Abstract
Introduction
Physical activity (PA) promotes resilience with respect to cognitive decline, although the underlying mechanisms are not well understood. We examined the associations between objectively measured PA and resting‐state functional connectivity magnetic resonance imaging (rs‐fcMRI) across seven anatomically distributed neural networks.
Methods
rs‐fcMRI, amyloid beta (Aβ) positron emission tomography (PET), PA (steps/day × 1 week), and longitudinal cognitive (Preclinical Alzheimer's Cognitive Composite) data from 167 cognitively unimpaired adults (ages 63 to 90) were used. We used linear and linear mixed‐effects regression models to examine the associations between baseline PA and baseline network connectivity and between PA, network connectivity, and longitudinal cognitive performance.
Results
Higher PA was associated selectively with greater connectivity in three networks previously associated with cognitive decline (default, salience, left control). This association with network connectivity accounted for a modest portion of PA's effects on Aβ‐related cognitive decline.
Discussion
Although other mechanisms are likely present, PA may promote resilience with respect to Aß‐related cognitive decline, partly by increasing connectivity in a subset of cognitive networks.
Keywords: Alzheimer's disease, amyloid, cognition, functional connectivity, physical activity
1. INTRODUCTION
Alzheimer's disease (AD) pathology develops over decades, 1 and recent studies suggest that the eventual emergence of cognitive impairment in people with AD pathology is strongly influenced by individual risk and lifestyle factors. 2 , 3 , 4 , 5 The long preclinical phase of AD has become a major focus of clinical research, as intervention at this stage of disease may prevent the widespread synaptic loss and neurodegeneration that is characteristic of symptomatic phases of AD. Intervening on modifiable risk and lifestyle factors during the preclinical phase of disease may have a significant impact on the emergence of clinical cognitive impairment and dementia at low cost and with minimal risk. Physical activity (PA) level is a modifiable risk factor known to promote resilience with respect to developing dementia, 2 , 6 , 7 although the mechanisms underlying this effect are not well understood. Prior work suggests that greater levels of PA may be particularly protective in people with elevated brain amyloid β (Aβ) levels, both with respect to cognitive decline and to the progression of cortical atrophy, effects that appear to be independent of vascular risk factors. 6
Resting‐state functional connectivity magnetic resonance imaging (rs‐fcMRI) is a non‐invasive tool that can be used to quantify the integrity of distributed neural networks that are somewhat specialized for particular cognitive functions. Building on the observation that decreased functional connectivity in the default network is seen in AD as compared to normal aging, 8 a broad array of rs‐fcMRI–based measures have been examined as potential biomarkers and predictors of disease progression. Our group and others have demonstrated that lower levels of connectivity in the default, salience, and control networks presage cognitive decline in cognitively unimpaired older adults. 9 , 10 Our prior work also demonstrated that lower functional connectivity in these networks was most predictive of cognitive decline in cognitively unimpaired individuals with elevated Aβ, suggesting a synergistic effect of low connectivity and greater Aβ with respect to cognitive decline. 9 Furthermore, these networks, especially the default network, share significant anatomical overlap with structures involved in early AD pathophysiology, including the posterior cingulate and precuneus. Progressive decrements in connectivity in the default network correspond to worsening clinical impairment, including diagnoses of mild cognitive impairment and AD dementia. 11 The extent to which default and salience network connectivity is disrupted correlates with episodic memory performance, both in cognitively unimpaired adults and in adults challenged with administration of scopolamine, an anticholinergic drug that impairs memory. 12 Although more work is needed, these data suggest that rs‐fcMRI may be a useful non‐invasive tool in both predicting who may be at risk for future cognitive decline as well as evaluating interventions aimed at delaying or preventing the development of dementia.
In this study, we investigated whether levels of PA in older adults were associated with connectivity across a broad set of networks, including three networks (default, salience, and left control) shown previously to be related to cognitive performance on tasks typically affected in AD 13 , 14 and in which low connectivity predicts future cognitive decline. 9 In addition, we analyzed the primary visual, visual association, dorsal attention, and motor networks for comparison. Given the association of greater PA with less cognitive decline over time, we also investigated the extent to which increased network connectivity may account for the protective effects of PA against cognitive decline. To do this, we leveraged data from the Harvard Aging Brain Study (HABS), a longitudinal study of cognitive aging and preclinical AD. As HABS participants undergo Aβ positron emission tomography (PET) imaging, we are also able to examine the extent to which the effects of connectivity and PA may be different in older adults with elevated Aβ burden.
2. METHODS
2.1. Participants
A total of 167 cognitively unimpaired participants from HABS were included in the study. Inclusion criteria for HABS required a Clinical Dementia Rating (CDR) Scale of 0, a Mini‐Mental State Examination (MMSE) of 26 or greater, education‐adjusted normative performance on Logical Memory II delayed recall, and a Geriatric Depression Scale of less than 11 at the time of study entry. Persons with a history of stroke with persistent symptoms or unstable medical comorbidities were excluded. Baseline rs‐fcMRI and Aβ imaging with Pittsburg compound B (PiB) PET were assessed in all participants during baseline evaluations. Participants are administered neuropsychological evaluations at baseline and yearly thereafter. Study protocols were approved by the Mass General Brigham (formerly Partners) Institutional Review Board. All participants are provided written informed consent prior to the completion of any study procedures.
2.2. Physical activity
Baseline PA was measured by using a waist‐band–mounted pedometer (HJ‐72OITC; Omron Healthcare) worn for seven consecutive days during waking hours. We calculated mean steps per day for each participant, excluding days that registered less than 100 or greater than 30,000 steps, in accordance with previously published cutoffs for pedometer data quality assurance. 15 Only participants who had five or more days of recorded activity within these cutoffs were included in the analyses.
2.3. Imaging
2.3.1. MRI acquisition
A Siemens 3T Trio Tim MRI scanner with a 12‐channel phased‐array coil was used to collect structural and functional MRI data. Functional MRI acquisition employed gradient‐echo echoplanar imaging sequence sensitive to blood oxygen level–dependent contrast. Whole‐brain coverage was obtained using repetition time 3 seconds, flip angle 85 degrees, echo time 30 ms, matrix 72 × 72, field of view 216 × 216 mm, and 47 × 3 mm axial slices, which resulted in isotropic voxels of 3 mm. A total of 124 volumes were acquired in each run. During the scan, participants were asked to remain awake, lie flat with their eyes open, and with a visible crosshair projected on a screen.
2.3.2. rs‐fcMRI processing and analysis
RESEARCH IN CONTEXT
Systematic Review: The literature was searched using PubMed for articles on resting‐state functional connectivity magnetic resonance imaging (rs‐fcMRI), physical activity (PA), cognition, and Alzheimer's disease (AD).
Interpretation: Increased baseline PA, measured in steps per day, was associated with greater connectivity in three AD‐relevant networks (default, salience, and left control) in which low connectivity had been shown previously to predict cognitive decline. Four other non‐cognitive networks did not demonstrate the same association. Increased connectivity associated with increased PA may account for a small degree of the protective effects of PA against longitudinal cognitive decline in individuals with elevated amyloid beta (Aβ). Increased connectivity may be one mechanism by which greater PA confers resilience with respective to cognitive decline.
Future direction: The effects of differing intensity, frequency, duration, and longitudinal changes of PA on network connectivity should be studied to better understand what types of PA may be most effective in promoting resilience related to cognitive decline.
rs‐fcMRI data processing utilized statistical parametric mapping 12 (fil.ion.ucl.ac.uk/spm/) using methods described previously. 16 Briefly, each run was normalized to the Montreal Neurological Institute (MNI) 152 EPI template using SPM‐defined normalization parameters. We employed a 6‐mm Gaussian kernel for smoothing. Additional processing involved movement correction using co‐registration parameters and first derivatives to reduce movement artifacts, as well as removing frequencies outside of the 0.01 to 0.08 Hz band with temporal bandpass filtering. Additional details regarding rs‐fcMRI data processing have been published previously. 17
The left control network was chosen because connectivity in this lateralized network has been shown previously to have robust associations with cognition and resilience to decline in symptomatic AD. 18 , 19 , 20 Notably, the standard approach to network template generation 17 naturally results in the generation of separate left and right control network templates. Template based rotation (TBR) was used to derive quantitative rs‐fcMRI measures for the default, salience, left control, primary visual, visual association, dorsal attention, and motor networks. As described previously, TBR makes use of unbiased network templates derived out‐of‐sample as comparators to participant data. The derivation of template maps for all networks utilized the Brain Genomics Superstruct Project. Methods and reproducibility of the templates have been described previously in detail. 21 Tools for TBR analysis and the network template maps are publicly available (https://habs.mgh.harvard.edu/researchers/data‐tools/). As in prior studies, we calculated TBR connectivity values for each participant and each bilateral network by computing the average correlation strength of all voxels within a particular thresholded network mask (greater than 40% of the maximum value in the corresponding template map), producing a whole network average connectivity measure.
2.3.3. Amyloid PET
11C PiB‐PET was used to assess Aβ burden, using previously published procedures. 22 Briefly, PiB‐PET images were acquired using a dynamic acquisition protocol that generated 39 volumes during a 1‐hour period following an 8.5 to 15 mCI bolus injection of 11C‐PiB. Reconstruction, attenuation correction, and evaluation for head motion were performed prior to co‐registering each image to the corresponding T1 image for each participant using a rigid‐body registration employing six degrees of freedom. The FreeSurfer‐defined cerebellar gray region‐of‐interest was used as the reference region. As in previous studies from HABS, partial volume correction was applied using geometric transformation and a distribution volume ratio (DVR) of the frontal, lateral, and retrosplenial regions (FLR) was used as the primary measure of Aβ burden. 23 Based on prior work in HABS using a Gaussian mixture modeling approach, a PiB FLR DVR of greater than 1.3 was considered Aβ positive. 24
2.4. Cognitive measures
The primary cognitive measure used was the preclinical Alzheimer cognitive composite (PACC). 25 The PACC combines z‐scored performance across five cognitive tests: the Mini‐Mental Status Examination (or MMSE), 26 the Wechsler Adult Intelligence Scale‐Revised Digit Symbol Coding, 27 the Wechsler Adult Intelligence Scale‐Revised Logical Memory Delayed Recall, 28 and the Free and Cued Selective Reminding Test (free plus cued recall). 29 The PACC is administered at study baseline and annually thereafter.
2.5. Statistical analyses
2.5.1. Baseline physical activity and network connectivity
Linear regression models implemented in R (R Foundation for Statistical Computing, version 3.5.3) were used for primary analyses. PA (mean steps per day) was log‐transformed prior to entry into models. To assess the cross‐sectional relationship of PA to network connectivity, PA was used as a predictor of connectivity in the default, salience, and left control networks (model: network connectivity ∼ PA + covariates). We included age, sex, and apolipoprotein E ε4 (APOE4) carrier status as covariates. Mean movement during MRI and the number of usable volumes were included as additional covariates to control for the quality of rs‐fcMRI measurement. False discovery rate (FDR) was employed to adjust for the number of comparisons performed. To determine the network specificity of PA associations with default, salience, and left control network connectivity, we repeated these models in four networks (dorsal attention, primary visual, visual association networks, and motor) in which connectivity strength at study baseline was not associated with cognitive decline as well as with global connectivity.
Because PA and vascular risk are often correlated, 30 we performed additional sensitivity analyses to examine the degree to which PA is independently associated with connectivity measures. We first calculated a Pearson correlation between log mean steps with the well‐described and validated body mass index (BMI)‐based Framingham cardiovascular risk score (FHS‐CVD). 31 We then included the FHS‐CVD score as an additional covariate in the same models described above (connectivity ∼ PA + FHS‐CVD + covariates).
2.5.2. Physical activity, network connectivity, and longitudinal cognition
Next, we used linear mixed‐effects models (R version 3.5.3) to examine the extent to which the protective effects of PA on cognitive decline in those with high Aβ burden may be accounted for by the effects of PA on connectivity (Figure 2). To reduce the number of comparisons being made in the longitudinal analyses of cognitive data, we derived a composite measure of cognitive network connectivity by averaging the z‐scored connectivity values in the default, salience, and left control networks. Interactions with Aβ level at baseline (assessed using PiB‐PET) were included in these models based on prior work. 6 Model 1 (below) was used to assess the interaction of PA with baseline Aβ relative to longitudinal cognitive performance. Models 2 and 3 followed on results from Model 1 and assessed whether the effects of PA and baseline Aβ on longitudinal cognitive performance may be accounted for by the effects of PA on network connectivity. In addition to MR quality measures, years of education, age, sex, and APOE4 carrier status were included as covariates, as in prior work from the HABS cohort. 6
Cognition ∼ PA*Aβ*time + covariates*time.
Cognition ∼ composite connectivity*Aβ*time + covariates*time.
Cognition ∼ PA*Aβ*time + composite connectivity*Aβ*time + covariates*time.
FIGURE 2.
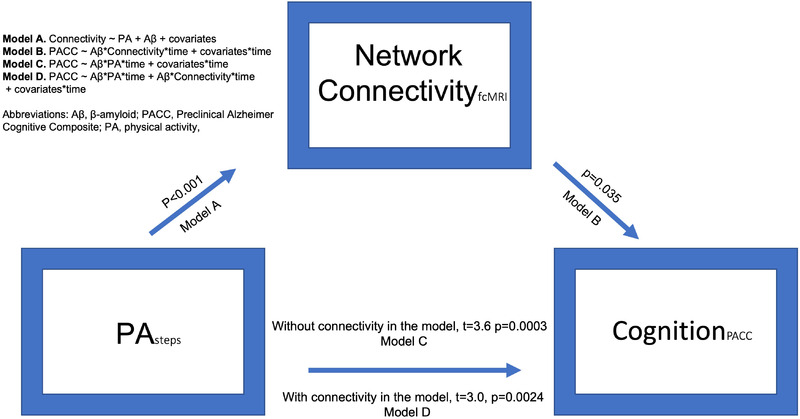
Inter‐relationships between physical activity, connectivity, and longitudinal cognitive performance. Greater physical activity (PA) in participants with elevated amyloid beta (Aβ) was associated both with lesser cognitive decline over time and with greater network connectivity. PA‐associated connectivity changes may account for a modest portion of the observed association of PA and Aβ with longitudinal cognitive performance.
3. RESULTS
3.1. Effect of physical activity on functional network connectivity
Baseline demographic and clinical characteristics are summarized in Table 1. We observed an association between greater PA and higher connectivity in the default, salience, and left control networks after correcting for multiple comparisons (default: t = 2.81(160), P = .009, salience: t = 2.60(160), P = .017, left control network: t = 3.91(160), P < .001) (Figure 1). As expected, the same association was not observed between PA levels and connectivity in the dorsal attention (P = .313), motor (P = .419), primary visual (P = .074), and visual association (P = .859) networks, or with global connectivity (Figure S1), indicating that PA relationships to connectivity were limited to the three networks shown previously to be associated with longitudinal cognitive changes in HABS.
TABLE 1.
Baseline demographic and clinical characteristics
| Characteristic | All participants (n = 167) |
|---|---|
| Age in years, mean (SD) | 74.1 (6.1) |
| Women, n (%) | 90, 53.9% |
| Education in years, mean (SD) | 15.6 (3) |
| Mean steps per day, mean (SD) | 5367 (2868) |
| Aβ PET FLR DVR (PVC) | 1.4 (0.4) |
| Aβ PET FLR DVR (PVC) > 1.3, n (%) | 56, 33.5% |
| Aβ PET FLR DVR (PVC) < 1.3, n (%) | 111, 66.5% |
| APOE ε4 carriers, n (%) | 52, 31% |
Abbreviations: APOE ε4, apolipoprotein E ε4, Aβ, amyloid beta; DVR, distribution volume ratio; FLR, frontal, lateral temporal and parietal, and retrosplenial regions; PET, positron emission tomography.
FIGURE 1.
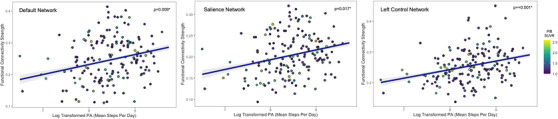
Associations between level of physical activity and network connectivity. Log‐transformed measures of mean steps per day were compared with connectivity within the default (left), salience (middle), left control (right), motor, primary visual, and visual association networks. Greater levels of physical activity were selectively associated with higher connectivity strengths in each of these networks.
In analyses performed to account for vascular risk factors, we found PA and FHS‐CVD to be weakly correlated in the anticipated direction (r = ‐0.16, P = .03). Associations of PA with connectivity in the default (P = .02), salience (P = .02), and control networks (P < .01) remained significant when including FHS‐CVD as a covariate, suggesting PA has an effect independent of systemic vascular risk on network connectivity.
3.2. Effect of physical activity, Aβamyloid, and network connectivity on longitudinal cognition
As both higher PA levels and higher connectivity in the default, salience, and control networks have been associated previously with less cognitive decline, 6 we next examined whether the potential protective effects of PA may be accounted for by increased mean network connectivity (Figure 2). As the effects of PA on longitudinal cognitive performance are seen largely in older adults with higher Aβ, interactions with Aβ levels were included for both PA and connectivity. As shown previously, 6 greater PA was associated with slower cognitive decline in individuals with higher Aβ (PA*Aβ, F[1,917] = 12.74, P < .001). Similarly, we observed a significant interaction of connectivity with Aβ in predicting longitudinal cognitive performance (P = .035). A third model in which both PA and connectivity were included suggests that PA effects on connectivity may account for a modest degree of the association of PA and Aβ with longitudinal cognitive performance (Figure 2, Supplementary table S2). Together, these results suggest that connectivity moderates the association of PA with cognition, although this result should be interpreted in the context of the Aβ and PA interaction included in these models.
4. DISCUSSION
We examined the associations between PA, network connectivity, Aβ, and longitudinal cognitive performance in a deeply phenotyped, longitudinal cohort of older adults. We demonstrate that objectively measured levels of PA are positively correlated with the integrity of resting state connectivity networks. Notably, the association of PA and connectivity was limited to a set of three networks—the default, salience, and left control networks—in which connectivity strength has been shown previously to predict longitudinal cognitive performance, particularly in individuals with elevated Aβ. 9 Next we observed suggestive evidence that the association between PA and network connectivity may account for a modest portion of the protective effects of PA on longitudinal cognitive change in preclinical AD, although this conclusion requires further confirmation. This study suggests that connectivity is one link between two previous findings in preclinical AD; first that increased PA is associated with resilience to cognitive decline, 6 , 7 and second that higher connectivity within a subset of cognitive networks may be associated with less cognitive decline over time, particularly in persons with higher Aβ levels. 9 Together, our findings suggest that one mechanism by which PA promotes resilience to cognitive decline may be through promoting the integrity of neural networks underlying key aspects of memory and attention.
Previous studies suggest that neurodegenerative diseases may involve the selective vulnerability and deterioration of large‐scale functional networks within the brain. 32 In AD, changes in default, salience, and control networks have been well described, 18 , 19 , 20 , 33 , 34 , 35 dovetailing with the observation here that increased connectivity is associated with PA in these same networks. A recent study demonstrated that interactive aberrant connectivity in the overlapping nodes of the default, salience, and control networks is seen in AD dementia and associated with worse scores on the MMSE. 36 These findings and those of the present study are consistent with the hypothesis that greater connectivity is one possible contributor to the protective effects of PA against cognitive decline and AD dementia. The effects of both PA and rs‐fcMRI on cognitive decline are most clear in individuals with higher levels of Aβ burden, perhaps because this population is at the highest risk of progressive neurodegeneration and loss of cognitive performance.
PA was associated only with increased connectivity in networks related to memory and attention, although the cause for this selectivity is unknown. Going forward, future studies that elucidate the mechanisms underlying this selectivity will be needed to further inform the relationship between PA and cognition. One possibility is through network or cell‐targeted molecular mechanisms via release of activity‐induced neurotrophic factors that act on proteins expressed in specific neurons, structures, or networks in the brain. For example, Fibronectin type III domain‐containing protein 5 (FNDC5)/irisin is an exercise‐associated myokin expressed in the hippocampus that when knocked down impairs long‐term potentiation, and conversely, when overexpressed, rescues memory impairment in AD mouse models. 37
Although more work is needed, our study suggests that rs‐fcMRI may be useful in evaluating non‐pharmacologic interventions in preclinical AD and mild cognitive impairment, particularly those with a PA‐related intervention. Boraxbekk et al. demonstrated a PA score to be positively associated with connectivity in the posterior default network in a study of healthy older individuals, consistent with our results. 38 Notably, the results here are consistent with a small prospective study of MCI participants engaged in a 12‐week walking exercise program, where significant increases in connectivity were observed in 10 brain regions (including the posterior cingulate and precuneus) over the course of the intervention. 39 Connectivity levels and changes in response to interventions in AD‐relevant brain regions could potentially be used to help determine or predict the intensity, frequency, and type of PA or other non‐pharmacologic interventions that have the greatest potential to benefit cognition and delay the onset of dementia.
There are important limitations in this study to consider, and this work should be interpreted in the context of the participants studied. HABS participants tend to be more highly educated than the general population, which may affect the generalizability of the results here. In addition, people with severe or unstable cerebrovascular disease are excluded from the study. Furthermore, this study makes use of baseline Aβ PET, PA, and rs‐fcMRI measures, and future work using longitudinal data may strengthen the causal inferences suggested by this study. Similarly, PA may need to be measured in greater detail (e.g., for intensity) or for longer periods to understand the PA more completely and rs‐fcMRI relationships. Future work should aim to understand these important variables to inform the design of non‐pharmacologic lifestyle interventions intended to prolong healthy cognition.
5. CONCLUSION
Although greater levels of PA are widely thought to confer resilience for late‐life cognitive decline, the linkage between PA and brain health have not been fully elucidated. By utilizing data in which objective measures of PA, functional connectivity, AD pathology, and longitudinal cognitive performance were available, we examined how variations in PA are reflected in the integrity of anatomically distributed neural networks known to be important for cognition. We observed an intriguing relationship between greater levels of PA and higher connectivity in a subset of neural networks in older adults at an elevated risk of developing AD. These relationships between PA and connectivity were limited to three networks (default, salience, and left control) that have been shown previously to be degraded in early AD and are linked to performance in memory, attention, and executive function intensive tasks, 9 , 10 , 11 , 12 , 13 , 14 suggesting that PA may confer resilience to cognitive decline, partly through increased integrity within a subset of AD‐sensitive neural networks. Together with prior work, the results here also suggest that rs‐fcMRI may be a useful tool in optimizing and evaluating the effectiveness of PA‐modifying interventions in cognitive aging and pre‐clinical AD.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
SUPPORTING INFORMATION
SUPPORTING INFORMATION
ACKNOWLEDGEMENTS
We thank the study participants of the Harvard Aging Brain Study. This work was supported by the National Institutes of Health (NIH; grant numbers P01AG036694 [Drs. Johnson and Sperling]; K23AG049087, and R01 AG062667 [Dr. Chhatwal]; Alzheimer's Association Clinical Scientist Fellowship Program (AACSF‐20‐685828) [Dr. Pruzin]; and the Doris Duke Charitable Foundation Clinical Scientist Development Award (Dr. Chhatwal). This research was carried out in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), NIH. This work also involved the use of instrumentation supported by the NIH Shared Instrumentation Grant Program and/or High‐End Instrumentation Grant Program; specifically, grant numbers S10RR021110, S10RR023401, and S10RR023043. Funding sources had no involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. This project was also supported by the National Institute on Aging (P01 AG036694).
Pruzin JJ, Klein H, Rabin JS, et al. Physical activity is associated with increased resting‐state functional connectivity in networks predictive of cognitive decline in clinically unimpaired older adults. Alzheimer's Dement. 2022;14:e12319. 10.1002/dad2.12319
Jeremy J. Pruzin and Hannah Klein contributed equally to this work.
REFERENCES
- 1. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9(1):119‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sofi F, Valecchi D, Bacci D, et al. Physical activity and risk of cognitive decline: a meta‐analysis of prospective studies. J Intern Med. 2011;269(1):107‐117. [DOI] [PubMed] [Google Scholar]
- 3. Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer's disease. Alzheimers Dement. 2015;11(9):1007‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuehn BM. Nearly half of dementia cases could be prevented or delayed. JAMA. 2020;324(11):1025. [DOI] [PubMed] [Google Scholar]
- 5. Wilson RS, Wang T, Yu L, Grodstein F, Bennett DA, Boyle PA. Cognitive activity and onset age of incident Alzheimer disease dementia. Neurology. 2021. 10.1212/WNL.0000000000012388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rabin JS, Klein H, Kirn DR, et al. Associations of physical activity and β‐Amyloid with longitudinal cognition and neurodegeneration in clinically normal older adults. JAMA Neurol. 2019;76(10):1203‐1210. 10.1001/jamaneurol.2019.1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchman AS, Yu L, Wilson RS, et al. Physical activity, common brain pathologies, and cognition in community‐dwelling older adults. Neurology. 2019;92(8):e811‐e822. 10.1212/WNL.0000000000006954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greicius MD, Srivastava G, Reiss AL, Menon V. Default‐mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637‐4642. 10.1073/pnas.0308627101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buckley RF, Schultz AP, Hedden T, et al. Functional network integrity presages cognitive decline in preclinical Alzheimer disease. Neurology. 2017;89(1):29‐37. 10.1212/WNL.0000000000004059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Butt OH, Meeker KL, Wisch JK, et al. Network dysfunction in cognitively normal APOE ε4 carriers is related to subclinical tau. Alzheimers Dement. 2021. 10.1002/alz.12375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hampton OL, Buckley RF, Manning LK, Schultz AP. Resting‐state functional connectivity and amyloid burden influence longitudinal cortical thinning in the default mode network in preclinical Alzheimer's disease. Neuroimage Clin. 2020;28:102407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chhatwal JP, Schultz AP, Hedden T, et al. Anticholinergic amnesia is mediated by alterations in human network connectivity architecture. Cereb Cortex. 2019;29(8):3445‐3456. 10.1093/cercor/bhy214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zuo N, Yang Z, Liu Y, Li J, Jiang T, Core networks and their reconfiguration patterns across cognitive loads. Hum Brain Mapp. 2018;39(9):3546‐3557. 10.1002/hbm.24193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grayson DS, Fair DA. Development of large‐scale functional networks from birth to adulthood: a guide to the neuroimaging literature. Neuroimage. 2017;160:15‐31. 10.1016/j.neuroimage.2017.01.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tudor‐Locke C, Burkett L, Reis JP, Ainsworth BE, Macera CA, Wilson DK. How many days of pedometer monitoring predict weekly physical activity in adults? Prev Med. 2005;40(3):293‐298. 10.1016/j.ypmed.2004.06.003 [DOI] [PubMed] [Google Scholar]
- 16. Chhatwal JP, Schultz AP, Johnson KA, et al. Dominantly Inherited Alzheimer Network. Preferential degradation of cognitive networks differentiates Alzheimer's disease from ageing. Brain. 2018;141(5):1486‐1500. 10.1093/brain/awy053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schultz AP, Chhatwal JP, Huijbers W, et al. Template based rotation: a method for functional connectivity analysis with a priori templates. Neuroimage. 2014;102(Pt 2(0 2)):620‐636. 10.1016/j.neuroimage.2014.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Franzmeier N, Hartmann JC, Taylor ANW, et al. Left frontal hub connectivity during memory performance supports reserve in aging and mild cognitive impairment. J Alzheimers Dis. 2017;59(4):1381‐1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Franzmeier N, Duering M, Weiner M, Dichgans M, Ewers M. Alzheimer's Disease Neuroimaging Initiative. Left frontal cortex connectivity underlies cognitive reserve in prodromal Alzheimer disease. Neurology. 2017;88:1054‐1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Franzmeier N, Düzel E, Jessen F, et al. Left frontal hub connectivity delays cognitive impairment in autosomal‐dominant and sporadic Alzheimer's disease. Brain. 2018;141(4):1186‐1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buckner RL, Roffman JL, Smoller JW. Brain Genomics Superstruct Project (GSP). V10. Harvard Dataverse; 2014. 10.7910/DVN/25833 [DOI] [Google Scholar]
- 22. Sperling RA, Laviolette PS, O'Keefe K, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178‐188. 10.1016/j.neuron.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mormino EC, Betensky RA, Hedden T, et al. Alzheimer's Disease Neuroimaging Initiative; Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing; Harvard Aging Brain Study. Amyloid and APOE ε4 interact to influence short‐term decline in preclinical Alzheimer disease. Neurology. 2014;82(20):1760‐1767. 10.1212/WNL.0000000000000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mormino EC, Betensky RA, Hedden T, et al. Synergistic effect of β‐amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol. 2014;71(11):1379‐1385. 10.1001/jamaneurol.2014.2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Donohue MC, Sperling RA, Salmon DP, et al. Australian Imaging, Biomarkers, and Lifestyle Flagship Study of Ageing; Alzheimer's Disease Neuroimaging Initiative; Alzheimer's Disease Cooperative Study. The preclinical Alzheimer cognitive composite: measuring amyloid‐related decline. JAMA Neurol. 2014;71(8):961‐970. 10.1001/jamaneurol.2014.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Folstein MF, Folstein SE, McHugh PR. Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 27. Wechsler D. WAIS‐R Manual: Wechsler Adult Intelligence Scale‐Revised. Psychological Corporation; 1981. [Google Scholar]
- 28. Wechsler D. WMS‐R: Wechsler Memory Scale‐Revised. Psychological Corporation; 1987. [Google Scholar]
- 29. Grober E, Lipton RB, Hall C, Crystal H. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54(4):827‐832. 10.1212/wnl.54.4.827 [DOI] [PubMed] [Google Scholar]
- 30. Lavie CJ, Ozemek C, Carbone S, Katzmarzyk PT, Blair SN. Sedentary behavior, exercise, and cardiovascular health. Circ Res. 2019;124(5):799‐815. 10.1161/CIRCRESAHA.118.312669 [DOI] [PubMed] [Google Scholar]
- 31. Sr D'AgostinoRB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743‐753. 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 32. Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large‐scale human brain networks. Neuron. 2009;62(1):42‐52. 10.1016/j.neuron.2009.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sheline YI, Raichle ME, Snyder AZ, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67(6):584‐587. 10.1016/j.biopsych.2009.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Elman JA, Madison CM, Baker SL, et al. Effects of beta‐amyloid on resting state functional connectivity within and between networks reflect known patterns of regional vulnerability. Cereb Cortex. 2016;26(2):695‐707. 10.1093/cercor/bhu259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Drzezga A, Becker JA, Van Dijk KR, et al. Neuronal dysfunction and disconnection of cortical hubs in non‐demented subjects with elevated amyloid burden. Brain. 2011;134(Pt 6). 10.1093/brain/awr066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li C, Li Y, Zheng L, et al, Alzheimer's Disease Neuroimaging Initiative . Abnormal brain network connectivity in a triple‐network model of Alzheimer's disease. J Alzheimers Dis. 2019;69(1):237‐252. 10.3233/JAD-181097 [DOI] [PubMed] [Google Scholar]
- 37. Lourenco MV, Frozza RL, de Freitas GB, et al. Exercise‐linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer's models. Nat Med. 2019;25(1):165‐175. 10.1038/s41591-018-0275-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boraxbekk CJ, Salami A, Wåhlin A, Nyberg L. Physical activity over a decade modifies age‐related decline in perfusion, gray matter volume, and functional connectivity of the posterior default‐mode network‐a multimodal approach. Neuroimage. 2016;131:133‐141. 10.1016/j.neuroimage.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 39. Chirles TJ, Reiter K, Weiss LR, Alfini AJ, Nielson KA, Smith JC. Exercise training and functional connectivity changes in mild cognitive impairment and healthy elders. J Alzheimers Dis. 2017;57(3):845‐856. 10.3233/JAD-161151 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION
SUPPORTING INFORMATION


