Abstract
Lately, scandals associated with the illegal addition of poisonous chemicals to food for commercial interests have been gradually disclosed to the public. Problems related to food safety do not only harm public health but also affect the stability of economic and social development. Food safety has become a common issue in society, and strengthening the related regulations have become increasingly important. Although conventional techniques are accurate and sensitive in the detection of the vast majority of illegal food additives, they rely on time-consuming, labor-intensive procedures that depend on expensive instruments. Thus, efficient and rapid identification of poisonous, illegal additives in food is a crucial task in analytical chemistry. Recently, in this context, gold nanoparticles (GNPs) have attracted considerable attention because of their optical, electronic, catalytic, and chemical properties. Their excellent properties have facilitated the widespread use of GNPs in different sensors. This review covers the two most common GNP-based sensors with colorimetric and electrochemical responses, which have proven to be effective in the detection of illegal additives. The GNP-based sensors comply with the requirement of modern analysis, such as high selectivity, sensitivity, simplicity, rapidity, and portability. Thus, they have great potential as powerful sensing tools for food safety screening. This review elucidates the utility and advances of GNP-based colorimetric and electrochemical sensors for the detection of illegal additives in the food industry and in the supervision of food quality and safety. Additionally, an outlook of the trends and future development of research on these sensors is provided.
Keywords: Gold nanoparticles, Illegal additives, Colorimetric sensors, Electrochemical sensors, Quick detection
1. Introduction
Food safety is a major global concern because of an increased public awareness of health and quality standards [1]. Food additives are natural or synthetic substances that are widely used in modern food industry during processing, packaging, and transport. They can improve the quality, durability, and stability of food products and adjust their color, smell, and flavor [2]. Illegal food additives are non-edible substances that are prohibited in human food because of the threat they pose to human health [3]. However, some illegal additives are still added to food, and this has become one of the most prominent food safety issues. Incidents caused by illegal additives in food have occurred recurrently, threatening public health and leading to a social distrust of the food industry and loss of public confidence in the regulatory system [4,5]. For instance, the following substances have been reported as illegal food additives: formaldehyde, nitrite, melamine, sodium formaldehyde sulfoxylate, alum, beef extract, clenbuterol, sulfur dioxide, Sudan red, diethylhexyl phthalate (DEHP), fluorescent bleacher, and talcum powder [5]. Because of the frequent food safety incidents, strengthening the food quality and safety regulation has become topical [6,7]. Accordingly, the development of methods to identify poisonous and illegal additives in food products are urgently required to minimize the public health hazards.
Recently, nanotechnology has emerged as a promising field of research in preventing food safety issues. With its rapid development, novel methods for detecting illegal food additives have been found.
Among the nanomaterials, gold nanoparticles (GNPs) have attracted considerable attention in food safety because of their unique, size-dependent properties, such as their optical, electric, catalytic, and magnetic behaviors, and because of their biocompatibility, ease of chemical modification, and dispersibility in water [8,9]. These properties make GNPs very promising for producing novel sensors for application in food safety control [10]. In colloidal solutions, GNPs can exhibit different optical properties, according to their dispersed or aggregated state. When the interparticle distance is approximately lower than the average GNP diameter, the color of the solution changes from red to blue, corresponding to a band shift of the surface plasmon from 523 nm to 610–670 nm that can be easily observed by simple naked-eye observation or spectroscopic measurements. Thus, they have been used as simple colorimetric sensors for the quick detection of illegal additives in food samples. Furthermore, GNPs have excellent conductivity and catalytic properties, which are appealing to optimize the sensitivity of electrochemical sensors by amplifying the electrode surface, enhancing the electron transfer between electroactive species and the electrode, and catalyzing electrochemical reactions [11]. In general, GNPs are used in electrochemical sensors as assisting components. Functionalized GNPs may act as both signal transducer and molecular receptor in a sensing platform, simplifying the design of the latter and enhancing its sensitivity. Sometimes, GNPs can act as electro-catalysts, especially for the redox reaction [10]. GNP-based colorimetric sensors are simple and rapid, and GNP-based electrochemical sensors have high sensitivity and selectivity, simultaneously being facile, robust, and easy to use. Thus, in food safety and quality assessment, these two kinds of sensors have been widely applied in rapid, real-time and on-site monitoring and detection of illegal food additives. This review presents a brief overview of the common applications and recent advances of GNP-based colorimetric and electrochemical sensors for detecting illegal food additives.
Because of the outbreak of publications in this field, we can not mention all the published reports and rather choose to discuss and summarize those that are most representative. Thus, the recent development of GNP-based colorimetric and electrochemical sensors for detecting illegal food additives and their applications in the last five years (2015–2019) are explained in detail (see Fig. 1 and Fig. 2). Future prospects and challenges are also discussed briefly. Other applications of GNP-based sensors such as fluorescent sensors, immunosensors, and optical-based biosensors are not discussed.
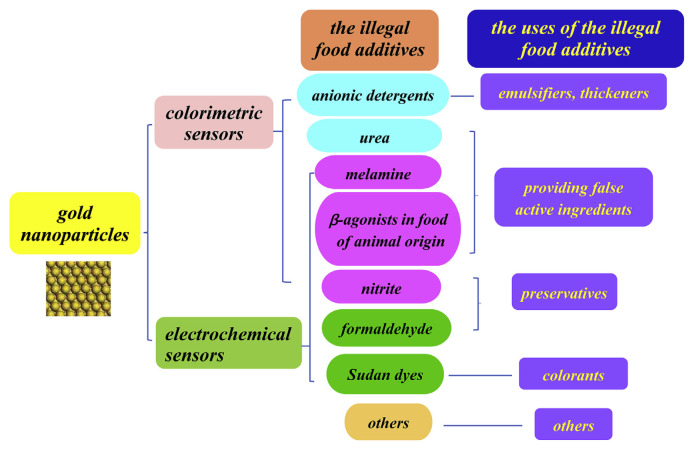
Fig. 1.
Systematic representation of application of GNP-based colorimetric and electrochemical sensors to detect illegal additives in food.
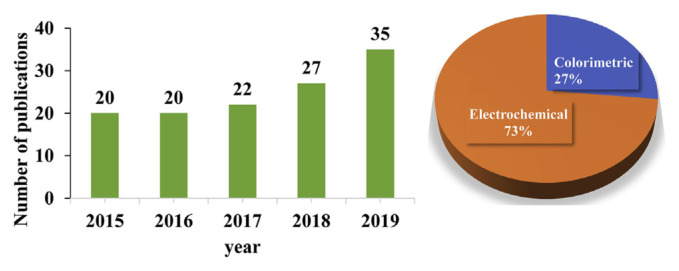
Fig. 2.
Systematic representation of application of GNP-based colorimetric and electrochemical sensors to detect illegal additives in food. Evolution of the number of publications concerning the keywords “gold nanoparticles”+“colorimetric sensors”+“additives” and “gold nanoparticles ”+“electrochemical sensors”+“additives” on indexed journals between 2015 and 2019. The insert pie graph exhibits the percentage of the available scientific reports which concerned the GNP-based sensors using colorimetric and electrochemical techniques from 2015 to 2019. The data comes from the research on “web of science”.
2. GNP-based colorimetric and electrochemical sensors of illegal food additives
Common illegal additives include melamine, Sudan dyes, β-agonists in food of animal origin, and nitrite. For the detection of these poisonous chemicals, different GNP-based colorimetric and electrochemical sensors have been developed.
2.1. Melamine detection
As an important nitrogen-heterocyclic industrial raw material, melamine (1,3,5-triazine-2,4,6-triamine), a trimer of cyanamide with a 1,3,5-triazine skeleton, is widely used in industry for the production of, e.g., plastics, amino resins, and flame retardants [12,13]. Melamine is not approved for application in food processing or food additives. However, because of its high nitrogen content (66% of its mass) and low cost, melamine has been illegally introduced by unethical manufacturers in infant milk, wheat gluten, and pet food to inflate the apparent content of crude proteins [14]. In fact, traditional protein detection methods, such as the Kjeldahl and Dumas methods, determine the nitrogen content without identifying its source. Thus, the presence of melamine can falsely increase the protein concentration. Unfortunately, melamine itself has acute toxicity and can gradually form insoluble reticulate crystals in the kidneys with its hydrolysate (cyanuric acid). A long-term and excessive intake of melamine-tainted food can cause urinary calculus, renal dysfunction, and even death, especially in babies and small pets [15,16]. Recently, melamine has represented a widespread concern in food safety. Therefore, effective, reliable, and highly sensitive methods for detecting melamine in food products with the sensitivity required by regulatory authorities are urgently needed.
In this context, GNP-based colorimetric and electrochemical sensors have become powerful detection tools for identifying melamine in food products.
2.1.1. GNP-based colorimetric sensors for melamine detection
Our group used bare GNPs as a colorimetric probe for melamine detection [17]. Because of the strong attraction between the amine groups of melamine and surface-bound ions, melamine can directly induce the aggregation of bare GNPs, resulting in an obvious color change from wine red to blue. However, colorimetric methods for melamine detection by naked GNPs require tedious sample preparation processes or complicated operation procedures, which are disadvantageous for on-site detection [18]. Moreover, bare GNPs are unstable in colloidal solution and can undergo aggregation due to salt-induced screening, resulting in false detection events [19]. To stabilize GNPs in aqueous media and improve the sensitivity of the GNP-based colorimetric assay, modified GNPs have been developed to detect melamine. Ligand-modified GNPs are characterized by high steric and hydration-based interparticle repulsion. Thus, they can modify the aggregation of GNPs in an oriented and controlled manner, and are more stable in high ionic strength conditions than bare GNPs [20,21].
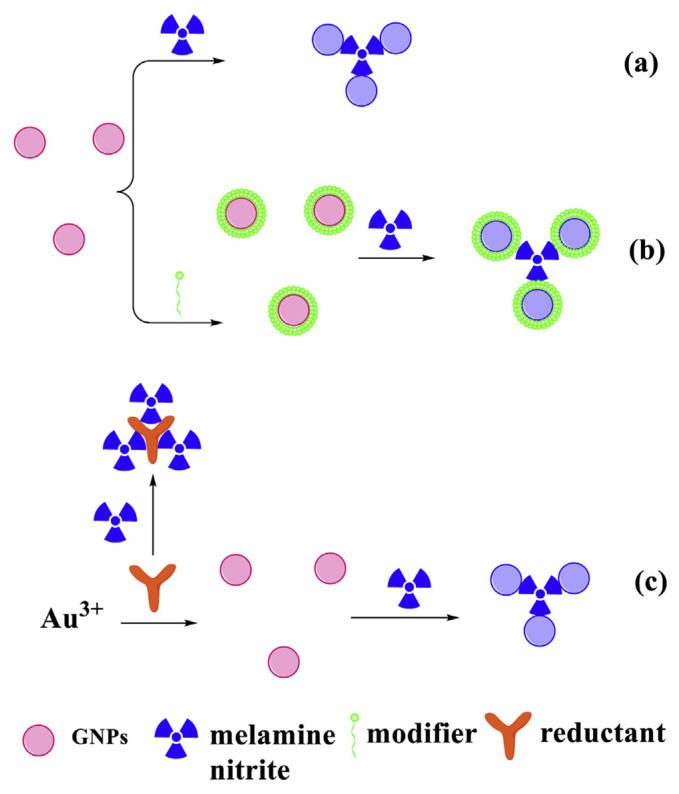
Because environmental factors such as the pH value, ionic strength, and temperature might influence the aggregation of GNPs, some new strategies have also been reported for the detection of melamine. For example, Xin’s group has developed a series of colorimetric sensors to detect melamine during the synthesis of GNPs [22]. Detailed mechanistic investigations indicate that melamine interrupts the process of synthesis of GNPs and stimulates the aggregation of formed GNPs (see Fig. 3(c)).
Fig. 3.
GNP-based colorimetric sensors for the detection of melamine and nitrite. (a) Bare GNP-based colorimetric sensors for the detection of melamine. (b) Modified GNP-based colorimetric sensors for the detection of melamine (or nitrite). (c) Sensing principle for melamine analysis during the synthesis of GNPs.
2.1.2. GNP-based electrochemical sensors for melamine detection
Recently, several reports described the successful use of GNPs in hybrid-modified electrodes for the electrochemical detection of melamine. For instance, Chen et al. have developed a sensor based on a glassy carbon electrode (GCE) modified by GNP/reduced graphene oxide (RGO) nanocomposites with a high melamine sensitivity [23]. In this electrochemical sensing system, RGO provides a platform for the uniform distribution of GNPs and enhances the electron transfer rate, improving the sensitivity of the sensor. For melamine measurement, hexacyanoferrate was used as electrochemical reporter. Melamine can be grafted on the surface of GNPs by the interaction between the amino groups of melamine and GNPs via Au–N bond, which leads to the suppression of the peak current of hexacyanoferrate because of the poor electrochemical activity of melamine. The suppression degree is related with the concentration of melamine and can be used for the quantitative determination.
2.2. Sudan dyes detection
Sudan dyes (comprising Sudan I, II, III, and IV) are a family of lipophilic azo dyes that are widely used in the chemical industry, and especially as coloring agents in solvents, oil, petrol, wax, and shoe polish [24,25]. Because they are classified as carcinogens by the International Agency for Research on Cancer, Sudan dyes are strictly banned as food additives by both the Food Standards Agency and the European Union [26]. However, because of their fascinating red color and low cost, they are illegally used as food additives by many unscrupulous manufacturers to improve the appearance of food products such as chili powders, beverages, sweets, palm oil-based products, frozen meat, etc. [27]. Therefore, for public health protection, monitoring the presence of Sudan dyes in food products is highly important, and the development of sensitive analytical methods to detect them is urgently required.
2.2.1. GNP-based electrochemical sensors for Sudan dye detection
Modified electrode systems have become research focus in electrochemical sensing because of their efficiency and accuracy [28]. For the electrochemical detection of Sudan dyes, the modifier is a key factor that can highly influence the sensitivity and selectivity of the detection [29]. GNPs are known to be efficient labels and viable materials to modify the surface of electrodes, thereby enhancing the detection limit of electrochemical sensors [30]. Thomas et al. have developed an electrochemical sensor based on the catalytic activity of the GNPs deposited on a GCE to detect Sudan I in chili powder and ketchup samples. The GNPs displayed excellent electrocatalytic activity in the oxidation of Sudan I [31]. The sensor exhibited two distinct linear response ranges of 4.0 × 10−5–1 × 10−3 mol/L and 2 × 10−5–7 × 10−7 mol/L. The lower detection limit of the sensor was 1.0 × 10−8 mol/L (Table 1).
Table 1.
Typical applications of gold nanoparticle-based colorimetric and electrochemical sensors for the detection of illegal food additives.
| Sample source | Adulterant | Biosensors | Nanomaterials | Concentration range | Detection limit | Pretreatment to real samples | Ref. |
|---|---|---|---|---|---|---|---|
| infant formula | melamine | colorimetric | bare GNPs | 5.0 × 10−6 2.0 × 10−4 g/L |
2.0 × 10−7 g/L | precipitate proteins by trichloroacetic solution, then collect the supernatant | [17] |
| milk | melamine | colorimetric | 1,4-dithiothreitol modified GNPs | 8.0 × 10−8 6.0 × 10−7 M; 6.0 × 10−7 1.5 × 10−6 M |
2.4 × 10−8 M | dilute 1000 times with distilled water | [19] |
| processed raw milk | melamine | colorimetric | asymmetrically PEGylated GNPs | 1.05 × 10−3 −1.00 × 103 μmol/L |
1.05 × 10−3 μmol/L | precipitate proteins with 10% trichloroacetic acid and acetonitrile, then sonicate, centrifuge, and filtrate | [21] |
| liquid milk | melamine | colorimetric | methanobactin-mediated synthesis of GNPs | 3.90 × 10−7 −3.97 × 10−6 mol/L |
2.38 × 10−7 mol/L | precipitate proteins with 10% trichloroacetic acid, then shake, centrifuge, and filtrate | [22] |
| food contact materials (plate or fruit tray) | melamine | electrochemical | GNPs/RGO/GCE | 5–50 nmol/L | 1.0 nmol/L | immerse in food simulants (water or 3% acetic acid) then heat and filtrate | [23] |
| ketchup and chilly sauce | Sudan I | electrochemical | GNPs/GCE | 4.0 × 10−5 −1 × 10−3; 2 × 10−5 −7 × 10−7 mol/L |
1.0 × 10−8 mol/L | ultrasonic extraction with ethanol and filtrate | [31] |
| chili and ketchup sauce | Sudan II | electrochemical | treated pencilgraphite electrode with DNA, o-phenylenediamine, and GNP bioimprinted polymer | 1.0–20.0; 20.0 −500.0 nmol/L |
0.3 nmol/L | ultrasonic extraction with ethanol and filtrate | [32] |
| chilli powder and ketchup sauce | Sudan I | electrochemical | GNPs/RGO/GCE | 0.01–70 μmol/L | 1 nmol/L | ultrasonic extraction with ethanol and filtrate | [37] |
| chopped red chili, tomato sauce; the apple juice and grape juice | Sudan I | electrochemical | ILRGO@GNPs/GCE | 1.0 × 10−10 −1.0 × 10−6 mol/L |
5.0 × 10−11 mol/L | for chopped red chili and tomato sauce, ultrasonic extraction with ethanol and filtrate; for the apple juice and grape juice, use directly without pretreatment | [39] |
| beef | ractopamine | colorimetric | Apt–GNPs | 0–400 ng/mL | 10 ng/g | homogenize with acetate ammonium buffer (pH = 5.2), then enzymatically digest, centrifuge and filtrate | [56] |
| pork | clenbuterol | electrochemical | MoS2–Au-PEI-hemin/GCE | 0.01–2 μg/mL | 0.00192 μg/mL | homogenize with 0.1 M HClO4 solution, then sonicate and centrifuge | [57] |
| meat products including ham sausage and red-braised pork | nitrite | colorimetric/fluorescent/SERS triple sensing | GNRs-Azo-GNPs | 0.5–100 μmol/L | 0.05 μmol/L | cut into 1 cm disk and 2 × 2 cm square piece | [65] |
| sausages | nitrite | colorimetric | Janus PEGylated GNP probe | 10.8–174 μmol/L | 10.8 μmol/L | shred, then dissolve with a saturated boric acid solution | [66] |
| packaged drinking water, processed meats and aquatic products | nitrite | electrochemical | ERGO/GNPs/SPCE | 1–6000 μmol/L | 0.13 μmol/L | homogenize, then ultrasonication, heat, cool to room temperature, filtrate, centrifuge and filtrate again | [68] |
| sausage | nitrite | electrochemical | GNP/p-ATP-modified gold electrode | 0.5–50 mg/L | 2.6 μmol/L | shred, add saturated borax solution, heat, precipitate proteins with 30% ZnSO4 solution, centrifuge and filtrate | [69] |
| pickled radish | nitrite | electrochemical | GNPs/GO-SH nanocomposites | 5–1000 μmol/L | 0.25 μmol/L | flush with ultrapure water, then squeeze into juice, ultrasonic treatment, heat and filtrate | [70] |
| milk | urea | colorimetric | GNPs–aptamer | 20–150 mmol/L | 20 mmol/L | precipitate proteins with methanol, vortex and centrifuge | [72] |
| milk | anionic detergents | colorimetric | bare GNPs | 92–900 μg/mL | 92 μg/mL | precipitate proteins using ice-cold methanol supplemented with NaCl, then vortex and centrifuge | [73] |
| fish | formalin | electrochemical | FDH/GNPs/[EMIM][Otf]/CHIT/GCE | 0.01–10 ppm | 0.1 ppm | thawed, then select the flesh to cut into small pieces, homogenize with Tris–HCl (pH 7, 0.5 M) and filtrate | [76] |
To date, many electrochemical sensors prepared by GNPs combined with other functional materials for detecting Sudan dyes have been reported. For example, an electrochemical sensor based on a molecularly imprinted polymer entrapped by double-stranded DNA (ds-DNA) and gold nanoparticles has been reported to detect Sudan II in chili and ketchup sauce [32]. The imprinted polymer provided great specific molecular recognition and high stability in harsh chemical and physical conditions [33]. In these kinds of polymers, molecular recognition relies on the intermolecular interaction, such as hydrogen, π-π, or ionic bonds and electrostatic, hydrophobic, dipole, or van der Waals interactions, between the functional monomers and the template [34]. The ds-DNA and GNPs enhance the selectivity and sensitivity of the measurement. In particular, the introduction of GNPs in molecularly imprinted polymers has been reported to increase the electrode specific area, enhance the electron transfer between the recognition sites and the electrochemical transducer, and act as catalysts that amplify the electrochemical reactions [35]. Additionally, GNP/RGO composites have proven to be good electrode materials in electrochemical sensing, because electrodes modified using these composites exhibited highly improved electrocatalytic activity and electrochemical stability [36]. For instance, a GNP–decorated RGO electrochemical sensor of high performance has been developed for the detection of Sudan I in chili powder and ketchup sauce [37]. Compared with the reported analogs, the GNP/RGO hybrid electrode displayed increased electrocatalytic activity in the oxidation of Sudan I because of the excellent catalysis and charge transfer ability of GNPs. Furthermore, the incorporation of ionic liquids into GNP/RGO-based electrochemical sensors can significantly improve their sensitivity, retaining their chemical stability and high catalytic activity [38]. Wang et al. reported GNP/RGO composites functionalized by an ionic liquid of 1-allyl-3-methylimidazolium chloride for the electrochemical detection of Sudan I in chopped red chili, tomato sauce, apple juice, and grape juice. Such fabricated Sudan I sensor reveals a wide linear range from 1.0 × 10−10–1.0 × 10−6 mol/L, low detection limit of 5.0 × 10−11 mol/L, high selectivity and long-term stability [39].
2.3. Detection of β-agonists in food of animal origin
β-agonists are a group of phenylethanolamine compounds with different substituents of the aromatic rings or aliphatic amino groups and include mainly clenbuterol (CLE), ractopamine (RAC), salbutamol (SAL), terbutaline, cimaterol, phenylethanolamine A (PEA) [40,41]. They are used in the clinical treatment of pulmonary diseases such as asthma [42,43] and chronic obstructive pulmonary disease [44]. Additionally, they can promote protein synthesis, increase muscular growth, and decrease the deposition of fats in livestock by stimulating β2-adrenergic receptors [45,46]. However, in livestock fed with β-agonists, the residues can stay in the tissues for a long time [47,48]. When people consume products of such livestock, acute poisoning can result, with symptoms such as muscular tremor or pain, cardiac palpitation, nervousness, headache, dizziness, nausea, vomiting, fever, chills, etc. [49]. Therefore, β-agonists have been banned as growth promoters for livestock in most countries, including the European Union and China [50–52]. Nevertheless, in some regions, β-agonists have been illegally used as food additives in feeding livestock for economic profit. To provide food safety to the consumer, such illegal food additives must be strictly monitored. However, the wide variety of βagonists and their extensive use in farming represent an overwhelming challenge for governments, requiring daily supervision and routine monitoring.
2.3.1. GNP-based colorimetric sensors for the detection of β-agonists in food of animal origin
Aptamers (Apts), which are single-stranded oligonucleotides exhibiting excellent biological recognition capability, can be used as recognition components in the design of biosensors and analytical applications [53–55]. Wang et al. reported a sensitive visual detection method for the identification of ractopamine (RAC) in beef using Apt–GNPs aptasensor [56]. The concentration of RAC could be quantified visually or using a UV–Vis spectrometer in the wide range from 10 to 400 ng/mL. The detection limit is as low as 10 ng/mL.
2.3.2. GNP-based electrochemical sensors for the detection of β-agonists in food of animal origin
Yang et al. developed an electrochemical non-enzyme sensor by fabricating layered MoS2–Au-PEI-hemin nanocomposites-modified GCE for detecting CLE in real pork samples [57]. Because of the unique physical and electrical properties correlated with its two-dimensional structure and high surface area, the MoS2 nanosheet is very interesting as supporting material for hierarchical composites in electrochemical systems [58]. The electrochemical sensor proposed in Ref. [57] combined the advantages of the MoS2 nanosheet, the high conductivity of GNPs, the presence of polyethyleneimine (PEI) amino-groups, and the electrochemical catalytic activity of hemin, exhibiting excellent detection performance to CLE. Such sensor reveals a wide linear ranging from 10 ng/mL to 2 μg/mL, low detection limit of 1.92 ng/mL CLE, favorable reproducibility and stability.
2.4. Detection of nitrite
Sodium nitrite is an industrial salt commonly known as nitrite. It is a pale yellow, granular crystal or powder, with a shape very similar to salt [59]. In the food processing industry, nitrite is used to improve the appearance (color), flavor, and texture of processed meat [60]. Additionally, it can be used as a food preservative for meat or meat products because it inhibits the growth of clostridium botulinum spores [61]. However, it has proven to be a threat to human health. In fact, nitrite is an essential precursor of carcinogenic N-nitrosamines, which can result in serious health issues even at very low concentrations [62,63]. Furthermore, nitrite can interfere with the oxygen transport system and may result in methaemoglobinaemia, which can cause tissue hypoxia and, possibly, death [59,63]. The World Health Organization has included nitrite in its list of carcinogens [64]. Consequently, the nitrite levels in food samples are globally strictly limited to protect public health. Because of the serious hazard to health and the frequent occurrence of food safety accidents related to nitrite, this is currently severely restricted or banned as a food additive in many countries [5,64]. However, to save costs, it is still used indiscriminately to replace allowed additives in the food processing industry. Therefore, the detection of nitrite in food is of great importance, especially for food quality control.
With the continuous development of technologies in analytical chemistry, the methods for detecting nitrite in food are increasingly diverse, and new methods are emerging. Rapid, cost-effective methods for on-site detection of nitrite in food products are becoming increasingly common because of their easy test protocols and instantaneous results. In this context, many researchers have attempted to develop novel sensors for the detection of nitrite.
2.4.1. GNP-based colorimetric sensors for the detection of nitrite
Li et al. developed the Griess reaction-based paper strips for colorimetric/fluorescent/surface enhanced Raman scattering (SERS) triple-mode sensing of nitrite in meat products [65]. The triple-mode sensor was based on hybrid gold nanorodsazo-GNPs (GNRs-Azo-GNPs) assemblies, which can not only be used as a naked-eye detector of nitrite, because of the color change from orange-yellow to purple upon addition of nitrite, but also as a highly selective fluorescence-quenching probe. Additionally, it can be used as a SERS substrate for reliable quantitative analysis of nitrite. Thus, the triple-mode sensor can be used as a paper-based test strip for on-site, fast screening of nitrite with a high sensitivity and selectivity.
Xiong et al. developed a Janus-type polyethylene glycol-based (PEGylated) GNP probe for colorimetric detection of nitrite in sausages with a surprisingly high analytical performance [66]. The PEGs and recognition ligands (e.g., 4-aminobenzenethiol, 4-ABT) were asymmetrically functionalized on the surface of GNPs to form a GNP-based probe. In this probe, the biocompatible and water-soluble PEGs were used to maintain the colloidal stability of GNPs against extreme conditions in real samples [67], whereas 4-ABT was used as a ligand for sensing nitrite. With this design, the probes showed high colloidal stability, signal robustness, specificity, and sufficient sensitivity in the detection of nitrite in different complex samples.
2.4.2. GNP-based electrochemical sensors for the detection of nitrite
Recently, different kinds of nitrite sensors have been fabricated by chemically modifying the electrodes to improve their performance. For example, Jian et al. developed a screen-printed carbon electrode (SPCE) modified with a composite of electrochemically reduced graphene oxide (ERGO) and GNPs for an effective electrocatalytic analysis of nitrite in several complex food samples, including packaged drinking water, processed meats, and aquatic products [68]. The optimized electrode exhibited excellent electroactive and electrocatalytic properties for the detection of nitrite, including a low oxidation potential (0.65 V), wide linear range (1–6000 μM), high sensitivity (0.3048 μA μM−1 cm−2), low detection limit of 0.13 μM (S/N π 3), and great selectivity.
Üzer et al. developed an electrochemical sensor with a GNP/p-aminothiophenol (p-ATP)-modified gold electrode to detect nitrite in sausage samples [69]. The GNP/PATP nanocomposites can form an electrocatalytic layer on the surface of the modified gold electrode, decreasing the overpotential and enabling faster electron-transfer kinetics for nitrite oxidation. Under the optimum conditions, the linear range for the detection of nitrite was 0.5–50 mg/L with a detection limit of 0.12 mg/L.
Pan et al. developed a GCE modified by a GNPs/l-cysteine functionalized graphene oxide (GNPs/GO-SH) nanocomposite for the electrochemical detection of nitrite in pickled radish [70]. Because of the low stability of GO, it is essential to improve the stability of this sensor and functionalize it by chemical modification. The surface of GO was modified using the sulfhydryl group of l-cysteine to improve the electrical conductivity of the electrode and the GNP loading [64,70]. As a result, the sensitivity and selectivity of this electrochemical sensor was enhanced.
2.5. Detection of other illegal food additives
2.5.1. GNP-based colorimetric sensors for the detection of other illegal additives
Recently, the economically motivated adulteration of milk and infant formula using hazardous chemicals like melamine, formalin, hydrogen peroxide, dichromate, urea, and detergents has increased [71]. To assure food safety and avoid health risks to consumers, novel analytical procedures have been proposed for the detection of these illegal additives. Kumar et al. developed an easy-to-use, non-enzymatic, dual readout aptasensor with both colorimetric and fluorescence sensing functions to detect urea adulteration in milk [72]. The urea aptasensor uses bareGNPs to transduce the signal of aptamer binding to urea in simultaneous intrinsic fluorescence and color change. Kumar et al. developed a simple method for the detection of anionic detergents (ADs, mainly consist of linear alkylbenzenesulfonate and have been widely used as surfactants) in milk using bare GNPs as colorimetric sensing platforms based on aggregation and dispersion of the GNPs [73].
2.5.2. GNP-based electrochemical sensors for the detection of other illegal additives
Formaldehyde is classified as a carcinogen by the International Agency for Research on Cancer (IARC) [74], and is prohibited as a food additive in China and the European Union [5, 75]. However, formaldehyde is sometimes used illegally as a food preservative in aquatic products. Recently, Aini et al. developed an electrochemical biosensor using a GCE modified with formaldehyde dehydrogenase (FDH)/GNPs/1-ethyl-3-methylimidazolium trifluoromethanesulfonate ([EMIM][Otf])/chitosan (CHIT) to detect formalin in fish samples [76]. The linear working range for the quantitation of formaldehyde was 0.01–10 ppm with a detection limit of 0.1 ppm.
3. The sensing mechanisms
3.1. The sensing mechanisms of GNP-based colorimetric sensors
The sensing mechanisms of GNP-based colorimetric sensors for the detection of illegal additives can be divided into two types: aggregation principle and anti-aggregation principle.
3.1.1. Aggregation principle
The localized surface plasmon resonance (LSPR) is one of the most remarkable features of GNPs [77]. A consequence of this unique optical property, analyte-induced aggregation of GNPs in aqueous media is accompanied with a color change, which has been employed for the colorimetric detection of illegal additives [78,79]. Currently, many studies have reported illegal additives detection in food products using naked or modified GNPs as colorimetric sensors, which can be easily observed either by naked eye or simple spectroscopy measurements (see Fig. 3(a) and (b)).
In addition, GNPs can readily interact with biomolecules, yielding improved signal amplification and targeted recognition. GNPs can adsorb small Apts by taking advantage of electrostatic attraction, hydrophobic absorption, and covalent bonding. The aptamer-conjugated GNPs (Apt–GNPs) is a promising tool for on-site detection in bioanalysis [80,81]. The mechanism of Apt–GNPs for the detection of illegal additives is shown in Fig. 4. In the absence of illegal additives, the Apt binds to GNPs, preventing salt-induced aggregation of the GNPs. If illegal additives is present, it selectively binds to the Apt, leaving the GNPs uncoated. This results in salt-induced aggregation of the GNPs along with a red-to-blue color change.
Fig. 4.
Schematic representation of aptamer–GNPs based methods for the detection of RAC and urea. (a) Without RAC (or urea). (b) With RAC (or urea).
3.1.2. Anti-aggregation principle
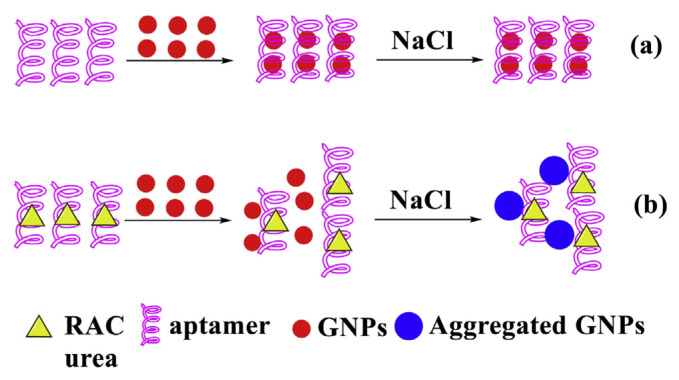
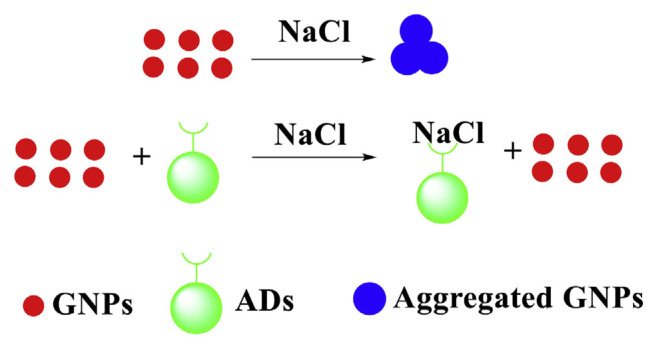
Addition of inducer (NaCl) to the GNPs neutralizes their surface charge and causes aggregation which is reflected in color change of the solution from red to purple. However, upon addition of illegal additives (such as ADs) with optimized NaCl concentration in GNPs solution, the solution color remains red, due to inhibitory effect on GNPs aggregation (Fig. 5). In Fig. 5, in the presence of ADs, the negatively charged ADs can neutralize the charge of sodium ions (Na+) of the NaCl, thus inhibiting the aggregation of GNPs induced by the NaCl.
Fig. 5.
Sensing principle for ADs analysis based on anti-aggregation of GNPs.
3.2. The sensing mechanism of GNP-based electrochemical sensors
GNPs have outstanding electrical conductivity, high surface area, and electrochemical activity that make them promising for electrochemical sensing [9]. Modifying the electrode of electrochemical sensors with GNPs and other functional materials can increase the electrochemically active surface, and exhibit strong biocompatibility, high electrical conductivity, and excellent catalytic activity [9,82–84]. For example, the mechanism for the electrocatalytic oxidation of nitrite at the ERGO/GNPs/SPCE is expected as below [68]: because of the large surface area of ERGO nanosheets, is firstly absorbed onto the ERGO surface to form a complex of [ ]. Then the neighboring Au0 is electrochemically oxidized to Au+ and nitrogen dioxide (NO2) is formed via the electron transferred through the conductive graphene network. Immediately, NO2 is further oxidized to in the presence of strongly active Au+. The GNPs were used as efficient electrocatalysts for the oxidation of nitrite [69,85], and the winkled ERGO sheets provided a three-dimensional scaffold for the attachment of GNPs and adsorption of abundant nitrite, promoting rapid and heterogeneous electron transfer [68].
The sensing mechanism of the electrochemical biosensor using FDH/GNPs/[EMIM][Otf]/CHIT modified GCE to detect formalin in fish samples is as follows [76]: FDH acts as the electron transfer to facilitate the addition of one hydrogen atom to NAD+ and reduced it to NADH, whereas formaldehyde converted to formic acid. CHIT has been used for the effective immobilization of molecules through electrostatic attraction in order to improve the stability and deposited onto the GCE. GNPs increased the surface area of immobilization. Meanwhile, the ionic liquid [EMIM][Otf] acts as an ionic solvent which diffuses easily due to its wide solubility in the solutions, especially during electrochemical measurements. Combining all of the characteristic above, FDH/GNPs/[EMIM][Otf]/CHIT indicated great potential in electrochemical activity for formaldehyde detection.
4. Conclusion and future perspectives
With the globalization of the food market, food safety issues have attracted great attention. As illegal additives are one of the main food safety issues, it is vital to develop simple, sensitive, and universal monitoring strategies of these additives to ensure the quality and safety of food.
Because of their excellent optical, electronic, catalytic, and chemical properties, GNPs have great potential in the development of colorimetric and electrochemical sensing technologies. This review has provided a brief overview of representative GNP-based colorimetric and electrochemical sensors for the detection of illegal additives in the food industry. Some typical applications of GNP-based colorimetric and electrochemical sensors for the detection of illegal food additives are briefly shown in Table 1. These techniques provide effective platforms for simpler, more rapid, cost-effective, and practical analysis of illegal additives compared to traditional analytical methods. However, these technologies are still emerging and facing several challenges. Firstly, colorimetric methods are intuitive, practical, simple and rapid, but require highly specific experimental conditions. Food products are multicomponent complex systems. some food sample matrices may be capable of interacting with gold nanoparticle, easily resulting in the aggregation of GNPs. Such non-specific reactions lead to a false positive result, seriously affecting the precision of measurements. After modifying GNPs with specific ligands such as biocompatible polymers, antibodies, functional small molecules, nucleic acids, peptide, and proteins, a highly selective for various analytes in complex food products may be achieved. Secondly, the introduction of recognition moieties on the surface of GNPs, can enhance the selectivity of target analytes. But sometimes instability of the modified GNPs frequently occur. Fine tuning the functionalization strategies could be the solution to introduce these recognition moieties on the GNPs without sacrificing their stability. Thirdly, driven by economic interests, numerous illegal additives may coexist in foodstuff. The simultaneous detection of different kinds of analytes is a new trend, but the colorimetric sensors based on GNPs are currently limited in this field. Electrochemical sensors can potentially be used to monitor different analytes simultaneously, therefore they are expected to be subject to further developments to this end. Fourthly, the electrochemical sensors suffer from the need for significant electroactivity of the analyte and the poor repeatability due to electrode fouling and charging. So there is an increasing demand for building up a complementary multi-mode sensing methods within one system because they can offer more than one kind of output signal simultaneously, thus making the detection results more convincing. Fifthly, both sensors usually need complicated sample pretreatment procedure to avoid matrix interference. Only few liquid samples such as the apple juice and grape juice were used directly without pretreatment. For most liquid samples such as milk, are usually treated as the following: precipitate proteins, then remove the precipitate by centrifugation, and at last, filter the supernatant. Solid complex samples such as chili powder or ketchup sauce are usually extracted with the solvent ethanol and the ultrasonic extractor, and then filtered. Solid complex samples such as meat usually need to be homogenized, then digested in a suitable solution, followed by centrifugation, dilution and/or filtration (Table 1). For the rapid and sensitive on-site quantitative detection of the analytes in complex matrix samples, portable pretreatment systems will be the future trend of development. Finally, along with the incessant research on the application of GNPs and other nanomaterials, several potential risks and toxicity issues associated with the application of these nanomaterials on environment and human health have been revealed [86,87], and should also be considered.
Supplementary Information
Funding Statement
This study was funded by the Natural Science Foundation for Colleges and Universities of Jiangsu Province (Grant No. 17KJD150005), the Jiangsu Overseas Research & Training Program for University Prominent Young & Middle-aged Teachers and Presidents Fund, the Qing Lan Project Fund of Jiangsu Province, Science and Technology Project Fund of Gaoxin District in Lianyungang (Grant No. HZ201906), the Fifth Phase of 521 Project Fund of Lianyungang, the Petrel Plan Project Fund of Lianyungang, and Science and Technology Funds of Kangda College of Nanjing Medical University (Grant No. KD2016GCCRCYJ01, KD2016GCCRCYJ02, KD201 8KYJJYB001 and KD2018KYJJYB010).
Footnotes
Funding
This study was funded by the Natural Science Foundation for Colleges and Universities of Jiangsu Province (Grant No. 17KJD150005), the Jiangsu Overseas Research & Training Program for University Prominent Young & Middle-aged Teachers and Presidents Fund, the Qing Lan Project Fund of Jiangsu Province, Science and Technology Project Fund of Gaoxin District in Lianyungang (Grant No. HZ201906), the Fifth Phase of 521 Project Fund of Lianyungang, the Petrel Plan Project Fund of Lianyungang, and Science and Technology Funds of Kangda College of Nanjing Medical University (Grant No. KD2016GCCRCYJ01, KD2016GCCRCYJ02, KD201 8KYJJYB001 and KD2018KYJJYB010).
Declaration of competing interest
There is no potential conflict of interest to declare.
References
- 1. Ye YL, Guo HY, Sun XL. Recent progress on cell-based biosensors for analysis of food safety and quality control. Biosens Bioelectron. 2019;126:389–404. doi: 10.1016/j.bios.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 2. Martins FCOL, Sentanin MA, De Souza D. Analytical methods in food additives determination: compounds with functional applications. Food Chem. 2019;272:732–50. doi: 10.1016/j.foodchem.2018.08.060. [DOI] [PubMed] [Google Scholar]
- 3. Xiao DL, Jiang Y, Bi YP. Molecularly imprinted polymers for the detection of illegal drugs and additives: a review. Microchim Acta. 2018;185:247. doi: 10.1007/s00604-018-2735-4. [DOI] [PubMed] [Google Scholar]
- 4. Li GH, Xu L, Wu WQ, Wang D, Jiang J, Chen XM, et al. Onsite ultrasensitive detection paper for multiclass chemical contaminants via universal bridge-antibody labeling: mycotoxin and illegal additives in milk as an example. Anal Chem. 2019;91:1968–73. doi: 10.1021/acs.analchem.8b04290. [DOI] [PubMed] [Google Scholar]
- 5. Zhang WJ, Xue JH. Economically motivated food fraud and adulteration in China: an analysis based on 1553 media reports. Food Contr. 2016;67:192–8. [Google Scholar]
- 6. Handford CE, Campbell K, Elliott CT. Impacts of milk fraud on food safety and nutrition with special emphasis on developing countries. Compr Rev Food Sci F. 2016;15:130–42. doi: 10.1111/1541-4337.12181. [DOI] [PubMed] [Google Scholar]
- 7. Inbaraj BS, Chen BH. Nanomaterial-based sensors for detection of foodborne bacterial pathogens and toxins as well as pork adulteration in meat products. J Food Drug Anal. 2016;24:15–28. doi: 10.1016/j.jfda.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yuan ZQ, Hu CC, Chang HT, Lu C. Gold nanoparticles as sensitive optical probes. Analyst. 2016;141:1611–26. doi: 10.1039/c5an02651b. [DOI] [PubMed] [Google Scholar]
- 9. Chen YP, Xianyu YL, Jiang XY. Surface modification of gold nanoparticles with small molecules for biochemical analysis. Accounts Chem Res. 2017;50:310–9. doi: 10.1021/acs.accounts.6b00506. [DOI] [PubMed] [Google Scholar]
- 10. Qin L, Zeng GM, Lai C, Huang DL, Xu PA, Zhang C, et al. Gold rush” in modern science: fabrication strategies and typical advanced applications of gold nanoparticles in sensing. Coord Chem Rev. 2018;359:1–31. [Google Scholar]
- 11. Scanlon MD, Smirnov E, Stockmann TJ, Peljo P. Gold nanofilms at liquid-liquid interfaces: an emerging platform for redox electrocatalysis, nanoplasmonic sensors, and electrovariable optics. Chem Rev. 2018;118:3722–51. doi: 10.1021/acs.chemrev.7b00595. [DOI] [PubMed] [Google Scholar]
- 12. Xie ZP, Lei JL, Yang MF, Li YJ, Geng XH, Liu SM, et al. Conical nanofluidic channel for selective quantitation of melamine in combination with beta-cyclodextrin and a single-walled carbon nanotube. Biosens Bioelectron. 2019;127:200–6. doi: 10.1016/j.bios.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 13. Jigyasa Rajput JK. Bio-polyphenols promoted green synthesis of silver nanoparticles for facile and ultra-sensitive colorimetric detection of melamine in milk. Biosens Bioelectron. 2018;120:153–9. doi: 10.1016/j.bios.2018.08.054. [DOI] [PubMed] [Google Scholar]
- 14. Rao HB, Chen M, Ge HW, Lu ZW, Liu X, Zou P, et al. A novel electrochemical sensor based on Au@PANI composites film modified glassy carbon electrode binding molecular imprinting technique for the determination of melamine. Biosens Bioelectron. 2017;87:1029–35. doi: 10.1016/j.bios.2016.09.074. [DOI] [PubMed] [Google Scholar]
- 15. Li N, Liu T, Liu SG, Lin SM, Fan YZ, Luo HQ, et al. Visible and fluorescent detection of melamine in raw milk with one-step synthesized silver nanoparticles using carbon dots as the reductant and stabilizer. Sensor Actuator B Chem. 2017;248:597–604. [Google Scholar]
- 16. Ge XS, Wu XQ, Liang SX, Su M, Sun HW. Trace residue analysis of dicyandiamide, cyromazine, and melamine in animal tissue foods by ultra-performance liquid chromatography. J Food Drug Anal. 2016;24:579–85. doi: 10.1016/j.jfda.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen W, Deng HH, Hong L, Wu ZQ, Wang S, Liu AL, et al. Bare gold nanoparticles as facile and sensitive colorimetric probe for melamine detection. Analyst. 2012;137:5382–6. doi: 10.1039/c2an35962f. [DOI] [PubMed] [Google Scholar]
- 18. Xie LP, Zi XY, Zeng HDL, Sun JJ, Xu LS, Chen S. Low-cost fabrication of a paper-based microfluidic using a folded pattern paper. Anal Chim Acta. 2019;1053:131–8. doi: 10.1016/j.aca.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 19. Xiao C, Zhang XF, Liu JF, Yang AK, Zhao H, Li XJ, et al. Sensitive colorimetric detection of melamine with 1,4-dithiothreitol modified gold nanoparticles. Anal Methods. 2015;7:924–9. [Google Scholar]
- 20. Chang CC, Chen CP, Wu TH, Yang CH, Lin CW, Chen CY. Gold nanoparticle-based colorimetric strategies for chemical and biological sensing applications. Nanomaterials. 2019;9:861. doi: 10.3390/nano9060861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen XY, Ha W, Shi YP. Sensitive colorimetric detection of melamine in processed raw milk using asymmetrically PEGylated gold nanoparticles. Talanta. 2019;194:475–84. doi: 10.1016/j.talanta.2018.10.070. [DOI] [PubMed] [Google Scholar]
- 22. Xin JY, Zhang LX, Chen DD, Lin K, Fan HC, Wang Y, et al. Colorimetric detection of melamine based on methanobactin-mediated synthesis of gold nanoparticles. Food Chem. 2015;174:473–9. doi: 10.1016/j.foodchem.2014.11.098. [DOI] [PubMed] [Google Scholar]
- 23. Chen NN, Cheng YX, Li C, Zhang CL, Zhao K, Xian YZ. Determination of melamine in food contact materials using an electrode modified with gold nanoparticles and reduced graphene oxide. Microchim Acta. 2015;182:1967–75. [Google Scholar]
- 24. Genualdi S, MacMahon S, Robbins K, Farris S, Shyong N, DeJager L. Method development and survey of Sudan I-IV in palm oil and chilli spices in the Washington, DC, area. Food Addit Contam. 2016;33:583–91. doi: 10.1080/19440049.2016.1147986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berlina AN, Zherdev AV, Dzantiev BB. ELISA and lateral flow immunoassay for the detection of food colorants: state of the art. Crit Rev Anal Chem. 2019;49:209–23. doi: 10.1080/10408347.2018.1503942. [DOI] [PubMed] [Google Scholar]
- 26. Tsai CF, Kuo CH, Shih DYC. Determination of 20 synthetic dyes in chili powders and syrup-preserved fruits by liquid chromatography/tandem mass spectrometry. J Food Drug Anal. 2015;23:453–62. doi: 10.1016/j.jfda.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The rapid alert system for food and feed–2017 annual report. Luxembourg: European Union; 2018. [Accessed 14 September 2019]. Available at: http://ec.europa.eu/food/safety/rasff/index_en.htm. [Google Scholar]
- 28. Luo H, Chen LX, Ge QM, Liu M, Tao Z, Zhou YH, et al. Applications of macrocyclic compounds for electrochemical sensors to improve selectivity and sensitivity. J Incl Phenom Macro. 2019;95:171–98. [Google Scholar]
- 29. Ibrahim H, Temerk Y. Novel sensor for sensitive electrochemical determination of luteolin based on In2O3 nanoparticles modified glassy carbon paste electrode. Sensor Actuator B Chem. 2015;206:744–52. [Google Scholar]
- 30. Rasheed PA, Sandhyarani N. Electrochemical DNA sensors based on the use of gold nanoparticles: a review on recent developments. Microchim Acta. 2017;184:981–1000. [Google Scholar]
- 31. Thomas D, Vikraman AE, Jos T, Kumar KG. Kinetic approach in the development of a gold nanoparticle based voltammetric sensor for Sudan I. LWT - Food Sci Technol (Lebensmittel-Wissenschaft-Technol) 2015;63:1294–300. [Google Scholar]
- 32. Rezaei B, Boroujeni MK, Ensafi AA. Development of Sudan II sensor based on modified treated pencil graphite electrode with DNA, o-phenylenediamine, and gold nanoparticle bioimprinted polymer. Sensor Actuat B-chem. 2016;222:849–56. [Google Scholar]
- 33. Ashley J, Shahbazi MA, Kant K, Chidambara VA, Wolff A, Bang DD, et al. Molecularly imprinted polymers for sample preparation and biosensing in food analysis: progress and perspectives. Biosens Bioelectron. 2017;91:606–15. doi: 10.1016/j.bios.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 34. Wackerlig J, Schirhagl R. Applications of molecularly imprinted polymer nanoparticles and their advances toward industrial use: a review. Anal Chem. 2016;88:250–61. doi: 10.1021/acs.analchem.5b03804. [DOI] [PubMed] [Google Scholar]
- 35. Ahmad R, Griffete N, Lamouri A, Felidj N, Chehimi MM, Mangeney C. Nanocomposites of gold nanoparticles @ molecularly imprinted polymers: chemistry, processing, and applications in sensors. Chem Mater. 2015;27:5464–78. [Google Scholar]
- 36. Yu H, Feng X, Chen XX, Wang SS, Jin J. A highly sensitive determination of sulfite using a glassy carbon electrode modified with gold nanoparticles-reduced graphene oxide nano-composites. J Electroanal Chem. 2017;801:488–95. [Google Scholar]
- 37. Li JH, Feng HB, Li J, Feng YL, Zhang YQ, Jiang JB, et al. Fabrication of gold nanoparticles-decorated reduced graphene oxide as a high performance electrochemical sensing platform for the detection of toxicant Sudan I. Electrochim Acta. 2015;167:226–36. [Google Scholar]
- 38. He ZQ, Alexandridis P. Ionic liquid and nanoparticle hybrid systems: emerging applications. Adv Colloid Interfac. 2017;244:54–70. doi: 10.1016/j.cis.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 39. Wang ML, Chen ZN, Chen YM, Zhan CJ, Zhao JW. New synthesis of self-assembly ionic liquid functionalized reduced graphene oxide-gold nanoparticle composites for electrochemical determination of Sudan I. J Electroanal Chem. 2015;756:49–55. [Google Scholar]
- 40. Zhang W, Wang PL, Su XO. Current advancement in analysis of β-agonists. Trac Trends Anal Chem. 2016;85:1–16. [Google Scholar]
- 41. Chen D, Yang M, Zheng NJ, Xie N, Liu DL, Xie CF, et al. A novel aptasensor for electrochemical detection of ractopamine, clenbuterol, salbutamol, phenylethanolamine and procaterol. Biosens Bioelectron. 2016;80:525–31. doi: 10.1016/j.bios.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 42. Anderson SD. Repurposing drugs as inhaled therapies in asthma. Adv Drug Deliv Rev. 2018;133:19–33. doi: 10.1016/j.addr.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 43. Sobieraj DM, Weeda ER, Nguyen E, Coleman CI, White M, Lazarus SC, et al. Association of inhaled corticosteroids and long-acting beta-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma A systematic review and meta-analysis. JAMA, J Am Med Assoc. 2018;319:1485–96. doi: 10.1001/jama.2018.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang MT, Lai JH, Tsai CL, Liou JT. Risk of adverse cardiovascular events with use of inhaled long-acting bronchodilators in management of chronic obstructive pulmonary disease. J Food Drug Anal. 2019;27:657–70. doi: 10.1016/j.jfda.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li CL, Li JY, Jiang WX, Zhang SX, Shen JZ, Wen K, et al. Development and application of a gel-based immunoassay for the rapid screening of salbutamol and ractopamine residues in pork. J Agric Food Chem. 2015;63:10556–61. doi: 10.1021/acs.jafc.5b04203. [DOI] [PubMed] [Google Scholar]
- 46. Lee HC, Chen CM, Wei JT, Chiu HY. Analysis of veterinary drug residue monitoring results for commercial livestock products in Taiwan between 2011 and 2015. J Food Drug Anal. 2018;26:565–71. doi: 10.1016/j.jfda.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang MY, Zhu W, Ma L, Ma JJ, Zhang DE, Tong ZW, et al. Enhanced simultaneous detection of ractopamine and salbutamol - via electrochemical-facial deposition of MnO2 nanoflowers onto 3D RGO/Ni foam templates. Biosens Bioelectron. 2016;78:259–66. doi: 10.1016/j.bios.2015.11.062. [DOI] [PubMed] [Google Scholar]
- 48. Kang JY, Zhang YJ, Li X, Miao LJ, Wu AG. A rapid colorimetric sensor of clenbuterol based on cysteamine-modified gold nanoparticles. Acs Appl Mater Inter. 2016;8:1–5. doi: 10.1021/acsami.5b09079. [DOI] [PubMed] [Google Scholar]
- 49. Yola ML, Atar N. Simultaneous determination of beta-agonists on hexagonal boron nitride nanosheets/multi-walled carbon nanotubes nanocomposite modified glassy carbon electrode. Mat Sci Eng C-Mater. 2019;96:669–76. doi: 10.1016/j.msec.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 50. Wang P, Wang Z, Su X. A sensitive and quantitative fluorescent multi-component immuno-chromatographic sensor for β-agonist residues. Biosens Bioelectron. 2015;64:511–6. doi: 10.1016/j.bios.2014.09.064. [DOI] [PubMed] [Google Scholar]
- 51. Lin YP, Lee YL, Hung CY, Huang WJ, Lin SC. Determination of multiresidue analysis of beta-agonists in muscle and viscera using liquid chromatograph/tandem mass spectrometry with quick, easy, cheap, effective, rugged, and safe methodologies. J Food Drug Anal. 2017;25:275–84. doi: 10.1016/j.jfda.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yan KP, Zhang HQ, Hui WL, Zhu HL, Li XB, Zhong FY, et al. Rapid screening of toxic salbutamol, ractopamine, and clenbuterol in pork sample by high-performance liquid chromatography-UV method. J Food Drug Anal. 2016;24:277–83. doi: 10.1016/j.jfda.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Justino CIL, Freitas AC, Pereira R, Duarte AC, Santos TAPR. Recent developments in recognition elements for chemical sensors and biosensors. Trac Trends Anal Chem. 2015;68:2–17. [Google Scholar]
- 54. Sharma R, Ragavan KV, Thakur MS, Raghavarao KSMS. Recent advances in nanoparticle based aptasensors for food contaminants. Biosens Bioelectron. 2015;74:612–27. doi: 10.1016/j.bios.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 55. Jiang HY, Ling K, Tao XJ, Zhang QQ. Theophylline detection in serum using a self-assembling RNA aptamer-based gold nanoparticle sensor. Biosens Bioelectron. 2015;70:299–303. doi: 10.1016/j.bios.2015.03.054. [DOI] [PubMed] [Google Scholar]
- 56. Wang PL, Su XO, Shi L, Yuan YW. An aptamer based assay for the β-adrenergic agonist ractopamine based on aggregation of gold nanoparticles in combination with a molecularly imprinted polymer. Microchim Acta. 2016;183:2899–905. [Google Scholar]
- 57. Yang Y, Zhang H, Huang C, Yang D, Jia N. Electrochemical non-enzyme sensor for detecting clenbuterol (CLB) based on MoS2-Au-PEI-hemin layered nanocomposites. Biosens Bioelectron. 2017;89:461–7. doi: 10.1016/j.bios.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 58. Huang YX, Guo JH, Kang YJ, Ai Y, Li CM. Two dimensional atomically thin MoS2 nanosheets and their sensing applications. Nanoscale. 2015;7:19358–76. doi: 10.1039/c5nr06144j. [DOI] [PubMed] [Google Scholar]
- 59. Gassara F, Kouassi AP, Brar SK, Belkacemi K. Green alternatives to nitrates and nitrites in meat-based products-A review. Crit Rev Food Sci. 2016;56:2133–48. doi: 10.1080/10408398.2013.812610. [DOI] [PubMed] [Google Scholar]
- 60. Nam J, Jung IB, Kim B, Lee SM, Kim SE, Lee KN, et al. A colorimetric hydrogel biosensor for rapid detection of nitrite ions. Sensor Actuator B Chem. 2018;270:112–8. [Google Scholar]
- 61. Balasubramanian P, Settu R, Chen SM, Chen TW, Sharmila G. A new electrochemical sensor for highly sensitive and selective detection of nitrite in food samples based on sonochemical synthesized Calcium Ferrite (CaFe2O4) clusters modified screen printed carbon electrode. J Colloid Interface Sci. 2018;524:417–26. doi: 10.1016/j.jcis.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 62. Li XL, He XL, Dong YZ, Jia LR, He Q. Analysis of N-nitrosodiethylamine by ion chromatography coupled with UV photolysis pretreatment. J Food Drug Anal. 2016;24:311–5. doi: 10.1016/j.jfda.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gill A, Zajda J, Meyerhoff ME. Comparison of electrochemical nitric oxide detection methods with chemiluminescence for measuring nitrite concentration in food samples. Anal Chim Acta. 2019;1077:167–73. doi: 10.1016/j.aca.2019.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li XJ, Ping JF, Ying YB. Recent developments in carbon nanomaterial-enabled electrochemical sensors for nitrite detection. Trac Trends Anal Chem. 2019;113:1–12. [Google Scholar]
- 65. Li D, Ma YD, Duan HZ, Deng W, Li DW. Griess reaction-based paper strip for colorimetric/fluorescent/SERS triple sensing of nitrite. Biosens Bioelectron. 2018;99:389–98. doi: 10.1016/j.bios.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 66. Xiong YM, Li MM, Liu HQ, Xuan ZH, Yang J, Liu DB. Janus PEGylated gold nanoparticles: a robust colorimetric probe for sensing nitrite ions in complex samples. Nanoscale. 2017;9:1811–5. doi: 10.1039/c6nr07879f. [DOI] [PubMed] [Google Scholar]
- 67. Li DA, Song Y, He JL, Zhang MZ, Ni PH. Polymer-doxorubicin prodrug with biocompatibility, pH response, and main chain breakability prepared by catalyst-free click reaction. ACS Biomater Sci Eng. 2019;5:2307–15. doi: 10.1021/acsbiomaterials.9b00301. [DOI] [PubMed] [Google Scholar]
- 68. Jian JM, Fu LF, Ji JY, Lin LW, Guo XS, Ren TL. Electrochemically reduced graphene oxide/gold nanoparticles composite modified screen-printed carbon electrode for effective electrocatalytic analysis of nitrite in foods. Sensor Actuator B Chem. 2018;262:125–36. [Google Scholar]
- 69. Üzer A, Sağlam Ş, Can Z, Erçağ E, Apak R. Electrochemical determination of food preservative nitrite with gold nanoparticles/p-aminothiophenol-modified gold electrode. Int J Mol Sci. 2016;17:1253. doi: 10.3390/ijms17081253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pan F, Chen DD, Zhuang XM, Wu XR, Luan F, Zhang S, et al. Fabrication of gold nanoparticles/L-cysteine functionalized graphene oxide nanocomposites and application for nitrite detection. J Alloys Compd. 2018;744:51–6. [Google Scholar]
- 71. Nascimento CF, Santos PM, Pereira-Filho ER, Rocha FRP. Recent advances on determination of milk adulterants. Food Chem. 2017;221:1232–44. doi: 10.1016/j.foodchem.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 72. Kumar P, Lambadi PR, Navani NK. Non-enzymatic detection of urea using unmodified gold nanoparticles based aptasensor. Biosens Bioelectron. 2015;72:340–7. doi: 10.1016/j.bios.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 73. Kumar P, Kumar P, Manhas S, Navani NK. A simple method for detection of anionic detergents in milk using unmodified gold nanoparticles. Sensor Actuator B Chem. 2016;233:157–61. [Google Scholar]
- 74. Chappell G, Pogribny IP, Guyton KZ, Rusyn I. Epigenetic alterations induced by genotoxic occupational and environmental human chemical carcinogens: a systematic literature review. Mutat Res-Rev Mutat. 2016;768:27–45. doi: 10.1016/j.mrrev.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Guzman JMCC, Tayo LL, Liu CC, Wang YN, Fu LM. Rapid microfluidic paper-based platform for low concentration formaldehyde detection. Sensor Actuator B Chem. 2018;255:3623–9. [Google Scholar]
- 76. Aini BN, Siddiquee S, Ampon K. Development of formaldehyde biosensor for determination of formalin in fish samples; malabar red snapper (lutjanus malabaricus) and longtail tuna (thunnus tonggol) Biosensors. 2016;6:32. doi: 10.3390/bios6030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Xie T, Jing C, Long YT. Single plasmonic nanoparticles as ultrasensitive sensors. Analyst. 2017;142:409–20. doi: 10.1039/c6an01852a. [DOI] [PubMed] [Google Scholar]
- 78. Priyadarshini E, Pradhan N. Gold nanoparticles as efficient sensors in colorimetric detection of toxic metal ions: a review. Sensor Actuator B Chem. 2017;238:888–902. [Google Scholar]
- 79. Li Y, Wang ZX, Sun L, Liu LQ, Xu CL, Kuang H. Nanoparticle-based sensors for food contaminants. Trac Trends Anal Chem. 2019;113:74–83. [Google Scholar]
- 80. Jiang Y, Shi ML, Liu Y, Wan S, Cui C, Zhang LQ, et al. Aptamer/AuNP biosensor for colorimetric profiling of exosomal proteins. Angew Chem Int Ed. 2017;56:11916–20. doi: 10.1002/anie.201703807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chavez JL, Hagen JA, Kelley-Loughnane N. Fast and selective plasmonic serotonin detection with aptamer-gold nanoparticle conjugates. Sensors. 2017;17:681. doi: 10.3390/s17040681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cui L, Wu J, Ju HX. Electrochemical sensing of heavy metal ions with inorganic, organic and bio-materials. Biosens Bioelectron. 2015;63:276–86. doi: 10.1016/j.bios.2014.07.052. [DOI] [PubMed] [Google Scholar]
- 83. Ben Messaoud N, Ghica ME, Dridi C, Ben Ali M, Brett CMA. Electrochemical sensor based on multiwalled carbon nanotube and gold nanoparticle modified electrode for the sensitive detection of bisphenol A. Sensor Actuator B Chem. 2017;253:513–22. [Google Scholar]
- 84. Guo W, Pi FW, Zhang HX, Sun JD, Zhang YZ, Sun XL. A novel molecularly imprinted electrochemical sensor modified with carbon dots, chitosan, gold nanoparticles for the determination of patulin. Biosens Bioelectron. 2017;98:299–304. doi: 10.1016/j.bios.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 85. Shankar S, Gowthaman NSK, John SA. Synthesis of albumin capped gold nanoparticles and their direct attachment on glassy carbon electrode for the determination of nitrite ion. J Electroanal Chem. 2018;828:33–40. [Google Scholar]
- 86. Bajpai VK, Kamle M, Shukla S, Mahato DK, Chandra P, Hwang SK, et al. Prospects of using nanotechnology for food preservation, safety, and security. J Food Drug Anal. 2018;26:1201–14. doi: 10.1016/j.jfda.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. He XJ, Deng H, Hwang HM. The current application of nanotechnology in food and agriculture. J Food Drug Anal. 2019;27:1–21. doi: 10.1016/j.jfda.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.