Abstract
The contribution of greenhouse gas (GHG) emissions from ruminant production systems varies between countries and between regions within individual countries. The appropriate quantification of GHG emissions, specifically methane (CH4), has raised questions about the correct reporting of GHG inventories and, perhaps more importantly, how best to mitigate CH4 emissions. This review documents existing methods and methodologies to measure and estimate CH4 emissions from ruminant animals and the manure produced therein over various scales and conditions. Measurements of CH4 have frequently been conducted in research settings using classical methodologies developed for bioenergetic purposes, such as gas exchange techniques (respiration chambers, headboxes). While very precise, these techniques are limited to research settings as they are expensive, labor-intensive, and applicable only to a few animals. Head-stalls, such as the GreenFeed system, have been used to measure expired CH4 for individual animals housed alone or in groups in confinement or grazing. This technique requires frequent animal visitation over the diurnal measurement period and an adequate number of collection days. The tracer gas technique can be used to measure CH4 from individual animals housed outdoors, as there is a need to ensure low background concentrations. Micrometeorological techniques (e.g., open-path lasers) can measure CH4 emissions over larger areas and many animals, but limitations exist, including the need to measure over more extended periods. Measurement of CH4 emissions from manure depends on the type of storage, animal housing, CH4 concentration inside and outside the boundaries of the area of interest, and ventilation rate, which is likely the variable that contributes the greatest to measurement uncertainty. For large-scale areas, aircraft, drones, and satellites have been used in association with the tracer flux method, inverse modeling, imagery, and LiDAR (Light Detection and Ranging), but research is lagging in validating these methods. Bottom-up approaches to estimating CH4 emissions rely on empirical or mechanistic modeling to quantify the contribution of individual sources (enteric and manure). In contrast, top-down approaches estimate the amount of CH4 in the atmosphere using spatial and temporal models to account for transportation from an emitter to an observation point. While these two estimation approaches rarely agree, they help identify knowledge gaps and research requirements in practice.
Keywords: estimates, greenhouse gas, livestock, measurements, quantification, sustainability
The contribution of greenhouse gas (GHG) emissions from ruminant production systems varies from country to country, and CH4 emissions from animals and their manure are highly variable. Variance in quantification methodologies for CH4 has also raised questions about the accuracy of current GHG emissions coefficients for national and international inventories and, perhaps more importantly, how best to mitigate CH4 emissions.
Introduction
The concept of sustainability continues to be a highly controversial discussion topic gaining tremendous traction within many different human activities worldwide, mainly when climate neutrality and global warming are part of the debate. Agriculture, including land-use change, and deforestation, is a particular focus in these discussions because, as reported by the Intergovernmental Panel on Climate Change (IPCC), it was responsible for 23% of total greenhouse gas (GHG) emissions globally in 2017, assessed using a global warming potential (GWP) of a 100-year horizon (IPCC, 2019b). According to Gerber et al. (2013), the livestock sector plays an important role in climate change representing 14.5% of human-induced GHG emissions. The share of the livestock sector in GHG emissions is region-specific and depends on the magnitude of other economic sectors, mainly the energy sector. For instance, the United States of America’s Environmental Protection Agency (EPA) reports that although agriculture is responsible for 9% to 10% of total GHG emissions, livestock contributes less than 4% of direct (not including GHG emissions from feed production and fuel) emissions (Dillon et al., 2021; Tedeschi, 2022). In Australia, livestock is responsible for about 10% of direct emissions (Henry et al., 2012). In Brazil, direct emissions from livestock from enteric fermentation and manure management accounted for 20.8% of national emissions in 2016, or 62% of the emissions of the agriculture sector (Brazilian Ministry of Science, 2021). In 2000, India had the highest methane (CH4) emission in the south-Asian countries, of which 61% was from the agriculture sector (40% was from enteric fermentation, 17% from rice cultivation, and 4% from manure management), and livestock was responsible for 11.8 Tg CH4 emissions (Garg et al., 2011). Furthermore, discrepancies among country’s estimates of the livestock sector’s relative contribution to their national GHG emissions might exist due to different assessment frameworks (e.g., life cycle assessment according to ISO 14044 vs. GHG inventory accounting based on IPCC guidelines), modeling approaches, updates in methodology (e.g., IPCC (2006) vs. IPCC (2019a)), and GHG emissions factors.
The concept of a sustainable production system is often confounded with the philosophy of a resilient system (Tedeschi et al., 2015), but regardless of its terminology or attributes, appropriate identification and quantification of GHG emissions of all players within a system of interest are of utmost importance for the implementation of management or mitigation strategies towards sustainable or resilient production conditions. For instance, livestock production systems have different sources of GHG, and although many methods exist to quantify the emissions, there are intrinsic methodological limitations that prevent broad recommendations without negative repercussions or unintended consequences. In part, the difficulties arise because of the complexity of livestock production systems (Ominski et al., 2021) and different management strategies specific to each situation.
Methane produced during enteric fermentation and manure handling and storage represents the largest source of GHG emissions from ruminant livestock production systems. However, there is no universally superior CH4 quantification method from animals or manure. Some methods are more appropriate for small-scale scenarios, mainly because they were developed with that intent, while others have been developed, since their conception, for large-scale use. Nevertheless, it does not mean that all methods agree or that their quantification can be scaled up or down without making assumptions that might not hold for all conditions without increasing uncertainties. Thus, it is challenging to provide a definitive assessment of a production system’s sustainability (or resiliency for the sake of inclusiveness) when discussing climate neutrality or global warming. Some methods work better for a few animals wholly or partially within an enclosure (e.g., classical bioenergetics methods) (Gerrits and Labussière, 2015), whereas others have been tested for large areas containing (or not) free-ranging animals (e.g., top-down approaches). Yet, when comparing broad methods like the top-down approaches, one needs to ensure that the assignment of the GHG emission from point (e.g., feedlot) and nonpoint (e.g., dam and peatland) sources is unequivocally carried out (Tedeschi, 2022). Therefore, it becomes imperative that the chosen quantification methodology is appropriate to the measurement purpose and provides sufficient evidence to support further investigation or recommendation without leaving much space for speculation or error, which creates distrust in science. Other publications have discussed existing techniques and methods to measure and estimate GHG emissions of ruminants (Cole et al., 2018; Bekele et al., 2022).
This review arose from a technical guidance document for the Food and Agriculture Organization of the United Nations (FAO) under the Livestock Environmental Assessment and Performance Partnership (LEAP) program. Our review provides a comprehensive and updated overview of the measurement and estimation of enteric and manure CH4 from ruminants. The goal is to present and discuss the advantages and limitations of existing methods and methodologies to: 1) measure enteric- and manure-based CH4 emissions and 2) estimate (i.e., predict through mathematical modeling) CH4 production of typical domesticated ruminants. A companion review paper addresses the potential mitigation of enteric CH4 (Beauchemin et al., 2022).
Measurements Using Animal-Based Techniques
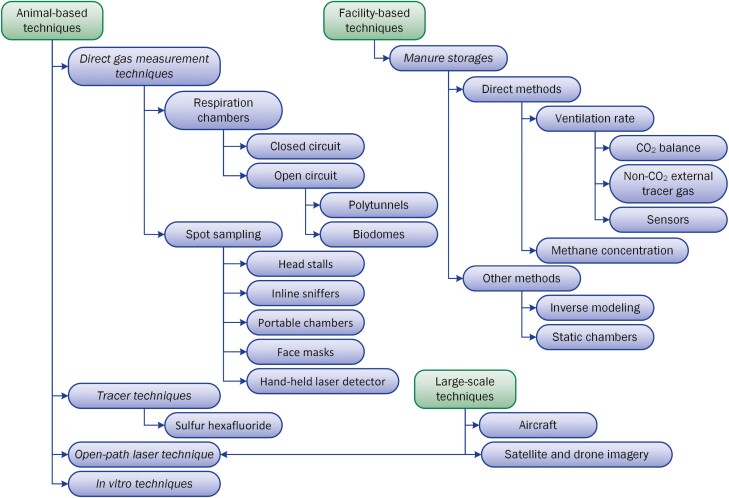
There are many different techniques and methodologies used to measure enteric CH4 emissions from ruminants, including gas exchange measurements (e.g., respiration chambers, headboxes, and face masks), air spot sampling, tracer gas, and micrometeorological technologies (Lassey, 2007; Storm et al., 2012; Hill et al., 2016; Hammond et al., 2016). Table 1 lists specific aspects of the different techniques, and Figure 1 depicts the flowchart of the categorization of the techniques. Each technique has specific requirements and assumptions that may limit its application outside of its intended purpose, and each has a different level of accuracy and precision that can be affected by the conditions of use. Incorrect use may overestimate or underestimate CH4 measurements if the conditions are inconsistent with original assumptions. For instance, some techniques are more suitable for grazing animals (e.g., sulfur hexafluoride—SF6—tracer gas technique), whereas others can mainly be used for confined animals (e.g., open-path laser).
Table 1.
Characteristics of different techniques to measure enteric methane1
| Techniques | Cost | Level | Environment | Application | Advantages | Disadvantages |
|---|---|---|---|---|---|---|
| Respiration and accumulation chambers | Generally high | Animal | Research | Highly accurate, controlled environment; information about individual animals; dry matter intake can be measured accurately; include emissions from hindgut fermentation | Results are different from free-range animals; configurations still vary from one research group to another; an animal adaptation period is required; every 1 to 3 h accumulation chambers must release CO2 that builds up | |
| Hood and/or headbox systems | Moderate to high | Animal | Grazing/pasture, indoors free stall, or tie stall | Research and Commercial | Portable and less expensive than chambers; requires less space | Do not measure hindgut emissions; an animal adaptation period is required; some may be designed for grazing situations |
| Tracers | Moderate | Animal | Research | Accurate; few interferences by other gases; the animal can free-range | Rely on SF6, which is a greenhouse gas itself; does not completely capture all tracer and, therefore, relies on spot concentration measurements; high contact with an animal, which can disrupt normal behavior | |
| Gas sensor capsules | Low | Animal | Research | Compatible with new electronic technologies; relies on small, low-cost sensors; continuous measurements | Information about the relation between concentration and flux (emission);isstill under development | |
| In vitro techniques | Low | In Vitro | Research and Commercial | High reproducibility but used to rank feeds for methanogenic potential and not for measurements of flux; allows different rumen microbial environments to be evaluated | Outcomes can be different from actual measurements;themethod relies on donor animals for rumen environment; standardization can be difficult | |
| Open-path laser | High | Pen; barn; building; pasture | Research | Information about groups of animals; data produced in a natural grazing or penned environment | Require expensiveequipment;data processingisheavily influenced by microclimatic conditions; loss of data can be high | |
| Unperson aerial/ground vehicles (UAV/UGV, drones) | Paddock/pasture | Research | ||||
| Satellite | Basin/Region | Research and Commercial | ||||
| Computer models | Low | Diverse | Research and Commercial | Estimate the distribution of production; not limited to any configuration | Can differ from real scenarios; relies on input data from animal measurement methods | |
| LiDAR | Moderate | Pasture | Grazing | Research | Airborne; detect CO2 and CH4 concurrently |
Adapted from Hill et al. (2016).
Figure 1.
A schematic flowchart of current techniques used to determine methane emissions at the animal, facility, and large-scale levels.
Direct gas measurement techniques
Direct techniques calculate the difference between the CH4 concentrations in ambient air with and without the animal present. Measurement of airflow (e.g., open circuit chambers) or volume (e.g., closed chambers) is used to calculate an emission rate.
Respiration chambers
Respiration chambers have been used for decades as the gold-standard technique to determine the energy expenditure of individual animals. They were previously viewed as the benchmark for measuring CH4 production from individual animals, but more recently, several other techniques have been shown to be equally valuable. Respiration chambers use the indirect calorimetry methodology that relies on gas exchange of mainly oxygen (O2), carbon dioxide (CO2), and CH4 either using open-circuit chambers that analyze the composition of inflow and outflow air or closed-circuit chambers that analyze the composition of air accumulated over some time (Johnson and Johnson, 1995). Some chambers are constructed using transparent polycarbonate, panels with polymethyl methacrylate (acrylic) windows, or metabolism crates covered with transparent polycarbonate walls. Respiration chambers provide accurate and precise measurements of CH4, including hindgut emissions but are costly and technically demanding (Goopy et al., 2016). However, limitations exist, and methods to overcome such limitations are impracticable or impossible to perform. There are limitations regarding altered metabolism rates such as gluconeogenesis, ketogenesis, or lipogenesis when animals are inside the respiration chambers (van den Borne et al., 2015). Another limitation of respiration chambers is that animals may not exhibit normal behaviors, e.g., feed consumption may decrease, thus resulting in an under-estimation of actual CH4 emissions compared to free-ranging animals under farm conditions (Huhtanen et al., 2019). In research studies, animals usually undergo a metabolism or performance trial, and CH4 is measured over 3 to 5 consecutive days by moving the trained animals to the chambers (Sakita et al., 2022). Several factors are essential when using this technology for controlled experiments, such as gas recovery routine maintenance, chamber temperature (<27 °C), relative humidity (<90%), CO2 concentration (<0.5%), ventilation rate (VR; 250 to 260 L/min), and constant gas flow as suggested by Pinares-Patiño and Waghorn (2014). The utility of respiration chambers is also limited to quantifying gaseous emissions from relatively few animals (12 or less) mainly to account for the emissions from manure when they are accumulated in the barn with and without the animals (Mathot et al., 2016). Respiration chambers are relatively expensive to build and maintain. The Global Research Alliance (GRA) on agricultural GHG has published respiration chamber designs from various countries around the world (Pinares-Patiño and Waghorn, 2018). Alternative low-cost systems exist (Abdalla et al., 2012; Hellwing et al., 2012; Canul Solis et al., 2017) that use the same principles as open-circuit indirect calorimetry, but with lower-cost materials available locally and simplified air-conditioning systems. These alternative chambers are typically set up in the daily environment of the cow (Hellwing et al., 2012; Canul Solis et al., 2017) or sheep (Abdalla et al., 2012; Sakita et al., 2022). With open-circuit chambers, gas concentrations are measured in the outlet using an infrared analyzer, gas chromatograph, or laser. Airflow is measured at the inlet, the exhaust, or both. Together, gas concentrations and airflow are used to calculate flux. To ensure measurement accuracy, it is critical to regularly calibrate the gas analyzers using gasses of known composition (zero and span) and to perform gas recovery tests before and after experiments. Heetkamp et al. (2015) and Pinares-Patiño and Waghorn (2018) detailed the design of respiration chambers for large and small ruminants. A simpler version of the respiration chamber is the polytunnel that consists of one large fixed or inflatable or tent type tunnel made of heavy-duty polyethylene or PVC film in which individuals or groups of cattle are housed for selected periods during which the amount of CH4 they produce is measured (Goopy et al., 2016). Polytunnels can be placed directly on pastures simulating semi-normal grazing conditions (Murray et al., 2001) or fixed close to the pastures where the daily allowance and intake of forages can be measured (Molina et al., 2016; Gaviria-Uribe et al., 2020). Typically, polytunnels have a volume ranging from 35 to 60 m3 per animal, and multiple animals are sometimes used to elevate the outlet gas concentrations, especially for small ruminants (Molina et al., 2016; Gaviria-Uribe et al., 2020). In addition to gas concentration measurements, the rate of flow-through air is calculated, and a daily CH4 emission is estimated. As with respiration chambers, polytunnels are used to measure the effects of treatment diets on CH4 emissions as dry matter intake (DMI) can be measured accurately. The main advantage of polytunnels is that they can be used in association with grazing studies. Similarly, feedyard conditions can be created to measure emissions from pen-fed beef cattle animals using a dome-like structure with dirt floors, pipe fencing, feed bunks, and water trough with similar principles of respiration chambers (Cooprider et al., 2011). Ventilated hoods and headboxes can also quantify gaseous exchange using principles similar to whole animal chambers. However, unlike whole animal respiration chambers, only enteric CH4 is measured. Animals have access to feed and water, and thus, these systems can be used to measure emissions continuously. Ventilated hoods and headboxes are considerably less expensive to construct than whole animal respiratory chambers, but not all animals can be trained to use these systems. Place et al. (2011) outlined configurations and schemes for constructing and operating ventilated hood chambers. For all techniques aimed at quantifying gas exchange, full system recovery tests of the relevant gas should be performed before and after the measurement period. Typical full systems tests, including CO2 recovery, use burning propane or ethanol, or nitrogen injection or release inside the respiration chambers (Lighton, 2008; Heetkamp et al., 2015). Should recoveries differ substantially from 100%, it is recommended that the source of error be identified and remedied. Additionally, the results of these tests should be included in published papers and reports.
Spot sampling
Spot sampling techniques measure the concentration of CH4 in the breath of individual animals for short periods of time. Some techniques combine the concentration measurements with airflow measurements to obtain a flux, as is the case with automated head chamber systems (AHCS), such as the GreenFeed Emission Monitoring system (C-Lock Inc., South Dakota) (Hristov et al., 2015). In contrast, sniffers (e.g., GASMET 4030 system) and handheld lasers that measure CH4 concentration must be calibrated against prediction equations to estimate a daily CH4 emission rate. The GreenFeed Emission Monitoring system is a stand-alone head chamber with an overhead hopper (in some cases double hopper) programmed to deliver a small amount of “bait” feed once the animal’s head is near the sensor. Allocating feed entices the animal to position its head near the sensors allowing the system to measure the increase in CH4 and CO2 concentration due to the animal’s breath compared with the background concentrations in ambient air. The increased CH4 and CO2 concentrations and the airflow rate in the collection pipe are used to calculate a flux each time the animal visits the system. The head needs to be in position for at least 3 min to obtain an accurate measurement. The fluxes determined at each visit are then averaged over the measurement period to determine the mean daily CH4 emission. Sound estimates of CH4 production from the GreenFeed Emission Monitoring system depend upon the animal visiting the system in a distributed pattern over the 24-h cycle to ensure the daily emission pattern is represented. To avoid bias toward daytime emissions, Manafiazar et al. (2016) recommended averaging the spot fluxes over the measurement period by six 4-h times of day bins. Hegarty (2013) proposed considering the circadian rhythm to minimize errors in CH4 estimates when using the GreenFeed Emission Monitor system. A sufficient number of days of data collection is also needed (Hammond et al., 2015; Thompson and Rowntree, 2020). An adequate sampling regime is easier to accomplish when the system is used with animals housed in stalls such that the system can be positioned in front of each animal at the desired sampling time. Using this approach, Hristov et al. (2015) recommended sampling eight times during a 24-h feeding cycle, staggered in time over 3 d. For group-housed animals, Gunter and Bradford (2017) recommended at least 2.4 visits per day for 6.3 d. Others recommended at least 20 visits over a 7- to 14-d measurement period (Manafiazar et al., 2016). Arbre et al. (2016) measured daily values and obtained repeatability of 70% in 17 d and could increase it to 90% in 40 d. Coppa et al. (2021) reported repeatability of 60% for a 1-wk measurement on daily CH4 and increased it to 78% for an 8-wk measurement period. Thus, AHCS estimates an average emission over several days in contrast to respiratory chambers that estimate an emission each day, which can be averaged over multiple days. Methane emissions determined with the GreenFeed Emission Monitoring system and empirical regressions developed from respiration chambers had a high correlation (r = 0.958) and low mean bias (12.9% of observed mean) for dairy cows (Huhtanen et al., 2019). The relationship between predicting CH4 using the GreenFeed Emission Monitoring system and respiration chamber seems to be high for grazing heifers. However, the trend might not be the same throughout the grazing period (Jonker et al., 2016), and feed intake might be needed to correct the estimates by the GreenFeed Emission Monitoring system (Alemu et al., 2017). The GreenFeed Emission Monitoring system can be used in research settings and on commercial farms with large and small ruminants (Zhao et al., 2020), and is suitable for grazing conditions, indoors and outdoors group-housing, and individually penned animals (e.g., tie-stalls). However, animal training is required, and not all animals will learn to use the system. The use of feed as bait in the system may interfere with the dietary treatments because animals consume the bait feed at different proportions of total DMI. In some cases, treatments can be delivered through the hopper system. Like respiration chambers, the gas sensors require calibration, and gas recovery tests must be performed routinely (Hristov et al., 2015). Although the components of the GreenFeed Emission Monitoring system can be calibrated, it is impossible to perform a whole-system evaluation using a release and recovery approach similar to that used for respiratory chambers (McGinn et al., 2021). The main advantages of the system are its ease of use, the ability to make measurements on a large number of animals, and the use of animals that are housed in conditions representative of commercial settings. However, head chambers do not consider CH4 emissions from hindgut fermentation like sniffers and gas tracers. Furthermore, intake of the bait feed can interfere with dietary treatment comparisons. Inline CH4 “sniffers” measure the concentration of CH4 in the animal’s breath at a feed bin usually located at an automatic milking station (Garnsworthy et al., 2012; Huhtanen et al., 2015). The set-up allows for repeated measurements over long periods and it can be installed in commercial dairy operations. The technique assumes a close relationship between daily CH4 production and CH4 concentration in the animal’s breath. The precision of sniffers is significantly less (Bell et al., 2014) than for respiration chambers (Yan et al., 2010) due to high within- and between-animal variation in the CH4 concentration in an animal’s breath. Muzzle movement and proximity to the sample intake and variable air-mixing conditions within the feed bin contribute to this variability. Ideally, the distance between the sniffers and the animal’s muzzle should be less than 30 cm (Huhtanen et al., 2015). Portable accumulation chambers can determine short-term CH4 emissions (1–2 h) in grazing sheep (Goopy et al., 2011). The principle is similar to that of closed-circuit respiration chambers. These chambers are bottomless boxes made with Plexiglass on the sides and top that are lowered down on animals and sealed (Thompson and Rowntree, 2020). Sampling ports located on the top of the box allow the operator to measure gas accumulation. Comparisons with respiration chambers have indicated moderate correlations (r < 0.6) for up to 2-h sampling durations (Goopy et al., 2011; Goopy et al., 2015). Face masks have been used to quantify CH4 emissions similar to closed-circuit animal chambers. Face masks do not permit animals to eat or drink during measurement, and thus they can only be used for short periods of time (e.g., 30 min) to obtain a spot measurement. These measurements can be repeated over time; however, the discomfort caused to the animal must be considered. Hand-held laser methane detectors (LMDs) can be used to measure CH4 concentration in the breath of individual animals (Chagunda, 2013). Although the LMD uses spot sampling of the animal’s breath, it can be used to calculate total emissions (g/d). The LMD uses infrared absorption spectroscopy, and other industries have used similar detection systems (van Well et al., 2005). The device is pointed toward the nostrils of the cow at a fixed distance of one to several meters, and thus, it does not disturb animal behavior. The instrument accounts for the thickness of the CH4 plumes (i.e., concentration), and the result is expressed as CH4 concentration. The LMD can segregate the CH4 concentration from dairy cows performing different physiological activities (e.g., ruminating, feeding, and sleeping). Other challenges relate to applying this approach to grazing animals because wind speed and direction, relative air humidity, and atmospheric pressure can significantly affect the resultant concentration of CH4. For example, wind speed negatively correlated with CH4 concentration (r = −0.41). The CH4 concentration at the animal’s muzzle is highly variable, depending upon respiration and eructation of the animals and air movement. The higher CH4 concentrations during eructation can be differentiated from the lower concentrations during respiration. An additional limitation is a correct distance the device should be from the animal (Sorg, 2022) to avoid contamination from the neighboring animal. The major disadvantage of the technique is that similarly to sniffers, only concentration and not flux are measured. Within a closed chamber, the flux can be estimated assuming the concentration differential and the volume, area, and temperature of the chamber, assuming the ideal gas law (Pedersen et al., 2010; Hüppi et al., 2018). However, it is not recommended for outdoors animals where air movement can dilute the concentration measurements. The CH4 measurement with the LMD demonstrated a strong agreement with measurements in respiration chambers (r = 0.8) in one study (Chagunda and Yan, 2011), but not in others (Ricci et al., 2014). Although the handheld laser is easy to use on commercial farms, studies are required to determine the precision and accuracy of the measurements.
Tracer techniques
Methane emissions can also be determined using a tracer gas (e.g., SF6) released from a bolus or permeation tube with a predetermined release rate in the animal’s rumen. The animal’s breath is sampled over time (usually every 24 h) into an evacuated cylinder by placing a tube near the nostril of the animal, usually positioned on a halter. The CH4 emission rate is computed by the known release rate of the tracer gas and the ratio of expired CH4 and tracer gas concentrations in the canister while accounting for background concentrations of CH4 and SF6 in ambient air (Johnson et al., 1994). Like AHCS and head chambers, the tracer gas technique only measures animal breath emissions and not CH4 from the rectum. Several factors can affect the accuracy of the technique, including the inconsistent release of SF6 from the permeation tubes, elevated background concentrations of SF6 and CH4 in ambient air, and equipment failure (breakage and leakage of the air collection canisters, and blockage of sampling tubes) (Lassey, 2007). There is also a need to handle animals frequently (daily) to exchange collection canisters. Several studies have reported that the tracer gas technique and respiratory chambers produced similar estimates of CH4 once an additional 3% correction for CH4 from the rectum is applied to the tracer estimates (Hammond et al., 2016). However, other studies have shown that the difference between SF6 and respiration chambers can be greater than 10% (Storm et al., 2012; Ramírez-Restrepo et al., 2020). Modifications to the SF6 method have been proposed to improve its predictability, such as continuous collection at a constant rate for 24 h and the incorporation of orifice plates rather than capillary tubes to restrict the rate of sample collection (Deighton et al., 2014). Arbre et al. (2016) suggested that a 3-d measurement period was needed to achieve a repeatability of 70% for CH4 emissions per unit of feed intake (i.e., CH4 yield), without any further increase in repeatability with more extended measurement periods. The SF6 tracer gas technique is suitable for large and small ruminants, and it can potentially be used outdoors (Ramírez-Restrepo et al., 2010) or indoors (Ramírez-Restrepo et al., 2016) in well-ventilated areas. In poorly ventilated buildings, background CH4 (and sometimes SF6) concentration in ambient air interferes with the CH4 calculation (Hristov et al., 2016). The technique cannot be used close to other CH4 sources (e.g., slurry, manure, other animals, and wet areas) and SF6 sources (e.g., electricity transformers and industrial sites) (Jonker and Waghorn, 2020). Although the SF6 technique is relatively inexpensive, it requires technical skill to operate. Adequate calibration of the release rate of the tracer gas from the permeation tube must be conducted in advance of placement in the rumen, with the experiment carried out soon after to ensure a constant release rate from the permeation tubes, as the rate can decline after 6 to 12 mo of use. For long-term trials, adjustments for the changing permeation rate should be performed (Jonker and Waghorn, 2020). Gas chromatography is also a fundamental and critical step in the tracer gas technique, which requires specific skills and facilities. Pinares-Patiño et al. (2015) provided the design and operation of the SF6 technique.
Madsen et al. (2010) suggested predicting CH4 from CO2 modeled from body weight, energy-corrected milk yield, and days of pregnancy, assuming that the energy utilization efficiency for maintenance and production is constant for dairy cows. Individual CH4 emission was recorded in an automatic milking system on dairy cows for 3 d, using a portable air sampler and analyzer unit, based on Fourier transform infrared detection and CO2 as a tracer gas (Lassen et al., 2012). Air was analyzed every 20 s when the animals were milked, and the ratio between CH4 and CO2 was used to measure CH4 emission. The repeatability of the measurement (CH4:CO2 ratio) was 0.39 and 0.34 for Holstein and Jersey cows, respectively (Lassen et al., 2012). These results suggested that the CH4:CO2 ratio could be used for the management and genetic evaluations of dairy cows (Lassen et al., 2012). However, efficient cows (i.e., more milk per feed consumed) produce less heat and consequently CO2 per unit of metabolic body weight and energy-corrected milk, thus overestimating their CH4 production. Hence, genetic selection for low CH4 emitters using this technique would favor inefficient dairy cows (Huhtanen et al., 2020).
Open-path laser technique
The open-path laser technique quantifies the dispersion of a specific gas from the source and the downwind concentration of the gas to establish the emission rate, using an “inverse dispersion” approach (McGinn et al., 2006). The technique has been used for CH4 (McGinn et al., 2006) and ammonia (NH3) (McGinn et al., 2007) emissions from groups of animals (e.g., feedlot and pastures). The open-path laser technique has been updated with different analyzers and atmospheric parameters integrated into aircraft (Hacker et al., 2016) and drones, showing reliable and promising results. Hacker et al. (2016) indicated that CH4 and NH3 could be detected for at least 25 and 7 km, respectively, from a high strength source (e.g., feedlot). Validation assays have shown limitations of the technique regarding the time of data collection (McGinn et al., 2006; McGinn et al., 2008); spot measurements made by aircraft and drones during the daytime, when emission rates are highest, may not reflect the 24-h period.
Tomkins et al. (2011) compared the daily CH4 emissions estimated using the open-path laser technique used on pasture to respiration chamber with animals fed freshly-cut Rhodes grass (Chloris gayana) from the same pasture. Daily estimates were 136 and 114 g CH4/d, respectively, and the authors suggested that further comparisons using different forages and herds were needed. Subsequently, Tomkins and Charmley (2015) tested the open-path laser technique around water points when animals were present. The authors concluded that the open-path laser technique is a good option when employed on aggregated grazing cattle for at least seven hours per day over 7 to 14 d. However, the 24-h pattern of CH4 emissions would not be fully represented. The open-path laser technique is helpful for directly measuring CH4 emissions from cattle at the herd scale in grazing conditions and in intensive livestock operations.
In vitro techniques
The in vitro fermentation technique has been used for many years to evaluate ruminal fermentation of feedstuffs and, more recently, to assess the effect of different nutritional strategies to mitigate CH4 production (Yáñez-Ruiz et al., 2016). Due to the complexity and cost of methodologies for evaluating enteric CH4 emissions directly from animals, the possibility of obtaining results through in vitro systems is a potential alternative, mainly to provide an initial screening of a larger number of samples with different alternatives for reducing methanogenesis such as tannins, plant secondary metabolites, and essential oils (Tedeschi et al., 2021). However, limitations exist if the fermentation end products are not adjusted for microbial mass (Makkar, 2005). Various in vitro techniques can be used, varying from batch culture systems (Pell and Schofield, 1993; Theodorou et al., 1994; Mauricio et al., 1999) to continuous fermenters such as RUSITEC (Czerkawski and Breckenridge, 1977) or dual-flow continuous culture system (Hoover and Stokes, 1991). Within the batch culture systems, the in vitro gas production technique has been widely adopted to determine the nutritive value of feeds through fermentation kinetics (Blümmel et al., 1997; Getachew et al., 1998; Tedeschi et al., 2009). Some systems measure CH4 production throughout the incubation, but CH4 production is determined at the end of the fermentation in other systems. An optimal fermentation time has not been established, and it is likely variable because the terminal CH4 production may represent the potential CH4 production rather than actual CH4 production if the incubation time used in vitro exceeds the mean retention time of feed in the rumen. Danielsson et al. (2017) reported a high correlation (r = 0.98) between in vitro and a head stall systems (GreenFeed Emission Monitoring system), though the values were underpredicted (399 vs. 418 L/d, respectively). Most in vitro techniques are derived from Tilley and Terry’s (1963) two-stage method, which consists of simulating rumen conditions (temperature, pH, and anaerobiosis) using a rumen inoculum (strained rumen fluid), buffer to avoid significant pH variation, and a media to provide necessary nutrients to the ruminal microbiota. The CH4 production is usually expressed per incubated or digested DM or organic matter basis and is more closely correlated with in vivo CH4 production expressed per unit of degraded material. Yáñez-Ruiz et al. (2016) discussed specific details about the in vitro techniques regarding experimental design, implementation and interpretation of in vitro experiments to assess enteric CH4, and factors that influence the results from in vitro fermentation techniques (e.g., donor animals and diet, inoculum collection and processing, different substrates, and incubation buffer and procedures).
Measurements Using Facility-Based Techniques
Manure storages
Three different approaches for the quantification of CH4 emissions (manure-only or manure and animal CH4) from housing are commonly used: direct measurement methods, inverse modeling (animal housing emissions), and chamber technique (manure emissions) (Hassouna and Eglin, 2016). At the barn level, removal of cattle to estimate emissions from manure has been performed (Mathot et al., 2012; Mathot et al., 2016; Edouard et al., 2019). Some methods are developed for measuring emissions from barn and manure storage at an experimental scale (Mathot et al., 2016) but are difficult to implement on commercial farms. To date, there is no international standardization of the methods for the animal house scale because of the considerable variability of the bedding conditions. Methodology to quantify the accuracy of the measurement is limited because of the complexity of the different measurement processes.
Direct methods
Direct methods are the most widely used. An emission rate is calculated as the product of the housing VR and the in-house CH4 concentration minus the background concentration (Hassouna et al., 2021). Methodology to quantify the uncertainty of aerial emissions for the direct methods has been outlined by Gates et al. (2009) and involves the statistical uncertainty of both the emissions concentration measurement and the VR measurement. Measurements associated with VR have been demonstrated to be the major contributor to the emissions rate uncertainty when utilizing direct methods.
Ventilation rate
For the VR quantification, three methods have been implemented mainly in studies and compared in the literature: internal tracer gas and external tracer gas (indirect methods) and sensor use (direct method). The first method is CO2 balance. For this method (Barreto-Mendes et al., 2014; Liu et al., 2016), the leading hypothesis is that VR determines the relationship between CO2 production in the barn and the difference in CO2 concentrations between the inside and outside of the barn (ΔCO2). CO2 is used as an internal tracer gas. In the barn, CO2 production comes from animals, litter, and gas or fuel heating systems, if applicable in the barn. Pedersen et al. (2008) did not recommend using this method to calculate VR in the animal house with deep litter because of its high and variable CO2 production. Animal CO2 production can be estimated from animal heat production, CO2 production per heat unit, and animal activity. In many studies, these parameters are calculated with models given by the International Commission of Agricultural Engineering (CIGR, 2002). According to Zhang et al. (2010), associated errors ranging from 10% to 20% and more recent models that take into account the progress of animal genetics should be taken into consideration to improve the accuracy of the VR estimations. Concerning the accuracy of VR, Calvet et al. (2011) demonstrated that it is necessary to consider the daily variation of CO2 production that depends on animal activity to estimate the daily variation of VR accurately. This CO2 balance method also requires ΔCO2. Van Ouverkerk and Pedersen (1994) suggested that ΔCO2 values should not be lower than 200 ppm for the method to yield reliable results, which can often be the case in very open barns.
The second method to estimate VR is using non-CO2 external tracer gas. The external tracer gas method for measurement of the emissions in Iivestock buildings refers to a technique that relies on the release of an external tracer gas (i.e., a gas that is not produced in the barn). This method is often used in naturally ventilated buildings (Ogink et al., 2013). The most widely used gas is SF6 because it is easy to detect, chemically inert, and is not produced in the building. The barn VR is calculated using the tracer gas injection rate and the tracer concentration gradient, assuming perfect mixing of the air inside the barn and steady-state conditions. Because of the high GWP of SF6, low concentrations of SF6 should be injected, and the concentration measurements have to be done with a sensor with a low detection limit. This method could be implemented in livestock buildings using two different approaches: a constant injection of the tracer gas or spot injections (concentration decay method). For the constant injection method, the tracer gas is dosed into the barn or, more generally, close to an emitting areal/point source. This tracer gas mimics the dynamic flow and the dilution of CH4 or other target gas such as N2O or NH3 (Schrade et al., 2012). For the tracer decay method (spot injections), a dose of tracer gas is injected and mixed into the housing until the desired threshold is achieved and uniform distribution of the tracer gas is reached. Then the injection is stopped, and the decrease of tracer gas concentration is monitored during a given period to calculate the VR (Mohn et al., 2018). This method requires a sensor or device to measure tracer concentration with a reasonably fast analysis frequency in highly ventilated barns like open barns and is not suitable for long-term airflow measurements (Ogink et al., 2013). Many studies have compared this method with the CO2 method in different livestock buildings. Edouard et al. (2016) found that both methods gave similar results being 10% to 12% lower with the CO2 mass balance method than SF6 tracer methods.
The third method for estimating VR is through sensors. In mechanically ventilated houses, continuous monitoring of the static pressure differential and each fan’s operating status (on-off) can be used to estimate the fan’s VR based on its theoretical or measured performance characteristics. Ideally, the in situ performance of each fan is determined first, and the house VR can be estimated by summing all operating fan flow rates (Gates et al., 2004). Gates et al. (2005) developed and improved a fan assessment numeration system (FANS) to measure ventilation fans’ in situ performance curve operating in a negative pressure mechanically ventilated animal house. This approach can provide ventilation estimates with uncertainties of less than 10% in low airflow conditions and less than 25% in higher airflow conditions when regular in situ calibration is conducted (Gates et al., 2009). In naturally ventilated houses, Joo et al. (2014) proposed a method that relies on implementing a high number of ultrasonic anemometers at the openings of the barn. In the methods they developed, any positive velocities indicated air outflows, whereas negative velocities denoted air flowing into the barns. The total air inflow rate was assumed as the sum of air inflows at the inlets, while the total air outflow rate was the sum of air outflow rates at the outlets.
Methane concentration
Methane concentrations also have to be measured inside and outside the barn to quantify the emission rate. The same device is often implemented for both measurements, implying that the device has to have the adapted detection range. Powers and Capelari (2016) listed many techniques commonly implemented for CH4 concentration measurements, including gas chromatography, infrared spectroscopy, Fourier transform infrared spectroscopy technologies, photoacoustic spectroscopy, mass spectroscopy, tunable diode laser absorption spectroscopy technology, and solid-state electrochemical technology. These techniques are mainly spectroscopic and portable, but only techniques with a very selective detection system, such as lasers, are preferable for continuous measurements. Hassouna et al. (2013) have highlighted interference problems with nonselective methods such as photoacoustic infrared spectroscopy (commonly used), leading to overestimated CH4 emissions. Gas chromatography can also be implemented, but continuous measurement is more complicated on commercial farms because regular calibration is required. Nevertheless, not all sensors and gas analyzers on the market are suitable for detecting CH4 in barns due to existing adverse conditions (e.g., dust, moisture, NH3, and animals). The reliability of measurements over time is not always guaranteed. Testing the new measuring equipment available is a process that can be quite long. Moreover, the available sensors and devices are typically costly.
Other methods
Inverse modeling (animal housing emissions) and chamber technique (manure emissions) comprise other methods to estimate CH4 emissions for housing and outdoor storage and spreading. Inverse modeling consists in determining the concentrations of CH4 in and around the area of interest and iteratively adjusting the sources to minimize the difference between measured and model-predicted concentrations. It has been employed on manure storage systems, spread slurry, manure of mineral fertilizers, or livestock buildings (Hassouna and Eglin, 2016). The inverse modeling technique has also been used to solve other problems (Vargas-Villamil and Tedeschi, 2014; Vargas-Villamil et al., 2020), and it shares some resemblance with the system dynamics methodology (Tedeschi, 2019). Both open and closed chambers are implemented for measuring CH4 emissions at a local scale, usually for areas less than a square meter (Wang et al., 2010) in various manure handling systems, including liquid and solid storage (M⊘ller et al., 2004; Kreuzer and Hindrichsen, 2006). Static chambers are used mainly to characterize the gaseous fluxes after spreading manure on fields (Norris et al., 2020), but they can be adapted for emissions from manure storage (e.g., slurry pits, lagoons, and manure heaps) and pasture land. This method estimates the fluxes from the manure based on the accumulation dynamics of CH4 inside the chamber placed on the surface of the manure (Hassouna and Eglin, 2016). Static flux chambers are intrusive, and for an accurate and reliable estimation of the emissions, a sampling strategy that relies on the implementation of several chambers has to be applied to reflect the variations in emissions over the area.
The principles for collection and measurement via chambers apply to both soils and manure storage systems. A solid or clear open-bottomed chamber of a known volume is fitted onto a permanently installed ring or collar. For closed or static chambers, CH4 builds up in the chamber’s headspace over time (e.g., 30 min), and the concentration in the chamber is sampled over a time series. For non-CO2 trace gases like N2O and CH4, more extended time series are often required due to these gases’ low, negligible, or negative fluxes (Collier et al., 2014). A small fan is often installed inside the chamber to mix the gases thoroughly. Gas samples can be collected via syringe and transferred into glass vials for offsite analysis (Sass et al., 1990; Sass et al., 1991) or in situ if using a dynamic system (Hall et al., 2014); these types of closed chambers are known as non-steady-state non-through-flow and non-steady-state through-flow chambers, respectively (Livingston and Hutchinson, 1995; Pumpanen et al., 2004). Open chambers, i.e., dynamic or steady-state chambers, replace air inside the headspace with ambient air through an inlet port, and CH4 flux is estimated as the difference between the gas concentrations at the inlet and outlet ports (Pumpanen et al., 2004). Like closed chambers, gas analysis can occur in situ or through collection in glass vials for offsite analysis.
Gas chromatography (GC) is the conventional method used to analyze CH4 concentrations in gas samples from soils and manure handling systems. Several types of GC detectors exist (Harvey et al., 2020), including mass spectrometry (Ekeberg et al., 2004), flame ionization detector (Weiss, 1981), and multiple gas analysis systems (Sitaula et al., 1992; Hedley et al., 2006). Laser technologies, Fourier-transform infrared, and other optical techniques continue to grow in popularity for analyzing CH4 concentrations because of their low detection limits, a higher degree of precision, and ability to measure multiple GHGs simultaneously at the sampling location (Brannon et al., 2016; Harvey et al., 2020). These include quantum cascade laser (QCL) (Nelson et al., 2002; Cowan et al., 2014), and other spectroscopic techniques with QCL like cavity ring-down spectroscopy (Christiansen et al., 2015; Brannon et al., 2016), and off-axis integrated cavity output (Brannon et al., 2016; Waldo et al., 2019; Harvey et al., 2020). Infrared adsorption measurement detectors are ideal for automated chamber systems and in situations that require frequent, high precision measurements. Although comparisons show good agreement between these methods, non-GC-based methods better capture diel variation and responses to experimental treatments. Other auxiliary measurements like soil and water temperature, air temperature inside and outside the chamber, and soil moisture should be collected at the time of collection (Pavelka et al., 2018) for use in seasonal and annual CH4 flux calculations. Regardless of chamber type, care should be taken to ensure that the collection of gas samples does not introduce artificial environments or conditions that alter CH4 flux. Collections rings or collars should be installed well in advance of sample collection, i.e., >24 h, to allow the diffusion of gas to the atmosphere from the soil or litter layer sufficient time to equilibrate after the disturbance event.
Both open and closed static chambers are widely accepted in the literature, but selecting between chamber types involves consideration of costs, labor availability, experimental design, and sampling conditions (e.g., site accessibility, climate, and soil type). Non-through-flow closed chambers are advantageous because they are low cost and simple to deploy, but they require greater manual labor investment (Savage et al., 2014), and both non-through-flow and through-flow types can alter temperature, moisture, and gas diffusion dynamics during sample collection (Husted, 1993) leading to errors in flux estimation (Pihlatie et al., 2013; Ueyama et al., 2015). For flux estimation with closed chambers, errors can be significantly reduced by increasing chamber size, i.e., height, area, and volume (Pihlatie et al., 2013). The long duration times needed for measurement with closed chambers can also alter diffusion gradients (Davidson et al., 2002; Savage et al., 2014). Open chambers, particularly through-flow systems, allow for more frequent, and less time and labor-intensive measurements (Savage et al., 2014; Ueyama et al., 2015). Furthermore, open chambers may be more appropriate for manure handling systems given the differences in gas diffusion dynamics relative to soils (Husted, 1993). However, these chambers require greater capital investments and maintenance, and may not be suitable in low infrastructure contexts (Collier et al., 2014).
Measurements Using Large-Scale Techniques
In addition to the open-path laser technique discussed above, there has been an increased use of aircraft, satellites, and unpersonned aerial vehicles (i.e., drones) in the last 5 yr to assist with GHG measurements and estimations primarily based on the top-down approach discussed below.
Aircraft
Airborne CH4 measurements of dairy farms have been conducted using a series of concentric, closed flight paths, and the emission rates were estimated with the application of Gauss’s Theorem (Conley et al., 2017). The CH4 mixing ratio, pressure, temperature, and horizontal wind are measured at the barn level while an aircraft is flying a series of concentric close paths around the farm facilities to calculate the whole-facility CH4 emissions. Aircraft measurements were compared with open-path measurements with inverse dispersion modeling, and vehicle measurements with the tracer flux ratio method in California dairies and estimated CH4 emission rates were compared on a whole-farm level and primary sources with a farm (e.g., animal housing and liquid manure lagoons) (Arndt et al., 2018; Daube et al., 2019). These measurement techniques are also sensitive to capturing CH4 emissions dynamics under different management systems, i.e., liquid slurry vs. dry manure storage (Arndt et al., 2018), with direct implications for GHG inventories and climate actions.
Satellite and drone imagery
Precision imagery, such as drone or satellite imagery, can be utilized to determine and monitor soil and crop health and estimate the yield of crops, given the good correlation between leaf area index and normalized difference vegetation index (NDVI) (Lamb et al., 2011; Nagy et al., 2018; Wahab et al., 2018). Drones could be used to track and count animals (Laradji et al., 2020) and have also been shown to detect CH4 leaks in natural gas pipelines (Tannant et al., 2018; Barchyn et al., 2019). There is potential to adapt these technologies to assess and benchmark livestock-related CH4 emissions on farms, but research is lacking in this field.
A new generation of remote sensing and satellite-based monitoring systems continues to support the quantification and monitoring of CH4 emissions. Satellite CH4 emission measurements provide better spatiotemporal coverage of emissions and hotspots than traditional in situ measurements. Early satellite measurements of global CH4 emissions were made with SCIAMACHY (Frankenberg et al., 2006) and later with GOSAT (Houweling et al., 2014; Kuze et al., 2016). The number of dedicated CH4 focused missions has increased over the past several years, including GHGSat (Varon et al., 2018), GOSAT-2 (Glumb et al., 2014), geoCARB (Polonsky et al., 2014), and MethaneSAT (Staebell et al., 2021; UNEP and CCAC, 2021). Satellite-based measurements rely on inverse modeling to understand and quantify CH4 emissions at regional and global scales (UNEP and CCAC, 2021). Under inverse modeling, the atmospheric measurements made with satellites are used to back-calculate both the location of an emissions source and the rate of emission (UNEP and CCAC, 2021; Houweling et al., 2014).
Methods to Estimate Methane
Bottom-up approaches
The so-called “bottom-up” approaches sum up estimates of all identified source components of a given region or boundary to achieve an estimate of the global source of CH4 emitters, including enteric, manure, and soil/crop. Lassey (2008) stated that many of these components are ill-quantified and that there is a lack of agreement among distinct estimates. The “bottom-up” approaches follow a more mechanistic, conceptual, build-up approach rather than a reconciliatory approach (e.g., “top-down”) that may be ill-equipped if the actual sources are not known; thus, incorrectly assigning estimate shares to known sources. Vibart et al. (2021) provided an extensive discussion about mathematical models predicting on-farm CH4 and N2O emissions.
Enteric modeling
There are many different types of mathematical modeling methods in agriculture; the most common ones are empirical vs. mechanistic, stochastic vs. deterministic, and static vs. dynamic (Thornley and France, 2007; France and Kebreab, 2008). Some mathematical nutrition models may incorporate different (and sometimes complementary) methods for predictability purposes, often called levels of solutions (Tedeschi and Fox, 2020a), or in other words, tiers of solutions. The simplicity of empirical models is often the dominant factor in decision-making when selecting models to predict CH4 emissions. In part, the model simplicity is brought up by the inputs required for the execution of the model (essentially derived from statistical regression models and methods), and it ends up favoring the selection of empirical models over more complex (and sometimes more complete) types of modeling such as mechanistic or even agent-based models. Empirical models, unfortunately, are not good explainers of the underlying biological mechanisms behind a natural phenomenon, but they serve their intended purpose of deterministic predictions (Tedeschi and Fox, 2020a) if all inputs (e.g., variables) are available and within the range of the original dataset used to develop the statistical regression. Another factor that is rarely considered is that the new inputs must have similar correlations among themselves as the inputs of the original dataset; otherwise, the variable’s coefficients might be incorrect, and the prediction will be biased. Therefore, cautionary notes should accompany model predictions because their limitations and intended use may not be the appropriate mathematical model for all types of production scenarios. Ideally, different alternatives for model predictability using contrasting modeling methods should be available and used. For instance, the Beef Cattle Nutrient Requirements Model (BCNRM) by the NASEM (2016) provided empirical and mechanistic options to predict the CH4 emissions of beef cattle. The BCNRM’s empirical option was developed based on selected empirical equations for typical beef cattle production scenarios in North America (Escobar-Bahamondes et al., 2017), whereas the BCNRM’s mechanistic option was developed based on mechanistic and empirical approaches to model the rumen functions (NRC, 2000; Fox et al., 2004), often called functional models because they simultaneously have empirical and mechanistic elements in support of a specific predictive goal (Tedeschi and Fox, 2020a). Unfortunately, few mathematical nutrition models have explicitly modeled the CH4 emission from the hindgut of ruminants, in part because the rumen represents close to 90% of the CH4 emission (Murray et al., 1976; Tedeschi and Fox, 2020a), and there is a lack of interest in predicting the fermentation dynamics in the hindgut because they contribute little, if any, to ruminant animal performance and production.
The gold standard for enteric CH4 determination is actual measurement using the methods described above. However, such measurements are resource-intensive. Bottom-up models to predict emissions have been used in place of actual measurement. These models use regional activity data to estimate emissions. The IPCC (1996) developed a standard predictive bottom-up model that has undergone several refinements to the current one. These models are generally stratified into tiers depending on the level of sophistication. Tier 1 uses default emission factors based on general literature due to the paucity of data in a region. This level, therefore, does not consider the characterization of livestock systems prevalent in a region, such as breed types, age of animals, physiological states, level of productivity (except for cattle and buffalo Tier 1a), and diet (intake and composition). Tier 2 is based on emission factors refined to consider feed and animal characterization. The emission factors for each livestock category are estimated based on the gross energy intake (GEI) and CH4 conversion factor (Ym, expressed as % of GEI converted to CH4). Tier 3 is region-specific based on years of extensive research in the region. The IPCC (2019a) model has been criticized for assuming ad libitum feed intake and that uncertainties accompanying the derived emission factors are ill-defined, which is often the case when prevailing conditions in a region are not considered (Goopy et al., 2018).
Many predictive models exist and are discussed in several reviews (Moraes et al., 2014; Niu et al., 2018; Benaouda et al., 2019; van Lingen et al., 2019). These models are based on dietary intake, proportions and compositions, and animal characteristics. The scientific community agrees that DMI is crucial in predicting CH4 production (and emission). For instance, Benaouda et al. (2019) reviewed 36 empirical models involving 16 dietary and animal variables and found that 56% of the models used DMI as the best predictor of enteric CH4 production, while 28% of the models selected GEI as the main predictor of CH4 production. Niu et al. (2018) developed 42 empirical models and reported that increased complexity improved prediction. They also reported that models with DMI only were as good as the complex models while other dietary variables, such as dietary fiber fractions and ether extract, improved the models’ prediction. These findings are consistent with those discussed by Appuhamy et al. (2016), who reviewed 40 models involving 20 variables and found that 43% of the models used DMI as a good predictor of CH4 production.
Determination of DMI for stall-fed and confined animals is straightforward, but many livestock systems involve ruminants grazing on native pastures supplemented with crop residues and cultivated fodder/forage in mixed crop-livestock systems. The determination of dietary amounts and composition in these systems is complicated. In part, voluntary feed intake depends on the digestibility of the diet (or digestible energy), which, in turn, depends on the intake level (Tedeschi et al., 2019). This complication becomes more convoluted because of the lack of proper characterization of the prevailing livestock systems (i.e., numbers, breeds, herd structures, body weight, physiological states, and level of productivity). General methods for estimating DMI include the use of empirical models such as those based on the net energy system (NRC, 2001; NRC, 2007; NASEM, 2016) and those utilizing animal characteristics, pasture conditions, and supplementation (CSIRO, 2007), use of internal and external markers and herbage disappearance (Macoon et al., 2003; Undi et al., 2008). These methods, being mere estimates, may inherit uncertainties that further compound and increase uncertainties in CH4 predictive models. In such cases, it would be advisable to adapt DMI estimates to local conditions as much as possible. One such adaptation is the use of “feed basket,” a term referring to proportions of feeds on offer in a given season in a given region, making up the seasonal diet of livestock in that locality (Gerber et al., 2013; Goopy et al., 2018; Marquardt et al., 2020). It is possible that the more region-specific the data and model, the lower the accompanying uncertainty and the better the resulting estimates. Predictive models are used to develop national emission inventories for monitoring, reporting, and verifying nationally determined contributions to the mitigation of GHG emissions (Bodansky et al., 2016).
As alluded above, mechanistic models represent the underlying processes that control emissions and their interactions. There are very few mechanistic models developed to predict CH4 emissions. A dynamic mechanistic model designed to simulate digestion, absorption, and outflow of nutrients in the rumen was developed by Dijkstra et al. (1992). The model contains 19 state variables representing N, carbohydrate, lipid, and volatile fatty acid (VFA) pools. Enteric CH4 production is estimated based on VFA stoichiometry developed by Bannink et al. (2006), which relates the VFA produced to the type of substrate fermented in the rumen. The assumption is that the hydrogen produced in the rumen from the fermentation of carbohydrates and protein is used: (1) to support rumen microbial growth, (2) for biohydrogenation of unsaturated fatty acids, and (3) for production of glucogenic VFA (i.e., propionate and valerate). The remaining hydrogen is used for the reduction of CO2 to CH4, and the prediction from rumen methanogenesis and hindgut fermentation is described by Mills et al. (2001). The model has been used to estimate enteric CH4 emissions mostly from dairy cattle (Kebreab et al., 2008; Alemu et al., 2011b; Morvay et al., 2011). A version with an updated VFA stoichiometry that includes the effect of rumen pH on the stoichiometry of VFA formed upon fermentation of soluble sugars and starch (Bannink et al., 2008) is used as a Tier 3 method for CH4 inventory accounting in The Netherlands (Bannink et al., 2011). Ellis et al. (2010) introduced modifications to the model in order to be able to handle predictions for beef cattle better.
MOLLY is another dynamic mechanistic model that simulates rumen digestion and whole-body metabolism in lactating dairy cows (Baldwin et al., 1987a; Baldwin et al., 1987b; Baldwin et al., 1987c; Baldwin, 1995). The model was constructed in a similar way as described above, but the VFA stoichiometry is based on the equations developed by Murphy et al. (1982) and later updated by Argyle and Baldwin (1988), which relate the amount of VFA produced to the type of substrate fermented in the rumen. In addition to the stoichiometric differences described above, the two mechanistic models differ in the number of microbial pools; MOLLY uses one microbial pool, whereas the model by Dijkstra et al. (1992) uses three pools (amylolytic, fibrolytic, and protozoa). The number of model pools (i.e., stock or state variables) is usually associated with different modeling concepts; it does not necessarily improve the model’s predictive ability because their purposes might differ (Tedeschi and Fox, 2020a, b).
Several studies have evaluated the predictive potential of empirical and mechanistic models for enteric CH4 production from cattle using independent data sources (Benchaar et al., 1998; Kebreab et al., 2006; Kebreab et al., 2008; Alemu et al., 2011b). Benchaar et al. (1998) compared the predictive capacity of two mechanistic and two linear models with a database constructed from literature. Predictions from linear equations were poor; the models explained between 42% and 57% of the variation. On the other hand, the mechanistic models explained more than 70% of the variation. Alemu et al. (2011a) compared empirical models and the VFA stoichiometry used in mechanistic models to estimate and assess trends in enteric CH4 emissions from western Canadian beef cattle. The authors concluded that a more robust approach might be to use mechanistic models to estimate regional Ym values, which are then used as input for IPCC models for inventory purposes.
Another mathematical model that can be used to forecast CH4 emission was developed by Pitt et al. (1996) and Pitt and Pell (1997) to predict VFA and ruminal pH within the Cornell Net Carbohydrate and Protein System framework. The assumptions in developing the model were based on the mass balance approach and included (1) ruminal degradation of real protein yields negligible amounts of VFA and CH4, (2) CH4 is the main sink of H2, (3) ruminal N balance is positive, and (4) the end products of ruminal fermentation are essentially computed as one minus bacteria yield, multiplied by the amount of ruminally degraded carbohydrate corrected for bacteria ash, crude protein derived from ammonia-N, and the carbon skeletons of noncarbohydrate sources (Tedeschi and Fox, 2020a, b). Further additions to Pitt’s model were discussed by Tedeschi and Fox (2020a, b) and incorporated into the NASEM (2016), including pectin impact on ruminal pH, adjustments for bacterial nitrogen, and optimization for ruminal pH given the rates of degradation and escape of carbohydrates, VFA, and lactate, and buffering capacity from saliva production and feed composition. Despite the limited evaluation of the VFA-pH-CH4 model conducted by Pitt et al. (1996), the CH4 emission has not been fully vetted. The model developed by the French Institute for Agricultural Research (INRA, 2018) serves as the base of a Tier 3 method to estimate CH4 emissions of indoor and grazing production systems, given available information on the type of animal, production level, and diet characteristics and consumption (Eugène et al., 2019).
Although mechanistic mathematical models represent a more advanced form of predicting CH4 production and emission by ruminants, additional, targeted inputs might further improve the adequacy of the predictability of such models. An example is the milk mid-infrared (MIR) spectra of milk components as a proxy to estimate individual CH4 emissions when using chemometrics models. Indeed, common metabolic processes will affect both the amount of eructated CH4 and the level of milk components (e.g., fatty acids). Milk mid-infrared spectra represent the chemical bonds from the components present in the milk. Moreover, milk MIR spectra can be obtained routinely at a reasonable cost (already collected for milk payment and/or milk recording). This proxy represents significant interest for large-scale studies (compare animals, herds, periods, geographical regions, and genetic studies) (Vanlierde et al., 2020), but information about the limitation and applicability of milk MIR is lacking.
Manure modeling
Like enteric models to estimate CH4, there are empirical and mechanistic models to estimate CH4 emissions from manure. For empirical models, as is the case for enteric CH4, IPCC’s (2019a) guidelines for National Greenhouse Gas inventories indicate three tiers of complexity to estimate CH4 produced during the storage and treatment of manure and from manure deposited on pasture. The Tier 1 approach is based on default emission factors per unit volatile solid (VS) by animal category and manure storage system. Tier 2 is based on country-specific estimates of VS and the impact of interactions between manure management systems and animal categories on total CH4 emissions during excretion and storage, including manure treatments such as biogas production. Recent emission factor databases may help refine the Tier 2 approach in line with the distribution of climate regions within a country (Vigan et al., 2019; van der Weerden et al., 2020; Beltran et al., 2021). Finally, Tier 3 requires specific modeling approaches tailored to country-specific methodologies or measurement-based approaches to quantify emission factors. Likewise, several models have been used to estimate the CH4 emissions from manure storage systems, which unfortunately possess a higher degree of uncertainties. For example, using the IPCC Tier 2 method, for the management of liquid manure in anaerobic lagoons and slurry storage systems, the reported CH4 emissions were in the range of 368 ± 193 and 101 ± 47 kg CH4 per head/year, respectively (Owen and Silver, 2015).
Mechanistic modeling of CH4 emissions from manure is challenging because of the complex data requirement and model parameterization (Li et al., 2012). Other limitations of most existing mechanistic modeling are the lack of microbial response to variations in manure temperature, substrate availability and age, and management system (Dalby et al., 2021) or the distinction between short- and long-term responses to environmental changes. Similar to enteric emissions, mechanistic models of manure emissions are scarce. Although some approaches are part of whole-farm models and can simulate and compare different manure systems, e.g., Manure-DNDC by Li et al. (2012) and Dairy-CropSyst by Khalil et al. (2019), others simulate specific manure systems, e.g., liquid manure storage by Huang et al. (2010), or treatments, e.g., anaerobic digestion (ADM1) by Batstone et al. (2002). The Manure-DNDC (Li et al., 2012) is an extended version of DeNitrification-DeComposition model (Li et al., 1992). The Manure-DNDC model was developed to simulate biogeochemical cycles of C, N, and phosphorus (P) in livestock farms and can be applied to simulate GHG, ammonia, and nitric oxide (NO) emissions from significant components of livestock production facilities. The model contains fundamental processes describing the turnover of manure’s organic matter. A relatively complete suite of biogeochemical processes, including decomposition, urea hydrolysis, ammonia volatilization, fermentation, methanogenesis, nitrification, and denitrification, have been embedded in the Manure-DNDC, which allows the model to compute the complex transfer and transformations of C, N, and P in livestock production systems. The model has been extensively calibrated for California cropping systems and has been used for developing California CH4 emission inventory from rice paddies and N2O emission inventory from synthetic fertilizers and crop residue (Deng et al., 2018a; Deng et al., 2018b). Nevertheless, there is still a need for simpler models that use fewer input parameters than mechanistic models but can adequately represent C and N flows dynamically and are sensitive to most of the factors influencing GHG emissions. Few mathematical models, to the best of our knowledge, have been successfully developed (Pardo et al., 2017b) and applied following these balanced and flexible principles (Pardo et al., 2017a).
Top-down approaches
“Top-down” approaches can provide more accurate estimates of global CH4 after mass balance is applied to global sources and sinks (Lassey, 2008). Measurements of CH4 emissions are made along a spectrum of spatial and temporal scales ranging from instantaneous (e.g., individual sources) to global assessments of annual CH4 emissions. As indicated above, “bottom-up” approaches typically involve measuring at a scale of individual CH4 emitters, such as livestock or manure storage facilities. It uses emissions factors developed based on data collected at individual, activity, and sometimes mechanistic models. “Top-down” approaches, in contrast, estimate emissions using observations of atmospheric CH4 concentrations and models that account for atmospheric transport from an emitter to an observation location (NASEM, 2018). The isotopic characterization of CH4 emission may provide robust discrimination between sources (Nisbet et al., 2020). The proportion of biogenic emissions (from wetlands, ruminants, or wastes) leads to a shift to negative values of δ13CCH4 (atmospheric CH4 changing carbon isotope ratio) (Nisbet et al., 2019). However, various “top-down” techniques are used for measuring CH4 emissions, including remote observations (e.g., atmospheric CH4 by infrared spectrometry), towers, aircraft, and satellites. Many modeling approaches are suitable for spatial scales of 10 to 100 m (Lassey, 2007). Another method is the airborne integrated-path differential-absorption LiDAR (Light Detection and Ranging) (Amediek et al., 2017), but more results are needed to confirm its usability and effectiveness given cloud coverage and different instrument settings for different regions.
Comparing bottom-up with top-down approaches
Comparing estimates produced from top-down and bottom-up techniques has helped identify information gaps and research needs. In some cases, top-down estimates of emissions and bottom-up inventories have significant differences, leading to a reexamination of estimates from both approaches (NASEM, 2018). The challenge for top-down approaches is that estimates include emissions from all sources but may have difficulty attributing emissions to specific sources. Bottom-up approaches, on the other hand, provide estimates from specific sources. Miller et al. (2013) used atmospheric CH4 observations, spatial datasets, and a high-resolution atmospheric transport model to estimate CH4 sources in the United States. The authors concluded that emissions due to ruminants and manure are up to twice the magnitude of the bottom-up approaches used by the US Environmental Protection Agency (EPA). Hristov et al. (2013) challenged Miller’s et al. (2013) top-down estimates and showed that the EPA estimates agree well with other more refined models used to quantify emissions at the individual scale. According to NASEM (2018), uncertainties in top-down CH4 emission estimates arise from uncertainties in atmospheric transport models. Further, NASEM (2018) reports that current global and regional atmospheric transport models are likely unable to accurately represent small-scale processes, making it difficult for them to simulate observed CH4 at continental sites accurately. Arndt et al. (2018) conducted contemporaneous top-down and bottom-up measurements. The authors showed that whole-facility CH4 emissions estimates were similar among open-path, vehicle, and aircraft measurements and to bottom-up estimates. Emissions from animal housing were similar to EPA estimates, but CH4 emissions from liquid manure storage were 3 to 6 times greater during the summer than during the winter measurement periods. Top-down and bottom-up methods could be complementary in identifying gaps and may lead to better characterization of CH4 emissions.
Uncertainty
Regardless of the method used to measure CH4, the measurement error associated with the quantification of aerial pollutants, such as CH4, comprises both systematic and random components. Uncertainty represents the quantification of the random component, and every technique has different sources of uncertainty. Because uncertainty establishes the range of values that the actual measurement value will be within, the uncertainty of emission measurements must be known when using the measurements to develop emission inventories or emission factors. Gates et al. (2009) reported how component error analysis could be used to quantify uncertainties associated with direct measurement of aerial pollutant emissions such as CH4. Hristov et al. (2018) examined the roots of uncertainties in predicting CH4 for inventory purposes. They reported that, at the animal level, animal inventory, feed dry matter intake, the chemical composition of the diets, CH4 emission factors, and predictions of enteric CH4 emissions are the main culprit. Uncertainty has not been evaluated for all published emissions values, making it difficult to compare the results between the different papers, evaluate the quality of the results, and the certification of emission reductions. One future challenge will be to provide a standard methodology for uncertainty assessment associated with emission measurements. Hristov et al. (2018) concluded that quantitative attribution of changes in atmospheric CH4 concentrations to CH4 sources based on δ13CH4 data (stable isotope signature, specifically 13C/12C used in top-down methodology) is at least questionable.
Final Remarks
The quality of CH4 measurements is critical, but special attention must also be paid to the information given in publications in relation to measurement context and methods to reasonably and comprehensively contextualize the results obtained (Webb et al., 2021). Every method or methodology to quantify CH4 emissions from livestock production has limitations brought about by their original intent of use. Della Rosa et al. (2021) assessed variations in technical procedures of respiration chambers, SF6, and Greenfeed Emission Monitoring System for measuring CH4 from ruminants and concluded that standardization within and between techniques could improve the reliability of the results. Therefore, using these technologies outside of their purpose is risky, and extrapolation of their estimates will undoubtedly result in unintended consequences. There is no one ideal method or methodology given the many different production scenarios worldwide, management strategies, and inherent assumptions associated with the method or methodology. Combining different methods might be the best approach, but more research is needed to validate individual methods, compare different methods in different production scenarios, and develop calibration and standardization protocols for existing methods and methodologies.
Acknowledgments
This work was supported by FAO Livestock Environmental Assessment and Performance (LEAP) Partnership (GCP/GLO/369/MUL). The views expressed in this publication are those of the author(s) and do not necessarily reflect the views or policies of the Food and Agriculture Organization of the United Nations. Article processing charges were funded by UC Davis Sesnon Endowed Chair program.
Glossary
Abbreviations
- AHCS
automated head-chamber system
- BCNRM
beef cattle nutrient requirements model
- CIGR
International Commission of Agricultural Engineering
- DMI
dry matter intake
- EPA
Environmental Protection Agency
- FANS
fan assessment numeration system
- FAO
Food and Agriculture Organization of the United Nations
- GC
gas chromatography
- GEI
gross energy intake
- GHG
greenhouse gas
- GRA
Global Research Alliance
- GWP
global warming potential
- IPCC
Intergovernmental Panel on Climate Change
- LiDAR
Light Detection and Ranging
- LMD
laser methane detector
- MIR
milk mid-infrared
- NASEM
National Academies of Sciences, Engineering, and Medicine
- NDVI
normalized difference vegetation index
- QCL
quantum cascade laser
- VFA
volatile fatty acid
- VR
ventilation rate
- VS
volatile solid
- Ym
methane conversion factor
Contributor Information
Luis Orlindo Tedeschi, Department of Animal Science, Texas A&M University, College Station, TX 77843-2471, USA.
Adibe Luiz Abdalla, Center for Nuclear Energy in Agriculture, University of Sao Paulo, Piracicaba CEP 13416.000, Brazil.
Clementina Álvarez, Department of Research, TINE SA, Christian Magnus Falsens vei 12, 1433 Ås, Norway.
Samuel Weniga Anuga, European University Institute (EUI), Via dei Roccettini 9, San Domenico di Fiesole (FI), Italy.
Jacobo Arango, International Center for Tropical Agriculture (CIAT), Km 17 Recta Cali-Palmira, A.A, 6713, Cali, Colombia.
Karen A Beauchemin, Agriculture and Agri-Food Canada, Lethbridge Research and Development Centre, Lethbridge, Alberta, T1J 4B1, Canada.
Philippe Becquet, International Feed Industry Federation, 51657 Wiehl, Germany.
Alexandre Berndt, Embrapa Southeast Livestock, Rod. Washington Luiz, km 234, CP 339, CEP 13.560-970. São Carlos, São Paulo, Brazil.
Robert Burns, Biosystems Engineering and Soil Science Department, The University of Tennessee, Knoxville, TN 37996, USA.
Camillo De Camillis, Animal Production and Health Division, Food and Agriculture Organization of the United Nations, Viale delle Terme di Caracalla, 00153 Rome, Italy.
Julián Chará, Centre for Research on Sustainable Agriculture, CIPAV, Cali 760042, Colombia.
Javier Martin Echazarreta, Centro Carnes—Instituto Nacional de Tecnología Industrial, INTI, Buenos Aires, Argentina.
Mélynda Hassouna, INRAE, Institut Agro Rennes Angers, UMR SAS, F-35042, Rennes, France.
David Kenny, Teagasc Animal and Grassland Research and Innovation Centre, Grange, Dunsany, Co. Meath, C15PW93, Ireland.
Michael Mathot, Agricultural Systems Unit, Walloon Agricultural Research Centre, rue du Serpont 100, B-6800 Libramont, Belgium.
Rogerio M Mauricio, Department of Bioengineering, Federal University of São João del-Rei, São João del-Rei, MG 36307-352, Brazil.
Shelby C McClelland, Animal Production and Health Division, Food and Agriculture Organization of the United Nations, Viale delle Terme di Caracalla, 00153 Rome, Italy; Soil and Crop Sciences, School of Integrative Plant Science, Cornell University, Ithaca, NY 14853, USA.
Mutian Niu, Institute of Agricultural Sciences, ETH Zurich, Universitaetstrasse 2, 8092 Zurich, Switzerland.
Alice Anyango Onyango, Mazingira Centre, International Livestock Research Institute (ILRI), Nairobi, Kenya; Department of Chemistry, Maseno University, Maseno, Kenya.
Ranjan Parajuli, EcoEngineers, Des Moines, IA 50309, USA.
Luiz Gustavo Ribeiro Pereira, Embrapa Dairy Cattle, Juiz de Fora, Minas Gerais, Brazil.
Agustin del Prado, Basque Centre For Climate Change (BC3), Leioa, Spain; IKERBASQUE, Basque Foundation for Science, Bilbao, Spain.
Maria Paz Tieri, Dairy Value Chain Research Institute (IDICAL) (INTA–CONICET), Rafaela, Argentina.
Aimable Uwizeye, Animal Production and Health Division, Food and Agriculture Organization of the United Nations, Viale delle Terme di Caracalla, 00153 Rome, Italy.
Ermias Kebreab, Department of Animal Science, University of California, Davis, CA 95616, USA.
Conflict of Interest Statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Abdalla, A. L., Louvandini H., Sallam S. M. A. H., Bueno I. C. D. S., Tsai S. M., and Figueira A. V. D. O.. . 2012. In vitro evaluation, in vivo quantification, and microbial diversity studies of nutritional strategies for reducing enteric methane production. Trop. Anim. Health Prod. 44 (5):953–964. doi: 10.1007/s11250-011-9992-0. [DOI] [PubMed] [Google Scholar]
- Alemu, A., Ominski K. H., and Kebreab E.. . 2011a. Estimation of enteric methane emissions trends (1990–2008) from Manitoba beef cattle using empirical and mechanistic models. Can. J. Anim. Sci. 91:305–321. doi: 10.4141/cjas2010-009. [DOI] [Google Scholar]
- Alemu, A. W., Dijkstra J., Bannink A., France J., and Kebreab E.. . 2011b. Rumen stoichiometric models and their contribution and challenges in predicting enteric methane production. Anim. Feed Sci. Technol. 16:761–778. doi: 10.1016/j.anifeedsci.2011.04.054. [DOI] [Google Scholar]
- Alemu, A. W., Vyas D., Manafiazar G., Basarab J. A., and Beauchemin K. A.. . 2017. Enteric methane emissions from low– and high–residual feed intake beef heifers measured using GreenFeed and respiration chamber techniques. J. Anim. Sci. 95:3727–3737. doi: 10.2527/jas.2017.1501. [DOI] [PubMed] [Google Scholar]
- Amediek, A., Ehret G., Fix A., Wirth M., Büdenbender C., Quatrevalet M., Kiemle C., and Gerbig C.. . 2017. CHARM-F—a new airborne integrated-path differential-absorption lidar for carbon dioxide and methane observations: measurement performance and quantification of strong point source emissions. Appl. Opt. 56:5182–5197. doi: 10.1364/AO.56.005182. [DOI] [PubMed] [Google Scholar]
- Appuhamy, J. A. D. R. N., France J., and Kebreab E.. . 2016. Models for predicting enteric methane emissions from dairy cows in North America, Europe, and Australia and New Zealand. Global Change Biol. 22:3039–3056. doi: 10.1111/gcb.13339. [DOI] [PubMed] [Google Scholar]
- Arbre, M., Rochette Y., Guyader J., Lascoux C., Gómez L. M., Eugène M., Morgavi D. P., Renand G., Doreau M., and Martin C.. . 2016. Repeatability of enteric methane determinations from cattle using either the SF6 tracer technique or the GreenFeed system. Anim. Prod. Sci. 56:238–243. doi: 10.1071/an15512. [DOI] [Google Scholar]
- Argyle, J. L., and Baldwin R. L.. . 1988. Modeling of rumen water kinetics and effects of rumen pH changes. J. Dairy Sci. 71:1178–1188. doi: 10.3168/jds.S0022-0302(88)79672-1. [DOI] [PubMed] [Google Scholar]
- Arndt, C., Leytem A. B., Hristov A. N., Zavala-Araiza D., Cativiela J. P., Conley S., Daube C., Faloona I., and Herndon S. C.. . 2018. Short-term methane emissions from 2 dairy farms in California estimated by different measurement techniques and US Environmental Protection Agency inventory methodology: a case study. J. Dairy Sci. 101:11461–11479. doi: 10.3168/jds.2017-13881. [DOI] [PubMed] [Google Scholar]
- Baldwin, R. L. 1995. Modeling ruminant digestion and metabolism. New York (NY):Chapman & Hall. [DOI] [PubMed] [Google Scholar]
- Baldwin, R. L., France J., Beever D. E., Gill M., and Thornley J. H. M.. . 1987a. Metabolism of the lactating cow. III. Properties of mechanistic models suitable for evaluation of energetic relationships and factors involved in the partition of nutrients. J. Dairy Res. 54:133–145. doi: 10.1017/S0022029900025243. [DOI] [PubMed] [Google Scholar]
- Baldwin, R. L., France J., and Gill M.. . 1987b. Metabolism of the lactating cow. I. Animal elements of a mechanistic model. J. Dairy Res. 54:77–105. doi: 10.1017/s002202990002522x. [DOI] [PubMed] [Google Scholar]
- Baldwin, R. L., Thornley J. H. M., and Beever D. E.. . 1987c. Metabolism of the lactating cow. II. Digestive elements of a mechanistic model. J. Dairy Res. 54:107–131. doi: 10.1017/S0022029900025231. [DOI] [PubMed] [Google Scholar]
- Bannink, A., Kogut J., Dijkstra J., France J., Kebreab E., Van Vuuren A. M., and Tamminga S.. . 2006. Estimation of the stoichiometry of volatile fatty acid production in the rumen of lactating cows. J. Theor. Biol. 238:36–51. doi: 10.1016/j.jtbi.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Bannink, A., Reijs J. W., and Dijkstra J.. . 2008. Integrated approaches to evaluate nutritional strategies for dairy cows. In: France J. and Kebreab E., editors. Mathematical modelling in animal nutrition. Wallingford, UK:CABI Publishing. p. 462–484. [Google Scholar]
- Bannink, A., van Schijndel M. W., and Dijkstra J.. . 2011. A model of enteric fermentation in dairy cows to estimate methane emission for the Dutch National Inventory Report using the IPCC Tier 3 approach. Anim. Feed Sci. Technol. 16:603–618. doi: 10.1016/j.anifeedsci.2011.04.043. [DOI] [Google Scholar]
- Barchyn, T. E., Hugenholtz C. H., and Fox T. A.. . 2019. Plume detection modeling of a drone-based natural gas leak detection system. Elem. Sci. Anth. 7. doi: 10.1525/elementa.379. [DOI] [Google Scholar]
- Barreto-Mendes, L., Ferreira-Tinoco I. D. F., Ogink N., Osorio-Hernandez R., and Osorio-Saraz J. A.. . 2014. A refined protocol for calculating air flow rate of naturally-ventilated broiler barns based on CO2 mass balance. DYNA. 81:189–195. doi: 10.15446/dyna.v81n185.38069 [DOI] [Google Scholar]
- Batstone, D. J., Keller J., Angelidaki I., Kalyuzhnyi S. V., Pavlostathis S. G., Rozzi A., Sanders W. T. M., Siegrist H., and Vavilin V. A.. . 2002. The IWA anaerobic digestion model No 1 (ADM1). Water Sci. Technol. 45:65–73. doi: 10.2166/wst.2002.0292. [DOI] [PubMed] [Google Scholar]
- Beauchemin, K. A., Ungerfeld E., Abdalla A., Álvarez C., Arndt C., Becquet P., Benchaar C., Berndt A., Mauricio R., McAllister T., . et al. 2022. INVITED REVIEW: Current enteric methane mitigation. J. Dairy Sci. [DOI] [PubMed] [Google Scholar]
- Bekele, W., Guinguina A., Zegeye A., Simachew A., and Ramin M.. . 2022. Contemporary methods of measuring and estimating methane emission from ruminants. Methane. 1(2):82–95. doi: 10.3390/methane1020008. [DOI] [Google Scholar]
- Bell, M. J., Potterton S. L., Craigon J., Saunders N., Wilcox R. H., Hunter M., Goodman J. R., and Garnsworthy P. C.. . 2014. Variation in enteric methane emissions among cows on commercial dairy farms. Animal 8:1540–1546. doi: 10.1017/S1751731114001530. [DOI] [PubMed] [Google Scholar]
- Beltran, I., van der Weerden T. J., Alfaro M. A., Amon B., de Klein C. A. M., Grace P., Hafner S., Hassouna M., Hutchings N., Krol D. J., . et al. 2021. DATAMAN: A global database of nitrous oxide and ammonia emission factors for excreta deposited by livestock and land-applied manure. J. Environ. Qual. 50:513–527. doi: 10.1002/jeq2.20186. [DOI] [PubMed] [Google Scholar]
- Benaouda, M., Martin C., Li X., Kebreab E., Hristov A. N., Yu Z., Yáñez-Ruiz D. R., Reynolds C. K., Crompton L. A., Dijkstra J., . et al. 2019. Evaluation of the performance of existing mathematical models predicting enteric methane emissions from ruminants: Animal categories and dietary mitigation strategies. Anim. Feed Sci. Technol. 255:114–207. doi: 10.1016/j.anifeedsci.2019.114207. [DOI] [Google Scholar]
- Benchaar, C., Rivest J., Pomar C., and Chiquette J.. . 1998. Prediction of methane production from dairy cows using existing mechanistic models and regression equations. J. Anim. Sci. 76:617–627. doi: 10.2527/1998.762617x. [DOI] [PubMed] [Google Scholar]
- Blümmel, M., Makkar H. P. S., and Becker K.. . 1997. In vitro gas production: a technique revisited. J. Anim. Physiol. Anim. Nutr. (Zeitschrift für Tierphysiologie Tierernährung und Futtermittelkunde). 77 (1):24–34. doi: 10.1111/j.1439-0396.1997.tb00734.x. [DOI] [Google Scholar]
- Bodansky, D. M., Hoedl S. A., Metcalf G. E., and Stavins R. N.. . 2016. Facilitating linkage of climate policies through the Paris outcome. Climate Policy. 16 (8):956–972. doi: 10.1080/14693062.2015.1069175 [DOI] [Google Scholar]
- Brannon, E. Q., Moseman-Valtierra S. M., Rella C. W., Martin R. M., Chen X., and Tang J.. . 2016. Evaluation of laser-based spectrometers for greenhouse gas flux measurements in coastal marshes. Limnol. Oceanogr. Methods. 14:466–476. doi: 10.1002/lom3.10105. [DOI] [Google Scholar]
- Brazilian Ministry of Science, Technology and Innovations, Secretariat for Research and Scientific Training. 2021. Fourth National Communication of Brazil to the United Nations Framework Convention on Climate Change/Secretariat for Research and Scientific Training. Ministry of Science, Technology and Innovations, Brasilia, Brazil. 620 p. Available at: https://www.gov.br/mcti/pt-br/acompanhe-o-mcti/sirene/publicacoes/comunicacoes-nacionais-do-brasil-a-unfccc/arquivos/4comunicacao/executive_summary-_4nc_brazil_web.pdf. [Google Scholar]
- Calvet, S., Estellés F., Cambra-López M., Torres A. G., and Van den Weghe H. F. A.. . 2011. The influence of broiler activity, growth rate, and litter on carbon dioxide balances for the determination of ventilation flow rates in broiler production. Poult. Sci. 90:2449–2458. doi: 10.3382/ps.2011-01580. [DOI] [PubMed] [Google Scholar]
- Canul Solis, J. R., Piñeiro Vázquez A. T., Arceo Castillo J. I., Alayón Gamboa J. A., Ayala Burgos A. J., Aguilar Pérez C. F., Solorio Sánchez F. J., Castelán Ortega O. A., Lachica López M., Quintana Owen P., . et al. 2017. Design and construction of low-cost respiration chambers for ruminal methane measurements in ruminants. Rev. Mex. Cienc. Pecu. 8:185–192. doi: 10.22319/rmcp.v8i2.4442 [DOI] [Google Scholar]
- Chagunda, M. G. G. 2013. Opportunities and challenges in the use of the laser methane detector to monitor enteric methane emissions from ruminants. Animal 7:394–400. doi: 10.1017/S1751731113000724. [DOI] [PubMed] [Google Scholar]
- Chagunda, M. G. G., and Yan T.. . 2011. Do methane measurements from a laser detector and an indirect open-circuit respiration calorimetric chamber agree sufficiently closely? Anim. Feed Sci. Technol. 165:8–14. doi: 10.1016/j.anifeedsci.2011.02.005. [DOI] [Google Scholar]
- Christiansen, J. R., Romero A. J. B., Jørgensen N. O. G., Glaring M. A., Jørgensen C. J., Berg L. K., and Elberling B.. . 2015. Methane fluxes and the functional groups of methanotrophs and methanogens in a young Arctic landscape on Disko Island, West Greenland. Biogeochemistry 122:15–33. doi: 10.1007/s10533-014-0026-7. [DOI] [Google Scholar]
- Cole, N. A., Parker D. B., Todd R. W., Leytem A. B., Dungan R. S., Hales K. E., Ivey S. L., and Jennings J.. . 2018. Use of new technologies to evaluate the environmental footprint of feedlot systems. Transl. Anim. Sci. 2 (1):89–100. doi: 10.1093/tas/txx001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier, S. M., Ruark M. D., Oates L. G., Jokela W. E., and Dell C. J.. . 2014. Measurement of greenhouse gas flux from agricultural soils using static chambers. J. Vis. Exp. e52110–e52110. doi: 10.3791/52110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commonwealth Scientific and Industrial Research Organization. 2007. Nutrient Requirements of Domesticated Ruminants. Commonwealth Scientific and Industrial Research Organization, Collingwood, VIC. [Google Scholar]
- Conley, S., Faloona I., Mehrotra S., Suard M., Lenschow D. H., Sweeney C., Herndon S., Schwietzke S., Pétron G., Pifer J., . et al. 2017. Application of Gauss’s theorem to quantify localized surface emissions from airborne measurements of wind and trace gases. Atmos. Meas. Tech. 10:3345–3358. doi: 10.5194/amt-10-3345-2017. [DOI] [Google Scholar]
- Cooprider, K. L., Mitloehner F. M., Famula T. R., Kebreab E., Zhao Y., and Van Eenennaam A. L.. . 2011. Feedlot efficiency implications on greenhouse gas emissions and sustainability. J. Anim. Sci. 89:2643–2656. doi: 10.2527/jas.2010-3539. [DOI] [PubMed] [Google Scholar]
- Coppa, M., Jurquet J., Eugène M., Dechaux T., Rochette Y., Lamy J. -M., Ferlay A., and Martin C.. . 2021. Repeatability and ranking of long-term enteric methane emissions measurement on dairy cows across diets and time using GreenFeed system in farm-conditions. Methods 186:59–67. doi: 10.1016/j.ymeth.2020.11.004. [DOI] [PubMed] [Google Scholar]
- Cowan, N. J., Famulari D., Levy P. E., Anderson M., Bell M. J., Rees R. M., Reay D. S., and Skiba U. M.. . 2014. An improved method for measuring soil N2O fluxes using a quantum cascade laser with a dynamic chamber. Eur. J. Soil Sci. 65:643–652. doi: 10.1111/ejss.12168. [DOI] [Google Scholar]
- Czerkawski, J. W., and Breckenridge G.. . 1977. Design and development of a long-term rumen simulation technique (RUSITEC). Br. J. Nutr. 38:371–384. doi: 10.1079/bjn19770102. [DOI] [PubMed] [Google Scholar]
- Dalby, F. R., Hafner S. D., Petersen S. O., VanderZaag A. C., Habtewold J., Dunfield K., Chantigny M. H., and Sommer S. G.. . 2021. Understanding methane emission from stored animal manure: a review to guide model development. J. Environ. Qual. 50:817–835. doi: 10.1002/jeq2.20252. [DOI] [PubMed] [Google Scholar]
- Danielsson, R., Ramin M., Bertilsson J., Lund P., and Huhtanen P.. . 2017. Evaluation of a gas in vitro system for predicting methane production in vivo. J. Dairy Sci. 100:8881–8894. doi: 10.3168/jds.2017-12675. [DOI] [PubMed] [Google Scholar]
- Daube, C., Conley S., Faloona I. C., Arndt C., Yacovitch T. I., Roscioli J. R., and Herndon S. C.. . 2019. Using the tracer flux ratio method with flight measurements to estimate dairy farm CH4 emissions in central California. Atmos. Meas. Tech. 12:2085–2095. doi: 10.5194/amt-12-2085-2019. [DOI] [Google Scholar]
- Davidson, E. A., Savage K., Verchot L. V., and Navarro R.. . 2002. Minimizing artifacts and biases in chamber-based measurements of soil respiration. Agric. For. Meteorol. 113:21–37. doi: 10.1016/S0168-1923(02)00100-4. [DOI] [Google Scholar]
- Deighton, M. H., Williams S. R. O., Hannah M. C., Eckard R. J., Boland T. M., Wales W. J., and Moate P. J.. . 2014. A modified sulphur hexafluoride tracer technique enables accurate determination of enteric methane emissions from ruminants. Anim. Feed Sci. Technol. 197:47–63. doi: 10.1016/j.anifeedsci.2014.08.003. [DOI] [Google Scholar]
- Della Rosa, M. M., Jonker A., and Waghorn G. C.. . 2021. A review of technical variations and protocols used to measure methane emissions from ruminants using respiration chambers, SF6 tracer technique and GreenFeed, to facilitate global integration of published data. Anim. Feed Sci. Technol. 279:115018. doi: 10.1016/j.anifeedsci.2021.115018. [DOI] [Google Scholar]
- Deng, J., Guo L., Salas W., Ingraham P., Charrier-Klobas J. G., Frolking S., and Li C.. . 2018a. Changes in irrigation practices likely mitigate nitrous oxide emissions from California cropland. Global Biogeochem. Cycles 32:1514–1527. doi: 10.1029/2018GB005961. [DOI] [Google Scholar]
- Deng, J., Li C., Burger M., Horwath W. R., Smart D., Six J., Guo L., Salas W., and Frolking S.. . 2018b. Assessing short-term impacts of management practices on N2O emissions from diverse Mediterranean agricultural ecosystems using a biogeochemical model. J. Geophys. Res. Biogeosci. 123:1557–1571. doi: 10.1029/2017JG004260. [DOI] [Google Scholar]
- Dijkstra, J., Neal H. D. S. C., Beever D. E., and France J.. . 1992. Simulation of nutrient digestion, absorption and outflow in the rumen: model description. J. Nutr. 122:2239–2256. doi: 10.1093/jn/122.11.2239. [DOI] [PubMed] [Google Scholar]
- Dillon, J. A., Stackhouse-Lawson K. R., Thoma G. J., Gunter S. A., Rotz C. A., Kebreab E., Riley D. G., Tedeschi L. O., Villalba J., Mitloehner F., . et al. 2021. Current state of enteric methane and the carbon footprint of beef and dairy cattle in the United States. Anim. Front. 11:57–68. doi: 10.1093/af/vfab043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edouard, N., Charpiot A., Robin P., Lorinquer E., Dollé J. B., and Faverdin P.. . 2019. Influence of diet and manure management on ammonia and greenhouse gas emissions from dairy barns. Animal 13:2903–2912. doi: 10.1017/S1751731119001368. [DOI] [PubMed] [Google Scholar]
- Edouard, N., Mosquera J., van Dooren H. J. C., Mendes L. B., and Ogink N. W. M.. . 2016. Comparison of CO2- and SF6-based tracer gas methods for the estimation of ventilation rates in a naturally ventilated dairy barn. Biosystems Eng. 149:11–23. doi: 10.1016/j.biosystemseng.2016.06.001. [DOI] [Google Scholar]
- Ekeberg, D., Ogner G., Fongen M., Joner E. J., and Wickstrøm T.. . 2004. Determination of CH4, CO2 and N2O in air samples and soil atmosphere by gas chromatography mass spectrometry, GC-MS. J. Environ. Monit. 6:621–623. doi: 10.1039/B401315H. [DOI] [PubMed] [Google Scholar]
- Ellis, J. L., Dijkstra J., Kebreab E., Archibeque S., France J., and Bannink A.. . 2010. Prediction of methane production in beef cattle within a mechanistic digestion model. In: Sauvant D., Van Milgen J., Faverdin P. and Friggens N., editors. Modelling nutrient digestion and utilisation in farm animals. Wageningen, The Netherlands:Wageningen Academic Publishers; p. 181–188. [Google Scholar]
- Escobar-Bahamondes, P., Oba M., Kröbel R., McAllister T. A., MacDonald D., and Beauchemin K. A.. . 2017. Estimating enteric methane production for beef cattle using empirical prediction models compared with IPCC Tier 2 methodology. Can. J. Anim. Sci. 97:599–612. doi: 10.1139/cjas-2016-0163. [DOI] [Google Scholar]
- Eugène, M., Sauvant D., Nozière P., Viallard D., Oueslati K., Lherm M., Mathias E., and Doreau M.. . 2019. A new Tier 3 method to calculate methane emission inventory for ruminants. J. Environ. Manage. 231:982–988. doi: 10.1016/j.jenvman.2018.10.086. [DOI] [PubMed] [Google Scholar]
- Fox, D. G., Tedeschi L. O., Tylutki T. P., Russell J. B., Van Amburgh M. E., Chase L. E., Pell A. N., and Overton T. R.. . 2004. The Cornell Net Carbohydrate and Protein System model for evaluating herd nutrition and nutrient excretion. Anim. Feed Sci. Technol. 112:29–78. doi: 10.1016/j.anifeedsci.2003.10.006. [DOI] [Google Scholar]
- France, J., and Kebreab E.. . 2008. Mathematical Modelling in Animal Nutrition. Wallingford, UK:CABI Publishing [Google Scholar]
- Frankenberg, C., Meirink J. F., Bergamaschi P., Goede A. P. H., Heimann M., Körner S., Platt U., van Weele M., and Wagner T.. . 2006. Satellite chartography of atmospheric methane from SCIAMACHY on board ENVISAT: analysis of the years 2003 and 2004. J. Geophys. Res. Atmos. 111 (D7). doi: 10.1029/2005JD006235. [DOI] [Google Scholar]
- Garg, A., Kankal B., and Shukla P. R.. . 2011. Methane emissions in India: Sub-regional and sectoral trends. Atmos. Environ. 45:4922–4929. doi: 10.1016/j.atmosenv.2011.06.004. [DOI] [Google Scholar]
- Garnsworthy, P. C., Craigon J., Hernandez-Medrano J. H., and Saunders N.. . 2012. On-farm methane measurements during milking correlate with total methane production by individual dairy cows. J. Dairy Sci. 95:3166–3180. doi: 10.3168/jds.2011-4605. [DOI] [PubMed] [Google Scholar]
- Gates, R. S., Casey K. D., Xin H., and Burns R. T.. . 2009. Building emissions uncertainty estimates. Trans. ASABE 52:1345–1351. doi: 10.13031/2013.27784. [DOI] [Google Scholar]
- Gates, R. S., Casey K. D., Xin H., Wheeler E. F., and Simmons J. D.. . 2004. Fan assessment numeration system (FANS) design and calibration specifications. Trans. ASAE 47:1709–1715. doi: 10.13031/2013.17613. [DOI] [Google Scholar]
- Gates, R. S., Xin H., Casey K. D., Liang Y., and Wheeler E. F.. . 2005. Method for measuring ammonia emissions from poultry houses. J. Appl. Poult. Res. 14:622–634. doi: 10.1093/japr/14.3.622. [DOI] [Google Scholar]
- Gaviria-Uribe, X., Bolivar D. M., Rosenstock T. S., Molina-Botero I. C., Chirinda N., Barahona R., and Arango J.. . 2020. Nutritional quality, voluntary intake and enteric methane emissions of diets based on novel cayman grass and its associations with two leucaena shrub legumes. Front. Vet. Sci. 7(764):1–12. doi: 10.3389/fvets.2020.579189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber, P. J., Steinfeld H., Henderson B., Mottet A., Opio C., Dijkman J., Falcucci A., and Tempio G.. . 2013. Tackling climate change through livestock—a global assessment of emissions and mitigation opportunities. Food and Agriculture Organization of the United Nations (FAO), Rome, Italy. 115 p. Available at: http://www.fao.org/docrep/018/i3437e/i3437e.pdf. [Google Scholar]
- Gerrits, W. J. J., and Labussière E.. . 2015. Indirect calorimetry: techniques, computations and applications. Wageningen, The Netherlands:Wageningen Academic Publishers. [Google Scholar]
- Getachew, G., Blümmel M., Makkar H. P. S., and Becker K.. . 1998. In vitro gas measuring techniques for assessment of nutritional quality of feeds: a review. Anim. Feed Sci. Technol. 72:261–281. doi: 10.1016/S0377-8401(97)00189-2. [DOI] [Google Scholar]
- Glumb, R., Davis G., and Lietzke C.. . 2014. The TANSO-FTS-2 instrument for the GOSAT-2 greenhouse gas monitoring mission. In: 2014 IEEE Geoscience and Remote Sensing Symposium. Quebec City, QC, Canada: IEEE; p. 1238–1240. doi: 10.1109/IGARSS.2014.6946656. [DOI] [Google Scholar]
- Goopy, J. P., Chang C., and Tomkins N.. . 2016. A comparison of methodologies for measuring methane emissions from ruminants. In: Rosenstock T. S., Rufino M. C., Butterbach-Bahl K., Wollenberg L. and Richards M., editors. Methods for measuring greenhouse gas balances and evaluating mitigation options in smallholder agriculture. Cham:Springer International Publishing; p. 97–117. doi: 10.1007/978-3-319-29794-1_5 [DOI] [Google Scholar]
- Goopy, J. P., Onyango A. A., Dickhoefer U., and Butterbach-Bahl K.. . 2018. A new approach for improving emission factors for enteric methane emissions of cattle in smallholder systems of East Africa—results for Nyando, Western Kenya. Agric. Syst. 161:72–80. doi: 10.1016/j.agsy.2017.12.004. [DOI] [Google Scholar]
- Goopy, J. P., Robinson D. L., Woodgate R. T., Donaldson A. J., Oddy V. H., Vercoe P. E., and Hegarty R. S.. . 2015. Estimates of repeatability and heritability of methane production in sheep using portable accumulation chambers. Anim. Prod. Sci. 56:116–122. doi: 10.1071/AN13370. [DOI] [Google Scholar]
- Goopy, J. P., Woodgate R., Donaldson A., Robinson D. L., and Hegarty R. S.. . 2011. Validation of a short-term methane measurement using portable static chambers to estimate daily methane production in sheep. Anim. Feed Sci. Technol. 16:219–226. doi: 10.1016/j.anifeedsci.2011.04.012. [DOI] [Google Scholar]
- Gunter, S. A., and Bradford J. A.. . 2017. Technical note: effect of bait delivery interval in an automated head-chamber system on respiration gas estimates when cattle are grazing rangeland. Prof. Anim. Sci. 33:490–497. doi: 10.15232/pas.2016-01593. [DOI] [Google Scholar]
- Hacker, J. M., Chen D., Bai M., Ewenz C., Junkermann W., Lieff W., McManus B., Neininger B., Sun J., Coates T., . et al. 2016. Using airborne technology to quantify and apportion emissions of CH4 and NH3 from feedlots. Anim. Prod. Sci. 56:190–203. doi: 10.1071/AN15513. [DOI] [Google Scholar]
- Hall, M. K. D., Winters A. J., and Rogers G. S.. . 2014. Variations in the diurnal flux of greenhouse gases from soil and optimizing the sampling protocol for closed static chambers. Commun. Soil Sci. Plant Anal. 45:2970–2978. doi: 10.1080/00103624.2014.956937. [DOI] [Google Scholar]
- Hammond, K. J., Crompton L. A., Bannink A., Dijkstra J., Yáñez-Ruiz D. R., O’Kiely P., Kebreab E., Eugène M. A., Yu Z., Shingfield K. J., . et al. 2016. Review of current in vivo measurement techniques for quantifying enteric methane emission from ruminants. Anim. Feed Sci. Technol. 219:13–30. doi: 10.1016/j.anifeedsci.2016.05.018. [DOI] [Google Scholar]
- Hammond, K. J., Humphries D. J., Crompton L. A., Green C., and Reynolds C. K.. . 2015. Methane emissions from cattle: estimates from short-term measurements using a GreenFeed system compared with measurements obtained using respiration chambers or sulphur hexafluoride tracer. Anim. Feed Sci. Technol. 203:41–52. doi: 10.1016/j.anifeedsci.2015.02.008. [DOI] [Google Scholar]
- Harvey, M. J., Sperlich P., Clough T. J., Kelliher F. M., McGeough K. L., Martin R. J., and Moss R.. . 2020. Global Research Alliance N2O chamber methodology guidelines: recommendations for air sample collection, storage, and analysis. J. Environ. Qual. 49:1110–1125. doi: 10.1002/jeq2.20129. [DOI] [PubMed] [Google Scholar]
- Hassouna, M., Calvet S., Hayes E., Gates R. S., and Schrade S.. . 2021. Measurement of gaseous emissions from animal housing. In: Holden N. M., Wolfe M. L., Ogejo, J. A. and Cummins E. J., editors. Introduction to biosystems engineering. Blacksburg (VA): ASABE and Virginia Tech Publishing; p. 1–21. doi: 10.21061/IntroBiosystemsEngineering. [DOI] [Google Scholar]
- Hassouna, M., and Eglin T.. . 2016. Measuring emissions from livestock farming: greenhouse gases, ammonia and nitrogen oxides. Ademe and INRA, Paris, France. p. 314—[accessed March 20, 2022] Available at: https://www6.inrae.fr/animal_emissions_eng/News/Measuring-gaseous-emissions-from-animal-farms [Google Scholar]
- Hassouna, M., Robin P., Charpiot A., Edouard N., and Méda B.. . 2013. Infrared photoacoustic spectroscopy in animal houses: effect of non-compensated interferences on ammonia, nitrous oxide and methane air concentrations. Biosystems Eng. 114 (3):318–326. doi: 10.1016/j.biosystemseng.2012.12.011. [DOI] [Google Scholar]
- Hedley, C. B., Saggar S., and Tate K. R.. . 2006. Procedure for fast simultaneous analysis of the greenhouse gases: methane, carbon dioxide, and nitrous oxide in air samples. Commun. Soil Sci. Plant Anal. 37:1501–1510. doi: 10.1080/00103620600709928. [DOI] [Google Scholar]
- Heetkamp, M. J. W., Alferink S. J. J., Zandstra T., Hendriks P., van den Brand H., and Gerrits W. J. J.. . 2015. Design of climate respiration chambers, adjustable to the metabolic mass of subjects. In: Gerrits W. J. J. and Labussière E., editors. Indirect calorimetry: techniques, computations and applications. Wageningen, The Netherlands:Wageningen Academic Publishers; p. 35–56. [Google Scholar]
- Hegarty, R. S. 2013. Applicability of short-term emission measurements for on-farm quantification of enteric methane. Animal. 7 (Supplements2):401–408. doi: 10.1017/S1751731113000839. [DOI] [PubMed] [Google Scholar]
- Hellwing, A. L. F., Lund P., Weisbjerg M. R., Brask M., and Hvelplund T.. . 2012. Technical note: test of a low-cost and animal-friendly system for measuring methane emissions from dairy cows. J. Dairy Sci. 95:6077–6085. doi: 10.3168/jds.2012-5505. [DOI] [PubMed] [Google Scholar]
- Henry, B., Charmley E., Eckard R. J., Gaughan J. B., and Hegarty R. S.. . 2012. Livestock production in a changing climate: adaptation and mitigation research in Australia. Crop Pasture Sci. 63:191–202. doi: 10.1071/CP11169. [DOI] [Google Scholar]
- Hill, J., McSweeney C., Wright A. G., Bishop-Hurley G., and Kalantar-zadeh K.. . 2016. Measuring methane production from ruminants. Trends Biotechnol. 34:26–35. doi: 10.1016/j.tibtech.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Hoover, W. H., and Stokes S. R.. . 1991. Balancing carbohydrates and proteins for optimum rumen microbial yield. J. Dairy Sci. 74:3630–3644. doi: 10.3168/jds.S0022-0302(91)78553-6. [DOI] [PubMed] [Google Scholar]
- Houweling, S., Krol M., Bergamaschi P., Frankenberg C., Dlugokencky E. J., Morino I., Notholt J., Sherlock V., Wunch D., Beck V., . et al. 2014. A multi-year methane inversion using SCIAMACHY, accounting for systematic errors using TCCON measurements. Atmos. Chem. Phys. 14:3991–4012. doi: 10.5194/acp-14-3991-2014. [DOI] [Google Scholar]
- Hristov, A. N., Kebreab E., Niu M., Oh J., Bannink A., Bayat A. R., Boland T. M., Brito A. F., Casper D. P., Crompton L. A., . et al. 2018. Symposium review: uncertainties in enteric methane inventories, measurement techniques, and prediction models. J. Dairy Sci. 101:6655–6674. doi: 10.3168/jds.2017-13536. [DOI] [PubMed] [Google Scholar]
- Hristov, A. N., Oh J., Giallongo F., Frederick T., Harper M. T., Weeks H., Branco A. F., Price W. J., Moate P. J., Deighton M. H., . et al. 2016. Short communication: comparison of the GreenFeed system with the sulfur hexafluoride tracer technique for measuring enteric methane emissions from dairy cows. J. Dairy Sci. 99:5461–5465. doi: 10.3168/jds.2016-10897. [DOI] [PubMed] [Google Scholar]
- Hristov, A. N., Oh J., Giallongo F., Frederick T., Weeks H., Zimmerman P. R., Harper M. T., Hristova R. A., Zimmerman R. S., and Branco A. F.. . 2015. The use of an automated system (Greenfeed) to monitor enteric methane and carbon dioxide emissions from ruminant animals. J. Vis. Exp. 52904. doi: 10.3791/52904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov, A. N., Oh J., Lee C., Meinen R., Montes F., Ott T., Firkins J. L., Rotz A., Dell C., Adesogan A. T., Yang W. Z., Tricarico J. M., Kebreab E., Waghorn G. C., Dijkstra J., and Oosting S.. . 2013. Mitigation of Greenhouse Gas Emissions in Livestock Production; A review of technical options for non-CO2 emissions. FAO Animal Production and Health Paper. No. 177. Food and Agriculture Organization, Rome, Italy. p. 206.—[accessed December 31, 2014]. Available at: http://www.fao.org/docrep/018/i3288e/i3288e.pdf [Google Scholar]
- Huang, Q., Wohlgemut O., Cicek N., France J., and Kebreab E.. . 2010. A mechanistic model for simulating methane emissions from unstirred liquid manure storages. Can. J. Soil Sci. 90:507–516. doi: 10.4141/cjss09094. [DOI] [Google Scholar]
- Huhtanen, P., Bayat A. R., Lund P., Hellwing A. L. F., and Weisbjerg M. R.. . 2020. Short communication: variation in feed efficiency hampers use of carbon dioxide as a tracer gas in measuring methane emissions in on-farm conditions. J. Dairy Sci. 103:9090–9095. doi: 10.3168/jds.2020-18559. [DOI] [PubMed] [Google Scholar]
- Huhtanen, P., Cabezas-Garcia E. H., Utsumi S., and Zimmerman S.. . 2015. Comparison of methods to determine methane emissions from dairy cows in farm conditions. J. Dairy Sci. 98:3394–3409. doi: 10.3168/jds.2014-9118. [DOI] [PubMed] [Google Scholar]
- Huhtanen, P., Ramin M., and Hristov A. N.. . 2019. Enteric methane emission can be reliably measured by the GreenFeed monitoring unit. Livest. Sci. 222:31–40. doi: 10.1016/j.livsci.2019.01.017. [DOI] [Google Scholar]
- Hüppi, R., Felber R., Krauss M., Six J., Leifeld J., and Fuß R.. . 2018. Restricting the nonlinearity parameter in soil greenhouse gas flux calculation for more reliable flux estimates. PLoS One 13:e0200876. doi: 10.1371/journal.pone.0200876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husted, S. 1993. An open chamber technique for determination of methane emission from stored livestock manure. Atmos. Environ. Part A 27:1635–1642. doi: 10.1016/0960-1686(93)90226-o. [DOI] [Google Scholar]
- Institut National de la Recherche Agronomique. 2018. INRA Feeding System for Ruminants. Wageningen, The Netherlands: Wageningen Academic Publishers. doi: 10.3920/978-90-8686-292-4 [DOI] [Google Scholar]
- Intergovernmental Panel on Climate Change. 1996. Revised 1996 IPCC Guidelines for National Greenhouse Gas Inventories: Workbook. vol. 2. Cambridge University Press, Cambridge, UK, and New York, NY. p. 335—[accessed November 16, 2014]. Available at: http://www.ipcc-nggip.iges.or.jp/public/gl/invs1.html [Google Scholar]
- Intergovernmental Panel on Climate Change. 2006. 2006 IPCC guidelines for National Greenhouse Gas Inventories: general guidance and reporting. In: Eggleston H. S., Buendia L., Miwa K., Ngara T. and Tanabe K., editors. vol. 1. Institute for Global Environmental Strategies (IGES), Hayama, Hanagawa, Japan. p. 309—[accessed November 16, 2014]. Available at: http://www.ipcc-nggip.iges.or.jp/public/2006gl/vol1.html [Google Scholar]
- Intergovernmental Panel on Climate Change. 2019a. 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories. In: Calvo Buendia E., Tanabe K., Kranjc A., Baasansuren J., Fukuda M., Osako N. S. A., Pyrozhenko Y., Shermanau P. and Federici S., editors. vol. 4. Agriculture, forestry and other land use. Geneva, Switzerland:IPCC. p. 824—[accessed April 25, 2021]. Available at: https://www.ipcc-nggip.iges.or.jp/public/2019rf/vol4.html [Google Scholar]
- Intergovernmental Panel on Climate Change. 2019b. Summary for policymakers. Climate Change and Land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. In: Shukla P. R., Skea J., Buendia E. C., Masson-Delmotte V., Pörtner H.-O., Roberts D. C., Zhai P., Slade R., Connors S., Diemen R. V., Ferrat M., Haughey E., Luz S., Neogi S., Pathak M., Petzold J., Pereira J. P., Vyas P., Huntley E., Kissick K., Belkacemi M. and Malley J., editors. Intergovernmental Panel on Climate Change, Geneva, Switzerland. p. 41—[accessed March 28, 2022]. Available at: https://www.ipcc.ch/srccl/chapter/summary-for-policymakers/ [Google Scholar]
- International Commission of Agricultural Engineering (CIGR). 2002. 4th report of working group on climatization of animal houses heat and moisture production at animal and house levels. In: Pedersen S. and Sällvik K., editors. International Commission of Agricultural Engineering, Section II. Research Centre Bygholm, Danish Institute of Agricultural Sciences, Horsens, Denmark. p. 45—[accessed.March 1, 2022 ]. Available at: https://www.cigr.org/sites/default/files/documets/CIGR_4TH_WORK_GR.pdf [Google Scholar]
- Johnson, K. A., Huyler M., Westberg H. H., Lamb B. K., and Zimmerman P.. . 1994. Measurement of methane emissions from ruminant livestock using a SF6 tracer technique. Environ. Sci. Techol. 28:359–362. doi: 10.1021/es00051a025 [DOI] [PubMed] [Google Scholar]
- Johnson, K. A., and Johnson D. E.. . 1995. Methane emissions from cattle. J. Anim. Sci. 73:2483–2492. doi: 10.2527/1995.7382483x. [DOI] [PubMed] [Google Scholar]
- Jonker, A., Molano G., Antwi C., and Waghorn G. C.. . 2016. Enteric methane and carbon dioxide emissions measured using respiration chambers, the sulfur hexafluoride tracer technique, and a GreenFeed head-chamber system from beef heifers fed alfalfa silage at three allowances and four feeding frequencies. J. Anim. Sci. 94:4326–4337. doi: 10.2527/jas.2016-0646. [DOI] [PubMed] [Google Scholar]
- Jonker, A., and Waghorn G. C.. . 2020. Guidelines for use of sulphur hexafluoride (SF6) tracer technique to measure enteric methane emissions from ruminants. MPI Technical Paper. No. 2020/06. New Zealand Agricultural Greenhouse Gas Research Centre, New Zealand. p. 86. Available at: http://www.mpi.govt.nz/news-and-resources/publications/ [Google Scholar]
- Joo, H. S., Ndegwa P. M., Heber A. J., Bogan B. W., Ni J. Q., Cortus E. L., and Ramirez-Dorronsoro J. C.. . 2014. A direct method of measuring gaseous emissions from naturally ventilated dairy barns. Atmos. Environ. 86:176–186. doi: 10.1016/j.atmosenv.2013.12.030. [DOI] [Google Scholar]
- Kebreab, E., France J., McBride B. W., Odongo N. E., Bannink A., Mills J. A. N., and Dijkstra J.. . 2006. Evaluation of models to predict methane emissions from enteric fermentation in North American dairy cattle. In: Kebreab E., Dijkstra J., Bannink A., Gerrits W. J. J. and France J., editors. Nutrient digestion and utilization in farm animals; modelling approaches. Wallingford, UK:CABI Publishing; p. 299–313. [Google Scholar]
- Kebreab, E., Johnson K. A., Archibeque S. L., Pape D., and Wirth T.. . 2008. Model for estimating enteric methane emissions from United States dairy and feedlot cattle. J. Anim. Sci. 86:2738–2748. doi: 10.2527/jas.2008-0960. [DOI] [PubMed] [Google Scholar]
- Khalil, T. M., Stöckle C. O., Carlson B. R., Uslar-Valle N., Nelson R. L., Frear C. S., Ma J., Higgins S. S., Leytem A. B., and Dungan R. S.. . 2019. Dairy-CropSyst: gaseous emissions and nutrient fate modeling tool. Comput. Electron. Agric. 162:962–978. doi: 10.1016/j.compag.2019.05.039. [DOI] [Google Scholar]
- Kreuzer, M., and Hindrichsen I. K.. . 2006. Methane mitigation in ruminants by dietary means: the role of their methane emission from manure. Int. Congr. Ser. 1293:199–208. doi: 10.1016/j.ics.2006.01.015. [DOI] [Google Scholar]
- Kuze, A., Suto H., Shiomi K., Kawakami S., Tanaka M., Ueda Y., Deguchi A., Yoshida J., Yamamoto Y., Kataoka F., . et al. 2016. Update on GOSAT TANSO-FTS performance, operations, and data products after more than 6 years in space. Atmos. Meas. Tech. 9:2445–2461. doi: 10.5194/amt-9-2445-2016. [DOI] [Google Scholar]
- Lamb, D. W., Schneider D. A., Trotter M. G., Schaefer M. T., and Yule I. J.. . 2011. Extended-altitude, aerial mapping of crop NDVI using an active optical sensor: a case study using a Raptor™ sensor over wheat. Comput. Electron. Agric. 77:69–73. doi: 10.1016/j.compag.2011.03.009. [DOI] [Google Scholar]
- Laradji, I., Rodriguez P., Kalaitzis F., Vazquez D., Young R., Davey E., and Lacoste A.. . 2020. Counting cows: Tracking illegal cattle ranching from high-resolution satellite imagery. arXiv. doi: 10.48550/arXiv.2011.07369. [DOI] [Google Scholar]
- Lassen, J., Løvendahl P., and Madsen J.. . 2012. Accuracy of noninvasive breath methane measurements using Fourier transform infrared methods on individual cows. J. Dairy Sci. 95:890–898. doi: 10.3168/jds.2011-4544. [DOI] [PubMed] [Google Scholar]
- Lassey, K. R. 2007. Livestock methane emission: from the individual grazing animal through national inventories to the global methane cycle. Agric. For. Meteorol. 142:120–132. doi: 10.1016/j.agrformet.2006.03.028. [DOI] [Google Scholar]
- Lassey, K. R. 2008. Livestock methane emission and its perspective in the global methane cycle. Austr. J. Exp. Agric. 48:114–118. doi: 10.1071/EA07220. [DOI] [Google Scholar]
- Li, C., Frolking S., and Frolking T. A.. . 1992. A model of nitrous oxide evolution from soil driven by rainfall events. 1. Model structure and sensitivity. J. Geophys. Res. Atmos. 97 (D9):9759–9776. doi: 10.1029/92JD00509 [DOI] [Google Scholar]
- Li, C., Salas W., Zhang R., Krauter C., Rotz A., and Mitloehner F.. . 2012. Manure-DNDC: a biogeochemical process model for quantifying greenhouse gas and ammonia emissions from livestock manure systems. Nutr. Cycl. Agroecosys. 93 (2):163–200. doi: 10.1007/s10705-012-9507-z [DOI] [Google Scholar]
- Lighton, J. R. B. 2008. Measuring metabolic rates: a manual for scientists. New York (NY):Oxford University Press. [Google Scholar]
- Liu, Z., Powers W., and Harmon J.. . 2016. Estimating ventilation rates of animal houses through CO2 balance. Trans. ASABE 59:321–328. doi: 10.13031/trans.59.10235. [DOI] [Google Scholar]
- Livingston, G. P., and Hutchinson G. L.. . 1995. Enclosure-based measurement of trace gas exchange: applications and sources of error. In: Matson P. A. and Harriss R. C., editors. Biogenic trace gases: measuring emissions from soil and water. Oxford, UK:Blackwell Science; p. 14–51. [Google Scholar]
- M⊘ller, H. B., Sommer S. G., and Ahring B. K.. . 2004. Biological degradation and greenhouse gas emissions during pre-storage of liquid animal manure. J. Environ. Qual. 33 (1):27–36. doi: 10.2134/jeq2004.2700. [DOI] [PubMed] [Google Scholar]
- Macoon, B., Sollenberger L. E., Moore J. E., Staples C. R., Fike J. H., and Portier K. M.. . 2003. Comparison of three techniques for estimating the forage intake of lactating dairy cows on pasture. J. Anim. Sci. 81:2357–2366. doi: 10.2527/2003.8192357x. [DOI] [PubMed] [Google Scholar]
- Madsen, J., Bjerg B. S., Hvelplund T., Weisbjerg M. R., and Lund P.. . 2010. Methane and carbon dioxide ratio in excreted air for quantification of the methane production from ruminants. Livest. Sci. 129:223–227. doi: 10.1016/j.livsci.2010.01.001. [DOI] [Google Scholar]
- Makkar, H. P. S. 2005. In vitro gas methods for evaluation of feed containing phytochemicals. Anim. Feed Sci. Technol. 123–124 (Part 1):291–302. doi: 10.1016/j.anifeedsci.2005.06.003 [DOI] [Google Scholar]
- Manafiazar, G., Zimmerman S., and Basarab J. A.. . 2016. Repeatability and variability of short-term spot measurement of methane and carbon dioxide emissions from beef cattle using GreenFeed emissions monitoring system. Can. J. Anim. Sci. 97:118–126. doi: 10.1139/cjas-2015-0190. [DOI] [Google Scholar]
- Marquardt, S., Ndung’u P. W., Onyango A. A., and Merbold L.. . 2020. Protocol for a Tier 2 approach to generate region-specific enteric methane emission factors (EF) for cattle kept in smallholder systems. ILRI Manual. No. 39. International Livestock Research Institute (ILRI), Nairobi, Kenya. Available at: https://cgspace.cgiar.org/handle/10568/109579. [Google Scholar]
- Mathot, M., Decruyenaere V., Lambert R., and Stilmant D.. . 2016. Deep litter removal frequency rate influences on greenhouse gas emissions from barns for beef heifers and from manure stores. Agric. Ecosys. Environ. 233:94–105. doi: 10.1016/j.agee.2016.08.022. [DOI] [Google Scholar]
- Mathot, M., Decruyenaere V., Stilmant D., and Lambert R.. . 2012. Effect of cattle diet and manure storage conditions on carbon dioxide, methane and nitrous oxide emissions from tie-stall barns and stored solid manure. Agric. Ecosys. Environ. 148:134–144. doi: 10.1016/j.agee.2011.11.012. [DOI] [Google Scholar]
- Mauricio, R. M., Mould F. L., Dhanoa M. S., Owen E., Channa K. S., and Theodorou M. K.. . 1999. A semi-automated in vitro gas production technique for ruminant feedstuff evaluation. Anim. Feed Sci. Technol. 79:321–330. doi: 10.1016/S0377-8401(99)00033-4. [DOI] [Google Scholar]
- McGinn, S. M., Chen D., Loh Z., Hill J., Beauchemin K. A., and Denmead O. T.. . 2008. Methane emissions from feedlot cattle in Australia and Canada. Austr. J. Exp. Agric. 48:183–185. doi: 10.1071/EA07204. [DOI] [Google Scholar]
- McGinn, S. M., Coulombe J. -F., and Beauchemin K. A.. . 2021. Technical note: validation of the GreenFeed system for measuring enteric gas emissions from cattle. J. Anim. Sci. 99:1–6. doi: 10.1093/jas/skab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinn, S. M., Flesch T. K., Crenna B. P., Beauchemin K. A., and Coates T.. . 2007. Quantifying ammonia emissions from a cattle feedlot using a dispersion model. J. Environ. Qual. 36:1585–1590. doi: 10.2134/jeq2007.0167. [DOI] [PubMed] [Google Scholar]
- McGinn, S. M., Flesch T. K., Harper L. A., and Beauchemin K. A.. . 2006. An approach for measuring methane emissions from whole farms. J. Environ. Qual. 35:14–20. doi: 10.2134/jeq2005.0250. [DOI] [PubMed] [Google Scholar]
- Miller, S. M., Wofsy S. C., Michalak A. M., Kort E. A., Andrews A. E., Biraud S. C., Dlugokencky E. J., Eluszkiewicz J., Fischer M. L., Janssens-Maenhout G., Miller B. R., Miller J. B., Montzka S. A., Nehrkorn T., and Sweeney C.. . 2013. Anthropogenic emissions of methane in the United States. Proc. Nat. Academies Sci. 110 (50):20018–20022. doi: 10.1073/pnas.1314392110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills, J. A. N., Dijkstra J., Bannink A., Cammell S. B., Kebreab E., and France J.. . 2001. A mechanistic model of whole-tract digestion and methanogenesis in the lactating dairy cow: Model development, evaluation, and application. J. Anim. Sci. 79:1584–1597. doi: 10.2527/2001.7961584x. [DOI] [PubMed] [Google Scholar]
- Mohn, J., Zeyer K., Keck M., Keller M., Zähner M., Poteko J., Emmenegger L., and Schrade S.. . 2018. A dual tracer ratio method for comparative emission measurements in an experimental dairy housing. Atmos. Environ. 179:12–22. doi: 10.1016/j.atmosenv.2018.01.057. [DOI] [Google Scholar]
- Molina, I. C., Angarita E. A., Mayorga O. L., Chará J., and Barahona-Rosales R.. . 2016. Effect of Leucaena leucocephala on methane production of Lucerna heifers fed a diet based on Cynodon plectostachyus. Livest. Sci. 185:24–29. doi: 10.1016/j.livsci.2016.01.009. [DOI] [Google Scholar]
- Moraes, L. E., Strathe A. B., Fadel J. G., Casper D. P., and Kebreab E.. . 2014. Prediction of enteric methane emissions from cattle. Global Change Biol. 20:2140–2148. doi: 10.1111/gcb.12471. [DOI] [PubMed] [Google Scholar]
- Morvay, Y., Bannink A., France J., Kebreab E., and Dijkstra J.. . 2011. Evaluation of models to predict the stoichiometry of volatile fatty acid profiles in rumen fluid of lactating Holstein cows. J. Dairy Sci. 94(6):3063–3080. doi: 10.3168/jds.2010-3995. [DOI] [PubMed] [Google Scholar]
- Murphy, M. R., Baldwin R. L., and Koong L. J.. . 1982. Estimation of stoichiometric parameters for rumen fermentation of roughage and concentrate diets. J. Anim. Sci. 55(2):411–421. doi: 10.2527/jas1982.552411x. [DOI] [PubMed] [Google Scholar]
- Murray, R. M., Bryant A. M., and Leng R. A.. . 1976. Rates of production of methane in the rumen and large intestine of sheep. Br. J. Nutr. 36:1–14. doi: 10.1079/BJN19760053. [DOI] [PubMed] [Google Scholar]
- Murray, P. J., Gill E., Balsdon S. L., and Jarvis S. C.. . 2001. A comparison of methane emissions from sheep grazing pastures with differing management intensities. Nutr. Cycl. Agroecosys. 60 (1):93–97. doi: 10.1023/A:1012654928177. [DOI] [Google Scholar]
- Nagy, A., Fehér J., and Tamás J.. . 2018. Wheat and maize yield forecasting for the Tisza river catchment using MODIS NDVI time series and reported crop statistics. Comput. Electron. Agric. 151:41–49. doi: 10.1016/j.compag.2018.05.035. [DOI] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. 2016. Nutrient Requirements of Beef Cattle. 8th ed. Animal Nutrition Series. Washington, DC:National Academy Press. doi: 10.17226/19014. [DOI] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. 2018. Improving characterization of anthropogenic methane emissions in the United States. National Academy Press, Washington, DC: —[accessed April 24, 2021]. Available at: http://nap.edu/24987 [PubMed] [Google Scholar]
- National Research Council. 2000. Nutrient requirements of beef cattle. Updated 7th ed. Nutrient requirements of domestic animals. Washington, DC:National Academy Press. doi: 10.17226/9791. [DOI] [Google Scholar]
- National Research Council. 2001. Nutrient requirements of dairy cattle 7th ed. Nutrient requirements of domestic animals. Washington, DC:National Academy Press. doi: 10.17226/9825. [DOI] [Google Scholar]
- National Research Council. 2007. Nutrient requirements of small ruminants: sheep, goats, cervids, and new world camelids. 7th ed. Animal Nutrition Series. Washington, DC:National Academy Press. doi: 10.17226/11654. [DOI] [Google Scholar]
- Nelson, D. D., Shorter J. H., McManus J. B., and Zahniser M. S.. . 2002. Sub-part-per-billion detection of nitric oxide in air using a thermoelectrically cooled mid-infrared quantum cascade laser spectrometer. Appl. Phys. B 75:343–350. doi: 10.1007/s00340-002-0979-4. [DOI] [Google Scholar]
- Nisbet, E. G., Fisher R. E., Lowry D., France J. L., Allen G., Bakkaloglu S., Broderick T. J., Cain M., Coleman M., Fernandez J., . et al. 2020. Methane mitigation: methods to reduce emissions, on the path to the Paris agreement. Rev. Geophys. 58:e2019–RG000675. doi: 10.1029/2019RG000675. [DOI] [Google Scholar]
- Nisbet, E. G., Manning M. R., Dlugokencky E. J., Fisher R. E., Lowry D., Michel S. E., Myhre C. L., Platt S. M., Allen G., Bousquet P., . et al. 2019. Very strong atmospheric methane growth in the 4 years 2014–2017: implications for the Paris agreement. Global Biogeochem. Cycles 33:318–342. doi: 10.1029/2018GB006009. [DOI] [Google Scholar]
- Niu, M., Kebreab E., Hristov A. N., Oh J., Arndt C., Bannink A., Bayat A. R., Brito A. F., Boland T., Casper D., . et al. 2018. Prediction of enteric methane production, yield, and intensity in dairy cattle using an intercontinental database. Global Change Biol. 24:3368–3389. doi: 10.1111/gcb.14094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris, A. B., Tedeschi L. O., Muir J. P., Foster J. L., Casey K. D., and Pinchak W. E.. . 2020. Effect of quebracho condensed tannin extract on fecal gas flux in steers. J. Environ. Qual. 49:1225–1235. doi: 10.1002/jeq2.20110. [DOI] [PubMed] [Google Scholar]
- Ogink, N. W. M., Mosquera J., Calvet S., and Zhang G.. . 2013. Methods for measuring gas emissions from naturally ventilated livestock buildings: developments over the last decade and perspectives for improvement. Biosystems Eng. 116 (3):297–308. doi: 10.1016/j.biosystemseng.2012.10.005. [DOI] [Google Scholar]
- Ominski, K., Gunte K., Wittenberg K., Legesse G., Mengistu G., and McAllister T.. . 2021. The role of livestock in sustainable food production systems in Canada. Can. J. Anim. Sci. 101:591–601. doi: 10.1139/cjas-2021-0005. [DOI] [Google Scholar]
- Owen, J. J., and Silver W. L.. . 2015. Greenhouse gas emissions from dairy manure management: a review of field-based studies. Global Change Biol. 21:550–565. doi: 10.1111/gcb.12687. [DOI] [PubMed] [Google Scholar]
- Pardo, G., del Prado A., Martínez-Mena M., Bustamante M. A., Martín J. A. R., Álvaro-Fuentes J., and Moral R.. . 2017a. Orchard and horticulture systems in Spanish Mediterranean coastal areas: is there a real possibility to contribute to C sequestration?. Agric. Ecosys. Environ. 238:153–167. doi: 10.1016/j.agee.2016.09.034. [DOI] [Google Scholar]
- Pardo, G., Moral R., and Prado A.. . 2017b. SIMSWASTE-AD—a modelling framework for the environmental assessment of agricultural waste management strategies: Anaerobic digestion. Sci. Total Environ. 574:806–817. doi: 10.1016/j.scitotenv.2016.09.096. [DOI] [PubMed] [Google Scholar]
- Pavelka, M., Acosta M., Kiese R., Altimir N., Brümmer C., Crill P., Darenova E., Fuß R., Gielen B., Graf A., . et al. 2018. Standardisation of chamber technique for CO2, N2O and CH4 fluxes measurements from terrestrial ecosystems. Int. Agrophys. 32:569–587. doi: 10.1515/intag-2017-0045. [DOI] [Google Scholar]
- Pedersen, S., Blanes-Vidal V., Joergensen H., Chwalibog A., Haeussermann A., Heetkamp M. J. W., and Aarnink A. J. A.. . 2008. Carbon dioxide production in animal houses: a literature review. Agricultural Engineering International: CIGR Journal. X (Manuscript BC 08 008):1–19. https://cigrjournal.org/index.php/Ejounral/article/view/1205. [Google Scholar]
- Pedersen, A. R., Petersen S. O., and Schelde K.. . 2010. A comprehensive approach to soil-atmosphere trace-gas flux estimation with static chambers. Eur. J. Soil Sci. 61:888–902. doi: 10.1111/j.1365-2389.2010.01291.x. [DOI] [Google Scholar]
- Pell, A. N., and Schofield P.. . 1993. Computerized monitoring of gas production to measure forage digestion in vitro. J. Dairy Sci. 76:1063–1073. doi: 10.3168/jds.S0022-0302(93)77435-4. [DOI] [PubMed] [Google Scholar]
- Pihlatie, M. K., Christiansen J. R., Aaltonen H., Korhonen J. F. J., Nordbo A., Rasilo T., Benanti G., Giebels M., Helmy M., Sheehy J., . et al. 2013. Comparison of static chambers to measure CH4 emissions from soils. Agric. For. Meteorol. 17:124–136. doi: 10.1016/j.agrformet.2012.11.008. [DOI] [Google Scholar]
- Pinares-Patiño, C., Lively F., and Lassey K. R.. . 2015. The sulphur hexafluoride tracer techique for estimating enteric methane emissions from ruminants. In: Gerrits W. J. J. and Labussière E., editors. Indirect calorimetry: techniques, computations and applications. Wageningen, The Netherlands:Wageningen Academic Publishers; p. 185–212. [Google Scholar]
- Pinares-Patiño, C., and Waghorn G.. . 2014. Technical manual on respiration chamber designs. Wellington, New Zealand:Ministry of Agriculture and Forestry. p. 118. Available at: http://www.globalresearchalliance.org/ [Google Scholar]
- Pinares-Patiño, C., and Waghorn G.. . 2018. Technical Manual on Respiration Chamber Designs. Wellington, New Zealand:Ministry of Agriculture and Forestry. p. 134—[accessed.May 18, 2022 ]. Available at: https://globalresearchalliance.org/library/livestock-research-group-technical-manual-respiration-chamber-designs-february-2014/ [Google Scholar]
- Pitt, R. E., and Pell A. N.. . 1997. Modeling ruminal pH fluctuations: interactions between meal frequency and digestion rate. J. Dairy Sci. 80:2429–2441. doi: 10.3168/jds.S0022-0302(97)76195-2. [DOI] [PubMed] [Google Scholar]
- Pitt, R. E., Van Kessel J. S., Fox D. G., Pell A. N., Barry M. C., and Van Soest P. J.. . 1996. Prediction of ruminal volatile fatty acids and pH within the net carbohydrate and protein system. J. Anim. Sci. 74:226–244. doi: 10.2527/1996.741226x. [DOI] [PubMed] [Google Scholar]
- Place, S. E., Pan Y., Zhao Y., and Mitloehner F. M.. . 2011. Construction and operation of a ventilated hood system for measuring greenhouse gas and volatile organic compound emissions from cattle. Animals 1:433–446. doi: 10.3390/ani1040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky, I. N., O’Brien D. M., Kumer J. B., O’Dell C. W., and the geo C. T.. . 2014. Performance of a geostationary mission, geoCARB, to measure CO2, CH4 and CO column-averaged concentrations. Atmos. Meas. Tech. 7:959–981. doi: 10.5194/amt-7-959-2014. [DOI] [Google Scholar]
- Powers, W., and Capelari M.. . 2016. Analytical methods for quantifying greenhouse gas flux in animal production systems. J. Anim. Sci. 94:3139–3146. doi: 10.2527/jas.2015-0017. [DOI] [PubMed] [Google Scholar]
- Pumpanen, J., Kolari P., Ilvesniemi H., Minkkinen K., Vesala T., Niinistö S., Lohila A., Larmola T., Morero M., Pihlatie M., . et al. 2004. Comparison of different chamber techniques for measuring soil CO2 efflux. Agric. For. Meteorol. 123:159–176. doi: 10.1016/j.agrformet.2003.12.001. [DOI] [Google Scholar]
- Ramírez-Restrepo, C. A., Barry T. N., Marriner A., López-Villalobos N., McWilliam E. L., Lassey K. R., and Clark H.. . 2010. Effects of grazing willow fodder blocks upon methane production and blood composition in young sheep. Anim. Feed Sci. Technol. 155:33–43. doi: 10.1016/j.anifeedsci.2009.10.003. [DOI] [Google Scholar]
- Ramírez-Restrepo, C. A., Clark H., and Muetzel S.. . 2016. Methane emissions from young and mature dairy cattle. Anim. Prod. Sci. 56:1897–1905. doi: 10.1071/AN15102. [DOI] [Google Scholar]
- Ramírez-Restrepo, C. A., Waghorn G. C., Gillespie H., and Clark H.. . 2020. Partition of dietary energy by sheep fed fresh ryegrass (Lolium perenne) with a wide-ranging composition and quality. Anim. Prod. Sci. 60:1008–1017. doi: 10.1071/AN19285. [DOI] [Google Scholar]
- Ricci, P., Chagunda M. G. G., Rooke J., Houdijk J. G. M., Duthie C. -A., Hyslop J., Roehe R., and Waterhouse A.. . 2014. Evaluation of the laser methane detector to estimate methane emissions from ewes and steers. J. Anim. Sci. 92:5239–5250. doi: 10.2527/jas.2014-7676. [DOI] [PubMed] [Google Scholar]
- Sakita, G. Z., Lima P. D. M. T., Abdalla Filho A. L., Bompadre T. F. V., Ovani V. S., Chaves C. D. M. E. S., Bizzuti B. E., Costa W. D. S. D., Paim T. D. P., Campioni T. S., . et al. 2022. Treating tropical grass with fibrolytic enzymes from the fungus Trichoderma reesei: Effects on animal performance, digestibility and enteric methane emissions of growing lambs. Anim. Feed Sci. Technol. 286:115253. doi: 10.1016/j.anifeedsci.2022.115253. [DOI] [Google Scholar]
- Sass, R. L., Fisher F. M., Harcombe P. A., and Turner F. T.. . 1990. Methane production and emission in a Texas rice field. Global Biogeochem. Cycles 4:47–68. doi: 10.1029/GB004i001p00047. [DOI] [Google Scholar]
- Sass, R. L., Fisher F. M., Turner F. T., and Jund M. F.. . 1991. Methane emission from rice fields as influenced by solar radiation, temperature, and straw incorporation. Global Biogeochem. Cycles 5:335–350. doi: 10.1029/91GB02586. [DOI] [Google Scholar]
- Savage, K., Phillips R., and Davidson E.. . 2014. High temporal frequency measurements of greenhouse gas emissions from soils. Biogeosciences 11:2709–2720. doi: 10.5194/bg-11-2709-2014. [DOI] [Google Scholar]
- Schrade, S., Zeyer K., Gygax L., Emmenegger L., Hartung E., and Keck M.. . 2012. Ammonia emissions and emission factors of naturally ventilated dairy housing with solid floors and an outdoor exercise area in Switzerland. Atmos. Environ. 47:183–194. doi: 10.1016/j.atmosenv.2011.11.015. [DOI] [Google Scholar]
- Sitaula, B. K., Luo J., and Bakken L. R.. . 1992. Rapid analysis of climate gases by wide bore capillary gas chromatography. J. Environ. Qual. 21:493–496. doi: 10.2134/jeq1992.00472425002100030030x. [DOI] [Google Scholar]
- Sorg, D. 2022. Measuring livestock CH4 emissions with the laser methane detector: A review. Methane. 1 (1). doi: 10.3390/methane1010004 [DOI] [Google Scholar]
- Staebell, C., Sun K., Samra J., Franklin J., Chan Miller C., Liu X., Conway E., Chance K., Milligan S., and Wofsy S.. . 2021. Spectral calibration of the MethaneAIR instrument. Atmos. Meas. Tech. 14:3737–3753. doi: 10.5194/amt-14-3737-2021. [DOI] [Google Scholar]
- Storm, I. M. L., Hellwing A. L. F., Nielsen N. I., and Madsen J.. . 2012. Methods for measuring and estimating methane emission from ruminants. Animals 2:160–183. doi: 10.3390/ani2020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannant, D., Smith K., Cahill A., Hawthorne I., Ford O., Black A., and Beckie R.. . 2018. Evaluation of a drone and laser-based methane sensor for detection of fugitive methane emissions. Vancouver, BC:University of British Columbia. p. 29—[accessed March 1, 2022]. Available at: https://www.bcogris.ca/sites/default/files/hs-2018-01-uav-fugitive-gas-emission-final-tannant-ubco.pdf [Google Scholar]
- Tedeschi, L. O. 2019. ASN-ASAS Symposium: Future of Data Analytics in Nutrition: mathematical modeling in ruminant nutrition: approaches and paradigms, extant models, and thoughts for upcoming predictive analytics. J. Anim. Sci. 97:1321–1944. doi: 10.1093/jas/skz092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi, L. O. 2022. A holistic perspective of the societal relevance of beef production and its impacts on climate change. Zenodo. doi: 10.5281/zenodo.5944758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi, L. O., and Fox D. G.. . 2020a. The ruminant nutrition system: Volume I—an applied model for predicting nutrient requirements and feed utilization in ruminants. 3rd ed. Ann Arbor (MI):XanEdu. [Google Scholar]
- Tedeschi, L. O., and Fox D. G.. . 2020b. The ruminant nutrition system: Volume II—tables of equations and coding. Ann Arbor (MI):XanEdu. [Google Scholar]
- Tedeschi, L. O., Kononoff P. J., Karges K., and Gibson M. L.. . 2009. Effects of chemical composition variation on the dynamics of ruminal fermentation and biological value of corn milling (co)products. J. Dairy Sci. 92:401–413. doi: 10.3168/jds.2008-1141. [DOI] [PubMed] [Google Scholar]
- Tedeschi, L. O., Molle G., Menendez H. M., Cannas A., and Fonseca M. A.. . 2019. The assessment of supplementation requirements of grazing ruminants using nutrition models. Transl. An. Sci. 3 (2):811–828. doi: 10.1093/tas/txy140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi, L. O., Muir J. P., Naumann H. D., Norris A. B., Ramírez-Restrepo C. A., and Mertens-Talcott S. U.. . 2021. Nutritional aspects of ecologically relevant phytochemicals in ruminant production. Front. Vet. Sci. 8. doi: 10.3389/fvets.2021.628445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi, L. O., Muir J. P., Riley D. G., and Fox D. G.. . 2015. The role of ruminant animals in sustainable livestock intensification programs. Int. J. Sustainable Dev. World Ecol. 22 (5):452–465. doi: 10.1080/13504509.2015.1075441 [DOI] [Google Scholar]
- Theodorou, M. K., Williams B. A., Dhanoa M. S., Mcallan A. B., and France J.. . 1994. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 48:185–197. doi: 10.1016/0377-8401(94)90171-6. [DOI] [Google Scholar]
- Thompson, L. R., and Rowntree J. E.. . 2020. Invited review: methane sources, quantification, and mitigation in grazing beef systems. Appl. Anim. Sci. 36 (4):556–573. doi: 10.15232/aas.2019-01951. [DOI] [Google Scholar]
- Thornley, J. H. M., and France J.. . 2007. Mathematical models in agriculture. 2nd ed. Wallingford, UK:CABI Publishing. [Google Scholar]
- Tilley, J. M. A., and Terry R. A.. . 1963. A two-stage technique for the in vitro digestion of forage crops. J. Br. Grassl. Soc. 18:104–111. doi: 10.1111/j.1365-2494.1963.tb00335.x. [DOI] [Google Scholar]
- Tomkins, N. W., and Charmley E.. . 2015. Herd-scale measurements of methane emissions from cattle grazing extensive sub-tropical grasslands using the open-path laser technique. Animal 9:2029–2038. doi: 10.1017/S1751731115001688. [DOI] [PubMed] [Google Scholar]
- Tomkins, N. W., McGinn S. M., Turner D. A., and Charmley E.. . 2011. Comparison of open-circuit respiration chambers with a micrometeorological method for determining methane emissions from beef cattle grazing a tropical pasture. Anim. Feed Sci. Technol. 166-167 (0):240–247. doi: 10.1016/j.anifeedsci.2011.04.014. [DOI] [Google Scholar]
- Ueyama, M., Takeuchi R., Takahashi Y., Ide R., Ataka M., Kosugi Y., Takahashi K., and Saigusa N.. . 2015. Methane uptake in a temperate forest soil using continuous closed-chamber measurements. Agric. For. Meteorol. 213:1–9. doi: 10.1016/j.agrformet.2015.05.004. [DOI] [Google Scholar]
- Undi, M., Wilson C., Ominski K. H., and Wittenberg K. M.. . 2008. Comparison of techniques for estimation of forage dry matter intake by grazing beef cattle. Can. J. Anim. Sci. 88:693–701. doi: 10.4141/CJAS08041. [DOI] [Google Scholar]
- United Nations Environment Programme, and Climate and Clean Air Coalition. 2021. Global Methane Assessment: Benefits and Costs of Mitigating Methane Emissions. United Nations Environment Programme, Nairobi, Kenya. 172p. Available at: https://www.unep.org/resources/report/global-methane-assessment-benefits-and-costs-mitigating-methane-emissions [Google Scholar]
- van den Borne, J. J. G. C., Heetkamp M. J. W., Alferink S. J. J., and Gerrits W. J. J.. . 2015. Moving from a complete energy balance towards substrate oxidation: use of stable isotopes. In: Gerrits W. J. J. and Labussière E., editors. Indirect calorimetry: techniques, computations and applications. Wageningen, The Netherlands:Wageningen Academic Publishers; p. 87–113. [Google Scholar]
- van der Weerden, T. J., Noble A. N., Luo J., de Klein C. A. M., Saggar S., Giltrap D., Gibbs J., and Rys G.. . 2020. Meta-analysis of New Zealand’s nitrous oxide emission factors for ruminant excreta supports disaggregation based on excreta form, livestock type and slope class. Sci. Total Environ. 732:139235. doi: 10.1016/j.scitotenv.2020.139235. [DOI] [PubMed] [Google Scholar]
- van Lingen, H. J., Niu M., Kebreab E., Valadares Filho S. C., Rooke J. A., Duthie C.-A., Schwarm A., Kreuzer M., Hynd P. I., Caetano M., . et al. 2019. Prediction of enteric methane production, yield and intensity of beef cattle using an intercontinental database. Agric. Ecosys. Environ. 283:106575. doi: 10.1016/j.agee.2019.106575 [DOI] [Google Scholar]
- Van Ouverkerk, E. N. J., and Pedersen S.. . 1994. Application of the carbon dioxide mass balance method to evaluate ventilation rates in livestock buildings. Pages 516–529 in XII World Congress Agricultural Engineering, v. 1. Milano, Italy. [Google Scholar]
- van Well, B., Murray S., Hodgkinson J., Pride R., Strzoda R., Gibson G., and Padgett M.. . 2005. An open-path, hand-held laser system for the detection of methane gas. J. Opt. A: Pure Appl. Opt. 7:S420–S424. doi: 10.1088/1464-4258/7/6/025. [DOI] [Google Scholar]
- Vanlierde, A., Dehareng F., Gengler N., Froidmont E., McParland S., Kreuzer M., Bell M., Lund P., Martin C., Kuhla B., . et al. 2020. Improving robustness and accuracy of predicted daily methane emissions of dairy cows using milk mid-infrared spectra. J. Sci. Food Agric. n/a (n/a). doi: 10.1002/jsfa.10969 [DOI] [PubMed] [Google Scholar]
- Vargas-Villamil, L. M., and Tedeschi L. O.. . 2014. Potential integration of multi-fitting, inverse problem and mechanistic modelling approaches to applied research in animal science: a review. Anim. Prod. Sci. 54:1905–1913. doi: 10.1071/AN14568. [DOI] [Google Scholar]
- Vargas-Villamil, L. M., Tedeschi L. O., Medina-Peralta S., Izquierdo-Reyes F., Navarro-Alberto J., and González-Garduño R.. . 2020. A multi-inverse approach for a holistic understanding of applied animal science systems. Animal 14:s238–s249. doi: 10.1017/S1751731120000877. [DOI] [PubMed] [Google Scholar]
- Varon, D. J., Jacob D. J., McKeever J., Jervis D., Durak B. O. A., Xia Y., and Huang Y.. . 2018. Quantifying methane point sources from fine-scale satellite observations of atmospheric methane plumes. Atmos. Meas. Tech. 11:5673–5686. doi: 10.5194/amt-11-5673-2018. [DOI] [Google Scholar]
- Vibart, R., de Klein C., Jonker A., van der Weerden T., Bannink A., Bayat A. R., Crompton L., Durand A., Eugène M., Klumpp K., . et al. 2021. Challenges and opportunities to capture dietary effects in on-farm greenhouse gas emissions models of ruminant systems. Sci. Total Environ. 769:144989. doi: 10.1016/j.scitotenv.2021.144989. [DOI] [PubMed] [Google Scholar]
- Vigan, A., Hassouna M., Guingand N., Brame C., Edouard N., Eglin T., Espagnol S., Eugène M., Génermont S., Lagadec S., . et al. 2019. Development of a database to collect emission values for livestock systems. J. Environ. Qual. 48:1899–1906. doi: 10.2134/jeq2019.01.0007. [DOI] [Google Scholar]
- Wahab, I., Hall O., and Jirström M.. . 2018. Remote sensing of yields: application of UAV imagery-derived NDVI for estimating maize vigor and yields in complex farming systems in Sub-saharan Africa. Drones. 2 (3):28. doi: 10.3390/drones2030028. [DOI] [Google Scholar]
- Waldo, S., Russell E. S., Kostyanovsky K., Pressley S. N., O’Keeffe P. T., Huggins D. R., Stöckle C. O., Pan W. L., and Lamb B. K.. . 2019. N2O emissions from two agroecosystems: high spatial variability and long pulses observed using static chambers and the flux-gradient technique. J. Geophys. Res. Biogeosci. 124:1887–1904. doi: 10.1029/2019JG005032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J., Duan C., Ji Y., and Sun Y.. . 2010. Methane emissions during storage of different treatments from cattle manure in Tianjin. J. Environ. Sci. 22:1564–1569. doi: 10.1016/S1001-0742(09)60290-4. [DOI] [PubMed] [Google Scholar]
- Webb, J., van der Weerden T. J., Hassouna M., and Amon B.. . 2021. Guidance on the conversion of gaseous emission units to standardized emission factors and recommendations for data reporting. Carbon Manage. 12:663–679. doi: 10.1080/17583004.2021.1995502. [DOI] [Google Scholar]
- Weiss, R. F. 1981. Determinations of carbon dioxide and methane by dual catalyst flame ionization chromatography and nitrous oxide by electron capture chromatography. J. Chromatogr. Sci. 19:611–616. doi: 10.1093/chromsci/19.12.611. [DOI] [Google Scholar]
- Yan, T., Mayne C. S., Gordon F. G., Porter M. G., Agnew R. E., Patterson D. C., Ferris C. P., and Kilpatrick D. J.. . 2010. Mitigation of enteric methane emissions through improving efficiency of energy utilization and productivity in lactating dairy cows. J. Dairy Sci. 93:2630–2638. doi: 10.3168/jds.2009-2929. [DOI] [PubMed] [Google Scholar]
- Yáñez-Ruiz, D. R., Bannink A., Dijkstra J., Kebreab E., Morgavi D. P., O’Kiely P., Reynolds C. K., Schwarm A., Shingfield K. J., Yu Z., . et al. 2016. Design, implementation and interpretation of in vitro batch culture experiments to assess enteric methane mitigation in ruminants—a review. Anim. Feed Sci. Technol. 216:1–18. doi: 10.1016/j.anifeedsci.2016.03.016. [DOI] [Google Scholar]
- Zhang, G., Pedersen S., and Kai P.. . 2010. Uncertainty analysis of using CO2 production models by cows to determine ventilation rate in naturally ventilated buildings. Pages 1–10 in XVII World Congress of the International Commission of Agricultural and Biosystems Engineering (CIGR). Quebec City, Canada. Canadian Society for Bioengineering—[accessed; May 8, 2021]. Available at: https://library.csbe-scgab.ca/all-publications/category/view/135-cigr-and-agm-quebec-city-2010 [Google Scholar]
- Zhao, Y., Nan X., Yang L., Zheng S., Jiang L., and Xiong B.. . 2020. A review of enteric methane emission measurement techniques in ruminants. Animals 10. doi: 10.3390/ani10061004. [DOI] [PMC free article] [PubMed] [Google Scholar]