Abstract
Nanozymes have become attractive in analytical and biomedical fields, mainly because of their low cost, long shelf life, and less environmental sensitivity. Particularly, nanozymes formed from nanomaterials having high surface area and rich active sites are interesting since their activities can be tuned through carefully controlling their size, morphology, and surface properties. This review article focuses on preparation of carbon dots (C dots) possessing peroxidase-like activity and their analytical applications. We highlight the important roles of the oxidation states and surface residues of C dots and their nanocomposites with metal, metal oxides, or metal sulfides playing on determining their specificity and sensitivity toward H2O2. Examples of C dot nanozymes (CDzymes) for developing sensitive and selective absorption, fluorescence, and electrochemical sensing systems in the presence of substrates are presented to show their potential in analytical applications. For example, CDzymes couple with glucose oxidase and cholesterol oxidase are specific and sensitive for quantitation of glucose and cholesterol, separately, when using 3,3′,5,5′-tetrame-thylbenzidine as the signal probe. This review article concludes with possible strategies for enhancing and tuning the catalytic activity of CDzymes.
Keywords: Carbon dots, CDzymes, Nanozymes, Peroxidase, Sensing
1. Introduction
Many natural enzymes with high specificities and catalytic activities are popular for analytical and biomedical applications [1, 2]. For example, horseradish peroxidase (HRP) is the most widely used enzyme for sensitive and selective detection of H2O2. The HRP general mechanism is initiated from the pentacoordinated ferric heme, binding H2O2. One of the H2O2 oxygen atoms then leaves as water, while the other is retained as a ferryl group to generate compound I, featuring an Fe(IV) center coupled to a porphyrin cation radical. Compound I then accepts one electron from a substrate molecule (typically an aromatic compound – phenolic or aminic), yielding Compound II, which still contains a ferryl group, but no porphyrin radical cation. Compound II then accepts one electron from a second substrate molecule, yielding the enzyme native state (ferric). As to the fate of the substrate, loss of one electron, usually accompanied by loss of a proton, leads to the formation of products with different absorbance, fluorescence and electrical properties from that of the substrates. More importantly, enzyme cascades of HRP combined with various enzymes are used in many sensitive and selective assays for many important analytes. For instance, HRP-glucose oxidase (GOx) and HRP-uricase are commonly employed to develop sensing systems for detection of glucose and uric acid, respectively [3].
Nowadays, immobilized oxidative enzymes are broadly accepted as a green way to face the challenge of high amounts of micropollutants in nature. Immobilized HRP are showed better stability, and reusability as well as easy separation from reaction mixture that make them more favorable and economic in compared to free enzymes [4]. Furthermore, the combination of enzyme immobilization with prodrugs was also considered as a promising approach for biomedical application of enzyme in cancer therapy [5]. However, use of natural enzymes for developing sensing systems is sometimes limited by their high cost and short shelf lifetime. In addition, their catalytic activities are usually very sensitive to environmental conditions [6]. For example, most enzymes reach maximal catalytic activities at temperature around 37°C and pH value at around 7.0. Therefore, inexpensive artificial enzymes with high catalytic activities and excellent stabilities for analytical and biomedical applications are highly demanded.
A number of organic materials and biomaterials like DNAzymes have been recognized for quantitation of various analytes with advantages of low cost, stability, and a wider working range (pH, ionic strength, and temperature) [7]. However, the specificity and turnover number of the artificial enzymes are usually not great as that of the natural ones. As an alternative to natural and artificial enzymes, nanozymes (nanomaterial-based artificial enzymes) with high activity have been prepared and applied for various analytical and biomedical applications [8–17]. In addition, nanozymes, when compared to DNAzymes, are usually cheaper and less sensitive to changes in pH, ionic strength, and temperature. Their activity is usually size dependent; small nanoparticles with greater surface area and higher density of defects (active sites) are more active than larger ones [18]. Many metal-based nanoparticles, including Pt, Pd, Au, and Ag exhibiting peroxidase-, oxidase-, and catalase-like activities have been used to develop sensitive and selective sensing assays for detection of various analytes, such as protein, heavy metal ions, and glucose [19–27]. Some relatively cheaper nanozymes, including metal oxide (Fe3O4, CuO, CeO2, MnO2, and V2O5) nanoparticles and metal sulfide (FeS, CuS, and MoS2) nanoparticles, have been employed to develop sensitive and selective sensing systems and to fabricate various logic gates [28–36]. With the advantages of high activity, low cost, and stability, nanozymes have become very popular materials in analytical chemistry and biomedical applications [37].
Having excellent biocompatible, catalytic, mechanical, electrical, optical, and thermal properties, many carbon nanomaterials such as carbon nanotubes, carbon dots (C dots), activated carbon, and graphite have become popular as energy materials, drug delivery, sensors, field emission devices, and water splitting [38–42]. For example, C dots with CuS, CoS, and NiS nanomaterials have been shown improved light conversion efficiency by taking advantages of the conductivity of C dots [43, 44]. Core–shell carbon nanomaterials prepared from red onion skins and boron have shown efficient for water splitting, with high oxygen reduction reaction efficiency and greater stability [45].
Owing to having high surface area, great number of surface defects, stability and biocompatibility, C dots are applied for various analytical and biomedical applications [46]. In addition, they can be prepared through green and environment friendly approaches, with large-scale production [47]. Thus, we focus our discussion on C dot nanozymes (CDzymes) with peroxidase mimic catalytic activity for analytical and biomedical applications in this review article, mainly because of our own interest and their importance for detection of important analytes such as H2O2, glucose, uric acid, glutathione and cholesterol. Further information regarding nanozymes and their applications are available from several excellent review papers published in the last three years [8, 9, 48]. We briefly discuss the preparation of CDzymes and their characteristics. Examples of their analytical applications are provided to highlight their advantages and drawbacks as artificial enzymes to replace natural peroxidases. This review article concludes with the discussion about the challenges and strategies for developing ideal CDzymes.
2. Preparation of C dots
C dots refer to carbon nanomaterials with photo-luminescence properties and they have received extensive attention as sensitive materials in sensing and imaging applications [49–52]. Photoluminescent carbon nanomaterials are also called in different names, including carbon quantum dots (carbon nanoparticles with sizes below 10 nm and some form of surface passivation) [53], carbon nanodots (carbon nanomaterials with sizes below 10 nm) [49], graphene quantum dots (graphene sheets with lateral size less than 100 nm) [54], carbogenic dots (discrete carbon nanoparticles of near spherical geometry with sizes below 10 nm) [55], and carbon nanocrystals (smaller crystals of around 2 nm in size) [56]. To make it easier for readers to follow, we use “C dots” to represent photo-luminescent carbon nanomaterials in this article.
Each C dot consists of a carbonaceous core and a surface passivation layer. The carbon core can be either sp2 hybridized graphene fragments or carbon composed of sp2 and sp3 hybridized carbon [57]. The size range of C dots is typically from 2 nm to 100 nm. Features of C dots include large surface area to volume ratios, insufficiently coordinated surface atoms, and many unsaturated bonds. In general, each C dot has a surface passivation layer on its core to reduce the system’s Gibbs free energy. When hydrophilic carbon precursors are used, hydrophilic C dots are usually obtained, mainly because of the existence of rich hydroxyl, amino, and carboxyl groups on their surfaces. On the other hand, C dots are generally hydrophobic when using hydrophobic carbon precursors. To modulate the polarity, optical properties, chemical reactivity, and selectivity of C dots, modifiers such as oligomers, polymers, and biomolecules are used to passivate their surfaces [58, 59]. Having the advantages of biocompatibility, brightness, negligible photoquenching and photo-blinking, stability against salt, and ease in preparation, low-cost C dots have become interesting materials for sensing, in vitro and in vivo imaging applications [60–67].
C dots can be prepared from various sources through top-down and bottom-up approaches [68, 69]. When a large size of solid or powder (graphite, carbon fiber, and carbon black) is available as a carbon source, a top-down approach through etching/oxidation is usually applied to obtain C dots. The structure of carbon source generally contains graphite crystallites or a large number of sp2 conjugated microdomains. Because of consumption of large amount of energy, need of an expensive synthetic system, and difficulty for a large-scale preparation of C dots, the top-down approach is less popular than the bottom-up approaches like hydrothermal route and electrochemical approach. Ever since the C dots preparation from amino acids through hydrothermal approaches were demonstrated [70], this approach has become most popular for the preparation of C dots from various carbon precursors such as carbohydrates, organic acids, organic amines, and polymers. The pioneering works for preparation of C dots with different biological activities from tea and used coffee powders have led to preparation of C dots from natural sources such as fruits, grass, and trees [71–75]. It has been suggested that C dots are formed from their precursors through four steps of dehydration, condensation, carbonization, and passivation [76]. When the preparation conditions are mild, C dots usually have no obvious crystal structures, with cores consisting of either amorphous carbon or non-conjugated polymers [77, 78]. The surface groups of C dots can be introduced either directly during the preparation process or through subsequent passivation treatment with functional organic ligands after the synthesis.
3. Artificial peroxidases of C dots
Owing to having high surface area and great number of surface defects, stable and biocompatible C dots are efficient for generation of reactive oxygen species inside cells [63, 71, 72]. C dots and their hybrids with nonmetal, metal, and metal oxide possess some apparent benefits such as resistance toward inhibition or digestion by proteases. When compared to natural enzymes, CDzymes have longer shelf lifetime, less working restriction, and lower cost, however, usually have lower catalytic activity [9, 79–81]. CDzymes possess peroxidase-like catalytic activities, which have been used to develop sensitive sensing systems for detection of various analytes and for environmental monitoring. Most of the sensing systems are based on the catalytic activities of the CDzymes to oxidize H2O2 to form hydroxyl radicals that convert substrates to form products with different absorbance, fluorescence and electrical properties from that of the substrates [81, 82].
3.1. C dots
Most sensing systems are based on the fact that C dots catalyzed H2O2-mediated oxidation of a peroxidase substrate 3,3′,5,5′-tetramethylbenzidine (TMB), leading to a change in color (colorless to blue) and an absorbance increase at 652 nm [83]. Generally, the catalytic pathway of C dots follows a ping-pong mechanism. According to the ping-pong mechanism, C dots and its intermediate C dots* are existent in the reaction system. Once an electron-donor substrate binds to C dots, C dots* are formed. For example, H2O2 molecules inside cells react with C dots to form C dots*, which then return to C dots and ROS is formed. When compared to C dots without containing heteroatoms, C dots synthesized from certain precursors containing heteroatoms usually possess higher catalytic activity, mainly because of their strong affinity toward substrates and high electron transfer rate.
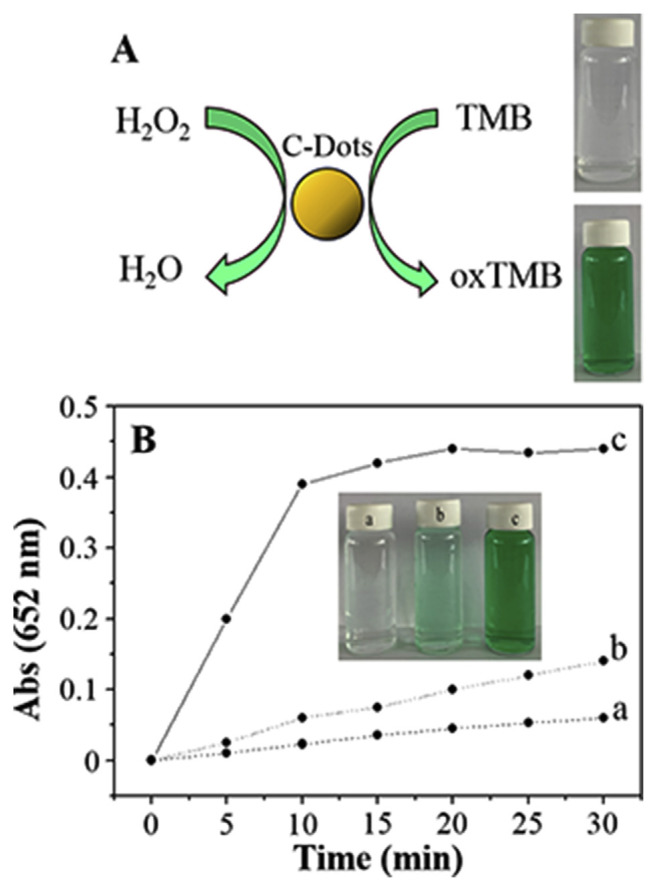
Similar to HRP, the activities of the CDzymes are highly dependent on their concentration, substrate concentration, pH, and temperature. C dots prepared from candle soot by oxidative treatment with 5 M HNO3 show peroxidase-like activity in sodium acetate buffer to catalyze the typical color reaction of H2O2 and TMB (Fig. 1). Electron transfer from the amino groups of TMB to the C dots has been suggested for the increase in the electron density and mobility in the C dots, which then accelerate electron transfer from the C dots to H2O2. As a result, the oxidation rate of TMB by H2O2 increases. Under the optimal conditions (pH 3.5, 35 °C, and 300 mM H2O2), a Michaelis-Menten constant (Km) value of the C dots for TMB is reported to be 0.039 ± 0.001 mM, showing its high affinity toward TMB. The absorbance (λmax: 652 nm) increases linearly with increasing H2O2 concentration in the range of 0.0010–0.1 mM, with a limit of detection (LOD) of 0.2 μM. When combined with GOx, the C-dot sensor is capable of determining the glucose concentration over a linear range of 0.001–0.5 mM and LOD of 0.4 μM. Having advantages of sensitivity and selectivity, the assay is applied for the quantitation of glucose in diluted serum samples.
Fig. 1.
(A) Schematic illustration of oxidation color reaction of TMB with H2O2 catalyzed by C dots. (B) Time-dependent absorbance changes at 652 nm of TMB in different reaction systems: (a) C dots + TMB, (b) TMB + H2O2 and (c) TMB + C dots + H2O2 in a pH 3.5 NaAc buffer (0.2 M) at 35°C. C dots are presented in C-Dots in the figure. Reproduced from Ref. [83] with permission from The Royal Society of Chemistry.
NaBH4-reduced C dots possessing peroxidase-like activity were applied for quantitation of H2O2, with a concentration range of 0.010 to 0.10 mM [84]. Their activity depends on the pH and temperature, as well as the concentrations of H2O2, TMB and reduced C dots. When separately combined with GOx and uricase, the C-dot sensor allows for the quantitation of glucose and uric acid, respectively. Under the optimized conditions, the assay provides a linear concentration range of 0.010–0.4 mM, with LOD of 2.0 μM for glucose, and a linear concentration range of 0.010–0.20 mM, with LOD of 3.0 μM for uric acid. When compared to C dots (without NaBH4 treatment), the reduced C dots provides lower catalytic activity toward H2O2, mainly because many ketonic carbonyl groups (−C=O) are converted to hydroxyl groups (−OH) in the reduced C dots. Relative to hydroxyl groups, ketonic carbonyl groups have higher catalytic activity for H2O2. C dots treated separately with phenylhydrazine, benzoic anhydride, and 2-bromo-1-phenlyethanone were used to investigate the role of the functional groups in the catalytic activity of C dots [85]. It is noted that the three reagents act as selective deactivation agents to react with the −C=O, −OH and carboxyl groups (O=C–O−) on the C dots, respectively. Based on the Km and the maximum initial velocity (Vmax) values for 2,2′-azinobis (3-ethylbenzthiazoline-6-sulfonate) (ABTS), the −C=O groups are suggested to act as the catalytically active sites, meanwhile the O=C–O− and the −OH groups serve separately as the substrate binding sites and catalytic-activity inhibiting residues.
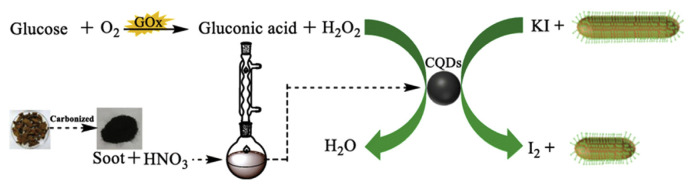
When combined with gold nanorods (GNRs), C dots enable colorimetric detection of glucose [86]. The C dots prepared from litchi rind through a carbonized treatment, followed by refluxing with 5 M HNO3 at 140 °C for 12 h exhibit peroxidase-like catalytic activity in the H2O2-mediated oxidation of iodide to form iodine that etches the GNRs along the longitudinal direction due to higher reaction activities at the tips of GNRs (Fig. 2). The etching of GNRs results in blue shifts in the maximum absorption wavelength from 953 to 645 nm. The shift in the maximum absorption wavelength decreases linearly upon increasing the glucose concentration in the range of 0.01–2.0 mM, with LOD of 3.0 μM.
Fig. 2.
Schematic presentation of the colorimetric method for glucose detection. GOx (glucose oxidase), O2 (oxygen), H2O2 (hydrogen peroxide), HNO3 (nitric acid), C dots, KI (potassium iodide), I2 (iodine). C dots are presented in CQD in the figure. Reproduced from Ref. [86] with permission from Springer Nature.
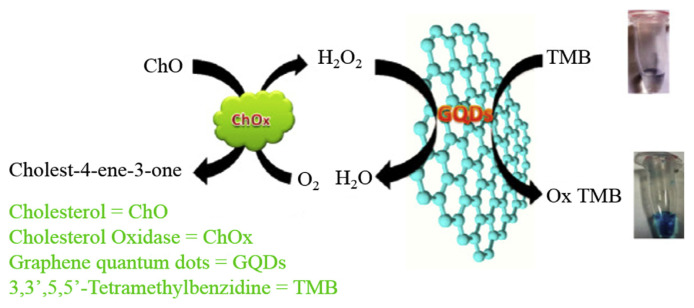
C dots prepared from wood charcoal through an electrochemical synthesis using (NH4)2S2O8 as an electrolyte possess peroxidase-like activity [87]. During electro-oxidation of wood charcoal, ions produce SO4•− radicals that function as sharp electrochemical scissors to cut down graphene sheets into very small intact sp2 structures through oxidation of the C–C bonds. The as-prepared C dots provide a Vmax of 7.2 × 10−7 M s −1 and Km of 12 μM for TMB oxidation reaction, showing their high peroxidase-like activity. When combined with GOx, the C dots in the presence of TMB allow a rapid and sensitive detection of glucose, with LOD of 6.0 μM and a linear range of 10–600 μM. Oxidative C dots prepared from multiwalled carbon nanotubes through a facile oxidation reflux approach show high peroxidase-like activity in a wide range of pH values, mainly because of abundant −C=O and O=C–O− groups, and negligible −OH groups found on their surfaces [88]. The oxidative C dots provide a Km value that is five times lower than those of C dots and even an order of magnitude lower than that of HRP. In the presence of TMB, C dots allow detection of H2O2 over a linear concentration range of 20.0 nM–5.0 μM. Using GOx to oxidize glucose to form H2O2, the system can determine glucose concentration in diluted blood samples from Balb/c mice, with high accuracy and precision. C dots prepared from graphite using a simple wet chemical method also show peroxidase-like activity [89]. When combined with cholesterol oxidase (ChOx), and TMB as substrate, C dots has been reported to detect cholesterol over a linear concentration range of 20–600 μM, with LOD of 6.0 μM (Fig. 3).
Fig. 3.
Schematic illustration of oxidation color reaction of TMB with H2O2 catalyzed by C dots. C dots are presented in GQDs in the figure. Reproduced from Ref. [89] with permission from Elsevier.
Photosensitization is a promising avenue of oxygen activation, which can overcome the spin selection rule to transform the ground state oxygen (3O2) into a highly reactive singlet oxygen (1O2). C dots are a promising type of carbon-based photosensitizer, and nitrogen doping can further improve the oxygen photosensitization performance. Wu et al. proposed a well-developed synthetic protocol of hydrothermal treatment of citric acid and ethylenediamine for the preparation of nitrogen-doped C dots (N-doped C dots) [90]. The oxygen photosensitization performances of the N-doped C dots were first confirmed by ROS investigation with TMB oxidation as the ROS probe and EPR. After XPS analysis of the surface nitrogen doping speciation, it was found that the changes of graphitic N and pyrrolic N correlated well with the oxygen photosensitization performances of N-doped C dots. The excellent photosensitized oxygen activation makes these N-doped C dots a promising oxidase-mimicking nanozyme for photodynamic antimicrobial chemotherapy and other applications [91]. In addition, N-doped C dots through strong acid oxidation of three dimensional N-doped graphene aerogel possess peroxidase-like activity for the oxidation of TMB [92]. The introduction of N atoms into benzene ring atoms can efficiently influence the spin density and the charge distribution of the carbon atoms, enhancing the density of the catalytic active sites on the graphene surface with low steric hindrance for binding TMB [93]. Meanwhile, the lone-pair of electrons in the amino groups of TMB are transferred to the surfaces of the N-doped C dots, which are also responsible for increasing their electron density and mobility. As a result, the electron transfer from the N-doped C dots to H2O2 is efficient, leading to high peroxidase-like activity of the CDzyme. The CDzyme/TMB sensing system with GOx is selective and sensitive for quantitation of glucose over a linear concentration range of 25–375 μM, in diluted serum and fruit juice samples. N-doped C dots prepared from organic amines, such as dimethylamine, ethylamine, and tripropylamine in the presence of H2SO4 through a microwave-assisted heating process also show peroxidase-like activity [94]. The N-doped C dots in the presence of TMB allows for the detection of H2O2 over a linear concentration range of 1–100 μM, with LOD of 0.4 μM. When combined with GOx, the colorimetric assay detects glucose with a linear concentration range of 1–5 μM and LOD of 0.5 μM. In addition, nitrogen-rich nucleobases are reported as good precursors for the large-scale and cost-effective synthesis of N-doped C dots through direct pyrolysis [95]. The dominant graphitic N species greatly boost the peroxidase-like activities of nucleobase-derived C dots. In the presence of TMB, the N-doped C dots allow for the H2O2 detection over a linear concentration range of 0.25–20 μM, with LOD of 115.5 nM. When combined with GOx, the colorimetric assay allows quantitation of glucose with a linear concentration range of 2–50 μM and LOD of 1.14 μM.
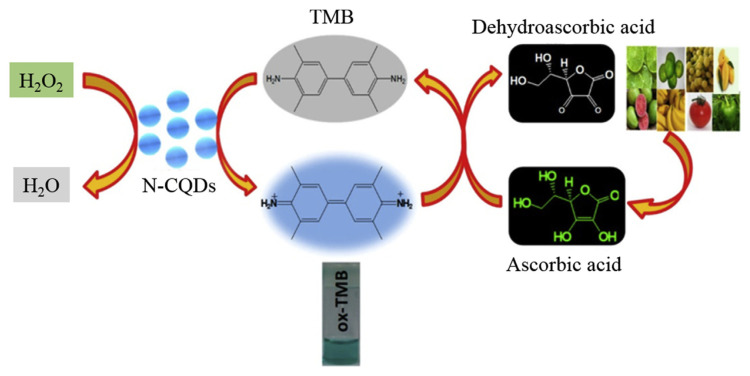
Assays based on analyte induced reduction of TMB products through H2O2 mediated oxidation have been developed for the quantitation of various analytes, such as glutathione (GSH) and ascorbic acid (AA), which possess hydrogen donating abilities [96–99]. In this case, the analyte induces a color change from blue to colorless and decreases in the absorbance at 653 nm. C dots prepared from disodium salt of ethylenediaminetetraacetic acid (Na2EDTA·2H2O) through pyrolysis were used for the quantitation of GSH [96]. The TMB oxidation products (cationic radicals) generated by H2O2 in the presence of C dots possessing peroxidase-like activity are reduced by GSH to form TMB. Upon increasing the GSH concentration, the absorbance at 653 nm decreases linearly over the concentration range of 0–7 μM. The assay exhibits an LOD of 0.3 μM for GSH and allows for the quantitation of GSH in human blood samples. C dots prepared from the latex of E. milii medicinal plant through a hydrothermal treatment at 180 °C for 3 h were used for the quantitation of GSH [97]. The prepared C dots with a quantum yield of 39.2% are resistant to high salt (ionic strength) and possess intrinsic peroxidase-like activity for TMB oxidation in the presence of H2O2. The C dots provide a small Km value of 0.427 mM toward TMB, with a higher Vmax (2.2 × 10−8 M s−1) when compared to HRP. Having LOD of 5.3 nM and a linear concentration range 0.02–0.1 μM for GSH, the assay is capable of quantitation of GSH in human blood serum samples. C dots prepared from carbon black were applied to detect GSH down to 0.5 μM in the presence of TMB [98]. Having high selectivity and sensitivity, the CDzyme/TMB sensing system allows quantitation of GSH in complicated biological samples like cell lysates. C dots prepared from leaf extracts of neem (Azadirachta indica) through a one-pot hydrothermal method were employed for quantitation of AA [90]. When compared to HRP for H2O2, the prepared C dots exhibit a smaller Km value (0.49 vs. 3.7 mM), revealing that the artificial nanozyme has greater affinity toward H2O2. The CDzyme allows for the detection of H2O2 down to 35.0 μM, with a linear concentration range of 0.1–0.5 mM. When TMB is the substrate, HRP and the CDzyme show similar Km values (0.43 vs. 0.51 mM). The result reveals that the CDzyme have less affinity toward TMB than H2O2. The results from the steady-state kinetic analysis suggest a ping-pong mechanism for the oxidation of TMB by the CDzyme. The CDzyme with TMB in the presence of H2O2 allow detection of AA in the concentration range of 5–40 μM, with LOD of 1.8 μM. The sensing system can be applied for the determination of AA in real samples such as common fruits, with good accuracy and precision (Fig. 4).
Fig. 4.
Schematic representation of oxidation of TMB by N-doped C dots and colorimetric detection of AA in a real sample. C dots are presented in N-CQDs in the figure. Reprinted with permission from Ref. [99]. Copyright (2019) American Chemical Society.
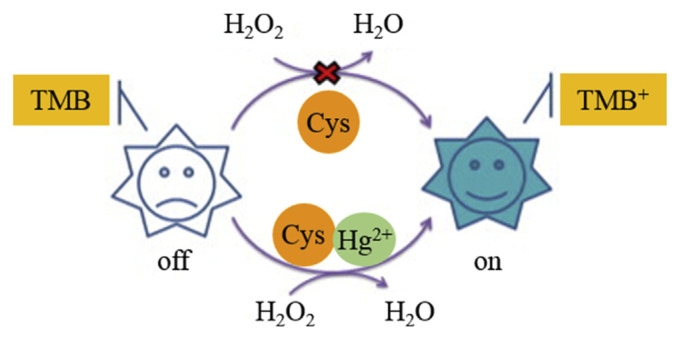
CDzyme/TMB sensing systems for the quantitation of some oxidative ions such as Fe3+ and Ag+ have been realized [93]. C dots prepared by refluxing a membranous carbonized β-cyclodextrin in HNO3 at 300 °C were used for quantitation of Ag+ and Fe3+ ions [100]. Because of the unique spatial structure of β-cyclodextrin, the carbonized blocks tend to form membranous structures, leading to the formation of high-quality C dots with a fluorescence quantum yield of 6.4%. The C dots possess peroxidase-like activity and allow colorimetric quantification of H2O2 in a linear concentration range of 2–500 μM and LOD of 1.0 μM. By taking the advantage of strong reduction strengths of Fe3+ and Ag+ to reduce the TMB oxidative products, the CDzyme/ TMB system is sensitive for the colorimetric detection of the two analytes, with LODs of 0.8 and 0.5 μM for Fe3+ and Ag+ ions, respectively. The assay is also selective towards the two analytes over the potential interfering ionic species (1 mM K+, Na+, Zn2+, Fe2+, Cu2+, Pb2+, Ni2+, Cd2+, Al3+, and Cr3+). A CDzyme/TMB system was developed for highly selective and sensitive detection of Hg2+ ions in the presence of cysteine (Cys) [101]. C dots prepared from Na2EDTA·2H2O exhibit peroxidase-like activity to catalyze TMB to form oxidative TMB products. Similar to GSH, Cys is a powerful anti-radical biomolecule, which can reduce the as–formed cationic free radicals. Because Hg2+ ions have strong affinity toward thiol compounds, the strength of Cys for reduction of the as–formed cationic free radicals is suppressed in the presence of Hg2+ (Fig. 5). The CDzyme/TMB system in the presence of Cys (5 μM) is sensitive for the quantitation of Hg2+ ions, with a linear concentration range of 0–0.31 μM and LOD of 23.0 nM. Other metal ions, such as Ag+, Cd2+, Cu2+, Co2+, Ni2+ and Pb2+ show negligible interference, revealing high selectivity of this assay.
Fig. 5.
Schematic representation of a colorimetric turn-on assay for mercury ion detection. Reproduced from Ref. [101] with permission from Elsevier.
3.2. C dot nanocomposites
C dot nanocomposites have been reported to possess enhanced peroxidase-like activity than that of C dots, due to the synergistic effects of different elements (N, Fe, Pt, Cu, Mo, S) [102–107]. Nitrogen-and iron-containing C dots (N,Fe–C dots) synthesized from a branched polyethylenimine (as a nitrogen source) and hemin (as an iron source) through a hydrothermal route at 180 °C show peroxidase-like activity [102]. The Fe species in N,Fe–C dots act like a Fenton’s reagent [103, 104], enhancing their catalytic activity. The N,Fe–C dots were used to develop colorimetric and fluorometric assays for the quantitation of dopamine (DA), based on DA-induced inhibition of the oxidation reaction of TMB. The colorimetric assay for DA has a LOD of 0.03 μM and a visual LOD of 0.05 μM. The analyte dependent signal response of the fluorometric assay is based on an inner filter effect from the oxidized TMB that absorbs the fluorescence (excitation/ emission wavelengths 360/452 nm) of N,Fe–C dots. The fluorescent assay exhibits LOD of 20.0 nM for the detection of DA. Having high sensitivity and selectivity, the assay allows for the quantitation of DA in human serum samples. For the preparation of Pt-modified C dots (Pt–C nanocomposites), C dots were firstly synthesized from L-AA by a hydrothermal process at 180 °C, which were then added into H2PtCl6 solution in the presence of NaBH4 under magnetic stirring for 48 h [105]. NaBH4 acted as a reducing agent to reduce H2PtCl6 to form Pt on the surface of C dots, leading to the formation of Pt–C nanocomposites. The reduction current intensity of H2O2 generated in the Pt–C nanocomposites modified electrode in the absence of TMB is the highest, which is 4-, 3-, and 2.5-fold larger than those of blank, C dots, and Pt-modified electrode, respectively, indicating the highest catalytic activity of the Pt–C nanocomposites for the reduction of H2O2. When TMB was added, the current signal generated from the reduction of H2O2 by the Pt–C nanocomposites modified electrode decreased sharply to just about 10% of its original value. At the same time, only slight decreases were observed when separately using blank, C dots, and Pt-modified electrode. This is because electrons transfer quickly form TMB to H2O2 on the Pt–C nanocomposites surface, leading to decreased electron transfer from the electrode to the solution. As a result, a sharp decrease in current was observed in the presence of TMB. Due to the synergistic effects between C dots and Pt, the peroxidase-like activity of the Pt–C nanocomposite is nine and five times higher than those of C dots and Pt nanoparticles, respectively. The Pt–C nanocomposites in the presence of TMB enable visual and colorimetric detection of H2O2, with LOD of 0.8 μM. In the presence of GOx, the sensing system allows detection of glucose down to 1.7 μM. A facile solid-phase synthesis strategy was developed to synthesize Cu-doped CDs (Cu-CDs) using citric acid as the carbon source and Cu(NO3)2 as the dopant, respectively [106]. The as-prepared Cu-CDs exhibited superior peroxidase-like activity and were stable under a wide range of pH and temperatures. Consequently, the Cu-CD-based chemiluminescence sensing was applied to sensitively detect glucose with a low detection limit of 0.32 μM, and the recoveries and the relative standard deviation of the serum sample are 87.2–112.2% and 8.16% (n = 6), respectively. Notably, the proposed chemiluminescence sensing was also successfully applied for label-free detection of glucose in complex biological samples. Mo, S codoped CQDs (Mo-CQDs) as a peroxidase mimic were used to fabricate a cascade colorimetric biosensor to detect cholesterol [107]. The Mo-CQDs possess a robust peroxidase-like activity. The Mo, S doping in the CQDs notably boosts the yield of CQDs and may facilitate the electron transfer between TMB and H2O2, which further enhances the catalytic activity of CQDs. The colorimetric biosensor based on Mo-CQDs and cholesterol oxidase exhibited excellent selectivity and high sensitivity for cholesterol in the range of 0.01–1.0 mM along with a detection limit as low as 7 μM. The total cholesterol concentration in the serum sample was measured with satisfactory results and read out by the naked eye, indicating the potential application in clinical diagnosis and portable test kits. Core-shell type Au nanoparticles@C nanocomposites prepared from chloroauric acid/sodium polyacrylate (precursor/soft template) and C dots (citric acid/urea as precursors under 900 W microwave irradiation) through a chemical reduction route show high peroxidase-like activity for H2O2 [108]. In the preparation process, Au nanoparticles are in situ reduced by C dots and assemble on the surface of sodium polyacrylate-C dots soft template. In addition, C dots act as both stabilizer and reducing agent. The Au nanoparticles@C nanocomposites have core-shell nanostructure, each with an ultrathin carbon layer of 1–2 nm. When compared to gold nanoparticles alone, the nanocomposite possesses higher peroxidase-like activity, mainly because of more efficient electron transfer between TMB and H2O2. Km value of the nanocomposite for TMB (0.059 mM) is much smaller than those provided by HRP (0.43 mM) and AuNPs (0.74 mM), revealing its higher affinity towards TMB.
By taking the advantages of large surface area, good adsorption ability, and anion exchange property of layered double hydroxides (LDH), C-dot/ NiAl–LDH nanocomposites exhibit high peroxidase-like activity for TMB oxidation [109]. The nanocomposite is formed through electrostatic self-assembly of negatively charged C dots and positively charged NiAl–LDH nanoplates. The peroxidase-like activity of the nanocomposite is higher than C dots and NiAl–LDH alone, showing the existence of a synergistic effect between C dots and NiAl–LDH. Compared to HRP, the nanocomposite exhibits a lower apparent Km value for TMB, revealing its higher affinity for TMB than that of HRP, mainly because of its larger surface area, stronger adsorption ability to TMB, and higher conductivity attributed from the C dots. The CDzyme has been used to develop a colorimetric method for the detection of H2O2, providing a linear concentration range of 0.2–20 μM and LOD of 0.1 μM. Also, the CDzyme/TMB sensing system can quantitate H2O2 in milk samples, with good accuracy and precision.
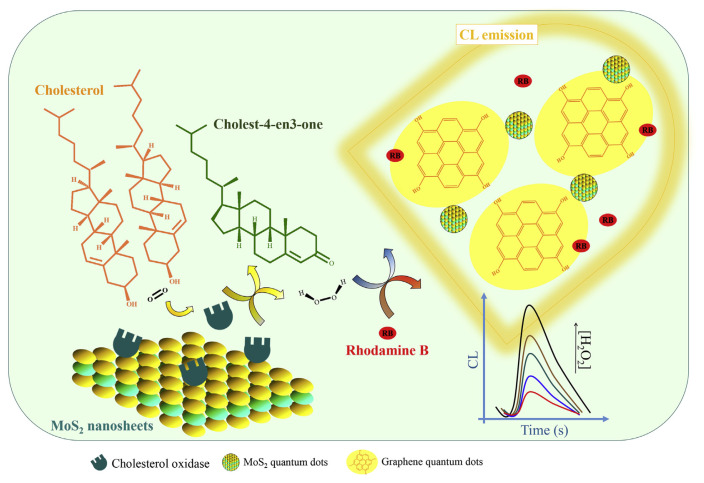
Nanocomposites formed from MoS2 quantum dots and C dots show a synergetic peroxidase-like activity for H2O2 [110]. MoS2 quantum dots with an average size of 1.3 nm were obtained by thermal treatment of MoS2 nanosheets that had been prepared from MoS2 powder through a simple exfoliation method. C dots with an average diameter of 14.5 ± 4.6 nm were prepared by thermal pyrolysis of glucose powder. The catalytic activity of the nanocomposite for H2O2 was determined by monitoring the chemiluminescence (CL) generated from the rhodamine B (RB)-H2O2 reaction. The nanocomposite has a catalytic activity that is 7.2- and 14.3-fold higher than that of the MoS2 and C dots alone, respectively. The assay allows quantitation of H2O2, with a linear concentration range of 1.5–460 nM and LOD of 0.4 nM. Because MoS2 nanosheets enhanced the activity of ChOx for the oxidation of cholesterol, the proposed CL system allows detection of cholesterol, with a linear concentration range of 0.08–300 μM and LOD of 35.0 nM (Fig. 6). Having high sensitivity and selectivity, the assay allows quantitation of cholesterol in human serum samples.
Fig. 6.
Schematic illustration of a chemiluminescence sensor for detection of total cholesterol. C dots are presented in graphene quantum dots in the figure. Reproduced from Ref. [110] with permission from Elsevier.
C dot/Fe3O4 nanocomposites also show peroxidase-like activity for sensitive detection of H2O2 and AA [111]. α-Fe2O3 nanofibers synthesized via electrospinning and C dots prepared from citric acid/ urea under microwave irradiation at a power of 750 W were used to prepare C dot/α-Fe2O3 hybrid nanofibers through a one-step hydrothermal reaction. The obtained C dot/α-Fe2O3 hybrid nanofibers were then subjected to calcination to form C dot/ Fe3O4 nanocomposites. When compared to individual C dots, α-Fe2O3 nanofibers, C dots/α-Fe2O3 hybrid nanofibers, and commercial Fe3O4 nanoparticles, the nanocomposite provides the highest peroxidase-like activity for TMB. The nanocomposite has a low Km value (0.06 mM) for TMB, showing its strong affinity toward TMB. Colorimetric assays using the nanocomposite and TMB allow for the detection of H2O2 and AA, with LODs of 0.9 and 0.3 μM, respectively. A simpler approach was developed for the preparation of C-dot/Fe3O4 nanocomposite from mixing solutions of C dots and Fe3O4 nanoparticles [112]. The nanocomposite is formed through the interaction of the OH groups on the Fe3O4 nanoparticles with the OH and CO2H groups on the surfaces of C dots. The nanocomposite in the presence of TMB exhibits Vmax and Km of 1.4 × 10−7 M s−1 and 3.5 mM for H2O2, respectively. The Km value is close to that of HRP. Relative to C dots and Fe3O4 nanoparticles, the nanocomposite provides 44.0- and 7.6-fold lower Km value, showing its stronger affinity toward H2O2. The CDzyme/TMB system is sensitive for the detection of H2O2, with a linear concentration range of 10.0 nM–1.0 mM and LOD of 1.0 nM.
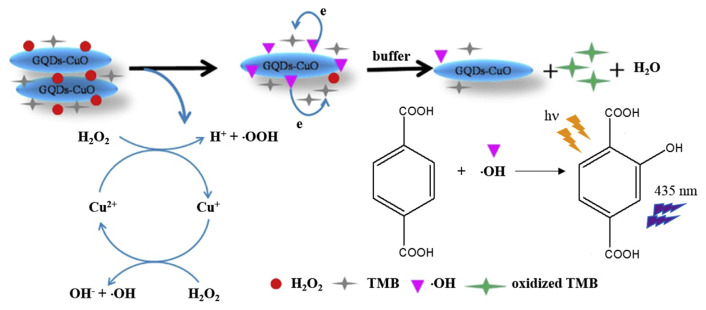
Nanocomposites of C dots with CuO nano-structures also show high peroxidase-like activity for H2O2 [113]. Graphene oxide (GO) was firstly prepared from graphite powder according to the Hummers and Offeman method [114]. Then, C dots were prepared from GO through a microwave-hydrothermal approach, which were then used as supports for the growth of CuO nanoneedles from copper acetate to produce C dot/CuO nanocomposites. When compared to the individual C dots and CuO nanoneedles, the nanocomposite provides a lower Km value (0.098 mM) and thus a stronger affinity toward H2O2, showing a synergistic effect from the two nanomaterials. H2O2 is adsorbed on the nanocomposite surface and then activated by the Cu2+ to generate hydroxyl species (•OH) that oxidizes TMB into its blue colored form (Fig. 7). The CDzyme/TMB sensing system shows a linear range of 0.5–10 μM and LOD of 0.2 μM for H2O2. In the presence of GOx, the sensing system allows detection of glucose in serum samples, with a linear concentration range of 2–100 μM and LOD of 0.6 μM.
Fig. 7.
Sensing mechanism for the C dots/CuO nanocomposites–H2O2–TMB system. C dots are presented in GQDs in the figure. Reproduced from Ref. [113] with permission from Elsevier.
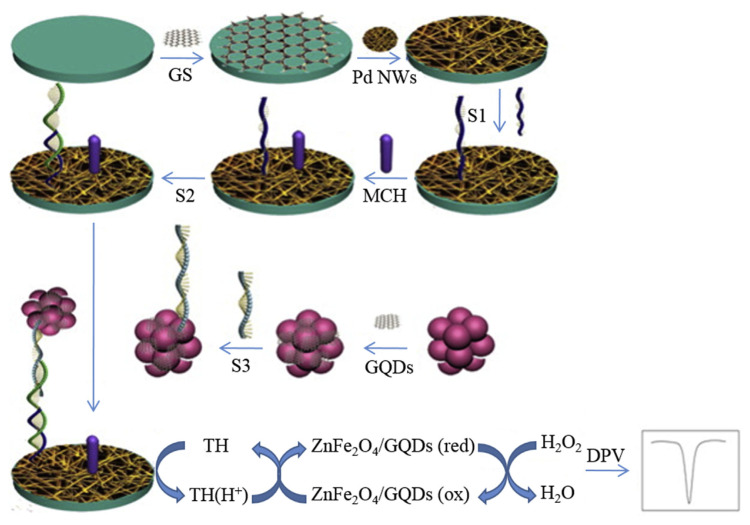
By taking advantage of electrical conductivity of C dots, nanocomposites were prepared from ZnFe2O4 and C dots to achieve high sensitivity for the electrochemical determination of DNA [115]. The ZnFe2O4 nanoparticles were prepared from ZnCl2 and FeCl3·2H2O through a hydrothermal route. Then C dots prepared from GO aqueous suspension through the Hummers and Offeman approach were assembled on the surface of ZnFe2O4 nanoparticles through a photo-Fenton reaction. During the photo-Fenton reaction, radicals (•OH and •O2H) were generated from Fe3+ and H2O2. Small graphene sheets erre formed through a simple exfoliation of GO in the presence of radicals and then adsorbed on the ZnFe2O4 surface as in situ nucleation of C dots. The resulting composites are spherical particles with diameter of about 100–120 nm, each with a thin layer (20 nm) on the particle surface. The ZnFe2O4/C dot nanocomposite labeled with complementary ssDNA (S3; 5′–NH2–(CH2)6-ATG TCC CTC AGA CCC TTT-3′) was then used as an enzyme mimic. In the presence of target DNA (S2; 5′-ACT GCT AGA GAT TTT CCA CAC TGA CTA AAA GGG TCT GAG GGA-3′), the nanocomposite is deposited on a Pd nanowire/graphene sheet-ssNDA (S1; 5′-TGG AAA ATC TCT AGC AGT CGT–(CH2)6–SH-3′) modified glassy carbon electrode through DNA hybridization. The ZnFe2O4/C dot nanocomposite-ssNDA (S3) interacts strongly with the functional electrode surface, providing stability and durability to the electrode. The functional electrode shows high peroxidase-like catalytic activity for detection of H2O2 as shown in Fig. 8. Using thionine as an electron mediator, the assay allows the detection of the target DNA (S2) with a linear concentration range of 0.1 fM– 5 nM and LOD of 6.2 × 10−17 M. The reason for such a high sensitivity for the target DNA sensor is mainly due to a good pathway for electron transfer provided by the Pd nanowire/graphene sheet and high surface density of the capture probe (S1) on the electrode surface. Having such a high sensitivity and selectivity, the assay is able to quantify target DNA in human serum samples, showing their great potential for gene diagnostics.
Fig. 8.
Schematic illustration of ZnFe2O4/C dots as a mimicking trace label for electrochemical detection of DNA. C dots are presented in GQDs in the figure. Reproduced from Ref. [115] with permission from Elsevier.
4. Conclusion and outlook
CDzymes possessing peroxidase-like activity have several attractive features, including catalytic activity, ease in preparation, (photo)chemical stability, cost-effectiveness, and good biocompatibility. They alone or in conjunction with various enzymes have been used to develop sensing systems (Table 1) for sensitive and selective quantitation of many analytes. When compared to HRP, the CDzymes are advantageous of low cost, long shelf life, and good thermal/pH stability. Compared to colorimetric methods, fluorescence and electrochemical methods using CDzymes are more sensitive. Nevertheless, colorimetric assays can be performed free of any instrumentation (i.e., by the naked eye). Like most nanozymes, most reported CDzymes are limited to samples without containing complicated matrixes, mainly because of loss in their activity once interfering species are adsorbed on their surfaces. In this case, surface modification of C dots with polymers are preferred to minimize nonspecific adsorption. When conducting surface modification, great attention must be paid to minimize the loss of the catalytic activity of C dots.
Table 1.
Comparison of nanocarbon-based artificial peroxidase for various analytes.
| Nanocarbon | Abbreviation in Ref. | Carbon source | Synthesis method | Detection method | Target | LOD | Real sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| C dots | C-Dots | Candle soot | Reflux with HNO3 at 140 °C for 12 h | Colorimetric | H2O2 | 0.2 μM | human serum | [83] |
| glucose | 0.4 μM | |||||||
| r-CDs | Lampblack | Reflux with HNO3 at 140 °C for 12 h, and then reduction with NaBH4 | Colorimetric | glucose | 2.0 μM | human serum | [84] | |
| uric acid | 3.0 μM | |||||||
| CQDs | Litchi rind | Reflux with HNO3 at 140°C for 12 h | Colorimetric | glucose | 3.0 μM | human serum | [86] | |
| E-GQDs | Wood charcoal | Electrochemical oxidation at 5 V in the presence of 0.01 M (NH4)2S2O8 | Colorimetric | H2O2 | 0.9 μM | - | [87] | |
| glucose | 6.0 μM | |||||||
| o-GQDs | Multiwalled carbon nanotubes | Reflux with HNO3 at 140 °C for 48 h | Colorimetric | H2O2 | 20 nM | blood from Balb/c mice | [88] | |
| glucose | 0.2 μM | |||||||
| GQDs | Graphite powder | Wet chemical oxidation method (sonicated for 2 h and 30 min at room temperature followed by stirring for 45 min at 90 °C.) | Colorimetric | H2O2 | 9.0 μM | - | [89] | |
| Cholesterol | 6.0 μM | |||||||
| N-GQDs | Graphite powder, dopamine | Hydrothermal treatment at 75 °C for 6 h | Colorimetric | H2O2 | 5.3 μM | human serum, commercial fruit juices | [92] | |
| glucose | 16.0 μM | |||||||
| CNDs | Dimethylamine | Microwave heat-treatment for 60 s | Colorimetric | H2O2 | 0.4 μM | - | [93] | |
| glucose | 0.5 μM | |||||||
| CDs | Na2EDTA | Pyrolysis at 400 °C for 2 h | Colorimetric | GSH | 0.3 μM | human whole blood | [96] | |
| CQDs | Latexes of E. milii plant | Hydrothermal treatment at 180 °C for 3 h | Colorimetric | GSH | 5.3 nM | human serum | [97] | |
| GDs | Carbon black | Reflux with HNO3 at 130 °C for 24 h | Colorimetric | H2O2 | 10 nM | cell lysate | [98] | |
| glucose | 0.5 μM | |||||||
| GSH | 0.5 μM | |||||||
| N-CQDs | Leaf extracts of neem (Azadirachta indica) | Hydrothermal treatment at 150 °C for 4 h | Colorimetric | H2O2 | 35.0 μM | fresh fruit juice | [99] | |
| AA | 1.8 μM | |||||||
| CDs | β-Cyclodextrin | Reflux with HNO3 for 12 h | Colorimetric | H2O2 | 1.0 μM | - | [100] | |
| Ag+ | 0.5 μM | |||||||
| Fe3+ | 0.8 μM | |||||||
| CDs | Na2EDTA | Pyrolysis at 400 °C for 2 h | Colorimetric | Hg2+ | 23 nM | river water sample | [101] | |
| C dot nanocomposites | N,Fe-CDs | BPEI, hemin | Hydrothermal treatment at 180 °C for 10 h | Colorimetric | DA | 0.03 μM | human serum | [102] |
| Fluorescence | 20 nM | |||||||
| Pt-CDs | L-ascorbic acid, H2PtCl6 | Hydrothermal treatment at 180 °C for 4 h | Colorimetric | H2O2 | 0.8 μM | - | [105] | |
| glucose | 1.7 μM | |||||||
| AuNPs@CDs | Citric acid, chloroauric acid | Microwave heat-treatment for 300 s and chemical reduction route | - | - | - | - | [108] | |
| C-dot/NiAl–LDH | Citric acid, Ni(NO3)2, Al(NO3)3, | Hydrothermal treatment at 200 °C for 3 h and simple mixing at room temperature | Colorimetric | H2O2 | 0.1 μM | milk | [109] | |
| MoS2 QDs, GQDs | Glucose, MoS2 nanosheets | Pyrolysis at 180 °C for GQDs and heated at 120°C for MoS2 QDs | Chemiluminometric | H2O2 | 0.4 nM | human serum | [110] | |
| Cholesterol | 35 nM | |||||||
| CDs/Fe3O4 | Citric acid, Fe(NO3)3 | Microwave heat-treatment for 300 s and hydrothermal treatment at 140 °C for 4 h, following calcined at 500 °C for 4 h | Colorimetric | H2O2 | 0.9 μM | [111] | ||
| AA | 0.3 μM | |||||||
| C-dots/Fe3O4 | Carbon soot, FeCl3 | Reflux with HNO3 and mixed together in acidic media for 30 min | Colorimetric | H2O2 | 1.0 nM | - | [112] | |
| GQDs/CuO | GO, copper acetate | Microwave heat-treatment at 200 °C for 8 min and simple mixing at room temperature | Colorimetric | H2O2 | 0.2 μM | - | [113] | |
| glucose | 0.6 μM | |||||||
| ZnFe2O4/GQDs | GO, ZnCl2, FeCl3 | Photo-Fenton reaction (365 nm, 1000 W) | Differential pulse voltammetry | DNA | 6.2 × 10−17 M | human serum | [115] |
Although most of reported CDzymes do not exhibit catalytic activity as high as that of HRP, their catalytic activity can be further enhanced by careful selection of carbon precursors and passivation of their surface with active ligands. Carbon precursors having high affinity towards electron-rich substrates shall be useful for preparation of CDzymes with high catalytic activity. Since N-doping C dots have shown higher activity than C dots, C dots containing heteroatoms such as S, P, and B, with different structures shall be tested. To further enhance their activity, C dots with high surface area and great amounts of surface defects are required. Treating C dots with strong inorganic acids/bases, photo-irradiation, and/or oxygen plasma can be efficient for varying their size, morphology, and structure.
Although CDzymes have become more popular in recent years, only ones with peroxidase-like activity have been used for developing sensitive and selective sensing systems. To expand their applications, nanocomposites formed from C dots with different nanozymes having activities such as oxidase and catalase are worthy to be tested. For example, nanocomposites formed from C dots and metal (oxide) nanoparticles, including Pt, Pd, Au, Ag, and BiOx, shall be good candidates. Based on the fact that the activity of metal nanoparticle can be tuned/ changed by deposition of different metal ions, nanocomposites of C dots with metal nanoparticles containing different metal ions such as Au–Ag and BiOx-Pt nanoparticles are also good to be tested. Once CDzymes with various enzymes-like activities are available, it will be interesting to develop C dots based enzyme cascades.
Recently, our research group has found that C dots with microstructures show light-induced peroxidase-like activity (unpublished results). Their activity is higher than individual C dots and depends on the wavelength of irradiation light. Their activity is also dependent on oxygen content, and thus it will be interesting to develop sensing systems for monitoring the oxygen content inside cells using C dot microstructures. When C dots with microstructures are used to entrap various chemicals such as metal, metal oxide, or oxidizing/reducing agents, CDzymes with different enzyme-like activities shall be available. In addition, their photo-induced catalytic activity shall be enhanced, enabling more sensitive sensing systems.
Supplementary Information
Acknowledgements
We are grateful to the Ministry of Science and Technology (MOST) of Taiwan for providing financial support for this study under contracts 107-2113-M-002-015-MY3, and MOST 108-2113-M-018-003.
Funding Statement
We are grateful to the Ministry of Science and Technology (MOST) of Taiwan for providing financial support for this study under contracts 107-2113-M-002-015-MY3, and MOST 108-2113-M-018-003.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1. Privman M, Guz N, Katz E. Enzyme-logic digital biosensors for biomedical applications. Int J Unconv Comput. 2018;13:435–76. [Google Scholar]
- 2. Rocchitta G, Spanu A, Babudieri S, Latte G, Madeddu G, Galleri G, Nuvoli S, Bagella P, Demartis MI, Fiore V, Manetti R, Serra PA. Enzyme biosensors for biomedical applications: strategies for safeguarding analytical performances in biological fluids. Sensors-Basel. 2016;16:780. doi: 10.3390/s16060780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azevedo AM, Martins VC, Prazeres DMF, Vojinović V, Cabral JMS, Fonseca LP. Biotechnology annual review. Vol. 9. Elsevier; 2003. Horseradish peroxidase: a valuable tool in biotechnology; pp. 199–247. [DOI] [PubMed] [Google Scholar]
- 4. Shakerian F, Zhao J, Li SP. Recent development in the application of immobilized oxidative enzymes for bioremediation of hazardous micropollutants - a review. Chemosphere. 2020;239:124716. doi: 10.1016/j.chemosphere.2019.124716. [DOI] [PubMed] [Google Scholar]
- 5. Sharifi M, Sohrabi MJ, Hosseinali SH, Hasan A, Kani PH, Talaei AJ, Karim AY, Nanakali NMQ, Salihi A, Aziz FM, Yan B, Khan RH, Saboury AA, Falahati M. Enzyme immobilization onto the nanomaterials: application in enzyme stability and prodrug-activated cancer therapy. Int J Biol Macromol. 2020;143:665–76. doi: 10.1016/j.ijbiomac.2019.12.064. [DOI] [PubMed] [Google Scholar]
- 6. Lin YH, Ren JS, Qu XG. Catalytically active nanomaterials: A promising candidate for artificial enzymes. Accounts Chem Res. 2014;47:1097–105. doi: 10.1021/ar400250z. [DOI] [PubMed] [Google Scholar]
- 7. Zhou WH, Ding JS, Liu JW. Theranostic dnazymes. Theranostics. 2017;7:1010–25. doi: 10.7150/thno.17736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang YY, Ren JS, Qu XG. Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem Rev. 2019;119:4357–412. doi: 10.1021/acs.chemrev.8b00672. [DOI] [PubMed] [Google Scholar]
- 9. Wu JJX, Wang XY, Wang Q, Lou ZP, Li SR, Zhu YY, Qin L, Wei H. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II) Chem Soc Rev. 2019;48:1004–76. doi: 10.1039/c8cs00457a. [DOI] [PubMed] [Google Scholar]
- 10. Golchin J, Golchin K, Alidadian N, Ghaderi S, Eslamkhah S, Eslamkhah M, Akbarzadeh A. Nanozyme applications in biology and medicine: an overview. Artif Cell Nanomed B. 2017;45:1069–76. doi: 10.1080/21691401.2017.1313268. [DOI] [PubMed] [Google Scholar]
- 11. Huang YY, Lin YH, Pu F, Ren JS, Qu XG. The current progress of nanozymes in disease treatments. Prog Biochem Biophys. 2018;45:256–67. [Google Scholar]
- 12. Li SR, Huang YC, Liu JR, Wang EK, Wei H. Nanozymes in analytical chemistry: from in vitro detection to live bioassays. Prog Biochem Biophys. 2018;45:129–47. [Google Scholar]
- 13. Liu BW, Liu JW. Surface modification of nanozymes. Nano Res. 2017;10:1125–48. [Google Scholar]
- 14. Wang XY, Hu YH, Wei H. Nanozymes in bionanotechnology: from sensing to therapeutics and beyond. Inorg Chem Front. 2016;3:41–60. [Google Scholar]
- 15. Yan XY. Nanozyme: A new type of artificial enzyme. Prog Biochem Biophys. 2018;45:101–4. [Google Scholar]
- 16. Zhou YB, Liu BW, Yang RH, Liu JW. Filling in the gaps between nanozymes and enzymes: challenges and opportunities. Bioconjugate Chem. 2017;28:2903–9. doi: 10.1021/acs.bioconjchem.7b00673. [DOI] [PubMed] [Google Scholar]
- 17. Wei H, Wang EK. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev. 2013;42:6060–93. doi: 10.1039/c3cs35486e. [DOI] [PubMed] [Google Scholar]
- 18. Peng YH, Wang ZY, Liu WS, Zhang HL, Zuo W, Tang HA, Chen FJ, Wang BD. Size- and shape-dependent peroxidaselike catalytic activity of MnFe2O4 nanoparticles and their applications in highly efficient colorimetric detection of target cancer cells. Dalton T. 2015;44:12871–7. doi: 10.1039/c5dt01585e. [DOI] [PubMed] [Google Scholar]
- 19. Lien CW, Huang CC, Chang HT. Peroxidase-mimic bismuth-gold nanoparticles for determining the activity of thrombin and drug screening. Chem Commun. 2012;48:7952–4. doi: 10.1039/c2cc32833j. [DOI] [PubMed] [Google Scholar]
- 20. Li CL, Huang CC, Chen WH, Chiang CK, Chang HT. Peroxidase mimicking DNA-gold nanoparticles for fluorescence detection of the lead ions in blood. Analyst. 2012;137:5222–8. doi: 10.1039/c2an35599j. [DOI] [PubMed] [Google Scholar]
- 21. Lien CW, Chen YC, Chang HT, Huang CC. Logical regulation of the enzyme-like activity of gold nanoparticles by using heavy metal ions. Nanoscale. 2013;5:8227–34. doi: 10.1039/c3nr01836a. [DOI] [PubMed] [Google Scholar]
- 22. Hsu CL, Lien CW, Wang CW, Harroun SG, Huang CC, Chang HT. Immobilization of aptamer-modified gold nanoparticles on biocl nanosheets: tunable peroxidase-like activity by protein recognition. Biosens Bioelectron. 2016;75:181–7. doi: 10.1016/j.bios.2015.08.049. [DOI] [PubMed] [Google Scholar]
- 23. Ju Y, Kim J. Dendrimer-encapsulated pt nanoparticles with peroxidase-mimetic activity as biocatalytic labels for sensitive colorimetric analyses. Chem Commun. 2015;51:13752–5. doi: 10.1039/c5cc06055a. [DOI] [PubMed] [Google Scholar]
- 24. Zhou NA, Zou SY, Zou L, Shen RD, Zhou YM, Ling LS. Peroxidase-like activity of palladium nanoparticles on hydrogen-bond supramolecular structures over a broader ph range and their application in glucose sensing. Can J Chem. 2019;97:317–23. [Google Scholar]
- 25. Jiang H, Chen ZH, Cao HY, Huang YM. Peroxidase-like activity of chitosan stabilized silver nanoparticles for visual and colorimetric detection of glucose. Analyst. 2012;137:5560–4. doi: 10.1039/c2an35911a. [DOI] [PubMed] [Google Scholar]
- 26. Wei H, Wang E. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal Chem. 2008;80:2250–4. doi: 10.1021/ac702203f. [DOI] [PubMed] [Google Scholar]
- 27. Wang WJ, Wu YH, Lin XL, Chen W, Liu AL, Peng HP. Synthesis of Au-WS2 nanocomposites and study on its peroxidase mimic activity. Chinese J Anal Chem. 2018;46:1545–51. [Google Scholar]
- 28. Hsu C-L, Lien C-W, Harroun SG, Ravindranath R, Chang H-T, Mao J-Y, Huang C-C. Metal-deposited bismuth oxyiodide nanonetworks with tunable enzyme-like activity: sensing of mercury and lead ions. Mater Chem Front. 2017;1:893–9. [Google Scholar]
- 29. Asati A, Santra S, Kaittanis C, Nath S, Perez JM. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew Chem Int Edit. 2009;48:2308–12. doi: 10.1002/anie.200805279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wan Y, Qi P, Zhang D, Wu JJ, Wang Y. Manganese oxide nanowire-mediated enzyme-linked immunosorbent assay. Biosens Bioelectron. 2012;33:69–74. doi: 10.1016/j.bios.2011.12.033. [DOI] [PubMed] [Google Scholar]
- 31. Sun JH, Li CY, Qi YF, Guo SL, Liang X. Optimizing colorimetric assay based on V2O5 nanozymes for sensitive detection of H2O2 and glucose. Sensors-Basel. 2016;16:584. doi: 10.3390/s16040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hsu KI, Lien CW, Lin CH, Chang HT, Huang CC. Immobilization of iron hydroxide/oxide on reduced graphene oxide: peroxidase-like activity and selective detection of sulfide ions. RSC Adv. 2014;4:37705–13. [Google Scholar]
- 33. Wu CW, Harroun SG, Lien CW, Chang HT, Unnikrishnan B, Lai IPJ, Chang JY, Huang CC. Self-templated formation of aptamer-functionalized copper oxide nanorods with intrinsic peroxidase catalytic activity for protein and tumor cell detection. Sensor Actuat B-Chem. 2016;227:100–7. [Google Scholar]
- 34. Song W, Zhao B, Wang C, Ozaki Y, Lu XF. Functional nanomaterials with unique enzyme-like characteristics for sensing applications. J Mater Chem B. 2019;7:850–75. doi: 10.1039/c8tb02878h. [DOI] [PubMed] [Google Scholar]
- 35.Shin HY, Park TJ, Kim MI.Recent research trends and future prospects in nanozymes J Nanomater 2015756278 10.1155/2015/756278. [DOI] [Google Scholar]
- 36. Gao LZ, Zhuang J, Nie L, Zhang JB, Zhang Y, Gu N, Wang TH, Feng J, Yang DL, Perrett S, Yan X. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2:577–83. doi: 10.1038/nnano.2007.260. [DOI] [PubMed] [Google Scholar]
- 37. Jiang D, Ni D, Rosenkrans ZT, Huang P, Yan X, Cai W. Nanozyme: new horizons for responsive biomedical applications. Chem Soc Rev. 2019;48:3683–704. doi: 10.1039/c8cs00718g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cardenas-Benitez B, Djordjevic I, Hosseini S, Madou MJ, Martinez-Chapa SO. Review-covalent functionalization of carbon nanomaterials for biosensor applications: an update. J Electrochem Soc. 2018;165:B103–17. [Google Scholar]
- 39. Yang ZB, Ren J, Zhang ZT, Chen XL, Guan GZ, Qin LB, Zhang Y, Peng HS. Recent advancement of nanostructured carbon for energy applications. Chem Rev. 2015;115:5159–223. doi: 10.1021/cr5006217. [DOI] [PubMed] [Google Scholar]
- 40. Jariwala D, Sangwan VK, Lauhon LJ, Marks TJ, Hersam MC. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem Soc Rev. 2013;42:2824–60. doi: 10.1039/c2cs35335k. [DOI] [PubMed] [Google Scholar]
- 41. Notarianni M, Liu JZ, Vernon K, Motta N. Synthesis and applications of carbon nanomaterials for energy generation and storage. Beilstein J Nanotech. 2016;7:149–96. doi: 10.3762/bjnano.7.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen TH, Chang HT. Stable and photoswitchable carbon-dot liposome. ACS Appl Mater Inter. 2017;9:44259–63. doi: 10.1021/acsami.7b14969. [DOI] [PubMed] [Google Scholar]
- 43. Gopi CVVM, Ravi S, Rao SS, Reddy AE, Kim HJ. Carbon nanotube/metal-sulfide composite flexible electrodes for high-performance quantum dot-sensitized solar cells and supercapacitors. Sci Rep-Uk. 2017;7:46519. doi: 10.1038/srep46519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang ZS, Chen CY, Liu CW, Li CL, Chang HT. Quantum dot-sensitized solar cells featuring CuS/CoS electrodes provide 4.1% efficiency. Adv Energy Mater. 2011;1:259–64. [Google Scholar]
- 45. Periasamy AP, Ravindranath R, Roy P, Wu WP, Chang HT, Veerakumar P, Liu SB. Carbon-boron core-shell microspheres for the oxygen reduction reaction. J Mater Chem A. 2016;4:12987–94. [Google Scholar]
- 46. Li MX, Chen T, Gooding JJ, Liu JQ. Review of carbon and graphene quantum dots for sensing. Acs Sensors. 2019;4:1732–48. doi: 10.1021/acssensors.9b00514. [DOI] [PubMed] [Google Scholar]
- 47. Wang ZH, Shen DK, Wu CF, Gu S. State-of-the-art on the production and application of carbon nanomaterials from biomass. Green Chem. 2018;20:5031–57. [Google Scholar]
- 48. Wang QQ, Wei H, Zhang ZQ, Wang EK, Dong SJ. Nanozyme: an emerging alternative to natural enzyme for biosensing and immunoassay. Trend Anal Chem. 2018;105:218–24. [Google Scholar]
- 49. Roy P, Chen PC, Periasamy AP, Chen YN, Chang HT. Photoluminescent carbon nanodots: synthesis, physicochemical properties and analytical applications. Mater Today. 2015;18:447–58. [Google Scholar]
- 50. Atabaev TS. Doped carbon dots for sensing and bioimaging applications: a minireview. Nanomaterials-Basel. 2018;8:342. doi: 10.3390/nano8050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zuo PL, Lu XH, Sun ZG, Guo YH, He H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim Acta. 2016;183:519–42. [Google Scholar]
- 52. Zhang J, Yu SH. Carbon dots: large-scale synthesis, sensing and bioimaging. Mater Today. 2016;19:382–93. [Google Scholar]
- 53. Zhan J, Geng BJ, Wu K, Xu G, Wang L, Guo RY, Lei B, Zheng FF, Pan DY, Wu MH. A solvent-engineered molecule fusion strategy for rational synthesis of carbon quantum dots with multicolor bandgap fluorescence. Carbon. 2018;130:153–63. [Google Scholar]
- 54. Zhang WF, Xu T, Liu ZW, Wu NL, Wei MD. Hierarchical TiO2-X imbedded with graphene quantum dots for high-performance lithium storage. Chem Commun. 2018;54:1413–6. doi: 10.1039/c7cc09406j. [DOI] [PubMed] [Google Scholar]
- 55. Bourlinos AB, Stassinopoulos A, Anglos D, Zboril R, Georgakilas V, Giannelis EP. Photoluminescent carbogenic dots. Chem Mater. 2008;20:4539–41. [Google Scholar]
- 56. Zheng LY, Chi YW, Dong YQ, Lin JP, Wang BB. Electrochemiluminescence of water-soluble carbon nanocrystals released electrochemically from graphite. J Am Chem Soc. 2009;131:4564–5. doi: 10.1021/ja809073f. [DOI] [PubMed] [Google Scholar]
- 57. Zhu SJ, Song YB, Zhao XH, Shao JR, Zhang JH, Yang B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective. Nano Res. 2015;8:355–81. [Google Scholar]
- 58. Luo PJG, Sahu S, Yang ST, Sonkar SK, Wang JP, Wang HF, LeCroy GE, Cao L, Sun YP. Carbon “quantum” dots for optical bioimaging. J Mater Chem B. 2013;1:2116–27. doi: 10.1039/c3tb00018d. [DOI] [PubMed] [Google Scholar]
- 59. Sun YP, Zhou B, Lin Y, Wang W, Fernando KAS, Pathak P, Meziani MJ, Harruff BA, Wang X, Wang HF, Luo PJG, Yang H, Kose ME, Chen BL, Veca LM, Xie SY. Quantumsized carbon dots for bright and colorful photo-luminescence. J Am Chem Soc. 2006;128:7756–7. doi: 10.1021/ja062677d. [DOI] [PubMed] [Google Scholar]
- 60. Wang X, Cao L, Yang ST, Lu FS, Meziani MJ, Tian LL, Sun KW, Bloodgood MA, Sun YP. Bandgap-like strong fluorescence in functionalized carbon nanoparticles. Angew Chem Int Edit. 2010;49:5310–4. doi: 10.1002/anie.201000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dimos K. Carbon quantum dots: surface passivation and functionalization. Curr Org Chem. 2016;20:682–95. [Google Scholar]
- 62. Li LB, Dong T. Photoluminescence tuning in carbon dots: surface passivation or/and functionalization, heteroatom doping. J Mater Chem C. 2018;6:7944–70. [Google Scholar]
- 63. Li CL, Ou CM, Huang CC, Wu WC, Chen YP, Lin TE, Ho LC, Wang CW, Shih CC, Zhou HC, Lee YC, Tzeng WF, Chiou TJ, Chu ST, Cang J, Chang HT. Carbon dots prepared from ginger exhibiting efficient inhibition of human hepatocellular carcinoma cells. J Mater Chem B. 2014;2:4564–71. doi: 10.1039/c4tb00216d. [DOI] [PubMed] [Google Scholar]
- 64. Roy P, Periasamy AP, Lin CY, Her GM, Chiu WJ, Li CL, Shu CL, Huang CC, Liang CT, Chang HT. Photoluminescent graphene quantum dots for in vivo imaging of apoptotic cells. Nanoscale. 2015;7:2504–10. doi: 10.1039/c4nr07005d. [DOI] [PubMed] [Google Scholar]
- 65. Yang ST, Cao L, Luo PGJ, Lu FS, Wang X, Wang HF, Meziani MJ, Liu YF, Qi G, Sun YP. Carbon dots for optical imaging in vivo. J Am Chem Soc. 2009;131:11308–9. doi: 10.1021/ja904843x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang CI, Wu WC, Periasamy AP, Chang HT. Electrochemical synthesis of photoluminescent carbon nanodots from glycine for highly sensitive detection of hemoglobin. Green Chem. 2014;16:2509–14. [Google Scholar]
- 67. Vedamalai M, Periasamy AP, Wang CW, Tseng YT, Ho LC, Shih CC, Chang HT. Carbon nanodots prepared from o-phenylenediamine for sensing of Cu2+ ions in cells. Nanoscale. 2014;6:13119–25. doi: 10.1039/c4nr03213f. [DOI] [PubMed] [Google Scholar]
- 68. Miao P, Han K, Tang YG, Wang BD, Lin T, Cheng WB. Recent advances in carbon nanodots: synthesis, properties and biomedical applications. Nanoscale. 2015;7:1586–95. doi: 10.1039/c4nr05712k. [DOI] [PubMed] [Google Scholar]
- 69. Li HT, Kang ZH, Liu Y, Lee ST. Carbon nanodots: synthesis, properties and applications. J Mater Chem. 2012;22:24230–53. [Google Scholar]
- 70. Hsu PC, Chang HT. Synthesis of high-quality carbon nanodots from hydrophilic compounds: role of functional groups. Chem Commun. 2012;48:3984–6. doi: 10.1039/c2cc30188a. [DOI] [PubMed] [Google Scholar]
- 71. Hsu PC, Shih ZY, Lee CH, Chang HT. Synthesis and analytical applications of photoluminescent carbon nanodots. Green Chem. 2012;14:917–20. [Google Scholar]
- 72. Hsu PC, Chen PC, Ou CM, Chang HY, Chang HT. Extremely high inhibition activity of photoluminescent carbon nanodots toward cancer cells. J Mater Chem B. 2013;1:1774–81. doi: 10.1039/c3tb00545c. [DOI] [PubMed] [Google Scholar]
- 73. Sahu S, Behera B, Maiti TK, Mohapatra S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: application as excellent bio-imaging agents. Chem Commun. 2012;48:8835–7. doi: 10.1039/c2cc33796g. [DOI] [PubMed] [Google Scholar]
- 74. Zhu LL, Yin YJ, Wang CF, Chen S. Plant leaf-derived fluorescent carbon dots for sensing, patterning and coding. J Mater Chem C. 2013;1:4925–32. [Google Scholar]
- 75. Sabet M, Mahdavi K. Green synthesis of high photo-luminescence nitrogen-doped carbon quantum dots from grass via a simple hydrothermal method for removing organic and inorganic water pollutions. Appl Surf Sci. 2019;463:283–91. [Google Scholar]
- 76. Liu ML, Chen BB, Li CM, Huang CZ. Carbon dots: synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019;21:449–71. [Google Scholar]
- 77. Siddique A, Pramanick AK, Chatterjee S, Ray M. Amorphous carbon dots and their remarkable ability to detect 2,4,6-trinitrophenol. Sci Rep-Uk. 2018;8:9770. doi: 10.1038/s41598-018-28021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhu SJ, Wang L, Zhou N, Zhao XH, Song YB, Maharjan S, Zhang JH, Lu LJ, Wang HY, Yang B. The crosslink enhanced emission (CEE) in non-conjugated polymer dots: from the photoluminescence mechanism to the cellular uptake mechanism and internalization. Chem Commun. 2014;50:13845–8. doi: 10.1039/c4cc05806b. [DOI] [PubMed] [Google Scholar]
- 79. Wang Y, Xia YS. Optical, electrochemical and catalytic methods for in-vitro diagnosis using carbonaceous nanoparticles: a review. Microchim Acta. 2019;186:50. doi: 10.1007/s00604-018-3110-1. [DOI] [PubMed] [Google Scholar]
- 80. Yao J, Wang H, Chen M, Yang M. Recent advances in graphene-based nanomaterials: properties, toxicity and applications in chemistry, biology and medicine. Microchim Acta. 2019;186:395. doi: 10.1007/s00604-019-3458-x. [DOI] [PubMed] [Google Scholar]
- 81. Attar F, Shahpar MG, Rasti B, Sharifi M, Saboury AA, Rezayat SM, Falahati M. Nanozymes with intrinsic peroxidase-like activities. J Mol Liq. 2019;278:130–44. [Google Scholar]
- 82. Shen XM, Gao XJ, Gao XF. Theoretical studies on the mechanisms of the enzyme-like activities of precious-metal and carbon nanomaterials. Prog Biochem Biophys. 2018;45:204–17. [Google Scholar]
- 83. Shi WB, Wang QL, Long YJ, Cheng ZL, Chen SH, Zheng HZ, Huang YM. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem Commun. 2011;47:6695–7. doi: 10.1039/c1cc11943e. [DOI] [PubMed] [Google Scholar]
- 84. Long YJ, Wang XL, Shen DJ, Zheng HZ. Detection of glucose based on the peroxidase-like activity of reduced state carbon dots. Talanta. 2016;159:122–6. doi: 10.1016/j.talanta.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 85. Sun HJ, Zhao AD, Gao N, Li K, Ren JS, Qu XG. Deciphering a nanocarbon-based artificial peroxidase: Chemical identification of the catalytically active and substrate-binding sites on graphene quantum dots. Angew Chem Int Edit. 2015;54:7176–80. doi: 10.1002/anie.201500626. [DOI] [PubMed] [Google Scholar]
- 86. Zhong QM, Chen YY, Qin X, Wang YL, Yuan CL, Xu YJ. Colorimetric enzymatic determination of glucose based on etching of gold nanorods by iodine and using carbon quantum dots as peroxidase mimics. Microchim Acta. 2019;186:161. doi: 10.1007/s00604-019-3291-2. [DOI] [PubMed] [Google Scholar]
- 87. Nirala NR, Khandelwal G, Kumar B, Vinita, Prakash R, Kumar V. One step electro-oxidative preparation of graphene quantum dots from wood charcoal as a peroxidase mimetic. Talanta. 2017;173:36–43. doi: 10.1016/j.talanta.2017.05.061. [DOI] [PubMed] [Google Scholar]
- 88. Wang H, Liu CQ, Liu Z, Ren JS, Qu XG. Specific oxygenated groups enriched graphene quantum dots as highly efficient enzyme mimics. Small. 2018;14:1703710. doi: 10.1002/smll.201703710. [DOI] [PubMed] [Google Scholar]
- 89. Nirala NR, Abraham S, Kumar V, Bansal A, Srivastava A, Saxena PS. Colorimetric detection of cholesterol based on highly efficient peroxidase mimetic activity of graphene quantum dots. Sensor Actuat B-Chem. 2015;218:42–50. [Google Scholar]
- 90. Wu SH, Zhou RH, Chen HJ, Zhang JY, Wu P. Highly efficient oxygen photosensitization of carbon dots: the role of nitrogen doping. Nanoscale. 2020;12:5543–53. doi: 10.1039/c9nr10986b. [DOI] [PubMed] [Google Scholar]
- 91. Zhang JY, Lu XM, Tang DD, Wu SH, Hou XD, Liu JW, Wu P. Phosphorescent carbon dots for highly efficient oxygen photosensitization and as photo-oxidative nanozymes. ACS Appl Mater Inter. 2018;10:40808–14. doi: 10.1021/acsami.8b15318. [DOI] [PubMed] [Google Scholar]
- 92. Lin LP, Song XH, Chen YY, Rong MC, Zhao TT, Wang YR, Jiang YQ, Chen X. Intrinsic peroxidase-like catalytic activity of nitrogen-doped graphene quantum dots and their application in the colorimetric detection of H2O2 and glucose. Anal Chim Acta. 2015;869:89–95. doi: 10.1016/j.aca.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 93. Hu YH, Gao XJJ, Zhu YY, Muhammad F, Tan SH, Cao W, Lin SC, Jin Z, Gao XF, Wei H. Nitrogen-doped carbon nanomaterials as highly active and specific peroxidase mimics. Chem Mater. 2018;30:6431–9. [Google Scholar]
- 94. Liu S, Tian JQ, Wang L, Luo YL, Sun XP. A general strategy for the production of photoluminescent carbon nitride dots from organic amines and their application as novel peroxidase-like catalysts for colorimetric detection of H2O2 and glucose. RSC Adv. 2012;2:411–3. [Google Scholar]
- 95. Lin SC, Zhang YH, Cao W, Wang XY, Qin L, Zhou M, Wei H. Nucleobase-mediated synthesis of nitrogen-doped carbon nanozymes as efficient peroxidase mimics. Dalton T. 2019;48:1993–9. doi: 10.1039/c8dt04499f. [DOI] [PubMed] [Google Scholar]
- 96. Shamsipur M, Safavi A, Mohammadpour Z. Indirect colorimetric detection of glutathione based on its radical restoration ability using carbon nanodots as nanozymes. Sensor Actuat B-Chem. 2014;199:463–9. [Google Scholar]
- 97. Bano D, Kumar V, Singh VK, Chandra S, Singh DK, Yadav PK, Talat M, Hasan SH. A facile and simple strategy for the synthesis of label free carbon quantum dots from the latex of euphorbia milli and its peroxidase-mimic activity for the naked eye detection of glutathione in a human blood serum. ACS Sustain Chem Eng. 2019;7:1923–32. [Google Scholar]
- 98. Zheng AX, Cong ZX, Wang JR, Li J, Yang HH, Chen GN. Highly-efficient peroxidase-like catalytic activity of graphene dots for biosensing. Biosens Bioelectron. 2013;49:519–24. doi: 10.1016/j.bios.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 99. Yadav PK, Singh VK, Chandra S, Bano D, Kumar V, Talat M, Hasan SH. Green synthesis of fluorescent carbon quantum dots from azadirachta indica leaves and their peroxidase-mimetic activity for the detection of H2O2 and ascorbic acid in common fresh fruits. ACS Biomater Sci Eng. 2019;5:623–32. doi: 10.1021/acsbiomaterials.8b01528. [DOI] [PubMed] [Google Scholar]
- 100. Zhu WF, Zhang J, Jiang ZC, Wang WW, Liu XH. High-quality carbon dots: Synthesis, peroxidase-like activity and their application in the detection of H2O2, Ag+ and Fe3+ RSC Adv. 2014;4:17387–92. [Google Scholar]
- 101. Mohammadpour Z, Safavi A, Shamsipur M. A new label free colorimetric chemosensor for detection of mercury ion with tunable dynamic range using carbon nanodots as enzyme mimics. Chem Eng J. 2014;255:1–7. [Google Scholar]
- 102. Wang B, Chen YF, Wu YY, Weng B, Liu YS, Li CM. Synthesis of nitrogen- and iron-containing carbon dots, and their application to colorimetric and fluorometric determination of dopamine. Microchim Acta. 2016;183:2491–500. [Google Scholar]
- 103. Cao SS, Kang FF, Li P, Chen RF, Liu H, Wei Y. Photo-assisted hetero-Fenton degradation mechanism of Acid Blue 74 by a γ-Fe2O3 catalyst. RSC Adv. 2015;5:66231–8. [Google Scholar]
- 104. Shete MD, Fernandes JB. A simple one step solid state synthesis of nanocrystalline ferromagnetic α-Fe2O3 with high surface area and catalytic activity. Mater Chem Phys. 2015;165:113–8. [Google Scholar]
- 105. Dong YM, Zhang JJ, Jiang PP, Wang GL, Wu XM, Zhao H, Zhang C. Superior peroxidase mimetic activity of carbon dots-Pt nanocomposites relies on synergistic effects. New J Chem. 2015;39:4141–6. [Google Scholar]
- 106. Duan Y, Huang YJ, Chen SY, Zuo WY, Shi BF. Cu-doped carbon dots as catalysts for the chemiluminescence detection of glucose. Acs Omega. 2019;4:9911–7. doi: 10.1021/acsomega.9b00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhao LJ, Wu ZP, Liu GN, Lu HY, Gao Y, Liu FM, Wang CG, Cui JW, Lu GY. High-activity Mo, S co-doped carbon quantum dot nanozyme-based cascade colorimetric biosensor for sensitive detection of cholesterol. J Mater Chem B. 2019;7:7042–51. doi: 10.1039/c9tb01731c. [DOI] [PubMed] [Google Scholar]
- 108. Zheng C, Ke WJ, Yin TX, An XQ. Intrinsic peroxidase-like activity and the catalytic mechanism of gold@carbon dots nanocomposites. RSC Adv. 2016;6:35280–6. [Google Scholar]
- 109. Guo YL, Liu XY, Wang XD, Iqbal A, Yang CD, Liu WS, Qin WW. Carbon dot/NiAl-layered double hydroxide hybrid material: facile synthesis, intrinsic peroxidase-like catalytic activity and its application. RSC Adv. 2015;5:95495–503. [Google Scholar]
- 110. Hassanzadeh J, Khataee A. Ultrasensitive chemiluminescent biosensor for the detection of cholesterol based on synergetic peroxidase-like activity of MoS2 and graphene quantum dots. Talanta. 2018;178:992–1000. doi: 10.1016/j.talanta.2017.08.107. [DOI] [PubMed] [Google Scholar]
- 111. Chen SH, Chi MQ, Yang ZZ, Gao M, Wang C, Lu XF. Carbon dots/Fe3O4 hybrid nanofibers as efficient peroxidase mimics for sensitive detection of H2O2 and ascorbic acid. Inorg Chem Front. 2017;4:1621–7. [Google Scholar]
- 112. Yousefinejad S, Rasti H, Hajebi M, Kowsari M, Sadravi S, Honarasa F. Design of C-dots/Fe3O4 magnetic nanocomposite as an efficient new nanozyme and its application for determination of H2O2 in nanomolar level. Sensor Actuat B-Chem. 2017;247:691–6. [Google Scholar]
- 113. Zhang L, Hai X, Xia C, Chen XW, Wang JH. Growth of CuO nanoneedles on graphene quantum dots as peroxidase mimics for sensitive colorimetric detection of hydrogen peroxide and glucose. Sensor Actuat B-Chem. 2017;248:374–84. [Google Scholar]
- 114. Hummers WS, Offeman RE. Preparation of graphitic oxide. J Am Chem Soc. 1958;80:1339. [Google Scholar]
- 115. Liu WY, Yang HM, Ma C, Ding YN, Ge SG, Yu JH, Yan M. Graphene-palladium nanowires based electrochemical sensor using ZnFe2O4-graphene quantum dots as an effective peroxidase mimic. Anal Chim Acta. 2014;852:181–8. doi: 10.1016/j.aca.2014.08.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.