Abstract
Platycodin D (PD) has been used as the quality control marker of Radix Platycodonis for its high content and various pharmacological properties. In this study, a specific polyclonal antibody against PD (PD–pAb) was developed, and PD–pAb-based indirect competitive enzyme-linked immunosorbent assay (icELISA) was established for the detection of PD in Radix Platycodonis. The 50% inhibition concentration (IC50) of PD was 2.70 μg/mL and the linearity range for PD was from 0.032 μg/mL to 100 μg/mL. No cross reactivity with PD–pAb was found in five PD analogs except for platycodin D2 (PD2, 0.93%). The average recovery of PD by icELISA was 97.14% (RSD = 1.17%). The icELISA was used for the detection of PD in different Radix Platycodonis samples and the results were confirmed by high performance liquid chromatography (HPLC). The correlation coefficient between the two assays was 0.9654. Taken together, the established icELISA might be a simple, cheap, rapid, sensitive, reliable and high-throughput method for determining the contents of PD in Radix Platycodonis.
Keywords: Platycodin D, Radix Platycodonis, Polyclonal antibody, Indirect competitive enzyme-linked immunosorbent assay (icELISA)
1. Introduction
Radix Platycodonis, the root of Platycodon grandiflorum A. DC (Campanulaceae), is a well-known traditional Chinese medicine used as an expectorant for pulmonary diseases and a remedy for respiratory disorders such as cough, colds, sore throat, tonsillitis, and chest congestion [1]. In addition, Radix Platycodonis are used to prepare salad and other traditional dishes in Korean [2].
Platycodin D (PD, Fig. 1), a triterpenoid saponin, is a major active ingredient in Radix Platycodonis. PD possessed multiple biological and pharmacological properties including anti-tumor, anti-inflammatory, anti-obesity, anti-atherosclerosis, antiviral, hepatoprotective and immunoregulatory activities [3]. Recently, PD has attracted considerable attention for its anti-tumor [4] and immune-adjuvant activity [5–8]. Meanwhile, PD is used as the quality control marker of Radix Platycodonis in the Pharmacopoeia of People’s Republic of China [9]. However, the quality of Radix Platycodonis varies with cultivation region, harvest time and processing technology. Thus, a reliable and accurate method is required for the purpose of quality control.
Fig. 1.
FT-IR and MALDI-TOF-MS spectra of PD, BSA, HSA, PD-BSA and PD-HSA. (A) FT-IR spectra. (B–F) MALDI-TOF-MS spectra of PD (B), BSA (C), PD-BSA (D), HSA (E) and PD-HSA (F).
Several chromatographic methods such as high performance liquid chromatography (HPLC) coupled with UV, evaporative light scattering detection (ELSD) and electrospray ionization mass spectrometry (ESI-MS) have been reported for the detection of PD in Radix Platycodonis [10–13]. These instrumental analytical methods not only are low throughput and time-consuming, but also require complex pretreatment and expensive and specialized equipment.
An indirect competitive enzyme-linked immunosorbent assay (icELISA) is a simple, sensitive, rapid and high throughput method for the analysis of saponins in crude drug [14–17]. All these reports used a monoclonal antibody (mAb) to develop icE-LISA. Compared to mAb, although the polyclonal antibody (pAb), obtained from the serum of immunized animals, has higher cross reactivity with analogs, it exhibits the advantages of simple processing, low cost and immunogen requirements as well as high stability and sensitivity. In this study, an icELISA based on pAb against PD (pAb-PD) was established to detect the contents of PD in Radix Platycodonis. Then, assay sensibility was analyzed by checking PD concentration range and the specificity was measured by the cross-reactivity of pAb-PD with PD and its structurally consecutive analogs in Radix Platycodonis. Finally, the established icE-LISA was used for the determining the contents of PD in Radix Platycodonis samples from different habitats, and were compared to HPLC.
2. Materials and methods
2.1. Reagents
Bovine serum albumin (BSA) and human serum albumin (HSA) were purchased by Pierce (Rockford, IL, USA); goat anti-rabbit IgG peroxidase conjugate (IgG-HRP) was from Abcam (West Chester, PA, USA); incomplete and complete Freund’s adjuvants were from Difco (Detroit, MI, USA); fetal calf serum (FCS) was from Gibco (Grand Island, NY, USA). All other chemicals were standard commercial products of analytical grade.
Six structurally consecutive saponins, platycodin D (PD), platycodin D2 (PD2), platycodin D3 (PD3), platycoside E (PE), deapioplatycoside E (DPE) and polygalacin D2 (PGD) were isolated from the root of P. grandiflorum as previously described [5,8]. Their chemical structures were identified based on chemical and spectral evidences including mass spectrometry and nuclear magnetic resonance spectroscopy (1H NMR, 13C NMR, 1H–1H COSY, HMQC and HMBC). The purity of each saponin was determined to be more than 98% by HPLC method.
2.2. Plants materials and sample preparation
Five batches of Radix Platycodonis were collected from Chishang Town, Boshan District, Zibo City, Shandong; Shanyang County, Shangluo City, Shaanxi; Niudachang Town, Shibing County, Guizhou; Pan’an County, Zhejiang; Meilin Town, karaqin banner, Chifeng City, Inner Mongolia, China in May 2018, and were authenticated by Prof. Yonghai Jiang from the Shaoxing Institute for Food and Drug Control, Zhejiang, China. A voucher specimen has been deposited at the Lab of Natural Medicine, College of Animal Sciences, Zhejiang University, China. The sample solution was prepared as described in the Pharmacopoeia of People’s Republic of China [9]. Briefly, the powdered material (10.0 g) was extracted with 70% ethanol (100 mL) under reflux for 2 h. After filtration, excess solvent was removed under reduced pressure. The ethanol extract was suspended in 50 mL water and extracted with equal volume of n-butanol saturated with water three times. The combined n-butanol layers were successively washed with equal volume of ammonia reagent and n-BuOH saturated with water, and then concentrated to dryness. The dried extract was separated on silica gel column eluting with a stepwise gradient of ascending polarity of CHCl3/MeOH (9:1), CHCl3/MeOH/H2O (60:20:3) and CHCl3/MeOH/H2O (60:29:6). The final eluate was concentrated to dryness. The residue was dissolve in methanol and filtered through a 0.22–μm Millipore membrane.
2.3. Antigen synthesis
The PD-carrier protein conjugates were synthesized as described previously [18]. Briefly, NaIO4 (4 mg) and PD (10 mg) were dissolved in 0.5 mL H2O and 0.7 mL 80% methanol, respectively. PD solution was dropwise added to NaIO4 solution and then the mixture was continuously stirred for 1 h at room temperature. The BSA and HSA (10 mg) were dissolved in 2.0 mL of carbonate buffer solution (0.01 M, pH 9.6). The protein solution was dropwise added to the above mixture and then was stirred for 5 h at room temperature. The resulting mixture was dialyzed and lyophilized to afford PD-BSA/HSA conjugate.
2.4. Fourier transform infrared spectroscopy (FTIR) analysis
FT-IR spectra of PD, BSA, HSA, PD-BSA and PD-HSA conjugates were collected using a Nicolet AVATAR 370 FT-IR Spectrometer (Thermo Scientific, USA). Each sample (1 mg) was put into agate mortar and well mixed with KBr (100 mg) and lapping. The mixture was compressed tightly to obtain a similar packing thickness about 5 mm. Each spectrum was an average of 32 scans with a resolution of 4 cm−1 across the wavenumbers 4000–400 cm−1. Each sample was scanned three times, and the average spectrum of three measurements was to build the model. The temperature was equilibrated at 25°C and the humidity was kept at 60%.
Second Derivative (DII) and Curve Fitting (Gaussian algorithm) procedures were performed to identify the precise position and absorbance of specific bands. By using OMNIC 8.0 software (Thermo Fisher Scientific), curve fitting was conducted on spectra in the range of 1700–1600 cm−1 after two points baseline linear fitted. In order to determine the underlying component bands, the number of peaks as well as their positions was identified based on DII results, resulting in the optimal reconstructed curve (residual close to zero).
2.5. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOFMS)
The molecular weights of PD-BSA and PD-HSA conjugates were determined using ultrafleXtreme MALDI-TOF/TOF mass spectrometry system (Bruker, USA). PD was dissolved in α-cyano-4- hydroxycinnamic acid (HCCA). BSA, HSA, PD-BSA and PD-HSA were mixed with the water solution containing 30% acetonitrile and 0.1% trifluoroacetic acid. The mixture was subjected to a Flex Control mass monitor and irradiated with a N2 lazer (355 nm, 130 ns pulse for PD; 450 ns pulse for protein and its conjugate). The ions formed by each pulse were accelerated by a 20 kV potential into a 2.0-m evacuated tube and detected using compatible computer.
2.6. Immunization and purification of polyclonal antibody against PD (pAb-PD)
New Zealand rabbits (2-month-old) were purchased from Zhejiang Experimental Animal Center (Hangzhou, China). Animals were acclimatized for 1 week before using. All the procedures were strictly in line with PR China legislation on the use and care of laboratory animals and guidelines of the Institute for Experimental Animals of Zhejiang University.
Rabbits were injected intramuscularly with PD-BSA (1 mg) four times at two-week intervals. For the first immunization, the PD-BSA dissolved in PBS (1 mg/mL) was emulsified with the equal volume of Freund’s complete adjuvant. The other immunizations were injected with PD-BSA solution emulsified in equal volume of Freund’s incomplete adjuvant. Two weeks after the last immunization, serum was collected by centrifugation. The serum was adjusted to pH 7 with 1 M sodium bicarbonate solution, and then subjected to Protein G column (0.46 × 11 cm, Ferrel biotech, Hangzhou, China). The column was washed with PBS (pH 7.0) and eluted with 200 mM glycine buffer (pH 2.7). The eluent was neutralized to pH 2.7 with 1Msodium bicarbonate solution, and then stored at −20 °C.
2.7. Indirect ELISA
The reactivity of pAb-PD to PD-HSA was determined by a direct ELISA as described previously [5]. In brief, microtiter plate wells were coated with 100 μL PD-HSA solution (50 mM carbonate-bicarbonate buffer, pH 9.6) for 24 h at 4°C. The wells were washed three times with PBS containing 0.05% (v/v) Tween 20 (PBS/Tween), and then blocked with 5% FCS/PBS at 37°C for 2 h. After three washings, 100 μl of a series of diluted pAb-PD or 0.5% FCS/PBS as control were added to triplicate wells. The plates were then incubated for 1 h at 37°C, followed by 3 times of washing. Aliquots of 100 μl of goat antirabbit IgG-HRP diluted with 0.5% FCS/PBS were added to each plate. The plates were further incubated for 1 h at 37°C. After washing, the peroxidase activity was assayed as following: 100 μl of substrate solution (10 mg of O-phenylenediamine and 37.5 μl of 30% H2O2 in 25 ml of 0.1 M citrate-phosphate buffer, pH5.0) was added to each well. The plate was incubated for 10 min at 37°C, and enzyme reaction was terminated by adding 50 μl/well of 2 N H2SO4. The optical density (OD) was measured in an ELISA reader at 490 nm.
2.8. Indirect competitive ELISA (icELISA)
The microtiter plate wells were coated with 100 μL PD-HSA solution (1 μg/mL in 50 mM carbonate-bicarbonate buffer, pH 9.6) for 24 h at 4°C. The wells were washed three times with PBS/Tween, and then blocked with 5% FCS/PBS at 37°C for 2 h. Fifty microliters of various concentrations of PD dissolved in PBS was incubated with 50 μL of pAb-PD solution (2.1 μg/mL) for 1 h. After three washings, 100 μl of the mixture were added to triplicate wells. The plates were then incubated for 1 h at 37°C, followed by 3 times of washing. Aliquots of 100 μl of goat anti-rabbit IgG-HRP diluted 110000: with 0.5% FCS/PBS were added to each plate. The plates were further incubated for 1 h at 37°C. After washing, the peroxidase activity was assayed as described above. The OD was measured in an ELISA reader at 490 nm. The relationship between the OD and concentration of PD was calculated by four parameter logistic curve fitting.
2.9. Assay specificity
The cross-reactivity (CR) of pAb-PD with PD and its analogs was detected to evaluate the assay specificity as described previously [19]. IC50 values of PD and its analogs as the inhibitors were determined by icELISA described above. CR was calculated using 50% displacement method as follows: CR (%) = (IC50 of PD)/IC50 of PD analogs) × 100%.
2.10. Recovery analysis
A series of PD standard solutions were spiked into the powered Radix Platycodonis for five replicates per concentration, and then extracted to prepare the samples as described above. The icELISA analysis of the samples was carried out under the established experimental condition as mentioned above. The recovery was calculated as follows: Recovery (%) = [(Measured amount − amount in the sample)/spiked amount] × 100%.
2.11. HPLC analysis
The contents of PD in Radix Platycodonis were determined using a Diamond C18 column (250 mm × 5 mm; i. d. 5 μm particle size) and Waters 2996 PDA detector on the Water 600E HPLC instrument. The mobile phase was H2O–CH3CN (75:25 v/v). The flow rate was 1 mL/min and the injection volume was 10 μL. Column temperature was constantly kept at 30°C.
2.12. Statistical analysis
The data were expressed as mean ± standard deviation (SD) and examined for their statistical significance of difference with Student’s t-test, ANOVA and the post hoc test. P-values of less than 0.05 were considered to be statistically significant.
3. Results and discussion
3.1. Synthesis and characterization of PD-BSA/HSA
PD has no immunogenicity due to its small molecular weight. Therefore, PD was conjugated with BSA or HSA to prepare the complete antigen. PD-BSA was used as the immunogen to produce rabbit polyclonal antibody against PD (pAb-PD), while PD-HSA was used as the coating antigen for ELISA. The complete antigens were identified and characterized by FT-IR and MALDI-TOF-MS.
The FT-IR spectra of PD, BSA, HSA, PD-BSA and PD-HSA at a frequency range of 4000–400 cm−1 were shown in Fig. 1A. PD was characteristic of three absorption peaks at 3385 cm−1 (free OH), 1732 cm−1 (COOR) and 1076 cm−1 (pyranose ring). Compared to the corresponding BSA and HSA, the presence of the absorption band at 1075 cm−1 (PD-BSA) and 1078 cm−1 (PD-HSA) indicated that PD was successfully coupled with BSA and HSA, respectively.
MALDI-TOF-MS analysis showed the quasi-molecular ion of PD at m/z 1247.606 in agreement with the molecular formula C57H92O28 (Fig. 1B). The molecular weights were determined to be 66424.46 and 66469.83 for BSA and HSA, respectively (Fig. 1C and D). The peaks coinciding with PD-BSA and PD-HSA appeared at m/z 68320.90 and 69013.88, respectively (Fig. 1E and F). Therefore, the PD-BSA and PD-HSA coupling ratios were 1.5:1 and 2:1, respectively.
3.2. Production and characterization of PD–pAb
The serum from the rabbit immunized with PD-BSA in combination incomplete and complete Freund’s adjuvants was collected to prepare a polyclonal antibody against PD (PD–pAb). The checkerboard titration method was performed to optimize the working condition of indirect ELISA using the various concentrations of coating antigen (PD-HSA) (1 – 200 μg/mL) and goat anti-rabbit IgG-HRP diluted from 1:5000 to 1:80000. The optimal combination of 1 μg/mL of PD-HSA and 1:10000 dilution of goat anti-rabbit IgG-HRP was used for the subsequent ELISA. The indirect ELISA reveals the higher titers of antibody against PD in the serum from immunized rabbits (Fig. 2A). In order to reduce non-specific response, the anti-PD rabbit serum was purified to prepare a polyclonal antibody against PD (PD–pAb) through Protein G column. The reactivity of PD–pAb with PD was determined using the various concentrations of PD–pAb, and the results were shown in Fig. 2B. The concentration of PD–pAb (2.1 μg/mL) at which the OD was about 1.0 was selected for icELISA.
Fig. 2.
Titer curve of rabbit serum against PD by indirect ELISA (A) and reactivity of PD–pAb against Platycodin D (B).
3.3. Assay sensitivity, specificity and accuracy
Assay sensitivity was investigated by the linear range of standard PD. The excessive PD–pAb following competition was bound to polystyrene microtiter plates pre-coated with PD-HSA. The 50% inhibition concentration (IC50) of PD was 2.70 μg/mL and the fitting curve range for PD was from 0.032 μg/mL to 100 μg/mL (Fig. 3).
Fig. 3.
Calibration curve of PD by icELISA.
Since the icELISA established in this study was used for detecting the contents of PD in Radix Platycodonis, the assay specificity was checked by determining the cross reactivity of PD–pAb to PD and its five structurally consecutive analogs with a triterpenoid aglycone and two unbranched sugar side chains in Radix Platycodonis. The differences in chemical structure of these saponins mainly exist in the aglycone, the linkage and number of glycosyl groups in the sugar chain attached to C-3, as well as the glycidic moieties attached to C-28 of the aglycone (Table 1). Compared to the other five saponin compounds, the major difference of PD was only one glucose unit at position C-3 of the aglycone. As shown in Table 1, no cross reactivity with PD–pAb was found in five analogs of PD except for PD2 (0.93%). It was evident that the sugar chain attached to C-3 rather than aglycone and the glycidic moieties attached to C-28 affected the cross reactivity of these saponins with PD–pAb, and that PD–pAb exhibited high specificity to PD. Therefore, PD–pAb could be routinely used for detecting the contents of PD in Radix Platycodonis.
Table 1.
Cross-reactivity of PD–pAb against PD and its analogs.
| Compounds | Aglycone | Composition of sugar chains | IC50 (μg/mL) | CR (%) | |
|---|---|---|---|---|---|
|
| |||||
| C-3 | C-28 | ||||
| Platycodin D | Platycodigenin | Glc- | Api-(1 → 3)-Xyl-(1 → 4)-Rha-(1 → 2)-Ara- | 2.70 | 100 |
| Platycodin D2 (PD2) | Glc-(1 → 3)-Glc- | Api-(1 → 3)-Xyl-(1 → 4)-Rha-(1 → 2)-Ara- | 291.8 | 0.93 | |
| Platycodin D3 (PD3) | Glc-(1 → 6)-Glc- | Api-(1 → 3)-Xyl-(1 → 4)-Rha-(1 → 2)-Ara- | NIa | NI | |
| Platycoside E (PE) | Glc-(1 → 6)-glc-(1 → 6)-glc- | Api-(1 → 3)-Xyl-(1 → 4)-Rha-(1 → 2)-Ara- | NI | NI | |
| Deapioplatycoside E (DPE) | Glc-(1 → 6)-glc-(1 → 6)-glc- | Xyl-(1 → 4)-Rha-(1 → 2)-Ara- | NI | NI | |
| Polygalacin D2 (PGD) | Polygalacic acid | Glc-(1 → 3)-Glc- | Api-(1 → 3)-Xyl-(1 → 4)-Rha-(1 → 2)-Ara- | NI | NI |
The data were expressed as mean ± SD (n = 6). Api: β-D-apiofuranosyl; Ara: α-L-arabinopyranosyl; Glc: β-D-glucopyranosyl; Rha: α-L-rhamnopyranosyl; Xyl: β-D-xylopyranosyl. NI: No inhibition was observed at up to 1 mg/mL of the compound.
The recovery experiment was conducted to evaluate the accuracy and reliability of the established icELISA. As shown in Table 2, the average recovery of PD was 97.14% and its RSD was 1.17% (n = 5).
Table 2.
The results of recovery test of PD.
| Spiked (mg/g) | Detected (mg/g) | Recovery (%) | Average recovery (%) | RSD (%) |
|---|---|---|---|---|
| 0 | 2.23 ± 0.17 | – | 97.14 | 1.17 |
| 1 | 3.15 ± 0.06 | 96.42 | ||
| 2 | 4.13 ± 0.05 | 95.56 | ||
| 4 | 6.17 ± 0.05 | 97.38 | ||
| 8 | 10.18 ± 0.12 | 98.05 | ||
| 10 | 12.19 ± 0.05 | 98.28 |
The data were expressed as mean ± SD (n = 5). RSD: relative standard deviation.
3.4. Analysis of PD in Radix Platycodonis
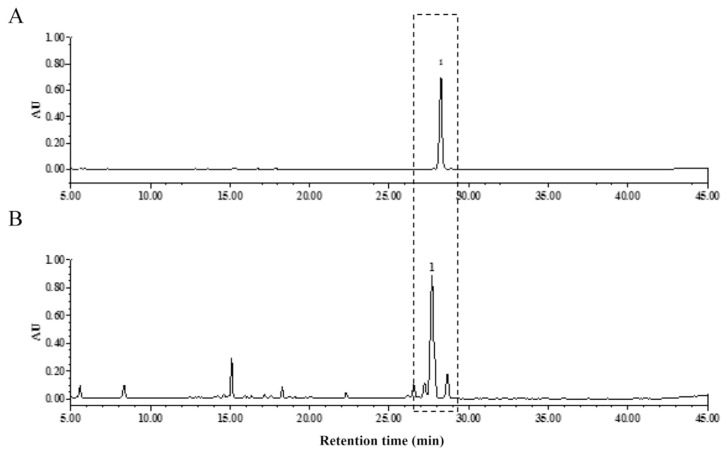
The contents of PD are used as the quality control marker of Radix Platycodonis by HPLC in Pharmacopoeia of People’s Republic of China [9]. The icELISA was used to determine the contents of PD in five Radix Platycodonis samples from different habitats in comparison with HPLC (Fig. 4). The correlation coefficient of the values analyzed by icELISA and HPLC was calculated to be 0.9654 using linear regression (Table 3), suggesting a good consistency between these two methods. The PD content detected by icELISA in the samples except for Inner Mongolia sample is higher than that estimated by HPLC. This might be related to the differences in the types and contents of compounds in Radix Platycodonis from the different habitats.
Fig. 4.
HPLC chromatograms of PD (A) and Radix Platycodonis (B).
Table 3.
The contents of platycodin D (PD) in Radix Platycodonis collected from different habitats.
| Sample | Content of PD (mg/g dr. wt) | |
|---|---|---|
|
| ||
| icELISA | HPLC | |
| Shandong | 1.45 ± 0.37 | 1.42 ± 0.03 |
| Shaanxi | 2.45 ± 0.62 | 2.42 ± 0.01 |
| Guizhou | 1.37 ± 0.22 | 1.32 ± 0.11 |
| Zhejiang | 2.23 ± 0.17 | 2.06 ± 0.03 |
| Inner Mongolia | 2.27 ± 0.36 | 2.50 ± 0.04 |
The data were expressed as mean ± SD (n = 6 for ELISA and n = 3 for HPLC). The purity of PD standard used in this study was 99.12% by HPLC.
4. Conclusions
In the present study, pAb–PD was developed and pAb–PD-based icELISA was established for the detection of PD in Platycodonis Radix. The linearity range for PD was from 0.032 μg/mL to 100 μg/mL. No cross reactivity with PD–pAb was found in five PD analogs except for PD2 (0.93%). The average recovery of PD by icELISA was 97.14% (RSD = 1.17%). There was a good consistency between the results by icELISA and HPLC. The established icE-LISA was suitable for detecting the contents of PD in Radix Platycodonis. This study provided a simple, cheap, rapid, sensitive, reliable and high-throughput method for the quality control of Radix Platycodonis.
Supplementary Information
Acknowledgment
This work was supported by Grant-in-Aid from the National Natural Science Foundation of China (Nos. 31472229, 31772783, and 31972726), the National Key R&D Program of China (2017YFD0501505).
Funding Statement
This work was supported by Grant-in-Aid from the National Natural Science Foundation of China (Nos. 31472229, 31772783, and 31972726), the National Key R&D Program of China (2017YFD0501505).
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1. Zhang L, Wang YL, Yang DW, Zhang CH, Zhang N, Li MH, Liu YZ. Platycodon grandiflorus - an ethnopharmacological, phytochemical and pharmacological review. J Ethnopharmacol. 2015;164:147–61. doi: 10.1016/j.jep.2015.01.052. [DOI] [PubMed] [Google Scholar]
- 2. Nyakudya E, Jeong JH, Lee NK, Jeong YS. Platycosides from the roots of Platycodon grandiflorum and their health benefits. Prev Nutr Food Sci. 2014;19:59–68. doi: 10.3746/pnf.2014.19.2.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang ZR, Chan CW. Multi-target pharmacological effects of platycodin D. Mini-rev Org Chem. 2017;14:342–7. [Google Scholar]
- 4. Khan M, Maryam A, Zhang H, Mehmood T, Ma T. Killing cancer with platycodin D through multiple mechanisms. J Cell Mol Med. 2016;20:389–402. doi: 10.1111/jcmm.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xie Y, Ye YP, Sun HX, Li D. Contribution of the glycidic moieties to the haemolytic and adjuvant activity of platycodigenin-type saponins from the root of Platycodon grandiflorum. Vaccine. 2008;26:3452–60. doi: 10.1016/j.vaccine.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 6. Xie Y, Sun HX, Li D. Platycodin D is a potent adjuvant of specific cellular and humoral immune responses against recombinant hepatitis B antigen. Vaccine. 2009;27:757–64. doi: 10.1016/j.vaccine.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 7. Xie Y, Sun HX, Li D. Platycodin D improves the immunogenicity of Newcastle disease virus-based recombinant avian influenza vaccine in mice. Chem Biodivers. 2010;7:677–89. doi: 10.1002/cbdv.200900183. [DOI] [PubMed] [Google Scholar]
- 8. Sun HX, Chen LQ, Wang JJ, Wang KW, Zhou JY. Structure-function relationship of the saponins from the roots of Platycodon grandiflorum for hemolytic and adjuvant activity. Int Immunopharmacol. 2011;11:2047–56. doi: 10.1016/j.intimp.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 9.National Commission of Chinese Pharmacopoeia. Pharmacopoeia of People’s Republic of China. Beijing, China: China Medical Science and Technology Press; 2015. [Google Scholar]
- 10. Ha YW, Na YC, Seo JJ, Kim SN, Linhardt RJ, Kim YS. Qualitative and quantitative determination of ten major saponins in Platycodi Radix by high performance liquid chromatography with evaporative light scattering detection and mass spectrometry. J Chromatogr A. 2006;1135:27–35. doi: 10.1016/j.chroma.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peng Y, Zhang F, Tao HX, Wang W, Sun LN, Chen WS, Wang CH. Simultaneous determination of multiple platycosides with a single reference standard in Platycodi Radix by high-performance liquid chromatography coupled with evaporative light scattering detection. J Sep Sci. 2015;38:3712–9. doi: 10.1002/jssc.201500542. [DOI] [PubMed] [Google Scholar]
- 12. Lu HY, Ju MZ, Chu SS, Xu T, Huang YZ, Chan QY, Peng HS, Gui SY. Quantitative and chemical fingerprint analysis for the quality evaluation of Platycodi Radix collected from various regions in China by HPLC coupled with chemometrics. Molecules. 2018;23:7. doi: 10.3390/molecules23071823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shan JJ, Zou JS, Xie T, Kang A, Zhou W, Xu JY, Shen CS, Du LN, Wang SC, Di LQ. Effects of Gancao on pharmacokinetic profiles of platycodin D and deapio-platycodin D in Jiegeng. J Ethnopharmacol. 2015;170:50–6. doi: 10.1016/j.jep.2015.04.056. [DOI] [PubMed] [Google Scholar]
- 14. Fukuda N, Tanaka H, Shoyama Y. Western blotting for ginseng saponins, ginsenosides using anti-ginsenoside Rb1 monoclonal antibody. Biol Pharm Bull. 1999;22:219–20. doi: 10.1248/bpb.22.219. [DOI] [PubMed] [Google Scholar]
- 15. Nah JJ, Song JY, Choi S, Kim SC, Rhim HW, Oh TH, Lee SM, Nah SY. Preparation of monoclonal antibody against ginsenoside Rf and its enzyme immunoassay. Biol Pharm Bull. 2000;23:523–6. doi: 10.1248/bpb.23.523. [DOI] [PubMed] [Google Scholar]
- 16. Zhao J, Li G, Wang BM, Liu W, Nan TG, Zhai ZX, Li ZH, Li QX. Development of a monoclonal antibody-based enzyme-linked immunosorbent assay for the analysis of glycyrrhizic acid. Anal Bioanal Chem. 2006;386:1735–40. doi: 10.1007/s00216-006-0780-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qu HH, Sai JY, Wang Y, Sun Y, Zhang Y, Li YF, Zhao Y, Wang QG. Establishment of an enzyme-linked immunosorbent assay and application on determination of ginsenoside Re in human saliva. Planta Med. 2014;80:1143–50. doi: 10.1055/s-0034-1382959. [DOI] [PubMed] [Google Scholar]
- 18. Fukuda N, Tanaka H, Shoyama Y. Formation of monoclonal antibody against a major ginseng component, ginsenoside Rg1 and its characterization. Cytotechnol. 2000;29:197–204. doi: 10.1023/A:1008162703957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang B, Nan TG, Zhan ZL, Kang LP, Yang J, Lai CJS, Yuan Y, Wang BM, Huang LQ. A monoclonal antibody-based enzyme-linked immunosorbent assay for the determination of chlorogenic acid in honeysuckle. J Pharmaceut Biomed Anal. 2018;148:1–5. doi: 10.1016/j.jpba.2017.09.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.