Abstract
Background
PD-L1 immunohistochemistry (IHC) is required to determine eligibility for pembrolizumab monotherapy in advanced non-small cell lung cancer (NSCLC) worldwide and for several other indications depending on the country. Four assays have been approved/CE-IVD marked, but PD-L1 IHC seems diversely implemented across regions and laboratories with the application of laboratory-developed tests (LDTs).
Method
To assess practice of PD-L1 IHC and identify issues and disparities, the IASLC pathology committee conducted a global survey for pathologists from January to May 2019, comprising multiple questions on pre-analytical, analytical and post-analytical conditions.
Result
344 pathologists from 64 countries participated with 41% from Europe, 24% from North America, and 18% from Asia. Besides biopsies and resections, cellblocks were used by 75% of the participants and smears by 11%. The clone 22C3 was most commonly used (69%) followed by SP263 (51%). They were applied as a LDT by 40% and 30% of the users, respectively, and 76% of the participants developed at least one LDT. A half of the participants reported turnaround time (TAT) of ≤ 2 days, while 13% reported that of ≥ 5 days. Additionally, quality assurance (QA), formal training for scoring and standardized reporting were not implemented by 18%, 16% and 14% of the participants, respectively.
Conclusion
Heterogeneity in PD-L1 testing is marked across regions and laboratories in terms of antibody clones, IHC assays, samples, TATs and QA measures. The lack of QA, formal training and standardized reporting stated by a significant minority identifies a need for additional QA measures and training opportunities.
Background
Immune checkpoint inhibitors (ICIs) targeting programmed cell death protein 1 (PD-1) / programmed cell death 1 ligand 1 (PD-L1) axis have drastically changed the treatment landscape in oncology. There are a few anti PD-1/PD-L1 agents approved for treatment of advanced non-small cell lung cancer (NSCLC) patients either alone or in combination with chemotherapy or an anti CTLA-4 agent in the first line setting or second line or beyond. Notably, pembrolizumab as a monotherapy or in combination with chemotherapy has been approved for the first line treatment of advanced NSCLC in many countries and changed the standard of care for those patients1–54. Pembrolizumab monotherapy is used in many countries1 to treat patients with tumors exhibiting a PD-L1 expression by immunohistochemistry (IHC) of at least 50% of tumor cells (Tumor Proportion Score [TPS] of ≥50%). A TPS of ≥1% has also been approved in this setting in the US and Japan4, 5. Although the combination of pembrolizumab and chemotherapy does not require a companion diagnostic, this combination is more likely reserved in current practice for patients with lower PD-L1 expression (<50%) or certain clinical factors (e.g., significant tumor or symptom burden). Tumors exhibiting high PD-L1 (≥50%) are commonly treated with pembrolizumab monotherapy given its less adverse effects6. Thus, PD-L1 IHC is now established as a predictive biomarker test to determine pembrolizumab as a monotherapy vs. in combination with chemotherapy for the first line treatment of advanced NSCLC. Further, PD-L1 IHC also serves as a companion diagnostic for pembrolizumab monotherapy in the second line setting and beyond, in the US, for atezolizumab monotherapy and for a combination of nivolumab and ipilimumab in the first line setting, and in Europe, for durvalumab therapy after chemoradiation in stage III NSCLC patients7–10. Consequently, PD-L1 IHC has been implemented in most pathology laboratories. However, the implementation of the test and participation in quality assurance (QA) programs and training for PD-L1 scoring appear variable across the regions and laboratories and may influence the test results and consequently, clinical care of patients. Therefore, the Immune Biomarker Working Group of the International Association for the Study of Lung Cancer (IASLC) pathology committee conducted an international survey for pathologists on PD-L1 testing in NSCLC. The aims of this survey were: 1) to determine the prevalence of PD-L1 testing worldwide; 2) to analyze differences in practice between different regions and laboratories; 3) to identify the issues that may influence the test results and consequently, clinical care of patients.
Method
The international online survey for pathologists on PD-L1 IHC testing in NSCLC was conducted from 2/1/2019 to 5/31/2019. The survey was advertised in the IASLC and Pulmonary Pathology Society websites, as well as at the 2019 annual meeting of the United States and Canadian Association of Pathology (USCAP). To increase the number of participants, we also contacted the president of individual national or regional pathology societies.
The survey consisted of more than 20 questions to encompass pre-analytical, analytical and post-analytical aspects of the PD-L1 IHC testing. They are summarized as: 1) the type of samples and tissue handling; 2) the availability/type of PD-L1 IHC assay(s); 3) participation in quality assurance program(s) and training course(s); 4) reporting of the results.
Regarding statistical analysis, quantitative results are presented as frequency (percent) with respondent as the unit of analysis. The Chi-squared test or Fischer exact bilateral test was used for regional comparisons. All quantitative analyses were conducted in SAS version 9.4. P-values < 0.05 were considered statistically significant.
Result
Participants
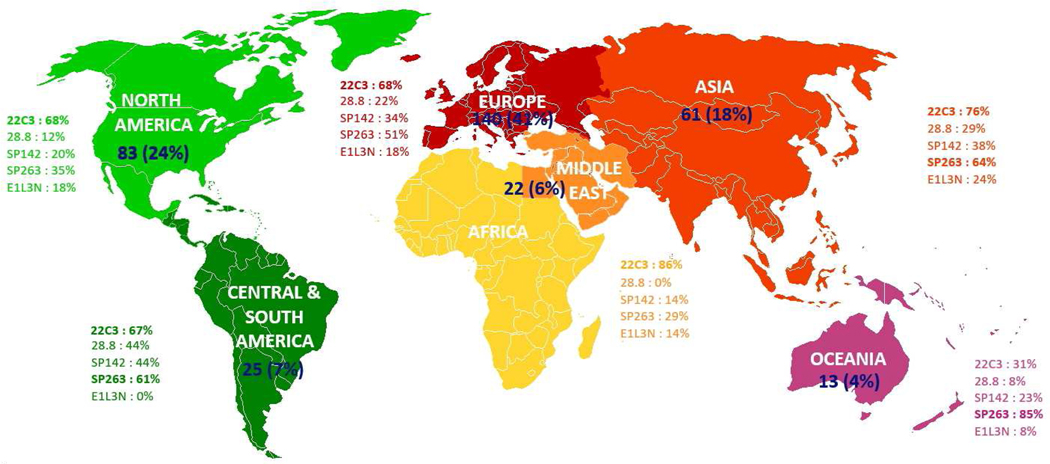
344 pathologists from 310 institutions in 64 countries participated in the survey. Of those, 140 (41%) were from Europe (including 45 from France, 14 from the UK and 13 from Spain), followed by 83 (24%) from North America (including 64 from the US and 19 from Canada), 61 (18%) from Asia (including 18 from Japan, 14 from China and 10 from India), 25 (7.3%) from Central & South America (including 10 from Argentina, 5 from Columbia and 4 each from Brazil and Mexico), 22 (6.4%) from Africa and Middle East (including 7 from Turkey and 5 from Saudi Arabia) and 13 (3.8%) from Oceania (including 10 from Australia and 3 from New Zealand) (Figure 1). As for the subspecialty, 109 (32%) were specialized in thoracic pathology, 102 (30%) in thoracic pathology and cytology, 22 (6%) in cytology, and 11 (3%) in other fields, while 100 (29%) practiced general pathology without specialization.
Figure 1.
Participation rate of pathologists and use of different PD-L1 clones by region
PD-L1 testing status
10 (2.9%) pathologists from 9 countries did not perform PD-L1 IHC for either clinical or research purpose. Additionally, two pathologists performed the IHC only for research. Another 34 (9.9%) sent out samples to other laboratories, in particular 25% of North American participants and 15% of those from Central & South America, with or without scoring the slide upon receiving (p<0.0001) (Table 1). Of 298 pathologists with clinical PD-L1 IHC available in their laboratories, 116 (39%) responded to perform the IHC with internal samples only and 171 (57%) with both internal and referral samples, and 11 (3.7%) belonged to a central or commercial laboratory.
Table 1:
Pre-analytical, analytical and post-analytical aspects of PD-L1 testing stratified by region
| Region | North America | Central & South America | Europe | Asia | Oceania | Africa and Middle East | World | |
|---|---|---|---|---|---|---|---|---|
| n=83 | n=25 | n=140 | n=61 | n=13 | n=22 | Comparison test | n=344 | |
|
| ||||||||
| PD-L1 testing status* | n=83 | n=20 | n=139 | n=59 | n=13 | n=20 | p†<0.0001 | n=334 |
| In house | 62 (75%) | 17 (85%) | 134 (96%) | 56 (95%) | 13 (100%) | 18 (90%) | 300 (90%) | |
| Send out | 21 (25%) | 3 (15%) | 5 (4%) | 3 (5%) | 0 | 2 (10%) | 34 (10%) | |
|
| ||||||||
| No. of sample types** | n=83 | n=20 | n=138 | n=59 | n=13 | n=19 | p†<0.0001 | n=332 |
| 1 | 2 (3%) | 5 (25%) | 5 (4%) | 5 (9%) | 1 (8%) | 6 (32%) | 24 (7%) | |
| 2 | 11 (13% | 11 (55%) | 21 (15%) | 22 (37%) | 1 (8%) | 1 (5%) | 67 (20%) | |
| 3 or more | 70 (84%) | 4 (20%) | 112 (81%) | 32 (54%) | 11 (84%) | 12 (63%) | 241 (73%) | |
| Sample types used** | ||||||||
| Biopsy | 80 (96%) | 20 (100%) | 138 (100%) | 57 (97%) | 13 (100%) | 16 (84%) | p†<0.0001 | 324 (98%) |
| Surgical | 80 (96%) | 15 (75%) | 128 (91%) | 55 (93%) | 11 (85%) | 16 (84%) | p†<0.0001 | 305 (92%) |
| Cell blocks | 73 (88%) | 4 (20%) | 116 (84%) | 32 (54%) | (92%) | 12 (63%) | p†<0.0001 | 249 (75%) |
| Cytology smear | 7 (8.4%) | 0 | 22 (16%) | 4 (6.8%) | 0 | 0 | 36 (11%) | |
|
| ||||||||
| Platform** | n=57 | n=19 | n=133 | n=54 | n=13 | n=18 | p†=0.07 | n=294 |
| Ventana | 31 (54%) | 14 (74%) | 95 (71%) | 42 (78%) | 10 (77%) | 9 (50%) | p†=0.10 | 201 (68%) |
| Dako | 29 (51%) | 10 (53%) | 48 (36%) | 35 (65%) | 1 (8%) | 10 (56%) | p†<0.0001 | 133 (45%) |
| Leica | 13 (23%) | 1 (5%) | 23 (17%) | 14 (26%) | 4 (31%) | 1 (6%) | p†=0.19 | 56 (19%) |
|
| ||||||||
| External QA** | n=59 | n=16 | n=134 | n=56 | n=13 | n=18 | p†=0.54 | n=296 |
| No | 8 (14%) | 5 (31%) | 23 (17%) | 11 (20%) | 1 (8%) | 4 (22%) | 52 (18%) | |
| Yes | 51 (86%) | 11 (69%) | 111 (83%) | 45 (80%) | 12 (92%) | 14 (78%) | 244 (82%) | |
| If Yes, | ||||||||
| Interlab validation | 28 (47%) | 6 (38%) | 39 (29%) | 29 (52%) | 5 (38%) | 7 (39%) | p†=0.04 | 114 (39%) |
| Formal external QA | 34 (58%) | 7 (44%) | 97 (72%) | 30 (54%) | 10 (77%) | 9 (50%) | p†=0.02 | 187 (63%) |
| Other | 2 (3%) | 1 (6%) | 2 (1 %) | 2 (4%) | 2 (15%) | 0 | p†=0.11 | 9 (3%) |
|
| ||||||||
| Training** | n=75 | n=21 | n=139 | n=60 | n=13 | n=21 | p†<0.0001 | n=329 |
| No | 23 (31%) | 5 (36%) | 9 (6%) | 8 (13%) | 0 | 7 (33%) | 52 (16%) | |
| Yes | 52 (69%) | 16 (64%) | 130 (94%) | 52 (87%) | 13 (100%) | 14 (67%) | 277 (84%) | |
| If yes, organized by | p†=0.02 | |||||||
| Companies | 28 (54%) | 10 (63%) | 91 (70%) | 43 (83%) | 11 (84%) | 8 (58%) | 191 (69%) | |
| IASLC | 5 (10%) | 2 (12%) | 11 (8%) | 7 (13%) | 1 (8%) | 3 (21%) | 29 (10%) | |
| Other societies | 19 (37%) | 4 (25%) | 28 (22%) | 2 (4%) | 1 (8%) | 3 (21%) | 57 (21%) | |
|
| ||||||||
| Guidelines used** | n=73 | n=21 | n=140 | n=59 | n=13 | n=20 | p†=0.07 | n=326 |
| No | 4 (5%) | 0 | 3 (2%) | 1 (2%) | 1 (8%) | 3 (15%) | 12 (4%) | |
| Yes | 69 (95%) | 21 (100%) | 137 (98%) | 58 (98%) | 12 (92%) | 17 (85%) | 314 (96%) | |
| If yes, | ||||||||
| Local or national | 53 (73%) | 5 (24%) | 95 (68%) | 36 (61%) | 7 (54%) | 6 (30%) | p†<0.0001 | 202 (62%) |
| IASLC | 26 (36%) | 18 (86%) | 78 (56%) | 34 (58%) | 9 (69%) | 15 (75%) | p†<0.0001 | 180 (55%) |
|
| ||||||||
| TAT** | p‡=0.0002 | |||||||
| Median | 1 –2 days | 2–3 days | 1 –2 days | 2–3 days | 1–2 days | 3–4 days | 1 –2 days | |
| Range | [1 ; ≤5] | [1 ; ≤5] | [1 ; ≤5] | [1 ; ≤5] | [1 ; ≤5] | [1 ; ≤5] | [1 ; ≤5] | |
|
| ||||||||
| Standardized report** | n=79 | n=19 | n=138 | n=58 | n=13 | n=18 | p†=0.53 | |
| No | 8 (10%) | 2 (11%) | 23 (17%) | 6 (10%) | 1 (8%) | 4 (22%) | 44 (14%) | |
| Yes | 71 (90%) | 17 (89%) | 115 (83%) | 52 (90%) | 12 (92%) | 14 (78%) | 281 (86%) | |
p†: Х2 or Fisher exact bilateral test; p‡: Mann-Whitney test; QA: quality assurance; TAT: turn-around time;
the numbers indicate participants whose lab offered clinical PD-L1 immunohistochemistry assessment;
the numbers indicate those of participants who responded to the specific question.
Type of specimens and preanalytical conditions
The vast majority of 332 participants, who had clinical PD-L1 testing available and responded to this question, used biopsies (98%), excision or resection samples (92%) and cell blocks (75%). Cell blocks were mainly used in North America (88%), Europe (84%) and Oceania (92%), while only 20%, 54% and 63% of the participants from Central & South America, Asia and Africa and Middle East, respectively, used them for PD-L1 testing (p<0.0001) (Table 1). Use of cytology smears was limited to 36 (11%) participants including 6.0% (5) of those from North America, 16% (22) from Europe, 6.8% (4) from Asia and 16% (3) from Africa and Middle East. No participants from Oceania used cytology smears for PD-L1 IHC.
As for the pre-analytical conditions, data were available form only 179 (54%) of 332 participants who performed clinical PD-L1 testing. Of those, 42%, 47% and 72% recorded delay before fixation, fixation duration and age of unstained slides, respectively, among others. Importantly, type of fixatives was recorded only by 6 (3.4%) responders (Supplementary Table 1).
PD-L1 Antibody Clones and Platforms used
Data relating to IHC platforms were available from 296 participants. The Ventana autostainers were most prevalent, with >70% of laboratories equipped in Central & South America, Europe, Asia and Oceania. Conversely, Dako and Leica autostainers were used in 45% and 19% of the laboratories, respectively. In particular, Dako platforms were available in only 8% of participating laboratories in Oceania and 36% in Europe (p<0.0001), while 31% used Leica platforms in Oceania (Table 1).
Of 302 participants with information about PD-L1 antibody clones available, 155 (51%) used more than one clone. The majority (69%) used the clone 22C3, followed by clone SP263 (51%), which was used by the majority of laboratories except in the North America (35%) and Africa & Middle East (29%) (p=0.001). The clones 28.8, SP142 and 73–10 were used only by 21%, 31% and 1.7% of participants, respectively (Figure 1 and Table 2). Interestingly, the clinical trial-validated, commercial assay was used in 60% of the laboratories that performed IHC with the 22C3 clone. The SP263 commercial assay was used in 86%, 69%, 64% and 100% of the laboratories using SP263 clone, in Europe, Asia, Oceania and Africa & Middle East, respectively, and in only 35% and 45% of laboratories in North America and Central & South America, respectively (p<0.0001) (Table 2). Overall, the 22C3, 28.8, SP142 and SP263 clones were applied as a laboratory developed test (LDT) by 84 (28%), 21 (7.0%), 36 (12%) and 46 (15%) of the 302 participants. In addition, a minority (18%) used a non-clinical trial clone, E1L3N (Table 2), while another non-clinical trial clone CE/IVD marked, QR1, was used by 7.3%, mainly in France. Six percent of the laboratories with clinical PD-L1 testing performed only LDTs.
Table 2:
PD-L1 antibody clones used stratified by region
| Region | North America | Central & South America | Europe | Asia | Oceania | Africa and Middle East | World | |
|---|---|---|---|---|---|---|---|---|
| n=83 | n=25 | n=140 | n=61 | n=13 | n=22 | Comparison test | n=344 | |
|
| ||||||||
| PD-L1 clone | ||||||||
| 22C3* | n=66 | n=18 | n=136 | n=55 | n=13 | n=14 | p†=0.03 | n=302 |
| Non used | 21 (32%) | 6 (33%) | 43 (32%) | 13 (24%) | 9 (69%) | 2 (14%) | 94 (31%) | |
| Used | 45 (68%) | 12 (67%) | 93 (68%) | 42 (76%) | 4 (31%) | 12 (86%) | 208 (69%) | |
| Clinical assay | 28 (62%) | 5 (42%) | 50 (54%) | 29 (69%) | 1 (25%) | 11 (92%) | p†=0.04 | 124 (60%) |
| LDT | 17 (38%) | 7 (58%) | 43 (46%) | 13 (31%) | 3 (75%) | 1 (8%) | 84 (40%) | |
| 28.8* | n=66 | n=18 | n=136 | n=55 | n=13 | n=14 | p†=0.005 | n=302 |
| Non used | 58 (88%) | 10 (56%) | 106 (78%) | 39 (71%) | 12 (92%) | 14 (100%) | 239 (79%) | |
| Used | 8 (12%) | 8 (44%) | 30 (22%) | 16 (29%) | 1 (8%) | 0 | 63 (21%) | |
| Clinical assay | 8 (100%) | 0 | 22 (73%) | 12 (75%) | 0 | - | p†<0.0001 | 42 (67%) |
| LDT | 0 | 8 (100%) | 8 (27%) | 4 (25%) | 1 (100%) | - | 21 (33%) | |
| SP142* | n=66 | n=18 | n=136 | n=55 | n=13 | n=14 | p†=0.09 | n=302 |
| Non used | 53 (80%) | 10 (56%) | 90 (66%) | 34 (62%) | 10 (77%) | 12 (86%) | 209 (69%) | |
| Used | 13 (20%) | 8 (44%) | 46 (34%) | 21 (38%) | 3 (23%) | 2 (14%) | 93 (31%) | |
| Clinical assay | 8 (62%) | 5 (63%) | 31 (67%) | 10 (48%) | 1 (33%) | 2 (100%) | p†=0.46 | 57 (61%) |
| LDT | 5 (38%) | 3 (37%) | 15 (33%) | 11 (52%) | 2 (67%) | 0 | 36 (39%) | |
| SP263* | n=66 | n=18 | n=136 | n=55 | n=13 | n=14 | p†=0.001 | n=302 |
| Non used | 43 (65%) | 7 (39%) | 67 (49%) | 20 (36%) | 2 (15%) | 10 (71%) | 149 (49%) | |
| Used | 23 (35%) | 11 (61%) | 69 (51%) | 35 (64%) | 11 (85%) | 4 (29%) | 153 (51%) | |
| Clinical assay | 8 (35%) | 5 (45%) | 59 (86%) | 24 (69%) | 7 (64%) | 4 (100%) | p†<0.0001 | 107 (70%) |
| LDT | 15 (65%) | 6 (55%) | 10 (14%) | 11 (31%) | 4 (36%) | 0 | 46 (30%) | |
| E1L3N* | n=66 | n=18 | n=136 | n=55 | n=13 | n=14 | p†=0.28 | n=302 |
| Non used | 54 (82%) | 18 (100%) | 111 (82%) | 42 (76%) | 12 (92%) | 12 (86%) | 249 (82%) | |
| Used (LDT only) | 12 (18%) | 0 | 25 (18%) | 13 (24%) | 1 (8%) | 2 (14%) | 53 (18%) | |
p†: X2 or Fisher exact bilateral test; LDT: laboratory developed test;
the numbers indicate those of participants who responded to the specific question.
External control
Information about “on slide” external control was provided by 296 participants. Of those, 99.7% used external (positive) control tissues, and tonsil was the most prevalent (71%) followed by placenta (38%), and lung cancer (31%). Commercial cell lines with known levels of PD-L1 expression were also used in 19% and the majority (52%) applied multiple tissue types as external control.
Quality assurance, Training and Guidelines
A total of 296 participants reported the status of quality assurance in their laboratories. While the vast majority (82%) of laboratories had external quality assessment (EQA) in place, 18% of participants reported a lack of EQA. Importantly, 63% of the laboratories participated in a formal EQA program(s), including three quarters of laboratories in Europe and Oceania, but only half of laboratories in the other regions (p=0.02) (Table 1). Of note, 39% of the laboratories performed only inter-laboratory validation. A total of 329 participants reported to score PD-L1 on clinical samples. Of those, 84% had undergone some training on the assessment of PD-L1 IHC. The rate was lower in the North America (69%), Africa and Middle East (67%) and Central & South America (64%). Of the 277 participants who had undergone training, the vast majority (89%) attended a training session(s) organized by venders, pharmaceutical companies, pathology societies or the IASLC (Table 1), but 11% had only undergone informal training, such as an intradepartmental session tutored by a colleague who had participated in a formal training session(s).
Some guidelines were applied in the vast majority of laboratories (96%). National or local guidelines were used by 62% of the participants, mainly in North America, Europe and Asia (73%, 68%, 61%, respectively) (Table 1), but 76% and 55% of laboratories in Central/South America and Africa and Middle East, respectively, only refered to the IASLC PD-L1 atlas (p<0.0001).
Turn-around-time and Reporting
Overall, the median turn-around time (TAT) from the acquisition of samples was 1–2 days, with a TAT of 2–3 days in South & Central America and Asia, and 3–4 days in Africa & Middle East (p<0.0001) (Table 1). The vast majority (76%) reported results within 3 days, while it took more than 5 days in 21% - 23% of laboratories in Asia, Central & South America and Africa & Middle East. TAT was the shortest in Europe. In North America, in particular, in the US, laboratories that sent out samples to other labs and scored them upon returning reported longer TAT. For reporting, the vast majority (86%) used a standardized report, but they were less frequently used in Africa & Middle East (78%) (Table 1).
Discussion
PD-L1 IHC is now routinely performed for advanced NSCLC patients to examine their eligibility for immune checkpoint blockade in a few indications. Due to the high running costs of the clinical tiral-validated assays, many laboratories utilize LDTs, leading to diverse implementation of PD-L1 testing across different regions as well as across different laboratories. To assess the current prevalence and practice of the PD-L1 testing globally and to identify potential issues and areas for improvement or disparities encountered in some countries, the Immune Biomarker Working Group of the IASLC pathology committee conducted an international survey for pathologists on PD-L1 testing in NSCLC.
This is the second survey conducted at the initiative of the IASLC to investigate the implementation of a theranostic test worldwide. The first survey, recently published by Smeltzer MP et al 11 and covering molecular testing in lung cancer, was also descriptive in nature, but was aimed at both clinicians and molecular pathologists. We deliberately chose herein to question pathologists only to assess issues related to the test itself in detail and to identify barriers for its implementation, without taking into account the perceptions of clinicians.
Like the survey on molecular testing, the worldwide dissemination was widespread with most responses coming from Europe, North America and Asia, while only 25 pathologists from Latin America, 22 from Africa and the Middle East and 13 from Oceania responded. Interestingly, the majority of the pathologists who responded were specializing in thoracic pathology, but 38% practiced other subspecialties or general pathology, which gave us a global vision of the real-life practice in pathology laboratories. Only 12 of the 344 responders did not conduct or offer clinical PD-L1 IHC for NSCLC, which is very encouraging in terms of test availability, and less than 10% of the laboratories outsourced the test. The vast majority tested biopsies and resection samples and 72% of our survey participants used cellblocks, in particular, 92%, 88% and 84% of those from Oceania, North America and Europe, in agreement with the good performance of cell blocks for PD-L1 testing compared to surgical samples 12–17. Although the use of cytology samples for PD-L1 IHC has not been validated in clinical trials, most of those studies reported high concordance in PD-L1 expression with a 50% cut-off between histology and cytology specimen irrespective of assays used 12–17.
Interestingly, 11% of the participants also conducted PD-L1 IHC on cytology smears, although there was a significant difference in the application of smears between regions. The quantification of PD-L1 expression on direct Papanicolaou-stained (PAP) cytology smears has been reported to be highly concordant with that on formalin-fixed, paraffin-embedded (FFPE) samples 16, 18, and in the study by Noll and Roy-Chowdhuri, PAP smears performed better than cell blocks as samples for PD-L1 IHC testing 16. Using smears will likely increase the availability of PD-L1 IHC for advanced NSCLC in which FNA may be the only sample procured for the diagnosis and biomarker testing, although there are no recommendations available yet for PD-L1 IHC on this type of sample 5 and large-scale studies are still warranted to confirm the performance of cytology smears in PD-L1 testing. While cytology samples can be used for PD-L1 IHC, many pathologists, in particular, non-cytopathology pathologists may find scoring PD-L1 expression in cytology samples challenging, partly due to the fragmented and scarce nature of tumor clusters in such specimens19. In the current survey, we failed to ask participnats how frequently they received both cytology and biopsy specimens from one procedure that may allow the pathologist to select a sample for PD-L1 IHC. It seems, however, that the practice varies to a large extent across different institutions and clinicians, but a combination of biopsy and cytology specimens are often obtained in one procedure, if the patient’s condition allows. Of those, biopies are preferred for PD-L1 IHC, but when biopsies do not contain adequate tumor cells or are not available, cytology samples, in particular, cellblocks are used for PD-L1 testing. Another issue associated with cytology specimens is their small sizes, given that PD-L1 expression is often under-scored in small samples 20, 21. While the size of biopsy was not recorded in the survey, bronchial biopsies are usually ~ 1mm; thus, combined with cytology specimens, a significant proportion of samples used for PD-L1 IHC are considered small. Awareness of the effect of small sample size on PD-L1 scoring around the 1% threshold is important, emphasizing the need for more or larger biopsies.
Optimal pre-analytical conditions are an important element for standardization of predictive biomarker teting. Unfortunately, however, only 54% of 332 participants who perform clinical PD-L1 IHC responded that they monitor pre-analytical conditions. Cold ischemia appears to have a significant impact on the performance of PD-L1 IHC22. Decalcification seems to slightly decrease the yield of staining, particularly when EDTA is used in combination with 22C3 clone 23. Furthermore, avoiding overfixation is of paramount importance since 20% and 10% of samples can be suboptimally stained with IHC using the SP142 and SP263 clones, respectively, when fixation duration is beyond 96 hours, while only 3% to 6% of samples may suffer suboptimal stainig with 12 to 72 hour fixation 24. Similarly, cellblock processing protocols affect PD-L1 staining. The Cellient automated system was reported to confer the strongest membranous staining with less cytoplasmic staining, while CytoLyt-based samples exhibited the poorest staining 25. Finally, PD-L1 protein can be degraded by time; thus, the age of FFPE slides, if not appropriatly stored after cutting, has been associated with a decrease in immunoreactivity 26, 27 leading to recommendations on the use of freshly cut slides for PD-L1 testing.
Among the antibodies used in practice, two clinical trial-validated clones, 22C3 and SP263, were most commonly used, by 69% and 51% of the participants respectively. However, only 60% of the participants with 22C3 IHC applied the clinical trial-validated, commercial assay, probably because the Dako IHC platform, which is required for the assay, is less prevalent across countries and the running costs are significantly higher with the clinical trial-validated assays than LDTs. In contrast, the SP263 commercial assay was more frequently used mainly in Europe and in Asia, but not as frequently in North America due in part to the less prevalent use of the Ventana platform in the region compared to others. In addition, a minority of participants used non-clinical trial clones, such as E1L3N or QR1. This is not surprising given that the clinical trial-validated clones are generally substantially more expensive with under reimbursement in some countries, and laboratories may not have access to the corresponding IHC platform required for the clinical trial–validated, commercial assay5. Thus, laboratories may, through choice or budget constrain, run their own PD-L1 IHC assay using a LDT, that has not been validated in a clinical trial. It is important to note, however, that any LDT will not necessarily deliver the same staining results as a commercial assay5. The variability in staining performance is not only due to the difference in antibody clones but also that in the ancillary chemistry and platform variables28. In addition, pre-analytical conditions can be critical for immunohistochemistry standardization, and the IHC protocol may need to be adjusted in accordance with the sample type 25, 29. Thus, thorough optimization and standardization of PD-L1 IHC 30, as well as quality control monitoring of the test, whether they are for an LDT or a commercial assay, are of paramount importance to achieve a constant staining performance. Unfortunately, the results of this survey have shown that a considerable minority (18%) of participating laboratories did not have QA in place, and only 63% of the laboratories participated in a formal EQA program(s) with significant regional disparities. The rate of formal EQA program participation reported was higher in Europe (72%) and Oceania (77%), while it was only 50% in the other regions. Of note, the Colleage of American Pathologists (CAP) has started offering PD-L1 proficiency tests 31 since the end of this survey; thus, it is likely that the majority of laboratories in the US currently have a formal EQA program(s) in place. Further, Nordic immunohistochemical Quality Control (NordiQC) has expanded proficiency testing for PD-L1 immunohistochemistry 32 that may have increased the rate of formal EQA participation.
Another important issue is the reproducibility of PD-L1 scoring since the assessment of PD-L1 IHC could be susceptible to inter- and intra-observer variability due to the semiquantitative nature of assay scoring. Although inter-observer agreements on PD-L1 tumor cell scoring have generally reported good interclass correlation coefficient [ICC] (0.8–0.9)5, 33–38 and intra-observer agreements were excellent (90% - 98%) in several studies36, 37, the question is whether the concordance of 80–90% is acceptable for a predictive biomarker testing. Considering the number of advanced NSCLC patients diagnosed per year (approximately 113,000 in 2018 in the US) and response rates stratified by PD-L1 tumor proportion score (TPS) based on the clinical trial data 1, 39–41, 10–20% of false positive results for the 50% cut-off could lead to treating 800 −1,500 patients with 1st line pembrolizumab alone, when additional chemotherapy might be helpful. Conversely, 10–20% of false negative results for the 50% cut-off could lead to combination therapy in 1,000 – 2,000 patients, 30% of which would have responded to the 1st line pembrolizumab only without a risk of additional side effects secondary to chemotherapy administration1, 41, 42.
To provide more reproducible PD-L1 IHC scoring, it is important for pathologists to attend training session(s) or gain more experience 5, 43. In this survey, 84% of participants reported attendance at some training on the assessment of PD-L1 IHC, but 11% of those had only undergone informal training. Since there are free training programs organized by vendors, pharmaceutical companies and pathology societies, either as formal hands-on sessions or via website, it is recommended that pathologists who score PD-L1 IHC participate in such a program(s)44. Alternatively, if PD-L1 IHC scoring is limited to thoracic patholologists or specific pathologists, they may be able to gain experience and achieve proficiency in a short period, and offer consistent scoring.
Adequate TAT and standardized reporting of the results are also important elements of PD-L1 testing. Overall, the median TAT was short (1–2 days), but there were some regional differences. It was longer in South & Central America, Asia and Africa & Middle East. While the vast majority (3/4) reported the results within 3 days, it took 5 or more days in 22% of laboratories in Asia, 21% in Central & South America and 30% in Africa & Middle East. Importantly, 25% of the US participants reported sending samples for PD-L1 IHC testing to other laboratories, adding to the TAT. In this survey, we did not ask the participants whether the PD-L1 IHC was performed in a reflex manner since we suspected it was as recommended in the IASLC PD-L1 IHC atlas. However, if it was not a reflex test, additional time spent on identifying the sample, etc. might have contributed to longer TAT. Similarly, while the vast majority of responding pathologists used a standardized report, this was not the case in one quarter of laboratories from Africa and the Middle East.
To our knowledge, this is the first comprehensive evaluation of pathologists’ perspectives on PD-L1 IHC in NSCLC; however, it is not without limitations. First, responses were received on a voluntary basis and there were multiple responses from the same institutions, albeit rare (data not shown), that may have resulted in duplicate or similar responses. Second, there appeared to be undersampling from Central & South America, Africa and the Middle East and Oceania. While it may not be possible to determine whether it is due to a lack of circulation of the questionnaire or a lack of available tests, regional oversampling and undersampling suggest our results may not accurately reflect the prevalence of PD-L1 testing across the globe. Although we advertised the survey on multiple society websites and contacted several specific pathology societies, we still may have failed to reach a large number of pathologists who assess PD-L1 IHC. However, although these laboratory and regional sampling issues limit our assessments, we believe it is still useful for identifying and understanding the prevalence and barriers to PD-L1 testing in NSCLC. We have now planned to conduct a novel survey that will be distributed to an extended (and exhausting) list of pathologists to involve various levels of organizations/Institutes and pathologists across different regions. We will add multiple precise pre-analytical and analytical questions along with the size of pathology practice and number of PD-L1 testing per year. We hope that the novel survey will allow us to understand the pre-analytical and analytical issues associated with PD-L1 IHC more in detail and come up with strategies to improve the quality of PD-L1 testing globally with standardization of PD-L1 IHC and high reproducibility of PD-L1 scoring among pathologists.
Conclusion
The results of this survey highlight the heterogeneity in PD-L1 testing practice across international regions as well as individual laboratories. The regional differences appear significant in PD-L1 testing status, PD-L1 antibody clones/assays used and TAT. In addition, a considerable minority reported a lack of QA, formal training and/or standardized reporting system. Given that PD-L1 IHC is predictive marker testing, constant and appropriate QA and pathologists’ participation in formal training sessions to achieve reproducible scoring are a key to improving the PD-L1 testing practice globally. In addition, despite the limitations of the study, with the majority of participants coming from Europe, North America and Asia with limited participation from Central & South America, Africa and the Middle East and Oceania, this survey clearly identified issues and disparities encountered in some countries regarding PD-L1 testing implementation. It highlights the need in some areas to set up actions to improve training and/or technical assistance to offer an optimized and standardized predictive biomarker for immunotherapies in lung cancer patients worldwide.
Supplementary Material
DISCLOSURES
Dr. Mino-Kenudson reports personal fees from H3 Biomedicine, personal fees from AstraZeneca, grants from Novartis, outside the submitted work.
Dr. NICHOLSON reports personal fees from MERCK, personal fees from BOEHRINGER INGELHEIM, grants and personal fees from PFIZER, personal fees from NOVARTIS, personal fees from ASTRA ZENECA, personal fees from BRISTOL MYER SQUIB, personal fees from ROCHE, personal fees from ASTRA ZENECA, personal fees from ABBVIE, personal fees from ONCOLOGICA, outside the submitted work.
Dr. PAPOTTI reports personal fees from Astra Zeneca, personal fees from Roche, personal fees from Pfizer, personal fees from MSD, personal fees from AbbVie, personal fees from EliLilly, outside the submitted work.
Dr. Motoi reports personal fees from AstraZeneca, personal fees from Chugai, grants from Ono, grants from NEC, personal fees from MSD, personal fees from Beckton Dickinson Japan, personal fees from Covidien Japan Inc, personal fees from Novartis, personal fees from Roche Diagnostics, personal fees from Miraca Life Science, personal fees from Taiho, outside the submitted work.
Dr. Dacic reports personal fees from Astra Zeneca, personal fees from Takeda, personal fees from Bayer, outside the submitted work.
Dr. Yatabe reports personal fees from MSD, personal fees from Chugai-pharma, personal fees from AstraZeneca, personal fees from Pfizer, personal fees from Roche/Ventana, from Agilent/Dako, personal fees from Thermo Fisher Science, personal fees from ArcherCD, personal fees from Novartis, personal fees from Elli-Lily, personal fees from Daiichi Sankyo, outside the submitted work.
Dr. Tsao reports personal fees from Merck, personal fees from BMS, personal fees from AstraZeneca, during the conduct of the study; grants and personal fees from Bayer, personal fees from Amgen, outside the submitted work.
Dr. Botling reports grants and personal fees from Bristol-Myers Squibb, personal fees from MSD, personal fees from Roche, personal fees from Pfizer, personal fees from Boehringer Ingelheim, personal fees from Novartis, personal fees from Illumina, outside the submitted work.
Dr. Bubendorf reports personal fees from BMS, personal fees from Astra Zeneca, grants and personal fees from Roche, grants and personal fees from MSD, personal fees from Bayer, personal fees from Boehringer Ingelheim, grants from Sanofi, personal fees from Pfizer, personal fees from Takeda, outside the submitted work.
Dr. Longshore reports personal fees from Amgen, grants and personal fees from AstraZeneca, personal fees from Bayer, personal fees from Blueprint Medicines, personal fees from Bristol-Myers Squibb, personal fees from Genentech, personal fees from GlaxoSmithKline, personal fees from Lilly, personal fees from Merck, personal fees from Novartis, personal fees from Pfizer, personal fees from Spectrum Pharmaceuticals, personal fees from Takeda, outside the submitted work.
Dr. Wistuba reports grants and personal fees from Genentech/Roche, grants and personal fees from Bayer, grants and personal fees from Bristol-Myers Squibb, grants and personal fees from AstraZeneca/Medimmune, grants and personal fees from Pfizer, grants and personal fees from HTG Molecular, grants and personal fees from Merck, personal fees from GlaxoSmithKline, grants and personal fees from Guardant Health, personal fees from MSD, grants from Oncoplex, grants from DepArray, grants from Adaptive, grants from Adaptimmune, grants from EMD Serono, grants from Takeda, grants from Amgen, grants from Karus, grants from Johnson & Johnson, grants from Iovance, grants from 4D, grants from Novartis, grants from Oncocyte, grants from Akoya, personal fees from Flame, outside the submitted work.
Dr. Lantuejoul reports personal fees from BMS, personal fees from Lilly, personal fees from Astra Zenenca, personal fees from MSD, outside the submitted work.
Dr. Hirsch reports serving on scientific advisory boards: Merck, Genentech/Roche, BMS, Regeneron/Sanofi, AstraZeneca/ Daiichi, and Novartis, outside the submitted work.
Dr. Kerr reports personal fees from BMS, Merck, AZ, Roche, Merck Serono, outside the submitted work.
Dr. Hwang reports grants and personal fees from Merck, grants from Astra-Zeneca, grants from Boehringer-Ingelheim, grants from Takeda, grants from EMD Serono, outside the submitted work.
Dr. Sholl reports grants from Genentech, personal fees from AstraZeneca, personal fees from EMD Serono, personal fees from Foghorn Therapeutics, outside the submitted work.
Remaining authors have nothing to disclose.
Dr. Lopez-Rios reports grants and personal fees from Lilly, grants and personal fees from Thermo Fisher, grants and personal fees from Roche, grants and personal fees from Pfizer, grants and personal fees from BMS, personal fees from Astra Zeneca, personal fees from Bayer, personal fees from MSD, outside the submitted work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 2.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078–2092. [DOI] [PubMed] [Google Scholar]
- 3.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040–2051. [DOI] [PubMed] [Google Scholar]
- 4.Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819–1830. [DOI] [PubMed] [Google Scholar]
- 5.Lantuejoul S, Sound-Tsao M, Cooper WA, et al. PD-L1 Testing for Lung Cancer in 2019: Perspective From the IASLC Pathology Committee. J Thorac Oncol 2020;15:499–519. [DOI] [PubMed] [Google Scholar]
- 6.Saxena P, Singh PK, Malik PS, et al. Immunotherapy Alone or in Combination with Chemotherapy as First-Line Treatment of Non-Small Cell Lung Cancer. Curr Treat Options Oncol 2020;21:69. [DOI] [PubMed] [Google Scholar]
- 7.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2019;381:2020–2031. [DOI] [PubMed] [Google Scholar]
- 8.Administration USFaD. FDA approves nivolumab plus ipilumab for first-line mNSCLC (PD-L1 tumor expression ≥ 1%). Available at https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-nivolumab-plus-ipilimumab-first-line-mnsclc-pd-l1-tumor-expression-1.
- 9.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919–1929. [DOI] [PubMed] [Google Scholar]
- 10.Administration USFaD. FDA approves atezolizumab for first-line treatment of metastatic NSCLC with high PD-L1 expression. Available at https://www.fda.gov/drugs/resourcesinformation-approved-drugs/fda-approves-atezolizumab-first-line-treatment-metastatic-nsclchigh-pd-l1-expression. [Google Scholar]
- 11.Smeltzer MP, Wynes MW, Lantuejoul S, et al. The International Association for the Study of Lung Cancer Global Survey on Molecular Testing in Lung Cancer. J Thorac Oncol 2020. [DOI] [PubMed] [Google Scholar]
- 12.Skov BG, Skov T. Paired Comparison of PD-L1 Expression on Cytologic and Histologic Specimens From Malignancies in the Lung Assessed With PD-L1 IHC 28–8pharmDx and PD-L1 IHC 22C3pharmDx. Appl Immunohistochem Mol Morphol 2017;25:453–459. [DOI] [PubMed] [Google Scholar]
- 13.Heymann JJ, Bulman WA, Swinarski D, et al. PD-L1 expression in non-small cell lung carcinoma: Comparison among cytology, small biopsy, and surgical resection specimens. Cancer Cytopathol 2017;125:896–907. [DOI] [PubMed] [Google Scholar]
- 14.Russell-Goldman E, Kravets S, Dahlberg SE, et al. Cytologic-histologic correlation of programmed death-ligand 1 immunohistochemistry in lung carcinomas. Cancer Cytopathol 2018;126:253–263. [DOI] [PubMed] [Google Scholar]
- 15.Ilie M, Juco J, Huang L, et al. Use of the 22C3 anti-programmed death-ligand 1 antibody to determine programmed death-ligand 1 expression in cytology samples obtained from non-small cell lung cancer patients. Cancer Cytopathol 2018;126:264–274. [DOI] [PubMed] [Google Scholar]
- 16.Noll B, Wang WL, Gong Y, et al. Programmed death ligand 1 testing in non-small cell lung carcinoma cytology cell block and aspirate smear preparations. Cancer Cytopathol 2018;126:342–352. [DOI] [PubMed] [Google Scholar]
- 17.Wang G, Ionescu DN, Lee CH, et al. PD-L1 testing on the EBUS-FNA cytology specimens of non-small cell lung cancer. Lung Cancer 2019;136:1–5. [DOI] [PubMed] [Google Scholar]
- 18.Lozano MD, Abengozar-Muela M, Echeveste JI, et al. Programmed death-ligand 1 expression on direct Pap-stained cytology smears from non-small cell lung cancer: Comparison with cell blocks and surgical resection specimens. Cancer Cytopathol 2019;127:470–480. [DOI] [PubMed] [Google Scholar]
- 19.Kerr K, Tsao M, Yatabe Y, et al. OA03.03 Phase 2B of Blueprint PD-L1 Immunohistochemistry Assay Comparability Study. Journal of Thoracic Oncology 2018;13:S325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bigras G, Mairs S, Swanson PE, et al. Small Biopsies Misclassify up to 35% of PD-L1 Assessments in Advanced Lung Non-Small Cell Lung Carcinomas. Appl Immunohistochem Mol Morphol 2018;26:701–708. [DOI] [PubMed] [Google Scholar]
- 21.Thunnissen E, Kerr KM, Dafni U, et al. Programmed death-ligand 1 expression influenced by tissue sample size. Scoring based on tissue microarrays’ and cross-validation with resections, in patients with, stage I-III, non-small cell lung carcinoma of the European Thoracic Oncology Platform Lungscape cohort. Mod Pathol 2020;33:792–801. [DOI] [PubMed] [Google Scholar]
- 22.van Seijen M, Brcic L, Gonzales AN, et al. Impact of delayed and prolonged fixation on the evaluation of immunohistochemical staining on lung carcinoma resection specimen. Virchows Arch 2019;475:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forest F, Cote G, Laville D, et al. Impact of delayed fixation and decalcification on PD-L1 expression: a comparison of two clones. Virchows Arch 2019;475:693–699. [DOI] [PubMed] [Google Scholar]
- 24.Barberà A, Marginet Flinch R, Martin M, et al. The Immunohistochemical Expression of Programmed Death Ligand 1 (PD-L1) Is Affected by Sample Overfixation. Appl Immunohistochem Mol Morphol 2020. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd IE, Zhou W, Witt BL, et al. Characterization of PD-L1 Immunohistochemical Expression in Cell Blocks With Different Specimen Fixation and Processing Methods. Appl Immunohistochem Mol Morphol 2019;27:107–113. [DOI] [PubMed] [Google Scholar]
- 26.Sato Y, Fujimoto D, Uehara K, et al. Reduced Tumour Proportion Scores for Programmed Cell Death Ligand 1 in Stored Paraffin Tissue Sections. Anticancer Res 2018;38:1401–1405. [DOI] [PubMed] [Google Scholar]
- 27.Giunchi F, Degiovanni A, Daddi N, et al. Fading With Time of PD-L1 Immunoreactivity in Non-Small Cells Lung Cancer Tissues: A Methodological Study. Appl Immunohistochem Mol Morphol 2018;26:489–494. [DOI] [PubMed] [Google Scholar]
- 28.Lawson NL, Dix CI, Scorer PW, et al. Mapping the binding sites of antibodies utilized in programmed cell death ligand-1 predictive immunohistochemical assays for use with immune-oncology therapies. Mod Pathol 2020;33:518–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain D, Nambirajan A, Borczuk A, et al. Immunocytochemistry for predictive biomarker testing in lung cancer cytology. Cancer Cytopathol 2019;127:325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torlakovic E, Albadine R, Bigras G, et al. Canadian Multicenter Project on Standardization of Programmed Death-Ligand 1 Immunohistochemistry 22C3 Laboratory-Developed Tests for Pembrolizumab Therapy in NSCLC. J Thorac Oncol 2020;15:1328–1337. [DOI] [PubMed] [Google Scholar]
- 31.Pathologists CoA. Proficiency Testing. Available at https://www.cap.org/laboratoryimprovement/proficiency-testing. 2020.
- 32.NordiQC. PD-L1. Available at https://www.nordiqc.org/epitope.php?id=107. 2020.
- 33.Tsao MS, Kerr KM, Kockx M, et al. PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J Thorac Oncol 2018;13:1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rimm DL, Han G, Taube JM, et al. A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol 2017;3:1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujimoto D, Sato Y, Uehara K, et al. Predictive Performance of Four Programmed Cell Death Ligand 1 Assay Systems on Nivolumab Response in Previously Treated Patients with Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:377–386. [DOI] [PubMed] [Google Scholar]
- 36.Cooper WA, Russell PA, Cherian M, et al. Intra- and Interobserver Reproducibility Assessment of PD-L1 Biomarker in Non-Small Cell Lung Cancer. Clin Cancer Res 2017;23:4569–4577. [DOI] [PubMed] [Google Scholar]
- 37.Phillips T, Simmons P, Inzunza HD, et al. Development of an automated PD-L1 immunohistochemistry (IHC) assay for non-small cell lung cancer. Appl Immunohistochem Mol Morphol 2015;23:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rehman JA, Han G, Carvajal-Hausdorf DE, et al. Quantitative and pathologist-read comparison of the heterogeneity of programmed death-ligand 1 (PD-L1) expression in non-small cell lung cancer. Mod Pathol 2017;30:340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 40.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2017, National Cancer Institute. 2020. [Google Scholar]
- 41.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 42.Boloker G, Wang C, Zhang J. Updated statistics of lung and bronchus cancer in United States (2018). J Thorac Dis 2018;10:1158–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adam J, Hofman V, Mansuet-Lupo A, et al. P2.09–17 Real-World Concordance Across Pathologists for PD-L1 Scoring in Non-Small Cell Lung Cancer: Results from a Large Nationwide Initiative. Journal of Thoracic Oncology 2019;14:S775. [Google Scholar]
- 44.AstraZeneca. iD PD-L1. Available at https://www.idpdl1.com/quality-andtraining.html#training-interpretation. 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.