Abstract
Three kinds of alkaliphilic bacteria able to utilize thiocyanate (CNS−) at pH 10 were found in highly alkaline soda lake sediments and soda soils. The first group included obligate heterotrophs that utilized thiocyanate as a nitrogen source while growing at pH 10 with acetate as carbon and energy sources. Most of the heterotrophic strains were able to oxidize sulfide and thiosulfate to tetrathionate. The second group included obligately autotrophic sulfur-oxidizing alkaliphiles which utilized thiocyanate nitrogen during growth with thiosulfate as the energy source. Genetic analysis demonstrated that both the heterotrophic and autotrophic alkaliphiles that utilized thiocyanate as a nitrogen source were related to the previously described sulfur-oxidizing alkaliphiles belonging to the gamma subdivision of the division Proteobacteria (the Halomonas group for the heterotrophs and the genus Thioalkalivibrio for autotrophs). The third group included obligately autotrophic sulfur-oxidizing alkaliphilic bacteria able to utilize thiocyanate as a sole source of energy. These bacteria could be enriched on mineral medium with thiocyanate at pH 10. Growth with thiocyanate was usually much slower than growth with thiosulfate, although the biomass yield on thiocyanate was higher. Of the four strains isolated, the three vibrio-shaped strains were genetically closely related to the previously described sulfur-oxidizing alkaliphiles belonging to the genus Thioalkalivibrio. The rod-shaped isolate differed from the other isolates by its ability to accumulate large amounts of elemental sulfur inside its cells and by its ability to oxidize carbon disulfide. Despite its low DNA homology with and substantial phenotypic differences from the vibrio-shaped strains, this isolate also belonged to the genus Thioalkalivibrio according to a phylogenetic analysis. The heterotrophic and autotrophic alkaliphiles that grew with thiocyanate as an N source possessed a relatively high level of cyanase activity which converted cyanate (CNO−) to ammonia and CO2. On the other hand, cyanase activity either was absent or was present at very low levels in the autotrophic strains grown on thiocyanate as the sole energy and N source. As a result, large amounts of cyanate were found to accumulate in the media during utilization of thiocyanate at pH 10 in batch and thiocyanate-limited continuous cultures. This is a first direct proof of a “cyanate pathway” in pure cultures of thiocyanate-degrading bacteria. Since it is relatively stable under alkaline conditions, cyanate is likely to play a role as an N buffer that keeps the alkaliphilic bacteria safe from inhibition by free ammonia, which otherwise would reach toxic levels during dissimilatory degradation of thiocyanate.
Thiocyanate (N C—S−) is a C1 sulfur species which can be produced both as a natural compound (mainly in biological cyanide detoxification processes) and as a waste product, largely by coke and metal plants (23, 46). Microorganisms can utilize thiocyanate as an energy, carbon, nitrogen, or sulfur source after it is hydrolyzed to sulfide, ammonia, and CO2. Like degradation of other C1 sulfur compounds, CNS− degradation requires the primary action of a specific enzyme(s) to release the sulfan atom for further microbial oxidation (23, 35). Currently, two distinct pathways of microbial degradation of thiocyanate are recognized, and either H2S or NH3 is the first product. For the autotrophic thiocyanate-oxidizing bacterium Thiobacillus thioparus (formerly known as Thiobacillus thiocyanooxidans) it has been postulated that thiocyanate is degraded via cyanate (N C O−), which is converted to ammonia and CO2 by the specific enzyme cyanase (13, 47). The liberated sulfide is utilized as an electron donor and energy source:
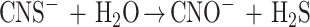 |
1 |
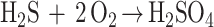 |
2 |
| 3 |
Apparently, the first enzyme in this pathway should be able to break the C—S bond. Nothing is known yet about the identity of such an enzyme(s). Moreover, no direct proof of production of cyanate as an intermediate during bacterial thiocyanate degradation has been obtained for this autotrophic bacterium so far. To our knowledge, formation of cyanate from thiocyanate has been observed only once in a mixed bacterial population from thiocyanate-degrading sludge (14). Another strain of T. thioparus degrades thiocyanate via carbonyl sulfide (O⩵C⩵S) by using the specific enzyme thiocyanate hydrolase, which has substantial homology to nitrile hydratase (19, 21, 22). Such homology is hardly surprising, assuming that both enzymes break the nitrile bond (N C). The COS produced is hydrolyzed to sulfide and CO2 (the enzymology of this reaction remains to be investigated), and sulfide is eventually oxidized to sulfate:
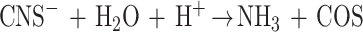 |
4 |
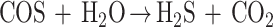 |
5 |
A similar two-stage hydrolysis via COS has been observed during carbon disulfide (S⩵C⩵S) degradation by T. thioparus TK-m, which is also able to oxidize thiocyanate (33). It seems likely that in this bacterium hydrolytic cleavage of CS2 and CNS− to sulfide proceeds through the same pathway (i.e., via COS).
Oxidation of thiocyanate to sulfate, ammonia, and CO2 yields eight electrons. Among the neutrophilic sulfur-oxidizing bacteria, the ability to grow with thiocyanate as an electron donor for energy generation and CO2 fixation is limited to a few strains of T. thioparus (7, 12, 13, 20, 32, 33, 47) and Thiobacillus denitrificans (7). The ability to utilize thiocyanate as an electron donor has recently been claimed for a newly described Paracoccus species, Paracoccus thiocyanatus (18), but it is difficult to analyze the evidence because no actual data for growth and oxidation kinetics were provided in the paper. The potential for active thiocyanate degradation has also been described for two bacterial consortia consisting of Pseudomonas and Acinetobacter species (3) and of Pseudomonas and Bacillus species (30). Both of these consortia were able to grow on thiocyanate mineral media at neutral pH values and produced sulfate, like the T. thioparus strains. However, no evidence concerning the ability of such consortia to grow autotrophically with other reduced sulfur compounds was presented. Although the possible existence of autotrophic thiocyanate specialists which utilize only thiocyanate as an energy source cannot be ruled out, so far all pure cultures of thiocyanate autotrophs are represented by sulfur bacteria able to grow on other reduced inorganic sulfur compounds. Therefore, whether the thiocyanate-oxidizing consortia may have contained a fraction of sulfur-oxidizing autotrophs morphologically indistinguishable from the heterotrophic components is an interesting question.
In addition to being oxidized for energy transduction purposes, CNS− can be metabolized as a nitrogen source. Several neutrophilic heterotrophic bacteria (Arthrobacter sp., Pseudomonas spp., Methylobacterium thiocyanatum) able to utilize the nitrogen atom from thiocyanate were isolated from different sources which may have contained thiocyanate (2, 11, 28, 41, 42, 45). It has been suggested that such bacteria employ the same primary thiocyanate degradation pathways as autotrophs (e.g., either cyanate pathways or COS pathways), but again, no direct proof of accumulation of these intermediates has been presented. In these cases, ammonium produced from thiocyanate is utilized as the nitrogen source, while the reduced sulfur can be utilized as a sulfur source but not as the energy source.
The thiocyanate-oxidizing T. thioparus strains are likely to be able to utilize the nitrogen of thiocyanate as an N source during growth solely on CNS−. However, there is no evidence concerning whether autotrophic sulfur bacteria or any other chemolithoautotrophs are able to assimilate thiocyanate nitrogen but are not able to use it as an electron donor, as is the case for the heterotrophic thiocyanate-utilizing bacteria.
CNS−-containing wastewaters can be treated by acclimated bacterial sludge containing a high density of T. thioparus-like thiocyanate-oxidizing autotrophs (3–5, 15, 16, 32) or heterotrophs if an alternative carbon source is available (17). Such biosystems proved to be able to remove millimolar amounts of CNS− at neutral or slightly alkaline pH values. The possibility of bioremoval of thiocyanate under highly alkaline conditions was not investigated.
This study demonstrated that thiocyanate can be used as the nitrogen source and as the energy source under highly alkaline conditions by alkaliphilic obligately organoheterotrophic and obligately lithoautotrophic sulfur-oxidizing bacteria, respectively, isolated from natural alkaline environments, such as those encountered in soda lakes.
MATERIALS AND METHODS
Samples.
Four composite samples were used for enrichment of thiocyanate-degrading alkaliphiles. Two soil samples were composed of 8 to 10 subsamples of soda solonchak soils collected near soda lakes in Burjatia (southeast Siberia) and Kenya (East African Rift Valley). The other two samples were composed of five to eight sediment subsamples collected from soda lakes in Burjatia and Kenya. The pH values of the subsamples varied from 9.7 to 11.0, and the salt contents ranged from 0.05 to 20% (wt/vol).
Bacterial strains.
Pure cultures of alkaliphilic heterotrophic and chemolithoautotrophic sulfur-oxidizing bacteria described previously (36–40) were tested for the ability to utilize CNS− as a nitrogen or energy source. The heterotrophs used are members of the Halomonas-Deleya cluster in the gamma subdivision of the division Proteobacteria (gamma-Proteobacteria). The autotrophs belong to the new genera Thioalkalimicrobium and Thioalkalivibrio, also in the gamma-Proteobacteria. Some of the properties of the alkaliphilic autotrophs are shown in Table 1.
TABLE 1.
Properties of the reference strains of obligately autotrophic sulfur-oxidizing bacteria belonging to the genus Thioalkalivibrio used in comparisons with the thiocyanate-utilizing autotrophic alkaliphilic isolates
| Thioalkalivibrio reference straina | Morphology
|
Nitrate reduction | Oxidation of trithionate | Use of thiocyanate as N source | Membrane-bound yellow pigment | Growth in the presence of 1.5 to 4 M Na+ | DNA G+C content (mol%)b | ||
|---|---|---|---|---|---|---|---|---|---|
| Vibrios | Spirilla | Rods | |||||||
| AL 2 | + | − | + | + | − | − | 63.7 ± 0.5 | ||
| ALJ 6 | + | − | − | − | − | − | 63.9 ± 0.5 | ||
| ALJ 10 | + | − | − | − | − | − | 65.0 ± 0.5 | ||
| ALJ 12 | + | + | − | + | − | − | 62.1 ± 0.5 | ||
| ALJ 15 | + | − | − | + | + | + | 64.9 ± 0.5 | ||
The general properties of the genus Thioalkalivibrio are as follows: obligately autotrophic alkaliphilic sulfur-oxidizing bacteria that are able to grow with sulfide and thiosulfate at pH 7.5 to 10.6 (optimum pH, approximately 10.0) and at salt (total Na+) concentrations of 0.3 to 4 M; strains oxidize sulfide, thiosulfate, sulfur, polysulfide, tetrathionate (some strains oxidize tri- and pentathionates), and sulfite to sulfate at pH values up to 11 to 11.5; and member of the gamma-Proteobacteria, whose nearest relatives are the purple sulfur bacteria belonging to the genus Ectothiorhodospira (39).
Determined by the melting temperature method.
Media and culture conditions.
Mineral base medium containing 0.6 M total Na+ as sodium carbonates and sodium chloride (pH 10) (38) was used in all growth experiments. It contained (per liter) 21 g of sodium carbonate, 9 g of sodium bicarbonate, 5 g of NaCl, 1 g of K2HPO4, and 0.5 g of KNO3. A trace elements solution (31) (2 ml/liter) and Mg salts (0.5 mM) were added after sterilization. KCNS, sodium thiosulfate, and sodium acetate were also supplied after sterilization from filter-sterilized 2 M stock solutions. CNS− was fairly stable under the alkaline conditions used; no chemical decomposition was observed during more than 1 month of incubation of uninoculated medium at pH 9.8 to 10.2. Media with higher salt contents (up to 4 M Na+; pH 10.0 to 10.1) were prepared by proportionally increasing the concentration of sodium carbonates.
Enrichment cultures and cultures grown with acetate or thiosulfate were incubated on a rotary shaker at 200 rpm. Cultures grown on mineral medium with thiocyanate were grown statically or on a rotary shaker at 100 rpm as specified below. All cultures were grown at 28°C. To grow cultures with 5 mM ammonium chloride as the nitrogen source at pH 10, it was necessary to employ bottles with rubber stoppers and a liquid phase/gas phase ratio of 1:10 to prevent loss of ammonia and oxygen limitation. A special test performed with sterile medium at pH 10 demonstrated that during incubation of open flasks on the rotary shaker at 200 rpm about 30% of the added ammonium was lost from the liquid phase over a 3-day period. The ability of isolated pure cultures to convert thiocyanate anaerobically in the presence of nitrate (20 mM) as the electron acceptor was studied by using 100-ml flasks with butyl rubber stoppers. Cultures were made anaerobic by repeated evacuation and flushing with argon (five cycles).
Enrichment procedure and isolation of pure cultures.
Heterotrophic alkaliphiles utilizing CNS− as a sole nitrogen source were enriched on a mineral base medium (pH 10.0) supplemented with 20 mM acetate as the carbon and energy source and 5 mM KCNS. Chemolithoautotrophic alkaliphilic bacteria utilizing the nitrogen from KCNS were enriched on the same medium, except that the acetate was replaced by 40 mM thiosulfate. After complete disappearance of CNS−, several subcultures (1:100 dilution) were made. The cultures exhibiting stable thiocyanate disappearance were plated onto solid medium having the same composition, and different colonies were then isolated and checked for the ability to use thiocyanate in liquid culture. Media without a nitrogen source were used as controls.
Chemolithoautotrophic thiocyanate-oxidizing alkaliphilic bacteria able to use CNS− as an electron donor were enriched on mineral base medium supplemented with 10 mM KCNS as the sole energy and nitrogen source. The same medium was suitable for growth of pure cultures. However, during isolation of pure cultures, it was found that most of the enrichment cultures were not able to form colonies on thiocyanate mineral medium. When thiosulfate (20 mM) was added together with 10 mM thiocyanate, several types of sulfur-producing colonies developed after 2 weeks of incubation. The smallest colonies usually were colonies of autotrophs able to grow on thiocyanate, and larger colonies were colonies of organisms able to use thiocyanate only as a nitrogen source.
Thiocyanate-oxidizing autotrophic strains were grown in thiocyanate-limited continuous cultures by using 1.5-liter laboratory fermentors equipped with pH and pO2 probes (Applicon, Schiedam, The Netherlands). The pH was controlled at 10.0, and the dissolved oxygen content was 50% of air saturation. The final medium composition was the same as that used for batch cultivation, and the final CNS− concentrations were 6 to 13 mM as specified below.
Oxygen uptake experiments.
Cells of autotrophic thiocyanate-oxidizing alkaliphiles were obtained from the cultures grown at pH 10.0 with thiocyanate or thiosulfate as the electron donor. After centrifugation, the cells were washed and resuspended at a protein concentration of about 10 mg ml−1 in sodium carbonate buffer (pH 10.0) (see below). The respiration activity was tested at pH values of 6.0 to 11.5 in buffers containing 0.6 M total Na+ and 50 mM KCl. For pH 6 to 8, 0.1 M HEPES–NaOH–NaCl was used; for pH 8.2, freshly prepared NaHCO3 was used; and for pH 9 to 11.5, a combination of Na2CO3 and NaHCO3 was used. The carbonate dependence of respiration was examined by using 0.1 M Tris-HCl–0.6 M NaCl at pH 9 to 10. The respiration rates were measured in a 5-ml thermostat-equipped chamber mounted on a magnetic stirrer and fitted with a Clark type of dissolved oxygen probe (Yellow Spring Instruments Co., Yellow Springs, Ohio). Stock solutions of sodium sulfide, polysulfide (S62−; prepared by autoclaving a 0.2 M sodium sulfide solution with a large excess of powdered sulfur), and sulfite were prepared anaerobically in 0.1 M Tris-HCl with 5 mM EDTA to prevent autooxidation and were introduced into the chamber at final concentrations of 25 to 50 μM. Elemental sulfur was added from a saturated solution in acetone at a final concentration of 70 μM. CS2 was added from a concentrated ethanol solution at final concentrations of 0.05 to 2 mM. COS, methane thiole (CH3SH), and dimethyl sulfide [(CH3)2S] were supplied as saturated water solutions at a final concentration of 100 μM. Thiosulfate and tetrathionate were added at final concentrations of 50 to 200 μM from freshly prepared concentrated stock solutions in water. Kinetic parameters (Vmax and Ks) were calculated from V-[S] plots.
Experiments with washed cells.
The kinetics of degradation of various substrates by washed cells obtained either from batch cultures or from chemostat cultures was studied by using 10-ml serum bottles containing 2 ml of suspension, in which the cell protein concentration ranged from 0.1 to 1 mg ml−1. Anaerobic experiments were conducted after removal of oxygen with evacuation and argon flushing (five cycles). When CS2 (2 mM) and COS (2 mM) were used as substrates, gray butyl rubber stoppers were used instead of black stoppers.
Analysis.
Thiocyanate was analyzed colorimetrically as ferric thiocyanate (34). The same method was employed to determine the elemental sulfur content after extraction with acetone and cyanolysis. Thiosulfate, tetrathionate, and trithionate contents were measured by cyanolysis (24). Sulfate content was measured by a turbidimetric method (6). Sulfide content was determined as described by Trüper and Schlegel (43) after precipitation as ZnS. NH4+ content was measured by a phenol-hypochlorite colorimetric procedure described by Weatherburn (44). Cell protein content was analyzed by the Lowry method. When elemental sulfur was produced, it was removed by extraction with acetone prior to alkaline digestion of the cell pellet for the protein assay.
Cyanate ion (OCN−) content was routinely measured as NH4+ after acidification of the solutions to pH 2 to 3 with 6 N HCl and subsequent heating in boiling water for 1 min. This procedure gave 95 to 97% recovery of pure cyanate added to standard sodium carbonate-containing media at pH 8 to 10. Final identification and quantitative measurements of cyanate in culture supernatants were performed by using a colorimetric reaction with anthranilic acid as described by Dorr and Knowles (9). The spectrum of the resulting complex (quinazoline-2,4-dione) was recorded with an HP 8453 UV-visible diode array spectrophotometer (Hewlett-Packard, Amsterdam, The Netherlands). Pure cyanate added to the sodium carbonate-containing media used for cultivation of the alkaliphilic bacteria and culture supernatants obtained after thiocyanate decomposition by autotrophic alkaliphiles gave products with identical spectral properties (absorption maximum at 310 nm).
Cyanase activity was measured with cell extracts obtained by sonification of washed cell suspensions in 0.5 M sodium bicarbonate buffer (pH 8.2). Incubation was started by adding a freshly prepared 2 mM potassium cyanate solution, and production of NH3-NH4+ was monitored at 5- to 10-min intervals.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the total cell protein was used to visualize expression of specific enzymes responsible for thiocyanate degradation. Autotrophic and heterotrophic cultures were grown at pH 10 with or without thiocyanate, and cells were collected, washed, and sonicated. The extracts were treated and analyzed by a standard procedure (26) by using 10% (wt/vol) polyacrylamide gels.
Electron microscopy.
For total-cell preparations, washed cells were directly fixed with formaldehyde (final concentration, 2.5%) in liquid medium and then positively stained with 1% phosphotungstic acid. Samples used for ultrathin sectioning were centrifuged, washed and resuspended in fresh 0.6 M NaHCO3 (pH 8), fixed with 1% (final concentration) OsO4 for 12 h at 4°C, dehydrated, and embedded in resin. Thin sections were stained with uranyl acetate and lead citrate. To detect intracellular accumulation of elemental sulfur, cells were sedimented, stained with a solution containing 2% AgNO3 and 2% glutaraldehyde for 10 h, and then fixed with OsO4. Postsectional staining was omitted in this case.
Genetic analysis.
Isolation of DNA, determination of the G+C contents of DNA preparations, and DNA-DNA hybridization were performed as described by Marmur (27) and De Ley et al. (8).
Amplification and sequencing of 16S rRNA genes.
For amplification and sequencing of 16S rRNA genes, DNA was obtained by standard phenol-chloroform extraction. The 16S rRNA genes were selectively amplified by using primers 5′-AGAGTTTGATCCTGGCTCAG-3′ (forward) and 5′-TACGGTTACCTTGTTACGACTT-3′ (reverse). PCR products were purified from low-melting-point agarose by using a Wizard PCR-Prep kit (Promega) according to the manufacturer's instructions. Almost complete sequencing (1,400 to 1,450 nucleotides) was performed by using a Silver Sequencing kit (Promega) according to the manufacturer's instructions, with minor modifications.
16S ribosomal DNA sequence analysis.
The sequences were aligned manually with sequences obtained from the database consisting of small-subunit rRNAs collected from the EMBL international nucleotide sequence library. The sequences were compared with the sequences of members of the Proteobacteria. Regions that were not sequenced in one or more reference organisms were omitted from the analyses. Pairwise evolutionary distances (expressed in estimated number of changes per 100 nucleotides) were computed by using the method of Jukes and Cantor. A phylogenetic tree was constructed by the neighbor-joining method. Bootstrap analysis (100 replications) was used to validate the reproducibility of the branching pattern of trees.
Nucleotide sequence accession numbers.
16S ribosomal DNA sequence data for strains ARh 1 and ARh 2 have been deposited in the EMBL and GenBank databases under accession numbers AF151432 and AF302081, respectively.
RESULTS
CNS− uptake in pure cultures of alkaliphilic sulfur-oxidizing bacteria.
Twenty-five strains of heterotrophic tetrathionate-forming alkaliphilic bacteria and 30 strains of obligately autotrophic sulfur-oxidizing alkaliphilic bacteria isolated previously from alkaline environments (35–39) were tested to determine their abilities to use thiocyanate as a sole source of nitrogen while they were growing with acetate and with thiosulfate, respectively, as the energy source.
Among the heterotrophs, strains AG 4 and AGJ 1-3 were capable of growth with acetate and thiocyanate. Thiocyanate consumption was coupled to acetate consumption. About 4 mM CNS− was consumed per 40 mM acetate. This ratio is within the correct order of magnitude that would be expected to be consumed for a normal bacterial biomass N content, assuming that the molar cell composition is CHON0.15 and that the C yield on acetate is about 35%.
None of the previously isolated strains of alkaliphilic autotrophic sulfur bacteria belonging to the genera Thioalkalimicrobium and Thioalkalivibrio were able to grow with thiocyanate as the energy and nitrogen source. Surprisingly, however, most of them grew well with thiosulfate as the energy source and thiocyanate as the N source instead of nitrate or NH3. Positive results were obtained with 7 of 10 Thioalkalimicrobium strains and with 16 of 20 Thioalkalivibrio representatives. The maximum amount of thiocyanate consumed was around 1.5 mM; again, given the lower yield on thiosulfate, the ratio between thiocyanate and thiosulfate was within the correct order of magnitude that would account for the N requirement for biomass formation. The Thioalkalivibrio strains consumed 1 mmol of CNS− per 24 mmol of thiosulfate oxidized, and the Thioalkalimicrobium strains needed twice as much thiosulfate because of their 1.5- to 1.8-fold-lower molar yield on thiosulfate. To obtain more specialized thiocyanate-utilizing alkaliphiles, direct enrichments with thiocyanate as the only nitrogen and/or energy source were prepared by using inocula from highly alkaline soda environments.
Enrichment and isolation of alkaliphilic bacteria utilizing CNS− as the nitrogen source. (i) Heterotrophic alkaliphiles.
Incubation of samples composed of subsamples of the Kenyan soda lake sediments and subsamples of the Kenyan and Siberian soda soils with 40 mM acetate and 5 mM thiocyanate at pH 10.0 resulted in complete disappearance of CNS− within 2 weeks. No consumption of thiocyanate was detected in cultures inoculated with composite samples obtained from the Siberian soda lake sediments. Plating of the cultures obtained after several successive passages in liquid medium resulted in domination by one or two morphological colony types in all three enrichments. Finally, we obtained five pure cultures (strains AGSCN 1 through AGSCN 5) that were able to utilize CNS− as a nitrogen source while growing with acetate at pH 10.
Morphologically, the five strains were similar to a dominant alkaliphilic acetate-utilizing aerobic bacterium, strain AGJ 1-3 (a motile coccobacillus that accumulates large amounts of polyhydroxybutyrate), found previously in Kenyan soda lakes (37). All strains grew with acetate at pH 7.5 to 10.5, and optimum growth occurred at 9.5 to 10.0 and at salt concentrations up to 2 M Na+. Some properties of the isolates are given in Table 2.
TABLE 2.
Heterotrophic alkaliphilic isolates that utilize CNS− as a nitrogen source
| Enrichment culturea | Strain | Oxidation of S2O32− to S4O62− | Denitrification | Utilization of NO3−NO2− as N source | G+C content of DNA, (mol%) | % DNA-DNA
homology withb:
|
|
|---|---|---|---|---|---|---|---|
| AG 4 | AGJ 1-3 | ||||||
| LK | AGCNS 1 | + | + | − | 64.9 | 52 | 81 |
| AGCNS 2 | + | + | + | 65.5 | 58 | 48 | |
| SK | AGCNS 3 | − | + | + | 65.2 | 50 | 76 |
| SS | AGCNS 4 | + | − | + | 65.4 | 43 | 53 |
| AGCNS 5 | + | + | + | 65.0 | 42 | 67 | |
LK, Kenyan lake sediments; SK, Kenyan soda soils; SS, Siberian soda soils.
The levels of DNA homology for strains AGCNS 1, AGCNS 3, and AGCNS 5 ranged from 70 to 90%, which indicated that these strains belong to the same species; strains AGCNS 2 and AGCNS 4 are less closely related to the other strains (40 to 50% DNA homology). Strains AG 4 and AGJ 1-3 are tetrathionate-forming heterotrophic alkaliphiles isolated previously from the Siberian and Kenyan soda lakes, respectively (35, 36).
During growth with acetate and thiocyanate at pH 10.0, the heterotrophs isolated consumed the two substrates simultaneously, with a minimal molar ratio of about 10:1. No NH4+, NH3, or cyanate was detectable in supernatants during utilization of thiocyanate by growing cultures or by washed cells. The presence of nitrate, nitrite, or urea in the growth medium at concentrations equal to the CNS− concentration did not inhibit utilization of the latter compound as a nitrogen source. Ammonia prevented CNS− utilization completely without influencing the growth yield. Under anaerobic conditions in the presence of nitrate or nitrite as an electron acceptor, CNS− consumption was inhibited. In contrast, when N2O was the electron acceptor, cultures consumed CNS− with the same efficiency as was observed for aerobic growth or CNS−.
(ii) Obligately autotrophic sulfur-oxidizing alkaliphiles using CNS− as the N source.
During incubation of the composite soda lake samples with thiocyanate as the sole source of energy and nitrogen at pH 10, two types of obligately lithoautotrophic sulfur-oxidizing alkaliphiles were enriched. One type was bacteria able to utilize thiocyanate as the N source during growth with thiosulfate as the energy source at pH 10. The other type was bacteria able to utilize thiocyanate as both the energy source and the nitrogen source (see below).
Bacteria that utilized thiocyanate as the N source formed large yellowish colonies on the alkaline agar medium containing thiosulfate and thiocyanate. In liquid medium at pH 10 no growth was observed without thiosulfate. Two strains isolated in pure culture from the sediments of the Kenyan and Siberian soda lakes were practically identical in terms of their phenotypic properties and were genetically very closely related (more than 90% DNA similarity). Cells of Kenyan isolate ALRh were small vibrios that were motile by means of one polar flagellum. The biomass grown on thiosulfate-CNS− was yellowish. The yellow pigment could be extracted with acetone and had absorption maxima at 397, 418, and 441 nm; these properties are similar to the properties of a specific subgroup of previously isolated strains of obligately autotrophic alkaliphilic sulfur bacteria belonging to the genus Thioalkalivibrio (38) which are unique because of their ability to grow at concentrations of sodium carbonate up to the saturation concentration. A special test confirmed that strain ALRh was similar to such strains in that it was able to grow in the presence of up to 4 M Na+ as sodium carbonate at pH 10. DNA-DNA hybridization with five reference strains of the genus Thioalkalivibrio demonstrated that strain ALRh is indeed specifically related to the yellow extremely natronotolerant members of this genus (Table 3). This strain has been deposited in the Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany) under accession number DSM 13533.
TABLE 3.
DNA-DNA homology between thiocyanate-utilizing strains ALRh, ARh 1, ARh 2, ARh 3, and ARh 4 and obligately autotrophic sulfur-oxidizing alkaliphiles belonging to the genus Thioalkalivibrio
| Strain | % DNA-DNA
homology with:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| ARh 1 | ARh 2 | AL 2 | AL 5 | ALJ 6 | ALJ 10 | ALJ 12 | ALJ 15 | |
| ALRh | 30 | 50 | 54 | 44 | 42 | 56 | 39 | 68 |
| ARh 1 | 100 | 30 | 21 | 20 | 16 | 21 | 26 | —a |
| ARh 2 | 30 | 100 | 45 | 42 | 33 | 51 | 33 | 65 |
| ARh 3 | 31 | 90 | — | — | — | — | — | 60 |
| ARh 4 | 28 | 61 | 60 | 44 | 45 | 58 | 27 | 48 |
—, no data.
Strain ALRh grew equally well on alkaline thiosulfate medium containing CNS− or ammonia as the N source. Much slower growth and heavy sulfur production were observed when nitrate was the N source. CNS− was consumed as the organism grew. After growth ceased, a small additional amount of thiocyanate was consumed, so that 1 mmol of CNS− was consumed per 13 to 15 mmol of thiosulfate oxidized. Assuming the maximal growth yield of ALRh (5.5 mg of protein ≈ 0.07 mmol of N/mmol of thiosulfate), the molar nitrogen demand should be approximately 1:14. Similar to thiocyanate consumption by the heterotrophic alkaliphiles, thiocyanate consumption in cultures and by washed cells of this autotroph was almost completely inhibited by the presence of ammonia at millimolar concentrations, and neither ammonia nor cyanate could be detected as an intermediate of thiocyanate degradation.
Alkaliphilic chemolithoautotrophic thiocyanate-oxidizing sulfur bacteria. (i) Enrichment and isolation of pure cultures.
Chemolithotrophic alkaliphilic bacteria able to grow solely on thiocyanate were enriched on mineral soda medium (pH 10.0) supplemented with 10 to 12 mM thiocyanate as the electron donor and source of nitrogen. At higher thiocyanate concentrations (20 to 40 mM) enrichments were negative. Positive enrichments were obtained with the sediments from Kenyan and Siberian soda lakes but not from the soil samples. The Kenyan culture developed more rapidly and consumed 11 mM thiocyanate within 10 days. The Siberian culture started to grow only after a long lag phase and consumed 10 mM thiocyanate within 18 days. After several 1:100 transfers, two stable enrichment cultures were obtained. Both the Kenyan and Siberian cultures included large nonmotile rod-shaped cells in which sulfur was deposited and two or three types of small, actively moving vibrios which were numerically dominant in subsequent serial dilutions on mineral medium with thiocyanate.
Pure cultures were isolated by using alkaline mineral agar with 10 mM CNS− or with 20 mM thiosulfate and 10 mM CNS−. Only the vibrio-shaped bacteria formed tiny transparent colonies on the CNS− agar after about 2 weeks of incubation. They also formed white refractile colonies containing sulfur on the thiosulfate-CNS− agar; these colonies gradually turned transparent, and some of them became yellowish. The large nonmotile rods observed in the enrichment cultures were not able to form colonies on the CNS− agar. They grew very slowly on the thiosulfate-CNS− agar, forming small, snow white, sulfur-containing colonies. However, as the numbers of these organisms were always much lower than the numbers of vibrios in the Siberian culture, only the Kenyan enrichment was suitable for isolating this bacterium in pure culture. Overall, we isolated three vibrio-shaped and one rod-shaped obligately chemolithoautotrophic bacteria able to grow solely on thiocyanate at pH 10.0 (Table 4).
TABLE 4.
Chemolithoautotrophic alkaliphilic sulfur bacteria able to grow on thiocyanate as an energy source
| Samplea | Strain | Morphology | Growth
with S2O32− at pH 10
|
G+C content of DNA (mol%)b | |
|---|---|---|---|---|---|
| N source(s) | 2-4 M Na+ | ||||
| LK | ARh 1 | Fat nonmotile rods with capsule, sulfur deposited inside cells, colorless | NH3 | − | 65.6 |
| ARh 2 | Thin vibrios, spirilla in old cultures, motile with one polar flagellum, yellow pigmented | NH3, NO3− | + | 66.2 | |
| ARh 3 | Same as ARh 2 | NH3 | + | 66.9 | |
| LS | ARh 4 | Short thick vibrios, motile with one polar flagellum, colorless | CNS−, NH3, NO3− | − | 66.3 |
LK, Kenyan lake sediments; LS, Siberian lake sediments.
Determined by the melting temperature method.
Rod-shaped isolate ARh 1 (= DSM 13531) was a minor component of the thiocyanate enrichment cultures from the Kenyan lake sediments. It differed morphologically from all previously isolated alkaliphilic sulfur-oxidizing autotrophs (39). Its cells were large, nonmotile, and barrellike (0.8 to 1 by 1.2 to 2 μm) and were covered by a thick capsule. During growth with thiocyanate and thiosulfate, elemental sulfur was produced both inside and outside the cells, and the intracellular sulfur globules were surrounded by a membrane, like purple sulfur bacteria. The cell morphology of the other strains grown with thiosulfate as a substrate was typical of the genus Thioalkalivibrio (39); each cell was a short vibrio (0.5 to 0.6 by 0.8 to 1.4 μm) with one polar flagellum and multiple carboxysomelike inclusions. The ultrastructure of the cells grown with thiocyanate as the electron donor was unusual in that the cell interior was clearly divided into compartments by internal membranes.
The biomass of vibrio strains ARh 2 (= DSM 13532) and ARh 3 was yellow. The pigment extracted with acetone had exactly the same optical properties as the pigment obtained from strain ALRh, an autotrophic sulfur alkaliphile that utilized thiocyanate as an N-source (see above) and was similar to members of a specific subgroup of extremely salt-tolerant Thioalkalivibrio strains (39). Therefore strains ARh 2 and ARh 3 were tested to determine their abilities to grow at pH 10 at sodium carbonate concentrations much higher than that used for routine cultivation (0.6 M Na+). With thiosulfate both strains were indeed able to grow at concentrations of Na+ (as carbonates) of at least 4 M, while with thiocyanate the highest salt concentration for growth was equivalent to 2.5 M total Na+. The upper salt limit for growth of strains ARh 1 and ARh 4 was not higher than 1.3 to 1.5 M Na+.
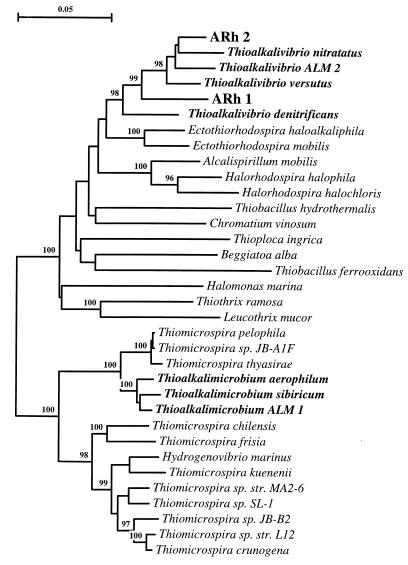
DNA-DNA hybridization between the thiocyanate-oxidizing strains (Table 3) demonstrated that vibrio-shaped isolates ARh 2 and ARh 3 belong to a single genospecies and are moderately closely related both to representatives of the yellow natronotolerant genus Thioalkalivibrio (ALJ 15 and ALRh) and to another vibrio strain, ARh 4. The similarity values (50 to 60%) indicate that they are different species. The similarity values obtained with other Thioalkalivibrio reference strains were lower but within the range observed for different strains of this genus (39). The low level of DNA similarity of rod-shaped strain ARh 1 with the other thiocyanate-utilizing autotrophs and with the reference strains of the genus Thioalkalivibrio (16 to 31%) (Table 3) correlated with a substantial morphological difference between this isolate and Thioalkalivibrio strains. Nevertheless, a 16S ribosomal DNA-based phylogenetic analysis demonstrated that strains ARh 1 and ARh 2 both are sulfur-oxidizing alkaliphilic sulfur bacteria belonging to the genus Thioalkalivibrio in the gamma-Proteobacteria (Fig. 1).
FIG. 1.
Phylogenetic tree showing the positions of thiocyanate-oxidizing alkaliphilic autotrophic strains ARh 1 and ARh 2 among the sulfur-oxidizing species in the gamma-Proteobacteria. The numbers at the branching points indicate the bootstrap values. Reference sequences were obtained from the GenBank, EMBL, and Ribosomal Database Project databases. Scale bar, 5 base substitutions per 100 bases.
(ii) Characteristics of growth of strain ARh 1 on thiocyanate.
Interestingly, strain ARh 1 grew faster with thiocyanate at pH 10.0 than with thiosulfate. A small amount of elemental sulfur, mostly intracellular, was produced during the active thiocyanate consumption phase. In the stationary phase, elemental sulfur disappeared. At this point about 90% of the thiocyanate sulfur was converted to sulfate. The bacterium was able to grow at initial thiocyanate concentrations of up to 30 mM but utilized no more than 10 to 15 mM. During growth on thiosulfate (with NH3 as the N source), strain ARh 1 produced much more elemental sulfur during the initial growth phase than it produced with thiocyanate. When most of the thiosulfate was consumed, elemental sulfur began to disappear concomitant with a more rapid increase in biomass. The maximum specific growth rate and the growth yield obtained with thiosulfate were lower than the values obtained in thiocyanate-grown cultures (Table 5). Stable growth in continuous cultures at pH 10.1 was achieved only with low influent thiocyanate concentrations (5 to 6 mM). The reason for such behavior is discussed below. The maximum specific growth rate obtained with low thiocyanate concentrations in chemostats was twofold higher than the maximum specific growth rate observed in batch cultures (Table 5). With 6 mM thiocyanate and a dilution rate of 0.09 h−1, the cultures started to produce intracellular sulfur (2 to 3 mM) but still oxidized all of the thiocyanate. Washout began at dilution rates greater than 0.11 h−1.
TABLE 5.
Parameters of autotrophic growth of thiocyanate-oxidizing alkaliphilic strains with thiocyanate and thiosulfate at pH 10 to 10.2a
| Strain | Maximum specific growth rate
(h−1)
|
Growth yield (mg of protein
mmol−1)
|
S0 formation
|
|||
|---|---|---|---|---|---|---|
| CNS− | S2O32− | CNS− | S2O32− | CNS− | S2O32− | |
| ARh 1 | 0.045 (0.09)b | 0.018 | 8–9 (9.2–11.3) | 6–7 | + | + |
| ARh 2 | 0.015 | 0.08 | 5.9 (6.8) | 4.0 | − | +/−c |
| ARh 3 | 0.015 | 0.07 | 5.7 | 7.5 | − | +/− |
| ARh 4 | 0.010 (0.042) | 0.10 | 4.1 (4.3–6.6) | 5.0 | − | − |
Strains ARh 1 and ARh 3 were grown with NH3 as the N source, and strains ARh 2 and ARh 4 were grown with NO3− as the N source.
The values in parentheses are values obtained from thiocyanate-limited continuous cultures; strain ARh 1 was grown with 6 mM thiocyanate at pH 10.1, and strain ARh 4 was grown with 10.5 mM thiocyanate at pH 10.2.
+/−, variable.
The potential for oxidation of thiocyanate and the other sulfur compounds was studied by using washed cells of strain ARh 1 grown either with thiocyanate, with thiosulfate, or with thiosulfate plus thiocyanate at pH 10.0. Only thiocyanate-grown cells were capable of thiocyanate-dependent oxygen consumption. Also, only thiocyanate-grown cells were able to oxidize carbon disulfide (CS2). Both CNS−- and thiosulfate-grown cells oxidized sulfide most actively (Table 6). Thiosulfate and polysulfide were oxidized less actively. Elemental sulfur was a very poor substrate. Tetrathionate, sulfite, formate, and dimethyl sulfide were not oxidized. In thiocyanate-grown cells the stoichiometry of oxygen consumption with all of the substrates corresponded to oxidation to the level of elemental sulfur, which accumulated in the respiration chamber when excessive substrate was supplied. Thiosulfate-grown cells oxidized the substrates to a mixture of sulfur and sulfate. The affinity constants for CNS−, S2O32−, HS−, and CS2, as measured with respiring cells at pH 10, were 25, 7, 5, and 350 μM, respectively. Strain ARh 1 exhibited a pH activity profile typical of alkaliphiles, with an optimum pH between 9.0 and 10.0. The optimum pH for thiosulfate oxidation was lower than that for the other substrates. Respiratory activity with all sulfur substrates at pH values lower than 7.5 was negligible. The upper pH limit for respiration was pH 11 to 11.5. Without any salt, the cells lysed immediately, and activity totally stopped. The presence of 0.4 to 0.5 M total Na+ was sufficient for maximal respiration activity; 1 M NaCl inhibited the thiocyanate oxidation activity by 50%, and complete inhibition occurred at 2 M NaCl. NH3 at concentrations up to 10 mM did not influence the rate of thiocyanate-dependent oxygen consumption at pH 10.0. CN− completely blocked CNS− oxidation at a concentration of 100 μM.
TABLE 6.
Substrate-dependent oxygen consumption by washed cells of thiocyanate-oxidizing alkaliphilic autotrophs grown with thiocyanate or thiosulfate at pH 10.0
| Sulfur compound | Maximum respiration rate (minus
endogenous rate) at pH 10.0 (nmol of O2 mg of
protein−1 min−1)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| ARh 1 grown
with:
|
ARh 2 grown with:
|
ARh 3 grown
with:
|
ARh 4 grown with:
|
|||||
| CNS− | S2O32− | CNS− | S2O32− | CNS− | S2O32− | CNS− | S2O32− | |
| CNS− | 130/160a | 0 | 180/300 | 0 | 220 | 10 | 260/400 | 0 |
| CS2 | 60/90 | 12 | 0/0 | NDb | 0 | ND | 0 | ND |
| S2O32− | 210/580 | 350 | 580/10 | 360 | 280 | 120 | 450/180 | 480 |
| HS− | 1,500/2,900 | 3,800 | 1,400c/820 | 850 | 720c | 570c | 810c/740 | 800c |
| S62− (polysulfide) | 400 | 2,600 | 960c | 450 | 650c | 400c | ND/220 | 490c |
| S8 | 25/50 | 50 | 450/250 | 330 | 160 | 110 | 160/180 | 220 |
| S4O62− (pH 9) | 0/0 | 0 | 90 | 200 | 0 | 30 | 90/0 | 80 |
Rate for cells from a batch culture/rate for cells from a CNS−-limited chemostat.
ND, not determined.
Initial rate.
Our experiments demonstrated that washed cells of strain ARh 1 were able to convert CS2 into HS− anaerobically at pH 10 at a rate of 5 to 7 nmol of HS− mg of protein−1 min−1. It was impossible, however, to demonstrate any intermediate COS accumulation, apparently because of rapid spontaneous hydrolysis of this compound in alkaline carbonate media. COS was much more stable in HEPES-NaCl buffer at pH 8. When this buffer was used, production of HS− from COS was observed under anaerobic conditions in the presence of washed cells of strain ARh 1 (8 to 10 nmol of HS− mg of protein−1 min−1, minus spontaneous rate in the absence of cells) but not in the presence of the cells of strain ARh 2 or ARh 5. Overall, these data suggest that strain ARh 1 is capable of degrading CS2 via primary hydrolysis to COS and then to HS−.
(iii) Characteristics of growth of the vibrio-shaped strains on thiocyanate.
Unlike rod-shaped strain ARh 1, the vibrio-shaped strains grew much more slowly with thiocyanate than with thiosulfate (Table 5). On the other hand, under certain conditions, the vibrio cultures utilized two to three times more thiocyanate than ARh 1 utilized. Maximum thiocyanate consumption was observed in cultures of strains ARh 2 and ARh 3 cultivated in the fed-batch mode. Neither of the vibrio strains produced elemental sulfur or other intermediate sulfur compounds during growth with thiocyanate. The sulfur from thiocyanate was almost quantitatively converted to sulfate. The growth efficiency of the alkaliphilic vibrios with thiocyanate was lower than that of strain ARh 1 (Table 5). Strain ARh 4 differed from the other ARh strains by its ability to grow fast on a thiosulfate-thiocyanate mixture. In thiocyanate-limited continuous cultures, stable growth of strain ARh 4 was achieved with 11 mM thiocyanate at pH 10.2. At a higher influent thiocyanate concentration (15 mM) the culture began to wash out at very low dilution rates (<0.02 h−1).
Like the CNS−-oxidizing activity of strain ARh 1, the CNS−-oxidizing activity of the vibrios was inducible (e.g., present in cells grown with thiocyanate as an energy source), but the maximum values were 1.5 to 2 times higher. In contrast to ARh 1, the vibrio strains were not able to oxidize CS2. On the other hand, they exhibited 5- to 10-fold-greater elemental sulfur-oxidizing activity than ARh 1 and also could use tetrathionate (Table 6). The stoichiometry of oxygen consumption with all of the oxidized sulfur compounds corresponded to complete oxidation of the compounds to sulfate. As for other alkaliphilic sulfur bacteria, sulfide and polysulfide were the most favorable substrates for the vibrio strains. The oxidation of sulfide and polysulfide was always biphasic. Usually, a first, short, high-rate stage was followed by a long, low-rate oxygen consumption stage. Such kinetics may be explained by initial rapid oxidation of HS− to zero-valence sulfur and subsequent slower oxidation of the latter to sulfate. Cells of vibrio strains ARh 2 and ARh 5 grown in thiocyanate-limited continuous cultures exhibited higher thiocyanate-oxidizing activities (30 to 40%) than cells grown in batch cultures. Also interesting was the finding that in contrast to batch-grown cells, cells from thiocyanate-limited chemostat cultures exhibited much lower thiosulfate-oxidizing activities. Strain ARh 2 even lost its thiosulfate-oxidizing capacity completely. On the other hand, the sulfide-oxidizing capacity remained high independent of the sulfur substrate used. The pH profiles for oxidation of sulfur compounds by washed cells of all three vibrio strains were typical for alkaliphiles, with an optimum pH around pH 10.0 and limits at pH 7.0 and 11 to 11.5. The pH profile for thiocyanate oxidation was narrower than those for the other compounds, with sharp decreases at pH values less than 9 and more than 10.
Thiocyanate degradation pathway in alkaliphilic bacteria. (i) Formation of cyanate from thiocyanate.
We indicate above that the alkaliphilic strains which utilized thiocyanate as an N source did not excrete any intermediate nitrogen compounds into the medium and that all of the thiocyanate nitrogen was apparently used for assimilation. In contrast, the N balance in cultures and cell suspensions of all ARh strains grown with thiocyanate as the electron donor was far from complete. A maximum of only about 20% of the converted thiocyanate could be accounted for by assimilation plus excreted ammonia. Part of the ammonia, of course, was lost by volatalization from the liquid at pH 10. However, special experiments with sterile media demonstrated that stripping of NH3 could have resulted in no more than 10 to 15% of the nitrogen loss that was not accounted for. Therefore, production of an intermediate nitrogen compound during thiocyanate dissimilation by alkaliphilic autotrophs had to be assumed. The most probable candidate species is cyanate (CNO−), which has been suggested as an intermediate in one of the microbial thiocyanate degradation pathways (see reaction 1 above). Cyanate is known to be reasonably stable at high pH values but decomposes rapidly under highly acidic conditions (see reaction 3 above). Indeed, acidification to pH 2 to 3 by HCl allowed almost complete recovery of nitrogen as ammonium in the supernatants after degradation of thiocyanate by the ARh strains. Pure cyanate added to a sterile carbonate buffer and to media reacted in a similar way, instantly decomposing to ammonium after acidification. A specific colorimetric reaction with anthranilic acid confirmed the identity of the intermediate N compound as cyanate in all samples of culture supernatants with substantial N disbalance (see above). The amounts of cyanate formed during utilization of thiocyanate by cultures and cell suspensions of ARh strains are shown in Table 7. Additional tests confirmed that in carbonate-based media at pH 10 to 10.5 spontaneous decomposition of cyanate to ammonia was relatively slow (5 to 10% with 10 mM cyanate at 30°C within 24 h).
TABLE 7.
Cyanate production by thiocyanate-oxidizing alkaliphilic bacteria at pH 10 after complete thiocyanate utilization
| Strain | Batch
culturea
|
Continuous
cultureb
|
Washed
cellsc
|
|||||
|---|---|---|---|---|---|---|---|---|
| N biomass concn (mM)d | NH3 concn (mM) | CNO− concn (mM) | N biomass concn (mM) | NH3 concn (mM) | CNO− concn (mM) | NH3 concn (mM) | CNO− concn (mM) | |
| ARh 1 | 1.4 | 0.3–0.5 | 10.5–11.5 | 0.6–1.25 | 0.1–1.9 | 4.6–8.5 | 0 | 4.8 |
| ARh 2 | 1.1 | 0.5–1.2 | 11.0–12.0 | 1.10 | 2.0–2.2 | 7.8–8.2 | 0 | 4.8 |
| ARh 4 | 0.9 | 1.2–1.6 | 11.0–12.0 | 0.60–0.72 | 1.25–2.9 | 7.4–9.0 | 0–0.2 | 4.8 |
The cultures were grown for 70 to 120 h with 15 mM CNS−.
The cultures were grown in CNS−-limited continuous cultures at dilution rates from 0.02 h−1 to 0.09 liter−1, with inflowing concentrations of 6 to 13 mM CNS−. Strain ARh 1 was grown with 6 to 13 mM thiocyanate, strain ARh 2 was grown with 13 mM thiocyanate, and strain ARh 4 was grown with 10.5 to 13 mM thiocyanate.
The cultures were incubated for 3 to 4 h with 5.4 mM CNS−.
We assumed that the N content of the cell protein is 15%.
(ii) Ammonia toxicity at pH 10.
The clear evidence that cyanate rather than ammonia accumulates during thiocyanate dissimilation by autotrophic alkaliphiles, in contrast to neutrophilic species, should have some explanations. One of the explanations could be that NH3, which is absolutely dominant over NH4+ at pH 10, is toxic and therefore accumulation of NH3 should somehow be avoided. For example, the sulfur-oxidizing alkaliphiles belonging to the genera Thioalkalimicrobium and Thioalkalivibrio were unable to grow at pH 10 in the presence of NH3 at concentrations higher than 2 to 3 mM (39). Therefore, the toxicity of ammonia for growth and activity of thiocyanate-utilizing alkaliphiles was tested at pH 10. While there was no inhibition of respiratory activity by NH3 or CNO− at concentrations up to 20 mM, ammonia inhibited growth of the autotrophic strains at relatively low concentrations (2 to 3 mM). Strain ARh 1 was the most sensitive ARh strain. The thiocyanate-oxidizing ARh strains were slightly more sensitive to ammonia than the heterotrophic alkaliphile AGCNS 1 was.
(iii) Cyanase activity.
The activities of cyanase (the enzyme which splits cyanate into ammonia and CO2 [see reaction 3 above]) were measured in cell extracts prepared from cells of different thiocyanate-utilizing alkaliphilic strains grown under different conditions. Considerably cyanase activity was found in (i) heterotrophic strain AGCNS 1 grown with thiocyanate as the N source and (ii) autotrophic strains ALRh and ARh 4 grown with thiosulfate as the energy source and ammonia, nitrate, or thiocyanate as the N source. Although constitutive, the cyanase activity in strain ALRh markedly increased in the presence of thiocyanate. In contrast, cyanase activity was undetectable in thiocyanate-dissimilating strains ARh 1, ARh 2, and ARh 3 and was extremely low in ARh 4 grown with thiocyanate as the energy source (Table 8). The activity was maximal at pH 8 and was HCO3− dependent (Ks = 2 mM). At pH 7 and 10 the activities were 40 and 88% of the maximal activity, respectively.
TABLE 8.
Cyanase activities in cell extracts prepared from cells of different thiocyanate-utilizing alkaliphiles grown with different substrates at pH 10
| Strain(s) | Maximum cyanase activity (nmol of
NH3 mg of protein−1
min−1)a
|
||
|---|---|---|---|
| Growth without CNS− | Growth with CNS− as N source | Growth with CNS− as energy source | |
| AGCNS | 20 (acetate) | 890 (acetate) | |
| ALRh | 100 (thiosulfate) | 625 (thiosulfate) | |
| ARh 1, ARh 2, ARh 3 | 0 (thiosulfate) | 0 | 0 |
| ARh 4 | 420 (thiosulfate) | 1,890 (thiosulfate) | 35 |
Cyanase activity was measured in 0.1 M HEPES–NaOH–10 mM NaHCO3 (pH 8.0) with 2 mM cyanate; the incubation time was 5 to 30 min, and the protein concentration was 0.03 to 0.1 mg ml−1.
(iv) Thiocyanate dissimilation.
Previous experiments demonstrated that the primary reaction in thiocyanate dissimilation by the alkaliphilic autotrophs should be hydrolysis to cyanate and HS−. In the neutrophilic T. thioparus strain, which may use the same thiocyanate degradation pathway, a substantial rate of sulfide production was observed when the cells were incubated with thiocyanate under anaerobic conditions. However, in our experiments performed with washed cells and cell extracts of the alkaliphilic ARh strains at pH 8.0 to 10.5, anaerobic thiocyanate degradation could not be detected. When ARh 1 and ARh 4 cells were crushed, the thiocyanate degradation activity decreased significantly. Nevertheless, it was still detectable after prolonged incubation (100 to 160 nmol mg of protein−1 h−1); 80 to 90% of this activity was recovered in the soluble fractions of the extracts after removal of the membranes by ultracentrifugation at 180,000 × g for 1 h. Thiocyanate was quantitatively converted to cyanate and elemental sulfur. As in whole-cell experiments, no thiocyanate degradation was observed under anaerobic conditions.
Denaturing gel electrophoresis of the total proteins from cells of different thiocyanate-utilizing alkaliphiles growing with or without thiocyanate revealed the presence of two major protein bands specific for thiocyanate-metabolizing cells. A band at ∼50 kDa was heavily expressed only by thiocyanate-dissimilating ARh strains grown with thiocyanate and therefore may be attributed to a thiocyanate-splitting enzyme. The intensity of this band suggests that it should be attributed to a dominant protein in these bacteria. The second specific band, at ∼40 kDa, for the most part correlated with the presence of cyanase activity. In the thiocyanate-assimilating heterotrophic alkaliphile AGCNS 1 it was present only in cells grown with thiocyanate. In autotrophic strains ARh 4 and ALRh with constitutive cyanase activity (present in cells grown with NH3), this band was present in cells grown without thiocyanate as well as in cells grown with thiocyanate as the N source, and its intensity was positively correlated with the observed cyanase activity. In the autotrophic strains grown with thiocyanate as the energy source, the 40-kDa band (and cyanase activity) was very weak (ARh 4) or totally absent (ARh 1, ARh 2, and ARh 3).
DISCUSSION
The results obtained in this study demonstrated for the first time that active thiocyanate biodegradation may occur under highly alkaline conditions. Thiocyanate can be used by heterotrophic and autotrophic alkaliphilic bacteria either as a nitrogen source or as an electron donor and energy source. Thiocyanate utilization either by pure bacterial cultures or by mixed populations in activated sludge has never been observed at pH values above 8.5. In fact, pH values higher than 8.0 negatively influenced thiocyanate degradation and growth of the neutrophilic bacteria (14, 25, 29), probably because of increased formation of undissociated NH3 instead of NH4+.
A substantial number of the previously isolated pure cultures of alkaliphilic sulfur-oxidizing autotrophic bacteria were able to utilize thiocyanate as a nitrogen source. While for heterotrophic bacteria this ability has been demonstrated previously more than once, no chemolithoautotrophs were known to grow with thiocyanate as a nitrogen source except for the known neutrophilic thiocyanate-oxidizing sulfur bacteria, which use thiocyanate as an electron donor and as a nitrogen source simultaneously. This is logical because in both assimilation and dissimilation pathways the thiocyanate molecule should first be split into sulfide and ammonium. In contrast, it is difficult to explain why many strains of alkaliphilic sulfur bacteria, which are able to utilize the nitrogen moiety, cannot grow solely with thiocyanate. The only way to obtain nitrogen from CNS− is to hydrolyze it and release ammonia. This, in turn, means that sulfur is released eventually as sulfide, which is a natural electron donor for the alkaliphilic sulfur autotrophs. Perhaps CNS− is transported inside the cells, where it cleaved to sulfide and ammonia. Then, if the sulfide-oxidizing system is located outside the cell membrane, difficulties with substrate oxidation might to be expected, whereas external thiosulfate or sulfide can be oxidized easily. Strong induction of the cyanase activity in heterotrophic (strain AGCNS 1) and autotrophic (strain ALRh) alkaliphiles during growth with thiocyanate as an N source could imply that they use a cyanate pathway for thiocyanate degradation, although the absence of any observed cyanate accumulation does not allow us to substantiate such a conclusion.
The thiocyanate-oxidizing alkaliphilic autotrophs can be enriched only when thiocyanate is used as the sole growth substrate. The presence of thiosulfate in addition to thiocyanate invariably resulted in enrichment of the sulfur-oxidizing alkaliphiles that were unable to grow with thiocyanate as an electron donor and grew faster than the thiocyanate specialists. All four alkaliphilic thiocyanate-oxidizing strains isolated were typical sulfur chemolithoautotrophs and were related to the other sulfur alkaliphiles belonging to the genus Thioalkalivibrio, which are unable to grow with thiocyanate (39). This supports the conclusion that the true electron donor in such bacteria is sulfide and, therefore, thiocyanate-oxidizing autotrophs are also sulfur-oxidizing autotrophs. Most of the previously described thiocyanate-degrading bacteria were isolated from thiocyanate-containing waste systems. The presence of thiocyanate-assimilating and thiocyanate-oxidizing bacteria in natural soda environments implies that there is a thiocyanate influx. Shallow soda lake sediments are usually rich in decaying organic material and reduced sulfur compounds. Perhaps thiocyanate can be formed from CN− and reduced sulfur, like polysulfide, in a well-known cyanolytic reaction or with thiosulfate by the action of the enzyme rhodanese (10, 46). Alkaliphilic representatives of the thiocyanate-oxidizing autotrophs described in this paper differed from the neutrophilic T. thioparus strains by their ability to grow and to oxidize thiocyanate and other sulfur compounds under highly alkaline conditions (optimum pH, around 10) in combination with high salt concentrations. Both previously described neutrophilic species of thiocyanate-oxidizing sulfur autotrophs (T. thioparus and T. denitrificans) belong to the beta-Proteobacteria, while the alkaliphilic isolates belong to the gamma-Proteobacteria.
In contrast to strains that utilize thiocyanate as the N source, thiocyanate-oxidizing alkaliphilic autotrophs accumulated a large amount of cyanate during thiocyanate dissimilation under alkaline conditions. In fact, cyanate was the major nitrogen species in cultures of thiocyanate-grown ARh strains. This finding correlated well with the absence (ARh 1, ARh 2, ARh 3) or suppression (strain ARh 4) of cyanase activity when these bacteria grew with thiocyanate as the electron donor. The cyanate accumulation observed can be taken as the first direct proof of the involvement of the cyanate pathway biodegradation of thiocyanate by pure bacterial cultures. Thus, it seems quite sensible for alkaliphiles to use this pathway in combination with the absence of cyanase activity to prevent ammonia toxicity at highly alkaline pH values. Nevertheless, even production of cyanate as an N buffer would not save these bacteria from intoxication when high concentrations of cyanate (8 to 10 mM) accumulate, as in the case of chemostat cultures grown at pH 10 at low dilution rates with 12 to 15 mM thiocyanate. In this case the residence time is sufficiently long to allow slow, spontaneous cyanate decomposition, which releases more toxic ammonia than the culture needs for assimilation. This was probably a major reason for the observed instability of the continuous cultures compared to batch cultures of the alkaliphilic thiocyanate-oxidizing ARh strains. Stable growth was achieved only with low influent thiocyanate concentrations (<10 mM), which allowed us to decrease the liquid residence time. In this case the steady-state NH3 concentration was kept below the toxicity level (0.1 to 1.3 mM).
Two specific enzyme activities and corresponding proteins associated with growth with thiocyanate were detected in alkaliphilic bacteria. The cyanase activity, detected in both thiocyanate-assimilating and dissimilating strains, resembled the enzyme activity of neutrophiles in the pH optimum (pH 8.0) and bicarbonate dependency (1). However, it was much more alkalitolerant. The catalysis of the breaking of the C—S bond of thiocyanate may be related to a protein with subunit mass of about 50 kDa which was heavily expressed only in ARh strains grown with thiocyanate as the energy source. It seems likely that this protein may be different from its analogue in neutrophilic T. thioparus strains in that it needs oxygen for activity. As nothing is known yet about this type of enzymes, it would be very interesting to purify this protein from the alkaliphilic bacteria.
The ability of alkaliphilic bacteria to degrade thiocyanate under highly alkaline conditions might also be important in improving bioremoval of thiocyanate from alkaline wastewater. Such wastewater, for example, can result from gold cyanidation, in which alkaline cyanide can react with polysulfide or reactive sulfur to form a less toxic alkaline thiocyanate-containing waste, which subsequently might be treated with the alkaliphiles.
ACKNOWLEDGMENTS
This research was supported by grant NWO 047.006.018 from the Netherlands Organization for Scientific Research.
We thank B. Jones for providing the samples from Kenyan soda lakes.
REFERENCES
- 1.Anderson P M. Purification and properties of the inducible enzyme cyanase. Biochemistry. 1980;19:2883–2887. doi: 10.1021/bi00554a010. [DOI] [PubMed] [Google Scholar]
- 2.Betts P M, Rinder D F, Fleeker J R. Thiocyanate utilization by an Arthrobacter. Can J Microbiol. 1979;25:1277–1282. doi: 10.1139/m79-201. [DOI] [PubMed] [Google Scholar]
- 3.Boucabeille C, Bories A, Ollivier P. Degradation of thiocyanate by a bacterial coculture. Biotechnol Lett. 1994;16:425–430. [Google Scholar]
- 4.Buczowska Z, Jarnuszkiewicz I. The biochemical activity of mixed bacterial cultures acclimated to thiocyanate. Bull Inst Mer Med Gdansk. 1968;19:201–210. [PubMed] [Google Scholar]
- 5.Catchpole J R, Cooper R L. The biological treatment of carbonization wastes. New advances in the biochemical oxidation of liquid wastes. Water Res. 1972;6:1459–1474. [Google Scholar]
- 6.Cypionka H, Pfennig N. Growth yield of Desulfotomaculum orientiswith hydrogen in chemostat culture. Arch Microbiol. 1986;143:396–399. [Google Scholar]
- 7.De Kruyff C D, van der Walt J I, Schwartz H M. The utilization of thiocyanate and nitrate by thiobacilli. Antonie Leeuwenhoek. 1957;23:305–316. doi: 10.1007/BF02545882. [DOI] [PubMed] [Google Scholar]
- 8.De Ley J, Caffon H, Reinaerts A. The quantitative measurements of hybridization DNA from renaturation rates. Eur J Biochem. 1970;12:133–140. doi: 10.1111/j.1432-1033.1970.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 9.Dorr P K, Knowles C J. Cyanide oxygenase and cyanase activities of Pseudomonas fluorescensNCIMB 11764. FEMS Microbiol Lett. 1989;60:289–294. [Google Scholar]
- 10.Drobnica L, Kristian P, Augustin J. The chemistry of the CNS group. In: Patai S, editor. Chemistry of cyanates and their derivatives. New York, N.Y: Wiley Press; 1977. pp. 1003–1221. [Google Scholar]
- 11.Grigor'eva N V, Avakyan Z A, Tourova T P, Kondrat'eva T F, Karavaiko G I. The search for and study of microorganisms that degrade cyanides and thiocyanates. Microbiology (Engl Trans Mikrobiologiya) 1999;68:453–460. [Google Scholar]
- 12.Happold F C, Johnstone K I, Roger H S, Youatt J B. The isolation and characteristics of an organism oxidizing thiocyanate. J Gen Microbiol. 1954;10:261–266. doi: 10.1099/00221287-10-2-261. [DOI] [PubMed] [Google Scholar]
- 13.Happold F C, Jones G L, Pratt D B. Utilization of thiocyanate by Thiobacillus thioparus and T. thiocyanooxidans. Nature. 1958;182:266–267. doi: 10.1038/182266a0. [DOI] [PubMed] [Google Scholar]
- 14.Hung C-H, Pavlostathis S. Aerobic biodegradation of thiocyanate. Water Res. 1997;31:2761–2770. [Google Scholar]
- 15.Hung C-H, Pavlostathis S. Kinetics and modeling of autotrophic thiocyanate biodegradation. Biotechnol Bioeng. 1999;62:1–11. doi: 10.1002/(sici)1097-0290(19990105)62:1<1::aid-bit1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Jones G L, Carrington E G. Growth of pure and mixed cultures of microorganisms concerned in the treatment of carbonization waste liquors. J Appl Bacteriol. 1972;35:395–404. doi: 10.1111/j.1365-2672.1972.tb03715.x. [DOI] [PubMed] [Google Scholar]
- 17.Karavaiko G I, Kondrat'eva T F, Savari E E, Grigor'eva N V, Avakyan Z A. Microbial degradation of cyanide and thiocyanate. Microbiology (Engl Trans Mikrobiologiya) 2000;69:209–216. [PubMed] [Google Scholar]
- 18.Katayama Y, Hiraishi A, Kuraishi H. Paracoccus thiocyanatus sp. nov., a new species of thiocyanate-utilizing facultative chemolithotroph, and transfer of Thiobacillus versutus to the genus Paracoccus as Paracoccus versutuscomb. nov. with emendation of the genus. Microbiology. 1995;141:1469–1477. doi: 10.1099/13500872-141-6-1469. [DOI] [PubMed] [Google Scholar]
- 19.Katayama Y, Kanagawa T, Kuraishi H. Emission of carbonyl sulfide by Thiobacillus thioparusgrown with thiocyanate in pure and mixed cultures. FEMS Microbiol Lett. 1993;114:223–228. [Google Scholar]
- 20.Katayama Y, Kuraishi H. Characteristics of Thiobacillus thioparusand its thiocyanate assimilation. Can J Microbiol. 1978;24:804–810. doi: 10.1139/m78-135. [DOI] [PubMed] [Google Scholar]
- 21.Katayama Y, Matsushita Y, Kaneko M, Kondo M, Mizuno T, Nyunoya H. Cloning of genes coding for the subunits of thiocyanate hydrolase of Thiobacillus thioparusTHI 115 and their evolutionary relationships to nitrile hydratase. J Bacteriol. 1998;180:2583–2589. doi: 10.1128/jb.180.10.2583-2589.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katayama Y, Narahara Y, Inoue Y, Amano F, Kanagawa T, Kuraishi H. A thiocyanate hydrolase of Thiobacillus thioparus. A novel enzyme catalyzing the formation of carbonyl sulfide from thiocyanate. J Biol Chem. 1992;267:9170–9175. [PubMed] [Google Scholar]
- 23.Kelly D P, Baker S C. The organosulfur cycle: aerobic and anaerobic processes leading to turnover of C1-sulfur compounds. FEMS Microbiol Rev. 1990;87:241–246. [Google Scholar]
- 24.Kelly D P, Chambers T A, Trudinger P A. Cyanolysis and spectrophotometric estimation of trithionate in mixture with thiosulfate and tetrathionate. Anal Chem. 1969;41:898–902. [Google Scholar]
- 25.Kim S-J, Katayama Y. Effect of growth conditions on thiocyanate degradation and emission of carbonyl sulfide by Thiobacillus thioparusTH115. Water Res. 2000;34:2887–2894. [Google Scholar]
- 26.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Marmur J. A procedure for isolation of DNA from microorganisms. J Mol Biol. 1961;3:208–214. [Google Scholar]
- 28.Mason F, Harped D, Larkin M. The microbial degradation of thiocyanate. Biochem Soc Trans. 1994;22:423S. doi: 10.1042/bst022423s. [DOI] [PubMed] [Google Scholar]
- 29.Neufeld R D, Mattson L, Lubon P. Thiocyanate bio-oxidation kinetics. J Environ Eng. 1981;108:1035–1049. [Google Scholar]
- 30.Paruchuri Y L, Shivaraman N, Kumaran P. Microbial transformation of thiocyanate. Environ Pollut. 1990;68:15–28. doi: 10.1016/0269-7491(90)90011-z. [DOI] [PubMed] [Google Scholar]
- 31.Pfennig N, Lippert K D. Über das Vitamin B12-bedürfnis phototropher Schwefel bacterien. Arch Microbiol. 1966;55:245–256. [Google Scholar]
- 32.Putilina N T. Bacteria of sewage waters of coke factories oxidizing thiocyanate and cyanide compounds. Microbiology (Engl Transl Mikrobiologiya) 1969;30:294–308. [Google Scholar]
- 33.Smith N A, Kelly D P. Oxidation of carbon disulfide as the sole source of energy for the autotrophic growth of Thiobacillus thioparusstrain TK-m. J Gen Microbiol. 1988;134:3041–3048. [Google Scholar]
- 34.Sörbo B. A colorimetric determination of thiosulfate. Biochem Biophys Acta. 1957;23:412–416. doi: 10.1016/0006-3002(57)90346-3. [DOI] [PubMed] [Google Scholar]
- 35.Sorokin D Y. Biological oxidation of sulfur atom in C1and organic compounds. Microbiology (Engl Transl Mikrobiologiya) 1993;62:575–581. [PubMed] [Google Scholar]
- 36.Sorokin D Y, Lysenko A M, Mityushina L L. Isolation and characterization of alkaliphilic heterotrophic bacteria capable of oxidation of inorganic sulfur compounds to tetrathionate. Microbiology (Engl Trans Mikrobiologiya) 1996;65:326–338. [Google Scholar]
- 37.Sorokin D Y, Mityushina L L. Ultrastructure of alkaliphilic heterotrophic bacteria oxidizing sulfur compounds to tetrathionate. Microbiology (Engl Transl Mikrobiologiya) 1998;67:93–101. [Google Scholar]
- 38.Sorokin D Y, Robertson L A, Kuenen J G. Isolation and characterization of obligately chemolithoautotrophic alkaliphilic sulfur-oxidizing bacteria. Antonie Leeuwenhoek. 2000;77:251–260. doi: 10.1023/a:1002445704444. [DOI] [PubMed] [Google Scholar]
- 39.Sorokin, D. Y., A. M. Lysenko, L. L. Mityushina, T. P. Tourova, B. E. Jones, F. A. Rainey, L. A. Robertson, and J. G. Kuenen.Thioalkalimicrobium sibericum, Thioalkalimicrobium aerophilum gen. nov., sp. nov., and Thioalkalivibrio versutus, Thioalkalivibrio nitratus, Thioalkalivibrio denitrificans gen. nov., sp. nov., new obligately alkaliphilic and obligately chemolithoautotrophic sulfur-oxidizing bacteria from soda lakes. Int. J. Syst. Evol. Microbiol., in press. [DOI] [PubMed]
- 40.Sorokin D Y, van Steenbergen R, Robertson L A, Jones B E, Kuenen J G. Isolation and characterization of alkaliphilic chemolithotrophs from soda lakes. In: Antramikian G, editor. First International Symposium on Extremophiles. Hamburg: Technical University Hamburg; 1996. p. 204. -Technologie GmbH, Hamburg, Germany. [Google Scholar]
- 41.Stafford D A, Calley A G. The utilization of thiocyanate by a heterotrophic bacterium. J Gen Microbiol. 1969;55:285–289. doi: 10.1099/00221287-55-2-285. [DOI] [PubMed] [Google Scholar]
- 42.Stratford J, Dias A E X O, Knowles C J. The utilization of thiocyanate as a nitrogen source by a heterotrophic bacterium: the degradative pathway involves formation of ammonium and tetrathionate. Microbiology. 1994;140:2657–2662. doi: 10.1099/00221287-140-10-2657. [DOI] [PubMed] [Google Scholar]
- 43.Trüper H G, Schlegel H G. Sulphur metabolism in Thiorhodaceae. Quantitative measurements on growing cells of Chromatium okeanii. Antonie Leeuvenhoek. 1964;30:225–237. doi: 10.1007/BF02046728. [DOI] [PubMed] [Google Scholar]
- 44.Weatherburn M V. Phenol-hypochlorite reaction for determination of ammonia. Anal Chem. 1967;39:971–974. [Google Scholar]
- 45.Wood A P, Kelly D P, McDonald I R, Jordan S L, Morgan T D, Khan S, Murell J C, Borodina E. A novel pink-pigmented facultative methylotroph, Methylobacterium thiocyanatumsp. nov., capable of growth on thiocyanate as sole nitrogen source. Arch Microbiol. 1998;169:148–158. doi: 10.1007/s002030050554. [DOI] [PubMed] [Google Scholar]
- 46.Wood J L. Biochemistry. In: Newman A A, editor. Thiocyanic acid and its derivatives. London, United Kingdom: Academic Press; 1975. pp. 156–252. [Google Scholar]
- 47.Youatt J B. Studies on the metabolism of Thiobacillus thiocyanooxidans. J Gen Microbiol. 1954;11:139–149. doi: 10.1099/00221287-11-2-139. [DOI] [PubMed] [Google Scholar]