Abstract
Cosmetic products containing hemp seed oil as permitted raw materials required the specific compound delta-9-tetrahydrocannabinol (THC) below 10 μg/g. THC was the main psychoactive constituent of cannabis. Since hemp seed oil became an increasingly popular ingredient in cosmetics over the last few years, an efficient and reliable analytical method for THC and other cannabinoids in cannabis-infused cosmetic products was in need. A liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for the determination of delta-9-tetrahydrocannabinol (THC), cannabidiol (CBD), and cannabinol (CBN) in hemp seed oil based cosmetic products was developed. Method validation was performed by fertilizing blank samples with analytes and internal standards (THC-d3, CBD-d3, and CBN-d3). Chromatographic method utilized a Xbridge BEH Shield RP18 column with gradient elution containing 10 mM ammonium formate in water and methanol provided successful separation of THC, CBD, and CBN in cosmetic matrix. The combination of MS detection in positive electrospray ionization (ESI) and multiple reaction monitoring (MRM) mode offered rapid run time 13 minutes with limit of quantification (LOQ) of 0.05 μg/g. The intra- and inter-day recoveries were 79.23–114.04% and 83.55–111.61% with spiking levels ranged between 0.05 μg/g and 0.5 μg/g, respectively. Surveillance results of 90 cosmetic products showed 22, 34, and 5 products containing THC (0.06–1777 μg/g), CBD (0.47–37217 μg/g), and CBN (2.2–25.2 μg/g), respectively. This validated method offered accurate, reliable, and fast way for the determination of drug contaminations including THC, CBD, and CBN in cosmetics. The surveillance results for commercial cosmetic products purchased in Taiwan between 2018–2020 provided valuable background references for THC, CBD, and CBN in hemp seed oil based cosmetic products, and could be used for administration purpose.
Keywords: Cannabidiol, Cannabinol, Cosmetics, Tetrahydrocannabinol
1. Introduction
The term “hemp”, or industrial hemp, is a variety of the Cannabis sativa plant species that is grown specifically for industrial use [1]. It can be refined into a variety of commercial items, including paper, rope, textiles, clothing, biofuel, food, and animal feed. These products require not more than 0.3 percent psychoactive component THC in general. However, the legality of industrial hemp varies widely between countries. Some area regulates THC and permits only hemp that is bred with an especially low THC content [1]. By contrast, another plant similar to hemp called “marijuana” or “weed” refers to the dried leaves, flowers, stems, and seeds from the Cannabis sativa or Cannabis indica plant, contained 5–20% THC was prohibited.
Hemp seed oil, obtained from the seed of Cannabis sativa L., is a naturally rich source of high quality protein and essential polyunsaturated fatty acids including omega-6 and omega-3 [2]. The health benefits of hemp seed oil are attributed mainly to its desirable omega-3 and omega-6 fatty acid ratio, 3:1, which is suggested as being optimal for human nutrition [3–5]. This unique composition of hemp seed oil differs from the other common seed oils and offers opportunities for the development of special nutritional formulations, personal care products, and cosmetics such as soap, lotion, shampoos and lipsticks [3,6–8]. Studies suggested that hemp seed and its oil may benefit skin disorders, improve itchiness, and reduce irritation [9, 10]. In recent years, researchers have also investigated its use for anti-inflammatory properties [11, 12]. The advantages to hair such as hair growth, and prevention of dry, split ends, and breakage were reported [13].
Nowadays, cosmetic regulatory compliance of cannabis-derived ingredients as plant extracts, and does not prohibit or restrict the use of them in cosmetics. However, the amounts of Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) are strictly regulated and limited. THC is the major psychoactive component of Cannabis sativa L. and may cause central nerve system disorders. In addition to THC, hemp seed oil contains many other minor cannabinoids including CBD and CBN. CBD is the second highest component in Cannabis sativa L., and accounts for up to 40% of the plant’s extract [14]. CBN was an oxidation byproduct of THC and was the first identified cannabinoid and commonly found in aged cannabis materials. Although CBN was not produced by the metabolism of the plant, it was easily formed from THC by degradation during drying, storing, and consumption (heating) of cannabis products. As a result, it may play a significant role in quality control attributed to Cannabis utilization. The commonly used detection methods for cannabinoids were gas chromatography-mass spectrometry (GC-MS) [15], HPLC-PDA [16], HPLC-fluorescence, and liquid chromatography-mass spectrometry (LC-MS/MS) [16–19]. Most of them used for forensic analysis of hair [18], blood [20], urine [21], and oral fluid [17], while others focused on natural products such as hemp seed oil [16] and herbal products [19], only a few were interested in cosmetics [22]. Cosmetic products contained high oils, surfactants, and oil-in-water emulsions, which made sample preparation difficult. Solvent system such as hexane, ethyl acetate, isopropyl alcohol, acetonitrile, methanol along with liquid-liquid extraction were developed for testing water or oil based samples [17–20, 22]. Solid phase extraction (SPE) may be applied to remove phospholipids (PLs) in blood samples [20].
This study aimed on the rapid determination of cannabinoids such as THC, CBD, and CBN in hemp seed oil based cosmetic products. A relatively high polarity solvent, methanol, was selected and tested for rapid sample preparation without further SPE purification. In this respect, multiple matrices were investigated including lotion, cream, essential oil, shampoo, hair colorant, shower gel, and lipstick products. A surveillance study consisted with 90 commercial cosmetics demonstrated the relationship between cannabinoid contents and product types was discussed.
2. Materials and methods
2.1. Chemicals, reagents and samples
Δ9-Tetrahydrocannabinol (THC, 1 mg/mL in methanol), cannabidiol (CBD, 1 mg/mL in methanol), cannabinol (CBN, 1 mg/mL in methanol), Δ9-tetrahydrocannabinol-d3 (THC-d3, 0.1 mg/mL in methanol), cannabidiol-d3 (CBD-d3, 0.1 mg/mL in methanol), and cannabinol-d3 (CBN-d3, 0.1 mg/mL in methanol) were purchased from Sigma Aldrich (St. Louis, MO, USA). Hemp seed oil based cosmetic products were either purchased from local market or supported from customs and prosecution.
2.2. Sample preparation
Cosmetic samples were homogenized, and then 0.2 g each was accurately weighed into a 10-mL volumetric flask. The flask was added approximately 8 mL methanol as extraction solvent and 25 μL of the internal standards (IS) mixed solution (10 μg/mL each in methanol). Analytes was extracted by ultrasound-assisted extraction with methanol for 30 min. The sample was dilute with methanol to 10 mL, filtered through a 0.22 μm PTFE membrane prior to LC-MS/MS analysis.
2.3. Separation and detection
Chromatographic separation was performed using ACQUITY UPLC H-Class system (Waters Corp., Milford, MA, USA) and a Xbridge BEH Shield RP18 column (3.0 mm × 100 mm, 2.5 μm particle size, Waters) at flow rate of 0.6 mL/min. The column was kept at 40°C and the sample was maintained at 15°C. The mobile phase of the LC system consisted of 10 mM ammonia formate buffer (A) and 10 mM ammonia formate in methanol (B). Linearly gradient was programed as follows: 0–5 min, 80% B; 5–6 min, 80–100% B; 6–8 min, 100% B; 8–8.1 min, 100–80% B; 8.1–13 min, 80% B. The sample injection volume was 3 μL. MS detection was performed by a Xevo TQ-XS triple quadrupole mass spectrometer (Waters) at positive electrospray ionization. Multiple reaction monitoring (MRM) parameters of THC, CBD, and CBN were listed as Table 1.
Table 1.
MS/MS conditions for THC, CBD, and CBN.
| Analytes | Polarity (ES) | Retention Time (min) | Quantification ion | Qualification ion | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Precursor ion > Product ion (m/z) | C.V. (V) | C.E. (eV) | Precursor ion > Product ion (m/z) | C.V. (V) | C.E. (eV) | |||
| THC | + | 3.82 | 315 > 193* | 40 | 20 | 315 > 259 | 40 | 15 |
| CBD | + | 2.34 | 315 > 193* | 40 | 20 | 315 > 259 | 40 | 15 |
| CBN | + | 3.42 | 311 > 223* | 30 | 15 | 311 > 241 | 30 | 18 |
| THC-d3 | + | 3.81 | 318 > 196 | 40 | 15 | |||
| CBD-d3 | + | 2.33 | 318 > 196 | 40 | 15 | |||
| CBN-d3 | + | 3.40 | 314 > 223 | 30 | 15 | |||
Abbreviations: C.V., cone voltage; C.E., collision energy.
2.4. Method validation
2.4.1. Limits of quantification (LOQs)
Blank cosmetic samples were spiked with selected concentrations of THC, CBD, and CBN at 5, 10, 20, 25, 50, and 100 ng/g. Limit of quantification (LOQ) was evaluated by measuring the lowest concentration level in spiked samples while the signal-to-noise ratios were least 10:1 or higher for quantification ions.
2.4.2. Linearity of calibration
Triplicate six-point calibration curves with concentration ranging from 1–50 ng/mL for THC, CBD, and CBN were prepared in methanol and fitted with a weighted (1/concentration) linear equation. The correlation of coefficient (r) should be above 0.99. For quantitative applications, individual standards should not deviate by more than 20% from the nominal value.
2.4.3. Accuracy and precision
A total of five replicate quality control (QC) samples at each of 4 different concentrations (50, 100, 250, 500 ng/g) in five different matrices (lotion, essential oil, shampoo, shower gel, and lip balm) were tested for intra-day and inter-day precision and accuracy. Inter-day studies were tested on three different dates. The requirement of repeatability precision of intra-day results must be within 15–20% coefficient of variation (CV) of the nominal value, and intermediate precision must within 18–22% CV.
2.4.4. Matrix effects and recovery studies
Matrix effects were measured by comparing the peak area of analytes in spiked sample extracts and in solvent. The percentage provided information about ion suppression or enhancement caused by cosmetic matrices. Sample type for matrix effect evaluation included lotion, essential oil, shampoo, shower gel and lip balm. For each compound, four concentrations (50, 100, 250, 500 ng/g) were tested with triplicates. Recoveries were calculated by comparing the mean peak areas of analytes in blank matrix and in solvent. Besides, internal standard method with isotopically labeled internal standards, THC-d3, CBD-d3, and CBN-d3 was also evaluated. The acceptable recoveries should be within 75–120% and precision must not exceed 10% CV.
2.5. Surveillance of THC, CBD, and CBN in cosmetics
Eighty-three samples were detained from customs and prosecutors. Seven samples were purchased from local department stores and drugstores. Sample types included lotions, creams, serums, essential oil, shampoos, treatments, shower gels, and lip balm. Each test samples were analyzed in duplicate using the validated method. Quality control (QC) samples were spiked with THC, CBD and CBN and internal standards in each of matrices.
3. Results and discussion
3.1. Sample preparation
Hemp seed oil based cosmetics contained relatively low polarity contents. Hence, relatively high polarity solvent such as isopropyl alcohol, ethyl alcohol and methyl alcohol were tested for cannabinoids extraction to reduce the interference of low polarity contents such as oils and fats. Among these solvents tested, methanol was found offered good extraction yield and less fat solubility. The followed ultrasonication assisted analytes releasing from samples. Satisfactory recovery rates were obtained using this simple and rapid sample preparation procedure without further cleaning and purification.
3.2. Optimized separation and MS detection
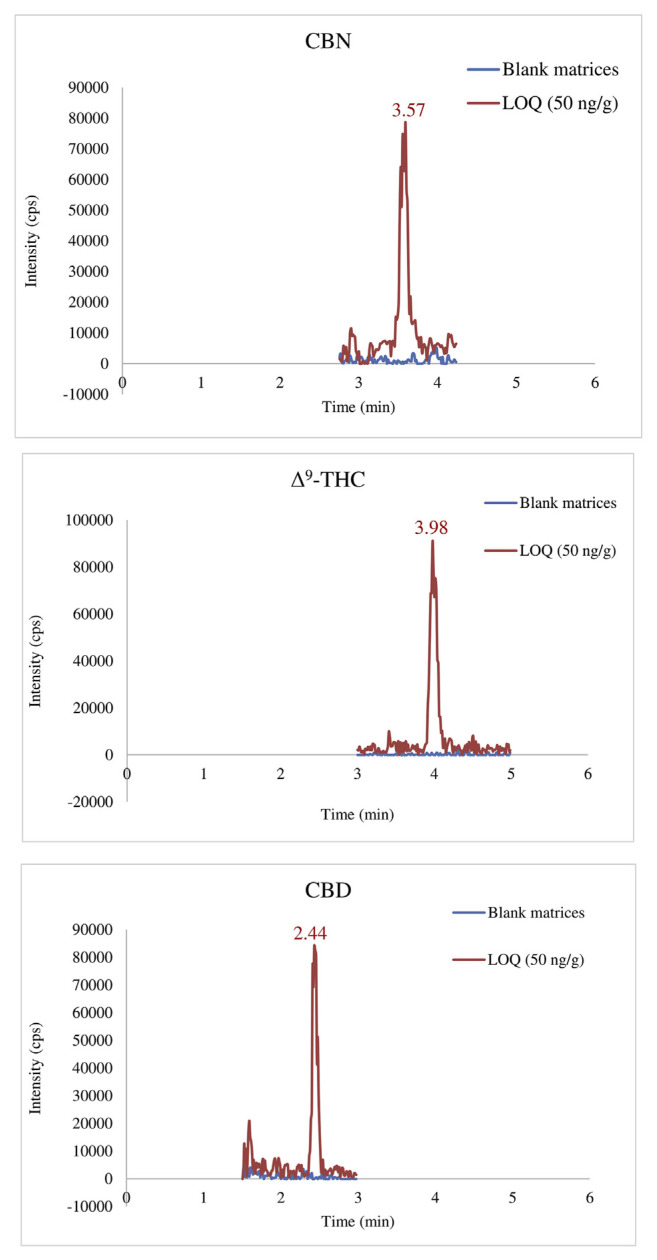
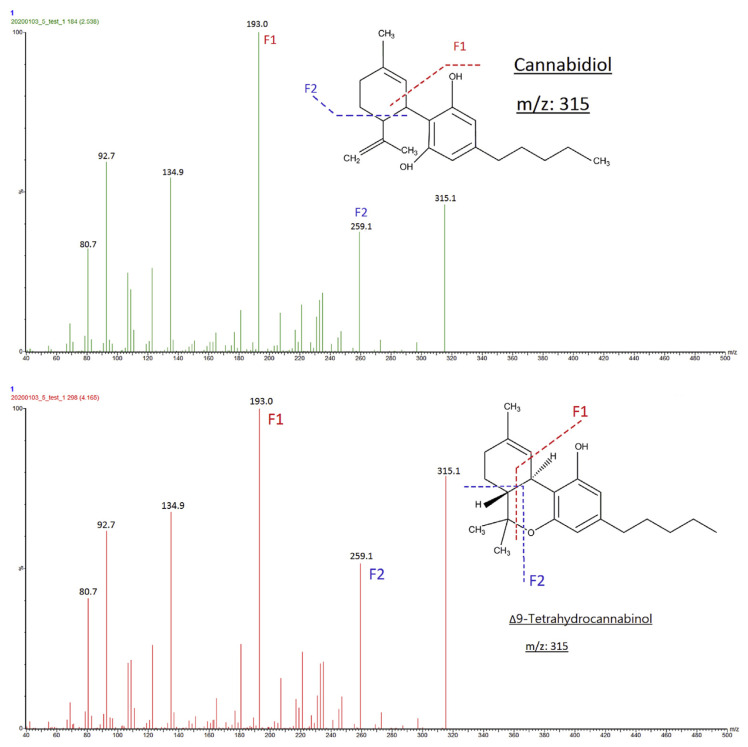
Conventional C18 columns could initially be used with an appropriate gradient. Therefore, LC separation was evaluated on Shiseido CAPCELL PAK C18 column (2.7 μm, 4.6 × 150 mm) and Xbridge BEH Shield RP18 column through isocratic and gradient elutions with mobile phases such as methanol and acetonitrile system. The results found that Xbridge BEH Shield RP18 column with solvent system contained 10 mM ammonium formate provided adequate separation. The retention time (RT) for CBD, THC, and CBN was 2.44, 3.98, and 3.57 min, respectively (Fig. 1). Total run time was 13 minutes in gradient mode. The mass spectrometer was in positive ESI mode. THC and CBD were isomers with the same monoisotope mass of 314.22 Dalton (Da) and showed an identical precursor to product ion transitions (315 /193 and 259, Fig. 2).
Fig. 1.
Chromatograms for blank matrices (dash) and spiked sample at limit of quantification 50 ng/g (solid).
Fig. 2.
Fragments spectra of standards CBD (A) and THC (B).
3.3. Method validation
3.3.1. Accuracy, precision, and recovery
Precision and accuracy data were summarized in Table 2. The coefficient of variation of QC samples was less than 10% of nominal concentrations for THC, CBD, and CBN. In most samples, accuracies were within 15% and CV were less than 10% (ranged between 4.4 and 8.7% for CBD, 3.2 and 9.3% for CBN, 1.8 and 9.0% for THC). Similar results were seen with other matrices of essential oil, shampoo, shower gel, and lip balm. The intra-day recoveries ranged from 79–113% for CBD, 87–106% for CBN, and 84–114% for THC (Table 2). The obtained results demonstrated the analytical method for the determination of THC, CBD, and CBN in hemp oil based cosmetics was validated in this work.
Table 2.
Intra- and inter-day precision and accuracy results in different sample type.
| Sample type | Spiked level (ng/g) | Intra-day (n = 5) | Inter-day (n = 15) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||||||
| Analyte | 50 | 100 | 250 | 500 | 50 | 100 | 250 | 500 | |||||||||
|
|
|
|
|
|
|
|
|
||||||||||
| REC* (%) | CV* (%) | REC (%) | CV (%) | REC (%) | CV (%) | REC (%) | CV (%) | REC (%) | CV | REC (%) (%) | CV (%) | REC (%) | CV (%) | REC (%) | CV (%) | ||
| Lotion | CBD | 98.80 | 7.14 | 87.73 | 4.43 | 87.03 | 8.67 | 113.47 | 7.87 | 101.50 | 8.71 | 91.92 | 7.46 | 90.85 | 8.31 | 104.00 | 8.57 |
| CBN | 90.20 | 8.42 | 86.70 | 7.84 | 92.43 | 4.13 | 95.37 | 4.52 | 92.27 | 7.69 | 90.75 | 9.33 | 94.87 | 5.91 | 92.55 | 5.13 | |
| THC | 106.54 | 7.00 | 87.23 | 5.31 | 84.17 | 4.70 | 106.68 | 7.47 | 110.63 | 8.66 | 94.68 | 8.77 | 88.88 | 6.36 | 98.08 | 8.50 | |
| Essential oil | CBD | 92.60 | 5.37 | 97.40 | 5.92 | 88.27 | 6.04 | 98.67 | 3.91 | 99.96 | 9.19 | 95.81 | 5.65 | 91.38 | 6.26 | 97.14 | 5.72 |
| CBN | 91.12 | 7.94 | 92.34 | 5.01 | 96.57 | 4.25 | 101.95 | 4.34 | 91.53 | 5.04 | 89.85 | 5.92 | 97.30 | 4.85 | 98.71 | 5.43 | |
| THC | 114.04 | 4.48 | 107.72 | 7.17 | 93.35 | 3.45 | 105.92 | 3.41 | 111.61 | 6.47 | 103.51 | 6.09 | 95.43 | 6.47 | 98.60 | 6.75 | |
| Shampoo | CBD | 86.38 | 4.36 | 84.55 | 3.45 | 82.51 | 4.15 | 100.06 | 3.01 | 90.63 | 7.10 | 91.06 | 8.45 | 88.91 | 8.61 | 95.52 | 9.92 |
| CBN | 96.74 | 6.64 | 100.61 | 7.73 | 95.28 | 5.05 | 99.96 | 3.61 | 91.82 | 5.79 | 93.38 | 9.35 | 97.60 | 4.62 | 101.21 | 3.54 | |
| THC | 90.90 | 5.95 | 102.65 | 6.06 | 92.92 | 2.20 | 108.84 | 1.90 | 97.41 | 7.71 | 101.48 | 6.12 | 93.31 | 5.23 | 101.06 | 6.28 | |
| Shower gel | CBD | 86.56 | 2.95 | 79.23 | 4.59 | 84.77 | 8.68 | 95.14 | 5.86 | 92.06 | 6.85 | 88.03 | 8.75 | 89.57 | 8.35 | 94.10 | 6.66 |
| CBN | 92.04 | 6.35 | 88.22 | 5.30 | 94.88 | 6.44 | 102.56 | 3.29 | 83.55 | 9.91 | 85.55 | 7.44 | 90.34 | 6.34 | 98.00 | 6.69 | |
| THC | 99.94 | 8.94 | 93.92 | 8.91 | 92.51 | 1.77 | 104.74 | 6.66 | 106.01 | 7.78 | 97.11 | 5.90 | 95.27 | 6.29 | 100.64 | 6.28 | |
| Lip balm | CBD | 100.68 | 6.06 | 92.26 | 5.33 | 91.85 | 5.01 | 101.61 | 8.42 | 101.65 | 7.24 | 95.12 | 5.59 | 93.04 | 5.95 | 96.78 | 8.51 |
| CBN | 106.08 | 9.31 | 97.19 | 2.79 | 102.77 | 3.21 | 102.12 | 5.52 | 104.46 | 9.82 | 98.65 | 7.49 | 97.50 | 6.07 | 104.68 | 7.42 | |
| THC | 98.12 | 3.34 | 95.89 | 2.95 | 90.87 | 3.93 | 103.71 | 2.75 | 102.40 | 6.30 | 97.79 | 3.41 | 93.95 | 5.61 | 98.02 | 5.57 | |
REC, recovery; CV, coefficient of variation.
3.3.2. Limits of quantification (LOQs)
Limit of quantification (LOQs) levels were established for each cannabinoid. The LOQs in this study was found to be 50 ng/g for THC, CBN, and CBD. Precision and accuracy at this concentration level was determined to be satisfactory (Fig. 1).
3.3.3. Linearity of calibration
Standard curves were made in triplicate for each concentration of THC, CBD, and CBN. Good linearity was achieved over the concentration of 1–50 ng/mL with the application of a weighting factor (1/x). The correlation coefficient values were r ≥ 0.999 for THC, r ≥ 0.998 for both CBN and CBD.
3.3.4. Matrix effects
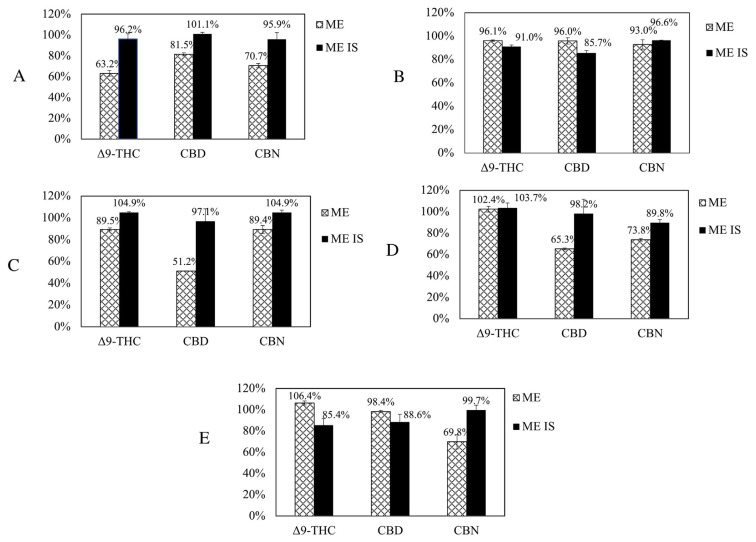
Matrix effect is due to the presence of coeluting matrix compounds that affect analyte ionizations leading to notable errors in quantification, and affects accuracy in LC-MS/MS method, unless adequately corrected or minimized. The use of internal standard is one of the most suitable for a reliable correction. Five matrix of cosmetics including lotion, essential oil, shampoo, shower gel, and lip balm were tested in this study, and the matrix effect was defined as the response ratio between analytes in matrix extract and analytes in solvent at the concentration of 0.5 μg/g. The results showed in Fig. 3 that signal suppression was observed higher in lotion than in essential oil, shampoo and lip balm. The use of internal standard correction observed better and satisfied results in most tested samples. In essential oil matrix (Fig. 2B), internal standard correction presented few negative effects. This may due to the complicate formula of essential oil and the higher content of solvents in essential oil products which may affect sample extraction. Consequently, the use of isotopically labeled internal standards was highly recommended for the analysis of cosmetic products.
Fig. 3.
Average matrix effects (ME, presented as recovery) for (A) lotion, (B) essential oil, (C) shampoo, (D) shower gel and (E) lip balm samples, before and after correction with internal standards (ME IS).
3.4. Surveillance results of cosmetics
According to regulations for the control of cosmetic hygiene, cosmetics are defined as products designed for topical use, such as moisturizers, deodorants, and makeup. A surveillance study consisted of 90 cosmetic samples was conducted. All of them claimed containing hemp seed oil or hemp seed extract as ingredient. Most of them were leave-on products (44 samples) including lotions and creams. Others were essential oils (20 samples), hair-care products (10 samples), rinse-off products (10 samples), and lipstick products (6 samples). Samples were collected between 2018 and 2020. Collection criteria of cosmetic samples in this study were ingredients labelled with “hemp seed oil” or “Cannabis sativa seed oil” as key words, or “CBD” or “Hemp” as specific product name. The test results showed 22 samples were found of THC (Table 3). The detected concentrations ranged between 0.063 and 1777 μg/g. Among them 11 samples (6, 4, 1 in leave-on, essential oil, and lipstick samples, respectively) were found THC concentrations above 10 μg/g which was the legal level in most of regulations such as EU, Canada, and Taiwan. Thirty four samples were detected of CBD ranged between 0.47 and 37,217 μg/g, and 5 samples were detected of CBN ranged between 2.22 and 25.17 μg/g. In leave-on products, an average level of CBD was obtained as high as 7,720 μg/g. Nineteen samples were found contained both THC and CBD. The present and concentration of THC and/or CBD in cosmetics in this study showed that there was no specific relation between them. Although the qualitative characterization of chemical phynotype in Cannabis sativa L. was based on the THC/CBD ratio [23]. The amounts of cannabinoids varied in hemp-seed based cosmetics might be due to the variation of ingredients from plants cultured on various climate condition, cultivation location, genetic variability and harvesting time [24]. It was suggested that cannabinoids contribute to the chemical variabilities in the final products [25]. The identification of other minor cannabinoids might be another approach to better understand the chemical profile of these hemp-based cosmetics.
Table 3.
CBD, CBN and THC concentrations detected in cosmetics.
| Analyte | sample type | Number of samples | Concentration Range (μg/g) | Average Conc. (μg/g) |
|---|---|---|---|---|
| CBD | Leave-on | 16 | 0.71–37,217 | 7,720 |
| Essential oil | 15 | 0.47–1,474 | 113 | |
| Hair | 0 | - | - | |
| Rinse-off | 0 | - | - | |
| Lip | 3 | 1.03–1,184 | 761 | |
| THC | Leave-on | 9 | 0.18–1,777 | 468 |
| Essential oil | 10 | 0.063–83.06 | 25.33 | |
| Hair | 0 | - | - | |
| Rinse-off | 0 | - | - | |
| Lip | 3 | 0.095–27.79 | 17.63 | |
| CBN | Leave-on | 1 | 23.81–23.81 | 23.81 |
| Essential oil | 2 | 24.81–25.17 | 25 | |
| Hair | 0 | - | ||
| Rinse-off | 0 | - | ||
| Lip | 2 | 2.22–6.53 | 4.35 |
Label accuracy of cannabinoid-based cosmetics was also an issue to be concerned. The labeling accuracy of CBD showed 13 of 34 CBD detected samples claimed to contain CBD (e.g., “Herbal Fracture CBD”, “CBD 750 mg”, “CBD LIP BALM”, “CBD DAY CREAM”, “CBD SERUM” and “CBD DAILY SOOTHING SERUM”). The concentration range of CBD in CBD claimed sample was between 10.9 and 37,217 μg/g, among them 7 articles also detected THC over 10 μg/g. Also, four of the 13 CBD claimed products did not specify how much CBD they contained. And in the 9 that did, only six had a CBD concentration within 10% of the amount listed on their labels. Of the 4 products that claimed to contain CBD but did not indicate the amount of it, CBD detected range was between 10.90 and 57.70 μg/g. The consumption of those undisclosed content of CBD products were for medical use or for other purpose remained to be elucidated. This developed method was successfully applied for the quantitative analysis of cannabinoids in commercial cosmetics products for a large range of matrix.
4. Conclusion
A presented method for the qualitative and quantitative determination of several cannabinoids including CBD, CBN, and THC, the main cannabinoids in hemp seed oil-based cosmetics, proved to be specific and selective. This validated method was applied into a surveillance consisted of 90 commercial cosmetics. In most of countries, the use of hemp seed oil as raw material in cosmetics was permitted as long as the product content of THC was below 10 μg/g. The examination result concluded that of the 90 products, 41% (37/90) contained cannabinoids (15 CBD only, 3 THC only, 19 both), though only 35% (13/37) claimed to contained CBD. A high ratio of products analyzed contained THC or cannabinoids, which were undisclosed on the label. The claimed amounts of cannabinoids versus the actual amounts were discovered to have 67% accurately labeled (6/9). Moreover, 50% (11/22) of THC detected samples were found to have noncompliance THC concentration over 10 μg/g. Though the sample size for this report is small, one should also consider the possibility that a product containing these substances may have both cosmetic and medical uses. Future work will include not only researches to cover more types of matrix of products, but also deeper investigations to other potential cannabinoids (analogues/derivatives) in hemp seed oil based cosmetics. The developed method for the quantification of cannabinoids was validated and surveillance reports could be used as a reference for administration to protect cosmetics product safety.
References
- 1.United Nations Office on Drug and Crime. Recommended methods for the identification and analysis of cannabis and cannabis products: Manual for Use by national drug analysis laboratories. United nations publication; 2009. [Google Scholar]
- 2. Callawa JC. Hempseed as a nutritional resource: An overview. Euphytica. 2004;140:65–72. [Google Scholar]
- 3. Mikulcova V, Kasparkova V, Humpolicek P, Bunkova L. Formulation, characterization and properties of hemp seed oil and its emulsions. Molecules. 2017;22:700. doi: 10.3390/molecules22050700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunford NT. 2-Hemp and flaxseed oil: Properties and applications for use in food. In: Talbot G, editor. Specialty oils and fats in food and nutrition. 1st ed. Cambridge: Woodhead Publishing; 2015. pp. 39–63. [Google Scholar]
- 5.Hazekamp A, Fischedick JT, Díez ML, Lubbe A, Ruhaak RL. Chemistry of Cannabis. In: Liu HW, Mander L, editors. Comprehensive Natural Products II. 1st ed. Oxford: Elsevier; 2010. pp. 1033–84. [Google Scholar]
- 6. Acosta E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr Opin Colloid Interface Sci. 2009;14:3–15. [Google Scholar]
- 7. Sun Y, Xia Z, Zheng J, Qiu P, Zhang L, McClements DJ, et al. Nanoemulsion-based delivery systems for nutraceuticals: Influence of carrier oil type on bioavailability of pterostilbene. J Funct Foods. 2015;13:61–70. [Google Scholar]
- 8. Raikos V, Ranawana V. Designing emulsion droplets of foods and beverages to enhance delivery of lipophilic bioactive components-a review of recent advances. Int J Food Sci Technol. 2017;52:68–80. [Google Scholar]
- 9. Callaway J, Schwab U, Harvima I, Halonen P, Mykkäanen O, Hyvöonen P, et al. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J Dermatolog Treat. 2005;16:87–94. doi: 10.1080/09546630510035832. [DOI] [PubMed] [Google Scholar]
- 10. Lodén M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003;4:771–88. doi: 10.2165/00128071-200304110-00005. [DOI] [PubMed] [Google Scholar]
- 11. Fichna J, Bawa M, Thakur GA, Tichkule R, Makriyannis A, McCafferty D-M, et al. Cannabinoids alleviate experimentally induced intestinal inflammation by acting at central and peripheral receptors. PLoS ONE. 2014;9:e109115. doi: 10.1371/journal.pone.0109115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mounessa JS, Siegel JA, Dunnick CA, Dellavalle RP. The role of cannabinoids in dermatology. J Am Acad Dermatol. 2017;77:188–90. doi: 10.1016/j.jaad.2017.02.056. [DOI] [PubMed] [Google Scholar]
- 13. Gavazzoni Dias MF. Hair cosmetics: An overview. Int J Trichology. 2015;7:2–15. doi: 10.4103/0974-7753.153450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimarães FS. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos Trans R Soc Lond B Biol Sci. 2012;367:3364–78. doi: 10.1098/rstb.2011.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grigoryev A, Savchuk S, Melnik A, Moskaleva N, Dzhurko J, Ershov M, et al. Chromatography-mass spectrometry studies on the metabolism of synthetic cannabinoids JWH-018 and JWH-073, psychoactive components of smoking mixtures. J Chromatogr B. 2011;879:1126–36. doi: 10.1016/j.jchromb.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 16. Citti C, Pacchetti B, Vandelli MA, Forni F, Cannazza G. Analysis of cannabinoids in commercial hemp seed oil and decarboxylation kinetics studies of cannabidiolic acid (CBDA) J Pharm Biomed Anal. 2018;149:532–40. doi: 10.1016/j.jpba.2017.11.044. [DOI] [PubMed] [Google Scholar]
- 17. Molnar A, Lewis J, Doble P, Hansen G, Prolov T, Fu S. A rapid and sensitive method for the identification of delta-9-tetrahydrocannabinol in oral fluid by liquid chromatography-tandem mass spectrometry. Forensic Sci Int. 2012;215:92–6. doi: 10.1016/j.forsciint.2011.01.045. [DOI] [PubMed] [Google Scholar]
- 18. Roth N, Moosmann B, Auwäarter V. Development and validation of an LC-MS/MS method for quantification of Delta 9-tetrahydrocannabinolic acid A (THCA-A), THC, CBN and CBD in hair. J Mass Spectrom. 2013;48:227–33. doi: 10.1002/jms.3152. [DOI] [PubMed] [Google Scholar]
- 19. Lebel P, Waldron KC, Furtos A. Rapid determination of 24 synthetic and natural cannabinoids for LC-MS-MS screening in natural products and drug inspection applications. LCGC. 2015;13:8–14. [Google Scholar]
- 20. Palazzoli F, Citti C, Licata M, Vilella A, Manca L, Zoli M, et al. Development of a simple and sensitive liquid chromatography triple quadrupole mass spectrometry (LC-MS/MS) method for the determination of cannabidiol (CBD), Δ9-tetrahydrocannabinol (THC) and its metabolites in rat whole blood after oral administration of a single high dose of CBD. J Pharm Biomed Anal. 2018;150:25–32. doi: 10.1016/j.jpba.2017.11.054. [DOI] [PubMed] [Google Scholar]
- 21. Leson G, Pless P, Grotenhermen F, Kalant H, ElSohly MA. Evaluating the impact of hemp food consumption on workplace drug tests. J Anal Toxicol. 2001;25:691–8. doi: 10.1093/jat/25.8.691. [DOI] [PubMed] [Google Scholar]
- 22. Meng Q, Buchanan B, Zuccolo J, Poulin M-M, Gabriele J, Baranowski DC. A reliable and validated LC-MS/MS method for the simultaneous quantification of 4 cannabinoids in 40 consumer products. PLoS ONE. 2018;13:e0196396. doi: 10.1371/journal.pone.0196396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas BF, ElSohly MA. Chapter 1: The Botany of Cannabis sativa L. In: Thomas BF, ElSohly MA, editors. The analytical chemistry of Cannabis Elsevier. 2016. pp. 1–26. [Google Scholar]
- 24. Menezes MMT, Andrade JF, Oliveira M, Tristäo H, Saczk A, Okumura L. Analysis of δ9-THC in cosmetics by high performance liquid chromatography with UV-Vis detection. Brazilian J Ana Chem. 2012;2:341–4. [Google Scholar]
- 25. Citti C, Linciano P, Panseri S, Vezzalini F, Forni F, Vandelli MA, et al. Cannabinoid Profiling of Hemp Seed Oil by Liquid Chromatography Coupled to High-Resolution Mass Spectrometry. Front Plant Sci. 2019;10:120. doi: 10.3389/fpls.2019.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]