acids.
Prebiotics, as well as exogenous short-chain fatty acids, improve energy and glucose homeostasis in rodent models of metabolic dysregulation.
What are the new findings in your manuscript?
Western diet-induced obesity is associated with reduced postprandial levels of short-chain fatty acids in the cecum and portal vein in rats, with no difference in fasting levels.
Supplementation of oligofructose in obesity increases postprandial short chain fatty acid levels compared to western diet alone and is associated with reduced adiposity and weight gain.
How might your results change the direction of research or the focus of clinical practice?
Obesity is associated with reduced SCFA levels, contrary to previous findings, but only in a postprandial setting.
These findings demonstrate that fasting and/or fecal measurements are not accurate indicators of endogenous short-chain fatty acid levels.
Therapeutic approaches to increase endogenous short-chain fatty acid levels may help alleviate obesity and insulin resistance.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH (R01DK121804). The authors declared no conflict of interest.
Data Availability Statement:
The microbiome sequencing data are available in the NIH National Center for Biotechnology Information (NCBI) at http://www.ncbi.nlm.nih.gov/bioproject/804890; accession number PRJNA804890.
References
- 1.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr 2005;81: 341–354. [DOI] [PubMed] [Google Scholar]
- 2.Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes 2006;55: 1484–1490. [DOI] [PubMed] [Google Scholar]
- 3.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nature Reviews Microbiology 2021;19: 55–71. [DOI] [PubMed] [Google Scholar]
- 4.Bauer PV, Duca FA, Waise TMZ, Rasmussen BA, Abraham MA, Dranse HJ, et al. Metformin Alters Upper Small Intestinal Microbiota that Impact a Glucose-SGLT1-Sensing Glucoregulatory Pathway. Cell Metab 2018;27: 101–117.e105. [DOI] [PubMed] [Google Scholar]
- 5.Ilhan ZE, DiBaise JK, Dautel SE, Isern NG, Kim YM, Hoyt DW, et al. Temporospatial shifts in the human gut microbiome and metabolome after gastric bypass surgery. NPJ Biofilms Microbiomes 2020;6: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agus A, Clément K, Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021;70: 1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TM, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes 2014;4: e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao F, Lv YW, Long J, Chen JM, He JM, Ruan XZ, et al. Butyrate Improves the Metabolic Disorder and Gut Microbiome Dysbiosis in Mice Induced by a High-Fat Diet. Front Pharmacol 2019;10: 1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.den Besten G, Bleeker A, Gerding A, van Eunen K, Havinga R, van Dijk TH, et al. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPARgamma-Dependent Switch From Lipogenesis to Fat Oxidation. Diabetes 2015;64: 2398–2408. [DOI] [PubMed] [Google Scholar]
- 10.Christiansen CB, Gabe MBN, Svendsen B, Dragsted LO, Rosenkilde MM, Holst JJ. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol 2018;315: G53–G65. [DOI] [PubMed] [Google Scholar]
- 11.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012;61: 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, et al. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 2016;534: 213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barczynska R, Litwin M, Slizewska K, Szalecki M, Berdowska A, Bandurska K, et al. Bacterial Microbiota and Fatty Acids in the Faeces of Overweight and Obese Children. Pol J Microbiol 2018;67: 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao L, Lou H, Peng Y, Chen S, Zhang Y, Li X. Comprehensive relationships between gut microbiome and faecal metabolome in individuals with type 2 diabetes and its complications. Endocrine 2019;66: 526–537. [DOI] [PubMed] [Google Scholar]
- 15.Brinkworth GD, Noakes M, Clifton PM, Bird AR. Comparative effects of very low-carbohydrate, high-fat and high-carbohydrate, low-fat weight-loss diets on bowel habit and faecal short-chain fatty acids and bacterial populations. Br J Nutr 2009;101: 1493–1502. [DOI] [PubMed] [Google Scholar]
- 16.Schwiertz A, Taras D, Schafer K, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18: 190–195. [DOI] [PubMed] [Google Scholar]
- 17.Valcheva R, Koleva P, Martinez I, Walter J, Ganzle MG, Dieleman LA. Inulin-type fructans improve active ulcerative colitis associated with microbiota changes and increased short-chain fatty acids levels. Gut Microbes 2019;10: 334–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Wang H, Howard AG, Meyer KA, Tsilimigras MCB, Avery CL, et al. Circulating Short-Chain Fatty Acids Are Positively Associated with Adiposity Measures in Chinese Adults. Nutrients 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar SA, Ward LC, Brown L. Inulin oligofructose attenuates metabolic syndrome in high-carbohydrate, high-fat diet-fed rats. Br J Nutr 2016;116: 1502–1511. [DOI] [PubMed] [Google Scholar]
- 20.Trapecar M, Communal C, Velazquez J, Maass CA, Huang YJ, Schneider K, et al. Gut-Liver Physiomimetics Reveal Paradoxical Modulation of IBD-Related Inflammation by Short-Chain Fatty Acids. Cell Syst 2020;10: 223–239.e229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Detman A, Laubitz D, Chojnacka A, Wiktorowska-Sowa E, Piotrowski J, Salamon A, et al. Dynamics and Complexity of Dark Fermentation Microbial Communities Producing Hydrogen From Sugar Beet Molasses in Continuously Operating Packed Bed Reactors. Front Microbiol 2020;11: 612344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 2012;6: 1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernesniemi A [East Bothnian treatment modalities of spinal manipulation and limb correction]. Duodecim 1989;105: 758–763. [PubMed] [Google Scholar]
- 24.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 2013;41: D590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin P, O’Hara B, et al. Vegan: Community Ecology Package. R Package Version 22–1 2015;2: 1–2. [Google Scholar]
- 26.Team R A language and environment for statistical computing. Computing 2006;1. [Google Scholar]
- 27.Wei T, Simko V, Levy M, Xie Y, Jin Y, Zemla J. corrplot: Visualization of a correlation matrix. R package version 073 2013;230: 11. [Google Scholar]
- 28.Caspi R, Altman T, Billington R, Dreher K, Foerster H, Fulcher CA, et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases. Nucleic Acids Res 2014;42: D459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cani PD, Neyrinck AM, Maton N, Delzenne NM. Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like Peptide-1. Obes Res 2005;13: 1000–1007. [DOI] [PubMed] [Google Scholar]
- 30.He J, Zhang P, Shen L, Niu L, Tan Y, Chen L, et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int J Mol Sci 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludwig DS, Pereira MA, Kroenke CH, Hilner JE, Van Horn L, Slattery ML, et al. Dietary fiber, weight gain, and cardiovascular disease risk factors in young adults. Jama 1999;282: 1539–1546. [DOI] [PubMed] [Google Scholar]
- 32.Wang H, Hong T, Li N, Zang B, Wu X. Soluble dietary fiber improves energy homeostasis in obese mice by remodeling the gut microbiota. Biochem Biophys Res Commun 2018;498: 146–151. [DOI] [PubMed] [Google Scholar]
- 33.Neyrinck AM, Possemiers S, Druart C, Van de Wiele T, De Backer F, Cani PD, et al. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS One 2011;6: e20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, Yi CX, Katiraei S, Kooijman S, Zhou E, Chung CK, et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 2018;67: 1269–1279. [DOI] [PubMed] [Google Scholar]
- 35.Richards P, Pais R, Habib AM, Brighton CA, Yeo GS, Reimann F, et al. High fat diet impairs the function of glucagon-like peptide-1 producing L-cells. Peptides 2016;77: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersson HB, Ellegård LH, Bosaeus IG. Nondigestibility Characteristics of Inulin and Oligofructose in Humans. The Journal of Nutrition 1999;129: 1428S–1430S. [DOI] [PubMed] [Google Scholar]
- 37.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007;50: 2374–2383. [DOI] [PubMed] [Google Scholar]
- 38.Bernini LJ, Simao AN, Alfieri DF, Lozovoy MA, Mari NL, de Souza CH, et al. Beneficial effects of Bifidobacterium lactis on lipid profile and cytokines in patients with metabolic syndrome: A randomized trial. Effects of probiotics on metabolic syndrome. Nutrition 2016;32: 716–719. [DOI] [PubMed] [Google Scholar]
- 39.Minami J, Kondo S, Yanagisawa N, Odamaki T, Xiao JZ, Abe F, et al. Oral administration of Bifidobacterium breve B-3 modifies metabolic functions in adults with obese tendencies in a randomised controlled trial. J Nutr Sci 2015;4: e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stenman LK, Waget A, Garret C, Klopp P, Burcelin R, Lahtinen S. Potential probiotic Bifidobacterium animalis ssp. lactis 420 prevents weight gain and glucose intolerance in diet-induced obese mice. Benef Microbes 2014;5: 437–445. [DOI] [PubMed] [Google Scholar]
- 41.Luo Y, Xiao Y, Zhao J, Zhang H, Chen W, Zhai Q. The role of mucin and oligosaccharides via cross-feeding activities by Bifidobacterium: A review. Int J Biol Macromol 2021;167: 1329–1337. [DOI] [PubMed] [Google Scholar]
- 42.Crovesy L, Ostrowski M, Ferreira D, Rosado EL, Soares-Mota M. Effect of Lactobacillus on body weight and body fat in overweight subjects: a systematic review of randomized controlled clinical trials. Int J Obes (Lond) 2017;41: 1607–1614. [DOI] [PubMed] [Google Scholar]
- 43.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017;5: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang WQ, Zhao TT, Gui DK, Gao CL, Gu JL, Gan WJ, et al. Sodium Butyrate Improves Liver Glycogen Metabolism in Type 2 Diabetes Mellitus. J Agric Food Chem 2019;67: 7694–7705. [DOI] [PubMed] [Google Scholar]
- 45.Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 2009;294: 1–8. [DOI] [PubMed] [Google Scholar]
- 46.Markowiak-Kopec P, Slizewska K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The microbiome sequencing data are available in the NIH National Center for Biotechnology Information (NCBI) at http://www.ncbi.nlm.nih.gov/bioproject/804890; accession number PRJNA804890.