Abstract
Objective:
To characterize the efficiency of screening through high-volume community health campaigns (CHCs) by comparing the costs and population reach and identify factors associated with gains in efficiency. Access to effective cervical cancer screening remains limited in low-resource settings, especially in rural areas. Periodic CHCs are a novel method of offering screening for HPV at lower costs and higher population coverage than health facilities.
Methods:
A micro-costing study was conducted within a cervical cancer screening trial to measure efficiency (cost per woman screened) and population uptake of HPV-based screening offered through CHCs in Migori County, Kenya between January and September 2016. Regression analysis assessed relationships between population size and efficiency. Structured observations and qualitative interviews identified implementation factors that affected efficiency in individual campaigns.
Results:
Communities screening through CHCs had costs per woman screened ranging from US $22.06 to $30.21. Efficiency was directly correlated to overall numbers of women screened, but not to proportion of population screened. Modifiable factors that acted as context-specific facilitators and barriers with a potential impact on efficiency were identified.
Conclusion:
There was substantial variation in efficiency among CHCs. Cultural factors, health beliefs, and poor coordination among implementation partners as potential key barriers to screening uptake were identified.
Keywords: Cervical cancer screening, Community-health campaigns, HPV testing, Implementation science, Kenya, Self-collection
1. INTRODUCTION
Cervical cancer is the fourth most common cancer in the world and the most common among women in sub-Saharan Africa [1]. East Africa has especially high risk, affecting an estimated 40.1 per 100 000 women annually [2]. Cervical cancer is highly preventable through early detection and treatment. Cytologic screening resulted in major declines in cervical cancer mortality in high-income countries over the past half century. However, most low-income countries still lack effective screening programs, resulting in almost no change in incidence of cervical cancer or rates of mortality over the same time period [3–5].
Screening based on HPV is now the primary WHO-recommended screening strategy for low-resource settings, but widespread implementation is hampered by expense and availability [3]. Technologies such as careHPV™ and HPVExpert™, testing systems developed for use in resource-constrained settings at lower costs, may expand availability [6, 7]. Further, self-sampling for HPV has been found to be accurate and preferred over clinician sampling, and may also raise access to screening and reduce costs [6, 8, 9]. A cost-effectiveness study comparing strategies for cervical cancer screening across five low- and middle-income countries found that strategies requiring fewer visits were also more efficient [10].
The community-based health fair, or community health campaigns (CHCs), is an effective and cost-effective strategy to deliver screening and preventive health services in low-resource contexts [11, 12]. CHCs may similarly be an effective strategy to deliver cervical cancer screening rates in high-burden communities because of high capacity and proximity to residential areas.
The aim of the present study was to examine cervical cancer screening uptake and costs through CHCs as compared with clinics, as well as contextual factors that may have contributed to differences in efficiency between CHCs. Practical suggestions on modifiable factors were offered that may increase efficiency of cervical cancer screening in low-resource settings.
2. METHODS
Between January and November 2016, A cluster-randomized trial was implemented in 12 communities in western Kenya, comparing uptake of HPV-based cervical cancer screening in two study arms: CHCs and government health clinics (six communities each). In each community, CHCs were held in approximately 10 villages over 2 weeks after 2 weeks of intensive mobilization. Women self-collected specimens for HPV testing, which was done using the careHPV system. Throughout the study, quantitative data was collected on campaign costs and the number of women screened, along with observational data on factors that may have facilitated or impeded uptake of screening.
Data collection
The number of women in each community in the target age range for screening (25–65 years) was obtained from health facilities census levels, confirmed by individual community health volunteer (CHV) household enumeration.
Costing data were collected in each community. Micro-costing techniques were used, as described in previous reports [13, 14]. In brief, all resources used were enumerated, whether fully paid, donated, or subsidized, and then multiplied by the unit price paid or from market quotes, adding up to estimate total unit costs per woman screened. Time and motion studies were also conducted to quantify patient and provider activities and thus quantify effort levels for costing. As previously reported, CHCs were found to be more efficient, mainly due to higher personnel costs in health facilities [13].
Observational data on factors impacting success of the campaign were collected through structured surveys augmented with field notes and semi-structured interviews. The observation surveys consisted of questions about the general impact of different activities on the flow of CHCs, mid-course adjustments in the implementation of CHCs, changes in staffing patterns, participants’ attitudes or any unusual reactions to screening, and the number of participants who left before completing screening including reasons for not completing screening. Detailed field notes were taken to record what was heard and seen. The field notes recorded the date and location, any other relevant contextual information, and were written in a narrative style. Qualitative semi-structured interviews were also conducted with the CHC lead. The guides for the semi-structured interview consisted of open-ended questions with specific inquiry on the facilitators for successful implementation of CHCs, problems encountered during the CHCs, and action plan for improving on the next CHCs.
Ethical approval was obtained from Duke University Institutional Review Board, the Kenya Medical Research Institute Scientific and Ethical Review Unit, and the University of California, San Francisco Committee for Human Research. Informed consent was obtained from women before screening, and from all participants in structured or semi-structured interviews.
Analysis
Efficiency across the six CHCs was compared using descriptive statistics and bivariate analysis. Sources were identified within each campaign that contributed to the greatest costs (capital goods, personnel, recurrent goods and services) overall and sought explanations for outlying values. Two multiple linear regression models were developed to look at the correlation between cost per woman screened and (1) overall number of women screened as well as (2) the proportion of women screened, controlling for community size in both models. The regression models controlled for clustering within communities. Finally, qualitative observational data were used to investigate contextual factors that may have influenced efficiency.
Descriptive analyses were performed in Excel (Microsoft, Seattle, WA, USA) and regression analysis was performed using Stata V15 (College Station, TX, USA).
3. RESULTS
A total of 2898 women received HPV-based cervical cancer screening in CHCs. Detailed results comparing CHC screening and treatment costs and uptake are presented elsewhere, and showed the main driver of the difference in efficiency was personnel time in the health facilities [13–15]. The cost per woman screened across the six CHCs ranged from US $22.06 to $30.21 (Table 1). Most costs were fixed across CHCs, including tents, vehicles, data collection tools, and careHPV machine, so variations in costs and efficiency were mostly attributable to differences in personnel numbers. Screening costs were in the range of $15.51–$21.95. The personnel costs for each woman reached during the screening phase among CHCs were in the range of $4.80–$9.00, including the compensation for CHVs of 3000 Kenyan Shillings ($30.00) per CHC. The compensation rates of personnel did not change across CHCs, although overall staff numbers were in the range of 9–11 per CHC, the ratio of higher-paid research assistants to CHVs ranged from 11:0 to 6:4. Likewise, the unit cost of capital goods did not change for different CHC communities. Variation in the cost of recurrent goods ($8.09–$8.82 per woman during the screening phase) was attributed to the observed differences in fuel costs, due to distance traveled to different CHCs, and the number of supplies required for screening. The cost of notifications varied because of the differences in notification options (texts, calls, and home visits) selected at each CHC.
Table 1.
Cost estimations, in 2016 USD, by phase and type of cost, per woman screened with self-collected HPV in CHCs in six communities in western Kenya in 2016.
| CHC community | ||||||
|---|---|---|---|---|---|---|
| Oyani | Ongito | Nyarongi | Nyamanga | Obware | Agenga | |
| Outreach | ||||||
| Capital goods a | $0.51 | $0.92 | $0.67 | $0.72 | $0.64 | $0.53 |
| Personnel b | $2.00 | $3.18 | $2.32 | $2.26 | $1.66 | $1.18 |
| Recurrent goods c | $0.01 | $0.02 | $0.02 | $0.56 | $0.24 | $0.25 |
| Services d | $0.32 | $0.51 | $0.35 | $0.40 | $0.43 | $1.01 |
| Outreach subtotal | $2.84 | $4.63 | $3.36 | $3.94 | $2.97 | $2.97 |
| Screening | ||||||
| Capital goods | $1.18 | $1.82 | $1.38 | $1.47 | $1.59 | $1.15 |
| Personnel | $5.09 | $9.00 | $7.72 | $6.40 | $4.91 | $4.80 |
| Recurrent goods | $8.11 | $8.82 | $8.42 | $8.70 | $8.21 | $8.09 |
| Services | $2.36 | $2.31 | $1.16 | $1.48 | $1.30 | $1.46 |
| Screening subtotal | $16.74 | $21.95 | $18.69 | $18.05 | $16.02 | $15.51 |
| Notification | ||||||
| Capital goods | $0.51 | $0.92 | $0.67 | $0.72 | $0.64 | $0.53 |
| Personnel | $3.47 | $2.17 | $3.47 | $3.28 | $3.66 | $2.78 |
| Recurrent goods | $0.08 | $0.02 | $0.08 | $0.07 | $0.04 | $0.05 |
| Services | $0.33 | $0.51 | $0.12 | $0.13 | $0.48 | $0.22 |
| Notification subtotal | $4.40 | $3.63 | $4.34 | $4.21 | $4.82 | $3.58 |
| Women screened | 601 | 337 | 461 | 430 | 483 | 586 |
| Total cost per woman screened | $23.98 | $30.21 | $26.39 | $26.21 | $23.81 | $22.06 |
Abbreviation: CHC, community health campaign; USD, US dollar.
Capital goods are items with more than 1 year of useful life and cost more than $250. They require an initial outlay but can then be used over a number of years, e.g. vehicles for transportation, tents for CHCs, and the careHPV test system.
Personnel are paid and unpaid staff who provide cervical cancer screening and prevention services. The value of unpaid volunteers was estimated using the market wage of staff serving similar functions.
Recurrent goods are items typically consumed within 1 year as well as longer-lived resources costing under $250 (e.g. office supplies).
Services are contracted support activities, e.g. consultant fees, IT support, utilities, and vehicle maintenance.
Outliers included recurrent costs in one community (Nyamanga) that were attributable to fuel costs, as this was the farthest site from Migori, and personnel costs in the community with the lowest uptake of screening (Ongito). The range of notification costs was not as wide ($3.63–$4.82) and was driven by communities with higher numbers of women preferring home visits over phone call-based strategies.
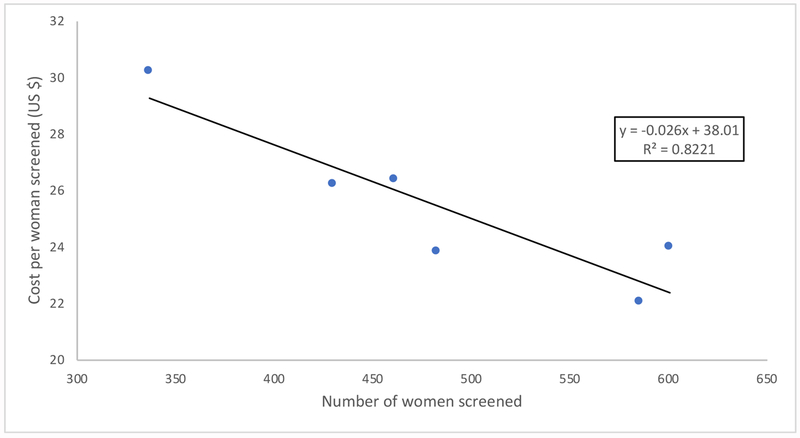
There was a significant relationship between greater efficiency and higher numbers of women screened (Fig. 1). The relationship between proportion of the population screened and efficiency was not significant (P=0.108). In a multivariate regression model controlling for overall community size and proportion of population reached, the relationship between efficiency and absolute screening uptake remained significant (−$0.03/woman, 95% confidence interval [CI] −0.01 to 0.04).
Figure 1.
The relationship between cervical cancer screening efficiency and number of women screened per community
Common facilitators across CHCs included door-to-door mobilization, key stakeholder engagement, logistics and technical support, and adequate staffing (Table 2). Use of a public address system was a facilitator in the more efficient campaigns. Scheduling of campaigns during non-harvest seasons was noted as a facilitator, but not necessarily seen in high-efficiency CHCs. Although conflict with market days was perceived as a barrier, the campaigns in which this occurred had the highest efficiency. The community with the lowest uptake and efficiency had two major barriers: religious beliefs and prior negative experiences with screening. Elucidation of these facilitators and barriers allowed for the development of proposed, context-specific solutions, including changing the timing of campaigns, pre-campaign community engagement, and broader Ministry of Health coordination among partners.
Table 2.
Facilitators, barriers and proposed solutions to increase CHC-based HPV testing in six communities in Migori County, Kenya.
| Oyani | Ongito | Nyarongi | Nyamanga | Obware | Agenga | |
|---|---|---|---|---|---|---|
| Sub-county | Suna East | Uriri | Suna East | Nyatike | Nyatike | Nyatike |
| Primary source of income | Farming | Farming | Farming | Farming + fishing | Farming | Farming + mining |
| Facilitators | ||||||
| Facilitators across all communities | Door-to-door mobilization Key stakeholder engagement Adequate staffing Proper logistics + technical support |
|||||
| Community-specific facilitators | Campaigns held in non-harvesting season | Campaigns held in non-harvesting season | Use of PA system | Use of PA system | Use of PA system | 1. Use of PA system 2. Other events/health campaigns complemented CHCs |
| Barriers | 1. CHCs occurred on market days 2. Cervical cancer screening campaigns by other groups a few months before CHCs |
1. Religious/cultural beliefs 2. Previous cervical cancer screening with VIA/VILI led to fear and reluctance to screen |
1. Farming activities during CHCs 2. Weather (rain) |
1. Fishing activities during CHCs 2. Cervical cancer screening campaigns by other groups a few months before CHCs |
1. CHCs occurred on market days 2. Weather (rain) |
CHCs occurred on market days |
| Potential future strategies | ||||||
| Solutions applicable to all communities | Involve CHVs early and through CHCs for logistical planning | |||||
| Community-specific solutions | 1. Hold CHCs on non-market days or adjacent to markets 2. Hold CHCs later in the day or on non-work days 3. Collaborate with other partners |
1. Greater engagement with religious/community leaders before CHC 2. Improve coordination with the CHMT and SCHMT before CHCs |
1. Hold CHCs later in the day or on non-work days 2. Plan CHCs during non-harvesting season 3. Screen through home visits or clinics in areas with low screening uptake |
1. Hold CHCs later in the day or on non-work days 2. Hold CHCs next to fishing sites 3. Collaborate with other partners |
1. Hold CHCs on non-market days or adjacent to markets 2. Hold CHCs later in the day or on non-work days 3. Screen through home visits or clinics in areas with low screening uptake |
1. Hold CHCs on non-market days or adjacent to markets 2. Hold CHCs later in the day or on non-work days |
| Cost per woman screened (USD) | 23.98 | 30.21 | 26.39 | 26.21 | 23.81 | 22.06 |
| Women screened (n) | 601 | 337 | 461 | 430 | 483 | 586 |
| Proportion of population (%) | 60 | 52 | 53 | 40 | 72 | 100 |
Abbreviations: CHC, community health campaign; CHMT, Community Health Management Team; CHV, community health volunteer; PA, public address; SCHMT, Sub-County Health Management Team.
4. DISCUSSION
The aim of the present study was to contextualize prior findings that HPV-based cervical cancer screening in CHCs both costs less per participant and yields higher rates of screening than screening offered in health facilities in order to further inform the design of cervical cancer screening programs conducted in rural areas of Kenya or similar countries. It was found that within this overall lower cost strategy of CHC-based screening, efficiency is most closely correlated with the absolute numbers of women screening in both large and small villages. Further, common and unique modifiable contextual factors were identified that seem to correlate with screening uptake and, therefore, efficiency. These findings offer concrete factors that can support the implementation of more successful screening campaigns, bolstering the promising evidence to suggest that the efficiency of a CHC-based screening program increases with increasing coverage, regardless of community size or proportion of women reached.
The study design facilitated identification of key characteristics that can be modified in partnership with the Ministry of Health, local program planners, and CHVs to increase the efficiency of cervical cancer screening in these settings. The low uptake associated with religious beliefs and prior negative experiences with speculum-based screening is an important observation and will push specific activities, such as earlier and broader engagement with religious leaders and improved communication with other partners offering screening services, ideally coordinated through the Ministry of Health for maximal sustainability. The positive impact of coordination with partners offering related health services was seen in the community with highest efficiency, where cervical cancer screening was integrated with other reproductive health services. Educational content will also be revised for specific situations where personal beliefs and prior healthcare experiences may negatively impact uptake of screening. While other identified factors may not have been correlated with efficiency, attempts will be made to address observed barriers, such as conflicts with market days and planning CHCs around farming or fishing activities in agricultural settings.
The detailed components of the individual costs of HPV-based screening will also be helpful in determining the program expenses if HPV testing would be integrated into other health services. While the CHC costs had significant variation based on uptake of screening, mainly driven by personnel, the actual cost of HPV testing did not have significant variation, staying within a narrow range of $8.11–$8.82, after the initial capital investment in the testing equipment. Programs that have the capacity to integrate screening for cervical cancer with other health services, leveraging personnel and mobilization costs, can use these estimates to determine the additive costs of HPV testing.
While this study identifies context-specific factors that correlate with efficiency of cervical cancer screening through CHCs, there are several limitations. First, the present study can only speculate on the causal relationship between contextual differences and changes in efficiency. The nature of these factors would make a prospective study with random assignment of factors impractical. Second, while rigorous micro-costing techniques were employed to capture the variation in implementation for each campaign in order to provide a range of estimated costs and efficiencies, the present study certainly cannot capture all of the intangibles that a campaign tailored to the specific needs of the target community would require, and therefore, cost.
Cost-effectiveness of this phase was not examined, but the greater efficiency is likely to reduce net cost per cervical cancer case or death averted. Treatment rates among HPV-positive women in Phase 1 were incredibly low. The present study will use similar methods to identify and modify gaps in implementation to increase uptake of treatment in Phase 2. A cost-effectiveness analysis of the full scope of services will be conducted. The lessons presented here suggest that while screening for cervical cancer in CHCs is more efficient than screening within health facilities, there is wide variation in uptake of screening and efficiency among communities offering CHC-based screening, which may be due to modifiable gaps in implementation. Structured observations of this screening experience helped to identify and address factors that had a negative impact on screening and replicate strategies from more successful communities. It is recommended that programs in novel settings such as this use similar strategies for evaluation.
Synopsis.
Community-health campaigns were an effective and efficient strategy to offer HPV-based cervical cancer screening using self-collected specimens in Kenya.
Acknowledgments
Research reported in this publication was funded by support from the National Cancer Institute of the National Institutes of Health, under award R01CA188248.
Footnotes
Conflicts of interest
The authors have no conflicts of interest.
References
- [1].Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM: Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127(12): 2893–2917. [DOI] [PubMed] [Google Scholar]
- [2].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68(6): 394–424. [DOI] [PubMed] [Google Scholar]
- [3].Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, et al. : HPV screening for cervical cancer in rural India. N Engl J Med 2009;360(14): 1385–1394. [DOI] [PubMed] [Google Scholar]
- [4].White HL, Mulambia C, Sinkala M, Mwanahamuntu MH, Parham GP, Moneyham L, et al. : ‘Worse than HIV’ or ‘not as serious as other diseases’? Conceptualization of cervical cancer among newly screened women in Zambia. Soc Sci Med 2012;74(10): 1486–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].IARC: “IARC Handbooks of Cancer Prevention: Cervix Cancer Screening.” International Agency for Research on Cancer. Lyon, France: IARC, 2005. https://www.iarc.fr/en/publications/pdfs-online/prev/handbook10/HANDBOOK10.pdf. [Google Scholar]
- [6].Qiao YL, Sellors JW, Eder PS, Bao YP, Lim JM, Zhao FH, et al. : A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. Lancet Oncology 2008;9(10): 929–936. [DOI] [PubMed] [Google Scholar]
- [7].Clavel C, Masure M, Bory JP, Putaud I, Mangeonjean C, Lorenzato M, et al. : Hybrid Capture II-based human papillomavirus detection, a sensitive test to detect in routine high-grade cervical lesions: a preliminary study on 1518 women. Brit J Cancer 1999;80(9): 1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Snijders PJF, Verhoef VMJ, Arbyn M, Ogilvie G, Minozzi S, Banzi R, et al. : High-risk HPV testing on self-sampled versus clinician-collected specimens: A review on the clinical accuracy and impact on population attendance in cervical cancer screening. Int J Cancer 2013;132(10): 2223–2236. [DOI] [PubMed] [Google Scholar]
- [9].Lazcano-Ponce E, Lorincz AT, Cruz-Valdez A, Salmeron J, Uribe P, Velasco-Mondragon E, et al. : Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (MARCH): a community-based randomised controlled trial. Lancet 2011;378(9806): 1868–1873. [DOI] [PubMed] [Google Scholar]
- [10].Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mahe C, et al. : Cost-effectiveness of cervical-cancer screening in five developing countries. New Engl J Med 2005;353(20): 2158–2168. [DOI] [PubMed] [Google Scholar]
- [11].Chamie G, Kwarisiima D, Clark TD, Kabami J, Jain V, Geng E, et al. : Leveraging Rapid Community-Based HIV Testing Campaigns for Non-Communicable Diseases in Rural Uganda. Plos One 2012;7(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Marseille E, Jiwani A, Raut A, Verguet S, Walson J, Kahn JG: Scaling up integrated prevention campaigns for global health: costs and cost-effectiveness in 70 countries. Bmj Open 2014;4(6): e003987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shen J, Olwanda E, Kahn JG, Huchko MJ: Cost of HPV screening at community health campaigns (CHCs) and health clinics in rural Kenya. Bmc Health Serv Res 2018;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Olwanda E, Shen J, Kahn JG, Bryant-Comstock K, Huchko MJ: Comparison of patient flow and provider efficiency of two delivery strategies for HPV-based cervical cancer screening in Western Kenya: a time and motion study. Glob Health Action 2018;11(1): 1451455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Huchko MJ, Ibrahim S, Blat C, Cohen CR, Smith JS, Hiatt RA, et al. : Cervical cancer screening through human papillomavirus testing in community health campaigns versus health facilities in rural western Kenya. Int J Gynaecol Obstet 2018;141(1): 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]