Abstract
We assessed mortality risks associated with source-specific fine particles (PM2.5) in a pooled European cohort of 323,782 participants. Cox proportional hazard models were applied to estimate mortality hazard ratios (HRs) for source-specific PM2.5 identified through a source apportionment analysis. Exposure to 2010 annual average concentrations of source-specific PM2.5 components was assessed at baseline residential addresses. The source apportionment resulted in the identification of five sources: traffic, residual oil combustion, soil, biomass and agriculture, and industry. In single-source analysis, all identified sources were significantly positively associated with increased natural mortality risks. In multisource analysis, associations with all sources attenuated but remained statistically significant with traffic, oil, and biomass and agriculture. The highest association per interquartile increase was observed for the traffic component (HR: 1.06; 95% CI: 1.04 and 1.08 per 2.86 μg/m3 increase) across five identified sources. On a 1 μg/m3 basis, the residual oil-related PM2.5 had the strongest association (HR: 1.13; 95% CI: 1.05 and 1.22), which was substantially higher than that for generic PM2.5 mass, suggesting that past estimates using the generic PM2.5 exposure response function have underestimated the potential clean air health benefits of reducing fossil-fuel combustion. Source-specific associations with cause-specific mortality were in general consistent with findings of natural mortality.
Keywords: source apportionment, fine particulate matter (PM2.5), absolute principal component analysis (APCA), mortality
Short abstract
Understanding particulate air pollution from which sources are of greater health impact than others would inform targeted policy controls. This study reports that residual fuel oil-related particles are most strongly associated with mortality risks.
1. Introduction
Epidemiological studies around the world have generally reported associations between fine particle mass (PM2.5) exposure and mortality and morbidity, with variations in the magnitude of effect estimates.1 Part of these effect size fluctuations per unit mass may be related to the fact that the composition of PM2.5 mass varies in time and space, depending on sources of emission and atmospheric chemistry, which may in turn result in differences in toxicity and risk to health of PM2.5 mass.2−5 Understanding which components of the PM mixture are of greater health impact than others would help inform targeted policies to control PM2.5 from those sources that contribute most of the toxic components in the PM mixture as well as allow more accurate assessments of source-specific health impacts.
To date, many studies have reported associations between adverse health outcomes and long-term exposure to a series of PM2.5 constituents, including secondary inorganic aerosols (sulfate, nitrate), black carbon (BC), metals, and organic components, with no consistent evidence of a single constituent being most strongly related to health effects.6−12 In our previous analyses within the Effects of Low-level Air Pollution: A Study in Europe (ELAPSE), we found that vanadium (V) within PM2.5 was most consistently associated with increased mortality risks in the pooled cohort of 323,782 participants from six European countries,13 whereas potassium (K) and silicon (Si) were most robustly associated with natural mortality in almost 27 million participants from six large administrative cohorts.14 However, analyses on a constituent basis may be difficult to interpret because individual elements can be linked to one or more specific sources (e.g., differing by location-specific source mixtures) and thus have different associated health effects, depending on what aerosol mixture they travel with (i.e., which source group they are in), while several covarying elemental markers may more reliably indicate the same source.15−17 For example, iron (Fe) may come from wind-blown soil, a steel mill operation, or brake wear, but it is associated with different elements, depending on which source (e.g., with Si for soil, Mn for steel, and Cu for brake wear).18 Looking at Fe in a source-specific tracer group can differentiate the mixture situations. This factor may help explain uncertainty as to which source/constituent is more strongly related to health effects in past PM2.5 constituent evaluations (e.g., US EPA, 2021).19
Observed health associations with individual PM2.5 trace elements are not necessarily causal but may rather indicate associations with the source-specific mixture they are part of. Furthermore, results from a model which includes multiple elements emitted from the same source (e.g., V and Ni from residual oil combustion) are difficult to interpret, as the model could bias the effect estimates for individual elements.20,21 To avoid this possibility, assessing health effects of PM components as a group (e.g., source-specific groupings of tracers) may provide more consistent and interpretable results across studies than trying to parse effects among individual elements. Source-specific associations are also more readily translatable into air quality policy than elemental associations. For example, an analysis within the American Cancer Society Cancer Prevention Study II (ACS CPS-II) suggested exposure to coal combustion-related air pollution explained most associations between PM2.5 mass and increased risk of mortality from all causes, ischemic heart diseases (IHDs), and lung cancer (LC) in the US.22,23 In the California Teachers study, associations with IHD mortality were observed for sources including gasoline- and diesel-fueled vehicles, meat cooking, and high-sulfur fuel combustion.6 The National Particle Component Toxicity (NPACT) studies identified that secondary sulfate and traffic sources were most consistently associated with adverse health effects.22,24
In the present study, we performed a further analysis within the ELAPSE pooled cohort to consider the mortality risks associated with long-term PM2.5 exposure on a source-specific basis. By comparing these source-specific results with our previous individual elemental analyses, we expected to have a more complete understanding of the health effects of PM2.5 mixtures from different sources.
2. Materials and Methods
2.1. Study Population
The ELAPSE pooled cohort consists of 14 subcohorts across six European countries. Detailed information on individual subcohorts has been extensively reported.13,25,26 The included subcohorts are: the Cardiovascular Effects of Air Pollution and Noise in Stockholm (CEANS) cohort in Sweden, which in turn includes the Stockholm Diabetes Prevention Program (SDPP),27 the Stockholm Cohort of 60-year-olds (SIXTY),28 Stockholm Screening Across the Lifespan Twin study (SALT),29 and Swedish National Study on Aging and Care in Kungsholmen (SNAC-K);30 the Diet, Cancer, and Health cohort (DCH)31 in Denmark; the Danish Nurse Cohort (DNC)32 in Denmark, consisting at baseline of two surveys conducted in 1993 and 1999; the European Prospective Investigation into Cancer and Nutrition-Netherlands (EPIC-NL) cohort in the Netherlands, including the Monitoring Project on Risk Factors and Chronic Diseases in the Netherlands (MORGEN) and Prospect;33 the Heinz Nixdorf Recall study (HNR) in Germany;34 the Etude Epidémiologique auprès de femmes de la Mutuelle Générale de l’Education Nationale (E3N) in France;35 the Cooperative Health Research in the Region of Augsburg (KORA) in Germany, consisting at baseline of two cross-sectional population-representative surveys conducted in 1994–1995 (S3) and 1999–2001 (S4); and the Vorarlberg Health Monitoring and Prevention Programme (VHM&PP) in Austria.36 Most cohorts covered a large city and its surrounding areas as study areas. The French E3N cohort and the Danish DNC cohort are national cohorts. All included cohort studies were approved by the medical ethics committees in their respective countries.
2.2. Source Apportionment and Exposure Assessment
Air pollution measurements for PM2.5 mass, NO2, BC, and PM2.5 elemental composition were derived from the ESCAPE monitoring campaign conducted in 19 study areas across Europe. Sampling and analysis methods have been described before.16,37 Briefly, measurements were made at 20 sites in each study area (40 in the large Catalunya and Netherlands/Belgium areas) for three 2 week periods in a 1 year period between October 2008 and April 2011. Monitoring sites were selected using a common sampling protocol to represent pollution levels at regional background, urban background, and street locations, with a focus on urban areas and in streets with only several regional background sites located outside of major urban areas.16 Eight components were a priori selected in the ESCAPE study: copper (Cu), Fe, K, nickel (Ni), sulfur (S), Si, V, and zinc (Zn).16,38 Annual average concentrations were calculated based on the measurements spread over the seasons (warm, cold, and intermediate) with temporal adjustment from a reference background site in each study area. Table D1, Supporting Information documents the distribution of air pollution measurements.
The 2010 annual average concentrations of air pollution from 397 sites were analyzed to estimate source apportioned PM2.5 mass exposures using absolute principal component analysis (APCA).39 The method involved the following: (a) applying PCA to the pollution data; (b) identifying source-related components based on key tracers in each component; (c) adjusting PC scores into absolute PC scores; and (d) regressing PM2.5 mass on the source-related components (i.e., the absolute PC scores), providing apportionments of PM2.5 mass to each identified source-related component. PM2.5 concentrations not attributable to the identified source components were incorporated into the model intercept. The APCA approach is further elaborated in Section A, Supporting Information. A five-factor PCA using Promax rotation (a type of oblique rotation)40 was chosen as the optimal solution. This decision was based on the goal to keep PCs having factor eigenvalues (i.e., the data variance explained by the identified component) greater than 1.0 after rotation, and the cumulative percentage of variance explained larger than 80%, as recommended by Hopke,41 as well as an examination of the source-related interpretability of the factors. We first tried a five-factor Varimax rotated approach (a type of orthogonal rotation) that resulted in 28.7% negative contributions estimated for the third component, indicating a non-optimal rotation of the PCs, which is likely due to the forcing of orthogonality (i.e., varimax rotation) when sources are actually correlated with each other in real world. We therefore instead applied an oblique rotation approach (i.e., Promax rotation) to allow the identified source components to be intercorrelated with one another, which is more realistic in real-world settings. The process to derive the optimal APCA solution is documented in Section B, Supporting Information.
Particulate K is an element enriched in biomass burning emissions but can also be in soil dust.15 In order to facilitate the interpretation of source-specific components, we attempted to obtain a more specific metric for K by looking at the non-soil K separately. We therefore adjusted the K concentrations with the tracer of crustal soil dust (i.e., Si) to exclude that K component before the source identification PCA. The adjusted K index was calculated by subtracting the soil dust-associated K from the total K concentration values (Kw = K – 0.42 × Si). The coefficient was calculated by regressing the K against Si concentrations for those samples with the lowest 10th percentile K/Si ratio samples.42 PM2.5 S was not included in the initial source identification analysis because S is considered as a general marker for fossil fuel sources and may complicate the separation of fine mass to specific sources.43 Also, excluding tracers of secondary formation (i.e., S) from the source apportionment analysis allows a clearer discrimination of the original primary sources of PM2.5.44 S was then apportioned among the sources by regressing it against the identified sources to calculate the “unexplained” secondary mass prior to the PM2.5 mass apportionment regression models.44 Based on the APCA, source-specific compositional profiles were assessed by regressing each pollutant against the mass contributions for all sources together in a linear model. Source profiles of S were similarly assessed, though S was not included in the PCA.
To evaluate the robustness of our source apportionment to the approach chosen, we performed sensitivity analyses, including: (1) fivefold robustness evaluation; (2) adding S into the source identification PCA; and (3) including total K concentrations in the PCA (i.e., without removing the dust soil-associated K component). In the fivefold robustness evaluation, the full set of measurements was randomly divided into five groups (20% each), stratified by site type (street, rural, and urban background) and region (north, west, central, and south). Five additional APCA analyses were performed, each based on a randomly selected 80% of the monitoring sites. We note that there is 60% overlap between any two training sets of the sites.
The APCA results were then applied to exposure estimates of NO2, BC, and individual elemental components to assess source-specific PM2.5 exposures. This involved first converting individual exposures to the absolute PC scores for all identified source components and then multiplied by the regression slopes derived from the PM2.5 mass measurement apportionment. Individual exposure to 2010 annual mean concentrations of PM2.5 mass, NO2, BC, and PM2.5 elemental composition was assessed at participants’ baseline residential addresses based on Europe-wide land use regression model estimates.45,46 The models were previously built on ground-based measurements with satellite-derived and chemical transport modeled air pollutant estimates, land use, road, and population data as predictors. The models explained a moderate to large fraction of the measured concentration variation at the European scale (i.e., 66% for PM2.5, 58% for NO2, 51% for BC, and 41 to 79% across elemental components).
2.3. Mortality Outcome Definition
Identification of outcomes was based upon linkage to mortality registries within each cohort. Based on the underlying cause of death recorded on death certificates according to the International Classification of Diseases, Ninth Revision (ICD-9)47 and the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10),48 we defined mortality from natural causes (ICD-9: 001-779, ICD-10: A00-R99), cardiovascular diseases (CVDs) (ICD-9: 400–440, ICD-10: I10–I70), non-malignant respiratory diseases (ICD-9: 460–519, ICD-10: J00–J99), and LC (ICD-9: 162, ICD-10: C34).
2.4. Statistical Analyses
Cox proportional hazard regression models were applied to evaluate associations of identified PM2.5 sources with natural and cause-specific mortality, following the general ELAPSE analytical framework applied in our previous paper of elemental exposures.13,26,49 Death from other causes, emigration, loss to follow-up for other reasons, or withdrawn alive at the end of follow-up were considered censoring events. The Cox models were stratified by subcohorts to account for differences in baseline hazards between the subcohorts unexplained by the available covariates and to relax the proportional hazards assumption. The decision to account for between subcohort heterogeneity with strata implies that we mostly evaluate within-cohort exposure contrasts. Three confounder models were a priori specified with increasing adjustment for individual and area-level covariates: model 1 adjusted for age (as the time axis), subcohort (as strata), sex (as strata), and year of enrollment; model 2 further added individual-level covariates including marital status (married/cohabiting, divorced/separated, single, and widowed), smoking status (never, former, and current), smoking duration (years of smoking) for current smokers, smoking intensity (cigarettes/day) for current smokers, squared smoking intensity, body mass index (BMI) categories (<18.5, 18.5–24.9, 25–29.9, and >30 kg/m2), and employment status (employed vs unemployed); and model 3 further adjusted for area-level mean income in 2001. Model 3 was considered as the main model. Participants with missing exposure or incomplete information on model 3 covariates were excluded from all main analyses to allow comparison between models with increasing covariate control.
Individual PM2.5 sources were included as linear functions in the Cox models as a reasonable summary of the association as well as to facilitate comparisons with previous studies. Besides the single-source analysis (where one PM2.5 source was evaluated at a time), multisource analyses were also performed with all identified PM2.5 sources included in the model simultaneously for comparison. Cumulative risks of all identified sources were estimated assuming additive effects of combined source exposures on mortality. Cumulative risk index (CRI) was defined as
where β̂′ = (β̂1, ... β̂p) are the log-hazard ratio (HR) for the P source exposures estimated at xp concentrations in a Cox model consisting of all P sources together.50 HRs and 95% confidence intervals (CIs) for interquartile range (IQR) increases in the estimated concentrations of each source were reported. In addition, HRs associated with per 1 μg/m3 increase are presented.
To evaluate the potential selection bias introduced by excluding participants with incomplete information on model 3 covariates, we compared model 1 HRs derived from analyses conducted in the model 3 population (i.e., with complete covariate information) and the model 1 population (i.e., with missing covariate information). To assess the impact of temporal misalignment of the exposure assessment, we performed sensitivity analyses starting the follow-up from 2000, 2005, 2008, and 2010, as per the previous analyses in the same cohort.13 To assess the uncertainty of source estimation effects on source-specific mortality results, we applied APCA results derived from the fivefold robustness evaluation analysis described above, in addition to the APCA results derived from the chosen optimal approach.
All analyses were performed in R version 3.4.0 using packages: survival, coxme, Matrix, foreach, multcomp, survey, splines, Hmisc, mfp, VIM, ggplot2, frailtySurv, survsim, eha, stamod, and psych. Statistical significance was based on a 95% CI of effect estimate not including unity.
3. Results and Discussion
3.1. Source Apportionment Results
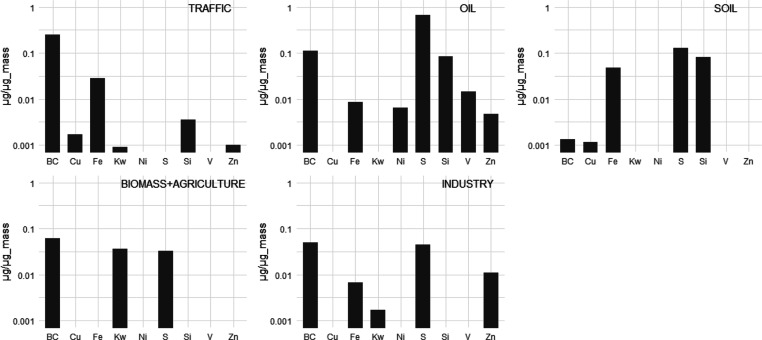
Table 1 provides the correlations between the identified source components and the individual air pollutants (i.e., factor loadings). PM2.5 mass and S were not included in the PCA, but their correlations with the identified factors were calculated to aid in the interpretation of the source components. Overall, the source apportionment resulted in a plausible identification of source contributions, with the possible exception of a biomass source, where we did not have access to a more specific marker, such as levoglucosan. The first factor was identified as traffic-related particles because of its high loadings on NO2, BC, Cu, and Fe. The principle sources of NO2 and BC include the combustion processes from motorized traffic and off-road machinery,51,52 whereas Cu and Fe are both considered as markers of brake wear.15,16 The interpretation of the first factor predominantly reflecting traffic is supported by the documentation of clearly higher concentrations of NO2, BC, Cu, and Fe at traffic locations compared to urban background locations of the monitoring database.16,37 The second factor was identified as particles from residual fuel oil combustion, based on its high loadings on both Ni and V, two elements known to be enriched in heavier fuel oils.17 Residual fuel oil, often burned by marine shipping, industry, and electric power plants, is the oil that remains after the removal of more valuable (and usually cleaner burning) distillates, such as gasoline, from petroleum. Ni and V have been mainly linked to shipping emissions in Europe.17 In the monitoring database, high concentrations of V and Ni were primarily measured in port cities including Rotterdam, Athens, Barcelona, and cities with significant industrial activities such as Turin and the German Ruhr area,16 suggesting marine shipping is likely a dominant source for this factor. The third factor was identifiable as crustal/soil particles because of its high loading on Si and moderate loading on Fe. Si is a specific tracer for the crustal material and Fe is also abundant in the crustal dust.15 The fourth component was first identified as particles from biomass burning because of its high loading on Kw, which is the most common element used to trace biomass burning.15 The identification of the fourth factor is rather uncertain, however, because of the high explained variance by S in the source profile (Figure 1), and the fact that there is no S in wood. We speculated that this factor may possibly also include windblown soil containing agricultural fertilizers because both K and S are in fertilizers widely used in Europe.53 We therefore identified the fourth factor as particles from biomass and agriculture. The last factor was identified as associated with industrial emissions because of its high loading on Zn. Even though Zn was selected as a tracer for non-tailpipe traffic emitted particles in ESCAPE,16 it was not found to correlate with other traffic tracers in this analysis (e.g., NO2, BC, and Cu). However, Zn is also a tracer for industrial emitted particles, as also supported by our Europe-wide models, where predictors representing industrial emitted Zn explained a predominant part of the variation in Zn measurements.45 Correlations at the monitoring sites between identified source-specific PM2.5 were low to moderate (Table D2, Supporting Information).
Table 1. Factor Loadings for the Five-Factor Promax Rotated Principal Component Analysis Solution (Kappa = 1.6) [*S and PM2.5 Not Included in Factor Analysis]a.
| traffic | oil | soil | biomass and agriculture | industry | |
|---|---|---|---|---|---|
| NO2 | 0.97 | 0.10 | –0.09 | –0.11 | 0.00 |
| BC | 0.88 | 0.03 | 0.00 | 0.22 | 0.10 |
| Cu | 0.86 | –0.02 | 0.22 | 0.06 | 0.04 |
| Fe | 0.71 | 0.02 | 0.46 | –0.06 | 0.09 |
| Kw | 0.02 | –0.02 | 0.00 | 0.99 | 0.03 |
| Ni | 0.10 | 0.91 | 0.10 | –0.01 | 0.03 |
| Si | 0.10 | 0.20 | 0.89 | 0.01 | 0.00 |
| V | –0.01 | 0.95 | 0.06 | –0.01 | 0.02 |
| Zn | 0.15 | 0.06 | 0.01 | 0.03 | 0.93 |
| *S | –0.01 | 0.43 | 0.38 | 0.27 | 0.20 |
| *PM2.5 | 0.44 | 0.04 | 0.17 | 0.46 | 0.25 |
| eigenvalue | 3.01 | 1.80 | 1.07 | 1.04 | 0.88 |
| cumulative var | 33.5% | 53.4% | 65.3% | 76.9% | 86.7% |
Kw is soil-adjusted K.
Figure 1.
Estimated fractional elemental source profiles of identified source-specific PM2.5.
The source compositional profiles show that S explained a large fraction of all the identified source-related mass, except for traffic-related particles (Figure 1). Again, S was not included in the initial source identification analysis and was therefore distributed over the identified sources. Sulfate is a secondary pollutant formed through a series of atmospheric reactions. Its precursor sulfur dioxide (SO2) is emitted by the burning of liquid and solid fuels that contain S. The estimation of trace elements associated with each source component aids in the identification of the source and provides perspective on the mass estimates.
Sensitivity analyses supported our main source apportionment approach (Section C, Supporting Information). The five additional APCA each based on 80% of the monitoring sites showed similar results to the main analysis, that is the same sources with similar loadings of individual elements were found (Tables C1 and C2, Figure C1, Supporting Information), suggesting our main results are robust. Including S in the PCA resulted in similar separation of source components to the main PCA approach (Table C3 and Figure C2, Supporting Information). We expected including S in the PCA would undermine the clear separation of the fossil fuel combustion sources (e.g., residual oil and traffic on one component) because particulate S results from transformation of sulfur dioxide emitted from many fossil fuel combustion sources and thus it is not unique to any one source. It is reassuring to see similar separation of source components using both approaches and it can be considered more a philosophical choice whether to include S in the PCA analysis or not. Including total K without adjusting for soil dust-associated K resulted in similar factor loadings but higher contributions in soil component from K (Table C4 and Figure C3,Supporting Information).
3.2. Population Characteristics and Source-Specific PM2.5 Exposure Estimation
Of the total population of 381,036 study participants, 323,782 (85%) had complete information on model 3 covariates and were included in the main analyses (Table 2). The participants were followed up for an average of 19.5 years, contributing to 6,317,235 person-years of follow-up. Most of the study cohorts started from mid-1990 and were followed-up till 2011–2015. The average age at baseline ranged from 42 to 73. Four subcohorts included only female participants, and the pooled cohort comprised 66% females. Differences across the cohorts were also observed for the population size, average years of follow-up, socioeconomic status (SES), and lifestyle factors, supporting our decision to account for difference in baseline hazards between subcohorts. Detailed baseline characteristics of study population in individual subcohorts can be found elsewhere.13,25,26
Table 2. Population Characteristics.
| cohort | population size (N)a | population in main model 3 [N (%)] | baseline period | follow-up | average years of follow-up | age at baseline (mean ± SD) | female (%) | current smokers (%) | married or living with partner (%) | employed (%) | mean area-level income, *1000€ (mean ± SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pooled cohort | 381,036 | 323,782 (85.0) | 19.5 | 48.7 ± 13.4 | 66 | 24 | 72 | 70 | 20.1 ± 5.8 | ||
| CEANS-SDPP | 7835 | 7716 (98.5) | 1992–1998 | 2011 | 15.9 | 47.1 ± 4.9 | 61 | 26 | 84 | 91 | 24.3 ± 4.2 |
| CEANS-SIXTY | 4180 | 3965 (94.9) | 1997–1999 | 2014 | 15.5 | 60.0 ± 0.0 | 52 | 21 | 74 | 68 | 24.7 ± 6.9 |
| CEANS-SALT | 6724 | 6174 (91.8) | 1998–2003 | 2011 | 10.4 | 57.8 ± 10.6 | 55 | 21 | 68 | 64 | 25.3 ± 6.6 |
| CEANS-SNACK | 3248 | 2830 (87.1) | 2001–2004 | 2011 | 7.4 | 72.9 ± 10.4 | 62 | 14 | 46 | 23 | 28.7 ± 2.2 |
| DCH | 56,308 | 52,779 (93.7) | 1993–1997 | 2015 | 18.2 | 56.7 ± 4.4 | 53 | 36 | 71 | 78 | 20.1 ± 3.4 |
| DNC-1993 | 19,664 | 17,017 (86.5) | 1993 | 2013 | 18.7 | 56.2 ± 8.4 | 100 | 37 | 68 | 70 | 19.2 ± 2.6 |
| DNC-1999 | 8769 | 8117 (92.6) | 1999 | 2013 | 14.4 | 47.9 ± 4.2 | 100 | 29 | 76 | 95 | 19.0 ± 2.4 |
| EPIC_NL-Morgen | 20,711 | 18,292 (88.3) | 1993–1997 | 2013 | 16.8 | 42.9 ± 11.3 | 55 | 35 | 65 | 69 | 12.2 ± 1.6 |
| EPIC_NL-Prospect | 16,194 | 14,570 (90.0) | 1993–1997 | 2013 | 16.4 | 57.7 ± 6.1 | 100 | 23 | 77 | 51 | 13.1 ± 1.4 |
| HNR | 4809 | 4733 (98.4) | 2000–2003 | 2015 | 12 | 59.7 ± 7.8 | 50 | 24 | 75 | 40 | 25.2 ± 8.2 |
| E3N | 53,521 | 38,537 (72.0) | 1989–1991 | 2011 | 16.8 | 53.0 ± 6.8 | 100 | 13 | 83 | 68 | 11.2 ± 3.0 |
| KORA-S3 | 4566 | 2572 (56.3) | 1994–1995 | 2011 | 15.6 | 49.4 ± 13.9 | 51 | 20 | 80 | 55 | 36.7 ± 4.4 |
| KORA-S4 | 4257 | 2281 (53.6) | 1999–2001 | 2014 | 12.9 | 49.3 ± 13.8 | 51 | 23 | 79 | 59 | 38.0 ± 7.3 |
| VHM&PP | 170,250 | 144,199 (84.7) | 1985–2005 | 2014 | 23.1 | 42.1 ± 15.0 | 56 | 20 | 69 | 70 | 22.9 ± 1.7 |
Population size is the number of subjects for which information was transferred to Utrecht University for construction of the pooled cohort.
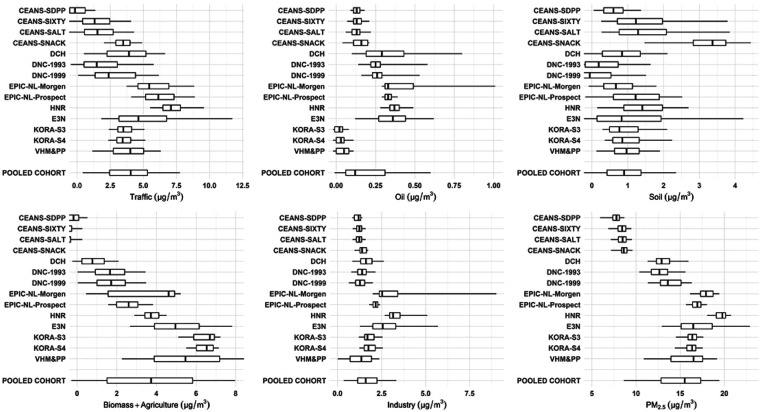
Figure 2 and Table D3,Supporting Information show the exposure distribution of the identified source-specific PM2.5 concentrations estimated for the study population in the individual subcohorts and the pooled cohort. Exposure concentrations generally showed a North–South increasing trend for PM2.5 from traffic and biomass and agriculture and the generic PM2.5 mass. The within-cohort exposure variability was large for PM2.5 from traffic, soil, and biomass and agriculture. PM2.5 exposures from residual oil burning and industrial sources were low in all subcohorts with relatively small within-cohort exposure contrasts, mainly because of the lack of sources in the study areas. An exception was observed for a small number of subjects within the Dutch EPIC-NL-Morgen cohort with high exposures in PM2.5 from both residual oil burning and industrial sources, likely related to shipping emissions from port cities and emissions from steel industries.54 Soil-related PM2.5 exposures ranged similarly across subcohorts, except the relatively high exposures observed for participants within the SNACK cohort located in Kungsholmen, Stockholm, which could be related to winter sanding of streets and road abrasion from studded tires. We estimated relatively low PM2.5 concentrations from soil, and relatively high PM2.5 concentrations from biomass and agriculture. This suggested that we may not be able to completely disentangle biomass burning-associated K from soil dust-associated K, even with the adjustment of K concentrations. However, the high estimate for biomass contribution was comparable with a PM2.5 source apportionment study previously conducted in Europe.55 Moreover, the windblown soil contribution to PM is predominately in the coarse fraction, and the traffic-associated soil mass from road dust may have been picked up by (i.e., attributed to) the traffic component and thus resulted in the low contribution of this non-traffic soil component.
Figure 2.
Exposure distribution of source-specific PM2.5 concentrations at participants’ baseline residential addresses. Subcohorts are shown from North to South; the boundary of the box closest to zero indicates P25; the boundary of the box furthest from zero, P75; the bold vertical line inside the box, P50; and the whiskers, P5 and P95. Exposure distribution for the pooled cohort is shown in Table D3,Supporting Information.
Correlations between source-specific PM2.5 exposures at residential addresses were moderate to low (Table D4, Supporting Information), allowing proper interpretation of the multisource analyses. We reported the median of cohort-specific correlations, as it is most relevant for our interpretations because analyses were stratified by subcohort. Correlations between sources varied across subcohorts. The values were not directly comparable to the correlations between identified sources at the monitoring sites presented in Table D2, Supporting Information as the correlations at monitoring sites were assessed at the European scale.
3.3. Mortality Risks Associated with Source-Specific PM2.5
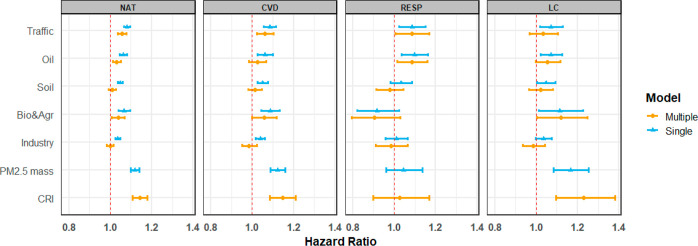
During the follow-up, we observed 46,640 (14.4%), 15,492 (4.8%), 2846 (0.9%), and 3776 (1.2%) deaths from natural causes, CVDs, non-malignant respiratory diseases, and LC respectively. Figure 3 and Table D5, Supporting Information show the associations between source-specific PM2.5 and natural and cause-specific mortality.
Figure 3.
We observed significantly positive associations with traffic-related PM2.5 for all assessed mortality endpoints in single-source models. In multisource models, associations decreased slightly for mortality from natural causes, CVDs and LC, and remained stable for respiratory mortality with slightly wider CIs. The HRs associated with a per IQR increase in source-specific PM2.5 concentrations were the highest for the traffic component for all assessed endpoints except LC mortality (HR: 1.06; 95% CI: 1.04 and 1.08 for natural mortality per 2.86 μg/m3 increase in traffic-related PM2.5). Consistent findings were reported by the ACS CPS-II and the California Teachers Study for strong associations between excess mortality and traffic-related exposures.6,56 In the previous elemental analyses within the ELAPSE pooled cohort, we observed significantly positive associations for mortality with Cu and Fe in single-pollutant models.13 The associations attenuated substantially in models with further adjustment for NO2, likely reflecting the common traffic source of these pollutants. Similar to this study, positive associations with mortality were reported in past studies analyzing individual tracers of traffic emissions, which became less stable after adjusting for other traffic-related components such as NO2 or organic carbon.7,8,14
A majority of the traffic-related PM2.5 was explained by BC (25.2%) in our study (Figure 1). There is mounting evidence on associations between long-term exposure to BC and adverse health outcomes.51,57,58 In the ELAPSE pooled cohort, we previously found significantly positive associations for BC exposure with mortality and incidence of stroke, asthma, and COPD.25,59−61 Health effects of BC were, however, difficult to disentangle from NO2 because of their high correlation in concentrations, reflecting conditions in developed countries, where an important source of both pollutants is diesel-powered vehicles. The source apportionment analysis conducted in the present study allowed us to consider the multiple air pollutants from the same source as a group and thus derived more interpretable results.
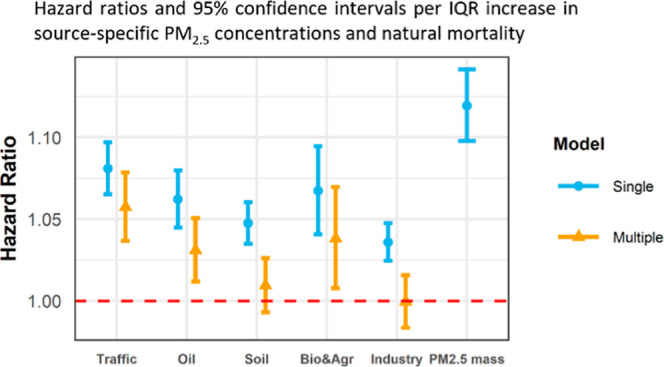
The oil combustion source component was significantly positively associated with all assessed mortality endpoints in single-source models. HRs reduced moderately and remained statistically significant (borderline significant for CVD mortality) in multisource models. This is consistent with our previous findings that V was most robustly associated with increased mortality risks.13 When considered on a per 1 μg/m3 comparable basis, the source-labeled residual oil combustion PM2.5 had the highest associations with all assessed mortality endpoints (HRs and 95% CIs 1.269 (1.189, 1.354) in the single-source model and 1.128 (1.047, 1.215) in the multisource model for natural mortality) (Table D6,Supporting Information). This suggests that the natural mortality risk estimate for residual oil PM2.5 may be about 5 times higher (2.3 if based on the lower and 7.2 based on the upper 95% CI limit) than that for PM2.5 mass in general. Previous findings have been mixed on mortality risks associated with residual oil component and its trace elements,23,62,63 likely because of the small exposure contrasts combined with low concentrations of residual oil-related particles. In quite a few study areas, residual oil is a less ubiquitous source than motorized traffic. The large population included in the current analysis likely allowed us to detect the potential associations better.
The soil component was positively associated with all assessed mortality endpoints in single-source models. However, these associations reduced to basically unity in multisource models. Crustal materials are often abundant in coarse particles. Our finding of null association is consistent with most previous studies showing little evidence for an association between long-term coarse PM exposure and adverse health effects.57,64 The 2019 Integrated Science Assessment (ISA) rated the association between PMcoarse exposure and natural-cause mortality as “suggestive”.65 A recent analysis conducted in the Medicare enrollees reported significantly positive, but much smaller, association with all-cause mortality for the soil component than for combustion-related components.66 The ACS CPS-II found no association with mortality for soil and its elemental tracers (calcium and Si).56 Si is a specific tracer for crustal material that is a major component of soil and resuspended road dust. However, a distinction between soil and road dust is often difficult because of the overlapping source profiles.15 The California Teachers Study reported adverse cardiovascular associations with long-term exposure to Si, yet the authors interpreted the Si exposure as a proxy either for toxic constituents found in road dust or for exposures to traffic-related pollutants.7 The relatively low concentrations of soil component observed in our study suggest that the traffic-associated soil mass from road dust may have been picked up by the traffic component, especially given the large percentage of elemental Si and Fe in the traffic PM2.5 profile in Figure 1. Nevertheless, the good practice statements recently provided by the WHO about particles originating from sand and dust storms were to continue monitoring programs and source apportionment activities, health effect analyses, and reduction of exposure.67
For the biomass and agriculture component, we observed significantly positive associations with mortality from natural causes, CVDs and LC, and non-significantly negative association with respiratory mortality in single-source models. In multisource models, HRs decreased but remained significantly positive for mortality from natural causes and CVDs, and remained stable for LC mortality. Different from other identified source components, HRs for biomass and agriculture increased from the crude model (model 1) to the model with further adjustment for individual level potential confounders (model 2) (Table D7,Supporting Information). The increase in HR is attributed to the negative correlations between the biomass and agriculture source component and the individual level covariates, indicating population with higher SES or healthier lifestyle tend to be exposed more to PM2.5 from biomass and agriculture. We identified the biomass and agriculture component because of its high loading on K (Table 1). However, K is not a unique indicator of wood combustion but can also derive from meat cooking, refuse incineration, and agriculture waste combustion.16,42 The California Teachers Study reported positive association between K and IHD mortality,7 whereas null association with mortality for K and biomass combustion source category was found in the ACS CPS-II and the Medicare cohort.56,62
The industry PM2.5 component was positively associated with all assessed mortality outcomes in single source models, but HRs were reduced to unity in multisource models. The observed null associations may be related to the low exposure level and small exposure contrasts exploited in our analyses. Consistently, no association between steel industry-related PM2.5 and mortality was found in the Medicare cohort.62 The ACS CPS-II reported positive but weak associations with IHD mortality for metal industrial combustion PM2.5 (tracers Pb and Zn).23
Overall, the present source-specific analysis and the previous individual elemental analysis revealed that particles from residual oil burning (tracers Ni and V) and traffic-related emissions (tracers NO2, BC, Cu, and Fe) were most consistently associated with mortality,13 agreeing with an analysis in the Netherlands based on dispersion model calculated particle source contributions.68
Cumulatively, we found significantly positive associations by air pollution with mortality from natural causes, CVDs and LC. Association with non-malignant respiratory mortality was positive though non-significant. The strongest cumulative risk estimate was for LC mortality (HR: 1.23; 95% CI: 1.10 and 1.38). The cumulative risk estimates were larger than any of the individual source-specific PM2.5 HRs resulting from single-source models, supporting that particles from multiple sources were associated with mortality. The cumulative risk was smaller than the sum of HRs, likely attributable to the correlations between PM2.5 components.
Sensitivity analyses confirmed the robustness of our findings. Model 1 HRs were almost identical for model 1 and model 3 populations, indicating little selection bias was introduced (Table D8,Supporting Information). When restricting analyses to the follow-up period starting from year 2000 (69% of total person-years at risk, 84% of total deaths), year 2005 (46% of total person-years at risk, 64% of total deaths), year 2008 (32% of total person-years at risk, 47% of total deaths), and year 2010 (23% of total person-years at risk, 33% of total deaths), we observed statistically significant associations between natural mortality and source-specific PM2.5, except for PM2.5 from biomass and agriculture emissions, which attenuated to unity (Table D9,Supporting Information). When restricting to follow-up starting in 2010, generally smaller HRs with wider CIs were found, likely related to the large reduction in follow-up time and the associated number of events. Applying APCA results derived from the fivefold robustness evaluation analysis resulted in similar source-specific PM2.5 associations with natural mortality (Table D10,Supporting Information).
3.4. Strengths and Limitations
One strength is the unique and standardized measurement data for PM2.5 elemental composition collected from 19 study areas across Europe used for source apportionment analysis. The number of studies on PM component-specific health effects has been small, partly because of the scarcity of measurements relative to regulated pollutants such as PM2.5 and NO2. Another strength is the large population included in this study, with detailed information on individual and area-level covariates. The pooling of 14 subcohorts and the harmonization of variables across cohorts allowed enhanced statistical power to detect source-specific associations with mortality, especially for source components that have small exposure contrasts. The source apportionment analysis allowed us to assess health effects of PM components in the group context and provided more interpretable results that are more readily translatable into generalizable air quality policy. The correct interpretation of results relies heavily on the reliability of the source apportionment approach. Although the APCA method applied does not quantitatively assess the uncertainties in the mass apportionments, our sensitivity analyses showed robustness of our main APCA approach. Furthermore, an intercomparison among several source apportionment methods has shown that the APCA source apportionment results are consistent across the various methods.69 Moreover, an assessment of the contribution by source apportionment to the variability in health effects is a relatively small portion (about 15%) of the uncertainty in the resulting epidemiological health effects estimates.70
While it is clear from our findings that source-specific PM2.5 differ in their associations with mortality, additional source-specific tracers are needed to be more definitive. We were able to include only nine pollutants in the PCA, whereas source apportionment analyses usually include more tracers. We cannot rule out health risks associated with sources that were not identified by our source apportionment analysis. For example, we could not identify coal burning source, likely because there was not arsenic (As) or selenium (Se) data available to consider in the PCA. Coal combustion PM2.5 was found to be most strongly and robustly associated with IHD mortality in the ACS CPS-II and the association between IHD mortality was about five times higher than that for generic PM2.5 mass.23 Also, more (likely organic) tracers such as levoglucosan need to be analyzed in future work to better separate out the biomass contribution.71 We were not able to incorporate organic carbon and heavy metals such as Pb, Cd, and Hg in the analysis, which were reported to be associated with increased mortality risks in a few studies.7,24
We had limited ability to investigate the spatial and temporal variability of source components. The air pollution data were collected from 397 monitoring sites across Europe and were clustered (20 or 40 sites per study area). Source of trace elements and their compositions may vary spatially. However, a sensitivity analysis by study area would be unstable and uninformative for our study because of the small number of monitoring sites within each area. Nevertheless, previous source apportionment analyses suggested that the sources of trace elements were relatively stable across European cities.15
Another limitation is that the exposure assessment was based on air pollution data in 2010, whereas most included cohorts started their follow-up from mid-1990. However, our sensitivity analyses by restricting recruitment to start years of 2000, 2005, 2008, and 2010 showed robust results, supporting our original analyses in the full cohort. We furthermore note that, for major air pollutants including NO2, spatial stability of exposure contrasts over a decade or more has been demonstrated in Europe.72−74 While the same data do not exist for source-specific PM, we suspect this holds as well because the spatial distributions of the identified sources have not changed substantially in the already highly developed European urban areas we consider.
Acknowledgments
We thank Marjan Tewis for the data management tasks in creating the pooled cohort database.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.2c01912.
Further elaboration on the APCA; documentation of the process to derive the optimal APCA solution; sensitivity analyses of APCA; factor loadings; percentage of negative source contributions; Spearman correlations between source-specific PM2.5 concentrations; estimated fractional elemental source profiles; descriptives of air pollution measurement data; exposure distribution of source-specific and generic PM2.5; associations of source-specific PM2.5 with mortality; HRs for mortality; associations of source-specific PM2.5 exposures and natural mortality with a restricted follow-up period; and associations between source-specific PM2.5 and natural-cause mortality (PDF)
Author Contributions
⊥⊥⊥⊥ B.B. and G.D.T. contributed equally to this work
The research described in this article was conducted under contract to the Health Effects Institute (HEI), an organization jointly funded by the United States Environmental Protection Agency (EPA) (Assistance Award No. R-82811201) and certain motor vehicle and engine manufacturers. The contents of this article do not necessarily reflect the views of HEI, or its sponsors, nor do they necessarily reflect the views and policies of the EPA or motor vehicle and engine manufacturers.
The authors declare no competing financial interest.
Supplementary Material
References
- Chen J.; Hoek G. Long-term exposure to PM and all-cause and cause-specific mortality: A systematic review and meta-analysis. Environ. Int. 2020, 143, 105974. 10.1016/j.envint.2020.105974. [DOI] [PubMed] [Google Scholar]
- Laden F.; Neas L. M.; Dockery D. W.; Schwartz J. Association of fine particulate matter from different sources with daily mortality in six US cities. Environ. Health Perspect. 2000, 108, 941–947. 10.1289/ehp.00108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özkaynak H.; Thurston G. D. Associations between 1980 US mortality rates and alternative measures of airborne particle concentration. Risk Anal. 1987, 7, 449–461. 10.1111/j.1539-6924.1987.tb00482.x. [DOI] [PubMed] [Google Scholar]
- Sarnat J. A.; Marmur A.; Klein M.; Kim E.; Russell A. G.; Sarnat S. E.; Mulholland J. A.; Hopke P. K.; Tolbert P. E. Fine particle sources and cardiorespiratory morbidity: an application of chemical mass balance and factor analytical source-apportionment methods. Environ. Health Perspect. 2008, 116, 459–466. 10.1289/ehp.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai F. C.; Apte M. G.; Daisey J. M. An exploratory analysis of the relationship between mortality and the chemical composition of airborne particulate matter. Inhalation Toxicol. 2000, 12, 121–135. 10.1080/08958378.2000.11463204. [DOI] [PubMed] [Google Scholar]
- Ostro B.; Hu J.; Goldberg D.; Reynolds P.; Hertz A.; Bernstein L.; Kleeman M. J. Associations of mortality with long-term exposures to fine and ultrafine particles, species and sources: results from the California Teachers Study Cohort. Environ. Health Perspect. 2015, 123, 549–556. 10.1289/ehp.1408565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B.; Lipsett M.; Reynolds P.; Goldberg D.; Hertz A.; Garcia C.; Henderson K. D.; Bernstein L. Long-term exposure to constituents of fine particulate air pollution and mortality: results from the California Teachers Study. Environ. Health Perspect. 2011, 118, 363–369. 10.1289/ehp.0901181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badaloni C.; Cesaroni G.; Cerza F.; Davoli M.; Brunekreef B.; Forastiere F. Effects of long-term exposure to particulate matter and metal components on mortality in the Rome longitudinal study. Environ. Int. 2017, 109, 146–154. 10.1016/j.envint.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Beelen R.; Hoek G.; Raaschou-Nielsen O.; Stafoggia M.; Andersen Z. J.; Weinmayr G.; Hoffmann B.; Wolf K.; Samoli E.; Fischer P. H.; Nieuwenhuijsen M. J.; Xun W. W.; Katsouyanni K.; Dimakopoulou K.; Marcon A.; Vartiainen E.; Lanki T.; Yli-Tuomi T.; Oftedal B.; Schwarze P. E.; Nafstad P.; De Faire U.; Pedersen N. L.; Östenson C.-G.; Fratiglioni L.; Penell J.; Korek M.; Pershagen G.; Eriksen K. T.; Overvad K.; Sørensen M.; Eeftens M.; Peeters P. H.; Meliefste K.; Wang M.; Bueno-de-Mesquita H. B.; Sugiri D.; Krämer U.; Heinrich J.; de Hoogh K.; Key T.; Peters A.; Hampel R.; Concin H.; Nagel G.; Jaensch A.; Ineichen A.; Tsai M.-Y.; Schaffner E.; Probst-Hensch N. M.; Schindler C.; Ragettli M. S.; Vilier A.; Clavel-Chapelon F.; Declercq C.; Ricceri F.; Sacerdote C.; Galassi C.; Migliore E.; Ranzi A.; Cesaroni G.; Badaloni C.; Forastiere F.; Katsoulis M.; Trichopoulou A.; Keuken M.; Jedynska A.; Kooter I. M.; Kukkonen J.; Sokhi R. S.; Vineis P.; Brunekreef B. Natural-cause mortality and long-term exposure to particle components: an analysis of 19 European cohorts within the multi-center ESCAPE project. Environ. Health Perspect. 2015, 123, 525–533. 10.1289/ehp.1408095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaschou-Nielsen O.; Beelen R.; Wang M.; Hoek G.; Andersen Z. J.; Hoffmann B.; Stafoggia M.; Samoli E.; Weinmayr G.; Dimakopoulou K.; Nieuwenhuijsen M.; Xun W. W.; Fischer P.; Eriksen K. T.; Sørensen M.; Tjønneland A.; Ricceri F.; de Hoogh K.; Key T.; Eeftens M.; Peeters P. H.; Bueno-de-Mesquita H. B.; Meliefste K.; Oftedal B.; Schwarze P. E.; Nafstad P.; Galassi C.; Migliore E.; Ranzi A.; Cesaroni G.; Badaloni C.; Forastiere F.; Penell J.; De Faire U.; Korek M.; Pedersen N.; Östenson C.-G.; Pershagen G.; Fratiglioni L.; Concin H.; Nagel G.; Jaensch A.; Ineichen A.; Naccarati A.; Katsoulis M.; Trichpoulou A.; Keuken M.; Jedynska A.; Kooter I. M.; Kukkonen J.; Brunekreef B.; Sokhi R. S.; Katsouyanni K.; Vineis P. Particulate matter air pollution components and risk for lung cancer. Environ. Int. 2016, 87, 66–73. 10.1016/j.envint.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Wang M.; Beelen R.; Stafoggia M.; Raaschou-Nielsen O.; Andersen Z. J.; Hoffmann B.; Fischer P.; Houthuijs D.; Nieuwenhuijsen M.; Weinmayr G.; Vineis P.; Xun W. W.; Dimakopoulou K.; Samoli E.; Laatikainen T.; Lanki T.; Turunen A. W.; Oftedal B.; Schwarze P.; Aamodt G.; Penell J.; De Faire U.; Korek M.; Leander K.; Pershagen G.; Pedersen N. L.; Östenson C.-G.; Fratiglioni L.; Eriksen K. T.; Sørensen M.; Tjønneland A.; Bueno-de-Mesquita B.; Eeftens M.; Bots M. L.; Meliefste K.; Krämer U.; Heinrich J.; Sugiri D.; Key T.; de Hoogh K.; Wolf K.; Peters A.; Cyrys J.; Jaensch A.; Concin H.; Nagel G.; Tsai M.-Y.; Phuleria H.; Ineichen A.; Künzli N.; Probst-Hensch N.; Schaffner E.; Vilier A.; Clavel-Chapelon F.; Declerq C.; Ricceri F.; Sacerdote C.; Marcon A.; Galassi C.; Migliore E.; Ranzi A.; Cesaroni G.; Badaloni C.; Forastiere F.; Katsoulis M.; Trichopoulou A.; Keuken M.; Jedynska A.; Kooter I. M.; Kukkonen J.; Sokhi R. S.; Brunekreef B.; Katsouyanni K.; Hoek G. Long-term exposure to elemental constituents of particulate matter and cardiovascular mortality in 19 European cohorts: results from the ESCAPE and TRANSPHORM projects. Environ. Int. 2014, 66, 97–106. 10.1016/j.envint.2014.01.026. [DOI] [PubMed] [Google Scholar]
- Hvidtfeldt U. A.; Geels C.; Sørensen M.; Ketzel M.; Khan J.; Tjønneland A.; Christensen J. H.; Brandt J.; Raaschou-Nielsen O. Long-term residential exposure to PM2.5 constituents and mortality in a Danish cohort. Environ. Int. 2019, 133, 105268. 10.1016/j.envint.2019.105268. [DOI] [PubMed] [Google Scholar]
- Chen J.; Rodopoulou S.; de Hoogh K.; Strak M.; Andersen ZJ.; Atkinson R.; Bauwelinck M.; Bellander T.; Brandt J.; Cesaroni G.; Concin H.; Fecht D.; Forastiere F.; Gulliver J.; Hertel O.; Hoffmann B.; Hvidtfeldt U. A.; Janssen N. A. H.; Jöckel K. H.; Jørgensen J.; Katsouyanni K.; Ketzel M.; Klompmaker J. O.; Lager A.; Leander K.; Liu S.; Ljungman P.; MacDonald C. J.; Magnusson P. K. E.; Mehta A.; Nagel G.; Oftedal B.; Pershagen G.; Peters A.; Raaschou-Nielsen O.; Renzi M.; Rizzuto D.; Samoli E.; van der Schouw Y. T..; Schramm S.; Schwarze P.; Sigsgaard T.; Sørensen M.; Stafoggia M.; Tjønneland A.; Vienneau D.; Weinmayr G.; Wolf K.; Brunekreef B.; Hoek G. Long-Term Exposure to Fine Particle Elemental Components and Natural and Cause-Specific Mortality-a Pooled Analysis of Eight European Cohorts within the ELAPSE Project. Environ. Health Perspect. 2021, 129, 47009. 10.1289/EHP8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodopoulou S.; Stafoggia M.; Chen J.; de Hoogh K.; Bauwelinck M.; Mehta A. J.; Klompmaker J. O.; Oftedal B.; Vienneau D.; Janssen N. A. H.; Strak M.; Andersen Z. J.; Renzi M.; Cesaroni G.; Nordheim C. F.; Bekkevold T.; Atkinson R.; Forastiere F.; Katsouyanni K.; Brunekreef B.; Samoli E.; Hoek G. Long-term exposure to fine particle elemental components and mortality in Europe: Results from six European administrative cohorts within the ELAPSE project. Sci. Total Environ. 2022, 809, 152205. 10.1016/j.scitotenv.2021.152205. [DOI] [PubMed] [Google Scholar]
- Belis C. A.; Karagulian F.; Larsen B. R.; Hopke P. K. Critical review and meta-analysis of ambient particulate matter source apportionment using receptor models in Europe. Atmos. Environ. 2013, 69, 94–108. 10.1016/j.atmosenv.2012.11.009. [DOI] [Google Scholar]
- Tsai M.-Y.; Hoek G.; Eeftens M.; de Hoogh K.; Beelen R.; Beregszászi T.; Cesaroni G.; Cirach M.; Cyrys J.; De Nazelle A.; de Vocht F.; Ducret-Stich R.; Eriksen K.; Galassi C.; Gražuleviciene R.; Gražulevicius T.; Grivas G.; Gryparis A.; Heinrich J.; Hoffmann B.; Iakovides M.; Keuken M.; Krämer U.; Künzli N.; Lanki T.; Madsen C.; Meliefste K.; Merritt A.-S.; Mölter A.; Mosler G.; Nieuwenhuijsen M. J.; Pershagen G.; Phuleria H.; Quass U.; Ranzi A.; Schaffner E.; Sokhi R.; Stempfelet M.; Stephanou E.; Sugiri D.; Taimisto P.; Tewis M.; Udvardy O.; Wang M.; Brunekreef B. Spatial variation of PM elemental composition between and within 20 European study areas - Results of the ESCAPE project. Environ. Int. 2015, 84, 181–192. 10.1016/j.envint.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Viana M.; Kuhlbusch T. A. J.; Querol X.; Alastuey A.; Harrison R. M.; Hopke P. K.; Winiwarter W.; Vallius M.; Szidat S.; Prévôt A. S. H.; Hueglin C.; Bloemen H.; Wåhlin P.; Vecchi R.; Miranda A. I.; Kasper-Giebl A.; Maenhaut W.; Hitzenberger R. Source apportionment of particulate matter in Europe: A review of methods and results. J. Aerosol Sci. 2008, 39, 827–849. 10.1016/j.jaerosci.2008.05.007. [DOI] [Google Scholar]
- Cooper J. A.; Watson J. G. Receptor Oriented Methods of Air Particulate Source Apportionment. J. Air Pollut. Control Assoc. 1980, 30, 1116–1125. 10.1080/00022470.1980.10465157. [DOI] [Google Scholar]
- USEPA . Supplement to the 2019 Integrated Science Assessment for Particulate Matter (External Review Draft, 2021). US Environmental Protection Agency, Washington, DC, EPA/600/R-21/198 2021, https://cfpubepagov/ncea/isa/recordisplaycfm?deid=352823 (last access April 28, 2022). [PubMed]
- Agier L.; Portengen L.; Chadeau-Hyam M.; Basagaña X.; Giorgis-Allemand L.; Siroux V.; Robinson O.; Vlaanderen J.; González J. R.; Nieuwenhuijsen M. J.; Vineis P.; Vrijheid M.; Slama R.; Vermeulen R. A systematic comparison of linear regression–based statistical methods to assess exposome-health associations. Environ. Health Perspect. 2016, 124, 1848. 10.1289/ehp172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R.; James G.; Witten D.; Hastie T.. An Introduction to Statistical Learning-With Applications in R; Springer: New York, NY, 2013. [Google Scholar]
- Lippmann M.; Chen L. C.; Gordon T.; Ito K.; Thurston G. D. National Particle Component Toxicity (NPACT) Initiative: integrated epidemiologic and toxicologic studies of the health effects of particulate matter components. Res. Rep.—Health Eff. Inst. 2013, 5–13. [PubMed] [Google Scholar]
- Thurston G. D.; Burnett R. T.; Turner M. C.; Shi Y.; Krewski D.; Lall R.; Ito K.; Jerrett M.; Gapstur S. M.; Diver W. R.; Pope C. A. Ischemic Heart Disease Mortality and Long-Term Exposure to Source-Related Components of U.S. Fine Particle Air Pollution. Environ. Health Perspect. 2016, 124, 785–794. 10.1289/ehp.1509777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedal S.; Campen M.; McDonald J.; Larson T.; Sampson P.; Sheppard L.; Simpson C.; Szpiro A. National Particle Component Toxicity (NPACT) initiative report on cardiovascular effects. Res. Rep.—Health Eff. Inst. 2013, 178, 5–8. [PubMed] [Google Scholar]
- Strak M.; Weinmayr G.; Rodopoulou S.; Chen J.; de Hoogh K.; Andersen Z. J.; Atkinson R.; Bauwelinck M.; Bekkevold T.; Bellander T.; Boutron-Ruault M.-C.; Brandt J.; Cesaroni G.; Concin H.; Fecht D.; Forastiere F.; Gulliver J.; Hertel O.; Hoffmann B.; Hvidtfeldt U. A.; Janssen N. A. H.; Jöckel K.-H.; Jørgensen J. T.; Ketzel M.; Klompmaker J. O.; Lager A.; Leander K.; Liu S.; Ljungman P.; Magnusson P. K. E.; Mehta A. J.; Nagel G.; Oftedal B.; Pershagen G.; Peters A.; Raaschou-Nielsen O.; Renzi M.; Rizzuto D.; van der Schouw Y. T.; Schramm S.; Severi G.; Sigsgaard T.; Sørensen M.; Stafoggia M.; Tjønneland A.; Verschuren W. M. M.; Vienneau D.; Wolf K.; Katsouyanni K.; Brunekreef B.; Hoek G.; Samoli E. Long term exposure to low level air pollution and mortality in eight European cohorts within the ELAPSE project: pooled analysis. BMJ [Br. Med. J.] 2021, 374, n1904. 10.1136/bmj.n1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunekreef B.; Strak M.; Chen J.; Andersen Z. J.; Atkinson R.; Bauwelinck M.; Bellander T.; Boutron M.; Brandt J.; Carey I.; Cesaroni G.; Forastiere F.; Fecht D.; Gulliver J.; Hertel O.; Hoffmann B.; de Hoogh K.; Houthuijs D.; Hvidtfeldt U. A.; Janssen N. A. H.; Jorgensen J.; Katsouyanni K.; Ketzel M.; Klompmaker J. O.; Krog N.; Liu S.; Ljungman P.; Mehta A.; Nagel G.; Oftedal B.; Pershagen G.; Peters A.; Raaschou-Nielsen O.; Renzi M.; Rodopoulou S.; Samoli E.; Schwarze P.; Sigsgaard T.; Stafoggia M.; Vienneau D.; Weinmayr G.; Wolf K.; Hoek G.. Mortality and Morbidity Effects of Long-Term Exposure to Low-Level PM2.5, Black Carbon, NO2 and O3: An Analysis of European Cohorts. Research Report; Health Effects Institute, 2021. [PMC free article] [PubMed]
- Eriksson A.-K.; Ekbom A.; Granath F.; Hilding A.; Efendic S.; Östenson C.-G. Psychological distress and risk of pre-diabetes and Type2 diabetes in a prospective study of Swedish middle-aged men and women. Diabetic Med. 2008, 25, 834–842. 10.1111/j.1464-5491.2008.02463.x. [DOI] [PubMed] [Google Scholar]
- Wändell P.-E.; Wajngot A.; De Faire U.; Hellénius M.-L. Increased prevalence of diabetes among immigrants from non-European countries in 60-year-old men and women in Sweden. Diabetes Metab. 2007, 33, 30–36. 10.1016/j.diabet.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Magnusson P. K. E.; Almqvist C.; Rahman I.; Ganna A.; Viktorin A.; Walum H.; Halldner L.; Lundström S.; Ullén F.; Långström N.; Larsson H.; Nyman A.; Gumpert C. H.; Råstam M.; Anckarsäter H.; Cnattingius S.; Johannesson M.; Ingelsson E.; Klareskog L.; de Faire U.; Pedersen N. L.; Lichtenstein P. The Swedish Twin Registry: establishment of a biobank and other recent developments. Twin Res. Hum. Genet. 2013, 16, 317–329. 10.1017/thg.2012.104. [DOI] [PubMed] [Google Scholar]
- Lagergren M.; Fratiglioni L.; Hallberg I. R.; Berglund J.; Elmståhl S.; Hagberg B.; Holst G.; Rennemark M.; Sjolund B.-M.; Thorslund M.; Wiberg I.; Winblad B.; Wimo A. A longitudinal study integrating population, care and social services data. The Swedish National study on Aging and Care (SNAC). Aging Clin. Exp. Res. 2004, 16, 158–168. 10.1007/bf03324546. [DOI] [PubMed] [Google Scholar]
- Tjønneland A.; Olsen A.; Boll K.; Stripp C.; Christensen J.; Engholm G.; Overvad K. Study design, exposure variables, and socioeconomic determinants of participation in Diet, Cancer and Health: a population-based prospective cohort study of 57,053 men and women in Denmark. Scand. J. Publ. Health 2007, 35, 432–441. 10.1080/14034940601047986. [DOI] [PubMed] [Google Scholar]
- Hundrup Y. A.; Simonsen M. K.; Jorgensen T.; Obel E. B. Cohort profile: the Danish nurse cohort. Int. J. Epidemiol. 2012, 41, 1241–1247. 10.1093/ije/dyr042. [DOI] [PubMed] [Google Scholar]
- Beulens J. W. J.; Monninkhof E. M.; Verschuren W. M. M.; Schouw Y. T. v. d.; Smit J.; Ocke M. C.; Jansen E. H. J. M.; Dieren S. v.; Grobbee D. E.; Peeters P. H. M.; Bueno-de-Mesquita H. B. Cohort profile: the EPIC-NL study. Int. J. Epidemiol. 2010, 39, 1170–1178. 10.1093/ije/dyp217. [DOI] [PubMed] [Google Scholar]
- Schmermund A.; Möhlenkamp S.; Stang A.; Grönemeyer D.; Seibel R.; Hirche H.; Mann K.; Siffert W.; Lauterbach K.; Siegrist J.; Jöckel K.-H.; Erbel R. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: rationale and design of the Heinz Nixdorf RECALL Study. Am. Heart J. 2002, 144, 212–218. 10.1067/mhj.2002.123579. [DOI] [PubMed] [Google Scholar]
- Clavel-Chapelon F.; Group E. N. S. Cohort profile: the French E3N cohort study. Int. J. Epidemiol. 2015, 44, 801–809. 10.1093/ije/dyu184. [DOI] [PubMed] [Google Scholar]
- Ulmer H.; Kelleher C. C.; Fitz-Simon N.; Diem G.; Concin H. Secular trends in cardiovascular risk factors: an age-period cohort analysis of 6 98 954 health examinations in 1 81 350 Austrian men and women. J. Intern. Med. 2007, 261, 566–576. 10.1111/j.1365-2796.2007.01779.x. [DOI] [PubMed] [Google Scholar]
- Eeftens M.; Tsai M.-Y.; Ampe C.; Anwander B.; Beelen R.; Bellander T.; Cesaroni G.; Cirach M.; Cyrys J.; de Hoogh K.; De Nazelle A.; de Vocht F.; Declercq C.; Dėdelė A.; Eriksen K.; Galassi C.; Gražulevičienė R.; Grivas G.; Heinrich J.; Hoffmann B.; Iakovides M.; Ineichen A.; Katsouyanni K.; Korek M.; Krämer U.; Kuhlbusch T.; Lanki T.; Madsen C.; Meliefste K.; Mölter A.; Mosler G.; Nieuwenhuijsen M.; Oldenwening M.; Pennanen A.; Probst-Hensch N.; Quass U.; Raaschou-Nielsen O.; Ranzi A.; Stephanou E.; Sugiri D.; Udvardy O.; Vaskövi É.; Weinmayr G.; Brunekreef B.; Hoek G. Spatial variation of PM2.5, PM10, PM2.5 absorbance and PMcoarse concentrations between and within 20 European study areas and the relationship with NO2 – Results of the ESCAPE project. Atmos. Environ. 2012, 62, 303–317. 10.1016/j.atmosenv.2012.08.038. [DOI] [Google Scholar]
- de Hoogh K.; Wang M.; Adam M.; Badaloni C.; Beelen R.; Birk M.; Cesaroni G.; Cirach M.; Declercq C.; Dėdelė A.; Dons E.; de Nazelle A.; Eeftens M.; Eriksen K.; Eriksson C.; Fischer P.; Gražulevičienė R.; Gryparis A.; Hoffmann B.; Jerrett M.; Katsouyanni K.; Iakovides M.; Lanki T.; Lindley S.; Madsen C.; Mölter A.; Mosler G.; Nádor G.; Nieuwenhuijsen M.; Pershagen G.; Peters A.; Phuleria H.; Probst-Hensch N.; Raaschou-Nielsen O.; Quass U.; Ranzi A.; Stephanou E.; Sugiri D.; Schwarze P.; Tsai M.-Y.; Yli-Tuomi T.; Varró M. J.; Vienneau D.; Weinmayr G.; Brunekreef B.; Hoek G. Development of land use regression models for particle composition in twenty study areas in Europe. Environ. Sci. Technol. 2013, 47, 5778–5786. 10.1021/es400156t. [DOI] [PubMed] [Google Scholar]
- Thurston G. D.; Spengler J. D. A quantitative assessment of source contributions to inhalable particulate matter pollution in metropolitan Boston. Atmos. Environ. 1985, 19, 9–25. 10.1016/0004-6981(85)90132-5. [DOI] [Google Scholar]
- Hendrickson A. E.; White P. O. Promax: A quick method for rotation to oblique simple structure. Br. J. Stat. Psychol. 1964, 17, 65–70. 10.1111/j.2044-8317.1964.tb00244.x. [DOI] [Google Scholar]
- Hopke P. K. Trace element concentrations in summer aerosols at rural sites in New York state and their possible sources and seasonal variations in the composition of ambient sulfatecontaining aerosols in the New York area. Atmos. Environ. 1982, 16, 1279–1280. 10.1016/0004-6981(82)90232-3. [DOI] [Google Scholar]
- Rahman M. M.; Begum B. A.; Hopke P. K.; Nahar K.; Thurston G. D. Assessing the PM2.5 impact of biomass combustion in megacity Dhaka, Bangladesh. Environ. Pollut. 2020, 264, 114798. 10.1016/j.envpol.2020.114798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston G. D.; Lioy P. J. Receptor modeling and aerosol transport. Atmos. Environ. 1987, 21, 687–698. 10.1016/0004-6981(87)90050-3. [DOI] [Google Scholar]
- Thurston G. D.; Ito K.; Lall R. A Source Apportionment of U.S. Fine Particulate Matter Air Pollution. Atmos. Environ. 2011, 45, 3924–3936. 10.1016/j.atmosenv.2011.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; de Hoogh K.; Gulliver J.; Hoffmann B.; Hertel O.; Ketzel M.; Weinmayr G.; Bauwelinck M.; van Donkelaar A.; Hvidtfeldt U. A.; Atkinson R.; Janssen N. A. H.; Martin R. V.; Samoli E.; Andersen Z. J.; Oftedal B. M.; Stafoggia M.; Bellander T.; Strak M.; Wolf K.; Vienneau D.; Brunekreef B.; Hoek G. Development of Europe-Wide Models for Particle Elemental Composition Using Supervised Linear Regression and Random Forest. Environ. Sci. Technol. 2020, 54, 15698. 10.1021/acs.est.0c06595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hoogh K.; Chen J.; Gulliver J.; Hoffmann B.; Hertel O.; Ketzel M.; Bauwelinck M.; van Donkelaar A.; Hvidtfeldt U. A.; Katsouyanni K.; Klompmaker J.; Martin R. V.; Samoli E.; Schwartz P. E.; Stafoggia M.; Bellander T.; Strak M.; Wolf K.; Vienneau D.; Brunekreef B.; Hoek G. Spatial PM2.5, NO2, O3 and BC models for Western Europe - Evaluation of spatiotemporal stability. Environ. Int. 2018, 120, 81–92. 10.1016/j.envint.2018.07.036. [DOI] [PubMed] [Google Scholar]
- WHO . International Classification of Diseases, Manual of the International Statistical Classification of Diseases, Injuries and Causes of Death; WHO: Geneva, Switzerland, 1997.
- WHO . International Statistical Classification of Diseases and Related Health Problems, 10th Revision. 2016, https://icd.who.int/browse10/2016/en (last access April 28, 2022).
- Samoli E.; Rodopoulou S.; Hvidtfeldt U. A.; Wolf K.; Stafoggia M.; Brunekreef B.; Strak M.; Chen J.; Andersen Z. J.; Atkinson R.; Bauwelinck M.; Bellander T.; Brandt J.; Cesaroni G.; Forastiere F.; Fecht D.; Gulliver J.; Hertel O.; Hoffmann B.; de Hoogh K.; Janssen N. A. H.; Ketzel M.; Klompmaker J. O.; Liu S.; Ljungman P.; Nagel G.; Oftedal B.; Pershagen G.; Peters A.; Raaschou-Nielsen O.; Renzi M.; Kristoffersen D. T.; Severi G.; Sigsgaard T.; Vienneau D.; Weinmayr G.; Hoek G.; Katsouyanni K. Modeling multi-level survival data in multi-center epidemiological cohort studies: Applications from the ELAPSE project. Environ. Int. 2021, 147, 106371. 10.1016/j.envint.2020.106371. [DOI] [PubMed] [Google Scholar]
- Crouse D. L.; Peters P. A.; Hystad P.; Brook J. R.; van Donkelaar A.; Martin R. V.; Villeneuve P. J.; Jerrett M.; Goldberg M. S.; Pope C. A. 3rd; Brauer M.; Brook R. D.; Robichaud A.; Menard R.; Burnett R. T. Ambient PM2.5, O3, and NO2Exposures and Associations with Mortality over 16 Years of Follow-Up in the Canadian Census Health and Environment Cohort (CanCHEC). Environ. Health Perspect. 2015, 123, 1180–1186. 10.1289/ehp.1409276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen N. A. H.; Hoek G.; Simic-Lawson M.; Fischer P.; van Bree L.; ten Brink H.; Keuken M.; Atkinson R. W.; Anderson H. R.; Brunekreef B.; Cassee F. R. Black Carbon as an Additional Indicator of the Adverse Health Effects of Airborne Particles Compared with PM10and PM2.5. Environ. Health Perspect. 2011, 119, 1691–1699. 10.1289/ehp.1003369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Air Quality Guidelines: Global Update 2005: Particulate Matter, Ozone, Nitrogen Dioxide, and Sulfur Dioxide; World Health Organization, 2006. [PubMed]
- EC . European Union: Agriculture Statistical Factsheet. 2021, European Commission, available at http://eceuropaeu/agriculture/statistics/factsheets_en (last access April 28, 2022).
- Keys A.; Van Hout M.; Daniels B.. Decarbonisation Options for the Dutch Steel Industry; PBL Netherlands Environmental Assessment Agency, 2019. [Google Scholar]
- Almeida S. M.; Manousakas M.; Diapouli E.; Kertesz Z.; Samek L.; Hristova E.; Sega K.; Šega R. P.; Belis C. A.; Eleftheriadis K.; Ambient particulate matter source apportionment using receptor modelling in European and Central Asia urban areas. Environ. Pollut. 2020, 266, 115199. 10.1016/j.envpol.2020.115199. [DOI] [PubMed] [Google Scholar]
- Thurston G.; Ito K.; Lall R.; Burnett R.; Turner M.; Krewski D.; Shi Y.; Jerrett M.; Gapstur S.; Diver W.. NPACT Study 4: Mortality and Long-Term Exposure to PM2. 5 and its Components in the American Cancer Society’s Cancer Prevention Study II Cohort. National Particle Component Toxicity (NPACT) Initiative: Integrated Epidemiologic and Toxicologic Studies of the Health Effects of Particulate Matter Components Health Effect Institute, 2013. [PubMed]
- Hoek G.; Krishnan R. M.; Beelen R.; Peters A.; Ostro B.; Brunekreef B.; Kaufman J. D. Long-term air pollution exposure and cardio- respiratory mortality: a review. Environ. Health: Global Access Sci. Source 2013, 12, 43. 10.1186/1476-069x-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungman P. L. S.; Andersson N.; Stockfelt L.; Andersson E. M.; Nilsson Sommar J.; Eneroth K.; Gidhagen L.; Johansson C.; Lager A.; Leander K.; Molnar P.; Pedersen N. L.; Rizzuto D.; Rosengren A.; Segersson D.; Wennberg P.; Barregard L.; Forsberg B.; Sallsten G.; Bellander T.; Pershagen G. Long-term exposure to particulate air pollution, black carbon, and their source components in relation to ischemic heart disease and stroke. Environ. Health Perspect. 2019, 127, 107012. 10.1289/ehp4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.; Jørgensen J. T.; Ljungman P.; Pershagen G.; Bellander T.; Leander K.; Magnusson P. K. E.; Rizzuto D.; Hvidtfeldt U. A.; Raaschou-Nielsen O.; Wolf K.; Hoffmann B.; Brunekreef B.; Strak M.; Chen J.; Mehta A.; Atkinson R. W.; Bauwelinck M.; Varraso R.; Boutron-Ruault M.-C.; Brandt J.; Cesaroni G.; Forastiere F.; Fecht D.; Gulliver J.; Hertel O.; de Hoogh K.; Janssen N. A. H.; Katsouyanni K.; Ketzel M.; Klompmaker J. O.; Nagel G.; Oftedal B.; Peters A.; Tjønneland A.; Rodopoulou S. P.; Samoli E.; Bekkevold T.; Sigsgaard T.; Stafoggia M.; Vienneau D.; Weinmayr G.; Hoek G.; Andersen Z. J. Long-term exposure to low-level air pollution and incidence of chronic obstructive pulmonary disease: The ELAPSE project. Environ. Int. 2021, 146, 106267. 10.1016/j.envint.2020.106267. [DOI] [PubMed] [Google Scholar]
- Liu S.; Jorgensen J. T.; Ljungman P.; Pershagen G.; Bellander T.; Leander K.; Magnusson P. K. E.; Rizzuto D.; Hvidtfeldt U. A.; Raaschou-Nielsen O.; Wolf K.; Hoffmann B.; Brunekreef B.; Strak M.; Chen J.; Mehta A.; Atkinson R. W.; Bauwelinck M.; Varraso R.; Boutron-Ruault M. C.; Brandt J.; Cesaroni G.; Forastiere F.; Fecht D.; Gulliver J.; Hertel O.; de Hoogh K.; Janssen N. A. H.; Katsouyanni K.; Ketzel M.; Klompmaker J. O.; Nagel G.; Oftedal B.; Peters A.; Tjonneland A.; Rodopoulou S. P.; Samoli E.; Kristoffersen D. T.; Sigsgaard T.; Stafoggia M.; Vienneau D.; Weinmayr G.; Hoek G.; Andersen Z. J. Long-term exposure to low-level air pollution and incidence of asthma: the ELAPSE project. Eur. Respir. J. 2021, 57, 2003099. 10.1183/13993003.030992020. [DOI] [PubMed] [Google Scholar]
- Wolf K.; Hoffmann B.; Andersen Z. J.; Atkinson R. W.; Bauwelinck M.; Bellander T.; Brandt J.; Brunekreef B.; Cesaroni G.; Chen J.; de Faire U.; de Hoogh K.; Fecht D.; Forastiere F.; Gulliver J.; Hertel O.; Hvidtfeldt U. A.; Janssen N. A. H.; Jørgensen J. T.; Katsouyanni K.; Ketzel M.; Klompmaker J. O.; Lager A.; Liu S.; MacDonald C. J.; Magnusson P. K. E.; Mehta A. J.; Nagel G.; Oftedal B.; Pedersen N. L.; Pershagen G.; Raaschou-Nielsen O.; Renzi M.; Rizzuto D.; Rodopoulou S.; Samoli E.; van der Schouw Y. T.; Schramm S.; Schwarze P.; Sigsgaard T.; Sørensen M.; Stafoggia M.; Strak M.; Tjønneland A.; Verschuren W. M. M.; Vienneau D.; Weinmayr G.; Hoek G.; Peters A.; Ljungman P. L. S. Long-term exposure to low-level ambient air pollution and incidence of stroke and coronary heart disease: a pooled analysis of six European cohorts within the ELAPSE project. Lancet Planet. Health 2021, 5, e620–e632. 10.1016/s2542-5196(21)00195-9. [DOI] [PubMed] [Google Scholar]
- Kazemiparkouhi F.; Honda T.; Eum K.-D.; Wang B.; Manjourides J.; Suh H. H. The impact of Long-Term PM2.5 constituents and their sources on specific causes of death in a US Medicare cohort. Environ. Int. 2022, 159, 106988. 10.1016/j.envint.2021.106988. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Shi L.; Lee M.; Liu P.; Di Q.; Zanobetti A.; Schwartz J. D. Long-term Exposure to PM2.5 and Mortality Among Older Adults in the Southeastern US. Epidemiology 2017, 28, 207–214. 10.1097/ede.0000000000000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adar S. D.; Filigrana P. A.; Clements N.; Peel J. L. Ambient coarse particulate matter and human health: a systematic review and meta-analysis. Curr. Environ. Health Rep. 2014, 1, 258–274. 10.1007/s40572-014-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA . Integrated Science Assessment (ISA) for Particulate Matter (Final Report, 2019). US Environmental Protection Agency, Washington, DC, EPA/600/R-19/188, 2019, https://cfpubepagov/ncea/isa/recordisplaycfm?deid=347534 (last access Jan 28, 2020).
- Wang Y.; Xiao S.; Zhang Y.; Chang H.; Martin R. V.; Van Donkelaar A.; Gaskins A.; Liu Y.; Liu P.; Shi L. Long-term exposure to PM2.5 major components and mortality in the southeastern United States. Environ. Int. 2022, 158, 106969. 10.1016/j.envint.2021.106969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, 2021. [PubMed]
- Fischer P. H.; Marra M.; Ameling C. B.; Velders G. J. M.; Hoogerbrugge R.; de Vries W.; Wesseling J.; Janssen N. A. H.; Houthuijs D. Particulate air pollution from different sources and mortality in 7.5 million adults - The Dutch Environmental Longitudinal Study (DUELS). Sci. Total Environ. 2020, 705, 135778. 10.1016/j.scitotenv.2019.135778. [DOI] [PubMed] [Google Scholar]
- Hopke P. K.; Ito K.; Mar T.; Christensen W. F.; Eatough D. J.; Henry R. C.; Kim E.; Laden F.; Lall R.; Larson T. V.; Liu H.; Neas L.; Pinto J.; Stölzel M.; Suh H.; Paatero P.; Thurston G. D. PM source apportionment and health effects: 1. Intercomparison of source apportionment results. J. Expo. Sci. Environ. Epidemiol. 2006, 16, 275–286. 10.1038/sj.jea.7500458. [DOI] [PubMed] [Google Scholar]
- Thurston G. D.; Ito K.; Mar T.; Christensen W. F.; Eatough D. J.; Henry R. C.; Kim E.; Laden F.; Lall R.; Larson T. V.; Liu H.; Neas L.; Pinto J.; Stölzel M.; Suh H.; Hopke P. K. Workgroup Report: Workshop on Source Apportionment of Particulate Matter Health Effects-Intercomparison of Results and Implications. Environ. Health Perspect. 2005, 113, 1768–1774. 10.1289/ehp.7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan T. B.; Seen A. J.; Jacobsen G. E. Levoglucosan as an atmospheric tracer for woodsmoke. Atmos. Environ. 2006, 40, 5316–5321. 10.1016/j.atmosenv.2006.03.023. [DOI] [Google Scholar]
- Cesaroni G.; Porta D.; Badaloni C.; Stafoggia M.; Eeftens M.; Meliefste K.; Forastiere F. Nitrogen dioxide levels estimated from land use regression models several years apart and association with mortality in a large cohort study. Environ. Health 2012, 11, 48. 10.1186/1476-069x-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeftens M.; Beelen R.; Fischer P.; Brunekreef B.; Meliefste K.; Hoek G. Stability of measured and modelled spatial contrasts in NO2 over time. Occup. Environ. Med. 2011, 68, 765–770. 10.1136/oem.2010.061135. [DOI] [PubMed] [Google Scholar]
- Gulliver J.; de Hoogh K.; Hansell A.; Vienneau D. Development and back-extrapolation of NO2 land use regression models for historic exposure assessment in Great Britain. Environ. Sci. Technol. 2013, 47, 7804–7811. 10.1021/es4008849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.