Abstract

This work reports on the synthesis and characterization of three tritopic receptors and their binding properties toward various anions, as their tetrabutylammonium salts, and three alkali metal–acetate salts by UV–vis, fluorescence, 1H, 7Li, 23Na, and 39K NMR in MeCN/dimethyl sulfoxide (DMSO) 9:1 (v/v). Molecular recognition studies showed that the receptors have good affinity for oxyanions. Furthermore, these compounds are capable of ion-pair recognition of the alkali metal–acetate salts studied through a cooperative mechanism. Additionally, molecular modeling at the density functional theory (DFT) level of some lithium and sodium acetate complexes illustrates the ion-pair binding capacity of receptors. The anion is recognized through strong hydrogen bonds of the NH– groups from the two urea sites, while the cation interacts with the oxygen atoms of the polyether spacer. This work demonstrates that these compounds are good receptors for anions and ion pairs.
1. Introduction
Cations and anions are involved in a wide variety of issues related to biological, chemical, and industrial processes, as well as various environmental and health problems, among others. Therefore, molecular recognition of cationic and anionic species has been a topic of great interest during the past few decades.1−10 In particular, anion recognition remains challenging due to the intrinsic properties of anions, such as their diverse geometry, pH dependence, and high solvation energies.11−15 The design of cyclic and acyclic neutral receptors for anion recognition usually includes amide, urea, or thiourea groups as recognition sites, since they can form strong and directional hydrogen bonds with anions.3,11−15 On the other hand, the inclusion in the structure of the receptors of an additional site capable of binding cations has been a strategy to improve the affinity toward their anionic targets. In this sense, there has been a significant increase in the last decade of reports related to heteroditopic and multitopic receptors for ion-pair recognition.16−21 The correct design of this type of compounds can result in a higher affinity and selectivity due to a cooperative effect regulated by a balance of enthalpic and entropic contributions.22 However, heteroditopic and multitopic compounds are generally based on macrocycles and thus often difficult to synthesize. For this reason, it is important to design efficient and easily obtainable systems for different purposes. Taking this into account, ion-pair recognition is a challenging topic in modern supramolecular chemistry.16−19,21,23−25
This work reports on the synthesis, characterization, and molecular recognition studies of three acyclic tritopic receptors containing two urea groups for anion recognition, linked through a polyether bridge as the cation recognition motif. Additionally, two 1- or 2-naphthyl chromophore units were incorporated for optical sensing (see Scheme 1). These compounds with flexible acyclic structures were designed to increase their versatility and adaptability to the size and geometry of various anions. Furthermore, with this structural design, both urea groups could simultaneously establish various strong hydrogen bonds with anions, thus allowing a pseudo ether-crown arrangement and the binding of the cation via ion–dipole and cation−π interactions. Anion and ion-pair recognition studies were performed in a mixture of MeCN/dimethyl sulfoxide (DMSO) 9:1 (v/v) by UV–vis, fluorescence, 1H, 7Li, 23Na, and 39K NMR techniques. The results from molecular recognition studies demonstrated that the receptors show, in general, good affinity for the anions, as their tetrabutylammonium (TBA) salts. Moreover, receptors can recognize alkali metal–acetate salts through a cooperative mechanism. Additional molecular modeling studies for some complexes were performed with a B3LYP/6-31G* density functional theory (DFT) level of theory. This work demonstrates that the acyclic tritopic compounds described here are good receptors for anion and ion-pair recognition.
Scheme 1. Chemical Structures of the Receptors Studied in this Work.
2. Results and Discussion
2.1. Synthesis and Structural Characterization
The new bis-urea compounds R1–R3 (Scheme 1) were synthesized in moderate to high yields from their corresponding diamine precursors (D1 or D2) previously reported by our research group;21 see Scheme 2. The receptors were characterized by IR and NMR (1H and 13C) spectroscopy (Figures S1–S6) using standard one- and two-dimensional techniques, mass spectrometry, and elemental analysis. In all cases, the spectroscopic data were consistent with the proposed structures. The formation of bis-urea receptors was first confirmed by 1H NMR spectra that gave signals corresponding to the −NH urea hydrogens at δ = 8.67 and 8.48 ppm for R1, 8.48 and 8.21 ppm for R2, and 8.63 and 8.46 ppm for R3. Furthermore, the 13C NMR spectra show signals consistent with urea carbonyl groups at δ = 154 ppm with one decimal difference between the three receptors. On the other hand, electrospray ionization (ESI) mass spectra in the positive mode gave peaks at m/z = 715.8 and 671.3 that correspond to the [M + H]+ ions of compounds R1/R2 and R3. Additionally, the formation of sodium complexes was also evidenced by this technique (see Section 4).
Scheme 2. Synthesis of Bis-Urea Receptors R1–R3.
2.2. Molecular Recognition Studies
2.2.1. UV–Vis Studies
Solution studies by UV–vis were carried out in a MeCN/DMSO 9:1 (v/v) mixture. As expected, R1 and R3 have a similar spectral behavior with an absorbance maximum at 325 nm. However, compound R2, with a different chromophore, has a structured spectrum with absorption maxima at 290, 310, and a broad shoulder between 320 and 360 nm. Titration experiments for R1–R3 were performed in the same medium, with several monovalent anions of different basicity and geometrical characteristics such as halides and oxyanions: F–, CH3COO– (OAc–), H2PO4–, and HP2O73– (PPi3–). Particularly, with the anions NO3– and HSO4–, it was not possible to monitor complexation due to negligible changes in the absorption spectra. The results obtained from the titrations for most of the complexes showed significant changes in the absorption spectra of the receptors. However, two general cases were observed. First, the acetate complexes: among these systems, only the spectra of R2 showed significant changes due to the presence of increasing concentrations of OAc– (Figure 1). Therefore, the data were adjusted by the method of least squares, considering a complex of a stoichiometry of 1:2 receptor/OAc–, which allowed obtaining binding constants K11 = 4058 and K12 = 1835 (with log β = 6.87). On the contrary, the titrations of R1 and R3 with this anion did not produce significant changes in the absorption spectra, so these data were not adequate to obtain binding constants with sufficient reliability (Figures S7 and S14).
Figure 1.

Absorption spectra of R2 (3 × 10–5 M) with increasing amounts of OAc– ((0–7.94) × 10–4 M) in MeCN/DMSO 9:1 (v/v) at 298 K. The upper right inset shows the abundance of species during titration, where H = receptor and G = anion guest. Dashed lines represent the theoretical profiles from data fit.
Second, for the complexes between all receptors (R1–R3) and the basic anions F–, H2PO4–, and PPi3–, both bathochromic and hyperchromic behaviors of the receptor spectra were observed as the anion concentration increased during the experiments, probably due to a combination of complexation and deprotonation equilibria. Fitting of these data, as described in Section 4.1.3, allowed us to obtain the binding constants with a good approximation but with an associated error greater than 10% (see Figures S8–S13 and S15–S17). Considering all of the above, it is evident that UV–vis is not a suitable technique to study the complexation process.
2.2.2. Fluorescence Studies
The fluorescence spectra of R1–R3 were recorded in MeCN/DMSO 9:1 (v/v). In this sense, R1 and R3 show two emission bands corresponding to the monomer and excimer bands with a maximum at 378 and 450 nm with λex = 307 nm. On the other hand, R2 has an unstructured emission band extending from 323 to 515 nm with an intensity maximum at 367 nm and no obvious excimer band, with λex = 298 nm; see Figure S18. Considering the results obtained for the receptor–acetate complexes by the UV–vis technique, titrations were carried out with receptors R1–R3 and OAc– by fluorescence.
As an example, Figure 2 shows the spectra for the R2–OAc– complex; the spectra for the rest of the experiments are shown in Figures S19 and S20. For all of these systems, the data were fitted considering a 1:2 receptor/OAc– stoichiometry. During the titration of R1 and R3 with OAc–, both receptors showed a decrease in their excimer bands, R1 being the one with the greatest decrease, indicating that this compound must undergo a conformational change to bind to the anion, which is unfavorable for excimer formation. The quenching of fluorescence for these systems can be attributed to the electron transfer (eT) from the carbonyl of the urea group to the excited naphthalene group. In addition, the anion OAc– caused a slight red shift (∼2–4 nm) of the receptor’s emission band after complexation. This can be attributed to the stabilization of the excited state due to hydrogen bonding of the anion. Both mechanisms have been reported for similar receptor–guest systems by Amendola et al. and Lopéz-Martínez et al.26,27 The binding constants of R3–OAc–, K11, and K12, are slightly lower when compared to the respective ones of R1 and R2; see Table 1. In this regard, the longer polyether chains of R1 and R2 (see Scheme 1) results in structures with better acetate complex stabilities.
Figure 2.

Fluorescence spectra of R2 (5.0 × 10–6 M) with increasing amounts of OAc– ((0–2.35) × 10–3 M) in MeCN/DMSO 9:1 (v/v) at 298 K and λex = 298 nm. The upper right inset shows the abundance of species during titration, where H = receptor and G = anion guest. Dashed lines represent the theoretical profiles from data fit.
Table 1. Logarithmic Binding Constants (log Ka) for Complexes between Receptors and OAc– Obtained by Fluorescence, in MeCN/DMSO 9:1 (v/v) at 298 K.
On the other hand, it is important to mention that the cooperativity factor α shown in Table 1 indicates that for R2 and R3, the cooperativity in binding to acetate is positive, while for R1 it is barely negative. In this context, it is likely that these values of α can be rationalized in terms of a balance between the conformational freedom of the receptors and the structural complementarity of the polyether chain to stabilize the TBA cation as a function of its length.
Therefore, considering the above, the structure of R2 has a large conformational freedom both in the polyether chain and in the regions of the urea units, where naphthalene can rotate freely, which in this case is apparently an advantage over R1 and R3, evidenced by the magnitude of the log β value of R2. On the other hand, although R1 has a polyether chain capable of stabilizing the TBA cation just like R2, its conformational freedom is due to the position of the naphthalene unit, which apparently hinders the conformational changes necessary for positive cooperativity. Nonetheless, in terms of complementarity, the union of R1 with TBA–OAc– is favored enthalpically. Finally, in the case of R3, although it has less conformational freedom than R1 and R2, it seems to favor the binding of two anions, which indicates that the conformational changes experienced by R3 favor hydrogen bonds, causing a loss of the interaction with the cation and therefore, a lower number of additive interactions, which is evidenced by its positive cooperativity but with a lower log β with respect to the other receptors.
To study the ion-pair recognition ability of compounds R1–R3, receptor titrations were performed with the OAc– anion in the presence of 1 equiv of Li+, Na+, or K+ (as their perchlorate salts). As a representative example, Figure 3 shows the spectra obtained for the titration of R3 + Na+ and OAc–, while the remaining results of these studies are shown in Figures S21–S28. Table 2 summarizes the binding constants and cooperativity factor (CF) of the various salt complexes. By simple inspection of the data, it can be seen that the receptors showed a higher affinity for acetate in the presence of alkali metals, with increases in log β ranging from 1.51 to 2.34, on a logarithmic scale, compared to the respective values in Table 1.
Figure 3.

Fluorescence spectra of a solution of R3 (5.0 × 10–6 M) and 1 equiv of Na+ in MeCN/DMSO 9:1 (v/v), at 298 K and λex = 307 nm, with increasing amounts of OAc– ((0–3.31) × 10–4 M). The upper right inset shows the abundance of species during titration, where H = receptor and G = anion guest. Dashed lines represent the theoretical profiles from the data fit.
Table 2. Logarithm of Apparent Binding Constants (log Ka) for Complexes between Receptors R1–R3 and OAc– b in the Presence of 1 equiv of Alkali Perchlorate Salt Obtained by Fluorescence, in MeCN/DMSO 9:1 (v/v) at 298 K.
| [R + Li+] |
[R + Na+] |
[R + K+] |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| receptor | log K11 | log K12 | log β | CFc | log K11 | log K12 | log β | CFc | log K11 | log K12 | log β | CFc |
| R1 | 4.44 | 3.82 | 8.26 | 52 | 4.29 | 3.77 | 8.05 | 32 | 4.51 | 3.86 | 8.37 | 68 |
| R2 | 4.61 | 4.39 | 9.00 | 155 | 4.73 | 4.37 | 9.10 | 195 | 4.66 | 4.30 | 8.96 | 141 |
| R3 | 4.39 | 3.68 | 8.06 | 69 | 4.51 | 3.96 | 8.47 | 178 | 4.62 | 3.95 | 8.56 | 219 |
Error less than 10%.
The acetate anion was added as its tetrabutylammonium salt.
The cooperativity factor in this case is given by CF = β(M+)/β(none) where β(M–2) is the overall constant in the presence (β(M+)) or absence (β(none)) of alkali metal cation.
On the other hand, considering the cooperativity factor obtained in this case, the relationship between β(M–2) in the presence and absence of alkali metal cations, it can be seen that in all receptors the acetate binding is favored in the presence of cations in the order R1 < R2 ≈ R3. In general, the CF values obtained for R1 indicate that their binding to acetate is more favored with potassium than with sodium, with CF values of 68 and 32, respectively.
In the case of R2, a higher affinity for acetate is observed in the presence of sodium with a cooperative factor of 195, while with potassium and lithium, these values are closer with cooperative factors of 155 and 141, respectively. The fact that R1 is more favored with potassium and R2 is more favored with sodium is in agreement with the structural design of the receptors and with the findings of our previous work.21 Regarding the latter, it was observed in the solid state that the corresponding precursor polyethers of R1 and R2 are capable of coordinating potassium, and complexes with sodium were also evidenced by mass spectrometry.
Regarding R3, a clear trend is observed where the cooperativity factor increases in the presence of alkali metal cations in the order Li < Na < K. This apparently contradicts our design criteria related to the length of the polyether chain. In this sense, these experiments allow it to affirm that the length of the polyether chain is an important factor to consider in the choice of an appropriate cation for the binding of a target anion. Although considering the findings for R3 as well as those previously discussed in conformational terms for the complexes between the receptors and TBA acetate, it is evident that a more global vision in enthalpic and entropic aspects should be considered.
In addition, it is important to highlight that the values obtained for the cooperative factor of the receptors R1–R3 are similar to those reported by Bunchuay et al.,30 and higher to those reported by Romański et al.,18,31,32 Piątek et al.,23 and by our group.21 However, care must be taken with these comparisons because other factors must be considered, such as the solvent and the host–guest binding models related to the calculation of the cooperativity factor.
2.2.3. NMR Studies
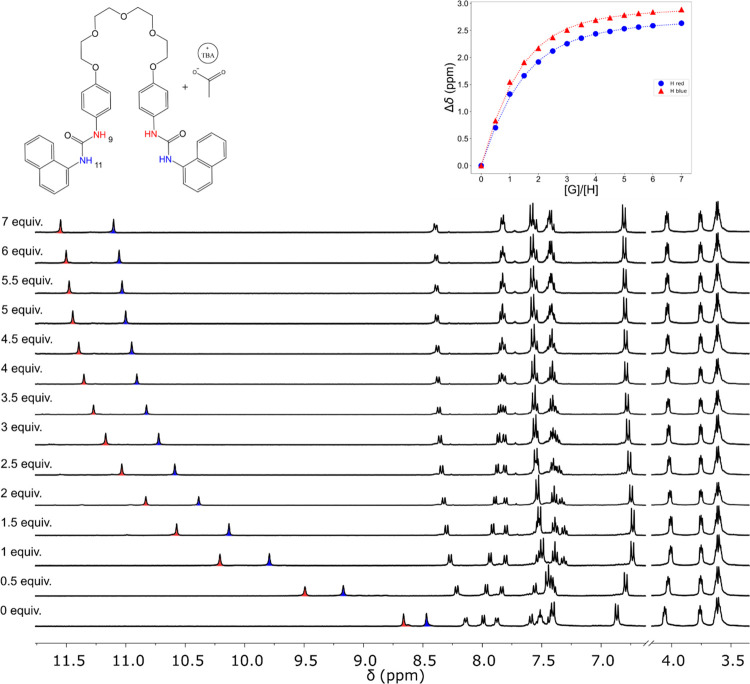
To obtain a better understanding of the anion complexes, compounds R1–R3 were titrated with different anions by the 1H NMR technique in CD3CN/DMSO-d6 9:1 (v/v). As shown in Figure 4, after the addition of OAc–, the signals of the N–H ureidic protons of the R1 receptor (colored in red and blue) were progressively and significantly downfield shifted. These results suggest that the acetate is bound through hydrogen bonds with the N–H hydrogens of both ureas; this behavior has been widely described in the literature.3,11−14,21 Additionally, the signals of the naphthyl hydrogens adjacent to ureidic oxygen, which are influenced by hydrogen bonding, also shift downfield. The rest of the signals of the naphthyl hydrogens experienced an upfield shift because of a higher electron density due to the recognition of the anion by urea, possibly accompanied by an anion−π interaction.33,34 It was observed that the phenyl protons of the receptors closest to the polyether chain experienced two behaviors in the cases of AcO–, BzO–, and H2SO4– titrations: first an upfield chemical shift change and then a downfield chemical shift change, thus indicating two equilibria in the complexation process. On the other hand, there were no significant changes for the methylene hydrogens of the polyether chain. Table 3 summarizes the highest chemical shift changes induced by complexation (Δδ) for R1, as well as those obtained for the respective titrations of compounds R2 and R3 (see Figures S34 and S41 for 1H NMR spectra from these titrations). A similar behavior was observed for all of the complexes formed between receptors and the OBz–, HSO4–, and NO3– anions although with minor chemical shift changes (Figure S61 compares differences in Δδ for the receptor ureidic proton between various complexes; see Figures S29–S31, S35–S37, and S42–S43 for data spectra). The stability constants summarized in Table 4 evidence a lower binding affinity of the receptors toward these anions compared to the more basic OAc– anion. These constants are in accordance with the tendency of the basicity, except for bisulfate, considering that the pKa values of the acid–base pair in acetonitrile are: OAc– (23.5), OBz– (21.5), F– (16.8), NO3– (10.6), and HSO4– (7.6).35−37
Figure 4.
1H NMR spectra of R1 (2.5 × 10–3 M) with increasing amounts of OAc– (0–0.05 M) in CD3CN/DMSO-d6 9:1 (v/v) at 298 K. The upper right inset shows the abundance of species during titration, where H = receptor and G = anion guest. Dashed lines represent the theoretical profiles from data fit.
Table 3. Chemical Shift Changes, Δδ (ppm), at Saturation for Different Protons of Compounds R1–R3 Induced by OAc–, in CD3CN/DMSO-d6 9:1 (v/v) at 298 Ka.
|
R1 |
R2 |
R3 |
|||
|---|---|---|---|---|---|
| proton | Δδ (ppm) | proton | Δδ (ppm) | proton | Δδ (ppm) |
| H-20 | 0.25 | H-21 | 0.21 | H-19 | 0.28 |
| H-11 | 2.63 | H-11 | 3.56 | H-10 | 3.08 |
| H-9 | 2.89 | H-9 | 3.62 | H-8 | 3.09 |
Δδ = δObs at saturation – δ of free receptor.
Table 4. Logarithmic Binding Constants (log Ka) for Complexes between Receptors and Diverse Anionsb Obtained by 1H NMR, in CD3CN/DMSO-d6 9:1 (v/v) at 298 Ke.
Furthermore, the geometric characteristics of the species that constitute the complexes are also important. For example, all three receptors showed the highest affinities for OAc– and the lowest affinities for NO3–. Taking all of these findings together, it can be concluded that strong hydrogen bonds are the driving force in the molecular recognition process between these receptor–anion systems.
The anomalous behavior of bisulfate and nitrate could be explained not only by the geometry but also with the stabilization energy of the anion determined through a calculation with a semiempirical level.38 In this sense, bisulfate has a better stabilization energy than nitrate, and this may explain the higher binding constant for bisulfate than for nitrate.
On the other hand, when comparing the log β values for the acetate complexes shown in Table 4 with those obtained by fluorescence (Table 1), it is evident that the values are considerably higher in the latter technique. This is not surprising considering that both techniques involve very different concentrations of the species studied.39 In this sense, another difference observed with respect to the studies of the fluorescence technique is the decrease in the cooperativity factor (α) for R1 and R2 (Table 4), which shows that the binding to acetate is not cooperative for R2 and negative for R1. Such differences, as mentioned above, can be mainly attributed to different concentrations used in both techniques. In this sense, some equilibria can be favored at higher concentrations in the case of multitopic systems, and therefore the formation of species that can interfere or compete with the receptor, thus affecting the observed property. Unfortunately, under the experimental conditions used in this work, it is not possible to accurately determine the origin of this complex phenomenon. Despite the differences mentioned, they are similar in trend, although the log β value is slightly higher in the case of R2.
A different expected behavior was observed for the rest of the receptor–anion systems. In these cases, the presence of the highly basic anions F–, H2PO4–, and PPi3– caused deprotonation of the −NH hydrogens of urea groups in all receptors; therefore, no data fitting was performed with them (see Figure 5 as a representative example and Figures S32, S33, S38–S40, and S44–S46 for spectra of titrations for the remaining systems). Fluoride and H2PO4– are special cases since they are not particularly strong bases in acetonitrile. In this less polar medium, the formation of the H-bond complex is favored. However, the deprotonation observed with F– and H2PO4– can be attributed to an excess of the anions that triggers the formation of the self-complex [HX···X] (X = F–, H2PO4–) released by the H-bond complex. Deprotonation by this type of anions has been reported by Fabbrizzi in several previous works.40,41 These findings are all in agreement with the results obtained by UV/vis and fluorescence techniques and validate the analysis discussed above.
Figure 5.
1H NMR spectra of R1 (2.5 × 10–3 M) with increasing amounts of F– (0–6.25 × 10–3 M) in CD3CN/DMSO-d6 9:1 (v/v) at 298 K.
On the other hand, considering the results obtained by fluorescence related to the ion-pair recognition capacity of the compounds, analogous experiments were carried out using this technique for titrations of the receptors with the OAc– anion in the presence of 1 equiv of Li+, Na+, or K+. The 1H NMR spectra obtained for these experiments are shown in Figures S52–S60. However, in all cases, the formation of the precipitate by complexation was observed, being more pronounced in R3. Therefore, it was not possible to determine reliable binding constants or further data analysis, under concentration conditions required by the 1H NMR technique.
To obtain direct evidence of the interaction between the alkali metal cations and the polyether chain of the receptors (R1–R3), 7Li, 23Na, and 39K NMR experiments were performed with equimolar mixtures of alkali metal perchlorate salts and receptors. Figure 6 shows the 7Li NMR spectra for free perchlorate as well as for its mixtures with 1 equiv of the receptors. In this figure, a downfield shift of lithium can be observed due to the presence of receptors. These chemical shift changes must be attributed to the interaction between the quadrupole of the cation and the dipole of the oxygen atoms of the polyether chain. The magnitude of these changes follows the trend R3 > R2 > R1, and according to them, it can be concluded that R3 coordinates more efficiently to lithium under these conditions. Furthermore, the lithium signal for the free salt is sharp and well defined, but in the presence of the receptors, the lithium signal appears broader and with a shoulder; this is more evident with R1, in this case unfolded and with a signal at −1.276 ppm corresponding to free lithium, thus evidencing a slow exchange of the cation in the NMR time scale.
Figure 6.
7Li NMR spectra of solutions of free LiClO4 (2.5 × 10–3 M) and its mixtures with 1 equiv of R1, R2, and R3; in the latter case, both the concentrations of the salt and the receptor were 2.0 × 10–3 M. All spectra were obtained in CD3CN/DMSO-d6 9:1 (v/v) at 298 K.
Regarding the 23Na NMR experiments shown in Figure S62, it can be seen that the sodium signal shows an upfield shift when R1 and R2 are present, indicating that sodium is positioned in the protective zone of the aromatic rings of the receptors, showing that the cation−π interaction plays an important role in the recognition, being more pronounced for R2 with a greater change than that for R1. On the other hand, in the case of the mixture of sodium perchlorate and R3, the sodium signal showed a downfield shift in a higher magnitude than those observed for the cases with R1 and R2. This shows that the additional oxygen atom in the polyether chain of these receptors influences the binding mode of the receptor to this cation.
Figure S63 shows the 39K NMR studies in which it can be seen that the potassium signal from free potassium perchlorate appears broad and low in intensity due to its quadrupolar nature and the concentrations used. In the case of the mixture solutions of this salt and the receptors, the potassium signal experienced downfield with small changes (>0.009 ppm), although this signal becomes sharper, probably indicating that the receptors compete for the cation against its perchlorate counterion and causing a rapid exchange of the potassium nucleus on the NMR time scale. This behavior has been widely described in the literature and also shows that the species of the ionic pair are in contact in the medium used.42
It is important to note that ion-pair experiments, such as those performed by fluorescence, must be designed using structural criteria of the receptor and considering the salt cannot interfere with the property observed, and that the counterions of the salts used are low coordinating, so as not to compete for the target guest.
Through the experiments with 7Li, 23Na, and 39K, we have verified that the conditions used were the ideal ones for the cases of R1 and R2, since it is shown that the perchlorate anion (as a counterion) complies with the adequate characteristics mentioned above and also because the results are consistent with those obtained by fluorescence. Nevertheless, this is not the case for R3, since the perchlorate counterion was shown to be able to bind the receptor, which was evidenced through the changes of the chemical shift of ureic protons when compared to the free receptor in R3, in the following order: R3–LiClO4 (Δδ = 0.215) > R3–NaClO4 (Δδ = 0.195) > R3–KClO4 (Δδ = 0.099); all values are in ppm.
On the other hand, these results mentioned for R3 agree with the trend shown for the cooperativity factor obtained by fluorescence, this being lower for lithium, intermediate for sodium, and higher for potassium, although this could go against some of the structural complementarity criteria, considering the size of the receptor and the cations. However, it makes sense considering that when a perchlorate salt is present in the medium and the titration is performed with TBA acetate, it should cause the displacement of the perchlorate anion, which, in energetic terms (as observed in NMR), leads to a greater penalty in the order LiClO4 > NaClO4 > KClO4.
2.3. Molecular Modeling
The geometry-optimized molecular structures obtained with the B3LYP/6-31G* level of theory for the ion-pair complexes formed between R2 and R3 with sodium and lithium acetate salts, respectively, are shown in Figure 7.
Figure 7.
Perspective view of the calculated molecular structures of (a) R2–sodium acetate and (b) R3–lithium acetate with the B3LYP/6-31G* level of theory.
According to the calculated molecular structure of R2–sodium acetate in Figure 7a, both urea groups of the R2 receptor interact with one acetate anion through four strong hydrogen bonds, which are indicated in the form of dashed lines, with N–H···O distances in the range from 1.5 to 2.0 Å. In addition, the sodium counterion interacts with the polyether spacer, with distances between four of the five oxygen atoms and the cation in the range from 2.7 and 2.9 Å.
On the other hand, Figure 7b shows the molecular structure of the complex formed between R3 and lithium acetate. Similar to the complex described above, both urea groups form strong hydrogen bonds with N–H···O distances in the range from 1.6 and 1.9 Å. As for the lithium cation, it interacts with the four oxygen atoms of the polyether with distances in the range from 2.1 and 2.3 Å. Finally, it is important to mention that these models are in agreement with the results obtained by fluorescence and 1H NMR studies, showing together that the receptors are capable of ion-pair recognition.
3. Conclusions
In summary, this work demonstrated that the design of the tritopic acyclic receptors described here is suitable for anion and ion-pair recognition. Molecular recognition studies showed that the receptors have good affinity for various oxyanions. Furthermore, they are capable of ion-pair recognition of alkali metal–acetate salts through a positive cooperative mechanism. The latter shows the feasibility of using simple acyclic compounds as ion-pair receptors. In this sense, the fine adjustment of their binding sites and/or chromophore units, among others, could improve the recognition of the desired target guest, as well as their spectroscopic characteristics for different applications. We are currently working on the synthesis of analogous compounds and their support in polymeric resins for various purposes.
4. Experimental Section
4.1. General Procedures and Materials
All reagents and solvents employed for the synthesis and molecular recognition studies were purchased from commercial suppliers and used without further purification.
4.1.1. Instruments
1H NMR studies were carried out on a Bruker Avance III 400 spectrometer, and standard references were used: tetramethylsilane (TMS) (δ 1H (400 MHz) = 0 and δ 13C (100 MHz) = 0). 7Li, 23Na, and 39K NMR experiments were carried out at 298 K using NaCl, KCl, and LiCl 0.5 M in D2O as an external reference, respectively. The parameters for the three metals were: 7Li recorded at 155.50 MHz with a spectral sweep (sw) of 24,000 Hz; 23Na at 105.86 MHz, sw of 20,000 Hz; and 39K at 18.67 MHz, sw 20,000 Hz. IR spectra were recorded on a PerkinElmer FTIR/FIR Spectrometer model FRONTIER using attenuated total reflection (ATR) and KBr pellet techniques. Mass spectrometry was conducted on an Agilent 6100 LC/MS using the ESI+ mode. UV–vis measurements were performed on an Agilent 8435 (Agilent Technologies) diode array with a quartz cell 1 cm optical path. Fluorescence studies were carried out on a Lambda LB-50 with a xenon lamp, quantifications consisting of 1–1000 light pulses per measurement, depending on the required reading quality and speed, and with a glass cell with 1 cm light path. Melting points were recorded on a Büchi melting point B-545 apparatus.
4.1.2. Solution Studies
As part of the preliminary studies, electronic absorption and emission spectra of the receptors were obtained at different concentrations ranging from 10–6 to 10–4 M in a MeCN/DMSO 9:1 (v/v) mixture and their spectral characteristics were determined, such as the absorption maximum wavelengths, molar extinction coefficients (ε), and the maximum excitation and emission wavelengths. On the other hand, molecular recognition studies of the receptors were carried out by UV–vis, fluorescence, 1H, 7Li, 23Na, and 39K NMR with different tetrabutylammonium and alkali metal salts of F–, C6H5COO– (OBz–), CH3COO– (OAc–), ClO4–, NO3–, HSO4–, H2PO4–, and HP2O73– (PPi3–). The typical titration procedure started from a solution with a fixed receptor concentration (3 × 10–5 M for UV–vis, 5 × 10–6 M for fluorescence, and 2.5 × 10–3 M for 1H NMR), and then increasing aliquots of a concentrated solution of the salt were added. Each titration was carried out in at least triplicate.
Ion-pair recognition studies were performed by fluorescence and 1H NMR. Regarding fluorescence studies, receptor solutions were prepared at a concentration of 5 × 10–6 M including 1 equiv of an alkali metal perchlorate salt (LiClO4, NaClO4, or KClO4), and then aliquots of a concentrated OAc– (as its tetrabutylammonium salt) solution were added. For the 1H NMR studies, the same procedure was performed, but the receptor solution concentrations were 2.5 × 10–3 M for R1 and R2 and 2 × 10–3 M for R3.
4.1.3. Data Analysis
Fitting of data obtained from UV–vis experiments was performed by minimizing the error term (E) of eq 1, using alternating least squares and penalty functions, as reported by Gemperline and Cash in 200343
| 1 |
where Y is the observed absorbance matrix, C is the species concentration matrix, AT is the molar absorptivity transpose matrix, and E is the associated error.
To use eq 1, it is necessary to establish an initial concentration profile or the molar absorptivities of the species in solution. The evolving factor analysis (EFA) methodology, described by Gampp et al.,44,45 was used to choose the fitting model and the initial parameters. The EFA values were visually chosen. However, in the case of an ambiguity, these values were chosen by the best fitting of the observed spectra. On the other hand, penalty functions to minimize the error of eq 1 were made based on the law of mass action and mass balance. For all of the described calculations, Python 3.8 was used.
The binding constants of the complexes studied by 1H NMR were determined by nonlinear fitting analyses of the titration curves according to the host–guest complex equations reported by Thordarson, using the BindFit package v0.05 available in supramolecular.org.46
4.2. Preparative Part
4.2.1. Synthesis of Diamine Precursors
Diamine precursors (D1 and D2) (see Scheme 2) were obtained as previously reported by our research group.21
4.2.2. Synthesis of Receptor R1
Receptor R1 was prepared according to the following
procedure: 0.170 g (1.00 mmol) of 1-naphthyl isocyanate dissolved
in 15 mL of anhydrous dichloromethane were added to a solution of
0.180 g (0.48 mmol) of diamine precursor D1 (see Scheme 2) in the same solvent
(15 mL), maintaining an inert atmosphere of N2. A brown
precipitate was observed after stirring the mixture over 12 h at room
temperature. The solid was filtered and washed with acetone and dichloromethane
and vacuum dried. Yield: 0.201 g (58.5%). Melting point: 219.3–220.3
°C. UV absorption: λmax(MeCN/DMSO 9:1 v/v)/327
nm (ε/dm3 mol–1 cm–1 = 15,000). IR (KBr): 3263, 3049, 2869, 2863, 1635, 1611, 1557, 1510,
1456, 1403, 1343, 1241, 1130, 1062 cm–1. 1H NMR (400 MHz, DMSO-d6, δ, ppm):
8.90 (s, 2H, H-9), 8.71 (s, 2H, H-11), 8.12 (d, J = 8.4 Hz, 2H. H-20), 8.02 (d, J = 7.2 Hz, 2H, H-13),
7.93 (d, J = 7.5 Hz, 2H, H-17), 7.63–7.56
(m, 4H, H-18 and H-15), 7.54 (t, J = 7.8, 6.9 Hz,
2H, H-19), 7.46 (t, J = 7.9 Hz, 2H, H-14), 7.41 (d, J = 9.0 Hz, 4H, H-7), 6.91 (d, J = 9.0
Hz, 4H, H-6), 4.05 (t, J = 4.3 Hz, 4H, H-4), 3.74
(t, J = 4.6, 3.8 Hz, 4H, H-3), 3.58 (dt, J = 5.7, 3.0, 2.7 Hz, 8H, H-1 and H-2). 13C NMR
(101 MHz, DMSO, δ): 154.1 (C-10), 153.5 (C-5), 135.0 (C-12),
134.2 (C-8), 133.4 (C-21), 128.9 (C-17), 126.4 (C-14), 126.3 (C-19),
126.2 (C-18), 126.1 (C-16), 123.1 (C-15), 121.8 (C-20), 120.2 (C-7),
117.5 (C-13), 115.1 (C-6), 70.4 (C-2), 70.3 (C-1), 69.5 (C-3), 67.7
(C-4). MS-ESI (+) m/z: 715.31 [M
+ H]+ (46.2%), 737.5 [M + Na]+ (6%). Elem. Anal.
Calcd for C42H42N4O7·H2O: C, 68.84; H, 6.05; N, 7.65; O, 17.47. Found: C, 68.72;
H, 5.88; N, 7.69.
4.2.3. Synthesis of Receptor R2
Receptor R2 was
prepared in an analogous manner to
that described for R1, using 0.188 g (0.5 mmol) of diamine
precursor D1 and 0.187 g (1.1 mmol) of 2-naphthyl isocyanate.
Yield: 0.220 g (51%). Decomposition point: 220.5–221.5 °C.
UV absorption: λmax(MeCN/DMSO 9:1 v/v)/297 nm (ε/dm3 mol–1 cm–1 = 23,300).
IR (KBr): 3282, 3046, 2920, 2863, 1636, 1600, 1555, 1502, 1455, 1433,
1347, 1284, 1228, 1127, 1069, 1049 cm–1. 1H NMR (400 MHz, DMSO-d6, δ, ppm):
8.83 (s, 2H, H-11), 8.58 (s, 2H, H-9), 8.09 (s, 2H, H-21), 7.86–7.78
(m, 4H, H-16 and H-19), 7.77 (d, J = 8.2 Hz, 2H,
H-14), 7.48 (d, J = 9.0 Hz, 2H, H-13), 7.43 (t, J = 7.3, 6.7 Hz, 2H, H-18), 7.38 (d, J =
8.5 Hz, 4H, H-7), 7.34 (t, J = 7.5 Hz, 2H, H-17),
6.89 (d, J = 8.5 Hz, 4H, H-6), 4.04 (t, J = 4.6 Hz, 4H, H-4), 3.73 (t, J = 4.7 Hz, 4H, H-3),
3.57 (dt, J = 4.5 Hz, 8H, H-1 and H-2). 13C NMR (101 MHz, DMSO, δ, ppm): 154.2 (C-10), 153.3 (C-5), 138.0
(C-12), 134.2 (C-20), 133.2 (C-8), 129.4 (C-14), 128.9 (C-15), 127.9
(C-16), 127.4 (C-19), 126.8 (C-18), 124.3 (C-17), 120.5 (C-7), 120.1
(C-13), 115.1 (C-6), 113.6 (C-21), 70.4 (C-2), 70.3 (C-1), 69.5 (C-3),
67.7 (C-4). MS-ESI (+) m/z: 715.31
[M + H]+ (46.2%), 737.5 [M + Na]+ (7.95%).
4.2.4. Synthesis of Receptor R3
Receptor R3 was prepared in an analogous manner to
that described for R1, using 0.040 g (0.120 mmol) of
diamine precursor D2 (see Scheme 2) and 0.040 g (0.24 mmol) of 1-naphthyl isocyanate.
In this case, the product was observed as a brown precipitate after
24 h. Yield: 0.0774 g (96.7%). Melting point: 229.6–230.8 °C.
UV absorption: λmax(MeCN/DMSO 9:1 v/v)/327 nm (ε/dm3 mol–1 cm–1 = 14,900).
IR (KBr): 3263, 3049, 2869, 2863, 1635, 1611, 1557, 1510, 1456, 1403,
1343, 1241, 1130, 1062 cm–1. 1H NMR (400
MHz, DMSO-d6, δ, ppm): 9.00 (s,
2H, H-8), 8.77 (s, 2H, H-10), 8.16 (d, J = 8.4 Hz,
2H, H-19), 8.02 (d, J = 6.8 Hz, 2H, H-12), 7.93 (d, J = 7.4 Hz, 2H, H-16), 7.64–7.57 (m, 4H, H-17 and
H-14), 7.54 (t, J = 7.0 Hz, 2H, H-18), 7.46 (t, J = 7.9 Hz, 2H, H-13), 7.41 (d, J = 9.0
Hz, 4H, H-6), 6.91 (d, J = 9.0 Hz, 4H, H-5), 4.06
(t, J = 5.6, 4.4 Hz, 4H, H-3), 3.75 (t, J = 6.5, 4.4 Hz, 4H, H-2), 3.63 (s, 4H, H-1). 13C NMR (101
MHz, DMSO, δ, ppm) 154.1 (C-9), 153.5 (C-4), 135.0 (C-11), 134.2
(C-7), 133.4 (C-20), 128.9 (C-16), 126.4 (C-13), 126.4 (C-18), 126.2
(C-17), 126.1 (C-15), 123.1 (C-14), 121.8 (C-19), 120.3 (C-6), 117.5
(C-12), 115.2 (C-5), 70.4 (C-1), 69.5 (C-2), 67.7 (C-3). MS-ESI (+) m/z: 671.3 [M + H]+ (45.0%).
Elem. Anal. Calcd for C40H38N4O6·H2O: C, 69.75; H, 5.85; N, 8.13; O, 16.26.
Found: C, 69.87; H, 5.35; N, 8.24.
4.3. Molecular Modeling
Compounds R2 and R3 were constructed with a molecular editor and then optimized by molecular mechanics methods. These models were then used to form their respective complexes. To determine the ground state of the complexes between receptors R2 and R3, lithium or sodium cation, and two species of acetate, a conformational search was performed. For each case, 20 initial structures with different geometries were used as the starting point, and then a PM6 level of theory was used to optimize them. Their frequencies were calculated to define whether the resulting structures were a local minimum or a transition state. In addition, the energy for all local minimal structures was calculated with a B3LYP/6-31G DFT level of theory. Finally, the consideration of these energy values allowed defining the ground state and its structural isomers. All of these calculations were performed with the Gaussian 09 (version B.01) software package47 using the ACARUS (High-Performance Computing Area of the University of Sonora) high-performance cluster. The molecular structures were visualized with Chemcraft program.48
Acknowledgments
This work received financial support from the National Council of Science and Technology of Mexico (CONACyT) in the form of a postgraduate grant for JMSC, and through projects CB-239581 and 281251 (Supramolecular Chemistry Thematic Network). J.G.-V. thanks the University of Sonora for the postdoctoral fellowship. The authors thank Prof. Ivan Castillo-Pérez and Q. María de la Paz Orta-Pérez for assistance with the Elemental Analysis and acknowledge access to “Laboratorios de Servicios Analíticos” of the Institute of Chemistry of the National Autonomous University of Mexico.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c00935.
1H and 13C NMR spectra of R1, R2, and R3; UV–vis, fluorescence, and 1H NMR titrations for complexes between the receptors (R1–R3) and various salts and 7Li, 23Na, and 39K NMR spectral data for perchlorate salts and their equimolar mixtures with the receptors (R1–R3); and chemical shifts (Δδ, ppm) for a −NH ureidic proton of the receptors (R1–R3) against the molar ratio [anion]/[receptor] (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Gale P. A.; Caltagirone C. Fluorescent and Colorimetric Sensors for Anionic Species. Coord. Chem. Rev. 2018, 354, 2–27. 10.1016/j.ccr.2017.05.003. [DOI] [Google Scholar]
- Olivari M.; Montis R.; Karagiannidis L. E.; Horton P. N.; Mapp L. K.; Coles S. J.; Light M. E.; Gale P. A.; Caltagirone C. Anion Complexation, Transport and Structural Studies of a Series of Bis-Methylurea Compounds. Dalton Trans. 2015, 44, 2138–2149. 10.1039/C4DT02893G. [DOI] [PubMed] [Google Scholar]
- Chen L.; Berry S. N.; Wu X.; Howe E. N. W.; Gale P. A. Advances in Anion Receptor Chemistry. Chem 2020, 6, 61–141. 10.1016/j.chempr.2019.12.002. [DOI] [Google Scholar]
- Langton M. J.; Serpell C. J.; Beer P. D. Anion Recognition in Water: Recent Advances from a Supramolecular and Macromolecular Perspective. Angew. Chem., Int. Ed. 2016, 55, 1974–1987. 10.1002/anie.201506589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans N. H.; Beer P. D. Advances in Anion Supramolecular Chemistry: From Recognition to Chemical Applications. Angew. Chem., Int. Ed. 2014, 53, 11716–11754. 10.1002/anie.201309937. [DOI] [PubMed] [Google Scholar]
- Araki K.; Shimizu H.; Shinkai S. Cation-π Interactions in Calix[4]Arene-Based Host Molecules. What Kind of Cavity-Shape Ls Favored for the Cation-Binding?. Chem. Lett. 1993, 22, 205–208. 10.1246/cl.1993.205. [DOI] [Google Scholar]
- Ludwig R.; Dzung N. T. K. Calixarene-Based Molecules for Cation Recognition. Sensors 2002, 2, 397–416. 10.3390/s21000397. [DOI] [Google Scholar]
- Terashima T.; Kawabe M.; Miyabara Y.; Yoda H.; Sawamoto M. Polymeric Pseudo-Crown Ether for Cation Recognition via Cation Template-Assisted Cyclopolymerization. Nat. Commun. 2013, 4, 2321 10.1038/ncomms3321. [DOI] [PubMed] [Google Scholar]
- Qiao B.; Sengupta A.; Liu Y.; McDonald K. P.; Pink M.; Anderson J. R.; Raghavachari K.; Flood A. H. Electrostatic and Allosteric Cooperativity in Ion-Pair Binding: A Quantitative and Coupled Experiment-Theory Study with Aryl-Triazole-Ether Macrocycles. J. Am. Chem. Soc. 2015, 137, 9746–9757. 10.1021/jacs.5b05839. [DOI] [PubMed] [Google Scholar]
- Oliveira M. T.; Lee J. W. Asymmetric Cation-Binding Catalysis. ChemCatChem 2017, 9, 377–384. 10.1002/cctc.201601441. [DOI] [Google Scholar]
- Dey S. K.; Basu A.; Chutia R.; Das G. Anion Coordinated Capsules and Pseudocapsules of Tripodal Amide, Urea and Thiourea Scaffolds. RSC Adv. 2016, 6, 26568–26589. 10.1039/C6RA00268D. [DOI] [Google Scholar]
- Bates G. W.; Gale P. A.. An Introduction to Anion Receptors Based on Organic Frameworks. In Recognition of Anions; Vilar R., Ed.; Structure and Bonding; Springer: Berlin, Heidelberg, 2007; Vol. 129, pp 1–44. [Google Scholar]
- Amendola V.; Fabbrizzi L.; Mosca L. Anion Recognition by Hydrogen Bonding: Urea-Based Receptors. Chem. Soc. Rev. 2010, 39, 3889–3915. 10.1039/b822552b. [DOI] [PubMed] [Google Scholar]
- Li A. F.; Wang J. H.; Wang F.; Jiang Y. B. Anion Complexation and Sensing Using Modified Urea and Thiourea-Based Receptors. Chem. Soc. Rev. 2010, 39, 3729–3745. 10.1039/b926160p. [DOI] [PubMed] [Google Scholar]
- Caltagirone C.; Bazzicalupi C.; Isaia F.; Light M. E.; Lippolis V.; Montis R.; Murgia S.; Olivari M.; Picci G. A New Family of Bis-Ureidic Receptors for Pyrophosphate Optical Sensing. Org. Biomol. Chem. 2013, 11, 2445–2451. 10.1039/c3ob27244c. [DOI] [PubMed] [Google Scholar]
- Miranda A. S.; Serbetci D.; Marcos P. M.; Ascenso J. R.; Berberan-Santos M. N.; Hickey N.; Geremia S. Ditopic Receptors Based on Dihomooxacalix[4]Arenes Bearing Phenylurea Moieties With Electron-Withdrawing Groups for Anions and Organic Ion Pairs. Front. Chem. 2019, 7, 758 10.3389/fchem.2019.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D.-H.; Smith B. D. Shape-Selective Recognition of Quaternary Ammonium Chloride Ion Pairs. J. Org. Chem. 2019, 84, 2808–2816. 10.1021/acs.joc.8b03197. [DOI] [PubMed] [Google Scholar]
- Jagleniec D.; Wilczek M.; Romański J. Tripodal, Squaramide-Based Ion Pair Receptor for Effective Extraction of Sulfate Salt. Molecules 2021, 26, 2751 10.3390/molecules26092751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell A. J.; Docker A.; Beer P. D. From Heteroditopic to Multitopic Receptors for Ion-Pair Recognition: Advances in Receptor Design and Applications. ChemPlusChem 2020, 85, 1824–1841. 10.1002/cplu.202000484. [DOI] [PubMed] [Google Scholar]
- He Q.; Vargas-Zúñiga G. I.; Kim S. H.; Kim S. K.; Sessler J. L. Macrocycles as Ion Pair Receptors. Chem. Rev. 2019, 119, 9753–9835. 10.1021/acs.chemrev.8b00734. [DOI] [PubMed] [Google Scholar]
- Gómez-Vega J.; Moreno-Corral R. A.; Santacruz Ortega H.; Corona-Martínez D. O.; Höpfl H.; Sotelo-Mundo R. R.; Ochoa-Terán A.; Escobar-Picos R. E.; Ramírez-Ramírez J. Z.; Juárez-Sánchez O.; Lara K. O. Anion, Cation and Ion-Pair Recognition by Bis-Urea Based Receptors Containing a Polyether Bridge. Supramol. Chem. 2019, 31, 322–335. 10.1080/10610278.2019.1578884. [DOI] [Google Scholar]
- Schneider H.-J. Efficiency Parameters in Artificial Allosteric Systems. Org. Biomol. Chem. 2016, 14, 7994–8001. 10.1039/C6OB01303A. [DOI] [PubMed] [Google Scholar]
- Zakrzewski M.; Załubiniak D.; Piątek P. An Ion-Pair Receptor Comprising Urea Groups and N-Benzyl-Aza-18-Crown-6: Effective Recognition and Liquid–Liquid Extraction of KCl Salt. Dalton Trans. 2018, 47, 323–330. 10.1039/C7DT03696E. [DOI] [PubMed] [Google Scholar]
- Zakrzewski M.; Załubiniak D.; Piątek P. Development of Effective Potassium Acetate Extractant. RSC Adv. 2021, 11, 10860–10865. 10.1039/D1RA00859E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse Y. C.; Docker A.; Zhang Z.; Beer P. D. Lithium Halide Ion-Pair Recognition with Halogen Bonding and Chalcogen Bonding Heteroditopic Macrocycles. Chem. Commun. 2021, 57, 4950–4953. 10.1039/D1CC01287H. [DOI] [PubMed] [Google Scholar]
- Amendola V.; Bergamaschi G.; Boiocchi M.; Fabbrizzi L.; Mosca L. The Interaction of Fluoride with Fluorogenic Ureas: An ON1–OFF–ON2 Response. J. Am. Chem. Soc. 2013, 135, 6345–6355. 10.1021/ja4019786. [DOI] [PubMed] [Google Scholar]
- Lopéz-Martínez L. M.; García-Elías J.; Ochoa-Terán A.; Ortega H. S.; Ochoa-Lara K.; Montaño-Medina C. U.; Yatsimirsky A. K.; Ramírez J. Z.; Labastida-Galván V.; Ordoñez M. Synthesis, Characterization and Anion Recognition Studies of New Fluorescent Alkyl Bis(Naphthylureylbenzamide)-Based Receptors. Tetrahedron 2020, 76, 130815 10.1016/j.tet.2019.130815. [DOI] [Google Scholar]
- Connors K. A.; Paulson A.; Toledo-Velasquez D. Complexing of.Alpha.-Cyclodextrin with Sym-4,4′-Disubstituted Biphenyls. J. Org. Chem. 1988, 53, 2023–2026. 10.1021/jo00244a033. [DOI] [Google Scholar]
- Thordarson P. Determining Association Constants from Titration Experiments in Supramolecular Chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. 10.1039/C0CS00062K. [DOI] [PubMed] [Google Scholar]
- Bunchuay T.; Docker A.; Eiamprasert U.; Surawatanawong P.; Brown A.; Beer P. D. Chalcogen Bond Mediated Enhancement of Cooperative Ion-Pair Recognition. Angew. Chem., Int. Ed. 2020, 59, 12007–12012. 10.1002/anie.202001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdanowski S.; Romański J. Ion Pair Binding by an L-Tyrosine-Based Polymerizable Molecular Receptor. New J. Chem. 2015, 39, 6216–6222. 10.1039/C5NJ01063B. [DOI] [Google Scholar]
- Ziach K.; Karbarz M.; Romański J. Cooperative Binding and Extraction of Sodium Nitrite by a Ditopic Receptor Incorporated into a Polymeric Resin. Dalton Trans. 2016, 45, 11639. 10.1039/C6DT02235A. [DOI] [PubMed] [Google Scholar]
- Jagleniec D.; Wilczek M.; Romański J. Tripodal, Squaramide-Based Ion Pair Receptor for Effective Extraction of Sulfate Salt. Molecules 2021, 26, 2751 10.3390/molecules26092751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoog P.; Gamez P.; Mutikainen I.; Turpeinen U.; Reedijk J. An Aromatic Anion Receptor: Anion−π Interactions Do Exist. Angew. Chem., Int. Ed. 2004, 43, 5815–5817. 10.1002/anie.200460486. [DOI] [PubMed] [Google Scholar]
- Cox B. G.Acids and Bases: Solvent Effects on Acid–Base Strength; Oxford University Press, 2013. [Google Scholar]
- Bessiere J.; Bazine F. Variation of Fluoride Ion Solvation and PF– Buffer Properties of HF2–/HF and HF/H+ Pairs in Acetonitrile-Water Mixtures. J. Fluorine Chem. 1989, 44, 45–58. 10.1016/S0022-1139(00)84370-5. [DOI] [Google Scholar]
- Muckerman J. T.; Skone J. H.; Ning M.; Wasada-Tsutsui Y. Toward the Accurate Calculation of PKa Values in Water and Acetonitrile. Biochim. Biophys. Acta, Bioenerg. 2013, 1827, 882–891. 10.1016/j.bbabio.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Ros-Lis J. V.; Martínez-Máñez R.; Sancenón F.; Soto J.; Rurack K.; Weißhoff H. Signalling Mechanisms in Anion-Responsive Push-Pull Chromophores: The Hydrogen-Bonding, Deprotonation and Anion-Exchange Chemistry of Functionalized Azo Dyes. Eur. J. Org. Chem. 2007, 2007, 2449–2458. 10.1002/ejoc.200601111. [DOI] [Google Scholar]
- Schneider H. J.; Yatsimirsky A. K.. Principles and Methods in Supramolecular Chemistry; Wiley, 2000. [Google Scholar]
- Esteban-Gómez D.; Fabbrizzi L.; Licchelli M.; Sacchi D. A Two-Channel Chemosensor for the Optical Detection of Carboxylic Acids, Including Cholic Acid. J. Mater. Chem. 2005, 15, 2670–2675. 10.1039/b502869h. [DOI] [Google Scholar]
- Boiocchi M.; Del Boca L.; Esteban-Gómez D.; Fabbrizzi L.; Licchelli M.; Monzani E. Anion-Induced Urea Deprotonation. Chem.—Eur. J. 2005, 11, 3097–3104. 10.1002/chem.200401049. [DOI] [PubMed] [Google Scholar]
- Schmidt E.; Popov A. I. Potassium-39 NMR Study of the Complexation Kinetics of Potassium(+) Ion by 18-Crown-6 in Some Nonaqueous Solvents. J. Am. Chem. Soc. 1983, 105, 1873–1878. 10.1021/ja00345a033. [DOI] [Google Scholar]
- Gemperline P. J.; Cash E. Advantages of Soft versus Hard Constraints in Self-Modeling Curve Resolution Problems. Alternating Least Squares with Penalty Functions. Anal. Chem. 2003, 75, 4236–4243. 10.1021/ac034301d. [DOI] [PubMed] [Google Scholar]
- Gampp H.; Maeder M.; Meyer C. J.; Zuberbühler A. D. Calculation of Equilibrium Constants from Multiwavelength Spectroscopic Data—III: Model-Free Analysis of Spectrophotometric and ESR Titrations. Talanta 1985, 32, 1133–1139. 10.1016/0039-9140(85)80238-1. [DOI] [PubMed] [Google Scholar]
- Gampp H.; Maeder M.; Meyer C. J.; Zuberbühler A. D. Calculation of Equilibrium Constants from Multiwavelength Spectroscopic Data—IV: Model-Free Least-Squares Refinement by Use of Evolving Factor Analysis. Talanta 1986, 33, 943–951. 10.1016/0039-9140(86)80233-8. [DOI] [PubMed] [Google Scholar]
- BindFit, v0.05, 2022. http://app.supramolecular.org/bindfit/ (accessed Jan 24, 2022).
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmylove A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam J. M.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratman R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochtersky J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas Ö.; Foresman J. B.; Ortiz J. V.; Cioslowsky J.; Fox D. J.. Gaussian 09; Gaussian, Inc.: Wallingford, CT, 2016.
- Chemcraft—Graphical Software for Visualization of Quantum Chemistry Computations. https://www.chemcraftprog.com.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.