Abstract
Discovering compounds that promote health during aging (“geroprotectors”) is key to the retardation of age-related pathologies and the prevention of chronic age-related diseases. In in-silico and model organisms’ lifespan screens, chondroitin sulfate has emerged as a geroprotective compound. Chondroitin sulfate is a glycosaminoglycan attached to extracellular matrix proteins and is naturally produced by our body. Oral supplementation of chondroitin sulfate shows a high tolerance in humans, preferable pharmacokinetics, a positive correlation with healthy human longevity, and efficacy in deceleration of age-related diseases in randomized clinical trials. We have recently shown that chondroitin sulfate supplementation increases the lifespan of C. elegans. Thus, chondroitin sulfate holds the potential to become a geroprotective strategy to promote health during human aging. This review discusses the two major potential mechanisms of action, extracellular matrix homeostasis and inhibition of inflammation, that counteract age-related pathologies upon chondroitin sulfate supplementation.
Keywords: chondroitin sulfate, supplement, healthy aging, longevity, anti inflammatory, extracellar matrix, drug discovery, matreotype
Introduction
Predicted Longevity Drug-Protein Targets Reveal Chondroitin
How to identify compounds that retard age-related pathologies and promote health during aging? There are many approaches to identify geroprotective compounds, spanning from in-silico and cell-based drug screening to direct lifespan assays in model organisms (Barardo et al., 2017; Bakula et al., 2018; Janssens et al., 2019; Kusumoto et al., 2021; Statzer et al., 2021). One of the largest screens assessed 1,300 compounds on about 20,000 mice performing full lifespans and yielded five longevity-promoting compounds (WO2018075641A1 and US 20200254006 A1). Using C. elegans, more than 100,000 compounds have been screened collectively across multiple studies, and about 100 compounds have been identified that increase C. elegans lifespan (Petrascheck et al., 2007; Lucanic et al., 2013; Ye et al., 2014; Kim and Lee, 2019; Statzer et al., 2021). Pathway analysis of these discovered longevity compounds showed enrichment for TGFβ pathway, chondroitin, and heparan sulfate biogenesis as potential drug-protein targets (Liu et al., 2016). In our drug screens, we also identified chondroitin sulfate (Statzer et al., 2021). Chondroitin sulfate is a naturally occurring sulfated glycosaminoglycan usually attached to extracellular matrix proteins (Figure 1). Thereby, it is abundantly found in connective tissues, such as cartilage that cushions the ends of the bones within the joints, skin, blood vessels, ligaments, and tendons, but also in other organs, such as the brain (Jerosch, 2011; Kwok et al., 2012). Chondroitin sulfate is a popular and widely used supplement that is well-tolerated, with no adverse effects above placebo, and is likely very safe, allowing long-term treatment (Hathcock and Shao, 2007). Thus, making chondroitin sulfate an ideal candidate to develop further into a geroprotective compound.
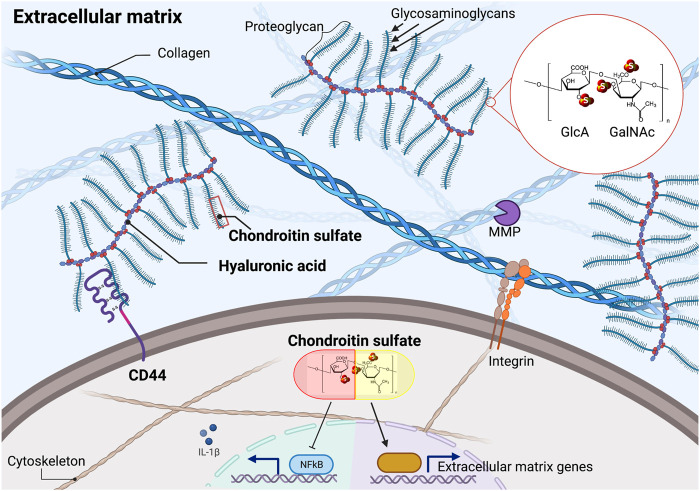
FIGURE 1.
Chondroitin sulfate in the extracellular matrix and cellular effects of supplementation. Chondroitin sulfate consists of repeating units of D-glucuronic acid (GlcA) and N-acetyl galactosamine (GalNAc). Naturally occurring chondroitin sulfate only shows one of the three sulfates at the indicated (S) positions, resulting in distinct isomers. Chondroitin sulfate polymers are the building blocks of proteoglycans that can be attached to the hyaluronic acid polymers. Supplementation of chondroitin sulfate blocks NF-ƙB mediated inflammation and stimulates extracellular matrix homeostasis. MMP = matrix metalloproteinase. Illustration not drawn to scale.
In-vivo Drug Screen C. Elegans Identifies Glycosaminoglycans to Extend Lifespan
While examining previously established longevity compounds, such as metformin and rapamycin, we noticed a robust extracellular matrix transcriptional signature as a cellular response to the drug (Statzer et al., 2021). Moreover, in 41 out of 47 known longevity compounds (i.e., almost 90%) we examined, extracellular matrix gene expression was significantly altered (Statzer et al., 2021), suggesting remodeling of the extracellular matrix elicited by these drugs (Ewald, 2020). Therefore, in contrast to previous drug screens, we centered our in-silico analysis around the extracellular matrix (Ewald, 2020). We analyzed the human Genotype-Tissue Expression (GTEx) transcriptomic data combined with eight different expression profile datasets to define the aging matreotype across tissues (Statzer et al., 2021). The aging matreotype is a list of 99 extracellular matrix genes that either decline or increase in expression during human aging (Statzer et al., 2021). Then we probed the cMap expression profiles of about 1,300 drugs for a “youthful” matreotype signature and identified 185 compounds, of which 24 have previously been shown to increase lifespan in model organisms (Statzer et al., 2021).
A challenge in validating compounds for healthy aging is to identify the beneficial drug dose. We developed a novel in-vivo surrogate marker for longevity, using collagen homeostasis as a read-out in C. elegans (Statzer et al., 2021). With this reporter, we examined the unexplored area of proteoglycans and glycosaminoglycans as they were identified as major drug targets across many geroprotective drugs (Liu et al., 2016). These glycosaminoglycans, such as hyaluronic acid, chondroitin sulfate, and glucosamine, are major components of extracellular matrix proteins, found in cartilage and synovial fluid, and are naturally produced by the body or can be supplemented by diet (Jerosch, 2011). We found that supplementing hyaluronic acid and chondroitin sulfate increased C. elegans lifespan by 25–35% and 23–28%, respectively (Statzer et al., 2021). Previous work by the Ristow lab had shown that supplementation with glucosamine increased mouse lifespan and also C. elegans lifespan by 8–12% (Weimer et al., 2014). Thus, oral uptake of these glycosaminoglycans promotes healthy aging and longevity in model organisms.
Biosynthesis of Chondroitin and Relationship to Glucosamine and Hyaluronic Acid
Glucosamine is a precursor and the rate-limiting step in the synthesis of chondroitin polymers, which are the building blocks of the side chains of several proteoglycans (Figure 1) (McCarty et al., 2000; Mikami and Kitagawa, 2013). In particular, chondroitin consists of long polysaccharides of 20–200 repeating units of N-acetyl galactosamine (GalNAc) and d-glucuronic acid (GlcA), which can be sulfated at three different positions resulting in distinct isomers (Figure 1) (Mikami and Kitagawa, 2013). Chondroitin sulfate is usually of 10–50 kDa molecular weight and is extracted from cartilaginous tissues from pigs, cows, birds, and sharks (Jerosch, 2011). Glucosamine and chondroitin sulfate may facilitate hyaluronic acid production (McCarty et al., 2000).
Following oral uptake, glucosamine, chondroitin sulfate, and hyaluronic acid get transported to the target tissue in animal studies; molecules are safe and show high-quality evidence for their effectiveness in randomized clinical trials (Balogh et al., 2008; Bruyère et al., 2008; Jerosch, 2011; Garantziotis and Savani, 2019). However, hyaluronic acid is a huge polymer that is poorly uptaken by the body and showed variable effects in randomized clinical trials (Kalman et al., 2008). Furthermore, hyaluronic acid needs to be broken down by hyaluronidase TMEM2 to protect against protein misfolded endoplasmic reticulum stress in human fibroblasts and promote C. elegans longevity (Schinzel et al., 2019). Glucosamine is a smaller monomer but impairs glucose metabolism and increases lifespan in part through glucose restriction and mitochondrial reactive oxygen species-induced hormesis (Weimer et al., 2014). On the other hand, chondroitin sulfate is a slow-acting drug (Bruyère et al., 2008) that has supportive evidence to modify cartilage structure in randomized clinical trials and is repeatedly recommended for the last 20 years by the European League Against Rheumatism (EULAR) (Jordan et al., 2003; Kloppenburg et al., 2018). Furthermore, the combination of glucosamine with chondroitin sulfate showed synergistic and additive effects for osteoarthritis in vitro, in vivo, and in randomized clinical trials (Clegg et al., 2006; Jerosch, 2011), suggesting distinct and overlapping modes-of-action. Hence, I focus here on the potential underlying mode-of-actions and molecular mechanisms promoted by supplementation with chondroitin sulfate.
Potential Mechanisms Promoted by Chondroitin Sulfate
Mechanism 1: Evidence of Chondroitin Sulfate Stimulating Extracellular Matrix Protein Homeostasis
During aging, the balance of extracellular matrix biosynthesis and degradation becomes dysregulated (Ewald, 2020). Genetic alterations of extracellular matrix genes cause diverse phenotypes and diseases (Huxley-Jones et al., 2008; Statzer and Ewald, 2020). While many chronic age-dependent diseases show increased inflammation and fibrotic collagen deposition (Wick et al., 2013; Bonnans et al., 2014; Teuscher et al., 2019; Haefke and Ewald, 2020; Karsdal et al., 2020), a general signature of extracellular matrix aging is the progressive decline in collagen biosynthesis and an increase of extracellular protease activity across species (Ewald, 2020). For instance, in human skin, collagen, elastic fibers, laminin, and integrin levels progressively decline during aging (Shuster et al., 1975; Bosset et al., 2003; Farage et al., 2008; Sugawara et al., 2008; Giangreco et al., 2009; Kanaki et al., 2016). The general decline of collagen biosynthesis might be driven by the senescence of fibroblasts and the loss of stem cell maintenance (Varani et al., 2006). Counteracting this age-dependent decline of collagen synthesis by prolonging collagen expression is sufficient to increase the lifespan of C. elegans (Ewald et al., 2015), suggesting that extracellular protein homeostasis is a novel yet unexplored mechanism to promote health during aging (Ewald, 2020).
Since domains of extracellular matrix proteins and remodeling enzymes are well conserved across species, particularly the active domains where drug target sites are preferentially located, the dynamic process of extracellular matrix remodeling has emerged as an attractive drug target (Huxley-Jones et al., 2008). In-silico analysis predicting chondroitin sulfate drug targets revealed mostly components for biosynthesis and degradation of chondroitins, such as chondroitinase (GALNS), sulfotransferase (CHST11), chondroitin/hyaluronic acid receptor (CD44), hyaluronidase (HYAL1, 2), and enzymes that remodel the extracellular matrix, such as matrix metalloproteinases (MMP1, 3, 16, 24) (Lila et al., 2018). In mice, deletion of chondroitin 6-sulfotransferase (CHST3) results in an abnormal extracellular matrix in the brain, accelerated brain aging, and memory impairments (Yang et al., 2021). Overexpression of CHST3 improved memory in old mice, suggesting the importance of maintaining proper chondroitin sulfate levels to promote neuroplasticity during aging (Yang et al., 2021). Furthermore, endogenous chondroitin sulfate is essential for maintaining embryonic stem cell pluripotency via binding to E-cadherin cell adhesion and RhoA and ERK1/2 downstream signaling (Izumikawa et al., 2014). Hence, endogenous chondroitin sulfate metabolism is linked to extracellular protein homeostasis.
In rats, endogenous chondroitin sulfate levels decline with age, probably due to the loss of chondrocytes (Honda et al., 1979). Interestingly, supplementing chondroitin sulfate increased chondrocyte cell proliferation in a dose-dependent manner (Jerosch, 2011; López-Senra et al., 2020). Moreover, supplementing C. elegans with chondroitin sulfate retarded the progressive decline of collagen renewal and increased lifespan (Statzer et al., 2021). In proteomics analysis of osteoarthritis patient-derived chondrocytes, ex-vivo chondroitin sulfate supplementation mainly resulted in remodeling of extracellular proteins and some inflammatory-associated proteins (Calamia et al., 2012a). This is consistent with many animal and human studies showing that chondroitin sulfate supplementation inhibits cartilage destruction and stimulates proteoglycan production for bolstering the connective tissues (Bassleer et al., 1998; Omata et al., 2000; Tahiri et al., 2007; Huskisson, 2008; Taniguchi et al., 2011; Martel-Pelletier et al., 2015; Sukhikh et al., 2020). Taken together, supplementing with chondroitin sulfate tips the balance towards prolonged extracellular matrix protein homeostasis, a requisite for healthy aging (Figure 1).
Mechanism 2: Evidence of Chondroitin Sulfate in Suppressing Inflammation
Endogenous chondroitin sulfate is essential to keep inflammation in check. A spontaneously-arisen deleterious mutation in the chondroitin sulfate synthase 1 (Chsy1) resulted in lower chondroitin sulfate levels leading to chronic inflammation and shortening of mouse lifespan (Macke et al., 2020). On the other hand, chondroitin sulfate supplementation reduces chronic inflammation. For instance, in a randomized, double-blind, placebo-controlled, clinical trial on 18 placebo and 18 glucosamine and chondroitin supplemented healthy adults, chondroitin with glucosamine significantly lowered serum inflammation biomarker C-reactive Protein and substantially remodeled the extracellular matrix detected by the blood plasma proteomic arrays (Navarro et al., 2015). Similarly, chondroitin sulfate intake was associated with a reduction in C-reactive Protein concentration in the blood (Kantor et al., 2020), suggesting a decrease in inflammation.
Induction of an inflammatory response by stimulating chondrocytes with interleukin IL-1β or lipopolysaccharides (LPS) increases protein levels of inflammatory-associated proteins, such as complement components, and also matrix metalloproteinases that degrade extracellular matrices. This increase is attenuated by chondroitin supplementation (Chan et al., 2005; Monfort et al., 2005; Tahiri et al., 2007; Legendre et al., 2008; Campo et al., 2009; Calamia et al., 2010; Imada et al., 2010; Calamia et al., 2012b), supporting a molecular role of chondroitin sulfate in blocking inflammation and extracellular matrix degradation (Figure 1). Similarly, in mice arthritis models, chondroitin sulfate slowed cartilage destruction and partially blocked inflammation (Omata et al., 2000). Mechanistically, chondroitin sulfate inhibits translocation of NF-ƙB, thereby decreasing NF-ƙB downstream signaling resulting in lower levels of pro-inflammatory cytokines and enzymes, such as IL-1β, IL-6, TNF-⍺, Cox-2, and Nos-2 (Jomphe et al., 2007; Campo et al., 2008; Xu et al., 2008; Souich et al., 2009; Vallières and Souich, 2010) (Figure 1).
Interestingly, across species, including humans, NF-ƙB increases in several tissues during aging (Tilstra et al., 2011). Genetic inhibitions of NF-ƙB delay several age-related pathologies in mice (Tilstra et al., 2011). Furthermore, in the hypothalamus of old mice, NF-ƙB activation decreases lifespan, whereas NF-ƙB inhibition increases lifespan (Zhang et al., 2013). Pharmacological inhibition of NF-ƙB also increases the lifespan of Drosophila (Moskalev and Shaposhnikov, 2011). This suggests that some beneficial effects of chondroitin sulfate supplementation might be mediated through inhibiting NF-ƙB age-related chronic inflammation resulting in improved health and longevity.
Taken together, supplementation of chondroitin sulfate increases lifespan via improving extracellular matrix homeostasis and via inhibiting chronic inflammation. Chronic inflammation can lead to upregulation of matrix metalloproteinase and extracellular matrix fragmentation and degradation as seen in osteoarthritis, suggesting a mechanistic link between disease progression and aging. However, chondroitin sulfate supplementation can increase the lifespan of model organisms via both mechanisms independently: i.e., extracellular matrix remodeling or inhibiting chronic inflammation, suggesting distinct but also overlapping modes of action.
Evidence of Chondroitin Sulfate Promoting Healthy Aging in Humans
Uptake of Chondroitin Sulfate
Is orally supplemented chondroitin sulfate being absorbed by the body, and does it reach the target tissue? In rats and dogs, 70% of the orally administered radioactive-labeled chondroitin sulfate was found within 2 hours in the bloodstream and showed the highest concentrations in the intestine, liver, kidneys, synovial fluid, and cartilage after 24 h (Conte et al., 1995). In humans, about 20 μg/ml endogenously produced chondroitin sulfate is found in the blood circulation, and this level is constantly maintained without any effects of circadian rhythm (Jackson et al., 2009). Supplementing chondroitin sulfate with a single dose of 1,200 mg, as clinically used, did not reach a significant increase above endogenous chondroitin sulfate levels in the bloodstream within 2–4 h (Jackson et al., 2009), but a single dose of 4,000 mg of chondroitin sulfate doubled the chondroitin sulfate levels in the blood plasma within 2–4 h (Volpi, 2002). By contrast, other studies have reported repeated application of exogenous chondroitin sulfate peaks during 2–8 h upon intravenous, intramuscular, or oral routes (Conte et al., 1991a; Ronca and Conte, 1993; Conte et al., 1995; Volpi, 2003). Furthermore, daily doses of 800–1,200 mg of orally taken chondroitin sulfate significantly increased chondroitin plasma concentration within 24 h (Ronca and Conte, 1993; Conrozier, 1998). Of the orally taken chondroitin sulfate, about 30% of chondroitin sulfate (full-length and degraded) is excreted by the urine, whereas about 10% of the full-length and 20% of the degraded lower-molecular weight chondroitin sulfate is absorbed by the body (Conte et al., 1991b; Ronca and Conte, 1993; Ronca et al., 1998). Intact full-length chondroitin sulfate is taken up by cells via pinocytosis (McCarty et al., 2000). Orally taken chondroitin sulfate is absorbed in the proximal part of the small intestine (Souich, 2014). Probably most of the chondroitin sulfate is degraded in the colon and the cecum (Souich, 2014). After the partial excretion in the urine, chondroitin sulfate is mainly retained by the kidney and the liver (Pecly et al., 2006). However, accumulation of orally administered chondroitin sulfate in the joint tissue has been detected (Souich, 2014). Summing up, orally supplemented chondroitin sulfate is taken up by the body and reaches target tissues.
Chondroitin Sulfate Impact on Age-Related Diseases
Endogenous chondroitin sulfate in the extracellular space affects growth factor and matrix metalloprotease reservoir storage, as well as their presentation and release. Chondroitin sulfate binds directly to cell surface receptors, such as L- and P-selectins and CD44 (Figure 1), and thereby modulates malignant transformation, metastasis, and tumor cell migration (Afratis et al., 2012). The use of chondroitin sulfate and glucosamine is also associated with a lower risk for various cancers, especially colorectal cancer, lung cancer, and adenocarcinomas (Satia et al., 2009; Kantor et al., 2016; Rani et al., 2018). Supplementation of chondroitin sulfate and glucosamine beneficially altered the gut microbiome (Navarro et al., 2019), suggesting a possible mode-of-action via improving microbiome composition to lower colorectal cancer incidences.
One of the most studied age-related diseases with regards to chondroitin sulfate supplementation is osteoarthritis, although it is still controversial how effective chondroitin sulfate is in slowing the disease progression. Osteoarthritis is a major health complication affecting >237 million middle-aged people worldwide (GBD 2015 Disease and Injury Incidence and Prevalence Collaborators, 2016). Osteoarthritis is the wear down of weight-bearing joint cartilage, such as knees, hips, and vertebrae. During aging, the self-repair of the extracellular matrix of joints declines resulting in insufficient repair upon mechanical stress and damage leading to cartilage degeneration, stiffness, pain, and chronic inflammation (Jerosch, 2011).
The consensus of many randomized clinical trials concluded that chondroitin sulfate supplementation showed a moderate effect on pain relief, larger efficacy on slowing the age-dependent shrinkage of the knee joint space, and improved knee function but also some considerable inconsistencies were observed across trials (Leeb et al., 2000; Jordan et al., 2003; Richy et al., 2003; Uebelhart et al., 2004; Michel et al., 2005; Clegg et al., 2006; Hathcock and Shao, 2007; Bruyère et al., 2008; Hochberg et al., 2008; Huskisson, 2008; Uebelhart, 2008; Kahan et al., 2009; Lee et al., 2009; Wildi et al., 2011; Zegels et al., 2013; Lomonte et al., 2018). A more detailed comparison is covered by these systematic reviews and meta-analyses (Wandel et al., 2010; Zhu et al., 2018; Honvo et al., 2019a; Fernández-Martín et al., 2021).
One contribution to the variable outcomes might be the manufacturing grade quality of chondroitin sulfate, which in some clinical trials, non-pharmaceutical grade chondroitin sulfate was included (Henrotin et al., 2014; Martel-Pelletier et al., 2015; Bruyère et al., 2017; Reginster and Veronese, 2021). Many tested food supplements had lower chondroitin sulfate concentration as indicated on their label and were less potent than pharmaceutical-grade chondroitin sulfate to inhibit inflammation markers in vitro (Stellavato et al., 2019). For instance, highly purified chondroitin sulfate administration reduced hand pain in a single-center, randomized, double-blind, placebo-controlled clinical trial (Gabay et al., 2011). Thus, it is imperative to use pharmaceutical-grade chondroitin sulfate for treatment (Martel-Pelletier et al., 2015; Honvo et al., 2019b; Reginster and Veronese, 2021). Another variable might be the time of application during the disease progression. For instance, for osteoarthritis, the sooner chondroitin sulfate was applied after diagnosis (i.e., earlier stages of the disease), the higher was the chance of success and beneficial response (Bruyère et al., 2020). Taken together, pharmaceutical-grade chondroitin sulfate might alleviate and slow age-related disease progression.
Chondroitin Sulfate Use is Associated With Human Longevity
The question becomes whether chondroitin sulfate might be applicable as a preventative and geroprotective strategy in humans. There are three large cohort studies that showed a reduction in all-cause mortality of chondroitin sulfate users.
The first two studies examined the VITAL (Vitamins and Lifestyle) prospective cohort, which included both men and women aged 50–76. 77,718 people were examined for their use of vitamins, minerals, and other supplements in relation to mortality. These studies revealed that after 5 and 6.8 years of follow-up that the multivariate-adjusted hazard ratio of chondroitin sulfate users (>4 days/week for >3 years) were 0.83 and 0.86 compared to non-users, suggesting a 17 and 14% significant decrease in risk of total mortality, respectively (Pocobelli et al., 2010; Bell et al., 2012).
The third study examined 16,686 people of the US NHANES cohort. This study found that after an 8.9-years follow-up, the multivariate-adjusted hazard ratio of a combinatory use of chondroitin sulfate with glucosamine was 0.73 for all-cause mortality and 0.42 for cardiovascular mortality compared to non-users, suggesting a 27% and a 58% lower likelihood of overall and cardiovascular mortality (King and Xiang, 2020). Thus, these longitudinal studies link chondroitin sulfate supplementation to human longevity.
Perspectives
Chondroitin sulfate supplementation is associated with reducing all-cause mortality in humans and increasing the lifespan of model organisms. But many gaps remain in our understanding of how chondroitin sulfate supplementation improves health during aging. Possible mechanisms include the reduction of chronic age-related inflammation and enhancement of extracellular matrix homeostasis. However, we need to define the missing steps in linking these two and possibly other mechanisms of chondroitin sulfate action to improve healthy aging. Such studies will provide new insights and help the development of therapeutic approaches that need to be tested in controlled clinical trials of chondroitin sulfate supplementation in age-matched individuals. Perhaps individualized assessment of chondroitin sulfate deficits might allow a personalized medical approach of chondroitin sulfate supplementation with other geroprotective drugs.
Acknowledgments
I thank Evelyne Bischof and the Ewald lab for critical comments on the manuscript. Figure 1 was created with Biorender (license number CT22PNT7QK). Chondroitin sulfate chemical structure backbone adapted from wikipedia.org.
Author Contributions
CE performed literature searches and wrote the manuscript.
Funding
Funding from the Swiss National Science Foundation PP00P3_190072 to CE.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Afratis N., Gialeli C., Nikitovic D., Tsegenidis T., Karousou E., Theocharis A. D., et al. (2012). Glycosaminoglycans: Key Players in Cancer Cell Biology and Treatment: GAG Targeting in Cancer Cell Biology. Febs J. 279, 1177–1197. 10.1111/j.1742-4658.2012.08529.x [DOI] [PubMed] [Google Scholar]
- Bakula D., Aliper A. M., Mamoshina P., Petr M. A., Teklu A., Baur J. A., et al. (2018). Aging and Drug Discovery. Aging 10, 3079–3088. 10.18632/aging.101646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh L., Polyak A., Mathe D., Kiraly R., Thuroczy J., Terez M., et al. (2008). Absorption, Uptake and Tissue Affinity of High-Molecular-Weight Hyaluronan after Oral Administration in Rats and Dogs. J. Agr Food Chem. 56, 10582–10593. 10.1021/jf8017029 [DOI] [PubMed] [Google Scholar]
- Barardo D., Thornton D., Thoppil H., Walsh M., Sharifi S., Ferreira S., et al. (2017). The DrugAge Database of Aging-Related Drugs. Aging cell 16, 594–597. 10.1111/acel.12585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassleer C. T., Combal J. P., Bougaret S., Malaise M. (1998). Effects of Chondroitin Sulfate and Interleukin-1 Beta on Human Articular Chondrocytes Cultivated in Clusters. Osteoarthr Cartil Oars Osteoarthr Res. Soc. 6, 196–204. 10.1053/joca.1998.0112 [DOI] [PubMed] [Google Scholar]
- Bell G. A., Kantor E. D., Lampe J. W., Shen D. D., White E. (2012). Use of Glucosamine and Chondroitin in Relation to Mortality. Eur. J. Epidemiol. 27, 593–603. 10.1007/s10654-012-9714-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnans C., Chou J., Werb Z. (2014). Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cel Biol. 15, 786–801. 10.1038/nrm3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosset S., Bonnet-Duquennoy M., Barre P., Chalon A., Lazou K., Kurfurst R., et al. (2003). Decreased Expression of Keratinocyte Beta1 Integrins in Chronically Sun-Exposed Skin In Vivo . Br. J Dermatol 148, 770–778. 10.1046/j.1365-2133.2003.05159.x [DOI] [PubMed] [Google Scholar]
- Bruyère O., Burlet N., Delmas P. D., Rizzoli R., Cooper C., Reginster J.-Y. (2008). Evaluation of Symptomatic Slow-Acting Drugs in Osteoarthritis Using the GRADE System. Bmc Musculoskelet. Di 9, 165. 10.1186/1471-2474-9-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyère O., Cooper C., Al-Daghri N. M., Dennison E. M., Rizzoli R., Reginster J.-Y. (2017). Inappropriate Claims from Non-Equivalent Medications in Osteoarthritis: A Position Paper Endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Aging Clin. Exp. Res. 30, 111–117. 10.1007/s40520-017-0861-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyère O., Dardenne N., Donneau A.-F., Reginster J.-Y. (2020). Responder Profile to Pharmaceutical-Grade Chondroitin Sulfate: An Analysis of the CONCEPT Trial. Adv. Ther. 37, 4641–4648. 10.1007/s12325-020-01484-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamia V., Fernández-Puente P., Mateos J., Lourido L., Rocha B., Montell E., et al. (2012a). Pharmacoproteomic Study of Three Different Chondroitin Sulfate Compounds on Intracellular and Extracellular Human Chondrocyte Proteomes. Mol. Cel Proteomics 11, M111.013417. 10.1074/mcp.m111.013417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamia V., Lourido L., Fernández-Puente P., Mateos J., Rocha B., Montell E., et al. (2012b). Secretome Analysis of Chondroitin Sulfate-Treated Chondrocytes Reveals Anti-Angiogenic, Anti-Inflammatory and Anti-Catabolic Properties. Arthritis Res. Ther. 14, R202. 10.1186/ar4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamia V., Ruiz-Romero C., Rocha B., Fernández-Puente P., Mateos J., Montell E., et al. (2010). Pharmacoproteomic Study of the Effects of Chondroitin and Glucosamine Sulfate on Human Articular Chondrocytes. Arthritis Res. Ther. 12, R138. 10.1186/ar3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo G. M., Avenoso A., Campo S., D’Ascola A., Traina P., Calatroni A. (2008). Chondroitin-4-Sulphate Inhibits NF-kB Translocation and Caspase Activation in Collagen-Induced Arthritis in Mice. Osteoarthr Cartilage 16, 1474–1483. 10.1016/j.joca.2008.04.002 [DOI] [PubMed] [Google Scholar]
- Campo G. M., Avenoso A., Campo S., D’Ascola A., Traina P., Samà D., et al. (2009). Glycosaminoglycans Modulate Inflammation and Apoptosis in LPS-Treated Chondrocytes. J. Cel Biochem 106, 83–92. 10.1002/jcb.21981 [DOI] [PubMed] [Google Scholar]
- Chan P.-S., Caron J. P., Orth M. W. (2005). Effect of Glucosamine and Chondroitin Sulfate on Regulation of Gene Expression of Proteolytic Enzymes and Their Inhibitors in Interleukin-1-Challenged Bovine Articular Cartilage Explants. Am. J. Vet. Res. 66, 1870–1876. 10.2460/ajvr.2005.66.1870 [DOI] [PubMed] [Google Scholar]
- Clegg D. O., Reda D. J., Harris C. L., Klein M. A., O’Dell J. R., Hooper M. M., et al. (2006). Glucosamine, Chondroitin Sulfate, and the Two in Combination for Painful Knee Osteoarthritis. New Engl. J. Med. 354, 795–808. 10.1056/nejmoa052771 [DOI] [PubMed] [Google Scholar]
- Conrozier T. (1998). Anti-Arthrosis Treatments: Efficacy and Tolerance of Chondroitin Sulfates (CS 4&6). Press. Méd. 27, 1862–1865. [PubMed] [Google Scholar]
- Conte A., Bernardi M. D., Palmieri L., Lualdi P., Mautone G., Ronca G. (1991a). Metabolic Fate of Exogenous Chondroitin Sulfate in Man. Arznei-forschung 41, 768–772. [PubMed] [Google Scholar]
- Conte A., Palmieri L., Segnini D., Ronca G. (1991b). Metabolic Fate of Partially Depolymerized Chondroitin Sulfate Administered to the Rat. Drug Exp. Clin. Res. 17, 27–33. [PubMed] [Google Scholar]
- Conte A., Volpi N., Palmieri L., Bahous I., Ronca G. (1995). Biochemical and Pharmacokinetic Aspects of Oral Treatment with Chondroitin Sulfate. Arznei-forschung 45, 918–925. [PubMed] [Google Scholar]
- Ewald C. Y., Landis J. N., Abate J. P., Murphy C. T., Blackwell T. K. (2015). Dauer-Independent insulin/IGF-1-Signalling Implicates Collagen Remodelling in Longevity. Nature 519, 97–101. 10.1038/nature14021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald C. Y. (2020). The Matrisome during Aging and Longevity: A Systems-Level Approach toward Defining Matreotypes Promoting Healthy Aging. Gerontology 66, 266–274. 10.1159/000504295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farage M. A., Miller K. W., Elsner P., Maibach H. I. (2008). Functional and Physiological Characteristics of the Aging Skin. Aging Clin. Exp. Res. 20, 195–200. 10.1007/bf03324769 [DOI] [PubMed] [Google Scholar]
- Fernández-Martín S., González-Cantalapiedra A., Muñoz F., García-González M., Permuy M., López-Peña M. (2021). Glucosamine and Chondroitin Sulfate: Is There Any Scientific Evidence for Their Effectiveness as Disease-Modifying Drugs in Knee Osteoarthritis Preclinical Studies?—A Systematic Review from 2000 to 2021. Animals 11, 1608. 10.3390/ani11061608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C., Medinger-Sadowski C., Gascon D., Kolo F., Finckh A. (2011). Symptomatic Effects of Chondroitin 4 and Chondroitin 6 Sulfate on Hand Osteoarthritis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial at a Single center. Arthritis Rheum. 63, 3383–3391. 10.1002/art.30574 [DOI] [PubMed] [Google Scholar]
- Garantziotis S., Savani R. C. (2019). Hyaluronan Biology: A Complex Balancing Act of Structure, Function, Location and Context. Matrix Biol. J Int Soc Matrix Biol. 78–79, 1–10. 10.1016/j.matbio.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators (2016). Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 310 Diseases and Injuries, 1990-2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Lond. Engl. 388, 1545–1602. 10.1016/s0140-6736(16)31678-6( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangreco A., Goldie S. J., Failla V., Saintigny G., Watt F. M. (2009). Human Skin Aging Is Associated with Reduced Expression of the Stem Cell Markers Beta1 Integrin and MCSP. J. Invest. Dermatol. 130, 604–608. 10.1038/jid.2009.297 [DOI] [PubMed] [Google Scholar]
- Haefke A., Ewald C. (2020). Tail Tendon Break Time for the Assessment of Aging and Longitudinal Healthspan in Mice. Bio-protocol 10, e3833. 10.21769/bioprotoc.3833 [DOI] [Google Scholar]
- Hathcock J. N., Shao A. (2007). Risk Assessment for Glucosamine and Chondroitin Sulfate. Regul. Toxicol. Pharm. 47, 78–83. 10.1016/j.yrtph.2006.07.004 [DOI] [PubMed] [Google Scholar]
- Henrotin Y., Marty M., Mobasheri A. (2014). What Is the Current Status of Chondroitin Sulfate and Glucosamine for the Treatment of Knee Osteoarthritis? Maturitas 78, 184–187. 10.1016/j.maturitas.2014.04.015 [DOI] [PubMed] [Google Scholar]
- Hochberg M. C., Zhan M., Langenberg P. (2008). The Rate of Decline of Joint Space Width in Patients with Osteoarthritis of the Knee: a Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials of Chondroitin Sulfate. Curr. Med. Res. Opin. 24, 3029–3035. 10.1185/03007990802434932 [DOI] [PubMed] [Google Scholar]
- Honda A., Abe M., Miirota S., Mori Y. (1979). The Effect of Aging on the Synthesis of Hexosamine-Containing Substances from Rat Costal Cartilage. J. Biochem. 85, 519–528. 10.1093/oxfordjournals.jbchem.a132359 [DOI] [PubMed] [Google Scholar]
- Honvo G., Bruyère O., Geerinck A., Veronese N., Reginster J.-Y. (2019a). Efficacy of Chondroitin Sulfate in Patients with Knee Osteoarthritis: A Comprehensive Meta-Analysis Exploring Inconsistencies in Randomized, Placebo-Controlled Trials. Adv. Ther. 36, 1085–1099. 10.1007/s12325-019-00921-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honvo G., Bruyère O., Reginster J.-Y. (2019b). Update on the Role of Pharmaceutical-Grade Chondroitin Sulfate in the Symptomatic Management of Knee Osteoarthritis. Aging Clin. Exp. Res. 31, 1163–1167. 10.1007/s40520-019-01253-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskisson E. (2008). Glucosamine and Chondroitin for Osteoarthritis. J. Int. Med. Res. 36, 1161–1179. 10.1177/147323000803600602 [DOI] [PubMed] [Google Scholar]
- Huxley-Jones J., Foord S. M., Barnes M. R. (2008). Drug Discovery in the Extracellular Matrix. Drug Discov. Today 13, 685–694. 10.1016/j.drudis.2008.05.005 [DOI] [PubMed] [Google Scholar]
- Imada K., Oka H., Kawasaki D., Miura N., Sato T., Ito A. (2010). Anti-Arthritic Action Mechanisms of Natural Chondroitin Sulfate in Human Articular Chondrocytes and Synovial Fibroblasts. Biol. Pharm Bull. 33, 410–414. 10.1248/bpb.33.410 [DOI] [PubMed] [Google Scholar]
- Izumikawa T., Sato B., Kitagawa H. (2014). Chondroitin Sulfate Is Indispensable for Pluripotency and Differentiation of Mouse Embryonic Stem Cells. Sci. Rep-uk 4, 3701. 10.1038/srep03701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C. G., Plaas A. H., Sandy J. D., Hua C., Kim-Rolands S., Barnhill J. G., et al. (2009). The Human Pharmacokinetics of Oral Ingestion of Glucosamine and Chondroitin Sulfate Taken Separately or in Combination. Osteoarthr Cartil Oars Osteoarthr Res. Soc. 18, 297–302. 10.1016/j.joca.2009.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens G. E., Lin X.-X., Millan-Ariño L., Kavšek A., Sen I., Seinstra R. I., et al. (2019). Transcriptomics-Based Screening Identifies Pharmacological Inhibition of Hsp90 as a Means to Defer Aging. Cel Rep. 27, 467–480. 10.1016/j.celrep.2019.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerosch J. (2011). Effects of Glucosamine and Chondroitin Sulfate on Cartilage Metabolism in OA: Outlook on Other Nutrient Partners Especially Omega-3 Fatty Acids. Int. J. Rheumatol. 2011, 1–17. 10.1155/2011/969012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomphe C., Gabriac M., Hale T. M., Héroux L., Trudeau L.-É., Deblois D., et al. (2007). Chondroitin Sulfate Inhibits the Nuclear Translocation of Nuclear Factor-Κb in Interleukin-1β-Stimulated Chondrocytes. Basic Clin. Pharmaco 102, 59–65. 10.1111/j.1742-7843.2007.00158.x [DOI] [PubMed] [Google Scholar]
- Jordan K. M., Arden N. K., Doherty M., Bannwarth B., Bijlsma J. W. J., Dieppe P., et al. (2003). EULAR Recommendations 2003: an Evidence Based Approach to the Management of Knee Osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann. Rheum. Dis. 62, 1145–1155. 10.1136/ard.2003.011742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan A., Uebelhart D., Vathaire F. D., Delmas P. D., Reginster J.-Y. (2009). Long-term Effects of Chondroitins 4 and 6 Sulfate on Knee Osteoarthritis: The Study on Osteoarthritis Progression Prevention, a Two-Year, Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Rheum. 60, 524–533. 10.1002/art.24255 [DOI] [PubMed] [Google Scholar]
- Kalman D. S., Heimer M., Valdeon A., Schwartz H., Sheldon E. (2008). Effect of a Natural Extract of Chicken Combs with a High Content of Hyaluronic Acid (Hyal-Joint) on Pain Relief and Quality of Life in Subjects with Knee Osteoarthritis: A Pilot Randomized Double-Blind Placebo-Controlled Trial. Nutr. J. 7, 3. 10.1186/1475-2891-7-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaki T., Makrantonaki E., Zouboulis C. C. (2016). Biomarkers of Skin Aging. Rev. Endocr. Metab. Disord 17, 433–442. 10.1007/s11154-016-9392-x [DOI] [PubMed] [Google Scholar]
- Kantor E. D., O’Connell K., Du M., Cao C., Zhang X., Lee D. H., et al. (2020). Glucosamine and Chondroitin Use in Relation to C-Reactive Protein Concentration: Results by Supplement Form, Formulation, and Dose. J. Altern. Complement. Med. N Y 27, 150–159. 10.1089/acm.2020.0283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor E. D., Zhang X., Wu K., Signorello L. B., Chan A. T., Fuchs C. S., et al. (2016). Use of Glucosamine and Chondroitin Supplements in Relation to Risk of Colorectal Cancer: Results from the Nurses’ Health Study and Health Professionals Follow-Up Study: Use of Glucosamine and Chondroitin in Relation to Colorectal Cancer Risk. Int. J. Cancer 139, 1949–1957. 10.1002/ijc.30250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsdal M. A., Daniels S. J., Nielsen S. H., Bager C., Rasmussen D. G. K., Loomba R., et al. (2020). Collagen Biology and Non‐Invasive Biomarkers of Liver Fibrosis. Liver Int. 40, 736–750. 10.1111/liv.14390 [DOI] [PubMed] [Google Scholar]
- Kim E. J. E., Lee S-J. V. (2019). Recent Progresses on Anti-Aging Compounds and Their Targets in Caenorhabditis elegans . Translational Med. Aging 3, 121–124. 10.1016/j.tma.2019.11.003 [DOI] [Google Scholar]
- King D. E., Xiang J. (2020). Glucosamine/Chondroitin and Mortality in a US NHANES Cohort. J. Am. Board Fam. Med. 33, 842–847. 10.3122/jabfm.2020.06.200110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppenburg M., Kroon F. P., Blanco F. J., Doherty M., Dziedzic K. S., Greibrokk E., et al. (2018). 2018 Update of the EULAR Recommendations for the Management of Hand Osteoarthritis. Ann. Rheum. Dis. 78, 16–24. 10.1136/annrheumdis-2018-213826 [DOI] [PubMed] [Google Scholar]
- Kusumoto D., Seki T., Sawada H., Kunitomi A., Katsuki T., Kimura M., et al. (2021). Anti-senescent Drug Screening by Deep Learning-Based Morphology Senescence Scoring. Nat. Commun. 12, 257. 10.1038/s41467-020-20213-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok J. C. F., Warren P., Fawcett J. W. (2012). Chondroitin Sulfate: A Key Molecule in the Brain Matrix. Int. J. Biochem. Cel Biol. 44, 582–586. 10.1016/j.biocel.2012.01.004 [DOI] [PubMed] [Google Scholar]
- Lee Y. H., Woo J.-H., Choi S. J., Ji J. D., Song G. G. (2009). Effect of Glucosamine or Chondroitin Sulfate on the Osteoarthritis Progression: A Meta-Analysis. Rheumatol. Int. 30, 357. 10.1007/s00296-009-0969-5 [DOI] [PubMed] [Google Scholar]
- Leeb B. F., Schweitzer H., Montag K., Smolen J. S. (2000). A Metaanalysis of Chondroitin Sulfate in the Treatment of Osteoarthritis. J. Rheumatol. 27, 205–211. [PubMed] [Google Scholar]
- Legendre F., Baugé C., Roche R., Saurel A. S., Pujol J. P. (2008). Chondroitin Sulfate Modulation of Matrix and Inflammatory Gene Expression in IL-1β-stimulated Chondrocytes – Study in Hypoxic Alginate Bead Cultures. Osteoarthr Cartilage 16, 105–114. 10.1016/j.joca.2007.05.020 [DOI] [PubMed] [Google Scholar]
- Lila A. M., Gromova O. A., Torshin I. Y., Montell E. (2018). Molecular Effects of Chondroitin Sulfate in Osteoarthritis and Herniated Discs. J. Rheumatol. Arthritic Dis 3, 1–11. 10.15226/2475-4676/3/3/00143 [DOI] [Google Scholar]
- Liu H., Guo M., Xue T., Guan J., Luo L., Zhuang Z. (2016). Screening Lifespan-Extending Drugs in Caenorhabditis elegans via Label Propagation on Drug-Protein Networks. BMC Syst. Biol. 10, 131. 10.1186/s12918-016-0362-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonte A. B. V., Mendonça J. A., Brandão G. de. C., Castro M. L. (2018). Multicenter, Randomized, Double-Blind Clinical Trial to Evaluate Efficacy and Safety of Combined Glucosamine Sulfate and Chondroitin Sulfate Capsules for Treating Knee Osteoarthritis. Adv. Rheumatol. 58, 41. 10.1186/s42358-018-0041-9 [DOI] [PubMed] [Google Scholar]
- López-Senra E., Casal-Beiroa P., López-Álvarez M., Serra J., González P., Valcarcel J., et al. (2020). Impact of Prevalence Ratios of Chondroitin Sulfate (CS)- 4 and -6 Isomers Derived from Marine Sources in Cell Proliferation and Chondrogenic Differentiation Processes. Mar. Drugs 18, 94. 10.3390/md18020094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucanic M., Lithgow G. J., Alavez S. (2013). Pharmacological Lifespan Extension of Invertebrates. Ageing Res. Rev. 12, 445–458. 10.1016/j.arr.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macke E. L., Henningsen E., Jessen E., Zumwalde N. A., Landowski M., Western D. E., et al. (2020). Loss of Chondroitin Sulfate Modification Causes Inflammation and Neurodegeneration in Skt Mice. Genetics 214, 121–134. 10.1534/genetics.119.302834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel-Pelletier J., Farran A., Montell E., Vergés J., Pelletier J.-P. (2015). Discrepancies in Composition and Biological Effects of Different Formulations of Chondroitin Sulfate. Mol. Basel Switz 20, 4277–4289. 10.3390/molecules20034277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty M. F., Russell A. L., Seed M. P. (2000). Sulfated Glycosaminoglycans and Glucosamine May Synergize in Promoting Synovial Hyaluronic Acid Synthesis. Med. Hypotheses 54, 798–802. 10.1054/mehy.1999.0954 [DOI] [PubMed] [Google Scholar]
- Michel B. A., Stucki G., Frey D., Vathaire F. D., Vignon E., Bruehlmann P., et al. (2005). Chondroitins 4 and 6 Sulfate in Osteoarthritis of the Knee: A Randomized, Controlled Trial. Arthritis Rheum. 52, 779–786. 10.1002/art.20867 [DOI] [PubMed] [Google Scholar]
- Mikami T., Kitagawa H. (2013). Biosynthesis and Function of Chondroitin Sulfate. Biochim. Biophys. Acta Bba - Gen Subj 1830, 4719–4733. 10.1016/j.bbagen.2013.06.006 [DOI] [PubMed] [Google Scholar]
- Monfort J., Nacher M., Montell E., Vila J., Verges J., Benito P. (2005). Chondroitin Sulfate and Hyaluronic Acid (500-730 Kda) Inhibit Stromelysin-1 Synthesis in Human Osteoarthritic Chondrocytes. Drug Exp. Clin. Res. 31, 71–76. [PubMed] [Google Scholar]
- Moskalev A., Shaposhnikov M. (2011). Pharmacological Inhibition of NF-Κb Prolongs Lifespan of Drosophila melanogaster . Aging 3, 391–394. 10.18632/aging.100314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro S. L., Levy L., Curtis K. R., Lampe J. W., Hullar M. A. J. (2019). Modulation of Gut Microbiota by Glucosamine and Chondroitin in a Randomized, Double-Blind Pilot Trial in Humans. Microorg 7, 610. 10.3390/microorganisms7120610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro S. L., White E., Kantor E. D., Zhang Y., Rho J., Song X., et al. (2015). Randomized Trial of Glucosamine and Chondroitin Supplementation on Inflammation and Oxidative Stress Biomarkers and Plasma Proteomics Profiles in Healthy Humans. Plos One 10, e0117534. 10.1371/journal.pone.0117534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata T., Itokazu Y., Inoue N., Segawa Y. (2000). Effects of Chondroitin Sulfate-C on Articular Cartilage Destruction in Murine Collagen-Induced Arthritis. Arzneimittelforschung 50, 148–153. 10.1055/s-0031-1300180 [DOI] [PubMed] [Google Scholar]
- Pecly I. M. D., Melo-Filho N. M., Mourão P. A. S. (2006). Effects of Molecular Size and Chemical Structure on Renal and Hepatic Removal of Exogenously Administered Chondroitin Sulfate in Rats. Biochim. Biophys. Acta Bba - Gen Subj 1760, 865–876. 10.1016/j.bbagen.2005.11.009 [DOI] [PubMed] [Google Scholar]
- Petrascheck M., Ye X., Buck L. B. (2007). An Antidepressant that Extends Lifespan in Adult Caenorhabditis elegans . Nature 450, 553–556. 10.1038/nature05991 [DOI] [PubMed] [Google Scholar]
- Pocobelli G., Kristal A. R., Patterson R. E., Potter J. D., Lampe J. W., Kolar A., et al. (2010). Total Mortality Risk in Relation to Use of Less-Common Dietary Supplements. Am. J. Clin. Nutr. 91, 1791–1800. 10.3945/ajcn.2009.28639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani A., Baruah R., Goyal A. (2018). Prebiotic Chondroitin Sulfate Disaccharide Isolated from Chicken Keel Bone Exhibiting Anticancer Potential against Human Colon Cancer Cells. Nutr. Cancer 71, 1–15. 10.1080/01635581.2018.1521446 [DOI] [PubMed] [Google Scholar]
- Reginster J.-Y., Veronese N. (2021). Highly Purified Chondroitin Sulfate: a Literature Review on Clinical Efficacy and Pharmacoeconomic Aspects in Osteoarthritis Treatment. Aging Clin. Exp. Res. 33, 37–47. 10.1007/s40520-020-01643-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richy F., Bruyere O., Ethgen O., Cucherat M., Henrotin Y., Reginster J.-Y. (2003). Structural and Symptomatic Efficacy of Glucosamine and Chondroitin in Knee Osteoarthritis: A Comprehensive Meta-Analysis. Arch. Intern. Med. 163, 1514–1522. 10.1001/archinte.163.13.1514 [DOI] [PubMed] [Google Scholar]
- Ronca F., Palmieri L., Panicucci P., Ronca G. (1998). Anti-inflammatory Activity of Chondroitin Sulfate. Osteoarthr Cartilage 6, 14–21. 10.1016/s1063-4584(98)80006-x [DOI] [PubMed] [Google Scholar]
- Ronca G., Conte A. (1993). Metabolic Fate of Partially Depolymerized Shark Chondroitin Sulfate in Man. Int. J. Clin. Pharm. Res. 13 (Suppl. l), 27–34. [PubMed] [Google Scholar]
- Satia J. A., Littman A., Slatore C., Galanko J., White E. (2009). Associations of Specialty Herbal Supplements with Lung and Colorectal Cancer Risk. Faseb J. 23, 222.3. 10.1096/fasebj.23.1_supplement.222.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinzel R. T., Higuchi-Sanabria R., Shalem O., Moehle E. A., Webster B. M., Joe L., et al. (2019). The Hyaluronidase, TMEM2, Promotes ER Homeostasis and Longevity Independent of the UPRER. Cell 179, 1306–1318. 10.1016/j.cell.2019.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster S., Black M. M., McVitie E. (1975). The Influence of Age and Sex on Skin Thickness, Skin Collagen and Density. Br. J. Dermatol. 93, 639–643. 10.1111/j.1365-2133.1975.tb05113.x [DOI] [PubMed] [Google Scholar]
- Souich P. D. (2014). Absorption, Distribution and Mechanism of Action of SYSADOAS. Pharmacol. Therapeut 142, 362–374. 10.1016/j.pharmthera.2014.01.002 [DOI] [PubMed] [Google Scholar]
- Souich P. D., García A. G., Vergés J., Montell E. (2009). Immunomodulatory and Anti-inflammatory Effects of Chondroitin Sulphate. J. Cel Mol Med 13, 1451–1463. 10.1111/j.1582-4934.2009.00826.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statzer C., Ewald C. Y. (2020). The Extracellular Matrix Phenome Across Species. Matrix Biol. Plus 8, 100039. 10.1016/j.mbplus.2020.100039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statzer C., Jongsma E., Liu S. X., Dakhovnik A., Wandrey F., Mozharovskyi P., et al. (2021). Youthful and Age‐related Matreotypes Predict Drugs Promoting Longevity. Aging Cell, e13441. [Epub ahead of print]. 10.1111/acel.13441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellavato A., Restaino O. F., Vassallo V., Finamore R., Ruosi C., Cassese E., et al. (2019). Comparative Analyses of Pharmaceuticals or Food Supplements Containing Chondroitin Sulfate: Are Their Bioactivities Equivalent? Adv. Ther. 36, 3221–3237. 10.1007/s12325-019-01064-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara K., Tsuruta D., Ishii M., Jones J. C. R., Kobayashi H. (2008). Laminin-332 and -511 in Skin. Exp. Dermatol. 17, 473–480. 10.1111/j.1600-0625.2008.00721.x [DOI] [PubMed] [Google Scholar]
- Sukhikh S., Babich O., Prosekov A., Patyukov N., Ivanova S. (2020). Future of Chondroprotectors in the Treatment of Degenerative Processes of Connective Tissue. Pharm 13, 220. 10.3390/ph13090220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiri K., Korwin-Zmijowska C., Richette P., Heraud F., Chevalier X., Savouret J.-F., et al. (2007). Natural Chondroitin Sulphates Increase Aggregation of Proteoglycan Complexes and Decrease Adamts-5 Expression in Interleukin 1 -treated Chondrocytes. Ann. Rheum. Dis. 67, 696–702. 10.1136/ard.2007.078600 [DOI] [PubMed] [Google Scholar]
- Taniguchi S., Ryu J., Seki M., Sumino T., Tokuhashi Y., Esumi M. (2011). Long-Term Oral Administration of Glucosamine or Chondroitin Sulfate Reduces Destruction of Cartilage and Up-Regulation of MMP-3 mRNA in a Model of Spontaneous Osteoarthritis in Hartley guinea Pigs: Oral Glucosamine or Chondroitin Sulfate in an Oa Model. J. Orthopaed Res. 30, 673–678. 10.1002/jor.22003 [DOI] [PubMed] [Google Scholar]
- Teuscher A. C., Statzer C., Pantasis S., Bordoli M. R., Ewald C. Y. (2019). Assessing Collagen Deposition During Aging in Mammalian Tissue and in Caenorhabditis elegans . Methods Mol. Biol. Clifton N J 1944, 169–188. 10.1007/978-1-4939-9095-5_13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilstra J. S., Clauson C. L., Niedernhofer L. J., Robbins P. D. (2011). NF-κB in Aging and Disease. Aging Dis. 2, 449–465. [PMC free article] [PubMed] [Google Scholar]
- Uebelhart D. (2008). Clinical Review of Chondroitin Sulfate in Osteoarthritis. Osteoarthr Cartilage 16, S19–S21. 10.1016/j.joca.2008.06.006 [DOI] [PubMed] [Google Scholar]
- Uebelhart D., Malaise M., Marcolongo R., DeVathaire F., Piperno M., Mailleux E., et al. (2004). Intermittent Treatment of Knee Osteoarthritis with Oral Chondroitin Sulfate: A One-Year, Randomized, Double-Blind, Multicenter Study versus Placebo. Osteoarthr Cartilage 12, 269–276. 10.1016/j.joca.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Vallières M., Souich P. D. (2010). Modulation of Inflammation by Chondroitin Sulfate. Osteoarthr Cartilage 18, S1–S6. 10.1016/j.joca.2010.02.017 [DOI] [PubMed] [Google Scholar]
- Varani J., Dame M. K., Rittie L., Fligiel S. E. G., Kang S., Fisher G. J., et al. (2006). Decreased Collagen Production in Chronologically Aged Skin Roles of Age-Dependent Alteration in Fibroblast Function and Defective Mechanical Stimulation. Am. J. Pathol. 168, 1861–1868. 10.2353/ajpath.2006.051302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi N. (2003). Oral Absorption and Bioavailability of Ichthyic Origin Chondroitin Sulfate in Healthy Male Volunteers. Osteoarthr Cartilage 11, 433–441. 10.1016/s1063-4584(03)00051-7 [DOI] [PubMed] [Google Scholar]
- Volpi N. (2002). Oral Bioavailability of Chondroitin Sulfate (Condrosulf) and its Constituents in Healthy Male Volunteers. Osteoarthr Cartil Oars Osteoarthr Res. Soc. 10, 768–777. 10.1053/joca.2002.0824 [DOI] [PubMed] [Google Scholar]
- Wandel S., Jüni P., Tendal B., Nüesch E., Villiger P. M., Welton N. J., et al. (2010). Effects of Glucosamine, Chondroitin, or Placebo in Patients with Osteoarthritis of Hip or Knee: Network Meta-Analysis. Bmj Clin. Res. Ed. 341, c4675. 10.1136/bmj.c4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer S., Priebs J., Kuhlow D., Groth M., Priebe S., Mansfeld J., et al. (2014). D-Glucosamine Supplementation Extends Life Span of Nematodes and of Ageing Mice. Nat. Commun. 5, 3563. 10.1038/ncomms4563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick G., Grundtman C., Mayerl C., Wimpissinger T.-F., Feichtinger J., Zelger B., et al. (2013). The Immunology of Fibrosis. Annu. Rev. Immunol. 31, 107–135. 10.1146/annurev-immunol-032712-095937 [DOI] [PubMed] [Google Scholar]
- Wildi L. M., Raynauld J.-P., Martel-Pelletier J., Beaulieu A., Bessette L., Morin F., et al. (2011). Chondroitin Sulphate Reduces Both Cartilage Volume Loss and Bone Marrow Lesions in Knee Osteoarthritis Patients Starting as Early as 6 Months after Initiation of Therapy: A Randomised, Double-Blind, Placebo-Controlled Pilot Study Using MRI. Ann. Rheum. Dis. 70, 982–989. 10.1136/ard.2010.140848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C.-X., Jin H., Chung Y.-S., Shin J.-Y., Lee K.-H., Beck G. R., et al. (2008). Chondroitin Sulfate Extracted from Ascidian Tunic Inhibits Phorbol Ester-Induced Expression of Inflammatory Factors VCAM-1 and COX-2 by Blocking NF-Κb Activation in Mouse Skin. J. Agr Food Chem. 56, 9667–9675. 10.1021/jf801578x [DOI] [PubMed] [Google Scholar]
- Yang S., Gigout S., Molinaro A., Naito-Matsui Y., Hilton S., Foscarin S., et al. (2021). Chondroitin 6-sulphate Is Required for Neuroplasticity and Memory in Ageing. Mol. Psychiatr. [Epub ahead of print]. 10.1038/s41380-021-01208-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X., Linton J. M., Schork N. J., Buck L. B., Petrascheck M. (2014). A Pharmacological Network for Lifespan Extension in Caenorhabditis elegans . Aging cell 13, 206–215. 10.1111/acel.12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegels B., Crozes P., Uebelhart D., Bruyère O., Reginster J. Y. (2013). Equivalence of a Single Dose (1200 Mg) Compared to a Three-Time a Day Dose (400 Mg) of Chondroitin 4&6 Sulfate in Patients with Knee Osteoarthritis. Results of a Randomized Double Blind Placebo Controlled Study. Osteoarthr Cartilage 21, 22–27. 10.1016/j.joca.2012.09.017 [DOI] [PubMed] [Google Scholar]
- Zhang G., Li J., Purkayastha S., Tang Y., Zhang H., Yin Y., et al. (2013). Hypothalamic Programming of Systemic Ageing Involving IKK-β, NF-Κb and GnRH. Nature 497, 211–216. 10.1038/nature12143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Sang L., Wu D., Rong J., Jiang L. (2018). Effectiveness and Safety of Glucosamine and Chondroitin for the Treatment of Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. J. Orthop. Surg. Res. 13, 170. 10.1186/s13018-018-0871-5 [DOI] [PMC free article] [PubMed] [Google Scholar]