Abstract
Age-associated diseases represent a growing burden for global health systems in our aging society. Consequently, we urgently need innovative strategies to counteract these pathological disturbances. Overwhelming generation of reactive oxygen species (ROS) is associated with age-related damage, leading to cellular dysfunction and, ultimately, diseases. However, low-dose ROS act as crucial signaling molecules and inducers of a vaccination-like response to boost antioxidant defense mechanisms, known as mitohormesis. Consequently, modulation of ROS homeostasis by nutrition, exercise, or pharmacological interventions is critical in aging. Numerous nutrients and approved drugs exhibit pleiotropic effects on ROS homeostasis. In the current review, we provide an overview of drugs affecting ROS generation and ROS detoxification and evaluate the potential of these effects to counteract the development and progression of age-related diseases. In case of inflammation-related dysfunctions, cardiovascular- and neurodegenerative diseases, it might be essential to strengthen antioxidant defense mechanisms in advance by low ROS level rises to boost the individual ROS defense mechanisms. In contrast, induction of overwhelming ROS production might be helpful to fight pathogens and kill cancer cells. While we outline the potential of ROS manipulation to counteract age-related dysfunction and diseases, we also raise the question about the proper intervention time and dosage.
Keywords: Aging, Reactive Oxygen Species, Mitohormesis, drugs, Pharmacology, pleiotropy
1 Highlights
• Age-related diseases are associated with defective ROS homeostasis.
• Approved drugs exhibit pleiotropic effects on ROS homeostasis.
• Enforcement of antioxidant defense mechanisms positively affects cardiovascular and neurodegenerative diseases.
• Overwhelming ROS production might be used to fight pathogens and kill cancer cells.
2 Introduction
2.1 Reactive Oxygen Species Modulation as a Potential Treatment Strategy
The global number of elderly over age 80 will triple from 2015 until 2050. Since aging is associated with the progressive decline of functionality and regenerative potential of tissues, there is an urgent need to prolong a healthy lifespan and to find strategies to delay the onset of age-related dysfunctions such as chronic inflammation and pain, diabetes, cardiovascular diseases (CVD), neurodegenerative diseases, and cancer (Sun et al., 2010; Tejero et al., 2019). On a cellular level, dysfunctions in cellular signaling, proteostasis, autophagy, and mitochondrial homeostasis are closely intertwined in a vicious circle leading to loss of cellular homeostasis and health. For instance, poor protein quality control leads to defective organelles that produce enhanced levels of reactive oxygen species (ROS). These highly reactive chemicals are based on reduced molecular oxygen and include superoxide, hydroxyl radicals, singlet oxygen, and peroxides (Sun et al., 2010; Tejero et al., 2019). In addition to superoxide, nitric oxide synthases (NOS) also produce nitric oxide that interacts with ROS to rapidly produce reactive nitrogen species (RNS) such as peroxynitrite (Sun et al., 2010; Tejero et al., 2019). Due to the electron transport chain (ETC), mitochondria are the cell’s main production sites of ROS (Sun et al., 2010; Tejero et al., 2019). Notably, a reduction in mitochondrial content was found to counteract the process of aging in vivo (Correia-Melo et al., 2016), suggesting that mitochondrial activity gets detrimental during aging. Besides, an enormous number of more than 200 clinical disorders, including type 2 diabetes mellitus (T2DM), degenerative brain impairments like Alzheimer’s disease (AD) and Parkinson’s disease (PD), cardiovascular dysfunction, and cancer, have been associated with early dysregulations in redox homeostasis through so-called ROS and NOS (Kaul et al., 2001; Farah et al., 2018). Besides, experiments in various species revealed an inverse (Schulz et al., 2007; Singh et al., 2011; Ristow and Schmeisser, 2014; Cai et al., 2017) correlation between ROS production rates and lifespan (Sun et al., 2010; Tejero et al., 2019). Align with these findings, increased formation of mitochondrial ROS (mtROS) was postulated as the primary cause of aging in (Harman, 1956) in the Free Radical Theory of Aging (Sun et al., 2010; Tejero et al., 2019). Consequently, in the hope of successfully counteracting age-related diseases, numerous clinical trials tested the impact of antioxidants, including natural or artificial ROS scavenging substances, on the development or progression of age-related diseases. Unfortunately, most clinical trials failed to reveal a benefit from the use of antioxidants or were even related to harmful side-effects on human health like cancer growth. For instance, a randomized controlled trial found that supplementation with vitamin E over 7 years does not prevent cancer or major cardiovascular events but increases the risk for heart failure in patients with vascular diseases or diabetes mellitus (Sun et al., 2010; Tejero et al., 2019). While the application of antioxidants failed to prove a beneficial effect in clinical trials, interventions boosting the body’s antioxidant defense mechanisms are associated with enhanced health and lifespan in various species. A transient ROS burst functions like a vaccine, thereby enabling an adaptational response with enhanced antioxidant defense mechanisms. Notably, behavioral interventions like caloric restriction or physical activity, both known to positively affect health and lifespan, as well as compounds like green tea catechins, long time associated with healthy aging, might trigger these vaccination-like ROS level rises (Merry and Ristow, 2016), (Schulz et al., 2007; Singh et al., 2011; Ristow and Schmeisser, 2014; Cai et al., 2017). Based on these reports, it might be essential to strengthen antioxidant defense mechanisms in advance by low ROS level rises to maintain a proper ROS homeostasis during age and to prevent age-related diseases (Ristow and Schmeisser, 2014). It remains questionable whether selectively targeting certain ROS species within the cell might be an intervention strategy when lacking the potential to induce antioxidant defense mechanisms, for instance, in the case of amyotrophic lateral sclerosis (ALS) (Carrera-Juliá et al., 2020). In contrast, overwhelming ROS production might also be desirable under certain conditions, including fighting off pathogens (Paiva and Bozza, 2014) or cancerous cells (Liou and Storz, 2010).
A strategy to delay the onset of age-related diseases by ROS modulation might require drug administration to still healthy and comparably young individuals for extended periods. Consequently, respective drug candidates are needed to be safe and exhibit a minimum of side effects in the long-term use. To identify drugs least likely to cause harm while still providing benefit, numerous classes of potential geroprotective compounds are currently tested in model organisms like Saccharomyces cerevisiae, Caenorhabditis elegans, and Drosophila melanogaster (Janssens and Houtkooper, 2020). According to the U.S. Food and Drug Administration (FDA), an experimental drug takes 12 years on average to progress from bench to market. Usually, only 5 out of 5000 pre-clinically tested compounds will be used in clinical trials, and only one out of these five clinically tested compounds will receive approval for therapeutic use (Kraljevic et al., 2004). Consequently, it might be worthwhile to evaluate whether approved drugs that are already characterized regarding their long-term safety and side effects might be repurposed to modulate ROS homeostasis. Notably, several drugs in clinical use exhibited pleiotropic antioxidative effects and were found to delay aging in various aging models (Steven et al., 2015; Janssens and Houtkooper, 2020). The current review provides an up-to-date overview of how approved drugs interfere with ROS homeostasis and evaluates their potential to counteract the age-related decline in cellular function.
2.2 Molecular targets
2.2.1 ROS production sites
2.2.1.1 Mitochondrial
Mitochondria are the main production site of ROS (Boveris et al., 1972). Superoxide and hydrogen peroxide are generated as a side product of mitochondrial respiration during electron leakage at the respiratory chain complexes I, II, and III in the inner mitochondrial membrane (Goncalves et al., 2015) ( Table 1). Since excessive mtROS production is associated with age-related dysfunction and pathologies, including tumor growth (Bell et al., 2011) and hypertension (Kimura et al., 2005; Dikalova et al., 2010), mtROS was long time seen as a harmful by-product of respiration only. However, moderate levels of mtROS are crucial for various signaling pathways (Collins et al., 2012), such as response to hypoxia (Guzy and Schumacker, 2006), cell differentiation (Mandal et al., 2011), autophagy (Lee et al., 2012a), inflammation (Zhou et al., 2011), and immune response (West et al., 2011). Notably, mitochondrial damage-associated molecular patterns (mtDAMP), which are indicators of mitochondrial dysfunction, play a critical role in ROS-mediated inflammatory processes. For example, oxidated mtDAMPs such as mitochondrial DNA (mtDNA) were shown to activate the NLR family pyrin domain containing 3 (NLRP3) inflammasome, an essential component of the innate immune system (Shimada et al., 2012; Zhong et al., 2013).
TABLE 1.
Source and localization of reactive oxygen species (ROS).
| Molecule | Oxidant formed | Enzymes | Localization |
|---|---|---|---|
| Molecular oxygen (O2) | Superoxide (O2 −) | NOX1-5, DUOX1-2 | Plasma membrane and intracellular membranes |
| XO, XDH | Cytoplasm | ||
| ETC | Inner mitochondrial membrane | ||
| Superoxide (O2 −) | Hydrogen Peroxide (H2O2) | SOD1 | Cytoplasm, peroxisomes, lysosomes, nucleus |
| SOD2 | Mitochondrial matrix | ||
| SOD3 | Extracellular space | ||
| Molecular oxygen (O2) | Hydrogen Peroxide (H2O2) | MAO | Outer mitochondrial membrane |
Monoamine oxidase (MAO): The mitochondrial monoamine oxidases (MAOs) A and B degrade a variety of neurotransmitters, including norepinephrine, serotonin, dopamine, and tyramine (Sub Laban and Saadabadi, 2021), by catalyzing the oxidative deamination of their amines (Tipton, 2018). Thereby, hydrogen peroxide is generated as a side product at the outer mitochondrial membrane that possibly contributes to oxidative stress (Table 1). Nonetheless, there is no clinical proof that MAO inhibitors reduce levels of toxic and prooxidant MAO products until now (Tipton, 2018). In the clinic, selective MAO-A inhibitors are used as amine-depleting drugs against depression (Kline, 1958; Zeller et al., 1959). In contrast, selective MAO-B inhibitors work as dopamine-sparing compounds against PD (selective MAO-B inhibitors) (Birkmayer et al., 1983).
2.2.1.2 Non-Mitochondrial
NADPH oxidase 1–7: NADPH oxidases (NOXs) are transmembrane enzymes that specifically generate radical superoxide anions by reducing molecular oxygen using nicotinamide adenine dinucleotide phosphate (NADPH) (Table 1). The NOX family consists of 7 members, including NOX1-5, as well as the dual oxidases 1 and 2 (DUOX1; DUOX2), which are expressed in endothelial cells, vascular smooth muscle cells, cardiac myocytes and fibroblasts, adipocytes, macrophages, stem cells, and adventitial fibroblasts (Lassègue et al., 2012). Although the prooxidant role of NOXs is crucial in cellular physiology, such as differentiation, proliferation, apoptosis, inflammatory responses, host defense, and redox signaling (Lassègue et al., 2012; Vermot et al., 2021), increased NOX activity was also correlated with several pathologies such as neurodegenerative diseases, including AD and PD (Sorce and Krause, 2009; Popa-Wagner et al., 2013; Vermot et al., 2021) and cancer (Juhasz et al., 2009; Meitzler et al., 2014; Wang et al., 2015; You et al., 2018). Targeting NOXs might therefore represent a suitable approach for disease-specific therapies (Spencer and Engelhardt, 2014).
Xanthine oxidoreductase: The xanthine oxidoreductase (XOR) belongs to the family of molybdoenzymes (Kisker et al., 1997) and is expressed in human tissues such as the liver, small intestine, mammary gland (Linder et al., 1999), and heart (Muxfeldt and Schaper, 1987; Abadeh et al., 1993; Vickers et al., 1998). XOR can be interconverted into xanthine dehydrogenase (XDH) and xanthine oxidase (XO) (Stirpe et al., 1969). Both XDH and XO are involved in the metabolism of hypoxanthine/xanthine to uric acid during purine degradation (Xu et al., 1996; Okamoto et al., 2013) upon generation of superoxide anion (Battelli et al., 2016) (Table 1). Besides the involvement of XOR in purine catabolism, there is evidence for a broader range of benefits. For example, it was speculated that XOR-derived uric acid positively impacts oxidative stress-associated aging and cancer and thus promotes the extension of life span in humans (Ames et al., 1981).
Nitric oxide synthase (NOS): The family of nitric oxide synthases includes neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS). These enzymes generate nitric oxide upon L-arginine utilization which plays an essential part in vascular regulation, inflammation, and intracellular signaling. Besides these physiological functions, nitric oxide also represents a harmful contributor to oxidative stress as it can react to cytotoxic peroxynitrite in the presence of oxygen (Adams et al., 2015).
2.2.2 The Antioxidant System
The cellular antioxidant system is subdivided into enzymatic antioxidants, including the glutathione and thioredoxin system, and nonenzymatic antioxidants, such as dietary vitamins. Polymorphisms in involved proteins such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX) were associated with various human metabolic disorders such as diabetes, CVD, and cancer (Crawford et al., 2012; Hebert-Schuster et al., 2012), emphasizing the critical role of enzymatic antioxidant systems.
Superoxide dismutase 1–3: The family of superoxide dismutases (SODs) consists of the copper-zinc superoxide dismutase (SOD1 gene) localized in the cytoplasm, peroxisomes, lysosomes, and the nucleus (Chang et al., 1988; Keller et al., 1991; Crapo et al., 1992; Liou et al., 1993), as well as the manganese superoxide dismutase (SOD2 gene) present in the mitochondrial matrix (Weisiger and Fridovich, 1973), and the extracellular superoxide dismutase (SOD3 gene) (Marklund, 1984). SODs are the only class of enzymes catalyzing the reduction of superoxide to hydrogen peroxide and O2 (Genestra, 2007) and thus playing a significant role in redox regulation (Table 1). Malfunction of SOD is associated with several clinical pathologies. For example, SOD1 mutations were able to mimic ALS-associated dysregulation in several mouse models (Bruijn et al., 2004). In addition, SOD2 mutations are associated with cardiomyopathy, sporadic motor neuron defects, and cancer (Hiroi et al., 1999). On the other hand, overexpression of SOD1 proved beneficial against neurological injuries and brain diseases in animal studies (Kamii et al., 1999; Morita-Fujimura et al., 2000; Sugawara et al., 2002; Yu et al., 2006; Endo et al., 2007).
Catalase: Human CAT is a peroxisomal enzyme that detoxifies hydrogen peroxide by catalyzing its reduction to H2O and O2 (Putnam et al., 2000). Overexpression of catalases in mitochondria results in extended life span with attenuated age-related pathologies (Schriner et al., 2005; Treuting et al., 2008; Dai et al., 2009; Olsen et al., 2013) and protects against CVD (Yang et al., 2003; Yang et al., 2009; Maiellaro-Rafferty et al., 2011) and diabetic nephropathy (Brezniceanu et al., 2007; Shi et al., 2013) in mice. In contrast, patients that lack catalase expression are prone to develop T2DM (Avraham et al., 1988).
Thioredoxin system: The thioredoxin system involves several players and types of redox reactions. In first-line are peroxiredoxins (PRX1-6), a family of peroxidases (Wood et al., 2003; Flohé et al., 2011; Rhee and Woo, 2011) that catalyze the reduction of hydrogen peroxide by oxidation of their active PRX disulfides. Regeneration of oxidized PRX is achieved by reduction through small thiol-disulfide oxidoreductases, so-called thioredoxins (TRX). Thioredoxins are eventually reduced by thioredoxin reductases (TR) upon utilization of the reductive potential of reduced NADPH (Lillig and Holmgren, 2007; Lu and Holmgren, 2014). Strengthening the thioredoxin system manifests in several health-beneficial alterations. For example, overexpression of PRX3 reduced cardiac failure upon myocardial infarction in murine heart mitochondria (Matsushima et al., 2006) and counteracted hyperglycemia and glucose intolerance of mice (Chen et al., 2008). TRX1 was detected in the plasma during inflammation and oxidative stress (Nakamura et al., 1996) and proposed as antioxidant therapy (Nakamura et al., 2009; Watanabe et al., 2010; Matsuo and Yodoi, 2013). Moreover, TRX1 and TRX80 displayed protective effects in AD in humans (Gil-Bea et al., 2012).
Glutathione system: Glutathione peroxidases (GPXs) use a reducing equivalence of glutathione (GSH) to reduce peroxides and hydroxy radicals into nontoxic derivatives (Brigelius-Flohé, 1999; Flohé et al., 2011). The resulting oxidized glutathione (GSSG) is reduced to GSH by glutathione reductase (GR) through the oxidation of NADPH to NADP+ (Birben et al., 2012). Another representative of the glutathione system is the class of glutaredoxins (GRXs), which are oxidoreductases mainly responsible for the reduction of GSH-disulfides (Holmgren, 1976) and deglutathionylation of S-glutathionylated proteins (Holmgren et al., 2005). The glutathione system is the principal regulator of the cellular redox balance, and the GSH/GSSG ratio is used as an oxidative stress biomarker for several diseases, including CVD, AD, PD, ALS, multiple sclerosis (MS), and cancer (Frijhoff et al., 2015). GPX1 knock-out increases oxidative stress and the prevalence of AD-associated neurotoxicity and heart ischemia-reperfusion injury in mice (Yoshida et al., 1997; Fu et al., 1999; Klivenyi et al., 2000; Crack et al., 2006; Lim et al., 2009). In contrast, GPX1 overexpression protected against oxidative stress, cerebral ischemia/reperfusion damage, and neurodegenerative pathologies such as PD (Weisbrot-Lefkowitz et al., 1998; Sheldon et al., 2004; Ridet et al., 2006). Several clinical trials linked polymorphisms of GPX and consequently decreased enzyme activity to an increased risk for T2DM (Ramprasath et al., 2012), cardiovascular dysregulations in T2DM patients (Hamanishi et al., 2004), breast cancer (Ravn-Haren et al., 2006), and colorectal adenomas (Hansen et al., 2005).
Nonenzymatic antioxidants: Nonenzymatic antioxidants include low molecular mass molecules such as carotenoids, vitamin A, ascorbic acid (vitamin C), α-tocopherol (vitamin E), polyphenols, minerals such as selenium and zinc, as well as various drugs including acetylcysteine (Pisoschi and Pop, 2015). The antioxidant potential of vitamin A, C, and E, as well as phenolic acids, is given by their ability to scavenge free radicals in the form of ROS and RNS (Burton and Ingold, 1984; Burton and Traber, 1990). In addition, carotenoids and vitamin E prevent lipid peroxidation by direct reduction of peroxyl radicals (Burton and Ingold, 1984). Minerals, instead, are crucial components of antioxidant enzymes and consequently crucial for their activity (Tabassum et al., 2010). Zinc, for example, is an inhibitor of NADPH oxidase and a part of superoxide dismutase and thus contributes to the antioxidant system. Although more than 100 clinical trials tested nutritional interventions with vitamins, polyphenols, and minerals have been conducted during the last twodecades (Bjelakovic et al., 2007; Bjelakovic et al., 2012), the vast majority failed to reveal the beneficial effects of dietary antioxidant supplementation (Goodman et al., 2011; Bjelakovic et al., 2012; Halliwell, 2013), questioning the total suppression of ROS generation as a therapeutical approach.
3 The implication of Reactive Oxygen Species in Age-Related Diseases
3.1 Cardiovascular Diseases
3.1.1 Clinical Significance
Cardiovascular diseases (CVD) like ischemic heart diseases and stroke are the leading disease burden worldwide, causing the majority of cases in global mortality and contributing to various disabilities. There has been a worrying trend, with cases of CVD roughly doubling from 1990 to 2019, reaching 18.6 million (Roth et al., 2020). Deterioration of ROS homeostasis is a common hallmark in CVD, and ROS-modified molecules might even serve as biomarkers for the progression of CVD. For instance, clinical trials have revealed an association between increased levels of circulating oxidative low-density lipoprotein (LDL) and atherosclerotic CVD (Gao and Liu, 2017). Moreover, 8-hydroxy-2-deoxyguanosine, a marker for oxidative DNA damage, is significantly increased in the serum patients with dilated cardiomyopathy (Kono et al., 2006), and the concentration in the urine correlates with heart failure (Kobayashi et al., 2011). Besides, 8-iso-prostaglandin F2α, a by-product of lipid peroxides generated during oxidative stress, is increased in patients with symptomatic heart failure and correlated with the functional severity of heart failure (Mallat et al., 1998). In contrast, another primary lipid peroxidation product, 4-hydroxy-2-nonenal-modified protein, was found to be elevated in the myocardium of hypertrophic cardiomyopathy patients (Nakamura et al., 2005). Despite the strong implication of ROS in CVD, clinical trials have failed to provide evidence for a therapeutic benefit of potent antioxidants in treating CVD so far (Panth et al., 2016), potentially due to blocking signaling function of ROS and by preventing ROS-induced upregulation of ROS defense mechanisms.
3.1.2 Cellular Mechanisms and Signaling
ROS have a crucial signaling function in the cardiovascular system. For instance, ROS contribute to the signal transduction pathway of angiotensin II, causing cardiac growth and hypertrophy in neonatal rat cardiomyocytes (Shih et al., 2001). Moreover, ROS adjust the iron homeostasis in response to catecholamines in cardiomyocytes, a process essential to maintaining proper metabolic activity (Costa et al., 2009). Notably, enhanced ROS production is associated with left ventricular hypertrophy, and heart failure in experimental guinea pig models with left ventricular hypertrophy exhibited a progressive expression increase in several NADPH oxidase subunits (Li et al., 2002). Notably, the expression of NOX2 was also found to be increased in infarcted areas but unchanged in unaffected regions of cardiac samples from patients who had died from acute myocardial infarction (Krijnen et al., 2003). Platelet-derived ROS function as signaling molecules but might induce a vicious circle resulting in a platelet procoagulant phenotype and apoptosis, enhancing the thrombotic risk (Masselli et al., 2020). Besides, a deterioration of ROS defense mechanisms might contribute to the genesis and progression of CVD. Experiments in a cardiomyocyte-specific SOD2 deficient mouse strain revealed that deficiency of SOD2 results in increased ROS levels and subsequent overproduction of electrophilic aldehydes, which serve as mediators of mitochondrial dysfunction and boost cardiomyopathy (Sharma et al., 2020). Besides, attenuated SOD2 activity resulted in enhanced mitochondrial oxidative stress and plaque instability in hyperlipidemic mice during aging (Vendrov et al., 2017). Enhanced ROS production and decreased ROS detoxification might also facilitate the formation of peroxynitrite that harms the vascular endothelium, smooth muscle, and myocardium (Pacher and Szabo, 2006). Besides, peroxynitrite formation reduces the amount of available nitric oxide that inhibits platelet activation and aggregation, cell adhesion molecule expression, and vascular smooth muscle proliferation and is, therefore, a crucial vasoprotective substance (Naseem, 2005).
3.2 Type 2 Diabetes Mellitus
3.2.1 Clinical Significance
Type 2 diabetes mellitus (T2DM) is characterized by insufficient insulin secretion, an uncontrolled rise in blood glucose levels, and insulin resistance of peripheral tissues (Rochette et al., 2014). Besides genetic components and age, obesity, diminished physical activity, chronic inflammation, and elevated cholesterol and triglyceride levels are the main primary risk factors of T2DM (Zeller et al., 2008; Olsson et al., 2011; Verdile et al., 2015), emphasizing the importance of functional metabolic regulations. An untreated T2DM condition and prolonged periods of high blood glucose may cause damage to the vascular system, subsequently leading to stroke, peripheral vascular diseases, neuropathy, retinopathy, and nephropathy (Wallace and Matthews, 2004; Asmat et al., 2016). Notably, the severity and T2DM-related mortality are linked to vascular complications and positively correlate with the degree of oxidative stress and ROS (Andreev and Rybakov, 1975; Domingueti et al., 2016). Cellular damage was associated with a ROS-dependent activation of stress pathways such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), c-jun N-terminal kinase (JNK)/stress-activated phosphor-kinase (SAPK), and p38 MAPK in T2DM with vascular complications (Evans et al., 2003). Other publications could measure elevated levels of NADPH and superoxide during vascular dysfunction in diabetic patients (Guzik et al., 2002; Ergul et al., 2005). Further evidence of increased ROS levels manifests in increased lipid peroxidation (Davì et al., 1999; Bandeira et al., 2012) and carbonylation of serum proteins of type 2 diabetes mellitus individuals (Pandey et al., 2010; Gradinaru et al., 2013). Besides, oxidative markers such as F2-isoprostane, nitrotyrosine (Ceriello et al., 2001; Odegaard et al., 2016), and glycated hemoglobin (HbA1c) (Ryden et al., 2013) are commonly found in plasma, urine, and tissues of T2DM patients and serve as biomarkers for hyperglycemia.
3.2.2 Cellular Mechanisms and Signaling
ROS signaling plays a significant role in the metabolic activity of pancreatic β-cells (Ahmed Alfar et al., 2017), as well as downstream insulin signaling and glucose uptake in adipocytes by activation of the phosphoinositol 3-kinase and protein kinase B (AKT) (Mahadev et al., 2001), most likely mediated by NOX4-dependent ROS generation (Mahadev et al., 2004). Thereby, the high ROS sensitivity of pancreatic β cells is crucial for a functional physiological metabolism, as well as β cell regeneration and proliferation (Ahmed Alfar et al., 2017; Wang and Wang, 2017). However, the comparably low levels of antioxidant proteins such as GPX, CAT, SOD, and TR (Evans et al., 2003; Newsholme et al., 2007), make β-cells highly vulnerable to oxidative stress and ROS overload provoked by stimulation of the respiratory chain activity or NOX activity (Newsholme et al., 2007; Newsholme et al., 2012). Chronic oxidative stress impairs the cellular function of β-cells. It results in apoptotic cell death via p38 MAPK, JNK, and NF-kB signaling (Heimberg et al., 2001; Gurzov and Eizirik, 2011), reducing insulin secretion and provoking hyperglycemia. In turn, hyperglycemia excessively generates electron donors in the tricarboxylic acid (TCA) cycle and thus, promotes hyperpolarization of the mitochondrial membrane potential (Ψ mito) and adenosine triphosphate (ATP) generation, followed by inhibition of complex III of the respiratory chain and electron accumulation at coenzyme Q. Consequently, oxygen is only partially reduced, boosting the production of superoxide radicals (Korshunov et al., 1997; Nishikawa et al., 2000; Brownlee, 2001). In addition, inflammation-associated ROS was shown to inhibit insulin receptor activity, the respective signaling, and consequently the response to insulin (Newsholme et al., 2014; Verdile et al., 2015), eventually leading to insulin resistance. Furthermore, it was shown that vascular homeostasis and anti-inflammatory processes are impaired in diabetes due to poor production of nitric oxide (Shi and Vanhoutte, 2017), potentially due to enhanced peroxynitrite formation (Caldwell et al., 2015). The interplay of poor NO levels, increased peroxynitrite concentrations, and elevated production of ROS does consequently further increase oxidative stress in diabetes (Pacher and Szabo, 2006).
3.3 Neurodegenerative Diseases
3.3.1 Clinical Significance
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by neuroinflammation, synaptic disruption, and abnormalities in mitochondrial structure and function (Hirai et al., 2001; Selkoe, 2002; Swerdlow and Khan, 2004; Barsoum et al., 2006; Wyss-Coray, 2006; Swerdlow et al., 2010), resulting in loss of memory and cognitive decline (Bertoni-Freddari et al., 1990; DeKosky et al., 1996; Tampellini and Gouras, 2010). (Walsh and Selkoe, 2007). As a consequence of an increasing global life expectancy (Jaul and Barron, 2017) and due to a growing population, the number of patients suffering from AD is predicted to increase in the future dramatically. While 35.6 million people were diagnosed with dementia in 2010, this number is estimated to reach 115.4 million people by 2050 (Prince et al., 2013) and thus strongly impacts worldwide health systems. One hallmark of AD is amyloid-β (Aβ) plaque formation due to abnormal processing of the amyloid precursor protein. Under pathological conditions, the amyloid precursor protein is cleaved into Aβ fragments (LaFerla et al., 2007), which oligomerize into soluble aggregates and subsequently accumulate to insoluble and toxic Aβ plaques. Consequently, oxidative damage of proteins, DNA, RNA, and lipids was found in AD patients’ brains (Reddy, 2006; Chaturvedi and Beal, 2008; Reddy and Beal, 2008). Thereby, oxidation productions such as 8-hydroxyguanosine and heme oxygenase serve as diagnostic markers in AD patients (Chaturvedi and Beal, 2008). Another important marker for AD is nitrotyrosine, a product of protein oxidation via nitric oxide (Good et al., 1996; Smith et al., 1997). Moreover, peroxynitrite production seems to be responsible for lysine, arginine, proline, and histidine oxidation in AD patients (Stadtman, 1990; Smith et al., 1997). Dysregulations in AD are undoubtedly associated with an imbalance in ROS homeostasis and subsequent oxidative damage in the brain of aged organisms (Migliaccio et al., 1999; Giorgio et al., 2005; Calkins et al., 2012). However, the molecular mechanisms are still elusive.
Parkinson’s disease (PD) is the fastest-growing neurodegenerative disease (Collaborators GBDN, 2019), and the number of people affected is predicted to double by 2040 (Dorsey and Bloem, 2018). PD is characterized by a progressive loss of dopaminergic neurons in the brain region substantia nigra (SN), leading to motor deficiencies such as tremor, rigidity, and bradykinesia. Another hallmark of PD is the development of insoluble inclusions consisting of aggregated α-synucleins, so-called Lewy bodies (Spillantini et al., 1997; Bellucci et al., 2012; Bellucci et al., 2016). While monomeric and tetrameric α-synucleins fulfill their physiological function as transport molecules, the formation of oligomers and fibrils is caused by α-synuclein mutation and contributes to pathological dysregulations (Bartels et al., 2011; Marques and Outeiro, 2012). Besides mutations in α-synuclein, familiar forms of PD might exhibit mutations in PTEN-induced kinase 1 (PINK) and E3 ubiquitin ligase (PARKIN), two proteins involved in autophagy (Lazarou et al., 2015; Pickrell and Youle, 2015), Parkinson’s disease protein 1 (PARK7 a redox-chaperone acting as oxidative stress sensor (Canet-Avilés et al., 2004), (Zondler et al., 2014), and leucine-rich repeat kinase 2 (LRRK2). Notably, these proteins are relevant for mitochondrial function and ROS homeostasis (Polymeropoulos et al., 1997; Bonifati et al., 2003; Valente et al., 2004; Gilks et al., 2005; Nichols et al., 2005). Indeed, the accumulation of damaged mitochondria is a further determinant for the mediation of PD pathology (Koentjoro et al., 2017). Neurons in the SN are highly vulnerable to oxidative stress and neurodegeneration, as seen in healthy elderly brains that contain double the number of oxidized proteins compared to other brain regions (Floor and Wetzel, 1998). Additionally, postmortem analyses of healthy aged brains compared to young controls revealed a decrease in SOD, GPX, and glutathione reductase activities (Venkateshappa et al., 2012), suggesting age-related loss of antioxidant enzyme activity. Another source of ROS represents the oxidative metabolism of dopamine, as hydrogen peroxide is produced as a side product of oxidative deamination of dopamine by MAO (Goldstein et al., 2013; Meiser et al., 2013). Furthermore, PD brains exhibit iron-enriched neurons in the SN (Dexter et al., 1987; Dexter et al., 1989; Michaeli et al., 2007; Pyatigorskaya et al., 2015), making this brain region highly vulnerable to oxidative stress (Brian J. Tabner et al., 2001; Jellen et al., 2013). Together with dopamine, labile iron is part of a prooxidant synergic interplay in aged SN (Hare and Double, 2016), leading to increased production of highly toxic dopamine-o-quinones (Tse et al., 1976; Graham, 1978; Zhou et al., 2010). The dopamine-o-quinones derivatives tetrahydroisoquinoline salsolinol and 6-hydroxydopamine were shown to increase ROS and oxidative stress by impairing ETC function (Su et al., 2013; Puspita et al., 2017). Chelation of iron as a treatment for PD was successful in animal models (Devos et al., 2014) and is currently tested in patients with early-stage PD by FAIRPARKII (ClinicalTrials.gov Identifier: NCT02655315).
3.3.2 Cellular Mechanisms and Signaling
Alzheimer’s disease (AD): Neuronal function is strongly dependent on a high energy supply. Based on calculations, neurons use up to 50% of their ATP for homeostasis and re-establishment of the ion gradient and 30% for synaptic transmission (Ames, 2000; Attwell and Laughlin, 2001). Consequently, neurons strongly rely on oxygen availability and functional mitochondrial oxidative phosphorylation for ATP generation. However, high oxygen levels and enhanced mitochondrial activity provoke the formation of ROS (Zorov et al., 2014). In addition, neurons contain elevated levels of lipids and comparably low amounts of antioxidant enzymes, making them highly vulnerable to oxidative stress (Jang et al., 2010). Also, the synaptic transmission that includes vesicle formation is energy-intense (Attwell and Laughlin, 2001). Therefore, ATP-generating mitochondria need to undergo axonal transport from the soma to the distant periphery to supply synapses with sufficient energy. Notably, impairment of this process was found in a range of neurodegenerative diseases such as ALS, Huntington’s disease, AD, and PD (Martin, 2011; Schon and Przedborski, 2011; Reddy and Shirendeb, 2012), once again emphasizing the importance of mitochondria for neuronal function. Several authors suggest that lack of mitochondrial transport represents an early event during neurodegeneration and the pathology of AD (Rui et al., 2006; Wang et al., 2010a; Du et al., 2010; Calkins and Reddy, 2011). Importantly, dysfunctional mitochondrial transport, enormous mitochondrial fragmentation, attenuated synaptic ATP, and synaptic dysfunction in AD neurons were associated with increased oxidative stress (Reddy et al., 2012) (Reddy, 2006). Similar observations were found in the brains of AD patients, which exhibited attenuated ATP levels, elevated levels of free radicals, OXPHOS disruptions, and mitochondrial dysfunctions (Gibson et al., 1998; Maurer et al., 2000; Wang et al., 2005; Devi et al., 2006; Wang et al., 2008; Reddy, 2009; Reddy et al., 2010).
Parkinson’s disease (PD): The susceptibility towards oxidative damage is based on several specific features of dopaminergic neurons. First, neurons of the SN are comparably large and unmyelinated (Pissadaki and Bolam, 2013), resulting in high demand for ATP to maintain the Ψ mito, action potential, and synaptic transmission. Secondly, SN dopaminergic neurons generate constant action potentials autonomously without dependency on synaptic input to maintain dopamine levels in surrounding brain regions (Grace and Bunney, 1983; Romo and Schultz, 1990). Thirdly, dopaminergic neurons are Ca2+ pacemakers with constant buffering activity (Olson et al., 2005). Increased Ca2+ levels might boost ETC activity leading to a pathological increase in ROS and the initiation of apoptotic pathways (Joza et al., 2001; Malhotra and Kaufman, 2007). In summary, all these processes contribute to an immense metabolic burden and the need for functional mitochondria, ROS′ major primary production site. Besides that, the accumulation of α-synuclein itself provokes the generation of mtROS. Data of transgenic mouse models could prove that α-synuclein aggregation diminishes complex I activity and increase ROS levels (Hsu et al., 2000; Martin et al., 2006) before dopaminergic neuron loss (Subramaniam et al., 2014). Inhibition of complex I, ROS production, and neuronal cell death via apoptosis and autophagy was also linked to the presence of PD-associated gene mutations [α-synuclein (SNCA), LRRK2, PARK7, PARK2, PINK1] (Müftüoglu et al., 2004; Yamada et al., 2004; Iaccarino et al., 2007; Hayashi et al., 2009; Ho et al., 2009; Gusdon et al., 2012; Venderova and Park, 2012; Dias et al., 2013; Blesa et al., 2015; Gegg and Schapira, 2016). Oxidative stress by complex I inhibition can be pharmacologically mimicked by environmental toxins, including 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridin (MPTP) and rotenone (Mizuno et al., 1987; Schapira et al., 1990; Ramsay et al., 1991; Richardson et al., 2005), leading to superoxide generation and reduction of ATP synthesis. Treatment with these substances shows similar upregulation of cell death pathways as seen in PD (Hayley et al., 2004; Clayton et al., 2005; Perier et al., 2005).
3.4 Cancer
3.4.1 Clinical Significance
While malignant tumors can occur at any age, cancer disproportionately strikes the elderly aged 65 years or older. The median age of cancer-related death is 71–77 years, independent of sex and race (Yancik, 2005). Consequently, in an aging society, the cancer burden is still expected to increase in the following centuries. The hallmarks of cancer include genome instability, mutations, replicative immortality, and cell death resistance, as well as angiogenesis, deregulation of cellular metabolism, tumor-promoting inflammation, and avoidance of immune destruction. Therefore, cancer cells exhibit high rates of proliferation, invasion, and metastasis (Somarelli et al., 2020). Mitochondria provide energy and building blocks for new cells and modulate ROS homeostasis, oncogenic signaling, and apoptosis. Consequently, these organelles are essential in cancer development and progression (Zong et al., 2016). While the Warburg effect suggested that cancer is accompanied by mitochondrial defects, forcing cancer cells into increased aerobic glycolysis, it was proven in the following years that cancer cells still exhibit functional mitochondria and also rely on mitochondrial respiration to obtain sufficient energy (Mazurek, 2011). Mitochondria also seem to play a crucial role in the development of tumors by triggering cell integrity loss through mutations in mtDNA and the generation of ROS (Badrinath and Yoo, 2018). In line with these in vitro findings, clinical trials revealed that oxidative stress correlates with the development and progression of various cancer types, including colorectal cancer (Boakye et al., 2020; Janion et al., 2020), bladder cancer (Wigner et al., 2021), breast cancer (Lee et al., 2017), and prostate cancer (Oh et al., 2016).
3.4.2 Cellular Mechanisms and Signaling
Cancer cells exhibit significantly higher levels of ROS than corresponding non-cancerous cells. This increase in ROS is triggered by enhanced metabolic rate, gene mutation, and hypoxia (Perillo et al., 2020). Adaption to these excessive ROS conditions is achieved by cancer cells through enhanced antioxidant capacity. Therefore, the main transcription factor involved in the antioxidant defense, the nuclear factor erythroid 2-related factor 2 (NRF2), is often upregulated in cancer cells and helps via boosting antioxidant defense mechanisms the proliferation of cancer cells (Jaramillo and Zhang, 2013). Besides, glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. For instance, inhibition of glutathione synthesis resulted in enhanced levels of thioredoxins. Consequently, just combined blockage of glutathione and thioredoxins resulted in cancer cell death in vitro and in vivo (Harris et al., 2015). Besides, the antioxidant potential might be further promoted through NADPH production. Interestingly, pyruvate kinase M2 was found to be predominantly present in a dimeric state in cancer cells, exhibiting less activity and forming a bottleneck in glycolysis. Consequently, the glucose flux gets redirected to the pentose phosphate pathway, where NADPH is produced (Mazurek et al., 2005; Hawk et al., 2016). Thereby, the antioxidant potential of cancer cells gets again improved.
3.5 Inflammation and Pain
3.5.1 Clinical Significance
Inflammation is the body’s response to pathogens, external injuries, damaged cells, or irritants. Thereby, immune cells release various inflammatory mediators to dilate small blood vessels. The thereby increased blood flow allows the transport of immune system cells to the affected tissues for repair (Abdulkhaleq et al., 2018). The release of proinflammatory and immune-active substances like cytokines and chemokines can result in nerve irritation and pain signals (Abdulkhaleq et al., 2018). For instance, the proinflammatory mediator histamine is well-known to induce itching (Shim and Oh, 2008). While injuries or infections might cause intermittent increases in inflammation, chronic systemic inflammation represents the main cause for diseases such as CVD, diabetes mellitus, cancer, chronic kidney and liver diseases, and autoimmune and neurodegenerative disorders, which strongly contribute to disability and mortality worldwide (Furman et al., 2019). Inflammatory processes induce oxidative stress and reduce cellular antioxidant capacity, causing loss of tissue integrity, impaired protein function, and DNA damage. Consequently, the combination of chronic inflammation and oxidative stress is a major hallmark of age-related diseases (Khansari et al., 2009). For instance, oxidative stress parameters like enhanced levels of malonaldehyde and superoxide anions and decreased SOD activity correlated with inflammation markers, including high sensitive C-reactive protein and fibrinogen, in patients with coronary heart disease (Kotur-Stevuljevic et al., 2007). Also, a correlation between inflammation and ROS levels was suggested as a universal parameter in patients with infective endocarditis (Ostrowski et al., 2012). Besides, spontaneous ROS production by neutrophils was associated with low-grade inflammation in the elderly (Ogawa et al., 2008), emphasizing that ROS production might represent a crucial aspect in age-associated immune dysregulation.
3.5.2 Cellular Signaling and Mechanisms
While pro-inflammatory processes increase ROS production (Yang et al., 2007), ROS are, in turn, also involved in the activation of pro-inflammatory processes such as mast cell activation and subsequent release of pro-inflammatory mediators (Son et al., 2006). For instance, hydrogen peroxide was shown to modulate the NF-kB signaling pathway associated with an inflammatory response. Several ROS-induced modifications were found at NF-kB, leading to activation of the NF-kB at the early phase of oxidative stress but attenuation of NF-kB activity in case of sustained stress (Lingappan, 2018). In addition, ROS are also utilized by inflammatory agonists as signaling molecules. For instance, oxidative stress produced by polymorphonuclear neutrophils during inflammation causes the opening of inter-endothelial junctions and thereby allows inflammatory cells to pass the endothelial barrier. Then, the migrated inflammatory cells contribute to the clearance of pathogens and also lead to tissue injury. Besides, ROS quickly react with nitric oxide to generate reactive nitrogen species, which induces nitrosative stress, and so adds to the pro-inflammatory impact of ROS (Mittal et al., 2014). Moreover, mitochondrial ROS production was found to convey lipopolysaccharide-driven production of proinflammatory cytokines (Bulua et al., 2011) and was identified as a major step in the activation of inflammasomes, multiprotein oligomers, which promote the secretion of pro-inflammatory cytokines (Yang et al., 2019). Acute inflammations are often induced by infections caused by bacteria, viruses, protozoa, or fungi. Thereby, ROS are used by the immune system to protect the host organism against infections. In response to inflammatory processes, phagocytes reside within the tissue to phagocyte microbes. Dependent on the microbe, phagocytes generate ROS signals to directly kill the microbes and activate ROS bursts within the cell to induce respective signaling cascades in order to eliminate pathogenes by non-oxidative mechanisms (Paiva and Bozza, 2014). Besides, ROS and peroxynitrite are also directly engaged in the nociceptive signaling by altering protein kinase A and calcium/calmodulin-dependent protein kinase type II-mediated signaling, glutamatergic neurotransmission, transient receptor potential cation channel subfamily V member 1 (TRPVI) sensitization, and cyclooxygenase enzyme (COX) activation (Salvemini et al., 2011). Interestingly, clinical trials revealed that administration of SOD1, known as the drug orgotein, reduced symptoms of osteoarthrosis of the knee joint significantly better than methylprednisolone acetate (Gammer and Brobäck, 1984), emphasizing the crucial role of ROS in the development of pain and the potential of ROS manipulation as a treatment of pain.
4 Reactive Oxygen Species Modulation by Approved Drugs
4.1 Cardioprotective Drugs
4.1.1 Beta-Blocker
Beta-blockers comprise compounds that inhibit the activation of β-adrenergic receptors by endogenous catecholamines. The first beta blocker, propranolol, was approved in the 1960s to treat angina pectoris and revolutionized the treatment of CVD. Nonselective β1 and β2 adrenoreceptor blockers like propranolol and carvedilol and specific β1 adrenoreceptor blockers such as atenolol, metoprolol, and bisoprolol are nowadays widely used to ameliorate cardiac function and reduce the mortality rate in heart failure patients (Srinivasan, 2019). Moreover, beta-blockers are widely used medications to treat hypertension. Stimulation of β1 receptors induces positive chronotropic and inotropic effects in the heart muscle and modulates arterial vasoconstriction by the release of renin in the kidney. β2-adrenergic receptors are located in various organs, including the liver and vascular smooth muscle, and β2 receptor activation causes smooth muscle relaxation (Farzam and January 2021). Beta-blockers affect ROS homeostasis indirectly via different mechanisms. First, inhibition of β1 adrenergic receptors prevents oxidative stress due to catecholamine-induced reactions. Notably, ROS might be essential in conveying the action of catecholamines by adjusting the homeostasis of mitochondrial iron, critical for rate-limiting enzymes of the TCA cycle and for the mitochondrial electron transport chain (Tapryal et al., 2015). However, elevated levels of the catecholamines adrenaline and noradrenaline are associated with enhanced oxidative stress and were found in various cardiovascular dysfunctions and diseases, including tachycardia, arrhythmias, heart failure, and ischemic reperfusion injury (Nakamura et al., 2011). For instance, incubation of freshly isolated rat cardiomyocytes with adrenaline boosted the activity of mitochondrial complexes and caused increased expression of SOD2 after 3 h of incubation, potentially due to enhanced electron leakage from the ETC and a boost in ROS production (Costa et al., 2009). Second, beta-blocker might reduce ROS production indirectly by lowering mechanical stress in vessels. Cyclic stretching increased ROS and a ROS-dependent activation of a signaling cascade, including extracellular signal-regulated kinases (ERK1/2) and JNK in neonatal rat ventricular myocytes (Pimentel et al., 2001). Third, the nonselective β1 and β2 adrenoreceptor blocker carvedilol was found to scavenge ROS directly and might also inhibit α1 stimulated hypertrophic signaling mediated by ROS (Nakamura et al., 2011). Due to its pleiotropic effects, including antioxidant actions or enhancement of insulin sensitivity (Nguyen et al., 2019), carvedilol was speculated to be more effective than other beta-blockers like metoprolol or bisoprolol in reducing the mortality rate in humans (Rain and Rada, 2015). However, a clinical trial in patients with chronic systolic heart failure revealed carvedilol to be less effective than bisoprolol in decreasing levels of troponin T, ameliorating inflammation, and increasing forced expiratory volume. Nevertheless, the impact of carvedilol on oxidative stress markers was more pronounced (Toyoda et al., 2020). For instance, carvedilol was applied in patients with dilated cardiomyopathy and enhanced oxidative DNA damage, significantly reducing oxidative DNA damage, lipid peroxidation and ameliorating heart failure (Nakamura et al., 2002; Kono et al., 2006). Besides carvedilol, also the β1-selective beta-blocker nebivolol was shown as a direct antioxidant either by scavenging free radicals or by acting as a chain breaker through proton donation or electron stabilization (Gao and Vanhoutte, 2012). Besides, nebivolol was found to inhibit ROS formation by reducing the activity and expression of the vascular NOX in angiotensin II-treated animals and cells (Oelze et al., 2006). Notably, the ratio of reduced glutathione to oxidized glutathione was significantly increased in patients with essential hypertension after treatment with carvedilol, while nebivolol-treated patients did not show significant differences in this parameter but showed increased nitrogen dioxide plasma concentrations (Zepeda et al., 2012). These reports suggest that the proper use of different beta-blockers might be dependent on the individual pathophysiology.
4.1.2 ACE Inhibitors/AT1 Antagonists
Angiotensin-converting enzyme (ACE) inhibitors prevent the conversion of angiotensin I into angiotensin II that binds to the angiotensin II receptor (AT1) in blood vessels to mediate its vasoconstrictive effect (Burnier, 2001), whereas AT1 receptor antagonists directly inhibit the binding of angiotensin II to AT1 receptors (Gradman, 2002). Consequently, ACE inhibitors are used to control blood pressure to reduce mortality in patients with congestive heart failure and in patients with high cardiovascular risk profiles, including diabetes. Moreover, ACE inhibitors are essential in delaying the progression of chronic renal diseases since they lower proteinuria. The first orally active ACE inhibitor, captopril, got approved by the FDA in 1981. Similar effects as for ACE inhibitors could be achieved by applying AT1 receptor antagonists, first approved by the FDA as losartan in 1995 (Ripley and Hirsch, 2010). ACE inhibitors and AT1 antagonists are supposed to diminish the angiotensin II-mediated generation of ROS and partly also directly scavenge ROS production. Angiotensin II was found to modulate the pressor effect through ROS signaling in the glutamatergic neuron in stress-induced hypertensive rats. Thereby, NAPDH oxidase-derived ROS activates the SAPK and the JNK, promoting the expression of AT1 receptors in glutamatergic neurons. Consequently, glutamate gets released into the spinal cord and leads to the pressor response (Jiang et al., 2018). Besides, the AT1 receptor antagonist candesartan was found to blunt the tumor necrose factor α (TNFα)-induced inflammatory cytokine production of embryonic kidney epithelial cells by inhibiting oxidative stress. Notably, knockdown of the AT1 receptor did not alter candesartan’s impact on ROS activity in humans (Yu et al., 2019). Angiotensin II was found to enhance ROS formation via AT1 receptor activation in old sheep, which was counteracted by the application of ACEII inhibitors (Gwathmey et al., 2010). Moreover, disruption of the AT1 receptor in mice caused reduced oxidative damage and significantly promoted longevity (Benigni et al., 2009). Application of the ACE inhibitor lisinopril attenuated ROS formation and counteracted cardiovascular remodeling in diabetic rats to the same extent as the antioxidant N-acetyl-l-cysteine (NAC) (Fiordaliso et al., 2006). Notably, combined application of the ACE inhibitor temocapril with the AT1 antagonist olmesartan induced a more pronounced suppression of ventricular hypertrophy and fibrosis in a diastolic heart failure rat model in comparison to the monotherapy with temocapril. This benefit was associated with an additive effect on the blockage of ROS generation and inflammation signaling (Yoshida et al., 2004). Besides, it was discussed whether thiol-carrying compounds like alacepril might function as direct ROS scavenging agents. For instance, 0.6–0.7 mM of alacepril reduced ROS production in bronchoalveolar lavage cells from chronic obstructive pulmonary disease patients by 50%, while 3–4 mM of thiol-free lisinopril was necessary to achieve the same effect (Teramoto et al., 2000), suggesting that Moreover, thiol-carrying captopril was more effective against copper-induced oxidative modification on lipids and proteins than the non-thiol ACE inhibitors enalapril and lisinopril (Fernandes et al., 1996). However, the non-thiol carrying AT1 receptor antagonist candesartan inhibited oxidative stress in embryonic kidney epithelial cells independent of AT1 receptor activity (Yu et al., 2019). Consequently, it remains questionable whether the thiol-group is necessary for the direct antioxidant properties of some ACE inhibitors and AT1 antagonists.
4.1.3 Statins
Statins inhibit the rate-limiting step in cholesterol synthesis by blocking the liver enzyme 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase that converts HMG-CoA to mevalonic acid. Consequently, cholesterol levels in the blood drop, and the potential side effects of hyperlipidemia are prevented. The first statin approved by the FDA as therapy for preventing coronary atherosclerotic events was lovastatin in 1987 (Harrington, 2017). HMG-CoA reductase is a crucial enzyme in the mevalonate pathway. Blocking of HMG-CoA reductase also reduces the bioavailability of various products of the mevalonate pathway, including heme A, ubiquinone, isoprenoids, corticosteroids, and vitamin D. Consequently, statins affect mitochondrial function indirectly via mevalonate pathway metabolite depletion. Besides, also direct impairment of the ETC activity was reported (Mollazadeh et al., 2021). Notably, statins exhibit opposite effects on mitochondria of cardiac and skeletal muscles (Sirvent et al., 2005; Bouitbir et al., 2012). A different effect on mitochondrial respiration is discussed as a potential reason. For instance, simvastatin was found to inhibit complex I of the ETC in human and rat skeletal muscle samples, while cardiomyocytes remained largely unaffected (Sirvent et al., 2005). Notably, statins were found to trigger a mitohormetic response by a transient ROS signaling in cardiac tissues, resulting in the upregulation of ROS detoxifying enzymes. At the same time, an enhanced level of ROS displayed harmful actions on skeletal muscle tissues (Bouitbir et al., 2012). In line with this finding, ROS production was decreased and oxidative capacities and peroxisome proliferator-activated receptor-gamma activator 1 (PGC-1) expression enhanced in the atrium of patients treated with atorvastatin, while statin-induced muscular myopathy was accompanied by reduced oxidative capacities, a side-effect counteracted by the application of antioxidant molecules (Bouitbir et al., 2012). Analysis of skeletal muscle biopsy samples from patients with statin-associated myopathy confirmed an enhanced hydrogen peroxide production after statin treatment. However, a difference between slow- and fast-twitching muscle fibers fueled by a greater extent of glycolysis could be detected: Atorvastatin treatment increased hydrogen peroxide accumulation, decreased GSH/GSSG ratios, and triggered apoptotic pathways in the glycolytic plantaris muscle of rats, while the oxidative soleus muscle was largely unaffected due to high antioxidative capacity (Bouitbir et al., 2016). Consequently, the response of different cell types to statin-induced initial ROS burst is probably dependent on the individual metabolic state and potential for antioxidant actions. Thereby, oxidative muscle fibers might be better adjusted to enhanced mitochondrial activity and to related side products like ROS. For instance, an increase in ROS is limited in cardiac myofibres due to their highly efficient antioxidant systems, causing PGC-1α activation and, thereby, mitochondrial biogenesis and function, while cells with lower antioxidant capacity face a giant ROS burst and cellular damage (Mollazadeh et al., 2021). Treatment with antioxidants might be a potential solution to counteract the destructive action of statins in muscle fibers. However, a clinical study revealed that tocopherol supplementation in addition to pravastatin treatment for half a year did not further improve lipid levels or the frequency of adverse effects, including muscle damage in older adults (Carlsson et al., 2002), highlighting that boosting cellular antioxidant defense mechanisms might be a more promising strategy to counteract potential side-effects of statins. Besides, lipophilic statins like cerivastatin, fluvastatin, atorvastatin, and simvastatin specifically decreased glutamate-driven state 3 respiration and induced mitochondrial swelling, cytochrome c release, and DNA fragmentation in rat skeletal muscle cells. In contrast, the hydrophilic pravastatin did not impair mitochondrial function (Kaufmann et al., 2006). Based on these results, administration of hydrophilic statins might prevent mitochondrial accumulation and, thus, harmful effects on the skeletal muscles.
4.1.4 Platelet Aggregation Inhibitors (“Antiplatelets”)
Antiplatelet medications are used in the primary and secondary prevention of thrombotic diseases by decreasing platelet aggregation and thrombus formation. Oral antiplatelets are classified regarding their mechanism of action, including platelet aggregation inhibitors aspirin and clopidogrel. Low-dose aspirin, 75–150 mg once a day (Cryer, 2002), is the most prominent antiplatelet drug, suppressing prostaglandin and thromboxane production through irreversible inactivation of COX. Thereby, the formation of the pro-thrombotic thromboxane A2 in platelets is blocked (Iqbal et al., 2021). Approved around 1900 to treat fever, pain, and inflammation, the compound experienced a revival in the last decades of the 20th century by widespread use as prevention against cardiovascular incidents (Tsoucalas et al., 2011). Nowadays, low-dose aspirin is one of the most popular antiplatelet therapies for the treatment of patients with the acute coronary syndrome (Amsterdam et al., 2014; Roffi et al., 20152016) and for the prevention of atherothrombotic complications in high-risk patients (Parekh et al., 2013; Patrono, 2015). Aspirin was found to inhibit ROS production by downregulation of NOX4 and the inducible nitric oxide synthase in human endothelial cells exposed to oxidized LDL (Chen et al., 2012). Besides, low doses of aspirin also increased the expression of SOD1 and SOD2 in rat astrocytes treated with the toxic peptide Aβ (Jorda et al., 2020). Notably, a double-blind, randomized study revealed that the combined application of low-dose aspirin with vitamin E caused a significant reduction in platelet adhesiveness compared to aspirin only. However, the incidence of hemorrhagic strokes increased in patients treated with both drugs, reaching no statistical significance due to the low number of cases (Steiner et al., 1995). These findings highlight the potential of antioxidant defense mechanisms in the prevention of platelet aggregation but once again question the usage of ROS scavengers.
4.2 Oral Antidiabetic Drugs
4.2.1 Metformin
Metformin was first approved in the United Kingdom in 1958 and got the first-line therapy for T2DM (Scarpello and Howlett, 2008). Metformin shows versatile anti-diabetic effects in various organs and tissues (Foretz et al., 2019). Firstly, metformin impairs glucose absorption in the intestine by increasing the secretion of glucose-lowering hormone glucagon-like peptide (GLP-1) (Mannucci et al., 2004). Secondly, metformin promotes glucose uptake in peripheral tissues by glucose transporter 4 (GLUT4) translocation to the plasma membrane, which allows increased blood glucose clearance and thus counteracts glycemia (Musi et al., 2002; Natali and Ferrannini, 2006; Boyle et al., 2011; Grzybowska et al., 2011; Lee et al., 2012b; Mummidi et al., 2016). Consequently, metformin attenuates fasting plasma insulin levels and helps to restore insulin sensitivity (Grzybowska et al., 2011). Thirdly, metformin inhibits complex I of the respiratory chain, causing an energy deficit leading to the activation of AMP-activated protein kinase (AMPK) (El-Mir et al., 2000; Owen et al., 2000; Zhou et al., 2001). AMPK signaling, in turn, hampers glucose production by inhibition of mitochondrial glycerol-3 phosphate dehydrogenase (Madiraju et al., 2014) and glucagon-stimulated hepatic gluconeogenesis (Miller et al., 2013). Besides that, pAMPK signaling regulates multiple other downstream pathways such as (Jaul and Barron, 2017) nutrient sensing by inhibition of mTORC1 and activation of SIRT1, as well as (Hayyan et al., 2016) initiation of mitochondrial biogenesis through PGC1α, (Tejero et al., 2019), inhibition of pro-inflammatory signaling via NFkB, and (Sun et al., 2010) the regulation of autophagy (Semancik and Vanderwoude, 1976; Suwa et al., 2006; Salminen and Kaarniranta, 2012; Aatsinki et al., 2014; Barzilai et al., 2016; Zhou et al., 2016; Herzig and Shaw, 2018). In addition, metformin-induced AMPK signaling stabilizes the transcription factor NRF2 (Onken and Driscoll, 2010), a master regulator of redox regulations, consequently initiating the expression of antioxidant genes such as CAT, GSH, SOD (Ashabi et al., 2015). The upregulation of antioxidant defense enzymes eventually leads to a decrease in ROS, an impairment of NOX, and a boost of SOD expression (Diniz Vilela et al., 2016; Shin et al., 2017). Inhibition of NOX by metformin highly contributes to the regulation of redox homeostasis as NOX-derived ROS represents the primary source of high glucose-induced oxidative stress (Inoguchi et al., 2000; Kim et al., 2002; Inoguchi et al., 2003). In addition, metformin interferes with RNS production and thereby diminishes nitro-oxidative stress. For instance, it was shown that the bioavailability of nitric oxide, a contributor to endothelial function, was improved by metformin, while levels of cytotoxic peroxynitrite were decreased in diabetic rats (Sambe et al., 2018).
In accordance with metformin-dependent activation of AMPK signaling and the consequent induction of redox regulatory processes, several in vitro studies presented antioxidant effects of metformin (Marycz et al., 2016; Ahangarpour et al., 2017; Smieszek et al., 2017; Algire et al., 2012; Abd-Elsameea et al., 2014). A randomized clinical trial investigating the impact of metformin on ROS homeostasis of T2DM patients found an improvement in the antioxidant status and a cardioprotective effect upon metformin treatment (Chakraborty et al., 2011). In addition to the interference with redox regulatory processes, metformin itself displays direct antioxidant actions by detoxifying hydroxyl radicals, as seen in murine in vitro and in vivo models of oxidative liver injury and cardiac fibrosis and human monocytes/macrophages (Mummidi et al., 2016; Dai et al., 2014; Buldak et al., 2014). Metformin supplementation was further linked to anti-inflammatory and anti-apoptotic processes in several studies investigating neurodegeneration and multiple sclerosis (Nath et al., 2009; Ullah et al., 2012; Alzoubi et al., 2014). Metformin interferes with the production of IL1β, a pro-inflammatory cytokine responsible for pancreatic β-cell apoptosis (Kelly et al., 2015). This mechanism counteracts ROS-dependent increases in IL1β expression in an AMPK-independent fashion (Bauernfeind et al., 2011). The same study revealed that metformin raises levels of the anti-inflammatory cytokine IL-10 (Bauernfeind et al., 2011). Clinical studies observed metformin’s role in preserving cognitive function (Ng et al., 2014), resulting in reduced depressive behavior (Guo et al., 2014) and decreased mortality in diabetic patients (Barzilai et al., 2016). Importantly, metformin-dependent health benefits go beyond glycemic control and include beneficial effects against various types of cancer (Heckman-Stoddard et al., 2017), CVD (Rena and Lang, 2018), neurodegenerative disorders (Rotermund et al., 2018), and autoimmune diseases (Ursini et al., 2018). Several preclinical and clinical studies have strong evidence for a geroprotective potential of metformin (Barzilai et al., 2016; Glossmann and Lutz, 2019; Piskovatska et al., 2019; Soukas et al., 2019). In addition, epidemiological and association studies show that metformin is linked to reduced incidences and all-cause mortalities in several age-related diseases, such as age-associated cancers and AD (Barzilai et al., 2016; Campbell et al., 2017; Valencia et al., 2017). Based on these promising results, the “targeting aging with metformin” (TAME, ClinicalTrials.gov Identifier: NCT02118727) study currently investigates metformin’s potential in aging and its therapeutical potential in age-related diseases (Campisi et al., 2019; Wang et al., 2020).
4.2.2 DPP4 Inhibitors and GLP-1 Agonists
Inhibitors of dipeptidyl peptidase 4 (DPP4), so-called gliptins, and glucagon-like protein 1 (GLP-1) receptor agonists (GLP-1RAs), also known as incretin mimetics, represent two drug classes that tackle the same pathway by acting in opposing ways. While gliptins increase the stability of GLP-1 by preventing its degradation, GLP-1RAs mimic GLP-1 and thus promotes glucagon suppression and insulin secretion (Deacon et al., 2012). DPP4 is a serine protease responsible for the degradation of proteins, including the incretins GLP-1 and gastric inhibitory peptide (GIP), two metabolic hormones involved in the attenuation of blood glucose levels. Elevated DPP4 activity is a risk factor for developing metabolic syndrome and T2DM (Zheng et al., 2014) and is associated with insulin resistance (Sell et al., 2013). As a result, DPP4 deficiency in mice manifests in improved glucose tolerance (Marguet et al., 2000) and decreased obesity and insulin resistance (Conarello et al., 2003). Besides lowering plasma insulin levels, treatment with the DPP4 inhibitors vildagliptin and sitagliptin successfully improved oxidative stress parameters in obese insulin-resistant rats (Apaijai et al., 2013). Another study reported that vildagliptin and sitagliptin positively affected mitochondrial oxidative stress and mitochondrial function, resulting in enhanced cognition and hippocampal brain function in high-fat diet-induced insulin-resistant Wistar rats (Pintana et al., 2013). Advanced glycation endproducts (AGEs) represent a measure of oxidative stress in T2DM. It was shown that crosstalk between AGEs, the receptor for AGEs (RAGE), and the DPP4-incretin system adds up to diabetic vascular complications (Yamagishi et al., 2015). Thereby, DPP4 positively correlates with ROS production and RAGE gene expression (Ishibashi et al., 2013). This process could be reversed by DPP4 inhibition via linagliptin supplementation in endothelial cells (Ishibashi et al., 2013). Similar results were obtained upon teneligliptin treatment resulting in reduced adverse effects of AGEs in mouse peritoneal macrophages and THP-1 cells (Terasaki et al., 2020). In general, oxidative stress markers and inflammatory cytokines were attenuated in T2DM patients receiving DPP4 inhibitor treatment for 4–16 weeks (Rizzo et al., 2012; Tremblay et al., 2014). Besides counteracting oxidative stress, DPP4 inhibitors improve mitochondrial function in rats on a high-fat diet (Apaijai et al., 2013; Pintana et al., 2013; Pipatpiboon et al., 2013) and increase mitochondrial biogenesis and exercise capacity in a mouse model for ischemic heart failure (Takada et al., 2016). Similar to this, GLP-1 agonists stimulated mitochondrial biogenesis and antioxidant defense systems by modulation of PPAR signaling in PC12 cells and mice treated with GLP-1RA (An et al., 2015). This manifests in increased mitochondrial mass and function associated with improved pancreatic β-cell function in INS-1 rat insulinoma cells (Kang et al., 2015). From a mechanistic point of view, treatment with the GLP-1 agonist extendin-4 resulted in upregulation of superoxide dismutase and protected against ROS-induced apoptosis in adipose-derived mesenchymal stem cells (Zhou et al., 2014). Moreover, the GLP-1 agonists, liraglutide, D-ser2-oxyntomodulin, a GLP-1/GIP dual receptor agonist, dAla (2)-GIP-GluPal, Val(8)GLP-1-GluPal and exendin-4 enhanced the expression of the autophagy-associated marker protein atg7 and pyruvate dehydrogenase and improved mitochondrial function in neuronal SH-SY5Y cells (Jalewa et al., 2016).
4.2.3 Glitazones
Glitazones, also known as thiazolidinediones (TZDs), are approved antidiabetic drugs and include compounds such as rosiglitazone and pioglitazone (Hauner, 2002). They represent specific agonists of the peroxisome proliferator-activated receptor γ (PPARγ) and thereby modulate its downstream metabolic regulations (Day, 1999). Their hypoglycemic and antidiabetic effects result from increased glucose absorption and insulin sensitivity in peripheral tissues (Hauner, 2002). Similar to metformin, TZDs were described to inhibit complex I of the respiratory chain in vitro activity assays (Brunmair et al., 2004) and promote cell survival by maintaining the Ψ mito via PPARγ signaling in lymphocytes (Wang et al., 2002). Pioglitazone was shown to counteract oxidative stress and inflammation, increase mitochondrial biogenesis in non-alcoholic fatty liver disease (Bogacka et al., 2005), and attenuate mitochondrial-induced oxidative damage in human subcutaneous adipose tissue human neuron-like cells (Bogacka et al., 2005; Ghosh et al., 2007). In accordance with these findings, pioglitazone increases the SOD1 activity and inhibits NOX expression in rat mesangial cells (Wang et al., 2013). Bolten et al. (Bolten et al., 2007) concluded that observed hypoglycemic effects are more likely a consequence of improved mitochondrial function rather than PPARγ signaling. In contrast, treatment of human hepatoma cells with troglitazone caused severe side effects and mitochondrial structure injuries, which were less potent upon treatment with rosiglitazone or pioglitazone in similar concentrations (Hu et al., 2015). As a consequence, troglitazone was withdrawn as an antidiabetic drug due to hepatotoxicity and mitochondrial toxicity side effects just 3 years after its approval in 2000 (Hu et al., 2015).
4.2.4 SGLT2 Inhibitors
Glucose is re-absorbed via active or passive transport processes during blood filtration in the proximal renal tubule of kidneys (Vallon and Thomson, 2017). One crucial player during this process is the sodium-glucose cotransporter 2 (SGLT2) (Kalra, 2014), which can be pharmacologically inhibited by SGLT2 inhibitors. Such compounds prevent the re-uptake of glucose and favor glucose secretion independent of insulin (Chao, 2014), thus representing antidiabetic drugs to counteract glycemia. Furthermore, SGLT2 inhibitors impair gluconeogenesis and increase insulin sensitivity and insulin secretion of β-cells (Han et al., 2008; Ferrannini et al., 2014; Wilding et al., 2014; Kern et al., 2016). More importantly, SGLT2 inhibitors comprise antioxidant properties by reducing free radical production and strengthening the antioxidant system (Osorio et al., 2012; Ishibashi et al., 2016). Experiments in mice revealed an improved redox state, diminished oxidative damage (Sugizaki et al., 2017), and enhanced mitochondrial function, eventually leading to a balanced ROS homeostasis in the brain (Sa-Nguanmoo et al., 2017). Mechanistically, SGLT2 inhibitors affect the activity and expression of prooxidant enzymes such as NOX, eNOS, and XO (Oelze et al., 2014; Kawanami et al., 2017). For instance, empagliflozin treatment in diabetic rat models led to the downregulation of NOX1 and NOX2 (Oelze et al., 2014). Moreover, NOX4 expression was shown to be impaired by dapagliflozin (Steven et al., 2017). In both cases, free-radical generation and oxidative damage are counteracted (Habibi et al., 2017; Steven et al., 2017). In addition to the depletion of prooxidant processes, SGLT2 inhibitors also strengthen the antioxidant defense system. Several studies show that expression of CAT, SOD, and GPX are increased in diabetic animal models in the presence of phlorizin (Osorio et al., 2012), dapagliflozin (Shin et al., 2016), and TA-1887 (Sugizaki et al., 2017), another SGLT2 inhibitor.
4.2.5 Alpha-Glucosidase Inhibitors
Alpha-glucosidase inhibitors, such as acarbose and miglitol, delay the digestion of carbohydrates by inhibiting alpha-glucosidase enzymes in the small intestines and thereby preventing postprandial hyperglycemia. The alpha-glucosidase inhibitor acarbose reduces inflammatory cytokine production, as seen in reduced levels of interferon-gamma induced protein 10 kD, monocyte chemoattractant protein-1, macrophage-derived chemokines, TNFα as well as NF-kB activity in THP-1 cells (Lin et al., 2019). Moreover, it was observed that acarbose co-treatment with insulin reduced inflammation and oxidative stress in diabetic individuals (Li et al., 2016). Reduced levels of superoxide might be the consequence of acarbose-dependent inhibition of NOXes in the aorta, heart, and kidney of obese diabetic rats (Rösen and Osmers, 2006). Furthermore, inhibition of NOX4 oxidase-dependent superoxide production was seen in rat aortic endothelial cells and is linked to anti-inflammatory regulations (Li et al., 2019).
4.2.6 Sulfonylurea and Glinide
Sulfonylurea inhibits ATP-sensitive K+ channels in the plasma membrane of β-cells and initiates insulin release and hypoglycemia (Groop, 1992). Sulfonylureas, including gliclazide, glibenclamide, and glimepiride, do also affect ATP-sensitive K+ channels in the inner mitochondrial membrane and thereby modify mitochondrial function (Inoue et al., 1991; Suzuki et al., 1997; Szewczyk et al., 1997; Argaud et al., 2009). Moreover, gliclazide treatment in rat models reduces oxidative stress and inflammation via several mechanisms, including upregulation of antioxidant enzymes such as SOD, CAT, and GPX1 (Del Guerra et al., 2007; Alp et al., 2012; Araújo et al., 2019).
4.3 Anti-degenerative Drugs
4.3.1l-Dopa (or Levodopa) and Dopamine Agonists
Until now, l-dopa is considered as “gold standard” for PD therapy (Nagatsu and Sawada, 2009). In the late 1960s, high-dose l-dopa treatment was shown to result in remarkable clinical efficacy in PD patients by restoring dopamine levels in the brain (Barbeau et al., 1961) and was first approved in 1970 (Abbott, 2010). Despite its effectiveness, long-term treatment with l-dopa often results in motor complications, including abnormal involuntary movements (Pahwa et al., 2006; Fabbrini et al., 2007). Similar to this, treatment with dopamine agonists is associated with a range of side effects, from mild to severe implications (Faulkner, 2014). It was shown that degradation of dopamine after l-dopa supplementation results in a dose-dependent increase in ROS and cell death of serotonergic neurons (Stansley and Yamamoto, 2013). These observations underline that both l-dopa and dopamine agonists are thought to act symptomatically only (Bonuccelli, 2003; Segawa et al., 2003; Nagatsu and Sawada, 2009; Blandini and Armentero, 2014), emphasizing the urgent need for more potent drugs that target early dysregulations of the disease, such as oxidative stress.
4.3.2 MAO-B Inhibitors
MAO-B-inhibitors against PD include the irreversible inhibitor selegiline (L-deprenyl), which was first approved by the FDA in 1996, followed by rasagiline in 2006 (Knudsen Gerber, 2011), as well as safinamide, the first reversible FDA-approved MAO-B inhibitor against PD available for clinical use since 2015 (Deeks, 2015). In preclinical models, selegiline was shown to increase levels of antioxidant enzymes such as glutathione and SOD, improve oxidative stress biomarkers, and reduce neuronal loss in rats (Kumar et al., 2018; Ahmari et al., 2020). Similar effects were obtained with rasagiline which attenuated oxidative stress in rats, as measured by levels of 7-ketocholesterol and GSSG/GSH ratio (Aluf et al., 2013).
Clinical trials with safinamide alone or in combination with levodopa or dopamine agonists (pergolide, ropinirole, pramipexole, cabergoline) confirmed improved PD symptoms (Martínez-Martín et al., 1994; Stocchi et al., 2006; Wasan et al., 2021). However, the clinical potential of MAO-B inhibitors to attenuate oxidative stress by inhibiting MAO-induced hydrogen peroxide production remains to be shown as clinical evidence of improved oxidant status in PD patients is lacking. Consequently, it is questionable whether observed positive effects with MAO-B inhibitors are due to neuroprotection or instead limited to symptomatic benefits such as maintaining dopamine levels (Schulzer et al., 1992; Shoulson, 1992; Stocchi et al., 2006).
4.3.3 Repurposing of Antidiabetics as Antidementia Drug
Notably, AD and PD show alterations in oxidative stress levels, hyperglycemia, mitochondrial dysfunction, glucose metabolism, insulin signaling, insulin resistance, and inflammatory processes (Baker et al., 2011; Moran et al., 2013; Willette et al., 2015; Morsi et al., 2018; Sergi et al., 2019; Cheng et al., 2020). Brains of AD individuals show a deficiency of GLUT1 and GLUT3 expression (Simpson et al., 1994) as a result of decreased activity of enzymes involved in glycolysis and TCA cycle (Manczak et al., 2004; Bubber et al., 2005; Manczak and Reddy, 2012). Moreover, AD brains often exhibit impaired insulin receptors activity (Frölich et al., 1998; Talbot et al., 2012), attenuated levels of insulin and insulin growth factor 1 as well as decreased levels of downstream proteins such as insulin receptor substrate 1 (Rivera et al., 2005; Moloney et al., 2010; Talbot et al., 2012). These characteristics positively correlate with cognitive impairments (Talbot et al., 2012) and the progression of AD (Rivera et al., 2005). In conclusion, T2DM and AD share common derangements in glucose metabolism, which led to the term “type III diabetes” and the classification of AD as a metabolic disease that might alternatively be treated with antidiabetics as a novel therapeutic strategy (de la Monte and Wands, 2005). Similar to AD, PD has overlapping dysregulations with diabetes. For example, 50–80% of PD patients have decreased glucose tolerance (Sandyk, 1993) and impaired glucose metabolism, which is considered an early event in the pathology of PD (Borghammer et al., 2012; Dunn et al., 2014). An integrative network analysis compared gene expression in PD and T2DM and elucidated dysregulation of 7 genes involved in insulin and IR signaling as a common mechanism of action (Santiago and Potashkin, 2013). Another common feature of PD and T2DM pathogenesis is mitochondrial dysfunction and impairment of the mitochondrial complex I (Esteves et al., 2008). Consequently, targeting metabolic dysregulations in neurodegenerative diseases has gained great therapeutical interest due to strong similarities to T2DM.
By strengthening the antioxidant system, metformin was shown to extinct ROS from brain tissues (Garg et al., 2017; Tang et al., 2017; Ruegsegger et al., 2019; Docrat et al., 2020) and thereby caught attention as a possible off-label treatment for AD. Indeed, preclinical animal studies suggest a role of metformin in preventing neuropathology in AD and T2DM models (Kickstein et al., 2010; Li et al., 2012; Cardoso and Moreira, 2020) as well as reducing the risk for AD development (Chin-Hsiao, 2019). Interestingly, metformin treatment interferes with amyloid plaque deposition and promotes hippocampal neurogenesis (Ou et al., 2018). In addition, it activates insulin signaling, inhibits structural changes under hyperinsulinemic conditions (Gupta et al., 2011), and restores mitochondrial function in an AMPK-dependent fashion (Chiang et al., 2016). A pilot clinical study observed that improved cognitive function, learning performance, and memory were positively correlated with metformin treatment (Koenig et al., 2017). However, these observations are controversial as other studies reported elevated Aβ levels (Chen et al., 2009) and an increased risk of AD (Imfeld et al., 2012). Thus, the effects of metformin on the pathology of AD remain to be investigated in more detail to conclude on its therapeutic potential. Metformin acts neuroprotective in the development and progression of PD (Paudel et al., 2020). These effects manifest in reduced neuroinflammation and dopaminergic cell death (Lu et al., 2016), reduced α-synuclein aggregation (Pérez-Revuelta et al., 2014; Saewanee et al., 2021), and improved cognitive and locomotor function in animals (Patil et al., 2014; Lu et al., 2016). However, clinical studies validating these metformin-specific effects in PD are still inconclusive or lacking, as metformin was most effective when combined with other antidiabetics, such as sulfonylurea (Wahlqvist et al., 2012).
4.4 Anti-cancer Drugs
ROS production plays an essential role in anticancer therapies. Depending on the actual level, ROS either act as tumor-suppressing or as a tumor-promoting agent (Sahoo et al., 2021). For instance, a moderate increase of intracellular ROS levels triggers cell proliferation and angiogenesis and inactivates tumor suppressor genes, promoting tumor progression (Kumari et al., 2018; Perillo et al., 2020). In contrast, overwhelming ROS levels that overcome the antioxidant defensive system of cancer cells induce cancer cell death (Galadari et al., 2017). Recent studies demonstrate that ROS levels exceeding the redox capacity might be used as anticancer therapies. Thereby, an accelerated accumulation of ROS by a selective triggering of a ROS burst or by inhibition of antioxidant processes disturbs the redox homeostasis and leads to extensive cellular damage and cell death (Wang and Yi, 2008; Trachootham et al., 2009). Enhanced ROS generation is either achieved through exogenous approaches or by endogenous ROS release (Van Loenhout et al., 2020). Several studies demonstrated that exogenous and endogenous ROS bursts actively contribute to the mechanism of action of anti-cancer therapies such as radiotherapy and chemotherapy and, therefore, enhance their efficacy (Ozben, 2007; Zhang et al., 2009; Kim et al., 2019). Physical interventions like radiotherapy or photodynamic therapy are exogenous ROS sources (Van Loenhout et al., 2020). Besides, various agents induce oxidative stress via endogenous ROS generation and accumulation. Compounds that generate high levels of ROS include anthracyclines including doxorubicin, platinum coordination complexes such as cisplatin, alkylating agents like cyclophosphamide, camptothecins, arsenic agents, and topoisomerase inhibitors (Weiner, 1979). Thereby, these agents either directly induce ROS generation or inhibit antioxidant defense mechanisms (Kim et al., 2019). For instance, motexafin gadolinium, doxorubicin, cisplatin, and 2-methoxyestradiol act by direct ROS generation. Thereby, motexafin gadolinium accepts electrons to form superoxide (Magda and Miller, 2006), doxorubicin induces chelation of iron to generate hydroxyl radicals (Kotamraju et al., 2002), cisplatin induces ROS generation by damaging mtDNA and the electron transport chain (Marullo et al., 2013), and 2-methoxyestradiol inhibits the ETC complex 1 (Hagen et al., 2004). Even though anticancer drugs with direct ROS-accumulating activity have been helpful in combating various cancers, their effects on normal cells remain controversial as they harm both cancer cells and normal cells (Francis et al., 2009; Cardinale et al., 2015). In contrast, the antioxidant process is, for example, inhibited by buthionine sulfoximine and imexon. Both disrupt GSH activity and disturb the Ψ mito, generating oxidative stress in cancer cells (Griffith and Meister, 1979; Moulder et al., 2010; Sheveleva et al., 2012). However, the inhibition of antioxidative enzymes also has side effects on normal cells and tissues (Dvorakova et al., 2002; Abdelhamid and El-Kadi, 2015). Consequently, it might be helpful to specifically target these agents to cancer cells in vivo, for instance, by using characteristic molecular signals of cancer cells. Notably, therapeutic strategies affecting ROS homeostasis are predominantly used to attack late-stage cancer cells since early-stage cancer cells are often able to actively counteract ROS disturbances by adjusting their redox status through the upregulation of antioxidant enzymes. Late-stage cancer cells already exhibit higher basal ROS levels, and additional ROS bursts are more effective to severe cytotoxic effects (Kim et al., 2019), inducing apoptosis, autophagic cell death, or necroptosis (Kim et al., 2019; Perillo et al., 2020).
4.5 Analgetics
Acetaminophen, also known as N-acetyl-p-aminophenol or paracetamol, is one of the most commonly used medications for pain worldwide. Approved in 2002 by the FDA, it is used as an analgesic and antipyretic agent and is recommended as first-line treatment in geriatric patients (Abdulla et al., 2013), pregnant women (Aitkin et al., 1996), and children (Cranswick and Coghlan, 2000; Purssell, 2002). Acetaminophen exerts its effects by blocking the synthesis of prostaglandins from arachidonic acid and via actions of its metabolite AM404 (Flower and Vane, 1972; Ghanem et al., 2016). Inhibition of prostaglandin synthesis is achieved by hampering the activity of COX-1 and COX-2 (Esh et al., 2021). COX-1 is expressed in most tissues and regulates basal levels of prostaglandins which control platelet activation and protect the lining of the gastrointestinal tract (Crofford, 1997). COX-2 is inducible and responsible for releasing prostaglandins after infection, in case of injury, or during cancer development. Prostaglandins mediate a number of biological effects, including the induction of an inflammatory immune response (Ornelas et al., 2017). In case of low levels of arachidonic acid and peroxide, therapeutic concentrations of acetaminophen inhibit COX activity sufficiently. However, acetaminophen has little effect when arachidonic acid or peroxide levels are high, as seen in severe inflammatory conditions such as rheumatoid arthritis (Boutaud et al., 2002). Accordingly, the anti-inflammatory action of acetaminophen is modest (Graham et al., 2013). Besides blocking prostaglandin synthesis, the metabolite of acetaminophen, AM404, displays analgetic effects. AMA404 is formed from 4-aminophenol by the action of fatty acid amide hydrolase and has been detected in cerebrospinal fluid of humans treated with acetaminophen (Ghanem et al., 2016; Sharma et al., 2017). AMA404 works as a weak agonist of cannabinoid receptors CB1 and CB2, as an inhibitor of endocannabinoid transporter, and a potent activator of TRPVI receptor (Anderson, 2008; Ghanem et al., 2016). Notably, acetaminophen also inhibits prostaglandin synthesis indirectly by scavenging peroxynitrite, an activator of COX (Schildknecht et al., 2008). When used in recommended doses, acetaminophen has few side effects, and iatrogenic complications are infrequent and minor (Cranswick and Coghlan, 2000; Warwick, 2008). However, in overdose, acetaminophen is hepatotoxic as it induces oxidative stress that subsequently causes mitochondrial impairment and hepatic necroptosis (Cranswick and Coghlan, 2000; Warwick, 2008). When acetaminophen is metabolized, the highly toxic acetaminophen metabolite N-acetyl-p-benzoquinone-imine is formed and gets conjugated to the hepatic store of reduced GSH. In case of acetaminophen overdose, N-acetyl-p-benzoquinone-imine reacts further with cellular proteins causing oxidative stress, lipid peroxidation, and excessive free radical production. Consequently, numerous studies in cells and animals have proven that oxidative stress plays an essential role in the toxic effects induced by acetaminophen (Wang et al., 2017). For example, 0.1 mM of acetaminophen decreased levels of cellular GSH and elevated levels of malondialdehyde, a highly reactive product from lipid peroxidation, in rat hepatocytes, while the antioxidant compound saponarin ameliorated acetaminophen-induced hepatoxicity by restoring GSH and malondialdehyde levels (Simeonova et al., 2013). Moreover, 6 mM of acetaminophen decreased GSH levels significantly and enhanced telomerase activity in rat embryonic liver cells (Bader et al., 2011). Even lower concentrations of acetaminophen, ranging from 0.05 to 0.3 mM, were found to increase ROS generation in mitochondria and induced the gene expression of NRF2, crucial for maintaining cellular redox homeostasis, in mouse hepatoma cells (Perez et al., 2011). Clinical trials revealed that acetaminophen treatment for more than 1 week decreases the antioxidative capacity in elderlies (Pujos-Guillot et al., 2012) and febrile children (Kozer et al., 2003). Moreover, a gradual decrease in serum antioxidant capacity, eventually by a reduction in GSH, was found in men and women after ingestion of maximum therapeutic doses of acetaminophen for 14 days (Nuttall et al., 2003). Combined administration of acetaminophen and N-acetyl-cysteine amide prevented the drop in levels of reduced GSH in liver mitochondria and reduced histopathologic hepatic lesions in C57BL/6 mice. Notably, the impact of N-acetylcysteine amide was superior to NAC, possible due to the derivative’s improved lipophilicity, membrane permeability, and antioxidant property (Khayyat et al., 2016). Moreover, recent studies also revealed hepatoprotective effects of the mitochondria-targeted antioxidant Mito-TEMPO in mice at late-stage acetaminophen overdose (Abdullah-Al-Shoeb et al., 2020), suggesting that scavenging of mtROS might be a promising approach to counteract acetaminophen-induced hepatotoxicity.
Acetylsalicylic acid, also known as aspirin and already introduced into the chapter “Antiplatelets”, is mainly used to reduce pain, fever, and inflammation. Moreover, acetylsalicylic acid is also specifically used to treat pericarditis, rheumatic fever, Kawasaki disease (Agarwal and Agrawal, 2017; Cortellini et al., 2017; Imazio et al., 2017). In addition to modulating the inflammatory response, acetylsalicylic acid affects the physiological function of platelets, counteracting the clotting. As discussed in the chapter “Antiplatelets”, low-dose acetylsalicylic acid is one of the most popular antiplatelet therapies for the treatment of patients with the acute coronary syndrome (Amsterdam et al., 2014), (Roffi et al., 20152016) and for the secondary prevention of atherothrombotic complications in high-risk patients (Parekh et al., 2013), (Patrono, 2015). Besides, several studies provided convincing evidence that regular low-dose acetylsalicylic acid use significantly lowers the risk of cancer (Rothwell et al., 2010; Algra and Rothwell, 2012; Chan et al., 2012; Ishikawa et al., 2013; Patrignani et al., 2017; Bosetti et al., 2020). Acetylsalicylic acid contains higher anti-inflammatory properties than acetaminophen (Mburu et al., 1990), probably because salicylic acid and its derivates also modulate signaling through NF-kB, which plays a crucial role in inflammation (McCarty and Block, 2006; Lawrence, 2009; Chen et al., 2018). It has also been suggested that aspirin converts COX-2 to lipoxygenase-like enzymes, which additionally results in the formation of mediators contributing to the anti-inflammatory effects of aspirin (Serhan and Chiang, 2013; Romano et al., 2015; Weylandt, 2016). Aspirin is the only non-steroidal anti-inflammatory drug (NSAID) not associated with increased cardiovascular events (Ghosh et al., 2015). Similar to acetaminophen and like the majority of NSAIDs, acetylsalicylic acid exerts its anti-inflammatory effects through inhibition of COX enzymes regulating the production of prostaglandins (Crofford, 1997). How acetylsalicylic acid influences ROS homeostasis seems partly unclear. Some studies show that aspirin decreases levels of ROS (Kim et al., 2017; Liu et al., 2019a; Liu et al., 2019b). For example, human hepatoma cells treated with 2- and 4-mM aspirin showed that aspirin remarkably decreased ROS levels (Liu et al., 2019a). Nucleus pulposus cells treated with 5 or 25 μg/ml aspirin significantly attenuated the production of NO and ROS (Liu et al., 2019b). Possibly, an application of rather low levels of aspirin or an application over short periods of time could eventually trigger mitohormetic responses (which is further discussed in chapter “Antiplatelets”) and thereby decrease ROS levels. Other studies propose an increase of ROS production in rat adipocytes, 1 µM of acetylsalicylic acid caused the activation of the NOX4 isoform of NADPH oxidase, boosting the generation of hydrogen peroxide (Vázquez-Meza et al., 2013). Moreover, 1 mM acetylsalicylic acid was found to increase lipid peroxidation in gastric small intestinal cells of rats (Nagano et al., 2012).
Opioids, which are often used as substitutes in the maintenance treatment for heroin addiction, modulate ROS homeostasis as well (Schiavone et al., 2019). Opioid use increases the production of ROS, which is suggested to play a role in opioid use disorders. It also leads to a decrease in the function of enzymatic antioxidants such as superoxide dismutase, catalase, and GPX and can elevate the risk of vitamin deficiency (Zahmatkesh et al., 2017; Salarian et al., 2018). Supplementation with antioxidants may help to restore the redox equilibrium. However, antioxidant therapy has not yet been proven to have a significant effect in randomized trials (Zahmatkesh et al., 2017).
4.6 Antibiotics
It has been demonstrated that major classes of bactericidal antibiotics, regardless of their molecular targets, trigger cell death in bacteria by acting as stressors leading to ROS overproduction (Dwyer et al., 2007; Kohanski et al., 2007; Wang et al., 2010b; Grant et al., 2012; Liu et al., 2012; Van Acker and Coenye, 2017). The mechanisms causing ROS overproduction involve the disruption of the TCA cycle and the ETC (Kohanski et al., 2007; Kohanski et al., 2008), as well as metabolism-related NADH depletion, damage of iron-sulfur clusters in proteins, and stimulation of the Fenton reaction (Dwyer et al., 2007; Kohanski et al., 2007; Van Acker and Coenye, 2017), mechanisms essential to maintain ROS homeostasis. Besides, studies suggested that low levels of ROS produced by sublethal levels of antibiotics might help bacteria to develop antibiotic resistance (Van Acker and Coenye, 2017; Rowe et al., 2020). Thereby, ROS might, for instance, trigger stress resistance mechanisms (Poole, 2012; Wu et al., 2012) or cause mutagenesis (Neeley and Essigmann, 2006; Kohanski et al., 2010; Jee et al., 2016; Van Acker and Coenye, 2017), helping bacteria to escape the bacteriocidic effect of antibiotics. According to the bacterial origin of mammalian cells’ mitochondria proposed by the endosymbiotic theory (Gray et al., 1999), it might be assumed that antibiotics target both pathogens and mitochondria of healthy cells. Indeed, bactericidal and bacteriostatic antibiotics have been shown to target mitochondrial components and function (Gootz et al., 1990; Hutchin and Cortopassi, 1994; McKee et al., 2006; Hobbie et al., 2008; Pochini et al., 2008; Lowes et al., 2009; Kalghatgi et al., 2013). For instance, chloramphenicol reversibly binds to the 50S subunit of the 70S ribosome in both prokaryotic organisms and mitochondria (Balbi, 2004), inhibiting peptidyl transferase, which catalyzes principal chemical reactions of protein synthesis. Align with this finding, tetracyclines like doxycycline and minocycline have been shown to impair mitochondrial biogenesis (Kroon and Van den Bogert, 1983), mitochondrial respiratory chain activity (Chatzispyrou et al., 2015), and mitochondrial protein synthesis (Fuentes-Retamal et al., 2020). Moreover, the macrolide antibiotic azithromycin has been shown to cause disruption of the Ψ mito (Xiao et al., 2019), ROS production (Jiang et al., 2019), and cytochrome c release (Salimi et al., 2016).
Regardless of their specific molecular targets, three major classes of bactericidal antibiotics—quinolones, aminoglycosides, and β-lactams—have been associated with cause mitochondrial dysfunction, which leads to DNA-, protein-, and lipid damage, causing ROS-induced damage in mammalian cells (Kalghatgi et al., 2013). Consequently, oxidative cellular damage induced by bactericidal antibiotics may cause adverse side effects in humans after long-term use, including ototoxicity, nephrotoxicity, and tendinopathy (Brummett and Fox, 1989; Mingeot-Leclercq and Tulkens, 1999; Khaliq and Zhanel, 2003; Kalghatgi et al., 2013). Patients with weakened antioxidant defense systems or people genetically disposed to developing a mitochondrial dysfunction disease (Schaefer et al., 2008) may be at higher risk from bactericidal antibiotic treatments. Notably, co-administration of bactericidal antibiotics and NAC reduced side effects without reducing the bacterial killing efficacy of antibiotics (Kalghatgi et al., 2013). Besides, bacteriostatic antibiotics, such as tetracycline, did not contribute to the overwhelming production of ROS in mammalian cells (Kohanski et al., 2007) and showed fewer side effects (Kalghatgi et al., 2013). Notably, it has been proposed that the usage of specific antibiotics such as tetracyclines might be even beneficial due to the generation of mild mitochondrial stress that leads to activation of the mitochondrial unfolded protein response and enhanced stress resistance (Suárez-Rivero et al., 2021). In addition, doxycycline was found to promote fitness and survival in a Leigh syndrome mouse model and to rescue cell death and inflammatory signatures in cells carrying mitochondrial mutations by inducing a mitohormetic response (Perry et al., 2021). In summary, it seems crucial to which extent antibiotics cause ROS production and whether treated individuals exhibit a functional antioxidant defense system.
5 Conclusion
The current review provides an overview of the implication of ROS in pathological dysregulations and age-related diseases and the mechanisms of how approved drugs modulate ROS homeostasis (Figure 1). Drug classes of antibiotics and anti-cancer agents induce overwhelming ROS production and might thereby help to trigger the death of pathogens or cancer cells. However, thereby may also harm healthy cells. In contrast, drugs against diabetes, neurodegeneration, and CVD, as well as anti-inflammatory compounds, are often associated with boosting antioxidant defense mechanisms and thus preventing ROS-mediated damage of DNA, RNA, lipids, and proteins. In addition, the review highlights the potential of repurposing drugs against metabolic diseases for the treatment of neurodegenerative diseases. For most described drugs, it remains to be questionable whether ROS manipulation is a desirable side-effect or suitable as a drug’s primary mode of action. Moreover, repurposing drugs in use might help to spare time in clinical trials since safety is already confirmed, but specific targeting of ROS sources or detoxification sites might be necessary to enhance the effect or avoid multiple targets and, thereby, side-effects. The individual ROS homeostasis and antioxidant potential undergo crucial alterations during aging (Beal, 2002; Suh et al., 2003; Choksi et al., 2008). Consequently, it might be essential to determine the proper intervention time and adjust the dosage of drugs dependent on the individual oxidative state. Besides, it still has to be clarified which intensity and duration of ROS signals are suitable to induce long-lasting effects in signaling. Moreover, fundamental questions must be solved, including determining the intensity and duration of ROS modulation to cause a long-lasting impact. Thereby, live-cell imaging methods enabling the real-time tracking of various ROS species in different cellular organelles are essential, and reliable blood markers for ROS generation and detoxification might have to be characterized to monitor patients. Manipulating ROS might be a promising strategy to induce toxic effects against pathogens or cancer cells. In addition, specific modulation of ROS levels during aging might be utilized to enhance defense mechanisms and, thereby, prevent the development and progression of cardiovascular and neurodegenerative diseases.
FIGURE 1.
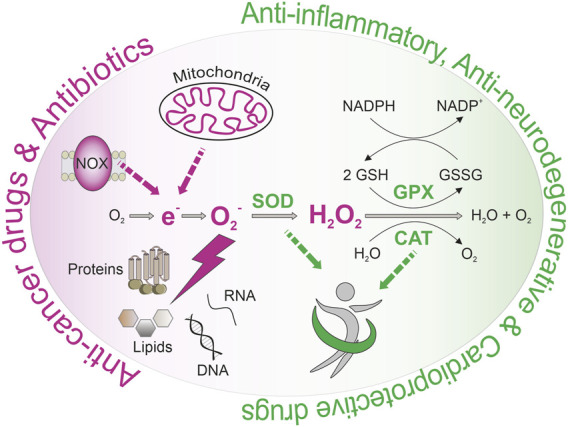
Intracellular ROS homeostasis affecting health and disease.
Author Contributions
Wrote and contributed to the writing of the manuscript: CT, LW, EM, MR, and CM-S. Designed and planned the manuscript: CM-S, MR.
Funding
The Madreiter laboratory is funded by the FWF (J4205-B27) and MEFOgraz. The Ristow laboratory is funded by the Swiss National Science Foundation (Schweizerischer Nationalsfonds, SNF 31003A_156031 and 310030_204511).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Aatsinki S.-M., Buler M., Salomäki H., Koulu M., Pavek P., Hakkola J. (2014). Metformin Induces PGC-1α Expression and Selectively Affects Hepatic PGC-1α Functions. Br. J. Pharmacol. 171 (9), 2351–2363. 10.1111/bph.12585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abadeh S., Case P. C., Harrison R. (1993). Purification of Xanthine Oxidase from Human Heart. Biochem. Soc. Trans. 21 (2), 99S. 10.1042/bst021099s [DOI] [PubMed] [Google Scholar]
- Abbott A. (2010). Levodopa: the Story So Far. Nature 466 (7310), S6–S7. 10.1038/466s6a [DOI] [PubMed] [Google Scholar]
- Abd-Elsameea A. A., Moustaf A. A., Mohamed A. M. (2014). Modulation of the Oxidative Stress by Metformin in the Cerebrum of Rats Exposed to Global Cerebral Ischemia and Ischemia/reperfusion. Eur. Rev. Med. Pharmacol. Sci. 18 (16), 2387–2392. [PubMed] [Google Scholar]
- Abdelhamid G., El-Kadi A. O. S. (2015). Buthionine Sulfoximine, an Inhibitor of Glutathione Biosynthesis, Induces Expression of Soluble Epoxide Hydrolase and Markers of Cellular Hypertrophy in a Rat Cardiomyoblast Cell Line: Roles of the NF-Κb and MAPK Signaling Pathways. Free Radic. Biol. Med. 82, 1–12. 10.1016/j.freeradbiomed.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Abdulkhaleq L. A., Assi M. A., Abdullah R., Zamri-Saad M., Taufiq-Yap Y. H., Hezmee M. N. M. (2018). The Crucial Roles of Inflammatory Mediators in Inflammation: A Review. Vet. World 11 (5), 627–635. 10.14202/vetworld.2018.627-635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulla A., Adams N., Bone M., Elliott A. M., Gaffin J., Jones D., et al. (2013). Guidance on the Management of Pain in Older People. Age Ageing 42 (Suppl. 1), i1–57. 10.1093/ageing/afs200 [DOI] [PubMed] [Google Scholar]
- Abdullah-Al-Shoeb M., Sasaki K., Kikutani S., Namba N., Ueno K., Kondo Y., et al. (2020). The Late-Stage Protective Effect of Mito-TEMPO against Acetaminophen-Induced Hepatotoxicity in Mouse and Three-Dimensional Cell Culture Models. Antioxidants (Basel) 9 (10). 10.3390/antiox9100965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams L., Franco M. C., Estevez A. G. (2015). Reactive Nitrogen Species in Cellular Signaling. Exp. Biol. Med. (Maywood) 240 (6), 711–717. 10.1177/1535370215581314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S., Agrawal D. K. (2017). Kawasaki Disease: Etiopathogenesis and Novel Treatment Strategies. Expert Rev. Clin. Immunol. 13 (3), 247–258. 10.1080/1744666x.2017.1232165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahangarpour A., Zeidooni L., Rezaei M., Alboghobeish S., Samimi A., Oroojan A. A. (2017). Protective Effect of Metformin on Toxicity of Butyric Acid and Arsenic in Isolated Liver Mitochondria and Langerhans Islets in Male Mice: an In Vitro Study. Iran. J. Basic Med. Sci. 20 (12), 1297–1305. 10.22038/IJBMS.2017.9567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmari M., Sharafi A., Mahmoudi J., Jafari-Anarkoli I., Gharbavi M., Hosseini M.-J. (2020). Selegiline (L-Deprenyl) Mitigated Oxidative Stress, Cognitive Abnormalities, and Histopathological Change in Rats: Alternative Therapy in Transient Global Ischemia. J. Mol. Neurosci. 70 (10), 1639–1648. 10.1007/s12031-020-01544-5 [DOI] [PubMed] [Google Scholar]
- Ahmed Alfar E., Kirova D., Konantz J., Birke S., Mansfeld J., Ninov N. (2017). Distinct Levels of Reactive Oxygen Species Coordinate Metabolic Activity with Beta-Cell Mass Plasticity. Sci. Rep. 7 (1), 3994. 10.1038/s41598-017-03873-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitkin L., Nelson J., Shepherd R. (1996). Development of Hearing and Vocalization in a Marsupial, the Northern Quoll,Dasyurus Hallucatus. J. Exp. Zool. 276 (6), 394–402. [DOI] [PubMed] [Google Scholar]
- Algire C., Moiseeva O., Deschênes-Simard X., Amrein L., Petruccelli L., Birman E., et al. (2012). Metformin Reduces Endogenous Reactive Oxygen Species and Associated DNA Damage. Cancer Prev. Res. 5 (4), 536–543. 10.1158/1940-6207.capr-11-0536 [DOI] [PubMed] [Google Scholar]
- Algra A. M., Rothwell P. M. (2012). Effects of Regular Aspirin on Long-Term Cancer Incidence and Metastasis: a Systematic Comparison of Evidence from Observational Studies versus Randomised Trials. Lancet Oncol. 13 (5), 518–527. 10.1016/s1470-2045(12)70112-2 [DOI] [PubMed] [Google Scholar]
- Alp H., Varol S., Celik M. M., Altas M., Evliyaoglu O., Tokgoz O., et al. (2012). Protective Effects of Beta Glucan and Gliclazide on Brain Tissue and Sciatic Nerve of Diabetic Rats Induced by Streptozosin. Exp. Diabetes Res. 2012, 230342. 10.1155/2012/230342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluf Y., Vaya J., Khatib S., Loboda Y., Finberg J. P. M. (2013). Selective Inhibition of Monoamine Oxidase A or B Reduces Striatal Oxidative Stress in Rats with Partial Depletion of the Nigro-Striatal Dopaminergic Pathway. Neuropharmacology 65, 48–57. 10.1016/j.neuropharm.2012.08.023 [DOI] [PubMed] [Google Scholar]
- Alzoubi K., Khabour O., Al-Azzam S., Tashtoush M., Mhaidat N. (2014). Metformin Eased Cognitive Impairment Induced by Chronic L-Methionine Administration: Potential Role of Oxidative Stress. Curr Neuropharmacol 12 (2), 186–192. 10.2174/1570159x11666131120223201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A., 3rd (2000). CNS Energy Metabolism as Related to Function. Brain Res. Brain Res. Rev. 34 (1-2), 42–68. 10.1016/s0165-0173(00)00038-2 [DOI] [PubMed] [Google Scholar]
- Ames B. N., Cathcart R., Schwiers E., Hochstein P. (1981). Uric Acid Provides an Antioxidant Defense in Humans against Oxidant- and Radical-Caused Aging and Cancer: a Hypothesis. Proc. Natl. Acad. Sci. U.S.A. 78 (11), 6858–6862. 10.1073/pnas.78.11.6858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam E. A., Wenger N. K., Brindis R. G., Casey D. E., Jr., Ganiats T. G., Holmes D. R., Jr., et al. (2014). 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-elevation Acute Coronary Syndromes. J. Am. Coll. Cardiol. 64 (24), e139–e228. 10.1016/j.jacc.2014.09.017 [DOI] [PubMed] [Google Scholar]
- An F.-M., Chen S., Xu Z., Yin L., Wang Y., Liu A.-R., et al. (2015). Glucagon-like Peptide-1 Regulates Mitochondrial Biogenesis and Tau Phosphorylation against Advanced Glycation End Product-Induced Neuronal Insult: Studies In Vivo and In Vitro . Neuroscience 300, 75–84. 10.1016/j.neuroscience.2015.05.023 [DOI] [PubMed] [Google Scholar]
- Anderson B. J. (2008). Paracetamol (Acetaminophen): Mechanisms of Action. Paediatr. Anaesth. 18 (10), 915–921. 10.1111/j.1460-9592.2008.02764.x [DOI] [PubMed] [Google Scholar]
- Andreev V. S., Rybakov V. N. (1975). Determination of the Indicators of Acid-Base Equilibrium by the Methods of Conductometric Titration. Lab. Delo (4), 208–214. [PubMed] [Google Scholar]
- Apaijai N., Pintana H., Chattipakorn S. C., Chattipakorn N. (2013). Effects of Vildagliptin versus Sitagliptin, on Cardiac Function, Heart Rate Variability and Mitochondrial Function in Obese Insulin-Resistant Rats. Br. J. Pharmacol. 169 (5), 1048–1057. 10.1111/bph.12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo A. A., Morais H. B., Medeiros C. A. C. X., Brito G. A. C., Guedes P. M. M., Hiyari S., et al. (2019). Gliclazide Reduced Oxidative Stress, Inflammation, and Bone Loss in an Experimental Periodontal Disease Model. J. Appl. Oral Sci. 27, e20180211. 10.1590/1678-7757-2018-0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaud L., Garrier O., Loufouat J., Gomez L., Couture-Lepetit E., Gateau-Roesch O., et al. (2009). Second-generation Sulfonylureas Preserve Inhibition of Mitochondrial Permeability Transition by the Mitochondrial K+ Atp Opener Nicorandil in Experimental Myocardial Infarction. Shock 32 (3), 247–252. 10.1097/shk.0b013e31819c3794 [DOI] [PubMed] [Google Scholar]
- Ashabi G., Khalaj L., Khodagholi F., Goudarzvand M., Sarkaki A. (2015). Pre-treatment with Metformin Activates Nrf2 Antioxidant Pathways and Inhibits Inflammatory Responses through Induction of AMPK after Transient Global Cerebral Ischemia. Metab. Brain Dis. 30 (3), 747–754. 10.1007/s11011-014-9632-2 [DOI] [PubMed] [Google Scholar]
- Asmat U., Abad K., Ismail K. (2016). Diabetes Mellitus and Oxidative Stress-A Concise Review. Saudi Pharm. J. 24 (5), 547–553. 10.1016/j.jsps.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D., Laughlin S. B. (2001). An Energy Budget for Signaling in the Grey Matter of the Brain. J. Cereb. Blood Flow. Metab. 21 (10), 1133–1145. 10.1097/00004647-200110000-00001 [DOI] [PubMed] [Google Scholar]
- Avraham K. B., Schickler M., Sapoznikov D., Yarom R., Groner Y. (1988). Down's Syndrome: Abnormal Neuromuscular Junction in Tongue of Transgenic Mice with Elevated Levels of Human Cu/Zn-Superoxide Dismutase. Cell 54 (6), 823–829. 10.1016/s0092-8674(88)91153-1 [DOI] [PubMed] [Google Scholar]
- Bader A., Oliver P., Keller M., Pavlica S. (2011). Paracetamol Treatment Increases Telomerase Activity in Rat Embryonic Liver Cells. Pharmacol. Rep. 63 (6), 1435–1441. 10.1016/s1734-1140(11)70707-1 [DOI] [PubMed] [Google Scholar]
- Badrinath N., Yoo S. Y. (2018). Mitochondria in Cancer: in the Aspects of Tumorigenesis and Targeted Therapy. Carcinogenesis 39 (12), 1419–1430. 10.1093/carcin/bgy148 [DOI] [PubMed] [Google Scholar]
- Baker L. D., Cross D. J., Minoshima S., Belongia D., Watson G. S., Craft S. (2011). Insulin Resistance and Alzheimer-like Reductions in Regional Cerebral Glucose Metabolism for Cognitively Normal Adults with Prediabetes or Early Type 2 Diabetes. Arch. Neurol. 68 (1), 51–57. 10.1001/archneurol.2010.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbi H. J. (2004). Chloramphenicol: a Review. Pediatr. Rev. 25 (8), 284–288. 10.1542/pir.25-8-284 [DOI] [PubMed] [Google Scholar]
- Bandeira Sde. M., Guedes Gda. S., da Fonseca L. J., Pires A. S., Gelain D. P., Moreira J. C., et al. (2012). Characterization of Blood Oxidative Stress in Type 2 Diabetes Mellitus Patients: Increase in Lipid Peroxidation and SOD Activity. Oxid. Med. Cell Longev. 2012, 819310. 10.1155/2012/819310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau A., Murphy G. F., Sourkes T. L. (1961). Excretion of Dopamine in Diseases of Basal Ganglia. Science 133 (3465), 1706–1707. 10.1126/science.133.3465.1706-b [DOI] [PubMed] [Google Scholar]
- Barsoum M. J., Yuan H., Gerencser A. A., Liot G., Kushnareva Y., Gräber S., et al. (2006). Nitric Oxide-Induced Mitochondrial Fission Is Regulated by Dynamin-Related GTPases in Neurons. EMBO J. 25 (16), 3900–3911. 10.1038/sj.emboj.7601253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels T., Choi J. G., Selkoe D. J. (2011). α-Synuclein Occurs Physiologically as a Helically Folded Tetramer that Resists Aggregation. Nature 477 (7362), 107–110. 10.1038/nature10324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N., Crandall J. P., Kritchevsky S. B., Espeland M. A. (2016). Metformin as a Tool to Target Aging. Cell Metab. 23 (6), 1060–1065. 10.1016/j.cmet.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelli M. G., Polito L., Bortolotti M., Bolognesi A. (2016). Xanthine Oxidoreductase-Derived Reactive Species: Physiological and Pathological Effects. Oxid. Med. Cell Longev. 2016, 3527579. 10.1155/2016/3527579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind F., Bartok E., Rieger A., Franchi L., Núñez G., Hornung V. (2011). Cutting Edge: Reactive Oxygen Species Inhibitors Block Priming, but Not Activation, of the NLRP3 Inflammasome. J. I. 187 (2), 613–617. 10.4049/jimmunol.1100613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal M. F. (2002). Oxidatively Modified Proteins in Aging and Disease1,2 1Guest Editor: Earl Stadtman 2This Article Is Part of a Series of Reviews on "Oxidatively Modified Proteins in Aging and Disease." the Full List of Papers May Be Found on the Homepage of the Journal. Free Radic. Biol. Med. 32 (9), 797–803. 10.1016/s0891-5849(02)00780-3 [DOI] [PubMed] [Google Scholar]
- Bell E. L., Emerling B. M., Ricoult S. J. H., Guarente L. (2011). SirT3 Suppresses Hypoxia Inducible Factor 1α and Tumor Growth by Inhibiting Mitochondrial ROS Production. Oncogene 30 (26), 2986–2996. 10.1038/onc.2011.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellucci A., Mercuri N. B., Venneri A., Faustini G., Longhena F., Pizzi M., et al. (2016). Review: Parkinson's Disease: from Synaptic Loss to Connectome Dysfunction. Neuropathol. Appl. Neurobiol. 42 (1), 77–94. 10.1111/nan.12297 [DOI] [PubMed] [Google Scholar]
- Bellucci A., Zaltieri M., Navarria L., Grigoletto J., Missale C., Spano P. (2012). From α-synuclein to Synaptic Dysfunctions: New Insights into the Pathophysiology of Parkinson's Disease. Brain Res. 1476, 183–202. 10.1016/j.brainres.2012.04.014 [DOI] [PubMed] [Google Scholar]
- Benigni A., Corna D., Zoja C., Sonzogni A., Latini R., Salio M., et al. (2009). Disruption of the Ang II Type 1 Receptor Promotes Longevity in Mice. J. Clin. Invest. 119 (3), 524–530. 10.1172/jci36703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoni-Freddari C., Fattoretti P., Casoli T., Meier-Ruge W., Ulrich J. (1990). Morphological Adaptive Response of the Synaptic Junctional Zones in the Human Dentate Gyrus during Aging and Alzheimer's Disease. Brain Res. 517 (1-2), 69–75. 10.1016/0006-8993(90)91009-6 [DOI] [PubMed] [Google Scholar]
- Birben E., Sahiner U. M., Sackesen C., Erzurum S., Kalayci O. (2012). Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 5 (1), 9–19. 10.1097/wox.0b013e3182439613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkmayer W., Knoll J., Riederer P., Youdim M. B. (1983). (-)-Deprenyl Leads to Prolongation of L-Dopa Efficacy in Parkinson's Disease. Mod. Probl. Pharmacopsychiatry 19, 170–176. 10.1159/000407513 [DOI] [PubMed] [Google Scholar]
- Bjelakovic G., Nikolova D., Gluud L. L., Simonetti R. G., Gluud C. (2012). Antioxidant Supplements for Prevention of Mortality in Healthy Participants and Patients with Various Diseases. Cochrane Database Syst. Rev. 3, CD007176. 10.1002/14651858.CD007176.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelakovic G., Nikolova D., Gluud L. L., Simonetti R. G., Gluud C. (2007). Mortality in Randomized Trials of Antioxidant Supplements for Primary and Secondary Prevention. JAMA 297 (8), 842–857. 10.1001/jama.297.8.842 [DOI] [PubMed] [Google Scholar]
- Blandini F., Armentero M.-T. (2014). Dopamine Receptor Agonists for Parkinson's Disease. Expert Opin. Investigational Drugs 23 (3), 387–410. 10.1517/13543784.2014.869209 [DOI] [PubMed] [Google Scholar]
- Blesa J., Trigo-Damas I., Quiroga-Varela A., Jackson-Lewis V. R. (2015). Oxidative Stress and Parkinson's Disease. Front. Neuroanat. 9, 91. 10.3389/fnana.2015.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boakye D., Jansen L., Schöttker B., Jansen E. H. J. M., Schneider M., Halama N., et al. (2020). Blood Markers of Oxidative Stress Are Strongly Associated with Poorer Prognosis in Colorectal Cancer Patients. Int. J. Cancer 147 (9), 2373–2386. 10.1002/ijc.33018 [DOI] [PubMed] [Google Scholar]
- Bogacka I., Xie H., Bray G. A., Smith S. R. (2005). Pioglitazone Induces Mitochondrial Biogenesis in Human Subcutaneous Adipose Tissue In Vivo . Diabetes 54 (5), 1392–1399. 10.2337/diabetes.54.5.1392 [DOI] [PubMed] [Google Scholar]
- Bolten C. W., Blanner P. M., McDonald W. G., Staten N. R., Mazzarella R. A., Arhancet G. B., et al. (2007). Insulin Sensitizing Pharmacology of Thiazolidinediones Correlates with Mitochondrial Gene Expression rather Than Activation of PPAR Gamma. Gene Regul. Syst. Bio 1, 73–82. 10.1177/117762500700100008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifati V., Rizzu P., van Baren M. J., Schaap O., Breedveld G. J., Krieger E., et al. (2003). Mutations in the DJ-1 Gene Associated with Autosomal Recessive Early-Onset Parkinsonism. Science 299 (5604), 256–259. 10.1126/science.1077209 [DOI] [PubMed] [Google Scholar]
- Bonuccelli U. (2003). Comparing Dopamine Agonists in Parkinsonʼs Disease. Curr. Opin. Neurology 16 (Suppl. 1), S13–S19. 10.1097/00019052-200312001-00004 [DOI] [PubMed] [Google Scholar]
- Borghammer P., Hansen S. B., Eggers C., Chakravarty M., Vang K., Aanerud J., et al. (2012). Glucose Metabolism in Small Subcortical Structures in Parkinson's Disease. Acta Neurol. Scand. 125 (5), 303–310. 10.1111/j.1600-0404.2011.01556.x [DOI] [PubMed] [Google Scholar]
- Bosetti C., Santucci C., Gallus S., Martinetti M., La Vecchia C. (2020). Aspirin and the Risk of Colorectal and Other Digestive Tract Cancers: an Updated Meta-Analysis through 2019. Ann. Oncol. 31 (5), 558–568. 10.1016/j.annonc.2020.02.012 [DOI] [PubMed] [Google Scholar]
- Bouitbir J., Charles A.-L., Echaniz-Laguna A., Kindo M., Daussin F., Auwerx J., et al. (2012). Opposite Effects of Statins on Mitochondria of Cardiac and Skeletal Muscles: a 'mitohormesis' Mechanism Involving Reactive Oxygen Species and PGC-1. Eur. Heart J. 33 (11), 1397–1407. 10.1093/eurheartj/ehr224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouitbir J., Singh F., Charles A.-L., Schlagowski A.-I., Bonifacio A., Echaniz-Laguna A., et al. (2016). Statins Trigger Mitochondrial Reactive Oxygen Species-Induced Apoptosis in Glycolytic Skeletal Muscle. Antioxidants Redox Signal. 24 (2), 84–98. 10.1089/ars.2014.6190 [DOI] [PubMed] [Google Scholar]
- Boutaud O., Aronoff D. M., Richardson J. H., Marnett L. J., Oates J. A. (2002). Determinants of the Cellular Specificity of Acetaminophen as an Inhibitor of Prostaglandin H 2 Synthases. Proc. Natl. Acad. Sci. U.S.A. 99 (10), 7130–7135. 10.1073/pnas.102588199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Oshino N., Chance B. (1972). The Cellular Production of Hydrogen Peroxide. Biochem. J. 128 (3), 617–630. 10.1042/bj1280617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. G., Logan P. J., Jones G. C., Small M., Sattar N., Connell J. M. C., et al. (2011). AMP-activated Protein Kinase Is Activated in Adipose Tissue of Individuals with Type 2 Diabetes Treated with Metformin: a Randomised Glycaemia-Controlled Crossover Study. Diabetologia 54 (7), 1799–1809. 10.1007/s00125-011-2126-4 [DOI] [PubMed] [Google Scholar]
- Brezniceanu M. L., Liu F., Wei C. C., Tran S., Sachetelli S., Zhang S. L., et al. (2007). Catalase Overexpression Attenuates Angiotensinogen Expression and Apoptosis in Diabetic Mice. Kidney Int. 71 (9), 912–923. 10.1038/sj.ki.5002188 [DOI] [PubMed] [Google Scholar]
- Brian J. Tabner B. S. P., Stuart Turnbull B. S. P., Omar M.A. El-Agnaf B. S. P., David Allsop B. S. P. (2001). Production of Reactive Oxygen Species from Aggregating Proteins Implicated in Alzheimers Disease, Parkinsons Disease and Other Neurodegenerative Diseases. Ctmc 1 (6), 507–517. 10.2174/1568026013394822 [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohé R. (1999). Tissue-specific Functions of Individual Glutathione Peroxidases. Free Radic. Biol. Med. 27 (9-10), 951–965. 10.1016/s0891-5849(99)00173-2 [DOI] [PubMed] [Google Scholar]
- Brownlee M. (2001). Biochemistry and Molecular Cell Biology of Diabetic Complications. Nature 414 (6865), 813–820. 10.1038/414813a [DOI] [PubMed] [Google Scholar]
- Bruijn L. I., Miller T. M., Cleveland D. W. (2004). Unraveling the Mechanisms Involved in Motor Neuron Degeneration in ALS. Annu. Rev. Neurosci. 27, 723–749. 10.1146/annurev.neuro.27.070203.144244 [DOI] [PubMed] [Google Scholar]
- Brummett R. E., Fox K. E. (1989). Aminoglycoside-induced Hearing Loss in Humans. Antimicrob. Agents Chemother. 33 (6), 797–800. 10.1128/aac.33.6.797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunmair B., Staniek K., Gras F., Scharf N., Althaym A., Clara R., et al. (2004). Thiazolidinediones, like Metformin, Inhibit Respiratory Complex I. Diabetes 53 (4), 1052–1059. 10.2337/diabetes.53.4.1052 [DOI] [PubMed] [Google Scholar]
- Bubber P., Haroutunian V., Fisch G., Blass J. P., Gibson G. E. (2005). Mitochondrial Abnormalities in Alzheimer Brain: Mechanistic Implications. Ann. Neurol. 57 (5), 695–703. 10.1002/ana.20474 [DOI] [PubMed] [Google Scholar]
- Buldak L., Labuzek K., Buldak R. J., Kozlowski M., Machnik G., Liber S., et al. (2014). Metformin Affects Macrophages' Phenotype and Improves the Activity of Glutathione Peroxidase, Superoxide Dismutase, Catalase and Decreases Malondialdehyde Concentration in a Partially AMPK-independent Manner in LPS-Stimulated Human Monocytes/macrophages. Pharmacol. Rep. 66 (3), 418–429. [DOI] [PubMed] [Google Scholar]
- Bulua A. C., Simon A., Maddipati R., Pelletier M., Park H., Kim K.-Y., et al. (2011). Mitochondrial Reactive Oxygen Species Promote Production of Proinflammatory Cytokines and Are Elevated in TNFR1-Associated Periodic Syndrome (TRAPS). J. Exp. Med. 208 (3), 519–533. 10.1084/jem.20102049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnier M. (2001). Angiotensin II Type 1 Receptor Blockers. Circulation 103 (6), 904–912. 10.1161/01.cir.103.6.904 [DOI] [PubMed] [Google Scholar]
- Burton G. W., Ingold K. U. (1984). β-Carotene: an Unusual Type of Lipid Antioxidant. Science 224 (4649), 569–573. 10.1126/science.6710156 [DOI] [PubMed] [Google Scholar]
- Burton G. W., Traber M. G. (1990). Vitamin E: Antioxidant Activity, Biokinetics, and Bioavailability. Annu. Rev. Nutr. 10, 357–382. 10.1146/annurev.nu.10.070190.002041 [DOI] [PubMed] [Google Scholar]
- Cai H., Rasulova M., Vandemeulebroucke L., Meagher L., Vlaeminck C., Dhondt I., et al. (2017). Life-Span Extension by Axenic Dietary Restriction Is Independent of the Mitochondrial Unfolded Protein Response and Mitohormesis in Caenorhabditis elegans . J. Gerontol. A Biol. Sci. Med. Sci. 72 (10), 1311–1318. 10.1093/gerona/glx013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell R. B., Toque H. A., Narayanan S. P., Caldwell R. W. (2015). Arginase: an Old Enzyme with New Tricks. Trends Pharmacol. Sci. 36 (6), 395–405. 10.1016/j.tips.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins M. J., Reddy P. H. (2011). Amyloid Beta Impairs Mitochondrial Anterograde Transport and Degenerates Synapses in Alzheimer's Disease Neurons. Biochimica Biophysica Acta (BBA) - Mol. Basis Dis. 1812 (4), 507–513. 10.1016/j.bbadis.2011.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins M., Manczak M., Reddy P. (2012). Mitochondria-Targeted Antioxidant SS31 Prevents Amyloid Beta-Induced Mitochondrial Abnormalities and Synaptic Degeneration in Alzheimer’s Disease. Pharmaceuticals 5 (10), 1103–1119. 10.3390/ph5101103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. M., Bellman S. M., Stephenson M. D., Lisy. K. (2017). Metformin Reduces All-Cause Mortality and Diseases of Ageing Independent of its Effect on Diabetes Control: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 40, 31–44. 10.1016/j.arr.2017.08.003 [DOI] [PubMed] [Google Scholar]
- Campisi J., Kapahi P., Lithgow G. J., Melov S., Newman J. C., Verdin E. (2019). From Discoveries in Ageing Research to Therapeutics for Healthy Ageing. Nature 571 (7764), 183–192. 10.1038/s41586-019-1365-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canet-Avilés R. M., Wilson M. A., Miller D. W., Ahmad R., McLendon C., Bandyopadhyay S., et al. (2004). The Parkinson's Disease Protein DJ-1 Is Neuroprotective Due to Cysteine-Sulfinic Acid-Driven Mitochondrial Localization. Proc. Natl. Acad. Sci. U.S.A. 101 (24), 9103–9108. 10.1073/pnas.0402959101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale D., Colombo A., Bacchiani G., Tedeschi I., Meroni C. A., Veglia F., et al. (2015). Early Detection of Anthracycline Cardiotoxicity and Improvement with Heart Failure Therapy. Circulation 131 (22), 1981–1988. 10.1161/circulationaha.114.013777 [DOI] [PubMed] [Google Scholar]
- Cardoso S., Moreira P. I. (2020). Antidiabetic Drugs for Alzheimer's and Parkinson's Diseases: Repurposing Insulin, Metformin, and Thiazolidinediones. Int. Rev. Neurobiol. 155, 37–64. 10.1016/bs.irn.2020.02.010 [DOI] [PubMed] [Google Scholar]
- Carlsson C. M., Papcke-Benson K., Carnes M., McBride P. E., Stein J. H. (2002). Health-related Quality of Life and Long-Term Therapy with Pravastatin and Tocopherol (Vitamin E) in Older Adults. Drugs & Aging 19 (10), 793–805. 10.2165/00002512-200219100-00008 [DOI] [PubMed] [Google Scholar]
- Carrera-Juliá S., Moreno M. L., Barrios C., de la Rubia Ortí J. E., Drehmer E. (2020). Antioxidant Alternatives in the Treatment of Amyotrophic Lateral Sclerosis: A Comprehensive Review. Front. Physiol. 11, 63. 10.3389/fphys.2020.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriello A., Mercuri F., Quagliaro L., Assaloni R., Motz E., Tonutti L., et al. (2001). Detection of Nitrotyrosine in the Diabetic Plasma: Evidence of Oxidative Stress. Diabetologia 44 (7), 834–838. 10.1007/s001250100529 [DOI] [PubMed] [Google Scholar]
- Chakraborty A., Chowdhury S., Bhattacharyya M. (2011). Effect of Metformin on Oxidative Stress, Nitrosative Stress and Inflammatory Biomarkers in Type 2 Diabetes Patients. Diabetes Res. Clin. Pract. 93 (1), 56–62. 10.1016/j.diabres.2010.11.030 [DOI] [PubMed] [Google Scholar]
- Chan A. T., Arber N., Burn J., Chia W. K., Elwood P., Hull M. A., et al. (2012). Aspirin in the Chemoprevention of Colorectal Neoplasia: an Overview. Cancer Prev. Res. 5 (2), 164–178. 10.1158/1940-6207.capr-11-0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. Y., Slot J. W., Geuze H. J., Crapo J. D. (1988). Molecular Immunocytochemistry of the CuZn Superoxide Dismutase in Rat Hepatocytes. J. Cell Biol. 107 (6 Pt 1), 2169–2179. 10.1083/jcb.107.6.2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao E. C. (2014). SGLT-2 Inhibitors: A New Mechanism for Glycemic Control. Clin. Diabetes 32 (1), 4–11. 10.2337/diaclin.32.1.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi R. K., Beal M. F. (2008). Mitochondrial Approaches for Neuroprotection. Ann. N. Y. Acad. Sci. 1147, 395–412. 10.1196/annals.1427.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzispyrou I. A., Held N. M., Mouchiroud L., Auwerx J., Houtkooper R. H. (2015). Tetracycline Antibiotics Impair Mitochondrial Function and its Experimental Use Confounds Research. Cancer Res. 75 (21), 4446–4449. 10.1158/0008-5472.can-15-1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Zhao J., Zhang S., Wu W., Qi R. (2012). Aspirin Inhibits the Production of Reactive Oxygen Species by Downregulating Nox4 and Inducible Nitric Oxide Synthase in Human Endothelial Cells Exposed to Oxidized Low-Density Lipoprotein. J. Cardiovasc. Pharmacol. 59 (5), 405–412. 10.1097/fjc.0b013e318248acba [DOI] [PubMed] [Google Scholar]
- Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J., et al. (2018). Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 9 (6), 7204–7218. 10.18632/oncotarget.23208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Na R., Gu M., Salmon A. B., Liu Y., Liang H., et al. (2008). Reduction of Mitochondrial H2O2by Overexpressing Peroxiredoxin 3 Improves Glucose Tolerance in Mice. Aging Cell 7 (6), 866–878. 10.1111/j.1474-9726.2008.00432.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhou K., Wang R., Liu Y., Kwak Y.-D., Ma T., et al. (2009). Antidiabetic Drug Metformin (Glucophage R ) Increases Biogenesis of Alzheimer's Amyloid Peptides via Up-Regulating BACE1 Transcription. Proc. Natl. Acad. Sci. U.S.A. 106 (10), 3907–3912. 10.1073/pnas.0807991106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Gang X., Liu Y., Wang G., Zhao X., Wang G. (2020). Mitochondrial Dysfunction Plays a Key Role in the Development of Neurodegenerative Diseases in Diabetes. Am. J. Physiology-Endocrinology Metabolism 318 (5), E750–E764. 10.1152/ajpendo.00179.2019 [DOI] [PubMed] [Google Scholar]
- Chiang M.-C., Cheng Y.-C., Chen S.-J., Yen C.-H., Huang R.-N. (2016). Metformin Activation of AMPK-dependent Pathways Is Neuroprotective in Human Neural Stem Cells against Amyloid-Beta-Induced Mitochondrial Dysfunction. Exp. Cell Res. 347 (2), 322–331. 10.1016/j.yexcr.2016.08.013 [DOI] [PubMed] [Google Scholar]
- Chin-Hsiao T. (2019). Metformin and the Risk of Dementia in Type 2 Diabetes Patients. Aging Dis. 10 (1), 37–48. 10.14336/ad.2017.1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choksi K. B., Nuss J. E., Deford J. H., Papaconstantinou J. (2008). Age-related Alterations in Oxidatively Damaged Proteins of Mouse Skeletal Muscle Mitochondrial Electron Transport Chain Complexes. Free Radic. Biol. Med. 45 (6), 826–838. 10.1016/j.freeradbiomed.2008.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton R., Clark J. B., Sharpe M. (2005). Cytochrome C Release from Rat Brain Mitochondria Is Proportional to the Mitochondrial Functional Deficit: Implications for Apoptosis and Neurodegenerative Disease. J. Neurochem. 92 (4), 840–849. 10.1111/j.1471-4159.2004.02918.x [DOI] [PubMed] [Google Scholar]
- Collaborators Gbdn (2019). Global, Regional, and National Burden of Neurological Disorders, 1990-2016: a Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18 (5), 459–480. 10.1016/S1474-4422(18)30499-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins Y., Chouchani E. T., James A. M., Menger K. E., Cochemé H. M., Murphy M. P. (2012). Mitochondrial Redox Signalling at a Glance. J. Cell Sci. 125 (Pt 4), 801–806. 10.1242/jcs.098475 [DOI] [PubMed] [Google Scholar]
- Conarello S. L., Li Z., Ronan J., Roy R. S., Zhu L., Jiang G., et al. (2003). Mice Lacking Dipeptidyl Peptidase IV Are Protected against Obesity and Insulin Resistance. Proc. Natl. Acad. Sci. U.S.A. 100 (11), 6825–6830. 10.1073/pnas.0631828100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia-Melo C., Marques F. D., Anderson R., Hewitt G., Hewitt R., Cole J., et al. (2016). Mitochondria Are Required for Pro-ageing Features of the Senescent Phenotype. EMBO J. 35 (7), 724–742. 10.15252/embj.201592862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortellini G., Caruso C., Romano A. (2017). Aspirin Challenge and Desensitization: How, when and Why. Curr. Opin. Allergy Clin. Immunol. 17 (4), 247–254. 10.1097/aci.0000000000000374 [DOI] [PubMed] [Google Scholar]
- Costa V. M., Silva R., Tavares L. C., Vitorino R., Amado F., Carvalho F., et al. (2009). Adrenaline and Reactive Oxygen Species Elicit Proteome and Energetic Metabolism Modifications in Freshly Isolated Rat Cardiomyocytes. Toxicology 260 (1-3), 84–96. 10.1016/j.tox.2009.03.012 [DOI] [PubMed] [Google Scholar]
- Crack P. J., Cimdins K., Ali U., Hertzog P. J., Iannello R. C. (2006). Lack of Glutathione Peroxidase-1 Exacerbates Aβ-Mediated Neurotoxicity in Cortical Neurons. J. Neural Transm. 113 (5), 645–657. 10.1007/s00702-005-0352-y [DOI] [PubMed] [Google Scholar]
- Cranswick N., Coghlan D. (2000). Paracetamol Efficacy and Safety in Children. Am. J. Ther. 7 (2), 135–142. 10.1097/00045391-200007020-00010 [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Oury T., Rabouille C., Slot J. W., Chang L. Y. (1992). Copper,zinc Superoxide Dismutase Is Primarily a Cytosolic Protein in Human Cells. Proc. Natl. Acad. Sci. U.S.A. 89 (21), 10405–10409. 10.1073/pnas.89.21.10405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A., Fassett R. G., Geraghty D. P., Kunde D. A., Ball M. J., Robertson I. K., et al. (2012). Relationships between Single Nucleotide Polymorphisms of Antioxidant Enzymes and Disease. Gene 501 (2), 89–103. 10.1016/j.gene.2012.04.011 [DOI] [PubMed] [Google Scholar]
- Crofford L. J. (1997). COX-1 and COX-2 Tissue Expression: Implications and Predictions. J. Rheumatol. Suppl. 49, 15–19. [PubMed] [Google Scholar]
- Cryer B. (2002). Gastrointestinal Safety of Low-Dose Aspirin. Am. J. Manag. Care 8 (22 Suppl. l), S701–S708. [PubMed] [Google Scholar]
- Dai D.-F., Santana L. F., Vermulst M., Tomazela D. M., Emond M. J., MacCoss M. J., et al. (2009). Overexpression of Catalase Targeted to Mitochondria Attenuates Murine Cardiac Aging. Circulation 119 (21), 2789–2797. 10.1161/circulationaha.108.822403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Liu M., Ai Q., Lin L., Wu K., Deng X., et al. (2014). Involvement of Catalase in the Protective Benefits of Metformin in Mice with Oxidative Liver Injury. Chemico-Biological Interact. 216, 34–42. 10.1016/j.cbi.2014.03.013 [DOI] [PubMed] [Google Scholar]
- Davì G., Ciabattoni G., Consoli A., Mezzetti A., Falco A., Santarone S., et al. (1999). In Vivo formation of 8-Iso-Prostaglandin F2alpha and Platelet Activation in Diabetes Mellitus: Effects of Improved Metabolic Control and Vitamin E Supplementation. Circulation 99 (2), 224–229. 10.1161/01.cir.99.2.224 [DOI] [PubMed] [Google Scholar]
- Day C. (1999). Thiazolidinediones: a New Class of Antidiabetic Drugs. Diabet. Med. 16 (3), 179–192. 10.1046/j.1464-5491.1999.00023.x [DOI] [PubMed] [Google Scholar]
- de la Monte S. M., Wands J. R. (2005). Review of Insulin and Insulin-like Growth Factor Expression, Signaling, and Malfunction in the Central Nervous System: Relevance to Alzheimer's Disease. Jad 7 (1), 45–61. 10.3233/jad-2005-7106 [DOI] [PubMed] [Google Scholar]
- Deacon C. F., Mannucci E., Ahrén B. (2012). Glycaemic Efficacy of Glucagon-like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors as Add-On Therapy to Metformin in Subjects with Type 2 Diabetes-A Review and Meta Analysis. Diabetes Obes. Metab. 14 (8), 762–767. 10.1111/j.1463-1326.2012.01603.x [DOI] [PubMed] [Google Scholar]
- Deeks E. D. (2015). Safinamide: First Global Approval. Drugs 75 (6), 705–711. 10.1007/s40265-015-0389-7 [DOI] [PubMed] [Google Scholar]
- DeKosky S. T., Scheff S. W., Styren S. D. (1996). Structural Correlates of Cognition in Dementia: Quantification and Assessment of Synapse Change. Neurodegeneration 5 (4), 417–421. 10.1006/neur.1996.0056 [DOI] [PubMed] [Google Scholar]
- Del Guerra S., Grupillo M., Masini M., Lupi R., Bugliani M., Torri S., et al. (2007). Gliclazide Protects Human Islet Beta-Cells from Apoptosis Induced by Intermittent High Glucose. Diabetes Metab. Res. Rev. 23 (3), 234–238. 10.1002/dmrr.680 [DOI] [PubMed] [Google Scholar]
- Devi L., Prabhu B. M., Galati D. F., Avadhani N. G., Anandatheerthavarada H. K. (2006). Accumulation of Amyloid Precursor Protein in the Mitochondrial Import Channels of Human Alzheimer's Disease Brain Is Associated with Mitochondrial Dysfunction. J. Neurosci. 26 (35), 9057–9068. 10.1523/jneurosci.1469-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos D., Moreau C., Devedjian J. C., Kluza J., Petrault M., Laloux C., et al. (2014). Targeting Chelatable Iron as a Therapeutic Modality in Parkinson's Disease. Antioxidants Redox Signal. 21 (2), 195–210. 10.1089/ars.2013.5593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter D. T., Wells F. R., Agid F., Agid Y., Lees A. J., Jenner P., et al. (1987). Increased Nigral Iron Content in Postmortem Parkinsonian Brain. Lancet 330 (8569), 1219–1220. 10.1016/s0140-6736(87)91361-4 [DOI] [PubMed] [Google Scholar]
- Dexter D. T., Wells F. R., Lee A. J., Agid F., Agid Y., Jenner P., et al. (1989). Increased Nigral Iron Content and Alterations in Other Metal Ions Occurring in Brain in Parkinson's Disease. J. Neurochem. 52 (6), 1830–1836. 10.1111/j.1471-4159.1989.tb07264.x [DOI] [PubMed] [Google Scholar]
- Dias V., Junn E., Mouradian M. M. (2013). The Role of Oxidative Stress in Parkinson's Disease. J. Park. Dis. 3 (4), 461–491. 10.3233/jpd-130230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikalova A. E., Bikineyeva A. T., Budzyn K., Nazarewicz R. R., McCann L., Lewis W., et al. (2010). Therapeutic Targeting of Mitochondrial Superoxide in Hypertension. Circulation Res. 107 (1), 106–116. 10.1161/circresaha.109.214601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz Vilela D., Gomes Peixoto L., Teixeira R. R., Belele Baptista N., Carvalho Caixeta D., Vieira de Souza A., et al. (2016). The Role of Metformin in Controlling Oxidative Stress in Muscle of Diabetic Rats. Oxid. Med. Cell Longev. 2016, 6978625. 10.1155/2016/6978625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docrat T. F., Nagiah S., Naicker N., Baijnath S., Singh S., Chuturgoon A. A. (2020). The Protective Effect of Metformin on Mitochondrial Dysfunction and Endoplasmic Reticulum Stress in Diabetic Mice Brain. Eur. J. Pharmacol. 875, 173059. 10.1016/j.ejphar.2020.173059 [DOI] [PubMed] [Google Scholar]
- Domingueti C. P., Dusse L. M. S. A., Carvalho M. d. G., de Sousa L. P., Gomes K. B., Fernandes A. P. (2016). Diabetes Mellitus: The Linkage between Oxidative Stress, Inflammation, Hypercoagulability and Vascular Complications. J. Diabetes its Complicat. 30 (4), 738–745. 10.1016/j.jdiacomp.2015.12.018 [DOI] [PubMed] [Google Scholar]
- Dorsey E. R., Bloem B. R. (2018). The Parkinson Pandemic-A Call to Action. JAMA Neurol. 75 (1), 9–10. 10.1001/jamaneurol.2017.3299 [DOI] [PubMed] [Google Scholar]
- Du H., Guo L., Yan S., Sosunov A. A., McKhann G. M., ShiDu Yan S. (2010). Early Deficits in Synaptic Mitochondria in an Alzheimer's Disease Mouse Model. Proc. Natl. Acad. Sci. U.S.A. 107 (43), 18670–18675. 10.1073/pnas.1006586107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L., Allen G. F., Mamais A., Ling H., Li A., Duberley K. E., et al. (2014). Dysregulation of Glucose Metabolism Is an Early Event in Sporadic Parkinson's Disease. Neurobiol. Aging 35 (5), 1111–1115. 10.1016/j.neurobiolaging.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorakova K., Payne C. M., Landowski T. H., Tome M. E., Halperin D. S., Dorr R. T. (2002). Imexon Activates an Intrinsic Apoptosis Pathway in RPMI8226 Myeloma Cells. Anti-Cancer Drugs 13 (10), 1031–1042. 10.1097/00001813-200211000-00007 [DOI] [PubMed] [Google Scholar]
- Dwyer D. J., Kohanski M. A., Hayete B., Collins J. J. (2007). Gyrase Inhibitors Induce an Oxidative Damage Cellular Death Pathway in Escherichia coli . Mol. Syst. Biol. 3, 91. 10.1038/msb4100135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mir M.-Y., Nogueira V., Fontaine E., Avéret N., Rigoulet M., Leverve X. (2000). Dimethylbiguanide Inhibits Cell Respiration via an Indirect Effect Targeted on the Respiratory Chain Complex I. J. Biol. Chem. 275 (1), 223–228. 10.1074/jbc.275.1.223 [DOI] [PubMed] [Google Scholar]
- Endo H., Nito C., Kamada H., Yu F., Chan P. H. (2007). Reduction in Oxidative Stress by Superoxide Dismutase Overexpression Attenuates Acute Brain Injury after Subarachnoid Hemorrhage via Activation of Akt/Glycogen Synthase Kinase-3β Survival Signaling. J. Cereb. Blood Flow. Metab. 27 (5), 975–982. 10.1038/sj.jcbfm.9600399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergul A., Johansen J. S., Strømhaug C., Harris A. K., Hutchinson J., Tawfik A., et al. (2005). Vascular Dysfunction of Venous Bypass Conduits Is Mediated by Reactive Oxygen Species in Diabetes: Role of Endothelin-1. J. Pharmacol. Exp. Ther. 313 (1), 70–77. 10.1124/jpet.104.078105 [DOI] [PubMed] [Google Scholar]
- Esh C. J., Chrismas B. C. R., Mauger A. R., Taylor L. (2021). Pharmacological Hypotheses: Is Acetaminophen Selective in its Cyclooxygenase Inhibition? Pharmacol. Res. Perspect. 9 (4), e00835. 10.1002/prp2.835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves A. R. F., Domingues A. F., Ferreira I. L., Januário C., Swerdlow R. H., Oliveira C. R., et al. (2008). Mitochondrial Function in Parkinson's Disease Cybrids Containing an Nt2 Neuron-like Nuclear Background. Mitochondrion 8 (3), 219–228. 10.1016/j.mito.2008.03.004 [DOI] [PubMed] [Google Scholar]
- Evans J. L., Goldfine I. D., Maddux B. A., Grodsky G. M. (2003). Are Oxidative Stress−Activated Signaling Pathways Mediators of Insulin Resistance and β-Cell Dysfunction? Diabetes 52 (1), 1–8. 10.2337/diabetes.52.1.1 [DOI] [PubMed] [Google Scholar]
- Fabbrini G., Brotchie J. M., Grandas F., Nomoto M., Goetz C. G. (2007). Levodopa‐induced Dyskinesias. Mov. Disord. 22 (10), 1379–1389. 10.1002/mds.21475 [DOI] [PubMed] [Google Scholar]
- Farah C., Michel L. Y. M., Balligand J.-L. (2018). Nitric Oxide Signalling in Cardiovascular Health and Disease. Nat. Rev. Cardiol. 15 (5), 292–316. 10.1038/nrcardio.2017.224 [DOI] [PubMed] [Google Scholar]
- Farzam K., Jan A. (2021). Beta Blockers. Treasure Island, FL: StatPearls. [PubMed] [Google Scholar]
- Faulkner M. A. (2014). Safety Overview of FDA-Approved Medications for the Treatment of the Motor Symptoms of Parkinson's Disease. Expert Opin. Drug Saf. 13 (8), 1055–1069. 10.1517/14740338.2014.931369 [DOI] [PubMed] [Google Scholar]
- Fernandes A. C., Filipe P. M., Freitas J. P., Manso C. F. (1996). Different Effects of Thiol and Nonthiol Ace Inhibitors on Copper-Induced Lipid and Protein Oxidative Modification. Free Radic. Biol. Med. 20 (4), 507–514. 10.1016/0891-5849(95)02086-1 [DOI] [PubMed] [Google Scholar]
- Ferrannini E., Muscelli E., Frascerra S., Baldi S., Mari A., Heise T., et al. (2014). Metabolic Response to Sodium-Glucose Cotransporter 2 Inhibition in Type 2 Diabetic Patients. J. Clin. Invest. 124 (2), 499–508. 10.1172/jci72227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiordaliso F., Cuccovillo I., Bianchi R., Bai A., Doni M., Salio M., et al. (2006). Cardiovascular Oxidative Stress Is Reduced by an ACE Inhibitor in a Rat Model of Streptozotocin-Induced Diabetes. Life Sci. 79 (2), 121–129. 10.1016/j.lfs.2005.12.036 [DOI] [PubMed] [Google Scholar]
- Flohé L., Toppo S., Cozza G., Ursini F. (2011). A Comparison of Thiol Peroxidase Mechanisms. Antioxidants Redox Signal. 15 (3), 763–780. 10.1089/ars.2010.3397 [DOI] [PubMed] [Google Scholar]
- Floor E., Wetzel M. G. (1998). Increased Protein Oxidation in Human Substantia Nigra Pars Compacta in Comparison with Basal Ganglia and Prefrontal Cortex Measured with an Improved Dinitrophenylhydrazine Assay. J. Neurochem. 70 (1), 268–275. 10.1046/j.1471-4159.1998.70010268.x [DOI] [PubMed] [Google Scholar]
- Flower R. J., Vane J. R. (1972). Inhibition of Prostaglandin Synthetase in Brain Explains the Anti-pyretic Activity of Paracetamol (4-acetamidophenol). Nature 240 (5381), 410–411. 10.1038/240410a0 [DOI] [PubMed] [Google Scholar]
- Foretz M., Guigas B., Viollet B. (2019). Understanding the Glucoregulatory Mechanisms of Metformin in Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 15 (10), 569–589. 10.1038/s41574-019-0242-2 [DOI] [PubMed] [Google Scholar]
- Francis D., Richards G. M., Forouzannia A., Mehta M. P., Khuntia D. (2009). Motexafin Gadolinium: a Novel Radiosensitizer for Brain Tumors. Expert Opin. Pharmacother. 10 (13), 2171–2180. 10.1517/14656560903179325 [DOI] [PubMed] [Google Scholar]
- Frijhoff J., Winyard P. G., Zarkovic N., Davies S. S., Stocker R., Cheng D., et al. (2015). Clinical Relevance of Biomarkers of Oxidative Stress. Antioxidants Redox Signal. 23 (14), 1144–1170. 10.1089/ars.2015.6317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frölich L., Blum-Degen D., Bernstein H. G., Engelsberger S., Humrich J., Laufer S., et al. (1998). Brain Insulin and Insulin Receptors in Aging and Sporadic Alzheimer's Disease. J. Neural Transm. (Vienna) 105 (4-5), 423–438. 10.1007/s007020050068 [DOI] [PubMed] [Google Scholar]
- Fu Y., Cheng W. H., Porres J. M., Ross D. A., Lei X. G. (1999). Knockout of Cellular Glutathione Peroxidase Gene Renders Mice Susceptible to Diquat-Induced Oxidative Stress. Free Radic. Biol. Med. 27 (5-6), 605–611. 10.1016/s0891-5849(99)00104-5 [DOI] [PubMed] [Google Scholar]
- Fuentes-Retamal S., Sandoval-Acuña C., Peredo-Silva L., Guzmán-Rivera D., Pavani M., Torrealba N., et al. (2020). Complex Mitochondrial Dysfunction Induced by TPP+-Gentisic Acid and Mitochondrial Translation Inhibition by Doxycycline Evokes Synergistic Lethality in Breast Cancer Cells. Cells 9 (2). 10.3390/cells9020407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman D., Campisi J., Verdin E., Carrera-Bastos P., Targ S., Franceschi C., et al. (2019). Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 25 (12), 1822–1832. 10.1038/s41591-019-0675-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galadari S., Rahman A., Pallichankandy S., Thayyullathil F. (2017). Reactive Oxygen Species and Cancer Paradox: To Promote or to Suppress? Free Radic. Biol. Med. 104, 144–164. 10.1016/j.freeradbiomed.2017.01.004 [DOI] [PubMed] [Google Scholar]
- Gammer W., Brobäck L.-G. (1984). Clinical Comparison of Orgotein and Methylpredisolone Acetate in the Treatment of Osteoarthrosis of the Knee Joint. Scand. J. Rheumatology 13 (2), 108–112. 10.3109/03009748409100372 [DOI] [PubMed] [Google Scholar]
- Gao S., Liu J. (2017). Association between Circulating Oxidized Low‐density Lipoprotein and Atherosclerotic Cardiovascular Disease. Chronic Dis. Transl. Med. 3 (2), 89–94. 10.1016/j.cdtm.2017.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Vanhoutte P. M. (2012). Nebivolol. J. Cardiovasc. Pharmacol. 59 (1), 16–21. 10.1097/fjc.0b013e3182073e27 [DOI] [PubMed] [Google Scholar]
- Garg G., Singh S., Singh A. K., Rizvi S. I. (2017). Antiaging Effect of Metformin on Brain in Naturally Aged and Accelerated Senescence Model of Rat. Rejuvenation Res. 20 (3), 173–182. 10.1089/rej.2016.1883 [DOI] [PubMed] [Google Scholar]
- Gegg M. E., Schapira A. H. V. (2016). Mitochondrial Dysfunction Associated with Glucocerebrosidase Deficiency. Neurobiol. Dis. 90, 43–50. 10.1016/j.nbd.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genestra M. (2007). Oxyl Radicals, Redox-Sensitive Signalling Cascades and Antioxidants. Cell. Signal. 19 (9), 1807–1819. 10.1016/j.cellsig.2007.04.009 [DOI] [PubMed] [Google Scholar]
- Ghanem C. I., Pérez M. J., Manautou J. E., Mottino A. D. (2016). Acetaminophen from Liver to Brain: New Insights into Drug Pharmacological Action and Toxicity. Pharmacol. Res. 109, 119–131. 10.1016/j.phrs.2016.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R., Alajbegovic A., Gomes A. V. (2015). NSAIDs and Cardiovascular Diseases: Role of Reactive Oxygen Species. Oxid. Med. Cell Longev. 2015, 536962. 10.1155/2015/536962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Patel N., Rahn D., McAllister J., Sadeghi S., Horwitz G., et al. (2007). The Thiazolidinedione Pioglitazone Alters Mitochondrial Function in Human Neuron-like Cells. Mol. Pharmacol. 71 (6), 1695–1702. 10.1124/mol.106.033845 [DOI] [PubMed] [Google Scholar]
- Gibson G. E., Sheu K. F., Blass J. P. (1998). Abnormalities of Mitochondrial Enzymes in Alzheimer Disease. J. Neural Transm. (Vienna) 105 (8-9), 855–870. 10.1007/s007020050099 [DOI] [PubMed] [Google Scholar]
- Gil-Bea F., Akterin S., Persson T., Mateos L., Sandebring A., Avila-Cariño J., et al. (2012). Thioredoxin-80 Is a Product of Alpha-Secretase Cleavage that Inhibits Amyloid-Beta Aggregation and Is Decreased in Alzheimer's Disease Brain. EMBO Mol. Med. 4 (10), 1097–1111. 10.1002/emmm.201201462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilks W. P., Abou-Sleiman P. M., Gandhi S., Jain S., Singleton A., Lees A. J., et al. (2005). A Common LRRK2 Mutation in Idiopathic Parkinson's Disease. Lancet 365 (9457), 415–416. 10.1016/s0140-6736(05)17830-1 [DOI] [PubMed] [Google Scholar]
- Giorgio M., Migliaccio E., Orsini F., Paolucci D., Moroni M., Contursi C., et al. (2005). Electron Transfer between Cytochrome C and p66Shc Generates Reactive Oxygen Species that Trigger Mitochondrial Apoptosis. Cell 122 (2), 221–233. 10.1016/j.cell.2005.05.011 [DOI] [PubMed] [Google Scholar]
- Glossmann H. H., Lutz O. M. D. (2019). Metformin and Aging: A Review. Gerontology 65 (6), 581–590. 10.1159/000502257 [DOI] [PubMed] [Google Scholar]
- Goldstein D. S., Sullivan P., Holmes C., Miller G. W., Alter S., Strong R., et al. (2013). Determinants of Buildup of the Toxic Dopamine Metabolite DOPAL in Parkinson's Disease. J. Neurochem. 126 (5), 591–603. 10.1111/jnc.12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves R. L. S., Quinlan C. L., Perevoshchikova I. V., Hey-Mogensen M., Brand M. D. (2015). Sites of Superoxide and Hydrogen Peroxide Production by Muscle Mitochondria Assessed Ex Vivo under Conditions Mimicking Rest and Exercise. J. Biol. Chem. 290 (1), 209–227. 10.1074/jbc.m114.619072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good P. F., Werner P., Hsu A., Olanow C. W., Perl D. P. (1996). Evidence of Neuronal Oxidative Damage in Alzheimer's Disease. Am. J. Pathol. 149 (1), 21–28. [PMC free article] [PubMed] [Google Scholar]
- Goodman M., Bostick R. M., Kucuk O., Jones D. P. (2011). Clinical Trials of Antioxidants as Cancer Prevention Agents: Past, Present, and Future. Free Radic. Biol. Med. 51 (5), 1068–1084. 10.1016/j.freeradbiomed.2011.05.018 [DOI] [PubMed] [Google Scholar]
- Gootz T. D., Barrett J. F., Sutcliffe J. A. (1990). Inhibitory Effects of Quinolone Antibacterial Agents on Eucaryotic Topoisomerases and Related Test Systems. Antimicrob. Agents Chemother. 34 (1), 8–12. 10.1128/aac.34.1.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace A. A., Bunney B. S. (1983). Intracellular and Extracellular Electrophysiology of Nigral Dopaminergic Neurons-2. Action Potential Generating Mechanisms and Morphological Correlates. Neuroscience 10 (2), 317–331. 10.1016/0306-4522(83)90136-7 [DOI] [PubMed] [Google Scholar]
- Gradinaru D., Borsa C., Ionescu C., Margina D. (2013). Advanced Oxidative and Glycoxidative Protein Damage Markers in the Elderly with Type 2 Diabetes. J. Proteomics 92, 313–322. 10.1016/j.jprot.2013.03.034 [DOI] [PubMed] [Google Scholar]
- Gradman A. H. (2002). AT(1)-receptor Blockers: Differences that Matter. J. Hum. Hypertens. 16 Suppl 3 (Suppl. 3), S9–S16. 10.1038/sj.jhh.1001434 [DOI] [PubMed] [Google Scholar]
- Graham D. G. (1978). Oxidative Pathways for Catecholamines in the Genesis of Neuromelanin and Cytotoxic Quinones. Mol. Pharmacol. 14 (4), 633–643. [PubMed] [Google Scholar]
- Graham G. G., Davies M. J., Day R. O., Mohamudally A., Scott K. F. (2013). The Modern Pharmacology of Paracetamol: Therapeutic Actions, Mechanism of Action, Metabolism, Toxicity and Recent Pharmacological Findings. Inflammopharmacol 21 (3), 201–232. 10.1007/s10787-013-0172-x [DOI] [PubMed] [Google Scholar]
- Grant S. S., Kaufmann B. B., Chand N. S., Haseley N., Hung D. T. (2012). Eradication of Bacterial Persisters with Antibiotic-Generated Hydroxyl Radicals. Proc. Natl. Acad. Sci. U.S.A. 109 (30), 12147–12152. 10.1073/pnas.1203735109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. W., Burger G., Lang B. F. (1999). Mitochondrial Evolution. Science 283 (5407), 1476–1481. 10.1126/science.283.5407.1476 [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. (1979). Potent and Specific Inhibition of Glutathione Synthesis by Buthionine Sulfoximine (S-N-Butyl Homocysteine Sulfoximine). J. Biol. Chem. 254 (16), 7558–7560. 10.1016/s0021-9258(18)35980-5 [DOI] [PubMed] [Google Scholar]
- Groop L. C. (1992). Sulfonylureas in NIDDM. Diabetes Care 15 (6), 737–754. 10.2337/diacare.15.6.737 [DOI] [PubMed] [Google Scholar]
- Grzybowska M., Bober J., Olszewska M. (2011). Metformina - Mechanizmy Działania I Zastosowanie W Terapii Cukrzycy Typu 2[i][/i]. Postepy Hig. Med. Dosw 65, 277–285. 10.5604/17322693.941655 [DOI] [PubMed] [Google Scholar]
- Guo M., Mi J., Jiang Q. M., Xu J. M., Tang Y. Y., Tian G., et al. (2014). Metformin May Produce Antidepressant Effects through Improvement of Cognitive Function Among Depressed Patients with Diabetes Mellitus. Clin. Exp. Pharmacol. Physiol. 41 (9), 650–656. 10.1111/1440-1681.12265 [DOI] [PubMed] [Google Scholar]
- Gupta A., Bisht B., Dey C. S. (2011). Peripheral Insulin-Sensitizer Drug Metformin Ameliorates Neuronal Insulin Resistance and Alzheimer's-like Changes. Neuropharmacology 60 (6), 910–920. 10.1016/j.neuropharm.2011.01.033 [DOI] [PubMed] [Google Scholar]
- Gurzov E. N., Eizirik D. L. (2011). Bcl-2 Proteins in Diabetes: Mitochondrial Pathways of β-cell Death and Dysfunction. Trends Cell Biol. 21 (7), 424–431. 10.1016/j.tcb.2011.03.001 [DOI] [PubMed] [Google Scholar]
- Gusdon A. M., Zhu J., Van Houten B., Chu C. T. (2012). ATP13A2 Regulates Mitochondrial Bioenergetics through Macroautophagy. Neurobiol. Dis. 45 (3), 962–972. 10.1016/j.nbd.2011.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik T. J., Mussa S., Gastaldi D., Sadowski J., Ratnatunga C., Pillai R., et al. (2002). Mechanisms of Increased Vascular Superoxide Production in Human Diabetes Mellitus. Circulation 105 (14), 1656–1662. 10.1161/01.cir.0000012748.58444.08 [DOI] [PubMed] [Google Scholar]
- Guzy R. D., Schumacker P. T. (2006). Oxygen Sensing by Mitochondria at Complex III: the Paradox of Increased Reactive Oxygen Species during Hypoxia. Exp. Physiol. 91 (5), 807–819. 10.1113/expphysiol.2006.033506 [DOI] [PubMed] [Google Scholar]
- Gwathmey T. M., Pendergrass K. D., Reid S. D., Rose J. C., Diz D. I., Chappell M. C. (2010). Angiotensin-(1-7)-angiotensin-converting Enzyme 2 Attenuates Reactive Oxygen Species Formation to Angiotensin II within the Cell Nucleus. Hypertension 55 (1), 166–171. 10.1161/hypertensionaha.109.141622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi J., Aroor A. R., Sowers J. R., Jia G., Hayden M. R., Garro M., et al. (2017). Sodium Glucose Transporter 2 (SGLT2) Inhibition with Empagliflozin Improves Cardiac Diastolic Function in a Female Rodent Model of Diabetes. Cardiovasc Diabetol. 16 (1), 9. 10.1186/s12933-016-0489-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen T., D’Amico G., Quintero M., Palacios-Callender M., Hollis V., Lam F., et al. (2004). Inhibition of Mitochondrial Respiration by the Anticancer Agent 2-methoxyestradiol. Biochem. Biophysical Res. Commun. 322 (3), 923–929. 10.1016/j.bbrc.2004.07.204 [DOI] [PubMed] [Google Scholar]
- Halliwell B. (2013). The Antioxidant Paradox: Less Paradoxical Now? Br. J. Clin. Pharmacol. 75 (3), 637–644. 10.1111/j.1365-2125.2012.04272.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanishi T., Furuta H., Kato H., Doi A., Tamai M., Shimomura H., et al. (2004). Functional Variants in the Glutathione Peroxidase-1 (GPx-1) Gene Are Associated with Increased Intima-Media Thickness of Carotid Arteries and Risk of Macrovascular Diseases in Japanese Type 2 Diabetic Patients. Diabetes 53 (9), 2455–2460. 10.2337/diabetes.53.9.2455 [DOI] [PubMed] [Google Scholar]
- Han S., Hagan D. L., Taylor J. R., Xin L., Meng W., Biller S. A., et al. (2008). Dapagliflozin, a Selective SGLT2 Inhibitor, Improves Glucose Homeostasis in Normal and Diabetic Rats. Diabetes 57 (6), 1723–1729. 10.2337/db07-1472 [DOI] [PubMed] [Google Scholar]
- Hansen R., Sæbø M., Skjelbred C. F., Nexø B. A., Hagen P. C., Bock G., et al. (2005). GPX Pro198Leu and OGG1 Ser326Cys Polymorphisms and Risk of Development of Colorectal Adenomas and Colorectal Cancer. Cancer Lett. 229 (1), 85–91. 10.1016/j.canlet.2005.04.019 [DOI] [PubMed] [Google Scholar]
- Hare D. J., Double K. L. (2016). Iron and Dopamine: a Toxic Couple. Brain 139 (Pt 4), 1026–1035. 10.1093/brain/aww022 [DOI] [PubMed] [Google Scholar]
- Harman D. (1956). Aging: a Theory Based on Free Radical and Radiation Chemistry. J. Gerontology 11 (3), 298–300. 10.1093/geronj/11.3.298 [DOI] [PubMed] [Google Scholar]
- Harrington R. A. (2017). Statins-Almost 30 Years of Use in the United States and Still Not Quite There. JAMA Cardiol. 2 (1), 66. 10.1001/jamacardio.2016.4709 [DOI] [PubMed] [Google Scholar]
- Harris I. S., Treloar A. E., Inoue S., Sasaki M., Gorrini C., Lee K. C., et al. (2015). Glutathione and Thioredoxin Antioxidant Pathways Synergize to Drive Cancer Initiation and Progression. Cancer Cell 27 (2), 211–222. 10.1016/j.ccell.2014.11.019 [DOI] [PubMed] [Google Scholar]
- Hauner H. (2002). The Mode of Action of Thiazolidinediones. Diabetes Metab. Res. Rev. 18 Suppl 2 (Suppl. 2), S10–S15. 10.1002/dmrr.249 [DOI] [PubMed] [Google Scholar]
- Hawk M. A., McCallister C., Schafer Z. T. (2016). Antioxidant Activity during Tumor Progression: A Necessity for the Survival of Cancer Cells? Cancers (Basel) 8 (10). 10.3390/cancers8100092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Ishimori C., Takahashi-Niki K., Taira T., Kim Y.-c., Maita H., et al. (2009). DJ-1 Binds to Mitochondrial Complex I and Maintains its Activity. Biochem. Biophysical Res. Commun. 390 (3), 667–672. 10.1016/j.bbrc.2009.10.025 [DOI] [PubMed] [Google Scholar]
- Hayley S., Crocker S. J., Smith P. D., Shree T., Jackson-Lewis V., Przedborski S., et al. (2004). Regulation of Dopaminergic Loss by Fas in a 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Model of Parkinson's Disease. J. Neurosci. 24 (8), 2045–2053. 10.1523/jneurosci.4564-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayyan M., Hashim M. A., AlNashef I. M. (2016). Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 116 (5), 3029–3085. 10.1021/acs.chemrev.5b00407 [DOI] [PubMed] [Google Scholar]
- Hebert-Schuster M., Fabre E. E., Nivet-Antoine V. (2012). Catalase Polymorphisms and Metabolic Diseases. Curr. Opin. Clin. Nutr. Metabolic Care 15 (4), 397–402. 10.1097/mco.0b013e328354a326 [DOI] [PubMed] [Google Scholar]
- Heckman-Stoddard B. M., DeCensi A., Sahasrabuddhe V. V., Ford L. G. (2017). Repurposing Metformin for the Prevention of Cancer and Cancer Recurrence. Diabetologia 60 (9), 1639–1647. 10.1007/s00125-017-4372-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg H., Heremans Y., Jobin C., Leemans R., Cardozo A. K., Darville M., et al. (2001). Inhibition of Cytokine-Induced NF-Κb Activation by Adenovirus-Mediated Expression of a NF-Κb Super-repressor Prevents β-Cell Apoptosis. Diabetes 50 (10), 2219–2224. 10.2337/diabetes.50.10.2219 [DOI] [PubMed] [Google Scholar]
- Herzig S., Shaw R. J. (2018). AMPK: Guardian of Metabolism and Mitochondrial Homeostasis. Nat. Rev. Mol. Cell Biol. 19 (2), 121–135. 10.1038/nrm.2017.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aliev G., Nunomura A., Fujioka H., Russell R. L., Atwood C. S., et al. (2001). Mitochondrial Abnormalities in Alzheimer's Disease. J. Neurosci. 21 (9), 3017–3023. 10.1523/jneurosci.21-09-03017.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi S., Harada H., Nishi H., Satoh M., Nagai R., Kimura A. (1999). Polymorphisms in the SOD2 and HLA-DRB1 Genes Are Associated with Nonfamilial Idiopathic Dilated Cardiomyopathy in Japanese. Biochem. Biophysical Res. Commun. 261 (2), 332–339. 10.1006/bbrc.1999.1036 [DOI] [PubMed] [Google Scholar]
- Ho C. C.-Y., Rideout H. J., Ribe E., Troy C. M., Dauer W. T. (2009). The Parkinson Disease Protein Leucine-Rich Repeat Kinase 2 Transduces Death Signals via Fas-Associated Protein with Death Domain and Caspase-8 in a Cellular Model of Neurodegeneration. J. Neurosci. 29 (4), 1011–1016. 10.1523/jneurosci.5175-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie S. N., Akshay S., Kalapala S. K., Bruell C. M., Shcherbakov D., Böttger E. C. (2008). Genetic Analysis of Interactions with Eukaryotic rRNA Identify the Mitoribosome as Target in Aminoglycoside Ototoxicity. Proc. Natl. Acad. Sci. U.S.A. 105 (52), 20888–20893. 10.1073/pnas.0811258106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A., Johansson C., Berndt C., Lönn M. E., Hudemann C., Lillig C. H. (2005). Thiol Redox Control via Thioredoxin and Glutaredoxin Systems. Biochem. Soc. Trans. 33 (Pt 6), 1375–1377. 10.1042/BST20051375 [DOI] [PubMed] [Google Scholar]
- Holmgren A. (1976). Hydrogen Donor System for Escherichia coli Ribonucleoside-Diphosphate Reductase Dependent upon Glutathione. Proc. Natl. Acad. Sci. U.S.A. 73 (7), 2275–2279. 10.1073/pnas.73.7.2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu L. J., Sagara Y., Arroyo A., Rockenstein E., Sisk A., Mallory M., et al. (2000). α-Synuclein Promotes Mitochondrial Deficit and Oxidative Stress. Am. J. Pathology 157 (2), 401–410. 10.1016/s0002-9440(10)64553-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D., Wu C.-q., Li Z.-j., Liu Y., Fan X., Wang Q.-j., et al. (2015). Characterizing the Mechanism of Thiazolidinedione-Induced Hepatotoxicity: An In Vitro Model in Mitochondria. Toxicol. Appl. Pharmacol. 284 (2), 134–141. 10.1016/j.taap.2015.02.018 [DOI] [PubMed] [Google Scholar]
- Hutchin T., Cortopassi G. (1994). Proposed Molecular and Cellular Mechanism for Aminoglycoside Ototoxicity. Antimicrob. Agents Chemother. 38 (11), 2517–2520. 10.1128/aac.38.11.2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaccarino C., Crosio C., Vitale C., Sanna G., Carrì M. T., Barone P. (2007). Apoptotic Mechanisms in Mutant LRRK2-Mediated Cell Death. Hum. Mol. Genet. 16 (11), 1319–1326. 10.1093/hmg/ddm080 [DOI] [PubMed] [Google Scholar]
- Imazio M., Battaglia A., Gaido L., Gaita F. (2017). Recurrent Pericarditis. La Rev. Médecine Interne 38 (5), 307–311. 10.1016/j.revmed.2016.12.006 [DOI] [PubMed] [Google Scholar]
- Imfeld P., Bodmer M., Jick S. S., Meier C. R. (2012). Metformin, Other Antidiabetic Drugs, and Risk of Alzheimer's Disease: a Population-Based Case-Control Study. J. Am. Geriatr. Soc. 60 (5), 916–921. 10.1111/j.1532-5415.2012.03916.x [DOI] [PubMed] [Google Scholar]
- Inoguchi T., Sonta T., Tsubouchi H., Etoh T., Kakimoto M., Sonoda N., et al. (2003). Protein Kinase C-dependent Increase in Reactive Oxygen Species (ROS) Production in Vascular Tissues of Diabetes: Role of Vascular NAD(P)H Oxidase. J. Am. Soc. Nephrol. 14 (8 Suppl. 3), S227–S232. 10.1097/01.asn.0000077407.90309.65 [DOI] [PubMed] [Google Scholar]
- Inoguchi T., Li P., Umeda F., Yu H. Y., Kakimoto M., Imamura M., et al. (2000). High Glucose Level and Free Fatty Acid Stimulate Reactive Oxygen Species Production through Protein Kinase C--dependent Activation of NAD(P)H Oxidase in Cultured Vascular Cells. Diabetes 49 (11), 1939–1945. 10.2337/diabetes.49.11.1939 [DOI] [PubMed] [Google Scholar]
- Inoue I., Nagase H., Kishi K., Higuti T. (1991). ATP-sensitive K+ Channel in the Mitochondrial Inner Membrane. Nature 352 (6332), 244–247. 10.1038/352244a0 [DOI] [PubMed] [Google Scholar]
- Iqbal A. M., Lopez R. A., Hai O. (2021). Antiplatelet Medications. Treasure Island (FL): StatPearls. [PubMed] [Google Scholar]
- Ishibashi Y., Matsui T., Yamagishi S. (2016). Tofogliflozin, A Highly Selective Inhibitor of SGLT2 Blocks Proinflammatory and Proapoptotic Effects of Glucose Overload on Proximal Tubular Cells Partly by Suppressing Oxidative Stress Generation. Horm. Metab. Res. 48 (3), 191–195. 10.1055/s-0035-1555791 [DOI] [PubMed] [Google Scholar]
- Ishibashi Y., Matsui T., Maeda S., Higashimoto Y., Yamagishi S.-i. (2013). Advanced Glycation End Products Evoke Endothelial Cell Damage by Stimulating Soluble Dipeptidyl Peptidase-4 Production and its Interaction with Mannose 6-phosphate/insulin-like Growth Factor II Receptor. Cardiovasc Diabetol. 12, 125. 10.1186/1475-2840-12-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Wakabayashi K., Suzuki S., Mutoh M., Hirata K., Nakamura T., et al. (2013). Preventive Effects of Low‐dose Aspirin on Colorectal Adenoma Growth in Patients with Familial Adenomatous Polyposis: Double‐blind, Randomized Clinical Trial. Cancer Med. 2 (1), 50–56. 10.1002/cam4.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalewa J., Sharma M. K., Hölscher C. (2016). Novel Incretin Analogues Improve Autophagy and Protect from Mitochondrial Stress Induced by Rotenone in SH-Sy5y Cells. J. Neurochem. 139 (1), 55–67. 10.1111/jnc.13736 [DOI] [PubMed] [Google Scholar]
- Jang Y. C., Lustgarten M. S., Liu Y., Muller F. L., Bhattacharya A., Liang H., et al. (2010). Increased Superoxide In Vivo Accelerates Age‐associated Muscle Atrophy through Mitochondrial Dysfunction and Neuromuscular Junction Degeneration. FASEB J. 24 (5), 1376–1390. 10.1096/fj.09-146308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janion K., Szczepańska E., Nowakowska-Zajdel E., Strzelczyk J., Copija A. (2020). Selected Oxidative Stress Markers in Colorectal Cancer Patients in Relation to Primary Tumor Location-A Preliminary Research. Med. Kaunas. 56 (2). 10.3390/medicina56020047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens G. E., Houtkooper R. H. (2020). Identification of Longevity Compounds with Minimized Probabilities of Side Effects. Biogerontology 21 (6), 709–719. 10.1007/s10522-020-09887-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo M. C., Zhang D. D. (2013). The Emerging Role of the Nrf2-Keap1 Signaling Pathway in Cancer. Genes Dev. 27 (20), 2179–2191. 10.1101/gad.225680.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaul E., Barron J. (2017). Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and over Population. Front. Public Health 5, 335. 10.3389/fpubh.2017.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee J., Rasouly A., Shamovsky I., Akivis Y., R. Steinman S., Mishra B., et al. (2016). Rates and Mechanisms of Bacterial Mutagenesis from Maximum-Depth Sequencing. Nature 534 (7609), 693–696. 10.1038/nature18313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellen L. C., Lu L., Wang X., Unger E. L., Earley C. J., Allen R. P., et al. (2013). Iron Deficiency Alters Expression of Dopamine-Related Genes in the Ventral Midbrain in Mice. Neuroscience 252, 13–23. 10.1016/j.neuroscience.2013.07.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Zhou X., Yang H., Guan R., Xin Y., Wang J., et al. (2018). Upregulation of AT1 Receptor Mediates a Pressor Effect through ROS-SAPK/JNK Signaling in Glutamatergic Neurons of Rostral Ventrolateral Medulla in Rats with Stress-Induced Hypertension. Front. Physiol. 9, 1860. 10.3389/fphys.2018.01860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Baucom C., Elliott R. L. (2019). Mitochondrial Toxicity of Azithromycin Results in Aerobic Glycolysis and DNA Damage of Human Mammary Epithelia and Fibroblasts. Antibiot. (Basel) 8 (3). 10.3390/antibiotics8030110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorda A., Aldasoro M., Aldasoro C., Guerra-Ojeda S., Iradi A., Vila J. M., et al. (2020). Action of Low Doses of Aspirin in Inflammation and Oxidative Stress Induced by Aβ1-42 on Astrocytes in Primary Culture. Int. J. Med. Sci. 17 (6), 834–843. 10.7150/ijms.40959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joza N., Susin S. A., Daugas E., Stanford W. L., Cho S. K., Li C. Y. J., et al. (2001). Essential Role of the Mitochondrial Apoptosis-Inducing Factor in Programmed Cell Death. Nature 410 (6828), 549–554. 10.1038/35069004 [DOI] [PubMed] [Google Scholar]
- Juhasz A., Ge Y., Markel S., Chiu A., Matsumoto L., van Balgooy J., et al. (2009). Expression of NADPH Oxidase Homologues and Accessory Genes in Human Cancer Cell Lines, Tumours and Adjacent Normal Tissues. Free Radic. Res. 43 (6), 523–532. 10.1080/10715760902918683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalghatgi S., Spina C. S., Costello J. C., Liesa M., Morones-Ramirez J. R., Slomovic S., et al. (2013). Bactericidal Antibiotics Induce Mitochondrial Dysfunction and Oxidative Damage in Mammalian Cells. Sci. Transl. Med. 5 (192), 192ra85. 10.1126/scitranslmed.3006055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra S. (2014). Sodium Glucose Co-transporter-2 (SGLT2) Inhibitors: A Review of Their Basic and Clinical Pharmacology. Diabetes Ther. 5 (2), 355–366. 10.1007/s13300-014-0089-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamii H., Kato I., Kinouchi H., Chan P. H., Epstein C. J., Akabane A., et al. (1999). Amelioration of Vasospasm after Subarachnoid Hemorrhage in Transgenic Mice Overexpressing CuZn-Superoxide Dismutase. Stroke 30 (4), 867–872. 10.1161/01.str.30.4.867 [DOI] [PubMed] [Google Scholar]
- Kang M. Y., Oh T. J., Cho Y. M. (2015). Glucagon-Like Peptide-1 Increases Mitochondrial Biogenesis and Function in INS-1 Rat Insulinoma Cells. Endocrinol. Metab. 30 (2), 216–220. 10.3803/enm.2015.30.2.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann P., Török M., Zahno A., Waldhauser K. M., Brecht K., Krähenbühl S. (2006). Toxicity of Statins on Rat Skeletal Muscle Mitochondria. Cell Mol. Life Sci. 63 (19-20), 2415–2425. 10.1007/s00018-006-6235-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M., Garden G. A., Lipton S. A. (2001). Pathways to Neuronal Injury and Apoptosis in HIV-Associated Dementia. Nature 410 (6831), 988–994. 10.1038/35073667 [DOI] [PubMed] [Google Scholar]
- Kawanami D., Matoba K., Takeda Y., Nagai Y., Akamine T., Yokota T., et al. (2017). SGLT2 Inhibitors as a Therapeutic Option for Diabetic Nephropathy. Int. J. Mol. Sci. 18 (5). 10.3390/ijms18051083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G. A., Warner T. G., Steimer K. S., Hallewell R. A. (1991). Cu,Zn Superoxide Dismutase Is a Peroxisomal Enzyme in Human Fibroblasts and Hepatoma Cells. Proc. Natl. Acad. Sci. U.S.A. 88 (16), 7381–7385. 10.1073/pnas.88.16.7381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly B., Tannahill G. M., Murphy M. P., O'Neill L. A. J. (2015). Metformin Inhibits the Production of Reactive Oxygen Species from NADH:Ubiquinone Oxidoreductase to Limit Induction of Interleukin-1β (IL-1β) and Boosts Interleukin-10 (IL-10) in Lipopolysaccharide (LPS)-activated Macrophages. J. Biol. Chem. 290 (33), 20348–20359. 10.1074/jbc.m115.662114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern M., Klöting N., Mark M., Mayoux E., Klein T., Blüher M. (2016). The SGLT2 Inhibitor Empagliflozin Improves Insulin Sensitivity in Db/db Mice Both as Monotherapy and in Combination with Linagliptin. Metabolism 65 (2), 114–123. 10.1016/j.metabol.2015.10.010 [DOI] [PubMed] [Google Scholar]
- Khaliq Y., Zhanel G. G. (2003). Fluoroquinolone-associated Tendinopathy: a Critical Review of the Literature. Clin. Infect. Dis. 36 (11), 1404–1410. 10.1086/375078 [DOI] [PubMed] [Google Scholar]
- Khansari N., Shakiba Y., Mahmoudi M. (2009). Chronic Inflammation and Oxidative Stress as a Major Cause of Age- Related Diseases and Cancer. Iad 3 (1), 73–80. 10.2174/187221309787158371 [DOI] [PubMed] [Google Scholar]
- Khayyat A., Tobwala S., Hart M., Ercal N. (2016). N -acetylcysteine Amide, a Promising Antidote for Acetaminophen Toxicity. Toxicol. Lett. 241, 133–142. 10.1016/j.toxlet.2015.11.008 [DOI] [PubMed] [Google Scholar]
- Kickstein E., Krauss S., Thornhill P., Rutschow D., Zeller R., Sharkey J., et al. (2010). Biguanide Metformin Acts on Tau Phosphorylation via mTOR/protein Phosphatase 2A (PP2A) Signaling. Proc. Natl. Acad. Sci. U.S.A. 107 (50), 21830–21835. 10.1073/pnas.0912793107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Lee K.-S., Kim J.-H., Lee D.-K., Park M., Choi S., et al. (2017). Aspirin Prevents TNF-α-Induced Endothelial Cell Dysfunction by Regulating the NF-κb-dependent miR-155/eNOS Pathway: Role of a miR-155/eNOS axis in Preeclampsia. Free Radic. Biol. Med. 104, 185–198. 10.1016/j.freeradbiomed.2017.01.010 [DOI] [PubMed] [Google Scholar]
- Kim S. J., Kim H. S., Seo Y. R. (2019). Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxid. Med. Cell Longev. 2019, 5381692. 10.1155/2019/5381692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. K., Lee M.-S., Son S. M., Kim I. J., Lee W. S., Rhim B. Y., et al. (2002). Vascular NADH Oxidase Is Involved in Impaired Endothelium-dependent Vasodilation in OLETF Rats, a Model of Type 2 Diabetes. Diabetes 51 (2), 522–527. 10.2337/diabetes.51.2.522 [DOI] [PubMed] [Google Scholar]
- Kimura S., Zhang G.-X., Nishiyama A., Shokoji T., Yao L., Fan Y.-Y., et al. (2005). Mitochondria-Derived Reactive Oxygen Species and Vascular MAP Kinases. Hypertension 45 (3), 438–444. 10.1161/01.hyp.0000157169.27818.ae [DOI] [PubMed] [Google Scholar]
- Kisker C., Schindelin H., Rees D. C. (1997). Molybdenum-cofactor-containing Enzymes: Structure and Mechanism. Annu. Rev. Biochem. 66, 233–267. 10.1146/annurev.biochem.66.1.233 [DOI] [PubMed] [Google Scholar]
- Kline N. S. (1958). Clinical Experience with Iproniazid (Marsilid). J. Clin. Exp. Psychopathol. 19 (2Suppl. 1), 72–79. [PubMed] [Google Scholar]
- Klivenyi P., Andreassen O. A., Ferrante R. J., Dedeoglu A., Mueller G., Lancelot E., et al. (2000). Mice Deficient in Cellular Glutathione Peroxidase Show Increased Vulnerability to Malonate, 3-nitropropionic Acid, and 1-Methyl-4-Phenyl-1,2,5,6-Tetrahydropyridine. J. Neurosci. 20 (1), 1–7. 10.1523/jneurosci.20-01-00001.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen Gerber D. S. (2011). Selegiline and Rasagiline: Twins or Distant Cousins? Guidelines. Consult. Pharm. 26 (1), 48–51. 10.4140/tcp.n.2011.48 [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Susa T., Tanaka T., Wada Y., Okuda S., Doi M., et al. (2011). Urinary 8-Hydroxy-2′-Deoxyguanosine Reflects Symptomatic Status and Severity of Systolic Dysfunction in Patients with Chronic Heart Failure. Eur. J. Heart Fail. 13 (1), 29–36. 10.1093/eurjhf/hfq178 [DOI] [PubMed] [Google Scholar]
- Koenig A. M., Mechanic-Hamilton D., Xie S. X., Combs M. F., Cappola A. R., Xie L., et al. (2017). Effects of the Insulin Sensitizer Metformin in Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 31 (2), 107–113. 10.1097/wad.0000000000000202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koentjoro B., Park J.-S., Sue C. M. (2017). Nix Restores Mitophagy and Mitochondrial Function to Protect against PINK1/Parkin-Related Parkinson's Disease. Sci. Rep. 7, 44373. 10.1038/srep44373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohanski M. A., DePristo M. A., Collins J. J. (2010). Sublethal Antibiotic Treatment Leads to Multidrug Resistance via Radical-Induced Mutagenesis. Mol. Cell 37 (3), 311–320. 10.1016/j.molcel.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohanski M. A., Dwyer D. J., Hayete B., Lawrence C. A., Collins J. J. (2007). A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics. Cell 130 (5), 797–810. 10.1016/j.cell.2007.06.049 [DOI] [PubMed] [Google Scholar]
- Kohanski M. A., Dwyer D. J., Wierzbowski J., Cottarel G., Collins J. J. (2008). Mistranslation of Membrane Proteins and Two-Component System Activation Trigger Antibiotic-Mediated Cell Death. Cell 135 (4), 679–690. 10.1016/j.cell.2008.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono Y., Nakamura K., Kimura H., Nishii N., Watanabe A., Banba K., et al. (2006). Elevated Levels of Oxidative DNA Damage in Serum and Myocardium of Patients with Heart Failure. Circ. J. 70 (8), 1001–1005. 10.1253/circj.70.1001 [DOI] [PubMed] [Google Scholar]
- Korshunov S. S., Skulachev V. P., Starkov A. A. (1997). High Protonic Potential Actuates a Mechanism of Production of Reactive Oxygen Species in Mitochondria. FEBS Lett. 416 (1), 15–18. 10.1016/s0014-5793(97)01159-9 [DOI] [PubMed] [Google Scholar]
- Kotamraju S., Chitambar C. R., Kalivendi S. V., Joseph J., Kalyanaraman B. (2002). Transferrin Receptor-dependent Iron Uptake Is Responsible for Doxorubicin-Mediated Apoptosis in Endothelial Cells. J. Biol. Chem. 277 (19), 17179–17187. 10.1074/jbc.m111604200 [DOI] [PubMed] [Google Scholar]
- Kotur-Stevuljevic J., Memon L., Stefanovic A., Spasic S., Spasojevic-Kalimanovska V., Bogavac-Stanojevic N., et al. (2007). Correlation of Oxidative Stress Parameters and Inflammatory Markers in Coronary Artery Disease Patients. Clin. Biochem. 40 (3-4), 181–187. 10.1016/j.clinbiochem.2006.09.007 [DOI] [PubMed] [Google Scholar]
- Kozer E., Evans S., Barr J., Greenberg R., Soriano I., Bulkowstein M., et al. (2003). Glutathione, Glutathione-dependent Enzymes and Antioxidant Status in Erythrocytes from Children Treated with High-Dose Paracetamol. Br. J. Clin. Pharmacol. 55 (3), 234–240. 10.1046/j.1365-2125.2003.01723.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraljevic S., Stambrook P. J., Pavelic K. (2004). Accelerating Drug Discovery. EMBO Rep. 5 (9), 837–842. 10.1038/sj.embor.7400236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krijnen P. A. J., Meischl C., Hack C. E., Meijer C. J., Visser C. A., Roos D., et al. (2003). Increased Nox2 Expression in Human Cardiomyocytes after Acute Myocardial Infarction. J. Clin. Pathology 56 (3), 194–199. 10.1136/jcp.56.3.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon A. M., Van den Bogert C. (1983). Antibacterial Drugs and Their Interference with the Biogenesis of Mitochondria in Animal and Human Cells. Pharm. Weekbl. Sci. Ed. 5 (3), 81–87. 10.1007/bf01960982 [DOI] [PubMed] [Google Scholar]
- Kumar S., Dang S., Nigam K., Ali J., Baboota S. (2018). Selegiline Nanoformulation in Attenuation of Oxidative Stress and Upregulation of Dopamine in the Brain for the Treatment of Parkinson's Disease. Rejuvenation Res. 21 (5), 464–476. 10.1089/rej.2017.2035 [DOI] [PubMed] [Google Scholar]
- Kumari S., Badana A. K., G M. M., Gg S. (2018). Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark. Insights 13, 1177271918755391. 10.1177/1177271918755391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla F. M., Green K. N., Oddo S. (2007). Intracellular Amyloid-β in Alzheimer's Disease. Nat. Rev. Neurosci. 8 (7), 499–509. 10.1038/nrn2168 [DOI] [PubMed] [Google Scholar]
- Lassègue B., San Martín A., Griendling K. K. (2012). Biochemistry, Physiology, and Pathophysiology of NADPH Oxidases in the Cardiovascular System. Circ. Res. 110 (10), 1364–1390. 10.1161/circresaha.111.243972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T. (2009). The Nuclear Factor NF- B Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 1 (6), a001651. 10.1101/cshperspect.a001651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarou M., Sliter D. A., Kane L. A., Sarraf S. A., Wang C., Burman J. L., et al. (2015). The Ubiquitin Kinase PINK1 Recruits Autophagy Receptors to Induce Mitophagy. Nature 524 (7565), 309–314. 10.1038/nature14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. D., Cai Q., Shu X. O., Nechuta S. J. (2017). The Role of Biomarkers of Oxidative Stress in Breast Cancer Risk and Prognosis: A Systematic Review of the Epidemiologic Literature. J. Women's Health 26 (5), 467–482. 10.1089/jwh.2016.5973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Giordano S., Zhang J. (2012). Autophagy, Mitochondria and Oxidative Stress: Cross-Talk and Redox Signalling. Biochem. J. 441 (2), 523–540. 10.1042/bj20111451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. O., Lee S. K., Kim J. H., Kim N., You G. Y., Moon J. W., et al. (2012). Metformin Regulates Glucose Transporter 4 (GLUT4) Translocation through AMP-Activated Protein Kinase (AMPK)-mediated Cbl/CAP Signaling in 3T3-L1 Preadipocyte Cells. J. Biol. Chem. 287 (53), 44121–44129. 10.1074/jbc.m112.361386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F.-F., Fu L.-Y., Xu X.-H., Su X.-F., Wu J.-D., Ye L., et al. (2016). Analysis of the Add-On Effect of α-glucosidase Inhibitor, Acarbose in Insulin Therapy: A Pilot Study. Biomed. Rep. 5 (4), 461–466. 10.3892/br.2016.744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.-M., Gall N. P., Grieve D. J., Chen M., Shah A. M. (2002). Activation of NADPH Oxidase during Progression of Cardiac Hypertrophy to Failure. Hypertension 40 (4), 477–484. 10.1161/01.hyp.0000032031.30374.32 [DOI] [PubMed] [Google Scholar]
- Li J., Deng J., Sheng W., Zuo Z. (2012). Metformin Attenuates Alzheimer's Disease-like Neuropathology in Obese, Leptin-Resistant Mice. Pharmacol. Biochem. Behav. 101 (4), 564–574. 10.1016/j.pbb.2012.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.-X., Ling S.-K., Hu M.-Y., Ma Y., Li Y., Huang P.-L. (2019). Protective Effects of Acarbose against Vascular Endothelial Dysfunction through Inhibiting Nox4/NLRP3 Inflammasome Pathway in Diabetic Rats. Free Radic. Biol. Med. 145, 175–186. 10.1016/j.freeradbiomed.2019.09.015 [DOI] [PubMed] [Google Scholar]
- Lillig C. H., Holmgren A. (2007). Thioredoxin and Related Molecules-From Biology to Health and Disease. Antioxidants Redox Signal. 9 (1), 25–47. 10.1089/ars.2007.9.25 [DOI] [PubMed] [Google Scholar]
- Lim C. C., Bryan N. S., Jain M., Garcia-Saura M. F., Fernandez B. O., Sawyer D. B., et al. (2009). Glutathione Peroxidase Deficiency Exacerbates Ischemia-Reperfusion Injury in Male but Not Female Myocardium: Insights into Antioxidant Compensatory Mechanisms. Am. J. Physiology-Heart Circulatory Physiology 297 (6), H2144–H2153. 10.1152/ajpheart.00673.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.-C., Chen Y.-C., Hsiao H.-P., Kuo C.-H., Chen B.-H., Chen Y.-T., et al. (2019). The Effects of Acarbose on Chemokine and Cytokine Production in Human Monocytic THP-1 Cells. Hormones 18 (2), 179–187. 10.1007/s42000-019-00101-z [DOI] [PubMed] [Google Scholar]
- Linder N., Rapola J., Raivio K. O. (1999). Cellular Expression of Xanthine Oxidoreductase Protein in Normal Human Tissues. Lab. Invest 79 (8), 967–974. [PubMed] [Google Scholar]
- Lingappan K. (2018). NF-κB in Oxidative Stress. Curr. Opin. Toxicol. 7, 81–86. 10.1016/j.cotox.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou G.-Y., Storz P. (2010). Reactive Oxygen Species in Cancer. Free Radic. Res. 44 (5), 479–496. 10.3109/10715761003667554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou W., Chang L., Geuze H., Strous G., Crapo J., Slot J. (1993). Distribution of CuZn Superoxide Dismutase in Rat Liver. Free Radic. Biol. Med. 14 (2), 201–207. 10.1016/0891-5849(93)90011-i [DOI] [PubMed] [Google Scholar]
- Liu Y., Lin J., Wu X., Guo X., Sun H., Yu B., et al. (2019). Aspirin-Mediated Attenuation of Intervertebral Disc Degeneration by Ameliorating Reactive Oxygen Species In Vivo and In Vitro . Oxid. Med. Cell Longev. 2019, 7189854. 10.1155/2019/7189854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.-x., Feng J.-y., Sun M.-m., Liu B.-w., Yang G., Bu Y.-n., et al. (2019). Aspirin Inhibits the Proliferation of Hepatoma Cells through Controlling GLUT1-Mediated Glucose Metabolism. Acta Pharmacol. Sin. 40 (1), 122–132. 10.1038/s41401-018-0014-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Liu X., Qu Y., Wang X., Li L., Zhao X. (2012). Inhibitors of Reactive Oxygen Species Accumulation Delay And/or Reduce the Lethality of Several Antistaphylococcal Agents. Antimicrob. Agents Chemother. 56 (11), 6048–6050. 10.1128/aac.00754-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonn E., Bosch J., Yusuf S., Sheridan P., Pogue J., Arnold J. M., et al. (2005). Effects of Long-Term Vitamin E Supplementation on Cardiovascular Events and Cancer: a Randomized Controlled Trial. JAMA 293 (11), 1338–1347. 10.1001/jama.293.11.1338 [DOI] [PubMed] [Google Scholar]
- Lowes D. A., Wallace C., Murphy M. P., Webster N. R., Galley H. F. (2009). The Mitochondria Targeted Antioxidant MitoQ Protects against Fluoroquinolone-Induced Oxidative Stress and Mitochondrial Membrane Damage in Human Achilles Tendon Cells. Free Radic. Res. 43 (4), 323–328. 10.1080/10715760902736275 [DOI] [PubMed] [Google Scholar]
- Lu J., Holmgren A. (2014). The Thioredoxin Antioxidant System. Free Radic. Biol. Med. 66, 75–87. 10.1016/j.freeradbiomed.2013.07.036 [DOI] [PubMed] [Google Scholar]
- Lu M., Su C., Qiao C., Bian Y., Ding J., Hu G. (2016). Metformin Prevents Dopaminergic Neuron Death in MPTP/P-Induced Mouse Model of Parkinson's Disease via Autophagy and Mitochondrial ROS Clearance. Int. J. Neuropsychopharmacol. 19 (9). 10.1093/ijnp/pyw047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madiraju A. K., Erion D. M., Rahimi Y., Zhang X.-M., Braddock D. T., Albright R. A., et al. (2014). Metformin Suppresses Gluconeogenesis by Inhibiting Mitochondrial Glycerophosphate Dehydrogenase. Nature 510 (7506), 542–546. 10.1038/nature13270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magda D., Miller R. A. (2006). Motexafin Gadolinium: a Novel Redox Active Drug for Cancer Therapy. Seminars Cancer Biol. 16 (6), 466–476. 10.1016/j.semcancer.2006.09.002 [DOI] [PubMed] [Google Scholar]
- Mahadev K., Motoshima H., Wu X., Ruddy J. M., Arnold R. S., Cheng G., et al. (2004). The NAD(P)H Oxidase Homolog Nox4 Modulates Insulin-Stimulated Generation of H 2 O 2 and Plays an Integral Role in Insulin Signal Transduction. Mol. Cell Biol. 24 (5), 1844–1854. 10.1128/mcb.24.5.1844-1854.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadev K., Wu X., Zilbering A., Zhu L., Lawrence J. T. R., Goldstein B. J. (2001). Hydrogen Peroxide Generated during Cellular Insulin Stimulation Is Integral to Activation of the Distal Insulin Signaling Cascade in 3T3-L1 Adipocytes. J. Biol. Chem. 276 (52), 48662–48669. 10.1074/jbc.m105061200 [DOI] [PubMed] [Google Scholar]
- Maiellaro-Rafferty K., Weiss D., Joseph G., Wan W., Gleason R. L., Taylor W. R. (2011). Catalase Overexpression in Aortic Smooth Muscle Prevents Pathological Mechanical Changes Underlying Abdominal Aortic Aneurysm Formation. Am. J. Physiology-Heart Circulatory Physiology 301 (2), H355–H362. 10.1152/ajpheart.00040.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra J. D., Kaufman R. J. (2007). Endoplasmic Reticulum Stress and Oxidative Stress: a Vicious Cycle or a Double-Edged Sword? Antioxidants Redox Signal. 9 (12), 2277–2294. 10.1089/ars.2007.1782 [DOI] [PubMed] [Google Scholar]
- Mallat Z., Philip I., Lebret M., Chatel D., Maclouf J., Tedgui A. (1998). Elevated Levels of 8-Iso-Prostaglandin F 2α in Pericardial Fluid of Patients with Heart Failure. Circulation 97 (16), 1536–1539. 10.1161/01.cir.97.16.1536 [DOI] [PubMed] [Google Scholar]
- Manczak M., Park B. S., Jung Y., Reddy P. H. (2004). Differential Expression of Oxidative Phosphorylation Genes in Patients with Alzheimer's Disease: Implications for Early Mitochondrial Dysfunction and Oxidative Damage. Nmm 5 (2), 147–162. 10.1385/nmm:5:2:147 [DOI] [PubMed] [Google Scholar]
- Manczak M., Reddy P. H. (2012). Abnormal Interaction of VDAC1 with Amyloid Beta and Phosphorylated Tau Causes Mitochondrial Dysfunction in Alzheimer's Disease. Hum. Mol. Genet. 21 (23), 5131–5146. 10.1093/hmg/dds360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S., Lindgren A. G., Srivastava A. S., Clark A. T., Banerjee U. (2011). Mitochondrial Function Controls Proliferation and Early Differentiation Potential of Embryonic Stem Cells. Stem Cells 29 (3), 486–495. 10.1002/stem.590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannucci E., Tesi F., Bardini G., Ognibene A., Petracca M. G., Ciani S., et al. (2004). Effects of Metformin on Glucagon-like Peptide-1 Levels in Obese Patients with and without Type 2 Diabetes. Diabetes Nutr. Metab. 17 (6), 336–342. [PubMed] [Google Scholar]
- Marguet D., Baggio L., Kobayashi T., Bernard A.-M., Pierres M., Nielsen P. F., et al. (2000). Enhanced Insulin Secretion and Improved Glucose Tolerance in Mice Lacking CD26. Proc. Natl. Acad. Sci. U.S.A. 97 (12), 6874–6879. 10.1073/pnas.120069197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. (1984). Extracellular Superoxide Dismutase and Other Superoxide Dismutase Isoenzymes in Tissues from Nine Mammalian Species. Biochem. J. 222 (3), 649–655. 10.1042/bj2220649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques O., Outeiro T. F. (2012). Alpha-synuclein: from Secretion to Dysfunction and Death. Cell Death Dis. 3, e350. 10.1038/cddis.2012.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L. J. (2011). Mitochondrial Pathobiology in ALS. J. Bioenerg. Biomembr. 43 (6), 569–579. 10.1007/s10863-011-9395-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L. J., Pan Y., Price A. C., Sterling W., Copeland N. G., Jenkins N. A., et al. (2006). Parkinson's Disease -Synuclein Transgenic Mice Develop Neuronal Mitochondrial Degeneration and Cell Death. J. Neurosci. 26 (1), 41–50. 10.1523/jneurosci.4308-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Martín P., Gil-Nagel A., Gracia L. M., Gómez J. B., Martínez-Sarriés J., Bermejo F. (1994). Unified Parkinson's Disease Rating Scale Characteristics and Structure. Mov. Disord. 9 (1), 76–83. 10.1002/mds.870090112 [DOI] [PubMed] [Google Scholar]
- Marullo R., Werner E., Degtyareva N., Moore B., Altavilla G., Ramalingam S. S., et al. (2013). Cisplatin Induces a Mitochondrial-ROS Response that Contributes to Cytotoxicity Depending on Mitochondrial Redox Status and Bioenergetic Functions. PLoS One 8 (11), e81162. 10.1371/journal.pone.0081162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marycz K., Tomaszewski K. A., Kornicka K., Henry B. M., Wroński S., Tarasiuk J., et al. (2016). Metformin Decreases Reactive Oxygen Species, Enhances Osteogenic Properties of Adipose-Derived Multipotent Mesenchymal Stem Cells In Vitro, and Increases Bone Density In Vivo . Oxid. Med. Cell Longev. 2016, 9785890. 10.1155/2016/9785890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masselli E., Pozzi G., Vaccarezza M., Mirandola P., Galli D., Vitale M., et al. (2020). ROS in Platelet Biology: Functional Aspects and Methodological Insights. Int. J. Mol. Sci. 21 (14). 10.3390/ijms21144866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo Y., Yodoi J. (2013). Extracellular Thioredoxin: a Therapeutic Tool to Combat Inflammation. Cytokine & Growth Factor Rev. 24 (4), 345–353. 10.1016/j.cytogfr.2013.01.001 [DOI] [PubMed] [Google Scholar]
- Matsushima S., Ide T., Yamato M., Matsusaka H., Hattori F., Ikeuchi M., et al. (2006). Overexpression of Mitochondrial Peroxiredoxin-3 Prevents Left Ventricular Remodeling and Failure after Myocardial Infarction in Mice. Circulation 113 (14), 1779–1786. 10.1161/circulationaha.105.582239 [DOI] [PubMed] [Google Scholar]
- Maurer I., Zierz S., Moller H. J. (2000). A Selective Defect of Cytochrome C Oxidase Is Present in Brain of Alzheimer Disease Patients. Neurobiol. Aging 21 (3), 455–462. 10.1016/s0197-4580(00)00112-3 [DOI] [PubMed] [Google Scholar]
- Mazurek S., Boschek C. B., Hugo F., Eigenbrodt E. (2005). Pyruvate Kinase Type M2 and its Role in Tumor Growth and Spreading. Seminars Cancer Biol. 15 (4), 300–308. 10.1016/j.semcancer.2005.04.009 [DOI] [PubMed] [Google Scholar]
- Mazurek S. (2011). Pyruvate Kinase Type M2: a Key Regulator of the Metabolic Budget System in Tumor Cells. Int. J. Biochem. Cell Biol. 43 (7), 969–980. 10.1016/j.biocel.2010.02.005 [DOI] [PubMed] [Google Scholar]
- Mburu D. N., Maitho T. E., Lökken P. (1990). Acetylsalicylic Acid or Paracetamol? East Afr. Med. J. 67 (5), 302–310. [PubMed] [Google Scholar]
- McCarty M. F., Block K. I. (2006). Preadministration of High-Dose Salicylates, Suppressors of NF-Κb Activation, May Increase the Chemosensitivity of Many Cancers: An Example of Proapoptotic Signal Modulation Therapy. Integr. Cancer Ther. 5 (3), 252–268. 10.1177/1534735406291499 [DOI] [PubMed] [Google Scholar]
- McKee E. E., Ferguson M., Bentley A. T., Marks T. A. (2006). Inhibition of Mammalian Mitochondrial Protein Synthesis by Oxazolidinones. Antimicrob. Agents Chemother. 50 (6), 2042–2049. 10.1128/aac.01411-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiser J., Weindl D., Hiller K. (2013). Complexity of Dopamine Metabolism. Cell Commun. Signal 11 (1), 34. 10.1186/1478-811x-11-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzler J. L., Antony S., Wu Y., Juhasz A., Liu H., Jiang G., et al. (2014). NADPH Oxidases: a Perspective on Reactive Oxygen Species Production in Tumor Biology. Antioxidants Redox Signal. 20 (17), 2873–2889. 10.1089/ars.2013.5603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merry T. L., Ristow M. (2016). Mitohormesis in Exercise Training. Free Radic. Biol. Med. 98, 123–130. 10.1016/j.freeradbiomed.2015.11.032 [DOI] [PubMed] [Google Scholar]
- Michaeli S., öz G., Sorce D. J., Garwood M., Ugurbil K., Majestic S., et al. (2007). Assessment of Brain Iron and Neuronal Integrity in Patients with Parkinson's Disease Using Novel MRI Contrasts. Mov. Disord. 22 (3), 334–340. 10.1002/mds.21227 [DOI] [PubMed] [Google Scholar]
- Migliaccio E., Giorgio M., Mele S., Pelicci G., Reboldi P., Pandolfi P. P., et al. (1999). The P66shc Adaptor Protein Controls Oxidative Stress Response and Life Span in Mammals. Nature 402 (6759), 309–313. 10.1038/46311 [DOI] [PubMed] [Google Scholar]
- Miller R. A., Chu Q., Xie J., Foretz M., Viollet B., Birnbaum M. J. (2013). Biguanides Suppress Hepatic Glucagon Signalling by Decreasing Production of Cyclic AMP. Nature 494 (7436), 256–260. 10.1038/nature11808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingeot-Leclercq M.-P., Tulkens P. M. (1999). Aminoglycosides: Nephrotoxicity. Antimicrob. Agents Chemother. 43 (5), 1003–1012. 10.1128/aac.43.5.1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal M., Siddiqui M. R., Tran K., Reddy S. P., Malik A. B. (2014). Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxidants Redox Signal. 20 (7), 1126–1167. 10.1089/ars.2012.5149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y., Sone N., Saitoh T. (1987). Effects of 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine and 1-Methyl-4-Phenylpyridinium Ion on Activities of the Enzymes in the Electron Transport System in Mouse Brain. J. Neurochem. 48 (6), 1787–1793. 10.1111/j.1471-4159.1987.tb05737.x [DOI] [PubMed] [Google Scholar]
- Mollazadeh H., Tavana E., Fanni G., Bo S., Banach M., Pirro M., et al. (2021). Effects of Statins on Mitochondrial Pathways. J. Cachexia, Sarcopenia Muscle 12 (2), 237–251. 10.1002/jcsm.12654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney A. M., Griffin R. J., Timmons S., O’Connor R., Ravid R., O’Neill C. (2010). Defects in IGF-1 Receptor, Insulin Receptor and IRS-1/2 in Alzheimer's Disease Indicate Possible Resistance to IGF-1 and Insulin Signalling. Neurobiol. Aging 31 (2), 224–243. 10.1016/j.neurobiolaging.2008.04.002 [DOI] [PubMed] [Google Scholar]
- Moran C., Phan T. G., Chen J., Blizzard L., Beare R., Venn A., et al. (2013). Brain Atrophy in Type 2 Diabetes. Diabetes Care 36 (12), 4036–4042. 10.2337/dc13-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita-Fujimura Y., Fujimura M., Gasche Y., Copin J.-C., Chan P. H. (2000). Overexpression of Copper and Zinc Superoxide Dismutase in Transgenic Mice Prevents the Induction and Activation of Matrix Metalloproteinases after Cold Injury-Induced Brain Trauma. J. Cereb. Blood Flow. Metab. 20 (1), 130–138. 10.1097/00004647-200001000-00017 [DOI] [PubMed] [Google Scholar]
- Morsi M., Maher A., Aboelmagd O., Johar D., Bernstein L. (2018). A Shared Comparison of Diabetes Mellitus and Neurodegenerative Disorders. J Cell. Biochem. 119 (2), 1249–1256. 10.1002/jcb.26261 [DOI] [PubMed] [Google Scholar]
- Moulder S., Dhillon N., Ng C., Hong D., Wheler J., Naing A., et al. (2010). A Phase I Trial of Imexon, a Pro-oxidant, in Combination with Docetaxel for the Treatment of Patients with Advanced Breast, Non-small Cell Lung and Prostate Cancer. Invest New Drugs 28 (5), 634–640. 10.1007/s10637-009-9273-1 [DOI] [PubMed] [Google Scholar]
- Müftüoglu M., Elibol B., Dalmızrak Ö., Ercan A., Kulaksız G., Ögüs H., et al. (2004). Mitochondrial Complex I and IV Activities in Leukocytes from Patients with Parkin Mutations. Mov. Disord. 19 (5), 544–548. 10.1002/mds.10695 [DOI] [PubMed] [Google Scholar]
- Mummidi S., Das N. A., Carpenter A. J., Kandikattu H., Krenz M., Siebenlist U., et al. (2016). Metformin Inhibits Aldosterone-Induced Cardiac Fibroblast Activation, Migration and Proliferation In Vitro, and Reverses Aldosterone+salt-Induced Cardiac Fibrosis In Vivo . J. Mol. Cell. Cardiol. 98, 95–102. 10.1016/j.yjmcc.2016.07.006 [DOI] [PubMed] [Google Scholar]
- Musi N., Hirshman M. F., Nygren J., Svanfeldt M., Bavenholm P., Rooyackers O., et al. (2002). Metformin Increases AMP-Activated Protein Kinase Activity in Skeletal Muscle of Subjects with Type 2 Diabetes. Diabetes 51 (7), 2074–2081. 10.2337/diabetes.51.7.2074 [DOI] [PubMed] [Google Scholar]
- Muxfeldt M., Schaper W. (1987). The Activity of Xanthine Oxidase in Heart of Pigs, guinea Pigs, Rabbits, Rats, and Humans. Basic Res. Cardiol. 82 (5), 486–492. 10.1007/bf01907096 [DOI] [PubMed] [Google Scholar]
- Nagano Y., Matsui H., Shimokawa O., Hirayama A., Tamura M., Nakamura Y., et al. (2012). Rebamipide Attenuates Nonsteroidal Anti-inflammatory Drugs (NSAID) Induced Lipid Peroxidation by the Manganese Superoxide Dismutase (MnSOD) Overexpression in Gastrointestinal Epithelial Cells. J. Physiol. Pharmacol. 63 (2), 137–142. [PubMed] [Google Scholar]
- Nagatsu T., Sawada M. (2009). L-Dopa Therapy for Parkinson's Disease: Past, Present, and Future. Park. Relat. Disord. 15 (Suppl. 1), S3–S8. 10.1016/s1353-8020(09)70004-5 [DOI] [PubMed] [Google Scholar]
- Nakamura H., De Rosa S., Roederer M., Anderson M. T., Dubs J. G., Yodoi J., et al. (1996). Elevation of Plasma Thioredoxin Levels in HIV-Infected Individuals. Int. Immunol. 8 (4), 603–611. 10.1093/intimm/8.4.603 [DOI] [PubMed] [Google Scholar]
- Nakamura H., Hoshino Y., Okuyama H., Matsuo Y., Yodoi J. (2009). Thioredoxin 1 Delivery as New Therapeutics. Adv. Drug Deliv. Rev. 61 (4), 303–309. 10.1016/j.addr.2009.01.003 [DOI] [PubMed] [Google Scholar]
- Nakamura K., Kusano K. F., Matsubara H., Nakamura Y., Miura A., Nishii N., et al. (2005). Relationship between Oxidative Stress and Systolic Dysfunction in Patients with Hypertrophic Cardiomyopathy. J. Cardiac Fail. 11 (2), 117–123. 10.1016/j.cardfail.2004.05.005 [DOI] [PubMed] [Google Scholar]
- Nakamura K., Kusano K., Nakamura Y., Kakishita M., Ohta K., Nagase S., et al. (2002). Carvedilol Decreases Elevated Oxidative Stress in Human Failing Myocardium. Circulation 105 (24), 2867–2871. 10.1161/01.cir.0000018605.14470.dd [DOI] [PubMed] [Google Scholar]
- Nakamura K., Murakami M., Miura D., Yunoki K., Enko K., Tanaka M., et al. (2011). Beta-Blockers and Oxidative Stress in Patients with Heart Failure. Pharmaceuticals 4 (8), 1088–1100. 10.3390/ph4081088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseem K. M. (2005). The Role of Nitric Oxide in Cardiovascular Diseases. Mol. Asp. Med. 26 (1-2), 33–65. 10.1016/j.mam.2004.09.003 [DOI] [PubMed] [Google Scholar]
- Natali A., Ferrannini E. (2006). Effects of Metformin and Thiazolidinediones on Suppression of Hepatic Glucose Production and Stimulation of Glucose Uptake in Type 2 Diabetes: a Systematic Review. Diabetologia 49 (3), 434–441. 10.1007/s00125-006-0141-7 [DOI] [PubMed] [Google Scholar]
- Nath N., Khan M., Paintlia M. K., Hoda M. N., Giri S., Giri S. (2009). Metformin Attenuated the Autoimmune Disease of the Central Nervous System in Animal Models of Multiple Sclerosis. J. Immunol. 182 (12), 8005–8014. 10.4049/jimmunol.0803563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeley W. L., Essigmann J. M. (2006). Mechanisms of Formation, Genotoxicity, and Mutation of Guanine Oxidation Products. Chem. Res. Toxicol. 19 (4), 491–505. 10.1021/tx0600043 [DOI] [PubMed] [Google Scholar]
- Newsholme P., Haber E. P., Hirabara S. M., Rebelato E. L., Procopio J., Morgan D., et al. (2007). Diabetes Associated Cell Stress and Dysfunction: Role of Mitochondrial and Non-mitochondrial ROS Production and Activity. J. Physiol. 583 (Pt 1), 9–24. 10.1113/jphysiol.2007.135871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme P., Cruzat V., Arfuso F., Keane K. (2014). Nutrient Regulation of Insulin Secretion and Action. J. Endocrinol. 221 (3), R105–R120. 10.1530/joe-13-0616 [DOI] [PubMed] [Google Scholar]
- Newsholme P., Gaudel C., Krause M. (2012). Mitochondria and Diabetes. An Intriguing Pathogenetic Role. Adv. Exp. Med. Biol. 942, 235–247. 10.1007/978-94-007-2869-1_10 [DOI] [PubMed] [Google Scholar]
- Ng T. P., Feng L., Yap K. B., Lee T. S., Tan C. H., Winblad B. (2014). Long-term Metformin Usage and Cognitive Function Among Older Adults with Diabetes. Jad 41 (1), 61–68. 10.3233/jad-131901 [DOI] [PubMed] [Google Scholar]
- Nguyen L. V., Ta Q. V., Dang T. B., Nguyen P. H., Nguyen T., Pham T. V. H., et al. (2019). Carvedilol Improves Glucose Tolerance and Insulin Sensitivity in Treatment of Adrenergic Overdrive in High Fat Diet-Induced Obesity in Mice. PLoS One 14 (11), e0224674. 10.1371/journal.pone.0224674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols W., Pankratz N., Hernandez D., Paisanruiz C., Jain S., Halter C., et al. (2005). Genetic Screening for a Single Common Mutation in Familial Parkinson's Disease. Lancet 365 (9457), 410–412. 10.1016/s0140-6736(05)70235-x [DOI] [PubMed] [Google Scholar]
- Nishikawa T., Edelstein D., Brownlee M. (2000). The Missing Link: a Single Unifying Mechanism for Diabetic Complications. Kidney Int. 58, S26–S30. 10.1046/j.1523-1755.2000.07705.x [DOI] [PubMed] [Google Scholar]
- Nuttall S. L., Khan J. N., Thorpe G. H., Langford N., Kendall M. J. (2003). The Impact of Therapeutic Doses of Paracetamol on Serum Total Antioxidant Capacity. J. Clin. Pharm. Ther. 28 (4), 289–294. 10.1046/j.1365-2710.2003.00493.x [DOI] [PubMed] [Google Scholar]
- Odegaard A. O., Jacobs D. R., Jr., Sanchez O. A., Goff D. C., Jr., Reiner A. P., Gross M. D. (2016). Oxidative Stress, Inflammation, Endothelial Dysfunction and Incidence of Type 2 Diabetes. Cardiovasc Diabetol. 15, 51. 10.1186/s12933-016-0369-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelze M., Daiber A., Brandes R. P., Hortmann M., Wenzel P., Hink U., et al. (2006). Nebivolol Inhibits Superoxide Formation by NADPH Oxidase and Endothelial Dysfunction in Angiotensin II-Treated Rats. Hypertension 48 (4), 677–684. 10.1161/01.hyp.0000239207.82326.29 [DOI] [PubMed] [Google Scholar]
- Oelze M., Kröller-Schön S., Welschof P., Jansen T., Hausding M., Mikhed Y., et al. (2014). The Sodium-Glucose Co-transporter 2 Inhibitor Empagliflozin Improves Diabetes-Induced Vascular Dysfunction in the Streptozotocin Diabetes Rat Model by Interfering with Oxidative Stress and Glucotoxicity. PLoS One 9 (11), e112394. 10.1371/journal.pone.0112394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K., Suzuki K., Okutsu M., Yamazaki K., Shinkai S. (2008). The Association of Elevated Reactive Oxygen Species Levels from Neutrophils with Low-Grade Inflammation in the Elderly. Immun. Ageing 5, 13. 10.1186/1742-4933-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh B., Figtree G., Costa D., Eade T., Hruby G., Lim S., et al. (2016). Oxidative Stress in Prostate Cancer Patients: A Systematic Review of Case Control Studies. Prostate Int. 4 (3), 71–87. 10.1016/j.prnil.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Kusano T., Nishino T. (2013). Chemical Nature and Reaction Mechanisms of the Molybdenum Cofactor of Xanthine Oxidoreductase. Cpd 19 (14), 2606–2614. 10.2174/1381612811319140010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. H. J., Johnson L. A., Zuloaga D. G., Limoli C. L., Raber J. (2013). Enhanced Hippocampus-dependent Memory and Reduced Anxiety in Mice Over-expressing Human Catalase in Mitochondria. J. Neurochem. 125 (2), 303–313. 10.1111/jnc.12187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson P. A., Tkatch T., Hernandez-Lopez S., Ulrich S., Ilijic E., Mugnaini E., et al. (2005). G-protein-coupled Receptor Modulation of Striatal CaV1.3 L-type Ca2+ Channels Is Dependent on a Shank-Binding Domain. J. Neurosci. 25 (5), 1050–1062. 10.1523/jneurosci.3327-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A. H., Rönn T., Elgzyri T., Hansson O., Eriksson K.-F., Groop L., et al. (2011). The Expression of Myosin Heavy Chain (MHC) Genes in Human Skeletal Muscle Is Related to Metabolic Characteristics Involved in the Pathogenesis of Type 2 Diabetes. Mol. Genet. Metabolism 103 (3), 275–281. 10.1016/j.ymgme.2011.03.017 [DOI] [PubMed] [Google Scholar]
- Onken B., Driscoll M. (2010). Metformin Induces a Dietary Restriction-like State and the Oxidative Stress Response to Extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One 5 (1), e8758. 10.1371/journal.pone.0008758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornelas A., Zacharias-Millward N., Menter D. G., Davis J. S., Lichtenberger L., Hawke D., et al. (2017). Beyond COX-1: the Effects of Aspirin on Platelet Biology and Potential Mechanisms of Chemoprevention. Cancer Metastasis Rev. 36 (2), 289–303. 10.1007/s10555-017-9675-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio H., Coronel I., Arellano A., Pacheco U., Bautista R., Franco M., et al. (2012). Sodium-glucose Cotransporter Inhibition Prevents Oxidative Stress in the Kidney of Diabetic Rats. Oxid. Med. Cell Longev. 2012, 542042. 10.1155/2012/542042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski S., Marcinkiewicz A., Nowak D., Zwoliński R., Jaszewski R. (2012). Comparison of the Clinical Application of Reactive Oxygen Species and Inflammatory Markers in Patients with Endocarditis. aoms 2 (2), 244–249. 10.5114/aoms.2012.28551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Z., Kong X., Sun X., He X., Zhang L., Gong Z., et al. (2018). Metformin Treatment Prevents Amyloid Plaque Deposition and Memory Impairment in APP/PS1 Mice. Brain, Behav. Immun. 69, 351–363. 10.1016/j.bbi.2017.12.009 [DOI] [PubMed] [Google Scholar]
- Owen M. R., Doran E., Halestrap A. P. (2000). Evidence that Metformin Exerts its Anti-diabetic Effects through Inhibition of Complex 1 of the Mitochondrial Respiratory Chain. Biochem. J. 348 (Pt 3), 607–614. 10.1042/bj3480607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozben T. (2007). Oxidative Stress and Apoptosis: Impact on Cancer Therapy. J. Pharm. Sci. 96 (9), 2181–2196. 10.1002/jps.20874 [DOI] [PubMed] [Google Scholar]
- Pacher P., Szabo C. (2006). Role of Peroxynitrite in the Pathogenesis of Cardiovascular Complications of Diabetes. Curr. Opin. Pharmacol. 6 (2), 136–141. 10.1016/j.coph.2006.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahwa R., Factor S. A., Lyons K. E., Ondo W. G., Gronseth G., Bronte-Stewart H., et al. (2006). Practice Parameter: Treatment of Parkinson Disease with Motor Fluctuations and Dyskinesia (An Evidence-Based Review): [RETIRED]. Neurology 66 (7), 983–995. 10.1212/01.wnl.0000215250.82576.87 [DOI] [PubMed] [Google Scholar]
- Paiva C. N., Bozza M. T. (2014). Are Reactive Oxygen Species Always Detrimental to Pathogens? Antioxidants Redox Signal. 20 (6), 1000–1037. 10.1089/ars.2013.5447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey K. B., Mishra N., Rizvi S. I. (2010). Protein Oxidation Biomarkers in Plasma of Type 2 Diabetic Patients. Clin. Biochem. 43 (4-5), 508–511. 10.1016/j.clinbiochem.2009.11.011 [DOI] [PubMed] [Google Scholar]
- Panth N., Paudel K. R., Parajuli K. (2016). Reactive Oxygen Species: A Key Hallmark of Cardiovascular Disease. Adv. Med. 2016, 9152732. 10.1155/2016/9152732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A. K., Galloway J. M., Hong Y., Wright J. S. (2013). Aspirin in the Secondary Prevention of Cardiovascular Disease. N. Engl. J. Med. 368 (3), 204–205. 10.1056/nejmp1213380 [DOI] [PubMed] [Google Scholar]
- Patil S. P., Jain P. D., Ghumatkar P. J., Tambe R., Sathaye S. (2014). Neuroprotective Effect of Metformin in MPTP-Induced Parkinson's Disease in Mice. Neuroscience 277, 747–754. 10.1016/j.neuroscience.2014.07.046 [DOI] [PubMed] [Google Scholar]
- Patrignani P., Sacco A., Sostres C., Bruno A., Dovizio M., Piazuelo E., et al. (2017). Low-Dose Aspirin Acetylates Cyclooxygenase-1 in Human Colorectal Mucosa: Implications for the Chemoprevention of Colorectal Cancer. Clin. Pharmacol. Ther. 102 (1), 52–61. 10.1002/cpt.639 [DOI] [PubMed] [Google Scholar]
- Patrono C. (2015). The Multifaceted Clinical Readouts of Platelet Inhibition by Low-Dose Aspirin. J. Am. Coll. Cardiol. 66 (1), 74–85. 10.1016/j.jacc.2015.05.012 [DOI] [PubMed] [Google Scholar]
- Paudel Y. N., Angelopoulou E., Piperi C., Shaikh M. F., Othman I. (2020). Emerging Neuroprotective Effect of Metformin in Parkinson's Disease: A Molecular Crosstalk. Pharmacol. Res. 152, 104593. 10.1016/j.phrs.2019.104593 [DOI] [PubMed] [Google Scholar]
- Perez M., Gonzalez-Sanchez E., Gonzalez-Loyola A., Gonzalez-Buitrago J., Marin J. (2011). Mitochondrial Genome Depletion Dysregulates Bile Acid- and Paracetamol-Induced Expression of the Transporters Mdr1, Mrp1 and Mrp4 in Liver Cells. Br. J. Pharmacol. 162 (8), 1686–1699. 10.1111/j.1476-5381.2010.01174.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Revuelta B. I., Hettich M. M., Ciociaro A., Rotermund C., Kahle P. J., Krauss S., et al. (2014). Metformin Lowers Ser-129 Phosphorylated α-synuclein Levels via mTOR-dependent Protein Phosphatase 2A Activation. Cell Death Dis. 5, e1209. 10.1038/cddis.2014.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perier C., Tieu K., Guégan C., Caspersen C., Jackson-Lewis V., Carelli V., et al. (2005). Complex I Deficiency Primes Bax-dependent Neuronal Apoptosis through Mitochondrial Oxidative Damage. Proc. Natl. Acad. Sci. U.S.A. 102 (52), 19126–19131. 10.1073/pnas.0508215102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perillo B., Di Donato M., Pezone A., Di Zazzo E., Giovannelli P., Galasso G., et al. (2020). ROS in Cancer Therapy: the Bright Side of the Moon. Exp. Mol. Med. 52 (2), 192–203. 10.1038/s12276-020-0384-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry E. A., Bennett C. F., Luo C., Balsa E., Jedrychowski M., O’Malley K. E., et al. (2021). Tetracyclines Promote Survival and Fitness in Mitochondrial Disease Models. Nat. Metab. 3 (1), 33–42. 10.1038/s42255-020-00334-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell A. M., Youle R. J. (2015). The Roles of PINK1, Parkin, and Mitochondrial Fidelity in Parkinson's Disease. Neuron 85 (2), 257–273. 10.1016/j.neuron.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel D. R., Amin J. K., Xiao L., Miller T., Viereck J., Oliver-Krasinski J., et al. (2001). Reactive Oxygen Species Mediate Amplitude-dependent Hypertrophic and Apoptotic Responses to Mechanical Stretch in Cardiac Myocytes. Circulation Res. 89 (5), 453–460. 10.1161/hh1701.096615 [DOI] [PubMed] [Google Scholar]
- Pintana H., Apaijai N., Chattipakorn N., Chattipakorn S. C. (2013). DPP-4 Inhibitors Improve Cognition and Brain Mitochondrial Function of Insulin-Resistant Rats. J. Endocrinol. 218 (1), 1–11. 10.1530/joe-12-0521 [DOI] [PubMed] [Google Scholar]
- Pipatpiboon N., Pintana H., Pratchayasakul W., Chattipakorn N., Chattipakorn S. C. (2013). DPP4-inhibitor Improves Neuronal Insulin Receptor Function, Brain Mitochondrial Function and Cognitive Function in Rats with Insulin Resistance Induced by High-Fat Diet Consumption. Eur. J. Neurosci. 37 (5), 839–849. 10.1111/ejn.12088 [DOI] [PubMed] [Google Scholar]
- Piskovatska V., Stefanyshyn N., Storey K. B., Vaiserman A. M., Lushchak O. (2019). Metformin as a Geroprotector: Experimental and Clinical Evidence. Biogerontology 20 (1), 33–48. 10.1007/s10522-018-9773-5 [DOI] [PubMed] [Google Scholar]
- Pisoschi A. M., Pop A. (2015). The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 97, 55–74. 10.1016/j.ejmech.2015.04.040 [DOI] [PubMed] [Google Scholar]
- Pissadaki E. K., Bolam J. P. (2013). The Energy Cost of Action Potential Propagation in Dopamine Neurons: Clues to Susceptibility in Parkinson's Disease. Front. Comput. Neurosci. 7, 13. 10.3389/fncom.2013.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochini L., Galluccio M., Scumaci D., Giangregorio N., Tonazzi A., Palmieri F., et al. (2008). Interaction of β-lactam Antibiotics with the Mitochondrial Carnitine/acylcarnitine Transporter. Chemico-Biological Interact. 173 (3), 187–194. 10.1016/j.cbi.2008.03.003 [DOI] [PubMed] [Google Scholar]
- Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., et al. (1997). Mutation in the α-Synuclein Gene Identified in Families with Parkinson's Disease. Science 276 (5321), 2045–2047. 10.1126/science.276.5321.2045 [DOI] [PubMed] [Google Scholar]
- Poole K. (2012). Stress Responses as Determinants of Antimicrobial Resistance in Gram-Negative Bacteria. Trends Microbiol. 20 (5), 227–234. 10.1016/j.tim.2012.02.004 [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A., Mitran S., Sivanesan S., Chang E., Buga A. M. (2013). ROS and Brain Diseases: the Good, the Bad, and the Ugly. Oxid. Med. Cell Longev. 2013, 963520. 10.1155/2013/963520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M., Bryce R., Albanese E., Wimo A., Ribeiro W., Ferri C. P. (2013). The Global Prevalence of Dementia: a Systematic Review and Metaanalysis. Alzheimer's & Dement. 9 (1), 63–75 e2. 10.1016/j.jalz.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Pujos-Guillot E., Pickering G., Lyan B., Ducheix G., Brandolini-Bunlon M., Glomot F., et al. (2012). Therapeutic Paracetamol Treatment in Older Persons Induces Dietary and Metabolic Modifications Related to Sulfur Amino Acids. Age 34 (1), 181–193. 10.1007/s11357-011-9218-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purssell E. (2002). Treating Fever in Children: Paracetamol or Ibuprofen? Br. J. Community Nurs. 7 (6), 316–320. 10.12968/bjcn.2002.7.6.10477 [DOI] [PubMed] [Google Scholar]
- Puspita L., Chung S. Y., Shim J.-w. (2017). Oxidative Stress and Cellular Pathologies in Parkinson's Disease. Mol. Brain 10 (1), 53. 10.1186/s13041-017-0340-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam C. D., Arvai A. S., Bourne Y., Tainer J. A. (2000). Active and Inhibited Human Catalase Structures: Ligand and NADPH Binding and Catalytic Mechanism 1 1Edited by R. Huber. J. Mol. Biol. 296 (1), 295–309. 10.1006/jmbi.1999.3458 [DOI] [PubMed] [Google Scholar]
- Pyatigorskaya N., Sharman M., Corvol J.-C., Valabregue R., Yahia-Cherif L., Poupon F., et al. (2015). High Nigral Iron Deposition in LRRK2 and Parkin Mutation Carriers Using R2* Relaxometry. Mov. Disord. 30 (8), 1077–1084. 10.1002/mds.26218 [DOI] [PubMed] [Google Scholar]
- Rain C., Rada G. (2015). Is Carvedilol Better Than Other Beta-Blockers for Heart Failure? Medwave 15 (Suppl. 1), e6168. 10.5867/medwave.2015.6168 [DOI] [PubMed] [Google Scholar]
- Ramprasath T., Murugan P. S., Kalaiarasan E., Gomathi P., Rathinavel A., Selvam G. S. (2012). Genetic Association of Glutathione Peroxidase-1 (GPx-1) and NAD(P)H:Quinone Oxidoreductase 1(NQO1) Variants and Their Association of CAD in Patients with Type-2 Diabetes. Mol. Cell Biochem. 361 (1-2), 143–150. 10.1007/s11010-011-1098-5 [DOI] [PubMed] [Google Scholar]
- Ramsay R. R., Krueger M. J., Youngster S. K., Gluck M. R., Casida J. E., Singer T. P. (1991). Interaction of 1-Methyl-4-Phenylpyridinium Ion (MPP+) and its Analogs with the Rotenone/piericidin Binding Site of NADH Dehydrogenase. J. Neurochem. 56 (4), 1184–1190. 10.1111/j.1471-4159.1991.tb11409.x [DOI] [PubMed] [Google Scholar]
- Ravn-Haren G., Olsen A., Tjønneland A., Dragsted L. O., Nexø B. A., Wallin H., et al. (2006). Associations between GPX1 Pro198Leu Polymorphism, Erythrocyte GPX Activity, Alcohol Consumption and Breast Cancer Risk in a Prospective Cohort Study. Carcinogenesis 27 (4), 820–825. 10.1093/carcin/bgi267 [DOI] [PubMed] [Google Scholar]
- Reddy P. H., Manczak M., Mao P., Calkins M. J., Reddy A. P., Shirendeb U. (2010). Amyloid-beta and Mitochondria in Aging and Alzheimer's Disease: Implications for Synaptic Damage and Cognitive Decline. J. Alzheimers Dis. 20 Suppl 2 (Suppl. 2), S499–S512. 10.3233/JAD-2010-100504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P. H. (2006). Mitochondrial Oxidative Damage in Aging and Alzheimer's Disease: Implications for Mitochondrially Targeted Antioxidant Therapeutics. J. Biomed. Biotechnol. 2006 (3), 31372. 10.1155/JBB/2006/31372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P. H. (2009). Amyloid Beta, Mitochondrial Structural and Functional Dynamics in Alzheimer's Disease. Exp. Neurol. 218 (2), 286–292. 10.1016/j.expneurol.2009.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P. H., Beal M. F. (2008). Amyloid Beta, Mitochondrial Dysfunction and Synaptic Damage: Implications for Cognitive Decline in Aging and Alzheimer's Disease. Trends Mol. Med. 14 (2), 45–53. 10.1016/j.molmed.2007.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P. H., Shirendeb U. P. (2012). Mutant Huntingtin, Abnormal Mitochondrial Dynamics, Defective Axonal Transport of Mitochondria, and Selective Synaptic Degeneration in Huntington's Disease. Biochimica Biophysica Acta (BBA) - Mol. Basis Dis. 1822 (2), 101–110. 10.1016/j.bbadis.2011.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P. H., Tripathi R., Troung Q., Tirumala K., Reddy T. P., Anekonda V., et al. (2012). Abnormal Mitochondrial Dynamics and Synaptic Degeneration as Early Events in Alzheimer's Disease: Implications to Mitochondria-Targeted Antioxidant Therapeutics. Biochimica Biophysica Acta (BBA) - Mol. Basis Dis. 1822 (5), 639–649. 10.1016/j.bbadis.2011.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rena G., Lang C. C. (2018). Repurposing Metformin for Cardiovascular Disease. Circulation 137 (5), 422–424. 10.1161/circulationaha.117.031735 [DOI] [PubMed] [Google Scholar]
- Rhee S. G., Woo H. A. (2011). Multiple Functions of Peroxiredoxins: Peroxidases, Sensors and Regulators of the Intracellular Messenger H2O2, and Protein Chaperones. Antioxidants Redox Signal. 15 (3), 781–794. 10.1089/ars.2010.3393 [DOI] [PubMed] [Google Scholar]
- Richardson J. R., Quan Y., Sherer T. B., Greenamyre J. T., Miller G. W. (2005). Paraquat Neurotoxicity Is Distinct from that of MPTP and Rotenone. Toxicol. Sci. 88 (1), 193–201. 10.1093/toxsci/kfi304 [DOI] [PubMed] [Google Scholar]
- Ridet J.-L., Bensadoun J.-C., Déglon N., Aebischer P., Zurn A. D. (2006). Lentivirus-mediated Expression of Glutathione Peroxidase: Neuroprotection in Murine Models of Parkinson's Disease. Neurobiol. Dis. 21 (1), 29–34. 10.1016/j.nbd.2005.06.003 [DOI] [PubMed] [Google Scholar]
- Ripley E., Hirsch A. (2010). Fifteen Years of Losartan: what Have We Learned about Losartan that Can Benefit Chronic Kidney Disease Patients? Ijnrd 3, 93–98. 10.2147/ijnrd.s7038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M., Schmeisser K. (2014). Mitohormesis: Promoting Health and Lifespan by Increased Levels of Reactive Oxygen Species (ROS). Dose Response 12 (2), 288–341. 10.2203/dose-response.13-035.Ristow [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera E. J., Goldin A., Fulmer N., Tavares R., Wands J. R., de la Monte S. M. (2005). Insulin and Insulin-like Growth Factor Expression and Function Deteriorate with Progression of Alzheimer's Disease: Link to Brain Reductions in Acetylcholine. Jad 8 (3), 247–268. 10.3233/jad-2005-8304 [DOI] [PubMed] [Google Scholar]
- Rizzo M. R., Barbieri M., Marfella R., Paolisso G. (2012). Reduction of Oxidative Stress and Inflammation by Blunting Daily Acute Glucose Fluctuations in Patients with Type 2 Diabetes. Diabetes Care 35 (10), 2076–2082. 10.2337/dc12-0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochette L., Zeller M., Cottin Y., Vergely C. (2014). Diabetes, Oxidative Stress and Therapeutic Strategies. Biochimica Biophysica Acta (BBA) - General Subj. 1840 (9), 2709–2729. 10.1016/j.bbagen.2014.05.017 [DOI] [PubMed] [Google Scholar]
- Roffi M., Patrono C., Collet J.-P., Mueller C., Valgimigli M., Andreotti F., et al. (20152016). 2015 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation. Eur. Heart J. 37 (3), 267–315. 10.1093/eurheartj/ehv320 [DOI] [PubMed] [Google Scholar]
- Romano M., Cianci E., Simiele F., Recchiuti A. (2015). Lipoxins and Aspirin-Triggered Lipoxins in Resolution of Inflammation. Eur. J. Pharmacol. 760, 49–63. 10.1016/j.ejphar.2015.03.083 [DOI] [PubMed] [Google Scholar]
- Romo R., Schultz W. (1990). Dopamine Neurons of the Monkey Midbrain: Contingencies of Responses to Active Touch during Self-Initiated Arm Movements. J. Neurophysiology 63 (3), 592–606. 10.1152/jn.1990.63.3.592 [DOI] [PubMed] [Google Scholar]
- Rösen P., Osmers A. (2006). Oxidative Stress in Young Zucker Rats with Impaired Glucose Tolerance Is Diminished by Acarbose. Horm. Metab. Res. 38 (9), 575–586. 10.1055/s-2006-950397 [DOI] [PubMed] [Google Scholar]
- Rotermund C., Machetanz G., Fitzgerald J. C. (2018). The Therapeutic Potential of Metformin in Neurodegenerative Diseases. Front. Endocrinol. 9, 400. 10.3389/fendo.2018.00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G. A., Mensah G. A., Johnson C. O., Addolorato G., Ammirati E., Baddour L. M., et al. (2020). Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 76 (25), 2982–3021. 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell P. M., Wilson M., Elwin C.-E., Norrving B., Algra A., Warlow C. P., et al. (2010). Long-term Effect of Aspirin on Colorectal Cancer Incidence and Mortality: 20-year Follow-Up of Five Randomised Trials. Lancet 376 (9754), 1741–1750. 10.1016/s0140-6736(10)61543-7 [DOI] [PubMed] [Google Scholar]
- Rowe S. E., Wagner N. J., Li L., Beam J. E., Wilkinson A. D., Radlinski L. C., et al. (2020). Reactive Oxygen Species Induce Antibiotic Tolerance during Systemic Staphylococcus aureus Infection. Nat. Microbiol. 5 (2), 282–290. 10.1038/s41564-019-0627-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegsegger G. N., Vanderboom P. M., Dasari S., Klaus K. A., Kabiraj P., McCarthy C. B., et al. (2019). Exercise and Metformin Counteract Altered Mitochondrial Function in the Insulin-Resistant Brain. JCI Insight 4 (18). 10.1172/jci.insight.130681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui Y., Tiwari P., Xie Z., Zheng J. Q. (2006). Acute Impairment of Mitochondrial Trafficking by Beta-Amyloid Peptides in Hippocampal Neurons. J. Neurosci. 26 (41), 10480–10487. 10.1523/jneurosci.3231-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryden L., Rydén L., Grant P. J., Anker S. D., Berne C., Cosentino F., et al. (2013). ESC Guidelines on Diabetes, Pre-diabetes, and Cardiovascular Diseases Developed in Collaboration with the EASD: the Task Force on Diabetes, Pre-diabetes, and Cardiovascular Diseases of the European Society of Cardiology (ESC) and Developed in Collaboration with the European Association for the Study of Diabetes (EASD). Eur. Heart J. 34 (39), 3035–3087. 10.1093/eurheartj/eht108 [DOI] [PubMed] [Google Scholar]
- Sa-Nguanmoo P., Tanajak P., Kerdphoo S., Jaiwongkam T., Pratchayasakul W., Chattipakorn N., et al. (2017). SGLT2-inhibitor and DPP-4 Inhibitor Improve Brain Function via Attenuating Mitochondrial Dysfunction, Insulin Resistance, Inflammation, and Apoptosis in HFD-Induced Obese Rats. Toxicol. Appl. Pharmacol. 333, 43–50. 10.1016/j.taap.2017.08.005 [DOI] [PubMed] [Google Scholar]
- Saewanee N., Praputpittaya T., Malaiwong N., Chalorak P., Meemon K. (2021). Neuroprotective Effect of Metformin on Dopaminergic Neurodegeneration and α-synuclein Aggregation in C. elegans Model of Parkinson's Disease. Neurosci. Res. 162, 13–21. 10.1016/j.neures.2019.12.017 [DOI] [PubMed] [Google Scholar]
- Sahoo B. M., Banik B. K., Borah P., Jain A. (2021). Reactive Oxygen Species (ROS): Key Components in Cancer Therapies. Anticancer Agents Med. Chem. [DOI] [PubMed] [Google Scholar]
- Salarian A., Kadkhodaee M., Zahmatkesh M., Seifi B., Bakhshi E., Akhondzadeh S., et al. (2018). Opioid Use Disorder Induces Oxidative Stress and Inflammation: The Attenuating Effect of Methadone Maintenance Treatment. Iran. J. Psychiatry 13 (1), 46–54. [PMC free article] [PubMed] [Google Scholar]
- Salimi A., Eybagi S., Seydi E., Naserzadeh P., Kazerouni N. P., Pourahmad J. (2016). Toxicity of Macrolide Antibiotics on Isolated Heart Mitochondria: a Justification for Their Cardiotoxic Adverse Effect. Xenobiotica 46 (1), 82–93. 10.3109/00498254.2015.1046975 [DOI] [PubMed] [Google Scholar]
- Salminen A., Kaarniranta K. (2012). AMP-activated Protein Kinase (AMPK) Controls the Aging Process via an Integrated Signaling Network. Ageing Res. Rev. 11 (2), 230–241. 10.1016/j.arr.2011.12.005 [DOI] [PubMed] [Google Scholar]
- Salvemini D., Little J. W., Doyle T., Neumann W. L. (2011). Roles of Reactive Oxygen and Nitrogen Species in Pain. Free Radic. Biol. Med. 51 (5), 951–966. 10.1016/j.freeradbiomed.2011.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambe T., Mason R. P., Dawoud H., Bhatt D. L., Malinski T. (2018). Metformin Treatment Decreases Nitroxidative Stress, Restores Nitric Oxide Bioavailability and Endothelial Function beyond Glucose Control. Biomed. Pharmacother. 98, 149–156. 10.1016/j.biopha.2017.12.023 [DOI] [PubMed] [Google Scholar]
- Sandyk R. (1993). The Relationship between Diabetes Mellitus and Parkinson's Disease. Int. J. Neurosci. 69 (1-4), 125–130. 10.3109/00207459309003322 [DOI] [PubMed] [Google Scholar]
- Santiago J. A., Potashkin J. A. (2013). Integrative Network Analysis Unveils Convergent Molecular Pathways in Parkinson's Disease and Diabetes. PLoS One 8 (12), e83940. 10.1371/journal.pone.0083940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpello J. H., Howlett H. C. (2008). Metformin Therapy and Clinical Uses. Diabetes Vasc. Dis. Res. 5 (3), 157–167. 10.3132/dvdr.2008.027 [DOI] [PubMed] [Google Scholar]
- Schaefer A. M., McFarland R., Blakely E. L., He L., Whittaker R. G., Taylor R. W., et al. (2008). Prevalence of Mitochondrial DNA Disease in Adults. Ann. Neurol. 63 (1), 35–39. 10.1002/ana.21217 [DOI] [PubMed] [Google Scholar]
- Schapira A. H. V., Cooper J. M., Dexter D., Clark J. B., Jenner P., Marsden C. D. (1990). Mitochondrial Complex I Deficiency in Parkinson's Disease. J. Neurochem. 54 (3), 823–827. 10.1111/j.1471-4159.1990.tb02325.x [DOI] [PubMed] [Google Scholar]
- Schiavone S., Neri M., Harvey B. H. (2019). Pathological Consequences of Drug Abuse: Implication of Redox Imbalance. Oxid. Med. Cell Longev. 2019, 4780852. 10.1155/2019/4780852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildknecht S., Daiber A., Ghisla S., Cohen R. A., Bachschmid M. M. (2008). Acetaminophen Inhibits Prostanoid Synthesis by Scavenging the PGHS‐activator Peroxynitrite. FASEB J. 22 (1), 215–224. 10.1096/fj.06-8015com [DOI] [PubMed] [Google Scholar]
- Schon E. A., Przedborski S. (2011). Mitochondria: the Next (Neurode)generation. Neuron 70 (6), 1033–1053. 10.1016/j.neuron.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriner S. E., Linford N. J., Martin G. M., Treuting P., Ogburn C. E., Emond M., et al. (2005). Extension of Murine Life Span by Overexpression of Catalase Targeted to Mitochondria. Science 308 (5730), 1909–1911. 10.1126/science.1106653 [DOI] [PubMed] [Google Scholar]
- Schulz T. J., Zarse K., Voigt A., Urban N., Birringer M., Ristow M. (2007). Glucose Restriction Extends Caenorhabditis elegans Life Span by Inducing Mitochondrial Respiration and Increasing Oxidative Stress. Cell Metab. 6 (4), 280–293. 10.1016/j.cmet.2007.08.011 [DOI] [PubMed] [Google Scholar]
- Schulzer M., Mak E., Calne D. B. (1992). The Antiparkinson Efficacy of Deprenyl Derives from Transient Improvement that Is Likely to Be Symptomatic. Ann. Neurol. 32 (6), 795–798. 10.1002/ana.410320614 [DOI] [PubMed] [Google Scholar]
- Segawa M., Nomura Y., Nishiyama N. (2003). Autosomal Dominant Guanosine Triphosphate Cyclohydrolase I Deficiency (Segawa Disease). Ann. Neurol. 54 (Suppl. 6), S32–S45. 10.1002/ana.10630 [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. (2002). Alzheimer's Disease Is a Synaptic Failure. Science 298 (5594), 789–791. 10.1126/science.1074069 [DOI] [PubMed] [Google Scholar]
- Sell H., Blüher M., Klöting N., Schlich R., Willems M., Ruppe F., et al. (2013). Adipose Dipeptidyl Peptidase-4 and Obesity. Diabetes Care 36 (12), 4083–4090. 10.2337/dc13-0496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semancik J. S., Vanderwoude W. J. (1976). Exocortis Viroid: Cytopathic Effects at the Plasma Membrane in Association with Pathogenic RNA. Virology 69 (2), 719–726. 10.1016/0042-6822(76)90500-6 [DOI] [PubMed] [Google Scholar]
- Sergi D., Renaud J., Simola N., Martinoli M.-G. (2019). Diabetes, a Contemporary Risk for Parkinson's Disease: Epidemiological and Cellular Evidences. Front. Aging Neurosci. 11, 302. 10.3389/fnagi.2019.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N., Chiang N. (2013). Resolution Phase Lipid Mediators of Inflammation: Agonists of Resolution. Curr. Opin. Pharmacol. 13 (4), 632–640. 10.1016/j.coph.2013.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma C., Long J. H., Shah S., Rahman J., Perrett D., Ayoub S., et al. (2017). First Evidence of the Conversion of Paracetamol to AM404 in Human Cerebrospinal Fluid. Jpr 10, 2703–2709. 10.2147/jpr.s143500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Bhattarai S., Ara H., Sun G., St Clair D. K., Bhuiyan M. S., et al. (2020). SOD2 Deficiency in Cardiomyocytes Defines Defective Mitochondrial Bioenergetics as a Cause of Lethal Dilated Cardiomyopathy. Redox Biol. 37, 101740. 10.1016/j.redox.2020.101740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon R. A., Jiang X., Francisco C., Christen S., Vexler Z. S., Täuber M. G., et al. (2004). Manipulation of Antioxidant Pathways in Neonatal Murine Brain. Pediatr. Res. 56 (4), 656–662. 10.1203/01.pdr.0000139413.27864.50 [DOI] [PubMed] [Google Scholar]
- Sheveleva E. V., Landowski T. H., Samulitis B. K., Bartholomeusz G., Powis G., Dorr R. T. (2012). Imexon Induces an Oxidative Endoplasmic Reticulum Stress Response in Pancreatic Cancer Cells. Mol. Cancer Res. 10 (3), 392–400. 10.1158/1541-7786.mcr-11-0359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Lo C.-S., Chenier I., Maachi H., Filep J. G., Ingelfinger J. R., et al. (2013). Overexpression of Catalase Prevents Hypertension and Tubulointerstitial Fibrosis and Normalization of Renal Angiotensin-Converting Enzyme-2 Expression in Akita Mice. Am. J. Physiology-Renal Physiology 304 (11), F1335–F1346. 10.1152/ajprenal.00405.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Vanhoutte P. M. (2017). Macro- and Microvascular Endothelial Dysfunction in Diabetes. J. Diabetes 9 (5), 434–449. 10.1111/1753-0407.12521 [DOI] [PubMed] [Google Scholar]
- Shields H. J., Traa A., Van Raamsdonk J. M. (2021). Beneficial and Detrimental Effects of Reactive Oxygen Species on Lifespan: A Comprehensive Review of Comparative and Experimental Studies. Front. Cell Dev. Biol. 9, 628157. 10.3389/fcell.2021.628157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih N.-L., Cheng T.-H., Loh S.-H., Cheng P.-Y., Wang D. L., Chen Y.-S., et al. (2001). Reactive Oxygen Species Modulate Angiotensin II-Induced β-Myosin Heavy Chain Gene Expression via Ras/Raf/Extracellular Signal-Regulated Kinase Pathway in Neonatal Rat Cardiomyocytes. Biochem. Biophysical Res. Commun. 283 (1), 143–148. 10.1006/bbrc.2001.4744 [DOI] [PubMed] [Google Scholar]
- Shim W. S., Oh U. (2008). Histamine-induced Itch and its Relationship with Pain. Mol. Pain 4, 29. 10.1186/1744-8069-4-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Crother T. R., Karlin J., Dagvadorj J., Chiba N., Chen S., et al. (2012). Oxidized Mitochondrial DNA Activates the NLRP3 Inflammasome during Apoptosis. Immunity 36 (3), 401–414. 10.1016/j.immuni.2012.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H.-S., Ko J., Kim D.-A., Ryu E.-S., Ryu H.-M., Park S.-H., et al. (2017). Metformin Ameliorates the Phenotype Transition of Peritoneal Mesothelial Cells and Peritoneal Fibrosis via a Modulation of Oxidative Stress. Sci. Rep. 7 (1), 5690. 10.1038/s41598-017-05836-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S. J., Chung S., Kim S. J., Lee E.-M., Yoo Y.-H., Kim J.-W., et al. (2016). Effect of Sodium-Glucose Co-transporter 2 Inhibitor, Dapagliflozin, on Renal Renin-Angiotensin System in an Animal Model of Type 2 Diabetes. PLoS One 11 (11), e0165703. 10.1371/journal.pone.0165703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoulson I. (1992). An Interim Report of the Effect of Selegiline (L-Deprenyl) on the Progression of Disability in Early Parkinson's Disease. The Parkinson Study Group. Eur. Neurol. 32 (Suppl. 1), 46–53. 10.1159/000116869 [DOI] [PubMed] [Google Scholar]
- Simeonova R., Vitcheva V., Kondeva-Burdina M., Krasteva I., Manov V., Mitcheva M. (2013). Hepatoprotective and Antioxidant Effects of Saponarin, Isolated from Gypsophila Trichotoma Wend. On Paracetamol-Induced Liver Damage in Rats. Biomed. Res. Int. 2013, 757126. 10.1155/2013/757126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson I. A., Chundu K. R., Davies-Hill T., Honer W. G., Davies P. (1994). Decreased Concentrations of GLUT1 and GLUT3 Glucose Transporters in the Brains of Patients with Alzheimer's Disease. Ann. Neurol. 35 (5), 546–551. 10.1002/ana.410350507 [DOI] [PubMed] [Google Scholar]
- Singh B. N., Shankar S., Srivastava R. K. (2011). Green Tea Catechin, Epigallocatechin-3-Gallate (EGCG): Mechanisms, Perspectives and Clinical Applications. Biochem. Pharmacol. 82 (12), 1807–1821. 10.1016/j.bcp.2011.07.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirvent P., Bordenave S., Vermaelen M., Roels B., Vassort G., Mercier J., et al. (2005). Simvastatin Induces Impairment in Skeletal Muscle while Heart Is Protected. Biochem. Biophysical Res. Commun. 338 (3), 1426–1434. 10.1016/j.bbrc.2005.10.108 [DOI] [PubMed] [Google Scholar]
- Smieszek A., Strek Z., Kornicka K., Grzesiak J., Weiss C., Marycz K. (2017). Antioxidant and Anti-senescence Effect of Metformin on Mouse Olfactory Ensheathing Cells (mOECs) May Be Associated with Increased Brain-Derived Neurotrophic Factor Levels-An Ex Vivo Study. Int. J. Mol. Sci. 18 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. A., Richey Harris P. L., Sayre L. M., Beckman J. S., Perry G. (1997). Widespread Peroxynitrite-Mediated Damage in Alzheimer's Disease. J. Neurosci. 17 (8), 2653–2657. 10.1523/jneurosci.17-08-02653.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somarelli J. A., Gardner H., Cannataro V. L., Gunady E. F., Boddy A. M., Johnson N. A., et al. (2020). Molecular Biology and Evolution of Cancer: From Discovery to Action. Mol. Biol. Evol. 37 (2), 320–326. 10.1093/molbev/msz242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son A., Nakamura H., Kondo N., Matsuo Y., Liu W., Oka S.-i., et al. (2006). Redox Regulation of Mast Cell Histamine Release in Thioredoxin-1 (TRX) Transgenic Mice. Cell Res. 16 (2), 230–239. 10.1038/sj.cr.7310031 [DOI] [PubMed] [Google Scholar]
- Sorce S., Krause K.-H. (2009). NOX Enzymes in the Central Nervous System: from Signaling to Disease. Antioxidants Redox Signal. 11 (10), 2481–2504. 10.1089/ars.2009.2578 [DOI] [PubMed] [Google Scholar]
- Soukas A. A., Hao H., Wu L. (2019). Metformin as Anti-aging Therapy: Is it for Everyone? Trends Endocrinol. Metabolism 30 (10), 745–755. 10.1016/j.tem.2019.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer N. Y., Engelhardt J. F. (2014). The Basic Biology of Redoxosomes in Cytokine-Mediated Signal Transduction and Implications for Disease-specific Therapies. Biochemistry 53 (10), 1551–1564. 10.1021/bi401719r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini M. G., Schmidt M. L., Lee V. M.-Y., Trojanowski J. Q., Jakes R., Goedert M. (1997). α-Synuclein in Lewy Bodies. Nature 388 (6645), 839–840. 10.1038/42166 [DOI] [PubMed] [Google Scholar]
- Srinivasan A. (2019). Propranolol: A 50-Year Historical Perspective. Ann. Indian Acad. Neurol. 22 (1), 21–26. 10.4103/aian.aian_201_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman E. R. (1990). Metal Ion-Catalyzed Oxidation of Proteins: Biochemical Mechanism and Biological Consequences. Free Radic. Biol. Med. 9 (4), 315–325. 10.1016/0891-5849(90)90006-5 [DOI] [PubMed] [Google Scholar]
- Stansley B. J., Yamamoto B. K. (2013). L-dopa-induced Dopamine Synthesis and Oxidative Stress in Serotonergic Cells. Neuropharmacology 67, 243–251. 10.1016/j.neuropharm.2012.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M., Glantz M., Lekos A. (1995). Vitamin E Plus Aspirin Compared with Aspirin Alone in Patients with Transient Ischemic Attacks. Am. J. Clin. Nutr. 62 (6 Suppl. l), 1381S–1384S. 10.1093/ajcn/62.6.1381S [DOI] [PubMed] [Google Scholar]
- Steven S., Münzel T., Daiber A. (2015). Exploiting the Pleiotropic Antioxidant Effects of Established Drugs in Cardiovascular Disease. Ijms 16 (8), 18185–18223. 10.3390/ijms160818185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven S., Oelze M., Hanf A., Kröller-Schön S., Kashani F., Roohani S., et al. (2017). The SGLT2 Inhibitor Empagliflozin Improves the Primary Diabetic Complications in ZDF Rats. Redox Biol. 13, 370–385. 10.1016/j.redox.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirpe F., Della Corte E., Lorenzoni E. (1969). The Regulation of Rat Liver Xanthine Oxidase. J. Biol. Chem. 244 (14), 3855–3863. 10.1016/s0021-9258(17)36428-1 [DOI] [PubMed] [Google Scholar]
- Stocchi F., Vacca L., Grassini P., De Pandis M. F., Battaglia G., Cattaneo C., et al. (2006). Symptom Relief in Parkinson Disease by Safinamide: Biochemical and Clinical Evidence of Efficacy beyond MAO-B Inhibition. Neurology 67 (7 Suppl. 2), S24–S29. 10.1212/wnl.67.7_suppl_2.s24 [DOI] [PubMed] [Google Scholar]
- Su Y., Duan J., Ying Z., Hou Y., Zhang Y., Wang R., et al. (2013). Increased Vulnerability of Parkin Knock Down PC12 Cells to Hydrogen Peroxide Toxicity: the Role of Salsolinol and NM-salsolinol. Neuroscience 233, 72–85. 10.1016/j.neuroscience.2012.12.045 [DOI] [PubMed] [Google Scholar]
- Suárez-Rivero J. M., Pastor-Maldonado C. J., Povea-Cabello S., Álvarez-Córdoba M., Villalón-García I., Talaverón-Rey M., et al. (2021). Mitochondria and Antibiotics: For Good or for Evil? Biomolecules 11 (7). 10.3390/biom11071050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sub Laban T., Saadabadi A. (2021). Monoamine Oxidase Inhibitors (MAOI). Island FL: StatPearls. Treasure. [PubMed] [Google Scholar]
- Subramaniam S. R., Vergnes L., Franich N. R., Reue K., Chesselet M.-F. (2014). Region Specific Mitochondrial Impairment in Mice with Widespread Overexpression of Alpha-Synuclein. Neurobiol. Dis. 70, 204–213. 10.1016/j.nbd.2014.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T., Lewén A., Gasche Y., Yu F., Chan P. H. (2002). Overexpression of SOD1 Protects Vulnerable Motor Neurons after Spinal Cord Injury by Attenuating Mitochondrial Cytochrome C Release. FASEB J. 16 (14), 1997–1999. 10.1096/fj.02-0251fje [DOI] [PubMed] [Google Scholar]
- Sugizaki T., Zhu S., Guo G., Matsumoto A., Zhao J., Endo M., et al. (2017). Treatment of Diabetic Mice with the SGLT2 Inhibitor TA-1887 Antagonizes Diabetic Cachexia and Decreases Mortality. NPJ Aging Mech. Dis. 3, 12. 10.1038/s41514-017-0012-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J. H. H., Heath T. M., Hagen T. M. (2003). Two Subpopulations of Mitochondria in the Aging Rat Heart Display Heterogenous Levels of Oxidative Stress. Free Radic. Biol. Med. 35 (9), 1064–1072. 10.1016/s0891-5849(03)00468-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Druhan L. J., Zweier J. L. (2010). Reactive Oxygen and Nitrogen Species Regulate Inducible Nitric Oxide Synthase Function Shifting the Balance of Nitric Oxide and Superoxide Production. Archives Biochem. Biophysics 494 (2), 130–137. 10.1016/j.abb.2009.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwa M., Egashira T., Nakano H., Sasaki H., Kumagai S. (2006). Metformin Increases the PGC-1alpha Protein and Oxidative Enzyme Activities Possibly via AMPK Phosphorylation in Skeletal Muscle In Vivo . J. Appl. Physiol. (1985) 101 (6), 1685–1692. 10.1152/japplphysiol.00255.2006 [DOI] [PubMed] [Google Scholar]
- Suzuki M., Kotake K., Fujikura K., Inagaki N., Suzuki T., Gonoi T., et al. (1997). Kir6.1: A Possible Subunit of ATP-Sensitive K+Channels in Mitochondria. Biochem. Biophysical Res. Commun. 241 (3), 693–697. 10.1006/bbrc.1997.7891 [DOI] [PubMed] [Google Scholar]
- Swerdlow R. H., Burns J. M., Khan S. M. (2010). The Alzheimer's Disease Mitochondrial Cascade Hypothesis. J. Alzheimers Dis. 20 (Suppl. 2), S265–S279. 10.3233/JAD-2010-100339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow R. H., Khan S. M. (2004). A "mitochondrial Cascade Hypothesis" for Sporadic Alzheimer's Disease. Med. Hypotheses 63 (1), 8–20. 10.1016/j.mehy.2003.12.045 [DOI] [PubMed] [Google Scholar]
- Szewczyk A., Wójcik G., Lobanov N. A., Nałecz M. J. (1997). The Mitochondrial Sulfonylurea Receptor: Identification and Characterization. Biochem. Biophysical Res. Commun. 230 (3), 611–615. 10.1006/bbrc.1996.6023 [DOI] [PubMed] [Google Scholar]
- Tabassum A., Bristow R. G., Venkateswaran V. (2010). Ingestion of Selenium and Other Antioxidants during Prostate Cancer Radiotherapy: a Good Thing? Cancer Treat. Rev. 36 (3), 230–234. 10.1016/j.ctrv.2009.12.008 [DOI] [PubMed] [Google Scholar]
- Takada S., Masaki Y., Kinugawa S., Matsumoto J., Furihata T., Mizushima W., et al. (2016). Dipeptidyl Peptidase-4 Inhibitor Improved Exercise Capacity and Mitochondrial Biogenesis in Mice with Heart Failure via Activation of Glucagon-like Peptide-1 Receptor Signalling. Cardiovasc Res. 111 (4), 338–347. 10.1093/cvr/cvw182 [DOI] [PubMed] [Google Scholar]
- Talbot K., Wang H.-Y., Kazi H., Han L.-Y., Bakshi K. P., Stucky A., et al. (2012). Demonstrated Brain Insulin Resistance in Alzheimer's Disease Patients Is Associated with IGF-1 Resistance, IRS-1 Dysregulation, and Cognitive Decline. J. Clin. Invest. 122 (4), 1316–1338. 10.1172/jci59903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampellini D., Gouras G. K. (2010). Synapses, Synaptic Activity and Intraneuronal Abeta in Alzheimer's Disease. Front. Aging Neurosci. 2. 10.3389/fnagi.2010.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G., Yang H., Chen J., Shi M., Ge L., Ge X., et al. (2017). Metformin Ameliorates Sepsis-Induced Brain Injury by Inhibiting Apoptosis, Oxidative Stress and Neuroinflammation via the PI3K/Akt Signaling Pathway. Oncotarget 8 (58), 97977–97989. 10.18632/oncotarget.20105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapryal N., Vivek G V., Mukhopadhyay C. K. (2015). Catecholamine Stress Hormones Regulate Cellular Iron Homeostasis by a Posttranscriptional Mechanism Mediated by Iron Regulatory Protein. J. Biol. Chem. 290 (12), 7634–7646. 10.1074/jbc.m114.592519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejero J., Shiva S., Gladwin M. T. (2019). Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiol. Rev. 99 (1), 311–379. 10.1152/physrev.00036.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto S., Suzuki M., Matsuse T., Ishii T., Fukuchi Y., Ouchi Y. (2000). Effects of Angiotensin-Converting Enzyme Inhibitors on Spontaneous or Stimulated Generation of Reactive Oxygen Species by Bronchoalveolar Lavage Cells Harvested from Patients with or without Chronic Obstructive Pulmonary Disease. Jpn. J. Pharmacol. 83 (1), 56–62. 10.1016/s0021-5198(19)30627-4 [DOI] [PubMed] [Google Scholar]
- Terasaki M., Yashima H., Mori Y., Saito T., Matsui T., Hiromura M., et al. (2020). A Dipeptidyl Peptidase-4 Inhibitor Inhibits Foam Cell Formation of Macrophages in Type 1 Diabetes via Suppression of CD36 and ACAT-1 Expression. Int. J. Mol. Sci. 21 (13). 10.3390/ijms21134811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton K. F. (2018). 90 Years of Monoamine Oxidase: Some Progress and Some Confusion. J. Neural Transm. 125 (11), 1519–1551. 10.1007/s00702-018-1881-5 [DOI] [PubMed] [Google Scholar]
- Toyoda S., Haruyama A., Inami S., Arikawa T., Saito F., Watanabe R., et al. (2020). Effects of Carvedilol vs Bisoprolol on Inflammation and Oxidative Stress in Patients with Chronic Heart Failure. J. Cardiol. 75 (2), 140–147. 10.1016/j.jjcc.2019.07.011 [DOI] [PubMed] [Google Scholar]
- Trachootham D., Alexandre J., Huang P. (2009). Targeting Cancer Cells by ROS-Mediated Mechanisms: a Radical Therapeutic Approach? Nat. Rev. Drug Discov. 8 (7), 579–591. 10.1038/nrd2803 [DOI] [PubMed] [Google Scholar]
- Tremblay A. J., Lamarche B., Deacon C. F., Weisnagel S. J., Couture P. (2014). Effects of Sitagliptin Therapy on Markers of Low-Grade Inflammation and Cell Adhesion Molecules in Patients with Type 2 Diabetes. Metabolism 63 (9), 1141–1148. 10.1016/j.metabol.2014.06.004 [DOI] [PubMed] [Google Scholar]
- Treuting P. M., Linford N. J., Knoblaugh S. E., Emond M. J., Morton J. F., Martin G. M., et al. (2008). Reduction of Age-Associated Pathology in Old Mice by Overexpression of Catalase in Mitochondria. Journals Gerontology Ser. A Biol. Sci. Med. Sci. 63 (8), 813–822. 10.1093/gerona/63.8.813 [DOI] [PubMed] [Google Scholar]
- Tse D. C. S., McCreery R. L., Adams R. N. (1976). Potential Oxidative Pathways of Brain Catecholamines. J. Med. Chem. 19 (1), 37–40. 10.1021/jm00223a008 [DOI] [PubMed] [Google Scholar]
- Tsoucalas G., Karamanou M., Androutsos G. (2011). Travelling through Time with Aspirin, a Healing Companion. Eur. J. Inflamm. 9 (1), 13–16. 10.1177/1721727x1100900102 [DOI] [Google Scholar]
- Ullah I., Ullah N., Naseer M. I., Lee H. Y., Kim M. O. (2012). Neuroprotection with Metformin and Thymoquinone against Ethanol-Induced Apoptotic Neurodegeneration in Prenatal Rat Cortical Neurons. BMC Neurosci. 13, 11. 10.1186/1471-2202-13-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursini F., Russo E., Pellino G., D’Angelo S., Chiaravalloti A., De Sarro G., et al. (2018). Metformin and Autoimmunity: A "New Deal" of an Old Drug. Front. Immunol. 9, 1236. 10.3389/fimmu.2018.01236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia W. M., Palacio A., Tamariz L., Florez H. (2017). Metformin and Ageing: Improving Ageing Outcomes beyond Glycaemic Control. Diabetologia 60 (9), 1630–1638. 10.1007/s00125-017-4349-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente E. M., Abou-Sleiman P. M., Caputo V., Muqit M. M. K., Harvey K., Gispert S., et al. (2004). Hereditary Early-Onset Parkinson's Disease Caused by Mutations in PINK1. Science 304 (5674), 1158–1160. 10.1126/science.1096284 [DOI] [PubMed] [Google Scholar]
- Vallon V., Thomson S. C. (2017). Targeting Renal Glucose Reabsorption to Treat Hyperglycaemia: the Pleiotropic Effects of SGLT2 Inhibition. Diabetologia 60 (2), 215–225. 10.1007/s00125-016-4157-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Acker H., Coenye T. (2017). The Role of Reactive Oxygen Species in Antibiotic-Mediated Killing of Bacteria. Trends Microbiol. 25 (6), 456–466. 10.1016/j.tim.2016.12.008 [DOI] [PubMed] [Google Scholar]
- Van Loenhout J., Peeters M., Bogaerts A., Smits E., Deben C. (2020). Oxidative Stress-Inducing Anticancer Therapies: Taking a Closer Look at Their Immunomodulating Effects. Antioxidants (Basel) 9 (12). 10.3390/antiox9121188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Meza H., de Piña M., Pardo J., Riveros-Rosas H., Villalobos-Molina R., Piña E. (2013). Non-steroidal Anti-inflammatory Drugs Activate NADPH Oxidase in Adipocytes and Raise the H2O2 Pool to Prevent cAMP-Stimulated Protein Kinase a Activation and Inhibit Lipolysis. BMC Biochem. 14, 13. 10.1186/1471-2091-14-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venderova K., Park D. S. (2012). Programmed Cell Death in Parkinson's Disease. Cold Spring Harb. Perspect. Med. 2 (8). 10.1101/cshperspect.a009365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrov A. E., Stevenson M. D., Alahari S., Pan H., Wickline S. A., Madamanchi N. R., et al. (2017). Attenuated Superoxide Dismutase 2 Activity Induces Atherosclerotic Plaque Instability during Aging in Hyperlipidemic Mice. J. Am. Heart Assoc. 6 (11). 10.1161/JAHA.117.006775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateshappa C., Harish G., Mythri R. B., Mahadevan A., Srinivas Bharath M. M., Shankar S. K. (2012). Increased Oxidative Damage and Decreased Antioxidant Function in Aging Human Substantia Nigra Compared to Striatum: Implications for Parkinson's Disease. Neurochem. Res. 37 (2), 358–369. 10.1007/s11064-011-0619-7 [DOI] [PubMed] [Google Scholar]
- Verdile G., Keane K. N., Cruzat V. F., Medic S., Sabale M., Rowles J., et al. (2015). Inflammation and Oxidative Stress: The Molecular Connectivity between Insulin Resistance, Obesity, and Alzheimer's Disease. Mediat. Inflamm. 2015, 105828. 10.1155/2015/105828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermot A., Petit-Härtlein I., Smith S. M. E., Fieschi F. (2021). NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants (Basel) 10 (6). 10.3390/antiox10060890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers S., Schiller H. J., Hildreth J. E. K., Bulkley G. B. (1998). Immunoaffinity Localization of the Enzyme Xanthine Oxidase on the outside Surface of the Endothelial Cell Plasma Membrane. Surgery 124 (3), 551–560. 10.1016/s0039-6060(98)70102-3 [DOI] [PubMed] [Google Scholar]
- Wahlqvist M. L., Lee M.-S., Hsu C.-C., Chuang S.-Y., Lee J.-T., Tsai H.-N. (2012). Metformin-inclusive Sulfonylurea Therapy Reduces the Risk of Parkinson's Disease Occurring with Type 2 Diabetes in a Taiwanese Population Cohort. Park. Relat. Disord. 18 (6), 753–758. 10.1016/j.parkreldis.2012.03.010 [DOI] [PubMed] [Google Scholar]
- Wallace T. M., Matthews D. R. (2004). Recent Advances in the Monitoring and Management of Diabetic Ketoacidosis. QJM 97 (12), 773–780. 10.1093/qjmed/hch132 [DOI] [PubMed] [Google Scholar]
- Walsh D. M., Selkoe D. J. (2007). A? Oligomers ? a Decade of Discovery. J. Neurochem. 101 (5), 1172–1184. 10.1111/j.1471-4159.2006.04426.x [DOI] [PubMed] [Google Scholar]
- Wang C., Chen B., Feng Q., Nie C., Li T. (2020). Clinical Perspectives and Concerns of Metformin as an Anti‐aging Drug. Aging Med. 3 (4), 266–275. 10.1002/agm2.12135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wang H. (2017). Oxidative Stress in Pancreatic Beta Cell Regeneration. Oxid. Med. Cell Longev. 2017, 1930261. 10.1155/2017/1930261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Xiong S., Xie C., Markesbery W. R., Lovell M. A. (2005). Increased Oxidative Damage in Nuclear and Mitochondrial DNA in Alzheimer's Disease. J. Neurochem. 93 (4), 953–962. 10.1111/j.1471-4159.2005.03053.x [DOI] [PubMed] [Google Scholar]
- Wang J., Yi J. (2008). Cancer Cell Killing via ROS: to Increase or Decrease, that Is the Question. Cancer Biol. Ther. 7 (12), 1875–1884. 10.4161/cbt.7.12.7067 [DOI] [PubMed] [Google Scholar]
- Wang P., Shi Q., Deng W. H., Yu J., Zuo T., Mei F. C., et al. (2015). Relationship between Expression of NADPH Oxidase 2 and Invasion and Prognosis of Human Gastric Cancer. Wjg 21 (20), 6271–6279. 10.3748/wjg.v21.i20.6271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Ye S. D., Sun W. J., Hu Y. Y. (2013). Pioglitazone Inhibits the Expression of Nicotinamide Adenine Dinucleotide Phosphate Oxidase and P38 Mitogen-Activated Protein Kinase in Rat Mesangial Cells. Chin. Med. J. Engl. 126 (21), 4054–4059. [PubMed] [Google Scholar]
- Wang X., Perry G., Smith M. A., Zhu X. (2010). Amyloid-beta-derived Diffusible Ligands Cause Impaired Axonal Transport of Mitochondria in Neurons. Neurodegener. Dis. 7 (1-3), 56–59. 10.1159/000283484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Su B., Fujioka H., Zhu X. (2008). Dynamin-like Protein 1 Reduction Underlies Mitochondrial Morphology and Distribution Abnormalities in Fibroblasts from Sporadic Alzheimer's Disease Patients. Am. J. Pathology 173 (2), 470–482. 10.2353/ajpath.2008.071208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wu Q., Liu A., Anadón A., Rodríguez J.-L., Martínez-Larrañaga M.-R., et al. (2017). Paracetamol: Overdose-Induced Oxidative Stress Toxicity, Metabolism, and Protective Effects of Various Compoundsin Vivo and In Vitro . Drug Metab. Rev. 49 (4), 395–437. 10.1080/03602532.2017.1354014 [DOI] [PubMed] [Google Scholar]
- Wang X., Zhao X., Malik M., Drlica K. (2010). Contribution of Reactive Oxygen Species to Pathways of Quinolone-Mediated Bacterial Cell Death. J. Antimicrob. Chemother. 65 (3), 520–524. 10.1093/jac/dkp486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. L., Frauwirth K. A., Rangwala S. M., Lazar M. A., Thompson C. B. (2002). Thiazolidinedione Activation of Peroxisome Proliferator-Activated Receptor γ Can Enhance Mitochondrial Potential and Promote Cell Survival. J. Biol. Chem. 277 (35), 31781–31788. 10.1074/jbc.m204279200 [DOI] [PubMed] [Google Scholar]
- Warwick C. (2008). Paracetamol and Fever Management. J. R. Soc. Promot. Health 128 (6), 320–323. 10.1177/1466424008092794 [DOI] [PubMed] [Google Scholar]
- Wasan H., Singh D., Kh R. (2021). Safinamide in Neurological Disorders and beyond: Evidence from Preclinical and Clinical Studies. Brain Res. Bull. 168, 165–177. 10.1016/j.brainresbull.2020.12.018 [DOI] [PubMed] [Google Scholar]
- Watanabe R., Nakamura H., Masutani H., Yodoi J. (2010). Anti-oxidative, Anti-cancer and Anti-inflammatory Actions by Thioredoxin 1 and Thioredoxin-Binding Protein-2. Pharmacol. Ther. 127 (3), 261–270. 10.1016/j.pharmthera.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Weiner D. B. (1979). The Apprenticeship of Philippe Pinel: a New Document, "observations of Citizen Pussin on the Insane". Am. J. Psychiatry 136 (9), 1128–1134. 10.1176/ajp.136.9.1128 [DOI] [PubMed] [Google Scholar]
- Weisbrot-Lefkowitz M., Reuhl K., Perry B., Chan P. H., Inouye M., Mirochnitchenko O. (1998). Overexpression of Human Glutathione Peroxidase Protects Transgenic Mice against Focal Cerebral Ischemia/reperfusion Damage. Brain Res. Mol. Brain Res. 53 (1-2), 333–338. 10.1016/s0169-328x(97)00313-6 [DOI] [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. (1973). Mitochondrial Superoxide Dismutase. J. Biol. Chem. 248 (13), 4793–4796. 10.1016/s0021-9258(19)43735-6 [DOI] [PubMed] [Google Scholar]
- West A. P., Shadel G. S., Ghosh S. (2011). Mitochondria in Innate Immune Responses. Nat. Rev. Immunol. 11 (6), 389–402. 10.1038/nri2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weylandt K.-H. (2016). Docosapentaenoic Acid Derived Metabolites and Mediators - the New World of Lipid Mediator Medicine in a Nutshell. Eur. J. Pharmacol. 785, 108–115. 10.1016/j.ejphar.2015.11.002 [DOI] [PubMed] [Google Scholar]
- Wigner P., Szymańska B., Bijak M., Sawicka E., Kowal P., Marchewka Z., et al. (2021). Oxidative Stress Parameters as Biomarkers of Bladder Cancer Development and Progression. Sci. Rep. 11 (1), 15134. 10.1038/s41598-021-94729-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding J. P., Woo V., Rohwedder K., Sugg J., Parikh S., Dapagliflozin D. S. G. (0062). Dapagliflozin in Patients with Type 2 Diabetes Receiving High Doses of Insulin: Efficacy and Safety over 2 Years. Diabetes Obes. Metab. 16 (2), 124–136. 10.1111/dom.12187 [DOI] [PubMed] [Google Scholar]
- Willette A. A., Modanlo N., Kapogiannis D., Alzheimer's Disease Neuroimaging I. (2015). Insulin Resistance Predicts Medial Temporal Hypermetabolism in Mild Cognitive Impairment Conversion to Alzheimer Disease. Diabetes 64 (6), 1933–1940. 10.2337/db14-1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood Z. A., Schröder E., Robin Harris J., Poole L. B. (2003). Structure, Mechanism and Regulation of Peroxiredoxins. Trends Biochem. Sci. 28 (1), 32–40. 10.1016/s0968-0004(02)00003-8 [DOI] [PubMed] [Google Scholar]
- Wu Y., Vulić M., Keren I., Lewis K. (2012). Role of Oxidative Stress in Persister Tolerance. Antimicrob. Agents Chemother. 56 (9), 4922–4926. 10.1128/aac.00921-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T. (2006). Inflammation in Alzheimer Disease: Driving Force, Bystander or Beneficial Response? Nat. Med. 12 (9), 1005–1015. 10.1038/nm1484 [DOI] [PubMed] [Google Scholar]
- Xiao Y., Xiong T., Meng X., Yu D., Xiao Z., Song L. (2019). Different Influences on Mitochondrial Function, Oxidative Stress and Cytotoxicity of Antibiotics on Primary Human Neuron and Cell Lines. J. Biochem. Mol. Toxicol. 33 (4), e22277. 10.1002/jbt.22277 [DOI] [PubMed] [Google Scholar]
- Xu P., Huecksteadt T. P., Hoidal J. R. (1996). Molecular Cloning and Characterization of the Human Xanthine Dehydrogenase Gene (XDH). Genomics 34 (2), 173–180. 10.1006/geno.1996.0262 [DOI] [PubMed] [Google Scholar]
- Yamada M., Iwatsubo T., Mizuno Y., Mochizuki H. (2004). Overexpression of Alpha-Synuclein in Rat Substantia Nigra Results in Loss of Dopaminergic Neurons, Phosphorylation of Alpha-Synuclein and Activation of Caspase-9: Resemblance to Pathogenetic Changes in Parkinson's Disease. J. Neurochem. 91 (2), 451–461. 10.1111/j.1471-4159.2004.02728.x [DOI] [PubMed] [Google Scholar]
- Yamagishi S.-i., Fukami K., Matsui T. (2015). Crosstalk between Advanced Glycation End Products (AGEs)-Receptor RAGE axis and Dipeptidyl Peptidase-4-Incretin System in Diabetic Vascular Complications. Cardiovasc Diabetol. 14, 2. 10.1186/s12933-015-0176-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancik R. (2005). Population Aging and Cancer. Cancer J. 11 (6), 437–441. 10.1097/00130404-200511000-00002 [DOI] [PubMed] [Google Scholar]
- Yang D., Elner S. G., Bian Z.-M., Till G. O., Petty H. R., Elner V. M. (2007). Pro-inflammatory Cytokines Increase Reactive Oxygen Species through Mitochondria and NADPH Oxidase in Cultured RPE Cells. Exp. Eye Res. 85 (4), 462–472. 10.1016/j.exer.2007.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Shi M., VanRemmen H., Chen X., Vijg J., Richardson A., et al. (2003). Reduction of Pressor Response to Vasoconstrictor Agents by Overexpression of Catalase in Mice. Am. J. Hypertens. 16 (1), 1–5. 10.1016/s0895-7061(02)03086-8 [DOI] [PubMed] [Google Scholar]
- Yang H., Zhou L., Wang Z., Roberts L. J., 2nd, Lin X., Zhao Y., et al. (2009). Overexpression of Antioxidant Enzymes in ApoE-Deficient Mice Suppresses Benzo(a)pyrene-Accelerated Atherosclerosis. Atherosclerosis 207 (1), 51–58. 10.1016/j.atherosclerosis.2009.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Wang H., Kouadir M., Song H., Shi F. (2019). Recent Advances in the Mechanisms of NLRP3 Inflammasome Activation and its Inhibitors. Cell Death Dis. 10 (2), 128. 10.1038/s41419-019-1413-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida J., Yamamoto K., Mano T., Sakata Y., Nishikawa N., Nishio M., et al. (2004). AT1 Receptor Blocker Added to ACE Inhibitor Provides Benefits at Advanced Stage of Hypertensive Diastolic Heart Failure. Hypertension 43 (3), 686–691. 10.1161/01.hyp.0000118017.02160.fa [DOI] [PubMed] [Google Scholar]
- Yoshida T., Maulik N., Engelman R. M., Ho Y. S., Magnenat J. L., Rousou J. A., et al. (1997). Glutathione Peroxidase Knockout Mice Are Susceptible to Myocardial Ischemia Reperfusion Injury. Circulation 96 (9 Suppl. l), II–21620. [PubMed] [Google Scholar]
- You X., Ma M., Hou G., Hu Y., Shi X. (2018). Gene Expression and Prognosis of NOX Family Members in Gastric Cancer. Ott 11, 3065–3074. 10.2147/ott.s161287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Sugawara T., Nishi T., Liu J., Chan P. H. (2006). Overexpression of SOD1 in Transgenic Rats Attenuates Nuclear Translocation of Endonuclease G and Apoptosis after Spinal Cord Injury. J. Neurotrauma 23 (5), 595–603. 10.1089/neu.2006.23.595 [DOI] [PubMed] [Google Scholar]
- Yu Y., Jiang H., Niu Y., Zhang X., Zhang Y., Liu X. I., et al. (2019). Candesartan Inhibits Inflammation through an Angiotensin II Type 1 Receptor Independent Way in Human Embryonic Kidney Epithelial Cells. Acad Bras Cienc 91 (2), e20180699. 10.1590/0001-3765201920180699 [DOI] [PubMed] [Google Scholar]
- Zahmatkesh M., Kadkhodaee M., Salarian A., Seifi B., Adeli S. (2017). Impact of Opioids on Oxidative Status and Related Signaling Pathways: An Integrated View. J Opioid Manag. 13 (4), 241–251. 10.5055/jom.2017.0392 [DOI] [PubMed] [Google Scholar]
- Zeller M., Steg P. G., Ravisy J., Lorgis L., Laurent Y., Sicard P., et al. (2008). Relation between Body Mass Index, Waist Circumference, and Death after Acute Myocardial Infarction. Circulation 118 (5), 482–490. 10.1161/circulationaha.107.753483 [DOI] [PubMed] [Google Scholar]
- Zeller P., Pletscher A., Gey K. F., Gutmann H., Hegedus B., Straub O. (1959). Amino Acid and Fatty Acid Hydrazides: Chemistry and Action on Monoamine Oxidase. Ann. N. Y. Acad. Sci. 80, 555–567. 10.1111/j.1749-6632.1959.tb49234.x [DOI] [PubMed] [Google Scholar]
- Zepeda R. J., Castillo R., Rodrigo R., Prieto J. C., Aramburu I., Brugere S., et al. (2012). Effect of Carvedilol and Nebivolol on Oxidative Stress-Related Parameters and Endothelial Function in Patients with Essential Hypertension. Basic Clin. Pharmacol. Toxicol. 111 (5), 309–316. 10.1111/j.1742-7843.2012.00911.x [DOI] [PubMed] [Google Scholar]
- Zhang B., Wang Y., Su Y. (2009). Peroxiredoxins, a Novel Target in Cancer Radiotherapy. Cancer Lett. 286 (2), 154–160. 10.1016/j.canlet.2009.04.043 [DOI] [PubMed] [Google Scholar]
- Zheng T., Gao Y., Baskota A., Chen T., Ran X., Tian H. (2014). Increased Plasma DPP4 Activity Is Predictive of Prediabetes and Type 2 Diabetes Onset in Chinese over a Four-Year Period: Result from the China National Diabetes and Metabolic Disorders Study. J. Clin. Endocrinol. Metab. 99 (11), E2330–E2334. 10.1210/jc.2014-1480 [DOI] [PubMed] [Google Scholar]
- Zhong Z., Zhai Y., Liang S., Mori Y., Han R., Sutterwala F. S., et al. (2013). TRPM2 Links Oxidative Stress to NLRP3 Inflammasome Activation. Nat. Commun. 4, 1611. 10.1038/ncomms2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., et al. (2001). Role of AMP-Activated Protein Kinase in Mechanism of Metformin Action. J. Clin. Invest. 108 (8), 1167–1174. 10.1172/jci13505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Yang J., Xin T., Li D., Guo J., Hu S., et al. (2014). Exendin-4 Protects Adipose-Derived Mesenchymal Stem Cells from Apoptosis Induced by Hydrogen Peroxide through the PI3K/Akt-Sfrp2 Pathways. Free Radic. Biol. Med. 77, 363–375. 10.1016/j.freeradbiomed.2014.09.033 [DOI] [PubMed] [Google Scholar]
- Zhou R., Yazdi A. S., Menu P., Tschopp J. (2011). A Role for Mitochondria in NLRP3 Inflammasome Activation. Nature 469 (7329), 221–225. 10.1038/nature09663 [DOI] [PubMed] [Google Scholar]
- Zhou Z., Tang Y., Jin X., Chen C., Lu Y., Liu L., et al. (2016). Metformin Inhibits Advanced Glycation End Products-Induced Inflammatory Response in Murine Macrophages Partly through AMPK Activation and RAGE/NFκB Pathway Suppression. J. Diabetes Res. 2016, 4847812. 10.1155/2016/4847812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z. D., Lan Y. H., Tan E. K., Lim T. M. (2010). Iron Species-Mediated Dopamine Oxidation, Proteasome Inhibition, and Dopaminergic Cell Demise: Implications for Iron-Related Dopaminergic Neuron Degeneration. Free Radic. Biol. Med. 49 (12), 1856–1871. 10.1016/j.freeradbiomed.2010.09.010 [DOI] [PubMed] [Google Scholar]
- Zondler L., Miller-Fleming L., Repici M., Gonçalves S., Tenreiro S., Rosado-Ramos R., et al. (2014). DJ-1 Interactions with α-synuclein Attenuate Aggregation and Cellular Toxicity in Models of Parkinson's Disease. Cell Death Dis. 5, e1350. 10.1038/cddis.2014.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong W.-X., Rabinowitz J. D., White E. (2016). Mitochondria and Cancer. Mol. Cell 61 (5), 667–676. 10.1016/j.molcel.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorov D. B., Juhaszova M., Sollott S. J. (2014). Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 94 (3), 909–950. 10.1152/physrev.00026.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]


