Abstract
Variation in nutrition is a key determinant of growth, body composition, and the ability of animals to perform to their genetic potential. Depending on the quality of feed available, animals may be able to overcome negative effects of prior nutritional restriction, increasing intake and rates of tissue gain, but full compensation may not occur. A 2 × 3 × 4 factorial serial slaughter study was conducted to examine the effects of prior nutritional restriction, dietary energy density, and supplemental rumen undegradable protein (RUP) on intake, growth, and body composition of lambs. After an initial slaughter (n = 8), 124 4-mo-old Merino cross wethers (28.4 ± 1.8 kg) were assigned to either restricted (LO, 500 g/d) or unrestricted (HI, 1500 g/d) intake of lucerne and oat pellets. After 8 wk, eight lambs/group were slaughtered and tissue weights and chemical composition were measured. Remaining lambs were randomly assigned to a factorial combination of dietary energy density (7.8, 9.2, and 10.7 MJ/kg DM) and supplemental RUP (0, 30, 60, and 90 g/d) and fed ad libitum for a 12- to 13-wk experimental period before slaughter and analysis. By week 3 of the experimental period, lambs fed the same level of energy had similar DMI (g/d) and MEI (MJ/d) (P > 0.05), regardless of prior level of nutrition. Restricted-refed (LO) lambs had higher rates of fat and protein gain than HI lambs (P < 0.05) but had similar visceral masses (P > 0.05). However, LO lambs were lighter and leaner at slaughter, with proportionally larger rumens and livers (P < 0.05). Tissue masses increased with increasing dietary energy density, as did DMI, energy and nitrogen (N) retention (% intake), and rates of protein and fat gain (P < 0.05). The liver increased proportionally with increasing dietary energy density and RUP (P < 0.05), but rumen size decreased relative to the empty body as dietary energy density increased (P < 0.05) and did not respond to RUP (P > 0.05). Fat deposition was greatest in lambs fed 60 g/d supplemental RUP (P < 0.05). However, lambs fed 90 g/d were as lean as lambs that did not receive supplement (P0, P > 0.05), with poorer nitrogen retention and proportionally heavier livers than P0 lambs (P < 0.05). In general, visceral protein was the first tissue to respond to increased intake during refeeding, followed by non-visceral protein and fat, highlighting the influence of differences in tissue response over time on animal performance and body composition.
Keywords: compensatory growth, feed intake, metabolism, nutrition, viscera
Visceral size and heat production are sensitive to both prior and current nutrition; differences in visceral size can alter the proportion of feed energy retained. In the present study, restricted-refed and unrestricted lambs had similar levels of ad-libitum intake, but restricted-refed lambs had proportionally larger viscera and were lighter and leaner at harvest. Diet quality during refeeding had different effects on rumen and liver size: lambs fed higher-energy (lower-forage) diets had proportionally smaller rumens but larger livers than lambs fed lower-energy diets with more forage. Exploration of the drivers of variation in these responses is key to improving the understanding of animal metabolism and feed requirements.
Introduction
Growing animals are often unable to consume sufficient nutrients to match their genetic potential for growth, leading to long-term impacts on productivity and efficiency (Oddy et al., 1997a; Greenwood et al., 2005; Keogh et al., 2015; Oddy et al. 2019). The impact of nutritional restriction on later growth and body composition depends on factors such as the duration and severity of restriction, the stage of maturity at which the restriction was imposed and the subsequent diet (Carstens et al., 1991; Oddy et al., 1997a; Oddy 1998, Greenwood et al., 2005). Viscera is the first tissue to respond to changes in nutrition, partly due to its high rate of protein turnover. Feed characteristics such as energy density, fiber, and nitrogen content have all been shown to influence visceral size and heat production, and therefore the amount of energy available to the rest of the body (Rompala et al., 1988, Sainz and Bentley, 1997; McLeod and Baldwin, 2000; Ferrell et al., 2001). The effects of dietary energy and protein supply on the fat and protein content and rate of compensatory growth are inconsistent. Furthermore, the effects of nutrient restriction and realimentation on visceral versus non-visceral tissues, which may contribute to variation in body composition, are also not well described (Oddy et al., 1997a; Oddy, 1998; Oddy and Sainz, 2002; Keogh et al., 2015).
This study follows from Hegarty et al. (1999), who examined the effect of energy intake and rumen undegradable protein (RUP) supply on growing lambs. Hegarty et al. (1999) found lasting effects of prior plane of nutrition on body composition; the study was constrained by the fixed levels of intake fed during the realimentation phase of the experiment. It was unclear if similar results would be observed when animals were allowed ad libitum access to feeds of different energy density. One particular goal of the present study was to explore if visceral and non-visceral tissues would respond differently when animals were allowed ad libitum access to feeds of different energy density, rather than the single diet fed in Hegarty et al. (1999).
The present study was designed to examine the impact of variation in dietary energy density and RUP on lambs that had been previously restricted. Rather than offer varying quantities of one diet, lambs were offered ad libitum intake of three diets of different energy density and four different levels of supplemental RUP. This allowed for exploration of the specific effects of variation in RUP supply and dietary energy on energy and protein supply to the animal, and their ensuing effects on visceral and non-visceral tissue growth and composition.
The objectives of the current study were therefore: 1) to examine the effect of dietary energy density and rumen undegradable protein on voluntary DMI and MEI in lambs previously subjected to a period of weight stasis; 2) to determine the effects of prior nutritional restriction and recovery with diets of different composition on liveweight, organ weight and chemical composition of the carcass and viscera; and 3) to investigate the implications of prior nutritional history, response to dietary energy density and rumen undegradable protein supply on energy and protein transactions in lambs.
Materials and Methods
All procedures involving the use of animals were approved by the New South Wales Department of Primary Industries Elizabeth McArthur Agricultural Institute Animal Ethics Committee and met all relevant state and federal legislation at the time the study was conducted.
Experimental design
The experiment was designed as a 2 × 3 × 4 factorial, with two rates of growth (HI and LO) during a preliminary period, followed by an experimental period where lambs were fed one of three dietary energy densities (E1, E2, and E3, 7.8, 9.2, and 10.7 MJ/kg DM, respectively) and one of four levels of supplemental rumen undegradable protein (RUP; 0, 30, 60, and 90 g/d). The experiment consisted of three phases: adaptation, preliminary, and experimental.
Animals and measurements
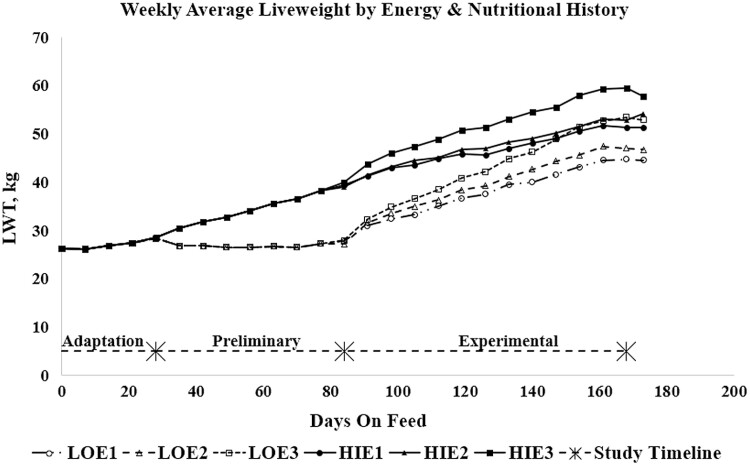
One hundred thirty-two crossbred castrated male lambs (from Border Leicester x Merino ewes joined to Poll Dorset rams) aged approximately 4 mo and with average initial weight of 26.2 (±2.12 SD) kg were transported to Elizabeth Macarthur Agricultural Institute Camden NSW, Australia. The lambs were treated for internal parasites with an oral dose of Ivermectin (Ivomec, Merial Australia, Parramatta, NSW), placed in individual pens, and offered 1 kg/d of a pelleted diet consisting of lucerne hay and oat grain (Table 1). Unshorn liveweights (LWT, kg) were recorded weekly (Figure 1, RUP levels not shown). Lambs were housed and fed individually throughout the entire experiment, and feed intake and refusals were measured individually on a daily basis. Accordingly, the experimental unit for all statistical analyses was the individual lamb.
Table 1.
Ingredients and nutritional composition of diets fed during the study
| Period | Adaptation and preliminary | Experimental | ||
|---|---|---|---|---|
| E1 | E2 | E3 | ||
| Ingredients, % as fed | ||||
| Lucerne hay | 75 | 57.3 | 43.2 | 9.6 |
| Oat grain | 25 | 19.2 | 33.6 | 67.2 |
| Wheat straw | − | 19.2 | 19.2 | 19.2 |
| Urea | − | 1.5 | 1.5 | 1.5 |
| Mineral mix1 | − | 2.5 | 2.5 | 2.5 |
| HCHO casein2 | − | + | + | + |
| Nutritional composition | ||||
| Dry matter, % | 90 | 93.2 | 93.4 | 91.1 |
| Crude protein, % DM | 15.2 | 10.9 | 13.3 | 16.5 |
| RDP, %DM | 11.6 | 8.6 | 10.0 | 12.7 |
| RUP, %DM | 3.6 | 2.3 | 3.3 | 3.8 |
| M/D, MJ ME/ kg DM | 10 | 7.8 | 9.2 | 10.7 |
Minerals: 1% NaCl, 1.5% ground limestone, and 0.05% trace mineral mixture (Hegarty et al, 1999).
Formaldehyde-treated casein (Hemsley et al. 1973); + Offered as 0,30,60,90 g/d with small amounts of molasses and lucerne chaff 2 h prior to offering pelleted diets.
Figure 1.
Weekly average liveweights over time by nutritional history and dietary energy concentration during the experimental period (weights include fleece). *Slaughter timepoints.
At the beginning of the adaptation period, lambs were weighed and given 28 d to adapt to the pelleted diet. The lambs were shorn during this period. Dye bands (Wheeler et al., 1977) were applied after initial shearing, prior to start of the experiment, at the end of the preliminary period, and at the end of the experimental period. At the end of the adaptation period, an initial group of eight lambs were slaughtered and tissue data were collected and analyzed as detailed below. The remaining 124 lambs were then randomly assigned to either a LO (550 g/d) or HI (1500 g/d) level of feeding during the 57-d preliminary period. These feeding levels were based on data from Hegarty et al. (1999). The LO feeding level represented estimated maintenance, while the HI level of feed intake was designed as a high feeding level, close to ad-libitum intake, but where lambs would still consume the entire daily ration offered. There were no refusals from lambs at either feeding level during the preliminary period.
At the end of the preliminary period, eight lambs from each of the HI and LO treatments were slaughtered and the remaining 108 lambs were randomly allocated to experimental diets as outlined above. Lambs were fed once daily at 0830 h in excess of appetite, and water was freely available at all times. Feed intake and refusals were recorded daily. At the end of the experimental period, lambs were allocated to slaughter day using a stratified random procedure to prevent biasing of treatment effects. Lambs were shorn 1 wk prior to slaughter. The average slaughter date was day 88 of the experimental period (range 83–93 d).
At slaughter, carcass and non-carcass components were separated and weighed as per Hegarty et al. (1999). Viscera was defined as the sum of the liver, kidneys, pluck (heart, lungs, and trachea), empty gut, gallbladder, spleen, and pancreas, excluding dissectible internal fat (omental, kidney, and mesenteric fat). Anything not in the visceral pool was considered part of the non-visceral empty body pool (NVEB), specifically defined as the sum of cold carcass weight, head and feet, skin (without fleece), and blood.
Diets
The diet fed during the adaptation and preliminary periods was a 75:25 mix (as-fed basis) of lucerne hay and oat grain (Table 1). For the experimental period, diets were formulated to provide energy densities of 7.8, 9.2, or 10.7 MJ ME/kg DM (E1, E2, and E3, respectively). All diets were pelleted through a 9-mm die. Energy density of the diet (MD, MJ ME/kg DM) was calculated from proximate analysis of the feed using the equations presented in Oddy et al. (1983).
Rumen undegradable protein (RUP) was provided in the form of formaldehyde-treated casein offered at levels of 0, 30, 60, or 90 g/d (levels P0, P30, P60, and P90, respectively). The treated casein supplement was prepared by thoroughly mixing 868 ml formalin solution (37% w/w Formaldehyde/water) per 50 kg casein powder, which was then dried prior to storage in bags (Hemsley et al., 1973). To facilitate consumption of supplemental protein, a small quantity of lucerne and molasses was mixed with the supplemental RUP and fed at 0800 h every morning, 30 min prior to feeding of E1, E2, or E3. Lambs that did not receive supplemental RUP (P0) received the lucerne and molasses at the same level as other lambs. Reported values for DMI and MEI include this supplement. The proportion of formaldehyde-treated casein that escaped ruminal degradation was approximately 67%, as assessed by the in situ methods described in Neutze et al. (1993).
Chemical analysis
Viscera and dissectible internal fat were pooled, minced, and stored frozen at −20 °C until analysis. Head and hocks were minced and a sub sample frozen at −20 °C until analysis. A sample of skin (without wool) was frozen at −20 °C until analysis. Samples of minced frozen tissues were homogenized prior to chemical analysis. Viscera, skin, head, hocks, and carcass tissues were analyzed as per the chemical analysis procedures described in Hegarty et al. (1999).
Calculations
Fleece-free empty body weight (FFEBW) was defined as fleece-free LWT at slaughter less gut fill measured at slaughter. Where lambs were not shorn immediately prior to slaughter, the weight of wool was calculated from measured growth rate from shearing. Average daily gain (ADG, g/d) was calculated as the sum of slaughter LWT plus fleece shorn before slaughter, minus unshorn LWT at the end of the preliminary period, divided by days on feed during the experimental period. Wool growth was estimated by the dye band technique of Wheeler et al. (1977). Dye band measurements of wool growth were added to the weight of fleece remaining on the lambs at slaughter to calculate total fleece production and gain. The ratio of clean to greasy wool was 0.7. Clean wool was assumed to be 100% protein with a retained energy of 23.8 MJ/kg clean wool protein.
Fleece-free liveweight (FFLWT) at the end of the preliminary period was calculated by subtracting individual rates of fleece growth during the experimental period from total fleece at slaughter. The corresponding FFEBW was calculated by multiplying FFLWT by the average ratio of FFEBW to FFLWT of animals slaughtered at the end of the preliminary period. Separate ratios were used for each nutritional history (HI or LO). Dye band data were not available for lambs slaughtered at the end of the preliminary period, but was available for both preliminary and experimental periods for lambs slaughtered at the end of the trial. Therefore, to estimate fleece growth and initial FFLWT in lambs slaughtered at the end of the preliminary period, fleece growth rates during the preliminary period were averaged by nutritional history (HI or LO, n = 44 and 43, respectively) and used to estimate initial FFLWT. Fleece-free EBW at the end of the adaptation period was calculated by multiplying initial FFLWT times the average ratio of FFEBW/FFLWT from lambs slaughtered at the end of the adaptation period (0.86).
The fat and protein content of the body at the end of the adaptation period were calculated by multiplying initial FFEBW by the average ratios of tissue pools in the body (viscera and NVEB, %FFEBW) as measured at slaughter, and the respective fat and protein percentages in each tissue pool. This approach was used to estimate fat and protein at the end of the preliminary period, using separate ratios for each nutritional history (HI or LO). Energy content of gain was calculated by dividing the change in retained energy (RE, MJ/d) in the FFEB by FFEB gain (kg/d) during each period, using 39.6 MJ/kg fat and 23.8 MJ/kg protein (Oddy et al. 2019).
Nitrogen retention was calculated as follows:
| (1) |
where NRetained is N retained (% of N consumed), NADG is N deposited in FFEB gain (g/d) as calculated from measurements at slaughter, NWool is N deposited in the clean fleece (g/d), NIntk is total N intake (g/d), and any N not retained in wool or the FFEB was assumed to be excreted. Percent N retained in the FFEB was calculated as daily accretion of N (g CP/6.25) in the FFEB divided by daily N intake (g/d). The ratio of N retention to retained energy (g N/MJ energy retained) was calculated from N retained in the NVEB and viscera (g/d) divided by total daily change in FFEB RE (MJ/d).
Statistical analysis
Data were analyzed in Minitab version 19.2020.1. Data from fifteen lambs were removed entirely from analysis of the final slaughter group due to missing values or gross outliers (>3 SD from mean). Specific data from a maximum of 11 lambs were omitted on a case-by-case basis within individual measurements; this was predominantly because of missing records. Data were split into two comparison groups for analysis: lambs slaughtered at the end of the adaptation period were compared with those slaughtered at the end of the preliminary period, and lambs slaughtered at the end of the experimental period were compared only to each other. Results for organs, carcass components, and chemical composition are presented as means of main effects (nutritional history, energy, and protein) within a comparison group (initial and end of preliminary, experimental period).
Daily records of DMI and MEI were averaged on a weekly basis during the experimental period (experimental period weeks 0 to 13). These average intakes along with weekly records of unshorn LWT and the ratio of DMI/LWT (within-week average daily DMI/weekly unshorn LWT) were analyzed using a mixed effects model procedure for repeated measures in Minitab. The potential mixed model included fixed effects of nutritional history (LO or HI), energy density (E1, E2, and E3), RUP level (0, 30, 60, and 90 g/d), week of experimental period (0 to 13), and their interactions between each other; individual lamb was included as a random effect.
Tissue, weight, composition, and average intake data were analyzed with a general linear model (GLM) approach within comparison groups. Initial liveweight (LWT0) was included as a covariate and nutritional history (initial kill, HI, and LO), energy density (E1, E2, and E3), and RUP level (0, 30, 60, and 90 g/d) as factors, with a stepwise selection process (α to enter or leave = 0.15) and Type III sums of squares. All potential interactions between factors were tested for, and AICc and BIC used as decision criteria. Levels of interaction or main effect were compared using a Bonferroni correction for multiple comparisons. Significance was declared at P < 0.05, and tendencies at 0.05 ≤ P < 0.10.
Results
Results (Tables 2–7) are presented as raw (unadjusted) means calculated from observed values. Due to unbalanced sample sizes from missing data, SEM is reported as the average of SEM values as within measure, main effect, and comparison group, and are calculated from the raw data rather than from fitted/adjusted means. There were few significant (P < 0.05) interactions; results are therefore presented by main effect, and significant interactions, where present, are discussed in the text. Data in figures are reported as unadjusted means by treatment and timepoint, and data in tables are reported as unadjusted means by level of main effect within timepoint to maximize utility of data as inputs for future modeling efforts.
Table 2.
Average intake of dry matter (DMI), ME(MEI), CP, RDP, and RUP intake by treatment within period
| Period | Adaptation | Preliminary | Experimental | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item/Treatment1 | Initial | LO | HI | LO | HI | SEM2 | E1 | E2 | E3 | SEM2 | P0 | P30 | P60 | P90 | SEM2 |
| N | 8 | 8 | 8 | 45 | 46 | 34 | 29 | 28 | 27 | 23 | 20 | 21 | |||
| DMI, kg/d | 0.90a | 0.55b | 1.5c | 1.40d | 1.50e | 0.029 | 1.33f | 1.48g | 1.57g | 0.032 | 1.49 | 1.45 | 1.46 | 1.39 | 0.042 |
| MEI, MJ/d | 9.00a | 4.95b | 13.5c | 13.04d | 13.75e | 0.47 | 10.36f | 13.72g | 16.77h | 0.31 | 13.92 | 13.51 | 13.53 | 12.49 | 0.66 |
| CP, g/d | 136.8a | 83.6b | 228c | 235.3d | 240.1e | 8.77 | 189.8f | 237.5g | 297.2h | 7.2 | 205.1j | 227.2k | 259.3l | 272.1m | 11.2 |
| RDP Intake, g/d3 | 103.97a | 63.54b | 173.28c | 161.6d | 168.0e | 5.92 | 129.2f | 161.2g | 211.9h | 3.78 | 157.5j | 161.4jk | 173.4kl | 169.9l | 8.4 |
| RUP Intake, g/d3 | 32.83a | 20.06b | 54.72c | 73.65d | 72.77e | 3.72 | 60.55f | 76.33g | 85.34h | 4.17 | 47.58j | 65.81k | 85.83l | 102.2m | 2.87 |
LO, low level of feeding during preliminary period; HI, high level of feeding during preliminary period; E1, E2, and E3, ad libitum intake of feed with M/D of 7.8, 9.2, and 10.7 MJ/kg DM, respectively; P0, P30, P60, P90, supplemented with 0, 30, 60, and 90 g/d of RUP, respectively.
Average of values of standard error of the mean (SEM) for levels within each comparison.
Sum of intake from basal feed + supplement.
Unlike superscripts within timepoints and main effects differ (P < 0.05). Data shown are unadjusted means.
Table 7.
Effect of treatments on average daily retention of energy and nitrogen within each period
| Period | Preliminary | Experimental | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item/Treatment1 | LO | HI | SEM2 | LO | HI | SEM2 | E1 | E2 | E3 | SEM2 | P0 | P30 | P60 | P90 | SEM1 |
| n | 7 | 8 | 41 | 40 | 32 | 25 | 27 | 25 | 22 | 20 | 17 | ||||
| Retained Energy3 in | |||||||||||||||
| FFEB, MJ/d 4 | 0.11a | 3.01b | 0.14 | 3.18c | 2.66d | 0.21 | l.53e | 2.89f | 4.46g | 0.12 | 2.79h | 2.90hj | 3.32j | 2.70h | 0.30 |
| NVEB, MJ/d 5 | 0.05a | 2.24b | 0.11 | 2.36c | l.90d | 0.15 | 1.15e | 2.l0f | 3.26g | 0.10 | 1.99h | 2.12hj | 2.42j | 2.04h | 0.22 |
| Viscera, MJ/d6 | 0.03a | 0.77b | 0.07 | 0.78 | 0.74 | 0.07 | 0.37e | 0.75f | 1.19g | 0.04 | 0.76hj | 0.71h | 0.90j | 0.66h | 0.09 |
| Fleece7 | 0.12a | 0.20b | 0.00 | 0.14c | 0.15d | 0.01 | 0.13e | 0.14e | 0.16f | 0.01 | 0.14 | 0.15 | 0.14 | 0.16 | 0.01 |
| Viscera, % energy retained in FFEB 4 | −4.50 | 25.54 | 13.00 | 24.02c | 27.59d | 0.94 | 24.54 | 26.10 | 26.76 | 1.21 | 26.96 | 24.48 | 27.40 | 23.72 | 1.40 |
| Retained Energy in FFEB, % of MEI | 2.21a | 22.32b | 1.84 | 23.30c | 18.l 7d | 0.98 | 14.83e | 21.14f | 26.87g | 0.87 | 19.04h | 20.62hj | 23.32j | 20.63hj | 1.48 |
| Energy density of gain, MJ RE/kg FFEB gain | −3.88a | 19.78b | 2.76 | 14.95c | 17.69d | 0.59 | 13.64e | 17.48f | 18.11f | 0.67 | 15.75h | 16.47hj | 18.14j | 14.56h | 0.85 |
| g N retained per MJ of energy retained in FFEB, g/MJ | −2.48 | 0.91 | 0.901 | 1.26c | 1.06d | 0.08 | 1.45e | l.07f | 0.94f | 0.08 | 1.17hj | 1.13hj | 0.98j | 1.40h | 0.11 |
| Nitrogen retained in the FFEB, % of Nitrogen Intake8 | −3.55a | 7.29b | 1.11 | 9.52c | 6.21d | 0.31 | 7.01e | 7.89ef | 8.87f | 0.48 | 8.60h | 8.35h | 6.88j | 7.50j | 0.54 |
LO, low level of feeding during preliminary period; HI, high level of feeding during preliminary period; E1, E2, and E3, ad libitum intake of feed with M/D of 7.8, 9.2, and 10.7 MJ/kg DM, respectively.
Average of values of standard error of the mean (SEM) for levels within each comparison.
Retained energy (RE), calculated from the energy content of protein (23.8 kJ/g) and fat (39.6 kJ/g).
FFEBW, fleece-free empty body.
NVEB, non-visceral empty body, defined as the sum of the weights of the carcass, blood, wool-free skin, head, and feet.
Viscera: sum of liver, kidneys, pluck (heart and lungs), the empty gut, gallbladder, spleen, and pancreas, including dissectible internal fat (sum of kidney and omental fat).
Assumes N content of clean wool is 16%, and a ratio of clean wool/greasy wool of 0.7 g/g, and energetic content of wool protein 23.8 MJ/kg protein.
Nitrogen retained in FFEB divided by total N intake.
Unlike superscripts within timepoints and main effects differ (P<0.05). Data shown are unadjusted means reported by kill and main effect of nutritional history or energy (effect of RUP not shown, no significant effects, P > 0.05.
Intake
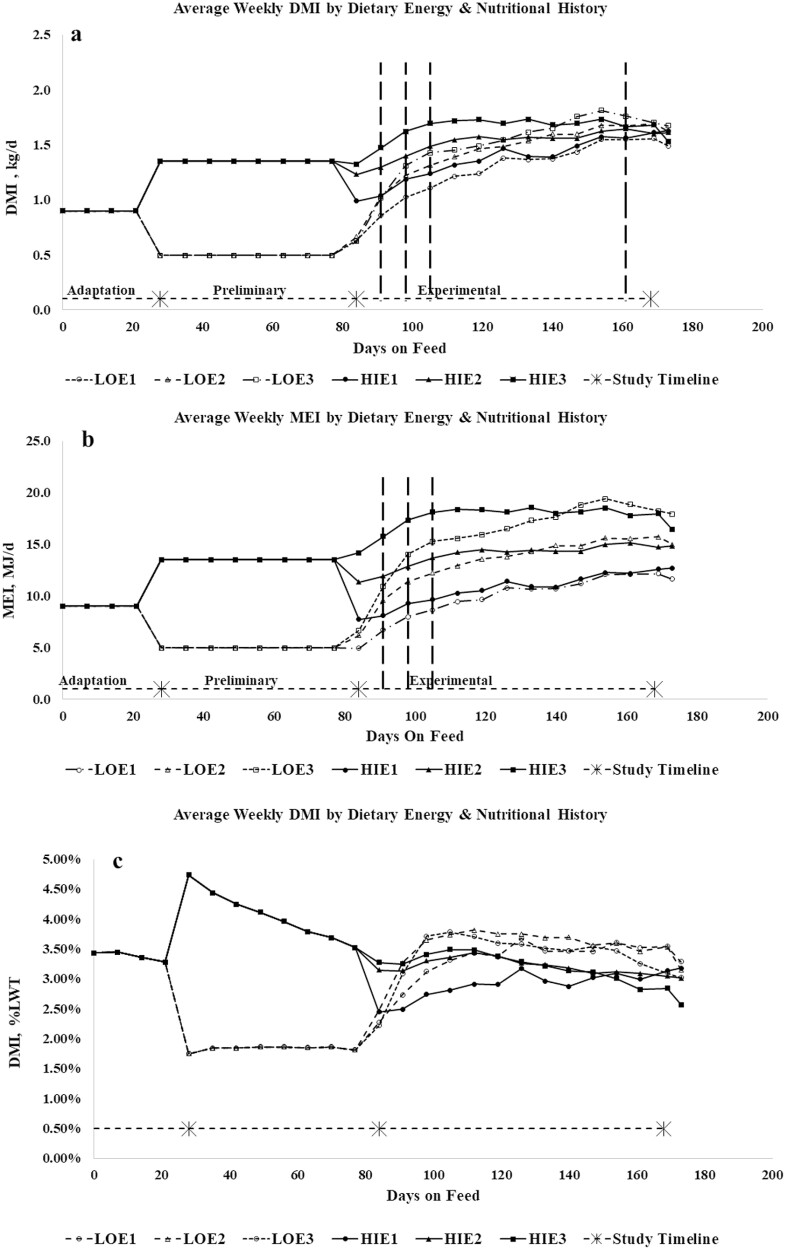
When intake was analyzed on a weekly basis (DMI, MEI, and DMI %LWT), there were significant interactions for week x nutritional history x dietary energy, energy x week, and nutritional history x week. There were no other significant interactions for intake.
During the experimental period, LO lambs had lower average DMI, MEI, and consumed less CP, RUP, and RDP (Table 2). However, differences between LO and HI lambs were transient: DMI (g/d) and MEI (MJ/d) of LO lambs increased rapidly at the start of the experimental period (Figure 2a and b). By week 3 of the experimental period, DMI and MEI of LO lambs did not differ from that of HI lambs fed the same level of dietary energy (M/D) when expressed on a g/d or MJ/d basis, and by week 11, DMI did not differ between lambs of any group when expressed on a g/d basis (Figure 2a). However, significant differences in MEI between levels of M/D persisted until the end of the trial. Lambs that had been previously restricted (LO) consumed proportionally more feed than HI lambs (Figure 2c), irrespective of energy intake level, and by week 4 of the experimental period, DMI (%LWT) did not differ between lambs of the same nutritional history.
Figure 2.
Weekly average DMI (kg/d) (a), MEI (MJ/d) (b), and DMI as a percentage of liveweight (DMI %LWT) (c), and by nutritional history and energy intake during the entire study (RUP not shown). Vertical dashed lines indicate point at which intakes converged and there were no significant (P > 0.05) differences between lambs of differing nutritional histories sharing the same dietary energy density (i.e., LOE1 and HIE1).
Neither weekly nor average DMI (kg/d or %LWT) differed between lambs fed the E2 and E3 diets, but E3 lambs had higher MEI. Intake relative to LWT was higher in E2 than E1 lambs, and E1 lambs had the lowest intakes, regardless of how intake was expressed. There was no effect of RUP on either DMI or MEI, either weekly or when averaged over the experimental period (Table 2); however, average DMI and MEI were numerically lower in P90 lambs, but the difference was not statistically significant.
Liveweight and non-visceral empty body
Non-visceral empty body tissues (NVEB) of restricted (LO) lambs generally did not change mass during the preliminary period (Table 3), with the following exceptions: compared to the initial group of lambs, the fleece-free empty body (FFEBW) and skin of LO lambs decreased in mass, and LO lambs had lower FFEBW/LWT at slaughter. Unrestricted lambs had higher ADG, and higher FFEB and wool gain.
Table 3.
Effect of treatments on growth performance and weights of the non-visceral empty body (NVEB)
| Period | Adaptation | Preliminary | Experimental | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item/Treatment1 | Initial | LO | HI | SEM2 | LO | HI | SEM2 | E1 | E2 | E3 | SEM2 |
| n | 7 | 8 | 8 | 43 | 43 | 31 | 27 | 27 | |||
| Final Liveweight, kg | 28.99a | 27.95a | 40.66b | 0.91 | 47.48d | 53.02e | 0.74 | 47.10f | 49.11g | 55.35h | 0.82 |
| Fleece Weight, kg greasy | 0.49a | 0.99b | 1.51c | 0.05 | 1.89d | 2.17e | 0.05 | l.94f | 1.99f | 2.19g | 0.06 |
| FFEBW, kg3 | 24.01a | 21.76b | 32.83c | 0.65 | 39.33d | 44.19e | 0.82 | 37.07f | 41.16g | 48.10h | 0.72 |
| Average Daily LWT Gain, g/d4 | n/a | -3.57a | 208.0b | 8.57 | 242.20d | 173.62e | 8.12 | 165.15f | 198.90g | 267.98h | 8.95 |
| Average Daily Wool Growth, g/d greasy | n/a | 7.50a | 11.81b | 0.27 | 8.34d | 9.27e | 0.29 | 8.06f | 8.60f | 9.82g | 0.34 |
| Average Daily Gain in FFEB, g/d4 | n/a | -33.83a | 154.73b | 5.80 | 206.10d | 140.70e | 9.29 | 114.71f | 167.73g | 247.60h | 7.87 |
| FFEBW/LWT5 | 0.84a | 0.78b | 0.81ab | 0.01 | 0.83d | 0.84e | 0.01 | 0.79f | 0.84g | 0.87h | 0.004 |
| NVEB pool components | |||||||||||
| Hot Carcass, kg | 12.92a | 12.35a | 18.64b | 0.43 | 22.83d | 25.80e | 0.50 | 21.45f | 24.00g | 28.19h | 0.44 |
| Cold Carcass, kg | 12.63a | 11.96a | 18.21b | 0.41 | 22.36d | 25.32e | 0.50 | 21.00f | 23.52g | 27.68h | 0.44 |
| Blood, kg | 1.37a | 1.32a | 1.75b | 0.06 | 2.00d | 2.20e | 0.05 | 1.90f | 2.05g | 2.39h | 0.05 |
| Wool-Free Skin, kg | 1.89a | 1.40b | 2.29c | 0.08 | 1.82d | 2.06e | 0.07 | 1.78f | 1.81f | 2.28g | 0.08 |
| Head and Feet, kg | 2.62a | 2.61a | 3.01b | 0.05 | 3.28d | 3.40e | 0.04 | 3.25f | 3.30f | 3.49g | 0.04 |
| Total NVEB Pool Weight, kg6 | 18.52a | 17.29b | 25.25c | 0.47 | 29.54d | 32.98e | 0.60 | 27.93f | 30.84g | 35.84h | 0.53 |
LO, low level of feeding during preliminary period; HI, high level of feeding during preliminary period; E1, E2, and E3, ad libitum intake of feed with M/D of 7.8, 9.2, and 10.7 MJ/kg DM, respectively.
Average of values of standard error of the mean (SEM) for levels within each comparison.
FFEBW, fleece-free empty body weight (kg).
Average daily gain of liveweight, greasy wool, and FFEBW of animals slaughtered at the end of each period, including fleece shorn during the experimental period.
FFEBW/LWT defined as the ratio of the weight of fleece-free empty body to liveweight at slaughter.
NVEB defined as the sum of the weights of the carcass, blood, wool-free skin, head, and feet.
Unlike superscripts within timepoints and main effects differ (P < 0.05). Data shown are unadjusted means reported by kill and main effect of nutritional history or energy (effect of RUP not shown, no significant effects, P > 0.05).
During the experimental period, LO lambs had higher rates of gain of FFEB, NVEB, and wool, but were still lighter at slaughter than HI lambs. Slaughter weight and NVEB weights increased with increasing M/D, as did FFEBW gain and ADG (including weight of fleece shorn prior to slaughter). Lambs fed the greatest level of M/D (E3) diet grew more wool than E1 and E2 lambs, and had heavier fleeces, skin, heads, and feet. Gut fill decreased as M/D increased, and LO lambs had a lower FFEBW/LWT than HI lambs. There was also a significant interaction between nutritional history and M/D for lambs fed the E2 diet: FFEBW/LWT was lower in LO lambs fed E2 (LOE2) than HIE2 lambs, but this was not seen for other levels of M/D. Lambs that had heavier initial liveweights (LWT0) had lower rates of wool growth during the experimental period (data not shown). There was no effect of RUP on any of the measures presented in Table 3, nor were there any other significant interactions.
Viscera
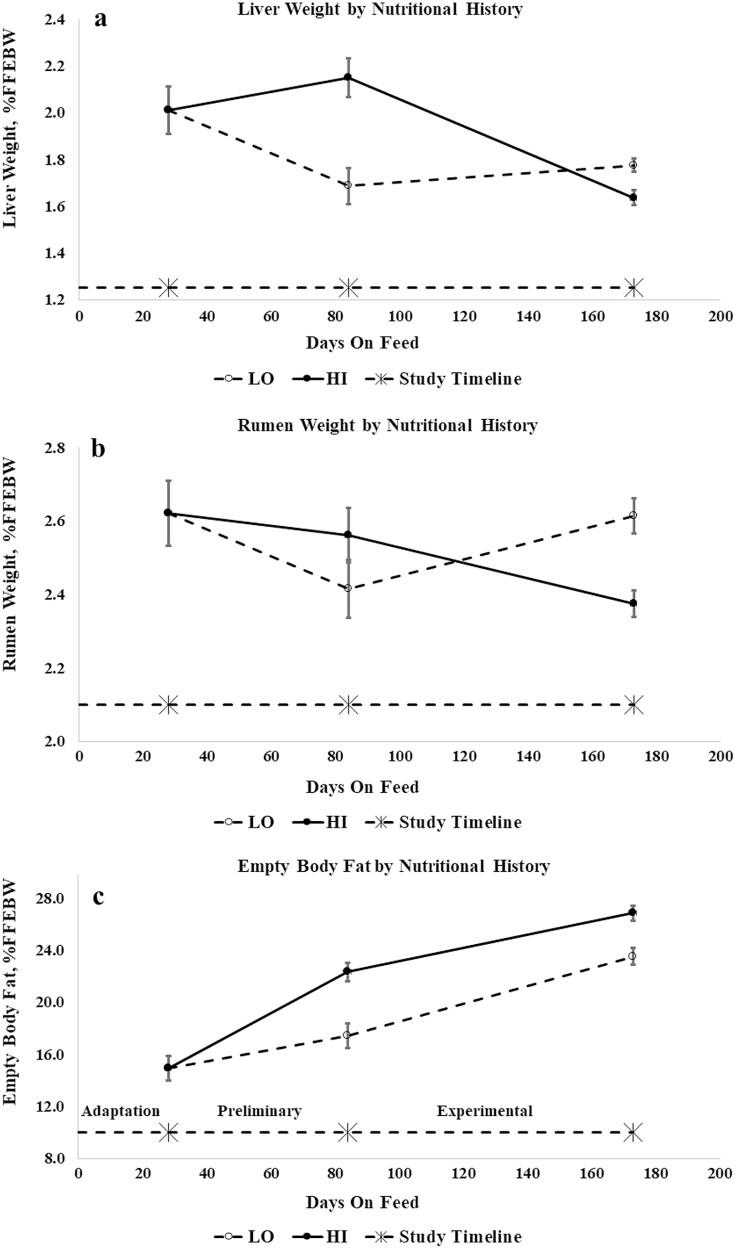
In contrast to the stasis experienced by the NVEB pool, nutritional restriction during the preliminary period was associated with losses in visceral mass (Table 4). Compared to the initial group of lambs, LO lambs lost weight in all visceral components except for the pluck, abomasum, and dissectible internal fat. While the gut tissue of LO lambs decreased in mass, there was no change in the mass of the gut contents. Unrestricted (HI) lambs had heavier gut tissue and total viscera than either initial or LO lambs, but the weight of the small and large intestines did not differ between HI and initial lambs. Lambs with a heavier LWT0 had heavier masses of the rumen, empty gut and contents at slaughter, and more internal fat. At the end of the preliminary period, LO lambs had proportionally smaller livers (%FFEBW, Figure 3a) and rumen contents were a higher proportion of LWT. The proportional size of the rumen did not change during the preliminary period, regardless of treatment (Figure 3b), but lambs with a heavier LWT0 had proportionally smaller livers and rumens.
Table 4.
Effect of treatments on weights of visceral organs, internal fat, and gut fill at the end of each period
| Period | Adaptation | Preliminary | Experimental | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item/Treatment1 | Initial | LO | HI | SEM2 | LO | HI | SEM2 | E1 | E2 | E3 | SEM2 |
| n | 7 | 7 | 8 | 44 | 44 | 33 | 28 | 27 | |||
| Pluck, g 3 | 757a | 762a | 931b | 43.00 | 927d | 991e | 17.05 | 880f | 960g | 1055h | 17.13 |
| Liver, g | 476a | 364b | 705c | 16.36 | 701d | 732e | 21.95 | 603f | 703g | 870h | 18.23 |
| Liver, %FFEBW4 | 2.01ab | 1.69a | 2.15b | 0.09 | 1.77d | l.64e | 0.03 | 1.62f | 1.71fg | 1.80g | 0.04 |
| Kidneys, g | 99a | 77b | 118c | 3.98 | 134 | 136 | 2.88 | 123f | 132g | 153h | 2.74 |
| Empty gut | |||||||||||
| Rumen, g | 625a | 524b | 839c | 18.13 | 1014 | 1046 | 17.7 | 1003f | 997f | 1098g | 20.7 |
| Rumen, %FFEBW4 | 2.62 | 2.42 | 2.56 | 0.08 | 2.61d | 2.38e | 0.04 | 2.71f | 2.44g | 2.28h | 0.05 |
| Omasum, g | 87a | 63b | 99a | 4.20 | 147 | 154 | 3.47 | 161f | 152f | 137g | 3.90 |
| Abomasum, g | 123a | 113a | 170b | 6.40 | 209 | 217 | 5.05 | 211 | 209 | 220 | 6.27 |
| Small Intestine, g | 603a | 399b | 638a | 23.93 | 748 | 753 | 15.00 | 717f | 743f | 799g | 17.53 |
| Large Intestine, g (including cecum) | 407a | 350b | 461a | 16.57 | 714d | 765e | 17.75 | 731 | 743 | 748 | 22.27 |
| Total Empty Gut, g | 1891a | 1448b | 2208c | 38.43 | 2831 | 2936 | 42.30 | 2823f | 2843fg | 3002g | 51.73 |
| Total Viscera, g5 | 3432a | 2780b | 4129c | 78.50 | 4891d | 5110e | 79.05 | 4664f | 4936g | 5478h | 78.93 |
| Dissectible Internal Fat, g6 |
716.2a | 644.1a | 1601b | 87.13 | 2428d | 3347e | 148.50 | 2044f | 2921g | 3878h | 146.5 |
| Gut Contents | |||||||||||
| Rumen Contents, g | 2796a | 3864ab | 4324b | 326.7 | 5199 | 5528 | 196.5 | 6461f | 5048g | 4396h | 176.33 |
| Rumen Contents, %LWT | 9.06a | 13.82b | 10.49a | 0.74 | 11.17d | 10.61e | 0.44 | 13.76f | 10.27g | 8.03h | 0.31 |
| Intestine Contents, g | 1060a | 942a | 1461b | 89.70 | 1870 | 1976 | 75.45 | 2223f | 1867g | 1606g | 79.97 |
| Total Gut Contents, g | 3996a | 5195ab | 6328b | 392.33 | 7679 | 8177 | 267.00 | 9355f | 7569g | 6557h | 242 |
LO, low level of feeding during preliminary period; HI, high level of feeding during preliminary period; E1, E2, and E3, ad libitum intake of feed with M/D of 7.8, 9.2, and 10.7 MJ/kg DM, respectively.
Average of values of standard error of the mean (SEM) for levels within each comparison.
Sum of weights of heart, lungs, and trachea.
FFEBW, fleece-free empty body weight.
Viscera defined as sum of liver, kidneys, pluck (heart and lungs), the empty gut, gallbladder, spleen, and pancreas not including dissectible internal fat;
Dissectible internal fat = sum of omental, mesenteric, and kidney fat.
Unlike superscripts within timepoints and main effects differ (P < 0.05). Data shown are unadjusted means reported by kill and main effect of nutritional history or energy (effect of RUP not shown, no significant effects, P > 0.05.
Figure 3.
Liver weight (a), rumen weight (b), and empty body fat (c) (%FFEBW) over time by nutritional history. Error bars represent SEM.
By the end of the experimental period, HI lambs had heavier total viscera and dissectible internal fat than LO lambs, and heavier livers. Weights of the total empty gut and its contents were similar between HI and LO lambs; only the large intestine was significantly heavier in HI lambs. However, LO lambs had proportionally heavier livers, rumens, and gut fill than HI lambs. Visceral mass increased with increasing M/D, as did dissectible internal fat. Mass of the empty gut did not differ between E1 and E2 lambs, but E1 lambs had heavier rumen, intestinal, and total gut contents than E2 and E3 lambs, and E2 lambs had heavier rumen, intestine, and total gut, but not intestinal contents, than E3 lambs. As M/D increased, the liver grew proportionally larger while the rumen decreased in proportion, as did its contents.
Lambs fed 60 g/d or 90 g/d of supplemental RUP had heavier kidneys than unsupplemented (P0) lambs (data not shown). Dissectible internal fat was heavier in P60 lambs than in P30 or P90 (data not shown), but unsupplemented lambs and supplemented lambs did not differ. Liver size increased in response to RUP supplementation (data not shown): P90 lambs had proportionally larger livers than P0 or P30 lambs (1.83% vs. 1.67% and 1.64%). There were no other effects of RUP on the viscera, nor were there any significant interactions between main effects.
Body composition
During the preliminary period, LO lambs lost empty body protein (Table 5), but deposited protein in wool such that whole-body nitrogen balance was positive, though much less than that of HI lambs. Despite losses of FFEB protein, the protein content of FFEB, NVEB, and viscera in LO lambs did not change during nutritional restriction and was higher than that of HI lambs. Protein content of the fat-free viscera increased during the preliminary period, but the fat-free NVEB was unaffected.
Table 5.
Effect of treatments on rates of protein gain and proportions of chemically determined crude protein at the end of each period
| Period | Adaptation | Preliminary | Experimental | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item/Treatment1 | Initial | LO | HI | SEM2 | LO | HI | SEM2 | E1 | E2 | E3 | SEM2 |
| n | 7 | 7 | 8 | 41 | 42 | 30 | 25 | 27 | |||
| Crude Protein gain in | |||||||||||
| FFEB, g/d3 | n/a | −2.97a | 16.61b | 1.43 | 22.84d | 15.08e | 1.11 | 13.02f | 18.74g | 25.80h | 1.20 |
| NVEB, g/d4 | n/a | −l.94a | 14.28b | 1.25 | 18.69d | 12.74e | 0.95 | 10.91f | 15.42g | 21.45h | 1.01 |
| Viscera, g/d5 | n/a | −1.19a | 2.33b | 0.36 | 3.95d | 2.31e | 0.19 | 2.09f | 3.07g | 4.35h | 0.22 |
| Wool, g/d6 | n/a | 5.25a | 8.27b | 0.19 | 5.84d | 6.49e | 0.21 | 5.64f | 6.02f | 6.88g | 0.24 |
| Protein Deposited in Viscera, % of Total FFEB Protein Gain | n/a | 31.40 | 14.27 | 9.11 | 17.82d | 15.28e | 0.70 | 15.89 | 16.37 | 17.51 | 0.90 |
| Crude Protein in | |||||||||||
| FFEB, %3 | 15.47a | 15.99a | 14.22b | 0.22 | 13.82d | 13.26e | 0.14 | 14.05f | 13.52g | 12.97h | 0.16 |
| NVEB, %4 | 17.46a | 17.61a | 15.93b | 0.25 | 15.82d | 15.34e | 0.14 | 16.14f | 15.57g | 14.90h | 0.15 |
| Viscera, %5 | 12.45ab | 12.83a | 11.28b | 0.32 | 10.65d | 9.91e | 0.13 | 10.85f | 10.10g | 9.78g | 0.16 |
| Fat-Free FFEB, %3,7 | 18.18a | 19.38b | 18.31a | 0.20 | 18.04 | 18.14 | 0.13 | 17.99 | 18.17 | 18.14 | 0.16 |
| Fat-Free NVEB, % | 20.68 | 21.20 | 20.42 | 0.25 | 20.67 | 20.87 | 0.12 | 20.67 | 20.88 | 20.79 | 0.15 |
| Fat-Free Viscera, %5,7 | 15.35a | 17.03b | 16.42b | 0.25 | 15.38d | 15.81e | 0.15 | 15.34f | 15.43f | 16.05g | 0.18 |
LO, low level of feeding during preliminary period; HI, high level of feeding during preliminary period; E1, E2, and E3, ad libitum intake of feed with M/D of 7.8, 9.2, and 10.7 MJ/kg DM, respectively.
Average of values of standard error of the mean (SEM) for levels within each comparison.
FFEBW, fleece-free empty body.
NVEB, non-visceral empty body, defined as the sum of the weights of the carcass, blood, wool-free skin, head, and feet.
Viscera: sum of liver, kidneys, pluck (heart and lungs), the empty gut, gallbladder, spleen, and pancreas, including dissectible internal fat (sum of kidney and omental fat).
Assuming protein content of clean wool is 100%, and a ratio of clean wool/greasy wool of 0.7 g/g.
Calculated as grams of crude protein divided by total tissue pool weight minus the weight of fat in the tissue pool.
Unlike superscripts within timepoints and main effects differ (P<0.05). Data shown are unadjusted means reported by kill and main effect of nutritional history or energy (effect of RUP not shown, no significant effects, P > 0.05.
In the experimental period, LO lambs had higher rates of protein gain in all FFEB, NVEB, viscera and wool, and deposited a higher proportion of total protein gain in the viscera. Tissue protein content was higher in LO lambs, but when expressed on a fat-free basis was similar to HI lambs. Protein content of the fat-free FFEB and NVEB during was unaffected by treatment during the experimental period. Rates of protein accretion rose as M/D increased, but the proportion of protein in the FFEB, NVEB, and viscera declined. While there was no effect of RUP on visceral protein content, protein content of the fat-free viscera was higher in P60 lambs than P90 (16.18% vs. 15.34% and 15.25%, data not shown). There were no other effects of RUP on protein gain or content, nor were there significant interactions between main effects.
Lambs restricted during the preliminary period (LO) gained fat and mobilized body protein (Tables 5 and 6). While LO lambs did deposit fat in all body pools during the preliminary period, overall fat gain was low and the proportion of fat in the FFEB at final slaughter did not differ between LO lambs and the initial group of lambs slaughtered at the beginning of the experiment.
Table 6.
Effect of treatments on rates of fat gain and proportions of chemically determined fat at the end of each period
| Period | Adaptation | Preliminary | Experimental | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item/Treatment1 | Initial | LO | HI | SEM2 | LO | HI | SEM2 | E1 | E2 | E3 | SEM2 | P0 | P30 | P60 | P90 | SEM1 |
| n | 7 | 7 | 8 | 42 | 44 | 30 | 27 | 27 | 26 | 21 | 19 | 17 | ||||
| Fat gain in | ||||||||||||||||
| FFEB, g/d 3 | n/a | 4.54a | 66.11b | 3.84 | 66.56d | 57.54e | 4.8 | 30.73f | 60.65g | 97.00h | 2.93 | 58.29j | 61.62jk | 72.87k | 55.82j | 6.79 |
| NVEB, g/d 4 | n/a | 2.36a | 47.99b | 3.01 | 48.32d | 40.43e | 3.46 | 22.37f | 43.78g | 69.47h | 2.29 | 41.24j | 43.96jk | 52.11k | 41.05j | 4.95 |
| Viscera, g/d 5 | n/a | 1.50a | 18.11b | 1.95 | 17.41 | 17.51 | 1.55 | 8.03f | 17.49g | 27.53h | 1.09 | 17.71jk | 16.22j | 20.76k | 14.78j | 2.15 |
| Fat Deposited in Viscera, % of Total Fat Gain | n/a | 3.80 | 27.29 | 9.42 | 25.52d | 30.69e | 1.14 | 27.25 | 28.78 | 28.29 | 1.49 | 30.26 | 26.45 | 29.13 | 25.59 | 1.70 |
| RE Deposited as Fat, % of Total RE6 | n/a | 136.90 | 86.51 | 13.38 | 81.22d | 84.26e | 1.13 | 78.40f | 84.12g | 86.00g | 1.18 | 82.54 | 83.20 | 85.39 | 79.14 | 1.56 |
| Fat in | ||||||||||||||||
| FFEB, % 3 | 14.91a | 17.45a | 22.36b | 0.86 | 23.53d | 26.89e | 0.60 | 21.87f | 25.88g | 28.50h | 0.62 | 25.04j | 25.49j | 27.20k | 23.06j | 0.88 |
| NVEB, %4 | 15.56a | 16.97a | 21.95b | 0.81 | 23.46d | 26.49e | 0.57 | 21.89f | 25.46g | 28.35h | 0.58 | 24.97j | 25.22jk | 27.01k | 23.03j | 0.85 |
| Viscera, %5 | 18.88a | 24.57ab | 31.25b | 2.03 | 30.63d | 37.32e | 0.92 | 29.18f | 34.79g | 38.93h | 1.05 | 34.21jk | 34.56j | 36.95k | 30.40j | 1.40 |
LO, low level of feeding during preliminary period; HI, high level of feeding during preliminary period; E1, E2, and E3, ad libitum intake of feed with M/D of 7.8, 9.2, and 10.7 MJ/kg DM, respectively.
Average of values of standard error of the mean (SEM) for levels within each comparison.
FFEB, fleece-free empty body.
NVEB, non-visceral empty body, defined as the sum of the weights of the carcass, blood, wool-free skin, head, and feet.
Viscera: sum of liver, kidneys, pluck (heart and lungs), the empty gut, gallbladder, spleen, and pancreas, including dissectible internal fat (sum of kidney and omental fat).
RE, retained energy (MJ).
Unlike superscripts within timepoints and main effects differ (P<0.05). Data shown are unadjusted means reported by kill and main effect of nutritional history or energy (effect of RUP not shown, no significant effects, P > 0.05.
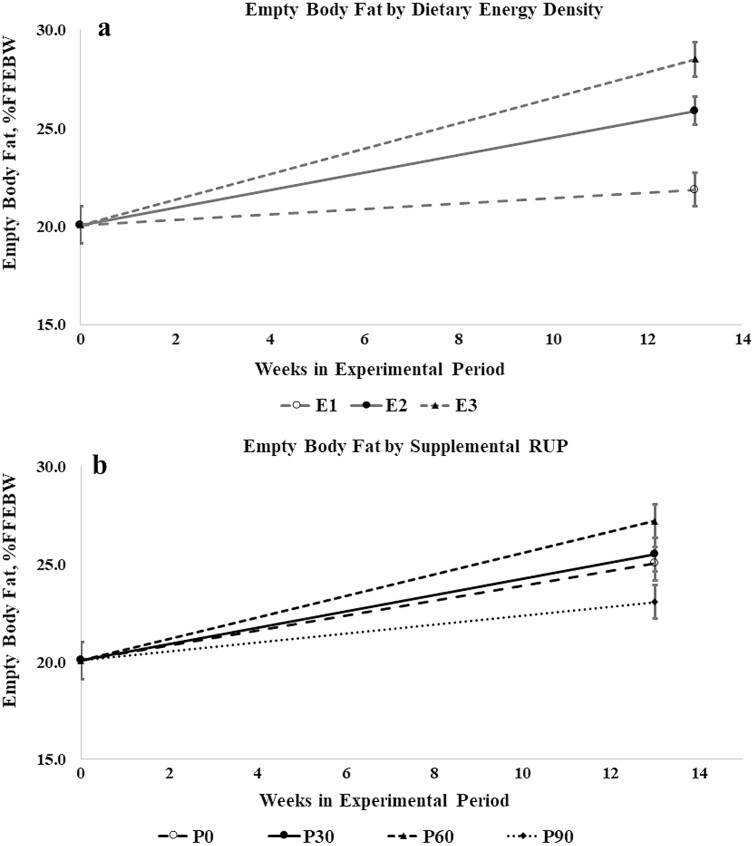
During the experimental period, LO lambs deposited more fat in the FFEB and NVEB than HI lambs, but deposited a lower proportion of retained energy (RE) as fat and were leaner at slaughter (Figure 3c). Nutritional history did not affect rates of visceral fat gain during the experimental period, but HI lambs deposited a greater proportion of fat gain in the viscera. Rates of fat deposition increased with increasing M/D, as did tissue fat content and the proportion of retained energy deposited as fat (Figure 4a). Fat deposition rates and fatness at slaughter (Figure 4b) responded curvilinearly to RUP supplementation, increasing as RUP increased and peaking at the P60 level of supplementation before declining in lambs from the P90 treatment. Lambs fed the P60 diet were fattest at slaughter, while P90 lambs had the same fat content as lambs receiving no supplemental RUP.
Figure 4.
Empty body fat (%FFEBW) over time by (a) dietary energy density and (b) supplemental RUP. Error bars represent SEM.
Energy and nitrogen retention
During the preliminary period, LO lambs lost protein and gained fat in the FFEB, with no change in total RE (Table 7). In contrast, HI lambs retained more energy than LO lambs in the FFEB, NVEB, viscera and wool, both in terms of MJ/d and as a proportion of MEI. Restricted and unrestricted lambs tended to differ in the ratio of N:RE retained during the preliminary period: LO lambs lost 2.5 g of N per MJ of RE while HI lambs deposited 1 g N/ MJ RE though inter-animal variation was high in LO lambs.
During the experimental period, LO lambs retained more energy in the FFEB, NVEB, and wool than HI lambs, but the proportion of fat gain and therefore energy density of gain was lower. There were no differences in the amount of energy retained in the viscera, but LO lambs deposited a higher proportion of RE in the viscera and retained higher proportions of MEI and N in the FFEB. The ratio of retained N:RE was higher in LO lambs. Tissue RE increased with increasing M/D, as did the proportion of MEI retained. Lambs fed the lowest energy diet retained more nitrogen per MJ of RE than E2 and E3 lambs, and had lower tissue energy density. Nitrogen retention was lower in lambs with heavier initial LWT0.
The effects of supplemental RUP on energy retention were similar to those on fat. Energy retention and tissue energy density were higher in P60 lambs than P0 and P90 lambs, and P60 lambs retained a higher proportion of MEI than P0 lambs. However, P90 lambs had a higher retained N:RE ratio than P60 lambs. In general, the proportion of N retained in the FFEB increased as M/D increased, and decreased with increasing RUP, but there was a significant interaction between M/D and RUP (data not shown) seen at the extremes of each main effect. For E3 lambs, lambs receiving no supplement (E3P0) retained more N than E3 lambs receiving the P90 level (10.36% vs. 6.79%). Within lambs receiving no supplemental RUP, E1P0 lambs retained less N than E3P0 (6.68% vs. 10.36%). There were no other significant differences within common levels of M/D or supplemental RUP. There was no effect of M/D or RUP on the proportion of RE deposited in viscera, nor were there other significant interactions between main effects.
Discussion
Effect of nutritional history
The objective of the preliminary period was to create lambs of different weights at the same age, allowing for comparison of the effects of differences in growth rate without potential confounding due to differences in age. Although the exact effects depend on both the timing and severity of restriction, nutritional restriction generates animals that are lighter at the same age, and therefore physiologically less mature, with the protein deposition potential of a younger animal (Eisemann et al., 1996; Oddy et al., 1997b; Hegarty et al., 1999; Oddy and Sainz 2002, Greenwood et al., 2005).
Although the growth of LO lambs was effectively paused on a whole-body level, individual tissues were not static. The majority of NVEB tissues did not change in weight, but the FFEB, NVEB, and viscera lost protein and gained fat, while wool growth continued. The overall effect was such that while retained energy was close to zero, rates of fat and protein gain were moving in opposite directions. Severe restriction leads animals to mobilise both protein and fat (Kabbali et al., 1992; Hornick et al., 2000), but the effects of feeding levels at or just below maintenance are less clear: animals may mobilise fat and spare or increase protein if the diet contains sufficient protein (Fattet et al., 1984), or may lose protein and gain fat (Aziz et al., 1992; Yambayamba et al., 1996). The results seen here agree with other work regarding ruminants at stasis (Graham and Searle, 1975a, b; Notter et al., 1983; Murray and Slezacek, 1988a, b; Yambayamba et al, 1996; Ball et al., 1997), but the exact point at which fat is deposited at the expense of protein, or vice versa, is poorly understood.
Visceral size and heat production are highly sensitive to changes in nutrition, reaching a new steady-state within 25 to 42 d after a change in diet (Ferrell et al., 1986; Burrin et al., 1990; Freetly et al., 1995; Sainz et al., 1995). One of the first responses to refeeding is an increase in intake (either in total or relative to liveweight), leading to a period where, although intake has increased, visceral size and basal heat production have not yet recovered to pre-restriction levels (Carstens et al., 1991; Ryan et al, 1993a, b; Keogh et al., 2015). In the present study, though LO lambs had lower average intake than HI lambs, differences in intake were transient and present only in the first few weeks. The subsequent increase in intake relative to both LWT and basal energy requirements indicated LO lambs were on a higher feeding level than HI lambs, as evidenced by higher energy retention and liveweight gain of LO lambs during the experimental period.
The increased rate of weight gain experienced by LO lambs due to increased relative intake was manifested in tissue gain with proportionately more protein, and water, than fat than HI lambs (Carstens et al., 1991; Ryan et al., 1993b; Hegarty et al., 1999). While LO lambs lost visceral protein during restriction, it was rapidly replenished during refeeding, accounting for the higher proportion of protein gain deposited in viscera. When growth is uninterrupted and not limited by intake, viscera grows and matures faster than the rest of the body (Butterfield 1988). During recovery, the viscera of LO lambs recovered tissue losses and then continued to grow in response to increased intake (Ryan et al., 1993a,b; Keogh et al., 2015). The net effect of this was such that LO lambs recovered visceral tissue mass to a disproportionate extent, consistent with the observed increase in DMI, and leading to similar masses of gut and gut contents between LO and HI lambs, but with a lower proportion of visceral fat and at a lighter LWT in LO lambs than HI.
Effect of dietary energy density
The effect of increasing M/D and therefore MEI was to increase visceral mass, but visceral organ growth relative to that of the empty body differed between organs: the liver increased in size relative to EBW and the rumen decreased relative to EBW. The liver is sensitive to energy and N intake, but is unaffected by physical form of the diet Reynolds et al., 1991a, b; (Sainz and Bentley, 1997; Lapierre et al., 2000; Dougherty et al., 2021). Conversely, the size of the rumen is sensitive to physical characteristics of feed. At similar DMI or MEI, the differences in retained energy between forage-fed and concentrate-fed animals can be accounted for by the effects of physical characteristics of the feed on differences in visceral mass and therefore heat production (Rompala et al., 1988, 1990; Oddy et al., 1997b; McLeod and Baldwin, 2000). Although the lambs in the present study converged in intake over time, lambs fed the higher-concentrate E3 diet had higher MEI, proportionally smaller rumens, less gut fill, and retained more energy, as also observed by Sainz et al. (1995), Oddy et al. (1997a), Sainz and Bentley (1997), and McLeod and Baldwin (2000).
Some of the differences in the responses of the rumen and liver may be due to physical differences between diets. Altering the ratio of wheat straw/lucerne hay/oat grain to create the different diets increased both dietary M/D and CP, and altered the ratios of forage: concentrate and of lucerne hay:wheat straw, changing the bulk effect of the diet and the relative quality of the forage fraction, as observed by Rompala et al. (1990) and Allen et al. (1996, 2019). While the feed used in the present study was ground and pelleted, and therefore had a lower bulk effect than if chopped forage had been fed (Allen 1996), the shift in composition of ingredients may have been sufficient to create changes in gut fill and in turn the proportion of rumen size and tissue weight to a greater extent than expected by change in M/D alone.
Differences in physical effect of diet on visceral mass are anticipated to lead to concomitant differences in visceral heat production, which, because of the relatively high rate of energy expenditure of viscera, affects energy available for retention in fat and protein in the fleece-free empty body(Sainz and Bentley, 1997; Oddy et al., 1997a). Visceral mass increases in response to increased MEI, but visceral mass will also increase in response to decreases in diet quality (M/D); the net effect of either of these responses is an increase in visceral heat production (Ferrell et al, 1988; Oddy et al, 2019). In the present study, the incremental increase in MEI exceeded the incremental increase in visceral heat production from increased MEI, as demonstrated by the proportion of MEI retained in tissue rising from 14.8% to 26.9% as M/D increased, primarily due to an increase in the percentage of RE deposited as fat. The proportion of total fat gain deposited in visceral tissues was unaffected by M/D and did not differ between treatments. Increased MEI would be expected to increase RE and fatness in general (Hegarty et al. 1999). Here, the effects of varying diet type and quality to achieve differences in M/D led to differences in each diet’s effects on visceral size, heat production, and RE. These effects led to a cascade of changes within the body of the lambs used in the present study, all with their own lag phases and time scales of response, as the effects of visceral size and heat production on growth rate and body composition in turn altered relative feeding level even at similar DMI (g/d), was well as the proportional size of the viscera, which in turn affected visceral heat production, and therefore the proportion of MEI remaining for deposition as RE and the ratio of fat to protein in RE. Therefore, by altering diet quality and M/D, which in turn affected ad-libitum DMI and MEI, the effects of these changes on visceral size, RE, and body composition are different from what would be seen simply from changing the MEI or relative feeding level without changing the composition of the diet itself (Hegarty et al., 1999).
Effect of supplemental RUP
Dietary RUP can be deposited as tissue protein or catabolized for energy or as a glucose precursor (Egan, 1970; Hunter and Siebert, 1987; Oddy et al., 1997b). It has also been suggested as an indirect source of microbial amino acids via urea recycling to the rumen (Hunter and Siebert, 1987; Oddy et al., 1997b; Archibeque et al., 2008; Atkinson et al., 2007, 2010a, 2010b). In the present study, where intake was not restricted during the refeeding phase, there was no specific effect of RUP on LO lambs, nor were there effects on intake or growth rates. The primary effects of RUP were instead on liver growth, nitrogen balance, and fatness. In the present study, fatness and liver size increased with increasing supplemental RUP, but lambs fed 60 g/d of RUP had significantly more fat than lambs fed 90 g/d or lambs receiving no supplement; results broadly consistent with those of Hegarty et al. (1999). However, RUP did not affect DMI or MEI, in contrast to Oddy et al. (1997b) who found that supplemental RUP increased intake in lambs fed low-quality diets but not in lambs fed higher-quality feed.
At any given weight and maturity, an increase in MEI allows for an increase potential protein deposition and N retention, as seen in the current study and elsewhere (Egan, 1970; Black and Griffiths, 1975; Ball et al., 1997; Oddy et al., 1997b; Hegarty et al., 1999). Unlike in Hegarty et al. (1999), the lambs in the present study were allowed ad libitum intake, and were able to consume sufficient N from the basal diet such that lambs were unable to retain the additional N from supplemental RUP, leading to a decrease in N retained relative to intake as RUP consumption increased.
Excess amino acids may be catabolized and used as an energy source, either directly or as glucose precursors, but which requires the disposal of excess N as urea, an energetically expensive process (Black et al., 1973; Ball et al., 1997; Oddy et al., 1997b). Liver size, energy usage, and ureagenesis are all sensitive to changes in MEI and N intake, and liver metabolism accounts for 20% to 25% of whole-body heat production (McBride and Kelly, 1990; Johnson et al., 1990; Ortigues and Durand, 1995; Ortigues and Doreau, 1995; Krehbiel et al., 2016; Dougherty et al., 2021). Similar to lambs in the fattening phase of Oddy et al. (1997b), it is possible that some of the surplus RUP was oxidized and contributed to both glucose supply and overall energy balance, although the inflection point seen in the present study was not observed by Oddy et al. (1997b). It is possible that at the greatest level of supplementation, the increased energetic demands of both a larger liver and of the disposal of excess N exceeded the potential increase in MEI from catabolised RUP, negating any benefits of the supplement. If lambs had been younger with more protein deposition potential, or if the basal diet had been limiting in RDP or total N, either in total or relative to protein deposition potential, it is possible that there would have been a beneficial effect of RUP on protein deposition or intake (Egan, 1970; Hunter and Siebert, 1987; Oddy et al., 1997b; Hegarty et al., 1999). The point where the energetic cost of removing excess protein exceeds the benefit obtained is likely dependent on the animal’s specific capacity for protein deposition, as determined by maturity and MEI, rather than N intake alone, as seen here and elsewhere (Black and Griffiths, 1975; Ball et al., 1997; Oddy et al. 1997a,b).
Implications and conclusions
Response to changes in nutrient intake and type of diet consumed involves a complex interplay of organs and their specific quantities of fat and protein. Each organ varies in its rate of change, energy use, and responses to hormones, nervous stimuli, and nutrient profiles in the cellular environment: all of which integrate at the animal level to determine current and future performance. The impacts of these underlying mechanisms are reflected in the effects of prior nutrition on the relative size of the viscera, final liveweight, and body composition at slaughter. This has implications for animals grazing pasture; when animals are coming off of low-quality pasture onto better feed, the effects of these diets on visceral size will play an important role in determining RE and the amount of fat and protein in the gain, as well as the amount of time and feed required to reach target weight and composition. It is now possible to include such effects as part of a dynamic, iterative approach to predicting growth and body composition (Oddy et al, 2019; Dougherty et al., 2021).
Tissues and organs vary in their response to the same stimuli, as seen in the differences in liver and rumen growth observed in the present study. These differences in liver growth and size in turn affected heat production by the liver and rumen, subsequently altering retained energy and body composition. Even at the same DMI, animals consuming lower M/D diets had lower MEI, but proportionally larger rumens, suggesting higher energy usage by gut tissue and a concomitant reduction in energy available for deposition in the rest of the body. Conversely, the liver grew in response to increases in nutrient supply, responding to both energy and protein. The change in liver size in response to RUP may partially explain a key finding of the present study: as RUP intake exceeded tissue deposition capacity, excess RUP may have been catabolized as an energy source. At lower levels, this was associated with an increase in fatness, but the greatest level of supplemental RUP, the incremental increases in liver size, heat production, and ureagenesis exceeded the incremental gains in energy from protein catabolism, effectively negating any potential benefits of the supplement.
Exploration and modeling of energy and protein metabolism is a key area for improving the understanding and prediction of animal metabolism and feed requirements. Specific modeling of visceral and non-visceral responses to nutrition and their ensuing impacts on heat production and body composition may lead to improvements in understanding of ruminant responses to change. Improved understanding of what determines these responses and how they vary is key to optimizing and predicting animal responses.
Acknowledgments
We acknowledge and thank the technical staff for their hard work and dedication, specifically Robyn Smith, Kris Riley, Christine Ewoldt, Helena Warren, and Allan Hendry. We also thank Dr. Ed Clayton, Dr. Linda Cafe, Dr. Ana Best, and Dr. Angela Lees for comments on a prior version of the manuscript. Financial support was provided by Meat and Livestock Australia Limited and New South Wales Department of Primary Industries.
Glossary
Abbreviations
- ADG
average daily gain;
- DMI
dry matter intake
- EBW
empty body weight
- FFEB
fleece-free empty body
- FFEBW
fleece-free empty body weight
- FFLWT
fleece-free liveweight
- LWT
liveweight
- M/D
energetic density of feed/diet (MJ ME/kg DM)
- MEI
metabolizable energy intake
- N
nitrogen
- NVEB
non-visceral empty body
- RDP
rumen degradable protein
- RUP
rumen undegradable protein
Contributor Information
Holland C Dougherty, Department of Animal Science, University of New England, Armidale, NSW 2351, Australia; NSW Department of Primary Industries, Livestock Industries Centre, University of New England, Armidale, NSW 2351, Australia.
Mark Evered, NSW Department of Primary Industries, Livestock Industries Centre, University of New England, Armidale, NSW 2351, Australia .
James W Oltjen, Department of Animal Science, University of California Davis, Davis, CA 95616, USA.
Roger S Hegarty, Department of Animal Science, University of New England, Armidale, NSW 2351, Australia.
Stephen A Neutze, Avalon Beach, NSW 2107, Australia.
V Hutton Oddy, NSW Department of Primary Industries, Livestock Industries Centre, University of New England, Armidale, NSW 2351, Australia .
Conflict of Interest Statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Allen, M. S. 1996. Physical constraints on voluntary intake of forages by ruminants. J. Anim. Sci. 74:3063–3075. doi: 10.2527/1996.74123063x [DOI] [PubMed] [Google Scholar]
- Allen, M. S., Sousa D. O., and VandeHaar M. J.. . 2019. Equation to predict feed intake response by lactating cows to factors related to the filling effect of rations. J. Dairy Sci. 102:7961–7969. doi: 10.3168/jds.2018-16166 [DOI] [PubMed] [Google Scholar]
- Archibeque, S. L., Freetly H. C., and Ferrell C. L.. . 2008. Feeding distillers grains supplements to improve amino acid nutriture of lambs consuming moderate-quality forages. J. Anim. Sci. 86:691–701. doi: 10.2527/jas.2007-0139 [DOI] [PubMed] [Google Scholar]
- Atkinson, R. L., Toone C. D., Robinson T. J., Harmon D. L., and Ludden P. A.. . 2007. Effects of supplemental ruminally degradable protein versus increasing amounts of supplemental ruminally undegradable protein on nitrogen retention, apparent digestibility, and nutrient flux across visceral tissues in lambs fed low-quality forage. J. Anim. Sci. 85:3331–3339. doi: 10.2527/jas.2006-417 [DOI] [PubMed] [Google Scholar]
- Atkinson, R. L., Toone C. D., and Ludden P. A.. . 2010b. Effects of ruminal protein degradability and frequency of supplementation on site and extent of digestion and ruminal fermentation characteristics in lambs fed low-quality forage. J. Anim. Sci. 88:718–726. doi: 10.2527/jas.2009-2245 [DOI] [PubMed] [Google Scholar]
- Atkinson, R. L., Toone C. D., Robinson T. J., Harmon D. L., and Ludden P. A.. . 2010a. Effects of ruminal protein degradability and frequency of supplementation on nitrogen retention, apparent digestibility, and nutrient flux across visceral tissues in lambs fed low-quality forage. J. Anim. Sci. 88:727–736. doi: 10.2527/jas.2009-2246 [DOI] [PubMed] [Google Scholar]
- Aziz, N. N., Murray D. M., and Ball R. O.. . 1992. The effect of live weight gain and live weight loss on body composition of merino wethers: dissected muscle, fat, and bone. J. Anim. Sci. 70:1819–1828. doi: 10.2527/1992.7061819x [DOI] [PubMed] [Google Scholar]
- Ball, A. J., Oddy V. H., and Thompson J. M.. . 1997. Nutritional manipulation of body composition and efficiency in ruminants. Rec. Adv. Anim. Nutr. Aust. 13:192–208. [Google Scholar]
- Black, J. L., and Griffiths D. A.. . 1975. Effects of live weight and energy intake on nitrogen balance and total N requirement of lambs. Br. J. Nutr. 33:399–413. doi: 10.1079/BJN19750044 [DOI] [PubMed] [Google Scholar]
- Black, J. L., Pearce G. R., and Tribe D. E.. . 1973. Protein requirements of growing lambs. Br. J. Nutr. 30:45–60. doi: 10.1079/BJN19730007 [DOI] [PubMed] [Google Scholar]
- Burrin, D. G., Ferrell C. L., and Bauer M.. . 1990. Level of nutrition and visceral organ size and metabolic activity in sheep. Br. J. Nutr. 64:439–438. doi: 10.1079/BJN19900044 [DOI] [PubMed] [Google Scholar]
- Butterfield, R. 1988. New concepts of sheep growth. The Department of Veterinary Anatomy, University of Sydney, Sydney, NSW, Australia. [Google Scholar]
- Carstens, G. E., Johnson D. E., Ellenberger M. A., and Tatum J. D.. . 1991. Physical and chemical components of the empty body during compensatory growth in beef steers. J. Anim. Sci. 69:3251–3264. doi: 10.2527/1991.6983251x [DOI] [PubMed] [Google Scholar]
- Dougherty, H. C., Evered M., Oltjen J. W., Hegarty R. S., and Oddy V. H.. . 2021. The effect of dietary energy density and supplemental RUP on visceral size & fat deposition in growing lambs. Proc. Rec. Adv. Anim. Nutr. 13:40–41. [Google Scholar]
- Eisemann, J. H., Huntington G. B., and Catherman D. R.. . 1996. Patterns of nutrient exchange and oxygen use among portal-drained viscera, liver, and hindquarters of beef steers from 235 to 525 kg body weight. J. Anim. Sci. 74:1812–1831. doi: 10.2527/1996.7481812x [DOI] [PubMed] [Google Scholar]
- Egan, A. R. 1970. Utilization by sheep of casein administered per duodenum at different levels of roughage intake. Aust. J. Agric. Res. 21:85–94. doi: 10.1071/AR9700085 [DOI] [Google Scholar]
- Fattet, I., Hovell F. D. DeB., Ørskov E. R., Kyle D. J., Pennie K., and Smart R. I.. . 1984. Undernutrition in sheep. The effect of supplementation with protein on protein accretion. Br. J. Nutr. 52:561–574. doi: 10.1079/BJN19840123 [DOI] [PubMed] [Google Scholar]
- Ferrell, C. L. 1988. Contribution of visceral organs to animal energy expenditures. J. Anim. Sci. 66:23–34. doi: 10.1093/ansci/66.Supplement_3.23 [DOI] [Google Scholar]
- Ferrell, C. J., Freetly H. C., Goetsch A. L., and Kreikemeier K. K.. . 2001. The effect of dietary nitrogen and protein on feed intake, nutrient digestibility, and nitrogen flux across the portal-drained viscera and liver of sheep consuming high-concentrate diets ad libitum. J. Anim. Sci. 79:1322–1328. doi: 10.2527/2001.7951322x [DOI] [PubMed] [Google Scholar]
- Ferrell, C. L., Koong L. J., and Nienaber J. A.. . 1986. Effects of previous nutrition on body composition and maintenance energy costs of growing lambs. Br. J. Nutr. 56:595–605. doi: 10.1079/BJN19860140 [DOI] [PubMed] [Google Scholar]
- Freetly, H. C., Ferrell C. L., Jenkins T. G., and Goetsch A. L.. . 1995. Visceral oxygen consumption during chronic feed restriction and realimentation in sheep. J. Anim. Sci. 73:843–852. doi: 10.2527/1995.733843x [DOI] [PubMed] [Google Scholar]
- Graham, N. McC., and Searle T. W.. . 1975a. Studies of weaner sheep during and after a period of weight stasis. I. Energy and nitrogen utilization. Aust. J. Agric. Res. 26:343–353. doi: 10.1071/AR9750343 [DOI] [Google Scholar]
- Graham, N. McC., and Searle T. W.. . 1975b. Studies of weaner sheep during and after a period of weight stasis. II. Body composition. Aust. J. Agric. Res. 26:355–361. doi: 10.1071/AR9750355 [DOI] [Google Scholar]
- Greenwood, P. L., Cafe L. M., Hearnshaw H., and Hennessy D. W.. . 2005. Consequences of nutrition and growth retardation early in life for growth and composition of cattle and eating quality of beef. Rec. Adv. Anim. Nutr. 15:183–195. https://www.researchgate.net/profile/Paul-Greenwood/publication/263167488_Consequences_of_nutrition_and_growth_retardation_early_in_life_for_growth_and_composition_of_cattle_and_eating_quality_of_beef/links/551a16130cf244e9a45853cf/Consequences-of-nutrition-and-growth-retardation-early-in-life-for-growth-and-composition-of-cattle-and-eating-quality-of-beef.pdf [Google Scholar]
- Hegarty, R. S., Neutze S. A., and Oddy V. H.. . 1999. Effects of protein and energy supply on the growth and carcass composition of lambs from differing nutritional histories. J. Agric. Sci. 132:361–375. doi: 10.1017/S0021859698006315 [DOI] [Google Scholar]
- Hemsley, J. A., Reis P. J., and Downes A. M.. . 1973. Influence of various formaldehyde treatments on the nutritional value of casein for wool growth. Aust. J. Biol. Sci. 26:961–972. doi: 10.1071/BI9730961 [DOI] [PubMed] [Google Scholar]
- Hornick, J. L., Van Eenaeme C., Gérard O., Dufrasne I., and Istasse L.. . 2000. Mechanisms of reduced and compensatory growth. Domest. Anim. Endocrinol. 19:121–132. doi: 10.1016/S0739-7240(00)00072-2 [DOI] [PubMed] [Google Scholar]
- Hunter, R. A., and Siebert B. D.. . 1987. The effect of supplements of rumen-degradable protein and formaldehyde-treated casein on the intake of low-nitrogen roughages by Bos taurus and Bos indicus steers at different stages of maturity. Aust. J. Agric. Res. 38:209–218. doi: 10.1071/AR9870209 [DOI] [Google Scholar]
- Johnson, D. E., Johnson K. A., and Baldwin R. L.. . 1990. Changes in liver and gastrointestinal tract energy demands in response to physiological workload in ruminants. J. Nutr. 120:649–655. doi: 10.1093/jn/120.6.649 [DOI] [PubMed] [Google Scholar]
- Kabbali, A., Johnson W. L., Johnson D. W., Goodrich R. D., and Allen C. E.. . 1992. Effects of undernutrition and refeeding on weights of body parts and chemical components of growing Moroccan lambs. J. Anim. Sci. 70:2859–2865. doi: 10.2527/1992.7092859x [DOI] [PubMed] [Google Scholar]
- Keogh, K., Waters S. M., Kelly A. K., and Kenny D. A.. . 2015. Feed restriction and subsequent realimentation in Holstein Friesian bulls. I. Effect on animal performance; muscle, fat, and linear body measurements; and slaughter characteristics. J. Anim. Sci. 93:3578–3589. doi: 10.2527/jas.2014-8470 [DOI] [PubMed] [Google Scholar]
- Krehbiel, C. R., Lopez R., and Hersom M. J.. . 2016. Net nutrient flux across the portal-drained viscera and liver in ruminants. In: Millen D., De Beni Arrigoni M., and Lauritano Pacheco R., editors, Rumenology. Springer, Cham. p. 243–263. doi: 10.1007/978-3-319-30533-2_9. [DOI] [Google Scholar]
- Lapierre, H., Bernier J. F., Dubreuil P., Reynolds C. K., Farmer C., Ouellet D. R., and Lobley G. E.. . 2000. The effect of feed intake level on splanchnic metabolism in growing beef steers. J. Anim. Sci. 78:1084–1099. doi: 10.2527/2000.7841084x [DOI] [PubMed] [Google Scholar]
- McBride, B. W., and Kelly J. M.. . 1990. Energy cost of absorption and metabolism in the ruminant gastrointestinal tract and liver: a review. J. Anim. Sci. 68:2997–3010. doi: 10.2527/1990.6892997x [DOI] [PubMed] [Google Scholar]
- McLeod, K. R., and Baldwin R. L. IV. 2000. Effects of diet forage:concentrate ratio and metabolizable energy intake on visceral organ growth and in vitro oxidative capacity of gut tissues in sheep. J. Anim. Sci. 78:760–770. doi: 10.2527/2000.783760x [DOI] [PubMed] [Google Scholar]
- Murray, D. M., and Slezacek O.. . 1988a. The effect of weight stasis on the dissected carcass composition of crossbred sheep. Aust. J. Agric. Res. 39:645–651. doi: 10.1071/AR9880645 [DOI] [Google Scholar]
- Murray, D. M., and Slezacek O.. . 1988b. The effect of weight stasis on the non-carcass components of crossbred sheep. Aust. J. Agric. Res. 39:653–658. doi: 10.1071/AR9880653 [DOI] [Google Scholar]
- Neutze, S. A., Smith R. L., and Forbes W. A.. . 1993. Application of an inhibitor in vitro method for estimating rumen degradation of feed protein. Anim. Feed Sci. Tech. 40:251–265. doi: 10.1016/0377-8401(93)90161-C [DOI] [Google Scholar]
- Notter, D. R., Ferrell C. L., and Field R. A.. . 1983. Effects of breed and intake level on allometric growth patterns in ram lambs. J. Anim. Sci. 56:380–395. doi: 10.2527/jas1983.562380x [DOI] [PubMed] [Google Scholar]
- Oddy, V. H. 1998. Is food intake pushed or pulled? In: Proceedings of the Beef Products Conference. NSW Agriculture, Armidale, NSW, Australia. [Google Scholar]
- Oddy, V. H., Ball A. J., and Pleasants A. B.. . 1997a. Understanding body composition and efficiency in ruminants: a non-linear approach. Rec. Adv. Anim. Nutr. Aust. 11:209–222. [Google Scholar]
- Oddy, V. H., Dougherty H. C., and Oltjen J. W.. . 2019. Integration of energy and protein transactions in the body to build new tools for predicting performance and body composition of ruminants. Anim. Prod. Sci. 59:1970–1979. doi: 10.1071/AN19229 [DOI] [Google Scholar]
- Oddy, V. H., Edwards S. R., Warren H. M., Speck P. A., Nichols P. J., and Neutze S. A.. . 1997b. Interrelationships between amino acid and glucose metabolism in lambs of different dietary history supplemented with rumen escape protein. J. Agric. Sci. (Camb.) 128:105–116. doi: 10.1017/S0021859696003917 [DOI] [Google Scholar]
- Oddy, V. H., Robards G. E., and Low S. G.. . 1983. Prediction of in vivo dry matter digestibility from the fibre and nitrogen content of a feed. In: Proceedings of the Second Symposium of the International Network of Feed Information Centers, Commonwealth Agricultural Bureaux. p. 35–398. [Google Scholar]
- Oddy, V. H., and Sainz R. D.. . 2002. Chapter 11: Nutrition for sheep-meat production. In: Freer M., and Dove H., editors, Sheep nutrition. CSIRO Publishing, Canberra, ACT, Australia. p. 237–262. [Google Scholar]
- Ortigues, I., and Doreau M.. . 1995. Responses of the splanchnic tissues of ruminants to changes in intake: absorption of digestion end products, tissue mass, metabolic activity and implications to whole energy metabolism. Ann. Zootech. 44:321–346. doi: 10.1051/animres:19950401 [DOI] [Google Scholar]
- Ortigues, I., and Durand D.. . 1995. Adaptation of energy metabolism to undernutrition in ewes. Contribution of portal-drained viscera, liver and hindquarters. Br. J. Nutr. 73:209–226. doi: 10.1079/BJN19950024 [DOI] [PubMed] [Google Scholar]
- Reynolds, C. K., Tyrell H. F., and Reynolds P. J.. . 1991a. Effects of diet forage-to-concentrate ratio and intake on energy metabolism in growing beef heifers: whole body energy and nitrogen balance and visceral heat production. J. Nutr. 121:994–1003. doi: 10.1093/jn/121.7.994 [DOI] [PubMed] [Google Scholar]
- Reynolds, C. K., Tyrell H. F., and Reynolds P. J.. . 1991b. Effects of diet forage-to-concentrate ratio and intake on energy metabolism in growing beef heifers: net nutrient metabolism by visceral tissues. J. Nutr. 121:1004–1015. doi: 10.1093/jn/121.7.1004 [DOI] [PubMed] [Google Scholar]
- Ryan, W. J., Williams I. H., and Moir R. J.. . 1993a. Compensatory growth in sheep and cattle. I. Growth pattern and feed intake. Aust. J. Agric. Res. 44:1609–1621. doi: 10.1071/AR9931609 [DOI] [Google Scholar]
- Ryan, W. J., Williams I. H., and Moir R. J.. . 1993b. Compensatory growth in sheep and cattle. II. Changes in body composition and tissue weights. Aust. J. Agric. Res. 44:1623–1633. doi: 10.1071/AR9931623 [DOI] [Google Scholar]
- Rompala, R. E., Hoagland T. A., and Meister J. A.. . 1988. Effect of dietary bulk on organ mass, fasting heat production, and metabolism of the small and large intestine in sheep. J. Nutr. 118:1553–1557. doi: 10.1093/jn/118.12.1553 [DOI] [PubMed] [Google Scholar]
- Rompala, R. E., Hoagland T. A., and Meister J. A.. . 1990. Modifications in growth and morphology of ovine jejunal and ruminal epithelia as affected by inert dietary substances. J. Anim. Sci. 68:2530–2535. doi: 10.2527/1990.6882530x [DOI] [PubMed] [Google Scholar]
- Sainz, R. D., and Bentley B. E.. . 1997. Visceral organ mass and cellularity in growth-restricted and refed beef steers. J. Anim. Sci. 75:1229–1236. doi: 10.2527/1997.7551229x [DOI] [PubMed] [Google Scholar]
- Sainz, R. D., De la Torre F., and Oltjen J. W.. . 1995. Compensatory growth and carcass quality in growth-restricted and refed beef steers. J. Anim. Sci. 73:2971–2979. doi: 10.2527/1995.73102971x [DOI] [PubMed] [Google Scholar]
- Wheeler, J. L., Hedges D. A., and Mulcahy C.. . 1977. The use of dyebanding for measuring wool production and fleece tip wear in rugged and unrugged sheep. Aust. J. Agric. Res. 28:721–735. doi: 10.1071/AR9770721 [DOI] [Google Scholar]
- Yambayamba, E. S. K., Price M. A., and Jones S. D. M.. . 1996. Compensatory growth of carcass tissues and visceral organs in beef heifers. Livest. Prod. Sci. 46:19–32. doi: 10.1016/0301-6226(96)00014-0 [DOI] [Google Scholar]