Abstract
We examined nine Aspergillus japonicus isolates and 10 Aspergillus aculeatus isolates by using molecular and biochemical markers, including DNA sequences of the ITS1-5.8S rRNA gene-ITS2 region, restriction fragment length polymorphisms (RFLP), and secondary-metabolite profiles. The DNA sequence of the internal transcribed spacers (ITS1 and ITS2) and the 5.8S rRNA gene could not be used to distinguish between A. japonicus and A. aculeatus but did show that these two taxa are more closely related to each other than to other species of black aspergilli. Aspergillus niger pyruvate kinase (pkiA) and pectin lyase A (pelA) and Agaricus bisporus 28S rRNA genes, which were used as probes in the RFLP analysis, revealed clear polymorphism between these two taxa. The A. niger pkiA and pelA probes placed six strains in an A. japonicus group and 12 isolates in an A. aculeatus group, which exhibited intraspecific variation when they were probed with the pelA gene. The secondary-metabolite profiles supported division of the isolates into the two species and differed from those of other black aspergilli. The strains classified as A. japonicus produced indole alkaloids and a polar metabolite, while the A. aculeatus isolates produced neoxaline, okaramins, paraherquamidelike compounds, and secalonic acid. A. aculeatus CBS 114.80 showed specific RFLP patterns for all loci examined. The secondary-metabolite profile of strain CBS 114.80 also differed from those of A. japonicus and A. aculeatus. Therefore, this strain probably represents a third taxon. This study provides unambiguous criteria for establishing the taxonomic positions of isolates of black aspergilli, which are important in relation to industrial use and legal protection of these organisms.
Representatives of the black aspergilli are commonly used by the fermentation industry to produce extracellular enzymes and metabolites such as citric acid. It is important to clearly establish the taxonomic positions of such Aspergillus isolates due to the use of their products in the food and feed industry and for legal protection purposes. Traditionally, morphological criteria like color, shape, size, and ornamentation of conidia have been used to classify such strains (1, 17, 23, 27, 31, 32).
However, the black aspergilli also vary significantly in their morphological and physiological characteristics. Therefore, unambiguous identification of an isolate requires molecular and biochemical identification techniques. The major taxonomic efforts with the black aspergilli have focused on the Aspergillus niger aggregate (20, 21, 26, 34–36). Using mitochondrial DNA (mtDNA) and nuclear DNA restriction fragment length polymorphisms (RFLPs) and randomly amplified polymorphic DNA patterns, workers have proposed that the strains of the A. niger aggregate (1) should be divided into four taxa: A. niger, Aspergillus tubingensis (20), Aspergillus brasiliensis (35), and Aspergillus foetidus (26). Similar studies have been carried out with isolates of Aspergillus carbonarius (15, 25) and with isolates of the uniseriate (i.e., metulae are not present) species, Aspergillus japonicus and Aspergillus aculeatus, as these species cannot be identified reliably on the basis of morphological features (9). A high degree of mtDNA polymorphism was found among the A. aculeatus and A. japonicus isolates, and this polymorphism correlated with randomly amplified polymorphic DNA patterns. Therefore, it also is difficult to use mtDNA polymorphisms to discriminate between these two taxa.
The objectives of this study were (i) to establish reliable methods for discriminating between A. japonicus and A. aculeatus isolates, (ii) to compare the results obtained with those obtained with well-characterized representatives of the other black aspergilli, and (iii) to provide guidelines for identification of all black aspergilli. We compared 19 A. japonicus and A. aculeatus strains by using three different approaches; we compared sequences of the ITS1-5.8S rRNA gene-ITS2 region, RFLPs of nuclear DNA, and secondary-metabolite profiles. A. aculeatus has been reported to produce the secondary metabolites emodin, secalonic acids D and F (2, 18), aculeasins (22, 28), and okaramins A, B, H, and I (13), while in A. japonicus festuclavine and cycloclavine (8), E-64 (10, 11), and neoxaline (14, 16) have been identified. By combining RFLP analysis of nuclear DNA, DNA sequencing, and secondary-metabolite profile analysis we could discriminate between isolates of these two taxa and improved the method by which strains of black aspergilli are identified. The accuracy obtained is important for characterizing and protecting industrial strains, for studying fungal biodiversity, and for providing a rationale for selecting fungal strains when screening for specific metabolites or novel enzymes.
MATERIALS AND METHODS
Strains and plasmids.
The strains used (Table 1) were obtained from the Centraalbureau voor Schimmelcultures, Baarn, The Netherlands. Plasmids carrying the pyruvate kinase-encoding pkiA gene of A. niger (pGW1100) (4), the pectin lyase A-encoding pelA gene of A. niger (pGW820) (12), a 0.9-kb EcoRI fragment of the 28S rRNA of Agaricus bisporus (pIM2131) (29), and the whole A. bisporus ribosomal DNA (rDNA) unit (pIM2132) (P. J. Schaap, unpublished results) were propagated in Escherichia coli DH5α (38).
TABLE 1.
Final grouping of the A. japonicus and A. aculeatus isolates after RFLP analysis of nuclear DNA and ITS1-5.8S rRNA gene-ITS2 sequencing
| Group | Strain | EMBL accession no.a | Pattern based on:
|
|||||
|---|---|---|---|---|---|---|---|---|
| rDNA (SmaI) | pkiA (KpnI-XhoI) | 26S rRNA (KpnI-XhoI) | 26S rRNA (PstI-SalI) | pelA (PstI-SalI) | ITS1-5.8S rRNA gene-ITS2 | |||
| A. japonicus | A. japonicus CBS 568.65 | AJ279991 | D | F | A | L | H | I |
| A. japonicus CBS 522.89 | AJ279990 | D | F | A | L | H | I | |
| A. japonicus CBS 101.14b | AJ279983 | D | F | A | M | H | I | |
| A. atroviolaceus CBS 113.48Tc | AJ279984 | D | F | A | M | H | I | |
| A. japonicus CBS 114.51T | AJ279985 | D | F | A | N | H | I | |
| A. aculeatus CBS 611.78 | AJ279992 | D | F | A | N | I | I | |
| A. aculeatus | A. japonicus CBS 114.34 | AJ279996 | D | G | B | I | L | II |
| A. lucknowensis CBS 119.49 | AJ279986 | D | H | B | I | M | I | |
| A. luchuensis CBS 116.80 | AJ279997 | D | G | B | P | L | II | |
| A. luchuensis CBS 101.43 | AJ279995 | D | H | B | I | M | II | |
| A. japonicus CBS 620.78 | AJ280002 | D | G | B | H | J | II | |
| A. japonicus CBS 312.80 | AJ279989 | D | I | B | F | K | I | |
| A. aculeatus CBS 172.66 | AJ279988 | D | J | B | H | N | I | |
| A. aculeatus CBS 610.78 | AJ280001 | D | G | B | G | O | II | |
| A. aculeatus CBS 186.67 | AJ279998 | D | G | B | J | P | II | |
| A. aculeatus CBS 308.80 | AJ279999 | D | G | B | K | Q | II | |
| A. aculeatus CBS 313.89 | AJ280000 | D | G | B | P | R | II | |
| A. bruneo-violaceus CBS 621.78T | AJ280003 | D | G | B | O | T | II | |
| ? | A. aculeatus CBS 114.80 | AJ280005 | D | K | C | Q | S | III |
| A. niger | A. niger CBS 120.49 | AJ280006 | B | B I | B | B | B | |
| A. tubingensis | A. tubingensis CBS 643.92 | AJ280008 | C | B II | A | C | D | |
| A. tubingensis | A. tubingensis CBS 127.49 | AJ280007 | C | NDd | A | C | D | |
| A. foetidus | A. foetidus var. acidus CBS 564.65T | AJ280009 | C | B II | A | D | C | |
| A. brasiliensis | A. brasiliensis IMI 381727 | AJ280010 | F | E | D | B | G | |
| A. carbonarius | A. carbonarius CBS 111.26Te | AJ280011 | A | A | C | A | A | |
| A. heteromorphus | A. heteromorphus CBS 117.55T | AJ280013 | E | D | A | E | F | |
| A. ellipticus | A. ellipticus CBS 707.79T | AJ280014 | E | C | A | E | E | |
EMBL accession number for the ITS1-5.8S rRNA gene-ITS2 region sequence.
Reclassified by Pařenicová et al. (25).
T = type strain.
ND, not determined.
Neotype strain.
Isolation of genomic DNA, digestion, and gel electrophoresis.
Genomic DNA isolation, digestion with the SmaI, XhoI-KpnI, and PstI-SalI restriction enzymes, and agarose gel electrophoresis were carried out essentially as described previously (26). Instead of 0.7% (wt/vol) agarose gels, a 0.8% (wt/vol) agarose gel was used.
Southern blotting and hybridization.
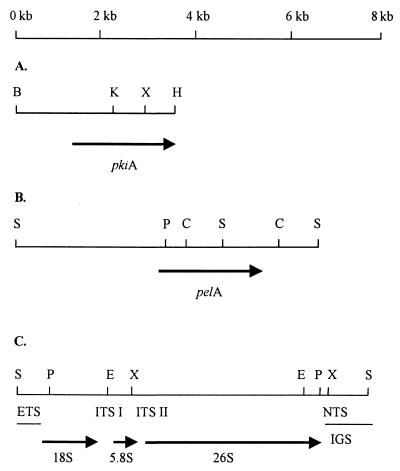
Transfer of DNA from agarose gels has been described previously (26). Radioactive labeling of the probes was carried out as described by Kusters-van Someren et al. (19). To identify DNA polymorphisms, the following probes were used for Southern blot hybridization: a 3.5-kb BamHI-HindIII fragment of the A. niger pkiA gene comprising the entire coding region and the 5′ and 3′ noncoding regions; a 10-kb BamHI fragment carrying the whole rDNA repeat of A. bisporus; a 0.9-kb EcoRI fragment containing the 3′ end of the 28S rRNA of A. bisporus and a downstream sequence; and a 1.6-kb ClaI fragment of the A. niger pelA gene comprising part of the coding region and some of the adjacent 3′ noncoding sequence (Fig. 1).
FIG. 1.
Restriction endonuclease maps of the BamHI-HindIII fragment of the A. niger pkiA gene (A), the SalI fragment of the A. niger pelA gene (B), and the rDNA repeat from A. niger as described by O'Connell et al. (24) (C). NTS, nontranscribed spacer; ETS, external transcribed spacer; ITS, internal transcribed spacer; IGS, intergenic spacer region consisting of nontranscribed spacer and external transcribed spacer. Restriction endonuclease sites: S, SalI; P, PstI; E, EcoRI; X, XhoI; H, HindIII; K, KpnI; B, BamHI; C, ClaI.
Hybridization and washing were carried out as described previously (26). After probing with the pelA probe, the blots carrying PstI-SalI-digested chromosomal DNA were stripped at 100°C in a 0.1% (wt/vol) sodium dodecyl sulfate solution and subsequently used for reprobing with the 28S rRNA probe.
PCR amplification.
The ribosomal ITS1-5.8S rRNA gene-ITS2 region (length, approximately 600 bp) was amplified by using primers ITS4 and ITS5 (37). PCR amplification was performed in 50-μl reaction mixtures containing 5 μl (5 to 10 ng) of genomic DNA template, 2.5 U of AmpliTaq DNA polymerase (PE Corporation, Norwalk, Conn.), 1 μM primer ITS4, 1 μM primer ITS5, each deoxynucleoside triphosphate at a concentration of 50 μM, 50 mM KCl, 50 mM Tris-HCl (pH 8.3), 0.1 mg of bovine serum albumin per ml, 3 mM MgCl2, 0.25% (vol/vol) Tween 20, and 10% (vol/vol) dimethyl sulfoxide. Amplification was performed with a GeneAmp 2400 PCR system (PE Corporation) by using the following temperature profile: initial denaturation at 94°C for 1 min, followed by 40 cycles of 15 s at 94°C, 1 min at 53°C, and 1 min at 72°C. Following amplification, PCR products were cleaned by using MicroSpin S-400 HR columns (Amersham Pharmacia Biotech, Uppsala, Sweden).
DNA sequencing and analysis.
PCR products were sequenced by using primers ITS1, ITS2, ITS3, and ITS4 (37), a Thermo Sequenase fluorescent labeled primer cycle sequencing kit (Amersham Pharmacia Biotech, Little Chalfort, United Kingdom), and an automated sequencer (A.L.F. Express; Amersham Pharmacia Biotech) according to the manufacturer's instructions. Sequences were generated from both strands, and they were edited and initially aligned by using the CLUSTAL W multiple-sequence alignment program, version 1.6 (33). Manual corrections were included to improve the alignment by using the MacClade program (version 3.05; Sinauer Associates, Sunderland, Mass.).
The data matrix consisted of 541 aligned nucleotide characteristics, some of which were scored as deletions or unknowns for one or more taxa. Phylogenetic analysis was performed with the PAUP software (version 4.0b2; Sinauer Associates, Sunderland, Mass.) by using neighbor joining, maximum-likelihood distances, and bootstrapping as described previously (30).
Isolation of secondary metabolites and identification of these metabolites.
The Aspergillus strains were cultured on the following media optimized for production of secondary metabolites: Czapek yeast autolysate agar and yeast extract sucrose agar (6). All cultures were incubated for 10 days in the dark at 30°C. For metabolite analysis the content of each plate was extracted as previously described (5) and analyzed by high-performance liquid chromatography with diode array detection (7). The metabolites found were compared to a spectral UV library made from authentic standards examined under the same conditions, and the retention indices were compared with those of standards.
Nucleotide sequence accession numbers.
The ITS1-5.8S rRNA gene-ITS2 sequences of the A. japonicus and A. aculeatus strains reported in this paper have been deposited in the EMBL Nucleotide Sequence Database under the accession numbers shown in Table 1. The EMBL accession numbers for the other black aspergilli that we used are as follows: A. niger CBS 120.49, AJ280006; A. tubingensis CBS 127.49, AJ280007; A. tubingensis CBS 643.92, AJ280008; A. foetidus var. acidus CBS 564.65T, AJ280009; A. brasiliensis IMI 381727, AJ280010; A. carbonarius CBS 111.26T, AJ280011; Aspergillus heteromorphus 117.55T, AJ280013; and Aspergillus ellipticus CBS 707.79T, AJ280014. The following other species were included in the study: Aspergillus fumigatus (accession no. AFO078889), Aspergillus flavus (AF138287), Petromyces albertensis (AJ005673), Petromyces muricatus (AJ005674), and Neosartorya fischeri (U18355).
RESULTS
Sequence analysis of the ITS1-5.8S rRNA gene-ITS2 region.
The ITS1-5.8S rRNA gene-ITS2 sequences from the 19 A. japonicus and A. aculeatus strains differed at only three nucleotide positions. Based on the ITS sequence differences, we divided the 19 A. japonicus and A. aculeatus strains into three distinct sequence types. Sequence type I comprised nine strains, sequence type II contained nine strains, and the third sequence type consisted of only one strain, A. aculeatus CBS 114.80. All sequence type I strains differed from the sequence type II strains by having a T instead of a C at positions 211 and 229. (The numbers refer to positions in the ITS1-5.8S rRNA gene-ITS2 sequence of sequence type I strain A. japonicus CBS 114.51T, which starts at the 5′ end of conserved primer ITS5 [37].) The ITS sequence of A. aculeatus CBS 114.80 (sequence type III) was identical to the sequences of the type I strains except for a single nucleotide (C instead of T) at position 189. Except for three strains (A. japonicus CBS 312.80, Aspergillus lucknowensis CBS 119.49, and A. aculeatus CBS 172.66) which had a type I sequence but which on the basis of RFLP and secondary-metabolite analysis data belonged to A. aculeatus, the sequence type I strains were A. japonicus isolates. Sequence type II strains were found only in the A. aculeatus taxon, not in the A. japonicus taxon. Since the region sequenced is so highly conserved, sequence data for the ITS1-5.8S rRNA gene-ITS2 region are indicative but not definitive.
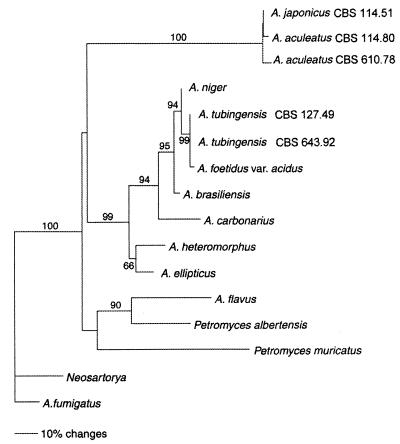
We identified a significant number of differences when the ITS1-5.8S rRNA gene-ITS2 sequences of the 19 A. japonicus and A. aculeatus strains were compared with the corresponding sequences of other black aspergilli (Table 1). The highest degree of ITS sequence dissimilarity (approximately 19%) was found when we compared the A. japonicus and A. aculeatus strains with the strains belonging to the A. niger aggregate (1). We inferred phylogenetic relationships (Fig. 2) among the black aspergilli based on a neighbor-joining analysis of the aligned ITS sequences. The closely related species A. fumigatus and N. fisheri were designated outgroups based on comparisons of 18S rRNA gene sequences with ARB (http://www.mikro.biologie.tu-muenchen.de / pub / ARB / documentation/arb.ps). A. flavus and two Petromyces species were included in the analysis based on comparisons of morphology and secondary-metabolite profiles. The analysis showed that the A. japonicus group and A. aculeatus CBS 114.80 form a well-supported clade along with the A. aculeatus group. This clade represents the uniseriate black aspergilli and was clearly separated from the biseriate black aspergilli; i.e., high bootstrap support (99%) was found for the branch leading to the cluster including the A. niger aggregate strains, A. brasiliensis, A. carbonarius, and the A. heteromorphus-A. ellipticus clade.
FIG. 2.
Neighbor-joining tree based on phylogenetic analysis of the ITS1-5.8S rRNA gene-ITS2 sequences. The numbers at branch points are the percentages of 1,000 bootstrapped data sets that supported the specific internal branches. Bootstrap values less than 50% are not shown. For the phylogenetic analysis, A. japonicus CBS 114.51T was used as a representative of sequence type I, A. aculeatus CBS 610.78 was used as a representative of sequence type II, and A. aculeatus CBS 114.80 was the sequence type III strain. Bar = 10% estimated sequence divergence.
RFLP analysis: SmaI digestion and rDNA polymorphism.
The rDNA repeat (Fig. 1C) is present at a level of 100 to 300 copies per haploid fungal genome (3). We previously reported (26) that an SmaI digest of nuclear DNA yielded five distinct rDNA band patterns. That analysis did not include A. brasiliensis. We used the 10-kb rDNA repeat of A. bisporus in a Southern blot analysis of SmaI-digested nuclear DNA to further characterize these patterns and identified six band patterns (Table 2). All the A. japonicus and A. aculeatus isolates produced the same pattern (pattern D). The SmaI-generated rDNA band pattern of A. brasiliensis (Table 2, pattern F) (35), is also clearly different from those established previously (26).
TABLE 2.
Band patterns of nuclear rDNA obtained after SmaI digestion
| Pattern | Species | Sizes of UV-visualized and hybridizing bands (kb) |
|---|---|---|
| A | A. carbonarius | 4.1, 2.3a |
| B | A. niger | 3.2, 2.3, 1.8 |
| C | A. tubingensis, A. foetidus var. acidus | 2.6, 1.9, 1.8 |
| D | A. japonicus, A. aculeatus | 1.9, 1.8, 0.9 |
| E | A. ellipticus, A. heteromorphus | 1.9, 1.8, 1.6 |
| F | A. brasiliensis | 2.1, 2.1, 1.8 |
Boldface type indicates bands that gave a hybridization signal in the Southern blot analysis.
Analysis of the 26S rRNA locus.
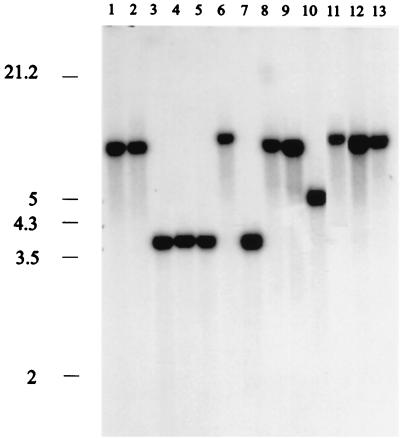
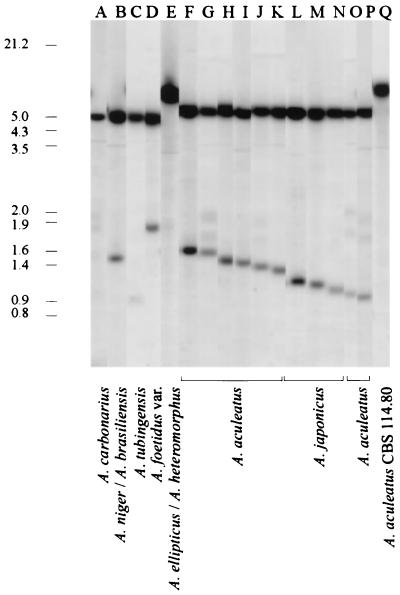
We also used the 3′ portion of the A. bisporus 28S rRNA gene that included a downstream sequence of the nontranscribed spacer region as a probe. When we digested the DNA with XhoI and KpnI and probed the preparation with the A. bisporus 0.9-kb 28S rRNA EcoRI fragment, the A. japonicus and A. aculeatus isolates were separated into two groups (Table 1). We detected four patterns (patterns A to D), each containing a single hybridizing band (7.5, 7, 5, or 4 kb) (Fig. 3). Three A. japonicus isolates, one Aspergillus atroviolaceus isolate, and one A. aculeatus isolate produced pattern A, as did CBS 101.14. Since type strain A. japonicus CBS 114.51 also produced pattern A, we concluded that these strains represent the A. japonicus taxon. The remaining 12 strains, (five A. aculeatus strains, three A. japonicus strains, two Aspergillus luchuensis strains, one A. lucknowensis strain, and one Aspergillus bruneo-violaceus strain) all produced pattern B. These strains were grouped into the A. aculeatus taxon. One A. aculeatus isolate, CBS 114.80, produced a third pattern, pattern C. If we used the same 0.9-kb EcoRI 28S rRNA probe to identify the RFLP patterns in PstI-SalI digests of nuclear DNA, then more variation was detected (Fig. 4 and Table 1). A 6.0-kb hybridizing band was found in each of the 11 patterns typical of the A. japonicus and A. aculeatus isolates (patterns F to P), and this band distinguished the uniseriate taxa from the other black aspergilli (Fig. 4, lanes A to E). The small hybridizing bands detected in the different patterns resulted from DNA sequence variation in the nontranscribed spacer of the rDNA repeat (Fig. 1C). Thus, digestion with PstI plus SalI highlighted the intragroup variations among the isolates of A. japonicus and A. aculeatus. It is obvious that the nontranscribed spacer region of the A. japonicus and A. aculeatus isolates frequently undergoes DNA rearrangements, including introduction of small insertions or deletions, given the numerous small size variations in the patterns. A. aculeatus CBS 114.80 produced only one hybridizing band at 8.0 kb (Fig. 4, lane Q).
FIG. 3.
RFLP patterns obtained after digestion of nuclear DNA with KpnI and XhoI and probing with the 0.9-kb 28S rRNA EcoRI fragment of A. bisporus. The sizes (in kilobases) of λ DNA fragments digested with EcoRI and HindIII are indicated on the left. The hybridizing bands correspond to the following isolates: lane 1, A. japonicus CBS 114.51T; lane 2, A. aculeatus CBS 611.78; lane 3, A. luchuensis CBS 101.43; lane 4, A. aculeatus CBS 308.80; lane 5, A. bruneo-violaceus CBS 621.78T; lane 6, A. aculeatus CBS 114.80; lane 7, A. niger CBS 120.49; lane 8, A. tubingensis CBS 643.92; lane 9, A. foetidus var. acidus CBS 564.65T; lane 10, A. brasiliensis IMI 381727; lane 11, A. carbonarius CBS 111.26T; lane 12, A. heteromorphus CBS 117.55T; and lane 13, A. ellipticus CBS 707.79T. The patterns in Table 1 correspond to the following sizes of hybridizing bands: pattern A, 7.0 kb; pattern B, 4.0 kb; pattern C, 7.5 kb; and pattern D, 5.0 kb.
FIG. 4.
RFLP patterns for different black aspergilli (lanes A to E) and A. japonicus and A. aculeatus isolates (lanes F to Q) after digestion of nuclear DNA with PstI plus SalI and probing with the 0.9-kb 28S rRNA EcoRI fragment of A. bisporus. The sizes (in kilobases) of bands corresponding to bands of λ DNA digested with HindIII and EcoRI are indicated on the left. The estimated sizes of the small hybridizing bands in A. aculeatus and A. japonicus band patterns F to P in Table 1 are as follows: pattern F, 1.65 kb; pattern G, 1.60 kb; pattern H, 1.55 kb; pattern I, 1.50 kb; pattern J, 1.45 kb; pattern K, 1.40 kb; pattern L, 1.35 kb; pattern M, 1.30 kb; pattern N, 1.20 kb; pattern O, 1.10 kb; and pattern P, 1.05 kb. The black aspergilli that produce patterns A to P (Table 1) are shown at the bottom.
Analysis of the pkiA locus.
The A. japonicus and A. aculeatus isolates produced patterns distinct from those of the other black aspergilli (Table 1) when they were probed with the 3.5-kb BamHI-HindIII fragment (Fig. 1A) containing the entire coding region of the A. niger pkiA gene and the 5′ and 3′ noncoding sequences. Isolates identified as A. japonicus based on the .26S rRNA RFLPs produced a unique pattern, pattern F (5.3, 4.8, and 0.5 kb) (Table 1). All of the RFLP patterns for the remaining uniseriate strains (Table 1) contained two (7.5 and 0.4 kb) of three hybridizing bands (Table 1). The 0.4-kb hybridizing fragment probably corresponded to a conserved internal KpnI-XhoI fragment (Fig. 1A) of the pkiA gene equivalent, so the differences among the patterns were probably due to changes in one of the restriction sites in the 5′ or 3′ noncoding regions. The A. brasiliensis pki pattern also was different, although it had the 7.5- and 0.4-kb hybridizing bands. A. aculeatus CBS 114.80 produced a pattern that was different from the A. japonicus and A. aculeatus patterns and contained three hybridizing pki bands (6.7, 1.2, and 0.5 kb) (Table 1, pattern K).
DNA polymorphism at the pelA locus.
We used the 1.6-kb ClaI fragment of the A. niger pelA gene in Southern blot hybridization of PstI-SalI-digested nuclear DNA. The A. japonicus and A. aculeatus isolates produced 13 different patterns. Five of the strains considered to belong to A. japonicus (Table 1) produced pattern H, which shared five of six hybridizing bands with pattern I produced by A. aculeatus CBS 611.78. In the final grouping (Table 1) this strain was classified as an A. japonicus isolate. The remaining pelA patterns were produced by isolates classified as A. aculeatus. They all contained a 0.8-kb hybridizing band.
Profiles of secondary metabolites.
The strains grouped into the A. japonicus taxon, all produce several unknown secondary metabolites (Table 3). All of the strains except CBS 114.51T produce several polar metabolites with end absorption near 200 nm. One of these metabolites may be the thiol protease inhibitor E-64 previously described by Hanada et al. (10, 11). This result should be confirmed by comparison to an authentic standard. Three strains of A. japonicus produce festuclavine (Table 3), as reported previously for another strain of this species (8). The remaining strains produce other indole alkaloids, but we could not determine whether these metabolites included cycloclavin (8). A. japonicus CBS 114.51T and CBS 568.65, A. japonicus CBS 522.89, and A. aculeatus CBS 611.78 all produce a secondary metabolite with the same characteristic chromophore.
TABLE 3.
Classification of isolates of A. aculeatus and A. japonicus taxa based on production of known secondary metabolitesa
| Strain(s) | Secondary metabolite(s) |
|---|---|
| A. japonicus taxon | |
| A. japonicus CBS 101.14 | Unknown indole alkaloidb |
| A. atroviolaceus CBS 113.48T | Festuclavin |
| A. japonicus CBS 114.51T | Unknown indole alkaloidb |
| A. japonicus CBS 568.65 | No known secondary metabolitesc |
| A. aculeatus CBS 611.78 | Festuclavin |
| A. japonicus CBS 522.89 | Unknown indole alkaloidb |
| A. aculeatus taxon | |
| A. bruneo-violaceus CBS 621.78 | Neoxaline, okaraminsd |
| A. lucknowensis CBS 119.49 | Neoxaline, secalonic acid D, okaramins, paraherquamidelike compounds |
| A. luchuensis CBS 116.80 | Neoxaline, secalonic acid D, okaramins, paraherquamidelike compounds |
| A. luchuensis CBS 101.43 | Neoxaline, secalonic acid D, okaramins, paraherquamidelike compounds |
| A. japonicus CBS 620.78 | Secalonic acid D |
| A. japonicus CBS 312.80 | No known secondary metabolitesc |
| A. japonicus CBS 114.34 | No known secondary metabolitesc |
| A. aculeatus CBS 610.78 | Neoxaline, secalonic acid D, paraherquamidelike compounds |
| A. aculeatus CBS 186.67 | Neoxaline, secalonic acid D, paraherquamidelike compounds |
| A. aculeatus CBS 308.80 | Neoxaline, secalonic acid D |
| A. aculeatus CBS 313.89 | Secalonic acid D, paraherquamidelike compounds |
| New species | |
| A. aculeatus CBS 114.80 | Secalonic acid D, okaramins A, B, H, and Ie |
| Other taxa in section Nigri | |
| A. niger CBS 120.49 | Tetracyclic compounds, naphtho-4-pyrones |
| A. niger CBS 554.65 | Tetracyclic compounds, naphtho-4-pyrones, orlandin |
| A. niger CBS 618.78 and CBS 139.52 | Tetracyclic compounds, naphtho-4-pyrones, orlandin, ochratoxin A |
| A. tubingensis CBS 643.92 | Tetracyclic compounds, naphtho-4-pyrones, nigragillin, orlandin |
| A. foetidus var. acidus CBS 564.65T | No known secondary metabolitesc |
| A. brasiliensis IMI 381727 | Tetracyclic compounds |
| A. carbonarius CBS 111.26T | Tetracyclic compounds, naphtho-4-pyrones, ochratoxin A |
| A. heteromorphus CBS 117.55T | No known secondary metabolitese |
| A. ellipticus CBS 707.79T | Austdiol, terphenyllins, candidusin |
All isolates produce several additional unknown secondary metabolites.
Production of cycloclavine is uncertain.
The isolate produces members of more than three different chromophore families of unknown secondary metabolites.
The okaramins produced by A. aculeatus also appear to be okaramins A, B, H, and I (13), but the new species represented by CBS 114.80 is a particularly good producer of these secondary metabolites.
CBS 114.80 and CBS 117.55T each produce members of several chromophore families of secondary metabolites that are unique to the isolate.
The strains in the A. aculeatus group also produce several unknown secondary metabolites, but they have the same secondary-metabolite profile, which allows them to be grouped together. The profiles of these strains are characterized by the presence of nitrogen-containing secondary metabolites (e.g., neoxaline, okaramins, and paraherquamidelike compounds) and secalonic acid (2). Three of the isolates, A. japonicus CBS 620.78, A. japonicus CBS 312.80, and A. aculeatus CBS 172.66, do not produce any nitrogenous secondary metabolites, but they all produce, large quantities of an unknown secondary metabolite with a characteristic chromophore. In contrast to the report of Hirano et al. (14), neoxaline is produced by A. aculeatus but not by A. japonicus.
The grouping of the strains based on the secondary-metabolite analysis is the same as that based on the RFLP analyses. A. aculeatus CBS 114.80 produces some of the same secondary metabolites as both A. aculeatus and A. japonicus, but it also produces a series of secondary metabolites with unique chromophores. Taking all of the data into account, we think that this strain probably represents a new species.
DISCUSSION
Using a combination of restriction enzymes (viz., SmaI, KpnI-XhoI, and PstI-SalI) and the rDNA repeat, 28S rRNA, and the pkiA and pelA genes as probes, we distinguished 10 different taxa among the black aspergilli (Table 1). The number of hybridizing bands which we observed when the rDNA repeat of the basidiomycete A. bisporus was used as a probe differed from the numbers of bands previously reported for the black aspergilli (9, 21). We think that the difference was due to the origin of the rDNA probe, since the two other groups of workers used the Aspergillus nidulans rDNA repeat in their hybridization experiments. The A. brasiliensis IMI 381727 RFLP patterns are clearly distinct from those of A. niger, A. tubingensis, and representatives of the A. foetidus varieties and thus support previous suggestions (35) that isolate IMI 381727 should be classified as a new species. Furthermore, the uniseriate A. japonicus and A. aculeatus isolates can be clearly separated into two distinct taxa. However, this division does not support the classification of individual isolates based on morphological criteria. Based on the sequence data for the ITS1-5.8S rRNA gene-ITS2 region and the data from the RFLP and secondary-metabolite analyses, we identified a third uniseriate taxon among the black aspergilli, and this taxon was represented by CBS 114.80. The 0.8-kb band hybridizing with the pelA probe probably corresponds to the pelA equivalent in A. aculeatus (Fig. 1B). The remaining hybridizing bands probably reflect DNA polymorphism in the 5′ and 3′ pelA noncoding regions and in other pectin lyase-encoding genes that also hybridize (19, 20). Of all the black aspergilli, the A. aculeatus taxon has the highest degree of variation in the pectin lyase-encoding genes. This taxon is well known for its ability to produce a variety of pectinases.
The other common taxa in section Nigri typically produce one or several of the following secondary metabolites: tetracyclic compounds, naphtho-4-pyrones, orlandin, nigragillin, and ochratoxin A. None of these compounds have been found in A. aculeatus, A. japonicus, or strain CBS 114.80 (Table 3). A. foetidus var. acidus CBS 564.65 and A. niger produce identical unknown compounds, whereas A. heteromorphus and A. ellipticus have a unique position in section Nigri; each of the latter species has only one secondary metabolite biosynthetic family in common with A. niger and/or related species and A. aculeatus or related species. For example, A. heteromorphus produces members of seven chromophore families of secondary metabolites; one of these is also produced by A. ellipticus, CBS 114.80, and A. japonicus, and another is also produced by A. niger and A. foetidus var. acidus. It is important to note that even though several additional isolates of each taxon were examined by high-performance liquid chromatography, the carcinogenic nephrotoxin ochratoxin A was detected only in isolates of A. niger and A. carbonarius.
In addition to A. niger, other black aspergilli also are important in biotechnological processes. During screening and commercialization of new Aspergillus isolates as metabolite and enzyme producers, their taxonomic positions must be firmly established. Morphological criteria are unreliable and inconsistent for identifying isolates of closely related species. This study demonstrates the power of combined molecular and biochemical methods and provides criteria for precise identification for this economically important group of fungi.
ACKNOWLEDGMENTS
We thank Dorte Lauritsen, Biotechnological Institute, for sequencing the ITS1-5.8S rRNA gene-ITS2 regions of the black aspergilli and for help in aligning the ITS sequence data and János Varga, Attila Jozsef University, Szeged, Hungary, for providing A. brasiliensis IMI 381727. We also thank Jacques Benen for useful comments.
REFERENCES
- 1.Al-Musallam A. Revision of the black Aspergillus species. Ph.D. thesis. Utrecht, The Netherlands: Rijksuniversiteit Utrecht; 1980. [Google Scholar]
- 2.Andersen R, Büchi G, Kobbe B, Demain A L. Secalonic acid D and F are toxic metabolites of Aspergillus aculeatus. J Org Chem. 1977;42:352–353. doi: 10.1021/jo00422a042. [DOI] [PubMed] [Google Scholar]
- 3.Borsuk P A, Nagięc M M, Stępieñ P P, Bartnik E. Organization of the ribosomal RNA gene cluster in Aspergillus nidulans. Gene. 1982;17:142–152. doi: 10.1016/0378-1119(82)90067-1. [DOI] [PubMed] [Google Scholar]
- 4.de Graaff L H, van den Broek H, Visser J. Isolation and expression of the Aspergillus nidulans pyruvate kinase gene. Curr Genet. 1988;13:315–321. doi: 10.1007/BF00424425. [DOI] [PubMed] [Google Scholar]
- 5.Frisvad J C, Thrane U. Standardized high-performance liquid chromatography of 182 mycotoxins and other fungal metabolites based on alkylphenone indices and UV-VIS spectra (diode array detection) J Chromatogr. 1987;404:195–214. doi: 10.1016/s0021-9673(01)86850-3. [DOI] [PubMed] [Google Scholar]
- 6.Frisvad J C, Filtenborg O. Terverticillate penicillia: chemotaxonomy and mycotoxins production. Mycologia. 1989;81:837–861. [Google Scholar]
- 7.Frisvad J C, Thrane U. Liquid column chromatography of mycotoxins. J Chromatogr Lib. 1993;54:253–372. [Google Scholar]
- 8.Furuta T, Koike M, Abe M. Isolation of cycloclavine from the culture broth of Aspergillus japonicus Saito. Agric Biol Chem. 1982;46:1921–1922. [Google Scholar]
- 9.Hamari Z, Kevei F, Kovács É, Varga J, Kozakiewicz Z, Croft J H. Molecular and phenotypic characterization of Aspergillus japonicus and Aspergillus aculeatus strains with special regard to their mitochondrial DNA polymorphisms. Antonie Leeuwenhoek. 1997;72:337–347. doi: 10.1023/a:1000578913759. [DOI] [PubMed] [Google Scholar]
- 10.Hanada K, Tamai M, Yamagishi M, Ohmura S, Sawada J, Tanaka I. Isolation and characterization of E-64, a new thiol protease inhibitor. Agric Biol Chem. 1978;42:523–528. [Google Scholar]
- 11.Hanada K, Tamai M, Ohmura S, Sawada J, Seki T, Tanaka I. Synthesis and structure of E-64, a new thiol protease inhibitor. Agric Biol Chem. 1978;42:529–536. [Google Scholar]
- 12.Harmsen J A M, Kusters-van Someren M A, Visser J. Cloning and expression of a second Aspergillus niger pectin lyase gene (pelA): indications of a pectin lyase gene family in Aspergillus niger. Curr Genet. 1990;18:161–166. doi: 10.1007/BF00312604. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi H, Furutsuka K, Shiono Y. Okaramines H and I, new okaramine congeners, from Aspergillus aculeatus. J Nat Prod (Lloydia) 1999;62:315–317. doi: 10.1021/np9802623. [DOI] [PubMed] [Google Scholar]
- 14.Hirano A, Iwai Y, Masuma R, Tei K, Omura S. Neoxaline, a new alkaloid produced by Aspergillus japonicus. Production, isolation and properties. J Antibiot. 1979;32:781–785. doi: 10.7164/antibiotics.32.781. [DOI] [PubMed] [Google Scholar]
- 15.Kevei F, Hamari Z, Varga J, Kozakiewicz Z, Croft J H. Molecular polymorphism and phenotypic variation in Aspergillus carbonarius. Antonie Leeuwenhoek. 1996;70:59–66. doi: 10.1007/BF00393570. [DOI] [PubMed] [Google Scholar]
- 16.Konda Y, Onda M, Hirano A, Omura S. Oxaline and neoxaline. Chem Pharm Bull (Tokyo) 1980;28:2987–2995. [Google Scholar]
- 17.Kozakiewicz Z. Aspergillus species on stored products. Mycol Pap. 1989;161:1–188. [Google Scholar]
- 18.Kurobane I, Vining L C, McInnes A G. Biosynthetic relationships among secalonic acids. Isolation of emodin, endocrocin and secalonic acids from Pyrenochaeta terrestris and Aspergillus aculeatus. J Antibiot. 1979;32:1256–1266. doi: 10.7164/antibiotics.32.1256. [DOI] [PubMed] [Google Scholar]
- 19.Kusters-van Someren M A, Samson R A, Visser J. Variation in pectinolytic enzymes of the black aspergilli: a biochemical and genetic approach. In: Samson R A, Pitt J I, editors. Modern concepts in Penicillium and Aspergillus classification. New York, N.Y: Plenum Press; 1990. pp. 321–334. [Google Scholar]
- 20.Kusters-van Someren M A, Samson R A, Visser J. The use of RFLP analysis in classification of the black aspergilli: reinterpretation of the Aspergillus niger aggregate. Curr Genet. 1991;19:21–26. [Google Scholar]
- 21.Megnegneau B, Debets F, Hoekstra R F. Genetic variability and relatedness in the complex group of black aspergilli based on random amplification of polymorphic DNA. Curr Genet. 1993;23:323–329. doi: 10.1007/BF00310893. [DOI] [PubMed] [Google Scholar]
- 22.Mizuno K, Yagi A, Satoi S, Takada M, Hayashi M, Asano K, Matsuda T. Studies on aculeacin. I. Isolation and characterization of aculeacin A. J Antibiot. 1977;30:297–302. doi: 10.7164/antibiotics.30.297. [DOI] [PubMed] [Google Scholar]
- 23.Mosseray R. Les Aspergillus de la section “Niger” Thom et Church. Cellule. 1934;43:203–285. [Google Scholar]
- 24.O'Connell M J, Dowzer C E A, Kelley J M. The ribosomal repeat of Aspergillus niger and its effects on transformation frequency. Fungal Genet Newsl. 1990;37:29–30. [Google Scholar]
- 25.Pařenicová L, Suykerbuyk M E G, Samson R A, Visser J. Evaluation of restriction fragment length polymorphism for the classification of Aspergillus carbonarius. Afr J Mycol Biotechnol. 1996;4:13–19. [Google Scholar]
- 26.Pařenicová L, Benen J A E, Samson R A, Visser J. Evaluation of RFLP analysis for the classification of selected black aspergilli. Mycol Res. 1997;101:810–814. [Google Scholar]
- 27.Raper K B, Fennell D I. The genus Aspergillus. Baltimore, Md: Williams and Wilkins Company; 1965. [Google Scholar]
- 28.Satoi S, Yagi A, Asano K, Mizuno K, Watanabe T. Studies of aculeasin. II. Isolation and characterization of aculeasins B, C, D, E, F and G. J Antibiot. 1977;30:303–307. doi: 10.7164/antibiotics.30.303. [DOI] [PubMed] [Google Scholar]
- 29.Schaap P J, Müller Y, Baars J J, Op den Camp H J, Sonnenberg A S, van Griensven L J, Visser J. Nucleotide sequence and expression of the gene encoding NADP+-dependent glutamate dehydrogenase (gdhA) from Agaricus bisporus. Mol Gen Genet. 1996;250:339–347. doi: 10.1007/BF02174392. [DOI] [PubMed] [Google Scholar]
- 30.Skouboe P, Frisvad J C, Taylor J W, Lauritsen D, Boysen M, Rossen L. Phylogenetic analysis of nucleotide sequences from the ITS region of terverticillate Penicillium species. Mycol Res. 1999;103:873–881. [Google Scholar]
- 31.Thom C, Church M B. The aspergilli. Baltimore, Md: Williams and Wilkins; 1926. [Google Scholar]
- 32.Thom C, Raper K B. A manual of the aspergilli. Baltimore, Md: Williams and Wilkins; 1945. [Google Scholar]
- 33.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varga J, Kevei F, Fekete C, Coenen A F, Kozakiewicz Z, Croft J H. Restriction fragment length polymorphisms in the mitochondrial DNAs of the Aspergillus niger aggregate. Mycol Res. 1993;97:1207–1212. [Google Scholar]
- 35.Varga J, Kevei F, Vriesema A, Debets F, Kozakiewicz Z, Croft J H. Mitochondrial DNA restriction fragment polymorphisms in field isolates of the Aspergillus niger aggregate. Can J Microbiol. 1994;40:612–621. doi: 10.1139/m94-098. [DOI] [PubMed] [Google Scholar]
- 36.Varga J, Kevei F, Vágvölgyi C. Double-stranded RNA mycoviruses in section Nigri of the Aspergillus genus. Can J Microbiol. 1994;40:325–329. doi: 10.1139/m94-054. [DOI] [PubMed] [Google Scholar]
- 37.White T J, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M A, Gelfand D H, Sninsky J J, White T J, editors. PCR protocols: a guide to methods and applications. London, United Kingdom: Academic Press; 1990. pp. 315–322. [Google Scholar]
- 38.Woodcock D M, Crowther P J, Doherty J, Efferson S, DeCruz E, Noyer-Weidner M, Smith S S, Michael M Z, Graham M W. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 1989;17:3469–3478. doi: 10.1093/nar/17.9.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]