Abstract
An eco-friendly green bio-organic catalyst and low-cost 3,4-dihydropyrimidin-2(1H)-ones/thione derivatives 4–7 have been synthesized using a high-yield, synthetic method via a one-pot, three-component process between 4-formylphenyl-4-methylbenzenesulfonate (1), thiourea, or urea and ethyl acetoacetate or acetylacetone under microwave irradiation in aqueous media of water and ethanol (3:1 ratio) as a green solvent in the presence of cysteine as a new green bio-organic catalyst. The reaction between compound 1, 4-(carbamothioylhydrazono) methyl]phenyl 4-methyl benzenesulfonate (3c), and ethyl acetoacetate or acetylacetone under the same condition afforded novel pyrimidines. Similarly, compound 1 was allowed to react with a mixture of 4-(carbamothioylhydrazono)methyl]phenyl 4-methyl benzenesulfonate (3c) and ethyl acetoacetate or acetylacetone under the same condition to afford pyrimidine derivatives 8 and 9. Excellent yields (90–98%) were obtained within short reaction times, and problems associated with the toxic solvents used (cost, safety, and pollution) were avoided. The structures of the new compounds were elucidated by elemental and spectral analyses. All compounds were studied using molecular docking, and their antifungal activity was investigated.
Introduction
In biology, cysteine (Cys) is the principal organic source of sulfide and the source of important cofactors including glutathione (GSH) and co-enzyme A (CoA).1 It is also a crucial ligand in ancient iron–sulfur proteins,2−4 with roles in catalysis, redox sensing, and electron transfer. It seems almost inconceivable that cysteinyl thiols were not present during the early Earth’s development of nascent biological processes, but this is not the widely held belief. Attempts to produce and isolate Cys’s under prebiotically plausible settings have failed numerous times,5−8 and it has led to the widespread opinion that Cys is both a biological creation and a late addition to the genetic code.9−12 In this paper, we provide a high-yielding prebiotic Cys synthesis and show that Cys peptides catalyze non-enzymatic CPL in neutral water. Our findings support the idea that Cys was available as a byproduct of serine nitrile production at the dawn of life, and that Cys’s were a key component of early catalytic activity. In the field of green chemistry, the main goal in the field of catalysis is to produce environmentally acceptable, commercially available, low-cost, selective, and efficient catalyst separation and recycling technologies.13 In recent years, organocatalysts have become one of the important environmentally catalysts, which are defined as a small organic molecule that can catalyze the chemical reaction in the absence of metals or metal ions.14
Biginelli synthesized dihydropyrimidin-2(1H)-(thi)one (DHPM) derivatives by combining three distinct aromatic aldehydes, a -keto ester, and urea or thiourea in a three-component reaction. The use of the DHPM moiety in numerous pharmacological prospects, such as the first cell-permeable anticancer scaffold, monastrol, the modified analogue (R)-mon-97,15 and antihypertensive agent (R)-SQ 32,926,16 continues to garner research interest. DNA and RNA, which are made up of pyrimidine derivatives, are the foundations of human life. Three of the five primary nucleic acid bases are pyrimidine derivatives: cytosine (found in DNA and RNA), uracil (found in RNA), and thymine (found in DNA). They have become extremely essential in the area of synthetic organic chemistry due to their role as DNA and RNA bases. These structures sparked the development of a wide range of synthesis and chemical transformation technologies. Moreover, pyrimidine derivatives are included in a number of essential vitamins.17 As a result of the importance of pyrimidone chemicals in human life, a synthetic approach that is particularly fascinating in organic chemistry has been developed. Some catalysts have been used to improve reaction conditions and yields in the Biginelli reaction in recent years, including mesoporous NH4H2PO4/MCM-41,18 sulfated polyborate,19 sulfated tungstate,20 zinc oxide nanoparticles,21N,O-bis(trimethylsilyl)acetamide, dicyclohexyl carbodiimide,22 Taurine catalyst,23 organosilane sulfonated graphene oxide,24 FeCl3 immobilized in Al-MCM 41,25 and so on, and have been used to improve reaction conditions and yields.
We have been working on the development of novel tools and methodology for bioactive molecules synthesized utilizing green methodologies for the past few years.26−35
For the one-pot multicomponent reaction synthesis of bio-active 3,4-dihydropyrimidin-2(1H)-ones/thiones (Biginelli reaction) in an aqueous media, we used a green bio-organic and recoverable catalyst Cys.
Results and Discussions
The group worked on specific objectives, including the creation of new pyrimidine and thiazole derivatives (Biginelli) with significant bioactivity. As a result, it was decided to investigate the use of Cys as a green bio-organic catalyst for the synthesis of highly substituted 3,4-dihydropyrimidin-2(1H)-ones/thiones 4–9 in high-yield, simple, mild, low-cost, expeditious, and environmentally friendly method with acceptable reaction times and microwave irradiation. The parent material 4-formylphenyl4-methylbenzenesulfonate (1) has been prepared while shaking 4-toluene sulfonyl chloride with 4-hydroxybenzaldehyde in the presence of sodium bicarbonate (eq 1)
 |
1 |
Equation 1: Synthesis of 4-formylphenyl 4-methylbenzenesulfonate (1)
4-formylphenyl4-methylbenzenesulfonate (1) was reacted with ethyl acetoacetate or acetylacetone 2a, b and urea/thiourea/thiourea derivatives 3a–c in a mixture of water and ethanol (3:1 ratio) using microwave irradiation (Scheme 1).
Scheme 1. Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones/thiones 4–9.
The chemical structures of the newly synthesized compounds 4–7 were confirmed by their spectral (IR, 1H, and 13C NMR) and elemental analyses.
IR spectra revealed the disappearance of the CHO group, and new absorption bands corresponding to two NH groups in the range 3368–3105 cm–1 and at 1740–1659 due to carbonyl groups were found. The 1H NMR spectra of compounds 4 and 5 indicated the disappearance of the CHO signal and appearance of the NH group signal in the range 10.28–9.58 and aromatic proton signal in the region 8.38–7.01 ppm and a single signal in the range 5.16 and 5.15 ppm due to the aliphatic proton attached to the sp3 carbon; quartet and triplet signals were observed at 4.34–4.29 and 1.32–1.28 ppm for the ester group in compound 4. Their 13CNMR spectra showed the appearance of the C=O groups at 184.75 and 162.04 ppm, respectively. A negative signal of the CH2 group at 62.94 and at 63.49 ppm in the DEPT 135 spectrum appeared in the case of compounds 4 and 5, respectively.
Compound 1 was allowed to react with thiosemicarbazide under irradiation via microwave to yield 4-(carbamothioylhydrazono)methyl]phenyl 4-methyl benzenesulfonate (3c) (eq 2).
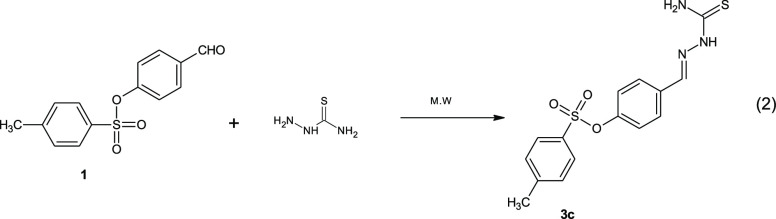 |
2 |
Equation 2: Synthesis of 4-(carbamothioylhydrazono)methyl]phenyl 4-methyl benzenesulfonate
Similarly, compound 1 was allowed to react with a mixture of compound 3c and ethyl acetoacetate or acetylacetone under the same condition via one-pot, three-component condensation under irradiation by ultrasonic waves in the presence of 15% mole Cys to afford pyrimidine derivatives. This reaction is so interesting when we use compound 4-(carbamothioylhydrazono)methyl]phenyl 4-methyl benzenesulfonate as a new thiourea derivative which can be cyclic with active methylene and aldehyde to afford a new pyrimidine ring.
IR spectra of compounds 8, 9 revealed the disappearance of the NH2 group and the appearance of only one NH group at 3250, 3211 cm–1, in addition to C=O groups in the range 1741, 1688 cm–1. 1H NMR spectra of compounds 8, 9 showed that besides the increase of the aromatic proton signals, new absorbance singlet signals were observed corresponding to NH groups at 10.29, 9.48 ppm (disappeared on deuteration); N=CH groups at 8.37, 8.36 ppm; and CHpyrimidine groups at 5.13, 5.23 ppm; quartet and triplet signals at 4.35–4.32 and 1.33–1.29 ppm were also observed for the ester group in compound 9 and for the CH3 group at 2.43 ppm. Furthermore,13C NMR and elemental analyses confirm the correct structure.
3,4-Dihydropyrimidin-2(1H)-ones/thiones 4–9 were synthesized using a high-yield, commercially available, simple, mild, low-cost, expeditious, and environmentally method with acceptable reaction times via a one-pot, three-component process between compound 1, thiourea, or urea and ethyl acetoacetate or acetylacetone in the presence of a Cys catalyst under irradiation by microwaves in an aqueous solution of water and ethanol (3:1 ratio) for 3–5 min to afford pyrimidines.
Another fascinating aspect of green chemistry is the design of organic reactions in watery media. Water is a plentiful solvent that is also environmentally friendly. This approach allows for the adjustment of complexity and molecular diversity. The reactions were fairly instantaneous, and a pure product was produced by recrystallization from ethanol without the need of any chromatographic techniques.
In order to standardize the reaction compound 4, compound 1, (1 mmol), ethyl acetoacetate 2a (1 mmol), and urea 3a (1 mmol) were dissolved in the solvent [a mixture of water and ethanol (3:1 ratio)] under irradiation by microwaves.
Effect of Catalyst Loading
Table 1 shows the link between catalyst doses and product yields. For example, increasing the catalyst loading from 3 to 15 mol % increased product yield from 21 to 98 per cent (Table 1). Product yield improves when the amount of the Cys catalyst is increased, possibly due to the increased number of accessible active sites. This results in a brooded catalyst surface that comes into direct connection with four reactants, allowing them to collide and start the reaction. Notably, increasing the loaded catalyst concentration from 15 to 16 mol % had no discernible effect on yield or reaction time (Table 1). As a result, the ideal quantity of catalyst required is 15 mol percent.
Table 1. Amounts of the Cys Catalyst Used in Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones/thiones (4–9).
| entry | cat. mol % | yield %a | entry | cat. mol % | yield %a |
|---|---|---|---|---|---|
| 1 | 3 | 21 | 5 | 13 | 84 |
| 2 | 6 | 41 | 6 | 15 | 98 |
| 3 | 9 | 58 | 7 | 16 | 98 |
| 4 | 11 | 76 | 8 | 17 | 98 |
Isolated yields based on 4, 1(1 mmol), 2a (1.5 mmol), and 3a (1 mmol) in a mixture of water and ethanol (3:1 ratio) by microwave irradiation condition, 3 min.
Effect of Solvents
We must identify the influence of many recognized solvents that were chosen as a medium for comparison in order to demonstrate the effectiveness of the process (Table 2). With the model reaction 4, the role of the solvents was assessed. Table 2 shows that polar protic solvents (MeOH, EtOH, AcOH, and H2O) performed much better than aprotic solvents (DCM, DMF, THF, CH3CN, and CHCl3). This result might be due to the fact that reactants are more soluble in polar solvents. Also, for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones, it is evident that the reaction carried out in a mixture of solvents (water/ethanol; v/v; 3/1) is the optimum option (4–9). In comparison to organic solvents, we chose this combination since it is ecologically benign, safe, and inexpensive.
Table 2. Effect of Solvents in Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones/thiones (4–9).
| solvent | time (min) | yield (%)a |
|---|---|---|
| DCM | 30 | 39 |
| DMF | 30 | 46 |
| THF | 30 | 52 |
| CH3CN | 30 | 57 |
| CHCl3 | 30 | 52 |
| MeOH | 20 | 79 |
| ACOH | 20 | 76 |
| EtOH | 6 | 90 |
| H2O | 6 | 89 |
| H2O/EtOH | 3 | 98 |
Isolated yields based on 4, 1 (1 mmol), 2a (1.5 mmol), and 3a (1 mmol) in a mixture of water and ethanol (3:1 ratio) by microwave irradiation condition, 3 min.
Comparative Study with Other Lewis Acid and Lewis Base Catalysts
Cys has garnered a lot of interest recently as a weak Lewis acid catalyst for a variety of chemical reactions. At the same circumstances (Table 3, entries 1–15), the reaction of compound 1 (1 mmol), ethyl acetoacetate 2a (1 mmol), and urea 3a (1 mmol) was modified, but with different catalysts. At optimal circumstances, AlCl3, MgCl2, PdCl2, Pd(OAc)2, FeCl3·6H2O, Fe(OTf)3, ZnBr2, CuCl2, TiCl4 p-TsOH, Et3N, and TBABrc were investigated (reported in the previous part). When compared to the Cys catalyst (not treated to microwave) (Table 3, entry 16, 17), the Cys catalyst (subjected to microwave) was the most effective catalyst, yielding the desired product 4 in 98% of cases (Table 3, entry 16).
Table 3. Use of Different Lewis Acid and Lewis Base by Microwave Irradiation (M.W) For Compound 4.
| entry | cat (mol %) | conditionsb | yield (%)a |
|---|---|---|---|
| 1 | no catalyst no M.W | water/ethanol, 1 day | trace |
| 2 | no catalyst | water/ethanol, 1 h | 45 |
| 3 | AlCl3 (15) | water/ethanol, 3 min | 49 |
| 4 | MgCl2 (15) | water/ethanol, 3 min | 47 |
| 5 | FeCl3·6H2O (15) | water/ethanol, 3 min | 56 |
| 6 | Fe(OTf)3 (15) | water/ethanol, 3 min | 69 |
| 7 | ZnBr2 (15) | water/ethanol, 3 min | 74 |
| 8 | CuCl2 (15) | water/ethanol, 3 min | 66 |
| 9 | PdCl2 (15) | water/ethanol, 3 min | 87 |
| 10 | Pd(OAC)2(15) | water/ethanol, 3 min | 89 |
| 11 | p-TsOH (15) | water/ethanol, 3 min | 75 |
| 12 | Et3N (15) | water/ethanol, 3 min | 67 |
| 13 | TBABrc (15) | water/ethanol, 3 min | 66 |
| 14 | TiCl4 (15) | water/ethanol, 3 min | 71 |
| 15 | l-Proline (15) | water/ethanol, 3 min | 84 |
| 16 | Cys (15) | water/ethanol, 3 min | 98 |
| 17 | Cys (15) | ||
| No M.W | water/ethanol, 3 min | 88 |
Isolated yields based on 4.
Reaction conditions: 1 (1 mmol), 2a (1.5 mmol), and 3a (1 mmol) in a mixture of water and ethanol (3:1 ratio) by microwave irradiation conditions, 3 min.
Recycling of the Cys Catalyst
The environmentally friendly and cost-effective characteristics of this synthetic technique were investigated further by looking into the potential of the recovering Cys catalyst for reuse in subsequent synthesis cycles. In the presence of Cys, a progressive reaction model with four reactants [compound 1, (1 mmol), ethyl acetoacetate 2a (1 mmol), and urea 3a (1 mmol)] was performed five times in the presence of Cys to synthesize 4. As a result, it was assumed that catalyst would be lost during the healing process. The results of the Cys recovery trials were graphed (Figure 1), and they showed that Cys could be reused for five consecutive cycles without losing catalytic activity. However, when we performed the next two runs (6 and 7) under the identical circumstances, we found low catalytic activity.
Figure 1.

Recyclability of Cys in the model reaction.
Plausible Mechanism for the Catalytic Pathway
In the presence of a catalytic quantity of Cys, a suggested process for the synthesis of 4–9 products is indicated in (Scheme 2). Cys, as a bifunctional hydrogen donor–acceptor reagent, may simultaneously activate the carbonyl group of aldehyde through its protonation to form intermediate I, which followed to react with active methylene compounds to form the hydrated intermediate II with elimination of Cat-SH. The intermediate II eliminate water molecules to form arylidene intermediate III (Scheme 2). Furthermore, the formed intermediate undergoes nucleophilic attack of the amino group (thiourea or urea), followed by elimination of water molecules to give the target products of 3,4dihydropyrimidin-2(1H)-(thio)ones 4–9.
Scheme 2. Suggestion Mechanism for the Synthesis of Compounds 4–9.
Antifungal Activity
Antifungal activity31 was tested for the selected compounds in vitro against Penicilliumsp. and Aspergillus niger. Sabouraud Dextrose media was used for growing the tested fungi. About 1.6 × 104–6 × 104 c.f.u./mL of spore suspension was poured on the surface of sterile agar plates. 100 μg/mL of each tested compound was dissolved in 5 mL of dimethyl sulfoxide (DMSO) and loaded on 6 mm filter paper discs. DMSO was used as a negative control. Loaded discs were placed on the surface of the media and incubated at 37 °C for 48 h (Table 4).
Table 4. Antifungal Activity of Selected Compoundsa.
| compound (DMSO) | Penicillium sp. | Aspergillus niger |
|---|---|---|
| 4 | +++ | +++ |
| 5 | +++ | ++ |
| 6 | -- | -- |
| 7 | ++ | + |
| 8 | ++ | ++ |
| 9 | ++ | +++ |
| DMSO | -- | -- |
(−) no activity (0.0 cm), (+) weak activity (0.1–0.6 cm), (++) moderate activity (0.7–2.0 cm), (+++) high activity (2.1–3.0).
Table 4 shows that the compounds 4, 5, 7, 8, and 9 are the most active where compound 6 has no activity on both types of fungi.
In Silico Docking Study
In this study, the newly constructed pyrimidine derivatives 4–9 were subjected to docking study inside dihydrofolate reductase enzyme (DHFR) to propose the mode of action of these novel candidates.36−38 We utilized MOE software, 2005.06, and the crystal structure of DHFR with the cocrystallized ligand was downloaded from a protein data bank (code: 6DTC). The target compounds were docked, and the obtained data are recorded in Table 5.
Table 5. Docking Results and Binding Interactions of Compounds 4–9 within the DHFR Active Region.
| compound | docking scores kcal/mol | no. of hydrogen bonds | distance (Å) from the main residue | bound groups | |
|---|---|---|---|---|---|
| 4 | –14.08 | 2 | Ser69 | 3.01 | SO2 |
| Arg36 | 2.98 | C=O | |||
| 5 | –13.69 | 3 | Thr66 | 3.06 | pyrimidine NH |
| Thr66 | 2.93 | pyrimidine CO | |||
| IIe156 | 2.72 | pyrimidine NH | |||
| 6 | –10.54 | 0 | |||
| 7 | –11.28 | 2 | Arg80 | 3.07 | C=O |
| Arg36 | 2.83 | CH3–C=O | |||
| 8 | –11.59 | 2 | IIe26 | 2.83 | NH |
| Ala12 | 2.17 | CH3–C=O | |||
| 9 | –13.22 | 2 | Arg36 | 2.88 | C=O |
| Ser69 | 2.97 | OSO2 | |||
| H9G | –13.41 | 4 | Arg80 | 2.89 | tetrazole N |
| Asp40 | 3.09 | pyrimidine NH | |||
| Asp40 | 2.50 | NH2 | |||
| IIe10 | 2.94 | NH2 | |||
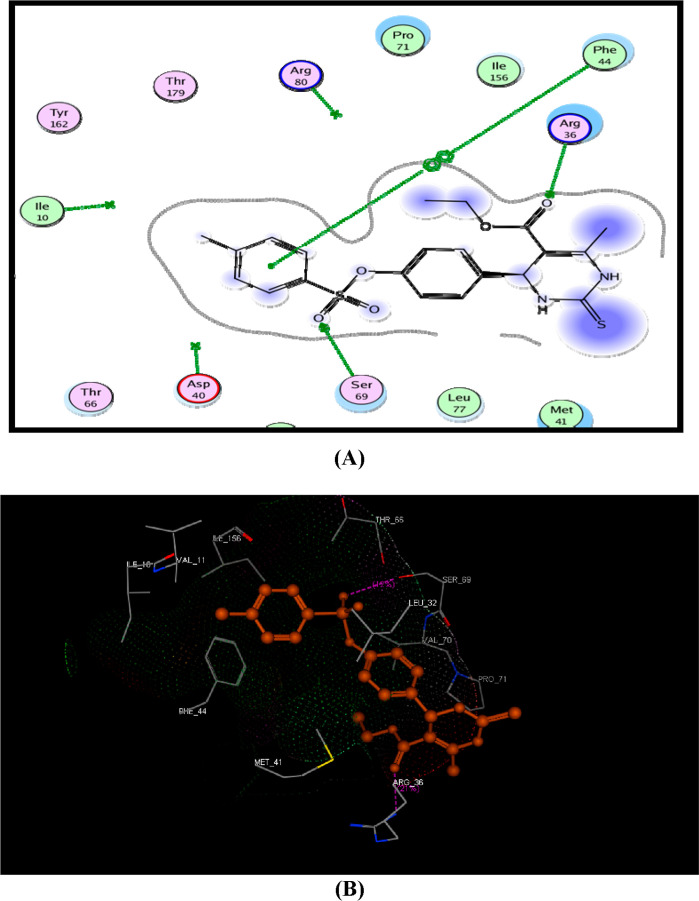
Compound 4 showed good binding with DHFR enzyme with a high binding energy score (−14.08 kcal/mol). It displayed two H-bonds with Arg36 and Ser69 amino acid residues through binding with carbonyl and sulphonyl groups. Moreover, the phenyl moiety made hydrophobic interaction with Phe44 (Figure 2).
Figure 2.
(A) Suggested 2D binding mode of compound 4 inside DHFR and (B) 3D suggested binding of compound 4 with Ser69 and Arg36.
Regarding ethyl 6-methyl-4-(2-[(4-methylphenyl)sulfonyl]oxy-phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (5), it recorded three hydrogen bonding interactions with a binding energy score = −13.69 kcal/mol as follows: (i) pyrimidine NH with IIe156, (ii) pyrimidine NH with Thr66, and (iii) pyrimidine C=O with Thr66 (Figure 3).
Figure 3.
(A) Suggested 2D binding mode of compound 5 inside DHFR and (B) 3D suggested binding of compound 5 with Thr66 and IIe156.
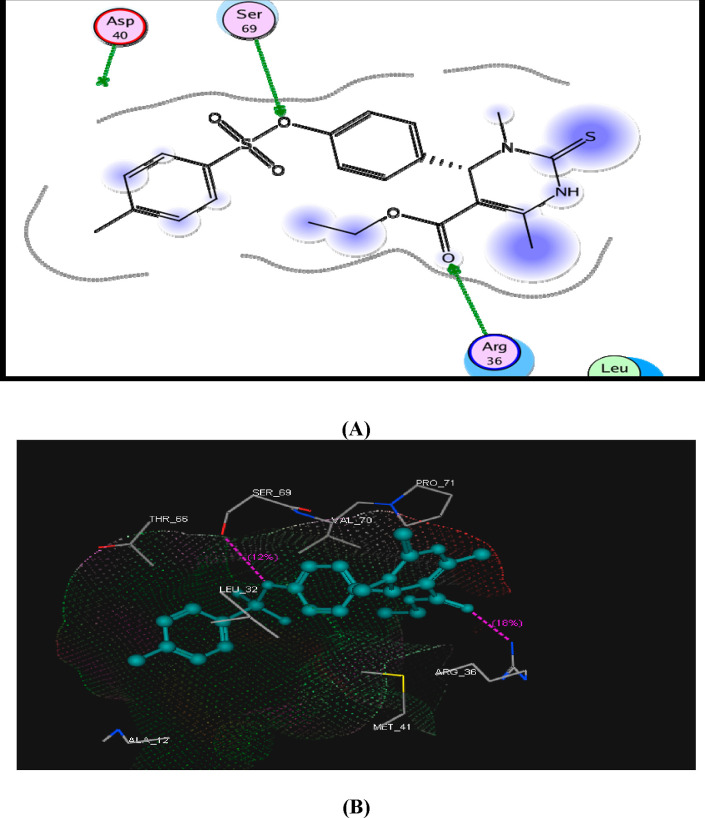
On the other hand, compound 9 displayed two hydrogen bonds with Ser69 and Arg36 amino acid residues with energy score = −13.22 kcal/mol (Figure 4).
Figure 4.
(A) Suggested 2D binding mode of compound 9 inside DHFR and (B) 3D suggested binding of compound 5 with Arg36 and Ser69.
Conclusions
In conclusion, the one-pot multicomponent synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thione derivatives 4–9, the very stable green bio-organic catalyst Cys has been proven as an efficient and ecologically friendly catalyst. This research has numerous important benefits over previous methods, including the avoidance of harmful organic solvents, the use of a metal-free bio-organic and non-toxic catalyst, the catalyst’s reusability, quick reaction durations, high yields, and gentle reaction conditions. All compounds were studied molecular docking and investigated in antifungal activity.
Experimental Section
All melting points were recorded on Melt-Temp II melting point apparatus. IR spectra were measured as KBr pellets on a Shimadzu DR-8001 spectrometer. 1H NMR and 13C NMR spectra were recorded on a Bruker at 400 MHz and 100 MHz using TMS as an internal reference and DMSO-d6 as solvent. The elemental analyses were carried out on a Perkin-Elmer 240C Micro analyzer. Microwave irradiations were carried out in a Kenstar OM9925E MW oven (2450 MHz, 800 W). All compounds were checked for their purity on TLC plates and recyclability of Cys by evaporating the water from the filtrate.
Synthesis of 4-Formylphenyl 4-Methylbenzenesulfonate (1)
A mixture of 4-toluene sulfonyl chloride (2 mmol, 3.81 g) with 4-hydroxybenzaldehyde (2 mmol, 2.45 g) and sodium bicarbonate (2 mmol, 1.68 g) in acetonitrile 10 mL was taken. The mixture was shaken for 10 min and poured into ice-cold water; the formed product was filtered off, washed with water several times, dried, and crystallized from ethanol.
Synthesis of 4-(Carbamothioylhydrazono)methyl]phenyl4-methylbenzenesulfonate (3c)
A mixture of 4-formylphenyl-4-methylbenzenesulfonate (1) (5 mmol, 1.38 g) and thiosemicarbazide (5 mmol, 0.46 g) in an aqueous solution of water and ethanol (1:2 ratio) was taken, and then the reaction mixture was irradiated in an MW oven for 5 min. The reaction mixture was allowed to cool to room temperature; the solid precipitate was collected by filtration and crystallized from ethanol.
mp: 236–238 °C; IR (KBr) 3320, 3287, 3162 (NH + NH2), 1399, 1163 (SO2), cm–1; 1H NMR: δ 11.43 (s, 1H, NH), 8.18 (s, 1H, CH=N), 8.01 (s, 2H, NH2), 7.82–7.80 (d, 2H), 7.76–7.74 (d, 2H), 7.48–7.46 (d, 2H), 7.05–7.03 (d, 2H),2.43 (s, 3H, CH3); 13C NMR: δ 150.20, 146.36, 141.08, 133.90, 131.83, 130.70, 129.24, 128.72, 122.76, 21.63 (CH2), Anal. Calcd. for C15H15N3O3S2 (349.43): C, (51.57%); H, (4.34%); N, (12.05%); S, (18.36%) Found: C, (51.67%); H, (4.36%); N, (12.03%); S, (18.96%).
General Procedure for the Synthesis of Compounds 4–9
Synthesis of Pyrimidine 2–5
An equimolar amount (1 mmol) of 4-formylphenyl-4-methylbenzenesulfonate (1), ethyl acetoacetate/acetylacetone, urea, thiourea or 4-(carbamothioylhydrazono)methyl]phenyl 4-methyl benzenesulfonate was taken, and the Cys catalyst was added (15 mmol) in an aqueous solution of water and ethanol (3:1 ratio); then the reaction mixture was irradiated in an MW oven for 3–5 min, as shown in Table 1. After cooling to room temperature, the precipitates were washed with cold water several times and recrystallized from an aqueous solution of water and ethanol (3:1 ratio), while the filtrate contains only Cys, which we can recycle by evaporating the water from the filtrate, a solid.
Ethyl-6-methyl-4-(2-[(4-methylphenyl)sulfonyl]oxy-phenyl)-2-thioxo-1,2,3,4-tetrahydro Pyrimidine-5-carboxylate (4)
mp: 175–177 °C; IR (KBr) 3368, 3247 (2NH) 1716, 1659 (2C=O), 1357, 1162 (SO2) cm–1; 1H NMR: δ 10.28, 9.55 (s, 2H, 2NH, D2O exchangeable), 8.36–7.01 (m, 8H, Ar–H), 5.16 (s, 1H, CHpyrimidine), 4.34–4.29 (q, 2 H, CH2), 2.42 (s, 3H, CH3), 2.28 (s, 3H, CH3), 1.32–1.28 (t, 3 H, CH3); 13C NMR: δ 162.04, 153.91, 152.27, 145.73, 133.13, 132.05, 131.90, 130.71, 128.66, 123.30, 122.57, 104.15, 62.99, 54.02, 21.63, 17.58, 14.37; Anal. Calcd. for C20H20N2O6S (430.47): C, (58.59%); H, (5.15%); N, (6.51%); S, (7.45%). Found: C, (58.41%); H, (5.50%); N, (6.34%); S, (7.76%).
Ethyl-6-methyl-4-(2-[(4-methylphenyl)sulfonyl]oxy-phenyl)-2-thioxo-1,2,3,4-tetrahydro Pyrimidine-5-carboxylate (5)
mp: 205–207 °C; IR (KBr) 3292, 3105 (2NH), 1740 (C=Oester), 1366, 1174 (SO2) cm–1; 1H NMR: δ 10.27, 9.58 (s, 2H, 2NH, D2O exchangeable), 8.38–7.02. (m, 8H, Ar–H), 5.15 (s, 1H, CH pyrimidine), 4.35–3.30 (q, 2 H, CH2), 2.44 (s, 3H, CH3), 1.33–1.29 (t, 3 H, CH3), 1.07 (s, 3H, CH3); 13C NMR: δ 184.74, 153.91,152.31, 145.76, 133.17, 131.76, 130.96, 130.86, 130.71, 128.69, 128.59, 123.33, 122.59, 62.94, 60.03, 21.64, 17.87, 14.40 DEPT; 133.16, 130.85, 130.70, 128.69, 128.58, 123.33, 122.58, 62.94 (negative side, CH2ester), 54.04, 21.63, 17.60, 14.38 Anal. Calcd. for C21H22N2O5S2 (446.54): C, (56.47%); H, (4.89%); N, (6.29%); S, (14.39%). Found: C, (56.56%); H, (4.99%); N, (6.40%); S, (14.31%).
2-(5-Acetyl-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidin-4-yl)-Phenyl 4-methylbenzene Sulfonate (6)
mp: 234–236 °C; IR (KBr) 3250, 3176 (2NH), 1689 (C=O), 1343, 1187 (SO2) cm–1; 1H NMR: δ10.26 (s, H, NH, D2O exchangeable), 9.69 (s, H, NH, D2O exchangeable), 7.77–7.01 (m, 8H, Ar–H), 5.28 (s, 1H, CHpyrimidine), 2.43 (s, 3H, COH3), 2.33 (s, 3H, CH3), 2.16 (s, 3H, CH3); 13C NMR: δ 187.12, 152.07, 151.15,140.54, 136.26, 133.27, 127.76, 121.97, 109.34, 49.63, 30.64, 21.73, 19.57; Anal. Calcd. for C20H20N2O5S (400.44): C, (59.99%); H, (5.03%); N, (7.00%); S, (8.01%). Found: C, (60.05%); H, (4.99%); N, (7.08%); S, (8.11%).
2-(5-Acetyl-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidin-4-yl)-phenyl 4-Methylbenzene Sulfonate (7)
mp: 167–169 °C; IR (KBr) 3193, 3105 (2NH), 1670, 1613, 1375, 1176 (SO2) cm–1; 1H NMR: δ 9.32, (s, H, NH, D2O exchangeable), 7.98–7.96 (m, 9H, Ar–H + NH), 5.57 (s, 1H, CHpyrimidine), 2.46 (s, 3H, CH3), 2.32 (s, 3H, CH3), 1.98 (s, 3 H, CH3); 13C NMR: δ 164.47, 158.40, 156.20, 142.50, 137.86, 134.43, 132.95, 129.24, 128.53, 122.41, 114.91, 110.47, 103.65, 89.54, 61.52, 18.81, 13.97; Anal. Calcd. for C20H20N2O6S (430.47): C, (58.59%); H, (5.15%); N, (6.51%); S, (7.45%). Found: C, (58.41%); H, (5.20%); N, (6.58%); S, (7.36%).
Ethyl-3-(iminomethyl)phenyl4-methylbenzenesulfonate-4-(4-{[(4-Methylphenyl)sulfonyl] oxy}phenyl)-2-thioxo-1,2,3,4-tetrahydro Pyrimidine-5-carboxylate (8)
mp: 267–269 °C; IR (KBr); 3250 (NH), 3054, 2971, 1741 (C=O), 1678, 1373, 1162 (SO2) 1H NMR: δ 9.28 (s, H, NH, D2O exchangeable), 8.37–7.27. (m, 17H, Ar–H), 5.23 (s, 1H, CHpyrimidine), 4.35–4.30 (q, 2 H, CH2), 2.43 (s, 3H, CH3), 2.42 (s, 3H, CH3), 1.33–1.29 (t, 3 H, CH3); 13C NMR: δ; 174.67, 165.41, 162.02, 153.90, 152.30, 146.63, 145.75, 133.16, 131.76, 130.85, 130.70, 128.68, 128.59, 123.32, 122.58, 116.75, 104.11, 62.94, 60.04, 54.04, 21.64, 17.60, 14.40; Anal. Calcd. for C34H31N3O8S3 (705.83): C, (57.88%); H, (4.42%); N, (5.96%); S, (13.64%). Found: C, (57.79%); H, (4.38%); N, (5.99%); S, (13.69%).
2-(5-Acetyl-6-methyl-2-thioxo-3-(iminomethyl)phenyl4-methylbenzenesulfonate-4-(4-{[(4-methylphenyl)sulfonyl] oxy}phenyl)-1,2,3,4-tetrahydro Pyrimidine-5-carboxylate (9)
mp: 284–286 °C; IR (KBr); 3211 (NH), 1688 (C=O), 1603, 1366, 1152 (SO2) cm–1; 1H NMR: δ 10.29 (s, H, NH, D2O exchangeable), 8.36–7.00. (m, 17H, Ar–H), 5.13 (s, 1H, CH pyrimidine), 2.48 (s, 3H, CH3), 2.42 (s, 3H, CH3), 2.27 (s, 3H, CH3), 1.28 (s, 3 H, CH3),1.02 (s, 3H, CH3), 13C NMR: δ 164.12, 162.02, 156.69, 153.90, 152.83, 152.30, 149.15, 147.48, 146.64, 146.20, 133.16, 131.76, 130.85, 130.68, 128.68, 128.40, 123.33, 122.40, 116.08, 104.11, 62.95, 54.01, 21.64, 18.35, 14.41; Anal. Calcd. for C34H31N3O7S3 (689.82): C, (59.20%); H, (4.53%); N, (6.09%); S, (13.94%). Found: C, (59.27%); H, (4.46%); N, (6.01%); S, (14.01%).
In Silico Docking Study
In this study, dihydrofolate reductase enzyme with the cocrystallized ligand was downloaded from the protein data bank (code: 6DTC). We docked compounds 4–9 within dihydrofolate reductase by the use of MOE, 2005.06 applying the previously reported methods.32−34
Acknowledgments
The authors extend their appreciation to Jouf University at Saudi Arabia, Sohag University, at Egypt and Aswan University at Egypt.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c02449.
All spectra data IR, 1HNMR, and 13CNMR for the synthesized compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Poole L. B. The basics of thiols and cysteines in redox biology and chemistry. Free Radical Biol. Med. 2015, 80, 148–157. 10.1016/j.freeradbiomed.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldford J. E.; Hartman H.; Smith T. F.; Segrè D. Remnants of an ancient metabolism without phosphate. Cell 2017, 168, 1126–1134. 10.1016/j.cell.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Eck R. V.; Dayhoff M. O. Evolution of the structure of ferredoxin based on living relics of primitive amino acid sequences. Science 1966, 152, 363–366. 10.1126/science.152.3720.363. [DOI] [PubMed] [Google Scholar]
- Bonfio C.; et al. UV-light-driven prebiotic synthesis of iron–sulfur clusters. Nat. Chem. 2017, 9, 1229–1234. 10.1038/nchem.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalayel I.; et al. Cysteine chemistry in connection with abiogenesis. Eur. J. Org. Chem. 2020, 3019–3023. 10.1002/ejoc.202000089. [DOI] [Google Scholar]
- Khare B. N.; Sagan C. Synthesis of cystine in simulated primitive conditions. Nature 1971, 232, 577–579. 10.1038/232577a0. [DOI] [PubMed] [Google Scholar]
- Weber A. L.; Miller S. L. Reasons for the occurrence of the twenty coded protein amino acids. J. Mol. Evol. 1981, 17, 273–284. 10.1007/bf01795749. [DOI] [PubMed] [Google Scholar]
- Parker E. T.; et al. Primordial synthesis of amines and amino acids in a 1958 Miller H2S-rich spark discharge experiment. Proc. Natl. Acad. Sci. U.S.A. 2006, 108, 5526–5531. 10.1073/pnas.1019191108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerwald A.; et al. RNA-dependent cysteine biosynthesis in Archaea. Science 2005, 307, 1969–1972. 10.1126/science.1108329. [DOI] [PubMed] [Google Scholar]
- Fujishima K.; et al. Reconstruction of cysteine biosynthesis using engineered cysteine-free enzymes. Sci. Rep. 2018, 8, 1776. 10.1038/s41598-018-19920-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J. T.-F. A co-evolution theory of the genetic code. Proc. Natl. Acad. Sci. U.S.A. 1975, 72, 1909–1912. 10.1073/pnas.72.5.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifonov E. N. Consensus temporal order of amino acids and evolution of the triplet code. Gene 2000, 261, 139–151. 10.1016/s0378-1119(00)00476-5. [DOI] [PubMed] [Google Scholar]
- Anastas p. t.; Warner J. C.. Green Chemistry, Theory and Practice; Oxford University, 1998. [Google Scholar]
- Govender T.; Arvidsson P. I.; Maguire G. E. M.; Kruger H. G.; Naicker T. Enantioselective Organocatalyzed Transformations of β-Ketoesters. Chem. Rev. 2016, 116, 9375–9437. 10.1021/acs.chemrev.6b00156. [DOI] [PubMed] [Google Scholar]
- Garcia-Saez I.; DeBonis S.; Lopez R.; Trucco F.; Rousseau B.; Thuéry P.; Kozielski F. Structure of Human Eg5 in Complex with a New Monastrol-based Inhibitor Bound in the R Configuration. J. Biol. Chem. 2007, 282, 9740–9747. 10.1074/jbc.m608883200. [DOI] [PubMed] [Google Scholar]
- Atwal K. S.; Swanson B. N.; Unger S. E.; Moreland S.; Hedberg A.; O’Reilly B. C. Dihydropyrimidine calcium channel blockers. 3. 3-Carbamoyl-4-aryl-1,2,3,4-tetrahydro-6-methyl-5-pyrimidinecarboxylic acid esters as orally effective antihypertensive agents. J. Med. Chem. 1991, 34, 806–811. 10.1021/jm00106a048. [DOI] [PubMed] [Google Scholar]
- Bienaymé H.; Hulme C.; Oddon G.; Schmitt P. Maximizing Synthetic Efficiency: Multi-Component Transformations Lead the Way. Chem.—Eur. J. 2000, 6, 3321–3329. . [DOI] [PubMed] [Google Scholar]
- Tayebee R.; Ghadamgahi M. Solvent free one-pot multi-component synthesis of 3,4-dihydropyrimidin-2(1H)-ones catalyzed by mesoporous NH4H2PO4/MCM-41 as an environmentally friendly, cheap, and effective catalyst. Arabian J. Chem. 2017, 10, S757–S764. 10.1016/j.arabjc.2012.12.001. [DOI] [Google Scholar]
- Khatri C. K.; Rekunge D. S.; Chaturbhuj G. U. Sulfated polyborate: a new and eco-friendly catalyst for one-pot multi-component synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones via Biginelli reaction. New J. Chem. 2016, 40, 10412–10417. 10.1039/c6nj03120j. [DOI] [Google Scholar]
- Salim S. D.; Akamanchi K. G. Sulfated tungstate: An alternative, eco-friendly catalyst for Biginelli reaction. Catal. Commun. 2011, 12, 1153–1156. 10.1016/j.catcom.2011.02.018. [DOI] [Google Scholar]
- Hassanpour A.; Abolhasani J.; Khanmiri R. H. One-Pot and Green Procedure for the Synthesis of 3,4-Dihydropyrimidin-2(1H)-(thio)ones Using ZnO Nanoparticles as a Solid Acid Catalyst. J. Korean Chem. Soc. 2014, 58, 445–449. 10.5012/jkcs.2014.58.5.445. [DOI] [Google Scholar]
- Murthy Y. L. N.; Rajack A.; Yuvaraj K. Solvent free synthesis of 3,4-dihydropyrimidine-2-(1H)-ones/thiones catalyzed by N,O-bis(trimethylsilyl)acetamide and dicyclohexyl carbodimide. Arabian J. Chem. 2016, 9, S1740–S1746. 10.1016/j.arabjc.2012.04.046. [DOI] [Google Scholar]
- Abd El Aleem Ali Ali El-Remaily M.; Elhady O. M. Green Bio-organic and Recoverable Catalyst Taurine (2-aminoethanesulfonic acid) for Synthesis of Bio-active Compounds 3,4-Dihydropyrimidin Derivatives in Aqueous Medium. ChemistrySelect 2020, 5, 12098–12102. 10.1002/slct.202002575. [DOI] [Google Scholar]
- Safari J.; Gandomi-ravandi S.; Ashiri S. Organosilane sulfonated graphene oxide in the Biginelli and Biginelli-like reactions. New J. Chem. 2016, 40, 512–520. 10.1039/c5nj01741f. [DOI] [Google Scholar]
- Oskooie H. A.; Heravi M.; Karimi M.; Monjezy N. H.; Heravi M. M.; Karimi N. FeCl3 Immobilized in Al-MCM 41: An Efficient Catalyst System for the Biginelli Reaction. Synth. Commun. 2013, 41, 826–831. 10.1080/00397911003668596. [DOI] [Google Scholar]
- a Abd El Aleem Ali Ali El-Remaily M.; Abu-Dief A. M.; Elhady O. M. Green synthesis of TiO2 nanoparticles as an efficient heterogeneous catalyst with high reusability for synthesis of 1,2-dihydroquinoline derivatives. Appl. Organomet. Chem. 2019, 33, 5005. 10.1002/aoc.5005. [DOI] [Google Scholar]; b Abd El Aleem Ali Ali El-Remaily M.; Abu-Dief A. M.; El-Khatib R. M. A robust Synthesis and Characterization for Superparamagnetic CoFe2O4 Nanoparticles as an Efficient and Reusable Catalyst for Synthesis of some Heterocyclic rings in aqueous media. Appl. Organomet. Chem. 2016, 30, 1022–1029. 10.1002/aoc.3536. [DOI] [Google Scholar]; c Abd El Aleem Ali Ali El-Remaily M.; Soliman A. M. M.; Elhady O. M. Green Method for the Synthetic Ugi Reaction by Twin Screw Extrusion without a Solvent and Catalyst. ACS Omaga 2020, 5, 6194–6198. 10.1021/acsomega.0c00369. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Abd El Aleem Ali Ali El-Remaily M.; Hamad H. A.; Elhady O. M. Boosting the catalytic performance of manganese (III)-porphyrin complex MnTSPP for facile one-pot green synthesis of 1,4-dihydropyridine derivatives under mild conditions. Appl. Organomet. Chem. 2021, 35, e6238. [Google Scholar]; e Shokr E. K.; Kamel M. S.; Abdel-Ghany H.; Abd El Aleem Ali Ali El-Remaily M. Optoelectronic characteristics of as-deposited, annealed and I2–Treated thin films of newly synthesized organic dye based on pyrrolo [2, 3-b] pyrrole. Curr. Res. Green Sustainable Chem. 2021, 4, 100090. 10.1016/j.crgsc.2021.100090. [DOI] [Google Scholar]; f Hamad H. A.; Nageh H.; El-Bery H. M.; Kasry A.; Carrasco-Marín F.; Elhady O. M.; Soliman A. M. M.; Abd El Aleem Ali Ali El-Remaily M. Unveiling the exceptional synergism-induced design of Co-Mg-Al layered triple hydroxides (LTHs) for boosting catalytic activity toward the green synthesis of indol-3-yl derivatives under mild conditions. J. Colloid Interface Sci. 2021, 599, 227–244. 10.1016/j.jcis.2021.04.083. [DOI] [PubMed] [Google Scholar]; g Shokr E. K.; Kamel M. S.; Abdel-Ghany H.; Abd El Aleem Ali Ali El-Remaily M. Optical characterization and effects of iodine vapor and gaseous HCl adsorption investigation of Novel Synthesized Organic dye Based on Thieno[2,3-b]thiophene. Optik 2021, 243, 167385. 10.1016/j.ijleo.2021.167385. [DOI] [Google Scholar]
- a Abd El Aleem Ali Ali El-Remaily M.; Soliman A. M. M.; Khalifa M. A.; El-Metwaly N. M.; Alsoliemy A.; El-Dabea T.; Abu-Dief A. M. Rapidly, highly yielded and green synthesis of dihydrotetrazolo[1,5-a]pyrimidine derivatives in aqueous media using recoverable Pd (II) thiazole catalyst accelerated by ultrasonic: Computational studies. Appl. Organomet. Chem. 2022, 36, e6320 10.1002/aoc.6238. [DOI] [Google Scholar]; b Elsayed A. M.; El-Remaily M. A. E. A. A. A.; Salama K. S. M.; Abdelhamid A. A. Utility of pyrrole-2-thioacetohydrazide in synthesis of new heterocyclic compounds with promising antimicrobial activities and molecular docking studies. J. Heterocycl. Chem. 2022, 59, 449–465. 10.1002/jhet.4392. [DOI] [Google Scholar]; c Abdelhamid A. A.; Salama K. S. M.; Elsayed A. M.; Gad M. A.; Abd El Aleem Ali Ali El-Remaily M. Synthesis and Toxicological Effect of Some New Pyrrole Derivatives as Prospective Insecticidal Agents against the Cotton Leafworm, Spodoptera littoralis (Boisduval). ACS Omega 2022, 7, 3990–4000. 10.1021/acsomega.1c05049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein B. R. M.; Ali A. M. Multicomponent Reaction for Synthesis of Novel 2-Tosyloxyphenylpyridines. J. Heterocycl. Chem. 2019, 56, 1420–1425. 10.1002/jhet.3521. [DOI] [Google Scholar]
- Khodairy A.; Ali A. M.; El-Wassimy M. T. 4-Toluenesulfonamide as a Building Block for Synthesis of Novel Triazepines, Pyrimidines, and Azoles. J. Heterocycl. Chem. 2016, 53, 1544–1553. 10.1002/jhet.2461. [DOI] [Google Scholar]
- Khodairy A.; Ali A. M.; Aboelez M. O.; El-Wassimy M. T. One-Pot Multicomponent Synthesis of Novel 2-Tosyloxyphenylpyrans under Green and Conventional Condition with Anti-inflammatory Activity. J. Heterocycl. Chem. 2017, 54, 1442–1449. 10.1002/jhet.2730. [DOI] [Google Scholar]
- Mourad A.-F. E.; Amer A. A.; El-Shaieb K. M.; Ali M. A.; Aly A. A. 4-Hydroxy-1-phenylquinolin-2 (1H)-one in One-pot Synthesis of Pyrimidoquinolines and Related Compounds under Microwave Irradiation and Conventional Conditions. J. Heterocycl. Chem. 2016, 53, 383–388. 10.1002/jhet.2286. [DOI] [Google Scholar]
- Khodairy A.; Shaaban K. M.; Ali M. A.; El-Wassimy M. T.; Nagwa S. A. Eco-friendly and efficiently synthesis, anti-inflammatory activity of 4-tosyloxyphenylpyrans via multi-component reaction under ultrasonic irradiation and room temperature conditions. J. Chem. Pharm. Res. 2015, 7, 332–340. [Google Scholar]
- Khodairy A.; Ali M. A.; El-Wassimy M. T. Synthesis of Novel Chromene, Pyridine, Pyrazole, Pyrimidine, and Imidazole Derivatives via One-pot Multicomponent Reaction. J. Heterocycl. Chem. 2017, 54, 3342–3349. 10.1002/jhet.2954. [DOI] [Google Scholar]
- Khodairy A.; Ali M. A.; El-Wassimy M. T. Synthesis and Reactions of New Thiazoles and Pyrimidines Containing Sulfonate Moiety. J. Heterocycl. Chem. 2018, 55, 964–970. 10.1002/jhet.3126. [DOI] [Google Scholar]
- Ahmed E. A.; Soliman A. M. M.; Ali M. A.; Abd El Aleem Ali Ali El-Remaily M. Boosting the catalytic performance of zinc linked amino acid complex as an eco-friendly for synthesis of novel pyrimidines in aqueous medium. Appl. Organomet. Chem. 2021, 35, e6197 10.1002/aoc.6197. [DOI] [Google Scholar]
- Bakr R. B.; Azab I. H. E.; Elkanzi N. A. A. Thiochromene candidates: design, synthesis, antimicrobial potential and in silico docking study. J. Iran. Chem. Soc. 2022, 19, 1413–1423. 10.1007/s13738-021-02391-w. [DOI] [Google Scholar]
- El Azab I. H.; Bakr R. B.; Elkanzi N. A. A. Facile one-pot multicomponent synthesis of pyrazolo-thiazole substituted pyridines with potential anti-proliferative activity: synthesis, in vitro and in silico studies. Molecules 2021, 26, 3103. 10.3390/molecules26113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shammri K. N.; Elkanzi N. A.; Arafa W. A.; Althobaiti I. O.; Bakr R. B.; Moustafa S. M. N. Novel indan-1,3-dione derivatives: Design, green synthesis, effect against tomato damping-off disease caused by Fusarium oxysporum and in silico molecular docking study. Arabian J. Chem. 2022, 11, 10373. 10.1016/j.arabjc.2022.103731. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.