Abstract
Acarbose (an α-glucosidase inhibitor) has been demonstrated to reduce the progression of atherosclerosis without affecting serum levels of glucose in rabbits fed a high cholesterol diet. The main focus of recent atherosclerosis studies has been microRNA targets. However, the mechanism by which acarbose targets miRNA-mediated atherosclerosis remains unclear. This study aimed to evaluate the effect of acarbose on microRNA-related regulation of rat aortic vascular smooth cell line (A7r5 cell) migration and proliferation induced by diabetic conditions. We reported that acabose exhibit significantly inhibits proliferative and cell migration abilities in A7r5 cells. The expression of protein and levels of mRNA were measured by Western blot analysis and real-time PCR. Acarbose inhibited the phosphorylation of focal adhesion kinase (FAK) and phosphoinositide 3-kinases (PI3K)/protein kinase B (Akt), Ras signals, small GTPase proteins expression to attenuate cell migration and proliferation. Furthermore, acarbose upregulated the expression of miR-143, and transfected miR-143 mimic and its inhibitor to explore its mechanism. In conclusion, acarbose reduces VSMC migration and proliferation via upregulating miR-143 to inhibit Ras-related signaling, and potentially prevention of atherosclerosis.
Keywords: Acarbose, Atherosclerosis, VSMC migration, VSMC proliferation, miR-143
1. Introduction
Cardiovascular disease (CVD) is a major cause of mortality worldwide, accounting for around 17.9 million deaths each year [1]. Excess consumption of saturated fatty acids, sugar-sweetened beverages, and alcohol is among the dietary and lifestyle behaviors associated with CVD development [2]. Risk factors of CVD (including obesity, hypertension, dyslipidemia and diabetes mellitus) have been shown to affect development and progression of the disease process [3,4]. Complications of CVD include angina, stroke, myocardial infarction, hypertensive heart disease, and heart failure [5]. However, atherosclerosis is one of the forms of CVD and has an association with arterial inflammation and other metabolic alterations [6,7].
The initial steps in the pathogenesis of atherosclerosis are modification of low-density lipoprotein cholesterol (LDL-c) in the vascular walls and then change in cellular permeability to damage the arterial walls [8]. These conditions trigger an inflammatory response in monocytes that adhere to the arterial endothelium. Next, endothelial cells express adhesion molecules to increase monocyte migration to the sub-endothelial space and then differentiation into macrophages that take up cholesterol esters and transform into foam cells that infiltrate the arterial walls [9]. Foam cells secrete some growth factors to promote VSMC proliferation leading to fatty streak formation [10]. Ras regulates the activation of the PI3K/Akt and the ERK pathways to control the proliferation of VSMCs [11,12]. FAK-Src complex plays an important role in the cell motility, and phosphorylation of FAK-Src complex increases VSMC migration [13,14]. FAK-Src complex regulates the activation of GTPases such as RhoA, Rac1, and Cdc42, which have roles in cell migration. Matrix metalloproteinase (MMP) facilitates migration and metastasis by damaging barriers formed by extracellular matrix [15].
The α-glucosidase inhibitor acarbose prevents postprandial hyperglycemia by delaying the absorption of complex carbohydrates and disaccharides from the small intestine [16]. Type 2 diabetes is associated with increasing risk for atherosclerosis [17]. Postprandial hyperglycemia may lead to atherosclerosis by increasing endothelial dysfunction, inflammatory reactions, and oxidative stress [18]. By decreasing postprandial hyperglycemia, acarbose decreases the incidence of silent myocardial infarctions and CVD in patients with impaired glucose tolerance [19]. In our previous study utilized Mulberry 1-Deoxynojirimycin (DNJ), the α-glucosidase inhibitor has been indicated to inhibit glucose-induced VSMCs migration by down-regulation of FAK and activation of AMPK/RhoB [20]. In this study, we investigated the inhibitory effect of acarbose in oleic acid/glucose-induced VSMCs migration and whether it is similar to DNJ. Acarbose also reduces the progression of intima-media thickening in glucose intolerant patients and improves carotid plaque echogenicity in patients with acute coronary syndrome [21].
MicroRNAs (miRNAs or miRs) are small noncoding RNAs, approximately 25 nucleotides in length, that regulate gene expression post-transcriptionally. Recently, miRNAs with an important role in cardiovascular biology, including miR-1, miR-133, miR-21, miR-195, and miR-143/145, have been identified in VSMCs [22,23]. In a previous study, miR-1 has been linked to whole heart development [24]; miR-133 expression has been shown to influence VSMC proliferation by downregulating the ERK1/2 kinase-dependent pathway [25]; and miR-21 by promoting VSMC differentiation and proliferation has been shown to lead to fibrosis and blood vessel wall thickening [26]. Wang et al. revealed that miR-195 reduced oxLDL-induced VSMC proliferation via inhibition of Cdc42 protein expression [27]. In addition, previous studies demonstrated that miR-143/145 modulates Ras-MAPK pathway signaling and thereby inhibits cellular proliferation of cultured VSMCs [28].
Moreover, it has been demonstrated that miR-143 has been demonstrated to modulate vascular injury, plays a critical role in VSMC phenotypic switching, promotes VSMC differentiation, and enhances actin accumulation. In addition, a number of clinical studies have shown that miR-143 dysregulation is a cause of many CVDs involving hypertension, coronary artery disease, and atherosclerosis [29–31]. We hypothesized that targeting miR-143 with acarbose may be a promising therapeutic strategy to improve CVD and atherosclerosis. Therefore, in the present study, we used the acarbose-induced decrease in VSMC motility under diabetic conditions (oleic acid and high glucose) as a model to investigate the potential role of miR-143 in this mechanism.
2. Materials and methods
2.1. Chemicals and reagents
The acarbose was purchased from Sigma–Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM), glutamine, Penicillin-Streptomycin-Amphotericin B (PSA), fetal bovine serum (FBS), pyruvate and trypsin EDTA were purchased from Hyclone (Logan, UT, USA) and Gibco (Grand Island, NY, USA) for cell culture. The ammonium persulfate (APS), sodium dodecyl sulfate (SDS), bisacrylamide, TEMED, PVDF membranes, and NC membranes were purchased from Gibco (Grand Island, NY, USA) and Hyclone (Logan, UT, USA) for Western blot analysis. The antibodies to Ras, phospho-ERK, ERK, PCNA, PI3K, phospho-AKT, AKT, Rac1, RhoA, Cdc42, phospho-FAK, FAK, MMP9, MMP2, phospho-Src, Src and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and Novus Biological (Littleton, CO, USA).
2.2. Cell culture
The rat aortic vascular smooth cell line A7r5 (Bio-resource Collection and Research Center [BCRC] cell bank, cell number 60082) was cultured in DMEM containing 10% FBS, 1 mmoL/l sodium pyruvate, 4.5 g/L glucose, 4 mmol/l l-glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin, and 1.5 g/L sodium bicarbonate at 37 °C in an incubator with a humidified atmosphere of 95% air and 5% CO2.
2.3. Cytotoxicity assay
The viability of A7r5 cells was determined using the 3-(4,5-di-methylthiazol-2-yl)-2,5-diphen-yltetrazolium bromide (MTT) assay. After treatment, 0.5 mg/mL MTT reagent was added to each well and the assay plate was incubated at 37 °C for 4 h. The medium was aspirated, and formazan crystal was dissolved in 100% isopropanol. The absorbance at 563 nm was measured in a spectrophotometric plate reader (Hitachi, Japan).
2.4. BrdU cell proliferation assay
A7r5 cells (1 × 104 in 100 μl per well) were seeded in a 96-well plate, cultured with (a) 150 μM oleic acid (OA), (b) 25 mM high glucose (HG), or (c) OA and HG together (OH), and then treated with acarbose (1 and 3 μM) for 24 h. The bromodeoxyuridine (BrdU) assay (Millipore, Billerica, MA, USA) was carried out by adding 20 μl of working solution (BrdU by dilute BrdU Label 1:2000) to each well, incubating plates 2–24 h at 37 °C, aspirating the contents of each well before adding 200 μl of fixative/denaturing solution, incubating 0.5 h at room temperature (RT) before aspirating the well contents, incubating the plate at RT for 1 h after adding 100 μl of anti-BrdU antibody (100X antibody diluted 1:100 in dilution buffer) to each well, washing the wells three times with 1 X wash buffer (25 mL of the 20X concentrated solution to 475 mL of deionized water) before pipetting 100 μl of solution (peroxidase goat anti-mouse IgG HRP conjugate in the conjugate diluent), incubating the plate at RT for 0.5 h before washing the wells three times, adding 100 μl of substrate solution to each well before incubating the plate 15 min at RT in the dark, and then adding 100 μl of stop solution to each well before measuring absorbance at a dual wavelength of 450–540 nm in a spectrophotometric plate reader (Hitachi, Japan).
2.5. Trypan blue staining assay
To evaluate cell proliferation, A7r5 cells were seeded at a density of 2 × 105 cells/mL in a 12-well plate. After attachment, cells were incubated with OA, HG, OH and then treated with acarbose (1 and 3 μM) for 24 h. The trypan blue exclusion assay was direct identification and calculation of non-viable and viable cells, respectively. Centrifuge the cell suspension at 1000×g for 3–5 min and resuspend the cell pellets in 1 mL PBS. A 100 μL sample of each cell suspension was an equal amount of 100 μL trypan blue (Invitrogen) to evaluate live (unstained) and dead (blue) cells using the Counting chambers (Paul Marienfeld GmbH & Co. KG) and assessed under the microscope.
2.6. Transwell migration assay
Cell migration assays were performed using 24-well plates with Millicell Hanging Cell Culture Insert with pore size of 8.0 μm (Cat. No. MCEP24H48). Cells seeded in upper chamber of each migration well (1 × 104 cells/well) were induced by (a) OA, (b) HG, or (c) OA and HG together, treated with various concentrations of acarbose (0, 1, 3 μM), and then allowed to migrate for 24 h at 37 °C in 5% CO2. After 24 h, the nonmigratory cells from the upper chambers were removed and the cells that migrated to the lower surface were fixed and stained in 5% Giemsa solution for 1 h. Each plate was placed under a microscope and the number of cells per well migrating to the lower surface was counted in a fixed area.
2.7. Wound healing assay
A7r5 cells were seeded in six-well plates (1 × 106 cells/well) for 24 h and grown until 90–95% confluence was reached. Using serum starvation medium for 2 h before the monolayer of each well was straight-scratched using a sterile 200 μL pipette tip. The unattached cells were removed by washing with PBS and the remaining cells were treated with (a) OA, (b) HG, or (c) OA and HG together. Then, the cells were treated with different concentrations of acarbose (1 and 3 μM), observed after wounding at 0, 24, and 48 h, and photographed under a microscope.
2.8. miRNA extraction and real-time PCR
Total RNA of cells was extracted using Nucleo-ZOL reagent (Macherey–Nagel, Düren, Germany) according to the manufacturer’s protocol. To quantify the expression of miR-143-3p, the TaqMan Small RNA Assay kit (Applied Biosystems, Carlsbad, CA) was used to translate RNA to cDNA. The purity and quantity of miR-143-3p were determined using Light Cycler Fast Start SYBR Green I Master Mix (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s protocol. The primers used to amplify miR-143-3p were the miRNA sequence (UGAGAUGAAGCACUGUAGCUCA), RT primer (GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGACACAGAGCCAACTGAGCT), and forward primer (GAACGTGAGATGAAGCACTGT). RNU6B was used as an endogenous (internal) control in each sample. After amplification, the expression level of miR-143-3p was analyzed using the Light Cycler software.
2.9. Transfection with miRNA-143 inhibitor or miRNA-143 mimic
The A7r5 cells were transfected using a Custom RNA system, the TOOLS Water DNA & RNA extraction kit (Biotools Co., Ltd., New Taipei, Taiwan) according to manufacturer’s instructions. In brief, 20 pmol of miR-143 inhibitor/mimic were respectively added to serum-free media (SFM) and then mixed with 3 μl/well of T-Pro Non-liposome transfection Reagent II (NTR II) for 15 min at room temperature. The mixture was added to each cell, and the cells were cultured 24 h at 37 °C in a humidified atmosphere of 95% air and 5% CO2.
2.10. Western blot analysis
Total protein was extracted from post-treatment cell samples by RIPA lysis buffer and centrifuged at 12,000 rpm for 20 min at 4 °C. Protein concentrations were determined using BCA protein assay kit (Thermo Scientific). The proteins were separated by 8–12% SDS-PAGE and transferred to nitrocellulose or PVDF membranes. The membranes were treated with blocking buffer to block non-specific protein binding, incubated with primary antibodies at 4 °C overnight, washed three times with TBST, and incubated with secondary antibodies at room temperature for 1 h. The protein bands were visualized with the ECL method, imaged using an LAS-4000 imaging system, and analyzed by densitometry scanning of image using Multi Gauge V3.0.
2.11. Fluorescence analysis
A7r5 (3 × 105) cells were seeded in six-well plates, cultured with OA, HG, or OA and HG, and then treated with acarbose for 24 h, fixed with paraformaldehyde (4%) in PBS for 15–20 min at RT, permeabilized with Triton X-100 in PBS for 3–5 min, blocked with bovine serum albumin (BSA, 3%) for 60 min, stained with TRITC-conjugated phalloidin (50 μg/mL) in PBS for 1 h, washed three times with PBS, and stained to visualize Factin microfilaments by confocal fluorescence microscopy (Nikon, Tokyo, Japan) at 400×or 100×magnification.
2.12. Statistical analysis
All experiments were performed at least three times. The results are expressed as the mean ± SD for each group. The Student t-test was performed using Graph Pad Prism to compare two different groups. Multiple groups were compared by one-way ANOVA and the Duncan’s new multiple range test. using SPSS software (SPSS, Inc., Chicago, IL, USA). P values < 0.05 were considered statistically significant.
3. Results
3.1. Effect of acarbose on cell viability in normal-A7r5 cells
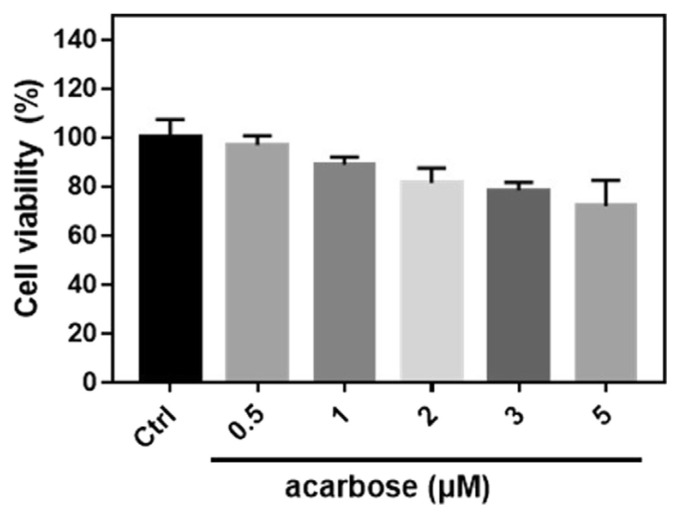
The cytotoxic effects of acarbose were evaluated using the MTT assay. Acarbose (0.5, 1, 2, 3, and 5 μM) for 24 h was not cytotoxic (Fig. 1). Hence, 1 and 3 μM were used to treat normal-A7r5 cells subsequently.
Fig. 1.
Cell viability of normal-A7r5 cells treated with different concentrations of acarbose.
A7r5 cells were treated with 0.5, 1, 2, 3 and 5 μM acarbose for 24 h and analyzed by MTT assay. The data are presented as the mean ± SD from three independent experiments.
3.2. Effect of acarbose on migration and cytoskeleton of A7r5 cells
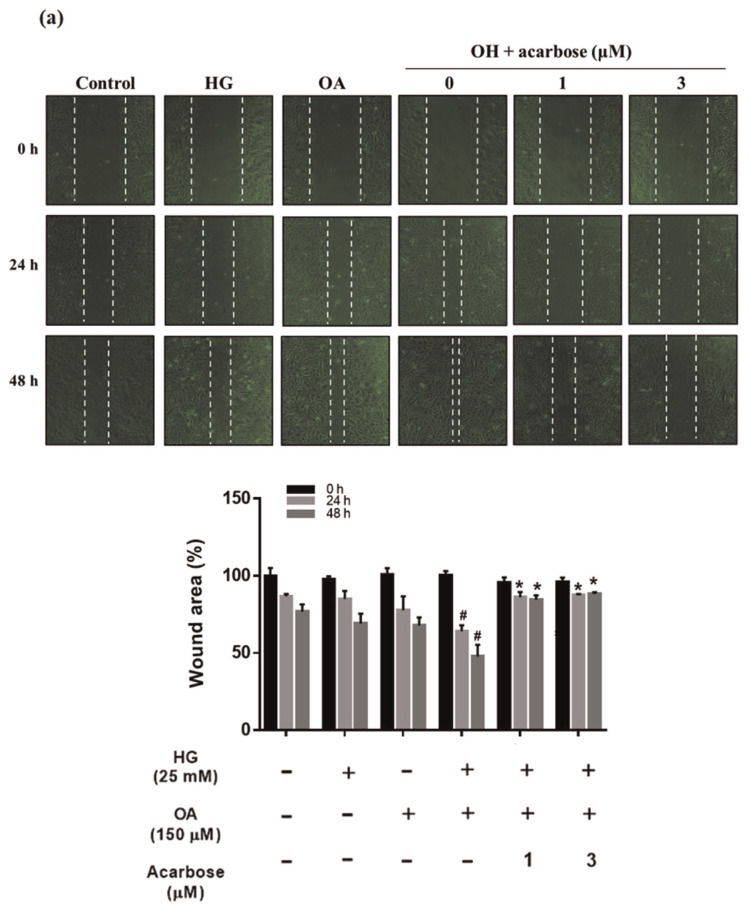
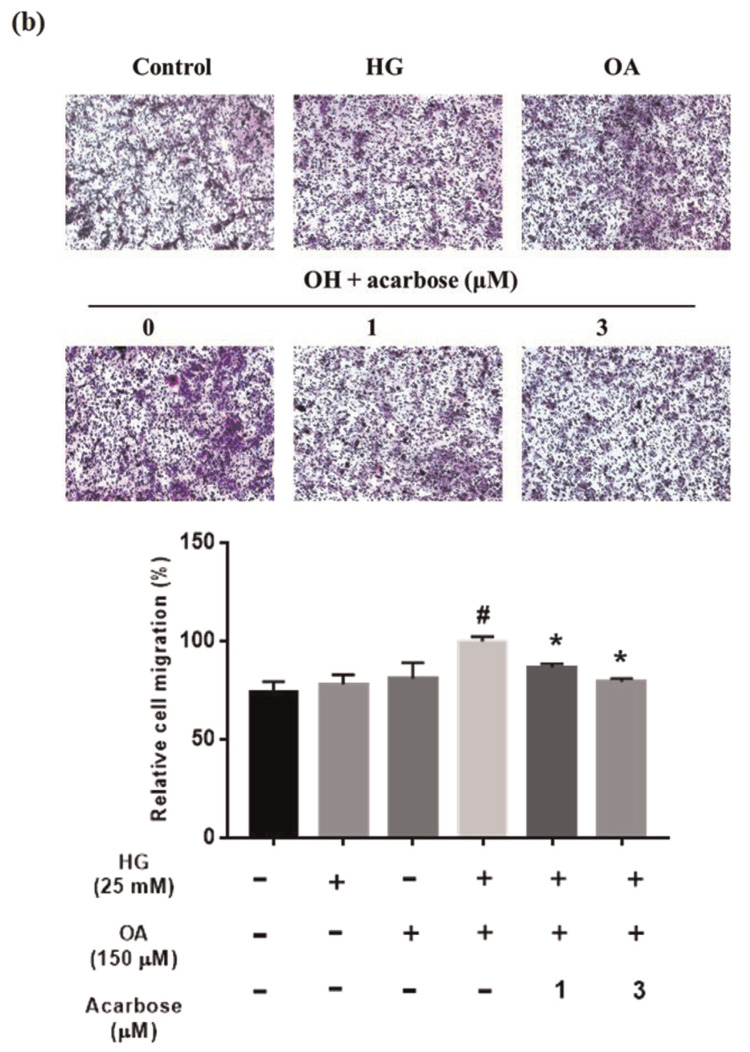
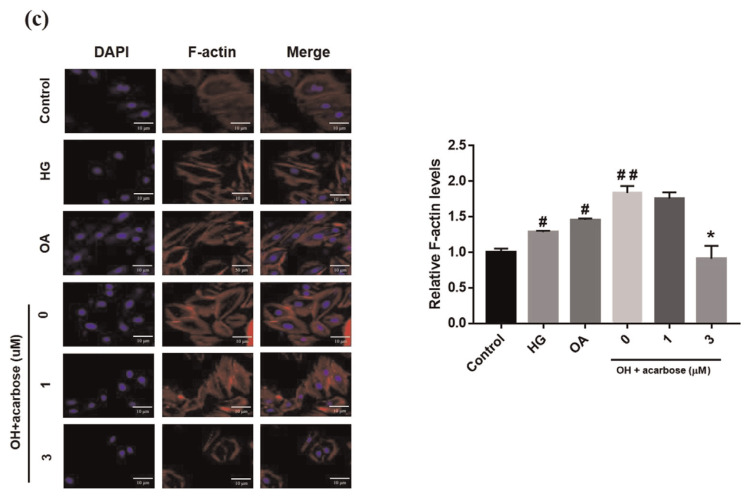
The VSMC migration is a critical aspect of atherosclerosis pathogenesis [32]. The effect of acarbose on normal-A7r5 cell migration under HG, OA, or OA + HG (OH) conditions was investigated. Migration was significantly inhibited by acarbose (3 μM), increasing wound closure time but significantly increasing wound closure in the OH group (Fig. 2a). The transwell assay was used to further confirm cell migration is induced by HG, OA, or OH over a 24-h period. The migration ability cells under OA + HG (OH) conditions was significantly reduced 13.25% and 20.65% after treatment with 1 and 3 μMacarbose, respectively (Fig. 2b). A previous study indicated that changes in the actin cytoskeleton regulated cell migration [33]. To evaluate whether acarbose mediates cellular motility and cytoskeletal change, we used phalloidin (F-actin) and DAPI (nuclei) staining to monitor acarbose-induced change in cell migration behavior and redistribution. Confocal fluorescence microscopy indicated a marked increase in phalloidin staining after treatment under OH conditions and a decrease in phalloidin staining after acarbose treatment (Fig. 2c). These results indicated that acarbose reduced diabetic condition-induced A7r5 cell migration.
Fig. 2.
Effect of acarbose on migration of A7r5 cells.
Normal-A7r5 cells were treated with HG, OA, or OH and acarbose (1 and 3 μM) for 24 h. Migration was evaluated with the (a) scratch wound assay and (b) Transwell assay. The results of B were consistent with those of A. In the Transwell assay, cells were seeded in the upper chamber, and cell migration to the lower surface of the membrane was assessed after 24 h. After membrane fixation and staining with Giemsa, the number of migrated cells was counted under a light microscope. (c) Confocal micrographs showing A7r5 cells fixed with paraformaldehyde and stained with DAPI (blue, nucleic acid) and phalloidin-TRITC (red, F-actin). The scale bars represent 10 μm. Representative images of F-actin staining and quantified with fluorescence intensity ratio using ImageJ. The data are presented as the mean ± SD from three independent experiments. #p < 0.05, # #p < 0.01 compared with Ctrl. *p < 0.05 compared with OH group.
3.3. Acarbose decreases the FAK phosphorylation signal in A7r5 cells
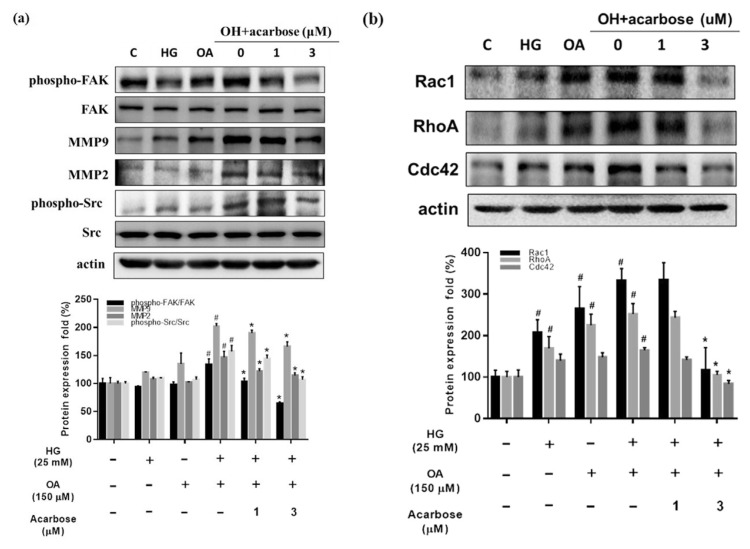
Cell migration is controlled by FAK-related signals, MMPs, Src activation, and small GTPase expression [34]. We investigated whether acarbose acts through the same signaling pathway to decrease OH-induced A7r5 cell migration. Acarbose treatment for 24 h reduced FAK phosphorylation and the protein levels of MMP2, MMP9, phosphorylated Src (Fig. 3a), and small GTPases (Rac1, RhoA, and Cdc42) (Fig. 3b) in a dose-dependent manner, indicating that acarbose reduces migration-related signals in diabetic condition-induced A7r5 cells.
Fig. 3.
Acarbose reduced the expression of migration-related protein in A7r5 cells.
Normal-A7r5 cells were co-treated with acarbose (1 or 3 μM) and HG, OA, or OH for 24 h. After the cells were harvested, Western blot analyses were conducted with (a) phosphorylated FAK (p-FAK), FAK, phosphorylated Src (p-Src), Src, MMP 2/9, and β-actin antibodies as described in Materials and methods. Densitometry was used to quantify p-FAK relative to total FAK, p-Src relative to total Src, and MMP 2/9 relative to the β-actin. (b) Small GTPase protein (Rac1, Rho A, Cdc42), and β-actin were detected using specific antibodies for each protein. Densitometry was used to quantify Rac1, Rho A, and Cdc42 relative to the β-actin. The Western blot data are presented as the mean ± SD from three independent experiments. #p < 0.05, compared with Ctrl. *p < 0.05 compared with OH group.
3.4. Acarbose inhibits the proliferation of A7r5 cells
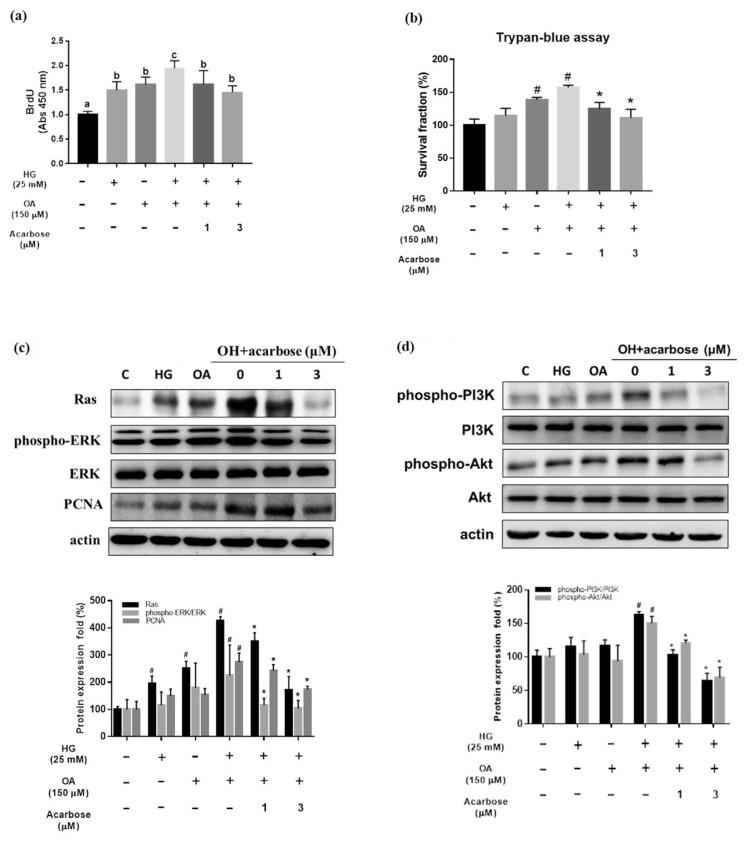
VSMC proliferation plays an important role in the pathophysiological course of atherosclerosis [35]. The BrdU assay and Trypan blue assay were used to assess the relationship between acarbose and the proliferation of A7r5 cells under diabetic conditions. Treatment with HG, OA, or OH for 24 h significantly increased the proliferation of A7r5 cells. However, acarbose (1 and 3 μM) significantly inhibited the proliferation of A7r5 cells treated with OH for 24 h (Fig. 4a and b).
Fig. 4.
Acarbose inhibits oleic acid/high glucose-induced proliferation and related protein expression in A7r5 cells.
Normal-A7r5 cells were co-treated with acarbose (1 or 3 μM) and HG, OA, or OH for 24 h and then analyzed by (a) the BrdU assay, (b) Trypan blue assay and (c and d) Western blot. (a) The BrdU data are presented as the mean ± SD from three independent experiments. P < 0.05 considered statistically significant using one-way ANOVA, followed by Duncan’s new multiple range test. Bars not sharing a common small letter are significantly different from each other. (b) Cell survival fraction was measured by trypan blue staining to measure viable cells against control. #p < 0.05, compared with Ctrl. *p < 0.05 compared with OH group. (c) Acarbose downregulated expression of Ras, phosphorylated ERK1/2 (p-ERK), ERK, PCNA and β-actin. Densitometry was used to quantify p-ERK relative to total ERK, Ras and PCNA relative to the β-actin. (d) phosphorylated PI3K (p-PI3K), PI3K, phosphorylated Akt (p-Akt), Akt, and β-actin. Densitometry was used to quantify p-PI3K relative to total PI3K, p-Akt relative to total Akt. The Western blot data are presented as the mean ± SD from three independent experiments. #p < 0.05, compared with Ctrl. *p < 0.05 compared with OH group.
3.5. Acarbose acts through Ras, ERK, and PI3K/Akt signaling pathway
The activation of ERK and PI3K/AKT signaling pathways mediated by Ras protein plays a critical role in the proliferation of VSMCs [11,36]. Western blotting showed the levels of Ras, phosphorylated ERK, and PCNA in OH-treated A7r5 cells were plainly increased in the absence of acarbose and unequivocally decreased in its presence (Fig. 4c). In OH-stimulated A7r5 cells treated or not with acarbose for 24 h, the levels of PI3K and phosphorylated Akt were decreased, and OH-induced upregulation was nullified by acarbose (Fig. 4d).
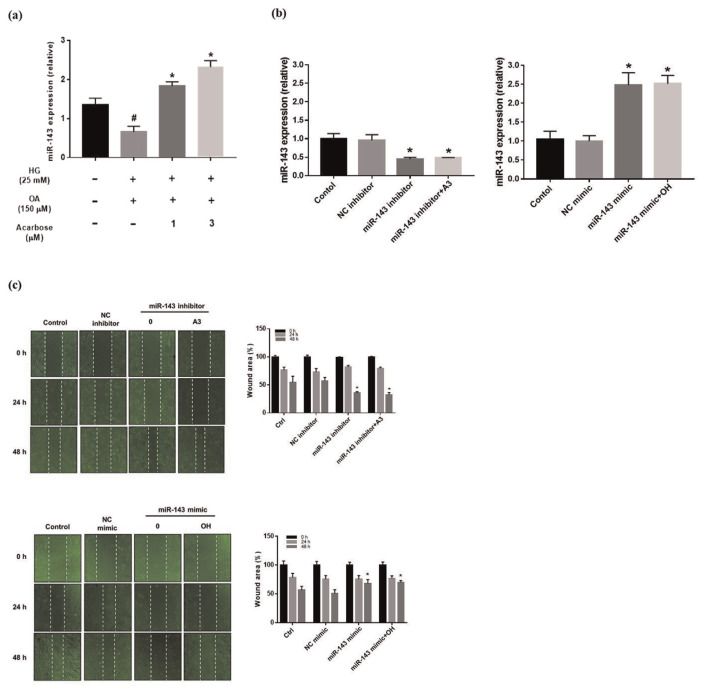
3.6. Upregulation of miR-143 expression by acarbose
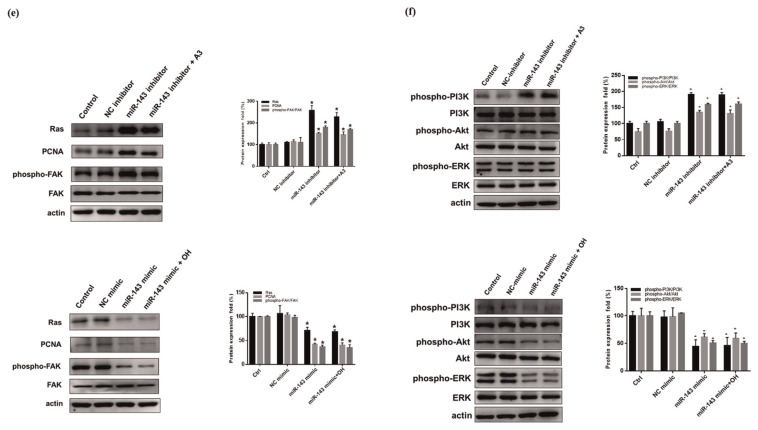
The potential relevance of miR-143 upregulation was investigated in OH-induced A7r5 cells before and after acarbose treatment. As shown in Fig. 5a, miR-143 expression was clearly increased by acarbose. In addition, we used transfected miR-143 inhibitor and mimic to the vascular smooth cell to regulate the expression of miR-143. The results show that processing miR-143 mimic increased miR-143 expression. Conversely, transfected miR-143 inhibitor decreased miR-143 expression (Fig. 5b).
Fig. 5.
Effect expression of miR-143 on migration in normal-A7r5 cells.
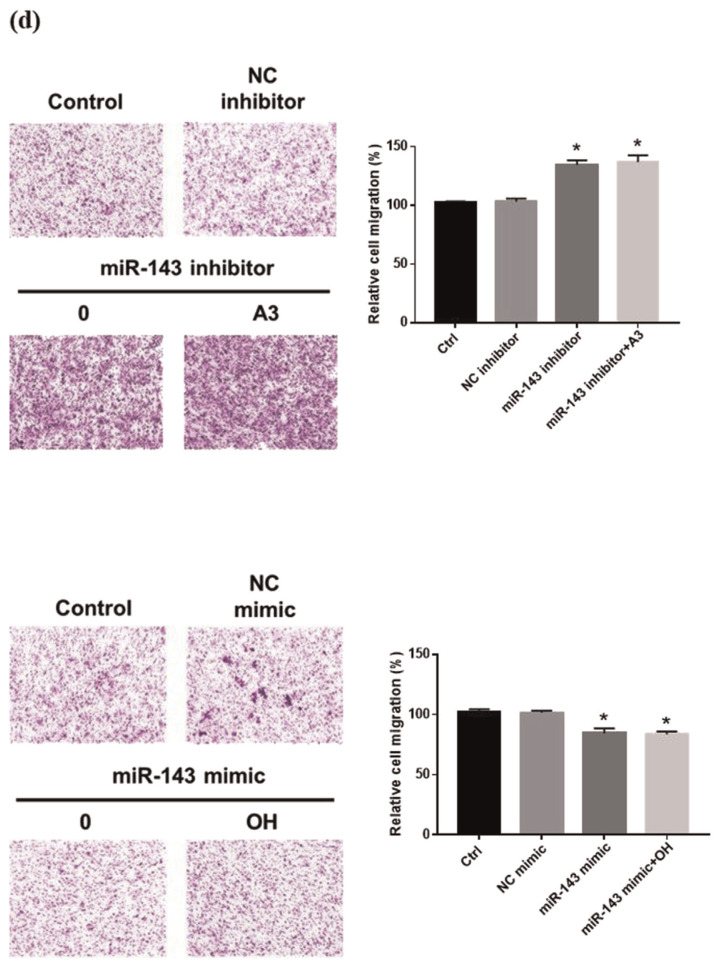
(a) Relative expression of miR-143 in normal-A7r5 cells analyzed using real-time PCR. Each bar represents the mean ± SD from three independent experiments. #p < 0.05, compared with Ctrl. *p < 0.05 compared with OH group. The migration of normal-A7r5 cells transfected or not transfected with miRNA inhibitor/mimic in the presence of OH and acarbose measured by the (b) real-time PCR (c) Wound healing assay and (d) Transwell assay. (e) Western blot analysis of Ras, PCNA and FAK protein expression of normal-A7r5 cells in the presence of OH and A3 (acarbose 3 μM), or with a miR-143 inhibitor/mimic. Densitometry was used to quantify p-FAK relative to total FAK, Ras, and PCNA relative to the β-actin. (f) phosphorylated PI3K (p-PI3K), PI3K, phosphorylated Akt (p-Akt), Akt, phosphorylated ERK (p-ERK), ERK and β-actin. Densitometry was used to quantify p-PI3K relative to total PI3K, p-Akt relative to total Akt, p-ERK relative to total ERK. The Western blot data are presented as the mean ± SD from three independent experiments. *p < 0.05 compared with NC (inhibitor or mimic) group.
3.7. Inhibition of miR-143 upgrade VSMC migration
miR-143 has been demonstrated to regulate VSMC proliferation and migration [37]. In this study, miR-143 inhibitor obviously increased cell migration 24 h after monolayer wounding. Conversely, the addition of miR-143 mimic inhibits the ability of cell migration (Fig. 5c). A comparison of the migration ability of normal-A7r5 cells in the transwell system before and after transfection with a miR-143 inhibitor showed that the miR-143 inhibitor significantly increased cell migration, while the cells in the miR-143 mimic group had decreased cell migration (Fig. 5d).
3.8. miR-143 down-regulates Ras signaling
Ras-mediated signaling is regulated via miR-143, which is highly expressed in mesenchymal cells such as smooth muscle cells and fibroblasts [31]. To verify the impact of miR-143 on Ras expression. Normal-A7r5 cells were treated with miR-143 inhibitor/mimic. As shown in Fig. 5e, acarbose prevented the miR-143 inhibitor from upregulating the expression of Ras and phosphorylated FAK, while the miR-143 mimic group had downregulating the expression of Ras and phosphorylated FAK when compared with control group and NC group. Furthermore, we also evaluate the mechanism of PI3K, Akt and ERK in the presence of miR 143 mimic or inhibitor (Fig. 5f). The data demonstrate that miR-143 regulates the Ras signaling pathway to affected PI3K/Akt and ERK expression, thereby regulating A7r5 cell migration and proliferation.
4. Discussion
Atherosclerosis is a chronic inflammatory disease. During the pathogenesis of atherosclerosis, cytokines or inflammatory factors are produced that enhance vascular smooth cell proliferation and migration from the arterial media to the intima. In recent years, obesity in patients with the characteristics of diabetes has led to an increase in the severity of atherosclerosis [3,38]. Moreover, micro-RNAs have been identified as critical players in VSMC dysfunction and biology [23]. Since high levels of blood sugar and lipids are responsible for atherosclerosis pathology, the present study used oleic acid and high glucose-treated A7r5 cells experimentally as a diabetic condition model (in vitro) to identify the specific microRNA regulating VSMC proliferation and migration.
VSMC is a highly differentiated cell type present in the medial region of the arteries and arterioles. In addition, VSMC is considered to play an important role in the progression of atherosclerosis, including phenotypic switching, cell proliferation, migration [32]. In the study, we utilized the transwell migration assay to demonstrate that acarbose reduces the migration of diabetic condition-induced A7r5 cells and fluorescence analysis of Factin to demonstrate that acarbose restores cytoskeletal organization. So we illustrated that acarbose could regulate migration-related pathways under experimental diabetic conditions in vitro. The FAK/Src pathway is a critical regulator of cell migration. FAK promotes motility by increasing Src phosphorylation and MMP expression [13]. In our study, acarbose reduced the protein expression of phosphorylated FAK, Src, and MMP. Therefore, acarbose might have attenuated the migration of diabetic condition-induced A7r5 cells via FAK/Src signaling. In addition, a small GTPase protein also participates in cell migration [39]. In this study, by showing that acarbose decreased RhoA, Rac1, and Cdc42 signaling, we proved FAK signals and small GTPases were the targets of acarbose-induced inhibition of VSMC migration under diabetic conditions.
Several studies showed that VSMC proliferation facilitates atherosclerotic plaque formation [32]. We used the BrdU ELISA assay and Trypan Blue staining assay to show that acarbose for 24 h significantly reduced VSMC proliferation. The PI3K/Akt pathway is a key regulator of cell proliferation [36]. In the current study, acarbose inhibited activated PI3K and Akt signals. According to previous studies, Ras is important in cell migration and proliferation, while ERK activation and PCNA are important in proliferation [40]. In the present study, acarbose inhibited Ras and PCNA expression and inactivated ERK signaling. Collectively, these findings indicate that acarbose leads to change in VSMC proliferation under diabetic conditions.
As shown in a 2018 article, miRNAs play a role in the regulation of critical aspects of atherosclerotic lesion formation and regression [41]. Previous studies revealed that miR-143 in VSMCs is atheroprotective, while overexpressed miR-143 can decrease the proliferation and migration of human bladder carcinoma cells [42]. However, Kent et al. reported that inhibition of miR-143 expression by oncogenic Ras initiated the tumor-promoting feed-forward pathway [43]. Furthermore, silencing of miR-143 could promote cell migration [44]. Our study indicated that miR-143 was significantly decreased in diabetic condition-induced cells and that acarbose could increase miR-143 expression. Moreover, the normal-A7r5 cells in the miR-143 inhibitor group had promoted cell migration, while in miR-143 mimic group had reduced cell migration. Therefore, elevated miR-143 can prevent cell migration. In addition, Chen et al. demonstrated that Ras upregulation and miR-143 inhibition could enhance cell migration and proliferation [45]. Taken together, these findings demonstrate that miR-143 negatively regulates Ras to enhance VSMC proliferation and migration through downstream FAK signaling.
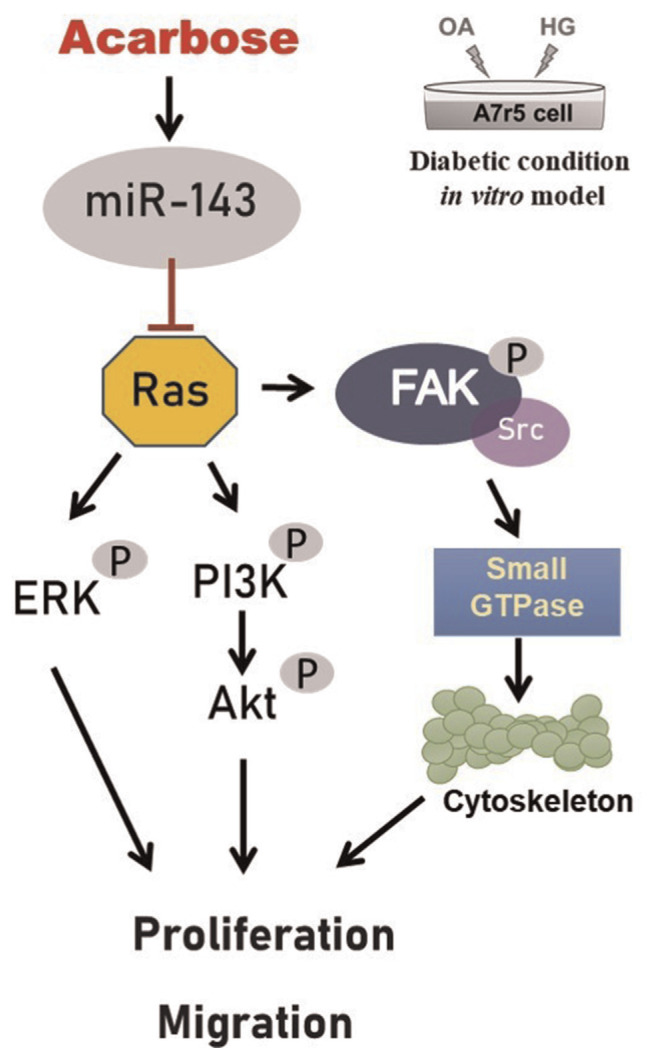
In this study we provide notable evidence that acarbose up-regulation of miR-143 reduces VSMC migration and proliferation via Ras expression and activation of the FAK and PI3K/Akt signaling pathways (Fig. 6). These results suggest that miR-143 could be a new target for the treatment of atherosclerosis.
Fig. 6.
Schematic diagram of acarbose attenuation of vascular smooth muscle cell proliferation and migration via miR-143 targeting of signals.
Supplementary Information
Acknowledgements
This study was supported by a grant from Chung Shan Medical University Hospital, Taichung, Taiwan (CSH-2017-C-003).
Funding Statement
This study was supported by a grant from Chung Shan Medical University Hospital, Taichung, Taiwan (CSH-2017-C-003).
Footnotes
Conflict of interest
The authors declare no competing financial interests.
References
- 1. Redfern J, Neubeck L. e-Health in Cardiovascular medicine. Med Sci (Basel Switzerland) . 2019:7. doi: 10.3390/medsci7060072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sacks FM, Lichtenstein AH, Wu JH, Appel LJ, Creager MA, Kris-Etherton PM, et al. Dietary fats and cardiovascular disease: a presidential advisory from the American heart association. Circulation. 2017;136:e1–23. doi: 10.1161/CIR.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 3. Piché M-E, Poirier P, Lemieux I, Després J-P. Overview of epidemiology and contribution of obesity and body fat distribution to cardiovascular disease: an update. Prog Cardiovasc Dis. 2018;61:103–13. doi: 10.1016/j.pcad.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 4. Lopez-Candales A, Burgos PMH, Hernandez-Suarez DF, Harris D. Linking chronic inflammation with cardiovascular disease: from normal aging to the metabolic syndrome. J Nat Sci. 2017;3 [PMC free article] [PubMed] [Google Scholar]
- 5. Standl E, Schnell O, McGuire DK, Ceriello A, Ryden L. Integration of recent evidence into management of patients with atherosclerotic cardiovascular disease and type 2 diabetes. Lancet Diab Endocrinol. 2017;5:391–402. doi: 10.1016/S2213-8587(17)30033-5. [DOI] [PubMed] [Google Scholar]
- 6. Katakami N. Mechanism of development of atherosclerosis and cardiovascular disease in diabetes mellitus. J Atherosclerosis Thromb. 2017:RV17014. doi: 10.5551/jat.RV17014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tesauro M, Mauriello A, Rovella V, Annicchiarico-Petruzzelli M, Cardillo C, Melino G, et al. Arterial ageing: from endothelial dysfunction to vascular calcification. J Intern Med. 2017;281:471–82. doi: 10.1111/joim.12605. [DOI] [PubMed] [Google Scholar]
- 8. Peng J, Luo F, Ruan G, Peng R, Li X. Hypertriglyceridemia and atherosclerosis. Lipids Health Dis. 2017;16:233. doi: 10.1186/s12944-017-0625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rahman K, Fisher E. Insights from pre-clinical and clinical studies on the role of innate inflammation in atherosclerosis regression. Front Cardiovas Med. 2018;5:32. doi: 10.3389/fcvm.2018.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Di Pietro N, Formoso G, Pandolfi A. Physiology and pathophysiology of oxLDL uptake by vascular wall cells in atherosclerosis. Vasc Pharmacol. 2016;84:1–7. doi: 10.1016/j.vph.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 11. Zhang F, Ren X, Zhao M, Zhou B, Han Y. Angiotensin-(1–7) abrogates angiotensin II-induced proliferation, migration and inflammation in VSMCs through inactivation of ROS-mediated PI3K/Akt and MAPK/ERK signaling pathways. Sci Rep. 2016;6:34621. doi: 10.1038/srep34621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu M-H, Lin M-C, Huang C-N, Chan K-C, Wang C-J. Acarbose inhibits the proliferation and migration of vascular smooth muscle cells via targeting Ras signaling. Vasc Pharmacol. 2018;103:8–15. doi: 10.1016/j.vph.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 13. Yu M-H, Yang T-Y, Ho H-H, Huang H-P, Chan K-C, Wang C-J. Mulberry polyphenol extract inhibits FAK/Src/PI3K complex and related signaling to regulate the migration in A7r5 cells. J Agric Food Chem. 2018;66:3860–9. doi: 10.1021/acs.jafc.8b00958. [DOI] [PubMed] [Google Scholar]
- 14. Suki B, Parameswaran H, Imsirovic J, Bartolák-Suki E. Regulatory roles of fluctuation-driven mechanotransduction in cell function. Physiology. 2016;31:346–58. doi: 10.1152/physiol.00051.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17:611. doi: 10.1038/nrm.2016.87. [DOI] [PubMed] [Google Scholar]
- 16. Patel S. Cerebrovascular complications of diabetes: alpha glucosidase inhibitor as potential therapy. Horm Metab Res. 2016;48:83–91. doi: 10.1055/s-0035-1565181. [DOI] [PubMed] [Google Scholar]
- 17. Patel TP, Rawal K, Bagchi AK, Akolkar G, Bernardes N, da Silva Dias D, et al. Insulin resistance: an additional risk factor in the pathogenesis of cardiovascular disease in type 2 diabetes. Heart Fail Rev. 2016;21:11–23. doi: 10.1007/s10741-015-9515-6. [DOI] [PubMed] [Google Scholar]
- 18. Ceriello A, Genovese S. Atherogenicity of postprandial hyperglycemia and lipotoxicity. Rev Endocr Metab Disord. 2016;17:111–6. doi: 10.1007/s11154-016-9341-8. [DOI] [PubMed] [Google Scholar]
- 19. Chan K-C, Yu M-H, Lin M-C, Huang C-N, Chung D-J, Lee Y-J, et al. Pleiotropic effects of acarbose on atherosclerosis development in rabbits are mediated via upregulating AMPK signals. Sci Rep. 2016;6:38642. doi: 10.1038/srep38642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chan K-C, Lin M-C, Huang C-N, Chang W-C, Wang C-J. Mulberry 1-deoxynojirimycin pleiotropically inhibits glucose-stimulated vascular smooth muscle cell migration by activation of AMPK/RhoB and down-regulation of FAK. J Agric Food Chem. 2013;61:9867–75. doi: 10.1021/jf403636z. [DOI] [PubMed] [Google Scholar]
- 21. Standl E, Theodorakis MJ, Erbach M, Schnell O, Tuomilehto J. On the potential of acarbose to reduce cardiovascular disease. Cardiovasc Diabetol. 2014;13:81. doi: 10.1186/1475-2840-13-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paul P, Chakraborty A, Sarkar D, Langthasa M, Rahman M, Bari M, et al. Interplay between miRNAs and human diseases. J Cell Physiol. 2018;233:2007–18. doi: 10.1002/jcp.25854. [DOI] [PubMed] [Google Scholar]
- 23.Shoeibi S.Diagnostic and theranostic microRNAs in the pathogenesis of atherosclerosis Acta Physiol 2019e13353 [DOI] [PubMed] [Google Scholar]
- 24. Muntean I, Togănel R, Benedek T. Genetics of congenital heart disease: past and present. Biochem Genet. 2017;55:105–23. doi: 10.1007/s10528-016-9780-7. [DOI] [PubMed] [Google Scholar]
- 25. Samidurai A, Kukreja RC, Das A. Emerging role of mTOR signaling-related miRNAs in cardiovascular diseases. Oxidat Med Cellul Longev. 20182018 doi: 10.1155/2018/6141902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shi L, Liao J, Liu B, Zeng F, Zhang L. Mechanisms and therapeutic potential of microRNAs in hypertension. Drug Discov Today. 2015;20:1188–204. doi: 10.1016/j.drudis.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y-S, Wang H-YJ, Liao Y-C, Tsai P-C, Chen K-C, Cheng H-Y, et al. MicroRNA-195 regulates vascular smooth muscle cell phenotype and prevents neointimal formation. Cardiovasc Res. 2012;95:517–26. doi: 10.1093/cvr/cvs223. [DOI] [PubMed] [Google Scholar]
- 28. Davis-Dusenbery BN, Chan MC, Reno KE, Weisman AS, Layne MD, Lagna G, et al. Down-regulation of Krüppel-like Factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-β and bone morphogenetic protein 4. J Biol Chem. 2011;286:28097–110. doi: 10.1074/jbc.M111.236950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rangrez AY, Massy ZA, Metzinger-Le Meuth V, Metzinger L. miR-143 and miR-145: molecular keys to switch the phenotype of vascular smooth muscle cells. Circulation: Cardiovas Genet. 2011;4:197–205. doi: 10.1161/CIRCGENETICS.110.958702. [DOI] [PubMed] [Google Scholar]
- 30. Zhang M-J, Zhou Y, Chen L, Wang Y-Q, Wang X, Pi Y, et al. An overview of potential molecular mechanisms involved in VSMC phenotypic modulation. Histochem Cell Biol. 2016;145:119–30. doi: 10.1007/s00418-015-1386-3. [DOI] [PubMed] [Google Scholar]
- 31. Avalle L, Incarnato D, Savino A, Gai M, Marino F, Pensa S, et al. MicroRNAs-143 and-145 induce epithelial to mesenchymal transition and modulate the expression of junction proteins. Cell Death Differ. 2017;24:1750. doi: 10.1038/cdd.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Basatemur GL, Jørgensen HF, Clarke MC, Bennett MR, Mallat Z. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 2019;1 doi: 10.1038/s41569-019-0227-9. [DOI] [PubMed] [Google Scholar]
- 33. Zeng Y-N, Kang Y-L, Rau L-R, Hsu F-Y, Tsai S-W. Construction of cell-containing, anisotropic, three-dimensional collagen fibril scaffolds using external vibration and their influence on smooth muscle cell phenotype modulation. Biomed Mater. 2017;12:045019. doi: 10.1088/1748-605X/aa766d. [DOI] [PubMed] [Google Scholar]
- 34. Wang D, Uhrin P, Mocan A, Waltenberger B, Breuss JM, Tewari D, et al. Vascular smooth muscle cell proliferation as a therapeutic target. Part 1: molecular targets and pathways. Biotechnol Adv. 2018;36:1586–607. doi: 10.1016/j.biotechadv.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 35. Tsioufis C, Mantzouranis E, Kalos T, Konstantinidis D, Tousoulis D. Risk factors of atherosclerosis: pathophysiological mechanisms. Coron Artery Dis: Biol Clin Prac. 2017:43. [Google Scholar]
- 36. Asati V, Mahapatra DK, Bharti SK. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: structural and pharmacological perspectives. Eur J Med Chem. 2016;109:314–41. doi: 10.1016/j.ejmech.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 37. Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, et al. The knockout of miR-143 and-145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009;16:1590. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Elagizi A, Kachur S, Lavie CJ, Carbone S, Pandey A, Ortega FB, et al. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2018;61:142–50. doi: 10.1016/j.pcad.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 39. Hodge RG, Ridley AJ. Regulating Rho GTPases and their regulators. Nat Rev Mol Cell Biol. 2016;17:496. doi: 10.1038/nrm.2016.67. [DOI] [PubMed] [Google Scholar]
- 40. Jiang C, Song T, Li J, Ao F, Gong X, Lu Y, et al. Ras promotes proliferation and resistances to apoptosis in meningioma. Mol Neurobiol. 2017;54:779–87. doi: 10.1007/s12035-016-9763-z. [DOI] [PubMed] [Google Scholar]
- 41. Parahuleva MS, Lipps C, Parviz B, Hölschermann H, Schieffer B, Schulz R, et al. MicroRNA expression profile of human advanced coronary atherosclerotic plaques. Sci Rep. 2018;8:7823. doi: 10.1038/s41598-018-25690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang H, Li Q, Niu X, Wang G, Zheng S, Fu G, et al. miR-143 inhibits bladder cancer cell proliferation and enhances their sensitivity to gemcitabine by repressing IGF-1R signaling. Oncol Lett. 2017;13:435–40. doi: 10.3892/ol.2016.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kent OA, Chivukula RR, Mullendore M, Wentzel EA, Feldmann G, Lee KH, et al. Repression of the miR-143/145 cluster by oncogenic Ras initiates a tumor-promoting feed-forward pathway. Genes Dev. 2010;24:2754–9. doi: 10.1101/gad.1950610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu X, Zhang Y, Wang S, Liu G, Ruan L. Loss of miR-143 and miR-145 in condyloma acuminatum promotes cellular proliferation and inhibits apoptosis by targeting NRAS. Royal Soc Open Sci. 2018;5:172376. doi: 10.1098/rsos.172376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen X, Guo X, Zhang H, Xiang Y, Chen J, Yin Y, et al. Role of miR-143 targeting KRAS in colorectal tumorigenesis. Oncogene. 2009;28:1385. doi: 10.1038/onc.2008.474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.