Abstract
As a muscular pump that contracts incessantly throughout life, the heart must constantly generate cellular energy to support contractile function and fuel ionic pumps to maintain electrical homeostasis. Thus, mitochondrial metabolism of multiple metabolic substrates such as fatty acids, glucose, ketones and lactate are essential to ensuring an uninterrupted supply of ATP. Multiple metabolic pathways converge to maintain myocardial energy homeostasis. The regulation of these cardiac metabolic pathways has been intensely studied for many decades. Rapid adaptation of these pathways is essential for mediating the myocardial adaptation to stress, and dysregulation of these pathways contribute to myocardial pathophysiology as occurs in heart failure and in metabolic disorders such as diabetes. The regulation of these pathways reflects the complex interactions of cell-specific regulatory pathways, neurohumoral signals and changes in substrate availability in the circulation. Significant advances have been made in the ability to study metabolic regulation in the heart, and animal models have played an central role in contributing to this knowledge. This review will summarize metabolic pathways in the heart and describe their contribution to maintaining myocardial contractile function in health and disease. The review will summarize lessons learned from animal models with altered systemic metabolism, and those in which specific metabolic regulatory pathways have been genetically altered within the heart. The relationship between intrinsic and extrinsic regulators of cardiac metabolism and the pathophysiology of heart failure and how these have been informed by animal models will be discussed.
Keywords: Animal Models, Cardiac Metabolism, Substrate Metabolism, Mitochondria
Subject Terms: Animal Models of Human Disease, Basic Science Research, Cardiovascular Disease, Metabolism
Introduction
To enable cardiac contraction, cardiac myocytes convert chemical energy derived from substrate utilization to mechanical work. Thus, the heart consumes more energy than other organs to meet the energetic needs of maintaining continuous pump function.1 This inextricable link between energy metabolism and contractile function is reflected by the heart’s ability to turn over its entire ATP pool within a few seconds to match the constantly high energetic demand. Defects and changes in cardiac energy metabolism have been widely reported in subjects suffering heart failure (HF) with reduced ejection fraction (HFrEF), including impaired mitochondrial oxidative capacity, reduced cardiac efficiency, and alterations in the substrate utilization pattern.2 The resulting energy deprivation is widely accepted to contribute to onset and progression of HF. In addition, alterations in systemic substrate availability determine changes in myocardial substrate utilization that are associated with altered cardiac structure and function, as exemplified in the metabolic syndrome (MetS).3 Thus, dysregulation of cardiac energy metabolism as a primary cause or secondary consequence may have detrimental consequences for cardiac structure and function.
Powerful non-invasive tools exist in clinical practice to evaluate cardiac metabolism, such as magnetic resonance spectroscopy (MRS) and positron emission tomography (PET). However, due to limitations in performing mechanistic experiments in human subjects and biomaterials, elucidating underlying mechanisms for altered cardiac energetics and development of therapeutic strategies to modulate myocardial energy metabolism remain challenging. Thus, investigators have generated and explored numerous animal models of dysregulated cardiac metabolism to facilitate mechanistic insights into the regulation of cardiac metabolism and to understand their relation to cardiac function and disease. Indeed, numerous findings from these models have been confirmed in human cardiac tissue thereby providing the rationale for clinical trials that partially reported beneficial clinical effects of targeted modulation of cardiac substrate utilization. Given the growing interest in developing metabolic therapies for the heart, we will present and discuss changes in cardiac metabolism in animal models of the metabolic syndrome and of HF and will review genetically engineered and disease-mimicking animal models useful to examine cardiac metabolism and related effects on cardiac function and structure. The large number of reported models precludes the inclusion of extensive details and citations in the main manuscript. Thus, we refer the reader to Supplemental Tables S1-S4 for detailed and comprehensive information on animal models and the relevant citations.
Overview of cardiac metabolism
Cardiac energy metabolism and its regulation has been the subject of intense investigation for several decades and has been reviewed in great detail by others.2 In brief, about 95% of ATP generated in cardiac muscle originates from oxidative phosphorylation within mitochondria, and 5% of ATP is directly generated by glycolysis. To meet the high energy demands of the heart, cardiomyocytes possess a high density of mitochondria, comprising up to 40% cellular volume, depending on the species. Approximately 40-60% of ATP generated within mitochondria result from the oxidation of fatty acids (FAs), and the remaining from oxidation of glucose, lactate, ketone bodies and amino acids, based in part on their availability. Following uptake, predominantly by FA transporters (CD36, fatty acid transport protein (FATP)), FA are esterified to generate acyl-CoA by acyl-CoA synthetase, imported into mitochondria by the carnitine palmitoyltransferase (CPT) shuttle, and oxidized in the beta oxidation spiral to yield acetyl-CoA. Glucose is taken up via glucose transporters 1 (GLUT1; insulin-independent) and 4 (GLUT4; insulin-dependent), metabolized via glycolysis to generate pyruvate, which is imported into mitochondria via the mitochondrial pyruvate carrier and decarboxylated by pyruvate dehydrogenase (PDH) to also yield acetyl-CoA. Acetyl-CoA is oxidized in the TCA cycle, thereby mainly generating NADH2 for delivery of electrons into the mitochondrial electron transport chain (ETC). Electrons are also delivered by FADH2 generated by beta oxidation. Electrons passing through the ETC ultimately reduce oxygen to water, a process coupled to the regeneration of ATP from ADP by the FoF1-ATP synthase (ATPsyn). Other substrates enter this main pathway of substrate oxidation at different sites. Lactate can be imported into cardiomyocytes and is converted into pyruvate via lactate dehydrogenase (LDH) to enter substrate oxidation via PDH, accounting for < 3% of ATP in healthy hearts.4
Ketone bodies are predominantly generated in the liver, yielding acetoacetate which is converted to b-hydroxybutyrate (βOHB). βOHB is imported into mitochondria and converted to acetyl-CoA for oxidation in the TCA cycle by βOHB dehydrogenase (BDH1), succinyl-CoA:3-oxoacid-CoA transferase (SCOT), and mitochondrial thiolase, accounting for 6-7% of ATP.5 The major amino acid species oxidized by the heart are branched chain amino acids (BCAA, i.e., leucine, valine, isoleucine). BCAAs enter the cell via the BCAA:cation symporter family (LIVCS) and are converted to ketoacids by mitochondrial branched chain aminotransferase (BCATm). In a second reaction, branched chain alpha-keto acid dehydrogenase (BCKDH) converts these ketoacids to acetyl CoA and succinyl CoA which serve as oxidative or anaplerotic substrates, respectively.4, 6 The contribution to ATP synthesis is estimated at 4-5% of total ATP produced in healthy hearts.4, 6
Regulation of oxidative capacity and metabolic substrate utilization is coordinated by a number of transcriptional, translational, and posttranslational mechanisms.2, 7, 8 The fetal heart predominantly relies on glycolysis for ATP production (accounting for up to 44% of total ATP), with low rates of oxidative ATP regeneration.9 Following birth, increased availability of oxygen and dietary FAs is accompanied by a 3-fold increase in mitochondrial density and a 10-fold increase in FA oxidation.10-12 This increased mitochondrial content is driven by induction of the transcriptional coactivator and master regulator of mitochondrial biogenesis, peroxisome proliferator-activated receptor (PPAR)γ co-activator 1a (PGC1α), thereby increasing mitochondrial number, size and enzymatic content.12 PGC1α coactivates and/or increases the expression of powerful transcription factors that drive FA oxidation and OXPHOS gene expression, including estrogen-related receptor (ERR) α and γ, and nuclear respiratory factor (NRF) 1 and 2, that increase mtDNA replication and transcription (mitochondrial transcription factor A, TFAm). Orchestration of mitochondrial biogenesis, the response to stress, adaptation to increased energy demands, regulation of OXPHOS function, maintenance of mitochondrial structure, and quality control require a balance of mitochondrial dynamics, a conserved process regulated by mitochondrial fusion (mitofusin 1/2 (MFN1/2), optic atrophy 1 (OPA1)) and fission proteins (dynamin-related protein 1 (DRP1), fission 1 (FIS1)). The predominant regulators of the expression of most enzymes involved in FA uptake and oxidation are the nuclear receptors and transcription factors, PPARα, γ, and β/δ.8 Another important regulator of cardiomyocyte energy homeostasis is the serine-threonine kinase, adenosine monophosphate-activated protein kinase (AMPK). AMPK is activated by a low AMP/ATP ratio (e.g. during exercise) and predominantly promotes catabolic metabolism by increasing glucose uptake via GLUT4 translocation to the sarcolemma, enhancing glycolysis by activating phospho-fructokinase-2 (PFK2), increasing FA uptake via CD36 translocation to the sarcolemma, and amplifying PGC1α signaling.13 These mechanisms are complemented by the classical glucose-FA cycle (also termed Randle cycle) wherein increased FA utilization leads to an inhibition of glucose utilization. Conversely, this competitive mechanism for fuel selection also applies when increased glucose utilization suppresses FA utilization (i.e., reverse Randle cycle). The molecular mechanisms for this regulation is detailed elsewhere.14 An important metabolite inhibiting CPT1β thereby limiting FA oxidation, is malonyl-CoA, which is generated by acetyl-CoA carboxylase (ACC) and catabolized by malonyl-CoA decarboxylase (MCD). These enzymes are also regulated by AMPK. Significant posttranslational regulation of energy metabolism is also mediated by the Sirtuin family of NAD+-dependent lysine deacetylases (Sirtuins 1-7 (SIRT1-7)), which are localized in the nucleus, cytosol and mitochondria and which remove a variety of modifications (e.g., acetyl-, succinyl-, malonyl, glutaryl-residues) to mainly activate their target enzymes and proteins. Figures 1 and 2 provides an overview of cardiac energy metabolism and mechanisms that regulate mitochondrial structure and function.
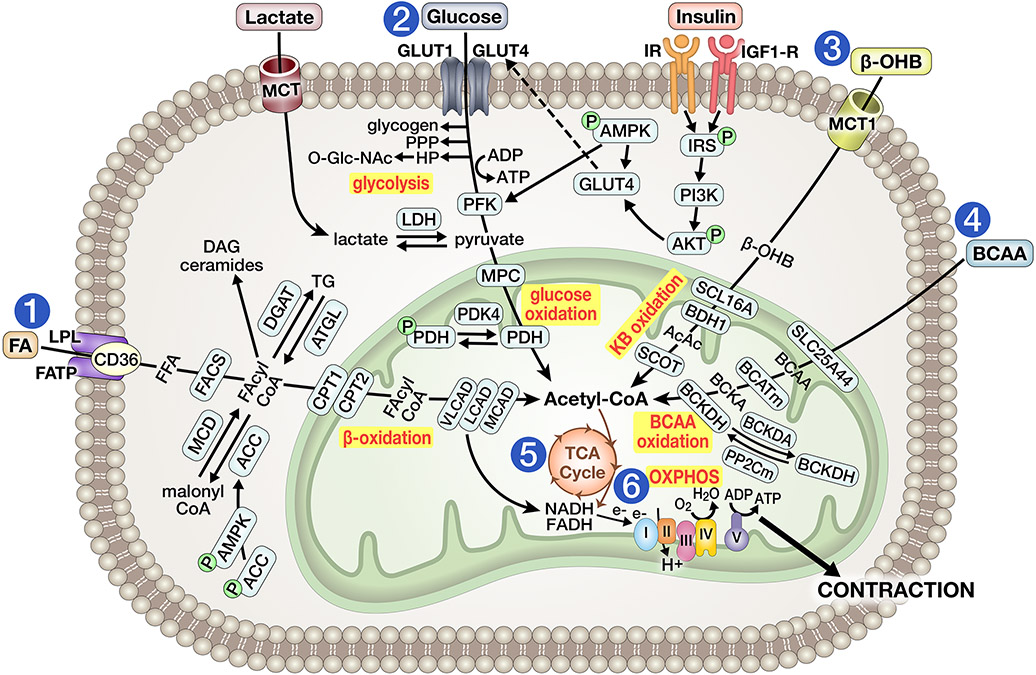
Figure 1A. Overview of energy substrate metabolism in the adult non-stressed heart.
1) Fatty acid (FA) uptake is facilitated by lipoprotein lipase (LPL) and mediated by FA transport proteins (FATP1/6) or CD36. FA are then esterified by acyl-CoA synthetase (ACS) to fatty acyl-CoA (FA-CoA), imported into mitochondria via the carnitine palmitoyltransferase (CPT) shuttle, and introduced for oxidation by medium-chain, long-chain or very long-chain acyl-CoA dehydrogenase (MCAD, LCAD, VLCAD). Activity of CPT1 is inhibited by malonyl CoA, which is generated by acetyl-CoA carboxylase (ACC) activity or decarboxylated by malonyl-CoA decarboxylase (MCD), an enzymatic system regulated by AMP-activated protein kinase (AMPK). Alternatively, FA-CoA may enter the cycle of synthesis and lipolysis of TG, regulated by diacylglycerol O-Acyltransferase (DGAT) and adipose triglyceride lipase (ATGL), or may be converted into other lipid intermediates (e.g., ceramides, DAG). 2) Glucose is taken up via glucose transporters (GLUT1/4), processed through glycolysis to yield pyruvate, which is imported into mitochondria by mitochondrial pyruvate carrier (MPC) and metabolized to acetyl-CoA by pyruvate dehydrogenase (PDH), the latter enzyme being inhibited by PDH kinase 4 (PDK4) activity. Alternatively, early glycolytic intermediates can be diverted into glycogen synthesis or into the pentose phosphate pathway (PPP) or hexosamine pathway (HP). Glucose uptake is increased by insulin signaling via insulin receptor (IR) and insulin-like growth factor 1 receptor (IGF1-R), thereby increasing GLUT4 translocation to the sarcolemma. Lactate is taken up by monocarboxylic acid transporter (MCT) and converted to pyruvate by lactate dehydrogenase (LDH). 3) Following uptake by monocarboxylate transporter (MCT) 1, ketones (predominantly β-hydroxybutyrate, βOHB) are converted to acetoacetate (AcAc) by βOHB dehydrogenase 1 (BDH1) and further oxidized to acetyl-CoA by succinyl-CoA:3 oxoacid-CoA transferase (SCOT) and thiolase. 4) Branched chain amino acids (BCAA) are imported into mitochondria by the solute carrier family 25 member 44 (SLC25A44), converted to branched chain ketoacids (BCKA) by mitochondrial branched chain aminotransferase (BCATm), and then to acetyl-CoA by branched chain alpha-keto acid dehydrogenase (BCKDH). BCKDH activity is inhibited by BCKD kinase and activated by mitochondrial protein phosphatase 2C (PP2Cm). 5) Acetyl CoA yielded from FA, carbohydrate, ketone or BCAA utilization enter the tricarboxylic acid (TCA) cycle to produce NADH2. NADH2, and FADH2 generated in β-oxidation, deliver electrons into the electron transport chain (ETC) to sustain oxidative phosphorylation (OXPHOS). The resulting ATP maintains cardiac contractility and fuels ionic pumps.
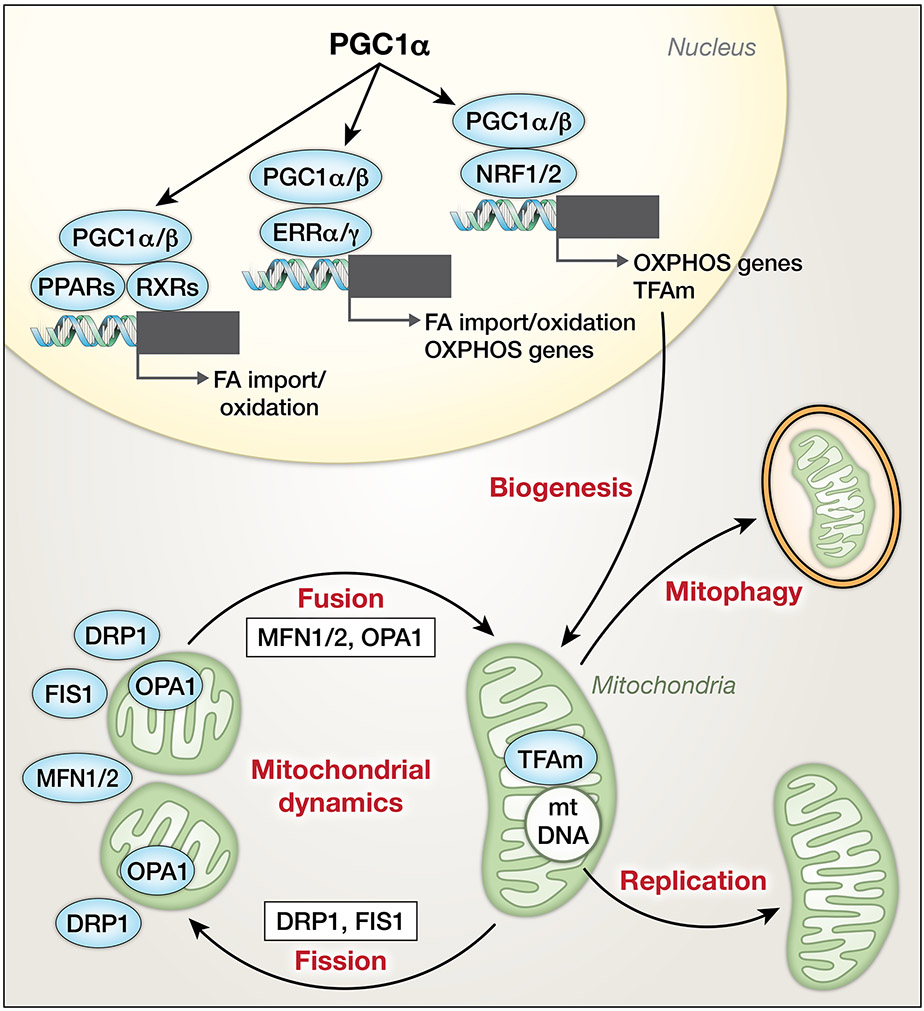
Figure 2. Mitochondrial life cycle.
Peroxisome proliferator-activated receptor gamma coactivators 1-α/β (PGC1α/β) coactivate peroxisome proliferator-activated receptors (PPARs), estrogen-related receptors (ERRs) and nuclear respiratory factors (NRFs) to orchestrate expression of proteins of FA utilization and OXPHOS during mitochondrial biogenesis. PGCs also drive mitochondrial transcription factor A (TFAm) expression to regulate protein/RNA expression and replication of mtDNA. Mitochondrial dynamics are balanced by mitochondrial fission, driven by dynamin-related protein 1 (DRP1) and fission 1 (FIS1), and mitochondrial fusion, promoted by mitofusin 1/2 (MFN1/2) and optic atrophy 1 (OPA1). Removal of mitochondria is accomplished by autophagy of mitochondria (i.e., mitophagy).
Relationship between cardiac metabolism and function
A bidirectional dependency exists between cardiac metabolism and function where changes in contractility demand adaptations in energy metabolism, and where changes in cardiac metabolism alter contractile function. Following an acute increase in workload (e.g., during acute exercise or increased blood pressure), the heart may increase its work by up to 3-fold, and in trained athletes by up to 6-fold. This contractile reserve requires an increase in coronary blood flow to match delivery of oxygen and substrates to increased energy demand.15 Simultaneously, the heart can increase substrate flux and oxidative phosphorylation, which operates at only 15-25% of its maximal capacity under resting conditions.16 However, under maximal workload, the heart may utilize up to 90% of its ATP-generating capacity within mitochondria. In addition, the heart utilizes cardiac metabolic reserve present in the form of phosphocreatine, TG and glycogen. Phosphocreatine serves as a rapidly claimable source of phosphate to regenerate ATP, and enzymatic breakdown of glycogen can rapidly feed glucose-6-phosphate into glycolysis for glucose oxidation.17
The general dependency of contractility on ATP availability is supported by studies using animal models with selected genetic manipulations. For example, mice lacking PGC1α have diminished content and activity of mitochondrial energy-metabolic proteins, resulting in mildly impaired contractile function in ex vivo beating Langendorff-perfused hearts, but clearly impaired ability to increase work output in response to dobutamine stress.18 These mice revealed a 20% reduction of ATP content under non-stressed conditions and impaired ability to increase ATP turnover that likely limited cardiac contractile performance in response to dobutamine stress. These observations provide evidence that a primary dysregulation of energetics (induced by PGC1α deficiency) is sufficient to impair cardiac contractility and reserve. Accordingly, reduced expression and signaling of PGC1α and the related decrease in OXPHOS expression in pathological hypertrophy and failing hearts have been proposed to contribute to onset or progression of heart failure.19-22 Although defects in cardiac energetics can induce or exacerbate contractile dysfunction, changes in cardiac structure and function are also correlated with and may be mechanistically linked to changes in metabolism.
Cardiac metabolism and function are also impacted by changes in systemic metabolism. As an omnivore the heart oxidizes a variety of substrates dependent on their availability in the circulation. This flexibility allows the heart to sustain pump function even under metabolically unfavorable conditions to mitigate energy deficiency. This ability to switch substrate preference for ATP generation (e.g., from FA utilization to glucose utilization) is mediated by acute and chronic signaling within cardiomyocytes that adapts the activity and capacity of metabolic enzymes to accomplish the shift in substrate utilization, a concept described as metabolic flexibility. For example, the heart increases the relative utilization of glucose during fed conditions when serum glucose and insulin levels are high, whereas starvation induces lipolysis leading to increased serum FA levels and cardiac FA utilization. Furthermore, high serum levels of FAs and triglycerides in obesity and diabetes mellitus cause a predominant use of FAs as the primary source for ATP regeneration. Loss of metabolic flexibility, as occurs in heart failure or the cardiomyopathy of obesity and diabetes mellitus, contributes to decreased cardiac efficiency and myocardial energy deficiency.
Overview of the metabolic syndrome and impact on cardiac metabolism, structure and function.
Recent decades, have been characterized by a global obesity epidemic, accompanied by increased incidence of the metabolic syndrome (MetS).23 This is characterized by the presence of insulin resistance (i.e., impaired fasting glucose, impaired glucose tolerance, or type 2 diabetes mellitus (T2DM)), combined with at least two other risk factors, including obesity, dyslipidemia (hypertriglyceridemia, low high-density lipoprotein cholesterol), or hypertension.24 While the pathogenesis of the MetS is complex and incompletely understood, genetic predisposition and the interaction with environmental factors, and sedentary lifestyle (lack of exercise, excess caloric intake) leading to visceral adiposity are highly associated with the MetS.25 Insulin resistance in adipose tissue increases lipolysis and circulating FAs that may impair glucose uptake and induce insulin resistance in skeletal muscle, induce hepatic insulin resistance and thus lipogenesis and gluconeogenesis and also impair pancreatic beta cells function resulting in decreased insulin secretion.25, 26 While insulin resistance may initially be compensated by hyperinsulinemia, reduced beta cell reserve ultimately leads to hyperglycemia and type 2 diabetes in susceptible individuals. Dysregulation of adipokine secretion (decreased adiponectin and increased leptin release), activation of the renin-angiotensin-aldosterone system (RAAS) reflected by increased secretion of angiotensin II, and release of proinflammatory cytokines (e.g. TNF-α, IL-1β, IL-6) contribute to insulin resistance in peripheral tissues by direct and indirect mechanisms such as oxidative stress, fibrosis, metabolic derangements, and inflammation.26, 27 Loss of the vasodilator effect of insulin, vasoconstriction caused by FFAs, increased RAAS and sympathetic nervous system activation, and induction of endothelial dysfunction may contribute to development of hypertension in the MetS.28
While increasing the risk for atherosclerotic cardiovascular disease (CVD), the MetS also promotes the development of a cardiomyopathy of obesity and diabetes. Some individuals may develop concentric hypertrophy and diastolic dysfunction, possibly progressing to heart failure with preserved ejection fraction (HFpEF), whereas others may develop eccentric hypertrophy, occasionally accompanied by systolic defects in contractility and potentially progressing to heart failure with reduced ejection fraction (HFrEF).29, 30 Numerous studies using rodent models of obesity, IR and T2DM provide evidence that these metabolic disorders may promote the development of cardiac hypertrophy with increased cardiomyocyte size, myocardial fibrosis, and functional defects such as impaired contractility, diastolic dysfunction and impaired cardiac efficiency (i.e. cardiac work/oxygen consumption).31-36 It is important to note that these models are generally resistant to the development of atherosclerosis in the absence of predisposing genetic mutations (e.g., ApoE or LDLR deficiency), thus emphasizing that metabolic perturbations per se could be sufficient to induce cardiac dysfunction. As summarized in Figure 3, studies investigating cardiac metabolism in these models revealed increased uptake and oxidation of long chain FAs, decreased glucose utilization, and diminished metabolic responsiveness upon insulin stimulation compatible with cardiac insulin resistance.33, 37, 38 In addition, impaired rates of mitochondrial ATP synthesis have been reported in hearts of various animal models of metabolic disease.32, 37 Underlying mechanisms include transcriptional, translational and posttranslational repression of OXPHOS activity, mitochondrial oxidative stress, mitochondrial uncoupling, dysregulation of mitochondrial biogenesis, perturbation of mitochondrial dynamics, and altered mitophagy, among others.19, 32, 39, 40 Of note, some changes described in rodent models have been confirmed in hearts of obese, insulin resistant and/or type 2 diabetic subjects, including increased FA utilization, decreased cardiac efficiency, increased mitochondrial ROS generation, and defects in mitochondrial respiratory function.30, 41-44
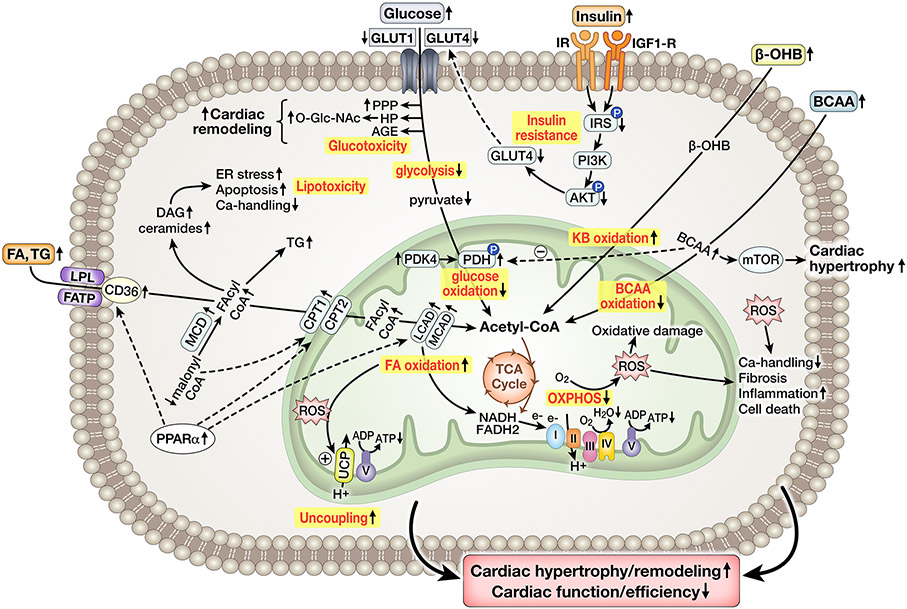
Figure 3: Simplified overview of cardiac energy metabolism in the Metabolic Syndrome.
Increased delivery of FA and TG, as well as activation of PPARα signaling, increase FA oxidation and generation of reactive lipid intermediates (acyl-CoA, ceramide, DAG). Glycolysis and glucose oxidation (GO) are decreased, contributed to by decreased glucose uptake due to impaired insulin stimulated glucose uptake. Early glycolysis intermediates are shifted into the PPP, AGE generation, and HBP pathway, driving O-Glc-Nacylation that could contribute to cardiac remodeling. KB oxidation may be increased, whereas BCAA oxidation is decreased, contributing to PDH inhibition and cardiac hypertrophy by mTOR activation. Mitochondrial ATP synthesis is impaired due to defects in the respiratory chain and ROS-mediated mitochondrial uncoupling. Mitochondrial and cytosolic ROS impair Ca2+ handling, induce fibrosis, promote inflammation and increase cell death. Collectively, impaired energetics contribute to cardiac remodeling, dysfunction and inefficiency.
Animal models of the metabolic syndrome
A variety of animal models exhibit dysregulated cardiac metabolism resulting from systemic changes associated with the MetS (summarized with detailed phenotypes in Supplemental Table S1). Specific mechanisms by which perturbed whole-body metabolism and changes in circulating levels of glucose, FA, cholesterol and insulin, influence cardiac energy metabolism, structure and function varies considerably between animal models.
Role of species differences
The murine and human genomes are similar in size, number of genes (>99%) and conservation of gene order.45 Since mouse strains are mostly inbred, genetic differences among these strains and also their sub-strains cause marked differences in systemic biochemical and metabolic phenotypes. These changes in turn may contribute to differences in the metabolic, structural and functional responses of the heart.46, 47 These inter-strain genetic differences have been exploited to explore the contribution of specific gene products to phenotypic abnormalities, including systemic dysmetabolism and their effects on cardiac function and structure.47, 48 Additional considerations such as choice of controls and effects of mixed backgrounds have been reviewed in detail elsewhere.47 Due to their size, measurement of some metabolic and cardiac parameters may be easier in rat models of MetS or cardiac disease. For both mice and rats, the age of animals and specific treatment regimens (e.g., type and duration of a diet) need to be considered among different rodent species. Thus, careful selection of the rodent model is advised depending on the research questions to be addressed, and care should be taken when extrapolating findings from rodent studies to humans with large genetic heterogeneity.
High-fat diet
High-fat diets utilized in rodent models primarily range from 45-60% of total energy (kcal) from fat sources, mainly from saturated FAs. In rats, short-term (3 weeks) feeding with high-fat diet (HFD) increases plasma FA and cholesterol levels, accompanied by obesity. Hearts display increased myocardial O2 consumption (MVO2) without an increase in cardiac function, resulting in decreased cardiac efficiency, likely related to FA-induced uncoupling of ATP synthesis from O2 consumption.37 Longer HFD feeding (7-8 weeks) induces systemic insulin resistance, accompanied by cardiac hypertrophy, and diastolic and mild systolic dysfunction; however, most HFDs fail to induce profound alterations in cardiac remodeling and function in rats. Increased FA uptake by relocation of CD36 to the plasma membrane and concurrent impairment in mitochondrial FA import and oxidation may result in FAs being shifted away from oxidation to storage.49 One study demonstrated increased FAO in response to 48 weeks of HFD (60%), whereas a Western diet with less fat (45%) initially increased FAO but finally decreased oxidation rates, accompanied by cardiac dysfunction.50 Selective cardiac dysfunction induced by Western diet may be related to inadequate stimulation of PPARα-driven expression of FAO genes, or inadequately increased FA-induced mitochondrial uncoupling.
In mouse models, 8-12 weeks of HFD increases body weight and circulating glucose, FA, cholesterol, TG and insulin levels.51 Similar to rats, minimal consequences on LV hypertrophy/remodeling and function are observed. While exhibiting enhanced FAO and reduced glucose oxidation, sustained mitochondrial function suggests cardiac energy status to be unaffected. Inhibition of PDH indicative of metabolic inflexibility may develop as early as day 1 of a HFD, although development of metabolic insulin resistance of the heart may require several weeks of HFD.52 Along with cardiac TG accumulation, increased content of reactive long-chain acyl-CoAs, ceramide, cholesterol, DAG and acylcarnitines is also observed, suggesting the presence of cardiac lipotoxicity in these models. With longer duration of HFD (> 20 weeks), myocardial levels of OXPHOS proteins may also decrease but do not seem to impair respiratory function or induce energy depletion, and ketogenic enzymes appear to be affected, although whether they are induced or repressed appears to be conflicting.53 In some studies cardiac hypertrophy and dysfunction, systolic dysfunction is absent following long-term HFD,54 although in other studies LV dysfunction has been reported.55 The basis for these disparities is not understood. Additional mechanisms such as increased oxidative stress, apoptosis, fibrosis and impaired Ca2+-handling may contribute to cardiac remodeling and functional defects in these models.
High-sugar or High-fat/high-sugar diet
Unlike HFD, high-sugar diets (high-sucrose or high-fructose) do not appear to induce obesity in rats in the first 9-12 weeks, but only beyond 30 weeks.56 However, hyperlipidemia and systemic insulin resistance are observed early on. High-sugar diets induce systolic and diastolic dysfunction in some studies, although the development of cardiac hypertrophy and myocardial fibrosis appears to be less obvious.56 Reduced cardiac ATP/ADP ratio and PCr content suggests an energetic deficit, possibly caused by reduced expression of genes involved in FA transport and oxidation. An association with increased cardiac lyso-phosphatidyl choline and FA content was also observed, which may induce cardiomyopathy by increasing apoptosis.
Increasing the fat content of the high sugar diet in mice, is insufficient to induce an overt cardiac phenotype following short-term feeding (1-5 weeks). However, longer (> 4 months) duration may result in the development of cardiac hypertrophy, dysfunction, and impaired contractile reserve.57 This diet induces a pattern of substrate utilization that mimics changes observed in longstanding obesity or T2DM, characterized by an early reduction of cardiac glycolysis and glucose oxidation rates (likely mediated by decreased GLUT4 content and translocation), increased FAO rates, and blunted cardiac metabolic response to insulin, accompanied by impaired ATP regeneration indicative of myocardial energy deprivation.58 The latter may be related to oxidative damage of energy metabolic proteins involved in FA utilization, glycolysis, TCA cycle or OXPHOS. As in HFD models, the early decrease in glucose utilization may be the initial molecular defect leading to a shift in the pattern of myocardial substrate oxidation.
Chemical-induced models of diabetes and MetS
Despite potential toxic side effects, chemical-induced diabetes may be a convenient model when exploring effects in rodents with genetic manipulations. Treatment with the beta-cell toxin, streptozotocin (STZ), leads to insulin-deficient diabetes with hyperglycemia and hyperlipidemia but not obesity. Cardiac size is reduced in STZ-treated mice, likely due to the increased protein degradation resulting from insulinopenia, and cardiac contractility is often impaired.59 In most studies, hearts typically display reduced glucose uptake, increased FA uptake and oxidation, and increased incorporation of FA into cardiac phospholipids, TG and FA.60, 61 When FA utilization is increased, MVO2 tends to increase as well, leading to reduced cardiac efficiency.36 STZ-induced diabetes increases circulating ketone body (KB) levels, particularly the ratio of βOHB to acetoacetate. While it remains unclear whether cardiac βOHB uptake is increased or decreased in STZ-induced diabetes, reduced KB utilization observed in some studies may be related to decreased expression and activity of BDH1, or to tyrosine nitration of SCOT.53 Of note, STZ treatment has also been combined with HFD in few studies to model severe, uncontrolled type 2 diabetes and to recapitulate additional traits (e.g. obesity, insulin resistance) of that characterize human type 2 diabetes in this model.
Genetic models of MetS
Several genetic animal models have provided useful insight to understanding the interaction between perturbed systemic metabolism, altered cardiac substrate utilization and development of cardiac dysfunction. Ob/ob mice carry a recessive mutation in the leptin gene resulting in deficiency of a functionally active leptin protein. Compared to their lean counterparts, ob/ob mice develop obesity and systemic insulin resistance as early as 4 weeks of age, with progressive increases in FA, TG and insulin levels as they age. The cardiac phenotype includes development of cardiac hypertrophy, variable degrees of systolic dysfunction, increased FAO and reduced glucose oxidation as early as 4 weeks of age.34 Studies using Langendorff heart perfusions demonstrated that the presence of palmitate in the perfusion buffer (but not glucose alone) resulted in reduced cardiac efficiency, associated with impaired ATP synthesis and less ATP generated per oxygen consumed when subsequently measured in saponin-permeabilized myocardial fibers, suggesting that FA-induced mitochondrial uncoupling may have reduced cardiac efficiency.37 In addition, ob/ob hearts display metabolic insulin resistance, evidenced by the inability of insulin to increase cardiac glucose utilization, likely due to impaired AKT signaling and subsequent GLUT4 translocation, and to suppress FA oxidation.33 Altogether, studies in ob/ob hearts revealed impaired metabolic flexibility and impaired conversion of ATP into mechanical work as a central characteristic of obesity and diabetes-associated cardiomyopathy .
Db/db mice carry a leptin receptor defect and on a susceptible genetic background, are characterized by obesity, hyperglycemia, increased circulating FA, TG, cholesterol and insulin, and impaired glucose tolerance as early as 8 weeks of age. In most studies, hearts were reported to be hypertrophied with some degree of systolic and diastolic dysfunction.36 Similar to ob/ob mice, myocardial FA uptake, FAO and also TG accumulation are increased, whereas basal glycolysis, glucose oxidation, and insulin-stimulated glucose utilization are impaired. FA-induced mitochondrial uncoupling and impaired cardiac efficiency was also confirmed in db/db hearts.32 Furthermore, db/db hearts displayed impaired ATP regeneration, in part from reduced expression and posttranslational modification (acetylation, deamidation, oxidation) of OXPHOS subunits thereby supporting the concept that mitochondria are important targets of diabetes-induced cardiac injury.
Another commonly used genetic rodent model of obesity is the Zucker fatty (ZF) rat which carries a non-functioning leptin receptor secondary to a missense mutation in the leptin receptor gene. These rats are characterized by obesity and increased serum levels of FA, TG and insulin. Selective breeding of hyperglycemic ZF rats yielded the inbred sub-strain of Zucker diabetic fatty rats (ZDF rats), which show early insulin resistance and later (10-12 weeks) develop, hypoinsulinemia and hyperglycemia in contrast to ZF rats. ZF rats develop cardiac hypertrophy, whereas cardiac function was reported to be either reduced or increased.62 Increased FA uptake is associated with increased TG storage, FA oxidation is not to increased, which may explain the absence of impaired cardiac efficiency in this model. In ZDF rats, cardiac hypertrophy is more consistently accompanied by contractile defects, and rates of FA oxidation are increased, whereas glucose oxidation was lower.63 ZDF rats have also been crossed with spontaneously-hypertensive (SHR) rats (ZSF1), a model with similar systemic metabolic abnormalities as observed in ZDF rats with additional hypertension that develop cardiac hypertrophy and diastolic dysfunction. While data on actual metabolic rates in hearts of ZSF1 rats are lacking, pathway analysis of the cardiac transcriptome revealed enrichment of genes encoding for enzymes of FA and BCAA utilization, including induction of CPT-1 and PDK4, suggesting increased FAO and decreased glucose oxidation as observed in ZDF rats.64
When comparing mouse and rat models of impaired leptin action, the ZF rat is the only model that does not develop hyperglycemia, thus allowing analysis of the long-term effects of obesity without confounding effects of hyperglycemia. Relative to ob/ob and db/db mice, serum lipid levels appear to be altered more dramatically in the ZDF rat. Given that ZF rats are an outbred strain with genetic heterogeneity, the model may more closely resemble the human disease compared to inbred mouse and rat strains. Of note, in all mouse and rat models with leptin deficiency or leptin receptor dysfunction, specific effects on the systemic and cardiac phenotype that could be a direct consequence of impaired leptin action cannot be excluded.65
Another genetic model of elevated blood glucose is the OVE26 mouse, in which overexpression of calmodulin in pancreatic beta cells causes cellular damage, insulin-deficient diabetes and hypertriglyceridemia. While data on substrate oxidation rates are lacking, unchanged levels of GLUT4 and insulin-stimulated AKT phosphorylation but increased phosphorylation of the PDH subunit E1 and impaired rates of pyruvate-supported respiration suggest intact myocardial insulin signaling but impaired glucose oxidation in this model.66 In fact, impaired mitochondrial respiration with glutamate or succinate indicates a general oxidative defect, likely related to oxidative stress. While OVE26 mice display cardiac hypertrophy without functional impairment at 14 weeks of age, dilated cardiomyopathy becomes apparent at 6 months of age.
The βV59M mouse develops insulin deficiency due to beta-cell specific expression of a human activating KATP channel mutation. Of note, insulin deficiency and hyperglycemia develop 24h after transgene induction, whereas hyperlipidemia does not develop until 4 weeks following transgene induction.67 In the absence of hyperlipidemia, hearts of βV59M mice have decreased cardiac output and show decreased PDH flux, while FA content was decreased and content of glycogen or lipid was unchanged, suggesting ATP production preferentially from FAO and uncoupling of glycolysis from glucose oxidation. Proteomics revealed increased expression of PDK4 and of proteins involved in FAO and lipolysis, while expression of LDH, and glycolytic and TCA cycles enzymes were reduced. Thus, compared to other models, βV59M mice develop changes in cardiac function and metabolism that are at least in part independent of increased circulating FA. Akita mice develop insulin-deficient diabetes secondary to a defect in insulin processing. Hearts of these mice exhibit reduced glucose utilization, increased FAO myocellular lipid accumulation and mitochondria dysfunction, despite preserved insulin sensitivity.68, 69
Of note, models have been generated that combine metabolic traits of MetS with risk for spontaneous atherosclerosis, to more closely parallel human MetS. Recently, ApoE-KO mice were crossed with hypertensive BPH/2J Schlager mice (BPHxApoE-KO), which display elevated blood pressure, impaired glucose tolerance, and increased serum levels of cholesterol, TG, FA and insulin upon feeding a Western diet.70 Data on cardiac function and energy metabolism remain to be reported.
Lessons learned from genetic models of altered cardiac metabolism or metabolic signaling
Numerous models with global or cardiomyocyte-restricted genetic manipulations of energy metabolic proteins or their regulators have been generated to mimic specific traits of metabolic disease and CVD, or to allow insights into their role in metabolic and cardiovascular pathologies. These models will be briefly discussed below, sorted by their attribution to specific biological pathways (Table 1). Detailed phenotypes can be found in Supplemental Table S2. While genetic models provide valuable insights into pathophysiologic mechanisms, it remains a challenge to relate a genetic model (where a protein/enzyme has been completely deleted) to a pathological condition relevant to humans. Thus, studying animal models of human disease, as opposed to diseased animal models, would be generally preferable. At this point, we would also like to remind the reader that transcriptional changes do not always correlate with protein levels and/or protein function or enzyme activity. This is particularly true in energy metabolism and requires careful interpretation of data provided in tables and throughout the manuscript. We also refer the reader to a scientific statement from the American Heart Association elaborating on methods and models that can be used to investigate cardiac metabolism.71 Finally, this review focuses mainly on metabolic pathways in cardiomyocytes. Insights in non-myocytes such as endothelial cells72 and fibroblasts as recently reviewed73 are now emerging and will likely contribute additional perspectives in the future.
Table 1:
Overview of genetically engineered mouse models mimicking or exhibiting dysregulated cardiac metabolism.
| Metabolic Pathway | Loss-of-function | Gain-of-function |
|---|---|---|
| FA uptake | CD36-KO, cmCD36-KO (+ aging); ACSL1-KO, cmACSL1-KO | CD36-OE (in SHR rats), cmFATP1-OE, ACSL1-OE, cmACSL1-OE |
| Triglyceride synthesis and lipolysis | cmDGAT1-KO; ATGL-KO (+ STZ), cmATGL-KO; LPL-KO, cmLPL-KO | cmDGAT1-OE; cmATGL-OE (+ HFD/high-sugar diet or STZ); LpL-OE, cmLpL-OE |
| Mitochondrial FA transport | ACC2-KO, ACC2-KI (mutant), cmACC2-KO; MCD-KO (+ HFD or aging); CPT1b-KO | L-CPT1-OE |
| FA oxidation: | VLCAD-KO, cmVLCAD-KO; LCAD-KO | |
| Regulators of energy metabolism | PGC1α-KO (+ HFD or in ob/ob mice); PGC1β-KO; PGC1α/β-KO (in ob/ob mice), cPGC1α/β-KO; TFAm-KO, cmTFAm-KO; POLGA-KO, cmMTERF3-KO; cmTFB1M-KO; NRF1-KO, efNRF2-KO; ERRα-KO, ERRγ-KO, cm ERRγ-KO, ERRα/cmERRγ-KO; PPARα-KO (+ STZ or HFD or in UCP-DTA mice); cmPPARδ-KO; PPARγ-KO, cmPPARγ-KO; AMPKα2-KO (+ calorie restriction); AMPKα2-OE (mutant), AMPKγ2-OE (mutant: N488I) | cmPGC1α-OE; TFAm-OE; cERRγ-OE; cmERRγ-OE; cmPPARα-OE (+ HFD or STZ); cmPPARγ-OE |
| Glucose utilization | PDK4-KO (+ HFD); cmPDK4-KO; cmGLUT1-KO, GLUT4-KO, cmGLUT4-KO (+ exercise), cmMPC1-KO, cm-MPC2-KO | cPDK4-OE; cmGLUT1-OE (+ HFD), GLUT4-OE (in db/db mice), cmGLUT4-OE (+ STZ); cPFK2-OE |
| Insulin/IGF1 signaling | CIRKO (+ exercise or STZ), cIRS1/2-KO, cIRS1-KO (+ exercise), cIRS2-KO (+ exercise); cmPI3K-KO; AKT1-KO (+ exercise), AKT2-KO (+ exercise), cmAKT-KO; cmIGF1R-KO (+ aging or + exercise); CIGF/IR-KO | cPI3K-OE; cmAKT-OE; IGF1R-OE; IGF-IIRα-OE |
| Ketone body utilization | cmBDH1-KO; SCOT-KO, cmSCOT-KO | cmBDH1-OE |
| BCAA utilization | cmBCATm-KO; KLF15-KO; PP2Cm-KO | SLC25A44-OE |
| Posttranslational regulation | SIRT1-KO (+ exercise), SIRT1-KO (+ HFD), SIRT3-KO (+ STZ or HFD/DOCA or HFD/L-NAME), ecSIRT3-KO, CrAT/SIRT3-KO; SIRT4-KO; SIRT5-KO, cmSIRT5-KO; SIRT6-KO, cmSIRT6-KO; SIRT7-KO, cmSIRT7-KO; cmOGT-KO, cmOGA-KO | SIRT1-OE, SIRT1/PPARα-OE, SIRT3-OE (in db/db mice); SIRT4-OE; SIRT6-OE (+ HFHS diet), cmSIRT6-OE |
| Mitochondrial dynamics | MFN1/2-KO, cmMFN1/2-KO, cmMFN1-KO, cmMFN2-KO; cmDRP1-KO; OPA1-KO; cmTME1L-KO; DRP1/MFN1/2-KO | cmDRP1-OE |
See extended version and references in Supplemental Table S2.
Abbreviations: ACC, acetyl-CoA carboxylase; AKT, protein kinase B; AMPK, adenosine monophosphate-activated protein kinase; ACSL, acyl-CoA synthetase, long chain; BCAA, branched chain amino acid; BCATm, branched-chain aminotransferase, mitochondrial; BDH, β-hydroxybutyrate dehydrogenase; CD36, fatty acid transporter; cm, cardiomyocyte; CIRKO – cardiomyocyte KO of insulin receptor, cIRS(1/2)1KO cardiomyocyte KO of insulin receptor substrates (1/2); CPT, carnitine palmitoyltransferase; CrAT, carnitine palmitoyl transferase; DGAT, diacylglycerol acyltransferases; DOCA, deoxycorticosterone acetate; DRP, dynamin-related protein; EF, embryonic fibroblast; ERR, estrogen-related receptor ; FA, fatty acid; GLUT, glucose transporter; IGF, insulin-like growth factor; IGF1R, IGF-1 receptor; IR, insulin receptor; KI, knock-in; KLF15, Krüppel-like factor 15 ; KO, knockout; L-CPT, CPT liver isoform; LCAD, long-chain acyl CoA dehydrogenase; MCAD, medium-chain acyl-CoA dehydrogenase; MCD, malonyl-CoA decarboxylase; MFN, mitofusin; NRF, nuclear respiratory factor; MPC, mitochondrial pyruvate carrier; OE, overexpression; OGA, O-GlcNAcase; OGT, O-GlcNAc Transferase; OPA, optic atrophy; PDK, pyruvate dehydrogenase kinase; PFK, phosphofructokinase; PGC,1 PPARγ co-activator 1; PI3K, phosphoinositide 3-kinase; POLGA, DNA polymerase gamma, subunit A; PP2Cm, protein phosphatase 2C , mitochondrial; PPAR, peroxisome proliferator-activated receptor; SCOT, succinyl-CoA:3-oxoacid-CoA transferase; SIRT, sirtuin; SLC, solute carrier family; STZ, streptozotocin; TFAm, mitochondrial transcription factor A; TFB1M, transcription factor B1; UCP, uncoupled protein; VLCAD, very long-chain acyl-CoA dehydrogenase; YME1L, ATP-dependent metalloprotease
FA uptake and activation:
Several models have been engineered to explore the mechanisms of cardiac FA uptake and activation. Mice with CD36 deficiency revealed that CD36 mediates a major fraction of the FA uptake by myocardial tissue and is required to maintain oxidative ATP regeneration and cardiac function in response to chronic pressure overload.74, 75 However, in the aged heart, increased CD36 levels may promote myocardial lipid accumulation and cardiac dysfunction.76 Studies in mice with combined CD36 deficiency and PPARα overexpression showed that CD36-mediated FA uptake is necessary for PPARα-driven lipotoxicity, and knockdown of CD36 was shown to protect from HFD-induced lipid accumulation, oxidative stress and cardiac dysfunction, collectively suggesting that inhibiting CD36-mediated FA uptake may be a promising strategy to prevent FA-induced cardiac dysfunction observed in obesity and diabetes.77, 78 Significance of FATP for FA uptake and oxidation was underscored by studies in mice with cardiomyocyte-selective overexpression of FATP, which consequently develop diastolic dysfunction.79 Studies in mice with deficiency or overexpression of ACSL1 provided evidence that ACSL1 also regulates FA uptake but additionally activates FAs for mitochondrial oxidation by intracellular trapping of acyl units via esterification to CoA.10, 80-82 Since increased FA uptake by ACSL1 overexpression in cardiomyocytes dose-dependently induced hypertrophy or dilated cardiomyopathy, this model evolved as a convenient model to study consequences of cardiac lipotoxicity, including ROS-driven perturbation of mitochondrial dynamics as a novel mechanism of lipotoxicity.10 Recent analysis of low-level ACSL1 overexpression suggested that these mitochondrial adaptations could redirect long-chain Acyl CoAs into cardioprotective ceramides, which limited LV remodeling following pressure overload.83
Mitochondrial FA import and oxidation:
Models with deficiency of FAO enzymes provided insights into underlying mechanisms of cardiomyopathy observed in some humans with inherited disorders of FAO. Studies in VLCAD−/− and LCAD−/− mice suggest energy deficiency due to impaired FAO and TCA cycle anaplerosis, and/or lipotoxicity due to accumulation of lipid intermediates such as ceramides as underlying mechanisms of associated cardiomyopathy.84, 85 Studies in mice with increased activity of LPL on the surface of cardiomyocytes revealed that LPL can increase uptake and storage of lipids derived from circulating TG, which may lead to cardiomyopathy.86 Conversely, cardiomyocyte-restricted knockout of LPL was necessary to maintain cardiac FAO and contractile function, collectively indicating a physiological role of LPL in regulating cardiac energy metabolism and function.87
Allosteric inhibition of CPT1B by malonyl-CoA is also a potent mechanism to inhibit FAO in the heart, as demonstrated in a knock in mouse model expressing the CPT1BE3A mutant enzyme, which has reduced sensitivity to malonyl-CoA.88 While ACC2 catalyzes the conversion of acetyl-CoA to malonyl-CoA, the reverse reaction is driven by MCD. Models with deletion of ACC or MCD have provided valuable insights into cardiac metabolism. Mice with ACC2 deletion confirmed that decreasing malonyl-CoA levels disinhibits CPT1B and thereby increases myocardial FAO. Of note, this metabolic intervention resulted in a correction of the typical switch towards increased glucose utilization observed following TAC and attenuated cardiac hypertrophy, suggesting that the reversion to the fetal metabolic profile observed in pathological hypertrophy is not necessarily beneficial.89 Of note, while AMPK-mediated phosphorylation and inhibition of ACC has been proposed to increase FAO, studies in mice with mutated AMPK phosphorylation sites on ACC1 and ACC2 showing no effect of FAO rates suggest that AMPK-dependent inactivation of ACC may not be essential for the control of myocardial FAO and subsequent cardiac function during conditions of stress known to activate AMPK such as increased workload or myocardial ischemia.90 Mice with MCD deletion revealed increased glucose oxidation and cardioprotection following ischemia, suggesting chronic pharmacological MCD inhibition as a therapeutic strategy for cardioprotection.91 In diet-induced obesity, intracellular accumulation of lipid intermediates is thought to contribute to insulin resistance in skeletal and cardiac muscle. However, using mice with germline deletion of MCD paradoxically revealed that despite reduced induction of FAO and accumulation of FA intermediates insulin-stimulated glucose oxidation was not impaired in response to diet-induced obesity.92 These findings imply that myocardial accumulation of lipid intermediates might not be sufficient to impair glucose utilization and that inhibition of FAO by MCD inhibition may instead have therapeutic potential in preventing obesity-induced insulin resistance in the heart. Of note, inhibition of FAO in mice with CPT1B deficiency leads to accumulation of TG and ceramides in the heart, and this lipotoxicity may cause aggravation of cardiac hypertrophy and dysfunction of these mice in response to pressure overload.93 Thus, underlying cardiac disease mode of FAO inhibition and potentially differences in lipid species that accumulate, may determine whether beneficial or detrimental effects will be achieved by interrogating FA utilization. Expression of the liver isoform of CPT1 (CPT1a) is induced in the failing heart and adenoviral overexpression of CPT1a in rat hearts reduced rates of FAO, indicating that reduced FAO in heart failure might be due in part to CPT isoform switching.94
Triglyceride synthesis and lipolysis:
Fluctuations in myocardial TG content reflect or induce changes in cardiac energy metabolism. Simultaneous cardiomyocyte-selective deletion of diacylglycerol acyl transferase 1 and 2 (DGAT1 and 2) suppressed TG turnover and synthesis, demonstrating the significance of DGATs in the synthesis of cardiac TG.95 In mice with increased FA uptake due to ACSL1 overexpression, simultaneous overexpression of DGAT1 lead to increased TG storage, lowering of toxic lipid intermediates and improvement of cardiac function, indicating a protective role of TG in states of lipid overload.96 In contrast, lipid accumulation due to impaired lipolysis in adipose triglyceride lipase (ATGL)-deficient mice lead to a strong reduction of PPARα–regulated gene expression, defects in mitochondrial substrate oxidation, severe cardiac dysfunction and premature death. Treatment with a PPARα agonist reversed mitochondrial defects and normalized cardiac dysfunction, indicating that energy depletion due to impaired PPARα/PGC-1α signaling may have caused cardiomyopathy.97, 98 Collectively, these findings revealed a potential treatment for humans with neutral lipid storage disease and showed that endogenous or exogenous FA may first need to undergo esterification to TG and subsequent hydrolysis before becoming signaling lipids capable of activating PPARα. Of note, ATGL overexpression also protects from TG accumulation and cardiac dysfunction in models of obesity, diabetes, and pressure overload hypertrophy, by decreasing reliance of FAO on exogenous lipid and normalizing increased PPARα signaling.99. Thus, the balance between oxidation of exogenous versus endogenous TG play an important role in the pathogenesis of lipotoxic cardiomyopathy.
Regulators of energy metabolism
Several transcriptional activators and coactivators participate in the regulation of cardiac energy metabolism. Transgenic mouse models of the PPARγ coactivators (PGC1s), PGC1α and PGC1β, provided insights into their role in cardiac physiology and metabolism. PGC-1 coactivators appear to be particularly important during postnatal growth since mice with combined deletion of PGC-1α and PGC-1β develop severe defects in mitochondrial dynamics and respiratory function, leading to contractile dysfunction, dilated cardiomyopathy and premature death .100 During adulthood, although PGC1α may be required to maintain mitochondrial ATP-regenerating capacity by sustaining expression of genes involved in OXPHOS, TCA cycle and FAO, PGC1α KO mice do not develop contractile deficits under physiologic conditions.18, 21, 101 However, PGC-1α deficiency blunts the increase in ATP turnover and limits the increase in cardiac contractility following dobutamine stress and accelerates the progression to heart failure following TAC.18, 21 The latter phenotype may additionally be related to impaired induction of antioxidant gene expression in PGC1α-deficient mice as evidenced by attenuation of cardiac dysfunction in response to mitochondria-targeted ROS scavenging in this model. 102 Furthermore, PGC1β deficiency induces a shift towards decreased FAO and increased GO, which has been proposed to mediate the metabolic switch towards glucose utilization observed in pressure overload induced cardiac hypertrophy or models of heart failure.101 Mice deficient in PGC1β also show decreased OXPHOS expression and respiratory dysfunction, and accelerated progression towards heart failure in response to TAC, related to increased oxidative stress and the failure to increase glucose utilization.103
Models with gain-of-function of PGC-1α provided evidence that PGC-1 coactivators can impressively stimulate mitochondrial biogenesis with a rapid and profound increase in mitochondrial mass, although accompanied by structural abnormalities of mitochondria and unexpected dilated cardiomyopathy.12, 104 This phenotype may be related to uncoordinated mitochondrial biogenesis due to strong activation of PGC-1α signaling or simply to excess mitochondrial mass within cardiomyocytes that spatially interferes with the contraction process. Thus, models with genetic manipulation of PGC coactivators support their role in regulating OXPHOS capacity, mitochondrial biogenesis, ROS homeostasis, and energy substrate preference in the heart. They also promote the postnatal increase in cardiomyocyte mitochondrial content and the switch from glycolysis towards predominant oxidation of FA for cardiac ATP regeneration. However therapeutic strategies utilizing PGC-1 stimulation to increase mitochondrial biogenesis in the heart may depend on the extent and speed of PGC-1 stimulation (reviewed in 105).
Both PGC-1α and PGC-1β mediate effects on gene transcription and mitochondrial biogenesis by coactivation of transcription factors, predominantly including estrogen-related receptors (ERRα, ERRγ), nuclear respiratory factors (NRF1, NRF2) and mitochondrial transcription factor A (TFAm). While mice with homozygous deletion of TFAm show embryonic lethality due to severe mtDNA depletion and abolished OXPHOS activity, heterozygous deletion or conditional deletion in heart and muscle reduce mtDNA copy number, impaired transcription of mitochondria-encoded OXPHOS subunits and precipitated respiratory dysfunction, and dilated cardiomyopathy with lethality within one week of deletion. In contrast, mice with overexpression of human TFAm gene show increased mtDNA copy number, but no alterations of OXPHOS function or cardiac structure and function. Deficiency of additional factors regulating transcription and replication of mtDNA (mtDNA polymerase gamma, mitochondrial transcription termination factor 3, mitochondrial transcription factor 1 B) phenocopy TFAm deficiency such as embryonic lethality, mtDNA depletion, impaired OXPHOS expression, structural abnormalities, and cardiomyopathy, although not coactivated by PGC-1 coactivators (for specific details on these models see Supplemental Table S2).
NRF1 and NRF2 promote the expression of OXPHOS genes and frequently work in conjunction to optimize mitochondrial oxidative capacity. Mice lacking NRF1 established their essential role in the maintenance of mtDNA and respiratory chain function during early embryogenesis.106 Mice with loss of NRF2 in mouse embryonic fibroblasts displayed decreased mitochondrial mass, ATP production, oxygen consumption, and mitochondrial protein synthesis without changing mitochondrial morphology or the expression of several genes previously reported to be NRF2 targets.107 Effects of deletion of NRF1 or 2 in the heart and on cardiac energy metabolism have not been reported to our knowledge.
The orphan receptors, estrogen-related receptors (ERRs) transcriptionally regulate expression of genes involved in mitochondrial biogenesis and substrate utilization.108 Mice with combined deficiency of ERRα and ERRγ show markedly decreased expression of FAO and OXPHOS genes, a strong defect in OXPHOS complex activities, display cardiac energy depletion, and die from dilated cardiomyopathy within the first month of life, illustrating that the ERR transcriptional pathway is essential for energy metabolism and maintenance of cardiac function.109 In addition, expression of genes involved in FA oxidation, glucose uptake, TCA cycle, OXPHOS, and phosphate transfer is dysregulated in ERRα−/− hearts at baseline and, in part, to a greater degree following pressure overload, thus identifying ERRα (a downstream component of PGC-1α signaling) as an important regulator of the bioenergetic and functional adaptation of the heart to hemodynamic stress imposed by pressure overload.108 Mice with germline deletion of ERRγ display neonatal cardiac defects and die within 48 hours, which may be related to dysregulated expression of genes involved in OXPHOS and FA uptake and oxidation in the embryonic heart. These findings imply that ERRγ may govern the postnatal transition towards oxidative metabolism in the heart.110 Of note, cardiomyocyte-restricted ERRγ overexpression also induces early lethality due to dilated cardiomyopathy, which correlates with the extent of ERRγ overexpression. Expression of FA oxidation and OXPHOS genes was (unexpectedly) not significantly increased within the first three weeks of life in these mice but markedly repressed after onset of cardiac dysfunction. These studies underscore the importance of stoichiometry in transcription factor regulation of mitochondrial metabolism and biogenesis, as similarly observed in PGC-1 transgenic models.
PPARα is a nuclear hormone receptor and serves as the major transcriptional regulator of cardiac FA utilization by binding to response elements, predominantly in a complex with RXRα and coactivated by PGC-1α.111 In global PPARα-KO mice, fasting induced TG accumulation in liver and heart due to inadequate induction of FA oxidation genes, thus defining a critical role for PPARα in a transcriptional regulatory response to fasting and identifying PPARα−/− mice as a useful model of inborn and acquired abnormalities of human fatty acid utilization. In the heart, PPARα deficiency markedly impairs FAO and reciprocally increases glucose and lactate utilization, however this switch in substrate utilization is unable to maintain high-energy phosphate content under high workload conditions and thus leads to contractile failure, unless glucose uptake and utilization is further increased by overexpression of GLUT1 .112 These findings from PPARα−/− mice revealed that adult hearts with decreased capacity for FAO may be more susceptible to progressive deterioration of cardiac function during hemodynamic overload, and that metabolic interventions aiming at increasing glucose utilization may prevent energy depletion and cardiac deterioration in the setting of hemodynamic stress or pathological cardiac hypertrophy. Conversely, mice overexpressing PPARα specifically in cardiomyocytes showed increased myocardial FA uptake and oxidation, enhanced lipid content, a reciprocal decrease in glucose utilization, and impaired insulin-stimulated glucose uptake, thus serving as a model that mimics the cardiac metabolic phenotype commonly attributed to rodent or human diabetic cardiomyopathy.113
Other members of the PPAR family, PPARδ and PPARγ, also participate in myocardial lipid utilization. PPARδ deletion in cardiomyocytes impairs FA oxidation and increases myocardial lipid accumulation, leading to heart failure and reduced survival.114 This model revealed PPARδ as an important determinant of myocardial FA utilization and its necessity to maintain energy balance and cardiac function. In addition, mice with inducible PPARδ deletion in cardiomyocytes showed a strong suppression of mitochondrial biogenic signaling and evidence of oxidative stress, elucidating an additional role of PPARδ in mitochondrial protection and biogenesis in the heart.115 Germline knockout of PPARγ leads to embryonic lethality, however mice with cardiomyocyte-specific deletion of PPARγ are viable but develop cardiac hypertrophy with preserved systolic function, due in part to NFkB activation.116 While data on substrate metabolism are not available from this study, mice overexpressing PPARγ have increased myocardial TG uptake, FA oxidation, and lipid accumulation, but GLUT4 expression and glucose uptake were not decreased, and glycogen was even increased, accompanied by development of dilated cardiomyopathy.117 Thus, genetic modulation of PPARγ revealed that PPARγ is necessary to balance cardiac growth, and that overactivation results in cardiac glucolipotoxicity due to inappropriately high storage of TG and glycogen, potentially explaining cardiotoxic side effects of PPARγ agonist treatment in humans.
AMPK exists as a heterotrimeric complex consisting of a catalytic subunit α and two regulatory β and γ subunits and is activated by an increased AMP/ATP ratio or by phosphorylation of upstream regulators such as LKB1 or CaMKKIIb (reviewed in detail elsewhere118). In the heart, AMPK functions primarily as a metabolic sensor to coordinate anabolic and catabolic activities but can also affect other cellular processes such as mitochondrial function, posttranslational modifications, autophagy, ER stress, or apoptosis. AMPKα2-KO mice or mice with a kinase-dead or dominant-negative mutation of the α2 catalytic subunit show only mild or no defect in cardiac function under non-stressed conditions. Although AMPKα2 may be necessary to sustain mitochondrial cardiolipin content and OXPHOS complex I activity, effects on myocardial substrate utilization seem to be minor under non-stressed conditions, and unchanged ATP content and PCr/ATP ratio imply negligible compromise of myocardial energy status.119, 120 Instead, activation of AMPK in cardiomyocytes stimulates FAO, glucose utilization and mitochondrial biogenesis in cardiac muscle during conditions of unmatched energy demand such as exercise, ischemia, and pressure overload, indicating a predominant role of AMPK in regulating the adaptation of myocardial energetics during stress.120, 121 In contrast to the α2 subunit, transgenic mice with cardiomyocyte expression of a γ2 subunit (N488I) resulting in aberrant induction of AMPK activity, showed selective glycogen storage in concert with increased FAO and cellular glucose entry, and revealed that activation of mTOR and inactivation of FoxO signaling may underlie excessive hypertrophy in this model.122, 123 This model thus provided mechanistic insights into human glycogen storage cardiomyopathy induced by γ2 subunit mutation.
Glucose utilization:
Uptake and utilization of serum glucose requires trans-sarcolemmal transport of glucose via glucose transporters, as evidenced by data generated in mice with genetic manipulation of GLUT1 or GLUT4.124, 125 Increasing insulin-independent glucose uptake in mice with lifelong cardiomyocyte-selective overexpression of GLUT1 protects hearts with pressure-overload induced hypertrophy against contractile dysfunction and LV dilation, thereby providing experimental data supporting therapeutic strategies aiming at increasing glucose utilization in failing hearts.126 Interestingly, short-term induction of GLUT1 at start of pressure overload was not sufficient to prevent LV contractile dysfunction, despite beneficial effects on mitochondrial, metabolic and structural remodeling, indicating that attenuation of structural and mitochondrial remodeling is offset by glucose-dependent mechanisms that limit contractility underscoring that optimal levels of increased glucose utilization may need to be defined to avoid detrimental effects of excess glucose availability.127 In addition, increased FA availability as during high fat feeding may interfere with metabolic flexibility in hearts with increased GLUT1 expression and thus render the heart susceptible to contractile dysfunction.128
Cardiomyocyte-specific GLUT4-KO abolishes insulin-stimulated glucose uptake but leads to compensatory increase in GLUT1-mediated basal glucose uptake.129 While these metabolic changes only induce compensated hypertrophy, subjecting these mice to TAC exacerbated cardiac hypertrophy and promoted cardiac dysfunction, indicating that GLUT4 is required for maintenance of cardiac function and structure in response to chronic pressure overload.130 Maintaining myocardial glucose and normalizing FA utilization via GLUT4 overexpression prevented cardiac dysfunction in transgenic db/db-hGLUT4 mice, providing evidence that altered energy metabolism contributes to cardiac dysfunction in models of type 2 diabetes.131 Combining cardiac GLUT4 overexpression with models of diabetes was also useful to reveal that increasing glucose utilization may suppress cardiac ketone utilization, and induce glucose- and O-GlcNAc-mediated mitochondrial dysfunction, thus identifying mitochondria as a target of glucotoxicity.53, 132
Insights into the relationship between glycolytic flux and cardiac function were provided by mice with cardiomyocyte-specific overexpression of kinase-deficient PFK2, an important positive regulator of glycolysis. This mutation reduced glycolysis rates as expected but also revealed impaired insulin mediated Akt activation suggesting a direct link between glycolytic metabolism and Akt activation. Impaired insulin action could also be related to the accumulation of UDP-GlcNAc indicating increased flux into the hexosamine biosynthetic pathway which originates upstream of fructose-1,6-bisphosphate. The redirection of glycolysis intermediates in this model contributed to mild hypertrophy and impaired contractility.133
The ability of PDKs (predominantly PDK4 in the heart) to inhibit glucose oxidation by PDH phosphorylation has been evidenced in mice with cardiomyocyte-specific overexpression of PDK4.134 Conversely, mice with germline or conditional KO of PDK4 exhibit increased glucose oxidation and decreased FAO resulting in attenuation of both acute IR injury and postinfarction remodeling, with the latter beneficial effect being related to induction of cardiomyocyte proliferation. These studies underscore myocardial metabolism as a target for cardioprotective therapeutic strategies.
Oxidation of pyruvate by PDH requires mitochondrial pyruvate import. Recent studies revealed the significance of the mitochondrial pyruvate carrier (MPC) for TCA cycle metabolism of glucose-derived substrates and its role in cardiac structure and function. Cardiomyocyte-restricted deletion of subunit 1 of MPC (cmMPC1-KO) leads to age-related cardiac hypertrophy that progresses to dilated cardiomyopathy, associated with accumulation of upstream metabolites such as lactate, pyruvate and glycogen, and increased protein O-GlcNAc.135 Interestingly, bypassing MPC1 by providing non-glucose substrates (KB, HFD) reversed the structural, metabolic and functional remodeling of cmMPC1-KO hearts by reducing flux of glucose-derived metabolites into pathways such as the hexosamine biosynthetic pathway that amplified ventricular remodeling. Similar findings were obtained in mice with cardiomyocyte-selective deletion of MPC2 (cmMPC2-KO) or mice with combined deletion or reduction of MPC1 and MPC2,136, 137 whereas overexpression of MPC attenuated isoproterenol-induced cardiac hypertrophy.137 Moreover, increasing lactate conversion of pyruvate ameliorated ventricular remodeling in an isoproterenol heart failure model.138 Collectively indicating that MPC-mediated mitochondrial pyruvate utilization is essential for the partitioning of glucose-derived cytosolic metabolic intermediates in the adaptation to myocardial stress.
Insulin and IGF signaling
Insulin signaling regulates glucose utilization in the heart and mediates its effects by binding to the insulin receptor and with less affinity to the IGF-1 receptor. Insulin receptor binding increases its autophosphorylation, which increases receptor tyrosine kinase activity for other substrates such as insulin receptor substrates (IRS) proteins. Consequently, IRS proteins and other binding partners activate a network of signaling components, including PI3K/AKT and MAP kinases.139
Mice with cardiomyocyte-specific deletion of insulin receptors (CIRKO mice) enabled an assessment of myocardial insulin receptor signaling in the absence of confounding systemic metabolic derangements. Absence of cardiomyocyte insulin receptor signaling induced modest age-dependent cardiac dysfunction.140 Underlying mechanisms may include cardiac metabolic insulin resistance, dysfunction and proteomic remodeling of mitochondria, and ROS-mediated mitochondrial uncoupling induced by FA exposure that can lead to impaired cardiac efficiency.38 Based on these findings, it has been proposed that mitochondrial dysfunction and impaired cardiac efficiency observed in hearts of Type 2 diabetic rodents may be related to cardiac insulin resistance in these diabetes models.61 Insulin receptor and IGF-1R exhibit signaling redundancy in the heart.141 Indeed, mice with combined loss of insulin and IGF-1 receptors develop a lethal cardiomyopathy, indicating a partial protective effect of preserved IGF-1R signaling in CIRKO mouse hearts.
The significance of insulin signaling in regulating mitochondrial biology was confirmed in mice with cardiomyocyte-specific deletion of IRS1 and IRS2 that also develop mitochondrial dysfunction which precedes development of cardiac dysfunction. 142 Furthermore, this model provided valuable insight that insulin action in early life mediates the physiological postnatal suppression of autophagy, thereby linking nutrient sensing to postnatal cardiac development. Studies in single IRS knockouts revealed divergent roles in the regulation of cardiac size, however exercise-induced hypertrophy and the accompanying increase in oxidative capacity observed in wildtype mice were blunted both in mice with deletion of IRS1 or IRS2, indicating that both isoforms are required for the adaptation to chronic exercise training.
PI3K and AKT are downstream components of insulin signaling and regulate energy metabolism and cellular/organ growth. Mice with long-term gain-of-function and loss-of-function of PI3K revealed that PI3K increases cardiac growth.143 Short-term activation of PI3K/AKT by insulin increases glucose uptake and decreases FAO, whereas sustained PI3K activation is sufficient to increase FA oxidative capacity independently of Akt and to suppress insulin-stimulated glucose uptake by impairing GLUT4 translocation.144 These studies demonstrate that long-term metabolic effects of PI3K can be dissociated from downstream Akt signaling, and identified a role for PI3K in coordinating myocardial FAO capacity in exercise-induced cardiac hypertrophy. Data from mice with long-term activation or repression of Akt signaling demonstrated the potential of Akt to induce cardiac hypertrophy which may or may not progress to heart failure, partly related to the degree and duration of overexpression, insufficient induction of angiogenesis and repression of mitochondrial oxidative capacity.145, 146 Persistent Akt signaling may also be deleterious due to feedback inhibition of IRS and PI3K signaling.147 Models with altered isoform-specific Akt expression informed Akt1 to promote physiological cardiac hypertrophy while antagonizing pathological hypertrophy, whereas Akt2 may not affect cardiac growth but instead mediate insulin-stimulated glucose utilization.148
The IGF-1 receptor shares structural homology and overlapping downstream signaling components with the IR signaling cascade, however the affinity of the receptor is much higher for IGF-1 than for insulin. Cardiomyocyte-selective deletion of IGF-1 receptor prevents aging-associated cardiac hypertrophy due to inhibition of PI3K/AKT signaling, indicating a role for IGF-1 signaling in age-associated cardiac hypertrophy.149 In addition, deletion of IGF-1 receptor prevents physiological cardiac hypertrophy in response to swimming exercise, whereas overexpression of IGF-1 receptor promotes physiological hypertrophy due to activation of the PI3K/AKT signaling pathway.150 Detailed data on myocardial energy-metabolic rates are currently lacking. Of note, mice with combined deficiency of the insulin receptor and insulin-like growth factor 1 (IGF-1) receptor in cardiac and skeletal muscle develop early-onset dilated cardiomyopathy and die from heart failure within the first month of life, associated with a coordinated down-regulation of genes encoding for OXPHOS and FA oxidation proteins, suggesting that intact signaling via both receptors is required for normal cardiac metabolism and function
Ketone body and BCAA metabolism
Cardiac ketone metabolism has been investigated in mice with manipulation of BDH1 or SCOT expression. Deletion of BDH1 in cardiomyocytes impairs βOHB utilization and increases FAO leading to cardiac dysfunction, whereas BDH1 overexpression enhances both KB and FA utilization thereby attenuating cardiac hypertrophy and dysfunction induced by TAC.151 Cardiomyocyte-specific loss of SCOT increases FAO but does not affect cardiac structure or function during ketolytic challenge, whereas myocardial ROS levels and cardiac dysfunction were enhanced in response to TAC.152 Collectively, these findings highlight KB utilization as part of the coordinated adaptation to pressure overload and suggest KB utilization might protect against heart failure development, or may reflect induction of pathways that bypasses CPT1, such as induction of short chain fatty acid utilization, which also occurs in the failing heart.153 Cardiomyocyte-specific deletion of BCAT revealed that BCAA accumulation due to defective BCAA oxidation induces cardiac hypertrophy, and that an accompanying decrease in BCKA levels may enhance myocardial metabolic insulin sensitivity.154 Suppressing BCAA oxidation by deletion of mitochondrial PP2C (an activator of BCKDH) induced systolic dysfunction and exacerbated TAC-induced cardiac hypertrophy and dysfunction.155 Collectively, BCAA oxidation seems to be required to sustain cardiac function and allow adaptation to pressure overload, implying that defective BCAA oxidation in failing hearts may causally contribute to heart failure development. Of note, another recent study demonstrated that the major fate of BCKA may not be oxidation but instead reamination to BCAA, thereby driving protein synthesis and cardiac hypertrophy via this mechanism. 156 These findings allow the speculation that increased circulatory levels of BCAA and BCKA, as occurs in obesity, diabetes and early stages of heart failure, may contribute to hypertrophy development by diversion of BCKA to the reamination pathway.
Post-translational modifications
Sirtuins (SIRTs) are NAD+-dependent lysine deacetylases whose activity might modulate transcriptional pathways that regulate adaptations to changes in energy availability, aging, and stress resistance. While SIRT1, 2, 6 and 7 are predominantly localized in the nucleus and/or cytosol, SIRT3-5 are predominantly expressed in mitochondria. Mice expressing a mutant SIRT1 protein with a deleted catalytic domain or mice with germline SIRT1 deficiency, were severely growth retarded and developed cardiac developmental defects, which precluded post-natal survival.157 In another study, germline knockout of SIRT1 led to dilated cardiomyopathy in adults, possibly due to morphological aberrations and functional defects of mitochondria. Interestingly, mild to moderate overexpression of SIRT1 retards aging-induced histological changes and LV dysfunction in the heart, whereas higher levels of SIRT1 expression leads to dilated cardiomyopathy, possibly through induction of mitochondrial dysfunction and ATP depletion.158 Besides regulating mitochondrial oxidative metabolism, SIRT1 also participates in myocardial glucose utilization and insulin signaling via deacetylation and activation of Akt.
Several transgenic models have addressed the role of mitochondrial sirtuins. Global deletion of SIRT3 leads to progressive age-related deterioration of cardiac function in response to chronic pressure overload, which was proposed to result from increased acetylation and thus inhibition of various enzymes involved in energy substrate oxidation and OXPHOS, although the inhibitory effect of this acetylation has only been proven for few enzymes to date.159 Conversely, overexpression of SIRT3 protects from angiotensin II-induced cardiac hypertrophy or doxorubicin-induced cardiomyopathy by suppressing cellular levels of ROS via FoxO3a-dependent expression of antioxidant proteins and by protecting from mitochondrial DNA damage, respectively, suggesting SIRT3 activation as a cardioprotective therapeutic strategy.160 Opposite observations were made in animal models with altered expression of SIRT4 where overexpression of SIRT4 exacerbated and deletion of SIRT4 attenuated agonist-induced cardiac hypertrophy and dysfunction, possibly related to modulation of mitochondrial ROS via SOD2.161 Of note, SIRT4 also suppresses FAO, glucose oxidation and TCA cycle anaplerosis by deacetylation of MCD, delipoamidylation of PDH, and ADP ribosylation of glutamate dehydrogenase in extracardiac tissues, respectively (for review see 162). However, detailed analysis of effects of SIRT4 on cardiac energy metabolism remain to be reported. Finally, systematic proteome profiling in mice lacking SIRT5 revealed numerous post translational modifications of mitochondrial proteins involved in mitochondrial metabolic pathways including desuccinylation and demalonylation of SIRT5 targets .162 While suppression of cardiac energetics has been proposed to underlie exaggerated hypertrophy and increased mortality in global SIRT5−/− mice, the same group later reported that inducible and cardiomyocyte-restricted deletion of SIRT5 did not increase mortality or alter the hypertrophic and contractile response to TAC, leaving the role of SIRT5 in the heart to be further explored.163
The role of SIRT6 and SIRT7 in cardiac metabolism have only been marginally explored. Cardiomyocyte-selective deletion of SIRT6 leads to cardiac hypertrophy and dysfunction, whereas overexpression protects from TAC-induced cardiac hypertrophy and dysfunction.164 Mechanistically, SIRT6 may bind to and suppress the promoter of IGF signaling-related genes via interaction with c-Jun and deacetylation of histone 3, and may attenuate aging-associated alterations such as NAD+ depletion, mtDNA lesions or accumulation of 8-oxo-dG adducts.164, 165 In the context of MetS, SIRT6 overexpression preserves cardiac insulin sensitivity and attenuates cardiac hypertrophy, fibrosis and mitochondrial dysfunction, implying a potential for SIRT6 agonism in ameliorating diabetic cardiomyopathy. Mice with germline or cardiomyocyte-selective deletion of SIRT7 develop mild hypertrophy at older ages and exaggerated remodeling following TAC, likely related to p53-mediated excessive apoptosis and diminished resistance to oxidative/genotoxic stress.166 Data on cardiac metabolism remain to be reported.
Another important posttranslational modification derived from glucose is the reversible attachment of O-linked N-acetylglucosamine moieties to Ser and Thr residues termed O-GlcNAc. Mice with deletion of O-GlcNAc transferase (OGT) and thus less O-GlcNAc demonstrated that this modification is necessary for cardiac maturation and induces cardiomyopathy.167 Overexpression of OGT and increased O-GlcNAc also leads to dilated cardiomyopathy, likely due to disruption of OXPHOS complex I activity, indicating that importance of balanced O-GlcNAc to maintain cardiac integrity.168 Of note, attenuation of O-GlcNAc by enhancing O-GlcNAcase activity is well tolerated and protects from pressure overload–induced pathologic remodeling, suggesting that this intervention may have therapeutic potential to attenuate cardiomyopathy. Of note, increasing flux through the hexosamine biosynthetic pathway by overexpression of the rate-limiting enzyme, glutamine:fructose-6-phosphate amidotransferase 1 (GFAT1) promotes cardiac hypertrophy through activation of mTOR, which appears to be mediated by increased O-GlcNAcylation.169
Models of mitochondrial dynamics
Although mitochondria of the adult heart might be perceived as static organelles, models with genetic modulation of mitochondrial dynamics proteins advanced our understanding of fusion and fission for heart health. Studies in mice with deletion of both MFN1 and MFN2 exhibit impaired mitochondrial biogenesis, mtDNA replication, structural abnormalities, defects in mitochondrial gene transcription, and respiratory defects in the heart, overall leading to cardiomyopathy and severely reduced lifespan and indicating that mitochondrial fusion proteins are essential for mitochondrial remodeling during postnatal cardiac development and in the adult heart.170, 171 Comparative studies of mitochondrial fission- and fusion-defective hearts (deletion of MFN1, MFN2, or DRP1) suggested that mitochondrial fission may serve to segregate healthy and damaged components and to protect against transition pore-mediated cell death, whereas mitochondrial fusion may serve to repair and regenerate mitochondria, with both processes needed to be intact for proper elimination of impaired organelles and renewal of healthy mitochondria.172 Additional studies showed that MFN1/MFN2/DRP1 triple knockout mice have hearts with adynamic mitochondria that exhibit extended life span compared to their fission- or fusion-defective parents, but develop mitochondrial senescence and heart failure due to impaired mitophagy, mitochondrial superabundance, and sarcomere distortion.173 Furthermore, proteolytic cleavage of the fusion protein, OPA1, drives mitochondrial fragmentation and cardiomyopathy development, accompanied by a switch from FA to glucose utilization, indicating an intimate link between mitochondrial morphology and energy metabolism in the heart.174
Crosstalk between heart and peripheral metabolism
Although viewed as an obligate omnivore and a net consumer of metabolic energy, recent studies have shed novel insight into the regulation of systemic metabolism by factors that originate in the heart. One of the most dramatic descriptions of this concept was reported from the Olson laboratory, demonstrating that modulating the expression of MED13, a subunit of the mediator complex and its regulatory microRNA mir-208A, selectively in cardiomyocytes in vivo, led to significant regulation of systemic energy expenditure and modified susceptibility to diet-induced obesity and the metabolic syndrome,175 via secreted factors, that remain to be characterized.176 Knockout of the transcription factor KL5 in cardiomyocytes increased susceptibility to diet-induced obesity, which was prevented by simultaneous deletion in cardiomyocytes of the myokine fibroblast growth factor 21.177 Inhibition of GRK2 activity in cardiomyocytes increased the susceptibility of mice to diet-induced obesity, whereas overexpression of GRK2 in cardiomyocytes, rendered animals resistant to diet-induced obesity. Metabolomics profiling revealed changes in myocardial and circulating levels of branch chain amino acids and endocannabinoid metabolites, which enhanced adipocyte differentiation in vitro.178 Natriuretic peptides are secreted from hypertrophied and failing hearts. Adipose tissues express natriuretic peptide receptors,179 activation of which may lead to browning of adipose tissue and increased energy expenditure.180 Thus, natriuretic peptide secretion from the failing heart could contribute to altered systemic metabolism, and potentially cachexia in heart failure patients. Finally, novel mechanisms of crosstalk between peripheral metabolic organs and the heart, such as those mediated by exosomes have been recently described.181, 182
Cardiac energy metabolism and models of HFrEF
Energy starvation contributes to the pathogenesis of HFrEF, linked in part to impaired ATP regeneration due to defects in mitochondrial oxidative capacity.183 Underlying mechanisms of mitochondrial dysfunction include: increased mitochondrial ROS generation and oxidative stress which may induce mitochondrial DNA damage, damage to OXPHOS and other proteins, and lipid peroxidation, all of which may impair the ATP generating pathways.184 Altered mitochondrial Ca2+ may further increase mitochondrial ROS production and alter mitochondrial NADH/NAD and NADPH/NADP ratios, thereby impairing the activity of Ca2+-dependent dehydrogenases (in particular of the TCA cycle) and triggering cell death pathways.185 Excess mitochondrial fission leading to mitochondrial fragmentation, decreased antioxidative capacity, increased production of ROS, and cell death; 186 sustained or impaired removal of damaged mitochondria by autophagy (i.e. mitophagy), thereby reducing the number of functional mitochondria or compromising the removal of damaged mitochondria, respectively, also contribute to mitochondrial dysfunction and its sequela including increased cell death in heart failure.187
In addition, fuel preference is altered in the failing heart (Figure 4). Many studies demonstrated that during the development of pathologic hypertrophy or early in the course of cardiac failure, FA oxidation remains preserved, whereas in more severe stages of the disease, FA oxidation rates are mostly decreased.188, 189 These observations are consistent across various animal models of HF such as pressure overload-induced or MI-induced HF in rodents, pacing-induced HF in canines, or rats prone to develop hypertensive cardiomyopathy and HF. The decrease in FAO rates has been attributed to decreased expression of PPARα, RXRα, PGC1α, ERRs, and proteins of FA uptake and oxidation (e.g., CPT1b, MCAD, CD36, FATP). While these findings were also confirmed in human failing hearts, some studies reported no decrease or even an increase in FAO rates, likely related to differences in disease severity or to systemic metabolic comorbidities such as obesity and metabolic syndrome which secondarily influences cardiac substrate selection. 190, 191
Figure 4: Simplified overview of energy metabolism in failing hearts.
Following transition from compensated hypertrophy to heart failure, both FA and glucose oxidation are impaired, mainly due to decreased transcriptional signaling (PPARα, PGC1α) and reduced expression of energy metabolic genes. While glucose oxidation and insulin stimulated glucose uptake is impaired in HF, glycolysis may be induced, driven in part by increased HIF1α signaling. A mismatch between glycolysis and glucose oxidation increases lactate levels and intracellular proton accumulation, and early intermediates of glycolysis drive cardiac remodeling by promoting flux into the PPP and HBP. KB oxidation is increased, whereas BCAA oxidation is decreased, resulting in myocardial and mitochondrial BCAA accumulation that promotes ROS generation and suppresses OXPHOS function. BCAA accumulation might also contribute to mTOR activation to increase cardiac hypertrophy. Downregulation and remodeling of OXPHOS subunits impair ATP synthesis and increases ROS generation, the latter promoting intramitochondrial oxidative damage, impaired Ca2+ handling, fibrosis, inflammation and cell death. ATP depletion may be further exaggerated by increased UCP expression and mitochondrial uncoupling. Collectively, these metabolic alterations contribute to cardiac remodeling and dysfunction in HF.
For detailed discussion of distinct alterations observed during compensated and decompensated stages of cardiac hypertrophy see refs2, 183, 190.
Despite differences among studies and models, myocardial uptake and oxidation of glucose and thus the relative preference for ATP production is predominantly increased in the failing heart, in some models even starting at early stages of cardiac hypertrophy. While the adaptive or maladaptive nature of this shift towards preferential glucose utilization in the development of HF remains a subject of debate, it is generally presumed that switching to glucose may enable more oxygen-efficient production of ATP. However, at later stages of HF, glucose oxidation is generally decreased, due in part to decreased expression of PDH, MPC1, and pyruvate/alanine aminotransferases, or as a consequence of overall impairment of mitochondrial oxidative capacity and mitochondrial energetics. The impaired oxidation of all energy metabolic substrates leads to myocardial energy depletion. 137 Furthermore, increased expression of UCP3 may induce mitochondrial uncoupling in some models (e.g., MI-induced HF), thereby compromising cardiac efficiency (=cardiac work/oxygen consumption). Decreased glucose oxidation and mitochondrial oxidative capacity is partially compensated by an induction of glycolysis which, only mildly increases ATP regeneration and may shift glucose into alternative pathways such as the pentose phosphate and hexosamine pathway, thereby further enhancing intracellular signaling that promotes adverse cardiac remodeling.192 In addition, uncoupling of glycolysis from glucose oxidation results in lactate production and proton accumulation in some HFrEF models, which renders glucose utilization less efficient and may secondarily increase cardiomyocyte Ca2+ accumulation. Increased glycolysis could be related in part to increased expression of GLUT1, and modification in the activity or expression of regulators of glycolytic flux, which may be mediated by increased HIF-1α levels as observed in rodent and human HF. 193, 194 HIF-1α is a hypoxia-inducible transcription factor, binding to the promoters of GLUT1 and other glycolytic pathway genes that contain HIF-1α response elements. A persistently activated hypoxic response in the heart as occurs during pressure overload or tachypacing may stabilize HIF-1α levels. HIF-1α transgenic overexpressing hearts develop age-dependent HF, accompanied by increased glucose uptake, increased expression of GLUT1 and PFK, a concomitant decrease of PPARα expression and a trend towards decreased FA oxidation.194
HF is a ketosis-prone state. KB oxidation has been proposed to be more oxygen-efficient than oxidation of a mixture of FAs and glucose.195 However, KB oxidation is a cataplerotic process (i.e. leading to loss of TCA cycle intermediates) and may ultimately compromise substrate oxidation and thus requires anaplerosis (oxaloacetate, malate) from oxidation of glucose or glucogenic amino acids to maintain TCA cycle activity. Plasma ketone levels and myocardial KB metabolism are increased in HF, but it remains a matter of debate whether enhanced myocardial KB utilization causes cardiac dysfunction, is caused by cardiac dysfunction, or simply is an epiphenomenon.5 The increased reliance on ketone body utilization in HF may result from increased systemic availability due to increased ketogenesis serving as an alternative fuel and possibly increasing oxygen-efficiency of ATP production.196
Accumulation of amino acids has been observed in animal models of HF and in human failing hearts, which may result from impaired BCAA catabolism.197 A transcriptome analysis of mice with pressure overload-induced HF revealed suppression of BCAA catabolic gene expression, in particular of pathways regulating catabolic pathways of valine, leucine, and isoleucine, including BCATm, BCKDH subunits E1α, E1β and E2, and the mitochondrially localized BCKDH phosphatase, protein phosphatase 2C (PP2C). As a result, BCKA metabolites accumulate in the myocardium, which can suppress respiration and induce superoxide production within mitochondria.155 Importantly, mice lacking PP2C developed both accumulation of BCKA and systolic dysfunction and showed accelerated development of cardiac remodeling and dysfunction in response to pressure overload, suggesting that impaired BCAA catabolism contributes to HF pathogenesis. 155, 197
Given the large number of studies performed in models of HFrEF, we provide an overview of frequently used HFrEF models and related characteristics of cardiac metabolism in Supplemental Table S3. For further details on these and other models of HFrEF, we refer to other excellent reviews.192, 198-200
Novel models of HFpEF
HFpEF is a clinical syndrome with heterogenous etiologies, leading to distinct changes in myocardial structure and associated molecular pathways.201 Comorbidities such as obesity, T2DM and hypertension are considered important causes of myocardial hypertrophy, ventricular stiffening, diastolic dysfunction and exertional dyspnea in human subjects suffering HFpEF. In human subjects and animal models of the MetS, the heart relies predominantly on FA utilization, coupled with impaired glucose utilization and mitochondrial oxidative defects, as described above. With regards to HFpEF pathophysiology, these models have however been criticized because they exhibit traits that are not necessarily observed in the human HFpEF syndrome. Importantly, some models may disqualify as bona fide HFpEF models since they may develop HFrEF at advanced stages of cardiac disease, which per definition is not compatible with a HFpEF diagnosis. For example, mice subjected to HFD, or aortic banding can ultimately lead to measurable systolic contractile defects, depending on the time point of investigation during follow-up and on variable factors (e.g., diet composition, degree of aortic constriction, genetic background, etc.), meaning that some models may only serve as a time-delimited model but not as bona fide model for human HFpEF. Further limitations of HFpEF modeling have been recently discussed in other excellent reviews.202. These considerations need to be considered when evaluating animal models that are intended to mimic human HFpEF. Detailed phenotypes of models discussed below are summarized in Supplemental Table S4.
“Two hit” and “three hit” mouse models
Recently, the “two hit” model of HFpEF has been introduced, induced by the combination of HFD and inhibition of nitric oxide synthesis by L-NAME, which revealed increased body weight, visceral obesity, impaired glucose tolerance and insulin sensitivity, and increased blood pressure.203 As a result, hearts were reported to develop cardiac hypertrophy, diastolic dysfunction, myocardial fibrosis and increased pulmonary congestion, without evidence of systolic dysfunction up to 15 weeks of follow-up after treatment initiation, collectively matching a phenotype resembling that of clinical HFpEF. Metabolic studies revealed impaired glucose oxidation, unchanged expression of FAO genes, and impaired respiration using palmitoyl carnitine or pyruvate as substrates, indicating altered energy metabolism and mitochondrial dysfunction. As observed in models of HFrEF, treating the “two hit” mouse model with β-OHB protected against the progression of HF by increasing cardiac regulatory T cells by modulating NOX2/GSK-3β signaling, suggesting that this metabolic intervention may have merit in treating HFpEF by attenuating cardiac inflammation.204
In the “three hit” mouse model, an additional single bolus of desoxycorticosterone pivalate (DOCA) was administered in the last month of 13 months of HFD to accentuate hypertension and systemic inflammation.205 These mice develop myocardial fibrosis, inflammation, pulmonary edema, and diastolic dysfunction, in conjunction with increased body weight, reduced glucose tolerance, and elevated blood pressure. The study identified an interplay between mitochondrial protein hyperacetylation and NLRP3-related inflammation as a driver in the pathogenesis of HF. As in the “two hit” model, increasing circulating β-OHB also ameliorated the HF phenotype, likely by interrupting the vicious circuit of mitochondrial dysfunction and inflammation, reducing the acetyl-CoA pool (that drives protein acetylation) due to suppression of FA uptake and activation of citrate synthase. Of note though, while ejection fraction was not impaired, dP/dt was significantly reduced after 13 months, indicating subtle impairment in systolic function and indicating that this model may not be a bona fide model of HFpEF but instead shows a time-delimited HFpEF phenotype. Since mice from the ‘two hit” model were only observed over 15 weeks, development of systolic function can also not be excluded in this model.
Non-rodent models of HFpEF
Several non-rodent models of HFpEF have been recently reported. In a feline model, slow-progressive pressure overload induced by TAC using pre-shaped customized bands in 2 months-old kittens induced a progressive increase in concentric LV hypertrophy, enlargement and dysfunction of the left atrium, and diastolic dysfunction in the absence of systolic defects, subsequently leading to elevated LV filling pressures, elevated NT-proBNP levels, and pulmonary hypertension with impaired oxygenation and stiffening of the lungs.206 In addition, evidence for increased LV fibrosis, cardiomyocyte hypertrophy, perivascular cuff formation, impaired lung expansion, and alveolar-capillary membrane thickening was provided. Based on this cardiopulmonary analysis, this model might meet the diagnostic criteria of HFpEF. Studies of cardiac metabolism have not yet been performed.
Two models recapitulating the phenotype of HFpEF have been described in pigs where HFpEF was induced by inflation of aortic cuffs or by aortic banding with or without the addition of a western diet (high-fat/high-cholesterol).207, 208 Both models show increased body weight, plasma cholesterol, TG, insulin resistance, and aortic systolic BP, associated with cardiac hypertrophy and evidence of diastolic dysfunction, but no systolic defects. While the banding model exhibits mitochondrial dysfunction, mice with inflatable aortic cuffs showed increased PDH flux measured by magnetic resonance spectroscopy compatible with the typical switch from FA towards glucose utilization. As in rodent models, no data are currently available about systolic function after long-term treatment in these non-rodent models.
Translational potential of targeting cardiac energy metabolism
Preferential utilization of glucose
Promoting preferential use of glucose and non-FA substrates away from FAs, to increase oxygen efficiency of ATP regeneration is believed to mitigate or prevent energy depletion of the failing heart. Such metabolic modulation is associated with an improvement of the cardiac phosphocreatine:ATP ratio by up to 33%, which correlates with a parallel increase of left ventricular performance.209 Treatment with activators of glucose utilization (e.g. dichloroacetate) or inhibitors of FA utilization (e.g. trimetazidine, perhexiline, etomoxir, ranolazine) in animal studies, revealed improvements in ejection fraction and promoted structural reverse remodeling in experimental heart failure.210 In humans, most evidence has been gained for trimetazidine, a drug with a favorable clinical safety profile causing partial inhibition of FA oxidation via long-chain 3-ketoacyl-CoA thiolase, increasing glucose oxidation, and inhibiting excessive glycolysis.211 While most clinical trials had limited patient size, meta-analyses of these trials revealed a reduction in hospitalization rates for cardiac causes and cardiovascular events, an improvement of ejection fraction, total exercise time, and NYHA function class, and a decrease in LV volumes and proBNP.212-214 All-cause mortality was reduced in one analysis (RR 0.29) as well,213 but remained non-significant despite clear trends (risk ratio < 0.50 in both analyses) towards a reduced risk ratio in the other two studies. Of note, an increase in cardiac function was associated with preferential cardiac glucose utilization in idiopathic dilated cardiomyopathy patients, confirming that trimetazidine indeed modulates cardiac metabolism in humans.215 Of note, trimetazidine treatment not only improved systolic function and NYHA class, but also insulin sensitivity in HFrEF patients with concomitant insulin resistance or T2DM, suggesting that trimetazidine may also improve cardiac substrate utilization indirectly by improving systemic glucose metabolism. 215 Despite some beneficial effects in small size clinical trials, less enthusiasm exists for other drugs with greater potency in shifting metabolism towards increased glucose utilization and away from FA metabolism. Oxfenicine an irreversible CPT1 inhibitor is not approved for human use. The potential risk of significant hepatotoxicity (etomoxir; reversible CPT1 inhibitor) or neuropathic toxicity (dichloroacetate; PDK inhibitor) has also precluded the use of these agents.216, 217 Increasing the coupling of glycolysis to glucose oxidation, thereby preventing accumulation of glycolytic intermediates that could promote LV remodeling and also reducing proton accumulation thereby increasing the efficiency of ATP production from glucose, may be another promising concept which could be achieved by inhibition of either MCD or ACC. Clinical data on these agents are not yet available.198
Modulation of BCAA and KB utilization
Accumulation of amino acids has been observed in HF, which may result from impaired BCAA catabolism.197, 218 Enhancing BCAA catabolism by pharmacological inhibition of BCKDH kinase blunted systolic and diastolic dysfunction after pressure overload,219 suggesting that enhancement of BCAA catabolism (by activation of catabolic enzymatic activity) could serve as a potentially efficacious treatment option in HF.197, 219 Cardiac and serum levels of BCAA are also increased in obesity, IR and T2DM, and circulating levels of BCAAs are independently associated with incident HF in subjects with T2DM.220 Dietary restriction of BCAAs has been shown to shift cardiac metabolism towards FA catabolism relative to glucose metabolism and eliminated a 2-fold increase in myocardial triglycerides. This also improved insulin resistance both in obese and/or diabetic rodents and humans, suggesting that both direct and indirect mechanisms (secondary to improved systemic metabolism) may modulate cardiac energy metabolism.221, 222 While increasing utilization or dietary restriction of BCAAs in HF and T2DM may exert beneficial effects, more studies in humans are required to further evaluate this therapeutic strategy.
Several studies evaluated increasing KB oxidation as a therapeutic strategy in the failing heart. A recent study demonstrated that a ketogenic diet (high fat/low carbohydrate) attenuated the extent of adverse cardiac structural remodeling in a combined TAC/MI model, and that chronically increasing delivery of ketones (4 weeks) in the canine tachypacing model of HF markedly improved cardiac function and remodeling.223 In this canine model of HF, ketone body uptake was increased compared to controls, and was further doubled under increased KB delivery. The beneficial response is possibly due to increased mitochondrial respiratory efficiency when utilizing ketones as a substrate. Also, direct application of βOHB (as ketone salt) by intravenous infusion increased cardiac output in HFrEF patients, and a dose-response relationship between β-OHB levels and cardiac output was shown.224 Furthermore, genetically induced elevation of circulating ketone bodies, without a coinciding reduction in glucose levels, protected against the development of pressure-overload induced HF by impairing NLRP3 inflammasome activation, suggesting that ketone bodies may prevent cardiac inflammation.225 While these studies may suggest that increased ketone utilization in HF may be an adaptive stress response, increasing myocardial KB utilization did not improve cardiac function in all studies.226 The durability of increasing ketone utilization to improve cardiac dysfunction in heart failure remains to be determined. Moreover, the method of delivery (duration, route of application, ketone chemistry) to increase myocardial ketone oxidation, and the underlying mechanisms how ketones may benefit the failing heart remain to be elucidated. Of note, certain routes of ketone administration (e.g., infusions) might not be feasible for clinical practice.
Weight loss strategies and impact on cardiac metabolism in heart failure
Weight loss, achieved by caloric restriction (CR) or intermittent fasting, improves systemic metabolism to mitigate cardiovascular risk factors that could reduce the onset and progression of CVD. CR or time-restricted feeding may induce sustained weight loss, improve insulin sensitivity, lower blood pressure, improve CRP levels, and lower oxidative stress in non-obese, obese, prediabetic or diabetic subjects.227, 228 CR has been shown to increase peak MVO2 in obese patients with HFpEF, and to improve diastolic dysfunction and to lower myocardial TG content in obese and diabetic subjects.229, 230 In rodents, CR and intermittent fasting also improved LV remodeling in HFrEF models.231 Studies in mice with pressure-overload hypertrophy and subjected to HFD suggest that these beneficial cardiac effects correlate with altered cardiac energy metabolism, including enhanced cardiac insulin signaling and insulin-stimulated glucose oxidation, decreased cardiac FA oxidation, reduced AMPK signaling and acetylation of ß-oxidation enzymes.232 In diabetic Long-Evans Tokushima Otsuka rats or in db/db mice, beneficial effects of CR may be related to increased SIRT1 expression, AMPK phosphorylation, FoxO1 phosphorylation, and PGC1α signaling, that correlate with improved diastolic and systolic LV function, and reverse remodeling.233 While weight loss strategies seem to indirectly improve cardiometabolic health, reduced compliance for lifelong CR and the potential risk of inducing essential nutrient deficiency, are limitations of weight loss-based approaches to heart failure , which would require additional study. Intermittent fasting could potentially be more feasible but remains to be studied in the context of heart failure.
Resveratrol
Resveratrol is a natural polyphenol that mimics effects of dietary restriction and/or every-other-day feeding. In rodent models of HF, resveratrol treatment increased median survival, attenuated cardiac fibrosis and structural remodeling, and improved or prevented cardiac dysfunction.234 While resveratrol attenuated oxidative stress, the functional improvements may be related to restoration of glucose oxidation (by shifting substrate preference towards glucose), restoration of OXPHOS subunit expression and mitochondrial respiratory function, normalization of AMPK phosphorylation, increased mitochondrial biogenesis and enhanced PPARα and SIRT1 signaling.235, 236 Specific underlying mechanisms remain incompletely understood. In rodent models of the metabolic syndrome, administration of Resveratrol protects against the development of diet-induced insulin resistance and obesity, and lowers circulating concentrations of triglycerides, FAs, insulin and leptin. These effects on systemic metabolism would be expected to beneficially affect cardiac energetics, structure and function, as observed in rat models of diabetic cardiomyopathy subjected to resveratrol treatment.237 While administration of resveratrol in humans is clinically feasible and safe, effects on diabetic or failing hearts in human subjects have not yet been reported.
SGLT2 inhibitors (SGLT2i)
SGLT2i, which are approved for the treatment of diabetes, lower blood glucose levels by inhibiting renal glucose reabsorption via inhibition of the sodium glucose cotransporter. Importantly, SGLT2i treatment decreased HF hospitalization and/or cardiovascular death in patients with HFrEF with and without T2DM.238 Inhibition of SGLT2 results in a mild hyperketonemia, which led to the proposal that improved myocardial ATP regeneration due to increased KB oxidation may be responsible for beneficial effects of SGLT2i. However, while empagliflozin treatment improves cardiac respiratory function and ATP production, and attenuated cardiac dysfunction and remodeling in rodent models of T2DM, KB oxidation remained unaffected by the treatment.239, 240 In MI-induced HF in pigs, empagliflozin treatment resulted in a switch towards utilization of KB, FA, and BCAA, which was associated with increased myocardial ATP content, amelioration of cardiac function and structure, and improved cardiac efficiency,241 however this was not observed in pressure-overload induced HF in mice, despite improved cardiac function.242 Thus additional research is required to understand if and how SGLT2i may exert beneficial cardiovascular effects by modulation of cardiac energy metabolism.
Conclusions
This comprehensive review and its supplementary materials, provides a comprehensive overview of a large body of work examining cardiac metabolism in a range of animal models ranging from those with altered systemic metabolism to those with specific perturbations in metabolic pathways or their signaling regulators. We have also summarized the cardiac adaptations to metabolic conditions such as diabetes and obesity, the metabolic and mitochondrial maladaptations that characterize heart failure and the interaction between these conditions. Lessons learned from these studies include the multiple and redundant regulatory mechanisms that exist in the heart to ensure metabolic homeostasis. The importance of maintaining metabolic flexibility has been underscored. Constraints on these mechanisms may contribute to functional maladaptations that lead to the progression of heart failure. The models reveal that in addition to ATP generation, metabolic substrates also regulate multiple signaling pathways that regulate myocardial homeostasis, disturbances of which may contribute to myocardial dysfunction. To the extent that animal models have provided significant and novel insights into metabolic regulation in health and disease, future studies should focus on determining the relevance of these findings to humans and evaluate how these findings can be translated into therapies for cardiovascular disease and heart failure.
Supplementary Material
SOURCES OF FUNDING
This work received no funding. Authors are grateful for research grants from the Austrian Science Fund (FWF) to H.B. (P-33874-B) and the following grants from the NIH (HL127764, HL112413, HL108379, DK092065, HL087947, HL73167, HL70525) and the grants from the American Heart Association (20SFRN35120123, 16SFRN31810000) to EDA, who is an established investigator of the AHA. NJB was supported by a postdoctoral fellowship grant issued by the Peter und Traudl Engelhorn Foundation.
Abbreviations:
- 4EBP1
4E-binding protein
- 8-OHdG
8-hydroxydeoxyguanosine
- AA
amino acid
- ABCA
ATP-binding cassette transporter
- Ac
acetylated
- ACAA2
acetyl-CoA acyltransferase 2
- AcAc
acetoacetate
- ACAD
Acyl-CoA dehydrogenase
- ACADL
long-chain ACAD
- ACADM
medium-chain ACAD
- ACAT
acetyl-CoA acetyltransferase
- ACC
acetyl-CoA carboxylase
- ACO
ACC oxidase
- ACON
aconitate hydratase
- ACOT
acyl-CoA thioesterase
- ACS
acyl-CoA synthetase
- AcylCN
acylcarnitine
- ADP
adenosine diphosphate
- ADP/O
ADP:oxygen ratio
- ADRP
adipose differentiated related protein
- AGPAT
1-acylglycerol-3-phosphate O-acyltransferase
- AKAP
A-kinase anchoring protein
- aKGDH
α-ketoglutarate dehydrogenase
- AKT
protein kinase B
- ALT
alanine aminotransferase
- AMP
adenosine monophosphate
- AMPK
adenosine monophosphate-activated protein kinase
- ANP
atrial natriuretic peptide
- ANT
adenine nucleotide translocase
- AOX
alternative oxidase
- ApoB
apolipoprotein B
- ASCL
acyl-CoA synthetase, long chain
- ASNS
asparagine synthetase
- ATGL
adipose triglyceride lipase
- ATP
adenosine triphosphate
- ATP/O
ATP:oxygen ratio
- ATP5A1
ATP synthase α-subunit
- ATP5F1
ATP synthase F1
- ATP5PD
ATP synthase peripheral stalk subunit D
- ATPsyn
ATP synthase
- BCAA
branched chain amino acid
- BCAT
branched-chain aminotransferase
- BCATm
branched-chain aminotransferase, mitochondrial
- BCKDH
branched chain alpha-keto acid dehydrogenase
- BDH
β-hydroxybutyrate dehydrogenase
- BPHx
hypertensive BPH/2J Schlage
- BT2
3,6-Dichlorobenzo[b]thiophene-2-carboxylic acid
- BW
body weight
- CASP
caspase
- CD36
fatty acid transporter
- CGP
choline glycerophospholipid
- ChE
cholesteryl esters
- CHO
carbohydrate
- CK
creatine kinase
- CL
cardiolipin
- CM
cardiomyocyte
- CoA
coenzyme A
- complex I
NADH-Q oxidoreductase
- complex II
succinate dehydrogenase
- complex III
Coenzyme Q:cytochrome c reductase
- complex IV
cytochrome c oxidase
- complex V
F0F1 ATP synthase
- COX
cytochrome c oxidase
- CPT
carnitine palmitoyltransferase
- CrAT
carnitine polmitoyl transferase
- CS
citrate synthase
- CT
CTP:phosphocholine cytidylyltransferase
- CYT
cytochrome
- DAG
diacylglycerol
- DBP
diastolic blood pressure
- DCA
dichloroacetate
- DGAT
diacylglycerol acyltransferases
- DHQ
dihydroquercetin
- DOCA
deoxycorticosterone acetate
- DRP
dynamin-related protein
- DSS
Dahl salt-sensitive
- EC
endothelial cell
- ECH
enoyl-CoA hydratase
- ECHA
trifunctional enzyme α subunit
- eEF
eukaryotic elongation factor
- EF
embryonic fibroblast
- EKG
electrocardiogram
- EP
ethanolamine phospholipids
- ER
endoplasmic reticulum
- ERK
extracellular Receptor Kinase
- ERR
estrogen-related receptor
- ESC
embryonic stem cell
- ETC
electron transport chain
- ETFA
electron transfer flavoprotein alpha
- F6P
fructose 6-phosphate
- FA
fatty acid
- FABP
FA binding protein
- FABPpm
FABP, plasma membrane
- FADH2
flavin adenine dinucleotide
- FAO
FA oxidation
- FAS
FA synthase
- FATP
FA transport protein
- FBP
fructose-1,6-bisphosphatase
- FFA
free FA
- FGF
fibroblast growth factor
- FIS
fission
- FOXO
forkhead box O
- Fru-2,6-P(2)
fructose 2,6-bisphosphate
- G1P
glucose-1-phosphate
- G6P
glucose-6-phosphate
- GABA
gamma-aminobutyric acid
- GABPA
GA repeat-binding protein α
- GATP
ATP free energy
- GCN5L1
GCN5-like protein
- GDF
growth differentiation factor
- GLDH
glutamate dehydrogenase
- GLUT
glucose transporter
- GPAM
glycerol-3-phosphate acyltransferase, mitochondrial
- GPAT
glycerol-3-phosphate acyltransferase
- GS
glycogen synthase
- GSH
glutathione
- GSK
glycogen synthase kinase
- H202
hydrogen peroxide
- HADH
3-hydroxyacyl-CoA dehydrogenase
- HbA1c
hemoglobin A1c
- HFD
high-fat diet
- HFHS
high-fat/high-sugar
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HIF
hypoxia-inducible Factor
- HK
hexokinase
- HMG-CoA
β-Hydroxy β-methylglutaryl-CoA
- HMGB
high mobility group box protein
- HMGSC
3-hydroxy-3-methylglutaryl-CoA synthase
- HOMA-IR
homeostasis model assessment-estimated insulin resistance
- HR
heart rate
- HR-LPL
heparin-releasable LPL
- HSL
hormone-sensitive lipase
- HSP
heart shock protein
- HW
heart weight
- ICHD
isocitrate dehydrogenase
- IFM
interfibrillar mitochondria
- IGF
insulin-like growth factor
- IGF1R
IGF-1 receptor
- IL
interleukin
- IL1RL
interleukin 1 receptor-like
- IR
insulin receptor
- IRS
IR substrate
- JNK
c-Jun N-terminal kinase
- KB
ketone body
- KEAP
kelch-like ECH-associated protein
- KI
knock-in
- KIV
ketoisovalerate
- KLF15
Krüppel-like factor 15
- KO
knockout
- L-CPT
CPT liver isoform
- L-NAME
Nitro-l-arginine methyl ester hydrochloride
- LAD
left anterior descending
- LC
long-chain
- LC-MS/MS
liquid chromatography–mass spectrometry
- LCAC
long-chain acylcarnitine
- LCAD
long-chain acyl CoA dehydrogenase
- LCFA
long-chain FA
- LCTH
long-chain 3-ketoacyl-CoA thiolase
- LDH
lactate dehydrogenase
- LDL
low-density lipoprotein
- LDLR
low-density lipoprotein receptor
- LIVCS
BCAA:cation symporter family
- LpL
lipoprotein lipase
- LPS
lipopolysaccharide
- LV
left ventricle
- LVRI
left ventricular remodelling index
- lycoPC
lysophosphatidylcholines
- LysAc
lysine acetylation
- MAP
mean arterial pressure
- MAPK
mitogen-activated protein kinase
- MCAD
medium-chain acyl-CoA dehydrogenase
- MCD
malonyl-CoA decarboxylase
- MCT
monocarboxylate transporter
- MEF
mouse embryonic fibroblasts
- MetS
metabolic syndrome
- MFN
mitofusin
- MHC
myosin heavy chain
- MI
myocardial infarction
- MMP
mitochondrial membrane potential
- MnSOD
manganese superoxide dismutase
- mPTP
mitochondrial permeability transition pore
- MRS
magnetic resonance spectroscopy
- mtDNA
mitochondrial DNA
- MTE
mitochondrial thioesterase
- MTERF
mitochondrial transcription termination factor
- MTHFD
methylenetetrahydrofolate dehydrogenase
- mTOR
mammalian target of rapamycin
- MUFA
monounsaturated fatty acids
- MVo2
myocardial O2 consumption
- NAD
nicotinamide adenine dinucleotide
- NADH
reduced NAD
- NADPH
nicotinamide adenine dinucleotide phosphate
- NDUFS
NADH-ubiquinone oxidoreductase, mitochondrial
- NDUFS3
mitochondrial complex I subunit
- NDUFV
NADH dehydrogenase [ubiquinone] flavoprotein, mitochondrial
- NMNAT
nicotinamide mononucleotide adenylyltransferase
- NRF
nuclear respiratory factor
- NYHA
New York Heart Association
- O-GlcNA
O-GlcNAcylation
- OE
overexpression
- OGA
O-GlcNAcase
- OGG
oxoguanine-DNA glycosylase
- OGT
O-GlcNAc Transferase
- OPA
optic atrophy
- OXPHOS
oxidative phosphorylation
- PC
phosphatidylcholines
- PCr
phosphocreatine
- PDK
pyruvate dehydrogenase kinase
- PDL1
programmed death-ligand
- PET
positron emission tomography
- PFK
phosphofructokinase
- PFKFB
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3
- PG
phosphatidylglycerol
- PGC
PPARy co-activator 1a
- PGI
phosphoglucose isomerase
- PGK
phosphoglycerate kinase
- Pi
phosphatidylinositol
- PI3K
phosphoinositide 3-kinase
- PKC
protein kinase C
- PL
phospholipid
- PLA
lipoprotein-associated phospholipase A
- PLB
phospholamban
- PLIN
perilipin protein
- POLGA
DNA polymerase gamma, subunit A
- PP
pulse pressure
- PP2A
protein phosphatase 2A
- PP2Cm
protein phosphatase 2C , mitochondrial
- PPAR
peroxisome proliferator-activated receptor
- PRAS
proline-rich Akt substrate
- PRC
PGC-1-related coactivator
- proBNP
pro b-type natriuretic peptide
- PRX
peroxiredoxin
- PTP
permeability transition pore
- PTX3
pentraxin-3
- RAAS
renin-angiotensin-aldosterone system
- RNA
ribonucleic acid
- ROS
reactive oxygen species
- RV
right ventricle
- RXR
retinoid X receptor
- RyR
ryanodine receptor
- S6
ribosomal protein s6
- S6K
S6 kinase
- S6K1
ribosomal protein S6 kinase beta
- SBP
systolic blood pressure
- SCAD
short-chain
- SCOT
succinyl-CoA:3-oxoacid-CoA transferase
- SERCA
sarcoplasmic reticulum Calcium-ATPase pump
- SFA
saturated fatty acid
- SGLT2i
sodium-glucose cotransporter-2 inhibitor
- SH
spontaneously-hypertensive
- SHR
spontaneously hypertensive rat
- SIRT
sirtuin
- SLC
solute carrier family
- SM
sphingomyelins
- SP
specificity protein
- SREBP
sterol element binding regulatory proteins
- SSM
subsarcolemmal mitochondria
- STAT
signal transducer and activator of transcription
- STZ
streptozotocin
- T2DM
type 2 diabetic
- TAC
transverse aortic constriction
- TCA
tricarboxylic acid cycle
- TFAm
mitochondrial transcription factor A
- TFB
transcription factor B
- TFB1M
TFB1M transcription factor B1
- TG
triglyceride
- TGF
transforming growth factor
- TIGAR
TP53-induced glycolysis and apoptosis regulator
- TnC
troponin C
- TNF
tumor necrosis factor
- TnT
troponin T
- TRIB
tribbles homolog
- TRPC
transient receptor potential cation channel
- TTN
titin
- UCP
uncoupled protein
- UDP
uridine diphosphate
- UDPG-PPL
UDP-glucose pyrophosphorylase
- UNSAT
unsaturated fat
- UPR
unfolded protein response
- UQCRC
ubiquinol-cytochrome c reductase core protein
- VDAC
voltage-dependent anion-selective channel
- VEGF
vascular endothelial growth factor
- VLCAD
very long-chain acyl-CoA dehydrogenase
- VLDL
very low density lipoprotein
- WAT
white adipose tissue
- WD
western diet
- YME1L
ATP-dependent metalloprotease
- ZDF
Zucker diabetic fatty
- ZF
Zucker fatty
- α-KGDH
alpha-ketoglutarate dehydrogenase
- βOHB
β-hydroxybutyrate
Footnotes
DISCLOSURES
None.
References
- 1.Taegtmeyer H. Energy metabolism of the heart: from basic concepts to clinical applications. Curr Probl Cardiol. 1994;19:59–113. [DOI] [PubMed] [Google Scholar]
- 2.Stanley WC, Recchia FA and Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–129. [DOI] [PubMed] [Google Scholar]
- 3.Maack C, Lehrke M, Backs J, Heinzel FR, Hulot JS, Marx N, Paulus WJ, Rossignol P, Taegtmeyer H, Bauersachs J, B, et al. Heart failure and diabetes: metabolic alterations and therapeutic interventions: a state-of-the-art review from the Translational Research Committee of the Heart Failure Association-European Society of Cardiology. Eur Heart J. 2018;39:4243–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, Rabinowitz JD, Frankel DS and Arany Z. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science. 2020;370:364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdul Kadir A, Clarke K and Evans RD. Cardiac ketone body metabolism. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165739. [DOI] [PubMed] [Google Scholar]
- 6.Fillmore N, Wagg CS, Zhang L, Fukushima A and Lopaschuk GD. Cardiac branched-chain amino acid oxidation is reduced during insulin resistance in the heart. Am J Physiol Endocrinol Metab. 2018;315:E1046–e1052. [DOI] [PubMed] [Google Scholar]
- 7.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS and Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–58. [DOI] [PubMed] [Google Scholar]
- 8.Huss JM and Kelly DP. Nuclear receptor signaling and cardiac energetics. Circ Res. 2004;95:568–78. [DOI] [PubMed] [Google Scholar]
- 9.Lopaschuk GD, Spafford MA and Marsh DR. Glycolysis is predominant source of myocardial ATP production immediately after birth. Am J Physiol. 1991;261:H1698–705. [DOI] [PubMed] [Google Scholar]
- 10.Tsushima K, Bugger H, Wende AR, Soto J, Jenson GA, Tor AR, McGlauflin R, Kenny HC, Zhang Y, Souvenir R, et al. Mitochondrial Reactive Oxygen Species in Lipotoxic Hearts Induce Post-Translational Modifications of AKAP121, DRP1, and OPA1 That Promote Mitochondrial Fission. Circ Res. 2018;122:58–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itoi T and Lopaschuk GD. The contribution of glycolysis, glucose oxidation, lactate oxidation, and fatty acid oxidation to ATP production in isolated biventricular working hearts from 2-week-old rabbits. Pediatr Res. 1993;34:735–41. [DOI] [PubMed] [Google Scholar]
- 12.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM and Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bairwa SC, Parajuli N and Dyck JR. The role of AMPK in cardiomyocyte health and survival. Biochim Biophys Acta. 2016;1862:2199–2210. [DOI] [PubMed] [Google Scholar]
- 14.Hue L and Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab. 2009;297:E578–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonow RO. Contractile reserve and coronary blood flow reserve in collateral-dependent myocardium. J Am Coll Cardiol. 1999;33:705–7. [DOI] [PubMed] [Google Scholar]
- 16.Fell DA. Increasing the flux in metabolic pathways: A metabolic control analysis perspective. Biotechnol Bioeng. 1998;58:121–4. [DOI] [PubMed] [Google Scholar]
- 17.Kassiotis C, Rajabi M and Taegtmeyer H. Metabolic reserve of the heart: the forgotten link between contraction and coronary flow. Prog Cardiovasc Dis. 2008;51:74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–71. [DOI] [PubMed] [Google Scholar]
- 19.Bugger H, Schwarzer M, Chen D, Schrepper A, Amorim PA, Schoepe M, Nguyen TD, Mohr FW, Khalimonchuk O, Weimer BC, et al. Proteomic remodelling of mitochondrial oxidative pathways in pressure overload-induced heart failure. Cardiovasc Res. 2010;85:376–84. [DOI] [PubMed] [Google Scholar]
- 20.Garnier A, Fortin D, Deloménie C, Momken I, Veksler V and Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol. 2003;551:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A and Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci U S A. 2006;103:10086–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oka SI, Sabry AD, Cawley KM and Warren JS. Multiple Levels of PGC-1α Dysregulation in Heart Failure. Front Cardiovasc Med. 2020;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beltrán-Sánchez H, Harhay MO, Harhay MM and McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999-2010. J Am Coll Cardiol. 2013;62:697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rochlani Y, Pothineni NV, Kovelamudi S and Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis. 2017;11:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H, Li X, Adams H, Kubena K and Guo S. Etiology of Metabolic Syndrome and Dietary Intervention. Int J Mol Sci. 2018;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samson SL and Garber AJ. Metabolic syndrome. Endocrinol Metab Clin North Am. 2014;43:1–23. [DOI] [PubMed] [Google Scholar]
- 27.Monteiro R and Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A and Dandona P. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52:2882–7. [DOI] [PubMed] [Google Scholar]
- 29.Seferović PM and Paulus WJ. Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J. 2015;36:1718–27, 1727a-1727c. [DOI] [PubMed] [Google Scholar]
- 30.Levelt E, Mahmod M, Piechnik SK, Ariga R, Francis JM, Rodgers CT, Clarke WT, Sabharwal N, Schneider JE, Karamitsos TD, et al. Relationship Between Left Ventricular Structural and Metabolic Remodeling in Type 2 Diabetes. Diabetes. 2016;65:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bugger H and Abel ED. Rodent models of diabetic cardiomyopathy. Dis Model Mech. 2009;2:454–66. [DOI] [PubMed] [Google Scholar]
- 32.Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, et al. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–66. [DOI] [PubMed] [Google Scholar]
- 33.Mazumder PK, O'Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S and Abel ED. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53:2366–74. [DOI] [PubMed] [Google Scholar]
- 34.Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, Cooksey RC, Litwin SE and Abel ED. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–9. [DOI] [PubMed] [Google Scholar]
- 35.Aasum E, Hafstad AD, Severson DL and Larsen TS. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes. 2003;52:434–41. [DOI] [PubMed] [Google Scholar]
- 36.How OJ, Aasum E, Severson DL, Chan WY, Essop MF and Larsen TS. Increased myocardial oxygen consumption reduces cardiac efficiency in diabetic mice. Diabetes. 2006;55:466–73. [DOI] [PubMed] [Google Scholar]
- 37.Boudina S, Sena S, O'Neill BT, Tathireddy P, Young ME and Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation. 2005;112:2686–95. [DOI] [PubMed] [Google Scholar]
- 38.Boudina S, Bugger H, Sena S, O'Neill BT, Zaha VG, Ilkun O, Wright JJ, Mazumder PK, Palfreyman E, Tidwell TJ, et al. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation. 2009;119:1272–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gollmer J, Zirlik A and Bugger H. Mitochondrial Mechanisms in Diabetic Cardiomyopathy. Diabetes Metab J. 2020;44:33–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bugger H and Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson RH, Thompson RP and Kern CB. Development of aortic valves with 2 and 3 leaflets. J Am Coll Cardiol. 2009;54:2319–20. [DOI] [PubMed] [Google Scholar]
- 42.Anderson EJ, Rodriguez E, Anderson CA, Thayne K, Chitwood WR and Kypson AP. Increased propensity for cell death in diabetic human heart is mediated by mitochondrial-dependent pathways. Am J Physiol Heart Circ Physiol. 2011;300:H118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–6. [DOI] [PubMed] [Google Scholar]
- 44.Rijzewijk LJ, van der Meer RW, Lamb HJ, de Jong HW, Lubberink M, Romijn JA, Bax JJ, de Roos A, Twisk JW, Heine RJ, et al. Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: studies with cardiac positron emission tomography and magnetic resonance imaging. J Am Coll Cardiol. 2009;54:1524–32. [DOI] [PubMed] [Google Scholar]
- 45.Waterston RH, Lander ES and Sulston JE. On the sequencing of the human genome. Proc Natl Acad Sci U S A. 2002;99:3712–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Champy MF, Selloum M, Zeitler V, Caradec C, Jung B, Rousseau S, Pouilly L, Sorg T and Auwerx J. Genetic background determines metabolic phenotypes in the mouse. Mamm Genome. 2008;19:318–31. [DOI] [PubMed] [Google Scholar]
- 47.Fontaine DA and Davis DB. Attention to Background Strain Is Essential for Metabolic Research: C57BL/6 and the International Knockout Mouse Consortium. Diabetes. 2016;65:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nickel AG, von Hardenberg A, Hohl M, Löffler JR, Kohlhaas M, Becker J, Reil JC, Kazakov A, Bonnekoh J, Stadelmaier M, et al. Reversal of Mitochondrial Transhydrogenase Causes Oxidative Stress in Heart Failure. Cell Metab. 2015;22:472–84. [DOI] [PubMed] [Google Scholar]
- 49.Ouwens DM, Diamant M, Fodor M, Habets DDJ, Pelsers M, El Hasnaoui M, Dang ZC, van den Brom CE, Vlasblom R, Rietdijk A, et al. Cardiac contractile dysfunction in insulin-resistant rats fed a high-fat diet is associated with elevated CD36-mediated fatty acid uptake and esterification. Diabetologia. 2007;50:1938–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson CR, Tran MK, Salazar KL, Young ME and Taegtmeyer H. Western diet, but not high fat diet, causes derangements of fatty acid metabolism and contractile dysfunction in the heart of Wistar rats. Biochem J. 2007;406:457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ge F, Zhou S, Hu C, Lobdell Ht and Berk PD. Insulin- and leptin-regulated fatty acid uptake plays a key causal role in hepatic steatosis in mice with intact leptin signaling but not in ob/ob or db/db mice. Am J Physiol Gastrointest Liver Physiol. 2010;299:G855–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L, Ussher JR, Oka T, Cadete VJ, Wagg C and Lopaschuk GD. Cardiac diacylglycerol accumulation in high fat-fed mice is associated with impaired insulin-stimulated glucose oxidation. Cardiovasc Res. 2011;89:148–56. [DOI] [PubMed] [Google Scholar]
- 53.Brahma MK, Ha CM, Pepin ME, Mia S, Sun Z, Chatham JC, Habegger KM, Abel ED, Paterson AJ, Young ME, et al. Increased Glucose Availability Attenuates Myocardial Ketone Body Utilization. J Am Heart Assoc. 2020;9:e013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tadinada SM, Weatherford ET, Collins GV, Bhardwaj G, Cochran J, Kutschke W, Zimmerman K, Bosko A, O'Neill BT, Weiss RM, et al. Functional resilience of C57BL/6J mouse heart to dietary fat overload. Am J Physiol Heart Circ Physiol. 2021;321:H850–H864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Q, Liu Y, Fu Q, Xu B, Zhang Y, Kim S, Tan R, Barbagallo F, West T, Anderson E, et al. Inhibiting Insulin-Mediated beta2-Adrenergic Receptor Activation Prevents Diabetes-Associated Cardiac Dysfunction. Circulation. 2017;135:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang JP, Cheng ML, Wang CH, Shiao MS, Chen JK and Hung LM. High-fructose and high-fat feeding correspondingly lead to the development of lysoPC-associated apoptotic cardiomyopathy and adrenergic signaling-related cardiac hypertrophy. Int J Cardiol. 2016;215:65–76. [DOI] [PubMed] [Google Scholar]
- 57.Wright JJ, Kim J, Buchanan J, Boudina S, Sena S, Bakirtzi K, Ilkun O, Theobald HA, Cooksey RC, Kandror KV, et al. Mechanisms for increased myocardial fatty acid utilization following short-term high-fat feeding. Cardiovasc Res. 2009;82:351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luptak I, Qin F, Sverdlov AL, Pimentel DR, Panagia M, Croteau D, Siwik DA, Bachschmid MM, He H, Balschi JA, et al. Energetic Dysfunction Is Mediated by Mitochondrial Reactive Oxygen Species and Precedes Structural Remodeling in Metabolic Heart Disease. Antioxid Redox Signal. 2019;31:539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hall JL, Stanley WC, Lopaschuk GD, Wisneski JA, Pizzurro RD, Hamilton CD and McCormack JG. Impaired pyruvate oxidation but normal glucose uptake in diabetic pig heart during dobutamine-induced work. Am J Physiol. 1996;271:H2320–9. [DOI] [PubMed] [Google Scholar]
- 60.Hasselbaink DM, Glatz JF, Luiken JJ, Roemen TH and Van der Vusse GJ. Ketone bodies disturb fatty acid handling in isolated cardiomyocytes derived from control and diabetic rats. Biochem J. 2003;371:753–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bugger H, Riehle C, Jaishy B, Wende AR, Tuinei J, Chen D, Soto J, Pires KM, Boudina S, Theobald HA, et al. Genetic loss of insulin receptors worsens cardiac efficiency in diabetes. J Mol Cell Cardiol. 2012;52:1019–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Young ME, Guthrie PH, Razeghi P, Leighton B, Abbasi S, Patil S, Youker KA and Taegtmeyer H. Impaired long-chain fatty acid oxidation and contractile dysfunction in the obese Zucker rat heart. Diabetes. 2002;51:2587–95. [DOI] [PubMed] [Google Scholar]
- 63.Wang P, Lloyd SG, Zeng H, Bonen A and Chatham JC. Impact of altered substrate utilization on cardiac function in isolated hearts from Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2005;288:H2102–10. [DOI] [PubMed] [Google Scholar]
- 64.Summer G, Kuhn AR, Munts C, Miranda-Silva D, Leite-Moreira AF, Lourenco AP, Heymans S, Falcao-Pires I and van Bilsen M. A directed network analysis of the cardiome identifies molecular pathways contributing to the development of HFpEF. J Mol Cell Cardiol. 2020;144:66–75. [DOI] [PubMed] [Google Scholar]
- 65.Sloan C, Tuinei J, Nemetz K, Frandsen J, Soto J, Wride N, Sempokuya T, Alegria L, Bugger H, Abel ED. Central leptin signaling is required to normalize myocardial fatty acid oxidation rates in caloric-restricted ob/ob mice. Diabetes. 2011;60:1424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen X, Zheng S, Thongboonkerd V, Xu M, Pierce WM Jr., Klein JB and Epstein PN. Cardiac mitochondrial damage and biogenesis in a chronic model of type 1 diabetes. Am J Physiol Endocrinol Metab. 2004;287:E896–905. [DOI] [PubMed] [Google Scholar]
- 67.Rohm M, Savic D, Ball V, Curtis MK, Bonham S, Fischer R, Legrave N, MacRae JI, Tyler DJ, Ashcroft FM. Cardiac Dysfunction and Metabolic Inflexibility in a Mouse Model of Diabetes Without Dyslipidemia. Diabetes. 2018;67:1057–1067. [DOI] [PubMed] [Google Scholar]
- 68.Bugger H, Chen D, Riehle C, Soto J, Theobald HA, Hu XX, Ganesan B, Weimer BC , Abel ED. Tissue-specific remodeling of the mitochondrial proteome in type 1 diabetic akita mice. Diabetes. 2009;58:1986–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bugger H, Boudina S, Hu XX, Tuinei J, Zaha VG, Theobald HA, Yun UJ, McQueen AP, Wayment B, Litwin SE, et al. Type 1 diabetic akita mouse hearts are insulin sensitive but manifest structurally abnormal mitochondria that remain coupled despite increased uncoupling protein 3. Diabetes. 2008;57:2924–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dragoljevic D, Veiga CB, Michell DL, Shihata WA, Al-Sharea A, Head GA, Murphy AJ, Kraakman MJ and Lee MKS. A spontaneously hypertensive diet-induced atherosclerosis-prone mouse model of metabolic syndrome. Biomed Pharmacother. 2021;139:111668. [DOI] [PubMed] [Google Scholar]
- 71.Taegtmeyer H, Young ME, Lopaschuk GD, Abel ED, Brunengraber H, Darley-Usmar V, Des Rosiers C, Gerszten R, Glatz JF, Griffin JL, et al. A Scientific Statement From the American Heart Association. Circ Res. 2016;118:1659–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Son NH, Basu D, Samovski D, Pietka TA, Peche VS, Willecke F, Fang X, Yu SQ, Scerbo D, Chang HR, et al. Endothelial cell CD36 optimizes tissue fatty acid uptake. J Clin Invest. 2018;128:4329–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gibb AA, Lazaropoulos MP and Elrod JW. Myofibroblasts and Fibrosis: Mitochondrial and Metabolic Control of Cellular Differentiation. Circ Res. 2020;127:427–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coburn CT, Knapp FF Jr., Febbraio M, Beets AL, Silverstein RL and Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem. 2000;275:32523–9. [DOI] [PubMed] [Google Scholar]
- 75.Sung MM, Byrne NJ, Kim TT, Levasseur J, Masson G, Boisvenue JJ, Febbraio M and Dyck JR. Cardiomyocyte-specific ablation of CD36 accelerates the progression from compensated cardiac hypertrophy to heart failure. Am J Physiol Heart Circ Physiol. 2017;312:H552–H560. [DOI] [PubMed] [Google Scholar]
- 76.Koonen DP, Febbraio M, Bonnet S, Nagendran J, Young ME, Michelakis ED and Dyck JR. CD36 expression contributes to age-induced cardiomyopathy in mice. Circulation. 2007;116:2139–47. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y, Bao M, Dai M, Wang X, He W, Tan T, Lin D, Wang W, Wen Y and Zhang R. Cardiospecific CD36 suppression by lentivirus-mediated RNA interference prevents cardiac hypertrophy and systolic dysfunction in high-fat-diet induced obese mice. Cardiovasc Diabetol. 2015;14:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang J, Sambandam N, Han X, Gross RW, Courtois M, Kovacs A, Febbraio M, Finck BN and Kelly DP. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ Res. 2007;100:1208–17. [DOI] [PubMed] [Google Scholar]
- 79.Chiu HC, Kovacs A, Blanton RM, Han X, Courtois M, Weinheimer CJ, Yamada KA, Brunet S, Xu H, Nerbonne JM, et al. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res. 2005;96:225–33. [DOI] [PubMed] [Google Scholar]
- 80.Ellis JM, Mentock SM, Depetrillo MA, Koves TR, Sen S, Watkins SM, Muoio DM, Cline GW, Taegtmeyer H, Shulman GI, Willis MS, et al. Mouse cardiac acyl coenzyme a synthetase 1 deficiency impairs Fatty Acid oxidation and induces cardiac hypertrophy. Mol Cell Biol. 2011;31:1252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE and Schaffer JE. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goldenberg JR, Wang X and Lewandowski ED. Acyl CoA synthetase-1 links facilitated long chain fatty acid uptake to intracellular metabolic trafficking differently in hearts of male versus female mice. J Mol Cell Cardiol. 2016;94:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goldenberg JR, Carley AN, Ji R, Zhang X, Fasano M, Schulze PC and Lewandowski ED. Preservation of Acyl Coenzyme A Attenuates Pathological and Metabolic Cardiac Remodeling Through Selective Lipid Trafficking. Circulation. 2019;139:2765–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bakermans AJ, Geraedts TR, van Weeghel M, Denis S, Joao Ferraz M, Aerts JM, Aten J, Nicolay K, Houten SM and Prompers JJ. Fasting-induced myocardial lipid accumulation in long-chain acyl-CoA dehydrogenase knockout mice is accompanied by impaired left ventricular function. Circ Cardiovasc Imaging. 2011;4:558–65. [DOI] [PubMed] [Google Scholar]
- 85.Tucci S, Flogel U, Hermann S, Sturm M, Schafers M and Spiekerkoetter U. Development and pathomechanisms of cardiomyopathy in very long-chain acyl-CoA dehydrogenase deficient (VLCAD(−/−)) mice. Biochim Biophys Acta. 2014;1842:677–85. [DOI] [PubMed] [Google Scholar]
- 86.Yagyu H, Chen G, Yokoyama M, Hirata K, Augustus A, Kako Y, Seo T, Hu Y, Lutz EP, Merkel M, et al. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest. 2003;111:419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Augustus AS, Buchanan J, Park TS, Hirata K, Noh HL, Sun J, Homma S, D'Armiento J, Abel ED and Goldberg IJ. Loss of lipoprotein lipase-derived fatty acids leads to increased cardiac glucose metabolism and heart dysfunction. J Biol Chem. 2006;281:8716–23. [DOI] [PubMed] [Google Scholar]
- 88.van Weeghel M, Abdurrachim D, Nederlof R, Argmann CA, Houtkooper RH, Hagen J, Nabben M, Denis S, Ciapaite J, Kolwicz SC Jr., et al. Increased cardiac fatty acid oxidation in a mouse model with decreased malonyl-CoA sensitivity of CPT1B. Cardiovasc Res. 2018;114:1324–1334. [DOI] [PubMed] [Google Scholar]
- 89.Kolwicz SC Jr., Olson DP, Marney LC, Garcia-Menendez L, Synovec RE and Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circ Res. 2012;111:728–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zordoky BN, Nagendran J, Pulinilkunnil T, Kienesberger PC, Masson G, Waller TJ, Kemp BE, Steinberg GR and Dyck JR. AMPK-dependent inhibitory phosphorylation of ACC is not essential for maintaining myocardial fatty acid oxidation. Circ Res. 2014;115:518–24. [DOI] [PubMed] [Google Scholar]
- 91.Dyck JR, Hopkins TA, Bonnet S, Michelakis ED, Young ME, Watanabe M, Kawase Y, Jishage K and Lopaschuk GD. Absence of malonyl coenzyme A decarboxylase in mice increases cardiac glucose oxidation and protects the heart from ischemic injury. Circulation. 2006;114:1721–8. [DOI] [PubMed] [Google Scholar]
- 92.Ussher JR, Koves TR, Jaswal JS, Zhang L, Ilkayeva O, Dyck JR, Muoio DM and Lopaschuk GD. Insulin-stimulated cardiac glucose oxidation is increased in high-fat diet-induced obese mice lacking malonyl CoA decarboxylase. Diabetes. 2009;58:1766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.He L, Kim T, Long Q, Liu J, Wang P, Zhou Y, Ding Y, Prasain J, Wood PA and Yang Q. Carnitine palmitoyltransferase-1b deficiency aggravates pressure overload-induced cardiac hypertrophy caused by lipotoxicity. Circulation. 2012;126:1705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lewandowski ED, Fischer SK, Fasano M, Banke NH, Walker LA, Huqi A, Wang X, Lopaschuk GD and O'Donnell JM. Acute liver carnitine palmitoyltransferase I overexpression recapitulates reduced palmitate oxidation of cardiac hypertrophy. Circ Res. 2013;112:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roe ND, Handzlik MK, Li T and Tian R. The Role of Diacylglycerol Acyltransferase (DGAT) 1 and 2 in Cardiac Metabolism and Function. Sci Rep. 2018;8:4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu L, Shi X, Bharadwaj KG, Ikeda S, Yamashita H, Yagyu H, Schaffer JE, Yu YH and Goldberg IJ. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J Biol Chem. 2009;284:36312–36323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haemmerle G, Moustafa T, Woelkart G, Buttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med. 2011;17:1076–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kienesberger PC, Pulinilkunnil T, Nagendran J, Young ME, Bogner-Strauss JG, Hackl H, Khadour R, Heydari E, Haemmerle G, Zechner R, et al. Early structural and metabolic cardiac remodelling in response to inducible adipose triglyceride lipase ablation. Cardiovasc Res. 2013;99:442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pulinilkunnil T, Kienesberger PC, Nagendran J, Waller TJ, Young ME, Kershaw EE, Korbutt G, Haemmerle G, Zechner R and Dyck JR. Myocardial adipose triglyceride lipase overexpression protects diabetic mice from the development of lipotoxic cardiomyopathy. Diabetes. 2013;62:1464–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martin OJ, Lai L, Soundarapandian MM, Leone TC, Zorzano A, Keller MP, Attie AD, Muoio DM and Kelly DP. A role for peroxisome proliferator-activated receptor gamma coactivator-1 in the control of mitochondrial dynamics during postnatal cardiac growth. Circ Res. 2014;114:626–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lehman JJ, Boudina S, Banke NH, Sambandam N, Han X, Young DM, Leone TC, Gross RW, Lewandowski ED, Abel ED, et al. The transcriptional coactivator PGC-1alpha is essential for maximal and efficient cardiac mitochondrial fatty acid oxidation and lipid homeostasis. Am J Physiol Heart Circ Physiol. 2008;295:H185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu Z, Xu X, Hu X, Fassett J, Zhu G, Tao Y, Li J, Huang Y, Zhang P, Zhao B, et al. PGC-1 alpha regulates expression of myocardial mitochondrial antioxidants and myocardial oxidative stress after chronic systolic overload. Antioxid Redox Signal. 2010;13:1011–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Riehle C, Wende AR, Zaha VG, Pires KM, Wayment B, Olsen C, Bugger H, Buchanan J, Wang X, Moreira AB, et al. PGC-1beta deficiency accelerates the transition to heart failure in pressure overload hypertrophy. Circ Res. 2011;109:783–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Russell LK, Mansfield CM, Lehman JJ, Kovacs A, Courtois M, Saffitz JE, Medeiros DM, Valencik ML, McDonald JA and Kelly DP. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res. 2004;94:525–33. [DOI] [PubMed] [Google Scholar]
- 105.Di W, Lv J, Jiang S, Lu C, Yang Z, Ma Z, Hu W, Yang Y and Xu B. PGC-1: The Energetic Regulator in Cardiac Metabolism. Curr Issues Mol Biol. 2018;28:29–46. [DOI] [PubMed] [Google Scholar]
- 106.Huo L and Scarpulla RC. Mitochondrial DNA instability and peri-implantation lethality associated with targeted disruption of nuclear respiratory factor 1 in mice. Mol Cell Biol. 2001;21:644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang ZF, Drumea K, Mott S, Wang J and Rosmarin AG. GABP transcription factor (nuclear respiratory factor 2) is required for mitochondrial biogenesis. Mol Cell Biol. 2014;34:3194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huss JM, Imahashi K, Dufour CR, Weinheimer CJ, Courtois M, Kovacs A, Giguere V, Murphy E and Kelly DP. The nuclear receptor ERRalpha is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab. 2007;6:25–37. [DOI] [PubMed] [Google Scholar]
- 109.Wang T, McDonald C, Petrenko NB, Leblanc M, Wang T, Giguere V, Evans RM, Patel VV and Pei L. Estrogen-related receptor alpha (ERRalpha) and ERRgamma are essential coordinators of cardiac metabolism and function. Mol Cell Biol. 2015;35:1281–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, Jonker JW, Giles W, Naviaux RK, Giguere V, et al. ERRgamma directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab. 2007;6:13–24. [DOI] [PubMed] [Google Scholar]
- 111.Leone TC, Weinheimer CJ and Kelly DP. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci U S A. 1999;96:7473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Luptak I, Balschi JA, Xing Y, Leone TC, Kelly DP and Tian R. Decreased contractile and metabolic reserve in peroxisome proliferator-activated receptor-alpha-null hearts can be rescued by increasing glucose transport and utilization. Circulation. 2005;112:2339–46. [DOI] [PubMed] [Google Scholar]
- 113.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N, Evans RM, Schneider MD, Brako FA, Xiao Y, et al. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med. 2004;10:1245–50. [DOI] [PubMed] [Google Scholar]
- 115.Wang P, Liu J, Li Y, Wu S, Luo J, Yang H, Subbiah R, Chatham J, Zhelyabovska O and Yang Q. Peroxisome proliferator-activated receptor {delta} is an essential transcriptional regulator for mitochondrial protection and biogenesis in adult heart. Circ Res. 2010;106:911–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Duan SZ, Ivashchenko CY, Russell MW, Milstone DS and Mortensen RM. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-gamma both induce cardiac hypertrophy in mice. Circ Res. 2005;97:372–9. [DOI] [PubMed] [Google Scholar]
- 117.Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS and Goldberg IJ. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J Clin Invest. 2007;117:2791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Viollet B, Athea Y, Mounier R, Guigas B, Zarrinpashneh E, Horman S, Lantier L, Hebrard S, Devin-Leclerc J, Beauloye C, et al. AMPK: Lessons from transgenic and knockout animals. Front Biosci (Landmark Ed). 2009;14:19–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Athea Y, Viollet B, Mateo P, Rousseau D, Novotova M, Garnier A, Vaulont S, Wilding JR, Grynberg A, Veksler V, et al. AMP-activated protein kinase alpha2 deficiency affects cardiac cardiolipin homeostasis and mitochondrial function. Diabetes. 2007;56:786–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Russell RR 3rd, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ and Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xing Y, Musi N, Fujii N, Zou L, Luptak I, Hirshman MF, Goodyear LJ and Tian R. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative alpha2 subunit of AMP-activated protein kinase. J Biol Chem. 2003;278:28372–7. [DOI] [PubMed] [Google Scholar]
- 122.Luptak I, Shen M, He H, Hirshman MF, Musi N, Goodyear LJ, Yan J, Wakimoto H, Morita H, Arad M, et al. Aberrant activation of AMP-activated protein kinase remodels metabolic network in favor of cardiac glycogen storage. J Clin Invest. 2007;117:1432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim M, Hunter RW, Garcia-Menendez L, Gong G, Yang YY, Kolwicz SC Jr., Xu J, Sakamoto K, Wang W and Tian R. Mutation in the gamma2-subunit of AMP-activated protein kinase stimulates cardiomyocyte proliferation and hypertrophy independent of glycogen storage. Circ Res. 2014;114:966–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Katz EB, Stenbit AE, Hatton K, DePinho R and Charron MJ. Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4. Nature. 1995;377:151–5. [DOI] [PubMed] [Google Scholar]
- 125.Pereira RO, Wende AR, Olsen C, Soto J, Rawlings T, Zhu Y, Riehle C and Abel ED. GLUT1 deficiency in cardiomyocytes does not accelerate the transition from compensated hypertrophy to heart failure. J Mol Cell Cardiol. 2014;72:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liao R, Jain M, Cui L, D'Agostino J, Aiello F, Luptak I, Ngoy S, Mortensen RM and Tian R. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation. 2002;106:2125–31. [DOI] [PubMed] [Google Scholar]
- 127.Pereira RO, Wende AR, Olsen C, Soto J, Rawlings T, Zhu Y, Anderson SM and Abel ED. Inducible overexpression of GLUT1 prevents mitochondrial dysfunction and attenuates structural remodeling in pressure overload but does not prevent left ventricular dysfunction. J Am Heart Assoc. 2013;2:e000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yan J, Young ME, Cui L, Lopaschuk GD, Liao R and Tian R. Increased glucose uptake and oxidation in mouse hearts prevent high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation. 2009;119:2818–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy J, Doetschman T, Minnemann T, Boers ME, Hadro E, Oberste-Berghaus C, et al. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest. 1999;104:1703–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wende AR, Kim J, Holland WL, Wayment BE, O'Neill BT, Tuinei J, Brahma MK, Pepin ME, McCrory MA, et al. Glucose transporter 4-deficient hearts develop maladaptive hypertrophy in response to physiological or pathological stresses. Am J Physiol Heart Circ Physiol. 2017;313:H1098–H1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Semeniuk LM, Kryski AJ and Severson DL. Echocardiographic assessment of cardiac function in diabetic db/db and transgenic db/db-hGLUT4 mice. Am J Physiol Heart Circ Physiol. 2002;283:H976–82. [DOI] [PubMed] [Google Scholar]
- 132.Wende AR, Schell JC, Ha CM, Pepin ME, Khalimonchuk O, Schwertz H, Pereira RO, Brahma MK, Tuinei J, Contreras-Ferrat A, et al. Maintaining Myocardial Glucose Utilization in Diabetic Cardiomyopathy Accelerates Mitochondrial Dysfunction. Diabetes. 2020;69:2094–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Donthi RV, Ye G, Wu C, McClain DA, Lange AJ and Epstein PN. Cardiac expression of kinase-deficient 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase inhibits glycolysis, promotes hypertrophy, impairs myocyte function, and reduces insulin sensitivity. J Biol Chem. 2004;279:48085–90. [DOI] [PubMed] [Google Scholar]
- 134.Chambers KT, Leone TC, Sambandam N, Kovacs A, Wagg CS, Lopaschuk GD, Finck BN and Kelly DP. Chronic inhibition of pyruvate dehydrogenase in heart triggers an adaptive metabolic response. J Biol Chem. 2011;286:11155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang Y, Taufalele PV, Cochran JD, Robillard-Frayne I, Marx JM, Soto J, Rauckhorst AJ, Tayyari F, Pewa AD, Gray LR, T et al. Mitochondrial pyruvate carriers are required for myocardial stress adaptation. Nat Metab. 2020;2:1248–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McCommis KS, Kovacs A, Weinheimer CJ, Shew TM, Koves TR, Ilkayeva OR, Kamm DR, Pyles KD, King MT, Veech RL, et al. Nutritional modulation of heart failure in mitochondrial pyruvate carrier-deficient mice. Nat Metab. 2020;2:1232–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fernandez-Caggiano M, Kamynina A, Francois AA, Prysyazhna O, Eykyn TR, Krasemann S, Crespo-Leiro MG, Vieites MG, Bianchi K, Morales V, et al. Mitochondrial pyruvate carrier abundance mediates pathological cardiac hypertrophy. Nat Metab. 2020;2:1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cluntun AA, Badolia R, Lettlova S, Parnell KM, Shankar TS, Diakos NA, Olson KA, Taleb I, Tatum SM, Berg JA, et al. The pyruvate-lactate axis modulates cardiac hypertrophy and heart failure. Cell Metab. 2021;33:629–648 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Abel ED. Insulin signaling in the heart. Am J Physiol Endocrinol Metab. 2021;321:E130–e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, Zhang D, Cooksey RC, McClain DA, Litwin SE, et al. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest. 2002;109:629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ikeda H, Shiojima I, Ozasa Y, Yoshida M, Holzenberger M, Kahn CR, Walsh K, Igarashi T, Abel ED and Komuro I. Interaction of myocardial insulin receptor and IGF receptor signaling in exercise-induced cardiac hypertrophy. J Mol Cell Cardiol. 2009;47:664–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Riehle C, Wende AR, Sena S, Pires KM, Pereira RO, Zhu Y, Bugger H, Frank D, Bevins J, Chen D, et al. Insulin receptor substrate signaling suppresses neonatal autophagy in the heart. J Clin Invest. 2013;123:5319–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, Cantley LC and Izumo S. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J. 2000;19:2537–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.O'Neill BT, Kim J, Wende AR, Theobald HA, Tuinei J, Buchanan J, Guo A, Zaha VG, Davis DK, Schell JC, et al. A conserved role for phosphatidylinositol 3-kinase but not Akt signaling in mitochondrial adaptations that accompany physiological cardiac hypertrophy. Cell Metab. 2007;6:294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R and Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem. 2002;277:22896–901. [DOI] [PubMed] [Google Scholar]
- 146.Wende AR, O'Neill BT, Bugger H, Riehle C, Tuinei J, Buchanan J, Tsushima K, Wang L, Caro P, Guo A, et al. Enhanced cardiac Akt/protein kinase B signaling contributes to pathological cardiac hypertrophy in part by impairing mitochondrial function via transcriptional repression of mitochondrion-targeted nuclear genes. Mol Cell Biol. 2015;35:831–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nagoshi T, Matsui T, Aoyama T, Leri A, Anversa P, Li L, Ogawa W, del Monte F, Gwathmey JK, Grazette L, et al. PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J Clin Invest. 2005;115:2128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M and Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation. 2006;113:2097–104. [DOI] [PubMed] [Google Scholar]
- 149.Ock S, Lee WS, Ahn J, Kim HM, Kang H, Kim HS, Jo D, Abel ED, Lee TJ and Kim J. Deletion of IGF-1 Receptors in Cardiomyocytes Attenuates Cardiac Aging in Male Mice. Endocrinology. 2016;157:336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.McMullen JR, Shioi T, Huang WY, Zhang L, Tarnavski O, Bisping E, Schinke M, Kong S, Sherwood MC, Brown J, et al. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110alpha) pathway. J Biol Chem. 2004;279:4782–93. [DOI] [PubMed] [Google Scholar]
- 151.Uchihashi M, Hoshino A, Okawa Y, Ariyoshi M, Kaimoto S, Tateishi S, Ono K, Yamanaka R, Hato D, Fushimura Y, et al. Cardiac-Specific Bdh1 Overexpression Ameliorates Oxidative Stress and Cardiac Remodeling in Pressure Overload-Induced Heart Failure. Circ Heart Fail. 2017;10. [DOI] [PubMed] [Google Scholar]
- 152.Schugar RC, Moll AR, Andre d'Avignon D, Weinheimer CJ, Kovacs A and Crawford PA. Cardiomyocyte-specific deficiency of ketone body metabolism promotes accelerated pathological remodeling. Mol Metab. 2014;3:754–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Carley AN, Maurya SK, Fasano M, Wang Y, Selzman CH, Drakos SG and Lewandowski ED. Short-Chain Fatty Acids Outpace Ketone Oxidation in the Failing Heart. Circulation. 2021;143:1797–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Uddin GM, Karwi QG, Pherwani S, Gopal K, Wagg CS, Biswas D, Atnasious M, Wu Y, Wu G, Zhang L, et al. Deletion of BCATm increases insulin-stimulated glucose oxidation in the heart. Metabolism. 2021;124:154871. [DOI] [PubMed] [Google Scholar]
- 155.Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z, Jeyaraj D, Youn JY, Ren S, Liu Y, et al. Catabolic Defect of Branched-Chain Amino Acids Promotes Heart Failure. Circulation. 2016;133:2038–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Walejko JM, Christopher BA, Crown SB, Zhang GF, Pickar-Oliver A, Yoneshiro T, Foster MW, Page S, van Vliet S, Ilkayeva O, et al. Branched-chain alpha-ketoacids are preferentially reaminated and activate protein synthesis in the heart. Nat Commun. 2021;12:1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW and Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100:10794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF and Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–21. [DOI] [PubMed] [Google Scholar]
- 159.Koentges C, Pfeil K, Schnick T, Wiese S, Dahlbock R, Cimolai MC, Meyer-Steenbuck M, Cenkerova K, Hoffmann MM, Jaeger C, et al. SIRT3 deficiency impairs mitochondrial and contractile function in the heart. Basic Res Cardiol. 2015;110:36. [DOI] [PubMed] [Google Scholar]
- 160.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A and Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Luo YX, Tang X, An XZ, Xie XM, Chen XF, Zhao X, Hao DL, Chen HZ and Liu DP. SIRT4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur Heart J. 2017;38:1389–1398. [DOI] [PubMed] [Google Scholar]
- 162.Bugger H, Witt CN and Bode C. Mitochondrial sirtuins in the heart. Heart Fail Rev. 2016;21:519–28. [DOI] [PubMed] [Google Scholar]
- 163.Hershberger KA, Abraham DM, Liu J, Locasale JW, Grimsrud PA and Hirschey MD. Ablation of Sirtuin5 in the postnatal mouse heart results in protein succinylation and normal survival in response to chronic pressure overload. J Biol Chem. 2018;293:10630–10645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sundaresan NR, Vasudevan P, Zhong L, Kim G, Samant S, Parekh V, Pillai VB, Ravindra PV, Gupta M, Jeevanandam V, et al. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat Med. 2012;18:1643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pillai VB, Samant S, Hund S, Gupta M and Gupta MP. The nuclear sirtuin SIRT6 protects the heart from developing aging-associated myocyte senescence and cardiac hypertrophy. Aging (Albany NY). 2021;13:12334–12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, Braun T and Bober E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102:703–10. [DOI] [PubMed] [Google Scholar]
- 167.Watson LJ, Long BW, DeMartino AM, Brittian KR, Readnower RD, Brainard RE, Cummins TD, Annamalai L, Hill BG and Jones SP. Cardiomyocyte Ogt is essential for postnatal viability. Am J Physiol Heart Circ Physiol. 2014;306:H142–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Umapathi P, Mesubi OO, Banerjee PS, Abrol N, Wang Q, Luczak ED, Wu Y, Granger JM, Wei AC, Reyes Gaido OE, et al. Excessive O-GlcNAcylation Causes Heart Failure and Sudden Death. Circulation. 2021;143:1687–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tran DH, May HI, Li Q, Luo X, Huang J, Zhang G, Niewold E, Wang X, Gillette TG, Deng Y, et al. Chronic activation of hexosamine biosynthesis in the heart triggers pathological cardiac remodeling. Nat Commun. 2020;11:1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Papanicolaou KN, Kikuchi R, Ngoh GA, Coughlan KA, Dominguez I, Stanley WC and Walsh K. Mitofusins 1 and 2 are essential for postnatal metabolic remodeling in heart. Circ Res. 2012;111:1012–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen Y, Liu Y and Dorn GW 2nd. Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res. 2011;109:1327–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Song M, Mihara K, Chen Y, Scorrano L and Dorn GW 2nd. Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab. 2015;21:273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Song M, Franco A, Fleischer JA, Zhang L and Dorn GW 2nd. Abrogating Mitochondrial Dynamics in Mouse Hearts Accelerates Mitochondrial Senescence. Cell Metab. 2017;26:872–883 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wai T, Garcia-Prieto J, Baker MJ, Merkwirth C, Benit P, Rustin P, Ruperez FJ, Barbas C, Ibanez B and Langer T. Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science. 2015;350:aad0116. [DOI] [PubMed] [Google Scholar]
- 175.Grueter CE, van Rooij E, Johnson BA, DeLeon SM, Sutherland LB, Qi X, Gautron L, Elmquist JK, Bassel-Duby R and Olson EN. A cardiac microRNA governs systemic energy homeostasis by regulation of MED13. Cell. 2012;149:671–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Baskin KK, Grueter CE, Kusminski CM, Holland WL, Bookout AL, Satapati S, Kong YM, Burgess SC, Malloy CR, Scherer PE, et al. MED13-dependent signaling from the heart confers leanness by enhancing metabolism in adipose tissue and liver. EMBO Mol Med. 2014;6:1610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pol CJ, Pollak NM, Jurczak MJ, Zacharia E, Karagiannides I, Kyriazis ID, Ntziachristos P, Scerbo DA, Brown BR, Aifantis I, et al. Cardiac myocyte KLF5 regulates body weight via alteration of cardiac FGF21. Biochim Biophys Acta Mol Basis Dis. 2019;1865:2125–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Woodall BP, Gresham KS, Woodall MA, Valenti MC, Cannavo A, Pfleger J, Chuprun JK, Drosatos K and Koch WJ. Alteration of myocardial GRK2 produces a global metabolic phenotype. JCI Insight. 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shi F, Simandi Z, Nagy L and Collins S. Diet-dependent natriuretic peptide receptor C expression in adipose tissue is mediated by PPARgamma via long-range distal enhancers. J Biol Chem. 2021;297:100941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ceddia RP and Collins S. A compendium of G-protein-coupled receptors and cyclic nucleotide regulation of adipose tissue metabolism and energy expenditure. Clin Sci (Lond). 2020;134:473–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Crewe C, Joffin N, Rutkowski JM, Kim M, Zhang F, Towler DA, Gordillo R and Scherer PE. An Endothelial-to-Adipocyte Extracellular Vesicle Axis Governed by Metabolic State. Cell. 2018;175:695–708 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Crewe C, Funcke JB, Li S, Joffin N, Gliniak CM, Ghaben AL, An YA, Sadek HA, Gordillo R, Akgul Y, et al. Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes. Cell Metab. 2021;33:1853–1868 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007;356:1140–51. [DOI] [PubMed] [Google Scholar]
- 184.Tsutsui H, Kinugawa S and Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2181–90. [DOI] [PubMed] [Google Scholar]
- 185.O'Rourke B, Ashok D and Liu T. Mitochondrial Ca(2+) in heart failure: Not enough or too much? J Mol Cell Cardiol. 2021;151:126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chaanine AH, Joyce LD, Stulak JM, Maltais S, Joyce DL, Dearani JA, Klaus K, Nair KS, Hajjar RJ and Redfield MM. Mitochondrial Morphology, Dynamics, and Function in Human Pressure Overload or Ischemic Heart Disease With Preserved or Reduced Ejection Fraction. Circ Heart Fail. 2019;12:e005131. [DOI] [PubMed] [Google Scholar]
- 187.Shires SE and Gustafsson ÅB. Mitophagy and heart failure. J Mol Med (Berl). 2015;93:253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Barger PM and Kelly DP. Fatty acid utilization in the hypertrophied and failing heart: molecular regulatory mechanisms. Am J Med Sci. 1999;318:36–42. [DOI] [PubMed] [Google Scholar]
- 189.Tuunanen H, Engblom E, Naum A, Någren K, Hesse B, Airaksinen KE, Nuutila P, Iozzo P, Ukkonen H, Opie LH, et al. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation. 2006;114:2130–7. [DOI] [PubMed] [Google Scholar]
- 190.Sack MN, Rader TA, Park S, Bastin J, McCune SA and Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–42. [DOI] [PubMed] [Google Scholar]
- 191.Taylor M, Wallhaus TR, Degrado TR, Russell DC, Stanko P, Nickles RJ and Stone CK. An evaluation of myocardial fatty acid and glucose uptake using PET with [18F]fluoro-6-thia-heptadecanoic acid and [18F]FDG in Patients with Congestive Heart Failure. J Nucl Med. 2001;42:55–62. [PubMed] [Google Scholar]
- 192.Doenst T, Nguyen TD and Abel ED. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res. 2013;113:709–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lei B, Lionetti V, Young ME, Chandler MP, d'Agostino C, Kang E, Altarejos M, Matsuo K, Hintze TH, Stanley WC, et al. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J Mol Cell Cardiol. 2004;36:567–76. [DOI] [PubMed] [Google Scholar]
- 194.Hölscher M, Schäfer K, Krull S, Farhat K, Hesse A, Silter M, Lin Y, Pichler BJ, Thistlethwaite P, El-Armouche A, et al. Unfavourable consequences of chronic cardiac HIF-1α stabilization. Cardiovasc Res. 2012;94:77–86. [DOI] [PubMed] [Google Scholar]
- 195.Cahill GF Jr. and Veech RL. Ketoacids? Good medicine? Trans Am Clin Climatol Assoc. 2003;114:149–61; discussion 162-3. [PMC free article] [PubMed] [Google Scholar]
- 196.Bedi KC Jr., Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, et al. Evidence for Intramyocardial Disruption of Lipid Metabolism and Increased Myocardial Ketone Utilization in Advanced Human Heart Failure. Circulation. 2016;133:706–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sun H and Wang Y. Branched chain amino acid metabolic reprogramming in heart failure. Biochim Biophys Acta. 2016;1862:2270–2275. [DOI] [PubMed] [Google Scholar]
- 198.Lopaschuk GD, Karwi QG, Tian R, Wende AR and Abel ED. Cardiac Energy Metabolism in Heart Failure. Circ Res. 2021;128:1487–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Huss JM and Kelly DP. Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest. 2005;115:547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Riehle C and Bauersachs J. Small animal models of heart failure. Cardiovasc Res. 2019;115:1838–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.De Jong KA and Lopaschuk GD. Complex Energy Metabolic Changes in Heart Failure With Preserved Ejection Fraction and Heart Failure With Reduced Ejection Fraction. Can J Cardiol. 2017;33:860–871. [DOI] [PubMed] [Google Scholar]
- 202.Valero-Munoz M, Backman W and Sam F. Murine Models of Heart Failure with Preserved Ejection Fraction: a "Fishing Expedition". JACC Basic Transl Sci. 2017;2:770–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Schiattarella GG, Altamirano F, Tong D, French KM, Villalobos E, Kim SY, Luo X, Jiang N, May HI, Wang ZV, et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature. 2019;568:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liao S, Tang Y, Yue X, Gao R, Yao W, Zhou Y and Zhang H. beta-Hydroxybutyrate Mitigated Heart Failure with Preserved Ejection Fraction by Increasing Treg Cells via Nox2/GSK-3beta. J Inflamm Res. 2021;14:4697–4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Deng Y, Xie M, Li Q, Xu X, Ou W, Zhang Y, Xiao H, Yu H, Zheng Y, Liang Y, J et al. Targeting Mitochondria-Inflammation Circuit by beta-Hydroxybutyrate Mitigates HFpEF. Circ Res. 2021;128:232–245. [DOI] [PubMed] [Google Scholar]
- 206.Wallner M, Eaton DM, Berretta RM, Borghetti G, Wu J, Baker ST, Feldsott EA, Sharp TE 3rd, Mohsin S, Oyama MA, et al. A Feline HFpEF Model with Pulmonary Hypertension and Compromised Pulmonary Function. Sci Rep. 2017;7:16587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Charles CJ, Lee P, Li RR, Yeung T, Ibraham Mazlan SM, Tay ZW, Abdurrachim D, Teo XQ, Wang WH, de Kleijn DPV, et al. A porcine model of heart failure with preserved ejection fraction: magnetic resonance imaging and metabolic energetics. ESC Heart Fail. 2020;7:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Olver TD, Edwards JC, Jurrissen TJ, Veteto AB, Jones JL, Gao C, Rau C, Warren CM, Klutho PJ, Alex L, et al. Western Diet-Fed, Aortic-Banded Ossabaw Swine: A Preclinical Model of Cardio-Metabolic Heart Failure. JACC Basic Transl Sci. 2019;4:404–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fragasso G, Perseghin G, De Cobelli F, Esposito A, Palloshi A, Lattuada G, Scifo P, Calori G, Del Maschio A, et al. Effects of metabolic modulation by trimetazidine on left ventricular function and phosphocreatine/adenosine triphosphate ratio in patients with heart failure. Eur Heart J. 2006;27:942–8. [DOI] [PubMed] [Google Scholar]
- 210.Steggall A, Mordi IR and Lang CC. Targeting Metabolic Modulation and Mitochondrial Dysfunction in the Treatment of Heart Failure. Diseases. 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Paganetti PA, Lis M, Klafki HW and Staufenbiel M. Amyloid precursor protein truncated at any of the gamma-secretase sites is not cleaved to beta-amyloid. J Neurosci Res. 1996;46:283–93. [DOI] [PubMed] [Google Scholar]
- 212.Zhang L, Lu Y, Jiang H, Zhang L, Sun A, Zou Y and Ge J. Additional use of trimetazidine in patients with chronic heart failure: a meta-analysis. J Am Coll Cardiol. 2012;59:913–22. [DOI] [PubMed] [Google Scholar]
- 213.Gao D, Ning N, Niu X, Hao G and Meng Z. Trimetazidine: a meta-analysis of randomised controlled trials in heart failure. Heart. 2011;97:278–86. [DOI] [PubMed] [Google Scholar]
- 214.Zhou X and Chen J. Is treatment with trimetazidine beneficial in patients with chronic heart failure? PLoS One. 2014;9:e94660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tuunanen H, Engblom E, Naum A, Någren K, Scheinin M, Hesse B, Juhani Airaksinen KE, Nuutila P, Iozzo P, Ukkonen H, et al. Trimetazidine, a metabolic modulator, has cardiac and extracardiac benefits in idiopathic dilated cardiomyopathy. Circulation. 2008;118:1250–8. [DOI] [PubMed] [Google Scholar]
- 216.Schmidt-Schweda S and Holubarsch C. First clinical trial with etomoxir in patients with chronic congestive heart failure. Clin Sci (Lond). 2000;99:27–35. [PubMed] [Google Scholar]
- 217.Lewis JF, DaCosta M, Wargowich T and Stacpoole P. Effects of dichloroacetate in patients with congestive heart failure. Clin Cardiol. 1998;21:888–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kato T, Niizuma S, Inuzuka Y, Kawashima T, Okuda J, Tamaki Y, Iwanaga Y, Narazaki M, Matsuda T, Soga T, et al. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ Heart Fail. 2010;3:420–30. [DOI] [PubMed] [Google Scholar]
- 219.Chen M, Gao C, Yu J, Ren S, Wang M, Wynn RM, Chuang DT, Wang Y and Sun H. Therapeutic Effect of Targeting Branched-Chain Amino Acid Catabolic Flux in Pressure-Overload Induced Heart Failure. J Am Heart Assoc. 2019;8:e011625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lim LL, Lau ESH, Fung E, Lee HM, Ma RCW, Tam CHT, Wong WKK, Ng ACW, Chow E, Luk AOY, et al. Circulating branched-chain amino acids and incident heart failure in type 2 diabetes: The Hong Kong Diabetes Register. Diabetes Metab Res Rev. 2020;36:e3253. [DOI] [PubMed] [Google Scholar]
- 221.Fontana L, Cummings NE, Arriola Apelo SI, Neuman JC, Kasza I, Schmidt BA, Cava E, Spelta F, Tosti V, et al. Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell Rep. 2016;16:520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.McGarrah RW, Zhang GF, Christopher BA, Deleye Y, Walejko JM, Page S, Ilkayeva O, White PJ and Newgard CB. Dietary branched-chain amino acid restriction alters fuel selection and reduces triglyceride stores in hearts of Zucker fatty rats. Am J Physiol Endocrinol Metab. 2020;318:E216–e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Horton JL, Davidson MT, Kurishima C, Vega RB, Powers JC, Matsuura TR, Petucci C, Lewandowski ED, Crawford PA, Muoio DM, et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Nielsen R, Møller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, Harms HJ, Frøkiær J, Eiskjaer H, Jespersen NR, et al. Cardiovascular Effects of Treatment With the Ketone Body 3-Hydroxybutyrate in Chronic Heart Failure Patients. Circulation. 2019;139:2129–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Byrne NJ, Soni S, Takahara S, Ferdaoussi M, Al Batran R, Darwesh AM, Levasseur JL, Beker D, Vos DY, Schmidt MA, et al. Chronically Elevating Circulating Ketones Can Reduce Cardiac Inflammation and Blunt the Development of Heart Failure. Circ Heart Fail. 2020;13:e006573. [DOI] [PubMed] [Google Scholar]
- 226.Ho KL, Zhang L, Wagg C, Al Batran R, Gopal K, Levasseur J, Leone T, Dyck JRB, Ussher JR, Muoio DM, et al. Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency. Cardiovasc Res. 2019;115:1606–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Barnosky AR, Hoddy KK, Unterman TG and Varady KA. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Transl Res. 2014;164:302–11. [DOI] [PubMed] [Google Scholar]
- 228.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E and Peterson CM. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018;27:1212–1221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hammer S, Snel M, Lamb HJ, Jazet IM, van der Meer RW, Pijl H, Meinders EA, Romijn JA, de Roos A and Smit JW. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol. 2008;52:1006–12. [DOI] [PubMed] [Google Scholar]
- 230.Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J and Nicklas BJ. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. Jama. 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.de Lucia C, Gambino G, Petraglia L, Elia A, Komici K, Femminella GD, D'Amico ML, Formisano R, Borghetti G, Liccardo D, et al. Long-Term Caloric Restriction Improves Cardiac Function, Remodeling, Adrenergic Responsiveness, and Sympathetic Innervation in a Model of Postischemic Heart Failure. Circ Heart Fail. 2018;11:e004153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Karwi QG, Zhang L, Altamimi TR, Wagg CS, Patel V, Uddin GM, Joerg AR, Padwal RS, Johnstone DE, Sharma A, et al. Weight loss enhances cardiac energy metabolism and function in heart failure associated with obesity. Diabetes Obes Metab. 2019;21:1944–1955. [DOI] [PubMed] [Google Scholar]
- 233.Waldman M, Cohen K, Yadin D, Nudelman V, Gorfil D, Laniado-Schwartzman M, Kornwoski R, Aravot D, Abraham NG, Arad M, et al. Regulation of diabetic cardiomyopathy by caloric restriction is mediated by intracellular signaling pathways involving 'SIRT1 and PGC-1α'. Cardiovasc Diabetol. 2018;17:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sung MM, Das SK, Levasseur J, Byrne NJ, Fung D, Kim TT, Masson G, Boisvenue J, Soltys CL, Oudit GY, et al. Resveratrol treatment of mice with pressure-overload-induced heart failure improves diastolic function and cardiac energy metabolism. Circ Heart Fail. 2015;8:128–37. [DOI] [PubMed] [Google Scholar]
- 235.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–22. [DOI] [PubMed] [Google Scholar]
- 237.Fang WJ, Wang CJ, He Y, Zhou YL, Peng XD and Liu SK. Resveratrol alleviates diabetic cardiomyopathy in rats by improving mitochondrial function through PGC-1α deacetylation. Acta Pharmacol Sin. 2018;39:59–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 239.Shao Q, Meng L, Lee S, Tse G, Gong M, Zhang Z, Zhao J, Zhao Y, Li G and Liu T. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2019;18:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Verma S, Rawat S, Ho KL, Wagg CS, Zhang L, Teoh H, Dyck JE, Uddin GM, Oudit GY, Mayoux E, et al. Empagliflozin Increases Cardiac Energy Production in Diabetes: Novel Translational Insights Into the Heart Failure Benefits of SGLT2 Inhibitors. JACC Basic Transl Sci. 2018;3:575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, Ishikawa K, Watanabe S, Picatoste B, Flores E, Garcia-Ropero A, Sanz J, Hajjar RJ, et al. Empagliflozin Ameliorates Adverse Left Ventricular Remodeling in Nondiabetic Heart Failure by Enhancing Myocardial Energetics. J Am Coll Cardiol. 2019;73:1931–1944. [DOI] [PubMed] [Google Scholar]
- 242.Byrne NJ, Parajuli N, Levasseur JL, Boisvenue J, Beker DL, Masson G, Fedak PWM, Verma S and Dyck JRB. Empagliflozin Prevents Worsening of Cardiac Function in an Experimental Model of Pressure Overload-Induced Heart Failure. JACC Basic Transl Sci. 2017;2:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.