Abstract
Central activation in response to emotion and cognitive stress induces perturbations in the heart and the peripheral vasculature that differ in physiology and clinical manifestations when compared to exercise induced changes. While our conventional framework of epicardial coronary artery disease (CAD) is foundational in cardiology, an expanded paradigm is required to address the cardiovascular (CV) response to mental stress (MS) and its associated risks, thus addressing the intersection of the patient’s ecologic and psychosocial experience with CV biology. To advance the field of MS in CV health, certain core challenges must be addressed. These include differences in the trigger activation between exercise and emotion, identification and interpretation of imaging cues as measures of pathophysiologic changes, characterization of the vascular response, and identification of central and peripheral treatment targets. Gender and psychosocial determinants of health are important in understanding the emerging overlap of mental stress induced myocardial ischemia (MSIMI) with microvascular dysfunction and symptoms in the absence of obstructive disease. In overcoming these critical knowledge gaps. Integration of the field of MS will require implementation studies to guide use of MS testing, to support diagnosis of MS induced cardiac and vascular pathophysiology, to assess prognosis, and understand the role of endotying to direct therapy.
Keywords: mental stress provoked ischemia, coronary artery disease, microvascular disease, cardiovascular imaging
Subject Terms: Cardiovascular Disease, Mental Health, Imaging, Risk Factors, Prognosis, Atherosclerosis
Introduction
Advanced cardiac imaging serves to assist clinicians in the diagnosis of cardiac conditions, assess prognosis, and guide treatment. With the maturation of this discipline, there has been a concurrent reinforcement of constructs that promote certain understandings and improve efficiencies, which has contributed to a decline in morbidity and mortality. The nature of successful endeavors often are a result of refinements and consolidation that become broadly accepted. The imaging constructs of the past must expand to rally the strengths of new technology and the transformative power inherent in multidisciplinary translational research.
The standard paradigm, epicardial coronary artery disease (CAD) resulting in ischemia, fixates in a locality and a concept: the obstructive epicardial coronary lesion and a binary cue of ischemia. While this framework will remain integral, an ‘ischemic phenomenon’ due to emotion may fall outside of the this construct. Very often there is clinically meaningful reduction in myocardial blood flow (MBF) in the absence of a localized ischemic signal which may be due to triggers that are not associated with exertion. The effects of emotion and cognitive stress on cardiac physiology may be pervasive, diffuse, and influenced by multiple biologic and environmental inputs.
Thus, the discipline of cardiac imaging is challenged to expand observations and approaches to promote a better understanding of perturbations in cardiac and systemic biology in response to stress experienced in daily life. The stress of daily life has been modeled in the laboratory to study changes in MBF and function. This has resulted in the term mental stress (MS) and its resultant ischemic imaging cue as mental stress induced myocardial ischemia (MSIMI).
The Impact of Stress on Cardiac health
The contribution of stress and emotional factors to incident cardiovascular (CV) disease and mortality has been described through the centuries, from Ovid to Osler to important work that demonstrated emotional stress experienced with anger as a trigger to myocardial infarction (MI).1 Epidemiologic work from INTERHEART and others demonstrate the important association of cumulative and daily psychosocial stress with CV risk. 2, 3 Responses to emotional stress during the course of daily life can provoke transient changes in myocardial perfusion along with regional and global systolic dysfunction, and is associated with the development of atherosclerosis, hypertension, and heart failure. 4 5 1 6 7 The relationship of life stressors and cardiac physiology has been modeled and standardized through laboratory MS testing, providing affirmation of the association between social determinants of health (socioeconomic status, gender) and psychosocial factors (anger reactivity, depression) with prevalence of MSIMI.
MSIMI is common, occurring in approximately half of patients with stable coronary artery disease (CAD), and has a strong association with increased CV morbidity and mortality. 1 The totality of work has demonstrated that patients who evidence MSIMI are at increased risk for cardiac events and early mortality, above that predicted by exercise provoked ischemia, regardless of the modality used to assess MSIMI. 4, 8, 9 A meta-analysis of 5 studies incorporating outcomes on 555 patients, reported a pooled relative risk of 2.24 (95% CI 1.59-1.35) for cardiac events or total mortality associated with change in LVEF or new RWMA in response to a MS trigger, a finding that has been affirmed in studies using a perfusion cue. 8, 10
Overcoming obstacles in the field of Mental Stress and Cardiovascular disease
Despite the well-established and accepted concept of MSIMI based upon the relationship between stress and CV pathophysiology, MSIMI is an under-identified disease with a resultant failure of diagnosis and treatment within our standard clinical practice. When we apply the framework of exercise physiology and demand-induced ischemia to that of emotion and cognitive triggers, the imaging cues of locality and threshold bias our observations and understandings.
The two cardinal distinction between triggers of exercise and that of emotion are: a difference in brain/central nervous system (CNS) hyperactivation with attendant neurohormonal stimulation and activation of peripheral vasculature in the opposite vectors. Within the broad and multidisciplinary stress literature,11 mental stress is recognized as systemic with initiation of central and peripheral processes that are transduced through multiple overlapping and interacting biologic pathways with terminal prominent systemic vascular vasoconstriction. Despite the systemic effects of stress, our measures of MS are often organ-specific, compartment specific, and more so, pathway-specific. The imaging cues must be reconsidered for cognitive triggers to address distinctions in regionality, threshold, target processes, and the interplay of peripheral and cardiac perturbations.
The current approaches in cardiac imaging, informed by a model of coronary obstruction, may not be sufficient to advance an understanding of the effect of emotion/cognitive stress on CV physiology and to promote development of novel treatments. Below we discuss several core challenges to our current approach with respect to MSMI:
Differences in ordinate activation of exercise and emotion triggers
Imaging cues
Characterization of the vascular response
Identification of treatment targets
Implementation into standard care
1. Differences in Ordinate Activation of Exercise and Emotion Triggers
Brain activation patterns suggest a phenomenological context defined by a cognitive-emotional trigger that is transduced centrally, and communicated to the periphery through both direct innervation of target organs - the myocardium, the vascular system - and through the circulation, by way of release by the adrenals of both catecholamines and cortisol. The autonomic ‘fight-flight response’ and the hypothalamic-pituitary-adrenal (HPA) axis response - have implications for provocation of MSIMI and thus provide potential targets for intervention.
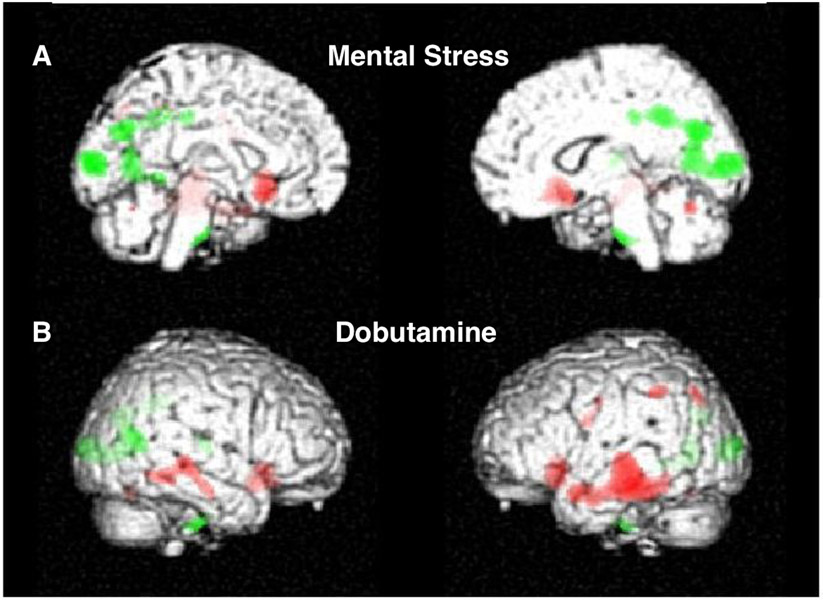
Simultaneous imaging of heart-brain activity in response to MS triggers by positron emmison tomography (PET) in CAD patients has shown specific patterns of CNS hyperactivation in association with MSIMI that are distinct from that observed with demand induced challenge with dobutamine and in non-CAD patients. FIGURE 112 Patients with MSIMI demonstrate hyperactivation in frontolimbic circuits associated with neurohormonal and autonomic regulation, emotion, memory, fear, and anxiety. There is deactivation in the prefrontal areas, along with hyperactivation in with the central autonomic network, which is strongly associated with the limbic system and involved in the negative feedback circuit between the limbic system and the prefrontal cortex. This circuit appears essential to the regulation of autonomic responses to stressful stimuli. A situational imbalance in the activity between these areas, or a fixed inability of the prefrontal cortex to attenuate limbic responses, may be linked with autonomic dysregulation which, during MS, can result in a net shift towards increased sympathetic tone and/or withdrawal of parasympathetic tone.13, 14 In comparison, demand/dobutamine-induced ischemiais associated with brain activation in the peripheral sensorimotor and areas referable to situational awareness.
Figure 1:
Subjects with CAD underwent simultaneous measurement of cerebral blood flow and cardiac function during (A-top panel) arithmetic mental stress (MS) and (B-bottom panel) dobutamine stress (DS). Cerebral hyperactivation (green) and deactivation (red) in response to MS (A-top panel) and DS-demand (B-bottom panel).12 Activation (green) during MS occurs in deep limbic structures, which transduce visceral effectors to the heart and significantly heightens neurohormonal activation. Concurrently, there is deactivation (red) in the frontal lobes. Activation during DS does not occur in deep limbic structures, instead occurs in the somatosensory areas referable to situation and environmental awareness, without activation of areas related to emotion and memory. Reproduced with permission from Springer Nature and Copyright Clearance Center.
The results of this transduction is an alteration in sympatho-vagal balance (an increase in sympathetic nervous system activity and a withdrawal of cardiac vagal control), along with additional communication to the periphery as reflected in an increase in downstream inflammatory activity, an increase in both inotrophic and chronotrophic drive, and, likely a consequence of both systemic (e.g., endothelial dysfunction) and local (e.g., altered vasomotor responsivity to autonomic stimulation in the coronary tree) aspects of CAD, a reduction in MBF - MSIMI.15
In the current era we have recognized the importance of gender interaction in regard to triggers that culiminate in myocardial ischemia. In regard to MS triggers, female gender is associated with bilateral activation of the anterior cingulate gyrus, which is linked to parasympathetic acetylcholine mediated vasoconstriction, a thought-provoking connection to the gender interaction with INOCA (ischemia and no obstructive coronary artery disease) and microvascular disease.
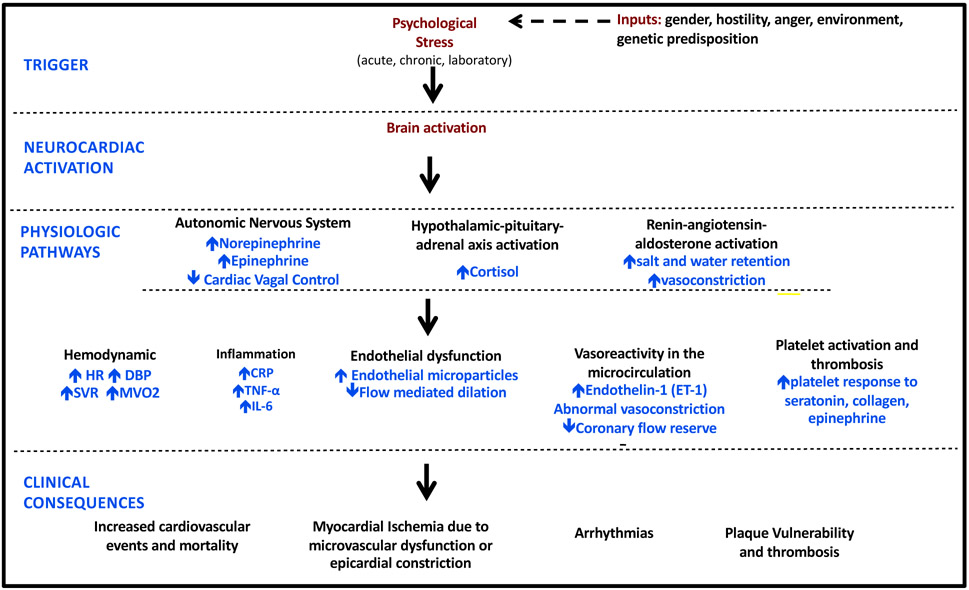
Understanding the multiple inputs that contribute to the heterogenous response to stress and identifying treatment targets must be informed by the totality of the stress provoked CNS activation, the multiple and overlapping physiologic pathways by which stress is transduced to the periphery, and the terminal effects on vasomotion, arterial compliance, endothelial (dys)function, and clinical CV phenomena. Figure 2. The marked hyperactivation of neurohormonal centers responsible for catecholamine driven responses overlaps with other emotion induced CV dysfunction. Heretofore, MSIMI has been considered to be distinct from Takotsubo cardiomyopathy, however there are similaritines of CNS activation with catecholamine driven terminal vascular changes.16
FIGURE 2: Proposed pathophysiologic mechanism of MSIMI.
HR, heart rate, SBP, DBP: diastolic blood pressure, SVR: systemic vascular resistance, CRP: C reactive protein, TNF-α: tumor necrosis factor alpha, IL-6: interleukin-6.
2. Challenges in Using Imaging Cues to Define MSIMI
Numerous studies have demonstrated that MSIMI has distinctions in clinical presentation and prevalence from exercise-induced angina. Early studies using ECG monitoring suggested that physiologic factors perturbed in exercise-induced ischemia differ from that due to emotional triggers, though they have some overlap.17 In ecological Holter studies, over 75% of the total ischemic episodes in CAD patients occurred during routine activities, without associated anginal symptoms, and at a significantly lower heart rate (HR) than observed during exercise stress.18 19, 20
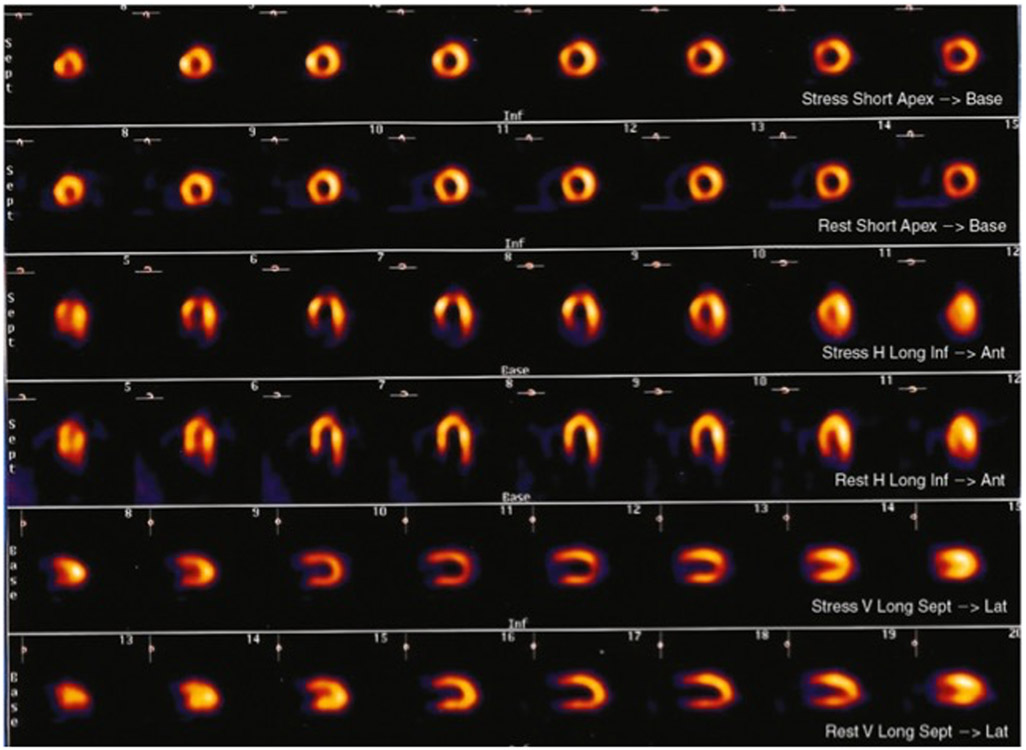
There has been a consistent and reproducible observations that the cue of ischemia differs in extent, location, degree, and prevalence in response to MS and to demand triggers within and across subjects.21 22 23 In a series of stable CAD patients undergoing echo imaging to stress triggers, more often patients will show ischemia to MS (up to a quarter) without demonstrating ischemia to demand.24 This finding was affirmed in the REMIT study wherein ischemia to MS was more common than exercise induced ischemia (43.5% vs. 33.8%, p=0.003) and 70.4% of CAD subjects that demonstrated ischemia to exercise also had MSIMI while only half of those with MSIMI demonstrated ischemia to exercise.25 Sensitive imaging studies using PET evaluated patients with chronic stable angina who underwent sequential arithmetic stress and exercise stress protocols with resulting perfusion heterogeneity to both stressors, though the ischemic distribution did not demonstrate strict concordance of size or location of defects between the two methods of stress.26 FIGURE 327
FIGURE 3:
Mental stress technetium-99m tetrofosmin single-photon emmision tomography (low-dose rest, high-dose stress) images from a patient who had an apical perfusion defect, consistent with mental stress-induced ischemia (top) compared with resst images (bottom). Ant, anterior; Inf, inferior; Lat, lateral; Sept, septum. 27 Reproduced with permission from Elsevier and Copyright Clearance Center.
Despite MSIMI being common, detection of MSIMI using conventional stress testing imaging tools has resulted in reports of variable prevalence and differences in regional vs. global localization. Work by our group demonstrated a 46% discordance in the detection of MSIMI in patients undergoing both echocardiography and MPI with MS trigger resulting in concordant detection of MSIMI by both modalities in 11%, only perfusion defects on MPI in 32%, and only regional or global LV dysfunction on echo in 21% of subjects.28 While this may be attributed to differences in test characteristics, it is more likely due to a difference in the physiologic target probed by the imaging cue. First, the regionality of wall motion abnormalities or perfusion is particularly problematic as it suggests a differential effect on epicardial territories or myocardial segments despite a well-based foundation that stress globally affects myocardial physiology, if not also systemic biology. Second, a stress induced drop in LVEF on conventional stress testing is considered a high risk feature indicative of severe diffuse obstructive epicardial disease. As such, global LV systolic function in response to MS would be suggestive of a threshold of ischemia that is out of proportion to what is suggested by a lack of overt symptoms and the mild nature of perfusion defects in MSIMI. Third, discordance of imaging cues may represent individual variability in the relative impact of different biologic pathways, highlighting a role for endotyping.
A single imaging cue, as a measure of a physiologic response to stress, may not provide a wide enough lens to assess the totality of the CV effects. Cues can be categorized by regionality (change in epicardial caliber, regional wall motion abnormalities (RWMA), regional perfusion defect) vs. global myocardial (change in LV systolic function or size, global coronary flow reserve (CFR)), coronary macrovascular vs. microvascular vasomotion, and cardiac vs. peripheral vascular effects. Table 1 Given an appreciation of the multiple ways in which MS alters CV measures and imaging cues 29 and the limited applicability of the concept of the ischemic cascade that is anchored in the construct of epicardial CAD, the naming of this phenomenon as MSIMI may not be sufficient to describe the breath of MS-induced perturbations of the CV system. While we will continue to use the MSIMI term in this conversation for familiarity, we recognize that the imaging cues of MSIMI-perfusion heterogeneity and LV function changes- may reflect physiologic alterations that represent both ischemia and non-ischemic phenomenon.
Table 1:
Characteristics of Imaging Cues of MSIMI and Associated Physiologic Target
| Measure of MSIMI |
Diagnostic technique |
Probable target | Vascular compartment | Regionality | Setting |
|---|---|---|---|---|---|
| ST segment depression | Holter monitoring ECG Wearable devices |
Cardiac ischemia | Cardiac
|
Regional or Global Non-transmural |
Ecologic Laboratory Non-invasive |
| Fall in LVEF >5% | Nuclear VEST ERNA Echocardiography |
Systolic dysfunction Due to: (1) Global Ischemia (epicardial or microvascular) (2) Peripheral vascular tone and SVR |
Cardiac
|
Global Transmural or non-transmural |
Ecologic Laboratory Non-invasive |
| New regional wall motion abnormality | Echocardiography | Regional ischemia | Cardiac
|
Regional Transmural or non-transmural |
Laboratory Non-invasive |
| Reversible segmental hypoperfusion | Nuclear perfusion imaging (SPECT, PET), qualitative | Segmental ischemia or segmental heterogeneity in vasomotor response | Cardiac
|
Regional Likely non-transmural | Laboratory Non-invasive |
| Dynamic epicardial vasoconstriction | Coronary angiography | Epicardial artery reactivity/spasm | Cardiac
|
Regional | Laboratory Invasive |
| Coronary flow at peak stress and reserve | Quantitative PET (MRI perfusion, CT perfusion) Invasive measure of intracoronary blood velocity | Global or segmental ischemia or alteration in vasomotor response Segmental or global abnormal change in flow | Cardiac
Exclude systemic effect by adjustment by RPP |
Regional and Global | Laboratory Non-invasive or invasive |
| Coronary microvascular resistance | Index of Microcirculatory Resistance (IMR) | Coronary microvascular tone | Cardiac
|
Regional | Laboratory Invasive |
| Systemic vascular tone | Calculated SVR Peripheral arterial tonometry |
Peripheral macrovascular and microvascular tone | Systemic
|
Laboratory Ecologic? | |
| Endothelial Function | Brachial artery reactivity Peripheral arterial tonometry |
Endothelium | Systemic
|
Laboratory |
3. Characterization of the vascular response
Studies of MSIMI have focused on cardiac changes using tools interpreted through the framework of demand ischemia in epicardial obstructive CAD, though the systemic effect of stress transduces changes throughout the vascular tree. Unlike patterns seen in conventional stress testing, recent work has affirmed a lack of concordance of MS provoked myocardial perfusion heterogeneity with severity of epicardial obstruction.8, 30 Angiographic assessment of the epicardial artery demonstrated that MS provocation resulted in dynamic epicardial vasoconstriction in minimally diseased arteries with non-obstructive plaque, as compared to the expected vasodilation in normal epicardial arteries.31 The vasomotor response of the epicardial arteries correlated with acetylcholine challenge, linking endothelial dysfunction with abnormal vasoconstriction. Perhaps, dynamic changes in the epicardial artery may account for the regionality of the MSIMI signal in certain individuals.
Further work in the invasive laboratory using Doppler measures of intracoronary blood velocity demonstrated that the magnitude of the reduction in coronary flow in the setting of MS was likely secondary to an increase in resistance in the coronary microvascular bed.32 The differential effects of MS and vasodilator stress on perfusion in coronary distributions subtended by angiographically defined epicardial arteries without obstructive disease was assessed non-invasively using N-13 ammonia (NH3) PET with measures of absolute MBF, CFR, and derived distal pressures.33 There was a decrease in MBF at peak MS in regions subtended by arteries with non-obstructive CAD, as compared to an increase in regions subtended by epicardial arteries with a moderate or greater stenosis or normal controls (0.68 ml/min/g vs. 0.75 ml/min/g vs. 0.8, p<0.05) and a decrease in CFR at peak MS (1.1 vs. 1.3 vs. 1.5, p<0.05). In contrast, there was augmentation in absolute MBF and CFR in response to vasodilator stress in all 3 groups (p<0.05). Thus, the role of the microvasculature, likely the pre-arteriolar vessels, was highlighted by an increase in derived distal pressures during MS only in territories subtended by vessels with non-obstructive CAD.
While much of the previous work in MSIMI has used qualitative assessment of flow heterogeneity on MPI, MS-induced changes in sub-endocardial blood flow may be missed due to limited spatial resolution and dependence of partial volume effect.34 MPI, notably using PET, can be used to measure absolute MBF during MS which may provide increased sensitivity to detect regional and global reductions in flow in response to MS. Alternative techniques such quantitative CT myocardial perfusion, quantitative MR myocardial perfusion, or hybrid nuclear imaging with first pass CT perfusion particularly with use of particular PET tracers (19F-Flupiridaz or 13N-NH3) may allow measurement of the transmural perfusion gradient (TPG) and ratio (TPGR), to relate sub-endocardial and sub-epicardial perfusion at rest and stress.35, 36 To understand use of MBF, CFR, TPG, and TPGR, further work needs to be performed to identify normal values and reproducibility of measures under a variety of triggers.
Identification of sub-endocardial hypoperfusion or of systolic dysfunction with MS may not necessarily be indicative of myocardial ischemia due to insufficient MBF, but rather alterations in peripheral vascular tone in response to MS. Work from the PIMI investigators and our group demonstrated that MS was associated with a significant increase in systemic vascular resistance (SVR) - afterload, with a strong inverse correlation between change in SVR and change in LVEF during MS - as SVR increased, LVEF was reduced. 37 Notably, some healthy individuals demonstrated up to 15% decrease in LVEF during MS, with women showing the biggest effect. The drop in LVEF and increase in SVR occurred without discrete regional perfusion deficits, suggesting a global effect on the myocardium notably due to alterations in peripheral vasomotion which is a prominent feature of MS.37 38. Even in lessons from conventional stress testing, abnormal rise in blood pressure to an exercise trigger is associated with adverse prognosis, perhaps identifying a marker of abnormal peripheral vasomotion.39
Endothelial dysfunction may underlie the systemic vascular response to stress and this peripheral response tracks with myocardial response, thus identifying a peripheral correlate for MS effects on coronary endothelial function and a potential diagnostic target for risk stratification in MSIMI. Initial studies in the periphery demonstrated that MS is associated with flow mediated dilation (FMD) at the brachial artery in healthy vasculature but is blunted in those with hypercholesterolemia and psychosocial features that predispose to MSIMI (hostility, depression in women), suggesting a mediating role of peripheral endothelial function.40, 41
Using acetylcholine as a probe of endothelial function in both the coronary and peripheral circulation, investigators in the Mental Stress Ischemia Prognosis study demonstrated that the change in MBF to MS correlates with MBF changes provoked by acetylcholine challenge (r=0.38, P=0.03), while there is a lack of correlation with nitroprusside, a probe of both smooth muscle and nonendothelial dependent vasodilation.42 This small mechanistic study (n=38) affirms that the predominant global effect of MS is facilitated through endothelial-mediated vasoconstriction in the coronary microvasculature. Additionally, investigators demonstrated a relationship between MS-provoked peripheral vasoconstriction, as assessed by peripheral arterial tonometry, and demand-adjusted measure of coronary microvascular tone (r=−0.60, P=0.004). This peripheral vasoconstriction in response to MS-trigger is associated with a higher risk of adverse CV outcomes (1.77 [95% CI, 1.12-2.80]), a finding that also correlated with norepinephrine, implicating the role of the neurohormonal cascade and sympathetic nervours system.43 Similarily, peripheral endothelial health is associated with CV events and correlates with epinephrine levels as demonstrated in a cohort of 569 patients with stable CAD in whom brachial artery FMD was assessed before and after MS testing. A MS provoked change in brachial artery FMD was associated with a 78% increase in CV events at 3 years.44
Similar to the extensive work in the area of ischemia with INOCA and microvascular disease, the binary or threshold signal used to interpret standard-of-care MPI studies fails to capture global and more subtle reductions in MBF that may occur along the spectrum of physiologic changes due to MS-a spectrum inclusive of degree, quality, chronicity, and the vascular response. The model of dynamic endothelial-dependent MS–induced microcirculatory dysfunction in MSIMI aligns with the current understanding of symptomatic nonobstructive CAD in women,45 and there is an emerging intersection between the association of heightened ischemic response to MS triggers in young women with CAD and the presentation and prevalence of INOCA in these patients.46 Studies such as CorMicA, which stratified patients based on invasive coronary functional testing with acetylcholine and thermodilution for flow reserve/microvascular resistance, have shown that tailoring medical therapy based on specific INOCA endotypes improves symptoms,47 and hopefully long-term outcomes.48 As we begin to unravel the overlapping syndromes of MSIMI, INOCA, and microvascular dysfunction, MS testing might be a useful probe of the microvasculature and abnormalities in endothelial function that could further phenotype patients with anginal syndromes. Similarly the identification of specific endotypes in MSIMI may offer a promising construct for personalization of therapies given the presumed heterogeneity in the relative contribution of the multiple biologic pathways.
4. Potential Targets and Treatments
There have been several promising clinical intevention trials to reduce the likelihood of MSIMI under stressful circumstances. This approach using cognitive behavior therapy for stress management has shown promise for reducing the likelihood of MSIMI under controlled laboratory conditions and in the natural environment, and for reducing the risk of major adverse cardiac events that has been found to accompany MSIMI. In a series of studies with moderately sized samples of 100-150 CAD patients, Blumenthal and colleagues have repeatedly shown that augmentation of a standard cardiac rehabilitation-based exercise program with cognitive behavioral stress management training conferred impressive relative risk reduction of events compared to standard cardiac rehabilitation (9.1% x 20.6% at 3 years, RR, 0.26; 95% CI, 0.07-0.90; p=0.03) and stress management training compared to standard cardiac rehabilitation (18% versus 33% at 5 years; hazard ratio=0.49; 95% confidence interval, 0.25–0.95; P=0.035). 49, 50 51 FIGURE 451 There was also significantly lower 5-year healthcare utilization and cost associated with the stress management treatment arm. Despite these impressive results, testing of implementation strategies for dissemination of these practices into routine clinical care remains to be accomplished.
FIGURE 4: Association of cardiac rehabilitation and stress management with cardiovascular outcomes in patients following acute coronary syndrome.
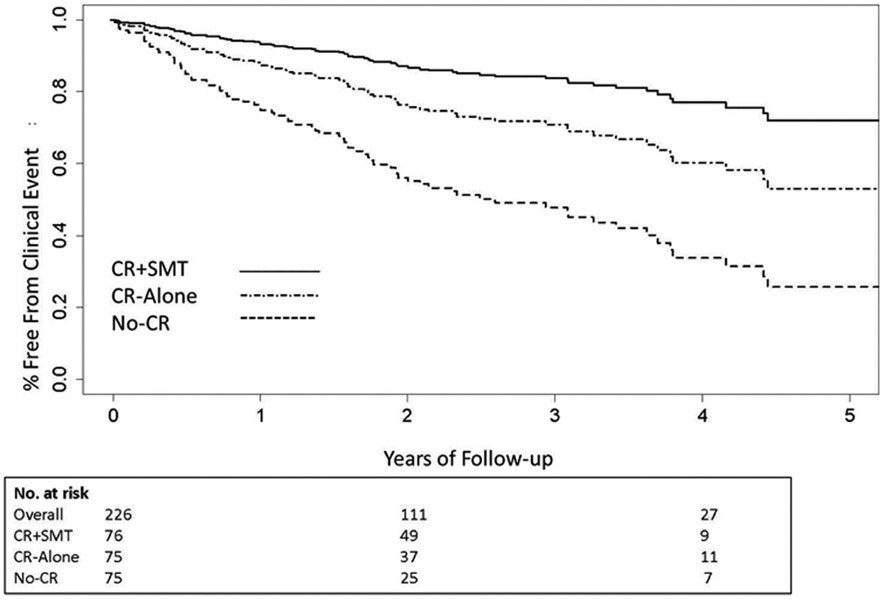
Cumulative time-to-event curves for clinical events in the CR+SMT, CR-alone, and No-CR groups. Clinical events included all-cause mortality, myocardial infarction, cardiac or peripheral vascular intervention, stroke/TIA, or unstable angina requiring hospitalization. Participants in the CR+SMT were at significantly lower risk of clinical events compared with the CR-alone group (HR= 0.47 [0.24, 0.91], P = 0.025). Both CR groups had lower event rates compared with a non-randomized, matched No-CR control group (HR = 0.35 [0.22, 0.56], P < .001). Number at risk represents participants with follow-up data for clinical events who had not yet had an event at years 0, 2, and 4.51 Reproduced with permission from Wolters Kluwer Health, Inc. and Copyright Clearance Center.
The REMIT study demonstrated an alternate, pharmacologic approach that targets central processes in MSIMI. In this study, CAD patients with MSIMI diagnosed by global or regional systolic dysfunction on echocardiography demonstrated that randomization to escitalopram, an SSRI, reduced MSIMI at 6 weeks compared to placebo (OR of not having MSIMI at 6 weeks 2.62 [95% CI, 1.06 to 6.44]; P=.04).50 The statistical significance of this finding was lost with adjustment for resting systolic dysfunction and gender. Additionally, a number of study participants were re-classified as not meeting the key inclusion criterion of baseline MSIMI when baseline MS-echocardiography was reviewed. In a randomized controlled trial (n=300) of post-ACS patients with depression, 24 weeks of SSRI treatment was associated with lower risk of events at 8 years as compared to placebo (hazard ratio 0.69; 95% CI, 0.49-0.96; P = .03)52, though more research is needed to demonstrate efficacy and safety in a population without depression and in the context of dual anti-platelet therapy. Use of a serotonin modulating agent highlights the wide and overlapping physiologic changes induced by central processes which may also may produce an effect on a peripheral/terminal processes. Beyond neurons, serotonin reuptake transporter (5-HTT) is active in platelets, a pathway by which serotonin affects platelet aggregation. There have been limited controversial reports of excess bleeding and risks associated with SSRI use, though use of SSRIs are generally shown to be safe for CAD patients.53, 54
Potential peripheral targets are also suggested by the consequences of the fight-flight autonomic and HPA axis responses to stress. Autoregulation of MBF and microvascular resistance is mediated by the sympathetic nervous system, which directly innervates the coronary arterioles via α-adrenergic receptors, and the myocardium via β-adrenergic receptors.14 MBF and microvascular resistance are also under the influence of the circulating catecholamines, which in response to MS among patients with known CAD can precipitate coronary vasoconstriction.55 The effect of MS on peripheral endothelial function are pronounced and prolonged, with impaired brachial artery hyperemic flow being observed for up to 90 minutes.56, 57 This effect is accompanied by an increase in circulating endothelial microparticles and a decrease in circulating endothelial precursor cells – again, evidence of endothelial cell damage and reduced restorative capacity58 and appears in part to be mediated by endothelin-1 (ET-1).59 Overlapping pathophysiologic pathways in MS affect ET-1 through, 1) ET-1 can potentiate vasoconstriction to norepinephrine,60 and 2) withdrawal of cardiac vagal control may disinhibit the release of ET-1 into the circulation.61 Additionally, MS is associated with an increase in inflammatory and prothrombotic markers, factors that are associated with endothelial dysfunction and atherosclerotic plaque development.
Despite the broad and varied physiologic processes that connect central activation with peripheral changes, each of which may be a potential therapeutic target, investigation into pharmacotherapeutic interventions directed to the peripheral processes that appear to play a role in MSIMI are required. Small, older cross-sectional studies assessed the effect of metoprolol, atenolol and nifedipine on MS induced RWMA without definitive results.62, 63 With much of the MSIMI research having been conducted with patients remaining on their optimal medical therapy, and use of standard agents, beta-blockers, ACE inhibitors, statins does not appear to affect the prevalence of this ischemic presentation.
With the expansion in our understanding of MS and its broad effects on the CV system, further work is needed to identify possible targets along the physiologic pathways that transduce central stress on the CV system. Candidate therapeutic may target traditional CV risk factors, associated with endothelial dysfunction, catecholamaine responsiveness notably alpha and beta blockers, traditional vasomotion targets (calcium channel blockers, nitrates, phosphodiesterase inhibitors), novel targets of vasomotor tone (ET-1 blockers, rho kinase inhibitors), platelet activation and aggregation, and inflammatory pathways.
As we consider the role of therapeutic interventions, approaches may vary from assessment of single agent to a “kitchen sink” approach to a tailored, personalized approach that targets the specific physiologic pathways that have been demonstrated to be perturbed under MS provocation. In order to move forward to consider therapeutic intervention and assessment of efficacy of the intervention, diagnostic identification of MSIMI must be implemented in our clinical practice despite the well-recognized limitations of our traditional imaging techniques.
5. Implementation into standard care
In order to advance implementation of this science and build the framework for mitigation of CV risk associated with MS, we must develop a better understanding of the relationship between (1) the biologic substrate-the patient, (2) the patient’s ecologic experience, and (3) their response to stress in relation to symptoms, underlying CV physiology, and broader systemic biology. Notably, arguments have been put forth that there is little merit in assessment of MSIMI as effective treatments have not been established. Rather, the foundations of conventional stress testing began with its power to diagnose disease and provide prognosis.
And so, a path forward in MSIMI must apply familiar concepts that have shaped conventional stress testing for epicardial obstructive CAD: (1) define the target disorder, (2) apply Bayes Theorem with established models of pretest probability of disease, (4) measure stress trigger, (3) identify imaging cue with established relationship of imaging cue to prognosis, patient-reported outcome, and target disorder, and (4) use of imaging cue to inform management decisions.64
Definition of the target disorder should begin with an untangling and recognition of overlaps and differences between syndromes/presentations of MSIMI, atypical angina, microvascular dysfunction, and INOCA. Much of the initial basis of MSIMI studies evaluated cohorts of patients with stable CAD or post-ACS, who have evidence of MSIMI that is often clinically silent. In this population, as described in the CLARIFY study, patient-reported anginal symptoms are associated with worse clinical outcomes regardless of the presence of myocardial ischemia using conventional stress testing, whereas ischemia, as a marker of epicardial obstructive disease, alone was not.65
The clinical cohort in whom microvascular dysfunction and/or INOCA is described have been referred for conventional evaluation because of a cardiac symptoms and in whom obstructive epicardial CAD has been excluded. This may have particular relevance in women as over half of women in the WISE study with symptoms suggestive of myocardial ischemia did not have obstructive CAD, thus suggesting alternative mechanisms for symptoms.66 Additionally, there is a broad and increasing population of patients referred to conventional stress testing with atypical symptoms for whom diagnosis and management remain unclear. A sequential cohort of 39,515 patients undergoing MPI from 1991 to 2009 demonstrated a shift in presenting symptoms with a decline in typical angina (12.7% vs. 1.9%) and an increase in atypical angina (24.8% vs. 59.6%).67 Gender may be a key factor in these overlapping disorders. In a cohort study of patients undergoing both conventional stress testing and MS testing, women experiencing angina in the month prior to enrollment demonstrated almost double the probability of MSIMI (19% vs 10%, adjusted prevalence rate ratio, 1.90; 95% confidence interval, 1.04-3.46), as compared to those without anginal symptoms: a finding not seen in men.68
The next important steps towards clinical implementation is the development of a model of pretest probability of MSIMI to both identify an at-risk patient and to inform interpretation of imaging cues. Use of previously identified variables and broad collection of triggers, stress exposure, narrative description of symptoms obtained at the time of presentation, along with results of diagnostic imaging and comorbidities should be combined to develop a predictive model. Beyond predicting probability of MSIMI, a useful model will also predict likelihood of an alternative diagnosis. The fitness of the model will need to be assessed in a range of patient cohorts and care settings.
A risk model is intrinsic in the design of studies to compare a MS-informed strategy to standard of care in which this MS-informed strategy incorporates the risk model ‘upfront’, to inform test selection: MS-testing as initial test vs. conventional stress testing vs. MS-testing in addition to conventional stress testing. As described earlier, the challenge is recognized that there are intrinsic differences in the MS-provoked imaging cues obtained in the variety of diagnostic tests, though the association with outcomes remains consistent. Outcomes of interest beyond prediction of events may include patient-reported outcomes, use of goal-directed medical therapy, and resource utilization.
With demonstration that a MS-informed strategy can be implemented and is prognostically relevant, there is the opportunity to probe the multiple biologic pathways that transduce MS. Phenotypes can be developed through assessment of broad variables incorporated in pretest probability models. Endotyping can be developed through focused cardiac and peripheral physiologic studies in patients with elevated risk for MSIMI which may inform the use of targeted, personalized treatment pathways. The understanding of the relative heterogeneity or homogeneity of MS-induced perturbation will inform therapeutic approaches to risk mitigation. These diagnostic pathways must be built before we can perform studies of therapies for MSIMI.
We expect shared lessons from studies of microvascular disease and MSIMI. While many of the past and the proposed MSIMI studies utilized cardiac imaging, further studies may elucidate less invasive and less expensive diagnostics that can assess likelihood of MS-induced perturbations of CV function and, perhaps, will identify those at risk for microvascular dysfunction.
Education and adoption of a multi-disciplinary scope is integral to the success in implementing constructs derived from our understanding of MSIMI. Medical training, along with primary and secondary CV prevention programs, should incorporate health psychology and public health to address the intersection of biology and psychosocial determinants of health, particularly given evidence supporting stress management training in the cardiac rehabilitation setting.
Conclusion:
The spectrum of the scientific enterprise from fundamental physics to various clinical translational scenarios is based upon a similar challenge: a phenomenon is defined by how it is observed and how it is measured.69 It is an uncommon that the tools to identify, target, and treat the unknown health needs within the population match the calling of a particular professional community. In the absence of that community’s involvement, more people suffer. A substantial portion of stable CAD patients do not exhibit ischemia to exercise yet have myocardial ischemia in response to standard laboratory induced MS protocol and have a worse prognosis. The persistence of the framework of exercise induced ischemia applied to the stressful cognitive trigger biases our understanding of expected outcomes and imaging cues. Herein we outlined the consideration from the brain to cardio/peripheral vascular responses to support this needed paradigm change. A recognition of the core differences between emotion and exercise triggers, that of central neurohormonal activation and systemic vascular perturbations require a tailored strategic approach and is supported by emerging studies.44 Paired with assessment of psychosocial determinants of health, an understanding of gender difference, diagnostic identification and treatment of MS-induced cardiovascular perturbations, a foundation based upon broad empiric evidence will facilitate the integration into standard care to address a pervasive, often subclinical determinant of outcome.
Acknowledgments
Sources of Funding
This work was supported by grants from the National Heart, Lung and Blood Institute to Dr. Soufer (R01HL 59619) and Dr. Burg (R01HL126770), and Dr. Soufer’s Merit Review Award from the Dept. Veteran Affairs.
Abbreviations:
- ACS
acute coronary syndrome
- CAD
coronary artery disease
- CFR
coronary flow reserve
- CNS
central nervous system
- CV
cardiovascular
- ET-1
endothelin
- FMD
flow mediated dilation
- HR
heart rate
- INOCA
ischemia and no obstructive coronary artery disease
- LV
left ventricle
- LVEF
left ventricular ejection fraction
- MBF
myocardial blood flow
- MI
myocardial infarction
- MPI
myocardial perfusion imaging
- MS
mental stress
- MSIMI
mental stress induced myocardial ischemia
- 13N-NH3
13N-Ammonia
- PET
positron emission tomography
- RWMA
regional wall motion abnormality
- SPECT
single photon emission computed tomography
- SVR
systemic vascular resistance
- TPG
transmural perfusion gradient
- TPGR
transmural perfusion gradient ratio
Footnotes
Disclosures: No conflicts exist
References:
- 1.Mittleman MA, Maclure M, Sherwood JB, Mulry RP, Tofler GH, Jacobs SC, Friedman R, Benson H and Muller JE. Triggering of acute myocardial infarction onset by episodes of anger. Determinants of Myocardial Infarction Onset Study Investigators. Circulation. 1995;92:1720–5. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. [DOI] [PubMed] [Google Scholar]
- 3.Burroughs Pena MS, Mbassa RS, Slopen NB, Williams DR, Buring JE and Albert MA. Cumulative Psychosocial Stress and Ideal Cardiovascular Health in Older Women. Circulation. 2019;139:2012–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheps DS, McMahon RP, Becker L, Carney RM, Freedland KE, Cohen JD, Sheffield D, Goldberg AD, Ketterer MW, Pepine CJ, et al. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: Results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation. 2002;105:1780–4. [DOI] [PubMed] [Google Scholar]
- 5.Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ and Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–48. [DOI] [PubMed] [Google Scholar]
- 6.Lampert R, Joska T, Burg MM, Batsford WP, McPherson CA and Jain D. Emotional and physical precipitants of ventricular arrhythmia. Circulation. 2002;106:1800–5. [DOI] [PubMed] [Google Scholar]
- 7.Kupper N, Denollet J, Widdershoven J and Kop WJ. Cardiovascular reactivity to mental stress and mortality in patients with heart failure. JACC Heart Fail. 2015;3:373–382. [DOI] [PubMed] [Google Scholar]
- 8.Jain D, Burg M, Soufer R and Zaret BL. Prognostic implications of mental stress-induced silent left ventricular dysfunction in patients with stable angina pectoris. Am J Cardiol. 1995;76:31–5. [DOI] [PubMed] [Google Scholar]
- 9.Krantz DS, Santiago HT, Kop WJ, Bairey Merz CN, Rozanski A and Gottdiener JS. Prognostic value of mental stress testing in coronary artery disease. Am J Cardiol. 1999;84:1292–7. [DOI] [PubMed] [Google Scholar]
- 10.Wei J, Rooks C, Ramadan R, Shah AJ, Bremner JD, Quyyumi AA, Kutner M and Vaccarino V. Meta-analysis of mental stress-induced myocardial ischemia and subsequent cardiac events in patients with coronary artery disease. Am J Cardiol. 2014;114:187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEwen BS and Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–101. [PubMed] [Google Scholar]
- 12.Soufer R, Jain H and Yoon AJ. Heart-brain interactions in mental stress-induced myocardial ischemia. Curr Cardiol Rep. 2009;11:133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soufer R, Bremner JD, Arrighi JA, Cohen I, Zaret BL, Burg MM and Goldman-Rakic P. Cerebral cortical hyperactivation in response to mental stress in patients with coronary artery disease. Proc Natl Acad Sci U S A. 1998;95:6454–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soufer R and Burg MM. The heart-brain interaction during emotionally provoked myocardial ischemia: implications of cortical hyperactivation in CAD and gender interactions. Cleve Clin J Med. 2007;74 Suppl 1:S59–62. [DOI] [PubMed] [Google Scholar]
- 15.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. [DOI] [PubMed] [Google Scholar]
- 16.Akashi YJ, Goldstein DS, Barbaro G and Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation. 2008;118:2754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiffer F, Hartley LH, Schulman CL and Abelmann WH. The quiz electrocardiogram: a new diagnostic and research technique for evaluating the relation between emotional stress and ischemic heart disease. Am J Cardiol. 1976;37:41–7. [DOI] [PubMed] [Google Scholar]
- 18.Pepine CJ and Schang SJ. Antianginal response of coronary heart disease patients on long-term perhexiline maleate. Am Heart J. 1975;35:168. [Google Scholar]
- 19.Schang SJ Jr. and Pepine CJ. Transient asymptomatic S-T segment depression during daily activity. Am J Cardiol. 1977;39:396–402. [DOI] [PubMed] [Google Scholar]
- 20.Imperi GA and Pepine CJ. Silent myocardial ischemia during daily activities: studies in asymptomatic patients and those with various forms of angina. Cardiol Clin. 1986;4:635–42. [PubMed] [Google Scholar]
- 21.Ramachandruni S, Fillingim RB, McGorray SP, Schmalfuss CM, Cooper GR, Schofield RS and Sheps DS. Mental stress provokes ischemia in coronary artery disease subjects without exercise- or adenosine-induced ischemia. J Am Coll Cardiol. 2006;47:987–91. [DOI] [PubMed] [Google Scholar]
- 22.Rozanski A, Bairey CN, Krantz DS, Friedman J, Resser KJ, Morell M, Hilton-Chalfen S, Hestrin L, Bietendorf J and Berman DS. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 1988;318:1005–12. [DOI] [PubMed] [Google Scholar]
- 23.Stone PH, Krantz DS, McMahon RP, Goldberg AD, Becker LC, Chaitman BR, Taylor HA, Cohen JD, Freedland KE, Bertolet BD, et al. Relationship among mental stress-induced ischemia and ischemia during daily life and during exercise: the Psychophysiologic Investigations of Myocardial Ischemia (PIMI) study. J Am Coll Cardiol. 1999;33:1476–84. [DOI] [PubMed] [Google Scholar]
- 24.Vashist A, Burg MM, Arrighi JA, Jadbabaie F, Blumenfeld H, Lampert R, Graeber B and Soufer R. Central nervous system correlates of myocardial ischemia: Neurocardiac distinctions between mental stress and dobutamine provocation. Psychosom Med. 2005;67:A8–9. [Google Scholar]
- 25.Jiang W, Samad Z, Boyle S, Becker RC, Williams R, Kuhn C, Ortel TL, Rogers J, Kuchibhatla M, O'Connor C, et al. Prevalence and clinical characteristics of mental stress-induced myocardial ischemia in patients with coronary heart disease. J Am Coll Cardiol. 2013;61:714–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deanfield JE, Shea M, Kensett M, Horlock P, Wilson RA, de Landsheere CM and Selwyn AP. Silent myocardial ischaemia due to mental stress. Lancet. 1984;2:1001–5. [DOI] [PubMed] [Google Scholar]
- 27.Soufer R BM, Fernandez A. Mechanistic and Methodological Considerations for the Imaging of Mental Stress Ischemia. In: Zaret B BG, ed. Clinical Nuclear Cardiology: State of the Art and Future Directions. 4th Edition ed.: Mosby; 2010: 896. [Google Scholar]
- 28.Arrighi JA, Burg M, Cohen IS and Soufer R. Simultaneous assessment of myocardial perfusion and function during mental stress in patients with chronic coronary artery disease. J Nucl Cardiol. 2003;10:267–74. [DOI] [PubMed] [Google Scholar]
- 29.Strike PC and Steptoe A. Systematic review of mental stress-induced myocardial ischaemia. Eur Heart J. 2003;24:690–703. [DOI] [PubMed] [Google Scholar]
- 30.Ramadan R, Quyyumi AA, Zafari AM, Binongo JN and Sheps DS. Myocardial ischemia and angiotensin-converting enzyme inhibition: comparison of ischemia during mental and physical stress. Psychosom Med. 2013;75:815–21. [DOI] [PubMed] [Google Scholar]
- 31.Yeung AC, Vekshtein VI, Krantz DS, Vita JA, Ryan TJ, Jr., Ganz P and Selwyn AP. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med. 1991;325:1551–6. [DOI] [PubMed] [Google Scholar]
- 32.Kop WJ, Krantz DS, Howell RH, Ferguson MA, Papademetriou V, Lu D, Popma JJ, Quigley JF, Vernalis M and Gottdiener JS. Effects of mental stress on coronary epicardial vasomotion and flow velocity in coronary artery disease: relationship with hemodynamic stress responses. J Am Coll Cardiol. 2001;37:1359–66. [DOI] [PubMed] [Google Scholar]
- 33.Arrighi JA, Burg M, Cohen IS, Kao AH, Pfau S, Caulin-Glaser T, Zaret BL and Soufer R. Myocardial blood-flow response during mental stress in patients with coronary artery disease. Lancet. 2000;356:310–1. [DOI] [PubMed] [Google Scholar]
- 34.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danad I, Raijmakers PG, Harms HJ, Heymans MW, van Royen N, Lubberink M, Boellaard R, van Rossum AC, Lammertsma AA and Knaapen P. Impact of anatomical and functional severity of coronary atherosclerotic plaques on the transmural perfusion gradient: a [15O]H2O PET study. Eur Heart J. 2014;35:2094–105. [DOI] [PubMed] [Google Scholar]
- 36.Pan J, Huang S, Lu Z, Li J, Wan Q, Zhang J, Gao C, Yang X and Wei M. Comparison of myocardial transmural perfusion gradient by magnetic resonance imaging to fractional flow reserve in patients with suspected coronary artery disease. Am J Cardiol. 2015;115:1333–40. [DOI] [PubMed] [Google Scholar]
- 37.Becker LC, Pepine CJ, Bonsall R, Cohen JD, Goldberg AD, Coghlan C, Stone PH, Forman S, Knatterud G, Sheps DS, et al. Left ventricular, peripheral vascular, and neurohumoral responses to mental stress in normal middle-aged men and women. Reference Group for the Psychophysiological Investigations of Myocardial Ischemia (PIMI) Study. Circulation. 1996;94:2768–77. [DOI] [PubMed] [Google Scholar]
- 38.Jain D, Shaker SM, Burg M, Wackers FJ, Soufer R and Zaret BL. Effects of mental stress on left ventricular and peripheral vascular performance in patients with coronary artery disease. J Am Coll Cardiol. 1998;31:1314–22. [DOI] [PubMed] [Google Scholar]
- 39.Kim D and Ha JW. Hypertensive response to exercise: mechanisms and clinical implication. Clin Hypertens. 2016;22:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO and Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol. 1994;480:361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D'Urzo KA, La Rocque CL, Williams JS, Stuckless TJR, King TJ, Plotnick MD, Gurd BJ, Harkness KL and Pyke KE. The impact of acute mental stress on brachial artery flow-mediated dilation in women diagnosed with depression. Int J Psychophysiol. 2019;135:113–120. [DOI] [PubMed] [Google Scholar]
- 42.Hammadah M, Kim JH, Al Mheid I, Samman Tahhan A, Wilmot K, Ramadan R, Alkhoder A, Khayata M, Mekonnen G, Levantsevych O, et al. Coronary and Peripheral Vasomotor Responses to Mental Stress. J Am Heart Assoc. 2018;7:e008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim JH, Almuwaqqat Z, Hammadah M, Liu C, Ko YA, Lima B, Sullivan S, Alkhoder A, Abdulbaki R, Ward L, et al. Peripheral Vasoconstriction During Mental Stress and Adverse Cardiovascular Outcomes in Patients With Coronary Artery Disease. Circ Res. 2019;125:874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lima BB, Hammadah M, Kim JH, Uphoff I, Shah A, Levantsevych O, Almuwaqqat Z, Moazzami K, Sullivan S, Ward L, et al. Association of Transient Endothelial Dysfunction Induced by Mental Stress With Major Adverse Cardiovascular Events in Men and Women With Coronary Artery Disease. JAMA Cardiol. 2019;4:988–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wokhlu A and Pepine CJ. Mental Stress and Myocardial Ischemia: Young Women at Risk. J Am Heart Assoc. 2016;5:e004196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J Am Coll Cardiol. 2018;72:2841–2855. [DOI] [PubMed] [Google Scholar]
- 48.Ford TJ, Stanley B, Sidik N, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, et al. 1-Year Outcomes of Angina Management Guided by Invasive Coronary Function Testing (CorMicA). JACC Cardiovasc Interv. 2020;13:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blumenthal JA, Jiang W, Babyak MA, Krantz DS, Frid DJ, Coleman RE, Waugh R, Hanson M, Appelbaum M, O'Connor C, et al. Stress management and exercise training in cardiac patients with myocardial ischemia. Effects on prognosis and evaluation of mechanisms. Arch Intern Med. 1997;157:2213–23. [PubMed] [Google Scholar]
- 50.Jiang W, Velazquez EJ, Kuchibhatla M, Samad Z, Boyle SH, Kuhn C, Becker RC, Ortel TL, Williams RB, Rogers JG, et al. Effect of escitalopram on mental stress-induced myocardial ischemia: results of the REMIT trial. JAMA. 2013;309:2139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blumenthal JA, Sherwood A, Smith PJ, Watkins L, Mabe S, Kraus WE, Ingle K, Miller P and Hinderliter A. Enhancing Cardiac Rehabilitation With Stress Management Training: A Randomized, Clinical Efficacy Trial. Circulation. 2016;133:1341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim JM, Stewart R, Lee YS, Lee HJ, Kim MC, Kim JW, Kang HJ, Bae KY, Kim SW, Shin IS, et al. Effect of Escitalopram vs Placebo Treatment for Depression on Long-term Cardiac Outcomes in Patients With Acute Coronary Syndrome: A Randomized Clinical Trial. JAMA. 2018;320:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maurer-Spurej E Serotonin reuptake inhibitors and cardiovascular diseases: a platelet connection. Cell Mol Life Sci. 2005;62:159–70. [DOI] [PubMed] [Google Scholar]
- 54.Rieckmann N, Kronish IM, Shapiro PA, Whang W and Davidson KW. Serotonin reuptake inhibitor use, depression, and long-term outcomes after an acute coronary syndrome: a prospective cohort study. JAMA Intern Med. 2013;173:1150–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lampert R, Jain D, Burg MM, Batsford WP and McPherson CA. Destabilizing effects of mental stress on ventricular arrhythmias in patients with implantable cardioverter-defibrillators. Circulation. 2000;101:158–64. [DOI] [PubMed] [Google Scholar]
- 56.Harris CW, Edwards JL, Baruch A, Riley WA, Pusser BE, Rejeski WJ and Herrington DM. Effects of mental stress on brachial artery flow-mediated vasodilation in healthy normal individuals. Am Heart J. 2000;139:405–11. [DOI] [PubMed] [Google Scholar]
- 57.Gottdiener JS, Kop WJ, Hausner E, McCeney MK, Herrington D and Krantz DS. Effects of mental stress on flow-mediated brachial arterial dilation and influence of behavioral factors and hypercholesterolemia in subjects without cardiovascular disease. Am J Cardiol. 2003;92:687–91. [DOI] [PubMed] [Google Scholar]
- 58.Shimbo D, Rosenberg LB, Chaplin W, Zhao S, Goldensohn ER, Cholankeril M, Fu J, Hong SB, Jelic S and Burg MM. Endothelial cell activation, reduced endothelial cell reparative capacity, and impaired endothelial-dependent vasodilation after anger provocation. Int J Cardiol. 2013;167:1064–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spieker LE, Hurlimann D, Ruschitzka F, Corti R, Enseleit F, Shaw S, Hayoz D, Deanfield JE, Luscher TF and Noll G. Mental stress induces prolonged endothelial dysfunction via endothelin-A receptors. Circulation. 2002;105:2817–20. [DOI] [PubMed] [Google Scholar]
- 60.Yang ZH, Richard V, von Segesser L, Bauer E, Stulz P, Turina M and Luscher TF. Threshold concentrations of endothelin-1 potentiate contractions to norepinephrine and serotonin in human arteries. A new mechanism of vasospasm? Circulation. 1990;82:188–95. [DOI] [PubMed] [Google Scholar]
- 61.Burg MM, Soufer A, Lampert R, Collins D and Soufer R. Autonomic contribution to endothelin-1 increase during laboratory anger-recall stress in patients with coronary artery disease. Mol Med. 2011;17:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bairey CN, Krantz DS, DeQuattro V, Berman DS and Rozanski A. Effect of beta-blockade on low heart rate-related ischemia during mental stress. J Am Coll Cardiol. 1991;17:1388–95. [DOI] [PubMed] [Google Scholar]
- 63.Andrews TC, Parker JD, Jacobs S, Friedman R, Cummings N, MacCallum G, Mannting F, Tofler GH, Carlson W, Muller JE, et al. Effects of therapy with nifedipine GITS or atenolol on mental stress-induced ischemic left ventricular dysfunction. J Am Coll Cardiol. 1998;32:1680–6. [DOI] [PubMed] [Google Scholar]
- 64.Genders TS, Steyerberg EW, Hunink MG, Nieman K, Galema TW, Mollet NR, de Feyter PJ, Krestin GP, Alkadhi H, Leschka S, et al. Prediction model to estimate presence of coronary artery disease: retrospective pooled analysis of existing cohorts. BMJ. 2012;344:e3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steg PG, Greenlaw N, Tendera M, Tardif JC, Ferrari R, Al-Zaibag M, Dorian P, Hu D, Shalnova S, Sokn FJ, et al. Prevalence of anginal symptoms and myocardial ischemia and their effect on clinical outcomes in outpatients with stable coronary artery disease: data from the International Observational CLARIFY Registry. JAMA Intern Med. 2014;174:1651–9. [DOI] [PubMed] [Google Scholar]
- 66.Sharaf BL, Pepine CJ, Kerensky RA, Reis SE, Reichek N, Rogers WJ, Sopko G, Kelsey SF, Holubkov R, Olson M, et al. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women's Ischemia Syndrome Evaluation [WISE] Study Angiographic Core Laboratory). Am J Cardiol. 2001;87:937–41; A3. [DOI] [PubMed] [Google Scholar]
- 67.Rozanski A, Gransar H, Hayes SW, Min J, Friedman JD, Thomson LE and Berman DS. Temporal trends in the frequency of inducible myocardial ischemia during cardiac stress testing: 1991 to 2009. J Am Coll Cardiol. 2013;61:1054–65. [DOI] [PubMed] [Google Scholar]
- 68.Pimple P, Hammadah M, Wilmot K, Ramadan R, Al Mheid I, Levantsevych O, Sullivan S, Garcia EV, Nye J, Shah AJ, et al. Chest Pain and Mental Stress-Induced Myocardial Ischemia: Sex Differences. Am J Med. 2018;131:540–547 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Manning AG, Khakimov RI, Dall RG and Truscott AG. Wheeler's delayed-choice gedanken experiment with a single atom. Nature Physics. 2015;11:539–542. [Google Scholar]